3 Management of Acute Pain in the Intensive Care Unit
Critically ill patients frequently experience acute pain, but assessment rates for pain remain below 40% in mechanically ventilated patients.1 Pain and discomfort can have multiple causes in the intensive care unit (ICU) setting, including surgical and posttraumatic wounds, the use of invasive monitoring devices and mechanical ventilators, prolonged immobilization, and routine nursing care (e.g., dressing changes, airway suctioning).1–3 Pain is defined by the International Association for the Study of Pain (IASP-1979) as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage.”4 The experience of pain differs among patients, but the physiologic sequelae of inadequately treated pain are relatively predictable and potentially deleterious. Some physiologic responses to acute pain and stress are mediated by neuroendocrine activation and increased sympathetic tone. As a consequence, patients develop tachycardia, increased myocardial oxygen consumption, immunosuppression, hypercoagulability, persistent catabolism, and numerous other metabolic alterations.5 Additional morbidity may be incurred by pain-related functional limitations such as impaired pulmonary mechanics6 or delayed ambulation.
 Acute Pain Assessment
Acute Pain Assessment
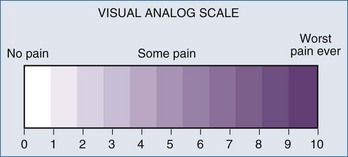
The past decade has seen an increase in the number of scales and assessment tools for the evaluation of sedation and analgesia in ICU patients. Several sedation scales—the Richmond Agitation Sedation Scale (RASS), Adaptation to the Intensive Care Environment (ATICE) tool, and the Minnesota Sedation Assessment Tool (MSAT)—as well as tools for assessment of analgesia in the ICU, such as the visual analog scale, the numeric rating scale, behavioral pain scale,7,8 and critical care pain observation scale, have been developed (Figure 3-1). The actual percentage of ICUs implementing formal sedation and analgesia protocols is approximately 50% in the United States. Unfortunately, many ICU patients cannot provide full (or even partial) information regarding their pain. However, the inability of ventilated, sedated ICU patients to report pain should not preclude pain management and does not rule out the possibility that the patients are experiencing pain.9 Caregivers sometimes must use signs of heightened sympathetic activity like hypertension, tachycardia, lacrimation, diaphoresis, and restlessness as surrogate indicators for the presence of pain. Trends in such signs provide a measure of the success of a given intervention.
 Options for Acute Pain Therapy
Options for Acute Pain Therapy
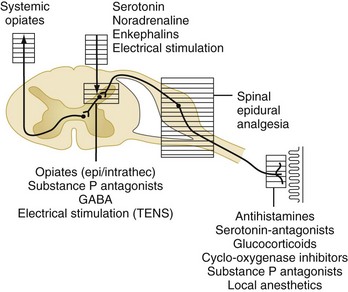
Acute pain is triggered by stimulation of peripheral nociceptors in the skin or deeper structures and is a complex process involving multiple mediators at various levels of the neuraxis (Figure 3-2).4 Different parts of the pain pathway can be targeted either individually or as part of a comprehensive strategy aimed at multiple sites for additive or synergistic effects. Thus, nociception can be influenced (1) peripherally by the use of nonsteroidal antiinflammatory drugs (NSAIDs) and nerve blocks, (2) at the spinal cord level by the use of epidural or intrathecal medications, and (3) centrally by the use of systemic medications.
Nonsteroidal Antiinflammatory Drugs
Drugs in this class inhibit cyclooxygenase (COX) enzymes, which are involved in synthesis of prostaglandins and related inflammatory mediators in response to injury. COX-1 is a constitutive enzyme that is present in most tissues and, through the production of prostaglandins E2 and I2, serves homeostatic and protective functions.10 COX-2 is an inducible enzyme that is expressed in response to inflammation. NSAIDs are commonly used in conjunction with other agents such as opioids to take advantage of different side-effect profiles and possible synergistic efficacy. As a class, NSAIDs can cause adverse effects that include nausea, gastrointestinal (GI) bleeding, inhibition of platelet function, operative site bleeding, renal insufficiency, and bronchospasm in aspirin-sensitive patients (triad of asthma, nasal polyposis, and aspirin allergy).2,4
Ketorolac tromethamine (Toradol) is the only parenteral NSAID available in the United States. It has been shown to reduce postoperative opioid requirements and does not cause respiratory depression.11 However, prolonged use has been associated with a significant incidence of the aforementioned side effects (primarily GI bleeding and renal failure)12; consequently, ketorolac therapy should be limited to a maximum of 5 days.2 In addition, ketorolac should be used at decreased dosages or avoided altogether in patients at higher risk of such complications (e.g., advanced age, hypovolemia, preexisting renal insufficiency). This caution also applies to enterally administered NSAIDs.
Selective COX-2 inhibitors like celecoxib (Celebrex) are available for enteral administration, and injectable COX-2 agents are being studied primarily for the management of acute postoperative pain.10 The main advantage of these agents over their nonselective relatives lies in the promise of decreased GI side effects.10 A joint meeting of the U.S. Food and Drug Administration (FDA) Arthritis Advisory Committee and the Drug Safety and Risk Management Advisory Committee reaffirmed that COX-2 inhibitors are important treatment options for pain management and that the preponderance of data demonstrate that the cardiovascular risk associated with celecoxib is similar to that associated with commonly used older nonspecific NSAIDs.13
Acetaminophen is a paraaminophenol derivative with analgesic and antipyretic properties similar to those of aspirin. The mechanism of action of acetaminophen is still poorly defined. Recent evidence has suggested that it may selectively act as an inhibitor of prostaglandin synthesis in the central nervous system (CNS) rather than in the periphery. A meta-analysis of randomized controlled trials of acetaminophen for postoperative pain revealed that this analgesic induced a morphine-sparing effect of 20% (9 mg) over the first 24 hours postoperatively but did not reduce the incidence of morphine-related adverse effects.14 It was concluded that acetaminophen may be a viable alternative to NSAIDs in high-risk patients because of the lower incidence of adverse effects. Therefore, it may be appropriate to administer acetaminophen with NSAIDs or COX-2 inhibitors, since the analgesics in these two classes may act additively or synergistically to improve analgesia.15
Opioid Analgesics
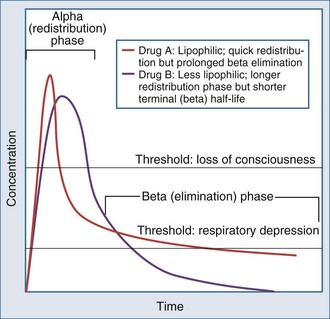
A number of opioids are available (Table 3-1), and this drug class remains the mainstay of ICU analgesia. Morphine, hydromorphone (Dilaudid), and fentanyl are commonly used in ICUs in the United States and have been recommended as first-line narcotic analgesics.2 Opioids bind to a variable degree with opioid receptor subtypes (µ, δ, κ) located in the brain, spinal cord, and peripheral sites and modulate the transmission and processing of nociceptive signals.4 The clinical and pharmacologic properties of opioids depend on several variables: chemical and solubility properties, dosing regimen, patient characteristics (Box 3-1), and presence of active metabolites. Drugs that are often thought of as short acting (e.g., fentanyl) actually have a markedly prolonged duration of action if given repeatedly or as an infusion (Figure 3-3).
Opioids are most commonly administered intravenously in critically ill patients and titrated to effect, either on a scheduled, intermittent basis or as a continuous infusion following a loading dose to achieve analgesia.2 This strategy avoids concerns regarding unpredictable bioavailability associated with intramuscular, enteral, or transdermal administration and favors more stable analgesic drug concentrations. The benefits of administering analgesics (and sedatives) in such a fashion must be balanced against the possibility of unintentional excessive dosing, which may result in prolonged mechanical ventilation and longer hospital stays.1 It has been reported, however, that scheduled daily interruption of sedative-analgesic drug infusions can help minimize this problem and may actually lead to a shorter duration of mechanical ventilation and a shorter ICU stay.16,17
Morphine is a naturally occurring narcotic analgesic.2 It is metabolized mainly by the liver to an active compound (morphine-6-glucuronide) that can cause a prolonged drug effect in patients with renal insufficiency. Onset of action after intravenous (IV) administration is relatively slow (5-10 minutes) owing to low lipid solubility, and the duration of clinical effect is long enough to permit its use as either an intermittent injection or an infusion. Dosing requirements vary significantly from patient to patient and must be individualized (see Table 3-1).
Hydromorphone is a semisynthetic narcotic. Compared to morphine, hydromorphone has a similar duration of action, is a more potent analgesic, does not release histamine, and lacks an active metabolite. These properties make it an attractive alternative to morphine in patients with hemodynamic instability or significant renal impairment.2 Hydromorphone is also best administered by either infusion or intermittent injection.
Fentanyl is a synthetic narcotic with a potency about 100 times that of morphine. Fentanyl has no active metabolites and generally has minimal effects on hemodynamics. It is very lipophilic, leading to a rapid onset of action. Fentanyl can accumulate in fat, giving rise to a prolonged drug effect, if it is given in very high doses or for a lengthy period, even in patients without significant renal or hepatic dysfunction.2
N-Methyl-D-Aspartate Receptor Antagonist
Ketamine in combination with either parenteral or epidural opioids not only reduces postoperative opioid consumption but also prolongs and improves analgesia.18,19,20 However, despite the opioid-sparing effect observed with the administration of ketamine, no reduction in opioid-related side effects has been documented.
Alpha-2 Adrenergic Agonists
Clonidine was originally used to control blood pressure (BP) and heart rate. It binds to α2-adrenergic and imidazole receptors in the CNS. It has been hypothesized that clonidine acts at α2-adrenergic receptors in the spinal cord to stimulate acetylcholine release, which acts at both muscarinic and nicotinic receptor subtypes for postoperative pain relief. Clonidine can be administered by oral, IV, or transdermal routes.21
Neuraxial Analgesic Techniques
The administration of narcotics, local anesthetics, and other agents via intrathecal or epidural catheters targets the processing of pain signals at the level of the spinal cord or nerve root.4 The use of epidural catheters for regional analgesia in ICU patients may be quite useful, assuming that the pain pattern is regionalized and that there are no contraindications to catheter placement (e.g., coagulopathy, uncontrolled infection, unstable spinal skeletal structures). In some patients, epidural analgesia may be preferable to intravenously administered medications, because this approach affords dense regional pain control4,22 while largely avoiding the sedative and respiratory side effects of systemic medications.22,23
Peripheral Nerve Blocks
Peripheral nerve blocks are an attractive method of providing postoperative analgesia for many orthopedic surgical procedures. Compared with general anesthesia, the use of peripheral nerve blocks achieved by either a single injection or by continuous infusion via a catheter for orthopedic anesthesia/analgesia has been associated with faster recovery times and decreased hospital readmission rates.24
On the basis of a recent meta-analysis,25,26 continuous peripheral analgesic techniques provide superior analgesia, reduce opioid consumption, and reduce opioid-related side effects (nausea and vomiting, sedation, pruritus). This technique is not commonly used in the ICU setting, but it opens a wide range of possibilities for the future treatment of acute pain in critically ill patients.
Payen JF, Bosson JL, Chanques G, Mantz J, Labarere J. Pain assessment is associated with decreased duration of mechanical ventilation in the intensive care unit. A post hoc analysis of the DOLOREA Study. for the DOLOREA investigators. Anesthesiology. 2009;111(6):1187-1188.
Kumar A, Brennan T. Pain assessment, sedation, and analgesic administration in the intensive care unit. Anesthesiology. 2009;111(6):1308-1316.
Richman JM, Liu SS, Courpas G, et al. Does continuous peripheral nerve block provide superior pain control to opioids? A meta-analysis. Anesth Analg. 2006;102(1):248-257.
Gilron I, Milne B, Hong M. Cyclooxygenase-2 inhibitors in postoperative pain management: current evidence and future directions. Anesthesiology. 2003;99(5):1198-1208.
An up-to-date review of COX-2 inhibitors for analgesia in the postoperative period.
Jacobi J, Fraser GL, Coursin DB, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30(1):119-141.
Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342(20):1471-1477.
1 Payen JF, Bosson JL, Chanques G, Mantz J, Labarere J. Pain assessment is associated with decreased duration of mechanical ventilation in the intensive care unit. A post hoc analysis of the DOLOREA study. for the DOLOREA investigators. Anesthesiology. 111(6), 2009.
2 Jacobi J, Fraser GL, Coursin DB, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30(1):119-141.
3 Higgins TL, Jodka PG, Farid A. Pharmacologic approaches to sedation, pain relief and neuromuscular blockade in the intensive care unit. Part II. Clin Intensive Care. 2003;14(3-4):91-98.
4 Stevens DS, Edwards WT. Management of pain in the critically ill. In: Irwin RS, Rippe JM, editors. Intensive Care Medicine. Philadelphia: Lippincott Williams & Wilkins; 2003:1732-1750.
5 Ready LB. Acute perioperative pain. In: Miller RD, editor. Anesthesia. Philadelphia: Churchill Livingstone; 2000:2323-2350.
6 Desai PM. Pain management and pulmonary dysfunction. Crit Care Clin. 1999;15:151-166.
7 Terai T, Yukioka H, Asada A. Pain evaluation in the intensive care unit: Observer-reported faces scale compared with self-reported visual analog scale. Reg Anesth Pain Med. 1998;23:147-151.
8 Puntillo KA, Miaskowski C, Kehrle K, et al. Relationship between behavioral and physiological indicators of pain, critical care patients’ self-reports of pain, and opioid administration. Crit Care Med. 1997;25:1159-1166.
9 Kumar A, Brennan T. Pain assessment, sedation, and analgesic administration in the intensive care unit. Anesthesiology. 111(6), Dec 2009.
10 Gilron I, Milne B, Hong M. Cyclooxygenase-2 inhibitors in postoperative pain management: Current evidence and future directions. Anesthesiology. 2003;99:1198-1208.
11 Ready LB, Brown CR, Stahlgren LH, et al. Evaluation of intravenous ketorolac administered by bolus or infusion for treatment of postoperative pain: A double-blind, placebo-controlled, multicenter study. Anesthesiology. 1994;80:1277-1286.
12 Feldman HI, Kinman JL, Berlin JA, et al. Parenteral ketorolac: The risk for acute renal failure. Ann Intern Med. 1997;126:193-199.
13 Young D. FDA labors over NSAID decisions: panel suggests COX-2 inhibitors stay available. Am J Health Syst Pharm. 2005;62:668-672.
14 Hyllested M, Jones S, Pedersen JL, Kehlet H. Comparative effect of paracetamol, NSAIDs, or their combination in postoperative pain management: a qualitative review. Br J Anaesth. 2002;88:199-214.
15 Sinatra R. Role of COX-2 inhibitors in the evolution of acute pain management. J Pain Symptom Manage. 2002;24:S18-S27.
16 Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471-1477.
17 Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): A randomized controlled trial. Lancet. 2008;371:126-134.
18 Schmid RL, Sandler AN, Katz J. Use and efficacy of low-dose ketamine in the management of acute postoperative pain: a review of current techniques and outcomes. Pain. 1999;82:111-125.
19 Subramaniam K, Subramaniam B, Steinbrook RA. Ketamine as adjuvant analgesic to opioids: A quantitative and qualitative systematic review. Anesth Analg. 2004;99:482-495.
20 Adam F, Chauvin M, Du Manoir B, Langlois M, Sessler DI, Fletcher D. Small-dose ketamine infusion improves postoperative analgesia and rehabilitation after total knee arthroplasty. Anesth Analg. 2005;100:475-480.
21 Hidalgo MP, Auzani JA, Rumpel LC, et al. The clinical effect of small oral clonidine doses on perioperative outcomes in patients undergoing abdominal hysterectomy. Anesth Analg. 2005;100:795-802.
22 Parker MJ, Handoll HH, Griffiths R. Anesthesia for hip fracture in adults. Cochrane Database Syst Rev 2001;4:CD 000521.
23 Scheini H, Virtanen T, Kentala E, et al. Epidural infusion of bupivacaine and fentanyl reduces perioperative myocardial ischemia in elderly patients with hip fracture—a randomized controlled trial. Acta Anaesthesiol Scand. 2000;44:1061-1070.
24 Matot I, Oppenhein-Eden A, Ratrot R, et al. Preoperative cardiac events in elderly patients with hip fracture randomized to epidural or conventional analgesia. Anesthesiology. 2003;98:156-163.
25 McCartney CJL, Brull R, Chan VS, et al. Early but no long-term benefit of regional compared with general anesthesia for ambulatory hand surgery. Anesthesiology. 2004;101:461-467.
26 Richman JM, Liu SS, Courpas G, et al. Does continuous peripheral nerve block provide superior pain control to opioids? A meta-analysis. Anesth Analg. 2006;102:248-257.