Chapter 27 Management of Acute Knee Dislocation before Surgical Intervention
CLASSIFICATION
A patient with two or more torn major ligaments may have sustained a knee dislocation.54 This definition accounts not only for those knees that are dislocated at the time of evaluation but also for those that have spontaneously reduced. Clinically, the degree of ligament disruption can be misleading. Because ligaments can stretch and may not appear completely disrupted, especially on MRI, the severity of these injuries can be easily underestimated.
The following variables are the basis for classification and complete description of a dislocated knee: (1) direction of dislocation, (2) open or closed, (3) high- or low-energy trauma, and (4) time from dislocation.16 Schenck50 has also recommended that the extent of ligamentous injury be included in the classification of knee dislocations (see Chapter 26, Classification of Knee Dislocations).
The direction of dislocation may be unidimensional (anterior, posterior, medial, or lateral) or a rotatory combination. If the knee has spontaneously reduced, classification is based on direction of instability.46 Anterior dislocations caused by hyperextension of the knee are the most common, occurring in 40% according to Green and Allen’s review17 of 245 knee dislocations. Posterior dislocations, often caused by high-energy dashboard mechanisms, accounted for 33% of the cases. Medial and lateral dislocations were present in 18% and 4% of the cases, respectively.
In 1963, Kennedy’s biomechanical study26 in cadavers elucidated the mechanisms for both anterior and posterior dislocations. This investigation demonstrated that by progressively hyperextending the knee, the posterior capsule ruptured followed by the PCL and the ACL. Without adequate ligamentous restraint, the knee dislocated in an anterior direction. A high posteriorly directed force on the proximal tibia in knee flexion caused a posterior dislocation.
Posterolateral dislocations are the most common type of rotatory dislocations. The flexed and non–weight-bearing knee undergoes a sudden rotatory moment that abducts and internally rotates the lower leg on the femur.44 This causes the medial femoral condyle to “buttonhole” through the anteromedial capsule, which may be irreducible. Inspection of the knee will reveal a transverse groove in the skin along the medial joint line secondary to invagination of the medial capsule. This “dimple sign” becomes more prominent with attempted reduction and is pathognomonic of the irreducible posterolateral dislocation.16,38,45,48,49
Open dislocations of the knee are emergencies that require antibiotics and urgent irrigation and débridement in the operating room. Open injuries, present in 19% to 35% of knee dislocations, are most commonly seen with anterior and posterior mechanisms.36,55
VASCULAR INJURIES
Arterial trauma associated with knee dislocations represents limb-threatening injuries. The incidence of popliteal artery disruption during a knee dislocation is 32% to 45%.17,24 Delay in diagnosis longer than 6 to 8 hours is associated with an increasing amputation rate.17,24 Because of the high rate of injury and the devastating consequences associated with a delay in diagnosis, it is imperative to recognize vascular injuries early. An abnormal vascular examination should prompt an immediate vascular surgery consultation.
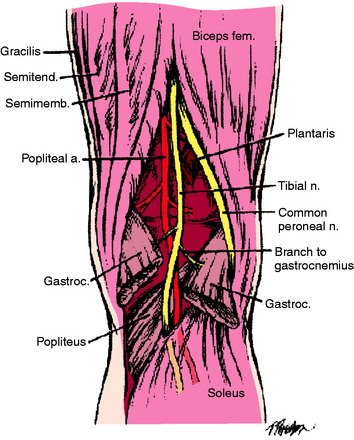
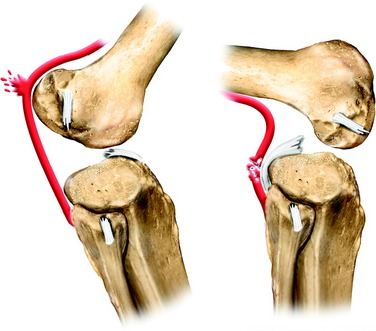
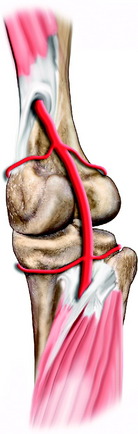
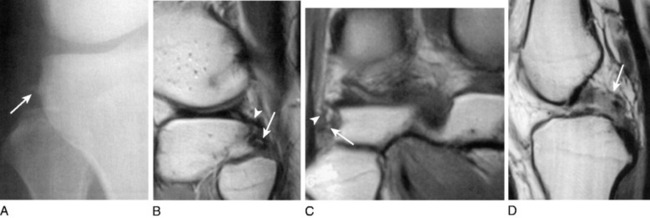
The popliteal artery is at risk of injury during a knee dislocation owing to its anatomic location (Fig. 27-1). The popliteal artery is tethered proximally by the adductor hiatus and distally by the soleus arch. The artery bifurcates into the anterior and posterior branches at the soleus arch. Collateral circulation in the form of the genicular arteries originates within the popliteal fossa. The geniculate arterial system is usually not sufficient to perfuse the limb should there be obstruction or transection of the popliteal artery.16,40 Anterior dislocations of the knee can place significant traction on the popliteal artery, leading to extensive intimal injury and/or vasospasm (Fig. 27-2).17 Posterior dislocations can result in complete transection of the artery (Fig. 27-3).41
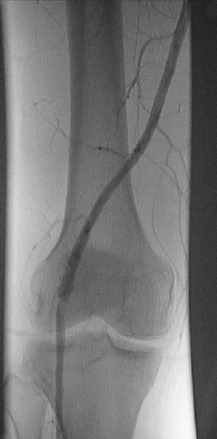
FIGURE 27-2 Angiogram demonstrates diffuse irregularity in the popliteal artery with intimal dissection.
Critical Points VASCULAR INJURIES
The diagnosis of a vascular injury should be made on the assimilation of a variety of clinical and diagnostic tests including physical examination, ankle/brachial index (ABI), and arteriography. The dorsalis pedis arterial pulse is palpated on dorsum of the foot, and the posterior tibial arterial pulse is palpable posterior to the medial malleolus. The presence of these pulses with a warm foot is not a guarantee that an arterial injury is not present. There are multiple reports of vascular occlusion or intimal tears with normal distal pulses; the incidence is reported to be 5% to 15%.1,24,33,61 In 2004, Barnes and coworkers4 performed a meta-analysis that included 284 multiligamentous knee injuries in seven studies. Seven of 52 patients requiring vascular surgery had normal pulses on presentation. Thirteen of 140 patients with normal pulses who underwent arteriography had nonocclusive intimal defects that were safely observed. Abnormal pulses had a sensitivity rate of 0.79 (95% confidence interval [CI], 0.64–0.89), specificity rate of 0.91 (95% CI, 0.78–0.96), positive predictive value of 0.75 (95% CI, 0.61–0.83), and a negative predictive value of 0.93 (95% CI, 0.85–0.96). The low sensitivity and specificity may be associated with hypotension in the acutely traumatized patient, as well as intimal lesions that may initially present with normal pulses.
The ABI is the second diagnostic tool to be used. ABIs are determined by measuring the systolic blood pressure for all four extremities using a Doppler probe and a standardized blood pressure cuff. For the lower extremity, the systolic pressure is measured at the posterior tibial and dorsalis pedis arteries. ABI is then calculated by dividing the highest measured pressure in the ankle or foot by the higher of the brachial arterial pressures. An ABI of less than 0.90 is considered abnormal and should prompt arteriography. In 2004, Mills and associates37 demonstrated that 11 of 11 patients with ABIs less than 0.90 required a reverse saphenous vein graft for popliteal artery injury (3 popliteal artery transections, 6 popliteal artery occlusions, 1 common femoral and peroneal artery thrombosis, and 1 high-grade superficial femoral artery occlusion with intimal flap altering popliteal artery flow). None of the 27 patients with ABIs greater than 0.90 had evidence of vascular injury by serial examination or duplex ultrasonography. The sensitivity and specificity was 100%. In 1998, Cole and colleagues8 evaluated 70 consecutive patients with blunt trauma and found no arterial injury with ABI greater than 0.90, whereas an ABI lower than 0.90 had a positive predictive value of 88%. Lynch and Johansen31 studied blunt and penetrating trauma in 100 consecutive patients and found an ABI less than 0.90 predicted vascular surgery with a sensitivity of 87%, specificity of 97%, and positive predictive value of 91%.
Arteriography, developed in the 1960s, has improved outcomes and reduced the number of negative explorations. Many authors have advocated routine use of arteriography to evaluate the limb after a suspected knee dislocation.1,9,17,25,26,30,32,33,67 The routine use of arteriograms for suspected knee dislocations is justified by the relative low morbidity of the test, the high incidence of popliteal injury, and the devastating consequences of a delay in diagnosis.46 However, clinicians should be aware that not all arterial trauma is detectable with arteriography. Stretch injuries of the intimal wall and small intimal flap tears may not be visualized on the arteriogram, but may subsequently clot and result in arterial occlusion. Those patients who worsen usually do so within the first 3 months, but more commonly, within a week after injury. McDonough and Wojtys34 reported on three patients with normal initial arteriograms. Two patients who had loss of pulse after tourniquet release during ligament reconstruction were found to have chronic intimal flaps with thrombi at immediate revascularization. One patient developed a pseudoaneurysm, which was recognized by a second arteriorgram after there was significant postoperative swelling.34
Arteriograms can be performed in the operating room if there are obviously diminished pulses or if the delay in obtaining a formal arteriogram in the angiography suite would compromise limb survival. Six to 8 hours of warm ischemic time will significantly compromise a lower extremity.17 Formal angiography is performed with a femoral artery puncture followed by insertion of a pigtail catheter. Next, a bolus of contrast is injected and an angiographic unit is used to capture images. The length of the segment to be imaged determines the number of acquisitions needed. Intraoperative arteriogram can be performed by injecting 45 mL of contrast through an 18-gauge catheter with a single film exposure35 or a vascular fluoroscopic unit.
Unfortunately, arteriography is not without risks, costs, and false-negative results. The complication rate has been reported to be 1.7% and includes thrombosis, arteriovenous fistulae, bleeding, renal failure, reaction to contrast material, and pseudoaneurysm formation.3,27,37,61,66 The reported false-positive results have ranged from 2.4% to 7%, which could lead to unnecessary surgical intervention.3,58,61,66 The cost of the arteriograms ranged from $750 to $1,500 per study in 1990; a more recent figure was $5,240 in 2003.3,58,61
In addition to formal angiography, new techniques such as computed tomographic angiography (CTA) and magnetic resonance angiography (MRA) are being developed. These techniques make use of readily available imaging techniques often with a bolus of contrast to define the lower extremity arterial system. Potter and coworkers43 demonstrated 100% correlation between conventional angiography and MRA in a small cohort of patients (six patients: four normal, one intimal tear, one decreased flow).
NEUROLOGIC INJURIES
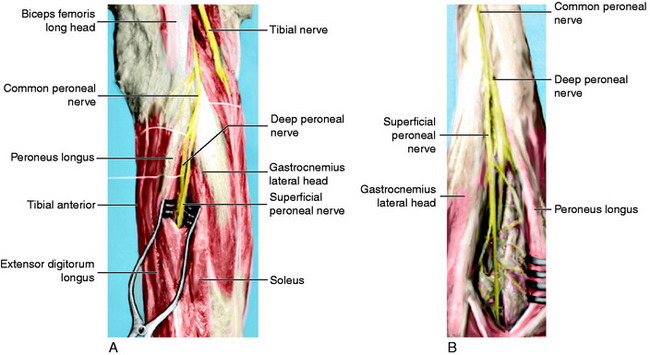
Neurologic damage occurs in 16% to 40% of knee dislocations.2,57 This may range from a stretch injury (neurapraxia) to a complete transection (neurotmesis). The peroneal nerve is most commonly injured as it is stretched over the fibular neck during a posterolateral dislocation (Fig. 27-4).14,47,60,62,67 A common presentation for peroneal nerve injuries is a patient with a drop foot and sensory deficit over the dorsum of the foot. Tibial nerve injury associated with a popliteal artery injury has also been reported.67 Although difficult, a complete neurologic examination of both lower extremities should be performed both before and after any attempt at reducing a knee dislocation. Repeated examinations are recommended during the course of treatment because there is a potential for nerve compromise due to soft tissue swelling and compartment syndrome, pressure, immobilization, and/or positioning.
Critical Points NEUROLOGIC INJURIES
In the posterior midthigh, the sciatic nerve divides into the common peroneal nerve and the tibial nerve (Fig. 27-5).29 The common peroneal nerve courses obliquely through the popliteal fossa, then traverses along the fibular neck in an exposed subcutaneous fibro-osseous tunnel.52,60 This subcutaneous position adjacent to the fibula makes the nerve susceptible to direct contact during sports and to indirect injury during a forced varus stress as it is stretched over the fibular neck.19 The tibial nerve lies lateral to the popliteal vessels within the popliteal fossa. As it courses distally, it crosses posterior to the vessels and then lies medially. It passes between the two heads of the gastrocnemius muscle and anterior to the arch of the soleus muscle to lie in the deep posterior compartment of the lower leg. Within the popliteal fossa, the tibial nerve gives off several branches. The medial sural cutaneous nerve joins the peroneal communicating branch of the common peroneal nerve to form the sural nerve. The posterior articular branch pierces the posterior capsule to innervate the contents of the knee. Finally, there is a motor branch to the popliteus muscle.29
A complete neurologic examination including motor and sensory evaluation of the peroneal and tibial nerve should be well documented before and after any intervention including reductions, surgical procedures, splinting, or casting. The peroneal nerve is divided into superficial and deep branches. The superficial peroneal nerve—lumbar nerve root 5 (L5) and sacral nerve roots 1 and 2 (S1, S2)—controls the peroneal muscles of the foot, which provide foot eversion. The superficial peroneal nerve provides sensation to lateral aspect of the leg and dorsum of the foot. The deep peroneal nerve (L4, L5, and S1) controls the tibialis anterior, extensor hallucis longus, and extensor digitorum longus muscles. This function can be tested manually by having the patient perform resisted ankle dorsiflexion and great and lesser toe extension, respectively. Sensory function of the deep peroneal nerve is located primarily within the first web space on the dorsum of the foot. The tibial nerve (L5, S1, and S2) innervates the muscles of the superficial and deep posterior compartments of the leg. The gastrocnemius and soleus muscles are tested by resisted plantar flexion of the foot. The flexor hallicus longus and flexor digitorum longus are tested by having the patient curl his or her great and lesser toes respectively, then performing resisted plantar flexion. The tibialis posterior is tested by resisted plantar flexion and inversion of the foot. Sensory distribution for the tibial nerve is located on the plantar aspect of the foot.21
Manual muscle testing is graded on a five-point scale with 0 indicating no muscle activation; 1, muscle activation without joint movement; 2, motion observed when gravity eliminated; 3, motion against gravity; 4, resisted submaximal contraction; and 5, maximal resisted motion. As with the vascular examination, the patient should have frequent neurologic examinations that are well documented. A progressive deterioration in the neurologic examination may indicate compartment syndrome or ischemia.46 Compartment pressure testing is indicated in this scenario.
Ultrasonography of the peroneal nerve is performed with the patient in the prone position and the knee flexed 20° with the ankle positioned in a cushion to allow access to the popliteal region. A 12.5-MHz and 15.7-MHz broadband linear array transducer and a silicon standoff pad are used. Gruber and associates in 200519 demonstrated that sonography by experienced technicians reliably demonstrated the state of the nerve and the surrounding soft tissues. Specifically, the level of the lesion and any scar tissue or hematoma that was surrounding the nerve were identified. It correlated precisely with intraoperative findings. It is important to identify a treatment program for peroneal nerve injury associated with dislocation.13 Nerve injuries from sharp laceration should be repaired acutely, those from blunt laceration should be delayed 3 to 4 weeks to delineate proximal and distal stump damage. Most commonly, nerve injury from a knee dislocation is a stretch injury or lesion in continuity. These injuries are observed for 4 to 6 months to allow for spontaneous recovery and/or delineation of the zone of injury.28 Within this time interval, splinting and dedicated therapy with nerve stimulation and reeducation should be instituted. An ankle-foot orthrosis (AFO) should be used to maintain the ankle in a neutral position and prevent contracture of the Achilles tendon. Initiating stretching exercises to correct the equinovarus foot positions may help prevent the development of contractures. Baseline electrodiagnostic studies should be performed at 6 weeks and again at 3 months if there has been no clinical evidence of tibialis anterior muscle function.60
With ongoing absence of clinical signs of recovery, nerve exploration may be indicated.16,60 At the time of surgery, nerve action potentials across the lesion are assessed. If conduction is demonstrated, neurolysis can be performed with 80% to 88% recovery of grade 3 muscle strength (peroneal and anterior tibial muscle contract against gravity and some resistance).27,28 End-to-end suturing or nerve grafting is recommended when there is no conduction across the deficit. Resection and suturing of short-segment deficits with partial fibulectomies may result in 84% chance of grade 3 recovery.28 Kim and colleagues28 also found grade 3 functional return in 42% of grafts overall; 75% of those that were less than 6 cm in length had good outcome. The outcome depends strongly on the degree of neural damage and the surgical technique used.19 When sufficient return of function does not occur, an anterior transfer of the posterior tibial tendon or other technique can maintain neutral ankle dorsiflexion and eliminate the need for the AFO.12,20,23,42,56,63–65
LIGAMENTOUS INJURIES
Critical Points LIGAMENTOUS INJURIES
A systematic approach to the evaluation of knee stability should be undertaken. The most accurate examination for identification of ACL integrity is the Lachman test.11 This is performed by applying an anteriorly directed force on the tibia with the knee in 20° to 30° of flexion. With the knee at 90°, a posterior drawer or posterior force on the tibia is used to assess the PCL. Translation and endpoint are evaluated. Evaluation of posterior sag, or the position of the medial femoral condyle with respect to the tibial plateau, is also useful to identify PCL injuries.
The MCL and FCL are evaluated by applying a valgus or varus stress, respectively, at both 0° and 30° of flexion. Increased medial or lateral joint space opening at 30° typically indicates a collateral ligament injury. A posteromedial or posterolateral complex injury can be identified with additional joint space opening at 0° of flexion. The posterolateral corner can be assessed further with the dial test. Increased external rotation of the tibia, greater than 10° to 15° side-to-side thigh-foot angle at 30° of knee flexion, indicates a posterolateral corner injury. If there is additional external rotation of the dial test at 90° of flexion, a PCL and lateral corner injury is present. Twaddle and coworkers62 in 2003 presented 63 dislocatable knees in 60 patients. These authors reported bicruciate ligament injuries in 71% of patients. The ACL was injured in 84%; the PCL, 87%; the MCL, 44%; and the FCL, 62%.
REDUCTION AND IMMOBILIZATION
Knees that are dislocated at the time of presentation should be reduced immediately. Reduction in the field by knowledgeable medical personnel is preferred. The neurovascular status of the limb must be assessed prior to reduction. The use of intravenous adjuvant medications is usually sufficient anesthesia. Reduction is accomplished by applying traction to the tibia while manipulating the proximal tibia in the appropriate direction. The presence of a dimple sign, indication of a posterolateral dislocation, may be a contraindication to closed reduction. Imaging should be pursued if possible. Reevaluation of joint congruence and neurovascular status must follow reduction.16 The limb should then be immobilized in a long-leg splint. Adequate reduction and maintenance of tibiofemoral alignment must be documented in both the anteroposterior (AP) and the lateral planes. If reduction cannot be maintained in a splint, the knee may be transfixed temporarily with cross pins (through the notch) or an external fixator until sufficient capsular healing occurs.
IMAGING
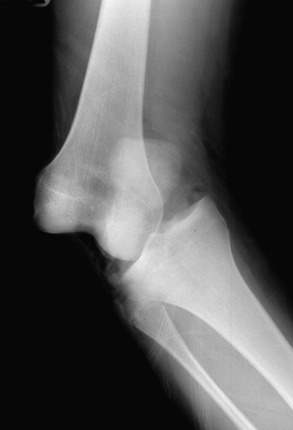
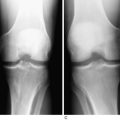
Radiographs of the knee should be obtained for all suspected knee dislocations (Fig. 27-6). Radiographs may be delayed until a clinically apparent knee dislocation has been adequately reduced. The basic knee series consists of an AP and a lateral plain radiograph. In cases in which fracture is noted or suspected, oblique views or stress views of the knee may be indicated. These views can determine the direction of dislocation as well as assess the adequacy of reduction and note any residual subluxation. In addition, the identification of fractures may direct treatment. Often, femoral and/or tibial fractures will require stabilization before the dislocation can be addressed.
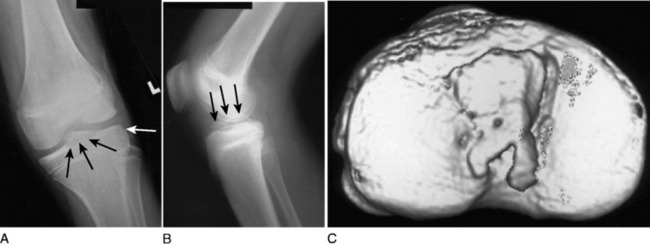
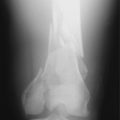
A tibial eminence fracture and lateral capsular avulsion fractures from the tibia, also known as a Segond fracture, indicate an ACL injury (Fig. 27-7).5,10,53 The tibial eminence fractures may be complete, incorporating the entire ACL footprint, or incomplete, primarily affecting the anteromedial bundle.18 The Segond fracture, best detected on the AP projection, is indicative of detachment of the middle third of the lateral capsular ligament and is associated with anterolateral rotational instability (Fig. 27-8). The fracture originates from the lateral cortex of the proximal tibia, superior, and posterior to Gerdy’s tubercle, approximately 2 to 10 mm distal to the articular surface.15 Tears of the ACL are present in 75%8 to 100%15 of patients with Segond fractures.
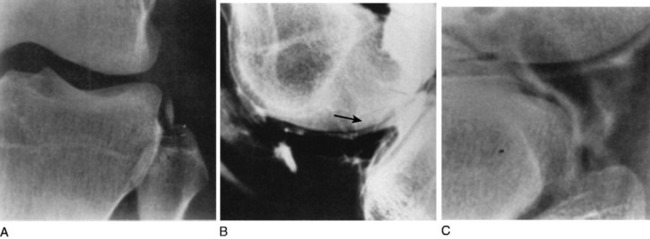
PCL and posterolateral ligament injuries can also be identified on plain radiographs (Fig. 27-9). PCL injuries may present as posterior avulsion fractures of the proximal tibia. These fractures will usually involve the entire PCL and may be comminuted or split sagitally.18 A cortical avulsion of the middle medial tibial plateau, a “reverse” Segond fracture, caused by a valgus and external rotation force, corresponds to an avulsion of the deep MCL. This is usually associated with a PCL injury and medial meniscal tear. This may be the only radiologic suggestion of a knee dislocation/multiligamentous injury.12
On the lateral side, the “arcuate sign” describes an avulsion fracture of the fibular head consisting of the FCL, popliteal fibular ligament, and fabellofibular ligament may present with this fragment. Huang and associates22 found that in addition to the injury to the posterolateral stabilizers, 13 of 13 patients with the arcuate sign had PCL disruptions (6 tibial avulsions, and 7 midsubstance) and MCL injuries (5 acutely abnormal, 8 chronic fibrous thickening). Also identified were medial and lateral meniscal tears (5 medial, 6 lateral).
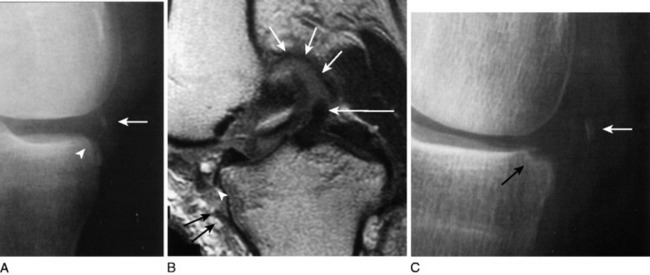
After a knee dislocation has been reduced, the patient stabilized, and plain radiographs confirm reduction, MRI can be obtained subacutely (Fig. 27-10). MRI will further define soft tissue and cartilage injuries and aid in planning definitive care. Whereas the physical examination is best for determining degrees of abnormal translation and rotation, MRI can help determine the location of the injury (e.g., midsubstance tear). This information can be useful in deciding the need for repair, augmentation versus reconstruction.
EMERGENT SURGERY
Emergent surgery is indicated for vascular injury, compartment syndrome, open injury, or irreducible dislocations.46 Vascular reconstruction, often performed with reverse saphenous vein grafts, should be performed within 6 hours to minimize ischemia to the muscles and increase the chances for limb survival. The orthopaedic surgeon may need to stabilize the limb with an external fixator to allow access to the limb during reconstruction and for postoperative wound management. Fasciotomies are often required after revascularization to prevent compartment syndrome.
Acute compartment syndrome is a condition in which the pressure within a fascial compartment rises to a level that decreases tissue perfusion leading to cellular anoxia, muscle ischemia, and death. Diagnosis is primarily clinical with supplementation by compartment pressure measurements. Clinical signs of compartment syndrome include pain out of proportion, pain aggravated by passive stretch, paresthesias, and late signs that include pulselessness, paralysis, and pallor. Compartment pressure measurements of 20 mm Hg below diastolic pressure or 30 mm Hg below the mean arterial blood pressure suggest muscle ischemia. Urgent restoration of perfusion and normalization of compartment pressures is accomplished by complete fasciotomy of all compartments.39
Critical Points EMERGENT SURGERY
When open knee dislocations are encountered, wound management principles should be employed. Irrigation and débridement of the open wound should be performed urgently. The patient should be started on appropriate antibiotics. Acute ligament reconstruction is contraindicated in the face of an open wound. Soft tissue issues may delay reconstruction for several months.46
DEFINITIVE MANAGEMENT
Most, if not all, surgeons would agree that soft tissue dissection, ligament, and capsular repairs are easier in the acute setting, less than 2 weeks after the injury. Unfortunately, this is when the knee is usually swollen and range of motion is poor with decreased or absent muscle control of the limb. All of these factors can increase the risk of a permanently stiff knee. Even in the environment of a high-volume knee trauma center,6,59 loss of motion with a multiple ligament reconstruction is difficult to prevent. Therefore, these decisions must take into account this risk of stiffness along with other patient factors such as complicating injuries, medical comorbidities, participation in rehabilitation efforts, ability to maintain knee reduction, soft tissue coverage and skin condition, and patient expectations. Fortunately, reconstructive options today surpass those available even a few years ago, thereby decreasing the need for acute repair. For patients with high physical demands, optimizing the condition of the knee (muscle function, range of motion, and swelling) and relying on reconstruction rather than acute repair may yield a superior functional result. Although time from injury dictates options such as repair versus reconstruction, it is the condition of the knee that may ultimately determine stiffness and functional results.
Arthroscopically assisted surgery should usually be delayed a minimum of 10 to 14 days after the injury to allow the joint capsule to heal and therefore minimize the risk of fluid extravasation and subsequent compartment syndrome.51 In addition, this delay allows for monitoring of the vascular status, decreased swelling, improvement in quadriceps tone, and range of motion.7
Shelbourne and colleagues54 in 1991 demonstrated a significantly increased incidence of arthrofibrosis in patients undergoing ACL reconstruction within 1 week of injury compared with those delayed 21 days or more. These authors also believed that delayed surgery gave the patient the opportunity to ask questions, as well as improve her or his psychological outlook on the injury and rehabilitation. Stannard and coworkers59 compared acute repair (within 3 wk) of the posterolateral corner with reconstruction. These investigators found significantly inferior results of repair (37% failure) to reconstruction (9% failure) in 56 patients with 57 posterolateral corner injuries at 2 years.
1 Alberty R.E., Goodfried G., Boyden A.M. Popliteal artery injury with fractural dislocation of the knee. Am J Surg. 1981;142:36-40.
2 Almekinders L.C., Logan T.C. Results following treatment of traumatic dislocations of the knee joint. Clin Orthop Relat Res. 1992;284:203-207.
3 Applebaum R., Yellin A.E., Weaver F.A., et al. Role of routine arteriography in blunt lower-extremity trauma. Am J Surg. 1990;160:221-224. discussion 224–225
4 Barnes C.J., Pietrobon R., Higgins L.D. Does the pulse examination in patients with traumatic knee dislocation predict a surgical arterial injury? A meta-analysis. J Trauma. 2002;53:1109-1114.
5 Campos J.C., Chung C.B., Lektrakul N., et al. Pathogenesis of the Segond fracture: anatomic and MR imaging evidence of an iliotibial tract or anterior oblique band avulsion. Radiology. 2001;219:381-386.
6 Chhabra A., Cha P.S., Rihn J.A., Cole B., et al. Surgical management of knee dislocations. Surgical technique. J Bone Joint Surg Am. 2005;87(suppl 1 pt 1):1-21.
7 Cole B.J., Harner C.D. The multiple ligament injured knee. Clin Sports Med. 1999;18:241-262.
8 Cole P.A., Campbell R., Swiontkowski M.F., Johansen K.H. Doppler arterial measurements reliably exclude occult arterial injury in blunt extremity trauma. Abstract. Orthopaedic Trauma Association. October 1998.
9 Dart C.H.Jr., Braitman H.E. Popliteal artery injury following fracture or dislocation at the knee. Diagnosis and management. Arch Surg. 1977;112:969-973.
10 Dietz G.W., Wilcox D.M., Montgomery J.B. Segond tibial condyle fracture: lateral capsular ligament avulsion. Radiology. 1986;159:467-469.
11 Donaldson W.F.3rd, Warren R.F., Wickiewicz T. A comparison of acute anterior cruciate ligament examinations. Initial versus examination under anesthesia. Am J Sports Med. 1985;13:5-10.
12 Escobedo E.M., Mills W.J., Hunter J.C. The “reverse Segond” fracture: association with a tear of the posterior cruciate ligament and medial meniscus. AJR Am J Roentgenol. 2002;178:979-983.
13 Ferraresi S., Garozzo D., Buffatti P. Common peroneal nerve injuries: results with one-stage nerve repair and tendon transfer. Neurosurg Rev. 2003;26:175-179.
14 Goitz R.J., Tomaino M.M. Management of peroneal nerve injuries associated with knee dislocations. Am J Orthop. 2003;32:14-16.
15 Goldman A.B., Pavlov H., Rubenstein D. The Segond fracture of the proximal tibia: a small avulsion that reflects major ligamentous damage. AJR Am J Roentgenol. 1988;151:1163-1167.
16 Good L., Johnson R.J. The dislocated knee. J Am Acad Orthop Surg. 1995;3:284-292.
17 Green N.E., Allen B.L. Vascular injuries associated with dislocation of the knee. J Bone Joint Surg Am. 1977;59:236-239.
18 Griffith J.F., Antonio G.E., Tong C.W., Ming C.K. Cruciate ligament avulsion fractures. Arthroscopy. 2004;20:803-812.
19 Gruber H., Peer S., Meirer R., Bodner G. Peroneal nerve palsy associated with knee luxation: evaluation by sonography—initial experiences. AJR Am J Roentgenol. 2005;185:1119-1125.
20 Hoover G.H., Frost H.M. Dynamic correction of spastic rocker-bottom foot. Peroneal to anterior tibial tendon transfer and heel-cord lengthening. Clin Orthop Relat Res. 1969;65:175-182.
21 Hoppenfeld S., editor. Physical Examination of the Spine and Extremeties. Norwalk, CT: Appleton-Century-Crofts, 1976.
22 Huang G.S., Yu J.S., Munshi M., et al. Avulsion fracture of the head of the fibula (the “arcuate” sign): MR imaging findings predictive of injuries to the posterolateral ligaments and posterior cruciate ligament. AJR Am J Roentgenol. 2003;180:381-387.
23 Jackson C.T., Weighill F.J. A combined peroneal tendon transfer and subtalar fusion using excised fibular bone. Br J Clin Pract. 1973;27:329-330.
24 Jones R.E., Smith E.C., Bone G.E. Vascular and orthopedic complications of knee dislocation. Surg Gynecol Obstet. 1979;149:554-558.
25 Kendall R.W., Taylor D.C., Salvian A.J., O’Brien P.J. The role of arteriography in assessing vascular injuries associated with dislocations of the knee. J Trauma. 1993;35:875-878.
26 Kennedy J.C. Complete dislocation of the knee joint. J Bone Joint Surg Am. 1963;45:889-904.
27 Kim D.H., Kline D.G. Management and results of peroneal nerve lesions. Neurosurgery. 1996;39:312-319. discussion 319–320
28 Kim D.H., Murovic J.A., Tiel R.L., Kline D.G. Management and outcomes in 318 operative common peroneal nerve lesions at the Louisiana State University Health Sciences Center. Neurosurgery. 2004;54:1421-1428. discussion 1428–1429
29 Kim T.K., Savino R.M., McFarland E.G., Cosgarea A.J. Neurovascular complications of knee arthroscopy. Am J Sports Med. 2002;30:619-629.
30 Lefrak E.A. Knee dislocation. An illusive cause of critical arterial occlusion. Arch Surg. 1976;111:1021-1024.
31 Lynch K., Johansen K. Can Doppler pressure measurement replace “exclusion” arteriography in the diagnosis of occult extremity arterial trauma? Ann Surg. 1991;214:737-741.
32 McCoy G.F., Hannon D.G., Barr R.J., Templeton J. Vascular injury associated with low-velocity dislocations of the knee. J Bone Joint Surg Br. 1987;69:285-287.
33 McCutchan J.D., Gillham N.R. Injury to the popliteal artery associated with dislocation of the knee: palpable distal pulses do not negate the requirement for arteriography. Injury. 1989;20:307-310.
34 McDonough E.B., Wojtys E.M. Multi-ligamentous injuries of the knee and associated vascular injuries. Am J Sports Med. 2009;37:156-159.
35 Merrill K.D. Knee dislocations with vascular injuries. Orthop Clin North Am. 1994;25:707-713.
36 Meyers M.H., Moore T.M., Harvey J.P.Jr. Traumatic dislocation of the knee joint. J Bone Joint Surg Am. 1975;57:430-433.
37 Mills W.J., Barei D.P., McNair P. The value of the ankle-brachial index for diagnosing arterial injury after knee dislocation: a prospective study. J Trauma. 2004;56:1261-1265.
38 Nystrom M., Samimi S., Ha’Eri G.B. Two cases of irreducible knee dislocation occurring simultaneously in two patients and a review of the literature. Clin Orthop Relat Res. 1992;277:197-200.
39 Olson S.A., Glasgow R.R. Acute compartment syndrome in lower extremity musculoskeletal trauma. J Am Acad Orthop Surg. 2005;13:436-444.
40 Ottolenghi C.E. Vascular complications in injuries about the knee joint. Clin Orthop Relat Res. 1982;165:148-156.
41 Peck J.J., Eastman A.B., Bergan J.J., et al. Popliteal vascular trauma. A community experience. Arch Surg. 1990;125:1339-1343. discussion 1343–1344
42 Pinzur M.S., Kett N., Trilla M. Combined anteroposterior tibial tendon transfer in post-traumatic peroneal palsy. Foot Ankle. 1988;8:271-275.
43 Potter H.G., Weinstein M., Allen A.A., et al. Magnetic resonance imaging of the multiple-ligament injured knee. J Orthop Trauma. 2002;16:330-339.
44 Quinlan A.G. Irreducible posterolateral dislocation of the knee with button-holing of the medial femoral condyle. J Bone Joint Surg Am. 1966;48:1619-1621.
45 Reckling F.W., Peltier L.F. Acute knee dislocations and their complications. 1969. Clin Orthop Relat Res. 2004;422:135-141.
46 Rihn J.A., Groff Y.J., Harner C.D., Cha P.S. The acutely dislocated knee: evaluation and management. J Am Acad Orthop Surg. 2004;12:334-346.
47 Rios A., Villa A., Fahandezh H., et al. Results after treatment of traumatic knee dislocations: a report of 26 cases. J Trauma. 2003;55:489-494.
48 Roman P.D., Hopson C.N., Zenni E.J.Jr. Traumatic dislocation of the knee: a report of 30 cases and literature review. Orthop Rev. 1987;16:917-924.
49 Samimi S., Shahriaree H. Arthroscopic view of an irreducible knee dislocation. Arthroscopy. 1993;9:322-326.
50 Schenck R.C.Jr. The dislocated knee. Instr Course Lect. 1994;43:127-136.
51 Schenck R.C.Jr., Hunter R.E., Ostrum R.F., Perry C.R. Knee dislocations. Instr Course Lect. 1999;48:515-522.
52 Sedel L., Nizard R.S. Nerve grafting for traction injuries of the common peroneal nerve. A report of 17 cases. J Bone Joint Surg Br. 1993;75:772-774.
53 Segond P. Recherches cliniques et experimentales sur les epanchements sanguins du genou par entorse. Prog Med. 1879;7:297-299. 319–321, 340–341
54 Shelbourne K.D., Porter D.A., Clingman J.A., et al. Low-velocity knee dislocation. Orthop Rev. 1991;20:995-1004.
55 Shields L., Mital M., Cave E.F. Complete dislocation of the knee: experience at the Massachusetts General Hospital. J Trauma. 1969;9:192-215.
56 Simonka J.A. [Management of a foot deformity, caused by paralysis of the peroneal nerve, by transfer of the tendon of the posterior tibial muscle]. Magy Traumatol Orthop Helyreallito Seb. 1991;34:230-232.
57 Sisto D.J., Warren R.F. Complete knee dislocation. A follow-up study of operative treatment. Clin Orthop Relat Res. 1985;198:94-101.
58 Stannard J.P., Sheils T.M., Lopez-Ben R.R., et al. Vascular injuries in knee dislocations: the role of physical examination in determining the need for arteriography. J Bone Joint Surg Am. 2004;86:910-915.
59 Stannard J.P., Riley R.S., Sheils T.M., et al. Anatomic reconstruction of the posterior cruciate ligament after multiligament knee injuries. A combination of the tibial-inlay and two-femoral-tunnel techniques. Am J Sports Med. 2003;31:196-202.
60 Tomaino M., Day C., Papageorgiou C., et al. Peroneal nerve palsy following knee dislocation: pathoanatomy and implications for treatment. Knee Surg Sports Traumatol Arthrosc. 2000;8:163-165.
61 Treiman G.S., Yellin A.E., Weaver F.A., et al. Examination of the patient with a knee dislocation. The case for selective arteriography. Arch Surg. 1992;127:1056-1062. discussion 1062–1063
62 Twaddle B.C., Bidwell T.A., Chapman J.R. Knee dislocations: where are the lesions? A prospective evaluation of surgical findings in 63 cases. J Orthop Trauma. 2003;17:198-202.
63 Vertullo C.J., Nunley J.A. Acquired flatfoot deformity following posterior tibial tendon transfer for peroneal nerve injury: a case report. J Bone Joint Surg Am. 2002;84:1214-1217.
64 Vigasio A., Marcoccio I., Patelli A., et al. New tendon transfer for correction of drop-foot in common peroneal nerve palsy. Clin Orthop Relat Res. 2008;466:1454-1466. Epub 2008; April 15
65 Wapner K.L., Taras J.S., Lin S.S., Chao W. Staged reconstruction for chronic rupture of both peroneal tendons using Hunter rod and flexor hallucis longus tendon transfer: a long-term followup study. Foot Ankle Int. 2006;27:591-597.
66 Wascher D.C. High-velocity knee dislocation with vascular injury. Treatment principles. Clin Sports Med. 2000;19:457-477.
67 Welling R.E., Kakkasseril J., Cranley J.J. Complete dislocations of the knee with popliteal vascular injury. J Trauma. 1981;21:450-453.