34 Management of Acute Ischemic Stroke
Stroke is currently recognized as the third most common cause of death and the leading cause of adult morbidity in the United States, affecting nearly 795,000 people annually.1 Acute ischemic stroke is a true medical emergency and must be treated in a swift yet pragmatic approach. The rationale for acute ischemic stroke treatment is based on the concept of the ischemic penumbra. When an arterial occlusion occurs, an area of irreversibly infarcted brain (i.e., core infarct) is surrounded by a region that has reduced blood flow impairing function (i.e., ischemic penumbra), although not of sufficient severity to result in irreversible infarction. If adequate blood flow can be restored within a critical time frame, this area of at-risk tissue may be salvageable and return to normal function. Experimental models of stroke indicate that lower levels of blood flow are tolerated for brief periods, whereas slightly higher blood flow can be maintained for several hours without developing infarction.2,3 The precise relationships between blood flow levels and duration for human stroke are still being elucidated, but the prevailing concept is that the more quickly restoration of blood flow occurs, the greater the probability that the salvageable tissue will be spared from permanent damage.
In 1995, the National Institute of Neurological Disorders and Stroke (NINDS) rt-PA Stroke Study Group showed for the first time an improvement in ischemic stroke outcome with acute treatment.4 At present, intravenous (IV) recombinant tissue plasminogen activator (tPA) is the only treatment that has been approved by the U.S. Food and Drug Administration (FDA) for acute ischemic stroke patients presenting within 3 hours of symptom onset. Other treatments for acute ischemic stroke—intraarterial thrombolysis, devices for mechanical clot disruption, and neuroprotective agents—continue to be investigated.
 Emergent Stroke Evaluation
Emergent Stroke Evaluation
A stroke team typically consists of individuals from multiple disciplines with specialized knowledge and interest in acute stroke care and often includes a vascular neurologist, nursing coordinator, and where available, a neurointerventionalist. A neurologist performs a National Institutes of Health Stroke Scale (NIHSS) (Table 34-1) assessment as an additional rapid neurologic assessment tool to better localize and ascertain the degree of clinical deficit. The score may impact which therapies may be available to patients. For patients presenting with stroke-like symptoms while already hospitalized in an intensive care unit (ICU) or other hospital floor, the algorithm should be identical.
| 1A. Level of Consciousness (LOC) | 1B. LOC Questions | 1C. LOC Commands |
|---|---|---|
| 0 = Alert 1 = Not alert, but arousable 2 = Not alert, obtunded 3 = Coma |
Ask the month and his/her age. 0 = Answers both correctly 1 = Answers one correctly 2 = Answers neither correctly |
Open and close the eyes. Open and close the non-paretic hand. 0 = Performs both tasks correctly 1 = Performs one task correctly 2 = Performs neither task correctly |
| 2. Best Gaze (Horizontal) | 3. Visual Fields | 4. Facial Palsy |
| 0 = Normal 1 = Partial gaze palsy 2 = Forced deviation or total gaze paresis |
0 = No visual loss 1 = Partial hemianopia 2 = Complete hemianopia 3 = Bilateral hemianopia |
0 = Normal 1 = Minor paralysis 2 = Partial paralysis (total or near total paralysis of lower face) 3 = Complete paralysis of upper and lower face |
| 5. Motor Arm | 6. Motor Leg | 7. Limb Ataxia |
| Right Arm extended with palms down 90 degrees (if sitting) or 45 degrees (if supine) for 10 seconds 0 = No drift 1 = Drift; limb drifts down from position and does not hit bed or support in 10 sec 2 = Some effort against gravity 3 = No effort against gravity 4 = No movement Left |
Right Leg extended at 30 degrees, always tested supine for 5 seconds 0 = No drift 1 = Drift; limb drifts down from position and does not hit bed or support in 5 sec 2 = Some effort against gravity 3 = No effort against gravity 4 = No movement Left |
The finger-nose-finger and heel-shin tests 0 = Absent 1 = Present in one limb 2 = Present in two limbs |
| 8. Sensory | 9. Best Language | 10. Dysarthria |
| To pinprick or noxious stimuli 0 = Normal 1 = Mild to moderate sensory loss 2 = Severe to total sensory loss |
0 = No aphasia, normal 1 = Mild to moderate aphasia 2 = Severe aphasia 3 = Mute, global aphasia, coma |
0 = Normal 1 = Mild to moderate 2 = Severe (including mute/anarthric due to aphasia) Do not score if intubated. |
| 11. Extinction and Inattention | ||
| 0 = No abnormality 1 = Present 2 = Profound (2 modalities) |
TOTAL SCORE: | |
Ischemic strokes generally are classified as large artery atherosclerosis, small vessel occlusion, cardioembolism, stroke of other determined etiology, or stroke of undetermined etiology.5 In the first few minutes to hours after ischemic stroke, identification of stroke mechanisms may be difficult or impossible. Emergent diagnosis is enhanced significantly by imaging modalities, including computed tomography (CT) and magnetic resonance imaging (MRI).
 Imaging of Acute Stroke
Imaging of Acute Stroke
It is necessary to differentiate ischemic from hemorrhagic stroke before deciding on thrombolytic administration, and imaging obviously plays a key role in this regard. However, imaging may provide much more information. At most stroke centers, time from symptom onset (i.e., time when patient was last confirmed to be seen at normal baseline) is a major determining factor in whether a patient may be a candidate for IV thrombolysis (i.e., up to 3 hours) or intraarterial therapy (i.e., 3 to 6 hours). One emerging concept gaining more acceptance is that physiology rather than time should be used to decide on eligibility for treatment.6 For example, some patients within the 3-hour time window may already have established infarction that would not reverse with thrombolysis and may result in hemorrhage due to reperfusion of infarcted brain. Conversely, some patients may have salvageable brain tissue despite presentation well after the 3-hour time window. A physiologic estimate of tissue viability would be preferable to a fixed time interval if a study were found that reliably predicted viability of brain after stroke. CT and MRI have the potential to provide this measurement.7
Computed Tomography
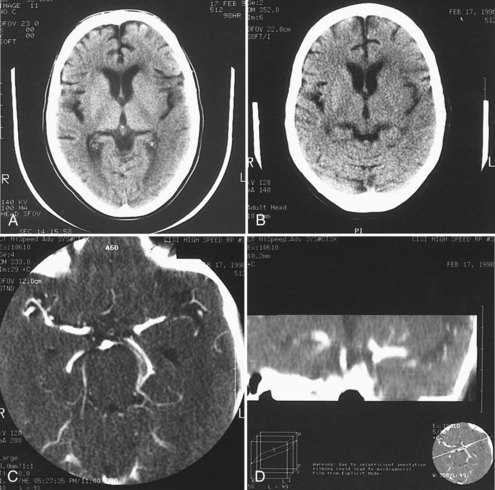
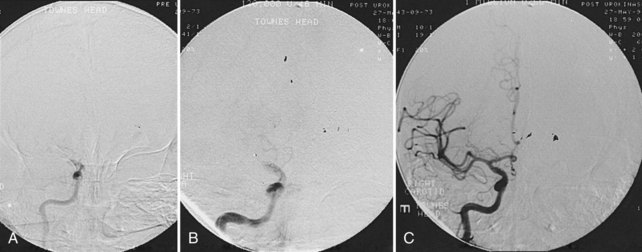
A noncontrast head CT is the initial imaging modality of choice for patients with suspected stroke for two main reasons. The foremost is the expediency with which one can obtain a CT scan because of its widespread availability, and the second is the ability of CT to exclude intracranial hemorrhage. However, in addition to differentiating ischemic stroke from hemorrhage, CT may demonstrate subtle parenchymal abnormalities indicative of early edema or infarction. It was previously believed that these changes did not occur on CT for at least 6 hours after ischemic stroke. More recent studies indicate, however, that early changes of ischemia frequently occur within a few hours of stroke onset and have been seen as soon as 1 hour after stroke.8 These changes include reduced attenuation in the basal ganglia,2 loss of gray-white differentiation particularly in the insular region,9 low density in the cortex and subcortical white matter, and loss of sulcal markings, suggesting early mass effect and edema (Figure 34-1, A and B).10
A hyperdense middle cerebral artery occurs in 20% to 37% of cases,11 indicating acute thrombus within the artery. It rarely occurs without at least one other early CT abnormality. Hyperdensity in the basilar artery associated with thrombosis also has been reported.12 In 100 patients studied within 14 hours (mean 6.4 hours) of stroke onset, multiple early CT abnormalities correlated with size of subsequent infarct and poor outcome.11 In the ECASS I trial of tPA for acute stroke, early CT changes correlated with larger subsequent infarct volume and a greater likelihood of hemorrhagic conversion after tPA.13 Quantitative assessment of CT changes using the Alberta Stroke Program Early CT Score (ASPECTS) scale in patients treated with IV tPA also showed a relationship between early CT hypodensity (ASPECTS < 8) and hemorrhage (Table 34-2).14,15 Based on these results, some experts recommend withholding thrombolytic therapy in patients with extensive early CT changes, particularly in patients later in the thrombolytic time window,16 although this practice is somewhat controversial. For example, subsequent analysis of the NINDS rt-PA trial data discovered that early ischemic changes did not predict symptomatic hemorrhage or response to treatment,17 and more recent evidence reports no association between early ischemic CT changes and outcome.18
TABLE 34-2 ASPECTS Measurement Tool for Early Changes on Computed Tomography
| 10 Regions of Interest*: | |
|---|---|
| At the Level of the Basal Ganglia and Thalamus | At the Level Just Rostral to Deep Nuclei |
| Anterior middle cerebral artery (MCA) cortex | Superior to anterior MCA cortex |
| MCA cortex lateral to insula | Superior to MCA cortex lateral to insula |
| Posterior MCA cortex | Superior to posterior MCA cortex |
| Caudate | |
| Lentiform nucleus Internal capsule Insular ribbon |
**1 point is subtracted for each defined area of early ischemic change, such as focal swelling or parenchymal hypoattenuation. Score varies from 0 to 10. |
* One point is subtracted for each defined area of early ischemic change, such as focal swelling or parenchymal hypoattenuation. Score varies from 0-10.
Adapted from Pexman JH, Barber PA, Hill MD, et al. Use of the Alberta Stroke Program Early CT Score (ASPECTS) for assessing CT scans in patients with acute stroke. AJNR Am J Neuroradiol. 2001;22(8):1534-1542.
Computed Tomography Angiography
CT angiography (CTA) can be performed using spiral CT technology, allowing for imaging of the intracranial and extracranial circulation. Optimally, CTA of the neck should include visualization of the aortic arch as well. The typical single bolus of iodine contrast material is approximately 70 mL of iodine. Owing to this injection, CTA is of limited use in patients with renal failure or contrast hypersensitivity. In acute stroke, CTA of the head and neck has been shown to be highly reliable for diagnosis of intracranial occlusions and correlates with other imaging modalities.19,20 Three-dimensional reconstruction images can also be created using this technology and can provide additional views and information about the carotid bifurcation and carotid lesions, showing eccentric lesions or ulceration not visualized by conventional angiography (see Figure 34-1, C and D).
Computed Tomography Perfusion
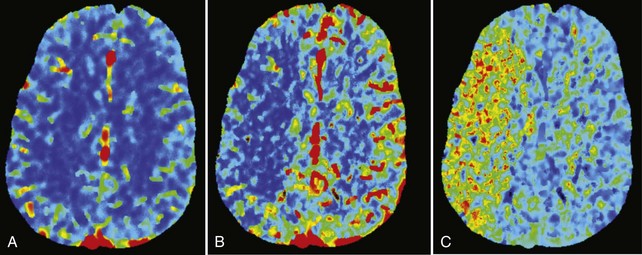
In addition to imaging the brain parenchyma with a noncontrast head CT and the cerebral vasculature with CTA, CT perfusion (CTP) adds assessment of cerebral blood volume (CBV) and cerebral blood flow (CBF). Using a helical scanner during a bolus of IV contrast, the time-dependent concentration curve of contrast in each pixel can be acquired. Mean transit time (MTT) and subsequently CBF can be calculated (Figure 34-2). In patients with acute stroke, CTP has been correlated with final infarct size and outcome, particularly after recanalization.21 CTP maps combining CBV and CBF information identify brain tissue that progresses to infarction if not reperfused, consistent with ischemic penumbra.22 Recent evidence suggests that the inclusion of CTP in a stroke imaging protocol increases diagnostic performance.21,23,24
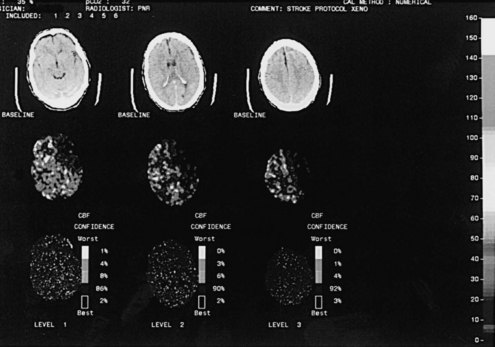
Whereas CTP serves as a qualitative measure of blood flow, there have been recent investigations into using xenon CT as a quantitative measure of blood flow.25 Stable xenon is an inert gas inhaled as a mixture of 27% xenon and 73% oxygen. During inhalation over a few minutes, rapid scanning is performed and pixel-by-pixel blood flow values are calculated at different brain levels (Figure 34-3). In a series of patients with middle cerebral artery (MCA) occlusion studied with xenon CT, areas of penumbra were present in all patients, and the percentage of MCA territory in the penumbral range (i.e., cerebral blood flow 8 to 20 mL/100 g/min) remained relatively constant across the group. In contrast, the percentage of MCA territory with CBF values representing infarcted tissue (i.e., cerebral blood flow <8 mL/100 g/min) varied greatly. Outcome was highly correlated with the area of infarcted MCA territory, not the amount of ischemic penumbra. These results suggest that after the first few hours, the size of the core infarcted tissue, not the amount of penumbral tissue, may be the most important imaging parameter to determine suitability for acute stroke therapy.26
Magnetic Resonance Imaging
Compared to CT modalities, MRI brain imaging is advantageous because it is more sensitive to cerebral infarction, especially in the brainstem and deep white matter. Typical sequences included in a MRI stroke protocol include diffusion-weighted imaging (DWI) and apparent diffusion coefficient (ADC) to evaluate for potential acute ischemia, multiplanar gradient-recalled (MPGR) or gradient recalled echo (GRE) to evaluate for hemorrhage, and fluid attenuated inversion recovery (FLAIR) to evaluate for important signs in both hyperacute and acute stages of stroke (i.e., assessment for absence of flow void in major cerebral arteries, which suggests occlusion or slow flow in that artery). Perfusion-weighted imaging (PWI) is also a sequence often used to determine abnormal tissue perfusion based on transit times for contrast material through brain parenchyma (Figure 34-4).
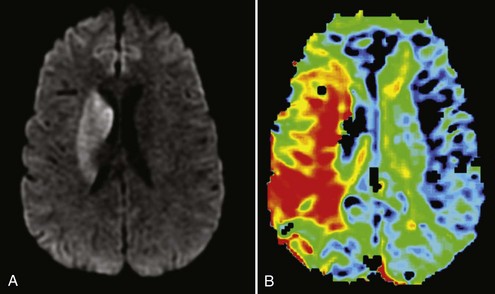
Figure 34-4 Magnetic resonance imaging of the same patient in Figure 34-2. A, Diffusion-weighted imaging (DWI) showing right basal ganglia stroke. B, Perfusion-weighted imaging (PWI) showing enhanced mean time to enhancement. These sequences together suggest a large ischemic penumbra in the right MCA territory.
DWI shows parenchymal abnormalities earlier than conventional T2-weighted images in patients with acute stroke.27 DWI detects the diffusion of water in the brain and shows hyperintensity in areas of reduced diffusion (see Figure 34-4). As water moves from the extracellular to the intracellular space, there is less movement of water and loss of signal, resulting in hyperintensity.28 DWI provides advantages in the evaluation of acute stroke. Early detection of lesions helps differentiate cerebral ischemia from other conditions that mimic stroke, such as seizures or toxic metabolic states. Additionally, combining DWI with PWI may identify reversibly ischemic tissue. If there is a large area of PWI abnormality indicating reduced blood flow but limited established infarction as evidenced by DWI abnormality, penumbral tissue is likely present, indicating areas of impaired flow at risk of undergoing infarction.
In stroke patients, the size of the DWI lesion and the growth of these abnormal DWI regions are strong predictors of outcome. In acute stroke, a marker of tissue viability is needed, and some investigators have suggested that the extent of mismatch between lesions on DWI and PWI could serve as this marker. The concept of DWI/PWI mismatch has been used as an inclusion criterion in several clinical trials (i.e., DIAS, DIAS-2, DEDAS, DEFUSE, EPITHET) assessing thrombolytic agents and is being employed more frequently to select patients that may ultimately benefit from reperfusion therapy.29–34 Patients with mismatch might be more likely to respond to reperfusion therapy.35 Patients with large areas of DWI abnormality or large severe PWI abnormalities may in fact be at greater risk for hemorrhage if reperfusion therapy is pursued.36
Magnetic Resonance Angiography
Magnetic resonance angiography (MRA) of the head and neck offers a noninvasive method of imaging the intracranial and extracranial vasculature. MRA typically uses gadolinium contrast in appropriate patients, but important information can be obtained based on time-of-flight techniques not utilizing contrast.37,38 Detection of dissection or occlusion in the circle of Willis and the extracranial vertebral and carotid arteries can be examined with MRA, but occlusions of small peripheral branch arteries may not be detected. Artifact may also wreak havoc in some cases by obscuring proper identification of arterial pathology. Signal dropout may occur at the site of arterial stenosis, owing to the effects of turbulent flow. If an artery is tortuous, it may extend out of the imaging section and appear occluded. MRI tends to overestimate the severity of stenosis, and evidence of severe stenosis should be confirmed with another modality. MRA is better for localizing the site of stenotic lesions than determining severity of stenosis. Similarly, differentiation between severe stenosis and occlusion is unreliable with MRI, and apparent occlusions by MRA should also be confirmed with angiography.
 Treatment of Acute Stroke
Treatment of Acute Stroke
Intravenous Thrombolysis
Acute stroke trials using IV thrombolytic agents date back to the early 1960s with use of streptokinase,39 fibrinolysin,40 and urokinase,41 showing either no benefit or a higher mortality in patients treated with thrombolysis. These studies preceded CT imaging, so patients with hemorrhage were not excluded. The discouraging results hindered the development of more acute stroke trials until the 1980s, when several case reports showed favorable outcomes with intraarterial thrombolytic therapy within a few hours of stroke onset.42,43 These reports resulted in small randomized trials and feasibility studies of IV thrombolytics44,45 which ultimately gave rise to the pivotal NINDS rt-PA trial that showed for the first time a beneficial effect of thrombolytic therapy for acute stroke treatment when administered within 3 hours of symptom onset.4
Tissue Plasminogen Activator Within 3 Hours
The NINDS trial included more than 600 patients with acute ischemic stroke. All patients were treated within 3 hours, and half of them were treated within 90 minutes. Patients were randomly assigned to receive either IV tPA at a dose of 0.9 mg/kg to a maximum of 90 mg, or IV placebo. Primary outcome measures were favorable outcomes at 90 days measured by the NIHSS, Barthel Index, Glasgow Outcome Scale, and modified Rankin Scale (mRS). By all four measures, significantly more patients had a favorable outcome at 90 days in the tPA group compared with placebo. Treatment with tPA resulted in an 11% to 13% absolute increase in good outcomes and a minor, non-significant decrease in mortality at 3 months. The benefit was sustained at 12 months.46 Intracerebral hemorrhage with clinical deterioration occurred in 6.4% of patients treated with tPA as compared to only 0.6% of placebo patients. Despite the increased hemorrhage rate, there was no significant increase in mortality or severe disability in the tPA group compared with placebo. When strokes were classified according to initial impression of stroke subtype, all types of strokes had more favorable outcomes with tPA. There were no clear factors that predicted response to tPA.47 Patients with large strokes as measured by NIHSS score higher than 20 and evidence of early low density or edema on CT had a higher rate of hemorrhage after tPA.48
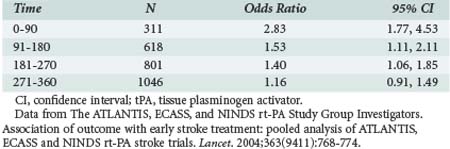
On the strength of these results, in June 1996 the FDA approved IV tPA for treatment of stroke within 3 hours of onset. This recommendation was supported by the results of an analysis of patients treated within 3 hours of onset in the ATLANTIS trial.49 A subsequent pooled analysis of NINDS rt-PA, ECASS, and ATLANTIS data showed that clinical benefit with tPA is greatest when given early, especially if started within 90 minutes (Table 34-3).50 It was noted that not all patients recanalize with IV tPA. In a dose escalation trial of IV tPA, angiography was performed before thrombolysis in all patients, documenting the site of arterial occlusion and repeated 2 hours later. Proximal occlusions in the MCA opened less frequently than distal branch occlusions, and only 8% of carotid occlusions recanalized.51
Tissue Plasminogen Activator Beyond 3 Hours
Several subsequent tPA trials attempted to extend the window for treatment beyond 3 hours. The ECASS I and II trials and the ATLANTIS trial treated patients with IV tPA up to 6 hours after stroke onset but failed to show a significant benefit compared with placebo.52–54 Pooled analysis of NINDS rt-PA, ECASS, and ATLANTIS data suggested a potential benefit beyond 3 hours. The ECASS III trial recently revealed that IV alteplase administered between 3 and 4.5 hours after symptom onset significantly improved clinical outcomes in patients with acute ischemic stroke, thereby potentially extending the therapeutic window in which patients may receive IV tPA. In addition to standard IV tPA exclusion criteria (Table 34-4), ECASS III exclusion criteria includes combination of previous stroke and diabetes, NIHSS score greater than 25, oral anticoagulant treatment, or age older than 80 years.55 Whether patients in this time window with these exclusions also benefit from IV tPA is unknown.
TABLE 34-4 Inclusion and Exclusion Criteria for Intravenous tPA
| Inclusion Criteria: |
CT, computed tomography; MCA, middle cerebral artery; NIH, National Institutes of Health; PT, prothrombin time; PTT, partial thromboplastin time; tPA, tissue plasminogen activator.
Other Thrombolytic Options
Desmoteplase (i.e., Desmodus rotundus salivary plasminogen activator) is a recombinant form of vampire bat saliva that is more potent than tPA. Desmoteplase possesses high fibrin selectivity, allowing it to dissolve a clot locally with less effect on the blood coagulation system. This property is thought to potentially reduce the risk of intracranial and systemic bleeding as compared to less fibrin-specific plasminogen activators like tPA. Desmoteplase was investigated in multiple trials to determine whether it could extend the treatment window for IV thrombolysis up to 9 hours.30,32 Unfortunately, no benefit of desmoteplase was realized between 3 and 9 hours after stroke symptom onset.31
Tenecteplase is a modified form of human tPA designed to achieve more effective thrombolysis. The half-life of tenecteplase is significantly longer, allowing administration as a single bolus. Similar to desmoteplase, tenecteplase has greater fibrin specificity and less fibrinogen depletion than tPA.56 A pilot safety study of tenecteplase for acute ischemic stroke was initiated but was recently discontinued due to slow enrollment; therefore no convincing conclusions at this time can be made about the promise of future study of tenecteplase in acute stroke.57,58
Reteplase is another recombinant form of human tPA that has been shown to be effective in the treatment of acute myocardial infarction.59 Reteplase also possesses a longer half-life compared to tPA, and a small case series found that in patients treated 9 hours after stroke onset with intraarterial reteplase, 88% completely recanalized and 44% achieved clinical improvement at 24 hours.60 Intraarterial reteplase has also been studied in conjunction with IV abciximab, a glycoprotein IIb/IIIa inhibitor, in a phase 1 study administering the combination therapy to stroke patients presenting between 3 and 6 hours.61 Abciximab may direct its effect through powerful antiplatelet effects or by direct thrombolysis. Abciximab monotherapy as emergent stroke treatment has also been evaluated in a phase 2 trial, with improved clinical outcome at 3 months in patients with mild to moderate strokes.62 Subsequently, a phase 3 trial was initiated but stopped prematurely due to an unfavorable benefit-risk profile.63
Sonothrombolysis is also currently being evaluated as an advantageous strategy for improving acute thrombolytic efficacy. The CLOTBUST trial indicated that continuous 2-MHz transcranial Doppler enhances tPA-induced arterial recanalization with a trend towards increased recovery from stroke.64 More recently, the TUCSON trial evaluated whether the addition of microspheres MRX-801 (ImaRx Therapeutics Inc., Round Rock, Texas) may further enhance the process of recanalization. Microspheres are a blend of phospholipids encapsulating a mixture of air and octafluoropropane gas (C3F8) that has the property of cavitation (i.e., rapid expansion and collapse) when exposed to ultrasound waves. The microspheres are administered IV, and when they reach intracranial occlusions, they transmit energy momentum from an ultrasound wave to residual flow and therefore promote recanalization. In TUCSON, it was concluded that microspheres could be safely combined with systemic tPA and ultrasound at a dose of 1.4 mL; however, there were safety concerns in the second dose tier of 2.8 mL that resulted in early termination of the trial. In both dose tiers, sonothrombolysis with microspheres and tPA showed a trend toward higher rates of early recanalization and clinical recovery compared to standard IV tPA therapy.65
Intraarterial Therapy
Intraarterial Thrombolysis
An alternative approach to IV thrombolysis is direct delivery of thrombolytic agents by a microcatheter embedded in the clot (Figure 34-5). The advantage of the intraarterial approach is direct visualization of the occluded artery and knowledge of the recanalization status as thrombolysis proceeds. Theoretically, delivery of the thrombolytic agent to the site of the clot should be more effective than IV infusion. The disadvantage is the additional time needed to bring the patient to the angiography suite, prepare the groin, catheterize the femoral artery, and guide the catheter from the femoral artery to the intracranial circulation before the thrombolytic agent can be administered.
Urokinase was used in early studies of intraarterial thrombolysis but is no longer available.66 Recombinant prourokinase was evaluated formally in clinical trials,67–69 and the PROACT II study was the first acute stroke trial to show a statistically significant improvement in clinical outcome when administered within 6 hours of stroke symptom onset. The median time to treatment was 5.5 hours, and most patients were treated after 5 hours.68 The clinical benefit was apparent despite this late time to treatment, and possibly a greater benefit would have been found had patients been treated earlier or mechanical manipulation also been allowed. Symptomatic hemorrhage occurred in 10% of patients treated with recombinant prourokinase and in 2% of controls.
Although the hemorrhage rate was higher than previous IV thrombolytic studies, the median NIHSS score of 17 indicates that the patients in the PROACT II study had more severe strokes treated at a later time interval. A higher hemorrhage rate would be expected in these scenarios. Based on factors predicting outcome in this group of patients, the treatment and control groups can be stratified according to risk. There was no differential effect of recombinant prourokinase across risk strata, indicating that all patients, regardless of risk, benefit equally from recombinant prourokinase.69 Despite these results, prourokinase has not been FDA approved to date, and tPA tends to be more often used in cases of intraarterial thrombolysis. The exact dose, efficacy, and safety profile of intraarterial tPA is limited, but recent studies have suggested doses up to 40 mg are reasonably safe for use.70
Mechanical Devices
The revolutionary Merci Retriever clot retrieval device (Concentric Medical Inc., Mountain View, California) received FDA approval for the removal of blood clots from the brain in patients experiencing an ischemic stroke after it was shown to be effective in restoring vascular patency in patients within 8 hours of symptom onset and could serve as an alternative therapy for patients who are otherwise ineligible for thrombolytic administration.71 The Merci device is a flexible nickel titanium (i.e., nitinol) wire that obtains a helical shape once it is passed through the tip of the guidance catheter. In practice, the catheter/wire is passed distal to the thrombus, the catheter is removed, and a helical configuration is assumed by the wire. The clot is then trapped in the helix and withdrawn from the vasculature. Second-generation Merci devices (e.g., L5 Retriever) have been developed and recently studied for recanalization efficacy. These new devices were associated with higher rates of recanalization, although these differences did not achieve statistical significance. They were also noted to produce lower mortality and a higher proportion of good clinical outcomes.72 An even newer generation of devices known as retrievable stents, specifically the Trevo System (Concentric Medical Inc., Mountain View, California) and Solitaire FR Revascularization System (ev3 Neurovascular, Irvine, California), have been developed and have been used in Europe, with very promising results.
Mechanical embolectomy using an aspiration platform was the basis for the creation of the Penumbra System (Penumbra Inc., Alameda, California). This device uses a microcatheter and separator-based debulking approach that allows for continuous aspiration of thrombus. A recent trial found that the Penumbra System resulted in safe and effective revascularization in patients who present with large-vessel occlusive disease within 8 hours of stroke onset, as 81.6% of patients achieved a Thrombolysis In Myocardial Infarction (TIMI) grade of 2 or 3.73
Angioplasty and stent placement without the use of thrombolytics have become routine modalities for treatment of acute coronary syndromes, with the intent to achieve timely reperfusion. The same principle could be applied to acute ischemic stroke therapy and might decrease hemorrhagic complications. One recent study found that this approach could be safely performed and improved neurologic status in patients without the use of thrombolysis. TIMI grade 3 was achieved in 88.9% of patients, and the mean 30-day NIHSS score improvement was 15.5 ± 5.6.74
Multimodal Approach
Many stroke investigators believe that a combined approach of IV thrombolysis and intraarterial thrombolysis may prove to be more beneficial for acute ischemic stroke patients with severe deficits and persistent arterial occlusion who present within 3 hours from symptom onset. Two initial studies demonstrated that a combined IV and intraarterial approach to recanalization may be more effective than standard IV rt-PA alone.75,76 The ongoing IMS III trial is a phase 3 study to formally evaluate this strategy. Patients are randomized into either an IV rt-PA only group treated with the standard 0.9 mg/kg dose, or IV/intraarterial therapy with a lower IV tPA dose of 0.6 mg/kg followed by intraarterial therapy. In the combined IV-IA group, patients may receive intraarterial tPA or one of the two FDA-approved devices, the Merci clot retriever or Penumbra device. The trial is using an mRS score of 0 to 2 at 3 months as its primary outcome measure, and mortality at 3 months and symptomatic intracerebral hemorrhage within 24 hours of randomization as its primary safety measures.77
Neuroprotection
The extent of ischemic injury in the brain depends on the level of cerebral blood flow in the affected territory. Cerebral blood flows less than 10 mL/100 g/min are probably tolerated only for minutes, whereas intermediate levels of blood flow of 20 to 30 mL/100 g/min may be tolerated for several hours before irreversible changes occur.78 During ischemia, there is insufficient energy for maintenance of normal membrane pump activity. Sodium diffuses into the cell across its gradient, causing neuronal depolarization and impairing the ability of the neuron to generate an action potential. In addition, there is an outpouring of excitatory neurotransmitters, particularly glutamate. Glutamate activates N-methyl-D-aspartate and non–N-methyl-D-aspartate receptors, causing influx of calcium into neurons.79 This influx results in production of toxic products including nitric oxide, free radicals, and activation of phospholipases. The duration of reversibility of ischemia is uncertain, but animal models of focal stroke suggest it is only a few hours.2
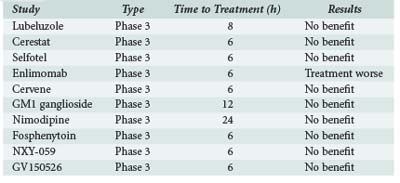
In animal models of stroke, many drugs have shown promise in reducing infarct size and improving function.80 Unfortunately, attempts to extrapolate these findings to human stroke in phase 3 trials have largely failed to provide any evidence of efficacy. One prominent example studied in recent years was NXY-059, a nitrone spin-trap agent that showed improved outcomes in nonhuman models of acute stroke. The SAINT I and SAINT II trials assessed the efficacy of IV NXY-059 within 6 hours of stroke onset. A pooled analysis showed that NXY-059 was ineffective for treatment of stroke patients within 6 hours, and this finding applied to all subgroups.81,82 Despite this negative result, as well as those for other neuroprotection agents (Table 34-5), there has been no deterrence to the continued investment in investigating neuroprotection agents for their efficacy in stroke.
Albumin
Albumin is the protein of highest concentration in plasma. Albumin transports many small molecules in blood and is of prime importance in keeping the fluid from blood from leaking out into the tissues. Albumin thus may help to minimize damage related to ischemia. The ALIAS trial is an ongoing trial evaluating whether high-dose serum albumin (2 g/kg, administered over 2 hours) given to patients within 5 hours of stroke onset improves clinical outcome at 3 months.83,84 The mechanism by which albumin provides neuroprotection is unclear. Although albumin causes hemodilution and increased cerebral blood flow, it also has many other effects, including reduction of cerebral edema and diminished platelet aggregation. In addition, albumin may act as an oxygen radical scavenger and antioxidant.
Magnesium
Within in vitro models, magnesium has been shown to relax vascular smooth muscle and result in vasodilation of vascular beds and increased cerebral blood flow, replete an ischemia-induced magnesium-deficient state, inhibit excitatory neurotransmitters from presynaptic vesicles, and block the NMDA receptor, among its intrinsic properties. The IMAGES trial evaluated whether IV magnesium given within 12 hours of stroke onset could significantly reduce the chances of death or disability. Unfortunately, these outcome measures were not realized, although a benefit in lacunar strokes was suggested.85 The ongoing FAST-MAG trial is looking at whether hyperacute paramedic-initiated IV magnesium sulfate administration improves long-term functional outcome. Half of the participants randomized to magnesium therapy will receive treatment within 1 hour of stroke onset with a 4-g bolus dose over 15 minutes, followed by an in-hospital infusion of 16 g over 24 hours. The other half of the randomized treatment group will be treated within 1 to 2 hours.86
Minocycline
Minocycline is a semisynthetic tetracycline that has antibacterial and antiinflammatory effects. There is strong preclinical data that minocycline can effectively reduce infarct size and improve functional outcome in animal stroke models.87–90 Minocycline also inhibits matrix metalloproteinase 9 (MMP-9), which helps mediate tissue injury during human ischemic stroke and is also associated with intracranial hemorrhage after tPA. Once the efficacy of IV doses (i.e., 3 mg/kg) of minocycline tolerable to the human body was established,91 subsequent human clinical trials using minocycline were initiated. A trial of oral minocycline within 6 to 24 hours of symptom onset improved outcome at 7, 30, and 90 days after stroke.92 The MINO trial is an early-phase trial underway to determine the safety of 4 escalating doses (i.e., 3 mg/kg to 10 mg/kg) of minocycline in acute ischemic stroke patients.
Hypothermia
Hypothermia has been recognized to reduce cerebral edema and intracranial pressure in patients with traumatic brain injury (TBI), and its efficacy for improving outcome in patients with post cardiac arrest hypoxic-ischemic brain injury is well documented.93–96 Mild to moderate hypothermia (i.e., core temperature > 32.0°C) appears to be the most accepted therapeutic range for focal or global ischemia. Adverse systemic effects at this therapeutic range are limited to confusion, shivering, catecholamine release, peripheral vasoconstriction, and cold-induced diuresis.97
There have been a few small pilot studies evaluating hypothermia as a treatment for acute ischemic stroke. The COOL-AID feasibility trial of endovascular cooling randomized acute ischemic stroke patients within 12 hours from symptom onset between a hypothermia group and a placebo group. An endovascular cooling device was inserted into the inferior vena cava of those patients randomized to hypothermia. A core body temperature of 33.0°C was targeted for 24 hours. Induced moderate hypothermia was found to be feasible in most patients with acute ischemic stroke.98 The ICTuS-L study recently confirmed that endovascular hypothermia after stroke can be safely combined with IV tPA in patients within 6 hours from stroke onset, but it was noted that pneumonia occurred more frequently after hypothermia treatment.99 There are also two currently ongoing international parallel studies (i.e., CHIL and CHILI) examining whether mild hypothermia administered either by systemic or local head cooling attenuates infarct expansion and salvages penumbral brain tissue, using imaging outcome parameters. Hypothermia is also being studied in both animal models and human clinical trials in combination with minocycline and magnesium.89,100
Neurorestoration
Citicoline
Citicoline is an exogenous choline precursor that once ingested is converted to choline in the body. Choline fosters the maintenance, repair, and de novo formation of cell membrane phospholipids as well as acetylcholine and dopamine.101,102 In a meta-analysis of hemorrhagic or ischemic stroke trials using citicoline over extended periods of treatment, there was a statistically significant reduction in the rate of death or dependency at long-term follow-up.101 Citicoline has also been shown to have a significant impact on reducing lesion volume growth in ischemic stroke, based on MRI outcome measures from baseline to week 12 of treatment.103 The ICTUS trial in Europe is ongoing and involves administering 1000 mg of citicoline IV every 12 hours during the first 3 days and orally from day 4 until the end of the 6-week treatment period.104 The results of this trial will be highly anticipated when available.
Stem Cell Therapy
Stem cells are present to a limited extent in adult tissue and may offer a new frontier into neurorestorative stroke therapy if their pluripotency can be harnessed. Many different cell types are available, ranging from embryonic stem cells to neural progenitor cells and immortalized tumor cells. Potential sources of stem cells include bone marrow, umbilical cord blood, and embryonic sources. Cells can now be reengineered to return to a more primitive pluripotent state and later differentiate into neuronal cell types. In animal models of stroke, stem cells stereotactically injected into the area of stroke reduce infarct size and improve function.105–107 Rodent studies in TBI models have shown that stem cells remain in tissues 2 weeks after being incorporated, with improvement in motor function tests.108 Several small human safety studies have been completed. Using immortalized tumor cells injected into the basal ganglia, Kondziolka et al. found no significant cell-related complications and a suggestion of clinical improvement in some patients.109,110 A study of five patients treated with porcine xenografts was halted because of two complications causing transient neurologic worsening.111 Another small trial of IV bone marrow stem cells also demonstrated safety.112 A study examining children with acute TBI treated with autologous stem cells is completed, with results pending. Drawing from these findings, there is a current trial assessing the safety and feasibility of autologous mononuclear bone marrow stem cell treatment in adult ischemic stroke patients. The study design calls for bone marrow aspiration and subsequent infusion of autologous stem cells in patients who have recently (i.e., 24 to 72 hours) suffered an acute ischemic stroke. Many important questions regarding cell therapy for stroke remain, including the optimal cell type, route of administration, timing of treatment, adjuvant therapies, number of cells, and selection of patients.
Surgical Options
Hemicraniectomy is the first and most commonly performed procedure. It involves removal of a generous bone flap ipsilateral to the side of the infarction. Often a durotomy is performed in order to allow outward herniation of the brain to decrease ICP and prevent downward herniation. For large MCA infarctions, timing of surgery, side of lesion, presence of signs of herniation prior to surgery, and involvement of other vascular territories were analyzed but were found to not significantly affect outcome.113 This analysis was obtained from uncontrolled, retrospective data; therefore, no formal meta-analysis could be completed.
The optimal timing of hemicraniectomy in patients with malignant MCA infarction is unclear. If herniation is already in progress, irreversible brainstem damage may occur, thereby limiting the benefit of the operation. More recent evidence suggests that surgical intervention should occur early regardless of whether signs of herniation are present. Three concurrent European trials (i.e., DECIMAL, DESTINY, HAMLET) including patients undergoing hemicraniectomy for malignant MCA infarction were combined in a pooled analysis. The three trials had similar inclusion/exclusion criteria, except for time from stroke onset to surgery. The time from stroke onset to surgery was 30 hours, 36 hours, and 99 hours in DECIMAL, DESTINY, and HAMLET, respectively.114–116 In the pooled analysis, thresholds were established for 45 hours to randomization and 48 hours to surgery from stroke onset. The combined results showed that decompressive surgery undertaken within 48 hours of stroke onset significantly decreased mortality and increased the number of patients with a favorable functional outcome.117
There has been a report of four patients with cerebral edema after stroke with impending herniation who experienced a “strokectomy” based on results of xenon CT CBF studies indicating areas of nearly absent flow.118 The imaging studies help guide surgical removal by providing information to avoid areas of intact cortex. This procedure prevents fatal herniation, but whether long-term outcome is truly improved must be determined by future randomized clinical trials.
Surgical decompression for hemispheric infarction should be considered for younger patients with a greater potential for recovery from massive stroke or patients with large non-dominant hemispheric strokes. Cerebellar infarction is a special case that clearly requires urgent surgical intervention.119 Compression of the brainstem and fourth ventricle leading to hydrocephalus or severe pontomedullary compromise can be reversed by rapid surgical decompression of the infarcted cerebellum.
Other Medical Therapies
Anticoagulation
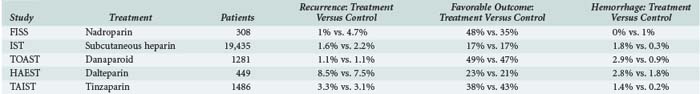
The use of anticoagulants in acute stroke is controversial, although several randomized clinical trials provide information regarding its efficacy. Retrospective data previously suggested a significant incidence of early recurrences after ischemic stroke, with reported rates of 20%. These studies also suggested that anticoagulation with heparin reduced recurrences. Hemorrhagic complications were acceptably low, particularly when patients with large strokes and uncontrolled hypertension were excluded from treatment. The results of recent randomized clinical trials have challenged these findings and call into question the value of anticoagulation for treatment of acute stroke.120 However, more recent studies indicate that for cardioembolic stroke, warfarin can be safely started shortly after stroke without bridging therapy with heparin or enoxaparin.121 The major results of these studies are summarized in Table 34-6.
The studies do not support a reduced recurrence rate or improved outcome with anticoagulation when administered within 24 to 48 hours of stroke onset. Hemorrhage rates ranged from 1% to 2.5%. The results suggest that there is little value in anticoagulation for all patients with acute stroke, but it remains possible that some subgroups benefit. The TOAST study suggested that patients with large vessel disease may achieve better functional outcome with anticoagulation.122 The relatively high hemorrhage rate in some studies also may have obscured some benefit. In the International Stroke Trial (IST), a significant reduction in recurrent strokes from 3.8% in the control group to 2.9% in patients treated with subcutaneous heparin (P < 0.01) was offset by an increase in hemorrhagic stroke from 0.4% in controls to 1.2% in patients receiving heparin (P < 0.00001).123 Even in patients with atrial fibrillation, the value of early anticoagulation is uncertain, with some studies showing benefit and others showing lack of benefit in reducing recurrent stroke.120 If anticoagulation is to be started, it should only be given more than 24 hours after IV thrombolysis and following imaging confirmation that no hemorrhagic transformation of the ischemic stroke has occurred. The roles of newer anticoagulant drugs in development (e.g., rivaroxaban, apixaban, dabigatran) in the acute stroke setting will need to be addressed as they become available.
Antiplatelet Therapy
There is less uncertainty about the benefit of aspirin in acute stroke. Two large randomized controlled trials, CAST124 and IST,123 showed a small but significant improvement in outcome in patients treated with aspirin. In IST, patients received 300 mg of aspirin daily for 14 days. There was a significant reduction in stroke recurrence within 14 days in the aspirin group (2.8%) versus nonaspirin groups (3.9%) and a significant decrease in the risk of death or nonfatal recurrent stroke in the aspirin group (11.3%) versus nonaspirin groups (12.4%). In CAST, 160 mg of aspirin was given per day for 4 weeks or until hospital discharge. In the aspirin group, there was a significant reduction in death within 4 weeks (3.3%) versus placebo (3.9%) and a significant reduction in death or nonfatal stroke during hospitalization. There also was a significant reduction in recurrent ischemic strokes in the aspirin group (1.6%) versus placebo (2.1%), which was offset only by a non-significant trend of excess hemorrhagic strokes (aspirin 1.1% versus placebo 0.9%).
CAST and IST were designed to be considered together and include more than 40,000 patients. Combining the results of both studies shows a significant reduction in recurrent stroke of 7 per 1000 (P < 0.000001) and reduction of death or dependency of 12 per 1000 (P = 0.01).125 The risk of aspirin in the absence of thrombolytics is minimal, and the small but significant benefit argues in favor of routine treatment, but only after 24 hours if IV thrombolysis has been used and there is confirmation that there is no hemorrhagic transformation.
Statin Therapy
Statins have been shown to reduce the incidence of strokes among patients who are at increased risk for cardiovascular disease. However, whether statins reduce the risk of stroke after a recent stroke or TIA was not firmly established until the SPARCL trial was completed. In SPARCL, patients who had a stroke or TIA within 1 to 6 months prior to randomization, had LDL of 100 to 190, and had no known coronary artery disease were randomized to receive 80 mg of atorvastatin or placebo. The primary endpoint was a first nonfatal or fatal stroke. In the cohort receiving high-dose atorvastatin, the overall incidence of strokes and cardiovascular events was significantly reduced. These findings argue that high-dose atorvastatin should be administered in the setting of acute ischemic stroke.126
 Special ICU Management Considerations
Special ICU Management Considerations
General Assessment
Blood Pressure
Hypertension commonly accompanies ischemic stroke, and in most cases abrupt lowering of blood pressure (BP) is not advised because of the risk of causing further impairment of perfusion in the ischemic region.127 When a systemic or cardiac reason for reducing BP is present, such as aortic dissection or acute myocardial infarction, the relative importance of the systemic and neurologic issues must be considered. Hypertensive encephalopathy is a syndrome of extreme hypertension, papilledema, altered mental status, microangiopathic hemolytic anemia, and renal insufficiency that responds to lowering BP. In the absence of papilledema or systemic features, it is unlikely that acute neurologic deficits are due to hypertensive encephalopathy, and acutely lowering BP is more likely to worsen deficits rather than improve them.
When thrombolytic therapy is considered, reducing BP within the prescribed limits is necessary. Before thrombolytic therapy is administered, systolic BP should be less than 185 mm Hg and diastolic less than 110 mm Hg.16 Labetalol typically is administered in increasing doses every 5 to 10 minutes to control BP. If beta-blockers cannot be used, enalapril is a reasonable alternative. Sublingual nifedipine should be avoided because of the potential to lower BP precipitously. If these agents do not provide adequate control, a nicardipine drip could be considered, although such patients may not be good candidates for thrombolysis. Following thrombolysis, BP should be aggressively controlled, keeping systolic BP below 185 mm Hg and diastolic below 110 mm Hg for the first 24 hours.
Fluids
There has been some literature on the role of hypertonic saline, ranging from 3% to 23% concentration, in TBI patients. However, its role in the treatment of acute ischemic stroke and its ability to minimize cerebral edema remains controversial. Those who oppose its use cite that it can lead to rebound parenchymal swelling once it is weaned off. Proponents will usually use a goal serum sodium range of 145 to 150 mEq/L and a serum osmolality goal of 315 to 320 mOsm/L. Serum sodium and osmolality levels are usually checked every 6 hours.128, 129
Glucose
Evidence from animal models of stroke suggests that hyperglycemia increases the severity of ischemic injury.130 Increased glucose concentration in the area of ischemia causes higher lactate concentrations and local acidosis, which increases free radical formation and thus damages neurons. Hyperglycemia also may increase ischemic edema, release excitatory amino acid neurotransmitters, and damage blood vessels in the ischemic area.
Studies of stroke in humans show an inconsistent association between stroke outcome and initial blood glucose; however, admission glucose concentration correlates with initial stroke severity. Initial hyperglycemia also has been associated with higher mortality rates after stroke.131 Some authors have suggested that hyperglycemia in acute stroke is a stress reaction, but the relationship between initial blood glucose concentration and outcome is independent of initial stroke severity, arguing against a stress phenomenon.
The GIST-UK trial investigated whether treatment with a glucose-potassium-insulin (GKI) infusion to maintain euglycemia immediately after the acute stroke event had an impact on mortality at 90 days. This trial was stopped due to slow enrollment but concluded that GKI infusions significantly reduced plasma glucose concentrations and BP, but treatment within the trial protocol was not associated with significant clinical benefit. It is notable that the study was underpowered, and alternative results should not be dismissed.132 The GRASP pilot trial found that insulin infusion for patients with acute ischemic stroke is feasible and safe. In this trial, three treatment arms were used utilizing tight control (70 to 110 mg/dL), loose control (70 to 200 mg/dL), and usual control (70 to 300 mg/dL).133 Additional comparative studies are being pursued, and results from these trials should help clarify future treatment regimens in an effort to improve functional outcomes.
Key Points
Chen ZM, Sandercock P, Pan HC, et al. Indications for early aspirin use in acute ischemic stroke: a combined analysis of 40,000 randomized patients from the Chinese Acute Stroke Trial and the International Stroke Trial. On behalf of the CAST and IST collaborative groups. Stroke. 2000;31:1240-1249.
Smith WS, Sung G, Starkman S, et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke. 2005;36(7):1432-1438.
Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. JAMA. 1999;282(21):2003-2011.
Amarenco P, Bogousslavsky J, Callahan A3rd, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355(6):549-559.
Vahedi K, Hofmeijer J, Juettler E, et al. Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurol. 2007;6(3):215-222.
Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317-1329.
The ATLANTIS, ECASS and NINDS rt-PA Study Group Investigators. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS and NINDS rt-PA stroke trials. Lancet. 2004;363(9411):768-774.
The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581-1587.
1 Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart Disease and Stroke Statistics–2010 Update. A Report From the American Heart Association. Circulation. 2009 Dec 17.
2 Jones TH, Morawetz RB, Crowell RM, et al. Thresholds of focal cerebral ischemia in awake monkeys. J Neurosurg. 1981 Jun;54(6):773-782.
3 Baron JC. Perfusion thresholds in human cerebral ischemia: historical perspective and therapeutic implications. Cerebrovasc Dis. 2001;11(Suppl 1):2-8.
4 Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995 Dec 14;333(24):1581-1587.
5 Adams HPJr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993 Jan;24(1):35-41.
6 Nogueira RG, Smith WS. Emergency treatment of acute ischemic stroke: expanding the time window. Curr Treat Options Neurol. 2009 Nov;11(6):433-443.
7 Molina CA, Saver JL. Extending reperfusion therapy for acute ischemic stroke: emerging pharmacological, mechanical, and imaging strategies. Stroke. 2005 Oct;36(10):2311-2320.
8 Tomura N, Uemura K, Inugami A, Fujita H, Higano S, Shishido F. Early CT finding in cerebral infarction: obscuration of the lentiform nucleus. Radiology. 1988 Aug;168(2):463-467.
9 Truwit CL, Barkovich AJ, Gean-Marton A, Hibri N, Norman D. Loss of the insular ribbon: another early CT sign of acute middle cerebral artery infarction. Radiology. 1990 Sep;176(3):801-806.
10 Moulin T, Cattin F, Crepin-Leblond T, et al. Early CT signs in acute middle cerebral artery infarction: predictive value for subsequent infarct locations and outcome. Neurology. 1996 Aug;47(2):366-375.
11 Jaillard A, Cornu C, Durieux A, Moulin T, et al. Hemorrhagic transformation in acute ischemic stroke. The MAST-E study. MAST-E Group. Stroke. 1999 Jul;30(7):1326-1332.
12 Tan X, Guo Y. Hyperdense basilar artery sign diagnoses acute posterior circulation stroke and predicts short-term outcome. Neuroradiology. 2010 Apr 1. [Epub ahead of print.]
13 Larrue V, von Kummer R, del Zoppo G, Bluhmki E. Hemorrhagic transformation in acute ischemic stroke. Potential contributing factors in the European Cooperative Acute Stroke Study. Stroke. 1997 May;28(5):957-960.
14 Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet. 2000 May 13;355(9216):1670-1674.
15 Pexman JH, Barber PA, Hill MD, et al. Use of the Alberta Stroke Program Early CT Score (ASPECTS) for assessing CT scans in patients with acute stroke. AJNR Am J Neuroradiol. 2001 Sep;22(8):1534-1542.
16 Adams HPJr, Brott TG, Furlan AJ, et al. Guidelines for Thrombolytic Therapy for Acute Stroke: a Supplement to the Guidelines for the Management of Patients with Acute Ischemic Stroke. A statement for healthcare professionals from a Special Writing Group of the Stroke Council, American Heart Association. Stroke. 1996 Sep;27(9):1711-1718.
17 Patel SC, Levine SR, Tilley BC, et al. Lack of clinical significance of early ischemic changes on computed tomography in acute stroke. JAMA. 2001 Dec 12;286(22):2830-2838.
18 Aronovich BD, Reider GII, Segev Y, Bornstein NM. Early CT changes and outcome of ischemic stroke. Eur J Neurol. 2004 Jan;11(1):63-65.
19 Shrier DA, Tanaka H, Numaguchi Y, Konno S, Patel U, Shibata D. CT angiography in the evaluation of acute stroke. AJNR Am J Neuroradiol. 1997 Jun-Jul;18(6):1011-1020.
20 Knauth M, von Kummer R, Jansen O, Hahnel S, Dorfler A, Sartor K. Potential of CT angiography in acute ischemic stroke. AJNR Am J Neuroradiol. 1997 Jun-Jul;18(6):1001-1010.
21 Lev MH, Segal AZ, Farkas J, et al. Utility of perfusion-weighted CT imaging in acute middle cerebral artery stroke treated with intra-arterial thrombolysis: prediction of final infarct volume and clinical outcome. Stroke. 2001 Sep;32(9):2021-2028.
22 Wintermark M, Reichhart M, Thiran JP, et al. Prognostic accuracy of cerebral blood flow measurement by perfusion computed tomography, at the time of emergency room admission, in acute stroke patients. Ann Neurol. 2002 Apr;51(4):417-432.
23 Ezzeddine MA, Lev MH, McDonald CT, et al. CT angiography with whole brain perfused blood volume imaging: added clinical value in the assessment of acute stroke. Stroke. 2002 Apr;33(4):959-966.
24 Hopyan J, Ciarallo A, Dowlatshahi D, et al. Certainty of stroke diagnosis: incremental benefit with CT perfusion over noncontrast CT and CT angiography. Radiology. 2010 Apr;255(1):142-153.
25 Gupta R, Jovin TG, Yonas H. Xenon CT cerebral blood flow in acute stroke. Neuroimaging Clin N Am. 2005 Aug;15(3):531-542. x
26 Jovin TG, Yonas H, Gebel JM, et al. The cortical ischemic core and not the consistently present penumbra is a determinant of clinical outcome in acute middle cerebral artery occlusion. Stroke. 2003 Oct;34(10):2426-2433.
27 Moseley ME, Kucharczyk J, Mintorovitch J, et al. Diffusion-weighted MR imaging of acute stroke: correlation with T2-weighted and magnetic susceptibility-enhanced MR imaging in cats. AJNR Am J Neuroradiol. 1990 May;11(3):423-429.
28 Mintorovitch J, Yang GY, Shimizu H, Kucharczyk J, Chan PH, Weinstein PR. Diffusion-weighted magnetic resonance imaging of acute focal cerebral ischemia: comparison of signal intensity with changes in brain water and Na+,K(+)-ATPase activity. J Cereb Blood Flow Metab. 1994 Mar;14(2):332-336.
29 Donnan GA, Baron JC, Ma H, Davis SM. Penumbral selection of patients for trials of acute stroke therapy. Lancet Neurol. 2009 Mar;8(3):261-269.
30 Hacke W, Albers G, Al-Rawi Y, et al. The Desmoteplase in Acute Ischemic Stroke Trial (DIAS): a phase II MRI-based 9-hour window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke. 2005 Jan;36(1):66-73.
31 Hacke W, Furlan AJ, Al-Rawi Y, et al. Intravenous desmoteplase in patients with acute ischaemic stroke selected by MRI perfusion-diffusion weighted imaging or perfusion CT (DIAS-2): a prospective, randomised, double-blind, placebo-controlled study. Lancet Neurol. 2009 Feb;8(2):141-150.
32 Furlan AJ, Eyding D, Albers GW, et al. Dose Escalation of Desmoteplase for Acute Ischemic Stroke (DEDAS): evidence of safety and efficacy 3 to 9 hours after stroke onset. Stroke. 2006 May;37(5):1227-1231.
33 Albers GW, Thijs VN, Wechsler L, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol. 2006 Nov;60(5):508-517.
34 Davis SM, Donnan GA, Parsons MW, et al. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol. 2008 Apr;7(4):299-309.
35 Parsons MW, Barber PA, Chalk J, et al. Diffusion- and perfusion-weighted MRI response to thrombolysis in stroke. Ann Neurol. 2002 Jan;51(1):28-37.
36 Singer OC, Humpich MC, Fiehler J, et al. Risk for symptomatic intracerebral hemorrhage after thrombolysis assessed by diffusion-weighted magnetic resonance imaging. Ann Neurol. 2008 Jan;63(1):52-60.
37 Debrey SM, Yu H, Lynch JK, et al. Diagnostic accuracy of magnetic resonance angiography for internal carotid artery disease: a systematic review and meta-analysis. Stroke. 2008 Aug;39(8):2237-2248.
38 Ruggieri PM, Masaryk TJ, Ross JS. Magnetic resonance angiography. Cerebrovascular applications. Stroke. 1992 May;23(5):774-780.
39 Meyer JS, Gilroy J, Barnhart MI, Johnson JF. Anticoagulants Plus Streptokinase Therapy in Progressive Stroke. JAMA. 1964 Aug 3;189:373.
40 Meyer JS, Gilroy J, Barnhart MI, Johnson JF. Therapeutic Thrombolysis in Cerebral Thromboembolism. Double-Blind Evaluation of Intravenous Plasmin Therapy in Carotid and Middle Cerebral Arterial Occlusion. Neurology. 1963 Nov;13:927-937.
41 Fletcher AP, Alkjaersig N, Lewis M, et al. A pilot study of urokinase therapy in cerebral infarction. Stroke. 1976 Mar-Apr;7(2):135-142.
42 del Zoppo GJ, Ferbert A, Otis S, et al. Local intra-arterial fibrinolytic therapy in acute carotid territory stroke. A pilot study. Stroke. 1988 Mar;19(3):307-313.
43 Hacke W, Zeumer H, Ferbert A, Bruckmann H, del Zoppo GJ. Intra-arterial thrombolytic therapy improves outcome in patients with acute vertebrobasilar occlusive disease. Stroke. 1988 Oct;19(10):1216-1222.
44 Haley ECJr, Brott TG, Sheppard GL, et al. Pilot randomized trial of tissue plasminogen activator in acute ischemic stroke. The tPA Bridging Study Group. Stroke. 1993 Jul;24(7):1000-1004.
45 Mori E, Yoneda Y, Tabuchi M, et al. Intravenous recombinant tissue plasminogen activator in acute carotid artery territory stroke. Neurology. 1992 May;42(5):976-982.
46 Kwiatkowski TG, Libman RB, Frankel M, et al. Effects of tissue plasminogen activator for acute ischemic stroke at one year. National Institute of Neurological Disorders and Stroke Recombinant Tissue Plasminogen Activator Stroke Study Group. N Engl J Med. 1999 Jun 10;340(23):1781-1787.
47 Generalized efficacy of t-PA for acute stroke. Subgroup analysis of the NINDS t-PA Stroke Trial. Stroke. 1997 Nov;28(11):2119-2125.
48 Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. The NINDS t-PA Stroke Study Group. Stroke. 1997 Nov;28(11):2109-2118.
49 Albers GW, Clark WM, Madden KP, Hamilton SA. ATLANTIS trial: results for patients treated within 3 hours of stroke onset. Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. Stroke. 2002 Feb;33(2):493-495.
50 Hacke W, Donnan G, Fieschi C, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004 Mar 6;363(9411):768-774.
51 del Zoppo GJ, Poeck K, Pessin MS, et al. Recombinant tissue plasminogen activator in acute thrombotic and embolic stroke. Ann Neurol. 1992 Jul;32(1):78-86.
52 Clark WM, Wissman S, Albers GW, Jhamandas JH, Madden KP, Hamilton S. Recombinant tissue-type plasminogen activator (Alteplase) for ischemic stroke 3 to 5 hours after symptom onset. The ATLANTIS Study: a randomized controlled trial. Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. JAMA. 1999 Dec 1;282(21):2019-2026.
53 Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA. 1995 Oct 4;274(13):1017-1025.
54 Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet. 1998 Oct 17;352(9136):1245-1251.
55 Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008 Sep 25;359(13):1317-1329.
56 Van De Werf F, Adgey J, Ardissino D, et al. Single-bolus tenecteplase compared with front-loaded alteplase in acute myocardial infarction: the ASSENT-2 double-blind randomised trial. Lancet. 1999 Aug 28;354(9180):716-722.
57 Haley ECJr, Lyden PD, Johnston KC, Hemmen TM. A pilot dose-escalation safety study of tenecteplase in acute ischemic stroke. Stroke. 2005 Mar;36(3):607-612.
58 Haley ECJr, Thompson JL, Grotta JC, et al. Phase IIB/III trial of tenecteplase in acute ischemic stroke: results of a prematurely terminated randomized clinical trial. Stroke. 2010 Apr;41(4):707-711.
59 Randomised, double-blind comparison of reteplase double-bolus administration with streptokinase in acute myocardial infarction (INJECT): trial to investigate equivalence. International Joint Efficacy Comparison of Thrombolytics. Lancet. 1995 Aug 5;346(8971):329-336.
60 Qureshi AI, Pande RU, Kim SH, Hanel RA, Kirmani JF, Yahia AM. Third generation thrombolytics for the treatment of ischemic stroke. Curr Opin Investig Drugs. 2002 Dec;3(12):1729-1732.
61 Qureshi AI, Harris-Lane P, Kirmani JF, et al. Intra-arterial reteplase and intravenous abciximab in patients with acute ischemic stroke: an open-label, dose-ranging, phase I study. Neurosurgery. 2006 Oct;59(4):789-796. discussion 96-7
62 Emergency administration of abciximab for treatment of patients with acute ischemic stroke: results of a randomized phase 2 trial. Stroke. 2005 Apr;36(4):880-890.
63 Adams HPJr, Effron MB, Torner J, et al. Emergency administration of abciximab for treatment of patients with acute ischemic stroke: results of an international phase III trial: Abciximab in Emergency Treatment of Stroke Trial (AbESTT-II). Stroke. 2008 Jan;39(1):87-99.
64 Alexandrov AV, Molina CA, Grotta JC, et al. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med. 2004 Nov 18;351(21):2170-2178.
65 Molina CA, Barreto AD, Tsivgoulis G, et al. Transcranial ultrasound in clinical sonothrombolysis (TUCSON) trial. Ann Neurol. 2009 Jul;66(1):28-38.
66 Barr JD, Mathis JM, Wildenhain SL, Wechsler L, Jungreis CA, Horton JA. Acute stroke intervention with intraarterial urokinase infusion. J Vasc Interv Radiol. 1994 Sep-Oct;5(5):705-713.
67 del Zoppo GJ, Higashida RT, Furlan AJ, Pessin MS, Rowley HA, Gent M. PROACT: a phase II randomized trial of recombinant pro-urokinase by direct arterial delivery in acute middle cerebral artery stroke. PROACT Investigators. Prolyse in Acute Cerebral Thromboembolism. Stroke. 1998 Jan;29(1):4-11.
68 Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA. 1999 Dec 1;282(21):2003-2011.
69 Wechsler LR, Roberts R, Furlan AJ, et al. Factors influencing outcome and treatment effect in PROACT II. Stroke. 2003 May;34(5):1224-1229.
70 Qureshi AI, Suri MF, Shatla AA, et al. Intraarterial recombinant tissue plasminogen activator for ischemic stroke: an accelerating dosing regimen. Neurosurgery. 2000 Aug;47(2):473-476. discussion 7-9
71 Smith WS, Sung G, Starkman S, et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke. 2005 Jul;36(7):1432-1438.
72 Smith WS, Sung G, Saver J, et al. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke. 2008 Apr;39(4):1205-1212.
73 Penumbra Pivotal Stroke Trial Investigators. The penumbra pivotal stroke trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke. 2009 Aug;40(8):2761-2768.
74 Abou-Chebl A, Vora N, Yadav JS. Safety of angioplasty and stenting without thrombolysis for the treatment of early ischemic stroke. J Neuroimaging. 2009 Apr;19(2):139-143.
75 The IMS Study Investigators. Combined intravenous and intra-arterial recanalization for acute ischemic stroke: the Interventional Management of Stroke Study. Stroke. 2004 Apr;35(4):904-911.
76 IMS II Trial Investigators. The Interventional Management of Stroke (IMS) II Study. Stroke. 2007 Jul;38(7):2127-2135.
77 Khatri P, Hill MD, Palesch YY, et al. Methodology of the Interventional Management of Stroke III Trial. Int J Stroke. 2008 May;3(2):130-137.
78 Astrup J, Siesjo BK, Symon L. Thresholds in cerebral ischemia – the ischemic penumbra. Stroke. 1981 Nov-Dec;12(6):723-725.
79 Simon RP, Swan JH, Griffiths T, Meldrum BS. Blockade of N-methyl-D-aspartate receptors may protect against ischemic damage in the brain. Science. 1984 Nov 16;226(4676):850-852.
80 Labiche LA, Grotta JC. Clinical trials for cytoprotection in stroke. NeuroRx. 2004 Jan;1(1):46-70.
81 Lees KR, Zivin JA, Ashwood T, et al. NXY-059 for acute ischemic stroke. N Engl J Med. 2006 Feb 9;354(6):588-600.
82 Diener HC, Lees KR, Lyden P, et al. NXY-059 for the treatment of acute stroke: pooled analysis of the SAINT I and II Trials. Stroke. 2008 Jun;39(6):1751-1758.
83 Ginsberg MD, Hill MD, Palesch YY, Ryckborst KJ, Tamariz D. The ALIAS Pilot Trial: a dose-escalation and safety study of albumin therapy for acute ischemic stroke–I: Physiological responses and safety results. Stroke. 2006 Aug;37(8):2100-2106.
84 Hill MD, Moy CS, Palesch YY, et al. The albumin in acute stroke trial (ALIAS); design and methodology. Int J. Stroke. 2007 Aug;2(3):214-219.
85 Muir KW, Lees KR, Ford I, Davis S. Magnesium for acute stroke (Intravenous Magnesium Efficacy in Stroke trial): randomised controlled trial. Lancet. 2004 Feb 7;363(9407):439-445.
86 Saver JL, Kidwell C, Eckstein M, Starkman S. Prehospital neuroprotective therapy for acute stroke: results of the Field Administration of Stroke Therapy-Magnesium (FAST-MAG) pilot trial. Stroke. 2004 May;35(5):e106-e108.
87 Yrjanheikki J, Tikka T, Keinanen R, Goldsteins G, Chan PH, Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci U S A. 1999 Nov 9;96(23):13496-13500.
88 Yrjanheikki J, Keinanen R, Pellikka M, Hokfelt T, Koistinaho J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci U S A. 1998 Dec 22;95(26):15769-15774.
89 Nagel S, Su Y, Horstmann S, et al. Minocycline and hypothermia for reperfusion injury after focal cerebral ischemia in the rat: effects on BBB breakdown and MMP expression in the acute and subacute phase. Brain Res. 2008 Jan 10;1188:198-206.
90 Wang CX, Yang T, Noor R, Shuaib A. Delayed minocycline but not delayed mild hypothermia protects against embolic stroke. BMC Neurol. 2002 Apr 18;2:2.
91 Xu L, Fagan SC, Waller JL, et al. Low dose intravenous minocycline is neuroprotective after middle cerebral artery occlusion-reperfusion in rats. BMC Neurol. 2004 Apr 26;4:7.
92 Lampl Y, Boaz M, Gilad R, et al. Minocycline treatment in acute stroke: an open-label, evaluator-blinded study. Neurology. 2007 Oct 2;69(14):1404-1410.
93 Abate MG, Cadore B, Citerio G. Hypothermia in adult neurocritical patients: a very ‘ ‘hot’ ’ strategy not to be hibernated yet!. Minerva Anestesiol. 2008 Jul-Aug;74(7-8):425-430.
94 Rincon F, Mayer SA. Therapeutic hypothermia for brain injury after cardiac arrest. Semin Neurol. 2006 Sep;26(4):387-395.
95 Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002 Feb 21;346(8):549-556.
96 Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005 Oct 13;353(15):1574-1584.
97 Hammer MD, Krieger DW. Acute ischemic stroke: is there a role for hypothermia? Cleve Clin J Med. 2002 Oct;69(10):770. 3-4, 6-7 passim
98 De Georgia MA, Krieger DW, Abou-Chebl A, et al. Cooling for Acute Ischemic Brain Damage (COOL AID): a feasibility trial of endovascular cooling. Neurology. 2004 Jul 27;63(2):312-317.
99 Hemmen TM, Raman R, Gomez JA, et al. Intravenous Thrombolysis Plus Hypothermia for Acute Treatment of Ischemic Stroke (ICTuS-L): Final Results. 2010 February 26, 2010.
100 Meloni BP, Campbell K, Zhu H, Knuckey NW. In search of clinical neuroprotection after brain ischemia: the case for mild hypothermia (35 degrees C) and magnesium. Stroke. 2009 Jun;40(6):2236-2240.
101 Saver JL. Citicoline: update on a promising and widely available agent for neuroprotection and neurorepair. Rev Neurol Dis. 2008 Fall;5(4):167-177.
102 Secades JJ, Lorenzo JL. Citicoline: pharmacological and clinical review, 2006 update. Methods Find Exp Clin Pharmacol. 2006 Sep;28(Suppl B):1-56.
103 Warach S, Pettigrew LC, Dashe JF, et al. Effect of citicoline on ischemic lesions as measured by diffusion-weighted magnetic resonance imaging. Citicoline 010 Investigators. Ann Neurol. 2000 Nov;48(5):713-722.
104 Gutierrez M, Merino JJ, de Lecinana MA, Diez-Tejedor E. Cerebral protection, brain repair, plasticity and cell therapy in ischemic stroke. Cerebrovasc Dis. 2009;27(Suppl 1):177-186.
105 Kim DY, Park SH, Lee SU, et al. Effect of human embryonic stem cell-derived neuronal precursor cell transplantation into the cerebral infarct model of rat with exercise. Neurosci Res. 2007 Jun;58(2):164-175.
106 Rice HE, Hsu EW, Sheng H, et al. Superparamagnetic iron oxide labeling and transplantation of adipose-derived stem cells in middle cerebral artery occlusion-injured mice. AJR Am J Roentgenol. 2007 Apr;188(4):1101-1108.
107 Roitberg BZ, Mangubat E, Chen EY, et al. Survival and early differentiation of human neural stem cells transplanted in a nonhuman primate model of stroke. J Neurosurg. 2006 Jul;105(1):96-102.
108 Harting MT, Sloan LE, Jimenez F, Baumgartner J, Cox CSJr. Subacute neural stem cell therapy for traumatic brain injury. J Surg Res. 2009 May 15;153(2):188-194.
109 Kondziolka D, Wechsler L. Stroke repair with cell transplantation: neuronal cells, neuroprogenitor cells, and stem cells. Neurosurg Focus. 2008;24(3-4):E13.
110 Kondziolka D, Wechsler L, Goldstein S, et al. Transplantation of cultured human neuronal cells for patients with stroke. Neurology. 2000 Aug 22;55(4):565-569.
111 Savitz SI, Dinsmore JH, Wechsler LR, Rosenbaum DM, Caplan LR. Cell therapy for stroke. NeuroRx. 2004 Oct;1(4):406-414.
112 Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005 Jun;57(6):874-882.
113 Gupta R, Connolly ES, Mayer S, Elkind MS. Hemicraniectomy for massive middle cerebral artery territory infarction: a systematic review. Stroke. 2004 Feb;35(2):539-543.
114 Vahedi K, Vicaut E, Mateo J, et al. Sequential-design, multicenter, randomized, controlled trial of early decompressive craniectomy in malignant middle cerebral artery infarction (DECIMAL Trial). Stroke. 2007 Sep;38(9):2506-2517.
115 Juttler E, Schwab S, Schmiedek P, et al. Decompressive Surgery for the Treatment of Malignant Infarction of the Middle Cerebral Artery (DESTINY): a randomized, controlled trial. Stroke. 2007 Sep;38(9):2518-2525.
116 Hofmeijer J, Kappelle LJ, Algra A, Amelink GJ, van Gijn J, van der Worp HB. Surgical decompression for space-occupying cerebral infarction (the Hemicraniectomy After Middle Cerebral Artery infarction with Life-threatening Edema Trial [HAMLET]): a multicentre, open, randomised trial. Lancet Neurol. 2009 Apr;8(4):326-333.
117 Vahedi K, Hofmeijer J, Juettler E, et al. Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurol. 2007 Mar;6(3):215-222.
118 Kalia KK, Yonas H. An aggressive approach to massive middle cerebral artery infarction. Arch Neurol. 1993 Dec;50(12):1293-1297.
119 Heros RC. Surgical treatment of cerebellar infarction. Stroke. 1992 Jul;23(7):937-938.
120 Adams HPJr. Emergent use of anticoagulation for treatment of patients with ischemic stroke. Stroke. 2002 Mar;33(3):856-861.
121 Hallevi H, Albright KC, Martin-Schild S, et al. Anticoagulation after cardioembolic stroke: to bridge or not to bridge? Arch Neurol. 2008 Sep;65(9):1169-1173.
122 Low molecular weight heparinoid, ORG 10172 (danaparoid), and outcome after acute ischemic stroke: a randomized controlled trial. The Publications Committee for the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) Investigators. JAMA. 1998 Apr 22-29;279(16):1265-1272.
123 International Stroke Trial Collaborative Group. The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. Lancet. 1997 May 31;349(9065):1569-1581.
124 CAST (Chinese Acute Stroke Trial) Collaborative Group. CAST: randomised placebo-controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke. Lancet. 1997 Jun 7;349(9066):1641-1649.
125 Chen ZM, Sandercock P, Pan HC, et al. Indications for early aspirin use in acute ischemic stroke: A combined analysis of 40 000 randomized patients from the chinese acute stroke trial and the international stroke trial. On behalf of the CAST and IST collaborative groups. Stroke. 2000 Jun;31(6):1240-1249.
126 Amarenco P, Bogousslavsky J, Callahan A3rd, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006 Aug 10;355(6):549-559.
127 Lisk DR, Grotta JC, Lamki LM, et al. Should hypertension be treated after acute stroke? A randomized controlled trial using single photon emission computed tomography. Arch Neurol. 1993 Aug;50(8):855-862.
128 Steiner T, Ringleb P, Hacke W. Treatment options for large hemispheric stroke. Neurology. 2001;57(5 Suppl 2):S61-S68.
129 Bhardwaj A, Ulatowski JA. Hypertonic saline solutions in brain injury. Curr Opin Crit Care. 2004 Apr;10(2):126-131.
130 Lindsberg PJ, Roine RO. Hyperglycemia in acute stroke. Stroke. 2004 Feb;35(2):363-364.
131 Kruyt ND, Biessels GJ, Devries JH, Roos YB. Hyperglycemia in acute ischemic stroke: pathophysiology and clinical management. Nat Rev Neurol. 2010 Mar;6(3):145-155.
132 Gray CS, Hildreth AJ, Sandercock PA, et al. Glucose-potassium-insulin infusions in the management of post-stroke hyperglycaemia: the UK Glucose Insulin in Stroke Trial (GIST-UK). Lancet Neurol. 2007 May;6(5):397-406.
133 Johnston KC, Hall CE, Kissela BM, Bleck TP, Conaway MR. Glucose Regulation in Acute Stroke Patients (GRASP) trial: a randomized pilot trial. Stroke. 2009 Dec;40(12):3804-3809.