Chapter 6 Mammographic and Ultrasound-Guided Breast Biopsy Procedures
Biopsy of nonpalpable imaging-detected breast lesions is an important part of the breast imaging service. The advantage of percutaneous biopsy is that it can provide a diagnosis with a minimum of patient trauma, and the diagnosis can guide appropriate follow-up, including definitive surgery. If the diagnosis is cancer, the patient can decide on lumpectomy versus mastectomy. Furthermore, patients with invasive cancer can have both tumor excision and axillary lymph node biopsy at the first surgery. This chapter describes percutaneous x-ray– and ultrasound-guided breast needle biopsy techniques, preoperative needle localization, and imaging–pathology correlation. Magnetic resonance imaging (MRI)-guided breast procedures are covered in Chapter 7.
Prebiopsy Patient Workup
Nothing substitutes for complete imaging workup of nonpalpable breast lesions. The radiologist must have the lesion’s location within the breast firmly entrenched in his or her mind to plan an approach that will be successful in biopsying the lesion with safety and accuracy. For mammography, this means visualization of the lesion in craniocaudal and mediolateral orthogonal views (Box 6-1). When the finding is not seen definitively in craniocaudal (CC) and mediolateral views, the radiologist locates the lesion with fine-detail mammographic views, views with skin markers, triangulation, stereotactic targeting, ultrasound, and physical examination. This is to make sure the lesion is real and to determine its location in the breast. For ultrasound, this means the lesion is visualized on orthogonal scans. Do not attempt to biopsy a breast lesion if you do not know whether it is real or if you do not know its location in the breast!
Box 6-1 Requirements for Nonpalpable Breast Lesion Biopsy
Lesion is seen in orthogonal views on mammography or is seen by ultrasound or MRI
Lesion can be accessed with safety and accuracy
Patient can cooperate and hold still during the procedure
Patient is not allergic to medications used in the biopsy procedure
Patient can follow postbiopsy instructions to diminish bleeding and other complications
Patient will comply with postbiopsy imaging or surgical follow-up
For a nonpalpable lesion to be biopsied with safety and accuracy, the patient must be able to cooperate and hold still during the procedure, have no allergies to medications used during the procedure, be able to follow postbiopsy instructions to diminish bleeding and other complications, and be compliant with postbiopsy follow-up (see Box 6-1).
Informed Consent
Informed consent is an important part of any procedure (Box 6-2). For percutaneous needle biopsy, the radiologist informs the patient of the risks, benefits, and alternatives to percutaneous biopsy (e.g., surgical biopsy), as well as the risks and benefits of any alternatives. The most common complication after core or vacuum needle biopsy is hematoma formation, but it is rarely significant. Other rare complications include untoward bleeding (very rarely requiring surgical intervention), infection (with mastitis very rare), pneumothorax, pseudoaneurysm formation, implant rupture, milk fistula (if the patient is pregnant or nursing), and vasovagal reactions (see Box 6-2). The patient is told that later surgical excision will be needed if the biopsy reveals a malignancy, high-risk lesion, or discordant benign lesion, or if the needle biopsy cannot be completed because of technical limitations (see Box 6-2). She is told that the postbiopsy metallic marker may end up in a suboptimal location. The patient is informed about wound management after the biopsy and about when and how to obtain biopsy results.
Preoperative Needle Localization
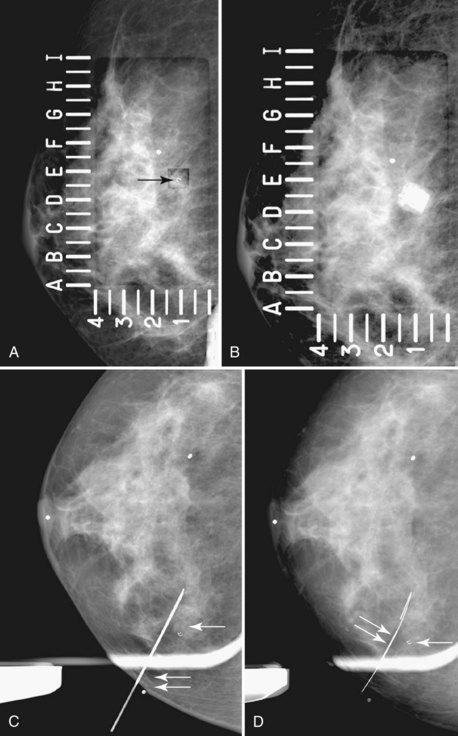
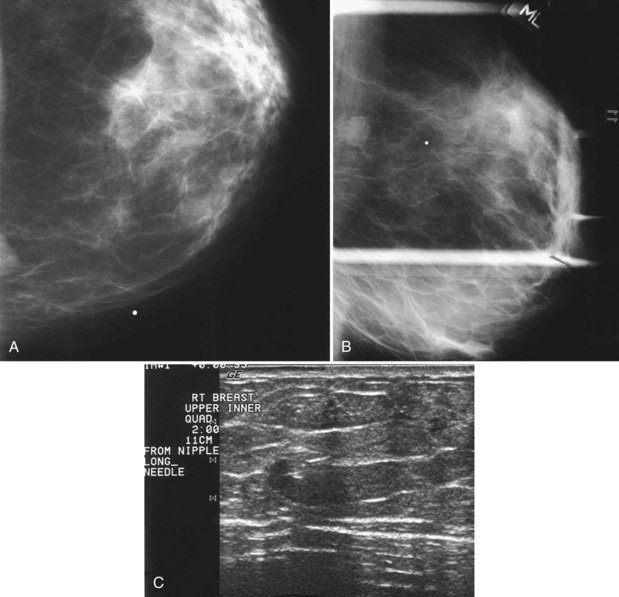
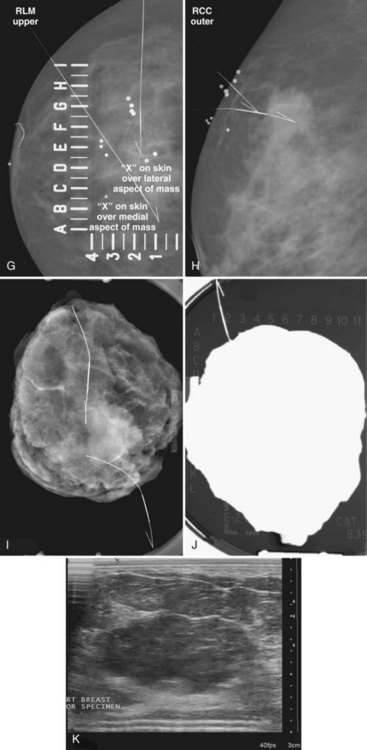
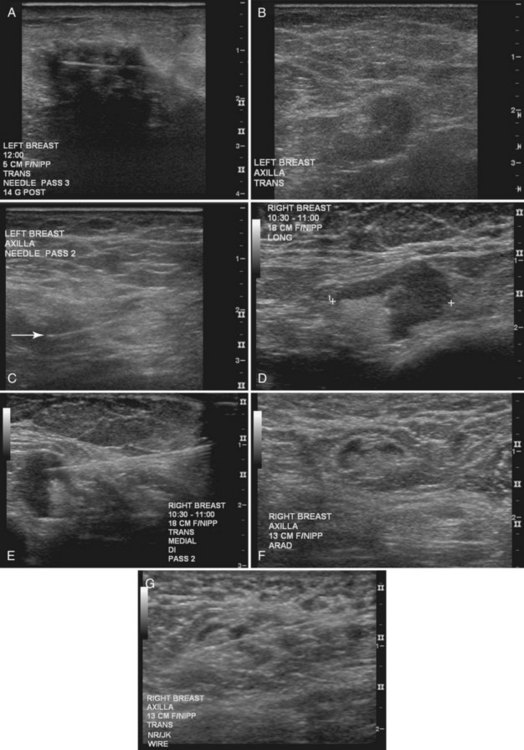
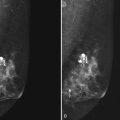
The surgeon uses the wire and mammograms to guide him or her to the lesion and excises the lesion and hookwire. The excised tissue is called a breast specimen. The technologist radiographs the breast specimen. The radiologist reviews the specimen radiograph to see if the lesion and the entire hookwire (with an intact hook) are included (Fig. 6-1A to G).
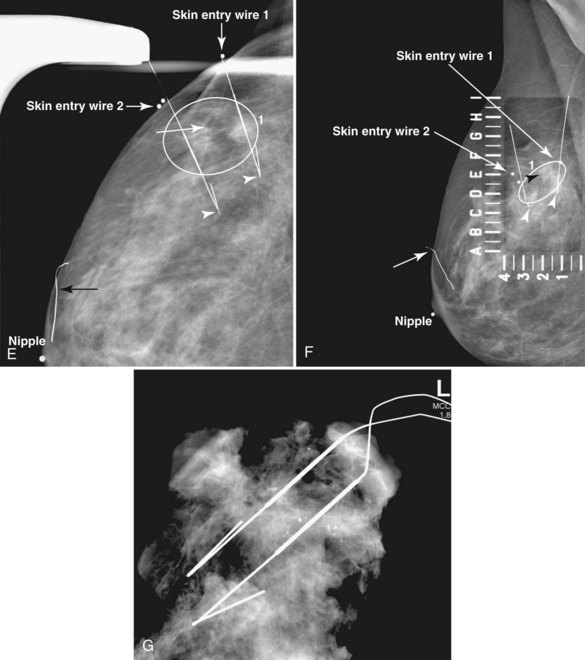
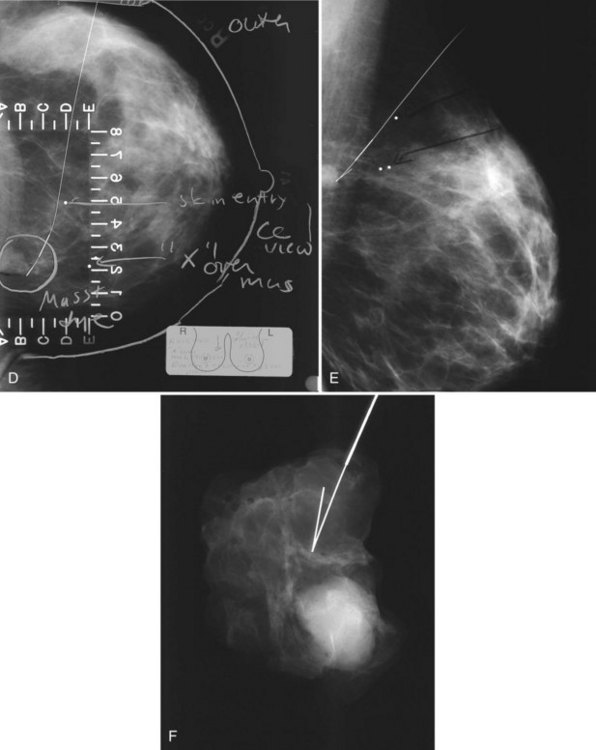
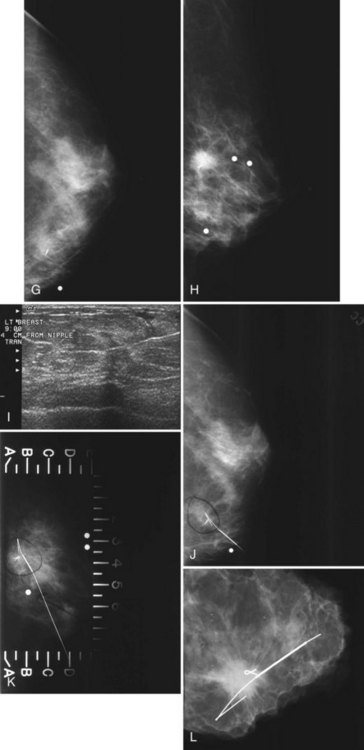
A special scenario regarding needle localizations occurs when surgeons use “bracketing” wires to remove a large area of breast tissue (Fig. 6-2), a scenario that happens when the mass or calcifications extend over too wide an area to be localized by one wire. In this situation, the radiologist places two wires in the breast, with one wire at one end of the lesion and the other wire at the other end of the lesion. The “brackets” help the surgeon remove the lesion between the two wires in toto. These “bracketed” breast specimens should include the two wires and the mass or calcifications between them.
Ultrasound Guidance
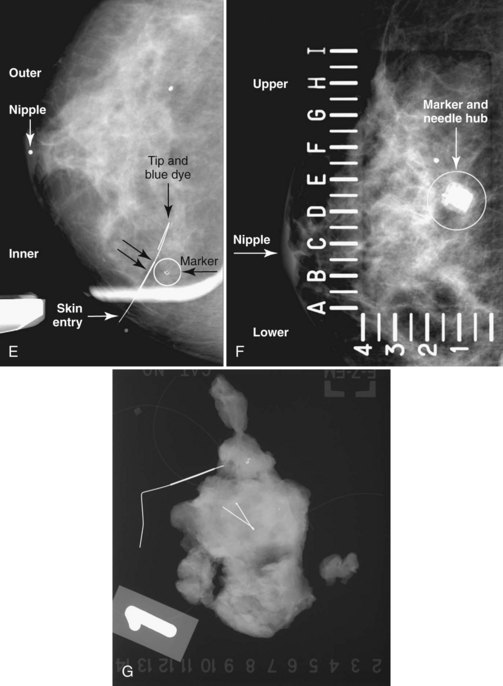
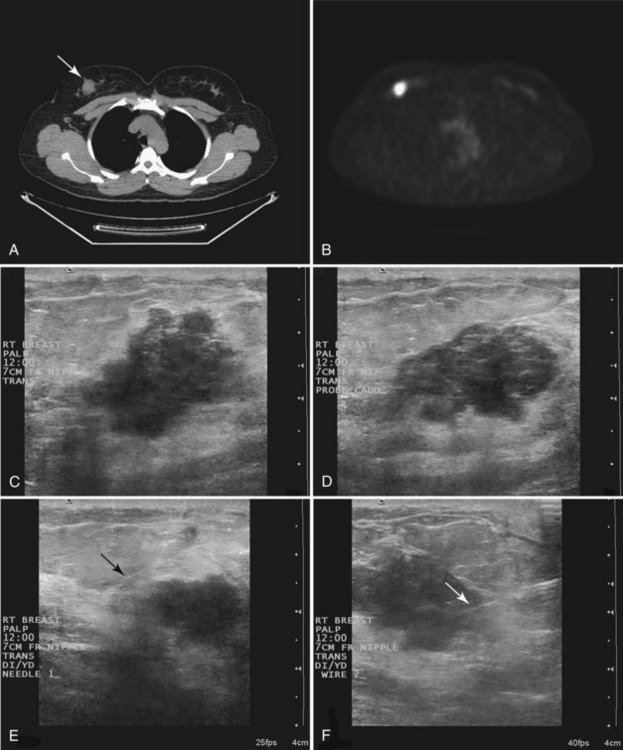
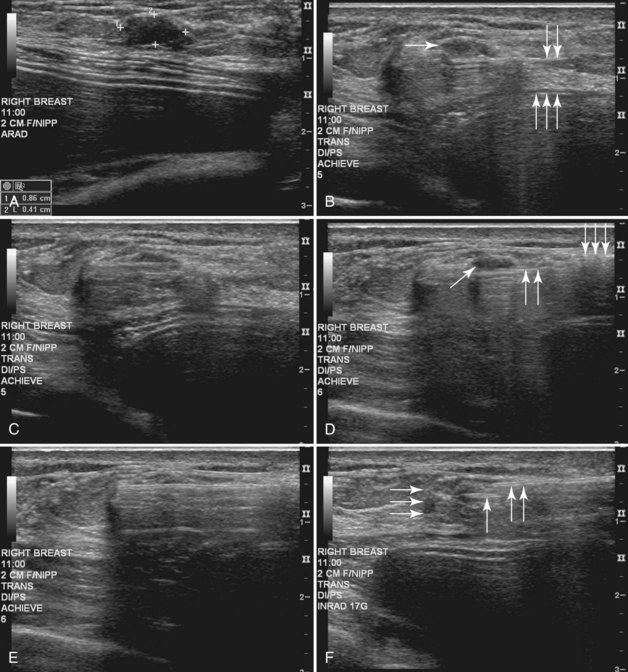
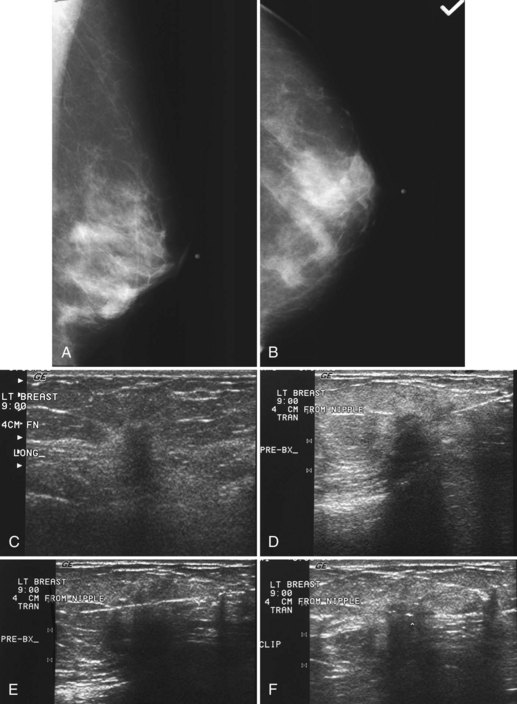
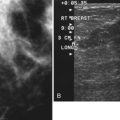
Real-time hand-held ultrasound units with a small transducer provide guidance for preoperative needle localization for ultrasonographically detected breast lesions (Fig. 6-3). To do the localization, the patient is placed in the supine position and the radiologist plans the needle path to the lesion. The radiologist rolls or angles the patient on the table until the needle path is directed safely away from the chest wall to prevent pneumothorax. Using sterile technique and under direct ultrasound visualization, the radiologist anesthetizes the skin and inserts a longer needle for deep anesthesia, keeping the entire shaft of the needle, the needle tip, and the target in the same plane. The anesthesia needle can be used as a “trial run” to judge the safety of the needle path and the difficulty of needle insertion. Then the radiologist inserts the preoperative localization needle into the lesion under real-time ultrasound guidance. Blue dye, if used, and a hookwire are inserted.
Specimen Radiography
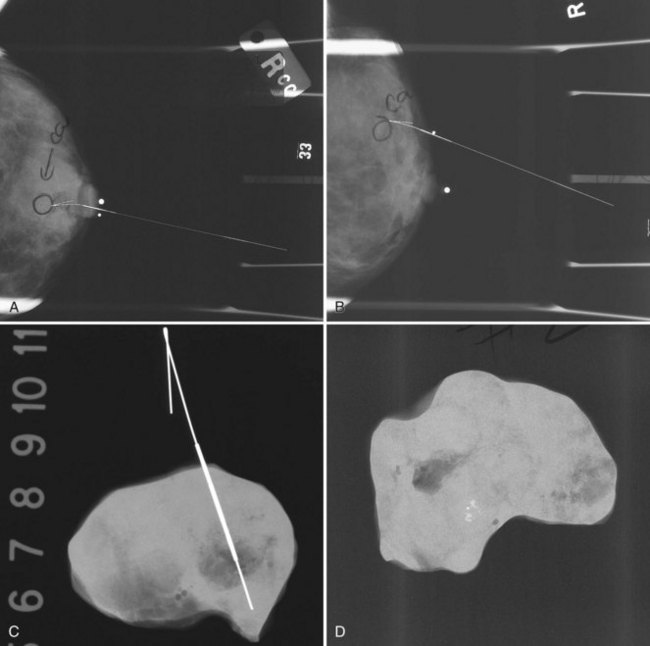
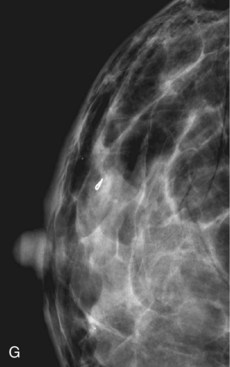
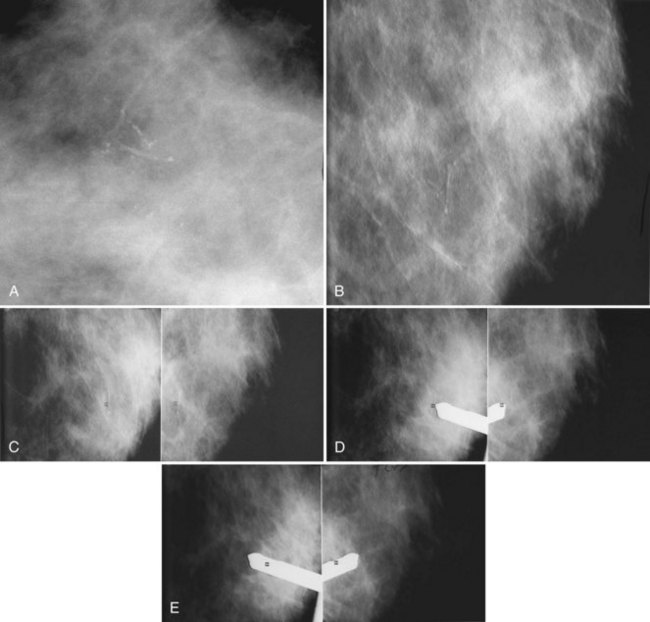
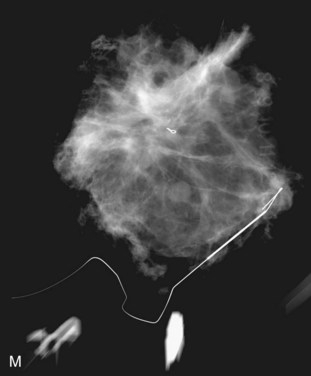
The needle localization procedure is not over until the specimen radiograph is taken by the technologist and reviewed by the radiologist. The radiologist reports whether the specimen contains the entire lesion, how far the lesion is away from the specimen edge, if the lesion was transected, and whether the hookwire, hookwire tip, and any markers are included (Box 6-3). The radiologist then calls these findings to the surgeon in the operating room. If the lesion is not in the specimen, the radiologist directs the surgeon to the expected location by using landmarks in the excised tissue and on the mammogram and waits for a second specimen (Fig. 6-4). If subsequent specimen radiographs still do not contain the lesion, the surgeon may close the breast and obtain a mammogram to determine whether the targeted lesion is still in the breast. The mammogram is usually done a few weeks after the biopsy.
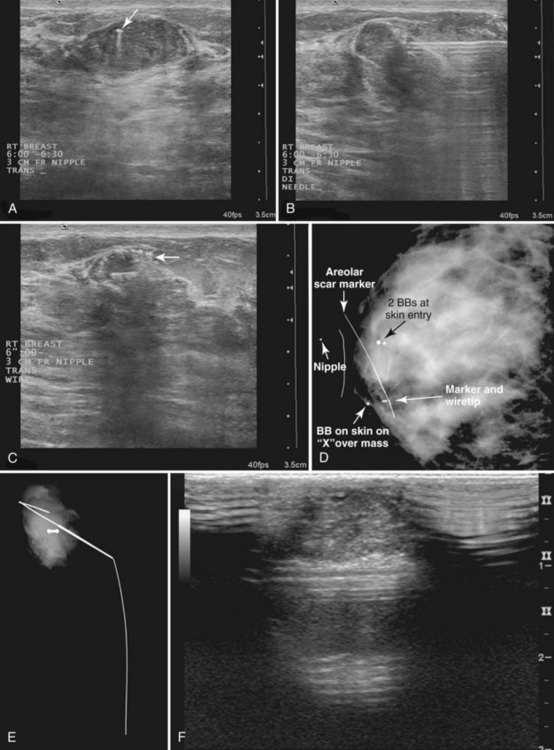
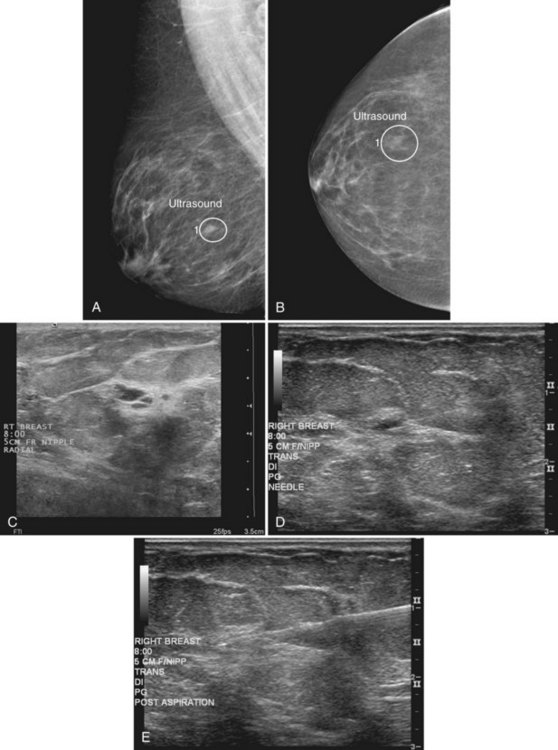
Tissue excised at ultrasound-guided preoperative localizations also undergoes specimen radiography, even if the finding cannot be seen on mammogram. The specimen radiograph may or may not show the ultrasound-localized finding, but will show if the entire hookwire or its tip, as well as any metallic markers, was excised. If the specimen radiograph does not show the ultrasound-localized finding, the radiologist can perform specimen ultrasound to see if the tissue contains the mass (Figs. 6-5 and 6-6).
Pathology Correlation
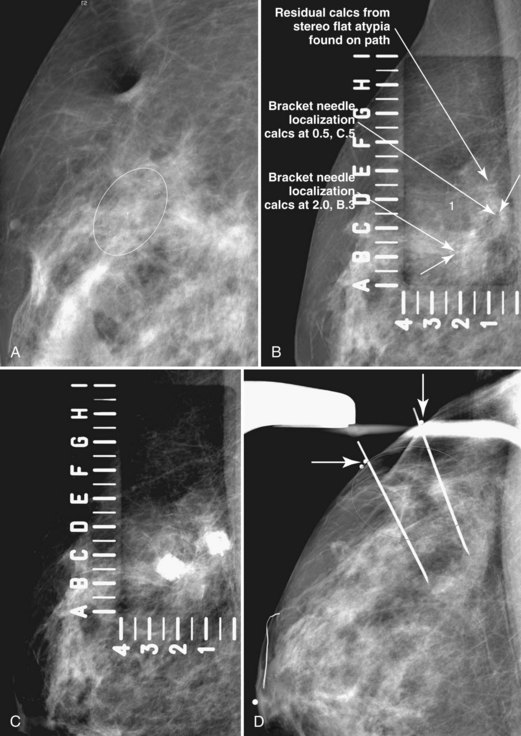
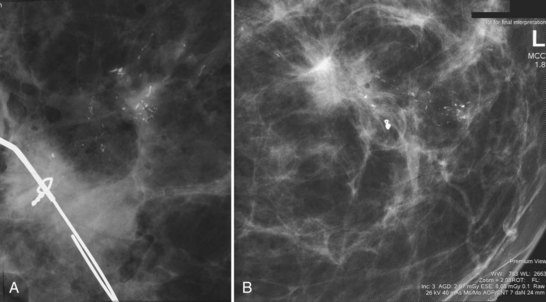
Later, the radiologist reviews the pathology report to see if the pathology reflects what the radiologist expected, based on the lesion’s imaging characteristics. Radiologic–pathologic correlation ensures that the targeted lesion analyzed at pathologic evaluation is concordant with the imaging finding and, specifically, that the pathology report describes a histologic finding that is known to correlate with the imaging findings. For example, if the targeted lesion shows fine pleomorphic calcifications, a diagnosis of malignancy, high-risk lesion, or benign lesion would all be concordant if the targeted calcifications were definitely seen in the specimen radiograph and preferably also on the pathology slides (Fig. 6-7). If the pathology report showed an uncalcified fibroadenoma when the targeted radiographic finding was fine pleomorphic calcifications, the pathologic–radiologic correlation would be discordant, and the case would warrant additional investigation.
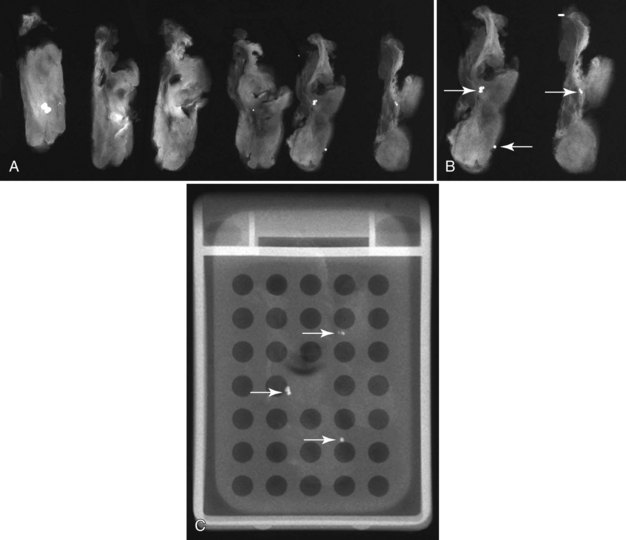
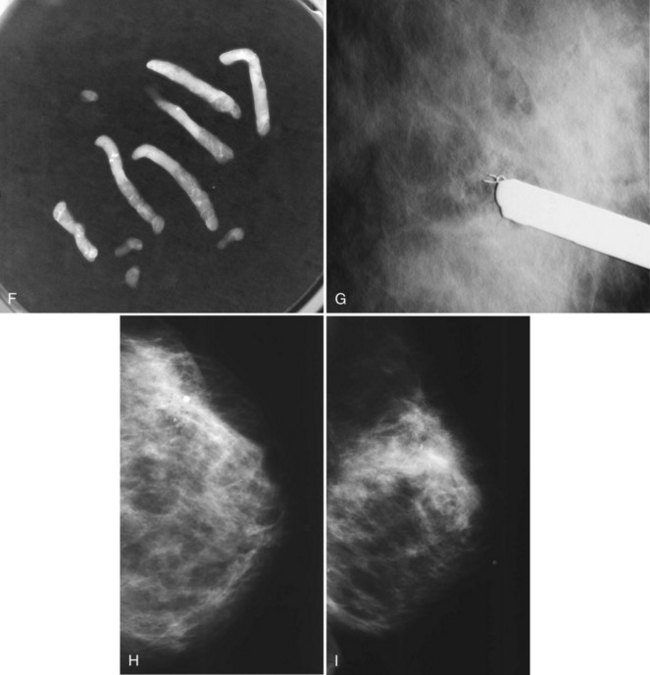
Second, the calcifications may be in the paraffin blocks. During specimen processing, thin breast tissue samples are embedded in paraffin blocks, which are then sliced and placed on slides for staining. Each block is several millimeters thick, but each slide contains only micromillimeters of paraffin and tissue. The calcifications may still be in the block and may never have been placed on a slide for review. A radiograph of the blocks may show the calcifications, and re-sectioning of that particular block will show the calcifications (Fig. 6-8).
Percutaneous Needle Biopsy of Cysts, Solid Masses, or Calcifications
Breast lesions can be classified as cysts, solid masslike lesions (which include true masses, asymmetries, and areas of architectural distortion), and calcifications. Needle types and cyst aspirations are discussed here, followed by needle biopsies guided by palpation, ultrasound, and stereotactic techniques. Needle biopsies guided by MRI are discussed in Chapter 7. This section then discusses core specimen radiography, marker placement, carbon marking, patient safety and comfort after biopsy, complete lesion removal, calcification and epithelial displacement, pathology correlation, high-risk lesions, follow-up of benign lesions, complications, differences between core and vacuum needle biopsies, and patient follow-up, audits, and noncompliance.
Needle Types
The types of biopsy needles used for specific breast lesions and guidance methods vary around the world. A trend toward progressively larger needles and more tissue samples per biopsy site has been noted, especially in the United States. Three main types of needles are used for percutaneous biopsies (Table 6-1). Fine-needle aspiration (FNA) needles, usually 25- to 20-gauge, are used for cyst aspirations and for solid breast masses. The aspirated material requires interpretation by expert cytopathologists. FNA is usually done with ultrasound or palpation guidance with at least four needle passes. FNA is less commonly done in the United States compared to Europe and Asia.
| Needle Type | Usual Gauge | Biopsy Use |
|---|---|---|
| Fine-needle aspiration | 25- to 20-gauge | Cyst aspiration. Solid mass highly likely to be either benign or malignant |
| Automated large-core | 18- to 14-gauge | Ultrasound-guided biopsy. Uncommon for stereotactic biopsy |
| Directional vacuum-assisted | 14- to 7-gauge | Stereotactic biopsy. Uncommon but growing use for ultrasound-guided biopsy |
Automated large-core (core) needles in 18- to 14-gauge (Fig. 6-9A and B) commonly are used to biopsy masses with ultrasound or palpation guidance. In some facilities, especially outside the United States, core needles are used with stereotactic guidance to biopsy masses or calcifications. An automated large-core biopsy needle obtains a single specimen with each pass of the needle, and 2 to 12 specimens are obtained by firing the needle multiple times. Pathologists who are comfortable interpreting surgically excised breast biopsy tissue can interpret the histologic material obtained.
Directional vacuum-assisted (vacuum) needles (see Fig. 6-9C) are available in 7- to 14-gauge and are used for stereotactic, ultrasound-guided, and MRI-guided biopsies. Depending on the manufacturer, vacuum biopsy can be done with just one needle pass, and multiple specimens are obtained by rotating the collection aperture of the needle to obtain between 6 and 18 specimens. Other directional vacuum-assisted needles obtain single vacuum specimens with each pass, requiring multiple insertions. In some facilities, vacuum biopsies are used to excise benign lesions such as fibroadenomas to avoid the need for surgical excision or imaging follow-up, once the fibroadenoma has been diagnosed by core needle biopsy and adequate sampling.
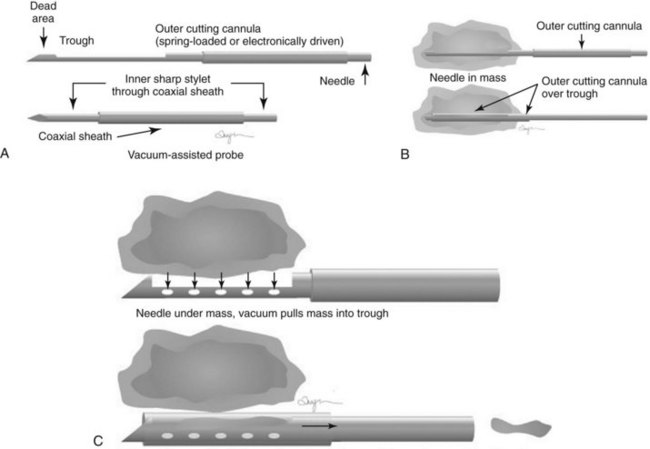
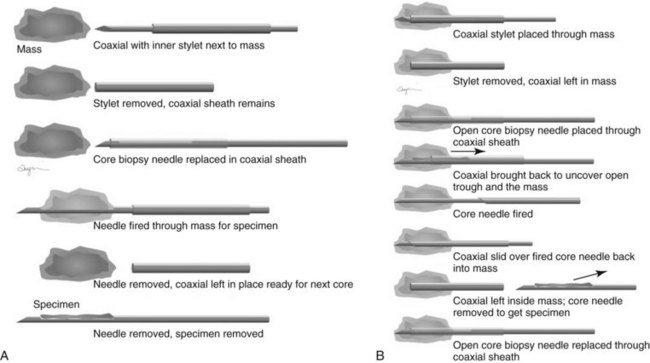
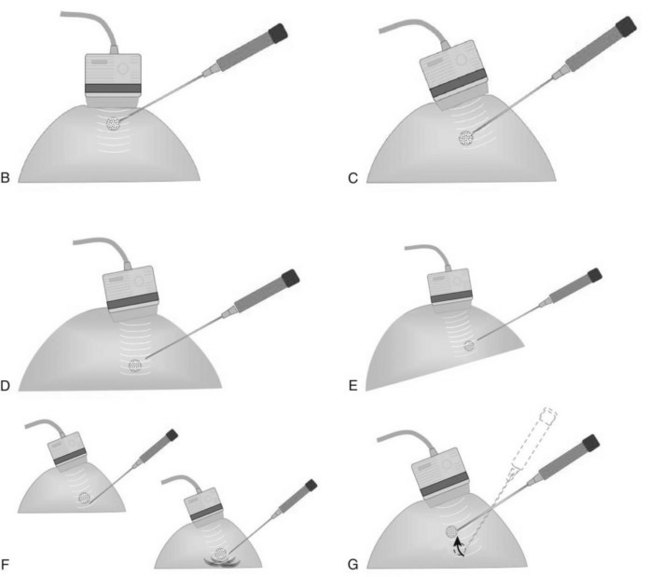
Both single-insertion and multi-insertion needles can be used with or without a coaxial guide (Fig. 6-10A). The coaxial guides are usually used with ultrasound or MRI guidance. The purpose of the coaxial guide is to provide a path to the target that the radiologist can use again and again without retraumatizing the breast tissue. The coaxial device consists of an inner sharp stylet and an outer sheath. The coaxial device is placed through the tissue so that the stylet tip/sheath edge is at or in the lesion. Then the radiologist removes the stylet, leaving a sheath that provides a “tunnel” through the breast tissue directly to the lesion. The radiologist then places the biopsy needle through the sheath into the lesion and takes samples. The radiologist can repeatedly place the biopsy needle through the sheath without having to disturb the surrounding breast tissue. Coaxial biopsies can be done with the sheath near the mass or through the mass (see Fig. 6-10B).
Cyst Aspiration
Masses on mammograms often prompt requests for breast ultrasound and cyst aspiration. To do a cyst aspiration the radiologist advances a fine needle into the cyst by palpation or image guidance. If the cyst is tense, fluid wells up into the needle hub. To aspirate the cyst, the radiologist attaches a syringe to the needle and draws fluid into the syringe until no more fluid can be obtained. Cyst aspiration can be done by ultrasound (Fig. 6-11) or, less commonly, by x-ray guidance using a fenestrated compression plate and mammography. If cyst aspiration is done under ultrasound, the radiologist should be able to watch the cyst disappear in real time.
Pneumocystograms are mammograms obtained after the radiologist injects air into a cyst cavity. The pneumocystogram shows the air-filled cyst cavity on the mammogram, enabling the radiologist to make sure that a mass prompting biopsy on the mammogram corresponds to the aspirated cyst and to exclude an intracystic mass. Air is thought to be therapeutic in preventing cyst recurrence (Fig. 6-12). To do a pneumocystogram, the radiologist aspirates the cyst first. Once the fluid has been aspirated completely, the radiologist disengages the syringe while carefully holding the needle tip in the decompressed, flattened cyst cavity. The radiologist attaches an air-filled syringe to the needle, injects a small amount of air into the cyst cavity, takes the needle out, and obtains CC and mediolateral mammograms immediately. A normal pneumocystogram should show an air-filled, thin-walled, round or oval cavity without intracystic solid masses or mural nodules.
Although radiologists can usually tell if a cyst on ultrasound corresponds to a specific mammographic mass, this correlation can be tricky. When the correlation is unclear and the radiologist has chosen not to do a pneumocystogram, the radiologist orders a postaspiration mammogram to see if the “cyst” disappears. The mass should be gone on the postaspiration mammogram if the aspirated cyst is the mammographic mass. If the mass still shows on the postaspiration mammogram, the mammographic finding is separate from the cyst and needs further investigation (Fig. 6-13).
Ultrasound Guidance
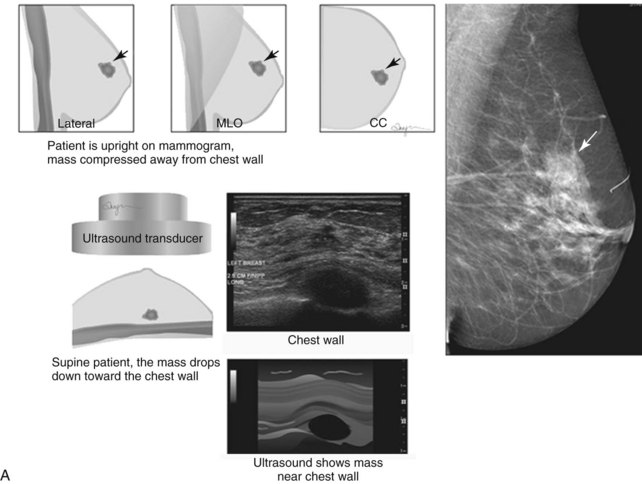
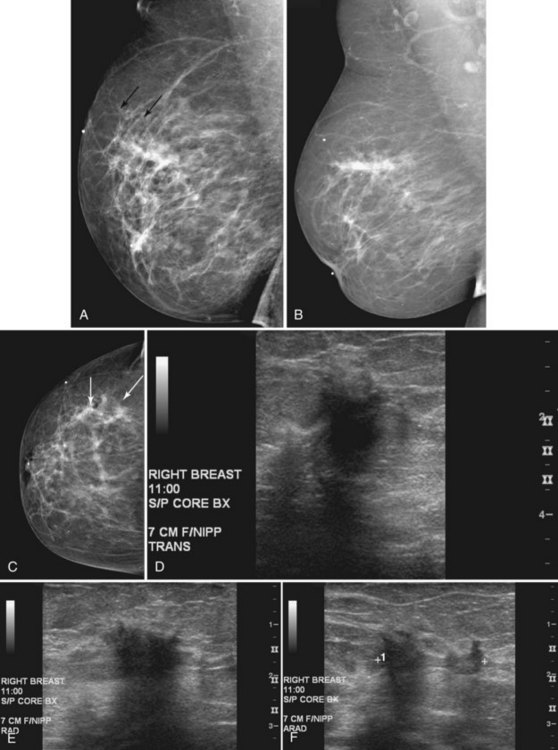
When compared with stereotactic biopsy, ultrasound-guided biopsy has the advantage of using readily available equipment and is fast and cost-effective. The first step in ultrasound-guided biopsy is to find the questioned lesion for biopsy. This commonly occurs when a mass on the mammogram prompts an ultrasound to further characterize the mass and localize it for biopsy. When correlating the mammogram to the ultrasound, the mass can be far away from the chest wall on the mammogram and lie next to the pectoralis muscle on the ultrasound. This occurs because the breast tissue is compressed far away from the chest wall when the patient stands up for the mammogram. On ultrasound, the breast falls dependently onto the chest wall when the patient lies down (Fig. 6-14A).
When planning ultrasound-guided needle biopsies, it is important to keep the needle tip away from the chest wall to prevent pneumothorax. Unlike upright preoperative x-ray–guided needle localization or prone stereotactic localization, the ultrasound-guided biopsy is done supine and the needle is not necessarily parallel to the chest wall. Further complicating matters, some core biopsy needles “throw” the cutting trough 2.5 cm further into the tissue beyond the needle tip. Thus, planning a safe ultrasound-guided needle biopsy trajectory must take into account both the needle tip and the needle “throw” trajectory. To plan a safe procedure, the radiologist rolls the patient on the table so that the needle trajectory is as parallel to the chest wall as possible and not at a steep angle aiming toward the lungs. Patient positioning can take some time, but it is worth the few minutes to position the patient accurately to avoid an untoward complication. Another way to keep the needle away from the chest wall is to inject anesthetic underneath the targeted mass to lift it away from the pectoralis muscle. Alternatively, in some cases, the radiologist can stick the biopsy needle tip into the mass and lift it into a safer trajectory before firing the needle (see Fig. 6-14B to G).
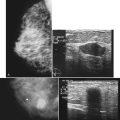
For ultrasound-guided FNA, the radiologist introduces a needle in the plane of the transducer axis to show the entire shaft of the needle, its tip, and the lesion. Once the needle is within the lesion, the radiologist aspirates the mass with a vigorous to-and-fro movement to obtain material for cytologic evaluation and then withdraws the needle. At least four passes should be performed; optimally, the material should be analyzed immediately to ensure that adequate cellular material has been obtained for diagnosis. After aspiration, direct pressure is applied to the site (Fig. 6-15).
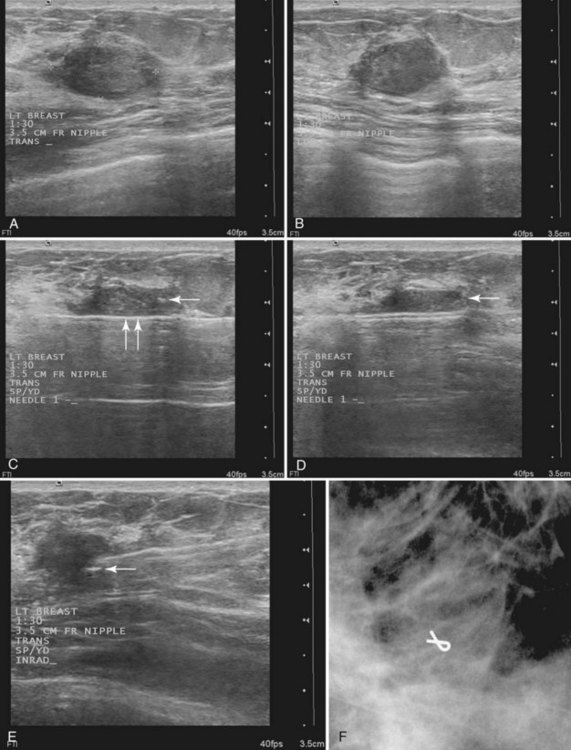
Then, under direct ultrasound visualization, the radiologist introduces the core biopsy needle into the breast. If the lesion is large enough, the radiologist introduces the needle into the edge of the lesion to hold it in place. Otherwise, the radiologist may choose to fire the needle through the mass with or without a coaxial system. In any case, the radiologist fires the biopsy core needle under direct visualization and harvests the cores. Optimally, at least three to five tissue specimens are obtained from different parts of the mass. After sampling, the radiologist places a metallic marker into the mass, and the technologist holds direct pressure on the breast to establish hemostasis. After hemostasis is established, the technologist bandages the wound and takes orthogonal mammograms to show the marker and any residual mass (Fig. 6-16).
A vacuum biopsy is similar to an automated multifire core biopsy, but the vacuum needle is usually placed under or occasionally inside the lesion. The probe “vacuums” tissue into the trough to be sampled. The vacuum technique carries a special caveat regarding the skin. If the probe is too close to the skin, the skin can be “vacuumed” into the trough and sampled, causing skin injury, requiring a suture or, in extreme cases, a skin graft. During a needle biopsy using vacuum technique, the radiologist obtains several samples, concentrating on aiming the trough at the mass (Fig. 6-17). Afterward, the radiologist can place a marker in the mass either through the probe or (depending on the manufacturer) by using a marker that has its own separate needle.
If a mass previously cored under ultrasound guidance must be removed, the radiologist localizes the mass or marker under ultrasound, places a wire, then takes orthogonal mammograms to show the wire and marker, and waits for the excised tissue specimen (Fig. 6-18).
Stereotactic Guidance
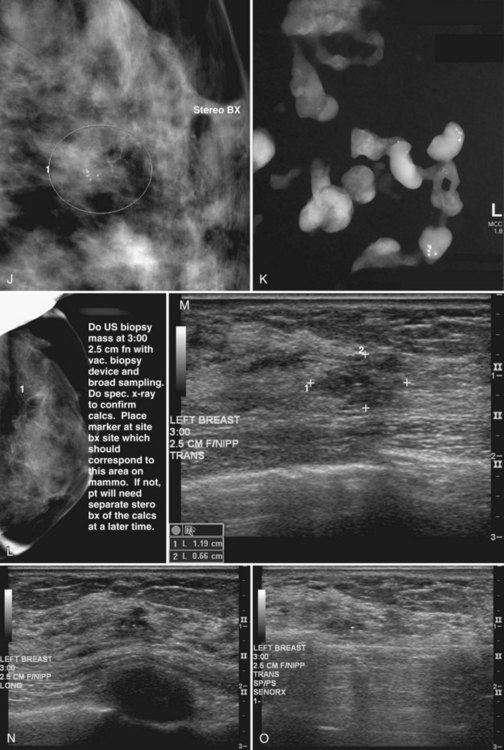
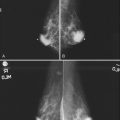
This method uses a compression device with a small aperture and an x-ray tube that has the ability to take two stereotactic views about 15 degrees off perpendicular (Fig. 6-19A to I). The patient is in a prone, upright, or decubitus position with the breast compressed by a fenestrated compression paddle for stereotactic needle biopsy. The radiologist reviews prebiopsy CC and mediolateral mammograms to determine the lesion’s location on orthogonal views. The breast is then firmly compressed with the compression paddle aperture placed on the skin surface closest to the breast lesion. After taking a straight-on scout view that visualizes the lesion, the stereotactic technologist takes two stereo views of the lesion. The radiologist locates the lesion on the stereo views and passes a needle into the breast to a calculated depth. Prefire stereotactic images, which should show the tip of the biopsy needle at the edge of the lesion, are obtained. The radiologist then fires the needle deeper into the breast and reviews postfire stereotactic images to ensure that the trough of the needle is within the breast lesion. After the radiologist collects multiple specimens, he or she reviews the core specimen radiograph to make sure that the calcifications or mass has been sampled. The tissue specimens are labeled and sent to the pathology laboratory. At this point the radiologist decides whether to deploy a metallic marker into the biopsy cavity. If the radiologist deploys a marker, the technologist takes additional stereotactic images to confirm marker deployment before releasing the patient from compression. The technologist maintains direct pressure on the biopsy site after release of the compression paddle to achieve hemostasis, places a bandage, and obtains immediate postbiopsy upright CC and mediolateral mammograms. These show the biopsy cavity, confirm removal of all or a portion of the calcifications or mass, and show the location of the marker and its position relative to the targeted findings.
Core Specimen Radiography
Magnification specimen radiography is mandatory after stereotactic biopsy of calcifications and optional after biopsy of a mass to ensure that the lesion that prompted biopsy has been adequately sampled or removed (see Fig. 6-19J and K). If the targeted lesion is not present in the specimen, the radiologist obtains more specimens. Specimen radiography is not usually done after ultrasound-guided core biopsy unless the biopsy is targeting radiographically detectable calcifications (see Fig. 6-19L to R).
Marker Placement, Movement, and Compatibility with Ultrasound and MRI
A variety of problems are associated with the markers (Box 6-4). The first potential problem, nondeployment, is uncommon. It was anecdotally noted that some plugs may get stuck during deployment, making it impossible to push the plunger in or difficult to withdraw or close the needle. This problem is possibly due to the plugs filling with fluid, expanding in the deployment device, and getting stuck in the trough. For vacuum-assisted needles, the deployment device can get stuck on a retained fragment in the vacuum needle.
If the marker was inaccurately deployed or has later migrated away from an original biopsy site, the radiologist determines the location of the original targeted lesion by using breast architecture and landmarks. The goal of subsequent localization is to remove the targeted biopsy site, including any residual cancer (Fig. 6-20). Whether it is necessary to also localize and remove the inaccurately positioned marker is controversial, but it should be considered if the needle biopsy revealed cancer. If the needle biopsy revealed a high-risk lesion or a discordant benign lesion, the inaccurately positioned marker presumably does not need to be removed. When the marker is in an inaccurate position, some facilities use presurgical or intraoperative ultrasound to try to identify the needle biopsy site.
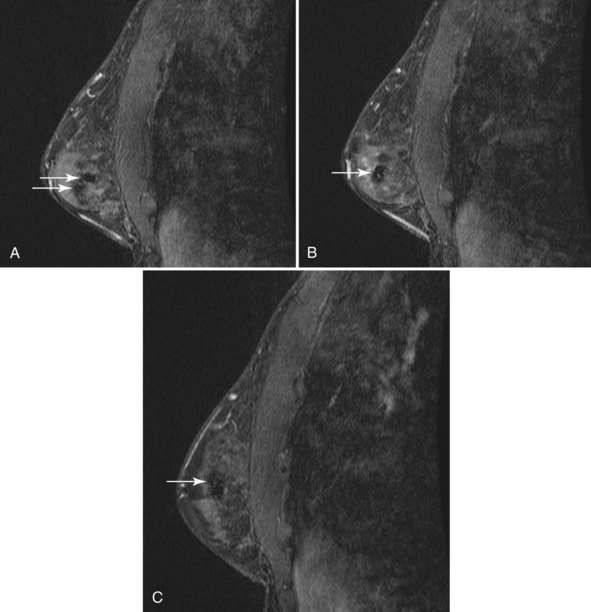
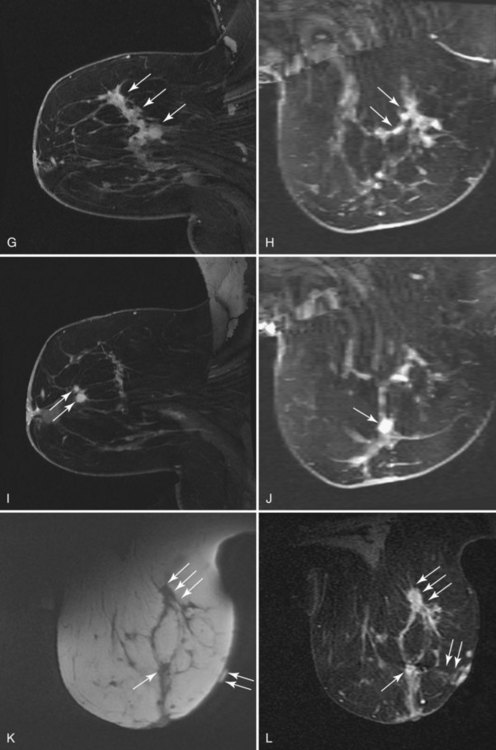
MRI is increasingly being used to stage the breast for cancer and to plan surgical management. Because biopsy site markers placed by stereotaxis, ultrasound, or MRI may be imaged by subsequent MRI studies, understanding of marker MRI compatibility and safety, and of marker artifacts, is becoming increasingly important. Accordingly, all facilities should use MRI-compatible metallic markers when markers are placed with any modality because MRI might be performed later. Metallic markers cause a signal void on MRI, and the size of the signal void varies according to the marker type and pulse sequence (Fig. 6-21). There is a difference between MRI marker compatibility and safety. MRI compatibility means that the marker can be used in the MRI magnet and will cause little artifact. Marker safety means that the marker will produce no harm to the patient in the magnet. Some markers are MRI compatible but still cause large artifacts of up to 2 cm, thus rendering the MRI less readable than when using other markers. MRI testing of markers for artifact by using phantoms on the facility’s pulse sequences is a simple way to determine the marker artifact and the size of the signal void. This should be done before inserting metallic markers for marking tumors or biopsy sites. However, correlation between MRI, ultrasound, and mammography can be challenging, when markers or even wires are placed for preoperative localization (Fig. 6-22).
Pathology Correlation
Liberman and colleagues emphasized in 2000 that benign histologic diagnoses that do not explain the imaging findings must be considered discordant and should lead to repeat biopsy done by surgical excision or a more aggressive needle core biopsy (Box 6-5). American College of Radiology Breast Imaging Reporting and Data System (BI-RADS®) category 5 lesions with a benign histologic diagnosis are discordant. Specific benign diagnoses for mass lesions include fibroadenomas, lymph nodes, and benign cysts. There are no specific diagnoses for nonmass lesions whether the nonmass lesions are calcified or uncalcified. With nonspecific benign diagnoses, one relies on the quality of the needle biopsy to determine if the diagnosis is concordant. As discussed, biopsy of calcifications must include adequate sampling or removal of the calcifications as determined on specimen radiographs of the core or vacuum biopsy samples (looking for calcifications) and postbiopsy stereotactic or upright images (looking for reduction or absence of calcifications seen before biopsy compared to after biopsy).
High-Risk Lesions, Including Controversies
More controversy exists about the need for surgical excision of non-ADH lesions. Most authors in the scientific literature report that lobular carcinoma in situ (LCIS), atypical lobular hyperplasia (ALH), and any other lesions with atypia (such as flat epithelial atypia, papillary lesions with atypia, and radial scar with atypia) need excision because cancer is often found at surgical excision (Box 6-6).
Box 6-6 High-Risk Lesions Diagnosed at Needle Biopsy and Need for Surgical Excision
Papillary lesions without atypia might be safe to follow without excision if the biopsy criteria outlined for radial scars are met, but there are no large studies to prove that. Currently individual decisions are made to either excise or follow papillary lesions, mucocele-like lesions, and pseudoangiomatous stromal hyperplasia (PASH), if they have no atypia (see Box 6-6). However, PASH or hemangiomas that have possible features of angiosarcoma need surgical excision.
Complications
Complications are discussed in the “Informed Consent” section of this chapter and are also listed in Box 6-2.
Differences between Core and Vacuum Needle Biopsies
MRI-guided needle biopsies are done almost exclusively with vacuum needles, but can be done with core biopsy needles. MRI-guided biopsies are covered in Chapter 7.
Key Elements
Know the location of the target lesion in three dimensions. Do not attempt biopsy of a lesion not known to be genuine or one whose location in the breast is not known.
Most nonpalpable lesions are now diagnosed by image-guided percutaneous needle biopsy and not by diagnostic surgical excision.
Specimen radiography of x-ray– or ultrasound-localized surgical specimens should show the lesion, hookwire, hookwire tip, and any associated metallic markers. The findings are called to the surgeon in the operating room.
For ultrasound-localized surgical specimens, specimen ultrasound can be done if the lesion is not seen on the specimen radiograph.
Reasons that calcifications may not be visualized on the histologic slides include nonremoval, calcium oxalate, location in the paraffin block, or displacement out of the specimen by the microtome.
Risks of core biopsy include hematoma (fairly common but rarely significant), and rarely untoward bleeding, infection, pneumothorax, pseudoaneurysm formation, implant rupture, milk fistula (if the patient is pregnant or nursing), and vasovagal reactions.
Correlation between the pathology results and imaging studies establishes concordance.
If the lesion targeted was calcifications, the specimen radiograph shows no calcifications, and the pathology report describes calcifications, the pathology report has described tiny, serendipitously found 100-micron calcifications that have nothing to do with the target, and the patient needs to undergo rebiopsy.
Markers are fairly often inaccurately deployed in the breast after needle biopsy but rarely migrate significantly after placement.
Surgical excisional biopsy is recommended for core or vacuum biopsy specimens showing invasive cancer, DCIS, ADH, LCIS, ALH, any atypical lesions (including flat epithelial atypia, atypical radial scar lesions, and atypical papillary lesions), phyllodes tumors, and discordant benign lesions.
It is controversial whether surgical excisional biopsy should always be performed after obtaining core or vacuum biopsy samples showing radial scar, papillary lesions, and PASH without atypia.
Adler DD, Ligut RJ, Granstrom P, et al. Follow-up of benign results of stereotactic core breast biopsy. Acad Radiol. 2000;7:248-253.
Agoff SN, Lawton TJ. Papillary lesions of the breast with and without atypical ductal hyperplasia: can we accurately predict benign behavior from core needle biopsy? Am J Clin Pathol. 2004;122:440-443.
Arora S, Menes TS, Moung C, et al. Atypical ductal hyperplasia at margin of breast biopsy—is re-excision indicated? Ann Surg Oncol. 2008;15:843-847. Epub 7 Nov 2007
Arpino G, Allred DC, Mohsin SK, et al. Lobular neoplasia on core-needle biopsy—clinical significance. Cancer. 2004;101:242-250.
Arpino G, Laucirica R, Elledge RM. Premalignant and in situ breast disease: biology and clinical implications. Ann Intern Med. 2005;143:446-457.
Bates T, Davidson T, Mansel RE. Litigation for pneumothorax as a complication of fine-needle aspiration of the breast. Br J Surg. 2002;89:134-137.
Bazzocchi M, Francescutti GE, Zuiani C, et al. Breast pseudoaneurysm in a woman after core biopsy: percutaneous treatment with alcohol. AJR Am J Roentgenol. 2002;179:696.
Becker L, Trop I, David J, et al. Management of radial scars found at percutaneous breast biopsy. Can Assoc Radiol J. 2006;57:72-78.
Berg WA. Image-guided breast biopsy and management of high-risk lesions. Radiol Clin North Am. 2004;42:935-946.
Berg WA, Krebs TL, Campassi C, et al. Evaluation of 14- and 11-gauge directional, vacuum-assisted biopsy probes and 14-gauge biopsy guns in a breast parenchymal model. Radiology. 1997;205:203-208.
Berkowitz JE, Gatewood OM, Donovan GB, et al. Dermal breast calcifications: localization with template-guided placement of skin marker. Radiology. 1987;163:282.
Birdwell RL, Ikeda DM, Brenner RJ. Methods of compliance with Mammography Quality Standards Act regulations for tracking positive mammograms: survey results. AJR Am J Roentgenol. 1999;172:691-696.
Birdwell RL, Jackman RJ. Clip or marker migration 5–10 weeks after stereotactic 11-gauge vacuum-assisted breast biopsy: report of two cases. Radiology. 2003;229:541-544.
Bober SE, Russell DG. Increasing breast tissue depth during stereotactic needle biopsy. AJR Am J Roentgenol. 2000;174:1085-1086.
Bolivar AV, Alonso-Bartolome P, Garcia EO, et al. Ultrasound-guided core needle biopsy of nonpalpable breast lesions: a prospective analysis in 204 cases. Acta Radiol. 2005;46:690-695.
Bonnett M, Wallis T, Rossmann M, et al. Histopathologic analysis of atypical lesions in image-guided core breast biopsies. Mod Pathol. 2003;16:154-160.
Brem RF, Lechner MC, Jackman RJ, et al. Lobular neoplasia at percutaneous breast biopsy: variables associated with carcinoma at surgical excision. AJR Am J Roentgenol. 2008;190:637-641.
Brem RF, Schoonjans JM. Local anesthesia in stereotactic, vacuum-assisted breast biopsy. Breast J. 2001;7:72-73.
Brenner RJ. Lesions entirely removed during stereotactic biopsy: preoperative localization on the basis of mammographic landmarks and feasibility of freehand technique—initial experience. Radiology. 2000;214:585-590.
Brenner RJ. Percutaneous removal of postbiopsy marking clip in the breast using stereotactic technique. AJR Am J Roentgenol. 2001;176:417-419.
Brenner RJ, Bassett LW, Fajardo LL, et al. Stereotactic core-needle breast biopsy: a multi-institutional prospective trial. Radiology. 2001;218:866-872.
Brenner RJ, Jackman RJ, Parker SH, et al. Percutaneous core needle biopsy of radial scars of the breast: when is excision necessary? AJR Am J Roentgenol. 2002;179:1179-1184.
Burbank F. Stereotactic breast biopsy: its history, its present, and its future. Am Surg. 1996;62:128-150.
Burbank F. Mammographic findings after 14-gauge automated needle and 14-gauge directional, vacuum-assisted stereotactic breast biopsies. Radiology. 1997;204:153-156.
Burbank F. Stereotactic breast biopsy: comparison of 14- and 11-gauge Mammotome probe performance and complication rates. Am Surg. 1997;63:988-995.
Burbank F, Forcier N. Tissue marking clip for stereotactic breast biopsy: initial placement accuracy, long-term stability, and usefulness as a guide for wire localization. Radiology. 1997;205:407-415.
Burbank F, Parker SH. Methods for evaluating the quality of an image-guided breast biopsy program. Sem Breast Dis. 1998;1:71-83.
Burbank F, Parker SH, Fogarty TJ. Stereotactic breast biopsy: improved tissue harvesting with the Mammotome. Am Surg. 1996;62:738-744.
Burnside ES, Sohlich RE, Sickles EA. Movement of a biopsy-site marker clip after completion of stereotactic directional vacuum-assisted breast biopsy: case report. Radiology. 2001;221:504-507.
Carder PJ, Garvican J, Haigh I, et al. Needle core biopsy can reliably distinguish between benign and malignant papillary lesions of the breast. Histopathology. 2005;46:320-327.
Carr JJ, Hemler PF, Halford PW, et al. Stereotactic localization of breast lesions: how it works and methods to improve accuracy. Radiographics. 2001;21:463-473.
Cassano E, Urban LA, Pizzamiglio M, et al. Ultrasound-guided vacuum-assisted core breast biopsy: experience with 406 cases. Breast Cancer Res Treat. 2007;102:103-110. Epub 13 Jul 2006
Charles M, Edge SB, Winston JS, et al. Effect of stereotactic core needle biopsy on pathologic measurement of tumor size of T1 invasive breast carcinomas presenting as mammographic masses. Cancer. 2003;97:2137.
Cho N, Moon WK, Cha JH, et al. Sonographically guided core biopsy of the breast: comparison of 14-gauge automated gun and 11-gauge directional vacuum-assisted biopsy methods. Korean J Radiol. 2005;6:102-109.
Cohen MA. Cancer upgrades at excisional biopsy after diagnosis of atypical lobular hyperplasia or lobular carcinoma in situ at core-needle biopsy: some reasons why. Radiology. 2004;231:617-621.
Collins LC, Connolly JL, Page DL, et al. Diagnostic agreement in the evaluation of image-guided breast core needle biopsies: results from a randomized clinical trial. Am J Surg Pathol. 2004;28:126-131.
Crowe JPJr, Patrick RJ, Rybicki LA, et al. Does ultrasound core breast biopsy predict histologic finding on excisional biopsy? Am J Surg. 2003;186:397-399.
Crystal P, Koretz M, Shcharynsky S, et al. Accuracy of sonographically guided 14-gauge core-needle biopsy: results of 715 consecutive breast biopsies with at least two-year follow-up of benign lesions. J Clin Ultrasound. 2005;33:47-52.
Dahlstrom JE, Sutton S, Jain S. Histologic–radiologic correlation of mammographically detected microcalcification in stereotactic core biopsies. Am J Surg Pathol. 1998;22:256-259.
de Lucena CE, Dos Santos Junior JL, de Lima Resende CA, et al. Ultrasound-guided core needle biopsy of breast masses: how many cores are necessary to diagnose cancer? J Clin Ultrasound. 2007;35:363-366.
Dershaw DD. Does LCIS or ALH without other high-risk lesions diagnosed on core biopsy require surgical excision? Breast J. 2003;9:1-3.
Dershaw DD, Morris EA, Liberman L, et al. Nondiagnostic stereotaxic core breast biopsy: results of rebiopsy. Radiology. 1996;198:323-325.
Deutch BM, Schwartz MR, Fodera T, et al. Stereotactic core breast biopsy of a minimal carcinoma complicated by a large hematoma: a management dilemma. Radiology. 1997;202:431-433.
Diaz LK, Wiley EL, Venta LA. Are malignant cells displaced by large-gauge needle core biopsy of the breast? AJR Am J Roentgenol. 1999;173:1303-1313.
Dillon MF, Hill AD, Quinn CM, et al. The accuracy of ultrasound, stereotactic, and clinical core biopsies in the diagnosis of breast cancer, with an analysis of false-negative cases. Ann Surg. 2005;242:701-707.
Dondalski M, Bernstein JR. Disappearing breast calcifications: mammographic–pathologic discrepancy due to calcium oxalate. South Med J. 1992;85:1252-1254.
Duchesne N, Parker SH, Lechner MC, et al. Multicenter evaluation of a new ultrasound-guided biopsy device: improved ergonomics, sampling and rebiopsy rates. Breast J. 2007;13:36-43.
Elsheikh TM, Silverman JF. Follow-up surgical excision is indicated when breast core needle biopsies show atypical lobular hyperplasia or lobular carcinoma in situ: a correlative study of 33 patients with review of the literature. Am J Surg Pathol. 2005;29:534-543.
Fahrbach K, Sledge I, Cella C, et al. A comparison of the accuracy of two minimally invasive breast biopsy methods: a systematic literature review and meta-analysis. Arch Gynecol Obstet. 2006;274:63-73. Epub 6 Apr 2006
Fine RE, Boyd BA, Whitworth PW, et al. Percutaneous removal of benign breast masses using a vacuum-assisted hand-held device with ultrasound guidance. Am J Surg. 2002;184:332-336.
Frenna TH, Meyer JE, Sonnenfeld MR. US of breast biopsy specimens. Radiology. 1994;190:573.
Friedman PD, Sanders LM, Menendez C, et al. Retrieval of lost microcalcifications during stereotactic vacuum-assisted core biopsy. AJR Am J Roentgenol. 2003;180:275-280.
Golub RM, Bennett CL, Stinson T, et al. Cost minimization study of image-guided core biopsy versus surgical excisional biopsy for women with abnormal mammograms. J Clin Oncol. 2004;22:2430-2437.
Goodman KA, Birdwell RL, Ikeda DM. Compliance with recommended follow-up after percutaneous breast core biopsy. AJR Am J Roentgenol. 1998;170:89-92.
Grady I, Gorsuch H, Wilburn-Bailey S. Ultrasound-guided, vacuum-assisted, percutaneous excision of breast lesions: an accurate technique in the diagnosis of atypical ductal hyperplasia. J Am Coll Surg. 2005;201:14-17.
Grin A, Horne G, Ennis M, et al. Measuring extent of ductal carcinoma in situ in breast excision specimens: a comparison of 4 methods. Arch Pathol Lab Med. 2009;133:31.
Guerra-Wallace MM, Christensen WN, White RLJr. A retrospective study of columnar alteration with prominent apical snouts and secretions and the association with cancer. Am J Surg. 2004;188:395-398.
Harris AT. Clip migration within 8 days of 11-gauge vacuum-assisted stereotactic breast biopsy: case report. Radiology. 2003;228:552-554.
Harvey JA, Moran RE, DeAngelis GA. Technique and pitfalls of ultrasound-guided core-needle biopsy of the breast. Semin Ultrasound CT MR. 2000;21:362-374.
Helvie MA, Ikeda DM, Adler DD. Localization and needle aspiration of breast lesions: complications in 370 cases. AJR Am J Roentgenol. 1991;157:711-714.
Hoda SA, Rosen PP. Practical considerations in the pathologic diagnosis of needle core biopsies of breast. Am J Clin Pathol. 2002;118:101-108.
Huber S, Wagner M, Medl M, et al. Benign breast lesions: minimally invasive vacuum-assisted biopsy with 11-gauge needles—patient acceptance and effect on follow-up imaging findings. Radiology. 2003;226:783-790.
Husien AM. Stereotactic localization mammography: interpreting the check film. Clin Radiol. 1992;45:387-389.
Ikeda DM, Helvie MA, Adler DD, et al. The role of fine-needle aspiration and pneumocystography in the treatment of impalpable breast cysts. AJR Am J Roentgenol. 1992;158:1239-1241.
Irfan K, Brem RF. Surgical and mammographic follow-up of papillary lesions and atypical lobular hyperplasia diagnosed with stereotactic vacuum-assisted biopsy. Breast J. 2002;8:230-233.
Ivan D, Selinko V, Sahin AA, et al. Accuracy of core needle biopsy diagnosis in assessing papillary breast lesions: histologic predictors of malignancy. Mod Pathol. 2004;17:165-171.
Jackman RJ, Birdwell RL, Ikeda DM. Atypical ductal hyperplasia: can some lesions be defined as probably benign after stereotactic 11-gauge vacuum-assisted biopsy, eliminating the recommendation for surgical excision? Radiology. 2002;224:548-554.
Jackman RJ, Burbank F, Parker SH, et al. Stereotactic breast biopsy of nonpalpable lesions: determinants of ductal carcinoma in situ underestimation rates. Radiology. 2001;218:497-502.
Jackman RJ, Lamm RL. Stereotactic histologic biopsy in breasts with implants. Radiology. 2002;222:157-614.
Jackman RJ, Marzoni FAJr. Needle-localized breast biopsy: why do we fail? Radiology. 1997;204:677-684.
Jackman RJ, Marzoni FAJr. Stereotactic histologic biopsy with patients prone: technical feasibility in 98% of mammographically detected lesions. AJR Am J Roentgenol. 2003;180:785-794.
Jackman RJ, Marzoni FAJr, Rosenberg J. False-negative diagnoses at stereotactic vacuum-assisted needle breast biopsy: long-term follow-up of 1,280 lesions and review of the literature. AJR Am J Roentgenol. 2009;192:341-351.
Jackman RJ, Nowels KW, Rodriguez-Soto J, et al. Stereotactic, automated, large-core needle biopsy of nonpalpable breast lesions: false-negative and histologic underestimation rates after long-term follow-up. Radiology. 1999;210:799-805.
Jackman RJ, Rodriguez-Soto J. Breast microcalcifications: retrieval failure at prone stereotactic core and vacuum breast biopsy—frequency, causes, and outcome. Radiology. 2006;239:61-70.
Jacobs TW, Connolly JL, Schnitt SJ. Nonmalignant lesions in breast core needle biopsies: to excise or not to excise? Am J Surg Pathol. 2002;26:1095-1110.
Jang M, Cho N, Moon WK, et al. Underestimation of atypical ductal hyperplasia at sonographically guided core biopsy of the breast. AJR Am J Roentgenol. 2008;191:1347-1351.
Kass R, Kumar G, Klimberg S, et al. Clip migration in stereotactic biopsy. Am J Surg. 2002;184:325-331.
Kettritz U, Rotter K, Schreer I, et al. Stereotactic vacuum-assisted breast biopsy in 2,874 patients: a multicenter study. Cancer. 2004;100:245-251.
Kim HS, Kim MJ, Kim EK, et al. US-guided vacuum-assisted biopsy of microcalcifications in breast lesions and long-term follow-up results. Korean J Radiol. 2008;9:503-509.
Kim JY, Han BK, Choe YH, et al. Benign and malignant mucocele-like tumors of the breast: mammographic and sonographic appearances. AJR Am J Roentgenol. 2005;185:1310-1316.
Kim MJ, Kim EK, Kwak JY, et al. Nonmalignant papillary lesions of the breast at US-guided directional vacuum-assisted removal: a preliminary report. Eur Radiol. 2008;18:1774-1783.
Ko ES, Cho N, Cha JH, et al. Sonographically guided 14-gauge core needle biopsy for papillary lesions of the breast. Korean J Radiol. 2007;8:206-211.
Lagios MD. Prognostic features of breast carcinoma from stereotactic biopsy material. Sem Breast Disease. 1998;1:101.
Lai JT, Burrowes P, MacGregor JH. Diagnostic accuracy of a stereotaxically guided vacuum-assisted large-core breast biopsy program in Canada. Can Assoc Radiol J. 2001;52:223-227.
Lamm RL, Jackman RJ. Mammographic abnormalities caused by percutaneous stereotactic biopsy of histologically benign lesions evident on follow-up mammograms. AJR Am J Roentgenol. 2000;174:753-756.
Lannin DR, Ponn T, Andrejeva L, et al. Should all breast cancers be diagnosed by needle biopsy? Am J Surg. 2006;192:450-454.
Lee CH, Carter D, Philpotts LE, et al. Ductal carcinoma in situ diagnosed with stereotactic core needle biopsy: can invasion be predicted? Radiology. 2000;217:466-470.
Lee CH, Egglin TK, Philpotts L, et al. Cost-effectiveness of stereotactic core needle biopsy: analysis by means of mammographic findings. Radiology. 1997;202:849-854.
Lee CH, Philpotts LE, Horvath LG, et al. Follow-up of breast lesions diagnosed as benign with stereotactic core-needle biopsy: frequency of mammographic change and false-negative rate. Radiology. 1999;212:189-194.
Lee SG, Piccoli CW, Hughes JS. Displacement of microcalcifications during stereotactic 11-gauge directional vacuum-assisted biopsy with marking clip placement: case report. Radiology. 2001;219:495-497.
Lehman CD, Shook JE. Position of clip placement after vacuum-assisted breast biopsy: is a unilateral two-view postbiopsy mammogram necessary? Breast J. 2003;9:272-276.
Liberman L. Percutaneous image-guided core breast biopsy. Radiol Clin North Am. 2002;40:483-500.
Liberman L, Benton CL, Dershaw DD, et al. Learning curve for stereotactic breast biopsy: how many cases are enough? AJR Am J Roentgenol. 2001;176:721-727.
Liberman L, Bracero N, Vuolo MA, et al. Percutaneous large-core biopsy of papillary breast lesions. AJR Am J Roentgenol. 1999;172:331-337.
Liberman L, Dershaw DD, Glassman JR. Analysis of cancers not diagnosed at stereotactic core breast biopsy. Radiology. 1997;203:151-157.
Liberman L, Dershaw DD, Rosen PP, et al. Percutaneous removal of malignant mammographic lesions at stereotactic vacuum-assisted biopsy. Radiology. 1998;206:711-715.
Liberman L, Drotman M, Morris EA, et al. Imaging–histologic discordance at percutaneous breast biopsy. Cancer. 2000;89:2538-2546.
Liberman L, Ernberg LA, Heerdt A, et al. Palpable breast masses: is there a role for percutaneous imaging-guided core biopsy? AJR Am J Roentgenol. 2000;175:779-787.
Liberman L, Feng TL, Dershaw DD, et al. US-guided core breast biopsy: use and cost-effectiveness. Radiology. 1998;208:717-723.
Liberman L, Kaplan J, Van Zee KJ, et al. Bracketing wires for preoperative breast needle localization. AJR Am J Roentgenol. 2001;177:565-572.
Liberman L, Kaplan JB, Morris EA, et al. To excise or to sample the mammographic target: what is the goal of stereotactic 11-gauge vacuum-assisted breast biopsy? AJR Am J Roentgenol. 2002;179:679-683.
Liberman L, Sama M, Susnik B, et al. Lobular carcinoma in situ at percutaneous breast biopsy: surgical biopsy findings. AJR Am J Roentgenol. 1999;173:291-299.
Liberman L, Smolkin JH, Dershaw DD, et al. Calcification retrieval at stereotactic, 11-gauge, directional, vacuum-assisted breast biopsy. Radiology. 1998;208:251-260.
Liberman L, Tornos C, Huzjan R, et al. Is surgical excision warranted after benign, concordant diagnosis of papilloma at percutaneous breast biopsy? AJR Am J Roentgenol. 2006;186:1328-1334.
Liberman L, Vuolo M, Dershaw DD, et al. Epithelial displacement after stereotactic 11-gauge directional vacuum-assisted breast biopsy. AJR Am J Roentgenol. 1999;172:677-681.
Liberman L, Zakowski MF, Avery S, et al. Complete percutaneous excision of infiltrating carcinoma at stereotactic breast biopsy: how can tumor size be assessed? AJR Am J Roentgenol. 1999;173:1315-1322.
Lomoschitz FM, Helbich TH, Rudas M, et al. Stereotactic 11-gauge vacuum-assisted breast biopsy: influence of number of specimens on diagnostic accuracy. Radiology. 2004;232:897-903. Epub 23 Jul 2004
Londero V, Zuiani C, Linda A, et al. Lobular neoplasia: core needle breast biopsy underestimation of malignancy in relation to radiologic and pathologic features. Breast. 2008;17:623-630.
Lourenco AP, Mainiero MB, Lazarus E, et al. Stereotactic breast biopsy: comparison of histologic underestimation rates with 11- and 9-gauge vacuum-assisted breast biopsy. AJR Am J Roentgenol. 2007;189:W275-W279.
Mahoney MC, Robinson-Smith TM, Shaughnessy EA. Lobular neoplasia at 11-gauge vacuum-assisted stereotactic biopsy: correlation with surgical excisional biopsy and mammographic follow-up. AJR Am J Roentgenol. 2006;187:949-954.
Mainiero MB, Koelliker SL, Lazarus E, et al. Ultrasound-guided large core needle biopsy of the breast: frequency and results of repeat biopsy. J Women’s Imaging. 2002;4:52-57.
March DE, Coughlin BF, Barham RB, et al. Breast masses: removal of all US evidence during biopsy by using a handheld vacuum-assisted device—initial experience. Radiology. 2003;227:549-555.
McNamara MPJr, Boden T. Pseudoaneurysm of the breast related to 18-gauge core biopsy: successful repair using sonographically guided thrombin injection. AJR Am J Roentgenol. 2002;179:924-926.
Meloni GB, Dessole S, Becchere MP, et al. Ultrasound-guided mammotome vacuum biopsy for the diagnosis of impalpable breast lesions. Ultrasound Obstet Gynecol. 2001;18:520-524.
Melotti MK, Berg WA. Core needle breast biopsy in patients undergoing anticoagulation therapy: preliminary results. AJR Am J Roentgenol. 2000;174:245-249.
Mercado CL, Hamele-Bena D, Oken SM, et al. Papillary lesions of the breast at percutaneous core-needle biopsy. Radiology. 2006;238:801-808.
Mercado CL, Hamele-Bena D, Singer C, et al. Papillary lesions of the breast: evaluation with stereotactic directional vacuum-assisted biopsy. Radiology. 2001;221:650-655.
Michalopoulos NV, Zagouri F, Sergentanis TN, et al. Needle tract seeding after vacuum-assisted breast biopsy. Acta Radiol. 2008;49:267-270.
Morris EA, Liberman L, Trevisan SG, et al. Histologic heterogeneity of masses at percutaneous breast biopsy. Breast J. 2002;8:187-191.
Mullen DJ, Eisen RN, Newman RD, et al. The use of carbon marking after stereotactic large-core-needle breast biopsy. Radiology. 2001;218:255-260.
Nagi CS, O’Donnell JE, Tismenetsky M, et al. Lobular neoplasia on core needle biopsy does not require excision. Cancer. 2008;112:2152-2158.
Pal S, Ikeda DM, Birdwell RL. Compliance with recommended follow-up after fine-needle aspiration biopsy of nonpalpable breast lesions: a retrospective study. Radiology. 1996;201:71-74.
Parker SH, Burbank F, Jackman RJ, et al. Percutaneous large-core breast biopsy: a multi-institutional study. Radiology. 1994;193:359-364.
Parker SH, Jobe WE, Dennis MA, et al. US-guided automated large-core breast biopsy. Radiology. 1993;187:507-511.
Parker SH, Klaus AJ, McWey PJ, et al. Sonographically guided directional vacuum-assisted breast biopsy using a handheld device. AJR Am J Roentgenol. 2001;177:405-408.
Parker SH, Lovin JD, Jobe WE, et al. Nonpalpable breast lesions: stereotactic automated large-core biopsies. Radiology. 1991;180:403-407.
Perez-Fuentes JA, Longobardi IR, Acosta VF, et al. Sonographically guided directional vacuum-assisted breast biopsy: preliminary experience in Venezuela. AJR Am J Roentgenol. 2001;177:1459-1463.
Pfarl G, Helbich TH, Riedl CC, et al. Stereotactic 11-gauge vacuum-assisted breast biopsy: a validation study. AJR Am J Roentgenol. 2002;179:1503-1507.
Philpotts LE, Hooley RJ, Lee CH. Comparison of automated versus vacuum-assisted biopsy methods for sonographically guided core biopsy of the breast. AJR Am J Roentgenol. 2003;180:347-351.
Philpotts LE, Lee CH, Horvath LJ, et al. Canceled stereotactic core-needle biopsy of the breast: analysis of 89 cases. Radiology. 1997;205:423-428.
Philpotts LE, Shaheen NA, Jain KS, et al. Uncommon high-risk lesions of the breast diagnosed at stereotactic core-needle biopsy: clinical importance. Radiology. 2000;216:831-837.
Pijnappel RM, Peeters PH, van den Donk M, et al. Diagnostic strategies in nonpalpable breast lesions. Eur J Cancer. 2002;38:550-555.
Pijnappel RM, van den Donk M, Holland R, et al. Diagnostic accuracy for different strategies of image-guided breast intervention in cases of nonpalpable breast lesions. Br J Cancer. 2004;90:595-600.
Pisano ED, Fajardo LL, Caudry DJ, et al. Fine-needle aspiration biopsy of nonpalpable breast lesions in a multicenter clinical trial: results from the Radiologic Diagnostic Oncology Group V. Radiology. 2001;219:785-792.
Pisano ED, Fajardo LL, Tsimikas J, et al. Rate of insufficient samples for fine-needle aspiration for nonpalpable breast lesions in a multicenter clinical trial: the Radiologic Diagnostic Oncology Group 5 Study. The RDOG5 investigators. Cancer. 1998;82:679-688.
Poellinger A, Bick U, Freund T, et al. Evaluation of 11-gauge and 9-gauge vacuum-assisted breast biopsy systems in a breast parenchymal model. Acad Radiol. 2007;14:677-684.
Rebner M, Helvie MA, Pennes DR, et al. Paraffin tissue block radiography: adjunct to breast specimen radiography. Radiology. 1989;173:695-696.
Renshaw AA. Can mucinous lesions of the breast be reliably diagnosed by core needle biopsy? Am J Clin Pathol. 2002;118:82-84.
Renshaw AA, Cartagena N, Derhagopian RP, et al. Lobular neoplasia in breast core needle biopsy specimens is not associated with an increased risk of ductal carcinoma in situ or invasive carcinoma. Am J Clin Pathol. 2002;117:797-799.
Renshaw AA, Derhagopian RP, Martinez P, et al. Lobular neoplasia in breast core needle biopsy specimens is associated with a low risk of ductal carcinoma in situ or invasive carcinoma on subsequent excision. Am J Clin Pathol. 2006;126:310-313.
Renshaw AA, Derhagopian RP, Tizol-Blanco DM, et al. Papillomas and atypical papillomas in breast core needle biopsy specimens: risk of carcinoma in subsequent excision. Am J Clin Pathol. 2004;122:217-221.
Rizzo M, Lund MJ, Oprea G, et al. Surgical follow-up and clinical presentation of 142 breast papillary lesions diagnosed by ultrasound-guided core-needle biopsy. Ann Surg Oncol. 2008;15:1040-1047.
Rosen EL, Bentley RC, Baker JA, et al. Imaging-guided core needle biopsy of papillary lesions of the breast. AJR Am J Roentgenol. 2002;179:1185-1192.
Rosen EL, Vo TT. Metallic clip deployment during stereotactic breast biopsy: retrospective analysis. Radiology. 2001;218:510-516.
Ross BA, Ikeda DM, Jackman RJ, et al. Milk of calcium in the breast: appearance on prone stereotactic imaging. Breast J. 2001;7:53-55.
Sauer G, Deissler H, Strunz K, et al. Ultrasound-guided large-core needle biopsies of breast lesions: analysis of 962 cases to determine the number of samples for reliable tumor classification. Br J Cancer. 2005;92:231-235.
Schnitt SJ, Connolly JL, Tavassoli FA, et al. Interobserver reproducibility in the diagnosis of ductal proliferative breast lesions using standardized criteria. Am J Surg Pathol. 1992;16:1133-1143.
Schnitt SJ, Vincent-Salomon A. Columnar cell lesions of the breast. Adv Anat Pathol. 2003;10:113-124.
Schoonjans JM, Brem RF. Fourteen-gauge ultrasonographically guided large-core needle biopsy of breast masses. J Ultrasound Med. 2001;20:967-972.
Schueller G, Jaromi S, Ponhold L, et al. US-guided 14-gauge core-needle breast biopsy: results of a validation study in 1352 cases. Radiology. 2008;248:406-413.
Shin HJ, Kim HH, Kim SM, et al. Papillary lesions of the breast diagnosed at percutaneous sonographically guided biopsy: comparison of sonographic features and biopsy methods. AJR Am J Roentgenol. 2008;190:630-636.
Shin SJ, Rosen PP. Excisional biopsy should be performed if lobular carcinoma in situ is seen on needle core biopsy. Arch Pathol Lab Med. 2002;126:697-701.
Sickles EA. Periodic mammographic follow-up of probably benign lesions: results in 3,184 consecutive cases. Radiology. 1991;179:463-468.
Sickles EA. Management of probably benign breast lesions. Radiol Clin North Am. 1995;33:1123-1130.
Simon JR, Kalbhen CL, Cooper RA, et al. Accuracy and complication rates of US-guided vacuum-assisted core breast biopsy: initial results. Radiology. 2000;215:694-697.
Smathers RL. Marking the cavity site after stereotactic core needle breast biopsy. AJR Am J Roentgenol. 2003;180:355-356.
Smith DN, Rosenfield Darling ML, et al. The utility of ultrasonographically guided large-core needle biopsy: results from 500 consecutive breast biopsies. J Ultrasound Med. 2001;20:43-49.
Smith LF, Henry-Tilman R, Rubio T, et al. Intraoperative localization after stereotactic breast biopsy without a needle. Am J Surg. 2001;182:584-589.
Sneige N, Lim SC, Whitman GJ, et al. Atypical ductal hyperplasia diagnosis by directional vacuum-assisted stereotactic biopsy of breast microcalcifications. Considerations for surgical excision. Am J Clin Pathol. 2003;119:248-523.
Soo MS, Baker JA, Rosen EL, et al. Sonographically guided biopsy of suspicious microcalcifications of the breast: a pilot study. AJR Am J Roentgenol. 2002;178:1007-1015.
Soo MS, Baker JA, Rosen EL. Sonographic detection and sonographically guided biopsy of breast microcalcifications. AJR Am J Roentgenol. 2003;180:941-948.
Stomper PC, Davis SP, Weidner N, et al. Clinically occult, noncalcified breast cancer: serial radiologic–pathologic correlation in 27 cases. Radiology. 1988;169:621-626.
Sydnor MK, Wilson JD, Hijaz TA, et al. Underestimation of the presence of breast carcinoma in papillary lesions initially diagnosed at core-needle biopsy. Radiology. 2007;242:58-62.
Tabar L, Pentek Z, Dean PB. The diagnostic and therapeutic value of breast cyst puncture and pneumocystography. Radiology. 1981;141:659-663.
Tartter PI, Bleiweiss IJ, Levchenko S. Factors associated with clear biopsy margins and clear reexcision margins in breast cancer specimens from candidates for breast conservation. J Am Coll Surg. 1997;185:268-273.
Teh WL, Wilson AR, Evans AJ, et al. Ultrasound guided core biopsy of suspicious mammographic calcifications using high frequency and power Doppler ultrasound. Clin Radiol. 2000;55:390-394.
Thompson WR, Bowen JR, Dorman BA, et al. Mammographic localization and biopsy of nonpalpable breast lesions. A 5-year study. Arch Surg. 1991;126:730-734.
Tornos C, Silva E, el-Naggar A, Pritzker KP. Calcium oxalate crystals in breast biopsies. The missing microcalcifications. Am J Surg Pathol. 1990;14:961-968.
Verkooijen HM. Core Biopsy after Radiological Localisation (COBRA) Study Group. Diagnostic accuracy of stereotactic large-core needle biopsy for nonpalpable breast disease: results of a multicenter prospective study with 95% surgical confirmation. Int J Cancer. 2002;99:853-859.
Verkooijen HM, Peterse JL, Schipper ME, et al. Interobserver variability between general and expert pathologists during the histopathological assessment of large-core needle and open biopsies of nonpalpable breast lesions. Eur J Cancer. 2003;39:2187-2191.
Whaley DH, Adamczyk DL, Jensen EA. Sonographically guided needle localization after stereotactic breast biopsy. AJR Am J Roentgenol. 2003;180:352-354.
Yang JH, Lee WS, Kim SW, et al. Effect of core-needle biopsy vs. fine-needle aspiration on pathologic measurement of tumor size in breast cancer. Arch Surg. 2005;140:125.
Yeh IT, Dimitrov D, Otto P, et al. Pathologic review of atypical hyperplasia identified by image-guided breast needle core biopsy. Correlation with excision specimen. Arch Pathol Lab Med. 2003;127:49-54.
Youk JH, Kim EK, Kim MJ, et al. Sonographically guided 14-gauge core needle biopsy of breast masses: a review of 2,420 cases with long-term follow-up. AJR Am J Roentgenol. 2008;190:202-207.
Youk JH, Kim EK, Kim MJ. Atypical ductal hyperplasia diagnosed at sonographically guided 14-gauge core needle biopsy of breast mass. AJR Am J Roentgenol. 2009;192:1135-1141.
Zografos GC, Zagouri F, Sergentanis TN, et al. Diagnosing papillary lesions using vacuum-assisted breast biopsy: should conservative or surgical management follow? Onkologie. 2008;31:653-656.
6-1. Fill in the requirements for nonpalpable percutaneous breast lesion biopsy.
_________________________________________________
_________________________________________________
_________________________________________________
_________________________________________________
_________________________________________________
6-2. Fill in the possible complications from breast biopsy.
_________________________________________________
_________________________________________________
_________________________________________________
_________________________________________________
_________________________________________________
_________________________________________________
_________________________________________________
6-3. Fill in the types of benign discordant needle biopsies.
_________________________________________________
_________________________________________________
_________________________________________________
_________________________________________________
_________________________________________________
_________________________________________________
_________________________________________________
_________________________________________________
_________________________________________________