Chapter 4 Mammographic and Ultrasound Analysis of Breast Masses
Mammographic Technique and Analysis
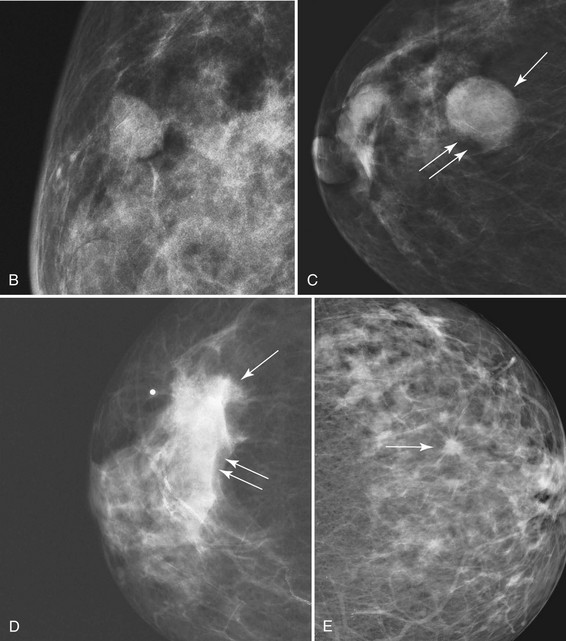
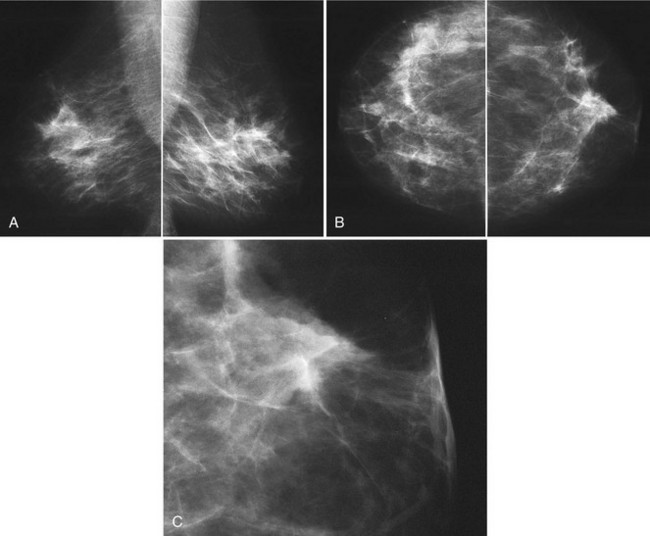
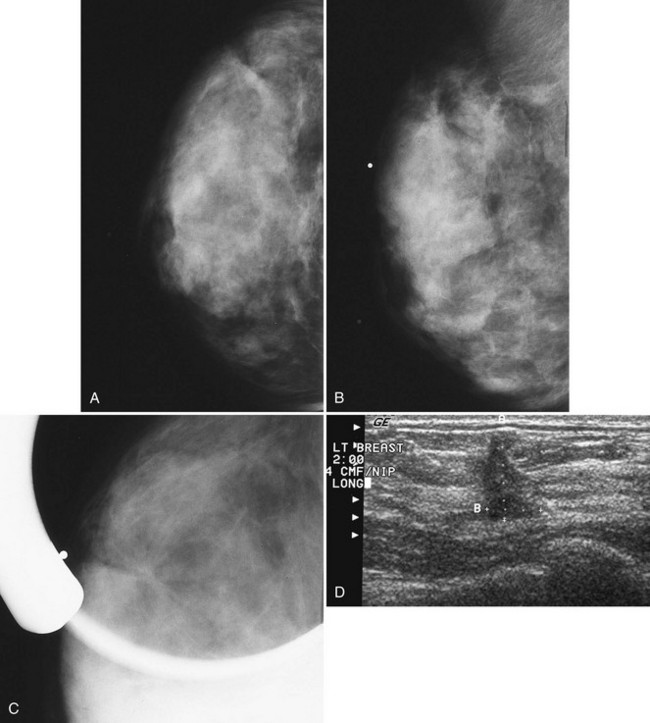
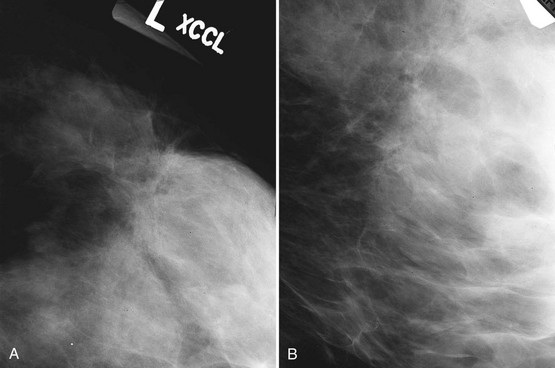
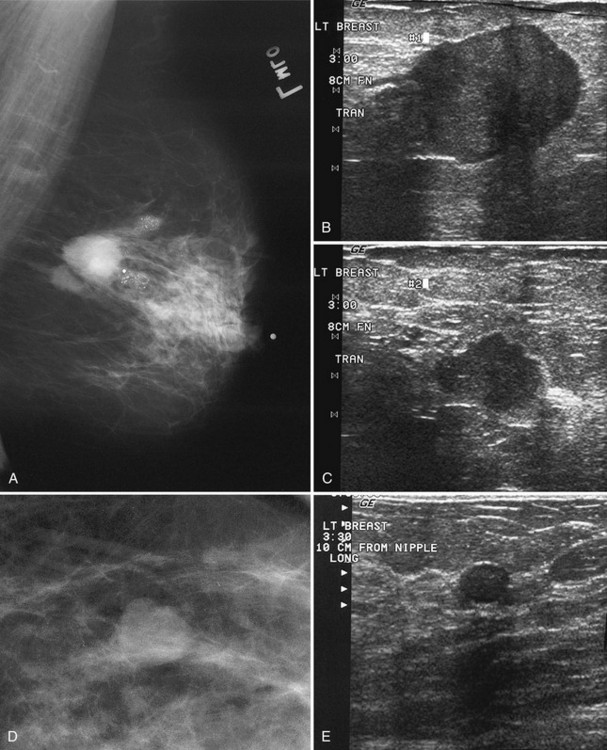
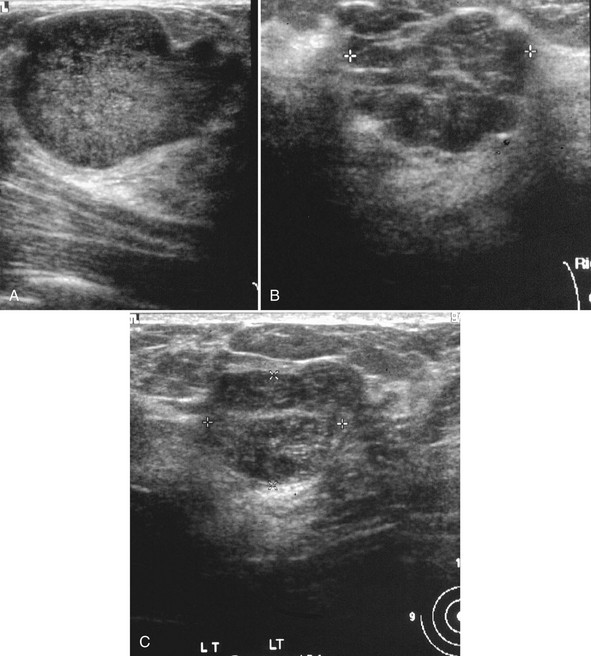
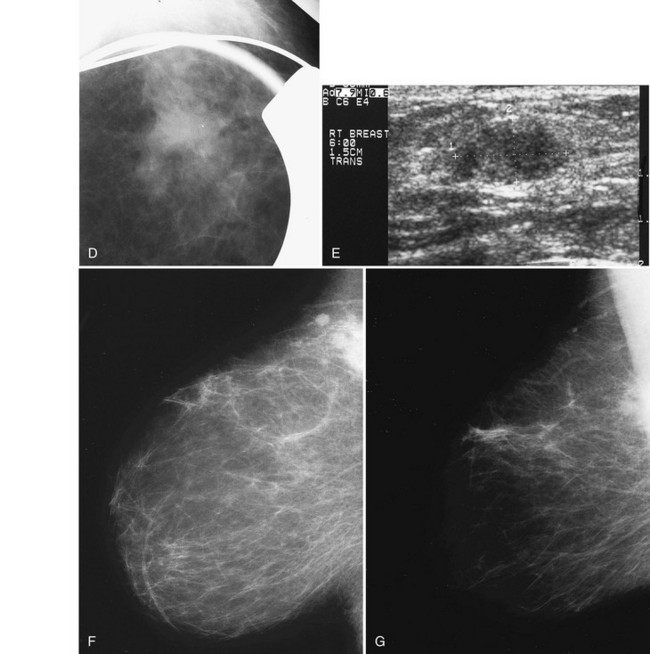
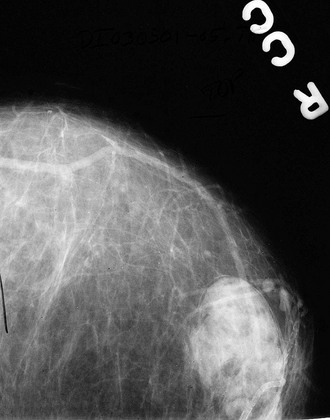
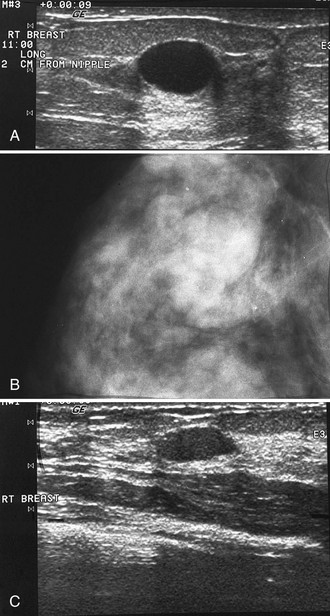
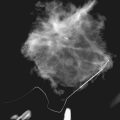
The ACR BI-RADS® lexicon (Table 4-1) defines mass shapes as round, oval, lobular, or irregular. As the mass shape becomes more irregular, the probability of cancer increases (Fig. 4-1A).
Table 4-1 American College of Radiology BI-RADS® Mass Descriptors
| Shape | Margin | Density |
|---|---|---|
BI-RADS®, Breast Imaging Reporting and Data System.
From American College of Radiology: ACR BI-RADS®—mammography, ed 4, In ACR Breast Imaging and Reporting and Data System, breast imaging atlas, Reston, VA, 2003, American College of Radiology.
The ACR BI-RADS® lexicon describes mass margins as circumscribed (well-defined or sharply defined), microlobulated, obscured by surrounding glandular tissue, indistinct, or spiculated (see Fig. 4-1B to E). As with mass shape, as the mass margin becomes more spiculated the probability of cancer increases. Masses with well-circumscribed borders are likely to be benign, and less than 10% of cancers are smooth. Microlobulated masses have small undulations, like petals on a flower, and are more worrisome for cancer than are smooth masses. An obscured mass has a border hidden by overlapping adjacent fibroglandular tissue; as a result, that border cannot be assessed. An indistinct mass is worrisome for carcinoma because it suggests that the surrounding glandular tissue is infiltrated by malignancy. Finally, spiculated masses are characterized by thin lines radiating from the central portion of the mass and are especially worrisome for cancer. When caused by cancer, spiculations are due to productive tumor fibrosis or growth of tumor into the surrounding glandular tissue.
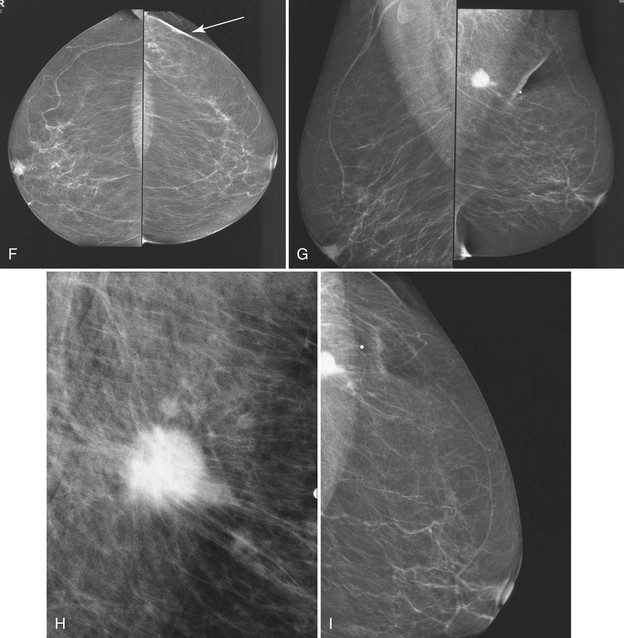
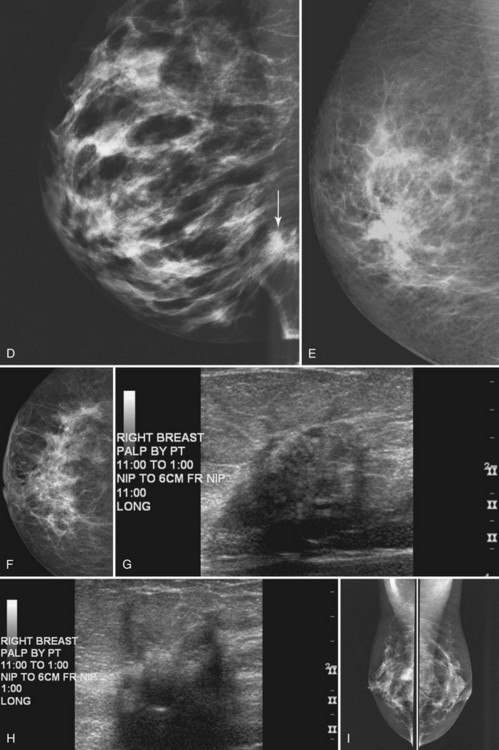
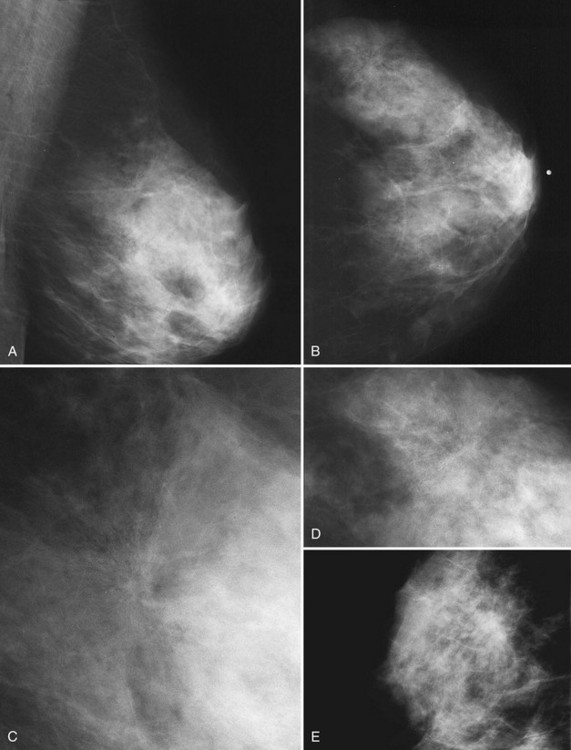
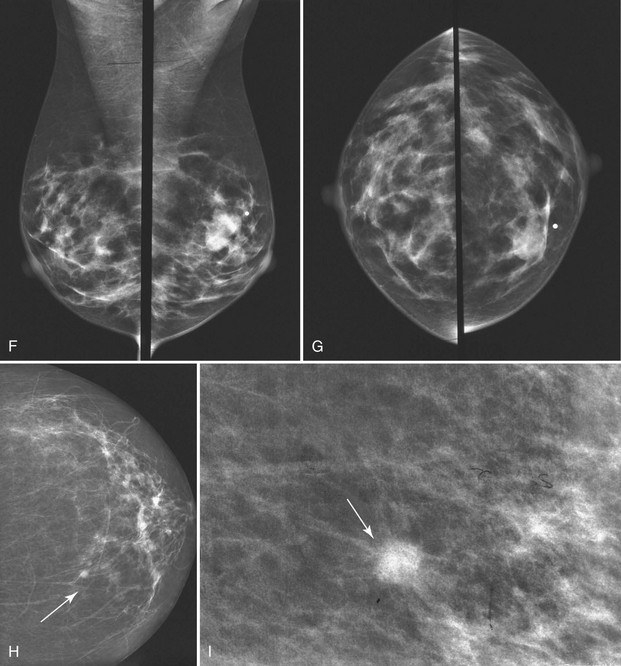
Masses can have associated findings that can indicate cancer (listed in Box 4-1). Associated findings worrisome for cancer include skin or nipple retraction, skin or trabecular thickening, axillary adenopathy, architectural distortion, and calcifications (see Fig. 4-1F to I).
Box 4-1
American College of Radiology BI-RADS® Associated Findings
BI-RADS®, Breast Imaging Reporting and Data System.
From American College of Radiology: ACR BI-RADS®—mammography, ed 4, In ACR Breast Imaging and Reporting and Data System, breast imaging atlas, Reston, VA, 2003, American College of Radiology.
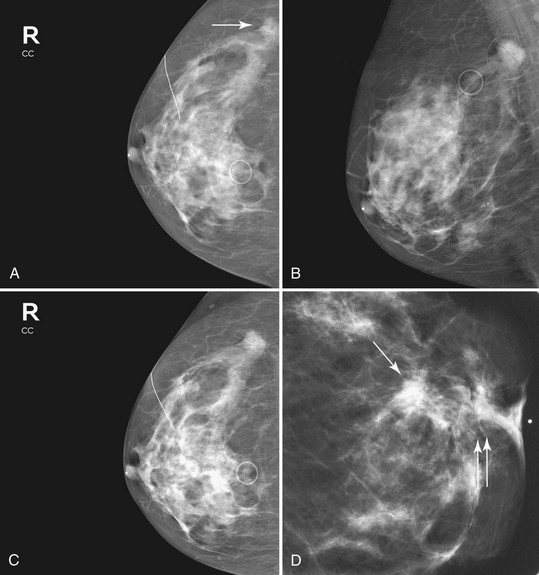
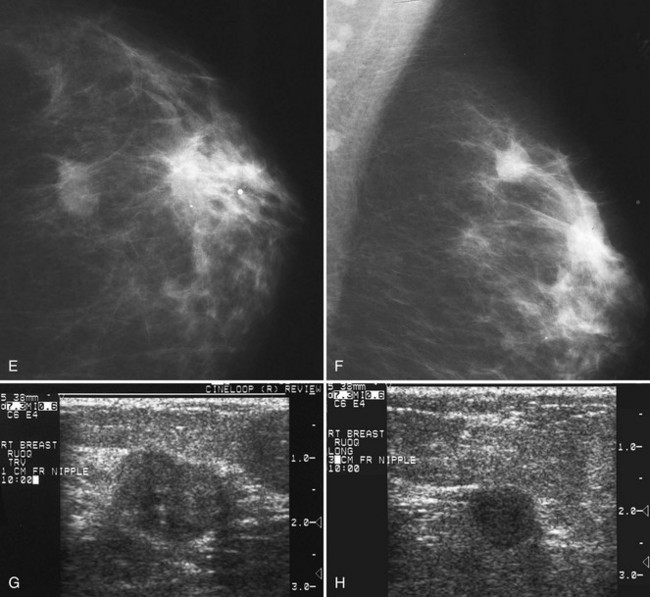
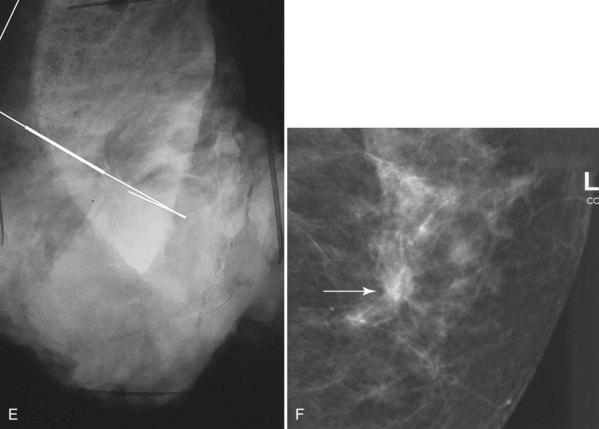
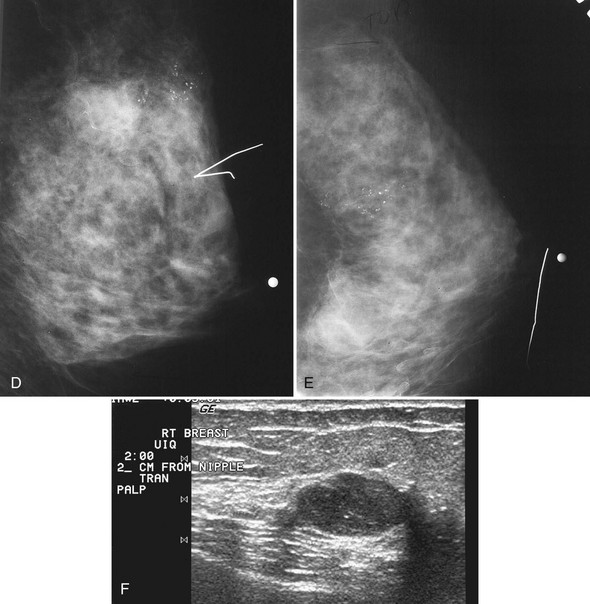
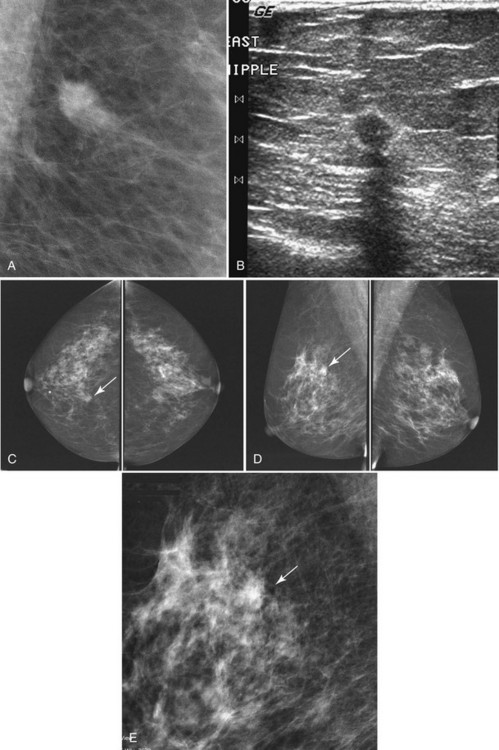
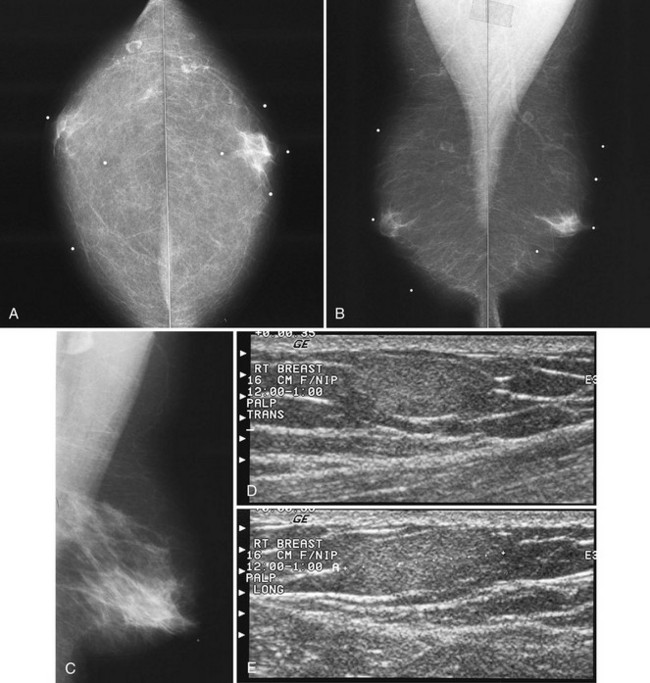
Associated calcifications in or around a suspicious mass are important for two reasons. If the mass is cancer, calcifications around it may represent ductal carcinoma in situ (DCIS). Subsequent excisional biopsy must remove both the mass and all surrounding suspicious calcifications to excise the entire malignancy (Box 4-2). Knowing the extent of the suspicious calcifications helps the surgeon plan the excision (Fig. 4-2). Second, suspicious calcifications inside a mass may be the only clue that the mass is a cancer.
Ultrasound Technique and Analysis of Masses
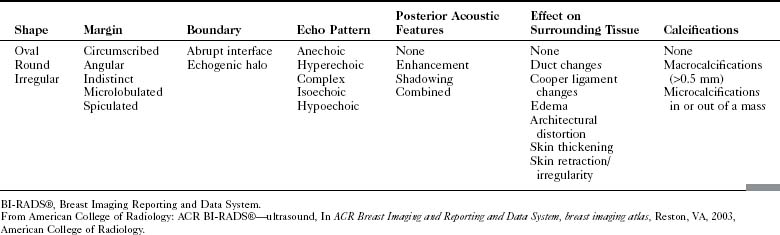
The ACR BI-RADS® ultrasound lexicon describes terms and features of breast masses that are key for the diagnosis of cancer (Table 4-2). Stavros and colleagues established another set of terms that are often used in evaluating breast masses (Box 4-3). Illustrations of these features are shown in Chapter 5.
Box 4-3
Ultrasound Features of Solid Breast Masses
From Stavros AT, Thickman D, Rapp CL, et al: Solid breast nodules: use of sonography to distinguish between benign and malignant lesions, Radiology 196:123–134, 1995.
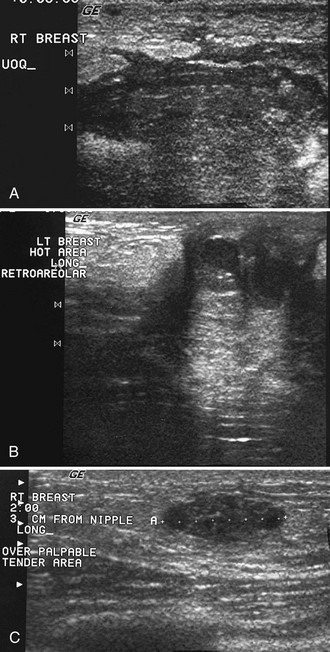
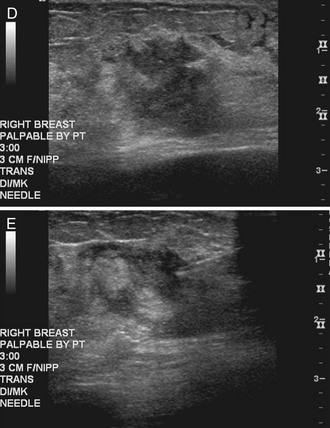
After scanning, the technologist takes representative pictures of the mass and labels the images to clarify the mass’s location in the breast. This makes the mass easier to find on repeat ultrasound examinations. Ultrasound labeling includes which breast was scanned (left or right), position of the mass in terms of breast clock face or quadrant, location in centimeters from the nipple, scan angle (radial or antiradial, transverse or longitudinal), and the technologist’s initials. The technologist captures the image without and with measuring calipers on the muscle (Box 4-4). It is also helpful to indicate whether the mass is palpable or nonpalpable.
Correlating Palpable and Nonpalpable Masses on Mammography and Ultrasound
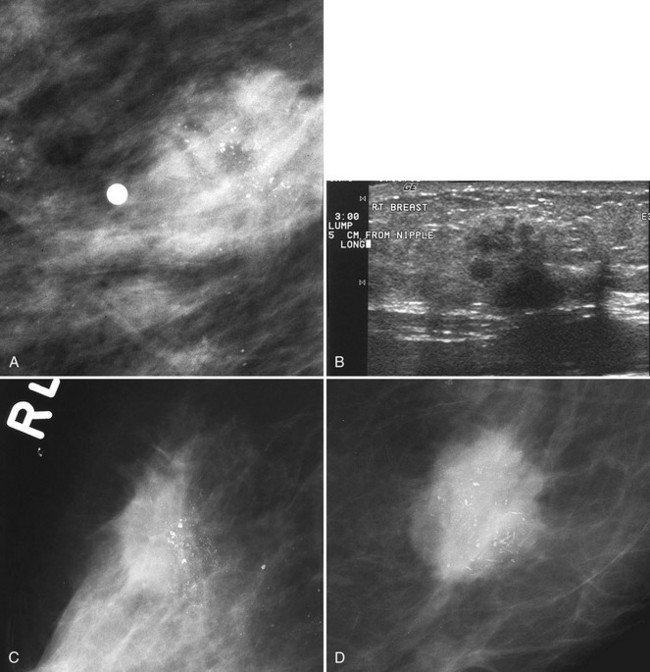
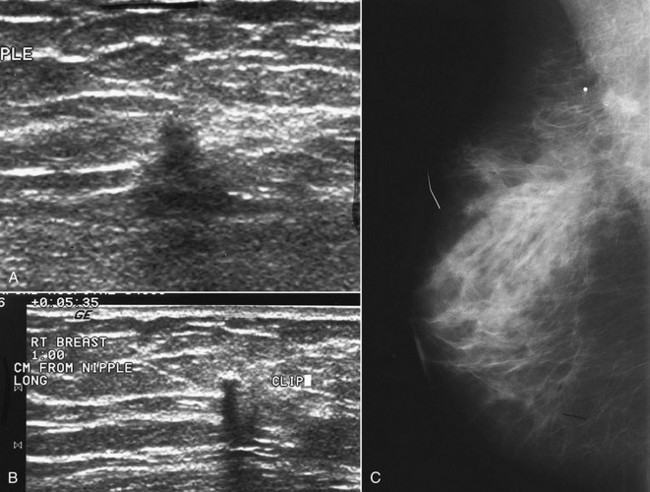
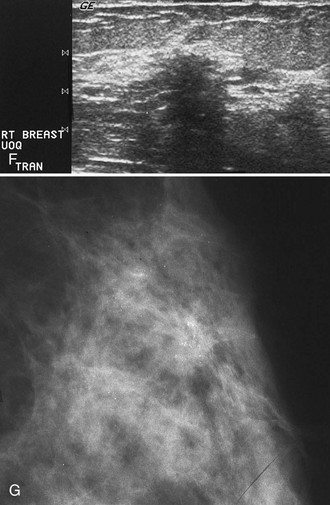
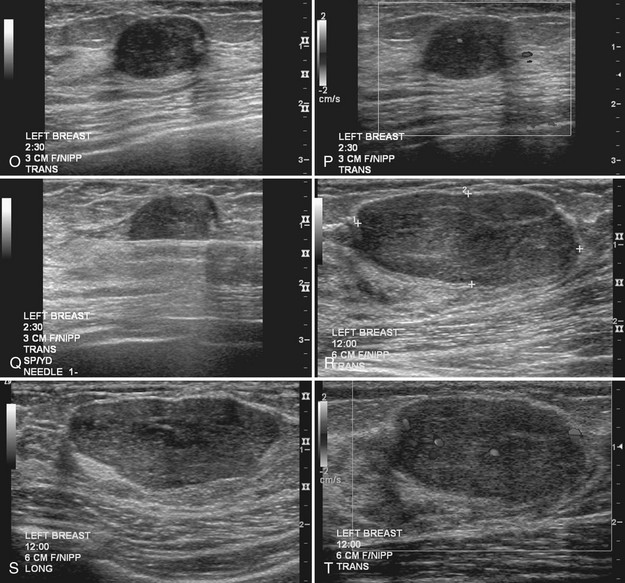
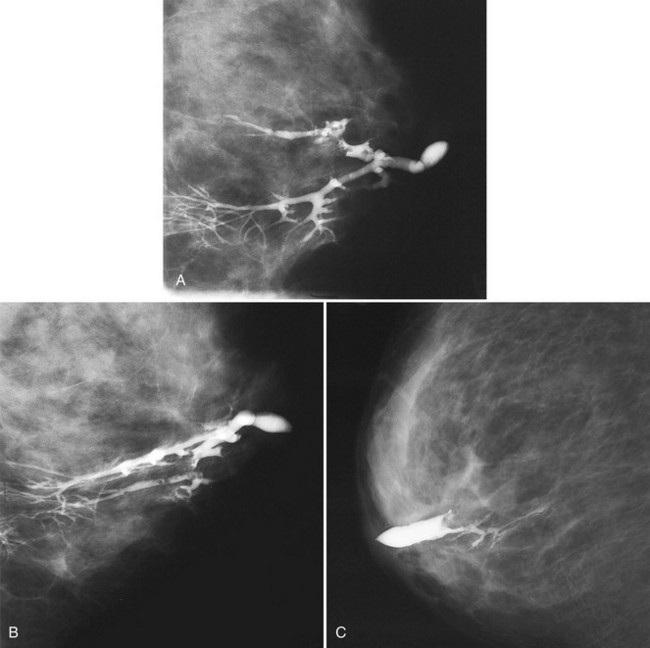
Sometimes it is still uncertain whether an ultrasound and mammographic finding are one and the same. If the patient agrees to a biopsy of the ultrasound finding, the radiologist places a metallic marker into the mass using an ultrasound-guided, percutaneously placed needle after the biopsy (Fig. 4-3). Repeat mammograms should show the marker in the mass if the two findings are the same. Alternatively, a retractable hookwire may be placed in the mass. A mammogram with the wire in place will show that the ultrasound finding and the mammographic finding represent the same mass. The radiologist can subsequently remove the retractable hookwire.
The mammography and ultrasound report for a breast mass should describe if the mass is palpable; the size, shape, margin, and density of the mass; its location and associated findings; and any change from previous examinations, if known. The report should also include ultrasound finding descriptors and whether it correlates with the mammographic finding. Finally, each report that includes a mammogram should be assigned an ACR BI-RADS® final assessment code indicating the level of suspicion for cancer and follow-up management recommendations (Box 4-5).
Box 4-5
American College of Radiology BI-RADS® Mass Reporting
BI-RADS®, Breast Imaging Reporting and Data System.
From American College of Radiology: ACR BI-RADS®—mammography, ed 4, In ACR Breast Imaging and Reporting and Data System, breast imaging atlas, Reston, VA, 2003, American College of Radiology.
Masses with Spiculated Borders and Sclerosing Features (Box 4-6)
Cancer
Invasive Ductal Cancer
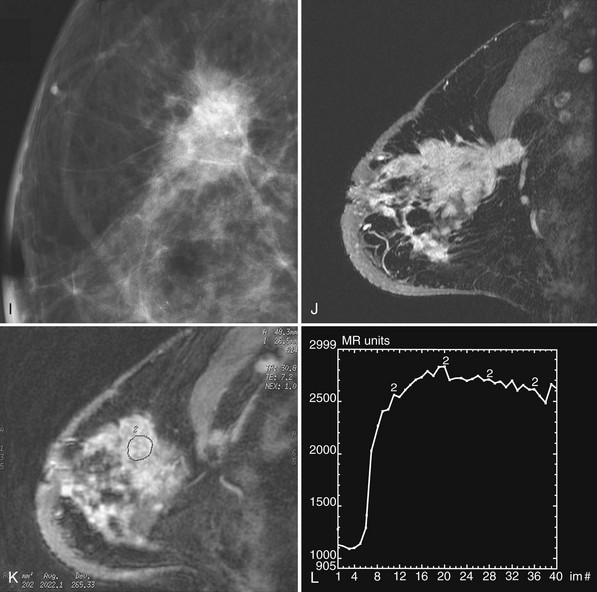
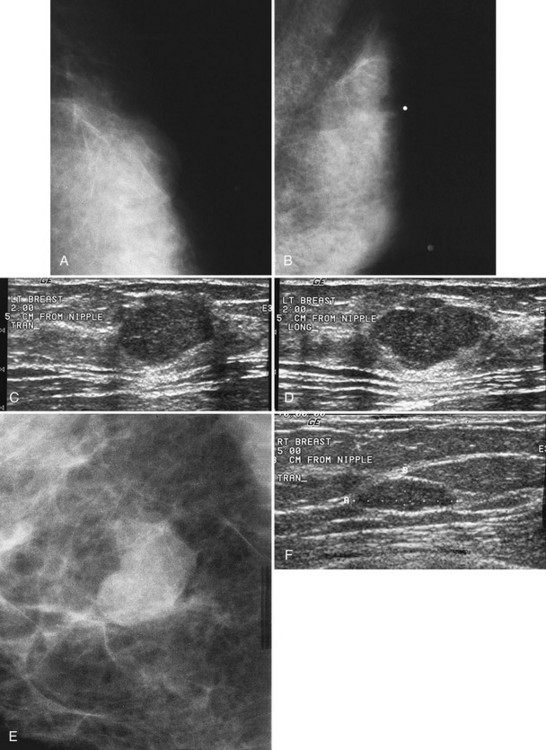
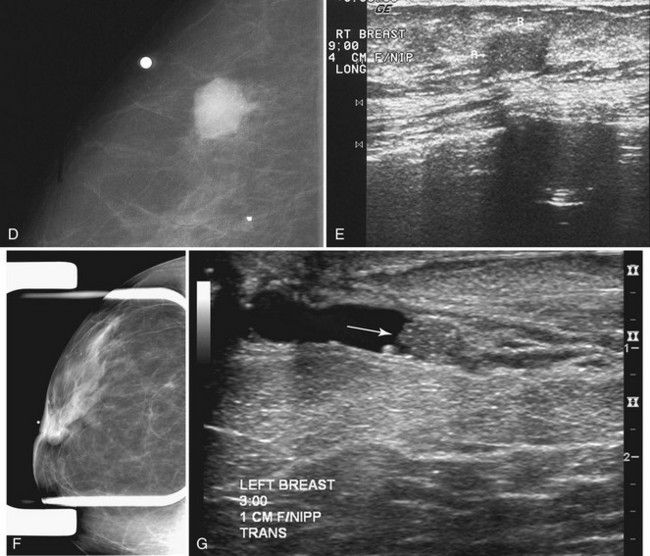
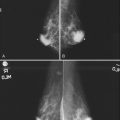
Invasive ductal carcinoma is the most common breast cancer and accounts for approximately 90% of all cancers. Also known as invasive ductal carcinoma not otherwise specified (NOS), invasive ductal cancer usually grows as a hard irregular mass in the breast (Fig. 4-4). The classic appearance of invasive ductal cancer is a dense irregular or spiculated mass, occasionally containing pleomorphic calcifications or having adjacent pleomorphic calcifications representing DCIS. On the mammogram, the mass should be about the same size and density on two orthogonal mammographic views. Spot compression magnification views may show unsuspected calcifications in or around the mass or unsuspected irregular borders.
Invasive Lobular Carcinoma
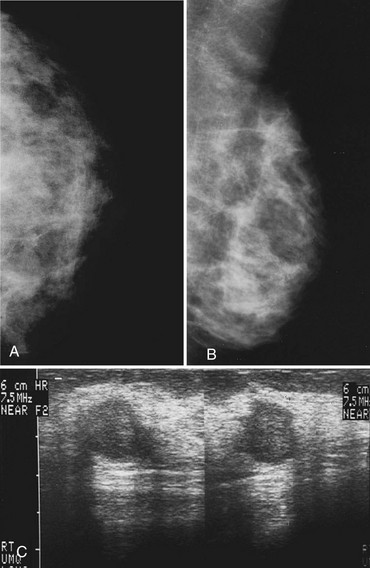
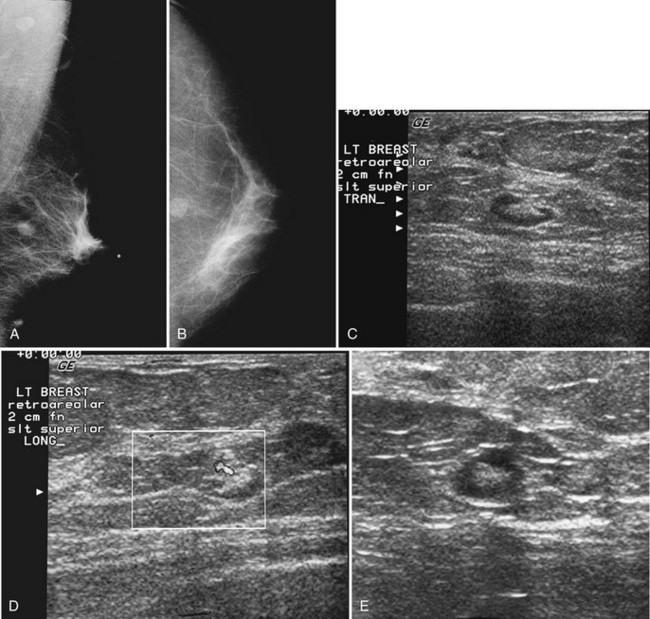
Invasive lobular carcinoma (ILC) is most commonly seen as an equal- or high-density noncalcified mass, occasionally showing spiculations or ill-defined borders. ILC has a higher rate of bilaterality and multifocality than does invasive ductal cancer. ILC accounts for less than 10% of all invasive cancers, but historically is the most difficult breast cancer to see on mammograms (Box 4-7). ILC is the cancer that gives radiologists a bad name because it can be missed by mammography, at a rate reported by Brem and colleagues to be as high as 21%. This failure can be partly explained by the growth pattern of the carcinoma. Classically, ILC grows in single lines of tumor cells infiltrating the surrounding glandular tissue and may not produce a mass, making it difficult to see by mammography and difficult to feel by physical examination. ILC usually does not contain microcalcifications. It infiltrates the breast, is often seen on only one view, and may cause subtle distortion of the surrounding glandular tissue. When actually seen on the mammogram, ILC masses are often of equal or higher density than fibroglandular tissue and are seen because of the mass itself or its effect on surrounding tissue, such as architectural distortion and straightening of Cooper ligaments. As with any mass, distortion and tenting of glandular tissue caused by ILC are most easily seen in locations where Cooper ligaments extend out into surrounding fat, such as in the retroglandular fat or along the edge of the normal, scalloped fibroglandular tissue (Fig. 4-5).
Tubular Cancer
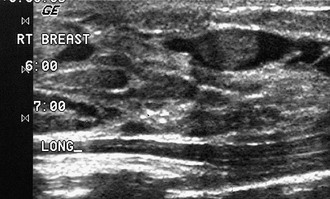
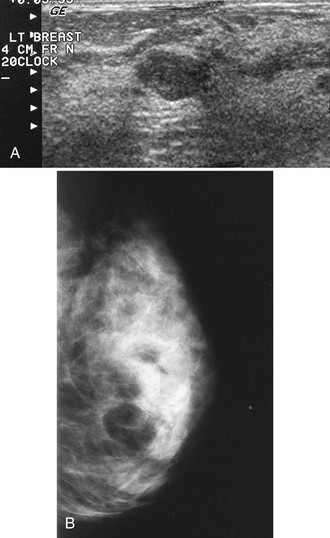
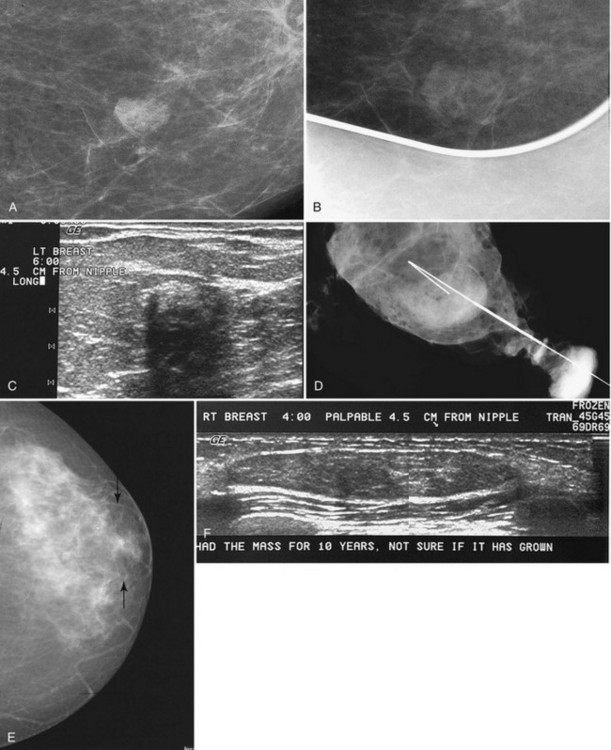
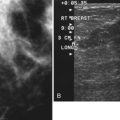
Tubular carcinoma is a generally slow-growing tumor with a bilateral incidence of 12% to 40%. On mammography, tubular cancer is a dense or equal-density spiculated mass with occasional microcalcifications. On occasion it may be apparent on the previous mammogram because of its slow growth. Although controversial, some pathologists believe that radial scars may be a precursor to tubular carcinoma. In general, tubular carcinoma has a good prognosis and a lower incidence of metastases than does invasive ductal cancer. On ultrasound, tubular cancers are hypoechoic, irregular masses that occasionally produce acoustic shadowing (Fig. 4-6).
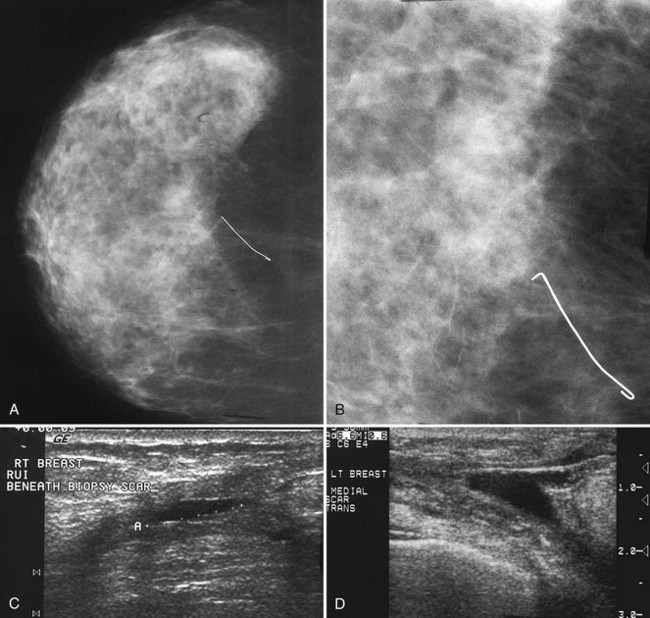
Postbiopsy Scar
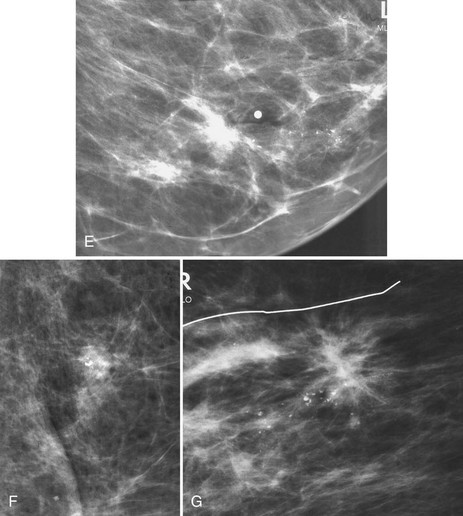
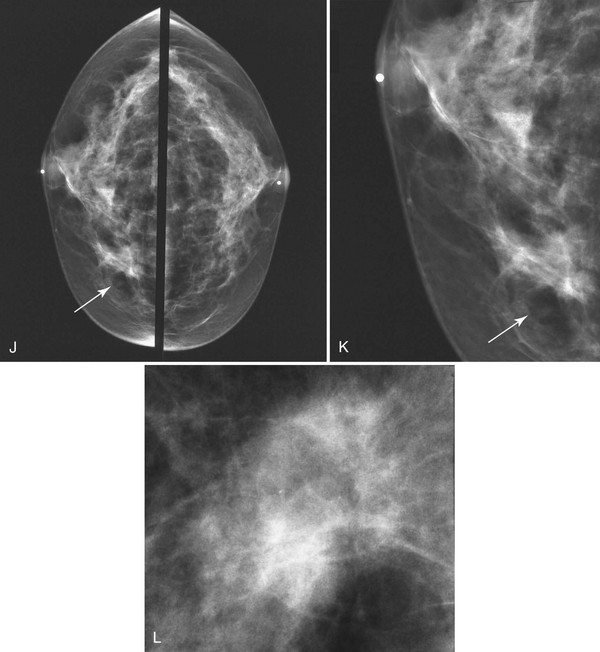
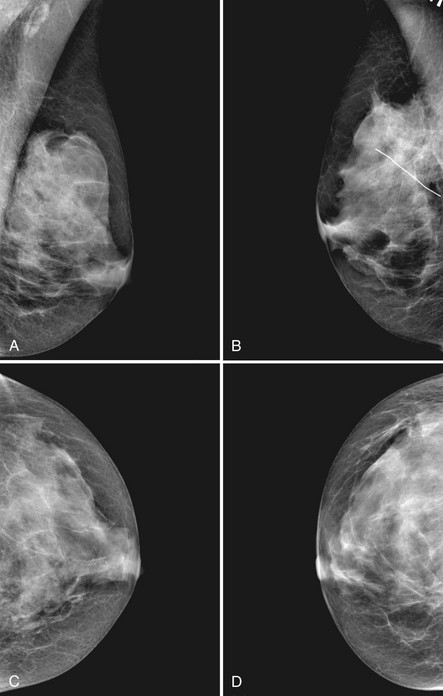
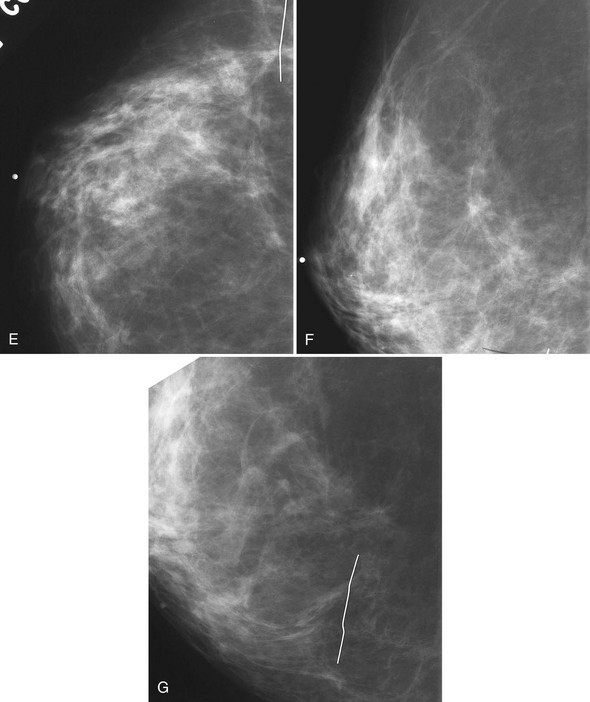
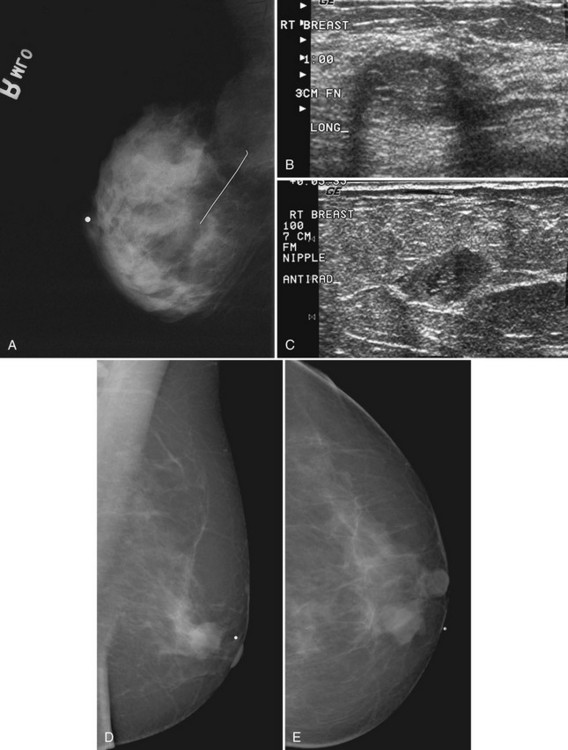
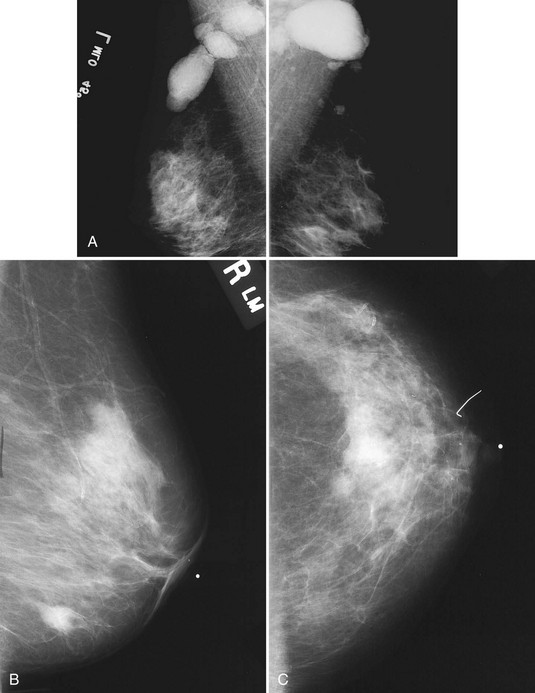
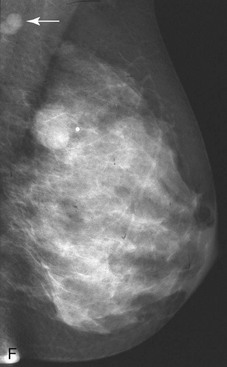
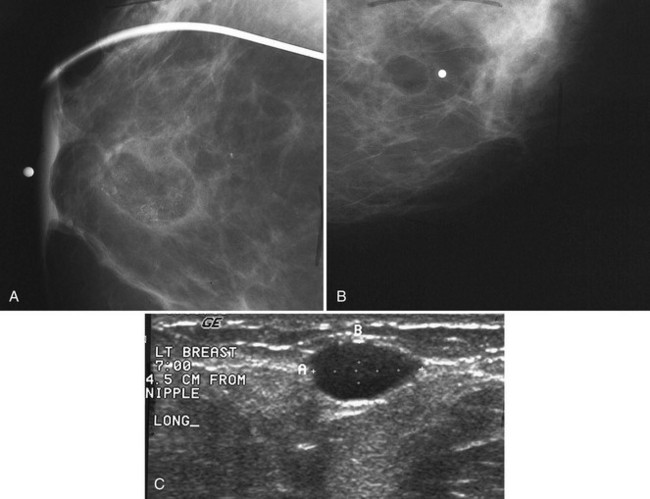
The scar is not of concern for cancer if it occupies a surgical site (Box 4-8). To distinguish postbiopsy scars from cancer, the radiologist looks at the previous biopsy locations on the breast history form and reviews older films to see if the “scar” is at the same location. Some facilities place a radiopaque linear metallic scar marker on the patient’s skin scar to show the location on the mammogram (Fig. 4-7A to D). The metallic linear scar marker should be on top of the “scar” (see Fig. 4-7E and F). If the “scar” does not correspond to a postbiopsy site, it is not a scar. Because spiculated masses may represent cancer, they should be considered suspicious and should undergo biopsy.
Fat Necrosis, Sclerosing Adenosis, and Other Benign Breast Disease
Both sclerosing adenosis and proliferative fibrocystic change may have a slightly spiculated appearance on mammography; they occasionally also contain calcifications and can simulate cancer. When spiculated and associated with calcifications, fat necrosis, sclerosing adenosis and proliferative fibrocystic disease undergo biopsy and are a cause of false-positive biopsies (Fig. 4-8).
Radial Scar
On mammography, a radial scar appears as a spiculated mass with either a dark or white central area that may or may not have associated microcalcifications (Fig. 4-9). It is a myth that radial scars have dark centers in the mass on mammography (Fig. 4-10) and can be distinguished from breast cancers, which have white-centered masses. Scientific studies have shown that radial scars and breast cancer can both have either white or dark centers on mammograms. This means that all spiculated masses not representing a postbiopsy scar should be sampled histologically (Box 4-9). On ultrasound, a radial scar is a hypoechoic mass, with or without acoustic shadowing.
Solid Masses with Rounded or Expansile Borders (Box 4-10)
Malignant Tumors
Invasive Ductal Cancer
Invasive ductal cancer is the most common round breast cancer (Fig. 4-11). The “round” invasive ductal cancer is an uncommon form of the most common cancer. Invasive ductal cancer represents approximately 90% of all invasive breast cancers. So even though invasive ductal cancers are not often “round,” there are so many invasive ductal cancers that the uncommon round form of the most frequent breast cancer is the most common histologic type (Box 4-11).
Box 4-10 Differential Diagnosis of Round Masses
Medullary Cancer
Medullary cancer is an invasive breast cancer that commonly has a round or pushing border. Medullary cancers occasionally have a surrounding lymphoid infiltrate and have a better prognosis than infiltrating ductal cancer (NOS). They are common in BRCA1-associated carcinomas. Atypical medullary cancers have the same prognosis as infiltrating ductal cancer. On screening mammography, medullary cancer is a high- or equal-density round mass whose margins may appear well circumscribed, suggestive of a cyst or fibroadenoma (Fig. 4-12). On ultrasound, medullary cancers are round, solid, and homogeneous. Because they are homogeneous, medullary cancers occasionally cause posterior acoustic enhancement. Because medullary cancers can simulate a breast cyst by their homogeneous nature and posterior acoustic shadowing, it is important to pay careful attention to technical details during scanning to show that the internal features are hypoechoic rather than anechoic. Color or power Doppler ultrasound may show internal vascularity, unlike an anechoic simple cyst. The pushing expansile growth of medullary cancer may produce well-circumscribed mass borders, similar to fibroadenoma, and is a cause for misdiagnosis. This means that a new round solid mass should be biopsied even if it is well-circumscribed.
Mucinous (Colloid) Carcinoma
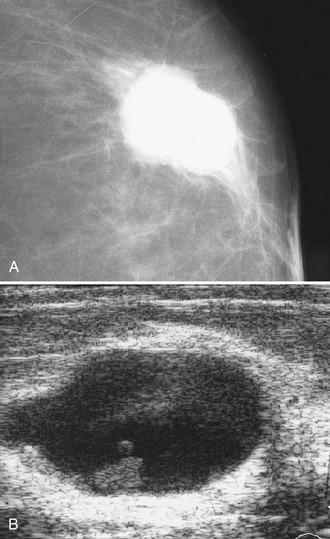
This rare, round or oval cancer contains malignant tumor cells that float in mucin within a solid tumor rim. The mucinous portion can have fibrovascular bands segregating the mucinous compartments that comprise the tumor and give it its name. On mammography, mucinous cancers with a large volume of mucin show a well-circumscribed low-density round mass that can suggest a cyst or fibroadenoma and can occasionally have lobulated margins. Mixed type mucinous carcinomas may have poorly defined or spiculated margins. On ultrasound, the tumor mass is round and may be isoechoic to fat, occasionally contains fluid-filled hypoechoic spaces, and may have posterior acoustic enhancement (Fig. 4-13). The mass can simulate a cyst on ultrasound, but unlike the cyst it will not be entirely anechoic. Thus, new round masses on a mammogram that have solid components or do not meet all the specific criteria for a simple cyst on ultrasound should be considered for biopsy.
Papillary Carcinoma
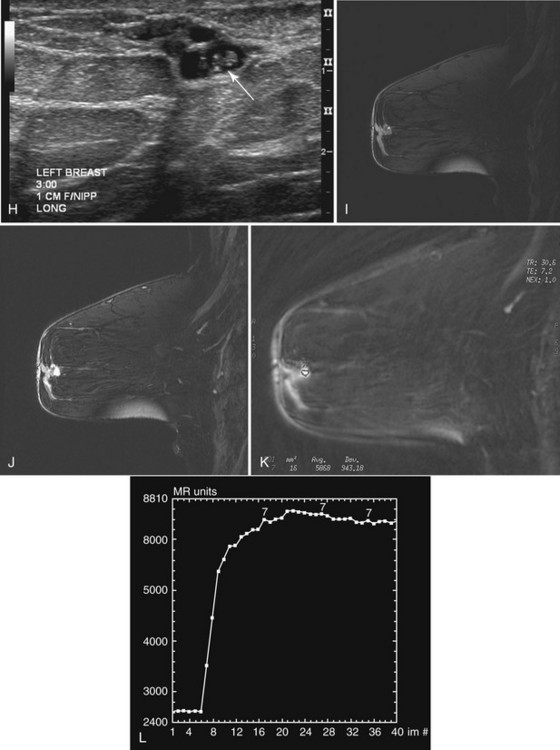
This rare tumor accounts for only 1% to 2% of all cancers and is the malignant form of benign intraductal papilloma. Papillary cancers may be single or multiple (Box 4-12), and DCIS is sometimes seen in surrounding breast tissue. Classically, these masses are round, oval, or lobulated on mammography, sometimes containing calcifications, and are solid on ultrasound. If associated with nipple discharge and detected by ultrasound, papillary cancers are solid intraductal masses outlined by a fluid-filled structure and are difficult to distinguish from a benign intraductal papilloma (Fig. 4-14).
Intracystic Carcinoma
This extremely rare tumor produces a solid mass in a cyst wall, and the mass looks like a cyst on mammography. Because the tumor is mostly mucin, the mammographic mass is low density unless it has a denser solid component or has bled into the cystic portion to produce a dense mass (Fig. 4-15A). On ultrasound, an intracystic carcinoma is a solid mural mass surrounded by cystic fluid that yields fresh or old blood on aspiration (see Fig. 4-15B). On pneumocystography, the air inside the cyst wall will outline a solid mass along the border of the wall. Intracystic carcinomas must be excised, just as all other cancers are excised. The differential diagnosis for a solid intracystic mass includes intracystic carcinoma, intracystic papilloma, and a cyst with debris adherent to the cyst wall.
Breast Metastasis
Axillary or intramammary lymph node metastasis from breast cancer, lymphoma, or other malignancy makes the normal benign lymph node change its appearance from a well-defined oval or lobular mass with a central radiolucency to a rounder, bigger, and denser mass with loss of the fatty hilum (Fig. 4-16). This results in a round, dense mass. Metastases can occur from breast cancer, lymphoma, leukemia, melanoma, other adenocarcinomas, and even mesothelioma.
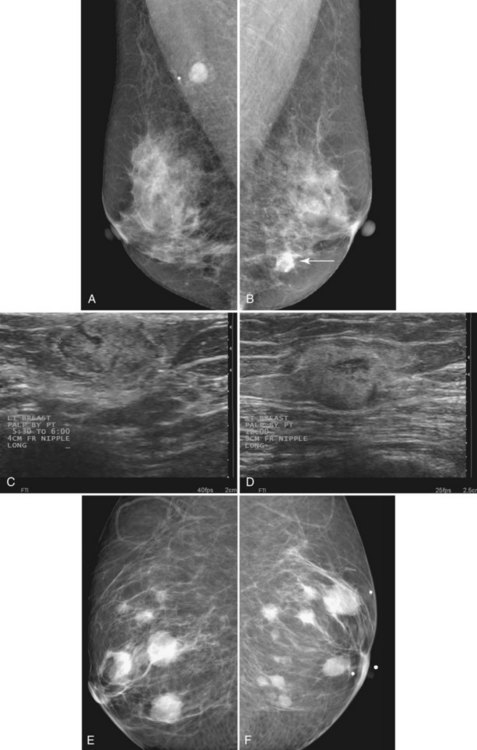
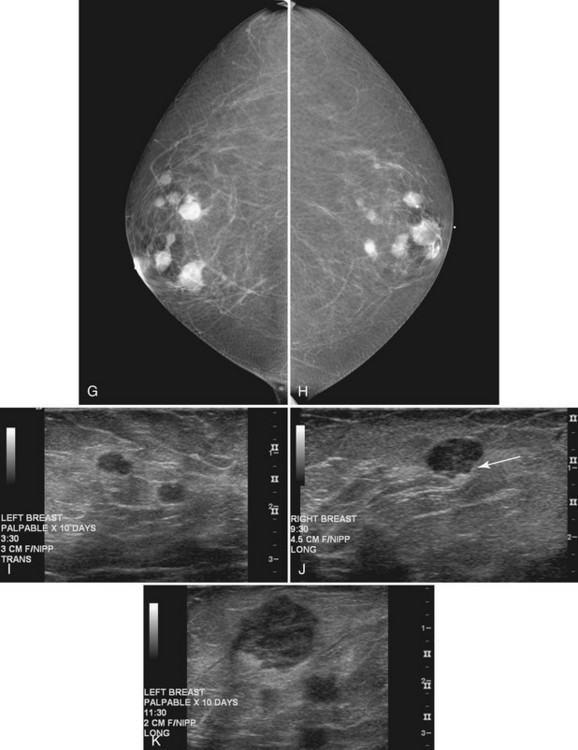
Hematologically spread metastases are single or multiple, round, usually circumscribed, and very dense masses in one or both breasts (see Fig. 4-16). The masses vary in size as a result of the various lengths of time that the metastases have had to grow in breast tissue. Typically, the appearance of multiple new solid masses all over the breast in a nonductal pattern is worrisome for hematogenous spread of cancer from a nonbreast site, similar to pulmonary metastases. Melanoma and renal cell carcinoma were reported to metastasize to the breast in this manner. The differential diagnosis of multiple solid breast masses includes multiple fibroadenomas or papillomas, or metastases when there are no old films for comparison.
Benign Tumors
Fibroadenoma
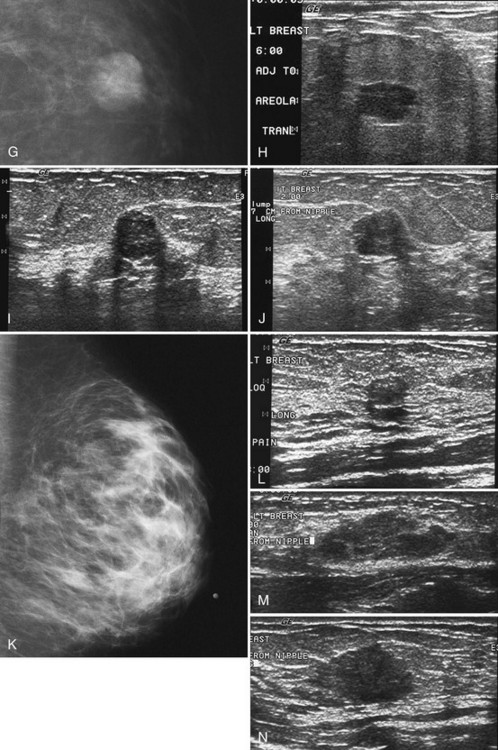
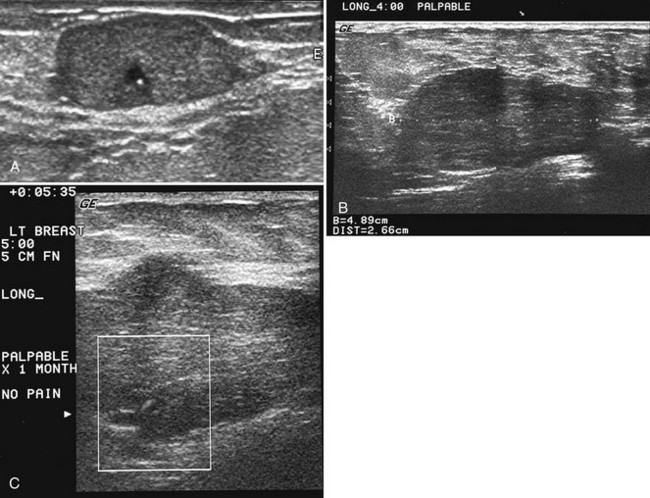
On ultrasound, fibroadenomas are oval, well-circumscribed homogeneous masses, usually wider than tall, with up to four gentle lobulations. They are hypoechoic but may occasionally contain cystic spaces. Posterior acoustic enhancement is increased, equal, or shadowing (Fig. 4-17). Fibroadenomas occasionally display irregular borders or heterogeneous internal characteristics, so biopsy is necessary to distinguish these atypical-appearing fibroadenomas from cancer.
Phyllodes Tumor
On mammography, a phyllodes tumor is a dense round, oval, or lobulated noncalcified mass with smooth borders. On ultrasound, a phyllodes tumor is a smoothly marginated inhomogeneous mass that occasionally contains cystic spaces producing acoustic posterior enhancement, and it can be mistaken for a fibroadenoma or circumscribed cancer (Fig. 4-18). The goal of imaging is to identify the tumor for wide excision and to search for recurrence in the biopsy bed after surgery when the patient returns for routine follow-up.
Papilloma
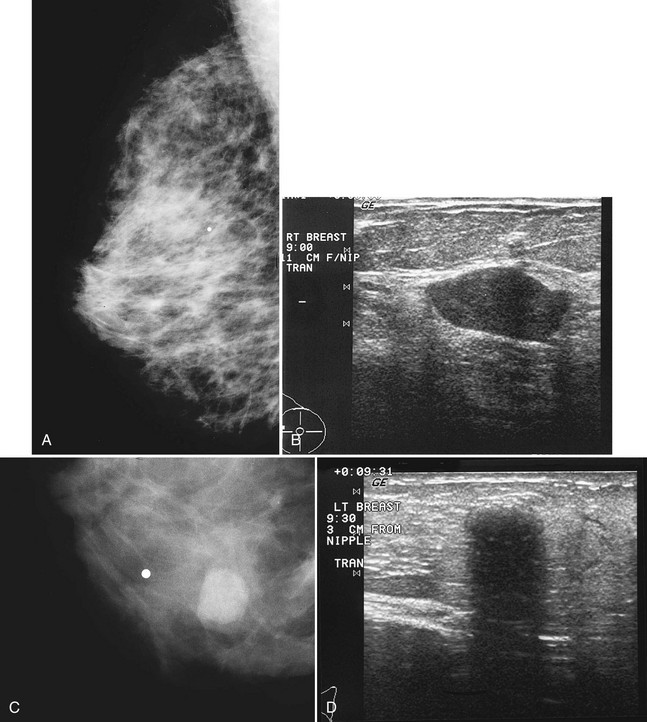
On mammography, papillomas are round, well-circumscribed, equal-density masses that occasionally contain calcifications. They are usually located in the subareolar region. In papillomatosis, papillomas can be multiple and peripherally located (Fig. 4-19). Occasionally, papillomas are not seen on mammography or ultrasound at all despite the symptom of bloody or clear nipple discharge. On ultrasound, papillomas are solid round, oval, or microlobulated hypoechoic masses. Small internal cystic spaces are seen occasionally in juvenile papillomatosis. In patients with nipple discharge, ultrasound may show papilloma as a solid mass in a fluid-filled subareolar duct. On galactography, the papilloma produces an intraductal filling defect.
Lactating Adenoma
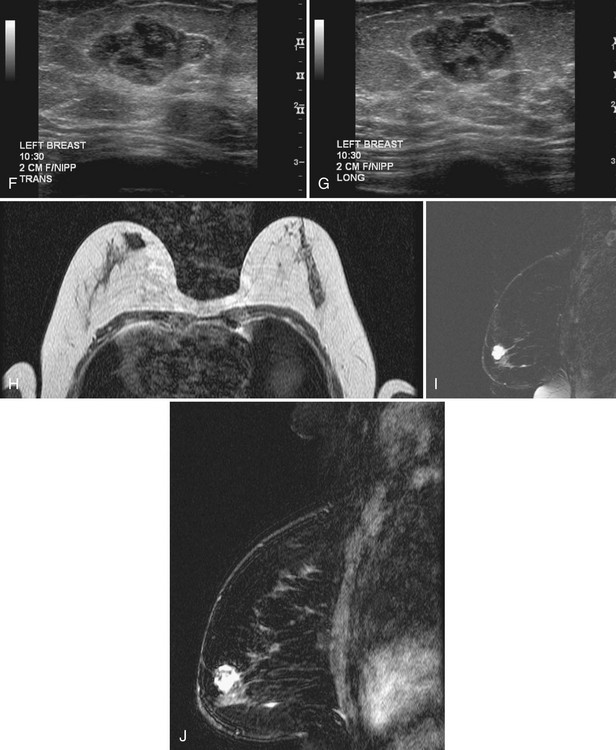
Occurring in young pregnant patients in the second or third trimester, lactating adenomas are solid well-circumscribed masses that can enlarge rapidly during pregnancy. Patients seek clinical evaluation because of a growing palpable mass not previously felt. On ultrasound, a lactating adenoma is oval or lobular, smoothly marginated, and can contain cystic or necrotic spaces; biopsy by either fine-needle aspiration or core needle biopsy is used to evaluate it (Fig. 4-20). The mass may regress in size in the postpartum period.
Solid Masses with Indistinct Margins (Box 4-13)
Invasive Ductal Cancer
On mammography, the indistinct margins of invasive ductal cancer are due to infiltration of the surrounding glandular tissue by tumor. The mass margin can appear unsharp or smudged, similar to a line partially erased by a pencil eraser. The indistinct margin is best seen on spot magnification views against a fatty background. On ultrasound, the indistinct tumor mass occasionally has an echogenic rim or halo that suggests the diagnosis (Fig. 4-21).
Lymphoma
Lymphoma can involve breast lymph nodes or can occur as a primary or secondary site in the breast parenchyma. Lymphadenopathy is the most common appearance of lymphoma involving the breast and is seen on the mammogram as large dense lymph nodes in the axilla that have lost their fatty hila and become bigger and rounder (Fig. 4-22A). Primary or secondary breast lymphoma is usually caused by non-Hodgkin lymphomatous infiltration into breast tissue and not into a lymph node. It is a rare cause of an ill-defined mass that looks just like invasive ductal cancer on mammography (see Fig. 4-22B to F). The borders of the mass are indistinct because of lymphomatous infiltration into the surrounding glandular tissue, but it can occasionally be well-circumscribed or lobulated. On ultrasound, primary or secondary breast lymphomas in the breast tissue appear as hypoechoic masses.
Pseudoangiomatous Stromal Hyperplasia
Pseudoangiomatous stromal hyperplasia (PASH) is a rare benign cause of a growing ill-defined noncalcified round or oval mass. It occurs in premenopausal women or in postmenopausal women receiving exogenous hormone therapy (Fig. 4-23). Occasionally, the mass may be well-circumscribed. This entity is of unknown etiology and is composed of stromal and epithelial proliferation; it occasionally shows rapid growth on mammography and requires biopsy. On ultrasound, PASH is a mixed or hypoechoic mass with ill-defined borders in 62%, as reported by Wieman and colleagues. It is thought that there is a hormonal influence on its development, and PASH is more often seen in premenopausal women or postmenopausal women receiving hormone therapy. Fine-needle aspiration can be inconclusive, as can core needle biopsy. Because low-grade angiosarcoma can mimic PASH on core biopsy, excisional biopsy is recommended if the mass grows.
Masses Containing Fat (Box 4-14)
Lymph Nodes
The lymph nodes are typically seen in the axilla. They are round or oval and contain a radiolucent fatty center. Benign lymph nodes may be of any size, have a smooth solid cortex, and contain a fatty hilum (Fig. 4-24). An intramammary lymph node has the same appearance as lymph nodes in the axilla; it is often located in the upper outer quadrant of the breast along blood vessels and should not be mistaken for a malignancy. In questionable cases, spot magnification views demonstrate a well-circumscribed oval or lobulated mass and, importantly, its fatty hilum. On breast ultrasound, the lymph node is hypoechoic and bean-shaped and contains a fatty center. On color Doppler ultrasound, the lymph node hilum or fatty center will contain a pulsating blood vessel (see Fig. 4-24D). On MRI, the lymph node kinetics show rapid initial enhancement with late washout, similar to cancer, due to its central blood vessel. However, its typical appearance on MRI, which shows a solid mass with the fatty hilum and high signal on T2-weighted images, should distinguish it from cancer, which has no fatty hilum and commonly has low signal on T2-weighted images.
Hamartoma
This entity, also known as a fibroadenolipoma, is a benign mass that contains fat and other elements found in the breast. On physical examination, a hamartoma may not be felt distinctly if it contains mostly fat and glandular tissue. The classic appearance is that of an oval mass containing fat and fibroglandular tissue with a thin capsule or rim, the “breast within a breast” appearance (Fig. 4-25). Breast hamartomas have a variable appearance, depending on the amount of fat and stromal elements that they contain. On occasion, a hamartoma may have mostly stromal and glandular elements and appear as a dense mass rather than one containing mostly fat and glandular elements (Fig. 4-26). Because cancer can develop in breast elements and ducts, cancer can develop in hamartomas. Biopsy should be performed on hamartomas if suspicious microcalcifications are developing within it. Otherwise, a classic “breast within a breast” hamartoma is benign and should be left alone.
Oil Cyst
An oil cyst is a sequela of fat necrosis after blunt trauma or surgery. A benign oil cyst is a radiolucent oval or round mass containing fatty fluid with a thin radiodense rim (Fig. 4-27). Oil cysts may subsequently calcify and result in eggshell-type calcifications. On ultrasound, oil cysts are round or oval and contain liquefied fat that is usually hypoechoic or isoechoic. The oil cyst is benign and is one of the “don’t touch” lesions that should be left alone.
Lipoma
Breast lipomas are similar to lipomas elsewhere in the body. They produce a soft mass or a mass that may not be felt at all. On mammography, a lipoma is a fatty mass containing a radiolucent center that may have a distinguishable thin discrete rim separating it from the surrounding glandular tissue. Unlike a post-traumatic oil cyst, a lipoma never calcifies. Typically, a lipoma is discovered because the patient feels a mass. If a technologist places a skin marker over the palpable lipoma, a spot compression view of the mass shows only fat (Fig. 4-28). Ultrasound of a lipoma shows only fatty tissue in a well-circumscribed oval or round mass. Lipomas are benign and should be left alone.
Fluid-Containing Masses (Box 4-15)
Cyst
Breast ultrasound shows an anechoic mass with imperceptible walls, a sharp back wall, and enhanced posterior transmission of sound (Fig. 4-29). Cysts may have internal echoes as a result of debris. Cysts may be left alone (Box 4-16) or can be aspirated by palpation or under ultrasound guidance if symptomatic, but they have no malignant potential. If a cyst is causing a palpable mass, the palpable finding should resolve after cyst aspiration. On MRI cysts have a high T2-weighted signal and show no enhancement. Occasionally inflamed cysts show rim enhancement and can be a cause of a false-positive MRI. However, the bright T2-weighted signal, no enhancement after contrast injection, and a thin rim should differentiate the inflamed cyst from cancer.
Hematoma/Seroma
Breast hematomas and seromas occur after biopsy or trauma, and their diagnosis is established by correlating the hematoma/seroma to the clinical history. On mammography, a hematoma has an irregular or ill-defined border and may be high-density or equal density (Fig. 4-30). In the acute phase, surrounding hemorrhage may obscure hematomas. A hematoma will become smaller with time as the blood resorbs. Initially on ultrasound, a hematoma is a fluid-filled mass. Later, as the hematoma evolves, ultrasound shows that the previously hypoechoic blood-filled mass changes to serous fluid. Subsequently, the seroma may contain thin movable septa that move on real-time ultrasound, or it may contain debris or fluid/fluid levels.
Intracystic Carcinoma
See the section “Solid Masses with Rounded or Expansile Borders” in this chapter.
Abscess
On ultrasound, an abscess is an irregular fluid-filled mass occasionally containing debris or septations (Fig. 4-31). The surrounding edema blurs the normal adjacent breast structures, makes the adjacent fat gray, and causes skin thickening. A breast abscess usually does not contain air. Bright echoes or specular reflectors may represent air in the abscess after attempted drainage.
Sebaceous and Epidermal Inclusion Cysts
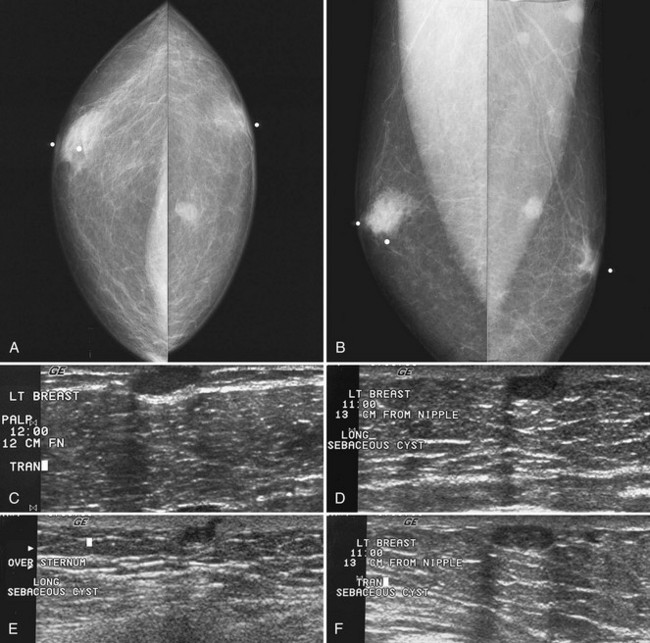
Clinically, sebaceous cysts can produce a palpable mass, a “blackhead” that when squeezed will yield cheesy yellow or white material. On mammography, sebaceous and epidermal inclusion cysts are identical, with subcutaneous oval or round well-defined masses that are often overexposed because of their location near the skin surface; they occasionally contain calcifications (Fig. 4-32A to C). Ultrasound shows an oval, well-circumscribed, hypoechoic or anechoic mass in a subcutaneous location with a little tail extending into the skin, representing the dilated hair follicle (see Fig. 4-32D to F).
Galactocele
Typically seen in lactating women, a galactocele represents a focal collection of breast milk that occasionally causes a palpable mass. On mammography, a galactocele is a low- or equal-density, oval or round, well-circumscribed mass (Fig. 4-33A and C), but it can be of higher density, depending on resorption of its fluid contents and the residual solid component. On an upright mammogram, a classic, but rarely seen finding of a fat/fluid level in the mass represents fat rising to the top of the galactocele while the other milk components layer dependently below. On ultrasound, a galactocele may look like a well-defined hypoechoic cystlike mass. Galactoceles containing more solid elements simulate a solid mass that occasionally displays posterior acoustic shadowing (see Fig. 4-33B and D). On aspiration, milky fluid will be obtained. Atypical galactoceles with a round or irregular shape, nonparallel orientation, indistinct or microlobulated noncircumscribed margin, but with a relatively sharp convex anterior or posterior echogenic rim, requiring biopsy, have been reported by Kim and colleagues.
Key Elements
A mass is a three-dimensional object seen on at least two mammographic projections.
On mammography, the mass repeat includes a description of the shape, margins, density, location, the mass’s associated findings, and how it has changed if previously present.
Mass shapes are round, oval, lobular, and irregular, with the probability of cancer increasing with increasing irregularity of the shape.
Mass margins are circumscribed, microlobulated, obscured, indistinct, or spiculated, with the probability of cancer increasing with increasing spiculation of the margin.
Fat-containing masses are almost never malignant.
Mass density is lower, equal to, or higher than an equal amount of fibroglandular tissue. High-density masses are suspicious for cancer.
The differential diagnosis for spiculated masses includes invasive ductal cancer, invasive lobular cancer, tubular cancer, postbiopsy scar, radial scar, fat necrosis, and sclerosing adenosis.
To determine whether a spiculated mass represents a postbiopsy scar, correlate the postbiopsy mammogram with the prebiopsy study showing where the finding was removed.
Spiculated masses that do not represent postbiopsy scars should undergo biopsy to exclude cancer.
Radial scars cannot be distinguished from spiculated breast cancer on mammography.
Invasive lobular cancer accounts for approximately 10% of all cancers but is one of the hardest to see on mammography because of its single-file cellular growth pattern.
The differential diagnosis for solid masses with round or expansile borders includes fibroadenoma, cancer, phyllodes tumor, papilloma, lactating adenoma, tubular adenoma, metastases, sebaceous cyst, and epidermal inclusion cyst.
The most common round cancer is invasive ductal cancer, an uncommon form of the most common breast cancer.
Medullary and mucinous breast cancers are commonly round in shape, but they are much rarer than invasive ductal cancer.
Fat-containing masses include lymph nodes, hamartoma, oil cyst, lipoma, and the rare liposarcoma.
Normal lymph nodes are oval, have an echogenic fatty hilum, and may contain a central pulsating blood vessel on color or power Doppler ultrasound in the fatty hilum.
Abnormal lymph nodes lose their fatty hilum and become larger and rounder than previously.
Fluid-containing masses include cysts, hematoma/seroma, necrotic cancer, intracystic carcinoma, intracystic papilloma, abscess, and galactocele.
Hamartomas look like a “breast within a breast” and should be left alone.
Galactoceles may rarely show a fat/fluid level on upright mammographic views.
Know the typical appearance of “don’t touch” benign lymph nodes, hamartomas, oil cysts, lipomas, galactoceles, cysts, and postbiopsy scars.
Adler DD, Helvie MA, Oberman HA, et al. Radial sclerosing lesion of the breast: mammographic features. Radiology. 1990;176:737-740.
Adler DD, Hyde DL, Ikeda DM. Quantitative sonographic parameters as a means of distinguishing breast cancers from benign solid breast masses. J Ultrasound Med. 1991;10:505-508.
American College of Radiology. Illustrated Breast Imaging Reporting and Data System (BI-RADS®), ed 3. Reston, VA: American College of Radiology; 1998.
Baker JA, Soo MS. Breast US: assessment of technical quality and image interpretation. Radiology. 2002;223:229-238.
Baker TP, Lenert JT, Parker J, et al. Lactating adenoma: a diagnosis of exclusion. Breast J. 2001;7:354-735.
Bilgen IG, Ustun EE, Memis A. Fat necrosis of the breast: clinical, mammographic and sonographic features. Eur J Radiol. 2001;39:92-99.
Brem RF, Ioffe M, Rapelyea JA, et al. Invasive lobular carcinoma: detection with mammography, sonography, MRI, and breast-specific gamma imaging. AJR Am J Roentgenol. 2009;192:379-383.
Brookes MJ, Bourke AG. Radiological appearances of papillary breast lesions. Clin Radiol. 2008;63:1265-1273.
Cardenosa G, Doudna C, Eklund GW. Mucinous (colloid) breast cancer: clinical and mammographic findings in 10 patients. AJR Am J Roentgenol. 1994;162:1077-1079.
Castro CY, Whitman GJ, Sahin AA. Pseudoangiomatous stromal hyperplasia of the breast. Am J Clin Oncol. 2002;25:213-216.
Cawson JN, Law EM, Kavanagh AM. Invasive lobular carcinoma: sonographic features of cancers detected in a BreastScreen Program. Australas Radiol. 2001;45:25-30.
Chao TC, Lo YF, Chen SC, Chen MF. Sonographic features of phyllodes tumors of the breast. Ultrasound Obstet Gynecol. 2002;20:64-71.
Chapellier C, Balu-Maestro C, Bleuse A, et al. Ultrasonography of invasive lobular carcinoma of the breast: sonographic patterns and diagnostic value: report of 102 cases. Clin Imaging. 2000;24:333-336.
Cheung YC, Wan YL, Chen SC, et al. Sonographic evaluation of mammographically detected microcalcifications without a mass prior to stereotactic core needle biopsy. J Clin Ultrasound. 2002;30:323-331.
Chopra S, Evans AJ, Pinder SE, et al. Pure mucinous breast cancer—mammographic and ultrasound findings. Clin Radiol. 1996;51:421-424.
Cohen MA, Morris EA, Rosen PP, et al. Pseudoangiomatous stromal hyperplasia: mammographic, sonographic, and clinical patterns. Radiology. 1996;198:117-120.
Cole-Beuglet C, Soriano RZ, Kurtz AB, Goldberg BB. Fibroadenoma of the breast: sonomammography correlated with pathology in 122 patients. AJR Am J Roentgenol. 1983;140:369-375.
Conant EF, Dillon RL, Palazzo J, et al. Imaging findings in mucin-containing carcinomas of the breast: correlation with pathologic features. AJR Am J Roentgenol. 1994;163:821-824.
Darling ML, Smith DN, Rhei E, et al. Lactating adenoma: sonographic features. Breast J. 2000;6:252-256.
Denison CM, Ward VL, Lester SC, et al. Epidermal inclusion cysts of the breast: three lesions with calcifications. Radiology. 1997;204:493-496.
Domchek SM, Hecht JL, Fleming MD, et al. Lymphomas of the breast: primary and secondary involvement. Cancer. 2002;94:6-13.
Doyle EM, Banville N, Quinn CM, et al. Radial scars/complex sclerosing lesions and malignancy in a screening programme: incidence and histological features revisited. Histopathology. 2007;50:607-614.
Dupont WD, Page DL, Pari FF, et al. Long-term risk of breast cancer in women with fibroadenoma. N Engl J Med. 1994;351(1):10-15.
Eisinger F, Nogues C, Birnbaum D, et al. BRCA1 and medullary breast cancer. JAMA. 1998;280:1227-1228.
Elson BC, Helvie MA, Frank TS, et al. Tubular carcinoma of the breast: mode of presentation, mammographic appearance, and frequency of nodal metastases. AJR Am J Roentgenol. 1993;161:1173-1176.
Elson BC, Ikeda DM, Andersson I, Wattsgard C. Fibrosarcoma of the breast: mammographic findings in five cases. AJR Am J Roentgenol. 1992;158:993-995.
Estabrook A, Asch T, Gump F, et al. Mammographic features of intracystic papillary lesions. Surg Gynecol Obstet. 1990;170:113-116.
Fornage BD, Lorigan JG, Andry E. Fibroadenoma of the breast: sonographic appearance. Radiology. 1989;172:671-675.
Gordon PB, Goldenberg SL. Malignant breast masses detected only by ultrasound. A retrospective review. Cancer. 1995;76:626-630.
Gunhan-Bilgen I, Memis A, Ustun EE. Metastatic intramammary lymph nodes: mammographic and ultrasonographic features. Eur J Radiol. 2001;40:24-29.
Gunhan-Bilgen I, Zekioglu O, Ustun EE, et al. Invasive micropapillary carcinoma of the breast: clinical, mammographic, and sonographic findings with histopathologic correlation. AJR Am J Roentgenol. 2002;179:927-931.
Harnist KS, Ikeda DM, Helvie MA. Abnormal mammogram after steering wheel injury. West J Med. 1993;159:504-506.
Harvey JA, Moran RE, Maurer EJ, DeAngelis GA. Sonographic features of mammary oil cysts. J Ultrasound Med. 1997;16:719-724.
Hashimoto BE, Kramer DJ, Picozzi VJ. High detection rate of breast ductal carcinoma in situ calcifications on mammographically directed high-resolution sonography. J Ultrasound Med. 2001;20:501-508.
Hilton SV, Leopold GR, Olson LK, Willson SA. Real-time breast sonography: application in 300 consecutive patients. AJR Am J Roentgenol. 1986;147:479-486.
Homer MJ. Proper placement of a metallic marker on an area of concern in the breast. AJR Am J Roentgenol. 1996;167:390-391.
Jorge Blanco A, Vargas Serrano B, Rodriguez Romero R, Martinez Cendejas E. Phyllodes tumors of the breast. Eur Radiol. 1999;9:356-360.
Kim MJ, Kim EK, Park SY, et al. Galactoceles mimicking suspicious solid masses on sonography. J Ultrasound Med. 2006;25:145-151.
Lee CH, Giurescu ME, Philpotts LE, et al. Clinical importance of unilaterally enlarging lymph nodes on otherwise normal mammograms. Radiology. 1997;203:329-334.
Levrini G, Mori CA, Vacondio R, et al. MRI patterns of invasive lobular cancer: T1 and T2 features. Radiol Med. 2008;113:1110-1125.
Lindfors KK, Kopans DB, Googe PB, et al. Breast cancer metastasis to intramammary lymph nodes. AJR Am J Roentgenol. 1986;146:133-136.
Memis A, Ozdemir N, Parildar M, et al. Mucinous (colloid) breast cancer: mammographic and US features with histologic correlation. Eur J Radiol. 2000;35:39-43.
Meyer JE, Amin E, Lindfors KK, et al. Medullary carcinoma of the breast: mammographic and US appearance. Radiology. 1989;170:79-82.
Ohlinger R, Frese H, Schwesinger G, et al. Papillary intracystic carcinoma of the female breast—role of ultrasonography. Ultraschall Med. 2005;26:325-328.
Paramagul CP, Helvie MA, Adler DD. Invasive lobular carcinoma: sonographic appearance and role of sonography in improving diagnostic sensitivity. Radiology. 1995;195:231-234.
Ribeiro-Silva A, Mendes CF, Costa IS, et al. Metastases to the breast from extramammary malignancies: a clinicopathologic study of 12 cases. Pol J Pathol. 2006;57:161-165.
Rodriguez-Pinilla SM, Rodriguez-Gil Y, Moreno-Bueno G, et al. Sporadic invasive breast carcinomas with medullary features display a basal-like phenotype: an immunohistochemical and gene amplification study. Am J Surg Pathol. 2007;31:501-508.
Rosen EL, Soo MS, Bentley RC. Focal fibrosis: a common breast lesion diagnosed at imaging-guided core biopsy. AJR Am J Roentgenol. 1999;173:1657-1662.
Salvador R, Salvador M, Jimenez JA, et al. Galactocele of the breast: radiologic and ultrasonographic findings. Br J Radiol. 1990;63:140-142.
Samardar P, de Paredes ES, Grimes MM, Wilson JD. Focal asymmetric densities seen at mammography: US and pathologic correlation. Radiographics. 2002;22:19-33.
Schneider JA. Invasive papillary breast carcinoma: mammographic and sonographic appearance. Radiology. 1989;171:377-379.
Schrading S, Kuhl CK. Mammographic, US, and MR imaging phenotypes of familial breast cancer. Radiology. 2008;246:58-70.
Sheppard DG, Whitman GJ, Huynh PT, et al. Tubular carcinoma of the breast: mammographic and sonographic features. AJR Am J Roentgenol. 2000;174:253-257.
Sickles EA. Mammographic features of 300 consecutive nonpalpable breast cancers. AJR Am J Roentgenol. 1986;146:661-663.
Sickles E. Practical solutions to common mammographic problems: tailoring the examination. AJR Am J Roentgenol. 1988;151:31-39.
Sickles EA, Herzog KA. Intramammary scar tissue: a mimic of the mammographic appearance of carcinoma. AJR Am J Roentgenol. 1980;135:349-352.
Soo MS, Dash N, Bentley R, et al. Tubular adenomas of the breast: imaging findings with histologic correlation. AJR Am J Roentgenol. 2000;174:757-761.
Sperber F, Blank A, Metser U. Adenoid cystic carcinoma of the breast: mammographic, sonographic, and pathological correlation. Breast J. 2002;8:53-54.
Stavros AT, Thickman D, Rapp CL, et al. Solid breast nodules: use of sonography to distinguish between benign and malignant lesions. Radiology. 1995;196:123-134.
Sumkin JH, Perrone AM, Harris KM, et al. Lactating adenoma: US features and literature review. Radiology. 1998;206:271-274.
Tabar L, Pentek Z, Dean PB. The diagnostic and therapeutic value of breast cyst puncture and pneumocystography. Radiology. 1981;141:659-663.
Venta LA, Wiley EL, Gabriel H, Adler YT. Imaging features of focal breast fibrosis: mammographic-pathologic correlation of noncalcified breast lesions. AJR Am J Roentgenol. 1999;173:309-316.
Vo T, Xing Y, Meric-Bernstam F, et al. Long-term outcomes in patients with mucinous, medullary, tubular, and invasive ductal carcinomas after lumpectomy. Am J Surg. 2007;194:527-531.
Wahner-Roedler DL, Sebo TJ, Gisvold JJ. Hamartomas of the breast: clinical, radiologic, and pathologic manifestations. Breast J. 2001;7:101-105.
Walsh R, Kornguth PJ, Soo MS, et al. Axillary lymph nodes: mammographic, pathologic, and clinical correlation. AJR Am J Roentgenol. 1997;168:33-38.
Weigel RJ, Ikeda DM, Nowels KW. Primary squamous cell carcinoma of the breast. South Med J. 1996;89:511-515.
Wieman SM, Landercasper J, Johnson JM, et al. Tumoral pseudoangiomatous stromal hyperplasia of the breast. Am Surg. 2008;74:1211-1214.
Woods ER, Helvie MA, Ikeda DM, et al. Solitary breast papilloma: comparison of mammographic, galactographic, and pathologic findings. AJR Am J Roentgenol. 1992;159:487-491.
4-1. Name the differential diagnosis for fat-containing masses.
1 _____________________________________________
2 _____________________________________________
3 _____________________________________________
4 _____________________________________________
5 _____________________________________________
4-2. Name the differential diagnosis for fluid-containing masses.
1 _____________________________________________
2 _____________________________________________
3 _____________________________________________
4 _____________________________________________
5 _____________________________________________
6 _____________________________________________
4-3. Name “don’t touch” benign masses that should be left alone.
1 _____________________________________________
2 _____________________________________________
3 _____________________________________________
4 _____________________________________________
5 _____________________________________________
6 _____________________________________________
7 _____________________________________________
8 _____________________________________________
| MALIGNANT | BENIGN |
|---|---|
| 1. ________________ | 1. __________________ |
| 2. ________________ | 2. __________________ |
| 3. ________________ | 3. __________________ |
| 4. ________________ | 4. __________________ |
| 5. ________________ | |
| 6. ________________ | |
| 7. ________________ | |
| 8. ________________ |
4-5. Name the differential diagnosis for spiculated masses.
1 _____________________________________________
2 _____________________________________________
3 _____________________________________________
4 _____________________________________________
5 _____________________________________________
4-6. Name the elements of ultrasound image labeling.
1 _____________________________________________
2 _____________________________________________
3 _____________________________________________
4-7. Name the differential diagnosis for round masses.
1 _____________________________________________
2 _____________________________________________
3 _____________________________________________
4 _____________________________________________
5 _____________________________________________
6 _____________________________________________
7 _____________________________________________
8 _____________________________________________
9 _____________________________________________
10 _____________________________________________
11 _____________________________________________
12 _____________________________________________
4-8. Name the differential diagnosis for multiple round masses.
1 _____________________________________________
2 _____________________________________________
3 _____________________________________________
4 _____________________________________________
4-9. Name the differential diagnosis for solid indistinct masses.