Chapter 3 Mammographic Analysis of Breast Calcifications
Technique for Finding Calcifications
On the other hand, air-gap magnification mammography with a 0.1-mm focal spot actually increases the resolution power of the imaging system by about 1.8 times normal and shows more calcifications than were present on the original image. Air-gap magnification separates closely grouped calcifications into their individual forms, displays faint calcifications not detected at screening, and sharpens the image (see Fig. 1-2). Thus, air-gap magnification mammography is an integral part of calcification analysis and should be obtained on all calcifications requiring further analysis.
Anatomy
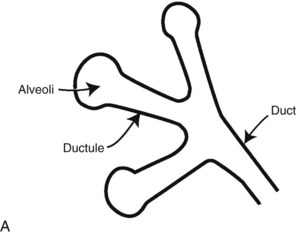
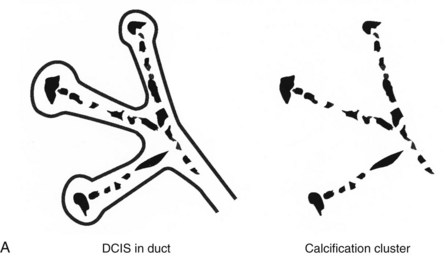
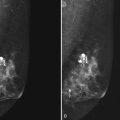
Calcifications form in breast ducts (Fig. 3-1), in lobules (Fig. 3-2A), or within breast tumors. Calcifications forming in the interlobular stroma, in periductal locations, or in blood vessels, fat, or skin are usually benign. Recognizing the location is important because calcifications within the skin, muscle, or nipple are almost invariably benign. Skin calcifications are especially important to recognize because they can easily be mistaken for intraparenchymal calcifications, leading to unnecessary biopsy. Skin calcifications are usually tiny, about the size of the skin pore on the mammogram, and often occur in skin folds where skin touches skin (e.g., axilla, inframammary fold, or in between the breasts). They are classically eggshell-type or contain a lucent center.
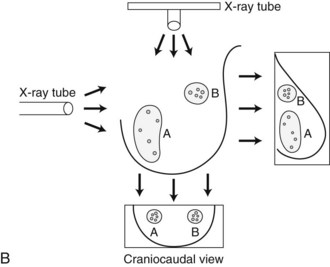
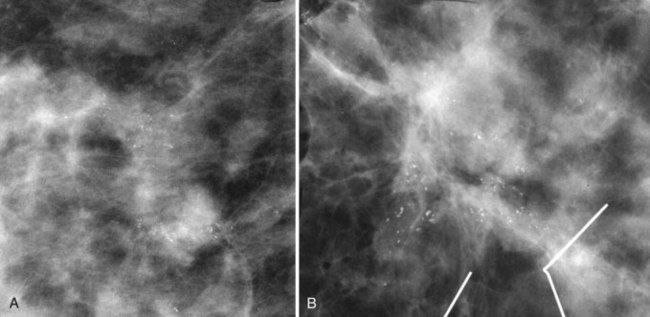
In general, clustered intraparenchymal calcifications are more suspicious for cancer than scattered calcifications. To be considered a suspicious intraparenchymal cluster, the finding must represent a true cluster and not simply be scattered calcifications superimposing on one another. The cluster must be tightly grouped on two orthogonal views to prove it is not a fake cluster (see Fig. 3-2B). To prove that clustered calcifications are truly grouped together, the radiologist looks for similar-appearing clustered calcifications over the same volume of tissue on orthogonal views. If the cluster is tightly packed on orthogonal views, it is a true cluster and should be assessed further. If the cluster is tightly packed on one view and scattered on the other view, it represents a superimposition of calcifications, is a fake cluster, and can be dismissed.
BI-RADS® Lexicon for Calcifications and Individual Calcification Shapes
The American College of Radiology (ACR) Breast Imaging Reporting and Data System (BI-RADS®) lexicon has a good section on description and assessment of calcifications. In the mammography report, radiologists use BI-RADS® terms to describe calcification forms, distribution, location, associated findings, and whether any change has occurred since the previous study. BI-RADS® terms are powerful descriptors that help the clinician understand the seriousness of the finding, such as fine linear branching in cancers. The BI-RADS® terms also help the radiologist classify calcifications into BI-RADS® assessment categories, which prompt patient management (Boxes 3-1 and 3-2). For example, the BI-RADS® term pleomorphic, which is suspicious for cancer, would prompt the radiologist to classify the calcifications into a BI-RADS® category 4, which calls for biopsy to be performed, whereas the term large rodlike, which indicates benign secretory disease, would be classified as a BI-RADS® category 2 and would be dismissed.
Box 3-1
Calcification Report
Size of the cluster or calcific group
Location (right or left breast, quadrant or clock position, centimeters from the nipple)
Overall characteristic of the worst-looking individual calcifications in the group
BI-RADS®, Breast Imaging Reporting and Data System.
From American College of Radiology: ACR BI-RADS®—mammography, ed 4, In ACR Breast Imaging and Reporting and Data System, breast imaging atlas, Reston, VA, 2003, American College of Radiology.
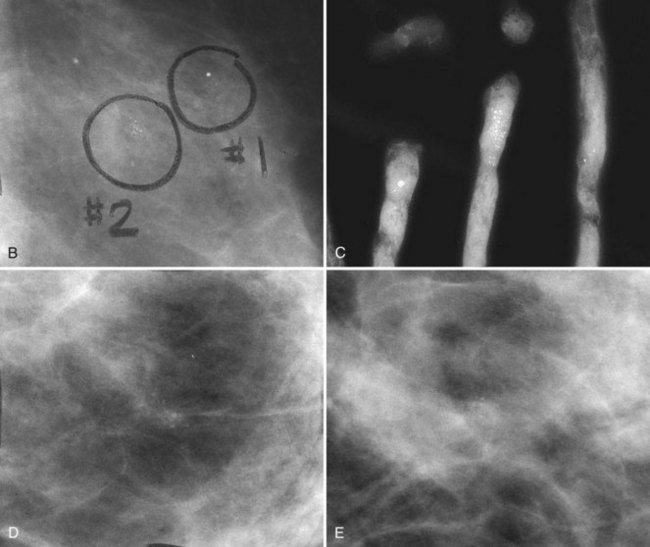
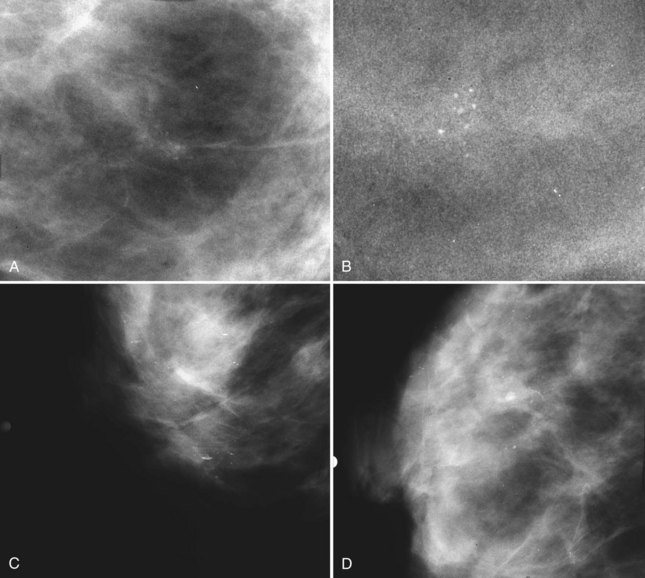
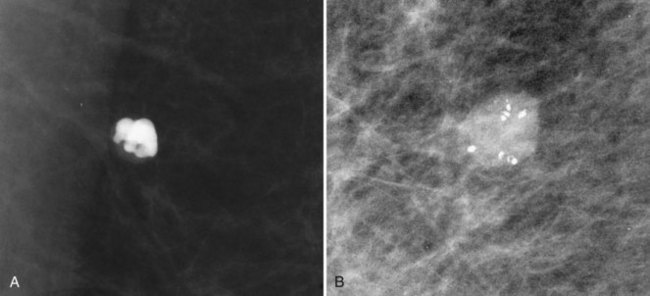
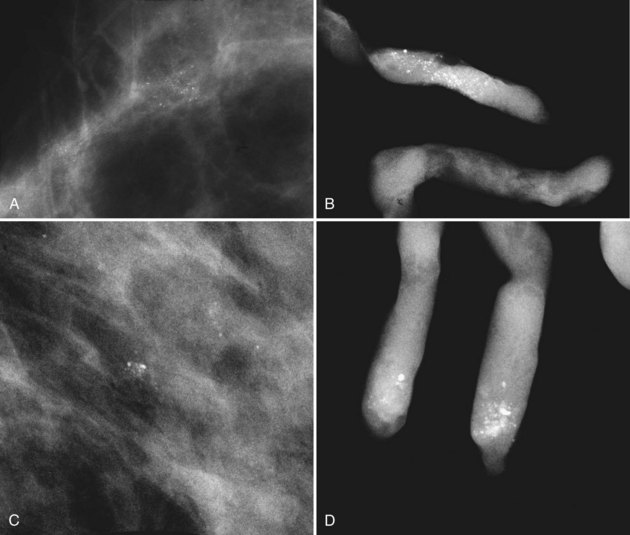
BI-RADS® terms are based on calcification morphology. Knowing the underlying anatomic structure in which calcific particles form helps the radiologist to understand why some calcific shapes have specific morphologies and why they suggest benign or malignant disease. An example of how anatomic structures influence calcification shapes are the round calcifications that form in round benign terminal breast acini or lobules. These benign calcifications take on the round shape of the acini in which they form, and hence, are round, regular in shape, densely calcified, and sharply marginated (Fig. 3-3A to C). The BI-RADS® term for these calcifications is punctate; they are usually benign.
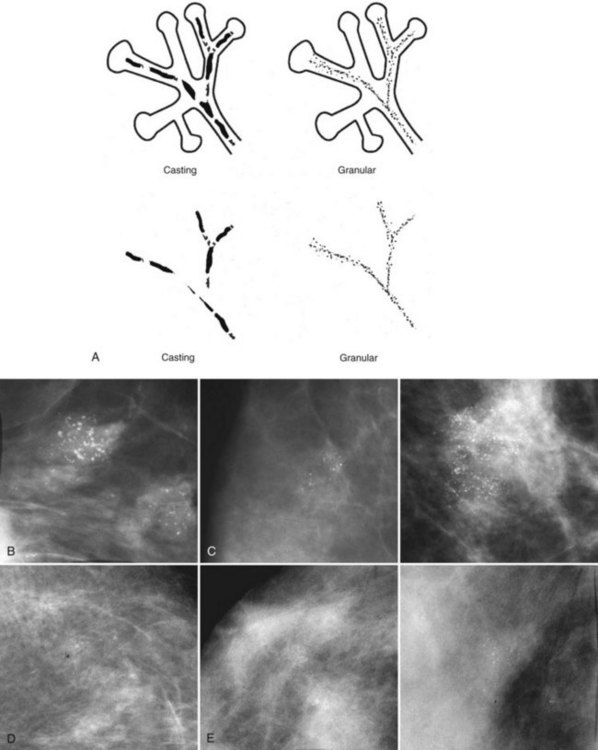
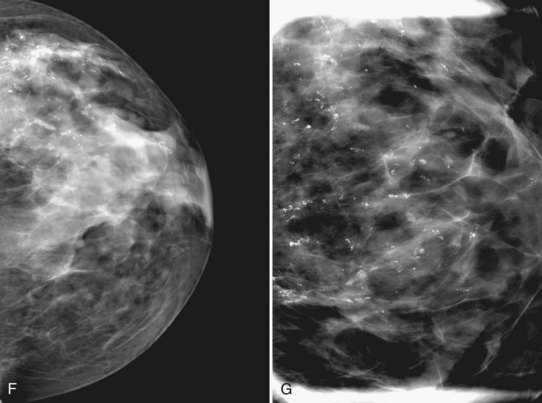
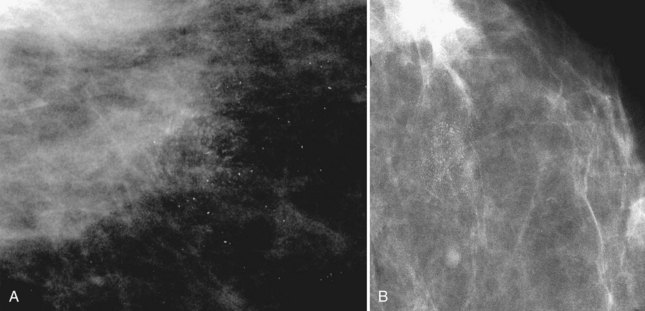
The BI-RADS® term amorphous or indistinct describes indeterminate calcifications that are tiny, roundish, flake-shaped particles that are too small and vague to allow further characterization. Both benign and malignant processes produce this type of calcification (Box 3-3). Benign fibrocystic disease and sclerosing adenosis produce blunt duct extension and ductal dilatation that result in indeterminate amorphous or indistinct calcifications (see Fig. 3-3D to F). However, some amorphous or indistinct calcifications can also form in ductal carcinoma in situ (DCIS) (see Fig. 3-3G to J). This overlap between benign- and malignant-appearing calcifications results in “false-positive” biopsies and accounts for up to 75% of benign biopsy results from procedures prompted by calcifications.
Box 3-3
Terms for Suspicious Calcifications
From American College of Radiology: ACR BI-RADS®—mammography, ed 4, In ACR Breast Imaging and Reporting and Data System, breast imaging atlas, Reston, VA, 2003, American College of Radiology.
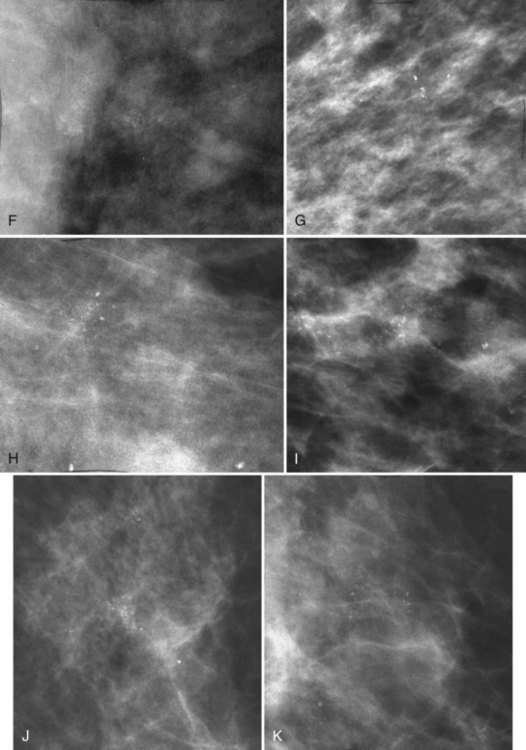
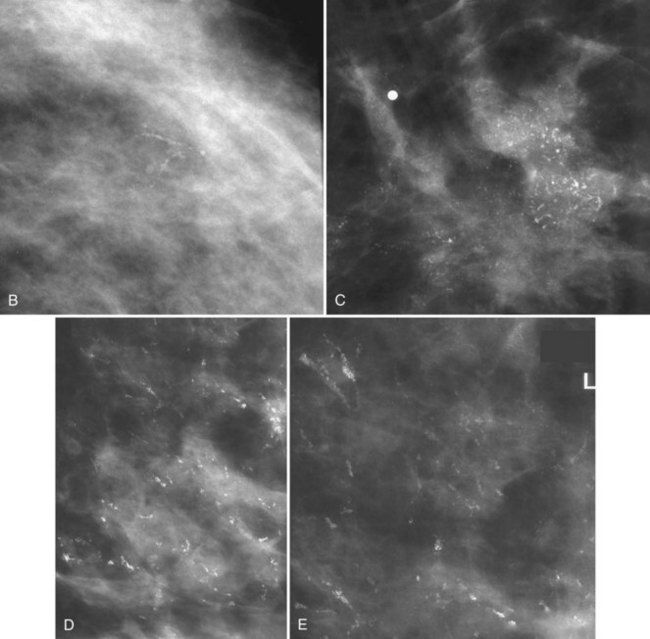
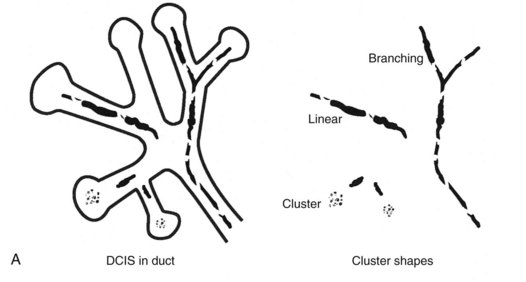
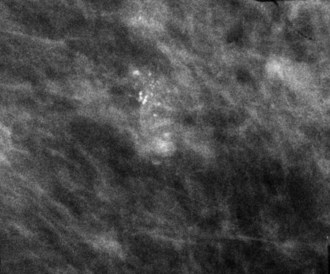
Calcifications that develop in DCIS or invasive ductal cancer grow in breast ducts and have classic appearances (Fig. 3-4A and B). The ACR BI-RADS® term for these calcifications is fine linear or fine linear branching (casting) calcifications. These calcifications have linear forms because DCIS grows in branching ducts and the calcifications form within the DCIS, making tiny irregular casts of the duct. These calcifications may look like little broken needles with pointy ends or may have a “dot-dash” appearance with both round and linear shapes. Calcific casts of tumors growing in duct branches form X-, Y– or Z-shaped calcifications. Radiologists describe these classic calcifications as fine linear, fine linear branching, casting, or pleomorphic in the report to reflect their concern for cancer. This is in contradistinction to benign-appearing round, punctate calcifications (see Fig. 3-4C to E).
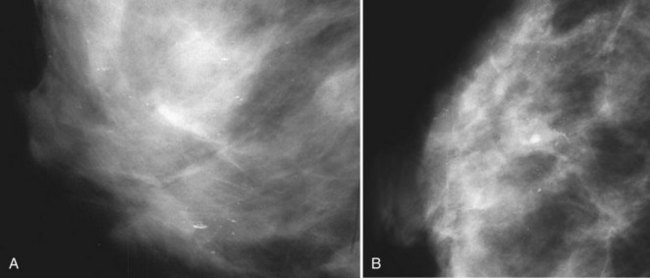
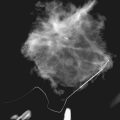
Another suspicious calcification form described by the ACR BI-RADS® lexicon is pleomorphic or heterogeneous (granular). This term reflects very tiny, irregularly shaped calcific particles that look like bizarre broken glass shards forming inside pockets of necrotic tumors, such as the micropapillary or cribriform forms of DCIS (Fig. 3-5). The individual calcifications are roughly round in shape but have irregular borders, are faint, smaller than 0.5 mm, and vary in size and density. A cluster containing granular calcifications may not exhibit casting or linear forms but should still be considered suspicious even in their absence. Occasionally, granular calcifications form in a duct and look like sand stuffed in a plastic straw. Unfortunately, benign disease occasionally mimics DCIS and also forms granular calcifications (see Fig. 3-5B to G).
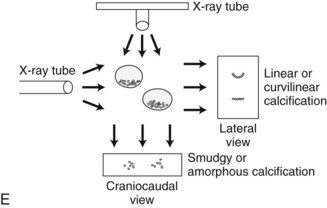
Calcification Group Shape or Distribution within the Breasts
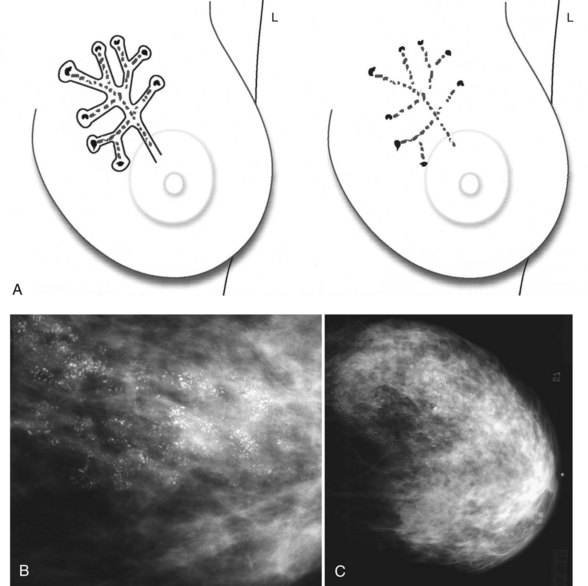
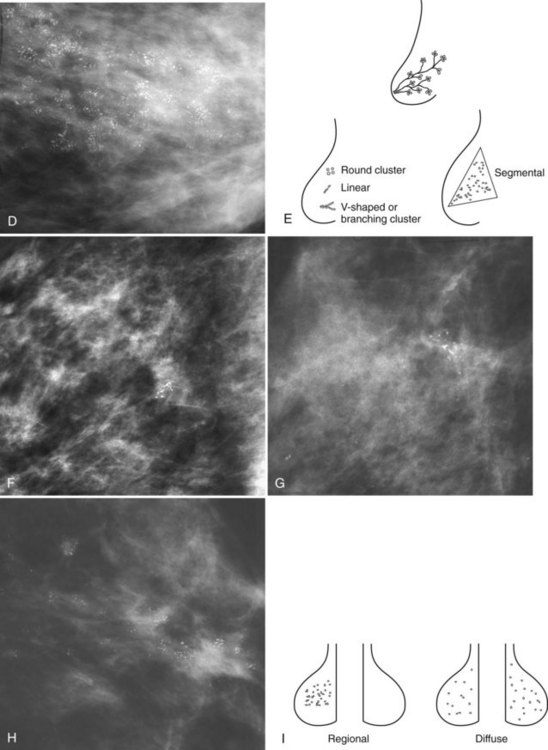
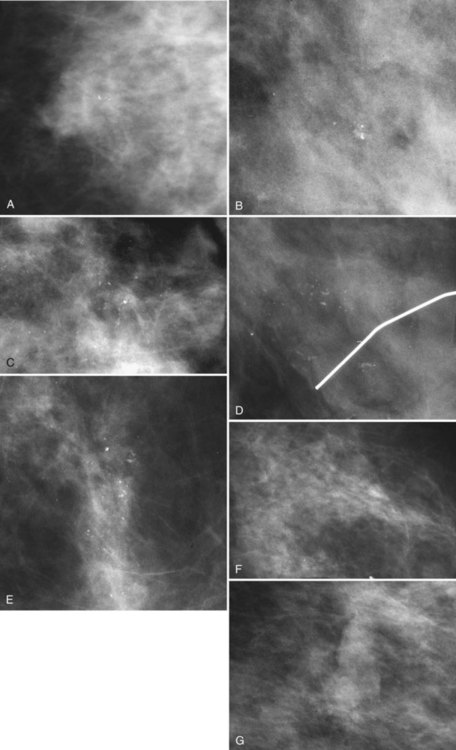
Calcification distributions that may represent cancer in the ACR BI-RADS® lexicon are those described as clustered or grouped, linear, branching (calcifications in a line that may show branching) (Fig. 3-6), and segmental (Fig. 3-7). Cancer forms in diseased ducts within a breast lobe, or the so-called “sick lobe,” as described by Tot and Gere.
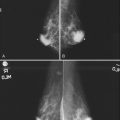
Isolated calcification clusters suggest an isolated disease process in a small volume of tissue. This may represent DCIS, invasive cancer, fibrocystic change, papilloma, or sclerosing adenosis. For clusters, one analyzes both the individual calcification forms and the overall cluster shape, which may be a clue to whether the cluster is benign or malignant. Lanyi suggested that the overall cluster shape is especially suspicious if it has a swallowtail, or V, shape, because it suggests cancer in tumor-packed branching ducts. On the other hand, calcifications forming in round clusters may be forming in acini and be benign, especially if the individual calcifications within the cluster are also benign-appearing (see Fig. 3-7E).
The ACR BI-RADS® terms linear branching and segmental describe suspicious findings because they suggest a process within a duct and its branches. The term linear describes calcifications in a line and can represent tumor in a duct or a focal benign process. A segmental distribution is suspicious for cancer because it suggests a process within a branch and its ducts. Segmental calcifications cover slightly less than a quadrant and form in a triangle with its apex pointing at the nipple (see Fig. 3-7F to H).
BI-RADS® terms suggesting benign calcification distributions include regional and diffuse/scattered (see Fig. 3-7I). This pattern suggests innumerable scattered and occasionally clustered calcifications widely dispersed over the breasts and often reflects benign processes, which are also often spread widely throughout both breasts. Calcifications widely distributed in both breasts are usually due to fibrocystic change. Regional calcifications extend over more than one ductal distribution (Box 3-4). Diffuse extensive benign-appearing calcifications in both breasts rarely represent breast cancer. The decision to biopsy calcifications is based on their distribution within the breasts, the worst features of the individual calcification clusters, change over time, the clinical scenario, and common sense.
Benign Calcifications
The specific benign calcifying entities covered in the following subsections require no further workup and should prompt no further action (Box 3-5).
Box 3-5 Typically Benign Calcifications
Artifacts (deodorant, hair, fingerprints)
Skin artifacts: antiperspirant, material in moles
Eggshell-type calcifications, radiolucent centers
Fat necrosis (postbiopsy, post-trauma)
Vascular calcifications (tram-track appearance)
Fibroadenoma (mass with round, coarse peripheral calcifications)
Plasma cell mastitis or secretory disease (needle-like or sausage-shaped calcifications pointing toward the nipple; found in middle-aged women; benign entity, usually asymptomatic)
Milk of calcium (linear on the mediolateral view, smudgy on the craniocaudal view)
Dystrophic calcifications (be alert for such calcifications in women after biopsy for cancer)
Suture calcifications (cat gut, postradiation)
Calcifications in the fibrous implant capsule
Artifacts Simulating Calcifications
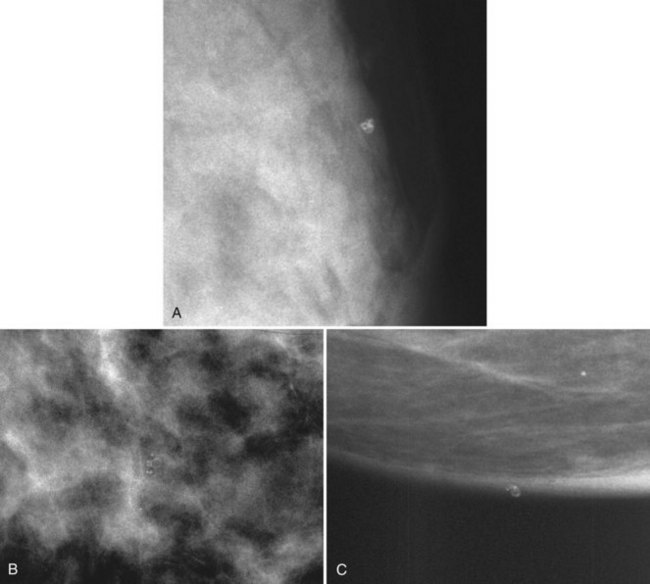
Radiopaque materials on the skin can simulate calcifications. This commonly occurs with deodorant and antiperspirant in the axilla or with radiopaque salves used on dermal skin tags or warts. Sometimes the deodorant or powder will produce dense particles in skin creases, suggesting the diagnosis. If one suspects deodorant artifact, have the patient wash her armpit to remove any deodorant. The deodorant should disappear on repeat films after the armpit has been washed (Fig. 3-8).
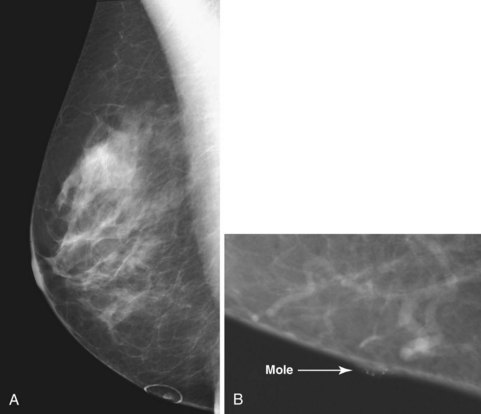
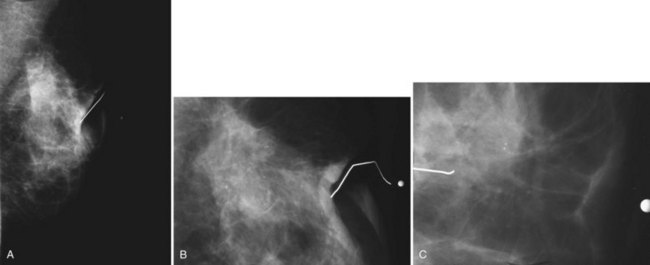
Dermal lesions or warts can hide radiopaque salves in their crevices, simulating calcifications in a mass. To distinguish the common dermal lesion from intraparenchymal breast masses, some facilities put radiopaque skin markers on all moles or skin tags. The skin marker on the “mass” distinguishes dermal lesions from masses inside the breast (Fig. 3-9).
Hair artifacts are white, thin, linear, strandlike opacities near the chest wall (Fig. 3-10) that occur when patients with long hair lean forward for their mammogram. Long hair overlaps into the field of view, causing the artifact. A repeat mammogram with the patient’s hair pulled away from the field of view will eliminate the strandlike artifacts on the mammogram.
Fingerprints are an artifact seen on SFM. This artifact has a characteristic whorled appearance caused by sticky fingers handling sensitive mammographic films or film-intensifying screens (Fig. 3-11). Sometimes this artifact simulates the linear calcifications in DCIS. A repeat film in the same projection after the screen has been cleaned will have no fingerprints in the same location, thereby resolving the issue.
Round Calcifications (0.5 mm or Less Is Punctate)
Small, 2- to 4-mm round benign intraparenchymal breast calcifications can be seen in any location within the breast. Punctate calcifications form in acini and breast lobules and take on their shape. Punctate calcifications are round, sharply marginated, and densely calcified. Their characteristic appearance suggests a benign diagnosis, and they are often seen in fibrocystic change (Fig. 3-12).
Calcifications with Radiolucent Centers
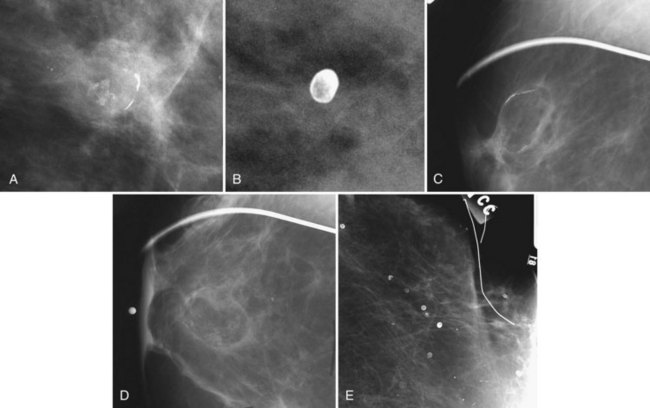
Radiolucent oil cysts or fat necrosis can form in patients who have sustained trauma or have undergone surgery. The center of oil cysts or fat necrosis is dark and fatty on the mammogram, and calcifies along its edge from saponification. Rimlike calcifications surrounding a radiolucent center of a fatty or oil-like substance suggest an oil cyst (Fig. 3-13). On edge, the curvilinear portion of the oil cyst or fat necrosis looks like a long, thin, curved calcification. En face, in the middle of the oil cyst, the calcifications appear amorphous and sheetlike. Calcified oil cysts distributed diagonally across the breast may be caused by blunt trauma from a seat belt or a steering wheel injury from an automobile accident. In any case, when suspecting fat necrosis the radiologist should look at the old mammogram and history to confirm that trauma or surgery has occurred in that area, and that there was fat in the area where the “calcifying fat necrosis” has now formed.
Dermal Calcifications
Skin calcifications are quite small, about the size of skin pores, are single or clustered, and often (but not always) have a calcific rim surrounding a radiolucent center. These eggshell-type skin calcifications are the same size as the skin pores on the mammograms (Fig. 3-14). This entity deserves special attention because dermal calcifications simulate grouped intraparenchymal calcifications when they have no lucent center, prompting biopsy. Any attempt to needle localize dermal calcifications will result in a dismal failure because the hookwire tip will never project onto the calcifications (because the calcifications are in the skin and not in the breast).
The radiologist suspects skin calcifications when the calcifications appear in a peripheral location or close to the skin, when other skin calcifications are present, or when they occur at sites where skin touches skin, such as in the axilla, the inframammary fold, or the medial portion of the breast (Box 3-6).
Box 3-6 Reasons to Suspect Skin Calcifications*
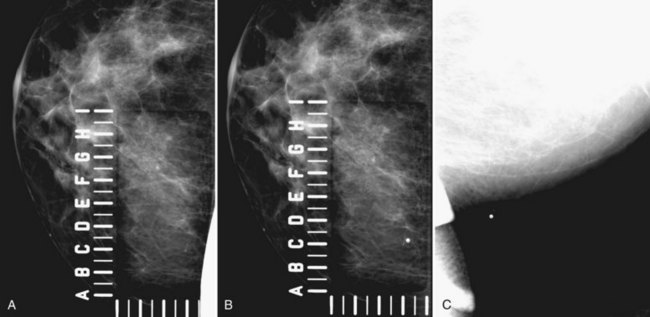
Special tangential views confirm calcifications within the skin and virtually exclude malignancy. To localize skin calcifications easily, the technologist does a “skin calcification study,” which is similar to a needle localization study, but without the needle. To localize the calcifications, the technologist uses a mammographic compression plate that contains perforations or an alphanumeric grid around a hole cut in the compression plate (Fig. 3-15). The technologist positions the breast so that the hole is directly over the skin containing the calcifications; the tangential view will not work if the localizing device is placed over the upper part of the breast in the craniocaudal (CC) view and the calcifications are in the skin on the underside. Therefore, it is important to look at both CC and lateral views to determine which portion of the breast skin contains the calcifications before starting the skin calcification study.
Milk of Calcium
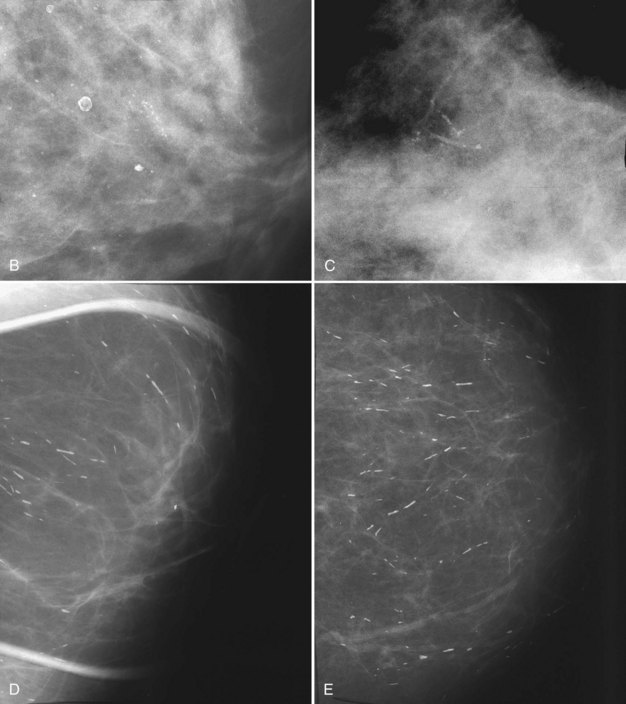
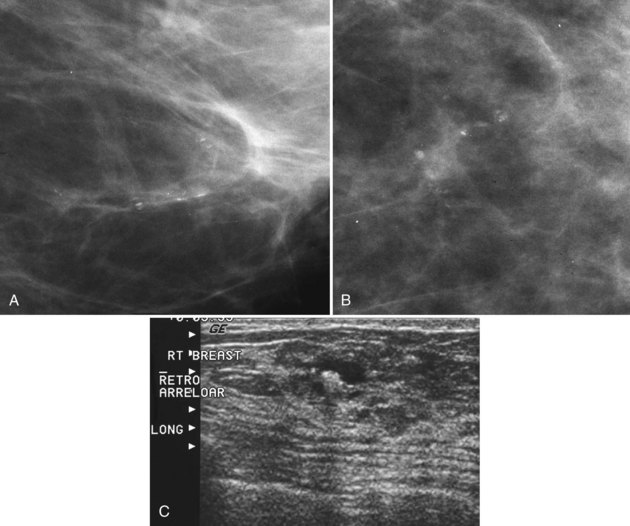
Milk of calcium has a classic appearance of sedimented calcifications within tiny benign cysts. Its appearance is pathognomonic on mediolateral and CC mammograms and should be left alone. The calcifications are not fixed to the cyst wall and float around the cyst like fake snow swirling in a snow globe. On the mediolateral mammogram, curvilinear dependently layered milk of calcium settles to the bottom of tiny imperceptible cysts. The layering calcifications are seen as dense, linear, or curvilinear in an upright patient on the lateral view (Fig. 3-16). On the CC projection, the calcifications will have a cloudlike or smudgy appearance like tea leaves in the bottom of a teacup.
The cysts in microcystic milk of calcium are too small to be seen, and only the characteristic linear appearance of calcifications on the lateral view indicates their presence. Microcystic milk of calcium should be suspected when calcifications plainly seen on the lateral or mediolateral oblique view cannot be seen at all on the CC view or just look like a vague smudge (Fig 3-17). Thus, linear calcifications on the lateral view that disappear on the CC view should suggest milk of calcium, particularly if the calcifications are never found on the CC view after a hard search or even after magnification.
The differential for linear calcifications includes DCIS, which can mimic milk of calcium; both are linear or curvilinear on the lateral and mediolateral views. DCIS can be distinguished from milk of calcium on the CC mammogram. Milk of calcium is amorphous or smudgy on the CC projection whereas DCIS retains a linear shape (Table 3-1).
| Milk of Calcium | DCIS | |
|---|---|---|
| Lateral-medial mammogram | Linear | Linear |
| Craniocaudal mammogram | Smudgy | Linear |
DCIS, ductal carcinoma in situ.
Even though milk of calcium is typically benign and should not undergo biopsy, unrecognized milk of calcium may be recommended for biopsy by accident. Because milk of calcium changes its shape in varying projections, the biopsy may be stopped if the condition is recognized in time. Patients undergoing stereotactic core biopsy for unrecognized milk of calcium lie prone on the stereotactic table, and the milk of calcium layers dependently in the prone patient. The “linear” calcifications change their position within the microcysts and are parallel (rather than perpendicular) to the chest wall. Calcifications that appear much different on stereotactic views compared to the prebiopsy lateral view and layer dependently on the prone view may suggest milk of calcium, thus precluding the need for biopsy (Fig. 3-18).
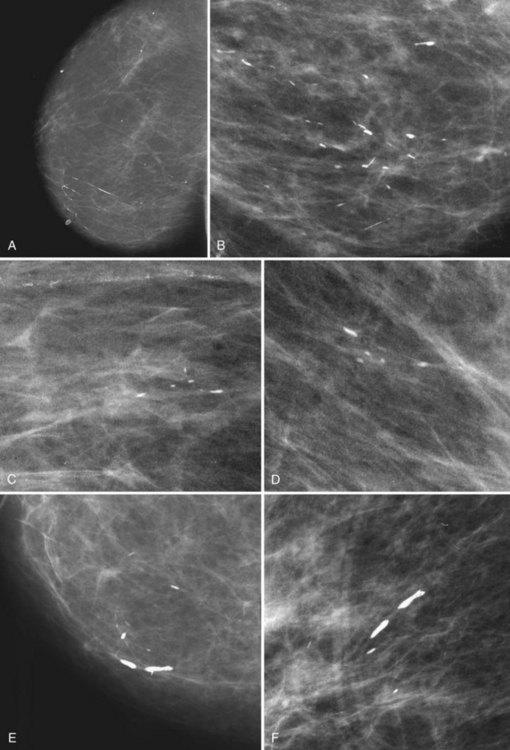
Large Rodlike Calcifications (Plasma Cell Mastitis, Secretory Disease, Duct Ectasia)
Plasma cell mastitis is a benign asymptomatic inflammation of the breast in older women. The inflammation is periductal or intraductal. Periductal inflammation results in dense, sausage-like calcifications with radiolucent centers oriented along the breast ducts and pointing at the nipple. Intraductal inflammation results in calcifications filling the ducts, producing a solid, large rodlike or a thinner, needle-like calcification (Fig. 3-19) oriented along the ducts and pointing toward the nipple. The calcifications usually are quite dense, have sharp margins, line up along the duct like cars in a train, and may be unilateral or bilateral.
Benign secretory disease, also known as plasma cell mastitis, is a DCIS look-alike. Because benign secretory disease forms in ducts, it also forms linear calcifications like those formed in DCIS (see Fig. 3-4C), but unlike DCIS, the calcifications are due to an inflammatory process either within or around the duct. Thus, benign secretory disease calcifications are large, coarse, and “rodlike”; if periductal, they can be sausage-shaped calcifications with a central lucency. Secretory disease calcifications are so big that they can be seen on the mammogram without a magnifier. On the other hand, DCIS calcifications are much smaller, less dense, and finer than those of secretory disease. DCIS calcifications branch quickly and are tightly packed compared to secretory disease calcifications, which branch widely over the breast and can be separated by centimeters (Table 3-2).
| Plasma Cell Mastitis | DCIS |
|---|---|
| Lined up along ducts | Lined up along ducts |
| Point at nipple | Point at nipple |
| Linear, sometimes branching | Linear, sometimes branching, pleomorphic |
| Branches over a wide area | Branches many times over 1 cm (ducts are smaller) |
| No tiny additional calcifications | Magnification shows many more small calcifications |
| Big calcifications—can be seen without a magnifier | Big and small calcifications |
| Coarse, rodlike calcifications | Fine, linear calcifications |
| Sharply marginated | Indefinite margins |
DCIS, ductal carcinoma in situ.
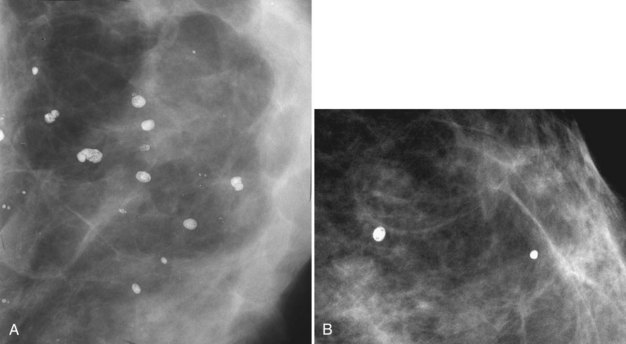
Coarse or “Popcorn-like” Calcifications (Calcifying Fibroadenomas)
Fibroadenomas most commonly occur between puberty and age 30. They are benign oval or lobulated solid masses equal in density to breast tissue on the mammogram. They occasionally calcify with classic coarse, dense “popcorn-like” features; they should be left alone (Fig. 3-20A). Fibroadenomas are multiple in approximately 10% to 20% of cases and result from epithelial and intraductal proliferation of breast elements. Fibroadenomas have intracanalicular and pericanalicular forms. They are often well-circumscribed, low-density masses that are impossible to distinguish from cysts on the mammogram when they do not calcify.
Calcifications within a fibroadenoma usually start at its periphery. The calcifications are large and dense, although at an early stage they may be somewhat irregular and small (see Fig. 3-20B). Periodic mammography may show slow progression of the calcifications from the tiny irregular forms to the more classic, dense, popcorn-like appearance, occasionally totally replacing the fibroadenoma with calcium (see Fig. 3-20). If the calcifications are not typical of fibroadenoma or if the mass shape or other features are not characteristic, consideration should be given to needle biopsy to establish a histologic diagnosis. However, fibroadenoma with the classic, characteristic mass shape and typical calcifications should be left alone.
Dystrophic Calcifications
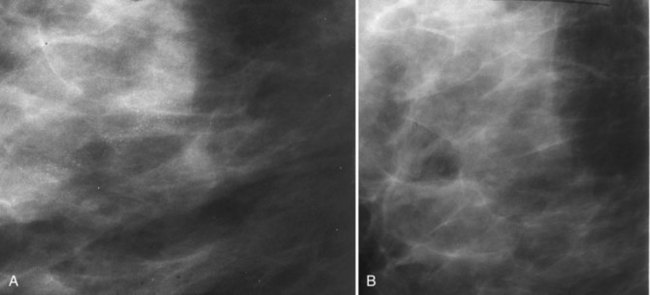
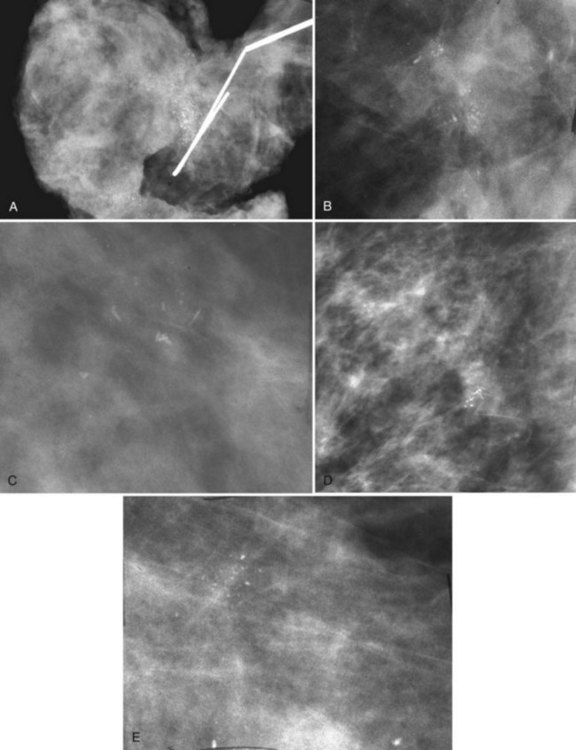
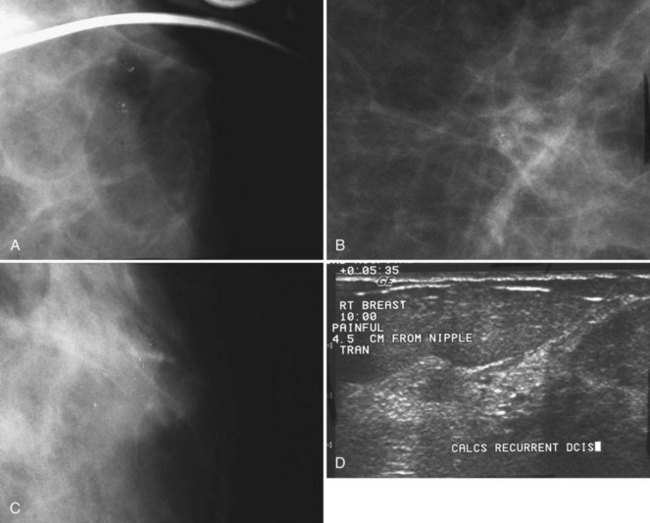
After breast biopsy, linear and amorphous calcifications can form in the surgical bed, occasionally accompanied by fat necrosis, causing pleomorphic and bizarre calcifications. A history of previous surgical biopsy or trauma, when correlated with a surgical scar, should reveal the true nature of these calcifications as dystrophic (Fig. 3-21). Some facilities use a metallic linear scar marker directly on the patient’s skin scar. This helps radiologists correlate the surface skin scar with underlying postbiopsy scarring (Box 3-7). Postbiopsy calcifications developing in a cancer biopsy site deserve special consideration because they may be forming in an in-breast tumor recurrence.
Calcifications in an irradiated breast due to calcifying sutures or benign fat necrosis usually develop 2 years or later after surgery and radiation therapy. Dystrophic calcifications in this setting may be difficult to distinguish from cancer (see Fig. 3-21). In the immediate postoperative period, comparing the current study with the prebiopsy, postbiopsy, and specimen mammograms can help determine whether the calcifications represent unresected tumors missed at the initial surgery, residual benign-appearing calcifications, or new calcifications.
Vascular Calcifications
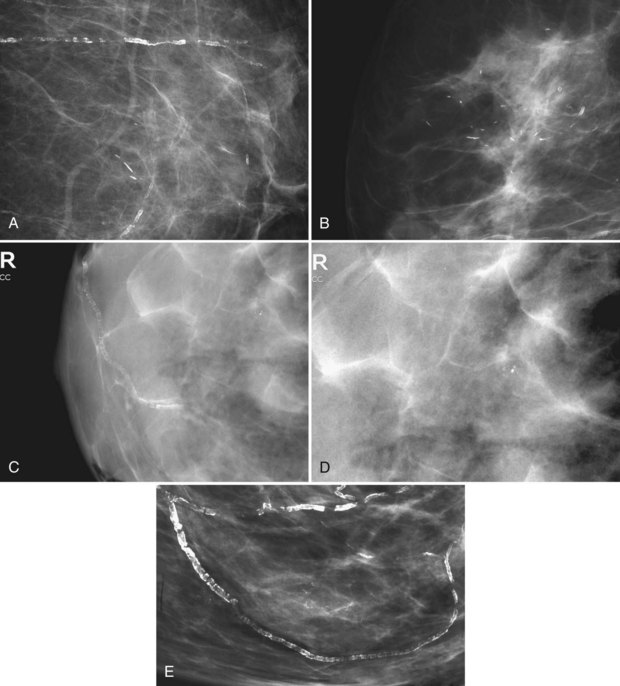
Arterial calcification from atherosclerosis has a characteristic appearance of two parallel calcified lines, representing calcification in the arterial wall (Fig. 3-22). Early arterial calcification along vascular walls may simulate the suspicious linear calcifications of DCIS. Seeing a noncalcified vessel leading to the calcifications may establish that the calcifications are in a calcified part of the blood vessel. Magnification views of vascular calcifications will show arterial tram-track calcifications in two parallel lines, with coarse calcifications en face in the vessel wall between them.
Calcifications from Foreign Bodies (Silicone Injections, Other)
Classically associated with the injection of silicone or a paraffin-like substance into the breast for purposes of augmentation, foreign-body granulomas in the breast have a characteristic eggshell-type appearance (see Fig. 9-9). The eggshell calcifications are innumerable and are packed closely together where the injection occurred. They are usually a few millimeters in size, but occasionally are larger because of fat necrosis or inflammation; they may have surrounding white fibrotic reaction that causes a palpable mass. Silicone injection calcifications obscure the underlying breast on both mammography and ultrasound, making it difficult to evaluate the breast by physical examination or imaging.
Calcifications can also form in the fibrous capsules around breast implants. These are characteristically dense, dystrophic calcifications that parallel the implant (Fig. 3-23).
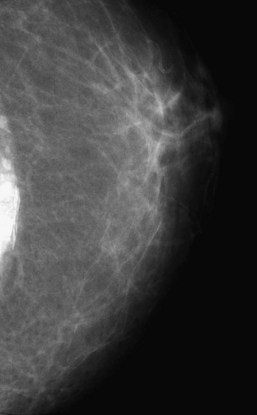
Figure 3-23 When a breast implant is removed, the fibrous capsule that forms around the implant is often left in place. The dystrophic calcifications forming in the fibrous capsule are typically seen near the chest wall in the deflated capsule, as shown here. In Figure 9-9, a craniocaudal mammogram shows multiple round tiny eggshell-type calcifications representing calcification around silicone injection granulomas. Silicone and other substances were injected directly into the breast for augmentation in Southeast Asia, but they cause dense calcifications. Direct injections of silicone are easily distinguished from dystrophic calcifications in the implant capsule after implant removal, shown here.
Calcifications Developing in Malignancy
Calcifications that do not fulfill all the criteria for benign entities require further evaluation. Their shapes, location, and distribution must be analyzed carefully to make sure they are not cancer. After establishing that the calcifications are not in the skin, the radiologist finds the calcifications in two orthogonal projections. This establishes their location within the breast and can determine that the finding of a cluster is indeed a cluster (and not a fake cluster caused by the superimposition of dispersed calcific particles) (Box 3-8).
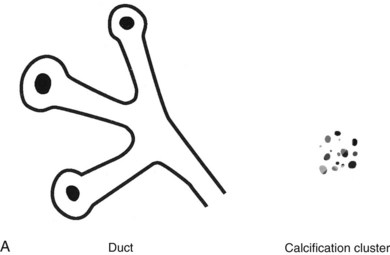
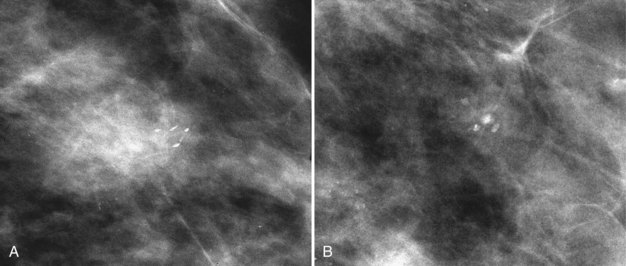
Calcifications in a localized malignancy are tightly clustered, vary in size and shape, and have bizarre branching irregular or linear forms. A suspicious cluster consists of at least five discrete particles smaller than 0.5 mm distributed over a 1-cm3 region (Box 3-9). Calcifications that meet these criteria and do not have a characteristic benign appearance should be viewed with suspicion and biopsied (Figs. 3-24 and 3-25).
Calcifications in malignancy are most commonly seen in invasive ductal carcinoma and DCIS. Calcifications are also seen in papillary carcinoma, but this is a more rare form of cancer. The extremely rare osteogenic sarcoma of the breast contains calcifications that look like bone. Calcifications are rarely seen in lobular carcinoma in situ (LCIS) which usually has no mammographic findings and is an incidental finding on a biopsy performed for another radiologic abnormality or patient symptom (Box 3-10). Calcifications are also not usually a feature of invasive lobular carcinoma.
Indeterminate Calcifications (Including Bi-Rads® Category 3) and Management
Even after careful analysis, some calcifications are indeterminate for malignancy (Figs. 3-26 to 3-28). At this point, surgical biopsy, percutaneous biopsy, or periodic short-term mammographic follow-up may be recommended, depending on the clinical history, the desires of the patient, and those of the referring physician.
On the other hand, periodic mammographic follow-up to confirm stability is an accepted method of follow-up for lesions thought to be benign if the calcifications have less than a 2% chance of malignancy. Brenner and Sickles showed that periodic follow-up for lesions thought to be benign is an acceptable management strategy if the findings fulfill all criteria for the probably benign lesion (Box 3-11). Specifically, the finding must be nonpalpable and one of three types: (1) a solitary cluster of round or punctate benign-appearing calcifications, (2) a well-defined round or oval solitary solid mass, or (3) a focal asymmetry without other associated mammographic abnormality. Periodic follow-up at 6 months and then yearly follow-up for 2 to 3 years is standard of care if the calcifications are round or punctate, have no malignant features, and are stable.
New, palpable, increasing, or pleomorphic calcifications do not fulfill criteria for the “probably benign” BI-RADS® category 3 and should be biopsied (Figs. 3-29 and 3-30). In fact two studies, one by Lehman and colleagues and another by Rosen and colleagues, showed that cancers diagnosed in patients sorted to BI-RADS® category 3 were findings that were either suspicious in the first place; calcifications that were new or increasing in number; masses that were suspicious, new, or were growing; findings that were palpable; or asymmetries that had spiculation or calcifications.
Ultrasound in Evaluation of Calcifications
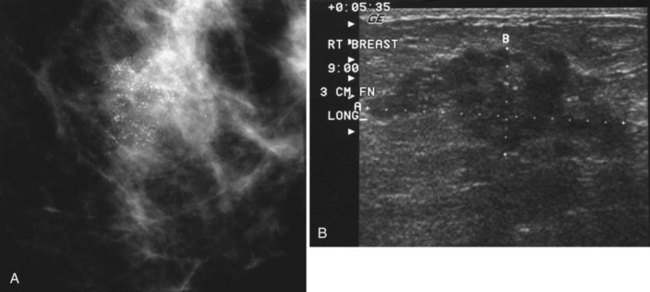
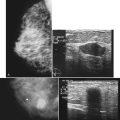
Scientific studies show that high-resolution ultrasound can detect calcifications in the breast or find the masses that have caused the calcifications. Often, however, the normal background speckle of breast parenchyma on ultrasound masks calcifications, if the calcifications alone are the only finding and there is no mass associated with them. Some investigators suggest that ultrasound scans in the region of calcifications are helpful if the scan shows a suspicious mass representing invasive ductal cancer or if it pinpoints the calcifications for percutaneous biopsy or needle localization (Fig. 3-31).
Key Elements
Pleomorphic calcifications without a mass are significant because they may represent DCIS or invasive ductal cancer.
Calcifications are not a feature of invasive lobular carcinoma and are rarely a feature of LCIS.
Use a magnifier to seek calcifications on standard mammograms.
Use air-gap magnification views with a 0.1-mm focal spot to work up calcifications.
“Don’t touch” calcifications include dermal calcifications, milk of calcium, secretory disease (plasma cell mastitis), degenerating fibroadenomas, dystrophic calcifications, vascular calcifications, calcifying implant capsules, and silicone injection calcifications.
Recognize “don’t touch” calcifications and leave them alone.
Tangential views identify and diagnose skin calcifications.
Calcifications in malignancy occur in breast parenchyma.
Both the individual calcification forms and the shape/distribution of the overall cluster are clues for finding cancer.
Fine linear, fine linear branching, amorphous, and pleomorphic individual calcification forms are worrisome for malignancy.
Clustered, linear, and segmental calcification distributions are suspicious for cancer, depending on the individual calcification forms within them.
Calcifications that have linear forms within the cluster need biopsy unless they are milk of calcium or secretory disease.
Know the BI-RADS® terms for calcification shapes and distribution.
Adair FE. Plasma cell mastitis—lesion simulating mammary carcinoma: clinical and pathologic study with a report of 10 cases. Arch Surg. 1933;26:735-749.
Adair FE, Munzer JT. Fat necrosis of the female breast. Am J Surg. 1947;74:117-128.
Bassett LW, Gold RH, Mirra JM. Nonneoplastic breast calcifications in lipid cysts: development after excision and primary irradiation. AJR Am J Roentgenol. 1982;138:335-338.
Berkowitz JE, Gatewood OM, Donovan GB, Gayler BW. Dermal breast calcifications: localization with template-guided placement of skin marker. Radiology. 1987;163:282.
Black JW, Young B. A radiological and pathological study of the incidence of calcification in diseases of the breast and neoplasms of other tissues. Br J Radiol. 1965;38:596-598.
Brenner RJ, Sickles EA. Acceptability of periodic follow-up as an alternative to biopsy for mammographically detected lesions interpreted as probably benign. Radiology. 1989;171:645-646.
Coren GS, Libshitz HI, Patchefsky AS. Fat necrosis of the breast: mammographic and thermographic findings. Br J Radiol. 1974;47:758-762.
Dershaw DD, Abramson A, Kinne DW. Ductal carcinoma in situ: mammographic findings and clinical implications. Radiology. 1989;170:411-415.
Dershaw DD, Chaglassian TA. Mammography after prosthesis placement for augmentation or reconstructive mammoplasty. Radiology. 1989;170(Pt 1):69-74.
Dershaw DD, Shank B, Reisinger S. Mammographic findings after breast cancer treatment with local excision and definitive irradiation. Radiology. 1987;164:455-461.
DiPiro PJ, Meyer JE, Frenna TH, Denison CM. Seat belt injuries of the breast: findings on mammography and sonography. AJR Am J Roentgenol. 1995;164:317-320.
D’Orsi CJ, Feldhaus L, Sonnenfeld M. Unusual lesions of the breast. Radiol Clin North Am. 1983;21:67-80.
Egan RL, McSweeney MB, Sewell CW. Intramammary calcifications without an associated mass in benign and malignant diseases. Radiology. 1980;137(Pt 1):1-7.
Gershon-Cohen J, Ingleby H, Hermel MB. Calcification in secretory disease of the breast. AJR Am J Roentgenol. 1956;76:132-135.
Gunhan-Bilgen I, Oktay A. Management of microcalcifications developing at the lumpectomy bed after conservative surgery and radiation therapy. AJR Am J Roentgenol. 2007;188(2):393-398.
Harnist KS, Ikeda DM, Helvie MA. Abnormal mammogram after steering wheel injury. West J Med. 1993;159:504-506.
Helvie MA, Rebner M, Sickler EA, Oberman HA. Calcifications in metastatic breast carcinoma in axillary lymph nodes. AJR Am J Roentgenol. 1988;151:921-922.
Holland R, Hendriks JH. Microcalcifications associated with ductal carcinoma in situ: mammographic-pathologic correlation. Semin Diagn Pathol. 1994;11:181-192.
Hunter TB, Roberts CC, Hunt KR, Fajardo LL. Occurrence of fibroadenomas in postmenopausal women referred for breast biopsy. J Am Geriatr Soc. 1996;44:61-64.
Ikeda DM, Sickles EA. Mammographic demonstration of pectoral muscle microcalcifications. AJR Am J Roentgenol. 1988;151:475-476.
Kang SS, Ko EY, Han BK, Shin JH. Breast US in patients who had microcalcifications with low concern of malignancy on screening mammography. Eur J Radiol. 2008;67(2):285-291.
Kopans DB, Meyer JE, Homer MJ, Grabbe J. Dermal deposits mistaken for breast calcifications. Radiology. 1983;149:592-594.
Kopans DB, Nguyen PL, Koerner FC, et al. Mixed form, diffusely scattered calcifications in breast cancer with apocrine features. Radiology. 1990;177:807-811.
Krishnamurthy R, Whitman GJ, Stelling CB, Kushwaha AC. Mammographic findings after breast conservation therapy. Radiographics. 1999;19(Spec No):S53-S63.
Lanyi M. Diagnosis and differential diagnosis of breast calcifications. Berlin, NY: Springer-Verlag; 1988.
Lehman CD, Rutter CM, Eby PR, et al. Lesion and patient characteristics associated with malignancy after a probably benign finding on community practice mammography. AJR Am J Roentgenol. 2008;190(2):511-515.
Leung JW, Sickles EA. Developing asymmetry identified on mammography: correlation with imaging outcome and pathologic findings. AJR Am J Roentgenol. 2007;188(3):667-675.
Lev-Toaff AS, Feig SA, Saitas VL, et al. Stability of malignant breast microcalcifications. Radiology. 1994;192(1):153-156.
Linden SS, Sickles EA. Sedimented calcium in benign breast cysts: the full spectrum of mammographic presentations. AJR Am J Roentgenol. 1989;152:967-971.
Mercado CL, Koenigsberg TC, Hamele-Bona D, Smith SJ. Calcifications associated with lactational changes of the breast: mammographic findings with histologic correlation. AJR Am J Roentgenol. 2002;179:685-689.
Millis RR, Davis R, Stacey AJ. The detection and significance of calcifications in the breast: a radiological and pathological study. Br J Radiol. 1976;49:12-26.
Murphy WA, DeSchryver-Kecskemeti K. Isolated clustered microcalcifications in the breast: radiologic-pathologic correlation. Radiology. 1978;127:335-341.
Orson LW, Cigtay OS. Fat necrosis of the breast: characteristic xeromammographic appearance. Radiology. 1983;146:35-38.
Pinsky RW, Rebner M, Pierce LJ, et al. Recurrent cancer after breast-conserving surgery with radiation therapy for ductal carcinoma in situ: mammographic features, method of detection, and stage of recurrence. AJR Am J Roentgenol. 2007;189(1):140-144.
Rebner M, Pennes DR, Adler DD, et al. Breast microcalcifications after lumpectomy and radiation therapy. Radiology. 1989;170(Pt 1):691-693.
Rosen EL, Baker JA, Soo MS. Malignant lesions initially subjected to short-term mammographic follow-up. Radiology. 2002;223(1):221-228.
Ross BA, Ikeda DM, Jackman RJ, Nowels KW. Milk of calcium in the breast: appearance on prone stereotactic imaging. Breast J. 2001;7:53-55.
Sickles EA. Further experience with microfocal spot magnification mammography in the assessment of clustered breast microcalcifications. Radiology. 1980;137(Pt 1):9-14.
Sickles EA. Mammographic detectability of breast microcalcifications. AJR Am J Roentgenol. 1982;139:913-918.
Sickles EA. Breast calcifications: mammographic evaluation. Radiology. 1986;160:289-293.
Sickles EA. Mammography screening and the self-referred woman. Radiology. 1988;166(Pt 1):271-273.
Sickles EA, Abele JS. Milk of calcium within tiny benign breast cysts. Radiology. 1981;141:655-658.
Sigfusson BF, Andersson I, Aspegren K, et al. Clustered breast calcifications. Acta Radiol Diagn. 1983;24:273-281. (Stockholm)
Spring DB, Kimbrell-Wilmot K. Evaluating the success of mammography at the local level: how to conduct an audit of your practice. Radiol Clin North Am. 1987;25:983-992.
Stomper PC, Connolly JL. Ductal carcinoma in situ of the breast: correlation between mammographic calcification and tumor subtype. AJR Am J Roentgenol. 1992;159:483-485.
Tot T, Gere M. Radiological-pathological correlation in diagnosing breast carcinoma: the role of pathology in the multimodality era. Pathol Oncol Res. 2008;14(2):173-178.
Tse GM, Tan PH, Pang AL, et al. Calcification in breast lesions: pathologists’ perspective. J Clin Pathol. 2008;61(2):145-151.
Witten D. The breast. An atlas of tumor radiology. Chicago: Year Book; 1969.
| ______________________________________________________________________________________________________ |
| ______________________________________________________________________________________________________ |
| ______________________________________________________________________________________________________ |
| ____________________________________________________________________________________________________ |
| ____________________________________________________________________________________________________ |
| ____________________________________________________________________________________________________ |
| ____________________________________________________________________________________________________ |
| ______________________________________________________________________________________________________ |
| ______________________________________________________________________________________________________ |
| ______________________________________________________________________________________________________ |
| ______________________________________________________________________________________________________ |
| ______________________________________________________________________________________________________ |
| ______________________________________________________________________________________________________ |
| ______________________________________________________________________________________________________ |
| ______________________________________________________________________________________________________ |
| ______________________________________________________________________________________________________ |
| ______________________________________________________________________________________________________ |
| MILK OF CALCIUM | DCIS | |
|---|---|---|
| Lateral view | ________________ | ________________ |
| CC view | ________________ | ________________ |
3-3. List BI-RADS® terms for suspicious calcifications.
_________________________________________________
_________________________________________________
Answers: pleomorphic, fine linear, fine linear-branching, amorphous.