Malrotation
The earliest descriptions of intestinal development were from Mall in 1898, and later expanded upon by Frazer and Robbins in 1915.1,2 Eight years later, Dott translated these preliminary embryologic observations into problems encountered clinically.3 In his 1932 landmark paper, Ladd described the evaluation and operative treatment of malrotation.4 He described a relatively simple solution to a complicated problem.5 Over 200 postmortem studies had been reported previous to Ladd’s paper, yet he was the first to emphasize the importance of placing the duodenum along the right abdominal wall, widening the mesenteric base, and moving the cecum to the left upper abdomen. With the exception of the laparoscopic approach, Ladd’s original technique has remained relatively unchanged.
Embryology
The development of the midgut begins with the differentiation of the primitive intestinal tract into the foregut, midgut, and hindgut at the fourth week of gestation.6 The mature alimentary tract and all associated digestive organs are formed from this primitive tube. The most accepted model of midgut maturation involves four distinct stages: (1) herniation; (2) rotation; (3) retraction; and (4) fixation. Normal fixation of the duodenum and colon is illustrated in Figure 31-1. The intestinal loop can be divided into the cephalic (duodenojejunal) limb and the caudal (cecocolic) limb, which rotate separately but in parallel. The SMA serves as the fulcrum with the omphalomesenteric duct at the apex. Due to the disproportional growth and elongation of the midgut during the fourth gestational week, the intestinal loop herniates into the extraembryonic coelom. Next, the bowel enters a critical period of rotation when the prearterial and postarterial limbs make three separate 90° turns, all in the counterclockwise direction around the SMA. The first 90° rotation occurs outside the abdomen. The second 90° turn commences during the return of the intestine into the abdominal cavity during the 10th gestational week. The duodenojejunal junction now passes posterior to the SMA. The last rotation occurs in the abdomen. The primitive intestine has thus completed a 270° counterclockwise rotation, allowing the duodenojejunal limb to be positioned to the left of the SMA while the cecocolic limb is on the right. Fixation of the ascending and descending colon then occurs. Disruption of any of these vital steps leads to the spectrum of malrotation encountered clinically.
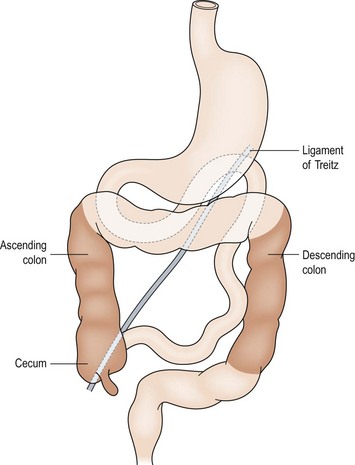
FIGURE 31-1 Normal intestinal anatomy results in fixation of the duodenojejunal junction in the left upper quadrant and the cecum in the right lower quadrant. This allows a wide breadth to the mesentery of the small bowel.
The most common forms of rotational disorders include nonrotation (Fig. 31-2), incomplete rotation (Fig. 31-3), and reversed rotation. Right and left mesocolic hernias can also occur. In nonrotation, there is failure of the normal intestinal 270° counterclockwise rotation around the SMA. Thus, the duodenojejunal limb lies in the right hemi-abdomen with the cecocolic limb in the left hemi-abdomen. Midgut volvulus due to a narrow mesenteric pedicle and extrinsic duodenal obstruction secondary to abnormally positioned cecal attachments are the most common symptomatic consequences. In cases of incomplete rotation, normal rotation has been arrested at or near 180°. The cecum will usually reside in the right upper abdomen. Obstructing peritoneal bands over the duodenum are present. With reversed rotation, an errant 90° clockwise rotation occurs, which leaves a tortuous transverse colon to the right of the SMA, passing through a retroduodenal tunnel dorsal to the artery and in the small bowel mesentery.7,8 The duodenum will assume an anterior position. Reverse rotation with volvulus may occur with obstruction of the transverse colon. Paraduodenal hernias are rare and result from failure of the right or left mesocolon to fuse to the posterior body wall. A potential space is created. Subsequently, the small intestine may become sequestered and potentially obstructed.
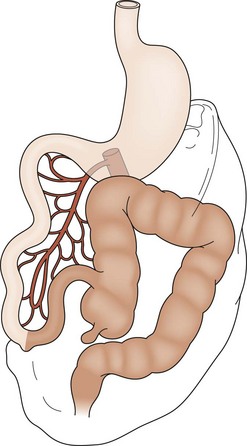
FIGURE 31-2 Nonrotation. The prearterial midgut (lightly shaded) is found on the right side of the abdomen, while the postarterial midgut (darkly shaded) remains on the left. Neither segment has undergone appropriate rotation. Volvulus is a risk.
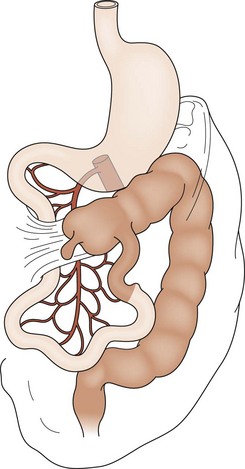
FIGURE 31-3 Incomplete rotation. Both the prearterial (lightly shaded) and postarterial (darkly shaded) segments have undergone partial, yet not complete, rotation. Ladd’s bands are seen attaching the cecum to the right posterior abdominal wall. The duodenum becomes compressed and possibly obstructed. Volvulus is a risk.
Presentation
The incidence of malrotation has been estimated at 1 in 6000 live births. An increased incidence of 0.2% has been found in barium swallow studies,9 whereas autopsy studies estimate that the true incidence may be as high as 1% of the total population.10 Associated anomalies are common (Table 31-1).11
Classic malrotation with midgut volvulus is often discovered in a previously healthy term neonate. Up to 75% of patients present during the first month of life, while another 15% will present within the first year.12–14 However, volvulus and mortality have been reported at all ages.15 Sudden onset of bilious vomiting is the cardinal sign of neonatal intestinal obstruction, and malrotation with volvulus must be the presumed diagnosis until proven otherwise. Physical examination findings will vary. Initially, the patient may have a scaphoid abdomen or only mild upper abdominal distension. However, if vascular compromise to the completely obstructed bowel develops, the abdomen will become progressively more distended and peritonitis will ensue. Late signs include abdominal wall erythema and shock. Similarly, laboratory data are often nonspecific and of limited diagnostic value. Thus, the clinician must have a high index of suspicion in a previously healthy baby who presents with bilious emesis. Furthermore, if signs of bowel ischemia are present, operative intervention must occur without delay.
Patients with chronic obstruction will present less dramatically. Nonspecific presenting problems such as failure to thrive, gastroesophageal reflux, early satiety, and mild abdominal discomfort are often seen. Partial volvulus can lead to mesenteric venous and lymphatic obstruction and subsequently impaired nutrient absorption. The diagnosis becomes even more challenging with the older child or teenager because the symptoms are often vague and may sometimes seem unrelated to the abdomen.16
Diagnosis
Radiologic studies play a critical role in establishing a diagnosis of intestinal malrotation. Initial evaluation will usually begin with a plain anteroposterior abdominal film combined with a lateral decubitus or upright view (Fig. 31-4). Nonspecific findings ranging from gastric distention to a gasless abdomen are common. Upper gastrointestinal contrast study remains the gold standard and is needed to document the position of the ligament of Treitz to the left of the spinal pedicles, and rising to the level of the gastric outlet. Additionally, the lateral film will show the duodenum in a retroperitoneal, posterior position.17 Findings of abnormal rotation include a low-lying ligament of Treitz or failure of the ligament of Treitz to be located left of the spine. If volvulus exists, the contrast study may show the ‘coil spring’ or ‘corkscrew’ configuration with incomplete obstruction and the “beak” appearance in the duodenum with complete obstruction (Figs 31-5 and 31-6).
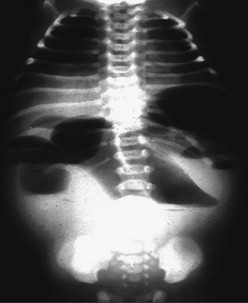
FIGURE 31-4 Upright abdominal film in an infant demonstrating proximal small bowel dilatation. This infant had a midgut volvulus.
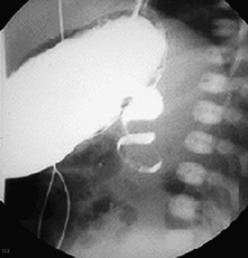
FIGURE 31-5 Lateral image on upper gastrointestinal series in an infant with malrotation and midgut volvulus showing the ‘corkscrew’ appearance of the obstructed duodenum.
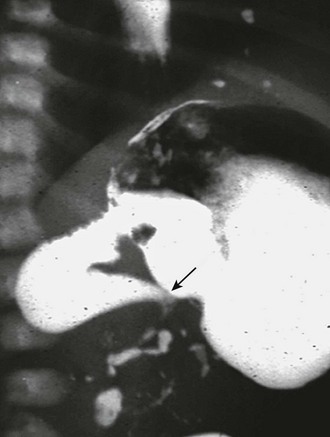
FIGURE 31-6 Lateral image on upper gastrointestinal series in infant with malrotation and midgut volvulus showing the ‘beak’ (arrow) appearance of the obstructed duodenum. Note that a small amount of contrast agent has progressed through the volvulus.
In some institutions, ultrasound (US) is being trialed to make the diagnosis of malrotation. Color Doppler ultrasound imaging may reveal a dilated duodenum with inversion of the SMA and vein (the whirlpool sign) in cases of acute volvulus.18–21 Additionally, Yousefzadeh and colleagues have proposed that ultrasound be used to diagnose malrotation without volvulus based on the position of the duodenum and the SMA. Because the third portion of the duodenum assumes a retroperitoneal position anterior to the aorta and posterior to the SMA in individuals with normal intestinal rotation, verification of this position by ultrasound potentially obviates the need for further imaging. This group prospectively validated this technique in 33 neonates at their institution.22 However, application of this technique prospectively in multiple institutions is likely needed prior to widespread acceptance.
Management
Open Approach
There has been little change from Ladd’s original description of the operative technique for correction of malrotation with or without volvulus. The six steps are summarized in Box 31-1. A right upper abdominal transverse incision is typically used. Chylous ascites secondary to lymphatic obstruction and rupture of mesenteric lacteals is often initially found. The bowel and mesentery should then be completely eviscerated. In patients with volvulus, the surgeon will commonly encounter two or three complete clockwise revolutions. Prompt, gentle counterclockwise detorsion is performed (Fig. 31-7). Once detorsion has been accomplished, warm soaked lap pads are placed on the bowel, and the surgeon should patiently observe for reperfusion. If perfusion remains in question, the surgeon has several options, including assessment of the antimesenteric vascular integrity with the use of a Doppler probe and intravenous administration of fluorescein with Wood’s lamp evaluation.
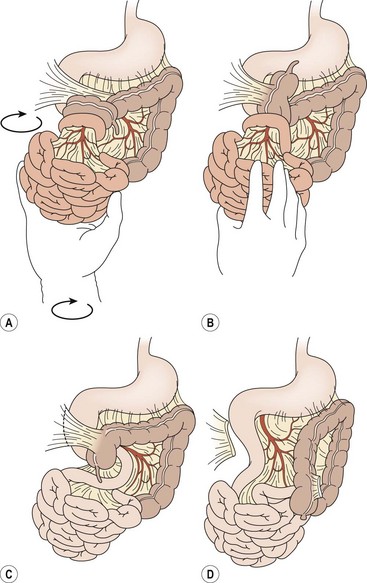
FIGURE 31-7 Open correction of malrotation. (A,B) Initial appearance of the malrotated bowel during exploration. The surgeon first will perform counterclockwise detorsion of the midgut. (C) Next, Ladd bands from the cecum to the right abdominal wall are divided. (D) Last is the critical step of broadening the small bowel mesentery. An appendectomy is also performed.
Bowel that is frankly necrotic should be resected. However, when longer segments are of questionable viability, a temporary closure or silo should be placed and the patient re-evaluated at a second-look operation 24 to 48 hours later. Unfortunately, an occasional patient will have complete infarction of the midgut (Fig. 31-8). Closure of the abdomen without bowel resection may be a reasonable choice after intraoperative discussion with the family. However, the development and sophistication of intestinal and multi-visceral transplantation makes the best option much less clear.
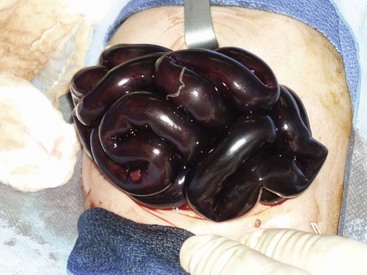
FIGURE 31-8 Unfortunately, on occasion, complete infarction of the midgut due to malrotation and volvulus will be found. Management options vary depending on surgeon and the wishes of the parents.
Once the bowel has been detorsed, the breadth of the mesentery must then be widened. This goal is accomplished by first dividing the Ladd bands traversing the duodenum (Fig. 31-9). Next, a generous Kocher maneuver is performed to straighten the duodenum. It is imperative that a nasogastric tube is passed through the now straightened duodenum so that intrinsic obstruction can be ruled out. The dissection then continues to the base of the SMA and vein by incising the anterior mesenteric leaflet. When properly opened, the mesentery is maximally broadened and the possibility of postoperative volvulus is presumably reduced, although not completely eliminated.
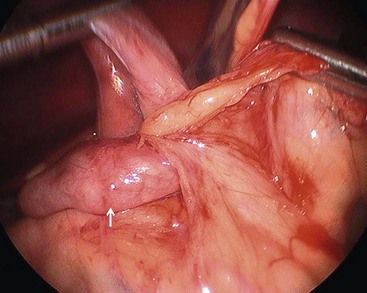
FIGURE 31-9 Laparoscopic view of retroperitoneal bands extending from the cecum and partially obstructing the distal duodenum and proximal jejunum (arrow).
Most surgeons will complete the procedure with an appendectomy, owing to the subsequent malposition of the cecum to the left side of the abdomen. Standard or inversion appendectomy are equally acceptable. Finally, the duodenum and small intestine are positioned toward the right abdomen with the colon on the left. Suture fixation of the bowel to the lateral abdominal wall is not recommended.23,24
Laparoscopic Approach
The laparoscopic treatment for intestinal rotation anomalies in neonates, infants, and children with or without midgut volvulus was first proposed by van der Zee and colleagues in 1995.25 Subsequent publications have been primarily single-institution case reports or small case series.26–36 Clinical outcomes for the laparoscopic technique have generally been favorable.
The patient is placed supine in the reversed Trendelenburg position and on a shortened operating room table, if available. The surgeon will stand at the child’s feet with the assistant positioned to the surgeon’s left. An oro- or nasogastric tube is inserted. A port is introduced at the umbilicus and insufflation is established to a pressure of 8–12 mmHg.26–28 The surgeon will work with two instruments while the assistant may use one to help with exposure. The surgeon’s first goal is to determine if malrotation is actually present and thus confirm the preoperative imaging studies (Figs 31-10 and 31-11). Tilting the table may help identify the ligament of Treitz and the ileocecal junction. Findings of malrotation include a high and medial cecal position, an elongated, tortuous duodenum, and a malpositioned ligament of Treitz. Although there are no specific guidelines established to predict whether a future volvulus will occur, a mesenteric base extending more than half the diameter of the abdomen is generally sufficient to prevent volvulus.29 If a rotational anomaly is felt to be present, the operation is then performed in the same stepwise fashion as the open Ladd procedure.
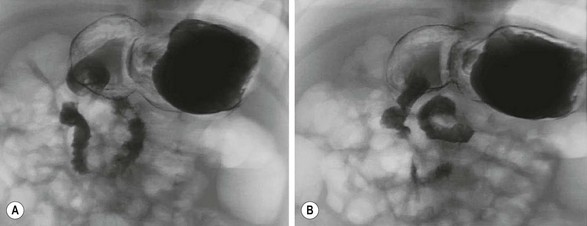
FIGURE 31-10 An upper gastrointestinal study was performed in this infant who presented with vomiting. The radiologic interpretation was that the duodenum was entirely on the patient’s right side and the ligament of Treitz was at the level of pylorus. Also, the ligament of Treitz did not cross the midline. There was no evidence of obstruction. This patient underwent diagnostic laparoscopy and was found to have normal anatomy with correct positioning of the ligament of Treitz and the cecum (see Fig. 31-11).
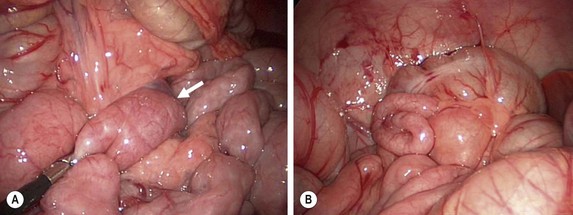
FIGURE 31-11 At times, the upper gastrointestinal study can be equivocal for possible malrotation (see Fig. 31-10). In such patients, diagnostic laparoscopy is a useful technique to ascertain whether the patient actually has malrotation. (A) The ligament of Treitz (arrow) is seen to be to the left of the patient’s midline. (B) In addition, the location of the cecum in the right lower quadrant also helps verify that the patient does not have malrotation.
Laparoscopy for malrotation can present unique challenges. If volvulus is encountered (Fig 31-12), the surgeon may continue laparoscopically provided rapid detorsion can be achieved. However, dilated bowel may make visualization and proper orientation difficult. Further, dilated bowel can be injured by laparoscopic instruments. A second scenario occurs in older children with long- standing duodenal obstruction. In these children, the proximal bowel may be molded into a cocoon-like deformity (Fig. 31-13), which may also make laparoscopic repair challenging.27 Lastly, appendectomy may be performed either extracorporeally via the umbilicus (Fig. 31-14) or intracorporeally using endoloops or a stapling device. Instruments are then removed under direct visualization, and the incisions are closed with absorbable suture. Postoperative management is similar to that for the open approach. Most children may begin clear liquids on the day of surgery. Discharge on day 1 or 2 is expected.28
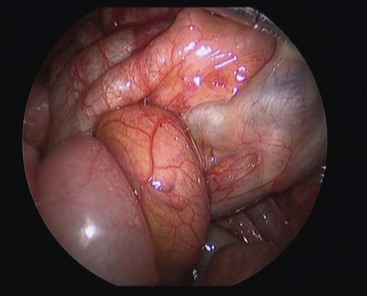
FIGURE 31-12 At laparoscopy, a midgut volvulus is noted. The bowel appears healthy. The operation was converted and an open Ladd procedure was performed.
FIGURE 31-13 In older children with long-standing partial obstruction or internal herniation, the Ladd bands may cause the bowel to form into a cocoon-like deformity. (From Moir CR. Laparoscopic Ladd procedure. In: Holcomb GW III, Georgeson KE, Rothenberg SS, editors. Atlas of Pediatric Laparoscopy and Thoracoscopy. Philadelphia: Elsevier; 2008. p. 55–60.)
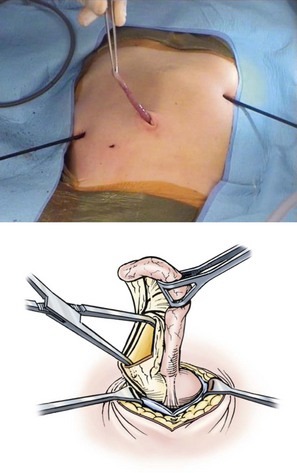
FIGURE 31-14 When performing a laparoscopic Ladd procedure, an extracorporeal appendectomy eliminates the need for a large port for the endoscopic stapler. The appendix is grasped with one of the intracorporeal instruments and brought into view through the umbilical fascial defect, where it is grasped and exteriorized. (From Moir CR. Laparoscopic Ladd procedure. In: Holcomb GW III, Georgeson KE, Rothenberg SS, editors. Atlas of Pediatric Laparoscopy and Thoracoscopy. Philadelphia: Elsevier; 2008. p. 55–60.)
Since van der Zee’s initial report, other authors have described variations in technique and have published their positive results.29–32 Successful laparoscopic management of a 15-day-old with acute midgut volvulus has been described.33 However, the majority of patients undergoing laparoscopic repair of intestinal malrotation have been elective cases without volvulus.30,31,34 Operative times have been reported to average 110 to 120 minutes.31,35 In one report, the time to a regular diet was two days (median) and resolution of symptoms was found in five of seven patients at 15 months.30
In another series, 12 neonates and infants, weighing 3–7 kg, underwent a three-port laparoscopic Ladd procedure.29 Presentation included intermittent upper intestinal obstruction, and the diagnosis was eventually confirmed by contrast study. Three 3.5 mm ports were placed in the infraumbilical ring and in the right and left abdomen. Operative time was equivalent to published open results, ranging from 35 to 120 minutes (mean, 58 minutes). Feedings were started on postoperative day 1 or 2, and the patients were discharged at a mean of two days. All symptoms resolved on postoperative evaluation.
The formation of intra-abdominal adhesions has generally been viewed as necessary to prevent postoperative volvulus. Through open exploration and direct manipulation of the bowel, adhesions are created. Concerns have been raised that adhesion formation may be limited with laparoscopy, as is seen in other laparoscopic procedures. Initial reports have not validated these concerns. In fact, in a retrospective study which compared open and laparoscopic Ladd procedures, the only patients in which postoperative volvulus occurred were in the open group.36
Laparoscopic correction of malrotation is an accepted procedure but still can be a technical challenge. In patients with acute volvulus, the working space may be limited owing to bowel edema and chylous ascites, and the surgeon should have a low threshold to convert to laparotomy, which can be done by slightly extending the umbilical incision.36 Although the current literature supports consideration of laparoscopy (especially in older patients with malrotation as an incidental finding), further prospective studies with large sample sizes, possible randomization, and long-term follow-up should be conducted to confirm the proper role for the laparoscopic approach.
Postoperative Management
In patients without evidence of volvulus or obstruction, a nasogastric tube is not generally used. Bowel function generally returns in 1 to 5 days. However, older patients with chronic obstruction are likely to have a prolonged ileus, and thus nasogastric drainage and parenteral support may be required. Antibiotics are not uniformly needed. Feedings can be advanced per the surgeon’s discretion. Patients with extended or subtotal small bowel resection pose special problems. Total parenteral nutrition is essential to sustain these patients until adaptation and compensatory growth of the residual bowel can occur.37 Small bowel or multiple-viscera transplantation should be considered. Postoperative intussusception has been noted in 3.1% of all patients who underwent a Ladd procedure, compared with 0.05% following other laparotomies.38 The incidence of recurrent volvulus is low. At the author’s institution, the recurrent volvulus rate has approximated 1%. Finally, up to 10% of patients may develop a postoperative adhesive small bowel obstruction requiring an operation.23,24,39
Special Considerations
The Older Patient
Malrotation is occasionally diagnosed in the teenager. The presenting symptoms are often vague, including vomiting, diarrhea, early satiety, bloating, and dyspepsia.40–43 The patient may have undergone multiple imaging modalities, including contrast studies, computed tomography (Fig. 31-15), and magnetic resonance imaging. The imaging modalities may, however, help delineate vascular and/or hepatobiliary anatomic irregularities that are associated with rotational disorders.44 Patients who are found to have symptoms from rotational anomalies should undergo operative intervention. Patients and their families should be cautioned, though, that when compared with younger patients, older patients (>16 years of age) endured a higher percentage of postoperative complications and required a higher percentage of reoperation.45
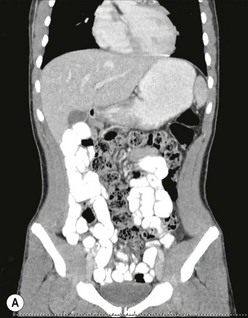
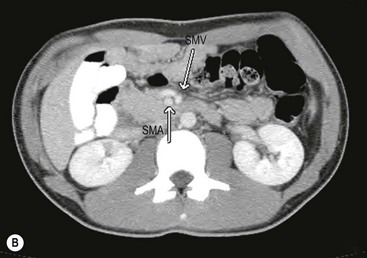
FIGURE 31-15 This 15-year-old presented with chronic abdominal pain and underwent a CT scan for diagnosis. (A) On this coronal view, the small bowel is filled with contrast and most of it is located along the right paracolic gutter. (B) On transverse section, note that the superior mesenteric artery (SMA) and superior mesenteric vein (SMV) are malpositioned, and are reversed in orientation to each other. This patient underwent a laparoscopic Ladd procedure with resolution of her symptoms.
The question arises whether the incidental discovery of malrotation in the older child or young adult should be repaired at all. The principle of ‘watchful waiting’ has been suggested.46 However, up to 20% of adult patients undergoing operative repair of malrotation will have an acute volvulus or bowel ischemia, both potentially life-threatening presentations.47 Furthermore, even in patients who are believed to be asymptomatic, preoperative symptoms attributable to malrotation have retrospectively been noted to improve or resolve.31,43,48 Thus, operative correction in the asymptomatic older patient is currently recommended.
Atypical Malrotation
The dilemma arises when an abnormality is discovered on imaging, and the child is labeled as a ‘malrotation variant.’ To exclude malrotation, the duodenojejunal flexure must be located to the left of the spine at the level of the duodenal bulb. Defined as the ligament of Treitz, this flexure can be in an equivocal position. Symptoms are generally mild and can easily be attributed to other diagnoses such as gastroesophageal reflux. Volvulus or internal hernia is usually not seen with malrotation variants. This clinical scenario leaves the surgeon with the dilemma of deciding whether the symptoms are due to the radiographic abnormality. Given the higher rate of persistence of symptoms after intervention and an overall increase in postoperative complications, close observation or repeated contrast study has been suggested in these equivocal cases.49,50 If operative intervention is decided upon, laparoscopy is an ideal approach.
Heterotaxy
Rotational disorders are known to coexist with heterotaxy syndrome (HS).51 Thus, in many institutions, screening upper gastrointestinal contrast studies are performed. Patients that are symptomatic from these anomalies should undergo a Ladd operation. However, management of the asymptomatic patient remains controversial with literature available to support either observation or elective operation. Recently, a retrospective study documented a higher morbidity rate when heterotaxy patients, who underwent prophylactic Ladd procedure, were compared to those without heterotaxy. Despite the small sample size, they concluded that asymptomatic patients should be observed.52 Conversely, a group from Boston noted similar outcomes when HS patients were compared to patients without HS that underwent Ladd procedures.53 Interestingly, 27% of the HS patients that had abdominal symptoms were found to have volvulus at exploration, emphasizing the potential hazards of observational management. Multi-institutional, prospective studies are needed to make more definitive recommendations for this challenging group of patients.
References
1. Mall, FP. Development of the human intestine and its position in the adult. Johns Hopkins Hosp Bull. 1898; 9:197–208.
2. Frazer, TE, Robbins, RF. On the factors concerned in causing rotation of the intestine in man. J Anat Physiol. 1915; 50:74–110.
3. Dott, NM. Anomalies of intestinal rotation: Their embryology and surgical aspects, with the report of five cases. Br J Surg. 1923; 11:251–286.
4. Ladd, W. Congenital obstruction of the duodenum in children. N Engl J Med. 1932; 206:277–283.
5. Ladd, WE, Gross, RE. Intestinal obstruction resulting from malrotation of the intestines and colon. Abdominal Surgery of Infancy and Childhood. Philadelphia: WB Saunders; 1941.
6. Kluth, D, Jaeschke-Melli, S, Fiegel, H. The embryology of gut rotation. Semin Pediatr Surg. 2003; 12:275–279.
7. Kanazawa, T, Kasugai, K, Miyata, M, et al. Midgut malrotation in adulthood. Intern Med. 2000; 39:626–631.
8. Wang, C, Welch, CE. Anomalies of intestinal rotation in adolescents and adults. Surgery. 1963; 54:829–854.
9. Warner, BR. Malrotation. In: Oldham KT, Colombani PM, Foglia RP, eds. Surgery of Infants and Children. Philadelphia: Lippincott-Raven; 1996:1229–1240.
10. Kapfer, SA, Rappold, JF. Intestinal malrotation—not just the pediatric surgeon’s problem. J Am Coll Surg. 2004; 199:628–635.
11. Aiken, JJ, Oldham, KT. Malrotation. In: Ashcraft KW, Holcomb GW, III., Murphy JP, eds. Pediatric Surgery. Philadelphia: Elsevier Saunders; 2005:435–447.
12. Gross, RE. Malrotation of the intestine and colon. In: Gross R.E., ed. The Surgery of Infancy and Childhood. Philadelphia: WB Saunders; 1953:192–203.
13. Ford, EG, Senac, MO, Jr., Srikanth, MS, et al. Malrotation of the intestine in children. Ann Surg. 1992; 212:172–178.
14. Stewart, DR, Colodny, AL, Daggett, WC. Malrotation of the bowel in infants and children: A 15-year review. Surgery. 1976; 79:716–720.
15. Powell, DM, Othersen, HB, Smith, CD. Malrotation of the intestine in children: The effect of age on presentation and therapy. J Pediatr Surg. 1989; 24:777–780.
16. Maxson, RT, Franklin, PA, Wagner, CW. Malrotation in the older child: Surgical management, treatment, and outcome. Am Surg. 1995; 61:135–138.
17. Sizemore, AW, Rabbani, KZ, Ladd, A, et al. Diagnostic performance of the upper gastrointestinal series in the evaluation of children with clinically suspected malrotation. Pediatr Radiol. 2008; 38:518–528.
18. Shimanuki, Y, Aihara, T, Takano, H. Clockwise whirlpool sign at color Doppler ultrasound: An objective and definite sign of midgut volvulus. Radiology. 1996; 199:261–264.
19. Pracros, JP, Sann, L, Genin, G, et al. Ultrasound diagnosis of midgut volvulus: The “whirlpool” sign. Pediatr Radiol. 1992; 22:18–20.
20. Chao, JC, Kong, MS, Chen, JY, et al. Sonographic features related to volvulus in neonatal intestinal malrotation. J Ultrasound Med. 2000; 19:371–376.
21. Orzech, N, Navarro, OM, Langer, JC. Is ultrasonography a good screening test for intestinal malrotation? J Pediatr Surg. 2006; 41:1005–1009.
22. Yousefzadeh, DK, Kang, L, Tessicini, L. Assessment of retromesenteric position of the third portion of the duodenum: An ultrasound feasibility study in 33 newborns. Pediatr Radiol. 2010; 40:1476–1484.
23. Stauffer, UG, Herman, P. Comparison of late results in patients with corrected intestinal malrotation with and without fixation of the mesentery. J Pediatr Surg. 1980; 15:9–12.
24. Rescorla, FJ, Shedd, FJ, Grosfeld, JL, et al. Anomalies of intestinal rotation in childhood: Analysis of 447 cases. Surgery. 1990; 108:710–715.
25. van der Zee, DC, Bax, NMA. Laparoscopic repair of acute volvulus in a neonate with malrotation. Surg Endosc. 1995; 9:1123–1124.
26. Bax, K, van der Zee, DC. Intestinal malrotation. In: Klaas N, Bax MA, Georgeson KE, et al, eds. Endoscopic Surgery in Infants and Children. New York: Springer; 2007:299–304.
27. Moir, CR. Laparoscopic Ladd procedure. In: Holcomb GW, III., Georgeson KE, Rothenberg SS, eds. Atlas of Pediatric Laparoscopy and Thoracoscopy. Philadelphia: WB Saunders; 2008:55–60.
28. McLean, SE, Minkes, RK. Intestinal Rotation Abnormalities. In: Langer JC, Albanese CT, eds. Pediatric Minimal Access Surgery. Boca Raton, FL: Taylor & Francis; 2005:271–283.
29. Bass, KD, Rothenberg, SS, Chang, JH. Laparoscopic Ladd’s procedure in infants with malrotation. J Pediatr Surg. 1998; 33:279–281.
30. Mazziotti, MV, Strasberg, SM, Langer, JC. Intestinal rotation abnormalities without volvulus: The role of laparoscopy. J Am Coll Surg. 1997; 185:172–176.
31. Lessin, MS, Luks, FI. Laparoscopic appendectomy and duodenocolonic dissociation (Ladd) procedure for malrotation. Pediatr Surg Int. 1998; 13:184–185.
32. Gross, E, Chen, MK, Lobe, TE. Laparoscopic evaluation and treatment of intestinal malrotation in infants. Surg Endosc. 1996; 10:936–937.
33. Martinez-Ferro, M, Bignon, H, Figueroa, M. Ladd laparoscopic procedure in the neonate. Cir Pediatr. 2006; 19:182–184.
34. Draus, JM, Jr., Foley, DS, Bond, SJ. Laparoscopic Ladd procedure: A minimally invasive approach to malrotation without midgut volvulus. Am Surg. 2007; 73:693–696.
35. Waldhausen, JH, Sawin, RS. Laparoscopic Ladd’s procedure and assessment of malrotation. J Laparoendosc Surg. 1996; 6:S103–S105.
36. Fraser, JD, Aguayo, P, Sharp, SW, et al. The role of laparoscopy in the management of malrotation. J Surg Res. 2009; 156:80–82.
37. Georgeson, KE, Breaux, CW, Jr. Outcome and intestinal adaptation in neonatal short-bowel syndrome. J Pediatr Surg. 1992; 27:344–350.
38. Kidd, J, Jackson, R, Wagner, C, et al. Intussusception following the Ladd procedure. Arch Surg. 2000; 135:713–715.
39. Murphy, FL, Sparnon, AL. Long-term complications following intestinal malrotation and the Ladd’s procedure: A fifteen-year review. Pediatr Surg Int. 2006; 22:326–329.
40. Hsu, SD, Yu, JC, Chou, SJ, et al. Midgut volvulus in an adult with congenital malrotation. Am J Surg. 2008; 195:705–707.
41. Gamblin, TC, Stephens, RE, Jr., Johnson, RK, et al. Adult malrotation: A case report and review of the literature. Curr Surg. 2003; 60:517–520.
42. von Flue, M, Herzog, U, Ackermann, C, et al. Acute and chronic presentation of intestinal nonrotation in adults. Dis Colon Rectum. 1994; 37:192–198.
43. Fu, T, Tong, WD, He, YJ, et al. Surgical management of intestinal malrotation in adults. World J Surg. 2007; 31:1797–1803.
44. Pickhardt, PJ, Bhalla, S. Intestinal malrotation in adolescents and adults: Spectrum of clinical and imaging features. AJR Am J Roentgenol. 2002; 179:1429–1435.
45. Durkin, ET, Lund, DP, Shaaban, AF, et al. Age-related differences in diagnosis and morbidity of intestinal malrotation. J Am Coll Surg. 2008; 206:658–663.
46. Malek, MM, Burd, RS. The optimal management of malrotation diagnosed after infancy: A decision analysis. Am J Surg. 2006; 191:45–51.
47. Malek, NM, Burd, RS. Surgical treatment of malrotation after infancy: A population-based study. J Pediatr Surg. 2005; 40:285–289.
48. Rao, KM, Kiran, PR. Midgut malrotation presenting in adult life. Br J Surg. 1994; 81:1173–1174.
49. McVay, MR, Kokoska, ER, Jackson, RJ, et al. The changing spectrum of intestinal malrotation: Diagnosis and management. Am J Surg. 2007; 194:712–717.
50. Smith, SD. Disorders of intestinal rotation and fixation. In: Grosfeld JL, O’Neill JA, Jr., Fonkalsrud EW, Coran AG, eds. Pediatric Surgery. 6th ed. Philadelphia: Mosby; 2006:1342–1357.
51. Chang, J, Brueckner, M, Touloukian, RJ. Intestinal rotation and fixation abnormalities in heterotaxia: Early detection and management. J Pediatr Surg. 1993; 28:1281–1284.
52. Pockett, CR, Dicken, B, Rebeyka, IM, et al. Heterotaxy Syndrome: Is a prophylactic Ladd procedure necessary in asymptomatic patients? Pediatr Cardiol. 2013; 34:59–63.
53. Yu, DC, Thiagarajan, RR, Laussen, PC, et al. Outcomes after the Ladd procedure in patients with heterotaxy syndrome, congenital heart disease, and intestinal malrotation. J Pediatr Surg. 2009; 44:1089–1095.






