CHAPTER 107 Malignant Tumors of the Larynx
Cancer of the larynx is the second most common malignancy of the upper aerodigestive tract (UADT), with over 11,000 cases annually in the United States alone.1 Although a large variety of malignancies may occur in the larynx, 85% to 95% of laryngeal malignancies are squamous cell carcinoma (SCC), arising from the epithelial lining of the larynx.2 The successful management of laryngeal malignancy requires accurate diagnosis, staging, assessment of patient wishes, and the selection of the most appropriate treatment for the individual patient, with close post-treatment surveillance. Treatment options have expanded and become more complex as new surgical procedures have been developed, advanced radiation therapy (RT) modalities have evolved, and new chemotherapeutic drugs have become available. These treatments still have significant morbidity and pose risks of adverse impact on voice quality, airway integrity, and swallowing. In recent decades, major efforts have been made to develop therapeutic strategies to preserve the larynx anatomically and functionally using RT, conservation laryngeal surgery, and chemoradiotherapy (CRT). These techniques are now important tools in the armamentarium of the head and neck oncologist. This chapter focuses on the principles of the diagnosis, evaluation, and treatment of SCC of the larynx. Non–squamous cell malignancies form a small but important subset of laryngeal malignancies and are also covered in detail. More specific details of surgical techniques are discussed in subsequent chapters.
Anatomy and Embryology
Embryology
The embryologic development of the larynx determines the pattern of metastatic spread of laryngeal cancer. The supraglottic larynx is derived from the buccopharyngeal primordium, which develops from the third and fourth branchial arches. The glottis and subglottis are derived from the tracheobronchial primordium from the sixth branchial arch and are formed by the union of lateral furrows that develop on each side of the tracheobronchial primordium. Therefore the larynx has a dual blood supply and lymphatic drainage. The supraglottis is supplied by the superior laryngeal arteries, and its lymphatic drainage follows these vessels to the carotid sheath to drain into deep cervical chain nodes in levels II and III. The glottis and subglottis are supplied by the inferior laryngeal arteries, and similarly, lymphatic drainage from these two regions follows these arteries to drain into prelaryngeal and pretracheal nodes (Level VI), before reaching the deep cervical chain nodes in level IV.3
The glottic region is formed by paired structures that fuse in the midline. The lymphatics drain unilaterally. The vocal folds have sparse lymphatics. Therefore, glottic cancers must invade deeply before getting access to lymphatic channels. These factors explain the lower incidence of lymphatic metastasis in glottic SCC, as well as the propensity for unilateral metastases. Because the supraglottis is formed without a midline union, its lymphatics drain bilaterally and the increased likelihood of bilateral lymphatic metastases from supraglottic carcinoma is ascribed to this embryologic factor, as described by Frazer in 1909.4 Pressman and colleagues5 noted that the inferior extent of supraglottic injection was the inferior false vocal cord, with the ventricle defining an anatomic barrier and halting further inferior flow of the dye.
Anatomy
A complete description of the anatomy of the larynx is beyond the scope of this chapter, so the following discussion is limited to a description of the boundaries of the larynx, its compartments, and its lymphatic drainage. The laryngeal framework is formed by three unpaired cartilages (epiglottis, thyroid, cricoid) and the paired arytenoid cartilages. The superior boundary of the larynx consists of the superior surface of the epiglottis and the superior edge of the aryepiglottic folds. The anterosuperior limit is formed by the lingual surface of the suprahyoid epiglottis and the hyoepiglottic ligament, which forms the superior boundary of the pre-epiglottic space. The anterior boundary of the larynx is formed by the thyrohyoid membrane and thyroid cartilage in the supraglottis, thyroid cartilage in the glottis, and cricothyroid membrane and anterior arch of the cricoid cartilage in the subglottis. The inferior limit of the larynx is defined by the horizontal plane, which passes through the inferior edge of the cricoid cartilage.6 The posterior and lateral borders of the larynx are composed of the laryngeal surface of the aryepiglottic folds, arytenoid cartilages, interarytenoid space, and posterior surface of the subglottic space (which is defined as the mucosa covering the surface of the cricoid cartilage).
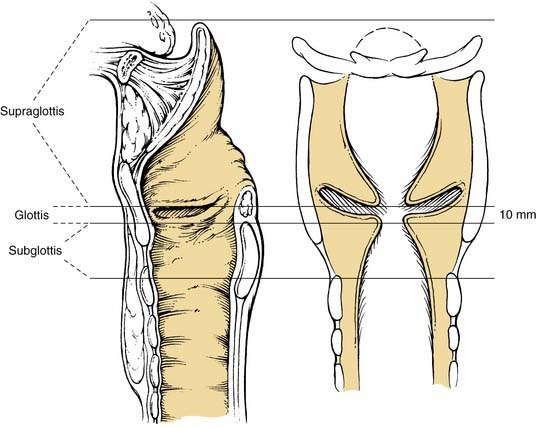
The larynx is divided into three regions or sites: the supraglottis, the glottis, and the subglottis (Fig. 107-1). This division reflects the embryologic structure of the larynx and the anatomic barriers to spread of laryngeal cancer outlined earlier. The TNM staging system further subdivides the supraglottis and glottis of the larynx into multiple subsites, used to define the T stage (Table 107-1).6
| Site | Subsite |
|---|---|
| Supraglottis |
From Greene F, Page D, Fleming I, et al. AJCC Cancer Staging Manual. 6th ed. New York: Springer; 2002.
The supraglottis is composed of the suprahyoid and infrahyoid epiglottis (both the lingual and the laryngeal surfaces), aryepiglottic folds (laryngeal surfaces only), arytenoids, and ventricular bands (false vocal cords). The boundary between the suprahyoid and infrahyoid epiglottis is a horizontal plane passing through the hyoid bone. The inferior limit of the supraglottis is a horizontal plane through the lateral margin of the ventricle at its junction with the superior surface of the true vocal cord. The glottis is composed of the true vocal cords (both the superior and inferior surfaces) and includes the anterior and posterior commissures. The inferior boundary of the glottis is a horizontal plane 1 cm inferior to the inferior limit of the supraglottis (defined as the lateral margin of the ventricle at its junction with the superior surface of the vocal cord). The subglottis extends from the inferior limit of the glottis to the inferior edge of the cricoid cartilage. It is not divided further into any subsites.6
The mucosal lining of the larynx differs in the three regions. The epithelium of the supraglottis is predominantly of the pseudostratified columnar type, except at the edges of the aryepiglottic folds and the lateral borders of the epiglottis, which are stratified squamous epithelium. The supraglottic mucosa has an abundance of mucus glands and lymphatic vessels. The true vocal cords have a unique structure: stratified squamous epithelium covers a three-layered lamina propria (composed of superficial, intermediate, and deep layers). The intermediate and deep layers of the lamina propria make up the vocal ligament, which forms the superior border of the conus elasticus and interdigitates with the vocalis muscle. The true vocal cords (and thus the glottis) have few lymphatic vessels. The subglottis is lined by pseudostratified columnar epithelium, which is in close approximation to the cricoid cartilage and cricothyroid membrane.7
The laryngeal cartilages, hyoepiglottic ligament, thyrohyoid membrane, quadrangular membrane, conus elasticus, anterior commissure, and cricothyroid membrane form natural barriers to the spread of tumor. Within the larynx, the pre-epiglottic space (PES) and the paraglottic space (PGS) provide pathways for spread of laryngeal tumors (Fig. 107-2). The boundaries of the PES are: anteriorly, the thyroid cartilage and thyrohyoid membrane; superiorly, the hyoid bone, hyoepiglottic ligament, and valleculae; posteriorly, the anterior surface of the epiglottic cartilage and the thyroepiglottic ligament; and laterally, the PES is open and continuous with each of the two PGSs. The PES contains fat and areolar tissue7 and is frequently invaded by tumors because the cartilage of the epiglottis has multiple small fenestrations through which cancers arising from the infrahyoid epiglottis may pass. Superiorly, the hyoepiglottic ligament provides a barrier to spread of tumor to the tongue base (Fig. 107-3). The lymphatics of the PES drain through the thyrohyoid membrane, spreading to lymph nodes on both sides of the neck, primarily in zones II and III (Fig. 107-4).5 Supraglottic tumors with PES involvement are staged as T3 lesions.
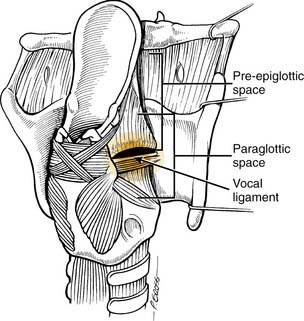
Figure 107-2. Posterior oblique view of larynx showing confluence of pre-epiglottic and paraglottic spaces.
(Reprinted with permission from Myers EN, Suen JY, Myers JN, Hanna EYN. Cancer of the Head and Neck. 4th ed. Philadelphia: WB Saunders; 2003, Fig. 15-21.)
The PGS lies lateral to the true and false vocal folds and extends laterally to the thyroid cartilage (Fig. 107-5). The boundaries of each PGS are: medially (from superior to inferior), the quadrangular membrane, laryngeal ventricle, and conus elasticus; laterally, the thyroid cartilage (anteriorly) and the mucosa of the medial wall of the pyriform sinus (posteriorly); and inferolaterally, the cricothyroid membrane.8 Anteriorly, each PGS is continuous with the PES, and tumors may spread along this pathway (see Fig. 107-2). Paraglottic space involvement in either a glottic or supraglottic tumor is staged as T3 and is significant because the extent of the PGS means that tumors in this space may spread to involve any or all of the three regions of the larynx (Fig. 107-6).
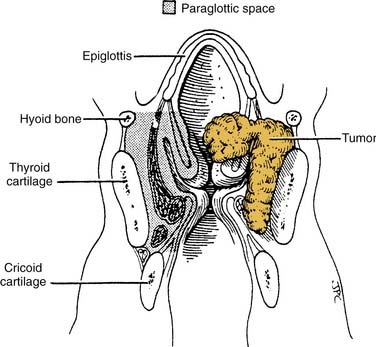
Figure 107-5. Dimensions of the paraglottic space located between the mucosa of the larynx and its cartilaginous framework.
(Reprinted with permission from the American Academy of Otolaryngology—Head and Neck Surgery; from Myers EN, Alvi A. Management of carcinoma of the supraglottic larynx: evolution, current concepts, and future trends. Laryngoscope. 1996;106:561.)
Conservation laryngeal surgery is undertaken on the basis of the theory of compartmentalization of the larynx, which evolved from the work of Frazer,4 Pressman and colleagues,5 and Tucker and Smith.9 Pressman and colleagues found that this separate embryologic derivation explains why supraglottic tumors of substantial bulk do not spread across the laryngeal ventricle to the vocal cord. In experiments using submucosal vital dyes and radioisotopes, Pressman also noted that the inferior extent of supraglottic injection was the inferior false vocal cord. The ventricle was an anatomic barrier to the inferior flow of the dye and thus was confirmed as a barrier to tumor spread. Using animals, cadavers, and whole organ serial sections of human tumor specimens, Tucker and Smith confirmed that elastic tissue barriers within the larynx explained the findings of the dye studies. Although these studies confirm compartmentalization of the larynx and clinically supraglottic tumors are observed to invade the glottis (and vice versa) uncommonly, there is no true anatomic barrier separating the supraglottis from the glottis.
Transglottic Cancer
Transglottic tumors are an important subset of laryngeal tumors with aggressive behavior and high risk of lymphatic metastasis. The term transglottic was first used by McGavran and associates in 1961.10 It is not used in the American Joint Commission and associates for Cancer (AJCC) staging system and is defined by Kirchner and colleagues and colleagues as a tumor that crosses the ventricle in a vertical direction.11 LeRoux-Robert probably first described this type of cancer, proposing that the site of origin was the ventricle and was the only tumor that invaded supraglottic and subglottic areas.12 Kirchner and colleagues11 have shown that transglottic tumors do not necessarily arise from the ventricle. Tumors can become transglottic in four ways: by crossing the ventricle directly; by crossing at the anterior commissure; by spreading through the paraglottic space; and by spreading along the arytenoid cartilage posterior to the ventricle.11,13 The latter form of spread does not predict deep invasion: in Kirchner’s series of 50 transgottic tumors studied in whole organ preparations, none of the 8 tumors with transglottic spread along the arytenoid demonstrated laryngeal cartilage invasion. In the same series, invasion of the laryngeal framework was seen in over half of transglottic tumors over 2 cm. Cervical metastases were seen in 30% of cases; and in primary tumors greater than 4 cm in dimension, 55% of tumors had nodal metastases.11
Anterior Commissure
The anterior commissure is the part of the glottis in which the true vocal cords meet anteriorly. The anterior commissure tendon is a band of fibrous tissue 1 mm wide and 10 mm in length extending from the vocal ligaments to the midline of the inner surface of the thyroid cartilage. At this insertion, the thyroid cartilage is devoid of perichondrium.14 In this area the true vocal cords are therefore in close approximation to the thyroid cartilage. Kirchner has studied factors associated with spread of tumors along the anterior commissure.15 The anterior commissure tendon forms a strong barrier to spread of cancer. Tumors crossing from one true vocal cord to the opposite cord do not necessarily have deep invasion. Thyroid cartilage invasion or extralaryngeal spread of cancer at the anterior commissure requires significant supraglottic or infraglottic extension. Superiorly, tumors have access to the petiole of the epiglottis and pre-epiglottic space, while inferiorly they have access to subglottic lymphatics, the thyroid cartilage, and the cricothyroid membrane. Inferiorly, the thyroid cartilage is more likely to be ossified, and ossified cartilage is not as strong a barrier to invasion as cartilage.16
Classification of Malignant Tumors of the Larynx
Although the vast majority of malignant tumors of the larynx originate from squamous epithelium, a small number arise from other tissues within the larynx. Because of the differing clinical behavior of these tumors, an accurate histologic diagnosis is crucial. Recognizing the importance of this issue and the need for standardization in the histologic nomenclature of laryngeal tumors, the World Health Organization (WHO) has published a histologic classification of laryngeal tumors.17 This classification system is outlined in Table 107-2. Non–squamous cell laryngeal malignancies are described individually later in the chapter.
Table 107-2 Differential Diagnosis of a Laryngeal Mass/Lesion
|
Primary Laryngeal Malignancies |
Staging
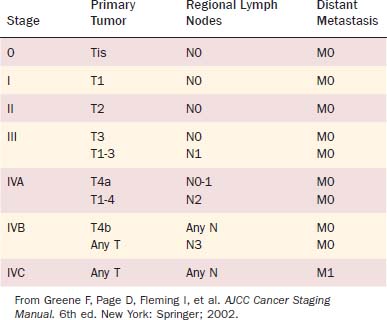
For the staging of malignant tumors, the AJCC and the International Union against Cancer (UICC) use the TNM classification system, which was developed by Pierre Denoix in 1943.6,18 The TNM system takes into account the extent of the primary tumor (T), the absence or presence and extent of regional lymph node metastasis (N), and the absence or presence of distant metastases (M). The 2002 AJCC/UICC TNM definitions for epithelial laryngeal malignancies are given in Table 107-3. Using this system, tumors with varying combinations of T, N, and M are grouped into stages, which are described in Table 107-4. A tumor may be classified clinically, designated cTNM or simply TNM, or pathologically, designated pTNM. The clinical classification is based on evaluation of the patient before treatment is commenced and includes information obtained from physical examination, radiologic imaging, endoscopy, and biopsy.
Table 107-3 Tumor Node Metastasis System for the Larynx (Epithelial Malignancies)6
| Primary Tumor (T) | |
| Tx | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| Supraglottis | |
| T1 | Tumor limited to one subsite of supraglottis with normal vocal cord mobility |
| T2 | Tumor invades mucosa of more than one adjacent subsite of supraglottis or glottis or region outside the supraglottis (e.g., mucosa of base of tongue, vallecula, medial wall of pyriform sinus) without fixation of the larynx |
| T3 | Tumor limited to larynx with vocal cord fixation and/or invades any of the following: postcricoid area, pre-epiglottic tissues, paraglottic space, and/or minor thyroid cartilage erosion (e.g., inner cortex) |
| T4a | Tumor invades through the thyroid cartilage and/or invades tissues beyond the larynx (e.g., trachea, soft tissues of neck including deep extrinsic muscles of the tongue, strap muscles, thyroid, or esophagus) |
| T4b | Tumor invades prevertebral space, encases carotid artery, or invades mediastinal structures |
| Glottis | |
| T1 | Tumor limited to the vocal cord(s) (may involve anterior or posterior commissure) with normal mobility |
| T1a | Tumor limited to one vocal cord |
| T1b | Tumor involves both vocal cords |
| T2 | Tumor extends to supraglottis and/or subglottis, or with impaired vocal cord mobility |
| T3 | Tumor limited to the larynx with vocal cord fixation, and/or invades paraglottic space, and/or minor thyroid cartilage erosion (e.g., inner cortex) |
| T4a | Tumor invades through the thyroid cartilage and/or invades tissues beyond the larynx, (e.g., trachea, soft tissues of neck including deep extrinsic muscles of the tongue, strap muscles, thyroid, or esophagus) |
| T4b | Tumor invades prevertebral space, encases carotid artery, or invades mediastinal structures |
| Subglottis | |
| T1 | Tumor limited to the subglottis |
| T2 | Tumor extends to vocal cord(s) with normal or impaired mobility |
| T3 | Tumor limited to larynx with vocal cord fixation |
| T4a | Tumor invades cricoid or thyroid cartilage and/or invades tissues beyond larynx (e.g., trachea, soft tissues of neck including deep extrinsic muscles of the tongue, strap muscles, thyroid, or esophagus) |
| T4b | Tumor invades prevertebral space, encases carotid artery, or invades mediastinal structures |
| Regional Lymph Nodes (N) | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in a single ipsilateral lymph node, ≤3 cm in greatest dimension |
| N2 | Metastasis in a single ipsilateral lymph node, >3 cm but not >6 cm in greatest dimension, or in multiple ipsilateral lymph nodes, none >6 cm in greatest dimension, or in bilateral or contralateral lymph nodes, none >6 cm in greatest dimension |
| N2a | Metastasis in a single ipsilateral lymph node, >3 cm but not >6 cm in greatest dimension |
| N2b | Metastasis in multiple ipsilateral lymph nodes, none >6 cm in greatest dimension |
| N2c | Metastasis in bilateral or contralateral lymph nodes, none >6 cm in greatest dimension |
| N3 | Metastasis in a lymph node, >6 cm in greatest dimension |
| Distant Metastasis (M) | |
| MX | Distant metastasis cannot be assessed |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
From Greene F, Page D, Fleming I, et al. AJCC Cancer Staging Manual. 6th ed. New York: Springer; 2002.
An additional comment regarding the T staging of glottic tumors should be made. The T2 classification for glottic tumors encompasses a wide variety of lesions including those with normal and impaired vocal cord mobility (vocal cord fixation denotes a T3 tumor). Because the local control rate of RT for T2 glottic tumors with impaired vocal cord mobility has been observed to be lower than that of T2 tumors with normal vocal cord mobility, many authors distinguish between these two subsets of the T2 group by using T2a to denote those tumors with normal vocal cord mobility and T2b for those with impaired mobility.19 This division of the T2 stage has not been incorporated into the AJCC/UICC TNM system.
Premalignant Laryngeal Lesions and Carcinoma in Situ
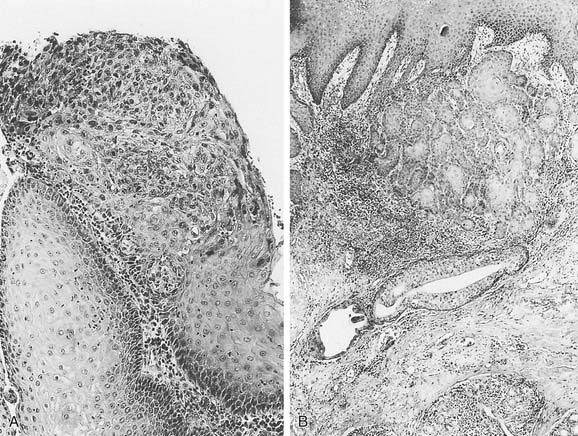
The World Health Organization (WHO) classifies premalignant laryngeal lesions as hyperplasia; keratosis; mild, moderate, or severe dysplasia; and carcinoma in situ (CIS).17 Very early lesions may demonstrate hyperkeratosis or parakeratosis without cellular atypia or dysplasia. Squamous cell dysplasia is a premalignant lesion of squamous epithelium characterized by cellular atypia and loss of normal maturation and stratification short of CIS. The cellular abnormalities of mild dysplasia are minor and are limited to the basal one third of the epithelium; moderate dysplasia displays more marked cellular abnormalities involving up to two thirds of the epithelial thickness; and severe dysplasia has cellular abnormalities involving more than two thirds of the epithelial thickness. Carcinoma in situ is an intraepithelial neoplasm in which the full thickness of the squamous epithelium shows the cellular features of carcinoma without violation of the basement membrane and stromal invasion (Fig. 107-7).17 Severe dysplasia and CIS are classified as different lesions, but clinically they behave in a similar fashion and may be considered as one.20 There is widespread variability in pathologic interpretation and diagnosis, which is inherently subjective.21,22
Malignant transformation of mild dysplasia has been reported to be as high as 11%.23 The malignant transformation rates for severe dysplasia and CIS show significant variability (up to 45%), but 30% is the generally accepted figure. A recent review noted that 30% of patients with dysplastic lesions that progressed to invasive cancer eventually underwent total laryngectomy.23 The likelihood of progression is related to the extent of disease, and at least one series observed that almost all diffuse lesions showing CIS developed invasive cancer.24 Because some lesions showing mild dysplasia and even those without dysplasia may progress to invasive cancer, long-term follow-up of all premalignant laryngeal lesions is warranted. Malignant transformation is a multistep process that takes many years. Blackwell and colleagues25 observed a 3.9-year interval between initial biopsy to progression to invasive cancer. In a large series of over 1000 keratotic larynx lesions, the mean time interval to malignant transformation was 3.1 years.26 The latency was longer for earlier lesions, and 7% of lesions transformed to invasive carcinoma more than 10 years after the initial biopsy.
The visual appearance of a premalignant laryngeal lesion does not predict its histologic nature. Nor does laryngeal videostroboscopy reliably differentiate premalignant from malignant lesions.27 Biopsy is the gold standard for diagnosis, and adequate sampling is important because an insufficient biopsy will result in sampling error.
Adjunctive techniques have been developed to improve the clinician’s ability to characterize these lesions and to guide biopsies. The use of vital dyes including toluidine blue and methylene blue as a diagnostic aid have been explored.28,29 Lundgren and coworkers used toluidine blue to detect dysplasia or malignant changes and noted 91% sensitivity, but only 52% specificity.29 Contact endoscopy provides magnification of 60 to 50× and, with methylene blue staining, provides histologic information and an assessment of vascular patterns.30 However, subsurface visualization is limited to 150 to 200 microns and is inadequate for characterizing thicker lesions. Widespread adoption of this technique has been hindered by the need for extensive experience to interpret the findings and for special equipment.
Human tissue has many compounds that fluoresce when exposed to blue light. The differing fluorescence of abnormal tissues has been exploited as a diagnostic aid for laryngeal malignancy. With experience, this technique can be useful. Limitations include false-positive and false-negative examinations in scarring, hyperkeratotic lesions, and inflammation.31 Induced autofluorescence using 5-aminolevulinic acid (5-ALA) also shows potential diagnostic utility. 5-ALA selectively induces accumulation of protoporphyrin IX fluorescence in tumors. Using a nebulizer, it is topically applied 1 to 2 hours before the examination and a light source emitting short wavelength visible light (375 to 440 nm) is used to induce fluorescence. Protoporphyrin IX fluorescence appears red during imaging. A series of 16 patients with suspected or proven laryngeal malignancy subjected to 5-ALA–induced fluorescence endoscopy demonstrated a sensitivity of 95% and a specificity of 80%.28
A prospective evaluation of autofluorescence and 5-ALA–induced fluorescence in 56 patients demonstrated that the two techniques have similar sensitivities (94% and 97%) and specificities (82% and 64%) to distinguish hyperplasia or mild dysplasia from moderate dysplasia, severe dysplasia, CIS, and invasive SCC.32 Autofluorescence was unreliable when scarring was present, whereas 5-ALA–induced fluorescence resulted in false-positive findings in inflamed lesions.
The combined use of autofluorescence and contact endoscopy (“compact endoscopy”) has also been explored. A pilot study by Arens and colleagues of 83 patients with hyperkeratotic, dysplastic, and invasive laryngeal lesions found correlation with histology in 88% of cases.33 Inflammation and scarring resulted in overestimation of disease, whereas underestimation of disease was seen in hyperkeratotic lesions.
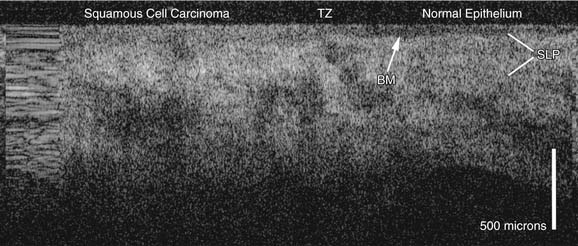
Current noninvasive diagnostic modalities do not reliably distinguish premalignant lesions from invasive SCC. Optical and microscopic imaging is limited by the inability to see below the first few layers of epithelial cells to evaluate the submucosal architecture. Infrared light has increased tissue penetrance and can provide diagnostic information about subsurface tissues. Optical coherence tomography (OCT) is a new diagnostic modality under investigation to examine the epithelial and subepithelial architecture that uses near-infrared light waves to produce cross-sectional images of tissue in vivo, with a resolution approaching that of histology.34,35 In the larynx, OCT clearly identifies basement membrane violation from laryngeal cancer and is able to identify transition zones at the margins of the cancer (Fig. 107-8).36 OCT has been used to assess the larynx intraoperatively to guide the extent of transoral laser resections for laryngeal cancer to ensure that excision is complete.37 The primary disadvantage of OCT is the limited depth of penetration (<2 mm), which limits its utility in bulky lesions. However, OCT has potential as a useful tool in the management of laryngeal cancer: for the diagnosis of smaller epithelial lesions, for guiding surgical biopsies and resections, and for monitoring larynges post treatment.36,37
Treatment of Premalignant Lesions
The treatment of a premalignant lesion should aim to eradicate the lesion while preserving voice quality and laryngeal function. Accurate diagnosis is critical for proper decision making, and a number of factors related to the characteristics of the lesion, as well as patient factors, affect the treatment decision-making process (Table 107-5).
Table 107-5 Factors to Consider in Treatment of Premalignant Laryngeal Lesions
| Patient Factors | Tumor Factors |
|---|---|
| General health/comorbidities |
Hyperplastic and dysplastic lesions are excised with microlaryngoscopic techniques to remove the visible lesion. Close follow-up is required due to the risk of recurrence of the lesion and possible malignant transformation. Carcinoma in situ may be treated with either surgery or RT. Surgical management is microlaryngoscopic excision. Critical to this is high-power magnification, fine microsurgical instruments, and meticulous surgical technique to remove the entire lesion and preserve underlying lamina propria. The carbon dioxide (CO2) laser is useful for precise excision, although thermal injury to the underlying lamina propria may result. Vocal cord stripping is the removal of the mucosa of the vocal fold including the lesion from the vocal process up to, but not across, the anterior commissure. It has significant risk of producing scarring and has been superseded by the more controlled microsurgical excision. Surgery provides tissue for pathologic analysis and can be repeated as often as needed. Voice quality is preserved in most cases. The recurrence rate for CIS treated with surgery is greater than those treated with RT and is reported to be approximately 20% after initial excision.23 With repeated surgical excision the ultimate local control rate is excellent and is at least equivalent to that of RT.38,39 Surgical treatment is preferred for focal lesions in patients who are reliable and will attend routine follow-up.
Radiation therapy is also an effective treatment for CIS: a recent review of 16 studies of CIS treated with RT determined that the local control rate was 93.5%.23 Radiation therapy is particularly useful for multiple recurrences following surgical excision; for diffuse lesions extending beyond the vocal cords that cannot be excised without inducing significant morbidity; for patients who are unlikely or unable to attend follow-up; and for medically infirm patients unfit for general anesthesia. Voice quality is well preserved following RT.40,41 The primary disadvantage of RT is that it cannot be repeated if there is a recurrence or second tumor in the irradiated field. The successful management of premalignant laryngeal lesions also requires implementation of tobacco and alcohol cessation strategies and treatment of laryngopharyngeal reflux when present.
In-Office Treatment of Premalignant Lesions
The 585nm PDL and 532nm pulsed KTP lasers are the two most widely used lasers for the in-office treatment of laryngeal epithelial lesions including leukoplakia, keratosis, and dysplasia. In a pilot study, Franco and colleagues42 used PDL for the treatment of dysplasia in the operating room under general anesthesia. In most cases the treated lesions were then excised and sent for histologic examination. This treatment was effective: 81% of patients had greater than 70% regression of their lesions. The authors also noted that regression occurred in treated lesions that were not excised. A subsequent report from the same group demonstrated the efficacy of PDL delivered via a flexible endoscope in the office for the treatment of dysplasia.43 Koufman and colleagues found that 64% (16/25) of their patients with dysplasia required no further treatment during the follow-up period of their study.44 Franco45 has also described favorable experience with the use of PDL for dysplasia. Zeitels and colleagues46 have subsequently adopted the pulsed KTP laser for in-office use, citing smaller fiber diameter, greater reliability, improved intralesional energy absorption, and improved hemostasis as advantages of the pulsed KTP system over PDL.
The energy from these lasers is preferentially absorbed by oxyhemoglobin, with the absorption of energy from the pulsed KTP being superior to that of PDL, causing photoangiolysis of the sublesional blood vessels.46 Preferential destruction of intraepithelial desmosome junctions and separation of the treated epithelial cells from the basement membrane have also been observed microscopically.47 Apart from the obvious advantages of in-office procedures (avoidance of general anesthesia, lower cost, improved efficiency, and patient preference), these lasers have minimal effect on the surrounding tissue and the scarring is uncommon. The bilateral simultaneous treatment of lesions in and around the anterior commissure is possible, with minimal risk of web formation.44
One of the major criticisms of in-office procedures for the treatment of laryngeal lesions, especially dysplasia, is that there is no specimen obtained for pathologic examination, and therefore a definitive assessment of the tumor extent and margin status is not possible. In the reports published to date, all patients have been biopsied before the commencement of treatment.44,46 A biopsy may be obtained in the operating room and initial treatment with the laser given at this time, or it may be taken in the office via the endoscope. Potential complications of in-office treatment include poor exposure of the lesion or poor patient tolerance of the procedure; however, these issues only arise in a small minority of cases.44 Further studies with long-term follow-up are awaited to ensure that this novel technology has similar outcomes for patients with dysplastic laryngeal lesions.
Photodynamic therapy uses nontoxic chemicals that are taken up preferentially by dysplastic or malignant cells. These photoreactive chemicals are activated by light of a specific frequency range unique to the compound used. The light activation of tissues at the target site in the presence of oxygen results in cell death that is confined to the cells that have selectively accumulated the chemical.48 Several agents have been used in the head and neck including 5-ALA, Photofrin, hematoporphyrin derivative, and Foscan. In the United States several photosenstitizers have been approved for use by the Food and Drug Administration, but treatment of head and neck malignancies is an off-label use at the present time. In the European Union, Foscan has been approved for treatment of early head and neck cancer. At present, data are available for approximately 1500 subjects treated with PDT for head and neck SCC. Biel48 reported on 115 patients with Tis to T2 larynx SCC treated with Photofrin PDT. With a mean follow-up of 91 months, the 5-year cure was 91%. All recurrences were salvaged successfully.
Photodynamic therapy has several useful characteristics: it is performed in an outpatient setting and is repeatable. The effects on voice quality are minimal, and scarring of the vocal folds has not been observed.48 Careful light-avoiding precautions are required because light sensitivity lasts up to 4 weeks. There is no pathologic specimen, and care must be taken to accurately assess tumor depth. PDT may have a particularly useful role for diffuse dysplasia or CIS. Schweitzer49 reported 80% local control with PDT in 10 cases of diffuse laryngeal CIS previously treated with RT. Published reports show promise for this modality, but currently it is used by a small number of practitioners and is not widely available.
Chemoprevention of Laryngeal Cancer
Carcinogenesis is a multistep accumulation of genetic damage, the end result of which is the development of cancer.50,51 The early arrest or reversal of carcinogenesis offers the opportunity to make a meaningful impact on cancer in the premalignant disease state, or before development of a second tumor.52,53 Chemoprevention is the use of specific chemical agents to reverse, suppress, or prevent carcinogenesis before invasive cancer develops.54 In numerous studies a low -fat diet and an increase in the consumption of fruits, vegetables, and fiber were associated with a protective effect against a number of cancers.55,56
13-cis-retinoic acid (13-cRA) has been studied extensively in oral leukoplakia.57,58 In randomized placebo-controlled trials, 13-cRA showed encouraging results against oral premalignant lesions. In the first major human trial of 13-cRA completed in 1986, Hong and colleagues58 observed a 67% response rate of oral leukoplakia in the treatment arm versus a 10% response rate for the placebo group. Significant side effects were seen and, unfortunately, mucosal lesions recurred in half of the patients receiving 13-cRA within 3 months following discontinuation of 13-cRA. In an effort to improve effectiveness and decrease toxicity, a multiagent trial using 13-cRA, alpha tocopherol, and interferon-α was conducted at the same institution. A complete remission was observed in 50% (7/14) of laryngeal lesions at 12 months. Subsequent analysis of biomarkers found the laryngeal lesions tended to have a lower level of chromosomal polysomy, and the level of chromosomal polysomy correlated with higher response rates to the three agents. There was also a trend toward a better response rate in cases without abnormal p53 accumulation, presumably reflecting less advanced disease.
A number of other agents show potential as chemopreventive agents for head and neck cancers including green tea polyphenols, sulindac, COX II inhibitors, curcumin, and protease inhibitors.54 None have undergone the same level of study as the retinoids, and considerable effort will be required to determine the ultimate potential of promising agents.
Squamous Cell Carcinoma of the Larynx
Squamous cell carcinoma is the most common malignant tumor of the larynx, responsible for between 85% and 95% of all laryngeal malignancies.2,59 This tumor arises from stratified squamous epithelium or from respiratory epithelium that has undergone squamous metaplasia. The incidence of SCC in each of the three regions of the larynx—the supraglottis, glottis, and subglottis—varies according to the patient population. In the United States, Canada, England, and Sweden, glottic SCC is more common than supraglottic SCC, whereas the reverse is true in France, Italy, Spain, Finland, and the Netherlands. In Japan, glottic and supraglottic SCC have a similar incidence. Primary subglottic SCC is rare in all populations.2 In a large review of nearly 160,000 cases of laryngeal SCC in the United States, the glottis was the site of origin in 51%, the supraglottis in 33%, and the subglottis in 2% (with 14% of lesions unable to be categorized accurately).60
Epidemiology
In the United States, approximately 11,300 cases of laryngeal cancer were diagnosed in 2007 and there will be approximately 3660 deaths. The male-to-female incidence ratio is 3.8:1.1 The epidemiology and risk factors for laryngeal cancer largely parallel those for head and neck cancer overall. Over 90% of cancers occur in those older than 40 years of age, and 85% to 95% of these are SCC. The higher incidence in males is due to an increased exposure to risk factors rather than an inherent gender predilection, and therefore as the number of females smoking has increased over the past 60 years, the gap has narrowed from over 15:1 to approximately 4:1. There does not appear to be a racial predilection to laryngeal cancer.61
Risk Factors
Tobacco and Alcohol
Tobacco and alcohol use are the two primary risk factors for cancer of the larynx. The International Agency for Research on Cancer (IARC) has concluded that there is enough evidence to state that there is a causal link between tobacco use and alcohol use with development of head and neck cancer. The risk is proportional to the intensity and duration of tobacco or alcohol consumption, and the risk decreases slowly after cessation but does not return to the baseline rate for at least 20 years. There are variations in risk with the type of tobacco exposure (e.g., cigar vs. cigarette, filtered vs. nonfiltered cigarettes), but the most important factors are the amount of tobacco consumed and the duration of exposure. Tobacco and alcohol act synergistically to increase the risk of cancer.62–64 The relative contribution of alcohol and tobacco varies by site. Alcohol consumption is a more important risk factor for supraglottic carcinoma, whereas tobacco use is strongly associated with glottic carcinoma.
Laryngopharyngeal Reflux
Chronic irritation of the larynx has been proposed as a risk factor for laryngeal cancer and may be a contributing factor for those who do not smoke or drink. Concern that laryngopharyngeal reflux (LPR) of acid could cause cancer was raised in the 1980s.65,66 It is difficult to determine if the relationship is causal or merely an association. A large case-control study of U.S. veterans concluded that there is a moderately increased risk for laryngeal or pharyngeal cancer associated with LPR independent of tobacco and alcohol use.67 A critical review of the literature indicates that the relationship between LPR and larynx cancer is unknown. LPR could be an associated factor, a cocarcinogen, or possibly an independent risk factor.68 Alkaline bile reflux may also be a causative factor. One study identified a significantly higher incidence of laryngeal carcinoma in patients who underwent gastrectomy compared with controls.69 The significance of asbestos exposure is controversial. A meta-analysis published in 1999 concluded there was a weak association between asbestos exposure and laryngeal cancer.70
Other Toxins
Occupational exposure to toxins is another risk factor for laryngeal cancer. The incidence of laryngeal cancer is higher in unskilled manual workers who have both a high alcohol and tobacco intake and are exposed to disproportionate levels of potential toxins.71–73 Numerous agents including diesel exhaust, asbestos, organic solvents, sulfuric acid, mustard gas, certain mineral oils, metal dust, asphalt, wood dust, stone dust, mineral wool, and cement dust have been implicated as risk factors for laryngeal cancer. Establishing a link between any one toxin and larynx cancer has been difficult because the studies have been underpowered and plagued with confounding variables.74
Human Papillomavirus
Human papillomavirus (HPV) has been recognized as a causative factor for cervical cancer. The role of HPV in cervical cancer is well studied, and several HPV vaccines are undergoing evaluation for prevention of cervical cancer. The link between HPV and head and neck SCC was first suggested in the early 1980s.75 Further studies have brought to light that HPV is a significant and increasing risk factor for head and neck cancer, particularly in the oropharynx. HPV is an independent risk factor for head and neck SCC.76 The association of sexual practices and risk of head and neck cancer has become clear over the past decade, and a recent study has emphasized the role of HPV on a subset of head and neck cancer patients.77 Vaccination against HPV has been shown to be highly effective at preventing cervical HPV infection.78 If the vaccine can also prevent oropharyngeal infection, then the vaccination of children has the potential for significantly reducing the risk of oropharyngeal cancer and possibly cancer at other sites in the head and neck. The mechanism for carcinogenesis is believed to be the E6 and E7 viral protein-mediated degradation of p53, which is involved in maintaining genomic integrity, controlling cellular proliferation, and apoptosis. A recent review shows a site-specific link between HPV and head and neck cancer: the magnitude of the association is strongest for the tonsil (Odds Ratio [OR] 15.1), intermediate for other subsites in the oropharynx (OR 4.3), and weakest for the oral cavity (OR 2.0) and the larynx (OR 3.0, 95%, confidence interval [CI] 1 to 4.2).79 The influence of HPV as a risk factor for larynx cancer is lower than for the oropharynx. Nevertheless, in one series, HPV was isolated in 36% of laryngeal cancers.80 Evidence suggests that the presence of HPV in a head and neck SCC is associated with a better prognosis.81 This observation is supported by a recent meta-analysis of the effect of HPV on head and neck cancer survival, but the effect is limited to oropharyngeal SCC.82
Genetic Susceptibility
Most smokers will die prematurely as a result of tobacco use, but only a minority will develop cancer. Genetic susceptibility to cancer clearly plays a role in risk for development of head and neck, as well as other cancers, but the risks are not clearly delineated. Epidemiologic studies can stratify risk for developing head and neck cancer and other cancers, and biomarkers are under development to identify molecular changes associated with cancer development. These assays provide markers for genetic susceptibility to cancer (e.g., mutagen sensitivity) and have the potential to provide prognostic information about response to treatment.83 Polymorphisms of phase I and phase II detoxifying enzymes are also associated with risk of developing head and neck cancer, as well as polymorphisms of DNA repair enzymes. These studies are useful for understanding the pathogenesis of cancer but currently do not provide assistance to guide the clinician managing individual patients.
The discovery of familial cancer syndromes drew attention to the role of genetic susceptibility to developing cancer.84 Only a small proportion of head and neck cancers arise from familial syndromes (e.g., xeroderma pigmentosum); however, studies of head and neck cancer demonstrate an elevated risk of head and neck cancer in family members. Copper and colleagues found a relative risk (RR) of 14.6 for respiratory tract cancers in the siblings of cancer patients and an overall RR of 3.5 for first-degree relatives.85 The host ability to tolerate exposure to carcinogens is highly variable and is related to the ability to prevent activation of procarcinogens, inactivate active carcinogens, repair deoxyribonucleic acid (DNA) damage, and maintain immune surveillance. These factors are highly variable among individuals and are still poorly understood.
Diet
There is evidence linking dietary factors to the risk of head and neck cancer.86,87 An increased intake of fruit and vegetables and a decreased intake of meat and fat have a protective effect and are associated with a reduced incidence of head and neck cancer, as well as colon cancer and cardiovascular disease. However, these benefits may take 20 years or longer to accrue, and at present these epidemiologic observations have not led to the successful development of a cancer reduction program.88,89
Second Primary Tumors
The single greatest risk factor for head and neck SCC is prior head and neck SCC. The annual risk of a second primary tumor (SPT) following an index head and neck SCC is 1% to 7%, with the risk persisting for at least 10 years.57,90–92 The cumulative risk of developing an SPT is at least 20% and is greater for those who continue to use tobacco and alcohol. An individual with stage I or II head and neck SCC is more likely to die from an SPT than from the index tumor. The majority of SPTs develop in the head and neck, but a significant proportion occur in the esophagus or lung. Second primary tumors may be synchronous (identified within 6 months of the index tumor) or metachronous (diagnosed more than 6 months after the index tumor). In a study of 875 patients with SCC of the head and neck, 207 had SPTs develop within 5 years of their index tumor.93 Of these patients, 31% had a third primary malignancy develop and 10% had a fourth primary malignancy develop. For laryngeal cancer, the lung is a significant site for synchronous and metachronous SPTs. An isolated lung malignancy in a patient with larynx cancer is more likely to be an SPT than a metastasis from the laryngeal cancer. Therefore an isolated pulmonary nodule should be considered an SPT until proven otherwise.92 Prior RT is associated with SPTs in a small number of cases.74 Slaughter and associates94 examined clinically normal tissue adjacent to head and neck cancers and identified many histologic changes seen in the malignant cells in the adjacent normal appearing tissue, leading him to propose the concept of “field cancerization.” Improved understanding of head and neck carcinogenesis has provided a molecular explanation for Slaughter’s observations.50,95 Bedi and colleagues examined X-chromosome inactivation and performed microsatellite analysis to evaluate allelic loss at chromosomes 3p and 9p in females with multiple primary head and neck cancers. Both the original cancer and the second malignancy arose from a single clone.95 Califano and coworkers similarly observed that tissues adjacent to malignant and premalignant lesions shared common genetic changes.50 Multiple tumors generally do not arise from multiple transforming events, but instead a single transforming event produces a cell with growth advantage that spreads throughout the mucosal surface. The tumor may accumulate further genetic damage, resulting ultimately in additional malignancies that are geographically distinct but genetically related to the original cancer.95
Molecular Biology
The molecular biology of laryngeal SCC is similar to that of SCC in other sites of the head and neck. Carcinogenesis is a long process occurring over many years preceding the development of cancer. Disruption of multiple genes is required to induce malignant transformation, with 6 to 12 distinct mutations generally necessary to produce malignancy. Understanding the molecular biology of head and neck cancer is useful to predict who is likely to develop cancer, to measure response to preventive agents, to identify novel targets for treatment, and to predict a patient’s response to RT or chemotherapy. The molecular biology of head and neck cancer is discussed in Chapter 76.
Pathology
Squamous differentiation is the hallmark of SCC, which is characterized by the formation of keratin and/or the presence of intercellular bridges.17 SCC is graded by its histologic appearance into three categories: well, moderately, and poorly differentiated. Well-differentiated SCC resembles normal squamous epithelium and contains basal-type cells and squamous cells with keratinization and intercellular bridges. The nuclei are hyperchromatic and irregular in size and shape (pleomorphic), and the nuclear-cytoplasmic ratio is reduced. Atypical mitoses are rare. Moderately differentiated SCC has less keratinization, more atypical mitoses, and more nuclear pleomorphism. Intercellular bridges are present. Poorly differentiated SCC has minimal (if any) keratinization, minimal intercellular bridges, and numerous atypical mitoses.17,59 The histologic grade has been reported as having prognostic value; however, grading is subjective, and sampling error may influence the grade assigned.
SCC breaches the basement membrane of the epithelium to invade the underlying tissue. The interface between the tumor and the adjacent normal tissue varies according to the pattern of invasion, which may be expansive, characterized by well-defined pushing margins, or infiltrative, characterized by poorly defined margins with occasional cells or tongues of tumor found in the tissue adjacent to the tumor. The latter pattern of invasion is associated with a worse prognosis.96 A lesion in which the entire thickness of the epithelium shows the cellular features of carcinoma without invasion of the underlying stroma is termed SCC in situ. Microinvasive SCC refers to SCC in which limited tumor invasion is confined to the area just deep to the basement membrane.17
SCC expresses epithelial markers such as cytokeratin and epithelial membrane antigen.59 These markers are detected by immunohistochemistry, which is used to differentiate between malignant tumors of similar histologic appearance.
The pathologic diagnosis of SCC is usually straightforward; however, two entities in particular can be difficult to distinguish from SCC. Pseudoepitheliomatous hyperplasia (PEH) is characterized by an overgrowth of squamous epithelium that histologically mimics carcinoma. It can be a primary process or a secondary histologic finding associated with chronic irritation, trauma, infection, or giant cell tumor. The epithelium does not demonstrate cytologic evidence of malignancy; however, elongation of the rete ridges may simulate invasion when specimens are cut tangentially. Properly oriented specimens and careful examination will usually distinguish PEH from SCC, but immunostaining may aid differentiation of the two entities.17,97
Necrotizing sialometaplasia is thought to be the result of infarction of salivary tissue. It is extremely uncommon in the larynx, but a few cases have been reported.98 It may develop in the larynx after ischemia or trauma and is characterized by squamous metaplasia of the ducts and acini of seromucinous glands, which may be confused with SCC or mucoepidermoid carcinoma. This lesion resolves spontaneously. Immunohistochemistry may be required to differentiate necrotizing sialometaplasia from SCC and certain nonsquamous malignancies such as mucoepidermoid carcinoma, neuroendocrine carcinoma, malignant melanoma, or lymphoma, which may have a similar appearance when examined histologically.17,99
Clinical Presentation
The symptoms of laryngeal SCC depend on the site from which the primary tumor originates. The cardinal symptom of glottic SCC is dysphonia, which develops early in the natural history of the disease as the normal vibratory characteristics of the vocal cord are altered by even a small lesion. Therefore patients with glottic SCC usually present with earlier stages of disease, although if the early symptoms are ignored or attributed to other diagnoses, symptoms of advanced disease such as dyspnea and stridor may arise. Glottic tumors remain localized in the glottis for prolonged periods, owing to the natural barriers to tumor spread (ligaments, membranes, and cartilages) and to the relative paucity of glottic lymphatics. Supraglottic tumors may cause dysphonia (often an alteration in vocal resonance), dysphagia, odynophagia, otalgia, stridor, dyspnea, or hemoptysis. Patients with supraglottic SCC may also present with metastatic cervical adenopathy, without obvious laryngeal symptoms, because the supraglottic larynx has a rich lymphatic drainage network. SCC of the subglottis often presents with advanced-stage disease. Dyspnea and stridor are the most common symptoms of subglottic SCC. Because their onset is usually gradual and insidious, subglottic SCC may be misdiagnosed as asthma or other pulmonary diseases.100,101
On examination of the larynx, SCC may appear as an ulcerative, exophytic, sessile, or polypoid lesion. However, in cases of ventriculosaccular SCC (which is uncommon), an epithelial lesion may not be seen in the early stages because the carcinoma arises from the ventriculosaccular system, which lies within the paraglottic space.102 Fullness of the vestibular fold may be the only obvious examination finding. In this scenario, deep biopsies of the vestibular fold are necessary to confirm or exclude the diagnosis of malignancy. A second clinical presentation, in which occult laryngeal SCC may be the underlying etiology, is that of the laryngocele. An association between laryngoceles and SCC has been recognized for some time.103 In patients with laryngoceles, direct laryngoscopy with careful inspection of the ventricle using angled endoscopes is mandated to exclude an SCC of the ventricle.
Cervical Nodal Metastases
The incidence of cervical metastases from SCC of the larynx, as well as the nodal groups involved, vary according to the site of the primary tumor (Fig. 107-9). Due to its rich network of lymphatics, supraglottic SCC has the highest incidence of regional metastases—both clinically apparent and occult metastases. Cervical metastasis has been confirmed pathologically in 10% of T1 supraglottic lesions, 29% of T2, 38% of T3, and 57% of T4.104 The incidence of occult metastases (cN0, pN+) in supraglottic SCC varies from 12% to 40% for all T stages.105–108 The incidence of occult metastases is greater in tumors with a higher T stage: T1 0% to 14%, T2 20% to 21%, T3 28% to 35%, and T4 40% to 75%.106,109 Supraglottic SCC usually metastasizes to levels II, III, and IV. Levels I and V are involved by metastases rarely and only when other nodal levels are also involved.110,111 Bilateral metastases (palpable and occult) occur frequently in supraglottic SCC and are more common in midline or bilateral tumors.104,112 Therefore, in cases of supraglottic SCC, surgical treatment of the N0 and N1 neck is usually with a bilateral selective neck dissection (levels II-IV). For N2 or N3 disease, a comprehensive neck dissection (levels I-V) is indicated.
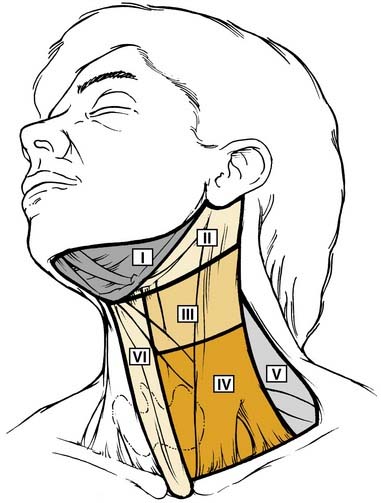
Figure 107-9. The six nodal levels of the neck.
(Redrawn from Robbins KT, Clayman G, Levine PA et al. Neck dissection classification update: revisions proposed by the American Head and Neck Society and the American Academy of Otolaryngology–Head and Neck Surgery. Arch Otolaryngol-Head Neck Surg. 2002;128:751-758, Figure 1).
Glottic SCC has a low risk of cervical metastasis: in a series of 910 patients, the overall incidence of pathologically confirmed nodal metastasis was 5.9%, with an incidence of occult metastasis of 18%.113 Similar to supraglottic SCC, the incidence of regional metastasis correlates with T stage. In the same series, nodal metastases were found in 0.1% of T1 tumors (one patient only), 5% of T2, 18% of T3, and 32% of T4.113 The nodes at risk of metastasis from glottic SCC are those in levels II, II, and IV, as well as level VI (prelaryngeal, pretracheal, and paratracheal nodes). Bilateral or contralateral metastases are rare.
Primary subglottic carcinoma is rare, and descriptions of the clinical behavior of these tumors are based on a small number of patients. The paratracheal nodes (level VI) are most frequently involved with metastases, including contralateral or bilateral metastases. Metastases to levels III, IV and V are uncommon. Although these tumors are aggressive and have a poor prognosis, the incidence of cervical metastasis is generally reported to be low, with a range of 4% to 27%.101,114 However, Harrison115 detected metastatic tumor in approximately 50% of serially sectioned paratracheal nodes. Metastases to mediastinal lymph nodes are common (up to 46%), but they are classified as distant metastases.116
Distant Metastases
Distant metastases from laryngeal SCC include not only hematogenous metastases to distant organs, but also lymphatic metastases to nodal groups outside the neck.59 The most common site for distant hematogenous metastases is the lung. The liver and skeletal system (ribs, vertebrae, and skull) are affected less often. The mediastinum is the most common site for distant lymphatic metastases.117 Distant metastases are uncommon at initial presentation. Patients who develop distant metastases have almost always had regional metastases diagnosed at some stage in the course of their disease. The incidence of distant metastasis varies according to the site of the primary tumor: 3.1% to 8.8% in glottic SCC, and 3.7% to 15% in supraglottic SCC.117–121 Supraglottic SCC usually has a higher incidence of distant metastases compared with glottic SCC. The frequency of distant metastases from subglottic SCC is less certain because primary tumors in this site are rare; however, Spector and colleagues117 observed that 14.3% of subglottic SCCs developed distant metastases. Clinical and pathologic factors associated with an increased risk of distant metastases include advanced stage of primary tumor (especially T4 stage); presence of cervical metastases (especially N2 and N3 disease); duration, level, and extracapsular spread of cervical metastases; and locoregional recurrence.117,121,122 Lymphatic metastasis to the skin is also a sign of advanced disease and, similar to distant metastases, has a grave prognosis.123
Diagnosis and Evaluation
History and Physical Examination
Indirect laryngoscopy (IDL) using a mirror may provide an excellent three-dimensional view of the larynx and any lesions. However, images are not recorded, the hypopharynx is not adequately visualized, and in patients who do not tolerate IDL, the larynx cannot be evaluated completely. Flexible fiberoptic laryngoscopy permits photographic and video documentation, as well as examination during dynamic maneuvers (e.g., Valsalva’s maneuver, coughing, swallowing). Laryngeal videostroboscopy is useful for the documentation of small lesions on the vocal fold and assessment of the mucosal wave before and after treatment. However, videostroboscopy cannot reliably distinguish intraepithelial neoplasia from invasive carcinoma, nor is it reliable for determining the depth of invasion of vocal cord carcinoma.124
Imaging
Radiologic imaging is a critical part of the evaluation of a patient with a suspected laryngeal malignancy.125 When feasible, imaging is performed before operative endoscopy and biopsy to obtain images before potential edema and distortion from biopsy and manipulation of the larynx. Information obtained with imaging can be integrated with endoscopic findings and, in some cases, can guide the surgeon to focus attention on deeper areas not normally visible on endoscopy requiring a biopsy. Cross-sectional imaging, using either computed tomography (CT) scanning or magnetic resonance imaging (MRI), yields the most useful information in the evaluation of tumors of the larynx.
Computed tomography is the most commonly used radiologic investigation in the evaluation of laryngeal cancer, although MRI is superior in some situations. CT is superior to MRI for imaging bony structures (such as ossified cartilages) and calcifications.125 MRI is superior for detecting cartilage invasion and has enhanced ability to discriminate soft tissues. The disadvantages of MRI of the larynx compared with CT include increased imaging time (resulting in motion artifact), decreased spatial resolution, inferior ability to detect bone invasion, and increased cost.126 MRI is also contraindicated for patients with pacemakers or other ferromagnetic foreign bodies, and false-positive findings are common because MRI is often unable to distinguish clearly between reactive inflammatory changes and tumor.127
Evaluation with CT includes a standard neck scan to evaluate extralaryngeal structures and lymph nodes, and finely cut images (≈1 mm) through the larynx to avoid partial volume averaging of the vocal folds, and allow multiplanar reconstruction.128 The axial images should be aligned parallel to the true vocal cords to allow correct interpretation of tumor location and size.125 Intravenous contrast is especially important because the primary tumor usually enhances with contrast, improving its visualization, and because the contrast will differentiate between nodal disease and blood vessels in the neck. Contrast (gadolinium)-enhanced, T1-weighted MRI images improve the definition of pathologic tissue, although some authors have found the use of contrast adds no information when assessing laryngeal tumors.129,130 Special MRI series such as fat suppression are often helpful, especially when assessing the paraglottic and pre-epiglottic spaces.125
Positron emission tomography (PET), using radiolabeled glucose (18 F-fluorodeoxyglucose, FDG), is able to distinguish between malignancy and other pathologic processes such as inflammation.131 PET is particularly useful in the head and neck when performed in conjunction with CT (PET/CT), giving superior anatomic localization of suspicious areas.132 PET has several potential uses: the staging of malignant tumors (especially for the detection of regional and distant metastases); the diagnosis of the unknown primary; the detection of synchronous lesions in addition to the index malignancy; and in the diagnosis of residual or recurrent cancer, which is particularly challenging in the irradiated larynx (see later).133 PET is being used more commonly in the head and neck as our understanding of its abilities and applications improves.
Radiology plays an important role in the staging of laryngeal SCC because deep invasion by the primary tumor is assessed poorly by clinical and endoscopic examination. Several characteristics of the primary tumor used for staging cannot be determined without imaging including invasion of the PES, the PGS, the thyroid or cricoid cartilage, the extralaryngeal tissues, the prevertebral space, mediastinal structures, and encasement of the carotid.6
Invasion of the PES is diagnosed on CT by a loss of fat in the PES and replacement with a moderately enhancing mass.128 On MRI, tumor has a low to intermediate signal on T1-weighted images, with a high signal on T2, and enhances with contrast on T1.128 MRI is highly sensitive in the diagnosis of PES and base of tongue invasion and is superior to CT.134 The radiologic appearance of the thyroid and cricoid cartilages depends on the degree of ossification. Although CT is superior to MRI for imaging bone and calcium, MRI is superior to CT in the detection of laryngeal cartilage invasion.126,127,135 MRI is highly sensitive for cartilage invasion and has a high negative predictive value; however, it is not good at distinguishing between reactive inflammation and tumor, resulting in a lower specificity and a positive predictive value of approximately 70%.126,127,135 CT is less sensitive than MRI in the diagnosis of cartilage invasion. Findings on CT such as extralaryngeal spread, sclerosis, erosion, and lysis suggest cartilage invasion.136 MRI is also better than CT at detecting cricoid invasion in subglottic tumors.137 The accurate diagnosis of cartilage invasion is important because it will upstage a carcinoma to T3 or T4a and is associated with a worse response to radiotherapy, poorer local control, and an increased risk of chondroradionecrosis.126,138
Imaging is also important to stage the neck accurately and detect distant metastases. In one series, CT and MRI were equivalent in the detection of cervical metastases in larynx cancer and superior to clinical examination and ultrasound. However, ultrasound-guided FNA and PET were the most accurate.139 PET has not been used extensively in the pretreatment evaluation of laryngeal cancer because standard PET lacks anatomic detail. However, fused PET/CT scanning addresses this issue and in all likelihood will become more widely used for this indication.140
The lungs are the most common site of metastasis for lung cancer, and second primary tumors commonly occur in the lungs as well. A chest radiograph or a chest CT should be obtained to exclude pulmonary lesions. In patients with advanced primary tumors and low cervical metastases, PET/CT is useful for identifying distant metastases or SPTs that would alter patient management.140
Operative Endoscopic Examination
Unless there are medical contraindications, all patients with suspected laryngeal cancer should undergo an endoscopic examination under general anesthesia. Direct laryngoscopy allows the clinician to examine the larynx in greater detail, palpate the larynx, and obtain a biopsy for histologic analysis. Angled endoscopes are useful to evaluate the laryngeal surface of the epiglottis, anterior commissure, ventricles, and undersurface of the true vocal cords. If vocal cord mobility is noted to be abnormal on preoperative examination, palpation of the vocal cord and arytenoid cartilage will differentiate arytenoid fixation from vocalis muscle invasion causing cord immobility. Invasion of adjacent sites in the oropharynx or hypopharynx is assessed with direct pharyngoscopy and palpation of the tongue base. The neck may be palpated under general anesthetic to assess nodal status. The complete examination should be documented with photos, video, and a diagram of the extent of the primary tumor. Esophagoscopy is performed to exclude an SPT in the esophagus. Bronchoscopy is not required routinely if the preoperative chest imaging is normal.141 Biopsies of the primary tumor and any suspicious areas are taken to confirm the histologic diagnosis and to determine the extent of tumor.
Caution should be exercised when performing direct laryngoscopy for patients presenting with laryngeal tumors obstructing the airway. Maintenance of a secure airway at all times is the paramount concern, and for severely compromised airways a tracheostomy under local anesthetic may be necessary. However, if possible, debulking of the obstructing tumor (using either cup forceps, a microdebrider, or the CO2 laser) is the preferred technique to avoid a tracheostomy and to maintain the airway so that thorough evaluation and staging can be performed before selection of the definitive treatment.142 If a tracheostomy is necessary, a high tracheostomy is performed so that any subsequent resection allows preservation of as much of the trachea as is oncologically possible. Surgical treatment is usually required for patients requiring tracheostomy because significant destruction of the larynx is usually present. The traditional treatment of an obstructing laryngeal SCC requiring a tracheostomy was an emergency laryngectomy (after a biopsy and frozen section biopsy had confirmed the diagnosis). However, emergency laryngectomy does not offer a survival advantage over tracheostomy with delayed laryngectomy and does not allow the patient to be counseled and prepared both psychologically and nutritionally before such radical laryngeal surgery.142,143 Tracheostomy has not been found to increase the risk of peristomal recurrence, which is more strongly associated with advanced local disease, especially subglottic tumor extension.143–146
Differential Diagnosis
The differential diagnosis of laryngeal SCC includes non-neoplastic conditions, benign tumors, premalignant squamous cell lesions, and non–squamous cell malignant tumors (see Table 107-2).
Treatment of Squamous Cell Carcinoma
A number of treatment options are available for patients with laryngeal cancer. Surgery and RT have long been the two most important treatment modalities. In the past 30 years, further therapeutic options have become available with the introduction of new surgical techniques for conservation laryngeal surgery (partial laryngectomy) and of chemotherapy combined with RT (CRT), which may be used in a neoadjuvant, concurrent, or adjuvant role. In general, early laryngeal cancer (stage I or II) is treated with single-modality therapy, either surgery or RT. Advanced laryngeal cancer (stage III or IV) is treated with combined-modality therapy, either primary surgery followed by RT or CRT, or primary CRT or RT with surgery for salvage. The selection of which modality to use, or (in advanced laryngeal cancer) which modality to use initially, should be made after careful consideration of a number of factors, which can be grouped into patient factors, disease factors, and institution factors (Table 107-6). For early-stage disease, voice quality, swallowing function, the duration of therapy, and patient preference are the major factors considered when selecting treatment.
Table 107-6 Prognostic Factors in Treatment of Laryngeal Cancer
| Patient Factors | Tumor Factors | Institution Factors |
|---|---|---|
| Age | Histologic diagnosis | Surgical expertise & experience |
| Comorbidities & general health | Site of primary tumor | Oncologic expertise & experience |
| Performance status | Stage (T,N,M) | |
| Occupation | Histologic features: | |
| Vocal demands |
The following general discussion of the therapeutic options available emphasizes decision making and treatment selection. Subsequent chapters will describe the specifics of the treatment of early glottic cancer (Chapter 108), transoral laser microsurgery (Chapter 109), conservation laryngeal surgery (Chapter 110), total laryngectomy (Chapter 111), radiation therapy (Chapter 112), and vocal rehabilitation following total laryngectomy (Chapter 113).
Treatment of Glottic Squamous Cell Carcinoma
Treatment of the Early Primary Tumor in Glottic Squamous Cell Carcinoma
Early glottic SCC (defined as stage I or II disease, i.e., T1-2N0) may be treated with either RT or surgery, without the need for elective treatment of the neck.147 Primary RT for T1 glottic SCC provides 5-year local control rates of 81% to 90% and laryngeal preservation in 90% to 98% of patients.148–150 For T2 tumors with normal vocal cord mobility, RT achieves local control in 64% to 87%, with laryngeal preservation rates of 75% to 87%.148,151,152 The effectiveness of RT may be overestimated due to complete excision of the tumor at the time of the initial biopsy. In 12 of 60 patients undergoing partial laryngectomy for early glottic SCC, Stutsman and McGavran found that there was no tumor in the pathologic specimen.153,154
Surgical treatment of early glottic SCC also aims to preserve the larynx and is referred to as conservation laryngeal surgery or partial laryngectomy. Traditionally, these limited laryngeal resections were performed via an external approach: Cordectomy and vertical hemilaryngectomy (VHL) are the two classic open procedures for the treatment of early glottic carcinoma. Cordectomy is the removal of the diseased true vocal cord via a laryngofissure. Vertical hemilaryngectomy removes the ipsilateral true and false vocal cords, extending laterally to the perichondrium of the thyroid cartilage. The lamina of the thyroid may be removed to allow the soft tissues adjacent to the larynx to collapse medially to reconstitute the glottis for phonation, or it may be preserved, with transposition of soft tissue (such as a strap muscle) medial to the lamina to recreate the glottis. Variations of the VHL (extended VHL) have been described to include the resection of the anterior commissure, contralateral true vocal cord, arytenoid, and supraglottic or subglottic tumor extension. Open surgical treatment oncologic results are reported as having 90% to 98% local control rates and 93% to 98% larynx preservation rates.155,156
In the past 30 years, endoscopic approaches analogous to the open procedures have been developed to accomplish the same resection without disruption of the supporting structures of the larynx. The oncologic results for transoral laser microsurgery (TLM) have been reviewed by Ambrosch.157 For Tis-T2 tumors, local control rates are 80% to 94% with a greater than 94% laryngeal preservation rate.158 In the hands of experienced surgeons, local control and laryngeal preservation rates following TLM equal those following open surgical techniques.158 In comparison with open surgical techniques, TLM avoids a tracheostomy, the hospital stay is shorter, the cost is reduced, and there is a lower incidence of dysphagia postoperatively.157
Lesions of the middle third of the true vocal cord have the best local control rates and may be treated by either TLM, open cordectomy or RT; local control approaches 100% after surgical excision, whereas RT achieves a 95% local control rate.159 Radiation failures may be caused by unrecognized deep invasion. Following surgical excision, repeat surgery or RT may be used to treat residual or recurrent tumor. Although RT alone has excellent results, a second course of radiation for a recurrence or for a second tumor cannot be offered. Recurrent tumors may not be amenable to conservation surgery after previous RT.
T2 glottic lesions with impaired vocal cord mobility warrant special consideration. Although classified as T2 lesions by the TNM system, glottic tumors with impaired vocal cord mobility have a worse prognosis than tumors that are classified as T2 on the basis of supraglottic or infraglottic invasion. Impaired vocal cord mobility is usually secondary to either tumor bulk or deep invasion. Radiotherapy is less effective in controlling these lesions, and this is probably often due to tumor volume. Dickens and colleagues148,160 noted that 4% of tumors less than 15 mm recurred after radiotherapy, whereas 26% of larger lesions of a similar stage recurred, even when only one true vocal cord was involved. T2 tumors managed by primary RT showed a 30% local failure rate, which, when surgically salvaged, improved to 94%.19,161 Harwood and DeBoer19 observed that impaired vocal cord mobility resulted in lower control rates in T2 lesions, and they suggested the classification be divided into T2a and T2b on the basis of mobility. In this analysis, a 70% local control rate was noted for the former category versus 51% in the latter. McLaughlin and colleagues162 noted recurrence rates of 11% and 26% for T2a and T2b tumors, respectively.
Voice quality following either surgery or RT is influenced by the extent of tumor and depth of invasion. Small superficial tumors will have excellent voice quality with either surgery or RT. Deeper tumors with muscular invasion will have inferior voice outcomes with either treatment modality. Furthermore, the control rate with RT will be lower. Surgical treatment provides a better assessment of tumor extent and in some cases may result in upstaging of the tumor. For small superficial tumors, voice quality with surgery or RT is generally good with comparable voice results.163
Early Glottic Squamous Cell Carcinoma and the Anterior Commissure
Anterior commissure involvement has been associated with decreased local control rates with surgery and RT.164,165 The topic has been controversial, and several explanations for the poorer control rates have been offered. One hypothesis for the decreased effectiveness of RT has been underdosage with supervoltage RT at the tumor-air interface. Increased dose fractions (to >2 Gy) are believed to have solved this problem. The anterior commissure is also a difficult region to assess, and deep invasion may not be recognized, resulting in understaging and thus undertreatment. The lack of perichondrium at the insertion of the anterior commissure tendon was thought to increase the risk of cartilage invasion. Kirchner and Carter166 examined laryngeal carcinoma with anterior commissure invasion using whole organ sections of the larynx and discovered that the anterior commissure tendon is a strong barrier to cancer spread. Deep invasion was seen only in cases where the tumor had invaded the supraglottis superiorly or the subglottis inferiorly. Spread of tumor across the anterior commissure did not increase the risk of deep invasion. Kirchner concluded that supraglottic spread provides access to the PES and that subglottic spread provides access to the thyroid cartilage and the cricothyroid membrane.
Frontolateral VHL obtains local control rates of 80% to 90% for T1 carcinomas involving the anterior commissure.167,168 Supracricoid partial laryngectomy (SCPL) is a more extensive procedure that removes the anterior commissure and the anterior two thirds of the vocal folds. Laccourreye and colleagues have reported a 5-year local control rate of 98% for T1 and T2 glottic tumors with anterior commissure involvement.169 Bron and colleagues170 reported local control of 94.5% for 45 previously untreated laryngeal SCCs involving the anterior commissure treated with SCPL. Although oncologically effective, voice quality is significantly impaired following this technique.
Early reports of TLM for glottic carcinoma considered anterior commissure involvement to be a contraindication. Krespi and colleagues noted a high rate of failure at the anterior commissure.171 This region may be difficult to visualize at the time of surgical resection. With improved understanding of the anatomy, instrumentation, and technique, excellent control rates have been obtained.172,173 Pearson and Salassa165 reported their initial experience of 39 patients with anterior commissure involvement. They had no local failures among 17 pT1 and pT2a tumors. The majority (19/22) of advanced tumors with anterior commissure involvement (pT2b, pT3-4) tumors were controlled with endoscopic surgery. Steiner and colleagues158 reported results on 263 patients with early glottic tumors treated over a 10-year period and observed a modest decrease in local control and laryngeal preservation rates, with equivalent 5-year survival. For T1a tumors, local control was 90% when the anterior commissure was not involved and 84% with anterior commissure involvement. The corresponding laryngeal preservation rates were 99% versus 93%. Similar findings were seen with T1b and T2a tumors.
Treatment of the Advanced Primary Tumor in Glottic Squamous Cell Carcinoma
Advanced glottic SCC (stage III or IV disease) is associated with vocal cord fixation, cartilage invasion, transglottic spread of tumor, subglottic extension, laryngeal framework invasion, extralaryngeal spread, lymph node metastases, and distant metastases. These features portend worse prognosis. The treatment of choice for T3 and T4 glottic tumors remains controversial because of the heterogeneity of the tumors and lack of reliable studies comparing surgery and radiotherapy for T3 and T4 carcinoma of the larynx.174 T3 and T4 glottic tumors are discussed separately in this chapter. T3 glottic carcinomas are unusual because despite their advanced T stage, in general they have a low risk of nodal metastasis. Furthermore, the spectrum of disease with T3 lesions is variable, ranging from low-volume tumors invading the vocalis muscle to cause fixation to very large transglottic tumors. Tumor volume and transglottic spread of T3 tumors predict increased aggressiveness, increased rate of lymph node metastasis, and poorer response to treatment. Tumors larger than 1.5 cm, subglottic extension, and lymph node metastasis laterally or to paratracheal or anterior pretracheal nodes predict failure above the clavicle.175,176
Traditionally, T3 tumors have been treated with total laryngectomy as single-modality therapy. Open VHL and more extensive partial laryngectomies are used in carefully selected cases. Kirchner and Som177 reported a 2-year survival rate of 60% following open partial laryngectomy and noted that failures occurred when there was inferior tumor extension in the larynx. Biller and Lawson reported a 73% absolute 2-year, tumor-free control rate following partial laryngectomy (with resection extended to include a portion of the cricoid cartilage when there was >5 mm of subglottic extension).178 Pearson and coworkers have published extensive experience of near-total laryngectomy (NTL) for patients with tumors not suitable for other conservation procedures.179 This procedure preserves one arytenoid and a portion of the cricoid cartilage to create a diversionary voice shunt from the trachea. Patients remain tracheotomy dependent for breathing and use the shunt to produce speech.
Radiation therapy has a local control rate of approximately 50% for T3 tumors, which is lower than that of surgery.180 For T3 tumors, the return of vocal cord mobility following RT predicts a good response. In a small series of 14 patients with T2b-3 laryngeal carcinomas, all five patients who did not have return of cord mobility and none of nine patients who had return of vocal cord mobility developed recurrent cancer.181 Tumor volume may predict the response to irradiation, with poor results in larger tumors.182 For small T3 tumors that would be amenable to partial laryngectomy, primary RT could be considered in those not wishing to pursue surgical option, although surgical resection generally has higher local control.183,184 Intensified RT regimens including twice-daily treatments and the use of intensity-modulated radiotherapy (IMRT) may improve local control.185 Further discussion on RT can be found in Chapter 112. Total laryngectomy or CRT is recommended for bulky T3 tumors or tumors not suitable for conservation laryngeal surgery.
In general, T4 glottic carcinoma is not considered amenable to conservative laryngeal resection. Options for T4 glottic carcinoma include total laryngectomy (usually with postoperative RT or CRT), near-total laryngectomy, or primary CRT in selected low-volume disease with limited cartilage destruction to preserve the larynx. Near-total laryngectomy may be considered in selected cases with limited subglottic extension and no interarytenoid involvement.186 Recent encouraging results for TLM for T3-4 laryngeal cancer support its use in carefully selected cases by experienced surgeons for organ preservation.187
Primary RT for T4 glottic carcinoma has poor local control rates. A historical review of primary RT for laryngeal SCC found that only 2 of 25 T4N0 glottic carcinomas treated with primary RT survived 5 years.188 In patients unable or unwilling to undergo concurrent CRT and unwilling to have a total laryngectomy, primary RT may provide a chance of local control. In some patients, the addition of newer agents such as cetuximab may increase the effectiveness of RT with acceptable risk of increased toxicity.189
Although attempts to avoid total laryngectomy are worthwhile, RT may result in significant local tissue destruction, scarring, and persistent edema. Thus the larynx may be preserved, but the patient may be left with a severely compromised organ with a restricted airway, a poor voice, dysphagia, and/or aspiration. If RT is selected for primary management, close follow-up is required as the success of this approach relies on the early detection of residual or recurrent disease, which may be challenging in a larynx scarred from irradiation. A total laryngectomy is usually required for salvage if recurrent disease is diagnosed. The successful salvage rate for recurrences after RT is approximately 60%. Persistent postradiation edema predicts persistent disease: 45% of patients with edema persisting for longer than 6 months after RT had a deep recurrence.190 In these situations close follow-up with endoscopy and imaging is vital.191 Distinguishing between recurrence and chondroradionecrosis of the larynx can be challenging. Deep biopsies required to obtain an accurate diagnosis can induce or exacerbate chondroradionecrosis. Positron emission tomography (PET) scanning has been helpful to resolve this dilemma and assists surveillance for tumor recurrence.192
Selected T4a tumors may be considered for nonsurgical organ preservation trials (see “Nonsurgical Organ Preservation” later). The success of organ-preservation protocols for bulky T4a tumors is lower than for T3 tumors because cartilage destruction predicts poor response to organ preservation protocols. These patients were excluded from the RTOG 91-11 trial comparing concurrent CRT, induction chemotherapy followed by RT, and RT alone.193
When total laryngectomy is undertaken for T4 disease, hemithyroidectomy or subtotal thyroidectomy is recommended for cases of palpable abnormality, subglottic tumors, or glottic tumors with greater than 1 cm of subglottic extension.194 Thyroid gland invasion may be predicted if a positive Delphian node or cartilage destruction is present. Cancer is found in 3% to 8% of thyroid specimens.
Treatment of the Neck in Glottic Squamous Cell Carcinoma
Occult metastases from T1-2 glottic SCCs are uncommon, and elective treatment of the N0 neck is not required.195 T3 glottic SCC is more controversial. In general, occult nodal metastases are uncommon from T3 glottic carcinomas unless there is transglottic spread of the tumor, which has a higher rate of occult metastasis.196 A national survey of otolaryngologists published in 2003 reported that 87% of respondents treated the neck of patients with T3N0 glottic SCC, and 90% treated the neck of those with T4N0 glottic SCC.197 T4 glottic carcinomas have a higher risk of occult metastases, approximately 20%, and treatment of the neck is recommended. If the primary tumor is being treated surgically, an ipsilateral selective neck dissection is recommended. For glottic carcinoma, the paratracheal nodes and levels II-IV are dissected. Adjuvant treatment with RT or CRT is used depending on pathologic findings in the neck dissection specimen.198,199 If RT is used to treat the primary tumor, the central compartment and ipsilateral lateral neck are included in the field.
Postoperative RT is recommended when multiple nodes, extracapsular spread, extralaryngeal invasion, and perineural or lymphovascular invasion is present. Recently, two large studies have demonstrated improved locoregional control with use of platinum-based chemotherapy given concurrently with RT.200,201 Cooper and colleagues200 found a 10% increase in the 2-year locoregional control rate (82% vs. 72%), although there was an associated 43% increase in acute grade III or higher toxicity (34% vs. 77%). Similarly, Bernier and associates observed an 11% increase in 5-year progression-free survival, which was also associated with a higher rate of acute toxicity.201 A comparative analysis of the combined results of these two studies found that extracapsular spread and microscopically positive surgical margins were the only risk factors for which adjuvant chemotherapy enhanced the efficacy of RT in both studies.202 Other variables trended toward statistical significance, but patients who had two or more positive lymph nodes (without extracapsular spread) as their only risk factor did not seem to benefit from the addition of chemotherapy.
Improved locoregional control rate with primary concurrent CRT has led to the addition of chemotherapy to RT as an adjuvant treatment for patients with adverse pathologic findings in the surgical specimen. The decision to use chemotherapy in this setting requires careful consideration of the ability of the patient to tolerate the treatment. The success of this therapy is dependent on completion of the treatment regimen without significant breaks.203–206 Encouragingly, a recent analysis of the tolerability of concurrent CRT in patients older than 70 years of age demonstrated high compliance with a regimen of concurrent carboplatin and RT.207
Treatment of Supraglottic Squamous Cell Carcinoma
Sessions and colleagues208 reported the results of 653 patients with supraglottic SCC treated with a number of modalities (except chemotherapy). Overall 5-year, disease-specific survival for all stages was 66%, with rates of 77%, 74%, 64% and 50% for stages I, II, III, and IV, respectively. No treatment modality was found to result in superior survival. The larynx was preserved in 86% of patients treated with open partial laryngectomy, and in 73% of RT patients. In another large series of patients (n = 903) treated with conservation surgery, the 5-year uncorrected actuarial survival for stage I, II, III, and IV disease was 84%, 81%, 76%, and 55%, respectively.209
An important point to highlight is that survival from laryngeal SCC has diminished over the past 20 to 30 years in the United States. A review of the National Cancer Database by Hoffman and colleagues60 found that 5-year relative survival from supraglottic SCC has decreased from 52.2% in 1985-1987 to 47.3% in 1994-1996. The greatest decline in survival for patients with supraglottic SCC occurred in those with T1N0 and T2N0 tumors, with a lesser but still significant decline in survival for T3N0 tumors. The authors noted that the frequency of use of the various treatment modalities has changed during this period, with an increase in RT and CRT, and hypothesized that the decreased survival observed in early supraglottic cancer could be explained by less aggressive surgical treatment of the primary and of the neck.
Treatment of the Early Primary Tumor in Supraglottic Squamous Cell Carcinoma
The traditional OSGL was first described by Alonso in 1947 and subsequently refined by Ogura and Som.210–212 The oncologic validity of OSGL as a treatment for supraglottic SCC comes from the principle that the supraglottis is embryologically separate from the glottis and subglottis, and for this reason, supraglottic SCC remains localized to the supraglottis in most cases, despite the lack of an anatomic structure preventing invasion of the glottis.213 OSGL removes all laryngeal structures superior to the floor of the ventricle, preserving both true vocal cords, both arytenoids, the base of tongue, and the hyoid bone (Fig. 107-10). Indications for OSGL are T1, T2, or selected T3 supraglottic tumors. T3 tumors with PES and no transglottic spread and/or vocal cord impairment are amenable to OSGL. Contraindications to OSGL include poor general physical condition (e.g., advanced age, lung disease, neurologic disease, preexisting dysphagia or aspiration), glottic involvement, impaired mobility or fixation of the vocal cord, thyroid or cricoid cartilage invasion, involvement of the base of tongue to within 1 cm of the circumvallate papilla, or involvement of the deep muscles of the tongue.214 The functional morbidity of OSGL is significant, and almost all patients will experience some aspiration postoperatively. Therefore careful patient selection is crucial to the overall success of this procedure: Candidates for OSGL must have good pulmonary function to tolerate the expected aspiration. An OSGL is referred to as an extended OSGL when a more extensive resection is carried out for the treatment of SCC involving the lingual surface of the epiglottis, the base of tongue, or one of the arytenoids.214
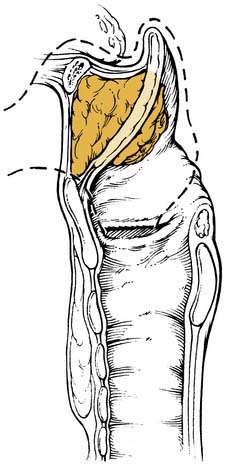
Figure 107-10. Outline of resection for open supraglottic laryngectomy. Note inclusion of entire pre-epiglottic space.
(Redrawn from Som ML. Conservation surgery for carcinoma of the supraglottis. J Laryngol Otol. 1970;84:657.)
The functional and oncologic outcomes of OSGL are well documented. Because OSGL disrupts the pharyngeal muscles, the strap muscles, and the sensory innervation of the pharynx and larynx, swallowing is markedly impaired, especially in the early postoperative period. A tracheostomy is required in all patients to provide a safe airway and to protect the lower airway from aspiration. Suarez and colleagues215 reported that 10% of patients in their series required a completion total laryngectomy for chronic aspiration. Advanced age (older than 65 years) was the major risk factor for intractable aspiration. A further 24% of subjects required permanent tracheostomy. Bron and colleagues216 noted approximately one quarter of their patients aspirated in the early postoperative period. The median duration until normal oral feeding was established was 16 days, and the median duration until decannulation was 17 days.
OSGL yields excellent local control in the treatment of early supraglottic cancer in the range of 80% to 100%.217–222 In patients treated with OSGL for T1-3 supraglottic SCC, Bron and colleagues216 reported 5-year local control, locoregional control, and overall survival rates of 92.5%, 90%, and 92%, respectively. Adjuvant RT was given in 30% for positive surgical margins or adverse pathologic findings in the lymph nodes. Local control and survival were poor when cartilage invasion was present or when there was extralaryngeal extension of disease (i.e., pT4). Suarez and colleagues222 treated 193 patients with supraglottic SCC with OSGL and ND. Nearly half of the subjects received adjuvant RT. Local control was 98% for T1 tumors and 91% for T2 tumors. No improvement in overall survival was noted with the addition of RT. In Bocca’s series of 537 patients with supraglottic cancer (predominantly T2), local control was 94% for stage I and 82% for stage II. Overall 5-year survival was 78%.218
Robbins and colleagues220 treated 139 T2 and T3 supraglottic SCCs with OSGL if technically possible, or with total laryngectomy or radiotherapy if not. Local control at 3 years was 100% for the OSGL group and 91% for the total laryngectomy group. Those subjects treated with primary RT had a 69% local control rate, which improved to 85% with salvage total laryngectomy. Five-year survival was 89% for those subjects treated with OSGL primarily, 78% with TL, and 70% with RT.
Transoral laser microsurgery for supraglottic carcinoma, first described by Vaughan in 1978, has become an accepted alternative to OSGL for supraglottic SCC.223 The indications for TLM are similar to those for OSGL (i.e., T1-2 and selected T3 tumors), although some institutions will use TLM for more advanced lesions such as T4.224,225 Few contraindications to TLM exist: incomplete exposure of the tumor; tumor involving the great vessels of the neck; and tumor location and/or bulk requiring an extensive resection that would place the patient at high risk of aspiration (e.g., extensive tongue base involvement).225 The indications for neck dissection are not changed with TLM, and both sides of the neck may be dissected at the same time the primary is resected, or more commonly performed in a staged fashion several weeks after the initial TLM.226 The local control rates of TLM are similar to those of OSGL; however, the functional morbidity is much less following TLM because the extrinsic muscles of the larynx, pharyngeal muscles, cartilaginous framework of the larynx, and superior laryngeal nerves are left intact.227 The functional advantages of TLM include the possible avoidance of a temporary or permanent tracheostomy; less impairment of swallowing postoperatively including less aspiration; and a lower incidence of pharyngocutaneous fistulae.224,228
Steiner’s group reported their experience with TLM for early supraglottic carcinoma in 1998.224 Only 4% of subjects were treated with adjuvant RT—96% underwent TLM only. No tracheostomies were performed at the time of the resection, although one patient required a temporary tracheostomy to manage aspiration. The average duration of nasogastric tube feeding was 6 days, and overall swallowing was excellent postoperatively. The oncologic results were equivalent to OSGL: 5-year local control rates were 100% for pT1 and 89% for pT2 tumors, which were salvaged with either a second TLM or with OSGL to give an ultimate local control rate of 97%. Overall 5-year survival was 76%.
Iro and colleagues229 reported the results of TLM in a series of 141 supraglottic cancers, which were predominantly T1-2. Half of the subjects also had neck dissections, and 45% received adjuvant RT. Tracheostomies were required in 13% of subjects during the course of treatment and were permanent in 9%. All but one of the subjects requiring a permanent tracheostomy were staged as pT4 and underwent extensive resection of the tongue base. Five-year local control rates were 86%, 75%, 75%, and 78% for stages I, II, III, and IV, respectively. Five-year, disease-free survival was reported as 66% (75% for stages I and II, 56% for stages III and IV).
Motta and colleagues230 performed TLM on 124 subjects with T1-3N0 supraglottic carcinoma and observed local control rates of 82%, 63%, and 77% for T1, T2, and T3 lesions. Laryngeal preservation rates were 89%, 85%, and 95%. Overall survival rates were 91%, 88%, and 81%. Advanced supraglottic tumors were also included in a series of TLM reported by Rudert and associates.231 The majority of patients (24 of 34) received RT and nearly 20% required tracheostomies. Local control was 100%, 75%, 78%, and 37% for T1, T2, T3, and T4 tumors; overall survival was 71% for stages I and II and 50% for stages III and IV tumors.
The functional outcomes of patients who underwent either TLM or OSGL for predominantly T1-2 supraglottic SCC were compared by Peretti and colleagues.227 A temporary tracheostomy was required in 14% of TLM patients for an average of 4.5 days compared with 100% of OSGL patients who were decannulated after an average of 35 days. No permanent tracheostomies were required in either group. A feeding tube was used in 21% of the TLM patients for an average of 5 days, compared with 100% of OSGL patients, for an average of 19 days. No permanent feeding tubes were placed in either group. The average period of hospitalization was 11 days for the TLM group versus 26 days for the OSGL group. No significant difference in voice quality between the two groups occurred.
The role of adjuvant RT in TLM has been unclear, with up to 94% of subjects receiving adjuvant RT after TLM in some series.232
Zeitels and colleagues233 concluded that small T1-2 supraglottic lesions in subsites amenable to endoscopic resection (suprahyoid epiglottis, aryepiglottic fold, vestibular fold) could be treated successfully with TLM without RT but recommended that RT should be given after endoscopic resections of larger lesions (T2-3) in less favorable sites (e.g., infrahyoid epiglottis). In this series there was a high rate of local failure after RT in those patients in whom negative margins were not achieved at TLM, suggesting that RT was unable to control residual disease at the primary site. Davis and coworkers described the concept of TLM as a neoadjuvant treatment to remove all carcinoma at the primary site (with negative margins), followed by RT to treat any residual microscopic disease and treat the neck.234 Using this approach for T2 supraglottic SCC, TLM (and neck dissections for N+ subjects) was performed, with 83% of subjects receiving adjuvant RT. Over one third of subjects were staged as pT3 secondary to PES invasion. Local control was 100% in the surgery-only group and 97% in the combined-modality group. Regional control was 96% in the N0 subjects who received RT to the necks and 91% in the N+ subjects who received surgery and RT to the necks. Overall survival was low at 63% for the TLM/RT group and 50% for the TLM-only group due to a high rate of non–cancer-related deaths. A permanent feeding tube was required in 3%, but there were no permanent tracheostomies. The average time until normal swallowing was established was less than 14 days.
Because RT generally has an inferior local control rate compared with surgery, especially for bulky lesions, Agrawal and colleagues232 proposed that a TLM procedure to debulk the primary tumor may improve the oncologic outcome. In a prospective trial of TLM with adjuvant RT for T1-2N0-1 supraglottic SCC, the 3-year local control rate was 97%, with one recurrence that was salvaged by TL. The overall survival rate was 88%. The functional results were not as good as in other TLM series, and this was probably due to the addition of the adjuvant RT: 21% of subjects experienced a prolonged period of dysphagia (3 to 10 months), and 9% required a permanent gastrostomy tube. Subject accrual for this trial became difficult as some coinvestigators were reluctant to use RT for all patients because they became increasingly comfortable in the use of surgery as single-modality treatment for these patients.
Radiation therapy plays an important role in the treatment of patients whose tumors are not amenable to partial laryngectomy, who are medically unfit for surgery, or who prefer to avoid surgery. In general, surgical excision has a higher local control rate for early supraglottic tumors than RT.235 Radiation therapy alone is also less effective than surgery (with adjuvant RT) or CRT in patients with advanced laryngeal carcinoma; however, it may be used to treat patients who are not eligible for CRT protocols but wish to attempt to preserve their larynx. However, RT is not without complications including dysphagia, aspiration, laryngeal edema, and chondronecrosis, which may require tracheostomy or total laryngectomy.235
Harwood reported initial local control rates with RT of 70% for T1N0 tumors, 68% for T2N0, and 54% for T3-4N0.236 Mendenhall and colleagues235 treated 209 supraglottic carcinomas with primary RT and obtained initial local control rates of 100%, 85%, 64%, and 36% for T1, T2, T3, and T4 lesions. Patients with recurrent disease were salvaged with either a total laryngectomy or, if possible, an OSGL, to give an ultimate local control rate of 100%, 88%, 81%, and 57%. The cause-specific survival for the series was 100%, 92%, 75%, 47%, and 32% for stages I, II, III, Iva, and IVb. Tumor volume (<6 cm3) and vocal cord mobility were significant predictors of local control. Hinerman and coworkers237 reported on 274 patients with supraglottic SCC treated with RT with or without neck dissection. The 5-year local control rate after RT was 100%, 86%, 62%, and 62% for T1, T2, T3, and T4 tumors. The 5-year cause-specific survival was 100%, 93%, 81%, 50%, and 49% for stages I, II, III, Iva, and IVb.
Primary RT was compared with partial laryngectomy (and neck dissection) by Spriano and colleagues238 for the treatment of 166 stage I and II supraglottic SCC. The surgical group had a superior 5-year, disease-free survival rate of 88% versus 76% for the RT group. Salvage surgery was effective in 66% of the partial laryngectomy failures and in 50% of the RT failures. Altered fractionation regimens have also been used to treat supraglottic SCC, and several reports have suggested improved local control rates with twice-daily hyperfractionated RT compared with once-daily RT.239–241
Treatment of the Advanced Primary Tumor in Supraglottic Squamous Cell Carcinoma
Advanced primary supraglottic tumors (i.e., T3 or T4) have traditionally been treated with total laryngectomy, bilateral neck dissections, and postoperative RT. However, laryngeal preservation has become an important aim in the treatment of laryngeal cancer in an attempt to improve the quality of life of patients.242 The therapeutic options for these advanced lesions currently include surgical excision (total laryngectomy or partial laryngectomy in selected cases) with bilateral neck dissections, followed by adjuvant RT (or concurrent CRT); primary RT with surgical salvage (usually total laryngectomy); or concurrent CRT with surgical salvage.243
The Department of Veterans Affairs (VA) Laryngeal Cancer Study, published in 1991, was a landmark trial in the development of nonsurgical organ preservation and chemotherapy for the treatment of advanced laryngeal cancer.244 Nearly two thirds of the subjects (208 of 332) had supraglottic SCC, with the majority of tumors classified as T3. The section on nonsurgical organ preservation later in this chapter describes the design and results of the important studies of nonsurgical treatment of advanced laryngeal cancer.
A second important trial investigating the nonsurgical treatment of advanced laryngeal SCC, the Head and Neck Intergroup Study (RTOG 91-11), was reported in 2003.193 Over two thirds of the subjects (356 of 518) had supraglottic tumors. Large-volume T4 tumors that extended into the tongue base by more than 1 cm or penetrated through cartilage were excluded. The concurrent CRT group had statistically significant higher rates of locoregional control (78%) and laryngeal preservation (88%) compared with the induction chemotherapy– and radiation therapy–alone arms. The findings of this study have led to concurrent CRT becoming the standard nonsurgical organ preservation protocol for advanced laryngeal cancers with T2, T3, and low-volume T4 tumors (without gross cartilage destruction). Further information on nonsurgical preservation is discussed later.
The surgical excision of advanced primary tumors has traditionally been achieved by total laryngectomy, and although this remains the most commonly used procedure, conservative laryngeal resections such as OSGL, extended OSGL, supracricoid partial laryngectomy (SCPL), or TLM may be used for selected cases. Supracricoid partial laryngectomy, first described in 1959 by Majer and Rieder, is an organ-preserving surgical technique in which the aim is to preserve function without the need for a stoma of any kind.245 It may be used as a primary treatment or as a salvage procedure after RT.246 This procedure resects both true vocal cords, both false vocal cords, both paraglottic spaces, the entire thyroid cartilage, and the epiglottis. The hyoid bone and the cricoid cartilage are preserved. One of the arytenoids may also be included in the resection, but at least one intact cricoarytenoid unit must be preserved for postoperative function.245 Following the resection of a supraglottic cancer, the surgical defect is closed by tightly apposing the hyoid bone and base of the tongue to the cricoid cartilage, termed cricohyoidopexy (CHP). A SCPL (with CHP) is used for selected T2, T3, and T4 supraglottic and transglottic tumors: T2 tumors not amenable to OSGL because of involvement of the true vocal cords or anterior commissure, extension to the floor of the ventricle, and/or impaired motion of the true vocal cord; T3 transglottic and supraglottic tumors with true vocal cord fixation and/or pre-epiglottic space invasion; and T4 transglottic and supraglottic tumors with limited invasion of the thyroid cartilage without extension to the outer thyroid perichondrium, or extralaryngeal spread.245 Contraindications to SCPL include poor general health, poor pulmonary function, invasion of the cricoarytenoid joint, invasion of the cricoid cartilage, involvement of the posterior commissure, extension to the subglottis, invasion of the hyoid bone, extension of tumor to the outer perichondrium of the thyroid cartilage, or extralaryngeal spread.214,247
The oncologic results of SCPL with CHP are excellent, with 5-year survival rates of 67% to 95% and local control rates of 88% to 95%.245,248 Total laryngectomy is the recommended salvage procedure for recurrent disease. In general, laryngeal function is good after SCPL. A tracheostomy and feeding tube are required for all patients initially but are temporary in most.246,249 A completion total laryngectomy may be required on occasion for intractable aspiration.170,246 The major disadvantage of SCPL is poor voice quality.245
Treatment of the Neck in Supraglottic Squamous Cell Carcinoma
Supraglottic SCC has a high incidence of clinically apparent and occult regional metastases. Supraglottic SCC usually metastasizes to levels II, III, and IV. Bilateral metastases occur frequently in supraglottic SCC. Elective treatment of the clinically negative neck is indicated in all supraglottic SCCs except T1 lesions. The choice of either surgery or RT for the neck will vary according to how the primary tumor is to be treated. For early-stage tumors (T1-2), either surgery or RT may be used as a single modality to treat both the primary site and the neck. The indications for neck dissection do not alter when the primary tumor is treated with a partial laryngeal resection. For advanced primary lesions (T3-4), combined modality therapy is usually indicated. Often, nonsurgical organ preservation such as CRT or RT is used initially, with surgery to the primary and/or neck for salvage. Alpert and colleagues250 investigated the use of RT for the treatment of clinically negative necks in SCC: for patients staged N0, both necks were treated with RT; and for patients with ipsilateral nodal metastases, the ipsilateral neck was treated with surgery and the contralateral neck was treated with RT. RT was effective in this role because failure was observed in only 3% of node-negative necks.
The standard surgical treatment of the neck in N0 supraglottic SCC is bilateral selective neck dissection of levels II to IV. Several studies have observed that levels I and V are rarely involved by metastases when clinically apparent metastases are present in levels II-IV and are never involved when occult metastases are present in the lateral neck.104,251,252 A selective neck dissection (levels II to IV) is as effective as a comprehensive neck dissection (levels I to V) for N0 (and N1) disease.253 Further studies of N0 supraglottic (and glottic) SCC have observed that metastases are not common in sublevel IIb and level IV, and therefore Ferlito and colleagues have suggested that a more targeted selective neck dissection of sublevel IIa and level III may be adequate for the elective surgical treatment of the neck in these cases.111 An exception to these findings was a study that found occult metastases were present in level I in 82% of supraglottic SCC staged cN0.108
Treatment of the N+ neck is less controversial. If surgery is the primary treatment modality, a selective neck dissection (levels II to IV) is as effective as a comprehensive neck dissection for N1 disease, but for more extensive nodal disease (N2-3), a comprehensive neck dissection should be undertaken.253,254 Bilateral neck dissection has been found to decrease the regional failure rate of the surgical treatment of supraglottic SCC from 20% of cases (in which the contralateral undissected neck was the most common site for recurrence) to 8%.255 Adjuvant RT is indicated for neck dissections showing unfavorable pathologic findings including multiple involved nodes, extracapsular spread, or soft tissue extension of tumor.209,218,256
Subglottic Squamous Cell Carcinoma
The treatment of subglottic SCC usually requires total laryngectomy and postoperative RT. Total laryngectomy is necessary because laryngeal framework invasion is frequent, and because of the difficulty of laryngeal reconstruction when a portion of the cricoid cartilage is resected in a partial laryngectomy. Ipsilateral thyroidectomy and paratracheal node dissection is also required (Fig. 107-11). Distal tracheal spread may occur, requiring a low tracheal resection and manubrial removal, and posterior extension into the cervical esophagus may be encountered. For patients with nodal metastases or extralaryngeal invasion, adjuvant RT (including the superior mediastinum) or concurrent CRT is used to decrease the likelihood of stomal recurrence.101
Despite such extensive treatment, survival is poor. Vermund reported 36% survival in pooled reports of subglottic SCC treated with primary RT compared with 42% for those treated surgically.188 Stell and Tobin reported 44% cancer mortality in their series.257 More recently, Garas and McGuirt reported a 25% three-year survival rate in 15 patients, the majority of whom presented with advanced disease.101 Radiation therapy can be considered for early-stage lesions, but there are limited data to guide management.
Nonsurgical Organ Preservation Treatment
A number of studies in the 1980s found that the addition of chemotherapy before RT for surgery for advanced head and neck cancer resulted in high rates of complete tumor regression associated with prolonged survival.258–260 Response to chemotherapy predicted tumor radiosensitivity. However, this improved response did not translate into increased survival.261
These early studies provided the rationale for the VA laryngeal cancer study reported in 1991.244 This was the first large prospective randomized study to demonstrate the effectiveness of the use of chemotherapy to define the more favorable patients and avoid laryngectomy in responders. This multi-institutional study reported on 332 patients with stage III or IV previously untreated laryngeal SCC. Subjects were randomized to receive either induction chemotherapy (IC) followed by RT, or surgery (total laryngectomy) and RT (Fig. 107-12). Two cycles of IC were given to the first group: nonresponders to the IC underwent immediate total laryngectomy, whereas responders received a third cycle of chemotherapy followed by RT (with salvage total laryngectomy for residual disease) (see Fig. 107-12). The laryngeal preservation rate was 64% for patients assigned to the IC/RT group, although only 39% had a fully functioning larynx. The 2-year survival for both groups was 68%. In a follow-up study, a further 10% decrease in the number of those with a functioning larynx was noted at 5 years, and there were no statistically significant differences in survival between the two groups.262
In an effort to determine the relative contributions of chemotherapy and RT to laryngeal preservation, as well as to determine whether concurrent chemotherapy as a radiosensitizer would improve the likelihood of organ preservation, the Head and Neck Intergroup conducted a prospective randomized study that included 518 patients randomly assigned to three arms (Fig. 107-13).193 Subjects received either IC followed by RT, concurrent CRT, or RT alone. Previously untreated stage III and IV glottic or supraglottic carcinomas requiring total laryngectomy for surgical treatment were eligible. This study excluded subjects with T1 tumors and bulky T4 tumors with laryngeal cartilage destruction, invasion through the laryngeal cartilages, or extensive tongue base invasion. At 2 years, the larynx was preserved in 88% of patients in the concurrent CRT group, compared with 75% and 70% in the IC/RT- and RT-only groups. The overall survival rate for all three groups was similar (5-year survival 54% to 56%). The high rate of laryngeal preservation in the concurrent CRT group was associated with an 82% incidence of high-grade toxicity compared with an incidence of 81% and 62% in the IC/RT and RT groups, respectively. In those who failed nonsurgical organ preservation therapy, total laryngectomy salvaged approximately three quarters of the patients.
Adelstein and associates compared RT with concurrent CRT for stages III and IV laryngeal cancer and observed a significant difference in the recurrence-free interval (51% vs. 62%).263 The 5-year overall survival was similar for the two groups (48% vs. 50%), which the authors believed was due to the efficacy of salvage surgery and non–cancer-related deaths. Most other studies of concurrent CRT in the head and neck do not address the larynx specifically and do not have large numbers of laryngeal cancer patients.243 The utility of IC has been re-examined in a recent report by Urba and colleagues,264 in which a single cycle of chemotherapy was used to select patients with advanced laryngeal cancer for treatment with concurrent CRT. The 3-year, cause-specific survival and overall survival rates were 87% and 85%, respectively, with a laryngeal preservation rate of 70%.
Despite the encouraging results, nonsurgical organ preservation is not without disadvantages, especially with regard to its toxicity, which must be taken into consideration when deciding on treatment. In the RTOG 91-11 study, acute toxicity was more common and severe with concurrent CRT when compared with the other two groups and included chemotherapy-related side effects (e.g., neutropenia, nausea, vomiting) and increased rates of severe radiation induced mucositis.193 The total rate of severe toxic effects, acute and late, was 82% in the concurrent CRT group. There was no difference between the three groups with regard to speech, with 3% to 8% reporting moderate or worse speech impairment at 2 years. However, the incidence of dysphagia was high: 26% of patients treated with concurrent CRT had problems swallowing at 1-year post treatment. At 2 years, there was no significant difference between the groups, with 15% of the concurrent CRT group reporting ongoing dysphagia. Previous RT and CRT may also pose problems for both the patient and the surgeon if surgery (usually total laryngectomy) is required after nonsurgical organ preservation for residual or recurrent disease, chondronecrosis, or intractable aspiration. In a subsequent report on the RTOG 91-11 trial, Weber and colleagues265 concluded that the morbidity of TL after organ preservation was acceptable but observed that up to 30% of patients developed a pharyngocutaneous fistula. The incidence was highest in the concurrent CRT group, but this difference was not statistically significant.
Therefore two important principles must be adhered to when nonsurgical larynx preservation treatment protocols are undertaken. First, if neoadjuvant chemotherapy or concurrent CRT is used, total laryngectomy must be performed for salvage in patients who fail to respond if survival is not to be compromised.266 Second, the success of CRT is dependent on the completion of the treatment protocol. The efficacy of RT is significantly diminished if treatment breaks are taken due to toxicity.267 Because nonsurgical larynx preservation therapy has significant toxicity (and mortality of 2% to 4%), patients with comorbidities and poor social support to help them through this challenging treatment regimen are less likely to complete the treatment and thus benefit from the addition of chemotherapy.
The success of nonsurgical larynx preservation treatment outside closely supervised clinical trials has also been questioned. Hoffman and colleagues60 observed a concerning decrease in larynx cancer survival between 1985 and 2001, in patients with advanced glottic cancer, early supraglottic cancer, and T3N0M0 supraglottic cancer. During this same period there was a marked increase in use of nonsurgical larynx preservation treatment. Recently Chen and colleagues268 analyzed 7019 larynx cancer cases from the National Cancer Database and observed that total laryngectomy was associated with a statistically significant increase in survival compared with CRT for stage IV laryngeal cancer.
Management of the Neck after Nonsurgical Organ Preservation Treatment
In all nonsurgical larynx preservation protocols, management of the neck and primary site must be considered both individually and together. Experts do not uniformly agree on the management of the neck in patients when there has been a complete response to treatment at both the primary site and in the neck. Traditionally, if any residual disease is detected in the neck after completion of RT or CRT, either by clinical examination or by radiographic evaluation, dissection of the neck is indicated.244 In the initial VA study of the 106 patients who underwent IC and RT, 46 had N2 or N3 disease. A complete response to treatment was obtained in 18 of 46 patients (39%) with advanced neck disease and a partial response in 16 of 46 patients (35%). There was no response in 12 patients (26%). Fifty percent of the complete responders had N2 disease, and 33% had N3 disease. These data demonstrate that there is not a direct correlation between initial node size and response. Of the 18 patients who obtained a complete response in the neck, 5 eventually required a neck dissection. Of the 28 patients who had less than a complete response, 19 underwent neck dissection. When there was a delayed recurrence in the neck, survival was poor—less than 30%. When a poor response was obtained in the neck to the combined therapy, salvage by surgery still resulted in a poor outcome.
Further experience with CRT protocols for laryngeal preservation has focused attention on the cervical metastases, and a paradox has been identified. When routine neck dissection is performed after CRT, various series have reported a 14% to 39% incidence of positive findings in patients who had a complete clinical response to CRT.269 In patients with less than a complete response, the majority of specimens did not demonstrate pathologic evidence of tumor.270,271 Furthermore, in studies in which no neck dissection had been performed following CRT, the rate of recurrence in the neck was low at 0% to 6%.272 It is hypothesized that in many patients with pathologic findings of tumor in the neck specimen, the cells are not viable but have not yet been cleared by the body. In a study of patients with persistent tumor after completion of treatment for nasopharyngeal cancer, serial biopsies every 2 weeks following treatment determined that positive biopsies showing tumor were seen for up to 12 weeks in patients who ultimately had complete response.273 Increasingly, a PET scan 3 months after the completion of treatment is used to assess the effectiveness of treatment. A negative PET scan at 3 months reliably indicates the absence of persistent disease. For patients with N1-2 disease, the use of PET shows promise for predicting which patients can avoid a neck dissection post treatment.
Treatment of Recurrent Squamous Cell Carcinoma
The detection of recurrent (or residual) disease is challenging and has become particularly important since the advent or nonsurgical organ preservation therapies. The status of the larynx may be difficult to assess following RT or CRT due to inflammation, edema, fibrosis and/or scarring, which develop as the result of the treatment. The symptoms of recurrence may vary according to the site of the original primary tumor and the nature of the recurrence. Signs of local recurrence include increasing edema, thickening of the true vocal cords, impairment of true vocal cord mobility, vocal cord fixation, leukoplakia, a mass lesion, and/or ulceration.274 Recurrent cancer may be submucosal, and deep biopsies may be required because superficial biopsies of the epithelium may fail to diagnose recurrent tumor. A deep biopsy should be undertaken with caution in the irradiated larynx because it may cause infection, perichondritis, or chondroradionecrosis.275 Recurrent disease must be differentiated from radiation-induced edema, perichondritis, or chondronecrosis because the clinical presentation of these entities may be similar, and a biopsy remains the gold standard for the diagnosis of recurrent disease.
PET scanning has been used successfully to distinguish between chondronecrosis and residual or recurrent tumor in the larynx following organ preservation therapy and helps to avoid unnecessary biopsies.192,276 The sensitivity and specificity of PET for the diagnosis of residual or recurrent carcinoma in the larynx was found to be 80% and 81%, respectively, compared with 58% and 100% for CT.277 In another report, the accuracy of PET for the diagnosis of recurrent cancer was superior to CT and clinical examination: 79% versus 61% versus 43%, respectively.278 The utility of PET for the diagnosis of recurrent disease is still being evaluated, and a prospective trial comparing PET (followed by a direct laryngoscopy and biopsy if the PET scan is positive) and direct laryngoscopy (and biopsy) has been proposed.279
Almost half of the patients diagnosed with recurrent SCC have transglottic rT3 or rT4 tumors.280 The standard treatment for recurrent laryngeal SCC is total laryngectomy. However, partial laryngectomy has been used to treat recurrent SCC for several decades and is particularly useful for early tumors that have failed RT. Som reported the use of an open approach (laryngofissure) for the salvage of radiation failures in the 1950s.281 In 1970 Biller and colleagues282 proposed criteria that would preclude the use of partial laryngectomy as surgical salvage, which included subglottic extension greater than 5 mm, cartilage invasion, contralateral vocal cord involvement, arytenoid involvement (except the vocal process), vocal cord fixation, and recurrence that did not correlate with the original primary lesion. In Ballantyne and Fletcher’s series of 78 early glottic cancers that recurred following RT, 75% could be salvaged. Total laryngectomy was performed in 85% of cases and partial laryngectomy in the remainder.274 A more recent report from the same institution observed that even though a greater variety of conservation laryngeal surgical procedures (such as TLM and SCPL) are available as treatment options, total laryngectomy is still required in approximately 70% of recurrences of early-stage (T1-2) glottic cancer.283
Therefore, in the minority of cases of early-stage recurrent disease, conservation laryngeal surgery may be possible. The results of open partial laryngectomy have been reported by several authors over the past 50 years.281,282,284,285 The more recently described procedure of SCPL has good oncologic results in the small numbers of subjects reported to date.246,286,287 Transoral laser microsurgery is effective for early-stage recurrences and, in the hands of experienced surgeons, for selected advanced stage recurrences.283,288–290
Nonsurgical organ-preservation therapies have become increasingly commonplace as a primary treatment modality for laryngeal SCC.60 However, surgery still plays an important role and may be indicated for the salvage of residual or recurrent disease at the primary site, for residual or recurrent disease in the neck, for chondroradionecrosis, or for functional problems such as intractable aspiration, laryngeal stenosis, or pharyngoesophageal stenosis. These functional problems result from the adverse effects of RT on the soft tissue of the larynx, particularly decreased tissue vascularity and fibrosis.291,292 Although it improves the oncologic efficacy of treatment, the addition of chemotherapy to RT also appears to increase the incidence and severity of these adverse soft tissue effects and there is a high risk of postoperative complications when surgery is required for these patients following RT or CRT.193,265 Poor wound healing and high rates of pharyngocutaneous fistula formation were observed in patients in the RTOG 91-11 trial undergoing salvage surgery by Weber and colleagues.265 Although a greater proportion of patients in the concurrent CRT group developed a fistula (30%), this was not statistically significant. There was no correlation between the timing of surgery and the incidence of complications.
Davidson and colleagues reported a series of 88 patients undergoing salvage surgery after RT without chemotherapy.293 The 5-year survival rate was 35%, which did not correlate with the TNM stage of the original tumor or with the timing of the diagnosis of the recurrence. As expected, the highest rate of surgical complications occurred in patients undergoing pharyngectomy (48%), with pharyngocutaneous fistulae occurring in 27% of the subjects. Sassler and colleagues294 studied patients undergoing surgery after nonsurgical organ preservation protocols and noted an overall complication rate of 61%. The rate of complications was 77% when surgery was performed within 1 year of treatment and only 20% when performed after 1 year. This was in contrast to the Davidson study. In this study (and in the literature in general), the risk of surgical complications was greatly increased when the pharynx was entered. Neck dissection alone (without entry into the UADT) has a much lower complication rate. Sassler observed a pharyngocutaneous fistula rate of 50%, with a mean time until closure of 7.7 months. The liberal use of free tissue transfer for reconstruction following total laryngectomy or laryngopharyngectomy for the treatment of RT or CRT failures decreases the incidence of pharyngocutaneous fistulae, decreases the duration of any fistulae that do occur, and decreases the rate of stricture formation and dependence on feeding tubes.295,296
Stomal Recurrence
After total laryngectomy, recurrence of SCC around the stoma is a grave sign. Stomal recurrence is often insidious and only discovered after the disease has become extensive. Recurrent disease may be secondary to paratracheal node involvement, invasion of the thyroid gland, intraoperative tumor spill with implantation of cells in the stoma, or incomplete excision of tracheal invasion by subglottic spread. Subglottic SCC is most frequently associated with stomal recurrence.297,298
The treatment of stomal recurrence is morbid and often unsuccessful, so prevention of stomal recurrence is paramount. When the risk of stomal recurrence is great (i.e., in primary subglottic SCC, glottic SCC with greater than 1 cm of subglottic extension, or T4 glottic tumors), the ipsilateral thyroid lobe is removed together with the larynx and bilateral paratracheal node dissections are performed. Adjuvant RT is also given, and the treatment field should include the upper mediastinum. In a large series reported by Rubin and colleagues,146 the majority of stomal recurrences (12 of 15) were from primary subglottic tumors.
Dissection of the mediastinum and wide local resection for stomal recurrence was pioneered by Sisson, who proposed a four-category classification system (Fig. 107-14).299 Significant morbidity and mortality are associated with the surgical treatment of stomal recurrence including injury to the great vessels, mediastinitis, hypocalcemia, and fistula formation. Aggressive treatment of stage III and IV stomal recurrence is usually not indicated because the prognosis for these patients is poor.300 Sisson concluded that mediastinal failure was an important factor in advanced head and neck cancer and should be considered for T4 glottic and subglottic tumors in which mediastinoscopy was positive for nodes.299
Prognosis and Prognostic Predictors
The 5-year relative survival rate for laryngeal SCC is 64%.60 The 5-year relative survival rates for the individual sites are 47% for supraglottic SCC, 79% for glottic SCC, and 30% to 50% for subglottic SCC.60,114 The prognosis for patients with laryngeal SCC depends on many variables, which may be classified as either disease factors or patient factors.
Disease Factors
Clinical Features
The most important predictor of prognosis for patients with laryngeal cancer is the clinical stage: An increase in T stage or in N stage is associated with a decrease in survival.301,302 However, the N stage is more important than the T stage in predicting survival.303 Overall survival is significantly better for patients who are N0 compared with patients who are N+.302 In patients with cervical metastases, the presence of extracapsular spread (ECS) on histologic examination is an unfavorable prognostic sign and is associated with a significant further reduction in survival.304,305 The prognosis for patients with distant metastases is poor, with a 5-year survival of less than 10%.117 The site of the primary tumor is the second most important predictor of prognosis. Glottic SCC has the best prognosis, and subglottic SCC has the worst.60,114
Local control of laryngeal SCC with RT is worse with tumors of larger volume.306,307 Fixation of the vocal cord upstages an SCC to T3; however, impaired vocal cord mobility is also an important clinical sign. Having observed lower local control rates after RT for T2 tumors with impaired vocal cord mobility, Harwood and DeBoer19 proposed that the classification for T2 tumors be divided into T2a (normal vocal cord mobility) and T2b (impaired vocal cord mobility). A recent study reported that the failure of return of vocal cord movement in a small series (n = 14) of T2b and T3 tumors treated with RT plus or minus chemotherapy was associated with locoregional recurrence in all cases, while none of the patients with return of vocal cord mobility had a recurrence.181
Radiologic Characteristics
Several reports have examined whether characteristics of the primary tumor observed on imaging can predict the outcome of treatment, particularly RT or CRT. Using CT or MRI, involvement of particular regions or structures of the larynx by tumor such as the anterior commissure, the PES, the laryngeal cartilages, or even having tumor adjacent to the thyroid cartilage has been found to be a predictor of local control.308–312 Tumor volume measured by CT, MRI, or even PET is also a reliable predictor of local control, although this is not always an independent factor.312–315 PET is the most accurate modality for determining tumor volume, which may be overestimated by CT or MRI.316 Another potential prognostic indicator determined by PET has recently been described: A high pretreatment standard uptake value of FDG by the primary tumor was associated with poorer local control and disease-free survival.317,318 At present there are no standardized criteria to predict local control or failure on the basis of CT, MRI, or PET.125
Histopathologic Characteristics
Several histologic features have been reported to be predictive of prognosis. The most important is the extracapsular spread of metastatic tumor in cervical nodes, which is associated with a significant decrease in survival in SCC of the head and neck including the larynx.304,305 The histologic type of tumor is also important. There are several variants of SCC, some of which have a different biologic behavior to conventional SCC. Verrucous carcinoma is less aggressive and has a better prognosis than conventional SCC, whereas the opposite is observed in cases of adenosquamous carcinoma. These variants are discussed in more detail in a subsequent section of this chapter.
The histologic grade of the tumor (well vs. moderately vs. poorly differentiated),319,320 the pattern of invasion (a pushing vs. infiltrative interface between the tumor and the normal tissue),96,321 and the presence of perineural and/or vascular invasion322 may also influence local control and survival.
The risk of local recurrence in the larynx is increased if the excision margins of the primary tumor are positive after open partial laryngectomy.323,324 Although complete excision of the primary tumor remains the most important principle of oncologic surgery, excision margins may be close without compromising local control, as reports of endoscopic laser resections have demonstrated.325
The prognostic usefulness of DNA ploidy is controversial. DNA ploidy status has been shown to be a marker for nodal metastases in laryngeal carcinoma and predictive of overall survival following RT.326 Aneuploid tumors have an increased risk of local recurrence after RT.327 However, some authors have not found DNA ploidy to be a significant prognostic factor.328
Patient Factors
Several factors independent of the stage or histologic characteristics of the tumor may affect the outcome of laryngeal SCC. Patient variables that have consistently been found to be important predictive factors include comorbidity (general health), age, and performance status. Comorbidity may be measured using one of several validated scales such as the Washington University Head and Neck Comorbidity Index (developed specifically for head and neck cancer patients)319 and is an independent prognostic factor for patients with laryngeal cancer.329,330 Advanced age (older than 65 years) is associated with a worse prognosis for SCC of the head and neck including laryngeal carcinoma.302,319 Patients with a higher performance status, as measured by scales such as the Karnofsky Performance Status, have a more favorable outcome.331,332 The significance of gender as a prognostic factor is controversial, with some reports finding no difference319,331 and others reporting a worse prognosis for women with laryngeal cancer.333,334 For patients undergoing RT for laryngeal SCC, a normal hemoglobin level is associated with increased overall survival.335
Molecular Markers of Prognosis
Clinicians and researchers alike are searching for molecular markers that will allow the identification of patients with laryngeal cancer who have adverse prognostic features so that more aggressive and successful treatment may be offered. The most commonly mutated gene in human cancer including laryngeal cancer is p53; however, its prognostic value in laryngeal cancer is uncertain.336–338 Epidermal growth factor receptor (EGFR) overexpression has been found to be a marker for aggressiveness, nodal metastasis, and survival.338,339 EGFR overexpression is also associated with radioresistance and poor local control of laryngeal cancer,340 and therefore a tumor’s EGFR status may be used to predict radiosensitivity and chemosensitivity.338 Vascular endothelial growth factor (VEGF) positivity is associated with increased overall survival in patients irradiated for laryngeal SCC.341 Cyclin D1 and cyclin D3 overexpression are reported to be significant predictors of disease-free survival, and determining the cyclin D1/D3 coexpression status of laryngeal carcinomas has allowed stratification of patients into prognostic groups, with patients overexpressing both cyclins having the worst prognosis.342 Markers of epithelial differentiation such as type 2 cyclo-oxygenase (Cox-2) and galectin-3, may also have predictive value. Low expression of Cox-2 in laryngeal SCC is associated with aggressive biologic behavior (including invasion), whereas galectin-3 expression is associated with improved overall survival.338 At present, much of the data regarding molecular markers are conflicting and no reliable prognostic molecular markers exist for clinical use.