Chapter 70 Malignant Pleural Mesothelioma
Pathogenesis
1. Mesothelioma cells have increased or dysregulated growth through both production of and response to various growth factors.
2. Mesothelioma cells express telomerase and therefore avoid telomere shortening (i.e., become “immortalized”).
3. Tumor suppressor genes such as p16 and p14, which are important in the Rb and p53 pathways, are absent in mesothelioma, allowing the tumor to avoid antigrowth signals.
4. Tumor cells evade apoptosis through increased activity of the antiapoptosis molecule Bcl-2.
5. Increased angiogenesis occurs through production of angiogenic factors such as vascular endothelial growth factor.
6. Malignant mesothelioma exists in a collagenous environment, and matrix interactions and regulation of this environment likely are related to tumor growth.
Pathology
Histology
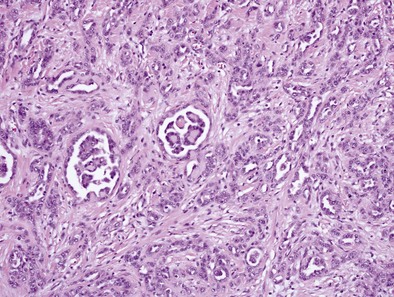
Epithelial Subtype
Epithelioid MPMs are the most common subtype and can be further characterized to a number of patterns. The tubulopapillary pattern contains a mixture of tubules and papillary structures (Figure 70-1). Adenomatoid and acinar patterns contain glandlike structures that should be differentiated from adenocarcinomas of other origin (e.g., lung). The solid, well-differentiated pattern has “nests” or sheets of cells that may resemble reactive mesothelium. Differentiating features from reactive mesothelial hyperplasia include stromal invasion, presence of necrosis and expansile nodules, as well as immunohistochemical staining patterns. Other types include solid, poorly differentiated, clear cell, and others.
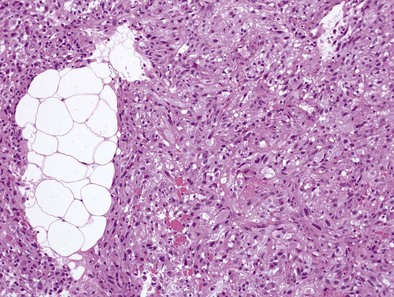
Sarcomatous Subtype
Sarcomatoid MPMs usually show spindle cells, often have more necrosis and atypical cells than epithelioid patterns, may contain anaplastic or giant cells, and include the lymphohistiocytoid and desmoplastic patterns (Figure 70-2). The desmoplastic pattern contains dense areas of collagen with small amounts of tumor cells, without significant atypia, and can be difficult to differentiate from nonmalignant pleural conditions such as fibrosing pleuritis; differentiating features include stromal invasion and disorganized growth with uneven thickness.
Immunohisto/Cytochemistry
• Reactive mesothelial hyperplasia and epithelioid MPM may be differentiated by a combination of morphologic features and IHC staining; desmin is often positive in reactive hyperplasia, and epithelial membrane antigen (EMA) and p53 may be positive in epithelial MPM.
• Malignant mesothelioma (MM) versus metastatic malignancy (often adenocarcinoma) is a common differential diagnosis. Adenocarcinomas often contain intracellular mucin on periodic acid–Schiff stain after digestion, which is only rarely seen in MM. As a general approach, initial IHC workup may include two mesothelial markers and two markers for the malignancy being considered, as well as pancytokeratin, which is positive in almost all MPMs, except less common sarcomatoid variants. If the tumor is keratin negative, panels for other cancers (e.g., melanoma, lymphoma) would be considered.
• Positive mesothelioma markers include calretinin, keratin 5/6, WT-1 protein, and podoplanin (D2-40). Markers that favor lung adenocarcinoma include Ber-EP4, which is positive in 95% to 100% of cases, compared to less than 20% of mesotheliomas. Carcinoembryonic antigen (CEA), TTF-1, MOC-31, BG8, B72.3, and CD15 are often positive in adenocarcinoma but only in up to 10% of mesotheliomas.
• When lung squamous cell carcinoma (SCC) is suspected, keratin 5/6 is not useful, being positive in both mesothelioma and lung SCC, but p63 is useful as it is expressed in almost all SCCs and in 7% of mesotheliomas. MOC-31, BG8, and Ber-EP4 are also often positive in lung SCC.
• If initial IHC panel results are conflicting, these can be expanded to include additional markers.
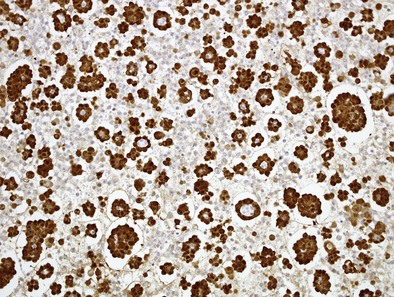
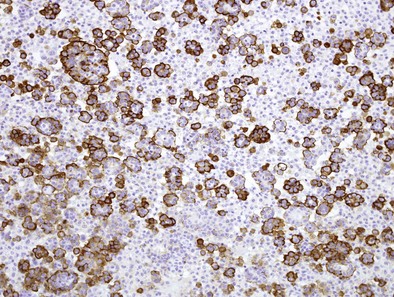
Immunocytochemistry (ICC) follows the same logic: determine if the cells are of mesothelial origin (e.g., using calretinin; Figure 70-3), then determine if they are malignant (e.g., using EMA to determine if there is a dense peripheral staining pattern; Figure 70-4). It is essential that the correct anti-EMA antibody is used because some give better results than others, partly contributing to dissent on the role of ICC in diagnosing mesothelioma.
Diagnosis
Imaging
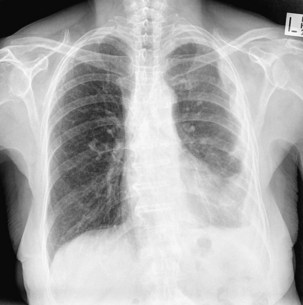
The most common finding with MPM on plain chest radiography is a unilateral pleural effusion (Figure 70-5). Pleural thickening may also be seen, or in more advanced cases, an encircling “rind” of tumor or lobulated pleural masses. Signs of asbestos exposure, such as pleural plaques, may be present but are not a precursor to mesothelioma.
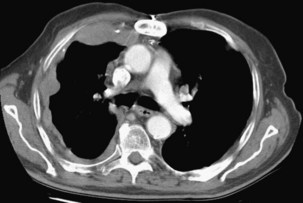
Computed tomography of the chest may show only a pleural effusion (74%). Pleural-based masses with or without thickening of the interlobular septa are not seen in most patients at presentation but develop later in the disease course (Figure 70-6). Lower lobe collapse may result from pleural encasement, pulmonary nodules (60%), or lymphadenopathy affecting the hilar and mediastinal lymph nodes.
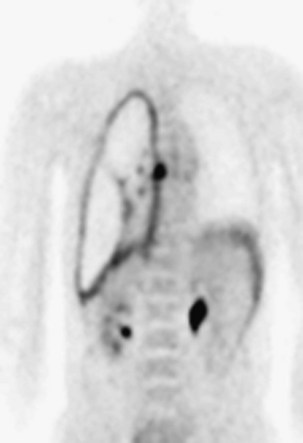
Positron emission tomography with fluorodeoxyglucose (FDG-PET) may be helpful to distinguish MPM from benign pleural disease (Figure 70-7). PET may also be useful in excluding extrathoracic disease in patients being considered for surgery, but is less accurate for defining locoregional spread to mediastinal lymph nodes. Integrated PET-CT may be more reliable in defining extent of disease than PET, CT, or MRI alone. Changes in PET results may predict response to chemotherapy.
Biomarkers
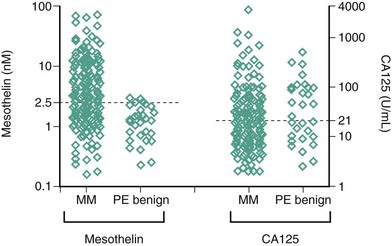
Two biomarkers, soluble mesothelin-related peptides (SMRPs) and osteopontin, have been extensively investigated as potential diagnostic or treatment monitoring markers. Mesothelin is a 40-kDa glycoprotein expressed on the surface of mesothelial cells and is overexpressed in mesothelioma. Serum SMRPs were increased in 84% of 44 patients with MPM, and in only three (2%) of 160 control patients. SMRPs measured in pleural effusions have also been investigated. In 192 patients presenting to respiratory clinics, elevated SMRP levels in effusions had a sensitivity of 67% and specificity of 98% for a diagnosis of mesothelioma, often several months before a definitive diagnosis was eventually made (Figure 70-8). Their utility has been validated, and SMRPs are now widely available. Of note, the terms mesothelin and SMRP are often used interchangeably in the literature.
Staging
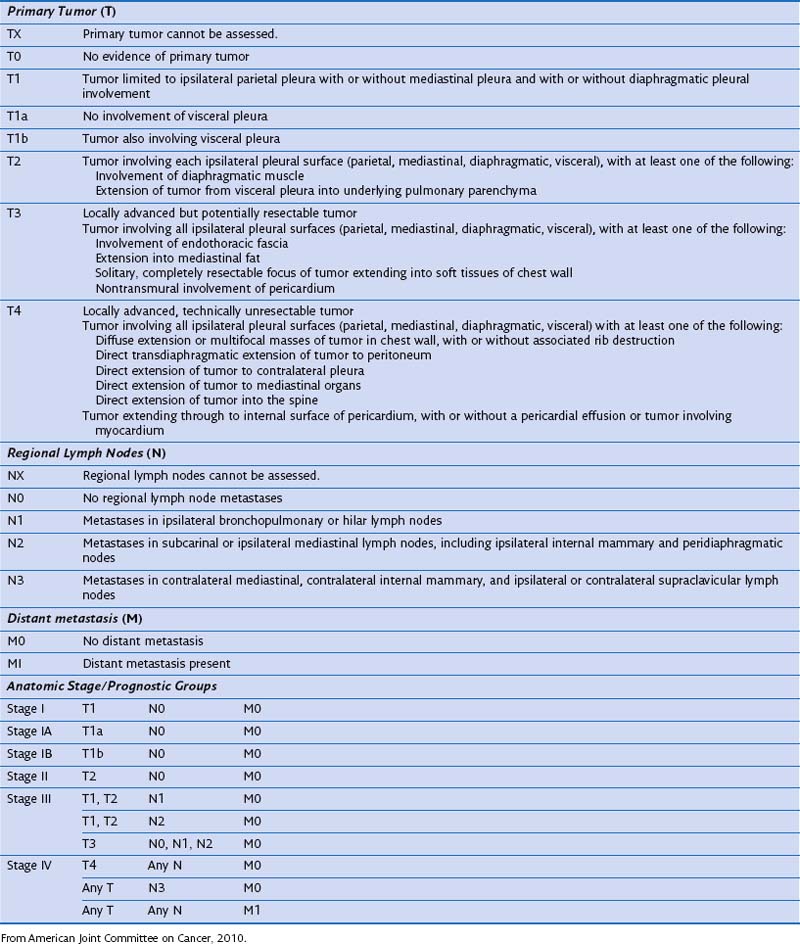
The current staging system of tumor (T), node (N), metastasis (M) is used by both the International Union against Cancer (UICC) and the American Joint Committee on Cancer (AJCC) and was adopted from the staging system proposed by IMIG in 1995. The AJCC TNM staging system for mesothelioma was published in 2010 (Table 70-1). A limitation of staging is that accurate staging, particularly of the T stage, can be determined only by surgical exploration, although initial staging with CT or PET-CT remains valuable.
Management
Cagle PT, Allen TC. Pathology of the pleura: what the pulmonologists need to know. Respirology. 2011;16:430–438.
Creaney J, Francis RJ, Dick IM, et al. Serum soluble mesothelin concentrations in malignant pleural mesothelioma: relationship to tumor volume, clinical stage and changes in tumor burden. Clin Cancer Res. 2011;17:1181–1189.
Creaney J, Yeoman D, Demelker Y, et al. Comparison of osteopontin, megakaryocyte potentiating factor, and mesothelin proteins as markers in the serum of patients with malignant mesothelioma. J Thorac Oncol. 2008;3:851–857.
Edwards JG, Abrams KR, Leverment JN, et al. Prognostic factors for malignant mesothelioma in 142 patients: validation of CALGB and EORTC prognostic scoring systems. Thorax. 2000;55:731–735.
Flores RM, Pass HI, Seshan VE, et al. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J Thorac Cardiovasc Surg. 2008;135:620–626. e1–e3
King J, Thatcher N, Pickering C, et al. Sensitivity and specificity of immunohistochemical antibodies used to distinguish between benign and malignant pleural disease: a systematic review of published reports. Histopathology. 2006;49:561–568.
Price B, Ware A. Time trend of mesothelioma incidence in the United States and projection of future cases: an update based on SEER data for 1973 through 2005. Crit Rev Toxicol. 2009;39:576–588.
Robinson BW, Lake RA. Advances in malignant mesothelioma. N Engl J Med. 2005;353:1591–1603.
Robinson BW, et al. Mesothelin-family proteins and diagnosis of mesothelioma. Lancet. 2003;362:1612–1616.
Whitaker D. The cytology of malignant mesothelioma. Cytopathology. 2000;11:139–151.