CHAPTER 88 Malignant Neoplasms of the Salivary Glands
Malignant neoplasms of the major and minor salivary glands are rare, comprising approximately 3% of all head and neck malignancies.1 The estimated incidence is only 0.9 per 100,000 in the United States, but the rate increases with age, peaking at ages 65 to 74 years.2 Less than 5% of all salivary gland tumors occur in the pediatric age group; however, salivary gland tumors in children are much more likely to be malignant than those of adults.
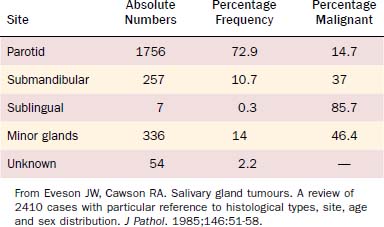
Of all salivary neoplasms (benign and malignant), the vast majority occurs in the parotid gland and the fewest in the sublingual gland. There is an interesting inverse relationship between the overall incidence of neoplasms by site and the percentage that are malignant (Table 88-1). In a review of 2410 cases of salivary gland tumors,3 73% occurred in the parotid, and of those, only 15% were malignant. On the other hand, minor salivary gland tumors constituted only 14% of the total number of cases but 46% were malignant. Likewise, submandibular gland neoplasms constituted 11% of the cases with 37% being malignant; sublingual gland neoplasms constituted only 0.3% with 86% being malignant.
The frequency of the different histologic types of salivary gland malignancy also varies depending on the gland and site. A number of studies have found the most common primary malignancy presenting in the salivary glands to be mucoepidermoid carcinoma.1,2,4 In one of the largest reviews of salivary gland neoplasms (2807 total), Spiro examined 1278 cases of malignant salivary gland tumors1 and reported that 34% were mucoepidermoid carcinoma (Table 88-2). The next most common type was adenoid cystic carcinoma (22%), followed by adenocarcinoma (a mixture of tumors which has more recently been subdivided, as described below) (18%), malignant mixed tumor (13%), acinic cell carcinoma (7%), and squamous cell (epidermoid) carcinoma (4%). When considering the types by anatomic site, mucoepidermoid carcinoma was the most frequent malignancy of the parotid, but adenoid cystic carcinoma was the most frequent of the submandibular and minor salivary glands. One exception to the latter was malignant minor salivary gland tumors arising in the nasal cavity and paranasal sinuses, in which case adenocarcinoma was the most common type as opposed to adenoid cystic carcinoma.
Table 88-2 Relative Incidence of Malignant Salivary Gland Neoplasms
| Histologic Type | Number | Percent |
|---|---|---|
| Mucoepidermoid | 439 | 34 |
| Adenoid cystic carcinoma | 281 | 22 |
| Adenocarcinoma NOS | 225 | 18 |
| Malignant mixed tumors | 161 | 13 |
| Acinic cell carcinoma | 84 | 7 |
| Squamous cell carcinoma | 53 | 4 |
| Other (anaplastic etc.) | 35 | 3 |
| TOTAL | 1278 |
From Spiro RH. Salivary neoplasms: overview of a 35-year experience with 2,807 patients. Head Neck Surg. 1986;8:177-184.
Evaluation of the Patient
Physical Examination
The clinical presentation of malignant salivary gland neoplasms can range from indolent asymptomatic masses to rapidly growing painful masses with progressive facial nerve paralysis. In Spiro’s review of 2807 salivary gland tumors, pain was a symptom in only 10% of malignant cases1 but was more frequently seen with malignant neoplasms than with benign ones. In general, episodic swelling and pain most often indicates salivary gland obstruction and inflammation, whereas constant pain is more worrisome for malignancy. However, sialadenitis can be secondary to obstruction by a salivary gland neoplasm; thus the latter must be considered in the evaluation of any salivary gland swelling with pain. Approximately 10% of parotid gland malignancies present with associated facial paralysis, and this portends a poor prognosis.5,6
Submandibular Gland
Bimanual (intraoral and external) palpation of any submandibular gland tumor should be performed to assess the extent of the tumor and to determine if there is fixation to adjacent structures such as the mandible or skin. A careful neurologic examination should also be performed to assess nerve involvement. In particular, worrisome signs of malignancy include numbness of the tongue (suggesting lingual nerve involvement), weakness of the tongue (suggesting hypoglossal nerve involvement), or weakness of the lower lip (suggesting facial nerve involvement). Careful examination of the neck is also important because 25% to 28% of submandibular malignancies will have metastases to regional lymph nodes.1,7
Sublingual Gland
The pair of sublingual glands is located in the floor of the mouth, one on each side of the frenulum, lateral to Wharton’s duct in the submucosal compartment. The drainage of these glands is via Bartholin’s ducts, which empty into Wharton’s ducts. As with submandibular gland tumors, bimanual palpation of the floor of the mouth is important to assess the extent and possible fixation of sublingual gland tumors to the mandible. Because the sublingual gland is intimately associated with the lingual and hypoglossal nerves, a careful neurologic examination is important as previously stated. Although tumors in this area are usually painless, the vast majority (86%) of sublingual gland tumors are malignant.1
Minor Salivary Glands
Minor salivary gland tissue is plentiful with estimates between 500 and 1000 glands along the upper aerodigestive tract. Although they are located in the submucosa throughout the oral cavity, oropharynx, nasal cavities, paranasal sinuses, pharynx, and larynx, the majority of them are located in the oral cavity, with the highest concentration being in the submucosa of the hard palate. As such, the site most frequently involved with minor salivary gland malignancies is the hard palate.8 Compared with the major salivary glands, minor salivary glands have minimal capsular tissue, making local invasion of tumors into surrounding tissue common. Patients with malignancies of the minor salivary glands most often present with a painless submucosal swelling, but there is frequently fixation of the overlying mucosa and ulceration. Approximately one quarter of patients will complain of local pain,9 and pain or paresthesia/anesthesia is concerning for nerve invasion. Because minor salivary glands are distributed throughout the mucosalized surfaces of the head and neck, malignancies of these glands may present in diverse ways depending on the location. Symptoms may include nasal airway obstruction, sinusitis, eustachian tube dysfunction, or hoarseness. A thorough head and neck evaluation including fiberoptic examination and cross-sectional imaging should be performed in all cases.
Radiologic Studies
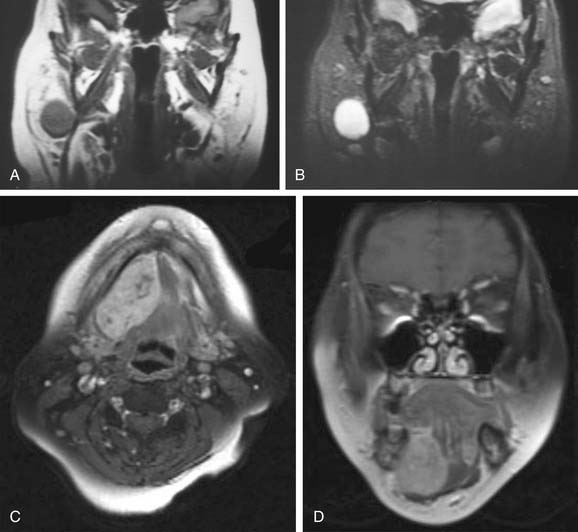
Magnetic resonance imaging (MRI) is the radiologic modality most often recommended to assess salivary gland neoplasms if there are no contraindications. Benign and malignant neoplasms of the parotid gland are well visualized on T1-weighted images because they are easily distinguished from the fatty parenchyma of the gland, which appears hyperintense. In general, benign epithelial neoplasms (such as pleomorphic adenomas) and low-grade malignancies have low T1-weighted and high T2-weighted signal intensities (Fig. 88-1A and B). High-grade carcinomas tend to have low-to-intermediate signal intensities on both T1-weighted and T2-weighted imaging (discussed later). The use of contrast material such as gadolinium and fat saturation of T1-weighted imaging provides additional information regarding the extent of a salivary gland malignancy (Fig. 88-1C and D). This is particularly useful in terms of assessing bone involvement and perineural spread. Bone marrow and cortex appear hypointense on fat-saturated images, and infiltrating tumor tissue appears hyperintense when enhanced with gadolinium. Enlarged foramina at the skull base and the presence of hyperintense enhanced tumor tissue are suggestive of perineural spread.
Some have proposed that T2-weighted imaging is helpful in distinguishing benign from malignant salivary gland neoplasms. Som and Biller10 examined 35 parotid tumors by MRI and made the following observations. Benign tumors and low-grade malignancies had low T1-weighted and high T2-weighted signal intensities, and they had clearly defined margins. High-grade malignancies, on the other hand, had low T1- and T2-weighted signal intensities and poorly defined margins. Freling and colleagues,204 however, did not make the same observations. They examined 116 patients with parotid masses, 30 of which were malignant tumors, and found no correlation between malignancy and signal intensity, heterogeneity, or radiographic margins on MRI. Malignant lesions could be discriminated from benign ones only when there was infiltration into adjacent structures. Among the malignant lesions, there was no correlation between tumor grade and MRI features. Thus MRI provides useful information about the extent of the disease, but histopathologic diagnosis is still required to distinguish benign from malignant processes.
Biopsy
Pretreatment diagnosis has been made possible by the use of fine-needle aspiration (FNA) biopsies. This diagnostic tool was established in the mid-to-late 1960s11–16 and has become a mainstay in the workup of salivary gland neoplasms. It is an extremely safe procedure that is well tolerated by patients and is considered to have no significant risk of tumor seeding to surrounding tissue.17
Although FNA biopsies of salivary neoplasms have proven to be extremely useful for preoperative planning, the interpretation of these biopsies can be difficult, resulting in diagnostic ambiguity and inaccuracy. A few entities have classic, pathognomonic features on needle aspiration. However, the variety of different tumor types and their widely overlapping histology usually necessitates the acquisition of biopsy or resection tissue for definitive diagnosis. Also, and most importantly, many malignant salivary gland tumors can only be diagnosable as such when the growth pattern (e.g., infiltration beyond the capsule into soft tissue, perineural invasion) is taken into consideration. In these cases, tumors can only be definitively diagnosed as malignant on surgical specimens, which adequately demonstrate the tumor architecture and its relationship to peripheral tissue. This is particularly true for low-grade malignant neoplasms such as polymorphous low-grade adenocarcinoma,18 myoepithelial carcinoma,19,20 and basal cell adenocarcinoma,21 which usually have bland-looking tumor cells and lack necrosis or abundant mitoses. Among tumors of the head and neck, FNA biopsies of major salivary gland tumors are considered to have the highest rate of error.22 By most reports, the sensitivity of FNA biopsies to diagnose a malignant neoplasm is much lower than the specificity.22–26 In other words, it is more common to misdiagnose a malignant tumor as benign than the reverse. A 5-year review of data from 6249 participant responses from the College of American Pathologists Interlaboratory Comparison Program in nongynecologic cytology revealed that FNA biopsies had a 68% sensitivity for diagnosing a salivary gland neoplasm as malignant, with an overall false-negative rate of 32%.27 The greatest number of false-negative diagnoses occurred in cases of lymphoma (false-negative rate = 57%), followed by acinic cell carcinoma (false-negative rate = 49%), low-grade mucoepidermoid carcinoma (false-negative rate = 43%), and adenoid cystic carcinoma (false-negative rate = 33%). In cases of benign neoplasms from the same study, the specificity of FNA biopsies was 91%, with an overall false-positive rate of 8%. The greatest false-positive rate was in cases of monomorphic adenoma (53%), which were most often diagnosed as adenoid cystic carcinoma. Pleomorphic adenomas, when diagnosed as malignant, were most often misinterpreted as adenoid cystic carcinoma, and Warthin’s tumors as lymphoma.
Although the utility and ease of FNA biopsies have resulted in a decline in the use of frozen section analysis intraoperatively, there are still several indications for frozen section studies, as reviewed by Seethala and colleagues28 and Westra.22 These include assessing the extent of tumor spread to local/regional tissues such as the nerves and lymph nodes, assessing surgical margins, and confirming/establishing the diagnosis in cases where the preoperative FNA biopsy was not diagnostic or was equivocal.
Staging
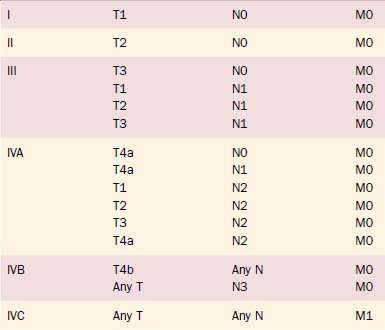
Clinical staging of salivary gland cancers is important for prognosis and treatment decisions. The 2003 TNM staging classification for major salivary gland cancers established by the American Joint Committee on Cancer (AJCC) is the classification most commonly used in the United States (Tables 88-3A and B). Minor salivary gland carcinomas are staged according to the anatomic site of origin (e.g., oral cavity, sinus, larynx). The staging guidelines are applicable to all forms of carcinoma; any nonepithelial tumor type is excluded.
Table 88-3A 2003 AJCC TNM Staging for Major Salivary Gland Cancer
| Primary Tumor (T) | |
| Tx | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| T1 | Tumor is ≤2 cm in greatest dimension without extraparenchymal extension* |
| T2 | Tumor is >2 cm but not >4 cm in greatest dimension without extraparenchymal extension* |
| T3 | Tumor is >4 cm in greatest dimension and/or has extraparenchymal extension* |
| T4a | Tumor invades skin, mandible, ear canal, and/or facial nerve |
| T4b | Tumor invades skull base and/or pterygoid plates and/or encases carotid artery |
| Regional Lymph Nodes (N) | |
| Nx | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in a single ipsilateral lymph node, ≤3 cm in greatest dimension |
| N2a | Metastasis in a single ipsilateral lymph node, >3 cm but not >6 cm in greatest dimension |
| N2b | Metastases in multiple ipsilateral lymph nodes, none >6 cm in greatest dimension |
| N2c | Metastases in bilateral or contralateral lymph nodes, none >6 cm in greatest dimension |
| N3 | Metastasis in a lymph node, >6 cm in greatest dimension |
| Distant Metastasis (M) | |
| Mx | Distant metastasis cannot be assessed |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
AJCC, American Joint Committee on Cancer.
* Extraparenchymal extension is clinical or macroscopic evidence of invasion of soft tissues. Microscopic evidence alone does not constitute extraparenchymal extension for classification purposes.
From Greene FL. AJCC Cancer Staging Manual. 6th ed. New York: Springer-Verlag, 2002.
Histopathology
Because salivary gland malignancies are remarkably diverse and heterogeneous, their behavior and resulting clinical management are highly dependent on their histologic type and, quite often, their grade. Thus knowledge of the types of tumors and their pathologic classification is critical for the clinician in order to provide proper treatment. Table 88-4 lists most of the critical pathology-related issues that the clinician needs to consider. The following section is intended to provide a succinct, but sufficient, discussion of the pathology of these tumors.
Table 88-4 Critical Pathologic Features for Assessing Malignant Salivary Gland Tumors
| Tumor Type | Unique Pathologic Issue(s) |
|---|---|
| Mucoepidermoid carcinoma | Grade |
| Adenoid cystic carcinoma |
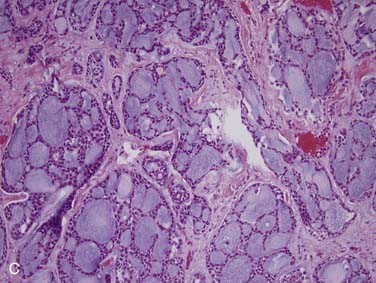
First, it is important to consider the normal histology of the glands because most tumors differentiate into the same cell types that are present in the normal gland. Salivary glands contain acini composed of either serous or mucous cells or a mixture of both. Serous cells are rounded or polygonal in shape and characteristically have abundant blue cytoplasmic granules, which are periodic acid–Schiff (PAS) positive. Mucous cells consist almost entirely of lightly basophilic intracytoplasmic mucus. The fluid secreted by the parotid gland is almost exclusively serous, while that from the sublingual gland is almost exclusively mucous and that from the submandibular gland is a mixture of serous and mucous. The ducts have cuboidal to columnar lining cells with abundant eosinophilic cytoplasm. They form tubular structures within the glands (Fig. 88-2). Both the acini and ducts have supportive cells called myoepithelial cells along their periphery. The parotid glands normally contain, on average, 10 to 20 intraglandular lymph nodes, a feature of great importance because many parotid masses represent metastases to these lymph nodes from primary skin or other cancers of the head and neck. These nodes have an otherwise typical appearance to any other nodal tissue in the body. The submandibular (also referred to as submaxillary) and sublingual glands have no intraglandular lymph nodes.
Neoplasms of the salivary gland can be roughly classified on the basis of the type of normal salivary gland cell toward which they differentiate. Neoplasms can differentiate toward the acinar, ductal, or myoepithelial cells. Practically speaking, though, most of them have dual differentiation—specifically, most salivary gland neoplasms have some myoepithelial differentiation.29 Also, most of the benign neoplasms have a malignant counterpart (e.g., pleomorphic adenoma and carcinoma ex pleomorphic adenoma, basal cell adenoma and basal cell adenocarcinoma, myoepithelioma and myoepithelial carcinoma). The number of different malignant epithelial tumors in the World Health Organization (WHO) classification has increased greatly over the past 50 years and now includes 24 entities.21 Between 21% and 46%3 of salivary gland tumors are malignant, including 15% to 32% of parotid, 41% to 45% of submandibular, 70% to 90% of sublingual, and 50% of minor salivary gland tumors.21 It is important to note that the incidence of particular histologic types is dependent on the site. For example, polymorphous low-grade adenocarcinoma virtually never occurs in the major glands.18,30
Mucoepidermoid Carcinoma
Mucoepidermoid carcinoma is the most common salivary gland malignancy.3,31–33 The majority of cases occur in the major salivary glands,34 but mucoepidermoid carcinoma can also arise from minor salivary glands in the oral cavity, particularly in the hard palate, buccal mucosa, lip, and retromolar trigone.35 Rarely, they can also arise intraosseously in the mandible and maxilla, but mucoepidermoid carcinomas of this location are considered odontogenic in origin and have a less aggressive clinical behavior.36,37 Clinically, mucoepidermoid carcinomas are slightly more common in women and have a mean age of approximately 45 years, but they can also occur in children.31 In fact, they are the most common pediatric salivary gland carcinoma.38 Patients usually present with a painless, slow-growing mass. Intraoral tumors may mimic a mucocele or vascular lesion clinically by presenting as a blue-red superficial nodule.3
Grossly, these tumors are not distinct. They usually have both solid and cystic components, often with mucinous material within the cysts.39 This is what sometimes imparts a bluish color to them, which can mimic the appearance of a mucocele in the oral cavity. Microscopically, their hallmark is the presence of three cell types: mucous, squamoid (or epidermoid), and intermediate (Fig. 88-3A). The architecture is usually a mixture of cystic (see Fig. 88-3B) and solid elements, the latter with sheets (see Fig. 88-3C), nests, or ductlike structures. The mucous cells have abundant, light blue mucin in their cytoplasm and nuclei displaced to the periphery. The mucin is usually obvious, but, in cases where it is scant, special stains such as PAS, mucicarmine, or Alcian blue can be used to highlight it. The squamoid cells are large with abundant pink cytoplasm, and, although they look somewhat squamous in appearance, true keratinization in mucoepidermoid carcinoma is rare.40 Intermediate cells typically have more modest amounts of pink or clear cytoplasm. The proportion of cell types varies quite a bit among tumors. Intermediate cells usually predominate and mucous cells usually line cystic spaces. Cytologic atypia varies from minimal to quite prominent.41–43 Immunohistochemistry is of limited to no utility in the diagnosis.39
The differential diagnosis, particularly in tumors arising along mucosal sites, includes necrotizing sialometaplasia, an uncommon non-neoplastic lesion of the hard palate with reactive changes in minor salivary glands44 and, more importantly, adenosquamous carcinoma, which is an aggressive variant of squamous cell carcinoma. Adenosquamous carcinomas are high grade, have definitive squamous differentiation usually with keratinization (unlike mucoepidermoid carcinoma), and often have surface mucosal squamous dysplasia, a feature that mucoepidermoid carcinoma does not have.39
Grading of mucoepidermoid carcinoma is important and correlates strongly with clinical behavior, although reproducibility and consistency are major issues, and no particular system has been universally accepted.41–4345 Clinical staging is as important as histologic grade, so the two should be considered in tandem. In general, low-grade lesions have a prominent cystic component and abundant well-differentiated mucous cells with little cytologic atypia and low mitotic activity. High-grade lesions are more solid with squamoid and intermediate cells predominating. They also have cytologic atypia, mitotic activity, necrosis, and infiltrative growth. A number of different grading schemes (well reviewed by Luna39) have been reported over the years. The most used grading system, originally designed by Auclair and colleagues,41,43 uses a three-tiered score based on a number of histologic features (Table 88-5). The original grading system was initially criticized for a tendency to “under grade” the tumors when others demonstrated that a significant number of low-grade mucoepidermoid carcinomas developed progressive disease. The later modification by Brandwein and colleagues42 (see Table 88-5) refined the grading system such that none of the tumors classified as low grade in their study went on to have progressive disease. Low-grade mucoepidermoid carcinoma, as strictly defined by their criteria, rarely ever metastasizes or results in death of the patient.41–43
| Parameter (Auclair et al, 1992) | Point Value |
|---|---|
| Cystic component <20% | +2 |
| Neural invasion | +2 |
| 4+ mitoses/10 HPF | +3 |
| Necrosis | +3 |
| Anaplasia | +4 |
| Grade | Point Score |
|---|---|
| Low (I) | 0-4 |
| Intermediate (II) | 5-6 |
| High (III) | 7 or more |
| Parameter (Brandwein et al, 2001) | Point Value |
|---|---|
| Cystic component <25% | +2 |
| Tumor front invades in small nests and islands | +2 |
| Pronounced nuclear atypia | +2 |
| Lymphatic and/or vascular invasion | +3 |
| Neural invasion | +3 |
| Necrosis | +3 |
| 4+ mitoses/10 HPF | +3 |
| Bony invasion | +3 |
| Grade | Point Score |
|---|---|
| Low (I) | 0 |
| Intermediate (II) | 2-3 |
| High (III) | 4 or more |
From Auclair PL, Goode RK, Ellis GL. Mucoepidermoid of intraoral salivary glands. Cancer. 1992;69:2021-2030; Brandwein MS, Ivanov K, Wallace DI, et al. Mucoepidermoid carcinoma. A clinicopathologic study of 80 cases with special reference to histological grading. Am J Surg Pathol. 2001;25(7):835-845.
In addition to grade, location of the primary tumor is potentially important in predicting clinical behavior. Several studies have shown that low-grade mucoepidermoid carcinomas of the submandibular gland recur and metastasize much more frequently than those of the parotid or minor salivary glands.40,43 Whether or not this represents truly different biology, it merits the aggressive and thorough resection of any submandibular gland primary malignancy, especially for known mucoepidermoid carcinoma.42
Adenoid Cystic Carcinoma
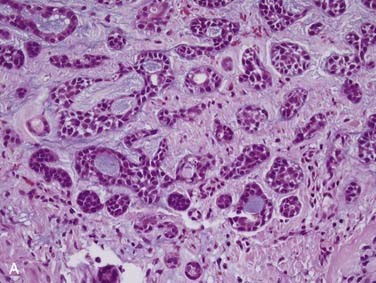
Adenoid cystic carcinoma (ACC) is one of the more common,3,32,33 and certainly more recognizable salivary gland tumors, being notorious for its infiltrative growth and slowly progressive behavior with recurrences and spread over a protracted course of many years.46 These tumors essentially occur with an even distribution among all salivary gland sites,32 although the total number of minor salivary gland cases outnumber those of major salivary glands when all sites are considered together.47–50 These malignancies occur with an equal incidence in men and women over a wide age range with the peak incidence between 50 and 60 years of age.47–4951
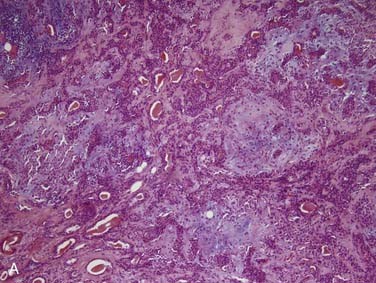
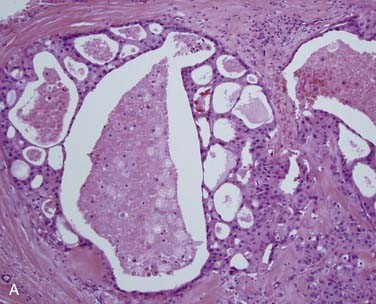
Grossly, ACCs are solid, light tan, firm and well-circumscribed, but unencapsulated, tumors. Microscopically, there are three growth patterns: tubular, cribriform, and solid. The tubular form consists of small tubules sitting in a pink, hyalinized, and hypocellular stroma (Fig. 88-4A). The solid variant has only rounded lobules of tumor cells with no, or almost no, glandlike structures and without a defined architecture (see Fig. 88-4B). The classic and most easily recognized pattern, though, is the cribriform (“Swiss cheese”-appearing) variant. Nests of cells are arranged around glandlike spaces that consist of PAS-positive blue or pink material (see Fig. 88-4C). The central spaces look like glandular lumina but are actually extracellular cavities containing reduplicated basement membrane (or ground substance) material52,53 and myxoid material that is produced by the tumor cells, rather than true epithelial mucin. The cells in ACC are markedly basaloid in appearance, with little cytoplasm and round-to-oval nuclei that are dark and hyperchromatic without nucleoli (see Fig. 88-4A).48 They are quite uniform in size and show little mitotic activity, except for the solid type, where mitotic activity can range from barely to extremely prominent. A rim of inconspicuous myoepithelial cells with clear cytoplasm is also usually present.29,54
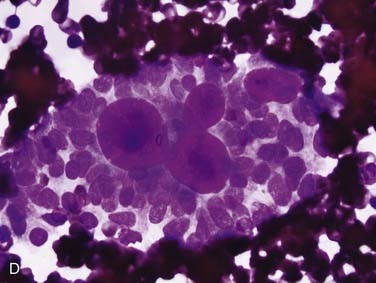
FNA frequently yields characteristic results in ACC. Aspirates have tumor cells with scant cytoplasm and round, regular nuclei in sheets and clusters. These features by themselves are not characteristic and actually are so bland that they might suggest a benign lesion. However, the finding of well-defined, round “cylinders” and/or spheres of acellular stroma accompanying these cells is typical, but not 100% specific, for ACC. The cells often “cling” to these rounded structures (see Fig. 88-4D).
Histologic grading has yielded conflicting results in the literature for predicting prognosis. Clinical stage, on the other hand, holds a great deal of prognostic information and, as such, should be considered as much or more than grade in clinical management.51 Also, as with mucoepidermoid carcinoma, ACC of the submandibular gland has a much more aggressive clinical course than ACC of other sites.49,51 The most accurate prognostic information has been garnered when tumors are not only graded by the predominant pattern (tubular, cribriform, or solid) but are more specifically graded as follows: tubular with or without some cribriform areas (grade I), cribriform with no tubular areas and with less than 30% solid areas (grade II), and cribriform with greater than 30% solid areas (grade III).49,55 A frequent histologic feature of ACC is perineural invasion, which is identified in approximately 70% to 75% of cases.49,56 Although somewhat inconsistent in the literature, many have correlated this finding with a worse prognosis, particularly when involving a major nerve trunk.46,57–59
The diagnosis of ACC is generally straightforward from routine hematoxylin and eosin examination. However, it can be difficult on small biopsies because one may not be able to appreciate the architecture and infiltrative growth. The differential diagnosis includes other salivary gland neoplasms (such as polymorphous low-grade adenocarcinoma or pleomorphic adenoma), basaloid squamous cell carcinoma, or high-grade neuroendocrine carcinoma.54 Although beyond the scope of this text, immunohistochemistry is not usually necessary but can occasionally be helpful in differentiating among these tumor types.60,61
Polymorphous Low-Grade Adenocarcinoma
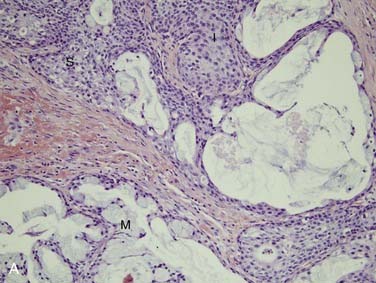
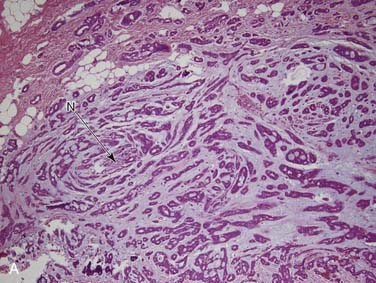
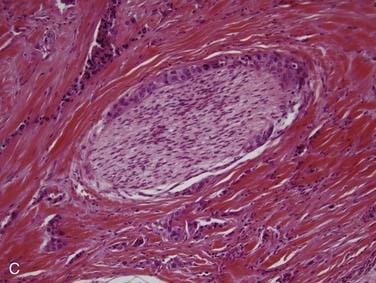
Polymorphous low-grade adenocarcinoma (PLGA) is a unique, low-grade neoplasm first recognized as a distinct entity in the mid 1980s.62 PLGAs arise almost exclusively from the minor salivary glands and, in most series, are the second most common minor salivary gland carcinoma.35,46 The most common site for these neoplasms is the palate,63 particularly at the junction of the hard and soft palates. Other sites include the upper lip, buccal mucosa, and posterior third of the tongue.18 There are only rare reports of PLGA arising in major salivary glands.30 PLGA is twice as common in women and tends to present in the fourth to sixth decades as a slow-growing mass that may have been present for years.18,63 They are often asymptomatic.18 On gross examination, they are circumscribed, unencapsulated, pale yellow or tan masses that range from 1 to 3 cm. Microscopically, the architectural features of this tumor are quite variable, as the name would suggest. They are well circumscribed but unencapsulated and may appear as solid nests in lobules, cribriform glandlike structures, or ductlike arrangements. A common appearance within the tumor is concentric whorling of the nests around each other in a single-file arrangement. This has been termed the “eye of the storm” pattern (Fig. 88-5A). Stromal hyalinization with a slate-gray coloration is also characteristic.18 Tumor cells are quite regular with moderate eosinophilic cytoplasm and characteristic round-to-oval, extremely regular nuclei with open chromatin (see Fig. 88-5B). There is little mitotic activity and no necrosis. The periphery of the tumor shows markedly infiltrative growth and most cases have perineural invasion (see Fig. 88-5C).18,63,64
The differential diagnosis includes pleomorphic adenoma and ACC. The myxochondroid areas of pleomorphic adenoma are not seen in PLGA, and, although minor salivary gland pleomorphic adenomas are unencapsulated, they will not show the infiltrative growth pattern of PLGA and will not have perineural invasion. One particular immunostain that may be useful in difficult cases is worth mentioning here. Curiously, it has been recognized that almost all pleomorphic adenomas will be positive for glial fibrillary acidic protein (GFAP), whereas almost all PLGA are negative.65–68 ACC is important to differentiate from PLGA. This is done primarily by cytologic features because ACCs have basaloid cells with dark chromatin and little cytoplasm, whereas PLGA cells have moderate eosinophilic cytoplasm and nuclei with open chromatin.
PLGA is a low-grade malignancy, and histologic grading is not applicable. Conservative resection is the treatment of choice. Local recurrence occurs in 10% to 15% of patients.18,63,69 Because lymph node metastases are distinctly uncommon (literature rates of around 10% or less), neck dissection is only recommended with significant clinical adenopathy or needle-proven metastasis. This likely overestimates the occurrence, however. With strict histologic criteria for PLGA, nodal metastases are extremely rare.18 Distant metastases are equally as uncommon,70 and patients have an excellent long-term prognosis.18,63,69 In fact, few patients have been documented to die from this tumor and only after prolonged periods.18,63
Acinic Cell Carcinoma
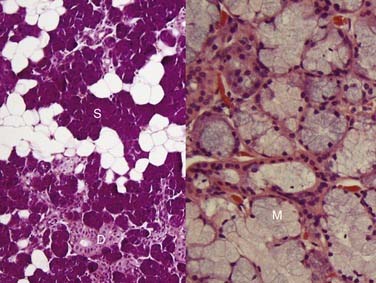
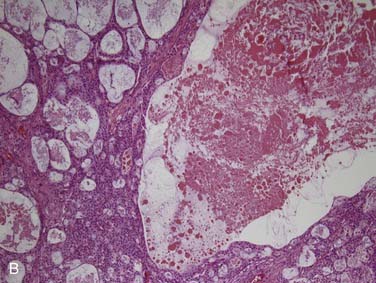
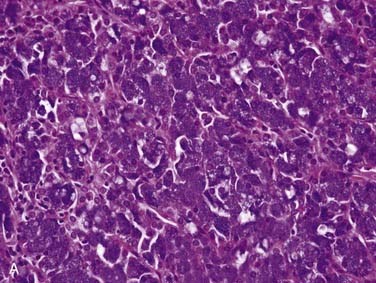
Acinic cell carcinoma, as the name implies, is a tumor with cells showing differentiation toward cells of the normal salivary gland acini (see Fig. 88-2). However, these tumors also show evidence of ductal and myoepithelial differentiation, an important feature to keep in mind. They are uncommon, comprising 1% to 3% of all tumors and approximately 10% of all malignant ones.3,32,33,71 More than 90% occur in the parotid,72,73 with the rest scattered among minor salivary gland sites and rarely the submandibular gland.71,74 Occurring over a wide age range from children to the elderly, they are relatively evenly distributed in the second through seventh decades75 with a peak in the third decade.73 Of note, these tumors are the second most common childhood salivary gland malignancy. They present as a slowly growing mass, which is only occasionally painful and rarely associated with a facial palsy.71,73
Grossly, acinic cell carcinoma presents as a single, usually circumscribed, rubbery, solid mass with up to one third showing cystic degeneration.75 Microscopically, they are highly variable, a feature of these tumors that has always led to angst in the diagnosing pathologist. The four principal histologic patterns are solid/lobular, microcystic, papillary-cystic, and follicular. Small tumors can be easily missed because the acinar cells are so well differentiated that they blend into the surrounding normal gland. Two features are classic, however. The first is the characteristic acinic cell, which has blue cytoplasm with abundant serous-type granules and a small, round, eccentrically placed nucleus (Fig. 88-6A). PAS stains will be strongly positive in these cells. A number of other cell types are seen including pink, clear, and vacuolated cells, such that most tumors are a mixture of different cell types (see Fig. 88-6A). The second classic feature is a dense lymphoid infiltrate with germinal centers (see Fig. 88-6B). The periphery of the tumors may or may not be infiltrative but are often “pushing” in nature (see Fig. 88-6C). Although one would assume that lack of an infiltrative border might signal a benign or low-grade lesion, there is no benign equivalent for this lesion (i.e., there is no known benign acinic cell neoplasm). “Dedifferentiation” has been described in a small subset of acinic cell carcinomas. This consists of tumors with areas of classic, “conventional” acinic cell carcinoma and an intermixed undifferentiated carcinoma in sheets with large, pleomorphic cells, brisk mitotic activity, and often necrosis.76–78
FNA biopsy of acinic cell carcinoma is fraught with difficulty because of the resemblance to normal tissue.27 The diagnosis rests on finding a cellular specimen with sheets and clusters of large acinar cells with abundant granular cytoplasm and central round, regular nuclei. One should not see normal ductal epithelial cells or significant amounts of adipose tissue (both of these latter findings are from normal salivary gland tissue), but there is often a prominent background component of lymphocytes.
Surgical resection with negative margins is the most important therapy. These tumors will recur in approximately one third of cases.71,73 Although classically regarded as low-grade malignancies, 10% to 15% of these tumors will metastasize locally to regional lymph nodes or distantly to the lung and bones. Acinic cell carcinomas are also notorious for recurrence and spread years beyond the primary presentation and for a protracted clinical course such that survival curves do not flatten out until after a decade.79 Survival is approximately 80% at 5 years and 70% at 10 years.71,75,79 Grading of these tumors has not correlated well with behavior, and there is not good agreement on histologic features within the proposed grading systems. Very bland, low-grade-appearing tumors can metastasize almost as frequently as tumors with more aggressive features. However, “dedifferentiation” (where there is a mixture of typical acinic cell carcinoma with a much higher-grade carcinoma) has consistently been associated with a poor outcome.76–78
Malignant Mixed Tumors
“Malignant mixed tumor” is the broad term that is used to encompass three different salivary gland malignancies: true malignant mixed tumor (or carcinosarcoma), carcinoma ex pleomorphic adenoma, and metastasizing mixed tumor. As a group, these account for approximately 3% to 5% of all salivary gland malignancies,3,32 and carcinoma ex pleomorphic adenoma is by far the most common of these.
True Salivary Gland Malignant Mixed Tumor (or Carcinosarcoma)
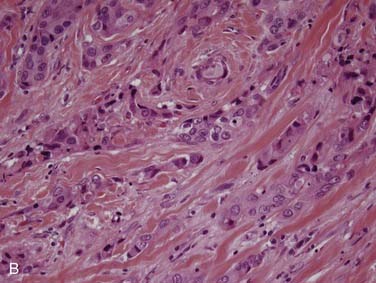
True salivary gland malignant mixed tumor (or carcinosarcoma) is a malignant neoplasm that consists of distinct carcinomatous and sarcomatous components. These tumors are rare,46 comprising approximately 1% of all salivary gland malignancies.32,35 The mean age of presentation is 58 years. Two thirds of cases arise in the parotid gland, approximately 15% in the submandibular gland, and 15% in the palate.80–84 Grossly, the findings are not distinct. The mass may be circumscribed or ill defined. It is commonly firm and tan-white with hemorrhage and necrosis and, on occasion, grittiness or calcification. Microscopically, there is an intimate admixture of the two components (carcinoma and sarcoma), but the amounts of each can vary widely. The carcinoma component can have any type of differentiation but typically takes the form of high-grade salivary duct carcinoma or undifferentiated carcinoma (Fig. 88-7A).80 The sarcomatous component is usually a chondrosarcoma (see Fig. 88-7B) or osteosarcoma,80 but fibrosarcoma, leiomyosarcoma,83 or even liposarcoma85 is possible. Treatment consists of wide local excision combined with radiotherapy. The tumors are aggressive with up to two thirds of patients dying of either local recurrence or, commonly, distant metastasis to the lungs or bone, usually within a period of 30 months.80
Carcinoma ex Pleomorphic Adenoma
Carcinoma ex pleomorphic adenoma is defined as a pleomorphic adenoma (or mixed tumor) in which, or with which, a carcinoma is present. It accounts for more than 95% of malignant mixed tumors but is still relatively uncommon. The critical thing to remember is that this term encompasses a heterogeneous group of tumors because the carcinoma component can be of any form, from very low grade to the highest grade types.86 These tumors are most common in the parotid gland, followed by the submandibular gland, the minor salivary glands, and the sublingual gland.87 Although there is a wide age range, most patients are in their sixth and seventh decades, approximately 1 decade later than the peak age for pleomorphic adenomas. The classic history is a patient with a longstanding mass that suddenly undergoes rapid growth over a period of several months.87,88
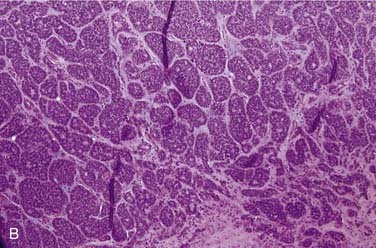
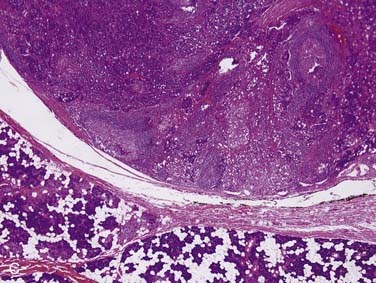
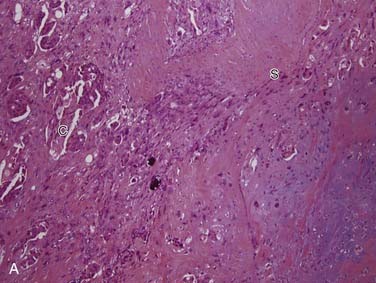
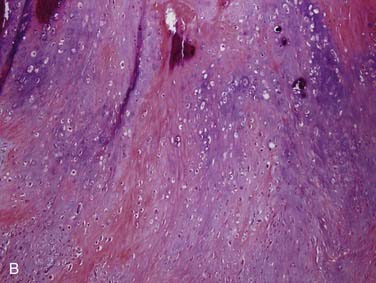
Grossly, these tumors can reach up to 25 cm and the average is more than twice that for pleomorphic adenomas.86 They usually consist of a tan-yellow, firm mass with ill-defined, infiltrative borders, and there may be a component of nodular, translucent blue or gray tumor representing the preexisting pleomorphic adenoma.88 Microscopically, the proportions of the two components vary quite a bit from tumor to tumor. The pleomorphic adenoma component has typical features with myxoid, blue stromal areas, a variable proliferation of epithelial elements, including ductlike structures and sheets of myoepithelial cells, and chondroid areas reminiscent of cartilage (Fig. 88-8A). This may be replaced by scarring or be overgrown by the malignant component. The malignant component is often distinguished by its collagenous stromal hyalinization (see Fig. 88-8B). It most commonly takes the form of poorly differentiated adenocarcinoma NOS, salivary duct carcinoma, or undifferentiated carcinoma.88 However, essentially any form of carcinoma can be found. As one can imagine, the prognosis and management are highly dependent on the type of carcinoma.86
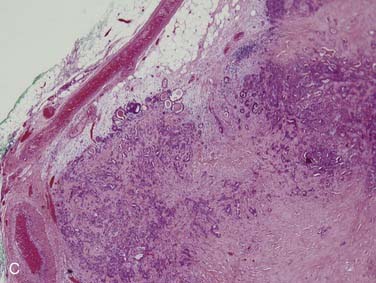
The extent of invasion is a critical histologic feature for prognostication.86,88–92 The malignant component should be classified as noninvasive (intracapsular), minimally invasive (≤1.5 mm beyond the capsule), or invasive (>1.5 mm). For those tumors where there is no invasion beyond the capsule/rounded edge of the tumor (so-called carcinoma ex pleomorphic adenoma “in-situ”) (see Fig. 88-8C) or where there is less than 1.5 mm invasion beyond it, there is essentially no risk of recurrence or metastasis from a completely resected tumor such that the behavior approaches that of benign pleomorphic adenoma.92 For invasive tumors, those with high-grade features do poorly, and those that are widely invasive have a survival ranging from 26% to 65% at 5 years and 0% to 38% at 20 years.86,88,89 Wide resection with lymph node dissection and radiation therapy is the treatment of choice for widely invasive tumors or obvious cervical lymph node metastases.
Metastasizing Mixed Tumor/Pleomorphic Adenoma
This is the least common form of malignant mixed tumor. These are tumors having the exact, bland morphology of a pleomorphic adenoma with a mixture of myoepithelial and ductlike epithelial elements and myxoid and/or chondroid surrounding matrix (see Fig. 88-8A). They show no significant cytologic atypia or mitotic activity, but they metastasize either to local lymph nodes (30%) or to distant sites including bone (50%) and lung (30%).93–95 Often, there is a prior protracted clinical course with recurrences at the primary site.96 The mean time between presentation of the primary tumor and detection of metastatic disease is 12 years.96 Although rare, metastasizing mixed tumor serves as an important reminder to treat pleomorphic adenomas properly at the time of initial therapy.
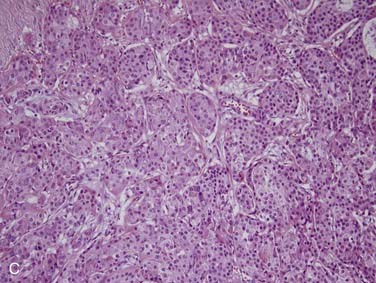
Salivary Duct Carcinoma
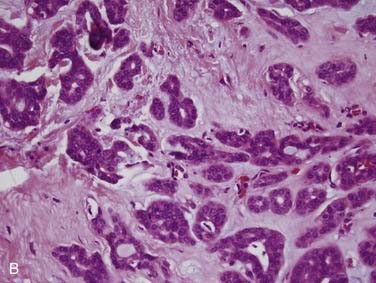
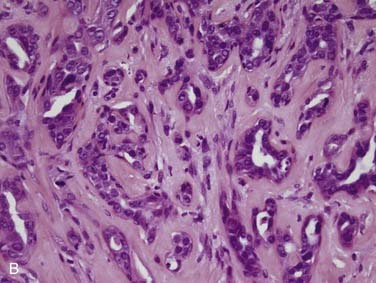
Salivary duct carcinoma is a relatively recently described tumor that was separated from the generic category of salivary adenocarcinoma.97 It is one of the most aggressive primary salivary gland tumors and has a similar histologic appearance to high-grade ductal carcinoma of the breast.97–99 It accounts for less than 10% of salivary gland tumors. Males are more commonly affected at a rate of 4:1.98,100–105 Patients generally present in the sixth decade with a rapidly growing parotid mass; a significant minority have facial nerve involvement and occasional facial skin ulceration.105 Between 70% and 90% of cases arise in the parotid, with the remaining few occurring in the submandibular gland and oral minor salivary glands.98,101,104–106 Grossly, these tumors are ill defined, solid and white, with hemorrhage, necrosis, and occasionally cystic areas.101,105 Infiltration of the surrounding tissue is usually quite obvious. Histologically, most salivary duct carcinomas have a large and prominent ductal carcinoma in-situ component with a cribriform pattern101 similar to what is seen in breast ductal carcinomas (Fig. 88-9A). This is the classic histologic finding. There is often central comedo-type necrosis. The invasive component consists of small nests, cords, and single large cells with abundant pink cytoplasm and large, round nuclei with vesicular chromatin and prominent nucleoli (see Fig. 88-9B).101,105 There is marked tissue infiltration with stromal desmoplasia and brisk mitotic activity. Vascular and perineural invasion (see Fig. 88-9C) are common.101,105,106 Immunohistochemistry is not particularly useful for diagnosis, but salivary duct carcinomas are positive for low- and high-molecular-weight cytokeratins, carcinoembryogenic antigen (CEA), androgen receptors, and, in a significant minority, Her2/neu,99,107,108 with distinct membrane staining for the latter. A minority of the tumors have actual amplification of the Her2/neu gene. Unlike the histologically similar breast carcinoma, almost all salivary duct carcinomas are negative for estrogen and progesterone receptors.99 The differential diagnosis includes metastatic breast carcinoma, poorly differentiated squamous cell carcinoma, and mucoepidermoid carcinoma. Intraductal cribriform carcinoma is the most important feature that argues for a diagnosis of primary salivary duct carcinoma and rules out these other possible lesions.
Salivary duct carcinoma is high grade by definition. It is the most aggressive salivary gland carcinoma with 30% to 40% of patients developing local recurrence and between 50% and 75% developing distant metastases and dying of their disease, most within 4 years of diagnosis.101,104,105 Wide local excision with neck dissection and postoperative radiotherapy is the treatment of choice.
Primary Squamous Cell Carcinoma
Squamous cell carcinomas are only rarely primary in the salivary gland, representing less than 1% of all malignancies in this site.109 Metastases to intraparotid lymph nodes from primary skin cancers of the head and neck (particularly of the scalp, ear, and face) are much more common, as is direct invasion from adjacent tissues. Most patients are in their sixth through eighth decades, and sometimes there is a history of prior radiation therapy. By definition, the diagnosis of primary squamous cell carcinoma is restricted to the major salivary glands because those arising in the minor salivary glands cannot be distinguished from primary squamous carcinoma of the surrounding mucosa. Of these, 80% to 90% arise in the parotid gland, and 10% to 20% in the submandibular gland.109,110 These tumors typically are high stage at the time of diagnosis.
Grossly, they appear as a firm, white, infiltrative, and nonencapsulated mass. Microscopically, they are identical to squamous cell carcinomas of the upper aerodigestive tract, although they tend to be better differentiated (most are moderately to well differentiated with abundant keratinization).109–112 There is usually prominent desmoplasia and perineural invasion, with extension of the tumor into periglandular soft tissue.109–112
Primary squamous cell carcinomas are aggressive tumors, with a 5-year survival of approximately 20% to 50%.112 Histologic tumor grade does not correlate well with behavior. Treatment involves radical surgery, neck dissection, and radiotherapy.
Primary Small Cell Carcinoma
Small cell carcinoma (or high-grade neuroendocrine carcinoma) can occur as a primary tumor in the salivary glands, but this is quite uncommon, representing approximately 2% of salivary gland malignancies.113,114 The majority occur in major salivary glands, particularly the parotid.113,114 They present as a painless mass of several months, typically in patients in their sixth and seventh decades, many with associated cervical lymphadenopathy and/or facial nerve palsy.
Grossly, they are not distinct, being poorly circumscribed, infiltrative masses that are white or tan with frequent necrosis.114 Microscopically, they consist of large sheets or trabeculae of “small blue cells” (having moderate-sized nuclei and minimal cytoplasm). The nuclei characteristically have a punctate, granular (“salt and pepper”) chromatin without nucleoli.113,114 There is nuclear molding where nuclei look pressed against each other due to the minimal amount of cytoplasm (Fig. 88-10A). There is brisk mitotic activity, frequent apoptosis, and often areas of necrosis.
Immunohistochemistry shows staining for neuroendocrine markers such as synaptophysin, chromogranin-A, and CD56 (N-CAM).113 There is typically punctate or “dotlike” staining for pan-cytokeratin. In addition, specific cytokeratin staining patterns have been described including positive staining for cytokeratin 20 (see Fig. 88-10B) and absence of staining for cytokeratins 34βE12 and 5/6.61
The differential diagnosis includes the solid variant of ACC and poorly differentiated squamous cell carcinoma, both of which can be ruled out by neuroendocrine marker staining (synaptophysin, chromogranin-A, neural cell adhesion molecule or N-CAM). Importantly, for both the pathologist and clinician, one must rule out metastases from primary lung small cell carcinoma or skin Merkel’s cell carcinoma. Lung primary tumors should be detectable clinically and also would show positive staining for TTF-1 and lack of staining for cytokeratin 20. Metastatic Merkel’s cell carcinoma, on the other hand, is virtually indistinguishable from primary salivary small cell carcinoma113,115,116; thus clinical findings are critical for these diagnoses.
The prognosis for small cell carcinomas is always relatively poor. However, for primary salivary small cell carcinoma, it may be slightly better than for pulmonary small cell, with reported 5-year survival rates between 13% and 50% and an average across studies of approximately 30% to 40%.113,114,117 Lymph node metastases are frequent, as are distant metastases to the liver, lung, brain, and bones.113
Other Malignant Tumors
The salivary glands are the primary site of a large number of additional (and heterogeneous) tumors, only a few of which are discussed here. Most of the additional carcinomas are low grade, with relatively frequent local recurrences, but uncommon or rare metastases. These include epithelial-myoepithelial carcinoma,118 clear cell carcinoma, cystadenocarcinoma, basal cell adenocarcinoma,119 and myoepithelial carcinoma,19,20 although the latter is somewhat more aggressive.
Basal cell adenocarcinoma is the rare, malignant counterpart to basal cell adenoma. The vast majority arises in the parotid gland119 and has a typical morphology consisting of basaloid cells in tubular, trabecular, solid, or membranous patterns. The latter is called such because of the thick, hyalinized collagen that surrounds the nests of tumor cells. It is usually a low-grade carcinoma with histology showing a modest degree of cellular atypia and can only really be diagnosed as malignant on the basis of infiltrative growth into the surrounding tissue with or without perineural invasion.119 Local recurrence occurs in a minority of patients, and metastases to regional lymph nodes in less than 10%. The prognosis is thus excellent.
Myoepithelial carcinoma is the uncommon malignant counterpart to myoepithelioma. About 60% occur in the parotid gland.19 Like myoepitheliomas, these can have diverse cell types including epithelioid, spindled, plasmacytoid or hyaline, or clear cell. They have infiltrative peripheral borders, and one third of patients will die of metastatic disease, one third will have multiple local recurrences, and one third will be disease free after resection.
Adenocarcinoma NOS is a “salivary gland carcinoma that exhibits ductal differentiation but otherwise lacks the histologic features that define other types of salivary carcinoma.” This should not be considered a “wastebasket” diagnosis; it is simply a gland-forming carcinoma outside of the usual “herd.” There is an almost equal distribution between major and minor salivary glands.120,121 They can range from bland but infiltrative tumors to pleomorphic and high-grade tumors with necrosis and wide infiltration. Thus given this heterogeneity, tumors should be graded into three categories: low grade, intermediate grade, and high grade.121 In general, the prognosis for adenocarcinoma NOS is worse than for most other salivary gland carcinoma with reported 10-year survival rates of 55%120 and 15-year survival rates of 41%, 34%, and 28% for low, intermediate, and high grade tumors, respectively.121
Metastases
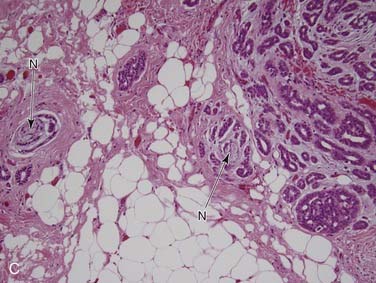
Metastasis to the salivary glands or, more commonly, to intraglandular or periglandular lymph nodes, is a frequent occurrence. The parotid gland contains an average of 20 intraparenchymal lymph nodes, whereas the submandibular and sublingual glands do not contain any.122 For this reason, the vast majority of metastases to the parotid gland are to intraparenchymal lymph nodes and thus from head and neck primaries,123,124 while approximately 85% of those to the submandibular gland are from non–head and neck primaries. The parotid lymph nodes drain from the scalp, face, and ear skin and from the external auditory canal and tympanic membrane, so skin tumors such as squamous cell carcinoma and melanoma account for approximately 80% to 90% of metastases.123,124 Merkel’s cell carcinomas also spread to this area but are uncommon. The remaining are from non–head and neck primary tumors, most commonly kidney, lung, and breast carcinomas.125 The opposite distribution is seen for the submandibular gland, with 85% of metastases coming from infraclavicular primary tumors, most commonly breast, kidney, and lung carcinomas (small cell carcinoma of the lung in particular).125–128
Lymphoma
Primary lymphoma of the major salivary glands comprises approximately 5% of extranodal lymphomas and approximately 2% of all salivary gland tumors.129,130 It is defined as arising in the salivary gland when there is absence of lymphoma in a noncontiguous site based on clinical evaluation.
Primary non-Hodgkin’s lymphoma (NHL) occurs either as a de novo process unrelated to other diseases or as a secondary process related to lymphoepithelial sialadenitis. De novo NHL can be large B-cell lymphoma (35%), follicular lymphoma (35%), or another low-grade lymphoma (30%), with half of the latter being secondary forms of the extranodal marginal zone B cell lymphomas of the mucosa-associated lymphoid tissue (MALT) type or just MALT lymphomas.129,131 Most patients are older with the average age between 50 and 70130 and present with a solitary, painless mass in de novo cases. In contrast, there is often a history of waxing and waning enlargement of several glands in the secondary forms.132 Most cases of salivary gland Hodgkin’s lymphoma occur in the parotid gland and probably just represent disease involvement of intraparotid lymph nodes.
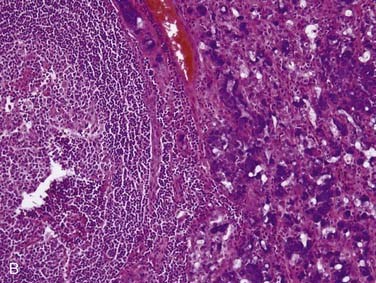
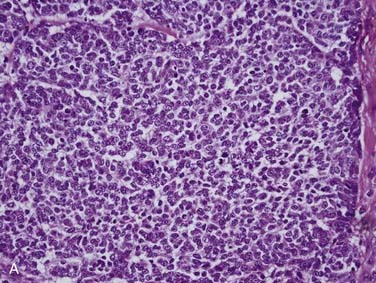
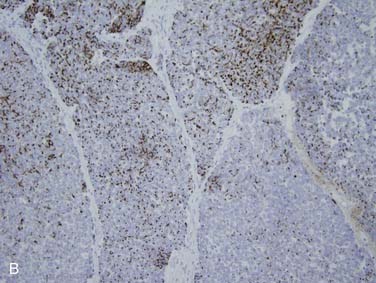
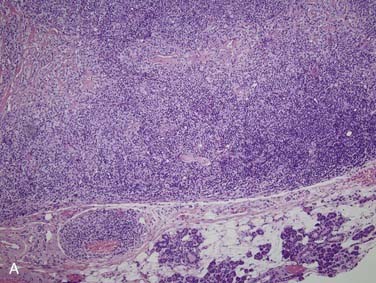
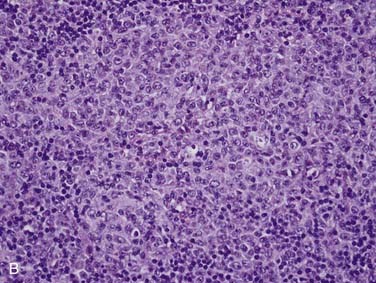
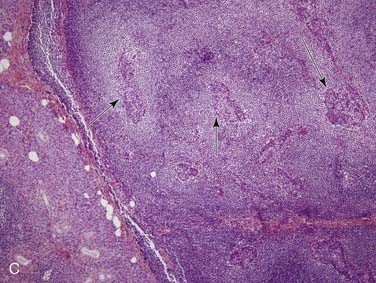
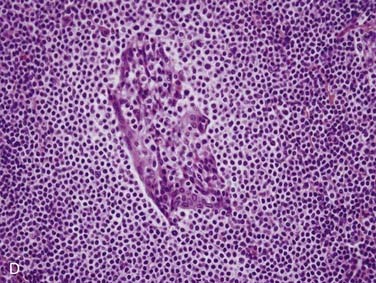
Grossly, salivary glands involved with lymphoma usually have a tan-white to yellow-tan color and are diffusely and homogeneously involved (so-called “fish-flesh” appearance). The microscopic appearance depends a great deal on the type of lymphoma. Primary diffuse large B cell lymphoma consists of sheets of large lymphocytes diffusely effacing the normal gland and infiltrating (with little tissue reaction) into and around anything that is left (Fig. 88-11A). The cells have modest amounts of cytoplasm, round to oval nuclei with pale chromatin, one to three prominent nucleoli, and brisk mitotic activity (see Fig. 88-11B). Other primary lymphomas will have a similar growth pattern but with different cells depending on the type. MALT lymphomas occur in the setting of lymphoepithelial sialadenitis, with this being prominent in the background. There is a somewhat heterogeneous infiltrate of B cells with sheets of medium-sized lymphocytes with abundant pale cytoplasm, uniform nuclei, and distinct cell membranes. These form “halos” around the epithelial cell nests and extend away from them in broad strands (see Fig. 88-11C).129 There is also a mixture of small cleaved lymphocytes, plasma cells, and lymphoplasmacytic cells that have cytologic features of both of the former two cell types (see Fig. 88-11D). The lymphoma cells markedly expand the spaces between the epithelial islands and also infiltrate into these islands along with the non-neoplastic lymphocytes from the background non-neoplastic sialadenitis. FNA is quite useful for diagnosing salivary gland lymphoma with high sensitivity and specificity for the diagnosis, as well as for providing material for flow cytometry.133
Pediatric Salivary Gland Tumors
Pediatric salivary gland tumors are uncommon. The most common is the hemangioma followed by a long list of tumors, most of which are identical to adult forms such as mucoepidermoid carcinoma, acinic cell carcinoma, and ACC. Two are unique to the congenital period and bear mentioning here. Sialoblastoma is an extremely rare, potentially aggressive neoplasm that recapitulates the embryonic stage of salivary gland development and has been suggested to represent retained blastomatous cells. Local recurrence is relatively common, occurring in up to 30% of cases. Metastasis, however, is uncommon, and only one fatality has been reported. Salivary gland anlage tumor is a peculiar and unique hamartoma occurring in the perinatal period as a pedunculated mass attached to the wall of the nasopharynx.134 These may obstruct the airway and, in this way, be clinically significant. Interestingly, they may be incidentally suctioned out of the newborn’s airway or spontaneously expelled.
Prognostic Variables
The overall 10-year, disease-free survival rate of patients with salivary gland malignancies ranges from 47% to 74%.4,32,135–139 Multiple studies have examined clinical, histologic, and molecular variables that may predict clinical outcome and survival. There is considerable variability in the results, and this is probably due to the fact that most of these studies have been of small numbers of patients. In addition, these data have been obtained entirely from retrospective studies; no prospective studies examining potentially prognostic variables have been reported. Nevertheless, some common themes can be gleaned from the available data (Table 88-6).
Table 88-6 Factors Associated with Occult Metastases and/or Poor Prognosis
| Feature | Comment |
|---|---|
| Primary tumor site | Tumors of the submandibular and sublingual glands tend to metastasize more frequently than those of the parotid glands |
| Primary tumor size and extraglandular extension | T3 and T4 tumors tend to have a higher incidence of occult metastases and worse outcome |
| Age | Patients older than 50 years of age have a worse outcome |
| Presenting symptoms | Facial nerve paralysis and pain are associated with lymph node metastases |
| Histologic type/grade | Neoplasms with higher incidence of nodal metastases and poorer prognosis include high-grade mucoepidermoid carcinoma, undifferentiated carcinoma, squamous cell carcinoma, adenocarcinoma NOS, and salivary duct carcinoma |
| Local tissue invasion | Perineural invasion and local bone invasion portend a shorter disease-free and overall survival |
| Positive resection margins | Associated with worse locoregional control and lower survival rate |
| High Ki-67 and low p27 expression | Associated with shorter disease-free survival in adenoid cystic and mucoepidermoid carcinoma |
| HER-2/neu overexpression | Associated with shorter disease-free survival in salivary duct carcinoma and mucoepidermoid carcinoma |
Clinical Variables
In general, several clinical characteristics of the primary tumor have been proposed to be predictive of outcome. Not surprisingly, advanced tumor (T) stage, which takes into account both tumor size and the presence of extraglandular involvement, has been widely reported as being associated with the presence of metastases and poor survival.138–146 The site of the primary tumor has also been proposed to have an association with poor survival. In particular, patients with malignant tumors of the submandibular and sublingual glands tend to have more frequent metastases and a worse outcome than patients with malignant tumors of the parotid gland.1,145,147,148 The relationship between the presence of metastatic disease and the tumor’s site and stage is reviewed later in “Surgery.”
The age and gender of the patient have also been suggested to be associated with prognosis. In a multivariate analysis by Terhaard and colleagues,138 more advanced age was an independent predictor of poor overall survival and male gender was associated with a 17% increased risk for distant metastasis. Hocwald and colleagues142 also observed that patients older than the age of 50 years presented with more aggressive tumors and that men presented with higher T-stage tumors than women (53% vs. 26%). Similarly, O’Brien and colleagues149 reported that patients 60 years or older had a significantly shorter survival but they did not find gender to be a significant factor for prognosis.
Presenting symptoms have also been examined for prognostic value. Specifically, facial nerve dysfunction and pain have been suggested in multiple studies to portend a poor prognosis. In the study by Terhaard and colleagues,138 complete facial paralysis was an independent predictor of failed regional control, with a relative risk of 6.1. Likewise, in a multivariate analysis by North and colleagues,150 facial nerve paresis was predictive of poor outcome with a 3-year, relapse-free survival of only 13%. In addition to its association with poor survival, facial nerve involvement has also been shown to be highly predictive of lymph node metastases.146,151 Pain is another symptom that has been found to be associated with a poor clinical outcome in some studies152; however, due in part to its subjective measurement, pain has not always been highly predictive.138
Histologic Variables
When referring to histologic grade, high-grade malignant tumors are considered to include squamous cell carcinoma, undifferentiated carcinoma, high-grade mucoepidermoid carcinoma, and carcinoma ex-pleomorphic adenoma.137,153 Low-grade malignancies include acinic cell carcinoma, low-grade mucoepidermoid carcinoma, low-grade adenocarcinoma (including basal cell and mucinous adenocarcinoma), and papillary cystadenocarcinoma. Intermediate-grade malignancies include adenoid cystic carcinoma and epithelial-myoepithelial carcinoma. In a multivariate analysis of 470 malignant tumors of the major salivary glands, Spiro and colleagues137 found that histologic grade was an independent prognosticator of survival. This has been corroborated in another retrospective review by Chen and colleagues139 of 207 patients with carcinomas of the major salivary glands that were treated with surgery alone. In this study, high histologic grade was also found to be an independent predictor of locoregional recurrence. Results such as these have led to the suggested role for postoperative radiation therapy in the treatment of high-grade salivary gland malignancies (discussed in “Radiation Therapy” later).
When considering a single histologic type of salivary gland malignancy, subtype grading of some malignancies is also highly correlated with clinical outcome. In general, grading seems to be most useful in predicting the clinical behavior of mucoepidermoid carcinoma and adenocarcinoma NOS.1,154–157 In a review of 108 patients with mucoepidermoid carcinoma of the major and minor salivary glands by Guzzo and colleagues,158 high-grade tumors were associated with a significantly reduced, 5-year, disease-free survival rate (22.5%) compared with low-grade tumors (97%, P <0.0001). Similarly, a recent review of 60 patients with mucoepidermoid carcinoma by Aro and colleagues159 found patients with low-grade tumors to have a significantly better disease-free survival than those with either intermediate-grade or high-grade tumors. There was, however, no difference in disease-free survival between the intermediate and high-grade groups. Although adenoid cystic carcinoma has one of the more varied histologic phenotypes (tubular vs. cribriform vs. solid) allowing for easier grading classification, there is controversy over the utility of tumor grade in predicting the prognosis of patients with this malignancy. Some have strongly advocated its utility,160,161 but studies by Spiro and colleagues1,47,51 did not find grade to be predictive of clinical outcome in patients with this tumor type.
Local tissue invasion, regardless of histologic type, appears to portend a worse clinical outcome. In particular, perineural invasion and spread has been found to be a predictor of a more aggressive tumor behavior. In the multivariate analysis by Terhaard and colleagues,138 perineural invasion was an independent prognostic factor associated with the risk of distant metastasis (relative risk = 2.2). Similarly, Hocwald and colleagues142 found perineural invasion to be an independent predictor of shorter disease-free survival in their multivariate analysis. Local bone invasion has also been shown to be independently associated with an increased risk of local recurrence and shorter overall survival.138,162
Not surprisingly, the presence of positive surgical margins has also been shown to be associated with a worse clinical outcome. Therkildsen and colleagues135 found this variable to be independently associated with both worse locoregional control and lower survival rates. Terhaard and colleagues138 also found the status of the resection margin to be independently associated with poor local control with a relative risk of 3.5. These observations are consistent with other studies in which poor local control was associated with positive resection margins.56,163,164
Molecular Markers
Markers of cell proliferation have been suggested to be prognostic for patients with salivary gland malignancy. Early studies looking at the proliferative fraction in salivary gland tumors using flow cytometry noted an association between proliferative activity and survival.165 Two of the more commonly studied markers of proliferation are proliferating cell nuclear antigen (PCNA) and Ki-67. The expression of both PCNA and Ki-67 by tumor cells has been shown to correlate with higher-tumor grade and shorter, disease-free survival of patients with ACC and mucoepidermoid carcinoma.166–171 In general Ki-67 is considered to be a more accurate marker of proliferating cells because it has a shorter half-life and hence more specific staining. In mucoepidermoid carcinoma, investigators have found that tumors with high expression of Ki-67 and low expression of p27 (a cyclin dependent kinase inhibitor, the action of which slows cell cycle progression) have a worse prognosis.172,173
Another potential indicator of prognosis is a specific translocation, t(11;19), which brings together the MECT1 and MAML1 genes. This translocation has been identified in up to 70% of mucoepidermoid carcinomas.174 Tumors with this translocation were found to be better differentiated and were shown to have a better prognosis. Although provocative, this has not garnered enough attention nor proven useful enough to be instituted in practice in the management of patients with these tumors. Whether or not it adds information beyond grade and clinical stage is unclear.
Another marker that has been investigated as a potential prognosticator is a receptor tyrosine kinase of the ErbB family. HER-2/neu (also known as ErbB2) is frequently expressed in a subset of salivary gland malignancies, salivary duct carcinoma in particular, and expression correlates significantly with prognosis.104,175 This oncogene is also overexpressed in approximately one third of mucoepidermoid carcinomas, and its overexpression appears to correlate with poorer prognosis.176,177 Prognostic data such as these have become the basis for targeted therapy against HER-2/neu, as discussed later in “Chemotherapy.”
Treatment
Surgery
Parotidectomy
The parotid gland can be divided into a superficial (or lateral) and a deep “lobe” by a sagittal plane defined by branches of the facial nerve. The main trunk of the facial nerve, after it exits the stylomastoid foramen, gives off branches to the posterior belly of the digastric muscle and then runs through the parenchyma of the parotid tissue. It divides into an upper temporofacial division and a lower cervicofacial division at the pes anserinus, which is located approximately 1 to 2 cm from the stylomastoid foramen. The upper division divides further into the temporal, zygomatic, and buccal branches (Fig. 88-12). The lower division divides into the marginal mandibular and cervical branches. It is important to note that the parotid gland is actually unilobular and that the plane created by the fanning branches of the facial nerve is not a true anatomic separation of the gland into two discrete lobes. That being said, it is useful from a surgical standpoint to use the facial nerve as a plane of dissection.
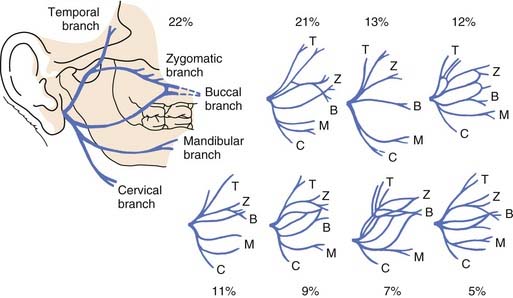
Figure 88-12. Branches of the facial nerve. Variations of the facial nerve branching in the parotid gland are depicted.
(Adapted from Davis RA, Anson BJ, Budinger JM, et al. Surgical anatomy of the facial nerve and parotid gland based upon a study of 350 cervicofacial halves. Surg Gynecol Obstet. 1956;102:385-412).
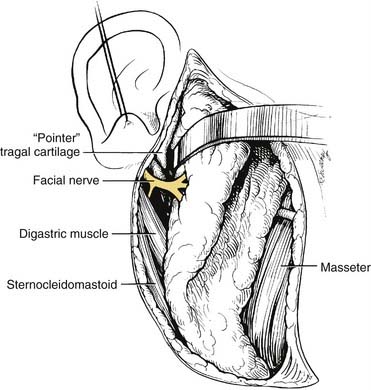
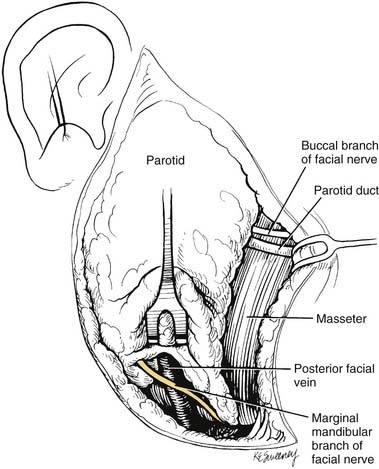
When performing a parotidectomy, one begins by making a modified version of the Blair incision typically used in rhytidectomies (Fig. 88-13). It begins at the root of the helix and extends inferiorly in the preauricular crease, curving posteriorly just below the lobule and then extending inferiorly and anteriorly to lie in a skin crease approximately two fingerbreadths below the inferior border of the mandible at the level of the hyoid bone. The inferior part of the incision can be extended to provide exposure for a lymph node dissection should one be indicated. An anteriorly based skin flap is then elevated in the subcutaneous plan immediately over the superficial musculoaponeurotic system (SMAS) layer. The greater auricular nerve is identified and traced inferiorly. It is divided superiorly and reflected inferiorly, preserving as much length as possible in the event that the facial nerve is resected and a cable graft is required. The tail of the parotid is elevated off of the sternocleidomastoid muscle, and the posterior belly of the digastric muscle is identified. The parotid gland tissue is then elevated off of the anterior aspect of the tragal cartilage, and the dissection is carried into the deeper aspects of the gland. The main trunk of the facial nerve is identified approximately 1 cm medial to the tragal pointer (Fig. 88-14). The nerve exits the stylomastoid foramen immediately posterior to the styloid process and enters the parotid gland immediately anterior to the insertion of the digastric muscle into the mastoid tip. In this region the posterior auricular artery coming from the external carotid artery is typically encountered and should be ligated once the facial nerve is identified. The main trunk of the nerve is traced anteriorly to the pes anserinus, and the branches of the upper and lower divisions are then followed, separating the lateral lobe of the gland from the nerve. Deep to the lower branches of the facial nerve, the retromandibular (posterior facial) vein may be encountered and should be ligated if a deep lobe parotidectomy is to be performed. If the location of the tumor prevents the identification of the main trunk of the facial nerve, one can identify a distal branch and trace the branch in a retrograde fashion back to the main trunk. The marginal mandibular branch is often used for this and can be found by following the posterior facial vein superiorly (Fig. 88-15).
While performing a deep lobe parotidectomy, multiple intraglandular and periglandular vessels will be encountered. Specifically, branches of the external carotid artery must be identified and controlled, taking great care to identify and preserve the internal carotid artery. The external carotid artery is found just superior to the digastric muscle, and it should be controlled and/or ligated just before it enters the parotid gland. As mentioned earlier, the retromandibular (posterior facial) vein will often also need to be ligated to obtain access to the deep lobe. Once these vessels are controlled, the main trunk and branches of the facial nerve should be carefully elevated off of the deep lobe tumor (Fig. 88-16). Once this is accomplished, the gland can be dissected off of the deep musculature, mandible, and temporal bone. Intraglandular branches of the external carotid that will be encountered include the transverse facial artery and the internal maxillary artery. These should be ligated at the anterior aspect of the gland, at the posterior border of the mandibular ramus. If the tumor extends into the parapharyngeal space, it is dissected free using blunt dissection. Occasionally, the stylomandibular ligament will need to be divided so that the mandible can be retracted anteriorly to provide better access.
Management of the N0 Neck
The decision of whether or not to treat a clinically N0 neck is controversial. Although some have advocated elective treatment of the neck in all patients with salivary gland malignancies, the majority have recommended that treatment of the neck be performed only in selected patients with tumors having poor prognostic features for metastasis (see Table 88-6).
The site of the primary tumor appears to be associated with the frequency of occult nodal involvement. Armstrong and colleagues145 reviewed 474 cases of patients with major salivary gland malignancies in order to define the indications for elective neck dissections. Of the cases, 407 (86%) had clinically N0 necks, and of these, an elective neck dissection was performed in 90 of the 407 (22%) patients. Occult metastases were discovered on histopathologic analysis in 34 of the 90 (38%) of the patients undergoing neck dissections. Albeit, these cases tended to be higher grade and more advanced. Tumors of the submandibular gland had a significantly higher incidence of occult metastasis to the cervical lymph nodes (21%) than those of the parotid gland (9%). These findings are corroborated by other studies. Spiro and colleagues1 reviewed 2807 patients with salivary malignancies and also found that submandibular cancers had a higher incidence of lymph node metastases (28%) compared with parotid (18%) and minor salivary gland malignancies (15%). Yu and Ma147 also found that submandibular and sublingual cancers tended to metastasize more frequently than cancers of other sites. Thus the site of the primary malignancy appears to be an important feature when considering treatment of the N0 neck.
The size of the primary tumor and the presence of extraglandular extension have also been correlated with risk of metastases. Armstrong and colleagues145 demonstrated that tumors greater than or equal to 4 cm in size had a 20% risk of occult metastases compared with a 4% risk associated with smaller tumors (P < 0.00001). In a multivariate analysis of risk factors associated with occult metastases from parotid malignancies, Frankenthaler and colleagues146 found that extraparotid tumor extension was among the most predictive variables. The T stage, which takes into account both the size of the primary and the presence of extraglandular extension, has been shown in multiple other studies to be associated with the risk of metastatic disease.143,144 Thus results such as these have led to the suggestion that patients with high T stage (T3 and T4) tumors should undergo routine neck dissections even if they do not have clinical evidence of cervical lymph node metastases.
Other features that have been associated with a higher risk of occult metastases are the histologic type and grade of the primary tumor.145–147,151,178–180 In general, histologic types considered to be at higher risk for occult nodal involvement include undifferentiated carcinoma, squamous cell carcinoma, high-grade mucoepidermoid carcinoma, adenocarcinoma NOS, carcinoma ex pleomorphic adenoma, and salivary duct carcinoma. Histologic types that have been deemed of lower risk include acinic cell carcinoma, adenoid cystic carcinoma, and low-grade mucoepidermoid carcinoma. The grade of the primary tumor has been reported to correlate with the incidence of occult nodal disease. Armstrong and colleagues145 found that high-grade tumors had a 49% risk of occult metastases compared with a 7% risk for intermediate- and low-grade tumors. Similarly, Bhattacharyya and Fried151 reported that high grading conferred a 1.99 odds ratio (95% CI 1.64 to 2.40) of having occult nodal involvement. These authors concluded that elective treatment of the neck is indicated in patients with high-grade histologic types such as adenocarcinoma and squamous cell carcinoma and high-grade subtypes such as high-grade mucoepidermoid carcinoma.
In sharp contrast to the authors of the studies cited earlier, Stennert and colleagues143 found a much higher incidence of occult cervical lymph node metastases in all salivary gland malignancies, regardless of histologic type or tumor stage. As such, they have strongly advocated the elective treatment of the neck in all patients, raising controversy in the management of N0 necks. Their study of 160 consecutive patients was unique in that they treated all patients with an ipsilateral neck dissection in a nondiscriminatory manner. They found that of the 139 patients who were initially staged as having an N0 neck, 45% had occult metastases. The rates of occult metastases were particularly high for undifferentiated carcinoma (75%), squamous cell carcinoma (64%), and adenocarcinoma NOS (58%). These authors also found occult metastases to be surprisingly frequent in what has traditionally been considered “lower-risk” histologic types: acinic cell carcinoma (44%) and adenoid cystic carcinoma (36%). In addition, whereas occult metastases have most often been reported with larger tumors, Stennert and colleagues143 found that even T1 and T2 lesions had frequent metastases (29% and 54%, respectively). From these data, the authors concluded that an ipsilateral neck dissection should be performed on all patients with salivary gland malignancies, regardless of histologic type or stage.
Another area of controversy has to do with how the neck should be treated once it has been actually decided to do so. Some have advocated the use of radiation therapy to treat an N0 neck because many of the features associated with occult disease are the same features that indicate the need for postoperative radiation of the primary site.181 Others have advocated surgery in order to spare patients with pathologically N0 necks the morbidity of radiation and to obtain accurate staging of the disease. The arguments in favor of surgery also state that, in most cases, the exposure for the neck dissection is already established and/or partially completed during the resection of the primary tumor.
When a neck dissection is performed in a patient with a clinically N0 neck, a selective neck dissection is considered the treatment of choice. The levels of the neck to be dissected are determined by the site of the primary tumor. For parotid tumors, Armstrong and colleagues145 demonstrated that 3 out of 30 patients (10%) had metastases in level I, 8 of 30 (27%) in level II, 7 of 30 (23%) in level III, 6 of 30 (20%) in level IV, and 1 of 30 (3%) in level V. They show that dissection of levels I through IV would have detected all occult metastases. The one patient with a positive level V node also had node-positive disease in levels II through IV. As such, the dissection of at least levels I through IV for parotid malignancies has been recommended. For submandibular and sublingual malignancies, occult metastases can be detected in N0 necks addressing levels I through III alone.145,182 If occult disease is detected in the first echelon dissection for either parotid or submandibular primary malignancies, however, a comprehensive neck dissection is recommended to treat possible additional disease in the other levels.
Management of the N+ Neck
A consensus regarding treatment of the clinically N+ neck is more established. It is well accepted that an ipsilateral neck dissection should be performed in patients with palpable or radiographically enlarged cervical lymph nodes.182 In addition, the use of radiation therapy following a cervical lymphadenectomy has been shown to improve locoregional control and survival (discussed in detail later).183,184 Because salivary gland malignancies that have metastasized to regional lymph nodes can involve any of the five levels in the neck and can skip levels,145 it has been recommended to perform a comprehensive modified radical neck dissection in N+ necks. However, because the incidence of nodal involvement in the contralateral neck is low, only the ipsilateral side should be treated.144
Radiation Therapy
Adjuvant Radiation Therapy
In general, the use of postoperative radiation therapy appears to improve locoregional control rates following resection of salivary gland malignancies. In a recent multivariate analysis of 140 patients with ACC, Chen and colleagues185 observed that the omission of postoperative radiation therapy was an independent predictor of local recurrence with a hazard ratio of 5.82 (95% CI 1.96-17.26, P = 0.002). Although the rationale for the use of postoperative radiation is based entirely on retrospective studies such as this one, a number of these studies have revealed certain features where postoperative radiation is particularly useful in controlling locoregional recurrence (Table 88-7). These features include advanced-stage tumors, the presence of positive margins following resection, high-grade tumors, neural involvement, and bone involvement.
Table 88-7 Indications for Adjuvant Radiation Therapy to the Primary Site
| Feature | Reference |
|---|---|
| Advanced stage | Terhaard et al.138,183; Armstrong et al.184 |
| Positive margins following resection | Garden et al.56,188; Hosokawa et al.189; Silverman et al.190 |
| High-grade histologic types: squamous cell carcinoma, undifferentiated carcinoma, high-grade mucoepidermoid carcinoma, carcinoma expleomorphic adenoma, salivary duct carcinoma | Matsuba et al.187; Renehan et al.153 |
| Local tissue invasion: perineural or bone invasion | Terhaard et al.183 |
Local control following surgical resection of advanced stage tumors has been shown to be better when postoperative radiation is given. This improved local control, however, is less clear for earlier-stage tumors. In one of the largest retrospective multivariate analyses, Terhaard and colleagues138 examined the outcomes and prognostic variables of 565 patients treated for a heterogeneous mixture of salivary gland malignancies in centers of the Dutch Head and Neck Oncology Cooperative Group. They found that patients who were treated by surgery alone had a relative risk of local recurrence of 9.7 and a relative risk of regional recurrence of 2.3 when compared with patients treated with surgery followed by radiation therapy. The local control of T3 and T4 tumors treated with surgery followed by radiation was 84% at 10 years, and this was significantly improved compared with cases treated with surgery alone (18% at 10 years, P < 0.001).183 This improvement in local control was not observed for T1 tumors (95% for surgery plus radiation vs. 83% for surgery alone) or T2 tumors (91% for surgery plus radiation vs. 88% for surgery alone). Furthermore, there did not appear to be any influence of radiation therapy on the development of distant metastases or overall survival. In a study by Armstrong and colleagues,184 a retrospective matched-pair analysis also found that postoperative radiation improved local control in patients with stage III and stage IV disease. The 5-year local control rate in patients treated with surgery and postoperative radiation was 51%, and that of patients treated with surgery alone was 17%. The corresponding 5-year determinate survival rates were 51.2% and 9.5%, respectively. In comparison, there was not much difference in the outcomes between the two treatment groups among patients with stage I and II disease. These recent studies are consistent with previous observations demonstrating advantages of combining surgery and radiation.136,150,186,187 Thus for advanced salivary gland cancers, there appears to be improved locoregional control and possibly an improvement in survival in patients treated with a surgical resection followed by radiation therapy.
In cases with positive margins, there tend to be increased rates of local recurrences overall (as discussed earlier in “Prognostic Variables”), and therefore it is not surprising that the addition of radiation therapy, when surgical margins are positive, appears to result in better local control. Garden and colleagues56 retrospectively reviewed 198 patients with adenoid cystic carcinoma of the head and neck, with a median follow-up period of 93 months, and found that 18% of patients with positive margins developed local recurrences. In contrast, only 9% with close or uncertain margins and 5% with negative margins developed local recurrences (P = 0.02). In a separate study, Garden and colleagues188 reviewed 166 patients with malignancies of the parotid gland and found that there was a trend for patients with positive margins to have improved local control when treated with radiation doses greater than 60 Gy. Similarly, Hosokawa and colleagues189 reviewed 61 cases of mucoepidermoid carcinoma treated with either surgery alone or surgery followed by radiation and found that the use of higher doses of radiation (>55 Gy) resulted in better local control despite positive margins. More recently, a review of 75 patients with adenoid cystic carcinoma by Silverman and colleagues190 demonstrated a statistically significant improvement in locoregional control of cases with positive margins when radiation therapy is administered following surgery. Thus postoperative radiation therapy is indicated when there are positive margins following tumor resection.
High-grade salivary gland malignancies have a propensity for locoregional recurrence, and postoperative radiation appears to be beneficial as adjuvant therapy. A study of high-histologic-grade malignancies of the parotid gland by Matsuba and colleagues187 demonstrated a significantly improved 5-year local control rate when postoperative radiation was given (70%) compared with that of surgery alone (20%). Likewise, a retrospective multivariate analysis of 103 parotid gland carcinoma patients by Renehan and colleagues153 found that the addition of postoperative radiation significantly reduced locoregional recurrence (15%) compared with surgery alone (43%) at 10 years and that this improved survival was seen mainly in patients with high-grade tumors. In contrast, postoperative radiation of low-grade malignancies did not affect locoregional control. Thus postoperative radiation therapy is recommended in cases of high-histologic-grade malignancies of the salivary glands.
Postoperative radiation is also recommended in cases where there is invasion into local tissues. Both perineural invasion and bone invasion are unfavorable histologic features and are associated with a poor clinical outcome. In the Terhaard study of 565 patients treated in centers of the Dutch Head and Neck Oncology Cooperative Group, postoperative radiation significantly improved the 10-year local control rates of cases with perineural or bone invasion compared with outcomes of surgery alone.183
Radiation of the Neck
The addition of postoperative radiation therapy of the neck in cases where there are lymph nodes positive for metastases from salivary gland cancers has been shown to improve locoregional control and survival. In a study by Armstrong and colleagues184 comparing 16 patients with lymph node metastases treated with surgery alone with 23 patients treated with surgery and radiation, there was an improvement in 5-year locoregional control from 40% to 69% (P = 0.05). The corresponding 5-year survival improved from 19% to 49% (P = 0.015). The utility of postoperative radiation of the neck has been corroborated by the Terhaard and colleagues study.183 They observed an 83% regional neck node control rate at 10 years in patients (n = 22) with pathologic N1 disease treated with postoperative locoregional radiation. In contrast, the 10-year regional neck node control rate was 57% in patients (n = 10) treated with no or only local postoperative radiation to the primary site (P = 0.04). Thus radiation therapy following surgical ablation of node-positive disease appears to improve outcome.
The use of radiation in the management of N0 necks, however, is less clear. In the Terhaard study, no significant benefit to regional control of the pathologic N0 neck was seen with the addition of postoperative radiation.183 In contrast, a more recent study by Chen and colleagues205 examined 251 patients with clinically N0 carcinomas of the salivary glands that were treated with surgery and postoperative radiation therapy. Elective neck irradiation was employed in 131 patients (90 ipsilateral and 41 bilateral). There was a significant reduction in the 10-year nodal failure rate from 26% to 0% (P = 0.0001). Of note, in the patients who did not receive elective neck irradiation, the nodal relapse occurred in cases of the more high-grade histologic types: squamous cell carcinoma, undifferentiated carcinoma, adenocarcinoma NOS, and mucoepidermoid carcinoma. No nodal recurrence was seen in cases of adenoid cystic or acinic cell carcinoma.
Neutron Beam Radiotherapy
Fast neutrons deliver more energy and thus result in greater radiation damage than what is created by conventional photon/electron radiotherapy. Because salivary gland malignancies have traditionally been considered to be fairly radioresistant, fast neutron therapy was proposed, and subsequently shown, to be effective in these cancers. In the only prospective randomized trial of radiation therapy of salivary gland neoplasms, fast neutron radiotherapy was compared with conventional photon radiotherapy in the treatment of unresectable and/or recurrent tumors. This study conducted jointly by the Radiation Therapy Oncology Group (RTOG) of the United States and the Medical Research Council (MRC) of Great Britain demonstrated a significant improvement in locoregional control with fast neutron radiotherapy.191,192 Overall survival, however, was not significantly different between the two groups. In this and subsequent studies, it appeared that patients tended to succumb to distant metastases despite the improvement in locoregional control and that this pattern of failure was the reason for the lack of improved survival, especially in cases of ACC.193–196
Chemotherapy
The use of systemic chemotherapy in the treatment of salivary gland malignancies is currently limited to palliative treatment of locally advanced unresectable, recurrent, and metastatic disease. A variety of agents have been explored; however, the studies that have been reported are nonrandomized and of small numbers of patients, making it difficult to assess the efficacy of these drugs. In addition, many of the reports are case series and retrospective reviews of heterogeneous types of malignancies and treatments. In order to circumvent the limitations of the available data, Rizk and colleagues197 recently reported a meta-analysis of data from 205 patients obtained from 17 phase II or phase III trial studies. The chemotherapeutic agents used in these studies included cisplatin, carboplatin, epirubicin, cyclophosphamide, doxorubicin hydrochloride (Adriamycin), mitoxantrone, paclitaxel, 5-fluorouracil, vinorelbine, methotrexate, and bleomycin. All patients were treated for cancer of the major or minor salivary glands with either single-agent or multi-agent first-line palliative chemotherapy. Using meta-analysis linear regression, the authors found that patients treated with either platinum- or anthracycline (e.g., mitoxantrone)-based chemotherapy had an increase in their median survival. When looking at the data using a multivariate analysis, only platinum-based chemotherapy was found to be significantly associated with increased survival, but there appeared to be a positive interaction between platinum agents and anthracycline. Thus although the field is in need of prospective randomized trials, the available data suggest that these two agents may have a useful role, not only in palliative treatment, but also in definitive multimodality treatment of salivary gland malignancies prone to distant metastases.
Recently, attention has been directed to the possible role for directed therapy against biologic targets expressed in these cancers. These targets include receptor tyrosine kinases such as c-KIT and members of the ErbB family (the epidermal growth factor receptor (EGFR), Her2/neu). Adenoid cystic carcinoma has been shown to overexpress c-KIT in more than 90% of cases, and expression of c-KIT has been correlated with tumor grade.198,199 EGFR (also known as ErbB1) is expressed in 79% of all parotid malignancies,199 leading to the investigation of directed therapy against this target. Her2/neu (also known as ErbB2) is frequently expressed in a subset of salivary gland malignancies, namely salivary duct carcinoma, and expression correlates significantly with prognosis.104,175 Observations such as these have established the rationale for investigating strategies to target these kinases in multiple phase II trials.200–202 Unfortunately, no objective response was found when using either small molecule inhibitors or antibodies as monotherapy in patients with unresectable, recurrent, or metastatic disease. However, the therapeutics did appear to stabilize the disease in a substantial number of the patients in these trials, suggesting that inhibition of these targets was having some effect on the biology of these tumors. Indeed, there are promising data indicating that targeted therapy in combination with conventional chemotherapy may have a synergistic effect.203
Armstrong JG, Harrison LB, Thaler HT, et al. The indications for elective treatment of the neck in cancer of the major salivary glands. Cancer. 1992;69:615-619.
Bhattacharyya N, Fried MP. Nodal metastasis in major salivary gland cancer: predictive factors and effects on survival. Arch Otolaryngol Head Neck Surg. 2002;128:904-908.
Brandwein MS, Ivanov K, Wallace DI, et al. Mucoepidermoid carcinoma: a clinicopathologic study of 80 patients with special reference to histological grading. Am J Surg Pathol. 2001;25:835-845.
Chen AM, Granchi PJ, Garcia J, et al. Local-regional recurrence after surgery without postoperative irradiation for carcinomas of the major salivary glands: implications for adjuvant therapy. Int J Radiat Oncol Biol Phys. 2007;67:982-987.
Garden AS, Weber RS, Morrison WH, et al. The influence of positive margins and nerve invasion in adenoid cystic carcinoma of the head and neck treated with surgery and radiation. Int J Radiat Oncol Biol Phys. 1995;32:619-626.
Frankenthaler RA, Byers RM, Luna MA, et al. Predicting occult lymph node metastasis in parotid cancer. Arch Otolaryngol Head Neck Surg. 1993;119:517-520.
Laramore GE, Krall JM, Griffin TW, et al. Neutron versus photon irradiation for unresectable salivary gland tumors: final report of an RTOG-MRC randomized clinical trial. Radiation Therapy Oncology Group. Medical Research Council. Int J Radiat Oncol Biol Phys. 1993;27:235-240.
Renehan A, Gleave EN, Hancock BD, et al. Long-term follow-up of over 1000 patients with salivary gland tumours treated in a single centre. Br J Surg. 1996;83:1750-1754.
Spiro RH. Salivary neoplasms: overview of a 35-year experience with 2,807 patients. Head Neck Surg. 1986;8:177-184.
Stennert E, Kisner D, Jungehuelsing M, et al. High incidence of lymph node metastasis in major salivary gland cancer. Arch Otolaryngol Head Neck Surg. 2003;129:720-723.
Terhaard CH, Lubsen H, Rasch CR, et al. The role of radiotherapy in the treatment of malignant salivary gland tumors. Int J Radiat Oncol Biol Phys. 2005;61:103-111.
Terhaard CH, Lubsen H, Van der Tweel I, et al. Salivary gland carcinoma: independent prognostic factors for locoregional control, distant metastases, and overall survival: results of the Dutch head and neck oncology cooperative group. Head Neck. 2004;26:681-692.
1. Spiro RH. Salivary neoplasms: overview of a 35-year experience with 2,807 patients. Head Neck Surg. 1986;8:177-184.
2. Pinkston JA, Cole P. Incidence rates of salivary gland tumors: results from a population-based study. Otolaryngol Head Neck Surg. 1999;120:834-840.
3. Eveson JW, Cawson RA. Salivary gland tumours. A review of 2410 cases with particular reference to histological types, site, age and sex distribution. J Pathol. 1985;146:51-58.
4. Hunter RM, Davis BW, Gray GFJr, et al. Primary malignant tumors of salivary gland origin. A 52-year review. Am Surg. 1983;49:82-89.
5. Eneroth CM. Facial nerve paralysis. A criterion of malignancy in parotid tumors. Arch Otolaryngol. 1972;95:300-304.
6. Witt RL. Major salivary gland cancer. Surg Oncol Clin North Am. 2004;13:113-127.
7. Bhattacharyya N. Survival and prognosis for cancer of the submandibular gland. J Oral Maxillofac Surg. 2004;62:427-430.
8. Ma DQ, Yu GY. Tumours of the minor salivary glands. A clinicopathologic study of 243 cases. Acta Otolaryngol. 1987;103:325-331.
9. Vander Poorten VL, Balm AJ, Hilgers FJ, et al. Stage as major long term outcome predictor in minor salivary gland carcinoma. Cancer. 2000;89:1195-1204.
10. Som PM, Biller HF. High-grade malignancies of the parotid gland: identification with MR imaging. Radiology. 1989;173:823-826.
11. Eneroth CM, Franzen S, Zajicek J. Aspiration biopsy of salivary gland tumors. A critical review of 910 biopsies. Acta Cytol. 1967;11:470-472.
12. Eneroth CM, Frazen S, Zajicek J. Cytologic diagnosis of aspirate from 1000 salivary-gland tumours. Acta Otolaryngol. 1966;Suppl 224:168.
13. Eneroth CM, Zajicek J. Aspiration biopsy of salivary gland tumors. II. Morphologic studies on smears and histologic sections from oncocytic tumors (45 cases of papillary cystadenoma lymphomatosum and 4 cases of oncocytoma). Acta Cytol. 1965;9:355-361.
14. Eneroth CM, Zajicek J. Aspiration biopsy of salivary gland tumors. IV. Morphologic studies on smears and histologic sections from 45 cases of adenoid cystic carcinoma. Acta Cytol. 1969;13:59-63.
15. Mavec P, Eneroth CM, Franzen S, et al. Aspiration biopsy of salivary gland tumours. I. Correlation of cytologic reports from 652 aspiration biopsies with clinical and histologic findings. Acta Otolaryngol. 1964;58:471-484.
16. Zajicek J, Eneroth CM. Cytological diagnosis of salivary-gland carcinomata from aspiration biopsy smears. Acta Otolaryngol. 1969;263:183-185.
17. Engzell U, Esposti PL, Rubio C, et al. Investigation on tumour spread in connection with aspiration biopsy. Acta Radiol Ther Phys Biol. 1971;10:385-398.
18. Castle JT, Thompson LD, Frommelt RA, et al. Polymorphous low grade adenocarcinoma: a clinicopathologic study of 164 cases. Cancer. 1999;86:207-219.
19. Savera AT, Sloman A, Huvos AG, et al. Myoepithelial carcinoma of the salivary glands: a clinicopathologic study of 25 patients. Am J Surg Pathol. 2000;24:761-774.
20. Skalova A, Jakel KT, Barnes L, et al. Myoepithelial Carcinoma Pathology and Genetics of Head and Neck Tumors. Lyon, France: IARC Press; 2005.
21. Eveson JW, Auclair P, Gnepp R, et al. Tumors of the Salivary Glands. Lyon, France: IARC Press; 2005.
22. Westra WH. Diagnostic difficulties in the classification and grading of salivary gland tumors. Int J Radiat Oncol Biol Phys. 2007;69:S49-S51.
23. Cristallini EG, Ascani S, Farabi R, et al. Fine needle aspiration biopsy of salivary gland, 1985-1995. Acta Cytol. 1997;41:1421-1425.
24. Jayaram N, Ashim D, Rajwanshi A, et al. The value of fine-needle aspiration biopsy in the cytodiagnosis of salivary gland lesions. Diagn Cytopathol. 1989;5:349-354.
25. Qizilbash AH, Sianos J, Young JE, et al. Fine needle aspiration biopsy cytology of major salivary glands. Acta Cytol. 1985;29:503-512.
26. Zurrida S, Alasio L, Tradati N, et al. Fine-needle aspiration of parotid masses. Cancer. 1993;72:2306-2311.
27. Hughes JH, Volk EE, Wilbur DC. Pitfalls in salivary gland fine-needle aspiration cytology: lessons from the College of American Pathologists Interlaboratory Comparison Program in Nongynecologic Cytology. Arch Pathol Lab Med. 2005;129:26-31.
28. Seethala RR, LiVolsi VA, Baloch ZW. Relative accuracy of fine-needle aspiration and frozen section in the diagnosis of lesions of the parotid gland. Head Neck. 2005;27:217-223.
29. Prasad AR, Savera AT, Gown AM, et al. The myoepithelial immunophenotype in 135 benign and malignant salivary gland tumors other than pleomorphic adenoma. Arch Pathol Lab Med. 1999;123:801-806.
30. Nagao T, Gaffey TA, Kay PA, et al. Polymorphous low-grade adenocarcinoma of the major salivary glands: report of three cases in an unusual location. Histopathology. 2004;44:164-171.
31. Ellis GL, Auclair PL. Atlas of Tumor Pathology, Tumors of the Salivary Glands. Washington DC: Armed Forces Institute of Pathology; 1996.
32. Renehan A, Gleave EN, Hancock BD, et al. Long-term follow-up of over 1000 patients with salivary gland tumours treated in a single centre. Br J Surg. 1996;83:1750-1754.
33. Spitz MR, Batsakis JG. Major salivary gland carcinoma. Descriptive epidemiology and survival of 498 patients. Arch Otolaryngol. 1984;110:45-49.
34. Nascimento AG, Amaral LP, Prado LA, et al. Mucoepidermoid carcinoma of salivary glands: a clinicopathologic study of 46 cases. Head Neck Surg. 1986;8:409-417.
35. Waldron CA, el-Mofty SK, Gnepp DR. Tumors of the intraoral minor salivary glands: a demographic and histologic study of 426 cases. Oral Surg Oral Med Oral Pathol. 1988;66:323-333.
36. Namin AK, Moshref M, Shahoon H, et al. Intraosseous mucoepidermoid carcinoma of the maxilla in a teenager: a case report and review of literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:e93-e96.
37. Zaharopoulos P. Primary intraosseous (central) salivary gland neoplasms in jaw bones: report of a mucoepidermoid carcinoma of the mandible diagnosed by fine-needle aspiration cytology. Diagn Cytopathol. 2004;31:271-275.
38. Krolls SO, Trodahl JN, Boyers RC. Salivary gland lesions in children. A survey of 430 cases. Cancer. 1972;30:459-469.
39. Luna MA. Salivary mucoepidermoid carcinoma: revisited. Adv Anat Pathol. 2006;13:293-307.
40. Spiro RH, Huvos AG, Berk R, et al. Mucoepidermoid carcinoma of salivary gland origin. A clinicopathologic study of 367 cases. Am J Surg. 1978;136:461-468.
41. Auclair PL, Goode RK, Ellis GL. Mucoepidermoid carcinoma of intraoral salivary glands. Evaluation and application of grading criteria in 143 cases. Cancer. 1992;69:2021-2030.
42. Brandwein MS, Ivanov K, Wallace DI, et al. Mucoepidermoid carcinoma: a clinicopathologic study of 80 patients with special reference to histological grading. Am J Surg Pathol. 2001;25:835-845.
43. Goode RK, Auclair PL, Ellis GL. Mucoepidermoid carcinoma of the major salivary glands: clinical and histopathologic analysis of 234 cases with evaluation of grading criteria. Cancer. 1998;82:1217-1224.
44. Brannon RB, Fowler CB, Hartman KS. Necrotizing sialometaplasia. A clinicopathologic study of sixty-nine cases and review of the literature. Oral Surg Oral Med Oral Pathol. 1991;72:317-325.
45. Evans HL. Mucoepidermoid carcinoma of salivary glands: a study of 69 cases with special attention to histologic grading. Am J Clin Pathol. 1984;81:696-701.
46. Beckhardt RN, Weber RS, Zane R, et al. Minor salivary gland tumors of the palate: clinical and pathologic correlates of outcome. Laryngoscope. 1995;105:1155-1160.
47. Spiro RH, Huvos AG, Strong EW. Adenoid cystic carcinoma of salivary origin. A clinicopathologic study of 242 cases. Am J Surg. 1974;128:512-520.
48. Spiro RH, Huvos AG. Stage means more than grade in adenoid cystic carcinoma. Am J Surg. 1992;164:623-628.
49. Szanto PA, Luna MA, Tortoledo ME, et al. Histologic grading of adenoid cystic carcinoma of the salivary glands. Cancer. 1984;54:1062-1069.
50. Matsuba HM, Thawley SE, Simpson JR, et al. Adenoid cystic carcinoma of major and minor salivary gland origin. Laryngoscope. 1984;94:1316-1318.
51. Spiro RH, Huvos AG, Strong EW. Adenoid cystic carcinoma: factors influencing survival. Am J Surg. 1979;138:579-583.
52. Tandler B. Ultrastructure of adenoid cystic carcinoma of salivary gland origin. Lab Invest. 1971;24:504-512.
53. Cheng J, Saku T, Okabe H, et al. Basement membranes in adenoid cystic carcinoma. An immunohistochemical study. Cancer. 1992;69:2631-2640.
54. El-Naggar AK, Huvos AG, Barnes L, et al. Adenoid Cystic Carcinoma Pathology and Genetics of Head and Neck Tumors. Lyon, France: IARC Press; 2005.
55. Batsakis JG, Luna MA, el-Naggar A. Histopathologic grading of salivary gland neoplasms: III. Adenoid cystic carcinomas. Ann Otol Rhinol Laryngol. 1990;99:1007-1009.
56. Garden AS, Weber RS, Morrison WH, et al. The influence of positive margins and nerve invasion in adenoid cystic carcinoma of the head and neck treated with surgery and radiation. Int J Radiat Oncol Biol Phys. 1995;32:619-626.
57. Fordice J, Kershaw C, el-Naggar A, et al. Adenoid cystic carcinoma of the head and neck: predictors of morbidity and mortality. Arch Otolaryngol Head Neck Surg. 1999;125:149-152.
58. Rapidis AD, Givalos N, Gakiopoulou H, et al. Adenoid cystic carcinoma of the head and neck. Clinicopathological analysis of 23 patients and review of the literature. Oral Oncol. 2005;41:328-335.
59. Vrielinck LJ, Ostyn F, van DB, et al. The significance of perineural spread in adenoid cystic carcinoma of the major and minor salivary glands. Int J Oral Maxillofac Surg. 1988;17:190-193.
60. Emanuel P, Wang B, Wu M, et al. p63 Immunohistochemistry in the distinction of adenoid cystic carcinoma from basaloid squamous cell carcinoma. Mod Pathol. 2005;18:645-650.
61. Serrano MF, El-Mofty SK, Gnepp DR, et al. Utility of high molecular weight cytokeratins, but not p63, in the differential diagnosis of neuroendocrine and basaloid carcinomas of the head and neck. Hum Pathol. 2008;39:591-598.
62. Evans HL, Batsakis JG. Polymorphous low-grade adenocarcinoma of minor salivary glands. A study of 14 cases of a distinctive neoplasm. Cancer. 1984;53:935-942.
63. Evans HL, Luna MA. Polymorphous low-grade adenocarcinoma: a study of 40 cases with long-term follow up and an evaluation of the importance of papillary areas. Am J Surg Pathol. 2000;24:1319-1328.
64. Perez-Ordonez B, Linkov I, Huvos AG. Polymorphous low-grade adenocarcinoma of minor salivary glands: a study of 17 cases with emphasis on cell differentiation. Histopathology. 1998;32:521-529.
65. Anderson C, Krutchkoff D, Pedersen C, et al. Polymorphous low grade adenocarcinoma of minor salivary gland: a clinicopathologic and comparative immunohistochemical study. Mod Pathol. 1990;3:76-82.
66. Curran AE, White DK, Damm DD, et al. Polymorphous low-grade adenocarcinoma versus pleomorphic adenoma of minor salivary glands: resolution of a diagnostic dilemma by immunohistochemical analysis with glial fibrillary acidic protein. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:194-199.
67. Gnepp DR, el-Mofty S. Polymorphous low-grade adenocarcinoma: glial fibrillary acidic protein staining in the differential diagnosis with cellular mixed tumors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:691-695.
68. Nishimura T, Furukawa M, Kawahara E, et al. Differential diagnosis of pleomorphic adenoma by immunohistochemical means. J Laryngol Otol. 1991;105:1057-1060.
69. Vincent SD, Hammond HL, Finkelstein MW. Clinical and therapeutic features of polymorphous low-grade adenocarcinoma. Oral Surg Oral Med Oral Pathol. 1994;77:41-47.
70. Tanaka F, Wada H, Inui K, et al. Pulmonary metastasis of polymorphous low-grade adenocarcinoma of the minor salivary gland. Thorac Cardiovasc Surg. 1995;43:178-180.
71. Spiro RH, Huvos AG, Strong EW. Acinic cell carcinoma of salivary origin. A clinicopathologic study of 67 cases. Cancer. 1978;41:924-935.
72. Guimaraes DS, Amaral AP, Prado LF, et al. Acinic cell carcinoma of salivary glands: 16 cases with clinicopathologic correlation. J Oral Pathol Med. 1989;18:396-399.
73. Ellis GL, Corio RL. Acinic cell adenocarcinoma. A clinicopathologic analysis of 294 cases. Cancer. 1983;52:542-549.
74. Hamper K, Mausch HE, Caselitz J, et al. Acinic cell carcinoma of the salivary glands: the prognostic relevance of DNA cytophotometry in a retrospective study of long duration (1965-1987). Oral Surg Oral Med Oral Pathol. 1990;69:68-75.
75. Perzin KH, Livolsi VA. Acinic cell carcinomas arising in salivary glands: a clinicopathologic study. Cancer. 1979;44:1434-1457.
76. Stanley RJ, Weiland LH, Olsen KD, et al. Dedifferentiated acinic cell (acinous) carcinoma of the parotid gland. Otolaryngol Head Neck Surg. 1988;98:155-161.
77. Piana S, Cavazza A, Pedroni C, et al. Dedifferentiated acinic cell carcinoma of the parotid gland with myoepithelial features. Arch Pathol Lab Med. 2002;126:1104-1105.
78. Henley JD, Geary WA, Jackson CL, et al. Dedifferentiated acinic cell carcinoma of the parotid gland: a distinct rarely described entity. Hum Pathol. 1997;28:869-873.
79. Lewis JE, Olsen KD, Weiland LH. Acinic cell carcinoma. Clinicopathologic review. Cancer. 1991;67:172-179.
80. Stephen J, Batsakis JG, Luna MA, et al. True malignant mixed tumors (carcinosarcoma) of salivary glands. Oral Surg Oral Med Oral Pathol. 1986;61:597-602.
81. Garner SL, Robinson RA, Maves MD, et al. Salivary gland carcinosarcoma: true malignant mixed tumor. Ann Otol Rhinol Laryngol. 1989;98:611-614.
82. Gandour-Edwards RF, Donald PJ, Vogt PJ, et al. Carcinosarcoma (malignant mixed tumor) of the parotid: report of a case with a pure rhabdomyosarcoma component. Head Neck. 1994;16:379-382.
83. Carson HJ, Tojo DP, Chow JM, et al. Carcinosarcoma of salivary glands with unusual stromal components. Report of two cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:738-746.
84. Kwon MY, Gu M. True malignant mixed tumor (carcinosarcoma) of parotid gland with unusual mesenchymal component: a case report and review of the literature. Arch Pathol Lab Med. 2001;125:812-815.
85. Tanahashi J, Daa T, Kashima K, et al. Carcinosarcoma ex recurrent pleomorphic adenoma of the submandibular gland. APMIS. 2007;115:789-794.
86. Tortoledo ME, Luna MA, Batsakis JG. Carcinomas ex pleomorphic adenoma and malignant mixed tumors. Histomorphologic indexes. Arch Otolaryngol. 1984;110:172-176.
87. Spiro RH, Huvos AG, Strong EW. Malignant mixed tumor of salivary origin: a clinicopathologic study of 146 cases. Cancer. 1977;39:388-396.
88. Olsen KD, Lewis JE. Carcinoma ex pleomorphic adenoma: a clinicopathologic review. Head Neck. 2001;23:705-712.
89. Livolsi VA, Perzin KH. Malignant mixed tumors arising in salivary glands. I. Carcinomas arising in benign mixed tumors: a clinicopathologic study. Cancer. 1977;39:2209-2230.
90. Gerughty RM, Scofield HH, Brown FM, et al. Malignant mixed tumors of salivary gland origin. Cancer. 1969;24:471-486.
91. Thomas WH, Coppola ED. Distant metastases from mixed tumors of the salivary glands. Am J Surg. 1965;109:724-730.
92. Brandwein M, Huvos AG, Dardick I, et al. Noninvasive and minimally invasive carcinoma ex mixed tumor: a clinicopathologic and ploidy study of 12 patients with major salivary tumors of low (or no?) malignant potential. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:655-664.
93. Qureshi AA, Gitelis S, Templeton AA, et al. “Benign” metastasizing pleomorphic adenoma. A case report and review of literature. Clin Orthop Relat Res. 1994:192-198.
94. Hoorweg JJ, Hilgers FJ, Keus RB, et al. Metastasizing pleomorphic adenoma: a report of three cases. Eur J Surg Oncol. 1998;24:452-455.
95. Bradley PJ. “Metastasizing pleomorphic salivary adenoma” should now be considered a low-grade malignancy with a lethal potential. Curr Opin Otolaryngol Head Neck Surg. 2005;13:123-126.
96. Nouraei SA, Ferguson MS, Clarke PM, et al. Metastasizing pleomorphic salivary adenoma. Arch Otolaryngol Head Neck Surg. 2006;132:788-793.
97. Chen KT, Hafez GR. Infiltrating salivary duct carcinoma. A clinicopathologic study of five cases. Arch Otolaryngol. 1981;107:37-39.
98. Barnes L, Rao U, Krause J, et al. Salivary duct carcinoma. Part I. A clinicopathologic evaluation and DNA image analysis of 13 cases with review of the literature. Oral Surg Oral Med Oral Pathol. 1994;78:64-73.
99. Barnes L, Rao U, Contis L, et al. Salivary duct carcinoma. Part II. Immunohistochemical evaluation of 13 cases for estrogen and progesterone receptors, cathepsin D, and c-erbB-2 protein. Oral Surg Oral Med Oral Pathol. 1994;78:74-80.
100. Afzelius LE, Cameron WR, Svensson C. Salivary duct carcinoma—a clinicopathologic study of 12 cases. Head Neck Surg. 1987;9:151-156.
101. Brandwein MS, Jagirdar J, Patil J, et al. Salivary duct carcinoma (cribriform salivary carcinoma of excretory ducts). A clinicopathologic and immunohistochemical study of 12 cases. Cancer. 1990;65:2307-2314.
102. Garland TA, Innes DJJr, Fechner RE. Salivary duct carcinoma: an analysis of four cases with review of literature. Am J Clin Pathol. 1984;81:436-441.
103. Hanna DC. Salivary duct carcinoma—a clinicopathologic study of 12 cases. Head Neck Surg. 1988;10:204-205.
104. Jaehne M, Roeser K, Jaekel T, et al. Clinical and immunohistologic typing of salivary duct carcinoma: a report of 50 cases. Cancer. 2005;103:2526-2533.
105. Lewis JE, McKinney BC, Weiland LH, et al. Salivary duct carcinoma. Clinicopathologic and immunohistochemical review of 26 cases. Cancer. 1996;77:223-230.
106. Colmenero RC, Patron RM, Martin PM. Salivary duct carcinoma: a report of nine cases. J Oral Maxillofac Surg. 1993;51:641-646.
107. Johnson CJ, Barry MB, Vasef MA, et al. Her-2/neu expression in salivary duct carcinoma: an immunohistochemical and chromogenic in situ hybridization study. Appl Immunohistochem Mol Morphol. 2008;16:54-58.
108. Nabili V, Tan JW, Bhuta S, et al. Salivary duct carcinoma: a clinical and histologic review with implications for trastuzumab therapy. Head Neck. 2007;29:907-912.
109. Batsakis JG. Primary squamous cell carcinomas of major salivary glands. Ann Otol Rhinol Laryngol. 1983;92:97-98.
110. Shemen LJ, Huvos AG, Spiro RH. Squamous cell carcinoma of salivary gland origin. Head Neck Surg. 1987;9:235-240.
111. Batsakis JG, McClatchey KD, Johns M, et al. Primary squamous cell carcinoma of the parotid gland. Arch Otolaryngol. 1976;102:355-357.
112. Gaughan RK, Olsen KD, Lewis JE. Primary squamous cell carcinoma of the parotid gland. Arch Otolaryngol Head Neck Surg. 1992;118:798-801.
113. Nagao T, Gaffey TA, Olsen KD, et al. Small cell carcinoma of the major salivary glands: clinicopathologic study with emphasis on cytokeratin 20 immunoreactivity and clinical outcome. Am J Surg Pathol. 2004;28:762-770.
114. Gnepp DR, Corio RL, Brannon RB. Small cell carcinoma of the major salivary glands. Cancer. 1986;58:705-714.
115. Scott MP, Helm KF. Cytokeratin 20: a marker for diagnosing Merkel cell carcinoma. Am J Dermatopathol. 1999;21:16-20.
116. Chan JK, Suster S, Wenig BM, et al. Cytokeratin 20 immunoreactivity distinguishes Merkel cell (primary cutaneous neuroendocrine) carcinomas and salivary gland small cell carcinomas from small cell carcinomas of various sites. Am J Surg Pathol. 1997;21:226-234.
117. Gnepp DR, Wick MR. Small cell carcinoma of the major salivary glands. An immunohistochemical study. Cancer. 1990;66:185-192.
118. Seethala RR, Barnes EL, Hunt JL. Epithelial-myoepithelial carcinoma: a review of the clinicopathologic spectrum and immunophenotypic characteristics in 61 tumors of the salivary glands and upper aerodigestive tract. Am J Surg Pathol. 2007;31:44-57.
119. Muller S, Barnes L. Basal cell adenocarcinoma of the salivary glands. Report of seven cases and review of the literature. Cancer. 1996;78:2471-2477.
120. Wahlberg P, Anderson H, Biorklund A, et al. Carcinoma of the parotid and submandibular glands—a study of survival in 2465 patients. Oral Oncol. 2002;38:706-713.
121. Spiro RH, Huvos AG, Strong EW. Adenocarcinoma of salivary origin. Clinicopathologic study of 204 patients. Am J Surg. 1982;144:423-431.
122. Martinez-Madrigal F, Micheau C. Histology of the major salivary glands. Am J Surg Pathol. 1989;13:879-899.
123. Malata CM, Camilleri IG, McLean NR, et al. Malignant tumours of the parotid gland: a 12-year review. Br J Plast Surg. 1997;50:600-608.
124. Malata CM, Camilleri IG, McLean NR, et al. Metastatic tumours of the parotid gland. Br J Oral Maxillofac Surg. 1998;36:190-195.
125. Seifert G, Hennings K, Caselitz J. Metastatic tumors to the parotid and submandibular glands—analysis and differential diagnosis of 108 cases. Pathol Res Pract. 1986;181:684-692.
126. Solomon MP, Rosen Y, Gardner B. Metastatic malignancy in the submandibular gland. Oral Surg Oral Med Oral Pathol. 1975;39:469-473.
127. Brodsky G, Rabson AB. Metastasis to the submandibular gland as the initial presentation of small cell (“oat cell”) lung carcinoma. Oral Surg Oral Med Oral Pathol. 1984;58:76-80.
128. Bouquot JE, Weiland LH, Kurland LT. Metastases to and from the upper aerodigestive tract in the population of Rochester, Minnesota, 1935-1984. Head Neck. 1989;11:212-218.
129. Harris NL. Lymphoid proliferations of the salivary glands. Am J Clin Pathol. 1999;111:S94-S103.
130. Gleeson MJ, Bennett MH, Cawson RA. Lymphomas of salivary glands. Cancer. 1986;58:699-704.
131. Royer B, Cazals-Hatem D, Sibilia J, et al. Lymphomas in patients with Sjögren’s syndrome are marginal zone B-cell neoplasms, arise in diverse extranodal and nodal sites, and are not associated with viruses. Blood. 1997;90:766-775.
132. Jaehne M, Ussmuller J, Jakel KT, et al. The clinical presentation of non-Hodgkin lymphomas of the major salivary glands. Acta Otolaryngol. 2001;121:647-651.
133. Chhieng DC, Cangiarella JF, Cohen JM. Fine-needle aspiration cytology of lymphoproliferative lesions involving the major salivary glands. Am J Clin Pathol. 2000;113:563-571.
134. Dehner LP, Valbuena L, Perez-Atayde A, et al. Salivary gland anlage tumor (“congenital pleomorphic adenoma”). A clinicopathologic, immunohistochemical and ultrastructural study of nine cases. Am J Surg Pathol. 1994;18:25-36.
135. Therkildsen MH, Christensen M, Andersen LJ, et al. Salivary gland carcinomas—prognostic factors. Acta Oncol. 1998;37:701-713.
136. Fitzpatrick PJ, Theriault C. Malignant salivary gland tumors. Int J Radiat Oncol Biol Phys. 1986;12:1743-1747.
137. Spiro RH, Armstrong J, Harrison L, et al. Carcinoma of major salivary glands. Recent trends. Arch Otolaryngol Head Neck Surg. 1989;115:316-321.
138. Terhaard CH, Lubsen H, Van der Tweel I, et al. Salivary gland carcinoma: independent prognostic factors for locoregional control, distant metastases, and overall survival: results of the Dutch head and neck oncology cooperative group. Head Neck. 2004;26:681-692.
139. Chen AM, Granchi PJ, Garcia J, et al. Local-regional recurrence after surgery without postoperative irradiation for carcinomas of the major salivary glands: implications for adjuvant therapy. Int J Radiat Oncol Biol Phys. 2007;67:982-987.
140. Theriault C, Fitzpatrick PJ. Malignant parotid tumors. Prognostic factors and optimum treatment. Am J Clin Oncol. 1986;9:510-516.
141. Lima RA, Tavares MR, Dias FL, et al. Clinical prognostic factors in malignant parotid gland tumors. Otolaryngol Head Neck Surg. 2005;133:702-708.
142. Hocwald E, Korkmaz H, Yoo GH, et al. Prognostic factors in major salivary gland cancer. Laryngoscope. 2001;111:1434-1439.
143. Stennert E, Kisner D, Jungehuelsing M, et al. High incidence of lymph node metastasis in major salivary gland cancer. Arch Otolaryngol Head Neck Surg. 2003;129:720-723.
144. Kelley DJ, Spiro RH. Management of the neck in parotid carcinoma. Am J Surg. 1996;172:695-697.
145. Armstrong JG, Harrison LB, Thaler HT, et al. The indications for elective treatment of the neck in cancer of the major salivary glands. Cancer. 1992;69:615-619.
146. Frankenthaler RA, Byers RM, Luna MA, et al. Predicting occult lymph node metastasis in parotid cancer. Arch Otolaryngol Head Neck Surg. 1993;119:517-520.
147. Yu GY, Ma DQ. Carcinoma of the salivary gland: a clinicopathologic study of 405 cases. Semin Surg Oncol. 1987;3:240-244.
148. Batsakis JG. Carcinomas of the submandibular and sublingual glands. Ann Otol Rhinol Laryngol. 1986;95:211-212.
149. O’Brien CJ, Soong SJ, Herrera GA, et al. Malignant salivary tumors—analysis of prognostic factors and survival. Head Neck Surg. 1986;9:82-92.
150. North CA, Lee DJ, Piantadosi S, et al. Carcinoma of the major salivary glands treated by surgery or surgery plus postoperative radiotherapy. Int J Radiat Oncol Biol Phys. 1990;18:1319-1326.
151. Bhattacharyya N, Fried MP. Nodal metastasis in major salivary gland cancer: predictive factors and effects on survival. Arch Otolaryngol Head Neck Surg. 2002;128:904-908.
152. Vander Poorten VL, Balm AJ, Hilgers FJ, et al. The development of a prognostic score for patients with parotid carcinoma. Cancer. 1999;85:2057-2067.
153. Renehan AG, Gleave EN, Slevin NJ, et al. Clinico-pathological and treatment-related factors influencing survival in parotid cancer. Br J Cancer. 1999;80:1296-1300.
154. Storey MR, Garden AS, Morrison WH, et al. Postoperative radiotherapy for malignant tumors of the submandibular gland. Int J Radiat Oncol Biol Phys. 2001;51:952-958.
155. Frankenthaler RA, Luna MA, Lee SS, et al. Prognostic variables in parotid gland cancer. Arch Otolaryngol Head Neck Surg. 1991;117:1251-1256.
156. Hicks MJ, el-Naggar AK, Flaitz CM, et al. Histocytologic grading of mucoepidermoid carcinoma of major salivary glands in prognosis and survival: a clinicopathologic and flow cytometric investigation. Head Neck. 1995;17:89-95.
157. Pedersen D, Overgaard J, Sogaard H, et al. Malignant parotid tumors in 110 consecutive patients: treatment results and prognosis. Laryngoscope. 1992;102:1064-1069.
158. Guzzo M, Andreola S, Sirizzotti G, et al. Mucoepidermoid carcinoma of the salivary glands: clinicopathologic review of 108 patients treated at the National Cancer Institute of Milan. Ann Surg Oncol. 2002;9:688-695.
159. Aro K, Leivo I, Makitie AA. Management and outcome of patients with mucoepidermoid carcinoma of major salivary gland origin: a single institution’s 30-year experience. Laryngoscope. 2008;118:258-262.
160. Santucci M, Bondi R. Histologic-prognostic correlations in adenoid cystic carcinoma of major and minor salivary glands of the oral cavity. Tumori. 1986;72:293-300.
161. Santucci M, Bondi R. New prognostic criterion in adenoid cystic carcinoma of salivary gland origin. Am J Clin Pathol. 1989;91:132-136.
162. Lopes MA, Santos GC, Kowalski LP. Multivariate survival analysis of 128 cases of oral cavity minor salivary gland carcinomas. Head Neck. 1998;20:699-706.
163. Prokopakis EP, Snyderman CH, Hanna EY, et al. Risk factors for local recurrence of adenoid cystic carcinoma: the role of postoperative radiation therapy. Am J Otolaryngol. 1999;20:281-286.
164. Poulsen MG, Pratt GR, Kynaston B, et al. Prognostic variables in malignant epithelial tumors of the parotid. Int J Radiat Oncol Biol Phys. 1992;23:327-332.
165. Hicks MJ, el-Naggar AK, Byers RM, et al. Prognostic factors in mucoepidermoid carcinomas of major salivary glands: a clinicopathologic and flow cytometric study. Eur J Cancer. 1994;30B:329-334.
166. Frankenthaler RA, el-Naggar AK, Ordonez NG, et al. High correlation with survival of proliferating cell nuclear antigen expression in mucoepidermoid carcinoma of the parotid gland. Otolaryngol Head Neck Surg. 1994;111:460-466.
167. Hellquist HB, Sundelin K, Di Bacco A, et al. Tumour growth fraction and apoptosis in salivary gland acinic cell carcinomas. Prognostic implications of Ki-67 and bcl-2 expression and of in situ end labelling (TUNEL). J Pathol. 1997;181:323-329.
168. Nordgard S, Franzen G, Boysen M, et al. Ki-67 as a prognostic marker in adenoid cystic carcinoma assessed with the monoclonal antibody MIB1 in paraffin sections. Laryngoscope. 1997;107:531-536.
169. Hicks J, Flaitz C. Mucoepidermoid carcinoma of salivary glands in children and adolescents: assessment of proliferation markers. Oral Oncol. 2000;36:454-460.
170. Yin HF, Okada N, Takagi M. Apoptosis and apoptotic-related factors in mucoepidermoid carcinoma of the oral minor salivary glands. Pathol Int. 2000;50:603-609.
171. Lim JJ, Kang S, Lee MR, et al. Expression of vascular endothelial growth factor in salivary gland carcinomas and its relation to p53, Ki-67 and prognosis. J Oral Pathol Med. 2003;32:552-561.
172. Okabe M, Inagaki H, Murase T, et al. Prognostic significance of p27 and Ki-67 expression in mucoepidermoid carcinoma of the intraoral minor salivary gland. Mod Pathol. 2001;14:1008-1014.
173. Choi CS, Choi G, Jung KY, et al. Low expression of p27(Kip1) in advanced mucoepidermoid carcinomas of head and neck. Head Neck. 2001;23:292-297.
174. Okabe M, Miyabe S, Nagatsuka H, et al. MECT1-MAML2 fusion transcript defines a favorable subset of mucoepidermoid carcinoma. Clin Cancer Res. 2006;12:3902-3907.
175. Glisson B, Colevas AD, Haddad R, et al. HER2 expression in salivary gland carcinomas: dependence on histological subtype. Clin Cancer Res. 2004;10:944-946.
176. Giannoni C, el-Naggar AK, Ordonez NG, et al. c-erbB-2/neu oncogene and Ki-67 analysis in the assessment of palatal salivary gland neoplasms. Otolaryngol Head Neck Surg. 1995;112:391-398.
177. Press MF, Pike MC, Hung G, et al. Amplification and overexpression of HER-2/neu in carcinomas of the salivary gland: correlation with poor prognosis. Cancer Res. 1994;54:5675-5682.
178. Regis De Brito Santos I, Kowalski LP, Cavalcante De Araujo V, et al. Multivariate analysis of risk factors for neck metastases in surgically treated parotid carcinomas. Arch Otolaryngol Head Neck Surg. 2001;127:56-60.
179. Ferlito A, Shaha AR, Rinaldo A, et al. Management of clinically negative cervical lymph nodes in patients with malignant neoplasms of the parotid gland. ORL; J Otorhinolaryngol Rel Spec. 2001;63:123-126.
180. McGuirt WF. Management of occult metastatic disease from salivary gland neoplasms. Arch Otolaryngol Head Neck Surg. 1989;115:322-325.
181. Medina JE. Neck dissection in the treatment of cancer of major salivary glands. Otolaryngol Clin North Am. 1998;31:815-822.
182. Gold DR, Annino DJJr. Management of the neck in salivary gland carcinoma. Otolaryngol Clin North Am. 2005;38:99-105.
183. Terhaard CH, Lubsen H, Rasch CR, et al. The role of radiotherapy in the treatment of malignant salivary gland tumors. Int J Radiat Oncol Biol Phys. 2005;61:103-111.
184. Armstrong JG, Harrison LB, Spiro RH, et al. Malignant tumors of major salivary gland origin. A matched-pair analysis of the role of combined surgery and postoperative radiotherapy. Arch Otolaryngol Head Neck Surg. 1990;116:290-293.
185. Chen AM, Bucci MK, Weinberg V, et al. Adenoid cystic carcinoma of the head and neck treated by surgery with or without postoperative radiation therapy: prognostic features of recurrence. Int J Radiat Oncol Biol Phys. 2006;66:152-159.
186. Shingaki S, Ohtake K, Nomura T, et al. The role of radiotherapy in the management of salivary gland carcinomas. J Craniomaxillofac Surg. 1992;20:220-224.
187. Matsuba HM, Thawley SE, Devineni VR, et al. High-grade malignancies of the parotid gland: effective use of planned combined surgery and irradiation. Laryngoscope. 1985;95:1059-1063.
188. Garden AS, el-Naggar AK, Morrison WH, et al. Postoperative radiotherapy for malignant tumors of the parotid gland. Int J Radiat Oncol Biol Phys. 1997;37:79-85.
189. Hosokawa Y, Shirato H, Kagei K, et al. Role of radiotherapy for mucoepidermoid carcinoma of salivary gland. Oral Oncol. 1999;35:105-111.
190. Silverman DA, Carlson TP, Khuntia D, et al. Role for postoperative radiation therapy in adenoid cystic carcinoma of the head and neck. Laryngoscope. 2004;114:1194-1199.
191. Griffin TW, Pajak TF, Laramore GE, et al. Neutron vs photon irradiation of inoperable salivary gland tumors: results of an RTOG-MRC Cooperative Randomized Study. Int J Oncol Biol Phys. 1988;15:1085-1090.
192. Laramore GE, Krall JM, Griffin TW, et al. Neutron versus photon irradiation for unresectable salivary gland tumors: final report of an RTOG-MRC randomized clinical trial. Radiation Therapy Oncology Group. Medical Research Council. Int J Radiat Oncol Biol Phys. 1993;27:235-240.
193. Douglas JG, Koh WJ, Austin-Seymour M, et al. Treatment of salivary gland neoplasms with fast neutron radiotherapy. Arch Otolaryngol Head Neck Surg. 2003;129:944-948.
194. Douglas JG, Laramore GE, Austin-Seymour M, et al. Treatment of locally advanced adenoid cystic carcinoma of the head and neck with neutron radiotherapy. Int J Radiat Oncol Biol Phys. 2000;46:551-557.
195. Douglas JG, Laramore GE, Austin-Seymour M, et al. Neutron radiotherapy for adenoid cystic carcinoma of minor salivary glands. Int J Radiat Oncol Biol Phys. 1996;36:87-93.
196. Huber PE, Debus J, Latz D, et al. Radiotherapy for advanced adenoid cystic carcinoma: neutrons, photons or mixed beam? Radiother Oncol. 2001;59:161-167.
197. Rizk S, Robert A, Vandenhooft A, et al. Activity of chemotherapy in the palliative treatment of salivary gland tumors: review of the literature. Eur Arch Otorhinolaryngol. 2007;264:587-594.
198. Holst VA, Marshall CE, Moskaluk CA, et al. KIT protein expression and analysis of c-kit gene mutation in adenoid cystic carcinoma. Mod Pathol. 1999;12:956-960.
199. Sorensen KB, Godballe C, de Stricker K, et al. Parotid carcinoma: expression of kit protein and epidermal growth factor receptor. J Oral Pathol Med. 2006;35:286-291.
200. Hotte SJ, Winquist EW, Lamont E, et al. Imatinib mesylate in patients with adenoid cystic cancers of the salivary glands expressing c-kit: a Princess Margaret Hospital phase II consortium study. J Clin Oncol. 2005;23:585-590.
201. Agulnik M, Cohen EW, Cohen RB, et al. Phase II study of lapatinib in recurrent or metastatic epidermal growth factor receptor and/or erbB2 expressing adenoid cystic carcinoma and non adenoid cystic carcinoma malignant tumors of the salivary glands. J Clin Oncol. 2007;25:3978-3984.
202. Haddad R, Colevas AD, Krane JF, et al. Herceptin in patients with advanced or metastatic salivary gland carcinomas. A phase II study. Oral Oncol. 2003;39:724-727.
203. Bruce IA, Slevin NJ, Homer JJ, et al. Synergistic effects of imatinib (STI 571) in combination with chemotherapeutic drugs in head and neck cancer. Anticancer Drugs. 2005;16:719-726.
204. Freling NJ, Molenaar WM, Vermey A, et al. Malignant parotid tumors: clinical use of MR imaging and histologic correlation. Radiology. 1992;185:691-696.
205. Chen AM, Garcia J, Lee NY, et al. Patterns of nodal relapse after surgery and postoperative radiation therapy for carcinomas of the major and minor salivary glands: what is the role of elective neck irradiation? Int J Radiat Oncol Biol Phys. 2007;67:988-994.