CHAPTER 96 Malignant Neoplasms of the Oral Cavity
Etiology
Tobacco and alcohol consumption are considered the most common preventable risk factors associated with the development of oral cavity squamous cell carcinoma. In addition, this relationship is synergistic, with alcohol serving as a promoter for the carcinogenic effects of tobacco. When compared with nonsmokers, smoking confers a 1.9-fold risk to males and 3-fold risk to females for development of head and neck squamous cell carcinoma. The risk is directly proportional to the years spent smoking and the number of cigarettes smoked per day. Alcohol alone confers a 1.7-fold risk to males drinking 1 to 2 drinks per day compared with nondrinkers. This risk rises to more than 3-fold for heavy drinkers. Individuals who both smoke (two packs per day) and drink (four units of alcohol per day) are 35 times more likely to develop cancer when compared with controls.1 The reported incident rates of developing head and neck cancer based on smoking status are higher in males than females, respectively (nonsmokers 24.4 : 4.8, former smokers 36.9 : 17.2, and current smokers 147.3 : 75.7); however, hazard ratios suggest smoking leads to a higher proportion of females (12.96 for females, 5.45 for males) eventually developing head and neck cancer with the same risk exposure.2 Users of smokeless tobacco have a 4-fold increased risk of oral cavity carcinoma compared with nonusers.3
Tobacco is the leading preventable cause of death in the United States and is responsible for one out of every five deaths.4 As of 1999, approximately one quarter of U.S. adults used tobacco products (25.7% of males and 21.5% of females).5 The evidence supporting the benefit for head and neck cancer patients to cease smoking is compelling. In a study by Moore, 40% of patients who continued to smoke after definitive treatment for an oral cavity malignancy went on to recur or develop a second head and neck malignancy. For patients who stopped smoking after treatment, only 6% went on to develop a recurrence.6 Induction of specific p53 mutations within upper aerodigestive tract tumors has been noted in patients with a history of tobacco and alcohol use.7
When smoking status in patients with head and neck squamous cell carcinoma is assessed, differences exist between the two populations (smoker vs. nonsmoker). Koch and colleagues noted that nonsmokers were represented by a disproportionate number of women and were more frequently at the extremes of age (younger than 30 or older than 85 years of age). Tumors from nonsmokers presented more frequently in the oral cavity, specifically within the oral tongue, buccal mucosa, and alveolar ridge. Smokers presented more frequently with tumors of the larynx, hypopharynx, and floor of the mouth (Fig. 96-1). Common genetic alterations such as loss of heterozygosity at 3p, 4q, and 11q13 and the overall number of chromosomal microsatellite (repeated base sequences) losses were significantly more likely in the tumors of smokers. In addition, the rate of p53 mutations was markedly increased in this group of patients.8 Smoking and habitual use of alcohol have also been associated with p15 gene methylation.9 Former smokers, defined as those individuals who had quit more than 10 years earlier, demonstrate a genetic profile more consistent with nonsmokers.8 Additionally, multiple mesenchyme-associated, stroma-specific, genetic alterations may have a significant role in the development and progression of smoking-related head and neck cancers.10
In India and Southeast Asia, the product of the Areca catechu tree, known as a betel nut, is chewed in a habitual manner and acts as a mild stimulant similar to that of coffee. The nut is chewed in combination with lime and cured tobacco as a mixture known as a quid. The long-term use of the betel nut quid can be destructive to oral mucosa and dentition and has been demonstrated to be highly carcinogenic.11 Another habit associated with oral malignancy is that of reverse smoking, where the lighted portion of the tobacco product is within the mouth during inhalation. The risk of hard palate carcinoma is 47 times greater in reverse smokers compared with nonsmokers.12
The human papillomavirus (HPV) is an epitheliotropic virus detected to varying degrees within samples of oral cavity squamous cell carcinoma. Infection alone is not considered sufficient for malignant conversion; however, results of multiple studies suggest a role of HPV in a subset of head and neck squamous cell carcinoma.13
Environmental ultraviolet light exposure has been associated with the development of lip cancer. The projection of the lower lip, as it relates to this solar exposure, has been implicated in the pathogenesis of squamous cell carcinomas arising on the vermilion border of the lower lip (Fig. 96-2).14 Pipe smoking has also been associated with the development of lip carcinoma. Mechanical irritation, thermal injury, and chemical exposure are suspected etiologic factors contributing to lower lip cancer development in pipe-smoking patients.15
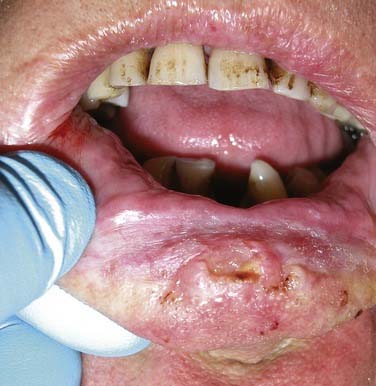
Figure 96-2. Lower lip ulcerative squamous cell carcinoma extending toward the gingivobuccal sulcus.
Other entities associated with oral malignancy include Plummer-Vinson syndrome (achlorhydria, iron deficiency anemia, and mucosal atrophy of the mouth, pharynx, and esophagus); chronic infection with syphilis; ill-fitting dentures; and long-term immunosuppression (30-fold increase with renal transplant). Although evidence linking HIV infection to squamous cell carcinoma of the head and neck is lacking, Kaposi’s sarcoma may arise in the oral cavity.16
Molecular Biology
H-ras, a member of the ras gene family, encodes a plasma protein (p21), which has a role in the transduction of mitogenic signals to the intracellular environment. A synergism between H-ras and HPV is believed to play a role in the induction of squamous cell carcinoma. In human studies, 22% of oral squamous cell carcinomas expressed H-ras, whereas 11% expressed H-ras and HPV. All specimens of oral verrucous carcinoma that expressed H-ras (25%) demonstrated evidence of HPV DNA.17
HPV is a mucosotropic virus that has been closely associated with cervical carcinoma in women, such that HPV 16 and 18 are considered carcinogenic. In oral cavity specimens, variable detection has been reported with rates ranging from 19% to 78%.18,19 Findings of HPV 16 within normal tissue at the margins of tumor specimens have suggested that infection is not a sole event preceding malignant transformation. Rather, it has been postulated that HPV may play a role in the early events of carcinogenesis. HPV oncoproteins E6 and E7 have the ability to bind and degrade tumor suppressor gene products of p53 and pRB, respectively. This binding can impair the capacity of the cell cycle to arrest for the repair of DNA damage and results in an accumulation of genetic changes assisting transformation.13
Smith and colleagues detected a significant increase in the presence of HPV DNA within oral cavity carcinoma samples (15%) compared with controls (5%). In addition, they noted that HPV (odds ratio [OR]-3.7) was a risk factor for carcinoma, independent of tobacco (OR-2.63) and alcohol use (OR-2.57).20 Maden and coworkers noted an increased risk for oral cavity carcinoma with the presence of HPV 6 and HPV 16. Similar to Smith and colleagues, they also demonstrated HPV infection to be a risk factor, independent of age and tobacco and alcohol use.21
Costa and colleagues examined resectable oral cavity carcinomas for biomarkers predictive of chemosensitivity before primary surgical treatment. They found that tumors negative for glutathione S-transferase (GST-π) were more responsive to cisplatin and 5-fluorouracil than tumors that were GST-π-positive. They also noted that lack of bcl-2 expression was consistent with an improved 3-year disease-free survival. No relationship between p53 activity and chemotherapeutic response was observed.22
Overexpression of mutant p53 has been associated with carcinogenesis at multiple sites. Point mutations in p53 have been reported in up to 45% of head and neck carcinomas. Brennan and colleagues have evaluated the concept of primary site margin assessment of specimens on the basis of p53 staining to guide the need for further treatment.23,24 Koch and others have noted that p53 mutation is a key event in the malignant transformation of greater than 50% of head and neck squamous cell carcinomas in smokers.8
Her2-Neu overexpression has been demonstrated in a subset of squamous cell carcinomas.25 Expression of Her2-Neu and heat shock protein (HSP)-27 have been associated with regional treatment recurrence in patients with oral cavity and oropharyngeal carcinomas treated with primary radiotherapy.26
Koch’s tumor progression model has demonstrated that 3p is a site of early loss of heterozygosity, 4q is a late event, and 11q13 is the site of the cyclin D1 gene. Reviews of the head and neck literature have suggested the role for p53, EGFR, TGF-α, and cyclin D1 in predicting clinical outcome for patients with squamous cell carcinoma.27
The Akt receptor has been correlated with the histologic progression of oral premalignant lesions to oral carcinoma. Massarelli and colleagues28 demonstrated that phosphylated-Akt, when highly expressed, is associated with recurrence and shorter disease-free survival independent of a patient’s overall staging.
Epidemiology
Oral cavity malignancies (excluding lip lesions) account for 14% of all head and neck cancers.29 In a review (1985-1996) of the National Cancer Data Base (NCDB), patients diagnosed with an oral cavity cancer had a mean age at presentation of 64 years with a male predominance (60%). Squamous cell carcinoma (SCC) represented the majority of lesions (86.3%), and adenocarcinoma (5.9%), verrucous carcinoma (2.0%), lymphoma (1.5%), and Kaposi’s sarcoma (1.5%) accounted for the remainder. At the time of diagnosis, 55% of patients had early-stage lesions (stage I-II). Patients 35 years old or younger were more likely to present with oral tongue SCC than older patients (76.1% vs. 33%) and less likely to present with a floor-of-mouth SCC (10.5% vs. 35.9%). Of note, the NCDB data did not reveal a poorer overall prognosis for those patients diagnosed at a younger age. In fact, younger patients demonstrated a better 5-year survival (63.7%) than older stage-matched patients (51% for 36 to 65 years of age and 47.6% for older than 65 years of age). Blacks and lower income patients more frequently presented with advanced-stage lesions. Five-year survival was found to be worse for males, blacks, and older patients (older than 65 years). Adenocarcinoma presented more commonly in women than men and was most frequently diagnosed on the hard palate.30
A study by Schantz and Yu31 noted that the incidence for oral tongue carcinoma in patients younger than 40 years of age increased 60% from 1973 to 1984 and has since stabilized. During this same timeframe the incidence of carcinoma at all other oral cavity subsites remained constant or decreased.32 The rise was related to patients born during the years 1938 to 1948. Despite this increase, the 5-year determinant survival of these patients was better than that for older individuals. Although the exact cause for the increase was considered unclear, etiologies of increased smokeless tobacco and marijuana use and human papillomavirus (HPV) infection were considered possible factors.19
Anatomy
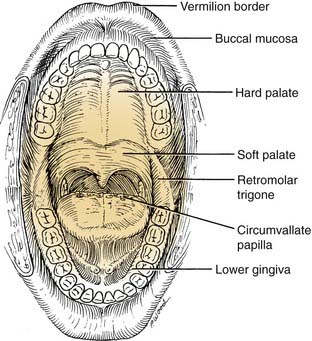
Because of the pivotal role the oral anatomy has on articulation and deglutition, treatment of an oral malignancy can have a significant impact on a patient’s quality of life. An understanding of the complex anatomic relationships among the salivary glands, maxilla, mandible, tongue function, and dentition are paramount to achieving satisfactory oncologic and functional results. The oral cavity extends from the vermilion border of the lip, posterosuperiorly to the hard palate–soft palate junction, inferiorly to circumvallate papillae (linea terminalis), and laterally to the anterior tonsillar pillars. It is divided into seven specific subsites. These subsites are the lips, dentoalveolar ridges, oral tongue, retromolar trigone, floor of mouth, buccal mucosa, and hard palate (Fig. 96-3).
The local, regional, and distant spread of oral cavity malignancies is dependent on the course of the neurovascular anatomy, lymphatic pathways, and the fascial planes of the head and neck. The latter serves as a barrier to the direct spread of tumor and can influence the pattern of local and regional lymphatic spread. In addition, perineural and angioinvasion can act as a conduit for the spread of head and neck malignancies. When present, these histologic findings can have a profound impact on the patient’s prognosis and long-term survival.33,34 Patients previously treated with either surgery or radiation for an oral malignancy may demonstrate atypical patterns of local invasion and regional spread of tumor.35 A brief discussion of the vascular and lymphatic anatomy pertinent to the oral cavity while reviewing the unique attributes of the oral cavity subsites is necessary before considering diagnostic and treatment options.
Arteriovenous Anatomy
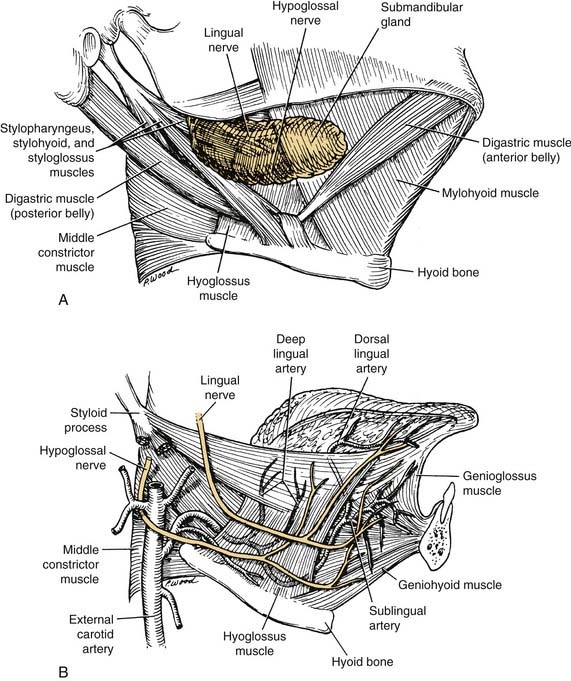
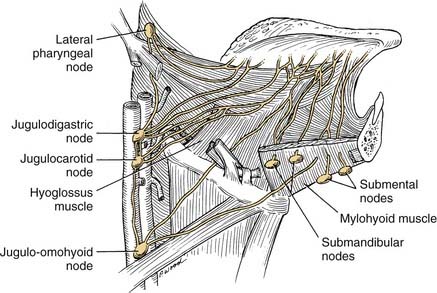
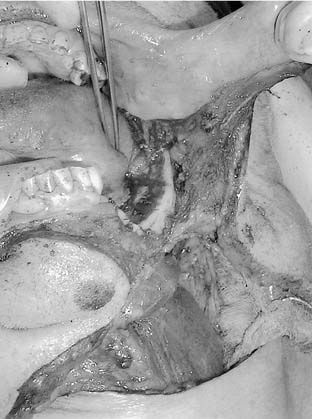
The arterial supply to the oral cavity includes multiple contributions from the external carotid artery. The lingual artery provides the majority of the vascular supply to the oral tongue and tongue base. Identification of the artery within the neck requires the exposure of the floor of the submandibular region. The artery is found deep to the hyoglossus muscle and requires the division of the muscle for maximal exposure. Superficial to the hyoglossus and deep to the mylohyoid muscle is the hypoglossal nerve and lingual veins (Fig. 96-4). An understanding of this relationship can aid in the localization of the artery when necessary for microvascular reconstruction or selective ligation. The vein, which closely approximates the nerve in this region, is prone to injury during dissection and necessitates care when attempting to obtain hemostasis if hypoglossal nerve injury is to be avoided.
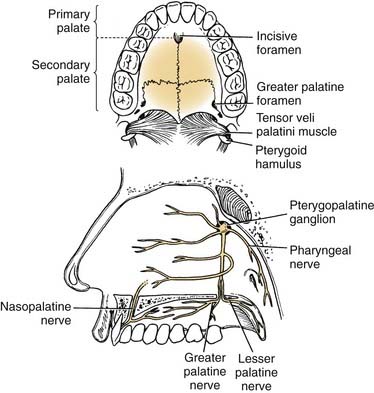
The hard palate blood supply is derived from the greater palatine and the superior alveolar arteries. After branching off the descending palatine artery at the greater palatine foramen, in the region medial to the second maxillary molar, the artery runs anteromedially within the soft tissue of the hard palate (Fig. 96-5). The venous drainage is to the pterygoid plexus and subsequently to the internal jugular venous system. The superior alveolar arteries (anterior, middle, posterior) arise as terminal branches after the transition of the internal maxillary artery to sphenopalatine artery at the pterygopalatine fossa. These arteries provide blood supply to the maxillary gingiva, alveolar ridge, and dentition.
Subsites of the Oral Cavity
Lips
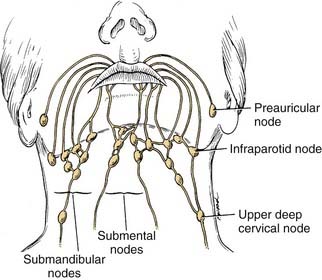
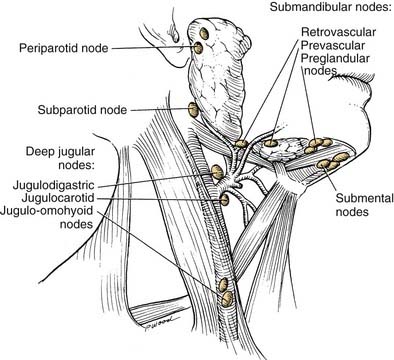
The lips represent a transition from external skin to internal mucous membrane that occurs at the vermilion border. The underlying musculature of the orbicularis oris, innervated by the facial nerve, creates a circumferential ring that allows the mouth to have a sphincter-like function. The sensation for the upper lip is supplied by the infraorbital nerve (CN V2), whereas the lower lip is provided by the mental nerve (CN V3). Lymphatics from both the upper and lower lips drain primarily to the submandibular lymph nodes, yet midline lower lip lesions may present with submental lymphatic spread. In addition, the upper lip may drain to preauricular, infraparotid, and perifacial lymph nodes (Fig. 96-6).
Alveolar Ridge
Oral Tongue
The lymphatic drainage of the oral tongue varies by the region within the tongue. The tip drains preferentially to submental nodes, whereas the lateral tongue drains primarily to the levels I and II (Fig. 96-7). However, it is important to note that a defined lymphatic pathway from the lateral tongue does exist and drains directly to the level III/IV nodal group. The base of the tongue drains to the upper cervical lymphatics. The lack of anastomoses between the anterior tongue lymphatics results in lateralized oral tongue lesions tending to drain ipsilaterally. This is not the case with base-of-tongue lesions where crossover and bilateral cervical lymphatic spread may readily occur.
Retromolar Trigone
This region is represented by the mucosa overlying the ascending ramus of the mandible toward the coronoid process. It is continuous with the buccal mucosa laterally and the anterior tonsillar pillar medially. The superior extent is the maxillary tuberosity and the anterior margin is the posterior aspect of the second mandibular molar. The same considerations that were true for alveolar lesions exist for this anatomic site, given the close approximation of mucosa to the underlying mandible. Lower lip paresthesia may be an indication of perineural invasion at the level of the mandibular foramen with these lesions. The sensation to this region is provided by the lesser palatine nerve and branches of the glossopharyngeal nerve. It is the presence of CN IX that causes patients with lesions within this area to present with referred otalgia. The primary lymphatic drainage for this region is to the upper cervical-jugulodigastric nodal group (Fig. 96-8).
Pathology
Certain histopathologic findings have significant implications on treatment. Tumor thickness, in particular with oral tongue carcinoma, has been the subject of many reports. Tumor thickness has been shown to have a direct relationship to the incidence of regional metastatic spread and survivorship.36 The degree of differentiation and the presence of vascular or perineural invasion have important prognostic implications and may warrant the use of postoperative radiation therapy.
Premalignant Lesions
Leukoplakia is white, mucosal-based keratotic plaque that cannot be wiped free from the underlying tissue. This is a clinical term without a definitive histologic definition. Leukoplakic lesions may demonstrate parakeratosis, hyperkeratosis, and acanthosis on histologic examination. Paradoxically, an increased risk of malignant transformation of leukoplakic lesions is seen more commonly in nonsmokers compared with smokers. If a leukoplakic lesion is associated with an area of dysplasia (in 1% to 3% of all lesions), the risk of progression to malignancy increases seven-fold. Banoczy followed 670 patients with leukoplakic lesions for 3 years and noted that 31% of lesions disappeared, 30% improved, 25% experienced no change, and 7.5% demonstrated local spread. Only 6% of lesions demonstrated eventual progression to squamous cell carcinoma.37 Syphilitic leukoplakias seen in tertiary syphilis have a higher reported rate of malignant transformation.38
Erythroplakia is a red mucosal plaque that does not arise from any obvious mechanical or inflammatory cause and persists after removal of possible etiologic factors. The associated risk for progression to carcinoma is significantly greater than that for leukoplakic lesions. Shafer and Waldron39 resected erythroplakic lesions in 58 patients and found that 91% had evidence of invasive, carcinoma in situ, or severe dysplasia within specimens.
Lichen planus, in particular the erosive subtype, has also been associated with the development of oral carcinoma. The etiology of lichen planus is unknown. T-cell lymphocyte infiltration is noted in this lesion, yet the development of lichen planus is not typically associated with immunologic disorders. The typical patient is a female in the fourth decade of life. Lesions demonstrate a lacy pattern of white striae. Atrophic lesions are red and smooth, whereas erosive lesions have depressed margins and are covered by a layer of fibrinous exudate. An estimated 1% risk of malignant change over 10 years has been reported.38
Squamous Cell Carcinoma and Variants
Several variants of squamous cell carcinoma exist and may be encountered in the oral cavity.
Sarcomatoid squamous cell carcinoma demonstrates a heterogeneous appearance with spindle-shaped cells interwoven with squamous cells. The cytologic behavior of this variant is considered aggressive with a reported metastatic rate of 37%.40 Basaloid carcinoma is also considered a high-grade variant with reported 64% incidence of regional metastases and 44% incidence of distant metastases, and it is associated with 38% mortality at 17 months.41 However, one report notes that when clinical T and N staging are matched, the 5-year disease-free survival associated with oral basaloid carcinoma is similar to that of poorly differentiated and moderately differentiated oral carcinomas.42
Other Pathologies
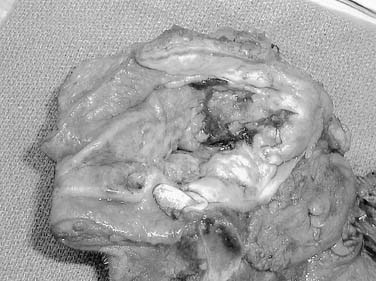
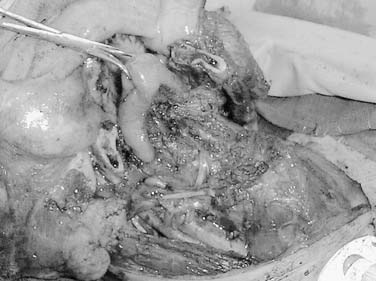
Sarcomas of the oral cavity usually arise in the mandible or hard palate and include osteosarcoma (Fig. 96-9), chondrosarcoma, malignant fibrous histiosarcoma, rhabdomyosarcoma, and liposarcoma. Patel and colleagues demonstrated a 5-year local control rate for head and neck osteosarcoma of 78% and overall survival of 70%. Patients presented with osteosarcoma within the maxilla and mandible with approximately equal frequency, 45% to 41%, respectively. Surgery was performed in all patients, and neoadjuvant chemotherapy was used in 68% of patients. Positive surgical margins were found to be the only significant negative predictor with respect to disease-specific survival.43
Kaposi’s sarcoma (KS) is the most common HIV-associated malignancy. The head and neck is the site for 63% of all presentations. KS may present as a mucosal or cutaneous lesion with a nodular or macular appearance and most commonly occurs on the hard palate. Despite being considered an incurable disorder, a variety of treatment options that may offer remission for limited disease are available. Local therapies include radiation, intralesional injection of chemotherapy, cryotherapy, alitretinoin gel, laser therapy, and surgical excision. Systemic chemotherapy is reserved for advanced disease.16
Desmoplastic neurotrophic melanoma is a nonpigmented lesion of the lower lip that presents as an ulceration and has a high incidence for perineural invasion. Mucosal melanoma typically presents as a pigmented lesion of the oral cavity that may be associated with preexisting melanosis. Pigmented lesions of the oral cavity should be considered for biopsy to exclude this diagnosis.44
Diagnostic Evaluation
History and Physical Examination
The head and neck physical examination should allow for accurate staging of the tumor, assess the patient’s functional capacity before treatment, and include a careful search for synchronous upper aerodigestive tract cancers. The physician should evaluate the dimensions of the index lesion and the potential anatomy involved by direct spread of tumor (Fig. 96-10). The lesion should be palpated to assess for fixation to the underlying periosteum suggesting potential mandibular or maxillary involvement. Determination of midline extension, regional lymphatic spread, and the need for reconstruction should be considered. The appropriateness of specific donor sites for free and/or pedicled flap reconstruction should also be assessed.
Future screening for oral cancer may be possible with outpatient biomarker laboratory assays. St. John and colleagues45 have described the use of an assay for salivary interleukin (IL)-8 and serum IL-6 with promising results in patients with oral cavity and oropharyngeal carcinoma.
Preoperative Assessment
Imaging to assess the primary extent of tumor and cervical lymph nodes includes either computed tomography (CT) or magnetic resonance imaging (MRI). CT is best for demonstrating cortical bone erosion and lymph node metastases. MRI is favored for demonstrating soft tissue invasion by tumor and extension into medullary bone. A Panorex may be obtained preoperatively in patients with suspected mandibular invasion and can assist dental evaluation. Dentascan imaging, using a dental CT-based software program, can be helpful in assessing the likelihood of mandibular invasion, with a reported sensitivity of 95%.46 Goerres and colleagues noted the assessment of cortical invasion of the mandible was best assessed by conventional contrast-enhanced CT (as well as the CT component of PET-CT) with a sensitivity of 100% and accuracy of 97%.47 The extent of imaging is frequently driven by physician preference and the complexity of the presenting lesion. Patients with T1 lesions, without clinical lymphadenopathy, may not require extensive imaging to aid operative decision making.
Staging
Staging for oral cavity malignancies is defined by the American Joint Committee on Cancer (AJCC)48 and follows TNM (primary Tumor, regional Nodal metastases, distant Metastasis) staging format (Table 96-1).
Prognosis
Patients with tumors that display “pushing” margins (when the peripheral invasive margin is assessed as it relates to the surrounding soft tissue) as opposed to “infiltrative” growth patterns have better outcomes.49 In addition, the depth of invasion of oral squamous cell carcinoma and its relationship to the risk of regional metastasis and 5-year survival has been examined. For lesions with a depth of invasion less than 2 mm, 13% of patients demonstrated regional metastases and a 95% 5-year survival was noted. For lesions with a 2- to 9-mm depth of invasion, 46% of patients had regional metastases and the 5-year survival rate decreased to 85%. When depth of invasion increased to greater than 9 mm, the risk of lymph node metastasis increased to 65% and the 5-year survival decreased to 65%.50
Loss of cadherin expression (E-cad and P-cad) as it relates to oral cavity squamous carcinoma correlates with the invasive potential of a cancer and locoregional disease recurrence. In particular, P-cad expression is considered an independent prognostic marker.51 Marcus and colleagues52 demonstrated that monocyte chemoattractant protein 1 was up-regulated in oral cavity carcinoma and the resultant increase in macrophage content was statistically associated with regional metastasis, extracapsular spread, and advanced-stage disease. Tissue inhibitor of metalloproteinase 1 and collagen type 11 alpha-1 have also been demonstrated to be increased in expression in oral cavity and oropharyngeal cancers that are metastatic.53
Laimer and colleagues54 noted that a high STAT1 activation (>35%) in oral cavity carcinoma specimens was associated with a negative lymph node status and a better prognosis in patients receiving adjuvant chemotherapy when compared with nonexpressing counterparts. Xie and colleagues55 noted that patients with higher numeric aberrations of chromosome X and 11 had shorter disease-specific survival. Podoplanin, a glycoprotein important in lymphangiogenesis, is expressed preferentially in oral tongue carcinoma. High levels of podoplanin have been shown to be predictive of lymph node metastasis and shortened disease-specific survival.56 Additionally, intratumoral lymphangiogenesis in patients with early-stage oral carcinoma has been correlated with locoregional recurrence.57
The 5-year distant metastatic rate in patients with oral carcinoma after treatment is approximately 10%. This rate increases from 6.6% in patients with locoregional control to 21.4% in patients who develop a locoregional recurrence. Additional risk factors noted at the time of initial treatment associated with the development of distant metastasis in the two groups were greater than or equal to five positive lymph nodes, extracapsular spread, poor differentiation, and advanced pathologic stage (III or IV).58
In a study examining whether oral tongue carcinoma was associated with a worse prognosis when compared with other oral cavity subsites, Bell and colleagues demonstrated no significant difference in disease-free survival between the groups. Risk factors that were found to statistically affect overall and disease-specific survival were tumor grade and overall staging.59
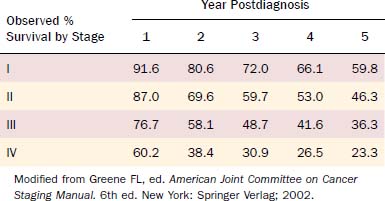
Table 96-2 demonstrates the 5-year observed survival rates for patients with oral cavity squamous cell carcinoma stratified by stage as reported by the AJCC for 1985 to 1991.
Second Primary Tumors
The overall incidence for synchronous and metachronous lesions for patients diagnosed with a primary head and neck malignancy is approximately 14%. Of these malignancies, 80% are metachronous cancers with 50% of cases presenting within the first 2 years of initial treatment of the primary tumor. For patients with oral cavity and oropharyngeal malignancies, the site of a second malignancy is most frequently in the cervical esophagus. New symptoms of dysphagia or odynophagia in this patient population should prompt a diagnostic evaluation with barium swallow or esophagoscopy.60 There appears to be a bimodal pattern for second primary tumors in the esophagus, with an early group developing tumors within 2 years of treatment of the index primary tumor and a later group that develops esophageal cancer greater than 5 years after primary treatment.61
Treatment Considerations
Perioperative antibiotics, in cases with aerodigestive tract exposure to the operative field, can decrease infectious complications associated with surgical intervention. Prophylactic antibiotics are most effective when administered immediately before surgery and for up to 24 hours postoperatively. Extended use of antibiotics in postsurgical patients has not been shown to decrease the risk of infectious complications or fistula formation. The inappropriate use of antibiotics increases the risk for pseudomembranous colitis and the emergence of resistant organisms. Selection of a prophylactic antibiotic for clean-contaminated head and neck surgery should provide adequate gram-positive in addition to anaerobic coverage.62
Multiple general statements can be made concerning the surgical management of oral cancer as a whole. A detailed discussion of the treatment of each oral cavity subsite follows within this chapter. For early-stage lesions of the oral cavity (T1/T2), transoral resection tends to be possible with complete tumor removal and adequate margin control. Primary closure, healing by secondary intention, or placement of a split-thickness skin graft may be used for reconstruction of small defects. Larger and more posteriorly located lesions may require a pull-through or mandibulotomy-based technique to aid exposure for resection and reconstruction. When the tumor closely approximates the mandible, marginal versus segmental mandibulectomy needs to be considered. Reconstructive options for advanced T-stage lesions include microvascular free tissue or pedicled flap reconstruction. Traditionally, T4b lesions have been considered inoperable. Liao and colleagues demonstrated equivalent results with T4a and selected T4b lesions (without carotid encasement and skull base extension) when aggressive surgical management of the primary site and regional lymphatics with postoperative radiation or chemoradiation therapy was performed. Free tissue reconstruction was necessary in 95% of patients. Local control, disease-free, and overall survival numbers were similar.63
Surgery—Treatment of the Primary Lesion
Lip
The majority of neoplastic lesions that affect this subsite present on the lower (88% to 95%) as opposed to the upper lip (2% to 7%) or the commissure (1%) (Fig. 96-11). For the lower lip, squamous cell carcinoma predominates, whereas basal cell carcinoma disproportionately arises on the upper lip. In addition to basal and squamous cell carcinoma, the differential diagnosis of a lip lesion includes keratoacanthoma, minor salivary gland tumors, malignant melanoma, and tumors of mesenchymal origin (malignant fibrous histiocytoma, leiomyosarcoma, fibrosarcoma, angiosarcoma, and rhabdomyosarcoma).
Clinical findings in lip cancer usually include an ulcerated lesion on the vermilion or cutaneous surface or, less commonly, on the mucosal surface. The majority of patients present with early-stage lesions and without evidence of neck metastases.64 A nodular or sclerotic lesion may infiltrate into the deeper tissues, and careful palpation is important for determining the actual size of the lesion. Minor salivary gland tumors present as a submucosal nodule on the inner surface of the lower lip. The presence of paresthesias or dysesthesias in the area adjacent to the lesion indicates possible mental nerve involvement.65
Neck metastases are an infrequent finding with lower lip squamous cell carcinomas and occur in only 10% of cases. Lower lip lesions may metastasize bilaterally because of the communicating pattern of the lymphatics. An increased risk of regional metastasis is associated with commissure and upper lip lesions. Adverse prognostic features of lip primaries include perineural invasion, bony involvement, cancer arising on the upper lip or commissure, regional lymphatic metastasis, and age younger than 40 years at onset.66 Lip cancer results in fewer than 200 patient deaths annually and is stage dependent. Diagnosed early and with adequate treatment, cure rates are high. Obtaining an adequate cosmetic result and functional outcome without microstomia can be challenging with advanced-staged lesions.
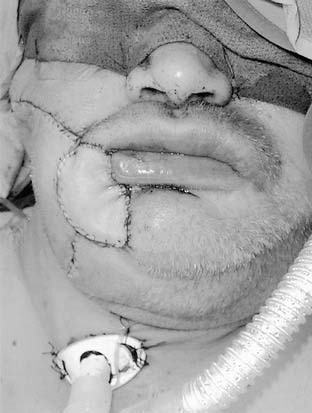
The surgeon should consider several factors when planning a resection and reconstruction for a patient with a lip carcinoma (Fig. 96-12). The typical lip length is 6 to 8 cm. Reconstructive algorithms are based on the proportion of lip resected. Realignment of the vermilion border during the reconstruction and preservation of the oral commissure (when possible) are important principles in attempting to attain an acceptable cosmetic and functional result.
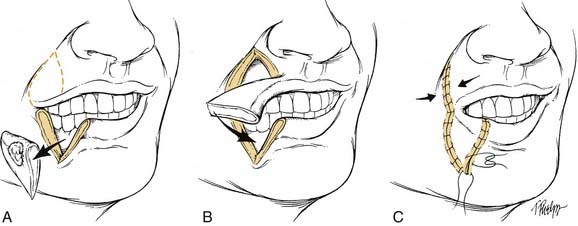
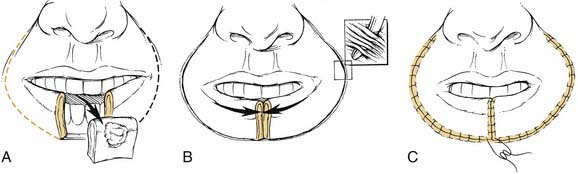
Small primary lesions may be treated with surgery or radiation with equal success and acceptable cosmetic results. However, surgical excision with histologic confirmation of tumor-free margins is the preferred modality. The reconstruction of lip defects after tumor excision requires innovative techniques to provide oral competence, maintenance of dynamic function, and acceptable cosmesis. With small lesions, defects up to one third of the lip’s length, simple excision with primary closure is possible. When lesions require resection of up to one third to two thirds of a lip’s length, reconstructive options include a lip-switch (Abbe-Estlander) (Fig. 96-13) or a Johansson stepladder flap.67 For tumors requiring resection of more than two thirds of the lip, the reconstructive options are the Gilles fan flap, bilateral advancement flaps, Karapandzic, or a free radial forearm with palmaris longus tendon. The Karapandzic flap (Fig. 96-14) is a sensate, neuromuscular flap that includes the remaining orbicularis oris muscle. The blood supply for this flap is the corresponding branches of the labial artery. Microstomia is a potential complication with these methods of lip reconstruction.68 For large defects, Webster or Bernard procedures using lateral nasolabial flaps with buccal advancement have been described.69 In addition, for aggressive and advanced-staged lesions, an evaluation for perineural spread should be performed at the time of resection. Potential biopsy of the mental nerve with a retrograde dissection in an attempt to obtain a negative margin should be considered. With extensive perineural invasion, a drill-out of the mental nerve or hemimandibulectomy may be required.
The 5-year survival for stage I and II lesions is 90%. For patients with cervical metastases, the survival decreases by 50%.67 Patients with oral commissure and upper lip carcinomas have a worse overall prognosis when compared with similar tumors of the lower lip. Postoperative radiation therapy is considered for patients with close or positive margins, lymph node metastases, or perineural invasion.
Alveolar Ridge
In a review of 155 patients with lower alveolar ridge carcinoma, the mean age at presentation was 66.7 years. In this group, 63% of tumors were located over the mandibular body and 25% arose over the symphysis. More than half of all lesions were diagnosed in edentulous patients. The majority of patients (67%) were noted to have early-stage lesions (T1/T2).70
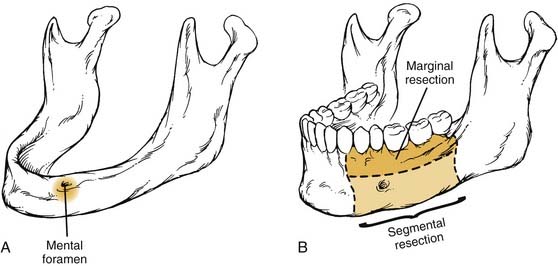
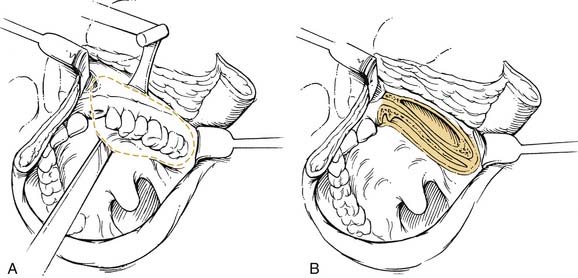
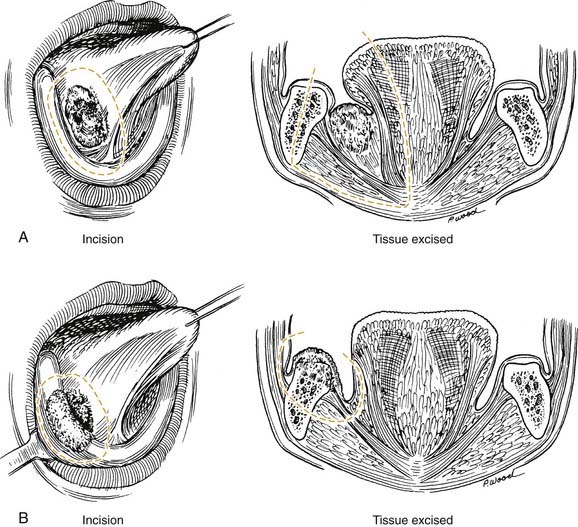
Given that the mucosa closely approximates the underlying bone of the alveolus, osseous erosion and/or invasion at presentation is common. Tumors extending within adjacent dental sockets are associated with a higher likelihood of bone invasion. In this situation, preoperative radiographic evaluation can significantly aid in treatment planning. Close and colleagues71 noted that the CT scan was more sensitive and specific than the bone scan and Panorex in predicting mandibular invasion. The need to address periosteal and bone invasion may be seen in up to 50% of patients. With mandibular invasion, the inferior alveolar nerve may become secondarily involved by perineural invasion. Treatment options of marginal versus segmental mandibulectomy are dependent on whether the tumor invades the periosteum, cortical, or medullary bone (Fig. 96-15). When tumors approach but do not invade the periosteum, a subperiosteal resection with mandibular preservation is possible with primary closure or split-thickness skin graft reconstruction. When the periosteum is invaded, marginal mandibulectomy is indicated. Marginal resection may be performed in two planes. The classic “rim” or coronal marginal mandibulectomy removes the superior aspect of the involved mandible, whereas a lingual sagittal marginal mandibulectomy removes the lingual cortex of the mandible contacting the tumor (Fig. 96-16). With lesions surrounding intact dentition, extraction and alveolar resection are required to obtain an adequate margin. When the tumor extends down the tooth socket into the medullary bone, segmental mandibulectomy is frequently necessary. With segmental mandibular defects of the symphysis, reconstruction with vascularized free tissue transfer with an osseous flap remains the standard of care. For lateral mandibular defects, the options are more varied and may include primary closure, pedicled and/or vascularized free flap soft tissue reconstruction, or vascularized osseous free tissue transfer.
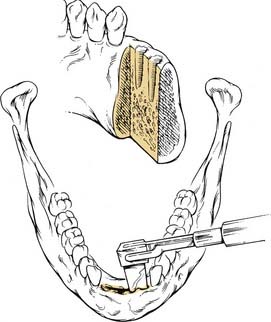
Figure 96-16. Cuts for a sagittal lingual marginal mandibulectomy at the symphysis.
(Adapted from Donald PJ. Head and Neck Cancer: Management of the Difficult Case. Philadelphia: WB Saunders; 1984.)
General guidelines for neck dissection with oral cavity malignancies in the N0 patient include treatment of the neck when the risk of occult metastatic spread to the regional lymphatics exceeds 20%. Upper alveolar ridge tumors tend to regionally metastasize to jugulodigastric lymph nodes, whereas lower alveolar ridge tumors drain to submandibular lymphatics. Among patients with clinical evidence of lymphadenopathy (N+), regional treatment typically requires a modified radical neck dissection (levels I-V). Eicher and colleagues noted that levels IV and V harbored metastases in 20.9% of patients with inferior alveolar ridge cancer undergoing therapeutic neck dissection for clinically evident adenopathy. Patients undergoing elective dissection demonstrated occult metastases in levels I (53.3%), II (40%), and III (6.7%). Presence of an advanced T-stage (T3, T4) lesion and bony mandibular invasion have been correlated with the development of regional metastases. As such, elective neck dissection removing levels I to III is advocated for all advanced-staged primaries (T3, T4) and T1-T2 lesions at the mandibular symphysis or for tumors with moderate to poorly differentiated tissue pathology.70 The overall risk of metastases with alveolar ridge carcinoma is 25%.70,72 In patients with a clinically N0 neck, occult metastases have been noted in 15% of operative specimens. The 2- and 5-year survival for patients with occult metastases who underwent END is better than for patients who underwent therapeutic neck dissection after the eventual development of metastatic adenopathy.70
The 5-year survival ranges from 85% (T1, T2) to 65% (T3, T4) for patients without metastatic spread. Among patients with regional or distant metastases, the 5-year survival has been reported to range from 35% to 59%.73,74 Decreased local control rates are seen with lesions greater than 3 cm and positive surgical margins. Significant prognostic factors include advanced T stage (T3, T4), margin status, mandibular invasion, and the presence of regional metastases. Neither local control nor survival are affected by the extent of mandibular resection, dental intervention near the site of the primary, perineural invasion, or histologic grade of the tumor.72 Postoperative radiation therapy is advocated for patients with T4 tumors, positive margins, perineural invasion, and regional metastasis.70,72
Oral Tongue
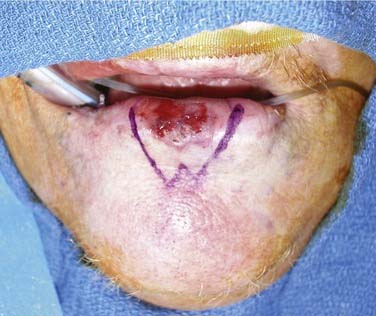
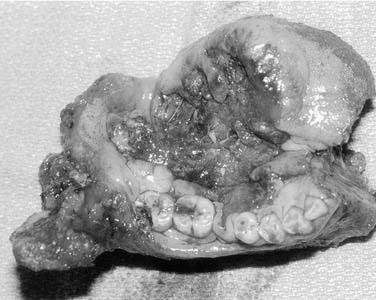
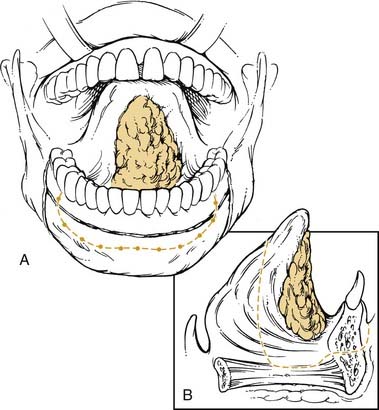
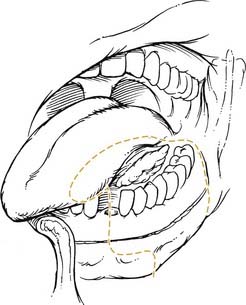
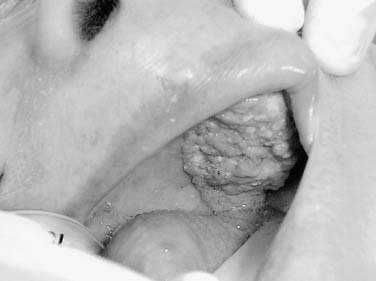
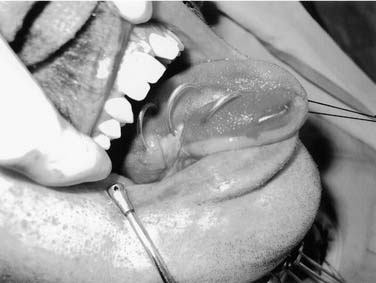
The oral tongue is a muscular structure with overlying nonkeratinizing squamous epithelium. The posterior limit of the oral tongue is the circumvallate papillae, whereas the ventral portion is contiguous with the anterior floor of mouth. Subsites include the lateral tongue, the anterior tip, the ventral tongue, and the dorsal oral tongue. Carcinomas of the tongue arise in the epithelium and invade into the deeper musculature. The most common presentation is that of a painful ulcerated or exophytic mass (Fig. 96-17).
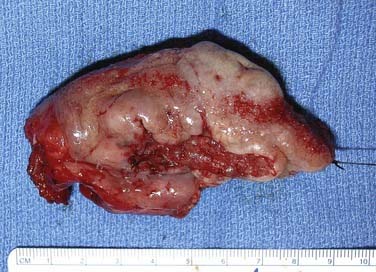
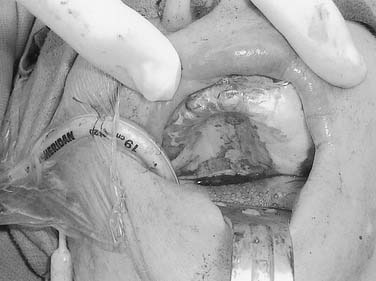
Figure 96-17. Hemiglossectomy specimen demonstrating an ulcer along the lateral edge from a tumor invading toward the floor of mouth.
Oral tongue carcinoma typically presents in males with a history of tobacco and alcohol use in their sixth or seventh decade of life. The majority is squamous cell carcinoma and approximately 75% occur on the posterolateral aspect of the oral tongue. The second most common site (20% of lesions) is the anterolateral and ventral surface of the tongue. The direct contact of carcinogens from tobacco or other agents is considered causative in the development of cancer at this site. Tumors may present on the dorsal surface of the tongue; however, this represents a minority of lesions (3% to 5%). The differential diagnosis for dorsal lesions of the tongue is broad and should include amyloidosis, median rhomboid glossitis, granular cell myoblastoma, and erosive lichen planus. Because of the rarity of carcinomas of the dorsal tongue, a delay in diagnosis is not infrequent.75 The differential diagnosis of submucosal oral tongue tumors is those derived from mesenchymal tissues including leiomyomas, leiomyosarcoma, rhabdomyosarcoma, and neurofibromas.
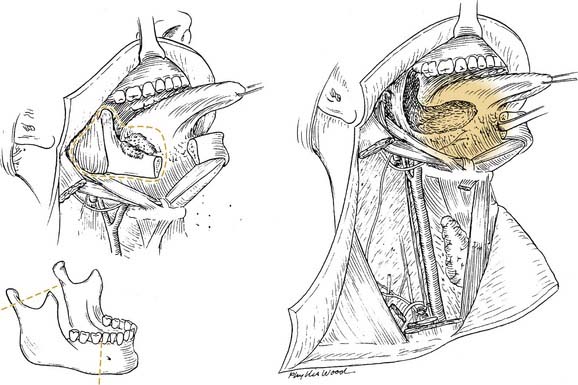
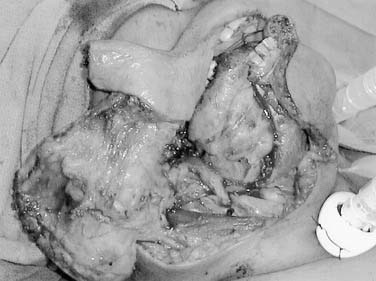
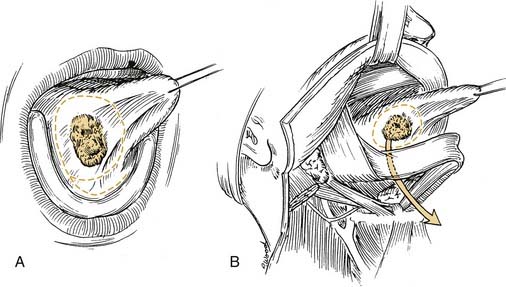
Local invasion by tongue carcinoma may progress by various routes depending on the site of origin. A tumor of the anterolateral tongue may spread medially across the central raphe to the contralateral side, posteriorly to the tongue base, and inferiorly into the suprahyoid muscles and the muscular “root” of the tongue. Lateral spread to involve a significant portion of the floor of mouth is not uncommon. The lingual and hypoglossal nerves may be invaded directly by tumors. Their involvement produces the clinical findings of loss of sensation of the dorsal tongue surface and deviation on tongue protrusion, fasciculations, and atrophy. In addition, the patient may complain of referred otalgia. The extreme of lateral tumor extension, beyond floor of mouth, includes direct invasion of the mandible, requiring composite resection (Figs. 96-18, 96-19, and 96-20).
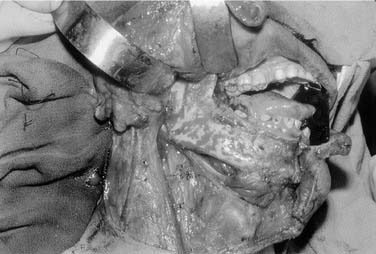
Figure 96-19. Approach for a composite resection for a T4 tongue squamous cell carcinoma invading the lateral mandible.
Kurokawa and colleagues76 noted that in patients with moderately differentiated squamous cell carcinoma of the oral tongue, tumor depth of invasion equal to or greater than 4 mm was associated with occult cervical lymph node metastases. Spiro and colleagues77 demonstrated that increasing tumor thickness, and not T stage, correlated with treatment failure and survival. Disease-related death was uncommon for patients with depth of invasion less than 2 mm. The depth of invasion can be used to guide the necessity for elective neck dissection in N0 patients with early-stage lesions.
At initial presentation, 40% of patients with oral tongue carcinoma demonstrate evidence of cervical metastases. For patients with T1 and T2 tumors with clinically N0 neck examination, 20% to 30% of elective neck dissection specimens are pathologically positive. Byers and colleagues noted that 15.8% of lateral tongue carcinoma patients demonstrated “skip” metastases to lymph nodes, bypassing levels I and II, and presenting with level III or IV metastatic disease. They concluded that elective supraomohyoid neck dissection was inadequate for the control of regional lymphatics of oral tongue carcinoma patients and advocated for levels I to IV neck dissection in this situation.78 When lesions approach the midline, the likelihood for bilateral regional metastasis is high and requires bilateral lymphadenectomy.
Patients with advanced-stage lesions (III or IV) require surgery and postoperative radiation therapy to achieve the best locoregional control rates. Franceschi and colleagues noted an increase in locoregional control for stage III and IV patients from 57% to 71% with the addition of postoperative radiotherapy. In this study the only factor predictive of locoregional recurrence was a positive surgical margin. The majority of patients (78%) recurred within 2 years.79 Among patients developing a local recurrence, attempts at surgical salvage to control disease are frequently unsuccessful.
The 5-year survival for stage I or II tumors is 75% and less than 40% for stage III or IV tumors.79,80
Retromolar Trigone
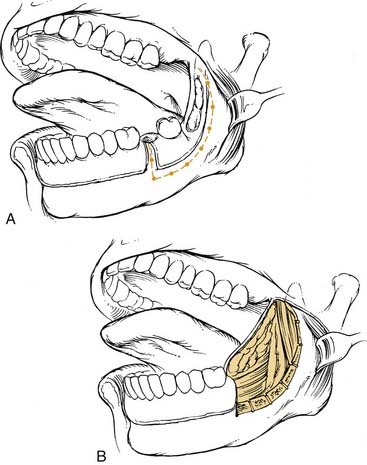
Given the proximity of the mucosa to the underlying ramus of the mandible, surgical resection of early-stage retromolar trigone lesions may require a resection of a portion of the mandible. Treatment options include an L-shaped coronal marginal mandibulectomy, segmental mandibulectomy, and hemimandibulectomy. With the L-shaped marginal mandibulectomy, the superior aspect of the body—extending toward the angle and ascending the ramus—is resected (Figs. 96-21 and 96-22). Segmental mandibulectomy is performed for more extensive lesions with gross bone destruction.
Huang and colleagues demonstrated a 5-year, disease-free survival for T1 lesions of 76%, which declined to 54% for T4 disease. Patients with N0 disease had a 5-year survival of 69%. Survival fell to 56% for those who were N1 and to 26% for patients with N2 disease. The recurrence rate for patients treated with radiation therapy alone was 44% compared with 23% for patients undergoing surgical resection with postoperative radiation therapy. Complications reported in patients undergoing treatment with radiation included osteoradionecrosis, soft tissue necrosis, or severe trismus.81
Floor of Mouth
Carcinomas of the floor of mouth typically present in males in their sixth decade of life. Approximately 35% of these patients present with an advanced T-stage (T3, T4) lesion on initial diagnosis.50 The floor of mouth musculature plays an important role in the modes of tumor spread. The floor of mouth is composed of the slinglike genioglossus, mylohyoid, and the hyoglossus muscles, which serve as a barrier to the spread of disease. Invasion into these muscles can lead to tongue hypomobility and dysarthria. In addition, tumors of the anterior floor of mouth may extend posteriorly into the ventral aspect or “root” of the tongue, causing fixation.
For lesions without evidence of bony invasion but demonstrating involvement with the lingual periosteum, a coronal partial mandibulectomy may be required (Fig. 96-23). For massive lesions associated with mandibular destruction, composite resection with segmental mandibulectomy is necessary (Fig. 96-24).
Cervical metastases are noted in approximately 50% of patients with floor of mouth carcinoma. The submandibular lymph nodes (level IB) are most frequently involved.50 Bilateral metastases are common with lesions of the anterior floor of mouth.
The 5-year survival for carcinomas of the floor of mouth are 90% for stage I, 80% for stage II, 65% for stage III, and 30% for stage IV disease.50,82
Buccal Mucosa
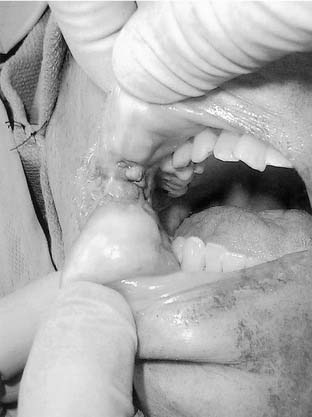
Carcinomas of the buccal mucosa represent 5% to 10% of all oral cavity malignancies (Fig. 96-25). There is a 4 : 1 male predominance and the typical patient is in the sixth decade of life. An association between smokeless tobacco use and buccal carcinomas has been noted. An increased incidence of these tumors is seen in the Southeastern U.S. population. In India, betel nut use is associated with a high incidence of buccal carcinoma.
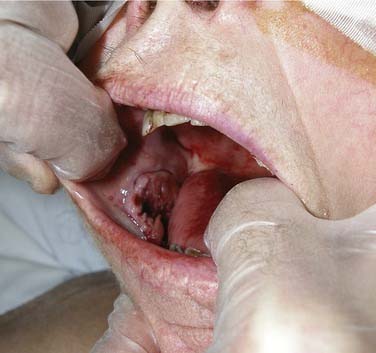
Figure 96-25. Ulcerated buccal mucosal lesion in a patient with oral pain and trismus noted to be squamous cell carcinoma at biopsy.
Verrucous carcinoma usually arises on the buccal mucosa and is considered a low-grade malignancy. Poor oral hygiene, tobacco use, and viral infection have all been suggested as potential etiologies. Although considered low grade, foci of invasive carcinoma and areas of dedifferentiation may be found within an individual specimen. Surgical resection is the treatment of choice.83 Elective treatment of the regional lymphatics is not necessary given the low likelihood of regional metastasis. The differential diagnosis for epithelial-based lesions presenting in the buccal mucosa should also include squamous papilloma and pseudoepitheliomatous hyperplasia. Minor salivary gland tumors may also present as a submucosal mass within this region.
Small lesions can be excised transorally. Intermediate-staged primary tumors may be resected transorally or through a lip-splitting incision. With the exception of superficial lesions, the buccinator muscle should be resected in continuity, thus providing an adequate deep margin. Although primary closure or healing by secondary intention is acceptable for small primary tumors, larger defects should be repaired with a fasciocutaneous flap to avoid scar contracture and trismus, which are frequent sequelae of skin grafting. Local intraoral spread may necessitate resection of the alveolar ridge of the mandible or maxilla. For invasive squamous cell carcinomas and minor salivary gland tumors, the buccinator muscle should be included with the specimen at the time of resection. Deep invasion into the cheek may require through-and-through resection (Figs. 96-26, 96-27, and 96-28). Ideal reconstruction to provide both internal and external lining is best accomplished with a folded fasciocutaneous free flap. Surgery as a primary modality is advocated for buccal carcinoma, whereas combination therapy is advised for advanced lesions. As with most lesions of the oral cavity, tumor thickness has a direct correlation to prognosis. In T1/T2 N0 tumors, depth of invasion greater than 5.17 mm at the primary site has been correlated with a risk of ipsilateral regional lymphatic spread.84
Diaz and colleagues reviewed a series of 119 (previously untreated) patients with buccal carcinoma undergoing treatment at the MD Anderson Cancer Center. Primary tumors were staged as follows: T1—21%, T2—38%, T3—23%, and T4—15%. The majority of patients (72%) were N0 at presentation. The majority of patients were treated exclusively with surgery (71%). No patients were treated with radiation therapy alone, and postoperative radiation therapy (dosage 50 to 60 Gy) was used for patients with extracapsular nodal spread or positive margins. Transoral excision was performed in the majority of patients with only 27% of patients requiring a lip-splitting incision and cheek flap for resection. Modified radical neck dissection was performed for patients with N+ necks, and supraomohyoid neck dissection was used for those with N0 necks. The 5-year survival for all patients within the series was 63%. Five-year survival declined from 70% for N0 patients to 49% for patients with metastatic lymphadenopathy. Within the series of patients, 45% experienced recurrence (23% local, 11% regional, 9% local and regional) and salvage therapy was successful in only 22%. Stensen’s duct involvement and buccinator muscle invasion had no effect on locoregional recurrence.85
Reported 5-year survival is 75% to 78% for stage I, 65% to 66% for stage II, 30% to 62% for stage III, and 20% to 50% for stage IV disease.82,85,86
For patients who are not considered appropriate candidates for extensive surgical resection and reconstruction, primary radiation or chemoradiation is a viable treatment option. The reported results on the use of primary radiation therapy in patients with locally advanced disease demonstrate high recurrence rates (45%) and compromised overall survival (38%) after treatment.87
Hard Palate
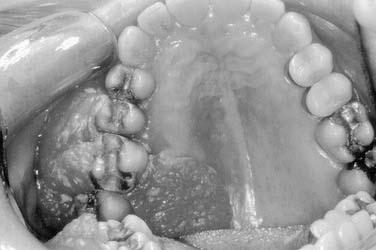
Squamous cell carcinoma is the most common malignancy of the palate (Fig. 96-29). A close second in frequency are minor salivary gland tumors that include adenoid cystic carcinoma, mucoepidermoid carcinoma, adenocarcinoma, and polymorphous low-grade adenocarcinoma.88 Mucosal melanoma most commonly arises on the hard palate and the maxillary gingiva and presents as a nonulcerated, pigmented plaque. The typical patient is 40 to 50 years of age, and the prognosis is poor because of the locoregional aggressiveness of the tumor and propensity for distant metastases. Kaposi’s sarcoma of the palate is the most common intraoral site for this tumor.89 Chronic irritation from ill-fitting dentures may also play a causal role in the development of dysplasia. Inflammatory lesions arising on the palate may mimic malignancy and can be differentiated by biopsy. Necrotizing sialometaplasia appears on the palate as a butterfly-shaped ulcer and resembles a malignancy. Treatment is symptomatic and biopsy confirms its benign nature. Torus palatini are exostoses or bony outgrowths of the midline palate and maxillary bone that do not require surgical treatment unless patients are symptomatic.
Squamous carcinoma of the hard palate is treated surgically. Small lesions may be treated with transoral wide local excision. The resection may be performed to the level of the bony portion of the hard palate with anticipated re-epithelialization. Because the periosteum of the palatal bone acts as a barrier to spread, excision with bony preservation is adequate for small lesions. Involvement of the periosteum requires removal of a portion of the bony palate. Partial or subtotal maxillectomy is required for larger lesions or those involving the maxillary antrum (Fig. 96-30). For malignancies extending along the greater palatine nerve, biopsy of this nerve is important for identifying neurotropic spread. Small- to medium-sized defects may be reconstructed with the aid of local advancement flaps or a buccal fat pad flap. Oronasal postresection fistulas of the palate require a dental prosthesis for rehabilitation of speech and swallowing. For lesions resulting in a palatal defect after resection, prosthetic rehabilitation can offer the patient an excellent functional result. In this circumstance a maxillofacial prosthodontist may construct a splint preoperatively for use at the time of resection. A split-thickness skin graft is used to cover edges of bone from osteotomy and replace sites within the sinuses where mucosa was removed as part of the resection. The cavity is then packed with petroleum gauze, and a lag screw can be used to secure the prosthesis to the maxilla, providing support to the underlying repair (Figs. 96-31, 96-32, and 96-33). The temporary prosthesis is removed approximately 1 week later and the packing is evacuated. An interim prosthesis is worn until a more permanent option can be tailored to the resultant defect.90 Adjuvant radiation is indicated for advanced staged tumors, perineural invasion, and positive margins.
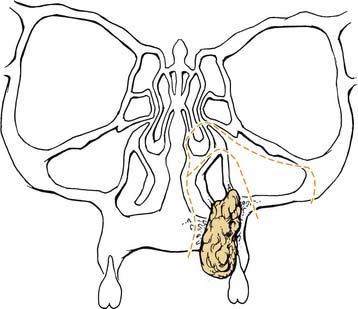
Figure 96-30. Bone cuts on an infrastructure maxillectomy for a hard palate malignancy.
(From Silver CE, Rubin JS. Atlas of Head and Neck Surgery. 2nd ed. New York: Churchill Livingstone; 1999.)
Primary radiotherapy has been investigated as a therapy for hard palate carcinomas with an 80% 5-year local control rate reported for T1 and T2 tumors; however, this control rate falls to 24% for T3 and T4 lesions. Nevertheless, surgery is the favored primary modality for carcinoma of the hard palate. In this study, 5-year survival was 48% for squamous cell carcinomas and 63% for minor salivary gland carcinomas.91
For patients with hard palate malignancies, 10% to 25% present with cervical metastases, most frequently to the submandibular and upper jugular cervical nodes.92 Given the low incidence of occult metastases, elective neck dissection is not indicated in most cases.
The reported 5-year survival for patients ranges from 40% to 60%.92,93
Surgery—Treatment of the Mandible
The management of the mandible as it applies to oral cavity malignancies has evolved over time. Initially, squamous cell carcinoma of the oral cavity was believed to invade the mandible through the adjacent lymphatics. Direct invasion is now recognized as the most common pathway. In a paper by Marchetta and colleagues in 1971,94 it was demonstrated that tumors involve the mandible by first invading the periosteum. When the periosteum was free of direct tumor invasion, a mandible-sparing operation was considered feasible.
Mandible-sparing procedures including vertical and horizontal marginal mandibulectomy (Fig. 96-34) and pull-through techniques have gained popularity as organ conservation philosophies have emerged within the field (Figs. 96-35 and 96-36). In addition, pull-through techniques offer the advantage of avoiding a lip-splitting incision and allow adequate exposure for free tissue reconstruction. A pull-through is contraindicated in patients with gross mandibular destruction but may be performed in continuity with resection of the lingual plate of the mandible.
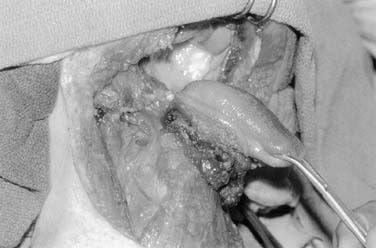
Figure 96-35. Pull-through technique for extensive floor of mouth carcinoma extending into the oral tongue.
Mandibulotomy for access in oncologic resection and reconstruction of oral cavity malignancies is typically reserved for advanced-stage primaries with extensive oropharyngeal spread. Placement of the osteotomy in the midline or paramedian locations (with mental nerve preservation) is the preferred site. The paramedian mandibulotomy offers the advantage of ipsilateral geniohyoid and genioglossus muscle preservation, whereas midline osteotomy requires transection of these muscles. At times, sacrifice of a mandibular incisor or canine tooth may be required to perform an osteotomy. Creation of a mild to moderate malocclusion, independent of technique, has been shown to be a common finding.95 With the bone cut for the mandibulotomy, loss of a fine column of cortical bone occurs. This can be lessened by partially sawing through the lingual cortex and greenstick fracturing the posterior table with an osteotome. Placement of a titanium plate before actual osteotomy is a strategy that will allow for retention of the patient’s normal occlusion when the mandible is reapproximated at the end of the procedure. The placement of drill holes for the plate at the inferior aspect of the mandible in patients with intact dentition avoids iatrogenic tooth root injury. If a lag screw technique is used, care needs to be exercised to avoid overcorrection with fixation of the mandibulotomy ends and creation of a malocclusion. Lateral mandibulotomy, at the level of the body or ramus, should be avoided because of poor healing at the osteotomy site and the interruption of the inferior alveolar artery and nerve at the time of osteotomy.
Complications from mandibulotomy have been extensively reported in the literature and range from 23.2% to 47.6%.96,97 These include plate exposure, lip contracture, ankyloglossia, cellulitis-abscess, temporomandibular joint dysfunction, nonunion, and osteoradionecrosis. When mandibulotomy is performed in the setting of a marginal mandibulectomy, the risk of nonunion and infection associated with devascularization of the involved mandible has led some surgeons to abandon combining the two techniques.96 Eisen and colleagues examined the morbidity of midline mandibulotomy in patients requiring postoperative radiation therapy. They found a complication rate of 11% in patients whose mandibulotomy site was included in the radiation field.98
Assessing the need for segmental mandibulectomy can be a difficult treatment decision to make (Table 96-3). Although gross mandibular invasion clearly mandates a segmental mandibulectomy, situations in which periosteal adherence and subtle cortical invasion are suggested (by clinical examination or radiographic imaging) require the experience and judgment of the surgeon as to whether to proceed with a marginal mandibulectomy versus segmental mandibular resection. Marginal resection in the setting of alveolar ridge carcinoma has been advocated by some authors when radiographically detected bony erosion fails to extend below the level of the mandibular canal.99
Table 96-3 Indications for Surgical Treatment of the Mandible
| Type of Presentation | Intervention Required |
|---|---|
| Mucosal lesion with mandibular approximation, freely mobile | Wide local excision with removal of adjacent periosteum |
| Mucosal lesion with isolated adherence to the mandible, lingual aspect | Lingual-sagittal marginal mandibulectomy |
| Mucosal lesion with isolated adherence to mandible, gingival aspect | “Rim”-coronal marginal mandibulectomy |
| Gross cortical invasion, inferior alveolar nerve involvement | Segmental mandibulectomy |
Surgery—Treatment of the Neck
Patterns of spread from primary tumor sites in the head and neck to cervical lymphatics are well described. The location and incidence of metastasis vary according to the primary site. Primary tumors within the oral cavity and lip typically metastasize to the nodes in levels I, II, and III (Fig. 96-37).
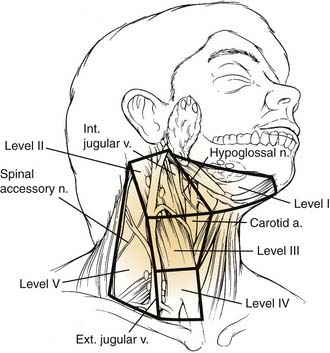
Figure 96-37. Levels of the regional lymphatics of the neck.
(Adapted from Silver CE, Rubin JS. Atlas of Head and Neck Surgery. 2nd ed. New York: Churchill Livingstone; 1999.)
The need to perform a neck dissection in the N0 patient has undergone considerable investigation. Lymphoscintigraphy with sentinel node biopsy in the setting of the N0 oral carcinoma patient has been reported to have a negative predictive value of 100%.100 Even in previously treated oral carcinoma patients, sentinel node biopsy has a high reported negative predictive value (91%).101 Although initial results appear promising, most reported series reflect the experience of only a limited number of patients. Sentinel node biopsy is not currently considered standard of care for oral cavity carcinoma. Myo and colleagues reported on the use of cyclin D1 gene numeric aberrations assessed on FNA samples in stage I and II oral cancer patients. Cluster-type amplification of the cyclin D1 gene correlated with future regional recurrence in patients when the neck was not treated electively.102
For a patient with an oral cavity malignancy who is clinically and radiographically N0, with a risk of occult metastases exceeding 20%, many consider elective treatment to the regional lymphatics necessary. Options for treatment of the regional lymphatics include elective neck irradiation versus neck dissection.103,104 With time, modifications of neck dissection have evolved from radical neck dissection through modified radical neck dissection to selective neck dissection. Surgeons have traditionally treated the N0 neck with surgery.105 Selective neck dissection can serve as a staging procedure to determine the need for postoperative adjuvant radiotherapy. For the clinically N+ neck, the surgical treatment of choice has traditionally been modified radical neck dissection (MRND) or radical neck dissection (RND), although some authors advocate selective neck dissection (SND) for the treatment of limited N1 disease.106 Kuntz and Weymuller examined quality-of-life data for patients undergoing RND, MRND, and SND. Shoulder disability at 6 months was the least for SND patients and similar for SND and MRND patients at 12 months. Additionally, MRND and SND patients were noted to have fewer problems with pain than RND patients.107
The most common selective neck dissection performed for the management of the regional lymphatics in patients with oral cavity cancers is the supraomohyoid neck dissection (SOHND), which includes removal of lymph node levels I, II, and III. The reported incidence of regional recurrence after elective SOHND is as low as 3%.108 Inclusion of level IIb nodes within the neck dissection is advocated for oral cavity primaries because of a reported 10% incidence of spread to this region109; however, some authors disagree that the inclusion of level IIb is necessary in the setting of elective neck dissection.110 The occurrence of skip metastases with oral tongue lesions makes possible the involvement of nodes in levels III or IV without involvement of higher echelons of levels I or II. Byers and colleagues78 noted that patients with lateralized oral tongue squamous cell carcinoma with an ipsilateral N0 neck had a 15.8% rate of “skip metastases.” As a result, Byers and colleagues advocated a level I to IV neck dissection rather than a standard supraomohyoid neck dissection for patients with oral tongue carcinoma and an N0 neck. Level V nodes are involved in some cases and are seen with concomitant involvement of higher echelon nodes.111 Midline lesions have a high incidence of bilateral spread to cervical lymphatics and treatment of both necks is indicated in these scenarios. When considering the routes of spread, extensive hard palate lesions must be viewed with concern. Given the predilection for retropharyngeal spread of soft palate tumors, hard palate lesions with posterior extension should be considered for postoperative radiation therapy to address this region that might otherwise go untreated with standard neck dissection. For all sites, the actual presence of cervical metastasis has a negative impact on survival and, as such, aggressive preoperative investigation and close follow-up for head and neck cancer patients is necessary.112
The decision for surgery versus radiation therapy for treatment of the neck is determined by the modality of treatment chosen for the primary tumor. If the primary tumor is treated with surgery, the neck is generally managed surgically. If the primary tumor is treated with radiation, then the neck is treated similarly.105 For advanced cervical lymphatic disease (N2, N3) or metastasis with extracapsular spread, surgical management of the neck alone is inadequate and postoperative adjuvant radiation therapy is necessary.
Surgically debulking of metastatic disease does not improve survival and is not advocated. Surgical salvage for recurrent neck disease after comprehensive neck dissection or radiation is associated with poor survival.112
Radiation
Role of External Beam Radiation
Of note, two of the indications for postoperative radiation therapy, perineural invasion and vascular invasion, may be identified less frequently than is actually present with standard hematoxylin and eosin staining. Kurtz and colleagues113 demonstrated an increase in the identification of these pathologic findings when immunohistochemical staining with S-100 (for perineural invasion) and CD31 (for vascular invasion) was used.
Conventional dosing is 1.8 to 2 gray (Gy) per fraction, once a day, 5 days a week, to a dose of 62 to 70 Gy. For locally advanced head and neck cancer, radiation delivered in hyperfractionation and accelerated fractionation with concomitant boost protocols has been demonstrated to provide better locoregional control than conventional radiation dosing.114
Package Time
Rosenthal and colleagues115 examined treatment package time, defined as the length of time from surgery to completion of radiation therapy, as it related to outcome for patients with locally advanced squamous cell carcinoma of the head and neck. Their findings supported the concept that a total treatment package time of less than 100 days was associated with improved locoregional control and survival. These findings emphasize the importance of developing a surgical treatment plan that minimizes the risks of complications that would delay the onset of radiation therapy and using a radiation protocol that minimizes the need for treatment breaks.
Brachytherapy
Brachytherapy catheter placement for localized radiation boost has a limited role in the treatment of oral malignancies. Brachytherapy has been used for oral tongue and floor of mouth cancers surgically resected with close surgical margins on final histopathologic interpretation. Intraoperative placement is necessary and tracheostomy may not be required (Fig. 96-38). Loading of the catheters occurs within a hospital setting in a protected room after placement. The use of brachytherapy does require a prolonged hospital stay compared with standard surgical treatment requirements. Brachytherapy as a primary treatment has not been advocated because of the creation of post-treatment fibrosis limiting monitoring for recurrence of disease, as well as the risk of post-treatment ORN.
Adjunctive Chemotherapy
Induction chemotherapy has been advocated to decrease the magnitude of resection; however, clinical trials supporting this approach are lacking. Induction (neoadjuvant) chemotherapy is not currently considered standard of care and potentially delays the timing of the definitive therapeutic procedure. Robbins and colleagues have reported on the use of a neoadjuvant RADPLAT protocol (intra-arterial infusion of cisplatin and intravenous infusion of sodium thiosulfate with concurrent radiation therapy to 50 Gy) in the setting of T2 and T3 oral carcinomas with varying levels of N staging. Surgery was described as a tumor nidusectomy performed after 8 weeks of therapy. Completed response was noted in 80% of the primary sites and 79% of the regional lymphatic fields. Five-year disease-specific survival rate was 64%, with a locoregional control rate of 74%.116
Increased survival with postoperative methotrexate and 5-fluorouracil (5-FU) has been demonstrated in patients with extracapsular nodal spread.117,118 Evidence suggests a response to chemotherapy is predictive of the likelihood of response to radiation therapy.119,120
Cooper and colleagues examined patients at high risk for locoregional recurrence that would benefit from the concurrent addition of cisplatin to postoperative radiation. High-risk patients were identified as having two or more metastatic lymph nodes, presence of extracapsular nodal spread, or positive surgical margins. In reviewing trial data from the Radiation Therapy Oncology Group (RTOG) #85-03 and RTOG #88-24, a benefit in locoregional control was suggested in the concomitant chemoradiation patients defined as “high risk” as opposed to those who received postoperative radiation alone. A difference in survival benefit was not seen between the two groups; however, factors such as lack of adequate randomization, limited group size, and length of follow-up were sited as reasons necessitating additional study.120
The role of induction polychemotherapy followed by radiotherapy or chemoradiotherapy for advanced-stage squamous cell cancer of the oral cavity, oropharynx, hypopharynx, or larynx has recently been reported in two randomized, phase three clinical trials. The European study (EORTC 24971/TAX 323) compared docetaxel, cisplatin, and fluorouracil (TPF) to cisplatin and fluorouracil (PF) as induction chemotherapy followed by radiation therapy for patients with previously untreated, unresectable squamous cell carcinoma.121 Patients in the TPF group demonstrated a significant improvement in progression-free and overall survival as compared with patients in the PF group. A similar study by Posner and colleagues122 (TAX 324) compared induction chemotherapy with TPF or PF followed by concomitant chemoradiotherapy with weekly carboplatin. The study population included previously untreated patients with either unresectable disease or advanced-stage disease where organ preservation therapy was being considered. The results were similar to the TAX 323 study, with patients receiving TPF demonstrating a significant improvement in progression-free and overall survival. Unfortunately, neither study included a control group of chemoradiotherapy alone for comparison to the induction chemotherapy group.
Additional Therapies
Photodynamic Therapy
Photodynamic therapy (PDT) may have a potential role in the treatment of widespread premalignant and superficial oral carcinoma. Use for residual tumor at positive resection margins has also been considered.123 This modality has been used for palliative treatment of gastrointestinal tract, bladder, and lung cancers. Photofrin, a light-activated dye, which theoretically localizes to tumor tissue, is activated by exposure to a 620-nm light and produces oxidizing free radicals. Through disruption of the vasculature, tumor necrosis occurs.124 Ideally, PDT should preferentially affect tumor tissue; however, histologic examination of post-PDT specimens have demonstrated the resultant tissue damage is not absolutely tumor-specific because tissues separate from the tumor also show evidence of necrosis.125 Advantages of PDT include the potential for multiple treatments with favorable functional and cosmetic results. A disadvantage of PDT includes the prolonged skin photosensitivity that may last up to 6 weeks. Newer generations of photosensitizers may be more specific for neoplastic tissue and result in less long-term photosensitization.
In a review of the use of PDT for the treatment of carcinoma in situ (CIS) and early-stage lesions of the head and neck, Biel examined the response of CIS and T1 recurrent squamous carcinomas of the oral cavity.123 A complete response to therapy was noted after one treatment in the 29 patients; however, 5 patients developed recurrences within a follow-up period of 70 months.126 Intraoperative adjuvant treatment with PDT for advanced-stage and recurrent tumors at the tumor’s primary site or regional lymphatic bed has also been advocated.123
Organ Preservation Protocols
An organ preservation approach to oral cavity carcinoma similar to laryngeal preservation treatment has been explored. Chemoradiation protocols have demonstrated effective organ preservation for advanced cancers and would be an attractive alternative for patients facing the need for total glossectomy if bony involvement is absent. Attempts at intra-arterial chemotherapy for advanced disease have demonstrated promising results; however, the toxicity and technical ability to perform this therapy remain obstacles to widespread utilization.127
Chemoprevention
The concept of chemoprevention has been investigated in clinical studies. The goals of treatment are to reverse oral premalignancy and prevent emergence of second primary tumors. Several agents have been used including peroxisome proliferator-activated receptor (PPAR), isotretinoin, and COX-2 inhibitors.128 Because of the side effects associated with some agents (in particular with retinoids) and lack of proven efficacy, general application in the clinical setting has not occurred. Investigation into the use of a “biofilm” created with a mucosal adhesive film to allow for topical administration of tretinoin has been demonstrated to be safe in the animal model and has the potential to decrease the toxicities associated with conventional use of the medication.129
In animal models, lyophilized black raspberries and vitamins C and E can reduce the number of dimethylbenz(a)anthracene (DMBA)-induced tumors within a hamster cheek pouch and may prove to have a role in chemoprevention.130,131 However, in a randomized controlled trial, research examining the potential protective effects of vitamin E and beta-carotene supplementation failed to demonstrate a protective effect on the development of oral cancers in male smokers.132 Studies have also suggested the Bowman-Birk protease inhibitor may be an agent of interest for treating premalignancy. A response was noted in approximately one third of patients treated for leukoplakia with low associated toxicity.133
Regular use of aspirin has been shown in a case-control study to decrease the risk of developing head and neck cancer. The effect was noted to be more significant in women and low to moderate users of alcohol and tobacco products. Heavy smokers and users of alcohol failed to demonstrate a chemoprotective benefit from regular aspirin use.134
Reconstruction
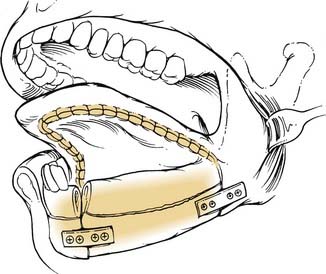
The method chosen for reconstruction depends on the nature of the defect and the patient’s comorbidities. Options, after the resection of small lesions, include allowing the wound to heal by secondary intention, primary closure, and split-thickness skin or dermal grafting. Depending on the size and location of the defect, various advancement and rotation flaps serve as reconstructive options. Use of buccal fat, temporoparietal fascia, or pectoralis major flaps each offer unique options for soft tissue reconstruction. Reconstruction of the mandible is a more significant challenge. Anterior mandibular defects mandate osseous reconstruction (Fig. 96-39). The expanded reconstructive armamentarium provided by free-tissue transfer affords a one-stage method for restoring form and function in patients with complex defects. Reliability of free-tissue transfer is greater than 95% at experienced centers.135 The possibility of distraction osteogenesis to address smaller mandibular defects is currently under investigation and may provide an alternative to free-tissue transfer in selected patients.136
Indications for Free Flap Reconstruction
Pectoralis major pedicled flaps and microvascular soft tissue reconstruction allow for the correction of the mucosal and soft tissue defects associated with tumor resection. For full-thickness buccal defects and extensive glossectomy defects, the pectoralis major flap can be partially de-epithelialized or rotated back on itself to provide resurfacing of the oral cavity components and the external lower one third of the face. However, the optimal treatment for many of these complex defects often requires microvascular free tissue reconstruction. The radial forearm free flap allows for a reconstruction that is tailored in shape to the oral defect without the burden of excessive bulk; offers the option for neural anastomosis, thus providing sensate reconstruction; and is associated with a limited donor site morbidity. When additional soft tissue is required, the anterolateral thigh,137 rectus, and parascapular-scapular flaps may be harvested with larger skin paddles and associated muscle for additional bulk.
As discussed earlier in this chapter, reconstruction of the mandible depends on the location of the defect. In a review of their experience, Urken and colleagues reported on 210 cases requiring mandibular reconstruction. The need for segmental mandibulectomy was necessary for the treatment of benign and malignant tumors of the oral cavity and in the management of stage III osteoradionecrosis. They compared four groups of patients that included those reconstructed with iliac crest, patients who were not reconstructed (lateral defects), normal denture wearers, and normal dentate patients. The study demonstrated that speech and swallowing function were determined by the soft tissue reconstruction. Bite force was best if osteointegrated implants were achieved in patients with bony reconstructions. In addition, oral competence was improved with reconstructed patients.135
Indications for a fibula osteocutaneous free flap reconstruction include total-subtotal mandibular reconstruction, reconstruction of “bone-only” defects (osteoradionecrosis), reconstruction of the atrophic mandible, pediatric mandibular reconstruction, and secondary reconstruction of the subcondylar-condylar complex (Fig. 96-40).135
Patients with advanced-staged complex primary lesions often present reconstructive dilemmas. For defects requiring additional soft tissue reconstruction, double free-tissue transfer with a soft tissue flap and an osseous flap have been advocated.135 A scapular composite flap offers the advantage of multiple skin paddles with bone.138 In addition, the presence of a musculoskeletal abnormality of the lower extremities may preclude a fibular free tissue transfer. Use of two microvascular free flaps, the fibula free flap combined with radial forearm free flap, has also been described to accomplish a reconstruction consistent with the needs of the specific defect. When external skin resurfacing is necessary, a cervicofacial advancement flap is a desirable option to consider in combination with a separate intraoral reconstructive technique.
Long-Term Management and Rehabilitation
Speech and Swallowing
As a rule, the more limited the surgical resection, the less likely a patient is to experience significant functional impact on speech and swallowing. Patients requiring wide surgical resections of the retromolar trigone or procedures that extend deep within the tongue base experience the most significant impact on swallowing. Floor of mouth resections are associated with a detrimental effect on chewing.139 Radiation, alone or in combination with surgery, has a side effect profile including problems such as xerostomia, lymphedema, and fibrosis with scar contracture. Postoperatively, the speech and swallowing therapist will assess the patient’s ability to progress to normal oral intake. Prevention of aspiration pneumonia in these patients is vital. An early postoperative evaluation of swallowing function with a flexible laryngoscope (with a fiberoptic endoscopic evaluation of swallowing protocol) or modified barium swallow can guide the direction of swallowing rehabilitation.
Follow-up
Follow-up occurs in two phases for patients undergoing surgery and radiation for oral malignancies. In the initial phase, when the patient is recovering from surgery or radiation therapy, weekly follow-up may be necessary to assess nutritional status and monitor rehabilitation goals. The second phase is cancer surveillance. See Table 96-4 for the American Head and Neck Society guidelines for cancer surveillance.140
Table 96-4 American Head and Neck Society Guidelines for Cancer Surveillance
| Year Post-Treatment | Interval (Mo) |
|---|---|
| 1st | 1-3 |
| 2nd | 2-4 |
| 3rd | 3-6 |
| 4th and 5th | 4-6 |
| After 5th | Every 12 |
From Deschler DG, Hayden RE. The optimum method for reconstruction of complex lateral oromandibular-cutaneous defects. Head Neck. 2000;22:674-679.
Long-term survival in the face of disease recurrence is limited. Argiris reported on the experience of 399 patients in Eastern Cooperative Oncology Group trials (ECOG 1393 and 1395) receiving palliative chemotherapy in the setting of locoregionally recurrent or metastatic disease. At 1 year, the overall survival was 32%. At 2 years, 49 patients (12%) were alive. Only 6 patients lived to 5 years after the diagnosis of recurrence.141
Argiris A, Li Y, Forastiere A. Prognostic factors and long-term survivorship in patients with recurrent or metastatic carcinoma of the head and neck. Cancer. 2004;101:2222-2229.
Brennan JA, Boyle JO, Koch WM, et al. Association between cigarette smoking and mutation of the p53 gene in squamous cell carcinoma of the head and neck. N Engl J Med. 1995;332:712-717.
Brockenbrough JM, Petruzzelli GJ, Lomasney L. DentaScan as an accurate method of predicting mandibular invasion in patients with squamous cell carcinoma of the oral cavity. Arch Otolaryngol Head Neck Surg. 2003;129:113-117.
Deschler DG, Hayden RE. The optimum method for reconstruction of complex lateral oromandibular-cutaneous defects. Head Neck. 2000;22:674-679.
Diaz EM, Holsinger FC, Zuniga ER, et al. Squamous cell carcinoma of the buccal mucosa: one institution’s experience with 119 previously untreated patients. Head Neck. 2003;25:267-273.
Eicher SA, Overholt SM, el-Naggar AK, et al. Lower gingival carcinoma: clinical and pathologic determinants of regional metastases. Arch Otolaryngol Head Neck Surg. 1996;122:634-638.
Freedman ND, Abnet CC, Leitzman MF, et al. Prospective investigation of the cigarette smoking-head and neck cancer association by sex. Cancer. 2007;110:1593-1601.
Funk GF, Karnell LH, Robinson RA. Presentation, treatment and outcome of oral cavity cancer: a National Cancer Data Base report. Head Neck. 2002;24:165-180.
Goerres GW, Schmid DT, Schuknecht B, et al. Bone invasion in patients with oral cavity cancer: comparison of conventional CT with PET/CT and SPECT/CT. Radiology. 2005;237:281-287.
Hart RD, Nasser JG, Trites JR, et al. Sentinel node biopsy in N0 squamous cell carcinoma of the oral cavity and oropharynx. Arch Otolaryngol Head Neck Surg. 2005;131:34-38.
Huang CJ, Chao KS, Tsai J, et al. Cancer of retromolar trigone: long-term radiation therapy outcome. Head Neck. 2001;23:758-763.
Koch WM, Lango M, Sewell D, et al. Head and neck cancer in nonsmokers: a distinct clinical and molecular entity. Laryngoscope. 1999;109:1544-1551.
Kurtz KA, Hoffman HT, Zimmerman MB, et al. Perineural and vascular invasion in oral cavity squamous carcinoma: increased incidence on re-review of slides and by using immunohistochemical enhancement. Arch Pathol Lab Med. 2005;129:354-359.
Liao CT, Wang HM, Chang JT, et al. Analysis of risk factors for distant metastases in squamous cell carcinoma of the oral cavity. Cancer. 2007;110:1501-1508.
Marcus B, Arenberg D, Lee J, et al. Prognostic factors in oral cavity and oropharyngeal carcinoma. Cancer. 2004;101:2779-2787.
Massarelli E, Liu DD, Lee JJ, et al. Akt activation correlates with adverse outcomes in tongue cancer. Cancer. 2005;104:2430-2436.
Medina JE, Byers RM. Supraomohyoid neck dissection: rationale, indications and surgical technique. Head Neck. 1989;11:111-122.
Munoz-Guerra MF, Marazuela EG, Fernandez-Contreras ME, et al. P-cadherin expression reduced in squamous cell carcinoma of the oral cavity: an indicator of poor prognosis. Cancer. 2005;103:960-969.
Myers EN, Fagan JJ. Treatment of the N+ neck in squamous cell carcinoma of the upper aerodigestive tract. Otolaryngol Clin North Am. 1998;31:671-686.
Nicoletti G, Soutar DS, Jackson MS, et al. Chewing and swallowing after surgical treatment for oral cancer: functional evaluation in 196 selected cases. Plast Reconstr Surg. 2004;114:329-338.
Robbins KT, Samant S, Vieira F, et al. Presurgical cytoreduction of oral cancer using intra-arterial cisplatin and limited concomitant radiation therapy (Neo-RADPLAT). Arch Otolaryngol Head Neck Surg. 2004;130:28-32.
Rosenthal DI, Liu L, Lee JH, et al. Importance of the treatment package time in surgery and postoperative radiation therapy for squamous carcinoma of the head and neck. Head Neck. 2002;24:115-126.
Shah JP. Patterns of cervical lymph node metastasis from squamous carcinomas of the upper aerodigestive tract. Am Surg. 1990;160:405-409.
Urken ML, Buchbinder D, Costantino PD, et al. Oromandibular reconstruction using microvascular composite flaps: report of 210 cases. Arch Otolaryngol Head Neck Surg. 1998;124:46-55.
Weber F, Xu Y, Zhang L, et al. Microenvironmental genomic alterations and clinicopathological behavior in head and neck squamous cell carcinoma. JAMA. 2007;297:187-195.
1. Blot WJ, McLoughlin JK, Winn DM, et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988;48:3282-3287.
2. Freedman ND, Abnet CC, Leitzman MF, et al. Prospective investigation of the cigarette smoking-head and neck cancer association by sex. Cancer. 2007;110:1593-1601.
3. Winn DM, Blot WJ, Shy CM, et al. Snuff dipping and oral cancer among women in the southern United States. N Engl J Med. 1986;305:745-749.
4. Tobacco use—United States, 1900-1999. MMWR Morb Mortal Wkly Rep. 1999;48:986-993.
5. Cigarette smoking among adults—United States, 1999. MMWR Morb Mortal Wkly Rep. 2001;50:869-873.
6. Moore C. Cigarette smoking and cancer of the mouth, pharynx and larynx. JAMA. 1971;218:553.
7. Brennan JA, Boyle JO, Koch WM, et al. Association between cigarette smoking and mutation of the p53 gene in squamous cell carcinoma of the head and neck. N Engl J Med. 1995;332:712-717.
8. Koch WM, Lango M, Sewell D, et al. Head and neck cancer in nonsmokers: a distinct clinical and molecular entity. Laryngoscope. 1999;109:1544-1551.
9. Chang CW, Ling GS, Wei WI, et al. Smoking and drinking can induce p15 methylation in the upper aerodigestive tract of healthy individuals and patients with head and neck squamous carcinoma. Cancer. 2004;101:125-132.
10. Weber F, Xu Y, Zhang L, et al. Microenvironmental genomic alterations and clinicopathological behavior in head and neck squamous cell carcinoma. JAMA. 2007;297:187-195.
11. Jusawalla DJ, Despandi VA. Evaluation of cancer risk in tobacco chewers and smokers: an epidemiological assessment. Cancer. 1971;28:244-252.
12. Baden E. Prevention of cancer of the oral cavity and pharynx. CA Cancer J Clin. 1987;37:49-62.
13. Snijders PJF, Scholes AGM, Hart CA, et al. Prevalence of mucosa tropic human papillomaviruses in squamous cell carcinomas of the head and neck. Int J Cancer. 1996;66:464-469.
14. Ju DMC. On the etiology of cancer of the lower lip. Plast Reconstr Surg. 1973;52:151-154.
15. Wurman LH, Adams GL, Meyerhoff WL. Carcinoma of the lip. Am J Surg. 1975;130:470-474.
16. Munck K, Goldberg AN. HIV and head and neck cancer. Curr Opin Otolaryngol Head Neck Surg. 2002;10:85-90.
17. Anderson JA, Irish JC, McLachlin CM, et al. H-ras oncogene mutation and human papillomavirus infection in oral carcinomas. Arch Otolaryngol Head Neck Surg. 1994;120:755-760.
18. Holladay EB, Gerald WL. Viral gene detection in oral neoplasms using the polymerase chain reaction. Amer J Clin Pathol. 1993;100:36-40.
19. Woods KV, Shillitoe EJ, Spitz MR, et al. Analysis of human papillomavirus DNA in oral squamous cell carcinoma. J Oral Pathol Med. 1993;22:101-108.
20. Smith EM, Hoffman H, Summersgill KS, et al. Human papillomavirus and risk of oral cancer. Laryngoscope. 1998;108:1098-1103.
21. Maden C, Beckmann AM, Thomas DB. Human papillomaviruses, herpes simplex viruses, and the risk of oral cancer in men. Am J Epidemiol. 1992;135:1093-1102.
22. Costa A, Licitra L, Veneroni S, et al. Biological markers as indicators of pathological response to primary chemotherapy in oral-cavity cancers. Int J Cancer. 1998;79:619-623.
23. Boyle JO, Koch W, Hrubin PA, et al. The incidence of P53 mutations increase with progression of head and neck cancer. Cancer Res. 1993;53:4477-4480.
24. Brennan JA, Mao L, Hruban RH, et al. Molecular assessment of histopathologic staging in squamous cell carcinoma of the head and neck. N Engl J Med. 1995;332:429-435.
25. Beckhardt RN, Kiyokawa N, Xil L, et al. Her-2/Neu oncogene characterization in head and neck squamous cell carcinoma. Arch Oto Head Neck Surg. 1995;121:1265-1270.
26. Fortin A, Raybaud-Diogene H, Tetu B, et al. Markers of neck failure in oral cavity and oropharyngeal carcinomas treated with radiotherapy. Head Neck. 2001;23:87-93.
27. Quon H, Liu FF, Cummings BJ. Potential molecular prognostic markers in head and neck squamous cell carcinoma. Head Neck. 2001;23:147-159.
28. Massarelli E, Liu DD, Lee JJ, et al. Akt activation correlates with adverse outcomes in tongue cancer. Cancer. 2005;104:2430-2436.
29. Hoffman HT, Karnell LH, Funk GF, et al. The National Cancer Data Base report on cancer of the head and neck. Arch Otolaryngol Head Neck Surg. 1998;124:951-962.
30. Funk GF, Karnell LH, Robinson RA. Presentation, treatment and outcome of oral cavity cancer: a National Cancer Data Base report. Head Neck. 2002;24:165-180.
31. Schantz SP, Yu GP. Head and neck cancer incidence trends in young Americans, 1973-1997, with a special analysis for tongue cancer. Arch Otolaryngol Head Neck Surg. 2002;128:268-274.
32. Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20-44 years. Cancer. 2005;103:1843-1849.
33. Close LG, Burns DK, Reisch J, et al. Microvascular invasion in cancer of the oral cavity and oropharynx. Arch Otolaryngol Head Neck Surg. 1987;113:1191-1195.
34. Fagan J, Collins B, Barnes L, et al. Perineural invasion in squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 1998;124:637-640.
35. Fleming ID, Cooper JS, Henson ED, et al, editors. AJCC Cancer Staging Manual, ed 5, Philadelphia: Lippincott-Raven, 1998.
36. Morton RD, Ferguson CM, Lambie NK, et al. Tumor thickness in early tongue cancer. Arch Otol Head Neck Surg. 1994;120:717-720.
37. Banoczy J. Follow-up studies in oral leukoplakia. J Maxillofac Surg. 1977;5:69-75.
38. Cawson RA, Odell EW. Oral pathology, ed 2. Philadelphia: Churchill Livingstone; 1999.
39. Shafer WG, Waldron CA. Erythroplakia of the oral cavity. Cancer. 1975;36:1021-1028.
40. Batsakis JG, Rice DH, Howard DR. The pathology of head and neck tumors: spindle cell lesions (sarcomatoid carcinoma, nodular fasciitis, and fibrosarcomas) of the aerodigestive tracts, part 14. Head Neck. 1982;4:499-513.
41. Coppola D, Catalano E, Tang CK, et al. Basaloid squamous cell carcinoma of the floor of mouth. Cancer. 1993;72:2299-2305.
42. de Sampaio Goes FC, Oliviera DT, Dorta RG, et al. Prognoses of oral basaloid squamous cell carcinoma and squamous cell carcinoma: a comparison. Arch Otolaryngol Head Neck Surg. 2004;130:83-86.
43. Patel SG, Meyers P, Huvos AG, et al. Improved outcomes in patients with osteogenic sarcoma of the head and neck. Cancer. 2002;95:1495-1503.
44. Patel SG, Prasad ML, Escrig M, et al. Primary mucosal malignant melanoma of the head and neck. Head Neck. 2002;24:247-257.
45. St John MA, Li Y, Zhou X, et al. Interleukin 6 and interleukin 8 as potential biomarkers for oral cavity and oropharyngeal squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2004;130:929-935.
46. Brockenbrough JM, Petruzzelli GJ, Lomasney L. DentaScan as an accurate method of predicting mandibular invasion in patients with squamous cell carcinoma of the oral cavity. Arch Otolaryngol Head Neck Surg. 2003;129:113-117.
47. Goerres GW, Schmid DT, Schuknecht B, et al. Bone invasion in patients with oral cavity cancer: comparison of conventional CT with PET/CT and SPECT/CT. Radiology. 2005;237:281-287.
48. American Joint Committee on Cancer. American Joint Committee for Cancer Staging Manual. ed 6. Chicago; 2002.
49. Bundgaard T, Rossen K, Henriksen SD, et al. Histopathologic parameters in the evaluation of T1 squamous cell carcinomas of the oral cavity. Head Neck. 2002;24:656-660.
50. Shaha AR, Spiro RH, Shah JP, et al. Squamous carcinoma of the floor of mouth. Am J Surg. 1984;148:455-459.
51. Munoz-Guerra MF, Marazuela EG, Fernandez-Contreras ME, et al. P-cadherin expression reduced in squamous cell carcinoma of the oral cavity: an indicator of poor prognosis. Cancer. 2005;103:960-969.
52. Marcus B, Arenberg D, Lee J, et al. Prognostic factors in oral cavity and oropharyngeal carcinoma. Cancer. 2004;101:2779-2787.
53. Schmalbach CE, Chepeha DB, Giordano TJ, et al. Molecular profiling and the identification of genes associated with metastatic oral cavity/pharynx squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2004;130:295-302.
54. Laimer K, Spizzo G, Obrist P, et al. STAT1 activation in squamous cell cancer of the oral cavity: a potential predictive marker of response to adjuvant chemotherapy. Cancer. 2007;110:326-333.
55. Xie X, Clausen OP, Boysen M. Correlation of numerical aberrations of chromosome X and 11 and poor prognosis in squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 2006;132:511-515.
56. Yuan P, Temam S, el-Naggar A, et al. Overexpression of podoplanin in oral cancer and its association with poor clinical outcome. Cancer. 2006;107:563-569.
57. Munoz-Guerra MF, Marazuela EG, Fernandez-Contreras ME, et al. Prognostic significance of intratumoral lymphangiogenesis in squamous cell carcinoma of the oral cavity. Cancer. 2004;100:553-560.
58. Liao CT, Wang HM, Chang JT, et al. Analysis of risk factors for distant metastases in squamous cell carcinoma of the oral cavity. Cancer. 2007;110:1501-1508.
59. Bell RB, Kademani D, Homer L, et al. Tongue cancer: Is there a difference in survival compared with other subsites in the oral cavity? J Oral Maxillofac Surg. 2007;65:229-236.
60. Weber RS, Duffey DC. Head and neck. In Townsend CM, editor: Sabiston’s Textbook of Surgery, ed 16, Philadelphia: WB Saunders, 2000.
61. Haughey BH, Gates GA, Arfken CL, et al. Meta-analysis of second malignant tumors in head and neck cancer: the case for an endoscopic screening protocol. Ann Otol Rhinol Laryngol. 1992;101:105-112.
62. Weber RS, Raad I, Frakenthaler R, et al. Ampicillin-sulbactam vs clindamycin in head and neck oncologic surgery: the need for gram-negative coverage. Arch Otolaryngol Head Neck Surg. 1992;118:1159-1163.
63. Liao CT, Chang JT, Wang HM, et al. Surgical outcomes of T4a and resected T4b oral cavity cancer. Cancer. 2006;107:337-344.
64. Heller KS, Shah JP. Carcinoma of the lip. Am J Surg. 1979;138:600-603.
65. Weber RS, Palmar JM, Adel E, et al. Minor salivary gland tumors of lip and buccal mucosa. Laryngoscope. 1989;99:6-9.
66. Zitsch RP, Park CW, Renner FJ, et al. Outcome analysis for lip carcinoma. Otolaryngol Head Neck Surg. 1995;113:589-596.
67. Baker SR. Current management of cancer of the lip. Oncology (Huntings). 1990;4:107-120.
68. Calhoun K. Reconstruction of small- and medium-sized defects of the lower lip. Am J Otolaryngol. 1992;13:16-22.
69. Conley JJ, Donovan DT. A new technique for total reconstruction of the lower lip in a patient with malignant melanoma. Otolaryngol Head Neck Surg. 1986;94:393-397.
70. Eicher SA, Overholt SM, el-Naggar AK, et al. Lower gingival carcinoma: clinical and pathologic determinants of regional metastases. Arch Otolaryngol Head Neck Surg. 1996;122:634-638.
71. Close LG, Merkel M, Burns DK, et al. Computed tomography in the assessment of mandibular invasion by intraoral carcinoma. Ann Otol Rhinol Laryngol. 1986;95:383-388.
72. Overholt SM, Eicher SA, Wolf P, et al. Prognostic factors affecting outcome in lower gingival carcinoma. Laryngoscope. 1996;106:1335-1339.
73. Byers RM. Results of treatment of squamous carcinoma of the lower gum. Cancer. 1981;47:2236-2238.
74. Soo KC, Spiro RH, King W, et al. Squamous carcinoma of the gums. Am J Surg. 1998;156:281-285.
75. Goldenberg D, Ardekian L, Rachmiel A, et al. Carcinoma of the dorsum of the tongue. Head Neck. 2000;22:190-194.
76. Kurokawa H, Yamashita Y, Takeda S, et al. Risks factors for late cervical lymph node metastases in patients with stage I or II carcinoma of the tongue. Head Neck. 2002;24:731-736.
77. Spiro RH, Huvos AG, Wong GY, et al. Predictive value of tumor thickness in squamous cell carcinoma confined to the tongue and floor of mouth. Am J Surg. 1986;152:345-350.
78. Byers RM, Weber RS, Andrews T, et al. Frequency and therapeutic implications of “skip metastases” in the neck from squamous carcinoma of the oral tongue. Head Neck. 1997;19:14-19.
79. Franceschi D, Gupta R, Spiro RH, et al. Improved survival in the treatment of squamous carcinoma of the oral tongue. Am J Surg. 1993;166:360-365.
80. Lydiatt DD, Robbins KT, Byers RM, et al. Treatment of stage I and II oral cancer. Head Neck. 1993;15:308-312.
81. Huang CJ, Chao KSC, Tsai J, et al. Cancer of retromolar trigone: long-term radiation therapy outcome. Head Neck. 2001;23:758-763.
82. Rodgers LWJr, Stringer SP, Mendenhall WM, et al. Management of squamous cell carcinoma of the floor of mouth. Head Neck. 1993;15:16-19.
83. Medina JE, Dichtel W, Lune MA. Verrucous carcinoma of the oral cavity: a clinicopathological study of 104 cases. Arch Otolaryngol. 1984;110:26-38.
84. Jing J, Li L, He W, et al. Prognostic predictors of squamous cell carcinoma of the buccal mucosa with negative surgical margins. J Oral Maxillofac Surg. 2006;64:896-901.
85. Diaz EM, Holsinger FC, Zuniga ER, et al. Squamous cell carcinoma of the buccal mucosa: one institution’s experience with 119 previously untreated patients. Head Neck. 2003;25:267-273.
86. Bloom ND, Spiro RH. Carcinoma of the cheek mucosa: a retrospective analysis. Am J Surg. 1987;154:411-414.
87. Pop LA, Eijkenboom WM, de Boer MF, et al. Evaluation of treatment results of squamous cell carcinoma of the buccal mucosa. Int J Radiat Oncol Biol Phys. 1989;162:483-487.
88. Beckhardt RN, Weber RS, Zane R, et al. Minor salivary gland tumors of the palate: clinical and pathologic correlates of outcome. Laryngoscope. 1995;105:1155-1160.
89. Jindal JR, Campbell BH, Ward TO, et al. Kaposi’s sarcoma of the oral cavity in a non-AIDS patient: case report and review of the literature. Head Neck. 1990;17:233-237.
90. Gullane P, O’Dwyer TP. Cancer of the hard palate. In Gates G, editor: Current Therapy in Otolaryngology: Head and Neck Surgery, ed 4, Toronto: BC Decker, 1990.
91. Yorozu A, Sykes AJ, Slevin NJ. Carcinoma of the palate treated with radiotherapy: a retrospective review of 31 cases. Oral Oncol. 2001;37:493-497.
92. Evans JF, Shah JP. Epidermoid carcinoma of the palate. Am J Surg. 1981;142:451-455.
93. Konrad HR, Canalis RF, Calcaterra TC, et al. Epidermoid carcinoma of the palate. Arch Otolaryngol. 1978;104:208-212.
94. Marchetta FC, Saro K, Murphy JB. The periosteum of the mandible and intraoral carcinoma. Am J Surg. 1971;122:711-713.
95. Riddle SA, Anderson PE, Everts EC, et al. Midline mandibular osteotomy: an analysis of functional outcomes. Laryngoscope. 1997;107:893-896.
96. Dai TS, Hao SP, Chang KP, et al. Complications of mandibulotomy: midline versus paramidline. Otolaryngol Head Neck Surg. 2003;128:137-141.
97. Shah JP, Kumaraswamy SV, Kulkarni V. Comparative evaluation of fixation methods after mandibulotomy for oropharyngeal tumors. Am J Surg. 1993;166:431-434.
98. Eisen MD, Weinstein GS, Chalian A, et al. Morbidity after midline mandibulotomy and radiation therapy. Am J Otolaryngol. 2000;21:312-317.
99. Tei K, Totsuka Y, Lizuka T, et al. Marginal resection for carcinoma of the mandibular alveolus and gingiva where radiographically detected bone defects do not extend beyond the mandibular canal. J Oral Maxillofac Surg. 2004;62:834-849.
100. Hart RD, Nasser JG, Trites JR, et al. Sentinel node biopsy in N0 squamous cell carcinoma of the oral cavity and oropharynx. Arch Otolaryngol Head Neck Surg. 2005;131:34-38.
101. Hart RD, Henry E, Nasser JG, et al. Sentinel node biopsy in N0 squamous cell carcinoma of the oral cavity and oropharynx in patients previously treated with surgery or radiation therapy: a pilot study. Arch Otolaryngol Head Neck Surg. 2007;133:806-809.
102. Myo K, Uzawa N, Miyamoto R, et al. Cyclin D1 gene numerical aberration is a predictive marker for occult cervical lymph node metastasis in TNM stage I and II squamous cell carcinoma of the oral cavity. Cancer. 2005;104:2709-2716.
103. Byers RM, Wolf PF, Ballantyne AJ. Rationale of elective modified neck dissection. Head Neck. 1988;10:160-167.
104. Shah JP, Candela FC, Poddar AK. The patterns of cervical lymph node metastases from squamous cell carcinoma of the oral cavity. Cancer. 1990;66:109-113.
105. Eicher SA, Weber RS. Surgical management of cervical lymph node metastases. Curr Opin Oncol. 1996;8:215-220.
106. Medina JE, Byers RM. Supraomohyoid neck dissection: rationale, indications and surgical technique. Head Neck. 1989;11:111-122.
107. Kuntz AL, Weymuller EAJr. Impact of neck dissection on quality of life. Laryngoscope. 1999;109:1334-1338.
108. Chone CT, Silva AR, Crespo AN, et al. Regional tumor recurrence after supraomohyoid neck dissection. Arch Otolaryngol Head Neck Surg. 2003;129:54-58.
109. Villaret AB, Piazza C, Peretti G, et al. Multicentric prospective study on the prevalence of sublevel IIb metastases in head and neck cancer. Arch Otolaryngol Head Neck Surg. 2007;133:897-903.
110. Lim YC, Song MH, Kim SC, et al. Preserving level IIb lymph nodes in elective supraomohyoid neck dissection for oral cavity squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2004;130:1088-1091.
111. Shah JP. Patterns of cervical lymph node metastasis from squamous carcinomas of the upper aerodigestive tract. Am J Surg. 1990;160:405-409.
112. Myers EN, Fagan JJ. Treatment of the N+ neck in squamous cell carcinoma of the upper aerodigestive tract. Otolaryngol Clinic North Am. 1998;31:671-686.
113. Kurtz KA, Hoffman HT, Zimmerman MB, et al. Perineural and vascular invasion in oral cavity squamous carcinoma: increased incidence on re-review of slides and by using immunohistochemical enhancement. Arch Pathol Lab Med. 2005;129:354-359.
114. Fu KK, Pajak TF, Trotti A, et al. A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: first report of RTOG 9003. Int J Radiat Oncol Biol Phys. 2000;48:7-16.
115. Rosenthal DI, Liu L, Lee JH, et al. Importance of the treatment package time in surgery and postoperative radiation therapy for squamous carcinoma of the head and neck. Head Neck. 2002;24:115-126.
116. Robbins KT, Samant S, Vieira F, et al. Presurgical cytoreduction of oral cancer using intra-arterial cisplatin and limited concomitant radiation therapy (Neo-RADPLAT). Arch Otolaryngol Head Neck Surg. 2004;130:28-32.
117. Ensley JF, Jacobs JR, Weaver A, et al. Correlation between response to cisplatinum-combination chemotherapy and subsequent radiotherapy in previously untreated patients with advanced squamous cell cancers of the head and neck. Cancer. 1984;54:811-814.
118. Johnson JT, Wagner RL, Myers EN. A long-term assessment of adjuvant chemotherapy on outcome of patients with extracapsular spread of cervical metastases from squamous cell carcinoma of the head and neck. Cancer. 1996;77:181-185.
119. Cognetti F, Pinnaro P, Ruggeri EM, et al. Prognostic factors for chemotherapy response and survival using combination chemotherapy as initial treatment of advanced head and neck squamous cell cancer. J Clin Oncol. 1989;7:829-837.
120. Cooper JS, Pajak TF, Forastiere A, et al. Precisely defining high-risk operable head and neck tumors based on RTOG #85-03 and #88-24: targets for postoperative radiochemotherapy? Head Neck. 1998;20:588-594.
121. Vermorken JB, Remenar E, van Herpen C, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357:1695-1704.
122. Posner MR, Hershock DM, Blajman CR, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357:1705-1715.
123. Biel MA. Photodynamic therapy and the treatment of head and neck neoplasia. Laryngoscope. 1998;108:1259-1268.
124. Gluckman JL, Zitsch RP. Early detection and screening for head and neck cancer. Cancer Treatment Research. 1995;74:141-157.
125. Grant WE, Speight PM, Hopper C, et al. Photodynamic therapy: an effective, but non-selective treatment for superficial cancers of the oral cavity. Int J Cancer. 1997;71:937-942.
126. Hopper C. Photodynamic therapy: a clinical reality in the treatment of cancer. Lancet Oncol. 2000;1:212-219.
127. Los G, Blommaert A, Barton R, et al. Selective intra-arterial infusion of high-dose cisplatin in patients with advanced head and neck cancer results in high tumor platinum concentrations and cisplatin-DNA adduct formation. Cancer Chemother Pharm. 1995;37:150-154.
128. Hong W, Endicott S, Itri LM, et al. 13-cis retinoic acid in the treatment of oral leukoplakia. N Engl J Med. 1986;315:1501.
129. Wang Z, Polavaram R, Fuentes CF, et al. Topical chemoprevention of oral cancer with tretinoin “biofilm”. Arch Otolaryngol Head Neck Surg. 2003;129:869-873.
130. Casto BC, Kresty LA, Kraly CL, et al. Chemoprevention of oral cancer by black raspberries. Anticancer Res. 2002;22:4005-4015.
131. Sawant SS, Kandarkar SV. Role of vitamins C and E as chemopreventive agents in the hamster cheek pouch treated with the oral carcinogen-DMBA. Oral Dis. 2000;6:241-247.
132. Wright ME, Virtamo J, Hartman AM, et al. Effects of alpha-tocopherol and beta-carotene supplementation on upper aerodigestive tract cancers in a large, randomized controlled trial. Cancer. 2007;109:891-898.
133. Armstrong WB, Kennedy AR, Wan XS, et al. Clinical modulation of oral leukoplakia and protease activity by Bowman-Birk inhibitor concentrate in a phase IIa chemoprevention trial. Clin Cancer Res. 2000;6:4684-4691.
134. Jayaprakesh V, Rigual NR, Moysich KB, et al. Chemoprevention of head and neck cancer with aspirin: a case-control study. Arch Otolaryngol Head Neck Surg. 2006;132:1231-1236.
135. Urken ML, Buchbinder D, Costantino PD, et al. Oromandibular reconstruction using microvascular composite flaps: report of 210 cases. Arch Otolaryngol Head Neck Surg. 1998;124:46-55.
136. Levin L, Carrasco L, Kazemi A, et al. Enhancement of the fibula free flap by alveolar distraction for dental implant restoration: report of a case. Facial Plast Surg. 2003;19:87-94.
137. Chuang HC, Su CY, Jeng SF, et al. Anterior lateral thigh flap for buccal mucosal defect after resection of buccal cancer. Otolaryngol Head Neck Surg. 2007;137:632-635.
138. Deschler DG, Hayden RE. The optimum method for reconstruction of complex lateral oromandibular-cutaneous defects. Head Neck. 2000;22:674-679.
139. Nicoletti G, Soutar DS, Jackson MS, et al. Chewing and swallowing after surgical treatment for oral cancer: functional evaluation in 196 selected cases. Plast Reconstr Surg. 2004;114:329-338.
140. The American Society for Head and Neck Surgery and Society for Head and Neck Surgeons. Clinical Practice Guidelines for the Diagnosis and Management of Cancer of the Head and Neck. Alexandria: Virginia; 1996.
141. Argiris A, Li Y, Forastiere A. Prognostic factors and long-term survivorship in patients with recurrent or metastatic carcinoma of the head and neck. Cancer. 2004;101:2222-2229.