Malignant lesions of the biliary tract
Introduction
Malignant lesions of the biliary tract, specifically arising from the gallbladder or biliary epithelium, are rare and only account for approximately 15% of hepatobiliary neoplasms. Gallbladder cancer is the most common site, accounting for 60% of all biliary tract cancers, while the remaining 40% are distributed throughout the extrahepatic and intrahepatic biliary tree, with the next most common site occurring at the extrahepatic biliary confluence.1 Complete resection is associated with the best survival and is the most effective therapy, but is usually only possible in a minority of patients. Palliating the effects of biliary obstruction is thus often the primary therapeutic goal. Chemotherapy and radiation therapy have not been proved to reduce the incidence of recurrence after resection nor to improve survival. Unfortunately, due to the rarity of these tumours and their frequently advanced stage at presentation, randomised prospective trials assessing different treatment regimens have not been performed. This chapter focuses on the current management of cholangiocarcinoma, specifically hilar, intrahepatic and distal bile duct cancers, as well as gallbladder carcinoma.
Cholangiocarcinoma
Epidemiology
Cholangiocarcinoma is an uncommon cancer with an incidence of 1–2 per 100 000 in the USA and approximately 5000–8000 new cases diagnosed each year.2 Overall, men are affected 1.5 times as often as women and the majority of patients are greater than 65 years of age, with the peak incidence occurring in the eighth decade of life.2 Cholangiocarcinomas are classified according to their site of origin within the biliary tree, with those involving the biliary confluence, or hilar cholangiocarcinoma, being the most common and accounting for approximately 60% of all cases.3–6 Twenty to thirty per cent of cholangiocarcinomas originate in the distal bile duct, while approximately 10% arise within the intrahepatic biliary tree.7–9 Rarely, patients will present with multifocal or diffuse involvement of the biliary tree.10 More recent studies have documented the marked increase in incidence of intrahepatic cholangiocarcinoma, which may become the more common variant (see below).11–14
Natural history
The vast majority of patients with unresectable bile duct cancer die within 6 months to 1 year of diagnosis, usually from liver failure or infectious complications secondary to biliary obstruction.3,15–17 The prognosis is often worse for hilar lesions and better for lesions of the distal bile duct, which probably reflects the greater complexity and difficulty in effectively managing proximal lesions, more so than differences in tumour biology. Indeed, it has been shown that location within the biliary tree (proximal versus distal) has no impact on survival provided that complete resection is performed.4 That being said, patients with intrahepatic cholangiocarcinoma often present with advanced lesions due to the absence of symptoms, such as jaundice or biliary tract-related sepsis.
Aetiology
Most cases of cholangiocarcinoma in the West are sporadic and have no obvious risk factors. However, certain pathological conditions are associated with an increased incidence, the most common of which is primary sclerosing cholangitis (PSC). The majority of patients (70–80%) with PSC have associated ulcerative colitis, while a minority of patients with ulcerative colitis develop PSC.18 The natural history of PSC is variable, and the true incidence of cholangiocarcinoma is unknown. In a Swedish series of 305 patients followed over several years, 8% of patients eventually developed cancer. On the other hand, occult cholangiocarcinoma has been reported in up to 40% of autopsy specimens and in up to 36% of liver explants from patients with PSC.18,19 Patients with cholangiocarcinoma associated with PSC are often not candidates for resection because of multifocal disease or severe underlying hepatic dysfunction.
Congenital biliary cystic disease (i.e. choledochal cysts) is also associated with an increased risk for the development of biliary tract cancer.20,21 This appears to be related to the finding that these patients have an abnormal choledochopancreatic duct junction, which predisposes to reflux of pancreatic secretions into the biliary tree, chronic inflammation and bacterial contamination.21–24 A similar mechanism may also explain the increased incidence of cholangiocarcinoma reported in patients subjected to transduodenal sphincteroplasty or endoscopic sphincterotomy. In a series of 119 patients subjected to this procedure for benign conditions, Hakamada et al. found a 7.4% incidence of cholangiocarcinoma over a period of 18 years.25
Hepatolithiasis is a well-known risk factor for the development of cholangiocarcinoma in Japan and parts of southeast Asia, arising in 10% of those affected. Chronic portal bacteraemia and portal phlebitis lead to intrahepatic pigment stone formation, obstruction of intrahepatic ducts, and recurrent episodes of cholangitis and stricture formation.26,27 This recurrent inflammatory state is likely the main contributing factor to cholangiocarcinogenesis. Biliary parasites (Clonorchis sinensis, Opisthorchis viverrini) are also prevalent and endemic in parts of Asia, such as Thailand, and are similarly associated with an increased risk of cholangiocarcinoma.19 Finally, exposure to several radionuclides and chemical carcinogens, such as thorium, radon, nitrosamines, dioxin and asbestos, may also increase the risk of cholangiocarcinoma.
Histopathology
Three macroscopic subtypes of extrahepatic cholangiocarcinoma are described: sclerosing, nodular and papillary, of which the first two are often combined into one (i.e. nodular-sclerosing) since features of both types are often seen together.28 The histopathology is distinct between cholangiocarcinomas arising from the extrahepatic and intrahepatic biliary system. For extrahepatic cholangiocarcinoma, the overwhelming majority is adenocarcinoma, and most are firm, sclerotic tumours with a paucity of cellular components within a dense fibrotic, desmoplastic background. As a consequence, a non-diagnostic preoperative biopsy is not uncommon.2,28,29 Papillary tumours represent a less common morphological variant, accounting for approximately 10% of tumours arising from the extrahepatic biliary tree.28 Papillary tumours are soft and friable, may be associated with little transmural invasion, and are characterised by a mass that expands rather than contracts the duct (Fig. 12.1). Although papillary tumours may grow to significant size, they often arise from a well-defined stalk, with the bulk of the tumour mobile within the ductal lumen. Despite this histological variant being the minority of cases, recognition of this entity is important since they are more often resectable and have a more favourable prognosis than the other types.19,30
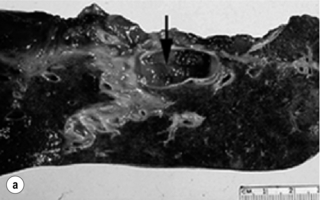
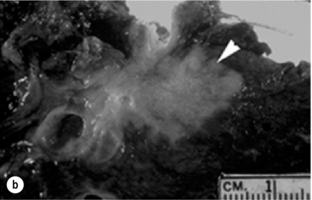
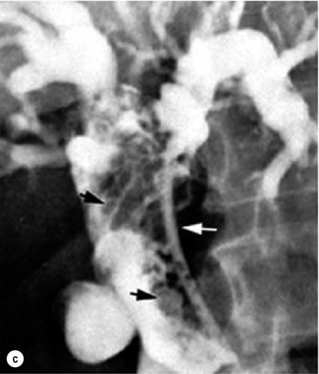
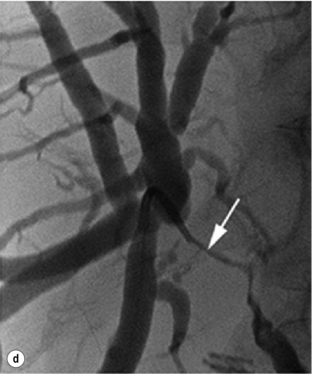
Figure 12.1 Gross and cholangiographic appearance of a papillary cholangiocarcinoma (a,c) and a nodular-sclerosing tumour (b,d). In (a) and (c), note that the papillary tumour occupies the lumen and expands the duct (black arrow). A biliary stent is visualised (white arrow). In (b) and (d), the nodular-sclerosing variant constricts the lumen, nearly obliterating it (white arrow). Reprinted with permission from Blumgart LH (ed.) Surgery of the liver, biliary tract, and pancreas, 4th edn. Elsevier Saunders, 2006.
Hilar cholangiocarcinoma is typically highly invasive within the hepatoduodenal ligament. Direct invasion of the liver or perihepatic structures, such as the portal vein or hepatic artery, is a common feature and has important clinical implications regarding resectability.28 The liver is also a common site of metastatic disease, as are the regional lymph node basins, but spread to distant extra-abdominal sites is uncommon at initial presentation.3,31 These tumours also have a propensity for longitudinal spread along the duct wall and periductal tissues, which is an important pathological feature of cholangiocarcinomas as it pertains to the margin of resection.28 There may be substantial extension of tumour beneath an intact epithelial lining, as much as 2 cm proximally and 1 cm distally, thus predisposing to a radiographic underestimation of tumour extent.32 This predilection for submucosal extension underscores the difficulty in achieving a complete resection. Frozen section analysis of the duct margin during operation may be helpful in this regard but caution is necessary when interpreting the results. Indeed, the authors recently analysed their experience with intraoperative frozen sections and found a substantial false-negative rate. In addition, the benefits of extending the resection with a positive frozen section result were questionable.33
Gross examination of intrahepatic cholangiocarcinoma reveals a grey scirrhous mass, often infiltrative into the liver parenchyma.34 These tumours are adenocarcinomas and the diagnosis of intrahepatic or peripheral cholangiocarcinoma should be considered in all patients presenting with a presumptive diagnosis of metastatic adenocarcinoma with an unknown primary, particularly in the setting of a large, solitary hepatic mass. A small number show different patterns with focal areas of papillary carcinoma with mucous production, signet-ring cells, squamous cell, mucoepidermoid and spindle cell variants.35 The Liver Cancer Study group of Japan established a subclassification of these tumours based on morphology: (1) mass forming type; (2) periductal infiltrating type; (3) intraductal growth type.36 Although some studies have suggested a correlation with outcome based on morphological subtype, this classification scheme has not gained wide acceptance. Positive immunohistochemical staining usually includes carcinoembryonic antigen (CEA), and tumour markers CA50 and CA19-9. K-ras mutations have also been detected in up to 70% of intrahepatic cholangiocarcinomas, although the frequency of this mutation is quite variable.37,38 Metastatic disease at the time of exploration is not an infrequent finding. Tumours with both hepatocellular and cholangiocellular differentiation (combined tumours) are rare but well described. Their clinical behaviour more closely approximates that of cholangiocarcinoma than hepatocellular carcinoma, and they tend to display aggressive biology.39
Cholangiocarcinoma involving the proximal bile ducts (hilar cholangiocarcinoma)
Clinical presentation and diagnosis
The early symptoms of hilar cholangiocarcinoma are often non-specific, with abdominal pain, discomfort, anorexia, weight loss and/or pruritus seen in about one-third of patients.6,19,40,41 Most patients present with jaundice or incidentally discovered abnormal liver function tests. Pruritus may precede jaundice by some weeks, and this symptom should prompt an evaluation, especially if associated with abnormal liver function tests. Patients with papillary tumours of the hilus may give a history of intermittent jaundice, perhaps due to the ball-valve effect of a pedunculated mass within the lumen or, more likely, small fragments of tumour having passed into the common bile duct. Clinical findings are often non-specific but may provide some useful information. Jaundice is usually obvious, and patients with pruritus often have multiple excoriations of the skin. The liver may be enlarged and firm as a result of biliary tract obstruction. The gallbladder is usually decompressed and non-palpable with hilar obstruction. Thus, a palpable gallbladder suggests a more distal obstruction or an alternative diagnosis. Rarely, patients with long-standing biliary obstruction and/or portal vein involvement may have findings consistent with portal hypertension.
In patients with no previous biliary intervention, cholangitis is rare at initial presentation, despite a 30% incidence of bacterial contamination.42,43 Endoscopic or percutaneous instrumentation significantly increases the incidence of bacterial contamination and the subsequent risk of clinical infection. In fact, the incidence of bacterobilia approaches 100% after endoscopic biliary intubation, thus making cholangitis more common.43 It should be noted that bacterial contamination of the biliary tract in partial obstruction is not always clinically apparent. The presence of overt or subclinical infection at the time of surgery is a major source of postoperative morbidity and mortality. Thus, endoscopic and percutaneous intubations are both associated with greater morbidity and mortality following surgical resection or palliative bypass for hilar cholangiocarcinoma. In an analysis of 71 patients who underwent either resection or palliative biliary bypass for proximal cholangiocarcinoma, all patients stented endoscopically and 62% of those stented percutaneously had bacterobilia. Postoperative infectious complications were doubly increased in patients stented before operation compared to non-stented patients, while non-infectious complications were equal in both groups.43 Enterococcus, Klebsiella, Escherichia coli, Streptococcus viridans and Enterobacter aerogenes are the most common organisms, and this spectrum of bacteria must be considered when administering perioperative antibiotics; it is imperative to take intraoperative bile specimens for culture in order to guide selection of postoperative antibiotic therapy.
While gallstones or even common bile duct stones may coexist with bile duct cancer, in the absence of certain predisposing conditions (e.g. PSC, recurrent pyogenic cholangitis (previously referred to as Oriental cholangiohepatitis)), it is uncommon for choledocholithiasis to cause obstruction at the biliary confluence. Furthermore, the degree of bilirubin elevation tends to be higher (e.g. 10–18 mg/dL) for malignant obstruction compared to benign stone disease (e.g. 2–10 mg/dL). That being said, other conditions may mimic hilar cholangiocarcinoma on imaging studies, such as benign idiopathic focal stenosis of the hepatic ducts (malignant masquerade), Mirizzi’s syndrome resulting from a large stone impacted in the neck of the gallbladder, and gallbladder cancer.44 Nevertheless, it is imperative to fully investigate and delineate the level and nature of any obstructing lesion causing jaundice to avoid missing the diagnosis of carcinoma.
However, the histopathological diagnosis of hilar cholangiocarcinoma is often not made until the specimen is removed at operation. As mentioned previously, due to the dense desmoplastic reaction associated with the sclerosing variant of cholangiocarcinoma, non-diagnostic preoperative biopsies or brushings are the usual clinical scenario. In the authors’ view, histological confirmation of malignancy is not mandatory prior to exploration. With no prior suggestive history (i.e. prior biliary tract operation, PSC, hepatolithiasis), the finding of a focal stenotic lesion combined with the appropriate clinical presentation is sufficient for a presumptive diagnosis of hilar cholangiocarcinoma, which is correct in most instances.45 Furthermore, the alternative conditions that one may encounter are often best assessed and treated at operation. It is dangerous to rely entirely on a negative result from a needle biopsy or biliary brush cytology, since they are often misleading, particularly in the face of compelling radiographic evidence of malignant disease.46 The use of spy glass technology via endoscopic guidance has facilitated direct visualisation of the bile duct lumen and allows for targeted biopsies of the affected area.
Radiological investigation
Direct cholangiography: Cholangiography demonstrates the location of the tumour and the extent of biliary disease, both of which are critical in surgical planning. Although endoscopic retrograde cholangiography (ERC) may provide helpful information, percutaneous transhepatic cholangiography (PTC) displays the intrahepatic bile ducts more reliably and has been the preferred approach. However, there is often a knee-jerk reflex to proceed with invasive cholangiography before a complete radiographic assessment has been made, which can lead to unnecessary patient morbidity and infectious complications.
Computed tomography: Cross-sectional imaging provided by CT remains an important study for evaluating patients with biliary obstruction and can provide valuable information regarding the level of obstruction, vascular involvement and liver atrophy. As portal venous inflow and bile flow are important in the maintenance of liver cell size and mass, segmental or lobar atrophy may be evident on CT that would suggest portal venous occlusion or biliary obstruction.47 CT angiography is particularly helpful for assessing portal venous and hepatic arterial involvement. However, CT imaging tends to underestimate the proximal extent of tumour within the bile duct and is thus not ideal as the primary determinant of resectability.48
Duplex ultrasonography: Ultrasonography is a non-invasive, but operator dependent, study that often precisely delineates the level of the tumour within the bile duct (Fig. 12.2). It can also provide information regarding tumour extension within the bile duct and in the periductal tissues.49–51 In a series of 19 consecutive patients with malignant hilar obstruction, ultrasonography with colour spectral Doppler technique was equivalent to angiography and CT portography in diagnosing lobar atrophy, level of biliary obstruction, hepatic parenchymal involvement and venous invasion.51 Duplex ultrasonography is particularly useful for assessing portal venous invasion. In a series of 63 consecutive patients from Memorial Sloan-Kettering Cancer Center (MSKCC), duplex ultrasonography predicted portal vein involvement in 93% of cases with a specificity of 99% and a 97% positive predictive value. In the same series, angiography with CT angio-portography had a 90% sensitivity, 99% specificity and a 95% positive predictive value.52
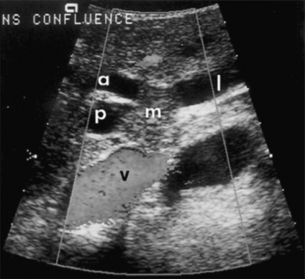
Figure 12.2 Ultrasonographic view of a hilar cholangiocarcinoma showing a papillary tumour (m) extending into the right anterior (a) and posterior (p) sectoral ducts and the origin of the left duct (l). The adjacent portal vein (v) is not involved and has normal flow. Reprinted with permission from Blumgart LH (ed.) Surgery of the liver, biliary tract, and pancreas, 4th edn. Elsevier Saunders, 2006.
Magnetic resonance cholangiopancreatography (MRCP): In the authors’ practice, MRCP has largely replaced endoscopic and percutaneous cholangiography to assess biliary tumour extent in hilar cholangiocarcinoma. Several studies have demonstrated its utility in evaluating patients with biliary obstruction.53–56 MRCP may not only identify the tumour and the level of biliary obstruction, but may also reveal obstructed and isolated ducts not appreciated at endoscopic or percutaneous study. By virtue of being an axial imaging modality, MRCP has further advantages over standard cholangiography by also providing information regarding the patency of hilar vascular structures, the presence of nodal or distant metastases, and the presence of lobar atrophy (Fig. 12.3). Furthermore, because it does not require biliary intubation, it is not associated with the same incidence of bacterobilia and infectious complications that is frequently associated with standard cholangiography.43
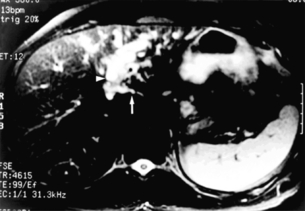
Figure 12.3 Cross-sectional MRCP from a patient with hilar cholangiocarcinoma extending into the left hepatic duct and left lobe atrophy. The bile ducts appear white. The left lobe is small with dilated and crowded ducts (arrowhead). The principal caudate lobe duct, seen joining the left hepatic duct, is also dilated (arrow). Reprinted with permission from Blumgart LH (ed.) Surgery of the liver, biliary tract, and pancreas, 4th edn. Elsevier Saunders, 2006.
Preoperative evaluation and assessment of resectability
The preoperative evaluation must address four critical determinants of resectability: extent of tumour within the biliary tree, vascular invasion, hepatic lobar atrophy and the presence of metastatic disease.3 The presence of lobar atrophy is often overlooked; however, its importance in determining resectability cannot be overemphasised, since it implies portal venous involvement, suggests a more locally advanced lesion, and compels the surgeon to perform a partial hepatectomy, if the tumour is indeed resectable.47 While long-standing biliary obstruction may cause moderate atrophy, concomitant portal venous compromise results in rapid and severe atrophy of the involved segments.
Appreciation of gross atrophy on preoperative imaging is important since it often influences both operative and non-operative therapy.47 If the tumour is not resectable, percutaneous biliary drainage through an atrophic lobe, unless necessary to control sepsis, should be avoided since it will not effect a reduction in bilirubin level. Atrophy is apparent on cross-sectional imaging as a small, often hypoperfused lobe with crowding of the dilated intrahepatic ducts (Fig. 12.3). Tumour involvement of the portal vein is usually present if there is compression/narrowing, encasement or occlusion seen on imaging studies.3,57
The staging systems currently used for hilar cholangiocarcinoma do not account fully for all of the tumour-related variables that influence resectability, namely biliary tumour extent, lobar atrophy and vascular involvement. The modified Bismuth–Corlette classification stratifies patients solely based on the extent of biliary duct involvement by tumour.58 Although useful to some extent, it is not indicative of resectability or survival. Similarly, the previous AJCC T-stage system (sixth edition) was based largely on pathological criteria and had little applicability for preoperative staging. The ideal staging system should accurately predict resectability and the likelihood of associated metastatic disease, and also correlate with survival. The authors have proposed such a preoperative staging system.3,57 This staging system places the finding of portal venous involvement and lobar atrophy into the proper context for determining resectability, especially when partial hepatectomy is an important component of the operative approach (Table 12.1). For example, a tumour with unilateral extension into second-order bile ducts that is associated with ipsilateral portal vein involvement and/or lobar atrophy would still be considered potentially resectable, while such involvement on the contralateral side would preclude a resection. The authors have found that this staging system correlated well with resectability, the likelihood of associated distant metastatic disease, and median survival (Table 12.2).57 Independent confirmation of the utility of this classification scheme (the Blumgart clinical staging system) was recently reported in a series of 85 patients from China.59 The authors’ criteria for unresectability are detailed in Box 12.1. This staging scheme is now incorporated in the seventh edition of the AJCC staging system for hilar cholangiocarcinoma.
Table 12.1
Proposed T-stage criteria for hilar cholangiocarcinoma
| Stage | Criteria |
| T1 | Tumour involving biliary confluence ± unilateral extension to second-order biliary radicles |
| T2 | Tumour involving biliary confluence ± unilateral extension to second-order biliary radicles |
| AND ipsilateral portal vein involvement ± ipsilateral hepatic lobar atrophy | |
| T3 | Tumour involving biliary confluence + bilateral extension to second-order biliary radicles |
| OR unilateral extension to second-order biliary radicles with contralateral portal vein involvement | |
| OR unilateral extension to second-order biliary radicles with contralateral hepatic lobar atrophy | |
| OR main or bilateral portal venous involvement |
Reprinted with permission from Jarnagin WR, Fong Y, DeMatteo RP et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg 2001; 234:507–19.
Table 12.2
Resectability, incidence of metastatic disease, and survival stratified by T stage
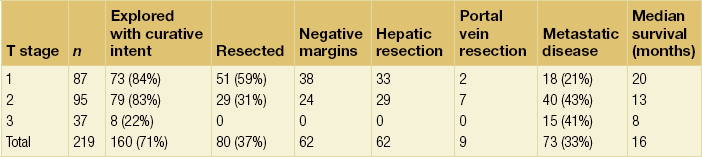
Reprinted with permission from Jarnagin WR, Fong Y, DeMatteo RP et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg 2001; 234:507–19.
Treatment options
Orthotopic liver transplantation has been attempted for unresectable hilar tumours. Klempnauer et al. reported four long-term survivors out of 32 patients who underwent transplantation for hilar cholangiocarcinoma.60 The same group also reported a 17.1% 5-year survival for their overall transplant group.61 Comparable results were reported by Iwatsuki et al.62 The results of transplantation have previously not been sufficiently adequate to justify its use, and most centres now do not perform liver transplantation for cholangiocarcinoma. More recently, data from the Mayo Clinic have emerged suggesting good results with transplantation in highly selected patients with low-volume unresectable disease and combined with an intensive pre-transplant treatment regimen.63,64 Although the data are compelling, routine use of vascular resection, even when there is no obvious tumour infiltration, will likely lead to higher perioperative morbidity; this approach would therefore seem applicable to a very small proportion of patients.
Resection
Resection is the most effective therapy for patients with potentially resectable tumours, with the primary objective being complete removal of all gross disease with clear histological margins (R0 resection). The importance of an R0 resection is clear from previous studies showing that incomplete (R1 or R2) resections do not improve survival beyond that of patients with unresectable tumours (Fig. 12.4).3,57 There is now overwhelming evidence to support the observation that partial hepatectomy, combined with excision of the extrahepatic biliary system, is usually required to achieve this goal (Table 12.3). A review of several series in the literature shows a close correlation between the proportion of patients who underwent concomitant partial hepatectomy and the proportion of R0 resections achieved. For tumours extending into the left hepatic duct, en bloc caudate lobectomy is usually necessary to obtain a complete resection, since the principal biliary drainage of the caudate lobe is via the left hepatic duct.65,66 A dilated caudate duct, suggesting tumour involvement, may occasionally be visualised on preoperative imaging (Fig. 12.3).
Table 12.3
Summary of selected studies showing the relationship between the rate of partial hepatectomy and proportion of negative histological margins achieved
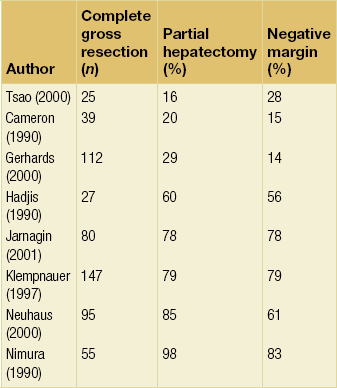
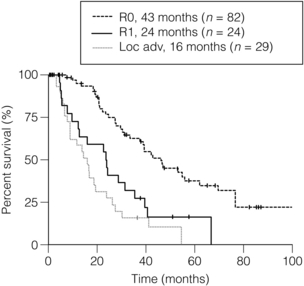
Figure 12.4 Survival curves after resection of hilar cholangiocarcinoma. R0 indicates complete resection with histologically negative resection margins (median survival 43 months). R1 indicates histologically involved resection margins (median survival 24 months; P < 0.001, R0 vs. R1). Loc Adv indicates patients explored, but found to have unresectable tumours owing to local invasion (no metastatic disease) (median survival 16 months; P < 0.19, R1 vs. Loc Adv). Reprinted with permission from Blumgart LH (ed.) Surgery of the liver, biliary tract, and pancreas, 4th edn. Elsevier Saunders, 2006.
Despite improvements in preoperative imaging, a considerable number of patients are still found to have unresectable disease at the time of exploration. In a recent report from MSKCC, this number approached 50% of patients with cholangiocarcinoma explored with curative intent.30 In an effort to minimise the number of non-curative laparotomies performed, staging laparoscopy has been utilised. Two recent studies specifically analysing patients with biliary cancer have shown that laparoscopy can identify a large proportion of patients with unresectable disease primarily in the form of radiographically occult metastases, the yield of which is greatest in locally advanced tumours.67,68 Weber et al. evaluated 56 patients with potentially resectable hilar cholangiocarcinomas; 33 were ultimately determined to have unresectable disease, of which 14 (42%) were identified at laparoscopy and spared an unnecessary laparotomy. Additionally, a number of recent reports have suggested a potential role for fluoro-2-D-glucose positron emission tomography (FDG-PET) scanning as a means of identifying occult metastatic disease. However, most of these studies include small numbers of patients, and further evaluation is needed before PET can be recommended as a routine screening study for this disease.69–71 In the authors’ experience with FDG-PET for all biliary tract cancer, the information provided influenced management in 24% of patients.72
Technical aspects of intraoperative tumour assessment, exposure and resection are outside the scope of this chapter. The reader is referred to specialty texts for a detailed description of surgical techniques.73 The authors’ general approach involves the use of staging laparoscopy, followed by a full exploration of the abdomen and pelvis, including intraoperative ultrasonography. Resection of the tumour involves, at a minimum, removal of the entire extrahepatic biliary tract from just above the pancreas distally to beyond the biliary confluence with a complete porta hepatis lymphadenectomy. Also, for the reasons cited above, en bloc partial hepatectomy is required in nearly every case in order to achieve complete tumour clearance. Tumour involvement of the main portal vein proximal to its bifurcation additionally requires a vascular resection and reconstruction if technically feasible. Some authors advocate a ‘no-touch’ technique where a hilar en bloc resection is performed that entails resection of the portal vein bifurcation with reconstruction.74 The authors report a 5-year survival advantage of 58% versus 29% (P = 0.02) associated with this approach compared to a conventional hepatectomy. This is clearly an aggressive surgical approach that is likely best applied to a select population.
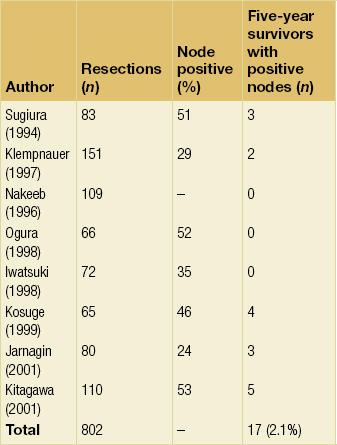
The extent of lymphadenectomy that should be performed remains an area of controversy. Some surgeons advocate an extended nodal dissection as some studies have demonstrated measurable 5-year actuarial survival in the presence of metastatic disease to distant nodal basins (e.g. para-aortic).75,76 However, an analysis of studies specifically reporting 5-year survival in patients would suggest that any nodal involvement is a powerful adverse factor and that very few patients benefit from such an aggressive approach (Table 12.4). Thus, while a complete porta hepatis lymphadenectomy should be routinely performed when attempting complete resection, the authors do not advocate an extended lymph node dissection. As is the case in other tumours, the clinical implication of a negative lymph node on histopathological analysis is likely dependent on the total number of lymph nodes sampled. A study from MSKCC reported that seven lymph nodes appears to be the target sampling number in order to accurately stage hilar cholangiocarcinoma.77 This must be weighed against the reality that, in most series, the median number of nodes sampled from a porta hepatis lymphadenectomy is usually around three.
Results of resection
Long-term survival after resection of hilar cholangiocarcinoma can be achieved and has improved over recent years.3,4,6,65,78,79 It is clear, however, that the results of resection depend critically on the status of the resection margins. The authors firmly believe that an increase in the use of hepatic resection is responsible for the increase in the percentage of R0 resections (negative histological margins) and the observed improvement in survival after resection. This point is emphasised by a reported series of 269 patients accumulated over a 20-year period demonstrating a progressive increase in the proportion of patients subjected to partial hepatectomy, with a corresponding increase in the incidence of negative histological margins and in survival.80 A more recent study from MSKCC reported results of resection in 106 consecutive patients and showed a median survival of 43 months in patients who underwent an R0 resection compared to 24 months in those with involved resection margins.30 Multivariate analysis showed that an R0 resection, a concomitant hepatic resection, well-differentiated histology and papillary tumour phenotype were independent predictors of long-term survival.
Adjuvant therapy
The rarity of cholangiocarcinoma has prevented any meaningful clinical trials evaluating the use of adjuvant therapy. Several small, single-centre studies have attempted to investigate the benefit of postoperative adjuvant chemoradiation therapy in patients with hilar cholangiocarcinoma. Cameron et al. and Pitt et al. from Johns Hopkins, in two separate reports, provided data suggesting no benefit of adjuvant external beam or intraluminal radiation therapy.81,82 In contrast, Kamada et al. suggested that radiation may improve survival in patients with histologically positive hepatic duct margins.83 Additionally, in a small series of patients, five with hilar cholangiocarcinoma, resectability was reportedly greater in patients given neoadjuvant radiation therapy prior to exploration.84 It must be noted, however, that none of these studies were randomised and most consisted of a small, heterogeneous group of patients. At the present time, there are no data to support the routine use of adjuvant or neoadjuvant radiation therapy, except in the context of a controlled trial.
The only phase III trial investigating adjuvant chemotherapy, which used mitomycin/5-fluorouracil (5-FU), included 508 patients with resected bile duct tumours (n = 139), gallbladder cancers (n = 140), pancreatic cancers (n = 173) and ampullary tumours (n = 56).85 On subset analysis, there were no significant differences in overall or disease-free survival for bile duct tumours. As with radiation therapy, there are no data to support the routine use of chemotherapy in the adjuvant setting, until newer agents, such as oxaliplatin, are tested in a randomised controlled fashion.
Palliation
All patients should be properly assessed for possible resection; however, unfortunately the majority of patients with hilar cholangiocarcinoma are not candidates for resection. In this setting, the management goals include biliary decompression and/or supportive care. Jaundice alone is not necessarily an indication for biliary decompression, given the associated morbidity and mortality. The indications for biliary decompression include intractable pruritus, recurrent cholangitis, the need for access for intraluminal radiotherapy and finally to allow recovery of hepatic parenchymal function in patients receiving chemotherapeutic agents. In fact, supportive care alone is probably the best approach for elderly patients with significant comorbid conditions, provided that pruritus is not a major feature. In patients who are found to be unresectable at operation, an operative biliary decompression can be performed and can be so constructed as to provide access to the biliary tree for postoperative irradiation.3,86
If the patient is deemed unresectable, the diagnosis should be confirmed with a biopsy. Biliary decompression can be obtained either by a percutaneous transhepatic route or by endoscopic stent placement, although hilar tumours are more difficult to transverse endoscopically. Moreover, the failure rates and incidence of subsequent cholangitis associated with endoscopic decompression are high.87 Thus, most are probably better palliated via a percutaneous approach.
Percutaneous biliary drainage: Although more difficult than in those with distal bile duct tumours, percutaneous transhepatic biliary drainage and subsequent placement of a self-expandable metallic endoprosthesis (Wallstent) can be successfully performed in most patients with hilar obstruction.88–90 Frequently, hilar tumours involve all three major hilar ducts (left hepatic, right anterior sectoral hepatic and right posterior sectoral hepatic), and thus may require two or more stents for adequate drainage.91 Jaundice secondary to portal vein occlusion without intrahepatic biliary dilatation, however, is not correctable with biliary stents. In addition, the presence of lobar atrophy is an important factor and biliary decompression of an atrophic lobe does not usually provide much palliative benefit.
The median patency of metallic endoprostheses placed at the hilus is approximately 6 months, which is significantly lower than that reported for similar stents placed in the distal bile duct.92 Becker et al. reported 1-year patency rates of 46% and 89% for Wallstents placed at the hilus and the distal bile duct, respectively.88 Due to this higher occlusion rate, 25% of patients will require re-intervention. This concurs with our findings of a mean patency of 6.1 months in 35 patients palliated for malignant high biliary obstruction by placement of expandable metallic endoprostheses. The periprocedural mortality was 14% at 30 days, and seven patients (24%) had documented stent occlusion requiring repeated intervention.92
Intrahepatic biliary-enteric bypass: Patients found to be unresectable at operation, particularly after the bile duct has been divided, may be candidates for intrahepatic biliary-enteric bypass. The segment III duct is usually the most accessible and is our preferred approach, but the right anterior or posterior sectoral hepatic ducts can also be used.93 Segment III bypass provides excellent biliary drainage and is less prone to occlusion since the anastomosis can be placed remote from the tumour. The 1-year bypass patency can approach 80% without any perioperative deaths.93 Decompression of only one-third of the functioning hepatic parenchyma is usually sufficient to relieve jaundice. Furthermore, provided that the undrained lobe has not been percutaneously drained or otherwise contaminated, communication between the right and left hepatic ducts is not necessary.94 As discussed for stenting, bypass to an atrophic lobe or a lobe heavily involved with tumour is generally not effective.
Radiation therapy: Patients with locally unresectable tumours without evidence of widespread disease may be candidates for palliative radiation therapy. Typically, external beam radiation (EBRT) alone is used, although a combination of EBRT (5000–6000 cGy) and intraluminal iridium-192 (2000 cGy) delivered percutaneously can be administered safely and may be more effective. However, despite its feasibility, improved survival compared to biliary decompression alone has not been documented in a controlled study.81,86,95,96 In a group of 12 patients treated with this regimen over a 3-year period at MSKCC, the median survival was 14.5 months. Episodes of cholangitis and intermittent jaundice were relatively common but the incidence of serious complications was low and there were no treatment-related deaths.86 Given the increased morbidity and minimal benefit associated with radiation therapy, it is clearly not indicated for most patients with unresectable hilar cholangiocarcinoma.
Photodynamic therapy: Ortner, as well as others, has evaluated the efficacy of photodynamic therapy in unresectable hilar cholangiocarcinoma and reported a median survival of 439 days.97,98 The technique involves first injecting a photosensitiser into the biliary tract, then direct illumination via cholangioscopy activates the compound, causing tumour cell death. Ortner treated nine patients in this fashion who had failed endoscopic stenting. No mortality was reported for the procedure; however, there was a 25% mortality related to the initial endoscopic stenting, which must be considered. The indication for biliary drainage or specific reasons for tumour unresectability were not mentioned, despite this information being important to assess the true extent of disease, thus making it difficult to interpret the extended survival with this palliative therapy. Since this study, two small randomised studies have reported an improvement in survival for patients with unresectable tumours treated with stenting and photodynamic therapy compared to stenting alone; however, the control groups were not comparable, since the biliary drainage procedures were suboptimal, which likely accounts for the differences in outcome.99,100
Chemotherapy: In cases of advanced biliary tract cancers where curative surgical resection is not an option, palliative chemotherapy has been used to potentially improve quality of life, reduce symptoms and increase survival. Only one randomised study has addressed such a role for chemotherapy, where 37 patients with advanced biliary tract cancers were randomised to receive chemotherapy (5-FU/leucovorin with or without etoposide) or best supportive care.101 Short-term improvements in survival (6.5 vs. 2.5 months) were noted among the chemotherapy group. In addition, the treatment group also demonstrated improvement in quality of life as measured by the EORTC QLQ-C30 instrument.
Many agents (5-FU, gemcitabine, capecitabine, cisplatin, oxaliplatin, interferon) alone or in combination continue to be evaluated in phase I and II trials. Partial disease responses are consistently in the range of 10–30%. Since no consensus had been reached regarding the standard use of chemotherapy in cases of advanced biliary tract cancer, gemcitabine as a single agent had emerged as the treatment regimen of choice given its more favourable profile in both toxicity and disease response.102 However, recently the ABC-02 Trial Investigators reported the superior survival of patients with advanced biliary cancers when treated with a doublet regimen of gemcitabine with cisplatin compared to gemcitabine alone (11.7 vs. 8.1 months; P < 0.001).103 The use of gemcitabine with a platinum agent, barring any contraindications, has now become the treatment regimen of choice for patients with advanced disease. This finding now raises the question of whether appropriately selected patients might benefit from this regimen in the adjuvant setting as well.
Cholangiocarcinoma involving the distal bile duct
Tumours of the lower bile duct, namely mid- and distal bile duct, are classified according to their anatomical location, although there may be considerable overlap. Mid-bile duct tumours arise between the upper border of the duodenum and the cystic duct, while distal bile duct tumours are those arising anywhere from the duodenum to the papilla of Vater.5 Tumours of the distal bile duct may represent approximately 20–30% of all cholangiocarcinomas and 5–10% of all periampullary tumours.6,104–106 True mid-duct tumours are distinctly uncommon, and thus Nakeeb et al. have proposed an alternative classification scheme that divides cholangiocarcinomas into intrahepatic, perihilar and distal subgroups, thereby eliminating the mid-duct group, which is often difficult to classify accurately.6 As is true throughout the biliary tree, adenocarcinoma is the principal histological type in the lower bile duct, and it has previously been suggested that the papillary variant is more common at this location compared to the biliary confluence.5
Clinical presentation and diagnosis
The clinical presentation of distal bile duct cancer is generally indistinguishable from that of hilar cholangiocarcinoma or other periampullary malignancies. Progressive jaundice is seen in 75–90% of patients, with serum bilirubin levels often exceeding 10 mg/dL.107 Abdominal pain, weight loss, fever or pruritus occurs in one-third or fewer.6,104 Distal bile duct tumours are frequently mistaken for adenocarcinoma of the pancreas, the most common periampullary malignancy. Endoscopic retrograde cholangiopancreatography (ERCP) can provide valuable information regarding the level of obstruction, may show that the obstruction is arising from the bile duct without involvement of the pancreatic duct, and can be both diagnostic and therapeutic in cases of choledocholithiasis. Percutaneous transhepatic cholangiography is generally less useful for tumours of the distal bile duct. A good-quality cross-sectional imaging study is also required, usually a CT with angiography to assess for vascular involvement and/or metastatic disease. It is not uncommon that CT does not reveal a mass given the frequent small tumour size at presentation. Increasingly, magnetic resonance cholangiopancreatography (MRCP) is being used to evaluate periampullary tumours. As is true for hilar lesions, MRCP can provide information of the distal bile duct previously obtainable only with the combination of ERCP and CT.108
In patients with a stricture of the distal bile duct and a clinical presentation consistent with cholangiocarcinoma (or any other periampullary malignancy), histological confirmation of malignancy is generally unnecessary, unless non-operative therapy is planned. Benign strictures do occur in the lower bile duct, but these are difficult to differentiate definitively from malignant strictures without resection. In addition, endoscopic brushings of the bile duct have an unacceptably low sensitivity, making a negative result virtually useless.109 Excessive reliance on the results of percutaneous or brush biopsies serves only to delay therapy.
Treatment options
Complete resection is the only effective therapy for cancers of the distal bile duct.4–6,104–106 Reported 5-year survival rates range from 14% to 40% after complete resection, and in most studies survival beyond 1 year was uncommon in patients with tumours not amenable to resection.5,38,104,105 Nearly all distal bile duct cancers require a pancreaticoduodenectomy for complete excision. In a series from MSKCC, 13% of patients (6 of 45) underwent bile duct excision alone, while in the Veterans Hospital study this figure was only 9% (3 of 34).104,105 In addition to resection margin status (i.e. an R0 resection), metastatic disease to regional lymph nodes is a critical determinant of outcome. Fong et al. found that lymph node status was the only independent predictor of long-term survival after complete resection, with positive nodes conferring a 6.7 times greater likelihood of recurrence and death.104 Ito et al. reported that 11 lymph nodes needed to be evaluated to accurately assess lymph node involvement for distal cholangiocarcinoma.77
Survival after resection of distal bile duct tumours is comparable to, and maybe better than, that for pancreatic cancer.6,104,105 Furthermore, it has been erroneously assumed that survival is greater than that after resection of hilar cholangiocarcinomas as well.5 However, if adjusted for stage and completeness of resection, the survival rates between the two are similar.4 Adjuvant therapy after resection (chemotherapy and radiation therapy) has not been proved to improve survival, although this issue has not been evaluated in a prospective fashion.6
Surgical bypass (hepaticojejunostomy or choledochojejunostomy) or biliary endoprostheses can be used for palliation of symptomatic biliary obstruction. Endoprostheses for distal biliary obstruction are easier to place and have a greater long-term patency than those placed for hilar obstruction.88 Surgical bypass provides excellent relief of jaundice, but is typically used when unresectability is found at laparotomy. The authors generally use biliary endoprostheses in patients with clear-cut unresectable disease, discovered preoperatively or at staging laparoscopy, and in those unfit for operation. Laparoscopic biliary enteric bypass is also an option, but the expertise needed to perform this procedure is not widely available.
Cholangiocarcinoma involving the intrahepatic bile ducts
Intrahepatic cholangiocarcinoma (IHC), also referred to as peripheral cholangiocarcinoma, originates from the intrahepatic biliary radicles. IHC is rare in Western countries, accounts only for approximately 10% of all cholangiocarcinomas and is less frequently associated with underlying liver parenchymal disease than hepatocellular carcinoma, although an association appears to exist. Recently, a marked increase in the incidence and age-adjusted mortality has been identified, the reasons for which are unclear but may be related to the rising incidence of obesity-related, non-alcoholic fatty liver disease or chronic hepatitis C infection.12,13 The presenting symptoms are subtle and often only include pain either directly or indirectly related to a large lesion. Malaise, weight loss and fever are uncommon, but jaundice and pruritus may be seen in up to one-third of cases, which is generally indicative of compression or invasion of the biliary confluence. Small lesions often present as incidental findings on imaging studies undertaken for unrelated symptoms.
Radiological investigations
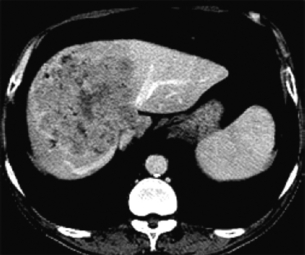
The radiological features of IHC on cross-sectional imaging are well described, and when combined with histological findings from a needle biopsy, can be virtually diagnostic. On MRI, these tumours are generally hypointense on T1-weighted images and heterogeneously hyperintense on T2-weighted images. These lesions demonstrate initial rim enhancement characterised by progressive and concentric enhancement post-administration of contrast material. Generally the lesions do not completely enhance post-contrast. In the absence of a separate primary source of disease, a lesion in the liver with this morphology on MRI evaluation can be considered virtually diagnostic of cholangiocarcinoma without a tissue diagnosis. On contrast-enhanced CT, variable rim-like enhancement is also seen, predominantly on the arterial phase images with gradual centripetal enhancement on delayed imaging (Fig. 12.5). Intrahepatic cholangiocarcinomas may only enhance completely on delayed imaging obtained hours after contrast administration, a finding related to the desmoplastic nature of the tumour. Capsular retraction may also be seen.110,111
Staging and assessment of resectability
Currently, there is no useful clinical staging system for intrahepatic cholangiocarcinoma. The AJCC TNM classification for primary liver cancers is applied both to hepatocellular carcinoma and IHC, but is of little clinical value. Because IHCs tend to be relatively silent lesions, they are often large at presentation. Thirty per cent of patients will have peritoneal or hepatic metastases at presentation and many of these will not be detected until staging laparoscopy or exploratory laparotomy is performed. In a review of 53 peripheral cholangiocarcinomas treated at MSKCC over an 8-year period, the median tumour diameter was 7.1 cm at presentation.112 Twenty patients were found to be unresectable at exploration for a 62% overall resectability rate. Operative findings precluding resection were intrahepatic metastases (35%), peritoneal metastases (30%), coeliac lymph node metastases (25%) and portal vein involvement (10%). Staging laparoscopy was conducted in 22 patients, of whom six were spared laparotomy secondary to findings of peritoneal and intrahepatic metastases. In a more recent review at the authors’ institution, a total of 270 IHC patients were seen over a 16-year period, representing an average annual increase of 14% in patients with this diagnosis over the study period. Of the patients treated at MSKCC, 54% had unresectable disease at presentation; ultimately, 34% of the entire cohort underwent a potentially curative resection (70% of those explored with curative intent).14
Treatment options
Hepatic resection with negative histological margins remains the only potentially curative treatment for this disease. Unfortunately, only about one-third to one-half of patients have potentially resectable lesions at the time of presentation. Additionally, a significant proportion of these patients will have findings at operation that preclude resection.14,112 Median survival after resection was approximately 36 months in a recent study by Endo et al., compared to 9 months for patients with advanced disease.14 Unfortunately, even after a complete resection, recurrence was common and was predicted by tumour size > 5 cm, the presence of multiple liver tumours or metastatic disease to regional lymph nodes; the liver was the single most common site of recurrence.
An international study group for IHC has recently advocated routine portal lymph node dissection at the time of resection, as approximately 30% of patients who underwent evaluation were found to have lymph node involvement.113 Although this does not seem to provide any therapeutic benefit, it may allow for better prognostication and patient selection for adjuvant therapy. The idea of adjuvant therapy needs to be revisited, given the promising results from the ABC-02 trial that reported improved survival with gemcitabine plus cisplatin.103 Appropriate patient selection will likely play a paramount role in the application of these data and practice to the adjuvant setting. Given the low yield of lymph nodes from a portal lymphadenectomy (median 3), the accuracy of lymph node evaluation is questionable, and thus its use as a selection criteria for adjuvant therapy is controversial. Perhaps pathological criteria from evaluation of the primary tumour, such as lymphovascular invasion and perineural invasion, should be used instead as selection criteria for adjuvant therapy, as the presence of these factors has been associated with poor survival that is similar to lymph node-positive disease.114
Orthotopic liver transplantation has been utilised in the management of some patients.115,116 However, many of these lesions are suitable for resection, which would likely produce similar results. Given the critical shortage of liver grafts, transplantation for IHC is not performed in most centres, unless it is done in the context of a clinical trial. The use of chemotherapy has not been shown to improve survival, either as adjuvant therapy following resection or in patients with unresectable lesions.117 However, as mentioned above, given the results of the ABC-02 trial for advanced disease, administration of the double regimen in the adjuvant setting needs to be evaluated. External beam radiation therapy, intraoperative radiation and intraluminal radiation therapy have all been evaluated as well, albeit in small, not well controlled, primarily retrospective studies. Similar to chemotherapy, none have shown a significant survival benefit in patients with unresectable disease. However, Ibrahim et al. reported a median survival of 31.8 months in patients with unresectable IHC treated with yttrium-90 (Y-90) who had a performance status of ECOG 0. This study included only 24 patients and a positive effect of Y-90 was not observed in patients with ECOG performance status of 1 or 2.118 The senior author has also reported the experience using hepatic arterial infusion pump therapy (FUDR) with and without bevacizumab for advanced disease. The median survival of patients treated with intra-arterial therapy was 29.5 months, better than that usually achieved with systemic chemotherapy.119 It also appeared that the addition of bevacizumab increased the incidence of biliary toxicity without any improvement in survival (31.1 vs. 29.5 months; P = NS).120
Gallbladder cancer
Gallbladder cancer is an uncommon malignancy with approximately 5000 new cases per year in the USA.1 Historically, clinical attitudes toward gallbladder cancer have been largely based on pessimism and nihilism. This frustration spawns from the usual late presentation, lack of effective therapy and the resultant dismal prognosis. In fact, most older series reported a median survival of 2–5 months for untreated gallbladder cancers, and a less than 5% 5-year survival for treated gallbladder cancers. However, improved understanding of the disease and its treatment has led to prolonged survival and cure in selected patients. Currently, the only chance of cure is with complete surgical extirpation of the cancer.
Epidemiology/aetiology
Worldwide, the highest incidence of gallbladder cancer is found among people indigenous to the Andes Mountains of South America. In North America, the incidence is approximately 1.2 per 100 000, the highest being among native American Indians and Mexican Americans. It occurs in women almost three times more often than in men across all populations studied.121
As with other biliary tract tumours, chronic inflammation leading to high cellular turnover is a common denominator of associated risk factors. The most common risk factor is cholelithiasis; other factors include the presence of a cholecystoenteric fistula, typhoid bacillus infection and an anomalous pancreaticobiliary junction.121,122 As with other gastrointestinal malignancies, the adenoma to carcinoma sequence has been demonstrated within adenomatous polyps of the gallbladder as well.123 Gallbladder polyps are noted in 3–6% of the population undergoing ultrasonography, although the vast majority are cholesterol polyps or adenomyomatosis, both of which are benign and have no malignant potential. However, about 1% of cholecystectomy specimens contain adenomatous polyps, which do have malignant potential.124 Conditions that increase the risk of malignancy include polyp size > 1 cm, patient age > 50 years and the presence of multiple lesions.125 The conservative recommendation is to perform a prophylactic cholecystectomy for polypoid lesions greater than 0.5 cm in size, although the likelihood of malignancy in polyps even up to 1 cm appears to be extremely low. This is in contrast to gallbladder polyps arising in the setting of primary sclerosing cholangitis, which are more often neoplastic.126 The authors’ practice is to recommend cholecystectomy for polyps > 1 cm, although carcinoma in such lesions appears to be much lower than previously thought. Polypoid lesions < 0.5 cm have an extremely low likelihood of harbouring malignancy and are safe to follow with serial ultrasounds for evidence of growth or change in character.123,124,127
A gallbladder with a calcified wall, also known as a ‘porcelain gallbladder’, is associated with an increased risk of developing cancer (Fig. 12.6). The deposition of calcium most likely reflects a state of chronic inflammation. Although the risk of malignancy in a porcelain gallbladder previously was considered to be extremely high (10–50%), recent studies demonstrate a much lower incidence (< 10%), with stippled calcification actually carrying a higher risk than diffuse intramural calcification.128,129 Nevertheless, the current recommendation for patients with a porcelain gallbladder is to perform a cholecystectomy, which in most cases can be safely done laparoscopically.
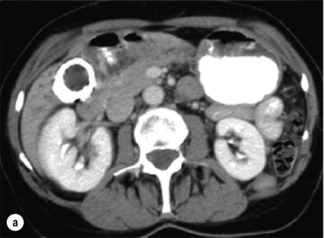
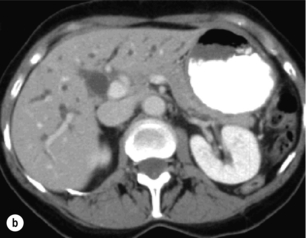
Figure 12.6 Axial CT images of a porcelain gallbladder. Note the marked circumferential calcification of the gallbladder wall (a) and the intrahepatic biliary ductal dilatation (b). This patient had a gallbladder cancer arising in the setting of a porcelain gallbladder, which had progressed to involve the common hepatic duct.
Clinical presentation and diagnosis
Many patients present late in the course of their disease, and 75% of patients present with unresectable disease.130 Two-thirds of patients present with abdominal pain/biliary colic. Approximately one-third will present with jaundice and 10% will have significant weight loss.131 For early stage cancers, the diagnosis is usually made on pathological examination of a cholecystectomy specimen resected for symptoms presumed to be benign biliary colic. Preoperative diagnosis should be suspected for any mass or irregularity of the gallbladder wall noted on radiological investigation (CT or ultrasound). In any patient suspected of having a gallbladder malignancy, a duplex ultrasound should be performed to evaluate the extent of disease and possible involvement of the portal vasculature. In addition, abdominal cross-sectional imaging (CT or MRI) should be performed to evaluate for nodal disease or M1 disease. For those patients suspected of having gallbladder cancer on preoperative imaging, a tissue diagnosis is not necessary prior to exploration, and both the surgeon and patient should be prepared for an appropriate resection, knowing that the final pathology may in fact reveal benign disease.
Histopathology and staging
The overwhelming majority of gallbladder cancers are adenocarcinomas, with a papillary subtype being associated with a relatively better prognosis compared to others.132 Other histological subtypes, such as adenosquamous carcinoma or pure squamous cell carcinoma, are seen in the gallbladder more commonly than at any other site within the biliary tree. The AJCC staging system was updated in 2002 (sixth edition) and was based on the standard TNM classification, of which the T stage has the greatest clinical impact on the extent of surgery performed, because it is dependent on the depth of invasion into the gallbladder wall and adjacent organs. The wall of the gallbladder consists of a mucosa and lamina propria, a thin muscular layer, perimuscular connective tissue and a serosa. However, it should be noted that the gallbladder wall lacks a serosal covering along its border with the liver and the perimuscular connective tissue is continuous with the liver connective tissue. T1 tumours are divided into T1a and T1b lesions, where the former is limited to the lamina propria and the latter has invaded the muscle layer. T2 tumours have invaded through the muscle layer into the perimuscular connective tissue. T3 tumours have penetrated the serosa and directly invade either the liver or another single extrahepatic organ. T4 tumours reflect locally unresectable tumours due to invasion into the main portal vein, hepatic artery or multiple extrahepatic organs. Of note, in patients with a new diagnosis of gallbladder cancer, the presence of jaundice is an ominous finding, generally implying advanced disease that is beyond resectability. Previously, the N stage was divided into locoregional and distant lymph node involvement, but due to the powerful adverse negative impact of any positive lymph node, the sixth edition staging system simply divided tumours into being either node negative or positive, i.e. N0 or N1, respectively. Metastatic disease refers to distant metastasis. This AJCC sixth edition staging system is detailed in Table 12.5. The seventh edition of the AJCC staging system has reverted back to stratifying nodal involvement based on location, thus creating an N1 and N2 designation, and considers T4 tumours as stage IV disease (Table 12.6). It should be noted that the majority of studies referenced in this chapter utilise previous editions of the staging system, where the major difference is that T4 tumours were not deemed as unresectable.
Table 12.5
AJCC staging system (sixth edition) for gallbladder cancer (TNM classification)
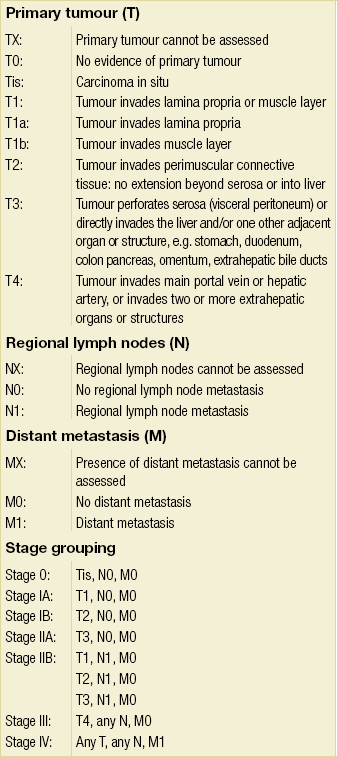
Reprinted with permission from Sobin LH, Wittekind C (eds) TNM classification of malignant tumours, 6th edn. Wiley-Liss, 2002.
Table 12.6
AJCC staging system for gallbladder cancer (TNM classification)
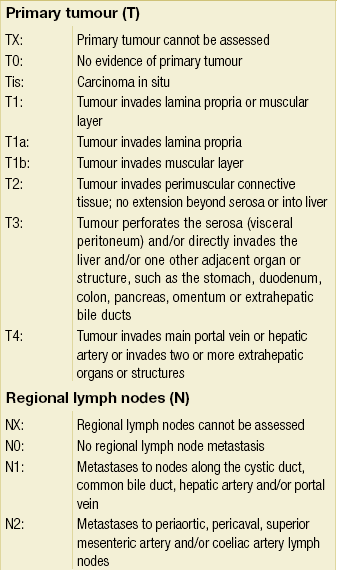
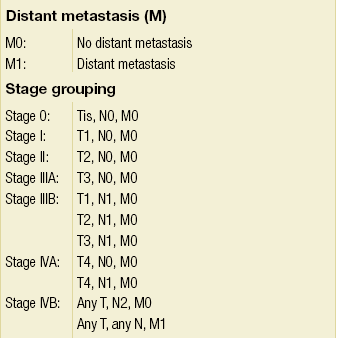
From Sobin LH, Wittekind C (eds) TNM Classification of Malignant Tumors, 6th edition. Wiley-Liss, 2002. This material is reproduced with permission of John Wiley & Sons, Inc.
Preoperative staging should be aimed at assessing the local extent of disease and excluding distant metastases. Cross-sectional imaging (CT or MRCP) is the mainstay of investigation, while duplex ultrasonography is helpful to assess the gallbladder lesion and can provide some insight as to the likelihood of a malignancy; in addition, US may be helpful in assessing possible vascular involvement. FDG-PET has been shown to be helpful in identifying additional disease that changes management; Corvera et al. reported such findings in nearly one-quarter of patients.72 In addition, staging laparoscopy is helpful for identifying distant disease, thereby avoiding non-therapeutic laparotomies.68
Evidence for an aggressive surgical approach
Over the past three decades, decreasing morbidity and mortality associated with radical en bloc resections including hepatectomy, bile duct resection and regional lymphadenectomy have allowed for broader application of surgical resection in selected patients.131,133 The current surgical approaches generally employ segmental resections (segments IVb/V) or major resections (hemihepatectomy or extended hepatectomy) when necessary. In most cases, it is the involvement of major hepatic vascular structures rather than actual depth of tumour invasion into the liver that dictates the extent of hepatic resection that must be performed.
In a series from MSKCC, Bartlett et al. reported on 149 cases in which complete surgical radical resection yielded an actuarial 5-year survival of 83% for stage II, 63% for stage III and 25% for stage IV.131 Many contemporary studies have reported similar results, even for stage III and IV disease.134–137 The improved survival reported in these studies relative to historical studies, in which the survival rates were dismal, demonstrates the importance of achieving negative margins at the time of resection.
Regional lymphadenectomy is currently employed as part of an aggressive surgical approach, but evidence to support an associated survival benefit is controversial. The chance of nodal involvement increases with increasing T stage. Bartlett et al. found nodal disease associated with 46% of resected T2 tumours and 54% of resected T3 tumours.131 Node status was found to be the most powerful predictor of outcome and no patient with node-positive disease experienced long-term survival. Poor outcome for node-positive disease has been consistently reported throughout the Western literature. Again, the value of a negative lymph node is dependent on the number of lymph nodes sampled, and a study from MSKCC suggests that six lymph nodes are needed to accurately assess for lymph node involvement.138
Surgical therapy
It is important to remember that the incidence of lymph node and distant metastases is directly related to T stage. Fong et al. reported a progressive increase in distant and nodal metastases from 16% to 79% and from 33% to 69%, respectively, on going from T2 to T4 tumours, which resulted in a progressive decline in resectability, from 58% to 13%.139
T1 tumours
T1a tumours, or those that are confined to the lamina propria, are most often discovered after, and adequately treated with, a simple cholecystectomy. Because the potential for nodal involvement is small, cure rates approach 85–100% if negative margins are achieved.140,141 T1b tumours, i.e. those tumours that have extended into, but not through, the muscle layer, in theory should be cured by a simple cholecystectomy as well. However, there have been reports in the literature documenting recurrence and death following a simple cholecystectomy for T1b tumours.142 Given the limited data regarding T1b gallbladder cancers in the literature, the decision to perform a simple cholecystectomy versus a more radical procedure should be made on a case-by-case basis.
T2 tumours
T2 lesions, or tumours that extend into the perimuscular connective tissues, should be treated with an aggressive resection, including removal of adjacent liver, lymphadenectomy of the hepatoduodenal ligament and a bile duct resection only if necessary to obtain a negative margin on the cystic duct. As discussed above, the extent of hepatic resection required depends on whether or not there is tumour involvement of the right portal pedicle (i.e. major inflow vascular structures or right hepatic duct). In the absence of such involvement, the authors prefer to perform a segmental resection of segments IVb and V, and most T2 tumours are amenable to such an approach. It should be noted that the normal plane of dissection of simple cholecystectomy, open or laparoscopic, is within the perimuscular connective tissue intimately associated with the liver. Thus, a simple cholecystectomy will not achieve tumour clearance with certainty. A lymphadenectomy is performed in the treatment of T2 tumours given that up to 50% of these lesions have associated lymph node metastases.131 The benefit of an extended resection over simple cholecystectomy is supported by data that demonstrate improved survival. This is underscored by the fact that liver involvement can be found after radical resection in up to a quarter of patients with presumed T2 disease after cholecystectomy alone, a finding that is associated with markedly reduced recurrence-free and disease-specific survival.138 De Aretxabala et al. reported 5-year survival rates of 70% compared with only 20% after simple cholecystectomy alone.143
T3 tumours
T3 tumours penetrate the serosa and may extend into the liver parenchyma or a single extrahepatic organ. These tumours require a hepatic resection and porta hepatis lymphadenectomy at a minimum. As with T2 tumours, if a limited partial hepatectomy can be performed to achieve the objectives, then this is preferred; however, one should not hesitate to perform a more extensive partial hepatectomy and/or bile duct resection if necessary. When a complete resection is achieved, 5-year survival rates of 30–50% can be obtained in this patient population.131,136,139
T4 tumours
T4 lesions, as defined by the sixth edition staging system, generally reflect unresectable disease.
Preoperative suspicion of malignancy
If gallbladder cancer is suspected on preoperative imaging studies, a staging laparoscopy prior to laparotomy is helpful to assess the abdomen for evidence of peritoneal spread or discontiguous liver disease. In general, however, performing a laparoscopic cholecystectomy should be avoided.67,68,144 One needs to be prepared to proceed with resection of an invasive malignancy, unless proven otherwise. It is not unreasonable to obtain intraoperative frozen section histology to prove malignancy before proceeding with hepatic resection.
Unsuspected malignancy at exploration
It should be routine to inspect the gallbladder mucosa after simple cholecystectomy. Suspicious lesions should be sent immediately for frozen section. If a carcinoma is diagnosed, the need to perform additional surgery is dictated by the T stage on frozen section, although the information will be limited since a full histopathological evaluation is not available at the time of operation. The authors prefer to perform an oncologically correct resection, suitable for an invasive lesion, at the time it is discovered, unless there are extenuating circumstances that mandate otherwise. However, if the surgeon is not comfortable with performing a radical cholecystectomy/hepatic resection, the patient is best served by transferring them to a centre/surgeon with experience in performing the appropriate operation. A delayed radical and appropriate resection does not negatively influence the patient’s outcome.139
Malignancy diagnosed post-cholecystectomy
When the cancer is diagnosed by postoperative histology, the need for a more radical resection will be based on T stage, as outlined above. As mentioned above, Fong et al. demonstrated a much improved 5-year survival rate in patients undergoing a second operation compared to those who did not. Five-year survival rates of 61% were achieved in patients who were re-resected compared to 19% for patients who did not undergo a radical second operation.139 However, prior to undertaking a second operation, high-quality cross-sectional imaging (CT/MRI) should be obtained to appropriately stage the disease. Postoperative inflammatory changes may be indistinguishable from tumour and thus may necessitate bile duct resection or a more aggressive hepatic resection to ensure complete tumour eradication.
Given that inadvertent cholecystotomy during cholecystectomy is rarely documented, it is difficult to predict who is at increased risk for peritoneal dissemination and, specifically, port site recurrence. In the past, routine resection of laparoscopic port sites was recommended, in an effort to ensure clearance of microscopic disease that may have implanted during the laparoscopic procedure. However, there is little evidence to support the efficacy of routine resection of all port sites at re-operation.145 In the authors’ experience, recurrence at the port sites is a harbinger of generalised peritoneal recurrence that will not be prevented with resection of these limited areas.
Adjuvant therapy
In order to provide a rational framework upon which to develop adjuvant therapies for patients having undergone resection, Jarnagin et al. investigated the initial pattern of recurrence after resection of biliary tract cancers. Sixty-six per cent of patients with gallbladder cancers who underwent a potentially curative resection recurred within a median follow-up of 24 months. Only 15% of patients developed a locoregional recurrence as the first site of failure, while the majority of patients (85%) had recurrence that involved a distant site.146 Thus, local therapies targeted at locoregional disease, such as radiotherapy, are unlikely to significantly impact the course of this disease, further emphasising the importance of developing effective systemic adjuvant therapies.
Most data for the use of adjuvant therapy are derived from phase II trials in which treated patients are compared with historical controls. Most of these trials are limited by small numbers, combine chemotherapy with radiation treatment, and are confounded by inclusion of patients with less than an R0 resection.147,148 Thus, minimal conclusions can be drawn regarding the use of external beam radiation/chemotherapy in the adjuvant setting. In cases of incomplete resection, there remains a theoretical benefit to adding an additional locoregional therapy such as external beam radiation therapy for disease control.
One phase III multi-institutional trial of adjuvant chemotherapy was performed in Japan as reported by Takada et al.85 It should be noted that this trial included 508 patients with biliary and pancreatic cancers. However, on subset analysis, this study included 140 patients with gallbladder cancer who were randomised to undergo surgical resection alone or resection plus adjuvant mitomycin and 5-FU. In considering only the patients with gallbladder cancer, the actuarial 5-year disease-free survival favoured the adjuvant chemotherapy group in comparison to the surgery-alone group (20.3% vs. 11.6%). From these data it is reasonable to offer adjuvant chemotherapy with 5-FU and mitomycin; however, no consensus has been reached regarding routine use of adjuvant chemotherapy.102
Palliation
Most gallbladder cancer patients present with advanced, incurable disease. Their symptoms may include pain, jaundice or gastrointestinal obstruction. Given the dismal prognosis of approximately 2–5 months’ survival, non-surgical methods of palliation including both percutaneous and endoscopic techniques to relieve intestinal or biliary obstruction should be considered first. If unresectable disease is discovered at the time of exploration, a segment III bypass can be performed to relieve jaundice, but in general patients are best served by avoiding a major operative procedure and proceeding with percutaneous biliary drainage postoperatively.149 Intestinal bypass should be performed only in patients who have symptomatic obstruction.
References
1. Landis, S.H., Murray, T., Bolden, S., et al, Cancer statistics, 1998. CA Cancer J Clin. 1998;48(1):6–29. 9449931
2. Carriaga, M.T., Henson, D.E., Liver, gallbladder, extrahepatic bile ducts, and pancreas. Cancer. 1995;75(1, Suppl.):171–190. 8000995
3. Burke, E.C., Jarnagin, W.R., Hochwald, S.N., et al, Hilar cholangiocarcinoma: patterns of spread, the importance of hepatic resection for curative operation, and a presurgical clinical staging system. Ann Surg. 1998;228(3):385–394. 9742921
4. Nagorney, D.M., Donohue, J.H., Farnell, M.B., et al, Outcomes after curative resections of cholangiocarcinoma. Arch Surg. 1993;128(8):871–879. 8393652
5. Tompkins, R.K., Thomas, D., Wile, A., et al, Prognostic factors in bile duct carcinoma: analysis of 96 cases. Ann Surg 1981; 194:447–457. 7283506
6. Nakeeb, A., Pitt, H.A., Sohn, T.A., et al, Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224(4):463–475. 8857851
7. Berdah, S.V., Delpero, J.R., Garcia, S., et al, A Western surgical experience of peripheral cholangiocarcinoma. Br J Surg. 1996;83(11):1517–1521. 9014664
8. Chu, K.M., Lai, E.C., Al-Hadeedi, S., et al, Intrahepatic cholangiocarcinoma. World J Surg. 1997;21(3):301–306. 9015175
9. Harrison, L.E., Fong, Y., Klimstra, D.S., et al, Surgical treatment of 32 patients with peripheral intrahepatic cholangiocarcinoma. Br J Surg. 1998;85(8):1068–1070. 9717998
10. Saunders, K., Longmire, W., Jr., Tompkins, R., et al, Diffuse bile duct tumors: guidelines for management. Am Surg. 1991;57(12):816–820. 1746801
11. Patel, T., Steer, C.J., Gores, G.J., Apoptosis and the liver: a mechanism of disease, growth regulation, and carcinogenesis. Hepatology. 1999;30(3):811–815. 10462391
12. Welzel, T.M., Graubard, B.I., El-Serag, H.B., et al, Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population-based case–control study. Clin Gastroenterol Hepatol. 2007;5(10):1221–1228. 17689296
13. Welzel, T.M., Mellemkjaer, L., Gloria, G., et al, Risk factors for intrahepatic cholangiocarcinoma in a low-risk population: a nationwide case–control study. Int J Cancer. 2007;120(3):638–641. 17109384
14. Endo, I., Gonen, M., Yopp, A.C., et al, Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248(1):84–96. 18580211
15. Kuwayti, K., Baggenstoss, A.H., Stauffer, M.H., et al, Carcinoma of the major intrahepatic and the extrahepatic bile ducts exclusive of the papilla of Vater. Surg Gynecol Obstet 1957; 104:357–366. 13422156
16. Sako, K., Seitzinger, G.L., Garside, E., Carcinoma of the extrahepatic bile ducts; review of the literature and report of six cases. Surgery. 1957;41(3):416–437. 13409219
17. Okuda, K., Kubo, Y., Okazaki, N., et al, Clinical aspects of intrahepatic bile duct carcinoma including hilar carcinoma: a study of 57 autopsy-proven cases. Cancer. 1977;39(1):232–246. 64293
18. Broome, U., Olsson, R., Loof, L., et al, Natural history and prognostic factors in 305 Swedish patients with primary sclerosing cholangitis. Gut. 1996;38(4):610–615. 8707097
19. Pitt, H.A., Dooley, W.C., Yeo, C.J., et al, Malignancies of the biliary tree. Curr Probl Surg. 1995;32(1):1–90. 7528652
20. Hewitt, P.M., Krige, J.E., Bornman, P.C., et al, Choledochal cysts in adults. Br J Surg. 1995;82(3):382–385. 7796017
21. Vogt, D.P., Current management of cholangiocarcinoma. Oncology (Williston Park). 1988;2(6):37–44 54. 2856323
22. Lipsett, P.A., Pitt, H.A., Colombani, P.M., et al, Choledochal cyst disease. A changing pattern of presentation. Ann Surg. 1994;220(5):644–652. 7979612
23. Tanaka, K., Ikoma, A., Hamada, N., et al, Biliary tract cancer accompanied by anomalous junction of pancreaticobiliary ductal system in adults. Am J Surg. 1998;175(3):218–220. 9560123
24. Jeng, K.S., Ohta, I., Yang, F.S., et al, Coexisting sharp ductal angulation with intrahepatic biliary strictures in right hepatolithiasis. Arch Surg. 1994;129(10):1097–1102. 7944942
25. Hakamada, K., Sasaki, M., Endoh, M., et al, Late development of bile duct cancer after sphincteroplasty: a ten- to twenty-two-year follow-up study. Surgery. 1997;121(5):488–492. 9142145
26. Chu, K.M., Lo, C.M., Liu, C.L., et al, Malignancy associated with hepatolithiasis. Hepatogastroenterology. 1997;44(14):352–357. 9164501
27. Kubo, S., Kinoshita, H., Hirohashi, K., et al, Hepatolithiasis associated with cholangiocarcinoma. World J Surg. 1995;19(4):637–641. 7676713
28. Weinbren, K., Mutum, S.S., Pathological aspects of cholangiocarcinoma. J Pathol. 1983;139(2):217–238. 6298394
29. Rodgers, C.M., Adams, J.T., Schwartz, S.I., Carcinoma of the extrahepatic bile ducts. Surgery. 1981;90(4):596–601. 7281000
30. Jarnagin, W.R., Bowne, W., Klimstra, D.S., et al, Papillary phenotype confers improved survival after resection of hilar cholangiocarcinoma. Ann Surg. 2005;241(5):703–714. 15849506
31. Tsuzuki, T., Ogata, Y., Iida, S., et al, Carcinoma of the bifurcation of the hepatic ducts. Arch Surg. 1983;118(10):1147–1151. 6615197
32. Shimada, H., Niimoto, S., Matsuba, A., et al, The infiltration of bile duct carcinoma along the bile duct wall. Int Surg. 1988;73(2):87–90. 2840410
33. Endo, I., House, M.G., Klimstra, D.S., et al, Clinical significance of intraoperative bile duct margin assessment for hilar cholangiocarcinoma. Ann Surg Oncol. 2008;15(8):2104–2112. 18543039
34. Craig, J.R., Peters, R.L., Edmondson, H.A. Tumor liver and intrahepatic ducts. Armed Forces Institute of Pathology; 1989.
35. The Liver Cancer Study Group of Japan, Primary liver cancer in Japan. Sixth report. Cancer. 1987;60(6):1400–1411. 3040216
36. Yamamoto, J., Kosuge, T., Shimada, K., et al, Intrahepatic cholangiocarcinoma: proposal of new macroscopic classification. Nippon Geka Gakkai Zasshi. 1993;94(11):1194–1200. 8272051
37. Levi, S., Urbano-Ispizua, A., Gill, R., et al, Multiple K-ras codon 12 mutations in cholangiocarcinomas demonstrated with a sensitive polymerase chain reaction technique. Cancer Res. 1991;51(13):3497–3502. 1675933
38. Nakeeb, A., Lipsett, P.A., Lillemoe, K.D., et al, Biliary carcinoembryonic antigen levels are a marker for cholangiocarcinoma. Am J Surg. 1996;171(1):147–153. 8554130
39. Jarnagin, W.R., Weber, S., Tickoo, S.K., et al, Combined hepatocellular and cholangiocarcinoma: demographic, clinical, and prognostic factors. Cancer. 2002;94(7):2040–2046. 11932907
40. Farley, D.R., Weaver, A.L., Nagorney, D.M., “Natural history” of unresected cholangiocarcinoma: patient outcome after noncurative intervention. Mayo Clin Proc. 1995;70(5):425–429. 7537346
41. Vatanasapt, V., Uttaravichien, T., Mairiang, E.O., et al, Cholangiocarcinoma in north-east Thailand. Lancet. 1990;335(8681):116–117. 1967402
42. McPherson, G.A., Benjamin, I.S., Hodgson, H.J., et al, Pre-operative percutaneous transhepatic biliary drainage: the results of a controlled trial. Br J Surg. 1984;71(5):371–375. 6372935
43. Heslin, M.J., Brooks, A.D., Hochwald, S.N., et al, A preoperative biliary stent is associated with increased complications after pancreatoduodenectomy. Arch Surg. 1998;133(2):149–154. 9484726
44. Corvera, C.U., Blumgart, L.H., Darvishian, F., et al, Clinical and pathologic features of proximal biliary strictures masquerading as hilar cholangiocarcinoma. J Am Coll Surg. 2005;201(6):862–869. 16310689
45. Wetter, L.A., Ring, E.J., Pellegrini, C.A., et al, Differential diagnosis of sclerosing cholangiocarcinomas of the common hepatic duct (Klatskin tumors). Am J Surg. 1991;161(1):57–63. 1846276
46. Rabinovitz, M., Zajko, A.B., Hassanein, T., et al, Diagnostic value of brush cytology in the diagnosis of bile duct carcinoma: a study in 65 patients with bile duct strictures. Hepatology. 1990;12(4, Pt 1):747–752. 16905310
47. Hadjis, N.S., Blumgart, L.H., Role of liver atrophy, hepatic resection and hepatocyte hyperplasia in the development of portal hypertension in biliary disease. Gut. 1987;28(8):1022–1028. 3666554
48. Tillich, M., Mischinger, H.J., Preisegger, K.H., et al, Multiphasic helical CT in diagnosis and staging of hilar cholangiocarcinoma. Am J Roentgenol. 1998;171(3):651–658. 9725291
49. Gibson, R.N., Yeung, E., Thompson, J.N., et al, Bile duct obstruction: radiologic evaluation of level, cause, and tumor resectability. Radiology. 1986;160(1):43–47. 3520654
50. Okuda, K., Ohto, M., Tsuchiya, Y., The role of ultrasound, percutaneous transhepatic cholangiography, computed tomographic scanning, and magnetic resonance imaging in the preoperative assessment of bile duct cancer. World J Surg. 1988;12(1):18–26. 2830726
51. Hann, L.E., Greatrex, K.V., Bach, A.M., et al, Cholangiocarcinoma at the hepatic hilus: sonographic findings. Am J Roentgenol. 1997;168(4):985–989. 9124155
52. Bach, A.M., Hann, L.E., Brown, K.T., et al, Portal vein evaluation with US: comparison to angiography combined with CT arterial portography. Radiology. 1996;201(1):149–154. 8816536
53. Itoh, K., Fujita, N., Kubo, K., et al, MR imaging of hilar cholangiocarcinoma – comparative study with CT. Nippon Igaku Hoshasen Gakkai Zasshi. 1992;52(4):443–451. 1321410
54. Guthrie, J.A., Ward, J., Robinson, P.J., Hilar cholangiocarcinomas: T2-weighted spin-echo and gadolinium- enhanced FLASH MR imaging. Radiology. 1996;201(2):347–351. 8888221
55. Schwartz, L.H., Coakley, F.V., Sun, Y., et al, Neoplastic pancreaticobiliary duct obstruction: evaluation with breath-hold MR cholangiopancreatography. Am J Roentgenol. 1998;170(6):1491–1495. 9609160
56. Lee, M.G., Lee, H.J., Kim, M.H., et al, Extrahepatic biliary diseases: 3D MR cholangiopancreatography compared with endoscopic retrograde cholangiopancreatography. Radiology. 1997;202(3):663–669. 9051013
57. Jarnagin, W.R., Fong, Y., DeMatteo, R.P., et al, Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234(4):507–519. 11573044
58. Bismuth, H., Nakache, R., Diamond, T., Management strategies in resection for hilar cholangiocarcinoma. Ann Surg. 1992;215(1):31–38. 1309988
59. Chen, R.F., Li, Z.H., Zhou, J.J., et al, Preoperative evaluation with T-staging system for hilar cholangiocarcinoma. World J Gastroenterol. 2007;13(43):5754–5759. 17963304
60. Klempnauer, J., Ridder, G.J., Werner, M., et al, What constitutes long-term survival after surgery for hilar cholangiocarcinoma? Cancer. 1997;79(1):26–34. 8988723
61. Pichlmayr, R., Weimann, A., Klempnauer, J., et al, Surgical treatment in proximal bile duct cancer. A single- center experience. Ann Surg. 1996;224(5):628–638. 8916878
62. Iwatsuki, S., Todo, S., Marsh, J.W., et al, Treatment of hilar cholangiocarcinoma (Klatskin tumors) with hepatic resection or transplantation. J Am Coll Surg. 1998;187(4):358–364. 9783781
63. Heimbach, J.K., Haddock, M.G., Alberts, S.R., et al, Transplantation for hilar cholangiocarcinoma. Liver Transpl. 2004;10(10, Suppl. 2):S65–S68. 15382214
64. Lazaridis, K.N., Gores, G.J., Cholangiocarcinoma. Gastroenterology. 2005;128(6):1655–1667. 15887157
65. Nimura, Y., Hayakawa, N., Kamiya, J., et al, Hepatic segmentectomy with caudate lobe resection for bile duct carcinoma of the hepatic hilus. World J Surg. 1990;14(4):535–544. 2166381
66. Mizumoto, R., Suzuki, H., Surgical anatomy of the hepatic hilum with special reference to the caudate lobe. World J Surg. 1988;12(1):2–10. 3344582
67. Vollmer, C.M., Drebin, J.A., Middleton, W.D., et al, Utility of staging laparoscopy in subsets of peripancreatic and biliary malignancies. Ann Surg. 2002;235(1):1–7. 11753036
68. Weber, S.M., DeMatteo, R.P., Fong, Y., et al, Staging laparoscopy in patients with extrahepatic biliary carcinoma. Analysis of 100 patients. Ann Surg. 2002;235(3):392–399. 11882761
69. Kluge, R., Schmidt, F., Caca, K., et al, Positron emission tomography with [18F]fluoro-2-deoxy-d-glucose for diagnosis and staging of bile duct cancer. Hepatology. 2001;33(5):1029–1035. 11343227
70. Fritscher-Ravens, A., Bohuslavizki, K.H., Broering, D.C., et al, FDG PET in the diagnosis of hilar cholangiocarcinoma. Nucl Med Commun. 2001;22(12):1277–1285. 11711897
71. Anderson, C.D., Rice, M.H., Pinson, C.W., et al, Fluorodeoxyglucose PET imaging in the evaluation of gallbladder carcinoma and cholangiocarcinoma. J Gastrointest Surg. 2004;8(1):90–97. 14746840
72. Corvera, C.U., Blumgart, L.H., Akhurst, T., et al, 18F-Fluorodeoxyglucose positron emission tomography influences management decisions in patients with biliary cancer. J Am Coll Surg. 2008;206(1):57–65. 18155569
73. Jarnagin, W.R., Blumgart, L.H., Saldinger, P. Cancer of the bile ducts. In: Blumgart L.H., Fong Y., eds. Surgery of the liver and biliary tract. London: WB Saunders; 2000:1017.
74. Neuhaus, P., Thelen, A., Jonas, S., et al, Oncological superiority of hilar en bloc resection for the treatment of hilar cholangiocarcinoma. Ann Surg Oncol. 2012;19(5):1602–1608. 21964888
75. Kitagawa, Y., Nagino, M., Kamiya, J., et al, Lymph node metastasis from hilar cholangiocarcinoma: audit of 110 patients who underwent regional and paraaortic node dissection. Ann Surg. 2001;233(3):385–392. 11224627
76. Tojima, Y., Nagino, M., Ebata, T., et al, Immunohistochemically demonstrated lymph node micrometastasis and prognosis in patients with otherwise node-negative hilar cholangiocarcinoma. Ann Surg. 2003;237(2):201–207. 12560778
77. Ito, K., Ito, H., Allen, P.J., et al, Adequate lymph node assessment for extrahepatic bile duct adenocarcinoma. Ann Surg. 2010;251(4):675–681. 20224368
78. Hadjis, N.S., Blenkharn, J.I., Alexander, N., et al, Outcome of radical surgery in hilar cholangiocarcinoma. Surgery. 1990;107(6):597–604. 2162082
79. Baer, H.U., Stain, S.C., Dennison, A.R., et al, Improvements in survival by aggressive resections of hilar cholangiocarcinoma. Ann Surg. 1993;217(1):20–27. 8380975
80. Saldinger, P.F., Blumgart, L.H., Resection of hilar cholangiocarcinoma – a European and United States experience. J Hepatobiliary Pancreat Surg. 2000;7(2):111–114. 10982601
81. Cameron, J.L., Pitt, H.A., Zinner, M.J., et al, Management of proximal cholangiocarcinomas by surgical resection and radiotherapy. Am J Surg. 1990;159(1):91–98. 1688486
82. Pitt, H.A., Nakeeb, A., Abrams, R.A., et al, Perihilar cholangiocarcinoma. Postoperative radiotherapy does not improve survival. Ann Surg. 1995;221(6):788–798. 7794082
83. Kamada, T., Saitou, H., Takamura, A., et al, The role of radiotherapy in the management of extrahepatic bile duct cancer: an analysis of 145 consecutive patients treated with intraluminal and/or external beam radiotherapy. Int J Radiat Oncol Biol Phys. 1996;34(4):767–774. 8598352
84. McMasters, K.M., Tuttle, T.M., Leach, S.D., et al, Neoadjuvant chemoradiation for extrahepatic cholangiocarcinoma. Am J Surg. 1997;174(6):605–609. 9409582
85. Takada, T., Amano, H., Yasuda, H., et al, Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer. 2002;95(8):1685–1695. 12365016
86. Kuvshinoff, B.W., Armstrong, J.G., Fong, Y., et al, Palliation of irresectable hilar cholangiocarcinoma with biliary drainage and radiotherapy. Br J Surg. 1995;82(11):1522–1525. 8535808
87. Liu, C.L., Lo, C.M., Lai, E.C., et al, Endoscopic retrograde cholangiopancreatography and endoscopic endoprosthesis insertion in patients with Klatskin tumors. Arch Surg. 1998;133(3):293–296. 9517743
88. Becker, C.D., Glattli, A., Maibach, R., et al, Percutaneous palliation of malignant obstructive jaundice with the Wallstent endoprosthesis: follow-up and reintervention in patients with hilar and non-hilar obstruction. J Vasc Interv Radiol. 1993;4(5):597–604. 7693074
89. Cheung, K.L., Lai, E.C., Endoscopic stenting for malignant biliary obstruction. Arch Surg. 1995;130(2):204–207. 7531431
90. Miyazaki, M., Ito, H., Nakagawa, K., et al, Aggressive surgical approaches to hilar cholangiocarcinoma: hepatic or local resection? Surgery. 1998;123(2):131–136. 9481397
91. Schima, E., Surgery of the biliary tract in geriatric patients (author’s transl.). Zentralbl Chir. 1977;102(14):858–868. 410197
92. Glattli, A., Stain, S.C., Baer, H.U., et al, Unresectable malignant biliary obstruction: treatment by self-expandable biliary endoprostheses. HPB Surg. 1993;6(3):175–184. 7683908
93. Jarnagin, W.R., Burke, E., Powers, C., et al, Intrahepatic biliary enteric bypass provides effective palliation in selected patients with malignant obstruction at the hepatic duct confluence. Am J Surg. 1998;175(6):453–460. 9645771
94. Baer, H.U., Rhyner, M., Stain, S.C., et al, The effect of communication between the right and left liver on the outcome of surgical drainage for jaundice due to malignant obstruction at the hilus of the liver. HPB Surg. 1994;8(1):27–31. 7993861
95. Bowling, T.E., Galbraith, S.M., Hatfield, A.R., et al, A retrospective comparison of endoscopic stenting alone with stenting and radiotherapy in non-resectable cholangiocarcinoma. Gut. 1996;39(6):852–855. 9038668
96. Vallis, K.A., Benjamin, I.S., Munro, A.J., et al, External beam and intraluminal radiotherapy for locally advanced bile duct cancer: role and tolerability. Radiother Oncol. 1996;41(1):61–66. 8961369
97. Ortner, M., Photodynamic therapy for cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2001;8(2):137–139. 11455469
98. Ortner, M., Photodynamic therapy in the biliary tract. Curr Gastroenterol Rep. 2001;3(2):154–159. 11276384
99. Ortner, M.E., Caca, K., Berr, F., et al, Successful photodynamic therapy for nonresectable cholangiocarcinoma: a randomized prospective study. Gastroenterology. 2003;125(5):1355–1363. 14598251
100. Zoepf, T., Jakobs, R., Arnold, J.C., et al, Palliation of nonresectable bile duct cancer: improved survival after photodynamic therapy. Am J Gastroenterol. 2005;100(11):2426–2430. 16279895
101. Glimelius, B., Hoffman, K., Sjoden, P.O., et al, Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol. 1996;7(6):593–600. 8879373
102. Daines, W.P., Rajagopalan, V., Grossbard, M.L., et al, Gallbladder and biliary tract carcinoma: a comprehensive update, Part 2. Oncology (Williston Park). 2004;18(8):1049–1059 discussion 1060, 1065–6, 1068. 15328897
103. Valle, J., Wasan, H., Palmer, D.H., et al, Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273–1281. 20375404
104. Fong, Y., Blumgart, L.H., Lin, E., et al, Outcome of treatment for distal bile duct cancer. Br J Surg. 1996;83(12):1712–1715. 9038548
105. Wade, T.P., Prasad, C.N., Virgo, K.S., et al, Experience with distal bile duct cancers in U.S. Veterans Affairs hospitals: 1987–1991. J Surg Oncol. 1997;64(3):242–245. 9121157
106. Yeo, C.J., Cameron, J.L., Sohn, T.A., et al, Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226(3):248–260. 9339931
107. Way, L.W., Biliary tract. Current surgical diagnosis and treatment. 10th ed. Appleton & Lange, East Norwark, CT, 1994. [p.537–66].
108. Georgopoulos, S.K., Schwartz, L.H., Jarnagin, W.R., et al, Comparison of magnetic resonance and endoscopic retrograde cholangiopancreatography in malignant pancreaticobiliary obstruction. Arch Surg. 1999;134(9):1002–1007. 10487597
109. Ryan, M.E., Cytologic brushings of ductal lesions during ERCP. Gastrointest Endosc. 1991;37(2):139–142. 1851708
110. Maetani, Y., Itoh, K., Watanabe, C., et al, MR imaging of intrahepatic cholangiocarcinoma with pathologic correlation. Am J Roentgenol. 2001;176(6):1499–1507. 11373220
111. Lim, J.H., Cholangiocarcinoma: morphologic classification according to growth pattern and imaging findings. Am J Roentgenol. 2003;181(3):819–827. 12933488
112. Weber, S.M., Jarnagin, W.R., Klimstra, D., et al, Intrahepatic cholangiocarcinoma: resectability, recurrence pattern, and outcomes. J Am Coll Surg. 2001;193(4):384–391. 11584966
113. de Jong, M.C., Nathan, H., Sotiropoulos, G.C., et al, Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol. 2011;29(23):3140–3145. 21730269
114. Patel, S.H., Kooby, D.A., Staley, C.A., 3rd., et al, The prognostic importance of lymphovascular invasion in cholangiocarcinoma above the cystic duct: a new selection criterion for adjuvant therapy? HPB (Oxford). 2011;13(9):605–611. 21843260
115. Schlinkert, R.T., Nagorney, D.M., Van Heerden, J.A., et al, Intrahepatic cholangiocarcinoma: clinical aspects, pathology and treatment. HPB Surg. 1992;5(2):95–102. 1319194
116. Ringe, B., Canelo, R., Lorf, T., Liver transplantation for primary liver cancer. Transplant Proc. 1996;28(3):1174–1175. 8658616
117. Falkson, G., MacIntyre, J.M., Moertel, C.G., Eastern Cooperative Oncology Group experience with chemotherapy for inoperable gallbladder and bile duct cancer. Cancer. 1984;54(6):965–969. 6235908
118. Ibrahim, S.M., Mulcahy, M.F., Lewandowski, R.J., et al, Treatment of unresectable cholangiocarcinoma using yttrium-90 microspheres: results from a pilot study. Cancer. 2008;113(8):2119–2128. 18759346
119. Jarnagin, W.R., Schwartz, L.H., Gultekin, D.H., et al, Regional chemotherapy for unresectable primary liver cancer: results of a phase II clinical trial and assessment of DCE-MRI as a biomarker of survival. Ann Oncol. 2009;20(9):1589–1595. 19491285
120. Kemeny, N.E., Schwartz, L., Gonen, M., et al, Treating primary liver cancer with hepatic arterial infusion of floxuridine and dexamethasone: does the addition of systemic bevacizumab improve results? Oncology. 2011;80(3–4):153–159. 21677464
121. Lazcano-Ponce, E.C., Miquel, J.F., Munoz, N., et al, Epidemiology and molecular pathology of gallbladder cancer. CA Cancer J Clin. 2001;51(6):349–364. 11760569
122. Serra, I., Serra, I., Diehl, A.K., Number and size of stones in patients with asymptomatic and symptomatic gallstones and gallbladder carcinoma. J Gastrointest Surg. 2002;6(2):272–273 author reply 273. 11992815
123. Kozuka, S., Tsubone, N., Yasui, A., et al, Relation of adenoma to carcinoma in the gallbladder. Cancer. 1982;50(10):2226–2234. 7127263
124. Fong, Y., Malhotra, S., Gallbladder cancer: recent advances and current guidelines for surgical therapy. Adv Surg 2001; 35:1–20. 11579805
125. Yeh, C.N., Jan, Y.Y., Chao, T.C., et al, Laparoscopic cholecystectomy for polypoid lesions of the gallbladder: a clinicopathologic study. Surg Laparosc Endosc Percutan Tech. 2001;11(3):176–181. 11444747
126. Buckles, D.C., Lindor, K.D., Larusso, N.F., et al, In primary sclerosing cholangitis, gallbladder polyps are frequently malignant. Am J Gastroenterol. 2002;97(5):1138–1142. 12014717
127. Yang, H.L., Sun, Y.G., Wang, Z., Polypoid lesions of the gallbladder: diagnosis and indications for surgery. Br J Surg. 1992;79(3):227–229. 1555088
128. Stephen, A.E., Berger, D.L., Carcinoma in the porcelain gallbladder: a relationship revisited. Surgery. 2001;129(6):699–703. 11391368
129. Kwon, A.H., Inui, H., Matsui, Y., et al, Laparoscopic cholecystectomy in patients with porcelain gallbladder based on the preoperative ultrasound findings. Hepatogastroenterology. 2004;51(58):950–953. 15239221
130. Adson, M. Carcinoma of the gallbladder. In: Moody F.G., ed. Advances in diagnosis and surgical treatment of biliary tract disease. Chicago: Year Book, 1983.
131. Bartlett, D.L., Fong, Y., Fortner, J.G., et al, Long-term results after resection for gallbladder cancer. Implications for staging and management. Ann Surg. 1996;224(5):639–646. 8916879
132. Henson, D.E., Albores-Saavedra, J., Corle, D., Carcinoma of the gallbladder. Histologic types, stage of disease, grade, and survival rates. Cancer. 1992;70(6):1493–1497. 1516000
133. Nakamura, S., Sakaguchi, S., Suzuki, S., et al, Aggressive surgery for carcinoma of the gallbladder. Surgery. 1989;106(3):467–473. 2772822
134. Shirai, Y., Yoshida, K., Tsukada, K., et al, Radical surgery for gallbladder carcinoma. Long-term results. Ann Surg. 1992;216(5):565–568. 1359844
135. Donohue, J.H., Nagorney, D.M., Grant, C.S., et al, Carcinoma of the gallbladder. Does radical resection improve outcome? Arch Surg. 1990;125(2):237–241. 2302063
136. Chijiiwa, K., Tanaka, M., Carcinoma of the gallbladder: an appraisal of surgical resection. Surgery. 1994;115(6):751–756. 7910985
137. Onoyama, H., Yamamoto, M., Tseng, A., et al, Extended cholecystectomy for carcinoma of the gallbladder. World J Surg. 1995;19(5):758–763. 7571677
138. Ito, H., Ito, K., D’Angelica, M., et al, Accurate staging for gallbladder cancer: implications for surgical therapy and pathological assessment. Ann Surg. 2011;254(2):320–325. 21617582
139. Fong, Y., Jarnagin, W., Blumgart, L.H., Gallbladder cancer: comparison of patients presenting initially for definitive operation with those presenting after prior noncurative intervention. Ann Surg. 2000;232(4):557–569. 10998654
140. Shirai, Y., Yoshida, K., Tsukada, K., et al, Inapparent carcinoma of the gallbladder. An appraisal of a radical second operation after simple cholecystectomy. Ann Surg. 1992;215(4):326–331. 1558412
141. Yamaguchi, K., Tsuneyoshi, M., Subclinical gallbladder carcinoma. Am J Surg. 1992;163(4):382–386. 1558279
142. Kimura, W., Shimada, H., A case of gallbladder carcinoma with infiltration into the muscular layer that resulted in relapse and death from metastasis to the liver and lymph nodes. Hepatogastroenterology. 1990;37(1):86–89. 2312046
143. De Aretxabala, X.A., Roa, I.S., Burgos, L.A., et al, Curative resection in potentially resectable tumours of the gallbladder. Eur J Surg. 1997;163(6):419–426. 9231853
144. Callery, M.P., Strasberg, S.M., Doherty, G.M., et al, Staging laparoscopy with laparoscopic ultrasonography: optimizing resectability in hepatobiliary and pancreatic malignancy. J Am Coll Surg. 1997;185(1):33–39. 9208958
145. Shoup, M., Fong, Y., Surgical indications and extent of resection in gallbladder cancer. Surg Oncol Clin North Am. 2002;11(4):985–994. 12607584
146. Jarnagin, W.R., Ruo, L., Little, S.A., et al, Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies. Cancer. 2003;98(8):1689–1700. 14534886
147. Kresl, J.J., Schild, S.E., Henning, G.T., et al, Adjuvant external beam radiation therapy with concurrent chemotherapy in the management of gallbladder carcinoma. Int J Radiat Oncol Biol Phys. 2002;52(1):167–175. 11777635
148. Mahe, M., Stampfli, C., Romestaing, P., et al, Primary carcinoma of the gall-bladder: potential for external radiation therapy. Radiother Oncol. 1994;33(3):204–208. 7536333
149. Kapoor, V.K., Pradeep, R., Haribhakti, S.P., et al, Intrahepatic segment III cholangiojejunostomy in advanced carcinoma of the gallbladder. Br J Surg. 1996;83(12):1709–1711. 9038546