Chapter 4 Malignancies of the Temporal Bone—Radical Temporal Bone Resection
Primary malignancies of the temporal bone were first recognized in the late 18th century and histologically first confirmed in the 1850s. These lesions are uncommon, with only 250 cases having been reported in the English literature by 1974.1 The overall prevalence in the general population is 6 cases per 1 million.2 Because of their infrequent occurrence, these tumors are often misdiagnosed and treated as chronic external otitis or chronic mastoiditis. They are usually discovered at a later stage, when more radical treatment is required. The infrequent occurrence of the disease poses a challenging obstacle to any attempt at a clinical study regarding treatment.
DIAGNOSTIC EVALUATION
Cerebral blood flow evaluation is indicated when involvement of the ICA is present. Patency of the anterior and posterior communicating arteries on angiography is an inadequate evaluation for collateral flow. Our current method of preoperative carotid artery testing is described.3 A 30-minute temporary balloon occlusion of the ICA allows identification of patients who would most likely tolerate carotid artery sacrifice. Transfemoral introduction of a nondetachable intravascular balloon, inflated in the ICA, is performed in the patient while sensory, motor, and higher cortical functions are assessed.
SURGICAL PROCEDURE
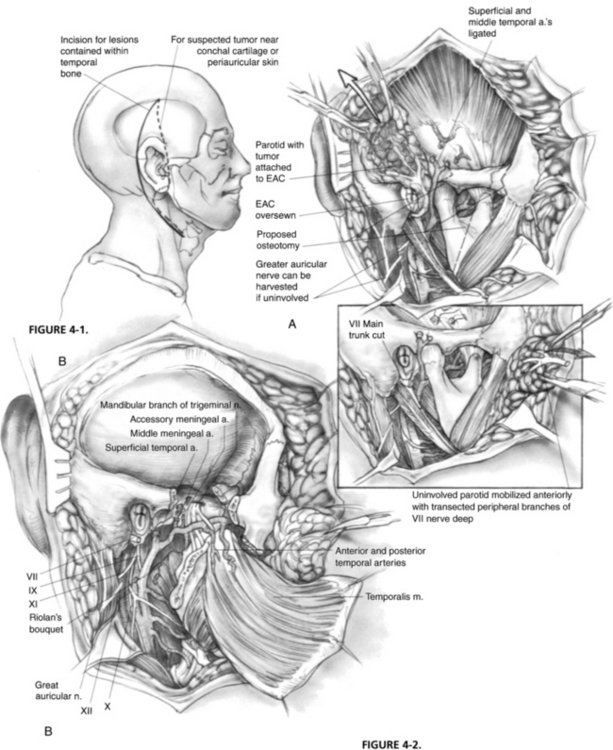
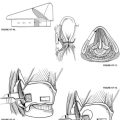
Incisions vary according to the extent of the tumor (Fig. 4-1). For lesions contained within the temporal bone, a C-shaped incision extending from the temporal fossa postauricularly into the neck is used. A blind-sac closure of the EAC helps contain the specimen. When tumor invasion of the conchal cartilage or periauricular skin is suspected, an appropriate skin island is incorporated into the overall design. The EAC skin is sutured shut to avoid tumor spillage. The outline of the incisions should preserve the blood supply to the remaining auricle. The anterior and posterior skin flaps are elevated (Fig. 4-2A). The superficial temporal fat pad is elevated with the anterior skin flap in a subperiosteal plane over the zygomatic arch. The superficial temporal and middle temporal arteries are ligated.
The masseter is detached from the zygomatic arch, allowing exposure of the zygoma and mandible. Zygomatic and mandibular osteotomies (see Fig. 4-2A) can then be performed. The meniscus of the temporomandibular joint is separated from the glenoid fossa, and the chorda tympani nerve emerging from the petrotympanic fissure is divided. The stylomandibular and sphenomandibular ligaments are divided and allow removal of the mandibular segment (Fig. 4-2B). The temporalis muscle is elevated in a subperiosteal fashion and reflected inferiorly. The temporalis muscle must be separated from the lateral pterygoid muscle, and care should be taken not to injure the deep temporal arteries supplying blood to the temporalis muscle. The lateral and medial pterygoid muscles are resected either en bloc with the specimen or separately, depending on tumor invasion.
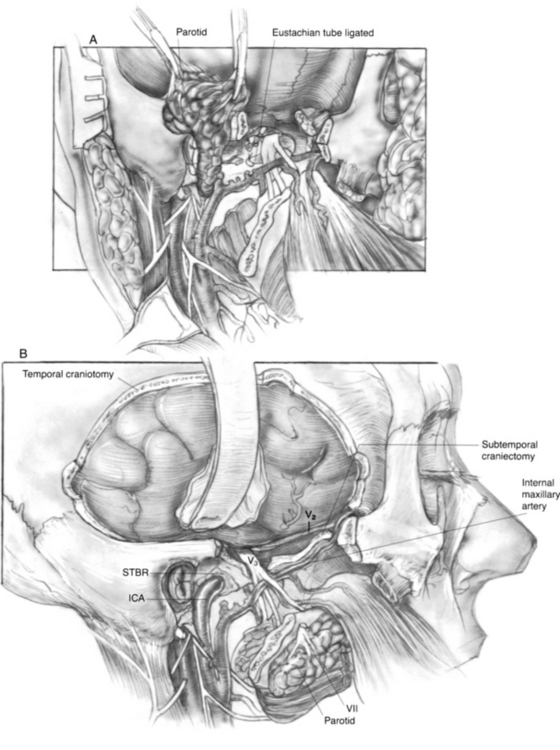
The stylohyoid, stylopharyngeus, and styloglossus muscles (Riolan’s bouquet) are detached from the styloid process, which is then rongeured away. The branches of the external carotid artery are dissected in the infratemporal fossa. The anterior tympanic and deep auricular branches of the internal maxillary artery are often divided before identification, and may require bipolar coagulation. The internal maxillary artery is preserved up to the branches of the deep temporal artery. When the internal maxillary artery must be sacrificed, brisk backflow from the anterior stump indicates that the temporalis muscle may derive its blood supply from reversed flow via the pterygoid system. If brisk backflow is not observed, the temporalis muscle cannot be relied on to reconstruct the surgical defect, and microvascular free flap options must be considered. The cartilaginous eustachian tube is divided, and the anterior end is sutured closed to prevent postoperative cerebrospinal fluid rhinorrhea (Fig. 4-3A).
The ICA is dissected toward the carotid canal, and care is taken not to injure CN IX, which crosses its anterior surface. Kerrison rongeurs are used to uncover the vertical and horizontal petrous segments of the carotid artery (Fig. 4-3B). Occasionally, bleeding from the pericarotid venous plexus requires bipolar coagulation. The caroticotympanic artery is also divided when the petrous carotid artery is separated from the specimen. The extent of petrous carotid mobilization depends on whether STBR or TTBR is performed. When STBR is performed, the vertical petrous carotid artery is mobilized from the carotid foramen and canal. When TTBR is performed, the vertical and horizontal petrous carotid artery is mobilized out of the carotid canal to the foramen ovale.
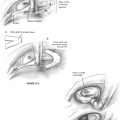
A temporal craniectomy is performed, and the intracranial portion of the middle meningeal vessels is coagulated (see Fig. 4-3B). The patient is hyperventilated to keep the Pco2 at 25 mm Hg for adequate brain relaxation. Mannitol and furosemide can improve brain relaxation. Subtemporal dural elevation proceeds in a posteroanterior direction. The greater superficial petrosal nerve and accompanying petrosal artery are coagulated and divided to lessen traction on the geniculate ganglion. The lesser petrosal nerve and superior tympanic artery are similarly divided. Subtemporal dural elevation proceeds as far medially as possible to expose the superior petrosal sinus. When carcinomatous involvement of the middle fossa dura is suspected, an intradural approach keeps the involved dura attached to the specimen.
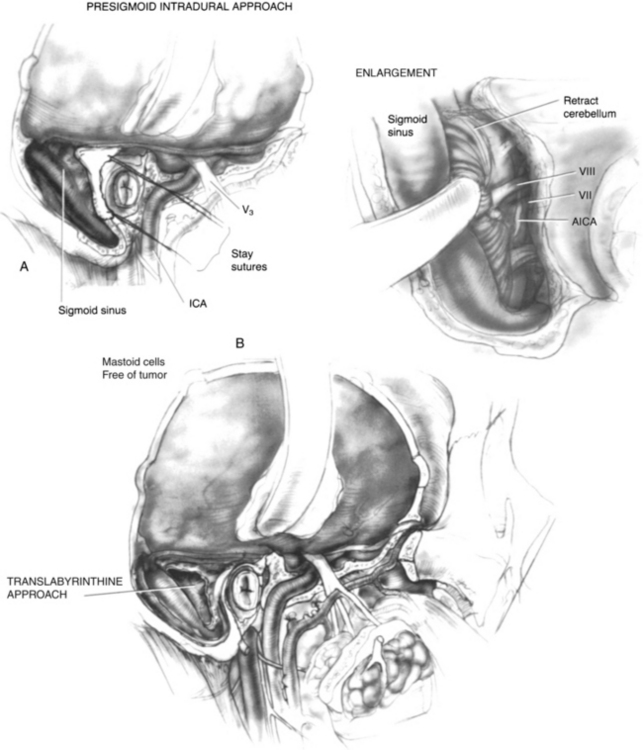
The extent of the mastoidectomy depends on whether tumor is present in the mastoid air cells. Care is taken to avoid exposure of tumor in the mastoid. In this case, the confluence of the transverse, sigmoid, and superior petrosal sinuses is decorticated to expose posterior fossa dura on either side of the sigmoid sinus. When posterior fossa dural involvement is suspected, a presigmoid intradural approach with gentle retraction of the cerebellum (Fig. 4-4A) allows exposure of the vessels and nerves in the cerebellopontine angle that are keeping the involved dura attached to the specimen. When the mastoid air cells are thought to be free of tumor, a translabyrinthine approach to the IAC is used (Fig. 4-4B). The anterior inferior cerebellar artery is retracted after the labyrinthine artery is bipolarly coagulated and divided. The superior and inferior vestibular nerves, cochlear nerve, facial nerve, and nervus intermedius (nerve of Wrisberg) are divided. A segment of the proximal facial nerve stump can be sent for frozen section pathologic examination if it is suspicious for carcinoma. The dome of the jugular bulb must be separated from the specimen when the dural venous sinuses are spared.
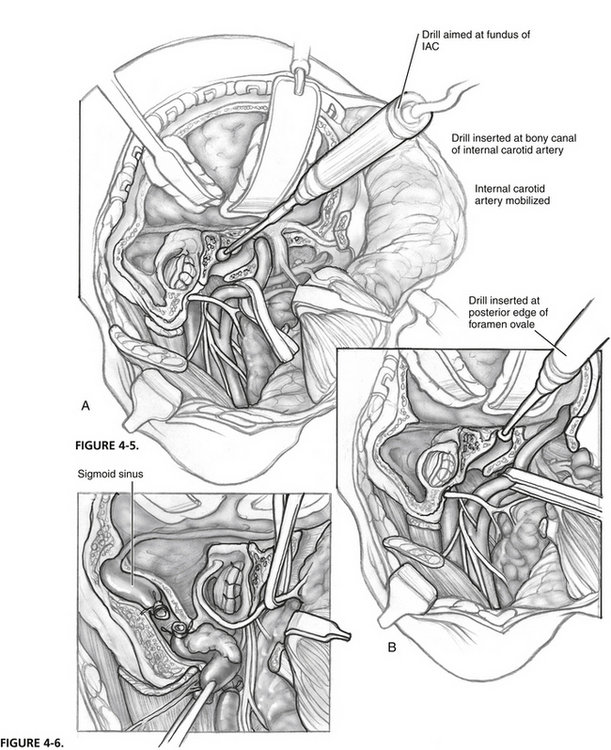
The surgical technique from here varies according to whether the dural venous sinuses or the ICA is preserved. When both are preserved, and STBR is being performed, the bone between the bony canal of the carotid artery at the junction of the vertical and horizontal segments (Fig. 4-5A) and the fundus of the IAC is removed with a high-powered drill. Separation of the petro-occipital synchondrosis sometimes requires insertion of an osteotome just above the jugular bulb. This step releases the specimen en bloc from attachment to the clivus. Packing oxidized cellulose intraluminally toward the cavernous sinus controls bleeding from the inferior petrosal sinus.
When both vascular structures are preserved, and TTBR is being performed, the bone between the posterior edge of the foramen ovale and spheno-occipital synchondrosis is removed (Fig. 4-5B). The petro-occipital synchondrosis may require an additional osteotomy before the specimen is delivered.
When the dural venous sinuses are sacrificed, the internal jugular vein is double ligated in the upper cervical area and mobilized toward the jugular bulb (Fig. 4-6). The superior petrosal sinus and sigmoid sinus are divided and intraluminally packed with oxidized cellulose. Care is taken not to overpack the sigmoid sinus proximally to avoid obstruction of the vein of Labbé; this can cause hemorrhagic necrosis of the temporal lobe. Similarly, excessive packing of the inferior petrosal sinus can lead to cavernous sinus thrombosis. The sigmoid sinus is opened toward the jugular bulb and resected. Intraluminal rather than extraluminal packing of the inferior petrosal sinus is preferred to avoid injury to cranial nerves traversing the jugular foramen.
REHABILITATION
Excessive packing of the inferior petrosal sinus can lead to lower cranial nerve dysfunction (CN IX, X, and XI) that can be debilitating. Some patients require temporary tracheostomy and gastrostomy, and others can be treated with polytetrafluoroethylene (Teflon) injection of the vocal fold or an Isshiki thyroplasty.4
RESULTS
Temporal bone neoplasms occur infrequently. The limited experience makes analysis of treatment difficult. Because of this difficulty, we reviewed the literature for all cases of squamous cell carcinoma of the temporal bone,5 analyzed the extent of disease present, studied the different treatment strategies employed, and reported on outcome. Our findings are presented.
All publications in the English language from 1915 to 1992 dealing with the treatment of squamous cell carcinoma of the temporal bone were reviewed; 96 publications1,6–100 were encountered, of which 26 articles75–100 contained enough information on 144 patients. Various parameters were then analyzed. The major reason for exclusion of a study was the lack of a descriptive table in which the extent of disease, type of treatment, and follow-up for each patient were carefully documented.
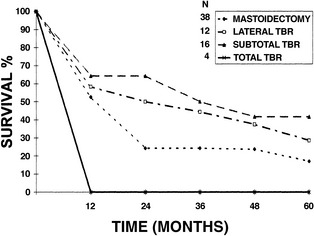
Several conclusions about overall survival could be made. When disease was confined to the EAC, no statistically significant difference in 5-year survival was found between patients treated with LTBR (48.6%) and patients treated with STBR (50%). When disease extended into the middle ear, patients who had STBR had better 5-year survival (41.7%) than patients who had LTBR (28.6%) (Fig. 4-7). The experience with carcinoma that invaded the petrous apex was limited. Four patients treated with TTBR had a 50% 1-year survival and 0% 2-year survival. One patient treated with STBR was dead of disease at 1 year.
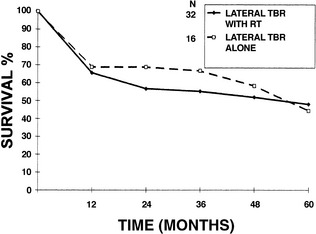
The value of preoperative or postoperative radiation therapy was also analyzed. When disease was confined to the EAC, the addition of either preoperative or postoperative radiation therapy to LTBR did not significantly improve 5-year survival (48% with radiation therapy, 44.4% without radiation therapy) (Fig. 4-8). This was the only group in which a conclusion regarding radiation treatment could be made.
SUMMARY
The indications for the operation are slowly evolving as we gain experience and collate data for analysis. From our literature review regarding squamous cell carcinoma,5 it seems that cancerous involvement of the middle ear is best treated by STBR rather than LTBR and gross removal of middle ear disease. When the tumor involves the petrous apex, we believe that TTBR can allow total tumor extirpation and possibly prolonged survival, although the latter remains to be proved. Prospective, randomized studies are needed to define the value of margin-free dural, carotid artery, and brain parenchymal resection. The value of adjunctive radiation therapy for extensive lesions also requires further study.
1. Johns M.E., Headington J.T. Squamous cell carcinoma of the external auditory canal: A clinicopathologic study of 20 cases. Arch Otolaryngol Head Neck Surg. 1974;100:45-49.
2. Kinney S.E. Tumors of the external auditory canal, middle ear, mastoid, and temporal bone. In: Thawley S.E., Panje W.R., Batsakis J.G., Lindberg R.D., editors. Comprehensive Management of Head and Neck Tumors. Philadelphia: Saunders; 1987:p 182.
3. Janecka I.P., Sekhar L.N., Horton J.A., Yonas H., et al. Cerebral blood flow evaluation. In: Cummings C.W., Frederickson J.M., Harlor L.A., editors. Otolaryngology Head and Neck Surgery: Update II. St Louis: Mosby–Year Book; 1990:54-63.
4. Sasaki C.T., Leder S.B., Petcu L., Freidman C.D. Longitudinal voice quality changes following Isshiki thyroplasty type I: The Yale experience. Laryngoscope. 1990;100:849-852.
5. Prasad S., Janecka I.P. Efficacy of surgical treatments for squamous cell carcinoma of the temporal bone. Otol Head Neck Surg. 1994;110:270-280.
6. Campbell E., Volk B.M., Burkland C.W. Total resection of the temporal bone for malignancy of the middle ear. Ann Surg. 1951;134:397-404.
7. Coleman C.C., Khuri A. A rational treatment for advanced cancer of the external ear and temporal bone. VA Med Monthly. 1959;86:21-24.
8. Hutcheon J.R. Experiences with aural carcinoma over the past six years. Med J Aust. 1966;2:406-407.
9. Figi F.A., Weisman P.A. Cancer and chemodectoma in the middle ear and mastoid. JAMA. 1954;156:1157-1162.
10. Wang C.C. Radiation therapy in the management of carcinoma of the external auditory canal, middle ear, or mastoid. Ther Radiat. 1975;116:713-715.
11. Sinha P.P., Aziz H.I. Treatment of carcinoma of the middle ear. Ther Radiat. 1978;126:485-487.
12. Boland J. The management of carcinomas of the middle ear. Radiology. 1963;80:285.
13. Holmes K.S. The treatment of carcinoma of the middle ear by the 4-MV linear accelerator. Proc R Soc Med. 1960;53:242-244.
14. Yamada S., Schuh F.D., Harvin J.S., Perot P.L. En bloc subtotal temporal bone resection for cancer of the external ear. J Neurosurg. 1973;39:370-379.
15. Hahn S.S., Kim J.A., Goodchild N., Constable W.C. Carcinoma of the middle ear and external auditory canal. Int J Radiat Oncol Biol Phys. 1983;9:1003-1007.
16. Sorenson H. Cancer of the middle ear and mastoid. Acta Radiol. 1960;54:460-468.
17. Frazer J.S. Malignant disease of the external acoustic meatus and middle ear. Proc R Soc Med. 1930;23:1235-1244.
18. Barnes E.B. Carcinoma of the ear. Proc R Soc Med. 1930;23:1231-1234.
19. Conley J.S. Cancer of the middle ear and temporal bone. N Y State J Med. 1974;74:1575-1579.
20. Kinney S.E., Wood B.G. Malignancies of the external ear canal and temporal bone: Surgical techniques and results. Laryngoscope. 1987;97:158-164.
21. Kinney S.E. Squamous cell carcinoma of the external auditory canal. Am J Otol. 1989;10:111-116.
22. Graham M.D., Sataloff R.T., Kemink J., McGillicuddy J.F. En bloc resection of the temporal bone and carotid artery for malignant tumors of the ear and temporal bone. Laryngoscope. 1984;94:528-533.
23. Clark L.J., Narola A.A., Morgan D.A.L., Bradley P.J. Squamous carcinoma of the temporal bone: A revised staging. J Laryngol Otol. 1991;105:346-348.
24. Corey J.P., Nelson E., Crawford M., et al. Metastatic vaginal carcinoma to the temporal bone. Am J Otol. 1991;12:128-131.
25. Hiraide F., Inonye T., Ishii T. Primary squamous cell carcinoma of the middle ear invading the cochlea: A histopathologic case report. Ann Otol Rhinol Laryngol. 1983;92:290-294.
26. Schusterman M.A., Kroll S.S. Reconstruction strategy for temporal bone and lateral facial defects. Ann Plastic Surg. 1983;26:233-294.
27. Haughey B.H., Gates G.A., Skerhut H.E., Brown W.E. Cerebral shift after lateral craniofacial resection and flap reconstruction. Otolaryngol Head Neck Surg. 1989;101:79-86.
28. Bergetedt H.F., Lind M.G. Temporal bone scintigraphy. Acta Otolaryngol (Stockh). 1980;89:465-473.
29. Jahn A.F., Farkashidy J., Berman J.M. Metastatic tumors in the temporal bone—a pathophysiologic study. J Otolaryngol. 1979;8:85-95.
30. Ruben R.J., Thaler S.U., Holzer N. Radiation-induced carcinoma of the temporal bone. Laryngoscope. 1977;87:1613-1621.
31. Katsarkas A., Seemayer T.A. Bilateral temporal bone metastases of a uterine cervix carcinoma. J Otolaryngol. 1976;5:315-318.
32. Ramsden R.T., Bulman C.H., Lorigan B.P. Osteoradionecrosis of the temporal bone. J Laryngol Otol. 1975;89:941-955.
33. Vize G. Laryngeal metastasis to the temporal bone causing facial paralysis. J Laryngol Otol. 1974;88:175-177.
34. Schuknecht H.F., Allam A.F., Murakami Y. Pathology of secondary malignant tumors of the temporal bone. Ann Otol Rhinol Laryngol. 1968;77:5-22.
35. Lewis J.S. Temporal bone resection: Review of 100 cases. Arch Otolaryngol Head Neck Surg. 1975;101:23-25.
36. Arriaga M., Curtin H., Takahashi H., et al. Staging proposal for external auditory meatus carcinoma based on preoperative clinical examination and computed tomography findings. Ann Otol Rhinol Laryngol. 1990;99:714-721.
37. Adams W.S., Morrison R. On primary carcinoma of the middle ear and mastoid. J Laryngol Otol. 1955;69:115-131.
38. Gacek R.R. Management of temporal bone carcinoma. Trans Penn Acad Otolaryngol Ophthalmol. 1978;32:67-71.
39. Conley J., Schuller D.E. Malignancies of the ear. Laryngoscope. 1976;86:1147-1163.
40. Greer J.A., Body D.T.R., Weiland L.H. Neoplasms of the temporal bone. J Otolaryngol. 1978;5:391-398.
41. Goodman M.L. Middle ear and mastoid neoplasms. Ann Otol Rhinol Laryngol. 1971;80:419-424.
42. Arena S., Keen M. Carcinoma of the middle ear and temporal bone. Am J Otol. 1988;9:351-356.
43. Hilding D.A., Selker R. Total resection of the temporal bone for carcinoma. Arch Otolaryngol Head Neck Surg. 1969;89:98-107.
44. Cundy R.L., Sando I., Hemenway W.G. Middle ear extension of nasopharyngeal carcinoma via the eustachian tube. Arch Otolaryngol Head Neck Surg. 1973;98:131-133.
45. Lewis J.S. Squamous carcinoma of the ear. Arch Otolaryngol Head Neck Surg. 1973;97:41-42.
46. Sekhar L.N., Pomeranz S., Janecka I.P., et al. Temporal bone neoplasms: A report on 20 surgically treated cases. J Neurosurg. 1992;76:578-587.
47. Lessor R.W., Spector G.J., Divinens V.R. Malignant tumors of the middle ear and external auditory canal: A 20-year review. Otolaryngol Head Neck Surg. 1987;96:43-47.
48. Kenyon G.S., Marks P.V., Scholtz C.L., Dhillon R. Squamous cell carcinoma of the middle ear: A 25-year retrospective study. Ann Otol Rhinol Laryngol. 1985;94:273-277.
49. Arthur K. Radiotherapy in carcinoma of the middle ear and auditory canal. J Laryngol Otol. 1976;90:753-762.
50. Tucker W.N. Cancer of the middle ear. Cancer. 1965;16:642-650.
51. Conley J.J., Novack A.J. The surgical treatment of malignant tumors of the ear and temporal bone. Arch Otolaryngol Head Neck Surg. 1960;71:635-652.
52. Conley J.J., Novack A.J. Surgical treatment of cancer of the ear and temporal bone. Trans Am Acad Ophthalmol Otolaryngol. 1960;64:83-92.
53. Wagenfield D.J.H., Keane T., Norstrand A.W.P., Bryce D.P. Primary carcinoma involving the temporal bone: Analysis of 25 cases. Laryngoscope. 1980;90:912-919.
54. Lewis J.S., Parsons H. Surgery for advanced ear cancer. Ann Otol Rhinol Laryngol. 1958;67:364-399.
55. Lewis J.S., Page R. Radical surgery for malignant tumors of the ear. Arch Otolaryngol Head Neck Surg. 1966;83:56-61.
56. Lewis J.S. Surgical management of tumors of the middle ear and mastoid. J Laryngol Otol. 1983;97:299-311.
57. Lederman M. Malignant tumors of the ear. J Laryngol Otol. 1965;79:85-119.
58. Frew I., Finney R. Neoplasms of the middle ear. J Laryngol Otol. 1963;77:415-421.
59. Goodwin W.J., Jesse R.H. Malignant neoplasms of the external auditory canal and temporal bone. Arch Otolaryngol Head Neck Surg. 1980;106:675-679.
60. Lindahl J.W.S. Carcinoma of the middle ear and meatus. J Laryngol Otol. 1955;69:457-467.
61. Bradley W.H., Maxwell J.H. Neoplasms of the middle ear and mastoid: Report of 54 cases. Laryngoscope. 1954;54:533-556.
62. Miller D. Cancer of the external auditory meatus. Laryngoscope. 1955;65:448-461.
63. Colledge L. Two cases of malignant disease of the temporal bone. J Laryngol Otol. 1943;58:251-254.
64. Peele J.C., Hauser G.H. Primary carcinoma of the external auditory canal and middle ear. Arch Otolaryngol Head Neck Surg. 1941;34:254-266.
65. Garnett-Passe E.R. Primary carcinoma of the eustachian tube. J Laryngol Otol. 1948;62:314-315.
66. Spencer F.R. Malignant disease of the ear. Arch Otolaryngol Head Neck Surg. 1938;28:916-940.
67. Means R.G., Gersten J. Primary carcinoma of the mastoid process. Ann Otol Rhinol Laryngol. 1953;62:93-100.
68. Spector J.G. Management of temporal bone carcinomas: A therapeutic analysis of two groups of patients and long-term follow-up. Otolaryngol Head Neck Surg. 1991;104:58-66.
69. Ariyan S., Sasaki C.T., Spencer D. Radical en bloc resection of the temporal bone. Am J Surg. 1981;142:443-447.
70. Arena S. Tumor surgery of the temporal bone. Laryngoscope. 1974;84:645-670.
71. Brooker G.B. Bilateral middle ear carcinomas associated with Waldenström’s macroglobulinemia. Ann Otol Rhinol Laryngol. 1982;91:299-303.
72. Towson C.E., Shofstall W.H. Carcinoma of the ear. Arch Otolaryngol Head Neck Surg. 1950;51:724-738.
73. Robinson G.A. Malignant tumors of the ear. Laryngoscope. 1931;41:467-473.
74. Kinney S.E., Wood B.G. Malignancies of the external ear canal and temporal bone: Surgical techniques and results. Laryngoscope. 1987;97:158-164.
75. Buckmann L.T., Barre W. Carcinoma of the middle ear and mastoid. Ann Otol Rhinol Laryngol. 1943;52:194-201.
76. Liebeskind M.M. Primary carcinoma of the external auditory canal, middle ear, and mastoid. Laryngoscope. 1951;61:1173-1187.
77. Stokes H.B. Primary malignant tumors of the temporal bone. Arch Otolaryngol Head Neck Surg. 1990;32:1023-1030.
78. Rosenwasser H. Neoplasms involving the middle ear. Arch Otolaryngol Head Neck Surg. 1940;32:38-53.
79. Grossman A.A., Donnelly W.A., Smithman M.F. Carcinoma of the middle ear and mastoid process. Ann Otol Rhinol Laryngol. 1947;56:709-721.
80. Mattick W.L., Mattick J.W. Some experience in management of cancer of the middle ear and mastoid. Arch Otolaryngol Head Neck Surg. 1951;53:610-621.
81. Wahl J.W., Gromet M.T. Carcinoma of the middle ear and mastoid. Arch Otolaryngol Head Neck Surg. 1953;58:121-126.
82. Crabtree J.A., Britton B.H., Pierce M.K. Carcinoma of the external auditory canal. Laryngoscope. 1976;86:405-415.
83. Hanna D.C., Richardson G.S., Gaisford J.C. A suggested technique for resection of the temporal bone. Am J Surg. 1967;114:553-558.
84. Adams G.L., Paparella M.M., Fiky F.M. Primary and metastatic tumors of the temporal bone. Laryngoscope. 1971;81:1273-1285.
85. Sataloff R.T., Myers D.L., Lowry L.D., Spiegel J.R. Total temporal bone resection for squamous cell carcinoma. Otolaryngol Head Neck Surg. 1987;96:4-14.
86. Nadol J.B., Schoknecht H.F. Obliteration of the mastoid in the treatment of tumors of the temporal bone. Ann Otol Rhinol Laryngol. 1984;93:6-12.
87. Arriaga M., Hirsch B.E., Kamerer D.B., Myers E.N. Squamous cell carcinoma of the external auditory meatus (canal). Otolaryngol Head Neck Surg. 1989;101:330-337.
88. Michaels L., Wells M. Squamous cell carcinomas of the middle ear. Clin Otolaryngol. 1980;5:235-248.
89. McCrea R.S. Radical surgery for carcinoma of the middle ear. Laryngoscope. 1972;82:1514-1523.
90. Gacek R.R., Goodman M. Management of malignancy of the temporal bone. Laryngoscope. 1977;87:1622-1634.
91. Scholl L.A. Neoplasms involving the middle ear. Arch Otolaryngol Head Neck Surg. 1935;22:548-553.
92. Clairmont A.A., Conley J.J. Primary carcinoma of the mastoid bone. Ann Otol Rhinol Laryngol. 1977;86:306-309.
93. Beal D.D., Lindsay J.R., Ward P.H. Radiation-induced carcinoma of the mastoid. Arch Otolaryngol Head Neck Surg. 1965;81:9-16.
94. Coleman C.C. Removal of the temporal bone for cancer. Am J Surg. 1966;112:583-590.
95. Parsons H., Lewis J.S. Subtotal resection of the temporal bone for cancer of the ear. Cancer. 1954;7:995-1001.
96. Miller D., Silverstein H., Gacek R.R. Cryosurgical treatment of carcinoma of the ear. Trans Am Acad Ophthalmol Otolaryngol. 1972;76:1363-1367.
97. Tabb H.G., Komet H., McLaurin J.W. Cancer of the external auditory canal: Treatment with radical mastoidectomy and irradiation. Laryngoscope. 1964;74:634-643.
98. Lodge W.O., Jones H.M., Smith M.E.N. Malignant tumors of the temporal bone. Arch Otolaryngol Head Neck Surg. 1955;61:535-541.
99. Ward G.E., Loch W.E., Lawrence W. Radical operation for carcinoma of the external auditory canal and middle ear. Am J Surg. 1951;82:169-178.
100. Newhart H. Primary carcinoma of the middle ear: Report of a case. Laryngoscope. 1917;27:543-555.