CHAPTER 76 Male reproductive system
The male reproductive system consists of the spermatic cord and scrotum, testes and epididymes, vas deferens, prostate, seminal vesicles and penis.
SPERMATIC CORD
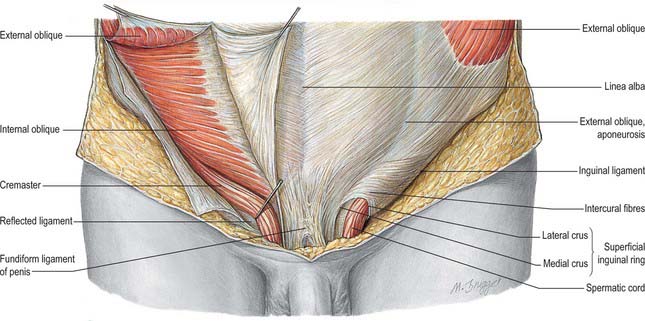
As the testis traverses the abdominal wall into the scrotum during early life, it carries its vessels, nerves and vas deferens with it. These meet at the deep inguinal ring to form the spermatic cord, which suspends the testis in the scrotum and extends from the deep inguinal ring to the posterior aspect of the testis (Fig. 76.1). The left cord is a little longer than the right. Between the superficial ring and testis the cord is anterior to the tendon of adductor longus. It is crossed anteriorly by the superficial external pudendal artery and posteriorly by the deep external pudendal artery. The ilioinguinal nerve lies inferior to the cord as it traverses the inguinal canal.
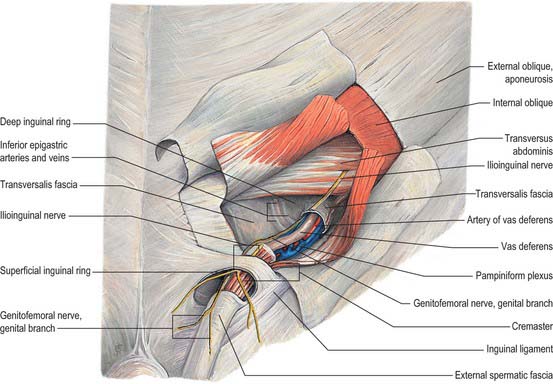
In the canal the cord acquires coverings from the layers of the abdominal wall that extend into the scrotal wall as the internal spermatic, cremasteric and external spermatic fasciae (Fig. 76.2). The internal spermatic fascia is derived from the transversalis fascia and forms a thin, loose layer around the spermatic cord. The cremasteric fascia contains fasciculi of skeletal muscle united by loose connective tissue to form the cremaster muscle which is continuous with internal oblique. The external spermatic fascia descends from the crura of the superficial ring and is a thin fibrous stratum continuous above with the aponeurosis of external oblique. The spermatic cord contains the vas deferens; the testicular artery and veins, cremasteric artery (a branch of the inferior epigastric artery) and artery to the vas deferens (from the superior vesical artery); the genital branch of the genitofemoral nerve and cremasteric nerve and the sympathetic components of the testicular plexus (which are joined by filaments from the pelvic plexus accompanying the artery to the vas deferens); 4–8 lymph vessels draining the testis. All of these structures are conjoined by loose connective tissue (Figs 76.2, 76.3A).
SCROTUM
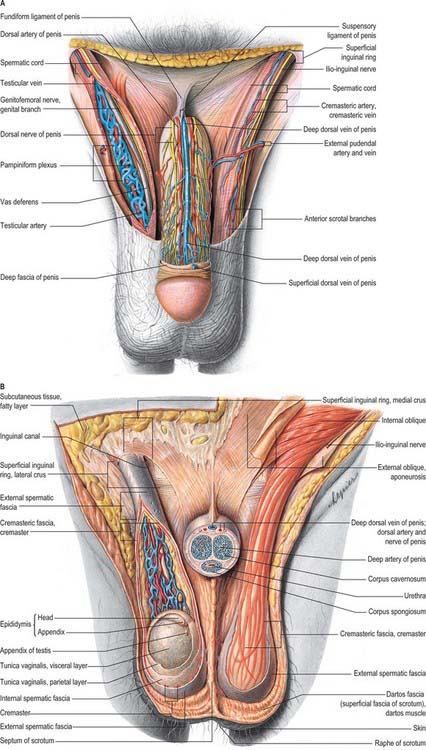
The scrotum is a cutaneous fibromuscular sac containing the testes and lower parts of the spermatic cords and hangs below the pubic symphysis between the anteromedial aspects of the thighs. It consists of skin, dartos muscle and external spermatic, cremasteric and internal spermatic fasciae. The internal spermatic fascia is loosely attached to the parietal layer of the tunica vaginalis (Fig. 76.3B). The scrotum is divided into right and left halves by a cutaneous raphe, which continues ventrally to the inferior penile surface and dorsally along the midline of the perineum to the anus. The raphe indicates the bilateral origin of the scrotum from the genital swellings. The left side of the scrotum is usually lower because the left spermatic cord is longer.
Vascular supply and lymphatic drainage
The arteries supplying the scrotum include the external pudendal branches of the femoral artery, the scrotal branches of the internal pudendal artery, and a cremasteric branch from the inferior epigastric artery. Dense subcutaneous plexuses of scrotal vessels carry a substantial blood flow, facilitating heat loss. Arteriovenous anastomoses of a simple but large-calibre type are also prominent. The veins follow the corresponding arteries. The skin of the scrotum is drained by vessels that accompany the external pudendal blood vessels to the superficial inguinal nodes (Fig. 76.3A).
TESTES AND EPIDIDYMIS
TESTES
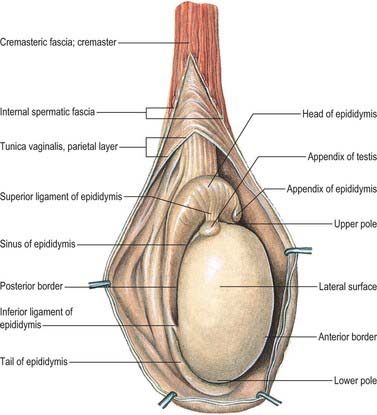
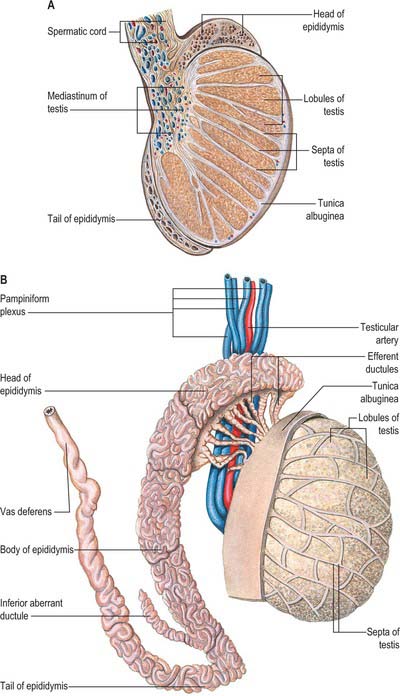
The testes are the primary reproductive organs or gonads in the male. They are ovoid, reproductive and endocrine organs responsible for sperm production and testosterone production. They are suspended in the scrotum by scrotal tissues including the dartos muscle and spermatic cords. Average testicular dimensions are 4–5 cm in length, 2.5 cm in breadth and 3 cm in anteroposterior diameter; their weight varies from 10.5–14 g. The left testis usually lies lower than the right testis. Each testis lies obliquely within the scrotum, the upper pole tilted anterolaterally and the lower posteromedially. The anterior aspect is convex, whereas the posterior aspect is nearly straight and has the spermatic cord attached to it. Anterior, medial and lateral surfaces and both poles are convex, smooth and covered by the visceral layer of the serosal tunica vaginalis, the parietal layer and the scrotal tissues, in that order from within outwards (Fig. 76.4). Each testis is separated from its fellow by a fibrous median raphe which is deficient superiorly. The posterior aspect of each testis is only partly covered by serosa; the epididymis adjoins its lateral part (see below). The testis is invested by three coats, which are, from outside inwards, the tunica vaginalis, tunica albuginea and tunica vasculosa (Fig. 76.4).
Tunica vaginalis
The tunica vaginalis is the lower end of the peritoneal processus vaginalis, whose formation precedes the descent of the fetal testis from the abdomen to the scrotum (see p. 1320). After this migration, the proximal part of the tunica, from the internal inguinal ring almost to the testis, contracts and is obliterated, leaving a closed distal sac into which the testis is invaginated. The tunica is reflected from the testis onto the internal surface of the scrotum, so forming the visceral and parietal layers of the tunica. The visceral layer covers all aspects of the testis except most of the posterior aspect. Posteromedially it is reflected forwards to the parietal layer. Posterolaterally it passes to the medial aspect of the epididymis and lines the epididymal sinus, and then passes laterally to its posterior border where it is reflected forwards to become continuous with the parietal layer. The visceral and parietal layers are continuous at both poles, but at the upper pole the visceral layer surmounts the head of the epididymis before reflexion. There is always a very fine film of fluid between the two layers of the tunica vaginalis. This fluid layer can increase in inflammatory and neoplastic conditions of the testis, leading to a hydrocele (see below).
Tunica albuginea
The tunica albuginea is a dense, bluish-white covering for the testis, composed mainly of interlacing bundles of collagen fibres. It is covered externally by the visceral layer of the tunica vaginalis, except at the epididymal head and tail and the posterior aspect of the testis, where vessels and nerves enter (Fig. 76.5A,B). The tunica vaginalis covers the tunica vasculosa and, at the posterior border of the testis, projects into the testicular interior as a thick, incomplete fibrous septum, the mediastinum testis, which extends from the upper to the lower end of the testis. Testicular vessels run within the mediastinum testis.
Vascular supply and lymphatic drainage
Testicular arteries
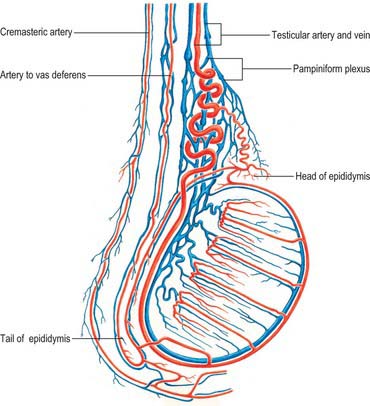
The testicular arteries are two long, slender vessels which arise anteriorly from the aorta a little inferior to the renal arteries. Each passes inferolaterally under the parietal peritoneum on psoas major. The right testicular artery lies anterior to the inferior vena cava and posterior to the horizontal part of the duodenum, right colic and ileocolic arteries, root of the mesentery and terminal ileum. The left testicular artery lies posterior to the inferior mesenteric vein, left colic artery and lower part of the descending colon. Each artery crosses anterior to the genitofemoral nerve, ureter and the lower part of the external iliac artery and passes to the deep inguinal ring to enter the spermatic cord and travel via the inguinal canal to enter the scrotum (Figs 76.3A, 76.6). At the posterosuperior aspect of the testis the testicular artery divides into two branches on its medial and lateral surfaces: these pass through the tunica albuginea and ramify in the tunica vasculosa. Terminal branches enter the testis over its surface. Some pass into the mediastinum testis and loop back before reaching their distribution. Capillaries lying next to seminiferous tubules penetrate the layers of interstitial tissue and may form part of the ‘blood–testis’ barrier. They run either parallel to the tubules or across them but do not enter their walls. They are separated from germinal and supporting cells by a basement membrane and variable amounts of fibrous tissue containing interstitial cells: selective exchange phenomena involving androgens and immune substances occur here.
The testis also receives blood from the cremasteric branch of the inferior epigastric artery, and from the artery to the vas deferens (Fig. 76.6). Interference with the testicular artery high in the abdomen therefore usually leaves the testis unharmed, whereas interruption in the region of the spermatic cord may interfere with all of these vessels and lead to infarction. Ligating both the testicular artery and vein high up interrupts the venae comitantes of the artery which anastomose with the internal spermatic veins and can be responsible for recurrence of a varicocele (see below).
Testicular veins
The testicular veins emerge posteriorly from the testis, drain the epididymis and unite to form the pampiniform plexus, a major component of the spermatic cord that ascends anterior to the vas deferens (Fig. 76.7). In the inguinal canal the pampiniform plexus is drained by three or four veins which run into the abdomen through the deep inguinal ring. Within the abdomen these veins coalesce into two veins, which ascend on each side of the testicular artery, anterior to psoas major and the ureter, and behind the peritoneum. The left veins pass behind the lower descending colon and inferior margin of the pancreas and are crossed by the left colic vessels, and the right veins pass behind the terminal ileum and horizontal part of the duodenum and are crossed by the root of the mesentery, ileocolic and right colic vessels. The veins join to form single right or left testicular veins: the right testicular vein opens into the inferior vena cava at an acute angle just inferior to the level of the renal veins, and the left testicular vein opens into the left renal vein at a right angle (Fig. 76.7). The testicular veins contain valves.
Microstructure
The surface of the testis is covered closely by the visceral tunica vaginalis, a layer of flat mesothelial cells similar to, and continuous with, the peritoneal lining. The visceral layer is separated from the parietal tunica vaginalis (the outer layer of the double fold of peritoneum that accompanies the descending testis (see p. 1320)), by a potential space containing serous fluid, which acts as a lubricant and allows movement of the testis within the scrotum. The testicular capsule proper, the tunica albuginea, is tough and collagenous and thickened posteriorly as the mediastinum testis. Beneath the tunica albuginea is a thin layer of connective tissue containing the superficial blood vessels. Blood vessels, lymphatics and the genital ducts enter or leave the body of the testis at the mediastinum (Fig. 76.8).
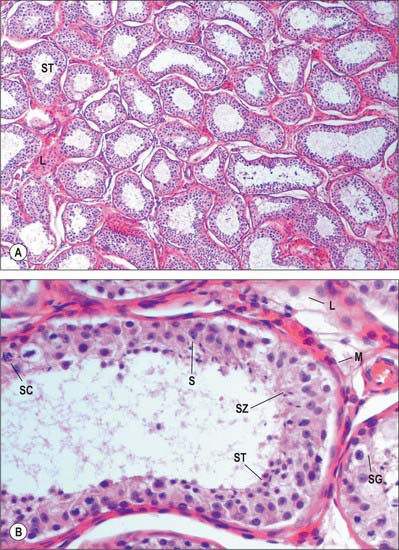
Septa from the mediastinum extend internally to partition the testis into approximately 250 lobules (Fig. 76.5A). These differ in size, and are largest and longest in the centre. Each lobule contains one to four convoluted seminiferous tubules, much-coiled loops whose free ends both open into channels (the rete testis) within the mediastinum. The loose connective tissue between seminiferous tubules contains several layers of contractile peritubular myoid cells and clusters of androgen-producing interstitial (Leydig) cells.
There are 400–600 seminiferous tubules in each testis, each 70–80 cm long and 0.12–0.3 mm. in diameter. They are pale in early life, but in old age they contain much fat and are deep yellow. Each tubule is surrounded by a basal lamina, on which rests a complex, stratified seminiferous epithelium containing spermatogenic cells and supportive Sertoli cells (Fig. 76.9A). When active, the spermatogenic cells include basally situated spermatogonia and their progeny in the adluminal compartment, spermatocytes, spermatids and mature spermatozoa. Among the spermatids may be residual bodies, spherical structures derived from surplus spermatid cytoplasm shed during maturation and phagocytosed by Sertoli cells.
Spermatogonia
Spermatogonia, the stem cells for all spermatozoa, are descended from primordial germ cells which migrate into the genital cords of the developing testis (Fig. 76.9B). In the fully differentiated testis they are located along the basal laminae of the seminiferous tubules. Several types of spermatogonia are recognized on the basis of cell and nuclear dimensions, distribution of nuclear chromatin (dark, condensed or pale, euchromatic) and histochemical and ultrastructural data. The three basic groups of spermatogonia are dark type A (Ad), pale type A (Ap), and type B. Ad cells divide mitotically to maintain the population of spermatogonia which, before puberty, is small but increases under androgenic stimulation. Some divisions give rise to Ap cells which also divide mitotically but remain linked in clusters by fine cytoplasmic bridges. These are the precursors of type B cells, which are committed to the spermatogenic sequence. At about the time that type B cells enter a final round of DNA synthesis, without undergoing cytokinesis, they leave the basal compartment and cross the blood–testis barrier to enter meiotic prophase as primary spermatocytes. These coordinated processes are under the control of Sertoli cells.
Primary and secondary spermatocytes
Primary spermatocytes have a diploid chromosome number but duplicated sister chromatids (DNA content is thus 4N, where N is the DNA content of haploid spermatozoa), and are all at some stage of a long meiotic prophase (p. 22) of approximately 3 weeks. Primary spermatocytes are large cells with large round nuclei in which the nuclear chromatin is condensed into dark, threadlike, coiled chromatids at different stages in the process of crossing over and genetic exchange between chromatids of maternal and paternal homologues. These cells give rise to secondary spermatocytes with a haploid chromosome complement (but 2N DNA content), the reduction division is designated as meiosis I. Few secondary spermatocytes are seen in tissue sections because they rapidly undergo the second meiotic (equatorial) division, where sister chromatids separate (DNA content now being N), to form haploid spermatids. Theoretically each primary spermatocyte produces four spermatids, but some degenerate during maturation so that the yield is lower.
Spermatids
Spermatids do not divide again but gradually mature into spermatozoa by a series of nuclear and cytoplasmic changes known as spermiogenesis. All of these maturational changes take place while the spermatids remain closely associated with Sertoli cells and linked by cytoplasmic bridges with each other. The first phase of spermiogenesis is the Golgi phase, when hydrolytic enzymes accumulate in Golgi vesicles that subsequently coalesce into a single large acrosomal vesicle close to the nucleus. The pair of centrioles migrates to the opposite posterior pole. The distal centriole begins to generate the axoneme, a circular arrangement of nine microtubule doublets surrounding a central pair. In the cap phase, the acrosomal vesicle flattens and envelops the anterior half of the nucleus to form an acrosomal cap which comes to occupy the presumptive anterior pole of the spermatozoon, furthest from the lumen of the tubule.
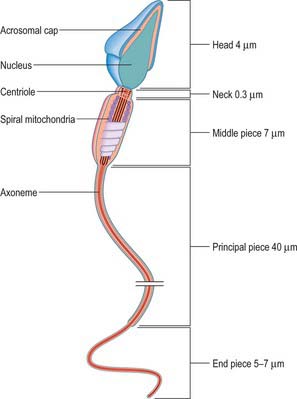
Spermatozoa
A spermatozoon that is released from the wall of the seminiferous tubule into the lumen is non-motile but structurally mature. Its expanded head contains little cytoplasm and is connected by a short constricted neck to the tail. The tail is a complex flagellum, divided into middle, principal and end pieces, that greatly exceeds the head region in volume. The head has a maximum length of approximately 4 μm and a maximum diameter of 3 μm. It contains an elongated, flattened nucleus with condensed, deeply staining chromatin, and is covered anteriorly by an acrosomal cap. The latter contains acid phosphatase, hyaluronidase, neuraminidase and proteases necessary for fertilization (see p. 168). The neck is approximately 0.3 μm long. In its centre is a well-formed centriole that corresponds to the proximal centriole of the spermatid from which it differentiated. The axonemal complex is derived from the distal centriole. A small amount of cytoplasm exists in the neck, covered by a plasma membrane continuous with that of the head and tail (Fig. 76.10).
Sertoli cells
The Sertoli cell nucleus is euchromatic and irregular or pear-shaped, contains one or two prominent nucleoli, and is usually aligned perpendicular to the basal lamina. The cytoplasm is rich in lysosomes, consistent with its phagocytic phenotype. Sertoli cells provide trophic support for the surrounding germ cells, secrete androgen-binding protein and play an important role in controlling spermatocyte and spermatid differentiation and maturation. The proteinaceous fluid they secrete into the tubule lumen provides nutrients and facilitates the transport of spermatozoa into the excurrent duct system. Sertoli cells change considerably during the spermatogenic cycle and respond to the hypophysial hormones, luteinizing hormone (LH) and follicle-stimulating hormone (FSH). They also produce a hormonal substance, inhibin B, which is thought to be involved in local paracrine functions.
Undescended testis
In the early fetal period the testes are located posteriorly in the abdominal cavity. Their descent to the scrotum appears to be under hormonal control (gonadotropins and androgens) (see p. 1320). Testicular descent may be arrested at any point along its route into the scrotum: a clinically undescended testis may be in the abdomen, at the deep inguinal ring, in the inguinal canal, or between the superficial inguinal ring and the scrotum. Occasionally, the testis may lie outside its normal path of descent and is termed an ectopic testis.
Testicular and epididymal appendices
At the upper extremities of the testis and epididymis are two small, stalked bodies, the appendix testis and appendix epididymis (Fig. 76.5A,B). They are developmental remnants of the paramesonephric (Müllerian) duct and the mesonephros, respectively. They may undergo torsion and produce symptoms that are difficult to differentiate from testicular torsion.
Male sub-fertility
Testicular failure is usually testicular in origin. It may have an underlying genetic aetiology, or may occur secondary to damage to the testis such as occurs following radiation therapy, torsion, trauma to the testis or undescended testis. Testicular failure resulting from these conditions may be associated with an increase in pituitary FSH and LH (hypergonadotrophic hypogonadism). Rarely, testicular failure may be the result of pituitary or hypothalamic damage leading to hypogonadotrophic hypogonadism. Histopathologically, testicular failure may be characterized by a spectrum of histopathological findings from Sertoli-cell-only syndrome (see Fig. 76.12) to varying degrees of hypospermatogenesis. Testicular sperm can be extracted from some men and injected into their partners’ eggs, a process known as intra-cytoplasmic sperm injection (ICSI).
EPIDIDYMIS
The epididymis lies posteriorly and slightly lateral to the testis, and the vas deferens lies along its medial side (Fig. 76.5A,B). It has an expanded head or globus major superiorly, a body (corpus) and a tail (cauda or globus minor). The epididymis is invested by tunica vaginalis, somewhat less closely applied than it is to the testis, except at its posterior margin. Laterally there is a deep groove, the sinus epididymis, between the epididymis and the testis. The vas deferens ascends from the tail of the epididymis to the deep inguinal ring, lying within the spermatic cord (see below).
Microstructure
Spermatogenesis occurs in the highly coiled parts of the seminiferous tubules. As the latter reach the apical part of the lobule towards the mediastinum they become much less convoluted and form the short tubuli recti, lined by cuboidal epithelium that lacks spermatogenic cells. Tubuli recti enter the fibrous tissue of the mediastinum testis as a close network of anastomosing tubes, the rete testis, lined by a flat epithelium. At the upper pole of the mediastinum, 12–20 efferent ductules (ductuli efferentes) perforate the tunica albuginea and leave the testis for the epididymis. The efferent ductules are lined by a ciliated columnar epithelium that also contains shorter, actively endocytic, non-ciliated cells. External to the epithelium, the ductules are surrounded by a thin circular coat of smooth muscle (Fig. 76.11).
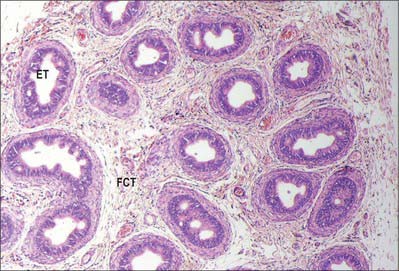
Fig. 76.11 Microstructure of the epididymis. ET, epididymal tubule; FCT, fibromuscular connective tissue.
VAS DEFERENS, PARADIDYMIS AND EJACULATORY DUCT
VAS DEFERENS
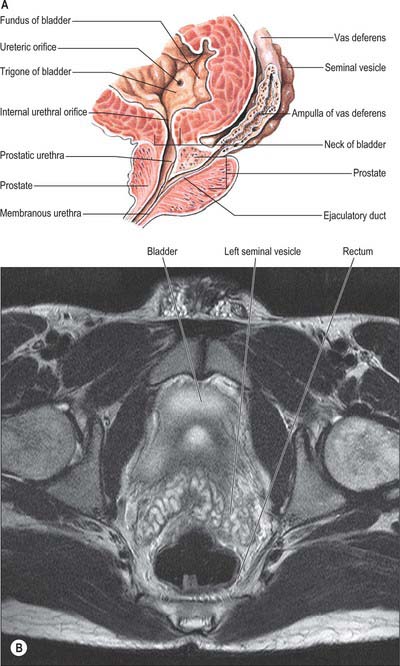
The vas deferens is the distal continuation of the epididymis, starting as a muscular tube at the epididymal tail. It is 45 cm long and conveys sperm to the ejaculatory ducts (Fig. 76.5A,B). Initially very tortuous, the convoluted portion of the vas becomes straighter as it ascends along the posterior aspect of the testis, medial to the epididymis. From the superior pole of the testis it ascends in the posterior part of the spermatic cord, and traverses the inguinal canal. At the deep inguinal ring the vas deferens leaves the cord, curves round the lateral side of the inferior epigastric artery and ascends for approximately 2.5 cm anterior to the external iliac artery. It then turns back and inclines slightly down and obliquely across the external iliac vessels to enter the lesser pelvis. In the pelvis it is situated retroperitoneally, coursing posteriorly and medially to the obliterated umbilical artery, the obturator nerve and vessels, and the vesical vessels. It crosses the ureter and bends acutely to pass anteromedially between the posterior surface of the bladder and the upper pole of the seminal vesicle. It then descends in contact with the seminal vesicle, gradually approaching the opposite duct, lying between the base of the bladder and the rectum, from which it is separated by Denonvillier’s fascia. At the base of the prostate, the vas deferens joins the duct of the seminal vesicle at an acute angle to form the ejaculatory duct. It feels cord-like when grasped because of its thick wall and small lumen. Posterior to the bladder the lumen becomes dilated and tortuous and is termed the ampulla; beyond this, where it joins the duct of the seminal vesicle, it is again greatly diminished in calibre. In men with azoospermia or no sperm in the ejaculate, testicular exploration may be undertaken to exclude an obstruction.
Aberrant ductules
A narrow, blind, caudal aberrant ductule often occurs, usually connected with the caudal part of the epididymal duct or with the start of the vas deferens. Uncoiled, it varies in length from 5 to 35 cm; it may be dilated near its end, but is otherwise uniform in calibre and in structure is similar to the vas deferens. Occasionally it is not connected with the epididymis. A rostral aberrant ductule may occur in the epididymal head, connected with the rete testis. Aberrant ductules are derived from mesonephric tubules (see p. 1305).
PARADIDYMIS
The paradidymis is a small collection of convoluted tubules, found anteriorly in the spermatic cord above the epididymal head. The tubules are lined by ciliated columnar epithelium and probably represent the remains of the mesonephros (see p. 1305).
EJACULATORY DUCTS
Microstructure
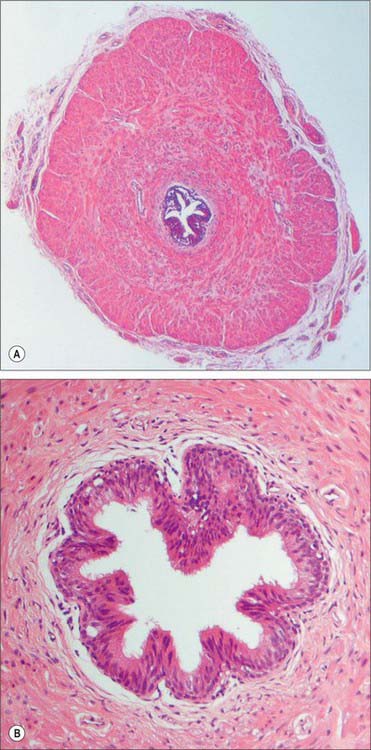
The wall of the vas deferens has loose connective tissue externally, a thick middle muscular layer and an internal mucosal layer. The muscular layer is composed of smooth muscle fibres arranged in external longitudinal and internal circular sheets. An additional internal longitudinal layer is present at the origin of the duct where it leaves the tail of the epididymis, and all muscle layers intermingle. The mucosa is folded longitudinally and its epithelium is columnar and non-ciliated through most of the duct. Towards its distal end a pseudostratified columnar epithelium appears, the tallest cells of which bear non-motile stereocilia (elongated microvilli), similar to those of the epididymis. The connective tissue of the lamina propria contains elastic fibres (Fig. 76.13A,B).
ACCESSORY GLANDULAR STRUCTURES
SEMINAL VESICLES
The two seminal vesicles are sacculated, contorted tubes located between the bladder and rectum. Each vesicle is approximately 5 cm long, somewhat pyramidal, the base being directed up and posterolaterally. In essence, the seminal vesicle is a single coiled tube with irregular diverticula, the coils and diverticula being connected by fibrous tissue. The diameter of the tube is 3–4 mm and its uncoiled length is 10–15 cm. The upper pole is a cul-de-sac, the lower narrows to a straight duct which joins the vas deferens to form the ejaculatory duct. The anterior surface contacts the posterior aspect of the bladder, and extends from near the entry of the ureter to the prostatic base: the prostate is intimately located to the seminal vesicles (Fig. 76.14A,B; see Fig. 74.21). The posterior surface is related to the rectum, from which it is separated by Denonvillier’s fascia. The seminal vesicles diverge superiorly. On each side they are related to the vas deferens and the termination of the ureter, and they are partly covered by peritoneum. Each has a dense, fibromuscular sheath. The ampulla of the vas deferens lies along the medial margin of each seminal vesicle and the veins of the prostatic venous plexus, which drain posteriorly to the internal iliac veins, lie laterally.
Microstructure
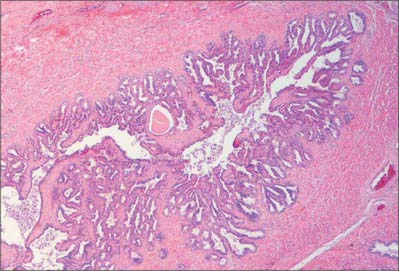
The wall of the seminal vesicle is composed of an external connective tissue layer, a middle smooth muscle layer (thinner than in the vas deferens and arranged in external longitudinal and internal circular layers) and an internal mucosal layer with a highly folded, labyrinthine structure (Fig. 76.15). The cuboidal to pseudostratified columnar epithelium of the mucosa shows features typical of protein-secreting cells. The vesicles are not reservoirs for spermatozoa as their name suggests, because spermatozoa are stored mainly in the epididymis. They contract during ejaculation, and their secretion forms most of the ejaculate.
PENIS
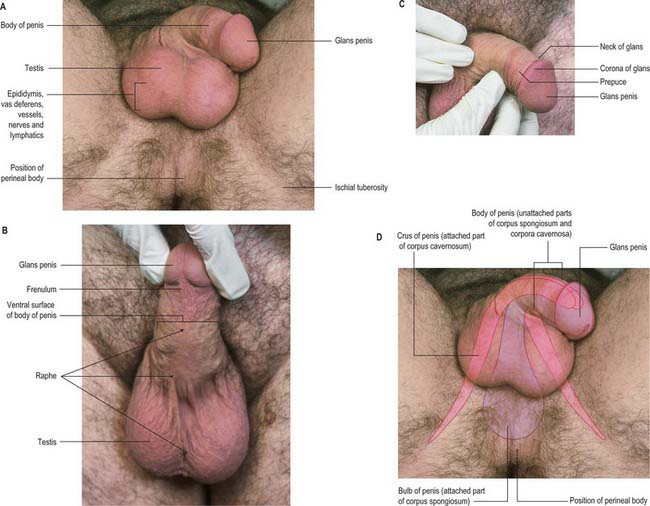
Skin
The penile skin is thin and loosely connected to the tunica albuginea. At the corona of the penis it is folded to form the prepuce or foreskin, which invariably overlaps the glans. The internal preputial layer is confluent at the neck with the thin skin covering and adhering firmly to the glans, and by this with the urethral mucosa at the external urethral orifice. On the urethral aspect of the glans a median fold, the frenulum, passes from the deep surface of the prepuce to the glans immediately proximal to the orifice. Cutaneous sensitivity is greatest over the glans penis. The prepuce and glans penis enclose a potential cleft, the preputial sac, and two shallow fossae flank the frenulum (Fig. 76.16).
Root
Each penile crus starts behind as a blunt, elongated but rounded structure, attached firmly to the everted edge of the ischiopubic ramus and covered by ischiocavernosus (Fig. 76.17). Anteriorly it converges towards the contra-lateral crus and is slightly enlarged posterior to this. Near the inferior symphysial border the two crura come together and continue as the corpora cavernosa of the body of the penis.
The bulb of the penis lies between the crura and is firmly connected to the inferior aspect of the perineal membrane, from which it receives a fibrous covering (Figs 76.16D, 76.17). Oval in section, the bulb narrows anteriorly into the corpus spongiosum, down and forwards at this point. Its convex superficial surface is covered by bulbospongiosus and its flattened deep surface is pierced above its centre by the urethra, which traverses it to reach the corpus spongiosum.
Body
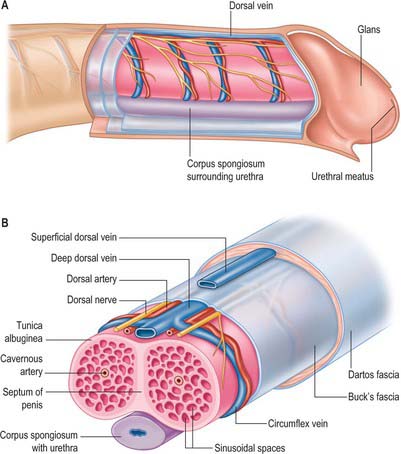
The main body of the penis consists of three masses of erectile tissue, the right and left corpora cavernosa, and the median corpus spongiosum, which are continuations of the crura and bulb of the penis respectively (Figs 76.17A, 76.18A,B). They become engorged with blood during penile erection (Fig. 76.19A). The penis is cylindrical when flaccid, but becomes triangular when erect. The surface which is posterosuperior during erection is termed the dorsum of the penis and the opposite aspect is the ventral surface.
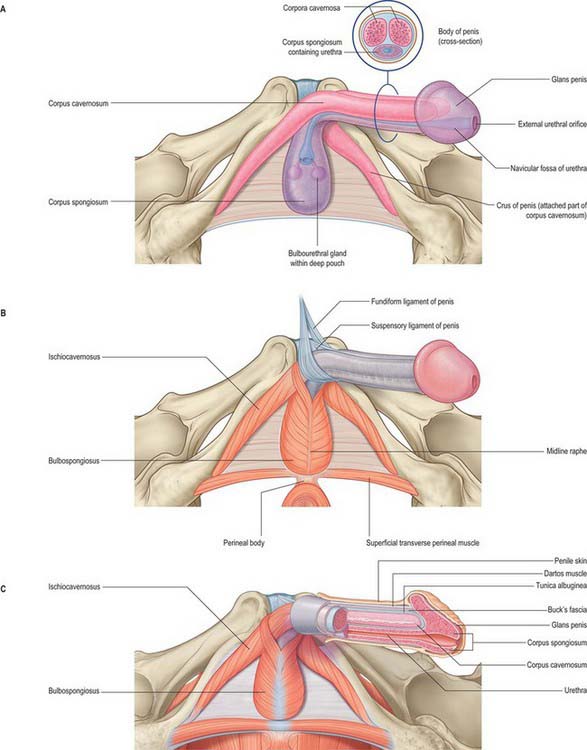
Corpora cavernosa
The corpora cavernosa form most of the body of the penis. They share a common fibrous envelope, the tunica albuginea, and are in close apposition throughout their length, although separated by a median fibrous septum. On the urethral surface their combined mass has a wide median groove that adjoins the corpus spongiosum (Figs 76.17–76.19); dorsally a similar but narrower groove contains the dorsal neurovascular bundle. The corpora end distally within the proximal aspect of the glans penis in a rounded cone, in which each has a small terminal projection (Fig. 76.16D).
Corpus spongiosum
The corpus spongiosum of the penis is traversed by the urethra (Figs 76.17A, 76.18B, 76.19). It adjoins the median groove on the urethral surface of the conjoined corpora cavernosa. It is cylindrical, tapering slightly distally, and surrounded by a tunica albuginea. Near the end of the penis it expands into the glans penis which projects dorsally over the end of the corpora cavernosa and has a shallow concave surface to which they are attached. The corona glandis projects from its base, overhanging an obliquely grooved neck of the penis. Numerous small preputial glands on the corona glandis and penile neck secrete sebaceous smegma. The navicular fossa of the urethra is in the glans and opens by a sagittal slit on or near its apex.
Superficial penile fascia
The superficial penile fascia is devoid of fat, and consists of loose connective tissue invaded by fibres of the dartos muscle from the scrotum. Clinically, it is commonly referred to as the dartos layer. As in the suprapubic abdominal wall, the deepest layer is condensed to form a distinct tough fascial sheath, Buck’s fascia (Fig. 76.18B). It surrounds both corpora cavernosa and splits to enclose the corpus spongiosum, separating the superficial and deep dorsal veins. At the penile neck it blends with the fibrous covering of all three corpora. Proximally, it is continuous with the dartos muscle and with the fascia covering the urogenital region of the perineum (see p. 1094).
Suspensory ligaments of penis
The body of the penis is supported by two ligaments, the fundiform and triangular ligaments, which are continuous with its fascia and consist largely of elastin fibres (Fig. 76.17B). The fundiform ligament stems from the lowest part of the linea alba and splits into two lamellae which skirt the penis and unite below with the scrotal septum. The triangular suspensory ligament, deep to the fundiform ligament, is attached above to the front of the pubic symphysis and blends below with the fascia penis on each side. Damage to the suspensory ligament may occur after trauma and results in ventral angulation of the body of the penis.
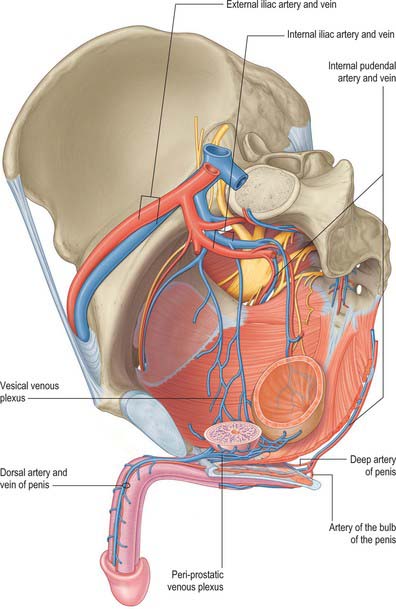
Vascular supply and lymphatic drainage
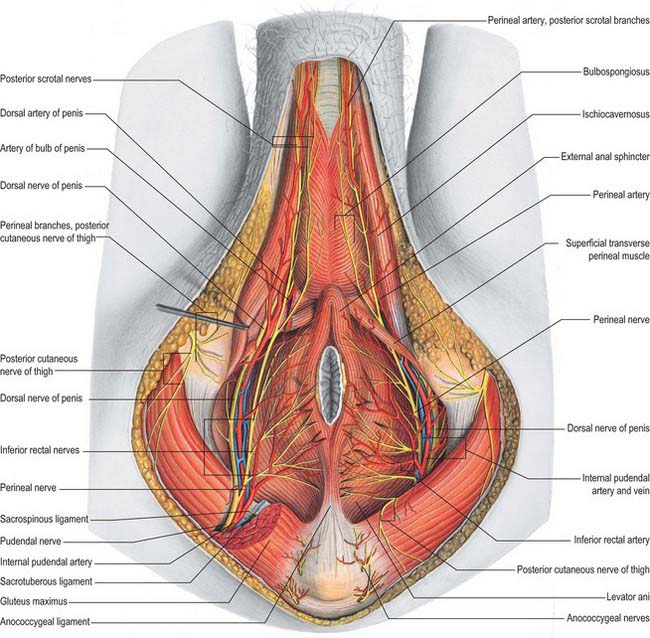
Perineal artery
The perineal artery (Fig. 76.20) leaves the internal pudendal artery near the anterior end of its canal and approaches the scrotum in the superficial perineal region, between bulbospongiosus and ischiocavernosus. Beyond the perineal membrane, and near its base, a small transverse branch passes medially, inferior to the superficial transverse perineal muscle, to anastomose with its contralateral fellow and with the posterior scrotal and inferior rectal arteries; collectively these vessels supply tissues between the anus and the penile bulb. The posterior scrotal arteries, distributed to the scrotal skin, dartos and perineal muscles, are usually terminal branches of the perineal artery but may also arise from its transverse branch.
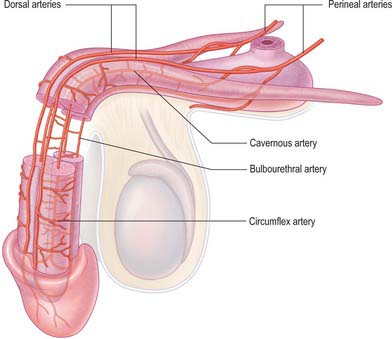
Artery of the bulb of the penis
The artery of the bulb of the penis is short but wide, and runs medially through the deep transverse perineal muscle to the penile bulb, which it penetrates (Fig. 76.20). It supplies the corpus spongiosum and the bulbourethral gland.
Cavernosal artery (deep artery of the penis)
The cavernosal artery is a terminal branch of the internal pudendal artery (Fig. 76.20). It passes through the perineal membrane, enters the crus penis on each side and runs the length of the corpus cavernosum, supplying the erectile tissue. Within the corpus, the cavernosal arteries divide into branches that either end directly in capillary networks which open into the cavernous spaces, or become convoluted and somewhat dilated helicine arteries, which also open into the cavernous spaces. Helicine arteries are most abundant in the posterior regions of the corpora cavernosa.
Dorsal artery of the penis
The dorsal artery of the penis is the other terminal branch of the internal pudendal artery (Figs 76.20, 76.21). It runs between the crus penis and pubic symphysis, and then pierces the suspensory ligament of the penis to run along its dorsum to the glans, where it forks into branches to the glans and prepuce. In the penis it lies deep to Buck’s fascia between the dorsal nerves and the deep dorsal vein, the latter being most medial. It supplies penile skin by branches that run through the dartos layer. It gives off circumflex branches that run laterally across the shaft of the penis, first deep to and then within Buck’s fascia, to supply the tunica albuginea of the corpus cavernosum, anastomosing through the tunica with the cavernosal system; these branches also supply the corpus spongiosum.
Dorsal veins of the penis
The veins that drain the corpora cavernosa leave the corpora by passing obliquely through the tunica albuginea via a series of small vessels called sub-tunical veins. These drain into the circumflex veins (Fig. 76.22) which run circumferentially around the shaft of the penis from its ventral aspect, where they receive tributaries from the corpus spongiosum, to its dorsal aspect, where they drain into the deep dorsal vein. The dorsal veins, superficial and deep, are unpaired. The superficial dorsal vein drains the prepuce and penile skin: it runs back in subcutaneous tissue and inclines either right or left before it opens into one of the external pudendal veins. The deep dorsal vein lies deep to Buck’s fascia. It receives blood from the glans penis and corpora cavernosa penis, and courses back in the midline between the paired dorsal arteries. Near the root of the penis it passes deep to the suspensory ligament and through a gap between the arcuate pubic ligament and anterior margin of the perineal membrane. It divides into right and left branches that connect below the symphysis pubis with the internal pudendal veins and ultimately enter the prostatic plexus.
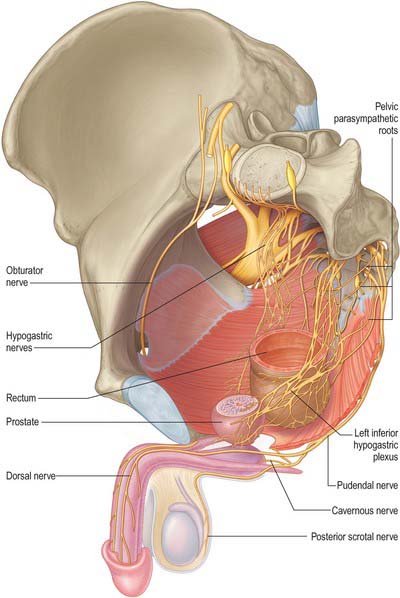
Innervation
The nerves to the corpora cavernosa mainly consist of the cavernous nerves, which arise from the pelvic plexus and contain both sympathetic and parasympathetic components (Fig. 76.23). The cavernous nerves pierce the fibrous penile sheath proximally to supply the erectile tissue of the corpus spongiosum and penile urethra. During their course they run postero-laterally to the apex of the prostate. The sympathetic supply to the male genital organs is derived from spinal cord segments T11–L2: stimulation produces vasoconstriction, contraction of the seminal vesicles and prostate, and seminal emission. Parasympathetic fibres are derived from spinal cord segments S2–4 via the pelvic plexuses: stimulation produces vasodilatation.
The main sensory nerve of the penis is the dorsal nerve of the penis. On the glans and bulb of the penis some cutaneous filaments innervate lamellated corpuscles. Afferent fibres from the glans penis and perigenital skin pass via the pudendal nerve to the spinal cord.
ERECTION AND EJACULATION
Motility of spermatozoa
It is now generally accepted that the spermatic tail executes undulatory movements in one plane. It has been suggested that a helical component is superimposed upon these movements, and that there are perhaps two separable mechanisms to account for sperm motility, one involving flat waves travelling along the tail, the other associated with torsional activity. The latter type of movement has been linked with the unequal size and distribution of the dense fibres, and the asymmetry of the spermatozoon head. The central pair of fine fibrils may act as axial stiffeners.
ERECTILE DYSFUNCTION AND PRIAPISM
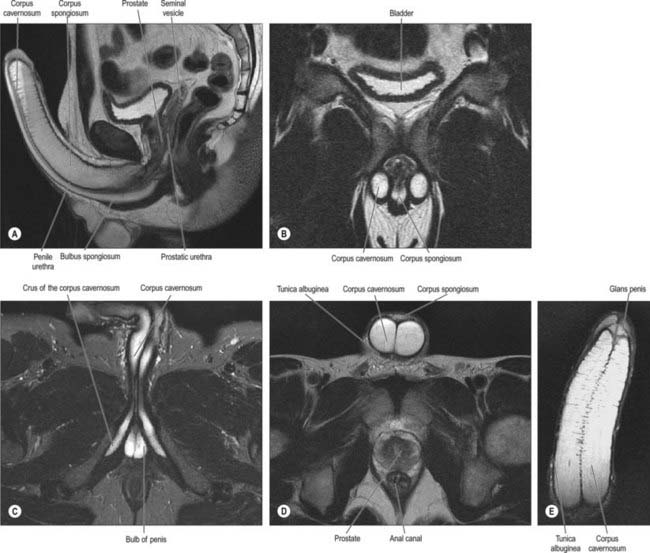
Peyronie’s disease may result in a bend in the erect penis that most commonly presents as a dorsal curvature that results from a localized thickening or plaque within the corpora cavernosa which prevents expansion of a segment during erection. Penile cancer is a rare malignancy of the penis and usually presents with a mass or ulceration of the penis. MRI scanning may be used in preoperative planning of Peyronie’s disease and local staging of penile cancer (see Fig. 76.19).
Mundy AR. Male sexual function. In: The Scientific Basis of Urology. London: Taylor and Francis; 2004.
Serhal P, Overton C. Clinical assessment and management of the infertile man. In: Good Clinical Practice in Assisted Reproduction. Cambridge: Cambridge University Press; 2004.
Walsh PC. Campbell-Walsh Urology, 9th edn. Edinburgh: Elsevier, 2007.