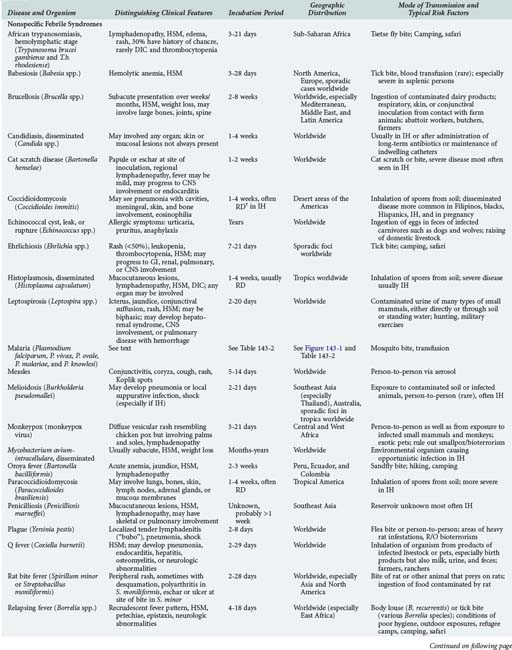
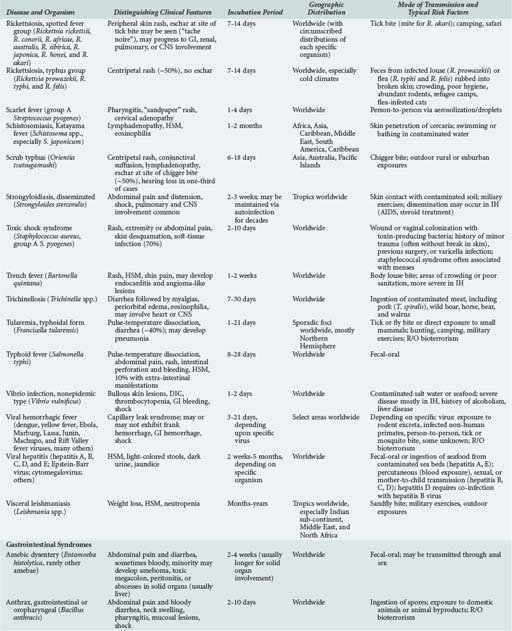
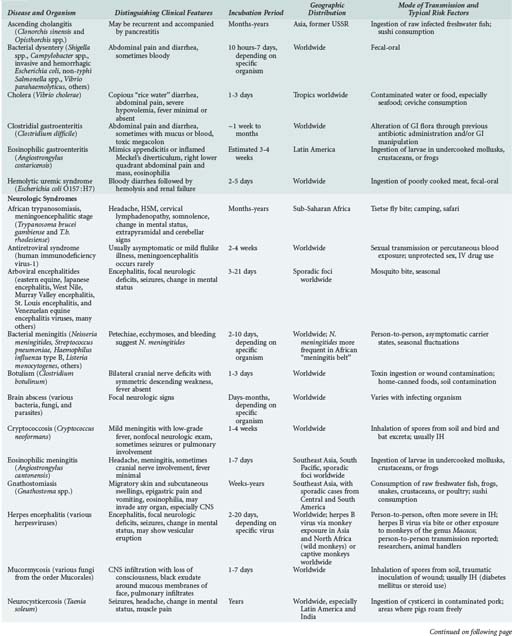
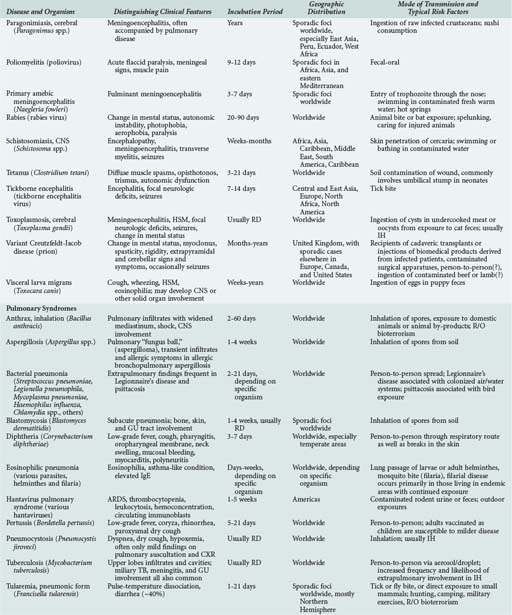
143 Malaria and Other Tropical Infections in the Intensive Care Unit
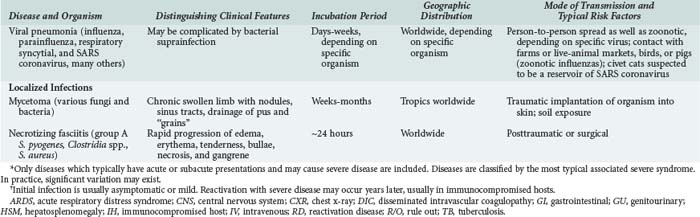
Although the spectrum of possible “tropical” infections in a patient with exposures overseas may initially seem daunting, a detailed history of the travel itinerary, activities, and exposures can often significantly narrow the differential diagnosis (Table 143-1). This must include more than simply recording the countries to which the patient traveled. Exposures of a business traveler staying at hotels and dining in fine restaurants in a major city may differ drastically from those of a student back-packing through rural areas of the same country. General knowledge of the diseases endemic in a given area and their incubation periods and drug resistance patterns is vital (Figure 143-1 and Table 143-2). In addition, most “non-tropical” infections are also common in developing countries. Thus, although the differential diagnosis must be expanded to include tropical pathogens, common illnesses seen in developing as well as industrialized countries must be considered.
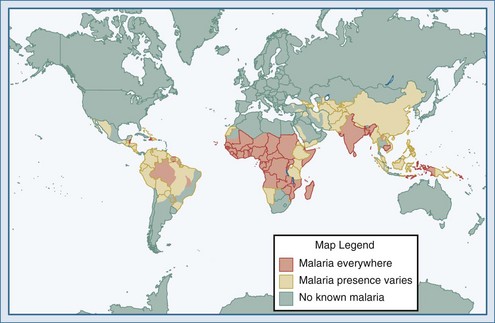
(From Health information for international travel 2010. Atlanta: Centers for Disease Control and Prevention; 2010. Available at: http://wwwnc.cdc.gov/travel/yellowbook/2010/chapter-2/malaria.aspx.)
Patients prone to tropical infections can be divided into three groups: (1) nonimmune persons who have no history of exposure to tropical pathogens, primarily tourists and young children, regardless of geographic origin, after the waning of maternal antibodies (around age 6 months); (2) immune or semi-immune persons residing in tropical countries who are repeatedly exposed; (3) those originally from tropical countries but now residing elsewhere who, in the absence of continued exposure, have waning immunity. The degree of immunity may exert profound effects on the presentation and severity of illness. For example, a returning traveler may develop severe malaria at a relatively low parasitemic load, whearas a resident of sub-Saharan Africa with the same degree of parasitemia may be asymptomatic. Genetic differences in susceptibility may also exist, such as resistance to Plasmodium vivax in blacks due to the absence of Duffy factor, which serves as the receptor, or the relative protection from severe malaria of any species afforded to those carrying the sickle cell trait.1,2,3
In returning travelers, knowledge of pre-travel vaccinations as well as prescribed and taken chemoprophylaxis (which often turn out not to be the same) is imperative. Nevertheless, these preventive measures do not confer 100% protection and should not be used to completely discard a given entity from the differential diagnosis. Both physicians and patients frequently err in the prescribing of and adherence to appropriate prophylactic regimens.4,5 Chemotherapy, complete or partial, may prolong the incubation period or alter the presentation of the illness. Those initially from tropical countries are often less likely to seek pre-travel medical advice before making a visit home and also often have considerably more exposures to tropical pathogens during their visit than do short-term travelers from industrialized countries.6
People living in resource-poor tropical countries may be more likely to have complicating health problems but less likely to have them previously diagnosed or controlled. Underlying diabetes, hypertension, malnutrition, chronic anemia, intestinal parasites, tuberculosis, HIV, or hepatitis virus infection may be discovered at the time of the acute illness.7 Infection with multiple tropical pathogens is common in those living in endemic areas. Thus the finding of a given pathogen cannot automatically be assumed to be the cause of the patient’s current illness.
 Epidemiology
Epidemiology
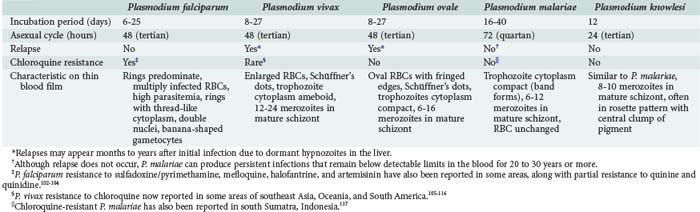
Malaria parasites are spread to humans by the bite of anopheline mosquitoes. Four species of Plasmodia commonly cause malaria in humans: Plasmodium falciparum, P. vivax, Plasmodium ovale, and Plasmodium malariae (see Table 143-2). A fifth species, Plasmodium knowlesi, is a zoonotic parasite of monkeys recently found to also cause disease in humans with exposure in forests of Southeast Asia.8,9 Furthermore, recent evidence suggests that there may be distinct species of P. vivax.10
Malaria is the most common serious infection in most tropical countries as well as in returning travelers, and it should therefore be considered in any patient reporting travel in malaria-endemic areas or with exposure to unscreened blood products (“transfusion malaria”) or blood-contaminated needles. Increased travel and immigration over the past several decades have resulted in increases in imported malaria in most industrialized countries.11,12 The risk of acquiring P. falciparum, the cause of most severe disease, is highest for those traveling to sub-Saharan Africa and New Guinea, moderate in India, and comparatively low in Southeast Asia and Latin America.13,14 Malaria is occasionally reported in individuals without reported travel, usually resulting from the carriage of malaria-infected passengers (who may be asymptomatic) or anopheline mosquitoes on aircraft arriving from endemic areas.15 The parasite may then be secondarily transmitted by anopheline mosquitoes endemic in some industrialized countries, including the United States.
 Pathophysiology
Pathophysiology
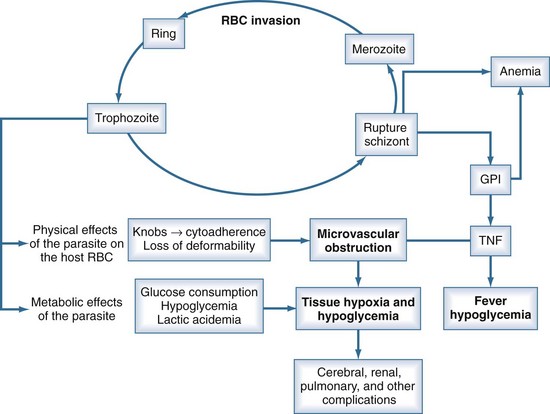
P. falciparum accounts for the vast majority of severe malaria because of (1) its ability to infect red blood cells (RBCs) of all ages, resulting in overwhelming parasitemia (up to 70% of RBCs); (2) its induction of adherence of parasitized RBCs to the microvascular wall, with consequent obstruction; (3) its induction of severe metabolic derangements directly through glucose consumption and lactate production and indirectly through the induction of cytokines; and (4) the high prevalence of chloroquine resistance to P. falciparum in many parts of the world (see Table 143-2). Nonimmune persons and pregnant women are at greatest risk. Human genetic as well as parasite strain differences probably play roles in the ultimate course of any given malaria infection.
Unlike the other species of malaria, P. falciparum causes decreased RBC deformability and the production of small protrusions or “knobs” on parasitized RBC membranes that mediate their adhesion to the venular endothelium (Figure 143-2). The rupture of schizont-stage parasites exposes glycosylphosphatidylinositol anchors on the parasite and RBC surface that induce macrophages and other inflammatory cells to release a host of inflammatory mediators including tumor necrosis factor alpha (TNF-α), interleukin-1, TNF-β, and various kinins and reactive nitrogen intermediates.16–18 These cytokines play a role in up-regulation and activation of endothelial adhesion molecules such as ICAM-1 and E-selectin, enhancing cytoadherence of parasitized cells as well as mediating pathologic processes such as hypoglycemia, lactic acidemia, shock, gut mucosal damage, and increased permeability and neutrophil aggregation in the lung. The sum total of this cascade is sequestration of parasitized RBCs in the microvasculature where they are not only sheltered from removal but cause sluggish flow and obstruction, resulting in impaired oxygen delivery and organ dysfunction.16,19 The most profound effects are usually on the cerebral capillaries, although a host of tissues may be affected, including the kidney, liver, spleen, placenta, intestine, lung, bone marrow, heart, and retina. Histopathologic changes are usually minimal, but ring hemorrhages and perivascular infiltrates sometimes develop at the sites of obstructed vessels, perhaps facilitated by thrombocytopenia due to splenic sequestration of platelets. Although subendocardial and epicardial hemorrhages have been noted at autopsy, myocarditis does not occur, and primary cardiac events are relatively rare in malaria.
 Clinical Presentation
Clinical Presentation
Although a classic periodicity is described for the different malaria species (see Table 143-2), this occurs only when the infection has persisted untreated long enough to allow for synchronization of schizont rupture. Furthermore, schizont rupture tends to be asynchronous in P. falciparum and in most primary infections of any plasmodium species. Therefore, malaria may often result in persistently spiking fevers difficult to distinguish from fever produced by many other infections. The absence of a classic paroxysm and periodicity therefore should not be used to exclude the diagnosis. Paroxysms may be accompanied by cough, sore throat, myalgias, back pain, postural hypotension, abdominal pain, nausea, vomiting, diarrhea, and weakness. These are more common in children and may lead to misdiagnoses. Rash and lymphadenopathy are not typical of malaria and suggest another diagnosis.
Severe and Complicated Malaria
Although all species of malaria may produce severe consequences in a debilitated patient, potentially fatal malaria which merits attention in an ICU can be grouped into three categories: (1) severe complications of P. falciparum in nonimmune children and adults, responsible for the vast majority of severe disease worldwide (Table 143-3); (2) splenic rupture, which occurs most frequently with P. vivax; and (3) chronic nephrotic syndrome due to immune-complex nephritis associated with P. malariae, usually seen in children and often complicated by overwhelming bacterial infection. There is emerging evidence that P. knowlesi can also cause severe fatal malaria and should be treated in an ICU setting.8
TABLE 143-3 Clinical and Laboratory Features That Classify a Patient as Suffering from Severe Plasmodium falciparum Malaria According to the World Health Organization
| Clinical Features |
Modified from Guidelines for the treatment of malaria 2010. Geneva: World Health Organization; 2010. Available at: http://whqlibdoc.who.int/publications/2010/9789241547925_eng.pdf.
Cerebral Malaria
This is the most frequent severe complication of plasmodium infection, accounting for most fatalities as well as chronic sequelae. It is most frequent in children of 3 to 5 years of age. Strictly defined, cerebral malaria implies unarousable coma due to P. falciparum.20,21 Hyperpyrexia and febrile convulsions in young children may produce transiently altered mental status without true involvement of the cerebral microvasculature and thus technically do not constitute cerebral malaria. However, in clinical practice, seizures or persistent changes in sensorium which cannot be attributed to other disease processes should be considered cerebral malaria until proven otherwise. Although cerebral malaria is classically attributed to cytoadhesion and microvascular obstruction in the brain, other ongoing processes including hypoglycemia, metabolic acidosis, and impaired oxygenation due to anemia and pulmonary edema likely contribute.
The altered sensorium of cerebral malaria may develop gradually within a few days of onset of illness or manifest as persistent coma after a generalized convulsion. Compared to adults, children with cerebral malaria have a shorter history of fever before progressing to coma (average about 2 days). The most common neurologic picture is of a symmetrical upper motor neuron lesion with hypertonia, hyperreflexia, clonus, absent abdominal reflexes, and extensor Babinski responses. Hypotonia and acute cerebellar ataxia are sometimes seen as well, especially in India and Sri Lanka. There is usually a diffuse symmetric encephalopathy, sometimes with signs of frontal lobe release such as a pout reflex or bruxism. There is usually no grasp reflex, and the gag reflex is normally maintained. Both decorticate as well as decerebrate posturing may occur.21 Meningismus, opisthotonos, and disconjugate gaze are frequently seen. Nystagmus and a sixth nerve palsy are rare. Pupils are usually symmetric with intact pupillary, corneal, oculocephalic, and oculovestibular reflexes. Photophobia, severe neck rigidity, and papilledema are almost never seen.
Convulsions may occur in up to 50% of cases of cerebral malaria. As a child ages above 3 to 4 years, seizures become more likely to represent cerebral malaria rather than febrile convulsions.22 Although generalized seizures are classically reported, partial motor seizures, with or without secondary generalization, may occur. 20 Although often showing only diffuse cortical dysfunction, EEG studies may sometimes reveal underlying status epilepticus even when it is not clinically evident.21
Pulmonary Edema and Acute Respiratory Distress Syndrome
Pulmonary edema, which may progress to acute lung injury (ALI) and acute respiratory distress syndrome (ARDS), is frequent and typically the most lethal of the complications of malaria. Recent evidence suggests that the mechanism may involve acute pulmonary hypertension precipitated by nitric oxide consumption by free plasma hemoglobin released from intravascular hemolysis.[Janka et al., in press] Endothelial injury leading to increased alveolar permeability and noncardiogenic pulmonary edema may also contribute. Interstitial edema and inflammatory cell infiltrates are seen at autopsy, but sequestration of parasitized RBCs in the lung is uncommon.23 Pulmonary complications occur in 5% to 30% of patients with severe malaria, especially pregnant women, nonimmune persons, and patients already suffering from other complications.23 The onset may be any time during the course of illness, even if the patient appears to be improving and parasitemia has decreased. Symptoms include dyspnea and cough, with rapid progression to hypoxia and respiratory distress.
Anemia and Hematologic Perturbations
Although some degree of anemia is common in all types of malaria, severe anemia (hemoglobin less than 5 g/100 mL) occurs almost exclusively with P. falciparum infections, owing to their high parasitemias. It is most common and often severe in pregnant women and young children (<1 year), in whom it may be the presenting sign.24 In addition to the acute hemolytic destruction of parasitized RBCs, the more chronic processes of removal of parasitized cells from circulation by the spleen and cytokine inhibition of erythropoiesis may contribute.25 Nonimmune subjects may develop anemia within days after infection, whereas anemia usually develops more slowly in those who are semi-immune. The degree of anemia generally correlates with bilirubin level and level of parasitemia. It may be exacerbated by underlying glucose-6-phosphate dehydrogenase (G6PD) deficiency in the setting of administration of oxidant antimalarial drugs (e.g., quinine, sulfadoxine) and iron-deficiency anemia due to malnutrition. Significant jaundice and hemoglobinuria may result. Thrombocytopenia, although frequent, is not usually associated with bleeding or correlated with disease severity. Disseminated intravascular coagulation (DIC) is seen in less than 10% of severe cases.
Acute Renal Failure
Blackwater fever refers to a severe syndrome characterized by low or absent parasitemia, intravascular hemolysis, hemoglobinuria, and ARF. It is classically seen in people of northern European descent chronically exposed to P. falciparum and irregularly taking the quinoline antimalarial drugs, quinine or quinidine, which together are known as the cinchona alkaloids. The syndrome virtually disappeared after 1950 when chloroquine superseded quinine. However, it is now said to be resurgent, albeit with lower mortality, in relation to mounting chloroquine resistance and consequent increased use of quinine and the newer quinolines, such as mefloquine and halofantrine.26
Shock and Bacterial and Other Suprainfection
So-called algid malaria, referring to hypotension and shock, may resemble and indeed sometimes be due to gram-negative sepsis from impaired flow in intestinal capillaries, with resultant mucosal erosion. Non-typhoidal salmonella septicemia is specifically associated with P. falciparum.27 Algid malaria is often seen in the setting of hyperparasitemia, with concomitant hypoglycemia and lactic acidemia, and may progress to multiorgan system failure and death. As with most malaria complications, severe hemodynamic derangements are most often seen in nonimmune persons.28 Whether bacteria are isolated or not, a classic septic shock picture is typical, with elevated cardiac index and decreased systemic vascular resistance.29 Hemodynamic decompensation due to splenic rupture may mimic algid malaria.
A host of other infectious complications, including aspiration pneumonia and parvovirus infection, may be related to falciparum malaria. Malaria occurs with increasing frequency and severity in those who are human immunodeficiency virus (HIV) infected, especially during pregnancy, and can also transiently up-regulate HIV replication.30–34 An association between severe malaria infection and hepatitis B surface antigen carriage has also been noted.35
Tropical Splenomegaly and Splenic Rupture
Splenomegaly is common in infection with all species of malaria. The tropical splenomegaly syndrome, also sometimes termed hyperreactive malarial syndrome (HMS), refers to a condition of massive splenomegaly, high titers of total serum IgM and malaria-specific antibodies, and scanty or absent parasitemia. It is seen in individuals with a history of residence in an endemic area and can be associated with any malaria species. Host genetic factors appear to play a role.36
Unlike virtually all the other complications of malaria that are most often associated with P. falciparum, acute splenic complications occur most commonly in P. vivax, especially with the first infection. Although the term spontaneous splenic rupture has traditionally been used, in reality a range of hematomas or tears of varying severity may occur. The rupture or tear usually occurs 2 to 3 months after infection, presumably due to increased intrasplenic tension, often precipitated by trauma of varying degrees or mechanical ventilation.37 Over-eager examiners have been suggested to play a role, although no cases of clear palpation-induced rupture have been reported. Fever, tachycardia, vomiting, prostration, abdominal pain or guarding, tender splenomegaly, hypovolemia, and rapidly worsening anemia are common presenting features. Abdominal pain may be localized or diffuse, mild or severe. Shock may ensue. Diaphragmatic irritation after rupture may cause referred pain to the left shoulder, supraclavicular, or scapular regions (“Kehr’s sign”). This is present in about one-half of cases and is said to have good specificity for rupture.
Malaria in Pregnancy and Children
In addition to being more susceptible to infection, malaria is particularly dangerous in pregnant women and their fetuses, with increased risk of pulmonary edema, hypoglycemia, severe anemia, premature delivery, low birth weight, and maternal and fetal death. Malaria parasites can often be found in the placenta and may impair oxygen and nutrient transport to the fetus. Disease is most severe in primiparae, especially if nonimmune. In contrast, women from endemic areas are usually asymptomatic, with the exception of the effects of anemia, again more severe in primiparae. Congenital malaria is rare except in those infants born to nonimmune mothers.38
 Diagnosis
Diagnosis
Clinical
Malaria often presents with nonspecific signs and symptoms, so making a clinical diagnosis may be difficult. Although almost all patients have a history of fever, they may frequently be afebrile at the time of examination.39 Physicians in industrialized countries who are unfamiliar with the disease may not initially include malaria in the differential diagnosis. Delayed diagnosis is frequent and associated with a poor outcome.6,40 Although patients with other species of malaria parasite may not present for months or even years after infection, the vast majority of those with P. falciparum will present within 6 months of exposure.4 The differential diagnosis includes most febrile illnesses found in the tropics (see Table 143-1). Babesiosis may present both clinically and microscopically similar to malaria in patients without travel to malaria-endemic areas. Cerebral malaria must be distinguished from bacterial meningitis, the viral meningoencephalitides, metabolic coma, and intoxications by lumbar puncture.41 In cerebral malaria, the cerebrospinal fluid (CSF) opening pressure is usually normal, although a few lymphocytes and moderate elevation of protein may be seen. High CSF lactate and low glucose indicate a poor prognosis.
Conventional Microscopy
Laboratory diagnosis has traditionally been made via the examination of thick and thin Giemsa-stained smears. Thick smears are more sensitive in diagnosing malaria, whereas thin smears allow identification of the specific parasite. Either smear can be used to quantify the level of parasitemia, but thick smears are theoretically more sensitive for this purpose.42,43 Simultaneous infections with multiple strains of P. falciparum are common in some areas of sub-Saharan Africa and also may occur with P. vivax in Southeast Asia and Latin America.44,45 Blood obtained by pricking a fingertip or earlobe is preferred because parasite densities are higher in these capillary-rich areas, although blood obtained by venipuncture collected in heparin or EDTA anticoagulant-coated tubes is acceptable if used shortly after being drawn (to prevent alteration in the morphology of white blood cells and malaria parasites).46 Smears should be taken as soon as the diagnosis of malaria is considered, without waiting for manifestation of a classic paroxysm. Parasitemia may be undetectable in the early stages of the illness, in those with partial immunity, and in those who have previously self-administered antimalarials, a common practice in malaria-endemic areas.47 Levels of parasitemia may fluctuate over time, necessitating repeated smears for diagnosis. Furthermore, P. falciparum–parasitized red blood cells may be sequestered in the deep capillaries of the spleen, liver, and bone marrow. Although a blood film is unlikely to be falsely negative in a patient with severe disease, negative smears should not prevent prompt administration of antimalarial therapy if the diagnosis is strongly suspected.14 Conversely, asymptomatic parasitemia is common in children from endemic areas, and thus a positive smear does not necessarily signify a clinical case under these circumstances.
Considerable expertise at reading malaria smears may be necessary to detect and distinguish the parasites (see Table 143-2). The most important point is to distinguish P. falciparum, with its concomitant risk of severe complications, from the other plasmodia. Superimposed platelets, particles of stain, pits in the slide, RBC inclusions such as Howell-Jolly bodies and those seen in siderocytes, and other intracellular pathogens such as Bartonella and Babesia must be distinguished from malaria parasites. Furthermore, alterations in parasite morphology may occur related to strain variation, drug pressure, and blood collection method.
Newer Laboratory Methods
Various new diagnostic techniques for malaria have been developed in recent years, including microscopy with fluorescent stains, dipstick antigen detection, DNA probes, polymerase chain reaction (PCR) assays, and automated blood cell analysis.42,43,48–52 Use of one of these new diagnostic modalities should be considered when a high suspicion of malaria remains despite repeatedly negative blood smears, especially if the microscopist has limited experience with reading malaria smears.43 Each technique has unique advantages and disadvantages, but the sensitivity and specificity for P. falciparum is generally similar or better than conventional microscopy. Because of its greater sensitivity (as low as 5 parasites/µL), PCR may be a particularly valuable tool in nonimmune persons. PCR also allows evaluation for possible infection with multiple malaria strains and determination of drug resistance. The U.S. Food and Drug Administration (FDA) recently approved a rapid diagnostic test—the BinaxNOW malaria test (Binax/Alere Inc., Scarborough, Maine)—that detects the HRP-2 protein of P. falciparum as well as an aldolase common to all plasmodia, with sensitivities of 100% and 97%, respectively.42 However, the sensitivity of this and other dipstick antigen tests is diminished when the parasitemia is less than 100 parasites/µL. Furthermore, the HRP-2 protein may persist in the bloodstream and give a false-positive test result for up to 4 weeks after successful treatment of malaria. Hence, it is still important to confirm the rapid diagnostic test with microscopy when possible.
Imaging
Computed tomography (CT) or magnetic resonance imaging (MRI) scanning of the abdomen is the usual diagnostic modality when splenic rupture is considered, although ultrasonography, arteriography, bleeding scans, or exploratory laparotomy may sometimes be needed. Findings such as increased brain volume and occasionally brain swelling have been noted in CT and MRI studies in cerebral malaria, but these tests are generally unhelpful clinically and are indicated only to rule out suspected mass lesions when the diagnosis of cerebral malaria is uncertain.53
 Clinical Management
Clinical Management
Indications for Admission to the Intensive Care Unit and General Management
Features that indicate severe disease meriting admission to an ICU and urgent IV therapy are noted in Table 143-3. In these critically ill patients, chloroquine-resistant P. falciparum should be assumed until proven otherwise. As per routine ICU management, the patient’s breathing and circulatory status should first be rapidly assessed, the airway secured, and the neurologic status scored on the Glasgow Coma Scale or other appropriate scoring system.54 For patients in profound shock, blood cultures should be drawn and broad-spectrum antibiotics begun unless the diagnosis of severe malaria has already been confirmed or if bacterial suprainfection is suspected. Unconscious patients should have a lumbar puncture to rule out bacterial meningitis.
Careful attention to fluid balance is imperative, especially considering the very poor prognosis once pulmonary edema or ARDS develops. Measurements of urine output and daily weights should be routinely performed. Monitoring of central venous pressure should be considered in delicate cases, such as those with respiratory distress or compromised renal function. Considering that the prognosis associated with pulmonary failure is considerably poorer than that of ARF, some authors recommend early use of inotropes rather than excessive fluids in the setting of hypotension, although a beneficial effect on the overall hemodynamic profile has yet to be conclusively demonstrated.39,55 Dialysis is indicated for ARF and may aid not only through improved fluid balance and control of acidemia but also via removal of circulating cytokine mediators of inflammation. Although observations are limited, the quinolines appear not to be dialyzed.56 Cautious transfusion of packed cells is usually indicated when the hematocrit falls below 20%. In addition to improved oxygen transport, blood transfusion may reduce the parasite load and cytokine mediators of inflammation.39,57 Concurrent administration of diuretics or low-dose dopamine may be warranted to avoid fluid overload.
Increasing respiratory distress may indicate the onset of ALI or ARDS. Arterial blood gas measurements may reveal hypoxemia, and chest x-rays bilateral infiltrates. Supplemental oxygen and mechanical ventilation may be required. In accordance with the NIH ARDS Network Trial, lung-protective ventilation, with tidal volume of 6 mL/kg predicted body weight and plateau pressures less than 30 cm H2O are indicated for improved survival.23 Extracorporeal oxygenation has also been employed.58 Metabolic acidosis should be treated by improving pulmonary gas exchange, correcting hypovolemia and hypoglycemia, and treating associated septicemia. Blood glucose should be checked frequently, especially in pregnant patients, and 50% dextrose administered when needed. Results of studies on the efficacy of continuous IV infusion of 5% dextrose have been mixed.59,60 Quinoline-induced hypoglycemia may be prevented by administering somatostatin analogs followed by glucagons.61 Acute seizures may be treated with benzodiazepines or paraldehyde, and prolonged seizures terminated with phenytoin.21 However, prophylactic anticonvulsants are not recommended and may be harmful.54 Although the risk of bleeding is low, aspirin should be avoided in the presence of thrombocytopenia. Many patients with splenic rupture can be managed conservatively with supportive therapy, although splenectomy may be necessary.36
Antimalarial Chemotherapy
Two classes of medicines are indicated for parenteral treatment: the artemisinin derivatives (artesunate, artemether, and others) and the cinchona alkaloids (Table 143-4). Randomized trials in Southeast Asia show artesunate to be superior to quinine for severe malaria in adults, although there is currently insufficient evidence to support this conclusion in children.54,62 Despite its use throughout much of the world, in the United States, intravenous (IV) artesunate has “investigational new drug” status and is only available through request to the Centers for Disease Control and Prevention (CDC).63,64 Because IV quinine is also unavailable in the United States, quinidine gluconate is often used.65 Cinchona alkaloids may also be considered for first-line treatment of patients infected in Southeast Asia, where resistance to artemisinin compounds has been documented, or if the patient has already received but not responded to an artemisinin-based therapy.66,67
TABLE 143-4 Treatment Guidelines for Severe Plasmodium falciparum Malaria
| Drug | Dose | Comments |
|---|---|---|
| Artemisinin Compound Regimens* | ||
| Artesunate | ||
* Various other artemisinin combined therapy regimens are in use around the world depending upon drug availability, national policy, and personal preference, including artesunate plus amodiaquine, artemether plus lumefantrine, dihydroartemisinin plus piperaquine.
According to CDC recommendations, the patient should receive at least 24 hours of parenteral therapy with quinidine gluconate even if there is immediate dramatic improvement.64 After 24 hours, patients may be transitioned to oral quinine only if they are able to tolerate oral medications and the parasite density is less than 1%. The IV quinidine/oral quinine treatment course is 7 days total if malaria was contracted in Southeast Asia and 3 days if in South America or Africa.
The patient should be given a 7-day oral course of second drug in addition to the IV artesunate or IV quinidine/oral quinine therapy (see Table 143-3). Artemisinin compounds should be followed by oral doxycycline, clindamycin, atovaquone/proguanil, or mefloquine, whereas either doxycycline or clindamycin are given concurrently with the cinchona alkaloids. Doxycycline is preferred to other tetracyclines because it can be given once daily and does not accumulate in renal failure. Mefloquine should be avoided if the patient presented initially with impaired consciousness; an increased incidence of neuropsychiatric complications associated with mefloquine following cerebral malaria has been documented. Chloroquine is no longer recommended for the treatment of severe malaria because of widespread resistance. Intramuscular sulfadoxine/pyrimethamine is no longer recommended.
Adverse Effects of Therapy
Side effects associated with artemisinin compounds are infrequent and generally mild and include abdominal pain, diarrhea, contact dermatitis, decreases in reticulocyte and neutrophil counts, and elevated hepatic transaminases.68 Severe allergic reactions and cerebellar dysfunction have been rarely reported.69
Side effects of quinine and quinidine, known as cinchonism, are common and typically include nausea, vomiting, headache, dysphoria, vasodilation, tinnitus, and changes in auditory and visual acuity. These alterations are dose related and reversible. Less common side effects include rash, urticaria, angioedema of the face, pruritus, agranulocytosis, hepatitis, blackwater fever, and psychiatric disorders. Overdoses are associated with depressed respiration, circulatory collapse, and CNS alterations including seizures and coma, which may be difficult to distinguish from cerebral malaria.70 Simultaneous use of two quinolines or retreatment with the same quinoline within a short period of time may predispose to severe side effects.71 The cinchona alkaloids are metabolized in the liver and excreted in the urine. Monitoring blood levels is recommended for persons with impaired renal or hepatic function, and dose reduction is necessary in those with severe renal impairment. Quinine metabolism appears to be decreased in children with kwashiorkor but increased in those with marasmus.72
Although rarely clinically significant, prolongation of the electrocardiographic QT interval with IV quinoline therapy is common.73 Severe conduction abnormalities may occur along with hypotension, blindness, and deafness.54,63,64,73 Dysrhythmias and hypotension may also result from overly rapid infusion. Coma may result when serum quinoline levels exceed 20 mg/L. Cardiac monitoring should be performed with IV quinoline use, especially with quinidine, which although more potent against the malaria parasite is also generally more toxic.73 Infusion rates of quinidine should be decreased if the QT interval increases by more than 25% of its baseline level.
Quinoline-induced stimulation of insulin release may elicit significant hypoglycemia, especially in pregnancy.60,74 Hypophosphatemia may also be precipitated by both quinoline and IV dextrose, causing CNS dysfunction.39 Levels of digoxin, mefloquine, neuromuscular blocking agents, and oral anticoagulants may all be increased with quinoline administration. Quinine can cause hemolysis in patients with G6PD deficiency. Because of their curare-like effect on skeletal muscle, quinolines are contraindicated in patients with myasthenia gravis.
Atovaquone/proguanil is usually well tolerated. Gastrointestinal symptoms, skin rash, headache, insomnia, and (rarely) hematologic and renal effects have been reported, especially at high levels.75,76
Ancillary Therapies
Various ancillary therapies have been proposed for severe malaria. In most cases, controlled data are not available to judge their efficacy. Exchange transfusion and erythrocytapheresis have been employed with apparent benefit in cases of severe disease with high parasitemia (>15%) and should be considered in such situations, especially if the patient’s condition is worsening despite adequate chemotherapy.77–80 The rationale for this form of therapy is based on (1) rapid reduction in parasite load; (2) removal of toxic substances; and (3) reducing microcirculatory sludging.77 In some studies, iron chelators such as desferrioxamine have been demonstrated to hasten malaria parasite clearance and shorten the duration of cerebral malaria coma.81,82 Proposed mechanisms include depriving the parasite of necessary iron, enhancing the T-helper immune response, and protecting against iron-mediated peroxidant cerebral tissue damage.58 Antioxidants such as pentoxifylline and inhaled nitric oxide have been used, but attempts to attenuate the immune response in malaria have generally met with mixed results.83–85 Monoclonal antibodies directed against TNF-α had no impact on mortality and may increase morbidity (neurologic sequelae), probably reflecting the participation of multiple cytokines in the pathogenesis of severe and complicated malaria.86,87 Dichloroacetate to counter lactic acidosis is also under study.88 Corticosteroids are detrimental in severe malaria and should not be used.89
Laboratory Monitoring
Findings in severe malaria may include profound hemolytic anemia and thrombocytopenia, leukocytosis with a left shift (although milder cases may show leukopenia), prolonged coagulation times (with increased fibrin split products and diminished fibrinogen reflecting DIC), hyponatremia, hypoalbuminemia, hypophosphatemia, hypoglycemia, lactic acidemia, and elevated hepatic enzymes, LDH, bilirubin, BUN, and creatinine. Urinalysis may reveal proteinuria, RBCs and RBC casts, and hemoglobinuria. Coagulation defects and thrombocytopenia often correlate with the degree of parasitemia. The level of parasitemia should be monitored via blood smear every 12 hours after initiation of therapy. A decrease of 75% should be noted within 48 hours. If this does not occur, drug resistance should be suspected, and the regimen should be changed accordingly (see Table 143-4).
 Prognosis
Prognosis
Case fatality rates in severe malaria range from 2% to 50%.20,21,90–92 Factors which correlate with a poor prognosis include the infecting species and resistance profile, CNS involvement, pulmonary edema, hypoglycemia, lactic acidosis, renal failure, severe anemia, younger age, pregnancy, and treatment in a rural health facility as opposed to an ICU.39,93–100 There is a semiquantitative relationship between level of parasitemia and risk of death, especially in nonimmune patients. Although less than 10% of adults with cerebral malaria have persistent neurologic sequelae, this number may be as high as 40% in children, especially if associated with hypoglycemia.59,90 Commonly seen sequelae include psychosis, hemiparesis, cerebellar ataxia, and extrapyramidal rigidity.20,21 Children who survive without obvious neurologic sequelae appear to then develop normally neuropsychologically.101 A postmalarial neurologic syndrome, usually associated with mefloquine use, of an acute confusional state, psychosis, convulsions, and tremors has been described but is usually self-limited.21
Key Points
Cox-Singh J, Davis TM, Lee KS, Shamsu SS, et al. Plasmodium knowlesi malaria in humans is widely distributed and potentially life threatening. Clin Infect Dis. 2008;46:165-171.
Griffith KS, Lewis LS, Mali S, Parise ME. Treatment of malaria in the United States: a systematic review. JAMA. 2007;297:2264-2277.
Mishra SK, Newton C. Diagnosis and management of the neurological complications of falciparum malaria. Nat Rev Neurol. 2009;5:189-198.
Mohan A, Sharma SK, Bollineni S. Acute lung injury and acute respiratory distress syndrome in malaria. J Vector Borne Dis. 2008;45:179-193.
Stauffer WM, Cartwright CP, Olson DA, et al. Diagnostic performance of rapid diagnostic tests versus blood smears for malaria in US clinical practice. Clin Infect Dis. 2009;49:908-913.
1 Frideman M. Erythrocytic mechanism of sickle cell resistance to malaria. Proc Natl Acad Sci. 1978 April;75(4):1994-1997.
2 Miller LH, Mason SJ, Clyde DF, McGinniss MH. The resistance factor to Plasmodium vivax in blacks. The Duffy-blood-group genotype, FyFy. N Engl J Med. 1976 Aug 5;295(6):302-304.
3 Hill AV, Allsopp CE, Kwiatkowski D, Anstey NM, Twumasi P, Rowe PA, et al. Common west African HLA antigens are associated with protection from severe malaria. Nature. 1991 Aug 15;352(6336):595-600.
4 Svenson JE, MacLean JD, Gyorkos TW, Keystone J. Imported malaria. Clinical presentation and examination of symptomatic travelers. Arch Intern Med. 1995 Apr 24;155(8):861-868.
5 Horowitz H, Carbonaro CA. Inhibition of the Salmonella typhi oral vaccine strain, Ty21a, by mefloquine and chloroquine. J Infect Dis. 1992 Dec;166(6):1462-1464.
6 Moore TA, Tomayko JFJr, Wierman AM, Rensimer ER, White ACJr. Imported malaria in the 1990s. A report of 59 cases from Houston, Tex. Arch Fam Med. 1994 Feb;3(2):130-136.
7 Adebajo AO, Smith DJ, Hazleman BL, Wreghitt TG. Seroepidemiological associations between tuberculosis, malaria, hepatitis B, and AIDS in West Africa. J Med Virol. 1994 Apr;42(4):366-368.
8 Cox-Singh J, Davis TM, Lee KS, Shamsul SS, Matusop A, Ratnam S, et al. Plasmodium knowlesi malaria in humans is widely distributed and potentially life threatening. Clin Infect Dis. 2008 Jan 15;46(2):165-171.
9 Cox-Singh J, Hiu J, Lucas SB, Divis PC, Zulkarnaen M, Chandran P, et al. Severe malaria—a case of fatal Plasmodium knowlesi infection with post-mortem findings: a case report. Malaria J. 2010;9:10.
10 Sutherland CJ, Tanomsing N, Nolder D, Oguike M, Jennison C, Pukrittayakamee S, et al. Two nonrecombining sympatric forms of the human malaria parasite Plasmodium ovale occur globally. J Infect Dis. 2010 May 15;201(10):1544-1550.
11 Lackritz EM, Lobel HO, Howell BJ, Bloland P, Campbell CC. Imported Plasmodium falciparum malaria in American travelers to Africa. Implications for prevention strategies. JAMA. 1991 Jan 16;265(3):383-385.
12 Yechouron A, Nguyen C, MacLean JD, Keystone J. The changing pattern of imported malaria. Can Dis Wkly Rep. 1988 Jul 30;14(30):133-136.
13 Lewis SJ, Davidson RN, Ross EJ, Hall AP. Severity of imported falciparum malaria: effect of taking antimalarial prophylaxis. BMJ. 1992 Sep 26;305(6856):741-743.
14 Anonymous. Recommendations for the prevention of malaria among travelers. MMWR Recomm Rep. 1990 Mar 9;39(RR-3):1-10.
15 Probable Locally Acquired Mosquito-Transmitted Plasmodium vivax Infection—Georgia, 1996. MMWR Morb Mortal Wkly Rep. 1997;46(12):264-267. 3-28-97
16 Miller LH, Good MF, Milon G. Malaria pathogenesis. Science. 1994 Jun 24;264(5167):1878-1883.
17 Barnwell JW. Cytoadherence and sequestration in falciparum malaria. Exp Parasitol. 1989 Nov;69(4):407-412.
18 Clark IA, Gray KM, Rockett EJ, Cowden WB, Rockett KA, Ferrante A, et al. Increased lymphotoxin in human malarial serum, and the ability of this cytokine to increase plasma interleukin-6 and cause hypoglycaemia in mice: implications for malarial pathology. Trans R Soc Trop Med Hyg. 1992 Nov-Dec;86(6):602-607.
19 Turner G. Cerebral malaria. Brain Pathol. 1997 Jan;7(1):569-582.
20 Mishra SK, Wiese L. Advances in the management of cerebral malaria in adults. Curr Opin Neurol. 2009 Jun;22(3):302-307.
21 Mishra SK, Newton CR. Diagnosis and management of the neurological complications of falciparum malaria. Nat Rev Neurol. 2009 Apr;5(4):189-198.
22 Wattanagoon Y, Srivilairit S, Looareesuwan S, White NJ. Convulsions in childhood malaria. Trans R Soc Trop Med Hyg. 1994 Jul-Aug;88(4):426-428.
23 Mohan A, Sharma SK, Bollineni S. Acute lung injury and acute respiratory distress syndrome in malaria. J Vector Borne Dis. 2008 Sep;45(3):179-193.
24 Allen SJ, O’Donnell A, Alexander ND, Clegg JB. Severe malaria in children in Papua New Guinea. QJM.. 1996 Oct;89(10):779-788.
25 Phillips R, Pasvol G. Anaemia of Plasmodium falciparum malaria. Baill Clin Haem.. 1993;5:315-330.
26 Bruneel F, Gachot B, Wolff M, Regnier B, Danis M, Vachon F. Resurgence of blackwater fever in long-term European expatriates in Africa: report of 21 cases and review. Clin Infect Dis. 2001 Apr 15;32(8):1133-1140.
27 Uneke CJ. Concurrent malaria and typhoid fever in the tropics: the diagnostic challenges and public health implications. J Vector Borne Dis. 2008 Jun;45(2):133-142.
28 Saissy JM, Seck M, Rouvin B, Diatta B, Ndiaye M, Angel G. Hemodynamic aspects and oxygenation variables in severe malaria of adults in Africa. Intensive Care Med. 2000 Oct;26(10):1449-1453.
29 Bruneel F, Gachot B, Timsit JF, Wolff M, Bedos JP, Regnier B, et al. Shock complicating severe falciparum malaria in European adults. Intensive Care Med. 1997 Jun;23(6):698-701.
30 Kublin JG, Patnaik P, Jere CS, Miller WC, Hoffman IF, Chimbiya N, et al. Effect of Plasmodium falciparum malaria on concentration of HIV-1-RNA in the blood of adults in rural Malawi: a prospective cohort study. Lancet. 2005 Jan 15-21;365(9455):233-240.
31 Whitworth J, Morgan D, Quigley M, Smith A, Mayanja B, Eotu H, et al. Effect of HIV-1 and increasing immunosuppression on malaria parasitaemia and clinical episodes in adults in rural Uganda: a cohort study. Lancet. 2000 Sep 23;356(9235):1051-1056.
32 Steketee RW, Wirima JJ, Bloland PB, Chilima B, Mermin JH, Chitsulo L, et al. Impairment of a pregnant woman’s acquired ability to limit Plasmodium falciparum by infection with human immunodeficiency virus type-1. Am J Trop Med Hyg. 1996;55(Suppl. 1):42-49.
33 Bloland PB, Wirima JJ, Steketee RW, Chilima B, Hightower A, Breman JG. Maternal HIV infection and infant mortality in Malawi: evidence for increased mortality due to placental malaria infection. AIDS. 1995 Jul;9(7):721-726.
34 Ayisi JG, van Eijk AM, ter Kuile FO, Kolczak MS, Otieno JA, Misore AO, et al. The effect of dual infection with HIV and malaria on pregnancy outcome in western Kenya. AIDS. 2003 Mar 7;17(4):585-594.
35 Thursz MR, Kwiatkowski D, Torok ME, Allsopp CE, Greenwood BM, Whittle HC, et al. Association of hepatitis B surface antigen carriage with severe malaria in Gambian children. Nat Med. 1995 Apr;1(4):374-375.
36 Zingman BS, Viner BL. Splenic complications in malaria: case report and review. Clin Infect Dis. 1993 Feb;16(2):223-232.
37 Sarkar PK, Ahluwalia G, Vijayan VK, Talwar A. Critical care aspects of malaria. J Intensive Care Med. 2009 Mar-Apr;25(2):93-103.
38 Silver HM. Malarial infection during pregnancy. Infect Dis Clin North Am. 1997 Mar;11(1):99-107.
39 Blumberg L, Lee RP, Lipman J, Beards S. Predictors of mortality in severe malaria: a two year experience in a non-endemic area. Anaesth Intensive Care. 1996 Apr;24(2):217-223.
40 Greenberg A, Lobel HO. Mortality from Plasmodium falciparum malaria in travelers from the United States, 1959-1987. Ann Intern Med. 1990;113:326-327.
41 Wright PW, Avery WG, Ardill WD, McLarty JW. Initial clinical assessment of the comatose patient: cerebral malaria vs. meningitis. Pediatr Infect Dis J. 1993 Jan;12(1):37-41.
42 Stauffer WM, Cartwright CP, Olson DA, Juni BA, Taylor CM, Bowers SH, et al. Diagnostic performance of rapid diagnostic tests versus blood smears for malaria in US clinical practice. Clin Infect Dis. 2009 Sep 15;49(6):908-913.
43 Parija SC, Dhodapkar R, Elangovan S, Chaya DR. A comparative study of blood smear, QBC and antigen detection for diagnosis of malaria. Indian J Pathol Microbiol. 2009 Apr-Jun;52(2):200-202.
44 Ntoumi F, Contamin H, Rogier C, Bonnefoy S, Trape JF, Mercereau-Puijalon O. Age-dependent carriage of multiple Plasmodium falciparum merozoite surface antigen-2 alleles in asymptomatic malaria infections. Am J Trop Med Hyg. 1995 Jan;52(1):81-88.
45 Kolakovich KA, Ssengoba A, Wojcik K, Tsuboi T, al-Yaman F, Alpers M, et al. Plasmodium vivax: favored gene frequencies of the merozoite surface protein-1 and the multiplicity of infection in a malaria endemic region. Exp Parasitol. 1996 Jun;83(1):11-19.
46 Gilles H. Diagnostic methods in malaria. In: Gilles HM, Warrell DA, editors. Essential malariology. 3rd ed. London: P. Edward Arnold; 1993:78.
47 Snow RW, Peshu N, Forster D, Mwenesi H, Marsh K. The role of shops in the treatment and prevention of childhood malaria on the coast of Kenya. Trans R Soc Trop Med Hyg. 1992 May-Jun;86(3):237-239.
48 Craig MH, Sharp BL. Comparative evaluation of four techniques for the diagnosis of Plasmodium falciparum infections. Trans R Soc Trop Med Hyg. 1997 May-Jun;91(3):279-282.
49 Hanscheid T. Diagnosis of malaria: a review of alternatives to conventional microscopy. Clin Lab Haematol. 1999 Aug;21(4):235-245.
50 Arora S, Gaiha M, Arora A. Role of the Parasight-F Test in the Diagnosis of Complicated Plasmodium falciparum Malrial Infection. Braz J Infect Dis. 2003 October;7(5):332-338.
51 Farcas GA, Zhong KJ, Lovegrove FE, Graham CM, Kain KC. Evaluation of the Binax NOW ICT test versus polymerase chain reaction and microscopy for the detection of malaria in returned travelers. Am J Trop Med Hyg. 2003 Dec;69(6):589-592.
52 Palmer CJ, Bonilla JA, Bruckner DA, Barnett ED, Miller NS, Haseeb MA, et al. Multicenter study to evaluate the OptiMAL test for rapid diagnosis of malaria in U.S. hospitals. J Clin Microbiol. 2003 Nov;41(11):5178-5182.
53 Looareesuwan S, Wilairatana P, Krishna S, Kendall B, Vannaphan S, Viravan C, et al. Magnetic resonance imaging of the brain in patients with cerebral malaria. Clin Infect Dis. 1995 Aug;21(2):300-309.
54 World Health Oorganization. Guidelines for the Treatment of Malaria. Geneva: WHO; 2010. 194
55 Day NP, Phu NH, Mai NT, Bethell DB, Chau TT, Loc PP, et al. Effects of dopamine and epinephrine infusions on renal hemodynamics in severe malaria and severe sepsis. Crit Care Med. 2000 May;28(5):1353-1362.
56 Sukontason K, Karbwang J, Rimchala W, Tin T, Na-Bangchang K, Banmairuroi V, et al. Plasma quinine concentrations in falciparum malaria with acute renal failure. Trop Med Int Health. 1996 Apr;1(2):236-242.
57 Meremikwu M, Smith HJ. Blood transfusion for treating malarial anaemia. Cochrane Database Syst Rev (Online) 2000(2):CD001475.
58 Losert H, Schmid K, Wilfing A, Winkler S, Staudinger T, Kletzmayr J, et al. Experiences with severe P. falciparum malaria in the intensive care unit. Intensive Care Med. 2000 Feb;26(2):195-201.
59 Taylor TE, Molyneux ME, Wirima JJ, Fletcher KA, Morris K. Blood glucose levels in Malawian children before and during the administration of intravenous quinine for severe falciparum malaria. N Engl J Med. 1988 Oct 20;319(16):1040-1047.
60 White NJ, Warrell DA, Chanthavanich P, Looareesuwan S, Warrell MJ, Krishna S, et al. Severe hypoglycemia and hyperinsulinemia in falciparum malaria. N Engl J Med. 1983 Jul 14;309(2):61-66.
61 Phillips RE, Warrell DA, Looareesuwan S, Turner RC, Bloom SR, Quantrill D, et al. Effectiveness of SMS 201-995, a synthetic, long-acting somatostatin analogue, in treatment of quinine-induced hyperinsulinaemia. Lancet. 1986 Mar 29;1(8483):713-716.
62 Jones KL, Donegan S, Lalloo DG. Artesunate versus quinine for treating severe malaria. Cochrane Database Syst Rev (Online) 2007(4):CD005967.
63 Rosenthal PJ. Artesunate for the treatment of severe falciparum malaria. N Engl J Med. 2008 Apr 24;358(17):1829-1836.
64 Malaria Diagnosis and Treatment in the United States. CDC, Atlanta. http://www.cdc.gov/malaria/diagnosis_treatment/index.html, February 8, 2010. [cited March 16, 2010]; Available from
65 Griffith KS, Lewis LS, Mali S, Parise ME. Treatment of malaria in the United States: a systematic review. JAMA. 2007 May 23;297(20):2264-2277.
66 Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009 Jul 30;361(5):455-467.
67 Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM. Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med. 2008 Dec 11;359(24):2619-2620.
68 Warshaw EM, Zug KA. Sesquiterpene lactone allergy. Am J Contact Dermat. 1996 Mar;7(1):1-23.
69 Miller LG, Panosian CB. Ataxia and slurred speech after artesunate treatment for falciparum malaria. N Engl J Med. 1997 May 1;336(18):1328.
70 Deleu D, Schmedding E. Acute psychosis as idiosyncratic reaction to quinidine: report of two cases. BMJ (Clin Res Ed). 1987 Apr 18;294(6578):1001-1002.
71 Phillips-Howard PA, ter Kuile FO. CNS adverse events associated with antimalarial agents. Fact or fiction? Drug Saf. 1995 Jun;12(6):370-383.
72 Treluyer JM, Roux A, Mugnier C, Flouvat B, Lagardere B. Metabolism of quinine in children with global malnutrition. Pediatr Res. 1996 Oct;40(4):558-563.
73 Bethell DB, Phuong PT, Phuong CX, Nosten F, Waller D, Davis TM, et al. Electrocardiographic monitoring in severe falciparum malaria. Trans R Soc Trop Med Hyg. 1996 May-Jun;90(3):266-269.
74 Davis TM, Suputtamongkol Y, Spencer JL, Wilson SG, Mekhton S, Croft KD, et al. Glucose turnover in pregnant women with acute malaria. Clin Sci (Lond). 1994 Jan;86(1):83-90.
75 Eriksson B, Bjorkman A, Keisu M. How safe is proguanil? A post-marketing investigation of side-effects. Scand J Infect Dis. 1991;23(4):489-493.
76 Emberger M, Lechner AM, Zelger B. Stevens-Johnson syndrome associated with Malarone antimalarial prophylaxis. Clin Infect Dis. 2003 Jul 1;37(1):e5-e7.
77 van Genderen PJ, Hesselink DA, Bezemer JM, Wismans PJ, Overbosch D. Efficacy and safety of exchange transfusion as an adjunct therapy for severe Plasmodium falciparum malaria in nonimmune travelers: a 10-year single-center experience with a standardized treatment protocol. Transfusion. 2010 Apr;50(4):787-794.
78 Macallan DC, Pocock M, Robinson GT, Parker-Williams J, Bevan DH. Red cell exchange, erythrocytapheresis, in the treatment of malaria with high parasitaemia in returning travellers. Trans R Soc Trop Med Hyg. 2000 Jul-Aug;94(4):353-356.
79 Weir EG, King KE, Ness PM, Eshleman SH. Automated RBC exchange transfusion: treatment for cerebral malaria. Transfusion. 2000 Jun;40(6):702-707.
80 Gulprasutdilog S, Chongkolwatana V, Buranakitjaroen P, Jaroonvesama N. Exchange transfusion in severe falciparum malaria. J Med Assoc Thai. 1999 Jan;82(1):1-8.
81 Mabeza GF, Biemba G, Gordeuk VR. Clinical studies of iron chelators in malaria. Acta Haematol. 1996;95(1):78-86.
82 Smith HJ, Meremikwu M. Iron chelating agents for treating malaria. Cochrane Database Syst Rev (Online) 2003(2):CD001474.
83 Di Perri G, Di Perri IG, Monteiro GB, Bonora S, Hennig C, Cassatella M, et al. Pentoxifylline as a supportive agent in the treatment of cerebral malaria in children. J Infect Dis. 1995 May;171(5):1317-1322.
84 Looareesuwan S, Wilairatana P, Vannaphan S, Wanaratana V, Wenisch C, Aikawa M, et al. Pentoxifylline as an ancillary treatment for severe falciparum malaria in Thailand. Am J Trop Med Hyg. 1998 Mar;58(3):348-353.
85 Looareesuwan S, Sjostrom L, Krudsood S, Wilairatana P, Porter RS, Hills F, et al. Polyclonal anti-tumor necrosis factor-alpha Fab used as an ancillary treatment for severe malaria. Am J Trop Med Hyg. 1999 Jul;61(1):26-33.
86 Kwiatkowski D, Molyneux ME, Stephens S, Curtis N, Klein N, Pointaire P, et al. Anti-TNF therapy inhibits fever in cerebral malaria. QJM. 1993 Feb;86(2):91-98.
87 van Hensbroek MB, Palmer A, Onyiorah E, Schneider G, Jaffar S, Dolan G, et al. The effect of a monoclonal antibody to tumor necrosis factor on survival from childhood cerebral malaria. J Infect Dis. 1996 Nov;174(5):1091-1097.
88 Krishna S, Supanaranond W, Pukrittayakamee S, Kuile FT, Ruprah M, White NJ. The disposition and effects of two doses of dichloroacetate in adults with severe falciparum malaria. Br J Clin Pharmacol. 1996 Jan;41(1):29-34.
89 Hoffman SL, Rustama D, Punjabi NH, Surampaet B, Sanjaya B, Dimpudus AJ, et al. High-dose dexamethasone in quinine-treated patients with cerebral malaria: a double-blind, placebo-controlled trial. J Infect Dis. 1988 Aug;158(2):325-331.
90 Genton B, al-Yaman F, Alpers MP, Mokela D. Indicators of fatal outcome in paediatric cerebral malaria: a study of 134 comatose Papua New Guinean children. Int J Epidemiol. 1997 Jun;26(3):670-676.
91 van Hensbroek MB, Palmer A, Jaffar S, Schneider G, Kwiatkowski D. Residual neurologic sequelae after childhood cerebral malaria. J Pediatr. 1997 Jul;131(1 Pt 1):125-129.
92 Jaffar S, Van Hensbroek MB, Palmer A, Schneider G, Greenwood B. Predictors of a fatal outcome following childhood cerebral malaria. Am J Trop Med Hyg. 1997 Jul;57(1):20-24.
93 Bruneel F, Hocqueloux L, Alberti C, Wolff M, Chevret S, Bedos JP, et al. The clinical spectrum of severe imported falciparum malaria in the intensive care unit: report of 188 cases in adults. Am J Respir Crit Care Med. 2003 Mar 1;167(5):684-689.
94 Mohapatra MK, Das SP. The malaria severity score: a method for severity assessment and risk prediction of hospital mortality for falciparum malaria in adults. J Assoc Physicians India. 2009 Feb;57:119-126.
95 Hanson J, Lee SJ, Mohanty S, Faiz MA, Anstey NM, Charunwatthana P, et al. A simple score to predict the outcome of severe malaria in adults. Clin Infect Dis. 2010 Mar 1;50(5):679-685.
96 Olumese PE, Sodeinde O, Gbadegesin RA, Nafiu O, Oguche S, Walker O. Respiratory distress adversely affects the outcome of childhood cerebral malaria. Trans R Soc Trop Med Hyg. 1995 Nov-Dec;89(6):634.
97 Mabeza GF, Moyo VM, Thuma PE, Biemba G, Parry D, Khumalo H, et al. Predictors of severity of illness on presentation in children with cerebral malaria. Ann Trop Med Parasitol. 1995 Jun;89(3):221-228.
98 Marsh K, Forster D, Waruiru C, Mwangi I, Winstanley M, Marsh V, et al. Indicators of life-threatening malaria in African children. N Engl J Med. 1995 May 25;332(21):1399-1404.
99 Zucker JR, Lackritz EM, Ruebush TK2nd, Hightower AW, Adungosi JE, Were JB, et al. Childhood mortality during and after hospitalization in western Kenya: effect of malaria treatment regimens. Am J Trop Med Hyg. 1996 Dec;55(6):655-660.
100 Day NP, Phu NH, Mai NT, Chau TT, Loc PP, Chuong LV, et al. The pathophysiologic and prognostic significance of acidosis in severe adult malaria. Crit Care Med. 2000 Jun;28(6):1833-1840.
101 Muntendam AH, Jaffar S, Bleichrodt N, van Hensbroek MB. Absence of neuropsychological sequelae following cerebral malaria in Gambian children. Trans R Soc Trop Med Hyg. 1996 Jul-Aug;90(4):391-394.
102 Basco LK, Le Bras J. In vitro activity of artemisinin derivatives against African isolates and clones of Plasmodium falciparum. Am J Trop Med Hyg. 1993 Sep;49(3):301-307.
103 Jelinek T, Schelbert P, Loscher T, Eichenlaub D. Quinine resistant falciparum malaria acquired in East Africa. Trop Med Parasitol. 1995 Mar;46(1):38-40.
104 Lege-Oguntoye L, Abua JU, Werblinska B, Ogala WN, Slotboom AB, Olurinola PF. Chloroquine-resistant Plasmodium falciparum with reduced sensitivity in vitro to mefloquine and quinine in Zaria, northern Nigeria. J Trop Med Hyg. 1991 Apr;94(2):73-75.
105 Phillips EJ, Keystone JS, Kain KC. Failure of combined chloroquine and high-dose primaquine therapy for Plasmodium vivax malaria acquired in Guyana, South America. Clin Infect Dis. 1996 Nov;23(5):1171-1173.
106 Longworth DL. Drug-resistant malaria in children and in travelers. Pediatr Clin North Am. 1995 Jun;42(3):649-664.
107 Baird JK, Basri H, Purnomo, Bangs MJ, Subianto B, Patchen LC, et al. Resistance to chloroquine by Plasmodium vivax in Irian Jaya, Indonesia. Am J Trop Med Hyg. 1991 May;44(5):547-552.
108 Arias AE, Corredor A. Low response of Colombian strains of Plasmodium vivax to classical antimalarial therapy. Trop Med Parasitol. 1989 Mar;40(1):21-23.
109 Murphy GS, Basri H, Purnomo, Andersen EM, Bangs MJ, Mount DL, et al. Vivax malaria resistant to treatment and prophylaxis with chloroquine. Lancet. 1993 Jan 9;341(8837):96-100.
110 Schuurkamp GJ, Spicer PE, Kereu RK, Bulungol PK, Rieckmann KH. Chloroquine-resistant Plasmodium vivax in Papua New Guinea. Trans R Soc Trop Med Hyg. 1992 Mar-Apr;86(2):121-122.
111 Fryauff DJ, Tuti S, Mardi A, Masbar S, Patipelohi R, Leksana B, et al. Chloroquine-resistant Plasmodium vivax in transmigration settlements of West Kalimantan, Indonesia. Am J Trop Med Hyg. 1998 Oct;59(4):513-518.
112 Baird JK, Wiady I, Fryauff DJ, Sutanihardja MA, Leksana B, Widjaya H, et al. In vivo resistance to chloroquine by Plasmodium vivax and Plasmodium falciparum at Nabire, Irian Jaya, Indonesia. Am J Trop Med Hyg. 1997 Jun;56(6):627-631.
113 Dua VK, Kar PK, Sharma VP. Chloroquine resistant Plasmodium vivax malaria in India. Trop Med Int Health. 1996 Dec;1(6):816-819.
114 Soto J, Toledo J, Gutierrez P, Luzz M, Llinas N, Cedeno N, et al. Plasmodium vivax clinically resistant to chloroquine in Colombia. Am J Trop Med Hyg. 2001 Aug;65(2):90-93.
115 Alecrim MDG, Alecrim W, Macedo V. Plasmodium vivax resistance to chloroquine (R2) and mefloquine (R3) in Brazilian Amazon region. Rev Soc Bras Med Trop. 1999 Jan-Feb;32(1):67-68.
116 Ruebush TK2nd, Zegarra J, Cairo J, Andersen EM, Green M, Pillai DR, et al. Chloroquine-resistant Plasmodium vivax malaria in Peru. Am J Trop Med Hyg. 2003 Nov;69(5):548-552.
117 Maguire JD, Sumawinata IW, Masbar S, Laksana B, Prodjodipuro P, Susanti I, et al. Chloroquine-resistant Plasmodium malariae in south Sumatra, Indonesia. Lancet. 2002 Jul 6;360(9326):58-60.