CHAPTER 105 Magnetic Resonance Imaging of Vascular Disorders of the Abdomen
INTRODUCTION
Gadolinium Agent Use and Safety
Concerns regarding the use of Gd-chelate contrast agents in the setting of renal disease have arisen since the initial description of a new disease, nephrogenic systemic fibrosis (NSF),1 and the subsequent correlation between prior Gd-chelate contrast agent exposure and development of NSF in patients with severe renal dysfunction.2 The current understanding of NSF is that nearly all cases have been attributed to exposure to gadodiamide (Omniscan) in more than 90% of peer-reviewed published cases, with the remainder of NSF cases associated with gadoversetamide (Optimark) or gadopentetate dimeglumine (Magnevist) exposure. Gadodiamide has relatively lower conditional stability, and it is believed that prolonged exposure to this agent, as occurs in the setting of reduced renal clearance, leads to greater chelate ligand dissociation and deposition of free gadolinium in tissues, including skin, leading to activation of the fibrotic process associated with NSF. NSF is primarily a disease affecting patients with severely impaired renal function (less than 15 mL/min) and mostly affecting patients on dialysis.1 Furthermore, NSF risk and severity appears to correlate with higher single and cumulative total gadodiamide dose.3 However, the risk of NSF using low dose, high relaxivity linear agents or high conditional stability cyclic agents appears immeasurably small; there remain no documented cases of NSF in the peer-reviewed literature with any of these other agents. Given concerns regarding NSF, we currently use a dose of 0.05 to 0.1 mmol/kg of a more stable high relaxivity linear or macrocyclic Gd-chelate contrast agent, mixed with an equal volume of normal saline. The contrast is then injected at a rate of 2 mL/s, followed by at least 20 mL of saline flush using a dual-chamber injector, which allows for a continuous influx of gadolinium during imaging. Our current understanding of NSF, and of contrast-induced nephropathy (CIN) associated with iodinated CT contrast, supports preferential use of contrast enhanced MRI over CT in patients with impaired renal function.4 Not yet published data documenting immeasurably low risk of higher cumulative dose administrations of high conditional stability cyclic agents may help to further define these dosage recommendations.
Cirrhosis and Portal Hypertension
Etiology and Pathophysiology
The parenchymal and vascular changes seen with MRI parallel the pathophysiologic changes occurring within the portal venous system, and are reflective of the underlying resistance to portal flow in the hepatic sinusoids. Chronic liver disease induces vasoconstriction within the hepatic microcirculation, owing to a relative lack of nitrous oxide (NO) and the action of potent vasoconstrictors.5 The larger portal venous branches dilate in a compensatory effort to increase portal flow. The reduction in NO within the hepatic microcirculation is reversed in the splanchnic vasculature,5 which also dilates in an attempt to increase portal flow and overcome developing resistance. An enlarged portal vein, superior mesenteric vein (SMV), and splenic vein are clear indicators of portal hypertension (Fig. 105-1). Flow in the portal vein, normally directed into the liver (hepatopetal) eventually reverses direction away from the liver (hepatofugal). These altered flow dynamics are most commonly assessed with ultrasound, although MRI has the capability to provide additional functional measures of portal flow that better correlate with the severity of cirrhosis and portal hypertension when compared with ultrasound.6 Continued increased resistance to portal flow in the hepatic sinusoids results in shunting of portal blood to the systemic venous system at predictable locations. Shunted blood pools at these sites of portosystemic anastomosis, causing the formation of varices.
Manifestations of Disease
Imaging Indications and Algorithm
MRI has the unique ability to characterize underlying hepatic parenchymal changes prior to the development of frank portal hypertension. The hepatic fibrosis caused by repeated liver injury is seen on delayed phase, 3D GRE images, showing a fine or coarse reticular pattern of enhancement in the underlying hepatic parenchyma (Fig. 105-2). This reticular parenchymal enhancement can identify fibrotic liver disease before morphologic changes of cirrhosis and portal hypertension develop. This is a powerful tool in that it allows for an earlier, more aggressive treatment plan in patients at high risk for chronic liver disease. Conventional catheter angiography assesses strictly vascular morphology, which only typically becomes abnormal with more advanced disease and cannot evaluate parenchymal changes of fibrosis. CTA can also provide a noninvasive method of evaluating both vessels and soft tissues; however, it lacks the underlying contrast resolution to adequately assess fibrotic changes in the hepatic parenchyma. In addition, CTA and catheter angiography employ the use of ionizing radiation, a concern especially in the setting of chronic liver disease, which often requires repeated, multiphase imaging. MR is the only modality with the contrast and spatial resolution to fully assess the vasculature and hepatic parenchyma, with the added benefit of not employing ionizing radiation. Currently, early detection of fibrotic liver disease is primarily diagnosed through biopsy, but the use of MR to grade liver fibrosis may reduce the need for liver biopsy, which carries risks of hepatic injury, bleeding, and infection.
Imaging Techniques and Findings
MR
The most dangerous consequence of portosystemic shunting is bleeding from esophageal varices (Fig. 105-3). Varices may be identified within the esophageal wall (termed esophageal varices) or surrounding the esophagus (termed paraesophageal varices), although both drain the portal system via the left gastric (cardinal) vein. Additional common sites of variceal formation within the abdomen due to portosystemic shunting include gastric and retroperitoneal varices, spontaneous splenorenal shunts, and recanalized paraumbilical veins. Recanalized paraumbilical veins drain into the epigastric veins along the undersurface of the abdominal wall, often forming a mesh of dilated varices surrounding the umbilicus termed caput medusa (Fig. 105-4). Identification of paraumbilical varices are important in that the draining meshwork of superficial veins along the anterior abdominal wall must be avoided during diagnostic and therapeutic paracenteses that are often performed in patients with chronic liver disease. Although less common, intrahepatic portosystemic (venovenous shunts) may also occur in the setting of chronic liver disease as a result of increased portal resistance. These shunts have been described at the microscopic level7 and can also be identified with MR (Fig. 105-5). Other etiologies of intrahepatic portosystemic shunting include congenital malformations and rupture of a portal vein aneurysm into a hepatic vein, whereas post-traumatic shunts are most often associated with surgical interventions such as transhepatic catheter placement.
In addition to portosystemic shunting, chronic liver injury and fibrosis also causes arterioportal shunting, either secondary to underlying tumor (hepatocellular carcinoma) or cased by the parenchymal changes of fibrosis itself. These arterioportal shunts manifest on MR as small, linear- or wedge-shaped blushes of enhancement on arterial phase imaging, most often located along a capsular surface (Fig. 105-6). These foci of enhancement may be mistaken for a small tumor on CT owing to a relative lack of contrast resolution. However, MRI can more easily differentiate these small arterioportal shunts (APS) from hepatocellular carcinoma (HCC) in the majority of cases because the APS will show a more linear- or wedge-shaped morphology and will not demonstrate the washout and rim enhancement typical of HCC. Arterioportal shunts may also occur in settings other than chronic liver disease, such as trauma, iatrogenic (postbiopsy or surgery), congenital anomalies, or aneurysm rupture.8 APS are associated with both benign (hemangiomas) and malignant (HCC) hepatic tumors.9 APS also contribute to the flow changes seen in portal hypertension. Studies examining flow characteristics in the portal vein with intermittent occlusion of the hepatic artery have demonstrated that APS are a factor in hepatofugal flow of the portal vein in the setting of chronic liver disease.10
Portal and Mesenteric Thrombosis
Imaging Techniques and Findings
MR
Intraluminal thrombus within the SMV is well demonstrated on MRI with 3D GRE images, providing a noninvasive method for diagnosis (Fig. 105-7). In addition, evaluation of the end-organ effects of mesenteric occlusion is important because the clinical picture varies between acute and chronic presentations, and not all patients require surgery. Bowel pathology can be evaluated concurrently with the vasculature using MRI, with ischemia demonstrating increased signal on fat-saturated T2W images secondary to inflammation and edema caused by venous congestion. The presence of bowel ischemia as identified by MR may prompt more aggressive therapy, with both percutaneous and intraoperative catheter-directed therapy demonstrating good results.11 Thrombosis of the splenic vein is a cause of isolated gastric varices and has been treated with splenectomy in the past, although percutaneous therapy has also been successful.
Synopsis of Treatment Options
Surgical/Interventional
Malignant Hepatic Neoplasms
Imaging Techniques and Findings
MR
MRI has been shown to be highly sensitive and specific for HCC,12 a tumor that is strongly associated with chronic liver disease. In the well-defined, focal type of HCC, defined MR features include an arterial enhancing lesion that demonstrates washout with rim enhancement on delayed images. The tumor washes out to become hypointense to adjacent liver parenchyma, a direct result of the differential vascular supply between tumor (fed primarily by hepatic artery) and adjacent liver parenchyma (fed primarily by portal vein). T2 signal, if elevated, supports the diagnosis, although this is not a necessary feature. These imaging features are distinct from intrahepatic cholangiocarcinoma (IHC), the second primary hepatic malignancy that tends to invade hepatic vasculature. MRI is also the imaging modality of choice for the evaluation of IHC. After the administration of gadolinium chelate, the tumor does not avidly enhance on arterial phase images, but demonstrates gradual accumulation of contrast on delayed imaging, likely due to leakage of gadolinium into the interstices of the tumor over time. IHC is usually associated with ill-defined T2 signal, and at least some degree of biliary ductal dilation is usually present. Both of these neoplasms invade intrahepatic portal and hepatic venous branches, a finding especially common in the infiltrative type of HCC. The distinction between tumor thrombus and bland thrombus in the setting of these neoplasms is important, especially in the setting of HCC when liver transplantation is considered. The diagnosis of tumor thrombus can be made with the use of 3D GRE sequences. Bland thrombus has no associated vascularity and will not have any signal on postgadolinium imaging, producing images of a black clot. Tumor thrombus, alternatively, will take up gadolinium on delayed imaging, which can be easily demonstrated with comparison to precontrast images. Any enhancement within a thrombus associated with these tumors is indicative of vascular tumor invasion (Fig. 105-8).
Synopsis of Treatment Options
Surgical/Interventional
Liver Transplantation
Manifestations of Disease
Clinical Presentation
Liver transplantation is the treatment of choice for patients with end-stage liver disease, and is also intended as a curative procedure for patients with HCC meeting specific imaging criteria.13 Patency of the portal and mesenteric vasculature is critical in the pretransplant cirrhotic patient because portal thrombosis will hasten liver failure. In addition, extension of thrombus into the SMV directly affects the technical feasibility of liver transplantation. If the patent portion of the SMV is large enough, the surgeon may attempt an interposition graft from the SMV to the transplanted liver. If, however, the patent SMV tributaries are small or replaced by varices, liver transplantation is not technically feasible. MRI is an excellent technique to evaluate mesenteric thrombosis and the technical feasibility of transplantation, providing accurate evaluation of vessel size and varices.
Imaging Techniques and Findings
MR
MR has been shown to be equivalent to CTA in the preoperative evaluation of hepatic vascular anatomy,14 but with the added benefits of improved parenchymal soft tissue evaluation compared to CT and also the lack of ionizing radiation, an especially important issue in the pediatric population. Of note, liver transplantation is usually performed with intent to cure and not as a palliative procedure. In this setting, cumulative radiation dose is a consideration, especially in patients with chronic liver disease who undergo surveillance imaging. MRI provides a superior imaging modality for evaluation of parenchymal and vascular changes associated with liver disease without contributing to cumulative radiation doses in a patient set to undergo curative surgery.
MRI performs quite well in the vascular assessment of the postoperative transplanted liver. In the immediate postoperative setting, complex and hemorrhagic blood products are often identified in and around the surgical bed. The most serious immediate postoperative complication is hepatic artery thrombosis, which occurs at variable rates from 3% to 9% and is more common with LDLT in centers with less experience.15 MRI not only directly detects hepatic artery thrombosis (HAT), but also the resultant changes in the hepatic parenchyma related to arterial devascularization. The consequences of acute hepatic artery thrombosis are severe and often require retransplantation to avoid graft loss. More commonly, HAT results in defined biliary complications because the sole vascular supply of the biliary system is via the hepatic artery in graft livers. These biliary complications include ductal dilation, biloma formation, and sepsis secondary to a resultant cholangitis (Fig. 105-9). MRI can also demonstrate the acute hepatic inflammation that is associated with bile stasis and infection16 better than any other imaging modality. Thromboses of the IVC, hepatic and portal veins are less common complications, but also are well assessed with 3D GRE imaging. Another rare vascular complication is the splenohepatic arterial steal syndrome. This is characterized by arterial malperfusion and ischemic damage of the hepatic graft caused by diversion of blood flow to a markedly enlarged spleen. Splenohepatic arterial steal syndrome may ultimately result in graft loss if it is recognized too late. A post-transplantation splenectomy represents a successful therapeutic approach for this condition. Even in the absence of pathology, a transplanted liver may be identified with MR by reproducible mild narrowing at the IVC and portal vein anastomosis with mild susceptibility artifact at the IVC anastomosis (Fig. 105-10).
Budd-Chiari Syndrome
Prevalence and Epidemiology
Budd-Chiari occurs equally in men and women, usually presenting in the 3rd and 4th decade.
Etiology and Pathophysiology
Sinusoidal congestion induces inflammation and fibrosis, leading to the common endpoint of hepatic fibrosis and portal hypertension from resistance to portal inflow. The etiology of this syndrome is variable, and includes vascular webs, venous tumor invasion (especially by HCC), inflammation (especially Behçet syndrome) and iatrogenic causes, although an underlying hypercoagulable disorder is present in most cases.17
Imaging Techniques and Findings
MR
The diagnosis of Budd Chiari depends on evaluation of the hepatic venous outflow system in addition to the associated parenchymal changes, and MR is the modality best suited for evaluation of both areas. Evaluation of the venous system is performed with postcontrast 3D GRE sequences in axial and coronal planes. Venous patency can also be assessed on additional imaging sequences, such as evaluating vascular signal on TFISP imaging and “black blood” flow void on T2W images. The ability to assess vessel patency on additional imaging sequences provides an inherent level of redundancy within the image acquisition that contributes to the high sensitivity and specificity of MR compared to CT. Parenchymal changes in Budd-Chiari are also well evaluated with MR. The most consistent feature is marked hypertrophy of the caudate lobe (sometimes quite massive) due to its separate drainage into the IVC, thus sparing the caudate from the effects of venous outflow obstruction (Fig. 105-11). In the acute setting, the liver is usually enlarged secondary to edema, whereas in the chronic setting there is atrophy of the peripheral aspects of the liver and sparing of the caudate. Postcontrast 3D GRE images demonstrate decreased perfusion of the peripheral aspects of the liver secondary to altered flow dynamics induced by liver injury. An additional parenchymal finding is the presence of focal nodules in the liver parenchyma. These nodules are termed “large regenerative nodules” or “multiacinar regenerative nodules”18 and correlate to nontumorous liver parenchyma surrounded by variable amounts of fibrous stroma.18 These nodules characteristically show no distinctive enhancement features compared with adjacent liver parenchyma or arterial phase images.
Splanchnic Artery Aneurysm
Prevalence and Epidemiology
True aneurysms represent a rare disorder, with an incidence between 0.1% to 2%. The visceral arteries most prone to true aneurysm formation are the splenic (Fig. 105-12) and hepatic arteries, having approximately 60% and 20% relative incidence, respectively. Splenic artery aneurysms are encountered in two distinct patient populations—women with multiple pregnancies and patients with portal hypertension. Other causes of splenic artery aneurysms include mycotic infection, fibromuscular dysplasia, and congenital causes. True aneurysms are most commonly located in the distal third of the splenic artery (75%), followed by the middle third (20)%. Hepatic artery aneurysms, in contrast, are seen most often in patients with hypertension, fibromuscular dysplasia, or polyarteritis nodosa. Seventy-seven percent of hepatic aneurysms are isolated to the segment proximal to the liver, whereas 20% have combined intra- and extraparenchymal involvement; 3% are localized exclusively within liver.20
Imaging Techniques and Findings
MR
MRI is highly sensitive for the detection of splanchnic artery aneurysms, which may be detected on CE 3D MRA or 3D GRE imaging. 3D GRE images better depict the aneurysm wall and associated thrombus, and are more helpful for distinguishing true from false aneurysms. Important preoperative information that is depicted with MR includes the type and location of the aneurysm, diameter and extent along the vessel, involvement of branch vessels, presence of mural thrombus, and whether the aneurysm has already ruptured.21 Morphologic imaging, including T2W images and TFISP, are also important for aneurysm assessment. Hepatic and gastroduodenal aneurysms may manifest with obstructive jaundice secondary to compression of the biliary tree, which is demonstrated well with T2W images (Fig. 105-13). Mycotic aneurysms show perianeurysmal inflammation, which is depicted as surrounding high signal on fat-suppressed T2W images.
Synopsis of Treatment Options
Surgical/Interventional
Because the natural course of splanchnic artery aneurysms (especially pseudoaneurysms) may lead to rupture, MRI is critical in providing the correct diagnosis to prompt therapy and treatment planning. Although therapy is clearly mandated in patients with asymptomatic aneurysms or contained rupture (pseudoaneurysms), the following asymptomatic lesions also warrant intervention: (1) splenic artery aneurysms in patients with the potential to become pregnant or requiring liver transplantation; (2) common or proper hepatic artery aneurysms in patients with polyarteritis nodosa or fibromuscular dysplasia; and (3) splenic or hepatic artery aneurysms greater than 2.0 cm in diameter.22 Percutaneous treatment with aneurysm coiling has demonstrated excellent results compared to surgery and should be the first line of therapy.
Pancreatic Pathology: Vascular Involvement
Manifestations of Disease
Imaging Indications and Algorithm
MRI of the pancreas continues to evolve with improvement in rapid-acquisition breath-hold or breathing-independent imaging techniques. MR should be considered essential in the imaging evaluation of pancreatic disease, and particularly for optimal presurgical identification, characterization, and staging of pancreatic masses. High resolution CE 3D MRA can easily define arterial vascular anatomy for presurgical planning, and MR assessment of vascular infiltration by tumor reaches a sensitivity and specificity of 83% and 96%, respectively, comparable to CT and endoscopic sonography evaluation.23 MR also offers concurrent, superior, soft tissue imaging that defines tumor size, invasion into adjacent soft tissue structures, pancreatic and biliary ductal obstruction, and also venous invasion by tumor.
Imaging Techniques and Findings
MR
The most common presentation is that of biliary ductal obstruction from tumor in the pancreatic head, at which point assessment of the adjacent portal vein, SMV and SMA are critical if surgical resection is attempted. Evaluation depends on a combination of the coronal MRA source data and 3D GRE images. Vascular tumor infiltration is assumed on the basis of either vessel caliber reduction, vessel encasement (90% circumferential involvement) or vessel occlusion.24 Extensive vascular collaterals may also be demonstrated. Pancreatic adenocarcinoma may also arise within the tail, typically presenting as a larger mass with local infiltration (Fig. 105-14) that commonly encases or occludes the splenic vein, and may invade the splenic hilum, leading to extensive infarction of the spleen.
Inflammatory processes of the pancreas also commonly involve the adjacent vasculature. This is seen in the setting of acute pancreatitis, which is a well-known cause of splenic vein thrombosis (Fig. 105-15), and has also been reported in at least 20% of patients with chronic pancreatitis. Occlusion of the splenic vein is often seen in association with collateralized venous flow into extensive peripancreatic and perisplenic varices. Splenic vein thrombosis is also a well-known cause of isolated gastric varices, and less commonly a cause of esophageal or colonic varices. Postcontrast 3D GRE imaging is the best sequence for depiction of splenic vein thrombosis and associated collateral flow from varices. The splenic artery may also be secondarily involved in cases of severe pancreatitis, with the formation of aneurysms and pseudoaneurysms secondary to vascular endothelial breakdown from the adjacent inflammatory process. Pseudocysts may erode into the adjacent splenic artery or vein, causing life-threatening episodes of hemorrhage.
Splenic Infarction
Prevalence and Epidemiology
Splenic infarcts are often seen in the setting of chronic liver disease, likely due to relative ischemia produced by a massively enlarged spleen. Other clinical settings with a predisposition to splenic infarction include arterial emboli (most commonly of cardiac origin), sickle cell anemia, infiltrative disorders such as Gaucher disease, hematological malignancies, collagen vascular diseases, and rarely, splenic torsion.25
Imaging Techniques and Findings
MR
On MRI, splenic infarcts are seen as peripheral wedge-shaped or linear defects that exhibit decreased signal intensity on both T1W and T2W images. These areas show no internal perfusion after the administration of Gd, most clearly defined on delayed postcontrast images (Fig. 105-16). Complications of splenic infarctions include abscess, splenic pseudocyst formation, splenic rupture and hemorrhage—all features that are easily depicted with MRI. Rarely, the entire spleen may be infarcted, demonstrating diffuse low-signal intensity on T1W images, inhomogeneous high-signal intensity on T2W, and an enhancing capsule on postgadolinium images.
Differential Diagnosis from Imaging Findings
Wedge-shaped perfusion defects within the spleen are highly specific for splenic infarction.
Polyarteritis Nodosum
Hereditary Hemorrhagic Telangiectasia
Imaging Techniques and Findings
MR
Hepatic involvement occurs in 8% to 31% of cases, and MR images can identify hepatic AVMs and associated features, such as a prominent feeding hepatic artery, dilated portal and/or hepatic veins, and collateral supply involving the gastric, pancreaticoduodenal, splenic, mesenteric and renal vasculature. MR imaging is also used in the staging and follow-up of these abnormalities in patients with HHT.27
AVMs can also uncommonly occur in the spleen. Reports of splenic AVMs have described patients presenting with massive diarrhea, ascites, abdominal pain, and signs of portal hypertension. MRI can demonstrate these lesions as multiple, serpiginous flow voids on T2W images and extensive, serpentine enhancement on 3D GRE images. Dilated feeding vessels in association with AVMs are also common, and are easily depicted on MR.20
Trauma
Manifestations of Disease
Imaging Indications and Algorithm
The role of MRI in patients with acute traumatic injury to the abdomen is less well defined, and CT remains the primary imaging modality in this scenario, primarily due to speed and ease of accessibility. However, MR may be useful in selected cases of traumatic injury. Settings that may benefit from an increased use of MR imaging include the pediatric population, in which trauma patients are often subject to pan-body CT in addition to multiple follow-up examinations. There is an increased lifetime radiation risk in children relative to adults, and the use of CT in the trauma setting may be overused, especially in the pediatric population.28 Other clinical settings include that of underlying allergy to iodinated contrast, or in the setting of chronic renal disease. The risk of contrast-induced nephropathy (CIN) is an important consideration in patients with chronic renal disease. CIN occurs at a rate of approximately 3% in the general population, but increases to 12% to 33% with underlying diabetes and chronic renal disease. In addition, CIN nephropathy has been shown to correlate with increased morbidity and mortality, even if renal dysfunction is transient.29 Consideration of MRI in selected cases such as these would limit patient morbidity induced by imaging and is also useful as a problem-solving modality.
Imaging Techniques and Findings
MR
MRI combined with MRA can also readily identify vascular complications of the abdominal vessels as a complication of different surgeries and procedures (Fig. 105-17). Bile duct injury in the setting of laparoscopic cholecystectomy is a well-known complication. Because biliary injuries sustained during laparoscopic cholecystectomy are known to occur more proximally compared to open cholecystectomy, a higher incidence of concomitant hepatic arterial injury has been described.30 This complication is potentially lethal and often requires partial hepatectomy or liver transplantation. MRA and magnetic resonance cholangiography (MRCP) can be performed emergently in patients with a suspicion of biliary and vascular injury, allowing the simultaneous evaluation of both the biliary tree and the hepatic vascular supply in these patients.
Synopsis of Treatment Options
Surgical/Interventional
KEY POINTS
 CT remains the first-line modality of choice in trauma patients, although MRI should be considered in select patient populations (such as children), chronic renal disease, and pregnancy.
CT remains the first-line modality of choice in trauma patients, although MRI should be considered in select patient populations (such as children), chronic renal disease, and pregnancy.Hogspiel KD, Leung DA, Angle JF, et al. MR angiography of the mesenteric vasculature. Radiol Clin North Am. 2002;40:867-886.
Martin DR, Brown MA, Semella RC. Primer on MR imaging of the abdomen and pelvis. Hoboken, NJ: John Wiley & Sons, Inc; 2005.
Nael K, Laub G, Finn JP. Three-dimensional contrast-enhanced MR angiography of the thoraco-abdominal vessels. Magn Reson Imaging Clin North Am. 2005;13:359-380.
1 Cowper SE, Su LD, Bhawan J, et al. Nephrogenic fibrosing dermopathy. Am J Dermatopathol. 2001;23:383-393.
2 Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17:2359-2362.
3 Lauenstein TC, Salman K, Morreira R, et al. Nephrogenic systemic fibrosis: center case review. J Magn Reson Imaging. 2007;26:1198-1203.
4 Martin DR. Nephrogenic systemic fibrosis: A radiologist’s practical perspective. Eur J Radiol. 2008;66(2):220-224.
5 Garcia-Tsao G. Portal hypertension. Current opinion in gastroenterology. 2006;22:254-262.
6 Annet L, Materne R, Danse E, et al. Hepatic flow parameters measured with MR imaging and Doppler US: correlations with degree of cirrhosis and portal hypertension. Radiology. 2003;229:409-414.
7 Villeneuve JP, Dagenais M, Huet PM, et al. The hepatic microcirculation in the isolated perfused human liver. Hepatology (Baltimore, Md). 1996;23:24-31.
8 Gabriel S, Maroney TP, Ringe BH. Hepatic artery-portal vein fistula formation after percutaneous liver biopsy in a living liver donor. Transplantation Proc. 2007;39:1707-1709.
9 Byun JH, Kim TK, Lee CW, et al. Arterioportal shunt: prevalence in small hemangiomas versus that in hepatocellular carcinomas 3 cm or smaller at two-phase helical CT. Radiology. 2004;232:354-360.
10 Wachsberg RH, Bahramipour P, Sofocleous CT, Barone A. Hepatofugal flow in the portal venous system: pathophysiology, imaging findings, and diagnostic pitfalls. Radiographics. 2002;22:123-140.
11 Kaplan JL, Weintraub SL, Hunt JP, et al. Treatment of superior mesenteric and portal vein thrombosis with direct thrombolytic infusion via an operatively placed mesenteric catheter. Am Surg. 2004;70:600-604.
12 Lauenstein TC, Salman K, Morreira R, et al. Gadolinium-enhanced MRI for tumor surveillance before liver transplantation: center-based experience. AJR Am J Roentgenol. 2007;189:663-670.
13 Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699.
14 Lee MW, Lee JM, Lee JY, et al. Preoperative evaluation of the hepatic vascular anatomy in living liver donors: comparison of CT angiography and MR angiography. J Magn Reson Imaging. 2006;24:1081-1087.
15 Salvalaggio PR, Modanlou KA, Edwards EB, et al. Hepatic artery thrombosis after adult living donor liver transplantation: the effect of center volume. Transplantation. 2007;84:926-928.
16 Martin DR, Seibert D, Yang M, et al. Reversible heterogeneous arterial phase liver perfusion associated with transient acute hepatitis: findings on gadolinium-enhanced MRI. J Magn Reson Imaging. 2004;20:838-842.
17 Zimmerman MA, Cameron AM, Ghobrial RM. Budd-Chiari syndrome. Clin Liver Dis. 2006;10:259-273. viii
18 Brancatelli G, Federle MP, Grazioli L, et al. Large regenerative nodules in Budd-Chiari syndrome and other vascular disorders of the liver: CT and MR imaging findings with clinicopathologic correlation. AJR Am J Roentgenol. 2002;178:877-883.
19 Grego FG, Lepidi S, Ragazzi R, et al. Visceral artery aneurysms: a single center experience. Cardiovasc Surg (London). 2003;11:19-25.
20 Pilleul F, Forest J, Beuf O. Magnetic resonance angiography of splanchnic artery aneurysms and pseudoaneurysms [in French]. Journal de radiologie. 2006;87:127-131.
21 Pilleul F, Beuf O. Diagnosis of splanchnic artery aneurysms and pseudoaneurysms, with special reference to contrast-enhanced 3D magnetic resonance angiography: a review. Acta Radiol. 2004;45:702-708.
22 Berceli SA. Hepatic and splenic artery aneurysms. Semin Vasc Surg. 2005;18:196-201.
23 Catalano C, Pavone P, Laghi A, et al. Pancreatic adenocarcinoma: combination of MR imaging, MR angiography and MR cholangiopancreatography for the diagnosis and assessment of resectability. Eur Radiol. 1998;8:428-434.
24 Pavone P, Laghi A, Catalano C, et al. MR imaging of pancreatic neoplasms. Tumori. 1999;85:S6-10.
25 Paterson A, Frush DP, Donnelly LF, et al. A pattern-oriented approach to splenic imaging in infants and children. Radiographics. 1999;19:1465-1485.
26 Adajar MA, Painter T, Woloson S, Memark V. Isolated celiac artery aneurysm with splenic artery stenosis as a rare presentation of polyarteritis nodosum: a case report and review of the literature. J Vasc Surg. 2006;44:647-650.
27 Willinek WA, Hadizadeh D, von Falkenhausen M, et al. Magnetic resonance (MR) imaging and MR angiography for evaluation and follow-up of hepatic artery banding in patients with hepatic involvement of hereditary hemorrhagic telangiectasia. Abdom Imaging. 2006;31:694-700.
28 Fenton SJ, Hansen KW, Meyers RL, et al. CT scan and the pediatric trauma patient—are we overdoing it? J Pediatr Surg. 2004;39:1877-1881.
29 Dangas G, Iakovou I, Nikolsky E, et al. Contrast-induced nephropathy after percutaneous coronary interventions in relation to chronic kidney disease and hemodynamic variables. Am J Cardiol. 2005;95:13-19.
30 Ragozzino A, Lassandro F, De Ritis R, Imbriaco M. Value of MRI in three patients with major vascular injuries after laparoscopic cholecystectomy. Emerg Radiol. 2007;14:443-447.

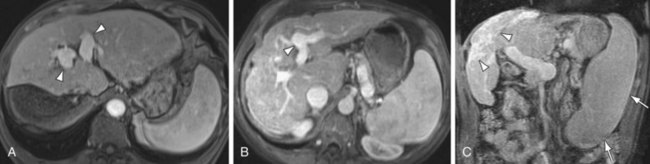
 FIGURE 105-1
FIGURE 105-1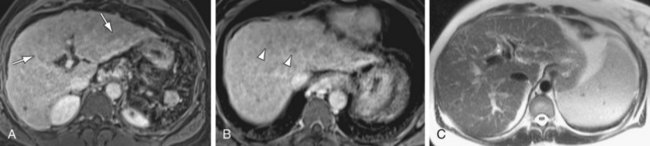
 FIGURE 105-2
FIGURE 105-2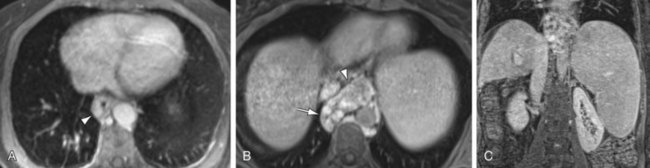
 FIGURE 105-3
FIGURE 105-3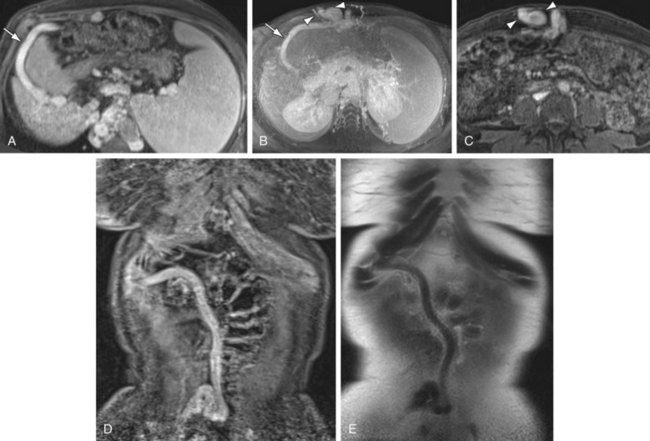
 FIGURE 105-4
FIGURE 105-4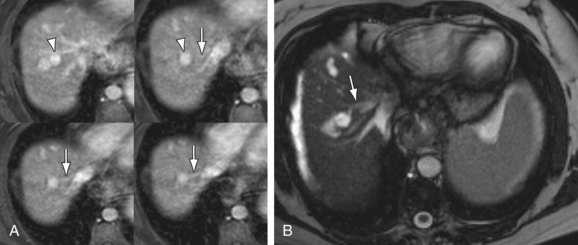
 FIGURE 105-5
FIGURE 105-5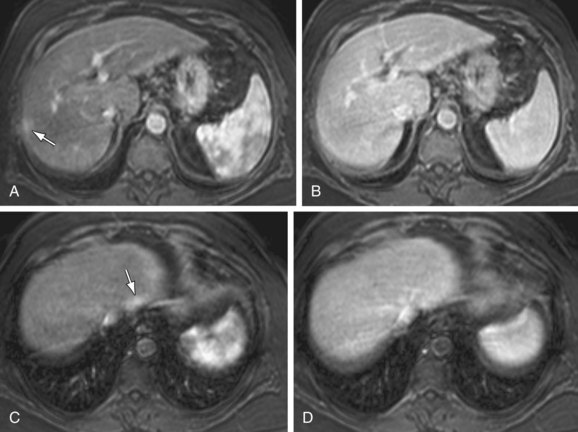
 FIGURE 105-6
FIGURE 105-6


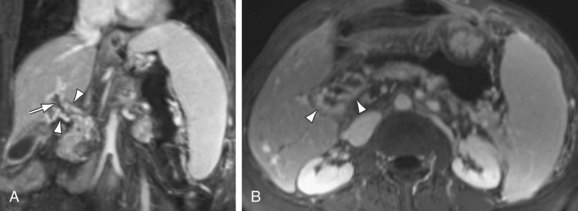
 FIGURE 105-7
FIGURE 105-7


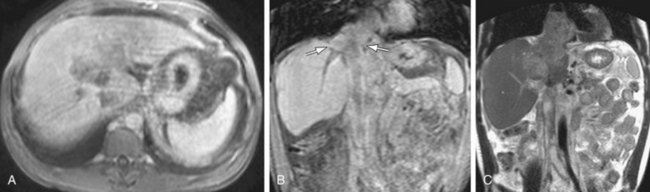
 FIGURE 105-8
FIGURE 105-8


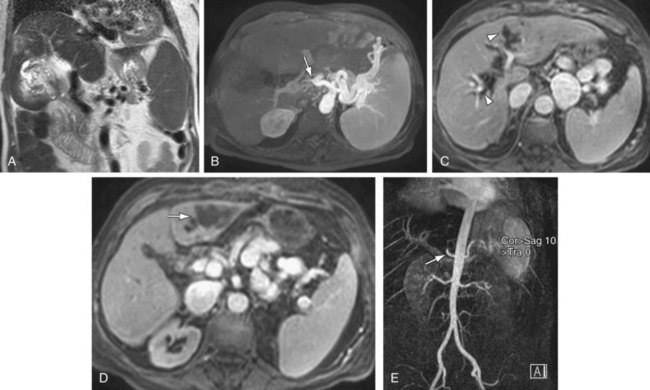
 FIGURE 105-9
FIGURE 105-9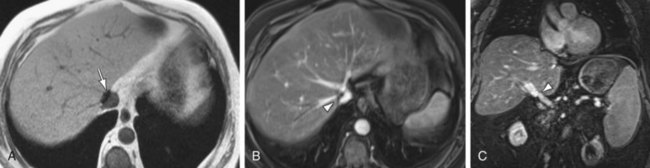
 FIGURE 105-10
FIGURE 105-10


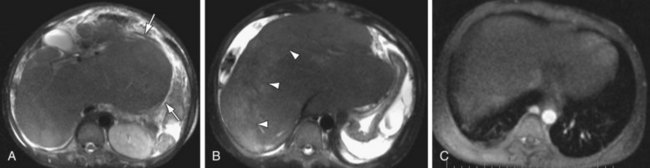
 FIGURE 105-11
FIGURE 105-11


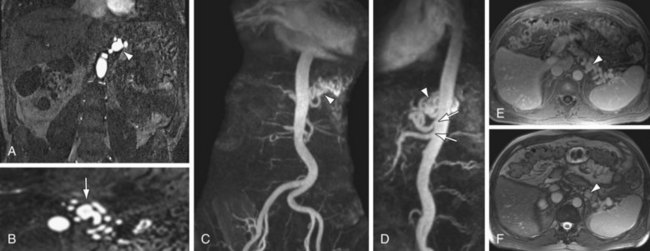
 FIGURE 105-12
FIGURE 105-12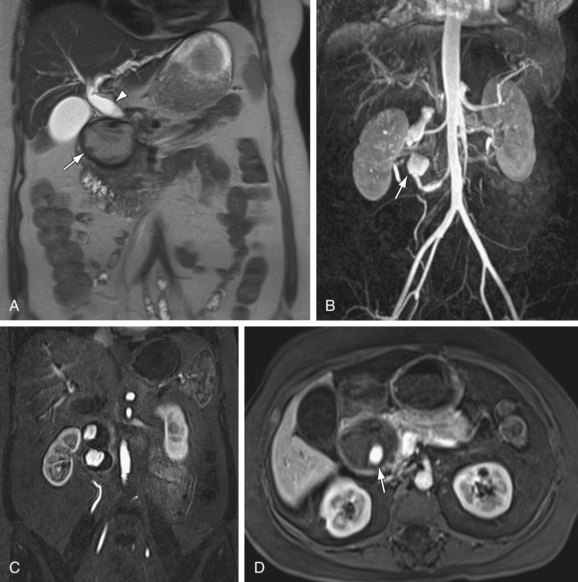
 FIGURE 105-13
FIGURE 105-13


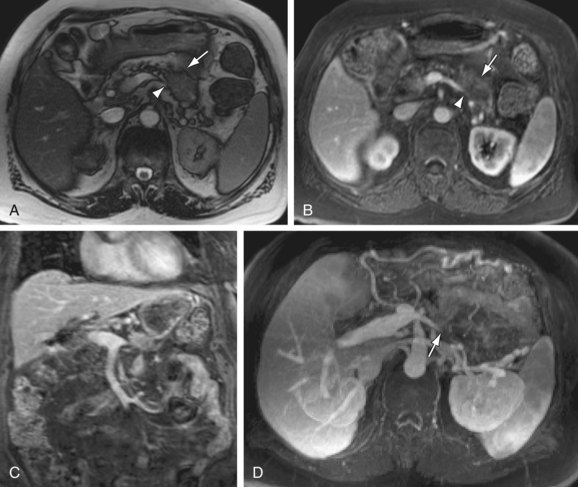
 FIGURE 105-14
FIGURE 105-14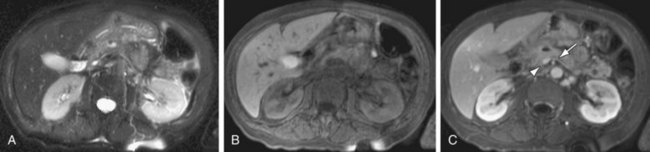
 FIGURE 105-15
FIGURE 105-15


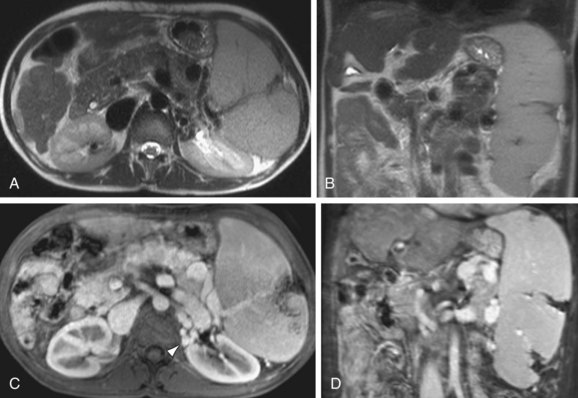
 FIGURE 105-16
FIGURE 105-16


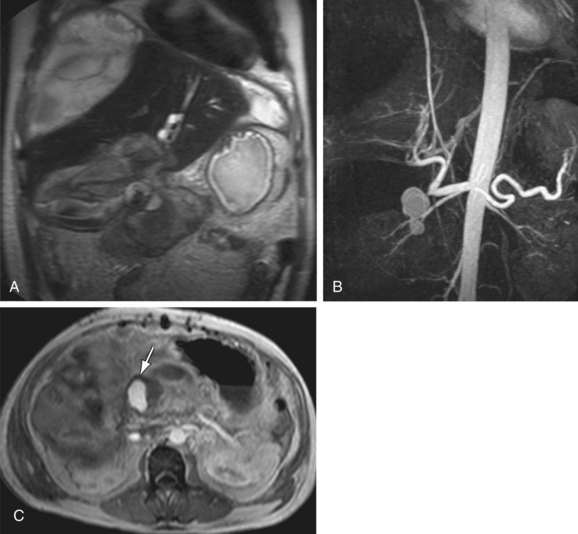
 FIGURE 105-17
FIGURE 105-17

