CHAPTER 57 Magnetic Resonance Imaging of Myocardial Viability
Heart failure is a complex clinical syndrome with high hospitalization and mortality rates. The incidence of heart failure approaches 10 per 1000 in the population older than 65 years.1 The most common etiology of heart failure in developed countries is coronary artery disease. In patients with left ventricular (LV) dysfunction, it is of great therapeutic and prognostic importance to differentiate irreversibly damaged scarred myocardium, dysfunctional but viable myocardium (hibernating or stunned), and LV dysfunction from causes not related to coronary artery disease. The assessment of myocardial viability is important to guide the management of patients with ischemic LV dysfunction when clinicians must weigh the risks and benefits of coronary revascularization in regard to the increased perioperative morbidity and mortality in this patient population.2,3
The meta-analysis by Allman and colleagues4 assessed the prognostic value of viability assessment across various nuclear and echocardiographic techniques. Determining the presence and extent of myocardial viability is crucial in guiding clinical decision making and influencing patient outcomes. Patients with significant viability assigned to be treated medically had higher rates of cardiac mortality (16% vs. 3.2 % per year) and nonfatal myocardial infarction (MI) (12.2% vs. 6 % per year) than patients who were assigned to undergo coronary revascularization. In patients without significant myocardial viability, there was no difference in annual mortality or nonfatal MI rates between coronary revascularization and medical therapy (7.7% vs. 6.2% and 10.2% vs. 8%).4 These findings underscore the importance of noninvasive assessment of viability in guiding appropriate treatment decision making.
VIABILITY DEFINITIONS
Stunned and hibernating myocardium can benefit from restoration of blood supply. Rahimtoola5 first reported the process of myocardial hibernation, and it was then observed that LV dysfunction resulting from myocardial hibernation is not an irreversible process. The process of myocardial hibernation is a dynamic process that involves a continuum of downregulation of myocyte metabolism, reduction in contractile elements, and dedifferentiation of myocardial cells with preserved cell membrane integrity. The following processes have been observed in hibernating myocardium: loss of sarcomeres, sarcoplasmic reticulum, and T tubules; intracellular buildup of glycogen; atrophic mitochondria; accumulation of extracellular matrix and fibrosis; and expression of fetal proteins.6
MAGNETIC RESONANCE IMAGING ASSESSMENT OF MYOCARDIAL VIABILITY
Several MRI techniques exist to assess myocardial viability. Common clinical techniques include assessment of LV end-diastolic wall thickness on resting cine MRI, myocardial contractile reserve and ischemia by dobutamine stress MRI (dobutamine MRI), and transmural extent of MI by contrast-enhanced myocardial delayed enhancement (MDE) imaging. Other MRI techniques, such as quantification of myocardial contractile reserve with MRI tagging7,8 and MR spectroscopy for assessment of high-energy phosphates metabolism (e.g., quantification of concentration and ratio of adenosine triphosphate and phosphocreatine using 31P spectroscopy within a small volume of myocardium), are currently not in wide clinical use and are beyond the scope of this chapter.9,10
Wall Thickness
In the presence of extensive chronic MI, wall thinning develops in the infarct zone because of infarct resorption and fibrotic contracture. Although autopsy studies have shown that wall thinning is frequently associated with transmural scar,11,12 end-diastolic wall thickness (EDWT) measured in millimeters has limited prediction for functional recovery after revascularization, which serves as the most common surrogate reference method for myocardial viability. Baer and coworkers13 compared resting MRI with 18FDG-PET in 35 patients. Regions with EDWT less than 5.5 mm had significantly reduced 18FDG uptake compared with regions with thickness 5.5 mm or greater that had preserved 18FDG uptake. The same investigators also tested the predictive value of EDWT for functional recovery after revascularization. A EDWT cutoff of 5.5 mm had 94% sensitivity, but only 52% specificity for predicting segmental functional recovery after revascularization.14
The low specificity of LV wall thinning can be attributed to the fact that myocardial segments even with preserved wall thickness can have substantial transmural scar. In addition, EDWT is a structural variable that does not assess physiologic response of the myocardium, and there is a wide variation of EDWT among individuals and among different myocardial segments in the same individual.15 Kim and Shah15 showed that regional wall thinning may occur early after MI even in the absence of transmural necrosis, and that the thinned myocardium can still improve its contractile function.
Dobutamine Magnetic Resonance Imaging Contractile Reserve
Improvement in wall thickening or contractile reserve in response to inotropic challenge such as a low-dose dobutamine infusion (5 to 15 µg/kg/min) has been well validated for assessment of myocardial viability in echocardiography and MRI literature.14,16–20 There are several advantages of MRI over the conventional echocardiography for detecting contractile reserve. Widely used cine steady-state free precession provides excellent spatial resolution and tissue contrast between the blood pool and LV myocardium. In contrast to echocardiography, which is limited by acoustic windows, MRI is able to provide multiplanar image acquisition for improved volumetric coverage of the heart. The quantification of LV volumes, mass, and ejection fraction with MRI does not require any geometric assumptions and is highly reproducible.21
Schmidt and colleagues22 assessed resting EDWT (5.5 mm cutoff), dobutamine-induced wall thickening of greater than 2 mm, and presence of normalized 18FDG uptake greater than 50% on PET in 40 patients with chronic MI to predict segmental functional recovery. The sensitivity and specificity for segmental functional recovery were 100% and 53% for resting EDWT 5.5 mm or greater alone, 96% and 87% for dobutamine-induced contractile reserve, and 100% and 73% for preserved 18FDG (>50%) uptake. These findings indicate that dichotomizing a threshold value of resting EDWT or 18FDG uptake is less accurate than the physiologic assessment of low-dose dobutamine challenge. This point is supported further by a study reported by Wellnhofer and associates,20 which showed higher specificity of dobutamine MRI compared with transmural extent of late hyperenhancement on MDE imaging to predict functional recovery, especially with 1% to 74% transmural extent of late hyperenhancement.
Contractile response during low-dose dobutamine challenge is specific, yet is only moderately sensitive in detecting segmental viability. Kaandorp and coworkers23 reported a weighted mean sensitivity and specificity for predicting functional recovery of 73% and 83% after pooling nine previously published dobutamine MRI studies. The relatively low sensitivity of dobutamine MRI can be attributed to the fact that in the presence of severe coronary stenosis, viable myocardial segments fail to show augmentation of segmental thickening because of rapid development of ischemia even with low dobutamine doses. We choose to infuse low-dose dobutamine using 5-minute per stage protocol. This prolonged infusion allows building up of the inotropic effects of the dobutamine, and the resultant display of augmentation of segmental thickening may enhance the sensitivity in detection of segmental viability.
Hyperenhancement on Myocardial Delayed Enhancement Imaging
The basis for development of MDE imaging was an observation that the differential between normal and infarcted myocardium can be accentuated by the use of gadolinium-chelate contrast agents on T1-weighted images.24 Gadolinium shortens the T1 relaxation time of tissues with increased gadolinium content. Nevertheless, the limitation in image quality had restricted the clinical application of contrast-enhanced MRI until recent years. The proposed mechanism of hyperenhancement on MDE (also known as late gadolinium enhancement) is related to a relatively large molecular size of conventional extracellular gadolinium-chelate contrast agents that is made metabolically inert by the chelation and unable to cross the cell membrane of a normal myocyte. When the cell membrane is damaged (e.g., acute MI), or if there is an increase in the extracellular space between myocytes (e.g., acute interstitial edema or chronic fibrosis), there is accumulation of gadolinium-chelate contrast agent in the extracellular space, and its washout is delayed after the injection (Fig. 57-1).
Areas of abnormal myocardium (e.g., infarcted, fibrotic, inflamed, or infiltrated myocardium) have elevated per-voxel gadolinium concentration and have a brighter signal (i.e., hyperenhancement) relative to normally enhancing myocardium, which appears nulled (i.e., dark) on the T1-weighted inversion recovery imaging sequence used for MDE imaging.25 Using this technique in a clinical setting of coronary artery disease, dysfunctional myocardium with normal nulled signal intensity suggests myocardial stunning or hibernation. In acute or chronic MI, myocardial hyperenhancement characteristically involves the subendocardium or progresses to involve the entire transmural extent in myocardial segments subtended by a coronary arterial territory (Fig. 57-2). Apart from illustration of myocardial hyperenhancement consistent with MI, other specific patterns of hyperenhancement (mid-wall, epicardial, focal, or diffuse) can also provide potential differentiation of other etiologies of cardiomyopathy, such as myocarditis, nonischemic idiopathic dilated cardiomyopathy, cardiac sarcoidosis, cardiac amyloidosis, or endomyocardial fibrosis (Fig. 57-3).26
Many animal and human studies have validated the use of this technique to detect the presence of or the extent of MI. Kim and colleagues27 used the inversion recovery MDE technique in 18 dogs with experimental MI and showed that MDE imaging accurately depicts the extent of histologically defined necrosis. The signal intensity of the infarcted myocardium was on average three to six times higher than the signal intensity of the normal myocardium during the acute and convalescence phases of infarction, allowing sensitive delineation of the transmural extent of myonecrosis. These authors also showed high agreement between the extent of scar tissue on MDE and histologic extent of necrosis across all time points during infarct healing (Fig. 57-4).27
Kim and colleagues28 went on to study the use of gadolinium-enhanced MDE MRI in 50 patients with LV dysfunction before undergoing surgical or percutaneous revascularization. They showed that the likelihood of improvement in segmental myocardial contractility after revascularization decreased progressively with the increased transmural extent of the myocardial hyperenhancement on MDE (Fig. 57-5). The major advantage contrast-enhanced MDE MRI had over other imaging modalities is the ability to assess the presence of subendocardial micromyonecrosis and the transmural extent of MI because of the excellent spatial resolution of this technique (1 to 3 mm in-plane).
Ricciardi and associates29 studied a small group of patients without history of infarction who had elevated creatine kinase-MB enzyme after percutaneous coronary intervention. They found that MDE MRI detected discrete areas of myonecrosis that were not detected by cine wall motion imaging. Wagner and colleagues30 compared the detection of MI by gadolinium-enhanced MDE MRI and SPECT in 12 dogs. Both imaging modalities detected all segments with greater than 75% transmural extent of MI. Gadolinium-enhanced MRI detected 92% of segments with less than 50% transmural infarction, whereas SPECT detected only 28% of those segments (Fig. 57-6).30
Klein and coworkers31 directly compared MDE MRI with 18FDG-PET in 31 patients with severe LV dysfunction. They found that myocardial hyperenhancement on MDE imaging correlated well with areas of PET scar defined as matched decrease in perfusion and metabolism. Of segments defined as viable by PET, 11% showed some degree of subendocardial hyperenhancement, reflecting the stronger capability of MRI in detecting small subendocardial infarction than PET because of its higher spatial resolution.
COMPUTED TOMOGRAPHY FOR ASSESSMENT OF MYOCARDIAL VIABILITY
Multidetector CT is a noninvasive imaging technique primarily used for evaluation of coronary artery anatomy and coronary stenoses. Coronary multidetector CT provides a three-dimensional volumetric data set of the entire heart after injection of iodinated contrast medium, and the data can be reconstructed at multiple phases of the cardiac cycle, able to provide additional functional information, such as LV volumes, mass, and ejection fraction. In small pilot clinical studies, comparison with MRI showed a good correlation in measuring LV volumes and global LV ejection fraction.32,33
Another emerging application of multidetector CT is evaluation of myocardial perfusion and viability. Similar to MRI, multidetector CT images are acquired during administration of an intravenous bolus of contrast agent. Similar to MRI first-pass perfusion technique, the hypoperfused areas on dynamic contrast-enhanced multidetector CT have lower attenuation compared with normally perfused myocardial segments.34 Nieman and associates35 investigated the ability of multidetector CT to differentiate recent and chronic MI based on CT attenuation measured in Hounsfield units (HU). Significantly lower CT attenuation values on the CT perfusion images were found in patients with long-standing MI (−13 ± 37 HU) than in patients with acute MI (26 ± 26 HU) or normal controls (73 ± 14 HU).
Using MDE multidetector CT in animal models of acute and chronic MI, Lardo and coworkers36 could identify the infarct size and its transmural extent when compared with histology (Fig. 57-7). The peak hyperenhancement of infarcted regions occurred approximately 5 minutes after contrast injection (see Fig. 57-7A1-A3). Acute and chronic infarcts by multidetector CT were characterized by hyperenhancement on MDE imaging, whereas regions of microvascular obstruction were characterized by hypoenhancement. Lessick and colleagues37 evaluated sensitivity of myocardial early perfusion defects and late enhancement with multidetector CT in 26 patients with acute MI to predict myocardial functional recovery. They concluded that in segments abnormal at baseline, the lack of functional recovery was related to the presence and size of the defect on early perfusion and late enhancement.
LIMITATIONS
Cardiac CT is a fast imaging technique, but has the associated concerns of ionizing radiation exposure and iodinated contrast agent use. The radiation dose for 64-slice coronary multidetector CT was reported to range from 15 to 21 mSv without the ECG gated dose modulation and 9 to 14 mSv with dose modulation. Organ-specific radiation doses are reported to range from 42 to 91 mSv for the lungs and 50 to 80 mSv for female breast, findings associated with non-negligible lifetime attributable risk of cancer.38 Other limitations of multidetector CT relate to use of iodinated contrast medium with increased risk of contrast-induced nephropathy or allergic reaction. Similar to MRI, the CT image quality is limited in patients unable to maintain breath-holding or in patients with fast or irregular heart rate. There is currently a paucity of data on validation and prognostic implications of CT-based perfusion and MDE imaging.
1 Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25-e146.
2 Alderman EL, Fisher LD, Litwin P, et al. Results of coronary artery surgery in patients with poor left ventricular function (CASS). Circulation. 1983;68:785-795.
3 Pepper J. Surgery for hibernation. Heart (British Cardiac Society). 2004;90:144-145.
4 Allman KC, Shaw LJ, Hachamovitch R, et al. Myocardial viability testing and impact of revascularization on prognosis in patients with coronary artery disease and left ventricular dysfunction: a meta-analysis. J Am Coll Cardiol. 2002;39:1151-1158.
5 Rahimtoola SH. The hibernating myocardium. Am Heart J. 1989;117:211-221.
6 Maes A, Flameng W, Nuyts J, et al. Histological alterations in chronically hypoperfused myocardium: correlation with PET findings. Circulation. 1994;90:735-745.
7 Geskin G, Kramer CM, Rogers WJ, et al. Quantitative assessment of myocardial viability after infarction by dobutamine magnetic resonance tagging. Circulation. 1998;98:217-223.
8 Sayad DE, Willett DL, Hundley WG, et al. Dobutamine magnetic resonance imaging with myocardial tagging quantitatively predicts improvement in regional function after revascularization. Am J Cardiol. 1998;82:1149-1151. A1110
9 Yabe T, Mitsunami K, Inubushi T, et al. Quantitative measurements of cardiac phosphorus metabolites in coronary artery disease by 31P magnetic resonance spectroscopy. Circulation. 1995;92:15-23.
10 Bottomley PA, Weiss RG. Non-invasive magnetic-resonance detection of creatine depletion in non-viable infarcted myocardium. Lancet. 1998;351:714-718.
11 Dubnow MH, Burchell HB, Titus JL. Postinfarction ventricular aneurysm: a clinicomorphologic and electrocardiographic study of 80 cases. Am Heart J. 1965;70:753-760.
12 Fishbein MC, Maclean D, Maroko PR. The histopathologic evolution of myocardial infarction. Chest. 1978;73:843-849.
13 Baer FM, Voth E, Schneider CA, et al. Comparison of low-dose dobutamine-gradient-echo magnetic resonance imaging and positron emission tomography with [18F]fluorodeoxyglucose in patients with chronic coronary artery disease: a functional and morphological approach to the detection of residual myocardial viability. Circulation. 1995;91:1006-1015.
14 Baer FM, Theissen P, Schneider CA, et al. Dobutamine magnetic resonance imaging predicts contractile recovery of chronically dysfunctional myocardium after successful revascularization. J Am Coll Cardiol. 1998;31:1040-1048.
15 Kim RJ, Shah DJ. Fundamental concepts in myocardial viability assessment revisited: when knowing how much is “alive” is not enough. Heart (British Cardiac Society). 2004;90:137-140.
16 Anselmi M, Golia G, Cicoira M, et al. Prognostic value of detection of myocardial viability using low-dose dobutamine echocardiography in infarcted patients. Am J Cardiol. 1998;81(12A):21G-28G.
17 Sandstede JJ, Bertsch G, Beer M, et al. Detection of myocardial viability by low-dose dobutamine cine MR imaging. Magn Reson Imaging. 1999;17:1437-1443.
18 Dendale PA, Franken PR, Waldman GJ, et al. Low-dosage dobutamine magnetic resonance imaging as an alternative to echocardiography in the detection of viable myocardium after acute infarction. Am Heart J. 1995;130:134-140.
19 Baer FM, Theissen P, Crnac J, et al. Head to head comparison of dobutamine-transesophageal echocardiography and dobutamine-magnetic resonance imaging for the prediction of left ventricular functional recovery in patients with chronic coronary artery disease. Eur Heart J. 2000;21:981-991.
20 Wellnhofer E, Olariu A, Klein C, et al. Magnetic resonance low-dose dobutamine test is superior to SCAR quantification for the prediction of functional recovery. Circulation. 2004;109:2172-2174.
21 Grothues F, Smith GC, Moon JC, et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002;90:29-34.
22 Schmidt M, Voth E, Schneider CA, et al. F-18-FDG uptake is a reliable predictor of functional recovery of akinetic but viable infarct regions as defined by magnetic resonance imaging before and after revascularization. Magn Reson Imaging. 2004;22:229-236.
23 Kaandorp TA, Lamb HJ, van der Wall EE, et al. Cardiovascular MR to access myocardial viability in chronic ischaemic LV dysfunction. Heart (British Cardiac Society). 2005;91:1359-1365.
24 McNamara MT, Tscholakoff D, Revel D, et al. Differentiation of reversible and irreversible myocardial injury by MR imaging with and without gadolinium-DTPA. Radiology. 1986;158:765-769.
25 Simonetti OP, Kim RJ, Fieno DS, et al. An improved MR imaging technique for the visualization of myocardial infarction. Radiology. 2001;218:215-223.
26 McCrohon JA, Moon JC, Prasad SK, et al. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation. 2003;108:54-59.
27 Kim RJ, Fieno DS, Parrish TB, et al. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992-2002.
28 Kim RJ, Wu E, Rafael A, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445-1453.
29 Ricciardi MJ, Wu E, Davidson CJ, et al. Visualization of discrete microinfarction after percutaneous coronary intervention associated with mild creatine kinase-MB elevation. Circulation. 2001;103:2780-2783.
30 Wagner A, Mahrholdt H, Holly TA, et al. Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study. Lancet. 2003;361:374-379.
31 Klein C, Nekolla SG, Bengel FM, et al. Assessment of myocardial viability with contrast-enhanced magnetic resonance imaging: comparison with positron emission tomography. Circulation. 2002;105:162-167.
32 Grude M, Juergens KU, Wichter T, et al. Evaluation of global left ventricular myocardial function with electrocardiogram-gated multidetector computed tomography: comparison with magnetic resonance imaging. Invest Radiol. 2003;38:653-661.
33 Belge B, Coche E, Pasquet A, et al. Accurate estimation of global and regional cardiac function by retrospectively gated multidetector row computed tomography: comparison with cine magnetic resonance imaging. Eur Radiol. 2006;16:1424-1433.
34 Hoffmann U, Millea R, Enzweiler C, et al. Acute myocardial infarction: contrast-enhanced multi-detector row CT in a porcine model. Radiology. 2004;231:697-701.
35 Nieman K, Cury RC, Ferencik M, et al. Differentiation of recent and chronic myocardial infarction by cardiac computed tomography. Am J Cardiol. 2006;98:303-308.
36 Lardo AC, Cordeiro MA, Silva C, et al. Contrast-enhanced multidetector computed tomography viability imaging after myocardial infarction: characterization of myocyte death, microvascular obstruction, and chronic scar. Circulation. 2006;113:394-404.
37 Lessick J, Dragu R, Mutlak D, et al. Is functional improvement after myocardial infarction predicted with myocardial enhancement patterns at multidetector CT? Radiology. 2007;244:736-744.
38 Einstein AJ, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA. 2007;298:317-323.

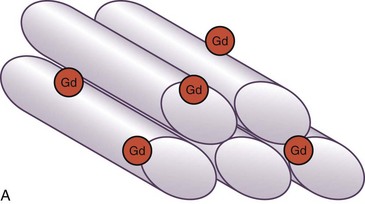
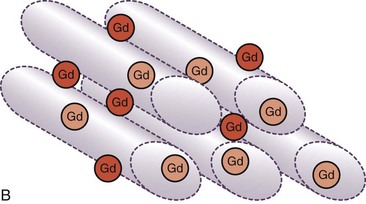
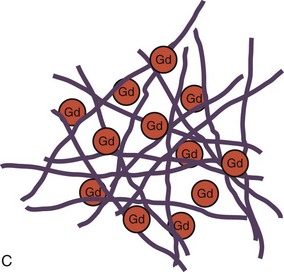
 FIGURE 57-1
FIGURE 57-1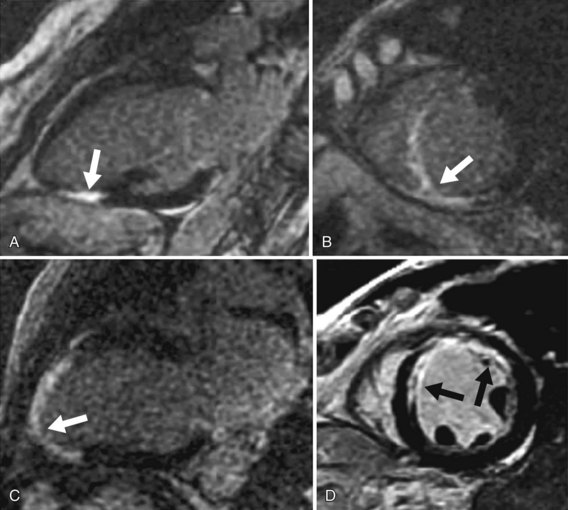
 FIGURE 57-2
FIGURE 57-2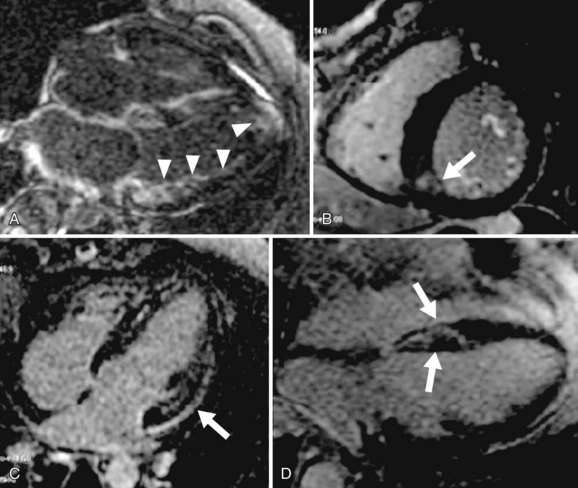
 FIGURE 57-3
FIGURE 57-3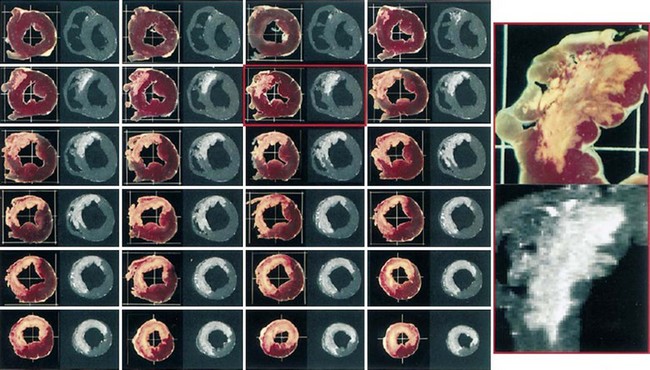
 FIGURE 57-4
FIGURE 57-4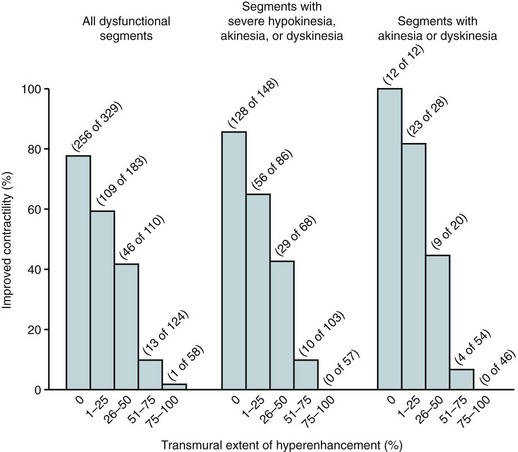
 FIGURE 57-5
FIGURE 57-5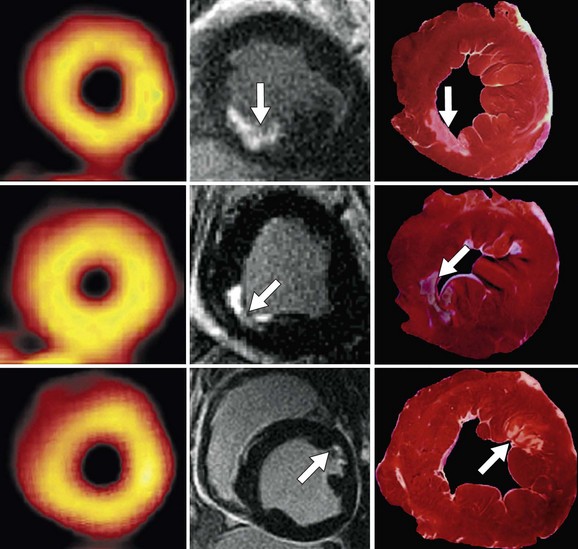
 FIGURE 57-6
FIGURE 57-6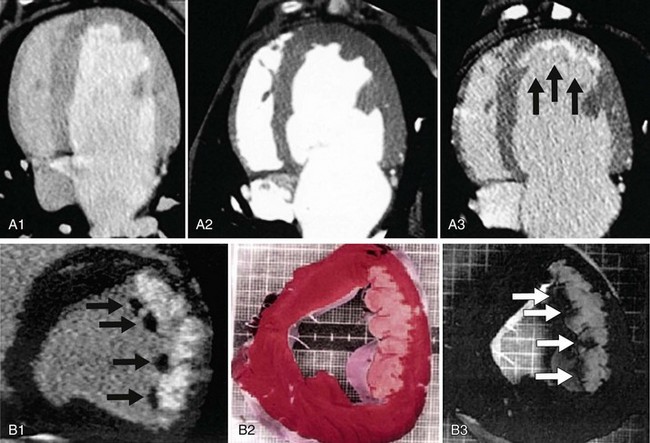
 FIGURE 57-7
FIGURE 57-7


