CHAPTER 50 Magnetic Resonance Imaging in the Postoperative Evaluation of the Patient with Congenital Heart Disease
POSTOPERATIVE ASSESSMENT
A number of MRI techniques are useful to the examination of the anatomy and physiology of the postoperative CHD patient. These techniques are detailed in Chapters 13 to 17. Here, their importance to this population is highlighted.
Cine Magnetic Resonance Imaging
ECG-gated gradient-echo sequences can be employed to provide multiple images throughout the cardiac cycle in prescribed anatomic locations. Display of these images in a cine mode permits visualization of the dynamic motion of the heart and vessels.1–3 Cine MRI techniques, at a minimum, allow assessment of anatomy. More important, such techniques allow qualitative and quantitative assessment of function. Specifically, cine MRI permits quantification of chamber volumes, myocardial mass, and ventricular function. Further, cine MRI allows qualitative assessment of focal and global wall motion abnormalities, qualitative and quantitative assessment of valve disease (including the mechanism and severity of valve regurgitation and the location and severity of valve stenoses), identification and quantification of intracardiac and extracardiac shunts, and visualization of other areas of flow turbulence.
Cine MRI is the principal tool used to quantitatively assess ventricular function. Such techniques, both fast gradient-echo4–7 and balanced steady-state free precession,1,2 have been extensively evaluated and validated.8,9 Briefly, evaluation of function begins with obtaining a series of contiguous cine slices along the short axis of the ventricles, extending from base to apex. The prescription of such slices should be performed from a true four-chamber view at end-diastole to ensure coverage of the entire ventricular mass (Fig. 50-1). These images are played back in a cine loop, and the end-systolic and end-diastolic phases are chosen. The endocardial borders are traced at both time points, and the epicardial borders are traced at one of the two time points (Fig. 50-2). Ventricular volumes are then calculated as the sum of the traced volumes (area × slice thickness). Myocardial mass is calculated as the myocardial muscle volume × 1.05 g/mm3 (density of myocardium). From these data, ventricular end-diastolic volume, end-systolic volume, stroke volume, ejection fraction, myocardial mass, and mass-to-volume ratio can be calculated for both the right and left ventricles. Most computer workstation software packages for cardiac MRI analysis provide semiautomated postprocessing tools to maximize efficiency.
Spin-Echo (Black Blood) Imaging
ECG-gated spin-echo sequences (black blood imaging) represent another important tool for imaging in the postoperative CHD patient. Despite providing only static information, black blood imaging has many benefits in this population. It allows assessment of anatomy with thin slices, high spatial resolution, and excellent blood-myocardium and blood–vessel wall contrast (Fig. 50-3). Black blood techniques are superb for evaluation of the spatial relationship between cardiovascular and other intrathoracic structures, such as the chest wall and the tracheobronchial tree. These features hold particular relevance in delineation of complicated postsurgical cardiac anatomy. Such techniques are also less susceptible to artifact from metallic implanted devices, such as stents, coils, occluder devices, clips, and sternal wires, which are commonly seen in the postoperative CHD patient.
Flow Quantification
Electrocardiography-gated gradient-echo sequences with flow-encoding gradients are used to quantify the velocity and flow of blood (Fig. 50-4).10 These sequences are referred to as velocity-encoded cine MRI or phase contrast MRI. Two-dimensional velocity-encoded cine MRI sequences are commonly used in clinical practice. They can be used to quantify cardiac output, pulmonary-to-systemic flow ratio (shunt), valvular regurgitation, differential lung perfusion, and coronary flow reserve. They can be used to observe the location and severity of flow obstruction. In addition, velocity-encoded cine MRI assessment of flow is useful for corroboration of volumetric data obtained with cine imaging to ensure the interpreting physician that the data obtained are accurate.
Newer velocity-encoded cine MRI sequences allow resolution of velocity vectors in three directions, with spatial coverage of a three-dimensional volume, temporally resolved throughout the cardiac cycle. Such techniques have been coined seven-dimensional flow encoding.11,12 These techniques have the advantage of providing complete spatial and temporal resolution of velocity with a higher signal-to-noise ratio than in two-dimensional methods. Postprocessing tools permit the construction of vector field plots that highlight the intracardiac and intravascular nature of flow. Although they are currently limited by long scan durations, faster imaging techniques will likely allow such methods to reach clinical practice in the near future.
Gadolinium-Enhanced Three-Dimensional Angiography
Three-dimensional magnetic resonance angiography (MRA) sequences are typically not ECG-gated and thus do not allow optimal assessment of intracardiac structures. Regardless, such techniques provide excellent depiction of arterial and venous vascular structures (Fig. 50-5). In the population of postoperative CHD patients, three-dimensional MRA fills a significant diagnostic role. It can be used to diagnose systemic arterial anomalies, such as aortopulmonary collaterals, shunts, vascular rings, and coarctation. It is useful in the diagnosis of pulmonary arterial abnormalities, such as focal and diffuse stenoses and abnormal distal arborization patterns. Three-dimensional MRA methods are also useful for investigation of systemic and pulmonary venous abnormalities, both congenital anomalies and postoperative abnormalities. Finally, three-dimensional MRA is useful for evaluation of the relation between vascular and other thoracic structures. With the development of faster imaging techniques, ECG-gated three-dimensional MRA sequences are becoming more practical, allowing evaluation of intracardiac anatomy and acquisition of time-resolved three-dimensional MRA data sets.13
Coronary Artery Imaging, Perfusion Imaging, and Myocardial Viability
Coronary artery abnormalities and ischemia are important issues to be investigated in postoperative CHD patients. Not only is this population of patients aging sufficiently to develop atherosclerotic coronary artery disease, they also commonly have congenitally abnormal or postoperatively acquired coronary artery lesions. It is not uncommon to find an anomalous origin or course of the left or right coronary artery, postsurgical coronary obstruction (i.e., after arterial switch for transposition of the great arteries), coronary artery thrombus (Fig. 50-6), or abnormal fistulous connections (i.e., pulmonary atresia with intact ventricular septum and right ventricle–dependent coronary circulation). Identification of such abnormalities is often critical to planning of reintervention or medical management. There is growing evidence to support that myocardial delayed hyperenhancement in a number of subsets of postoperative CHD patients is predictive of poor outcome, including patients with tetralogy of Fallot (Fig. 50-7).14,15 Delayed hyperenhancement has been observed in other postoperative patients with CHD as well, the significance of which is being explored and elucidated.16,17 In summary, although it is still not as robust as routine coronary artery angiography with x-ray fluoroscopy or ECG-gated CT angiography at investigating distal coronary artery lesions, MRI can image proximal coronary arteries well,18–21 evaluate myocardial perfusion and viability,22–26 and allow stress testing,27–30 all noninvasively without exposure to contrast agents and ionizing radiation.
IMAGING TECHNIQUE AND FINDINGS
Tetralogy of Fallot
Postoperative Management
Most patients with repaired TOF will have a certain degree of pulmonary regurgitation or stenosis, which has been shown to result in right ventricular dilation and dysfunction. In addition, progressive right ventricular dilation often leads to tricuspid valve annular stretch and ultimately regurgitation, which perpetuates continued right ventricular dilation. In addition, many patients with repaired TOF will have abnormal peripheral pulmonary vasculature and residual aortopulmonary collaterals, all of which contribute to their hemodynamic burden. Multiple studies in the recent literature have demonstrated that right ventricular dilation and dysfunction, as determined by MRI, predict adverse outcomes such as symptoms of right-sided heart failure, major arrhythmic events, and even death.31 Right ventricular dilation and dysfunction will often be detected by MRI before the onset of obvious clinical symptoms. In addition, delayed hyperenhancement has been shown to correlate with adverse outcomes in patients with repaired TOF.32
MRI for a patient with TOF may include the following:
Transposition of the Great Arteries
Postoperative Management
Coarctation of the Aorta
MRI is useful for delineation of anatomy and physiology in patients after repair of coarctation of the aorta.33,34 This can be monitored with serial MRI to identify the need for and to ensure appropriate timing of reintervention.
Echocardiographic assessment is often limited by poor acoustic windows. Although CT angiography provides anatomic detail, it is unable to characterize flow and the effect of the obstruction on the myocardium. MRI can noninvasively enhance our understanding of coarctation severity, and clinical assessment combined with MRI as a primary imaging modality can be more cost-effective than an approach using echocardiography as a primary imaging modality.35,36
Postoperative Management
MRI for a patient with repaired coarctation of the aorta may include the following:
Single Ventricle After Fontan Palliation
Patients born with complex CHD and single-ventricle physiology (i.e., tricuspid atresia, hypoplastic left heart syndrome, pulmonary atresia with intact ventricular septum) represent a spectrum of disease severity, depending on their initial anatomy. In the current era, they typically undergo several surgical procedures during the first several years of life to provide a stable cardiopulmonary physiology. The first of these procedures is usually performed in the neonatal period and is directed at recruiting the single ventricle as the systemic pumping chamber and providing controlled pulmonary blood flow. The subsequent procedures involve sequential conversion to a physiology of separated systemic and pulmonary circulations with passive pulmonary blood flow (elimination of intracardiac mixing). The final circulation is named the Fontan circulation after the French surgeon who developed and first performed it in humans.37 This palliation improves the patient’s cardiovascular efficiency and provides nearly normal systemic arterial oxygen saturations, but the physiology remains abnormal.
Postoperative Management
KEY POINTS
 The field of CHD is advancing rapidly, and as a result, patients with complex disease are surviving and patients in general are living longer.
The field of CHD is advancing rapidly, and as a result, patients with complex disease are surviving and patients in general are living longer. Despite repair, postoperative CHD patients uniformly have residual hemodynamic burdens that need to be monitored carefully. Reintervention is often necessary. Choice of appropriate timing for reintervention is often challenging.
Despite repair, postoperative CHD patients uniformly have residual hemodynamic burdens that need to be monitored carefully. Reintervention is often necessary. Choice of appropriate timing for reintervention is often challenging. MRI is ideally suited as a primary noninvasive imaging modality, given its ability to provide accurate and reproducible anatomic and functional information. Multiple MRI techniques can be combined to provide a complete evaluation of anatomy and function, including cine (steady-state free precession) imaging, black blood (spin-echo) imaging, flow quantification (velocity-encoded cine) MRI, gadolinium-enhanced three-dimensional MRA, coronary artery imaging, perfusion imaging, and viability (delayed hyperenhancement) MRI.
MRI is ideally suited as a primary noninvasive imaging modality, given its ability to provide accurate and reproducible anatomic and functional information. Multiple MRI techniques can be combined to provide a complete evaluation of anatomy and function, including cine (steady-state free precession) imaging, black blood (spin-echo) imaging, flow quantification (velocity-encoded cine) MRI, gadolinium-enhanced three-dimensional MRA, coronary artery imaging, perfusion imaging, and viability (delayed hyperenhancement) MRI. The postoperative CHD patients presenting for MRI most commonly include those with tetralogy of Fallot, transposition of the great arteries, coarctation of the aorta, or single ventricles after Fontan repair.
The postoperative CHD patients presenting for MRI most commonly include those with tetralogy of Fallot, transposition of the great arteries, coarctation of the aorta, or single ventricles after Fontan repair. MRI has advanced significantly during the past 3 decades and continues to advance at a rapid rate. Real-time imaging, MRI-guided interventions, myocardial tagging assessment of wall strain, and seven-dimensional flow techniques will reach the clinical armamentarium in the near future, making MRI an even more useful tool in evaluation and management of this population of patients.
MRI has advanced significantly during the past 3 decades and continues to advance at a rapid rate. Real-time imaging, MRI-guided interventions, myocardial tagging assessment of wall strain, and seven-dimensional flow techniques will reach the clinical armamentarium in the near future, making MRI an even more useful tool in evaluation and management of this population of patients.Gutierrez FR, Ho ML, Siegel MJ. Practical applications of magnetic resonance in congenital heart disease. Magn Reson Imaging Clin N Am. 2008;16:403-435.
Kellenberger CJ, Yoo SJ, Buchel ER. Cardiovascular MR imaging in neonates and infants with congenital heart disease. Radiographics. 2007;27:5-18.
Knauth Meadows A, Ordovas K, Higgins CB, Reddy GP. Magnetic resonance imaging in the adult with congenital heart disease. Semin Roentgenol. 2008;43:246-258.
Valente AM, Powell AJ. Clinical applications of cardiovascular magnetic resonance in congenital heart disease. Cardiol Clin. 2007;25:97-110.
1 Plein S, Bloomer TN, Ridgway JP, et al. Steady-state free precession magnetic resonance imaging of the heart: comparison with segmented k-space gradient-echo imaging. J Magn Reson Imaging. 2001;14:230-236.
2 Carr JC, Simonetti O, Bundy J, et al. Cine MR angiography of the heart with segmented true fast imaging with steady-state precession. Radiology. 2001;219:828-834.
3 Weiger M, Pruessmann KP, Boesiger P. Cardiac real-time imaging using SENSE. SENSitivity Encoding scheme. Magn Reson Med. 2000;43:177-184.
4 Hernandez RJ, Aisen AM, Foo TK, Beekman RH. Thoracic cardiovascular anomalies in children: evaluation with a fast gradient-recalled-echo sequence with cardiac-triggered segmented acquisition. Radiology. 1993;188:775-780.
5 Furber A, Balzer P, Cavaro-Menard C, et al. Experimental validation of an automated edge-detection method for a simultaneous determination of the endocardial and epicardial borders in short-axis cardiac MR images: application in normal volunteers. J Magn Reson Imaging. 1998;8:1006-1014.
6 Bax JJ, Lamb H, Dibbets P, et al. Comparison of gated single-photon emission computed tomography with magnetic resonance imaging for evaluation of left ventricular function in ischemic cardiomyopathy. Am J Cardiol. 2000;86:1299-1305.
7 Bellenger NG, Marcus NJ, Davies C, et al. Left ventricular function and mass after orthotopic heart transplantation: a comparison of cardiovascular magnetic resonance with echocardiography. J Heart Lung Transplant. 2000;19:444-452.
8 Pennell DJ. Ventricular volume and mass by CMR. J Cardiovasc Magn Reson. 2002;4:507-513.
9 Lorenz CH. The range of normal values of cardiovascular structures in infants, children, and adolescents measured by magnetic resonance imaging. Pediatr Cardiol. 2000;21:37-46.
10 Varaprasathan GA, Araoz PA, Higgins CB, Reddy GP. Quantification of flow dynamics in congenital heart disease: applications of velocity-encoded cine MR imaging. Radiographics. 2002;22:895-905.
11 Markl M, Alley MT, Pelc NJ. Balanced phase-contrast steady-state free precession (PC-SSFP): a novel technique for velocity encoding by gradient inversion. Magn Reson Med. 2003;49:945-952.
12 Markl M, Chan FP, Alley MT, et al. Time-resolved three-dimensional phase-contrast MRI. J Magn Reson Imaging. 2003;17:499-506.
13 Zhang H, Maki JH, Prince MR. 3D contrast-enhanced MR angiography. J Magn Reson Imaging. 2007;25:13-25.
14 Babu-Narayan SV, Kilner PJ, et al. Ventricular fibrosis suggested by cardiovascular magnetic resonance in adults with repaired tetralogy of Fallot and its relationship to adverse markers of clinical outcome. Circulation. 2006;113:405-413.
15 Oosterhof T, Mulder BJ, Vliegen HW, de Roos A. Corrected tetralogy of Fallot: delayed enhancement in right ventricular outflow tract. Radiology. 2005;237:868-871.
16 Harris MA, Johnson TR, Weinberg PM, Fogel MA. Delayed-enhancement cardiovascular magnetic resonance identifies fibrous tissue in children after surgery for congenital heart disease. J Thorac Cardiovasc Surg. 2007;133:676-681.
17 Prakash A, Powell AJ, Krishnamurthy R, Geva T. Magnetic resonance imaging evaluation of myocardial perfusion and viability in congenital and acquired pediatric heart disease. Am J Cardiol. 2004;93:657-661.
18 Weber OM, Martin AJ, Higgins CB. Whole-heart steady-state free precession coronary artery magnetic resonance angiography. Magn Reson Med. 2003;50:1223-1228.
19 Weber OM, Pujadas S, Martin AJ, Higgins CB. Free-breathing, three-dimensional coronary artery magnetic resonance angiography: comparison of sequences. J Magn Reson Imaging. 2004;20:395-402.
20 Spuentrup E, Katoh M, Buecker A, et al. Free-breathing 3D steady-state free precession coronary MR angiography with radial k-space sampling: comparison with cartesian k-space sampling and cartesian gradient-echo coronary MR angiography—pilot study. Radiology. 2004;231:581-586.
21 Danias PG, Roussakis A, Ioannidis JP. Diagnostic performance of coronary magnetic resonance angiography as compared against conventional x-ray angiography: a meta-analysis. J Am Coll Cardiol. 2004;44:1867-1876.
22 Plein S, Radjenovic A, Ridgway JP, et al. Coronary artery disease: myocardial perfusion MR imaging with sensitivity encoding versus conventional angiography. Radiology. 2005;235:423-430.
23 Nagel E, Klein C, Paetsch I, et al. Magnetic resonance perfusion measurements for the noninvasive detection of coronary artery disease. Circulation. 2003;108:432-437.
24 Kim RJ, Fieno DS, Parrish TB, et al. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992-2002.
25 Weinsaft JW, Klem I, Judd RM. MRI for the assessment of myocardial viability. Cardiol Clin. 2007;25:35-56.
26 Simonetti OP, Kim RJ, Fieno DS, et al. An improved MR imaging technique for the visualization of myocardial infarction. Radiology. 2001;218:215-223.
27 Akhtar M, Ordovas K, Martin A, et al. Effect of chronic sustained-release dipyridamole on myocardial blood flow and left ventricular function in patients with ischemic cardiomyopathy. Congest Heart Fail. 2007;13:130-135.
28 Jahnke C, Nagel E, Gebker R, et al. Prognostic value of cardiac magnetic resonance stress tests: adenosine stress perfusion and dobutamine stress wall motion imaging. Circulation. 2007;115:1769-1776.
29 Ingkanisorn WP, Kwong RY, Bohme NS, et al. Prognosis of negative adenosine stress magnetic resonance in patients presenting to an emergency department with chest pain. J Am Coll Cardiol. 2006;47:1427-1432.
30 Kim RJ, Chen EL, Lima JA, Judd RM. Myocardial Gd-DTPA kinetics determine MRI contrast enhancement and reflect the extent and severity of myocardial injury after acute reperfused infarction. Circulation. 1996;94:3318-3326.
31 Knauth AL, Gauvreau K, Powell AJ, et al. Ventricular size and function assessed by cardiac MRI predict major adverse clinical outcomes late after tetralogy of Fallot repair. Heart. 2008;94:211-216.
32 Geva T, Sandweiss BM, Gauvreau K, et al. Factors associated with impaired clinical status in long-term survivors of tetralogy of Fallot repair evaluated by magnetic resonance imaging. J Am Coll Cardiol. 2004;43:1068-1074.
33 Bogaert J, Kuzo R, Dymarkowski S, et al. Follow-up of patients with previous treatment for coarctation of the thoracic aorta: comparison between contrast-enhanced MR angiography and fast spin-echo MR imaging. Eur Radiol. 2000;10:1847-1854.
34 Pujadas S, Reddy GP, Weber O, et al. Phase contrast MR imaging to measure changes in collateral blood flow after stenting of recurrent aortic coarctation: initial experience. J Magn Resonance Imaging. 2006;24:72-76.
35 Nielsen JC, Powell AJ, Gauvreau K, et al. Magnetic resonance imaging predictors of coarctation severity. Circulation. 2005;111:622-628.
36 Therrien J, Thorne SA, Wright A, et al. Repaired coarctation: a “cost-effective” approach to identify complications in adults. J Am Coll Cardiol. 2000;35:997-1002.
37 Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax. 1971;26:240-248.

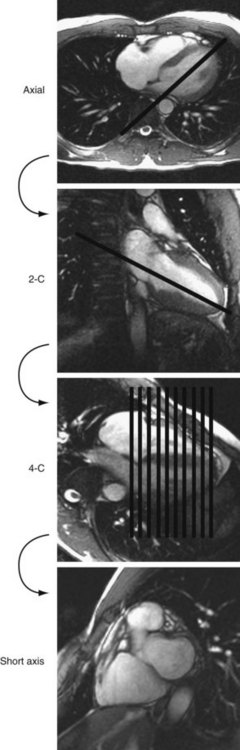
 FIGURE 50-1
FIGURE 50-1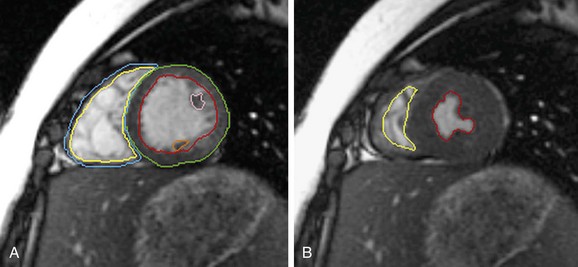
 FIGURE 50-2
FIGURE 50-2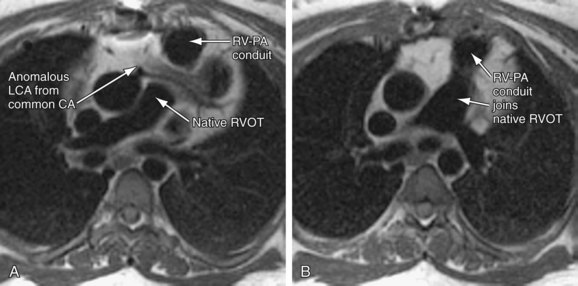
 FIGURE 50-3
FIGURE 50-3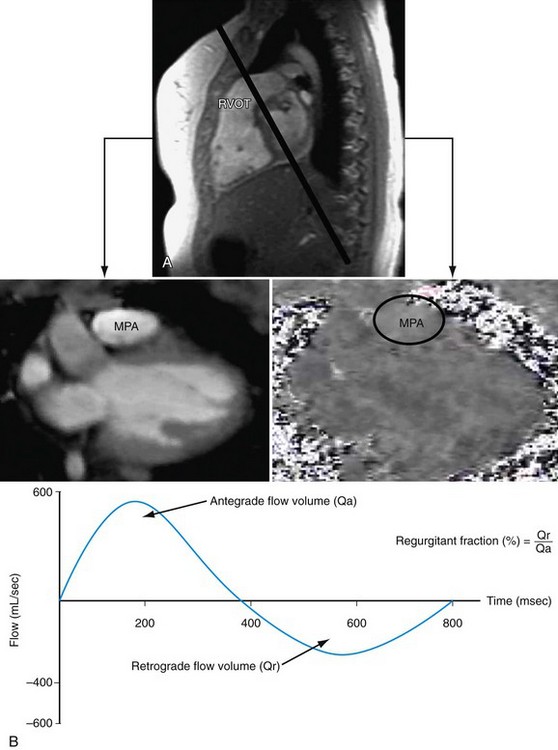
 FIGURE 50-4
FIGURE 50-4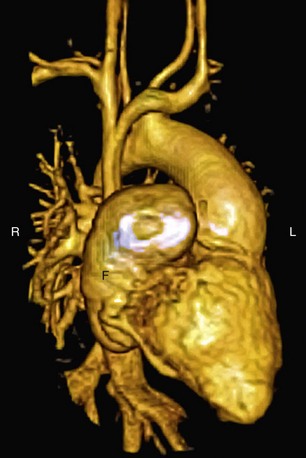
 FIGURE 50-5
FIGURE 50-5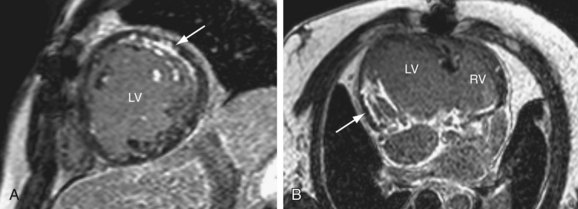
 FIGURE 50-6
FIGURE 50-6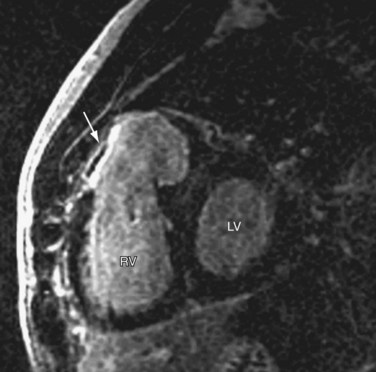
 FIGURE 50-7
FIGURE 50-7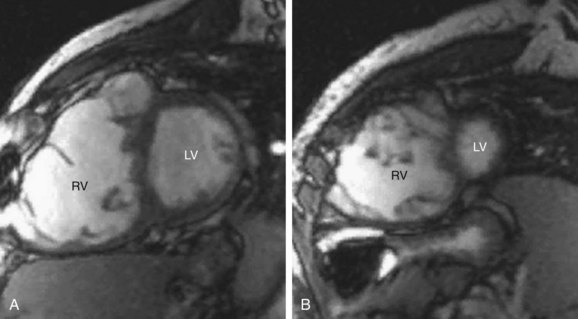
 FIGURE 50-8
FIGURE 50-8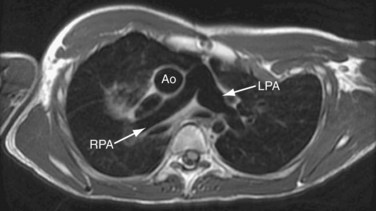
 FIGURE 50-9
FIGURE 50-9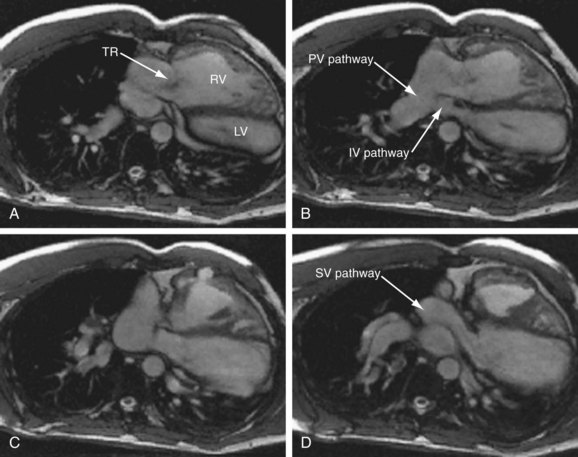
 FIGURE 50-10
FIGURE 50-10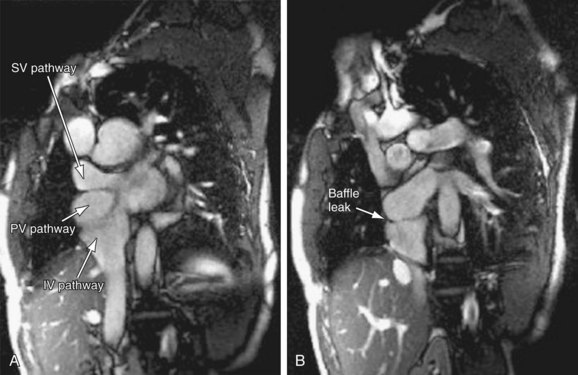
 FIGURE 50-11
FIGURE 50-11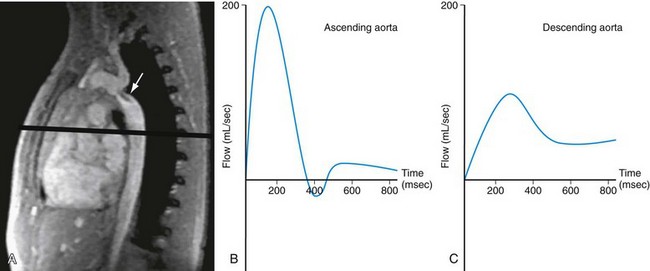
 FIGURE 50-12
FIGURE 50-12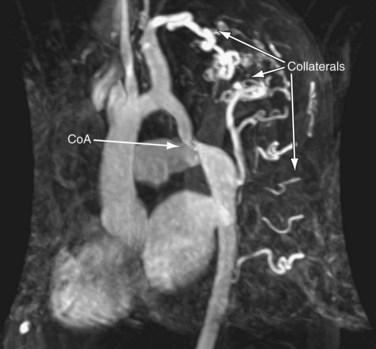
 FIGURE 50-13
FIGURE 50-13