CHAPTER 82 Magnetic Resonance Angiography
Clinical Techniques
DESCRIPTION OF TECHNICAL REQUIREMENTS
Field Strength
It has been established that signal-to-noise ratio (SNR) increases in an approximately linear fashion with magnetic field strength, which provides the opportunity for immensely superior vascular depiction with imaging at 3.0 T compared with 1.5 T. High field strength imaging is associated with a realm of potential challenges, however, which are relatively less significant at 1.5 T, including specific absorption rate considerations, T2* and dielectric resonance effects, and a greater incidence of clinically appreciable peripheral nerve stimulation.1 Use of a 3.0 T system demands familiarity with methods of avoiding and addressing these challenges such that compromised patient safety or image quality is not acceptable. Although higher field strength has advantages for MR angiography, it is not essential, and high-quality diagnostic examinations are routinely produced on 1.5 T MRI systems.
Gradient Coils
More recent developments in MRI hardware design have facilitated further improvements in gradient coil performance.2 High-performance gradient coils enable optimization of vascular SNR. These SNR improvements incur a penalty, however, in the form of energy deposition and increases in specific absorption rate. In practical terms, limitations in specific absorption rate often necessitate a compromise in attainable slice coverage for a particular repetition time (TR).
Phased-Array Surface Coils and Parallel Imaging Techniques
Comprising multiple integrated receiver coils, phased-array coils combine the advantages of high SNR achieved by smaller coils with the benefits of improved volume coverage, previously afforded only by large coil elements. Parallel imaging techniques (e.g., sensitivity encoding, or SENSE),3 whereby incomplete k-space sampling is tolerated by coil sensitivity profile calculation of the missing data, allow for significant improvements in temporal resolution, spatial resolution, or volume coverage. Parallel imaging depends on phased-array surface coils for its application. Parallel imaging improvements are attained at the expense, however, of reduced SNR. Such SNR loss may be offset, and even reversed, by imaging at higher field strengths (e.g., 3.0 T), allowing the benefit of ever-increasing acceleration factors to be realized without compromise in field of view (FOV) or spatial resolution.4
TIME OF FLIGHT MAGNETIC RESONANCE ANGIOGRAPHY
Repetitive successive radiofrequency pulses, if applied at a magnitude and rate sufficient to prevent interval T1 recovery, results in saturation of signal from tissue within the imaged volume.5 Time of flight (TOF) MR angiography exploits this saturation effect, providing untainted visualization of the signal produced by unsaturated entry of blood (i.e., through-plane blood flow) without the requirement for contrast agent administration. Unidirectional flow may be imaged through the use of presaturation pulses (also known as saturation bands) to eliminate signal from spins traveling in the opposite direction, with the effect of providing pure angiographic or venographic depiction, as desired. These attributes have made TOF MR angiography the most established MR angiography technique currently available, particularly with regard to the carotid, vertebral, and intracranial vascular territories.
Numerous potential implementations of this technique are available. Each varies in its degree of suitability, depending on the clinical indication. Two-dimensional TOF MR angiography involves the excitation of a single anatomic section and has proven useful for the evaluation of anatomic regions where respiratory or cardiac motion precludes useful volumetric evaluation (e.g., chest or abdomen). Multiple breath-holds and sequential, independent two-dimensional TOF MR angiography acquisitions may be used in this instance to provide diagnostic quality examinations, even in dyspneic patients (Fig. 82-1). Three-dimensional TOF MR angiography is preferred for intracranial evaluation in particular, permitting detailed volumetric data acquisition at submillimeter voxel resolution and the potential for subsequent postprocessing (Fig. 82-2). Multiple overlapping thin slab acquisition (MOTSA) represents a compromise in two-dimensional and three-dimensional techniques, integrating the advantages of three-dimensional imaging with the relatively fewer limitations of the two-dimensional approach. MOTSA combines multiple, relatively thin three-dimensional slabs to provide clinically useful volume coverage.6
Indications
Before the widespread introduction of contrast-enhanced MR angiography techniques for comprehensive large-volume anatomic vascular coverage, TOF MR angiography represented the cornerstone of MR angiography throughout the body. Contrast-enhanced MR angiography, however, required revision of many diagnostic algorithms in favor of this faster and typically higher quality method. Nonetheless, TOF MR angiography remains the technique of choice for intracranial vascular depiction, a reflection of its superb spatial and contrast resolution and its patient acceptability.7 Advances in MRI hardware, including the introduction of dedicated head and neck coils and resultant implementation of parallel imaging techniques, have enhanced the value of this approach in clinical practice further.
Pitfalls and Solutions
Despite its popularity and widespread implementation, TOF MR angiography may be extremely challenging to implement and interpret because of its numerous potential pitfalls.8
Saturation
As explained, successful TOF MR angiography depends on saturation of signal from static tissue, such that “fresh” through-plane vascular spins produce an appreciable signal. Saturation of blood signal occurs if blood flow is slow or persists within the imaging field (e.g., vessel coursing in-plane) and can result in suboptimal vascular visualization, to the point of potentially mimicking a vascular occlusion. This situation is of particular significance with regard to the use of three-dimensional TOF MR angiography, owing to the more extensive volume coverage required for most body applications. Numerous potential solutions to this dilemma exist, including optimization of TR and imaging plane, reduction of flip angle and echo time (TE), and use of thinner slices. Three-dimensional TOF MOTSA provides the advantages of three-dimensional TOF MR angiography, but by using thinner three-dimensional slabs, minimizes the saturation effects over that of a single large three-dimensional volume. If saturation effects persist in small, slow-flow vessels, administration of a small amount of T1-shortening paramagnetic contrast agent may prove effective, although at the risk of inducing adjacent soft tissue enhancement and venous contamination.9
PHASE CONTRAST MAGNETIC RESONANCE ANGIOGRAPHY
Phase contrast MR angiography is an unenhanced approach to imaging that employs bipolar phase-encoding gradient pairs to encode flow velocity in the gradient direction. Stationary background tissue accumulates a net phase shift of zero. Moving spins experience a net phase shift that produces signal and the image contrast necessary to distinguish between moving and stationary tissue (i.e., angiography).10 Phase contrast MR angiography requires the operator selection of a velocity encoding (VENC) in cm/s, which is responsible for determination of the flow sensitivity of the acquisition. Because assignment of phase shift is limited to a range of −180 degrees to +180 degrees, the VENC represents a flow velocity that would cause a maximal phase shift of 180 degrees. For optimal sensitivity, this VENC should be selected to correspond with or slightly exceed the highest velocity present within the vessel in question. For intracranial applications, a VENC of 70 to 80 cm/s is often sufficient for arterial imaging, whereas a factor of 20 to 30 cm/s should be applied for venous imaging.11 If the flow velocity exceeds the chosen VENC, aliasing results with the effect of apparent flow reversal.
Indications
Before the widespread availability of contrast-enhanced and increasingly impressive TOF techniques, phase contrast MR angiography was relatively successful in the evaluation of various vascular territories. In recent times, this approach has been relegated in importance to that of a “last resort,” should the other angiographic techniques discussed in this chapter be unsuccessful or contraindicated. Phase contrast MR angiography has regained some of its former popularity more recently because of its flow quantification capabilities. Our experience suggests that phase contrast flow quantification is a valuable, versatile tool in the noninvasive evaluation of flow characteristics within almost any vascular bed.12 Although this technique has not yet been incorporated into widespread clinical practice, its future potential remains encouraging.
THREE-DIMENSIONAL STEADY-STATE FREE PRECESSION MAGNETIC RESONANCE ANGIOGRAPHY
SSFP is a low flip angle gradient-recalled-echo (GRE) technique that induces a persistent level of tissue magnetization by means of a TR that is significantly shorter than the T2 of tissue. As a result, this approach permits bright blood vascular imaging, the signal from which is a reflection of the inherent T2/T1 ratio of blood, while precluding gadolinium-chelate contrast agent administration.13 Owing to a very short TR and large flip angle, two-dimensional SSFP techniques allow rapid subsecond image acquisition that does not require respiratory suspension, even when imaging the chest. These attributes have resulted in the adoption of SSFP as a cornerstone imaging technique in many aspects of cardiac imaging, including two-dimensional single-shot multiplanar morphologic and ECG gated cine functional myocardial assessment.
Many three-dimensional implementations of SSFP have been successfully evaluated for the purpose of vascular imaging, most notably with regard to the coronary and renal arteries.14,15 In exploiting the intrinsic T2/T1 signal of blood, three-dimensional SSFP MR angiography allows large FOV vascular coverage, while avoiding the data acquisition constraints because of the contrast bolus imposed during contrast-enhanced MR angiography. Combining three-dimensional SSFP MR angiography with navigator gating allows free-breathing nonenhanced chest and abdominal vascular depiction. Further addition of ECG gating has allowed the realization of free-breathing coronary MR angiography, although at the expense of often prolonged acquisition times (≥10 minutes) (Fig. 82-3). The potential of parallel imaging techniques to aid in reduction of these acquisition times has been evaluated, providing encouraging results to date. Implementation of this data-sharing technique does incur penalties with regard to SNR, however, with the effect of image degradation that may be poorly tolerated.
Pitfalls and Solutions
SSFP techniques are sensitive to off-resonance artifacts, manifesting as dark bands traversing the images acquired. These potentially detrimental regions may prove particularly difficult to avoid when imaging at 3.0 T. Preventive methods have been described, including small volume frequency scouting to select the optimal frequency at which the bands are eradicated.16
Whole heart coronary MR angiography using three-dimensional SSFP may represent a source of considerable frustration owing to its occasional production of poor-quality studies despite its apparently adequate implementation. In many cases, these suboptimal studies stem from poor delineation of the luminal and mural margins, with resultant blurring of the images obtained. Because successful three-dimensional SSFP demands careful coordination of numerous key components, knowledge of each is necessary so that the offending factor may be addressed.17
Image Interpretation
Postprocessing
The volumetric nature of three-dimensional SSFP MR angiography with near-isotropic voxel dimensions permits data reconstruction in any desired plane without incurring penalties with regard to image distortion (Fig. 82-4). Comprehensive multiplanar vascular evaluation is typically feasible on the basis of a single acquisition. Coronary artery assessment generally involves curved multiplanar reconstruction (MPR) processing, allowing visualization of the arterial lumen throughout its length, despite its tortuous course. Short-axis reconstructions (perpendicular to the vascular lumen) at sites of suspected pathology may also be derived from this data set. Renal artery evaluations generally involve coronal oblique and axial reconstructions.
TIME RESOLVED MAGNETIC RESONANCE ANGIOGRAPHY
Time resolved MR angiography is a dynamic approach to contrast-enhanced angiography that involves rapid sequential imaging of an anatomic volume during the luminal transit of a contrast bolus (Fig. 82-5). Although most commonly implemented as a two-dimensional technique for the purpose of “bolus timing” in preparation for subsequent high-resolution contrast-enhanced MR angiography, more recent three-dimensional applications of TR MR angiography have shown considerable promise with regard to functional vascular imaging and evaluation of visceral perfusion.
Regardless of the approach used, time resolved MR angiography involves the use of a T1-weighted GRE sequence with sufficiently short TR to allow repeated imaging at temporal resolutions of 1 to 2 seconds per frame. Achievement of such ultrashort temporal resolutions demands that compromises are made, however, most commonly with respect to spatial resolution. One approach is to allow concessions in the slice-select direction, as described by Finn and colleagues.18 Although this method precludes off-axis reconstruction of the volumetric data obtained, this is rarely of sufficient impact to limit the diagnostic utility of the studies performed. Alternatively, through-plane resolution comparable to that of the in-plane direction may be employed, although at the expense of less frequent data refreshment.19
Indications
Widespread appreciation of the potential utility of time resolved MR angiography has resulted in its successful application across a wide range of disciplines (Figs. 82-6 and 82-7). Our experience with this technique has been considerable and similarly encouraging. Table 82-1 summarizes proven indications for the use of time resolved MR angiography, including suggested applications that are based on our personal experience to date.
TABLE 82-1 Indications for the Use of Time Resolved Magnetic Resonance Angiography
| Discipline | Application |
|---|---|
| Neurovascular | Cerebral and spinal vascular malformations |
| Spinal arteriovenous shunts | |
| Subclavian steal syndrome | |
| Dural arteriovenous fistulas | |
| Carotid bifurcation imaging | |
| Postoperative assessment of extracranial to intracranial arterial bypass | |
| Intracranial venous sinus evaluation | |
| Assessment of bilateral symmetric cerebral parenchymal perfusion in the presence of internal carotid or vertebrobasilar steno-occlusive disease | |
| Cardiac | Work-up of complex pediatric and adult congenital heart disease |
| Evaluation of patency of intracardiac and extracardiac surgical shunts (e.g., Fontan, Glenn shunt) | |
| Detection of right-to-left intracardiac shunts | |
| Pulmonary | Assessment of pulmonary perfusion |
| Differentiation between idiopathic and thromboembolic pulmonary hypertension | |
| Evaluation of pulmonary arteriovenous malformations | |
| Primary screening of patients with suspected pulmonary embolism | |
| Pulmonary venous ostial evaluation for stenosis | |
| Pulmonary venous assessment for anomalous drainage | |
| Chest/abdomen | Assessment of differential flow in true and false lumens after aortic dissection |
| Evaluation for endoleaks after endograft repair of abdominal aortic aneurysm | |
| Preoperative evaluation of hepatic arterial and portal venous anatomy in potential liver donors | |
| Determination of adequacy of renal parenchymal perfusion in the presence of renal artery stenosis | |
| Extremity | Evaluation of the hemodynamic significance of infrageniculate arterial occlusive disease |
| Assessment of soft tissue enhancement in the presence of cellulitis | |
| Determination of degree of arteriovenous shunting in peripheral arteriovenous malformations | |
| Venous | Confirmation of venous patency |
| Determination of dominant venous collaterals in the presence of venous occlusion | |
| General (incidental detection) | Hypervascular thyroid nodules |
| Unknown arterial abnormalities (e.g., splenic/renal artery aneurysms) |
Contraindications
The potential role of time resolved MR angiography in clinical practice has been readdressed in recent times as a result of the emergence of a potential link between administration of gadolinium-chelate contrast agents and development of the potentially fatal condition nephrogenic systemic fibrosis in patients with severe, end-stage renal failure.20 Although some authors have questioned the justification for contrast-enhanced MR angiography and time resolved MR angiography in these patients, others have focused on the potential of time resolved MR angiography to replace its high-resolution three-dimensional counterpart in certain instances. Such preclusion of the requirement for contrast-enhanced MR angiography is wholly desirable, a reflection of the ability of time resolved MR angiography to provide highly diagnostic studies with the injection of only 0.01 to 0.02 mmol of gadolinium-chelate contrast agent per kilogram of patient body weight compared with 0.1 to 0.2 mmol/kg for contrast-enhanced MR angiography.21
THREE-DIMENSIONAL CONTRAST-ENHANCED MAGNETIC RESONANCE ANGIOGRAPHY
High-resolution three-dimensional contrast-enhanced MR angiography has become one of the most powerful diagnostic tools in diagnostic imaging. This is due in large part to the introduction of high field MRI systems with rapid high-performance gradient coils and the development of phased array coils and, subsequently, parallel imaging techniques.22 These advances have conspired to make large FOV high-resolution three-dimensional contrast-enhanced MR angiography during comfortable breath-hold times and in the absence of venous contamination a clinical reality.
Indications
The efficacy of three-dimensional contrast-enhanced MR angiography has been proven for almost every vascular territory, with the result that it has now replaced conventional diagnostic angiography as the imaging modality of choice for various clinical applications. The most common indication for contrast-enhanced MR angiography is the investigation of suspected atherosclerotic steno-occlusive disease.23 This approach allows comprehensive, large-volume, detailed evaluation for the presence of mural irregularity, stenosis, dissection, or aneurysmal dilation, and permits accurate assessment of their effect on the adjacent vessel lumen. The most common territories routinely evaluated using this technique are extracranial carotid, supra-aortic, thoracic and abdominal aortic, renal, mesenteric, and peripheral extremity vessels (Figs. 82-8, 82-9, and 82-10).
Pulmonary vascular imaging has also benefited significantly from developments in contrast-enhanced MR angiography; this technique allows depiction of branch vessels of calibers of 1 to 2 mm. Contrast-enhanced MR angiography has now become a valuable tool in the evaluation of suspected pulmonary hypertension and determination of its potential etiology, such as chronic thromboembolic disease. The efficacy of this technique in diagnosis of acute pulmonary embolism has also been shown, and this may be of particular value in patients in whom iodinated contrast media are undesirable or contraindicated.24
The reproducibility of contrast-enhanced MR angiography has also led to its widespread adoption in the follow-up of patients who have undergone open surgical intervention for atherosclerotic occlusive disease, most often in the form of bypass grafts, especially extra-anatomic bypass grafts. Although the presence of anastomotic hemostatic clips occasionally may preclude visualization of such conduits throughout their length owing to metallic susceptibility artifact, this is rarely of such severity as to limit diagnostic interpretation.25 Three-dimensional contrast-enhanced MR angiography has also proven to be of high diagnostic utility in the diagnosis of acute aortic events, such as dissection or intramural hematoma, vascular inflammatory conditions (e.g., Takayasu and giant cell arteritis), evaluation of coronary arterial course and patency, and pediatric vascular abnormalities, including aortic coarctation (Figs. 82-11 and 82-12) and truncus arteriosus (Fig. 82-13), among many other conditions (Fig. 82-14).
Pitfalls and Solutions
Compromise in any of these prerequisites may result in suboptimal contrast-enhanced MR angiography. Premature central k-space filling relative to the peak of the contrast bolus may result in intraluminal “pseudofilling defects” owing to inappropriate filling of the low spatial frequency data in this region of k-space. Failure of coordination of the contrast bolus and data acquisition such that the arterial phase has passed and venous contamination is present may also obscure vascular detail, which is a particular disadvantage when imaging the lower extremities. Dispersion of the contrast bolus or poor signal reception may result in inadequate luminal enhancement, making identification of subtle vascular abnormalities impossible. Motion artifact also may have a significantly detrimental effect on image quality, owing to the production of blurring of vessel margins.26
Preemptive avoidance of many of these potential adversities is often readily achievable, however. Numerous methods have been developed to aid in bolus timing, including use of a test bolus, “fluoroscopic” or automated bolus detection of contrast arrival, incorporation of a fixed delay between contrast injection and start of data acquisition, and repetitive scanning so that different phases of opacification are obtained.27 Although some methods (e.g., test bolus, real-time “fluoroscopy,” and automated bolus detection algorithm) may be expected to produce more consistent results than others (e.g., fixed delay and multiphase imaging), familiarity with more than one technique is recommended so that timing issues in uncharacteristic cases may be addressed. Optimization of intravascular signal should involve a combination of MRI-compatible power injector and appropriate surface receiver coils, such that image contrast between the lumen and extravascular soft tissues is maximal. Motion artifact may be difficult, or occasionally impossible, to address—particularly in dyspneic patients because of the relatively long breath-hold times required for chest and abdominal contrast-enhanced MR angiography (approximately 20 seconds). Nonetheless, preprocedural coaching of patient respiratory suspension and attention to patient fears should represent a routine part of any contrast-enhanced MR angiography examination.
Image Interpretation
Postprocessing
Three-dimensional contrast-enhanced MR angiography provides a complete volumetric data set amenable to a wide variety of image post-processing.28 MPRs are of significant practical value in the assessment of vascular patency, particularly in regions where overlapping vascular structures with tortuous courses are present (e.g., chest MR angiography). This holds particularly true when isotropic or near-isotropic voxels have been acquired because MPR allows reconstruction at any conceivable projection without the risk of image distortion or elongation. In certain instances, curved MPRs (whereby the axis of the reconstruction deviates from a single plane) may also be considerably valuable, most commonly in the determination of changes in caliber of long, tortuous vessels, such as the coronary arteries and thoracic aorta.
Reporting
KEY POINTS
 The term MR angiography encompasses a variety of imaging techniques (e.g., TOF MR angiography, phase contrast MR angiography, SSFP MR angiography, time resolved MR angiography, contrast-enhanced MR angiography), each unique in design, implementation, and clinical applicability.
The term MR angiography encompasses a variety of imaging techniques (e.g., TOF MR angiography, phase contrast MR angiography, SSFP MR angiography, time resolved MR angiography, contrast-enhanced MR angiography), each unique in design, implementation, and clinical applicability.Grobner T, Prischl FC. Gadolinium and nephrogenic systemic fibrosis. Kidney Int. 2007;72:260-264.
Ho VB, Foo TK, Czum JM, et al. Contrast-enhanced magnetic resonance angiography: technical considerations for optimized clinical implementation. Top Magn Reson Imaging. 2001;12:283-299.
Kramer H, Michaely HJ, Reiser MF, et al. Peripheral magnetic resonance angiography at 3.0 T. Top Magn Reson Imaging. 2007;18:135-138.
Meaney JF, Goyen M. Recent advances in contrast-enhanced magnetic resonance angiography. Eur Radiol. 2007;17(Suppl 2):B2-B6.
Michaely HJ, Dietrich O, Nael K, et al. MRA of abdominal vessels: technical advances. Eur Radiol. 2006;16:1637-1650.
Ozsarlak O, Van Goethem JW, Maes M, et al. MR angiography of the intracranial vessels: technical aspects and clinical applications. Neuroradiology. 2004;46:955-972.
Stuber M, Weiss RG. Coronary magnetic resonance angiography. J Magn Reson Imaging. 2007;26:219-234.
Yucel EK, Anderson CM, Edelman RR, et al. AHA scientific statement. Magnetic resonance angiography: update on applications for extracranial arteries. Circulation. 1999;100:2284-2301.
Zhang H, Maki JH, Prince MR. 3D contrast-enhanced MR angiography. J Magn Reson Imaging. 2007;25:13-25.
1 Bernstein MA, Huston J3rd, Ward HA. Imaging artifacts at 3.0 T. J Magn Reson Imaging. 2006;24:735-746.
2 Rohrer M, Geerts-Ossevoort L, Laub G. Technical requirements, biophysical considerations and protocol optimization with magnetic resonance angiography using blood-pool agents. Eur Radiol. 2007;17(Suppl 2):B7-B12.
3 Pruessmann KP, Weiger M, Scheidegger MB, et al. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 1999;42:952-962.
4 Frydrychowicz A, Bley TA, Winterer JT, et al. Accelerated time-resolved 3D contrast-enhanced MR angiography at 3T: clinical experience in 31 patients. MAGMA. 2006;19:187-195.
5 Bosmans H, Marchal G, Lukito G, et al. Time-of-flight MR angiography of the brain: comparison of acquisition techniques in healthy volunteers. AJR Am J Roentgenol. 1995;164:161-167.
6 Davis WL, Blatter DD, Harnsberger HR, et al. Intracranial MR angiography: comparison of single-volume three-dimensional time-of-flight and multiple overlapping thin slab acquisition techniques. AJR Am J Roentgenol. 1994;163:915-920.
7 Sadikin C, Teng MM, Chen TY, et al. The current role of 1.5T non-contrast 3D time-of-flight magnetic resonance angiography to detect intracranial steno-occlusive disease. J Formos Med Assoc. 2007;106:691-699.
8 Wilcock DJ, Jaspan T, Worthington BS. Problems and pitfalls of 3D TOF magnetic resonance angiography of the intracranial circulation. Clin Radiol. 1996;50:526-532.
9 Ishimaru H, Ochi M, Morikawa M, et al. Accuracy of pre- and post-contrast 3D time-of-flight MR angiography in patients with acute ischemic stroke: correlation with catheter angiography. AJNR Am J Neuroradiol. 2007;28:923-926.
10 Dumoulin CL, Souza SP, Walker MF, et al. Three-dimensional phase contrast angiography. Magn Reson Med. 1989;9:139-149.
11 Marks MP, Pelc MJ, Ross MR, et al. Determination of cerebral blood flow with a phase-contrast cine MR imaging technique: evaluation of normal subjects and patients with arteriovenous malformation. Radiology. 1992;182:467-476.
12 Baledent O, Gondry-Jouet C, Stoquart-Elsankari S, et al. Value of phase contrast magnetic resonance imaging for investigation of cerebral hydrodynamics. J Neuroradiol. 2006;33:292-303.
13 Reeder SB, Herzka DA, McVeigh ER. Signal-to-noise ratio behavior of steady-state free precession. Magn Reson Med. 2004;52:123-130.
14 Zagrosek A, Noeske R, Abdel-Aty H, et al. MR coronary angiography using 3D-SSFP with and without contrast application. J Cardiovasc Magn Reson. 2005;7:809-814.
15 Maki JH, Wilson GJ, Eubank WB, et al. Steady-state free precession MRA of the renal arteries: breath-hold and navigator-gated techniques vs CE-MRA. J Magn Reson Imaging. 2007;26:966-973.
16 Wansapura J, Fleck R, Crotty E, et al. Frequency scouting for cardiac imaging with SSFP at 3 Tesla. Pediatr Radiol. 2006;36:1082-1085.
17 Stuber M, Weiss RG. Coronary magnetic resonance angiography. J Magn Reson Imaging. 2007;26:219-234.
18 Finn JP, Baskaran V, Carr JC, et al. Thorax: low-dose contrast-enhanced three-dimensional MR angiography with subsecond temporal resolution—initial results. Radiology. 2002;224:896-904.
19 Korosec FR, Frayne R, Grist TM, et al. Time-resolved contrast-enhanced 3D MR angiography. Magn Reson Med. 1996;36:345-351.
20 Sieber MA, Peitsch H, Walter J, et al. A preclinical study to investigate the development of nephrogenic systemic fibrosis: a possible role for gadolinium-based contrast media. Invest Radiol. 2008;43:65-75.
21 Michaely HJ, Nael K, Schoenberg SO, et al. Renal perfusion: comparison of saturation-recovery TurboFLASH measurements at 1.5T with saturation-recovery TurboFLASH and time-resolved echo-shared angiographic technique (TREAT) at 3.0T. J Magn Reson Imaging. 2006;24:1413-1419.
22 Michaely HJ, Herrmann KA, Kramer H, et al. High-resolution renal MRA: comparison of image quality and vessel depiction with different parallel imaging acceleration factors. J Magn Reson Imaging. 2006;24:95-100.
23 Hansen T, Ahlstrom H, Johansson L. Whole-body screening of atherosclerosis with magnetic resonance angiography. Top Magn Reson Imaging. 2007;18:329-337.
24 Pleszewski B, Chartrand-Lefebvre C, Qanadli SD, et al. Gadolinium-enhanced pulmonary magnetic resonance angiography in the diagnosis of acute pulmonary embolism: a prospective study on 48 patients. Clin Imaging. 2006;30:166-172.
25 Loewe C, Cejna M, Schoder M, et al. Contrast material-enhanced, moving-table MR angiography versus digital subtraction angiography for surveillance of peripheral arterial bypass grafts. J Vasc Interv Radiol. 2003;14(9 Pt 1):1129-1137.
26 Spincemaille P, Hai ZX, Cheng L, et al. Motion artifact suppression in breath hold 3D contrast enhanced magnetic resonance angiography using ECG ordering. Conf Proc IEEE Eng Med Biol Soc. 2006;1:739-742.
27 Shetty AN, Bis KG, Kirsch M, et al. Contrast-enhanced breath-hold three-dimensional magnetic resonance angiography in the evaluation of renal arteries: optimization of technique and pitfalls. J Magn Reson Imaging. 2000;12:912-923.
28 Lell M, Fellner C, Baum U, et al. Evaluation of carotid artery stenosis with multisection CT and MR imaging: influence of imaging modality and postprocessing. AJNR Am J Neuroradiol. 2007;28:104-110.

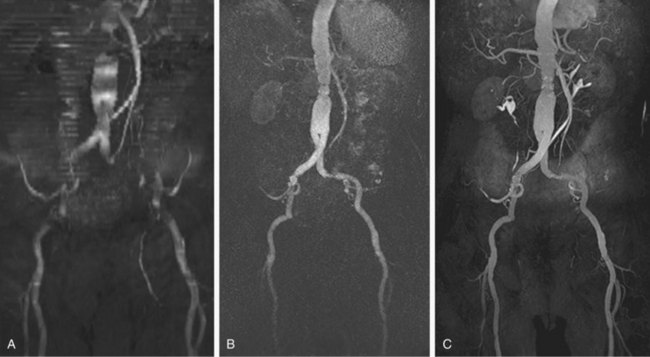
 FIGURE 82-1
FIGURE 82-1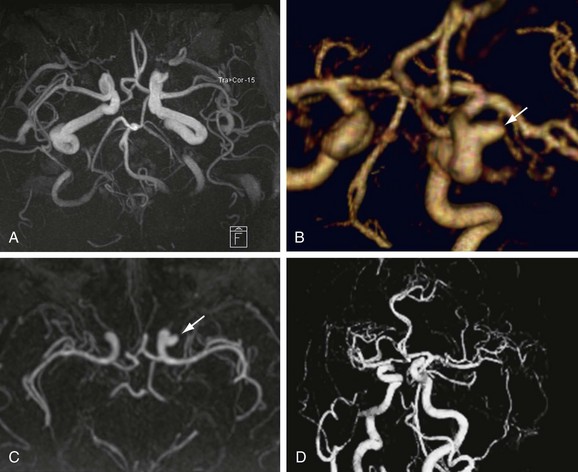
 FIGURE 82-2
FIGURE 82-2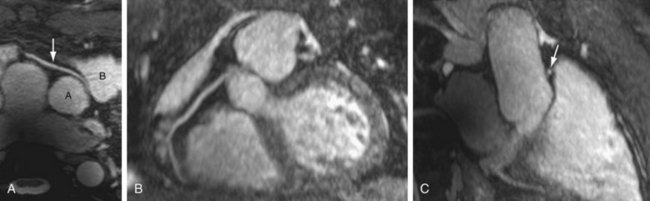
 FIGURE 82-3
FIGURE 82-3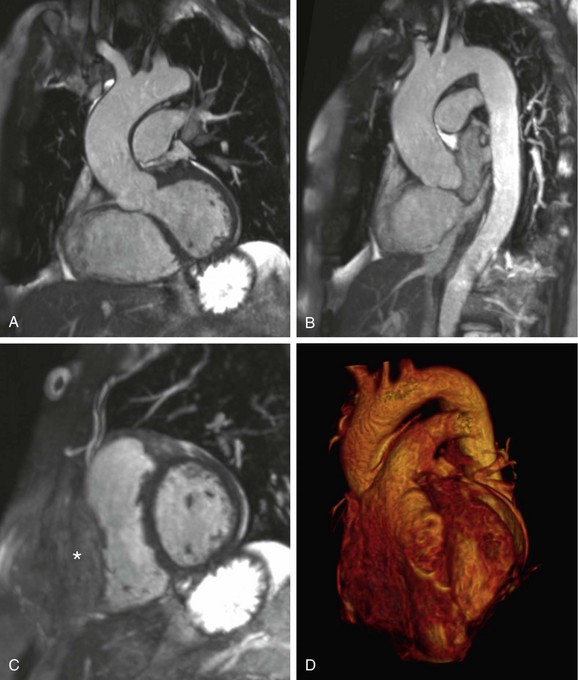
 FIGURE 82-4
FIGURE 82-4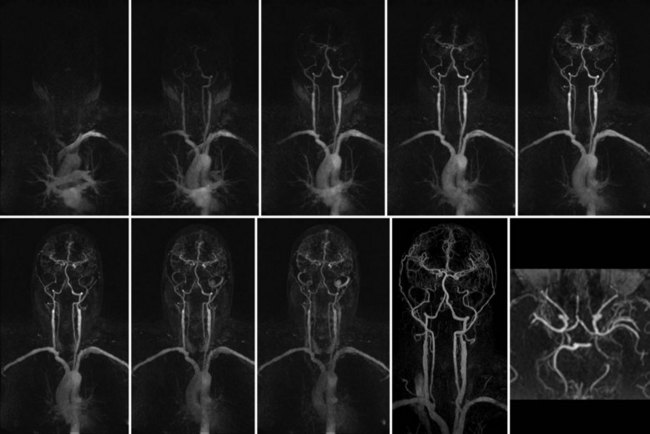
 FIGURE 82-5
FIGURE 82-5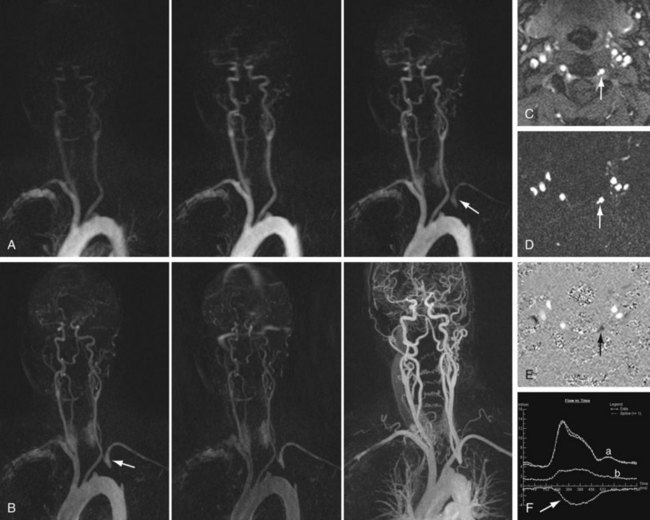
 FIGURE 82-6
FIGURE 82-6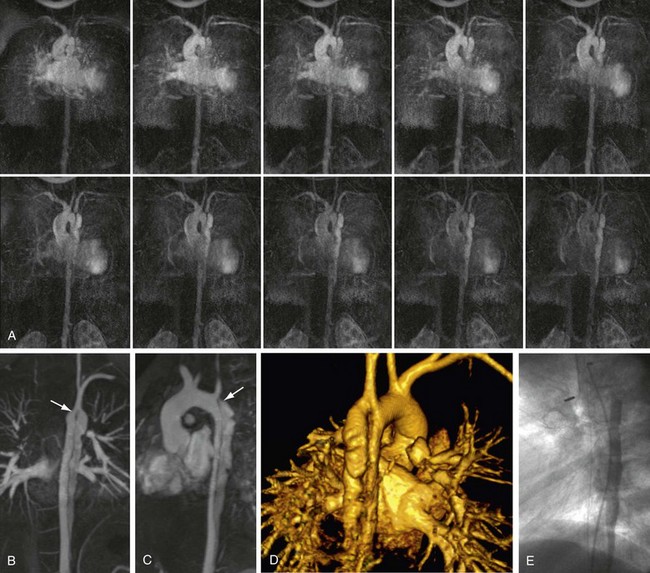
 FIGURE 82-7
FIGURE 82-7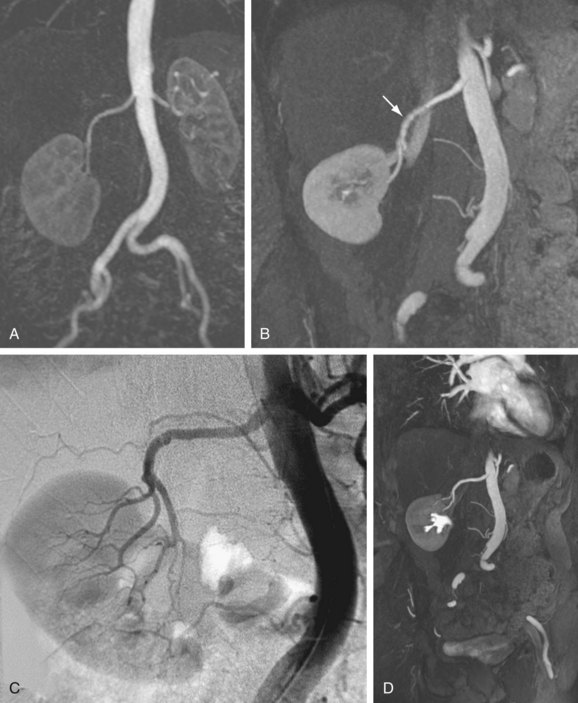
 FIGURE 82-8
FIGURE 82-8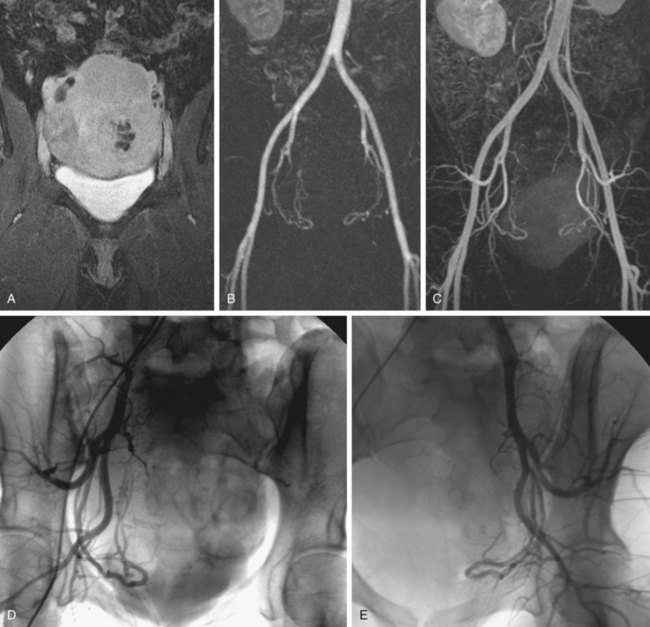
 FIGURE 82-9
FIGURE 82-9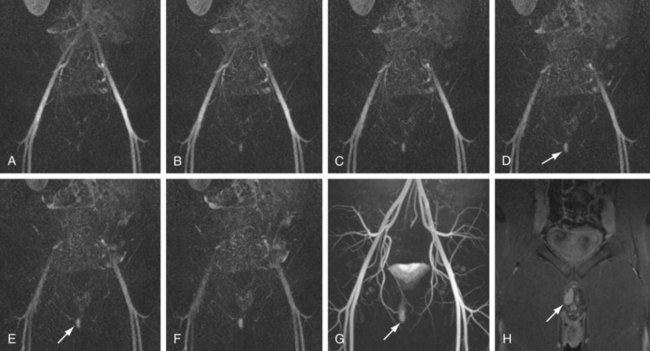
 FIGURE 82-10
FIGURE 82-10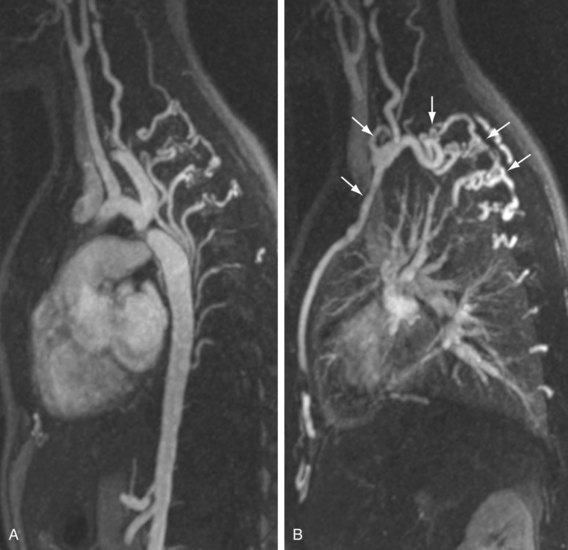
 FIGURE 82-11
FIGURE 82-11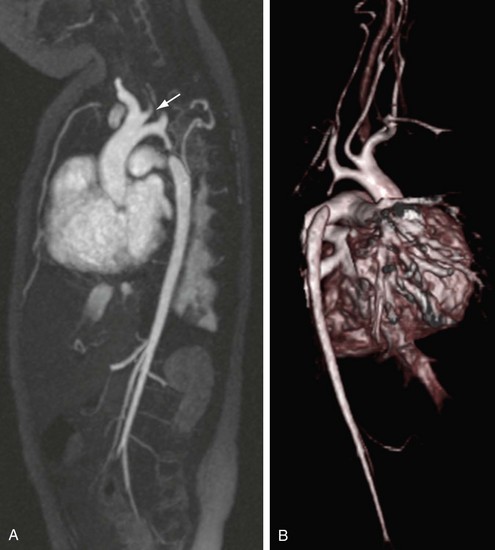
 FIGURE 82-12
FIGURE 82-12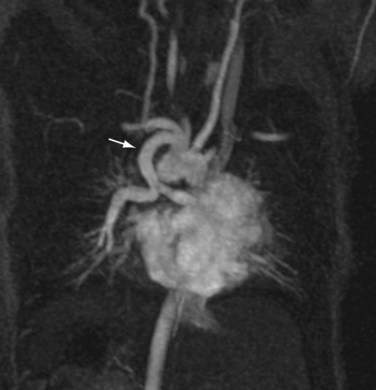
 FIGURE 82-13
FIGURE 82-13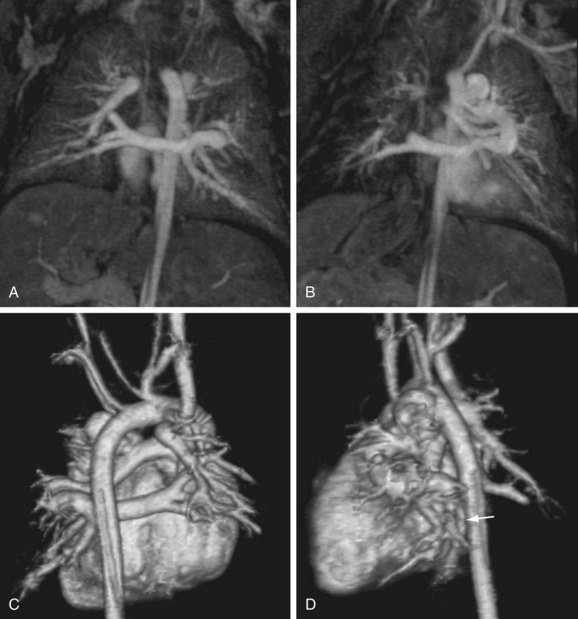
 FIGURE 82-14
FIGURE 82-14

