CHAPTER 53 Magnetic Resonance and Computed Tomographic Imaging of Myocardial Perfusion
Data from the National Health and Nutrition Examination Survey (1999-2004, National Heart, Lung and Blood Institute) estimate the prevalence of coronary heart disease to be 16 million individuals in the United States, and incidence of new and recurrent coronary events to be 1.2 million per year.1 Data from 44 years of follow-up in the original Framingham Heart Study cohort and 20 years of their offspring surveillance show the lifetime risk of developing coronary heart disease for individuals 40 years old to be 49% in men and 32% in women.2 With mortality statistics claiming that one out of every five deaths is the result of coronary artery disease (CAD),1 there is a clear need for improved diagnostic imaging strategies for detecting coronary heart disease and myocardium vulnerable to scenarios of reduced perfusion.
The clinical presentation of ischemic heart disease is incredibly diverse and includes asymptomatic or silent ischemia, chronic angina, unstable angina or infarction (i.e., ACS), new-onset or chronic heart failure, or sudden cardiac death. Although symptomatic patients are often identified by symptoms related to angina or heart failure, patients who have silent ischemia represent a diagnostic dilemma because they are less likely to be referred for testing. Patients who are particularly susceptible to silent ischemia include diabetics, elderly patients, and patients with prior MI or surgical revascularization.3
Noninvasive imaging is a crucial component in the evaluation of patients with suspected ischemic heart disease. Figure 53-1 illustrates an algorithm for the management of patients with potential ischemic heart disease. For patients presenting with a possible ACS who have a negative ECG and biomarkers (e.g., troponin), a stress test can be used to identify high-risk patients who would benefit from being admitted to the hospital for further evaluation and testing. The American College of Cardiology/American Heart Association guidelines for the management of unstable angina/non–ST segment elevation MI suggest that coronary CT angiography is a reasonable alternative to stress testing in patients with low to intermediate probability of CAD in whom initial ECG and initial biomarkers are unremarkable.4
Patients who are admitted with an ACS can be treated with an early invasive or early conservative strategy. High-risk patients have been shown to benefit from early interventions, and warrant early referral for invasive angiography. For low-risk patients, and in particular women, a conservative strategy that uses noninvasive testing is recommended.4
For patients with chronic stable angina, the American College of Cardiology/American Heart Association guidelines5 suggest that ejection fraction should be measured for all patients with a history of MI or signs suggesting heart failure. In the presence of a systolic murmur suggestive of aortic stenosis, mitral regurgitation, or hypertrophic cardiomyopathy, an echocardiogram should be obtained.
As is shown in Figure 53-1, all patients with chronic angina (i.e., stable angina) should have an evaluation for the presence of ischemic heart disease. This evaluation can be accomplished by ECG exercise testing, noninvasive stress imaging, or invasive angiography. Higher risk patients or patients with abnormal baseline ECG should be referred for an imaging-based test. The goal of such a test is to identify the extent, severity, and location of ischemia and when possible provide information about prognosis.
The role of cardiac imaging for the detection of silent ischemia is controversial. Patients with diabetes may benefit from such a strategy because they have a high risk of cardiovascular-related mortality, are more likely to have silent ischemia, and are less likely to survive an MI than nondiabetic patients.6 Nevertheless, the cost-effectiveness of screening all such at-risk patients with nuclear perfusion imaging is controversial.7
This chapter focuses on the evaluation of MRI and CT for the assessment of myocardial perfusion (Figs. 53-2 and 53-3). The advantages and disadvantages of other imaging modalities are discussed and compared with MRI and CT, and these imaging modalities are put in clinical perspective with descriptions of how and when they can facilitate the diagnosis and management of patients suspected to have CAD or with known CAD.
MAGNETIC RESONANCE IMAGING
Preclinical and Clinical Evaluation
Numerous animal studies have shown good correlation of MRI perfusion with tissue perfusion as measured by radioactive microspheres.8,9 Notable studies included work by Wilke and colleagues and Klocke and associates,8 in which porcine and canine models of left circumflex coronary artery stenosis were created that showed that MRI can detect different degrees of myocardial perfusion under adenosine stress.
Subsequently, Lee and colleagues9 used a canine model of left circumflex coronary artery stenosis and compared perfusion MRI with Tc 99m sestamibi and thallium 201. They showed that MRI could detect perfusion defects with a left circumflex coronary artery stenosis of 50% or greater, whereas SPECT perfusion defects were detected only with left circumflex coronary artery stenosis of 85% or greater.
Multiple clinical human studies testing the diagnostic accuracy and performance of stress perfusion MRI were performed comparing MRI with nuclear imaging and conventional coronary angiography. Table 53-1 summarizes published data with x-ray angiography as the reference standard. Nandalur and associates10 performed a meta-analysis of all stress MRI studies with two main techniques in use, perfusion imaging and imaging of stress-induced wall motion abnormalities, from January 1990 to January 2007 with a total of 37 studies involving 2191 patients. All studies used catheter x-ray angiography as the reference standard. Fourteen studies (N = 754 patients) using stress-induced wall motion abnormalities imaging showed 83% sensitivity and 86% specificity on a patient level. Perfusion imaging showed a sensitivity of 91% and specificity of 81% on a patient level (disease prevalence 57.4%).
TABLE 53-1 Diagnostic Accuracy of Stress Perfusion Magnetic Resonance Imaging Studies, Having Invasive Coronary Angiography as the Reference Standard
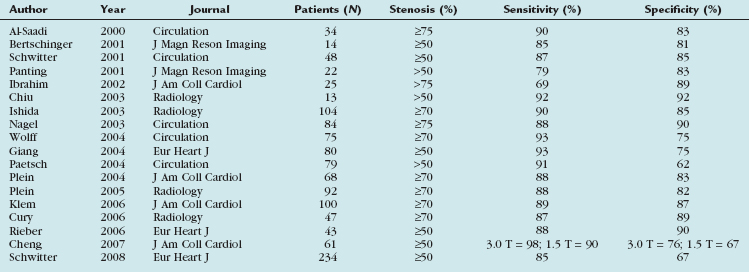
Two more recent studies by Cury and associates11 and Klem and coworkers12 (see Table 53-1) sought to improve the diagnostic accuracy of stress perfusion MRI by using a comprehensive imaging approach of first-pass contrast administration for myocardial perfusion at rest and stress with the addition of myocardial delayed enhancement (MDE) imaging for infarct detection and characterization. Our group showed that this combined approach has 87% sensitivity, 89% specificity, and 88% accuracy, and was superior to rest/stress perfusion MRI alone, which has 81% sensitivity, 87% specificity, and 85% accuracy, having invasive coronary angiography as the reference standard. Klem and coworkers12 showed similar results—that stress perfusion and MDE MRI can better detect inducible ischemia and fixed defects compared with stress/rest perfusion MRI. MDE MRI also provides the benefit of improved identification of regions of no reflow or microvascular obstruction, which is readily identified on MDE as regions of no hyperenhancement within the hyperenhancing infarction (see Figs. 53-2 and 53-3).
In another study evaluating patients presenting to the emergency department with acute chest pain, Ingkanisorn and colleagues13 evaluated the diagnostic value of adenosine stress myocardial perfusion MRI in 135 patients who presented to the emergency department with chest pain and a negative initial troponin value. The main study outcome was the detection of any evidence of significant CAD. Patients were contacted at 1 year to determine the incidence of significant CAD, defined as significant coronary artery stenosis (>50%) on invasive coronary angiography, abnormal correlative stress test, new MI, or death. Adenosine myocardial perfusion MRI abnormalities had 100% sensitivity and 93% specificity for detection of significant CAD, and an abnormal MRI added significant prognostic value in predicting a future diagnosis of CAD, MI, or death over clinical risk factors. No patients with a normal adenosine myocardial perfusion MRI had a subsequent diagnosis of CAD or an adverse outcome.
The first multicenter trial involving 18 centers in Europe and the United States (MR-IMPACT trial) comparing stress myocardial perfusion MRI with SPECT imaging and invasive coronary angiography was published more recently.14 When comparing perfusion MRI at 0.1 mmol/kg versus the entire SPECT population, the receiver operating curve analysis showed a better performance for perfusion MRI (n = 42, area under the curve [AUC] 0.86 ± 0.06) versus SPECT (n = 212, AUC 0.67 ± 0.05; P = .013 vs. MRI). The MRI performance at 0.1 mmol/kg was also superior in patients with multivessel disease (n = 32 and n = 161 for MRI and SPECT, AUC 0.89 ± 0.06 vs. 0.70 ± 0.05; P =.006). Overall, the authors concluded that the comparison of stress perfusion MRI with the entire SPECT population suggests superiority of MRI over SPECT, which warrants further evaluation in larger trials. These results are in concordance with a prior study from Ishida and associates14a that also showed superiority of MRI over SPECT in a single-center trial.
COMPUTED TOMOGRAPHY
Although multiple, more recent single-center and multicenter studies15 have established the diagnostic accuracy of cardiac CT for the detection of coronary artery stenosis, the functional significance of many coronary artery lesions identified by such techniques (or by invasive coronary angiography) is often unknown.16 MPI and angiography have the potential to provide complementary information by imaging ischemia and atherosclerosis. The potential of obtaining this information from a single imaging modality is very attractive.
Animal Studies: Rest Perfusion
The ability of CT to detect acute MI in explanted hearts and experimental animal models dates back to the late 1970s.17 More recently, multidetector CT has been used to assess myocardial perfusion in animal models of total coronary occlusion.18,19 Table 53-2 lists some of the main studies that characterized myocardial perfusion in animal models under rest and stress conditions.
Hoffmann and coworkers18 used four-slice multidetector CT and performed a quantitative analysis of CT attenuation and compared that with microsphere-determined blood flow and triphenyltetrazolium chloride (TTC)–stained tissue samples. The quantitative analysis by multidetector CT showed significant differences in the mean CT attenuation of infarct and reference areas (32.1 ± 8.5 Hounsfield units [HU] vs. 75.6 ± 16.7 HU; P < .001), and this correlated with changes in microsphere-determined blood flow. The volume of perfusion defect was similar to volume of tissue that lacked TTC staining (17 ± 6.4% vs. 13.6 ± 6%), with slight overestimation of infarct size by multidetector CT.
Mahnken and colleagues19 used a similar porcine model to assess the ability of multidetector CT to evaluate rest myocardial perfusion versus first-pass perfusion MRI with TTC staining serving as the gold standard. In their protocol, they used dynamic multidetector CT imaging by acquiring 64 scans at the apical level with a prospectively acquired ECG triggered examination protocol. Hypoenhanced regions on multidetector CT corresponded directly to perfusion defects visualized on MRI and areas of MI seen on TTC staining. The hypoenhanced regions detected by multidetector CT were again slightly larger than areas of acute MI as detected by MRI and TTC staining.
Human Studies of Rest Perfusion
Table 53-3 summarizes several key human studies of stress and rest myocardial perfusion using multidetector CT. Nikolaou and colleagues20 attempted to correlate rest multidetector CT to stress perfusion MRI and MDE MRI in 30 patients with chronic infarcts or suspected CAD or both. In this retrospective study, all patients previously underwent multidetector CT (16 detectors) and MRI within 10 ± 16 days. Multidetector CT was able to detect 13 of 17 perfusion defects correctly (sensitivity 76%, specificity 92%, accuracy 83%); however, when considering only the 6 perfusion defects not associated with chronic MI, the sensitivity decreased to 50%—not surprising given that multidetector CT was performed under resting conditions, whereas stress perfusion MRI used vasodilator-induced hyperemic blood flow. Comparing multidetector CT versus MDE MRI for detection of infarct resulted in a sensitivity of 91%, specificity of 79%, and accuracy of 83%. The attenuation values in the 10 infarcted areas correctly detected by multidetector CT were significantly lower than in noninfarcted areas of myocardium (53.7 ± 33.5 HU vs. 122.3 ± 25.5 HU; P < .01). In the volumetric assessment of infarct size, a strong correlation between the volumes of 16-multidetector CT and MDE MRI was found (r = 0.98), but 16-multidetector CT tended to underestimate the infarct volume as assessed by cardiac MRI by 19% (P < .01).
More recently, Nieman and coworkers21 retrospectively tested the hypothesis that 64-multidetector CT can differentiate recent (<7 days) versus old (>12 months) MI. They found significantly lower CT attenuation values in patients with long-standing MI (−13 ± 37 HU) than patients with acute MI (26 ± 26 HU) and normal controls (73 ± 14 HU; P < .001). The attenuation difference between infarcted and remote myocardium was larger in patients with long-standing MI than in patients with recent MI (89 ± 41 HU and 55 ± 33 HU; P < .001), probably owing to fatty replacement. As anticipated, long-standing MI was associated with wall thinning and ventricular dilation, whereas recent MI was not (P > .05).
Stress Multidetector Computed Tomography to Identify Ischemia
George and colleagues22 performed rest and adenosine-mediated stress multidetector CT on a canine model of left anterior descending artery stenosis and were able to achieve lesions that were non–flow-limiting at rest but flow-limiting during pharmacologic stress. They were able to identify perfusion defects in noninfarcted myocardium. By using microspheres to measure myocardial blood flow, they were able to show that the myocardial signal density ratio (myocardial signal density/left ventricular blood pool signal density) corresponded well with microsphere-derived myocardial blood flow.
Preliminary human studies investigating the feasibility and accuracy of multidetector CT stress myocardial perfusion have been presented only more recently. In 19 patients with an abnormal SPECT study, George and colleagues23 used 256-detector multidetector CT to assess for subendocardial perfusion defects during rest and intravenous adenosine infusion. When compared with 50% or greater stenosis by CT angiography, the sensitivity and specificity of multidetector CT stress MPI were 85% and 77% compared with 69% and 74% sensitivity and specificity of SPECT. When compared with a gold standard combining 50% or greater stenosis by CT angiography with a SPECT perfusion defect, the 256-row multidetector CT was 78% sensitive and 90% specific. Although these findings represent preliminary work, they suggest that ischemia can be detected with a modest sensitivity and reasonably high specificity.
TECHNIQUE DESCRIPTION
Magnetic Resonance Imaging
IMAGE INTERPRETATION
Postprocessing
Magnetic Resonance Imaging
The absence of significant hyperenhancement on MDE (Fig. 53-4) is consistent with viable myocardium, and suggests that the patient would likely benefit from myocardial revascularization (i.e., percutaneous coronary intervention or coronary artery bypass graft surgery). Lastly, left ventricular systolic function and wall motion under stress are assessed. A wall motion abnormality in a coronary territory that matches perfusion defects is further evidence of ischemic myocardium. The diagnosis of CAD is made in the presence of a stress-induced perfusion defect or if an MI is detected on MDE images.
REPORTING
KEY POINTS
 Myocardial perfusion CT can assess areas of perfusion defects at rest that represent myocardial infarct.
Myocardial perfusion CT can assess areas of perfusion defects at rest that represent myocardial infarct. CT evaluation of coronary arteries, myocardial perfusion, and myocardial function is possible with 64-detector multidetector CT.
CT evaluation of coronary arteries, myocardial perfusion, and myocardial function is possible with 64-detector multidetector CT.Berman DS, Hachamovitch R, Shaw LJ, et al. Roles of nuclear cardiology, cardiac computed tomography, and cardiac magnetic resonance: assessment of patients with suspected coronary artery disease. J Nucl Med. 2006;47:74-82.
Cury RC, Nieman K, Shapiro MD, et al. Comprehensive cardiac CT study: evaluation of coronary arteries, left ventricular function, and myocardial perfusion—is it possible? J Nucl Cardiol. 2007;14:229-243.
Gershlick AH, de Belder M, Chambers J, et al. Role of non-invasive imaging in the management of coronary artery disease: an assessment of likely change over the next 10 years. A report from the British Cardiovascular Society Working Group. Heart. 2007;93:423-431.
Jerosch-Herold M, Muehling O, Wilke N. MRI of myocardial perfusion. Semin Ultrasound CT MR. 2006;27:2-10.
Kellman P, Arai AE. Imaging sequences for first pass perfusion—a review. J Cardiovasc Magn Reson. 2007;9:525-537.
Mahnken AH, Mühlenbruch G, Günther RW, et al. Cardiac CT: coronary arteries and beyond. Eur Radiol. 2007;17:994-1008.
Nieman K, Shapiro MD, Ferencik M, et al. Reperfused myocardial infarction: contrast-enhanced 64-section CT in comparison to MR imaging. Radiology. 2008;247:49-56.
Pennell DJ. Cardiovascular magnetic resonance and the role of adenosine pharmacologic stress. Am J Cardiol. 2004;94:26D-31D.
1 Rosamond W, Flegal K, Friday G, et al. Heart disease and stroke statistics—2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69-e171.
2 Lerner DJ, Kannel WB. Patterns of coronary heart disease morbidity and mortality in the sexes: a 26-year follow-up of the Framingham population. Am Heart J. 1986;111:383-390.
3 Deedwania PC, Carbajal EV. Silent myocardial ischemia: a clinical perspective. Arch Intern Med. 1991;151:2373-2382.
4 Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients with Unstable Angina/Non ST-Elevation Myocardial Infarction): developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons: endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. Circulation. 2007;116:e148-e304.
5 Gibbons RJ, Abrams J, Chatterjee K, et al. ACC/AHA 2002 guideline update for the management of patients with chronic stable angina—summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients with Chronic Stable Angina). Circulation. 2003;107:149-158.
6 Bax JJ, Bonow RO, Tschope D, et al. The potential of myocardial perfusion scintigraphy for risk stratification of asymptomatic patients with type 2 diabetes. J Am Coll Cardiol. 2006;48:754-760.
7 Beller GA. Noninvasive screening for coronary atherosclerosis and silent ischemia in asymptomatic type 2 diabetic patients: is it appropriate and cost-effective? J Am Coll Cardiol. 2007;49:1918-1923.
8 Klocke FJ, Simonetti OP, Judd RM, et al. Limits of detection of regional differences in vasodilated flow in viable myocardium by first-pass magnetic resonance perfusion imaging. Circulation. 2001;104:2412-2416.
9 Lee DC, Simonetti OP, Harris KR, et al. Magnetic resonance versus radionuclide pharmacological stress perfusion imaging for flow-limiting stenoses of varying severity. Circulation. 2004;110:58-65.
10 Nandalur KR, Dwamena BA, Choudhri AF, et al. Diagnostic performance of stress cardiac magnetic resonance imaging in the detection of coronary artery disease: a meta-analysis. J Am Coll Cardiol. 2007;50:1343-1353.
11 Cury RC, Cattani CA, Gabure LA, et al. Diagnostic performance of stress perfusion and delayed-enhancement MR imaging in patients with coronary artery disease. Radiology. 2006;240:39-45.
12 Klem I, Heitner JF, Shah DJ, et al. Improved detection of coronary artery disease by stress perfusion cardiovascular magnetic resonance with the use of delayed enhancement infarction imaging. J Am Coll Cardiol. 2006;47:1630-1638.
13 Ingkanisorn WP, Kwong RY, Bohme NS, et al. Prognosis of negative adenosine stress magnetic resonance in patients presenting to an emergency department with chest pain. J Am Coll Cardiol. 2006;47:1427-1432.
14 Schwitter J, Wacker CM, van Rossum AC, et al. MR-IMPACT: comparison of perfusion-cardiac magnetic resonance with single-photon emission computed tomography for the detection of coronary artery disease in a multicentre, multivendor, randomized trial. Eur Heart J. 2008;29:480-489.
14a Ishida N, Sakuma H, Motoyasu M, et al. Non-infarcted myocardium: correlation between dynamic first-pass contrast-enhanced myocardial MR imaging and quantitative coronary angiography. Radiology. 2003;229:209-216.
15 Miller Jr RC, Dewey M, et al. Coronary artery evaluation using 64-row multidetector computed tomography angiography (CORE-64): results of a multicenter, international trial to assess diagnostic accuracy compared with conventional coronary angiography. Presented at American Heart Association 30th Annual Scientific Sessions, Orlando, November 3-7, 2007.
16 Hacker M, Jakobs T, Matthiesen F, et al. Comparison of spiral multidetector CT angiography and myocardial perfusion imaging in the noninvasive detection of functionally relevant coronary artery lesions: first clinical experiences. J Nucl Med. 2005;46:1294-1300.
17 Higgins CB, Sovak M, Schmidt W, et al. Uptake of contrast materials by experimental acute myocardial infarctions: a preliminary report. Invest Radiol. 1978;13:337-339.
18 Hoffmann U, Millea R, Enzweiler C, et al. Acute myocardial infarction: contrast-enhanced multi-detector row CT in a porcine model. Radiology. 2004;231:697-701.
19 Mahnken AH, Bruners P, Katoh M, et al. Dynamic multi-section CT imaging in acute myocardial infarction: preliminary animal experience. Eur Radiol. 2006;16:746-752.
20 Nikolaou K, Sanz J, Poon M, et al. Assessment of myocardial perfusion and viability from routine contrast-enhanced 16-detector-row computed tomography of the heart: preliminary results. Eur Radiol. 2005;15:864-871.
21 Nieman K, Cury RC, Ferencik M, et al. Differentiation of recent and chronic myocardial infarction by cardiac computed tomography. Am J Cardiol. 2006;98:303-308.
22 George RT, Silva C, Cordeiro MA, et al. Multidetector computed tomography myocardial perfusion imaging during adenosine stress. J Am Coll Cardiol. 2006;48:153-160.
23 George RT, Arbad-Zadeh A, Miller JM, et al. Adenosine stress 64- and 256-row detector computed tomography angiography and perfusion imaging: a pilot study evaluating the transmural extent of perfusion abnormalities to predict atherosclerosis causing myocardial ischemia. Circ Cardiovasc Imaging. 2009;2:174-182.
24 George RT, Jerosch-Herold M, Silva C, et al. Quantification of myocardial perfusion using dynamic 64-detector computed tomography. Invest Radiol. 2007;42:815-822.
25 Mahnken AH, Koos R, Katoh M, et al. Assessment of myocardial viability in reperfused acute myocardial infarction using 16-slice computed tomography in comparison to MRI. J Am Coll Cardiol. 2005;45:2042-2047.
26 Gerber BL, Belge B, Legros GJ, et al. Characterization of acute and chronic myocardial infarcts by multidetector computed tomography: comparison with contrast enhanced magnetic resonance. Circulation. 2006;113:823-833.

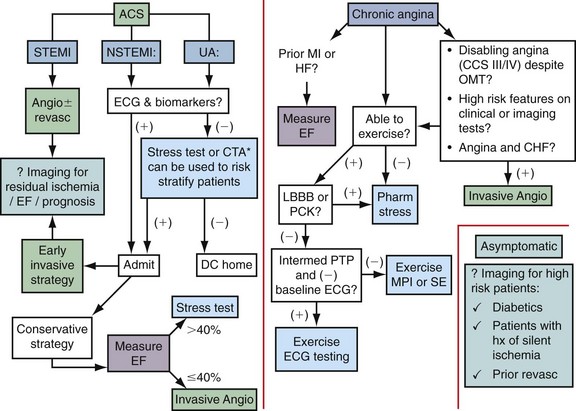
 FIGURE 53-1
FIGURE 53-1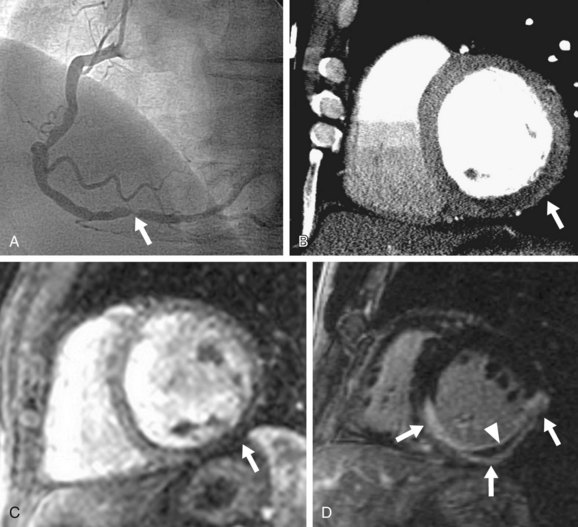
 FIGURE 53-2
FIGURE 53-2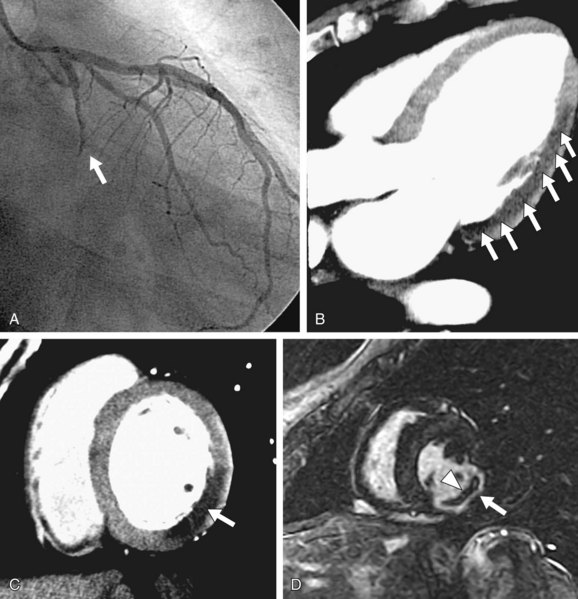
 FIGURE 53-3
FIGURE 53-3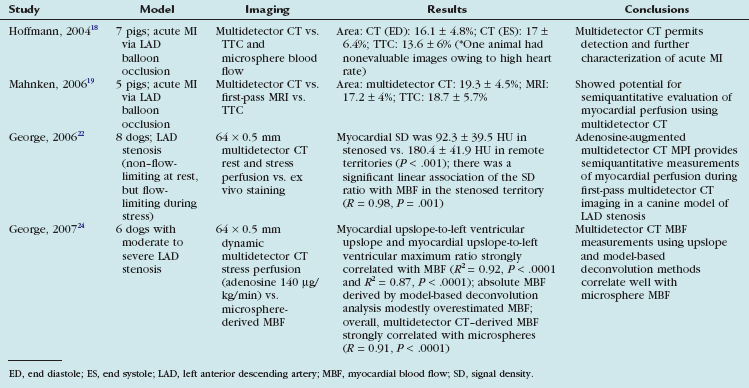
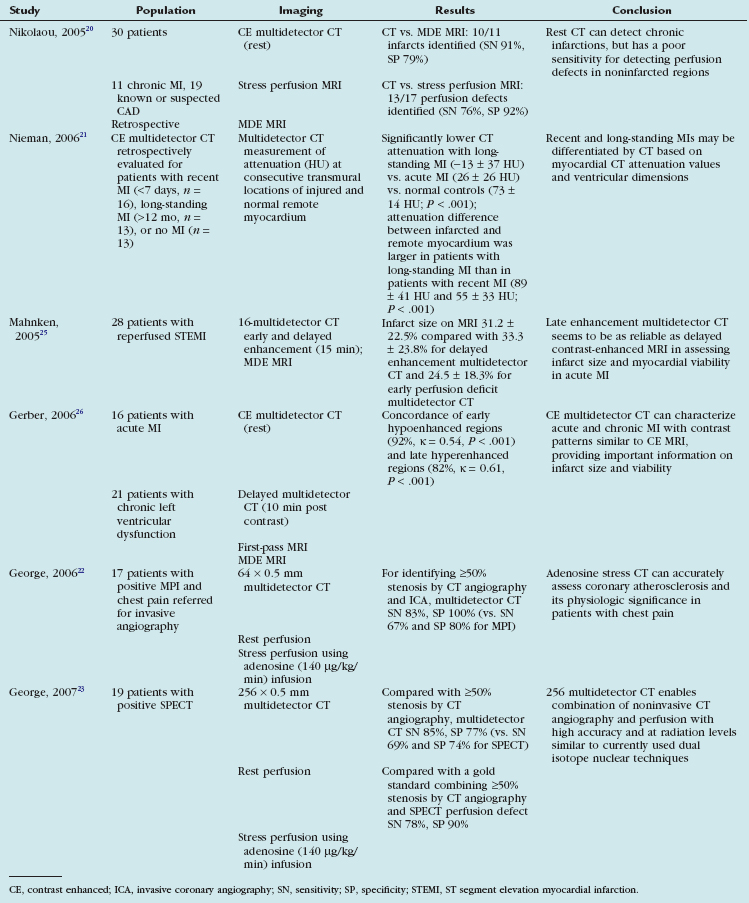
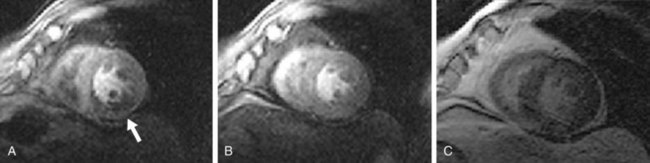
 FIGURE 53-4
FIGURE 53-4

