123 Macrolides
The macrolide class of antibiotics is based on the structure of erythromycin, the prototype natural macrolide isolated from Streptomyces erythreus.1 Commonly the term macrolide is expanded to include the azalide, azithromycin. The newly developed ketolides, owing to their similar structural bases, are close members of the macrolide family. There are many macrolides available throughout the world, the most commonly used being erythromycin, clarithromycin, and azithromycin. Roxithromycin is available in Europe and Asia. Telithromycin is the only currently available ketolide.
 Mechanism of Action
Mechanism of Action
The macrolides inhibit bacterial protein synthesis by binding to the 50S ribosomal subunit.2 The advanced macrolides have improved binding to ribosomes compared with erythromycin. Telithromycin, the ketolide, has a similar target site, but its structure allows for enhanced binding, even in the presence of ribosomal mutations.
 Mechanisms of Resistance
Mechanisms of Resistance
There are three major mechanisms of bacterial resistance to macrolides: drug efflux, ribosomal mutations, and enzymatic inactivation. Active efflux, mediated by mef genes, and ribosomal methylation of the target site, mediated by erm genes, are the most clinically important resistance mechanisms.3 Organisms containing the mef gene commonly express low-level resistance that can often be overcome with larger doses of the antibiotic. In contrast, erm-containing organisms, expressing phenotypic macrolide-lincosamide-streptogramin B resistance, often express high-level resistance, rendering macrolides clinically ineffective.
 Antimicrobial Spectrum of Activity
Antimicrobial Spectrum of Activity
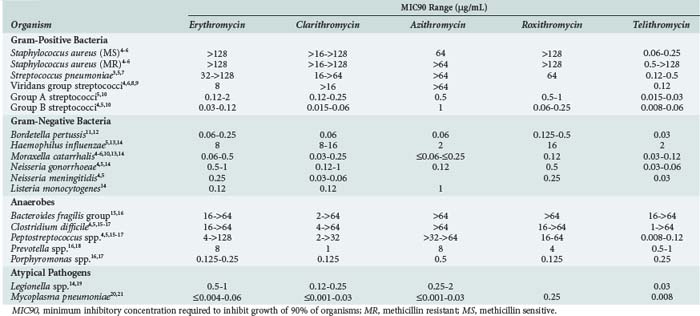
The macrolides have activity against many classes of bacteria but have only sporadic activity within each of these groups. Their primary microbiologic activity is directed against respiratory and intracellular pathogens (Table 123-1).3–21
Gram-Positive Aerobes
Among the gram-positive aerobes, erythromycin activity is limited to the streptococci with reasonable activity against Streptococcus pneumoniae. The advanced macrolides (azithromycin, clarithromycin, roxithromycin) have similar activity against S. pneumoniae. The utility of the macrolides against pneumococci is hampered by increasing resistance, commonly coupled with penicillin resistance. A 2001-2004 study from 40 countries reported 37% worldwide erythromycin resistance.3 In the United States, macrolide-resistant pneumococci are found in up to 35% of isolates.22 The predominant worldwide resistance mechanism is ermB-mediated high-level resistance (58%), but there is considerable international variability. Resistant North American isolates most commonly contain low-level mefA resistance, whereas most European and Far East countries report higher levels of ermB-containing pathogens. The prevalence of pneumococci expressing both mechanisms simultaneously is increasing.3
Resistance mechanisms for macrolides are important, because low-level resistance may possibly be overcome with conventional dosing of the macrolides.23 Pneumococcal resistance to one macrolide commonly confers resistance to all members of the macrolide class. The ketolides, however, maintain their activity against macrolide-resistant S. pneumoniae possessing both erm- and mef-mediated resistance.3
Gram-Negative Aerobes
The macrolides, with the exception of erythromycin, and the ketolide, telithromycin, have activity against Haemophilus influenzae. The activity of clarithromycin against H. influenzae is enhanced in the presence of its active metabolite.24 The macrolides and ketolide also display activity against Moraxella catarrhalis, Bordetella pertussis, Neisseria gonorrhoeae, and Neisseria meningitidis. Clarithromycin has been the most commonly used macrolide against Helicobacter pylori, although resistance rates are currently 13% in the United States, with higher rates reported worldwide.25 The macrolides are largely ineffective against the Enterobacteriaceae and other nosocomial pathogens. Though not directly active, limited auxiliary azithromycin activity has been noted against Pseudomonas spp.26
Miscellaneous
The macrolides and the ketolide attain high intracellular concentrations and are active against Legionella spp., Chlamydia spp., and Mycoplasma pneumoniae. In vitro activity is also present against Rickettsia, Bartonella, and Brucella spp.,27–29 as well as Borrelia burgdorferi, the agent of Lyme disease.30 In addition, azithromycin, clarithromycin, and telithromycin have activity against some strains of atypical nontuberculosis mycobacteria, including Mycobacterium avium complex.31–33
 Pharmacokinetics
Pharmacokinetics
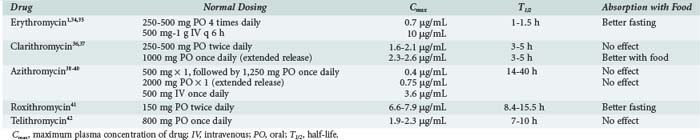
Erythromycin base is acid labile but still adequately absorbed from the gastrointestinal (GI) tract. Food can decrease absorption (Table 123-2).1,34–42 More stable oral formulations have complexed erythromycin with salts or esters to form erythromycin estolate, stearate, and ethylsuccinate. Erythromycin lactobionate has also been formulated to allow for intravenous (IV) delivery. Peak concentrations are 0.73 µg/mL after 250 mg base orally and 10 µg/mL after 500 mg IV.1,34 The half-life is 1 to 1.5 hours.35 Like all macrolides, erythromycin is widely distributed throughout the body, with higher tissue and intracellular concentrations compared with plasma. Erythromycin is not found in the cerebrospinal fluid in normal volunteers, but low levels have been reported in patients with meningitis.35 Erythromycin is metabolized by cytochrome P450 (CYP) enzymes in the liver and excreted as inactive metabolites, primarily in the feces.
Clarithromycin is well absorbed from the GI tract (bioavailability 52%-55%), with or without food. An IV lactobionate form is available in some countries. A peak concentration of 1.65 to 2.12 mg/mL is obtained after a 500-mg oral dose with a half-life of 3 to 5 hours.37 Similar pharmacokinetics are observed after dosing with the oral suspension, even in critically ill patients.43 An extended-release formulation is available that delays the time to peak concentrations, provides similar total drug exposure, and allows for once-daily dosing.36 Clarithromycin is well distributed throughout the body, with respiratory tract tissue and fluid concentrations 3 to 30 times that of the plasma and alveolar macrophage concentrations 102 to 103 higher than plasma.37 Cerebrospinal fluid concentrations are unknown. Hepatic metabolism is the major metabolic pathway and leads to the formation of 14-hydroxy-clarithromycin, an active metabolite with greater activity than the parent compound.24,37 Clarithromycin is extensively metabolized, with 18.4% and 4.4% of unchanged drug excreted in the urine and feces, respectively, after a 250-mg dose.37 Dosing changes are required in patients with moderate to severe renal dysfunction.
Azithromycin is 37% bioavailable when administered orally but is also available in an IV formulation.39,44 Food has little effect on bioavailability. Peak concentrations in the plasma after a 500-mg dose range from 0.4 µg/mL for the oral formulation to 3.6 µg/mL for the IV formulation.38,39 An extended-release formulation of a single 2-g azithromycin dose shows a similar overall pharmacokinetic profile to standard oral dosing.40 Azithromycin is unique in its extended half-life of 14 to 40 hours, thus providing low sustained plasma concentrations that persist after cessation of dosing.39 Whereas plasma concentrations are very low, azithromycin attains very high concentrations in tissues (100 times plasma) and phagocytes (3000-7000 times plasma).38 Little to no azithromycin can be recovered from the cerebrospinal fluid, but brain tissue concentrations well exceed those in the serum.45 Azithromycin is minimally metabolized and largely excreted via the biliary tract into the feces.
Roxithromycin is well absorbed orally, with peak plasma concentrations of 6.6 to 7.9 µg/mL after a 150-mg oral dose, and a half-life of 8.4 to 15.5 hours.41 Fasting prior to dosing improves absorption. Tissue concentrations exceed those of the plasma. Roxithromycin is metabolized by multiple mechanisms, with the majority of the dose excreted in feces.41,46
Telithromycin is 57% bioavailable, with peak plasma concentrations of 1.9 and 2.3 µg/mL after single or multiple 800-mg doses, respectively.42 The half-life of telithromycin is 7 to 10 hours. Like the macrolides, telithromycin achieves high concentrations in respiratory tissues, alveolar macrophages, and peripheral polymorphonuclear cells. Telithromycin is metabolized by CYP3A4 and non-CYP–related mechanisms. Fecal elimination of metabolites accounts for the majority of the excretion of telithromycin.
 Pharmacodynamics
Pharmacodynamics
The macrolides and ketolides appear to have time-dependent antibacterial activity that is slowly bactericidal or bacteriostatic.47,48 The relationship between drug concentration and bacterial effect that best explain drug activity is the free (unbound) drug area under the inhibitory curve (fAUC: MIC).47,48 Differences in the pharmacokinetics of the individual agents and limited analyses do not allow absolute determination of the best dosing strategy. It should be noted that pharmacodynamic principles for antibacterials have generally been related to plasma concentrations.49 As noted earlier, the plasma concentrations of the macrolides are usually lower than those of the tissues, where the majority of bacteria reside. Models looking at both epithelial lining fluid (a proxy for lung concentrations) and serum concentrations suggest that increasing bacterial resistance favors telithromycin in its ability to maintain favorable pharmacodynamics.50
 Immune Modulation
Immune Modulation
Increasing evidence suggests that antibacterial macrolides have antiinflammatory effects.51–53 In vitro studies have suggested macrolide effects on a number of cellular mechanisms within human cells.54 In addition to in vitro and animal models, clinical data suggest that macrolides may have activity in the treatment of inflammatory diseases including cystic fibrosis, diffuse panbronchiolitis, chronic sinusitis, and inflammatory skin diseases.51,53 Azithromycin has been cautiously recommended for use in cystic fibrosis for its antiinflammatory effects in patients colonized with Pseudomonas spp.55
 Adverse Effects
Adverse Effects
Gastrointestinal effects (nausea and diarrhea) are the most common adverse events observed with macrolide therapy.41,56 Erythromycin has the highest level of GI effects.1 Nausea with erythromycin may occur after IV dosing, as erythromycin is secreted into the GI tract via the bile.57 The advanced macrolides have a similar incidence of GI adverse events. A review of azithromycin safety data from over 4000 patients treated with the immediate-release formulation reported GI event rates of 4% for diarrhea and 3% for nausea.58 Gastrointestinal side effects in patients receiving the 2-g extended release azithromycin formulation were higher, with 12% nausea and 4% diarrhea.59 In 3800 patients receiving immediate-release clarithromycin, similar side-effect rates were observed for nausea (3.8%) and diarrhea (3.0%). Tolerability of the extended-release clarithromycin formulation was similar to those with the immediate-release product.36 Roxithromycin was reported to have a 4% incidence of side effects in 32,405 patients, with 75% being mild to moderate GI events.41 From data in clinical trials, GI side effects were reported frequently with telithromycin (10.8% diarrhea, 7.9% nausea), although considerable variability was observed across studies.56
More serious events associated with macrolide use include prolongation of the QT interval, with torsades de pointes. In vitro estimations of HERG blockade suggest that clarithromycin ≈ roxithromycin > erythromycin.60 In contrast, erythromycin was found to have a higher proarrhythmic potential than clarithromycin and azithromycin in animal models.61 Torsades de pointes has been reported in patients receiving macrolides. Sudden cardiac death was observed with greater frequency in patients taking erythromycin than amoxicillin in a large database study.62 Although the relative ability of the macrolides to cause arrhythmias is difficult to ascertain, arrhythmias associated with clarithromycin use were reported more frequently than with erythromycin.63
Severe hepatotoxicity associated with telithromycin was identified through post-marketing surveillance in the United States.64 Forty-two cases were available for full review, with 5 patients dying or requiring liver transplants. Clinical features included short time to hepatoxicity (as short as 1-2 days), fever, abdominal pain, and ascites. As a result of this toxicity, telithromycin use has been limited to more severely infected individuals with a careful review of risks and benefits.
 Prokinetic Activity
Prokinetic Activity
The intestinal prokinetic activity of the macrolides has been used to improve GI mobility. Erythromycin has been shown to improve gastric emptying in a dose-dependent manner.65 However, concerns have been raised over the potential to increase bacterial resistance with non-antibacterial macrolide use.66 In a comparative multidose trial in patients with enteral nutrition intolerability, erythromycin provided better results than metoclopramide, with both agents showing efficacy.67 Optimal use of erythromycin as a prokinetic agent is not yet confirmed.66–68
 Drug-Drug Interactions
Drug-Drug Interactions
Drug-drug interactions must be evaluated when considering macrolide therapy. The macrolides have variable degrees of inhibition of CYP3A4 and are also substrates of this enzyme. The use of macrolides with other drugs metabolized by CYP3A4 may result in increases in the second drug concentrations. Erythromycin is the most potent inhibitor of CYP3A4, followed by moderate inhibition with clarithromycin and roxithromycin and little to no inhibition by azithromycin.69 Erythromycin has been implicated in multiple drug interactions, including with benzodiazepines, carbamazepine, cyclosporine, digoxin, HMG-CoA inhibitors, tacrolimus, and theophylline. Case reports of interactions with warfarin have been documented for many of the macrolides.70 Clarithromycin, although in vitro a less potent inhibitor of CYP3A4, has been associated with a similar scope of clinical interactions.70 As expected by its limited CYP activity, few clinically important interactions have been reported with roxithromycin and azithromycin.39,41,70
The pharmacodynamic interaction between macrolides and other drugs known to increase the QT interval must not be overlooked (see Adverse Effects).
Key Points
Jenkins S, Farrell D. Increase in pneumococcus macrolide resistance, United States. Emerg Infect Dis. 2009;15:1260-1264.
Noreddin A, El-Khatib W, Aolie J, Salem AH, Zhanel GG. Pharmacodynamic target attainment potential of azithromycin, clarithromycin, and telithromycin in serum and epithelial lining fluid of community-acquired pneumonia patients with penicillin-susceptible, intermediate, and resistant Streptococcus pneumoniae. Int J Infect Dis. 2009;13:483-487.
Shaffer D, Singer S, Korvick J, Honig P. Concomitant risk factors in reports of torsades de pointes associated with macrolide use: review of the United States Food and Drug Administration Adverse Event Reporting System. Clin Infect Dis. 2002;35:197-200.
Ribeiro C, Hurd H, Wu Y, Martino ME, Jones L, Brighton B, et al. Azithromycin treatment alters gene expression in inflammatory, lipid metabolism, and cell cycle pathways in well-differentiated human airway epithelia. PLoS One. 2009;4:e5806.
Brinker A, Wassel R, Lyndly J, Serrano J, Avigan M, Lee WM, et al. Telithromycin-associated hepatotoxicity: clinical spectrum and causality assessment of 42 cases. Hepatology. 2009;49:250-257.
1 Chambers HF. Protein synthesis inhibitors and miscellaneous antibacterial agents. In: Hardman JG, Limbird LE, Goodman Gillman A, editors. The Pharmacological Basis of Therapeutics. 10th ed. New York: McGraw-Hill; 2001:1239-1271.
2 Douthwaite S, Hansen LH, Mauvais P. Macrolide-ketolide inhibition of MLS-resistant ribosomes is improved by alternative drug interaction with domain II of 23S rRNA. Mol Microbiol. 2000;36:183-193.
3 Felmingham D, Canton R, Jenkins SG. Regional trends in beta-lactam, macrolide, fluoroquinolone and telithromycin resistance among Streptococcus pneumoniae isolates 2001-2004. J Infection. 2007;55:111-118.
4 Andrews JM, Weller TM, Ashby JP, et al. The in vitro activity of ABT773, a new ketolide antimicrobial agent. J Antimicrob Chemother. 2000;46:1017-1022.
5 Boswell FJ, Andrews JM, Ashby JP, et al. The in-vitro activity of HMR 3647, a new ketolide antimicrobial agent. J Antimicrob Chemother. 1998;42:703-709.
6 McCloskey L, Moore T, Niconovich N, et al. In vitro activity of gemifloxacin against a broad range of recent clinical isolates from the USA. J Antimicrob Chemother. 2000;45(Suppl 1):13-21.
7 McGhee P, Clark C, Kosowska-Shick KM, et al. In vitro activity of CEM-101 against Streptococcus pneumoniae and Streptococcus pyogenes with defined macrolide resistance mechanisms. Antimicrob Agents Chemother. 2010;54:230-238.
8 Gershon AS, de Azavedo JC, McGeer A, et al. Activities of new fluoroquinolones, ketolides, and other antimicrobials against blood culture isolates of viridans group streptococci from across Canada, 2000. Antimicrob Agents Chemother. 2002;46:1553-1556.
9 Ieven M, Goossens W, De Wit S, et al. In vitro activity of gemifloxacin compared with other antimicrobial agents against recent clinical isolates of streptococci. J Antimicrob Chemother. 2000;45(Suppl 1):51-53.
10 Wootton M, Bowker KE, Janowska A, et al. In-vitro activity of HMR 3647 against Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis and beta-haemolytic streptococci. J Antimicrob Chemother. 1999;44:445-453.
11 Brett M, Short P, Beatson S. The comparative in-vitro activity of roxithromycin and other antibiotics against Bordetella pertussis. J Antimicrob Chemother. 1998;41(Suppl B):23-27.
12 Hoppe JE, Bryskier A. In vitro susceptibilities of Bordetella pertussis and Bordetella parapertussis to two ketolides (HMR 3004 and HMR 3647), four macrolides (azithromycin, clarithromycin, erythromycin A, and roxithromycin), and two ansamycins (rifampin and rifapentine). Antimicrob Agents Chemother. 1998;42:965-966.
13 Hoban D, Felmingham D. The PROTEKT surveillance study: Antimicrobial susceptibility of Haemophilus influenzae and Moraxella catarrhalis from community-acquired respiratory tract infections. J Antimicrob Chemother. 2002;50(Suppl Sl):49-59.
14 Nilius AM, Bui MH, Aimer L, et al. Comparative in vitro activity of ABT-773, a novel antibacterial ketolide. Antimicrob Agents Chemother. 2001;45:2163-2168.
15 Ednie LM, Jacobs MR, Appelbaum PC. Comparative antianaerobic activities of the ketolides HMR 3647 (RU 66647) and HMR 3004 (RU 64004). Antimicrob Agents Chemother. 1997;41:2019-2022.
16 Wexler HM, Molitoris E, Molitoris D, et al. In vitro activity of telithromycin (HMR 3647) against 502 strains of anaerobic bacteria. J Antimicrob Chemother. 2001;47:467-469.
17 Goldstein EJ, Citron DM, Merriam CV, et al. Comparative in vitro activities of ABT-773 against aerobic and anaerobic pathogens isolated from skin and soft-tissue animal and human bite wound infections. Antimicrob Agents Chemother. 2000;44:2525-2529.
18 Citron DM, Appleman MD. Comparative in vitro activities of ABT-773 against 362 clinical isolates of anaerobic bacteria. Antimicrob Agents Chemother. 2001;45:345-348.
19 Dunbar LM, Farrell DJ. Activity of telithromycin and comparators against isolates of Legionella pneumophila collected from patients with community-acquired respiratory tract infections: PROTEKT Years 1-5. Clin Microbiol Infect. 2007;13:743-746.
20 Kenny GE, Cartwright FD. Susceptibilities of Mycoplasma hominis, M. pneumoniae, and Ureaplasma urealyticum to GAR-936, dalfopristin, dirithromycin, evernimicin, gatifloxacin, linezolid, moxifloxacin, quinupristin-dalfopristin, and telithromycin compared to their susceptibilities to reference macrolides, tetracyclines, and quinolones. Antimicrob Agents Chemother. 2001;45:2604-2608.
21 Waites KB, Crabb DM, Duffy LB. In vitro activities of ABT-773 and other antimicrobials against human mycoplasmas. Antimicrob Agents Chemother. 2003;47:39-42.
22 Jenkins SG, Farrell DJ. Increase in pneumococcus macrolide resistance, United States. Emerging infectious diseases. 2009;15:1260-1264.
23 Noreddin AM, Roberts D, Nichol K, et al. Pharmacodynamic modeling of clarithromycin against macrolide-resistant [PCR-positive mef(A) or erm(B)] Streptococcus pneumoniae simulating clinically achievable serum and epithelial lining fluid free-drug concentrations. Antimicrob Agents Chemother. 2002;46:4029-4034.
24 Bergeron MG, Bernier M, L’Ecuyer J. In vitro activity of clarithromycin and its 14-hydroxy-metabolite against 203 strains of Haemophilus influenzae. Infection. 1992;20:164-167.
25 Duck WM, Sobel J, Pruckler JM, et al. Antimicrobial resistance incidence and risk factors among Helicobacter pylori-infected persons, United States. Emerging infectious diseases. 2004;10:1088-1094.
26 Mulet X, Macia MD, Mena A, et al. Azithromycin in Pseudomonas aeruginosa biofilms: bactericidal activity and selection of nfxB mutants. Antimicrob Agents Chemother. 2009;53:1552-1560.
27 Garcia-Rodriguez JA, Munoz Bellido JL, Fresnadillo MJ, et al. In vitro activities of new macrolides and rifapentine against Brucella spp. Antimicrob Agents Chemother. 1993;37:911-913.
28 Rolain JM, Maurin M, Bryskier A, et al. In vitro activities of telithromycin (HMR 3647) against Rickettsia rickettsii, Rickettsia conorii, Rickettsia africae, Rickettsia typhi, Rickettsia prowazekii, Coxiella burnetii, Bartonella henselae, Bartonella quintana, Bartonella bacilliformis, and Ehrlichia chaffeensis. Antimicrob Agents Chemother. 2000;44:1391-1393.
29 Rolain JM, Maurin M, Vestris G, et al. In vitro susceptibilities of 27 rickettsiae to 13 antimicrobials. Antimicrob Agents Chemother. 1998;42:1537-1541.
30 Dever LL, Jorgensen JH, Barbour AG. Comparative in vitro activities of clarithromycin, azithromycin, and erythromycin against Borrelia burgdorferi. Antimicrob Agents Chemother. 1993;37:1704-1706.
31 Fernandez-Roblas R, Esteban J, Cabria F, et al. In vitro susceptibilities of rapidly growing mycobacteria to telithromycin (HMR 3647) and seven other antimicrobials. Antimicrob Agents Chemother. 2000;44:181-182.
32 Rastogi N, Goh KS, Berchel M, et al. In vitro activities of the ketolides telithromycin (HMR 3647) and HMR 3004 compared to those of clarithromycin against slowly growing mycobacteria at pHs 6.8 and 7.4. Antimicrob Agents Chemother. 2000;44:2848-2852.
33 Steele-Moore L, Stark K, Holloway WJ. In vitro activities of clarithromycin and azithromycin against clinical isolates of Mycobacterium avium-M. intracellulare. Antimicrob Agents Chemother. 1999;43:1530.
34 Fraser DG. Selection of an oral erythromycin product. Am J Hosp Pharm. 1980;37:1199-1205.
35 Wilson JT, van Boxtel CJ. Pharmacokinetics of erythromycin in man. Antibiot Chemother. 1978;25:181-203.
36 Guay DR, Gustavson LE, Devcich KJ, et al. Pharmacokinetics and tolerability of extended-release clarithromycin. Clin Ther. 2001;23:566-577.
37 Rodvold KA. Clinical pharmacokinetics of clarithromycin. Clin Pharmacokinet. 1999;37:385-398.
38 Garey KW, Amsden GW. Intravenous azithromycin. Ann Pharmacother. 1999;33:218-228.
39 Lalak NJ, Morris DL. Azithromycin clinical pharmacokinetics. Clin Pharmacokinet. 1993;25:370-374.
40 Liu P, Allaudeen H, Chandra R, et al. Comparative pharmacokinetics of azithromycin in serum and white blood cells of healthy subjects receiving a single-dose extended-release regimen versus a 3-day immediate-release regimen. Antimicrob Agents Chemother. 2007;51:103-109.
41 Markham A, Faulds D. Roxithromycin: an update of its antimicrobial activity, pharmacokinetic properties and therapeutic use. Drugs. 1994;48:297-326.
42 Zeitlinger M, Wagner CC, Heinisch B. Ketolides–the modern relatives of macrolides: the pharmacokinetic perspective. Clin Pharmacokinet. 2009;48:23-38.
43 Fish DN, Abraham E. Pharmacokinetics of a clarithromycin suspension administered via nasogastric tube to seriously ill patients. Antimicrob Agents Chemother. 1999;43:1277-1280.
44 Luke DR, Foulds G, Cohen SF, et al. Safety, toleration, and pharmacokinetics of intravenous azithromycin. Antimicrob Agents Chemother. 1996;40:2577-2581.
45 Jaruratanasirikul S, Hortiwakul R, Tantisarasart T, et al. Distribution of azithromycin into brain tissue, cerebrospinal fluid, and aqueous humor of the eye. Antimicrob Agents Chemother. 1996;40:825-826.
46 Zhong D, Li X, Wang A, et al. Identification of the metabolites of roxithromycin in humans. Drug Metab Dispos. 2000;28:552-559.
47 Owens RCJr, Shorr AF. Rational dosing of antimicrobial agents: pharmacokinetic and pharmacodynamic strategies. Am J Health Syst Pharm. 2009;66(12 Suppl 4):S23-S30.
48 Tessier PR, Kim MK, Zhou W, et al. Pharmacodynamic assessment of clarithromycin in a murine model of pneumococcal pneumonia. Antimicrob Agents Chemother. 2002;46:1425-1434.
49 Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26:1-10. quiz 11-12
50 Noreddin AM, El-Khatib WF, Aolie J, et al. Pharmacodynamic target attainment potential of azithromycin, clarithromycin, and telithromycin in serum and epithelial lining fluid of community-acquired pneumonia patients with penicillin-susceptible, intermediate, and resistant Streptococcus pneumoniae. Int J Infect Dis. 2009;13:483-487.
51 Amsden GW. Anti-inflammatory effects of macrolides–an underappreciated benefit in the treatment of community-acquired respiratory tract infections and chronic inflammatory pulmonary conditions? J Antimicrob Chemother. 2005;55:10-21.
52 Leiva M, Ruiz-Bravo A, Jimenez-Valera M. Effects of telithromycin in in vitro and in vivo models of lipopolysaccharide-induced airway inflammation. Chest. 2008;134:20-29.
53 Shinkai M, Henke MO, Rubin BK. Macrolide antibiotics as immunomodulatory medications: proposed mechanisms of action. Pharmacol Ther. 2008;117:393-405.
54 Ribeiro CM, Hurd H, Wu Y, et al. Azithromycin treatment alters gene expression in inflammatory, lipid metabolism, and cell cycle pathways in well-differentiated human airway epithelia. PloS One. 2009;4:e5806.
55 Flume PA, O’Sullivan BP, Robinson KA, et al. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2007;176:957-969.
56 Zuckerman JM, Qamar F, Bono BR. Macrolides, ketolides, and glycylcyclines: azithromycin, clarithromycin, telithromycin, tigecycline. Infect Dis Clin North Am. 2009;23:997-1026. ix-x
57 Bowler WA, Hostettler C, Samuelson D, et al. Gastrointestinal side effects of intravenous erythromycin: Incidence and reduction with prolonged infusion time and glycopyrrolate pretreatment. Am J Med. 1992;92:249-253.
58 Zuckerman JM. The newer macrolides: Azithromycin and clarithromycin. Infect Dis Clin North Am. 2000;14:449-462.
59 Drehobl MA, De Salvo MC, Lewis DE, et al. Single-dose azithromycin microspheres vs clarithromycin extended release for the treatment of mild-to-moderate community-acquired pneumonia in adults. Chest. 2005;128:2230-2237.
60 Volberg WA, Koci BJ, Su W, et al. Blockade of human cardiac potassium channel human ether-a-go-go-related gene (HERG) by macrolide antibiotics. J Pharmacol Exp Ther. 2002;302:320-327.
61 Milberg P, Eckardt L, Bruns HJ, et al. Divergent proarrhythmic potential of macrolide antibiotics despite similar QT prolongation: Fast phase 3 repolarization prevents early afterdepolarizations and torsades de pointes. J Pharmacol Exp Ther. 2002;303:218-225.
62 Ray WA, Murray KT, Meredith S, et al. Oral erythromycin and the risk of sudden death from cardiac causes. N Engl J Med. 2004;351:1089-1096.
63 Shaffer D, Singer S, Korvick J, et al. Concomitant risk factors in reports of torsades de pointes associated with macrolide use: review of the United States Food and Drug Administration Adverse Event Reporting System. Clin Infect Dis. 2002;35:197-200.
64 Brinker AD, Wassel RT, Lyndly J, et al. Telithromycin-associated hepatotoxicity: clinical spectrum and causality assessment of 42 cases. Hepatology. 2009;49:250-257.
65 Boivin MA, Carey MC, Levy H. Erythromycin accelerates gastric emptying in a dose-response manner in healthy subjects. Pharmacotherapy. 2003;23:5-8.
66 Hawkyard CV, Koerner RJ. The use of erythromycin as a gastrointestinal prokinetic agent in adult critical care: benefits versus risks. J Antimicrob Chemother. 2007;59:347-358.
67 MacLaren R, Kiser TH, Fish DN, et al. Erythromycin vs metoclopramide for facilitating gastric emptying and tolerance to intragastric nutrition in critically ill patients. JPEN J Parenter Enteral Nutr. 2008;32:412-419.
68 Nguyen NQ, Chapman M, Fraser RJ, et al. Prokinetic therapy for feed intolerance in critical illness: one drug or two? Crit Care Med. 2007;35:2561-2567.
69 von Rosensteil NA, Adam D. Macrolide antibacterials: Drug interactions of clinical significance. Drug Saf. 1995;13:105-122.
70 Pai MP, Graci DM, Amsden GW. Macrolide drug interactions: an update. Ann Pharmacother. 2000;34:495-513.