Chapter 75 Lung Transplantation
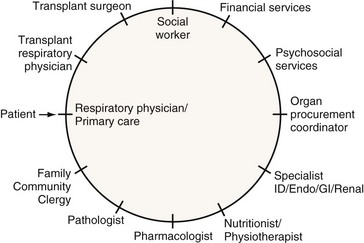
Pulmonary transplantation has become an effective and reliable means to improve survival and quality of life in carefully selected patients with end-stage pulmonary disease. The current success in transplantation is attributed to appropriate referral, early selection, careful evaluation, and improving management of lung allograft donors and recipients. The multidisciplinary approach (Figure 75-1), together with meticulous clinical care of each transplant candidate (including understanding of the underlying disease state and optimization of psychosocial status), is of the utmost importance. The concurrent advances in surgical techniques, combined immunosuppressive regimens, surveillance for rejection and institution of prophylaxis, and early treatment of infection have resulted in the excellent survival rates and quality of life witnessed today.
Current Trends in Lung Transplantation
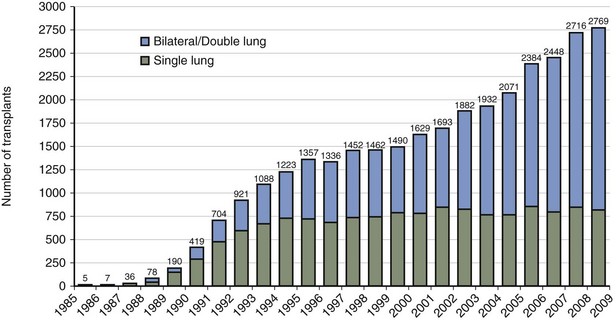
In the United States, the United Network for Organ Sharing (UNOS) has been operating the Organ Procurement and Transplantation Network (OPTN) since 1984. The International Society for Heart and Lung Transplantation (ISHLT), in collaboration with UNOS, created a worldwide registry of all heart and heart-lung transplant procedures in 1982. The Twenty-seventh Report documents that more than 32,000 lung transplant procedures have been performed since that time, with 2769 lung transplant procedures done in 2008 by 158 transplant centers worldwide. Most current transplant procedures involve bilateral sequential or double-lung transplants (Figure 75-2). Seventy-three heart-lung transplant procedures were done during that same year.
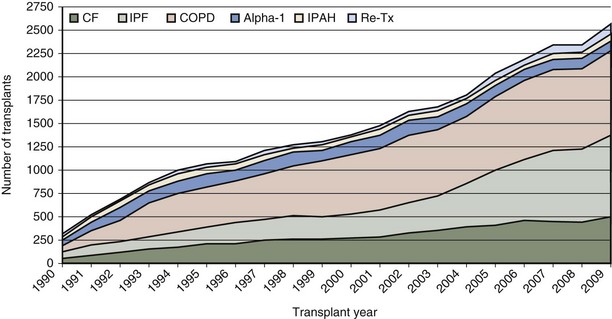
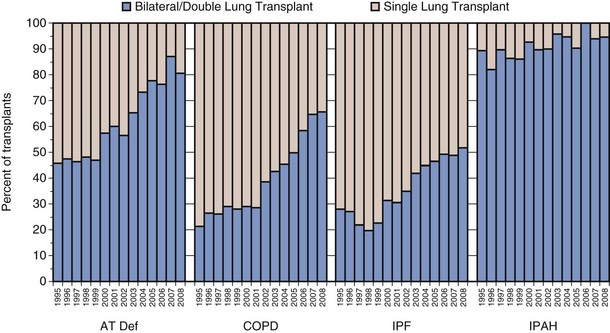
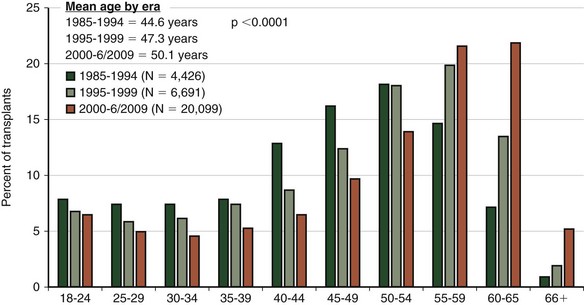
Although the number of lung transplant procedures has increased substantially in the past 2 decades, the leading indications for lung transplantation remain relatively unchanged: chronic obstructive pulmonary disease (COPD), idiopathic pulmonary fibrosis (IPF), cystic fibrosis (CF), α1-antitrypsin (A1A) deficiency with emphysema, and pulmonary arterial hypertension (PAH) (Figure 75-3). The type of transplant a patient receives is dictated in part by the recipient’s underlying disease: Recipients with COPD and IPF tend to receive single-lung as often as double-lung transplants, whereas those with CF and PAH almost always receive bilateral lung transplants (Figure 75-4). Over the past several years, a trend toward performing double-lung transplants and transplanting older people in the 55 to 65 age range has emerged (Figure 75-5).
Survival
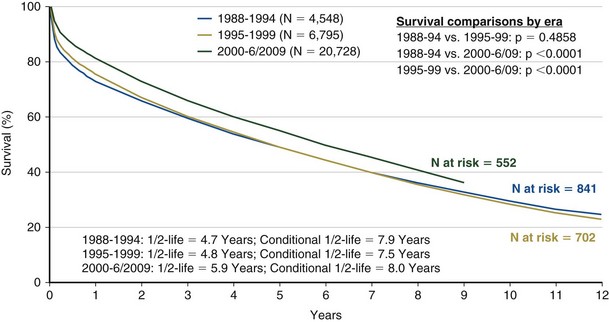
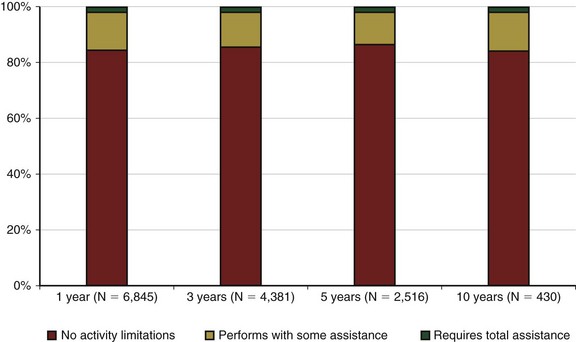
Achieving successful outcomes and maximal survival in the lung transplant population starts with the proper selection of transplant candidates. This entails an understanding of the natural history of the recipient’s lung disease and the projected survival with optimal medical and surgical therapy. Identifying potential candidates must be based on their current quality of life and the potential for improvement with and without transplantation. The median survival for lung transplant recipients has improved dramatically over the past several years. Transplant procedures performed from 2000 to 2004 were associated with a median survival of 5 years, significantly higher than for previous years (Figure 75-6). The survival curve is not linear, however, because approximately 20% of all recipients die in the first year after transplantation, with most occurring in the first 90 days. After this rapid decline, survival then stabilizes and follows a more linear trajectory, with an estimated 6% mortality rate per year. First-year mortality is attributable to postoperative graft complications, infection, cardiac failure, rejection, and early toxicity from immunosuppressive medications (Figure 75-7).
Several independent factors also seem to affect survival, including older age, higher body mass index (BMI), severe pulmonary hypertension, the type of transplant procedure performed, and the recipient’s native pulmonary disease. In recipients older than 65, the expected 5-year median survival is 3.4 years, considerably lower than the overall average 5-year median survival. This is one reason why age older than 65 is considered to be a relative contraindication (Table 75-1).
Table 75-1 General Contraindications to Lung Transplantation
| Absolute | Relative |
|---|---|
BCC, basal cell cancer; BMI, body mass index; HIV, human immunodeficiency virus; SCC, squamous cell cancer.
Modified from Orens JB, Estenne M, Arcasoy SM, et al: International guidelines for the selection of lung transplant candidates: 2006 update—a consensus report from the Pulmonary Scientific Council of the International Society of Heart and Lung Transplantation, J Heart Lung Transplant 25:745–755, 2006.
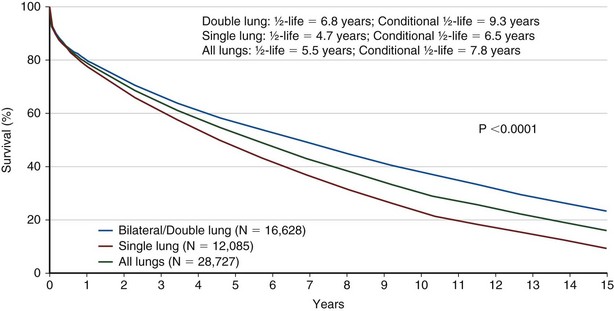
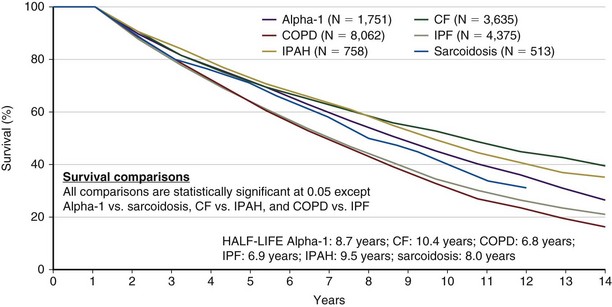
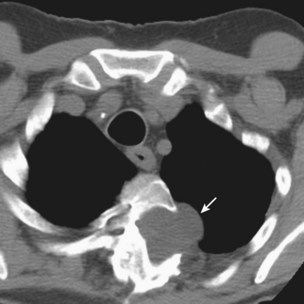
Bilateral lung transplantation (BLTx) is associated with a median survival of 5.6 years, compared with 4.3 years for single lung transplantation (SLTx) (Figure 75-8). Although heart-lung transplantation (HLTx) is associated with the lowest median survival rates of approximately 3 years, HLTx procedures also are the least commonly performed. Although it seems that bilateral lung transplant recipients have a better survival, specifically in the COPD group, this finding is controversial, because the observation is retrospective and uncontrolled. One contributing factor may be that older patients tend to receive single-lung transplants, whereas younger patients receive bilateral lung transplants. The highest survival rates are seen in patients with COPD and CF. The lowest survival rates are seen in patients with IPF and PAH, with a relative risk of death exceeding 2.0 in the first year after transplantation (Figure 75-9).
General Selection Criteria
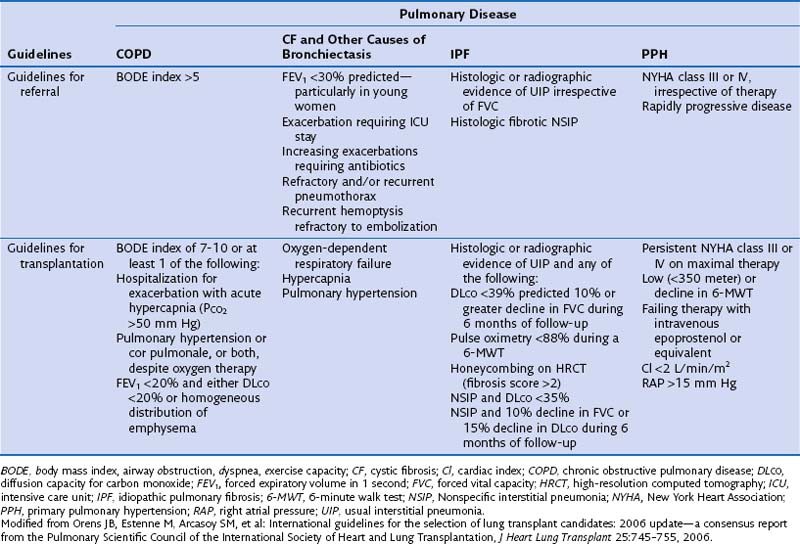
The International Consensus Guidelines for referral and selection of transplant candidates were published in 1998 as a joint effort from the American Society of Transplant Physicians, the American Thoracic Society, the European Respiratory Society, and the International Society for Heart and Lung Transplantation, to facilitate the appropriate timing of referral and proper selection of candidates most likely to benefit from transplantation while ensuring a fair allocation of limited organs. These guidelines were updated in 2006 (Table 75-2). In all instances, selected patients with end-stage pulmonary disease should have declining and irreversible lung function despite optimal medical and surgical management. To justify the risk of transplantation, patients should have an estimated survival of less than 2 years.
Disease-Specific Considerations for Referral and Selection
Chronic Obstructive Pulmonary Disease
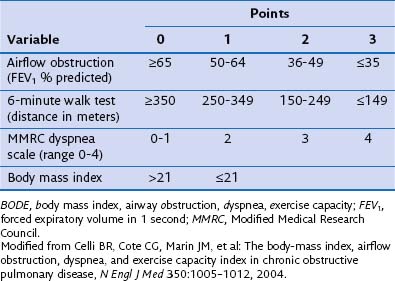
Survival benefit has not been documented in patients with COPD, even though transplantation confers substantial improvement in functional capacity and quality of life. One potential explanation for this discrepancy is that the forced expiratory volume in 1 second (FEV1) may not be as reliable a referral parameter as, for example, the BODE index (determined by scoring for body mass index, airway obstruction, dyspnea, and exercise capacity) (Table 75-3). Patients with a BODE index between 7 and 10 should be selected for transplantation. Other criteria for selecting candidates with COPD include severe worsening of pulmonary function, shorter 6-minute walking distance capacity (less than 100 yards), weight loss (BMI less than 20 kg/m2), need for hospital admission (to the intensive care unit [ICU] in particular), and radiographically homogeneous emphysema. More severely affected patients will show chronic hypoventilation (i.e., PaCO2 greater than 55 mm Hg and evidence of pulmonary hypertension despite oxygen therapy). All patients with COPD should be referred for pulmonary rehabilitation and treated with oxygen therapy, and use of an alternative approach such as lung volume reduction surgery should be either documented as not a viable option or proved to be ineffective.
Donor Selection and Management
The LAS assigns donor lungs to transplant candidates by use of a scoring system determined by medical urgency and net transplant benefit (predicted posttransplantation survival minus predicted wait list survival). Since its implementation in the United States in 2005, median waiting times have shortened markedly. Unfortunately, a scarcity of donated organs remains a limiting problem, leading to use of non–heart-beating donors (Table 75-4), ex vivo donor lung resuscitation, use of “bridges to transplant,” and development of different types of lung grafts (Table 75-5).
Table 75-4 Maastricht Classification for Non–Heart-Beating Donors*
| I | Uncontrolled | Brought in dead |
| II | Uncontrolled | Unsuccessful resuscitation |
| III | Controlled | Awaiting cardiac arrest |
| IV | Controlled | Cardiac arrest after brain death |
| V | Controlled | Cardiac arrest in a hospital inpatient |
* Controlled non–heart-beating donors may be eligible for lung donation.
Table 75-5 Types of Lung Grafts
| Living donor |
From Wang D, Zhou X, Liu X, et al: Wang-Zwische double-lumen cannula—toward a percutaneous and ambulatory paracorporeal artificial lung, ASAIO J 54(6):606–611, 2008.
Currently, the Ideal Donor Selection Criteria are more stringent than those used for selecting other solid organs. In light of the scarcity of available lung grafts, many suggest that donor criteria be made more flexible to expand the existing donor pool. Alternate criteria include donor age older than 55 years, an initial PaO2/FIO2 ratio less than 300, the presence of pulmonary infiltrates on chest imaging, purulent secretions, and a positive history of use of tobacco or other inhalant drug (Table 75-6 and Box 75-1). Retrospective studies suggest similar outcomes for ideal and extended lung allograft recipients, specifically with respect to perioperative complications, ICU stay, requirements for mechanical ventilation, and 1-year survival rate (higher than 80% for both groups). Improved donor management also is under careful investigation, because it may help improve marginal donors and optimize them into ideal candidates. Optimizing donor status with reversal of any limiting physiologic insults is becoming the standard of care in most transplant centers (Figure 75-10). Common respiratory insults are caused by aspiration, atelectasis, infection, pulmonary edema, and the hemodynamic instability associated with neurologic dysfunction. Ventilatory strategies to improve alveolar ventilation may be implemented, along with therapeutic bronchoscopy to suction excess secretions and reexpand atelectatic lung. Small-volume bronchoalveolar lavage (BAL) can minimize the amount of alveolar flooding. Adequate tissue perfusion and cardiac function should be aided with vasoactive medications when needed. Repletion of cortisol, vasopressin, and thyroid hormone also may be of benefit (Figure 75-11).
Table 75-6 Donor Selection Guidelines
| Feature | Ideal Donor | Extended Donor |
|---|---|---|
| Age (years) | <55 | >55 |
| ABO | Identical or compatible | Compatible |
| Chest radiograph | Clear | Unilateral or focal infiltrate |
| *PaO2 (mm Hg) | >300 | <300 on initial assessment; must be >300 after optimization |
| Tobacco (smoking) history | <20 pack-years | >20 pack-years |
| Trauma | Absence of chest trauma | Trauma without significant abnormality |
| Sputum | No purulent secretions | May be considered, therapeutic suctioning |
| Can be performed | ||
| Gram stain | Absence of organisms | Certain organisms may be considered (see Box 75-1) |
* Partial pressure of arterial oxygen tension (PaO2) is measured on a fraction of inspired oxygen of 1.0 and a positive end-expiratory pressure of 5 cm H2O.
Box 75-1
Donor-Related Infections/Factors That May Be Compatible with Donor Eligibility
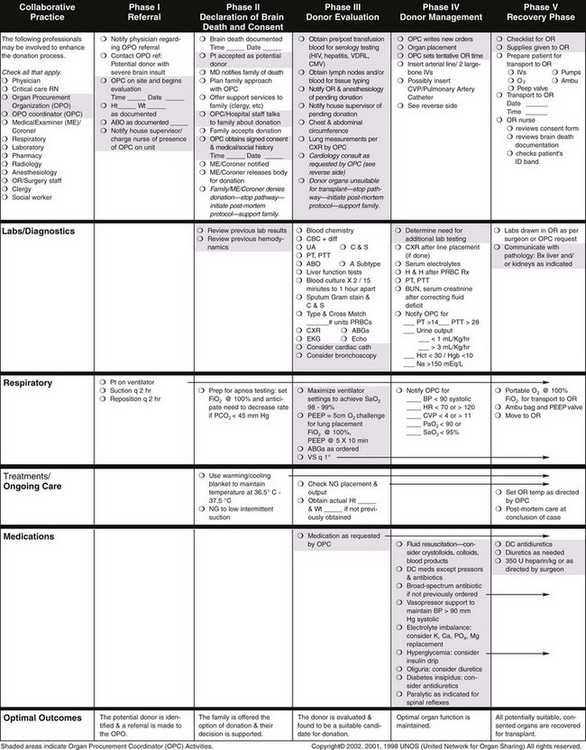
Figure 75-10 Critical pathway of organ donor management.
(From Critical pathway for the organ donor, Richmond, Va, United Network for Organ Sharing, 2006 [poster online]: http://www.Unos.Org/Resources/Pdfs/Criticalpathwayposter.pdf.)
Lung Allograft Implantation
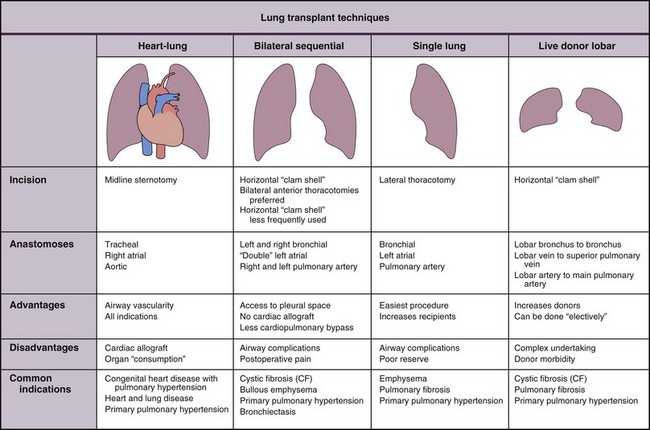
The implantation of a lung allograft (Figure 75-12) depends on the planned procedure, surgical experience, and the previous surgical history of the recipient. Before all transplant procedures, the recipient’s pulmonary function should be maximized, and management should continue through surgery (e.g., continuing pulmonary vasodilators).
Noninfectious Complications
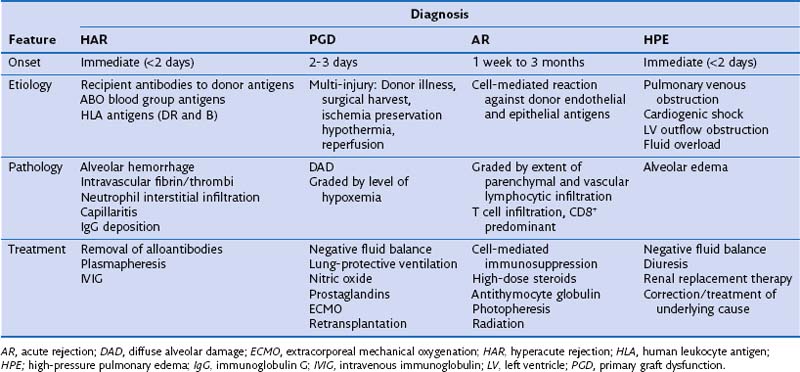
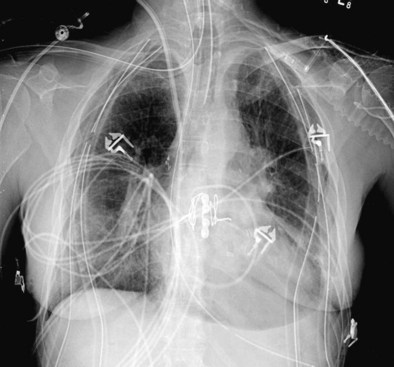
The most common problems in the immediate posttransplantation period are the same as those occurring in most postsurgical patients. Hypoxemia may stem from poor cardiac output, endotracheal tube displacement, or obstruction of uncleared secretions, leading to lobar atelectasis. Additional complications that are unique to lung transplantation include hyperacute rejection, graft dysfunction, and cardiac failure; each may be difficult to differentiate from the others (Table 75-7). A suggested reading by Ahya and Kawut has been listed.
Primary Graft Dysfunction
Primary graft dysfunction (PGD) is the current name for the syndrome of acute lung injury (ALI) after reperfusion of the lung allograft (Table 75-8). This entity previously was referred to as ischemia-reperfusion injury, reimplantation edema, or early graft dysfunction. PGD occurs 48 to 72 hours after transplantation and is characterized by development of bilateral diffuse alveolar infiltrates evident on plain chest radiograph coupled with hypoxemia resulting from capillary leak and alveolar flooding and is associated with prolonged ischemic time (i.e., longer than 550 minutes). In approximately 15% of patients, the degree of hypoxemia reaches ALI criteria (PaO2/FIO2 ratio less than 200) with an accompanying mortality rate of more than 60%. Exclusion of other causes of alveolar infiltrates, including high-pressure pulmonary edema, is necessary.
Table 75-8 Recommendations for Grading of Primary Graft Dysfunction Severity
| Grade | PaO2/FIO2 Ratio | Radiographic Infiltrates Consistent with Pulmonary Edema |
|---|---|---|
| 0 | >300 | Absent |
| 1 | >300 | Present |
| 2 | 200-300 | Present |
| 3 | <200 | Present |
From Christie JD, Carby M, Bag R, et al: Report of the ISHLT Working Group on Primary Lung Graft Dysfunction. Part II: definition. A consensus statement of the International Society for Heart and Lung Transplantation, J Heart Lung Transplant 24(10):1454–1459, 2005.
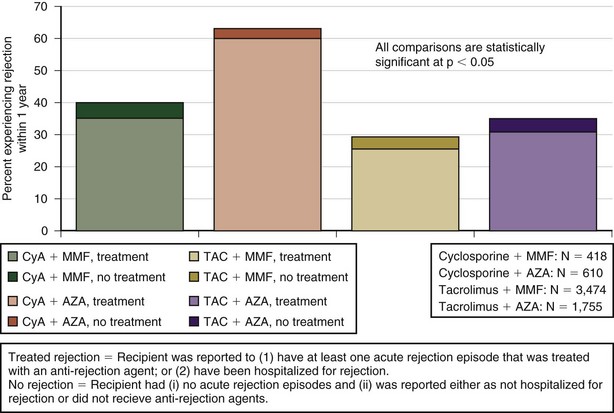
Acute Rejection
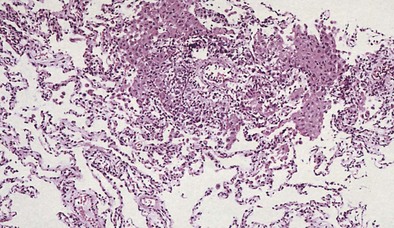
Acute rejection (AR) is a cell-mediated event activated by donor antigens. It occurs in about 40% of transplant recipients in the first year alone with the highest incidence at 6 months after lung transplantation and is irrespective of specific induction treatment. Figure 75-13 shows the reported rejection between discharge and 1-year follow-up stratified by immunosuppressive regimen. It manifests with presence of alveolar infiltrates on the chest radiograph, hypoxemia, and fever, but many patients may be asymptomatic. Histologic examination (Figure 75-14) is required to diagnose AR, because the clinical presentation mimics that in pulmonary infection, and increasing immunosuppression empirically can lead to toxicity, infection, and malignancy.
Bronchial Anastomotic Complications
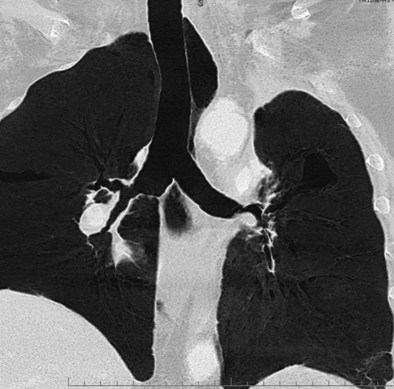
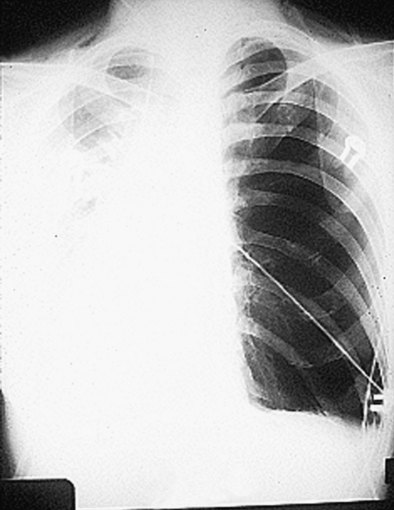
Bronchial anastomotic dehiscence may result from ischemic injury, infection, or poor healing. Bronchial artery interruption during the transplant procedure disrupts perfusion to the large airways. High doses of corticosteroids impair wound healing, potentially leading to bronchomalacia and stenosis (Figure 75-15). Dehiscence may manifest as pneumomediastinum, pneumopericardium, and/or pneumothorax (Figure 75-16). Fiberoptic bronchoscopy is necessary to ensure that all bronchial anastomoses are intact. Correction consists of treating any underlying infection and maintenance of adequate tissue perfusion. Bronchial stent placement, laser ablation, and balloon dilatation often are used in management; rarely is reoperation considered.
Hyperinflation
Hyperinflation of the native lung may be seen in patients with COPD and unilateral lung transplantation as a result of the higher lung compliance, more severe airway obstruction, and air trapping that occurs in the remaining emphysematous lung. The hyperinflated lung can compress the transplanted lung and/or the mediastinal structures, causing a substantial reduction in function (Figure 75-17).
Infectious Complications
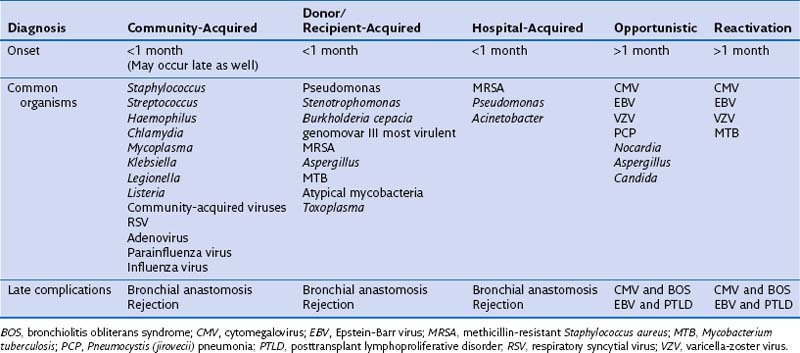
Infectious complications in the immediate postoperative period are the same as those associated with other major cardiothoracic surgery. Blood-borne infections from indwelling catheters, urinary tract, and wound complications are common. Pneumonia in the acute posttransplantation phase requires careful assessment of the donor’s and recipient’s microbiologic and clinical histories, including previous bacterial colonization or recent infections. Clinical history is relevant, because it may detect risks for aspiration, nosocomial, and ventilator-associated pathogens (Table 75-9). Physiologic abnormalities in the transplanted lung also predispose to infection. Impaired mucociliary clearance, denervation of the cough reflex, and disruption of the pulmonary lymphatic and vascular outflow all may act in combination. Infection of the allograft results in inflammation and graft damage. Although prophylaxis seems logical in preventing future infection, strong clinical evidence to support universal use of prophylaxis is lacking, with variable findings among different transplant centers worldwide.
Opportunistic Infections
Cytomegalovirus (Human Herpesvirus Type 5)
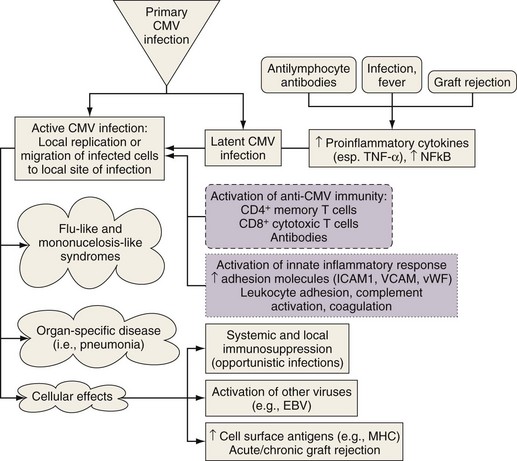
CMV pneumonitis can be diagnosed by the presence of intranuclear viral inclusion bodies on histologic examination. This condition may be an indirect cause of rejection and the bronchiolitis obliterans syndrome (Figure 75-18). CMV prophylaxis often is given routinely and may delay acute infection (Table 75-10). This practice, however, may result in the evolution of resistant strains and predispose the patient to antiviral toxicity, as well as increasing the current cost of care. Alternatively, preemptive monitoring and treatment of infection can be implemented. CMV replication can be detected early by rising serum CMV antigen levels or quantitative CMV polymerase chain reaction (PCR) assay results.
Table 75-10 Suggested CMV Prophylaxis After Lung Transplantation
| CMV Status | Recipient + | Recipient − |
|---|---|---|
| Donor + |
• Risk of CMV disease is increased with extensive blood transfusion
• Leukodepleted and CMV seronegative blood products are recommended
• If extensive transfusions are necessary, CMV prophylaxis should be considered
• Monitoring weekly for CMV PCR should be considered
• We use Acyclovir or Valacyclovir for HSV prophylaxis days 1-90
CMV, cytomegalovirus; HSV, herpes simplex virus; PCR, polymerase chain reaction.
* When used as prophylaxis, the dose of Valganciclovir is 900 mg daily, versus treatment dose which is 900 mg twice daily.
Aspergillus Spp.
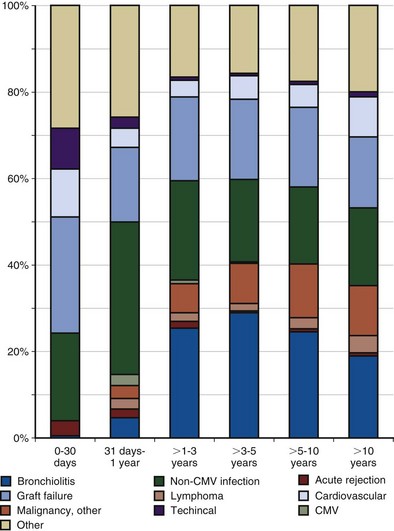
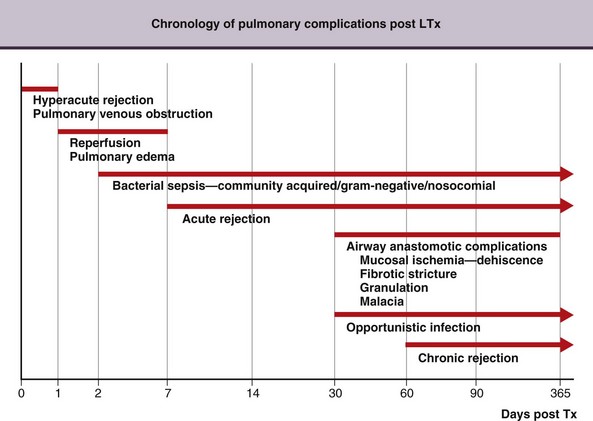
A chronology for the common infectious and noninfectious complications that occur after transplantation over time is presented in Figure 75-19.
Chronic Rejection
Chronic Lung Allograft Dysfunction
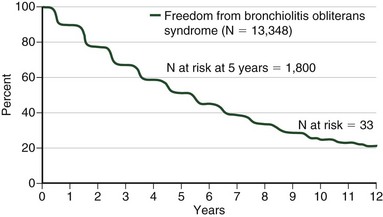
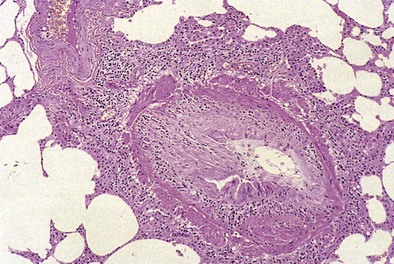
Bronchiolitis obliterans syndrome (BOS) is a common clinicopathologic syndrome of progressive and irreversible airway obstruction with a declining DLCO that occurs as a late complication in graft function with an incidence of almost 50% by the fifth year after transplantation (Figure 75-20). On histopathologic examination, BOS is represented by obliterative bronchiolitis (OB)—specifically, fibrosis of the small airways with intimal thickening and sclerosis of its accompanying vessel that lead to near-complete occlusion of the bronchiolar lumen and small airway obstruction (Figure 75-21). Chest imaging should yield normal findings in the early stages of BOS, which remains a diagnosis of exclusion at that time. Acute rejection, infection, drug-induced pneumotoxicity, and bronchial anastomotic obstruction must be excluded. Advanced BOS may appear as peripheral bronchiectasis with a loss of vascularity and atelectasis. A staging system has been created to aid in the diagnosis of BOS by the International Society of Heart and Lung Transplantation (Tables 75-11 and 75-12). Of importance, not all graft dysfunction is due to OB. A restrictive pattern is present in a significant minority of patients. Imaging may suggest peripheral encroachment of the lung volume and restrictive pulmonary function tests (PFTs). Therefore, the phraseology “chronic lung allograft dysfunction” (CLAD) is coming into use to account for more than one manifestation of chronic graft failure.
Table 75-11 Histologic Classification and Grading of Pulmonary Allograft Rejection
| Acute Rejection | |
| Grade 0—none | No significant abnormality |
| Grade 1—minimal | Infrequent perivascular mononuclear cell infiltrates mainly surrounding venules that are 2-3 cells deep |
| Grade 2—mild | More frequent infiltrates, 5 cells or more deep, involving venules and arterioles |
| Grade 3—moderate | More exuberant mononuclear cell infiltrate that extends from the perivascular space into the alveolar |
| Interstitium | |
| Grade 4—severe | Infiltrate extending into the alveolar space with pneumocyte damage and at times necrosis of vessels and lung parenchyma |
| Airway inflammation | Lymphocytic bronchitis/bronchiolitis; pathologist may grade |
| Chronic Rejection | |
| Chronic airway rejection Active Inactive |
|
| Chronic vascular rejection–accelerated graft vascular sclerosis | |
Table 75-12 Comparison of Acute Rejection and Bronchiolitis Obliterans Syndrome
| Feature | Acute Rejection | Bronchiolitis Obliterans Syndrome |
|---|---|---|
| Peak frequency | First 6 months | >3 months |
| Onset | Abrupt to subacute | Usually subtle |
| Symptoms/signs | Tightness in chest (immediate postoperative period) Cough (usually not productive) Dyspnea |
Dyspnea with heavy exertion Cough (often productive) |
| Physiologic | Restrictive impairment Desaturation of arterial blood |
Obstructive impairment Normoxia until late |
| Radiologic | Diffuse interstitial infiltrates Pleural effusions |
No abnormality until disorder is far advanced Computed tomography evidence of bronchiectasis and mosaic pattern |
| Hematologic | Leukocytosis | Normal white blood cell count |
| Histologic | Perivascular mononuclear cell infiltrates Airway inflammation is variable |
Obliterative bronchiolitis Atherosclerosis of pulmonary and bronchial arteries Pleural scarring |
| Response to treatment function | Majority of cases improve rapidly with intravenous corticosteroid | Forced expiratory volume in 1 second at best stabilized Majority of recipients show progressive decline in allograft |
Pitfalls of Immunosuppressive Therapy
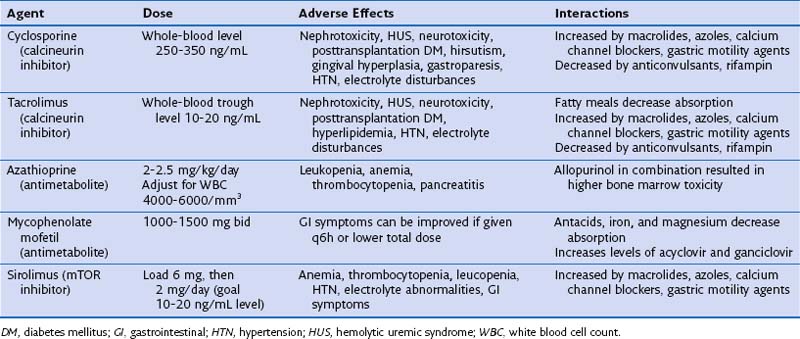
Common complications from the chronic use of immunosuppressive drugs vary, depending on the specific agent and the recipient’s predisposing characteristics (Table 75-13). Toxicities may be severe and irreversible, as is evident in cases of posttransplantation malignancy. Frequent checks for metabolic derangements and end-organ toxicity are critical. Unfortunately, serum drug levels do not correlate with the dosage of administered drugs and are not predictive of the potential toxic effects, because these factors vary from person to person.
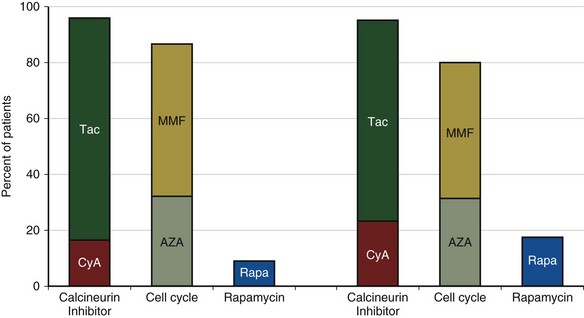
A current trend is for the use of tacrolimus, MMF, and sirolimus (Figure 75-22). A recent study comparing azathioprine and MMF in lung transplant recipients failed to show a significant difference in prevention of BOS. Although there are multiple reasons why a difference was not observed, there is still no definitive evidence to suggest superior outcomes with these newer drugs.
Medical Complications in Lung Transplant Survivors
Malignancy
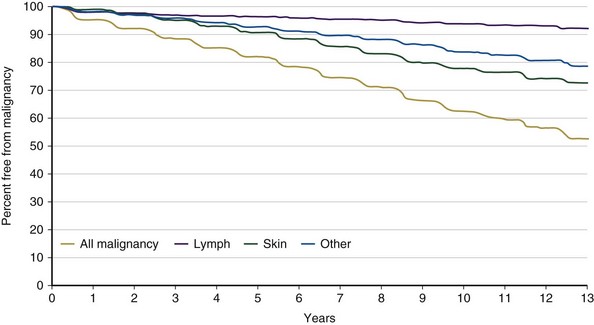
Posttransplantation malignancy (Figure 75-23) frequently is seen after solid organ transplantation. The calcineurin inhibitors and azathioprine have been associated with a higher risk of cancer. Currently, a 10% incidence of tumor development is reported in 5-year survivors, with lymphoid and skin cancers accounting for a majority. Newer immunosuppressive agents, such as the mTOR inhibitors, seem to have antineoplastic properties; evidence that these agents lead to lower cancer risk or decreased mortality is lacking, however.
Other Medical Complications after Transplantation
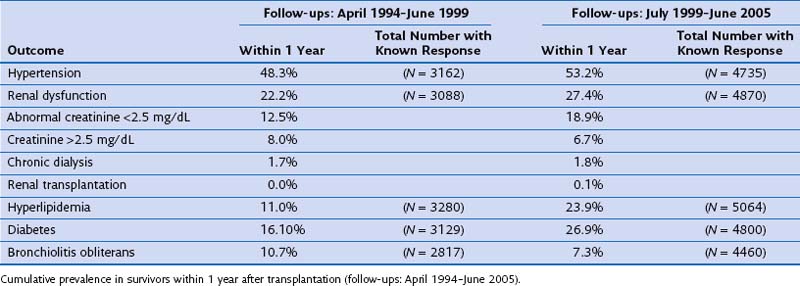
Acute and chronic nephrotoxicity is well described with use of the calcineurin inhibitors and is characterized by hyalinosis of afferent arterioles, vacuolization of proximal tubules, and focal areas of interstitial and glomerular fibrosis (Figure 75-24). Although renal dysfunction is rather common in 1-year survivors, renal failure requiring dialysis remains rare. Use of calcineurin inhibitors as well as corticosteroids also can lead to diabetes mellitus, hypertension, and dyslipidemia (Table 75-14).
Ahya VN, Kawut SM. Noninfectious pulmonary complications after lung transplantation. Clin Chest Med. 2005;26:613–622.
Christie JD, Edwards LB, Kucheryavaya AY, et al. Registry of the International Society for Heart and Lung Transplantation: Twenty-Seventh Official Adult Lung and Heart-Lung Transplantation Report. J Heart Lung Transplant. 2010;29:1104–1118.
De Soyza A, McDowell A, Archer L, et al. Burkholderia cepacia complex genomovars and pulmonary transplantation outcomes in patients with cystic fibrosis. Lancet. 2001;358:1780–1781.
Estenne M, Maurer JR, Boehler A, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21:297–319.
Kowalski R, Post D, Schneider MC, et al. Immune cell function testing: an adjunct to therapeutic drug monitoring in transplant patient management. Clin Transplant. 2003;17:77–88.
McNeil K, Glanville AR, Wahlers T, et al. Comparison of mycophenolate mofetil and azathioprine for prevention of bronchiolitis obliterans syndrome in de novo lung transplant recipients. Transplantation. 2006;81:998–1003.
Orens JB, Boehler A, Perot MD, et al. A review of lung transplant donor acceptability criteria. J Heart Lung Transplant. 2003;22:1183–1200.
Orens JB, Estenne M, Arcasoy SM, et al. International guidelines for the selection of lung transplant candidates: 2006 update—a consensus report from the Pulmonary Scientific Council of the International Society of Heart and Lung Transplantation. J Heart Lung Transplant. 2006;25:745–755.
Shargall Y, Guenther G, Ahya VN, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part VI: treatment. J Heart Lung Transplant. 2005;24:1489–1500.
Steen S, Sjoberg T, Pierre L, et al. Transplantation of lungs from a non-heart beating donor. Lancet. 2001;357:825–829.
Woodrow JP, Shlobin OA, Barnett SD, et al. Comparison of bronchiolitis obliterans syndrome to other forms of chronic lung allograft dysfunction after lung transplantation. J Heart Lung Transplant. 2010;29:1159–1164.