6 Lung Infections
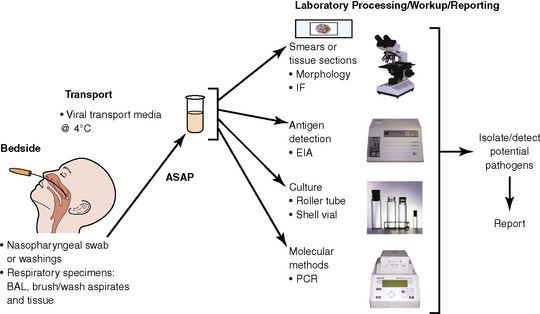
Lower respiratory tract infections constitute a leading cause of morbidity and death worldwide.1,2 Included in this category of infections are bronchitis and bronchiolitis, community-acquired and nosocomial pneumonias, and pneumonias in the immunocompromised patient. A relatively small percentage of these infections come to the attention of the surgical pathologist, because most are diagnosed in the microbiology laboratory. Nevertheless, as summarized in Box 6-1, the anatomic pathologist can play a pivotal role in the diagnosis of lung infections by identifying reaction patterns in tissue, and sometimes in the identification of an organism that microbiologic techniques fail to detect.3 Despite significant advances in laboratory techniques, culture diagnosis is not always possible; the organism may not reproduce in culture, a culture study may not have been requested, or the culture technique may have failed for any of various technical reasons. Even when culture is successful, the time frame for diagnostic purposes may not be clinically useful, or the culture result, in the absence of an expected tissue response, may not permit distinction of pathogens from innocent bystanders, be they colonizers or contaminants. For all of these reasons, the pulmonary infection for which biopsy is performed often is one that has eluded standard microbiologic techniques, has not responded to empirical therapy, or requires morphologic analysis for clarification of a critical aspect of the differential diagnosis. In these situations, the diagnostic pathologist is indispensable,4,5 if not for providing an immediate report intraoperatively (by frozen section or cytologic imprints or smears), then for dramatically improving diagnosis turnaround time with the use of newer rapid tissue-processing systems6 (Table 6-1).
Box 6-1 Role of the Diagnostic Pathologist
Modified from Watts J, Chandler F. The surgical pathologist’s role in the diagnosis of infectious disease. J Histotechnol. 1995;18:191–193.
Table 6-1 Diagnostic Tools of the Pathologist
| Activity | Objective |
|---|---|
| Pre-/intra-/postoperative consultation | Information exchange and strategies |
| Gross examination | Tissue handling and triage |
| Histopathologic examination | Organism morphology; cytopathic effect; host response |
| Histochemical stains | Detection and morphologic detail |
| Immunohistochemical stains | Detection of organisms; confirmation of genus/species |
| Electron microscopy | Selective use for virus, fungi, parasites, and bacteria |
| Molecular techniques: in situ hybridization, polymerase chain reaction | Sensitive and specific detection/identification of nonculturable organisms; stain-negative cases |
| Report | Clinicopathologic and microbiologic correlation |
Diagnostic Tools and Strategies
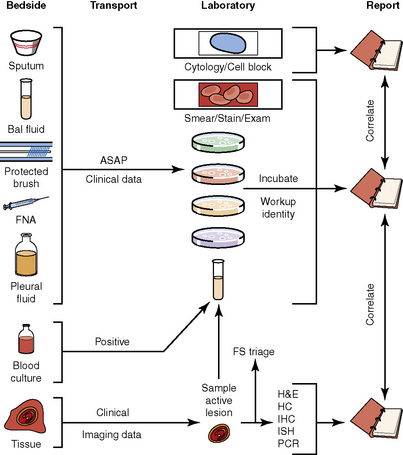
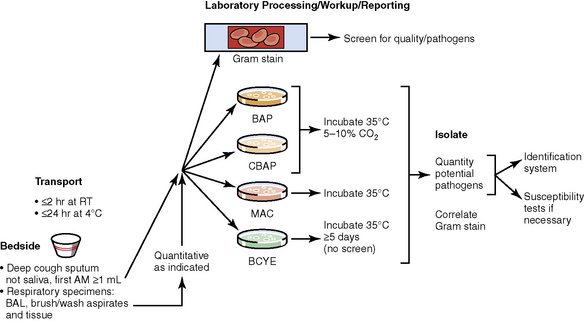
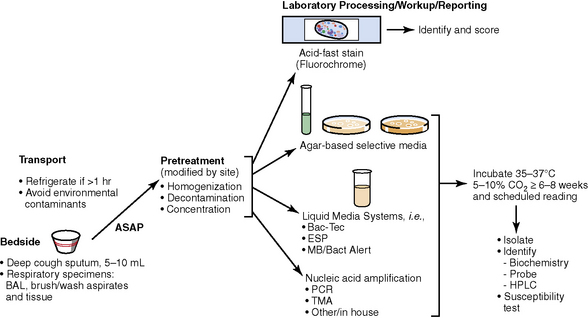
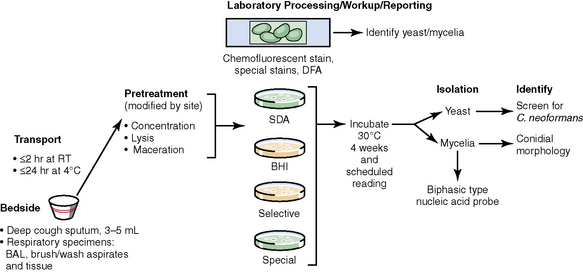
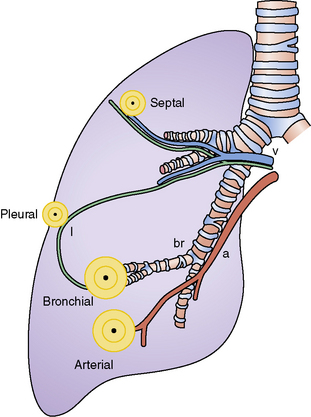
The history of the field of pathology is intertwined with the discovery of pathogenic bacteria and the development of the science of microbiology.7 Today, pathologists and microbiologists approach the diagnosis of infection with techniques and methods that share some aspects but have important differences.8 The surgical pathologist and the cytopathologist are in a position to apply the tools of both disciplines to achieve a clinically relevant diagnosis by correlating the histopathologic or cytopathologic examination findings with data obtained using microbiology techniques (Table 6-2). Unfortunately, the diagnostic workup and reporting of biopsy findings in surgical or cytopathology departments and those in the microbiology laboratory typically run along nonintersecting paths, often without one group knowing (or acknowledging) the findings of the other. An interdisciplinary approach that is based on mutual understanding and communication would seem to be a logical, if not ideal, scenario for optimal clinical management.9 Our concept of an integrated morphologic and microbiologic approach is presented schematically in Figure 6-1, and with greater detail for specific situations in which bacterial (Fig. 6-2), mycobacterial (Fig. 6-3), fungal (Fig. 6-4), or viral (Fig. 6-5) pathogens are suspected.
| Activity | Objective |
|---|---|
| Pre-/intra-/postoperative consultation | Information exchange and strategies |
| Direct visualization (smears and imprints) | Rapid detection |
| Culture | Identification of genus and species; susceptibility studies |
| Antigen detection | Rapid identification |
| Serologic testing | Specific antibody response |
| Molecular techniques | Sensitive and specific detection/identification |
| Report | Traditional versus interpretive format |
In current medical practice, identification of the genus or species of an infectious organism can have important prognostic and therapeutic implications. Because histopathologic examination alone rarely provides this information, the findings should always be correlated with results of cultures. Accordingly, foresight is required on the part of the intraoperative pathologist in obtaining and properly handling tissues for culture.10 The correlation of the morphologic and microbiologic data can be facilitated in the surgical pathology report by appending a comment that seeks to enhance the morphologic diagnosis by suggesting a specific etiologic disorder or agent, considerations for the differential diagnosis, or additional workup with culture, serology, or molecular studies. In certain situations, it is also appropriate to include the preliminary results of microbiology stains and cultures, and to correlate this information with the morphologic findings whenever possible.
Knowledge of the Clinical Setting
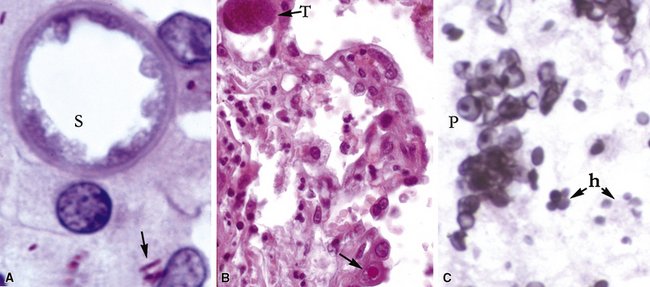
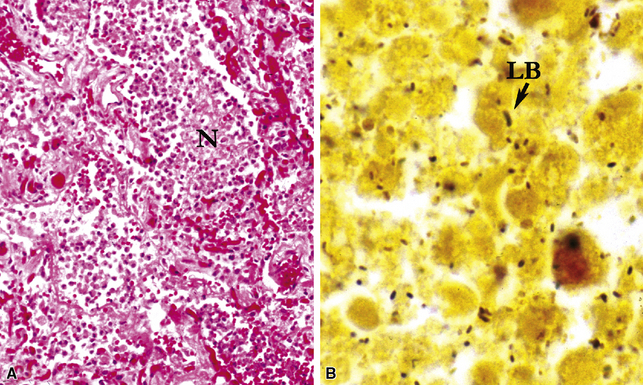
Identification of risk factors and determination of the immune status of the patient are of primary importance, because these parameters typically influence the spectrum of histopathologic changes and the type of etiologic agents and pathogen burden.11–16 Also, because the degree of immunosuppression often influences the burden of organisms, different efforts may be required to identify the pathogen. For example, organisms are less often found in lung tissues from patients with normal or near-normal immunity. In this setting, cultures, serologic studies, and epidemiologic data must be relied on to provide the diagnosis.17 By contrast, persons infected with the human immunodeficiency virus (HIV) in whom the acquired immunodeficiency syndrome (AIDS) or Mycobacterium avium infection develops typically manifest poorly formed granulomas, or simply histiocytic infiltrates, despite an overabundance of organisms identified by tissue acid-fast stains. Pneumocystis organisms may be easily identified in patients with AIDS, who manifest diffuse alveolar damage accompanied by abundant, foamy alveolar casts but when immunosuppression is less severe (such as that produced by corticosteroids therapy for arthritis), the morphologic features can be less typical, and the organisms sparse. The relationship among the level of immunity, burden of organisms, and patterns of disease is illustrated for cryptococcosis in Figure 6-6.
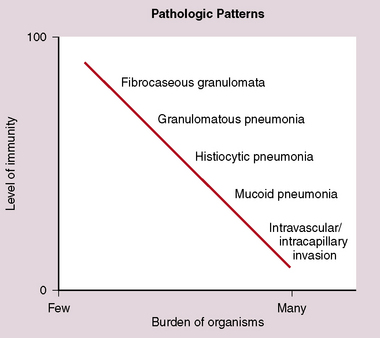
Figure 6-6 Cryptococcosis: Correlation of pathologic patterns with immunity level and organism burden.
(Data from Mark EJ. Case records of the Massachusetts General Hospital. N Engl J Med. 2002;347:518–524.)
In the immunocompromised patient, one must also consider a broader differential diagnosis. In addition to infection, other disorders come into consideration such as pulmonary involvement by pre-existing disease, drug-induced and treatment-related injury, noninfectious interstitial pneumonias, malignancy, and new pulmonary diseases unrelated to the patient’s immunocompromised state, such as aspiration, heart failure, and pulmonary embolism. When immunosuppression is intentional, as in transplant recipients, unique additional challenges come into play, such as transplant rejection, graft-versus-host disease, and Epstein-Barr virus (EBV)-associated lymphoproliferative disorders. Immunosuppressed persons are at risk for multiple simultaneous infections, so when one organism is found, a careful search for others is always warranted (Fig. 6-7).
A number of well-characterized genetic disorders of immunity and cellular function are known to predispose affected persons to lung infection.18–21 Cystic fibrosis bears special recognition in this context because it is associated with reproducible patterns of lung disease and susceptibility to a wide spectrum of infectious organisms. This geneticdisease of autosomal recessive inheritance involves mutation of the CFTR gene that affects the ability of epithelial cells to effectively transport chloride and, secondarily, water across cell membranes. As a result, many organs, including the lungs, develop excessively viscous mucous secretions, which cannot be cleared effectively from the airways. In the lung, retention of such secretions leads to progressive and widespread bronchiectasis with airway obstruction that in turn paves the way for recurrent infection (Fig. 6-8). Bacterial organisms commonly isolated include Pseudomonas aeruginosa (both mucoid and nonmucoid strains), Haemophilus influenzae, Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, Burkholderia cepacia complex, Stenotrophomonas maltophilia, and Achromobacter xylosoxidans.22 Polymicrobial infections are not uncommon, and some of these pathogens, especially certain subspecies within the B. cepacia complex, are linked to an adverse prognosis.23 Cystic fibrosis also is a risk factor for non-tuberculous mycobacterial infection and allergic bronchopulmonary fungal disease, and the condition is potentially exacerbated by superimposed viral infections.24–27
Pattern Recognition
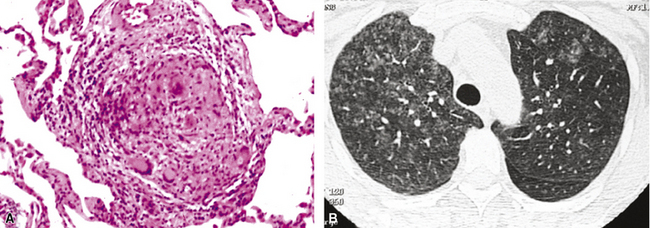
Knowledge of the radiologic pattern of infectious lung disease in a given patient often helps to narrow the scope of the differential diagnosis.28,29 Patterns of lung infection seen on high-resolution computed tomography (HRCT) typically are dominated by increased attenuation (opacity). Such opacities may occur as one or more localized densities (nodule, mass, or localized infiltrate) or as more extensive infiltrates referred to as either ground-glass opacities (attenuation that allows underlying lung structures to be visible) or consolidation (attenuation that overshadows underlying structure).30 Review of the chest imaging studies with the radiologist can be very helpful in arriving at a clinically relevant diagnosis. Correlating these data with the clinical history and pace of the disease under scrutiny (acute, subacute, chronic) allows a more accurate interpretation of the observed histopathologic pattern of disease in the tissue (Fig. 6-9). Fortunately, the recognized histopathologic patterns of lung infection are fairly limited (airway disease, acute lung injury, cellular infiltrates, alveolar filling, and nodules), and these typically correlate with a particular group of organisms (Table 6-3).
Table 6-3 Histopathologic Patterns and Most Agents of Pulmonary Infection
| Pattern | Most Common Agent(s) |
|---|---|
| Airway disease | |
| Bronchitis/bronchiolitis | Virus; bacteria; Mycoplasma |
| Bronchiectasis | Bacteria; mycobacteria |
| Acute exudative pneumonia | |
| Purulent (neutrophilic) | Bacteria |
| Lobular (bronchopneumonia | Bacteria |
| Confluent (lobar pneumonia) | Bacteria |
| With granules | Agents of botryomycosis (Staphylococcus aureus), actinomycosis (Actinomyces israelii) |
| Eosinophilic | Parasites |
| Foamy alveolar cast | Pneumocystis |
| Acute diffuse/localized alveolar damage | Virus; polymicrobial |
| Chronic pneumonia | |
| Fibroinflammatory | Bacteria |
| Organizing diffuse/localized alveolar damage | Virus |
| Eosinophilic | Parasite |
| Histiocytic | Mycobacteria |
| Interstitial pneumonia | |
| Perivascular lymphoid | Virus; atypical agents |
| Eosinophilic | Parasite |
| Granulomatous | Mycobacteria |
| Nodules | |
| Large | |
| Necrotizing | Fungi; mycobacteria |
| Granulomatous | Fungi; mycobacteria |
| Fibrocaseous | Fungi; mycobacteria |
| Calcified | Fungi; mycobacteria |
| Miliary | |
| Necrotizing | Viral; mycobacteria; fungi |
| Granulomatous | Fungi |
| Cavities and cysts | Fungi; mycobacteria |
| Intravascular/infarct | Fungi |
| Spindle cell pseudotumor | Mycobacteria |
| Minimal (“id”) reaction | Polymicrobial |
Useful Tissue Stains in Lung Infection
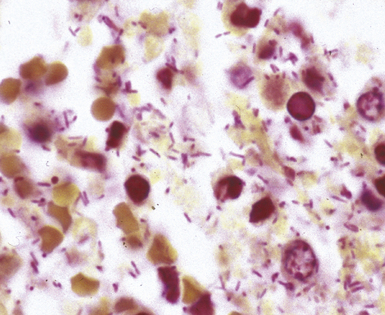
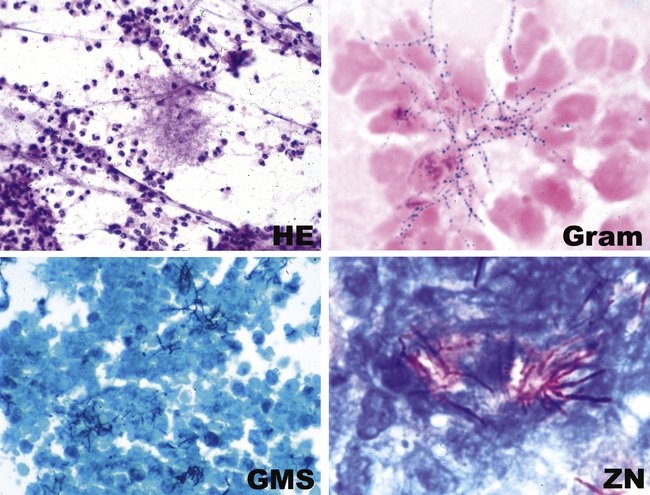
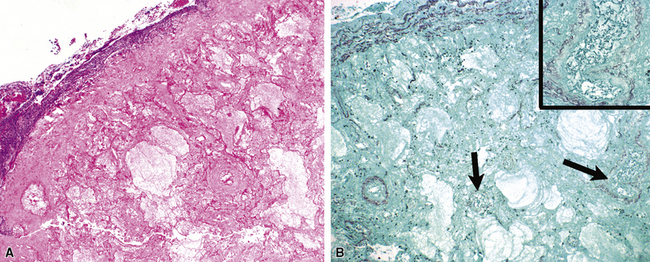
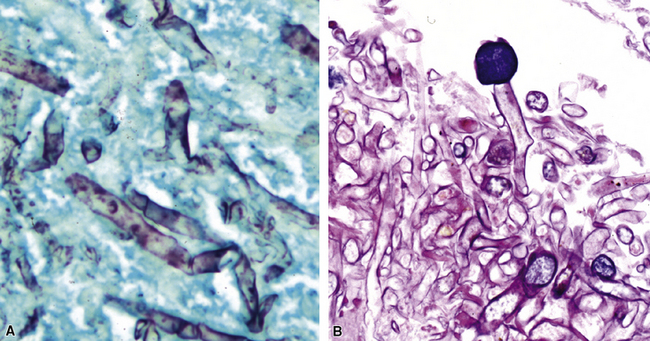
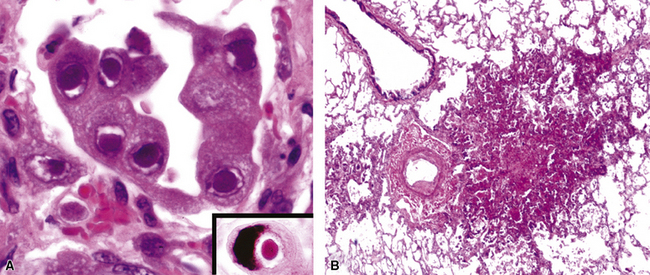
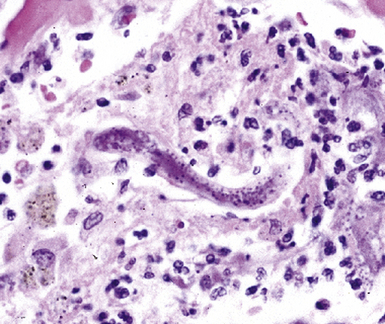
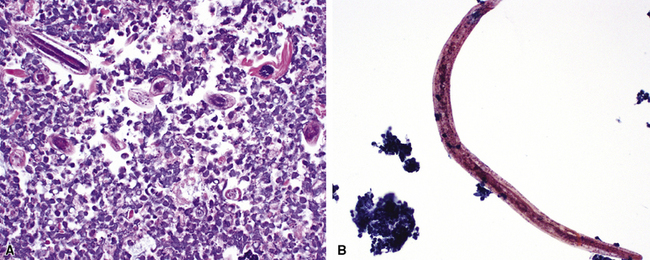
Many diagnostic pathologists have an aversion to the use of special stains for identifying organisms in tissue sections based on less than optimal specificity and sensitivity and the technical difficulty of performing some of these (especially silver impregnation methods, such as the Dieterle, Steiner, and Warthin-Starry stains). Nevertheless, several tissue section staining techniques are quite useful in detecting bacteria, mycobacteria, and fungi in tissue sections. A list of these is presented in Box 6-2. These stains should always be applied as part of an algorithmic strategy for acute lung injury, but especially in the immunocompromised patient.3 For example, when bacteria are being sought, some pathologists would prefer to begin with the tissue Gram stain (e.g., Brown and Hopps, Brown and Brenn) (Fig. 6-10), but silver impregnation techniques (e.g., Warthin-Starry) are actually more sensitive and a good starting point for approaching a suspected bacterial infection. By coating the bacteria with metallic silver, the bacterial silhouettes are enhanced (Fig. 6-11) and become more visible.3 Other stains (e.g., Giemsa) will sometimes detect bacteria that do not stain well with more conventional stains (Fig. 6-12). The Grocott methenamine silver (GMS) stain (Fig. 6-13) is the best stain for most fungi in tissue and also stains actinomycetes, Nocardia, Pneumocystis (cysts), free-living soil amebae, algal cells, the spores of certain microsporidia, and the cytoplasmic inclusions of cytomegalovirus (CMV).8
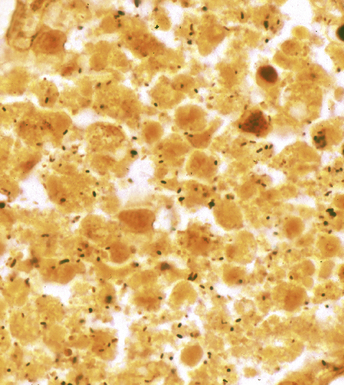
Figure 6-11 Black (silver-coated) bacilli (Legionella pneumophila) in alveolar exudate (Dieterle stain).
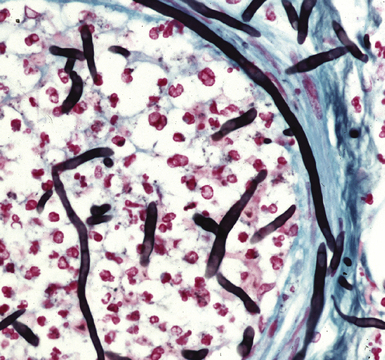
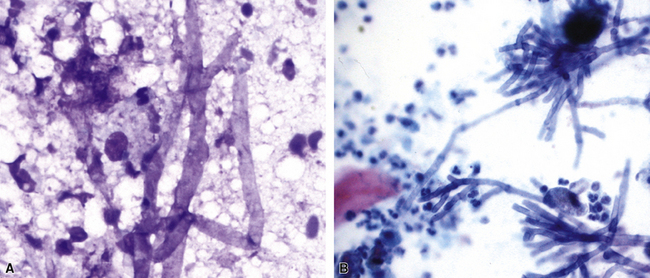
Figure 6-13 Angioinvasive Aspergillus species (Grocott methenamine silver stain).
(Courtesy of Dr. Francis Chandler, Augusta, GA.)
Most mycobacteria stain well with the Ziehl-Neelsen procedure (Fig. 6-14), but the auramine-rhodamine fluorescent procedure is superior in terms of sensitivity (Fig. 6-15). Nocardia organisms, Legionella micdadei, and Rhodococcus equi are weakly or partially acid-fast, and use of modified acid-fast stains such as in the Fite-Faraco technique is more satisfactory for identification of these organisms. Some mycobacterial species, such as M. avium complex (MAC), also are periodic acid/Schiff reagent (PAS)-positive, GMS-positive, and weakly gram-positive.
Finally, for completeness, it can be said that for identification of most protozoa and helminths, as well as viral inclusions, a good-quality hematoxylin and eosin (H&E)-stained section suffices; in fact, a well-prepared H&E section alone is diagnostic for many infectious diseases. This stain often can detect and even distinguish between bacterial cocci and bacilli when the burden of organisms is high (Fig. 6-16).
Immunologic and Molecular Techniques
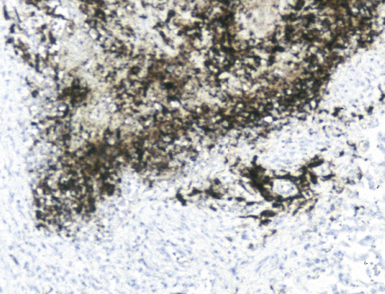
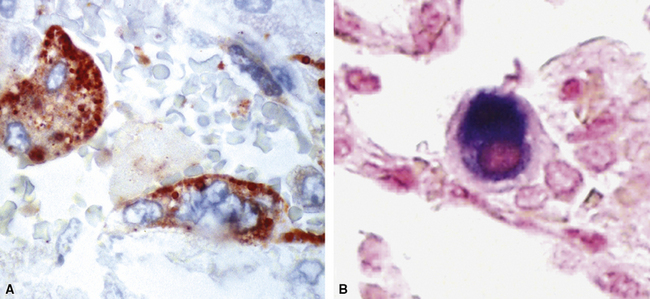
The application of ancillary studies, such as immunohistochemistry, in situ hybridization31 (Fig. 6-17), or nucleic acid amplification technology, can provide a specific etiologic diagnosis in certain cases. These techniques have the best chance for diagnosing infections caused by fastidious species that are difficult or impossible to culture from fresh samples, and also for situations in which only formalin-fixed, paraffin-embedded tissues are available. Immunohistochemical reagents for microbiological detection are becoming increasingly available and provide added power to determining specific diagnoses on formalin-fixed paraffin-embedded tissue32 (Fig. 6-18). Although these techniques provide the diagnostic equivalence of culture confirmation, they are not without limitations and diagnostic pitfalls. The PCR method first introduced in the 1980s has undergone a number of modifications. Non-PCR DNA amplification methods and methods based not on the amplification of the DNA target per se, but on amplification of the signal or probe have also been introduced.33 Among the more recently available technologies is the rapid-cycle “real-time” PCR assay, representing an especially powerful advance in that it is significantly more sensitive than culture. The adaptation of various amplification methods to real-time and multiplex formats enables laboratories to detect a wide range of respiratory pathogens. Furthermore, the transition from traditional and analyte-specific methods to more global technologies such as PCR arrays, liquid bead arrays, microarrays, and high-throughput DNA sequencing is under way. Over time, these methods will find a place in laboratories of all sizes, and dramatically impact the speed and accuracy of microbiologic testing practice for all types of microorganisms.34–37
Limiting Factors in Diagnosis
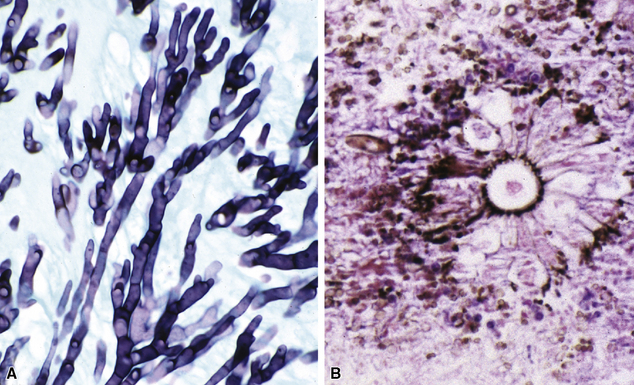
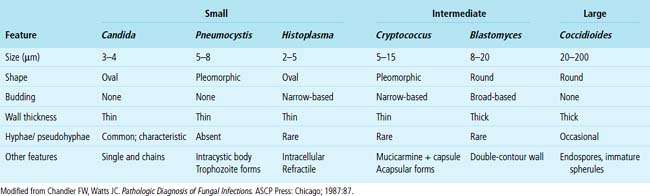
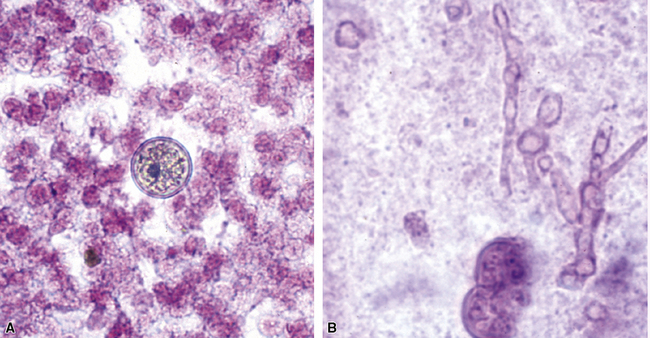
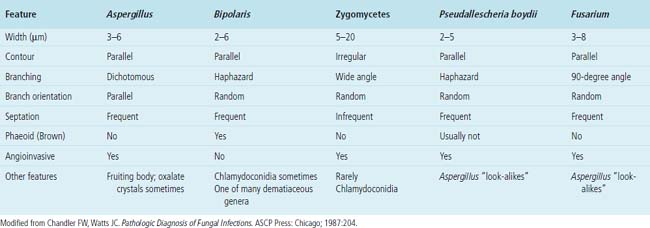
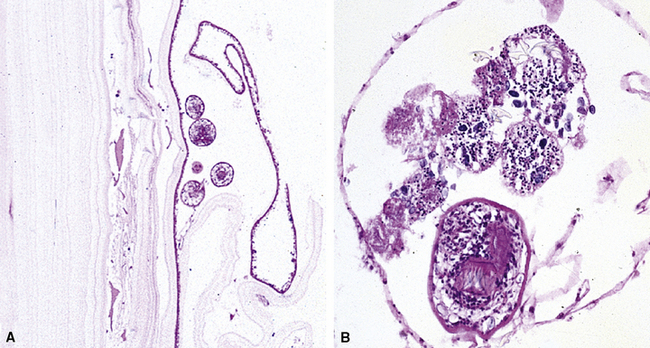
Needless to say, the diagnostic tools employed by both pathologists and microbiologists have their limitations, in terms of sensitivity and specificity.8 Some common tools are listed in Box 6-3. Culture alone cannot distinguish contamination from colonization, or in the case of viruses, asymptomatic shedding from true infection. Molecular tests also suffer from some of these problems; require specialized, often costly equipment; and are susceptible to false positive and false negative results.36 If a surgical biopsy is available, correlation of the histopathologic features can help assign an etiologic role to an agent recovered in culture. The host inflammatory pattern and morphologic features of an organism can be characteristic for certain types of infections, but often the organism’s morphology alone is not sufficient for a diagnosis at the genus or species level. Furthermore, the classic histopathologic findings for a given infection may be incomplete or lacking, making specific morphologic diagnosis possible for relatively few organisms. For example, the etiologic diagnosis is straightforward when large spherules with endospores characteristic of Coccidioides species are present, when small budding yeasts of Histoplasma capsulatum are seen, or yeasts with large mucoid capsules of Cryptococcus neoformans are identified. However, atypical forms of these organisms can be confusing.38 Similarly, hyphal morphology is helpful when it is characteristic of a specific genus or group, but the many look-alikes (Fig. 6-19) require separation by searching for subtle differences under high magnification (or oil immersion), or reliance on special techniques and culture.39
Box 6-3 Limitations of Diagnostic Tools
Morphology
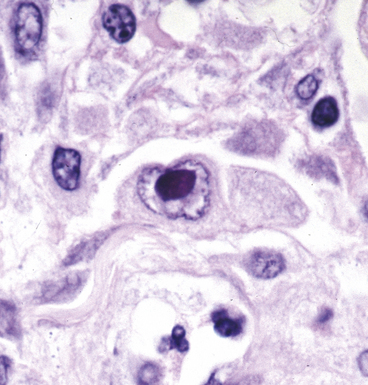
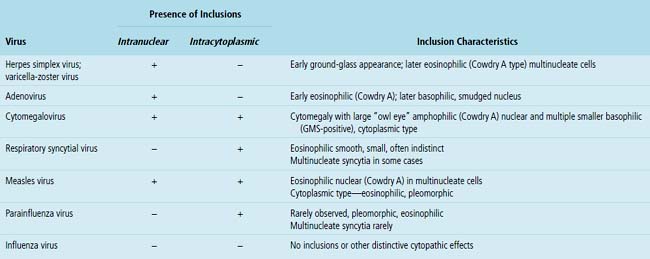
Certain viruses may have characteristic inclusions in tissue, but there are notable pitfalls. For example, eosinophilic intranuclear inclusions of adenovirus may resemble the early inclusions in herpes simplex virus or CMV, especially when the typical smudged cellular forms of adenovirus are absent. Also, simulators of viral cytopathic effect (CPE) can occur in a number of conditions and need to be recognized, such as macronucleoli, optically clear nuclei, and intranuclear cytoplasmic invaginations (Fig. 6-20).
Pseudo-microbe artifacts also have been recognized on routine and special stains for identification of bacteria and fungi. Such potential artifacts include fragmented reticulin fibers, pigments, calcium deposits, Hamazaki-Wesenberg (yellow-brown) yeast-like bodies (Fig. 6-21), pollen grains, and even lymphoglandular bodies.40 For all of these reasons, the pathologist must maintain a high threshold for diagnosing organisms on morphologic grounds. If any question remains, it is best to repeat special stains liberally on deeper levels or in different tissue blocks.
The Role of Cytopathologic Examination in Diagnosis of Lung Infection
A wide variety of infectious diseases of the lung, including bacterial, mycobacterial, fungal, viral, and parasitic, can be diagnosed through exfoliative or fine-needle aspiration cytologic techniques.41–44 Fine-needle aspiration is an especially powerful tool, compared with exfoliative cytology study of respiratory secretions—sputum samples, bronchial washings, brushings, and bronchoalveolar lavage (BAL) fluid samples. The usefulness of exfoliative cytology examination often is limited owing to problems associated with distinguishing colonizing or oral contaminant organisms in the airways from true pathogens. Nonetheless, both diagnostic techniques are complementary and have been used in recent years to evaluate pneumonias and pulmonary nodules in both immunocompetent and immunocompromised patients.
Mass-like infiltrates are often the target of aspiration biopsy needles when suspicion or exclusion of an infectious process ranks high in the differential diagnosis. Besides the morphologic features of the microorganism, important cytologic clues to the diagnosis include the accompanying cellular response and the presence and character of any necrotic debris present, as outlined in Table 6-4. Although nonspecific, such features can suggest certain possibilities to the cytopathologist and assist the microbiology laboratory in triaging the specimen.45 To this end, the presence of a cytopathologist, microscope, and staining setup during the aspiration process can be useful. The cytopathologist can correlate the clinical setting, radiologic features, and clues from the gross character of the aspirate (color, consistency, odor, and so on), thereby assisting in narrowing the diagnostic possibilities and avoiding false-positive and false-negative diagnoses.46 Also, immediate evaluation of smears by rapid stain procedures allows the cytopathologist to either make or suggest a specific diagnosis, as with preparation and evaluation of a frozen section during intraoperative consultation. Smears can be prepared for special stains, needle rinses can be performed for culture and other ancillary studies, and additional aspirations may be encouraged for these purposes.47 Special stains for bacteria, mycobacteria, and fungi should be used whenever the character of the aspirate and the clinical setting (e.g., compromised immune status) indicate that such studies may be useful.
Table 6-4 Fine-Needle Aspiration (FNA) Patterns of Pulmonary Infectious Diseases
| Pattern | Possible Etiologic Agent(s) |
|---|---|
| Acute purulent inflammation/ abscess | Bacteria Fungi |
| Granuloma pattern (epithelioid cells with or without necrosis): Caseous/necrotizing Suppurative Epithelial Mixed |
Mycobacteria Bacteria Parasite Fungi |
| Foamy alveolar cast pattern | Pneumocystis jiroveci |
| Histiocytic | Mycobacteria Bacteria Fungi |
| Chronic inflammation (lymphocyte and plasma cell) | Virus Other agent not otherwise specified |
| Null (“id”) reaction | Virus Any other |
Some interventionists prefer to provide only a needle core biopsy in lieu of an aspirate for a variety of reasons. These two techniques can be viewed as complementary; while needle core biopsies work well for neoplasms and many granulomas, the aspirate is often superior for diagnosing many types of infections, especially bacterial abscesses. Sometimes a rapid and specific etiologic diagnosis is possible at the bedside, based on the microscopic features of the organism itself. However, when the organism is not readily apparent or its features are inconclusive, the microbiology laboratory can be invaluable for its role in isolation and identification.47
Summary
The successful treatment of pulmonary infections depends on accurate identification of the pathogen involved. In turn, this requires collecting the best specimens, transporting them to the anatomic and microbiology sections of the laboratory under optimal conditions, and processing them with techniques appropriate for the spectrum of possible etiologic disorders. An interdisiplinary approach enhances this process. It is in the best interest of all parties involved that pathologists, clinicians, and microbiologists communicate frequently and recognize the strengths and weaknesses of their respective disciplines. Joint strategies can be developed for the approach to certain types of suspected infections, helping to foster the development of laboratory “foresight” in surgical colleagues and medical consultants. As methods of diagnosis, treatment, and antimicrobial prophylaxis change, the pathologist must remain vigilant to a changing spectrum of etiologic agents and tissue injury patterns. The pathologist capable of integrating clinical and imaging data with morphologic and microbiologic findings can construct a comprehensive report useful for patient managment. The microbiology laboratory can be instrumental in delivery of more effective and efficient patient management if the microbiologist can capture this information, in view of its optimal position for choosing the appropriate combination of diagnostic methods (morphologic, culture, immunologic, molecular) for a particular type of sample. An example of such an operational protocol is presented in Box 6-4.
Bacterial Pneumonias
The surgical pathologist rarely receives biopsy specimens from patients with community-acquired or nosocomial pneumonias. Most of these infections are suspected clinically by symptoms and physical and radiologic findings; some are confirmed immediately by Gram stains (or later by culture) performed on respiratory secretions in the microbiology laboratory. Serologic studies sometimes prove to be diagnostic. Even when conventional microbiologic approaches are applied, however, approximately 50% of bacterial pneumonias remain undiagnosed.48–50 Patients with mild disease often are not tested but simply treated empirically with antibiotic regimens following established guidelines. By contrast, patients with severe disease, whether immunocompromised or not, often become candidates for invasive procedures.
Etiologic Agents
Bacterial pneumonia may be classified according to various parameters including pathogenesis, epidemiology, anatomic pattern, clinical course, and organism type51 (Box 6-5). Using bacterial type as a starting point allows the pathologist to correlate anatomic and histopathologic patterns of lung injury with categories of etiologic agents.
The pyogenic bacteria most commonly associated with community-acquired pneumonias include S. pneumoniae, H. influenzae, and Moraxella catarrhalis.50 Other pathogens such as Legionella species, Chlamydia pneumoniae, and Mycoplasma pneumoniae (often referred to as the “atypical” group) are clinically important, but controversy exists with regard to the relative frequency of these organisms as etiologic agents. Although community-acquired pneumonia is considered to be fundamentally different in children and in adults, severe or complicated pneumonias in both of these age groups are of similar etiology.52 The enteric gram-negative bacilli cause relatively few community-acquired pneumonias, whereas they account for most of the nosocomial pneumonias, along with Pseudomonas species, Acinetobacter species, S. aureus, and anaerobes.53,54Most nosocomial pneumonias result from aspiration of these bacterial species that colonize the oropharynx of hospitalized patients, and they are often polymicrobial. Any of the bacterial organisms listed (including mixtures with fungi and viruses) can cause pneumonia in immunocompromised patients.15,55 Ventilator-associated pneumonia is a special subset of nosocomial pneumonia and an important cause of morbidity and mortality in the intensive care unit.56–58 The bacterial etiology in this setting is quite diverse and dependent on such factors as patient characteristics, underlying lung disease, and geographic location.59 Most recently, an increase in skin and soft tissue staphylococcal infections due to methicillin-resistant strains has led to the recognition of these organisms as an important cause of both community-acquired and nosocomial pneumonia with attendant morbidity and mortality.60 In rare nosocomial pneumonias, a number of unusual organisms, such as Salmonella, Rhodococcus, and Leptospira species, may be the etiologic agent.61,62
The atypical pneumonia agents are those that do not commonly produce lobar consolidation. Although this potentially implicates a wide variety of bacterial, viral, and protozoal pathogens, a selective list by convention includes Mycoplasma pneumoniae, Legionella species, and C. pneumoniae as the three dominant nonzoonotic pathogens, and Coxiella burnetii (the agent of Q fever), Chlamydia psittaci (causing psittacosis in people), and F. tularensis (causing tularemia) as the three more common zoonotic pathogens.63,64
The filamentous/granule group refers to those bacteria that form long, thin, branching filaments in tissues, such as Actinomyces (anaerobic actinomycetes) or Nocardia (aerobic actinomycetes).65 Botryomycosis is caused by nonfilamentous bacteria, especially Staphylococcus aureus, or gram-negative bacilli, such as P. aeruginosa and E. coli, which form organized aggregates referred to as grains or granules.66
Histopathology
Bacterial lung injury patterns will vary in accordance with the virulence of the organism and the host response. These patterns are further modulated by therapeutic or immunologic factors. Although some of the patterns presented in Box 6-6 are characteristic, none are diagnostic. Overlap and mixed patterns occur.
Acute Exudative Pneumonia
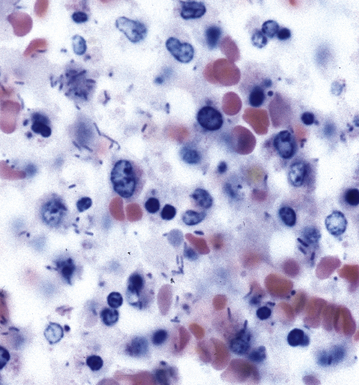
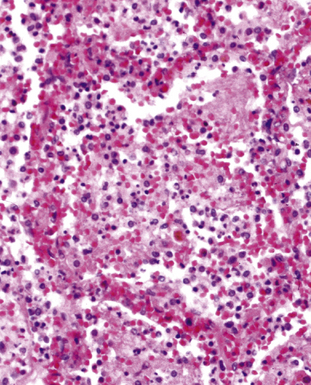
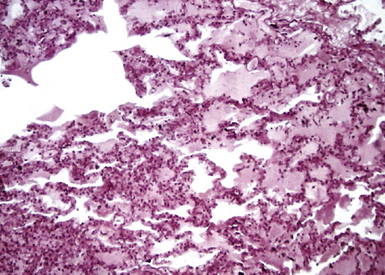
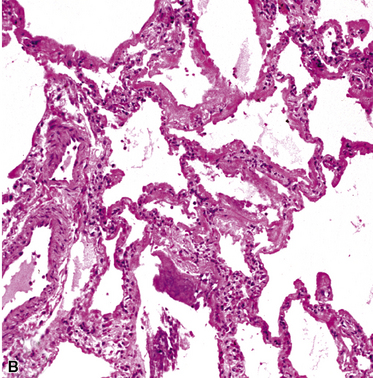
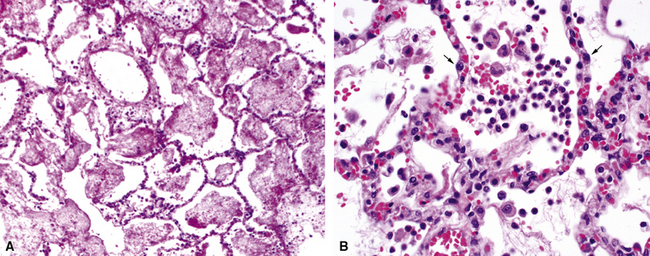
Acute exudative pneumonia most often is caused by pyogenic bacteria, such as streptococci, which typically produce a neutrophil-rich intra-alveolar exudate (i.e., alveolar filling) with variable amounts of fibrin and red cells. Pathologists recognize this constellation of findings as acute lobular pneumonia (Fig. 6-22), which usually correlates with patchy segmental infiltrates on the chest film (consolidation pattern on HRCT).29,67–69
With increasing organism virulence and disease severity, lobular exudates may become confluent (i.e., lobar pneumonia). In milder cases, the disease may be limited to the airways (bronchitis/bronchiolitis) with a mixed cellular infiltrate of mononuclear cells and neutrophils (Fig. 6-23). One very common manifestation of such airway-limited infection has been designated as “acute exacerbation of chronic obstructive pulmonary disease” (COPD). A majority of these exacerbations are caused by particular bacteria, specifically H. influenzae, S. pneumoniae, and M. catarrhalis, with approximately one third resulting from viral airway infections, typically resulting from rhinovirus, respiratory syncytial virus (RSV), and human metapneumovirus.70
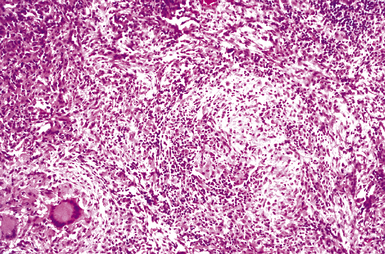
Nodular/Necrotizing Lesions
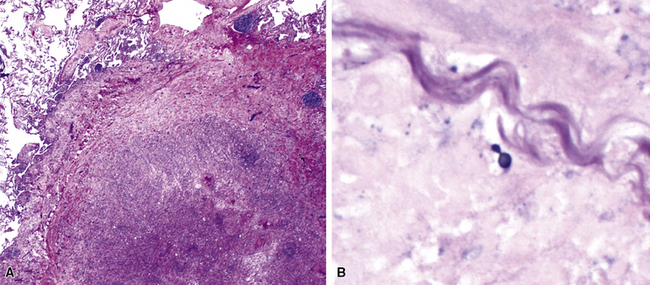
Nodular inflammatory infiltrates with or without necrotizing features (Fig. 6-24) are characteristic of infection by certain species, such as Rhodococcus equi (Fig. 6-25).71 Necrotizing pneumonias also may be produced by pyogenic bacteria such as Staphylococcus aureus, Streptococcus pyogenes, and the gram-negative bacilli—Klebsiella, Acinetobacter, Pseudomonas, and Burkholderia species.
Miliary Lesions
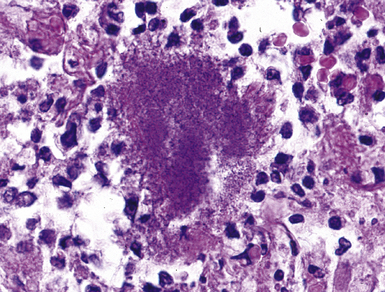
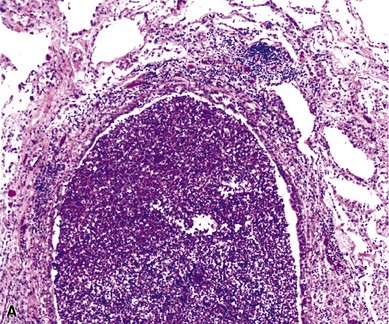
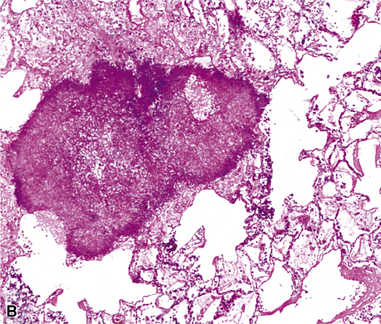
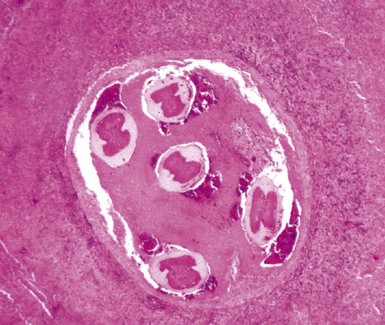
A subset of the nodular histopathologic pattern, miliary infection (Fig. 6-26), strongly implies pneumonia secondary to hematogenous spread of bacteria (septicemia). This pattern of infection can be seen with other organisms, such as Nocardia and the anaerobic actionomycetes. In these settings, histopathologic examination may show a hybrid reaction with both nodular disease and alveolar filling.
Aspiration Pneumonia and Lung Abscess
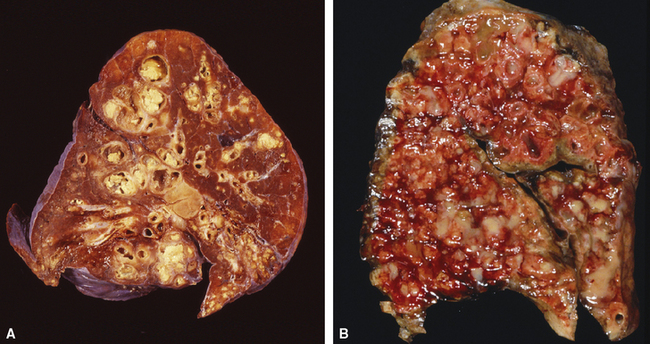
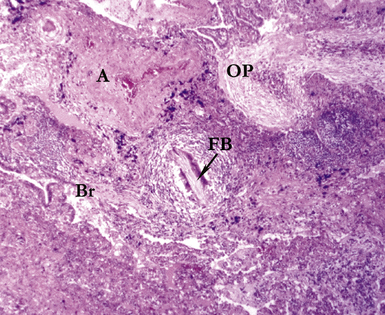
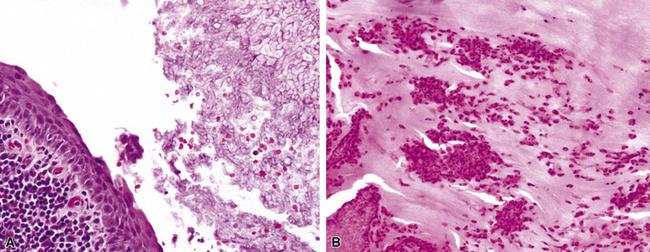
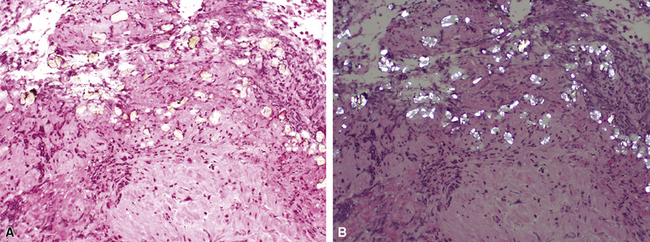
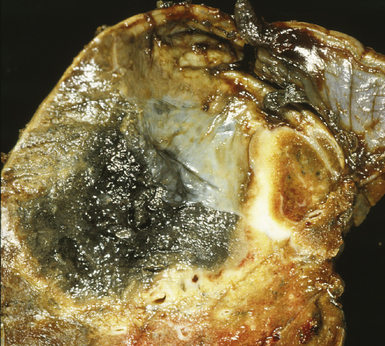
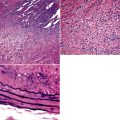
Several pulmonary aspiration scenarios are recognized, including those caused by chemical pneumonitis (so-called Mendelsson syndrome), airway obstruction, exogenous lipoid pneumonia, chronic interstitial fibrosis, diffuse bronchiolar disease, bacterial pneumonia, and lung abscess.72,73 Aspiration pneumonia refers specifically to aspiration of bacteria in oropharyngeal secretions and is classically a polymicrobial aerobic/anaerobic bacterial infection, with the bacterial species depending on whether the aspiration event occurs in the community or hospital setting. Recognition of food particles (so-called pulses) is key to the diagnosis. These may or may not be invested by giant cells but usually are found in purulent exudate or granulomatous foci. In the organizing phase of the pneumonia, food particles may be found within polyps of organizing pneumonia in the alveolar ducts and alveoli. Lobular pneumonia, lipoid pneumonia, organizing pneumonia, and bronchiolitis, alone or in combination, also may be seen.69,74 The pathogens in lung abscess (Fig. 6-27) usually encompass a polymicrobic mixture of aerobic and anaerobic bacteria,75 and formation of such abscesses most often is secondary to aspiration (Fig. 6-28). Infections due to Actinomyces species (Fig. 6-29) and Nocardia species also may manifest this pattern, as can those due to certain pyogenic bacteria, such as Staphylococcus aureus and the other organisms listed previously for necrotizing pneumonias. Granulomatous inflammation with foreign bodies may be present if aspiration is the cause (Fig. 6-30).
Chronic Bacterial Pneumonias
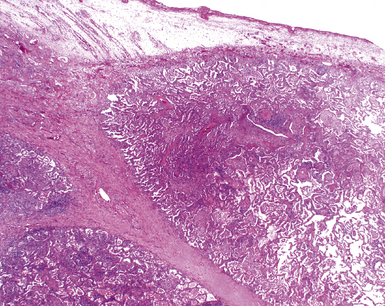
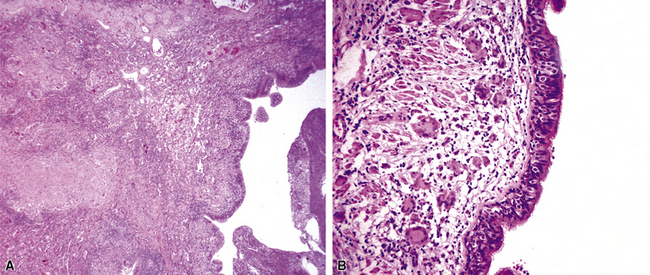
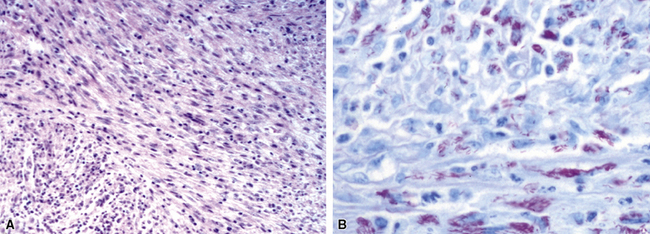
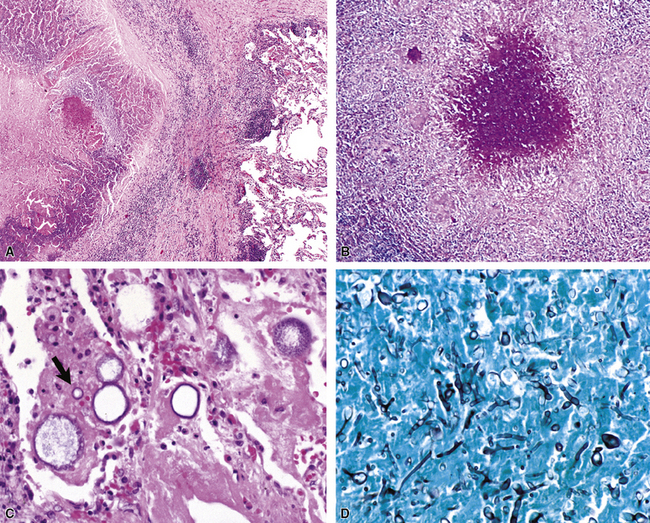
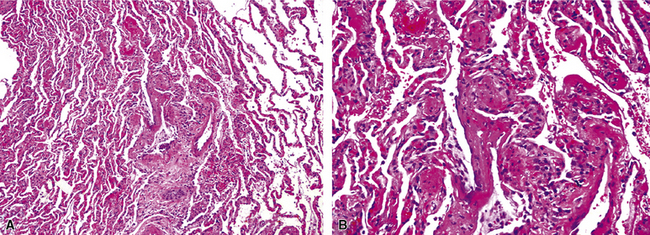
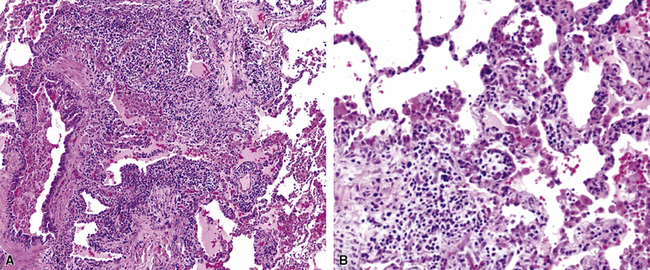
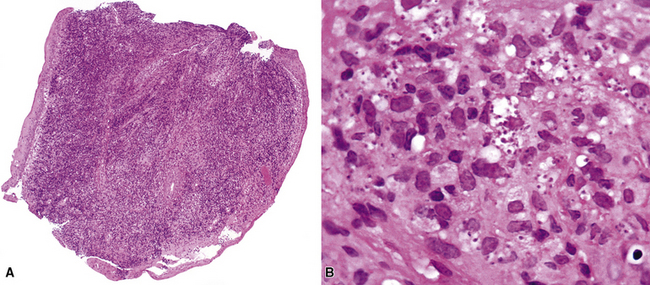
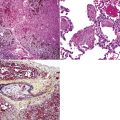
Chronic bacterial infections (Fig. 6-31) that are slow to resolve as a result of inappropriate initial therapy, involvement with certain microbial species, a noninfectious comorbid process, or an inadequate host response can produce a nonspecific fibroinflammatory pattern, with lymphoplasmacytic infiltrates, macrophages, or organization with polyps of immature fibroblasts in alveolar ducts and alveolar spaces.76–79 If not resorbed, polyps of air space organization may become polyps of intra-alveolar fibrosis, which sometimes ossify (dendriform ossification). Such scarring in chronic pneumonia often is associated with localized interlobular septal and pleural thickening (Fig. 6-32), producing a “jigsaw puzzle” pattern of scarring best seen at scanning magnification.
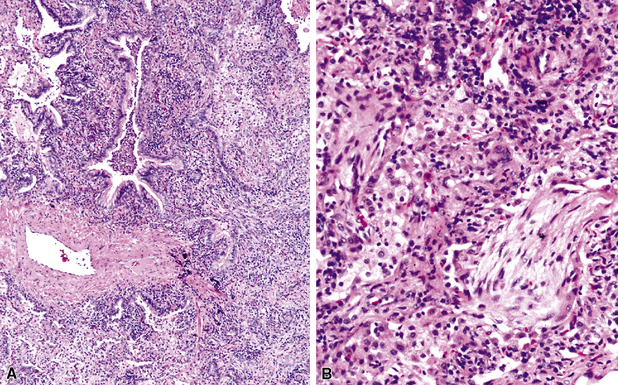
Figure 6-31 Chronic pneumonia.
A, lymphoplasmacytic infiltrate. B, fascicles of fibroblasts in alveolar ducts and spaces.
Diffuse alveolar damage is the histopathologic correlate of the acute repiratory distress syndrome (ARDS), and today, lung infection is the leading cause of diffuse alveolar damage and ARDS in the United States.80 Diffuse alveolar damage may coexist with any of the necroinflammatory patterns described earlier. The initial exudative phase of this process is accompanied by hyaline membranes (Fig. 6-33); the later organizing phase is attended by air space and interstitial fibroplasia. In clinical practice, diffuse alveolar damage accompanied by tissue necrosis is nearly always a manifestation of lung infection.
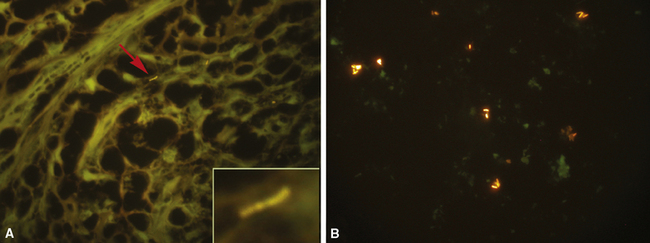
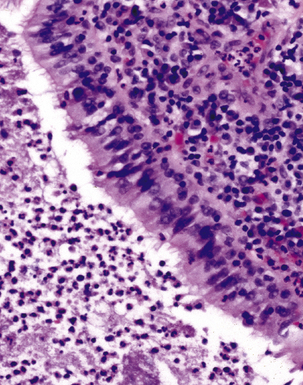
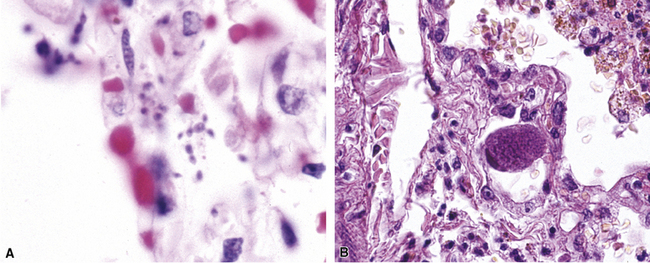
The atypical pneumonias include the well-described cases due to Legionella species and the less well-described cases caused by other organisims comprising the atypical group. Legionella infection typically results in an intensely neutrophilic acute fibrinopurulent lobular pneumonia64,67 (Fig. 6-34A). Legionella bacilli often can be identified in silver impregnation-stained sections (see Fig. 6-34B) or recovered in culture, but newer diagnostic methods, such as real-time PCR and in situ hybridization (Fig. 6-35) also can be applied when standard approaches fail.81 The histopathologic patterns associated with the other members of the atypical group (i.e., Chlamydia, Mycoplasma) are not well characterized, mainly because investigation of these pneumonias rarely includes biopsy. The few well-documented cases of Mycoplasma, Chlamydia, and Coxiella infections that have been described in the literature resemble viral bronchitis or bronchiolitis, with mixed inflammatory infiltrates in airway walls and in the adjacent interstitium82,83 (Fig. 6-36). Relative sparing of the peribronchiolar alveolar spaces has been described, although patchy organized fibrinous exudates are seen in some cases, and complications may superimpose additional findings.
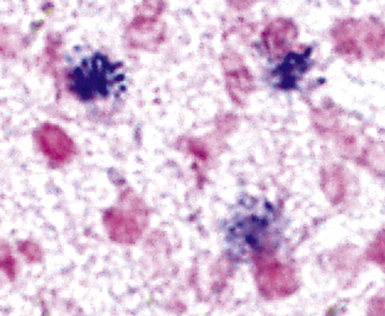
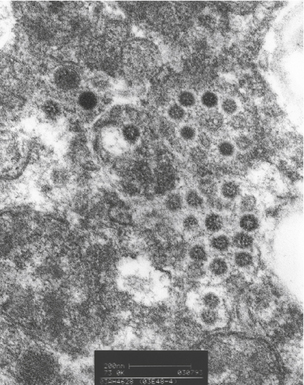
Figure 6-35 Legionnaire’s disease.
Detection of organisms by in situ DNA hybridization.
(Courtesy of R. V. Lloyd, MD, Rochester, MN.)
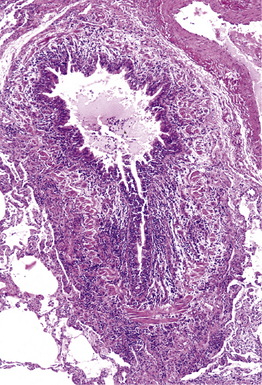
Figure 6-36 Mycoplasma pneumonia.
Bronchiolitis with patchy infiltrates in peribronchial interstitium.
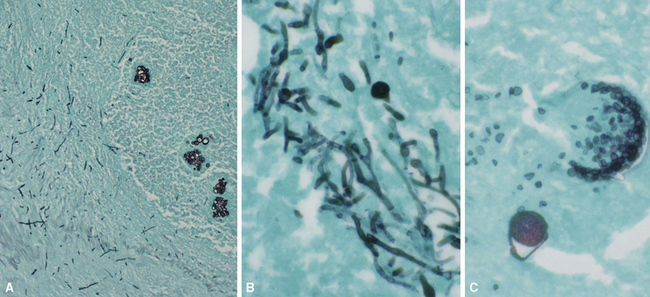
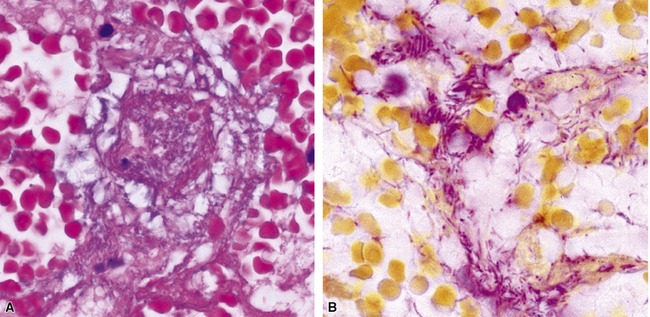
The grains and granules formed by the actinomycetes and bacteria of botryomycosis may have a uniform tinctorial hue on routine hematoxylin and eosin (H&E)–stained sections, but sometimes these bacterial aggregations display a distinctive body with a hematoxylinophilic core and an outer investment of eosinophilic material; formation of this array is referred to as the Splendore-Hoeppli phenomenon (Fig. 6-37). Actinomyces species tend to form similar-appearing granules, and both they and the bacteria of botryomycosis typically are found in the midst of purulent exudates.65,84–86 The Nocardia species may aggregate in colonies simulating granules, but with a much looser texture (Fig. 6-38) and more monochromatic tinctorial properties.87 Rarely, these colonies may be identical in appearance to the grains or granules of botryomycosis or actinomycosis in H&E sections.
Bacterial Agents of Bioterrorism
The potential for use of microbial pathogens as agents of bioterrorism requires that clinicians be alert to this possibility when community-acquired pneumonias are found to be caused by these agents. In turn, pathologists must become familiar with the histopathologic features these agents can produce.88 Respiratory disease caused by the inhalation of Bacillus anthracis, Yersinia pestis, and Franciscella tularensis is especially pertinent in this context and is discussed next.
Bacillus anthracis
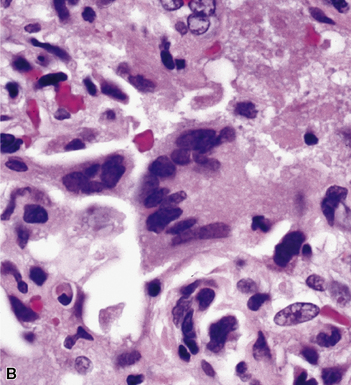
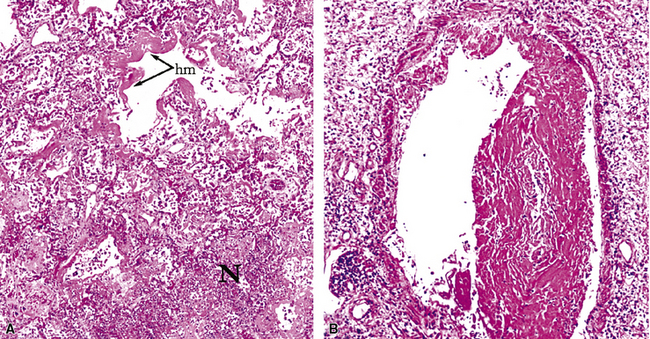
In 1877, Robert Koch’s conclusive demonstration that B. anthracis was the etiologic agent of anthrax revolutionized medicine by linking microbial cause and effect.7 Set against its historical importance to medicine, the recent use of anthrax as a bioterrorism agent represents a sad contrast. Inhalational anthrax causes a severe hemorrhagic mediastinitis.89–93 This pathologic process, in combination with the toxemia (B. anthracis produces an exotoxin with three potent components—protective antigen, lethal factor, and edema factor) from the ensuing massive bacteremia, severely compromises pulmonary function, leading to death in 40% or more of the cases. Pleural effusion may be present, but pneumonia generally is minor and secondary. In those patients in whom pulmonary parenchymal changes are found, the alveolar spaces contain a serosanguineous fluid with minimal fibrin deposits and some mononuclear cells, but few if any neutrophils.92 Large gram-positive bacilli (some may appear partially gram-negative) without spores, pervade the alveolar septal vessels, with a few in the alveolar spaces. This distribution suggests hematogenous rather than airway acquisition. Hemorrhagic mediastinitis in a previously healthy adult is essentially pathognomonic for inhalational anthrax. The lymph node parenchyma generally is teeming with intact and fragmented gram-positive bacilli, which can be identified as B. anthracis by immunohistochemical studies.91,92 Cultures of blood and pleural fluid, if available, are likely to yield the earliest positive diagnostic results.93 Sputum studies are much less useful in this regard.
Yersinia pestis
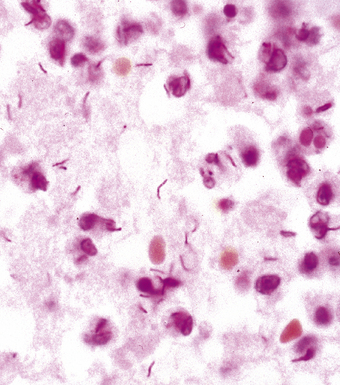
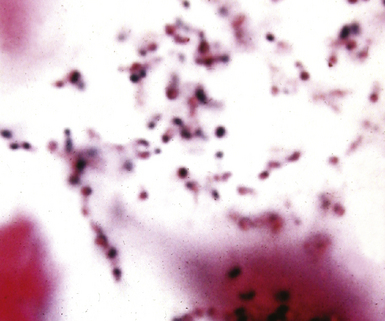
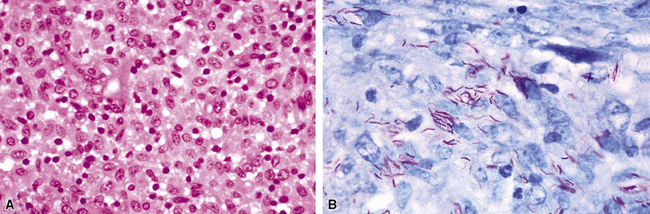
Primary pneumonic plague follows inhalation of Y. pestis bacilli in a potential bioterrorism scenario.94 The infection begins as bronchiolitis and alveolitis that progress to a lobular and eventual lobar consolidation.95 The histopathologic features evolve over time, beginning with serosanguineous intra-alveolar fluid accumulation with variable fibrin deposits (Fig. 6-39), progressing through a fibrinopurulent phase, and culminating in a necrotizing lesion.96 The presence of myriad bacilli in the intra-alveolar exudates, with significantly fewer organisms in the interstitium (a characteristic of primary pneumonia), is one of several pulmonary and extrapulmonary features used to distinguish primary from secondary pneumonic plague.97 These bacilli may be obvious in H&E-stained sections (Fig. 6-40) but generally are better visualized with Giemsa rather than Gram stain. Immunohistochemical staining provides a rapid and specific diagnosis.95 Unlike with inhalational anthrax, sputum Gram stain and culture are useful tests that are likely to yield a positive result at clinical presentation. Also, because sepsis is an integral component of the pneumonia, it is important to collect blood culture specimens.
Francisella tularensis
Inhalation of F. tularensis bacilli, following a bioterrorism aerosol release, generally is expected to result in a slowly progressing pneumonia, with a lower case-fatality rate than with either inhalational anthrax or plague.97 Initially, a hemorrhagic and ulcerative bronchiolitis is followed by a fibrinous lobular pneumonia with many macrophages but relatively few neutrophils (Fig. 6-41). Necrosis then supervenes and evolves into a granulomatous reaction. The small, gram-negative coccobacillary organisms are difficult to identify in a tissue Gram stain, and the use of silvering techniques (e.g., Steiner, Dieterle, Warthin-Starry) is required to enhance their silhouette.98 Specific fluorescent antibody testing for formalin-fixed tissue and immunohistochemical studies also are available through public health laboratories. In the microbiology laboratory, Gram stain and culture of respiratory secretions are useful for diagnosis, but blood culture results are not often positive. Antigen detection and molecular techniques, such as PCR amplification, can be used to identify F. tularensis. Serologic tests are available but probably would not provide timely information in an outbreak situation.97
Cytopathology
The stereotypic cellular response to pyogenic bacteria is acute inflammation, characterized by variable numbers of neutrophils. Bacteria may be visualized in various stained preparations made from respiratory tract secretions and washings using the Papanicolaou and Diff-Quik methods.43 The clinical significance is rather limited in these specimens owing to the potential contamination by oral flora and the problem of distinguishing colonization from infection. However, when the upper respiratory tract can be bypassed, by means of either transtracheal or transthoracic needle aspiration, the presence of bacteria becomes much more significant, especially when sheets of neutrophils or necroinflammatory debris are present (Fig. 6-42A), as would be the case with a typical lobar or lobular consolidation, lung abscess, or other complex pneumonia.49,86,99,100 In this context, transthoracic needle aspiration can establish the etiologic diagnosis of community-ascquired and nosocomial pneumonias in both children and adults when coupled with modern microbiologic methods.47,54,101,102 Proponents consider it an underutilized technique whose potential benefits, in experienced hands, outweigh the modest associated risks.
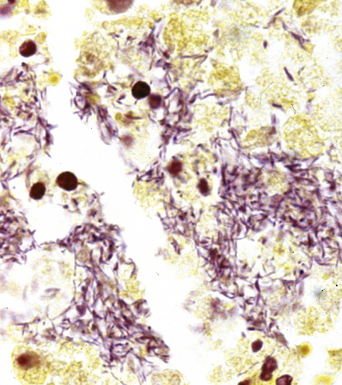
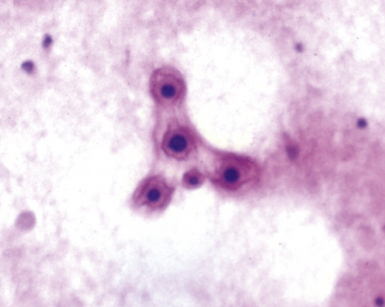
Many types of bacilli and cocci can be seen within and around neutrophils on Diff-Quik–stained smears (see Fig. 6-42B). A smear also can be prepared for Gram stain and the aspirate needle rinsed in nonbacteriostatic sterile saline or nutrient broths for culture. The size (length and width) and shape of organisms and the Gram reaction allow rough categorization of organisms into groups such as enteric-type bacilli, pseudomonads, fusiform anaerobic-type bacilli, tiny coccobacillary types suggestive of the Haemophilus–Bacteroides group (Fig. 6-43), or gram-positive cocci.103 Branching filamentous forms suggest actinomycetes or Nocardia organisms (Fig. 6-44), with the latter distinguished by being partially acid-fast.104,105 Extreme care must be exercised in the staining laboratory to prevent contamination of staining solutions, because this can be a cause of false-positive results.
Microbiology
Microbiology techniques in current use for the laboratory diagnosis of bacterial pneumonia are summarized in Box 6-7.106–108 The traditional morphologic and functional approach to microbiologic diagnosis is gradually shifting to molecular methods, but their routine application continues to be a hope for the near future.
The workup of respiratory secretions, such as sputum, in the microbiology laboratory may or may not be indicated, based on the clinical and immunologic status of the patient. Certainly, the value of this workup for community-acquired pneumonias has been questioned for some time, and the guidelines from two specialty societies—the American Thoracic Society and the Infectious Disease Society of America—differ in this regard.109–111 Nevertheless, when a carefully collected specimen reveals one or two predominant bacterial morphotypes on a well-prepared Gram stain (Fig. 6-45), especially in the presence of neutrophils and few or no squamous cells, a presumptive diagnosis can be offered and correlated with whatever grows on culture plates.112,113 A mixed bacterial population usually is considered nondiagnostic, especially in the absence of inflammation or the presence of many benign oral squamous cells. By contrast, pneumonia in the hospitalized or immunocompromised patient requires an aggressive strategy to collect a good sputum sample for Gram stain and culture. If this attempt is unsatisfactory or the findings are nondiagnostic, then use of invasive techniques beginning with fiberoptic bronchocopy and BAL with protected catheters should be considered.56,58,114 Anaerobic pulmonary infections, typically in the form of a lung abscess, also can be approached in this way or with transthoracic needle aspiration.75
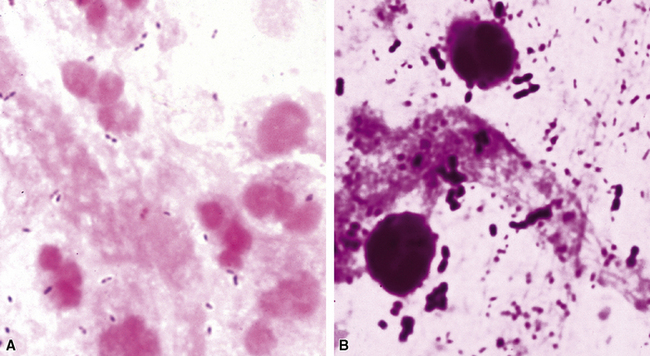
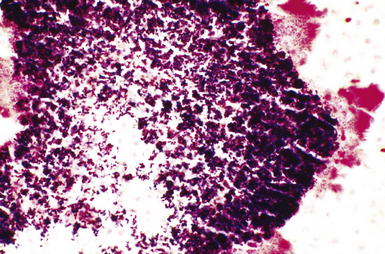
In those cases in which bacteria are visible on H&E-stained sections, the Gram stain is especially helpful in confirming a presumptive etiology. For example, pairs and chains of gram-positive cocci in a necroinflammatory background suggest a streptococcal pneumonia, whereas numerous slender gram-negative bacilli investing and infiltrating blood vessels are characteristic of a Pseudomonas pneumonia (Fig. 6-46). Other types of gram-negative pneumonias (Fig. 6-47) also can be confirmed with well-prepared Gram stains.77 In the case of an abscess, a mixture of gram-positive cocci and gram-negative bacilli in tissue (illustrated earlier in Fig. 6-28) is a useful finding that is helpful in supporting a diagnosis of an anaerobic infection.
When organisms are sparse, other stains such as Giemsa or silver impregnation may highlight the organisms in the exudates (Fig. 6-48). The Gram stain also is useful for evaluating infections with granules and allows differentiation of the agents of botryomycosis (the gram-positive cocci or gram-negative bacilli) from the filamentous Actinomyces organisms (Fig. 6-49).
Staining with methenamine silver is the best procedure for detecting Nocardia organisms. The modified Ziehl-Neelsen stain allows for differentiation of Nocardia (positive) from the anaerobic Actinomyces (negative).105
Commercially available immunohistochemical reagents exist for relatively few bacterial species. Immunohistochemistry testing for the potential bioterrorist agents discussed in this chapter is available through the Centers for Disease Control and Prevention (CDC) in Atlanta, Georgia. It is expected that commercial reagents will become increasingly available for the common etiologic agents in the near future.32
Culture media that will allow recovery of common bacterial species causing pneumonia from various types of respiratory samples (secretions, washings, brushings, aspirates, and tissues) include sheep blood agar, chocolate agar, and McConkey agar. These media also will support growth of B. anthracis and Y. pestis. Buffered charcoal yeast extract (BCYE) agar is the primary medium for Legionella species. Because Legionella organisms survive poorly in respiratory secretions, rapid transport and immediate plating is essential for recovery. BYE also is a good “all-purpose” medium for growing other fastidious species including F. tularensis. However, F. tularensis grows best in cysteine-enriched media.115
The actinomycetes are best isolated from invasive specimens such as needle aspirates and transbronchial and lung biopsy specimens. The laboratory should be alerted to search for these agents because special consideration must be given to culture setup and incubation conditions.85 The actinomycetes responsible for actinomycosis require anaerobic media and atmosphere as well as prolonged incubation. Nocardia, an aerobic actinomycete, grows well on most nonselective media but requires extended incubation. Determination of colonial morphology, Gram and acid-fast stains, and a few biochemical tests generally suffice to identify these organisms at the genus level. However, genotype rather than phenotype characteristics are required to identify newly emergent species.116
In general, the laboratory diagnosis of pneumonia caused by most of the atypical agents is difficult because systems are not routinely available or are costly, cumbersome, or unsafe. For the atypical agents (Mycoplasma, Chlamydia, and Coxiella species), serologic testing has been the method of choice for diagnosis.63,117 Classic cold agglutinin and complement fixation tests for these agents have largely been replaced by enzyme immunoassay and microimmunofluorescence testing.83,118,119 Serologic methods also are useful for diagnosis of tularemia because of the difficulty in culturing the fastidious bacterium.
Legionella pneumonia is a common form of severe pneumonia not readily diagnosed for a number of reasons, including the organism’s fastidiousness.120 In the microbiology laboratory, the direct fluorescent antibody test and culture on buffered BCYE agar have been the mainstays of diagnosis. Culture is considered the diagnostic gold standard but is only 60% sensitive. Serologic testing is available for most of the L. pneumophila serotypes, which account for 90% of the pneumonia cases; however, the need to collect paired sera weeks apart limits its usefulness in the acutely ill patient. Antigen detection in urine has become commercially available for both L. pnemophila and S. pneumoniae, and because the need to collect acute and convalesent sera is obviated, it has become a frequently used diagnostic test.120,121 Its advantage lies in its potential to effect early treatment decisions through rapid diagnosis. Its disadvantage lies in the fact that it identifies only patients infected with L. pneumophila serogroup 1 (LP1), the most prevalent species and serotype, but none of the non-LP1 serotypes, or cases due to other Legionella species.122–124
The use of molecular diagnostic tools (in situ hybridization and nucleic acid amplification by PCR or other methods) to detect these agents has been reported.81,124,125 Real-time PCR assay appears promising as a sensitive, specific, and rapid diagnostic technique that is likely to find routine clinical application. It provides a platform for the simultaneous amplification and detection of target DNA in a single tube through use of one of several types of fluorescence resonance transfer (FRET) fluorescent probe quencher techniques or melting curve analysis. Furthermore, it obviates the concern for amplicon contamination in the laboratory.126 The development of a multiplex assay, to detect multiple agents in a single reaction, would seem to be an ideal pursuit for the laboratory diagnosis of the most common community-acquired pneumonias including those due to the atypical pneumonia agents.35,127,128
Differential Diagnosis
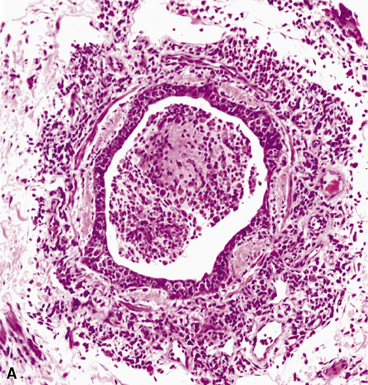
The key morphologic and microbiologic features of the bacterial pneumonias are summarized in Table 6-5. The presence of purulent exudates or significant numbers of neutrophils in biopsy or cytologic samples should always trigger a search for bacterial infection. Of note, however, because lung biopsies usually are performed late in the clinical course with respect to an evolving infiltrate, after many procedures have been performed and bacterial infections have been excluded or treated with antibiotics, neutrophilic exudates may not signify bacterial infection unless accompanied by necrosis, as in an abscess. Instead, consideration should be given to one of several noninfectious acute inflammatory diseases, with an immunologic basis, that can mimic bacterial infection. Some of these include Wegener granulomatosis, Goodpasture syndrome, systemic lupus erythematosus, and microscopic polyangiitis, all conditions that can produce acute inflammation predominantly involving alveolar septal blood vessels (“capillaritis”). On occasion, capillaritis can result in air space accumulation of neutrophils, further raising concern for bronchopneumonia. Centrally necrotic or cavitary neoplasms of various types may mimic abscesses grossly and microscopically, and exceptionally well-differentiated adenocarcinomas containing glands filled with detritus may mimic inflammatory and bacterial diseases. Suppurative granulomas can have a bacterial, mycobacterial, or fungal etiology. Even the miliary necroinflammatory lesion typical of bacterial infection can be produced by viruses, some fungi, and even protozoa (e.g., Toxoplasma gondii).
Table 6-5 Bacterial Pneumonias: Summary of Pathologic Findings
| Assessment Component | Findings |
|---|---|
| Pyogenic Bacteria | |
| Surgical pathology | Acute purulent inflammation with/without necrosis; organization; diffuse alveolar damage may be present |
| Cytopathology | Acute inflammation with/without visible bacteria on Diff-Quik–stained smear |
| Microbiology | Gram stain reactivity and morphology (visual detection requires heavy bacterial burden: 106 organisms/gram of tissue) Culture-sterile lung tissue on standard nonselective and selective media (blood, chocolate, MacConkey agars); anaerobic broth and agars for abscesses Urinary antigen for Streptococcus pneumoniae |
| Atypical Pneumonia Agents | |
| Surgical pathology | Legionella pneumonia: fibrinopurulent with bacilli visible in silver-stained (Dieterle; Warthin-Starry) sections DAD often present Chlamydia and Mycoplasma infection: polymorphous bronchiolar and interstitial infiltrate |
| Cytopathology | Acute inflammation with bacilli stained with silver or by immunofluorescence (Legionella pneumonia) |
| Microbiology | DFA for L. pneumophila serotypes Culture on selective (BCYE) agar for Legionella; urinary antigen for Legionella Serologic testing and/or PCR assay for Mycoplasma and Chlamydia |
| Filamentous-Granule Group | |
| Surgical pathology | Granules or loose filamentous aggregates in purulent exudate with abscess formation and poorly formed granuloma in some cases |
| Cytopathology | Filamentous tangles or aggregates or granules with neutrophils and/or necroinflammatory background |
| Microbiology | Gram-positive branching filaments: Nocardia (aerobic actinomycete) and Actinomyces (anaerobic actinomycete) Nocardia partially acid-fast and GMS-positive Gram-positive cocci or gram-negative bacilli (botryomycosis) Culture on standard nonselective media and selective (BCYE) media; anaerobic culture broths and media for Actinomyces |
BCYE, buffered charcoal yeast extract; DAD, diffuse alveolar damage; DFA, direct fluorescence assay; GMS, Grocott methenamine silver.
Mycobacterial Infections
The surgical pathologist tends to encounter mycobacterial infections in lung biopsies when standard clinical diagnostic approaches to pulmonary infiltrates are unsuccessful and the lesions persist or progress. Tuberculosis is but one of several different types of lung infection that can manifest clinically as community-acquired pneumonia, resulting in delay until an invasive procedure such as transbronchial biopsy, transthoracic needle biopsy, or surgical lung biopsy is performed, often a “last resort” effort.129,130 In recent years, delays in diagnosis of mycobacterial infection have markedly decreased, thanks in part to recommendations from the CDC for improving laboratory turnaround time and to the response of the diagnostics industry with better methods and technology. In fact, however, because direct acid-fast bacillary smears of respiratory specimens yield negative findings in at least one half of the cases,131 and because many mycobacterial species are fastidious and slow-growing, the biopsy results may be the first suggestion of a mycobacterial infection. The biopsy findings also can define the organism’s relationship to a histopathologic lesion, or host response. This is important in evaluating the significance of a culture result, because although an isolate of M. tuberculosis is always taken seriously, obtaining a single isolate of a nontuberculous mycobacterium from the respiratory tract does not necessarily implicate the organism as the cause of disease.132
Etiologic Agents
Mycobacterium tuberculosis
M. tuberculosis is the most virulent mycobacterial species and an unequivocal pathogen that is responsible for more deaths worldwide than any single microbe. This organism is the etiologic agent of tuberculosis worldwide in its various forms, which are listed in Box 6-8.
Box 6-8 Classification of Tuberculosis
Data from Allen E. Tuberculosis and other mycobacterial infections of the lung. In: Churg AM, Thurlbeck WM, eds. Pathology of the Lung, 2nd ed. New York: Thieme; 1995:233, Table 13-1.
Primary tuberculosis occurs in patients without previous exposure or loss of acquired immunity. Progressive primary tuberculosis occurs in patients with inadequate acquired immunity, that is, impaired cellular immunity. Post-primary tuberculosis, also referred to as secondary or reinfection-reactivation tuberculosis, occurs in patients with previous immunity to the organism and accounts for most clinical cases of tuberculosis.133,134 Many clinical experts consider that most cases of active tuberculosis in adults with normal immunity arise from reactivation of latent infection (post-primary tuberculosis), whereas reinfection with a new strain derived from the environment (primary or post-primary tuberculosis) can occur in the immunocompromised patient. More recently, DNA fingerprinting methods (genotyping) have challenged this dogma, however, by showing that exogenous reinfection accounts for a significant percentage of cases in some areas of the world.135 Miliary tuberculosis and extrapulmonary disease can occur with any of these forms.133,136
Primary tuberculosis usually is a mild illness that often is not clinically recognized. Of note, however, the bacillemia that occurs during its development can seed extrapulmonary organs and set the stage for subsequent reactivation. Approximately 5% of patients pass through latency to post-primary disease within 2 years of primary infection, and another 5% do so later in their lives.137
Non-Tuberculous Mycobacteria
Recognized NTM species number more than 125, many of which were identified during the past decade.138,139 However, relatively few cause pulmonary disease.132,140–142 These organisms are acquired from the environment, where they are ubiquitous. In contrast with M. tuberculosis, the NTM are not spread from person to person. In most instances, patients in whom NTM infection develops have chronic lung disease and other risk factors, such as AIDS, alcoholism, or diabetes. Reports of NTM infections in non-immunocompromised patients are increasing.17,143 MAC and then Mycobacterium kansasii are the most frequent isolates in all settings. Among a growing number of species causing lung disease are Mycobacterium abscessus, Mycobacterium fortuitum, Mycobacterium szulgai, Mycobacterium simiae, Mycobacterium xenopi, Mycobacterium malmoense, Mycobacterium celatum, Mycobacterium asiaticum, and Mycobacterium shimodii. These latter species manifest marked geographic variability with respect to prevalence and severity. Of note, however, since 1985, more MAC isolates than M. tuberculosis have been reported in the United States.132
Histopathology
The histopathologic patterns produced by mycobacteria are listed in Box 6-9. The radiologic, gross, and microscopic patterns of mycobacterial disease reflect the virulence of the various mycobacterial species, as well as the patient’s prior exposure and immune status.144–146
Box 6-9 Histopathologic Patterns in Mycobacterial Lung Injury
Primary Tuberculosis
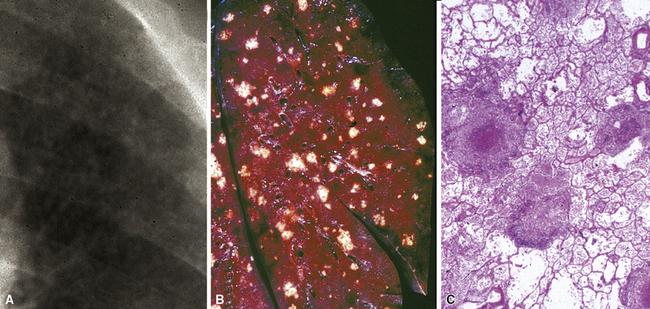
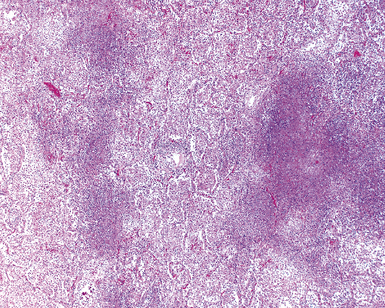
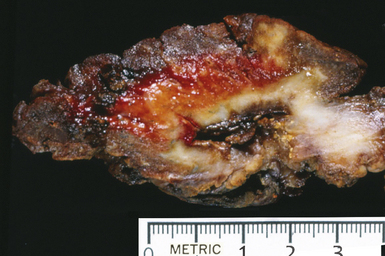
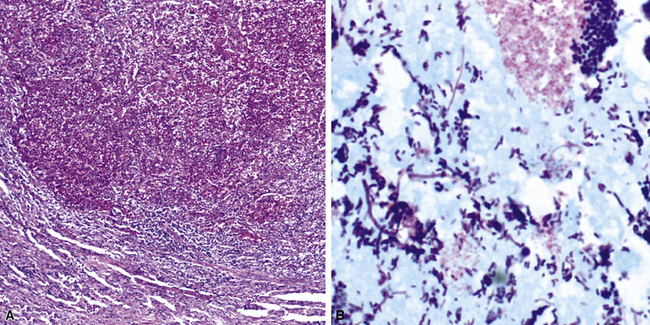
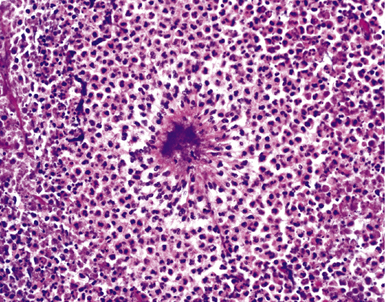
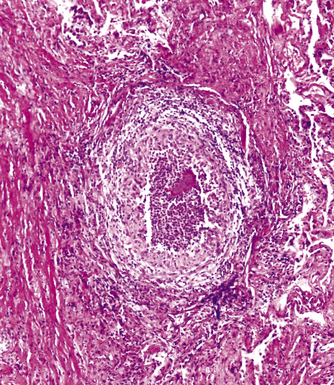
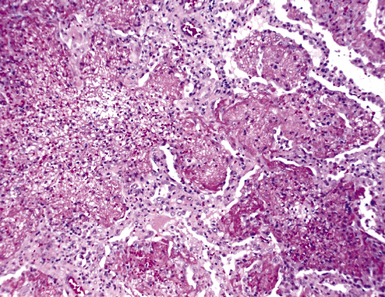
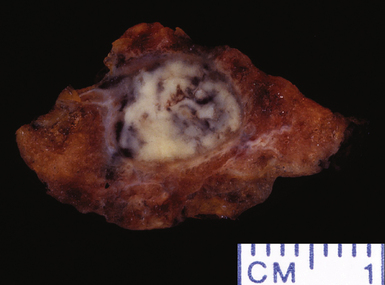
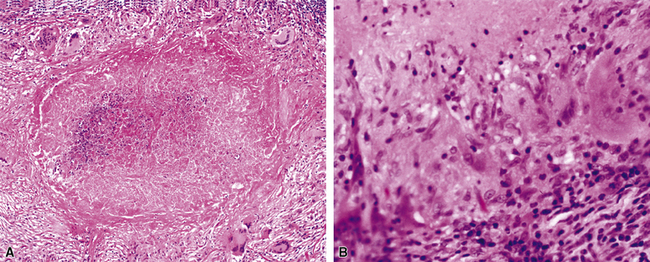
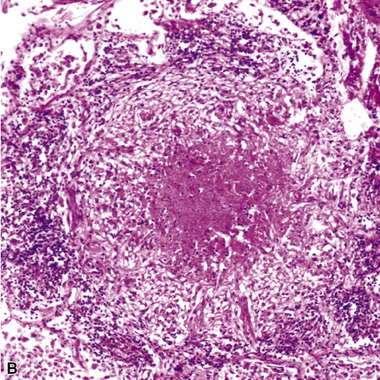
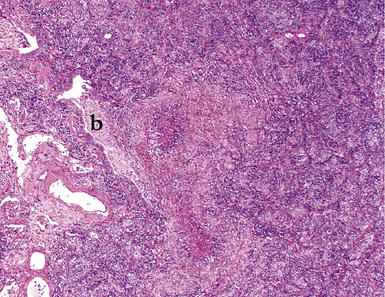
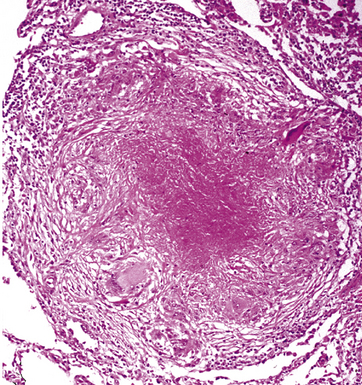
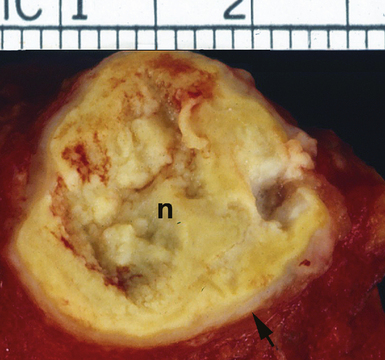
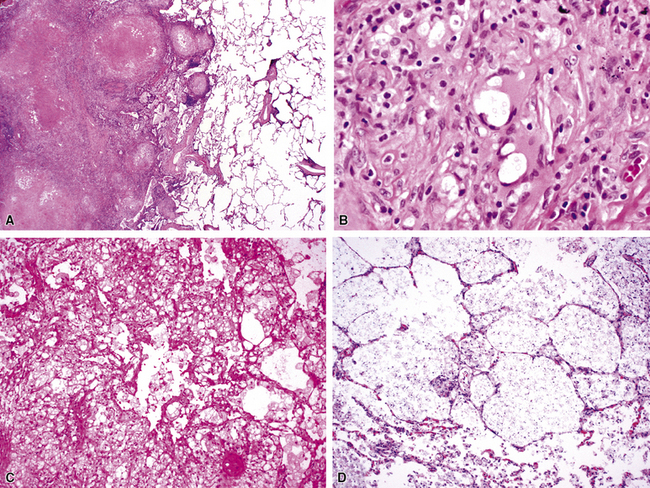
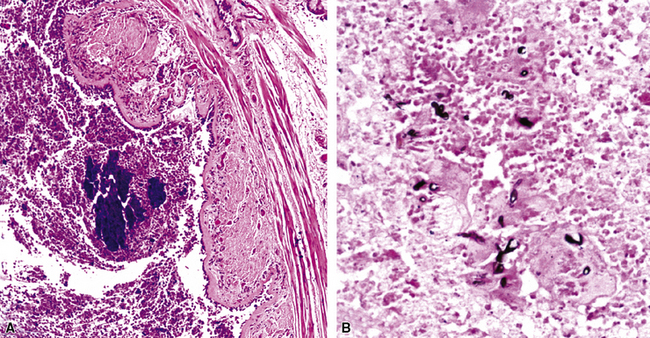
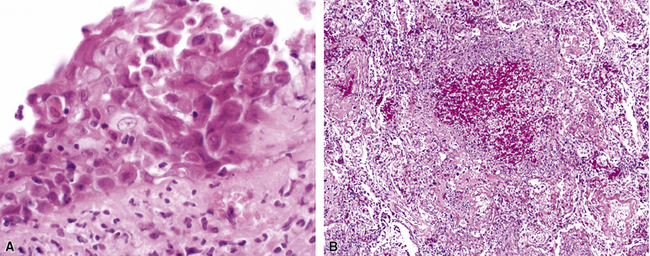
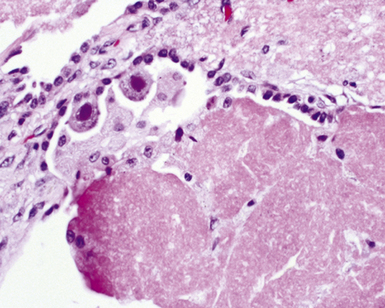
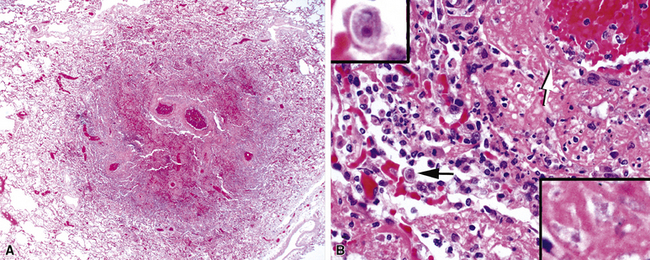
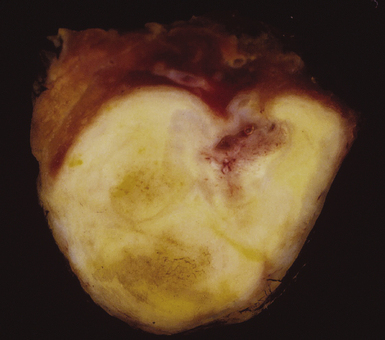
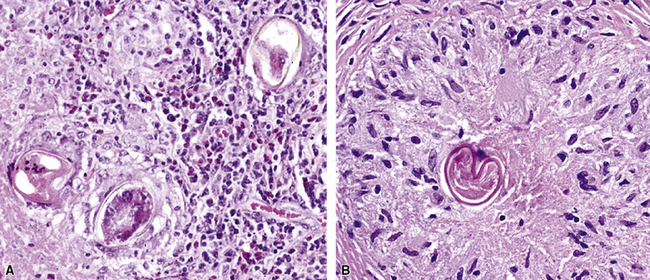
Mycobacterium tuberculosis occurs typically in the best-aerated lung regions (anterior segments of the upper lobes, lingua and middle lobe, or basal segments of lower lobes.145 The disease passes through progressive phases of exudation, recruitment of macrophages and T lymphocytes, and granuloma formation followed by repair with granulation tissue, fibrosis, and mineralization.134,147 Macrophage-laden bacilli also travel to the hilar lymph nodes, where the phases are repeated. This combination of events produces the classic Ghon complex, consisting of a peripheral 1- to 2-cm lung nodule (Fig. 6-50) and an enlarged, sometimes calcified hilar lymph node. In both locations, the histopathologic hallmark is a necrotizing granuloma (Fig. 6-51) composed of epithelioid cells with variable numbers of Langhans giant cells, a peripheral investment of lymphocytes, and a central zone of caseation necrosis, a form of necrosis attributed to apoptosis.133,148 A spectrum of lesions may be seen, from the tuberculoid “hard” granuloma without necrosis and rare organisms, to the multibacillary necrotic lesion with scant epithelioid cells.149 In a minority of patients the lesions enlarge and progress as a result of increased necrosis or liquifaction.
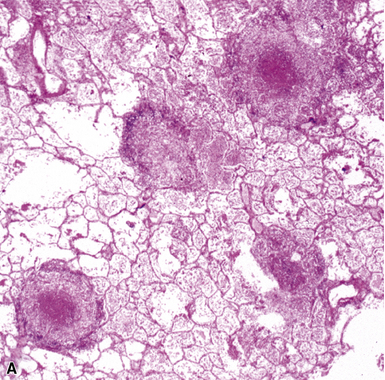
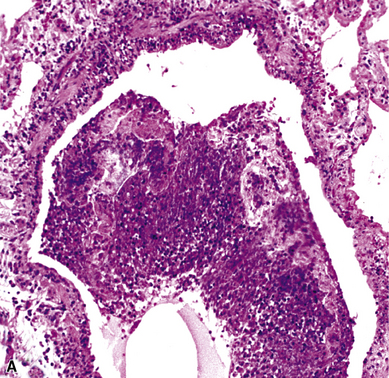
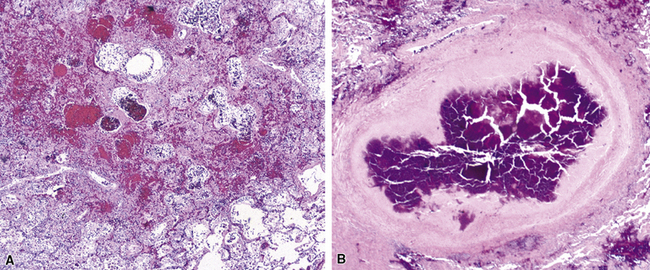
The complications of tuberculosis are listed in Box 6-10 and illustrated in Figure 6-52. Other complications may include extension into blood vessels with miliary (Fig. 6-53) or systemic dissemination, lymphatic drainage into the pleura with granulomatous pleuritis and effusions, or to bronchi with bronchocentric granulomatous lesions (Fig. 6-54) or tuberculous bronchopneumonia. Granulomas also may encroach upon blood vessels, mimicking a “granulomatous” vasculitis. The hemophagocytic syndrome, which has been implicated in a variety of bacterial, viral, and parasitic infections, also has been associated with tuberculosis.150
Post-primary Tuberculosis
Post-primary tuberculosis, the most common form in adults, typically involves the apices of the upper lobes, producing granulomatous lesions with greater caseation, often with cavities and variable degrees of fibrosis and retraction of the parenchyma.136 Fibrosis and bronchiectasis occurs with the healing of cavities and is the major cause of pulmonary disability in this disease.151 Recent studies have proposed that post-primary disease begins as a form of lipoid pneumonia, with bacilli-laden foamy alvolar macrophages and bronchiolar obstruction progressing to cavitary disease, as a result of caseation, and microvascular occusion due to delayed-type hypersensitivity.152 Extension to other lobes, hilar or mediastinal lymph nodes and miliary spread through the lungs and to extrapulmonary sites can complicate this form of disease. Other presentation patterns include acute and organizing diffuse alveolar damage with advanced or miliary disease, acute tuberculous bronchopneumonia, and the solitary pulmonary nodule (tuberculoma). A proximal endobronchial form may mimic a neoplasm and also is noteworthy for extensive necrosis and often large numbers of bacilli.153 Because characteristic granulomatous morphology may not be visible around the necrotic material, stains for mycobacteria should be considered for all necrotic endobronchial samples.
Nontuberculous Mycobacterial Infections
NTM infections may be similar to those due to M. tuberculosis, but certain differences have been noted. For example, the NTM pathogens do not cause the same sequence of primary or post-primary disease, and systemic dissemination does not occur except in the immunocompromised patient. M. kansasii is more virulent than MAC, and the infection-associated histopathologic pattern is more like that produced by M. tuberculosis.154
Infections due to MAC and other common pulmonary NTM pathogens generally manifest as one of five clinicopathologic entities: solitary pulmonary nodule, chronic progressive pulmonary disease, disseminated disease, chronic bronchiolitis with bronchiectasis, and hypersensitivity-like pneumonitis.134,155 Solitary pulmonary nodules generally exhibit granulomas resembling those caused by M. tuberculosis.
Chronic progressive disease also resembles tuberculosis, with upper lobe thin-walled cavities and granulomatous inflammation, with or without caseous necrosis (Fig. 6-55). Multiple confluent granulomas in fibrosis can mimic sarcoidosis. Organisms usually are sparse and more diffiult to find in the immunocompetent patient. This presentation most often is seen in patients with underlying chronic lung disease such as COPD, bronchiectasis, cystic fibrosis, pneumoconiosis, reflux disease, or pre-existing cavitary lung disease of any cause (including old tuberculous cavities).
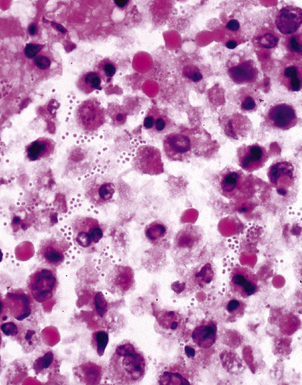
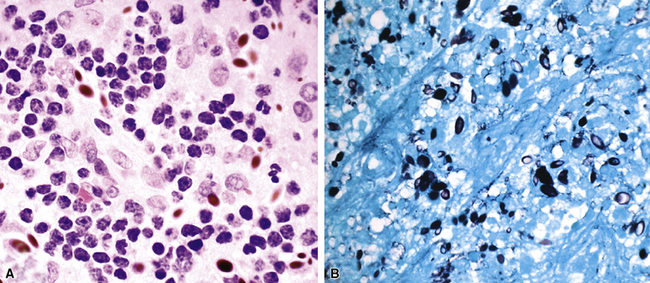
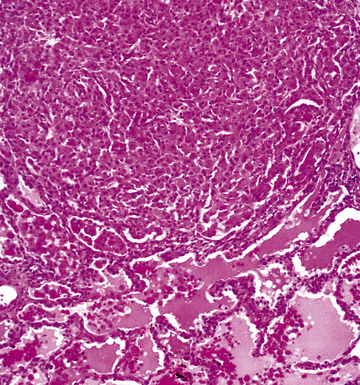
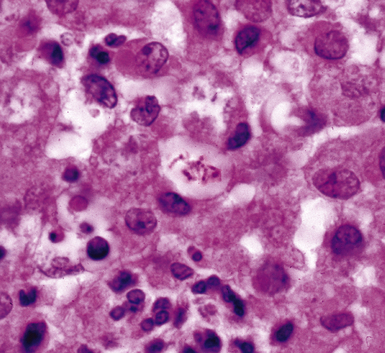
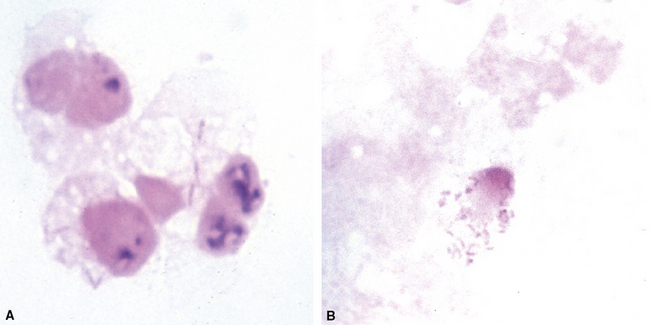
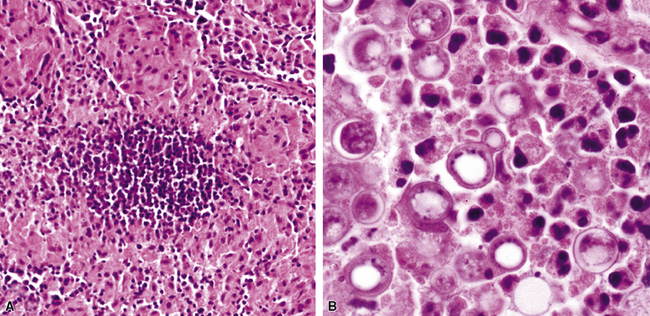
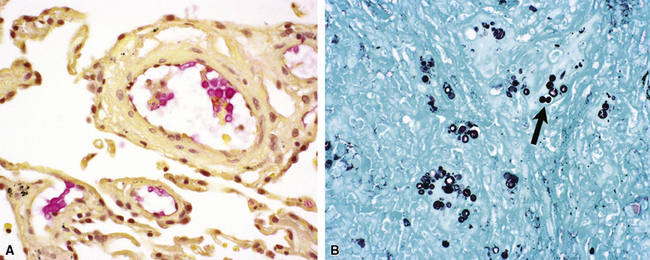
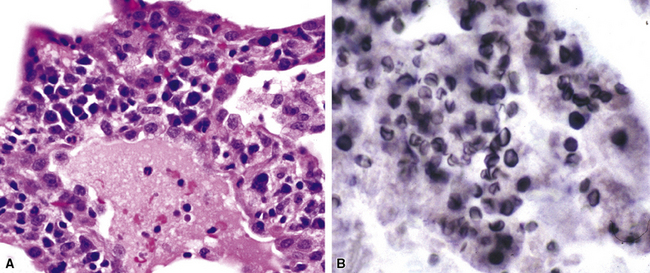
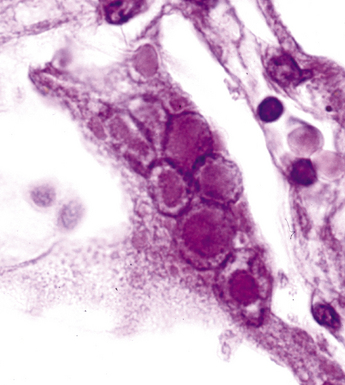
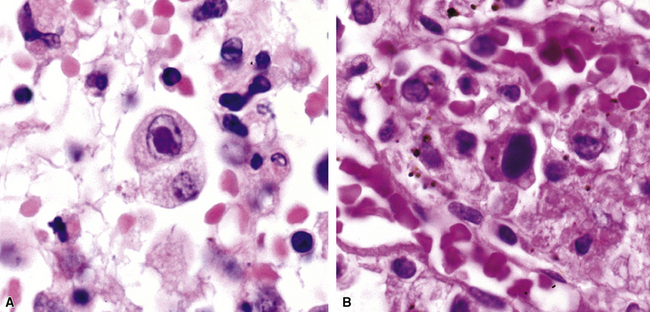
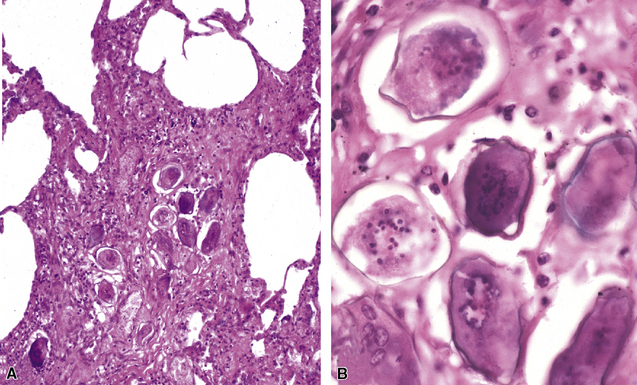
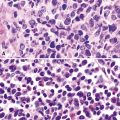
Disseminated disease typically is associated with the immunocompromise produced by HIV infection, in which the disease tends to target the gastrointestinal tract (the likely portal of entry), and pulmonary and reticuloendothelial disease signifies dissemination.156 In this setting, NTM bacilli (predominantly MAC) proliferate characteristically to high levels in poorly formed granulomas, or in sheets and clusters of plump, finely vacuolated macrophages (“pseudo-Gaucher” cells) containing abundant phagocytosed intracytoplasmic bacilli (Fig. 6-56).
A distinctive form of NTM disease occurs as the “Lady Windermere syndrome.” In the classic clinical scenario, an elderly, nonsmoking, immunocompetent woman of particular habits, demeanor, and body type presents with multiple pulmonary nodules, preferentially involving the middle lobe and lingula. The airway-centric granulomas and bronchiectasis can be subtle or pronounced (Fig. 6-57); this has been recognized as one of the patterns of middle lobe syndrome.157 NTM bacilli also can colonize bronchiectatic lung from any cause, with resultant granulomatous inflammation predominantly affecting the airway walls—presumably a result of localized decreased mucociliary clearance.
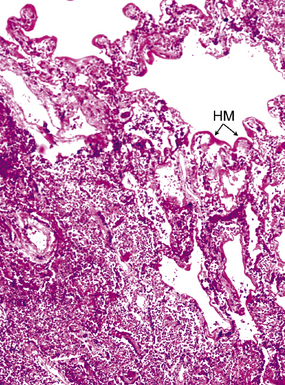
Hypersensitivity-like pulmonary disease recently has been associated with contaminated water in hot tubs (“hot tub lung”) and other environmental sources such as humidifiers and air conditioners.17 Biopsy reveals a miliary bronchiolocentric and interstitial granulomatous pattern, similar to that produced by hypersensitivity pneumonitis (Fig. 6-58). A similar infection-colonization-hypersensitivity syndrome has been described in workers exposed to metal-working fluid aerosols.158 The clinical, radiologic, and pathologic findings are similar to disease associated with hot tub use and other water sources except that a distinctive rapid-growing NTM species, M. immunogenum, has been recovered almost exclusively. Organisms are difficult to find in these cases but sometimes can be recovered in culture or with molecular techniques. Whether this entity represents an infection, a colonization, a hypersensitivity reaction, or a hybrid condition remains unresolved at this time.
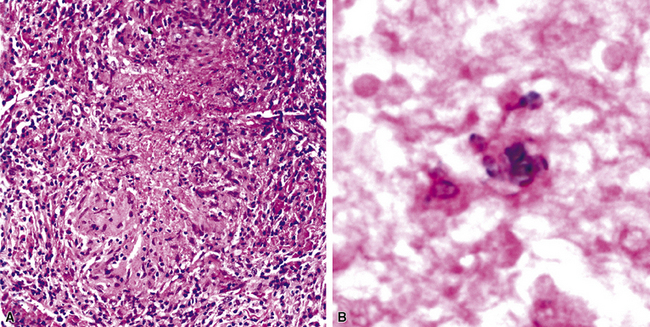
A rare morphologic manifestation of mycobacterial infection is the so called “spindle cell inflammatory pseudotumor” (Fig. 6-59) which may occur in lung, skin, lymph nodes, and a number of other sites in immunocompromised patients.159 The etiologic agents usually are NTM (MAC and M. kansasii), but M. tuberculosis has also been identified in some cases. Another uncommon variant is proximal endobronchial disease, discussed earlier in the spectrum of post-primary tuberculosis. Most cases are due to M. avium complex and manifest as polypoid lesions in immunocompromised HIV-infected patients, but this lesion also may be seen in immunocompetent persons.160
Certain species of rapidly growing mycobacteria (RGM) are capable of producing pulmonary disease, albeit infrequently.132,161 Nevertheless, M. abscessus is the third most frequently recovered NTM respiratory pathogen in the United States, after M. avium complex and M. kansasii. It accounts for 80% of respiratory tract isolates, making it the leading rapidly growing mycobacterial species recovered from the lung. M. abscessus produces chronic lung infection that has a striking clinical and pathologic similarity to M. avium complex infection, including the propensity to involve the lungs of patients with bronchiectasis. The RGM also have been thought to colonize lipoid pneumonia162; however, it is more likely that the pathogenesis of the lung injury pattern caused by the RGM is similar to that seen in skin and soft tissue cases, in which various combinations of suppurative foci, poorly formed or necrotizing granulomas, scattered multinucleated giant cells, and vacuoles are typical (termed “pseudocysts”).163 These combined features may mimic lipoid pneumonia and constitute an important clue to the presence of RGM infection.
Cytopathology
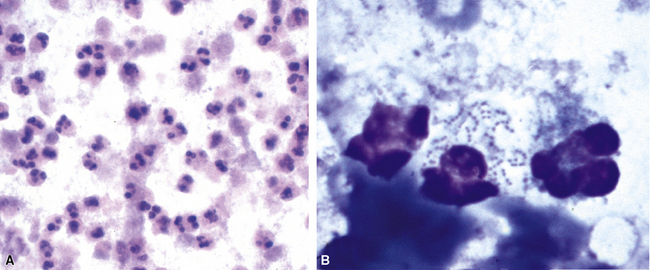
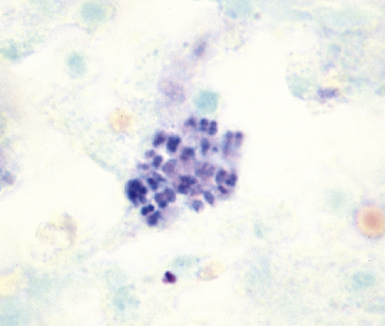
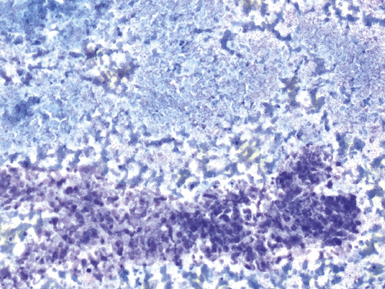
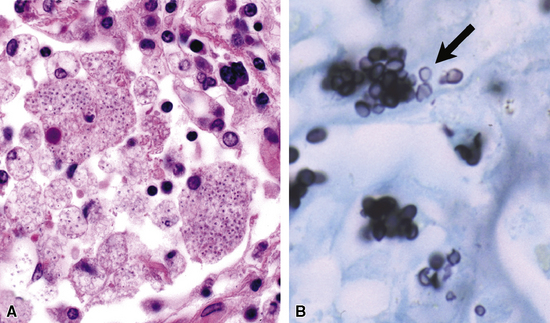
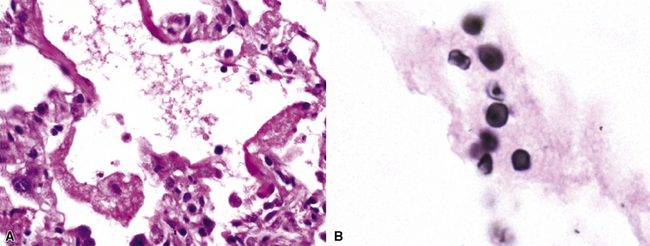
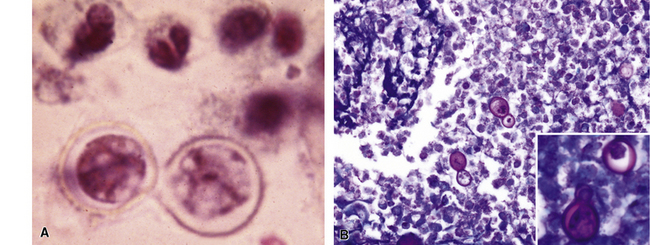
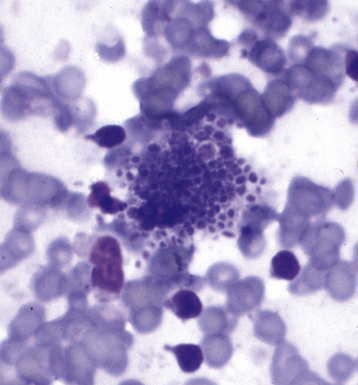
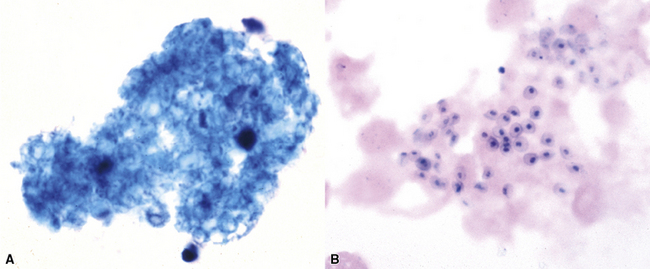
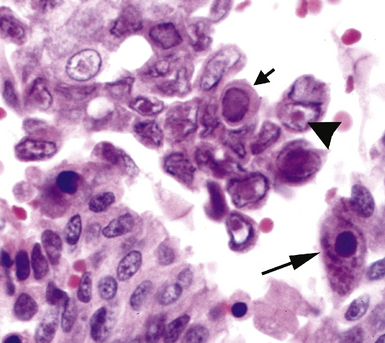
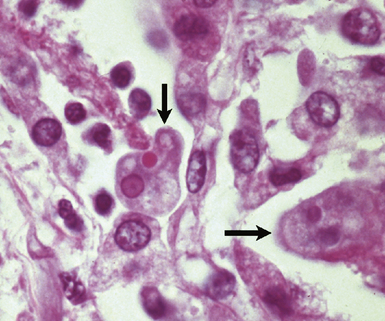
Fine-needle aspiration biopsy has been successfully used to diagnose both tuberculous pulmonary lesions and nontuberculous mycobacterial infections.164 The finding of finely granular amorphous necrotic debris associated with aggregates of epithelioid histiocytes (with or without multinucleate giant cells) (Fig. 6-60) is suggestive of a mycobacterial or fungal infection.165 In this setting, necrotic cancers must be excluded by a thorough search for atypical cells.
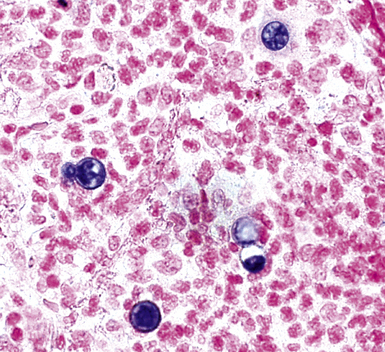
Special stains for acid-fast bacilli can be applied to aspirate smears, but culture of the aspirate is more likely to yield the etiologic agent when bacilli are sparse. Also, culture is still necessary for species identification and, if necessary, antimicrobial susceptibility testing. Epithelioid granulomas manifest a similar cellular pattern, but the granular necrotic debris is absent. Another pattern that may be seen, particularly in specimens from the immunocompromised patient, is a pure histiocytic or macrophage reaction with few or no epithelioid or multinucleate giant cells or necrotic debris. Numerous bacilli may be present in the distended cytoplasm of histiocytes and in the extracellular background. In air-dried (Diff-Quik) and alcohol-fixed (H&E- or Papanicolau-stained) smears, the bacilli may be recognized as negative images (Fig. 6-61).
Microbiology
The traditional as well as newer molecular approaches to the laboratory diagnosis of mycobacterial lung infection are outlined in Box 6-11. The mycobacterium is a slender but slightly curved bacillus, 4 μm in length, often with a beaded appearance; the length, curvature, and beadedness sometimes are accentuated in M. kansasii.166 In tissue sections or on smears, the Ziehl-Neelsen acid-fast stain or auramine-rhodamine fluorescent stains are most often recommended for best visualization. Organisms most often are found within the area of granulomatous reaction at the immediate periphery of the necrotic zone of the granulomas, or the cellular reactive process in the lining of cavities. Sections from several tissue blocks may be required to find organisms. Bacilli are rarely found in the absence of necrosis, except in smears from immunocompromised patients, in which they are visible and abundant within pseudo-Gaucher cells on H&E-stained sections, or as ghosted intracellular outlines with Giemsa-type stains. Dead bacilli lose their acid-fast character but sometimes may be identified with the GMS stain. The NTM, especially the RGM, may be more sensitive to acid alcohol decoloration and may not stain well or at all with the auramine-rhodamine method.132 A commercial immunohistochemical reagent for mycobacteria is now available but is effective only in cases in which traditional acid-fast stains yield a positive result.32 The differentiation of mycobacterial species in Ziehl-Neelsen–positive, formalin-fixed sections also has been achieved by in situ hybridization techniques with specific nucleic acid probes.167–169 PCR amplification plus identification is likely to be the most sensitive technique in those cases in which the lesion is suspected to harbor mycobacteria but yields a negative result on acid-fast staining.170 This technique may also be useful in cases in which the characteristic granulomatous pattern of inflammation is lacking, or mycobacteria have been identified in acid-fast–stained sections but culture results remain negative or cultures were not performed.171,172
Conventional wisdom states that culture is more sensitive than direct examination; however, the literature clearly documents cases in which acid-fast stains on tissue biopsies succeeded when cultures of tissue failed—an outcome that speaks to the virtue of perseverance in the face of compelling histopathologic findings.173 Furthermore, tissue culture is prone to sampling error unless more than one site is sampled.174 Specimens also may be smear positive and culture negative in patients whose disease has been treated. When only a rare bacillus is found, a strict criteria must be maintained and artifactual “pseudo” acid-fast bacilli excluded. As a general rule, a cutoff value of three organisms for a positive result seems prudent. False-positive smears also can result from contamination with local tap water, which may harbor mycobacteria.
Traditional solid media (Lowenstein-Jensen, Petragani, and Middlebrook agars) have given way to liquid media (radiometric and nonradiometric) as the first-line systems. Liquid media have demonstrated increased recovery of mycobacteria and decreased time to detection. They also facilitate rapid and accurate susceptibility testing.131,175 Some of these liquid systems are manual with visual inspection, whereas others are fully automated and continuously monitored. Most laboratories back up liquid systems with conventional media, because no system, at this time, is capable of identifying all isolates. Commercially available DNA probes that hybridize to the mycobacterial RNA have largely replaced traditional biochemical testing, and these methods have significantly shortened the time to identification of M. tuberculosis and selected NTM.176 For identification of the less frequently isolated species of NTM, for which probes are not available, it usually is necessary to send specimens to reference or state laboratories, where identification is accomplished by either biochemical testing, cell wall analysis using chromatographic techniques, or genotypic sequencing.138
The rapid differentiation of M. tuberculosis from NTM species is clinically very important, because the latter are much less infectious. In this context, molecular techniques have decreased the time to detection and identification of mycobacteria to less than three weeks in most instances. Direct nucleic acid amplification testing of clinical specimens using commercially available polymerase chain reaction (PCR) or transcription-mediated amplification (TMA) methods can reduce detection and identification times to less than 8 hours.174 Immunochromatographic techniques based on the detection of secreted mycobacterial proteins have the potential to reduce these times even further.177 Although NAA is faster, its overall accuracy is higher than that of smears but less than that of culture.176 In fact, no single test at this time has sufficient sensitivity and specificity to stand alone, and use of a combination of available techniques, depending on the clinical and economic setting, may be the best overall strategy.178,179
Interpretation of a culture isolate can sometimes be difficult. The presence of M. tuberculosis is always significant. M. kansasii is an important pathogen, and its isolation usually is also significant, although it may represent colonization. The significance of other NTM isolates is variable, depending on whether there is clinical and radiologic evidence of disease. It is in this setting that histopathologic examination plays an important role. M. avium complex can be isolated from the respiratory tract of otherwise healthy adults, as well as HIV-infected patients with no clinical or radiologic evidence of disease. The American Thoracic Society has proposed diagnostic criteria requiring that certain clinical, radiologic, and laboratory parameters be met in order to prove pathogenicity.132
Differential Diagnosis
A synopsis of the key morphologic and microbiologic attributes of mycobacterial lung infections is presented in Table 6-6. Mycobacteria produce a wide spectrum of inflammatory patterns, both granulomatous and nongranulomatous. Although the potential differential diagnostic listing is long, in practical terms, major considerations are fungal infections, sarcoidosis, Wegener granulomatosis, and bacterial infections that produce suppurative granulomas, such as those due to Nocardia, Actinomyces, Brucella, and Francisella species. Generally, the use of special stains and cultures will resolve most diagnostic dilemmas. Wegener granulomatosis can usually be excluded based on the lack of the characteristic tinctorial properties of the necrosis in the granulomas, and absence of vasculitis or capillaritis. When necrosis is absent or sparse in a mycobacterial infection, sarcoidosis can be difficult to exclude. Radiologic evidence of bilateral hilar adenopathy and other systemic findings of sarcoidosis often resolve the issue.
Table 6-6 Mycobacterial Pneumonias: Summary of Pathologic Findings
| Assessment Component | Findings |
|---|---|
| Mycobacterium tuberculosis | |
| Surgical pathology | Necrotizing (tuberculoid) granulomas |
| Cytopathology | Epithelioid cells and necroinflammatory debris Acid-fast bacilli detected with Ziehl-Neelsen or auramine O stains of cell block sections, more sensitive than smears |
| Microbiology | Acid-fast bacilli detected with Ziehl-Neelsen; Kinyon stains or fluorescent bacilli with auramine O stain Culture on Lowenstein-Jensen and Middlebrook selective and nonselective agar and/or liquid media systems DNA probes or NAA for identification |
| Nontuberculous Mycobacteria (MOTT) | |
| Surgical pathology | Granulomas generally with less necrosis; often epithelioid only Unusual patterns, e.g., pseudo-Gaucher and spindle cell proliferation in immunocompromised patients |
| Cytopathology | Epithelioid cells; pseudo-Gaucher or spindle cells with little or no necrosis Negative images in Diff-Quik, confirmed as acid-fast bacilli with Ziehl-Neelsen Organisms sparse, except in immunocompromised patient |
| Microbiology | As for Mycobacterium tuberculosis |
MOTT, mycobacteria other than M. tuberculosis; NAA, nucleic acid amplification.
Fungal Pneumonias
The pathologist examining tissue sections containing fungal forms is in a unique position to provide at least a provisional diagnosis at the group or genus level, and to make a judgment as to the significance of the organism in terms of its invasiveness or presence as a saprophobe or allergen. Indeed, often the most effective diagnostic strategy available is the rapid identification of fungi in tissue sections or cytologic samples.43,180,181 This is especially important when opportunistic infection is being considered in the immunocompromised patient. However, optimal performance also requires knowing when the morphologic features of a fungal organism are insufficient to permit group or genus level diagnosis, and when integration of microbiologic data and histopathologic findings is required.
Etiologic Agents
Nearly 70,000 fungi are known, and approximately 100 have been recovered from respiratory infections.182 Fortunately, only a small number are implicated as pathogenic on a consistent basis, and these are listed in Box 6-12.
Histopathology
Like mycobacterial species, fungal pathogens typically produce one or more nodular lesions in the normal host (Fig. 6-62) and these may become cavitary as the lesions evolve (Fig. 6-63). Inflammatory histopathologic patterns that suggest the presence of a fungal infection are summarized in Box 6-13. As is the case for other categories of etiologic agents, there are no absolutely characteristic or diagnostic patterns. Overlap is common and atypical reactions occur, ranging from overwhelming diffuse alveolar damage to little or no reaction in the immunocompromised patient. Proximal endobronchial disease mimicking neoplasm has also been described for various fungal species.183 Detection of the etiologic agent in tissue by microscopic examination, ancillary tests, or culture confers specificity and significance to the listed patterns. Large spherules with endospores characteristic of C. immitis or yeast with large mucoid capsules of C. neoformans can be diagnostic. However, atypical forms of these organisms can be misleading and challenging. For example, in aerated cavities or in the setting of bronchopleural fistula, Coccidioides species may produce branching septate and moniliform hyphae or immature morula-like spherules mimicking other fungi (e.g., hyaline molds and Blastomyces dermatitidis).38 Similarly, C. neoformans, H. capsulatum, and S. schenckii have been reported to produce hyphae or pseudohyphae in tissue, whereas acapsular C. neoformans may mimic other yeasts or Pneumocystis organisms.184
Mycelial morphology is helpful when it is characteristic of a specific genus or group. For example, broad, sparsely septate, nonparallel, twisted or irregular-diameter, thin-walled mycelia, with variable wide-angle branching, characterize zygomycetes, whereas progressively proliferating, regularly septate, 45-degree angle, dichotomously branching mycelia with parallel walls are typical of Aspergillus species (Fig. 6-64). In the case of Aspergillus, an important point is that only the presence of a fruiting body (conidiophore with sterigmata and conidia) permits diagnosis at the genus level, and there are many Aspergillus look-alikes in tissue, such as Fusarium, Paecilomyces, Acremonium, Bipolaris, Pseudallescheria boydii, and its asexual anamorph, Scedosporium apiospermum.184 Sometimes careful examination of tissue with special stains under high magnification or oil emersion will reveal clues, such as in situ sporulation, allowing a more definitive diagnosis.39 However, these clues often are subtle, even for experienced microscopists, and it is important to defer to culture whenever possible.185 Typical morphologic injury patterns and related etiologic agents are briefly highlighted below. The cited references should be consulted for further details.
Blastomycosis
Blastomycosis, the chronic granulomatous and suppurative infection produced by B. dermatitidis, is essentially a North American disease, concentrated in the Ohio and Mississippi river valleys. The prevalence of infection is particularly high in the state of Mississippi. Blastomycosis is the third most common endemic mycosis in North America, following histoplasmosis and coccidioidomycosis. It may occur in patients with normal immunity as well as those imunocompromised by diseases or medical therapy.186 The isolated nodular manifestation can simulate lung cancer, radiologically.187 The disease almost always begins in the lungs, although skin and bone are other common sites of involvement. In the lung, pathologic manifestations include focal or diffuse infiltrates; rare lobar consolidation; miliary nodules; solitary nodules; and acute or organizing diffuse alveolar damage186–189 (Box 6-14). Necrotizing granulomas are characteristic and often of the suppurative type (Fig. 6-65A), but non-necrotizing granulomas may be found as well.
The broad-based budding yeast forms of Blastomyces are refractile and have double-contoured walls. Multinucleate yeast cells typically are 8 to 15 μm in diameter, with some forms measuring up to 30 μm (see Fig. 6-65B). These large forms can mimic small Coccidioides spherules,190 whereas smaller forms (“microforms”) can mimic C. neoformans.189
Coccidioidomycosis
Endemic in the Lower Sonoran life zone of the southwestern United States, the soil fungus Coccidioides immitis and the more recently recognized, morphologically identical and genomically similar species Coccidioides posadasii191 may be encountered outside the endemic area as a result of fomite transmission of arthroconidia (e.g., Asian textile workers handling imported Arizona cotton) or in travelers who have returned from an endemic area. Most primary pulmonary infections are asymptomatic. The exceptionally wide spectrum of pulmonary pathology in patients with clinically evident disease is outlined in Box 6-15. The true prevelance of the disease is significantly underestimated in endemic regions of the southwest, where it is thought to account for nearly 30% of community-acquired pneumonias in some metropolitan areas.192–195 Granulomas are characteristic and may occur with or without necrosis. Intact spherules induce fibrocaseous granulomas (Fig. 6-66A) whereas ruptured spherules may incite suppurative and bronchocentric granulomatosis (BCG)-like reactions (see Fig. 6-66B).192
The large mature spherule (up to 40–60 μm in diameter) has a thick refractile wall lined by or filled with endospores and constitutes the key diagnostic finding (see Fig. 6-66C). This finding allows the distinction of coccidioidomycosis from other fungal infections such as blastomycosis and histoplasmosis, which are associated with similar histopathologic reaction patterns. In aerated cavities or the setting of bronchopleural fistula, mycelia resembling various hyaline molds may be seen with or without a variety of mature and immature spherules (see Figs. 6-19 and 6-66D). Coccidioides spherule look-alikes include large-variant B. dermatitidis, adiasporomycosis, pollen grains, and pulses (legume seeds).
Histoplasmosis
Histoplasmosis, the most common pulmonary fungal infection worldwide, is endemic in the Ohio and Mississippi river valleys of North America and is the most common endemic mycosis in AIDS.196 The clinical forms of H. capsulatum infection51,181,197 are presented in Box 6-16. The histopathologic correlates include a spectrum ranging from an exudative to a granulomatous process, influenced by such factors as the fungal burden and the immune status of the patient. In patients with normal defenses the characteristic histopathology is dominated by well-formed necrotizing and non-necrotizing granulomas occurring as solitary lesions indistinguishable from other granulomatous infections. Other presentations include miliary nodules (Fig. 6-67), cavitary lesions, and laminated fibrous solitary nodules (Fig. 6-68) that may be partially calcified (sometimes referred to as “residual granulomas”). In patients with impaired immunity, striking macrophage response with numerous intracellular yeasts is a characteristic pattern (Fig. 6-69A). The exudative lesion resembles acute lobular pneumonia with fibrinopurulent exudates.198
Box 6-16 Clinical Forms of Pulmonary Histoplasmosis
Reprinted with permission from Travis WD, Colby TV, Koss MN, et al. Lung infections. In: King D, ed. Atlas of Non-Tumor Pathology, Fascicle 2. Non-neoplastic Disorders of the Lower Respiratory Tract. Washington, DC: American Registry of Pathology; 2002:539–728, Table 12-6.
H. capsulatum organisms are yeasts (2–5 μm), with narrow-based unequal budding (see Fig. 6-69B). They may be seen on H&E-stained sections and, when numerous, appear as small refractile ovoid structures within macrophages. Yeasts typically occur in clusters but may be rare or very localized in old granulomas. A search for budding organisms in these situations may prove futile. Sometimes, yeasts may have dark-staining foci resembling pneumocystis organisms. Also, some yeast cells may be surrounded by a clear space and may be mistaken for Cryptococcus.51 Other look-alikes include Candida species, P. marneffei, capsule-deficient cryptococci, intracellular B. dermatitidis, and Hamazaki-Wesenberg bodies.
Paracoccidioidomycosis (South American Blastomycosis)
Seven clinical forms occur, but rarely cause lung infections in North America. The histopathology resembles other mycoses and can be exudative or granulomatous. Paracoccidioides braziliensis appears as a large spherical yeast (10–60 μm) with multiple buds attached by narrow necks (“steering wheel” or “ship’s wheel”).199 When budding is sparse, look-alikes include H. capsulatum with small intracellular forms, B. dermatitidis and capsule-deficient cryptococci for medium-size forms, and C. immitis or C. posadasii for large forms.
Sporotrichosis
Infection by Sporothrix schenckii usually is confined to the skin, subcutis, and lymphatic pathways, but the organism can disseminate to the lungs. Rarely, S. schenckii is a primary pulmonary pathogen. The organism can produce cavitary disease in the form of a single lesion. Infection may be bilateral and apical, progressive and destructive, or may be identified clinically as a solitary pulmonary nodule. Microscopically, caseous and suppurative type granulomas (Fig. 6-70A) occur with variable numbers of round to oval, small (2–3 μm) narrow budding yeast (see Fig. 6-70B) or cigar-shaped forms.200 Non-necrotizing granulomas also occur. Asteroid bodies are an important clue, especially when organisms are sparse, as is often the case. Look-alikes include H. capsulatum, acapsular cryptococci, Candida organisms, and Hamazaki-Wesenberg bodies.
Penicilliosis
Southeast Asia is the endemic setting of the unique dimorphic fungus Penicillium marneffei. The disease it produces is not seen in North America except in travelers, especially immunocompromised persons. It is one of the commonest opportunistic infections in AIDS patients in Southeast Asia and a significant clue to the presence of AIDS in that area. The respiratory tract is the portal of entry, with pulmonary infiltrates and disseminated disease, especially to skin. Microscopically, alveolar macrophages stuffed with spherical to oval yeast-like cells (2.5–5 μm) are seen, each with a single transverse septum; short hyphal forms and elongated, curved “sausage” forms may be formed in necrotic and cavitary lesions.201,202 The septum distinguishes it from its look-alike, H. capsulatum.
Cryptococcosis
C. neoformans is a ubiquitous, facultative intracellular yeast. Pulmonary cryptococcocosis occurs worldwide but has a particularly high incidence in the United States. The pathogenicity and histopathologic features of lung infection depends largely on the patient’s immune status, as illustrated earlier in Figure 6-7 and summarized in Box 6-17. In the normal host, a substantial proportion of cryptococcal infections are asymptomatic while the remainders have respiratory symptoms associated with infiltrates or nodules. Immunocompromised patients are almost invariably symptomatic and often develop disseminated disease with a predilection for the brain and meninges. Pulmonary injury patterns include single or multiple large nodules, segmental or diffuse infiltrates, cavitary lesions, and miliary nodules. Normal hosts most often develop nodules comprised of fibrocaseous granulomas (Fig. 6-71A), or granulomatous pneumonia (see Fig. 6-71B). Immunocompromised patients are more likely to have histiocytic (see Fig. 6-71C) or mucoid infiltrates without inflammation (see Fig. 6-71D).
Box 6-17 Histopathologic Patterns in Cryptococcal Lung Disease
Reprinted with permission from Mark EJ. Case records of the Massachusetts General Hospital. N Engl J Med. 2002;347:518–524.
The cryptococcal organisms are round yeast forms ranging in diameter from 2 to 15 μm, with an average size of 4 to 7 μm. Cryptococcal yeasts are visible on H&E-stained sections as pale gray to light blue structures, frequently with attached smaller buds. They often occur in clusters and sometimes can be found within giant cells.181 The mucicarmine stain highlights the capsule (Fig. 6-72A), but with capsule-deficient forms (see Fig. 6-72B), the pleomorphic appearance can be confused with that of other yeast forms (e.g., H. capsulatum, B. dermatitidis, S. schenckii) and sometimes Pneumocystis.
The lungs of patients with the most severe immunodeficiency may show myriad yeasts in alveolar septal capillaries (see Fig. 6-72A) with little if any intra-alveolar reaction203 and this form of the disease also may be associated with mucoid pneumonia.204 The mucoid pneumonia (Fig. 6-73A) of cryptococcal infection can be confirmed with mucin stains such as Alcian blue (see Fig. 6-73B). Another microscopic pattern recently described in HIV-infected patients is the so-called “inflammatory spindle cell pseudotumor,” a lesion much more commonly associated with mycobacterial infection.205
Candidiasis
Candida organisms are yeasts that can produce pseudohyphae and are the most common invasive fungal pathogens in humans. Secondary Candida pneumonia is relatively common, but primary Candida pneumonia is rare in other than immunocompromised patients in the intensive care unit.51 In general, C. albicans is the most frequently isolated of the more than 100 known species which include a few rare and emerging human pathogens. C. glabrata and C. tropicalis, together with C. albicans, account for 95% of bloodstream infections, the principal route for acquisition of Candida pneumonia.206 A non–blood-borne route to pneumonia results from aspiration of organisms from a heavily colonized or infected oropharynx. When blood-borne, miliary nodules with a necroinflammatory center and a hemorrhagic rim reflect an intravascular distribution of fungi. In the case of aspiration, the organisms may be found in the airways associated with an alveolar filling pattern of bronchopneumonia207 (Fig. 6-74A) or, much less commonly, a bronchocentric granulomatosis pattern.
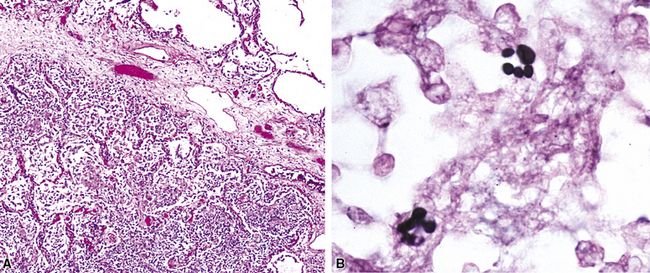
Figure 6-74 A, Candida bronchopneumonia. B, Candida yeast cells—blastoconidia (Grocott methenamine silver stain).
In tissue sections, oval budding yeast-like cells (blastoconidia) 2 to 6 μm in diameter may appear with pseudohyphae, which constrict at points of budding, creating the impression of bulging rather than parallel walls (see Fig. 6-74B). The pseudohyphae branch at acute angles and can overlap in width with the true hyphae of Aspergillus, from which they must be distinguished. Among the medically important species, C. glabrata (formerly Torulopsis glabrata) and C. parapsilosis produce only yeast cells in tissue, in contrast with most other Candida species, which produce both yeast and pseudohyphae.181
Other look-alikes include H. capsulatum, Trichosporon beigeli, and Malassezia furfur, depending on whether pseudohyphae or yeast forms alone are present. They can be distinguished from Histoplasma by their extracellular location and Gram stain positivity. T. beigelitends to be somewhat larger and more pleomorphic. Malasseziasis is clinically associated with parenteral nutrition, Intralipid, and indwelling catheters. Pulmonary lesions include pneumonia, mycotic thromboemboli, infarcts, and vasculitis. M. furfur may be found in small arteries, where the organisms appear as small, 2- to 5-μm yeast-like cells. They form distinctive unipolar broad-based buds but no pseudohyphae.51
Aspergillosis
Aspergillus species and other hyaline and dematiaceous molds have emerged as significant causes of morbidity and death in the immunocompromised host. Worldwide, species of Aspergillus are the most common invasive molds. They are the second most common fungal pathogens after Candida species but, in contrast with Candida, are more commonly isolated from the lung. Several species are recognized, but A. fumigatus is the one most often seen in the clinical laboratory and most often isolated from the lungs of immunocompromised patients.208 Respiratory aspergillosis can be classified into a colonizing or saprophytic form (intrabronchial and pre-existing cavity fungus ball) (Fig. 6-75A); hypersensitivity forms (allergic bronchopulmonary aspergillosis, including mucoid impaction of bronchi and hypersensitivity pneumonitis) (see Fig. 6-75B); and invasive disease (minimally invasive–chronic necrotizing or angioinvasive–disseminated), as outlined in Box 6-18.51,209–211 Invasive disease (Fig. 6-76) tends to occur in immunocompromised patients, including those with prolonged neutropenia, transplant recipients (especially hematopoietic stem cell and lung transplants), advanced AIDS, and and the inherited immune deficiency disorder referred to as “chronic granulomatous disease of childhood.” The clinicopathologic features of invasive disease reflect these host-associated risk factors.212 In patients with neutopenia, a charcteristic angioinvasive pattern occurs, with intravascular spread resulting in hemorrhagic infarcts (Fig. 6-77). In the non-neutropenic patient, the necroinflammatory pattern tends to lack this angioinvasive feature.213 Some cases defy categorization; are unique, e.g. bronchocentric and miliary patterns (Fig. 6-78); or may be hybrids of infection and hypersensitivity.214
Box 6-18 Histopathologic Patterns in Pulmonary Aspergillosis
Reprinted with permission from Travis WD, Colby TV, Koss MN, et al. Lung infections. In: King D, ed. Atlas of Non-Tumor Pathology, Fascicle 2. Non-neoplastic Disorders of the Lower Respiratory Tract. Washington, DC: American Registry of Pathology; 2002:539–728, Table 12-10.
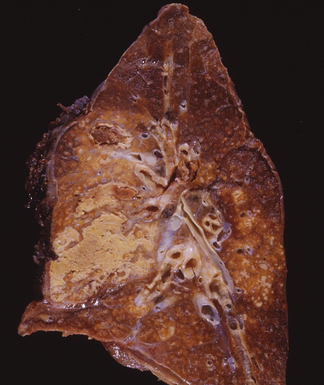
Figure 6-76 Resected lung specimen from an immunocompromised patient with necrotizing Aspergillus pneumonia.
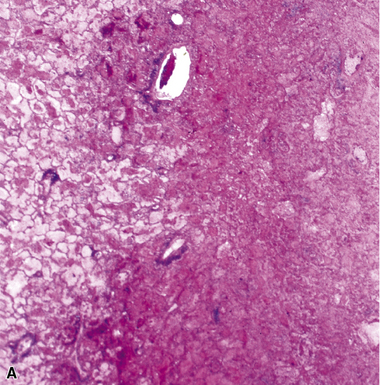
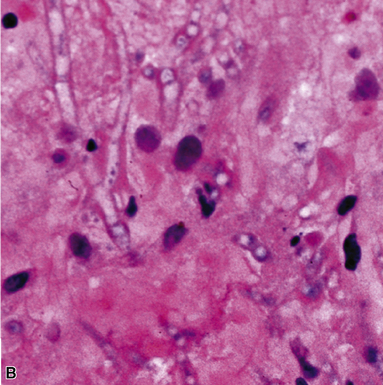
Figure 6-77 Invasive aspergillosis.
A, Hemorrhagic infarct. B, 45-degree angle branching septate hyphae.
Microscopically, septate hyphae, dichotomatously branched at 45-degree angle, have uniform, consistent width (3–6 μm) without constrictions at points of septation. When numerous, as in some angioinvasive lesions and fungus balls, these features can be readily appreciated in H&E-stained sections. Fruiting heads of Aspergillus (shown earlier in Fig. 6-64) are sometimes formed in cavities. Oxalate crystals, visible in plane-polarized light (Fig. 6-79), are an important clue to Aspergillus infection when hyphae cannot be identified.
Look-alikes include various hyaline molds such as zygomycetes and Candida species, as well as P. boydii.215 Another look-alike is Fusarium species. Fusariosis is an emerging mycosis in the immunocompromised host, and Fusarium is the second most common opportunist after Aspergillus species in immunosuppressed patients with hematologic malignancies.216 The clinical and pathological features in the lung and at sites of dissemination mimic those of aspergillosis, and the mycelia are essentially indistinguishable. Isolation in culture or by immunohistochemistry or molecular techniques, such as in situ hybridization or PCR amplification, is required for definitive diagnosis. Other previously uncommon but newly emerging hyaline molds that may be difficult to distinguish from Aspergillus in tissue are Paecilomyces, Acremonium, Scedosporium, and Basidiobolus.206,217,218
Zygomycosis
The taxonomic organization of the fungal phylum Zygomycota includes the class Zygomycetes, which is subdivided into two orders: Mucorales and Entomophthorales. These orders contain the agents of human zygomycosis.219 The order Mucorales includes the genera Absidia, Apophysomyces, Rhizopus, Rhizomucor, and Mucor, from which the often taxonomically incorrect term mucormycosis is derived. In fact, most infections are due to Rhizopus and Absidia species.220 The zygomycete species share clinical and pathologic features with invasive Aspergillus species, being angiotropic and capable of inducing hemorrhagic infarcts with sparse inflammation.
Clinical syndromes produced by these fungi include rhinocerebral, pulmonary, cutaneous, and gastrointestinal infections, with a predilection for neonates. Hematopoietic malignancies and diabetes mellitus with acidosis underlie most cases of pulmonary infection in children and adults.221 Box 6-19 lists a broad spectrum of pulmonary diseases that includes solitary or multiple and bilateral nodular lesions, segmental or lobar consolidation, cavitary lesions, fistulas, infarcts (Figs. 6-80 and 6-81); direct extension into mediastinal, thoracic soft tissue, chest wall and diaphragm; chronic tracheal and endobronchial infection; and fungus ball similar to aspergilloma.222 An endobronchial syndrome with a propensity for blood vessel ersoion also has been described, sometimes resulting in fatal hemoptysis.223
Hyphae are broad (6–25 μm), thin-walled, and pauciseptate (Fig. 6-82A). They display considerable variation in width, with twisted, nonparallel contours and random wide-angle branching, nearing 90 degrees.181 They also have a tendency to fragment more commonly than Aspergillus organisms, which tend to retain their elongate sweeping profiles. Additional features include variability in tinctorial staining in H&E sections, ranging from basophilia to eosinophilia. In frozen sections, hyphae may show weak staining, and they often have a bubbly or vacuolated appearance.222 In addition to being angiotropic, they are neurotropic.224 In lesions exposed to air, the hyphae may form ovoid or spherical thick-walled chlamydoconidia, within or at the terminal ends (see Fig. 6-82B).225 Look-alikes at the lower-width range include Aspergillus and other Aspergillus-like hyaline molds. The pseudohyphae of Candida species sometimes can be closely simulated.
Phaeohyphomycosis
A few genera of dematiaceous molds produce infections resembling those of Aspergillus, including allergic bronchopulmonary disease (Fig. 6-83A) and bronchocentric granulomatosis patterns.226,227 The more than 80 genera and species of these saprophytes, which occur naturally in wood, soil, and decaying matter, include Bipolaris, Exserohilum, Xylohypha, Alternaria, and Curvularia, among others.181 The unique appearance of these fungi is due to their cell wall melanin content. In the allergic mucin or other deposits of necroinflammatory debris, the phaeoid (dark brown– to black-pigmented) hyphae (2–6 μm in diameter) generally are sparse but can resemble Aspergillus and other hyaline molds, especially when lightly pigmented or nonpigmented. Typically, only small mycelial fragments are seen, which may be mistaken for artifacts, sometimes with terminal swellings resembling chlamydoconidia (see Fig. 6-83B). The dematiaceous agents of subcutaneous forms of chromoblastomycosis appear as pigmented muriform cells in granulomas, and they do not form mycelia. Chromoblastomycosis is rarely encountered in the lung. Another Aspergillus look-alike is P. boydii, an organism that is sometimes grouped with the dematiaceous fungi. P. boydii usually exhibits a more ragged, disorganized, and densely clustered pattern of mycelia. Clinically, localized disease may be cured by excision alone; systemic disease often is refractory to treatment.228
Pneumocystosis
The face of Pneumocystis pneumonia continues to change. Once considered to be a protozoan, this organism is now classified as a fungus, and the species infecting humans has been renamed Pneumocystis jiroveci (formerly Pneumocystis carinii).229 Once a disease primarily of malnourished children and occasionally occurring in the setting of treatment for childhood leukemia, today Pneumocystis infection is identified most commonly in patients with defective immunity, especially AIDS, or those on immunosuppressive therapies for hematopoietic malignancies, organ transplants, and collagen vascular diseases. With the success of contemporary therapy for AIDS, the pathologist is now more likely to encounter the disease in the latter group of patients in whom it is apt to be more subtle.230 The classic pattern during the HIV epidemic was the foamy alveolar cast (Fig. 6-84) with moderate to numerous organisms, type II pneumocyte hyperplasia, and a scant to moderate interstitial lymphoplasmacytic infiltrate.231,232
In recent years a number of atypical and unusual patterns have been described that are worth recognizing.51,233,234 These are listed in Box 6-20. Pneumocystis jiroveci infection can mimic any lung injury pattern, ranging from acute diffuse alveolar damage with hyaline membranes (Fig. 6-85) and minimal or no foamy exudates to an organizing phase with sparse organisms. There is also a spectrum of granulomatous infection, both non-necrotizing and necrotizing, that may overlap morphologically with mycobacterial or other fungal infections, particularly histoplasmosis (Fig. 6-86). Cavitary disease, solitary pulmonary nodules that may be relatively fibrotic, cysts, and dystrophic calcification also are described.234–236
Cytopathology
Many of the fungal pathogens involving the respiratory tract can be detected by cytologic techniques in sputum samples, bronchial washings and brushings, BAL fluid samples, and needle aspirates.44 The aspirates and other samples also can be submitted for culture and ancillary studies.237 The four most common yeast forms—C. neoformans, C. immitis or C. posadasii, H. capsulatum, and B. dermatitidis—must be distinguished from each other, and P. jiroveci also can enter the differential diagnosis.43 Morphologic features of these organisms are often better visualized in cytologic preparations than in tissue sections, usually permitting a rapid and definitive diagnosis on smears prepared using routine stains (Papanicolaou, Diff-Quik, and H&E). More specific fungal stains (Grocott methenamine silver, Gridley, and Fontana-Masson) often can be held in reserve.
Cytology of Common Yeast Forms
Morphologic features of some of the more common yeast forms that the pathologist may encounter in cytologic material are presented in Table 6-7.
C. neoformans organisms are seen as are single budding yeast forms with a narrow, pinched-off base, approximately 4 to 7 μm in diameter but ranging in size from 2 to 15 μm. In needle aspirates, the mucoid capsule investing the yeast imparts a “spare tire” appearance (Fig. 6-87).
B. dermatitidis organisms are refractile, double-contoured yeast forms and range in diameter from 8 to 15 μm with broad-based budding (Fig. 6-88). An internal amorphous mass can be appreciated in some stained preparations. Smaller or larger yeast cells can be mistaken for C. neoformans or C. immitis, respectively.
C. immitis/C. posadasii spherules exhibit a variety of sizes and shapes, ranging from large spherules packed with endospores (Fig. 6-89A) to empty collapsed spheres and small immature spherules.238 The latter may overlap with Blastomyces and other yeasts. Mycelial forms of Coccidioides species, with arthrospores, may be found in aspirates of cavitary nodules exposed to air (see Fig. 6-89B).
H. capsulatum yeast cells are small (2–5 μm) and stain poorly in routine smears, but presence of this pathogen can be suspected on the basis of the dot-like refractile appearance of these cells in the cytoplasm of macrophages. In Diff-Quik–stained smears, the characteristic purple, polarized yeast forms (Fig. 6-90) are discernible, and they are outlined entirely in GMS-stained smears.
P. jiroveci most commonly is identified in exfoliative samples and aspirates by the presence of the foamy alveolar cast, which varies from eosinophilic to basophilic and is highly characteristic (Fig. 6-91A). These organisms rarely occur singly. The GMS stain outlines the characteristic cysts (see Fig. 6-91B).
Cytology of Common Mycelial Forms
The cytopathologist’s most frequent challenge is the interpretation of mycelial forms in exfoliated material, especially the distinction between Aspergillus look-alikes—zygomycete and Candida hyphae. The morphologic features of some of the more common agents are compared in Table 6-8.
Aspergillus species are characterized by septate mycelia that branch at angles approaching 45 degrees (Fig. 6-92). Aspergillus hyphae lack constrictions at points of septation. However, Aspergillus organisms cannot be differentiated from one of their mimics by morphology alone unless accompanied by a fruiting body. A rapid in situ hybridization technique specific for Aspergillus species can be performed on pulmonary cytocentrifuge preparations, as well as on tissue.239 An additional advantage is that this technique may assist in this otherwise difficult differential diagnosis.
A potential pitfall in the evaluation of cytopathologic specimens in fungal infections (both exfoliative samples and needle aspirates) is the confounding presence of atypical reactive squamous cells and type II pneumocytes, which can mimic the cytologic atypia of malignant neoplasms.46 Furthermore, the pathologist interpreting lung biopsy findings, especially with transbronchial specimens, should always attempt to correlate such findings with samples that may have been collected for cytologic or microbiologic study. This is especially advisable because etiologic agents that escape detection in tissue, such as Pneumocystis, Aspergillus, and CMV, may be found in washings or lavage fluid.240
Microbiology
Complementary laboratory methods are often required for diagnosis of fungal infection, and these are listed in Box 6-21.182 Under the microscope, many fungi are readily apparent in H&E-stained sections, where they appear colorless (negative staining) or phaeoid (naturally pigmented). The GMS stain is the best histologic stain for demonstrating fungi when they are sparse or not visible on H&E sections. However, some fungi, notably the zygomycetes, may stain poorly with GMS. The GMS preparation can be counterstained with H&E, allowing co-evaluation of the host inflammatory response. The Fontana-Masson stain has been used to detect melanin in C. neoformans and phaeoid fungi, but many Aspergillus species and some zygomycetes also also will stain with this reagent.225,241 The PAS stain can be useful in select circumstances, and histochemical stains for mucin (Alcian blue or mucicarmine) are useful for C. neoformans infections. The PAS and mucin preparations also can be counterstained with GMS or Fontana-Masson to simultaneously highlight cell walls and capsules of cryptococci. It is important to recognize that not everything that stains with the silver methods is a fungus, and care must be taken to distinguish organisms from pseudomicrobes, such as overstained red cells, whiteblood cell nuclei, reticulin and elastic fibers, calcium deposits, and even Hamazaki-Wesenberg bodies.40
In the microbiology laboratory, the age-old technique of direct light microscopic visualization of fluids, exudates, and tissue homogenates treated with potassium hydroxide (KOH) is being replaced by chemofluorescent cotton-brightening agents (such as calcofluor white and fungiqual). Fluorescence microscopy with these reagents can detect a wide variety of fungi in wet mounts as well as frozen sections and paraffin-embedded tissue.242,243
The time-honored laboratory techniques for the identification of fungi (gross colonial and microscopic morphologic analysis after isolation on fungal media, followed by biochemical testing) may be the principal means to an etiologic diagnosis. For deep tissues, including the lung and other sterile sites, the Emmons modification of Saboraud glucose agar with chloramphenicol is recommended by many mycologists.244 Additional use of enriched media such as brain-heart infusion agar can improve recovery of C. neoformans, B. dermatitidis, and H. capsulatum. Selective media containing cyclohexamide are not recommended for normally sterile sites, because they are potentially inhibitory for yeasts, such as Cryptococcus and Candida species, and molds, such as Aspergillus and zygomycetes.
The interpretation of a positive fungal culture must be made in the clinical context. In the absence of proof of tissue invasion, or compelling ancillary data, the interpretation of laboratory results requires considerable judgement. This is because many fungi are ubiquitous in the environment, and most fungal isolates from nonsterile respiratory samples do not represent disease unless there are also significant risk factors such as HIV infection, organ transplantation, or immunocompromising drug therapy.245
The value of bringing multiple, often complementary laboratory methods to bear on inconclusive morphologic findings cannot be overemphasized. In this context, while culture has been considered the most reliable method for definitive diagnosis, and histopathology often the fastest, the greatest yield results from combining histopathology with traditional culture and one or more of the newer molecular methods.246,247 Culture may fail to yield an isolate even in the face of positive microscopic findings. In fact, the yield from tissue specimens, needle aspirates, BAL fluid samples, and bronchial washings is quite low for molds and other fungi, for reasons that are not entirely clear.47,248 Immunofluorescence testing using specific monoclonal antibodies can achieve rapid and specific diagnosis in selected infections, especially when tissue has not been submitted for culture. Antibodies directed against the antigens of Aspergillus species and selected other fungi have been described but most are not yet commercially available. For the problematic case, the mycology section of the CDC can provide assistance. Immunohistochemical identification of fungi can be accomplished fairly easily for those species for which reagents are commercially available.32,249,250
Molecular techniques, including in-situ hybridization and amplification technologies such as PCR, are other powerful tools that can provide rapid, accurate diagnosis for yeasts and molds which may be present in small numbers or manifest overlapping histologic features with one another.243,251–253 A few laboratories (including the CDC) are performing such assays. Use of quantitative real-time PCR assays on blood, body fluids, and other samples holds promise for relatively rapid definitive diagnosis when routine methods of isolation and identification fail in critical situations.254
Serologic tests can support a morphologic diagnosis when positive titers are present, but effective serodiagnosis of systemic fungal infections is not available for most fungi.255 Unfortunately, an antibody response does not necessarily correlate with invasive disease; and an antibody response may be lacking for various reasons. False-positive results due to cross reactions and false-negative results due to a variety of reasons plague many of these assays. Some of the most accurate serologic tests (with high sensitivity and specificity) for fungal infections are those for histoplasmosis and coccidioidomycosis, yet tests for both have limitations that must be recognized in interpreting results.256,257
The detection of macromolecular antigens shed into various body fluids requires a relatively large microbial burden which tends to limit sensitivity for most fungal infections except histoplasmosis and crytococcosis.246 For these two fungi, useful antigen detection techniques are available using serum, urine, cerebrospinal and BAL fluids. They are especially sensitive in patients with defective immunity.237,257 In patients with pneumonia and normal immunity, however, these tests may be positive in lavage fluid but negative in urine unless the disease has disseminated. Other assays designed to detect antigens or metabolites of invasive fungi include those for 1,3β-D-glucan, a cell wall component of several fungi such as Aspergillus, Candida, Fusarium, and others, and for galactomannin, a polysaccharide antigen in the cell wall of Aspergillus, have shown fair sensitivity and specificity.181,258
Differential Diagnosis
A synopsis of the key morphologic and mycologic features of the fungal pneumonias is presented in Table 6-9. When H&E and GMS stains fail to detect or clearly identify fungal elements in a suspected fungal infection, the use of ancillary procedures may provide the specific diagnosis. Sometimes, if tissue or other patient specimens have been submitted for culture, the answer may lie in the mycology section of the microbiology laboratory, as many species begin to grow in a matter of days. When fungi are not readily identified by any of these techniques or strategies, other granulomatous infections should be considered, especially mycobacterial, uncommon bacterial (e.g., tularemia, brucellosis), and parasitic infections. Noninfectious necrotizing and non-necrotizing granulomatous disorders also enter the differential diagnosis. These include Wegener granulomatosis, idiopathic bronchocentric granulomatosis, aspiration, sarcoidosis, rheumatoid nodules, pyoderma gangrenosum–like lung lesions in patients with inflammatory bowel disease, and Churg-Strauss syndrome.
| Assessment Component | Findings |
|---|---|
| Blastomycosis | |
| Surgical pathology | Suppurative granuloma most characteristic; also, tuberculoid (necrotizing) types Round, thick-walled (double-contour) yeast with broad-based budding |
| Cytopathology | Neutrophils and epithelioid cells with characteristic refractile yeast cell with double-contoured wall and broad-based budding |
| Microbiology | Characteristic yeast seen on wet mount, KOH- and calcofluor-stained smear Culture-sterile lung tissue on nonselective fungal media (e.g., Emmons modified Sabouraud) and enriched media (e.g., brain-heart infusion) Add selective media for bronchial/transbronchial samples Colonies produce oval conidia on terminal ends of conidiophore at right angle to mycelium Confirm with DNA probe Serologic studies not useful |
| Coccidioidomycosis | |
| Surgical pathology | Fibrocaseous granuloma Large intact and/or ruptured spherules, full or partially or completely empty of endospores Mycelial forms in aerated cavities and fistula |
| Cytopathology | Necroinflammatory debris with epithelioid histiocytes Intact, viable, colorless spherules with variable number of endospores and/or ruptured degenerating forms with stained wall; range in size from large mature to small immature types |
| Microbiology | Characteristic mature spherules in wet mount, KOH- and calcofluor-stained smear Culture of sterile lung tissue on nonselective fungal media yields mycelia with characteristic arthroconidia Confirm with DNA probe Serologic diagnosis with tests for IgG and IgM antibodies by immunodiffusion, EIA; complement fixation for titers |
| Histoplasmosis | |
| Surgical pathology | Macrophage reaction and/or granulomas, based on immunity, including miliary and solitary pulmonary, variably hyalinized nodule Small, thin-walled, oval yeasts with narrow-based buds, often refractile |
| Cytopathology | Macrophage and epithelioid cells with characteristic yeast cell, often intracellular, stained purple with Diff-Quik, black with GMS |
| Microbiology | Rarely detected by direct examination of most clinical specimens Culture sterile lung tissue on nonselective and enriched fungal media produces tuberculate macroconidia Confirm with DNA probe Antigen detection by EIA available for BAL fluid, CSF, serum, and urine |
| Paracoccidioidomycosis | |
| Surgical pathology | Exudative or granulomatous lesion with large, globose yeast cell with multiple buds |
| Cytopathology | Suppurative or granulomatous reaction with characteristic yeast cell |
| Microbiology | Direct detection in wet mount, KOH- and calcofluor-stained smear Culture-sterile lung tissue on standard nonselective fungal media Serologic testing by immunodiffusion, EIA; complement fixation for titer |
| Sporotrichosis | |
| Surgical pathology | Necrotizing granuloma, often cavitary with small, usually round, sometimes cigar-shape yeast with sparse, narrow buds |
| Cytopathology | Suppurative or necrotizing granuloma pattern Yeast cells generally sparse or absent |
| Microbiology | Rarely detected by direct examination of most clinical specimens Culture of sterile lung tissue on nonselective fungal media yield thin, hyphae-bearing conidia in a rosette pattern Converts to a yeast phase at 37°C on blood agar No serologic tests |
| Penicilliosis | |
| Surgical pathology | Alveolar macrophages stuffed with yeast cells resemble Histoplasma species, but with septum reflecting binary fission, not budding reproduction |
| Cytopathology | Macrophage with intracellular characteristic yeast forms |
| Penicilliosis | |
| Microbiology | Culture of sterile lung tissue on nonselective fungal media yields a mold with a red pigment evident as culture ages Erect conidiophores sometimes branched with metulae bearing one or several phialides with long, loose chains of oval conidia New urinary antigen test |
| Cryptococcosis | |
| Surgical pathology | Granulomas, histiocytic infiltrate or mucoid pneumonia, based on immunity with pale, round, budding pleomorphic yeast cells, often in clusters Mucoid capsules usually; acapsular types sometimes |
| Cytopathology | Yeast cell with mucoid capsular halo resembles “spare tire” Combination of mucicarmine and GMS or Fontana-Masson outlines capsule and cell wall Background of epithelioid cells or necroinflammatory debris may be sparse or absent |
| Microbiology | Oval to lemon-shaped calcofluor-positive yeast cell with capsule in India ink–stained touch imprint Culture on nonselective fungal media yields mucoid yeast-type colonies No pseudohyphae; germ tube–negative Dark brown pigment on birdseed (niger) agar Confirm with biochemical tests Antigen detection test (latex agglutination or EIA) on serum, BAL fluid, CSF, and needle aspirates |
| Candidiasis | |
| Surgical pathology | Miliary necroinflammatory lesions or bronchopneumonia with small, oval, budding yeasts with or without pseudohyphae C. glabrata yeast only |
| Cytopathology | Yeasts and/or pseudohyphae in a necroinflammatory background |
| Microbiology | Budding yeasts and pseudohyphae in wet mounts, KOH- and calcofluor-stained smears Cultures on selective and nonselective fungal media yield creamy tan to white yeast-type colonies Identification by germ tube production, carbohydrate assimilation, and cornmeal agar morphology |
| Aspergillosis | |
| Surgical pathology | Various forms include saprophytic (fungus ball), allergic (ABPA and mucoid impaction), hypersensitivity pneumonitis, and invasive disease, ranging in severity from minimal chronic necrotizing to extensive pneumonia Angiotrophic with necrotizing infarcts; also hybrid forms of disease Septate, dichotomous, 45-degree angle mycelia; oxalate crystals Presence of fruiting body is genus-specific |
| Cytopathology | Tangled clusters of septate mycelia in a necroinflammatory background May appear sparsely septate and twisted, mimicking zygomycetes |
| Microbiology | Positive staining of mycelia with calcofluor and GMS Culture of sterile lung tissue on nonselective fungal media produces mold-type colonies in a range of colors Species differentiation by conidial and conidiophore morphology |
| Zygomycosis | |
| Surgical pathology | Nodular lesions, lobar consolidations, cavitary lesions, fungus balls, and airway infections commonly necrotizing and ischemic secondary to angioinvasion Broad pauciseptate mycelia with 90-degree angle branching, often with twisted ribbon morphology |
| Cytopathology | Pauciseptate mycelia, often with twisted ribbon morphology in a necroinflammatory background |
| Microbiology | Positive staining of mycelia with calcofluor and GMS Rapidly growing cottony colonies are grown on most nonselective fungal media, but “controlled baiting” with bread sometimes necessary Identification based on presence and locations of rhizoids, shape of sporangia, presence of columellae, and shape of sporangiospores |
| Phaeohyphomycosis | |
| Surgical pathology | Allergic bronchopulmonary fungal disease similar to aspergillosis |
| Cytopathology | Similar to ABPA pattern—“allergic mucin” with eosinophils, Charcot-Leiden crystals in inspissated mucus Fungal mycelial fragments sparse or absent |
| Microbiology | Dematiaceous (phaeoid) dark brown to black colonies on nonselective fungal media Identified by shape and cross walls of multicell, pigmented conidia |
| Pneumocystosis | |
| Surgical pathology | Pneumonia with foamy alveolar cast is classic; other patterns include diffuse alveolar damage, granulomatous lesions, and minimal changes Variable numbers of cysts noted in GMS-stained sections |
| Cytopathology | Foamy alveolar cast with characteristic cysts outlined by GMS |
| Microbiology | Causative organism: formerly Pneumocystis carinii, classified as a fungus and renamed Pneumocystis jiroveci; cannot be cultured Detection is with fluorescent monoclonal antibody assay or GMS-stained smears |
ABPA, allergic bronchopulmonary aspergillosis; BAL, bronchoalveolar lavage; CSF, cerebrospinal fluid; EIA, enzyme immunoassay; GMS, Grocott methenamine silver; KOH, potassium hydroxide.
Viral Pneumonia
Viruses cause more infections than all other types of microorganisms combined, and involve the respiratory tract more commonly than other organ systems.259 Fortunately, the lung diseases produced by viruses usually are mild and self-limited. Nevertheless, viruses cause major public health illnesses and account for many of the new and emerging diseases in today’s headlines. At times, viruses also are capable of producing serious and life-threatening infections that come to the attention of pathologists in both immunocompromised patients and young, healthy persons.260 The viruses that commonly infect the lung are listed in Table 6-10.
| RNA Viruses | DNA Viruses |
|---|---|
| Influenza virus | Adenovirus |
| Parainfluenza virus | Herpes simplex virus |
| Respiratory syncytial virus | Varicella-zoster virus |
| Measles virus | Cytomegalovirus |
| Hantavirus | Epstein-Barr virus |
Etiologic Agents
The conventional respiratory viruses—influenza virus, parainfluenza virus, RSV, and adenovirus—cause outbreaks of respiratory illness in the general population each year. In infants, the elderly, and in those patients with chronic diseases, these pathogens can cause serious pneumonias. Pneumonia in immunocompromised persons usually is attributed to the herpesviruses (herpes simplex virus and CMV). Less appreciated is that the conventional respiratory viruses also are frequent causes of respiratory illness in these patients, and that such infections result in high rates of morbidity and mortality.261
Newly recognized respiratory viruses262,263 include a highly pathogenic strain of influenza, H5N1. First detected in 1997 in Hong Kong, it has since spread to Europe, the Middle East and Africa. Another unique, triple-reassortment swine-origin influenza virus A, H1N1 (S-OIV), emerged in 2009 as the cause of outbreaks sustained by person-to-person transmission in multiple countries. It was characterized by respiratory illness of variable severity ranging from self-limited disease resembling seasonal flu to severe illness requiring hospitalization and occasionally eventuating in death from respiratory failure.264 An acute cardiopulmonary syndrome in the southwest United States was etiologically linked to a new hantavirus referred to as Sin Nombre (“without a name”). The severe acute respiratory syndrome (SARS), which began in southern China and was carried by travelers to 33 other countries and 5 continents, was caused by a newly recognized coronavirus, SARS-CoV. Four other coronaviruses linked to respiratory illnesses (HCoV-229E; HCoV-NL63; HCoV-OC43; HCoV-HKU1) have since been reported.265 Human metapneumovirus, a paramyxovirus closely related to RSV, clinically and pathologically, has become recognized as one of the leading causes of respiratory illness in children and also can cause illness in adults and immunocompromised patients. Human bocavirus (h0bv) has been isolated in several countries from children with wheezing.266 Qther viruses such as the picornavirus group (rhinovirus and enterovirus) can cause pneumonia, as can BK polyoma virus.267 Other unusual viral lung infections have been attributed to henipah and hemorrhagic fever viruses.268 Parvovirus B19, an erythrovirus, has long been known to cause disease, primarily in maternal-fetal and pediatric patients. Recently, an autoimmune-type pneumonitis associated with serologic evidence of parvovirus B19 also has been described.269 The evolution of diagnostic laboratory methods and large-scale molecular screening suggests that more viruses will be linked to respiratory tract disease in the future.
Histopathology
The respiratory tract viruses have a tendency to target specific regions of the tracheobronchial tree and lungs, producing characteristic clinical syndromes. However, sufficient overlap clinically, radiologically, and pathologically often limits a strict interpretation of findings for a definitive diagnosis. Box 6-22 can sometimes be useful in narrowing the search for a specific etiologic agent. The microscopic findings in most pulmonary viral infections include the direct effect of the virus as well as the host’s inflammatory response. The clinical outcome depends upon the virulence of the organism and the nature of the host response, be it diffuse alveolar damage, diffuse or patchy bronchiolitis and interstitial pneumonits, giant cell reactions, or even minimal change.270
The histopathologic diagnosis of viral infection is impossible without identification of the characteristic CPE. The term cytopathic effect traditionally has been used by virologists to describe cellular changes in unstained cell culture monolayers seen by light microscopy,271,272 but it can be applied to all virus-associated nuclear and cytoplasmic alterations seen on H&E-stained slides or highlighted by immunohistochemical staining, molecular in situ–based methodology, or ultrastructural localization.273,274 Diffuse alveolar damage, often with bronchiolitis, is the most typical pattern of viral lung injury. As noted earlier, however, diffuse alveolar damage also occurs in bacterial, mycobacterial, and fungal pneumonias, so a careful search for specific viral CPE becomes important in this setting. For the surgical pathologist, CPE manifests mainly as the viral inclusion present in the nucleus or cytoplasm of an infected cell. Viral inclusions confer diagnostic specificity to the pathologic pattern of injury in which they are found, and for the common respiratory tract viruses, the features are presented in Table 6-11. Finally, it is worth mentioning that most clinically significant viral pneumonias that have CPE also show necrosis somewhere in the biopsy.
Influenza virus
Influenzaviruses are the most pathogenic of the respiratory viruses and predispose patients most commonly to secondary bacterial pneumonia. These viruses also account for the greatest public health burden. Annually, they cause epidemic outbreaks of respiratory disease that are often associated with considerable morbidity; periodically, they produce pandemics with high mortality rates. These viruses target the ciliated epithelium of the tracheobronchial tree, producing necrotizing bronchitis and bronchiolitis and a spectrum of changes that vary depending on the stage of the disease (early versus late), outcome (fatal versus nonfatal), and the presence or absence of secondary bacterial pneumonia. Uncomplicated influenza pneumonia is rarely biopsied today. Based on historical data from bronchoscopic biopsies performed in the 1950s and early 1960s, the histopathologic findings in nonfatal uncomplicated influenza are those of active tracheobronchitis.275 Necrosis and desquamation of the epithelial cells to the basement membrane is associated with a relatively scant lymphocytic infiltrate; however, in more severe cases, the virus and its attendant inflammatory response spread more distally into the respiratory bronchioles and alveoli, with hemorrhage, edema, fibrinous exudate with hyline membranes, and patchy interstitial cellular infiltrates (Fig. 6-93). This constellation of findings comprises the “lesion of characterization.”276 In contemporary pathologic terms this would correspond to diffuse alveolar damage, and clinically, a primary viral pneumonia. Depending on the clinical course and time of lung biopsy (or autopsy) within the first 2 weeks of illness, the process may be in the acute and/or organizing phase.277,278 Later, the airway epithelial damage may pave the way for secondary bacterial pneumonia, which accounts for much of the morbidity and mortality of influenza and which may obscure the features of primary viral pneumonia.
From 2003 through 2008, 391 human cases of highly pathogenic avian influenza involving the H5N1 strain were recorded, with 247 deaths.279 The histopathologic changes observed in the few autopsied cases fall within the spectrum of findings described during the pandemics of 1918, 1957, and 1968 and in fatal cases of interpandemic (seasonal) influenza.278 A characteristic feature of the the H5N1 and 1918 cases is the high mortality rate, especially among previouly healthy older children and young adults. Excessively high levels of cytokine and chemokines are thought to play an important role in the pathogenesis of the acute lung injury pattern seen in these fatal cases of influenza.280 Because these viruses produce no characteristic cellular inclusions, etiologic diagnosis is not possible by morphology alone, and requires antigen detection by immunofluorescence, immunohistochemistry, in situ hybridization, or culture.281
Parainfluenza Virus
Parainfluenza virus comprises four serotypes (I to IV) that typically target the upper respiratory tract, classically in the form of croup.282 Some cases involve distal airways, as in infections due to RSV and influenza virus, but are milder, with less morbidity and requiring fewer hospitalizations. A few documented cases have been described with a diffuse alveolar damage pattern or an interstitial pneumonitis with giant cells, the latter resembling those of measles and respiratory syncytial virus infection. The giant cells of parainfluenza tend to be larger and have more intracytoplasmic inclusions.51 Parainfluenza virus is a potential opportunist in immunocompromised patients, especially children with congenital immunodeficiency disorders283 in whom fatal pneumonitis with disseminated disease may occur.284
Respiratory Syncytial Virus
RSV causes more significant respiratory infections in early childhood than those attributable to either influenza viruses or parainfluenza viruses.285 The annual outbreaks of bronchiolitis and pneumonia in infants are especially severe during the first year of life, and in those of low birth weight or with cardiopulmonary disease.282 Considered primarily a childhood virus, RSV has more recently been recognized as the etiologic agent of pneumonia in community-dwelling and high-risk adults with chronic lung disease requiring hospitalization.283,286,287 Also, RSV is often an unsuspected opportunistic pathogen in immunocompromised patients.261,288 RSV targets the epithelium of the distal airway, producing bronchiolitis with disorganization of the epithelium and epithelial cell sloughing268 (Fig. 6-94A). In fatal cases, airway obstruction due to sloughed cell detritus, mucus, and fibrin is compounded by airway lymphoid hyperplasia.289 Diffuse alveolar damage may be seen in immunocompromised patients. Giant cells (syncytia), similar to the cytopathic changes seen in cell culture, may be present in alveolar ducts and air spaces around areas of bronchiolitis (see Fig. 6-94B). Eosinophilic inclusions in cytoplasm may be seen in tissues and cytology specimens from immunosuppressed patients, but these are difficult to confirm as diagnostic of RSV without immunohistochemistry.
Human Metapneumovirus
Human metapneumovirus, a newly recognized paramyxovirus, is a leading cause of respiratory tract disease in infants, with annual epidemics occurring during the winter and early spring months.290 The virus also causes disease in immunocompormised patients and likely explains some lower respiratory tract infections in the elderly. The clinical spectrum of croup, bronchiolitis, and pneumonia is similar to that for infections due to other paramyxoviruses such as RSV and parainfluenza virus. The pathologic features are not well characterized, because few well-documented cases have included biopsy in the evaluation. However, histopathologic assessment of lung tissue in severe cases has revealed acute and organizing diffuse alveolar damage, as well as smudge cell formation.291,292 The definitive identification of the virus can be established in tissue culture, but monoclonal antibody reagents and molecular techniques (real-time PCR assay) are the current diagnostic methods of choice.
Measles Virus
The measles virus causes a highly communicable childhood viral exanthema worldwide that, unlike varicella (chickenpox), leads to complications that are common and serious.293 Measels pneumonia accounts for the vast majority of measles-related deaths and most of these are a consequence of secondary pneumonia (bacterial or viral), or attributable to an aberrant immune response. Primary viral pneumonia occurs, but is uncommon, even in immunocompromised hosts. Microscopically, bronchial and bronchiolar epithelial degeneration and reactive hyperplasia with squamous metaplasia is typically accompanied by peribronchial inflammation. Diffuse alveolar damage may occur and quantitative immmunohistochemical studies have revealed severe immune dysfunction with loss of key effector cells and their cytokines.294 Characteristic giant cells show distintive intranuclear eosinophilic inclusions surrounded by halos (Fig. 6-95). This is the classic measles injury pattern268 and is referred to as Hecht giant cell pneumonia. Minute intracytoplasmic eosinophilic inclusions precede the development of the intranuclear inclusions, and are often difficult to identify. Pneumonia with giant cells should always suggest measles, but similar changes can be seen in RSV and parainfluenza pneumonias, and not all cases of measles pneumonia have these giant cells.268 Hard metal pneumoconiosis (giant cell interstitial pneumonia) is in the differential diagnosis, but the overall appearance of hard metal disease is one of a chronic disease with some fibrosis, and few if any acute changes. In the absence of giant cells, the cellular interstitial pneumonia must be differentiated from those caused by other viruses and atypical pneumonia agents, as well as from nonspecific interstitial pneumonia (NSIP).
Hantavirus
The recently identified hantavirus produces a rapidly evolving cardiopulmonary syndrome with a high mortality rate. This disorder first came to public attention as an emerging infection following an outbreak in the southwestern United States in 1993 that was causally linked to a previously unrecognized hantavirus. All members of this genus are zoonotic and are found in rodents around the world. The specific type responsible for the cardiopulmonary syndrome, designated Sin Nombre (“without a name”), is present in rodent feces and is acquired from the environment through inhalation. It produces florid pulmonary edema with pleural effusions, variable fibrin deposits, and focal wispy hyaline membranes295 (Fig. 6-96A). Immunoblast-like cells are present in vascular spaces and in the peripheral blood (see Fig. 6-96B). Morphologic diagnosis is presumptive, because hantaviral antigen in endothelial cells, detected by immunohistochemistry, is required for definitive diagnosis.296 In the appropriate clinical setting, clues to the diagnosis can sometimes be found in a constellation of morphologic findings on a peripheral blood smear, and confirmation can be achieved serologically by detection of hantavirus-specific immunoglobulin M (IgM) antibodies, or by detection of hantavirus RNA by PCR assay in peripheral blood leukocytes.297,298
Coronaviruses
Coronaviruses are ubiquitous RNA viruses known to cause disease in many animals. At least five different coronaviruses are known to infect humans and these cluster into two antigenic groups.265 They are responsible for a majority of common colds, along with the rhinoviruses. Co-infections with other respiratory viruses occur in infants and children presenting with more severe respiratory disease. In certain epidemiologic situations, they can cause pneumonia in children, frail elderly individuals, and immunocompromised adults.299,300
In November 2002, the appearance of an atypical pneumonia in China, subsequently labeled severe acute respiratory syndrome (SARS), became an alarming global health problem in the period of a few months.301 The disease was linked (Koch’s postulates were fulfilled) by means of tissue culture isolation, electron microscopy, and molecular analysis to an emergent novel coronavirus, proposed as the Urbani strain of SARS-associated coronavirus.302
Clinically, the disease ranges from a nonhypoxemic febrile respiratory disease (with minimal symptoms in some patients) to one of severe pulmonary dysfunction, manifesting as acute respiratory distress syndrome and eventuating in death for approximately 5% of the patients affected.303 In the reported cases, either the chest x-ray appearance on presentation was normal or the chest film showed unilateral, predominantly peripheral areas of consolidation that progressed to bilateral, patchy consolidation, the degree and extent of which correlated with the developmnent of respiratory failure. In patients who presented with normal x-ray appearance, CT scans often revealed bilateral ground-glass consolidation resembling that in bronchiolitis obliterans with organizing pneumonia (cryptogenic organizing pneumonia). Laboratory abnormalities in some but not all patients included leukopenia with lymphopenia and thrombocytopenia. The partial thromboplastin time and D-dimer levels were increased. Biochemical abnormalities included elevated lactate dehydrogenase (LDH), alanine aminotransferase, and creatinine levels. Lymphopenia and elevated LDH were helpful clues, but the clinical, radiologic, and laboratory features, although characteristic, were not distinguishable from those in patients with pneumonia caused by other viruses and bacteria and various atypical agents.
Histopathologic findings in lung biopsy and autopsy tissues included acute lung injury (diffuse alveolar damage) in various stages of organization.304,305 Lung biopsy specimens in milder cases showed relatively scant intra-alveolar fibrin deposits with some congestion and edema (Fig. 6-97). However, the spectrum of findings included acute fibrinous pneumonia, hyaline membrane formation, interstitial lymphocytic infiltrates, desquamation of alveolar pneumocytes, and areas undergoing organization of the acute phase injury.306 In some patients, multinucleate syncytial cells reminiscent of the CPE seen in influenza virus, RSV, and measles virus infections were noted. Viral inclusions were not identified, and initial immunohistochemical studies failed to reveal viral antigen. Subsequent investigations detected virus in epithelial cells (predominantly type II pneumocytes) and alveolar macrophages using immunohistochemical staining, in situ hybridization, RT-PCR methods, and electron microscopy. A unique coronavirus (Fig. 6-98) was finally implicated as the etiologic agent.306,307 Comparative histopathologic studies in fatal cases of SARS and H5N1 avian influenza reveal similarities and differences.308 Both infections feature acute and organizing diffuse alveolar damage, but SARS appears to be more frequently associated with subacute injury with intra-alveolar organization, whereas H5N1 virus causes a more fulminant diffuse alveolar damage pattern with patchy intersitial inflammation and paucicellular fibrosis.
Adenovirus
Adenovirus comprises several genera, with multiple serotypes that cause infections of the upper and lower respiratory tract, conjunctiva, and gut. Respiratory tract infections are most common and account for approximately 5% to 10% of pediatric pneumonias. These can be especially severe in neonates and children and in immunocompomised persons.268,309 In the lung, adenovirus infection produces two patterns of lung injury: diffuse alveolar damage, with or without necrotizing bronchiolitis, and pneumonitis with “dirty” or karyorrhectic necrosis310 (Fig. 6-99). These patterns may coexist in some cases and the pneumonia may be accompanied by hemorrhage secondary to adenovirus-induced endothelial cell damage.311 Two types of adenoviral CPE may be seen. Initially an eosinophilic (Cowdry A) intranuclear inclusion occurs surrounded by a halo with marginated chromatin, similar to herpes simplex virus (Fig. 6-100A). This later enlarges and becomes amphophilic and then more basophilic, obliterating the nuclear membrane, producing the characteristic “smudge cell” (see Fig. 6-100B).268
Herpes Simplex Viruses
Herpes simplex virus (HSV) type I and type II have had traditional assigned roles as etiologic agents of mucocutaneous disease of the head and neck (type I) and genitalia (type II). Considerable crossover has been documented, however, with both types isolated from patients with disease at either site. Tracheobronchitis and pneumonia due to these viruses are rare in healthy adults with intact immune systems. They occur primarily in patients with underlying pulmonary disease and in association with inhalational and intubational trauma. They also occur in neonates and in patients who are immunosuppressed or compromised by various chronic diseases. Characteristic lesions include tracheobronchitis (Fig. 6-101A) with ulcers and hemorrhagic diffuse alveolar damage. Necrosis in a miliary small, or rarely large, nodular pattern is a helpful clue and the best location to identify CPE69 (see Fig. 6-101B). Like adenovirus, HSV also has two types of CPE: Initially a ground-glass amphophilic intranuclear inclusion, Cowdry B, appears with marginated chromatin. Later, a single eosinophilic, Cowdry A inclusion (Fig. 6-102) surrounded by a halo, similar to that seen with adenovirus, develops. The Cowdry A inclusion is considered noninfectious, as it is devoid of nucleic acid protein and is thought to represent the nuclear “scar” of HSV infection.268 In the absence of smudge cells, HSV and adenoviral infections can look identical. Fortunately, immunohistochemistry or in situ hybridization can often resolve this differential diagnosis.
Varicella-Zoster Virus
Varicella-zoster virus (VZV) infection produces considerable morbidity in the newborn, the adult, and the immunocompromised host, both in its primary form (varicella) and in its reactivated form (zoster). Varicella pneumonia is rarely observed in otherwise healthy children but is a major complication of adult varicella, occurring in approximately 10% to 15% of adults with VZV. In affected adults without underlying diseases and normal immunity the course generally is mild and self-limited. Nevertheless, fatality rates of up to 10% have been reported.268 By contrast, high mortality rates (25% to 45%) have been noted among some cohorts of immunosuppressed patients. Microscopically, small, miliary, nodules of necrosis are seen, associated with interstitial pneumonitis, edema, fibrin deposits, or patchy hyaline membranes (Fig. 6-103A). HSV-like intranuclear inclusions are present but may be sparse and difficult to identify. A miliary pattern of calcified nodules (see Fig. 6-103B) may be present in the healed phase.312
Cytomegalovirus
CMV infections are acquired throughout life. This virus can cause considerable morbidity and even death in the neonate, but infection generally is asymptomatic in older healthy children and adults. Like other herpesviruses, primary infection is followed by latency which persists until immune deficiency or immunosuppressive therapy causes it to reactivate and disseminate. CMV has therefore become one of the most common opportunists in patients with AIDS and those who receive organ transplants. In these settings, CMV can produce a variety of patterns, including one with minimal changes where only scattered alveolar lining cells with typical viropathic changes are seen. The CPE of CMV produces cytomegalic cells with large, round to oval, smooth “owl eye” eosinophilic to basophilic intranuclear inclusions surrounded by a clear halo (Fig. 6-104A).
Later, multiple eosinophilic cytoplasmic inclusions develop that may be positive on staining with PAS and GMS (see Fig. 6-104A, inset). The more numerous the cytomegalic cells, the greater the clinical significance. In some cases, atypical inclusions may be seen in cells that are not significantly enlarged and the nuclei may contain dark-staining homogeneous inclusions that may lack a clear halo. Despite their atypical appearance, these inclusions usually will be highlighted with immunohistochemical stains.313 Another typical pattern that suggests viral infection is the presence of miliary small nodules with central hemorrhage surrounded by necrotic alveolar walls69 (see Fig. 6-104B). Interstitial pneumonitis is the least common pattern of CMV infection. Ulcers may be seen in the trachea and bronchi, but occur less often than in herpetic infections. In CMV pneumonias, it is advisable to look for other pathogens, typically P. jiroveci (Fig. 6-105), but bacteria, fungi, protozoa, and other viruses all are possible co-infecting organisms.314
Epstein-Barr Virus
EBV infections usually are acquired in childhood and generally are asymptomatic. The pathologist most often encounters this virus in the lung in the context of pulmonary lymphomas or in other EBV-associated lymphoproliferative disorders that can occur in transplant recipients and other immunocompromised patients. However, the most common symptomatic primary EBV infection is infectious mononucleosis. Most of these patients recover uneventfully but a few develop one or more complications. Pneumonitis is one of them, albeit rare and not well characterized. The few reports describing pathology indicate a nonspecific lymphocytic interstitial pneumonitis, which may be bronchiolocentric315,316 (Fig. 6-106). CPE is absent, and although serologic studies can be supportive of a clinicopathologic diagnosis, etiologic proof of EBV infection requires demonstration of the virus in lymphoid cells by in situ hybridization for EBV-encoded RNA-1 (EBER-1).
Cytopathology
The cytologic features of viral infections in the respiratory tract are most likely to be found in exfoliative specimens, such as bronchial washings and BAL fluid samples, rather than needle aspirates, although viral diagnosis has been achieved with this technique.317,318 This is because viral infections are less likely to produce radiologic mass–like infiltrates, which are the most common targets of needle biopsy procedures. Herpes simplex virus, CMV (Fig. 6-107), and adenovirus are the most commonly identified viral pathogens in respiratory cytologic specimens, but varicella virus, parainfluenza virus, RSV, human metapneumovirus, and measles virus also have been detected.
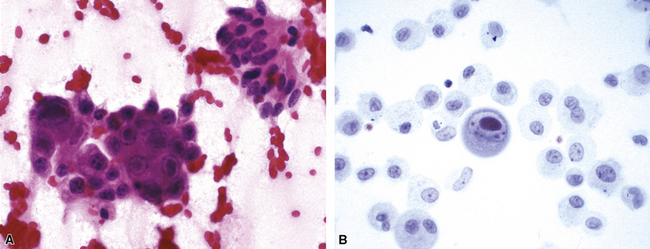
Figure 6-107 Cytomegalovirus pneumonitis with characteristic cytopathic effect.
A, Fine-needle aspirate. B, Bronchoalveolar lavage specimen.
Characteristic CPE produced by these viruses often is better appreciated in cytologic smears than in tissue sections, which may in fact yield a negative result. Therefore, review of any cytology sample taken at the time of biopsy can be valuable. Other, less specific changes may be found. These include ciliocytophoria (free cilia complexes with terminal bars) and cytologic atypia mimicking cancer.44
Microbiology
Diagnostic virology is the newest of the microbiology and infectious disease specialities to have benefited from the technologic revolution in laboratory medicine. Rapid and accurate diagnosis can often be achieved today using practical, convenient laboratory methods that employ reliable, commercially-available mammalian cells, media, and reagent systems.260,319,320 This has allowed many rural and small urban hospital laboratories to provide timely viral diagnostic services not possible a short time ago. It is predicted that self-contained, rapid-cycle real-time PCR methods will one day account for a majority of viral assays in laboratories of all sizes. As a result, the pathologist who suspects a viral infection will increasingly have a variety of tools to obtain an etiologic diagnosis when morphologic manifestations are suggestive of viral infection.
The basic approaches to viral diagnosis in the laboratory are listed in Box 6-23. In questionable cases, confirmation by immunohistochemical studies (Fig. 6-108A), in situ hybridization (see Fig. 6-108B), or electron microscopy may be helpful.31,321
In the microbiology laboratory, the diagnosis of viral respiratory infections is based primarily on antigen detection and culture (Fig. 6-109). Direct antigen detection in clinical specimens collected by nasopharyngeal swabs, nasal washings, and aspirates or BAL fluid (but not sputum samples or, with rare exception, throat swabs) is performed using monoclonal antibodies by either immunofluorescence microscopy or enzyme immunoassay. By using a single reagent containing the monoclonal antibodies against several viruses and dual fluorochromes, the common respiratory viruses can be rapidly screened by direct immunofluoresence testing. Positive specimens can then be tested with individual reagents to determine the specific etiologic agent, while negative specimens can be submitted for culture.322 Enzyme immunoassay includes methods that offer speed and convenience at the point of care. However, they are less sensitive than standard virologic methods, which still must be used to test negative specimens. Direct detection can also be accomplished in cellular samples, including tissue, by in situ hybridization or amplification techniques such as PCR. For RNA viruses, PCR amplification uses a reverse transcriptase (RT) step. Recently, PCR methodology has evolved into multiplex formats and novel systems have been introduced that combine multiplex PCR chemistry with electron microarray (DNA chip) technology or fluid microsphere-based systems, permitting the simultaneous detection of a wide array of respiratory viruses and other pathogens.323–327 These systems have the potential to more rapidly and accurately diagnose acute infections and also may allow the study of complex coinfections and the active monitoring of outbreaks of influenza and other viral illneses.328 Current molecular diagnostic approaches are more technically demanding than culture, antigen detection by immunofluorescence, or enzyme immunoassay; and few are approved by the U.S. Food and Drug Administration (FDA). At present, isolation still remains useful for many respiratory viral infections, and antigen detection methods offer the speed and immediacy of reporting that many molecular methods lack.
Traditional viral cultures in tubes with various types of cell monolayers are currently performed with greater sensitivity and turnaround time using the shell vial technique. This technique uses centrifugation of clinical specimen suspensions onto coverslipped cell monolayers, followed by brief incubation (1–2 days) and antigen detection.319 It is important therefore to preserve a portion of tissue from a bronchial or transbronchial biopsy or thoracotomy specimen in viral transport medium, especially in the immunocompromised patient, who may not have had BAL fluid submitted for culture.
Viral serologic testing commonly has been used for diagnosis but may be the least sensitive approach. A positive serodiagnosis typically is based on a fourfold rise in titer between acute and convalescent sera and therefore cannot be achieved by this means in the acutely ill patient; antigen detection or culture of respiratory tract specimens is much preferred. However, a serologic strategy, utilizing a panel of antigens in an immunofluorescence or enzyme immunoassay format on a single specimen, is useful in suspected EBV infections.329
A case also can be made for the benefit of CMV serologic testing for assessment of the antibody status of organ donors and recipients for predicting risk of post-transplantation CMV disease. When tissue is not available or findings are inconclusive, tests for the detection of actual disease in these transplant recipients, include the p65 antigenemia assay on peripheral blood leukocytes and amplification or quantitation of CMV DNA in various peripheral blood compartments (plasma, whole blood, and leukocytes).330 These assays may eventually replace culture of BAL fluid for surveillance of CMV infection in such patients.331 The detection of virus in respiratory secretions (including BAL fluid), urine, or blood establishes the presence of virus but does not necessarily implicate it as the etiologic agent of a pneumonia. Quantitation of viral load by real-time PCR amplification, however, can be useful in this regard by linking high viral load with infection.332
Differential Diagnosis
A synopsis of the key morphologic and microbiologic features of the viral pneumonias is presented in Table 6-12. In the absence of CPE, diffuse alveolar damage and other patterns of lung injury are not diagnostic of viral infection. Diffuse alveolar damage is a nonspecific response to many types of infection, including bacterial, mycobacterial, fungal and protozoal, all of which must be considered in the differential diagnosis. In addition, other noninfectious causes include reactions to drugs, radiation, toxic inhalants, and shock of any type. Occasionally, CPE may not be diagnostic; for example, the early inclusions of adenovirus, herpes simplex virus, and CMV may be quite similar. In most cases, immunohistochemistry or molecular techniques can resolve the diagnostic dilemma. Mimics of CPE that must be ruled out include macronuclei in both reactive processes and occult neoplastic infiltrates, and intranuclear cytoplasmic invaginations which can occur in a variety of cells. Cytoplasmic viral inclusions also can be simulated by aggregated altered protein and particulate matter.
| Assessment Component | Findings |
|---|---|
| Influenza Virus | |
| Surgical pathology | Diffuse alveolar damage, bronchitis and bronchiolitis Secondary acute purulent pneumonia Antigen detection by immunofluorescence, immunohistochemical, or in situ hybridization studies |
| Cytopathology | Nonspecific changes may include presence of reactive-type pneumocytes; ciliocytophoria |
| Microbiology | Antigen detection by DFA or EIA Culture on primary monkey kidney cells: noncytopathic Detection by hemadsorption |
| Respiratory Syncytial Virus | |
| Surgical pathology | Bronchiolitis with lumen detritus; may be associated with syncytial giant cells Diffuse alveolar damage in immunocompromised patients Confirm with immunohistochemistry |
| Cytopathology | Giant cell syncytia characteristic, but often not seenEosinophilic inclusions may be seen in bronchial epithelial cells of immunocompromised patients; rarely in those of normal hosts Rarely diagnosed by cytology alone |
| Microbiology | Antigen detection by DFA and EIA usually more sensitive than culture Cultures on continuous epithelial cell lines (Hep-2) and primary monkey kidney yield characteristic syncytial CPEs |
| Measles Virus | |
| Surgical pathology | Bronchitis, bronchiolitis, diffuse alveolar damage with giant cells containing Cowdry A inclusions and small cytoplasmic inclusions |
| Cytopathology | Eosinophilic intranuclear and cytoplasmic inclusions Rarely diagnosed by cytology |
| Assessment Component | Findings |
| Measles Virus | |
| Microbiology | Antigen detection by DFA and EIA Culture on primary monkey kidney produces spindle cell or multinucleate CPE Serologic testing (for measles-specific IgM) available |
| Hantavirus | |
| Surgical pathology | Pulmonary edema pattern with variable fibrin deposits Immunoblast-like cells in vascular spaces Confirm by immunohistochemistry |
| Cytopathology | Noncytopathic |
| Microbiology | Serology: Hantavirus-specific IgM or detection of specific RNA by PCR assay in peripheral blood leukocytes |
| Adenovirus | |
| Surgical pathology | Diffuse alveolar damage with or without necrotizing bronchiolitis and/or pneumonitis with necrosis and karyorrhexis |
| Cytopathology | Early Cowdry A intranuclear inclusions, later smudge cell Reactive and reparative-type atypia in background |
| Microbiology | Antigen detection by EIA and DFA Culture on continuous epithelial cell lines produces characteristic grape-like clustered cytopathic effect |
| Herpesvirus | |
| Surgical pathology | Tracheobronchitis; diffuse alveolar damage; miliary necroinflammatory lesions |
| Cytopathology | Ground-glass (Cowdry B) intranuclear inclusions; later Cowdry A inclusions in multinucleated cells, often with a “seeds in a pomegranate” appearance on Pap-, H&E-, and Diff-Quik–stained smears Background reactive and reparative atypia |
| Assessment Component | Findings |
| Herpesvirus | |
| Microbiology | Antigen detection by immunofluorescence Culture on diploid fibroblasts produces characteristic cytopathic effect, sometimes within 24 hours Serologic testing less useful |
| Varicella-Zoster Virus | |
| Surgical pathology | Miliary necroinflammatory lesions; calcified nodules in healed phase |
| Cytopathology | Intranuclear Cowdry A inclusions sparse and less well-defined than with herpes simplex |
| Microbiology | Antigen detection by immunofluorescence Culture on human embryonic lung or Vero cells produces CPE more slowly than for herpesviruses (3–7 days) Serologic testing available |
| Cytomegalovirus | |
| Surgical pathology | Minimal changes with scattered cytomegalic cells; miliary necroinflammatory lesions; interstitial pneumonitis |
| Cytopathology | Large “owl eye” Cowdry A inclusions with halo; cytoplasmic inclusions stained with GMS |
| Microbiology | Culture on human diploid fibroblasts produces characteristic CPE slowly in traditional tube cultures but more rapidly with use of shell vial technique p65 antigenemia assay; PCR assay Selective application of serology useful |
| Epstein-Barr Virus | |
| Surgical pathology | Polymorphous lymphoid interstitial pneumonitis Confirm by in situ hybridization |
| Cytopathology | Noncytopathic |
| Microbiology | No routine culture; diagnosis by serologic testing using panel of antibodies (EA; IgG and IgM VCA; EBNA) |
CPE, cytopathic effect; DFA, direct immunofluorescence antibody [test]; EA, early antigen; EBNA, Epstein-Barr virus–determined nuclear antigen; EIA, enzyme immunoassay; GMS, Grocott methenamine silver; H&E, hematoxylin-eosin; IgG, IgM, immunoglobulins G and M; Pap, Papanicolaou; PCR, polymerase chain reaction; VCA, viral capsid antigen.
Parasitic Infections
It is estimated that approximately 300 species of helminth worms and 70 species of protozoa have been acquired by humans during our short history on Earth.333 Most of these are rare, but approximately 90 are relatively common, and some of them have been found in the lung.334 A world made smaller by globalization and travel to endemic areas, in combination with the emergence (and re-emergence) of parasitic pathogens in immunocompromised patients, guarantees that pathologists will be increasingly challenged by diagnostic problems associated with these organisms.335 Nevertheless, pulmonary parasitic infections are relatively rare and continue to be exotic diseases for surgical pathologists and cytopathologists in the United States.
Etiologic Agents
Several parasite species migrate through the lungs as part of their normal life cycle, but few preferentially infect the human lung.336 Most are aberrant pulmonary localizations in the human host, where they become lost in transit or are part of a secondary disseminated infection from another organ system, often in the setting of compromised immunity. The etiologic listing in Box 6-24 is selective, based on the more common pathogens known to be associated with pulmonary involvement. The reader is encouraged to consult the References for a more comprehensive compilation.
Histopathology
When parasites, in the form of adult worms, larvae, or eggs, invade or become deposited in lung tissue, they usually provoke an intense inflammatory reaction with neutrophils, eosinophils, and various mononuclear cells. One or more of the patterns listed in Box 6-25 may be identified. When the predominant site of involvement is the bronchial mucosa, a bronchitis and bronchiolitis pattern is observed; when they become impacted in pulmonary arteries, a nodular angiocentric pattern is observed, although it may be overshadowed by thrombosis and infarction. Some parasites invade the alveolar parenchyma, resulting in a pattern of miliary small nodules or pneumonitis. Naturally, none of these patterns are consistently present and combinations of patterns may be seen. In some cases, an acute Loeffler-like eosinophilic pneumonia may reflect an allergic reaction to the transient passage of larvae through the pulmonary vasculature.
The various patterns, although nondiagnostic, can be suggestive of a parasitic infection, particularly when they incorporate a heavy eosinophilic infiltrate or granulomatous component. Eosinophilic lung disease, with or without blood eosinophilia, has a diverse etiology but is particularly characteristic of parasitic infection, especially in the tropics.334 In the United States, other infections such as coccidioidomycosis must be considered, in addition to the many noninfectious causes of pulmonary eosinophilia. The challenge for the pathologist is the identification of a parasite, distinguishing it from artifact or foreign body, and classifying it as precisely as possible based on its size and unique morphologic features. Once the presence of suggestive morphologic features has been confirmed, the patient’s travel history can help to further narrow the scope of the differential diagnosis. Of interest, a common “parasite” encountered in clinical practice is not a parasite at all but aspirated vegetable material simulating the complex structure of an organism.337
Toxoplasmosis
Toxoplasma gondii is an obligate, intracellular protozoan and a common opportunist in patients with AIDS, the disease underlying most cases of toxoplasmosis seen in recent years. The brain and retina are most commonly involved in these patients, but pulmonary lesions also may be present in cases of disseminated disease. These often take the form of miliary small nodules with fibrinous exudates, which may progress to a confluent fibrinopurulent pneumonia.338 Free forms (crescent-shaped tachyzoites) and cysts may be identified (Fig. 6-110). Pseudocysts packed with tachyzoites can be distinguished from true cysts with bradyzoites by staining of the latter with PAS and GMS.339
Amebiasis
Amebic dysentery becomes invasive in a small percentage of patients. When the trophozoites leave the gut, they most commonly travel to the liver. From the liver, either by direct extension, or rarely by hematogenous spread, the lungs may become involved. In this scenario, abscesses composed of liquifactive debris—with few neutrophils, distinguishable from bacterial abscess where neutrophils are dominant—may be seen, most often in the right lower lobe adjacent to the liver.340,341 Trophozoites can be best seen at the margin of viable tissue (Fig. 6-111). They resemble histiocytes but usually are larger, with a lower nucleocytoplasmic ratio. A tiny central karyosome within a round nucleus having vesicular chromatin is characteristic.342,343 Bronchial fistula formation and empyema can occur as complications; amebae may be found in sputum and pleural fluid, respectively, in these situations. For free-living amebic species (those of the genera Acanthamoeba, Balamuthia, Naegleria), the central nervous system is the principal focus of infection. However, disseminated disease including lung infection (Fig. 6-112) may occur in certain epidemiologic situations, especially those involving compromised immune status.344
Cryptosporidiosis
Ten species of the intracellular coccidian protozoa are currently recognized, but one of them, Cryptosporidium parvum, causes most human infections.345 Clinically, infection due to this organism may have three major manifestations: asymptomatic shedding, acute watery diarrhea that lasts for approximately 2 weeks, and persistent diarrhea that lasts several weeks. Patients with AIDS have a wider spectrum of disease severity and duration that includes a fulminant cholera-like illness.345 These patients are most likely to manifest extraintestinal disease. In the lung, the organism targets the epithelium of the airways just as it does the surface epithelium of the gut and biliary tract.346 In H&E sections, cryptosporidia appear as small (4–6 μm in diameter), round to oval protrusions from the cell surface. Electron microscopy reveals that they are intracellular but extracytoplasmic. In addition to H&E, they stain with Giemsa, PAS, GMS, and acid-fast stains. A mild to moderate chronic inflammatory cell infiltrate usually is present in the submucosa. Recognition of this disease in patients with AIDS can be challenging because the findings may be subtle and coexistent pneumonias caused by other pathogens can divert the pathologist’s attention.
Microsporidiosis
The microsporidia are obligate intracellular, spore-forming protozoa. More than 140 genera and 1200 species are recognized, but only seven genera and a few species have been confirmed as human pathogens.347 They are opportunists that have recently emerged in severely immunocompromised patients, especially people with AIDS and transplant recipients. They are found less often in persons with intact immunity. Clinically, they primarily cause chronic diarrhea and cholangitis. In the lung, they cause bronchitis or bronchiolitis (or both), usually in patients who also have intestinal infection or disease in other sites, especially the biliary tract.348 The predominant pathologic changes are in the airways, which show a mixed inflammatory cell infiltrate of mononuclear and polymorphonuclear leukocytes.349 The organisms are found within vacuoles in the apical portion of epithelial cells lining the airways. They appear as very small (1–1.5 μm in diameter) basophilic dots, whose recognition depends on organism load. However, even when heavy, the findings can be subtle. Also, as with cryptosporidiosis, their presence often is overlooked or obscured by coexistent pneumonias. Special stains, such as modified trichrome, Warthin-Starry–type silver, and Gram stains, are more sensitive and specific, especially when used in combination.350
Leishmaniasis
Leishmaniasis (Leishmania donovani infection) is transmitted to humans by several species of the Phlebotomus sand fly.351 Pulmonary leishmaniasis has been reported in HIV-infected patients and transplant recipients.334 The organisms (L. donovani amastigotes) can be found in the alveoli and alveolar septa and may be recovered in BAL fluid from these patients.352 They also can be found in bronchoscopic biopsies. (Fig. 6-113). Serologic testing for leishmaniasis has been suggested as part of the pre-transplantation workup in endemic areas.353 A rapid PCR-amplified diagnostic method has been described.354
Dirofilariasis
The zoonosis caused by Dirofilaria immitis, a parasite of dogs and other mammals, is transmitted by mosquitos and black flies to humans. Larvae injected by these insect vectors migrate from the subcutis into veins and travel to the heart, where they die before maturing into adult worms. They are then washed into the lungs by the pulmonary arterial blood flow, where they form the nidus of a thrombus. Formation of an infarct follows, typically manifesting as an asymptomatic solitary pulmonary nodule (“coin lesion”) in the lung periphery (Fig. 6-114) that may be visualized on a positron emission tomography (PET) scan.355–357 Microscopically, the nodule resembles a typical infarct with a core of coagulation necrosis but also containing degenerated worm fragments in the remnant of an arteriole (Fig. 6-115). A peripheral investment of chronic granulation tissue forms an interface with the alveolated parenchyma. “Step” sections and trichrome stains may be needed when H&E sections do not show the parasite.358
Strongyloidiasis
Strongyloides is a parasite most often found in patients or travelers in the tropics, but endemic foci are present in the southeastern United States. Rabditiform larvae of the nematode Strongyloides stercoralis, after hatching from ingested eggs, invade the small intestinal mucosa. At this site occult infection may remain asymptomatic for years. Dissemination typically follows debilitation brought on by immunocompromising diseases and therapies. When this occurs, filariform larvae leave the gut and travel through the pulmonary vasculature. When they penetrate alveoli (Fig. 6-116), they provoke hemorrhage and inflammation.359 Loeffler syndrome, eosinophilic pneumonia, and abscesses may develop. When migration is interrupted, filariform larvae may metamorphose in situ to adult worms, which can produce eggs and rabidiform larvae. Larvae identified in the sputum indicate hyperinfection.360 Disseminated stronglyloidiasis is but one example of an infection that may become manifest, particularly in immunocompromised patients, years after emigration from or travel to an endemic area harboring pathogens considered unusual or exotic by pathologists in the United States.
Echinococcosis
Echinococcosis is a zoonosis that occurs wherever sheep, dogs or other canids, and humans live in close contact. Ingested eggs of the tapeworm Echinococcus hatch in the gut, releasing oncospheres, which then invade the mucosa, enter the circulation, and travel to various sites, where they develop into hydatid cysts. In the lung, unilocular slow-growing cysts are produced by Echinococcus granulosus.361 Echinococcus multilocularis proliferates by budding, producing an alveolar pattern of microvesicles.343 The cyst of E. granulosus has a trilayered membrane (Fig. 6-117A) with an outer fibrous, middle-laminated hyaline, and inner germinal layer that gives rise to brood capsules containing infective protoscolices with hooklets and suckers (see Fig. 6-117B). The layers usually become separated in tissue, with the outer fibrous layer containing chronic inflammatory cells forming an interface with the alveolated parenchyma. Cysts that rupture into bronchi may be expectorated as debris with protoscolices or portions of the cyst wall. Abscesses and granulomas may also form in the lung, pleura, and chest wall.362
Paragonimoniasis
The parasite Paragonimus targets the lung and is acquired by the ingestion of freshwater crabs or crayfish infected with the metacercarial larvae of Paragonimus species. Most cases worldwide are due to P. westermani but several other species exist in Asia, Africa, South and Latin America. In the United States, infections due to P. kellicotti have been reported.336 The disease manifestations are related to the migratory route and the inflammatory response these hermaphroditic flukes stimulate as they enter lung parenchyma and travel to sites near larger bronchioles or bronchi. Typically, an area of eosinophil-rich inflammatory reaction surrounds them, and this reactive process may evolve to form a fibrous pseudocyst or capsule containing worms, exudate, and debris (Fig. 6-118A). Cysts rupturing into bronchioles may result in eggs, blood, and inflammatory cells being coughed up in the sputum. Alternatively, eggs may become embedded in parenchyma, producing nodular granulomatous lesions (see Fig. 6-118B) that progress to scars.363 The eggs are yellowish, ovoid, and operculated, measuring 75 to 110 μm by 45 to 60 μm. The opercula unfortunately are not easily seen in tissue; however, the eggs are birefringent under polarized light, which helps to distinguish them from nonbirefringent schistosome eggs.336
Schistosomiasis
The public health burden of schistosomiasis is enormous: This parasitic infection affects 200 million people in 74 countries while continuing to expand its geographic range.364,365 The life cycle and disease manifestations of the three major Schistosoma species—Schistosoma mansoni, Schistosoma haematobium, and Schistosoma japonicum—involve eggs, snail intermediate hosts, and free-swimming cercaria, which penetrate the skin of susceptible animals and people and develop into adult worms. The male and female worms eventually come to reside in various human venous plexuses, depending on the species, where egg deposition occurs. Pulmonary schistosomiasis comprises both acute and chronic forms. The acute disease, referred to as Katayama syndrome, manifests with fever, chills, weight loss, gastrointestinal symptoms, myalgia, and urticaria in patients with no previous exposure to the parasite. Acute larval pneumonitis and a Loeffler-like eosinophilic pneumonia may be seen in this setting.364,366 Chronic pulmonary disease is almost always secondary to severe hepatic involvement with portal hypertension. In this setting, the eggs of S. mansoni, and rarely S. japonicum or S. haematobium, may be shunted through portosystemic collateral veins to the lungs. The eggs lodge in arterioles, provoking a characteristic granulomatous endarteritis with pulmonary symptoms and radiologic infiltrates.367,368 When the endarteritis is accompanied by angiomatoid changes, the lesion is considered pathognomonic for pulmonary schistosomiasis.336
Eggs typically are surrounded by epithelioid cells and collagen (Fig. 6-119). Most schistosome eggs do not exhibit birefringence and are larger than Paragonimus eggs, with which they share a superficial resemblance. Adult schistosomes may rarely be found in pulmonary blood vessels.
Visceral Larva Migrans
The common parasites that cause visceral larva migrans are the dog tapeworm, Toxacara canis, and the less common cat tapeworm, Toxacara catis. When embryonated eggs are ingested by an intermediate host, typically a child with a history of pica, they hatch into infective larvae in the intestine. Subsequently, the larvae penetrate the intestinal wall, gain access to the circulation, and are carried to many organs, including the lungs. This is the end point, for their growth is arrested by a granulomatous reaction and they never mature into adult worms. The granulomatous reaction usually has a conspicuous eosinophilic component, and larvae may be seen.369
Cytopathology
The cytologic literature contains many reports of the successful identification of parasites in pulmonary specimens recovered by exfoliative (sputum, bronchial washing or brushing, BAL fluid, pleural fluid) and needle aspiration techniques. Some of these are listed in Box 6-26.352,355–357,362,370–381 Commonly cited in textbooks and reviews is the finding of Strongyloides stercoralis larvae in expectorated sputum or bronchial washings of patients with hyperinfections (Fig. 6-120). Also common are reports of Echinococcus protoscolices and hooklets in needle aspirates from patients with pleuropulmonary disease.43,44 Use of large-bore and cutting needle biopsies traditionally has been contraindicated in the setting of suspected Echinococcus infections; reports of success with fine-needle aspiration, without untoward reactions, suggest that this latter technique is a relatively safe procedure in which the benefits outweigh the risks.372
Microbiology
The laboratory diagnosis of parasitic disease depends on the collection of appropriate specimens, which in turn requires appropriate clinical evaluation. For example, just as stool examination is the most efficient means of diagnosing most intestinal protozoa and helminths, respiratory specimens (e.g., sputum samples, bronchial washings, BAL fluid samples, touch imprints of lung biopsy tissue) can provide a specific etiologic diagnosis when pulmonary infections are suspected.335 As in the case for cytologic samples, these specimens often reveal the characteristic microanatomic features of parasite larvae and eggs that usually cannot be readily seen when they are embedded in tissue. Moreover, the identification of organisms in respiratory specimens is diagnostic of pulmonary infection, whereas the presence of the organism in the feces of a patient suspected to have pulmonary disease provides only presumptive evidence.
Serodiagnosis with immunologic and molecular methods can be useful when parasites are located deep within tissue, such as the lung, and not easily accessible to biopsy or cytologic sampling.341 The effectiveness of serodiagnosis of parasitic diseases has been hampered by tests with low sensitivity and specificity, mainly as a result of the complex composition of parasitic antigens and the occurrence of frequent cross reactions.335 In recent years, however, significant refinements in antigenic preparations and improvements in technology have resulted in assays with greater predictive value. The newer tests are based on enzyme immunoassay and immunoblot methodology. Many test kits are commercially available, and diagnostic services are available from the CDC and other reference laboratories.382
With protozoal infections, serologic testing is especially useful for the diagnosis of toxoplasmosis. Several commercial kits are available for detection of immunoglobulin G (IgG) and IgM antibodies; however, false-negative results are possible in immunocompromised patients, and positive results must be interpreted with caution, especially when the index of clinical suspicion is low.383 Real-time PCR analysis has been used for the diagnosis of toxoplasmosis in the immunocompromised patient.384,385 Antibody determinations also have value in cases of pulmonary and other tissue-invasive forms of amebiasis, as compared with antigen detection methods, which are more useful for noninvasive amebic intestinal diseases. However, the best diagnostic approach to invasive disease may be the use of serologic testing, antigen detection, and PCR methods, in various combinations.386 For identification of cryptosporidia, the new immunofluorescence tests and enzyme immunoassays that have been developed for intestinal infections may have application in respiratory infections. Similar tests are not available for the microsporidia, and diagnosis of infection with these organisms continues to rely on direct staining techniques at this time. For the helminths, serodiagnosis is possible for Echinococcus, Paragonimus, Strongyloides, and Schistosoma species using enzyme immunoassay methods, which have fair sensitivity and specificity.334,382 The available tests for Dirofilaria suffer from poor sensitivity and specificity and are not clinically useful at this time.
Differential Diagnosis
The key morphologic and microbiologic features of selected parasitic lung infections are summarized in Table 6-13. In the absence of eggs, larvae, worms, or trophozoites, the various inflammatory patterns must be distinguished from those of other infections and various noninfectious processes due to toxins, drugs, and such entities as asthma, allergic bronchopulmonary aspergillosis, and pulmonary vasculitis syndromes including Churg-Strauss and hypereosinophilic syndromes.387 Acute and chronic forms of eosinophilic pneumonia, as previously emphasized, have a varied etiology that includes parasitic infections.388 False-positive morphologic diagnosis of a parasitic infection may be based on presence of objects resembling parasites337,389 such as lentils in aspiration pneumonia, pollen grains, or Liesegang rings. These ring-like structures can simulate various types of nematodes.390 Careful attention to the microanatomy of an apparent foreign body and comparison with parasites illustrated in atlases often can resolve such diagnostic dilemmas. Some cases, however, may require referral to pathologists with specialized training and experience in parasitic diseases.
Table 6-13 Parasitic Pneumonias: Summary of Pathologic Findings
| Assessment Component | Findings |
|---|---|
| Toxoplasmosis | |
| Surgical pathology | Miliary small necroinflammatory nodules with fibrin; fibrinous pneumonia |
| Cytopathology | Crescent-shaped tachyzoites, pseudocysts and true cysts |
| Microbiology | Serologic diagnosis by IFA or EIA Identification of tachyzoites or pseudocyst in tissue |
| Amebiasis | |
| Surgical pathology | Lung abscess |
| Cytopathology | Trophozoite in necroinflammatory debris resembles histiocytes Confirm with immunohistochemistry |
| Microbiology | Identification of trophozoite characteristics Serologic methods positive in most cases of extraintestinal disease DNA probes |
| Cryptosporidiosis | |
| Surgical pathology | Bronchitis and/or bronchiolitis with cryptosporidia seen on H&E sections as small, round protrusions along the epithelial surface of the mucosa |
| Cytopathology | Red oocysts in smears prepared from bronchial washes and BAL fluid stained with modified acid-fast stains |
| Microbiology | Findings on direct examination of specimens similar to those on cytologic examination Immunofluorescence and enzyme immunoassays developed for intestinal infection |
| Microsporidiosis | |
| Surgical pathology | Bronchitis and/or bronchiolitis Small basophilic dots in vacuoles may be visible in H&E-stained sections when burden of organism is heavy; highlighted with Gram and modified trichrome stains; toluidine blue stain on plastic sections; electron microscopy |
| Cytopathology | Characteristic pink capsule-shaped spores with dark band in modified trichrome-stained preparations of BAL fluid Giemsa, Gram, and chemofluorescence stains also useful |
| Microbiology | Findings on direct examination of fluids similar to those on cytologic examination Culture in research setting by special arrangement Molecular methods |
| Dirofilariasis | |
| Surgical pathology | Solitary pulmonary nodule with infarct pattern and worm fragments |
| Cytopathology | Intact or fragmented worm in necroinflammatory debris |
| Microbiology | Identification of characteristic roundworm in tissues Serologic studies not useful |
| Strongyloides Infection | |
| Surgical pathology | Eosinophilic pneumonia, abscess, Loeffler syndrome with filariform larvae |
| Cytopathology | Filariform larvae in sputum indicates hyperinfection |
| Microbiology | Primary diagnostic stage in stool is rhabitiform larvae; filariform larvae may be seen in sputum and lung tissue Eggs resemble hookworm eggs, but rarely seen |
| Echinococcus Infection | |
| Surgical pathology | Trilayered cyst with brood capsules containing protoscolices Fibrous wall forms interface with lung parenchyma; sometimes abscess and granulomas |
| Cytopathology | Protoscolices with sucker and hooklets or detached hooklets in granular background debris |
| Microbiology | Identification of hooklets and protoscolices in needle aspirates, pleural fluid, and sputum Serologic testing available |
| Paragonimiasis | |
| Surgical pathology | Eosinophilic pneumonia Fibrous pseudocysts containing worms and necroinflammatory debris Egg granulomas |
| Cytopathology | Yellow ovoid birefringent eggs with flattened operculum |
| Microbiology | Identification of characteristic egg in sputum or tissue Serologic testing available |
| Schistosomiasis | |
| Surgical pathology | Granulomatous endarteritis; eggs in epithelioid granulomas |
| Cytopathology | Characteristic nonbirefringent, nonoperculated eggs Presence and position of spine determines species |
| Microbiology | Embryonated eggs may be present in feces or urine; not sputum Serologic testing available |
BAL, bronchoalveolar fluid; EIA, enzyme immunoassay; H&E, hematoxylin-eosin; IFA, immmunofluoresence assay.
1 Fauci A.S. Infectious diseases: considerations for the 21st century. Clin Infect Dis. 2001;32(5):675-685.
2 Schmidt-Ioanas M. Treatment of pneumonia in elderly patient. Exp Opin Pharmacol. 2006;7:499-507.
3 Chandler F. Approaches to the pathologic diagnosis of infectious diseases. In: Chandler F., Connor D., Schwartz D., editors. Pathology of Infectious Disease. Stamford, CT: Appleton & Lange; 1997:3-7.
4 Watts J. The surgical pathologist’s role in the diagnosis of infectious disease. J Histotechnol. 1995;18:191-193.
5 Watts J.C. Surgical pathology and the diagnosis of infectious diseases. Am J Clin Pathol. 1994;102(6):711-712.
6 Morales A.R., Essenfeld H., Essenfeld E., et al. Continuous-specimen-flow, high-throughput, 1-hour tissue processing. A system for rapid diagnostic tissue preparation. Arch Pathol Lab Med. 2002;126(5):583-590.
7 Rosati L.A. The microbe, creator of the pathologist: an inter-related history of pathology, microbiology, and infectious disease. Ann Diagn Pathol. 2001;5(3):184-189.
8 Woods G.L., Walker D.H. Detection of infection or infectious agents by use of cytologic and histologic stains. Clin Microbiol Rev. 1996;9(3):382-404.
9 Procop G.W., Wilson M. Infectious disease pathology. Clin Infect Dis. 2001;32(11):1589-1601.
10 Braunstein H. The value of microbiologic culture of tissue samples in surgical pathology. Mod Pathol. 1989;2(3):217-221.
11 Travis W.D. Surgical pathology of pulmonary infections. Semin Thorac Cardiovasc Surg. 1995;7(2):62-69.
12 Colby T.V., Weiss R.L. Current concepts in the surgical pathology of pulmonary infections. Am J Surg Pathol. 1987;11(suppl 1):25-37.
13 Dunn D.L. Diagnosis and treatment of opportunistic infections in immunocompromised surgical patients. Am Surg. 2000;66(2):117-125.
14 Levine S.J. An approach to the diagnosis of pulmonary infections in immunosuppressed patients. Semin Respir Infect. 1992;7(2):81-95.
15 Dichter J.R., Levine S.J., Shelhamer J.H. Approach to the immunocompromised host with pulmonary symptoms. Hematol Oncol Clin North Am. 1993;7(4):887-912.
16 Wilson W.R., Cockerill F.R.3rd, Rosenow E.C.3rd. Pulmonary disease in the immunocompromised host (2). Mayo Clin Proc. 1985;60(9):610-631.
17 Khoor A., Leslie K.O., Tazelaar H.D., et al. Diffuse pulmonary disease caused by nontuberculous mycobacteria in immunocompetent people (hot tub lung). Am J Clin Pathol. 2001;115(5):755-762.
18 Gaspar H.B., Goldblatt D. Immunodeficiency syndromes and recurrent infection. Br J Hosp Med. 1997;58(11):565-568.
19 Ming J.E., Stiehm E.R., Graham J.M.Jr. Immunodeficiency as a component of recognizable syndromes. Am J Med Genet. 1996;66(4):378-398.
20 Paller A.S. Update on selected inherited immunodeficiency syndromes. Semin Dermatol. 1995;14(1):60-65.
21 Williams L.W. Congenital immunodeficiency syndromes. Chest Surg Clin N Am. 1999;9(1):239-257.
22 Davies J.C., Rudin B.K. Emerging and unusual gram-negative infections in cystic fibrosis. Semin Respir Crit Care Med. 2007;28:312-321.
23 Sibley C., Rabin H., Surette M. Cystic fibrosis: a polymicrobial infection. Future Microbiol. 2006;1:53-61.
24 Hayes D. Mycobacterium abscessus and other nontuberculosus mycobacteria: Evolving respiratory pathogens in cystic fibrosis: a case report and review. South Med J. 2005;98:657-661.
25 Simmonds E., Littlewood J., Evans E. Cystic fibrosis and allergic bronchopulmonary aspergillosis. Arch Dis Child. 1990;65:507-511.
26 van Ewijk B.E., van der Zalm M.M., Wolfs T.F., et al. Prevalence and impact of respiratory viral infections in young children with cystic fibrosis: prospective cohort study. Pediatrics. 2008;122:1171-1176.
27 Wat D., Gelder C., Hibbitts S., et al. The role of respiratory viruses in cystic fibrosis. J Cystic Fibros. 2008;7(4):320-328.
28 Colby T.V., Swensen S.J. Anatomic distribution and histopathologic patterns in diffuse lung disease: correlation with HRCT. J Thorac Imaging. 1996;11(1):1-26.
29 Leslie K.O. My approach to interstitial lung disease using clinical, radioological and histopathologic pattterns. J Clin Pathol. 2009;62(5):387-401.
30 Elicker B., Pereira C.A., Webb R., Leslie K.O. High-resolution computed tomography patterns of diffuse interstitial lung disease with clinical and pathological correlation. J Bras Pneumol. 2008;34(9):715-744.
31 Montone K.T., Park C. In situ hybridization with oligonucleotide probes: applications to infectious agent detection. In: Advances in Pathology. London: Year Book–Mosby; 1996::329-357.
32 Cartun R. Use of immunohistochemistry in the surgical pathology laboratory for the diagnosis of infectious disease. Pathol Case Rev. 1999;4:260-265.
33 Wolk D., Mitchell S., Patel R. Principles of molecular microbiology testing methods. Infect Dis Clin North Am. 2001;15(4):1157-1204.
34 Versalovic J. Arrays and medical microbiology. Arch Pathol Lab Med. 2009;133:537-541.
35 Benson R., Tondella M.L., Bhatnagar J., et al. Technology for molecular differential detection of bacterial respiratory disease pathogens. J Clin Microbiol. 2008;46:2074-2077.
36 Chan Y., Morris A. Molecular methods in pneumonia. Curr Opin Infect Dis. 2007;20:157-164.
37 Kumar S., Wang L., Fan J., et al. Detection of 11 common viral and bacterial pathogens causing community-acquired pneumonia or sepsis in assymptomatic patients by using a mutiplex reverse transcription–PCR assay with manual (enzyme hybridization) or automated (electronic microarray) detection. J Clin Microbiol. 2008;46:3063-3072.
38 Kaufman L., Valero G., Padhye A.A. Misleading manifestations of Coccidioides immitis in vivo. J Clin Microbiol. 1998;36(12):3721-3723.
39 Liu K., Howell D.N., Perfect J.R., Schell W.A. Morphologic criteria for the preliminary identification of Fusarium, Paecilomyces, and Acremonium species by histopathology. Am J Clin Pathol. 1998;109(1):45-54.
40 Gorelkin L., Chandler F.W. Pseudomicrobes: some potential diagnostic pitfalls in the histopathologic assessment of inflammatory lesions. Hum Pathol. 1988;19(8):954-959.
41 Al-Za’abi A.M., MacDonald S., Geddie W., Boerner S.L. Cytologic examination of bronchoalveolar lavage fluid from immunosuppressed patients. Diagn Cytopathol. 2007;35:710-714.
42 DeMay R.M. A micromiscellany. In: DeMay R.M., editor. The Art and Science of Cytopathology. Vol. 1, Exfoliative Cytology. Chicago: ASCP Press; 1996:53-58.
43 Powers C.N. Diagnosis of infectious disease: a cytopathologist’s perspective. Clin Microbiol Rev. 1998;11:341-365.
44 Johnson W., Elson C. Respiratory tract. In: Bibbo M., editor. Comprehensive Cytopathology. Philadelphia: Saunders; 1991:340-352.
45 Silverman J., Gay J. Fine-needle aspiration and surgical pathology of infectious disease: morphologic features and role of the clinical microbiology laboratory for rapid diagnosis. Clin Lab Med. 1995;15:251-278.
46 Crapanzano J.P., Zakowski M.F. Diagnostic dilemmas in pulmonary cytology. Cancer. 2001;93(6):364-375.
47 Granville L., Laucirica R., Verstovek G. Cinical significance of cultures collected from fine-needle aspirates. Diagn Cytopathol. 2008;36:85-88.
48 Fang G., Fine M., Orloff J. New and emerging etiologies for community-acquired pneumonias with implications for therapy. Medicine (Baltimore). 1990;69:307-316.
49 Ruiz-Gonzales A., Falquera M., Nogues A. Is Streptococcus pneumoniae the leading cause of pneumonia of unknown etiology? Am J Med. 1999;106:385-390.
50 Reimer L. Community-acquired bacterial pneumonia. Semin Respir Infect. 2000;15:95-100.
51 Travis W.D., Colby T.V., Koss M.N., et al. Lung infections. In: King D., editor. Atlas of Non-Tumor Pathology, Fascicle 2. Non-neoplastic Disorders of the Lower Respiratory Tract. Washington, DC: American Registry of Pathology; 2002:539-728.
52 McIntosh K. Community-acquired pneumonia in children. N Engl J Med. 2002;346:429-436.
53 Pennington J. Hospital acquired pneumonia. In: Pennington J., editor. Respiratory Infections. Diagnosis and Management. New York: Raven Press; 1994:207-227.
54 Mayer J. Laboratory diagnosis of nosocomial pneumonia. Semin Respir Infect. 2000;15:119-131.
55 Baselski V., Mason K. Pneumonia in the immunocompromised host: the role of bronchoscopy and newer diagnostic methods. Semin Respir Infect. 2000;15:144-161.
56 Muscedere J., Dodek P., Keenan S., et al. Comprehensive evidence-based clinical practice guidelines for ventilator-associated pneumonia: diagnosis and treatment. J Crit Care. 2008;23:132-147.
57 Wall R.J., Ely E.W., Talbot T.R., et al. Evidence-based algorithms for diagnosing and treating ventilator-associated pneumonia. J Hosp Med. 2008;3:409-422.
58 Rea-Neto A., Youssef N.C., Tuche F., et al. Diagnosis of ventilator-associated pneumonia: a systematic review of the literature. Crit Care. 2008;12:R56.
59 Park D. The microbiology of ventilator-associated pneumonia. Respir Care. 2005;50:742-763.
60 Rubinstein E., Kollef M., Nathwani D. Pneumonia caused by methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2008;46:S370-S385.
61 Genzen J.R., Towle D.M., Kravetz J.D., Campbell S.M. Salmonella typhimurium pulmonary infection in an immunocompetent patient. Conn Med. 2008;72:142-148.
62 Dolhnikoff M., Mauad T., Bethlem E.P., Carvalho C.R. Pathology and pathophysiology of pulmonary manifestations in leptospirosis. Braz J Infect Dis. 2007;11:142-148.
63 Hindizeh M., Carroll K. Laboratory diagnosis of atypical pneumonia. Semin Respir Infect. 2000;15:101-113.
64 Cunha B. The atypical pneumonias: clinical diagnosis and importance. Clin Microbiol Infect Dis. 2006;12:12-24.
65 Chandler F. Actinomycosis. In: Chandler F., Connor D., Schwartz D., editors. Pathology of Infectious Disease. Stamford, CT: Appleton & Lange; 1997:391-396.
66 de Montpreville V., Nashashibi N., Dulmet E. Actinomycosis and other bronchopulmonary infections with granules. Ann Diagn Pathol. 1999;3:67-74.
67 Winn W., LaSala P., Leslie K.O. Bacterial infections. In: Tomashefski J., Farver C., Cagle P., Fraire A., editors. Dail and Hammer’s Pulmonary Pathology. 3rd ed. New York: Springer; 2008:228-315.
68 Hazelton P. Pulmonary bacterial infections. In: Hazelton P., editor. Spencer’s Pathology of the Lung. New York: McGraw-Hill; 1996:189-256.
69 Lombard C., Yousem S., Kitaichi M., Colby T., editors. Atlas of Pulmonary Surgical Pathology. Philadelphia: Saunders, 1991.
70 Sethi S., Murphy T. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359:2355-2365.
71 Kwon K.Y., Colby T.V. Rhodococcus equi pneumonia and pulmonary malakoplakia in acquired immunodeficiency syndrome. Pathologic features. Arch Pathol Lab Med. 1994;118(7):744-748.
72 Manik P. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344:665-671.
73 Barnes T.W., Vassallo R., Tazelaar H.D., et al. Diffuse bronchiolar disease due to chronic occult aspiration. Mayo Clin Proc. 2006;81:172-176.
74 Mukhopadhyay S., Katzenstein A.L. Pulmonary disease due to aspiration of food and other particulate matter: a clinicopathologic study of 59 cases diagnosed on biopsy or resection specimens. Am J Surg Pathol. 2007;31(5):752-759.
75 Verma P. Laboratory diagnosis of anaerobic pleuropulmonary infections. Semin Respir Infect. 2000;15(2):114-118.
76 Corley D.E., Winterbauer R.H. Infectious diseases that result in slowly resolving and chronic pneumonia. Semin Respir Infect. 1993;8(1):3-13.
77 Belchis D.A., Simpson E., Colby T. Histopathologic features of Burkholderia cepacia pneumonia in patients without cystic fibrosis. Mod Pathol. 2000;13(4):369-372.
78 Low D., Massulli T., Marrie T. Progressive and nonresolving pneumonia. Curr Opin Pulm Med. 2005;11:247-252.
79 Weyers C., Leeper K. Nonresolving pneumonia. Clin Chest Med. 2005;26:143-158.
80 Bauer T., Ewig S., Rodloff A.C., Müller E.E. Acute respiratory distress syndrome and pneumonia: a comprehensive review of clinical data. Clin Infect Dis. 2006;43:748-756.
81 Hayden R.T., Uhl J.R., Qian X., et al. Direct detection of Legionella species from bronchoalveolar lavage and open lung biopsy specimens: comparison of LightCycler PCR, in situ hybridization, direct fluorescence antigen detection, and culture. J Clin Microbiol. 2001;39(7):2618-2626.
82 Rollins S., Colby T., Clayton F. Open lung biopsy in Mycoplasma pneumoniae pneumonia. Arch Pathol Lab Med. 1986;110(1):34-41.
83 Waites K., Tarkington D. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev. 2004;17:697-728.
84 Bartlett A.H., Rivera A.L., Krishnamurthy R., Baker C.J. Thoracic actinomycosis in children: case report and review of the literature. Pediatr Infect Dis J. 2008;27:165-169.
85 Yildz O., Doggoy M. Actinomycosis and nocardia pulmonary infections. Curr Opin Pulm Med. 2006;12:228-234.
86 Vera-Alvarez J., Marigil-Gómez M., García-Prats M.D., et al. Primary pulmonary botryomycosis diagnosed by fine needle aspiration biopsy: a case report. Acta Cytol. 2006;50:331-334.
87 Oddo D., Gonzalez S. Actinomycosis and nocardiosis. A morphologic study of 17 cases. Pathol Res Pract. 1986;181(3):320-326.
88 Dattwyler R. Community-acquired pneumonia in the age of bioterrorism. Allergy Asthma Proc. 2005;26:191-194.
89 Bush L.M., Abrams B.H., Beall A., Johnson C.C. Index case of fatal inhalational anthrax due to bioterrorism in the United States. N Engl J Med. 2001;345(22):1607-1610.
90 Borio L., Frank D., Mani V., et al. Death due to bioterrorism-related inhalational anthrax: report of 2 patients. JAMA. 2001;286(20):2554-2559.
91 Barakat L.A., Quentzel H.L., Jernigan J.A., et al. Fatal inhalational anthrax in a 94-year-old Connecticut woman. JAMA. 2002;287(7):863-868.
92 Grinberg L.M., et al. Quantitative pathology of inhalational anthrax I: quantitative microscopic findings. Mod Pathol. 2001;14(5):482-495.
93 Inglesby T.V., Henderson D.A., Bartlett J.G., et al. Anthrax as a biological weapon: medical and public health management. Working Group on Civilian Biodefense. JAMA. 1999;281(18):1735-1745.
94 Inglesby T.V., Dennis D.T., Henderson D.A., et al. Plague as a biological weapon: medical and public health management. Working Group on Civilian Biodefense. JAMA. 2000;283(17):2281-2290.
95 Guarner J., Shieh W.J., Greer P.W., et al. Immunohistochemical detection of Yersinia pestis in formalin-fixed, paraffin-embedded tissue. Am J Clin Pathol. 2002;117(2):205-209.
96 Smith J., Reisner B. Plague. In: Chandler F., Connor D., Schwartz D., editors. Pathology of Infectious Disease. Stamford, CT: Appleton & Lange; 1997:729-738.
97 Dennis D.T., Inglesby T.V., Henderson D.A., et al. Tularemia as a biological weapon: medical and public health management. JAMA. 2001;285(21):2763-2773.
98 Geyer S., Burkey A., Chandler F. Tularemia. In: Chandler F., Connor D., Schwartz D., editors. Pathology of Infectious Disease. Stamford, CT: Appleton & Lange; 1997:869-873.
99 Yang P.C., Luh K.T., Lee Y.C., et al. Lung abscesses: US examination and US-guided transthoracic aspiration. Radiology. 1991;180(1):171-175.
100 Grinan N., Lucerna F., Romero J. Yield of percutaneous aspiration in lung abscess. Chest. 1990;97:69-74.
101 Vuori-Holopainen E., Salo E., Saxén H., et al. Etiological diagnosis of childhood pneumonia by use of transthoracic needle aspiration and modern microbiological methods. Clin Infect Dis. 2002;34(5):583-590.
102 Garg S., Handa U., Mohan H., Janmeja A.K. Comparative analysis of various cytohistological techniques in diagnosis of lung diseases. Diagn Cytopathol. 2007;35:26-31.
103 Bartlett R. Medical microbiology: How far to go—how fast to go in 1982. In: Lorian V., editor. Significance of Medical Microbiology in the Care of Patients. Baltimore/London: Williams & Wilkins; 1982:12-44.
104 Busmanis I., Harney M., Hellyar A. Nocardiosis diagnosed by lung FNA: a case report. Diagn Cytopathol. 1995;12(1):56-58.
105 Mathur S., Sood R., Aron M., et al. Cytologic diagnosis of pulmonary nocardiosis: a report of 3 cases. Acta Cytol. 2005;49:567-570.
106 Saubolle M.A., McKellar P.P. Laboratory diagnosis of community-acquired lower respiratory tract infection. Infect Dis Clin North Am. 2001;15(4):1025-1045.
107 Carroll K.C. Laboratory diagnosis of lower respiratory tract infections: controversy and conundrums. J Clin Microbiol. 2002;40(9):3115-3120.
108 Stratton K. Usefulness of aetiological tests for guiding antibiotic therapy in community-acquired pneumonia. Int J Antimicrob Agents. 2008;31:3-11.
109 Barrett-Connor E. The nonvalue of sputum culture in the diagnosis of pneumococcal pneumonia. Am Rev Respir Dis. 1971;103(6):845-848.
110 American Thoracic Society. Diagnosis, treatment and prevention of non-tuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
111 Bartlett J.G., Breiman R.F., Mandell L.A., File T.M.Jr. Community-acquired pneumonia in adults: guidelines for management. The Infectious Diseases Society of America. Clin Infect Dis. 1998;26(4):811-838.
112 Rosón B., Carratalà J., Verdaguer R., et al. Prospective study of the usefulness of sputum Gram stain in the initial approach to community-acquired pneumonia requiring hospitalization. Clin Infect Dis. 2000;31:869-874.
113 Musher D., Montoya R., Wanahita A. Diagnostic value of microscopic examination of gram-stained sputum and sputum cultures in patients with bacteremic pneumococcal pneumonia. Clin Infect Dis. 2004;39:165-169.
114 Danés C., González-Martín J., Pumarola T., et al. Pulmonary infiltrates in immunosuppressed patients: analysis of a diagnostic protocol. J Clin Microbiol. 2002;40(6):2134-2140.
115 Ellis J., Oyston P.C., Green M., Titball R.W. Tularemia. Clin Microbiol Rev. 2002;15(4):631-646.
116 Conville P.S., Brown J.M., Steigerwalt A.G., et al. Nocardia veterana as a pathogen in North American patients. J Clin Microbiol. 2003;41(6):2560-2568.
117 Petitjean J., Vabret A., Gouarin S., Freymuth F. Evaluation of four commercial immunoglobulin G (IgG)- and IgM-specific enzyme immunoassays for diagnosis of Mycoplasma pneumoniae infections. J Clin Microbiol. 2002;40(1):165-171.
118 Blasi F., Tarsia P., Aliberti S., et al. Chlamydia pneumoniae and Mycoplasma pneumoniae. Semin Respir Crit Care Med. 2005;26:617-624.
119 Lee K.Y. Pediatric respiratory infection by Mycoplasma pneumoniae. Exp Rev Anti Infect Ther. 2008;6:509-521.
120 Fields B.S., Benson R.F., Besser R.E. Legionella and legionnaires’ disease: 25 years of investigation. Clin Microbiol Rev. 2002;15(3):506-526.
121 Boulware D.R., Daley C.L., Merrifield C., et al. Rapid diagnosis of pneumococcal pneumonia among HIV-infected adults with urine antigen detection. J Infect. 2007;55:300-309.
122 Muder R.R., Yu V.L. Infection due to Legionella species other than L. pneumophila. Clin Infect Dis. 2002;35(8):990-998.
123 Benin A.L., Benson R.F., Besser R.E. Trends in legionnaires disease, 1980–1998: declining mortality and new patterns of diagnosis. Clin Infect Dis. 2002;35(9):1039-1046.
124 Waring A.L., Halse T.A., Csiza C.K., et al. Development of a genomics-based PCR assay for detection of Mycoplasma pneumoniae in a large outbreak in New York State. J Clin Microbiol. 2001;39(4):1385-1390.
125 Reischl U., Linde H.J., Lehn N., et al. Direct detection and differentiation of Legionella spp. and Legionella pneumophila in clinical specimens by dual-color real-time PCR and melting curve analysis. J Clin Microbiol. 2002;40(10):3814-3817.
126 Krafft A., Kulesh D. Applying molecular biologic techniques in detection of biologic agents. Clin Lab Med. 2001;21:631-660.
127 Wittwer C., Hermann M., Gundry C. Real-time multiplex assays. Methods. 2001;25:132-143.
128 Loens K., Beck T., Ursi D., et al. Development of real-time multiplex nucleic acid sequence-based amplification for detection of Mycoplasma pneumoniae, Chlymydia pneumoniae and Legionella spp. in respiratory specimens. J Clin Microbiol. 2008;46:185-191.
129 Kunimoto D., Long R. Tuberculosis: still overlooked as a cause of community-acquired pneumonia—how not to miss it. Respir Care Clin N Am. 2005;11:25-34.
130 Storla D., Yimer S., Bjune G.A. A systematic review of delay in the diagnosis and treatment of tuberculosis. BMC Public Health. 2008;8:15.
131 Gardiner D.F., Beavis K.G. Laboratory diagnosis of mycobacterial infections. Semin Respir Infect. 2000;15(2):132-143.
132 Griffith D.E., Aksamit T., Brown-Elliott B.A., et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367-416.
133 Procop G.W., Tazelaar H.D. Tuberculosis and other mycobacterial infections of the lung. In: Churg A.M., Myers J.L., Tazelaar H.D., Wright J.L., editors. Thurlbeck’s Pathology of the Lung. 3rd ed. New York/Stuttgart: Thieme Medical Publishers; 2005:219-248.
134 Tomashefski J., Farver C. Tuberculosis and nontuberculous mycobacterial infections. In: Tomashefski J., Farver C., Cagle P., Fraire A., editors. Dail and Hammer’s Pulmonary Pathology. 3rd ed. New York: Springer; 2008:316-348.
135 Barnes P., Cave C. Molecular epidemiology of tuberculosis. N Engl J Med. 2003;12:1149-1156.
136 Lack E., Connor D. Tuberculosis. In: Chandler F., Connor D., Schwartz D., editors. Pathology of Infectious Disease. Stamford, CT: Appleton & Lange; 1997:857-868.
137 Small P.M., Fujiwara P.I. Management of tuberculosis in the United States. N Engl J Med. 2001;345(3):189-200.
138 Tortoli E. Impact of genotypic studies on mycobacterial taxonomy: the new mycobacteria of the 1990s. Clin Microbiol Rev. 2003;16(2):319-354.
139 Primm T.P., Lucero C.A., Falkinham J.O.3rd. Health impacts of environmental mycobacteria. Clin Microbiol Rev. 2004;17(1):98-106.
140 Glassroth J. Pulmonary disease due to nontuberculous mycobacteria. Chest. 2008;133:243-251.
141 Field S.K., Cowie R.L. Lung disease due to more common mycobacteria. Chest. 2006;129:1653-1672.
142 Johnson M.M., Waller E.A., Leventhal J.P. Nontuberculous mycobacterial pulmonary disease. Curr Opin Pulm Med. 2008;14:203-210.
143 Prince D.S., Peterson D.D., Steiner R.M., et al. Infection with Mycobacterium avium complex in patients without predisposing conditions. N Engl J Med. 1989;321(13):863-868.
144 Marchevesky A., Damster B., Gribetz A. The spectrum of pathology of nontuberculous mycobacteria in open lung biopsy. Am J Clin Pathol. 1982;78:755.
145 Van Dyck P., Vanhoenacker F.M., Van den Brande P., De Schepper A.M. Imaging of pulmonary tuberculosis. Eur Radiol. 2003;13:1771-1785.
146 Martinez S., McAdams H., Batchu C. The many faces of nontuberculous mycobacterial infection. Am J Roentgenol. 2007;189:177-186.
147 Pieters J. Mycobacterium tuberculosis and the macrophage. Cell Host Microbe. 2008;3:399-407.
148 Leong A.S., Wannakrairot P., Leong T.Y. Apotosis is a major cause of so-called “caseation necrosis” in mycobacterial granulomas in HIV-infected patients. J Clin Pathol. 2008;61:366-372.
149 Tang Y.W., Procop G.W., Zheng H., et al. Histologic parameters predictive of mycobacterial infection. Am J Clin Pathol. 1998;109(3):331-334.
150 Brastianos P.K., Swanson J.W., Torbenson M., et al. Tuberculosis-associated haemophagocytic syndrome. Lancet Infect Dis. 2006;6:447-454.
151 Dehda K., Booth H., Huggett J.F., et al. Lung remodeling in pulmonary tuberculosis. J Infect Dis. 2005;192:1201-1209.
152 Hunter R., Jagannath C., Actor J. Pathology of post primary tuberculosis in humans and mice: contradictions of long-held beliefs. Tuberculosis. 2007;87:267-278.
153 Hoheisel G., Chan B.K., Chan C.H., et al. Endobronchial tuberculosis: diagnostic features and therapeutic outcome. Respir Med. 1994;88(8):593-597.
154 Bloch K.C., Zwerling L., Pletcher M.J., et al. Incidence and clinical implications of isolation of Mycobacterium kansasii: results of a 5-year, population-based study. Ann Intern Med. 1998;129(9):698-704.
155 Rotterdam H. Mycobacterium avium complex infection. In: Chandler F., Connor D., Schwartz D., editors. Pathology of Infectious Disease. Stamford, CT: Appleton & Lange; 1997:657-669.
156 Horsburgh C.R.Jr. Mycobacterium avium complex infection in the acquired immunodeficiency syndrome. N Engl J Med. 1991;324(19):1332-1338.
157 Kwon K.Y., Myers J.L., Swensen S.J., Colby T.V. Middle lobe syndrome: a clinicopathological study of 21 patients. Hum Pathol. 1995;26(3):302-307.
158 Wilson R.W., Steingrube V.A., Böttger E.C., et al. Mycobacterium immunogenum sp. nov, a novel species related to Mycobacterium abscessus and associated with clinical disease, pseudo-outbreaks and contaminated metalworking fluids: an international cooperative study on mycobacterial taxonomy. Int J Syst Evol Microbiol. 2001;51:1751-1764.
159 Sekosan M., Cleto M., Senseng C., et al. Spindle cell pseudotumors in the lungs due to Mycobacterium tuberculosis in a transplant patient. Am J Surg Pathol. 1994;18(10):1065-1068.
160 Asano T., Itoh G., Itoh M. Disseminated Mycobacterium intracellulare infection in an HIV-negative, non-immunosuppressed patient with multiple endobronchial polyps. Respiration. 2002;69:175-177.
161 Brown-Elliott B.A., Wallace R.J.Jr. Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting rapidly growing mycobacteria. Clin Microbiol Rev. 2002;15(4):716-746.
162 Greenberger P., Katzenstein A. Lipoid pneumonia with atypical mycobacterial colonization. Association with allergic bronchopulmonary aspergillosis. Arch Intern Med. 1983;143:2003-2005.
163 Gable A.D., Marsee D.K., Milner D.A., Granter S.R. Suppurative inflammation with microabscess and pseudocyst formation is a characteristic histologic manifestation of cutaneous infections with rapid-growing Mycobacterium species. Am J Clin Pathol. 2008;130:514-517.
164 Das D. Fine needle aspiration cytology in the diagnosis of tuberculous lesions. Lab Med. 2000;31:625-632.
165 Dahlgren S.E., Ekstrom P. Aspiration cytology in the diagnosis of pulmonary tuberculosis. Scand J Respir Dis. 1972;53(4):196-201.
166 Smith M.B., Molina C.P., Schnadig V.J., et al. Pathologic features of Mycobacterium kansasii infection in patients with acquired immunodeficiency syndrome. Arch Pathol Lab Med. 2003;127(5):554-560.
167 Zerbi P., Schønau A., Bonetto S., et al. Amplified in situ hybridization with peptide nucleic acid probes for differentiation of Mycobacterium tuberculosis complex and nontuberculous Mycobacterium species on formalin-fixed, paraffin-embedded archival biopsy and autopsy samples. Am J Clin Pathol. 2001;116(5):770-775.
168 Cho S., Brennan R. Tuberculosis diagnostics. Tuberculosis. 2007;87:s14-s17.
169 Tiwari R.P., Hattikudur N.S., Bharmal R.N., et al. Modern approaches to a rapid diagnosis of tuberculosis: promises and challenges ahead. Tuberculosis. 2007;87:193-201.
170 Hardman W.J., Benian G.M., Howard T., et al. Rapid detection of mycobacteria in inflammatory necrotizing granulomas from formalin-fixed, paraffin-embedded tissue by PCR in clinically high-risk patients with acid-fast stain and culture-negative tissue biopsies. Am J Clin Pathol. 1996;106(3):384-389.
171 Park D.Y., Kim J.Y., Choi K.U., et al. Comparison of polymerase chain reaction with histopathologic features for diagnosis of tuberculosis in formalin-fixed, paraffin-embedded histologic specimens. Arch Pathol Lab Med. 2003;127(3):326-330.
172 Schulz S., Cabras A.D., Kremer M., et al. Species identification of mycobacteria in paraffin-embedded tissues: frequent detection of non tuberculous mycobacteria. Mod Pathol. 2005;18:274-282.
173 Renshaw A.A. The relative sensitivity of special stains and culture in open lung biopsies. Am J Clin Pathol. 1994;102(6):736-740.
174 O’Sullivan C.E., Miller D.R., Schneider P.S., Roberts G.D. Evaluation of Gen-Probe amplified Mycobacterium tuberculosis direct test by using respiratory and nonrespiratory specimens in a tertiary care center laboratory. J Clin Microbiol. 2002;40(5):1723-1727.
175 Bemer P., Palicova F., Rüsch-Gerdes S., et al. Multicenter evaluation of fully automated BacTec Mycobacteria Growth Indicator Tube 960 system for susceptibility testing of Mycobacterium tuberculosis. J Clin Microbiol. 2002;40(1):150-154.
176 Woods G.L. Molecular techniques in mycobacterial detection. Arch Pathol Lab Med. 2001;125(1):122-126.
177 Hasegawa N., Miura T., Ishii K., et al. New simple and rapid test for culture confirmation of Mycobacterium tuberculosis complex: a multicenter study. J Clin Microbiol. 2002;40(3):908-912.
178 Al Zahrani K., Al Jahdali H., Poirier L., et al. Accuracy and utility of commercially available amplification and serologic tests for the diagnosis of minimal pulmonary tuberculosis. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1323-1329.
179 Schluger N.W. Changing approaches to the diagnosis of tuberculosis. Am J Respir Crit Care Med. 2001;164(11):2020-2024.
180 Sabonya R. Fungal disease, including Pneumocystis. In: Churg A.M., Myers J.L., Tazelaar H.D., Wright J.L., editors. Thurlbeck’s Pathology of the Lung. 3rd ed. New York: Thieme Medical Publishers; 2005:283-314.
181 Haque A., McGinnis M.R. Fungal infections. In: Tomashefski J., Farver C., Cagle P., Fraire A., editors. Dail and Hammer’s Pulmonary Pathology. 3rd ed. New York: Springer; 2008:349-425.
182 Saubolle M.A. Fungal pneumonias. Semin Respir Infect. 2000;15(2):162-177.
183 Karnak D., Avery R.K., Gildea T.R., et al. Endobronchial fungal disease: an under-recognized entity. Respiration. 2007;74:88-104.
184 Chandler F., Watts J.C. Pathologic Diagnosis of Fungal Infections. Chicago: ASCP Press, 1987.
185 Watts J.C., Chandler F.W. Morphologic identification of mycelial pathogens in tissue sections. A caveat. Am J Clin Pathol. 1998;109(1):1-2.
186 Lemos L.B., Baliga M., Guo M. Blastomycosis: The great pretender can also be an opportunist. Initial clinical diagnosis and underlying diseases in 123 patients. Ann Diagn Pathol. 2002;6(3):194-203.
187 Taxy J. Blastomycosis: contributions of morphology to diagnosis. A surgical pathology, cytopathology and autopsy study. Am J Surg Pathol. 2007;31:615-623.
188 Lemos L.B., Guo M., Baliga M. Blastomycosis: organ involvement and etiologic diagnosis. A review of 123 patients from Mississippi. Ann Diagn Pathol. 2000;4(6):391-406.
189 Chandler F. Blastomycosis. In: Chandler F., Connor D., Schwartz D., editors. Pathology of Infectious Disease. Stamford, CT: Appleton & Lange; 1997:943-951.
190 Hussain Z., Martin A., Youngberg G.A. Blastomyces dermatitidis with large yeast forms. Arch Pathol Lab Med. 2001;125(5):663-664.
191 Fisher M.C., Koenig G.L., White T.J., Taylor J.W. Molecular and phenotypic description of Coccidioides posadasii sp. nov., previously recognized as the no-California population of Coccidioides immitis. Mycologia. 2002;94:73-84.
192 Pappagianis D., Chandler F. Coccidioidomycosis. In: Chandler F., Connor D., Schwartz D., editors. Pathology of Infectious Disease. Stamford, CT: Appleton & Lange; 1997:977-987.
193 DiTomasso J.P., Ampel N.M., Sobonya R.E., Bloom J.W. Bronchoscopic diagnosis of pulmonary coccidioidomycosis. Comparison of cytology, culture, and transbronchial biopsy. Diagn Microbiol Infect Dis. 1994;18(2):83-87.
194 Polesky A., Kirsch C.M., Snyder L.S., et al. Airway coccidioidomycosis—report of cases and review. Clin Infect Dis. 1999;28(6):1273-1280.
195 Valdivia L., Nix D., Wright M., et al. Coccidioidomycosis as a common cause of community-acquired pneumonia. Emerg Infect Dis. 2006;12:958-962.
196 Wheat J. Endemic mycoses in AIDS: A clinical review. Clin Microbiol Rev. 1995;8(1):146-159.
197 Goodwin R.A.Jr, Shapiro J.L., Thurman G.H., et al. Disseminated histoplasmosis: clinical and pathologic correlations. Medicine (Baltimore). 1980;59(1):1-33.
198 Chandler F., Watts J.C. Histoplasmosis capsulati. In: Chandler F., Connor D., Schwartz D., editors. Pathology of Infectious Disease. Stamford, CT: Appleton & Lange; 1997:1007-1015.
199 Londero A., Chandler F. Paracoccidioidomycosis. In: Chandler F., Connor D., Schwartz D., editors. Pathology of Infectious Disease. Stamford, CT: Appleton & Lange; 1997:1045-1053.
200 England D.M., Hochholzer L. Primary pulmonary sporotrichosis. Report of eight cases with clinicopathologic review. Am J Surg Pathol. 1985;9(3):193-204.
201 Deng Z.L., Connor D.H. Progressive disseminated penicilliosis caused by Penicillium marneffei. Report of eight cases and differentiation of the causative organism from Histoplasma capsulatum. Am J Clin Pathol. 1985;84(3):323-327.
202 McGinnis M.R., Chandler F. Penicilliosis marneffei. In: Chandler F., Connor D., Schwartz D., editors. Pathology of Infectious Disease. Stamford, CT: Appleton and Lange; 1997:1055-1058.
203 Mark E.J. Case records of the Massachusetts General Hospital. N Engl J Med. 2002;347:518-524.
204 Menefee J., Hutchins G.M. Pulmonary cryptococcosis. Hum Pathol. 1985;16:121-128.
205 Singh Y., Pratistadevi K. Cryptococcal inflammatory pseudotumor. Am J Surg Pathol. 2007;31:1521-1527.
206 Pfaller M., Diekema D. Rare and emerging opportunistic fungal pathogens: concerns for resistance beyond Candida albicans and Aspergillus fumigatus. J Clin Microbiol. 2004;42:4419-4431.
207 Lun M. Candidiasis. In: Chandler F., Connor D., Schwartz D., editors. Pathology of Infectious Disease. Stamford, CT: Appleton & Lange; 1997:953-964.
208 Latge J.P. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev. 1999;12(2):310-350.
209 Bosken C.H., Myers J.L., Greenberger P.A., Katzenstein A.L. Pathologic features of allergic bronchopulmonary aspergillosis. Am J Surg Pathol. 1988;12(3):216-222.
210 Yousem S.A. The histological spectrum of chronic necrotizing forms of pulmonary aspergillosis. Hum Pathol. 1997;28(6):650-656.
211 Scully R., Mark E., McNeeley W. Case records of the Massachusetts General Hospital. N Engl J Med. 2001;345:443-449.
212 Segal B. Aspergillosis. N Engl J Med. 2009;360:1870-1884.
213 Stergiopoulou T., Meletiadis J., Roilides E., et al. Host-dependent patterns of tissue injury in invasive pulmonary aspergillosis. Am J Clin Pathol. 2007;127:349-355.
214 Kradin R., Mark E.J. The pathology of pulmonary disorders due to Aspergillus spp. Arch Pathol Lab Med. 2008;132:606-614.
215 Tadros T.S., Workowski K.A., Siegel R.J., et al. Pathology of hyalohyphomycosis caused by Scedosporium apiospermum (Pseudallescheria boydii): an emerging mycosis. Hum Pathol. 1998;29(11):1266-1272.
216 Boutati E.I., Anaissie E.J. Fusarium, a significant emerging pathogen in patients with hematologic malignancy: ten years’ experience at a cancer center and implications for management. Blood. 1997;90(3):999-1008.
217 Gutiérrez F., Masiá M., Ramos J., et al. Pulmonary mycetoma caused by an atypical isolate of Paceilomyces species in an immunocompetent individual: case report and literature review of Paecilomyces lung infections. Eur J Clin Microbiol Infect Dis. 2005;24:607-611.
218 Bigliazzi C., Poletti V., Dell’Amore D., et al. Disseminated basidiobolomycosis in an immunocompetent woman. J Clin Microbiol. 2004;42:1367-1369.
219 Guarro J., Gene J., Stchigel A.M. Developments in fungal taxonomy. Clin Microbiol Rev. 1999;12(3):454-500.
220 Ribes J.A., Vanover-Sams C.L., Baker D.J. Zygomycetes in human disease. Clin Microbiol Rev. 2000;13(2):236-301.
221 Zaoutis T., Roilides E., Chiou C. Zygomycosis in children. Pediatr Infect Dis J. 2007;26:723-727.
222 Irwin R., Rinaldi M., Walsh T. Zygomycosis of the respiratory tract. In: Sarosi G., Davies S., editors. Fungal Diseases of the Lung. Philadelphia: Lippincott Williams & Wilkins; 2000:163-185.
223 Hansen L.A., Prakash U.B., Colby T.V. Pulmonary complications in diabetes mellitus. Mayo Clin Proc. 1989;64(7):791-799.
224 Frater J.L., Hall G.S., Procop G.W. Histologic features of zygomycosis: emphasis on perineural invasion and fungal morphology. Arch Pathol Lab Med. 2001;125(3):375-378.
225 Kimura M., Schnadig V.J., McGinnis M.R. Chlamydoconidia formation in zygomycosis due to Rhizopus species. Arch Pathol Lab Med. 1998;122(12):1120-1122.
226 Lake F.R., Froudist J.H., McAleer R., et al. Allergic bronchopulmonary fungal disease caused by Bipolaris and Curvularia. Aust N Z J Med. 1991;21(6):871-874.
227 Travis W.D., Kwon-Chung K.J., Kleiner D.E., et al. Unusual aspects of allergic bronchopulmonary fungal disease: report of two cases due to Curvularia organisms associated with allergic fungal sinusitis. Hum Pathol. 1991;22(12):1240-1248.
228 Revankar S. Therapy of infections caused by dematiaceous fungi. Exp Rev Anti Infect Ther. 2005;3:601-612.
229 Stringer J.R., Beard C.B., Miller R.F., Wakefield A.E. A new name (Pneumocystis jiroveci) for Pneumocystis from humans. Emerg Infect Dis. 2002;8(9):891-896.
230 Zahar J.R., Robin M., Azoulay E., et al. Pneumocystis carinii pneumonia in critically ill patients with malignancy: a descriptive study. Clin Infect Dis. 2002;35(8):929-934.
231 Schliep T.C., Yarrish R.L. Pneumocystis carinii pneumonia. Semin Respir Infect. 1999;14(4):333-343.
232 Wazir J., Ansari N. Pneumocystis carinii infection. Update and review. Arch Pathol Lab Med. 2004;128:1023-1027.
233 Travis W.D., Pittaluga S., Lipschik G.Y., et al. Atypical pathologic manifestations of Pneumocystis carinii pneumonia in the acquired immune deficiency syndrome. Review of 123 lung biopsies from 76 patients with emphasis on cysts, vascular invasion, vasculitis, and granulomas. Am J Surg Pathol. 1990;14(7):615-625.
234 Haque A., Adegboyega P. Pneumocystis jiroveci pneumonia. In: Tomashefski J., Farver C., Cagle P., Fraire A., editors. Dail and Hammar’s Pulmonary Pathology. 3rd ed. New York: Springer; 2008:426-475.
235 Hartz J.W., Geisinger K.R., Scharyj M., Muss H.B. Granulomatous pneumocystosis presenting as a solitary pulmonary nodule. Arch Pathol Lab Med. 1985;109(5):466-469.
236 Couples J.B., Blackie S.P., Road J.D. Granulomatous Pneumocystis carinii pneumonia mimicking tuberculosis. Arch Pathol Lab Med. 1989;113(11):1281-1284.
237 Liaw Y.S., Yang P.C., Yu C.J., et al. Direct determination of cryptococcal antigen in transthoracic needle aspirate for diagnosis of pulmonary cryptococcosis. J Clin Microbiol. 1995;33(6):1588-1591.
238 Raab S.S., Silverman J.F., Zimmerman K.G. Fine-needle aspiration biopsy of pulmonary coccidioidomycosis. Spectrum of cytologic findings in 73 patients. Am J Clin Pathol. 1993;99(5):582-587.
239 Zimmerman R.L., Montone K.T., Fogt F., Norris A.H. Ultra fast identification of Aspergillus species in pulmonary cytology specimens by in situ hybridization. Int J Mol Med. 2000;5(4):427-429.
240 Aubry M.C., Fraser R. The role of bronchial biopsy and washing in the diagnosis of allergic bronchopulmonary aspergillosis. Mod Pathol. 1998;11(7):607-611.
241 Kimura M., McGinnis M.R. Fontana-Masson–stained tissue from culture-proven mycoses. Arch Pathol Lab Med. 1998;122(12):1107-1111.
242 Monheit J.E., Cowan D.F., Moore D.G. Rapid detection of fungi in tissues using calcofluor white and fluorescence microscopy. Arch Pathol Lab Med. 1984;108(8):616-618.
243 Bialek R., Ernst F., Dietz K., et al. Comparison of staining methods and a nested PCR assay to detect Histoplasma capsulatum in tissue sections. Am J Clin Pathol. 2002;117(4):597-603.
244 Rosner E.R., Reiss E., Warren N.G., et al. Evaluation of the status of laboratory practices and the need for continuing education in medical mycology. Am J Clin Pathol. 2002;118(2):278-286.
245 Perfect J.R., Cox G.M., Lee J.Y., et al. The impact of culture isolation of Aspergillus species: a hospital-based survey of aspergillosis. Clin Infect Dis. 2001;33(11):1824-1833.
246 Yeo S.F., Wong B. Current status of nonculture methods for diagnosis of invasive fungal infections. Clin Microbiol Rev. 2002;15(3):465-484.
247 Challier S., Boyer S., Abachin E., Berche P. Development of a serum based Taqman real-time PCR assay for dignosis of invasive aspergillosis. J Clin Microbiol. 2004;42:844-846.
248 Tarrand J.J., Lichterfeld M., Warraich I., et al. Diagnosis of invasive septate mold infections. A correlation of microbiological culture and histologic or cytologic examination. Am J Clin Pathol. 2003;119(6):854-858.
249 Moskowitz L.B., Ganjei P., Ziegels-Weissman J., et al. Immunohistologic identification of fungi in systemic and cutaneous mycoses. Arch Pathol Lab Med. 1986;110(5):433-436.
250 Choi J., Mauger J., McGowan K. Immunohistochemical detection of Aspergillus species in pediatric tissue samples. Am J Clin Pathol. 2004;121:18-25.
251 Hayden R.T., Qian X., Roberts G.D., Lloyd R.V. In situ hybridization for the identification of yeastlike organisms in tissue section. Diagn Mol Pathol. 2001;10(1):15-23.
252 Sandhu G.S., Kline B.C., Stockman L., Roberts G.D. Molecular probes for diagnosis of fungal infections. J Clin Microbiol. 1995;33(11):2913-2919.
253 Lindsley M.D., Hurst S.F., Iqbal N.J., Morrison C.J. Rapid identification of dimorphic and yeast-like fungal pathogens using specific DNA probes. J Clin Microbiol. 2001;39(10):3505-3511.
254 Pham A.S., Tarrand J.J., May G.S., et al. Diagnosis of invasive mold infection by real-time quantitative PCR. Am J Clin Pathol. 2003;119(1):38-44.
255 Wheat J. Serologic diagnosis of infectious disease. In: Sarosi G., Davies S., editors. Fungal Disease of the Lung. Philadelphia: Lippincott Williams & Wilkins; 2000:17-29.
256 Pappagianis D., Zimmer B.L. Serology of coccidioidomycosis. Clin Microbiol Rev. 1990;3(3):247-268.
257 Wheat J. Laboratory diagnosis of histoplasmosis. Semin Respir Infect. 2001;16:141-148.
258 Kwak E.J., Husain S., Obman A., et al. Efficacy of galactomannan antigen in the Platelia Aspergillus antigen immunoassay for the diagnosis of invasive aspergillosis in liver transplants. J Clin Microbiol. 2004;42:435-438.
259 Treanor J. Respiratory infections. In: Richman R., Whitley R., editors. Clinical Virology. New York: Churchill Livingstone; 1997:5-34.
260 Storch G.A. Diagnostic virology. Clin Infect Dis. 2000;31(3):739-751.
261 Rabella N., Rodriguez P., Labeaga R., et al. Conventional respiratory viruses recovered from immunocompromised patients: clinical considerations. Clin Infect Dis. 1999;28(5):1043-1048.
262 Gilliam-Ross L. Emerging respiratory viruses: challenges and vaccine strategies. Clin Microbiol Rev. 2006;19:614-636.
263 Kahn J. Newly identified respiratory viruses. Pediatr Infect Dis J. 2007;26:745-746.
264 WHO Writing Committee. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med. 2010;362:1708-1719.
265 Papa A., Papadimitriou E. Coronaviruses in children. Greece. Emerg Infect Dis. 2007;13:447-449.
266 Vicente D., Cilla G., Montes M., et al. Human bocavirus, a respiratory and enteric virus. Emerg Infect Dis. 2007;13:636-637.
267 Galan A., Rauch C., Otis C. Fatal BK polyoma viral pneumonia associated with immunosuppression. Hum Pathol. 2005;36:1031-1034.
268 Zaki S.R., Paddock C.D. Viral infections of the lung. In: Tomashefski J., Farver C., Cagle P., Fraire A., editors. Dail and Hammar’s Pulmonary Pathology. 3rd ed. New York: Springer; 2008:426-475.
269 Magro C.M., Wusirika R., Frambach G.E., et al. Autoimmune-like pulmonary disease in association with parvovirus B19: a clinical, morphologic and molecular study of 12 cases. Appl Immunohist Mol Morphol. 2006;14:208-216.
270 Mizgard J. Acute lower respiratory tract infection. N Engl J Med. 2008;358:716-727.
271 Malherbe H., Strickland-Cholmley M. Viral Cytopathology. Boca Raton, FL: CRC Press, 1980.
272 Fields B., Knipe D. Virology. New York: Raven Press, 1990.
273 Shieh W.J., Hsiao C.H., Paddock C.D., et al. Immunohistochemical, in situ hybridization and ultrastructural localization of SARS-associated coronavirus in lung of a fatal case of severe acute respiratory syndrome in Taiwan. Hum Pathol. 2005;36:303-309.
274 Nuovo G. The utility of in situ-based methodologies including in situ polymerase chain reaction for the diagnosis and study of viral infections. Hum Pathol. 2007;38:1123-1136.
275 Walsh J.J., Dietlein L.F., Low F.N., et al. Broncheotracheal response in human influenza. Type A, Asian strain, as studied by light and electron microscopic esamination of bronchoscopic biopsies. Arch Intern Med. 1961;108:376-388.
276 Winternitz M., Wason I., McNamara F. The Pathology of Influenza. New Haven, CT: Yale University Press, 1920.
277 Anjuna V., Colby T. Pathologic features of lung biopsy specimens from influenza pneumonia cases. Hum Pathol. 1994;25:47-53.
278 Taubenberger J., Morens D. L The pathology of influenza virus infections. Ann Rev Pathol. 2008;3:499-522.
279 World Health Organization. Cumulative number of confirmed cases of avian influenza A/H5N1 reported to WHO. Available at: www.who.int/csr/disease/avian_influenza/country/cases_table_2008_06_19/en/index.html Accessed 25.12.09
280 de Jong M.D., Simmons C.P., Thanh T.T., et al. Fatal outcome of human influenza A (H5N1) is associated with viral load and hypercytokinemia. Nat Med. 2006;12:1203-1207.
281 Guarner J., Shieh W.J., Dawson J., et al. Immunohistochemical and in situ hybridization studies of influenza A virus infection in human lungs. Am J Clin Pathol. 2000;114(2):227-233.
282 Hall C.B. Respiratory syncytial virus and parainfluenza virus. N Engl J Med. 2001;344(25):1917-1928.
283 Griffin M.R., Coffey C.S., Neuzil K.M., et al. Winter viruses: influenza- and respiratory syncytial virus–related morbidity in chronic lung disease. Arch Intern Med. 2002;162(11):1229-1236.
284 Madden J., Burchette J., Hale L. Pathology of parainfluenza virus infection in patients with congenital immunodeficiency syndromes. Hum Pathol. 2004;35:594-603.
285 Hall C.B., Weinberg G. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588-598.
286 Falsey A., Walsh E.E. Viral pneumonia in older adults. Clin Infect Dis. 2006;42:518-524.
287 Falsey A.R., Formica M.A., Walsh E.E. Diagnosis of respiratory syncytial virus infection: Comparison of reverse transcription-PCR to viral culture and serology in adults with respiratory illness. J Clin Microbiol. 2002;40(3):817-820.
288 Krinzman S., Basgoz N., Kradin R., et al. Respiratory syncytial virus–associated infections in adult recipients of solid organ transplants. J Heart Lung Transplant. 1998;17(2):202-210.
289 Johnson J., Gonzales R., Olson S. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod Pathol. 2007;20:108-119.
290 Williams J.V., Harris P.A., Tollefson S.J., et al. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med. 2004;350:443-450.
291 Sumino K.C., Agapov E., Pierce R.A., et al. Detection of severe human metapneumovirus infection by real-time polymerase chain reaction and histopathologic assessment. J Infect Dis. 2005;192:1052-1060.
292 Vargas S.O., Kozakewich H.P., Perez-Atayde A.R., McAdam A.J. Pathology of human metapneumovirus infection: insights into pathogenesis of a newly identified respiratory virus. Pediatr Dev Pathol. 2004;7:478-486.
293 Duke T., Mgone C. Measles: not just another viral exanthem. Lancet. 2003;361:763-773.
294 Moussallem T., Guedes F., Fernandes E. Lung involvement in childhood measles: severe immune dysfunction revealed by quantitative immunohistochemistry. Hum Pathol. 2007;38:1239-1247.
295 Nolte K.B., Feddersen R.M., Foucar K., et al. Hantavirus pulmonary syndrome in the United States: a pathological description of a disease caused by a new agent. Hum Pathol. 1995;26(1):110-120.
296 Colby T.V., Zaki S.R., Feddersen R.M., Nolte K.B. Hantavirus pulmonary syndrome is distinguishable from acute interstitial pneumonia. Arch Pathol Lab Med. 2000;124(10):1463-1466.
297 Koster F., Foucar K., Hjelle B., et al. Rapid presumptive diagnosis of hantavirus cardiopulmonary syndrome by peripheral blood smear review. Am J Clin Pathol. 2001;116(5):665-672.
298 Peters C.J., Khan A.S. Hantavirus pulmonary syndrome: the new American hemorrhagic fever. Clin Infect Dis. 2002;34(9):1224-1231.
299 MacIntosh K. Coronaviruses. In: Richman D., Whitley R., editors. Clinical Virology. New York: Churchill Livingstone; 1997:1123-1130.
300 Falsey A.R., Walsh E.E., Hayden F.G. Rhinovirus and coronavirus infection–associated hospitalizations among older adults. J Infect Dis. 2002;185(9):1338-1341.
301 Wenzel R.P., Edmond M.B. Managing SARS amidst uncertainty. N Engl J Med. 2003;348(20):1947-1948.
302 Ksiazek T.G., Erdman D., Goldsmith C.S., et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348(20):1953-1966.
303 Booth C.M., Matukas L.M., Tomlinson G.A., et al. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289(21):2801-2809.
304 Lee N., Hui D., Wu A., et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348(20):1986-1994.
305 Chow K.C., Hsiao C.H., Lin T.Y., et al. Detection of severe acute respiratory syndrome–associated coronavirus in paneumocytes of the lung. Am J Clin Pathol. 2004;121:574-580.
306 Hwang D.M., Chamberlain D.W., Poutanen S.M., et al. Pulmonary pathology of severe acute respiratory syndrome in Toronto. Mod Pathol. 2005;18:1-10.
307 Ye J., Zhang B., Xu J., et al. Molecular pathology in the lungs of severe acute respiratory syndrome patients. Am J Pathol. 2007;170:538-545.
308 Ng W.F., To K.F., Lam W.W., et al. The comparative pathology of severe acute respiratory syndrome and avian influenza A subtype H5N1—a review. Hum Pathol. 2006;37:381-390.
309 Peled N., Nakar C., Huberman H., et al. Adenovirus infection in hospitalized immunocompetent children. Clin Pediatr (Phila). 2004;43:223-229.
310 Ohori N.P., Michaels M.G., Jaffe R., et al. Adenovirus pneumonia in lung transplant recipients. Hum Pathol. 1995;26(10):1073-1979.
311 Pham T., Burchette J., Hale L. Fatal disseminated adenovirus infection in immunocompromised patients. Am J Clin Pathol. 2003;120:575-583.
312 Floudas C.S., Kanakis M.A., Andreopoulos A., Vaiopoulos G.A. Nodular lung calcifications following varicella-zoster pneumonia. Q J Med. 2008;101:159.
313 Andrade Z.R., Garippo A.L., Saldiva P.H., Capelozzi V.L. Immunohistochemical and in situ detection of cytomegalovirus in lung autopsies of children immunocompromised by secondary interstitial pneumonia. Pathol Res Pract. 2004;200(1):25-32.
314 Landry M.L. Multiple viral infections in the immunocompromised host: recognition and interpretation. Clin Diagn Virol. 1994;2(6):313-321.
315 Schooley R.T., Carey R.W., Miller G., et al. Chronic Epstein-Barr virus infection associated with fever and interstitial pneumonitis. Clinical and serologic features and response to antiviral chemotherapy. Ann Intern Med. 1986;104(5):636-643.
316 Wick M.J., Woronzoff-Dashkoff K.P., McGlennen R.C. The molecular characterization of fatal infectious mononucleosis. Am J Clin Pathol. 2002;117(4):582-588.
317 Buchanan A.J., Gupta R.K. Cytomegalovirus infection of the lung: cytomorphologic diagnosis by fine-needle aspiration cytology. Diagn Cytopathol. 1986;2(4):341-342.
318 Feldman P., Covell J. Fine Needle Aspiration Cytology and Its Clinical Applications: Breast and Lung. Chicago: ASCP Press, 1985.
319 Leland D.S., Emanuel D. Laboratory diagnosis of viral infections of the lung. Semin Respir Infect. 1995;10(4):189-198.
320 Barenfanger J., Drake C., Leon N., et al. Clinical and financial benefits of rapid detection of respiratory viruses: an outcomes study. J Clin Microbiol. 2000;38(8):2824-2828.
321 Payne C. Electron microscopy in the diagnosis of infectious diseases. In: Chandler F., Connor D., Schwartz D., editors. Pathology of Infectious Disease. Stamford, CT: Appleton & Lange; 1997:9-34.
322 Murphy P., Roberts Z.M., Waner J.L. Differential diagnoses of influenza A virus, influenza B virus, and respiratory syncytial virus infections by direct immunofluorescence using mixtures of monoclonal antibodies of different isotypes. J Clin Microbiol. 1996;34(7):1798-1800.
323 Legoff J., Kara R., Moulin F., et al. Evaluation of the one-step multiplex real-time reverse transcription-PCR ProFlu-1 assay for the detection of influenza A and influenza B viruses in children. J Clin Microbiol. 2008;46:789-791.
324 Reijans M., Dingemans G., Klaassen C.H., et al. RespiFinder: a new multiparameter test to differentially identify fifteen respiratory viruses. J Clin Microbiol. 2008;46:1232-1240.
325 Mahony J., Chong S., Merante F., et al. Development of a respiratory virus panel test for detection of twenty human respiratory viruses by use of multiplex PCR and fluid microbead–based assay. J Clin Microbiol. 2007;45:2965-2970.
326 Nolte F.S., Marshall D.J., Rasberry C., et al. MultiCode-PLx system for multiplexed detection of seventeen respiratory viruses. J Clin Microbiol. 2007;45:2779-2786.
327 Takahashi H., Norman S.A., Mather E.L., Patterson B.K. Evaluation of the Nanochip 400 system for detection of influenza A and B, respiratory syncytial virus and parainfluenza virus. J Clin Microbiol. 2008;46:1724-1727.
328 Wang W., Ren P., Mardi S., et al. Design of mutiplexed detection assays for identification of avian influenza A virus subtypes pathogenic to humans by SmartCycler real-time reverse transcription-PCR. J Clin Microbiol. 2009;47:86-92.
329 Rea T.D., Ashley R.L., Russo J.E., Buchwald D.S. A systematic study of Epstein-Barr virus serologic assays following acute infection. Am J Clin Pathol. 2002;117(1):156-161.
330 Razonable R.R., Paya C.V., Smith T.F. Role of the laboratory in diagnosis and management of cytomegalovirus infection in hematopoietic stem cell and solid-organ transplant recipients. J Clin Microbiol. 2002;40(3):746-752.
331 Weinberg A., Schissel D., Giller R. Molecular methods for cytomegalovirus surveillance in bone marrow transplant recipients. J Clin Microbiol. 2002;40(11):4203-4206.
332 Chemaly R., Yen-Lieberman B., Castilla E.A., et al. Correlation between viral loads of cytomegalovirus in blood and bronchoalveolar lavage specimens from lung transplant recipients determined by histology and immunohistochemistry. J Clin Microbiol. 2004;42:2168-2172.
333 Cox F.E. History of human parasitology. Clin Microbiol Rev. 2002;15(4):595-612.
334 Vijuyan V. Tropical parasitic lung disease. Indian J Chest Dis Allied Sci. 2008;50:49-66.
335 Fritche T.R., Selvarangan R. Medical parasitology. In: McPherson R., Pincus M., editors. Henry’s Clinical Diagnosis and Management by Laboratory Methods. Philadelphia: Saunders/Elsevier; 2007:1119-1168.
336 Procop G.W., Marty A.M. Parasitic infections. In: Tomashefski J., Farver C., Cagle P., Fraire A., editors. Dail and Hammer’s Pulmonary Pathology. 3rd ed. New York: Springer; 2008:515-560.
337 Ali A., Hoda S. Vegetable matter in histology sections may simulate pathogenic microorganisms [Abstract]. Mod Pathol. 2002;15:272A.
338 Nash G., Kerschmann R.L., Herndier B., Dubey J.P. The pathological manifestations of pulmonary toxoplasmosis in the acquired immunodeficiency syndrome. Hum Pathol. 1994;25(7):652-658.
339 Frenkel J. Toxoplasmosis. In: Chandler F., Connor D., Schwartz D., editors. Pathology of Infectious Disease. Stamford, CT: Appleton & Lange; 1997:1261-1278.
340 Lyche K.D., Jensen W.A. Pleuropulmonary amebiasis. Semin Respir Infect. 1997;12(2):106-112.
341 Wilson M.R., Jorgensen J.H., Yolken R.H., et al. Diagnosis of parasitic infection: Immunologic and molecular methods. In Murray P., Baron E., Pfaller M., et al, editors: Manual of Clinical Microbiology, 6th ed., Washington, DC: ASM Press, 1995.
342 Ash L., Orihel T. Atlas of Human Parasitology, 4th ed. Chicago: ASCP Press, 1997.
343 Sun T. Parasitic Disorders: Pathology, Diagnosis and Management, 2nd ed. Baltimore: Williams & Wilkins, 1999.
344 Marciano-Cabral F., Cabral G. Acanthamoeba spp as agents of disease in humans. Clin Micro Rev. 2003;16:273-307.
345 Chen X.M., Keithly J.S., Paya C.V., LaRusso N.F. Cryptosporidiosis. N Engl J Med. 2002;346(22):1723-1731.
346 Pearl M., Villanueva T.G., Kauffman C.A. Respiratory cryptosporidiosis in the acquired immune deficiency syndrome. JAMA. 1984;252:1290-1301.
347 Garcia L.S. Laboratory identification of the microsporidia. J Clin Microbiol. 2002;40(6):1892-1901.
348 Weber R., Bryan R.T., Schwartz D.A., Owen R.L. Human microsporidial infections. Clin Microbiol Rev. 1994;7(4):426-461.
349 Schwartz D.A., Visvesvara G.S., Leitch G.J., et al. Pathology of symptomatic microsporidial (Encephalitozoon hellem) bronchiolitis in the acquired immunodeficiency syndrome: a new respiratory pathogen diagnosed from lung biopsy, bronchoalveolar lavage, sputum, and tissue culture. Hum Pathol. 1993;24(9):937-943.
350 Lamps L.W., Bronner M.P., Vnencak-Jones C.L., et al. Optimal screening and diagnosis of microsporidia in tissue sections: a comparison of polarization, special stains, and molecular techniques. Am J Clin Pathol. 1998;109(4):404-410.
351 Piscopo T., Mallia A. Leishmaniasis. Postgrad Med J. 2006;82:649-657.
352 Jokipii L., Salmela K., Saha H., et al. Leishmaniasis diagnosed from bronchoalveolar lavage. Scand J Infect Dis. 1992;24(5):677-681.
353 Morales P., Torres J.J., Salavert M., et al. Visceral leishmaniasis in lung transplantation. Transplant Proc. 2003;35:2001-2003.
354 Deborggraeve S., Boelaert M., Rijal S., et al. Diagnostic accuracy of a new Leishmania PCR for clinical visceral leishmaniasis in Nepal and its role in diagnosis of disease. Trop Med Int Health. 2008;13:1378-1383.
355 Ro J.Y., Tsakalakis P.J., White V.A., et al. Pulmonary dirofilariasis: the great imitator of primary or metastatic lung tumor. A clinicopathologic analysis of seven cases and a review of the literature. Hum Pathol. 1989;20(1):69-76.
356 Nicholson C.P., Allen M.S., Trastek V.F., et al. Dirofilaria immitis: a rare, increasing cause of pulmonary nodules. Mayo Clin Proc. 1992;67(7):646-650.
357 Akaogi E., Ishibashi O., Mitsui K., et al. Pulmonary dirofilariasis cytologically mimicking lung cancer. A case report. Acta Cytol. 1993;37(4):531-534.
358 Flieder D.B., Moran C.A. Pulmonary dirofilariasis: a clinicopathologic study of 41 lesions in 39 patients. Hum Pathol. 1999;30(3):251-256.
359 Byard R., Bourne A., Matthews N. Pulmonary strongyloidiasis in a child diagnosed on open lung biopsy. Surg Pathol. 1993;109:55-61.
360 Upadhyay D., Corbridge T., Jain M., Shah R. Pulmonary hyperinfection syndrome with Strongyloides stercoralis. Am J Med. 2001;111(2):167-169.
361 Baden L.R., Elliott D.D. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 4–2003. A 42-year-old woman with cough, fever, and abnormalities on thoracoabdominal computed tomography. N Engl J Med. 2003;348(5):447-455.
362 Redington A.E., Russell S.G., Ladhani S., et al. Pulmonary echinococcosis with chest wall involvement in a patient with no apparent risk factors. J Infect. 2001;42(4):285-1258.
363 Sinniah B. Paragonimiasis. In: Chandler F., Connor D., Schwartz D., editors. Pathology of Infectious Disease. Stamford, CT: Appleton & Lange; 1997:1527-1530.
364 Ross A.G., Bartley P.B., Sleigh A.C., et al. Schistosomiasis. N Engl J Med. 2002;346(16):1212-1220.
365 King C. Toward the elimination of schistosomiasis. N Engl J Med. 2009;360:106-108.
366 Cooke G.S., Lalvani A., Gleeson F.V., Conlon C.P. Acute pulmonary schistosomiasis in travelers returning from Lake Malawi, sub-Saharan Africa. Clin Infect Dis. 1999;29(4):836-839.
367 Bethlem E.P., Schettino Gde P., Carvalho C.R. Pulmonary schistosomiasis. Curr Opin Pulm Med. 1997;3(5):361-365.
368 Schwartz E., Rosenman J., Perlman M. Pulmonary manifestations of early schistosome infection among nonimmune travelers. Am J Med. 2000;9:718-722.
369 Despommier D. Toxacariasis: clinical aspects, epidemiology, medical ecology and molecular aspects. Clin Microbiol Rev. 2003;34:7-15.
370 Procop G.W., Marty A.M., Scheck D.N., et al. North American paragonimiasis. A case report. Acta Cytol. 2000;44(1):75-80.
371 Singh A., Singh Y., Sharma V.K., et al. Diagnosis of hydatid disease of abdomen and thorax by ultrasound guided fine needle aspiration cytology. Indian J Pathol Microbiol. 1999;42(2):155-156.
372 Handa U., Mohan H., Ahal S., et al. Cytodiagnosis of hydatid disease presenting with Horner’s syndrome: a case report. Acta Cytol. 2001;45(5):784-788.
373 Brown R.W., Clarke R.J., Denham I., Trembath P.W. Pulmonary paragonimiasis in an immigrant from Laos. Med J Aust. 1983;2(12):668-669.
374 Abdulla M.A., Hombal S.M., al-Juwaiser A. Detection of Schistosoma mansoni in bronchoalveolar lavage fluid. A case report. Acta Cytol. 1999;43(5):856-858.
375 Kramer M.R., Gregg P.A., Goldstein M., et al. Disseminated strongyloidiasis in AIDS and non-AIDS immunocompromised hosts: diagnosis by sputum and bronchoalveolar lavage. South Med J. 1990;83(10):1226-1229.
376 Kapila K., Verma K. Cytologic detection of parasitic disorders. Acta Cytol. 1982;26(3):359-362.
377 Didier E.S., Rogers L.B., Orenstein J.M., et al. Characterization of Encephalitozoon (Septata) intestinalis isolates cultured from nasal mucosa and bronchoalveolar lavage fluids of two AIDS patients. J Eukaryot Microbiol. 1996;43(1):34-43.
378 Weber R., Kuster H., Keller R., et al. Pulmonary and intestinal microsporidiosis in a patient with the acquired immunodeficiency syndrome. Am Rev Respir Dis. 1992;146(6):1603-1605.
379 Wheeler R.R., Bardales R.H., North P.E., et al. Toxoplasma pneumonia: cytologic diagnosis by bronchoalveolar lavage. Diagn Cytopathol. 1994;11(1):52-55.
380 Radosavljevic-Asic G., Jovanovic D., Radovanovic D., Tucakovic M. Trichomonas in pleural effusion. Eur Respir J. 1994;7(10):1906-1908.
381 Newsome A.L., Curtis F.T., Culbertson C.G., Allen S.D. Identification of Acanthamoeba in bronchoalveolar lavage specimens. Diagn Cytopathol. 1992;8(3):231-234.
382 Maddison S.E. Serodiagnosis of parasitic diseases. Clin Microbiol Rev. 1991;4(4):457-469.
383 Wilson M., Remington J.S., Clavet C., et al. Evaluation of six commercial kits for detection of human immunoglobulin M antibodies to Toxoplasma gondii. The FDA Toxoplasmosis Ad Hoc Working Group. J Clin Microbiol. 1997;35(12):3112-3115.
384 Remington J.S., Thulliez P., Montoya J. Recent developments for diagnosis of toxoplasmosis. J Clin Microbiol. 2004;42:941-945.
385 Petersen E., Edvinsson B., Lundgren B., et al. Diagnosis of pulmonary infection with Toxoplasma gondii in immunocompromised HIV-positive patients by real-time PCR. Eur J Clin Microbiol Infec Dis. 2006;25:401-404.
386 Tanyukjel M., Petri W. Laboratory diagnosis of amoebiasis. Clin Microbiol Rev. 2003;16:713-729.
387 Churg A. Recent advances in the diagnosis of Churg-Strauss syndrome. Mod Pathol. 2001;14(12):1284-1293.
388 Allen J.N., Davis W.B. Eosinophilic lung diseases. Am J Respir Crit Care Med. 1994;150(5 Pt 1):1423-1438.
389 Burgers J.A., Sluiters J.F., de Jong D.W., et al. Pseudoparasitic pneumonia after bone marrow transplantation. Neth J Med. 2001;59(4):170-176.
390 Tuur S.M., Nelson A.M., Gibson D.W., et al. Liesegang rings in tissue. How to distinguish Liesegang rings from the giant kidney worm, Diotophyma renale. Am J Surg Pathol. 1987;11:598-605.