Chapter 66 Lung Cancer
Clinical Evaluation and Staging
Symptomatic Presentation
Symptoms and Signs Related to the Primary Tumor
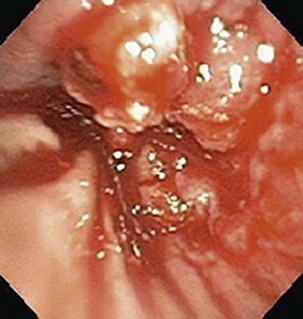
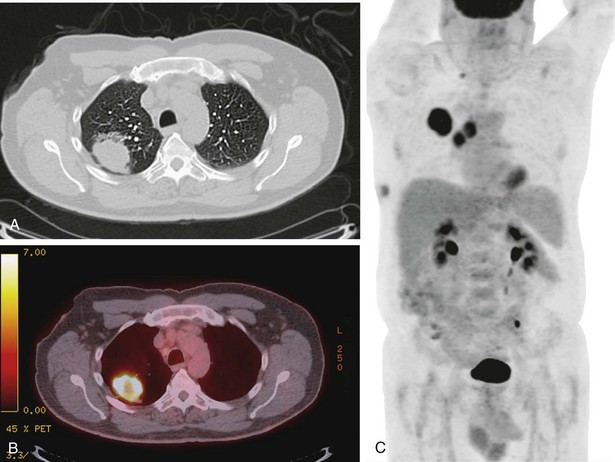
Currently, most patients with lung cancer have advanced disease at the time of presentation and are likely to present with symptoms, particularly if local extension, metastasis to distant sites, or a paraneoplastic syndrome is present. Obtaining a thorough history and a review of systems in patients suspected of having lung cancer constitute an important part of the initial evaluation, because the symptom history will influence the choice of imaging studies, the interpretation of those studies, and the institution of early palliative interventions. Symptoms and signs related to the primary tumor most commonly include cough, dyspnea, chest pain, and hemoptysis. Cough is present in up to 75% of patients with lung cancer and may be related to airway involvement, postobstructive pneumonitis, or bronchorrhea. Dyspnea may be associated with primary site–related problems, such as endobronchial or extrinsic airway obstruction, postobstructive atelectasis, infection, or increased airway secretions, or may be related to metastatic disease, with potential manifestations including pleural effusion, lymphangitic tumor spread, pericardial effusion with tamponade, and pulmonary thromboemboli in the setting of hypercoagulability. Hemoptysis with lung cancer usually manifests as intermittent or persistent bloody streaking of the sputum and rarely can be massive. In up to 9% of patients with hemoptysis and lung cancer, the chest radiograph will be normal in appearance, underscoring the need to include endobronchial tumor as a major consideration in the differential diagnosis in patients presenting with hemoptysis and lung cancer risk factors (Figure 66-1). With the exception of hemoptysis, these pulmonary symptoms may not necessarily prompt a patient with a history of cigarette use, chronic obstructive pulmonary disease, or interstitial lung disease to seek specific medical attention or, conversely, for the clinician to consider an alternative diagnosis. These may be contributing factors to the consistent observation of a delay of several months between the onset of symptoms and a definitive diagnosis of lung cancer.
Symptoms Related to Metastatic Spread
Paraneoplastic Syndromes
Paraneoplastic syndromes occur in approximately 10% of patients with lung cancer (Table 66-1). Such syndromes may be the initial presenting complaint triggering an evaluation but also can develop late in the course of disease. Paraneoplastic syndromes are unrelated to direct invasion or distant spread of tumor and in and of themselves do not preclude curative-intent therapy. Endocrine syndromes associated with lung cancers often are characterized by tumor production of biologically active hormones. Lung cancer is the most common cause of cancer-associated hypercalcemia, hyponatremia, and syndromes involving ectopic production of adrenal corticotropic hormone (ACTH).
Table 66-1 Paraneoplastic Syndromes Associated with Lung Cancer
| Endocrine | Syndrome of inappropriate secretion of antidiuretic hormone (SIADH)/hyponatremia; hypercalcemia; ectopic adrenocorticotropic hormone (ACTH) syndrome; Cushing syndrome; hyperglycemia; hypoglycemia; hyperthyroidism; carcinoid syndrome; gynecomastia; elevated growth hormone; elevated follicle-stimulating hormone (FSH); galactorrhea |
| Musculoskeletal | Hypertrophic osteoarthropathy (HOP); clubbing, myopathy; dermatomyositis; polymyositis |
| Neurologic | Lambert-Eaton myasthenic syndrome (LEMS); encephalomyelitis/subacute sensory neuropathy; cerebellar degeneration; opsoclonus-myoclonus; autonomic neuropathy; retinopathy; mononeuritis multiplex; peripheral neuropathy; myopathy |
| Skin | Acanthosis nigricans; pruritus and urticaria, erythema multiforme; erythroderma; exfoliative dermatitis; hyperpigmentation |
| Hematologic | Anemia, thrombocytosis, leukocytosis, hypercoagulable state |
Nonendocrinologic extrapulmonary syndromes include musculoskeletal abnormalities, most commonly asymptomatic digital clubbing, which can be seen in isolation or in the setting of hypertrophic pulmonary osteoarthropathy (HPO). The pathogenesis of HPO is unknown, but the disorder consists of a proliferative periostitis characterized by symmetric, painful arthropathy that typically involves the ankles, shins, knees, wrists, and elbows. The diagnosis usually is confirmed by the identification of new periosteal bone formation on plain radiographs of the long bones, or by demonstration of diffuse long bone uptake of radionuclide on bone scan or positron emission tomography (PET) imaging. HPO is more commonly seen with adenocarcinoma. Hematologic dyscrasias commonly occurring in patients with lung cancer include anemia, which may compound fatigue and dyspnea; thrombocytosis; and leukocytosis. Eosinophilia is seen only rarely. Lung cancer is the most common cause of hypercoagulability associated with malignancy, which usually declares itself as deep venous thrombosis or thromboembolism, or with classic Trousseau syndrome (migratory superficial thrombophlebitis). Lung cancer, and specifically SCLC, also is the most common cause of a clinically diverse group of paraneoplastic neurologic syndromes (see Table 66-1).
Asymptomatic Presentation
The Solitary Pulmonary Nodule
In evaluating SPNs, it is useful to distinguish small (8 mm or less in diameter) from larger nodules. Small SPNs are very common findings in patients who have participated in CT screening studies for lung cancer, as well as in those undergoing CT scanning for non-lung-related reasons, as noted earlier. In the feasibility study for the NLST performed within the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial, 21% of subjects had abnormalities identified on the baseline screening CT scan, most of which were small SPNs. Similarly, in the Mayo Clinic lung cancer screening study, 51% of subjects had abnormalities found on the baseline screening study, with an increase to 69% by the second annual screen, the vast majority of which were small SPNs. The enormous number of small SPNs generated by an increased volume of CT scanning prompted a position statement from the Fleischner Society proposing guidelines for the management of small (8 mm or less) SPNs detected on CT scans. The recommendations are outlined in Table 66-2. Appropriately applied, the guidelines outline algorithms for patients based on their risk of lung cancer, providing timelines for follow-up of incidentally discovered nodules that minimize the number of CT scans necessary in the course of evaluation. These practical recommendations will become even more relevant if CT screening for lung cancer becomes the standard of practice. Of note, these recommendations apply only to patients in whom SPNs are discovered incidentally, unrelated to any known underlying disease, and only for solid SPNs 8 mm or less in diameter. Specifically, the guidelines are not intended to apply to patients known to have or suspected of having malignant disease, patients younger than 35 years of age, or patients with unexplained fever. Furthermore, the recommendations are not fully applicable to persons with nonsolid (ground glass appearance) or partially solid nodules, who may require longer follow-up to permit exclusion of biologically indolent cancers with greater confidence.
Table 66-2 Fleischner Society Guidelines for Management of Small Pulmonary Nodules Detected on Computed Tomography (CT) Scans
| Nodule Size* | Low-Risk Patient† | High-Risk Patient‡ |
|---|---|---|
| ≤4 mm | No follow-up needed§ | Follow-up CT at 12 months; if unchanged, no further follow-up¶ |
| >4-6 mm | Follow-up CT at 12 months; if unchanged, no further follow-up¶ | Initial follow-up CT at 6-12 months, then at 18-24 months if no change¶ |
| >6-8 mm | Initial follow-up CT at 6-12 months, then at 18-24 months if no change | Initial follow-up CT at 3-6 months, then at 9-12 months and 24 months if no change |
| >8 mm | Follow-up CT at around 3, 9, and 24 months, then dynamic contrast-enhanced CT, PET, and/or biopsy | Same as for low-risk patient |
NOTE: Guidelines refer to newly detected indeterminate nodules in persons 35 years of age or older.
PET, positron emission tomography.
* Average of length and width.
† Minimal or absent history of smoking and of other known risk factors.
‡ History of smoking or of other known risk factors.
§ The risk of malignancy in this category (<1%) is substantially less than that determined with a baseline CT scan in an asymptomatic smoker.
¶ Nonsolid (ground glass) or partly solid nodules may require longer follow-up period to exclude indolent adenocarcinoma.
Modified from MacMahon H, Austin JH, Gamsu G, et al: Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society, Radiology 237:395–400, 2005.
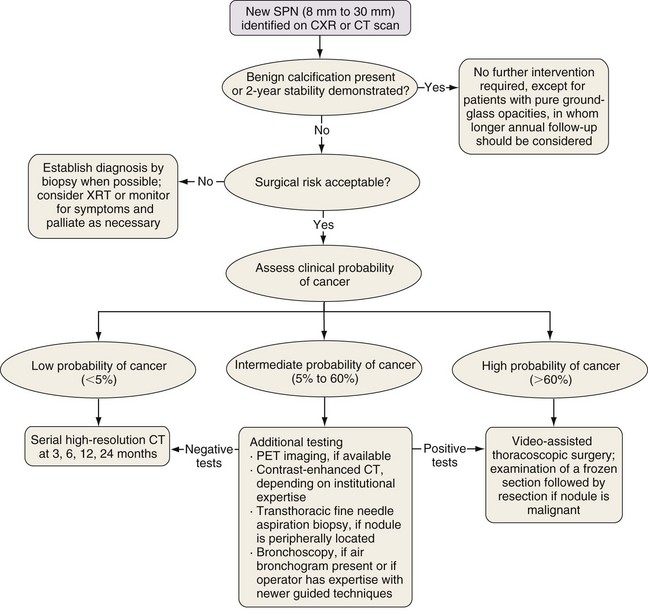
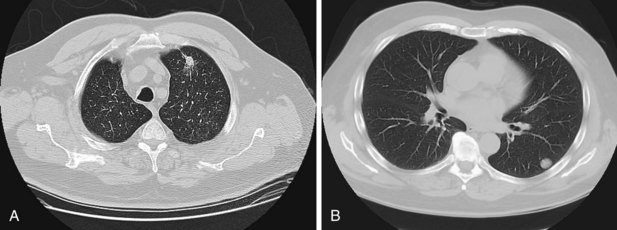
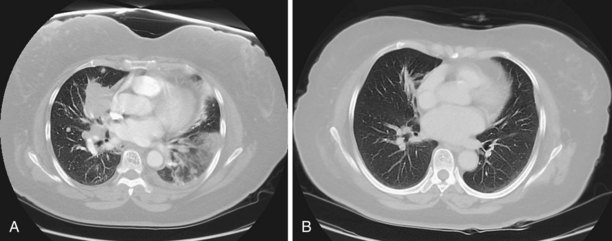
The larger the nodule, the more likely it is to be a cancer. A majority of asymptomatic pulmonary masses greater than 30 mm in diameter are malignant. Figure 66-2 outlines a management strategy for SPNs 8 to 30 mm in diameter recommended by the American College of Chest Physicians evidence-based clinical practice guidelines for lung cancer. The algorithm is appealing in its simplicity. The key step is the clinical definition of a pretest probability of malignancy for every patient with an SPN, because this will inform decisions about the extent of evaluation. SPN factors associated with malignancy include larger size, positive smoking history, older age, a previous history of cancer, the location within the lung (higher risk with upper lobe location), and nodule edge characteristics (higher risk with spiculated than with smooth borders) (Figure 66-3). Mathematical prediction models incorporating these factors typically perform similarly to the clinical acumen of experienced physicians. Patients with a low probability (less than 5%) of malignancy can be monitored by serial CT imaging. Patients with a high probability (more than 60%) of malignancy should proceed to definitive surgical biopsy or resection. For the large group of patients with an intermediate probability (5% to 60%) of malignancy, the use of a variety of noninvasive and invasive modalities should be considered in deciding whether the SPN should be observed, biopsied, or removed.
Lung Cancer Staging
Different types of staging may be used to describe a malignancy during a course of evaluation (Table 66-3). The most common staging assessments are the clinical stage (indicated by the prefix c), defined as the stage before treatment as determined by all available imaging and biopsy data, and the pathologic stage (indicated by the prefix p), defined as the stage determined on the basis of the available imaging and biopsy data, as well as analysis of tissue samples obtained after a surgical resection performed with intent to cure. The completeness of resection is determined histopathologically after surgery (Table 66-4). Clinical staging typically is associated with poorer outcomes than those achieved with pathologic staging, the latter being inherently more accurate. All patients will undergo clinical staging; only in a select number will their disease be able to be staged pathologically. Restaging may take place after treatment, at the time of a recurrence, or at autopsy, identified by the prefix y, r, or a, respectively, as outlined in Table 66-3.
| Prefix | Name | Definition |
|---|---|---|
| c | Clinical | Before initiation of any treatment, using any and all information available (e.g., including mediastinoscopy) |
| p | Pathologic | After resection, based on pathologic assessment |
| y | Restaging | After part or all of the treatment has been given |
| r | Recurrence | Stage at time of a recurrence |
| a | Autopsy | Stage as determined at autopsy |
From Detterbeck FC, Boffa DJ, Tanoue LT: The new lung cancer staging system, Chest 136:260–271, 2009.
Table 66-4 Staging Designation for Completeness of Surgical Resection
| Symbol | Name | Definition |
|---|---|---|
| R0 | No residual | No identifiable tumor remaining; negative surgical margins |
| R1 | Microscopic residual | Microscopically positive margins but no visible tumor remaining |
| R2 | Gross residual | Gross (visible or palpable) tumor remaining |
From Detterbeck FC, Boffa DJ, Tanoue LT: The new lung cancer staging system, Chest 136:260–271, 2009.
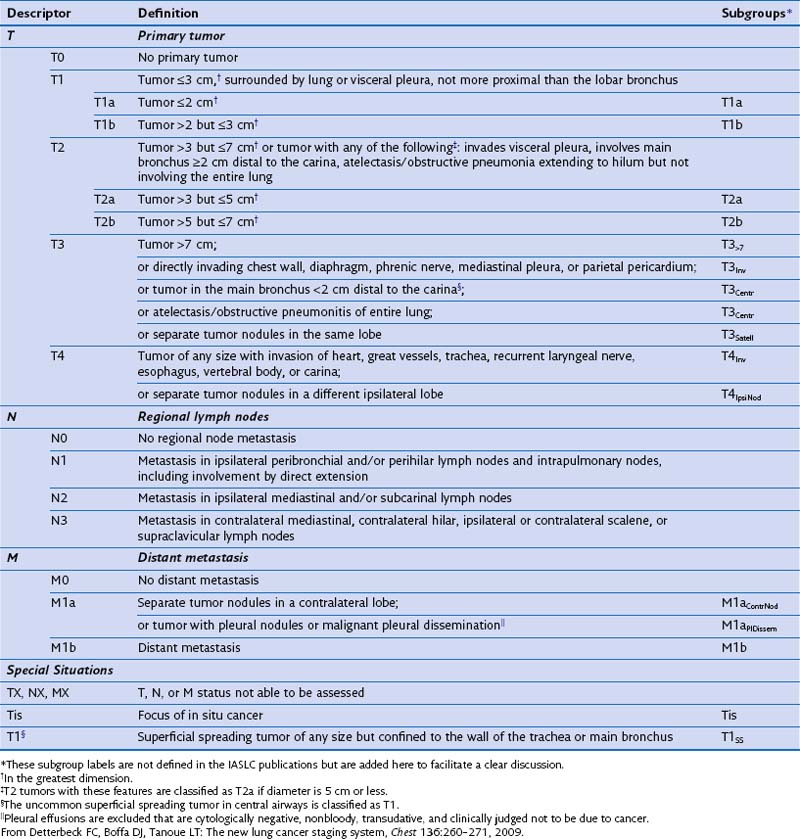
The TNM paradigm is based solely on anatomy (Table 66-5). The T descriptor characterizes the primary tumor, the N descriptor, involvement of the regional lymph nodes; and the M descriptor, the presence or absence of distant metastases. Combinations of these descriptors are grouped into stages. Even though limitations to the generalized application of the TNM system are increasingly appreciated, it serves as a consistent structure to describe extent of disease and to provide prognosis. Furthermore, staging provides a platform for comparisons between reasonably homogeneous populations in therapeutic clinical trials. It may be tempting to use the clinical stage as the major determinant of therapy, but it is important to recognize that the staging classification system is not intended to be an algorithm for assigning treatment. The designation of stage inherently portends prognosis, because the separation of TNM combinations into stages is fundamentally determined by survival. Two of the limitations of the TNM system become immediately evident. First, the anatomic descriptors yield no information relating to biologic behavior of the cancer. With a burgeoning recognition that the molecular characteristics of an individual tumor may drive outcomes and more specifically render some tumors very responsive to targeted treatments, the prognostic ability of the TNM system becomes less reliable. Second, patients who share similar survival outcomes may have clinically dissimilar cancers; this divergence becomes clear on examining the stage groupings outlined in Table 66-6. Within a given stage of disease, the patients often are not clinically homogeneous, even though they may have similar survival rates. Despite the large size of the IASLC database, outcomes for specific TNM groups with small numbers of patients could not be adequately evaluated, with an inevitable “lumping” rather than “splitting” of these groups. Other issues remain unresolved with use of the current system, including how to incorporate specific radiographic features such as the density of pulmonary nodules, or how to properly prognosticate in clinical situations that fall out of the staging paradigm, for example, the presence of multiple synchronous primary tumors, but the most important limitation is the absence of components reflecting tumor biology. As current understanding of molecular pathways important to neoplasia expands, it seems inevitable that the staging system must incorporate such information in the future.
Table 66-6 Lung Cancer Stage Groupings by Tumor-Node-Metastasis (TNM) Descriptors*
| Stage | TNM Grouping |
|---|---|
| 0 |
* As defined in the seventh edition of the American Joint Commission on Cancer and the Union Internationale Contre le Cancer classification.
From Edge SB, editor: The American Joint Commission on Cancer cancer staging manual, ed 7. Chicago, Springer, 2010.
Stage I
Patients with stage IB lung cancer have larger solitary tumors (ranging from less than 3 cm to more than 5 cm in diameter). In such cases, the primary tumor may be surrounded by normal lung but also may invade the visceral pleura, involve the main bronchus 2 cm or more distal to the carina, or be associated with atelectasis or obstructive pneumonia not involving the entire lung. The likelihood of finding regional nodal or distant metastasis in the setting of a larger primary lesion is higher than in stage IA, so these patients should undergo PET scanning and brain imaging (MRI or CT with contrast) as part of their staging evaluation (Figure 66-4). With the recognition that false-positive results on PET are frequent with nonmalignant inflammation or infection, tissue confirmation should be obtained whenever possible to establish the presence or absence of regional or distant metastasis, because patients should not be denied potentially curative treatments on the basis of imaging alone. Conversely, controversy continues regarding whether a negative result on PET scan in a patient with a T2 or T3 solitary pulmonary lesion and normal mediastinal nodes on CT scan should be followed by tissue biopsy to prove that the mediastinum is truly free of disease. In a metaanalysis evaluating this question, the probability of finding malignant lymph node involvement when both CT and PET findings were negative was approximately 9% (of note, the pretest probability of mediastinal lymph node metastasis was estimated at 35%, and patients were not differentiated by the size of the primary tumor). Clinicians ultimately must decide whether the overall 9% probability of mediastinal lymph node metastasis in patients with clinical stage IB disease and negative results on CT and PET imaging of the mediastinum warrants further invasive evaluation. This decision may be influenced by the recognition that certain subgroups, including patients with central or larger tumors, have an increased likelihood of mediastinal nodal involvement.
Stage III
Approximately one third of patients with lung cancer present with clinical stage III disease. All patients with evidence of stage III disease on chest CT scan should be evaluated for the presence of distant disease; this may be accomplished by a combination of (1) brain MRI or head CT and (2) PET imaging or abdominal CT scanning with bone scan. The presence of metastatic disease immediately changes the clinical stage to IV. As with stage II, stage III comprises a wide variety of TNM groupings (see Table 66-6). However, a majority and perhaps the most clinically challenging of these patients with such disease will be defined by involvement of the N2 nodes; careful evaluation of the mediastinum is thus vitally important. The IASLC lymph node map defining the lymph node stations is shown in Figure 66-5.
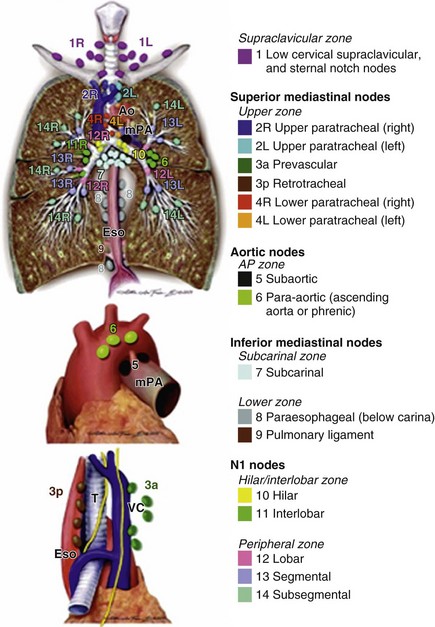
Figure 66-5 The International Association for the Study of Lung Cancer lymph node map.
(From Rusch VW, Asamura H, Watanabe H, et al: The IASLC Lung Cancer Staging Project: a proposal for a new international lymph node map in the forthcoming 7th edition of the TNM classification for lung cancer, J Thorac Oncol 4:568–577, 2009.)
Mediastinal Evaluation: Noninvasive Modalities
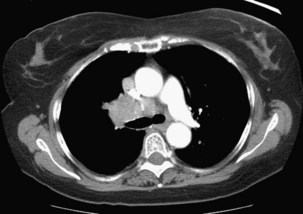
Chest CT remains the most accurate anatomic modality for evaluation of the mediastinum. Pathologic enlargement of the mediastinal nodes is defined as a short-axis diameter of 1 cm or greater. CT in the setting of lung cancer is a relatively inaccurate tool for identification of malignant involvement in the mediastinum. Many studies evaluating CT accuracy in this setting have demonstrated that approximately 20% of mediastinal nodes that are not enlarged are actually malignant; conversely, approximately 40% of enlarged mediastinal nodes are actually benign. For all patients with enlarged discrete mediastinal lymph nodes on CT, further evaluation of the mediastinum should be performed before a definitive treatment decision is reached, in view of the high false-positive rate. The exception to this approach would be in patients with bulky mediastinal disease, with lymph node infiltration around vessels and airways so extensive that discrete lymph node measurements are meaningless (Figure 66-6). In such cases, malignant involvement of the nodes would be assumed, and the establishment of cell type could be pursued by sampling the most accessible site, either the mediastinal nodes or the primary tumor.
Mediastinal Evaluation: Invasive Modalities
A variety of minimally invasive modalities are available for the evaluation of the mediastinal lymph nodes (Table 66-7). TTNA, EUS, extended cervical mediastinoscopy, and EBUS typically are utilized for the purpose of directed biopsy in patients with discrete abnormalities on CT or PET scanning. The choice between these procedures will be decided by the anatomic location of the abnormal nodes and by institutional expertise. The sensitivities of all of the directed minimally invasive techniques are very high, in part because they typically are done in situations in which the prevalence of malignancy in the abnormal nodes also is very high. In theory, all mediastinal lymph nodes are amenable to TTNA, although the individual anatomy will determine whether biopsy is feasible. Ultrasound-guided biopsy techniques using endoscopy or bronchoscopy are increasingly available and have high diagnostic yield.
Table 66-7 Mediastinal Lymph Node Stations: Accessibility to Minimally Invasive Modalities
| Procedure | Accessible Mediastinal Lymph Node Stations |
|---|---|
| Mediastinoscopy | Stations 2, 3, 4, 7 (anterior only) |
| Extended cervical mediastinoscopy | Station 6 |
| Video-assisted thorascopic surgery (VATS) | Ipsilateral stations 2, 3, 4, 7; station 6 with left-sided procedure |
| Parasternal mediastinotomy (Chamberlain procedure) | Station 5 (aortopulmonary window) |
| Endobronchial ultrasound (EBUS) | Stations 2, 3, 4, 7 |
| Endoscopic ultrasound (EUS) | Stations 4, 5, 7, 8, 9 |
| Transthoracic needle aspiration (TTNA) | All stations |
Future Directions
Even with the many advances accomplished in its newest edition, the current staging system is imperfect. Its most obvious deficiencies are a lack of incorporation of information related to tumor cell type and the absence of any indicators of biologic behavior. It is increasingly evident that distinguishing between cell types within the NSCLC classification is important in identifying populations of patients for the purposes of comparing clinical outcomes, for defining homogeneous groups of patients for clinical trials, and ultimately for assigning treatment. It also is clear that molecular profiling of cancers can play an important role in characterizing subgroups of patients who share common gene mutations or translocations that may drive the neoplastic process, particularly in never-smokers or light smokers with lung adenocarcinoma (Figure 66-7). In the future, these distinctions are likely to help define new staging classifications incorporating indicators of biologic activity, with the potential for effectively facilitating personalized patient care.
Aberle DR, Berg CD, Black WC, et al. The National Lung Screening Trial: overview and study design. Radiology. 2011;258:243–253.
Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest. 2009;136:260–271.
Edge SB, ed. The American Joint Commission on Cancer cancer staging manual, ed 7, Chicago: Springer, 2010.
Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for revision of the TNM stage groupings in the forthcoming seventh edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:706–714.
Gould MK, Fletcher J, Iannettoni MD, et al. Evaluation of patients with pulmonary nodules: when is it lung cancer? Chest. 2007;132:108S–130S.
Gould MK, Kuschner WG, Rydzak CE, et al. Test performance of positron emission tomography and computed tomography for mediastinal staging in patients with non-small cell lung cancer: a meta-analysis. Ann Intern Med. 2003;139:879–892.
MacMahon H, Austin JH, Gamsu G, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology. 2005;237:395–400.
Rusch VW, Asamura H, Watanabe H, et al. The IASLC Lung Cancer Staging Project: a proposal for a new international lymph node map in the forthcoming 7th edition of the TNM classification for lung cancer. J Thorac Oncol. 2009;4:568–577.
Silvestri G, Littenberg B, Colice G. The clinical evaluation for detecting metastatic lung cancer: a meta-analysis. Am J Respir Crit Care Med. 1995;152:225–230.
Silvestri GA, Gould MK, Margolis ML, et al. Noninvasive staging of non-small cell lung cancer. Chest. 2007;132:178S–201S.
Toloza E, Harpole L, McCrory DC. Noninvasive staging of non-small cell lung cancer: a review of the current evidence. Chest. 2003;123:137S–146S.
Toloza EM, Harpole L, Detterbeck F, et al. Invasive staging of non-small cell lung cancer: a review of the current evidence. Chest. 2003;123:157S–166S.