Chapter 6 Lung Cancer
Introduction
Lung cancer is a common malignancy, and imaging is used in these patients to determine anatomic extent of disease and the appropriate therapeutic management. Specifically, imaging has an integral part in the detection, diagnosis, and staging of the disease as well as in assessing response to therapy and monitoring for tumor recurrence after treatment. Typically, imaging with computed tomography (CT) is an important component in the clinical management of patients with lung malignancy. Positron-emission tomography (PET) using 18F-2-deoxy-D-glucose (FDG), a D-glucose analogue labeled with fluorine-18, complements conventional radiologic assessment in the evaluation of patients’ lung cancer and is routinely used to improve the detection of nodal and extrathoracic metastases. This chapter reviews the two major histologic categories of lung cancer—non–small cell lung carcinoma (NSCLC) and small cell lung carcinoma (SCLC)—and emphasizes the appropriate use of CT, magnetic resonance imaging (MRI), and PET imaging in patient management.
Epidemiology and Risk Factors
The American Cancer Society (ACS) estimates that 221,130 new cases of lung cancer will be diagnosed in the United States in the year 2011 (ACS www.cancer.org). Although the number of new cases in men has decreased from a high of 102 per 100,000 in 1984 to 71.8 in 2007, the incidence in women has continued to increase since the 1960s. However, the rate of increase in women is now approaching a plateau with 50.2 per 100,000 new cases in 2004. The annual mortality rate in men has decreased since the mid 1990s by approximately 2% annually, whereas the rate in women, after continuously increasing for several decades, has been stable since 2003. Nevertheless, lung cancer remains the leading cause of cancer-related deaths in both men and women in the United States, and the ACS estimates that it will account for 27% of all cancer deaths in the year 2011.
The strongest risk factor for the development of lung cancer is cigarette smoking; it is estimated that 85% to 90% of lung cancers in men and 80% in women are attributable to smoking.1 Involuntary smoke exposure is also associated with an increased risk of lung cancer, and a meta-analysis comprising 22 studies showed a 24% increase in lung cancer risk among workers exposed to environmental tobacco smoke.2 Environmental and occupational exposure to particulate and chemical substances are additional risk factors, and exposure to the naturally occurring radioactive gas radon is the most important lung cancer risk factor after cigarette smoking.1,3 Asbestos exposure is a risk factor for lung cancer; up to 33% of lung cancers that occur in smokers exposed to asbestos may be the result of the synergistic effect of the two carcinogens.4,5 Although the risk of lung cancer due to occupational exposure to asbestos depends on the duration, concentration, and fiber type, the increased risk may be largely limited to those with radiologic evidence of asbestosis or to cigarette smokers.
Additional risk factors for development of lung cancer include exposure to ionizing radiation, arsenic, chloromethyl ethers, chromium, isopropyl oil, mustard gas, nickel, beryllium, lead, copper, chloroprene, and vinyl chloride.6,7 Finally, genetic susceptibility to lung cancer may be an important risk factor. A number of different gene mutations are common in lung cancer and there is a strong association between KRAS mutations in adenocarcinoma and smoking.8 In addition, mutations of the epidermal growth factor receptor (EGFR) gene appears to have a strong association with adenocarcinoma, particularly bronchioloalveolar cell carcinoma subtype.9,10 Furthermore, although multigenic factors influence carcinogen metabolism, a region on chromosome 6q increases the risk for lung cancer, particularly in never and light smokers and there is compelling evidence that a locus at 15q25 predisposes to lung cancer.11
Pathology
Lung cancer is divided by the World Health Organization (WHO) Classification into two major histologic categories: non–small cell lung carcinoma (NSCLC) and small cell lung carcinoma (SCLC) (Table 6-1).12 NSCLC is subdivided into histologic types (squamous cell carcinoma [SCC], adenocarcinoma, and large cell carcinoma) according to the most differentiated portion of the tumor. In addition, some NSCLCs have immunohistochemical and/or ultrastructural features of neuroendocrine differentiation and are collectively referred to as NSCLC with neuroendocrine differentiation.12 These malignancies are distinguished from the neuroendocrine tumors of lung (typical carcinoid, atypical carcinoid, large cell neuroendocrine carcinoma, and SCLC) by the absence on microscopy of organoid nesting, rosette formation, peripheral palisading of tumor nests, and trabeculae and on immunohistochemistry by nonreactivity with neuroendocrine markers.13
Table 6-1 Histologic Classification of Lung Cancer
| Squamous Cell Carcinoma |
| Variants: papillary, clear cell, small cell, basaloid |
| Adenocarcinoma |
| Adenocarcinoma, mixed subtype Acinar adenocarcinoma |
| Papillary adenocarcinoma |
| Bronchioloalveolar carcinoma |
Solid Adenocarcinoma with Mucin ProductionVariants: fetal, clear cell, signet-ring, colloidLarge Cell CarcinomaVariants: large cell neuroendocrine, basaloid, lymphoepithelioma-like, clear cell, large cell with rhabdoid phenotypeAdenosquamous CarcinomaSmall Cell CarcinomaVariant: combined small cell carcinoma
Modified from Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC. Pathology and Genetics: Tumours of the Lung, Pleura, Thymus and Heart. Lyon, France: IARC; 2004.
Squamous Cell Carcinoma
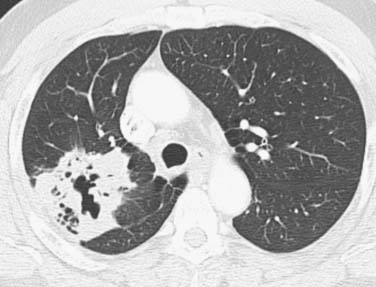
SCCs constitute approximately 30% of all lung cancers.14 They typically occur as a central endobronchial mass and frequently manifest as postobstructive pneumonia or atelectasis (Figure 6-1).15 Approximately one third of SCCs occur beyond the segmental bronchi and usually range in size from 1 to 10 cm.16 SCCs are more likely to cavitate than the other histologic cell types of lung cancer (Figure 6-2).16 Histologically, SCC grows in nests within a desmoplastic stroma and the tumor cells demonstrate keratinization, intercellular bridge formation, and squamous pearls.
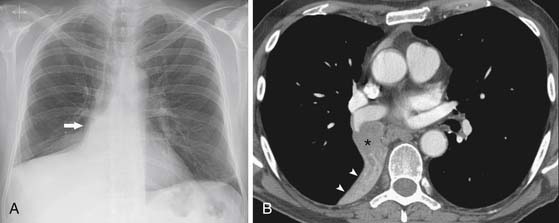
Figure 6-1 A 62-year-old man with squamous cell lung cancer manifesting as a central endobronchial mass. A, Posteroanterior chest radiograph shows complete atelectasis of the middle and right lower lobes. Convexity in the atelectatic lung (arrow) is the result of a central mass. B, Computed tomography (CT) scan confirms an endobronchial mass (asterisk) that occludes the bronchus intermedius and causes complete atelectasis of the middle and lower lobes distal to the mass (arrowheads).
Adenocarcinoma
Adenocarcinomas are the most frequent histologic type and constitute approximately 50% of all lung cancers.14 Adenocarcinoma commonly manifests as a peripheral, solitary pulmonary nodule with irregular or spiculated margins as a result of parenchymal invasion and associated fibrotic response. The nodules are usually of soft tissue attenuation and cavitation is rare (Figure 6-3).16 However, adenocarcinomas manifesting as purely ground-glass or solid or mixed (ground-glass and solid) attenuation are being detected with increasing frequency (Figures 6-4 and 6-5). Histologically, lung adenocarcinoma is classified according to the 2011 International Association for the Study of Lung Cancer (IASLC)/American Thoracic Society (ATS)/European Respiratory Society (ERS) classification.17 This new classification strategy is based on a multidisciplinary approach to the diagnosis of lung adenocarcinoma that incorporates clinical, molecular, radiologic, and surgical issues, but it is primarily based on histology. This new classification provides a uniform terminology and diagnostic criteria, especially for tumors formerly known as bronchioloalveolar carcinoma (BAC), as well as an approach to small biopsy specimens. For resection specimens, the new classification comprises preinvasive lesions (adenocarcinoma in situ [AIS] < 3 cm, solitary, pure lepidic growth, formerly bronchioloalveolar cell carcinoma), minimally invasive adenocarcinoma (MIA) < 3 cm with predominant lepidic growth and ≤ 5 mm invasion, invasive adenocarcinomas and variants of invasive adenocarcinoma. AIS and MIA are usually nonmucinous but rarely may be mucinous. Invasive adenocarcinomas are classified by predominant pattern: lepidic, acinar, papillary, micropapillary, and solid patterns. Variants include invasive mucinous adenocarcinoma (formerly mucinous BAC), colloid, fetal, and enteric adenocarcinoma. This classification provides guidance for small biopsies and cytology specimens, as approximately 70% of lung cancers are diagnosed in such samples.
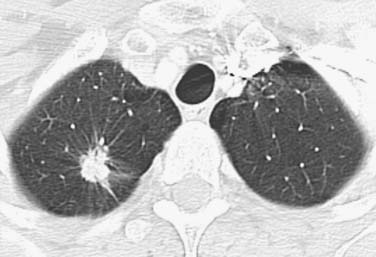
Figure 6-3 A 59-year-old woman with adenocarcinoma manifesting as small peripheral pulmonary nodule. CT scan shows a spiculated nodule in the right upper lobe. The spiculated margin is typical of lung cancer.
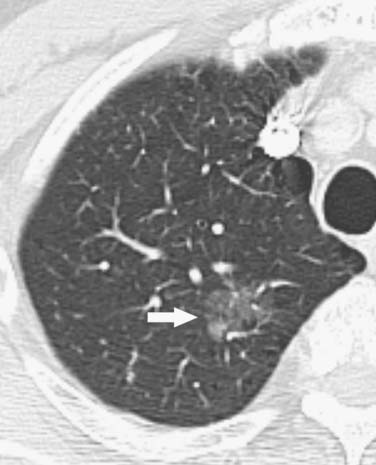
Figure 6-4 A 78-year-old man with adenocarcinoma manifesting as a pulmonary nodule with ground-glass attenuation. CT scan shows a poorly marginated ground-glass opacity in the left upper lobe (arrow). Note that these malignancies are typically indolent.
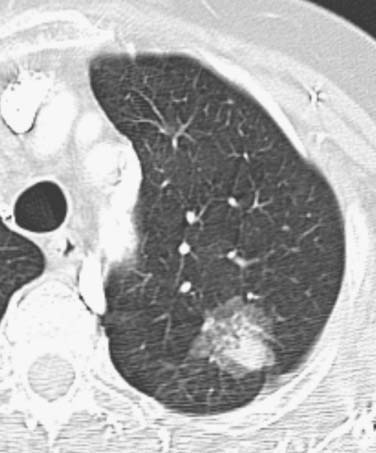
Figure 6-5 A 76-year-old woman with adenocarcinoma manifesting as a pulmonary nodule with mixed ground-glass and solid attenuation. CT scan shows a poorly marginated ground-glass opacity containing a focal solid component in the left upper lobe. The likelihood of invasive adenocarcinoma is high with mixed and solid opacities.
A histologic classification has also been proposed by Noguchi and coworkers,18 whereby small (≤2 cm) peripheral adenocarcinomas are classified into six types based on tumor growth patterns: type A, localized BACs that grow by replacement of alveolar lining cells, with minimal or mild thickening of the alveolar septa and fibrotic foci are absent; type B, localized BAC similar to type A except fibrotic foci due to alveolar collapse are present in the tumor; type C, localized BAC with replacement growth pattern and foci of active proliferating fibroblasts; type D, poorly differentiated adenocarcinomas that have a solid growth pattern with only minor components of papillary and tubular patterns of growth; type E, tubular adenocarcinoma is a specific type of adenocarcinoma that is derived from bronchial gland cells and consists of acinar, tubular, and cribriform structures; type F, papillary adenocarcinomas show papillary growth that is expansive and destructive (i.e., do not grow by replacing the alveolar lining cells).18 The soft tissue attenuation component tends to be absent or less than a third of the opacity with type A and greater in extent (more than two thirds) in types D to F.19 Thin-section CT has been reported to be useful in differentiating type C from types A and B.20 The likelihood of invasive adenocarcinoma and more advanced stage of lung cancer has been reported to be higher with mixed and solid opacities.21
Large Cell Carcinoma
Large cell carcinomas constitute 10% to 20% of all lung cancers.1,14 Most are peripheral, poorly marginated masses greater than 7 cm in diameter.15,16 Histologically, they are defined as undifferentiated tumors that lack the cytologic features of SCCs and have no glandular differentiation. The cells are usually relatively large and contain abundant cytoplasm and vesicular chromatin and occasional nucleoli.
Small Cell Lung Cancer
SCLCs constitute 15% to 20% of all lung cancers.1,15 The primary tumor is typically small and often central in location, and extensive hilar and mediastinal adenopathy is common (Figure 6-6).15 Rarely, SCLC manifests as a small, peripheral, solitary pulmonary nodule. SCLC is a neuroendocrine tumor with 10 mitoses/2 mm2 that manifests histologically as sheets of small, oval to slightly spindle-shaped cells with scant cytoplasm and hyperchromatic nuclei with small to absent nucleoli.
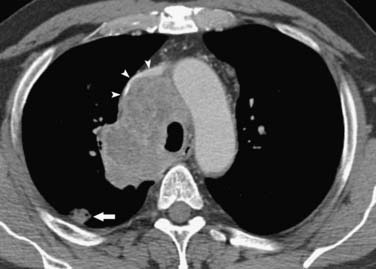
Figure 6-6 A 52-year-old man with small cell lung cancer (SCLC) manifesting as a small lung nodule and extensive mediastinal adenopathy. CT scan shows a small lung nodule (arrow) and mediastinal adenopathy that compresses and narrows the left brachiocephalic vein and superior vena cava (arrowheads).
Key Points Pathology
• Two major histologic categories are NSCLC and SCLC.
• NSCLC is subdivided into SCC, adenocarcinoma, and large cell carcinoma.
• Adenocarcinoma is the most common histologic subtype and is classified as AIS, MIA, invasive adenocarcinoma and variants of invasive adenocarcinoma.
• Noguchi and coworkers’ classification applies to small peripheral adenocarcinomas and is based on tumor growth patterns.
Clinical Manifestations
At presentation, most patients are in their fifth and sixth decades and are symptomatic. Symptoms are variable and depend on the local effects of the primary mass, the presence of regional or distant metastases, and the coexistence of paraneoplastic syndromes. Central endobronchial carcinomas can manifest as cough, hemoptysis, and dyspnea. Symptoms that can occur as a result of local growth and invasion of adjacent nerves, vessels, and mediastinal structures include superior vena cava syndrome (Figure 6-7), chest pain due to peribronchial nerve or chest wall involvement, vocal cord paralysis and hoarseness, dyspnea due to diaphragmatic paralysis (Figure 6-8), and Horner’s syndrome (ptosis, miosis, anhidrosis due to sympathetic chain, and stellate ganglion involvement by superior sulcus tumors).
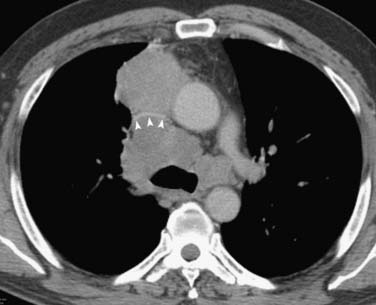
Figure 6-7 A 57-year-old man with non–small-cell lung cancer (NSCLC) presenting with superior vena cava syndrome (dyspnea, upper extremity and facial swelling). CT scan shows extensive, multicompartmental adenopathy and obstruction of the superior vena cava (arrowheads).
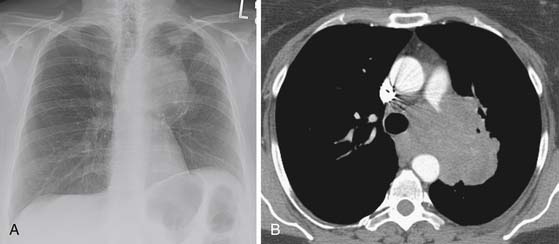
Figure 6-8 A 61-year-old woman with NSCLC presenting with hoarseness and dyspnea. A, Posteroanterior chest radiograph shows a left perihilar mass and opacities more peripherally in the left upper lobe due to obstructive atelectasis/consolidation. Note elevation of the left hemidiaphragm due to paralysis as a result of phrenic nerve invasion. B, CT scan reveals mediastinal invasion with extension of the mass into the aortopulmonary window (the anatomic location of the recurrent laryngeal nerve).
Many patients present with symptoms related to extrathoracic metastases, most commonly bone pain or central nervous system abnormalities. Clinical signs and symptoms can also be caused by tumor excretion of a bioactive substance or hormone or as a result of immune-mediated neural tissue destruction caused by antibody- or cell-mediated immune responses. These paraneoplastic syndromes occur in 10% to 20% of lung cancer patients and are usually associated with SCLC.22,23 Antidiuretic and adrenocorticotropin hormones are the more frequently excreted hormones and can result in hyponatremia and serum hypo-osmolarity and in Cushing’s syndrome (central obesity, hypertension, glucose intolerance, plethora, and hirsutism), respectively.22 Other hormones that can be elevated are calcitonin; growth hormone; and human chorionic gonadotropin, prolactin, and serotonin.
Neurologic paraneoplastic syndromes (Lambert-Eaton myasthenic syndrome, paraneoplastic cerebellar degeneration, paraneoplastic encephalomyelitis, and paraneoplastic sensory neuropathy) are rare and are usually associated with SCLC. The neurologic symptoms typically precede the diagnosis of lung cancer by up to 2 years, are incapacitating, and progress rapidly, although improvement can occur after treatment.24,25 Miscellaneous paraneoplastic syndromes associated with lung cancer include acanthosis nigricans, dermatomyositis, disseminated intravascular coagulation, and hypertrophic pulmonary osteoarthropathy.
Patterns of Tumor Spread
Lung cancers usually invade the pulmonary arterial and venous systems and, less commonly, the bronchial arteries. Hematogenously disseminated metastases to the lung, pleura, adrenals, liver, brain, and bones are common (Figure 6-9). There is evidence that dissemination of cells or fragments of tumor from the primary malignancy occurs at an early stage of the malignancy. Tumor emboli or micrometastasis in bone marrow and circulating cancer cells in blood have been detected in localized NSCLC.26 However, the clinical relevance of this minimal hematogenous tumor cell dissemination is controversial. Nonetheless, these shed cells may represent true micrometastasis because they are an independent prognostic factor for overall survival.27 The pathogenesis of metastatic disease once tumor emboli have disseminated is complex and multifactorial. Although there is considerable variation among tumors, there is a relationship of the incidence of hematogenously disseminated metastases and the cell type of the lung cancer. In this regard, SCCs tend to grow slowly and remain localized to the lung; hematogenous dissemination of extrathoracic metastases usually occurs late.16 Because adenocarcinomas are histologically a very diverse group of malignancies, there is variability in their propensity for hematogenous dissemination. Generally, the likelihood of early hematogenous dissemination of metastases is high with poorly differentiated, invasive adenocarcinomas (Noguchi types D, E, and F) and low with localized and indolent adenocarcinomas (Noguchi types A, B, and C).21,28 Large cell carcinoma and small cell carcinoma have a high propensity for early vascular invasion, and hematogenous dissemination of distant metastases to liver, bone marrow, adrenals, and brain is common.
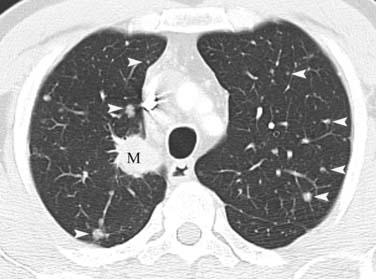
Figure 6-9 A 55-year-old man with NSCLC and hematogenously disseminated lung metastases. Chest CT scan shows the primary malignancy in the right upper lobe (M) and numerous small discrete nodules in both lungs (arrowheads). Note that sharp margination and variability in size are typical of hematogenous dissemination.
Similar to hematogenous dissemination, lymphatic dissemination is broadly related to tumor location and tumor cell type. There is a greater frequency of nodal metastasis in patients with central cancers than in those with peripheral tumors, and lymphatic dissemination of metastasis tends to occur late in patients with SCCs and early and frequently in patients with invasive adenocarcinomas, large cell carcinomas, and SCLCs.29 In addition, besides a higher prevalence of mediastinal nodal metastases in these malignancies compared with SCC, there is a much higher frequency of mediastinal metastases without lobar or hilar involvement reported in adenocarcinomas.29,30 In this regard, the mediastinal nodes are typically not involved with metastasis in patients with SCCs unless the lobar and/or hilar nodes are involved.29 It has been reported that anatomic lymphatic pathways can explain the likely pattern of spread of mediastinal nodal metastases based on the lobar location of the primary tumor.29,30 For instance, there is a high likelihood of involvement of the right paratracheal and aorticopulmonary nodes in right and left upper lobe malignancies, respectively. However, the unreliability of predicting the location of these lymphatically disseminated metastases requires extensive invasive sampling in most patients with potentially resectable NSCLCs.
Lymphatic dissemination of tumor emboli in the lungs, more frequently seen with adenocarcinomas, is termed lymphangitic carcinomatosis (Figure 6-10). Lymphangitic carcinomatosis is preceded by hematogenous dissemination of metastases. After hematogenous dissemination of metastasis in the lung parenchyma, tumor growth remains localized to the perivascular interstitium with subsequent growth toward the hilum or periphery of the lung along pulmonary lymphatics in the perivenous and bronchoarterial interstitium and interlobular septa. Typically, this mechanism of dissemination affects the lymphatics and surrounding interstitium of the bronchovascular bundles, interlobular septa, and pleura and is not accompanied by hilar or mediastinal nodal metastases. A less common mechanism for the development of lymphangitic carcinomatosis is retrograde spread of tumor within the lymphatics of the lung secondary to involvement of mediastinal and hilar nodes.
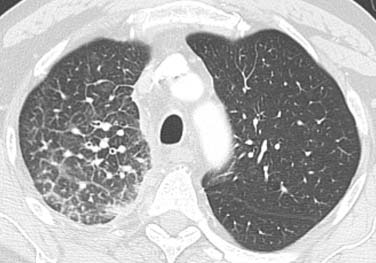
Figure 6-10 A 66-year-old man with a right upper NSCLC (not shown) and lymphangitic carcinomatosis. Chest CT scan shows thickening of the interlobular septa and peribronchovascular interstitium and visualization of the polygonal shape of the secondary pulmonary lobules.
Lastly, to account for the multifocality of BAC, dissemination by aerolization has been proposed. In this regard, the origin of BAC may be either monoclonal (with multifocality due to dissemination by aerosolization, intrapulmonary lymphatics, and intra-alveolar growth) or polyclonal (with multifocality due to de novo tumor growth at multiple sites) (Figure 6-11).
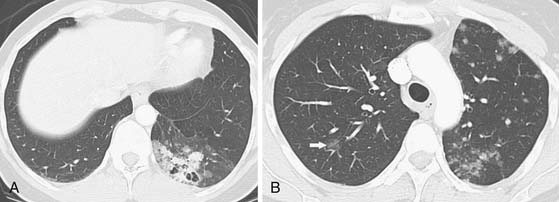
Figure 6-11 A 39-year-old woman with bronchioloalveolar cell carcinoma of the lung. A, Chest CT scan shows a poorly marginated cavitary mass with mixed solid and ground-glass attenuation. Note adjacent diffuse and nodular ground-glass opacities, consistent with multifocal malignancy. The patient underwent lower lobe resection confirming multifocal bronchioloalveolar cell malignancy. B, Chest CT scan 6 years after A shows interval development of multifocal discrete and ground-glass nodular opacities in the left upper lobe and a focal ground-glass nodular opacity in the right upper lobe (arrow), consistent with bronchioloalveolar malignancy. Note that dissemination of malignancy may be by aerosolization, intrapulmonary lymphatics, and intra-alveolar growth or due to de novo tumor growth at multiple sites.
Key Points Patterns of tumor spread
• Dissemination of cancer cells from the primary malignancy occurs early and tumor emboli are common and are a prognostic factor for overall survival.
• Incidence of hematogenously disseminated metastases is related to cell type.
• Hematogenous dissemination occurs late with SCC.
• Hematogenous dissemination is high with invasive adenocarcinoma, large cell carcinoma, and small cell carcinoma and low with localized/indolent adenocarcinoma.
• Lymphatic dissemination of metastasis occurs late with SCC and early and frequently in patients with invasive adenocarcinoma, large cell carcinoma, and SCLC.
• Lymphatic pathways explaining the likely pattern of spread of mediastinal nodal metastases based on the lobar location of the primary tumor are unreliable.
Imaging Evaluation
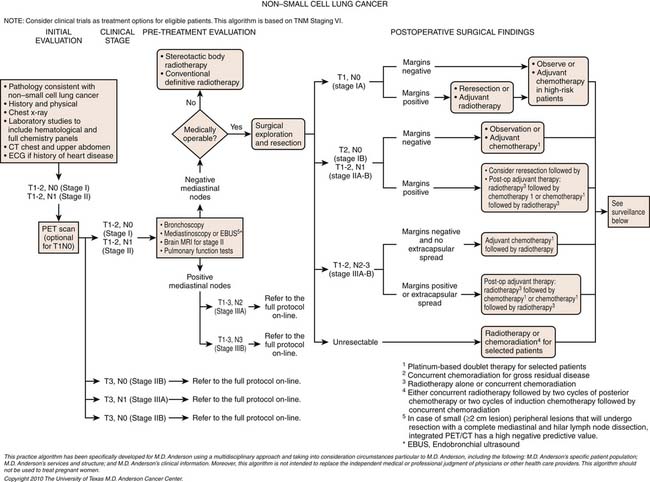
Non–Small Cell Lung Cancer Staging
The treatment and prognosis of patients with NSCLC depends on staging: the determination of the anatomic extent of disease at initial presentation. The tumor-node-metastasis (TNM) descriptors and stage-grouping revisions proposed by the International Association for the Study of Lung Cancer (IASLC) Lung Cancer Staging Project have been accepted and forthwith patients will be staged according to the seventh edition of the American Joint Committee on Cancer (AJCC) TNM staging system.31–33
Primary Tumor (T Status)
The T status defines the size, location, and extent of the primary tumor (Table 6-2 and Figure 6-12). There are numerous changes to the T descriptor in the seventh edition of the TNM classification of lung cancer that are based on differences in survival: (1) T1 is now subclassified as T1a (≤2 cm) or T1b (>2 to ≤3-cm); (2) T2 is now subclassified as T2a (>3 to ≤5 cm or T2 by other factors and ≤5 cm) or T2b (>5 to ≤7 cm); (3) T2 tumors greater than 7 cm are reclassified as T3; (4) T4 tumors by additional nodule(s) in the lung (primary lobe) are reclassified as T3; (5) M1 by additional nodule(s) in the ipsilateral lung (different lobe) is reclassified as T4; and (6) T4 pleural dissemination (malignant pleural effusions and pleural nodules) is reclassified as M1.31
Table 6-2 Definitions for Tumor-Node-Metastasis Descriptors
| STAGE | DEFINITION |
|---|---|
| T | Primary Tumor |
| TX | Primary tumor cannot be assessed, or tumor proven by the presence of malignant cells in sputum or bronchial washings but not visualized by imaging or bronchoscopy |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| T1 | Tumor 3 cm or less in greatest dimension, surrounded by lung or visceral pleura, without bronchoscopic evidence of invasion more proximal than the lobar bronchus (i.e., not in the main bronchus) |
| T1a | Tumor 2 cm or less in greatest dimension* |
| T1b | Tumor more than 2 cm but not more than 3 cm in greatest dimension |
| T2 | Tumor more than 3 cm but not more than 7 cm, or tumor with any of the following features†: |
T2aTumor more than 3 cm but not more than 5 cm in greatest dimensionT2bTumor more than 5 cm but not more than 7 cm in greatest dimensionT3Tumor more than 7 cm or one that directly invades any of the following: chest wall (including superior sulcus tumors), diaphragm, phrenic nerve, mediastinal pleura, parietal pericardium; or tumor in the main bronchus less than 2 cm distal to the carina* but without involvement of the carina; or associated atelectasis or obstructive pneumonitis of the entire lung or separate tumor nodule(s) in the same lobe as the primaryT4Tumor of any size that invades any of the following: mediastinum, heart, great vessels, trachea, recurrent laryngeal nerve, esophagus, vertebral body, carina; separate tumor nodule(s) in a different ipsilateral lobe to that of the primary NRegional Lymph NodesNX
N0
N1N2
N3
Regional lymph nodes cannot be assessed
No regional lymph node metastasis
Metastasis in ipsilateral peribronchial and/or ipsilateral hilar lymph nodes and intrapulmonary nodes, including involvement by direct extension
Metastasis in ipsilateral mediastinal and/or subcarinal lymph node(s)
Metastasis in contralateral mediastinal, contralateral hilar, ipsilateral or contralateral scalene, or supraclavicular lymph node(s)MDistant MetastasisM0
M1
M1a
M1bNo distant metastasis
Distant metastasis
Separate tumor nodule(s) in a contralateral lobe; tumor with pleural nodules or malignant pleural or pericardial effusion‡
Distant metastasis
* The uncommon superficial spreading tumor of any size with its invasive component limited to the bronchial wall, which may extend proximal to the main bronchus, is also classified as T1a.
† T2 tumors with these features are classified T2a if 5 cm or less or if size cannot be determined, and T2b if greater than 5 cm but not larger than 7 cm.
‡ Most pleural (pericardial) effusions with lung cancer are due to tumor. In a few patients, however, multiple microscopic examinations of pleural (pericardial) fluid are negative for tumor, and the fluid is nonbloody and is not an exudate. Where these elements and clinical judgment dictate that the effusion is not related to the tumor, the effusion should be excluded as a staging element and the patient should be classified as M0.
Reprinted with permission courtesy of the International Association for the Study of Lung Cancer. Copyright 2009 IASLC.
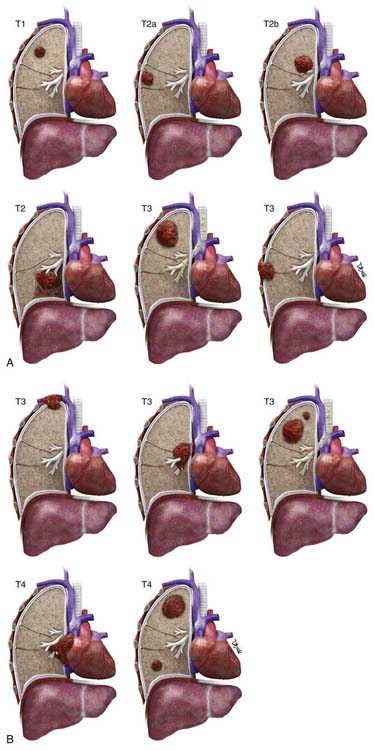
Figure 6-12 A and B, T1: Tumor ≤3 cm surrounded by lung or visceral pleura without evidence of invasion proximal to a lobar bronchus. T2: Tumor >3 to ≤7 cm or tumor with any of the following: invades visceral pleura (T2a), involves main bronchus >2 cm distal to the carina (T2b), or atelectasis/obstructive pneumonia but not involving the entire lung (T2). T3: Tumor >7 cm or tumor of any size invading the chest wall, including superior sulcus tumors, diaphragm and mediastinal pleura, parietal pericardium or tumor <2 cm from the carina or atelectasis/obstructive pneumonia involving the entire lung (not shown) or separate tumor nodule(s) in same lobe as the primary. T4: Tumor of any size with invasion of the mediastinum, trachea, heart, great vessels, esophagus, carina, vertebra, or separate tumor nodule(s) in a different ipsilateral lobe than the primary.
The International Staging System for Lung Cancer combines the T descriptors with the N and M descriptors into subsets or stages that have similar treatment options and prognosis (Table 6-3). However, it is important to realize that, although a T4 descriptor generally precludes resection, patients with cardiac, tracheal, and vertebral body invasion are designated in the seventh edition staging system as being potentially resectable (stage IIIa) in the absence of N2 and/or N3 disease.
Regional Lymph Nodes (N Status)
The presence and location of nodal metastasis are of major importance in determining management and prognosis in patients with NSCLC. To enable a consistent and standardized description of N status, nodal stations are defined by the American Thoracic Society in relation to anatomic structures or boundaries that can be identified before and during thoracotomy (Table 6-4; see also Table 6-2). The N descriptors in the seventh edition of the TNM classification of lung cancer have been maintained because there were no significant survival differences in analysis by station.32 However, lymph node stations will be grouped together in six zones within the current N1 and N2 patient subsets for further evaluation. Zones are defined as peripheral (stations 12, 13, and 14) or hila (stations 10 and 11) for N1 and upper mediastinal (stations 1, 2, 3, and 4), lower mediastinal (stations 8 and 9), aortopulmonary (stations 5 and 6), and subcarinal (station 7) for N2 nodes.32
Table 6-4 Regional Lymph Node Stations for Lung Cancer Staging
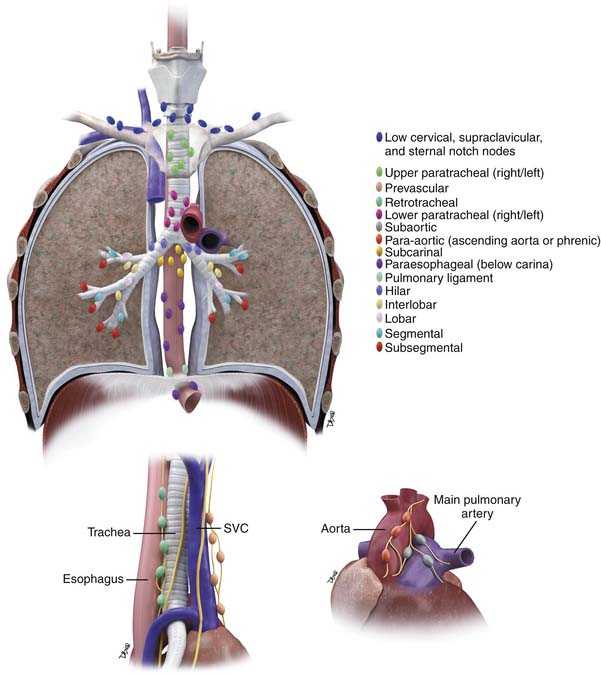 |
| Low Cervical, Supraclavicular, and Sternal Notch Nodes (Left/Right) |
Upper Paratracheal Nodes (Left/Right)
2R: Upper border: apex of the right lung and pleural space and, in the midline, the upper border of the manubrium
Lower border: intersection of caudal margin of innominate vein with the trachea
2L: Upper border: apex of the left lung and pleural space and, in the midline, the upper border of the manubrium
Lower border: superior border of the aortic arch
As for #4, in #2, the oncologic midline is along the left lateral border of the trachea.
Prevascular and Retrotracheal Nodes
Lower Paratracheal Nodes (Left/Right)
Right: includes right paratracheal nodes, and pretracheal nodes extending to the left lateral border of trachea
Upper border: intersection of caudal margin of innominate vein with the trachea
Lower border: lower border of azygos vein
Left: includes nodes to the left of the left lateral border of the trachea, medial to the ligamentum arteriosum
Subaortic (Aortopulmonary Window)
Subaortic lymph nodes lateral to the ligamentum arteriosum
Para-aortic Nodes Ascending Aorta or Phrenic
Lymph nodes anterior and lateral to the ascending aorta and aortic arch
Upper border: a line tangential to the upper border of the aortic arch
Subcarinal Nodes
Upper border: the carina of the trachea
Lower border: the upper border of the lower lobe bronchus on the left; the lower border of the bronchus intermedius on the right
Paraesophageal Nodes (Below Carina) (Left/Right)
Nodes lying adjacent to the wall of the esophagus and to the right or left of the midline, excluding subcarinal nodes
Upper border: the upper border of the lower lobe bronchus on the left; the lower border of the bronchus intermedius on the right
Pulmonary Ligament Nodes (Left/Right)
Hilar Nodes (Left/Right)
Includes nodes immediately adjacent to the mainstem bronchus and hilar vessels including the proximal portions of the pulmonary veins and main pulmonary artery
Upper border: the lower rim of the azygos vein on the right; upper rim of the pulmonary artery on the left
Interlobar Nodes
Between the origin of the lobar bronchi
*#11s: between the upper lobe bronchus and the bronchus intermedius on the right
*#11i: between the middle and the lower lobe bronchi on the right
Lobar Nodes
Segmental Nodes
Subsegmental Nodes
Reprinted and figure redrawn with permission courtesy of the International Association for the Study of Lung Cancer. Copyright 2009 IASLC.
Metastatic Disease (M status)
Patients with NSCLC commonly have metastases to the lung, adrenals, liver, brain, bones, and extrathoracic lymph nodes at presentation. There are numerous changes to the M descriptor in the seventh edition of the TNM classification of lung cancer that are based on differences in survival (see Table 6-2). The M1 descriptor is now subclassified into M1a (additional nodules in the contralateral lung) and M1b (distant metastases outside the lung and pleura).33 In addition, based on survival analysis, the current M descriptor is modified to reclassify pleural metastases (malignant pleural effusions and pleural nodules) from T4 to M1a.33
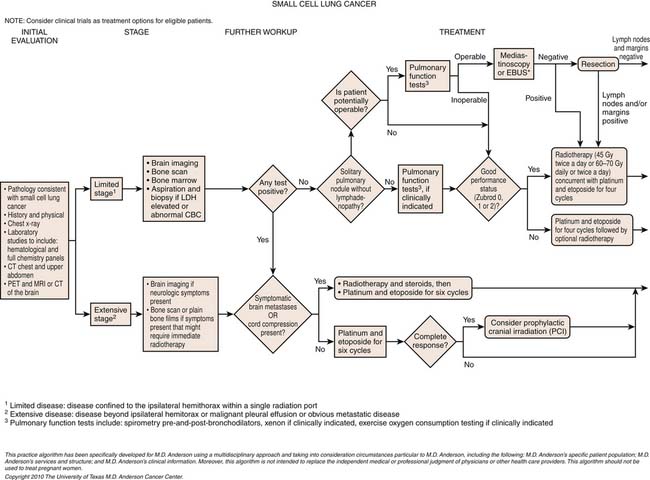
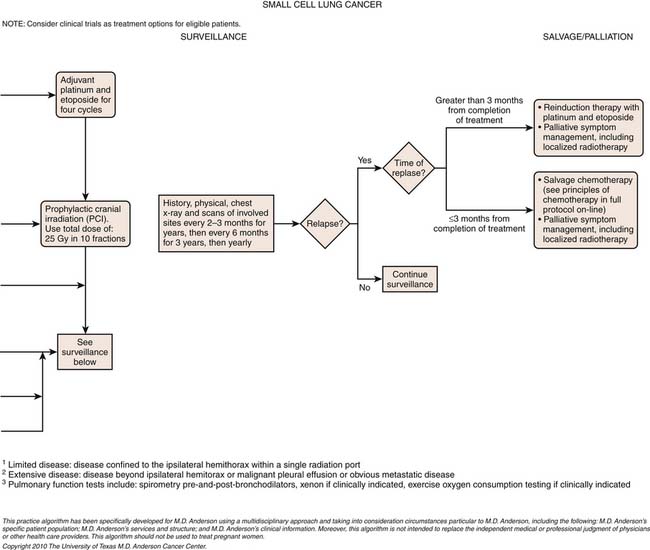
Small Cell Lung Cancer Staging
SCLC is generally staged according to the Veteran’s Administration Lung Cancer Study Group (VALG) recommendations as limited disease (LD) or extensive disease (ED).34 LD defines tumor confined to a hemithorax and the regional lymph nodes. Unlike the TNM classification for NSCLC,
Key Points Staging of non–small cell lung cancer
• Changes in TNM staging proposed by IASLC Lung Cancer Staging Project have been accepted.
• T descriptor includes changes: tumors greater than 7 cm are now reclassified as T3 (previously T2), additional nodule(s) in the lung (primary lobe) are reclassified as T3 (previously T4), additional nodule(s) in the ipsilateral lung (different lobe) are reclassified as T4 (previously M1), and malignant pleural effusions and pleural nodules are reclassified as M1 (previously T4).
• N descriptors are unchanged.
• M1 descriptor is subclassified into M1a (additional nodules in the contralateral lung) and M1b (distant metastases).
metastases to the ipsilateral supraclavicular, contralateral supraclavicular, and mediastinal lymph nodes are considered local disease. ED includes tumor with noncontiguous metastases to the contralateral lung and distant metastases.34
The TNM staging system of the AJCC is also applicable to SCLC, but is used less frequently in clinical practice because only a small percentage of patients with SCLC present at a stage for which surgery is appropriate. Nevertheless, small published series of resected SCLC have suggested that the TNM pathologic staging correlates with the survival of resected patients. A recent analysis of the 8088 cases of SCLC in the IASLC database demonstrated the usefulness of clinical TNM staging in this malignancy.35 Accordingly, the IASLC Lung Cancer Staging Project proposes that TNM staging should be the standard for all cases of SCLC.
Imaging
Non–Small Cell Lung Cancer
Imaging is a major component of clinical TNM staging. However, there is currently little consensus on the imaging that should be performed for appropriate staging evaluation in patients presenting with NSCLC. Recently, the American Society of Clinical Oncology (ASCO) published evidence-based guidelines for the diagnostic evaluation of patients with NSCLC.36 In the staging of locoregional disease, these guidelines recommend that a chest radiograph and contrast-enhanced chest CT that includes the liver and adrenals should be performed. In addition, the ASCO recommendations are that whole body FDG-PET should be performed when there is no evidence of distant metastatic disease on CT.36 This recommendation is based on the fact that FDG-PET imaging improves the detection of nodal and distant metastases and frequently alters patient management.37,38
CT and MRI are often performed to more optimally assess the primary tumor because the extent of the primary tumor can determine therapeutic management (surgical resection, palliative radiotherapy, or chemotherapy). Evaluation of the primary tumor (size, location, and proximity to critical structures) is important and provides information to surgeons and radiation oncologists that can affect therapeutic management. For instance, centrally located tumors close to the spinal cord impose radiation dose-volume constraints and determination of tumor margins is important and can affect the delivery of radiotherapy. This is especially important with the increasing use of conformal radiation therapy, a technique that uses multiple radiation beams to generate dose distributions that conform tightly to target volumes. The determination of the degree of pleural, chest wall, and mediastinal invasion, as well as involvement of the central airways and pulmonary arteries, is also important not only to radiation oncologists but also to surgeons evaluating the patients for resectability. For instance, involvement of the pulmonary artery may require a pneumonectomy rather than a lobectomy in order to obtain clear surgical margins. In addition, involvement of the origin of the lobar bronchus or main bronchus may require a sleeve resection or pneumonectomy. Because patients treated by medical oncologists generally have metastatic disease and treatment is directed both at local and for systemic disease, accurate determination of tumor location and extent is only important if there is a potential risk for a significant complication such as invasion of a vascular structure that could result in significant bleeding. However, CT is important to the medical oncologists in the determination of the effectiveness of treatment to determine whether the treatment regimen should be continued or changed (see “Monitoring Tumor Response”).
CT is useful in defining the T parameters of the primary tumor, but in many patients, this assessment has limitations. For instance, CT is useful in confirming gross chest wall invasion but is inaccurate in differentiating between anatomic contiguity and subtle invasion. Determining the presence and extent of chest wall invasion is important from a surgical perspective because the surgical approach may be altered to include an en bloc resection of the primary malignancy and chest wall. Although MRI offers superior soft tissue contrast resolution to that of CT, the sensitivity and specificity in identifying chest wall invasion is not optimal. Imaging with CT or MRI is also useful in confirming gross invasion of the mediastinum, but these modalities, similar to chest wall assessment, are inaccurate in determining subtle invasion. However, MRI is particularly useful in the evaluation of cardiac invasion and assessment of superior sulcus tumors. MRI is particularly useful in the assessment of the degree of involvement of the brachial plexus, subclavian vessels, and vertebral bodies in patients with superior sulcus tumors (Figure 6-13).39,40 Importantly, absolute contraindications to surgery (invasion of the brachial plexus roots or trunks above the level of T1, invasion > 50% of a vertebral body, and invasion of the esophagus or trachea) are often accurately assessed by MRI.41,42
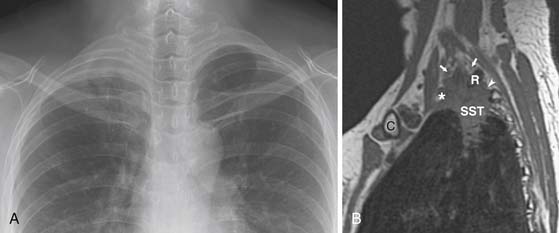
Figure 6-13 A 49-year-old woman with a superior sulcus NSCLC presenting with shoulder pain and Horner’s syndrome (ptosis, miosis, and anhidrosis). A, Posteroanterior chest radiograph shows a soft tissue mass in the right lung apex. There are no findings of rib or vertebral body invasion. B, Sagittal T1-weighted magnetic resonance imaging (MRI) study shows the superior sulcus tumor (SST) extending posteriorly into the T1-2 neurovertebral foramen (arrowhead) and causing obliteration of the exiting T1 nerve root. The C7 and C8 nerve roots are preserved (arrows). Note that limited involvement of the brachial plexus does not preclude surgical resection. C, clavicle, R, first rib; *, subclavian artery.
Because surgical resection and potential use of adjuvant therapy are dependent on the patient’s N descriptor, attempts have been made to improve the accuracy of detection of nodal metastases. Importantly, ipsilateral peribronchial or hilum (N1) nodes are usually resectable, and it is the presence of mediastinal adenopathy that has a major impact on resectability. Specifically, ipsilateral mediastinal or subcarinal adenopathy (N2) may be resectable (usually after induction chemotherapy), whereas contralateral mediastinal adenopathy and scalene or supraclavicular adenopathy (N3) are unresectable. The detection of nodal metastases is also important to the radiation oncologist because incorporation of these nodes into the radiation treatment plan is important in appropriate treatment. In the imaging evaluation of nodal metastasis, size is the only criterion used to diagnose metastases, with nodes greater than 1 cm in short-axis diameter considered abnormal. However, lymph node size is not a reliable parameter for the evaluation of nodal metastatic disease in patients with NSCLC. In a meta-analysis of 3438 patients evaluating CT accuracy for staging the mediastinum, there was a pooled sensitivity of 57%, specificity of 82%, positive predictive value of 56%, and negative predictive value of 83%.43 Furthermore, Prenzel and colleagues44 reported that, in 2891 resected hilar and mediastinal nodes obtained from 256 patients with NSCLC, 77% of the 139 patients with no nodal metastases had at least one node greater than 1 cm in diameter and 12% of the 127 patients with nodal metastases had no nodes greater than 1 cm.
FDG-PET improves the accuracy of nodal staging (Figure 6-14).45,46 In a recent meta-analysis (17 studies, 833 patients) comparing PET and CT in nodal staging in patients with NSCLC, the sensitivity and specificity of FDG-PET for detecting mediastinal lymph node metastases ranged from 66% to 100% (overall 83%) and 81% to 100% (overall 92%), respectively, compared with sensitivity and specificity of CT of 20% to 81% (overall 59%) and 44% to 100% (overall 78%), respectively.45 Because of the improvements of nodal staging when PET/CT is incorporated into the imaging algorithm of those patients with potentially resectable NSCLC, the performance of PET/CT should be considered in all patients without CT findings of distant metastasis, regardless of the size of mediastinal nodes, to direct nodal sampling as well as to detect distant occult metastasis. Importantly, although FDG-PET is cost-effective for nodal staging and can reduce the likelihood that a patient with mediastinal nodal metastases (N3) that would preclude surgery will undergo attempted resection, the number of false-positive results due to infectious or inflammatory etiologies is too high to preclude invasive sampling.
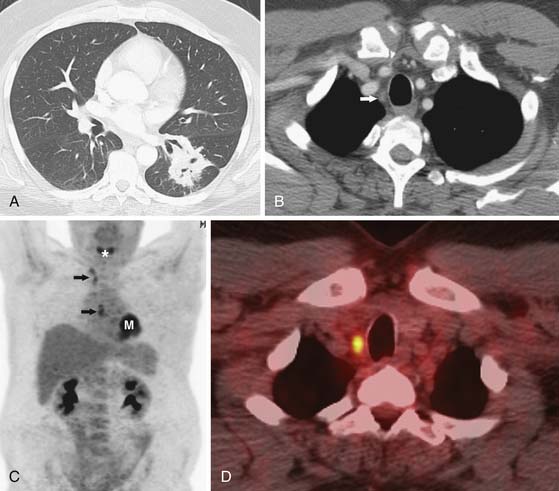
Figure 6-14 A 51-year-old man with a left lower lobe NSCLC being evaluated for surgical resection. A and B, CT scans show a left lower lobe mass and small node in the contralateral superior mediastinum (arrow in B). C, Whole body coronal positron-emission tomography (PET) scan shows focal increased 18F-2-deoxy-D-glucose (FDG) uptake in the left lower lobe mass (M) and in subcarinal and contralateral mediastinal nodes (arrows). *, normal uptake of FDG in vocal cords. D, PET/CT scan shows increased uptake of FDG in a right superior mediastinal node. Biopsy confirmed metastatic disease (N3) and the patient was treated palliatively.
The detection of metastases is important in determining whether the patient will be a candidate for surgical resection or receive palliative radiation and chemotherapy. For instance, the diagnosis of a malignant pleural effusion or pleural metastases is important in patient management because these metastases preclude surgical resection (Figure 6-15). However, the role of imaging in detecting M1 disease is not clearly defined. For instance, patients with early stage (T1, N0) NSCLC have a very low incidence of occult metastasis and extensive evaluation for metastasis in these patients is not warranted.47 However, in patients with more advanced disease, whole body FDG-PET can improve the accuracy of staging. FDG-PET has a higher sensitivity and specificity than CT in detecting metastases to the adrenals, bones, and extrathoracic lymph nodes (Figures 6-16 and 6-17). In this regard, the American College of Surgeons Oncology Trial38 reports a sensitivity, specificity, positive predictive value, and negative predictive value of 83%, 90%, 36% and 99%, respectively, for M1 disease. Whole body PET imaging stages intra- and extrathoracic disease in a single study and detects occult extrathoracic metastases in up to 24% of patients selected for curative resection.37,38,48 The incidence of detection of occult metastases has been reported to increase as the staging T and N descriptors increase, that is, 7.5% in early-stage disease to 24% in advanced disease.48 In two studies with a relatively high proportion of more advanced lung cancers considered resectable by standard clinical staging, PET imaging prevented nontherapeutic surgery in one in five patients.37,38 It is important to emphasize that, although whole body FDG-PET imaging improves the accuracy of staging, false-positive uptake of FDG can mimic distant metastases and, therefore, all focal lesions with increased FDG-uptake should be biopsied if they potentially would alter patient management.
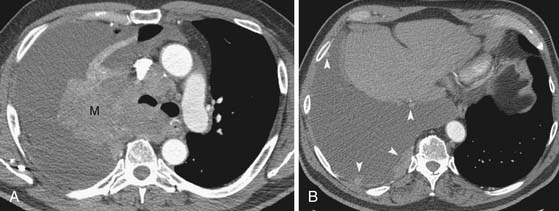
Figure 6-15 A 64-year-old man with primary NSCLC and a malignant pleural effusion at presentation manifesting as shortness of breath. A and B, Contrast-enhanced CT scans show a large right upper lobe mass (M) with invasion into the mediastinum, large right pleural effusion, and nodular pleural lesions consistent with metastases (arrowheads in B).
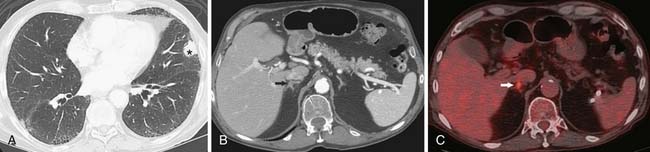
Figure 6-16 An 83-year-old man with NSCLC of the left upper lobe. A and B, Contrast-enhanced CT scans show a left upper lobe nodule (asterisk) and a small region of focal nodularity of the right adrenal gland (arrow in B). Note emphysema and fibrosis. C, PET/CT scan shows increased uptake of FDG in the right adrenal gland (arrow), suspicious for a metastasis. Repeat CT (not shown) revealed increase in size, consistent with progression of metastatic disease.
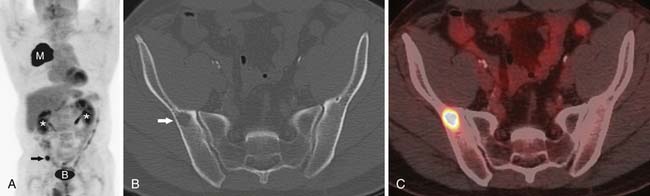
Figure 6-17 A 52-year-old man with NSCLC presenting with a solitary bone metastasis. A, Coronal whole body PET scan shows increased FDG uptake within the primary malignancy (M). There is focal abnormal FDG uptake (arrow) in the region of the pelvis, suspicious for a metastasis. B, accumulation of FDG in the bladder. *, renal excretion of FDG. CT (B) and PET/CT (C) scans show lytic lesion in the right iliac bone (arrow in B) with focal increased uptake of FDG. Biopsy confirmed metastatic disease and the patient was treated palliatively.
Summary of American Society of Clinical Oncology Guidelines for Evaluation of Patients with Non–Small Cell Lung Cancer36
1. A chest x-ray and contrast-enhanced CT are recommended to stage locoregional disease. The CT should include the liver and adrenals. In the absence of distant metastases on CT, FDG-PET is recommended.
2. For patients with clinically resectable disease, biopsy is recommended of mediastinal lymph nodes found on CT to be 1 cm or greater in short-axis diameter or positive on FDG-PET. A negative FDG-PET scan does not preclude biopsy of enlarged mediastinal lymph nodes.
3. A bone scan is optional in patients with evidence of bone metastases on FDG-PET, unless there are suspicious symptoms in regions not imaged by PET. In patients with resectable disease, bone lesions detected on bone scan or PET require histologic confirmation or corroboration by additional imaging studies (radiographs, CT, and/or MRI).
4. Brain CT or MRI with and without contrast material is recommended in patients with signs or symptoms of central nervous system disease, as well as asymptomatic patients with stage III disease being considered for aggressive local therapy (surgery or radiation therapy).
5. Isolated adrenal or liver mass on ultrasound, CT, or FDG-PET requires biopsy to rule out metastatic disease if the patient is otherwise considered to be potentially resectable.
Key Points Imaging of non–small cell lung cancer
What the Surgeon Needs to Know
• Size, location, and presence of locoregional invasion (T descriptor) is necessary to determine surgical resection plan.
• Degree of pleural, chest wall, and mediastinal invasion and involvement of central airways and pulmonary arteries is important in determining not only potential resectability but also surgical approach.
• Involvement of the pulmonary artery may require pneumonectomy rather than a lobectomy.
• Involvement of lobar or main bronchi may require a sleeve resection or pneumonectomy.
• Ipsilateral peribronchial/hila (N1) nodes are usually resectable.
• Ipsilateral (N2) nodes may be resectable after induction chemotherapy; contralateral or supraclavicular nodes (N3) preclude resection.
What the Radiation Oncologist Needs to Know
• Size and location of the primary tumor and the proximity of tumor to critical structures is important where radiation tolerance imposes dose-volume constraints.
• Determination of tumor margins is important because of increasing use of techniques in which dose distributions conform tightly to target volumes.
• Detection and incorporation of nodal metastasis into the radiation treatment are required for appropriate treatment.
Small Cell Lung Cancer
Most patients with SCLC have widely disseminated disease at presentation. Common sites of metastatic disease include the liver, bone, bone marrow, brain, and retroperitoneal lymph nodes (Figure 6-18). Although there is no consensus regarding the imaging and invasive procedures that should be performed in the staging evaluation of patients with SCLC, MRI has been advocated to assess the liver, adrenals, brain, and axial skeleton in a single study.49 Whole body PET imaging has also been reported to improve the accuracy of staging of patients with SCLC (Figure 6-19).50,51 Imaging evaluation of extrathoracic metastatic disease usually includes 99mTc-MDP (methylene diphosphate) bone scintigraphy, and MRI to detect bone metastases because these patients are often asymptomatic.34 However, because isolated bone and bone marrow metastases are uncommon, routine radiologic imaging for occult metastases is usually performed only if there are other findings of extensive disease. CT or MRI is also performed routinely to evaluate the central nervous system and abdomen because metastases are common at presentation and patients are often asymptomatic.34
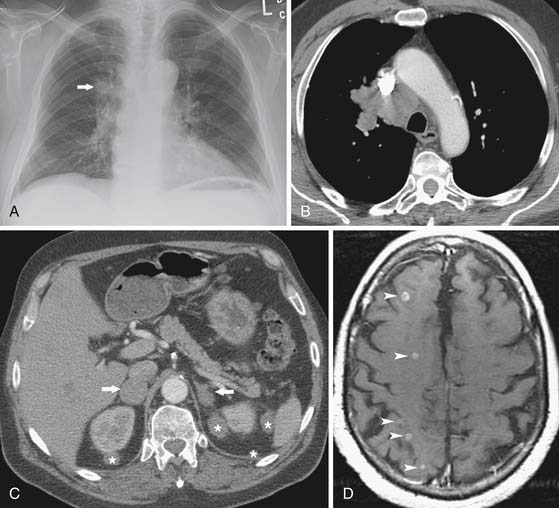
Figure 6-18 A 61-year-old man with extensive disease SCLC presenting with seizures. A, Posteroanterior chest radiograph shows a poorly marginated right lung mass (arrow). B, Chest CT scan confirms a poorly marginated right upper lobe mass and reveals confluent mediastinal adenopathy. C, Contrast-enhanced abdominal CT scan shows bilateral adrenal masses metastases (arrows) and perirenal soft tissue metastases (asterisks). D, T1-weighted contrast-enhanced axial MRI study of the brain reveals numerous small metastases (arrowheads).
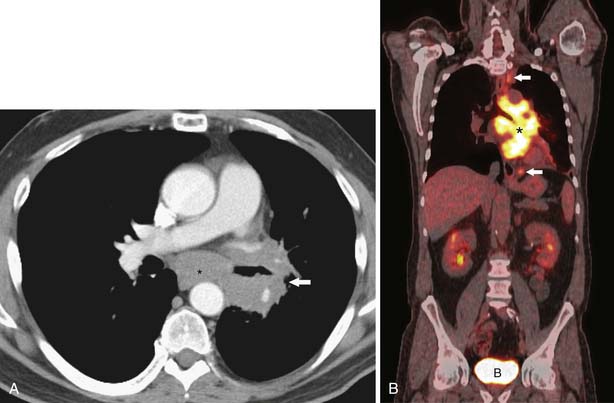
Figure 6-19 A 63-year-old man with SCLC who presented with a history of hemoptysis. A, Contrast-enhanced CT scan shows a large left lower lobe mass (arrow) surrounding the left lower lobe bronchus as well as subcarinal adenopathy (asterisk). Extensive multicompartmental mediastinal adenopathy was also present (not shown). B, Whole body coronal PET/CT scan shows increased uptake of FDG within confluent multicompartmental mediastinal nodes (asterisk) and superior paratracheal and periesophageal nodes (arrows). There are no FDG-avid extrathoracic metastases. Because the patient had limited disease (disease confined to the thorax), treatment was concurrent chemoradiation. B, accumulation of FDG in the bladder.
Treatment*
Non–Small Cell Lung Cancer
Surgical resection is the treatment of choice in patients with localized NSCLC. In those patients who are potential candidates for surgical resection, clinical and physiologic assessment should be performed to determine the patient’s ability to tolerate resection. Spirometry is a good initial test to quantify a patient’s pulmonary reserve and assess the ability to tolerate surgical resection. A postoperative forced expiratory volume in 1 second (FEV1) of less than 0.8 L or less than 35% of predicted is associated with an increased risk of perioperative complications, respiratory insufficiency, and death. Additional risk factors for lung resection include a predicted postoperative carbon monoxide diffusing capacity (DLCO) or maximum ventilatory ventilation (MVV) of less than 40%, hypercarbia (>45 mm CO2) or hypoxemia (<60 mm O2) on preoperative arterial blood gases.
Surgical resection can be curative in patients with early stage (stages I and II) NSCLC and in those who are physiologically fit, surgery alone is usually the treatment of choice (Figures 6-20 and 6-21). Unfortunately, most patients present with locally advanced (stage IIIa or IIIb) or metastatic (stage IV) disease for which surgery alone is not a therapeutic option. Locally advanced NSCLC (stages IIIa and IIIb) is composed of a heterogenous population of patients (see Table 6-3). Overall survival for this group of patients is poor because of the high risk of both locoregional and metastatic recurrence. Stage IIIa has been separated from stage IIIb because it has been believed to encompass a group of patients whose disease is resectable with a multidisciplinary approach (surgery, chemotherapy, and radiation therapy). For instance, patients with stage IIIa-N2 or T4 tumors with N0 or N1 nodes can benefit from resection.52,53 Postoperative adjuvant chemotherapy is the standard treatment for most patients with advanced NSCLC who undergo surgical resection.54 In these patients, administration of adjuvant chemotherapy improves long-term survival rates by a few percentage points.55,56 Efficacy of this adjuvant chemotherapy appears to be comparable whether it is administered before surgery (neoadjuvant chemotherapy) or postoperatively.56,57
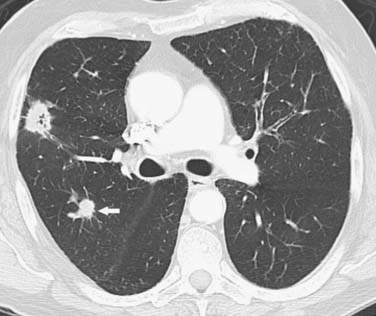
Figure 6-20 A 79-year-old man with NSCLC. Contrast-enhanced CT scan shows a cavitary nodule in the right upper lobe and a smaller satellite nodule (arrow). Note that, when there is an additional nodule in the same lobe as the primary tumor, the new tumor-node-metastasis (TNM) staging system designates a T3 descriptor (potentially resectable). The patient was T3 N0 on staging (stage IIb) and underwent curative surgical resection.
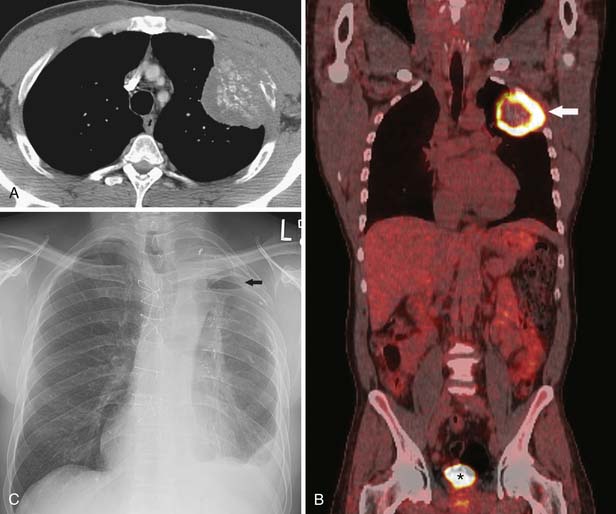
Figure 6-21 A 49-year-old man with primary NSCLC presenting with chest pain. A, CT shows a large left upper lobe lung mass containing amorphous calcification. There is locoregional chest wall invasion and focal destruction of the adjacent rib (T3). B, Whole body coronal PET/CT scan shows increased FDG uptake within the primary malignancy (arrow). FDG uptake in the mediastinum is normal and there is no focal abnormal extrathoracic FDG uptake. The findings are indicative of T3 N0 M0 disease (stage IIb). Mediastinoscopy confirmed absence of nodal metastasis and the patient underwent surgical resection. *, accumulation of FDG in the bladder. C, Posteroanterior chest radiograph shows postsurgical changes after left upper lobe lobectomy and en bloc chest wall resection of the second through fourth ribs. Note small air and fluid collection in the pleural space (arrow).
Patients with tumors involving the contralateral mediastinal nodes (N3) and T4 tumors that invade the mediastinum, heart, great vessels, trachea, esophagus, or vertebral body and who have ipsilateral mediastinal (N2) nodes are better treated with chemoradiation rather than surgery. Patients with metastatic disease typically are treated with chemotherapy and are treated only surgically in the unusual circumstance of an isolated brain metastasis with a node-negative lung primary. In metastatic NSCLC, chemotherapy can improve symptoms as well as the quality of life in up to 75% of patients.58,59 In addition, chemotherapy induces an objective response in approximately 35% of patients and modestly prolongs median survival.58,60 In front-line therapy of advanced NSCLC, a second agent (e.g., paclitaxel, docetaxel, pemetrexed, gemcitabine, or vinorelbine) is generally added to a platinum (cisplatin or carboplatin).55,61 Overall, combination chemotherapy is superior to single-agent chemotherapy, and cisplatin-based regimens are somewhat superior to regimens that do not include cisplatin.62,63
Recent evidence suggests that giving maintenance therapy with single-agent pemetrexed or other agents after completion of the initial standard four to six cycles of combination chemotherapy may improve time to tumor progression and overall survival time.64,65 In terms of second-line chemotherapy, docetaxel has been shown to be superior to best supportive care, and pemetrexed is at least as effective as docetaxel in adenocarcinomas, but somewhat less toxic.64,66 The antiangiogenic agent bevacizumab also modestly prolongs survival when added to front-line chemotherapy in adenocarcinomas, but it is generally not used in SCCs because it appears to increase the risk of fatal hemorrhage in that group.67 The EGFR tyrosine kinase inhibitor (TKI) erlotinib has also been shown to be superior to placebo when used in previously pretreated NSCLC patients.68 EGFR TKIs including erlotinib and gefitinib are most effective in patients with an activating EGFR mutation (an exon 19 deletion or an exon 21 L8585R point mutation) and are less effective in patients without activating EGFR mutations.69,70 Patients who have never smoked and East Asians are more likely to have an activating mutation than are other patients, and patients with an activating mutation can have very rapid dramatic responses that in some cases can be prolonged.71 Recent studies also indicate that single-agent EGFR inhibitors are more effective than combination chemotherapy as front-line therapy for metastatic NSCLC if an EGFR-activating mutation is present.72
Radiation therapy (RT) is an important modality in the management of patients with NSCLC, and it has been estimated that approximately 45% of patients with receive RT as initial treatment.73 Stereotactic body radiotherapy (SBRT) alone is being used for curative intent in medically inoperable patients with early stage NSCLC (stage I/II). It has also been suggested that SBRT may have a role in the treatment of medically operable patients with early-stage disease. In this regard, an ongoing randomized trial of surgery versus SBRT has shown equivalent results regarding 5-year overall survival.74 Patients with stage II NSCLC are typically treated with surgery followed by adjuvant chemotherapy. However, after resection, if the patients have positive mediastinal nodes (N2) or multiple or large hilar nodes (N1) with extracapsular involvement, postoperative RT is a therapeutic option (50 Gy in 5 wk) and adjuvant cisplatin-based chemotherapy is given before or after RT. Patients with stage II that are medically inoperable receive IMRT or proton RT with or without chemotherapy. Patients with stage IIIa (microscopic N2 or < 1.5-cm nodes) are usually treated by induction chemotherapy followed by surgery and postoperative RT. In patients with multiple-level positive mediastinal nodes or nodes larger than 1.5 cm or patients with T3-4 lesions requiring pneumonectomy or surgically unresectable stage IIIb, the treatment is concurrent chemoradiotherapy. Importantly, in the inoperable patients undergoing RT with curative intent, chemotherapy given concurrently with the RT increases cure rates by a few percentage points by reducing metastatic disease and increasing the efficiency of tumor cell killing by RT.75
Key Points Therapy of non–small cell lung cancer
• Surgical resection is the treatment of choice in patients with localized NSCLC.
• Resection may be an option in patients with locally advanced (T4) disease.
• Postoperative, adjuvant chemotherapy is the standard treatment for most patients with advanced NSCLC who undergo surgical resection.
• Surgery alone is not a therapeutic option for advanced (stage IIIa or IIIb) or metastatic (stage IV) disease.
• Chemotherapy is used to treat patients with distant metastasis.
• Cisplatin-based (cisplatin or carboplatin) regimens are standard therapy.
• Combination chemotherapy is superior to single-agent chemotherapy.
• EGFR TKI has a role in previously treated NSCLC patients.
• SBRT alone is being used for curative intent in medically inoperable patients with early-stage NSCLC (stage I/II).
• Stage II patients that are medically inoperable receive intensity-modulated radiation therapy (IMRT) or proton RT with or without chemotherapy.
• Concurrent chemoradiotherapy can be used to treat patients with advanced-stage disease.
Small Cell Lung Cancer
SCLC tends to be disseminated at the time of presentation and is, therefore, not typically amenable to cure with surgical resection. However, there is a small role for surgical resection of SCLCs. Solitary peripheral pulmonary nodules without distant metastatic disease can be treated with surgical resection. In these select patients, 5-year survivals of 50% have been achieved for T1 N0, T2 N0, and completely resected N1 disease (Figure 6-22). Generally, limited-stage SCLC is usually treated by concurrent chemoradiotherapy whereas patients with extensive SCLC are treated with systemic chemotherapy. Chemotherapy is very effective at inducing tumor regression in SCLC and can cure approximately 10% of patients with limited disease (confined to one hemithorax) and RT increases the probability of cure by approximately 5% when added to chemotherapy.76 In extensive SCLC (distant metastases), median survival is only approximately 6 weeks without chemotherapy, and increases to 7 to 11 months with chemotherapy, but long-term survival is uncommon.77,78 A large majority of SCLC patients will respond to chemotherapy, with symptomatic and radiologic improvement often seen within a few days of therapy initiation.79 The standard chemotherapy choice for SCLC is the combination of cisplatin or carboplatin with the topoisomerase II inhibitor etoposide.78 Addition of the topoisomerase I inhibitor irinotecan to a platinum gives outcomes comparable with those seen with the combination of etoposide with a platinum. New targeted therapies have to date not proved useful in SCLC.80
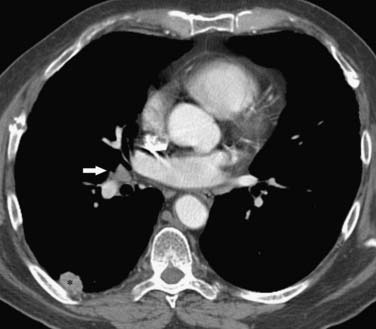
Figure 6-22 A 72-year-old man with SCLC. Contrast-enhanced CT scan shows a left lower nodule (asterisk) and 1- to 1.5-cm left infrahilar node (arrow). Using TNM descriptors, the patient was considered to have stage I disease and underwent surgical resection. The resected primary tumor showed visceral pleural invasion and the infrahilar nodes were negative for metastatic disease. Note bilateral posttraumatic rib fractures.
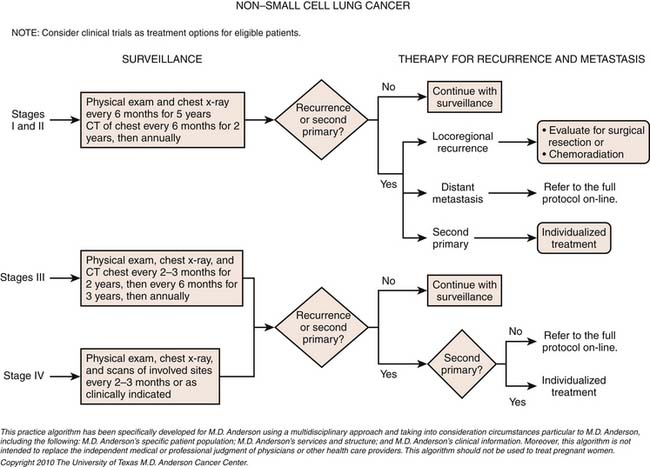
Surveillance
Monitoring Tumor Response
The monitoring of tumor response is important in patients with NSCLC who receive chemotherapy and/or RT for the treatment of advanced disease or who receive induction chemotherapy before surgery. Evaluation of the effectiveness of this treatment is important to determine whether the treatment regimen should be continued or changed. There are uniform criteria for reporting response, recurrence, disease-free interval, and toxicity. These criteria are the WHO criteria (largely based on the longest perpendicular diameters of the tumor) and Response Evaluation Criteria In Solid Tumors (RECIST; single measurement of the largest tumor diameter).81,82 Image-based serial measurements of tumor size before and after treatment based on the recommendations of the WHO or RECIST of tumor size are commonly used in determining response.81 RECIST is now the preferred method of assessing response and requires the identification of target lesions to be followed for a response to treatment. Using RECIST, treatment response is defined as complete response (no evidence of tumor), partial response (decrease in tumor size by 30%), stable disease (no change in tumor size), and progressive disease (increase in tumor size by 20%). However, measurements of lung tumor size on CT scans are often inconsistent and can lead to an incorrect interpretation of tumor response. Interobserver variability of measurements is greater than intraobserver variability and measurement differences are greatest when the edge of the lesion is irregular or spiculated and smallest when the edge is well-defined.83 In addition, morphologic alterations detected by CT may not correlate with pathologic response and tumor viability. In this regard, FDG-PET may allow an early and sensitive assessment of the effectiveness of anticancer chemotherapy because FDG uptake is a function of proliferative activity and viable tumor. However, unlike the WHO recommendations and RECIST applied to morphologic imaging, there is no clear consensus on when PET should be performed or the most appropriate criteria for assessment of tumor response by FDG-PET. There have been comparatively few studies assessing the value of early FDG-PET in assessing tumor response while patients are still receiving therapy. However, a decrease in FDG uptake before and after one cycle of chemotherapy may predict outcome with improved survival directly related to the magnitude of decreased uptake.84,85 FDG-PET has also been shown to be of value in patients with locally advanced but potentially resectable NSCLC who have completed neoadjuvant therapy by identifying those who have had a pathologic response to treatment and may benefit from further locoregional control.86–88
Key Points Monitoring tumor response
• Monitoring response is important in patients who receive chemotherapy and/or RT.
• WHO criteria and RECIST provide uniform criteria for reporting response, recurrence, disease-free interval, and toxicity
• RECIST (single measurement of the largest tumor diameter) is the preferred method of assessing response.
• RECIST defines complete response (no evidence tumor), partial response (decrease in size by 30%), stable disease (no change), and progressive disease (increase in size by 20%).
• Morphologic alterations detected by CT may not correlate with pathologic response and tumor viability.
• FDG-PET may allow an early and sensitive assessment of the effectiveness of anticancer chemotherapy.
• A decrease in FDG uptake before and after one cycle of chemotherapy may predict outcome.
• FDG-PET may be useful in determining response patients with locally advanced but potentially resectable NSCLC who have completed neoadjuvant therapy.
Detection of Recurrence
In an attempt to prolong survival of patients with recurrent malignancy after attempted curative treatment of NSCLC, patients can be treated with repeat surgery, salvage chemotherapy, or RT.89–91 However, relying on patient symptomatology to determine persistent or local recurrence of NSCLC can delay diagnosis and compromise retreatment. Furthermore, CT or MRI is unreliable in distinguishing persistent or recurrent tumor from necrosis, posttreatment scarring, or fibrosis. FDG-PET can be useful in detecting local recurrence of tumor after definitive treatment with surgery, chemotherapy, or RT before conventional imaging (Figure 6-23).92,93 However, diagnostic difficulties over the presence or absence of persistent or recurrent cancer are frequent after RT. In this regard, three-dimensional conformal RT is particularly likely to manifest as opacities on CT that can be difficult to differentiate from tumor recurrence and the associated radiation-induced inflammatory changes can result in false-positive uptake of FDG.
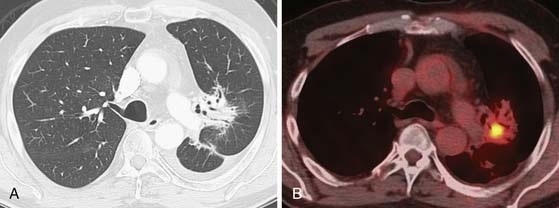
Figure 6-23 A 74-year-old woman with history of left upper lobe NSCLC 1 year after resection and chemoradiation therapy. A, Axial contrast-enhanced CT scan shows atelectatic and consolidative opacities in the left perihilar region due to radiation-induced lung injury (RILD). Note air bronchograms and no CT findings of recurrence of malignancy. B, Axial integrated PET/CT scan shows focal increased FDG uptake in radiation fibrosis. Recurrence of malignancy was confirmed by transthoracic needle aspiration biopsy.
At present, there is no standardized clinical algorithm established to monitor patients with NSCLC for recurrence of disease. Although imaging is an integral component of this evaluation, there is considerable variability in the imaging performed to evaluate for disease owing to a lack of evidence in the published literature regarding optimal follow-up after treatment. Nevertheless, specific follow-up strategies have been advocated and several guidelines including those from the ASCO, American College of Radiology, and National Comprehensive Cancer Network have been published that include recommendations for a posttreatment surveillance program. The guidelines and institutional practices are similar. Each recommends more frequent visits during the first 2 years after curative-intent therapy and a decrease to a minimal level after year 5. Colice and associates94 have suggested that a clinically reasonable and cost-effective surveillance approach would include a medical history, a physical examination, and an imaging study (either a chest radiograph or a chest CT scan) every 6 months for 2 years and then annually. However, little is known about the effectiveness of follow-up regimens. Walsh and coworkers,95 in a retrospective study following curative-intent surgical resection for NSCLC, concluded that intensive surveillance was not cost-effective and suggested a reduced surveillance approach consisting of a chest radiograph every 6 months for the first year after curative-intent surgery and annually thereafter. However, there are concerns regarding the validity of the conclusions of the studies advocating limited surveillance that include the limitations of retrospective analyses, the small size and heterogenous nature of the study groups, as well as the different treatments and the imaging evaluations that the patients received. Westeel and colleagues96 performed a prospective study to determine the feasibility of an intensive surveillance program and the influence on patient survival and concluded that intensive follow-up is feasible and may improve survival by detecting asymptomatic recurrences after surgery.
Key Points Detection of recurrence
• Recurrent malignancy can occasionally be treated with repeat surgery, salvage chemotherapy, or RT.
• CT and MRI can be unreliable in distinguishing persistent or recurrent tumor from necrosis and posttreatment fibrosis.
• FDG-PET can detect local recurrence of tumor before conventional imaging.
• No standardized clinical algorithm has been developed to monitor patients with NSCLC for recurrence of disease after treatment.
• Reasonable and cost-effective surveillance approach includes a medical history, a physical examination, and chest radiograph or chest CT every 6 months for 2 years and then annually.
Complications of Therapy
Complications after chemotherapy and/or RT are common. Radiation-induced lung disease (RILD) rarely occurs with fractionated total doses below 20 Gy. A pooled analysis of 24 studies of 1911 patients with both NSCLC and SCLC who received chemoradiation showed that the incidence of clinically significant radiation-induced pneumonitis (grade 2-3 according to the Radiation Therapy Oncology Group/European Organization for Research and Treatment of Cancer criteria) was 6% for total doses less than 45 Gy and 12% for doses greater than 55 Gy.97,98 Although the total dosage of radiation delivered is important in the development of RILD, other radiation technique factors that affect lung injury are the fractions into which the total dose is divided, the dose rate, and the volume of lung irradiated (Figure 6-24).97,99 In addition, improved RT techniques such as IMRT, three-dimensional conformal radiotherapy, and dose escalation (often employing a hyperfractionated schedule and resultant increased total doses up 70-80 Gy) can modify the development and extent of lung injury.100–104 Various factors can increase the degree of injury sustained by the lung after radiation including age, low performance status, cigarette smoking, preexisting lung disease, and prior RT. The effect of neoadjuvant or concurrent chemotherapy on RILD is not clear, with some studies reporting an increase whereas others have reported no significant association with pneumonitis.99,101,103,105 Furthermore, cytoprotectants such as amifostine, an organic thiophosphate, can reduce the incidence and severity of injury associated with RT.106
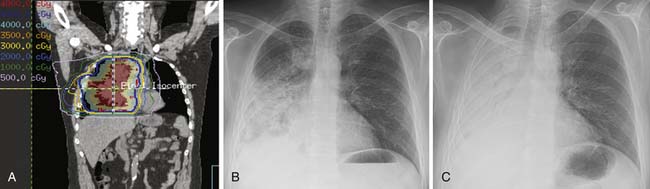
Figure 6-24 A 64-year-old man with NSCLC and RILD after palliative treatment of extensive malignancy in the right lung involving the central airways and mediastinum with intensity-modulated radiation therapy (IMRT) (45 Gy over 15 fractions). A, Computed dosimetric reconstruction coronal image used for planning IMRT shows most of the lung within blue color line (45 Gy) surrounding location of treated malignancy. B, Posteroanterior chest radiograph obtained 2 months after completion of radiation therapy shows extensive consolidation due to RILD. C, Posteroanterior chest radiograph obtained 2 months after B shows near-complete atelectasis and consolidation of the right lung due to RILD.
The most common complications after chemotherapy are infection and drug toxicity. Therapeutic agents used in the treatment of lung cancer such as gemcitabine, etoposide, paclitaxel, and gefitinib have been reported to cause lung injury.107–109 The diagnosis of drug toxicity requires a high index of suspicion because it can mimic infection, radiation pneumonitis, or recurrent tumor. The most common histopathologic patterns include noncardiogenic pulmonary edema, diffuse alveolar damage, nonspecific interstitial pneumonia, cryptogenic organizing pneumonia, and pulmonary hemorrhage. Radiologically, drug toxicity manifests as interstitial, ground-glass, and consolidative opacities or fibrosis.110
Key Points Complications of therapy
• Complications after chemotherapy and/or RT are common.
• Total dosage of radiation delivered is important in the development of RILD; it rarely occurs with fractionated total doses below 20 Gy.
• Improved radiation techniques can modify the development and extent of lung injury.
• Various factors can increase the degree of lung injury including age, low performance status, cigarette smoking, and preexisting lung disease.
• Most common complications after chemotherapy are infection and drug toxicity.
• Therapeutic agents used in the treatment of lung cancer such as gemcitabine, etoposide, paclitaxel, and gefitinib can cause lung injury.
Conclusion
TNM staging of lung cancer is important in determining therapeutic management and prognosis. CT, PET, and MRI are an integral component of this evaluation. However, there is considerable variability in the imaging performed to evaluate for nodal and extrathoracic disease. Chest CT is almost universally used to stage patients with lung cancer and is typically performed to assess the primary tumor, direct mediastinoscopy, and detect intra- and extrathoracic metastases. MRI is particularly useful in the evaluation of superior sulcus tumors. Otherwise, MRI is generally used as an adjunct to CT in evaluating patients whose CT findings are equivocal. PET complements conventional radiologic assessment of lung cancer and is routinely used to improve the detection of nodal and extrathoracic metastases.
1. Alberg A.J., Ford J.G., Samet J.M. Epidemiology of lung cancer: ACCP evidence-based clinical practice guidelines. 2nd ed. Chest. 2007;132(Suppl 3):29S-55S.
2. Samet J.M., Avila-Tang E., Boffetta P., et al. Lung cancer in never smokers: clinical epidemiology and environmental risk factors. Clin Cancer Res. 2009;15:5626-5645.
3. Al-Zoughool M., Krewski D. Health effects of radon: a review of the literature. Int J Radiat Biol. 2009;85:57-69.
4. Erren T.C., Jacobsen M., Piekarski C. Synergy between asbestos and smoking on lung cancer risks. Epidemiology. 1999;10:405-411.
5. Goodman M., Morgan R.W., Ray R., et al. Cancer in asbestos-exposed occupational cohorts: a meta-analysis. Cancer Causes Control. 1999;10:453-465.
6. Wild P., Bourgkard E., Paris C. Lung cancer and exposure to metals: the epidemiological evidence. Methods Mol Biol. 2009;472:139-167.
7. Clapp R.W., Jacobs M.M., Loechler E.L. Environmental and occupational causes of cancer: new evidence 2005-2007. Rev Environ Health. 2008;23:1-37.
8. Le Calvez F., Mukeria A., Hunt J.D., et al. TP53 and KRAS mutation load and types in lung cancers in relation to tobacco smoke: distinct patterns in never, former, and current smokers. Cancer Res. 2005;65:5076-5083.
9. Jackman D.M., Miller V.A., Cioffredi L.A., et al. Impact of epidermal growth factor receptor and KRAS mutations on clinical outcomes in previously untreated non–small cell lung cancer patients: results of an online tumor registry of clinical trials. Clin Cancer Res. 2009;15:5267-5273.
10. van Zandwijk N., Mathy A., Boerrigter L., et al. EGFR and KRAS mutations as criteria for treatment with tyrosine kinase inhibitors: retro- and prospective observations in non–small-cell lung cancer. Ann Oncol. 2007;18:99-103.
11. Hung R.J., McKay J.D., Gaborieau V., et al. A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature. 2008;452:633-637.
12. Travis W.D., Brambilla E., Muller-Hermelink H.K., Harris Curtis C. Pathology and Genetics of Tumours of the Lung Pleura, Thymus and Heart. Lyon, France: International Agency for Research on Cancer; 2004.
13. Franks T.J., Galvin J.R. Lung tumors with neuroendocrine morphology: essential radiologic and pathologic features. Arch Pathol Lab Med. 2008;132:1055-1061.
14. Wahbah M., Boroumand N., Castro C., et al. Changing trends in the distribution of the histologic types of lung cancer: a review of 4,439 cases. Ann Diagn Pathol. 2007;11:89-96.
15. Rosado-de-Christenson M.L., Templeton P.A., Moran C.A. Bronchogenic carcinoma: radiologic-pathologic correlation. Radiographics. 1994;14:429-446. quiz 447–448
16. Sider L. Radiographic manifestations of primary bronchogenic carcinoma. Radiol Clin North Am. 1990;28:583-597.
17. Travis W.D., Brambilla E., Noguchi M., et al. International association for the study of lung cancer/American thoracic society/European respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244-285.
18. Noguchi M., Morikawa A., Kawasaki M., et al. Small adenocarcinoma of the lung. Histologic characteristics and prognosis. Cancer. 1995;75:2844-2852.
19. Kishi K., Homma S., Kurosaki A., et al. Small lung tumors with the size of 1 cm or less in diameter: clinical, radiological, and histopathological characteristics. Lung Cancer. 2004;44:43-51.
20. Takashima S., Li F., Maruyama Y., et al. Discrimination of subtypes of small adenocarcinoma in the lung with thin-section CT. Lung Cancer. 2002;36:175-182.
21. Suzuki K., Kusumoto M., Watanabe S., et al. Radiologic classification of small adenocarcinoma of the lung: radiologic-pathologic correlation and its prognostic impact. Ann Thorac Surg. 2006;81:413-419.
22. Gandhi L., Johnson B.E. Paraneoplastic syndromes associated with small cell lung cancer. J Natl Compr Canc Netw. 2006;4:631-638.
23. McClelland M.T. Paraneoplastic syndromes related to lung cancer. Clin J Oncol Nurs. 2010;14:357-364.
24. Darnell R.B., Posner J.B. Paraneoplastic syndromes affecting the nervous system. Semin Oncol. 2006;33:270-298.
25. Vedeler C.A., Antoine J.C., Giometto B., et al. Management of paraneoplastic neurological syndromes: report of an EFNS Task Force. Eur J Neurol. 2006:13682-13690.
26. Pantel K., Izbicki J., Passlick B., et al. Frequency and prognostic significance of isolated tumour cells in bone marrow of patients with non-small-cell lung cancer without overt metastases. Lancet. 1996;347:649-653.
27. Passlick B. Micrometastases in non–small cell lung cancer (NSCLC). Lung Cancer. 2001;34(Suppl 3):S25-S29.
28. Kondo T., Yamada K., Noda K., et al. Radiologic-prognostic correlation in patients with small pulmonary adenocarcinomas. Lung Cancer. 2002;36:49-57.
29. Libshitz H.I., McKenna R.J.Jr., Mountain C.F. Patterns of mediastinal metastases in bronchogenic carcinoma. Chest. 1986;90:229-232.
30. Watanabe Y., Shimizu J., Tsubota M., Iwa T. Mediastinal spread of metastatic lymph nodes in bronchogenic carcinoma. Mediastinal nodal metastases in lung cancer. Chest. 1990;97:1059-1065.
31. Rami-Porta R., Ball D., Crowley J., et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the T descriptors in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol. 2007;2:593-602.
32. Rusch V.W., Crowley J., Giroux D.J., et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the N descriptors in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol. 2007;2:603-612.
33. Postmus P.E., Brambilla E., Chansky K., et al. The IASLC Lung Cancer Staging Project: proposals for revision of the M descriptors in the forthcoming (seventh) edition of the TNM classification of lung cancer. J Thorac Oncol. 2007;2:686-693.
34. Darling G.E. Staging of the patient with small cell lung cancer. Chest Surg Clin North Am. 1997;7:81-94.
35. Vallieres E., Shepherd F.A., Crowley J., et al. The IASLC Lung Cancer Staging Project: proposals regarding the relevance of TNM in the pathologic staging of small cell lung cancer in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol. 2009;4:1049-1059.
36. Pfister D.G., Johnson D.H., Azzoli C.G., et al. American Society of Clinical Oncology treatment of unresectable non–small-cell lung cancer guideline: update 2003. J Clin Oncol. 2004;22:330-353.
37. van Tinteren H., Hoekstra O.S., Smit E.F., et al. Effectiveness of positron emission tomography in the preoperative assessment of patients with suspected non-small-cell lung cancer: the PLUS multicentre randomised trial. Lancet. 2002;359:1388-1393.
38. Reed C.E., Harpole D.H., Posther K.E., et al. Results of the American College of Surgeons Oncology Group Z0050 trial: the utility of positron emission tomography in staging potentially operable non–small cell lung cancer. J Thorac Cardiovasc Surg. 2003;126:1943-1951.
39. Bruzzi J.F., Komaki R., Walsh G.L., et al. Imaging of non-small cell lung cancer of the superior sulcus: part 2: initial staging and assessment of resectability and therapeutic response. Radiographics. 2008;28:561-572.
40. Bruzzi J.F., Komaki R., Walsh G.L., et al. Imaging of non-small cell lung cancer of the superior sulcus: part 1: anatomy, clinical manifestations, and management. Radiographics. 2008;28:551-560. quiz 620
41. Bilsky M.H., Vitaz T.W., Boland P.J., et al. Surgical treatment of superior sulcus tumors with spinal and brachial plexus involvement. J Neurosurg. 2002;97(Suppl 3):301-309.
42. Dartevelle P., Macchiarini P. Surgical management of superior sulcus tumors. Oncologist. 1999;4:398-407.
43. Toloza E.M., Harpole L., Detterbeck F., McCrory D.C. Invasive staging of non-small cell lung cancer: a review of the current evidence. Chest. 2003;123(Suppl 1):157S-166S.
44. Prenzel K.L., Monig S.P., Sinning J.M., et al. Lymph node size and metastatic infiltration in non–small cell lung cancer. Chest. 2003;123:463-467.
45. Birim O., Kappetein A.P., Stijnen T., Bogers A.J. Meta-analysis of positron emission tomographic and computed tomographic imaging in detecting mediastinal lymph node metastases in nonsmall cell lung cancer. Ann Thorac Surg. 2005;79:375-382.
46. Gould M.K., Kuschner W.G., Rydzak C.E., et al. Test performance of positron emission tomography and computed tomography for mediastinal staging in patients with non–small-cell lung cancer: a meta-analysis. Ann Intern Med. 2003;139:879-892.
47. Tanaka K., Kubota K., Kodama T., et al. Extrathoracic staging is not necessary for non-small-cell lung cancer with clinical stage T1-2 N0. Ann Thorac Surg. 1999;68:1039-1042.
48. MacManus M.P., Hicks R.J., Matthews J.P., et al. High rate of detection of unsuspected distant metastases by PET in apparent stage III non–small-cell lung cancer: implications for radical radiation therapy. Int J Radiat Oncol Biol Phys. 2001;50:287-293.
49. Jelinek J.S., Redmond J., Perry J.J., et al. Small cell lung cancer: staging with MR imaging. Radiology. 1990;177:837-842.
50. Kut V., Spies W., Spies S., et al. Staging and monitoring of small cell lung cancer using [18F]fluoro-2-deoxy-D-glucose-positron emission tomography (FDG-PET). Am J Clin Oncol. 2007;30:45-50.
51. Niho S., Fujii H., Murakami K., et al. Detection of unsuspected distant metastases and/or regional nodes by FDG-PET [corrected] scan in apparent limited-disease small-cell lung cancer. Lung Cancer. 2007;57:328-333.
52. Mitchell J.D., Mathisen D.J., Wright C.D., et al. Resection for bronchogenic carcinoma involving the carina: long-term results and effect of nodal status on outcome. J Thorac Cardiovasc Surg. 2001;121:465-471.
53. Rendina E.A., Venuta F., De Giacomo T., et al. Sleeve resection and prosthetic reconstruction of the pulmonary artery for lung cancer. Ann Thorac Surg. 1999;68:995-1001. discussion 1002
54. Strauss G.M. Adjuvant vs neoadjuvant chemotherapy in resectable NSCLC: is that the real question? Oncology. 2009;23:534-536. 538
55. Higgins M.J., Ettinger D.S. Chemotherapy for lung cancer: the state of the art in 2009. Expert Rev Anticancer Ther. 2009;9:1365-1378.
56. Lim E., Harris G., Patel A., et al. Preoperative versus postoperative chemotherapy in patients with resectable non–small cell lung cancer: systematic review and indirect comparison meta-analysis of randomized trials. J Thorac Oncol. 2009;4:1380-1388.
57. Song W.A., Zhou N.K., Wang W., et al. Survival benefit of neoadjuvant chemotherapy in non–small cell lung cancer: an updated meta-analysis of 13 randomized control trials. J Thorac Oncol. 2010;5:510-516.
58. Vansteenkiste J. Improving patient management in metastatic non–small cell lung cancer. Lung Cancer. 2007;57(Suppl 2):S12-S17.
59. Dooms C., Verbeken E., Stroobants S., et al. Prognostic stratification of stage IIIA-N2 non–small-cell lung cancer after induction chemotherapy: a model based on the combination of morphometric-pathologic response in mediastinal nodes and primary tumor response on serial 18-fluoro-2-deoxy-glucose positron emission tomography. J Clin Oncol. 2008;26:1128-1134.
60. NSCLC Meta-Analyses Collaborative Group. Chemotherapy in addition to supportive care improves survival in advanced non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data from 16 randomized controlled trials. J Clin Oncol. 2008;26:4617-4625.
61. Grossi F., Aita M., Defferrari C., et al. Impact of third-generation drugs on the activity of first-line chemotherapy in advanced non–small cell lung cancer: a meta-analytical approach. Oncologist. 2009;14:497-510.
62. Rajeswaran A., Trojan A., Burnand B., Giannelli M. Efficacy and side effects of cisplatin- and carboplatin-based doublet chemotherapeutic regimens versus non–platinum-based doublet chemotherapeutic regimens as first line treatment of metastatic non–small cell lung carcinoma: a systematic review of randomized controlled trials. Lung Cancer. 2008;59:1-11.
63. Delbaldo C., Michiels S., Rolland E., et al. Second or third additional chemotherapy drug for non-small cell lung cancer in patients with advanced disease. Cochrane Database Syst Rev. 2007;4:CD004569.
64. Baldwin C.M., Perry C.M. Pemetrexed: a review of its use in the management of advanced non-squamous non–small cell lung cancer. Drugs. 2009;69:2279-2302.
65. Soon Y.Y., Stockler M.R., Askie L.M., Boyer M.J. Duration of chemotherapy for advanced non–small-cell lung cancer: a systematic review and meta-analysis of randomized trials. J Clin Oncol. 2009;27:3277-3283.
66. Scagliotti G., Hanna N., Fossella F., et al. The differential efficacy of pemetrexed according to NSCLC histology: a review of two phase III studies. Oncologist. 2009;14:253-263.
67. Sandler A., Gray R., Perry M.C., et al. Paclitaxel-carboplatin alone or with bevacizumab for non–small-cell lung cancer. N Engl J Med. 2006;355:2542-2550.
68. Shepherd F.A., Rodrigues Pereira J., Ciuleanu T., et al. Erlotinib in previously treated non–small-cell lung cancer. N Engl J Med. 2005;353:123-132.
69. Costa D.B., Kobayashi S., Tenen D.G., Huberman M.S. Pooled analysis of the prospective trials of gefitinib monotherapy for EGFR-mutant non–small cell lung cancers. Lung Cancer. 2007;58:95-103.
70. Gazdar A.F. Activating and resistance mutations of EGFR in non–small-cell lung cancer: role in clinical response to EGFR tyrosine kinase inhibitors. Oncogene. 2009;28(Suppl 1):S24-S31.
71. Riely G.J., Pao W., Pham D., et al. Clinical course of patients with non–small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefitinib or erlotinib. Clin Cancer Res. 2006;12:839-844.
72. Mok T.S., Wu Y.L., Thongprasert S., et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947-957.
73. Tyldesley S., Boyd C., Schulze K., et al. Estimating the need for radiotherapy for lung cancer: an evidence-based, epidemiologic approach. Int J Radiat Oncol Biol Phys. 2001;49:973-985.
74. Onishi H., Araki T., Shirato H., et al. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer. 2004;101:1623-1631.
75. Kepka L., Sprawka A., Casas F., et al. Combination of radiotherapy and chemotherapy in locally advanced NSCLC. Expert Rev Anticancer Ther. 2009;9:1389-1403.
76. Sandler A.B. Chemotherapy for small cell lung cancer. Semin Oncol. 2003;30:9-25.
77. Rosti G., Carminati O., Monti M., et al. Chemotherapy advances in small cell lung cancer. Ann Oncol. 2006;17(Suppl 5):v99-v102.
78. Murray N., Turrisi A.T.3rd. A review of first-line treatment for small-cell lung cancer. J Thorac Oncol. 2006;1:270-278.
79. Rosti G., Bevilacqua G., Bidoli P., et al. Small cell lung cancer. Ann Oncol. 2006;17(Suppl 2):ii5-ii10.
80. Pirker R., Filipits M. Targeted therapies in lung cancer. Curr Pharm Des. 2009;15:188-206.
81. Eisenhauer E.A., Therasse P., Bogaerts J., et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247.
82. Therasse P., Eisenhauer E.A., Verweij J. RECIST revisited: a review of validation studies on tumour assessment. Eur J Cancer. 2006;42:1031-1039.
83. Erasmus J.J., Gladish G.W., Broemeling L., et al. Interobserver and intraobserver variability in measurement of non–small-cell carcinoma lung lesions: implications for assessment of tumor response. J Clin Oncol. 2003;21:2574-2582.
84. Weber W.A., Petersen V., Schmidt B., et al. Positron emission tomography in non–small-cell lung cancer: prediction of response to chemotherapy by quantitative assessment of glucose use. J Clin Oncol. 2003;21:2651-2657.
85. Hoekstra C.J., Stroobants S.G., Smit E.F., et al. Prognostic relevance of response evaluation using [18F]-2-fluoro-2-deoxy-D-glucose positron emission tomography in patients with locally advanced non-small-cell lung cancer. J Clin Oncol. 2005;23:8362-8370.
86. Eschmann S.M., Friedel G., Paulsen F., et al. Repeat 18F-FDG PET for monitoring neoadjuvant chemotherapy in patients with stage III non–small cell lung cancer. Lung Cancer. 2007;55:165-171.
87. Cerfolio R.J., Bryant A.S., Ojha B. Restaging patients with N2 (stage IIIa) non–small cell lung cancer after neoadjuvant chemoradiotherapy: a prospective study. J Thorac Cardiovasc Surg. 2006;131:1229-1235.
88. Pottgen C., Levegrun S., Theegarten D., et al. Value of 18F-fluoro-2-deoxy-d-glucose-positron emission tomography/computed tomography in non–small-cell lung cancer for prediction of pathologic response and times to relapse after neoadjuvant chemoradiotherapy. Clin Cancer Res. 2006;12:97-106.
89. Campione A., Ligabue T., Luzzi L., et al. Late outcome and perioperative complications for surgery of locally recurrent bronchogenic carcinoma. J Cardiovasc Surg. 2005;46:515-518.
90. Milton D.T., Miller V.A. Advances in cytotoxic chemotherapy for the treatment of metastatic or recurrent non–small cell lung cancer. Semin Oncol. 2005;32:299-314.
91. Curran W.J.Jr., Herbert S.H., Stafford P.M., et al. Should patients with post-resection locoregional recurrence of lung cancer receive aggressive therapy? Int J Radiat Oncol Biol Phys. 1992;24:25-30.
92. Hicks R.J., Kalff V., MacManus M.P., et al. The utility of (18)F-FDG PET for suspected recurrent non–small cell lung cancer after potentially curative therapy: impact on management and prognostic stratification. J Nucl Med. 2001;42:1605-1613.
93. Hellwig D., Groschel A., Graeter T.P., et al. Diagnostic performance and prognostic impact of FDG-PET in suspected recurrence of surgically treated non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2006;33:13-21.
94. Colice G.L., Rubins J., Unger M. Follow-up and surveillance of the lung cancer patient following curative-intent therapy. Chest. 2003;123(Suppl 1):272S-283S.
95. Walsh G.L., O’Connor M., Willis K.M., et al. Is follow-up of lung cancer patients after resection medically indicated and cost-effective? Ann Thorac Surg. 1995;60:1563-1672.
96. Westeel V., Choma D., Clement F., et al. Relevance of an intensive postoperative follow-up after surgery for non–small cell lung cancer. Ann Thorac Surg. 2000;70:1185-1190.
97. Roach M.3rd, Gandara D.R., Yuo H.S., et al. Radiation pneumonitis following combined modality therapy for lung cancer: analysis of prognostic factors. J Clin Oncol. 1995;13:2606-2612.
98. Cox J.D., Stetz J., Pajak T.F. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31:1341-1346.
99. Mehta V. Radiation pneumonitis and pulmonary fibrosis in non–small-cell lung cancer: pulmonary function, prediction, and prevention. Int J Radiat Oncol Biol Phys. 2005;63:5-24.
100. Yorke E.D., Jackson A., Rosenzweig K.E., et al. Correlation of dosimetric factors and radiation pneumonitis for non–small-cell lung cancer patients in a recently completed dose escalation study. Int J Radiat Oncol Biol Phys. 2005;63:672-682.
101. Wang S., Liao Z., Wei X., et al. Analysis of clinical and dosimetric factors associated with treatment-related pneumonitis (TRP) in patients with non–small-cell lung cancer (NSCLC) treated with concurrent chemotherapy and three-dimensional conformal radiotherapy (3D-CRT). Int J Radiat Oncol Biol Phys. 2006;66:1399-1407.
102. Tsujino K., Hirota S., Kotani Y., et al. Radiation pneumonitis following concurrent accelerated hyperfractionated radiotherapy and chemotherapy for limited-stage small-cell lung cancer: dose-volume histogram analysis and comparison with conventional chemoradiation. Int J Radiat Oncol Biol Phys. 2006;64:1100-1105.
103. Kong F.M., Hayman J.A., Griffith K.A., et al. Final toxicity results of a radiation-dose escalation study in patients with non–small-cell lung cancer (NSCLC): predictors for radiation pneumonitis and fibrosis. Int J Radiat Oncol Biol Phys. 2006;65:1075-1086.
104. Chen G.Y., Jiang G.L., Qian H., et al. Escalated hyperfractionated accelerated radiation therapy for locally advanced non-small cell lung cancer: a clinical phase II trial. Radiother Oncol. 2004;71:157-162.
105. Hope A.J., Lindsay P.E., El Naqa I., et al. Modeling radiation pneumonitis risk with clinical, dosimetric, and spatial parameters. Int J Radiat Oncol Biol Phys. 2006;65:112-124.
106. Komaki R., Lee J.S., Milas L., et al. Effects of amifostine on acute toxicity from concurrent chemotherapy and radiotherapy for inoperable non–small-cell lung cancer: report of a randomized comparative trial. Int J Radiat Oncol Biol Phys. 2004;58:1369-1377.
107. Boiselle P.M., Morrin M.M., Huberman M.S. Gemcitabine pulmonary toxicity: CT features. J Comput Assist Tomogr. 2000;24:977-980.
108. Gurjal A., An T., Valdivieso M., Kalemkerian G.P. Etoposide-induced pulmonary toxicity. Lung Cancer. 1999;26:109-112.
109. Ramanathan R.K., Reddy V.V., Holbert J.M., Belani C.P. Pulmonary infiltrates following administration of paclitaxel. Chest. 1996;110:289-292.
110. J.J. Erasmus, H.P. McAdams, S.E. Rossi. Drug-induced lung injury. Semin Roentgenol.. 2002;37:72-81.
* Please refer to the abridged M.D. Anderson practice consensus algorithms at the end of this chapter and to the unabridged algorithms on expertconsult.com.