CHAPTER 51 Lumbar Total Disc Replacement
Lumbar spinal fusion is a time-tested, proven successful procedure for alleviating symptoms in patients with unremitting low back pain from various causes. Immobilizing an unstable motion segment, as in a degenerative or isthmic spondylolisthesis, is the most common and least controversial reason for performing a lumbar spinal fusion. Painful disc degeneration is a more controversial indication for spinal fusion because methods for identification of an intervertebral disc as the pain generator are imperfect. Multiple studies have shown successful pain relief of symptomatic lumbar degenerative disc disease with lumbar fusion.1–4 Fusion also creates an anatomic situation with potentially negative consequences for long-term lumbar function, however. By limiting motion of the affected motion segment, additional stress is created at adjacent motion segments, leading to an increased incidence of adjacent level degeneration.5–7 Often this degeneration becomes symptomatic and is a common cause of late failure after lumbar fusion.7–10 In addition, spinal fusion fixes the sagittal alignment at that motion segment, which can affect the adjacent segments over time and be responsible for adjacent segment degeneration.11 A third potential cause of adjacent segment degeneration is the potential iatrogenic injury of the adjacent level with surgery, especially injury to the cephalad facet joint with placement of pedicle screw instrumentation.
With these clinical and anatomic limitations on use, lumbar disc arthroplasty is appropriate only for a small percentage of overall lumbar fusion patients. Various studies have shown potential lumbar TDR candidates limited to 0% to 25% of fusion patients, depending on the specific demographics of the surgical practices being studied.12,13 Many TDR implants have been shown to retain flexion-extension motion at the operative motion segment over time.14–20 This chapter addresses the general concepts of lumbar total disc arthroplasty and focuses on the specifics of lumbar TDR implants that currently have U.S. Food and Drug Administration (FDA) approval or are completing FDA-sponsored multisite investigational device exemption (IDE) trials in preparation for FDA approval.
Historical Devices
The origins of disc replacement date back to the late 1950s, with the first early attempts at disc reconstruction being accomplished by the injection of acrylic bone cement within the nucleus after discectomy.21–23 Around the same time, Fernstrom24 began implanting stainless steel spheres into the intervertebral disc space through a posterior approach after laminotomy and discectomy. Low back pain was associated with progressive disc height loss, and so the goal of these early devices was to restore disc height and maintain spinal motion. Fernstrom24 published his results in 1966, showing fair clinical results at 2 years postoperatively. The long-term outcomes of this device showed a high failure rate, however, secondary to subsidence of the implant with loss of motion, foraminal narrowing, and pain.
In the 1980s, artificial discs were designed using metal endplates and an intervening elastomeric rubber or plastic core, creating metal-plastic and rubber articulations among unattached components. The elastomeric discs were developed by Stefee and Lee and colleagues.25–29 Only the Stefee disc (Acroflex, Acromed) was implanted clinically, but it was soon discontinued because of catastrophic failure of the rubber core.25 Articulating discs include the SB CHARITÉ and the Marnay disc (later renamed the Prodisc I, precursor to the current Prodisc-L). Both discs enjoyed clinical success and are discussed later in this chapter. Another older implant design that never progressed to clinical application included the use of hinged spring articulation in all metal components. The newer, “modern,” designs that are discussed in more detail in this chapter draw upon the continued Orthopaedic experience with large joint replacement with respect to articulating materials, component geometry, and component constraint to minimize the risk of mechanical failure of the arthroplasty device or late failure of the supporting structures of the motion segment.
Biomechanical Considerations
Most of the forces seen across the motion segment are transmitted through the disc space. Force distribution across the disc and facet joint is posture dependent, however. Ledet and colleagues30 performed real-time measurements of forces across the disc space using an interbody force transducer. In this study, forces across the intervertebral disc are highest when the center of gravity is shifted forward and during twisting motions. Forces across the intervertebral disc decrease when the center of gravity is shifted posteriorly. The corresponding postures show high forces across the disc space in sitting positions with the highest forces seen during sitting with the torso flexed and twisted. Standing and lying in the supine position resulted in progressively lower forces at the disc space. A fused segment has no effect on the force transmission to the adjacent level but increases the range of motion of the adjacent level.31 The body attempts to preserve the total range of motion of the lumbar spine by compensating through the adjacent levels.
The motion segment does not act as a pure hinge in that the axis of rotation changes slightly with flexion and extension.32,33 Each lumbar vertebral level has a different location of the instantaneous axis of rotation for a given movement. For the L1-5 disc spaces, the average instantaneous axis of rotation is slightly posterior to midline and just below the inferior endplate on a mid-sagittal image. At L5-S1, the instantaneous axis of rotation is within the disc space.34 Range of motion and type of motion vary by level. Flexion-extension ranges from 12.7 to 19.6 degrees with the highest motion occurring at the L5-S1 segment (Table 51–1).32 At the L4-5 level, the motion is mostly translational, whereas the motion is rotational at the L5-S1 segment. Most studies comparing disc arthroplasty with the normal motion segment focus on flexion and extension. The motion of each vertebral segment is not only multidimensional, but also rotation and translation are coupled to varying degrees by level.
| L1-2 | 12.7 degrees ± 2.47 |
| L2-3 | 16.6 degrees ± 2.43 |
| L3-4 | 16.7 degrees ± 2.36 |
| L4-5 | 18.3 degrees ± 2.67 |
| L5-S1 | 19.6 degrees ± 4.71 |
Implant Design Considerations
Constrained versus Unconstrained
The concept of constraint in TDR has evolved over time and is not the same as in other articulating joint replacements of the appendicular skeleton. Kostuik35 described constraint in terms of the implant’s potential to limit the natural range of motion of the vertebral motion segment for each of the various rotational and translational motions. An implant unconstrained for a certain motion would provide no limitation to that motion well beyond the normal physiologic range. Examples are the CHARITÉ, Prodisc-L, and Maverick discs, which all are ball-and-socket articulations that provide no inherent limitation of axial rotation. These implants do have differing constraint, however, for the other motions of the lumbar motion segment.
Generally, the effect of increasing constraint is to increase the overall stability of the motion segment. A consequence of increasing constraint may be to decrease range of motion and change the stresses seen at the surrounding structures. Increasing constraint has been shown to increase the forces seen at the endplates.34,36,37 Increased stress at the endplate can have an effect on loosening of the component over time, as is seen in large joint arthroplasty. The effect of increasing constraint on the facet joints is unclear. Finite element models have shown increasing stress on the facet joints with increasing constraint.36,38 In vitro analyses have shown increased facet joint stresses with decreased constraint.39 Although it is logical to think that decreased constraint would increase the stress at the surrounding structures, the inconsistency in the data suggest that other factors may also play a role. Increased stress at the facet joints may lead to degenerative changes and pain over time. Implant design must balance between stability of the implant and restoring normal biomechanics.
Bearing Surfaces
Although many potential bearing surfaces are available, the most commonly used are cobalt-chromium alloy on ultrahigh molecular weight polyethylene (UHMWPE) and cobalt-chromium alloy on cobalt-chromium alloy. Both bearing surfaces are well established in orthopaedics for their biocompatibility and good wear resistance. Both bearing surfaces are known, however, to have long-term failures. Experience with large joint arthroplasty has shown that wear particles can lead to osteolysis, subsidence, migration, and need for revision surgery.40–42 Nonetheless, the effect of wear particles within a joint have a different local effect compared with the epidural space.43 To date, significant problems with osteolysis owing to polyethylene debris have not been seen clinically with the metal-polyethylene articulating implants with more than 20 years of use. This may be due to the lack of true synovium surrounding the disc arthroplasty, which has been implicated in the osteolysis seen associated with loosening of hip and knee implants.
Wear patterns observed in retrieval analysis of cobalt-chromium alloy with UHMWPE disc arthroplasty designs show the same failure mechanisms that are seen in hip and knee arthroplasty.44,45 Cobalt-chromium alloy with cobalt-chromium alloy articulations also include the risk of systemic release of cobalt and chromium ions. Although the blood serum ion concentrations are similar to total hip arthroplasty, the long-term effect is unknown.46,47 Industrial exposure to heavy metals such as hexavalent chromium is known to increase systemic inflammation and carcinoma.48 The types of ions that are released from an implant are different and may not have the same effect. Sarcoma adjacent to metal implants is reported in the literature, but it is very rare.49 Metal hypersensitivity is a potential cause for persistent pain with this bearing surface.
Implant Stabilization
Material and implant design considerations are factors in implant stability. Early stability is generally provided for by implant design, including roughened surface coatings, spikes, keels, or screw fixation.50 Late stability is obtained through additional direct bony attachment to the implant via ongrowth or ingrowth into the surface coating. In addition, implants designed to maximize endplate surface area contact and promote force transfer to the peripheral aspect of the endplate, where the bone is strongest, minimize the risk of implant subsidence. Biologic surfaces have become the standard for noncemented acetabular fixation in total hip arthroplasty. Attempts at biologic coatings for total knee arthroplasty have failed secondary to loosening of the components.51 This high failure rate is likely due to micromotion at the bone-implant interface. Similar problems must be monitored for in the spine.
Clinical Indications for Lumbar Total Disc Replacement
In the United States, lumbar disc replacement is currently approved for the treatment of single-level lumbar degenerative disc disease in skeletally mature individuals with no more than grade I spondylolisthesis having failed at least 6 months of nonoperative treatment. Currently available implants are approved only for use from L3 to S1 (Prodisc L3-S1, CHARITÉ L4-S1). The contraindications for TDR are extensive (Table 51–2). A relative contraindication is significant facet joint degeneration, although this is difficult to grade.52 There are many reports of multilevel disc arthroplasty, including level 1 evidence in prospectively randomized clinical trials, but this is currently considered off-label use in the United States based on strict FDA-approved criteria.39,53
TABLE 51–2 Contraindications to Lumbar Total Disc Arthroplasty
Preoperative evaluation for total disc arthroplasty should include plain radiographs with flexion and extension films, magnetic resonance imaging (MRI), computed tomography (CT) scan, and dual-energy x-ray absorptiometry (DEXA) scan in patients at risk for osteopenia.39 Discography is considered by many authors to be an important diagnostic criterion for lumbar TDR but was not a requirement for many of the FDA-sponsored clinical trials. In addition, more recent reports not only have questioned the efficacy of discography, but also have implicated it in accelerating the development of disc degeneration.54–56
Surgical Approach and Discectomy
Implant designs of most lumbar TDR devices require a direct anterior exposure of the intervertebral space; this is true of both prostheses currently available for use in the United States. This exposure can be obtained through either a transperitoneal or a retroperitoneal approach. Currently, a retroperitoneal approach is preferred because it minimizes the risk of postoperative ileus, bowel obstruction secondary to adhesions, and retrograde ejaculation (in men).19 In most cases, the approach is performed with a vascular or general cosurgeon. The exposure must be wide enough to expose the entire anterior surface of the disc space, requiring greater mobilization of the great vessels than is necessary for fusion. The final view of the disc must be directly anterior. Other prostheses in development or available for use outside the United States (e.g., Oblique-Maverick) use an anterolateral or direct lateral approach to the disc space, minimizing or eliminating the need for mobilization of the great vessels.
After exposure of the disc space, the disc is removed in entirety, including the anterior anulus and anterior longitudinal ligament, the entire nucleus pulposus, and the posterior anulus. To restore posterior disc height in cases with significant disc collapse, the posterior longitudinal ligament must be either released or resected. The outer lateral anulus is preserved. The cartilaginous endplate is removed taking care to preserve the subchondral bone. To recreate the proper center of rotation for the lumbar motion segment, most of the devices are placed abutting the posterior edge of the vertebral body. Proper posterior placement also helps to decrease the forces seen at the facet joints.57 Position of the implant is confirmed using intraoperative anteroposterior and lateral fluoroscopy throughout the procedure.
Specific Devices
CHARITÉ Artificial Disc
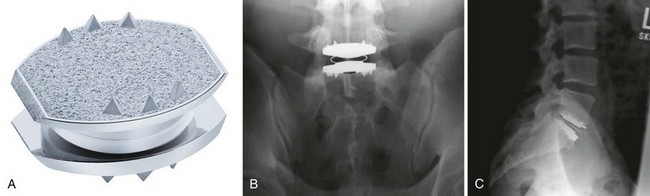
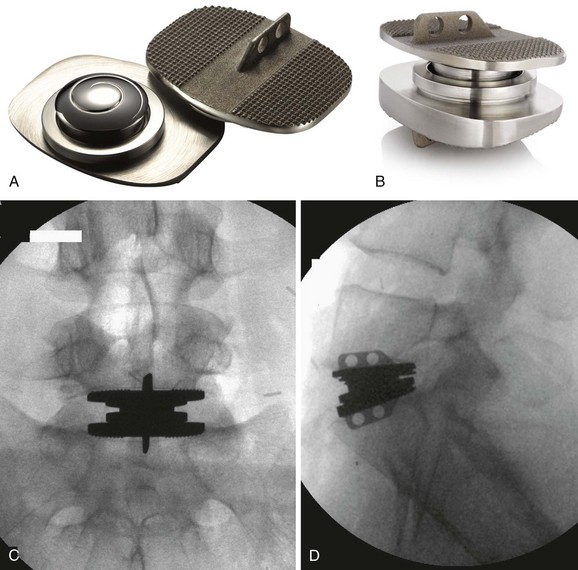
The CHARITÉ Artificial Disc (DePuy Spine Inc., Raynham, MA) is the first device to complete the U.S. FDA approval process and has the largest and the longest clinical experience. The device has a biconvex UHMWPE spacer that acts as a mobile core (Fig. 51–1). The two metallic endplates are made of a cobalt-chromium alloy, and each concave endplate articulates independently with the core. There are ventral and dorsal teeth on the endplates to aid in positioning and maintenance of final position, and the latest version of the device has a titanium and a hydroxyapatite coating.21
The FDA IDE study gave level 1 evidence showing the CHARITÉ disc replacement as equivalent to anterior interbody fusion with a BAK cage (Zimmer Spine, Minneapolis MN) filled with iliac crest autograft.14,15 In the study, 305 patients were randomly assigned to receive either the CHARITÉ lumbar disc replacement or anterior lumbar interbody fusion (ALIF) with a pair of cylindric threaded BAK cages and iliac crest autograft. Study levels included only L4-5 or L5-S1. For the CHARITÉ group, the Oswestry Disability Index (ODI) improved by 24.8 points from 50.6 preoperatively to 25.8 at 24 months. In the BAK fusion group, ODI improved by 22 points from 52.1 to 30.1. The visual analog score (VAS) improved from 72 points in the CHARITÉ group to 30.6 points at 24 months. For the BAK fusion group, the VAS improved from 71.8 to 36.3 points. These results were maintained at the 5-year follow-up.58 In this study, FDA success at 24 months was defined by success in all of four major criteria: (1) a 25% improvement in the ODI, (2) no device failure, (3) no major complication, and (4) and no neurologic deterioration. In the FDA IDE study, 57% of the CHARITÉ group and 46% of the fusion group met all four criteria.
Longer term retrospective level 3 clinical data are also available for TDR using the CHARITÉ device.59,60 Lemaire and colleagues59 reviewed 100 patients with a minimum 10-year follow-up. Of patients, 90% had a good to excellent outcome with 63% returning to heavy labor jobs and 91.6% overall returning to work. Mean flexion-extension was 10.3 degrees with 5.4 degrees of lateral bending. Five patients (5%) required arthrodesis secondary to either implant failure or facet arthrosis. There were two neurologic injuries (2%), one L5 nerve injury and one sexual dysfunction, both of which recovered. There were two cases of spontaneous fusion (2%), and two cases of adjacent segment degeneration (2%). David60 reviewed 106 patients implanted over a 6-year period with a minimum 10-year follow-up. Of patients, 82% had good to excellent outcomes; 89% returned to work with 77% returning to heavy labor. Eight patients (7.5%) required a posterior instrumented fusion. There were five cases (4.6%) of postoperative facet arthrosis, three cases (2.8%) of subsidence, three cases (2.8%) of adjacent level disease, and two cases (1.9%) of core subluxation.
Ross and colleagues61 found poorer quality long-term results using the CHARITÉ device. Kaplan-Meier analysis was performed on 226 CHARITÉ implants. Radiologic failure was defined as a broken wire, subsidence, or lack of movement. At 8 years, there was a 90% survival rate. This rate declined dramatically to 35% at 13 years. At final follow-up, ODI improved by 14 points on average, 1 point below the benchmark for clinical improvement. At final follow-up, 69% of patients believed that they were better or much better than preoperatively; 20% believed that they were worse than preoperatively.
Cunningham and colleagues62 showed that the CHARITÉ disc is effective in maintaining lumbar motion. They reviewed radiographs from the initial IDE study and assessed motion at the disc arthroplasty as a percentage of the total lumbar spine motion. These numbers were compared with cadaveric normal discs. At 2 years, the disc replacement maintained normal segmental lumbar motion and preserved the overall lumbar motion. Putzier and colleagues63 reviewed 63 CHARITÉ discs at 17-year follow-up. At 17 years, 60% of the implanted discs had spontaneously ankylosed, and only 17% were deemed functional. In the patients in whom the disc replacement was still functional, there was no evidence of adjacent segment degeneration, suggesting that a functional implant is effective in preventing adjacent segment degeneration. Clinically, the patients with spontaneous ankylosis did better than patients with functional implants. One suggested cause of the clinical failure is hypermobility leading to early facet joint degeneration at the arthroplasty level. Hypermobility from removal of the anterior longitudinal ligament has been shown in vitro.64 In contrast, Huang and colleagues65 assessed 51 TDRs at 8.5 years. Range of motion greater than 5 degrees corresponded to improved clinical outcome.
Prodisc-L
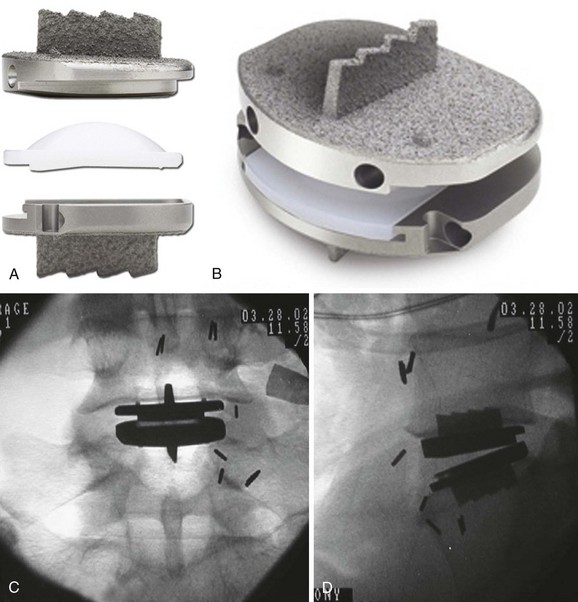
The Prodisc-L (Synthes Inc., Paoli, PA) is a modular metal and UHMWPE device with three components, two of which are locked together during insertion to create a two-piece implant (Fig. 51–2). The two endplates are made of cobalt-chromium alloy. The upper endplate has a concave articular surface. The lower metallic endplate has slots to accept a convex UHMWPE articular component. Both endplates are secured to the vertebral endplates by means of a central keel, small spikes, and a titanium plasma-sprayed rough surface.21 The polyethylene is inserted into the disc space and locked to the caudal endplate after the endplates are in their final position, resulting in a single ball-and-socket articulation with the center of rotation in the upper aspect of the caudal vertebra.
The only level 1 evidence for the Prodisc-L comes from the U.S. FDA IDE trial. In this study, the Prodisc-L was compared with circumferential fusion with femoral ring allograft anteriorly and instrumented posterolateral fusion with iliac crest autograft. The 2-year data have been published.20 VAS pain assessment showed statistically significant improvement from preoperative levels regardless of treatment. The average VAS score was 37 in the Prodisc-L group for a 39-mm average reduction and was 43 in the fusion group with a 32-mm average reduction from baseline and trended toward significance (P = .08). The FDA success criteria included ODI reduction by at least 15 points; improvement in short-form health survey (SF-36); device success; no adverse events; and multiple radiographic criteria including no migration or subsidence, no loosening or osteolysis, no loss of disc height, and no decrease in range of motion. Using the FDA success definition as success in all of these end points, 53% of Prodisc-L patients and 41% of control patients were considered successful, with a statistically significant difference favoring the Prodisc-L group. A more recent report of 5-year follow-up found no statistically significant difference in the ODI or VAS compared with 2 years.66 Successful radiographic range of motion was maintained by 93.7% of Prodisc-L implants.
Cakir and colleagues67 examined the clinical effect of TDR range of motion on clinical outcome at a minimum of 3 years after Prodisc-L implantation. They found no statistical difference in the preoperative and postoperative range of motion, with an increase in motion in 40% and a decrease in 30% of patients. The postoperative range of motion had no effect on clinical outcome. Bae and colleagues68 reviewed 219 patients from the IDE trial at 2-year follow-up. In contrast, these authors found that greater average postoperative range of motion correlated significantly with less VAS pain at 6 weeks at all time points. They also found that a greater body mass index correlated to a decrease in preoperative and postoperative range of motion at 1 and 2 years after arthroplasty.
Although Prodisc-L TDR is FDA approved only for single-level use in the United States, the results of multilevel surgeries have been reported. The U.S. FDA IDE study included a two-level arm, with adjacent two-level painful degenerative disc diseases cases prospectively randomized (in a similar 2 : 1 fashion as the one-level arm) to either a two-level Prodisc-L or circumferential fusion. These data were submitted to the FDA well after the one-level approval and have not yet been approved as a new amended indication for the use of Prodisc-L by the FDA as of the writing of this chapter. Results from the IDE trial for two-level Prodisc-L have been presented.69 In the trial, 237 patients were randomly assigned to receive either two-level Prodisc-L or circumferential fusion at two levels. Intraoperative data showed that Prodisc-L cases had a significantly shorter operative time, estimated blood loss, and length of hospital stay (P < .0001, P = .0006, P < .0001). At 24 months, 90% of Prodisc-L patients and 86.7% of fusion patients reported improvement in ODI from preoperative levels; 73.3% of Prodisc-L patients and 55.9% of fusion patients met the defined 15-point ODI improvement criteria. Overall neurologic success in Prodisc-L patients was superior to fusion patients (Prodisc-L 89.2%, fusion 77.9%; P = .0260). At all follow-up points, Prodisc-L patients recorded SF-36 scores significantly higher than fusion patients (P = .0523). VAS pain scores were significantly improved from preoperative scores regardless of treatment (P < .0001); at 24 months, the Prodisc-L group showed significantly higher pain reduction than the fusion group (P = .0466). VAS satisfaction at 24 months significantly favored Prodisc-L patients over fusion patients. At 24 months, a significant difference favored Prodisc-L patients over fusion patients (Prodisc-L 97.6%, fusion 91.8%, P = .0497) in device success—absence of any reoperation required to modify or remove implants and no need for supplemental fixation. Radiographic range of motion of patients was maintained within a normal functional range.
Siepe and colleagues70 reviewed the results of 218 patients implanted with the Prodisc II device. The patients were divided into groups by the number of levels that the device was implanted. They found that implanting more than one level leads to worsening results compared with a single level. The results of Siepe and colleagues70 are contradicted by another published study using the same device. Hannibal and colleagues53 compared 27 patients receiving Prodisc II at one level with 32 patients receiving Prodisc II at two levels. The number of patients in each group was small, but the investigators found no statistical difference between the two groups at 2 years. These results are consistent with other published series.71,72
Other Devices
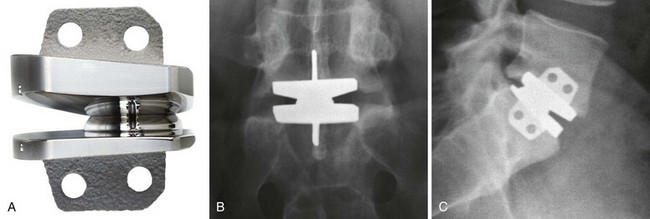
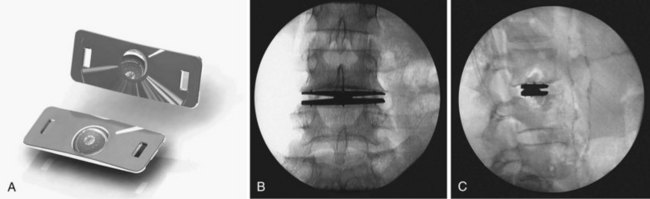
The Maverick lumbar TDR prosthesis (Medtronic Sofamor Danek Inc., Memphis, TN) is an all-metal two-piece design that uses a highly polished cobalt-chromium alloy ball-and-socket metal-on-metal articulation. The device has a central fin on both endplates and hydroxyapatite coating of all bony contact surfaces for increased stability after insertion (Fig. 51–3). The center of rotation is fixed posteriorly to the mid-portion of the prosthesis, in the upper aspect of the caudal vertebrae. The U.S. FDA IDE trial is currently ongoing; in this study, the Maverick TDR is compared with an anterior-only fusion using an LT cage with Infuse-BMP (Medtronic, Memphis, TN).
Published prospective level 2 evidence is available after European use. Le Huec and colleagues73 reported the results of 64 implanted devices with a minimum of 2-year follow-up. ODI scores preoperatively and at 2-year follow-up were 43.8 and 23.1 (P < .05). Low back pain improved from a mean VAS score of 7.7 preoperatively to 3.2 at 2 years. Le Huec and colleagues73 found that grade I and II facet joint degeneration did not influence the clinical outcome. They also found that paraspinal muscle atrophy resulted in a poorer outcome. The Maverick metal-on-metal articulation introduces the possibility of metal ion release into the bloodstream. Zeh and colleagues47,74 found increased levels of cobalt and chromium at implantation of the Maverick prosthesis. The increased levels were persistent at 3-year follow-up and were similar to levels with a metal-on-metal total hip prosthesis.
Preliminary results at 2-year follow-up have been presented from the U.S. FDA IDE trial.16 In the trial, 405 investigational (TDR) patients and 172 control (ALIF) patients with single-level degenerative disc disease (L4-S1) were treated after failing conservative care for 6 months. Mean (TDR and ALIF) operative time (1.8 hours and 1.4 hours) and blood loss (240.7 mL and 95.2 mL) differences were significant (P < .001); mean hospital stay (2.2 days and 2.3 days) and operative level distribution (73.6% and 78.5% L5-S1) were not. The mean ODI score improved dramatically for both groups at 12 months and 24 months (TDR 33.9 and 33.8 points; ALIF 29 and 29.3 points). At 24 months, 82.2% of TDR patients reported an ODI improvement of at least 15 points, an FDA-defined measure of success, versus 75.2% of ALIF patients.
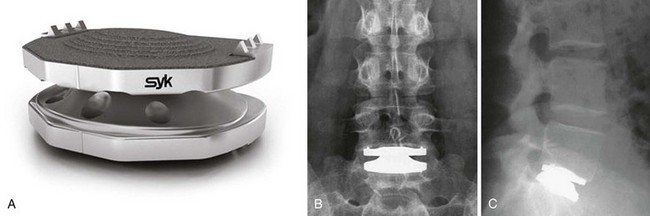
The FlexiCore lumbar prosthesis (Stryker Spine, Allendale, NJ) is a cobalt-chromium alloy highly polished ball-and-socket metal-on-metal prosthesis.21 The device is a one-piece design, which prevents separation and migration of a single endplate. The device also has a built-in rotational stop to prevent the facets from being overstressed (Fig. 51–4). There are fixation spikes on the upper and lower base with a titanium plasma spray coating to allow for bony ongrowth. There are currently no published clinical results with this device, but preliminary results have been presented. Adult patients with single-level discogenic pain who failed 6 months of nonoperative care were randomly assigned in a 2 : 1 ratio to FlexiCore disc replacement or circumferential fusion.19 A total of 140 FlexiCore subjects and 58 fusion subjects were available for review.
The Kineflex lumbar prosthesis (Spinal Motion, South Africa) is an unconstrained device that allows for the option of a metal-on-polyethylene or metal-on-metal articulation with a mobile core. The mobile core is designed to replicate more closely the dynamic center of rotation of the normal disc (Fig. 51–5). The endplates have serrations and a keel for increased fixation. There are currently no published clinical results with this device; however, preliminary results have been presented. Hahnle and colleagues17 reported their 2-year results with the Kineflex prosthesis. Using return to work, ODI, pain scoring, and patient satisfaction as the outcome measures, 100 patients were evaluated: 39 patients underwent an isolated single-level disc replacement; 25 patients, a two-level disc replacement; 1 patient, a double-level replacement adjacent to a previous fusion; and 7 patients, a fusion of another level at the time of the index procedure (hybrid cases). For 72 of 75 patients, 2-year clinical outcome was available (44 excellent, 17 good, 7 fair, and 4 poor). ODI score improved from 47.8 preoperatively to 14.5 (P ≤ .01) at the latest follow-up questionnaire. The pain score improved from 9.1 preoperatively to 2.9 at 2 years. As a group, the hybrid cases had the poorest outcome with two poor results out of seven.
Results at 12 months from the ongoing IDE trial have also been presented. In the trial, 58 patients were randomly assigned in a 1 : 1 ratio to receive either the Kineflex lumbar disc replacement or the CHARITÉ lumbar disc replacement (the control group).18 Overall complication rates did not differ significantly between groups. At 1 year postoperatively, both groups showed statistically and clinically significant reductions in ODI (investigational group 50.1% and control group 56.6%) and VAS (investigational group 56.7% and control group 71.8%). There were no significant differences between the two groups. The FDA defines individual clinical success for the study at 24 months. When the criteria were applied at 12 months, the results did not differ significantly between groups (57.1% investigational and 72% control). Both groups had high patient satisfaction scores. No significant difference in time to return to work was shown between groups (investigational group 140 days and control group 113 days). Only one patient working preoperatively failed to return to work within 12 months. In the investigational group, six patients eligible but not working preoperatively returned to work.
Hybrid Procedures
A hybrid construct refers to the use of a lumbar disc replacement adjacent to a fusion. The TDR may be performed along with fusion of an adjacent level, or it may be performed adjacent to a previously fused level (Fig. 51–6). Both of these clinical scenarios are considered off-label use for the prostheses currently available for use in the United States based on the inclusion and exclusion criteria from the FDA IDE trials. The theoretical advantages of preventing adjacent segment disease may have more utility when used in hybrid constructs. The advantages would be increased in longer fusions, which have a higher incidence of adjacent segment disease.75–78 Aunoble and colleagues79 implanted hybrid constructs in 42 patients and followed them prospectively. At 2 years, ODI decreased by 53%. No prospective randomized level 1 data or long-term prospective data exist to support the regular use of these hybrid constructs, however.
Complications and Revision Strategies
Complications after total disc arthroplasty can be early or delayed. Although there are complications associated with use of an anterior approach, these are not specifically reviewed here because they are not specific to total disc arthroplasty. The full-width, direct anterior exposure required for disc arthroplasty requires greater mobilization of the great vessels, which may increase the morbidity of the procedure compared with use of cage or bone graft, which can be inserted off midline or via an anterolateral approach if necessary. Early complications include endplate fracture, subsidence, nerve entrapment, and dislocation. Late complications include infection, osteolysis and loosening, hardware failure, facet arthrosis, fracture, and spontaneous fusion. The early complication rate ranges from 0% to 25%.39,60,61,80–83 This complication rate increases as the number of levels operated on increases.84 The late complication rate is 21%, although a true assessment of this number requires long-term data.59,85
Each individual device has its own unique mechanisms of failure, so each device design should be evaluated individually. The early CHARITÉ design was associated with rim fracture of the polyethylene insert.86 Alternative designs using a polyethylene core may not have the same problem. Implant revision is an important consideration because it is likely that many young patients require a revision in their lifetime. Prosthesis removal, when necessary, can often be accomplished via an anterolateral or direct lateral approach without full mobilization of the great vessels.
The limited life span for any motion-preserving device requires an assessment of revisability. In situ posterior fusion with retention of the components is possible for patients who experience subsidence, fracture, or facet arthrosis. It is still unclear whether this approach, with retention of the TDR implant, yields fusion or clinical results similar, better, or worse than strategies that include removal of the TDR device and interbody fusion. There are limited options for revision when removal of the components is required. Anatomic approaches include right and left retroperitoneal, transperitoneal, and direct lateral approach, either with reflection of the psoas muscle or with a trans–psoas muscle–splitting approach. Even keeled prostheses can be removed via an anterolateral or direct lateral approach.87,88 Ideally, a surgical approach different from that used in the primary implantation should be considered, unless revision is done for acute failure within 3 weeks of the index procedure.
A revision anterior surgical approach is associated with a significantly higher complication rate than a primary approach.80,89 Scar tissue complicates the anterior approach because mobilization of the great vessels becomes much more challenging and sometimes impossible. This is most problematic at the L4-5 level. Changing the approach minimizes the risk of injury to the large vessels that are encased in the scar tissue. McAfee and colleagues90 reviewed 24 patients who underwent revision using an anterior approach. Four patients (16.7%) sustained an injury to the large vessels. Leary and colleagues91 had a large vessel injury rate of 10% (2 of 20) after revision anterior exposure. Bumpass and colleagues92 performed cadaveric dissections of the lumbar plexus to determine retrievability through a posterior approach. The smallest root-to-root distance measured was 9.1 mm. The smallest measured root-to-tether distance was 39.2 mm. These measurements theoretically allow for removal of small implants posteriorly. No clinical studies assess the risk of nerve injury through a posterior approach.
Future Considerations
Only time and close study will tell if persistent motion at an operative segment (as opposed to fusion) minimizes adjacent degeneration and if specific motion patterns, based on TDR implant biomechanics, are more clinically beneficial than others. The true fate of the mobile facet joints at the TDR level over time for the various implants is also a clinical unknown that needs more long-term radiographic follow-up. Newer lumbar TDR devices are attempting to incorporate the viscoelastic properties of the native disc, which might also provide a role in dissipation of acute axial loads and protect adjacent segments. One example is the one-piece polymer core and metal endplate Freedom disc (Axiomed Spine Corporation, Cleveland, OH). Finally, devices designed for a lateral or posterior insertion into the disc space are being designed and tested, and these may improve the safety for revision or removal when needed. One example is the XL TDR (NuVasive, San Diego, CA), a ball-and-socket TDR device designed for direct lateral implantation (Fig. 51–7). This device is currently being implanted in limited use in patients outside of the United States.
Conclusion
Pearls
Pitfalls
Key Points
1 Eck JC, Humphreys SC, Hodges SD. Adjacent-segment degeneration after lumbar fusion: A review of clinical, biomechanical, and radiologic studies. Am J Orthop. 1999;28:336-340.
2 Carragee EJ, Paragioudakis SJ, Khurana S. 2000 Volvo Award winner in clinical studies: Lumbar high-intensity zone and discography in subjects without low back problems. Spine (Phila Pa 1976). 2000;25:2987-2992.
3 Putzier M, Funk JF, Schneider SV, et al. Charité total disc replacement—clinical and radiographical results after an average follow-up of 17 years. Eur Spine J. 2006;15:183-195.
4 Zigler J, Delamarter R, Spivak JM, et al. Results of the prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of the ProDisc-L total disc replacement versus circumferential fusion for the treatment of 1-level degenerative disc disease. Spine (Phila Pa 1976). 2007;32:1155-1162. discussion 1163
5 Guyer RD, McAfee PC, Banco RJ, et al. Prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of lumbar total disc replacement with the Charité artificial disc versus lumbar fusion: Five-year follow-up. Spine J. 2009;9:374-386.
1 O’Brien JP. The role of fusion for chronic low back pain. Orthop Clin North Am. 1983;14:639-647.
2 Fischgrund JS, Montgomery DM. Diagnosis and treatment of discogenic low back pain. Orthop Rev. 1993;22:311-318.
3 Wetzel FT, LaRocca SH, Lowery GL, et al. The treatment of lumbar spinal pain syndromes diagnosed by discography: Lumbar arthrodesis. Spine (Phila Pa 1976). 1994;19:792-800.
4 Thalgott JS, Giuffre JM, Klezl Z, Timlin M. Anterior lumbar interbody fusion with titanium mesh cages, coralline hydroxyapatite, and demineralized bone matrix as part of a circumferential fusion. Spine J. 2002;2:63-69.
5 Rao RD, David KS, Wang M. Biomechanical changes at adjacent segments following anterior lumbar interbody fusion using tapered cages. Spine (Phila Pa 1976). 2005;30:2772-2776.
6 Dekutoski MB, Schendel MJ, Ogilvie JW, et al. Comparison of in vivo and in vitro adjacent segment motion after lumbar fusion. Spine (Phila Pa 1976). 1994;19:1745-1751.
7 Eck JC, Humphreys SC, Hodges SD. Adjacent-segment degeneration after lumbar fusion: A review of clinical, biomechanical, and radiologic studies. Am J Orthop. 1999;28:336-340.
8 Lee CK. Accelerated degeneration of the segment adjacent to a lumbar fusion. Spine (Phila Pa 1976). 1988;13:375-377.
9 Whitecloud TS3rd, Davis JM, Olive PM. Operative treatment of the degenerated segment adjacent to a lumbar fusion. Spine (Phila Pa 1976). 1994;19:531-536.
10 Rahm MD, Hall BB. Adjacent-segment degeneration after lumbar fusion with instrumentation: A retrospective study. J Spinal Disord. 1996;9:392-400.
11 Djurasovic MO, Carreon LY, Glassman SD, et al. Sagittal alignment as a risk factor for adjacent level degeneration: A case-control study. Orthopedics. 2008;31:546.
12 Huang RC, Lim MR, Girardi FP, et al. The prevalence of contraindications to total disc replacement in a cohort of lumbar surgical patients. Spine (Phila Pa 1976). 2004;29:2538-2541.
13 Wong DA, Annesser B, Birney T, et al. Incidence of contraindications to total disc arthroplasty: A retrospective review of 100 consecutive fusion patients with a specific analysis of facet arthrosis. Spine J. 2007;7:5-11.
14 McAfee PC, Cunningham B, Holsapple G, et al. A prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of lumbar total disc replacement with the CHARITE artificial disc versus lumbar fusion: Part II. Evaluation of radiographic outcomes and correlation of surgical technique accuracy with clinical outcomes. Spine (Phila Pa 1976). 2005;30:1576-1583. discussion E388-E390
15 Blumenthal S, McAfee PC, Guyer RD, et al. A prospective, randomized, multicenter Food and Drug Administration investigational device exemptions study of lumbar total disc replacement with the CHARITE artificial disc versus lumbar fusion: Part I. Evaluation of clinical outcomes. Spine (Phila Pa 1976). 2005;30:1565-1575. discussion E387-E391
16 Gornet MF, Burkus JK, Mathews HH, et al. Maverick total disc replacement versus anterior lumbar interbody fusion with the INFUSE bone graft/LT-CAGE device: A prospective, randomized, controlled, multicenter IDE trial. Spine J. 2007;7(Suppl 1):1S.
17 Hahnle U, Weinberg IR, De Villiers M. Kineflex (Centurion) lumbar disc prosthesis: Two-year results. Spine J. 2006;6(Suppl 1):109S-110S.
18 Knight R, MacLennan B, Roh J, et al. A prospective, randomized, FDA IDE study of lumbar TDR with the Kineflex artificial disc vs. the Charité artificial disc: Evaluation of clinical outcomes at 12 months from a single site. Spine J. 2009;9(Suppl 1):69S.
19 Errico T, Sasso R, Mize G, et al. Total disc replacement for treating lumbar discogenic back pain: A prospective randomized multicenter study of FlexiCore vs. 360 spinal fusion. Spine J. 2008;8(Suppl 1):15S-16S.
20 Zigler J, Delamarter R, Spivak JM, et al. Results of the prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of the ProDisc-L total disc replacement versus circumferential fusion for the treatment of 1-level degenerative disc disease. Spine (Phila Pa 1976). 2007;32:1155-1162. discussion 1163
21 Errico TJ. Lumbar disc arthroplasty. Clin Orthop Relat Res. 2005;435:106-117.
22 Cleveland D. Interspace reconstruction and spinal stabilization after disk removal. Lancet. 1956;76:327-331.
23 Hamby WB, Glaser HT. Replacement of spinal intervertebral discs with locally polymerizing methyl methacrylate: Experimental study of effects upon tissues and report of a small clinical series. J Neurosurg. 1959;16:311-313.
24 Fernstrom U. Arthroplasty with intercorporal endoprothesis in herniated disc and in painful disc. Acta Chir Scand Suppl. 1966;357:154-159.
25 Steffee AD. The Steffee artificial disc. In: Weinstein JN, editor. Clinical efficacy and outcome in the diagnosis and treatment of low back pain. New York: Raven Press; 1992:245-247.
26 Lee CK, Langrana NA, Parsons JR, et al. Development of a prosthetic intervertebral disc. Spine (Phila Pa 1976). 1991;16(6 Suppl):S253-S255.
27 Enker P, Steffee A, Mcmillin C, et al. Artificial disc replacement: Preliminary report with a 3-year minimum follow-up. Spine (Phila Pa 1976). 1993;18:1061-1070.
28 Vuono-Hawkins M, Zimmerman MC, Lee CK, et al. Mechanical evaluation of a canine intervertebral disc spacer: In situ and in vivo studies. J Orthop Res. 1994;12:119-127.
29 Vuono-Hawkins M, Langrana NA, Parsons JR, et al. Materials and design concepts for an intervertebral disc spacer. II. Multidurometer composite design. J Appl Biomater. 1995;6:117-123.
30 Ledet EH, Tymeson MP, DiRisio DJ, et al. Direct real-time measurement of in vivo forces in the lumbar spine. Spine J. 2005;5:85-94.
31 Chow DH, Luk KD, Evans JH, et al. Effects of short anterior lumbar interbody fusion on biomechanics of neighboring unfused segments. Spine (Phila Pa 1976). 1996;21:549-555.
32 Yoshioka T, Tsuji H, Hirano N, et al. Motion characteristic of the normal lumbar spine in young adults: Instantaneous axis of rotation and vertebral center motion analyses. J Spinal Disord. 1990;3:103-113.
33 Pearcy MJ, Bogduk N. Instantaneous axes of rotation of the lumbar intervertebral joints. Spine (Phila Pa 1976). 1988;13:1033-1041.
34 Frelinghuysen P, Huang RC, Girardi FP, et al. Lumbar total disc replacement, part I: Rationale, biomechanics, and implant types. Orthop Clin North Am. 2005;36:293-299.
35 Kostuik JP. Intervertebral disc replacement: Experimental study. Clin Orthop Relat Res. 1997;337:27-41.
36 Chung SK, Kim YE, Wang KC. Biomechanical effect of constraint in lumbar total disc replacement: A study with finite element analysis. Spine (Phila Pa 1976). 2009;34:1281-1286.
37 Galbusera F, Bellini CM, Zweig T, et al. Design concepts in lumbar total disc arthroplasty. Eur Spine J. 2008;17:1635-1650.
38 Rundell SA, Auerbach JD, Balderston RA, et al. Total disc replacement positioning affects facet contact forces and vertebral body strains. Spine (Phila Pa 1976). 2008;33:2510-2517.
39 Rousseau MA, Bradford DS, Bertagnoli R, et al. Disc arthroplasty design influences intervertebral kinematics and facet forces. Spine J. 2006;6:258-266.
40 Mirra JM, Amstutz HC, Matos M, et al. The pathology of the joint tissues and its clinical relevance in prosthesis failure. Clin Orthop Relat Res. 1976;117:221-240.
41 Howie DW, Haynes DR, Rogers SD, et al. The response to particulate debris. Orthop Clin North Am. 1993;24:571-581.
42 Howie DW, Manthey B, Hay S, et al. The synovial response to intraarticular injection in rats of polyethylene wear particles. Clin Orthop Relat Res. 1993;292:352-357.
43 Cunningham BW. Basic scientific considerations in total disc arthroplasty. Spine J. 2004;4(6 Suppl):219S-230S.
44 Kurtz SM, van Ooij A, Ross R, et al. Polyethylene wear and rim fracture in total disc arthroplasty. Spine J. 2007;7:12-21.
45 Punt IM, Visser VM, van Rhijn LW, et al. Complications and reoperations of the SB Charite lumbar disc prosthesis: Experience in 75 patients. Eur Spine J. 2008;17:36-43.
46 Cobb AG, Schmalzreid TP. The clinical significance of metal ion release from cobalt-chromium metal-on-metal hip joint arthroplasty. Proc Inst Mech Eng H. 2006;220:385-398.
47 Zeh A, Planert M, Siegert G, et al. Release of cobalt and chromium ions into the serum following implantation of the metal-on-metal Maverick-type artificial lumbar disc (Medtronic Sofamor Danek). Spine (Phila Pa 1976). 2007;32:348-352.
48 Keegan GM, Learmonth ID, Case CP. A systematic comparison of the actual, potential, and theoretical health effects of cobalt and chromium exposures from industry and surgical implants. Crit Rev Toxicol. 2008;38:645-674.
49 Keel SB, Jaffe KA, Petur Nielsen G, et al. Orthopaedic implant-related sarcoma: A study of twelve cases. Mod Pathol. 2001;14:969-977.
50 Lee CK, Goel VK. Artificial disc prosthesis: Design concepts and criteria. Spine J. 2004;4(6 Suppl):209S-218S.
51 Fehring TK, Odum S, Griffin WL, et al. Early failures in total knee arthroplasty. Clin Orthop Relat Res. 2001;392:315-318.
52 Walraevens J, Liu B, Meersschaert J, et al. Qualitative and quantitative assessment of degeneration of cervical intervertebral discs and facet joints. Eur Spine J. 2009;18:358-369.
53 Hannibal M, Thomas DJ, Low J, et al. ProDisc-L total disc replacement: A comparison of 1-level versus 2-level arthroplasty patients with a minimum 2-year follow-up. Spine (Phila Pa 1976). 2007;32:2322-2326.
54 Carragee EJ, Paragioudakis SJ, Khurana S. 2000 Volvo Award winner in clinical studies: Lumbar high-intensity zone and discography in subjects without low back problems. Spine (Phila Pa 1976). 2000;25:2987-2992.
55 Carragee EJ, Lincoln T, Parmar VS, et al. A gold standard evaluation of the “discogenic pain” diagnosis as determined by provocative discography. Spine (Phila Pa 1976). 2006;31:2115-2123.
56 Carragee EJ, Alamin TF, Miller J, et al. Provocative discography in volunteer subjects with mild persistent low back pain. Spine J. 2002;2:25-34.
57 Dooris AP, Goel VK, Grosland NM, et al. Load-sharing between anterior and posterior elements in a lumbar motion segment implanted with an artificial disc. Spine (Phila Pa 1976). 2001;26:E122-E129.
58 Guyer RD, McAfee PC, Banco RJ, et al. Prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of lumbar total disc replacement with the CHARITE artificial disc versus lumbar fusion: Five-year follow-up. Spine J. 2009;9:374-386.
59 Lemaire JP, Carrier H, Sariali el-H, et al. Clinical and radiological outcomes with the Charite artificial disc: A 10-year minimum follow-up. J Spinal Disord Tech. 2005;18:353-359.
60 David T. Long-term results of one-level lumbar arthroplasty: Minimum 10-year follow-up of the CHARITE artificial disc in 106 patients. Spine (Phila Pa 1976). 2007;32:661-666.
61 Ross R, Mirza AH, Norris HE, et al. Survival and clinical outcome of SB Charite III disc replacement for back pain. J Bone Joint Surg Br. 2007;89:785-789.
62 Cunningham BW, McAfee PC, Geisler FH, et al. Distribution of in vivo and in vitro range of motion following 1-level arthroplasty with the CHARITE artificial disc compared with fusion. J Neurosurg Spine. 2008;8:7-12.
63 Putzier M, Funk JF, Schneider SV, et al. Charite total disc replacement—clinical and radiographical results after an average follow-up of 17 years. Eur Spine J. 2006;15:183-195.
64 Cunningham BW, Gordon JD, Dmitriev AE, et al. Biomechanical evaluation of total disc replacement arthroplasty: An in vitro human cadaveric model. Spine (Phila Pa 1976). 2003;28:S110-S117.
65 Huang RC, Girardi FP, Cammisa FPJr, et al. Correlation between range of motion and outcome after lumbar total disc replacement: 8.6-year follow-up. Spine (Phila Pa 1976). 2005;30:1407-1411.
66 Delamarter R, Zigler J, Spivak JM, et al. Five year results of the prospective randomized multicenter FDA IDE ProDisc-L clinical trial. Spine J. 2008;8(Suppl 1):62S-63S.
67 Cakir B, Schmidt R, Mattes T, et al. Index level mobility after total lumbar disc replacement: Is it beneficial or detrimental? Spine (Phila Pa 1976). 2009;34:917-923.
68 Bae H, Kanim LEA, Kropf M, et al. Radiographic range of motion is related to clinical outcomes in lumbar artificial disc replacement patients: One site analysis of 219 patients with minimum 2 year follow-up, USA-FDA IDE study. Spine J. 2009;9(Suppl 1):104S-105S.
69 Delamarter R, Zigler J, Balderston RA, et al. Results of the prospective randomized multicenter FDA IDE study of the ProDisc-L total disc replacement vs. circumferential fusion for the treatment of two level degenerative disc disease. Spine J. 2008;8(Suppl 1):94S-95S.
70 Siepe CJ, Mayer HM, Heinz-Leisenheimer M, et al. Total lumbar disc replacement: Different results for different levels. Spine (Phila Pa 1976). 2007;32:782-790.
71 Bertagnoli R, Yue JJ, Nanieva R, Fenk-Mayer A, et al. Lumbar total disc arthroplasty in patients older than 60 years of age: A prospective study of the ProDisc prosthesis with 2-year minimum follow-up period. J Neurosurg Spine. 2006;4:85-90.
72 Bertagnoli R, Yue JJ, Shah RV, et al. The treatment of disabling multilevel lumbar discogenic low back pain with total disc arthroplasty utilizing the ProDisc prosthesis: A prospective study with 2-year minimum follow-up. Spine (Phila Pa 1976). 2005;30:2192-2199.
73 Le Huec JC, Mathews H, Basso Y, et al. Clinical results of Maverick lumbar total disc replacement: Two-year prospective follow-up. Orthop Clin North Am. 2005;36:315-322.
74 Zeh A, Becker C, Planert M, et al. Time-dependent release of cobalt and chromium ions into the serum following implantation of the metal-on-metal Maverick type artificial lumbar disc (Medtronic Sofamor Danek). Arch Orthop Trauma Surg. 2009;129:741-746.
75 Gillet P. The fate of the adjacent motion segments after lumbar fusion. J Spinal Disord Tech. 2003;16:338-345.
76 Hilibrand AS, Robbins M. Adjacent segment degeneration and adjacent segment disease: The consequences of spinal fusion? Spine J. 2004;4(6 Suppl):190S-194S.
77 Park P, Garton HJ, Gala VC, et al. Adjacent segment disease after lumbar or lumbosacral fusion: Review of the literature. Spine (Phila Pa 1976). 2004;29:1938-1944.
78 Throckmorton TW, Hilibrand AS, Mencio GA, et al. The impact of adjacent level disc degeneration on health status outcomes following lumbar fusion. Spine (Phila Pa 1976). 2003;28:2546-2550.
79 Aunoble S, Meyrat R, Al Sawad Y, et al. Hybrid construct for two levels disc disease in lumbar spine. Eur Spine J. 2010;19:290-296.
80 Geisler FH, Guyer RD, Blumenthal SL, et al. Effect of previous surgery on clinical outcome following 1-level lumbar arthroplasty. J Neurosurg Spine. 2008;8:108-114.
81 Berg S, Tullberg T, Branth B, et al. Total disc replacement compared to lumbar fusion: A randomised controlled trial with 2-year follow-up. Eur Spine J. 2009;18:1512-1519.
82 Guyer RD, Geisler FH, Blumenthal SL, et al. Effect of age on clinical and radiographic outcomes and adverse events following 1-level lumbar arthroplasty after a minimum 2-year follow-up. J Neurosurg Spine. 2008;8:101-107.
83 Blumenthal S, Zigler J, Guyer RD, et al. A prospective randomized comparison of cervical disc replacement and anterior cervical fusion. Spine J. 2008;8(Suppl 1):147S-148S.
84 Di Silvestre M, Bakaloudis G, Lolli F, et al. Two-level total lumbar disc replacement. Eur Spine J. 2009;18(Suppl 1):64-70.
85 Park CK, Ryu KS, Jee WH. Degenerative changes of discs and facet joints in lumbar total disc replacement using ProDisc II: Minimum two-year follow-up. Spine (Phila Pa 1976). 2008;33:1755-1761.
86 Kurtz SM, Peloza J, Siskey R, et al. Analysis of a retrieved polyethylene total disc replacement component. Spine J. 2005;5:344-350.
87 Pimenta L, Diaz RC, Guerrero LG. Charite lumbar artificial disc retrieval: Use of a lateral minimally invasive technique. Technical note. J Neurosurg Spine. 2006;5:556-561.
88 Spivak JM, Petrizzo AM. Revision of a lumbar disc arthroplasty following late infection. Eur Spine J. 2010;19:677-681.
89 Brau SA, Delamarter RB, Kropf MA, et al. Access strategies for revision in anterior lumbar surgery. Spine (Phila Pa 1976). 2008;33:1662-1667.
90 McAfee PC, Geisler FH, Saiedy SS, et al. Revisability of the CHARITE artificial disc replacement: analysis of 688 patients enrolled in the U.S. IDE study of the CHARITE Artificial Disc. Spine (Phila Pa 1976). 2006;31:1217-1226.
91 Leary SP, Regan JJ, Lanman TH, et al. Revision and explantation strategies involving the CHARITE lumbar artificial disc replacement. Spine (Phila Pa 1976). 2007;32:1001-1011.
92 Bumpass DB, Keller TC, Robinson EP, et al. Implications of lumbar plexus anatomy for removal of total disc replacements through a posterior approach. Spine (Phila Pa 1976). 2008;33:E274-E278.