Lumbar Spine Disc Replacement
Derrick G. Sueki, Erin Carr and Babak Barcohana
Etiology
Low back pain is a potentially disabling condition with a lifetime prevalence of 60% to 80% in the U.S. adult population. Pain may be due to a simple muscle sprain, sciatica caused by a disc herniation, vertebral fractures, or a number of other conditions, but degeneration of the spine is often the number one cause.
Disc degeneration in the lumbar spine has a significant effect on the functional behavior of the lumbar discs. As the proteoglycan content is lost and the osmotic pressure decreases within the nucleus, there is a diminished ability to retain water within the disc. The loss of volume leads to a reduced disc height, which commonly results in intervertebral foraminal stenosis, inappropriate stress concentrations causing osteophyte formation, and central stenosis. This causes lumbar instability, axial back pain, and radicular leg pain, which can become disabling and chronic, resulting in depression, loss of work, and the inability to enjoy simple activities of daily living.
The goals of lumbar spinal surgery are to alleviate pain, restore stability, and improve neurologic injury. Lumbar disc degeneration is a continuum with a spectrum of etiologic conditions resulting in pain. Based on where in the spectrum the patient falls, various surgical techniques and approaches will be offered. This chapter focuses on artificial lumbar disc arthroplasty.
Surgical Indications and Considerations
Patients with pain related to the lumbar spine have various complaints ranging from axial low back pain, sensation of instability, difficulty bending forward, pain with prolonged sitting or standing, inability to lift heavy items, and/or radicular leg complaints. The goal of the clinician is to identify the cause of the pain.
Various diagnostic tests are employed in the detection of the pain generator. Initially, a thorough history and physical examination are of utmost importance. The goals are to rule out other causes of pain such as infections, tumors, and visceral conditions, which may result in back pain. A careful history is taken regarding trauma, fevers, chills, weight loss, cancer history, and other associated symptoms. Questions regarding the back are also obtained, including timing of injury, aggravating factors, description of pain (sharp, dull, numbness), radicular complaints, and weakness.
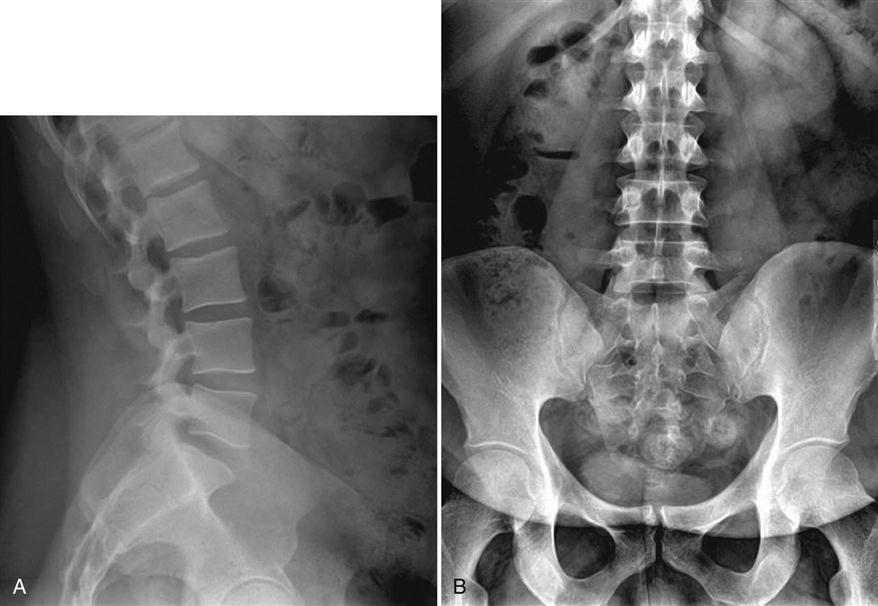
Subsequently, imaging studies are obtained, including plain radiographs (Fig. 17-1), MRI studies, and CT scans. Alignment, disc heights, signs of degenerative changes, and neural compression is noted. Electrodiagnostic studies, bone densitometry, and discograms are occasionally ordered.
Once the diagnosis is established, treatment is initiated. Absent neurologic deficits and unbearable pain, nonoperative treatment is recommended. This may include physical therapy for stretching, strengthening, and modalities. Back braces may be used for short periods of time to allow the muscles to relax. Medications are prescribed, including antiinflammatory medications, pain medications, and muscle relaxants. Acupuncture, chiropractic care, physical therapy, heat, ice, traction, epidural injections, and facet injections may be ordered as well.
When the diagnosis is certain and nonoperative measures have failed, surgery is recommended. Based on the diagnosis, various surgical procedures may be recommended. For example, for an isolated disc herniation, a lumbar microdiscectomy may be considered whereas decompression and instrumented fusion may be considered for an unstable spondylolisthesis.
There are a select group of patients for which lumbar disc arthroplasty is an option. These are patients for whom an isolated decompression or discectomy is insufficient. Additionally, these patients have symptoms arising from a single lumbar disc level with complaints of axial low back pain with disc dysfunction. Based on U.S. Food and Drug Administration (FDA) guidelines, lumbar disc replacement is approved only for one level procedures. These patients would otherwise be candidates for a lumbar fusion procedure.
Criteria for disc replacement includes degenerative disc disease with discogenic back pain isolated to one level from L3-S1 in skeletally mature patients who have failed 6 months of nonoperative treatment. Contraindications include spondylolisthesis greater than grade I, significant instability or facet arthrosis, infection, bony spinal stenosis, allergy or sensitivity to implant, compromised or small vertebral bodies, isolated radicular compression syndromes, or pars defects.
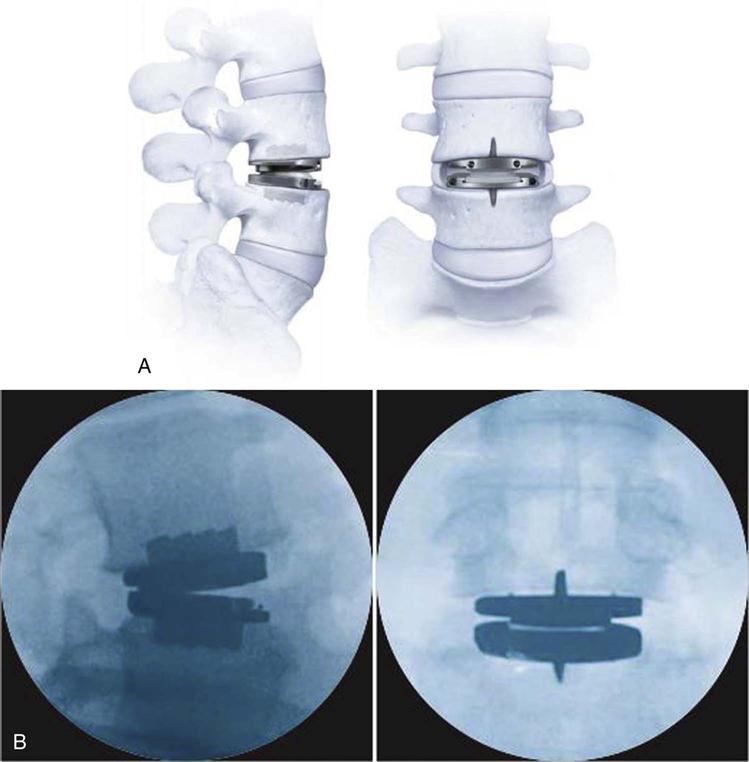
As of the writing of this chapter, two lumbar artificial disc replacement devices have been granted FDA approval in the United States, including Synthes ProDisc-L (Fig. 17-2) and Charité artificial discs. These implants consist of cobalt chromium, titanium, and ultra-high molecular weight polyethylene.

The goal and potential advantages of artificial disc replacement are to maintain motion at the operated level, thereby replicating the biomechanics of the normal disc. This serves to reduce the mechanical forces that would be transmitted to adjacent segments, which are seen with rigid fusion procedures and which may lead to early adjacent segment disease and degeneration. The device would serve to anatomic disc height while maintaining structural integrity. It would need to withstand lumbar forces with long-term stability and endurance. Given that it is not a fusion device but a motion sparing device, pseudarthrosis is not a concern. However, the implant requires integration of bone into its surfaces. There is a potential concern that fusion may still occur at the operated level.
Surgical Procedure
The surgical approach for artificial disc procedures is similar to that performed for an anterior lumbar interbody fusion. Currently available lumbar disc replacement devices are placed from an anterior lumbar approach.
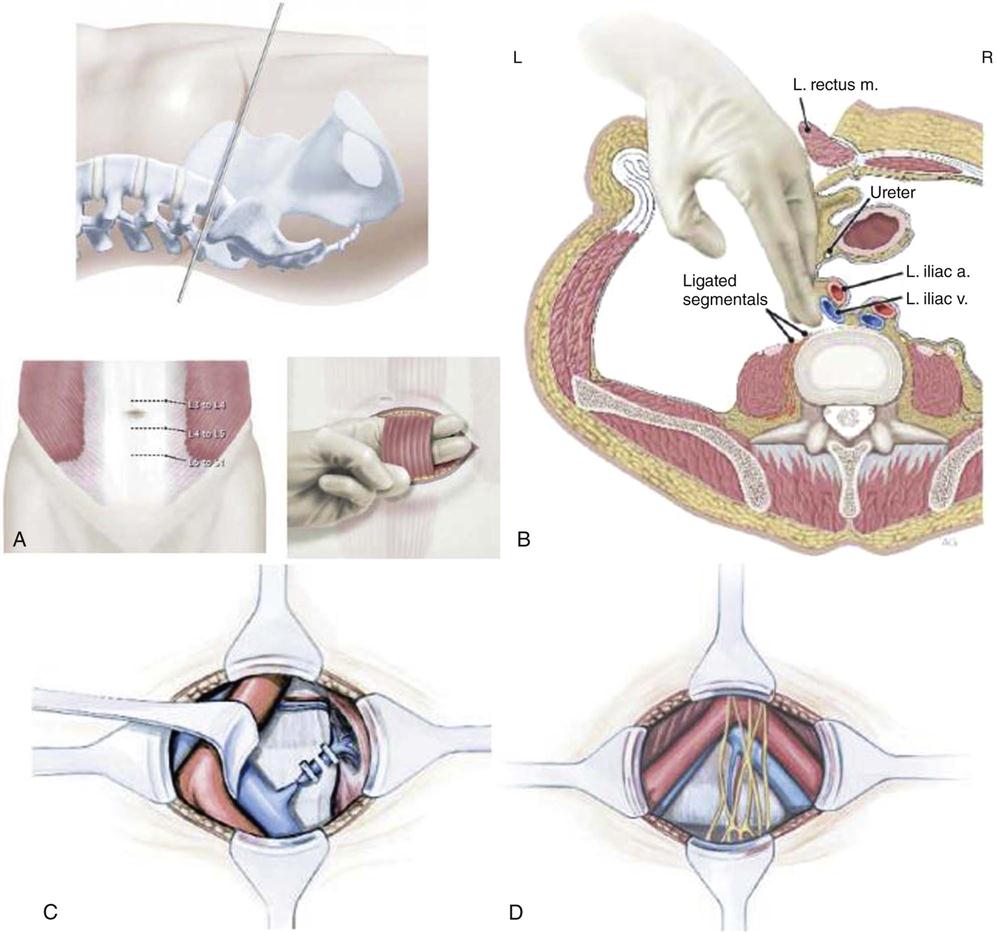
The patient is positioned on the operating room table in a supine position with all bony prominences well padded after induction of general endotracheal tube anesthesia (Fig. 17-3). Once the surgical level is identified with intraoperative C-arm fluoroscopic images, the skin is prepped and draped in the usual sterile fashion. A retroperitoneal approach to the spine is performed. A paramedian transverse or vertical skin incision is made. The rectus sheath is incised and the rectus muscle is retracted laterally. The posterior rectus sheath is encountered and incised to reach the preperitoneal space. The abdominal muscles, including the external oblique, internal oblique, and transverses abdominis, are divided. The transversalis fascia is then divided to allow exposure of the extra retroperitoneal space. The peritoneum and its contents are carefully retracted to allow access to the retroperitoneal space. Here, various neurovascular and visceral structures are encountered, including the ureter, genitofemoral nerve and branches, psoas musculature, aorta, vena cava, sympathetic chain, and iliac vessels (Fig. 17-4).
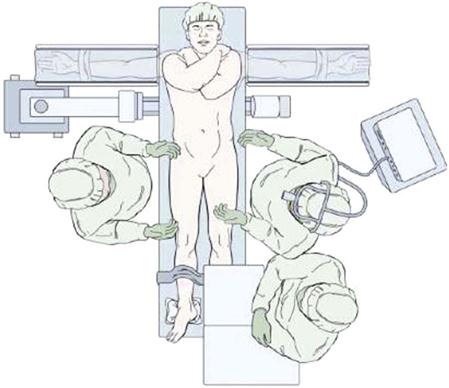
Injury to the sympathetic nerves in males can result in retrograde ejaculation. Deep venous thrombosis may occur in addition to injury to any of the neurovascular and visceral structures in this approach. Although many spine surgeons perform the anterior approach themselves, often general or vascular surgeons are employed to access the spine.
Once the spine is exposed and the adjacent structures are protected, a radical discectomy is performed. If necessary, neural decompression may also be performed. Next, various trials are placed to measure the size of the implant. Great care is taken to position the implant properly to maintain the appropriate center or rotation in the spine (Fig. 17-5). Multiple intraoperative images are obtained in addition to direct visualization to achieve this. The final implant is placed and is evaluated, ensuring that it is rigid with good contact (Fig. 17-6). The instruments and retractors are removed and a meticulous layered wound closure is performed.
Complications from this procedure include vascular injury, ureteral injury, wound infection, postoperative ileus, neurologic injury, dural tear, deep venous thrombosis, retrograde ejaculation, vertebral fracture, hardware failure or migration, subsidence, malpositioned implant, or fusion. The complication rate is reported to be less than 10%.
The outcomes of lumbar artificial disc replacements have been quite favorable. The results have been similar to lumbar fusion results with respect to functional outcomes and pain relief. Further research is necessary to determine whether disc replacement surgery reduces the rate of adjacent segment disease as compared with fusion procedures, but the early data are promising.
Physical therapy is key after all lumbar spinal procedures to strengthen and increase the flexibility of the spine with decreasing postoperative scar formation. Signs of infection should be watched for in the immediate postoperative period. If the patient exhibits increased pain, loss of pulses, leg pain, lower extremity swelling, or changes in neurologic examination, the physician should be contacted.
Various disc replacement products are being developed, not only for anterior approaches but also for placement through lateral or posterior approaches, which would eliminate the risks associated with the anterior approach. Research is being performed to evaluate various nucleus replacement devices to either replace or rejuvenate the nucleus of the disc. This will significantly alter the approach to and treatment of spinal related conditions.
Lumbar Disc Replacement Surgery
Therapy Guidelines for Rehabilitation
The lumbar spine can be one of the most challenging regions of the body to treat. There are many factors associated with the lumbar spine that contribute to the challenge of this region. Anatomically, the lumbar spine consists of 5 moving spinal segments and 10 articulating joints. Multiple ligaments give the region its passive stability while multiple muscles provide the active stability of the region. The nerve roots of the cauda equina run through the spinal canal in the lumbar region and exit through the intervertebral canal. These are just a few of the structural components of the lumbar spine that must work in concert to provide for pain-free and seamless movement within the region.1–3 Biomechanically, the lumbar spine is designed to provide motion as well as stability. It is a transitional zone that allows upper body motion on a relatively fixed sacrum. The sacrum in turn will transition the weight of the central axis outward into the hips and lower extremities.
The concept of replacing a lumbar disc is not new. Attempts were made in the 1950s and 1960s, but both attempts failed to produce successful results.4 In East Germany during the early 1980s, Shellnac and Buttner-Jans designed the first successful artificial disc, the SB Charité disc. Since developed, the artificial disc and surgical technique have been used in Europe, yet it wasn’t until 2004 that the SB Charité disc was even approved by the FDA in the United States. By comparison, in 1911, lumbar fusion, or arthrodesis, was first employed in the United States and is still considered the gold standard for lumbar surgery. Rehabilitation following lumbar fusion/arthrodesis has been well established. Clinical guidelines and empirical data validating rehabilitation have also been generated for the surgery. In comparison, very few clinical guidelines have been established for lumbar disc replacement surgery and no research currently exists validating any of the suggested protocols. The guidelines that follow will be a synthesis of established tissue healing guidelines, protocols for similar spinal surgeries, and treatment geared specifically for the attributes unique to lumbar disc replacement. They are not meant to replace or supplant clinical reasoning processes. Instead they are meant as a supplement to clinical reasoning and decision-making. Each patient who has undergone total lumbar disc replacement surgery is unique. The guidelines presented should be used as a point of departure from which the clinician can customize the program to the individual’s needs.
Principles of Tissue Healing5,6
A clinician must have a firm grasp of the tissue healing process if they are to effectively rehabilitate any patient. Variation exists in the categorization of healing; some clinicians prefer to use a system based upon symptom acuity. Acute symptoms are present for the first 3 weeks immediately following injury. The subacute phase begins at 3 weeks and continues until 2 to 3 months after injury. Symptoms lasting longer then 2 to 3 months are considered chronic. Conversely, other systems of classification are based upon the physiologic goal of the phase. This type of physiologic based system of classification will provide the framework of this chapter.
Phase 1 is considered the inflammation phase and is so named because of the phase’s physiologic goal of producing inflammation within the injured area. Inflammation is the body’s initial response to any injury or surgery. Immediately after surgery, the body begins the process of repair. Inflammation occurs and intensifies in the surgical region over the course of the next several days and reaches its peak production within the first 72 hours after injury. The generation of acute inflammation is generally completed within 14 days and during these first 14 days, several events occur.6,7 Clinically, rehabilitation during the inflammation phase of tissue healing should focus upon the prevention of blood loss, reduction of inflammation, and managing the pain that accompanies tissue damage.
The second phase of tissue healing is the reparative phase. The chief physiologic goal of this phase is to repair the injured tissue. Chronologically, this phase begins immediately after injury and concludes around 21 days after injury, running concurrently with the inflammation phase of healing. It is valuable for the clinician to know the exact surgical technique used by the surgeon. With the disc replacement surgery, the injured tissue is actually removed and replaced with an artificial disc. Healing of the disc is not an issue in this case. Instead reparation focuses on providing an environment of healing for the tissue that was incised in the process of replacing the disc. The surgical technique will influence the rehabilitation. The primary function of this phase is the formation of the dense connective scar tissue needed to repair the wound and reestablish structural continuity of the affected region. Most of the actual dense connective tissue development is completed by day 21. Clinically, the goal of rehabilitation in this phase should be to promote the development of the new dense connective reparative tissue.
The final phase of the healing process is the remodeling phase. The main purpose of this phase of healing is to strengthen the newly formed dense connective scar tissue. Classically, this phase is divided into two subphases, the consolidation subphase and the maturation subphase. While the purpose of the two subphases is essentially the same, they are characterized by several key factors. During the consolidation subphase, tissue is being formed and converted. Therefore, there are large quantities of fibroblast and angioblast cells present within the tissue. This subphase lasts from 22 to 60 days. Strengthening of the newly formed connective tissue should be the goal during this subphase. Care must be taken during this phase so as not to exceed the mechanical limits of the newly formed tissue, as overstress to the tissue will result in tissue injury and delayed healing. The second subphase, the maturation subphase, occurs from day 60 to 360 and is hallmarked by dense connective scar tissues that are fully fibrous in nature. For this reason, a progression in the strengthening of the affected tissues may begin more aggressively. As in the consolidation subphase, a rehabilitation programs must provide appropriate levels of stress to encourage dense connective scar tissue formation without creating or exacerbating tissue injury.
Summary Statement
Although guidelines can provide generalized timeframes for healing and recovery, it is important to realize that a firm grasp of the factors listed above will enable the clinician to individualize the rehabilitation program for each patient (also consideration is always given to the patient’s signs and symptoms). No two patients are identical. Therefore, no two rehabilitation programs should be identical. Solid clinical reasoning regarding the patient and the nature of his or her injury and surgery will ultimately drive the rehabilitation process. Table 17-1 summarizes the soft tissue healing timeframe for all three phases of healing. Adequate muscle activity and protection must accompany the healing process to progress activity levels. Healing tissues may be compromised because of increased levels of strain without adequate muscle support and protection.
TABLE 17-1
Soft Tissue Healing Timeframes
| Phase | Events | Timeframe |
| Phase I: Inflammation | Vasoconstriction in immediate area Vasodilation in surrounding areas Wound closure Removal of foreign and necrotic tissue |
0-14 days |
| Phase II: Reparative | Fibroblasts enter region to create dense connective tissue scars Angioblasts enter the region for revascularization |
0-21 days |
| Phase IIIa: Remodeling | Dense connective tissue is converted from cellular to fibrous | 22-60 days |
| Phase IIIb: Remodeling | Dense connective tissue is strengthened | 61-84 days |
| Phase IIIc: Remodeling | Dense connective tissue is strengthened | 85-360 days |
Data from Nitz A: Soft tissue injury and repair. In Placzek J, Boyce D, editors: Orthopaedic physical therapy secrets, Philadelphia, 2001, Hanley and Belfus; Frenkel S, Grew J: Soft tissue repair. In Spivak J, et al, editors: Orthopaedics: A study guide, New York, 1999, McGraw-Hill.
Attributes Unique to Lumbar Disc Replacement Surgery
The gold standard for surgical treatment of chronic low back pain is the lumbar fusion surgery. But like all surgeries, no surgical technique has 100% success rate and in the case of lumbar fusion surgery, 20% of patients will require additional surgery within 5 years after the initial surgical technique.8–12 See Box 17-1 for indicators that lumbar disc replacement surgery may be required. The most common reasons for failure of the surgery are bone graft donor morbidity, the formation of pseudoarthrosis, and adjacent spinal segment degeneration. One of the major factors believed to be associated with these failure factors is the loss of normal lumbar biomechanics following spinal fusion. The lumbar disc replacement surgery has been designed to eliminate these factors. The disc replacement is designed to maintain normal spinal biomechanics at the surgical site, decompression of the lumbar facets and neural structures, and restore the normal disc height between spinal segments.13–16 The disc replacement surgery is performed anteriorly and requires incisions through the rectus abdominis and the anterior aspect of the disc space. Following surgery, these two structures are weak and vulnerable to injury. Rehabilitation programs should address the unique aspects of this surgery and interventions designed accordingly.
Description of Rehabilitation and Rationale for Using Instrumentation
Phase I: Inflammatory Phase
TIME: Weeks 1 to 2 (Days 0 to 14)
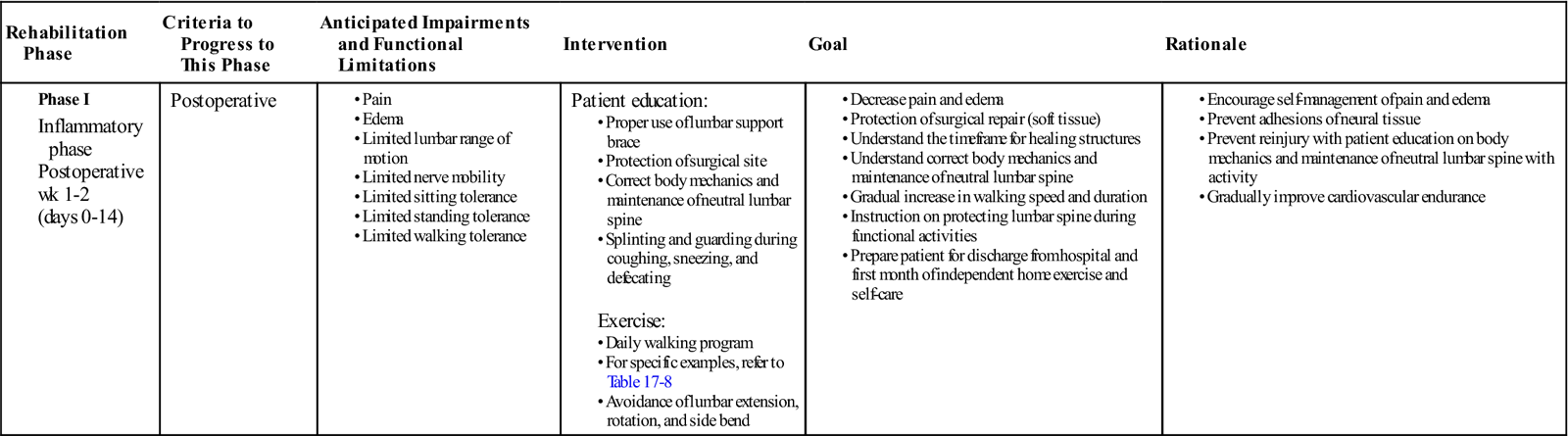
GOALS: Protection of the surgical site, decrease pain and inflammation, initiate patient education regarding neutral lumbar spine mechanics, begin walking program (Table 17-2)
TABLE 17-2
< ?comst?>
| Rehabilitation Phase | Criteria to Progress to This Phase | Anticipated Impairments and Functional Limitations | Intervention | Goal | Rationale |
|
Phase I |
Postoperative
|
Patient education:
Exercise:
|
|
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
Hospital Rehabilitation.
Immediately following surgery, the goals while in the hospital should focus on patient education, protection of the surgical site, reduction in pain and inflammation, and restoration of independent activities of daily living. The normal hospital stay is between 5 to 7 days, with discharge either to a home environment or a skilled nursing facility.14,15
While in the hospital setting, the patient will be instructed in how to protect the surgical site. This is accomplished by instructing the patient on maintaining proper neutral spine during motion. Additionally, a lumbar stabilization brace is issued to the patient for additional support and protection. Instructions regarding the duration of its use are determined by the physician and may vary on a case-by-case basis. Log rolling and abdominal bracing techniques are used to get into and out of bed, and transitioning from supine to a sitting position. Care should be taken to avoid overstressing the abdominal muscles since the rectus was surgically incised and is subsequently weak and subject to tearing or injury. Initial examination and evaluation should include assessment of the wound, hip passive range of motion (ROM) testing, bed mobility, and gait assessment.
Hospital rehabilitation should also include gentle abdominal activation/core strengthening. The goal is not strength, but muscle recruitment. Circulation exercises are also incorporated early in the rehabilitation process. Ankle pumping exercises and thromboembolic hose hose stockings are used to prevent pooling of blood in the lower extremities. Diaphragmatic breathing exercises can be used to mobilize the abdominal muscles and abdominal contents to stimulate the lymphatic system and encourage circulation. Since the abdominal region is the site for most of the surgery, it is not uncommon for inflammation and edema to accumulate in the abdomen.
Weight-bearing activities should also begin early in the rehabilitation process. Sit to stand and gait activities should be initiated. Initially, standing and gait training will be accomplished with the aid of a front wheel walker. By the end of the hospital stay, the patient should be ambulating with the aid of a single point cane. Ambulation to and from the restroom should begin immediately with assistance as needed. These activities should progress until the patient is independent. Gentle lumbar spinal ROM can also be initiated in the hospital. Lumbar flexion exercise is the only direction of motion allowable initially. Because the disc space and abdominal cavity was incised anteriorly, it is the weakest portion of the body. Overstressing these tissues should be avoided. ![]() The clinician should avoid excessive and repetitive extension exercises, as well as lateral flexion and rotation. These precautions are generally in place for 6 to 8 weeks. Prone lying should also be avoided during this time period because of weakness and sensitivity of the anterior tissues.
The clinician should avoid excessive and repetitive extension exercises, as well as lateral flexion and rotation. These precautions are generally in place for 6 to 8 weeks. Prone lying should also be avoided during this time period because of weakness and sensitivity of the anterior tissues.
Before discharge from the hospital, it is important that the clinician educates the patient on proper lumbar spine mechanics during activity and the need to avoid excessive trunk extension, side bend, or rotation. Refer to Box 17-2 for specific patient guidelines to follow after discharge. The patient should be advised to refrain from heavy lifting, bearing down during defecation, and abdominal splinting during coughing or sneezing. Before discharge, the need for a continued home exercise program should also be addressed. Patients can be discharged once they are able to walk unassisted or with minimal assistance depending on their postsurgical care and rehabilitation plans. They also must be free of complications, have normalized their bowel and bladder function, and show a good understanding of their surgical precautions and activity limitations.
Initial Posthospital Rehabilitation.
The second postoperative week will occur at home or a skilled nursing facility. Activities during this later stage of the inflammation phase are a continuation of the care received while in the hospital. During this time, activities should center on resuming protected normal daily activities. The patient should be encouraged to increase their daily sitting, standing, and walking tolerances. Pain and fatigue should guide the progression. The lumbar stabilization belt should be worn 24 hours a day unless otherwise ordered by the physician. Patient exercises may progress. The patient can begin gentle neutral spine lumbar stabilization exercises. Once again care must be taken to avoid overstressing the abdominal muscles. The goal is muscle recruitment, not strengthening. The patient may begin gentle lower extremity strengthening exercises, but care must be taken to stabilize the lumbar region. The clinician should keep in mind throughout this phase that the primary goal of this phase of rehabilitation is protection of the surgery, pain abatement, and restoration of protected daily activities.
Phase II: Reparative Phase
TIME: Week 3 (Days 0 to 21)
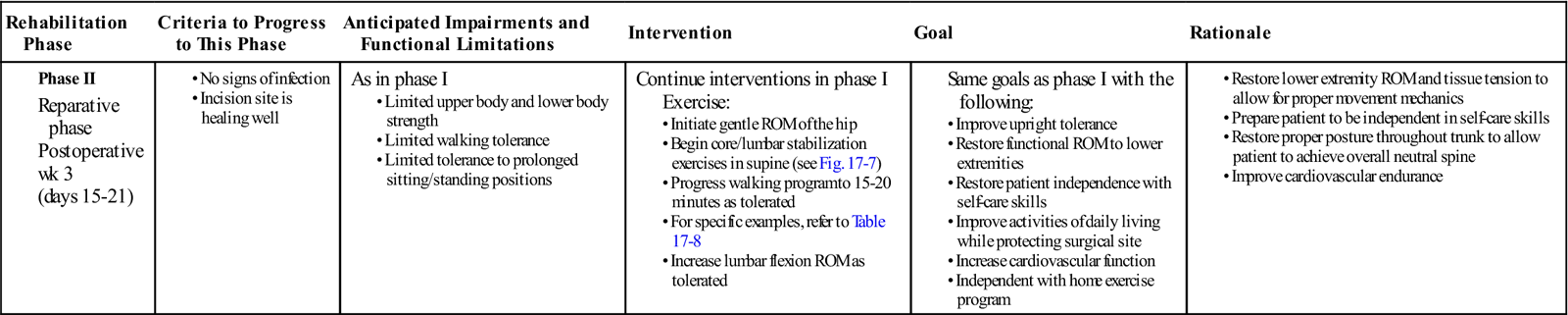
GOALS: Understand neutral spine concepts, increase lower extremity mobility, improve upright tolerance, improve protected activities of daily living, increase cardiovascular function (Table 17-3)
TABLE 17-3
< ?comst?>
| Rehabilitation Phase | Criteria to Progress to This Phase | Anticipated Impairments and Functional Limitations | Intervention | Goal | Rationale |
|
Phase II |
As in phase I
|
Continue interventions in phase I
Exercise: |
|
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
In many instances, phase II of the rehabilitation process will take place independently in the patient’s home. Home therapy is rarely indicated. Therefore, education regarding patient progression through the first month following surgery is an important aspect of hospital care. The clinician’s advice and instructions will be followed for the next 3 to 4 weeks. During the reparative phase of tissue healing, the body begins to form and lay down scar tissue at the surgical site, thus enhancing the integrity of the musculatures, ligaments, and capsule to withstand gradual increases in loads to the tissues. Therefore, as time progresses, increasing load can be placed upon the surgically repaired tissue. Rehabilitation should be a continuation of phase I and progress restoring lower extremity ranges of motion and independence with self-care skills. Movement improves circulation and prevents the formation of scar tissue adhesions between the nerve and the healing tissue surrounding the surgery. Following lumbar disc replacement surgery, scar tissue formation is inevitable in and around the surgical site. In certain instances, scar tissue can adhere to surrounding tissues, impacting mobility of any structure to which it attaches. Therefore, movement of the lower extremity and lumbar region should be encouraged to promote circulation and prevent adhesion formation. Throughout all activities and exercises, the patient should be encouraged to maintain a neutral lumbar spine. Activities should not increase symptoms. Protection of the surgical site and proper immobilization should continue until the physician has seen evidence that the prosthetic is well situated. At this time, the physician will approve additional lumbar motion and activities.
Phase IIIa: Remodeling Phase
TIME: Weeks 4 to 8 (Days 22 to 60)
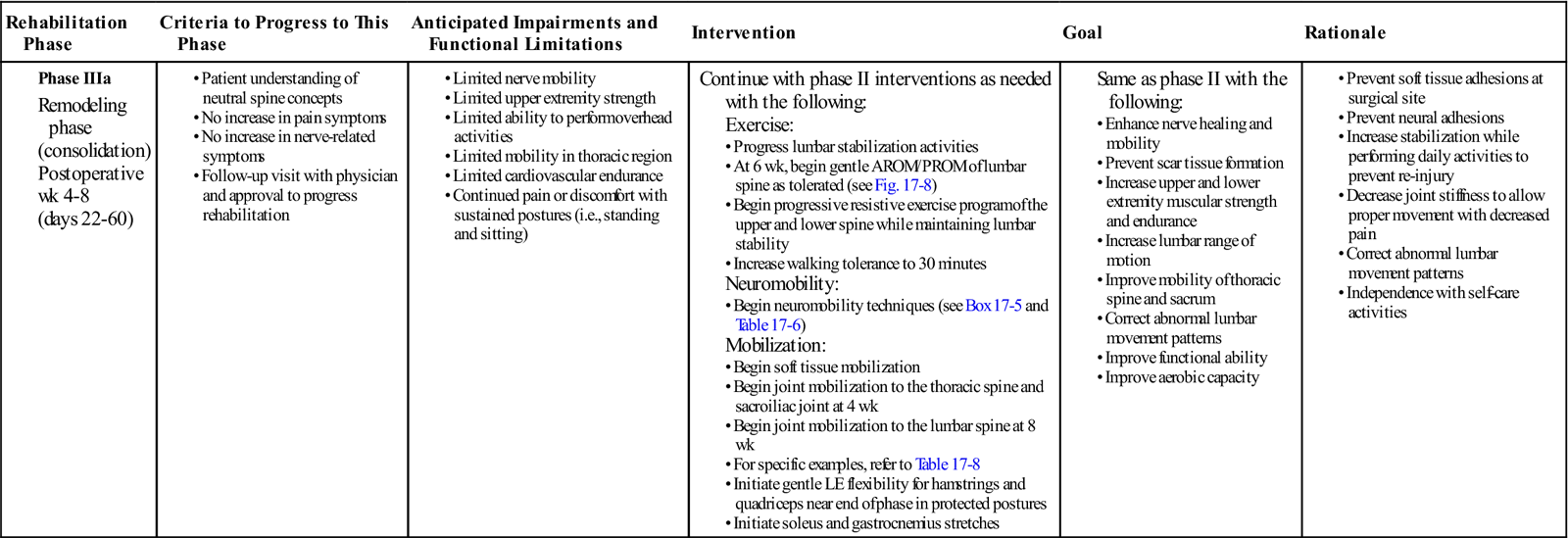
GOALS: Enhance nerve healing and mobility, prevent scar tissue formation, increase lower extremity strength and endurance, improve thoracic spine and sacral mobility, begin normalization of functional daily activities, restoration of lumbar ROM (Table 17-4)
TABLE 17-4
< ?comst?>
| Rehabilitation Phase | Criteria to Progress to This Phase | Anticipated Impairments and Functional Limitations | Intervention | Goal | Rationale |
|
Phase IIIa |
Continue with phase II interventions as needed with the following:
Exercise: |
|
|
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
AROM, Active range of motion; LE, lower extremity; PROM, passive range of motion.
Between the end of the fourth week and up to the sixth postoperative week, the patient’s physician will reassess the patient. Generally, this reassessment will include a new radiographic study. Most physicians will release the patient to begin outpatient rehabilitation following this reassessment. This decision will be dependent upon several factors, including patient symptoms and function. At 4 to 6 weeks following surgery, it is anticipated that the patient will continue to have mild (possibly moderate) low back pain and achiness. This will most likely be present in the morning and at the end of the day. Functionally, the patient should be walking limited community distances with a single point cane for balance. Neurologic symptoms that are the result of spinal compression or inflammation should be improving and stabilizing. Objectively, the physician will order a spinal radiograph to assess the position of the prosthetic. If all of these factors are acceptable, the physician will allow the patient to begin outpatient rehabilitation.
The first postoperative outpatient examination should include evaluation of the patient’s scar, assessment of posture and gait, balance testing, and active range of motion assessment. ROM can be tested in all directions of motion, but end range extension, rotation, and side bending must be avoided for 6 weeks. Additionally, the clinician should conduct a neurologic examination if nerve involvement is suspected. The clinician should also assess the patient’s lumbar soft tissue, looking for muscle guarding and atrophy. Quick screening of the patient’s lower extremity should also be completed. Once the patient has been screened and deemed appropriate for phase III rehabilitation, the clinician can begin to design his or her treatment plan.
Postural Rehabilitation.
Upon initial evaluation, observation of the patient’s posture will give the physical therapist a significant amount of information concerning weakness, elongation, and strength of specific musculature as well as the patient’s ability to maintain a neutral lumbar spine. According to Janda, a common postural alignment seen in people with lower quarter pathology is known as the lower cross syndrome.17 Regardless of the cause, this alignment will consist of a lower quarter muscle pattern in which certain muscles will be weakened and lengthened and others will be strong and shortened, resulting in an increased lumbar lordosis and increased hip flexion. More specifically, there is a weakening and lengthening of the gluteal and abdominal muscles. This is combined with a tightening and shortening of the hip flexors and lumbar extensors. Although a very common posture following spinal surgery, this position is not advisable for the patient because it places the lumbar spine in an extended or lordotic position. During gait, the clinician may notice that the patient walks with a shortened stride length because with long strides the spine is further extended during the terminal stance phase of gait if tight hip flexors are present. ![]() Physiologically, lumbar extension should be avoided because of the increased stress it places upon the prosthetic and the weakened anterior musculature.
Physiologically, lumbar extension should be avoided because of the increased stress it places upon the prosthetic and the weakened anterior musculature.
Postural rehabilitation should be implemented and interventions should focus upon the stretching of shortened hip flexor and lumbar extensor muscles and strengthening of the weakened gluteal and abdominal muscles of the lumbar region. ![]() Posturally, the patient should be instructed to avoid anterior pelvic tilt that will lead to increased lumbar lordosis.
Posturally, the patient should be instructed to avoid anterior pelvic tilt that will lead to increased lumbar lordosis.
Therapeutic Exercise.
While no research or clinical practice guidelines have been developed specifically for lumbar disc replacement surgery, systematic reviews and clinical practice guidelines have been developed for the rehabilitation after lumbar disc surgery and can be extended to rehabilitation after lumbar disc replacement surgery. While most studies are mixed in terms of intervention efficacy, the one intervention that is uniformly beneficial is therapeutic exercise.
Lumbar Stabilization.
Core stabilization, lumbar stabilization, transverse abdominis training, and multifidus training are all rehabilitation programs developed to activate local muscle groups, stabilize the lumbar region, and normalize the recruitment of lumbar musculature. Normal muscle activity involves a coordinated recruitment of both local and global muscle groups. Local muscles work to stabilize the region while global muscles function as movers of the body. In the lumbar spine, the local muscles, such as the multifidus and transverse abdominis, engage to stabilize spinal segments. The global muscles, such as the quadratus lumborum and hip flexors, function as primary movers of the lumbar region. When injury occurs, local muscle groups are inhibited, requiring global muscles to activate and stabilize the region. Theoretically, localized inflammation inhibits neuromuscular control systems. Richardson and associates performed a series of studies on the ability of deep lumbar muscles to stabilize spinal segments in patients with lumbar pain.18 Their findings suggest that deep muscle activation is a necessary component in the reestablishment of spinal control following a low back injury. Subjects that did not reestablish segmental control continued to experience low back pain. Therefore, whether the clinician chooses to use core stabilization, lumbar stabilization, transverse abdominis training, or multifidus training, the program should have a component of deep local muscle activation. Once the local muscle can be recruited, rehabilitation should progress to coordinating local and global muscle activation. Exercises focused upon the recruitment of local muscle groups are appropriate for this phase of healing.19–21 See Fig. 17-7 for examples of therapeutic exercises appropriate for this phase of healing.
Stretching.
Following most surgeries, tissue mobility in and around the surgical area will be tight and restricted. In the presence of tissue injury or damage, muscles play a primary role of protection. Muscle tightness is commonly found in the hip flexors, quadratus lumborum, and the erector spinae of disc replacement patients. Stretching and ROM exercises should target these muscles, because normalization of muscle length is a key component of the restoration of muscle function and of normal lumbar mechanics. Normalization of lumbar motion should occur by 8 weeks following disc replacement surgery. Although individual variations will occur, by 6 weeks, the patient should have exercises that actively and passively promote normal spinal motion in all directions, including rotation, side bend, and extension. Care should be taken when initiating each of these motions, and patients should be advised to stretch slowly and within pain tolerances. All stretches should be pain free. See Fig. 17-8 for examples of therapeutic exercises appropriate for this phase of healing. Typical ROM exercises for this phase of rehabilitation include single knee to chest, seated flexion, prone press ups, prayer stretch, supine piriformis stretch, hip flexor stretch, supine and seated trunk rotations, and lateral side bend exercises. See Boxes 17-3 and 17-4 for normal sequencing of motion during lumbar flexion and for normal ranges of motion in the lumbar spine during lumbar flexion.
Soft Tissue Mobilization.
As a lone intervention, massage and soft tissue mobilization have been shown largely to be ineffective at reducing a patient’s symptoms or improving a patient’s functional capacities. When used in conjunction with other intervention, soft tissue mobilization can be an effective adjunct to allow the body to recover mobility and function. Muscles will contract to protect any area of the body vulnerable to injury. Chronically, this protective contraction can result in postural changes and these postural changes can result in abnormal forces being placed upon normal tissue. Over time these alterations in posture and loads can result in tissue breakdown and pathology. Retraining and maintaining muscles and soft tissue tension is an important aspect of normalizing lumbar motion. Soft tissue mobilization to the hip flexors, quadratus lumborum, and the erector spinae can be used effectively as an adjunct to normalizing lumbar motion and mechanics.
Joint Mobilization.
Normalization of the biomechanics of the lumbar spine should be a large consideration in any rehabilitation program that is developed for disc replacement surgery. Mobilization techniques have taken on increased prominence in rehabilitation programs. In practice, they are used to increase ranges of motion within targeted regions by moving specific joints or specific muscles. Care must be taken in choosing the appropriate time to begin implementation of joint mobilization techniques because of the potential translational effect they may have on the lumbar spine and more specifically at the replacement site. Research has studied the effects of a posterior to anterior force placed on the spinous process of L3. Studies by Lee et al showed a force at L3 could result in movement as far away as T8, and in follow-up studies by the same group, the same posterior to anterior force resulted in an anterior rotation of the sacrum.22–25 The implications of these findings for the patient following a lumbar disc replacement surgery is that even mobilizations to distant segments may have a translatory impact upon the surgical site. Initially, the clinician must use caution when directly mobilizing the lumbar spine.![]() The anterior aspect of the joint and the prosthetic are particularly vulnerable to injury. Posterior to anterior mobilization of the lumbar spine is not advisable for the first 8 weeks following surgery. After that time, the lumbar spine can be mobilized in this direction. Posterior to anterior mobilization should focus on segments adjacent to the prosthetic. Mobilization of adjacent segments can be used to normalize motion in these segments and decrease the demands placed upon the replacement site.
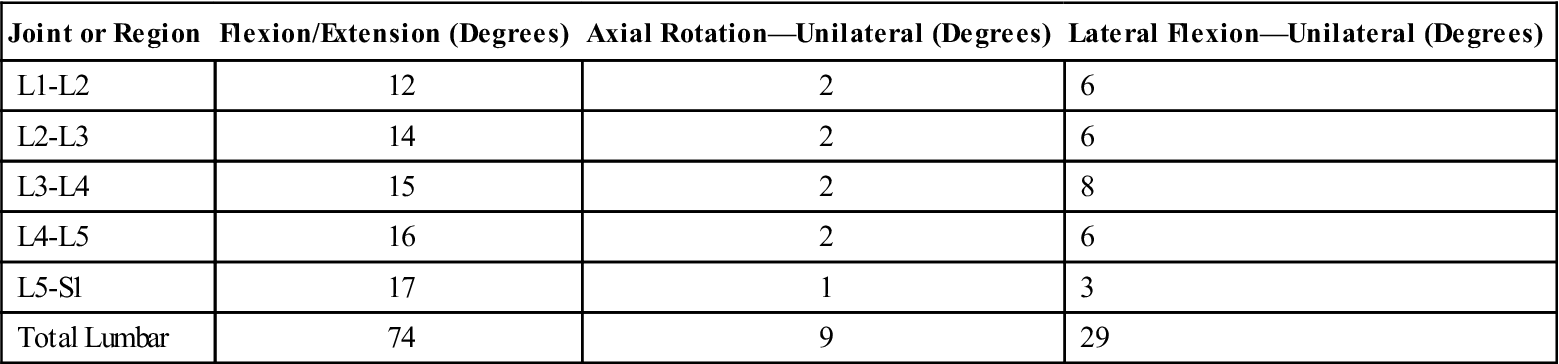
The anterior aspect of the joint and the prosthetic are particularly vulnerable to injury. Posterior to anterior mobilization of the lumbar spine is not advisable for the first 8 weeks following surgery. After that time, the lumbar spine can be mobilized in this direction. Posterior to anterior mobilization should focus on segments adjacent to the prosthetic. Mobilization of adjacent segments can be used to normalize motion in these segments and decrease the demands placed upon the replacement site. ![]() There is no need to mobilize the segment with the prosthetic in a posterior to anterior manner since the prosthetic is a fixed unit and posterior to anterior motion of the prosthetic does not occur. The more appropriate mobilizations for the prosthetic segment are techniques focused on improving segmental flexion, extension, and rotation, for these are the motions provided by the new prosthetic. Mobilization in flexion can begin before mobilization into other ranges of motion. As with any mobilization, the clinician should use patient symptoms and status as a guide for how much mobilization to use. See Table 17-5 for normal ranges of motion for lumbar spinal segments.
There is no need to mobilize the segment with the prosthetic in a posterior to anterior manner since the prosthetic is a fixed unit and posterior to anterior motion of the prosthetic does not occur. The more appropriate mobilizations for the prosthetic segment are techniques focused on improving segmental flexion, extension, and rotation, for these are the motions provided by the new prosthetic. Mobilization in flexion can begin before mobilization into other ranges of motion. As with any mobilization, the clinician should use patient symptoms and status as a guide for how much mobilization to use. See Table 17-5 for normal ranges of motion for lumbar spinal segments.
TABLE 17-5
Approximate Range of Motion for the Three Planes of Movement for the Joints of the Lumbar Region
< ?comst?>
| Joint or Region | Flexion/Extension (Degrees) | Axial Rotation—Unilateral (Degrees) | Lateral Flexion—Unilateral (Degrees) |
| L1-L2 | 12 | 2 | 6 |
| L2-L3 | 14 | 2 | 6 |
| L3-L4 | 15 | 2 | 8 |
| L4-L5 | 16 | 2 | 6 |
| L5-S1 | 17 | 1 | 3 |
| Total Lumbar | 74 | 9 | 29 |
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
Adapted from White AA III, Panjabi MM: The basic kinematics of the human spine: A review of past and current knowledge. Spine 2:12, 1978; White AA, Panjabi MM: Clinical biomechanics of the spine, Philadelphia, 1990, Lippincott.
Lumbar pain and pathology can lead to or be the result of movement dysfunction and compensations in other regions of the body. While mobilization of the lumbar spine may not be advised immediately because of postsurgical tissue weakness, mobilization of regions adjacent to the lumbar spine is appropriate. Initially, the clinician can begin to mobilize the thoracic spine or sacrum. Decreased flexibility in thoracic spine segments and the soft tissue of the thoracic region may prevent proper body alignment, including normal lumbar lordosis. Thus treatment should include soft tissue mobilization to the thoracic spine musculature and passive joint mobilization techniques to the thoracic spine.26,27 Mechanics and proper functioning of the sacrum can also directly impact the functioning of the lumbar spine. The sacrum is required to provide a stable base from which the rest of the spine can move. The sacrum and sacroiliac joint are an important link between the lower extremities and the spine. If they are not functioning correctly, the forces are transmitted to the lumbar spine. Mobilization of the thoracic and sacral regions can help to alleviate the pressure placed upon the disc. Both mobilizations are an appropriate early intervention for this phase of rehabilitation.
Neural Mobilization.
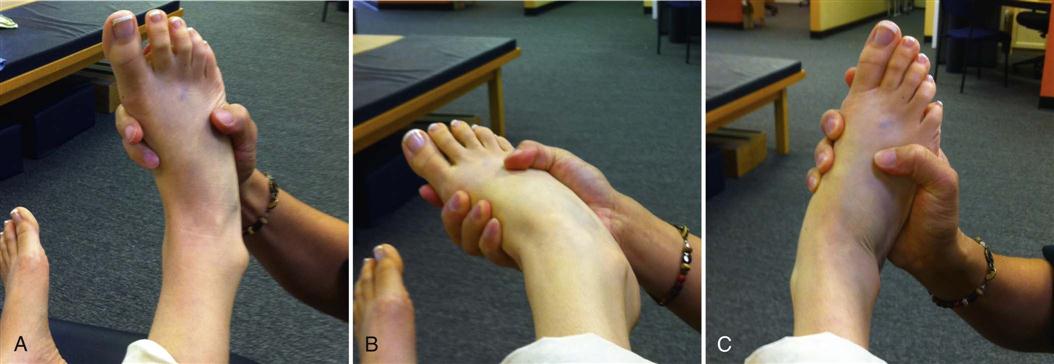
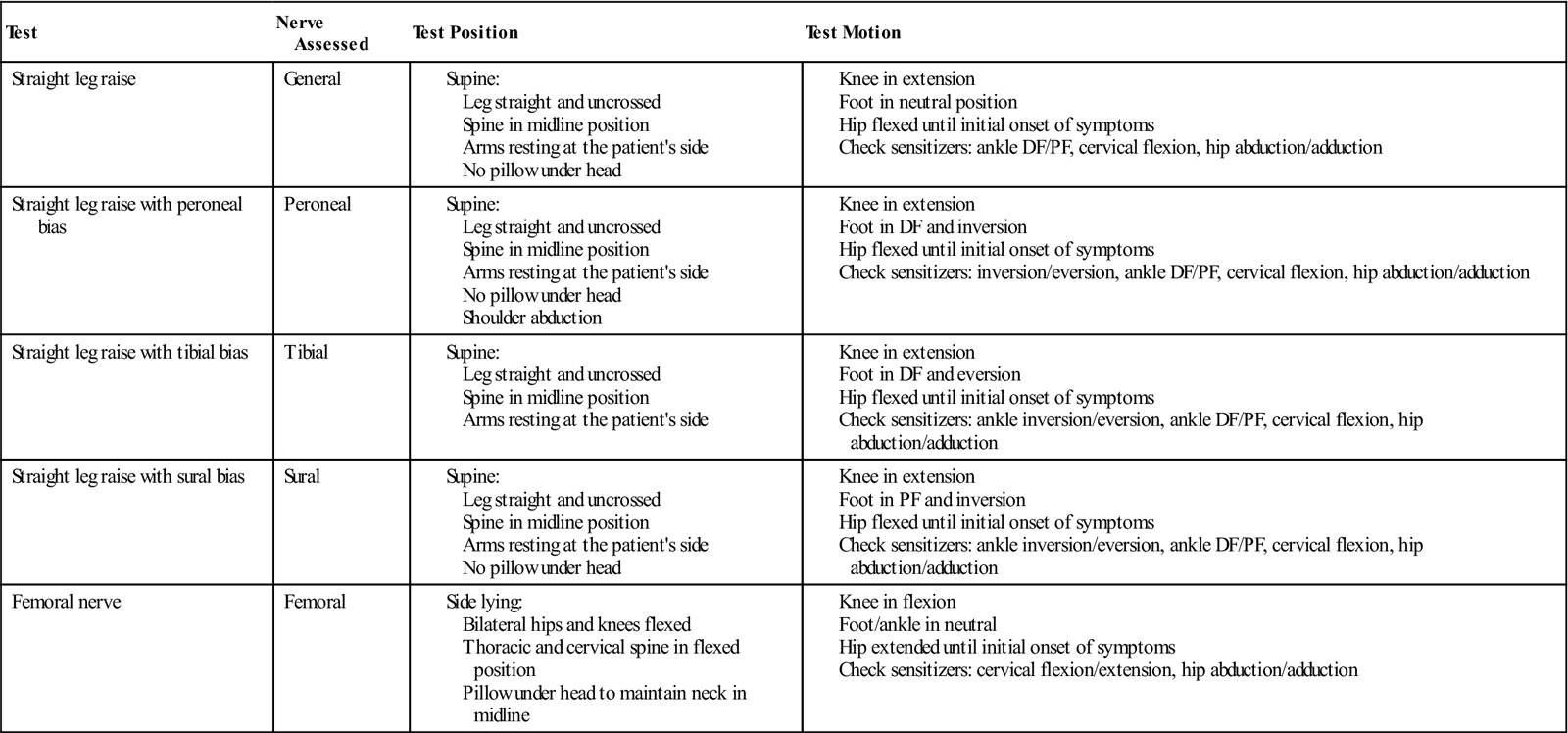
At this phase of rehabilitation, neural mobilization should show progress. The clinician should assess the mobility of neural structures. The common base test for neural mobility is the straight leg raise test. This test can be used as a starting point from which the clinician can test specific peripheral nerves of the lower extremity. Fibular, tibial, sural, and femoral all have specific test positions that place the nerve into a position of tension.28 See Figs. 17-9 through 17-11 for examples of neural mobility testing and testing sequences. If the nerve does not glide through its surrounding tissue, the nerve is stretched. In response to the tensile load, symptoms such as numbness, tightness, and tingling are produced. These symptoms lessen when tension is removed from the nerve. A positive test is indicative of restricted mobility in the nerve being tested. Using the test position to stretch the nerve and release any adhesions along its course is a common treatment philosophy. See Box 17-5 and Table 17-6 for lower limb nerve test positions and methods. Unlike muscles, neural tissue is not as elastic and responds adversely to stretching.29 Neural mobility techniques are commonly classified into two categories: techniques that glide the nerve and techniques that stretch the nerve. Sliding techniques produce a greater amount of nerve excursion through the surrounding tissue than tensioning techniques. Joint motions can influence the mobility of the nerve.30,31 Muscle activity in the test leg also increases when the ankle is placed in a tensile position.32,33 It has been hypothesized that increased muscle activity could indicate the muscles plays a protective role with regard to nerve mobility testing. When the nerve is placed in tension, muscles are recruited to protect the nerve and prevent injury to the structure. Nerves are sensitive structures and can be easily damaged.
TABLE 17-6
Lower Limb Nerve Testing Positions
< ?comst?>
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
DF, Dorsiflexion; PF, plantar flexion.
Adapted from Butler D: The sensitive nervous system, Adelaide Australia, 2000, Noigroup Publications.
![]() Treatment, therefore, should address the gliding and not stretching of nerves. Before gliding the nerve, soft tissue structures along the course of the nerve can be mobilized to allow for nerve mobility. Movement of the lower extremity can be combined with small movements of the neck to encourage gliding of the nerve rather than stretching. Finally, communication with the patient is essential since radicular pain or paresthesia are indications that the nerve is being stretched and potentially irritated.
Treatment, therefore, should address the gliding and not stretching of nerves. Before gliding the nerve, soft tissue structures along the course of the nerve can be mobilized to allow for nerve mobility. Movement of the lower extremity can be combined with small movements of the neck to encourage gliding of the nerve rather than stretching. Finally, communication with the patient is essential since radicular pain or paresthesia are indications that the nerve is being stretched and potentially irritated. ![]() The patient and therapist should work in ranges of motion that do not reproduce the patient’s radicular symptoms. Neural mobilization techniques, as well as all techniques mentioned in this book, should only be used by therapists specifically trained in the technique.
The patient and therapist should work in ranges of motion that do not reproduce the patient’s radicular symptoms. Neural mobilization techniques, as well as all techniques mentioned in this book, should only be used by therapists specifically trained in the technique.
Summary Comments.
At this stage of rehabilitation, the patient may find it difficult to perform activities that require prolonged sitting or standing postures. These limitations are normal. It is important to assist the patient in recognizing methods or activities that have the ability to relieve some of the pain or soreness. It is also important that they be assisted in the development of strategies to increase muscle endurance so that they may gradually build a tolerance to these positions. Strategies may include limiting the time spent in any one position, the use of cryotherapy to the back, or active lumbar ROM exercises to relieve stiffness and soreness. Improving protected functional abilities and normalizing nerve mobility are appropriate for this phase of healing. Cardiovascular endurance and strength should continue during this phase.
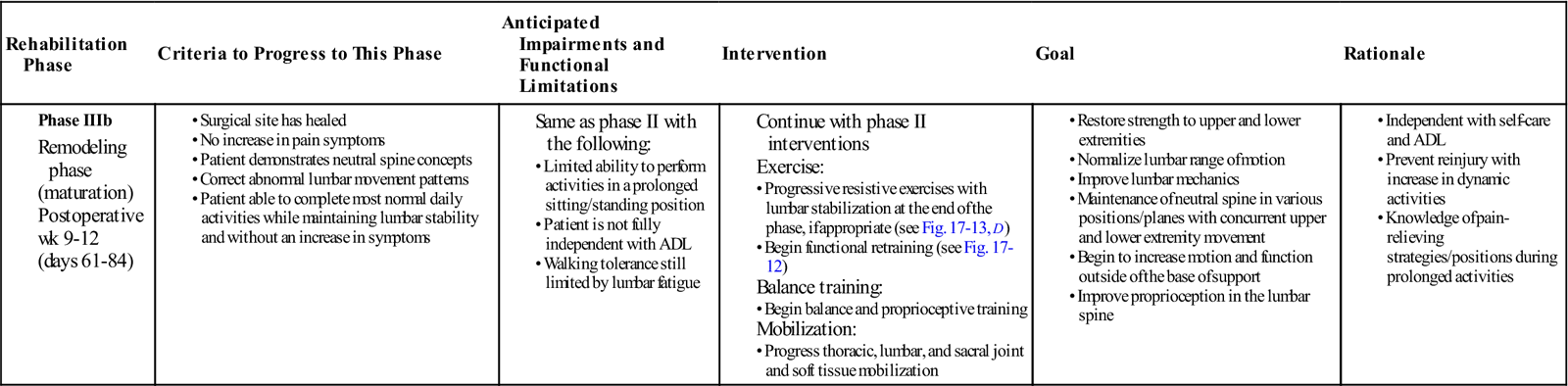
Phase IIIb: Remodeling Phase
TIME: Weeks 9 to 12 (Days 61 to 84)
GOALS: Restore strength and ROM in the lumbar spine, maintenance of neutral spine with concurrent upper and lower extremity movements, improve and normalize function movements, improve the coordinated recruitment of local and global muscle, address musculoskeletal issues that may have contributed to the patient’s low back pain (Table 17-7)
TABLE 17-7
< ?comst?>
| Rehabilitation Phase | Criteria to Progress to This Phase | Anticipated Impairments and Functional Limitations | Intervention | Goal | Rationale |
|
Phase IIIb |
|
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
The primary goal of this phase of rehabilitation is restoration of function. Progression to this phase of rehabilitation should begin once the patient is able to tolerate the exercises of phase IIIa without an increase in low back or lower extremity symptoms. Interventions from the previous phase have focused on loading the body in a manner that protected the lumbar spine. The purpose of this precaution was to prevent overstressing newly healed structures. By week 9 after surgery, most scar tissue should have been formed. Edema and inflammation should be minimal and patient function should be returning to normal. From a tissue perspective, the newly laid dense connective tissue requires appropriate loading to promote strength in the tissue. Basic strengthening and protection should be continued but progressed to include movement outside the basic framework of the core. The goal is protected restoration of function and functional motion.
Functional Retraining.
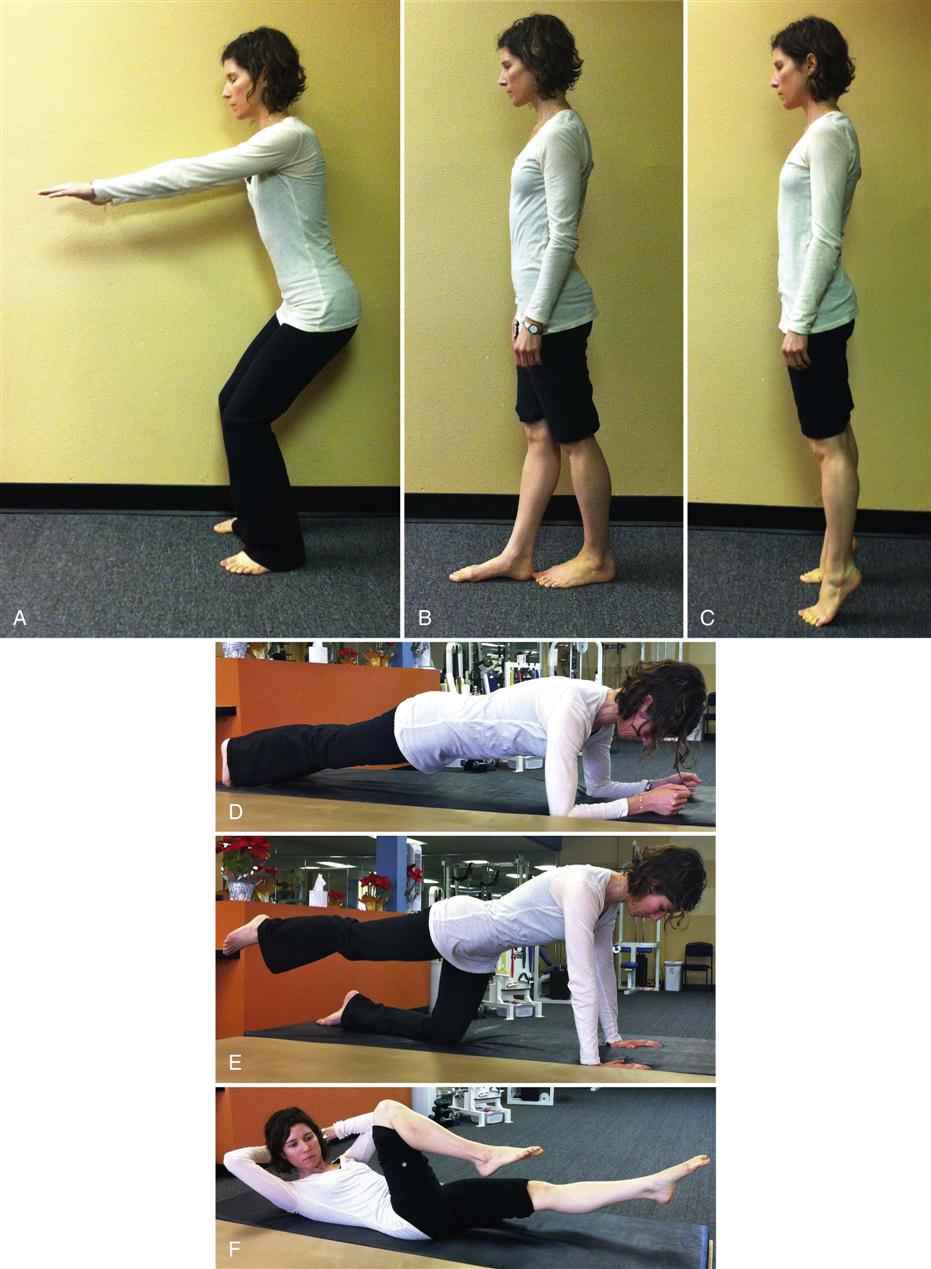
Functional retraining is not a new concept in rehabilitation. It challenges strength, balance, and coordination in functional positions. It involves systems of core training and lumbar stabilization in a weight-bearing, functional environment. At this phase of rehabilitation, it is appropriate to begin to train the body to work in outside of its center of mass (Fig. 17-12). Earlier in the chapter, it was discussed how local muscles are inhibited and global muscles recruited in response to injury. Initial exercises involved recruitment of local muscles such as the transverse abdominis and the multifidus muscles. As the patient continues his or her progress and rehabilitation, it becomes appropriate to start to challenge the patient outside of midline and in upright functional positions. Patients need to operate outside of a neutral spine and perform normal function where strength, flexibility, motor control, and proprioception are all required. Activities such as front lunges and side lunges can be progressed to include upper extremity motions and upper body rotation or side bends. Near the end of this phase, patients can be evaluated for advanced strengthening (i.e., planks, pointer dog, and supine bicycle). Squats and sit to stand can incorporate trunk rotation. Single-leg balance can be combined with single-leg squats and forward or side reaching. By changing position of the upper body and lumbar spine, and adding active functional based activities, the body can begin to recruit the local muscles while at the same time selectively targeting weakened groups of muscles. Various positions of the hip and trunk can force the selective recruitment of one muscle group while inhibiting others. See Fig. 17-13 for examples of therapeutic exercises appropriate for this phase of healing. Progression to this phase of rehabilitation should not be permitted until the patient shows good motor recruitment and control of the basic set of exercises and the patient’s symptoms are minimal.
Lumbar Proprioception.
Proprioceptive training has played a large role in the rehabilitation of individuals following injury. In the lower extremities, it has been established for quite some time that injury impacts joint proprioception. The mechanism of this impairment can vary and in some cases the exact physiologic mechanism behind the proprioceptive changes is not clear. Regardless of the exact physiologic mechanisms, research has shown that rehabilitation can improve joint proprioception. In response to these findings, rehabilitation experts have included proprioceptive training in rehabilitation programs to address and change the proprioceptive system.34
While a mainstay in many extremity rehabilitation programs, proprioceptive training has not factored into most spinal programs. Recently, research has surfaced that suggests that proprioception should play a larger role in spinal rehabilitation programs.35–39 In response to these findings, as a patient’s functional and physical capabilities progress, it is appropriate and necessary to begin to progress exercises in manners that challenge proprioception and balance. See Figures 17-12 and 17-13, F, for examples of therapeutic exercises appropriate for this phase of healing.
Summary Comments.
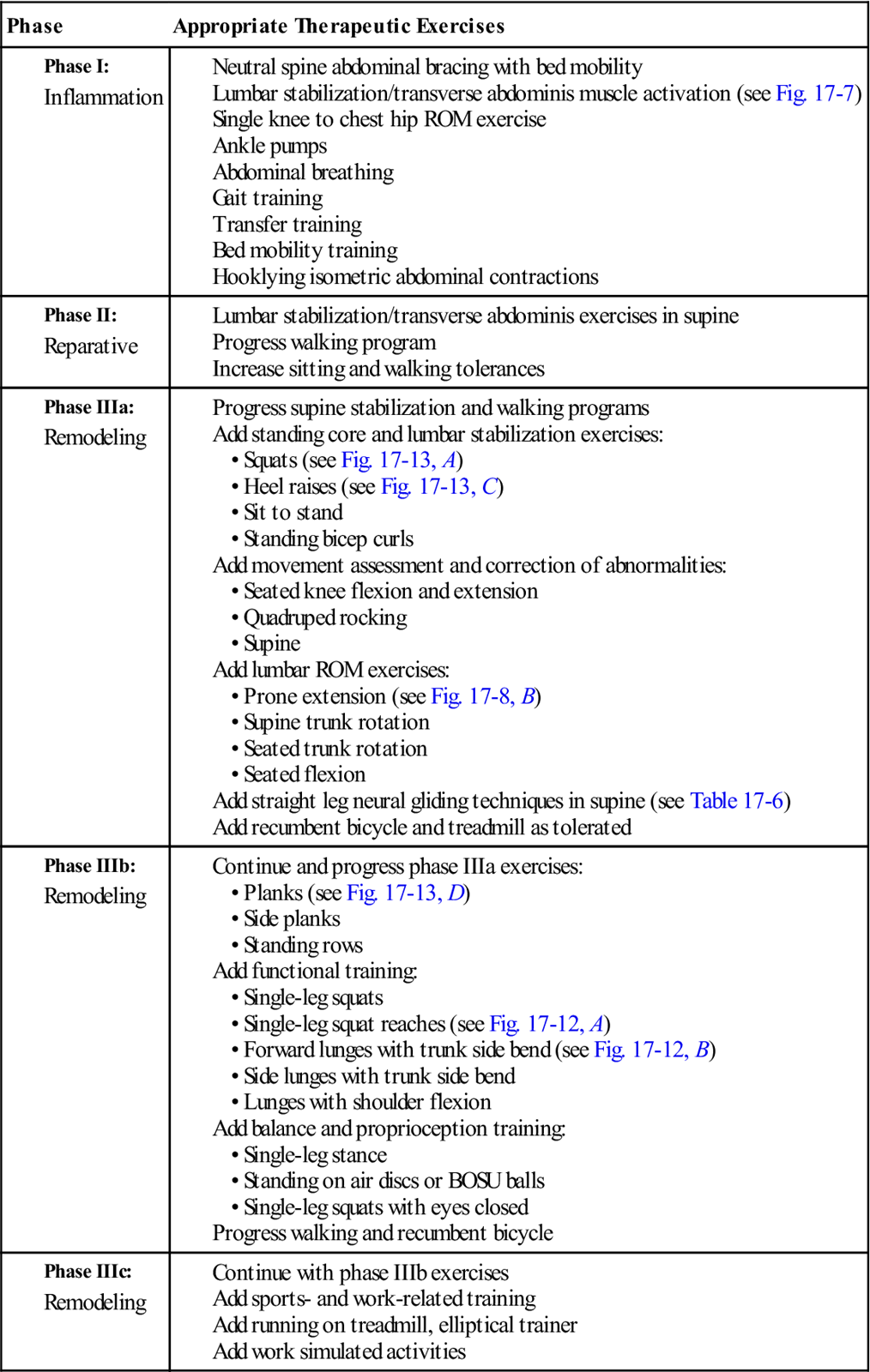
As the patient progresses, it is important for him or her to begin to normalize lumbar motion with functional activities. Functional activities that change balance and proprioception while at the same time challenging strength and endurance are the most appropriate types for this phase of healing. Patients do not function in a small, limited amount of lumbar motion. They must bend, twist, and rotate through large ranges of motion and their backs must be capable of functioning during these larger ranges of motion. See Table 17-8 for a list of therapeutic exercises for the various phases of rehabilitation.
TABLE 17-8
< ?comst?>
| Phase | Appropriate Therapeutic Exercises |
|
Phase I: |
|
|
Phase II: |
|
|
Phase IIIa: |
• Supine
|
|
Phase IIIb: |
|
|
Phase IIIc: |
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
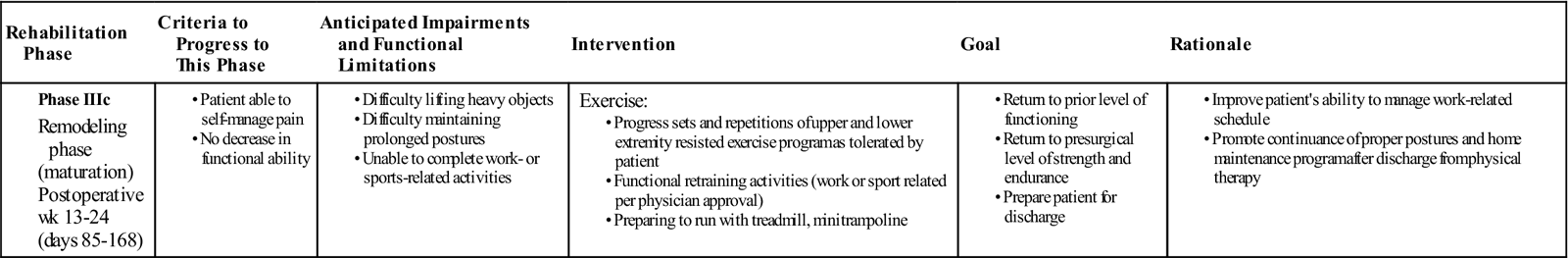
Phase IIIc: Remodeling Phase
TIME: Weeks 13 to 52 (Days 85 to 360)
GOALS: Return to work or sport, return to prior level of functioning, preparation for discharge from physical therapy (Table 17-9). Some of these goals may be reached at 6 months and some may be reached at 1 year.
TABLE 17-9
< ?comst?>
| Rehabilitation Phase | Criteria to Progress to This Phase | Anticipated Impairments and Functional Limitations | Intervention | Goal | Rationale |
|
Phase IIIc |
Exercise:
|
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
From a prognostic standpoint, it takes a full year for a patient to totally recover from lumbar surgery. That is not to say that it will take a full year for the patient to restore function and return to normal daily activities. It is simply that the remodeling phase takes a full year and that changes in tissue can take up to year to reach their ultimate strength. The remodeling phase of the rehabilitation process focuses on return to work, sport, or normal daily activities. By the end of this phase, the patient should be able to function independently at home and in the workplace. As the patient progresses through the rehabilitation process, functional retraining of work or sport-specific activities should be assessed. Activities that require increased loads on the lumbar spine should be evaluated and, pending physician approval, rehabilitation geared towards functional training can be initiated. Return to activities or sports that require contact between players or heavy lifting will require a physician’s approval. At this time, the clinician may implement a gym- or home-based exercise program to assist in maintenance of proper strength and muscle function. Discharge of the patient should occur once the patient, rehabilitation personnel, and physician have all determined that the patient has reached his or her functional goals and is able continue the rehabilitation process safely and independently.
Troubleshooting
Red Flags
There is a 30% to 40% chance complications will occur during and/or after lumbar spine surgery because of exposure and mobilization of anterior structures.40 Those requiring multilevel versus single level exposure are at a higher risk for such complications, as are the elderly, obese, and those with cardiovascular disease.40,41 It is important the clinician is aware of these potential complications to educate the patient on the importance of reporting any signs or symptoms to his or her physician immediately.
Infection
Infection has been reported in approximately 1% to 2.4% of patients undergoing lumbar spine surgery.42 Incisional hernias, sterile discharge, and other superficial wounds at the incision site can occur.43 Periincisional abdominal bulges can also occur because of intercostal denervation.40 Deeper infection can also occur, leading to more serious complications including bone destruction and resorption as well as osteomyelitis in vertebrae adjacent to the surgical site.42 Signs and symptoms of infection include fever, hypotension, tachycardia, tachypnea, increased pain, edema, wound drainage, tenderness, and general malaise.44
Vascular Complications
Injury to vascular structures is the most common complication when using an anterior approach for lumbar spine surgery.40,41,45 Potential damage to the arterial and venous systems occurs in 2.8% of cases because of mobilization of the aorta, inferior vena cava, and iliac arteries.46–48 Thrombosis is the most likely postoperative arterial complication, yet can be prevented with use of anticoagulants, compressive hoses, and calf pumps.40 When exposure of the L4-L5 lumbar spine segments is required, mobilization of renal, iliac, and iliolumbar veins is necessary, placing an increased risk for complications. With this in mind, intraoperative bleeding can occur when venous structures are damaged. Individuals who are diabetic, obese, elderly, or have cardiovascular disease are not only at a higher risk for experiencing such complications, but they are also at risk for postoperative ischemia.40 Signs and symptoms of arterial or venous damage may include calf pain, lower extremity edema, diminished pedal pulses, temperature changes, discoloration, and heaviness in the lower extremities.
Neural Complications
There are a variety of nerves that can be injured during lumbar spine surgery. The lumbar sympathetic chain runs along the spine, controlling genitourinary organs. If damaged, one may be left with a warm lower extremity, often mistaken for vascular damage, and a variation of incontinence.40 The iliohypogastric, ilioinguinal, and genitofemoral somatic nerves are also at risk for damage resulting in decreased sensation to the groin and external genitalia.40 After surgery, increased radicular pain may occur because of epidural fibrosis causing nerve root traction during surgery.49,50 This has been shown to resolve by the third month postoperatively.50
Genitourinary Complications
Genitourinary complications can occur from damage from the mobilization of the hypogastric sympathetic plexus. Injury to urinary tract organs, decreased genital sensation, retrograde ejaculation, and impotence in males can result.40,51 Damage to ureters occurs in 0.3% to 8.0% of cases and can be injured whether spine surgery requires an anterior or posterior approach.40 Retrograde ejaculation occurs in up to 28% of males undergoing lumbar spine surgery with an anterior approach.40,51 When this occurs during lumbar spine surgery it is often irreversible. Elderly men, diabetics, and those with vascular disease are at a higher risk for such complications.40
Spontaneous Fusion and Heterotropic Ossification
Unlike spinal fusion surgery, the goal of total disc replacement (TDR) surgery is to preserve movement and restore disc height and segmental lumbar lordosis. Spontaneous interbody fusion of segments above and below the surgical site has occurred in greater than 60% of patients after a 17-year follow-up.48,50 Approximately 1.4% to 15.2% of patients have also experienced heterotropic ossification with the use of ProDisc and SB Charité prostheses.48
Implant Materials
The polyethylene metal used in TDR is the same type of metal used in total knee and hip replacements. Although there haven’t been any reported cases of wear debris, creep, or osteolysis occurring in disc replacement surgery, there is a potential for permanent deformation and wear of the metal similar to total knee and hip replacements.45,48 This may take as little as 1 year or as long as 10 years to occur, both leading to a necessary anterior and posterior spinal fusion of the involved segments.45,48,50 Possible dislocation or loosening of the polyethylene metal can also occur over time, resulting in chronic pain and requiring spinal fusion because of biomechanical failure.52
Biomechanics
The purpose of having a TDR rather than spinal fusion is to preserve the normal movement of the lumbar spine.48 During surgery, malpositioning of the disc implant, whether anterior or posterior, can occur.48 This can ultimately cause decreased ROM and/or an increase in load to posterior structures. Malpositioning also affects the sagittal balance of the lumbar spine, resulting in decreased lumbar lordosis and possible degeneration of adjacent segments.48
Chronic Pain
Changes in the peripheral and central nervous system occur almost immediately following an injury. Some of these changes are reversible and other changes are nonreversible. It is beyond the scope of this chapter to describe all the neural changes that occur with injury, but from a clinician’s viewpoint it is important to realize that not all patients will have full resolution of symptoms following surgery. Surgery may have addressed the structures that were originally the source of the patient’s symptoms, but the adaptations that have occurred in the central and peripheral nervous system may not be reversible. It is important to realize that not all pain is a reflection of actual tissue damage. As a result, not all patients will have full resolution of symptoms.28,53,54 See Box 17-6 for factors that contribute to chronic pain.
Summary
Rehabilitation of a patient following a TDR is unique, considering its goal is to maintain lumbar ROM and overall mobility. Although it is pertinent to begin mobility exercises early on in the rehabilitation process, it is just as important to allow the surgical site to heal. Therefore, patient education regarding surgical protection guidelines immediately following surgery is a must. Aside from healing the surgical site, specific rehabilitation protocols and guidelines created for TDR surgery are similarly based on protocols for other lumbar spine surgeries (i.e., microdiscectomies and fusions). Balancing abdominal stability and lumbar mobility in conjunction with lower extremity strength and cardiovascular exercise is most important. Considering the variety of individuals requiring a lumbar disc replacement surgery, it is necessary to create and progress a program specific to each patient’s needs. Finally, since radicular pain and lower extremity paresthesia are often the symptoms driving the decision for lumbar disc replacement surgery, prevention of neural adhesions and promotion of nerve healing should be addressed appropriately.
Clinical Case Review
1Your patient is a 34-year-old male who underwent a L4-L5 lumbar disc replacement surgery 1 day ago. The physician states that the surgery was a complete success and that the patient is ready to begin in-hospital rehabilitation. You have been asked to evaluate and begin the rehabilitation process. What will your evaluation and initial treatment involve?
Initial evaluation in the hospital will involve taking the patient’s vital signs and assessing the patient’s wounds. Given that both of these objective measures are satisfactory, the clinician should have several goals on day 1. First is to educate the patient on neutral spine bracing and mechanics. The patient should be educated on how to move in bed and progress from lying to sitting with the lumbar region braced. The patient should be fitted with a lumbar support brace and shown how to use the brace. The brace is worn 24 hours a day unless otherwise ordered by the physician. Sit to stand mobility should be assessed using the front wheel walker for support. The patient should be able to walk from the hospital bed to the bathroom with minimal assistance. A front wheel walker will be used for support. The patient should be encouraged to sit, walk, and stand for limited times initially. No position should be held for greater than 15 minutes. The patient should be advised not to transfer, stand, or walk without assistance for the first day.
2Your patient is a 53-year-old male who underwent an L4-L5 TDR 4 weeks ago. He has type II diabetes and is 100 lb overweight. During his initial evaluation for outpatient physical therapy, he reports that he ran out of his blood thinners 1 week ago and has been experiencing right lower extremity leg pain and swelling for the past 5 days. Symptoms seem to be progressively worsening. The patient has not yet spoken with his physician.
Red Flag: This patient is at risk for a deep vein thrombosis. He is diabetic and overweight, both predisposing him to vascular complications during and after surgery. Symptoms including lower extremity pain, severe edema, discoloration, temperature changes, diminished pedal pulses, and heaviness are all signs of deep vein thrombosis. This patient should be advised to see his physician or go to the nearest hospital as soon as possible to prevent further complications, including a pulmonary embolus.
3Your patient is a 42-year-old female who underwent a lumbar disc replacement surgery 5 days ago. She is progressing well and without complications and is preparing for discharge from the hospital tomorrow. She will be returning to her home where her husband and family will help care for her. What must be done with the patient before her discharge tomorrow?
The patient will mostly likely not have home rehabilitation care. The instructions given in the hospital at time of discharge should be followed until the patient is released to physical therapy in approximately a month. Therefore, several instructions need to be purveyed to the patient. The patient should be given the hospital discharge instructions (See Box 17-2). The patient should be instructed to maintain the lumbar stabilization program and joint protection precautions. A progressively increasing walking program should be encouraged. Lumbar flexion should be promoted and lumbar extension, rotation, and side bend avoided. A lumbar brace should be worn 24 hours a day unless otherwise ordered by the physician. Lumbar core stabilization should be used with all daily activities.
4Your patient is once again the 42-year-old female who underwent a lumbar disc replacement surgery. She is now in an outpatient orthopedic clinic 5 weeks following her surgery. She saw her doctor yesterday and has been cleared to begin outpatient orthopedic rehabilitation. What will your evaluation and initial treatment involve?
The patient will guide your initial evaluation. Most patients will have a little low back pain and stiffness, but the symptoms are getting better. The initial evaluation will involve assessing posture and motion. ROM can be tested in all directions of motion, but end-range extension, rotation, and side bend should be avoided until 6 weeks. Lower extremity ROM and lower extremity strength should also be tested. Care must be taken not to overtax the patient’s injured regions. A neurologic examination should be completed to determine the amount of nerve damage sustained during surgery. The patient should avoid lying on her stomach for the first 6 weeks following surgery. Interventions will include reviewing the patient’s current exercises and the progression of exercises as needed. Walking tolerance should be encouraged. Lumbar and core stability exercises should be included and progressed at week 6. Soft tissue mobilization in side lying may begin at this time. The patient should be warned to avoid trunk extension, rotation, and side bend. The patient may begin to wean off of the lumbar brace at 6 weeks pending physician approval. The weaning process should involve coming out of the brace for 1 hour on day 1, 2 hours on day 2, 3 hours on day 3, etc.
5Your patient is a 29-year-old female who is 5 weeks postsurgical L4-L5 lumbar disc replacement. She is in your clinic for her first outpatient rehabilitation session. You are assessing her posture and ROM. She stands with increased hip flexion and increased lumbar lordosis. When asked to forward flex, her motion is guarded and she is unable to fully flex because of low back tightness and pain. What are the implications of these finding in your clinical decision-making process?
Pain and guarding are normal symptoms at 5 weeks postoperation. Lumbar lordosis should be avoided in patients following disc replacement. The patient should be taught abdominal bracing and lumbar protection strategies. Neutral lumbar spine positioning should be incorporated to avoid lumbar lordosis. With lumbar flexion, the patient should be able to bend forward and the lumbar spine should flatten or reverse its lordotic curve. The patient has a normal response for her phase of healing. The clinician should begin manual therapy and exercises to restore normal lumbar motion.
6Your patient is a 65-year-old male who underwent a TDR surgery with dynamic stabilization 5 weeks ago. He complains of shooting pain and numbness down his left lower extremity. He has difficulty when transitioning from supine to sit and sit to stand, and can only walk 100 feet before having to sit and rest because of pain. He asks why this is happening after surgery and wonders how long it will take for these symptoms to resolve.
Radicular symptoms into the lower extremity following disc replacement surgery are not an uncommon complaint. Multiple factors can be the cause of the symptoms. In certain instances, the radicular symptoms can be the result of nerve root traction during surgery secondary to epidural fibrosis. Such symptoms usually resolve by the twelfth week, postoperatively. It is important that the clinician educates the patient on such symptoms, so he also knows what to expect and to follow up with his physician in case symptoms worsen.
7Your patient is a 37-year-old male who is 6 weeks postsurgical L3-L4 lumbar disc replacement. He is progressing well but continues to experience limitation in ROM. Low back pain is improving, but stiffness in the lumbar region continues to persist. You decide to incorporate joint mobilization into your treatment plan. What joints and techniques should you use in your treatments?
At 6 weeks after surgery, prone techniques are appropriate for the patient. The clinician can begin mobilization of the thoracic region at 4 weeks and mobilization of the lumbar spine at 6 weeks. The patient’s symptoms will be your guide to mobilization intensity and duration. Posterior to anterior mobilization can be used at the thoracic and lumbar spine, but is not appropriate at the L3-L4 spinal segment. Sacral mobilization can also be incorporated. Rotational and flexion/extension mobilization techniques are most appropriate for the L3-L4 spinal segment.
8Your patient is a 55-year-old female who had L2-L3 lumbar disc replacement surgery 2 months ago. She is progressing well with her initial exercises and would like to begin further exercise progression. What and how can she progress her exercises?
Lumbar exercises can be progressed in the following manner:
• Increase the number of repetitions for each exercise
• Increase the amount of resistance the patient is using for each exercise
• Progress core stabilizing exercises from supine to more functional positions such as standing
• Decrease base of support while completing exercises and begin to challenge balance
• Begin to work outside of the patient’s base of support and begin functional-based exercises
9Your patient is a 35-year-old male who had lumbar disc replacement surgery 3 months ago. He is progressing well and would like to begin running. What is your advice for the patient and should he be allowed to begin running?
Generally, running can begin in some patients at 3 months following surgery, but most people will begin at a running regime at 5 to 6 months following surgery. Before that time, the patient can begin prerunning activities. Prerunning would include several weeks of walk/running on the treadmill, use of an elliptical trainer, and running on a minitrampoline before beginning running on the street. In the beginning, the patient should only begin running for 10 to 15 minutes and then increase the running time 5 minutes a day as tolerated. Ultimately the decision to begin running will need to be approved by the surgeon, but will be based upon the patient’s symptoms and whether he can complete daily activities with good lumbar control and stability.
10Your patient is a 34-year-old male who has been making steady progress in physical therapy. He is 9 weeks postlumbar disc replacement surgery at L4-L5 and wants to know when he can return to surfing and snowboarding.
Surfing and snowboarding are considered extreme sports. It is recommended to postpone participation in such activities for at least 6 months post-TDR and in most cases it is closer to 9 months. This time frame is variable and depends upon the nature of the sport and the patient’s functional and symptom progression. At 12 weeks, he is able to begin light running, lifting, twisting, and bending activities. Sport-specific activities can begin when the patient can run pain free. The patient will require clearance from his physician before returning to sports. This patient needs to be educated on the proper exercise progression to prepare for such recreational activities and requires clearance from his physician to participate in such activities.
11Your patient is a 38-year-old male who had lumbar disc replacement surgery 6 months ago. He is now running for short distances and has not noticed any increase in symptoms. His chief limitation is running greater than 30 minutes. The physician has approved him to return to work and discontinued outpatient orthopedic rehabilitation. What is his program and what is your advice to him in terms of his ultimate prognosis?
If the patient is running and has been approved to return to work. He is now appropriate for discharge. He will continue to heal and strengthen for up to 1 year following the surgery. So, he should continue to progress his exercises at home and in a gym setting. He can begin to return to playing sports at 6 months, but this will be dependent upon the nature of the sport and the patient’s symptoms. Higher impact sports that require contact or repetitive jumping may require additional time. These athletes may require 9 to 12 months of time before return to the sport. The patient should be given a home- and gym-based program to continue for the next 6 months. The patient should also be advised to respect his pain and symptoms, and to progress his home program in a pain-free manner.