CHAPTER 52 Lumbar Nucleus Replacement
Parallel to the development of lumbar artificial total disc replacement (TDR), physicians and researchers have worked on the development of lumbar nucleus replacement.1,2 TDR and nucleus replacement are part of disc arthroplasty, which is intended to provide an alternative to fusion for patients with discogenic back pain and sciatica. Although both procedures preserve index segment motion and reduce the stress on the adjacent motion segments, there are major differences between TDR and nucleus replacement, as follows: (1) Nucleus replacement is more tissue-preserving than TDR. (2) Nucleus replacement technology is simpler and theoretically easier for restoration of normal biomechanical functions than TDR because of the preservation of anulus and ligaments. (3) Nucleus replacement does not affect effective conversion to TDR or fusion if it fails for any reason. (4) Because of the smaller dimension of a nucleus replacement device than a TDR device, the nucleus replacement can be performed with multiple surgical approaches, including the traditional posterior approach, whereas TDR currently has to be performed via an anterior or anterolateral approach.
Clinical Challenge of Nucleus Replacement
Design and Material Options of Nucleus Replacement
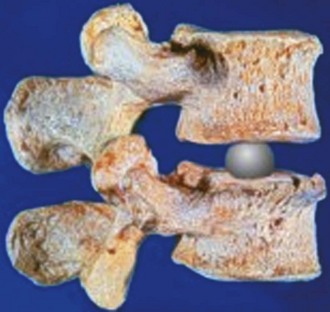
In contrast to the predominant ball-and-socket design with either metal-on-metal or metal-on-polyethylene for the current generation of TDR, the design and material options for nucleus replacement are quite variable. This variability is largely due to the fact that nucleus replacement preserves more of the anatomy of the motion segment, such as anulus and endplates, and more function. The first nucleus replacement with long-term clinical experience was the Fernstrom ball made of stainless steel (Fig. 52–1).3,4 Although this design offered bipolar articulation with the endplates to provide segment motion, it caused high contact stress because of point loading and implant subsidence.
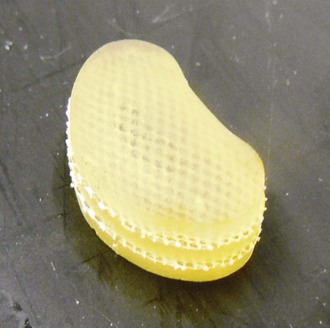
Aquarelle developed by Howmedica (later Stryker) was the first preformed polyvinyl alcohol hydrogel used for nucleus replacement.5 Subsequently, several other preformed hydrogel materials have been used for nucleus replacement, including prosthetic disc nucleus (PDN) (later redesigned and named HydraFlex) (Fig. 52–2) by RayMedica6 and NeuDisc by Replication Medical (Fig. 52–3).7 PDN and NeuDisc used the same type of base hydrogel, an acrylic copolymer hydrogel (HYPAN), with PDN having the hydrogel core encased in a polyethylene jacket and NeuDisc having the hydrogel sandwiched in between the Dacron knitted meshes. The main advantage of using preformed hydrogel materials for nucleus replacement is that they not only mimic the viscoelastic and physiologic properties (imbibing and releasing water during the cyclic loading) of the nucleus, but also they have the ability to bear the mechanical load.
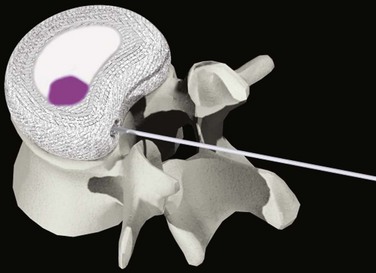
In addition to preformed hydrogel, some in situ curable hydrogel materials have also been used for nucleus replacement. Examples in this category are NuCore (Spine Wave)8 and BioDisc (Cryolife).9 NuCore is an injectable synthetic recombinant protein hydrogel, which is a sequential block copolymer of silk and elastin, with two silk blocks and eight elastin blocks per polymer sequence repeat (Fig. 52–4). The material has a water content and modulus similar to that of the natural nucleus. BioDisc is a biopolymer consisting of a protein-based hydrogel. During implantation, the dispensing device mixes the two components of predetermined ratio, and the glutaraldehyde cross-links the bovine serum albumin (BSA) molecules to each other and to the surrounding tissues.
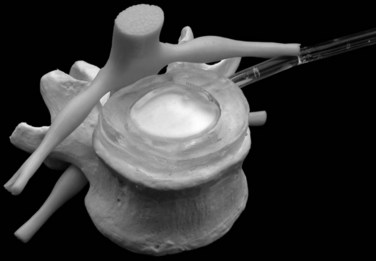
DASCOR (Disc Dynamics) is the most clinically advanced in situ cured nonhydrogel elastomer nucleus replacement and is the only injectable nucleus device with a balloon for containment (Fig. 52–5).10 The injectable polymer is a two-part in situ curable polyurethane. The balloon is also made from polyurethane to facilitate adhesion between the curable polymer and the balloon. There are several clear advantages of using the balloon. First, it allows the polymer to be injected under certain pressure to fill the disc cavity completely without the risk of polymer leaking through the defected anulus. Second, the balloon prevents the direct contact between the uncured or semicured polymer and the surrounding wet tissue, so it avoids the leach of uncured monomers. The isolation of body fluid from the uncured polymer also can ensure better polymerization and a more consistent and stronger mechanical property for the final implant.
Another in situ curable nucleus replacement device is Percutaneous Nucleus Replacement (PNR; Trans1).11 The PNR consists of two threaded titanium vertebral body anchors connected by a cylindric silicone rubber membrane. The two titanium anchors are fixed to the adjacent vertebrae via a trans-sacral approach, and the in situ curable silicone rubber is injected through the sacral anchor into the silicone membrane until the disc cavity is filled.
Some nonelastomeric materials have also been used for nucleus replacement based on the favorable clinical data of the Fernstrom ball. The main advantages of using these nonelastomers for nucleus replacement are their better mechanical strength and durability. The design of the Regain (Biomet) is similar to a modified Fernstrom ball and made of pyrolytic carbon (Fig. 52–6). Although the endplate contacting surfaces of Regain are still convex, they have a much larger radius so that the device has a larger initial contact area and smaller initial contact stress than the Fernstrom ball. Because of the mismatch of the surface contour between the implant and endplates, some subsidence is still inevitable.
To maintain the articulating feature of the Fernstrom ball while intending to increase the contact area, the NUBAC (Pioneer Surgical Technology) adopted the design similar to many TDR devices (Fig. 52–7).12 It consists of two plates with outer surfaces contoured to the general geometry of the endplates and has an inner ball-and-socket articulation. This inner ball-and-socket articulation allows free motion within all physiologic ranges of motion in all major axes. This articulation feature is also believed to be a major factor in minimizing the shear force between the endplate and implant surface during bending motion and reducing the risk of implant extrusion. The implant is made from polyetheretherketone (PEEK) OPTIMA, which has well established biocompatibility, superb biodurability, and a history of being used as permanent spinal implants.
Clinical Experience of Different Nucleus Replacement Devices
The early short-term and long-term clinical data of the Fernstrom ball have provided fundamental scientific evidence that nucleus replacement can be effective in relieving discogenic back pain. Fernstrom3 reported his relative short-term clinical data (6 months to 2.5 years) on 125 patients who received the device after discectomy and 100 patients with discectomy alone. He divided his patient population into two different groups: back pain only and back pain with leg pain. The success rates for patients with back pain only were 60% for the Fernstrom ball group versus 12% for the discectomy group. The success rates for patients with back pain with leg pain were 88% for the Fernstrom ball group versus 40% for the discectomy group. Motion, although not being quantitatively measured, was observed in the index level for patients with a Fernstrom ball. A different degree of subsidence of 1 to 3 mm was noticed for most of the Fernstrom ball group.
In 1995, McKenzie4 reported long-term (average 17 years) follow-up on 103 patients. The success rates were 75% for patients with back pain only and 83% for patients with back pain and leg pain. After the procedure, 95% of patients returned to work. Between these two studies with a total of 344 implants, only one implant extrusion was reported (0.3%). Although late criticism of the Fernstrom ball nucleus replacement device has been mainly on the subsidence, this does not seem to have a significant impact on the clinical success. The Fernstrom ball has shown similar results to other surgical treatments, including TDR and fusion, for either back pain only or back pain with leg pain.
Klara and Ray13 reported the clinical data of the first four phases from 1996 to early 2000. The surgical success, which has not been well defined by the authors but is different from the commonly used term clinical success, varied from 62% to 91% during these four phases with implant extrusion up to 26% in phase II when the design changed to HYPAN 80 (with a higher water content than the original HYPAN 68) and a pair of angle-shaped devices. In addition to the design change, the surgical approach was changed from the initial posterior approach to the anterolateral transPsoatic approach.
Bertagnoli and Schoenmayr14 reported a large cohort study with 243 patients having a good clinical outcome. The average Oswestry Disability Index (ODI) score declined from 52.7 preoperatively to 9 at 2-year follow-up, and the average visual analog scale (VAS) score declined from 7.1 preoperatively to 1.8 at 2-year follow-up. The average disc height increased from 8.1 mm to 10.2 mm at 2-year follow-up. Shim and colleagues15 reported similar clinical outcomes with their series of 48 patients at 1-year follow-up. They found 19.6% subsidence, however. They also observed 60.9% endplate sclerosis and 82.8% Modic change. In a separate study, Shim and colleagues16 reported good results on their 75 PDN-SOLO patients with ODI and VAS scores decreased from 53 and 7.3 preoperatively to 14 and 2.2 at 2-year follow-up and only 2.7% implant extrusion. Regardless of the high incidence of endplate sclerosis and Modic change, these changes did not seem to be correlated with the clinical outcome and could have been caused by restoring the mechanical load on the endplate in the nucleus region.
Other than the Fernstrom ball, PDN is the only other nucleus replacement device that has some long-term follow-up results. The long-term clinical results of the PDN have been mixed, however. Schoenmayr and Kliniken,17 who implanted the first series of 11 PDN devices, reported 8-year follow up on the original 11 patients using the first-generation PDN device with fairly good long-term results. The average ODI scores were 45 preoperatively and 14 8 years postoperatively. Of the 11 patients, only 1 had extruded (9%), and 8 were satisfied with the surgery. Pimenta and colleagues18 reported high adverse event rates with 9 years’ follow-up, however, on 80 patients with the more recent version of PDN-SOLO, even though there were still significant reductions in ODI and VAS scores for patients who were not revised. Of these 80 patients, 38 (47.5%) were revised during the 9-year follow-up, of which 23 (28.8%) were due to implant expulsion, and 15 (18.8%) were due to subsidence.
Since its first clinical use at the end of 2004, NuBac has progressed quickly among all other nucleus replacement devices and become the second most implanted nucleus device in more than 300 patients so far. At the 2009 Spine Arthroplasty Society annual meeting, Pioneer Surgical Technology reported the worldwide multicenter clinical study that included patients enrolled outside of the United States and within the United States as a part of an IDE feasibility study with up to 2 years’ follow-up.19 The study has enrolled 219 patients using all three different surgical approaches—posterior, lateral, and anterolateral. Significant improvement in function and pain relief were observed with ODI scores being reduced from 53 preoperatively to 30, 25, 24, 22, and 20 at 6 weeks, 3 months, 6 months, 12 months, and 24 months postoperatively. VAS scores were reduced from 77 preoperatively to 32, 29, 23, 31, and 32 at 6 weeks, 3 months, 6 months, 12 months, and 24 months postoperatively.
In a separate study, Balsano and colleagues20 reported the clinical data of 45 NuBac patients using only the posterior surgical approach. ODI score decreased from 51 preoperatively to 31, 27, 24, and 23 at 6 weeks, 3 months, 6 months, and 12 months postoperatively, and VAS score decreased from 76 preoperatively to 31, 31, 31, and 27 at 6 weeks, 3 months, 6 months, and 12 months postoperatively. In the U.S. IDE feasibility study, 20 patients were enrolled using the lateral surgical approach. Similar ODI improvement and VAS reduction were observed in these patients with no implant extrusions. Based on these clinical results, in 2008 NuBac became the first nucleus replacement device to gain the approval of the U.S. Food and Drug Administration (FDA) to start the pivotal study.
More than 200 DASCOR devices have been implanted since 2003, using anterolateral, lateral, posterolateral, and endoscopic approaches. Ahrens and colleagues reported an interim 2-year European clinical study of 85 patients using lateral and anterolateral approaches.21 Mean preoperative VAS and ODI scores improved significantly by the 6-week postoperative follow-up point throughout the 2 years. Mean VAS back pain scores showed a 56.6% reduction at 24 months from a preoperative mean of 7.6. Mean ODI score showed a 59.7% reduction at 24 months from a preoperative mean of 57.5. No expulsions were observed in this 2-year clinical cohort. DASCOR is presently undergoing the FDA IDE feasibility study with VAS and ODI results showing statistically significant similarity to the European cohort.
Several other nucleus replacement devices with smaller patient numbers and relatively short follow-up have been also reported. Berlemann and Schwarzenbach22 reported 2-year follow-up results on 12 NuCore patients who had back pain and leg pain. Decompression is part of the procedure to remove a herniated disc. Owing to the decompression, the patient’s leg pain was significantly reduced from 6.8 preoperatively to 1.1 at 2 years. Back pain VAS score was also reduced from 32 to 6. Pawulski and colleagues23 reported on 11 BioDisc patients with up to 2 years of follow-up with the average ODI and VAS scores reduced from 47.5 and 5.9 preoperatively to 17.7 and 2.3 at 2 years postoperatively. Two patients had recurrent herniation, and both had reherniation removed along with the device without incident.
Since 2005, NeuDisc has been undergoing a two-arm European pilot study.7 The first arm is an open approach using the anterolateral transPsoatic approach, and the second arm is a posterolateral endoscopic approach. In the pilot study, 15 patients were implanted, but the details of follow-up data were not reported. Based on the initial lessons learned from this pilot study, the device has been modified to a less hydrophilic and reconfigured kidney-shaped design. A new pilot study with posterior and endoscopic transforaminal approaches has been initiated.
Conclusion
Pearls
Key Points
1 Bao Q-B, Yuan HA. New technologies in spine: Nucleus replacement. Spine (Phila Pa 1976). 2002;27:1245-1247.
The authors reviewed various nucleus replacement designs and material options and clinical utility.
2 McKenzie AH. Fernstrom intervertebral disc arthroplasty: A long-term evaluation. Orthop Int Educ. 1995;3:313-324.
3 Davis RJ. PDN-SOLO and HydraFlex nucleus replacement system. In: Yue JJ, Bertagnoli R, McAfee PC, et al, editors. Motion Preservation Surgery of the Spine, Advanced Technologies and Controversies. Philadelphia: WB Saunders; 2008:407-410.
4 Ahrens M, Tsantrizos A, Le Huec J-C. DASCOR. In: Yue JJ, Bertagnoli R, McAfee PC, et al, editors. Motion Preservation Surgery of the Spine, Advanced Technologies and Controversies. Philadelphia: WB Saunders; 2008:393-406.
5 Bao Q-B, Songer MN, Pimenta L, et al. NUBAC disc arthroplasty. In: Yue JJ, Bertagnoli R, McAfee PC, et al, editors. Motion Preservation Surgery of the Spine, Advanced Technologies and Controversies. Philadelphia: WB Saunders; 2008:442-451.
1 Bao Q-B, Yuan HA. New technologies in spine: Nucleus replacement. Spine (Phila Pa 1976). 2002;27:1245-1247.
2 Di Martino A, Vaccaro AR, Lee JY, et al. Nucleus pulposus replacement: Basic science and indications for clinical use. Spine (Phila Pa 1976). 2005;30(16 Suppl):S16-S22.
3 Fernstrom U. Arthroplasty with intercorporal endoprothesis in herniated disc and in painful disc. Acta Scand Suppl. 1966;357:154-159.
4 McKenzie AH. Fernstrom intervertebral disc arthroplasty: A long-term evaluation. Orthop Int Educ. 1995;3:313-324.
5 Bao Q-B, Yuan HA. Aquarelle hydrogel disc nucleus. In: Yue JJ, Bertagnoli R, McAfee PC, et al, editors. Motion Preservation Surgery of the Spine, Advanced Technologies and Controversies. Philadelphia: WB Saunders; 2008:423-430.
6 Davis RJ. PDN-SOLO and HydraFlex nucleus replacement system. In: Yue JJ, Bertagnoli R, McAfee PC, et al, editors. Motion Preservation Surgery of the Spine, Advanced Technologies and Controversies. Philadelphia: WB Saunders; 2008:407-410.
7 Yeung AT, Prewell A, Yue JJ. NeuDisc artificial lumbar nucleus replacement. In: Yue JJ, Bertagnoli R, McAfee PC, et al, editors. Motion Preservation Surgery of the Spine, Advanced Technologies and Controversies. Philadelphia: WB Saunders; 2008:411-416.
8 Schwarzenbach O, Berlemann U, Wilson T. NuCore injectable nucleus: An in situ curing nucleus replacement. In: Yue JJ, Bertagnoli R, McAfee PC, et al, editors. Motion Preservation Surgery of the Spine, Advanced Technologies and Controversies. Philadelphia: WB Saunders; 2008:417-422.
9 Wardlaw D. BioDisc nucleus pulposus replacement. In: Yue JJ, Bertagnoli R, McAfee PC, et al, editors. Motion Preservation Surgery of the Spine, Advanced Technologies and Controversies. Philadelphia: WB Saunders; 2008:423-434.
10 Ahrens M, Tsantrizos A, Le Huec J-C. DASCOR. In: Yue JJ, Bertagnoli R, McAfee PC, et al, editors. Motion Preservation Surgery of the Spine, Advanced Technologies and Controversies. Philadelphia: WB Saunders; 2008:393-406.
11 Diaz R, Pimenta L, Nicole N, et al. Tran1 percutaneous nucleus replacement. In: Yue JJ, Bertagnoli R, McAfee PC, et al, editors. Motion Preservation Surgery of the Spine, Advanced Technologies and Controversies. Philadelphia: WB Saunders; 2008:435-441.
12 Bao Q-B, Songer MN, Pimenta L, et al. NUBAC disc arthroplasty. In: Yue JJ, Bertagnoli R, McAfee PC, et al, editors. Motion Preservation Surgery of the Spine, Advanced Technologies and Controversies. Philadelphia: WB Saunders; 2008:442-451.
13 Klara P, Ray D. Artificial nucleus replacement: Clinical experience. Spine (Phila Pa 1976). 2002;27:1374-1377.
14 Bertagnoli R, Schoenmayr R. Surgical and clinical results with the PDN prosthetic disc-nucleus device. Eur Spine J. 2002;11(Suppl 2):S143-S148.
15 Shim S, Lee H, Park C, et al. Partial disc replacement with the PDN prosthetic disc nucleus device: Early clinical results. J Spinal Dis Tech. 2003;16:324-330.
16 Shim S, Lee S-H, Bracth B, et al: Preliminary results of a prospective international multi-center study of the PDN-SOLO device. In Programs and Abstracts of the Seventh Annual Meeting of Spine Arthroplasty Society, Berlin, 2007.
17 Schoenmayr R, Kliniken HS: Ten year follow-up of disc height, ROM and clinical outcomes with the 1st generation PDN device. In Programs and Abstracts of the Sixth Annual Meeting of Spine Arthroplasty Society, Montreal, 2006.
18 Pimenta L, Lhamby J, Schaffa T, et al: PDN nucleus replacement: 9 year follow-up experiences. In Programs and Abstracts of the Ninth Annual Meeting of Spine Arthroplasty Society, London, 2009.
19 Coric, D, Songer M, Yuan H, et al: Up to 2-year follow-up results of a novel PEEK-on-PEEK disc arthroplasty: A prospective worldwide multicenter clinical study. In Programs and Abstracts of the Ninth Annual Meeting of Spine Arthroplasty Society, London, 2009.
20 Balsano M, Bucciero A, Agrillo U: Preliminary clinical evaluation of posterior disc arthroplasty. In Programs and Abstracts of the Ninth Annual Meeting of Spine Arthroplasty Society, London, 2009.
21 Ahrens M, Tsantrizos A, Sherman J, et al. Nucleus replacement with the DASCOR disc arthroplasty device: Interim two-year efficacy and safety results from two prospective, non-randomized multi-center European studies. Spine (Phila Pa 1976). 2009;34:1376-1384.
22 Berlemann U, Schwarzenbach O: Two-year clinical evaluation of an injectable in situ curing nucleus replacement. In Programs and Abstracts of the Annual Meeting of North American Spine Society, Toronto, 2008.
23 Pawulski A, Singh V, Nandakumar A, et al: 2-year clinical results of a safety pilot study evaluating a protein hydrogel nucleus replacement device. In Programs and Abstracts of the Ninth Annual Meeting of Spine Arthroplasty Society, London, 2009.