CHAPTER 46 Lumbar Disc Herniations
Lumbar disc herniations are a common manifestation of degenerative disease.1–3 They tend to occur early within the degenerative cascade, representing the tensile failure of the anulus to contain the gel-like nuclear portion of the disc. With improvements in advanced imaging techniques, lumbar disc herniations have been increasingly recognized in symptomatic and asymptomatic individuals.4
Treatment decision making for patients with herniated discs can be challenging. Nonoperative treatment can be effective in most cases.5–9 Other authors have indicated that surgery leads to superior results, especially in short-term pain relief.1,7–10 Several authors have highlighted the influence of fragment location and pattern and social and psychological factors on outcomes.7–9,11–13 The exact natural history and complex interaction of biologic, psychosocial, ergonomic, and cultural variables have not been well established.
Pathoanatomy
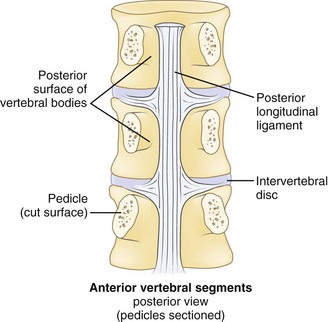
Effective evaluation is based on an intimate understanding of the relationship of the lumbar intervertebral disc to its surrounding structures. The disc is the anterior border of the spinal canal at the facet joint level. It is covered by the thin posterior longitudinal ligament, which is concentrated in the midline, from which small bands extend laterally to cover the inferior aspect of the disc (Fig. 46–1). This configuration leaves the superior part of the posterolateral disc bare and is thought to contribute to the fact that posterolateral (or paracentral) herniations are the most frequent location for herniations to occur. Cumulative degenerative changes occur in this region of the disc from concentration of torsional, axial loading, and flexion-induced biomechanical strains.
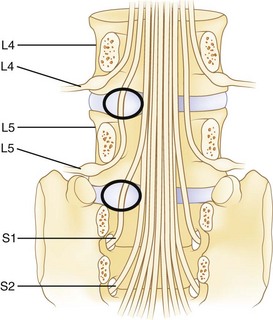
The spinal cord ends at approximately the L1 level in adults to form the conus medullaris. The cauda equina is located within the lumbar spinal canal. It contains the lumbar and sacral nerve roots bathed in cerebrospinal fluid contained, or encapsulated, by the pia, arachnoid, and dural membranes (meninges). Nerve roots branch from the cauda equina one level above their exiting foramen (Fig. 46–2). The L5 nerve root leaves the cauda equina approximately at the level of the L4 vertebral body. It descends inferolaterally to pass anterior to the L4-5 facet joint and posterior to the L4-5 disc. Intimately associated with the inferomedial aspect of the L5 pedicle, the root turns lateral to enter the L5-S1 intervertebral (neural) foramen just proximal to the L5-S1 disc. Within the foramen, sensory cell bodies form the dorsal root ganglion. The root, now termed a postganglionic spinal nerve, exits the neural foramen where it is in close proximity to the lateral aspect of the L5-S1 disc. Fibrous bands (called Hoffman ligaments) often tether the nerve to the disc in this region.14,15 After a short extraspinal course, the nerve divides into a ventral and dorsal primary ramus.
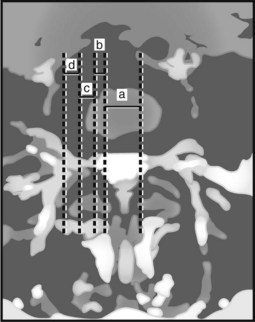
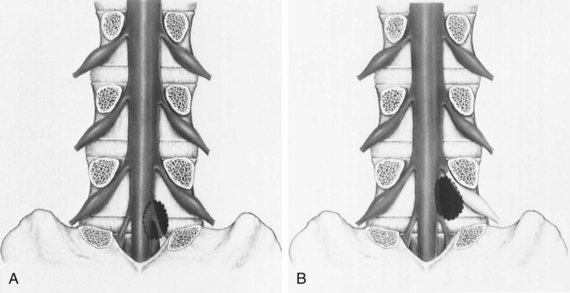
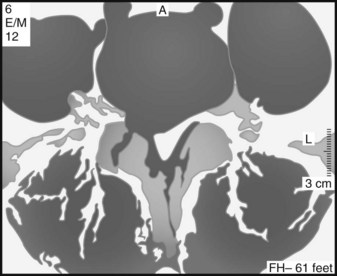
The location of the disc herniation determines which root is primarily affected. The spinal canal can be divided into longitudinal zones (Fig. 46–3). The central zone is delineated by the lateral borders of the cauda equina. The lateral recess is between the lateral border of the cauda equina and the medial border of the pedicle. Although the term lateral recess is frequently used to describe stenosis from bony encroachment (lateral recess stenosis), it sufficiently describes the location of paracentral, posterolateral, or juxtacentral herniations. Within the lateral recess, fragments medial to the nerve root, interposed between it and the cauda equina, are termed axillary herniations (Fig. 46–4). The foraminal zone is between the medial and lateral borders of the pedicle. Herniations beyond the lateral border of the pedicle are within the far-lateral or extraforaminal zone. Herniations in the foraminal or extraforaminal zones usually affect the exiting nerve.
Pathophysiology
Disc Degeneration and Herniation
Disc herniation is one stage of the lumbar degenerative cascade. It is considered one of the earlier stages, following internal disc disruption. Herniation occurs through a tear in the anulus fibrosus. The anulus is the thick outer layer that normally withstands tensile forces transferred from the compressed nucleus pulposus (Fig. 46–5).16,17 Force transfer works only if the nucleus-anulus-endplate complex acts as a closed volume system.18 Normally, compression across the disc space leads to increased pressure within the nucleus. The soft nucleus deforms and flattens, pushing against the annular fibers, which then generates tensile hoop stresses. The circumferential fibers are placed under tension, dissipating stresses and containing the anulus.
With disruption of the anulus, the soft nucleus can be pushed through (i.e., herniated) if placed under sufficient pressure. The nucleus must be fluid, or “dynamic,” enough to permit herniation to occur. Discs in younger individuals that have a well-hydrated nucleus are more likely to herniate. Older patients with desiccated discs are less prone to herniation. The ejected portion is typically a fibrocartilaginous fragment.19 In some cases, a piece of anulus or endplate fibrocartilage can be associated with it. In juveniles, an apparent herniation may represent a Salter type II fracture of the vertebral ring apophysis with its attached anulus.
When a portion of the nucleus is ejected, disc mechanics are altered. Frei and colleagues17 showed that nucleotomy alters the loading pattern across the disc space, with the anulus sustaining higher compression forces than normal. This situation can lead to increases in endplate pressures along the periphery where the anulus attaches to the bone. Chondro-osseous metaplastic changes such as osteophytes or sclerosis in these regions are a response to long-standing abnormal loading patterns.
Postural variations can influence intradiscal pressures. The highest pressures have been recorded in patients with the torso forward flexed with weight in hand. In an elegant biomechanical study, Wilder and colleagues16 found that combined lateral bend, flexion, and axial rotation with 15 minutes of exposure to vibration can lead to tears extending from the nucleus across the anulus. This finding may have significance for occupations with exposure to long periods of vibratory stimuli, such as truck drivers and machine workers.
Disc Herniation and Sciatica
In animals and humans, pure compression of a noninflamed nerve produces sensory and motor changes without pain, whereas pain is elicited with manipulation of inflamed nerves.20 These findings suggest that herniated discs large enough to cause mechanical compression of a nerve root may produce focal deficits, but that associated sciatic-type pain is produced only if the nerve root is concurrently irritated or inflamed. Inflammation may be produced by prolonged neuroischemia of the microvasculature of the nerve root from mechanical compression or by nonmechanical, possibly biochemical, factors. This phenomenon helps explain why some patients with small bulges or protrusions contacting inflamed nerves have pain that does not seem to be consistent with the “small” degree of neural compression. Additionally, these patients frequently do not have demonstrable sensory or motor deficit.
Neurochemical factors also have a role in the production of sciatic pain. This role may be related to initiation of an immune response locally or systemically or both. Spiliopoulou and colleagues21 examined IgG and IgM levels in discs excised from patients with sciatica and controls. Although IgG levels were equivalent, elevated levels of IgM were found in discs from sciatica patients but not in controls suggesting a local and humoral antigenic inflammatory reaction as a contributor to pain. Other investigators have shown the role of cytokines in the mediation of root pain. Olmarker and Rydevik22 studied the effects of selective inhibition of tumor necrosis factor-α in a herniated disc model in pigs. They found preservation of nerve conduction velocity and decreased nerve root injury in treated animals versus controls, suggesting a role of tumor necrosis factor-α in potentiating nerve dysfunction.
Similarly, research has suggested that matrix metalloproteinase, nitric oxide, prostaglandin E2, and interleukin-6 in discs excised from patients with herniation and radiculopathy may have a causative role in pain production.23 A more recent investigation was unable to confirm the presence of these inflammatory markers in the epidural space of patients with symptomatic disc herniations, however.24 Other investigators have shown that in extruded or sequestered discs a cellular inflammatory reaction may be locally mediated via T cells and macrophages25; this has been postulated to play a role in herniated disc regression.26
There is evidence of systemic inflammatory responses to disc herniations as well. Brisby and colleagues27 detected elevated levels of glycosphingolipid antibodies in the serum of patients with sciatica and disc herniation compared with healthy volunteers. Elevations were equivalent to those found in patients with autoimmune neurologic disorders such as Guillain-Barré syndrome. Brisby and colleagues27 suggested that a systemic autoimmune response to disc tissue may result in damage, or alteration, of nerve tissue. After age 12, the endplate apophyseal vessels close, which may facilitate an amnestic antigenic response to exposure to extruded nucleus pulposus tissue. These findings are helpful in considering patients who have severe sciatic pain with minimal mechanical compression and patients who seem to have persistent symptoms despite surgical decompression.
Disc Herniation and Back Pain
The concept of vertebrogenic pain has also been suggested. Jinkins and colleagues28 studied the contribution of anterior disc herniations to back pain. They believed that the pain was neurally mediated through branches of the ventral ramus and paravertebral autonomic plexus. Because the herniations were outside the spinal canal, they were not associated with compression of the cauda equina or nerve roots, but most patients complained of lower extremity paresthesias, mostly bilateral, in addition to low back pain. A direct causal relationship between anterior disc herniations and leg symptoms has not been clarified.
Classification of Disc Herniations
Classification of any disorder should be based on identifiable features that have some influence on prognosis or treatment decision making. Many classification systems have been proposed for lumbar disc herniations, although none are all-inclusive or ideal.29,30 It is more appropriate to consider them as tools to describe the herniation.
Morphology
Disc herniations can be described by their morphology. Before the introduction of advanced imaging, morphology was difficult to assess preoperatively. Currently, magnetic resonance imaging (MRI) and to a lesser extent computed tomography (CT) can differentiate disc morphology with reasonable reliability. Spengler and colleagues13 divided herniations into three types (Fig. 46–6). A protruded disc was defined as eccentric bulging through an intact anulus fibrosus. An extrusion was defined as disc material that crosses the anulus but is in continuity with the remaining nucleus within the disc space. A sequestered disc represents a herniation that is not continuous with the disc space; this is the typical “free fragment.”
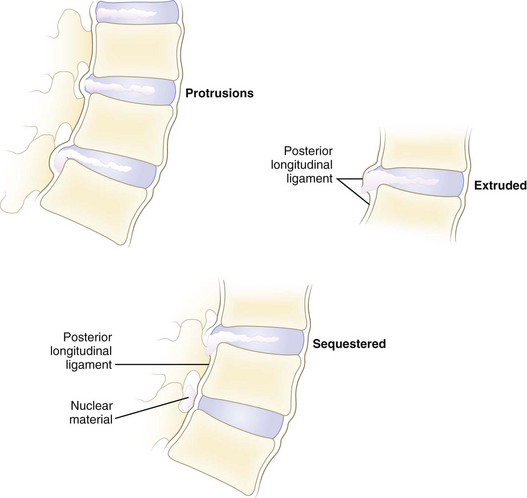
FIGURE 46–6 Classification of disc herniations as described by Spengler and colleagues.13 Disc protrusion is defined as bulging displaced nucleus that has not extended beyond limits of anulus fibrosus. Extrusion extends beyond anulus fibrosus but is still in continuity, at least partially, with parent disc. Sequestered disc herniation implies that fragment has broken free (i.e., free fragment) and is no longer in continuity with parent disc. In some cases, in which disc herniation lies immediately behind vertebral body, it is difficult to tell from which disc the herniated fragment originated.
Other authors have classified discs as either contained or uncontained.31 Contained disc herniations are subligamentous. It is presumed that they have not passed beyond the limits of the posterior longitudinal ligament or the outer layer of the anulus. Uncontained disc herniations have crossed this boundary. Advocates of this system describe contained and uncontained extrusions, with the former remaining beneath the outer layers of the anulus.31
Location
Herniations can be described topographically according to anatomic location (see Fig. 46–3). The herniation can be located within the central zone, lateral recess, foraminal, or extraforaminal regions. Herniations can also exhibit cranial or caudal migration in relation to the disc space.
Timing
Lumbar disc herniations can be organized according to the time from initial symptom onset. These may be arbitrarily divided as acute or chronic. Acute herniations are present for less than 3 to 6 months, whereas chronic discs cause symptoms for a longer time. Breakdown according to this time frame is based on the authors’ sense of what is a reasonable cutoff point. Because the results of disc excision seem to be influenced by the timing of surgery, this categorization is important. From a survey of the literature, it seems that the results of disc excision are compromised if delayed more than 2 to 16 months from symptom onset.12,32–34
History and Symptoms
Many patients describe a prodromal history of long-standing mild to moderate back pain. Although trauma is not the only component leading to a disc herniation, some patients describe a specific incident attributable to the onset of leg and back pain. This incident may be a fall, a twist, or lifting of a heavy item. Specific postures can lead to exponential increases in intradiscal pressure, which can predispose to disc injury.18 Exposure to vibrational energy combined with sustained lateral flexion and rotation may also predispose to herniation.16 The exact history of the incident and the presence of preexistent back or leg pain must be explored; this is particularly important in work-related injuries.
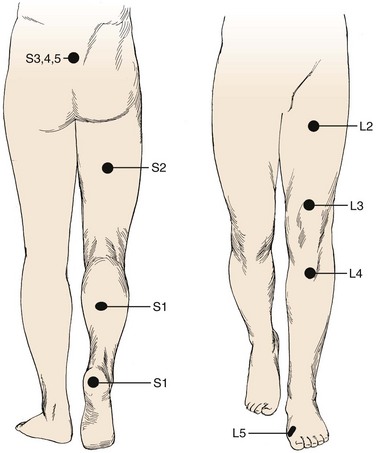
Pain is the most common complaint. Axial back pain is typically present, although some patients do not have this complaint. Radicular pain is more typical and often the more “treatable” of the complaints. The pattern of lower extremity radiation depends on the level of the herniation. Lower lumbar or lumbosacral disc herniations can lead to the classic symptoms of pain radiating below the knee. Often pain extends into the foot and can follow a dermatomal distribution. S1 radicular pain may radiate to the back of the calf or the lateral aspect or sole of the foot. L5 radicular pain can lead to symptoms on the dorsum of the foot (Fig. 46–7). Radiculopathy from involvement of the upper lumbar roots can lead to more proximal symptoms. L2 and L3 radiculopathy can produce anterior or medial thigh and groin pain. Groin pain may also be indicative of L1 pathology. Radicular pain can be difficult to discern and is often not “classic.” Many patients do not exhibit pain in a specific dermatomal distribution, or the radiation does not extend along the entire leg. It may radiate only into the hip region or just the foot or any portion of the leg.
The influence of social and psychological factors on the outcomes of disc surgery has been well documented. It is highly recommended to obtain a social and at least cursory psychiatric history. Prescription use of antidepressants is an important clue, although depression is often undiagnosed and untreated at the time of initial presentation. Other personality factors, such as chronic headaches, hysteria, hypochondriasis, nervous disorders, and impulsivity, can also be influential.13,35 Work history, pending litigation, and type of work should be obtained. A history of smoking is an independent risk factor for low back pain and a risk factor for a poor result after back surgery.35,36
Physical Examination
Inspection
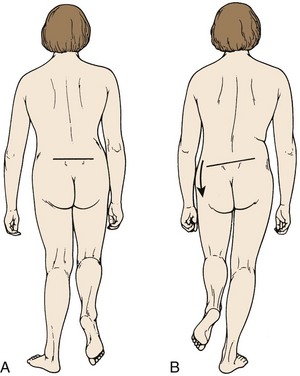
Inspection is the first step in the physical examination. As the patient walks into the examining room, gait should be observed. A sciatic list may be present, usually manifested as the patient leaning away from the side of leg pain. This sciatic list is thought to be associated with a paracentral herniation lateral to the nerve root. Axillary herniations may cause a list toward the side of herniation. The list is an attempt to relieve neuromeningeal tension by drawing the nerve root away from the herniated fragment. Another feature of gait that should be noted is a wide-based gait, indicative of lumbar or more cranial canal stenosis. A footdrop or foot slapping gait may occur with L4 or L5 paresis. A Trendelenburg gait can suggest hip abductor weakness (Fig. 46–8), which may be a clue to L5 nerve root compression because the gluteus medius is most often an L5 dominant muscle.
Neurologic Examination
A neurologic examination is required in all patients with suspected herniated discs. Sensation of light touch is tested along dermatomes from L1 to S1. Standard dermatomal charts can be helpful, but there is variability among individuals, and so this is highly subjective. In testing the upper lumbar roots, there is often a significant amount of overlap. The most discrete levels of testing are for L4, L5, and S1 nerve roots.37 These nerve roots are the most often affected by lumbar disc herniations (Table 46–1). L4 sensory function is tested at the medial ankle; L5, at the first webspace between the great and second toes; and S1, at the lateral aspect of the sole of the foot. Sensation is difficult to “grade.” It is more useful to document sensation as normal, diminished, or absent. Sensory function should be compared with the contralateral side because this may help detect differences. The examiner should be wary of the presence of a glove-and-stocking distribution sensory loss, which can indicate a peripheral neuropathy, such as associated with diabetes, or functional overlay—as it is not anatomic.
| Characteristic | Prevalence (%) |
|---|---|
| Any low back pain | 60-80 |
| Any low back pain persisting at least 2 wk | 14 |
| Low back pain persisting at least 2 wk at a given time (point prevalence) | 7 |
| Back pain with features of sciatica lasting at least 2 wk | 1.6 |
| Lumbar spine surgery | 1-2 |
From Deyo RA, Loeser J, Bigos S: Herniated lumbar intervertebral disc. Ann Intern Med 112:598-603, 1990.
The motor examination should proceed in a routine manner. In the lower extremity, it is better to test movements rather than specific muscles. S1 motor function is assessed by testing plantar flexion, whereas L5 is tested by toe dorsiflexion, particularly the great toe (extensor hallucis longus), and hip abduction. L4 involvement most often affects ankle dorsiflexion (anterior tibialis), although quadriceps function can be compromised. There is a significant amount of overlap of upper lumbar motor innervation. Knee extension can be considered L3 function (although L2 and L4 contribute); hip flexion, an assessment of L1-2 function; and hip adduction, an assessment of L2 function. Motor function is graded as 0 to 5, with 5 being full strength against active resistance (Table 46–2). In particular, S1 function should be assessed by asking the patient to toe raise repeatedly or toe-walk. Because of the enormous strength of the gastrocnemius complex, even a weakened muscle can overcome the examiner’s hand. Toe-walking can show smaller differences, however, from side to side by using the weight of the patient’s body as the resistance. Repetitive toe raising may help detect smaller differences.
TABLE 46–2 Motor Strength Grading System by Physical Examination
| Grade | Definition |
|---|---|
| 0 | No visible muscle contraction at all |
| 1 | Visible muscle contraction; no joint movement |
| 2 | Can move joint but not overcome gravity |
| 3 | Able to overcome gravity but cannot overcome any examiner resistance |
| 4 | Able to overcome some, but not full, examiner resistance |
| 5 | Full strength; able to resist full examiner force |
Specific Tests
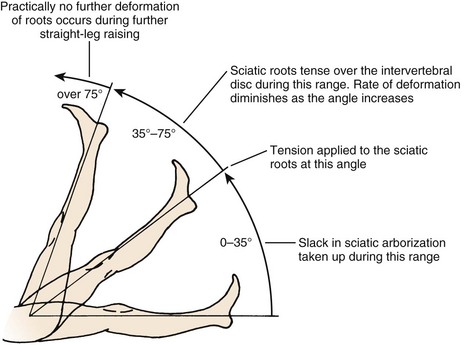
The straight-leg raise (SLR) test is an extremely useful provocative test in examining patients with a herniated disc (Fig. 46–9). The classic test is performed with the patient in the supine position. The heel of a relaxed leg is cupped by the examiner’s hand and elevated slowly. The knee is kept in extension while the hip is flexed. The test is considered positive if sciatic pain is reproduced between 35 degrees and 70 degrees of elevation. Studies have determined that in the first 35 degrees of elevation, the slack in the nerves is taken up, and at 35 degrees or more, tension is placed on the nerves. More than 70 degrees of elevation causes no further stretch of the nerve roots. The SLR test is best for eliciting L4, L5, or S1 radiculopathy. It is not useful for upper lumbar roots, for which a femoral stretch test should be used. A positive SLR test is indicative of nerve root compression in 90% of cases.38 It does not implicate a herniated disc as the source of compression, however, because foraminal encroachment or other mass lesions can lead to a positive SLR test as well.
The so-called slump test is a variant of the Lasègue test and the SLR test. This test is performed in the seated position; the patient is asked to flex the thoracic and lumbar spine while fully flexing the neck. Next, the SLR test is performed while the foot is dorsiflexed on the same side, as denoted by the Lasègue test. The combination of these maneuvers adds cephalad gliding of the spinal cord to the examination, whereas the SLR test and Lasègue test by themselves produce only caudal tension on the nerve roots. A more recent study found the slump test was more sensitive than the SLR test in patients with lumbar disc herniations, whereas the SLR test was more specific.39
Differential Diagnosis
Diagnostic Imaging
Magnetic Resonance Imaging
MRI is the most popular modality for advanced imaging of lumbar disc herniations. MRI is superior to CT in delineating soft tissues. The disc and fragments that may have herniated from it are readily visualized. Free fragments (sequestered) can be differentiated from extruded disc herniations (Fig. 46–10), and a symmetrical bulge can be differentiated from a contained protrusion. The neural elements themselves are well visualized. Neural encroachment can be detected within the spinal canal, the foramina, or extraforaminally. MRI is also useful in differentiating disc herniations from tumors, vascular anomalies, or bony compression.
Numerous features of a herniated disc can be noted on MRI. The size and type of disc herniation can be reliably determined using MRI, which may have prognostic significance.11,40,41 Carragee and Kim11 correlated outcomes with herniated fragment size and its effect on canal area. Larger discs (>6 mm) were more likely to have a positive SLR test or femoral stretch test (Wasserman sign). In the operative group, larger discs were predictive of a better outcome. The fair and poor outcomes in operative patients were in patients with small discs (<6 mm).
Attempts to correlate MRI findings with clinical symptoms have been made. In 33 patients in whom disc herniation was diagnosed clinically and 5 control patients with low back pain alone, Kikkawa and colleagues42 performed three-dimensional MRI using a fast low angle shot (FLASH) with gadolinium enhancement. Dorsal root ganglion enhancement was found to be nonspecific, occurring in controls and sciatica patients. Enhancement of the root proper was detected, however, in 11 of 30 symptomatic patients, with patients having a statistical tendency for more severe motor involvement. There was no significant association of diffuse versus local enhancement with the positivity of the SLR test or sensory changes. Central compression of the cauda equina did not lead to enhancement in any cases. Although these results are modest, they suggest a future use of gadolinium-enhanced MRI as a noninvasive method of determining the microvascular response to compression of neural structures.
Komori and colleagues41 studied the significance of enhancement around the herniated fragment itself. Patients with radiculopathy underwent initial and follow-up gadolinium-enhanced MRI to correlate clinical improvement with the degree of enhancement. Patients with marked decrease in size of the herniation showed good clinical resolution. This resolution was most significant in the “migrating”-type discs, which were closest to sequestered discs according to the authors’ description. Decrease in fragment size was associated with a gradual increase in the area of enhancement in 17 of 22 sequestered disc herniations, all of which had improvement of radicular pain. Five cases of sequestered discs without enhancement or size decrease had a poor clinical result. Enhancement was less marked in extruded versus sequestered herniations; however, herniations that did show enhancement had a significantly better clinical course. From these data, Komori and colleagues41 recommended this test as a prognostic tool in guiding the treatment of patients with extruded or sequestered herniated discs.
Of more recent interest is the influence of posture on the MRI appearance of discs and their relationship to the neural structures. Because images are traditionally acquired in the supine position, the spine is not axially loaded as it is during everyday activities. Weishaupt and colleagues43 performed positional MRI in patients with low back or leg pain for 6 weeks that was not responding to conservative treatment. Images were obtained in the usual supine position and with a seated flexed and extended posture. Changes in foraminal size and neural compression occurred with flexion and extension. Changes in foraminal size correlated with increased pain scores. These findings are probably most significant for low-grade herniations (i.e., bulges or protrusions) in which there is still a fixed-volume system within the disc space provided by an intact outer annular layer. Similar findings have been shown using dynamic functional plain myelography.44
Magnetic Resonance Imaging in the Postoperative Spine
As the modality of choice for imaging the neural structures, MRI is frequently obtained. Because of edema, hematoma, and formation of surgical scar, MRI is best delayed until 6 months after surgery,45 if symptoms allow. The main challenge is differentiating scar from new-onset disc. On standard T1-weighted sequences, this differentiation can be difficult. In the early days of MRI, T2-weighted images were not as useful because of longer scan times with inadequate magnet strength.46
Evidence suggests that sophisticated T2 image analysis might supplant the need for gadolinium-enhanced MRI. Barrera and colleagues47 compared different imaging sequences with and without gadolinium contrast agent. These investigators documented 100% sensitivity for detecting scar for T2-weighted turbo-spin echo (TSE) and fluid attenuated inversion recovery (FLAIR) sequences compared with T1-weighted images with gadolinium. Specificity was 94% and 92% for TSE T2 and FLAIR images. Barrera and colleagues47 concluded that standard TSE T2 images acquired using a rapid sequence are extremely sensitive and specific in distinguishing disc from scar in most cases and that the use of gadolinium contrast agent should be reserved for the rare situation in which that distinction cannot be made. These recommendations are supported by others.46
Grane and Lindqvist45 studied the role of gadolinium enhancement of the nerve roots after discectomy. These investigators found intradural (within the cauda equina) nerve root enhancement in 59% of patients with recurrent clinical symptoms. Recurrent symptoms occurred, however, in 84% of patients with focal (extradural, after the nerve root has existed the cauda equina) enhancement and 86% of patients with nerve root thickening. Enhancement occurred in patients with and without evidence of nerve root displacement by scar or disc. This finding indicates that although symptoms may correlate with MRI enhancement, it is not associated with a compressive mass lesion.
In an early report on the use of MRI without gadolinium, Bundschuh and colleagues48 studied 20 patients after failed disc surgery who had a strong likelihood of undergoing further surgery. In 14 patients, CT with contrast agent was also performed. The authors found that free fragments of disc had a mildly increased signal on T1 images compared with scar, whereas scar and disc were similarly hyperintense on T2 images. Overall, Bundschuh and colleagues48 believed that MRI was at least comparable to CT with contrast agent in differentiating scar from disc, confirmed by intraoperative findings.
Myelography
Plain myelography previously was the imaging modality of choice in detecting herniated discs. It involves injection of intrathecal contrast material to outline the boundaries of the subarachnoid space and silhouette the enclosed neural elements. It is invasive and cannot show compression beyond the confines of the subarachnoid space. Extradural compression caused by a foraminal or extraforaminal disc can be missed. Advantages of myelography are that it is a dynamic test because images can be made with the patient standing.44 Myelography should be reserved for cases in which noninvasive imaging, such as CT or MRI, are nondiagnostic, equivocal, or contraindicated. Currently, myelography is rarely used for the routine workup of herniated discs. When used, it is usually followed by a CT scan.
Computed Tomography
Some disc herniations can contain gas (Knuttson phenomena), noted on CT images. Mortensen and colleagues49 reported four such cases that responded well to surgical discectomy. It is unknown if the gas forms before or after herniation. The clinical significance of the gas is not well understood. Ford and colleagues50 determined that intradiscal gas is composed predominantly of nitrogen.
Natural History
In a widely quoted retrospective study, Saal and Saal5 found a 90% good or excellent outcome in patients treated nonoperatively for a lumbar disc herniation diagnosed by clinical examination and CT. Inclusion criteria were strict, including patients with SLR test positive at 60 degrees or less, leg pain greater than back pain, and electromyographic evidence of radiculopathy. Of patients, 92% returned to work. Nonoperative treatment consisted of aggressive physical therapy and back school education. A possible confounding factor is that many patients were referred for a second opinion regarding surgical versus nonsurgical treatment because they were anxious to avoid surgery. This may have introduced preselection bias error because the authors of the study were not surgeons. Concern has been raised about eventual fibrosis formation with nonoperative treatment of herniated discs. In a follow-up MRI study,6 the same investigators documented no increased risk for perineural fibrosis or adhesions with nonsurgical management.
Other authors have reported more modest results. In the nonoperative arm of Weber’s10 classic randomized study, the long-term outcome of lumbar disc herniations was observed in 49 patients. Inclusion criteria were clinical signs and symptoms of L5 or S1 radiculopathy in addition to myelographic evidence of nerve root compression. Treatment included full-time bed rest for 1 week followed by partial bed rest the 2nd week and back school instruction as an inpatient. At 1 year, 33% had good results, 49% had a fair result, and 18% had a poor result. At 4 years, good results were reported in 51%, fair results were reported in 39%, and poor or bad results were reported in 10%. Because the tiered system is slightly different than that used by Saal and Saal,5 a direct comparison of the studies is difficult. If Weber’s good and fair results are equated to Saal’s excellent and good results, an 89% success rate achieved in the former at 4 years may be considered comparable to the latter’s 90% success. Many of Saal and Saal’s patients ultimately dropped out of the study and underwent surgical discectomy so that their 90% success rate might represent an overestimation.
In another nonoperative arm of a comparative study, 10-year follow-up results from the prospective Maine Lumbar Spine Study showed 61% improvement in the predominant symptom, 40% resolution of low back symptoms, and 56% satisfaction rate.7 Work and disability status were comparable between operative and nonoperative groups in this investigation. Similar findings were reported for the observational cohort of the Spine Patient Outcomes Research Trial (SPORT).8
Methods of Nonoperative Treatment
Physiotherapy
Bed rest should be limited to no more than 2 to 3 days.51 Greater periods of inactivity can potentiate prolonged disability and continued or augmented pain. Exercise therapy and physical rehabilitation should be included in the nonoperative care of herniated discs. Treatment goals are to restore strength, flexibility, and function that were lost secondary to pain, splinting, and spasm. Postural education to avoid activities that can increase intradiscal pressure or neuromeningeal tension or both should be provided.
Adjunctive modalities can aid in relieving some associated symptoms. These modalities include ultrasound treatment, electrical stimulation, and massage. These may be helpful in short-term, symptomatic relief of back pain. Traction is also commonly prescribed. It theoretically may diminish intradiscal pressure, increase foraminal dimensions, and possibly relieve radicular pain secondary to herniated discs.52 The role of chiropractic manipulation is controversial. Although some patients believe that manipulations have “reversed” the herniation, there is no evidence to support the ability of chiropractic manipulation to alter the normal or pathologic morphology of the disc.53
Pharmacologic Treatment
In a prospective series touted as a natural history study, Bush and colleagues54 reported the results of 159 patients with CT-confirmed disc herniations treated with epidural steroid injections. Although 91% avoided surgery, this underscores a glaring problem of so-called natural history studies in that an interventional treatment was used. Although its exact biochemical effects are still being elucidated, steroid injections may be effective in “avoiding” surgery.
In a well-designed retrospective study, Wang and colleagues55 studied 69 patients who failed nonoperative (or, more accurately, noninvasive) care and had requested surgery as treatment. Instead, each patient was advised to undergo one or more transforaminal steroid injections at the affected root level. Of patients, 77% had clinical resolution and had not undergone surgery at an average follow-up of 1.5 years. In agreement with other studies, clinical success was not related to disc size, percentage canal compromise, or degree of motor weakness. From these findings, selective nerve root steroid injections seem to produce at least short-term relief of radicular symptoms from a herniated disc.
Operative Versus Nonoperative Treatment
The results of operative and nonoperative treatment for symptomatic herniated discs have been compared in numerous studies. The Maine Lumbar Spine Study group published 1-year and 5-year results of an ongoing comparison of surgically and nonsurgically treated patients.1,56 More than 500 patients were included in the prospective observational study without stringent clinical or radiographic criteria except for disc-related sciatica treated with at least 2 weeks of nonoperative care within 2 months of onset. The decision to undergo surgery was determined on an individual basis and was not randomized. At the 1-year follow-up, surgically treated patients were less symptomatic than patients in the nonoperative group, despite the former being more symptomatic at initial presentation. Relief of back or leg pain was reported by 71% of operated patients compared with 43% of the nonoperative group. High satisfaction levels and improved quality of life were documented for the operative group. For the workers’ compensation group, there was no difference in time to return to work between operative and nonoperative groups. A criticism of the study is a substantial attrition rate, with 24% of patients unavailable for final follow-up.
In the 5-year outcome report, 70% of surgical patients reported back or leg pain improvement, whereas 56% of nonoperatively treated patients reported improvement.1 As with the 1-year results, a similar percentage of patients were receiving workers’ compensation benefits in both groups with no difference in return to work at final follow-up. Reoperations were performed in 20% of the operative patients; 16% of patients initially treated nonsurgically went on to operation. The authors noted that the benefits of surgery versus nonoperative treatment were greatest in the early part of the study within the first 2 years and that at final follow-up these advantages were less apparent.
Most recently, the Maine Lumbar Spine Study Group published their 10-year follow-up results.7 Of the eligible patients initially enrolled, data were available for 85% of patients treated surgically and 82% of patients treated nonoperatively. A significantly larger percentage of surgical patients reported relief of low back and leg pain than patients treated nonoperatively. Similarly, surgical patients exhibited better function and satisfaction compared with nonoperative patients. Nonetheless, improvement in dominant symptom was reported for both treatment groups. Work and disability status were similar for both groups.
The 2006 SPORT investigation was designed to be a rigorous, randomized, prospective, controlled study.8,9,57 In its execution, there was a high rate of crossover between operative and nonoperative groups and a substantial portion of patients not willing to be randomized, resulting in a large observational group. The randomized and observational arms of the investigation reported improvement in bodily pain, physical function, and Oswestry Disability Index (ODI) scores regardless of intervention. In the randomized arm of the study, between-group differences favored surgical intervention, but these differences did not reach statistical significance in an intention-to-treat analysis. With the difficulties encountered with nonadherence to assigned treatments (i.e., crossover), the intention-to-treat analysis may not be reflective of the true outcomes. In an as-treated analysis, surgical treatment showed statistically superior results compared with nonoperative treatment. Similar results were found in the observational arm of SPORT.
The classic work by Weber10 reported similar results to these findings. As briefly discussed in the prior section on natural history, Weber10 compared surgery versus nonoperative care in a randomized, prospective study. There were three study groups: one group “required surgery”; another showed no indications for surgery; and the third was the “undecided” group, in which it was unclear if surgery would be beneficial. Only the third group was randomly assigned to surgical or nonsurgical treatment.
Alaranta and colleagues58 also prospectively compared operated versus nonoperated patients. In contrast to other similar studies, the nonoperative group was subdivided into cases with and without myelographic evidence of nerve root compression, whereas all operated patients had a positive myelogram. At 1-year follow-up, 91% of operated and 82% of nonoperatively treated patients with positive myelograms had improved pain levels. Only 51% of nonoperatively treated patients with negative myelograms had improvement. These data suggest that a distinctly worse natural history exists for patients with sciatica and no evidence of root compression. Although not specified by the investigators, this group probably represents sciatica from an extraspinal or non–disc-related origin. In a companion study published by the same authors, they further identified this group as having a high incidence of generalized pain (e.g., concomitant occipital headaches), more physically strenuous jobs, and lower pain thresholds.59 This study could possibly point to a preponderance of psychosocial and behavioral factors involved in the perpetuation of the symptom complex.
Operative Treatment
Indications
Only a few patients match such textbook descriptions. Most patients lack one or more of the supportive diagnostic clues, making it more difficult to support the decision to operate. This situation does not represent a contraindication to surgery, however, because many published series show approximately 85% success rates in patient groups with lesser percentages of objective motor and sensory findings.3,38,56,60–67 The slim chance of back pain relief in relation to leg pain with discectomy that surgeons obligatorily confess to their patients is also probably an underestimation because most series document at least modest improvements in back pain. It is incumbent on clinicians to discuss the advantages, disadvantages, risks, alternatives, and estimated expected outcomes with patients.
Available Techniques
A vast array of techniques exist for surgical treatment of herniated discs.68–73 Standard open discectomy is the most common surgical approach.60,65,74,75 It involves careful incision planning, laminotomy or partial laminectomy to provide adequate visualization of the pathology, gentle retraction of the neural elements, and direct excision of the herniation. As an adjunct to open discectomy, some surgeons advocate the use of a microscope for better visualization and minimizing incision size.31,67,71,76,77 The purported advantage of the microscope is the ability for the surgeon and the assistant to visualize the operative field equally through a smaller surgical wound.
Alternatives to interlaminar techniques have been developed for excision of foraminal and extraforaminal lateral disc herniations, which involve exposures between the transverse processes and lateral to the pars interarticularis.14,77 Although some surgeons have advocated the addition of fusion,64,78 this practice is unpopular.69 Advocates hold that fusion decreases the chance for reherniation; however, only a complete discectomy with interbody reconstruction can eliminate this risk. Long-term effects such as an adjacent segment degeneration and the additional morbidity and complication rates are potential disadvantages.64,69,78,79
With increased interest in smaller incisions and minimally invasive surgery, various percutaneous methods of treatment have been developed. Some methods entail placement of a cutting device intradiscally to decompress the disc space to retract the herniated fragment.80–83 Other methods involve percutaneous techniques of directly visualizing the neural elements and disc using an endoscope.68,84 Chemical digestion of the disc (i.e., chemonucleolysis) has enjoyed popularity; however, enzyme-related complications and results inferior to open discectomy have limited its continued popularity in the United States.80,85,86
Open Simple Discectomy
Timing
Rotheorl and colleagues33 stratified operatively treated patients according to time from presentation to surgery. Patients with symptom duration more than 2 months had a statistically significantly worse outcome than patients operated on within 2 months. There was no difference if surgery was performed within 1 or 2 months. Likewise, Hurme and Alaranta12 found the best results in patients operated on within 2 months of the onset of disabling sciatica. Nygaard and colleagues32 reported worse results in patients with leg pain for 8 months or more; Jansson and colleagues36 found similar results in their analysis of the Swedish registry data, which included greater than 27,000 patients. Sorensen and colleagues34 found that symptom duration greater than 16 months was predictive of poor results, but this was highly influenced by patient personality and social factors. The disparity between these findings is difficult to explain. Randomized prospective data comparing early versus late surgery are lacking. In the authors’ practice, surgery is performed within 6 months from symptom onset if nonoperative care has failed. The important message is that there is not an urgency to perform surgery. Nerve roots, as compared with the spinal cord, are fairly resilient.
Other factors can influence the time to surgery. Ito and colleagues40 found patients with uncontained herniations had surgery much earlier than patients with contained herniations. Specifically, 56% of patients with uncontained herniations and 21% of patients with contained herniations had surgery within 1 month. This finding was not correlated to outcomes but was influenced more by the severity of symptoms. Early surgery does not seem to affect the rate of neurologic recovery; objective improvements in motor and sensory deficits do not seem to correlate with symptomatic relief and overall success rates.87
Technique
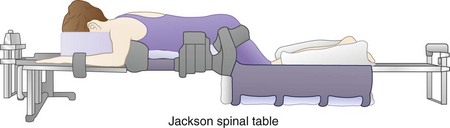
General endotracheal anesthesia is induced. Alternatively, spinal anesthesia can be used. The patient is carefully logrolled into the prone position. An Andrews frame can be attached to a regular operating table to facilitate the prone kneeling position. Special spine frames, such as the sling attachment for the Jackson (OSI) table (Mizuho OSI, Union City, CA), can be used. These frames allow hip flexion to produce some flexion of the lumbar spine, which widens the interlaminar space (Fig. 46–11). The kneeling position also allows the abdomen to hang free, which helps indirectly to reduce the epidural venous pressure and reduce intraoperative blood loss. One set of hip pads is placed just distal to the anterior superior iliac crest. Adequate padding protects against lateral femoral cutaneous nerve injury. The thighs are allowed to flex at the hip, with the knees resting within the sling. The sling should be positioned low enough to allow adequate hip and knee flexion and is padded with gel pads and pillows. A pad is placed between the knees and medial malleoli to prevent pressure necrosis. The transverse chest pad is placed just above the xiphoid process.
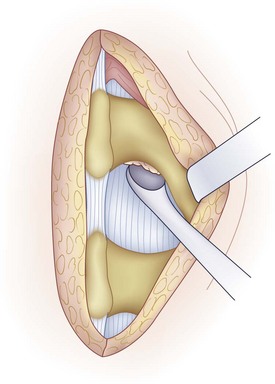
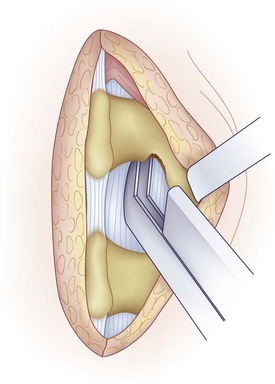
A medium-sized curet is used to detach the ligamentum flavum from the inferior aspect of the superior lamina (Fig. 46–12). Because the ligamentum inserts along the anterior aspect of the lamina, the curet needs to be introduced at an angle with the spoon facing cephalad. The instrument is worked from side to side to release all layers of the ligamentum. In some cases, it is difficult to release the ligamentum flavum completely without removing a portion of the inferior hemilamina (Fig. 46–13). Bone resection can be kept to a minimum but should not be avoided if it jeopardizes the exposure of the cauda equina and nerve root.
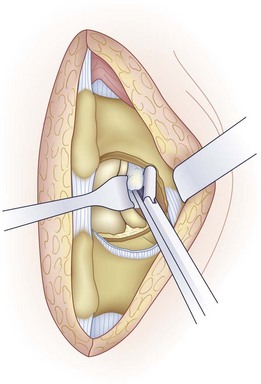
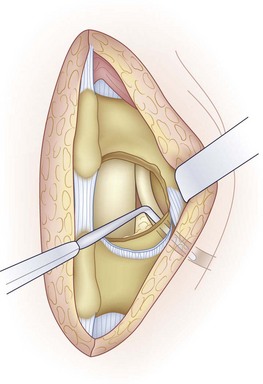
Before discectomy, a blunt-tipped probe is passed along the root and out the foramen to assess the amount of space available. Using a Penfield No. 4 elevator, the nerve root is mobilized medially. This mobilization can be facilitated using the bipolar cautery to lyse adhesions between the dura and the posterior disc. Retracting the nerve root medially shows the posterior anulus or the herniated fragment or both. A nerve root retractor is used to maintain retraction (Fig. 46–14).
The mobility of the nerve root is assessed using a Penfield No. 4 or Woodson elevator (Fig. 46–15). After discectomy, the nerve root should be freely mobile and under no tension or compression. If it is not, additional pathology should be suspected. Using a Woodson elevator, the epidural space must be systematically inspected in a clockwise fashion, checking the canal ventral to the thecal sac and the patency of the foramen. A concomitant lateral recess or foraminal stenosis from bony encroachment is decompressed with Kerrison rongeurs. Finally, the disc space is irrigated with normal saline to dislodge any remaining loose fragments.
If the ligamentum flavum was spared, it can be loosely replaced. A fat graft placed over the dura does not improve clinical results but does facilitate re-exploration and theoretically prevents adhesions from inhibiting normal neuromeningeal motion.88,89 The wound is copiously irrigated. Adequate epidural hemostasis is ensured before closure. In most cases, a drain is not needed. All retractors are removed, and the deep lumbar fascia is approximated with interrupted No. 1 absorbable suture. The subcutaneous layer is closed in two layers, and the skin is approximated with a subcuticular stitch or staples. The wound is cleaned, and a sterile dressing is applied.
Postoperative Care and Rehabilitation
After an uncomplicated simple open discectomy, the patient is discharged on postoperative day 1 or 2. Outpatient discectomy can be performed on selected patients with appropriate presurgical planning and postdischarge discussion.74 The level of activity in the 1st week after surgery usually is limited by incisional pain. If leg symptoms were predominant preoperatively, the patient typically reports immediate relief.
Unrestricted activity protocols have shown success rates comparable to other series. Carragee and colleagues75 conducted an uncontrolled, prospective trial of 152 patients who were allowed full activity after lumbar discectomy. Approximately one third of patients returned to work in 1 week, with most (97%) returning to work within 8 weeks. Recurrent sciatica occurred in 17 patients and was considered possible reherniation (11%). Nine of these patients experienced improvement, and eight underwent repeat surgery. This rate is similar to reherniation rates previously reported, which have ranged from 0% to 13%. Carragee and colleagues75 concluded that an unrestricted activity protocol does not negatively affect results after discectomy.
Other studies have focused on possible advantages to an early rehabilitation protocol after discectomy. Kjellby-Wendt and Styf90 performed a prospective, randomized, controlled study comparing an early active versus traditional training program. The focus of early activity was to reduce lumbar edema and maintain mobility of the neural elements through motion and trunk-strengthening exercises. A pain-coping program was included in the early active group that was not included in the traditional group. There was greater range of motion and decreased pain at 12 weeks in the early group. At 1-year follow-up, both groups were equivalent. Patient satisfaction was ultimately greater in the early active group (88%) versus the control group (67%), although this was not statistically significant. In critique of the study, it was unclear why the difference in subjective outcome was not accompanied by sustained objective benefits. Inclusion of the pain-coping program in the study group, which would have minimal influence on range of motion, may have positively influenced patients’ perception of their residual impairment. In support of the findings by Carragee and colleagues,75 there was no difference in reherniation rates between the two groups.
Alaranta and colleagues91 performed a randomized trial comparing an “immediate” versus “normal” rehabilitation program. Physical activities, such as sports and games, were encouraged in the immediate activity group; psychological and social counseling were also provided. Details of the normal rehabilitation protocol were not provided. At 1-year follow-up, there were no differences in subjective outcomes, postoperative impairments, or return to work statistics. These results further indicate that early activity programs do not seem to be harmful but offer only minimal, if any, long-term benefits. The current authors’ decision to initiate early return to unrestricted activity is made on an individual basis.
Outcomes
Carragee and Kim11 correlated operative outcomes with herniated fragment size and its effect on canal area. Using axial MRI, these authors recorded several parameters including disc area, canal area, anteroposterior disc length, and anteroposterior canal length. Patients with larger discs (>6 mm) were more likely to have positive SLR or femoral stretch tests. Comparing patients with operative and nonoperative treatment, the former had larger anteroposterior disc lengths and larger ratios of disc to canal area. In the operative group, larger discs were predictive of a better outcome. In the nonoperative group, symptom duration less than 6 months before presentation, no litigation, and younger age were predictive of a better outcome. All of the fair and poor outcomes in operative patients were in patients with small discs (<6 mm). Summarizing these findings, it seems that larger discs respond to surgery better than small discs and that disc size is less predictive of outcomes in the nonoperative group. A criticism of this study involves the existence of a selection bias because the decision to treat operatively was nonrandomized.
Knop-Jergas and colleagues92 analyzed the results of lumbar discectomy based on anatomic location. They characterized herniations as central, paracentral, intraforaminal, extraforaminal, or multiregional broad-based protrusions. The best outcomes were documented with paracentral and intraforaminal discs, with 80% yielding good or excellent results. Central discs and multiregional discs had worse results, with 47% and 54% good or excellent outcomes. There was only one extraforaminal disc, which had a poor outcome. The level of herniation was not predictive of outcome, with 59% occurring at L4-5.
In contrast, other authors have found that the level of herniation can effect operative outcomes. Dewing and colleagues93 reported that discectomy for L5-S1 herniations had significantly greater improvements in visual analog scale (VAS) leg and ODI scores compared with L4-5 herniations. These authors also found that patients with sequestered disc fragments had significantly better outcomes than patients with contained disc herniations. In a post-hoc analysis of SPORT trial data, Pearson and colleagues57 examined the influence of disc herniation location (central or lateral) and morphology (protrusion, extrusion, or sequestration) on the results of operative and nonoperative treatment. Their data suggest that the advantages of surgical treatment over nonoperative treatment were greatest for patients with lateral disc herniations. The magnitude of improvement from surgical treatment was not related to the location or morphology of the herniation.
Various preoperative and postoperative findings may also be predictive of results. Barrios and colleagues61 found that the use of (and positive response to) traction as part of preoperative conservative treatment was predictive of a good outcome with surgery. In agreement with other studies, patients with sedentary, non–physically demanding jobs had better results than patients with more strenuous occupations. Jansson and colleagues36 found that reduced preoperative walking distance and a history of back pain greater than 6 months were predictors of lower functional outcomes after discectomy. Jonsson and Stromqvist94 found a persistently positive SLR test to be a reliable indicator of inferior results; this was particularly evident in patients in whom the test was positive for more than 4 months after discectomy.
As part of an ongoing study of lumbar discectomy, the Maine group published an investigation comparing operative rates and outcomes between high versus low rate of surgery regions within the state.95 The data suggest that in the high rate region the results were inferior compared with the low rate region. These calculations were based on the rate of surgery per capita (population). The investigators concluded that this difference was most significantly related to the surgical indications used by the individual physicians and that in the higher rate areas the indications may have been less stringent. This might have been true, but an additional factor was not highlighted, which the current authors calculated from the data. In the high rate region, there were more surgeries done per capita, but the number of operations done per physician was lower, averaging 11 surgeries per surgeon. In the low rate region, surgeons averaged 26 operations each, although the overall rate of surgery was lower per capita. This factor directly supports previous analyses of the results of total joint arthroplasty, which indicate better results in high-volume hospitals because of concentrated experience. From these data, discectomy is optimally performed by an experienced, high-volume surgeon who employs strict indications.
Psychological and social factors have been shown by many investigators to influence profoundly the surgical results of lumbar discectomy.34,62 Sorensen and colleagues34 found that preoperative psychological assessment was 86% predictive of surgical results after discectomy. Cashion and Lynch62 found patients with a good outcome were more self-confident individuals, were only mildly depressed, and were generally optimistic about the outcome of surgery. Slover and colleagues35 found that a history of chronic headaches, smoking, depression, and self-rated poor health were associated with poor outcomes after discectomy. Jansson and colleagues36 found that smoking was the most significant risk factor in patients who failed to improve after surgery.
Spengler and colleagues13 calculated preoperative assessment scores for 84 patients before discectomy and correlated these with outcomes. The four components of the score were neurologic signs, clinical tension signs, psychological factors, and imaging evidence of neural compression. Imaging studies were most predictive of operative findings but not of outcome. The best predictor of outcome was the psychological score. This was based on the Minnesota Multiphasic Personality Inventory (MMPI).
Hurme and Alaranta12 prospectively studied 220 patients after discectomy, analyzing preoperative and perioperative factors. Optimistic patients had better results. Patients who preoperatively decided not to return to work had poorer outcomes. Patients who perceived their jobs to be physically strenuous had inferior results. The most predictive factors of a poor outcome were the decision not to return to work, marital status (divorced or widowed), age older than 40 years, a protracted period of sciatic pain, and multiple nonspecific somatic complaints. Predictors of good results were a high preoperative pain index, higher education level, overall satisfaction with life, and the perception that the patient’s job was of light or suitable duty. Patients with highly positive SLR test and younger patients with large disc herniations tended to have better results. This study highlights the importance of psychological, social, and objective physical factors in predicting the outcome of lumbar disc excision.
Dvorak and colleagues96 retrospectively applied a set of “accepted” operative indications, including radicular pain, positive SLR, contralateral SLR, dermatomal hypesthesia, motor deficit, and diminished deep tendon reflexes, to a series of patients who underwent discectomy. Of the patients, 65% fulfilled these criteria, and 35% did not. The long-term outcomes were not significantly different between these two groups. Patients who returned to work postoperatively were on disability compensation less than 2 months before surgery, whereas patients who did not return to work were on disability compensation an average of 4 months before surgery. Indications based on objective physical findings alone do not ensure a satisfactory outcome. The preoperative period of disability is a significant factor.
Some proponents of microscopically assisted discectomy purport that results are superior compared with standard techniques. In a published review of the literature by one of the strongest advocates of microdiscectomy, McCulloch76 concluded that the available data are insufficient to show its superiority. He noted that successful results have ranged from 80% to 96%, regardless of the technique used, and highlighted the importance of patient selection as the more important determinant of outcome. Series of microsurgical discectomy report comparable rates of complications, such as dural tear and recurrent herniations.97,98
There is disagreement regarding the efficacy of simple fragment excision (the so-called Williams sequestrectomy99) versus more extensive nucleus curettage. In a retrospective study of 200 patients, Faulhauer and Manicke65 showed a lower reherniation rate with fragment excision alone. This comparison was inherently flawed. The standard discectomy group, by definition, had herniations that were in continuity with the disc space and could be characterized as subligamentous (contained). The fragment excision group obligatorily had displaced fragments that were not contained within the disc space. The most useful information perhaps is that when an extruded or sequestered (uncontained) fragment is found, acceptable results with a low herniation rate can be expected with fragment excision alone. Although aggressive methods of removal of further nuclear material may not be warranted, meticulous examination of all quadrants of the epidural space surrounding the nerve root and cauda equina should be performed in each case to avoid missing disc fragments.
Balderston and colleagues60 compared 40 patients who underwent simple fragment excision in one center with 40 patients who underwent excision and curettage in another center. The reherniation rate was not significantly different: 12.5% in the former group and 11.6% in the latter group. There was also no difference in the rate of disc space narrowing between the two groups. The only difference was a higher rate of postoperative back pain in the curettage group at a minimum 2-year follow-up. In distinction to Faulhauer and Manicke’s work,65 the types of disc herniation were not different between the two groups.
Carragee and colleagues100 reported the results of a prospective, controlled study comparing subtotal versus limited discectomy. In the latter group, only the extruded disc material and loose fragments present in the disc space were removed. In the former, a more aggressive disc space curettage and débridement was performed in addition to removal of the extruded fragments. Early findings suggested a shorter convalescence in the limited discectomy group but decreased VAS and ODI scores in the subtotal group. These differences were not statistically significant at 2-year follow-up. Although the reherniation rate in the limited group was 18% compared with 9% in the subtotal group, this difference was not significant with the numbers available. Type II (beta) error cannot be excluded, however, because a significant difference may have been detected with greater patient numbers. The clinical significance of a 9% difference in reherniation rate may outweigh the lack of statistical significance.
Complications
Wound infections have been reported in 0% to 3% of cases.12,60,65,72,74,99 These may be superficial or deep. Superficial infections may be managed with local wound care and antibiotics. Deep infections should be surgically débrided and irrigated. Epidural abscess is rare, with reported rates of 0.3%, and should be managed with surgical evacuation.101 The microscope has been considered a possible source of contamination because of the exposed unsterile optics that are in close proximity over the wound.76,102 Infection rates are comparable, however, to cases performed without the microscope.
Pyogenic discitis may occur after discectomy 2.3% of the time.12,101 Early detection is crucial in avoiding extensive bone involvement. Intravenous antibiotic therapy is usually successful, with rare cases requiring surgical débridement. MRI findings, including increased bone edema near the endplates and loss of disc space height, are difficult to discern from typical degenerative Modic-type changes. Laboratory evidence, such as elevated erythrocyte sedimentation rates and C-reactive protein levels, is important in confirming the diagnosis.
Vascular injuries are exceedingly rare. Injury to anterior vessels from perforation of the disc space have been documented in a few case reports.103,104 Arteriovenous fistula has also been documented.105,106 The most important component of managing these complications is prompt recognition and aggressive treatment, including vascular repair. Excessive epidural bleeding is uncommon, although one series documented blood loss of more than 300 mL in 4% of cases.12
Incidental durotomy occurs 0% to 4% of the time.8,60,72,97,107 It has been associated with a poor outcome in some series. At an average of 10 years after surgery, Saxler and colleagues107 reported a lower rate of symptom resolution and a greater rate of chronic pain and headaches in patients who sustained an incidental durotomy. This study did not discern between primary and revision cases, which may have been a potential confounder. The potential for long-term clinical sequelae after incidental durotomy during discectomy is most likely influenced by the size of the tear, the ability to repair it, and the coexistence of neurologic injury.
Instability is quite rare after discectomy. Preservation of the facet joints is helpful in avoiding this sequela, although in the authors’ experience a large percentage of the facet joint can be resected unilaterally without adverse effect. Some authors have indicated that instability can occur 30% of the time. This percentage is highly dependent on the definition of instability. Padua and colleagues108 found radiographic evidence of instability, detected by flexion-extension films, in 20% of patients who underwent discectomy. Only 6% seemed to be symptomatic, however. In contrast, Faulhauer and Manicke65 defined instability by clinical findings, such as apprehension with flexion or extension, instead of radiographic measurements. These authors reported rates of 16% and 30% with two different operative techniques; 3% had severe enough symptoms to warrant a brace, and only one patient eventually went on to fusion. Kotilainen and Valtonen101 found that the presence of clinical apprehension postoperatively was predictive of a poor result after surgery. These data highlight the importance of documenting preoperative radiographic and clinical instability so that the effects of the discectomy itself can be better assessed in the postoperative period.109 Biomechanically, simple discectomy is not a destabilizing procedure if performed properly.
Recurrent Disc Herniations
The distinguishing histologic feature of recurrent disc herniation is the presence of large collagen bundles associated with a fibrillar framework.110 Granulation tissue found in recurrences is not present in primary disc herniations. This fact indicates that the pathophysiology of recurrent disc herniations may be different than in primary cases.
Depending on the definition, recurrent disc herniations can occur in 18% of cases. Clinical presentation is usually of recurrent sciatic leg pain. Jonsson and Stromqvist111 attempted to determine the relative frequency of clinical signs and symptoms after discectomy to differentiate better perineural fibrosis from true recurrent herniation. Pain reproduced by cough and a positive SLR were more frequent with recurrent discs. These findings were also present in many patients with fibrosis alone, however, so imaging modalities are crucial to the diagnosis. As discussed previously, MRI is best delayed for at least 6 months after surgery.
Important questions arise after the decision to proceed with surgery is made. The main focus of current investigations is whether or not comparable or inferior results can be expected after discectomy for a recurrent disc. Some authors have documented inferior results after repeat surgery,112 although most failures were reported in patients operated on without imaging evidence of neural compression.
Suk and colleagues113 retrospectively examined their results in a highly select group of patients. Recurrence was defined as a reherniation at the same level (ipsilateral or contralateral) after a pain-free interval of 6 months or more. These authors used gadolinium-enhanced MRI to confirm the diagnosis. They found that the second surgery was significantly longer and that recurrent disc herniations were typically larger. Clinical success was documented in 71.1% of cases, which was comparable to their results after primary discectomy (79.3%). In a similar study, Cinotti and colleagues114 examined their results with same-level contralateral reherniations only. At 2-year follow-up, surgery for recurrences resulted in satisfactory outcomes in 88% of cases and in 90% of primary discectomies. The only difference noted was more back pain in the reherniation group at 6 months, although this difference was not noted at 2 years.
Cinotti and colleagues115 also studied a group of patients with ipsilateral lumbar disc reherniation and compared these patients with patients after primary surgery as a control group. Satisfactory results were reported in 85% of patients with ipsilateral lumbar disc reherniation and 88% of control patients. Noted differences were a higher degree of disc degeneration in the recurrence group. Although epidural fibrosis was abundant in the study group, its presence or amount did not adversely influence outcome. There was no difference in the psychological profiles of recurrent versus primary discectomy groups. In a similar study, Papadopoulos and colleagues116 reported 85% improvement after discectomy for reherniations compared with 80% improvement for primary herniations at an average of 53.6 months follow-up. Herron117 reported 69% good and 24% fair results after laminectomy and discectomy for recurrences at the same and other levels.
Most authors do not recommend fusion after a first-time recurrent disc herniation.117,118 After any lumbar surgery involving facetectomy and bone resection, preoperative and intraoperative assessment of stability is a major determinant of the decision to fuse. Although investigational evidence is lacking, the authors consider fusion after a second reherniation at the same level.
Discectomy in Children
The role of trauma in causing disc herniations in children is believed to be more significant than in adults.3,119,120 Children are unlikely to have disc degeneration or the antecedent period of back pain before herniation. Clinical signs and symptoms are similar to adults but are much more acute in onset. The clinical and imaging evaluation is similar to adults.
The literature of discectomy in children reveals prolonged length of follow-up. Initial results are excellent, but results tend to deteriorate with time. At 1-year follow-up, Papagenlopoulos and colleagues121 reported 93% good or excellent results. At final follow-up ranging from 12 to 45 years, 92% were good or excellent; however, there was a 28% reoperation rate. Parisini and colleagues122 documented 95% success at short-term follow-up, whereas at long-term follow-up (average 12.4 years), good or excellent outcomes were documented in 87% of cases. These investigators found a 10% reoperation rate at 10 years. DeOrio and Bianco123 showed 96% good or excellent results at initial follow-up, trending down to 74% at final examination. Numerous other series document similar results.3,119,124
Discectomy in Elderly Patients
Maistrelli and colleagues125 reported results of discectomy in 32 patients older than 60 years. Clinical findings were similar, with 81% of patients having root tension signs such as a positive SLR. At an average follow-up of 50 months, good or excellent results were found in 87% of cases. None of the patients had evidence of neurogenic claudication, which is an important distinguishing feature when making the diagnosis of a disc herniation versus stenosis. Jonsson and Stromqvist126 also found disc surgery to be gratifying in patients older than 70 years, with good results documented at 2-year follow-up.
Foraminal and Extraforaminal (Far-Lateral) Herniations
So-called far-lateral disc herniations require special consideration. Surgical excision is more easily accomplished through modified operative techniques. Foraminal and extraforaminal disc herniations are more common in older patients. Symptoms are more likely to be isolated to one particular nerve root. In contrast to paracentral discs, they lead to compression of the exiting nerve root rather than the descending root. Diagnosis is best by MRI or CT (Fig. 46–16). Because the compression is usually extradural, myelography does not show a filling defect.
Different techniques have been advocated for the foraminal or extraforaminal herniation. Compression of the nerve root occurs outside of the spinal canal, making visualization of the herniation challenging through a standard laminotomy or laminectomy performed from within the canal. Garrido and Connaughton127 advocated a unilateral facetectomy. Bone is removed along the entire path of the nerve root from its exit from the cauda equina out and through the foramen; this necessitates complete removal of the facet on the side of exposure. Although symptom relief was not well documented, painful radiographic instability was detected in only 1 of 35 (3%) cases. This patient had pain relief after fusion.
Donaldson and colleagues128 advocated a less destabilizing procedure. They considered identification of the nerve within the intertransverse process “blind.” As a solution, they performed a partial hemilaminectomy at the level above the disc at the point where the root exited the cauda equina. They followed it laterally beneath the bone of the remaining pars interarticularis. By placing a probe over the root and advancing it laterally, they were able to identify the root and facilitate its dissection between the transverse processes before discectomy was performed through this interval. They reported good or excellent pain relief in 72% of patients. No cases of instability were reported.
Melvill and Baxter14 used the intertransverse approach for discectomy in 40 patients with extraforaminal herniations. They performed this using standard midline dissection that was extended subperiosteally out and over the facets to expose the transverse processes. This is in contrast to the classic description of the Wiltse paraspinal muscle–splitting approach. Complete resolution of leg pain was reported in 85% of cases. Melvill and Baxter14 noted a fibrous band that was present tethering the nerve root to the lateral aspect of the disc in an associated cadaveric study.
In an investigation with mid-term follow-up, satisfactory results were reported using the Wiltse paraspinal muscle–splitting approach for so-called lateral disc herniations.129 Satisfaction was reported by 85% of patients, with 60% of patients reporting complete resolution of pain. At 5 years’ follow-up, 20% of patients had developed some degree of instability, half of whom ultimately underwent fusion. Assuming uniformity of the definition of instability and indications for fusion in these cases, this finding is supportive of the hypothesis by McCulloch and Transfelt31 that lateral disc herniations were a precursor to the development of degenerative spondylolisthesis.
A thorough knowledge of the anatomy in this region is imperative to effective discectomy without undue bleeding or injury to the nerve root.77 The authors prefer to excise foraminal or extraforaminal discs through a true Wiltse paraspinal muscle–splitting approach to the intertransverse interval. It is easiest through a paramedian incision (Fig. 46–17). Identification of the correct level of incision is crucial in minimizing the length of the incision.
Cauda Equina Syndrome
Cauda equina syndrome most commonly occurs from a herniated lumbar disc. It is more common with central herniation (27% of central herniations), although it can occur with paracentral or lateral herniations as well.130,131 It is more frequent in men in their 4th decade. An L4-5 disc is the usual cause.130 Cauda equina syndrome should be considered a true surgical urgency because the neurologic results are affected by the time to decompression. The clinical diagnosis of cauda equina syndrome relies on many components, including perineal sensory deficit (so-called saddle anesthesia), bowel or bladder incontinence, new-onset lower extremity sensory deficit, and a new or progressive motor deficit (Fig. 46–18). In addition to a meticulous physical examination, evaluation of cauda equina syndrome should include measurement of a bladder postvoid residual. Normally, the postvoid residual should be less than 50 to 100 mL. The postvoid residual is often abnormal preoperatively and can be an important parameter to follow postoperatively.132
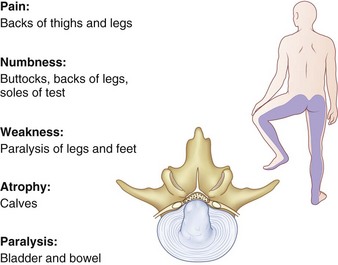
FIGURE 46–18 Cauda equina syndrome is characterized by saddle anesthesia, motor weakness, and loss of bowel and bladder control.
Decompression and discectomy can be via a laminotomy or through a formal laminectomy. Proponents of laminectomy believe this provides superior visualization of the dura and avoids excessive traction.133–135 Adequate exposure is particularly relevant for removal of central disc herniations. Discectomy is best performed within 48 to 72 hours of the onset of symptoms.132,135,136 This time frame leads to better sensory, motor, urinary, and rectal function recovery. Motor strength may continue to improve for 1 year after surgery.135 Although the postvoid residual usually decreases to less than 110 mL by 6 weeks, bladder function may continue to improve for 16 months.131 Early surgery does not seem to affect substantially the resolution of postoperative pain compared with delayed intervention.137 Preoperative neurologic status seems to be the greatest predictor of recovery,138 although many patients are found to have residual neurologic deficits despite timely and effective surgical intervention.137
Alternatives to Standard Discectomy
Endoscopic Discectomy
The use of the endoscope to perform lumbar discectomy has enthusiastic proponents. Improvements in equipment have made the technique more user-friendly and facile. Still, endoscopic discectomy remains a technically demanding procedure, and the results depend heavily on surgeon experience.139 Although it is not yet a replacement for standard discectomy techniques, more recent series have shown the ability to achieve comparable results in some surgeons’ hands.68,140
DeAntoni and colleagues84 reported results of translaminar epidural endoscopic discectomy in 190 patients. Using a lateral decubitus position, the technique uses a small paramedian incision for introduction of the instruments. Dilators are used to strip the muscles subperiosteally from the adjacent lamina as far lateral as the facet joint. The arthroscope (endoscope) is inserted through a 6-mm working cannula. An additional lateral paraspinal incision is made for an outflow cannula. A shaver is used to remove bone within the interlaminar window until the attachments of the ligamentum flavum can be visualized. The ligamentum flavum is elevated using a Penfield dissector and removed with a Kerrison rongeur, similar to a standard open approach. A root retractor pulls the nerve root and dura medially, allowing access to the disc space. The anulus is incised, and the herniation is removed. The endoscope can be inserted into the disc space to look for any additional loose fragments (so-called discoscopy). Good or excellent results were documented in 92% of cases with a minimum 2-year follow-up. There was only one dural laceration that did not need repair, there was no instability, and there were no neurologic injuries.
Yeung and Tsou68 reported results of 307 endoscopically assisted transforaminal disc herniations. The patient is positioned supine on a radiolucent table. Local anesthesia with light sedation is used. In this technique, intraoperative fluoroscopy is crucial. A standard discogram is performed first, injecting blue dye into the disc space to help identify the herniated tissue. Along the same path, a series of dilators and ultimately a single working cannula is inserted percutaneously through the intervertebral foramen (Fig. 46–19). One cannula is used for inflow, outflow, and instrument insertion. The cannula has a shielded tip that allows aggressive discectomy with the use of graspers and shavers while retracting the exiting nerve root. As stated, this is performed through the intervertebral foramen. The nerve root can be visualized by rotating the cannula, allowing assessment of its integrity and mobility. Satisfactory results were reported in 89.3% of patients. Six patients (2%) had lower extremity dysesthesia that lasted longer than 6 weeks, two had thrombophlebitis, two had discitis, and one had a dural tear that did not require repair.
FIGURE 46–19 Transforaminal endoscopic approach for disc excision.
(From Yeung AT, Tsou PM: Posterolateral endoscopic excision for lumbar disc herniation. Spine (Phila Pa 1976) 27:722-731, 2002.)
A more recent study showed that this technique is safe and effective for recurrent disc herniations as well.140 In a prospective evaluation of 262 consecutive cases of reherniation treated by an endoscopically assisted transforaminal technique, Hoogland and colleagues140 reported an 85% success rate and 3.8% complication rate at 2-year follow-up.
Several authors have sought to compare the results of microendoscopic discectomy with more traditional techniques.141–143 Ruetten and colleagues143 compared the results of endoscopic interlaminar and transforaminal discectomy with microsurgical discectomy. They showed no clinically significant differences between the endoscopic and microsurgical groups and identical reherniation rates (6.2% for each).143 Analogous findings have been reported with comparisons of endoscopic and standard open discectomy.141,142
Percutaneous Automated Discectomy
Results of automated discectomy are inferior to standard open discectomy. Shapiro81 showed only partial improvement of leg pain in 57% of patients undergoing this procedure. More disappointing was that only 5% had complete sciatica relief. All patients had disc bulges or protrusions, with none having free fragments. These results are less positive than the results documented with open discectomy.
In contrast, Hoppenfeld82 reported more successful results, with relief of sciatica and sensory deficit in 86% of patients. He determined, however, that patients with sequestered discs do not reliably respond well to the procedure. Kotilainen and Valtonen83 treated 41 patients with small protrusions or prolapses (bulges) with percutaneous automated discectomy. Sciatica had completely resolved in 78% of patients. From these data, it can be inferred that automated discectomy can be reasonably effective in patients with small disc bulges or protrusions that are in direct continuity with the remaining nucleus. Only in this select group of patients is there the possibility of relieving the neural compression. The procedure should not be used in patients with sequestered or extruded fragments (uncontained herniations) or most patients with disc herniations.
Chemonucleolysis
Chemonucleolysis has been rigorously examined in numerous clinical investigations. In a randomized prospective trial comparing chemonucleolysis and standard discectomy, Muralikuttan and colleagues86 concluded that it leads to inferior results. There was a high rate of conversion to open surgery in the chemonucleolysis group because of continued, unrelieved symptoms. Crawshaw and colleagues144 found extremely high failure rates—47% to 52%—after chemonucleolysis compared with 11% with open surgery. vanAlphen and colleagues70 documented increased radicular pain after chemonucleolysis in 22% of patients treated. Salvage discectomy after failed chemonucleolysis leads to worse results than primary surgery.70,86,144 In addition to these poor results, neurologic complications, such as transverse myelitis associated with the use of chymopapain, have all but eliminated the procedure from continued use in the United States.
Pearls
Pitfalls
Key Points
1 Atlas SJ, Keller RB, Wu YA, et al. Long-term outcomes of surgical and nonsurgical management of sciatica secondary to a lumbar disc herniation: 10 year results from the Maine Lumbar Spine Study. Spine (Phila Pa 1976). 2005;30:927-935.
2 Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical vs nonoperative treatment for lumbar disk herniation: The Spine Patient Outcomes Research Trial (SPORT) observational cohort. JAMA. 2006;296:2451-2459.
3 Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical vs nonoperative treatment for lumbar disk herniation: The Spine Patient Outcomes Research Trial (SPORT): A randomized trial. JAMA. 2006;296:2441-2450.
4 Weber H. Lumbar disc herniation: A controlled, prospective study with ten years of observations. Spine (Phila Pa 1976). 1983;8:131-140.
5 Carragee EJ, Kim D. A prospective analysis of magnetic resonance imaging findings in patients with sciatica and lumbar disc herniation: Correlation of outcomes with disc fragment and canal morphology. Spine (Phila Pa 1976). 1997;22:1650-1660.
1 Atlas SJ, Keller RB, Chang Y, et al. Surgical and nonsurgical management of sciatic secondary to a lumbar disc herniation: Five-year outcomes from the Maine Lumbar Spine Study. Spine (Phila Pa 1976). 2001;26:1179-1187.
2 DePalma AF, Rothman RH. Surgery of the lumbar spine. Clin Orthop Relat Res. 1969;63:162-170.
3 Fisher RG, Saunders RL. Lumbar disc protrusion in children. J Neurosurg. 1981;54:480.
4 Boden SD, Davis DO, Dina TS, et al. Abnormal magnetic resonance scans of the lumbar spine in asymptomatic subjects: A prospective investigation. J Bone Joint Surg Am. 1990;72:403-408.
5 Saal JA, Saal JS. Nonoperative treatment of herniated lumbar intervertebral disc with radiculopathy: An outcome study. Spine (Phila Pa 1976). 1989;14:431-437.
6 Saal JA, Saal JS, Herzog RJ. The natural history of lumbar intervertebral disc extrusions treated nonoperatively. Spine (Phila Pa 1976). 1990;15:683-686.
7 Atlas SJ, Keller RB, Wu YA, et al. Long-term outcomes of surgical and nonsurgical management of sciatica secondary to a lumbar disc herniation: 10 year results from the Maine Lumbar Spine Study. Spine (Phila Pa 1976). 2005;30:927-935.
8 Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical vs nonoperative treatment for lumbar disk herniation: The Spine Patient Outcomes Research Trial (SPORT) observational cohort. JAMA. 2006;296:2451-2459.
9 Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical vs nonoperative treatment for lumbar disk herniation: The Spine Patient Outcomes Research Trial (SPORT): A randomized trial. JAMA. 2006;296:2441-2450.
10 Weber H. Lumbar disc herniation: A controlled, prospective study with ten years of observations. Spine (Phila Pa 1976). 1983;8:131-140.
11 Carragee EJ, Kim D. A prospective analysis of magnetic resonance imaging findings in patients with sciatica and lumbar disc herniation: Correlation of outcomes with disc fragment and canal morphology. Spine (Phila Pa 1976). 1997;22:1650-1660.
12 Hurme M, Alaranta H. Factors predicting the results of surgery for lumbar intervertebral disc herniation. Spine (Phila Pa 1976). 1987;12:933-938.
13 Spengler DM, Ouellette EA, Battie M, et al. Elective discectomy for herniation of a lumbar disc: Additional experience with an objective method. J Bone Joint Surg Am. 1990;72:320-327.
14 Melvill RL, Baxter BL. The intertransverse approach to extraforaminal disc protrusion in the lumbar spine. Spine (Phila Pa 1976). 1994;19:2707-2714.
15 Grimes PF, Massie JB, Garfin SR. Anatomic and biomechanical analysis of the lower lumbar foraminal ligaments. Spine (Phila Pa 1976). 2000;25:2009-2014.
16 Wilder DG, Pope MH, Frymoyer JW. The biomechanics of lumbar disc herniation and the effect of overload and instability. J Spinal Disord. 1988;1:16-32.
17 Frei H, Oxland TR, Rathonyi GC, et al. The effect of nucleotomy on lumbar spine mechanics in compression and shear loading. Spine (Phila Pa 1976). 2001;26:2080-2089.
18 White A, Panjabi M. Clinical Biomechanics of the Spine, 2nd ed. Philadelphia: Lippincott-Raven; 1990.
19 Repanti M, Korovessis PG, Stamatakis MV, et al. Evolution of disc degeneration in lumbar spine: A comparative histological study between herniated and postmortem retrieved disc specimens. J Spinal Disord. 1998;11:41-45.
20 Smyth MJ, Wright VJ. Sciatica and the intervertebral disc: An experimental study. J Bone Joint Surg Am. 1958;40:1401.
21 Spiliopoulou I, Korovessis P, Konstantinou D, et al. IgG and IgM concentration in the prolapsed human intervertebral disc and sciatica etiology. Spine (Phila Pa 1976). 1994;19:1320-1323.
22 Olmarker K, Rydevik B. Selective inhibition of tumor necrosis factor-alpha prevents nucleus pulposus-induced thrombus formation, intraneural edema, and reduction of nerve conduction velocity: Possible implications for future pharmacologic treatment strategies of sciatica. Spine (Phila Pa 1976). 2001;26:863-869.
23 Kang JD, Stefanovic-Racic M, McIntyre LA, et al. Toward a biochemical understanding of human intervertebral disc degeneration and herniation: Contributions of nitric oxide, interleukins, prostaglandin E2, and matrix metalloproteinases. Spine (Phila Pa 1976). 1997;22:1065-1073.
24 Scuderi GJ, Brusovanik GV, Anderson DG, et al. Cytokine assay of the epidural space lavage in patients with lumbar intervertebral disk herniation and radiculopathy. J Spinal Disord Tech. 2006;19:266-269.
25 Arai Y, Yasuma T, Shitoto K, et al. Immunohistochemical study of intervertebral disc herniation of lumbar spine. J Orthop Sci. 2000;5:229-231.
26 Hatano E, Fujita T, Ueda Y, et al. Expression of ADAMTS-4 (aggrecanase-1) and possible involvement in regression of lumbar disc herniation. Spine (Phila Pa 1976). 2006;31:1426-1432.
27 Brisby H, Balague F, Schafer D, et al. Glycosphingolipid antibodies in serum in patients with sciatica. Spine (Phila Pa 1976). 2002;27:380-386.
28 Jinkins JR, Whittemore AR, Bradley WG. The anatomic basis of vertebrogenic pain and the autonomic syndrome associated with lumbar disc extrusion. AJR Am J Roentgenol. 1989;152:1277-1289.
29 Fardon DF, Milette PC. Nomenclature and classification of lumbar disc pathology. Recommendations of the combined task forces of the North American Spine Society, American Society of Spine Radiology, and American Society of Neuroradiology. Spine (Phila Pa 1976). 2001;26:E93-E113.
30 Fardon DF. Nomenclature and classification of lumbar disc pathology. Spine (Phila Pa 1976). 2001;26:461-462.
31 McCulloch JA, Transfelt EE. Macnab’s Backache. Baltimore: Williams & Wilkins; 1997.
32 Nygaard OP, Kloster R, Solberg T. Duration of leg pain as a predictor of outcome after surgery for lumbar disc herniation: A prospective cohort study with 1-year follow up. J Neurosurg. 2000;92:131-134.
33 Rothoerl RD, Woertgen C, Brawanski A. When should conservative treatment for lumbar disc herniation be ceased and surgery considered? Neurosurg Rev. 2002;25:162-165.
34 Sorensen LV, Mors O, Skovlund O. A prospective study of the importance of psychological and social factors for the outcome after surgery in patients with slipped lumbar disk operated upon for the first time. Acta Neurochir (Wien). 1987;88:119-125.
35 Slover J, Abdu WA, Hanscom B, et al. The impact of comorbidities on the change in Short-Form 36 and Oswestry scores following lumbar spine surgery. Spine (Phila Pa 1976). 2006;31:1974-1980.
36 Jansson KA, Nemeth G, Granath F, et al. Health-related quality of life in patients before and after surgery for a herniated lumbar disc. J Bone Joint Surg Br. 2005;87:959-964.
37 Weise MD, Garfin SR, Gelberman RH, et al. Lower-extremity sensibility testing in patients with herniated lumbar intervertebral discs. J Bone Joint Surg Am. 1985;67:1219-1224.
38 Kosteljanetz M, Espersen JO, Halaburt H, et al. Predictive value of clinical and surgical findings in patients with lumbago-sciatica: A prospective study (Part I). Acta Neurochir (Wien). 1984;73:67-76.
39 Majlesi J, Togay H, Unalan H, et al. The sensitivity and specificity of the slump and the straight leg raising tests in patients with lumbar disc herniation. J Clin Rheumatol. 2008;14:87-91.
40 Ito T, Takano Y, Yuasa N. Types of lumbar herniated disc and clinical course. Spine (Phila Pa 1976). 2001;26:648-651.
41 Komori H, Okawa A, Haro H, et al. Contrast-enhanced magnetic resonance imaging conservative management of lumbar disc herniation. Spine (Phila Pa 1976). 1998;23:67-73.
42 Kikkawa I, Sugimoto H, Saita K, et al. The role of Gd enhanced three dimensional MRI fast low-angle shot (FLASH) in the evaluation of symptomatic lumbosacral nerve roots. J Orthop Sci. 2001;6:101-109.
43 Weishaupt D, Schmid MR, Zanetti M, et al. Positional MR imaging of the lumbar spine: Does it demonstrate nerve root compromise not visible at conventional MR imaging. Radiology. 2000;215:247-253.
44 Botwin KP, Skene G, Tourres-Ramos FM, et al. Role of weightbearing flexion and extension myelography in evaluating the intervertebral disc. Am J Phys Med Rehabil. 2001;80:289-295.
45 Grane P, Lindqvist M. Evaluation of the postoperative lumbar spine with MR imaging: The role of contrast enhancement and thickening in nerve roots. Acta Radiol. 1997;38:1035-1042.
46 Heithoff KB. Recurrent disc herniation and gadolinium. SpineLine. 2002;Sept/Oct:23-26.
47 Barrera MC, Alustiza JM, Gervas C, et al. Postoperative lumbar spine: Comparative study of TSE T2 and turbo FLAIR sequences vs contrast-enhanced SE T1. Clin Radiol. 2001;56:133-137.
48 Bundschuh CV, Modic MT, Ross JR, et al. Epidural fibrosis and recurrent disc herniation in the lumbar spine: MR imaging assessment. AJR Am J Roentgenol. 1988;150:923-932.
49 Mortensen WW, Thorne TP, Donaldson WF. Symptomatic gas-containing disc herniation: Report of four cases. Spine (Phila Pa 1976). 1991;16:190-192.
50 Ford LT, Gilula LA, Murphy WA, et al. Analysis of gas in vacuum lumbar disc. AJR Am J Roentgenol. 1977;128:1056-1057.
51 Deyo RA, Diehl AK, Rosenthal M. How many days of bedrest for acute low back pain? A randomized clinical trial. N Engl J Med. 1986;315:1064-1070.
52 Krause M, Reshauge KM, Dessen M, et al. Lumbar spine traction: Evaluation of effects and recommended application for treatment. Man Ther. 2000;5:72-81.
53 Polkinghorn BS, Colloca CJ. Treatment of symptomatic lumbar disc herniation using activator methods chiropractic technique. J Manipulative Physiol Ther. 1998;21:187-196.
54 Bush K, Cowan N, Katz DE, et al. The natural history of sciatica associated with disc pathology: A prospective study with clinical and independent radiographic follow-up. Spine (Phila Pa 1976). 1992;17:1205-1212.
55 Wang JC, Lin E, Brodke DS, et al. Epidural injections for the treatment of symptomatic lumbar herniated discs. J Spinal Disord. 2002;15:269-272.
56 Atlas SJ, Deyo RA, Keller RB, et al. The Maine Lumbar Spine Study, Part II: 1-year outcomes of surgical and nonsurgical management of sciatica. Spine (Phila Pa 1976). 1996;21:1777-1786.
57 Pearson AM, Blood EA, Frymoyer JW, et al. SPORT lumbar intervertebral disk herniation and back pain: Does treatment, location, or morphology matter? Spine (Phila Pa 1976). 2008;33:428-435.
58 Alaranta H, Hurme M, Einola S, et al. A prospective study of patients with sciatica: A comparison between conservatively treated patients and patients who have undergone operation, Part II: Results after one year follow-up. Spine (Phila Pa 1976). 1990;15:1345-1349.
59 Hurme M, Alaranta H, Einola S, et al. A prospective study of patients with sciatica: A comparison between conservatively treated patients and patients who have undergone operation, Part I: Patient characteristics and differences between groups. Spine (Phila Pa 1976). 1990;15:1340-1344.
60 Balderston RA, Gilyeard GG, Jones AA, et al. The treatment of lumbar disc herniation: Simple fragment excision versus disc space curettage. J Spinal Disord. 1991;4:22-25.
61 Barrios C, Ahmed M, Arrotegui JI, et al. Clinical factors predicting outcome after surgery for herniated lumbar disc: An epidemiological multivariate analysis. J Spinal Disord. 1990;3:205-209.
62 Cashion EL, Lynch WJ. Personality factors and results of lumbar disc surgery. Neurosurgery. 1979;4:141-145.
63 Daneyemez M, Sali A, Kahraman S, et al. Outcome analyses in 1072 surgically treated lumbar disc herniations. Minim Invasive Neurosurg. 1999;42:63-68.
64 Eie N. Comparison of the results in patients operated for ruptured lumbar discs with and without spinal fusion. Acta Neurochir (Wien). 1978;41:107-113.
65 Faulhauer K, Manicke C. Fragment excision versus conventional disc removal in the microsurgical treatment of herniated lumbar disc. Acta Neurochir (Wien). 1995;133:107-111.
66 Jonsson B, Stromqvist B. Motor affliction of the L5 nerve root in lumbar nerve root compression syndromes. Spine (Phila Pa 1976). 1995;20:2012-2015.
67 Kulali A, vonWild K. Microsurgical management of the lumbar intervertebral disc-disease. Neurosurg Rev. 1995;18:183-188.
68 Yeung AT, Tsou PM. Posterolateral endoscopic excision for lumbar disc herniation. Spine (Phila Pa 1976). 2002;27:722-731.
69 White AH, vonRogov P, Zucherman J, et al. Lumbar laminectomy for herniated disc: A prospective controlled comparison with internal fixation fusion. Spine (Phila Pa 1976). 1987;12:305-307.
70 vanAlphen HA, Braakman R, Berfelo MW, et al. Chemonucleolysis or discectomy? Results of a randomized multicentre trial in patients with a herniated lumbar intervertebral disc (a preliminary report). Acta Neurochir Suppl (Wien). 1988;43:35-38.
71 Tullberg T, Isacson J, Weidenhielm L. Does microscopic removal of lumbar disc herniation lead to better results than the standard procedure? Results of a one-year randomized study. Spine (Phila Pa 1976). 1993;18:24-27.
72 Soldner F, Hoelper BM, Wallenfang T, et al. The translaminar approach to canalicular and craniodorsolateral lumbar disc herniations. Acta Neurochir (Wien). 2002;144:315-320.
73 Pointillart V, Broc G, Senegas J. A novel paraspinal surgical approach for lumbar lateral extraforaminal root entrapment. Eur Spine J. 1997;6:102-105.
74 An HS, Simpson JM, Stein R. Outpatient laminotomy and discectomy. J Spinal Disord. 1999;12:19-26.
75 Carragee EJ, Han MY, Yang B, et al. Activity restrictions after posterior lumbar discectomy: A prospective study of outcomes in 152 cases with no postoperative restrictions. Spine (Phila Pa 1976). 1999;24:2346-2351.
76 McCulloch JA. Focus issue on lumbar disc herniation: Macro- and microdiscectomy. Spine (Phila Pa 1976). 1996;21:45S-56S.
77 Reulen HJ, Muller A, Ebeling U. Microsurgical anatomy of the lateral approach to extraforaminal lumbar disc herniations. Neurosurgery. 1996;39:345-350.
78 Eie N, Solgaard T, Kleppe H. The knee-elbow position in lumbar disc surgery: A review of complications. Spine (Phila Pa 1976). 1983;8:897-900.
79 Miyamoto K. Long-term follow-up results of anterior discectomy and interbody fusion for lumbar disc herniation. J Jpn Orthop Assoc. 1991;65:1179-1190.
80 Revel M, Payan C, Vallee C, et al. Automated percutaneous lumbar discectomy versus chemonucleolysis in the treatment of sciatica: A randomized multicenter trial. Spine (Phila Pa 1976). 1993;18:1-7.
81 Shapiro S. Long-term followup of 57 patients undergoing automated percutaneous discectomy. J Neurosurg. 1995;83:31-33.
82 Hoppenfeld S. Percutaneous removal of herniated lumbar discs: 50 cases with ten-year follow-up periods. Clin Orthop Relat Res. 1989;238:92-97.
83 Kotilainen E, Valtonen S. Long-term outcome of patients who underwent percutaneous nucleotomy for lumbar disc herniation: Results after a mean follow-up of 5 years. Acta Neurochir (Wien). 1998;140:108-113.
84 DeAntoni DJ, Claro ML, Poehling GG, et al. Translaminar lumbar epidural endoscopy: Technique and clinical results. J South Orthop Assoc. 1998;7:61-62.
85 Fraser RD. Chymopapain for the treatment of intervertebral disc herniation: The final report of a double-blind study. Spine (Phila Pa 1976). 1984;9:815-818.
86 Muralikuttan KP, Hamilton A, Kernohan WG, et al. A prospective randomized trial of chemonucleolysis and conventional disc surgery in single level lumbar disc herniation. Spine (Phila Pa 1976). 1992;17:381-387.
87 Weber H. The effect of delayed disc surgery on muscular paresis. Acta Orthop Scand. 1975;46:631-642.
88 MacKay MA, Fischgrund JS, Herkowitz HN, et al. The effect of interposition membrane on the outcome of lumbar laminectomy and discectomy. Spine (Phila Pa 1976). 1995;20:1793-1796.
89 Bernsmann K, Kramer J, Ziozios I, et al. Lumbar micro disc surgery with and without autologous fat graft: A prospective randomized trial evaluated with reference to clinical and social factors. Arch Orthop Trauma Surg. 2001;121:476-480.
90 Kjellby-Wendt G, Styf J. Early active training after lumbar discectomy: A prospective, randomized, and controlled study. Spine (Phila Pa 1976). 1998;23:2345-2351.
91 Alaranta H, Hurme M, Einola S, et al. Rehabilitation after surgery for lumbar disc herniation: Results of a randomized clinical trial. Int J Rehabil Res. 1986;9:247-257.
92 Knop-Jergas BM, Zucherman JF, Hsu KY, et al. Anatomic position of a herniated nucleus pulposus predicts the outcome of lumbar discectomy. J Spinal Disord. 1996;9:246-250.
93 Dewing CB, Provencher MT, Rifenburgh RH, et al. The outcomes of lumbar microdiscectomy in a young, active population: Correlation by herniation type and level. Spine (Phila Pa 1976). 2008;33:33-38.
94 Jonsson B, Stromqvist B. Significance of a persistent positive straight leg raising test after lumbar disc surgery. J Neurosurg. 1999;91:50-53.
95 Keller RB, Atlas SJ, Soule DN, et al. Relationship between rates and outcomes of operative treatment for lumbar disc herniation and spinal stenosis. J Bone Joint Surg Am. 1999;81:752-762.
96 Dvorak J, Gauchat MH, Valach L. The outcome of surgery for lumbar disc herniation, I: A 4-17 years follow-up with emphasis on somatic aspects. Spine (Phila Pa 1976). 1988;13:1418-1422.
97 Kotilainen E, Valtonene S, Carlson CA. Microsurgical treatment of lumbar disc herniation: Follow-up of 237 patients. Acta Neurochir (Wien). 1993;120:143-149.
98 Goffin J. Microdiscectomy for lumbar disc herniations. Clin Neurol Neurosurg. 1994;96:130-134.
99 Wenger M, Mariani L, Kalbarczyk A, et al. Long-term outcome of 104 patients after lumbar sequestrectomy according to Williams. Neurosurgery. 2001;49:329-334.
100 Carragee EJ, Spinnickie AO, Alamin TF, et al. A prospective controlled study of limited versus subtotal posterior discectomy: Short-term outcomes in patients with herniated lumbar intervertebral discs and large posterior anular defect. Spine (Phila Pa 1976). 2006;31:653-657.
101 Kotilainen E, Valtonen S. Clinical instability of the lumbar spine after microdiscectomy. Acta Neurochir (Wien). 1993;125:120-126.
102 Tronnier V, Schneider R, Kunz U, et al. Postoperative spondylodiscitis: Results of a prospective study about the aetiology of spondylodiscitis after operation for lumbar disc herniation. Acta Neurochir (Wien). 1992;117:149-152.
103 Sande E, Myhre HO, Witsoe E, et al. Vascular complications of lumbar disc surgery (case report). Eur J Surg. 1991;157:141-143.
104 Ewah B, Calder I. Intraoperative death during lumbar discectomy. Br J Anaesth. 1991;66:712-723.
105 Christensen C, Bank A. Arteriovenous fistula complicating lumbar disc surgery (case report). Eur J Surg. 1991;157:145-147.
106 Farouk M, Murie JA. Postlaminectomy arteriovenous fistula formation: A continuing problem. J R Coll Surg Edinb. 1991;36:130-131.
107 Saxler G, Kramer J, Barden B, et al. The long-term clinical sequelae of incidental durotomy in lumbar disc surgery. Spine (Phila Pa 1976). 2005;30:2298-2302.
108 Padua R, Padua S, Romanini E, et al. Ten to 15 year outcome of surgery for lumbar disc herniation: Radiographic instability and clinical findings. Eur Spine J. 1999;8:70-74.
109 Kotilainen E. Long-term outcome of patients suffering from clinical instability after microsurgical treatment of lumbar disc herniation. Acta Neurochir (Wien). 1998;140:120-125.
110 Laus M, Bertoni F, Bacchini P, et al. Recurrent lumbar disc herniation: What recurs? (A morphological study of recurrent disc herniation). Chir Organi Mov. 1993;78:147-154.
111 Jonsson B, Stromqvist B. Clinical characteristics of recurrent sciatica after lumbar discectomy. Spine (Phila Pa 1976). 1996;21:500-505.
112 Vik A, Zwart JA, Hullberg G, et al. Eight year outcome after surgery for lumbar disc herniation: A comparison of reoperated and nonreoperated patients. Acta Neurochir (Wien). 2001;143:607-610.
113 Suk KS, Lee HM, Moon SH, et al. Lumbosacral scoliotic list by lumbar disc herniation. Spine (Phila Pa 1976). 2001;26:667-671.
114 Cinotti G, Gumina S, Giannicola G, et al. Contralateral recurrent lumbar disc herniation: Results of discectomy compared with those in primary herniation. Spine (Phila Pa 1976). 1999;24:800-806.
115 Cinotti G, Roysam GS, Eisenstein SM, et al. Ipsilateral recurrent lumbar disc herniation: A prospective, controlled study. J Bone Joint Surg Br. 1998;80:825-832.
116 Papadopoulos EC, Girardi FP, Sandhu HS, et al. Outcome of revision discectomies following recurrent lumbar disc herniation. Spine (Phila Pa 1976). 2006;31:1473-1476.
117 Herron L. Recurrent lumbar disc herniation: Results of repeat laminectomy and discectomy. J Spinal Disord. 1994;7:161-166.
118 Stambough JL. Recurrent same-level, ipsilateral lumbar disc herniation. Orthop Rev. 1994;23:810-816.
119 Kurihara A, Kataoka O. Lumbar disc herniation in children and adolescents: A review of 70 operated cases and their minimum 5-year follow-up studies. Spine (Phila Pa 1976). 1980;5:443-451.
120 Garrido E. Lumbar disc herniation in the pediatric patient. Neurosurg Clin N Am. 1993;4:149-152.
121 Papagenlopoulos PJ, Shaughnessy WJ, Ebersold MJ, et al. Long-term outcome of lumbar discectomy in children and adolescents sixteen years of age or younger. J Bone Joint Surg Am. 1998;80:689-698.
122 Parisini P, DiSilvestre M, Greggi T, et al. Lumbar disc excision in children and adolescents. Spine (Phila Pa 1976). 2001;26:1997-2000.
123 DeOrio JK, Bianco AJ. Lumbar disc excision in children and adolescents. J Bone Joint Surg Am. 1982;64:991-996.
124 Shillito J. Pediatric lumbar disc surgery: 20 patients under 15 years of age. Surg Neurol. 1996;46:14-18.
125 Maistrelli GL, Vaughan PA, Evans DC, et al. Lumbar disc herniation in the elderly. Spine (Phila Pa 1976). 1987;12:63-66.
126 Jonsson B, Stromqvist B. Lumbar spine surgery in the elderly: Complications and surgical results. Spine (Phila Pa 1976). 1994;19:1431-1435.
127 Garrido E, Connaughton PN. Unilateral facetectomy approach for lateral lumbar disc herniation. J Neurosurg. 1991;74:754-756.
128 Donaldson WF, Star MJ, Thorne RP. Surgical treatment for the lateral herniated lumbar disc. Spine (Phila Pa 1976). 1993;18:1263-1267.
129 Weiner BK, Dabbah M. Lateral lumbar disc herniations treated with a paraspinal approach: An independent assessment of longer-term outcomes. J Spinal Disord Tech. 2005;18:519-521.
130 Walker JL, Schulak D, Murtagh R. Midline disk herniations of the lumbar spine. South Med J. 1993;86:13-17.
131 Tay ECK, Chacha PB. Midline prolapse of a lumbar intervertebral disc with compression of the cauda equina. J Bone Joint Surg Br. 1979;61:43-46.
132 Nielsen B, deNully M, Schmidt K, et al. A urodynamic study of cauda equina syndrome due to lumbar disc herniation. Urol Int. 1980;35:167-170.
133 Kostuik JP, Harrington I, Alexander D, et al. Cauda equina syndrome and lumbar disc herniation. J Bone Joint Surg Am. 1986;68:386-391.
134 Choudry AR, Taylor JC. Cauda equina syndrome in lumbar disc disease. Acta Orthop Scand. 1980;51:493-499.
135 Shapiro S. Cauda equina syndrome secondary to lumbar disc herniation. Neurosurgery. 1993;32:743-747.
136 Ahn UM, Ahn NU, Buchowski JM, et al. Cauda equina syndrome secondary to lumbar disc herniation. Spine (Phila Pa 1976). 2000;25:1515-1522.
137 McCarthy MJ, Aylott CE, Grevitt MP, et al. Cauda equina syndrome: Factors affecting long-term functional and sphincteric outcome. Spine (Phila Pa 1976). 2007;32:207-216.
138 Chang HS, Nakagawa H, Mizuno J. Lumbar herniated disc presenting with cauda equina syndrome. Surg Neurol. 2002;53:100-105.
139 Yeung AT, Yeung CA. Minimally invasive techniques for the management of lumbar disc herniation. Orthop Clin North Am. 2007;38:363-372.
140 Hoogland T, van den Brekel-Dijstra K, Schubert M, et al. Endoscopic transforaminal discectomy for recurrent lumbar disc herniation: A prospective, cohort evaluation of 262 consecutive cases. Spine (Phila Pa 1976). 2008;33:973-978.
141 Wu X, Zhuang S, Mao Z, et al. Microendoscopic discectomy for lumbar disc herniation: Surgical technique and outcome in 873 consecutive cases. Spine (Phila Pa 1976). 2006;31:2689-2694.
142 Righesso O, Falavigna A, Avanzi O. Comparison of open discectomy with microendoscopic discectomy in lumbar disc herniations: Results of a randomized controlled trial. Neurosurgery. 2007;61:545-549.
143 Ruetten S, Komp M, Merk H, et al. Full-endoscopic interlaminar and transforaminal lumbar discectomy versus conventional microsurgical technique: A prospective, randomized, controlled study. Spine (Phila Pa 1976). 2008;33:931-939.
144 Crawshaw C, Frazer AM, Merriam WF, et al. A comparison of surgery and chemonucleolysis in the treatment of sciatica: A prospective randomized trial. Spine (Phila Pa 1976). 1984;9:195-198.