CHAPTER 45 Lumbar Disc Disease
The location of the anatomic pain generator in patients with low back pain is often difficult to discern. Pain can originate from several anatomic structures within the spine making it difficult for the patient and the physician to localize. Experimental and clinical studies suggest that the intervertebral disc (IVD) is an important source of back pain in 10% to 39%1,2 of cases of chronic low back pain.
Natural History
At some point during their lifetime, 60% to 80% of adults can be expected to experience low back pain. The annual incidence of back pain in adults is 15%, and its point prevalence is about 30%.3 By the age of 30 years, almost half of adults have experienced a substantive episode of low back pain.4 Most symptoms are short-lived; it is generally believed that 80% to 90% of episodes of low back pain resolve within 6 weeks of onset regardless of the type of treatment.5
Although resolution of symptoms is the common and expected outcome, there is also a high recurrence rate. Croft and colleagues6 reported that although 90% of subjects ceased to pursue consultation about symptoms within 3 months, most still had substantial low back pain and related disability. Additionally, only 25% of the patients who sought consultation for low back pain had fully recovered within 12 months. In a survey of the British general population, 38% of adults reported a significant episode of low back pain within a 1-year period, of which a third had experienced symptoms for longer than 4 weeks.7 Inability to return to work within 3 months of symptom onset is a poor prognostic indicator. Only 20% of patients still disabled after 1 year return to work, and only 2% return after 2 years.8
The clinical onset and course of low back pain may be prolonged for many patients and may best be represented as a continuum of back-related disability and distress.9 Numerous patients presenting with acute low back pain have a prior history of chronic back pain.10 The strongest predictive factor for a new episode of low back pain is a previous episode.11,12
The natural history of DDD is largely unknown. Smith and colleagues13 reported on the outcome of 25 patients with a positive discogram who were treated nonoperatively and found that 68% of patients improved by the 3-year minimum follow-up. Although 60% of the patients were involved with workers’ compensation and 32% were being treated for psychiatric diagnoses, this study suggests that at least two thirds of patients with discogenic pain improve with conservative therapy. The retrospective study design and small sample size limit the conclusions, and because only patients with significant symptoms typically undergo discography, the natural history of untreated symptomatic DDD is likely to be even better.
In their classic description, Kirkaldy-Willis and Farfan14 classified the degenerative process into three distinct phases: dysfunction, instability, and stabilization. In the first phase, the disc loses its normal function as the degenerative process begins. A period of relative instability ensues as degeneration progresses with intermittent episodes of pain. During the instability phase, abnormal motion occasionally can be seen on flexion-extension x-rays; however, more often the spinal segments during this phase of degeneration show no demonstrable radiographic instability. The final phase—stabilization—results when the spinal segment has restabilized because of loss of height and compression of disc tissue, and the patient typically no longer has episodes of back pain. The problem with this theory is that often patients who meet diagnostic criteria of DDD radiographically—loss of height, osteophytes, or even olisthesis—or by magnetic resonance imaging (MRI)—signal changes compared with adjacent levels—are completely symptom-free. Waris and colleagues,15 in a study with 17 years of follow-up MRI, showed that young patients with DDD did show radiographic evidence of progression, but it was not significantly associated with low back pain or a higher rate of surgery.
Relevant Anatomy
The disc is composed of the inner gelatinous nucleus pulposus surrounded by the collagenous anulus fibrosus. Sheets of interlacing lamellae of collagen within the anulus provide tensile strength, which limits the expansion of the viscoelastic nucleus. The nucleus pulposus consists of a matrix of collagen, glycosaminoglycans, and water, which provide compressive stiffness and allow the tissue to undergo reversible deformation. The anatomy of the IVD allows the disc to absorb and dissipate loads on the spinal column and allows motion between adjacent spinal segments. The IVD has sparse cellularity; cells comprise approximately 1% to 5% of the tissue volume. The disc is bordered above and below by a sheet of hyaline cartilage called the vertebral endplate. Pores in the endplates provide channels for diffusion of nutrients to the disc.16
The IVD is largely avascular and aneural, with vascularity and innervation in a healthy disc limited to the peripheral fibers of the anulus. The sinuvertebral nerve innervates the disc, posterior longitudinal ligament, ventral dura, posterior anulus, and blood vessels. It comprises a sensory branch from the ventral root and a sympathetic branch from the gray rami communicans near the distal pole of dorsal root ganglion. The sinuvertebral nerve is believed to have three segmental levels of overlap, which makes it difficult to localize pain originating in the disc, dura, and posterior longitudinal ligament. Nakamura and colleagues17 treated 33 patients with selective L2 nerve root block with good relief of back pain. The authors hypothesized that the main afferent pathways of pain from lower lumbar IVDs in patients with discogenic back pain are sympathetic in nature and are mediated through the L2 nerve root via the sinuvertebral nerve; however, this hypothesis has yet to be validated.
Changes in Disc Structure with Aging and Degeneration
The number of arterioles supplying the peripheral disc diminishes significantly as remaining blood vessels are obliterated by calcification of the cartilaginous endplates. Loss of endplate vascularity and porosity leads to a reduction in the influx of nutrients and efflux of waste products. Lactate levels increase locally within the hypovascular disc secondary to increased production and decreased removal. Cell apoptosis occurs as a result of decreased tissue pH,18 and the biosynthetic reparative capability of the disc is impaired further.
The degenerative process resulting from matrix changes and internal structural disruption sets the stage for abnormal motion at the degenerated segment. Changes in disc structure alter the loading response and alignment of the spinal column. These changes can influence the facet joints, ligaments, and paraspinal muscles, which may also become pain generators. Pain does not always correlate with morphologic changes in the disc and mechanical compression, however.19 Macnab20 described traction osteophytes around the vertebrae originating 2 mm from the anterior endplate, at the site of attachment of the outermost annular fibers. These osteophytes were thought to be signs of abnormal biomechanics, caused by traction at the insertion of the annular fibers into the vertebral bodies. Subsequent studies found these osteophytes to be inconsistently present.
Associated Factors
Various risk factors have been implicated in the pathogenesis of lumbar disc degeneration. In a review of factors associated with IVD degeneration in elderly adults, Hangai and colleagues21 cited increased age, high body mass index, occupational lifting, sporting activities, and factors associated with atherosclerosis as risk factors. Multiple studies show the genetic contribution to degenerative low back pain.22 Battie and colleagues23 estimated the familial contribution to IVD degeneration to be 34% to 61%. Cigarette smoking has also been implicated and seems to have an adverse vasoconstrictive and atherosclerotic effect on the nutrition of the IVD.24,25 Type of occupation has also been shown to have an adverse effect on lumbar spinal segment degeneration, increasing the risk of symptomatic DDD. Studies have implicated occupations that require repetitive lifting or pulling, prolonged sitting26 such as motor vehicle driving,27 and whole-body vibration.28
Arun and colleagues29 used serial postcontrast MRI to study the effect of prolonged mechanical load on diffusion into the IVD. The authors reported that 4.5 hours at a load corresponding to 50% body weight significantly retarded the diffusion of small solutes into the center of the IVD, and it required 3 hours in an unloaded recovery phase to return the diffusion rate to that seen in the unloaded disc. Prolonged mechanical load can cause a disruption of diffusion, which may accelerate disc degeneration; however, this hypothesis has not been confirmed clinically.
The genetic predisposition to lumbar DDD and lifetime exposures were studied in a classic monozygotic twin study by Battie and colleagues.23 These investigators reviewed 115 male identical twin pairs for exposures to common risk factors such as occupation, recreational activities, driving, and smoking. Disc degeneration was determined by MRI and clinical evaluation. In the upper lumbar spine, only 7% of the variability was explained by occupation, 16% was explained by age, and 77% was explained by familial aggregation. In the lower lumbar spine, recreational physical loading explained 2% of variability, age explained 9%, and familial aggregation explained 43%. Battie and colleagues23 concluded that primarily genetic and other unexplained factors result in DDD, whereas commonly implicated environmental factors have only modest effects. In a 5-year follow-up study of the same twin population, the same investigators reaffirmed that genetics have a dominant role in progression of DDD, whereas occupational lifting and leisure activity had only modest effects.30 The important role of genetic factors has been corroborated in other twin studies,31,32 but it seems to be less of an explanatory factor for back pain in older people.33
Several gene loci have been discovered that are associated with increased risk for DDD. Type IX collagen was one of the first gene loci identified with some aberrant alleles imparting a threefold or fourfold increase in relative risk.34–36 More recent publications also implicate collagen type XI, interleukin (IL)-1, aggrecan, vitamin D receptor, matrix metalloproteinase (MMP)-3, and cartilage intermediate layer protein (CILP) as candidate genes.37 The discovery of these genetic risk factors has yet to result in new useful diagnostic and treatment modalities, however.
Pathophysiology
Internal Disc Disruption
Crock19 coined the term internal disc disruption in 1970 and defined it as a painful increase in biologic activity of the IVD after injury with normal radiographic, computed tomography (CT), and myelogram examinations but an abnormal discogram. IDD as a cause of discogenic back pain is controversial. The advent of MRI has dramatically improved the detection of this entity—IDD manifests as a dark disc with relatively preserved height and contour on MRI. Pain in IDD is thought to be caused by mechanical and chemical stimulation of nociceptors within the anulus or on the surface layers of the anulus and the overlying ligamentous tissue. The hallmark of IDD is the absence of disc herniation, prolapsed disc material, segmental instability, or other radiographic abnormality.19,38 Nerve root irritation, radicular pain, and neurologic deficits are also absent.
Radiographic changes associated with DDD—significant disc space narrowing, endplate osteophyte formation, endplate sclerosis, and gas formation within the disc space—are not seen in IDD.39 MRI (dark disc) and positive discography (concordant pain in the abnormal level and not at normal adjacent levels) are required to make the diagnosis of IDD. Because of the poor sensitivity and specificity of discography, many clinicians question the existence of IDD as a clinical entity.
Degenerative Disc Disease
The precise pathophysiologic mechanism for chemically mediated induction of hyperalgesia within the disc has yet to be fully elucidated. Radial annular tears provide a route for nuclear material and noxious chemicals to leak from the disc and contact the dural sac and nerve roots; some studies have shown that autologous nucleus pulposus alone has the capacity to produce an inflammatory response. Additionally, degradative changes can occur within nerve roots exposed to nuclear material even in the absence of mechanical compression.38,40–42 Weinstein and colleagues43 investigated the reproduction of pain on discography and concluded that various neurochemical changes within the disc are expressed by sensitized annular nociceptors. These nociceptors are terminal nerve endings of sensory neurons that selectively respond to painful stimuli by the release of substance P.44 These chemicals are leaked into the epidural space and are transported into the axons of the exiting nerve roots. Within the nerve root, they alter the excitability of type C nerve fibers and initiate the production of inflammatory agents such as prostaglandins, which results in radicular pain.20,45,46
In addition to material from the nucleus pulposus, many other substances in the degenerated disc have been implicated in pain generation. The role of nitric acid and phospholipase A2 in irritation of nerve roots has been well documented.45,47–50 Phospholipase A2 has been implicated in multiple aspects: (1) direct activation of nociceptors, (2) nerve injury from degradation of cell membrane phospholipids, and (3) nerve injury from inflammatory mediators created from the arachidonic acid cascade (i.e., prostaglandins and leukotrienes).51–53 Burke and colleagues54 reported on the elevation of inflammatory mediators within the disc, such as IL-6, IL-8, and prostaglandin E2. Other studies have shown the presence of inflammatory cytokines in the facet joints,55 suggesting facet involvement as a pain generator via a biochemical mechanism as well. Ohtori and colleagues56 reported on ingrowth of nerve tissue immunoreactive for tumor necrosis factor and prostaglandin P in 18 surgically harvested vertebral endplates of patients with Modic stage I and II changes who had undergone surgery. Their findings suggest that axon ingrowth into the vertebral endplate in association with Modic changes was induced by tumor necrosis factor and may be related to pain generation.
Neurovascular proliferation within and around degenerated disc elements has been proposed as another mechanism of pain generation. Normal IVDs have sparse innervation and vascularity that is distributed mainly within the outer lamellae (3 mm) of the anulus fibrosus,57,58 whereas degenerated discs have significant neurovascular ingrowth within the inner anulus and nucleus pulposus.59 Immunoreactive staining and acetylcholinesterase studies have shown penetration of nerve fibers within the inner third of the anulus in association with neovascularized granulation tissue.47,57 Peng and colleagues60 reported a histologic study of 19 IVDs harvested from surgery compared with normal control discs. The distinctive histologic characteristic of painful discs was a zone of richly innervated vascular granulation tissue extending from the outer anulus to the nucleus along the edges of fissures. Proliferation of vascular channels and sensory nerve endings rich in calcitonin gene-related peptide has also been observed in the endplate region and vertebral body adjacent to the degenerated disc. These findings suggest a role for the vertebral endplate and body as additional pain generators in DDD.61
Other studies suggest that the sensory nerve supply within the IVD is similar to visceral innervation patterns,62 with calcitonin gene-related peptide immunoreactive fibers that pass through the sympathetic trunks.63 This visceral pattern of innervation is potentially susceptible to central sensitization, which may complicate chronic low back pain further with psychosomatic overtones.64 Psychosocial and chronic non–back pain syndromes have been implicated in more recent publications as having a significant effect in patients with low back pain.65–69
Clinical Picture
Internal Disc Disruption
The pain is characterized as a deep, dull ache in the lower lumbar region, exacerbated by rotation, flexion, and side-bending movements, and partially relieved by rest. Sitting intolerance may be a primary complaint, and pain is often relieved in a lateral recumbent position. Occasionally, there is a complaint of pain in the buttock or posterior thigh, but there is a conspicuous lack of radiculopathic symptoms. In the rare instances of associated leg pain, it is usually a late finding and pain does not follow any dermatomal pattern. In a study involving intradiscal electrothermal annuloplasty in 25 patients, O’Neill and colleagues70 showed that stimulation of the IVD may result in low back and referred leg pain in patients presenting with symptoms of IDD. The distal distribution of pain was found to depend on the intensity of stimulation, and occasionally pain extending below the knee was produced.
Diagnostic Imaging
Plain Radiography
Plain radiographs are the recommended initial imaging modality for patients with a complaint of low back pain. Classic comparative and cost benefit studies have been done to determine when and what radiographs to obtain.71,72 In 1982, Liang and Komaroff73 published a comparison study between performing radiographs on all patients versus performing radiographs only on patients whose pain did not improve within 8 weeks of presentation. They found that risks and costs did not justify obtaining radiographs on initial presentation. Scavone and colleagues74 reviewed the radiographs of 782 patients and found that spot lateral and oblique films added diagnostic information in only 2% of patients. They recommended that a spine series in patients with low back pain should consist only of anteroposterior and lateral films. Generally, flexion-extension and oblique views are necessary only in patients suspected to have instability or a pars fracture. The presence of “red flags” increases the chances of diagnostic radiographic findings and may prompt the physician to obtain early radiographic studies. These “red flag” indications are summarized in Table 45–1.75
|
Anteroposterior and lateral x-rays generally are not useful in the acute setting but may be warranted
|
From Institute for Clinical Systems Improvement (ICSI): Adult Low Back Pain. Bloomington, MN, ICSI, 2008.
Magnetic Resonance Imaging
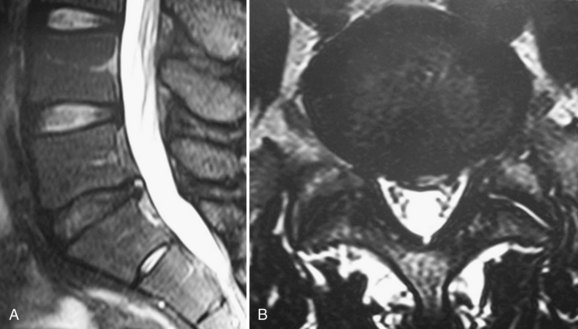
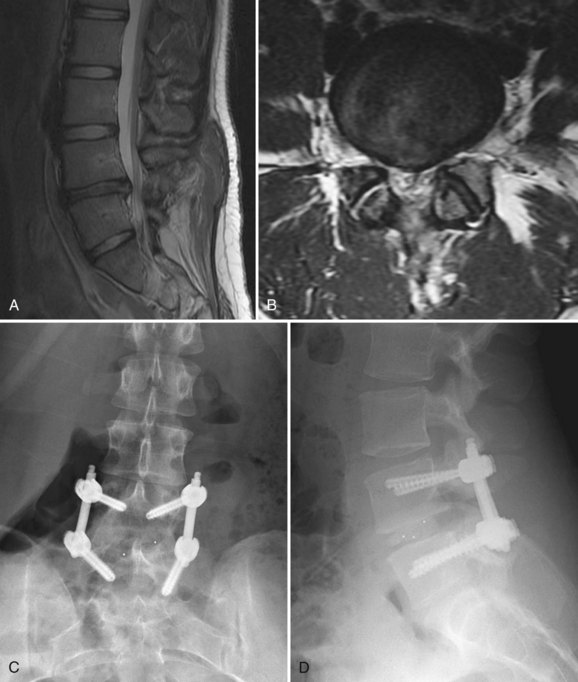
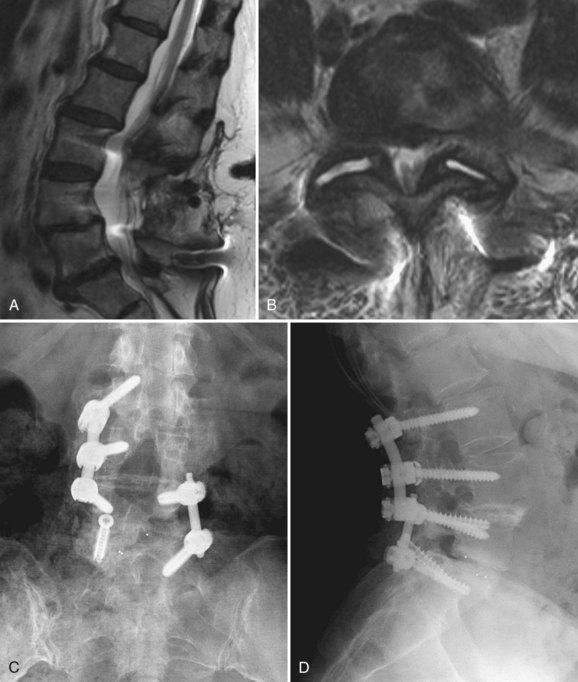
The MRI finding of a HIZ was originally described by Aprill and Bogduk76 in 1992 and is believed to be specific for an annular tear (Fig. 45–1). Postmortem studies have shown three types of tears that can occur in the anulus: concentric, transverse, or radial.77,78 A concentric tear is a crescentic or oval cavity created by a disruption in the short transverse fibers interconnecting the annular lamellae and is usually not visible on MRI. Concentric tears are occasionally referred to as delamination. A transverse tear represents a rupture of Sharpey fibers near their attachments to the ring apophysis at the disc periphery; these tears are typically thought to be clinically insignificant. A radial tear extending from the nucleus pulposus to the outermost surface of the posterior anulus is manifest on MRI as a HIZ.79 HIZ is visualized on spin-echo T2-weighted images as a high-intensity signal located within the anulus fibrosus and is clearly distinguishable from the nucleus pulposus.
Endplate changes (Fig. 45–2) that occur with disc degeneration have been well described by Modic and colleagues.80 Stage I change represents edema and is characterized by decreased signal on T1 and bright signal on T2 within the endplate. In stage II, fatty degeneration in the bone adjacent to the endplates is represented by bright signal on T1 and intermediate signal on T2 sequences. Stage III changes correspond with advanced degenerative changes and endplate sclerosis and are characterized on MRI by decreased signal intensity on T1-weighted and T2-weighted images.
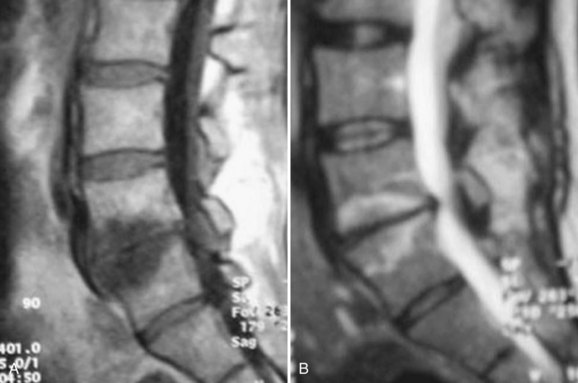
FIGURE 45–2 A and B, T1-weighted (A) and T2-weighted (B) sagittal MRI showing Modic stage I endplate changes.
When interpreting MRI findings, the clinician must be careful to consider the high prevalence of clinically false-positive findings. Abnormal disc findings on MRI are often found in clinically asymptomatic individuals. Boden and colleagues81 showed that approximately 30% of asymptomatic individuals have a major finding on lumbar MRI scans. In patients older than 60 years, such abnormal findings are almost universally present regardless of symptoms. Jensen and colleagues82 reported on 98 asymptomatic patients 20 to 80 years old and found that 52% overall had disc bulge in at least one level on MRI. Stadnik and colleagues83 showed an unusually high rate of disc bulge (81%) and annular tears (56%) on MRI in 30 asymptomatic volunteers.
Abnormal MRI findings in asymptomatic patients are not indicators of future problems. Borenstein and colleagues84 reported on 50 of the 67 patients from the study by Boden and colleagues81 at a 7-year follow-up interval and found that incidental MRI findings were not predictive of the development or duration of low back pain. Jarvik and colleagues85 studied 148 veterans with no symptoms of low back pain for at least 4 months. They found an incidence of moderate to severe desiccation in at least one disc in 83% of patients, disc bulge in 64%, and loss of disc height in 58%. In a 3-year follow-up of the same cohort, the investigators found no association between the development of new back pain and incidental MRI findings such as Modic changes, disc degeneration, annular tears, or facet degeneration. The greatest risk factor for developing low back pain in the 3-year interval was depression.65
Jarvik and colleagues86 also published a report on the use of early MRI in the primary care setting. They randomly assigned 380 patients with low back pain to receive initial spine imaging via rapid MRI or plain radiography. Jarvik and colleagues86 reported that substituting rapid MRI for x-ray studies in the primary care setting offered little additional benefit to patients in terms of secondary outcomes measures at 1 year and had the potential to increase the cost of care by $320 per patient (in 2002 dollar value). Carragee and colleagues87 performed a prospective study of 200 asymptomatic patients to determine the rate at which new episodes of low back pain are associated with changes on MRI. On follow-up MRI in 51 patients who developed an episode of low back pain, 84% had no new finding. The most common new findings were disc signal loss (dark disc), progressive facet arthrosis, and increased endplate changes. New findings were not more common in patients developing back pain after minor trauma. The conclusion was that new findings on MRI within 12 weeks of onset of a serious episode of low back pain were unlikely to represent any significant structural change and preexisted the onset.
Contrast-Enhanced Magnetic Resonance Imaging
Lappalainen and colleagues,88 in an animal study of surgically created annular tears, showed that gadolinium-enhanced MRI did not detect all tears; specifically, peripheral, small tears were not visualized, but these tears would still represent clinically significant disc disruption. Yoshida and colleagues89 investigated the relationship between T2-weighted gadolinium DTPA–enhanced MRI and a positive pain response with discography of 56 lumbar discs in 23 patients with chronic low back pain. The sensitivity, specificity, positive predictive value, and negative predictive value of the unenhanced T2-weighted images in detecting the symptomatic disc were 94%, 71%, 59%, and 97%, whereas the same values for gadolinium DTPA–enhanced images were 71%, 75%, 56%, and 86%. The findings of Yoshida and colleagues89 support the use of unenhanced T2-weighted MRI in detecting symptomatic disc pathology in appropriately selected patients, while avoiding unnecessary discography in patients with chronic low back pain.
High-Intensity Zone
In an attempt to find a noninvasive means of diagnosing IVD pathology with a high degree of certainty, several studies have investigated the correlation between positive provocative discography and various findings on MRI, such as HIZ, decreased disc intensity (dark disc), and Modic vertebral endplate changes. In their original publication, Aprill and Bogduk90 correlated the finding of a HIZ with CT discography and found an 86% positive predictive value for a positive discogram; however, the predictive value and clinical significance of HIZ on MRI has been brought into question more recently. Multiple authors91–94 have found a positive correlation between the finding of a HIZ and concordant pain on discography similar to the findings of Aprill and Bogduk,90 whereas others95,96 have documented the correlation but found unacceptably low sensitivity.
In a study of 62 patients 17 to 68 years old, Kang and colleagues97 found that only a HIZ in association with disc protrusion correlated with concordant pain on discography. Specificity was 98%, and positive predictive value was 87%; however, the sensitivity was still low at 46%. HIZ in association with either a normal or a bulging disc on MRI was not found to be associated with positive discogram. In a 30-patient study, Ricketson and colleagues98 were unable to find any correlation between the presence of a HIZ on MRI and a concordant pain response on discography; however, these authors noted that a HIZ was never visualized in a disc found to be morphologically normal on discography. Further studies49,92,99–101 attempting to correlate positive HIZ findings on MRI and painful discography suggest that although lumbar IVDs with posterior combined annular tears are likely to produce pain, the validity of these signs for predicting discogenic lumbar pain is limited.
Although the exact prevalence is unknown, a HIZ can be seen occasionally in asymptomatic individuals.44 Carragee and colleagues67 reported the prevalence of a HIZ in 59% of symptomatic patients and 24% of asymptomatic patients. In the asymptomatic group, 69% of the discs with a HIZ were positive on discography, whereas 10% of the discs without a HIZ were positive. Carragee and colleagues67 also reported that 50% of the discs with a HIZ were positive on discography in patients with normal psychometric testing compared with 100% positive discography results in patients with abnormal psychometric testing or chronic pain. They concluded that the presence of a HIZ does not reliably indicate the presence of symptomatic IDD because of the high prevalence of HIZ in asymptomatic patients.
In 2004, Mitra and colleagues102 published a study of 56 low back pain patients with the finding of a HIZ followed longitudinally for 6 to 72 months with MRI. Changes in HIZ on follow-up MRI—either an increase in intensity or spontaneous resolution—were not correlated to changes in visual analog scale (VAS) score, Oswestry Disability Index (ODI), or symptoms, which calls into question the clinical significance of HIZ. Although HIZ on MRI has been found in some studies to have good specificity and positive predictive value for concordant pain generation on discography, it has low sensitivity, high false-positive rates, and questionable clinical significance.
Dark Disc
Whether a dark disc by itself is painful is another controversial topic. Most patients with a dark disc are asymptomatic; however, in some patients, the disc can be a source of pain. Milette and colleagues103 found that loss of disc height and abnormal signal intensity were highly predictive of symptomatic tears extending beyond the anulus. Horton and Daftari104 reported a positive discogram in 50% of patients with dark discs without evidence of an annular tear. An isolated dark disc with concordant pain on provocative discography is often considered to be pathologic in the absence of other potential sources of pain and in the absence of confounding psychosocial issues; however, as discussed previously, this evidence is weak.
Modic Endplate Changes
The various stages of Modic changes are thought to be specifically linked with phases of the degenerative disc process. Toyone and colleagues105 evaluated MRI scans of 74 patients with Modic changes and found that stage I changes tended to be associated with complaint of low back pain and correlated to segmental hypermobility. Other investigators also described Modic stage I changes as specifically associated with low back pain.106,107 In a large retrospective review by Thompson and colleagues,108 Modic changes in 736 patients were correlated to provocative discogram. These authors found that Modic stage I changes had a high positive predictive value (0.81) for a positive discogram. Modic stage II changes had a lower positive predictive value (0.64), and the predictive value of Modic stage III changes was not statistically significant.
In the original description of vertebral body marrow changes by Modic and colleagues,80 the conversion between signal characteristics from stage I to stage II was described in five of six patients over the course of 14 months to 3 years. Mitra and colleagues109 performed a more recent prospective evaluation of 48 patients with Modic stage I changes. At 12 months to 3 years of follow-up, 37% were found to have progressed to Modic stage II, 15% partially progressed, and 40% had more extensive Modic stage I changes. Stage I changes are believed to represent the unstable, dynamic phase of the degenerative process and tend either to convert to a stage II pattern or to become more pervasive. Modic stage II changes are thought to be stable and less associated with painful episodes, but there have been reports of stage II changes converting back to stage I.110 Kuisma and colleagues111 reported the prevalence of Modic changes in 60 patients treated nonoperatively for sciatica to be 23%. In a longitudinal follow-up of the same patients at 3 years, 14% were noted to have changed type. The levels that did not convert were found to have more extensive Modic changes. Development of Modic change at previously unaffected levels was found in 6%.
Many authors have explored the correlation between Modic changes on MRI with positive concordant pain on discography. Sandhu and colleagues112 found that both were relatively specific for discogenic pain, with no significant correlation between them. Braithwaite and colleagues113 found the Modic changes did not predict positive response on discography; they concluded that Modic changes may represent a specific but relatively insensitive sign of discogenic low back pain. Kokkonen and colleagues114 observed that contrast injection during discography reflected well pain of discogenic origin, whereas the pain associated with endplate damage was usually not shown by CT discography. These authors found a stronger association between endplate degeneration and disc degeneration than between endplate degeneration and annular tears, which may explain why Modic changes have been found to be less sensitive for discogenic pain than discography.
Conversely, other studies have found better correlation between back pain and Modic changes than the correlation between back pain and discography. Carragee and colleagues66 reported on 100 prospectively followed asymptomatic patients who were at high risk for developing disabling back pain. Of all the incidental diagnostic findings, only moderate or severe Modic changes of the vertebral endplates were found to be weakly associated with subsequent development of a disabling episode of back pain. Other structural MRI findings and concordant pain with discography correlated only weakly with previous back pain episodes and had no association with future disability or medical consultations for back pain. Psychosocial, neurophysiologic (chronic nonlumbar pain), and occupational factors strongly predicted future disabling episodes and consultations for back pain.
In a cross-sectional study of 109 women from two groups, nursing or administrative professions, Schenk and colleagues115 found that Modic changes and nerve root compromise were the only MRI findings that were statistically significant predictors of low back pain. Signs of disc degeneration, disc herniation, HIZ, and facet arthritis were found in both groups but were not significant risk factors for low back pain.
Similar findings were reported in a study by Kjaer and colleagues,116 in which complaint of low back pain was correlated to MRI findings in a random selection of 412 Danish subjects. Although Modic changes occurred in less than 25% of subjects (16% Modic stage I and 7% Modic stage II), this finding had the strongest correlation with complaints of back pain. When the subjects were evaluated clinically, the authors found that patients with radiographic evidence of DDD and Modic changes had the best clinical evidence of disc disease. Clinical findings in patients with radiographic evidence of disc degeneration without Modic changes were not significantly different from the baseline population. Kjaer and colleagues117 concluded that Modic change was a critical finding in relation to history of low back pain and clinical findings. In a follow-up study of the same Danish population, Modic changes correlated with type of occupation, history of smoking, and overweight. The odds ratio for heavy labor combined with smoking was 4.9 for the presence of Modic changes on MRI.118
A meta-analysis review of Modic changes by Jensen and colleagues119 found that the median prevalence of Modic changes from all studies was 43% in patients with nonspecific low back pain. A positive association between low back pain and Modic changes was reported in 7 of 10 studies with odds ratios between 2.0 and 19.9.
Axially Loaded Magnetic Resonance Imaging
There has been interest more recently in the potential role of axially loaded MRI in evaluating patients with lumbar spinal diseases. The idea is to use axial loading to reproduce better the anatomy of the disc under physiologic load. The utility of axially loaded MRI has been studied much more extensively in patients with spinal stenosis and spondylolisthesis.120–123 Danielson and Willen124 observed a significant decrease in dural cross-sectional area between a psoas-relaxed position and axial compression in extension in 56% of asymptomatic individuals. The decrease was most pronounced at L4-5 and was worse in older individuals. Although the clinical role of axially loaded MRI in patients with discogenic back pain has not yet been established, Saifuddin and colleagues125 postulated that lumbar spine MRI with axial loading may increase the sensitivity for the detection of HIZs; however, this hypothesis has not been tested.
Discography
There is significant controversy in the literature surrounding the usefulness of discography for the evaluation of the integrity of the lumbar disc. Some investigators consider discography to be the most important tool in the diagnosis of IDD,43,126 but more recent outcome studies127 and a practice guideline by the American Pain Society128 have recommended against the use of provocative discography in the diagnosis of discogenic back pain.
Discography is the only physiologic modality used to determine if a specific disc is a pain generator. Although several attempts have been made to explain the pathogenesis of pain provocation during discography, the precise pathomechanism is not well understood. There are four components to the evaluation of a discogram: (1) the pressure and volume of fluid injected into the disc, (2) the morphology of the disc being injected, (3) the subjective pain response at the level of interest, and (4) the pain response when adjacent control levels are injected.129,130 The subjective pain response to low-pressure provocation is the most important determinant of disc derangement; reproduction of the patient’s symptoms on injection of the diseased level is essential to a positive test. A normal disc can accept 1 to 1.5 mL of contrast medium. If 2 mL or more of contrast agent is easily introduced, some degree of disc degeneration is assumed.
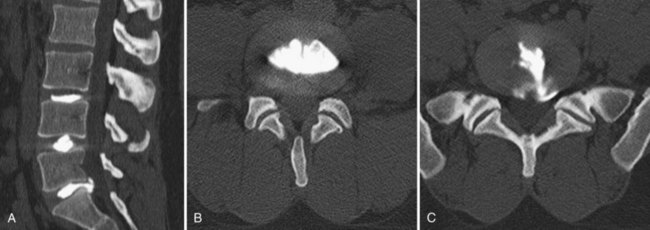
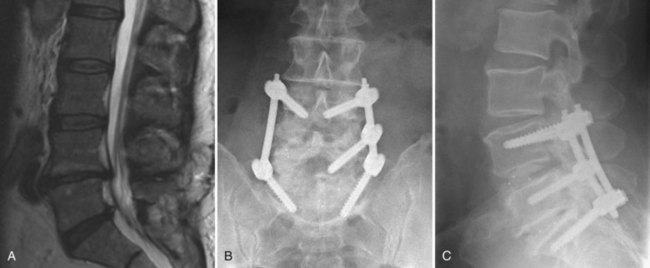
The use of postdiscography CT has also been reported to increase the sensitivity for the diagnosis of radial tears of the anulus.131 Because of low specificity and sensitivity, postdiscography CT is not as helpful, however, in the diagnosis of IDD. Most authors believe that to be diagnostic, not only should the pain be concordant on low-pressure injection, but also a normal control disc should be pain-free (Fig. 45–3).
Despite being used since 1948, discography remains controversial. Holt126 and Massie132 published in the 1960s on the high false-positive rate of lumbar discography, which was found to be 26% by Holt. Walsh and colleagues133 later published a rigorous study on the reliability of lumbar discography. Their study compared 10 normal volunteers with 7 symptomatic patients. Although 17% of the normal discs were found to be morphologically abnormal, there were no positive pain responses. Walsh and colleagues133 concluded that with modern techniques the false-positive rate of lumbar discography is not as high as reported by Holt.126
Derby and colleagues134 found similar results in a more recent study of 90 patients with low back pain and 16 controls. Morphologically, the prevalence of grade III annular tears was 58% among the asymptomatic control population. Presumably, asymptomatic discs in symptomatic individuals on pressure-controlled discography showed pain levels and responses similar to the control group, whereas patients with true-positive discography showed pain characteristics concordant with their usual symptoms. Derby and colleagues134 concluded that pressure-controlled discography can differentiate between asymptomatic discs and morphologically abnormal discs.
Carragee and colleagues68 studied the false-positive rate of low-pressure discography in a comparison of 69 volunteers with no significant low back pain and 52 patients undergoing discography in consideration for treatment of discogenic pain. Low-pressure discography was positive in at least one level in 27% of the patients with low back pain and in 25% of the controls. The false-positive rate of discography was 25% and correlated with psychosocial factors and history of chronic pain of a non-lumbar origin. In another publication from Carragee’s group,69 psychosocial factors and chronic nonlumbar pain, such as cervical pain and somatization disorder, also correlated with positive discography in patients without symptoms of low back pain. These authors concluded that false-positive rates can be low with strict application of the Walsh protocol133 in patients who do not have positive psychometric issues or other chronic pain syndromes.
In contrast to reports of high false-positive rates, two more recent meta-analyses of low-pressure discography report strong evidence to support the role of discography in identifying patients with discogenic pain.135,136 Combined data from all studies showed an overall false-positive rate of 9.3% per patient and 6% per disc. False-positive rates among asymptomatic patients were 3% per patient and 2.1% per disc. Chronic pain was not found to be a confounder, and strength of evidence was reported as level II-2 in support of the diagnostic accuracy of discography.
Finding a “gold standard” with which discography results can be compared remains a problem. Few studies have compared the use of discography and outcomes after surgical fusion, which is perhaps the best measure for the validity of discography. Colhoun and colleagues,137 in a study of 137 patients, reported 89% favorable outcomes in patients with positive concordant pain on discography versus 52% favorable outcomes among patients who had no painful response. Madan and colleagues138 had different findings; 81% of 41 patients who underwent fusion based on MRI findings had satisfactory outcomes versus 76% of 32 patients who had surgery based on discography. Perhaps the most rigorous study to date was published by Carragee and colleagues.139 In their study, success of surgical fusion was compared in 32 patients with single-level positive discogram and a matched cohort of 34 patients with single-level spondylolisthesis; 72% of the patients with spondylolisthesis met the highly effective success criteria for surgery versus only 27% of the patients with discogenic pain. Minimal acceptable success criteria were 91% and 43%. Carragee and colleagues139 calculated a best case positive predictive value for discography of 50% to 60% and concluded that provocative discography was not highly predictive of single-level discogenic back pain.
In an attempt to improve on the poor reliability of discography, interest has turned to functional anesthetic discograms, also called discoblocks. A discoblock is a modification of discography, in which a local anesthetic, usually bupivacaine, is infused with the contrast agent into the disc to enhance the diagnostic capability of the procedure. Relief of pain after discoblock is considered diagnostic for discogenic pain. Ohtori and colleagues140 published a randomized controlled study comparing standard provocative discogram with discoblock in diagnosing discogenic low back pain. Anterior lumbar interbody fusion (ALIF) procedures were performed in 15 patients whose discogenic pain was diagnosed with the aid of discography and 15 patients whose pain was diagnosed with the aid of discoblock. Outcome measures (ODI, VAS, and Japanese Orthopaedic Association score) at 3-year follow-up showed better results that were statistically significant in the group in which diagnosis was aided by discoblock.
Regardless of the details of how discography is performed, some authors have posed the question of potential ill effects resulting from perforating the lumbar disc. Carragee and colleagues127 more recently published a report on the effect of lumbar discography in precipitating accelerated degeneration in a matched cohort study. The 10-year follow-up showed that discs that had been punctured had a greater progression of disc degeneration—35% versus 14% in the control group. There were 55 new disc herniations in the discography group versus 22 in the control group. Carragee and colleagues127 concluded that despite using modern discography techniques with small-gauge needles, there is still an increased risk of disc degeneration, disc herniation, changes in disc and endplate signal, and loss of disc height when discography is performed.
Although discography has the potential to assist in diagnosing disc derangement, its reliance on the patient’s subjective pain response can also be problematic where secondary gain may be an issue. Psychosocial factors and chronic nonlumbar pain have also been shown to alter the diagnostic capabilities of the procedure. Finally, consideration of the consistent reports of the high false-positive rates and new findings of accelerated degeneration in discs that undergo discography make it difficult to recommend the procedure for the diagnosis of discogenic back pain. The validity of lumbar discography is very much in doubt, which is underscored by a more recent practice recommendation published by the American Pain Society. The society’s current recommendation is that provocative lumbar discography should not be used for making the diagnosis of a discogenic source of pain in the setting of nonradicular low back pain.128 The value of using discography to assess the levels to be operated on in patients with multilevel disc degeneration has not been adequately established scientifically.
Treatment
In 2009, the American Pain Society published five practice guidelines on the management of chronic nonradicular back pain based on the best available evidence for the various diagnostic and treatment modalities available. These recommendations are summarized in Table 45–2.128 These treatment modalities and others not mentioned in the treatment recommendations are discussed in detail along with brief summaries of the current supporting and opposing literature.
TABLE 45–2 Summary of Recommendations of American Pain Society Specifically in Regard to Management of Chronic Nonradicular Low Back Pain
| Recommendation #1 |
From Chou R, Loeser JD, Owens DK, et al: Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: An evidence-based clinical practice guideline from the American Pain Society. Spine (Phila Pa 1976) 34:1066-1077, 2009.
Nonoperative Treatment
Nonoperative treatment of lumbar disc disorders has been extensively discussed in the literature.141 Physical therapy, pharmacology, and spinal manipulations all have been supported by multiple studies of reasonable validity, but it is difficult to evaluate fully most of these studies because of a generalized lack of randomized control design, blind observers, compliance measures, and cointerventions. Additionally, very little of the literature on these nonoperative treatments is specific for the diagnosis of IDD or DDD but rather is generalized to chronic and acute low back pain, which may have multiple etiologies.
Bed Rest and Advice to Stay Active
The use of bed rest and its duration has long been debated in the literature. Treatment schedules ranging from 2 days to 6 weeks have been described.142–144 The currently accepted recommendation75 is limited bed rest for a maximum of 2 days because longer durations of bed rest may be detrimental to the patient’s general health while offering no benefit to the back pain. Allen and colleagues145 published a review of studies documenting bed rest as treatment for 15 different conditions and found that for patients with acute low back pain there was significant worsening of outcome measures. The updated Cochrane Review of bed rest for treatment of acute low back pain reported that there is high-quality evidence that advice to rest in bed is less effective than advice to stay active.146 Progressive return to activity and the initiation of a formal physical therapy or home exercise program are recommended after any initial short period of rest.
Verbunt and colleagues147 explored reasons why patients sometimes use prolonged bed rest in the setting of acute episodes of low back pain. Among the study population of 282 patients, 33% reported using bed rest, and 8% remained in bed for longer than 4 days. Behavioral factors, catastrophizing, and fear of injury were associated with use of prolonged bed rest. History of back pain and pain intensity were not associated with patient use of prolonged bed rest. Additionally, patients who used prolonged bed rest in the early phase of acute low back pain were more disabled after 1 year.
Patient education and advice to stay active is now the favored recommendation. A more recent Cochrane review148 of patient education and advice to stay active showed strong evidence that individual instructional sessions of 2.5 hours are more effective in returning patients to work than no intervention; however, in the setting of chronic back pain, patient education was less effective than more intensive interventions. Education sessions of shorter duration and written information were no more effective than no intervention. Another meta-analysis149 of 39 randomized controlled studies evaluated advice to stay active alone or as an adjunct to other interventions such as back school or specific exercise routines. Advice as an adjunct to a specific exercise program was the most common form of treatment implemented and the best supported of the treatments studied for chronic low back pain. Outcomes among patients with acute low back pain were generally poor, but advice to stay active alone was found to be the best recommendation.
Brox and colleagues150 published a systematic review of brief education in the clinical setting involving examination, information, reassurance, and advice to stay active. The authors found strong evidence that brief education was more effective for return to work but was no more effective than usual care for reduction of pain. There was limited evidence that dissemination of a back book or an Internet session was less effective than exercise. The authors concluded that a back book should not be distributed to patients as an alternative to another form of treatment.
Brace Wear and Other Orthotics
Another common conservative management technique involves the use of limited brace wear either with a recommendation to stay active or in conjunction with another form of nonoperative therapy. Calmels and colleagues,151 based on the results of a randomized clinical trial, recommended the limited use of a lumbar belt to improve functional status, pain, and medication use. Oleske and colleagues152 performed a randomized clinical trial of back supports and patient education in work-related back pain. These authors found no effect on patient self-report of recovery or lost work time between brace use and controls, but back supports were found to have some value in preventing recurrence of work-related back pain. A more recent Cochrane systematic review153 of brace treatment for low back pain failed, however, to find sufficient evidence to support the use of lumbar supports to treat low back pain. Moderate evidence was found that braces are no more effective than no treatment or physical training in preventing episodes of back pain.
Use of shoe insoles has been recommended in the past for treatment and prevention of nonspecific low back pain. A more recent Cochrane systematic review154 of six randomized controlled trials reported strong evidence that use of insoles does not prevent episodes of low back pain. There was limited evidence that insoles alleviated low back pain, but no conclusions or recommendations were made for use in the treatment of patients with low back pain.
Physical Therapy
There are multiple randomized controlled trials in the literature in support of many therapy routines or programs. Although a comprehensive review of all the various programs is not undertaken here, there have been some important updates in recent years worthy of discussion. More recent prospective randomized trials comparing physical therapy with fusion have emphasized the importance of a multidisciplinary approach with cognitive therapy, fear avoidance counseling, and intensive exercise programs.155–157 A systematic review150 found moderate evidence that fear avoidance training emphasizing exposure is more effective than graded activity increase for improvement of pain, disability, and fear avoidance.
Intensive interdisciplinary rehabilitation with emphasis on cognitive and behavioral intervention was one of the treatment recommendations made by the American Pain Society.128 Interdisciplinary rehabilitation was defined by the society as an integrated intervention with rehabilitation plus a psychological or social or occupational component. The American Pain Society recommended that interdisciplinary therapy should be offered as a viable alternative before proceeding to surgical treatment. Noninterdisciplinary or “traditional” physical therapy is also efficacious in this patient population, but no one specific program, method, or technique is significantly better than another.
Back schools are another commonly discussed therapy modality, and there is some indication that low-intensity back schools may have some efficacy. Heymans and colleagues,158 in a randomized controlled trial, found that patients who attended low-intensity back school experienced fewer sick leave days (68 days versus 75 days and 85 days) than usual care patients and patients who attended high-intensity back school. Functional status and kinesiophobia were improved at 3 months, but there was no difference in pain intensity and perception of recovery between the groups. In another randomized controlled trial, Kaapa and colleagues159 found no significant benefit of back school, however, compared with physical therapy combined with cognitive therapy at 6-month, 1-year, and 2-year follow-up.
A systematic review from the Cochrane Database in 2004160 concluded that there was moderate evidence suggesting that back schools in an occupational setting reduce pain and improve function and return-to-work status compared with other forms of therapy, such as exercises, manipulation, myofascial therapy, advice, placebo, and waiting list controls. Brox and colleagues150 published a separate systematic review of back schools and found moderate evidence that back schools were no better than waiting lists, no intervention, placebo, or general exercises for reduction of pain.
A European economic evaluation of a randomized controlled study161 of intensive group therapy found no significant cost difference between intensive group therapy and standard physiotherapy. There was also no difference in clinical effect between the groups at 1-year follow-up.162 To the authors’ knowledge, there are no economic studies to date of group therapy back schools in the United States. Although low-intensity back school and programs in a work setting may have benefit versus other forms of nonoperative treatment, most of the current literature shows that back schools offer little benefit over standard physical therapy and cognitive therapy.
Adjunctive Modalities
Another treatment option for low back pain includes adjunctive physical therapy modalities such as TENS, electrical muscle stimulation, ultrasound, and iontophoresis. Poitras and Brosseau163 reviewed randomized controlled data on the use of TENS and found that it may be useful for immediate short-term pain reduction but has little impact on patient perception of disability or on long-term pain control. A 2008 Cochrane systematic review of TENS versus placebo164 concluded that there is currently not enough evidence to support the routine use of TENS for management of chronic low back pain. Even less literature is available on the use of iontophoresis and ultrasound in the setting of discogenic back pain. The few randomized controlled trials that exist focus on ultrasound in conjunction with other physical therapy regimens. The efficacy of these modalities in isolation has not been determined.
Chiropractic and Complementary and Alternative Medicine Therapies
Several studies have reported the potential beneficial effects of chiropractic treatment for acute nonspecific low back pain.165–167 The role of chiropractic manipulations for the treatment of IDD or DDD of the lumbar spine has not been studied. Chiropractic manipulation is generally not considered effective in the treatment of chronic back pain resulting from disorders of the IVDs.168 A Cochrane systematic review169 failed to find evidence that spinal manipulative therapy was superior to general practitioner care, analgesics, physical therapy, exercises, or back school in the treatment of acute and chronic low back pain.
Eisenberg and colleagues170 published a randomized trial of usual care therapy versus the addition of the patient’s choice of alternative therapy—chiropractic, acupuncture, or therapeutic massage—in the treatment of acute low back pain. Outcomes based on the Roland-Morris scale and subjective assessment of symptoms showed no statistically significant improvement in patients who underwent alternative therapies compared with patients treated with the usual care of limited bed rest, nonsteroidal anti-inflammatory drugs (NSAIDs), education, and activity modification. The study did show, however, an increase in patient satisfaction with care, which came at an average $244 net increase in cost per patient.
Hurwitz and colleagues171 had similar findings in a randomized prospective study of 681 patients with chronic low back pain comparing chiropractic care with medical treatment with 18 months of follow-up. Although less than 20% of the patients overall experienced pain relief and differences in outcome measures were not clinically significant, patients in the chiropractic group were more likely to perceive that their symptoms had improved.
Other alternative medical therapies include acupuncture, prolotherapy, and massage. The Cochrane systematic review of acupuncture172 showed superiority to placebo sham therapy and a short-term benefit that did not extend beyond first follow-up when acupuncture was used in conjunction with other conventional therapies. A more recent systematic review by Ammendolia and colleagues173 questioned inconclusive evidence of the success of acupuncture versus sham acupuncture and called for further randomized trials to rule out the possibility of a placebo effect.
Prolotherapy is a technique that attempts to regenerate ligamentous and tendinous structures of the spine via injections of various irritant solutions. The treatment is usually performed in conjunction with spinal manipulation. There is no consensus on method, type of solution injected, or frequency of sessions. Most practitioners use various combinations of saline, dextrose, glycerin, phenol, and lidocaine. Many randomized trials and systematic reviews report conflicting efficacy of prolotherapy.174–176 No evidence has been reported for the efficacy of prolotherapy without cointerventions such as spinal manipulation or exercise.
Pharmacotherapy
Judicious use of narcotic pain medications, oral steroids, and NSAIDs in patients with severe, acute back pain can provide good pain relief. Most patients with painful degenerative discs can be treated adequately on an outpatient basis. NSAIDs and acetaminophen (Tylenol) are common over-the-counter medications used to treat back pain. A Cochrane review177 included 65 studies on NSAID use in low back pain. NSAIDs were found to be superior to placebo but had significantly more side effects. There is no documented difference between type of NSAID, including cyclooxygenase-2 inhibitors. Acetaminophen has an effect similar to NSAIDs but reduced risk of associated side effects when taken as directed and in general should be tried before NSAIDs. The Cochrane group concluded that NSAIDs are effective for short-term treatment of acute and chronic low back pain, but the size of the effect is small.
Opioid formulations are commonly used to treat back pain, but considering their widespread use there is a surprising paucity of high-quality randomized controlled data available on their efficacy. A Cochrane database meta-analysis178 of opioid use found only four studies, three of which focused on the use of tramadol. Pooled data found that tramadol, an atypical opioid, was more effective than placebo for pain relief and showed a slight improvement in functional scores. The only randomized controlled study of classic opioids179 was a comparison with naproxen. Opioids were found to be more effective for pain relief but were not more effective for improving function than naproxen. The Cochrane review authors concluded that the benefits of opioids for the treatment of chronic low back pain are questionable, and further well-designed randomized controlled studies need to be performed. Two subsequent systematic reviews180,181 of opioid use in the setting of chronic low back pain concluded that there is evidence to support the efficacy of opioids for short-term relief of pain only. There is little evidence for long-term opioid use, which is fraught with an incidence of aberrant consumptive behavior approaching 25%.
Use of opioid pain medication has many problems ranging from minor side effects such as constipation and nausea to severe complications including respiratory depression, altered mental status, and insidious issues with tolerance and addiction. Another more recent concern with opioid use is related to the combination of opioids and acetaminophen in commonly prescribed formulations.182 The maximum recommended daily dose of acetaminophen for adults and children older than 12 years is 4 g; thus concern arises when patients inadvertently take larger doses in the setting of prescription drug abuse. The potential to inadvertently take hepatotoxic or lethal doses can be a concern in the setting of prescription drug abuse. An advisory committee from the U.S. Food and Drug Administration (FDA)184 recommended the addition of a boxed warning on the risk of acetaminophen overdose and suggested elimination of combination opioid-acetaminophen formulations. Care should be exercised when prescribing opioid pain medications. They are best given for only a few days in the setting of severe acute back pain, and their use in patients with chronic back pain is not recommended.
Oral tapering courses of steroids have also been found useful for decreasing symptoms of low back pain, most specifically in patients with disc herniations.80,185 Steroids can cause gastrointestinal bleeding, and gastrointestinal protective agents should be used simultaneously with oral steroids to reduce the risk of this complication.
Muscle relaxants are another class of medication routinely used in the treatment of muscle spasm associated with low back pain. Their use should be limited to very short courses because of their addictive potential. The Cochrane review186 of muscle relaxants for the treatment of back pain included 30 trials evaluating the use of benzodiazepines, nonbenzodiazepines, and antispasmodic muscle relaxants. Strong evidence for the efficacy of muscle relaxants over placebo was reported for short-term pain relief in the setting of acute back pain. No difference between the various drugs and classes was discerned. More trials to determine the efficacy of muscle relaxants compared with other analgesics and NSAIDs were recommended.
The last class of medications commonly prescribed in the setting of back pain is antidepressants. Their use may be particularly beneficial in patients presenting with chronic low back pain in association with altered mental status, depression, anhedonia, sleep disturbances, agitation, and anorexia. Clinical studies187–189 supporting the use of tricyclic antidepressants (TCAs) have shown an improvement in mood and sleep patterns. Low doses of TCAs also affect membrane potentials of peripheral nerves, which may be a mechanism by which they produce pain reduction. A 2003 review190 of antidepressants in the treatment of chronic low back pain found that TCAs have a moderate effect on pain reduction in patients with no history of depression but reported conflicting evidence for improvement in functional outcomes. Physicians prescribing TCAs should be aware of potentially serious side effects involving orthostatic hypotension and cardiovascular perturbations. In a systematic review,191 selective serotonin reuptake inhibitors, another common class of antidepressants, failed to show efficacy in the treatment of chronic low back pain and should be reserved for emotional or psychiatric disturbances related to back pain and not used as a primary treatment for symptoms of back pain.
Keller and colleagues192 published a meta-analysis of nonsurgical management options for low back pain. These authors reported that behavioral therapy, exercise therapy, and NSAIDs had the largest effect of the modalities studied. Machado and colleagues193 published a separate large meta-analysis of placebo-controlled randomized trials of various forms of nonoperative treatment for nonspecific low back pain. Small improvements in complaints of pain were found in patients treated with traction, physical therapy, antidepressants, and NSAIDs; moderate improvements were found in patients treated with opioid analgesics, muscle relaxants, facet injections, and nerve blocks.
Nonsurgical Interventional Therapies
Epidural Spinal Injection
Administration of epidural steroids should be considered by the surgeon and patient before proceeding to a surgical intervention. The advantage of epidural injections over oral steroids is the ability to achieve higher concentrations of steroid at the site of pain while minimizing systemic effects. Epidural steroids typically work well when administered in the setting of radicular pain and do not work well in the setting of axial pain. Patients with foraminal stenosis secondary to loss of disc height may benefit from selective nerve root blocks either as a diagnostic or as a therapeutic tool. Many clinicians recommend epidural steroid injections as second-line therapy in the treatment of lumbar disc disorders. Epidural steroids are commonly administered by three different routes: caudal, interlaminar, and transforaminal. Although discogenic back pain with leg symptoms is considered an indication for all three modes of administration, the transforaminal approach is generally considered best because it achieves a better anterior epidural distribution. Complications from injection exist but are uncommon.194,195
Reports on the efficacy of epidural injections in the literature are contradictory. Manchikanti and colleagues196 published preliminary results of a randomized trial of serial caudal epidural injections in patients with discogenic pain without disc herniation or radiculitis. These authors reported greater than 50% pain relief in 72% to 81% of patients and 40% reduction in ODI scores in 81% of patients. Manchikanti and colleagues196 concluded that caudal epidural injections with or without steroid are effective in treating discogenic back pain in greater than 70% of patients. Two other observational studies by the same authors197,198 have similar findings for the beneficial effects of caudal epidural injections in the specific setting of discogenic low back pain.
Buttermann199 studied patients with DDD and back pain of greater than 1 year’s duration who were candidates for fusion. There was initial success of treatment in greater than 50% of patients, but success rate declined to 23% to 29% by the 1- to 2-year follow-up. The study was plagued by a high dropout rate with more than two thirds of the patients seeking another invasive treatment within 2 years. Buttermann199 concluded that patients with DDD without spinal stenosis may experience a short-term benefit from epidural injections with only one fourth to one third experiencing long-term improvement in pain and function. Other earlier studies of caudal and transforaminal approaches have reported similar good results for short-term efficacy in low back pain, with 59% of patients having greater than 50% improvement in symptoms and function at a 1-year interval.197,200
A more recent systematic review201 criticized the literature on epidural injections for a lack of careful control of route of administration and patient diagnosis. On evaluation of the pooled data, the only evidence found in support of epidural injections was for short-term symptom relief in nonspecific low back pain. No well-designed randomized trials were found specific to discogenic back pain. A 2008 Cochrane systematic review202 of injection therapy for low back pain failed to find sufficient evidence to make a recommendation. A systematic review by Chou and colleagues,203 as part of the American Pain Society practice recommendations, found fair evidence that epidural steroid injection is moderately effective for short-term pain relief; however, the literature supporting its use in nonradicular low back pain is sparse and has not shown significant benefit. No specific recommendation for the use of epidural steroid injections or the route of administration was made by the American Pain Society.
Intradiscal Injection
Direct intradiscal injection, usually with a steroid solution, is another intervention that has been described in the literature for IDD. The desired effect is suppression of an inflammatory process within the disc, which is thought to be the cause of the discogenic pain. Intradiscal steroid injections were reported in an early case series by Feffer,204 in which 47% of patients reportedly had remission of discogenic symptoms. Similar results were found by Wilkinson and Schuman.205 More recently, Fayad and colleagues206 reported a short-term improvement in VAS score at a 1-month follow-up with intradiscal steroid injection in patients with Modic stage I and I-2 changes on MRI, but there was no long-term benefit. The only two major prospective randomized trials207,208 of intradiscal steroid injection failed to find a statistically significant benefit versus placebo in the treatment of discogenic back pain. Other authors have attempted intradiscal injection of various other substances, including solutions of chondroitin and dextrose,209 hypertonic dextrose,210 methylene blue,211 and oxygen–ozone gas mixtures.212,213 Although these studies purport promising results, they have yet to be proven efficacious by rigorous randomized controlled trials.
Thermal Annuloplasty
The proposed mechanism of action for these procedures is twofold: (1) elimination of nociceptive pain fibers and aberrant painful responses to the disrupted disc and (2) collagen rearrangement in the anulus with resultant spinal segment stabilization. The biologic effects are not well understood, and there is a lack of clear consensus regarding the effects on neuronal deafferentation, collagen modulation, and spinal stability. Freeman and colleagues214 studied the effect of nociceptor destruction via IDET on experimentally created annular tears in a sheep model. The authors failed to find any difference in the amount of neoinnervation in the anulus between specimens that underwent IDET and specimens that did not, which calls into question the theory of deafferentation of the anulus. Whether collagen rearrangement with resultant shrinkage and stabilization of the discal element is a viable mechanism for IDET also is questioned.215,216 Cadaveric studies of the effect of IDET on annular collagen have been performed by Kleinstueck and colleagues,216 which showed a 10% to 16.7% reduction in tissue volume immediately adjacent to the electrode.
To destroy nociceptors in the anulus fibrosus, temperatures must be increased to a minimum of 42° C to 45° C.217,218 It is impossible to generate sufficient temperatures in the anulus with a radiofrequency probe placed in the center of the disc as shown by Houpt and colleagues.219 Temperature changes at distances farther than 11 mm were insufficient to increase the tissue temperature of the outer anulus to the 42° C needed for neuronal ablation. Ashley and colleagues220 compared temperature distribution in the disc between a radiofrequency needle and a navigable SPINECATH (Smith & Nephew, Memphis, TN). Using this method, they were able to deliver thermal energy to the anulus more effectively and achieved sufficient temperatures to cause denervation. Karasek and Bogduk221 recommended inserting the IDET electrode so as to remain within 5 mm of the outer surface of the anulus. Placement of the probe in the interlamellar plane rather than inside the innermost layer of the anulus allows for sufficient heat generation to destroy the nociceptors in the outer layers of the anulus.
Complications secondary to any of the thermal annuloplasty procedures are rare. There has been one reported case of postoperative cauda equina syndrome caused by inadvertent placement of the catheter in the spinal canal222 and a few reports of broken catheters with no resultant adverse effect. There have been no reports of infection, bleeding, or other equipment-related or technique-related complications.
Early uncontrolled clinical trials of IDET were promising, with improvement in 50% to 70% of patients,42,221–224 but randomized controlled trials have produced conflicting results. Freeman and colleagues225 found no significant improvement in outcome measures compared with sham surgery at 6 months’ follow-up. The opposite findings were reported by Pauza and colleagues226 in patients with discographically diagnosed low back pain of greater than 6 months’ duration. Pauza and colleagues226 found that 40% of their patients who underwent IDET experienced at least 50% relief of pain, whereas a significant portion of the control group experienced symptom progression. These authors concluded that the IDET procedure is an effective intervention for a selective patient population and reported a number needed to treat of 5 to achieve a 75% relief of pain. Barendse and colleagues227 reported on a trial of intradiscal radiofrequency thermocoagulation in patients with chronic discogenic back pain. An 8-week follow-up assessment showed no difference from sham surgery in VAS score, global perceived effect, and ODI outcome measures.
Andersson and colleagues228 published a systematic review of IDET versus spinal fusion in patients with disc degeneration and disruption. Similar median percentage improvement was noted between the two interventions for pain severity and quality of life outcomes. Fusion showed better functional improvement but had a higher rate of complications. Andersson and colleagues228 concluded that IDET offers similar symptom relief with less risk of complications compared with fusion. In a systematic review, Derby and colleagues229 concluded that IDET is generally safer and cheaper than more invasive surgical techniques despite the fact that the best evidence available shows only modest improvement in pain relief and functional outcomes.
Other systematic reviews of IDET have been more critical. Helm and colleagues230 reported level II-2 evidence in support of IDET in the setting of discogenic back pain based on two of the above-mentioned randomized trials and numerous observational studies. Two observational studies were found by these authors in support of radiofrequency intradiscal thermocoagulation for a II-3 level of evidence. Evidence in support of biacuplasty was lacking and was assigned level III. Freeman231 published a systematic review of the literature that criticized generally poor outcomes even among studies in support of IDET. Freeman231 concluded that evidence for the efficacy of IDET is weak and has not passed the standard of scientific proof.
Chou and colleagues203 published a systematic review summarizing all nonoperative interventional therapies as part of the American Pain Society practice recommendations published in 2009. These authors reported fair evidence that epidural steroid injections are effective for short-term pain relief. Good evidence was reported that prolotherapy, facet injection, intradiscal steroid, and intradiscal radiofrequency thermocoagulation are ineffective. For IDET, no conclusions were made because available randomized controlled trials are conflicting. IDET may best be indicated for patients with less functional impairment, with well-maintained disc heights, and with discogenic pain from annular tears.229 IDET is not universally successful, but roughly 50% of patients can expect significant reduction (>50%) in pain.
Surgical Treatment
Chou and colleagues232 published a systematic review of surgical treatment for nonradicular low back pain as part of the American Pain Society’s practice recommendations. These authors found fair evidence that surgical fusion is no better than intensive rehabilitation with a cognitive behavioral emphasis. Surgically treated patients were considered to be performing poorly, with less than 50% obtaining optimal outcome with fusion. The benefits of instrumented fusion compared with noninstrumented fusion were unclear. Fair evidence was found that for single-level DDD arthroplasty performs as well as fusion, but more long-term outcomes data are needed.
The American Pain Society128 practice recommendations, published in 2009, encourage clinicians to offer intensive interdisciplinary rehabilitation as an option with outcomes similar to surgery in the setting of nonradicular low back pain. Most patients with nonradicular pain who undergo surgery do not experience an optimal outcome, which was defined by the American Pain Society as (1) minimal or no pain, (2) discontinuation of or only occasional use of pain medications, and (3) return to high-level function. The society also suggested that there is insufficient evidence at this time to support disc arthroplasty for patients with nonradicular low back pain. Other treatment guidelines also take a cautious view on spinal fusion for DDD, but in some patients the symptoms are so severe that the chance for a good result makes surgical management particularly attractive, especially when nonoperative treatment has failed.
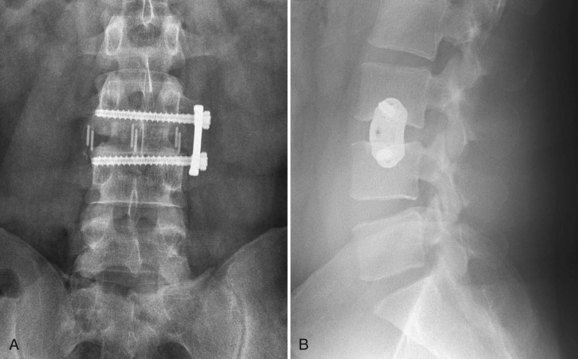
Spinal Fusion
Surgical treatment for unremitting discogenic back pain has traditionally been spinal fusion; however, fusion is not universally accepted as the “gold standard” for this condition. Most clinicians find that it is acceptable to perform spinal fusion for DDD in patients who have failed exhaustive conservative care. The role of spinal fusion in the management of IDD is more controversial.233
Three high-quality randomized controlled studies in the past decade have evaluated spinal fusion compared with nonoperative treatment in the setting of chronic low back pain and DDD. Fritzell and colleagues234 published a randomized controlled multicenter study of severe chronic low back pain comparing fusion of the lower lumbar spine with nonsurgical therapy. The study involved 222 operative and 72 nonoperative patients 25 to 65 years old with chronic low back pain of at least 2 years’ duration and radiologic evidence of disc degeneration at L4-5, L5-S1, or both. The nonsurgical group received physical therapy, patient education, and alternative pain control modalities, such as TENS units, acupuncture, and injections. Results at 2 years’ follow-up were found to be significantly better in the fusion group, with back pain reduced by 33% compared with 7% in the nonsurgical group. Pain improvement was most significant during the first 6 months postoperatively and then gradually deteriorated thereafter. Disability according to ODI was reduced by 25% compared with 6% among nonsurgical patients, and 63% of surgical patients rated themselves as “much better” or “better” compared with 29% of nonsurgical patients. The “net back to work rate” was 36% in the surgical group and 13% in the nonsurgical group. The early complication rate in the surgical group was 17%. Fritzell and colleagues234 concluded that surgical treatment of severe chronic low back pain provides improved results compared with nonoperative treatment in carefully selected patients.
Brox and colleagues155 published another randomized trial comparing outcomes of lumbar instrumented fusion versus cognitive intervention and exercise in 64 patients with chronic low back pain and DDD. The critical component of this study was the addition of cognitive therapy to an intensive rehabilitation program. The mean change in ODI for the surgical fusion group was from 41 preoperatively to 26 at 1-year follow-up and for the rehabilitation group from 42 to 30. The investigators reported no significant difference in back pain, use of analgesics, emotional distress, and life satisfaction between the groups. Return to work rate at 1 year was 22% in the surgical group and 33% in the rehabilitation group. The rehabilitation group experienced greater improvement in fear avoidance beliefs, and fingertip-to-floor distance, whereas the surgical group had greater improvement in associated symptoms of leg pain. The overall success rate for surgical intervention was 70% and for nonoperative cognitive therapy was 76%. Brox and colleagues155 concluded that there were near-equivalent outcomes between the groups, which was offset by an 18% complication rate among the surgical group.
Fairbank and colleagues157 published the last major randomized clinical trial of surgery versus nonoperative therapy. The Medical Research Council (MRC) spine stabilization trial was a randomized controlled trial comparing surgical treatment and intensive rehabilitation in 349 patients with chronic low back pain. Similar to the study by Brox and colleagues,155 the intensive physical therapy program in the MRC trial also incorporated principles of cognitive behavioral therapy. At 1-year follow-up, the mean ODI scores decreased from 46.5 to 34 in the surgical group and from 44.8 to 36.1 in the rehabilitation group. No significant differences were found between the groups in the shuttle walking test and Short Form-36 General Health Survey (SF-36) outcomes. The authors concluded that although the surgical group enjoyed a small but statistically significant benefit in one of the primary outcome measures (ODI), this was contradicted by the additional cost and potential risk of complication associated with surgery.
In a separate publication on the MRC trial, Rivero-Arias and colleagues235 performed a cost analysis at 2 years’ follow-up. The cost per patient over the study time frame in the surgical group was estimated to be £7830 ($12,450) versus £4526 ($7200) in the rehabilitation group. There was no significant difference in mean quality-adjusted life-years between the groups. The investigators concluded that surgical treatment was not a cost-effective use of health care funds compared with therapy, although the authors pointed out that ultimate costs could vary depending on the number of patients in either group that require subsequent intervention after the 2-year follow-up period.
Meta-analyses of surgical versus nonoperative treatment have paralleled the findings of Brox and colleagues155 and the MRC trial.157 Ibrahim and colleagues236 pooled the data from these three randomized trials and found that a modest improvement in mean ODI scores among surgical patients should not be used as justification for routine operative treatment in light of a 16% early complication rate. Mirza and Deyo,237 in a separate systematic review, concluded that surgical outcomes are equivalent to a structured rehabilitation program with cognitive behavioral therapy.
Posterolateral Fusion
Fusion rates for the lumbar spine vary in the literature and depending on the type and extent of procedure. For a single-level, uninstrumented PLF in the setting of DDD, fusion rates of 85% to 91% have been reported.238 McCulloch239 reported a 91% solid fusion rate with uninstrumented single-level PLF and a satisfactory clinical outcome in 78% of patients.
The improvement in fusion rates and outcomes with instrumentation is debated in the literature. France and colleagues240 reported radiographic fusion rates of 76% among instrumented patients and 64% among noninstrumented patients, but there was no significant difference in outcomes between the groups or any correlation between radiographic union and patient-reported improvement. Other studies have reported a 26% rate of pseudarthrosis in uninstrumented fusion,241,242 whereas addition of instrumentation has been reported to improve fusion rate, reduce symptoms of pain, and increase return-to-work rate.241–247 In a prospective study of one-level fusions with and without instrumentation for disabling back pain, Lorenz and colleagues243 reported superior results with instrumented fusion. There were no reports of pseudarthrosis among the instrumented group, and 75% of patients experienced improvement in pain and were able to return to work. In contrast, 58% of patients in the uninstrumented group had a nonunion, and only one third experienced pain relief and were able to return to work.
In contrast, Thomsen and colleagues,248 in a randomized clinical study, reported no statistical difference in the rate of fusion between instrumented and noninstrumented patients. Instrumentation was related to an increase in operative time, blood loss, and early reoperation rate and a 4.8% risk of pedicle screw misplacement. Bono and Lee249 performed a comprehensive review of studies published on lumbar fusion from 1979-2000 and noted a clear trend toward increasing use of instrumentation—23% of all fusions in the 1980s versus 41% of all fusions in the 1990s. These authors were unable to show any significant improvement in overall fusion rate or clinical outcome. The benefit of supplemental instrumentation in PLF is not clearly documented in the literature, particularly in light of newer biologic materials currently being used to enhance fusion rates.
Lumbar Interbody Fusion
PLF can lead to the persistence of discogenic pain in some patients despite solid fusion, presumably owing to the presence of micromotion and pain in the involved disc. The disc material itself may be a pain generator, which interbody fusion directly addresses by discectomy.250,251 Weatherley and colleagues250 reported resolution of pain after an ALIF in five patients with persistent back pain despite solid PLF. Barrick and colleagues252 also reported excellent pain relief after anterior interbody fusion in 20 patients who had persistent low back pain despite previous PLF. For these reasons, interbody fusion is thought by many to provide better and more predictable pain relief in patients with primarily a discogenic source of low back pain, but there are no high-quality studies supporting this view.
Anterior Lumbar Interbody Fusion
ALIF with bone grafting (Fig. 45–4) for the treatment of IDD was the treatment modality originally recommended by Crock38 when he described the disorder. ALIF classically is performed through either an intra-abdominal or a retroperitoneal approach. After the symptomatic disc levels have been exposed, the surgeon performs an annulotomy and complete discectomy. The discal segment is reconstructed with autograft bone, allograft bone, or a cage device.
Reports of success for ALIF in the literature are high; Loguidice and colleagues253 reported an 80% rate of fusion and an 80% clinical success rate with ALIF. Newman and Grinstead254 reported similar results with 89% fusion and 86% clinical success in patients with IDD. Other than infection, the early risks in ALIF are mainly associated with the surgical approach, including ileus, injury to the abdominal contents or vasculature,255,256 incisional hernia, muscular atony, and retrograde ejaculation in men secondary to injury to the autonomic plexus.257,258
Circumferential Fusion
Combined interbody and posterior fusion, so-called global or 360-degree fusion, is another technique described in the literature. Interbody fusion can be performed via a separate anterior approach (ALIF and PLF) (Fig. 45–5), but a posterior approach (PLIF or TLIF) is often simpler because it involves a single approach for both parts of the fusion procedure (these procedures are discussed subsequently). A combined ALIF and PLF procedure previously was reserved for situations in which the risk of pseudarthrosis was high, such as in patients undergoing revision surgery, patients with preexisting pseudarthrosis, smokers, and diabetic patients; however, today it is commonly used in primary cases as well.
Moore and colleagues259 reported a 95% arthrodesis rate and 86% clinical success rate with combined anterior and posterior fusion for patients with chronic low back pain and DDD who had failed prolonged nonoperative treatment. Gertzbein and colleagues260 reported 97% fusion rate and 77% good clinical outcome with global fusion in a challenging group of patients—62% had previously had surgery, 25% had pseudarthrosis, 55% had two or more levels fused, and 43% were heavy smokers. Kozak and O’Brien261 treated 69 patients with circumferential fusion through two incisions for discogram-positive, disabling low back pain. They reported greater than 90% good results with one-level and two-level fusions and 78% good results with three-level procedures. Similarly, Hinkley and Jaremko262 reported greater than 90% positive outcomes in 81 patients who were receiving workers’ compensation and were treated with 360-degree lumbar fusion. Videbaek and colleagues,263 in a randomized clinical trial involving 148 patients comparing the results of circumferential fusion with PLF at 5 to 9 years’ follow-up, found that the circumferential fusion group had significantly better outcomes as measured by the Dallas Pain Questionnaire (DPQ), ODI, and SF-36. The circumferential group also complained of less back pain than the PLF group.
Although combined ALIF and PLIF may reduce the rate of pseudarthrosis to less than 5%, the morbidity is higher than for PLF alone, which is often warranted in patients undergoing revision spine surgery, diabetics, and heavy smokers. Suratwala and colleagues264 reported retrospectively on 80 complicated patients who underwent circumferential fusion of three or more levels. Encountered complications included 19% pseudarthrosis rate per patient (12% per level), 14% symptomatic pseudarthrosis, and 14% rate of adjacent segment degeneration. Within the 2- to 7-year follow-up, 34% of patients underwent repeat surgery with 20% undergoing implant removal for pain. The rate of deep wound infection was 2.5%, and the rate of superficial infection was 3.8%. Excessive intraoperative bleeding (>3 L) was rare, but 50% of patients required transfusion. Despite the rate of perioperative complications in this complex patient population, the authors reported mean ODI improvement from 50 to 35 and statistically significant improvement in SF-36 and Roland Morris scores.
Posterior and Transforaminal Lumbar Interbody Fusion
Posterior techniques for performing interbody fusion, including PLIF and TLIF (Fig. 45–6), are typically performed with posterior instrumentation and fusion, which makes them by default circumferential fusions. TLIF and PLIF have become increasingly popular techniques for performing circumferential fusion because they can be performed through a single posterior incision, which considerably lessens the morbidity associated with combined anterior and posterior approaches.265
In PLIF, the IVD is approached through laminectomy, partial facetectomy, and retraction of the dura and its contents. Risks of PLIF include dural tears, conus injury from retraction, nerve root injury, and epidural fibrosis. Success rates of PLIF in the literature are mixed. Madan and Boeree266 reported no difference in the outcome of discogenic back pain treated by ALIF versus instrumented PLIF. Conversely, Vamvanij and colleagues267 compared four fusion procedures and found simultaneous anterior interbody and posterior facet fusion to be superior to PLIF, with an 88% fusion rate. Superior fusion rate did not correlate with a better clinical outcome, however, because only 63% of patients in their study experienced a satisfactory result. Other studies have reported even lower success rates with fusion for discogenic back pain; Knox and Chapman268 reported only 35% good clinical results with one-level fusion for IDD.
Lowe and colleagues269 performed a prospective analysis of 40 consecutive patients who had spinal fusion for degenerative diseases of the lumbar spine using unilateral TLIF with pedicle screw fixation. Universal improvement in segmental lordosis, solid fusion in 90% of patients, and excellent or good clinical outcomes in 85% of patients were reported in this study. Whitecloud and colleagues270 reported that the TLIF approach produces greater than $15,000 of cost savings compared with a combined anterior-posterior procedure. There were no major complications noted in either group in this study, and no patient required repeat surgery for a lumbar spinal complication at the authors’ hospital within the 1-year follow-up period.
Minimally Invasive Surgical Approaches
Endoscopically Assisted and Mini-open Anterior Lumbar Interbody Fusion
In 1991, Obenchain271 described the first interbody fusions performed via a laparoscopic assisted technique. From the time of its description, the anterior laparoscopic technique has been plagued with a steep learning curve; the requirement for significant technical skill; and increased risk of complications such as visceral injury, abdominal vessel injury, and sexual dysfunction. The approach is associated with significantly longer preparation and operative times272,273 and is usually still dependent on general surgery assistance.274
Zdeblick and David275 reported similar outcomes between traditional open and laparoscopic ALIF except for a significantly higher complication rate—4% versus 20% in the laparoscopic group. Kaiser and colleagues272 reported no benefit of laparoscopic assisted ALIF versus a mini-open approach. In a meta-analysis of laparoscopic assisted ALIF, Inamasu and Guiot276 reported that at the L5-S1 level there is no major benefit of laparoscopic versus an open approach with regard to operative time, blood loss, and hospital stay. At other levels, the literature is conflicting; however, there is a consistent association of laparoscopic assisted ALIF with increased risk of retrograde ejaculation at all levels. No conclusion was made with regard to superiority of laparoscopic ALIF over an open approach owing to a lack of evidence.
A mini-open retroperitoneal approach to ALIF has become more popular in part because of the high rate of complications with laparoscopic assisted ALIF. Brau277 published a large retrospective review involving 686 patients who underwent ALIF via a mini-open approach with specific focus on the complication rate. The rate of arterial and venous injury was 1.6%; retrograde ejaculation, 0.1%; ileus longer than 3 days, 0.6%; superficial wound infection, 0.4%; and compartment syndrome, 0.3%. The rate of complications in a mini-open approach was significantly less than in laparoscopic assisted ALIF and closely approximated complication rates in open ALIF without the associated morbidity of a full open peritoneal approach. The mini-open approach typically still requires general surgical support.
Minimally Invasive Transforaminal Lumbar Interbody Fusion and Posterior Lumbar Interbody Fusion
Various, less invasive modifications of the traditional TLIF technique have been described. One technique involves using pedicle instrumentation only on the side of the facetectomy and placing a transfacet screw on the contralateral side (Fig. 45–7). This technique minimizes dissection on the contralateral side and eliminates the risk of screw abutment on the adjacent facet joint. Operative time and cost of the instrumentation are slightly reduced without adversely affecting the rigidity of the construct.278 Slucky and colleagues279 reported on a biomechanical study of constructs using bilateral pedicle screws, unilateral pedicle screws, and unilateral pedicle screws with a contralateral facet screw. These authors found that unilateral pedicle screw constructs allowed significantly increased segmental motion, less stiffness, and off-axis movement, whereas the addition of a contralateral facet screw produced biomechanics similar to bilateral pedicle screw constructs. Constructs using unilateral pedicle screws with a contralateral facet screw are a viable minimally invasive option and reduce instrumentation costs. Sethi and colleagues280 reported that this technique reduces construct cost by nearly 50% and still has a rate of fusion as high as traditional constructs—100% in the authors’ patient population at 9 to 26 months of follow-up.
Other minimally invasive TLIF approaches employ fluoroscopic assisted percutaneous instrumentation (Fig. 45–8). Interbody fusion is typically performed through a paramedian incision with the assistance of a tubular retractor system. Early experience has been positive, but there is a steep learning curve, and the potential for neurologic injury exists. Schwender and colleagues281 reported good success with this minimally invasive TLIF approach in a population with mixed diagnoses; mean improvement of ODI was from 46 to 14, and the fusion rate was reported to be 100%.
Peng and colleagues282 reported on a comparative prospective analysis of 29 minimally invasive TLIF procedures versus 29 traditional open TLIF procedures. Intraoperatively and perioperatively minimally invasive TLIF was associated with less blood loss (150 mL vs. 681 mL) and shorter postoperative stays (4 days vs. 6.7 days) but greater fluoroscopic times (106 seconds vs. 35 seconds) and longer operative times (216 minutes vs. 171 minutes). Both groups had improvement in ODI and back pain at 6 months and 2 years of follow-up, with no significant difference between the groups. Radiographic evidence of fusion was 80% for minimally invasive TLIF and 87% for open TLIF. Similar results were reported by Scheufler and colleagues283 and Park and Ha.284
Stevens and colleagues285 performed an MRI analysis of patients who had undergone minimally invasive lumbar fusion versus traditional open PLF to determine the difference in effect on the paraspinal musculature. The measured maximal intramuscular pressure intraoperatively was significantly less with use of the minimally invasive tubular retractor systems versus open retractors. There was also significantly less edema of the paraspinal musculature on MRI in patients who underwent minimally invasive fusion, indicating that minimally invasive surgical techniques produce less muscle and tissue damage than traditional open fusion.
Percutaneously placed facet screws have also been described in the literature. Shim and colleagues286 described the use of fluoroscopic assisted percutaneously placed facet screws as a modification of the Magerl technique. These authors reported 11% violation of the laminar wall with no incidences of neural compression; they also reported 15% rate of imperfect pedicle screw placement. Jang and Lee287 reported on the efficacy of circumferential fusion with percutaneously placed facet screws compared with ALIF and PLF with pedicle screws and found no difference between the two groups in regard to operative outcomes. These authors concluded that percutaneously placed facet screws after ALIF are a viable alternative to PLF and pedicle screws.
Extreme Lateral Lumbar Interbody Fusion and Direct Lateral Lumbar Interbody Fusion
Another novel approach to lumbar interbody fusion is XLIF, sometimes called direct lateral lumbar interbody fusion (Fig. 45–9). This procedure involves an anterolateral interbody fusion performed through a transpsoas approach to the lateral aspect of the IVD. This approach was first described by Pimenta288 in 2001 as a modification of the retroperitoneal approach. In 2004, Bergey and colleagues289 described a similar endoscopically assisted transpsoas procedure.
Benglis and colleagues290 published an anatomic study of the lumbar plexus around the psoas muscle to clarify the approach and risk to the neural structures during a direct lateral approach. The nervous plexus has a progressive dorsal to ventral migration from the L1-2 posterior endplate edge, to a ratio of 0.28 of the width of the disc at the L4-5 level. Ventral migration was most pronounced at the L4-5 level, and risk of injury to the lumbosacral plexus is highest at this level if the dissector cannula is placed too far posterior.
Reports of outcomes for the XLIF procedure are sparse, and short-term data only are available. Knight and colleagues290a reported complication rates of direct lateral interbody fusion in a cohort of 58 patients; 8 of the 13 reported complications were mild and related to the approach, the most common being meralgia paresthetica. Two patients (3.4%) experienced L4 nerve root injury, one of which lasted longer than 1 year. In a separate, smaller series, Ozgur and colleagues291 reported no complications with the XLIF procedure.
Percutaneous Axial Lumbar Interbody Fusion
Another emerging fusion technique specifically aimed at the L5-S1 level involves a percutaneous presacral approach. The procedure was described by Marotta and colleagues292 and is referred to as AxiaLIF. Aryan and colleagues293 reported a 91% fusion rate in 33 patients, some of whom also required adjunctive stabilizing procedures during the index procedure. At this point, data on outcomes for AxiaLIF are sparse, and there are as yet only a few case series in the literature.
Radiation Exposure
The advent of minimally invasive surgical techniques has generally been accompanied by increases in the use of intraoperative fluoroscopy. The risks to the patient and physician are not inconsequential. Bindal and colleagues294 reported on intraoperative radiation exposure during minimally invasive TLIF. The average fluoroscopic time was 1.69 minutes (range 0.82 to 3.73 minutes), and radiation exposure per case was 76 mrem total, 27 mrem at the waist under a lead apron, and 32 mrem at the thyroid. Radiation-induced malignancy is a potential concern with increased intraoperative use of fluoroscopy associated with minimally invasive surgical techniques. Navigation systems may have a role in decreasing radiation exposure,295 and there are numerous safe operating techniques the surgeon should use to reduce radiation exposure.
Comparison of Fusion Techniques
As part of the Swedish Spine Study Group’s randomized clinical trial, Fritzell and colleagues296 compared the outcomes of the 222 patients in the surgical arm. Patients had been randomly assigned to three surgical groups: (1) PLF, (2) instrumented PLF, and (3) circumferential fusion. All three procedures showed statistically significant reduction in pain and disability with no significant differences between the groups. Radiographically determined fusion rates were 72%, 87%, and 91%. With increasing complexity of procedure (groups 2 and 3), operative times were significantly longer, blood transfusion requirements were greater, and hospital stays were longer. Early complication rates were 6%, 16%, and 31%. Complication rates at 2 years’ follow-up297 were 12%, 22%, and 40%. An odds ratio for risk of complication was 5.3 between circumferential fusion and PLF and 2.4 between circumferential fusion and instrumented posterior fusion. Kim and colleagues298 compared three fusion techniques: PLF, PLIF, and circumferential fusion via PLIF. At minimum 3 years’ follow-up, good or excellent results were reported in 81% of PLF cases, in 88% of PLIF cases, and 86% of circumferential fusion cases, and pseudarthrosis rates were 8%, 5%, and 4%.
Glassman and colleagues299 published a large multicenter retrospective analysis of 497 patients who underwent different types of fusion procedures. Despite the fact that patients who underwent ALIF or PLF had slightly better clinical outcomes, good efficacy was found for all fusion modalities with respect to pain relief and outcomes. The authors concluded that surgeons can select the fusion approach at which they are most comfortable without significantly affecting outcome results. Between the multiple techniques and approaches available to perform lumbar arthrodesis, there is no clear consensus on which is the most successful, despite multiple studies comparing outcome measures, cost, rates of pseudarthrosis, and complications. Certain procedures may be more successful in specific patient populations; interbody fusion may treat a discogenic pain generator more successfully in patients with IDD.
Materials and Osteobiologics
Use of BMP has many reported benefits. Results for fusion rates so far have been excellent,300,301 and reduction in pseudarthrosis rates results in decreased requirement for costly revision surgeries. Some centers have attempted placing BMP through the PLIF or TLIF approach; however, the benefit of this use is unclear.302 BMP seems to obviate the need for autograft bone grafting, eliminating the risk of donor site morbidity, decreasing operative time, and decreasing blood loss. Donor site morbidity associated with iliac crest autograph harvest is significant. Sasso and colleagues303 combined results from four randomized trials comparing iliac crest graft and recombinant human BMP-2 (rhBMP-2) as part of an ALIF procedure. The investigators evaluated 208 patients’ VAS score for intensity and frequency of pain. At 2 years’ follow-up, 31% of patients were still reporting persistent pain at the donor site, and 16% reported fair or poor appearance of the graft site.
Multiple randomized controlled trials have been performed on BMP-2; most have evaluated the effectiveness of the protein compared with iliac crest autograft. Dimar and colleagues304 published the results of a randomized prospective trial with 98 patients who underwent single-level PLF. Fusion rate at 2 years was 73% in the iliac crest autograph group and 88% in the rhBMP-2 group. Average operating time was 21% longer and blood loss was 81% greater in the autograft group compared with the rhBMP-2 group. No significant difference in outcome measures was noted between the groups at any of the follow-up intervals. A later study305 by the same group found decreased operative time and blood loss and higher rates of fusion but no difference in outcome measures. Fusion results in single-level ALIF with allograft dowels were similar as reported by Burkus and colleagues:306 100% in the rhBMP-2 group and 81.5% in the autologous bone graft group. A 6-year follow-up of patients in the Burkus study307 projected a worst-case scenario fusion rate of 91%, considering reoperation rate for pseudarthrosis within the 6-year postoperative interval.
Vaccaro and colleagues308 compared OP-1/BMP-7 with iliac crest autograft in PLF. This was the first large (335 patients), randomized controlled trial of BMP-7 for use in spine fusion. At 3 years’ average follow-up, there was no significant difference between the two groups with respect to outcome measures, success of fusion, reoperation rate, complications, and fusion. The iliac crest autograft group had statistically longer operative times and blood loss. Vaccaro and colleagues308 concluded that OP-1/BMP-7 was equivalent to iliac crest bone graft in PLF.
The use of BMP is not without complications.309 BMP used in minimally invasive TLIF is reported to be associated with postoperative radiculitis.310 Use of BMP is also linked to postoperative bleeding and seroma formation, heterotopic bone formation,311 and osteolysis. Rates of vertebral osteolysis associated with use of BMP-2 in interbody cages have been reported to be 5.8%.312 Two unpublished cases of vertebral osteolysis at the authors’ institution have resulted in cage dislodgment.
The high cost of BMP has led some authors to caution against routine use, whereas others report that the decreased risk of pseudarthrosis and associated revision surgery justifies the expense in certain patient populations. Glassman and colleagues313 reported on the success of rhBMP-2 in single-level posterolateral lumbar fusions in smokers. In the rhBMP-2 group, 20 of 21 patients (95%) achieved fusion, and in the iliac crest autograph group, 16 of 21 patients (76%) achieved fusion; however, the authors noted that other clinical outcomes were still adversely affected by smoking independent of fusion status. Carreon and colleagues314 published a cost utility study on BMP-2 versus iliac crest autograft in elderly patients. At 2 years’ follow-up, after accounting for complications, including a higher rate of nonunion (9.6% in the autograft group) and revision surgery, the mean cost in the rhBMP-2 group was $39,967 and in the iliac crest autograph group was $42,286.
A British meta-analysis published by the National Institute of Health Research (NIHR) Health Technology Assessment Programme315 evaluated the cost-effectiveness of BMP in spinal fusions. Garrison and colleagues315 found evidence that BMP-2 was more effective than autogenous bone graft for radiographic fusion in single-level DDD. BMP was also associated with decreased operative time, improved clinical outcomes, shorter hospital stays, and fewer secondary interventions. The probability that BMP was cost-effective in Great Britain, based on a cost per quality-adjusted life-year of less than £30,000 (roughly $50,000), was only 6.4%, however. Garrison and colleagues315 concluded that although BMP improved outcome measures and decreased morbidity associated with autologous graft harvesting, its use was not cost-effective.
Adjacent Segment Degeneration
Criticisms of spinal arthrodesis are that it alters endplate loading, increases intradiscal pressures,316 significantly alters lumbar mechanics,317 and potentially increases the rate of adjacent segment degeneration.318–322 The problem seems to be more than simple subacute preexisting degenerative disease exacerbated by fusion at an adjacent level. Willems and colleagues323 looked at prefusion status of adjacent segments on discography and found that preoperative findings of disc degeneration were not associated with poor outcomes after fusion secondary to symptomatic adjacent levels.
Schulte and colleagues324 published a study on the effect of circumferential fusion in relation to adjacent disc heights and outcome measures at 10 years’ follow-up. Among the 27 patients with a diagnosis of DDD, disc height loss for the immediate cephalad level averaged 21% and for the second cephalad level was 16%. Patients with a preoperative diagnosis of DDD tended to have a greater loss of adjacent disc height than patients with spondylolisthesis. Multilevel fusions also showed greater adjacent level loss of disc height that was statistically significant. No correlation was found between outcome measures and adjacent loss of disc height.
Wai and colleagues320 reported on 39 patients with a minimum of 20 years’ follow-up after ALIF. Of patients, 74% had some evidence of degenerative changes in the lumbar spine, 23% had advanced degeneration at a level adjacent to the fusion, and 18% had advanced degeneration at another level with preservation of the adjacent levels. There was no association found between radiographic degeneration and functional outcome. Only three patients (8%) had undergone additional surgery for adjacent level degeneration within the 20 years of follow-up. The fact that a large portion of the degenerative changes after fusion occurred at nonadjacent levels led Wai and colleagues320 to question adjacent level degeneration, suggesting that changes were more likely a result of constitutional factors (aging, preexisting degeneration) rather than alteration in loading from an adjacent fusion.
Dynamic Spinal Stabilization Techniques
Dynamic techniques for spinal stabilization have been described in the literature for many years, but newer techniques and instrumentation designs have made this an area of renewed interest. Several authors have attempted to use soft tissue stabilization techniques that restrict rather than completely eliminate motion, while still relieving mechanical back pain. One of the first dynamic systems was described by Graf325 and involved an artificial ligament reconstruction using four pedicle screws and two braided polyester bands to stabilize the painful segment in lordosis. Proponents of the technique state that pain is reduced by coaptation of the painful facets; posterior annular compression, which closes annular tears; and stabilization of the motion segment. Theoretically, the fixation relaxes over the first 6 months postoperatively allowing a return of motion after healing has occurred.326
Outcomes for Graf ligamentoplasty in the literature are sparse and mixed. Kanayama and colleagues327 reported on 10-year follow-up, with preservation of segmental motion in 80% of patients and improvement in VAS scores. Other studies have reported poor outcomes at 1 year and a high rate of revision at 2 years. Revision for ligamentoplasty has been found to have poor outcomes—similar to the outcomes seen after revision arthrodesis.328–331 One of the biggest arguments against the procedure has been that the Graf ligament significantly restricts flexion at the spinal segment, which increases load in the problematic posterior anulus of the disc.
Newer techniques and designs, such as the dynamic neutralization system for the spine (Dynesys Dynamic Stabilization System; Zimmer, Warsaw, IN), attempt to reduce movement equally in flexion and in extension. The Dynesys system consists of titanium pedicle screws connected by an elastic band, which controls motion in a more consistent manner than the Graf ligamentoplasty. Although an improvement over the Graf technique, the degree to which the Dynesys system successfully unloads the disc is still unpredictable.332 Reported outcomes by Grob and colleagues333 were poor with only half of patients achieving improved quality of life and less than half experiencing functional capacity improvement. These authors concluded that there was no support for superiority of dynamic stabilization to typical arthrodesis. Few randomized controlled clinical studies have been conducted with this technique, and long-term efficacy is not yet clearly established.334
Disc Arthroplasty
Although TDA designs have been used in Europe for quite a while, long-term results are still in dispute. The Charité device has the longest term information based on randomized controlled data. Guyer and colleagues335 reported on 5-year follow-up for 90 TDA patients in a noninferiority study compared with a control group of 43 ALIF patients. ODI and SF-36 data between the groups was comparable, but 66% of the TDA patients versus 47% of the ALIF patients were back to full-time work at the 5-year interval. Range of motion for the Charité device at 5 years’ follow-up was reported to be 6 degrees.
Blumenthal and colleagues336 published clinical results of a randomized controlled trial of the Charité disc and reported noninferiority to ALIF controls. Radiographic examination of study patients showed better restoration of disc height and less subsidence in TDA versus ALIF with BAK cages (Spine tech, Minneapolis, MN).337 Tropiano and colleagues338 had 75% excellent or good results at an average 8.7 years of follow-up. Lemaire and colleagues339 reported 90% good or excellent outcomes and 91.5% return-to-work rate at a minimum of 10 years of follow-up in 147 patients.
Randomized controlled device trials of ProDisc-L340 have also shown noninferiority to spinal fusion. Outcomes of TDA in this study show a statistically significant advantage of arthroplasty compared with fusion. Improvement criteria (≥15%) in ODI, VAS, and SF-36 were met in 77% of TDA patients versus 64% of fusion patients. Favorable initial randomized controlled clinical trial results have also been published in support of the FlexiCore system.341
Proponents of TDA suggest that preservation of motion at the operated level decreases the incidence of adjacent segment degenerative disease associated with fusion. One of the purported benefits of this technique is for patients who would otherwise require multilevel fusion because of asymptomatic or subacute adjacent level degenerative changes. A significant problem with fusion procedures is the risk of developing adjacent segment degeneration; however, in some reports TDA devices have been associated with increased adjacent segment degenerative changes. Kostuik342 reported that two of the most common complications necessitating revision surgery in TDA were facet degeneration and adjacent level disease.
Park and colleagues343 reported on radiographic evidence of adjacent level degeneration after ProDisc-L implantation; at 26 months postoperatively, progression of facet degeneration was noted in 29% of 32 patients. Adjacent level progression was positively correlated with female gender, malposition of the prosthesis, and two-level disc replacement. In a comparative study between Charité and ProDisc-L, Shim and colleagues344 found that although the clinical outcomes of both systems were good, the facet joints at the operative level (32% to 36%) and the discs at adjacent levels (19% to 29%) showed advancement of degeneration at 3 years’ follow-up regardless of device used. Harrop and colleagues345 published a systematic literature review comparing published reports of adjacent segment degeneration in lumbar fusion with TDA. In the fusion group, 173 of 1216 patients (14%) developed symptomatic adjacent segment disease compared with 7 of the 595 TDA patients. This study had the benefit of large patient numbers, but none of the published reports used for the analysis were of a high level of evidence.
Another problem with TDA systems is that it is very difficult to create a mechanical device that can mimic all of the properties of the native disc. Several patients who underwent TDA with good pain relief have been found to have experienced spontaneous fusion secondary to heterotopic bone formation. In some reports, heterotopic ossification and spontaneous fusion have been unusually high in cervical disc replacement.346 In lumbar disc replacement, Huang and colleagues347 reported a 13% rate of heterotopic ossification in 65 patients, and 2 patients went on to spontaneous fusion. These authors cited preoperative ossification of the anulus, bony endplate injury, component malposition, and subsidence as potential factors leading to postoperative heterotopic ossification. Tortolani and colleagues348 reported that the rate of heterotopic ossification in 276 Charité patients was only 4.3% and that regardless of the presence of heterotopic ossification, postoperative range of motion was still better than preoperative range of motion in all cases.
Another possible problem with TDA is the potential for a failed prosthesis to cause catastrophic complications and make revisions extremely complex. Reported reasons for failure in the literature include acquired spondylolysis,349 implant subsidence,350 implant loosening, malposition, displacement, early wear, and infection.342 TDA failure by catastrophic wear, similar to that seen in total joint arthroplasty, is a potential concern, but wear rates and behavior of polyethylene debris around the spine are still largely unknown. Managing revision surgeries has the potential to become incredibly complicated dealing with repeat anterior exposures, osteolysis, prosthesis subsidence, and bone loss. In a feasibility study of revision of the Charité device, McAfee and colleagues351 reported that TDA did not preclude further procedures at the index level of surgery. Of 589 TDA patients, 52 required revision (9%) compared with 10 of 99 ALIF patients (10%). Through a repeat anterior retroperitoneal approach, 22 of 24 TDA devices were removed successfully. Seven of the 24 (29%) removed discs were revised to another Charité device.
Another concern with TDA is the cost of the device and procedure. Levin and colleagues352 published a charge analysis of one-level and two-level TDA with ProDisc-L versus circumferential fusion. For one-level fusion, average implant cost was nearly identical at $13,990 for fusion and $13,800 for TDA. Operative time for TDA was about one half of the average time for fusion (185 minutes vs. 344 minutes). Total charge for a single level averaged $35,592 for TDA and $46,280 for fusion. For two-level procedures, implant costs were much less for fusion ($18,460) than for TDA ($27,600), and operative times were 242 minutes versus 387 minutes. In a smaller retrospective review, Patel and colleagues353 compared TDA with fusions and found that implant costs for TDA, ALIF, and TLIF were similar if rhBMP-2 was excluded from the analysis.
Nuclear Replacement
The first nuclear replacement procedures were performed by Fernstrom354 in the late 1950s. This technique involved an annulotomy, resection of the nucleus pulposus, placement of a steel ball bearing (dubbed the Fernstrom ball), and preservation of the anulus. Fernstrom354 claimed outcomes similar to fusion, but application of this technique became associated with unacceptable rates of implant subsidence.
Multiple modern nuclear replacement designs currently are under development and investigation, which fall under two main device types. The first type is a mechanical design and includes devices such as the original Fernstrom ball, and newer designs composed of PEEK and pyrolytic carbon. The second type is an elastomeric design usually made with either preformed or injectable materials such as polyurethane, silicone, and various other polymers.355
The Prosthetic Disc Nucleus (PDN) (Raymedica, Bloomfield, MN) is the most studied nuclear replacement device. Klara and Ray356 published a series of 423 patients treated with PDN since 1996. They reported a 90% survival rate and 10% rate of device explantation. Initially, the study was plagued with a high device migration rate, but newer designs have had improved results. Shim and colleagues357 reported 78% good results in 46 patients followed for longer than 6 months; 4 patients required revision surgery because of migration of the implant. Ahrens and colleagues358 published a 2-year prospective outcome study on the DASCOR device (Disc Dynamics, Eden Prairie, MN) involving 85 patients. These authors reported significant improvement in VAS and ODI outcome measures. The rate of explantation was 8%, most commonly for resumption of severe back pain. The outcome data for nucleus pulposus replacement technologies are short-term, and the technique is still in need of careful investigation.
Future Directions
Great interest has been generated in recent years for the development of biologic repair strategies and biopharmaceutical approaches for interception and prevention of the degenerative disc cascade. Several investigators359,360 have reported successful transplantation of disc chondrocyte cells in animal studies with regeneration of viable matrix and normalized distribution within the disc space. Nomura and colleagues361 hypothesized that the extracellular matrix may play a role in slowing the rate of disc degeneration. Meisel and colleagues362 published a canine model for autologous chondrocyte transplant repair of damaged IVDs. A randomized controlled trial is under way comparing chondrotransplant DISC with discectomy.
Another approach under investigation is the use of various growth factors alone or in combination to induce disc regeneration. Metabolically impaired cells in the IVD that exhibit degenerative or age-related changes have been shown to repair their own matrix and disc structure under the influence of OP-1/BMP-7. BMP-7 seems to have an anabolic effect on proteoglycan and collagen synthesis, particularly within the nucleus pulposus. Animal studies have been performed using BMP-7, and GDF-5 with results showing restoration of disc height, increased extracellular matrix, and increased proteoglycan synthesis,363–365 but human studies have not been performed yet.
Manipulation of genes that regulate the synthesis of specific RNA and protein molecules on a cellular level also has promising applications for prevention of DDD and disc regeneration. Gene delivery, typically by a viral vector, provides for local production of sustainable, high concentrations of the gene product for extended periods. Targeted delivery of a gene product maximizes therapeutic potential, while minimizing side effects. Endogenously produced proteins may also have greater biologic activity than exogenously administered recombinant proteins.366 The IVD is relatively avascular and has poorly characterized, slowly dividing cells, so injection of viral vectors could potentially be maintained for long periods in this encapsulated and immunoprotected environment.
Because of safety concerns, ex vivo methods of gene therapy with the help of a bioreactor or tissue scaffold may be preferable. Ex vivo studies showing successful incorporation, increased matrix production, and restoration of the IVD structure have now been published for multiple gene products, including SOX-9,367 TIMP-1,368 TGF-β1, IGF-1, and BMP-2.369 There are still several concerns, however; for example, the virus may leak through annular fissures in the degenerated disc and evoke an immune response. An ideal treatment program for DDD should also allow for repetitive administration of gene therapy injections at the same or different disc levels.370
Summary
Pearls
Pitfalls
Key Points
1 Aprill C, Bogduk N. High-intensity zone: A diagnostic sign of painful lumbar disc on magnetic resonance imaging. Br J Radiol. 1992;65:361-369.
This study focuses attention on HIZ as a diagnostic sign of an annular tear.
2 Boden S, McCowin P, Davis D, et al. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects: A prospective investigation. J Bone Joint Surg Am. 1990;72:403-408.
3 Brox J. Randomized clinical trial of lumbar instrumented fusion and cognitive intervention and exercises in patients with chronic low back pain and disc degeneration. Spine (Phila Pa 1976). 2003;28:1913-1921.
4 Carragee E. A gold standard evaluation of the “discogenic pain” diagnosis as determined by provocative discography. Spine (Phila Pa 1976). 2006;31:2115-2123.
5 Carragee E, Paragioudakis S, Khurana S. 2000 Volvo Award Winner in Clinical Studies: Lumbar high-intensity zone and discography in subjects without low back problems. Spine (Phila Pa 1976). 2000;25:2987-2992.
This article points out the low predictive value of lumbar HIZ and discography.
6 Chou R, Baisden J, Carragee EJ, et al. Surgery for low back pain: A review of the evidence for an American Pain Society Clinical Practice Guideline. Spine (Phila Pa 1976). 2009;34:1094-1109.
7 Chou R, Loeser JD, Owens DK, et al. American Pain Society Low Back Pain Guideline Panel: Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: An evidence-based clinical practice guideline from the American Pain Society. Spine (Phila Pa 1976). 2009;34:1066-1077.
This article reports the American Pain Society’s current practice recommendations for the treatment of chronic nonradicular low back pain (see Table 45–2).
8 Crock H. Internal disc disruption: A challenge to disc prolapsed fifty years on. Spine (Phila Pa 1976). 1986;11:650-653.
In this article, the concept of IDD was elaborated.
9 Fairbank J. Randomised controlled trial to compare surgical stabilisation of the lumbar spine with an intensive rehabilitation programme for patients with chronic low back pain: The MRC spine stabilisation trial. BMJ. 2005;330:1233.
10 Fritzell P, Hagg O, Wessberg P, et al. Swedish Lumbar Spine Study Group: 2001 Volvo Award Winner in Clinical Studies: Lumbar fusion versus nonsurgical treatment for chronic low back pain: A multicenter randomized controlled trial from the Swedish Lumbar Spine Study Group. Spine (Phila Pa 1976). 2001;26:2521-2532. discussion 2532-2534
11 Modic M, Steinberg P, Ross J, et al. Degenerative disk disease: Assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166(1 Pt 1):193-199.
The changes observed in vertebrae adjacent to the degenerative disc are described and classified.
1 Schwarzer AC, Aprill CN, Derby R, et al. The prevalence and clinical features of internal disc disruption in patients with chronic low back pain. Spine (Phila Pa 1976). 1995;20:1878-1883.
2 Manchikanti L, Singh V, Pampati V, et al. Evaluation of the relative contributions of various structures in chronic low back pain. Pain Physician. 2001;4:308-316.
3 Andersson G. Epidemiological features of chronic low-back pain. Lancet. 1999;354:581-585.
4 Papageorgiou AC, Croft PR, Ferry S, et al. Estimating the prevalence of low back pain in the general population: Evidence from the South Manchester Back Pain Survey. Spine (Phila Pa 1976). 1995;20:1889-1894.
5 Waddell G. 1987 Volvo award in clinical sciences: A new clinical model for the treatment of low-back pain. Spine (Phila Pa 1976). 1987;12:632-644.
6 Croft PR, Macfarlane GJ, Papageorgiou AC, et al. Outcome of low back pain in general practice: A prospective study. BMJ. 1998;316:1356-1359.
7 Walsh K, Cruddas M, Coggon D. Low back pain in eight areas of Britain. J Epidemiol Community Health. 1992;46:227-230.
8 Andersson GB, Svensson HO, Oden A. The intensity of work recovery in low back pain. Spine (Phila Pa 1976). 1983;8:880-884.
9 Wahlgren DR, Atkinson JH, Epping-Jordan JE, et al. One-year follow-up of first onset low back pain. Pain. 1997;73:213-221.
10 Carey TS, Garrett JM, Jackman AM. Beyond the good prognosis: Examination of an inception cohort of patients with chronic low back pain. Spine (Phila Pa 1976). 2000;25:115-120.
11 Roland MO, Morrell DC, Morris RW. Can general practitioners predict the outcome of episodes of back pain? BMJ (Clin Res Ed). 1983;286:523-525.
12 Papageorgiou AC, Croft PR, Thomas E, et al. Influence of previous pain experience on the episode incidence of low back pain: Results from the South Manchester Back Pain Study. Pain. 1996;66:181-185.
13 Smith SE, Darden BV, Rhyne AL, et al. Outcome of unoperated discogram-positive low back pain. Spine (Phila Pa 1976). 1995;20:1997-2000.
14 Kirkaldy-Willis WH, Farfan HF. Instability of the lumbar spine. Clin Orthop Relat Res. 1982;165:110-123.
15 Waris E, Eskelin M, Hermunen H, et al. Disc degeneration in low back pain: A 17-year follow-up study using magnetic resonance imaging. Spine (Phila Pa 1976). 2007;32:681-684.
16 Urban JP, Holm S, Maroudas A, et al. Nutrition of the intervertebral disc: Effect of fluid flow on solute transport. Clin Orthop Relat Res. 1982;170:296-302.
17 Nakamura SI, Takahashi K, Takahashi Y, et al. The afferent pathways of discogenic low-back pain: Evaluation of L2 spinal nerve infiltration. J Bone Joint Surg Br. 1996;78:606-612.
18 Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine (Phila Pa 1976). 1995;20:1307-1314.
19 Crock HV. Internal disc disruption: A challenge to disc prolapse fifty years on. Spine (Phila Pa 1976). 1986;11:650-653.
20 Macnab I. The traction spur: An indicator of segmental instability. J Bone Joint Surg Am. 1971;53:663-670.
21 Hangai M, Kaneoka K, Kuno S, et al. Factors associated with lumbar intervertebral disc degeneration in the elderly. Spine J. 2008;8:732-740.
22 Kalichman L, Hunter DJ. The genetics of intervertebral disc degeneration: Familial predisposition and heritability estimation. Joint Bone Spine. 2008;75:383-387.
23 Battie MC, Videman T, Gibbons LE, et al. 1995 Volvo Award in clinical sciences: Determinants of lumbar disc degeneration: A study relating lifetime exposures and magnetic resonance imaging findings in identical twins. Spine (Phila Pa 1976). 1995;20:2601-2612.
24 An HS, Silveri CP, Simpson JM, et al. Comparison of smoking habits between patients with surgically confirmed herniated lumbar and cervical disc disease and controls. J Spinal Disord. 1994;7:369-373.
25 Kauppila LI. Atherosclerosis and disc degeneration/low-back pain—a systematic review. Eur J Vasc Endovasc Surg. 2009;37:661-670.
26 Lis AM, Black KM, Korn H, et al. Association between sitting and occupational LBP. Eur Spine J. 2007;16:283-298.
27 Kelsey JL, Hardy RJ. Driving of motor vehicles as a risk factor for acute herniated lumbar intervertebral disc. Am J Epidemiol. 1975;102:63-73.
28 Wilder DG. The biomechanics of vibration and low back pain. Am J Ind Med. 1993;23:577-588.
29 Arun R, Freeman BJ, Scammell BE, et al. 2009 ISSLS prize winner: What influence does sustained mechanical load have on diffusion in the human intervertebral disc? An in vivo study using serial postcontrast magnetic resonance imaging. Spine (Phila Pa 1976). 2009;34:2324-2337.
30 Videman T, Battie MC, Ripatti S, et al. Determinants of the progression in lumbar degeneration: A 5-year follow-up study of adult male monozygotic twins. Spine (Phila Pa 1976). 2006;31:671-678.
31 MacGregor AJ, Andrew T, Sambrook PN, et al. Structural, psychological, and genetic influences on low back and neck pain: A study of adult female twins. Arthritis Rheum. 2004;51:160-167.
32 Hestbaek L, Iachine IA, Leboeuf-Yde C, et al. Heredity of low back pain in a young population: A classical twin study. Twin Res. 2004;7:16-26.
33 Hartvigsen J, Christensen K, Frederiksen H, et al. Genetic and environmental contributions to back pain in old age: A study of 2,108 Danish twins aged 70 and older. Spine (Phila Pa 1976). 2004;29:897-901.
34 Paassilta P, Lohiniva J, Goring HH, et al. Identification of a novel common genetic risk factor for lumbar disk disease. JAMA. 2001;285:1843-1849.
35 Jim JJ, Noponen-Hietala N, Cheung KM, et al. The TRP2 allele of COL9A2 is an age-dependent risk factor for the development and severity of intervertebral disc degeneration. Spine (Phila Pa 1976). 2005;30:2735-2742.
36 Solovieva S, Lohiniva J, Leino-Arjas P, et al. Intervertebral disc degeneration in relation to the COL9A3 and the IL-1ss gene polymorphisms. Eur Spine J. 2006;15:613-619.
37 Kalichman L, Hunter DJ. The genetics of intervertebral disc degeneration: Associated genes. Joint Bone Spine. 2008;75:388-396.
38 Crock HV. A reappraisal of intervertebral disc lesions. Med J Aust. 1970;1:983-989.
39 Haughton VM. MR imaging of the spine. Radiology. 1988;166:297-301.
40 Deyo RA, Battie M, Beurskens AJ, et al. Outcome measures for low back pain research: A proposal for standardized use. Spine (Phila Pa 1976). 1998;23:2003-2013.
41 Spruit M, Jacobs WC. Pain and function after intradiscal electrothermal treatment (IDET) for symptomatic lumbar disc degeneration. Eur Spine J. 2002;11:589-593.
42 Derby R, Eek B, Chen Y. Intradiscal electrothermal annuloplasty (IDET): A novel approach for treating chronic discogenic back pain. Neuromodulation. 2000;3:69-75.
43 Weinstein J, Claverie W, Gibson S. The pain of discography. Spine (Phila Pa 1976). 1988;13:1344-1348.
44 Weinstein J. Neurogenic and nonneurogenic pain and inflammatory mediators. Orthop Clin North Am. 1991;22:235-246.
45 Kawakami M, Tamaki T, Hayashi N, et al. Possible mechanism of painful radiculopathy in lumbar disc herniation. Clin Orthop Relat Res. 1998;351:241-251.
46 Byrod G, Olmarker K, Konno S, et al. A rapid transport route between the epidural space and the intraneural capillaries of the nerve roots. Spine (Phila Pa 1976). 1995;20:138-143.
47 Freemont AJ, Peacock TE, Goupille P, et al. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet. 1997;350:178-181.
48 Evans W, Jobe W, Seibert C. A cross-sectional prevalence study of lumbar disc degeneration in a working population. Spine (Phila Pa 1976). 1989;14:60-64.
49 Gibson MJ, Buckley J, Mawhinney R, et al. Magnetic resonance imaging and discography in the diagnosis of disc degeneration: A comparative study of 50 discs. J Bone Joint Surg Br. 1986;68:369-373.
50 Saal JS, Franson RC, Dobrow R, et al. High levels of inflammatory phospholipase A2 activity in lumbar disc herniations. Spine (Phila Pa 1976). 1990;15:674-678.
51 Nygaard OP, Mellgren SI, Osterud B. The inflammatory properties of contained and noncontained lumbar disc herniation. Spine (Phila Pa 1976). 1997;22:2484-2488.
52 O’Donnell JL, O’Donnell AL. Prostaglandin E2 content in herniated lumbar disc disease. Spine (Phila Pa 1976). 1996;21:1653-1655.
53 Franson RC, Saal JS, Saal JA. Human disc phospholipase A2 is inflammatory. Spine (Phila Pa 1976). 1992;17(6 Suppl):S129-S132.
54 Burke JG, Watson RW, McCormack D, et al. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J Bone Joint Surg Br. 2002;84:196-201.
55 Igarashi A, Kikuchi S, Konno S, et al. Inflammatory cytokines released from the facet joint tissue in degenerative lumbar spinal disorders. Spine (Phila Pa 1976). 2004;29:2091-2095.
56 Ohtori S, Inoue G, Ito T, et al. Tumor necrosis factor-immunoreactive cells and PGP 9.5-immunoreactive nerve fibers in vertebral endplates of patients with discogenic low back pain and Modic type 1 or type 2 changes on MRI. Spine (Phila Pa 1976). 2006;31:1026-1031.
57 Ashton IK, Walsh DA, Polak JM, et al. Substance P in intervertebral discs: Binding sites on vascular endothelium of the human annulus fibrosus. Acta Orthop Scand. 1994;65:635-639.
58 Palmgren T, Gronblad M, Virri J, et al. An immunohistochemical study of nerve structures in the anulus fibrosus of human normal lumbar intervertebral discs. Spine (Phila Pa 1976). 1999;24:2075-2079.
59 Coppes MH, Marani E, Thomeer RT, et al. Innervation of “painful” lumbar discs. Spine (Phila Pa 1976). 1997;22:2342-2349. discussion 2349-2350
60 Peng B, Wu W, Hou S, et al. The pathogenesis of discogenic low back pain. J Bone Joint Surg Br. 2005;87:62-67.
61 Brown MF, Hukkanen MV, McCarthy ID, et al. Sensory and sympathetic innervation of the vertebral endplate in patients with degenerative disc disease. J Bone Joint Surg Br. 1997;79:147-153.
62 Takebayashi T, Cavanaugh JM, Kallakuri S, et al. Sympathetic afferent units from lumbar intervertebral discs. J Bone Joint Surg Br. 2006;88:554-557.
63 Aoki Y, Ohtori S, Takahashi K, et al. Innervation of the lumbar intervertebral disc by nerve growth factor-dependent neurons related to inflammatory pain. Spine (Phila Pa 1976). 2004;29:1077-1081.
64 Edgar MA. The nerve supply of the lumbar intervertebral disc. J Bone Joint Surg Br. 2007;89:1135-1139.
65 Jarvik JG, Hollingworth W, Heagerty PJ, et al. Three-year incidence of low back pain in an initially asymptomatic cohort: Clinical and imaging risk factors. Spine (Phila Pa 1976). 2005;30:1541-1548. discussion 1549
66 Carragee EJ, Alamin TF, Miller JL, et al. Discographic, MRI and psychosocial determinants of low back pain disability and remission: A prospective study in subjects with benign persistent back pain. Spine J. 2005;5:24-35.
67 Carragee EJ, Paragioudakis SJ, Khurana S. 2000 Volvo Award winner in clinical studies: Lumbar high-intensity zone and discography in subjects without low back problems. Spine (Phila Pa 1976). 2000;25:2987-2992.
68 Carragee EJ, Alamin TF, Carragee JM. Low-pressure positive discography in subjects asymptomatic of significant low back pain illness. Spine (Phila Pa 1976). 2006;31:505-509.
69 Carragee EJ, Tanner CM, Khurana S, et al. The rates of false-positive lumbar discography in select patients without low back symptoms. Spine (Phila Pa 1976). 2000;25:1373-1380.
70 O’Neill CW, Kurgansky ME, Derby R, et al. Disc stimulation and patterns of referred pain. Spine (Phila Pa 1976). 2002;27:2776-2781.
71 Deyo RA, Diehl AK. Lumbar spine films in primary care: Current use and effects of selective ordering criteria. J Gen Intern Med. 1986;1:20-25.
72 Torgerson WR, Dotter WE. Comparative roentgenographic study of the asymptomatic and symptomatic lumbar spine. J Bone Joint Surg Am. 1976;58:850-853.
73 Liang M, Komaroff AL. Roentgenograms in primary care patients with acute low back pain: A cost-effectiveness analysis. Arch Intern Med. 1982;142:1108-1112.
74 Scavone JG, Latshaw RF, Weidner WA. Anteroposterior and lateral radiographs: An adequate lumbar spine examination. AJR Am J Roentgenol. 1981;136:715-717.
75 Institute for Clinical Systems Improvement (ICSI). Adult Low Back Pain. Bloomington, MN: ICSI; 2008.
76 Aprill C, Bogduk N. High-intensity zone: A diagnostic sign of painful lumbar disc on magnetic resonance imaging. Br J Radiol. 1992;65:361-369.
77 Yu SW, Sether LA, Ho PS, et al. Tears of the anulus fibrosus: Correlation between MR and pathologic findings in cadavers. AJNR Am J Neuroradiol. 1988;9:367-370.
78 Osti OL, Vernon-Roberts B, Moore R, et al. Annular tears and disc degeneration in the lumbar spine: A post-mortem study of 135 discs. J Bone Joint Surg Br. 1992;74:678-682.
79 Morgan S, Saifuddin A. MRI of the lumbar intervertebral disc. Clin Radiol. 1999;54:703-723.
80 Modic M, Steinberg P, Ross J, et al. Degenerative disk disease: Assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166(1 Pt 1):193-199.
81 Boden S, McCowin P, Davis D, et al. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects: A prospective investigation. J Bone Joint Surg Am. 1990;72:403-408.
82 Jensen MC, Brant-Zawadzki MN, Obuchowski N, et al. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med. 1994;331:69-73.
83 Stadnik TW, Lee RR, Coen HL, et al. Annular tears and disk herniation: Prevalence and contrast enhancement on MR images in the absence of low back pain or sciatica. Radiology. 1998;206:49-55.
84 Borenstein DG, O’Mara JWJr, Boden SD, et al. The value of magnetic resonance imaging of the lumbar spine to predict low-back pain in asymptomatic subjects: A seven-year follow-up study. J Bone Joint Surg Am. 2001;83:1306-1311.
85 Jarvik JJ, Hollingworth W, Heagerty P, et al. The Longitudinal Assessment of Imaging and Disability of the Back (LAIDBack) Study: Baseline data. Spine (Phila Pa 1976). 2001;26:1158-1166.
86 Jarvik JG, Hollingworth W, Martin B, et al. Rapid magnetic resonance imaging vs radiographs for patients with low back pain: A randomized controlled trial. JAMA. 2003;289:2810-2818.
87 Carragee E, Alamin T, Cheng I, et al. Are first-time episodes of serious LBP associated with new MRI findings? Spine J. 2006;6:624-635.
88 Lappalainen AK, Kaapa E, Lamminen A, et al. The diagnostic value of contrast-enhanced magnetic resonance imaging in the detection of experimentally induced anular tears in sheep. Spine (Phila Pa 1976). 2002;27:2806-2810.
89 Yoshida H, Fujiwara A, Tamai K, et al. Diagnosis of symptomatic disc by magnetic resonance imaging: T2-weighted and gadolinium-DTPA-enhanced T1-weighted magnetic resonance imaging. J Spinal Disord Tech. 2002;15:193-198.
90 Aprill C, Bogduk N. High-intensity zone: A diagnostic sign of painful lumbar disc on magnetic resonance imaging. Br J Radiol. 1992;65:361-369.
91 Lim CH, Jee WH, Son BC, et al. Discogenic lumbar pain: Association with MRI imaging and CT discography. Eur J Radiol. 2005;54:431-437.
92 Lam KS, Carlin D, Mulholland RC. Lumbar disc high-intensity zone: The value and significance of provocative discography in the determination of the discogenic pain source. Eur Spine J. 2000;9:36-41.
93 Schellhas KP, Pollei SR, Gundry CR, et al. Lumbar disc high-intensity zone: Correlation of magnetic resonance imaging and discography. Spine (Phila Pa 1976). 1996;21:79-86.
94 Peng B, Hou S, Wu W, et al. The pathogenesis and clinical significance of a high-intensity zone (HIZ) of lumbar intervertebral disc on MR imaging in the patient with discogenic low back pain. Eur Spine J. 2006;15:583-587.
95 Lei D, Rege A, Koti M, et al. Painful disc lesion: Can modern biplanar magnetic resonance imaging replace discography? J Spinal Disord Tech. 2008;21:430-435.
96 Saifuddin A, Braithwaite I, White J, et al. The value of lumbar spine magnetic resonance imaging in the demonstration of anular tears. Spine (Phila Pa 1976). 1998;23:453-457.
97 Kang CH, Kim YH, Lee SH, et al. Can magnetic resonance imaging accurately predict concordant pain provocation during provocative disc injection? Skeletal Radiol. 2009;38:877-885.
98 Ricketson R, Simmons JW, Hauser BO. The prolapsed intervertebral disc: The high-intensity zone with discography correlation. Spine (Phila Pa 1976). 1996;21:2758-2762.
99 Schneiderman G, Flannigan B, Kingston S, et al. Magnetic resonance imaging in the diagnosis of disc degeneration: Correlation with discography. Spine (Phila Pa 1976). 1987;12:276-281.
100 Ito M, Incorvaia KM, Yu SF, et al. Predictive signs of discogenic lumbar pain on magnetic resonance imaging with discography correlation. Spine (Phila Pa 1976). 1998;23:1252-1258.
101 Simmons JW, Emery SF, McMillin JN, et al. Awake discography: A comparison study with magnetic resonance imaging. Spine (Phila Pa 1976). 1991;16(6 Suppl):S216-S221.
102 Mitra D, Cassar-Pullicino VN, McCall IW. Longitudinal study of high intensity zones on MR of lumbar intervertebral discs. Clin Radiol. 2004;59:1002-1008.
103 Milette P, Fontaine S, Lepanto L, et al. Differentiating lumbar disc protrusions, disc bulges, and discs with normal contour but abnormal signal intensity: Magnetic resonance imaging with discographic correlations. Spine (Phila Pa 1976). 1999;24:44-53.
104 Horton WC, Daftari TK. Which disc as visualized by magnetic resonance imaging is actually a source of pain? A correlation between magnetic resonance imaging and discography. Spine (Phila Pa 1976). 1992;17(6 Suppl):S167-S171.
105 Toyone T, Takahashi K, Kitahara H, et al. Vertebral bone-marrow changes in degenerative lumbar disc disease: An MRI study of 74 patients with low back pain. J Bone Joint Surg Br. 1994;76:757-764.
106 Kuisma M, Karppinen J, Niinimaki J, et al. Modic changes in endplates of lumbar vertebral bodies: Prevalence and association with low back and sciatic pain among middle-aged male workers. Spine (Phila Pa 1976). 2007;32:1116-1122.
107 Albert HB, Manniche C. Modic changes following lumbar disc herniation. Eur Spine J. 2007;16:977-982.
108 Thompson KJ, Dagher AP, Eckel TS, et al. Modic changes on MR images as studied with provocative diskography: Clinical relevance—a retrospective study of 2457 disks. Radiology. 2009;250:849-855.
109 Mitra D, Cassar-Pullicino VN, McCall IW. Longitudinal study of vertebral type-1 end-plate changes on MR of the lumbar spine. Eur Radiol. 2004;14:1574-1581.
110 Marshman LA, Trewhella M, Friesem T, et al. Reverse transformation of Modic type 2 changes to Modic type 1 changes during sustained chronic low-back pain severity: Report of two cases and review of the literature. J Neurosurg Spine. 2007;6:152-155.
111 Kuisma M, Karppinen J, Niinimaki J, et al. A three-year follow-up of lumbar spine endplate (Modic) changes. Spine (Phila Pa 1976). 2006;31:1714-1718.
112 Sandhu HS, Sanchez-Caso LP, Parvataneni HK, et al. Association between findings of provocative discography and vertebral endplate signal changes as seen on MRI. J Spinal Disord. 2000;13:438-443.
113 Braithwaite I, White J, Saifuddin A, et al. Vertebral end-plate (Modic) changes on lumbar spine MRI: Correlation with pain reproduction at lumbar discography. Eur Spine J. 1998;7:363-368.
114 Kokkonen SM, Kurunlahti M, Tervonen O, et al. Endplate degeneration observed on magnetic resonance imaging of the lumbar spine: Correlation with pain provocation and disc changes observed on computed tomography diskography. Spine (Phila Pa 1976). 2002;27:2274-2278.
115 Schenk P, Laubli T, Hodler J, et al. Magnetic resonance imaging of the lumbar spine: Findings in female subjects from administrative and nursing professions. Spine (Phila Pa 1976). 2006;31:2701-2706.
116 Kjaer P, LeBoeuf-Yde C, Korsholm L, et al. Magnetic resonance imaging and low back pain in adults: A diagnostic imaging study of 40-year-old men and women. Spine (Phila Pa 1976). 2005;30:1173-1180.
117 Kjaer P, Korsholm L, Bendix T, et al. Modic changes and their associations with clinical findings. Eur Spine J. 2006;15:1312-1319.
118 Leboeuf-Yde C, Kjaer P, Bendix T, et al. Self-reported hard physical work combined with heavy smoking or overweight may result in so-called Modic changes. BMC Musculoskelet Disord. 2008;9:5.
119 Jensen TS, Karppinen J, Sorensen JS, et al. Vertebral endplate signal changes (Modic change): A systematic literature review of prevalence and association with non-specific low back pain. Eur Spine J. 2008;17:1407-1422.
120 Huang KY, Lin RM, Lee YL, et al. Factors affecting disability and physical function in degenerative lumbar spondylolisthesis of L4-5: Evaluation with axially loaded MRI. Eur Spine J. 2009;18:1851-1857.
121 Hansson T, Suzuki N, Hebelka H, et al. The narrowing of the lumbar spinal canal during loaded MRI: The effects of the disc and ligamentum flavum. Eur Spine J. 2009;18:679-686.
122 Jayakumar P, Nnadi C, Saifuddin A, et al. Dynamic degenerative lumbar spondylolisthesis: Diagnosis with axial loaded magnetic resonance imaging. Spine (Phila Pa 1976). 2006;31:E298-E301.
123 Hiwatashi A, Danielson B, Moritani T, et al. Axial loading during MR imaging can influence treatment decision for symptomatic spinal stenosis. AJNR Am J Neuroradiol. 2004;25:170-174.
124 Danielson B, Willen J. Axially loaded magnetic resonance image of the lumbar spine in asymptomatic individuals. Spine (Phila Pa 1976). 2001;26:2601-2606.
125 Saifuddin A, McSweeney E, Lehovsky J. Development of lumbar high intensity zone on axial loaded magnetic resonance imaging. Spine (Phila Pa 1976). 2003;28:E449-E451.
126 Holt EPJr. The question of lumbar discography. J Bone Joint Surg Am. 1968;50:720-726.
127 Carragee EJ, Don AS, Hurwitz EL, et al. 2009 ISSLS Prize Winner: Does discography cause accelerated progression of degeneration changes in the lumbar disc: A ten-year matched cohort study. Spine (Phila Pa 1976). 2009;34:2338-2345.
128 Chou R, Loeser JD, Owens DK, et al. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: An evidence-based clinical practice guideline from the American Pain Society. Spine (Phila Pa 1976). 2009;34:1066-1077.
129 Aprill C. Diagnostic disc injection. In: Frymoyer J, editor. The Adult Spine: Principles and Practice. New York: Raven Press, 1991.
130 Guyer RD, Ohnmeiss DD. Lumbar discography. Position statement from the North American Spine Society Diagnostic and Therapeutic Committee. Spine (Phila Pa 1976). 1995;20:2048-2059.
131 Bernard TNJr. Lumbar discography followed by computed tomography: Refining the diagnosis of low-back pain. Spine (Phila Pa 1976). 1990;15:690-707.
132 Massie W. A critical evaluation of discography. Scientific exhibit. In Proceedings of the American Academy of Orthopedic Surgeons. J Bone Joint Surg Am. 1967;49:1243-1244.
133 Walsh TR, Weinstein JN, Spratt KF, et al. Lumbar discography in normal subjects: A controlled, prospective study. J Bone Joint Surg Am. 1990;72:1081-1088.
134 Derby R, Kim BJ, Lee SH, et al. Comparison of discographic findings in asymptomatic subject discs and the negative discs of chronic LBP patients: Can discography distinguish asymptomatic discs among morphologically abnormal discs? Spine J. 2005;5:389-394.
135 Buenaventura RM, Shah RV, Patel V, et al. Systematic review of discography as a diagnostic test for spinal pain: an update. Pain Physician. 2007;10:147-164.
136 Wolfer LR, Derby R, Lee JE, et al. Systematic review of lumbar provocation discography in asymptomatic subjects with a meta-analysis of false-positive rates. Pain Physician. 2008;11:513-538.
137 Colhoun E, McCall IW, Williams L, et al. Provocation discography as a guide to planning operations on the spine. J Bone Joint Surg Br. 1988;70:267-271.
138 Madan S, Gundanna M, Harley JM, et al. Does provocative discography screening of discogenic back pain improve surgical outcome? J Spinal Disord Tech. 2002;15:245-251.
139 Carragee EJ, Lincoln T, Parmar VS, et al. A gold standard evaluation of the “discogenic pain” diagnosis as determined by provocative discography. Spine (Phila Pa 1976). 2006;31:2115-2123.
140 Ohtori S, Kinoshita T, Yamashita M, et al. Results of surgery for discogenic low back pain: A randomized study using discography versus discoblock for diagnosis. Spine (Phila Pa 1976). 2009;34:1345-1348.
141 Deyo RA. Conservative therapy for low back pain: Distinguishing useful from useless therapy. JAMA. 1983;250:1057-1062.
142 Deyo RA, Diehl AK, Rosenthal M. How many days of bed rest for acute low back pain? A randomized clinical trial. N Engl J Med. 1986;315:1064-1070.
143 Quinet RJ, Hadler NM. Diagnosis and treatment of backache. Semin Arthritis Rheum. 1979;8:261-287.
144 Rowe ML. Low back pain in industry. A position paper. J Occup Med. 1969;11:161-169.
145 Allen C, Glasziou P, Del Mar C. Bed rest: A potentially harmful treatment needing more careful evaluation. Lancet. 1999;354:1229-1233.
146 Hagen KB, Hilde G, Jamtvedt G, et al: Bed rest for acute low-back pain and sciatica. Cochrane Database Syst Rev CD001254, 2004.
147 Verbunt JA, Sieben J, Vlaeyen JW, et al. A new episode of low back pain: Who relies on bed rest? Eur J Pain. 2008;12:508-516.
148 Engers A, Jellema P, Wensing M, et al: Individual patient education for low back pain. Cochrane Database Syst Rev CD004057, 2008.
149 Liddle SD, Gracey JH, Baxter GD. Advice for the management of low back pain: A systematic review of randomised controlled trials. Man Ther. 2007;12:310-327.
150 Brox JI, Storheim K, Grotle M, et al. Evidence-informed management of chronic low back pain with back schools, brief education, and fear-avoidance training. Spine J. 2008;8:28-39.
151 Calmels P, Queneau P, Hamonet C, et al. Effectiveness of a lumbar belt in subacute low back pain: An open, multicentric, and randomized clinical study. Spine (Phila Pa 1976). 2009;34:215-220.
152 Oleske DM, Lavender SA, Andersson GB, et al. Are back supports plus education more effective than education alone in promoting recovery from low back pain? Results from a randomized clinical trial. Spine (Phila Pa 1976). 2007;32:2050-2057.
153 van Duijvenbode IC, Jellema P, van Poppel MN, et al: Lumbar supports for prevention and treatment of low back pain. Cochrane Database Syst Rev CD001823, 2008.
154 Sahar T, Cohen MJ, Ne’eman V, et al: Insoles for prevention and treatment of back pain. Cochrane Database Syst Rev CD005275, 2007.
155 Brox JI, Sorensen R, Friis A, et al. Randomized clinical trial of lumbar instrumented fusion and cognitive intervention and exercises in patients with chronic low back pain and disc degeneration. Spine (Phila Pa 1976). 2003;28:1913-1921.
156 Keller A, Brox JI, Gunderson R, et al. Trunk muscle strength, cross-sectional area, and density in patients with chronic low back pain randomized to lumbar fusion or cognitive intervention and exercises. Spine (Phila Pa 1976). 2004;29:3-8.
157 Fairbank J, Frost H, Wilson-MacDonald J, et al. Spine Stabilisation Trial Group: Randomised controlled trial to compare surgical stabilisation of the lumbar spine with an intensive rehabilitation programme for patients with chronic low back pain: The MRC Spine Stabilisation Trial. BMJ. 2005;330:1233.
158 Heymans MW, de Vet HC, Bongers PM, et al. The effectiveness of high-intensity versus low-intensity back schools in an occupational setting: A pragmatic randomized controlled trial. Spine (Phila Pa 1976). 2006;31:1075-1082.
159 Kaapa EH, Frantsi K, Sarna S, et al. Multidisciplinary group rehabilitation versus individual physiotherapy for chronic nonspecific low back pain: A randomized trial. Spine (Phila Pa 1976). 2006;31:371-376.
160 Heymans MW, van Tulder MW, Esmail R, et al: Back schools for non-specific low-back pain. Cochrane Database Syst Rev CD000261, 2004.
161 van der Roer N, van Tulder M, van Mechelen W, et al. Economic evaluation of an intensive group training protocol compared with usual care physiotherapy in patients with chronic low back pain. Spine (Phila Pa 1976). 2008;33:445-451.
162 van der Roer N, van Tulder M, Barendse J, et al. Intensive group training protocol versus guideline physiotherapy for patients with chronic low back pain: A randomised controlled trial. Eur Spine J. 2008;17:1193-1200.
163 Poitras S, Brosseau L. Evidence-informed management of chronic low back pain with transcutaneous electrical nerve stimulation, interferential current, electrical muscle stimulation, ultrasound, and thermotherapy. Spine J. 2008;8:226-233.
164 Khadilkar A, Odebiyi DO, Brosseau L, et al: Transcutaneous electrical nerve stimulation (TENS) versus placebo for chronic low-back pain. Cochrane Database Syst Rev CD003008, 2008.
165 Hoiriss KT, Pfleger B, McDuffie FC, et al. A randomized clinical trial comparing chiropractic adjustments to muscle relaxants for subacute low back pain. J Manipulative Physiol Ther. 2004;27:388-398.
166 Haas M, Groupp E, Kraemer DF. Dose-response for chiropractic care of chronic low back pain. Spine J. 2004;4:574-583.
167 McMorland G, Suter E. Chiropractic management of mechanical neck and low-back pain: A retrospective, outcome-based analysis. J Manipulative Physiol Ther. 2000;23:307-311.
168 Ernst E, Canter PH. A systematic review of systematic reviews of spinal manipulation. J R Soc Med. 2006;99:192-196.
169 Assendelft WJ, Morton SC, Yu EI, et al: Spinal manipulative therapy for low back pain. Cochrane Database Syst Rev CD000447, 2004.
170 Eisenberg DM, Post DE, Davis RB, et al. Addition of choice of complementary therapies to usual care for acute low back pain: A randomized controlled trial. Spine (Phila Pa 1976). 2007;32:151-158.
171 Hurwitz EL, Morgenstern H, Kominski GF, et al. A randomized trial of chiropractic and medical care for patients with low back pain: Eighteen-month follow-up outcomes from the UCLA low back pain study. Spine (Phila Pa 1976). 2006;31:611-621. discussion 622
172 Furlan AD, van Tulder MW, Cherkin DC, et al: Acupuncture and dry-needling for low back pain. Cochrane Database Syst Rev CD001351, 2005.
173 Ammendolia C, Furlan AD, Imamura M, et al. Evidence-informed management of chronic low back pain with needle acupuncture. Spine J. 2008;8:160-172.
174 Yelland MJ, Glasziou PP, Bogduk N, et al. Prolotherapy injections, saline injections, and exercises for chronic low-back pain: A randomized trial. Spine (Phila Pa 1976). 2004;29:9-16.
175 Dagenais S, Mayer J, Haldeman S, et al. Evidence-informed management of chronic low back pain with prolotherapy. Spine J. 2008;8:203-212.
176 Rabago D, Best TM, Beamsley M, et al. A systematic review of prolotherapy for chronic musculoskeletal pain. Clin J Sport Med. 2005;15:376-380.
177 Roelofs PD, Deyo RA, Koes BW, et al: Non-steroidal anti-inflammatory drugs for low back pain. Cochrane Database Syst Rev CD000396, 2008.
178 Deshpande A, Furlan A, Mailis-Gagnon A, et al: Opioids for chronic low-back pain. Cochrane Database Syst Rev CD004959, 2007.
179 Jamison RN, Raymond SA, Slawsby EA, et al. Opioid therapy for chronic noncancer back pain: A randomized prospective study. Spine (Phila Pa 1976). 1998;23:2591-2600.
180 Martell BA, O’Connor PG, Kerns RD, et al. Systematic review: Opioid treatment for chronic back pain: Prevalence, efficacy, and association with addiction. Ann Intern Med. 2007;146:116-127.
181 Schofferman J, Mazanec D. Evidence-informed management of chronic low back pain with opioid analgesics. Spine J. 2008;8:185-194.
182 Armstrong TA, Rohal GM. Potential danger from too much acetaminophen in opiate agonist combination products. Am J Health Syst Pharm. 1999;56:1774-1775.
183 Physicians Desk Reference, 63rd ed. Montvale, NJ: Thomson PDR. 2009.
184 Krenzelok EP. The FDA Acetaminophen Advisory Committee Meeting—what is the future of acetaminophen in the United States? The perspective of a committee member. Clin Toxicol. 2009;47:784-789.
185 Vamvanij V, Fredrickson BE, Thorpe JM, et al. Surgical treatment of internal disc disruption: An outcome study of four fusion techniques. J Spinal Disord. 1998;11:375-382.
186 van Tulder MW, Touray T, Furlan AD, et al: Muscle relaxants for non-specific low back pain. Cochrane Database Syst Rev CD004252, 2003.
187 Alcoff J, Jones E, Rust P, et al. Controlled trial of imipramine for chronic low back pain. J Fam Pract. 1982;14:841-846.
188 Pheasant H, Bursk A, Goldfarb J, et al. Amitriptyline and chronic low-back pain: A randomized double-blind crossover study. Spine (Phila Pa 1976). 1983;8:552-557.
189 Ward NG. Tricyclic antidepressants for chronic low-back pain: Mechanisms of action and predictors of response. Spine (Phila Pa 1976). 1986;11:661-665.
190 Staiger TO, Gaster B, Sullivan MD, et al. Systematic review of antidepressants in the treatment of chronic low back pain. Spine (Phila Pa 1976). 2003;28:2540-2545.
191 Chang V, Gonzalez P, Akuthota V. Evidence-informed management of chronic low back pain with adjunctive analgesics. Spine J. 2008;8:21-27.
192 Keller A, Hayden J, Bombardier C, et al. Effect sizes of non-surgical treatments of non-specific low-back pain. Eur Spine J. 2007;16:1776-1788.
193 Machado LA, Kamper SJ, Herbert RD, et al. Analgesic effects of treatments for non-specific low back pain: A meta-analysis of placebo-controlled randomized trials. Rheumatology. 2009;48:520-527.
194 Delaney TJ, Rowlingson JC, Carron H, et al. Epidural steroid effects on nerves and meninges. Anesth Analg. 1980;59:610-614.
195 Fairbank JC, Park WM, McCall IW, et al. Apophyseal injection of local anesthetic as a diagnostic aid in primary low-back pain syndromes. Spine (Phila Pa 1976). 1981;6:598-605.
196 Manchikanti L, Cash KA, McManus CD, et al. Preliminary results of a randomized, equivalence trial of fluoroscopic caudal epidural injections in managing chronic low back pain: Part 1. Discogenic pain without disc herniation or radiculitis. Pain Physician. 2008;11:785-800.
197 Manchikanti L, Singh V, Rivera JJ, et al. Effectiveness of caudal epidural injections in discogram positive and negative chronic low back pain. Pain Physician. 2002;5:18-29.
198 Manchikanti L, Pampati V, Rivera JJ, et al. Caudal epidural injections with sarapin or steroids in chronic low back pain. Pain Physician. 2001;4:322-335.
199 Buttermann GR. The effect of spinal steroid injections for degenerative disc disease 5, Sep-Oct 2004. Spine J, 4. 2004:495-505.
200 Rosenberg SK, Grabinsky A, Kooser C, et al. Effectiveness of transforaminal epidural steroid injections in low back pain: A one year experience. Pain Physician. 2002;5:266-270.
201 DePalma MJ, Slipman CW. Evidence-informed management of chronic low back pain with epidural steroid injections. Spine J. 2008;8:45-55.
202 Staal JB, de Bie R, de Vet HC, et al: Injection therapy for subacute and chronic low-back pain. Cochrane Database Syst Rev CD001824, 2008.
203 Chou R, Atlas SJ, Stanos SP, et al. Nonsurgical interventional therapies for low back pain: A review of the evidence for an American Pain Society clinical practice guideline. Spine (Phila Pa 1976). 2009;34:1078-1093.
204 Feffer HL. Therapeutic intradiscal hydrocortisone: A long-term study. Clin Orthop Relat Res. 1969;67:100-104.
205 Wilkinson HA, Schuman N. Intradiscal corticosteroids in the treatment of lumbar and cervical disc problems. Spine (Phila Pa 1976). 1980;5:385-389.
206 Fayad F, Lefevre-Colau MM, Rannou F, et al. Relation of inflammatory Modic changes to intradiscal steroid injection outcome in chronic low back pain. Eur Spine J. 2007;16:925-931.
207 Simmons JW, McMillin JN, Emery SF, et al. Intradiscal steroids: A prospective double-blind clinical trial. Spine (Phila Pa 1976). 1992;17(6 Suppl):S172-S175.
208 Khot A, Bowditch M, Powell J, et al. The use of intradiscal steroid therapy for lumbar spinal discogenic pain: A randomized controlled trial. Spine (Phila Pa 1976). 2004;29:833-836. discussion 837
209 Klein RG, Eek BC, O’Neill CW, et al. Biochemical injection treatment for discogenic low back pain: A pilot study. Spine J. 2003;3:220-226.
210 Miller MR, Mathews RS, Reeves KD. Treatment of painful advanced internal lumbar disc derangement with intradiscal injection of hypertonic dextrose. Pain Physician. 2006;9:115-121.
211 Peng B, Zhang Y, Hou S, et al. Intradiscal methylene blue injection for the treatment of chronic discogenic low back pain. Eur Spine J. 2007;16:33-38.
212 Gallucci M, Limbucci N, Zugaro L, et al. Sciatica: Treatment with intradiscal and intraforaminal injections of steroid and oxygen-ozone versus steroid only. Radiology. 2007;242:907-913.
213 Muto M, Ambrosanio G, Guarnieri G, et al. Low back pain and sciatica: Treatment with intradiscal-intraforaminal O(2)-O(3) injection: Our experience. Radiol Med. 2008;113:695-706.
214 Freeman BJ, Walters RM, Moore RJ, et al. Does intradiscal electrothermal therapy denervate and repair experimentally induced posterolateral annular tears in an animal model? Spine (Phila Pa 1976). 2003;28:2602-2608.
215 Shah RV, Lutz GE, Lee J, et al. Intradiskal electrothermal therapy: A preliminary histologic study. Arch Phys Med Rehabil. 2001;82:1230-1237.
216 Kleinstueck FS, Diederich CJ, Nau WH, et al. Acute biomechanical and histological effects of intradiscal electrothermal therapy on human lumbar discs. Spine (Phila Pa 1976). 2001;26:2198-2207.
217 Strohbeln JW. Temperature distributions from interstitial RF electrode hyperthermia systems: Theoretical predictions. Int J Radiat Oncol Biol Phys. 1983;9:1655-1667.
218 Troussier B, Lebas JF, Chirossel JP, et al. Percutaneous intradiscal radio-frequency thermocoagulation: A cadaveric study. Spine (Phila Pa 1976). 1995;20:1713-1718.
219 Houpt JC, Conner ES, McFarland EW. Experimental study of temperature distributions and thermal transport during radiofrequency current therapy of the intervertebral disc. Spine (Phila Pa 1976). 1996;21:1808-1812. discussion 1812-1813
220 Ashley J, Gharpuray V, Saal J. Temperature distribution in the intervertebral disc: A comparison of intranuclear radiofrequency needle to a novel heating catheter. Proceedings of the 1999 Bioengineering Conference. BED. 1999;42:77.
221 Karasek M, Bogduk N. Twelve-month follow-up of a controlled trial of intradiscal thermal anuloplasty for back pain due to internal disc disruption. Spine (Phila Pa 1976). 2000;25:2601-2607.
222 Hsia AW, Isaac K, Katz JS. Cauda equina syndrome from intradiscal electrothermal therapy. Neurology. 2000;55:320.
223 Bogduk N, Karasek M. Two-year follow-up of a controlled trial of intradiscal electrothermal anuloplasty for chronic low back pain resulting from internal disc disruption. Spine J. 2002;2:343-350.
224 Saal JA, Saal JS. Intradiscal electrothermal treatment for chronic discogenic low back pain: Prospective outcome study with a minimum 2-year follow-up. Spine (Phila Pa 1976). 2002;27:966-973. discussion 973-974
225 Freeman BJ, Fraser RD, Cain CM, et al. A randomized, double-blind, controlled trial: Intradiscal electrothermal therapy versus placebo for the treatment of chronic discogenic low back pain. Spine (Phila Pa 1976). 2005;30:2369-2377. discussion 2378
226 Pauza KJ, Howell S, Dreyfuss P, et al. A randomized, placebo-controlled trial of intradiscal electrothermal therapy for the treatment of discogenic low back pain. Spine J. 2004;4:27-35.
227 Barendse GA, van Den Berg SG, Kessels AH, et al. Randomized controlled trial of percutaneous intradiscal radiofrequency thermocoagulation for chronic discogenic back pain: Lack of effect from a 90-second 70 C lesion. Spine (Phila Pa 1976). 2001;26:287-292.
228 Andersson GB, Mekhail NA, Block JE. Treatment of intractable discogenic low back pain: A systematic review of spinal fusion and intradiscal electrothermal therapy (IDET). Pain Physician. 2006;9:237-248.
229 Derby R, Baker RM, Lee CH, et al. Evidence-informed management of chronic low back pain with intradiscal electrothermal therapy. Spine J. 2008;8:80-95.
230 Helm S, Hayek SM, Benyamin RM, et al. Systematic review of the effectiveness of thermal annular procedures in treating discogenic low back pain. Pain Physician. 2009;12:207-232.
231 Freeman BJ. IDET: A critical appraisal of the evidence. Eur Spine J. 2006;15(Suppl):S448-S457.
232 Chou R, Baisden J, Carragee EJ, et al. Surgery for low back pain: A review of the evidence for an American Pain Society Clinical Practice Guideline. Spine (Phila Pa 1976). 2009;34:1094-1109.
233 Deyo RA, Nachemson A, Mirza SK. Spinal-fusion surgery—the case for restraint. N Engl J Med. 2004;350:722-726.
234 Fritzell P, Hagg O, Wessberg P, et al. Swedish Lumbar Spine Study Group: 2001 Volvo Award Winner in Clinical Studies: Lumbar fusion versus nonsurgical treatment for chronic low back pain: A multicenter randomized controlled trial from the Swedish Lumbar Spine Study Group. Spine (Phila Pa 1976). 2001;26:2521-2532. discussion 2532-2534
235 Rivero-Arias O, Campbell H, Gray A, et al. Surgical stabilisation of the spine compared with a programme of intensive rehabilitation for the management of patients with chronic low back pain: Cost utility analysis based on a randomised controlled trial. BMJ. 2005;330:1239.
236 Ibrahim T, Tleyjeh IM, Gabbar O. Surgical versus non-surgical treatment of chronic low back pain: A meta-analysis of randomised trials. Int Orthop. 2008;32:107-113.
237 Mirza SK, Deyo RA. Systematic review of randomized trials comparing lumbar fusion surgery to nonoperative care for treatment of chronic back pain. Spine (Phila Pa 1976). 2007;32:816-823.
238 Steinmann JC, Herkowitz HN. Pseudarthrosis of the spine. Clin Orthop Relat Res. 1992;284:80-90.
239 McCulloch JA. Uninstrumented posterolateral lumbar fusion for single level isolated disc resorption and/or degenerative disc disease. J Spinal Disord. 1999;12:34-39.
240 France JC, Yaszemski MJ, Lauerman WC, et al. A randomized prospective study of posterolateral lumbar fusion: Outcomes with and without pedicle screw instrumentation. Spine (Phila Pa 1976). 1999;24:553-560.
241 Hellstadius A. Experiences gained from spondylo-syndesis operations with H-shaped bone transplantations in the case of degeneration of discs in the lumbar back. Acta Orthop Scand. 1955;24:207-215.
242 Shaw EG, Taylor JG. The results of lumbo-sacral fusion for low back pain. J Bone Joint Surg Br. 1956;38:485-497.
243 Lorenz M, Zindrick M, Schwaegler P, et al. A comparison of single-level fusions with and without hardware. Spine (Phila Pa 1976). 1991;16(8 Suppl):S455-S458.
244 Louis R. Fusion of the lumbar and sacral spine by internal fixation with screw plates. Clin Orthop Relat Res. 1986;203:18-33.
245 Schwab FJ, Nazarian DG, Mahmud F, et al. Effects of spinal instrumentation on fusion of the lumbosacral spine. Spine (Phila Pa 1976). 1995;20:2023-2028.
246 Dawson EG, Lotysch M3rd, Urist MR. Intertransverse process lumbar arthrodesis with autogenous bone graft. Clin Orthop Relat Res. 1981;154:90-96.
247 Wood GW2nd, Boyd RJ, Carothers TA, et al. The effect of pedicle screw/plate fixation on lumbar/lumbosacral autogenous bone graft fusions in patients with degenerative disc disease. Spine (Phila Pa 1976). 1995;20:819-830.
248 Thomsen K, Christensen FB, Eiskjaer SP, et al. 1997 Volvo Award winner in clinical studies: The effect of pedicle screw instrumentation on functional outcome and fusion rates in posterolateral lumbar spinal fusion: A prospective, randomized clinical study. Spine (Phila Pa 1976). 1997;22:2813-2822.
249 Bono CM, Lee CK. Critical analysis of trends in fusion for degenerative disc disease over the past 20 years: Influence of technique on fusion rate and clinical outcome. Spine (Phila Pa 1976). 2004;29:455-463. discussion Z5
250 Weatherley CR, Prickett CF, O’Brien JP. Discogenic pain persisting despite solid posterior fusion. J Bone Joint Surg Br. 1986;68:142-143.
251 Kozak JA, O’Brien JP. Simultaneous combined anterior and posterior fusion: An independent analysis of a treatment for the disabled low-back pain patient. Spine (Phila Pa 1976). 1990;15:322-328.
252 Barrick WT, Schofferman JA, Reynolds JB, et al. Anterior lumbar fusion improves discogenic pain at levels of prior posterolateral fusion. Spine (Phila Pa 1976). 2000;25:853-857.
253 Loguidice VA, Johnson RG, Guyer RD, et al. Anterior lumbar interbody fusion. Spine (Phila Pa 1976). 1988;13:366-369.
254 Newman MH, Grinstead GL. Anterior lumbar interbody fusion for internal disc disruption. Spine (Phila Pa 1976). 1992;17:831-833.
255 Baker JK, Reardon PR, Reardon MJ, et al. Vascular injury in anterior lumbar surgery. Spine (Phila Pa 1976). 1993;18:2227-2230.
256 Hackenberg L, Liljenqvist U, Halm H, et al. Occlusion of the left common iliac artery and consecutive thromboembolism of the left popliteal artery following anterior lumbar interbody fusion. J Spinal Disord. 2001;14:365-368.
257 Christensen FB, Bunger CE. Retrograde ejaculation after retroperitoneal lower lumbar interbody fusion. Int Orthop. 1997;21:176-180.
258 Flynn JC, Price CT. Sexual complications of anterior fusion of the lumbar spine. Spine (Phila Pa 1976). 1984;9:489-492.
259 Moore KR, Pinto MR, Butler LM. Degenerative disc disease treated with combined anterior and posterior arthrodesis and posterior instrumentation. Spine (Phila Pa 1976). 2002;27:1680-1686.
260 Gertzbein SD, Betz R, Clements D, et al. Semirigid instrumentation in the management of lumbar spinal conditions combined with circumferential fusion: A multicenter study. Spine (Phila Pa 1976). 1996;21:1918-1925. discussion 1925-1926
261 Kozak JA, O’Brien JP. Simultaneous combined anterior and posterior fusion: An independent analysis of a treatment for the disabled low-back pain patient. Spine (Phila Pa 1976). 1990;15:322-328.
262 Hinkley BS, Jaremko ME. Effects of 360-degree lumbar fusion in a workers’ compensation population. Spine (Phila Pa 1976). 1997;22:312-322. discussion 323
263 Videbaek TS, Christensen RB, Soegaard R, et al. Circumferential fusion improves outcome in comparison with instrumented posterolateral fusion: Long-term results of a randomized clinical trial. Spine (Phila Pa 1976). 2006;31:2875-2880.
264 Suratwala SJ, Pinto MR, Gilbert TJ, et al. Functional and radiological outcomes of 360 degrees fusion of three or more motion levels in the lumbar spine for degenerative disc disease. Spine (Phila Pa 1976). 2009;34:E351-E358.
265 Salehi SA, Tawk R, Ganju A, et al. Transforaminal lumbar interbody fusion: Surgical technique and results in 24 patients. Neurosurgery. 2004;54:368-374. discussion 374
266 Madan SS, Boeree NR. Comparison of instrumented anterior interbody fusion with instrumented circumferential lumbar fusion. Eur Spine J. 2003;12:567-575.
267 Vamvanij V, Fredrickson BE, Thorpe JM, et al. Surgical treatment of internal disc disruption: An outcome study of four fusion techniques. J Spinal Disord. 1998;11:375-382.
268 Knox BD, Chapman TM. Anterior lumbar interbody fusion for discogram concordant pain. J Spinal Disord. 1993;6:242-244.
269 Lowe TG, Tahernia AD, O’Brien MF, et al. Unilateral transforaminal posterior lumbar interbody fusion (TLIF): Indications, technique, and 2-year results. J Spinal Disord Tech. 2002;15:31-38.
270 Whitecloud TS3rd, Roesch WW, Ricciardi JE. Transforaminal interbody fusion versus anterior-posterior interbody fusion of the lumbar spine: A financial analysis. J Spinal Disord. 2001;14:100-103.
271 Obenchain TG. Laparoscopic lumbar discectomy: Case report. J Laparoendosc Surg. 1991;1:145-149.
272 Kaiser MG, Haid RWJr, Subach BR, et al. Comparison of the mini-open versus laparoscopic approach for anterior lumbar interbody fusion: A retrospective review. Neurosurgery. 2002;51:97-103. discussion 103-105
273 Regan JJ, Yuan H, McAfee PC. Laparoscopic fusion of the lumbar spine: Minimally invasive spine surgery: A prospective multicenter study evaluating open and laparoscopic lumbar fusion. Spine (Phila Pa 1976). 1999;24:402-411.
274 Lieberman IH, Willsher PC, Litwin DE, et al. Transperitoneal laparoscopic exposure for lumbar interbody fusion. Spine (Phila Pa 1976). 2000;25:509-514. discussion 515
275 Zdeblick TA, David SM. A prospective comparison of surgical approach for anterior L4-L5 fusion: Laparoscopic versus mini anterior lumbar interbody fusion. Spine (Phila Pa 1976). 2000;25:2682-2687.
276 Inamasu J, Guiot BH. Laparoscopic anterior lumbar interbody fusion: A review of outcome studies. Minim Invasive Neurosurg. 2005;48:340-347.
277 Brau S. Mini-open approach to the spine for anterior lumbar interbody fusion: Description of the procedure, results and complications. Spine J. 2002;2:216-223.
278 Schleicher P, Beth P, Ottenbacher A, et al. Biomechanical evaluation of different asymmetrical posterior stabilization methods for minimally invasive transforaminal lumbar interbody fusion. J Neurosurg Spine. 2008;9:363-371.
279 Slucky AV, Brodke DS, Bachus KN, et al. Less invasive posterior fixation method following transforaminal lumbar interbody fusion: A biomechanical analysis. Spine J. 2006;6:78-85.
280 Sethi A, Lee S, Vaidya R. Transforaminal lumbar interbody fusion using unilateral pedicle screws and a translaminar. Eur Spine J. 2009;18:430-434.
281 Schwender JD, Holly LT, Rouben DP, et al. Minimally invasive transforaminal lumbar interbody fusion (TLIF): Technical feasibility and initial results. J Spinal Disord Tech. 2005;18(Suppl):S1-S6.
282 Peng CW, Yue WM, Poh SY, et al. Clinical and radiological outcomes of minimally invasive versus open transforaminal lumbar interbody fusion. Spine (Phila Pa 1976). 2009;34:1385-1389.
283 Scheufler KM, Dohmen H, Vougioukas VI. Percutaneous transforaminal lumbar interbody fusion for the treatment of degenerative lumbar instability. Neurosurgery. 2007;60(4 Suppl 2):203-212. discussion 212-213
284 Park Y, Ha JW. Comparison of one-level posterior lumbar interbody fusion performed with a minimally invasive approach or a traditional open approach. Spine (Phila Pa 1976). 2007;32:537-543.
285 Stevens KJ, Spenciner DB, Griffiths KL, et al. Comparison of minimally invasive and conventional open posterolateral lumbar fusion using magnetic resonance imaging and retraction pressure studies. J Spinal Disord Tech. 2006;19:77-86.
286 Shim CS, Lee SH, Jung B, et al. Fluoroscopically assisted percutaneous translaminar facet screw fixation following anterior lumbar interbody fusion: Technical report. Spine (Phila Pa 1976). 2005;30:838-843.
287 Jang JS, Lee SH. Clinical analysis of percutaneous facet screw fixation after anterior lumbar interbody fusion. J Neurosurg Spine. 2005;3:40-46.
288 Pimenta L. Lateral endoscopic transpsoas retroperitoneal approach Proceedings of VIII Brazilian Spine Society Meeting, Minas Horizonte, Minas Gerais, Brazil. 2001.
289 Bergey DL, Villavicencio AT, Goldstein T, et al. Endoscopic lateral transpsoas approach to the lumbar spine. Spine (Phila Pa 1976). 2004;29:1681-1688.
290 Benglis DM, Vanni S, Levi AD. An anatomical study of the lumbosacral plexus as related to the minimally invasive transpsoas approach to the lumbar spine. J Neurosurg Spine. 2009;10:139-144.
290a Knight RQ, Schwaegler P, Hanscom D, Roh J. Direct lateral lumbar interbody fusion for degenerative conditions: early complication profile. J Spinal Disord Tech. 2009;22:34-37.
291 Ozgur BM, Aryan HE, Pimenta L, et al. Extreme lateral interbody fusion (XLIF): A novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006;6:435-443.
292 Marotta N, Cosar M, Pimenta L, et al. A novel minimally invasive presacral approach and instrumentation technique for anterior L5-S1 intervertebral discectomy and fusion: Technical description and case presentations. Neurosurg Focus. 2006;20:E9.
293 Aryan HE, Newman CB, Gold JJ, et al. Percutaneous axial lumbar interbody fusion (AxiaLIF) of the L5-S1 segment: Initial clinical and radiographic experience. Minim Invasive Neurosurg. 2008;51:225-230.
294 Bindal RK, Glaze S, Ognoskie M, et al. Surgeon and patient radiation exposure in minimally invasive transforaminal lumbar interbody fusion. J Neurosurg Spine. 2008;9:570-573.
295 Kim CW, Lee YP, Taylor W, et al. Use of navigation-assisted fluoroscopy to decrease radiation exposure during minimally invasive spine surgery. Spine J. 2008;8:584-590.
296 Fritzell P, Hagg O, Wessberg P, et al. Chronic low back pain and fusion: A comparison of three surgical techniques: A prospective multicenter randomized study from the Swedish lumbar spine study group. Spine (Phila Pa 1976). 2002;27:1131-1141.
297 Fritzell P, Hägg O, Nordwall A, Swedish Lumbar Spine Study Group. Complications in lumbar fusion surgery for chronic low back pain: Comparison of three surgical techniques used in a prospective randomized study. A report from the Swedish Lumbar Spine Study Group. Eur Spine J. 2003;12:178-189.
298 Kim KT, Lee SH, Lee YH, et al. Clinical outcomes of 3 fusion methods through the posterior approach in the lumbar spine. Spine (Phila Pa 1976). 2006;31:1351-1357.
299 Glassman S, Gornet MF, Branch C, et al. MOS Short Form 36 and Oswestry Disability Index outcomes in lumbar fusion: A multicenter experience. Spine J. 2006;6:21-26.
300 Burkus JK, Gornet MF, Dickman CA, et al. Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J Spinal Disord Tech. 2002;15:337-349.
301 Boden SD, Zdeblick TA, Sandhu HS, et al. The use of rhBMP-2 in interbody fusion cages: Definitive evidence of osteoinduction in humans: A preliminary report. Spine (Phila Pa 1976). 2000;25:376-381.
302 Haid RWJr, Branch CLJr, Alexander JT, et al. Posterior lumbar interbody fusion using recombinant human bone morphogenetic protein type 2 with cylindrical interbody cages. Spine J. 2004;4:527-538. discussion 538-539
303 Sasso RC, LeHuec JC, Shaffrey C. Spine Interbody Research Group: Iliac crest bone graft donor site pain after anterior lumbar interbody fusion: A prospective patient satisfaction outcome assessment. J Spinal Disord Tech. 2005;18(Suppl):S77-S81.
304 Dimar JR, Glassman SD, Burkus KJ, et al. Clinical outcomes and fusion success at 2 years of single-level instrumented posterolateral fusions with recombinant human bone morphogenetic protein-2/compression resistant matrix versus iliac crest bone graft. Spine (Phila Pa 1976). 2006;31:2534-2539. discussion 2540
305 Dimar JR2nd, Glassman SD, Burkus JK, et al. Clinical and radiographic analysis of an optimized rhBMP-2 formulation as an autograft replacement in posterolateral lumbar spine arthrodesis. J Bone Joint Surg Am. 2009;91:1377-1386.
306 Burkus JK, Sandhu HS, Gornet MF. Influence of rhBMP-2 on the healing patterns associated with allograft interbody constructs in comparison with autograft. Spine (Phila Pa 1976). 2006;31:775-781.
307 Burkus JK, Gornet MF, Schuler TC, et al. Six-year outcomes of anterior lumbar interbody arthrodesis with use of interbody fusion cages and recombinant human bone morphogenetic protein-2. J Bone Joint Surg Am. 2009;91:1181-1189.
308 Vaccaro AR, Lawrence JP, Patel T, et al. The safety and efficacy of OP-1 (rhBMP-7) as a replacement for iliac crest autograft in posterolateral lumbar arthrodesis: A long-term (>4 years) pivotal study. Spine (Phila Pa 1976). 2008;33:2850-2862.
309 Rihn JA, Patel R, Makda J, et al. Complications associated with single-level transforaminal lumbar interbody fusion. Spine J. 2009;9:623-629.
310 Mindea SA, Shih P, Song JK. Recombinant human bone morphogenetic protein-2-induced radiculitis in elective minimally invasive transforaminal lumbar interbody fusions: A series review. Spine (Phila Pa 1976). 2009;34:1480-1484. discussion 1485
311 Joseph V, Rampersaud YR. Heterotopic bone formation with the use of rhBMP2 in posterior minimal access interbody fusion: A CT analysis. Spine (Phila Pa 1976). 2007;32:2885-2890.
312 Lewandrowski KU, Nanson C, Calderon R. Vertebral osteolysis after posterior interbody lumbar fusion with recombinant human bone morphogenetic protein 2: A report of five cases. Spine J. 2007;7:609-614.
313 Glassman SD, Dimar JR3rd, Burkus K, et al. The efficacy of rhBMP-2 for posterolateral lumbar fusion in smokers. Spine (Phila Pa 1976). 2007;32:1693-1698.
314 Carreon LY, Glassman SD, Djurasovic M, et al. rhBMP-2 versus iliac crest bone graft for lumbar spine fusion in patients over 60 years of age: A cost-utility study. Spine (Phila Pa 1976). 2009;34:238-243.
315 Garrison KR, Donell S, Ryder J, et al. Clinical effectiveness and cost-effectiveness of bone morphogenetic proteins in the non-healing of fractures and spinal fusion: A systematic review. Health Technol Assess. 2007;11:1-150. iii-iv
316 Cunningham BW, Kotani Y, McNulty PS, et al. The effect of spinal destabilization and instrumentation on lumbar intradiscal pressure: An in vitro biomechanical analysis. Spine (Phila Pa 1976). 1997;22:2655-2663.
317 Lee CK, Langrana NA. Lumbosacral spinal fusion: A biomechanical study. Spine (Phila Pa 1976). 1984;9:574-581.
318 Lee CK. Accelerated degeneration of the segment adjacent to a lumbar fusion. Spine (Phila Pa 1976). 1988;13:375-377.
319 Okuda S, Iwasaki M, Miyauchi A, et al. Risk factors for adjacent segment degeneration after PLIF. Spine (Phila Pa 1976). 2004;29:1535-1540.
320 Wai EK, Santo ER, Morcom RA, et al. Magnetic resonance imaging 20 years after anterior lumbar interbody fusion. Spine (Phila Pa 1976). 2006;31:1952-1956.
321 Pellise F, Hernandez A, Vidal X, et al. Radiologic assessment of all unfused lumbar segments 7.5 years after instrumented posterior spinal fusion. Spine (Phila Pa 1976). 2007;32:574-579.
322 Yang JY, Lee JK, Song HS. The impact of adjacent segment degeneration on the clinical outcome after lumbar spinal fusion. Spine (Phila Pa 1976). 2008;33:503-507.
323 Willems PC, Elmans L, Anderson PG, et al. Provocative discography and lumbar fusion: Is preoperative assessment of adjacent discs useful? Spine (Phila Pa 1976). 2007;32:1094-1099. discussion 1100
324 Schulte TL, Leistra F, Bullmann V, et al. Disc height reduction in adjacent segments and clinical outcome 10 years after lumbar 360 degrees fusion. Eur Spine J. 2007;16:2152-2158.
325 Graf H. Surgical treatment without fusion. Rachis. 1992;412:123-137.
326 Kanayama M, Hashimoto T, Shigenobu K. Rationale, biomechanics, and surgical indications for Graf ligamentoplasty. Orthop Clin North Am. 2005;36:373-377.
327 Kanayama M, Hashimoto T, Shigenobu K, et al. A minimum 10-year follow-up of posterior dynamic stabilization using Graf artificial ligament. Spine (Phila Pa 1976). 2007;32:1992-1996. discussion 1997
328 Gardner A, Pande KC. Graf ligamentoplasty: A 7-year follow-up. Eur Spine J. 2002;11(Suppl 2):S157-S163.
329 Markwalder TM, Wenger M. Dynamic stabilization of lumbar motion segments by use of Graf’s ligaments: Results with an average follow-up of 7.4 years in 39 highly selected, consecutive patients. Acta Neurochir (Wien). 2003;145:209-214. discussion 214
330 Brechbühler D, Markwalder TM, Braun M. Surgical results after soft system stabilization of the lumbar spine in degenerative disc disease—long-term results. Acta Neurochir (Wien). 1998;140:521-525.
331 Hadlow SV, Fagan AB, Hillier TM, et al. The Graf ligamentoplasty procedure: Comparison with posterolateral fusion in the management of low back pain. Spine (Phila Pa 1976). 1998;23:1172-1179.
332 Mulholland RC, Sengupta DK. Rationale, principles and experimental evaluation of the concept of soft stabilization. Eur Spine J. 2002;11(Suppl 2):S198-S205.
333 Grob D, Benini A, Junge A, et al. Clinical experience with the Dynesys semirigid fixation system for the lumbar spine: Surgical and patient-oriented outcome in 50 cases after an average of 2 years. Spine (Phila Pa 1976). 2005;30:324-331.
334 Stoll TM, Dubois G, Schwarzenbach O. The dynamic neutralization system for the spine: A multi-center study of a novel non-fusion system. Eur Spine J. 2002;11(Suppl 2):S170-S178.
335 Guyer RD, McAfee PC, Banco RJ, Bitan FD, et al. Prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of lumbar total disc replacement with the CHARITE artificial disc versus lumbar fusion: Five-year follow-up. Spine J. 2009;9:374-386.
336 Blumenthal S, McAfee PC, Guyer RD, et al. A prospective, randomized, multicenter Food and Drug Administration investigational device exemptions study of lumbar total disc replacement with the CHARITE artificial disc versus lumbar fusion: Part I. Evaluation of clinical outcomes. Spine (Phila Pa 1976). 2005;30:1565-1575. discussion E387-E391
337 McAfee PC, Cunningham B, Holsapple G, et al. A prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of lumbar total disc replacement with the CHARITE artificial disc versus lumbar fusion: Part II. Evaluation of radiographic outcomes and correlation of surgical technique accuracy with clinical outcomes. Spine (Phila Pa 1976). 2005;30:1576-1583. discussion E388-E390
338 Tropiano P, Huang RC, Girardi FP, et al. Lumbar total disc replacement: Seven to eleven-year follow-up. J Bone Joint Surg Am. 2005;87:490-496.
339 Lemaire JP, Carrier H, Sariali el-H, et al. Clinical and radiological outcomes with the Charité artificial disc: A 10-year minimum follow-up. J Spinal Disord Tech. 2005;18:353-359.
340 Zigler J, Delamarter R, Spivak JM, et al. Results of the prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of the ProDisc-L total disc replacement versus circumferential fusion for the treatment of 1-level degenerative disc disease. Spine (Phila Pa 1976). 2007;32:1155-1162. discussion 1163
341 Sasso RC, Foulk DM, Hahn M. Prospective, randomized trial of metal-on-metal artificial lumbar disc replacement: Initial results for treatment of discogenic pain. Spine (Phila Pa 1976). 2008;33:123-131.
342 Kostuik JP. Complications and surgical revision for failed disc arthroplasty. Spine J. 2004;4(6 Suppl):289S-291S.
343 Park CK, Ryu KS, Jee WH. Degenerative changes of discs and facet joints in lumbar total disc replacement using ProDisc II: Minimum two-year follow-up. Spine (Phila Pa 1976). 2008;33:1755-1761.
344 Shim CS, Lee SH, Shin HD, et al. CHARITE versus ProDisc: A comparative study of a minimum 3-year follow-up. Spine (Phila Pa 1976). 2007;32:1012-1018.
345 Harrop JS, Youssef JA, Maltenfort M, et al. Lumbar adjacent segment degeneration and disease after arthrodesis and total disc arthroplasty. Spine (Phila Pa 1976). 2008;33:1701-1707.
346 Mehren C, Suchomel P, Grochulla F, et al. Heterotopic ossification in total cervical artificial disc replacement. Spine (Phila Pa 1976). 2006;31:2802-2806.
347 Huang DS, Liang AJ, Ye W, et al. The risk factors and preventive strategies of heterotopic ossification after artificial disc replacement in lumbar spine. Zhonghua Wai Ke Za Zhi. 2006;44:242-245.
348 Tortolani PJ, Cunningham BW, Eng M, et al. Prevalence of heterotopic ossification following total disc replacement: A prospective, randomized study of two hundred and seventy-six patients. J Bone Joint Surg Am. 2007;89:82-88.
349 Schulte TL, Lerner T, Hackenberg L, et al. Acquired spondylolysis after implantation of a lumbar ProDisc II prosthesis: Case report and review of the literature. Spine (Phila Pa 1976). 2007;32:E645-E648.
350 Marshman LA, Friesem T, Rampersaud YR, et al. Subsidence and malplacement with the Oblique Maverick Lumbar Disc Arthroplasty: Technical note. Spine J. 2008;8:650-655.
351 McAfee PC, Geisler FH, Saiedy SS, et al. Revisability of the CHARITE artificial disc replacement: Analysis of 688 patients enrolled in the U.S. IDE study of the CHARITE Artificial Disc. Spine (Phila Pa 1976). 2006;31:1217-1226.
352 Levin DA, Bendo JA, Quirno M, et al. Comparative charge analysis of one- and two-level lumbar total disc arthroplasty versus circumferential lumbar fusion. Spine (Phila Pa 1976). 2007;32:2905-2909.
353 Patel VV, Estes S, Lindley EM, et al. Lumbar spinal fusion versus anterior lumbar disc replacement: The financial implications. J Spinal Disord Tech. 2008;21:473-476.
354 Fernstrom U. Arthroplasty with intercorporal endoprosthesis in herniated disc and in painful disc. Acta Chir Scand Suppl. 1966;357:154-159.
355 Coric D, Mummaneni PV. Nucleus replacement technologies. J Neurosurg Spine. 2008;8:115-120.
356 Klara PM, Ray CD. Artificial nucleus replacement: Clinical experience. Spine (Phila Pa 1976). 2002;27:1374-1377.
357 Shim CS, Lee SH, Park CW, et al. Partial disc replacement with the PDN prosthetic disc nucleus device: Early clinical results. J Spinal Disord Tech. 2003;16:324-330.
358 Ahrens M, Tsantrizos A, Donkersloot P, et al. Nucleus replacement with the DASCOR disc arthroplasty device: Interim two-year efficacy and safety results from two prospective, non-randomized multicenter European studies. Spine (Phila Pa 1976). 2009;34:1376-1384.
359 Hutton W, Decatur G, Meisel H. Autologous disc chondrocyte transplantation for repair of acute disc herniation Presented at International Society for the Study of the Lumbar Spine, 29th annual meeting, Cleveland, OH. 2000.
360 Nishimura K, Mochida J. Percutaneous reinsertion of the nucleus pulposus: An experimental study. Spine (Phila Pa 1976). 1998;23:1531-1538. discussion 1539
361 Nomura T, Mochida J, Okuma M, et al. Nucleus pulposus allograft retards intervertebral disc degeneration. Clin Orthop Relat Res. 2001;389:94-101.
362 Meisel HJ, Siodla V, Ganey T, et al. Clinical experience in cell-based therapeutics: Disc chondrocyte transplantation: A treatment for degenerated or damaged intervertebral disc. Biomed Eng. 2007;24:5-21.
363 An HS, Takegami K, Kamada H, et al. Intradiscal administration of osteogenic protein-1 increases intervertebral disc height and proteoglycan content in the nucleus pulposus in normal adolescent rabbits. Spine (Phila Pa 1976). 2005;30:25-31. discussion 31-32
364 Kawakami M, Matsumoto T, Hashizume H, et al. Osteogenic protein-1 (osteogenic protein-1/bone morphogenetic protein-7) inhibits degeneration and pain-related behavior induced by chronically compressed nucleus pulposus in the rat. Spine (Phila Pa 1976). 2005;30:1933-1939.
365 Chujo T, An HS, Akeda K, et al. Effects of growth differentiation factor-5 on the intervertebral disc—in vitro bovine study and in vivo rabbit disc degeneration model study. Spine (Phila Pa 1976). 2006;31:2909-2917.
366 Kang R, Ghivizzani SC, Muzzonigro TS, et al. Orthopaedic applications of gene therapy: From concept to clinic The Marshall R. Urist Young Investigator Award. Clin Orthop Relat Res, 375. 2000:324-337.
367 Paul R, Hayden RC, Cheng H, et al. Potential use of Sox9 gene therapy for intervertebral degenerative disc disease. Spine (Phila Pa 1976). 2003;28:755-763.
368 Wallach CJ, Sobajima S, Watanabe Y, et al. Gene transfer of the catabolic inhibitor TIMP-1 increases measured proteoglycans in cells from degenerated human intervertebral discs. Spine (Phila Pa 1976). 2003;28:2331-2337.
369 Moon SH, Nishida K, Gilbertson LG, et al. Biologic response of human intervertebral disc cells to gene therapy cocktail. Spine (Phila Pa 1976). 2008;33:1850-1855.
370 Kang R, Boden S. Breakout Session 7: Spine. Clin Orthop Relat Res. 2000;279S:S256-S259.