55 Lumbar Disc Arthroplasty
Indications and Contraindications
KEY POINTS
Introduction
Total disc replacement (TDR) surgery began in Europe over 20 years ago and migrated to the United States in 2000 with the first TDR Food and Drug Administration (FDA) Investigational Device Exemption (IDE) trial of the Charité III disc. Results based on the long-term follow-up from European literature have been promising so far, as have the early results from our U.S. experience.1–5 Some of the first 5-year follow-up data from the FDA IDE trial comparing the Charité disc to lumbar fusion are now available.6 The results show no statistically significant differences in outcome measures (visual analog scales (VAS) assessing pain and the Oswestry Disability Index) between the groups at the five-year mark, substantiating the noninferiority of the disc arthroplasty group. Additionally, the Charité patients had a statistically greater rate of part-time and full-time employment and a lower rate of long-term disability at 5years. Furthermore, the range of motion of the prosthesis, as evaluated by radiographic criteria, remained the same at 5-years compared with the 2-year data, showing preservation of motion at the surgical level.
At the time of writing of this chapter, a recently published systematic review analyzing the association of symptomatic adjacent segment disease (as distinguished from asymptomatic adjacent segment degeneration) in lumbar arthroplasty compared to arthrodesis showed that 14% of arthrodesis patients developed adjacent segment disease, compared with 1% of arthroplasty patients.7 Given this positive trend for arthroplasty, it is reasonable to assume that disc replacement surgery will remain a valid tool in the spine surgeon’s armamentarium and will likely become more prevalent in years to come.
Clinical Practice Guidelines
Indications
The established indication initially set forth by the FDA IDE studies for lumbar disc arthroplasty is severe unremitting low back pain resulting from single-level degenerative disc disease that has failed to respond to a prolonged course of conservative measures. The trial period for conservative treatment is usually defined as a minimum of 6 months, although the exact time frame itself is less important than the extent to which nonoperative management has been attempted. Nonoperative treatments should incorporate the use of various antiinflammatory, nonnarcotic, and even narcotic medications if necessary; physical therapy, including active exercise and core stabilization; chiropractic modalities; and a trial of spinal injections, including epidural injections and facet injections as appropriate. The purpose of these conservative efforts is to ensure that the patient has been afforded every opportunity to obtain a satisfactory result without surgery. We know that MRI alone is not a reliable indicator in predicting whether a degenerative-appearing disc is truly symptomatic.8,9 In some cases, discography may be helpful in delineating whether the disc is responsible for the patient’s clinical symptoms. The exact role for discography as part of the clinical work-up for potential surgical candidates is not clearly established, and is still an issue of significant controversy. That being said, at our institution we incorporate the use of discography in all patients that we feel might be appropriate candidates for total disc replacement. Because the interpretation of results from discography can heavily depend on the skill of the technician performing the procedure, the established relationship between the surgeon and the physician performing the test cannot be overemphasized. In our opinion, a poorly-done discogram is worse than no discogram at all.
Clinical Case Examples
Case 1
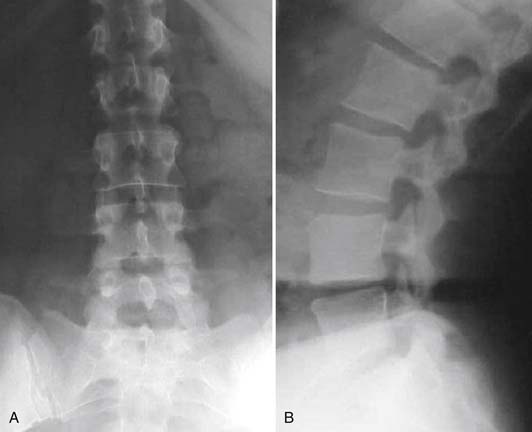
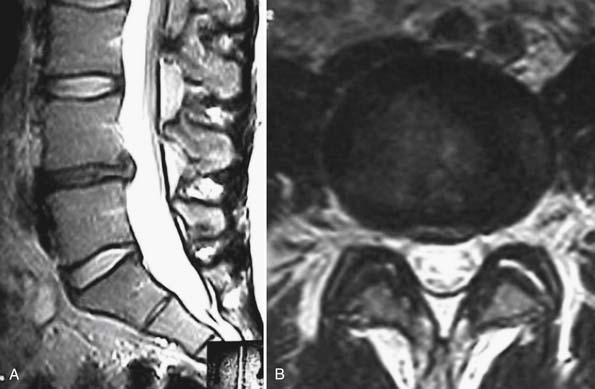
A 54-year-old woman presents to your clinic with complaints of severe low back pain that has progressed over the past 2 years. She has been through physical therapy focusing on core muscle strengthening and has had a trial of epidural steroid injections and various facet injections that did not improve her symptoms. She denies any radicular leg pain, but states that her low back pain is constant and is aggravated by prolonged standing, sitting, or walking. She has been on NSAIDs for the past few years, and has recently started to take narcotics because of increased pain. A complete neurological examination reveals no motor or sensory deficits. She has pain and limited motion with forward flexion of her lumbar spine. Her radiographs (Figures 55-1A and B) show normal spinal alignment with decreased disc space height at the L4-5 and L5-S1 level. No instability is present on flexion-extension views. A T2-weighted MRI (Figures 55-2A and B) reveals a dessicated L4-5 disc with preservation of hydration in the remaining lumbar discs. Given this scenario, what treatment option(s) would you discuss with this patient?
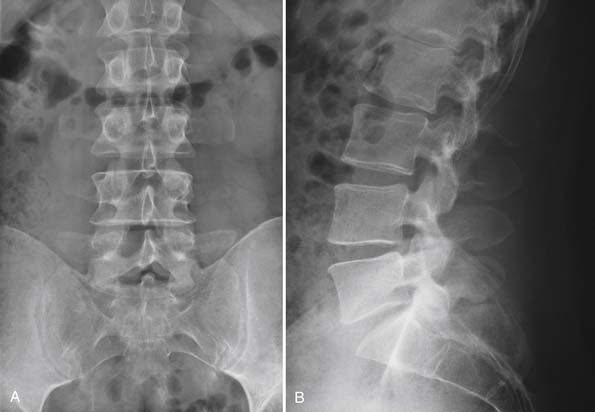
Case 2
A 56-year-old male presents to your clinic for evaluation of debilitating low back pain over the last 6 years. He reports daily pain that radiates across his low back, worse on the right side. His primary care physician sent him to physical therapy for 6 weeks, but he did not see any symptomatic improvement. He has been taking hydrocodone for the past 6 months in order to bring his pain to a tolerable level but is frustrated by the need to be on narcotic medications and wishes for something to be done surgically to address his pain. His physical examination is fairly unremarkable and he has normal motor and sensory function. His plain radiographs reveal only minimal narrowing at the L4-5 and L5-S1 levels (Figure 55-3A and B). A T2-weighted MRI reveals pronounced desiccation with a small posterior bulge, along with minimal disc desiccation at the L4-5 level (Figure 55-4). On axial MRI views, the facet joints at both levels appear normal or with minimal degenerative changes. He asks whether he is a candidate for the disc replacement surgery. What do you tell him?
Bertagnoli and Kumar stratified indications for disc arthroplasty into four categories based on remaining disc height, status of the facet joints, adjacent level degeneration, and stability of the posterior elements.10 The prime candidate for a disc replacement, based on their evaluation of clinical outcome in 108 patients who underwent implantation of a ProDisc II prosthesis, had at least 4 mm of remaining disc space height, no radiographic changes suggestive of facet arthritis, no adjacent level disc degeneration, and intact posterior elements.
Certainly, having competent, nondegenerative facets and posterior element stability are important inclusion criteria for a patient to be considered appropriate to undergo TDR. We will discuss the issue of facet arthrosis further as a part of contraindications to disc arthroplasty, but as far as a clinical evaluation is concerned, facets should be assessed with direct palpation and by having the patient demonstrate whether spinal extension (i.e., facet loading) reproduces pain. Radiographically, the facets should be evaluated by examining their appearance on plain films, CT scans, and/or axial MR images. There exist a few grading systems for facet joints, although none has gained universal acceptance. The first, proposed by Pathria, assigned a grade of 0 to 3, depending on the extent of facet joint narrowing.11 A “normal” facet joint is given a grade of 0, whereas a grade 1 is assigned for mild narrowing, 2 for moderate, and 3 for severe narrowing. Patients with grade 3 facets in this grading system should be excluded as candidates for disc arthroplasty. Fujiwara also proposed a grading system based on evaluation of the facet joints as they appear on axial MR images.12 In this system, a grade of 0 is again assigned to “normal” facets, grade 1 for moderately compressed facets with small osteophytes, grade 2 for facets with subchondral sclerosis and moderate osteophytes, and grade 3 for facets lacking articular joint space and with large osteophytes. Again, patients who meet the criteria for grade 3 facets in this classification system are not indicated for total disc arthroplasty.
Concern over the relationship between preoperative disc height and clinical outcome in disc arthroplasty with severely collapsed disc space (i.e., less than 4 mm) is somewhat controversial. Despite speculation that TDR is not appropriate for severely collapsed discs, there exist few data to support or refute this. At our institution, we set out to determine if there was a relationship between preoperative and/or postoperative disc height and clinical outcome at 2 years (Li, Guyer, et al., International Meeting on Advanced Spinal Techniques, 2008).13 For 117 patients (42 Charité and 75 ProDisc-L) undergoing a single-level TDR, we recorded disc height as a ratio of vertebral body height, thereby accounting for variation in individual size and radiographic magnification. Patients were categorized into four groups based on these ratios (most collapsed, second most collapsed, second least collapsed, least collapsed). For all groups, the mean VAS pain score improved significantly from preoperative values, but there were no statistically significant differences among the groups. We therefore concluded that there is no relationship between preoperative disc height and clinical outcome. If patients with severely collapsed discs otherwise meet the strict selection criteria for TDR, they can expect as favorable an outcome from the surgery as patients with discs that are not as collapsed.
Contraindications
It is often easier to define patients who are not good candidates for a given procedure than to accurately define those who would be suitable. To some extent, the same can be said for lumbar disc arthroplasty. In recent years, much attention has turned toward defining and understanding the established contraindications for TDR, and this, in turn, has generated some controversy. In an epidemiological study to investigate the contraindications to lumbar total disc arthroplasty in their patient population (that of an academic medical center), Huang et al reported that 95% of patients had at least one of ten contraindications to surgery.13 This finding was further substantiated in a recent publication by Wong et al,14 who retrospectively reviewed 100 consecutive lumbar spine surgery patients with specific analysis of facet arthrosis and noted that all patients had one or more of the aforementioned ten contraindications to the procedure. In their population (a private medical center), they found that 97% of patients had facet arthrosis as the contraindication against TDR, followed by spondylolisthesis (75%), and central spinal stenosis (72%).
For the purpose of our discussion, we will group contraindications into two categories: absolute or “hard” contraindications, and relative or “soft” contraindications. Absolute contraindications include osteopenia and osteoporosis, history of previous disc infection or ongoing infection, prior fusion at the level of consideration, severe posterior element pathology, instability at the operative segment, vertebral fracture, malignancy, curves of greater than 11 degrees, metal allergy, and a psychosocial state that places a given patient at increased risk for poor surgical outcome. Additionally, as this is entirely an elective procedure, pregnancy should be viewed as an absolute contraindication. Relative contraindications include history of prior abdominal surgery, and obesity. To better appreciate the reasons behind the various contraindications, we will discuss a number of them in further detail. The reader may also refer to Table 55-1, which gives a summary of the contraindications that will be discussed in the chapter.
Facet Joints
An appreciation of the status of the facet joints is vital in evaluating a patient for a TDR. Yet, this is arguably the area of greatest controversy in discussions of disc arthroplasty candidates. The question of “how much is too much?” remains to be answered definitively. In an earlier section of this chapter, we discussed that any patient under consideration for TDR should have no or minimal degenerative changes of the facet joints. Clearly, facet arthrosis follows a spectrum of degenerative processes, and there exists no reliable and universally accepted grading system by which to categorize the various stages of disease. Despite attempts by various authors at defining such stages, as was discussed earlier in the chapter,12,15 it remains unclear what the clinical implications of these stratifications are. Eventually, with more long-term data, it is likely that the issue will unfold more clearly; however, for the time being, we are left with many opinions and few hard data.
Prior Abdominal Surgery
Because the standard surgical approach to the disc space for TDR is through an anterior retroperitoneal approach, a history of prior abdominal surgery is a relative contraindication, since the patient is likely to have adhesions that can make the subsequent surgical approach fraught with difficulty and possible complications. Prior retroperitoneal surgical approach is an absolute contraindication, due to scarring of the great vessels. It is vital to have good communication with the approach surgeon who will be assisting in the case, and the ultimate decision as to whether the approach can be safely navigated should be his or hers.
Anatomic and Vascular Considerations
Discussion of Clinical Case Examples
Case 1
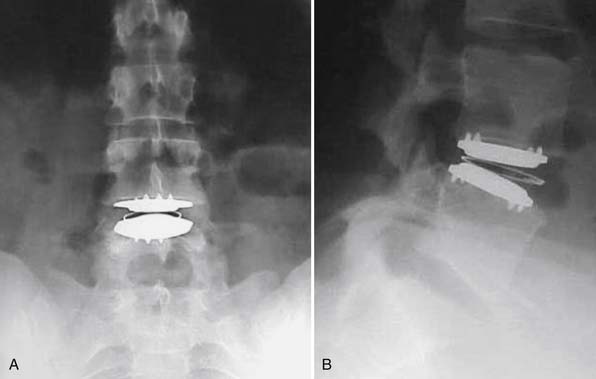
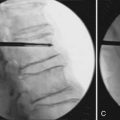
In our first case example, the patient is a middle-aged woman who has been through appropriate conservative management but has persistence of severe back pain. She has imaging studies suggestive of single-disc disease at the L4-5 level. If she is insistent upon having surgery, it is a reasonable option to discuss a one-level fusion at the suspected symptomatic L4-5 level. However, concern might exist that by fusing the L4-5 level, an increased amount of stress would be placed upon the L5-S1 level, possibly causing it to become symptomatic sooner than it otherwise would have done. She is also a very reasonable candidate to undergo total disc replacement, and given the aforementioned concern, we would likely be in favor of TDR over fusion. She ultimately decided to undergo TDR and had complete resolution of her back pain (Figure 55-5 A and B).
Case 2
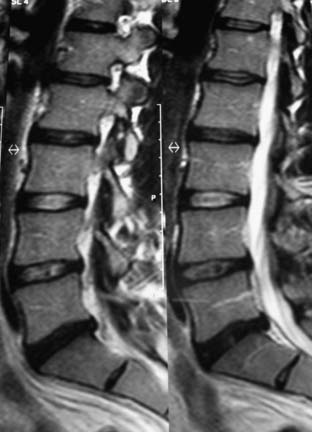
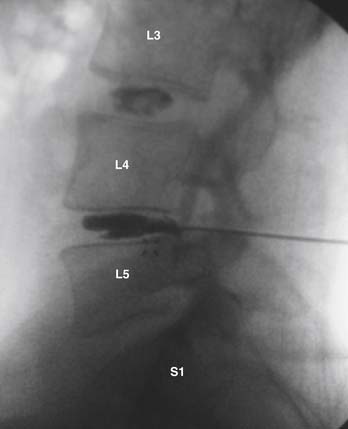
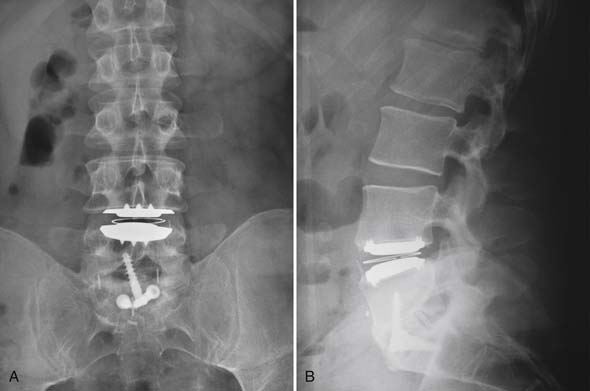
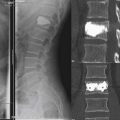
The gentleman presented in the second case represents more of a diagnostic and treatment challenge. He has two disc levels that could be symptomatic, with the L5-S1 level appearing the worst, based solely on imaging studies. However, knowing that imaging studies alone can be misleading, it would be helpful to ensure that the suspected level is truly the pain generator and determine whether the L4-5 level is also a contributing factor. We used discography to help with our decision-making (Figure 55-6). Both the L5-S1 and L4-5 levels reproduced concordant pain of 10/10 and 8/10 respectively, and the L3-4 level was completely asymptomatic. The options discussed with him included either fusion at both levels or consideration of fusion at the more caudal level and a disc replacement at the cranial level. At this point, two-level lumbar total disc replacement has not been FDA-approved. He decided to undergo a hybrid procedure, with L5-S1 fusion and an artificial disc at the L4-5 level (Figure 55-7 A and B). Within 6 weeks following surgery, he was able to discontinue use of all narcotic medications.
Psychosocial Factors
One of the strongest risk factors for poor surgical outcome relates to excessive pain sensitivity as assessed by the hysteria and hypochondriasis scales of the MMPI. Elevations in these scales have been shown to be associated with poor spine surgery outcome in numerous studies.16 Other studies have determined that patients who abuse narcotic medications and/or alcohol also have a high failure rate following spine surgery. Recently the use of PPS, specifically in lumbar TDR patients, was reported (Block et al, North American Spine Society, 2008). The authors found that the results of screening were significantly related to clinical outcome. In cases where the spine surgeon has suspicion that multiple psychosocial factors may exist and compromise outcome from the proposed surgery, it can be quite helpful to incorporate the use of PPS as part of the preoperative work-up.
Conclusions
As the population continues to age and remain active, the demand from our patients has moved toward expectations of maintaining function. Patients who are chronologically aged but remain physiologically young may be appropriate candidates for disc replacement. Age alone may not be an appropriate exclusion criterion in isolation, but should be taken in context with the many other factors we have discussed in the chapter. In other words, the “aging spine” may still be deserving of this new technology. In fact, data on patients enrolled in the IDE study of the Charité Artificial Disc were analyzed based on age, with groupings of patients aged 18 to 45 years compared with those aged 46 to 60 years.16 At 2-year follow-up, there was no significant difference between the groups with respect to changes in ODI scores, VAS scores, or SF-36 component scores compared to baseline values. Patient satisfaction was equivalent in both groups (87% and 85%, respectively), and no significant differences were noted as far as adverse events or reoperation. This reflects the fact that, given judicious application of inclusion and exclusion criteria, patients who are chronologically older can still expect equivalent outcomes to their younger counterparts. Bertagnoli et al prospectively evaluated a series of patients aged 60 years or older (range 61 to 71 years) who underwent TDR for discogenic low back pain.17 They noted statistically significant improvement in patient satisfaction and ODI scores by 3 months after surgery and maintenance of these improvements throughout the 24-month follow-up. Although the authors recommend cautious use of TDR in this population, their results suggest that if patients otherwise meet indications for TDR, with particular attention to spinal stenosis and bone quality, age greater than 60 years alone is not a factor that should preclude them from having this procedure.
1. David T. Long-term results of one-level lumbar arthroplasty: minimum 10-year follow-up of the CHARITÉ artificial disc in 106 patients. Spine. 2007;32:661-666.
2. Lemaire J.P., Carrier H., Sariali el H., Skalli W., Lavaste F. Clinical and radiological outcomes with the Charite artificial disc: a 10-year minimum follow-up. J. Spinal Disord. Tech.. 2005;18:353-359.
3. Tropiano P., Huang R.C., Girardi F.P., Cammisa F.P., Marnay T. Lumbar total disc replacement: seven to eleven-year follow-up. J. Bone Joint Surg. Am.. 2005;87:490-496.
4. Blumenthal S., McAfee P.C., Guyer R.D., Hochschuler S.H., Geisler F.H., Holt R.T., et al. A prospective, randomized, multicenter Food and Drug Administration investigational device exemptions study of lumbar total disc replacement with the CHARITÉ artificial disc versus lumbar fusion: part I: evaluation of clinical outcomes. Spine. 2005;30:1565-1575.
5. Zigler J., Delamarter R., Spivak J.M., Linovitz R.J., Danielson G.O., Haider T.T., et al. Results of the prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of the ProDisc-L total disc replacement versus circumferential fusion for the treatment of 1-level degenerative disc disease. Spine. 2007;32:1155-1162.
6. R.D. Guyer, P.C. McAfee, R.J. Banco, F.D. Bitan, A. Cappuccino, F.H. Geisler, et al., Prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of lumbar total disc replacement with the CHARITÉ Artificial Disc versus lumbar fusion: five-year follow-up, Spine J., in press.
7. Harrop J.S., Youssef J.A., Maltenfort M., Vorwald P., Jabbour P., Bono C.M., et al. Lumbar adjacent segment degeneration and disease after arthrodesis and total disc arthroplasty. Spine. 2008;33:1701-1707.
8. Boden S.D., Davis D.O., Dina T.S., Patronas N.J., Wiesel S.W. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects: a prospective investigation. J. Bone Joint Surg. Am.. 1990;72:403-408.
9. Boos N., Rieder R., Schade V., Spratt K.F., Semmer N., Aebi M: 1995 Volvo Award in clinical sciences. The diagnostic accuracy of magnetic resonance imaging, work perception, and psychosocial factors in identifying symptomatic disc herniations, Spine, 20:2613-2625, 1995.
10. Bertagnoli R., Kumar S. Indications for full prosthetic disc arthroplasty: a correlation of clinical outcome against a variety of indications. Eur. Spine J. 11 Suppl. 2002;2:S131-S136.
11. Pathria M., Sartoris D.J., Resnick D. Osteoarthritis of the facet joints: accuracy of oblique radiographic assessment. Radiology. 1987;164:227-230.
12. Fujiwara A., Tamai K., An H.S., Lim T.H., Yoshida H., Kurihashi A., et al. Orientation and osteoarthritis of the lumbar facet joint. Clin. Orthop. Relat. Res. 385. 2001:88-94.
13. Huang R.C., Lim M.R., Girardi F.P., Cammisa F.P. The prevalence of contraindications to total disc replacement in a cohort of lumbar surgical patients. Spine. 2004;29:2538-2541.
14. Wong D.A., Annesser B., Birney T., Lamond R., Kumar A., Johnson S., et al. Incidence of contraindications to total disc arthroplasty: a retrospective review of 100 consecutive fusion patients with a specific analysis of facet arthrosis. Spine J.. 2007;7:5-11.
15. Block A.R., Gatchel R.J., Deardorff W.W., Guyer R.D. The psychology of spine surgery. Washington, D.C: American Psychological Association; 2003.
16. Guyer R.D., Geisler F.H., Blumenthal S.L., McAfee P.C., Mullin B.B. Effect of age on clinical and radiographic outcomes and adverse events following 1-level lumbar arthroplasty after a minimum 2-year follow-up. J. Neurosurg. Spine. 2008;8:101-107.
17. Bertagnoli R., Yue J.J., Nanieva R., Fenk-Mayer A., Husted D.S., Shah R.V., et al. Lumbar total disc arthroplasty in patients older than 60 years of age: a prospective study of the ProDisc prosthesis with 2-year minimum follow-up period. J. Neurosurg. Spine. 2006;4:85-90.