Lower Large Airway Disease
Overview
Disorders of the lower large airways are common in the pediatric population and have the potential to be life threatening.1–3 Because these disorders are associated with nonspecific clinical symptoms, the diagnosis frequently is missed or delayed, particularly in infants and young children. After careful investigation of the clinical history and physical examination, imaging evaluation is the next management step. Imaging plays an important role in the diagnosis of congenital and acquired lower large airway disorders. By becoming familiar with the characteristic imaging findings of lower large airway disorders, radiologists can play an important role in ensuring prompt diagnosis and guiding appropriate management of these often acute and complex conditions in pediatric patients.
This chapter reviews the etiology, imaging findings, and management of the most frequently encountered congenital and acquired lower large airway disorders in the pediatric population. Large airway disorders due to primary benign neoplasms and extrinsic compression due to mediastinal vascular anomalies are not included in this chapter because they are discussed in detail in other chapters (Chapters 51 and 77, respectively) in this book.
Spectrum of Lower Large Airway Disease
Tracheobronchial Branching Anomalies
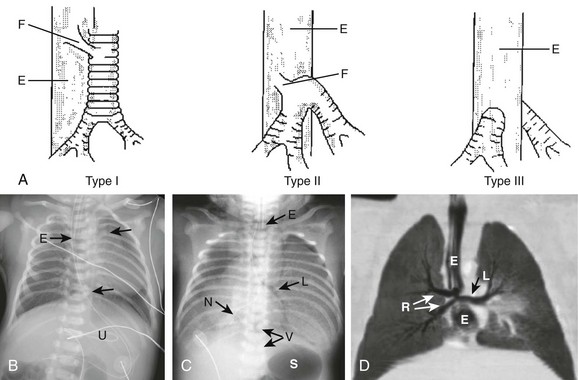
Etiology: Tracheal agenesis, which is a rare congenital anomaly of unknown etiology, is characterized by either partial or complete tracheal underdevelopment.4–6 This condition frequently is associated with maternal polyhydramnios, and a tracheoesophageal or bronchoesophageal fistula often is present concomitantly.4–7 Three main types of tracheal agenesis exist. Type 1 consists of absent upper trachea and connection of the lower trachea to the esophagus; type 2 consists of a common bronchus connecting bilateral main bronchi to the esophagus; and type 3 consists of independent bilateral main bronchi arising from the esophagus (Fig. 52-1, A). Of these three types, type 2 is the most common. Affected patients typically present with severe respiratory distress and absence of an audible cry, and the airway cannot be intubated below the larynx immediately after birth.4–7 The diagnosis of tracheal agenesis should be considered in any infant who demonstrates improved lung ventilation after placement of the endotracheal tube in the esophagus following an initial unsuccessful intubation attempt. Once tracheal agenesis is diagnosed, radiologists should look carefully for other congenital anomalies that frequently are associated with this condition, such as congenital heart disease, duodenal atresia, and radial ray anomalies.4–7
Imaging: Chest radiographic imaging findings of tracheal agenesis often are nonspecific, such as absent or decreased lung volume. However, the diagnosis of tracheal agenesis should be considered when absence of the normal tracheal air lucency, abnormal carinal position, and placement of the endotracheal tube in the esophagus are seen on chest radiographs of infants with severe respiratory distress immediately after birth (Fig. 52-1, B and C). The diagnosis is confirmed by computed tomography (CT) and/or bronchoesophagoscopy.4–8 These studies can show both the partial or complete tracheal underdevelopment and anomalous bronchi connected to the esophagus (Fig. 52-1, D).
Treatment and Follow-up: Because of difficulties related to both early diagnosis and treatment, tracheal agenesis usually is a fatal condition.4–7 The initial management of tracheal agenesis is aimed at early diagnosis at birth and immediate maintenance of airway patency, usually via the esophagus in the presence of a bronchoesophageal fistula. Although several surgical approaches have been proposed in the past, definitive treatment currently has not been established, and long-term survival of affected patients is rare. However, patients with short-segment tracheal agenesis may be amenable to direct tracheal anastomosis.5,7
Etiology: Tracheal bronchus is a congenital bronchial branching anomaly in which an ectopic (more frequently) or supernumerary bronchial branch arises from the lateral tracheal wall just above the carina.9–15 This condition also is known as bronchus suis because it is a normal finding in pigs. The incidence of tracheal bronchus in the pediatric population is between 0.1% and 5%.16 Although tracheal bronchus most frequently occurs on the right side, it also can present on the left side or bilaterally. Most patients with tracheal bronchus are asymptomatic, and it is usually an incidental finding detected on imaging studies obtained for the workup of other medical conditions. However, patients with tracheal bronchus also may present with symptoms such as persistent or recurrent upper lobe pneumonia, atelectasis, or air trapping.11 Additionally, tracheal bronchus may unexpectedly be discovered after intubation as a result of upper lobe atelectasis related to inadvertent occlusion of the ectopic upper lobe bronchial orifice by a low-lying endotracheal tube.14
Imaging: On chest radiographs, secondary imaging findings of tracheal bronchus such as upper lobe atelectasis or pneumonia can be detected, but the anomalous upper lobe bronchus cannot be reliably visualized. In the past, tracheal bronchus was evaluated with tracheobronchography. However, CT with two-dimensional (2D) and three-dimensional (3D) reconstructions is now the imaging technique of choice for evaluating anomalous tracheal bronchus and associated lung abnormalities17–19 (Fig. 52-2). Bronchoscopy can confirm the diagnosis of tracheal bronchus when necessary.
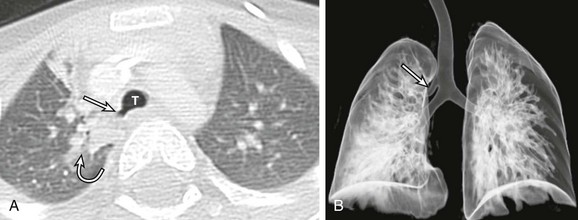
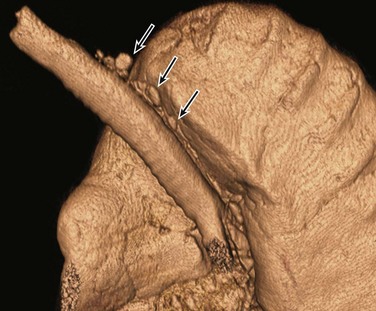
Figure 52-2 Tracheal bronchus in a 1-year-old boy with recurrent right upper lobe atelectasis.
A, An axial lung window computed tomography (CT) image shows an anomalous right upper lobe bronchus (straight arrow) directly arising from the trachea (T). Atelectasis (curved arrow) is present in the medial right upper lobe. B, A frontal three-dimensional (3D) volume-rendered image of the central airways and lungs shows an anomalous right upper lobe bronchus (arrow) directly arising from the trachea. The location, size, and course of this anomalous right upper lobe bronchus (i.e., tracheal bronchus) are better demonstrated on a 3D volume-rendered image than on an axial CT image (A).
Treatment and Follow-up: Pediatric patients with incidentally detected tracheal bronchus generally do not require any treatment. However, symptomatic children with recurrent upper lobe infection due to tracheal bronchus may require surgical resection, especially if permanent lung damage has developed or they are considered at risk for the development of permanent lung damage.1
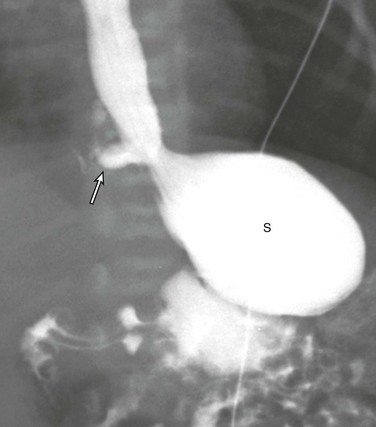
Etiology: Esophageal bronchus or lung is a rare congenital anomaly.11,20 The term “esophageal bronchus” refers to the condition in which a lobar bronchus, typically the medial basal segment of the right lower lobe, arises directly from the esophagus. The term “esophageal lung” is used when the main bronchus arises directly from the esophagus. This condition most commonly presents in infants but may be diagnosed at any age. It is associated with a wide spectrum of clinical presentations ranging from asymptomatic to recurrent severe pulmonary infections or even death depending on the size and location of the anomaly. In general, symptomatic pediatric patients with esophageal bronchus or lung typically present with feeding difficulties and recurrent respiratory tract infections. Other associated congenital anomalies include congenital heart disease, duodenal atresia, duodenal stenosis, distal tracheoesophageal fistula, and esophageal atresia.
Imaging: On chest radiographs, affected patients typically present as a result of aspiration during feeding, with air space opacification in the medial lower lobe in the case of esophageal bronchus and air space opacification that involves the entire lung in the case of esophageal lung.1,11 An esophagogram can provide a definitive diagnosis by allowing visualization of a direct communication between a bronchus and the esophagus (Fig. 52-3). CT may be helpful for evaluating associated lung parenchymal abnormalities and guiding surgery.
Congenital Tracheal Stenosis
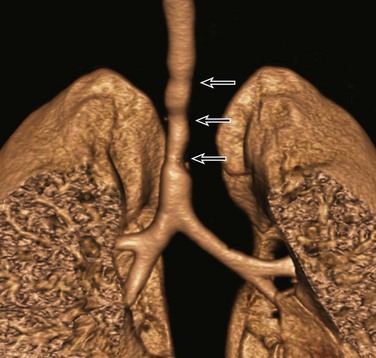
Etiology: Congenital tracheal stenosis is a rare condition characterized by intrinsic narrowing of the tracheal lumen, usually as a result of underlying complete cartilaginous rings.2,3,21,22 Such cartilaginous rings with an absent or deficient posterior membranous portion render the tracheal lumen smaller and less pliable. Affected patients present in the first year of life with expiratory stridor, wheezing, and respiratory distress.2,3,21,22 Congenital tracheal stenosis traditionally is classified into three types, including (1) focal (50%), (2) generalized (30%), and (3) funnel shaped (20%).23 Other congenital anomalies often associated with congenital tracheal stenosis are tracheoesophageal fistula, pulmonary agenesis or hypoplasia, pulmonary artery sling type 2, and bronchial stenosis.2,3,11,24
Imaging: Although neck and chest radiographs or fluoroscopy may lead to the suspicion of congenital tracheal stenosis when a narrowed trachea is encountered in pediatric patients with respiratory symptoms, CT is the imaging modality of choice for diagnosis and characterizion.2,4,24 With CT, the diagnosis of congenital tracheal stenosis is based on the identification of decreased caliber of the trachea without evidence of tracheal wall thickening. The size of the subglottic region (which does not contain tracheal cartilage) can serve as an internal reference standard. The use of 2D/3D reconstructed CT imaging is particularly helpful for increasing detection of subtle stenoses, improving measurement of craniocaudal extent of disease, and enhancing evaluation of its anatomic relationship with other mediastinal structures for preoperative assessment (Fig. 52-4).2,4,24 Virtual bronchoscopic images can confirm the diagnosis of complete rings by showing concentric rings extending along the posterior wall of the trachea.25 This appearance contrasts with the normal appearance in which the C-shaped rings do not extend to the posterior membranous wall.25 In addition, virtual bronchoscopy has the capability of evaluating the airways distal to high-grade trachea stenoses, beyond which a conventional bronchoscope cannot pass.2,4,24,25 CT also may aid in the detection of other associated anomalies that often have an abnormal lung component.
Treatment and Follow-up: Treatment of congenital tracheal stenosis mainly depends on the following three factors: (1) degree, (2) location, and (3) extent of the tracheal narrowing. A short tracheal stenosis may be treated by end-to-end anastomosis, whereas a longer lesion may require a patch or autograft repair.22 Other available options for short-segment tracheal stenosis include stent placement or balloon dilatation, although these methods are used more commonly to treat acquired tracheal stenosis.
Tracheobronchomegaly
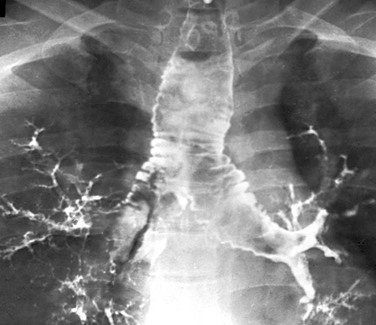
Etiology: Tracheobronchomegaly, also known as Mounier-Kuhn syndrome, is a rare disorder characterized by dilatation of the trachea and main bronchi.26–28 Although the exact etiology of this condition currently is unknown, a defect in the elastic and muscular tissues of the large airways is presumed to be a potential underlying cause. The increased compliance of the large airway walls as a result of the atrophy of longitudinal elastic fibers with thinning of the muscularis mucosa often results in the development of broad, diverticulum-like protrusions of redundant musculomembranous tissue between the cartilaginous rings. It may occur either as a familial condition or in association with a connective tissue disorder such as Ehlers-Danlos syndrome.29 It typically occurs in pediatric patients who have received prolonged ventilatory support or who have a chronic pulmonary infection such as cystic fibrosis. Although the clinical manifestations of tracheobronchomegaly are nonspecific, affected patients may present with a harsh cough, copious purulent sputum, occasional hemoptysis, and progressive dyspnea.
Imaging: Chest radiographs alone may be adequate to detect the enlargement of trachea and bronchi in severe cases (Fig. 52-5), but CT is the imaging modality of choice for diagnosing tracheobronchomegaly, potential tracheal diverticulum, and associated lung abnormalities (Fig. 52-6).1 Because of the increased incidence of tracheobronchomalacia (TBM) in patients with tracheobronchomegaly, a dynamic CT study consisting of both inspiratory and expiratory phase imaging may be beneficial for detecting concomitant TBM.
Treatment and Follow-up: Asymptomatic pediatric patients with tracheobronchomegaly require no specific treatment. For symptomatic patients, treatment usually is conservative, including chest physiotherapy for assistance with clearing of secretions and antibiotics for treatment of pulmonary infections.30,31 Unfortunately, the generalized nature of this disorder limits possible benefits from surgical management.
Acquired Abnormalities
Etiology: Foreign body aspiration into the tracheobronchial airway is a frequent cause of acute respiratory distress in pediatric patients, especially those between 6 months and 3 years of age.1–3,32,33 Each year, aspirated foreign bodies are responsible for approximately 160 deaths in children aged 14 years or younger in the United States alone, along with substantial additional morbidity.32,33 Although some pediatric patients may present with a clinical history of possible aspiration followed by cough, wheezing, respiratory distress, or decreased breath sounds, most affected pediatric patients present with a history that is either lacking or misleading.1–3,32,33 Therefore a high clinical suspicion and thorough investigation are required for any infants and young children with respiratory symptoms suspicious for possible foreign body aspiration.
Only approximately 10% of aspirated foreign bodies within the tracheobronchial airway are radiopaque.34 The remaining 90% of nonradiopaque foreign bodies are particularly difficult to diagnose early in pediatric patients. Nearly 70% of aspirated foreign bodies lodge in the bronchi, with the right side (52%) affected more frequently than the left side (18%).32 The remaining 30% of aspirated foreign bodies lodge in the trachea (13%) and less common locations (17%).32 In the early phase of foreign body aspiration, affected patients typically present with cough, wheezing, respiratory distress, or decreased breath sounds. During the late phase of missed foreign body aspiration, affected patients often present with episodic wheezing and/or recurrent pneumonias.
Imaging: Radiographic findings of foreign body aspiration depend on the size, location, duration, and nature of the aspirated foreign body (Fig. 52-7). Radiopaque foreign bodies usually are detected easily with radiographic studies, which should include frontal and lateral films encompassing the upper airway from the nasopharynx to the upper abdomen. When the foreign body is not radiopaque, careful inspection of the tracheobronchial airway with high-kilovoltage films or fluoroscopy may show a faintly visible opacity interrupting the air column within the large airways. If the foreign object is located in the trachea, the chest radiograph may be normal or may show bilateral hypoinflation or hyperinflation depending on the degree of obstruction. Many intratracheal foreign bodies escape detection without the use of CT.
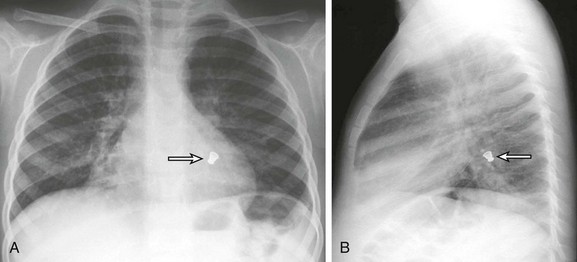
Figure 52-7 Radiopaque foreign body aspiration in a 4-year-old boy who presented with acute onset of coughing and respiratory distress.
After a chest radiograph was performed, the patient underwent bronchoscopy, which showed a metallic bottle cap lodged in the left lower lobe bronchus. A, A frontal chest radiograph shows a radiopaque foreign body (arrow) located in the left lower lobe, retrocardiac region. B, A lateral chest radiograph confirms the location of the foreign body (arrow) in a left lower lobe bronchus. (From Lee EY, Restrepo R, Dillman JR, et al. Imaging evaluation of pediatric trachea and bronchi: systematic review and updates, Semin Roentgenol. 47(2):183, 2012.)
Rather than lodging in the trachea, most foreign bodies lodge in the main bronchi. In approximately 20% of cases, the foreign body migrates into a segmental bronchial branch.32 The chest radiograph may show a variety of findings, the most common of which is a unilateral hyperlucent lung. If the bronchial obstruction becomes more complete, postobstructive atelectasis, pneumonia, or bronchiectasis may develop. A chest radiograph obtained at full inspiration can appear normal in approximately 20% to 30% of patients with bronchial foreign bodies; close inspection may show relatively increased volume on the normal side with a slight mediastinal shift toward the partially obstructed side. If a foreign body is suspected clinically, an expiratory chest radiograph always should be obtained, because it is critical for diagnosis. Lateral decubitus films may be diagnostic if satisfactory inspiration-expiration chest radiographs cannot be obtained. When air trapping is present in a dependent lung on the decubitus view, the affected lobe or segment tends to remain hyperlucent rather than deflating, as would normally occur (Fig. 52-8). Fluoroscopic examination of the chest also is valuable for detecting air trapping. It can show inspiratory mediastinal shift toward the affected side and restricted diaphragmatic excursion on the affected side.
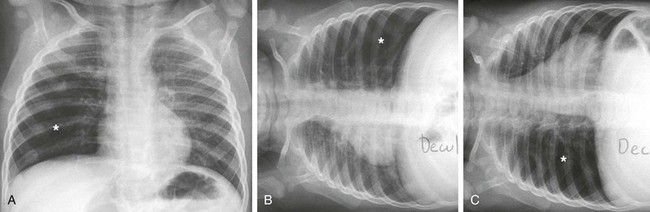
Figure 52-8 Nonradiopaque foreign body aspiration in a 5-year-old girl who presented with persistent coughing and respiratory distress while eating popcorn.
A, A frontal chest radiograph obtained at end-inspiration shows mild hyperinflation (asterisk) of the right lower lobe. B, A frontal chest radiograph obtained at end-inspiration in the left lateral decubitus position demonstrates normal volume loss in the left lung and mild hyperinflation (asterisk) of the right lower lobe. C, A frontal chest radiograph obtained at end-inspiration in the right lateral decubitus position shows persistent hyperinflation (asterisk) of the right lower lobe.
The sensitivity and specificity of chest radiographs for foreign body detection were only 74% and 45%, respectively, in a series of 93 patients examined by Silva et al35 and 68% and 67%, respectively, in a series of 83 patients examined by Svedstrom et al.36 Because of this relatively poor accuracy, Silva and colleagues have suggested that chest radiographs should not be relied upon for diagnosis, but rather that all patients with suspected foreign body aspiration should undergo bronchoscopy.35 This approach results in a false-negative bronchoscopy rate of at least 20% in most series, however.35
CT is the most sensitive diagnostic imaging technique, but in general it should be reserved for patients for whom chest radiography is either normal or nonspecific. With use of CT, the diagnosis of a foreign body can be established with nearly 100% accuracy.2 Either the foreign body is visualized or a focal pulmonary abnormality such as postobstructive air trapping, atelectasis, or consolidation is seen. If none of these findings is present, it is extremely unlikely that a foreign body is present. However, CT is less likely to visualize the foreign body directly if the lung is consolidated unless the foreign body is calcified or opaque.
Preliminary studies of low-dose multidetector computed tomography (MDCT) virtual bronchoscopy suggest that it may play a potential role in children with suspected foreign body aspiration by (1) identifying the precise location of a foreign body prior to bronchoscopy and (2) excluding a foreign body in children with a low level of suspicion and normal or nonspecific findings on chest radiography.36–39 Because CT is less expensive and less invasive than bronchoscopy, it may be a viable alternative for selected patients.
Treatment and Follow-up: Once an aspirated foreign body is diagnosed on imaging studies, bronchoscopy and removal of the aspirated foreign body should be performed. Although imaging findings may be nonspecific or normal, symptomatic pediatric patients with high clinical suspicion and a convincing history of possible foreign body aspiration require bronchoscopy for a complete assessment. CT after bronchoscopy also is valuable because it provides additional diagnostic information regarding the presence and pattern of bronchial obstruction in symptomatic children with suspected residual foreign body.34
Infection/Inflammation
Etiology: Tuberculosis (TB) is caused by Mycobacterium tuberculosis. Although advances in diagnosis and treatment have been made in the past two decades, TB continues to be a major cause of morbidity and mortality, particularly in infants, elderly persons, and immunocompromised patients.40,41 In developed countries, TB most commonly is transmitted to infants and children by an adult family member infected with active TB. Although most pediatric patients who have primary TB are asymptomatic, some patients may present with nonspecific symptoms such as a mild cough, a low-grade fever, weight loss, fatigue, and malaise. Respiratory distress may be the primary symptom in pediatric patients with large airway involvement from TB infection. The diagnosis usually can be made by TB skin testing, whereas sputum and gastric aspirates are confirmatory in anergic patients.
Imaging: Large airway involvement of TB infection mainly results from either extrinsic compression of airways by enlarged mediastinal and/or hilar infectious lymph nodes or direct infection of the airway wall through peribronchial lymphatic pathways.1–3,40–43 Although chest radiographs may show mediastinal and/or hilar lymphadenopathy with resultant large airway narrowing, the location, degree, and extent can be better evaluated with CT with 2D/3D reconstructions (Fig. 52-9).1–3 Direct tracheobronchial infection from TB eventually may result in irreversible large airway stricture/stenosis.1–3,40–43 Such narrowing of the large airway is best assessed with CT with 2D/3D reconstructions. Additionally, CT also can show lung abnormalities associated with TB infection such as “tree-in-bud” nodular opacities and airspace consolidation.
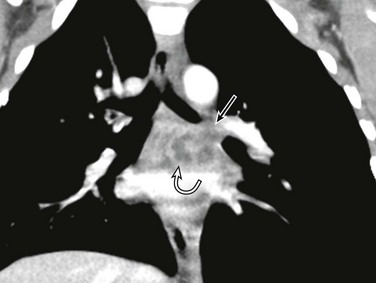
Figure 52-9 Left main bronchial obstruction due to subcarinal lymphadenopathy from tuberculosis infection in a 4-year-old girl.
A coronal enhanced computed tomography image shows left main stem bronchial obstruction (straight arrow) as a result of the necrotic subcarinal lymphadenopathy (curved arrow).
Treatment and Follow-up: Pediatric patients affected with TB currently are treated with 6 months of a combination of antibiotics containing rifampin along with isoniazid, pyrazinamide, and ethambutol for the first 2 months, followed by isoniazid alone for the following 4 months. Large airway narrowing due to extrinsic compression from the enlarged mediastinal lymph nodes often resolves after medical treatment, whereas irreversible stricture/stenosis from direct tracheobronchial TB infection may require surgical management, including primary end-to-end anastomosis after stricture resection.
Etiology: Histoplasmosis is caused by the dimorphic fungus Histoplasma capsulatum. Although H. capsulatum is found throughout the world, it is endemic in certain areas, including states bordering the Ohio River valley, the lower Mississippi River, and caves in southern and East Africa. Although acute histoplasmosis is attributed to airborne primary infection, chronic progressive histoplasmosis is the consequence of reactivation of a prior infection. Large airway involvement from histoplasmosis often results from fibrosing mediastinitis.44–46 Affected patients may present with respiratory or esophageal symptoms related to complete or partial obstruction of large airways and the esophagus.45,46 The definitive diagnosis of histoplasmosis is made by finding the fungus in samples taken from sputum, blood, or infected organs. It also can be diagnosed by identifying antigens in blood or urine samples by enzyme-linked immunosorbent assay polymerase chain reaction protocol.
Imaging: On chest radiographs, typical imaging findings of pediatric patients with histoplasmosis infection include multiple, ill-defined pulmonary nodules (1 to 3 cm in diameter) and hilar and/or mediastinal lymphadenopathy, both of which often are calcified.44 CT currently is the imaging modality of choice for evaluating large airway compromise, particularly related to underlying fibrosing mediastinitis.1–3 These patients typically show heterogeneous and often calcified mediastinal soft tissue densities resulting in complete or partial obstruction of mediastinal structures such as the tracheobronchial airway, superior vena cava, and esophagus (Fig. 52-10).1–3,45,46 CT also can demonstrate abnormal lung findings from histoplasmosis infection.
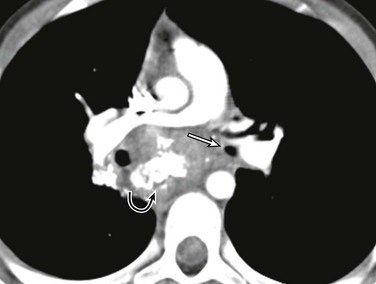
Figure 52-10 Fibrosing mediastinitis caused by histoplasmosis in a 9-year-old girl who presented with one month of cough and dyspnea.
An axial contrast-enhanced computed tomography image shows narrowing of the left main stem bronchus (straight arrow) from a heterogeneously enhancing and partially calcified subcarinal soft tissue mass (curved arrow). (From Lee EY, Greenberg SB, Boiselle PM. Multidetector computed tomography of pediatric large airway diseases: state-of-the-art, Radiol Clin North Am. 49[5]:886, 2011.)
Treatment and Follow-up: In severe cases, antifungal medications such as itraconazole and amphotericin B are used to treat acute, chronic, and disseminated histoplasmosis infection. Unfortunately, no pharmacologic treatment has been shown to affect the outcome of fibrosing mediastinitis related to histoplasmosis infection. Large airway occlusion due to fibrosing mediastinitis is particularly difficult to treat because surgery often is ineffective and placement of metallic airway stents is avoided because of recurrent obstruction from ingrowth of granulation tissue.
Neoplasm
Tracheobronchial tumors are rare in children. Two of the most common primary benign large airway neoplasms, subglottic hemangioma and recurrent respiratory papillomatosis, were discussed in Chapter 51. The most common malignant lower large airway primary neoplasm, carcinoid tumor, is discussed in the next section.
Etiology: A carcinoid tumor is a neuroendocrine neoplasm that encompasses a spectrum of histology ranging from slow-growing, locally infiltrative lesions to a metastasizing neoplasm.47–52 Carcinoid tumors traditionally have been classified into two types based on typical or atypical histologic findings. Affected patients typically present with a cough, wheezing, and recurrent pneumonia as a result of airway obstruction. Because of the underlying hypervascularity of carcinoid tumors, hemoptysis may be the presenting symptom. The carcinoid syndrome rarely occurs in pediatric patients with a large airway carcinoid tumor. The diagnosis is confirmed by bronchoscopy and biopsy.
Imaging: Imaging findings of a carcinoid tumor within the large airway depend on the size and location of the tumor.47–52 Most tracheobronchial carcinoid tumors are intrabronchial (Fig. 52-11). Centrally located carcinoid tumors may mimic foreign bodies and may cause postobstructive air trapping, atelectasis, recurrent infection, abscess, or bronchiectasis.51 Peripherally located carcinoid tumors usually are not associated with bronchial obstruction and have the appearance of a pulmonary nodule.51 Chest radiographs may be normal in 10% of cases or may show either a well-defined hilar or perihilar opacity representing an underlying tumor or secondary signs such as focal air trapping or consolidation.47–52 CT characteristically shows an enhancing spherical or ovoid mass with a well-defined lobulated contour.51,52 Although calcification may not be clearly visible on chest radiographs, either punctate or diffuse calcifications may be detected in up to 30% of cases on CT.52 The tumor may recur locally after resection, but distant metastases are rare.
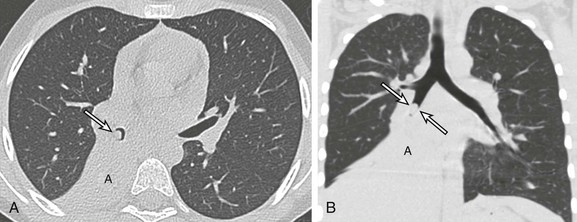
Figure 52-11 An endobronchial carcinoid tumor in a 17-year-old girl who presented with a cough and recurrent right lower lobe pneumonia for the past 18 months.
Surgical pathology confirmed the diagnosis of an endobronchial carcinoid tumor. A, An axial bone window computed tomography (CT) image shows an endobronchial mass (arrow) located within the right bronchus intermedius with postobstructive atelectasis (A). B, A coronal lung window CT image demonstrates an endobronchial mass (arrows) in the right bronchus intermedius. The overall shape, size, and location of the mass is better visualized on this coronal multiplanar reformatted CT image than on the axial CT image (A). Again, postobstructive atelectasis is seen (A).
Trauma
Acquired Tracheobronchial Stenosis:
Etiology: Acquired tracheobronchial stenosis in children usually is caused by previous instrumentation such as endotracheal intubation or use of a tracheostomy tube.1–3 Granulation tissue and fibrosis can develop at the stoma, at the tip of the tube, or at the site of the cuff. Scott and Kramer53 reported tracheostomy-related complications in 26% of intubated children. The duration of intubation is a major factor in determining the incidence and severity of complications. Microscopic lesions occur after approximately 48 hours of intubation. Epithelial metaplasia is seen in children intubated for longer than 7 days, although occasionally granuloma may develop after very brief periods of intubation. Bronchial stenosis, like tracheal stenosis, usually is acquired and occurs at the site of surgical anastomosis in pediatric patients after lung transplantation.1–3
Imaging: The diagnosis of tracheobronchial stenosis may be suspected on chest radiographs when large airway narrowing is observed. However, CT, which can measure cross-sectional areas of the large airways accurately, is the current choice for evaluating tracheobronchial stenosis, especially preoperatively.1–3 Such stenosis may be weblike or fusiform, or it may have an irregular shape (Fig. 52-12).1–3,54 Newer techniques such as paired inspiratory and expiratory CT or real-time dynamic four-dimensional CT can help differentiate a fixed tracheobronchial stenosis from TBM.54–59 In some patients, these conditions may coexist.
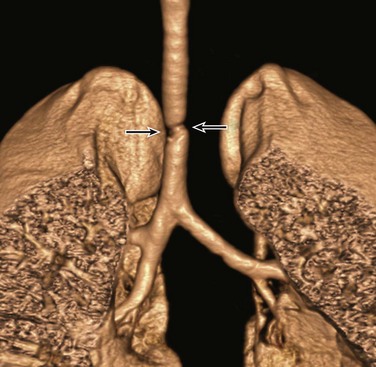
Figure 52-12 Acquired tracheobronchial stenosis in a 4-year-old girl with progressively worsening stridor after long-term endotracheal tube placement.
A frontal three-dimensional volume-rendered image of the central airways and lungs shows a focal irregular narrowing (arrows) at the level of the previous endotracheal tube placement.
Etiology: Tracheobronchial injury is a potentially life-threatening condition that may be caused by either penetrating or blunt chest trauma.60–66 Although tracheobronchial injury is relatively rare with a reported incidence between 0.7% and 2.9%, it is associated with a substantial mortality of 30%.60–71 Tracheobronchial injury typically occurs within 2.5 cm of the carina.60–69 In the setting of trauma, abnormal endotracheal tube position, rib fractures (particularly involving the anterior ends of the first three ribs), and persistent pneumothoraces and/or pneumomediastinum despite the presence of a well-functioning chest tube and/or mediastinal tube should raise the possibility of underlying tracheobronchial injury.70,71
Imaging: Radiographic imaging findings of a traumatic tracheobronchial injury depend on the location and degree of injury. In the setting of mild injury, a small amount of pneumothorax and/or pneumomediastinum may be the only findings. Such nonspecific and subtle radiographic findings often delay the accurate diagnosis of tracheobronchial injury, particularly in pediatric patients.60,61,66 Extensive pneumothorax and/or pneumomediastinum, often extending into subcutaneous tissues of the neck and chest wall, typically are seen with severe injuries such as a displaced laceration or transection.60–71 When the collapsed lung is observed in a dependent position, hanging on the hilum only by its vascular attachments (the “fallen lung sign”), a complete transaction or rupture of the main stem bronchus should be considered.1 MDCT with thin-section axial and 2D/3D reconstruction can provide accurate diagnosis and preoperative guidance in these patients (Fig. 52-13).1,68,70,71
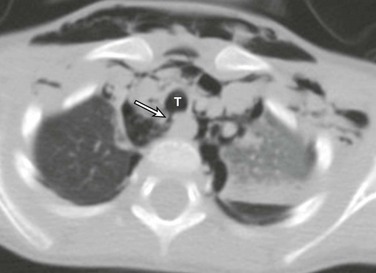
Figure 52-13 Traumatic tracheal injury in a 3-year-old boy as a result of a motor vehicle accident.
An axial computed tomography image shows a focal disruption (arrow) of the posterior wall of the trachea at the 7 o’clock position, with an adjacent collection of air within the mediastinum, indicating tracheal rupture. Extensive pneumomediastinum and subcutaneous emphysema also are seen. T, Trachea.
Dynamic Tracheobronchial Disorder
Etiology: TBM is attributed to an abnormal weakness of the underlying airway walls and/or supporting cartilage.57,58,72 Despite increasing recognition of this condition in recent years, diagnosing TBM continues to be challenging mainly because clinical symptoms of affected patients are nonspecific and overlap with those of other chronic respiratory disorders.57,58,72 TBM can be divided into two types: primary and secondary.58,72 Primary TBM often is seen in premature infants or children with a variety of syndromes and in persons with systemic disease affecting cartilage, such as Larsen syndrome and relapsing polychondritis.58,72 Secondary TBM is seen in infants or children with tracheoesophageal fistula, extrinsic pressure by vessels, and mediastinal masses.58,72 It also may arise as a result of tracheal injury, most commonly intubation. Affected patients typically present with expiratory stridor and a cough that often is described as barking or brassy. Undiagnosed TBM may result in chronic tracheobronchial and lung infections.
Imaging: Chest radiographs and airway fluoroscopy have been used to evaluate TBM in pediatric patients in the past.1 Fluoroscopy may show an exaggerated decrease (>50%) in the caliber of the trachea during expiration in patients with tracheomalacia1,2 (Fig. 52-14); however, evaluation of the bronchi is markedly limited with this technique. MDCT has become the imaging modality of choice for a complete assessment of TBM and underlying causes in pediatric populations.55–59 MDCT provides noninvasive evaluation of TBM with diagnostic accuracy that is similar to the historical reference standard of bronchoscopy, and 2D and 3D evaluation with MDCT in particular has become an important preoperative assessment of TBM because it offers information regarding the precise location, accurate degree and extent, and underlying predisposing conditions of TBM.57,58,72 For the diagnosis of TBM with MDCT in children, tracheobronchial collapse >50% currently is used (Fig. 52-15).57,58 The very large detector array CT scanners that have become available recently allow real-time evaluation of large airway collapsibility even in nonsedated infants and young children, which is a promising technique.1,2
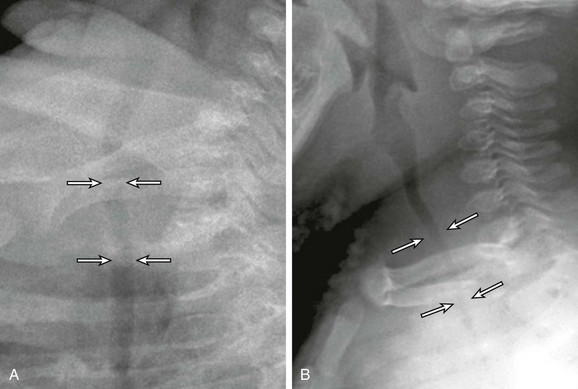
Figure 52-14 Tracheomalacia in a 2-year-old girl who presented with a chronic cough and recurrent pulmonary infections.
A, A lateral radiograph obtained at end-inspiration during airway fluoroscopy study shows a patent trachea (arrows). B, A lateral radiograph obtained at end-expiration during airway fluoroscopy study demonstrates marked (>75%) collapse of the trachea (arrows), consistent with tracheomalacia.
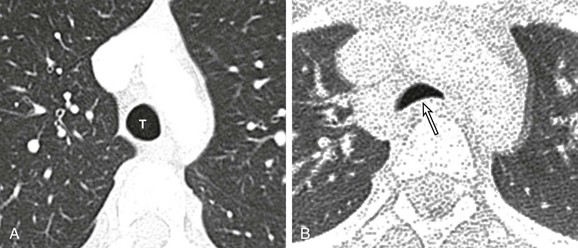
Figure 52-15 Tracheomalacia in a 15-year-old girl who presented with a chronic cough and recurrent pulmonary infections.
Bronchoscopy showed marked tracheomalacia. A, An axial computed tomography (CT) image obtained at end inspiration shows a normal patent trachea (T). B, An axial CT image obtained at end expiration demonstrates a collapse of the trachea (arrow) >50%, which is consistent with tracheomalacia. Increased attenuation of the lungs is a result of decreased lung volume related to end expiration.
Treatment and Follow-up: Conservative therapies such as treatment of underlying respiratory infections, humidified oxygen therapy, and pulmonary physiotherapy are the mainstay of management in pediatric patients with a mild to moderate degree of TBM.72–74 However, pediatric patients with severe and progressively worsening TBM may require more aggressive treatment including tracheostomy placement, stent placement, and surgical intervention such as a tracheoplasty.75–86
Carden, KA, Boisell, PM, Waltz, DA, et al. Tracheomalacia and tracheobronchomalacia in children and adults: an in-depth review. Chest. 2005;127:987–1005.
Lee, EY, Restrepo, R, Dillman, JR, et al. Imaging evaluation of pediatric trachea and bronchi: systematic review and updates. Semin Roentgenol. 2012;47(2):182–196.
Lee, EY, Greenberg, SB, Boiselle, PM. Multidetector computed tomography of pediatric large airway disease: state-of-the-art. Radiol Clin North Am. 2011;49:869–893.
Lee, EY, Siegel, MR. MDCT of tracheobronchial narrowing in pediatric patients. J Thorac Imaging. 2007;22:300–309.
Shin, SM, Kim, WS, Cheon, JE, et al. CT in children with suspected residual foreign body in airway after bronchoscopy. Am J Roentgenol. 2009;192:1744–1751.
References
1. Lee, EY, Restrepo, R, Dillman, JR, et al. Imaging evaluation of pediatric trachea and bronchi: systematic review and updates. Semin Roentgenol. 2012;47(2):182–196.
2. Lee, EY, Greenberg, SB, Boiselle, PM. Multidetector computed tomography of pediatric large airway disease: state-of-the-art. Radiol Clin North Am. 2011;49:869–893.
3. Lee, EY, Siegel, MR. MDCT of tracheobronchial narrowing in pediatric patients. J Thorac Imaging. 2007;22:300–309.
4. de Groot-van der Mooren, MD, Haak, MC, Lakeman, P, et al. Tracheal agenesis: approach towards this severe diagnosis. Case report and review of the literature. Eur J Pediatr. 2012;17(3):425–431.
5. Kanojia, RP, Parikh, M, Bhagat, H, et al. Tracheal agenesis: case report with review of the current scenario and management reported over the past two decades. Eur J Pediatr Surg. 2010;20(1):55–57.
6. Saccardi, C, Ludwig, K, Cosmi, E, et al. Tracheal agenesis with bifurcating common airway arising from midesophagus. Pediatr Dev Pathol. 2010;13(3):252–254.
7. Fuchimoto, Y, Mori, M, Takasato, F, et al. A long-term survival case of tracheal agenesis: management for tracheoesophageal fistula and esophageal reconstruction. Pediatr Surg Int. 2011;27(1):103–106.
8. Panthagani, ID, Santos, MC, D’Angio, CT. Use of computed tomography to categorize the type of tracheal agenesis. J Pediatr Surg. 2009;44(5):1044–1046.
9. Manjunatha, YC, Gupta, AK. Tracheal bronchus (pig bronchus). Indian J Pediatr. 2010;77(9):1037–1038.
10. Panigada, S, Sacco, O, Girosi, D, et al. Recurrent severe lower respiratory tract infections in a child with abnormal tracheal morphology. Pediatr Pulmonol. 2009;44(2):192–194.
11. Berrocal, T, Madrid, C, Novo, S, et al. Congenital anomalies of the tracheobronchial tree, lung, and mediastinum: embryology, radiology, and pathology. Radiographics. 2004;24(1):e17.
12. Bennett, EC, Holinger, LD. Congenital malformations of the trachea and bronchi. In Bluestone CD, Stool SE, eds.: Pediatric otolaryngology, 4th ed, Philadelphia: Saunders, 2002.
13. Ghaye, B, Szapiro, D, Fanchamps, JM, et al. Congenital bronchial anomalies revisited. Radiographics. 2001;21:105–119.
14. O’Sullivan, BP, Frassica, JJ, Rayder, SM. Tracheal bronchus: a cause of prolonged atelectasis in intubated children. Chest. 1998;113:537–540.
15. Doolittle, AM, Mair, EA. Tracheal bronchus: classification, endoscopic analysis, and airway management. Otolaryngol Head Neck Surg. 2002;126(3):240–243.
16. Landing, BH, Dixon, LG. Congenital malformation and genetic disorders of the respiratory tract. Am Rev Respir Dis. 1979;120:151–185.
17. Yasuda, M, Nagashima, A, Ichiki, Y, et al. Operation for trachea bronchus: 3-dimensional reconstruction imaging. Ann Thorac Surg. 2011;92(6):e127.
18. Akiba, T, Marushima, H, Takagi, M, et al. Preoperative evaluation of a tracheal bronchus by three-dimensional 64-row multidetector-row computed tomography (MDCT) bronchography and angiography: report of a case. Surg Today. 2008;38(9):841–843.
19. Ming, Z, Lin, Z. Evaluation of tracheal bronchus in Chinese children using multidetector CT. Pediatr Radiol. 2007;37(12):1230–1234.
20. Sugandhi, N, Sharma, P, Agarwala, S, et al. Esophageal lung: presentation, management, and review of literature. J Pediatr Surg. 2011;46:1634–1637.
21. Webb, WR. The trachea. In Webb WR, Higgins CB, eds.: Thoracic imaging, 1st ed, Philadelphia: Lippincott Williams and Wilkins, 2005.
22. Laing, MR, Albert, DM, Quinney, RE, et al. Tracheal stenosis in infants and young children. J Laryngol Otol. 1990;104:229–235.
23. Beasley, SW, Qui, BQ. Understanding tracheomalacia. J Paediatr Child Health. 34, 1998. [209-201].
24. Lee, EY, Boiselle, PM, Shamberger, RC. Multidetector computed tomography and 3-dimensional imaging: preoperative evaluation of thoracic vascular and tracheobronchial anomalies and abnormalities in pediatric patients. J Pediatr Surg. 2010;45(4):811–821.
25. Boiselle, PM, Ernst, A, DeCamp, MM. CT diagnosis of complete tracheal rings in an adult. J Thorac Imaging. 2007;22(2):169–171.
26. Karsh, S, Mahboubi, S. Tracheomegaly in children. Clin Imaging. 1989;13(1):77–81.
27. Menon, B, Malik, A, Chugh, A, et al. Radiological appearances in a rare case of tracheomegaly, tracheal diverticulosis, bronchomegaly and bronchiectasis. Indian J Chest Dis Allied Sci. 2005;47(1):39–41.
28. Woodring, JH, Barrett, PA, Rehm, SR, et al. Acquired tracheomegaly in adults as a complication of diffuse pulmonary fibrosis. AJR Am J Roentgenol. 1989;152(4):743–747.
29. Ayres, JG, Pope, FM, Reidy, JF, et al. Abnormalities of the lungs and thoracic cage in the Ehlers-Danlos syndrome. Thorax. 1985;40:300–305.
30. Schoor, JV, Joos, G, Pauwels, R. Tracheobronchomegaly: the Mounier-Kuhn syndrome: report of two cases and review of the literature. Eur Respir J. 1991;4:1301–1306.
31. Guest, JL, Anderson, JN. Tracheobronchomegaly (Mounier-Kuhn syndrome). JAMA. 1977;238:1754–1755.
32. Eren, S, Balci, AE, Dikici, B, et al. Foreign body aspiration in children: experience of 1160 cases. Ann Trop Paediatr. 2003;1:31–37.
33. Centers for Disease Control and Prevention. Nonfatal choking-related episodes among children—United States, 2001. MMWR Morb Mortal Wkly Rep. 2002;51:945–948.
34. Shin, SM, Kim, WS, Cheon, JE, et al. CT in children with suspected residual foreign body in airway after bronchoscopy. AJR Am J Roentgenol. 2009;192:1744–1751.
35. Silva, AB, Muntz, HR, Clary, R. Utility of conventional radiography in the diagnosis and management of pediatric airway foreign bodies. Ann Otol Rhinol Laryngol. 1998;107:834–838.
36. Svedstrom, E, Puhakka, H, Kero, P. How accurate is chest radiography in the diagnosis of tracheobronchial foreign bodies in children? Pediatr Radiol. 1989;19:520–522.
37. Sodhi, KS, Aiyappan, SK, Saxena, AK, et al. Utility of multidetector CT and virtual bronchoscopy in tracheobronchial obstruction in children. Acta Paediatr. 2010;99(7):1011–1015.
38. Adaletli, I, Kurugolglu, S, Ulus, S, et al. Utility of low-dose multidetector CT and virtual bronchoscopy in children with suspected foreign body aspiration. Pediatr Radiol. 2007;37(1):33–40.
39. Kosucu, P, Ahmetoglu, A, Koramaz, I, et al. Low-dose MDCT and virtual bronchoscopy in pediatric patients with foreign body aspiration. AJR Am J Roentgenol. 2004;183(6):1771–1777.
40. Marquez, L, Starke, JR. Diagnosis and management of TB in children: an update. Expert Rev Anti Infect Ther. 2011;9:1157–1168.
41. Cruz, AT, Starke, JR. A current review of infection control for childhood tuberculosis. Tuberculosis (Edinb). 2011;91(suppl 1):S11–S15.
42. Leung, AN, Muller, NL, Pineda, PR, et al. Primary tuberculosis in childhood: radiographic manifestations. Radiology. 1992;182:87–91.
43. Weber, AL, Bird, KT, Janower, ML. Primary tuberculosis in childhood with particular emphasis on changes affecting the tracheobronchial tree. AJR Am J Roentgenol. 1968;103:123–132.
44. Fischer, GB, Mocelin, H, Severo, CB, et al. Histoplasmosis in children. Paediatr Respir Rev. 2009;10:172–177.
45. Rossi, SE, McAdams, HP, Rosado-de-Christenson, ML, et al. Fibrosing mediastinitis. Radiographics. 2001;21:737–757.
46. Sherrick, AD, Brown, LR, Harms, GF, et al. The radiographic findings of fibrosing mediastinitis. Chest. 1994;106(2):484–489.
47. Bellah, RD, Mahboubi, S, Berdon, WE. Malignant endobronchial lesions of adolescence. Pediatr Radiol. 1992;22:563–567.
48. Chong, S, Lee, KS, Chung, MJ, et al. Neuroendocrine tumors of the lung: clinical, pathologic, and imaging findings. Radiographics. 2006;26:41–57.
49. Connor, GF, Fishman, EK. Endobronchial carcinoid in a child: depiction with three-dimensional volume rendering. Pediatr Radiol. 2004;34:1008–1011.
50. Parson, RB, Milestone, BN, Adler, LP. Radiographic assessment of airway tumors. Chest Surg Clin N Am. 2003;13:63–77.
51. Yikilmaz, A, Lee, EY. CT imaging of mass-like nonvascular pulmonary lesions in children. Pediatr Radiol. 2007;37:1253–1263.
52. Magid, D, Siegelman, SS, Eggleston, JC, et al. Pulmonary carcinoid tumors: CT assessment. J Comput Assist Tomogr. 1989;13:244–247.
53. Scott, JR, Kramer, SS. Pediatric tracheostomy. II. Radiographic features of difficult decannulations. AJR Am J Roentgenol. 1978;130(5):893–898.
54. Lee, EY. Advancing CT and MR imaging of the lungs and airways in children: imaging into practice. Pediatr Radiol. 2008;38(suppl 2):S208–S212.
55. Lee, EY, Mason, KP, Zurakowski, D, et al. MDCT assessment of tracheomalacia in symptomatic infants with mediastinal aortic vascular anomalies: preliminary technical experience. Pediatr Radiol. 2008;38(1):82–88.
56. Lee, EY, Zurakowski, D, Waltz, DA, et al. MDCT evaluation of the prevalence of tracheomalacia in children with mediastinal aortic vascular anomalies. J Thorac Imaging. 2008;23(4):248–265.
57. Lee, EY, Litmanovich, D, Boiselle, PM. Multidetector CT evaluation of tracheobronchomalacia. Radiol Clin North Am. 2009;47(2):261–269.
58. Lee, EY, Boiselle, PM. Tracheobronchomalacia in infants and children: multidetector CT evaluation. Radiology. 2009;252(1):7–22.
59. Ridge, CA, O’Donnell, CR, Lee, EY, et al. Tracheobronchomalacia: current concepts and controversies. J Thorac Imaging. 2011;26(4):278–289.
60. Ozdulger, A, Cetin, G, Erkmen Gulhan, S, et al. A review of 24 patients with bronchial ruptures: is delay in diagnosis more common in children? Eur J Cardiothorac Surg. 2003;23:379–383.
61. Nakayama, DK, Ramenofsky, ML, Rowe, MI. Chest injuries in childhood. Ann Surg. 1989;201:770–775.
62. Jackimczyk, K. Blunt chest trauma. Emerg Med Clin North Am. 1993;11:81–96.
63. Harvey-Smith, W, Bush, W, Northrop, C. Traumatic bronchial rupture. AJR Am J Roentgenol. 1980;134:1189–1193.
64. Ein, SH, Friedberg, J, Shandling, B, et al. Traumatic bronchial injuries in children. Pediatr Pulmonol. 1986;2:60–64.
65. Mahboubi, S, O’Hara, AE. Bronchial rupture in children following blunt chest trauma. Report of five cases with emphasis on radiologic findings. Pediatr Radiol. 1981;10:133–138.
66. Hrkac Pustahija, A, Vukelic Markovic, M, Ivanac, G, et al. An unusual case of bronchial rupture—pneumomediastinum appearing 7 days after blunt chest trauma. Emerg Radiol. 2009;16:163–165.
67. Scaglione, M, Romano, S, Pinto, A, et al. Acute tracheobronchial injuries: impact of imaging in diagnosis and management implications. Eur J Radiol. 2006;59:336–343.
68. Le Guen, M, Beigelman, C, Bouhemad, B, et al. Chest computed tomography with multiplanar reformatted images for diagnosing traumatic bronchial rupture: a case report. Crit Care. 2007;11:R94.
69. Wan, YL, Tsai, KT, Yeow, KM, et al. CT findings of bronchial transaction. Am J Emerg Med. 1997;15:176–177.
70. Moore, MA, Wallace, EC, Westra, SJ. Chest trauma in children: current imaging guidelines and techniques. Radiol Clin North Am. 2011;49(5):949–968.
71. Hammer, MR, Dillman, JR, Chong, ST, et al. Imaging of pediatric thoracic trauma. Semin Roentgenol. 2012;47(2):135–146.
72. Carden, KA, Boiselle, PM, Waltz, DA, et al. Tracheomalacia and tracheobronchomalacia in children and adults: an in-depth review. Chest. 2005;127(3):984–1005.
73. Greenholz, SK, Karrer, FM, Lilly, JR. Contemporary surgery of tracheomalacia. J Pediatr Surg. 1986;21(6):511–514.
74. Sommer, D, Forte, V. Advances in the management of major airway collapse: the use of airway stents. Otolaryngol Clin North Am. 2000;33(1):163–177.
75. Lee, KS, Lunn, W, Feller-Kopman, D, et al. Multislice CT evaluation of airway stents. J Thorac Imaging. 2005;20(2):81–88.
76. Weber, TR, Keller, MS, Fiore, A. Aortic suspension (aortopexy) for severe tracheomalacia in infants and children. Am J Surg. 2002;184(6):573–577.
77. Adler, SC, Isaacson, G, Balsara, RK. Innominate artery compression of the trachea: diagnosis and treatment by anterior suspension—25 year experience. Ann Otol Rhinol Laryngol. 1995;104(12):924–927.
78. Morabito, A, MacKinnon, E, Alizai, N, et al. The anterior mediastinal approach for management of tracheomalacia. J Pediatr Surg. 2000;35(10):1456–1458.
79. Baxter, JD, Dunbar, JS. Tracheomalacia. Ann Otol Rhinol Laryngol. 1963;72:1012–1023.
80. Kiely, EM, Spitz, L, Brereton, R. Management of tracheomalacia by aortopexy. Pediatr Surg Int. 1987;2:13–15.
81. Vasko, JS, Ahn, C. Surgical management of secondary tracheomalacia. Ann Thorac Surg. 1968;6(3):269–272.
82. Davis, S, Jones, M, Kisling, J, et al. Effect of continuous positive airway pressure on forced expiratory flows in infants with tracheomalacia. Am J Respir Crit Care Med. 1998;158(1):148–152.
83. Reiterer, F, Eber, E, Zach, MS, et al. Management of severe congenital tracheobronchomalacia by continuous positive airway pressure and tidal breathing flow-volume loop analysis. Pediatr Pulmonol. 1994;17(6):401–403.
84. Ferguson, GT, Benoist, J. Nasal continuous positive airway pressure in the treatment of tracheobronchomalacia. Am Rev Respir Dis. 1993;147(2):457–461.
85. Page, BA, Klein, EF, Jr. Tracheal stent as an aid in weaning from mechanical ventilation in tracheomalacia. Anesthesiology. 1977;47(3):300–301.
86. Altman, KW, Wetmore, FR, Marsh, RR. Congenital airway abnormalities in patients requiring hospitalization. Arch Otolaryngol Head Neck Surg. 1999;125(5):525–528.