Chapter 14 Lower Gastrointestinal Bleeding
Epidemiology
Acute severe lower GI bleeding occurs with an annual hospitalization rate of 22 per 100,000 adult population; this is based on a retrospective study of middle-class Americans who were members of Kaiser Permanente Health Care system in San Diego, California.1 Assuming that an average full-time clinical gastroenterologist is responsible for 50,000 adult lives, he or she would see more than 10 cases per year. Most cases occur in elderly patients, given the increased frequency and risk for diverticulosis, vascular disease, and colonic malignancy.1 Risk of lower GI bleeding is also associated with the use of aspirin and nonsteroidal antiinflammatory drugs (NSAIDs).2,3
Initial Approach to a Patient with Severe Hematochezia
The most important parts of the physical examination are the vital signs and the stool examination. The presence of bright red blood on rectal examination strongly suggests the possibility of colonic bleeding. Bright red blood per rectum is always a colonic source, unless it is accompanied by hypotension, which can occur during a severe upper GI or small bowel bleed with rapid transit of blood.4 In the setting of hematochezia without hypotension, placement of a diagnostic nasogastric (NG) tube is usually unnecessary because it is unlikely that there is a severe upper GI bleed without hypotension. If there is hypotension and hematochezia, a severe upper GI bleed is possible, and an NG tube should be placed. A clear NG tube lavage does not always imply a lower GI source because 16% of patients with duodenal ulcer bleeds have negative NG lavage.5 If bile is seen in the NG tube lavage, it is unlikely to be an upper GI bleed. Physical examination should also focus on abdominal tenderness, surgical scars, and stigmata of liver disease. Most patients with severe hematochezia do not need placement of an NG tube for diagnostic lavage, unless there is a strong suspicion for an upper GI source. At least one large-bore (14-gauge or 16-gauge) intravenous catheter should be placed, with two placed in the setting of ongoing bleeding.
Early Predictors of Severity in Acute Lower Gastrointestinal Bleeding
Early predictors (within 4 hours of admission) of severity for continued or recurrent bleeding after 24 hours of hospitalization include heart rate greater than 100 beats/min, systolic blood pressure less than 115 mm Hg, syncope, nontender abdominal examination, observed rectal bleeding during the first 4 hours of hospital evaluation, aspirin ingestion, and the presence of more than two comorbid conditions (Box 14.1).6,7 This prediction model has been prospectively validated; the low-risk group had 0% rebleeding, the moderate-risk group had 45% rebleeding, and the high-risk group had 77% risk of rebleeding.7 It is possible that factors such as these can be used to help triage patients to the appropriate level of care, such as ICU, hospital ward, or outpatient evaluation and urgent versus elective endoscopic evaluation.
Mortality in Severe Lower Gastrointestinal Bleeding
A large U.S. database study comprising 227,000 patients with discharge diagnoses of lower GI bleed in 2002 reported an overall mortality rate from lower GI bleeding of 3.9%.8 Multivariate analysis found that independent predictors of in-hospital mortality were age (>70 years), intestinal ischemia, presence of two or more comorbid illnesses, bleeding while hospitalized for a separate process, coagulopathies, hypovolemia, transfusion of packed red blood cells, and male gender. Colorectal polyps and hemorrhoids were associated with a lower mortality risk. Patients who develop severe lower GI bleeding while hospitalized for other lesions have a much higher mortality rate than patients admitted with lower GI bleeding. In a large Kaiser Permanente San Diego retrospective study, the in-hospital mortality rate for patients with lower GI bleeding who began as outpatients was 2.4% compared with 23% for patients with in-hospital lower GI bleeding (P < .001).1
Diagnostic Options
Flexible Sigmoidoscopy
Occasionally, flexible sigmoidoscopy may be performed to evaluate the left side of the colon quickly for any bleeding site stigmata rather than waiting for a full colonoscopy bowel preparation, and this results in a diagnosis in approximately 9% of cases.9 Flexible sigmoidoscopy may be especially useful in patients with strongly suspected diverticular bleeding or ischemic colitis.
Barium Enema
There is no role for emergency barium enema in a patient with severe lower GI bleeding. This test is rarely diagnostic because it cannot show vascular lesions and may be misleading if only diverticula are present. It also fails to detect 50% of polyps greater than 10 mm in size.10 Subsequent colonoscopy is needed for any suspicious lesions seen on barium enema, and no therapy can be performed.
Nuclear Medicine Scintigraphy
Nuclear medicine scintigraphy involves injecting a radiolabeled substance in the patient’s bloodstream and then performing serial scintigraphy to detect focal collections of radiolabeled material. It has been reported to detect bleeding at a rate of 0.1 mL/min.11 The overall positive diagnostic rate is approximately 45%, with a 78% accuracy in the localization of the true bleeding site.12 The most common false-positive result occurs when there is rapid transit of luminal blood such that labeled blood is detected in the colon, although it originated in the upper GI tract.
Angiography
Angiography is positive when the arterial bleeding rate is at least 0.5 mL/min.13 The diagnostic yield depends on patient selection, timing of the procedure, and the skill of the angiographer, with positive yields in 12% to 69% of cases. An advantage of angiography is that embolization can be performed to control some bleeding lesions. There is also a 3% rate of major complications, however, including hematoma formation, femoral artery thrombosis, contrast dye reactions, renal failure, and transient ischemic attacks.14
Computed Tomography Colonography
Computed tomography (CT) visualization of the colon is increasingly used to evaluate the colon for polyps and masses and may be of some benefit in lower GI bleeding. Faster scanners allow CT angiography, CT colonography, and CT evaluation of the small bowel to be performed. Use of CT potentially could allow diagnosis of mass lesions and vascular lesions, which would be an advantage compared with other radiologic imaging studies. One study from France reported that CT accurately diagnosed 17 of 19 lower GI bleeding sites, including diverticula, tumors, angiomas, and varices.15
Colonoscopy
Urgent colonoscopy using a rapid sulfate purge has been shown to be safe, to provide important diagnostic information, and sometimes to allow therapeutic intervention.16 Patients usually ingest 4 to 8 L of polyethylene glycol either orally or via NG tube over 3 to 5 hours until the rectal effluent is clear of stool, blood, and clots. Metoclopramide may be given intravenously before the purge and repeated every 3 to 4 hours to facilitate gastric emptying and reduce nausea.
The overall diagnostic yield of a presumed or definite etiology using colonoscopy in lower GI bleeding ranges from 48% to 90%, with an average of 68%, based on a review of 13 studies.12 The problem with interpreting these data is that it is often impossible to determine a definite diagnosis of the cause of the bleeding, unless bleeding stigmata are identified such as active bleeding, a visible vessel, an adherent clot, mucosal friability or ulceration, or the presence of fresh blood limited to a specific part of the colon. A presumptive diagnosis often is made, especially in the case of diverticulosis, in which no blood is seen but there is a potential bleeding site present.
The optimal time for performing urgent bowel preparation and colonoscopy is unknown. Theoretically, the sooner the endoscopy is performed, the higher the likelihood of finding a lesion that might be amenable to endoscopic hemostasis, such as a bleeding diverticulum or polyp stalk. However, a retrospective study from the Mayo Clinic suggested that there was no significant association between the time of endoscopy (0 to 12 hours, 12 to 24 hours, >24 hours) and the findings of active bleeding or other stigmata that would prompt colonoscopic hemostasis in patients with diverticular bleeding.17 Early colonoscopy has been associated with fewer hospitalization days.18 Active bleeding or lesions at risk for rebleeding can be treated with colonoscopic hemostasis; this mostly applies to postpolypectomy bleeding and diverticula and is discussed later.
Etiology and Pathogenesis of Severe Lower Gastrointestinal Bleeding
It is not always possible to visualize active bleeding during colonoscopy. The timing of endoscopy may influence visualization: Earlier colonoscopy should increase the chances of detecting an actively bleeding lesion. A definite diagnosis of a bleeding lesion can usually be made if active bleeding is seen or there is an obvious stigma, such as clot or visible vessel. A presumptive diagnosis can be made if there is a suspicious lesion and no other possible sources. Table 14.1 lists the frequency of various presumed or definite sites of acute colonic bleeding.19 Potential colonic lesions amenable to endoscopic hemostasis include diverticula, postpolypectomy sites, angiomas, hemorrhoids, Dieulafoy’s lesions, tumors, ulcers, and radiation proctitis.
Table 14.1 Etiology of Severe Lower Gastrointestinal Bleeding*
| Cause | Cases (%) |
|---|---|
| Diverticulosis | 33 |
| Cancer/polyps | 19 |
| Colitis | 18 |
| Unknown | 16 |
| Angiodysplasia | 8 |
| Other | 8 |
| Postpolypectomy | 6 |
| Anorectal | 4 |
* Summary of 1333 patients in seven published studies.
From Zuckerman GR, Prakash C: Acute lower intestinal bleeding. Part II. Etiology, therapy, and outcomes. Gastrointest Endosc 49:228–238, 1999.
Diverticular Bleeding
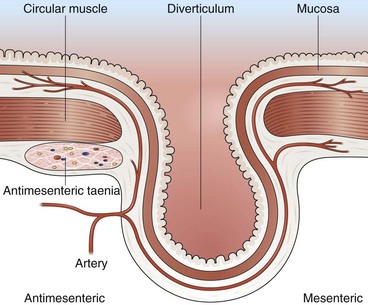
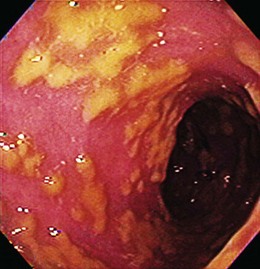
Most colonic diverticula are asymptomatic and remain uncomplicated. Bleeding may occur from vessels at the neck or base of the diverticulum (Fig 14.1).20 Diverticula are common in Western countries, with a prevalence of 50% in older adults.21 In contrast, less than 1% of people living in continental Africa and Asia have diverticula.22 This finding has led to the hypothesis that regional differences in prevalence can be explained by the low amounts of dietary fiber in Western diets. Presumably, a low-fiber diet results in less stool content, longer fecal transit time, increased colonic muscle contraction, and, ultimately, increased intraluminal pressure that results in the formation of propulsion diverticula. In addition, diverticula occur with increasing frequency with advanced age, which could be a result of weakening of the colonic wall and muscle tone.
It has been estimated that 3% to 5% of patients with diverticulosis develop diverticular bleeding.23 Although most diverticula are in the left colon, several series suggest that bleeding diverticula occur more often in the right colon.20,23–25 Patients with diverticular bleeding are typically elderly and present with painless hematochezia. They often have been taking aspirin or NSAIDs.2 In at least 75% of patients with diverticular bleeding, the bleeding stops spontaneously.24 Patients in whom bleeding stops usually require less than 4 U of blood. In one surgical series, surgical resection was needed in 60% of patients, most of whom had continued bleeding despite transfusion of 4 U of blood.24 Patients with successful resection of a bleeding diverticulum had a rebleed rate of 4%.24 Among patients in whom bleeding stopped spontaneously, the rebleeding rate from colonic diverticulosis was 25% to 38% over the next 4 years.1,24 Urgent colonoscopy after rapid bowel preparation often reveals that bleeding has stopped by the time of colonoscopy, and only nonbleeding diverticula are detected. These patients are given the diagnosis of “presumptive diverticular bleed” because the diverticula are the only likely source of bleeding, although no stigmata were identified.
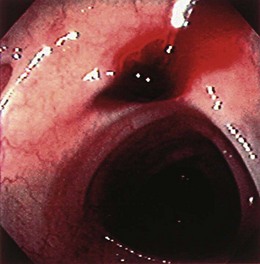
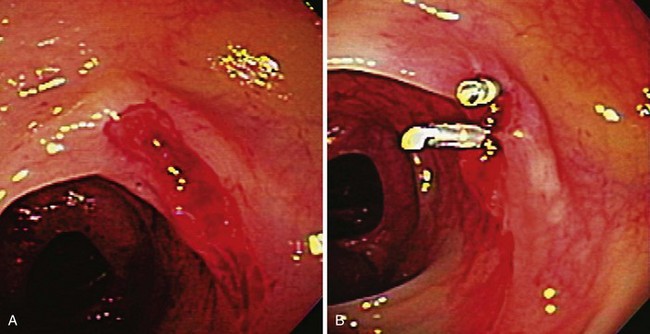
Occasionally, urgent colonoscopy reveals stigmata of recent bleeding, such as active bleeding, a visible vessel, clot, or blood limited to one segment of the colon (Fig. 14.2). It seems possible that earlier colonoscopy in lower GI bleeding would result in a greater frequency of finding stigmata of recent diverticular bleeding, although a small case series study from the Mayo Clinic did not find any difference if colonoscopy was performed between 0 and 12 hours, between 12 and 24 hours, or more than 24 hours from the time of hospital admission.17 There have been attempts to stratify patients with diverticular bleeding at increased risk for rebleeding employing the same endoscopic stigmata used in high-risk peptic ulcer bleeding (active bleeding, visible vessel, and clot), although the natural history for each of these untreated stigmata is unknown. The “pigmented protuberance” found on the edge of some diverticula at histopathology is usually clot at the edge of a ruptured blood vessel.26
The UCLA/CURE group17 found that among patients with stigmata of recent diverticular hemorrhage (six active bleeding, four visible vessels, and seven adherent clots), there was a very high rebleed rate of 53% and emergency surgery rate of 35%.27 Colonoscopic hemostasis of actively bleeding diverticula has been reported using bipolar probe coagulation, epinephrine injection, metallic clips, rubber band ligation, and fibrin glue.26–33 If fresh red blood is seen in a focal segment of colon, we try to examine this segment of bowel carefully to detect the exact bleeding site. If the bleeding is coming from the edge of the diverticulum or there is a pigmented protuberance on the edge, we initially inject 1 : 10,000 epinephrine in 1-mL aliquots using a sclerotherapy needle into four quadrants around the bleeding site. Then we use either an endoscopic clip or a bipolar probe at a low power setting (10 to 15 W) and light pressure for a 1-second pulse duration to cauterize the diverticular edge and stop bleeding or flatten the visible vessel. If there is a nonbleeding adherent clot, we inject around the clot with 1 : 10,000 epinephrine in four quadrants with 1 mL per quadrant and remove the clot in piecemeal fashion using a cold polyp snare. The clot is shaved down until it is 3 mm above the diverticulum, and then the underlying stigma is treated with either endoscopic clip or bipolar probe coagulation as discussed previously. After performing endoscopic hemostasis of a bleeding diverticulum, a permanent submucosal tattoo and a metal clip (if not previously placed) should be placed in the adjacent mucosa to identify the site in case colonoscopy, angiographic embolization, or surgery is required for recurrent bleeding. For long-term management after colonoscopic hemostasis, patients are told to avoid aspirin (if approved by their cardiologist) and NSAIDs and to take a daily fiber supplement.
In 2000, Jensen and colleagues in the UCLA/CURE group27 published their results on urgent colonoscopy for diagnosis and treatment of severe diverticular hemorrhage. The investigators found that 20% of patients with severe hematochezia had endoscopic stigmata suggesting a definite diverticular bleed. Compared with a historical control group with high-risk stigmata but no colonoscopic hemostasis, the group receiving colonoscopic hemostasis had a rebleed rate of 0% versus 53% and an emergency hemicolectomy rate of 0% versus 35%. After 3 years of follow-up, there were no rebleeding episodes in the patients who underwent colonoscopic hemostasis. In contrast to the UCLA experience, a smaller, retrospective review of the Duke University Medical Center Endoscopic Database revealed 13 patients with active bleeding or stigmata who received endoscopic treatment with epinephrine or bipolar coagulation or both.34 The 30-day rebleed rate was 38%; four of these patients underwent surgery. The long-term rebleed rate was 23% with a mean follow-up of 3 years.
Colon Cancer
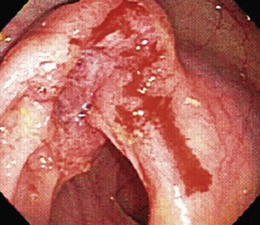
Most patients with colon cancer present with occult GI blood loss rather than hematochezia. For adult patients with hematochezia, determining the presence or absence of a colon cancer is imperative because early diagnosis improves survival. Because a cancer must ulcerate for overt bleeding to occur, most bleeding cancers are at a relatively advanced tumor stage (Fig. 14.3). Colon cancer can occur anywhere in the colon or rectum, but there is increased prevalence of right-sided tumors in elderly patients.
Colitis
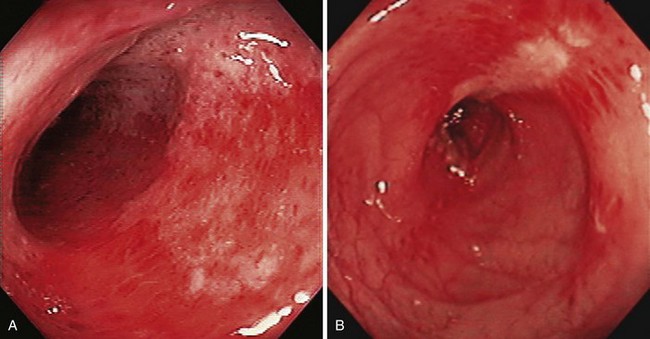
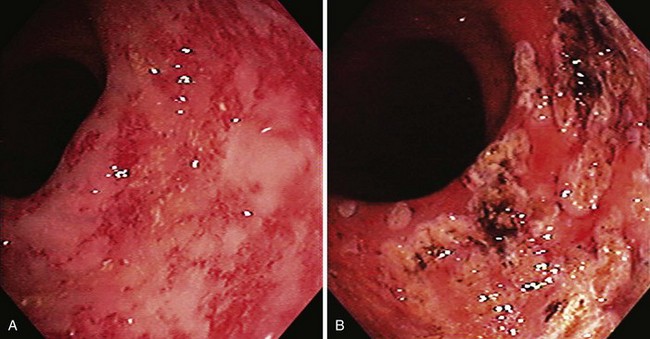
The term colitis refers to any form of inflammation of the colon. With regard to severe lower GI bleeding, this is usually ischemic colitis, inflammatory bowel disease, or possibly infectious colitis. Ischemic colitis generally manifests as hematochezia with mild left-sided abdominal discomfort. It results from mucosal hypoxia and is thought to be caused by hypoperfusion of the intramural vessels of the intestinal wall rather than by large vessel occlusion. Most cases do not have a recognizable cause, but associated conditions include recent aortic or cardiac surgery, vasculitis, and medications.35,36 Because of collateral circulation, ischemic involvement is usually segmental and primarily affects the mucosal aspect of the intestine. The colon is mostly affected in the watershed areas, such as the splenic flexure or rectosigmoid junction in which there is reduced collateral circulation, although ischemia can occur anywhere.
The diagnosis is usually confirmed by colonoscopy but can be suspected by “thumbprinting” on plain film radiographs or colonic wall thickening on CT scan. The colonoscopic appearance includes erythema, friability, and exudate (Fig. 14.4). Biopsy specimens may be suggestive of ischemic changes but more importantly are used to exclude infectious changes or Crohn’s disease. Ischemic colitis generally resolves in a few days and does not require colonoscopic hemostasis. In a large retrospective series from Kaiser Permanente San Diego, there were no episodes of rebleeding from ischemic colitis over a 4-year period.1 Inflammatory bowel disease affecting the colon can rarely cause severe acute lower GI bleeding. In a case series from the Mayo Clinic, most patients had Crohn’s disease.37 Most patients were successfully treated medically. Three of the 31 patients in the series received endoscopic therapy with epinephrine injection alone or with bipolar coagulation for adherent clots or oozing ulcers in Crohn’s disease. These patients had no rebleeding. Rebleeding occurred in 23% of patients a median of 3 days after the initial bleed (range 1 to 75 days). Of the patients with Crohn’s disease with severe bleeding, 39% required surgical management.
Infectious colitis should always be excluded in any patient with severe lower GI bleeding and colitis. Lower GI bleeding can occur with infection by Campylobacter jejuni, Salmonella, Shigella, invasive Escherichia coli, E. coli 0157, or Clostridium difficile (Fig. 14.5). Significant blood loss is rare. Diagnosis is made by stool cultures and flexible sigmoidoscopy.
Angiodysplasia
Colonic angiomas are also referred to as angiodysplasia, arteriovenous malformations, or vascular ectasias. They are generally uncommon: Less than 1% of asymptomatic patients undergoing screening colonoscopy were found to have angiodysplasia.38 The lesion seems to increase with age and may represent degeneration of previously normal blood vessels in the cecum and proximal ascending colon. Histopathology reveals a large, dilated, submucosal vein and, in advanced cases, dilated mucosal veins with small arteriovenous communications. Proposed explanations for angioma formation include the partial obstruction of submucosal veins passing through the colonic muscle layers, with eventual dilation of the submucosal and mucosal veins, and local mucosal ischemia.
Medical conditions associated with angiomas include chronic renal failure and hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu syndrome). There have been reports suggesting that aortic stenosis is associated with lower GI bleeding, presumably from colonic angiomas.39 The potential biologic explanation is that aortic stenosis causes defects in von Willebrand factor, which causes the patient to have decreased platelet adhesion and increased bleeding tendency, especially if there were preexisting mucosal GI lesions such as angiomas.40,41 Clinical studies do not support the association between aortic stenosis and the presence of angiomas, however.42,43 Bleeding from angiodysplasia is usually painless. The bleeding usually occurs from the right colon or cecum.
In the past 2 decades, it seems that the reported frequency of angiomas as the source of lower GI bleeding has decreased.16,27 This may be because of better recognition of angiomas with improved endoscope technology and increased attribution to presumed diverticular bleeding as the cause of hematochezia. Endoscopic hemostasis has been successfully reported using thermal modalities (e.g., bipolar probe, heater probe, laser) and injection therapy.44 Hormonal therapy has been reported as useful for decreasing bleeding from angiodysplasia, but a more recent randomized controlled trial found no benefit.45
Postpolypectomy Bleeding
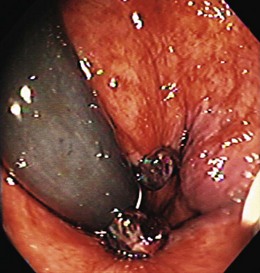
Postpolypectomy bleeding occurs after 1% to 6% of polypectomies, usually within the first 7 days.46 It is generally mild and self-limited. Reported risk factors for postpolypectomy bleeding include large polyps (>2 cm), thick stalks, sessile polyps, and right colon polyps.46 Endoscopic management techniques include resnaring the stalk (without cautery), epinephrine injection, thermal coagulation, hemoclips, and endoloops (Fig. 14.6).46–49 In a case series from the Mayo Clinic, the median time to bleeding after polypectomy was 5 days (range 0 to 17 days).48 Of patients, 65% received aspirin, NSAIDs, warfarin (Coumadin), heparin, or steroids after polypectomy; 76% required transfusions; and 96% were managed endoscopically with coagulation or epinephrine injection or both. The routine use of placing hemoclips after colonic polypectomy or endoscopic mucosal resection does not decrease the subsequent postpolypectomy bleeding rate.50 However, in selected patients who have had polypectomies and who were believed to be at increased risk for bleeding, prophylactic hemoclip placement or other endoscopic hemostasis may be considered.
Radiation Proctitis
Patients should be instructed to avoid all aspirin and NSAIDs and should be put on a high-fiber diet. Medical therapies with topical or oral 5-aminosalicylic acid, sucralfate, or steroids can be tried but are usually unsuccessful.51 Thermal therapy can be quite successful, including argon plasma coagulation, bipolar probe coagulation, radiofrequency ablation, and cryotherapy (Fig. 14.7).52–54 Topical formalin applied directly to the rectal mucosa can reduce bleeding.55 In refractory cases, hyperbaric oxygen can also be used successfully.56,57 A few pilot studies suggest that antioxidant vitamins, such as vitamins A, C, and E, can also decrease bleeding resulting from chronic radiation proctitis.58
Hemorrhoids
Hemorrhoids are a plexus of veins just above the rectal squamocolumnar junction. Internal hemorrhoids are located above the dentate line, and external hemorrhoids are located below the dentate line. Symptomatic hemorrhoids are common in adults, mostly associated with prolonged straining during bowel movements, chronic constipation, pregnancy, obesity, and low-fiber diet. Bleeding is characterized by bright red blood per rectum that can coat the outside of the stool, may drip into the toilet bowel, and is present when wiping with tissue. Usually this is mild bleeding, but occasionally severe bleeding may occur from hemorrhoids. Treatment of hemorrhoids usually starts with medical therapy consisting of fiber supplementation to soften stool, lubricant rectal suppositories (with or without steroids), and warm sitz baths. Anoscopic therapy can also be used, including injection sclerotherapy, rubber band ligation, cryosurgery, infrared photocoagulation, and bipolar and direct current electrocoagulation. The use of a flexible endoscope and esophageal band ligation devices has also been described (Fig. 14.8).59 Surgery is reserved for refractory bleeding not controlled with other mechanisms. Most patients respond to medical management.
Rectal Varices
In response to portal hypertension, varices can develop in the rectal mucosa between the superior hemorrhoidal veins (portal circulation) and the middle and inferior hemorrhoidal veins (systemic circulation). With anoscopy or sigmoidoscopy, rectal varices are seen as vascular structures located several centimeters above the dentate line. The incidence of rectal varices increases with the degree of portal hypertension. About 60% of patients with a history of bleeding esophageal varices have rectal varices. Rectal varices can be treated similarly to esophageal varices, with sclerotherapy, rubber band ligation, or portosystemic shunts.60,61
Rectal Dieulafoy’s Lesion
Dieulafoy’s lesions are large submucosal arteries without overlying mucosal ulceration, which can cause massive bleeding from anywhere in the GI tract, although bleeding is usually from the stomach. There have been reports of bleeding Dieulafoy’s lesions in the rectum that were treated successfully with endoscopic hemostasis.62,63
1 Longstreth GF. Epidemiology and outcome of patients hospitalized with acute lower gastrointestinal hemorrhage: A population-based study. Am J Gastroenterol. 1997;92:419-424.
2 Foutch PG. Diverticular bleeding: Are nonsteroidal anti-inflammatory drugs risk factors for hemorrhage and can colonoscopy predict outcome for patients? Am J Gastroenterol. 1995;90:1779-1784.
3 Laine L, Connors LG, Reicin A, et al. Serious lower gastrointestinal clinical events with nonselective NSAID or coxib use. Gastroenterology. 2003;124:288-292.
4 Zuckerman GR, Trellis DR, Sherman TM, et al. An objective measure of stool color for differentiating upper from lower gastrointestinal bleeding. Dig Dis Sci. 1995;40:1614-1621.
5 Gilbert DA, Silverstein FE, Tedesco FJ, et al. The national ASGE survey on upper gastrointestinal bleeding. III. Endoscopy in upper gastrointestinal bleeding. Gastrointest Endosc. 1981;27:94-102.
6 Strate LL, Orav EJ, Syngal S. Early predictors of severity in acute lower intestinal tract bleeding. Arch Intern Med. 2003;163:838-843.
7 Strate LL, Saltzman JR, Ookubo R, et al. Validation of a clinical prediction rule for severe acute lower intestinal bleeding. Am J Gastroenterol. 2005;100:1821-1827.
8 Strate LL, Ayanian JZ, Kotler G, et al. Risk factors for mortality in lower intestinal bleeding. Clin Gastroenterol Hepatol. 2008;6:1004-1010.
9 Richter JM, Christensen MR, Kaplan LM, et al. Effectiveness of current technology in the diagnosis and management of lower gastrointestinal hemorrhage. Gastrointest Endosc. 1995;41:93-98.
10 Winawer SJ, Stewart ET, Zauber AG, et al. A comparison of colonoscopy and double-contrast barium enema for surveillance after polypectomy. National Polyp Study Work Group. N Engl J Med. 2000;342:1766-1772.
11 Nicholson ML, Neoptolemos JP, Sharp JF, et al. Localization of lower gastrointestinal bleeding using in vivo technetium-99m-labelled red blood cell scintigraphy. Br J Surg. 1989;76:358-361.
12 Zuckerman GR, Prakash C. Acute lower intestinal bleeding. Part I. Clinical presentation and diagnosis. Gastrointest Endosc. 1998;48:606-617.
13 Baum S. Angiography and the gastrointestinal bleeder. Radiology. 1982;143:569-572.
14 Egglin TK, O’Moore PV, Feinstein AR, et al. Complications of peripheral arteriography: A new system to identify patients at increased risk. J Vasc Surg. 1995;22:787-794.
15 Ernst O, Bulois P, Saint-Drenant S, et al. Helical CT in acute lower gastrointestinal bleeding. Eur Radiol. 2003;13:114-117.
16 Jensen DM, Machicado GA. Diagnosis and treatment of severe hematochezia: The role of urgent colonoscopy after purge. Gastroenterology. 1988;95:1569-1574.
17 Smoot R, Gostout CJ, Rajan E, et al. Is early colonoscopy after admission for acute diverticular bleeding needed? Am J Gastroenterol. 2003;98:1996-1999.
18 Strate LL, Syngal S. Timing of colonoscopy: Impact on length of hospital stay in patients with acute lower intestinal bleeding. Am J Gastroenterol. 2003;98:317-322.
19 Zuckerman GR, Prakash C. Acute lower intestinal bleeding. Part II. Etiology, therapy, and outcomes. Gastrointest Endosc. 1999;49:228-238.
20 Meyers MA, Alonso DR, Gray GF, et al. Pathogenesis of bleeding colonic diverticulosis. Gastroenterology. 1976;71:577-583.
21 Stollman NH, Raskin JB. Diagnosis and management of diverticular disease of the colon in adults. Ad Hoc Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol. 1999;94:3110-3121.
22 Painter NS, Burkitt DP. Diverticular disease of the colon: A deficiency disease of Western civilization. BMJ. 1971;2:450-454.
23 McGuire HHJr, Haynes BWJr. Massive hemorrhage for diverticulosis of the colon: Guidelines for therapy based on bleeding patterns observed in fifty cases. Ann Surg. 1972;175:847-855.
24 McGuire HHJr. Bleeding colonic diverticula: A reappraisal of natural history and management. Ann Surg. 1994;220:653-656.
25 Wong SK, Ho YH, Leong AP, et al. Clinical behavior of complicated right-sided and left-sided diverticulosis. Dis Colon Rectum. 1997;40:344-348.
26 Foutch PG, Zimmerman K. Diverticular bleeding and the pigmented protuberance (sentinel clot): Clinical implications, histopathological correlation, and results of endoscopic intervention. Am J Gastroenterol. 1996;91:2589-2593.
27 Jensen DM, Machicado GA, Jutabha R, et al. Urgent colonoscopy for the diagnosis and treatment of severe diverticular hemorrhage. N Engl J Med. 2000;342:78-82.
28 Yen EF, Ladabaum U, Muthusamy VR, et al. Colonoscopic treatment of acute diverticular hemorrhage using endoclips. Dig Dis Sci. 2008;53:2480-2485.
29 Ramirez FC, Johnson DA, Zierer ST, et al. Successful endoscopic hemostasis of bleeding colonic diverticula with epinephrine injection. Gastrointest Endosc. 1996;43(2 Pt 1):167-170.
30 Savides TJ, Jensen DM. Colonoscopic hemostasis for recurrent diverticular hemorrhage associated with a visible vessel: A report of three cases. Gastrointest Endosc. 1994;40:70-73.
31 Lara LF, Bloomfeld RS. Endoscopic therapy for acute diverticular hemorrhage. Gastrointest Endosc. 2001;53:492.
32 Kim YI, Marcon NE. Injection therapy for colonic diverticular bleeding: A case study. J Clin Gastroenterol. 1993;17:46-48.
33 Andress HJ, Mewes A, Lange V. Endoscopic hemostasis of a bleeding diverticulum of the sigma with fibrin sealant. Endoscopy. 1993;25:193.
34 Bloomfeld RS, Rockey DC, Shetzline MA. Endoscopic therapy of acute diverticular hemorrhage. Am J Gastroenterol. 2001;96:2367-2372.
35 Brandt LJ, Boley SJ. AGA technical review on intestinal ischemia. American Gastrointestinal Association. Gastroenterology. 2000;118:954-968.
36 Brandt LJ, Boley SJ. Intestinal ischemia. In: Feldman M, Friedman LS, Sleisenger MH, editors. Gastrointestinal and liver disease. Philadelphia: Saunders; 2002:2321-2340.
37 Pardi DS, Loftus EVJr, Tremaine WJ, et al. Acute major gastrointestinal hemorrhage in inflammatory bowel disease. Gastrointest Endosc. 1999;49:153-157.
38 Foutch PG, Rex DK, Lieberman DA. Prevalence and natural history of colonic angiodysplasia among healthy asymptomatic people. Am J Gastroenterol. 1995;90:564-567.
39 Heyde EC. Gastrointestinal bleeding in aortic stenosis. N Engl J Med. 1958;259:196.
40 Vincentelli A, Susen S, Le Tourneau T, et al. Acquired von Willebrand syndrome in aortic stenosis. N Engl J Med. 2003;349:343-349.
41 Sadler JE. Aortic stenosis, von Willebrand factor, and bleeding. N Engl J Med. 2003;349:323-325.
42 Imperiale TF, Ransohoff DF. Aortic stenosis, idiopathic gastrointestinal bleeding, and angiodysplasia: Is there an association? A methodologic critique of the literature. Gastroenterology. 1988;95:1670-1676.
43 Bhutani MS, Gupta SC, Markert RJ, et al. A prospective controlled evaluation of endoscopic detection of angiodysplasia and its association with aortic valve disease. Gastrointest Endosc. 1995;42:398-402.
44 Foutch PG. Angiodysplasia of the gastrointestinal tract. Am J Gastroenterol. 1993;88:807-818.
45 Junquera F, Feu F, Papo M, et al. A multicenter, randomized, clinical trial of hormonal therapy in the prevention of rebleeding from gastrointestinal angiodysplasia. Gastroenterology. 2001;121:1073-1079.
46 Waye JD, Kahn O, Auerbach ME. Complications of colonoscopy and flexible sigmoidoscopy. Gastrointest Endosc Clin N Am. 1996;6:343-377.
47 Uno Y, Satoh K, Tuji K, et al. Endoscopic ligation by means of clip and detachable snare for management of colonic postpolypectomy hemorrhage. Gastrointest Endosc. 1999;49:113-115.
48 Sorbi D, Norton I, Conio M, et al. Postpolypectomy lower GI bleeding: Descriptive analysis. Gastrointest Endosc. 2000;51:690-696.
49 Parra-Blanco A, Kaminaga N, Kojima T, et al. Hemoclipping for postpolypectomy and postbiopsy colonic bleeding. Gastrointest Endosc. 2000;51:37-41.
50 Shioji K, Suzuki Y, Kobayashi M, et al. Prophylactic clip application does not decrease delayed bleeding after colonoscopic polypectomy. Gastrointest Endosc. 2003;57:691-694.
51 Baum CA, Biddle WL, Miner PBJr. Failure of 5-aminosalicylic acid enemas to improve chronic radiation proctitis. Dig Dis Sci. 1989;34:758-760.
52 Swaroop VS, Gostout CJ. Endoscopic treatment of chronic radiation proctopathy. J Clin Gastroenterol. 1998;27:36-40.
53 Taieb S, Rolachon A, Cenni JC, et al. Effective use of argon plasma coagulation in the treatment of severe radiation proctitis. Dis Colon Rectum. 2001;44:1766-1771.
54 Villavicencio RT, Rex DK, Rahmani E. Efficacy and complications of argon plasma coagulation for hematochezia related to radiation proctopathy. Gastrointest Endosc. 2002;55:70-74.
55 Parikh S, Hughes C, Salvati EP, et al. Treatment of hemorrhagic radiation proctitis with 4 percent formalin. Dis Colon Rectum. 2003;46:596-600.
56 Mayer R, Klemen H, Quehenberger F, et al. Hyperbaric oxygen—an effective tool to treat radiation morbidity in prostate cancer. Radiother Oncol. 2001;61:151-156.
57 Clarke RE, Tenorio LM, Hussey JR, et al. Hyperbaric oxygen treatment of chronic refractory radiation proctitis: A randomized and controlled double-blind crossover trial with long-term follow-up. Int J Radiat Oncol Biol Phys. 2008;72:134-143.
58 Kennedy M, Bruninga K, Mutlu EA, et al. Successful and sustained treatment of chronic radiation proctitis with antioxidant vitamins E and C. Am J Gastroenterol. 2001;96:1080-1084.
59 Berkelhammer C, Moosvi SB. Retroflexed endoscopic band ligation of bleeding internal hemorrhoids. Gastrointest Endosc. 2002;55:532-537.
60 Hosking SW, Smart HL, Johnson AG, et al. Anorectal varices, haemorrhoids, and portal hypertension. Lancet. 1989;1:349-352.
61 Firoozi B, Gamagaris Z, Weinshel EH, et al. Endoscopic band ligation of bleeding rectal varices. Dig Dis Sci. 2002;47:1502-1505.
62 Abdulian JD, Santoro MJ, Chen YK, et al. Dieulafoy-like lesion of the rectum presenting with exsanguinating hemorrhage: Successful endoscopic sclerotherapy. Am J Gastroenterol. 1993;88:1939-1941.
63 Meister TE, Varilek GW, Marsano LS, et al. Endoscopic management of rectal Dieulafoy-like lesions: A case series and review of literature. Gastrointest Endosc. 1998;48:302-305.