CHAPTER 114 Lower Extremity Operations and Interventions
Vascular surgical procedures have traditionally been the “gold standard” against which newer technologies and their long-term results have been measured. Whereas open vascular surgical procedures have continued to undergo refinements and expansions of clinical indications, endovascular or catheter-based interventions have had an almost explosive growth of new technology and broadened indications and have increasingly gained acceptance as the primary treatment in a variety of applications. There are now long-term data for many of these endovascular procedures that compare favorably with the traditional open surgical operations.1–6 Some of the newer technologies continue to evolve and are likely to have expanding indications. The decision process for selection of open versus endovascular treatment, as well as which endovascular option, involves consideration of the specific disease entity, the medical condition and age of the patient, the anatomic constraints, and the durability of the procedure in question.
REVASCULARIZATION PROCEDURE: VASCULAR BYPASS SURGERY
Description and Special Anatomic Considerations
Vascular bypass surgery involves placement of a conduit to serve as an alternative vascular pathway to a diseased or obstructed arterial bed. Vascular surgical conduits are anatomically classified on the basis of the locations of the proximal and distal anastomoses. The most common infrainguinal bypass is the femoropopliteal bypass, between the common femoral and the popliteal arteries (Fig. 114-1A,B). The distal anastomosis may be to either the above-knee or the below-knee segment of the popliteal artery (Fig. 114-1C). Conduits are further defined according to the material from which they are constructed. They may be native, such as an autogenous vein or artery, or they may be prosthetic, such as expanded polytetrafluoroethylene (ePTFE) or Dacron. The native greater saphenous vein is preferred for bypass surgery in the lower extremity because it performs better than any other conduit choice. However, it may not always be an available option because donor veins may be diseased or may have been previously harvested for other vascular procedures, such as coronary artery bypass surgery. Other autogenous veins used as vascular conduits include the short saphenous vein, the femoral vein (also known as the superficial femoral vein) within the thigh, and the basilic and cephalic veins of the upper extremity.
The lack of a completely satisfactory prosthetic substitute for the greater saphenous vein has led to the use of other biologic conduits, such as human umbilical vein, arterial or venous homografts, and xenografts. There are certain inherent problems, such as aneurysmal degeneration (Fig. 114-2), and long-term patency issues that are unique to these biografts, and results remain mixed compared with prosthetic grafts.7
Extra-anatomic bypass refers to grafts that are constructed in anatomic locations that are significantly different from the normal location of the diseased arteries that are being bypassed. Typical examples are the cross-femoral (femorofemoral) and axillofemoral bypass grafts (Figs. 114-3 to 114-5). These were originally designed for patients too ill to undergo direct aortofemoral bypass or to replace grafts that were infected; these are still the primary indications. In addition, they now often serve as adjuncts to endovascular repair of abdominal aortic aneurysms (EVAR), particularly in the category of aorto–uni-iliac EVAR. These grafts are usually constructed of prosthetic material and generally have somewhat lower long-term patency than more traditional vascular bypass grafts, such as the aortobifemoral bypass graft. The obturator bypass (Fig. 114-6) was developed to replace femoropopliteal bypass surgery in patients with groin infections involving the native arteries or previously placed grafts and in patients with other complicating circumstances in the groin, such as trauma or previous radiation treatment. This bypass can be constructed with prosthetics or with autogenous vein.8
Indications
Patients with peripheral arterial disease may be classified by both a clinical description of the symptoms and objective testing criteria by the Rutherford categories of chronic limb ischemia (Table 114-1). These aid in prognosis and treatment planning.
Outcomes and Complications
Complications and lesions that threaten vascular conduit longevity include infection, development of anastomotic stenoses (Fig. 114-7) or pseudoaneurysms, progression of disease distal to the graft resulting in inadequate outflow, failure to incise all valves or to ligate all venous side branches within an in situ bypass graft, intimal hyperplasia, poor conduit quality, and degeneration of the graft. Such complications will require open surgical or endovascular correction.
The outcomes of various infrainguinal bypass procedures with use of currently available vascular conduits are summarized in Tables 114-2 to 114-4. With regard to extra-anatomic bypass grafts, the long-term patency rates are typically lower than for the anatomically positioned grafts. The obturator bypass graft for infrainguinal occlusive disease has reported patency rates of 73% and 57% at 1 and 5 years, respectively, which are somewhat lower than with conventional femoropopliteal bypass.8
REVASCULARIZATION PROCEDURE: ENDOVASCULAR TREATMENT OF STENOSIS AND OCCLUSIONS
Description and Special Anatomic Considerations
The most frequently employed endovascular treatment options are angioplasty and intravascular stent placement. Angioplasty may be used as a stand-alone primary therapy (Figs. 114-8 and 114-9) or may be combined with stent placement. Stents provide an intravascular scaffold for the vessel lumen and are available in a variety of materials, configurations, and delivery systems. Stents may be constructed from stainless steel, platinum, Elgiloy, and nitinol and may be combined with ePTFE or Dacron to produce a covered stent endoprosthesis or “stent graft.” Noncovered stents may be constructed with “open” or “closed cell” designs, which influence stent flexibility, conformability, radial strength, fracture resistance, and restenosis rates. In addition, there are balloon-mounted and self-expanding stents available in both the noncovered and covered groups (Figs. 114-10 and 114-11). Balloon-mounted stents are typically sized to correspond to the desired diameter of the vessel lumen, whereas self-expanding stents are usually oversized and may require secondary angioplasty to achieve a satisfactory diameter.
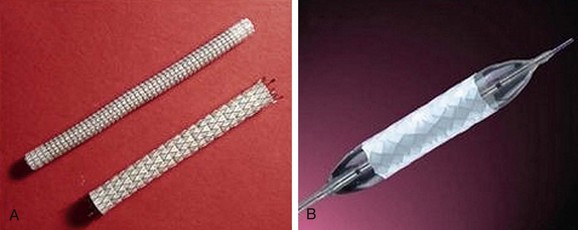
 FIGURE 114-11 Covered stents (stent grafts or vascular endoprostheses) are available as either self-expanding (A) or balloon mounted (B).
FIGURE 114-11 Covered stents (stent grafts or vascular endoprostheses) are available as either self-expanding (A) or balloon mounted (B).
Atherectomy involves a catheter-based atherosclerotic plaque excision system consisting of two components: a low-profile monorail excision catheter and a palm-sized power drive unit. A tiny rotating blade housed near the catheter tip is exposed when activated, removing and capturing thin shavings of plaque from the arterial wall into a collection chamber. Atherectomy thus permits “debulking” of atheroma from the lesion and may be used as a primary therapy or in combination with other endovascular treatment options. Catheters vary in diameter and tip length to accommodate various lesions (Figs. 114-12 and 114-13).
Cryoplasty is an angioplasty-based technology that uses liquid nitrous oxide as the balloon inflation medium, which lowers the balloon surface temperature to −10° C. Theoretically, cryoplasty causes an altered plaque response in which, as a result of freezing, microfractures form and weaken the plaque, contributing to a more uniform vessel dilation and less injury to the media. There may also be less elastic recoil and an induction of cellular apoptosis through freezing (Fig. 114-14).9,10
Another angioplasty-based technology is cutting balloon angioplasty, in which multiple small atherotomes (microsurgical blades) are fixed longitudinally on the outer surface of a noncompliant balloon. These expand radially during balloon inflation, delivering longitudinal incisions into the plaque and the vessel. Theoretically, there should be advantages to cutting balloon angioplasty through reduction of vascular injury by scoring of the vessel and the plaque longitudinally rather than by causing an uncontrolled disruption of the atherosclerotic plaque. However, in a randomized trial of 1385 coronary lesions, there was no significant difference between cutting and standard angioplasty at 6-month follow-up in angiographic and clinical results. The primary endpoint of angiographic restenosis at 6 months was 31.4% in the cutting balloon angioplasty group versus 30.4% in the standard group. This trial showed that cutting balloon angioplasty is equivalent in safety and efficacy endpoints to standard angioplasty, but it did not prove superiority for the general pool of percutaneous coronary intervention patients.11
Indications
The TransAtlantic Inter-Society Consensus Working Group (TASC) developed another classification system in 200012 and addressed both aortoiliac and infrainguinal occlusive disease, with the latter guidelines limited to femoropopliteal disease. These guidelines were updated in 200713 and reflect the expanded role of endovascular therapy as a primary option in the treatment of vascular occlusive disease (Table 114-5).
TABLE 114-5 TransAtlantic Inter-Society Consensus (TASC II) Recommendations (Femoropopliteal Arterial Disease)
| Lesion Category | Lesion Characteristics | Treatment Recommendation |
|---|---|---|
| Type A |
Recurrent stenoses or occlusions that need treatment after two endovascular interventions
Chronic total occlusion of popliteal artery and proximal trifurcation vessels
Data from Norgren L, Hiatt WR, Dormandy JA, et al; TASC II Working Group. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg 2007; 45(Suppl S):S5-S67.
Treatment choices may be influenced by the nature of the lesion to be treated. In general, short segmental stenoses respond well to angioplasty and may not require stent placement unless a significant arterial dissection or intimal flap occurs. Long-segment, diffusely diseased, or heavily calcified lesions may require stent placement to achieve and to maintain patency. Chronic total occlusions that have been successfully recanalized may also be treated with either angioplasty or intravascular stent placement (Fig. 114-15). Once again, the lesion length and other characteristics may dictate the choice of treatment. Furthermore, when intravascular stents are used for treatment of recanalized chronic total occlusions, the operator must determine whether the noncovered or covered stent option is appropriate. If a lengthy subintimal guidewire passage has occurred during the recanalization process, a covered stent may be a better treatment option. The decision of whether to use a covered or noncovered stent may also be influenced by the necessity of preserving collateral vessels or by potential stent encroachment on a side branch or vessel origin. Noncovered stents allow continued perfusion of collaterals or side branches, whereas covered stents will occlude these vessels.
Outcomes and Complications
Early experiences with stainless steel balloon-mounted stents in treating superficial femoral artery atherosclerotic disease showed no significant benefit over angioplasty alone, but more recent studies comparing angioplasty with self-expanding nitinol stents have shown decreased restenosis rates with primary stenting.1–3 Other studies have reported superiority of ePTFE-covered stents over angioplasty alone.4 Many centers now use endovascular revascularization procedures as a first-line therapy in appropriate patients with chronic occlusive disease and reserve open surgical bypass for endovascular failures.5,6 A recent randomized trial that compared use of covered stents with femoropopliteal bypass surgery using a prosthetic vascular conduit for treatment of superficial femoral artery occlusive disease showed no significant difference in 1-year patency rates.6
Imaging Findings
Understanding of the disease process that has resulted in the pathologic changes is also important in determining the optimal intervention. The appropriate treatment for embolic occlusion, for example, will differ considerably from the treatment of a chronic total occlusion (Fig. 114-16A,B). Various pathologic entities may have characteristic appearances (e.g., stenotic vs. aneurysmal disease), whereas others may appear similar (e.g., various vasculitides).
REVASCULARIZATION PROCEDURE: SURGICAL THROMBOEMBOLECTOMY
Indications
The clinical presentation of the patient and the severity of the limb ischemia usually dictate the appropriate therapy. A classification system for categorization of the acutely ischemic limb serves to direct the intervention. This classification is detailed in Table 114-6.
Outcomes and Complications
Surgical thromboembolectomy of an acutely ischemic limb with an inflatable balloon catheter is considered a relatively minor and technically simple operation. However, there may be high perioperative mortality that can be attributed to serious underlying cardiac disease or to the consequences of reperfusion of the ischemic limb and subsequent release of toxic metabolites. On occasion, pulmonary emboli may result from secondary venous thrombi, or there may be reactive hyperemia after successful revascularization that may increase cardiac workload of the heart and cause acute cardiac failure. There are studies that report a 30-day mortality exceeding 20% and amputation rates above 10% after arterial embolectomy. Several studies have also shown that older patients have a higher mortality rate,14 and mortality is greater with proximal occlusions than with distal occlusions.15–18 A short duration of symptoms before embolectomy has been reported to increase mortality, whereas other authors have found no effect on mortality. According to Levy and Butcher,14 severe ischemia did not increase the mortality rate, whereas Balas and colleagues19 concluded the opposite. Many studies report higher amputation rates when embolectomy was delayed14,15,19; other authors have found no adverse effect of delayed treatment.20–22 High amputation rates have also been associated with age, advanced arteriosclerotic disease,19 severe ischemic symptoms, and thrombotic compared with embolic occlusion.23 In addition, there is a worse prognosis the more distally the occlusion is located, and the prognosis is also worse with common femoral emboli than at other sites.
REVASCULARIZATION PROCEDURE: CATHETER-DIRECTED OR PHARMACOMECHANICAL THROMBOLYSIS
Description and Special Anatomic Considerations
For intra-arterial pharmacologic thrombolysis to be most effective, a catheter must first be advanced across the occluded segment; inability to cross the thrombosed segment generally indicates a more chronic process that will probably respond poorly to thrombolysis. A variety of infusion catheters are available; the majority are constructed with multiple side holes that allow maximal exposure of the surface area of the thrombus to the pharmacologic agent. Thrombolysis may be accelerated by the forceful pulsed injection of the fibrinolytic agent directly into the clot. Newer catheter systems combine pharmacologic with mechanical thrombolysis by use of ultrasound or rheolytic technologies to enhance drug activity (Fig. 114-16C,D).
EXCLUSION PROCEDURE: SURGICAL EXCISION AND VASCULAR BYPASS
Description and Special Anatomic Considerations
Arteriovenous Fistula and Malformation
Arteriovenous fistulas (AVFs) and vascular anomalies such as venous or arteriovenous malformations are also managed by exclusion procedures. AVFs may be managed surgically through ligation of the involved vessels, but endovascular treatment with embolization or covered stent placement has become the treatment of choice in many of these lesions (Fig. 114-17). Vascular anomalies are also generally treated by transcatheter delivery of embolic agents, although there are cases in which combined endovascular and surgical techniques have been effectively used.
Indications
Popliteal artery aneurysms are degenerative in more than 90% of cases and are bilateral in 60% to 70%. Thrombosis of the aneurysm or distal embolization of mural thrombus occurs far more frequently than rupture, which is the least frequent complication (Fig. 114-18). Popliteal artery aneurysms should be treated before the patient becomes symptomatic because nearly 50% of patients with asymptomatic aneurysms will develop distal ischemia within 5 years. Treatment is indicated in all symptomatic aneurysms and in asymptomatic aneurysms more than 2 cm in diameter. Surgical excision and bypass is the traditional treatment; the vascular conduit options include autogenous and prosthetic.
True degenerative common femoral artery aneurysms larger than 2.5 cm are typically treated with surgical excision and bypass. Other true degenerative aneurysms that occur in the lower extremities are much less common; when they are identified in the superficial femoral artery, profunda femoral artery, or tibial artery, an evaluation should be initiated for an unusual etiology, such as the Ehlers-Danlos syndrome (Fig. 114-19).
Contraindications
As previously noted, anastomotic pseudoaneurysms require surgical revision if they are sufficiently large. Currently, there are no satisfactory endovascular treatment options as pseudoaneurysms associated with vascular anastomoses result from disruption at the suture site. Pseudoaneurysms that occur spontaneously may be mycotic, and these would therefore also require surgical repair. Similarly, extremely large iatrogenic post-catheterization pseudoaneurysms that cause significant mass effect (e.g., claudication, neuropathy, limb ischemia, skin necrosis) should be treated surgically. Very small iatrogenic pseudoaneurysms (1.5 cm or less) associated with catheterization procedures may be observed for continued expansion. If they remain stable, without continued expansion, treatment may be unnecessary. Continued enlargement, however, generally mandates intervention. Larger pseudoaneurysms, with a sufficiently narrow “neck” between the true arterial lumen and the pseudoaneurysm, may be treated with thrombin injection when they involve the femoral arteries. If there is a very large or nonexistent neck to the pseudoaneurysm, this should be considered an arterial laceration and should be surgically repaired because it is unlikely that it can be effectively managed by more conservative means. Similarly, even relatively small pseudoaneurysms of the upper extremity arteries should be surgically treated, given the significant potential for adverse outcomes if they are observed or percutaneously treated.24
Outcomes and Complications
Symptomatic aneurysms of the lower extremities that present with either acute thrombosis or significant distal embolization have much worse surgical outcomes than those aneurysms that are electively treated. With regard to popliteal artery aneurysms, a recent review of consecutive patients who underwent surgical repair in two vascular surgery units between 1988 and 2006 in the United Kingdom reported 5-year primary graft patency, secondary graft patency, limb salvage, and patient survival rates of 75%, 95%, 98%, and 81%, respectively. The 10-year primary graft patency rates were significantly lower for emergency cases (59%) compared with elective cases (66%).9 There are increased amputation rates and substantially higher morbidity and mortality associated with emergency treatment of peripheral aneurysms. In addition, adjunctive procedures, such as preoperative or intraoperative thrombolysis, may be necessary. Thrombolysis may be problematic in a severely ischemic limb, however, as there may be insufficient perfusion to allow the time required for successful thrombolysis. Also, during the thrombolysis procedure because there is fragmentation of the clot and resultant passage of small fragments distally into the smaller runoff arteries, there may be further worsening of the ischemia. This may then necessitate emergent surgical intervention. Such complications of thrombolysis are typically seen more frequently in patients undergoing thrombolysis for acute thrombosis of a peripheral arterial aneurysm than in patients in whom thrombolysis is performed for thrombotic or embolic occlusion of atherosclerotic occlusive lesions or thrombosed bypass grafts. When complications occur in association with thrombolytic therapy for acutely thrombosed aneurysms, amputation rates are high. This is particularly true if there is significant runoff disease or the thrombolysis procedure is excessively lengthy.
Surgical treatment of anastomotic pseudoaneurysms has patency rates similar to those of the initial primary surgery, provided there has been no disease progression in the runoff anatomy at or distal to the anastomoses. Similarly, there are excellent surgical results for primary repair of a post-catheterization pseudoaneurysm.24
Imaging Findings
Preoperative imaging of lower extremity aneurysms or pseudoaneurysms allows both diagnosis and treatment planning. The length and extent of the aneurysm, the amount of intraluminal thrombus, the inflow and runoff anatomy, and the relationship to adjacent structures should be delineated. In cases of popliteal artery aneurysms presenting with acute thrombosis or distal embolization, clear definition of the runoff circulation is essential for treatment planning. Thrombolysis or intraoperative embolectomy may be necessary if no runoff vessels can be identified. In addition, imaging confirmation of a thrombosed popliteal artery aneurysm is necessary. Because angiography will demonstrate only the arterial lumen, a thrombosed aneurysm may not be suspected if there is insufficient mural calcium. Other imaging modalities, such as ultrasonography, MR, or CT, may be necessary for confirmation. Similarly, a popliteal artery aneurysm that contains significant intraluminal thrombus may not be clearly appreciated as aneurysmal without supplemental imaging (see Fig. 114-18D).
For post-catheterization pseudoaneurysms, imaging is necessary to identify the size and depth of the pseudoaneurysm and the depth, width, and length of the track that connects to the “feeding” artery from which the pseudoaneurysm arose. Adjacent venous structures should be evaluated for any evidence of deep venous thrombosis from expansion of the hematoma that may be associated with the pseudoaneurysm. A post-catheterization pseudoaneurysm has a typical color Doppler ultrasound appearance in which there is a turbulent swirling flow pattern within a sac that expands and contracts with the cardiac cycle. Pulsed wave Doppler demonstrates a to-and-fro waveform (Fig. 114-20). If management involves thrombin injection, imaging is generally performed both during and after treatment to ensure precise needle placement within the pseudoaneurysm, complete thrombosis of the sac, and preserved patency of the feeding artery.24
Adventitial cystic disease has a characteristic appearance on imaging studies as a result of cystic compression of the popliteal artery. Both ultrasound and MR images clearly demonstrate the cystic structures within the arterial wall and the mass effect on the adjacent artery. The angiographic appearance is that of a “spiral-shaped” stenosis and is unique to this entity (Fig. 114-21).
EXCLUSION PROCEDURE: ENDOVASCULAR METHODS OF EXCLUSION OR OCCLUSION
Description and Special Anatomic Considerations
In contrast, endovascular treatment of iatrogenic post-catheterization pseudoaneurysms is a well-established therapy, with excellent long-term data available.24 With certain exceptions noted in the section on surgical treatment of these pseudoaneurysms, percutaneous thrombin injection is the preferred management.
Outcomes and Complications
For endovascular repair of peripheral aneurysms to be accepted as a valid alternative to open repair, it must be equivalent or superior to the accepted standard.25–28 Mohan and coworkers29 reported a cumulative primary patency rate of 74.5% and a cumulative secondary patency rate of 83.2% at 24 and 36 months, respectively. Tielliu and colleagues30 reported overall 3- and 5-year patency rates of 77% and 70% for primary patency and 86% and 76% for secondary patency, respectively, in 73 popliteal artery aneurysms treated by endovascular means. This group found that the use of clopidogrel (75 mg daily) was a significant predictor of the success of a popliteal artery stent graft. In these two series, the use of versatile and flexible new stent grafts for endovascular popliteal artery aneurysm repair yielded patency results that are comparable to those of conventional open surgical repair, which has an average 5-year graft patency of 70% to 80%.29–31
There have been more than 45 series reporting the safety and efficacy of thrombin injection for post-catheterization pseudoaneurysms, with a 97% overall cumulative success rate. Potential complications include thrombosis of the underlying native artery, distal embolization of thrombotic material, deep venous thrombosis, allergic reaction to the thrombin, local erythema, and infected abscess. The complication rate is extremely low, however, with a reported overall incidence of 1.3% and an embolic rate of 0.5%.24
Imaging Findings
As with surgical exclusion procedures, successful endovascular treatment requires appropriate preoperative imaging. The morphology of the aneurysm and of the adjacent arterial segments and the distal runoff should be clearly defined. This will allow both appropriate sizing and optimal placement of the endoprosthesis. This can be accomplished with CTA, MRA, or conventional catheter angiography. If CTA is performed preoperatively, one may obtain volume rendered three-dimensional images of the aneurysm through the use of postprocessing and reformatting software (Fig. 114-22A). Images such as these allow one to view the aneurysm in multiple projections and to obtain “centerline” measurements that allow more accurate determination of the appropriate length of the endoprosthesis. Accurate intraoperative imaging is also essential for accurate endovascular aneurysm repair. Iodinated contrast medium is injected either through an intravascular sheath or through a catheter that has been positioned proximal to the aneurysm so that angiographic images are obtained. Anteroposterior and lateral angiographic views are generally obtained to delineate the aneurysm morphology intraprocedurally and to assess for any branch vessels that arise within the treatment zone. Angiography of the runoff arterial anatomy is also obtained to verify the quality and patency of the vasculature. After endovascular treatment, the proximal and distal attachments and the course of the prosthesis should be clearly demonstrated. Delayed images should be obtained to assess for an endoleak. The distal runoff anatomy should once more be evaluated; any compromise may significantly affect the long-term outcome (Fig. 114-22B,C and Fig. 114-23).
Iatrogenic or post-traumatic pseudoaneurysms must also be clearly defined, particularly the relationship of neck and the parent feeding artery. After treatment, one should document complete thrombosis of the pseudoaneurysm and patency of the adjacent native vascular structures (Fig. 114-24).
KEY POINTS
 Arterial bypass is indicated in patients with long-segment chronic total superficial femoral artery occlusion, chronic total popliteal artery or proximal trifurcation vessel occlusion, diffuse multiple stenoses or occlusions involving the entire superficial femoral artery, and recurrent stenoses or occlusions after two or more prior endovascular treatments.
Arterial bypass is indicated in patients with long-segment chronic total superficial femoral artery occlusion, chronic total popliteal artery or proximal trifurcation vessel occlusion, diffuse multiple stenoses or occlusions involving the entire superficial femoral artery, and recurrent stenoses or occlusions after two or more prior endovascular treatments. Surveillance of bypass grafts is critical to ensure long-term patency, given that a relatively high percentage of vascular conduits develop problems that threaten longevity. The simplest and most effective surveillance tool is duplex ultrasonography coupled with ankle-brachial index measurement.
Surveillance of bypass grafts is critical to ensure long-term patency, given that a relatively high percentage of vascular conduits develop problems that threaten longevity. The simplest and most effective surveillance tool is duplex ultrasonography coupled with ankle-brachial index measurement. Determination of which lesions are appropriate for endovascular treatment versus traditional surgical revascularization continues to be an evolving and sometimes controversial process.
Determination of which lesions are appropriate for endovascular treatment versus traditional surgical revascularization continues to be an evolving and sometimes controversial process. The characteristics of the lesion greatly affect outcomes of endovascular revascularization procedures as the aim of these procedures is essentially to repair a diseased arterial segment in situ as opposed to bypass or replacement of the diseased segment.
The characteristics of the lesion greatly affect outcomes of endovascular revascularization procedures as the aim of these procedures is essentially to repair a diseased arterial segment in situ as opposed to bypass or replacement of the diseased segment. In general, short segmental stenoses respond well to angioplasty and may not require stent placement, whereas long-segment, diffusely diseased, or heavily calcified lesions may require stent placement to achieve and to maintain patency.
In general, short segmental stenoses respond well to angioplasty and may not require stent placement, whereas long-segment, diffusely diseased, or heavily calcified lesions may require stent placement to achieve and to maintain patency.Bunting TA, Garcia LA. Peripheral atherectomy: a critical review. J Interv Cardiol. 2007;20:417-424.
Eskelinen E, Lepäntalo M. Role of infrainguinal angioplasty in the treatment of critical limb ischaemia. Scand J Surg. 2007;96:11-16.
Galland RB. Popliteal aneurysms: from John Hunter to the 21st century. Ann R Coll Surg Engl. 2007;89:466-471.
Lopera JE, Trimmer CK, Josephs SG, et al. Multidetector CT angiography of infrainguinal arterial bypass. Radiographics. 2008;28:529-548.
McCaslin JE, Macdonald S, Stansby G. Cryoplasty for peripheral vascular disease. Cochrane Database Syst Rev 2007; 4:CD005507.
Nelson PR, Anthony Lee W. Endovascular treatment of popliteal artery aneurysms. Vascular. 2006;14:297-304.
Perera GB, Lyden SP. Current trends in lower extremity revascularization. Surg Clin North Am. 2007;87:1135-1147. x
Ramaiah V. Endovascular infrainguinal revascularization: technical tips for atherectomy device selection and procedural success. Semin Vasc Surg. 2008;21:41-49.
Rogers JH, Laird JR. Overview of new technologies for lower extremity revascularization. Circulation. 2007;116:2072-2085.
Silvers LW, Royster TS, Mulcare RJ. Peripheral arterial emboli and factors in their recurrence rate. Ann Surg. 1980;192:232-236.
Wain RA, Hines G. A contemporary review of popliteal artery aneurysms. Cardiol Rev. 2007;15:102-107.
1 Haider SN, Kavanaugh EG, Forlee M, et al. Two-year outcome with preferential use of infrainguinal angioplasty for critical ischemia. J Vasc Surg. 2006;43:504-512.
2 Schillinger M, Sabeti S, Loewe C, et al. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N Engl J Med. 2006;354:1879-1888.
3 BASIL trial participants. Bypass versus angioplasty in severe ischemia of the leg (BASIL): multicenter randomized controlled trial. Lancet. 2005;366:1925-1934.
4 Schillinger M, Sabeti S, Loewe C, et al. Sustained benefit at 2 years of primary femoropopliteal stenting compared with balloon angioplasty with optional stenting. Circulation. 2007;115:2745-2749.
5 Saxon RR, Coffman JM, Gooding JM, et al. Long-term results of ePTFE stent-graft versus angioplasty in the femoropopliteal artery: single center experience from a prospective randomized study. Circulation. 2000;102:2694-2699.
6 Kedora J, Hohmann S, Garrett W, et al. Randomized comparison or percutaneous Viabahn stent graft vs. prosthetic femoral-popliteal bypass in the treatment of superficial femoral arterial occlusive disease. J Vasc Surg. 2007;45:10-16.
7 Tolva V, Bertoni GB, Trimarchi S, et al. Unreliability of depopulated bovine ureteric xenograft for infrainguinal bypass surgery: mid-term results from two vascular centres. Eur J Endovasc Surg. 2007;33:214-216.
8 Patel A, Taylor SM, Langan EM3rd, et al. Obturator bypass: a classic approach for the treatment of contemporary groin infection. Am Surg. 2002;68:653-659.
9 Laird JR, Biamino G, McNamara T, et al. Cryoplasty for the treatment of femoropopliteal arterial disease: extended follow-up results. J Endovasc Ther. 2006;13(Suppl 2):II52-II59.
10 Laird J, Jaff MR, Biamino G, et al. Cryoplasty for the treatment of femoropopliteal arterial disease: results of a prospective, multicenter registry. J Vasc Interv Radiol. 2005;16:1067-1073.
11 Bittl JA, Chew DP, Topol EJ, et al. Meta-analysis of randomized trials of percutaneous transluminal coronary angioplasty versus atherectomy, cutting balloon atherotomy, or laser angioplasty. J Am Coll Cardiol. 2004;43:936-942.
12 Dormandy JA, Rutherford RB. Management of peripheral arterial disease (PAD) TASC Working Group. TransAtlantic Inter-Society Consensus (TASC). J Vasc Surg. 2000;331(pt 2):S1-S296.
13 Norgren L, Hiatt WR, Dormandy JA, et al. TASC II Working Group. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg. 2007;45(Suppl S):S5-S67.
14 Levy F, Butcher HR. Arterial emboli: an analysis of 125 patients. Surgery. 1970;68:968-973.
15 Eriksson I, Holmberg T. Analysis of factors affecting limb salvage and mortality after embolectomy. Acta Chir Scand. 1977;143:237-240.
16 Galbraith K, Collin J, Morris PJ, Wood RFM. Recent experience with arterial embolism of the limbs in a vascular unit. Ann R Coll Surg Engl. 1985;67:30-33.
17 Thompson JE, Staler L, Raut PS, et al. Arterial embolectomy: a 20 year experience with 163 cases. Surgery. 1970;67:212-220.
18 Atiani B, Evans WE. Immediate prognosis and five year survival after arterial embolectomy following myocardial infarction. Surg Gynecol Obstet. 1980;150:41-44.
19 Balas P, Bonatsos G, Xeromeritas N, et al. Early surgical results on acute arterial occlusion of the extremities. J. Cardiovasc Surg. 1985;26:262-269.
20 Kairaluoma K, Larmi TK. Surgical treatment of arterial embolism. Ann Chir Gyn. 1976;65:163-167.
21 Kendrick J, Thompson BW, Campbell GS, et al. Arterial embolectomy in the leg. Am J Surg. 1981;142:739-743.
22 Szczepanski KP. Results of surgical treatment of arterial embolism. Scand J Thorac Cardiovasc Surg. 1979;13:71-75.
23 Dryksjim M, Swedenborg J. Acute thrombosis and embolism of the extremities: factors influencing the result of treatment. Acta Chir Scand. 1982;148:135-139.
24 Webber GW, Jang J, Gustavson S, Olin JW. Contemporary management of postcatheterization pseudoaneurysms. Circulation. 2007;115:2666-2674.
25 Curi MA, Geraghty PJ, Merino OA, et al. Mid-term outcomes of endovascular popliteal artery aneurysm repair. J Vasc Surg.. 2007;45:505-510.
26 Davies RS, Wall M, Rai S, et al. Long-term results of surgical repair of popliteal artery aneurysm. Eur J Endovasc Surg. 2007;34:714.
27 Rajasinghe HA, Tzilinis A, Keller T, et al. Endovascular exclusion of popliteal artery aneurysms with expanded polytetrafluoroethylene stent-grafts: early results. Vasc Endovascular Surg. 2006;40:460-466.
28 Laganà D, Carrafiello G, Mangini M, et al. Endovascular treatment of femoropopliteal aneurysms: a five-year experience. Cardiovasc Intervent Radiol. 2006;29:819-825.
29 Mohan IV, Bray PJ, Harris JP, et al. Endovascular popliteal aneurysm repair: are the results comparable to open surgery? Eur J Vasc Endovasc Surg. 2006;32:149-154.
30 Tielliu IF, Verhoeven EL, Zeebregts CJ, et al. Endovascular treatment of popliteal artery aneurysms: results of a prospective cohort study. J Vasc Surg. 2005;41:561-567.
31 Antonello M, Frigatti P, Battocchio P, et al. Open repair versus endovascular treatment for asymptomatic popliteal artery aneurysm: results of a prospective randomized study. J Vasc Surg. 2005;42:185-193.

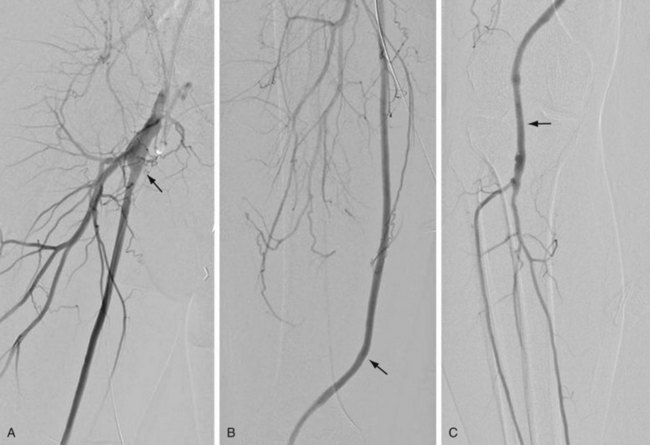
 FIGURE 114-1
FIGURE 114-1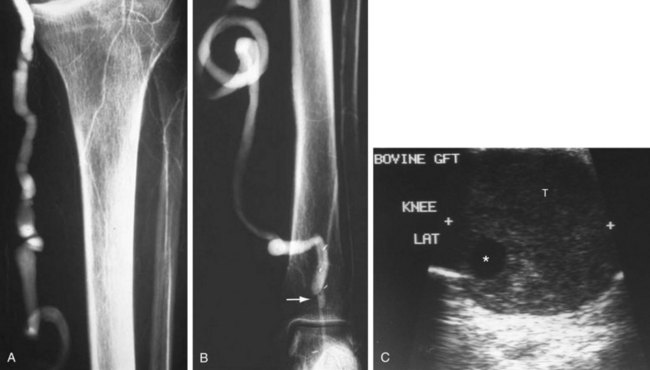
 FIGURE 114-2
FIGURE 114-2
 FIGURE 114-3
FIGURE 114-3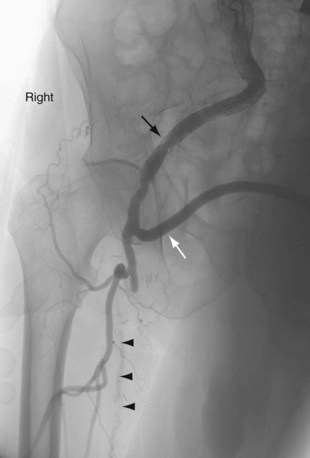
 FIGURE 114-4
FIGURE 114-4
 FIGURE 114-5
FIGURE 114-5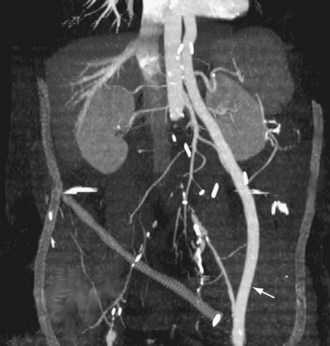
 FIGURE 114-6
FIGURE 114-6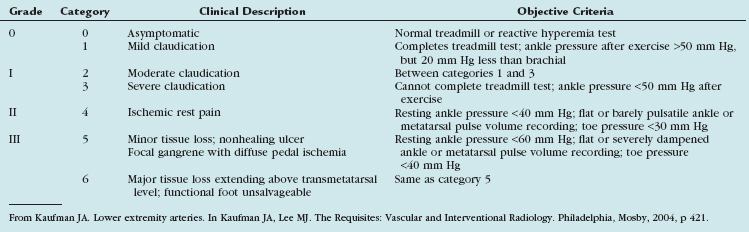
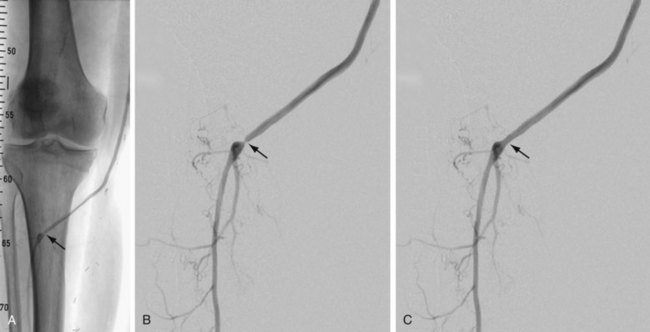
 FIGURE 114-7
FIGURE 114-7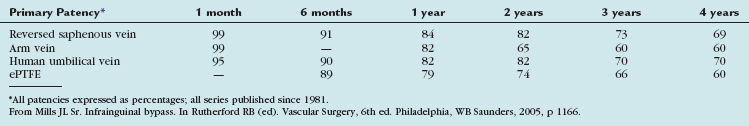
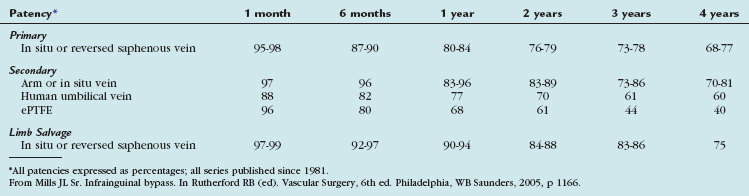
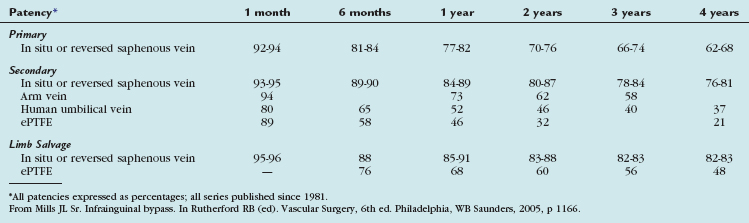

 FIGURE 114-8
FIGURE 114-8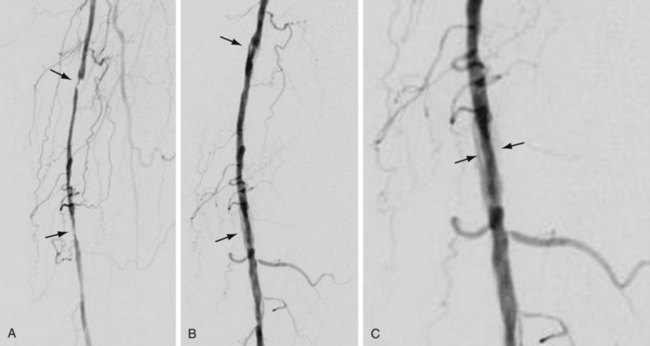
 FIGURE 114-9
FIGURE 114-9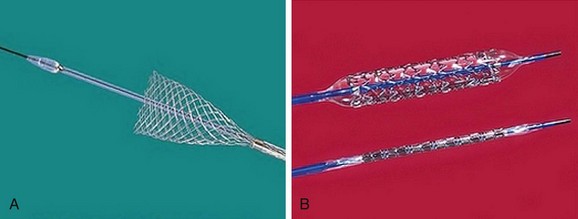
 FIGURE 114-10
FIGURE 114-10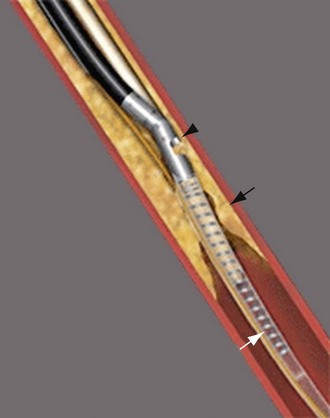
 FIGURE 114-12
FIGURE 114-12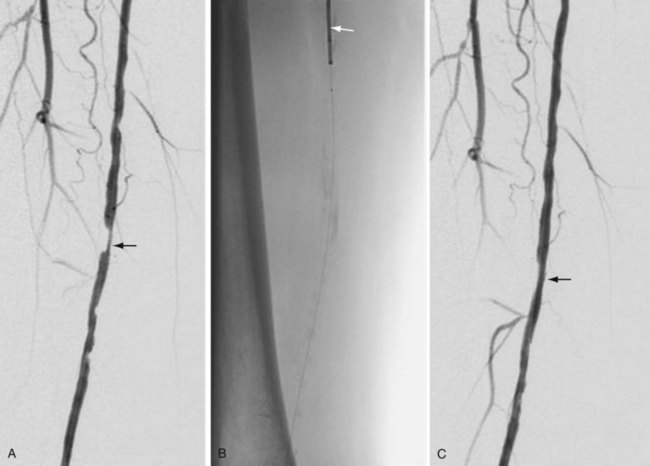
 FIGURE 114-13
FIGURE 114-13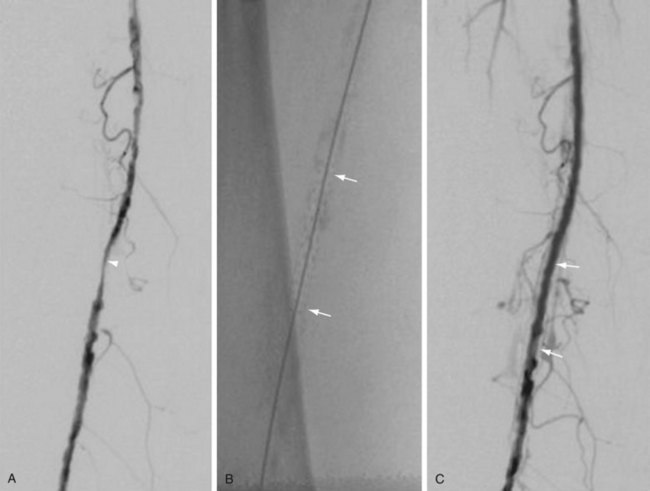
 FIGURE 114-14
FIGURE 114-14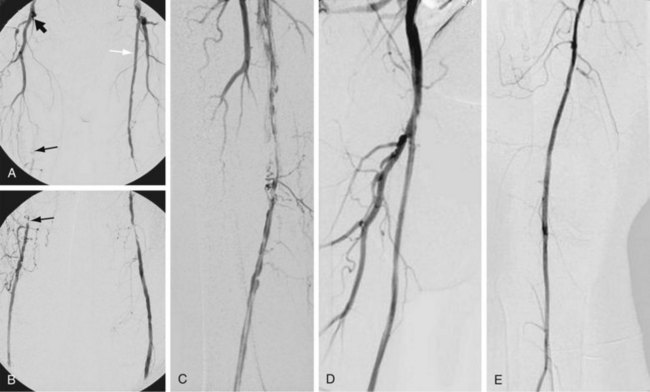
 FIGURE 114-15
FIGURE 114-15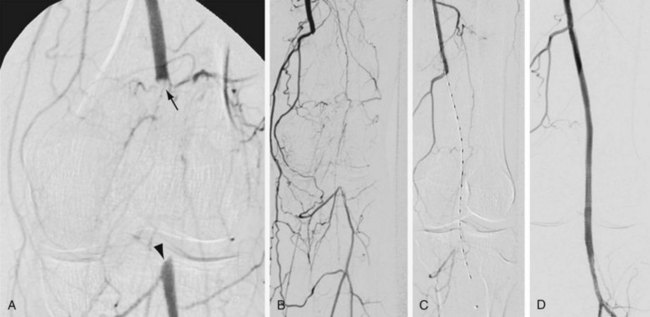
 FIGURE 114-16
FIGURE 114-16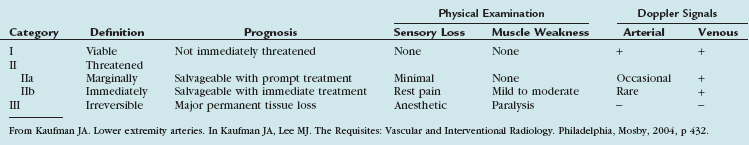
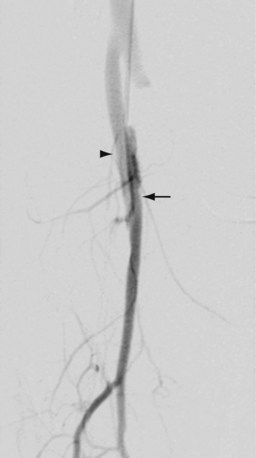
 FIGURE 114-17
FIGURE 114-17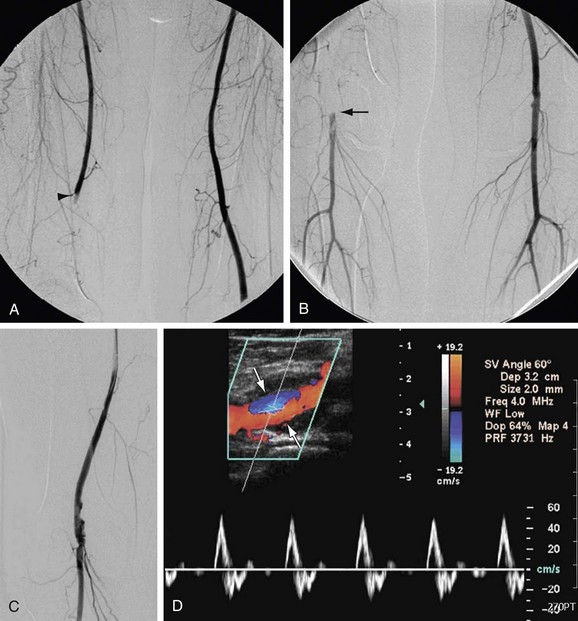
 FIGURE 114-18
FIGURE 114-18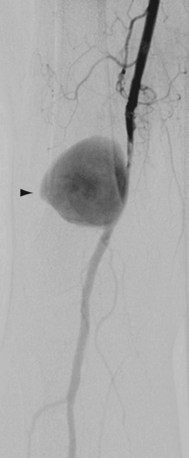
 FIGURE 114-19
FIGURE 114-19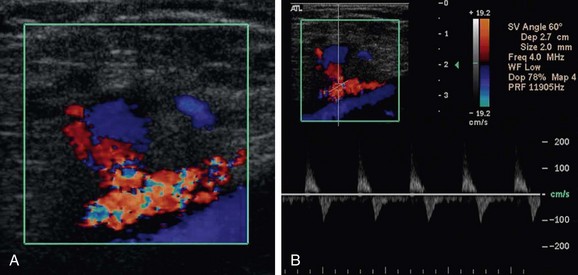
 FIGURE 114-20
FIGURE 114-20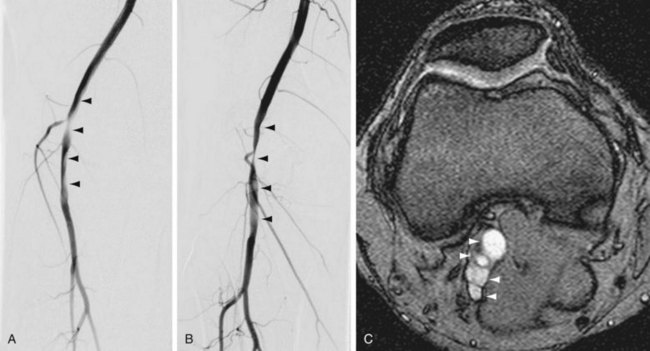
 FIGURE 114-21
FIGURE 114-21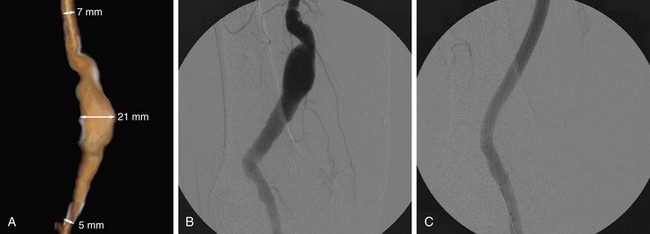
 FIGURE 114-22
FIGURE 114-22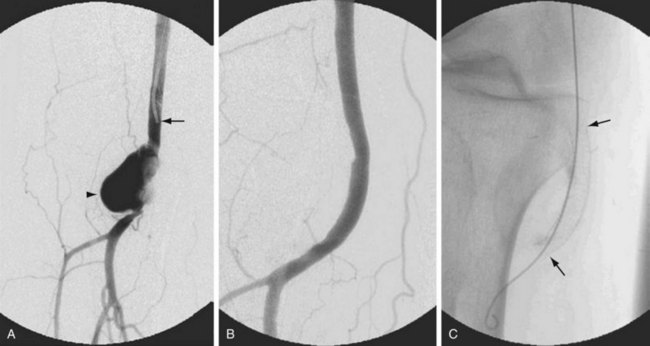
 FIGURE 114-23
FIGURE 114-23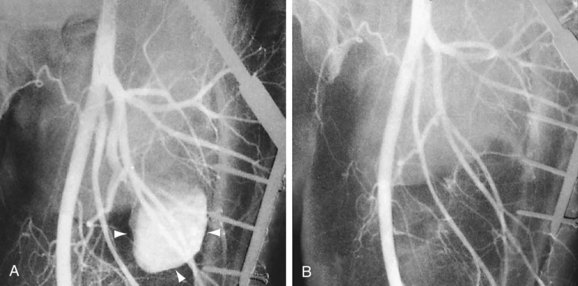
 FIGURE 114-24
FIGURE 114-24


