Chapter 15 Low back pain
Definitions
Back pain
In an effort to standardise the use of terms and to set standards of diagnostic practice, the International Association for the Study of Pain (IASP) published the second edition of its taxonomy.1 This document provides definitions of clinical terms used to describe pain and sets criteria for the diagnosis of specific entities.
With respect to presenting complaints, the taxonomy defines spinal pain topographically. It recognises ‘lumbar spinal pain’ and ‘sacral spinal pain’.1 Lumbar spinal pain is defined as pain perceived within a region bounded laterally by the lateral borders of the erector spinae, superiorly by an imaginary transverse line through the T12 spinous process, and inferiorly by a line through the S1 spinous process. Sacral spinal pain is defined as pain perceived within a region overlying the sacrum, bounded laterally by imaginary vertical lines through the posterior superior and posterior inferior iliac spines, superiorly by a transverse line through the S1 spinous process, and inferiorly by a transverse line through the posterior sacrococcygeal joints.
Somatic pain
Somatic pain is pain that results from noxious stimulation of one of the musculoskeletal components of the body.1,2 Neurophysiologically, the essential feature is that it arises as a result of the stimulation of nerve endings in a bone, ligament, joint or muscle.
Referred pain
Referred pain is pain perceived in a region innervated by nerves other than those that innervate the actual source of pain.1,2 As such, referred pain may be perceived in areas relatively remote from the source of pain, but often the distinction is blurred when the regions of local pain and referred pain are contiguous and the two pains appear to be confluent. A knowledge of the innervation of the affected regions, however, serves to make the distinction.
The physiological basis for referred pain is convergence.1 Within the spinal cord and in the thalamus, sensory neurones that subtend different peripheral sites converge onto common neurones that relay to higher centres. In the absence of any additional sensory information, the brain is unable to determine whether activity in the common neurone was initiated by one or the other of its peripheral inputs.
Clinically, the characteristic features of somatic referred pain are that it is perceived deeply, it is diffuse and hard to localise and it is aching in quality.2 Physiologically, the critical feature is that it is evoked by the stimulation of nerve endings in the structure that is the primary source of pain. The sensory nerves that innervate the region of referred pain are not activated by the primary stimulus, nor do they convey the referred pain. Referred pain occurs because of a misperception of the origin of the signal which reaches the brain by a convergent sensory pathway. These features underlie the distinction between somatic referred pain and radicular pain.
Radiculopathy
Radiculopathy is a neurological condition in which conduction is blocked in the axons of a spinal nerve or its roots.1,2 Conduction block in sensory axons results in numbness; conduction block in motor axons results in weakness. Radiculopathy can be caused by compression or ischaemia of the affected axons. Systematically, these causes are outlined in Table 15.1.
| Condition | Cause |
|---|---|
| Foraminal stenosis | Vertical subluxation of vertebrae3,4 |
| Osteophytes from disc5–10 | |
| Osteophytes from zygapophysial joint11–13 | |
| Buckled ligamentum flavum14 | |
| Cyst of ligamentum flavum15 | |
| Slipped inferior articular epiphysis16 | |
| Ganglion17–21 | |
| Synovial tumour22 | |
| Infections and tumours of vertebrae11,12 | |
| Paget’s disease11,23 | |
| Zygapophysial lipoma24,25 | |
| Epidural disorders | Lipoma; angioma11,12 |
| Infections11 | |
| Meningeal disorders | Cysts of the nerve root sleeve11,26–29 |
| Intradural ossification30 | |
| Neurological disorders | Diabetes31 |
| Cysts and tumours11 | |
| Infections; tabes dorsalis11 | |
| Disc herniation |
Radicular pain
Radicular pain is pain that arises as a result of irritation of a spinal nerve or its roots.1,2 Radicular pain may be associated with radiculopathy but not necessarily so. Radicular pain may occur without radiculopathy and radiculopathy may occur without radicular pain.
It was once believed that radicular pain was due to compression of nerve roots. This is patently untrue. Neurophysiological experiments have shown that compression of a nerve root does not evoke nociceptive activity; at best it evokes a brief discharge at the time of application of the compression stimulus, but thereafter the root becomes silent.32,33 It is only when dorsal root ganglia are compressed that sustained activity is evoked, but this activity occurs not only in nociceptive axons but also in β fibres.32,33 The sensation, therefore, must be more than just pain. This is borne out in clinical experiments.
Clinical experiments have shown that compressing normal nerve roots with urinary catheters evokes paraesthesia and numbness but not pain.34 Similarly, traction on a normal nerve root does not evoke pain.35 It is only when previously damaged nerve roots are squeezed by forceps36 or pulled with sutures,35 or when nerve roots are stimulated electrically,37 that a characteristic pain is evoked. The pain is shooting or lancinating in quality and travels down the lower limb along a band no more than 50 mm (2 inches) wide.35
In the face of this evidence, it is pertinent to consider the term ‘sciatica’. The implied basis for sciatica is nerve root compression or nerve root irritation, whereupon sciatica must be considered a form of radicular pain. However, the available physiological evidence dictates that radicular pain has a characteristic quality and distribution, and therefore the term sciatica should be restricted to this type of pain in the lower limb. The only type of pain that has ever been produced experimentally by stimulating nerve roots is shooting pain in a band-like distribution.2 There is no physiological evidence that constant, deep aching pain in the lower limb arises from nerve root irritation. This latter type of pain constitutes somatic referred pain and there is no justification for misrepresenting it as ‘sciatica’, or for inferring that it arises from nerve root irritation. Moreover, there is no evidence that back pain can be caused by nerve root irritation, especially in the absence of neurological signs indicative of nerve root irritation. Indeed, on clinical grounds it has been estimated that fewer than 30%, and perhaps as few as 5% or 1%, of presentations of low back pain are associated with nerve root irritation due to disc herniation.38–40 A formal survey in the USA established that no more than 12% of patients with low back pain had any clinical evidence of disc herniation.41
Disc herniation is the single most common cause of radicular pain, and there is increasing evidence that this condition causes pain by mechanisms other than simple compression. The evidence against compression is twofold. On myelography, CT or MRI, individuals can exhibit root compression by disc herniation but have no symptoms.42–45 Conversely, patients previously symptomatic can still exhibit root compression on medical imaging despite resolution of their symptoms.46,47 These observations indicate that some factor in addition to, or quite apart from, root compression operates to produce symptoms. The current evidence implicates some form of inflammation.
Inflammation was implicated initially on the grounds that surgeons have often seen signs of nerve root inflammation when operating on herniated discs.48,49 Some early post-mortem studies reported signs of inflammation around nerve roots obtained at autopsy,50 but others found no such signs.51,52 Subsequently, a variety of studies suggested that nuclear material was inflammatory49,53–55 and perhaps capable of eliciting an autoimmune response.56–62 However, clinical studies failed to reveal features of a classic autoimmune diathesis in patients with prolapsed discs.63 Nevertheless, belief in some form of inflammation has persisted and has been explored.
In animal studies, compression of lumbar nerve roots causes oedema and increased intraneural pressure,64,65 and application of nucleus pulposus to nerve roots induces inflammatory changes in the form of increased vascular permeability, oedema, and intravascular coagulation.66–69 The inflammation damages the nerve roots, blocks nerve conduction,67,70–73 and produces hyperalgesia and pain behaviour.74–76 The mediators of this inflammatory response are phospholipase A2, nitric oxide and tumour necrosis factor alpha (TNF-α).70,75–82
Studies in human patients have shown that herniated disc material attracts macrophages, fibroblasts and lymphocytes83–90; and that a variety of inflammatory chemicals are produced by these cells or the disc material itself. These chemicals include: phospholipase A2;91,92 metalloproteinases;93,94 prostaglandin E2;89,93,94 leukotriene B4 and thrombaxane;95 nitric oxide;80,81,93,94 interleukin 8;96,97 interleukin 12 and interferon γ;88 and TNF-α.96 The disc material stimulates the production of IgM and IgG antibodies,98 particularly to the glyco-sphingolipid of the nerve roots.99 These inflammatory changes are more pronounced in patients in whom disc material has penetrated the anulus fibrosus and posterior longitudinal ligament, i.e. when it has become exposed in the epidural space.
The evidence is, thus, abundant that disc material evokes a chemical inflammation. The resulting nerve root oedema causes conduction block and the features of radiculopathy. Ectopic impulses generated in the dorsal root ganglia are responsible for the radicular pain,100–102 and are probably produced by ischaemia.65
Back pain
Postulates
1 The structure should have a nerve supply, for without access to the nervous system it could not evoke pain.
2 The structure should be capable of causing pain similar to that seen clinically. Ideally, this should be demonstrated in normal volunteers, for inferences drawn from clinical studies may be compromised by observer bias or poor patient reliability.
3 The structure should be susceptible to diseases or injuries that are known to be painful. Ideally, such disorders should be evident upon investigation of the patient but this may not always be possible. Certain conditions may not be detectable using currently available imaging techniques, whereupon the next line of evidence stems from post-mortem studies or biomechanical studies which can provide at least prima facie evidence of the types of disorders or injuries that might affect the structure.
4 The structure should have been shown to be a source of pain in patients, using diagnostic techniques of known reliability and validity. From such data, a measure of the prevalence of the condition in question can be obtained to indicate whether the condition is a rarity or oddity, or a common cause of back pain.
Sources of back pain
Vertebrae
There is no doubt that the vertebral bodies of the lumbar vertebrae are innervated.103–105 Nerve fibres, derived from the plexuses of the anterior longitudinal ligament and posterior longitudinal ligament, supply the periosteum of the bones and penetrate deep into the vertebral bodies where they provide a possible substrate for bone pain. However, it is not known whether the intraosseous nerves are exclusively vascular (either vasomotor or vasosensitive) or whether the bone of the vertebral body itself receives a sensory innervation.
For understandable logistic reasons, no experiments have demonstrated whether back pain can be evoked directly from bone in the vertebral body, but like periosteum in general106 the vertebral periosteum is clearly pain sensitive. Needling the periosteum in the course of procedures such as lumbar sympathetic blocks is regularly associated with pain.
The vertebral body may be affected by painful, metabolic bone diseases such as Paget’s disease107 or osteitis fibrosa,108 and it may be the site of primary or secondary tumours109,110 or infections.111,112 There is no dispute that such conditions can be painful, but how they actually cause pain is not known.
Fractures of the vertebral body may or may not be painful,113,114 and it is difficult to determine whether the pain stems from the fracture itself or arises from abnormal stresses applied to adjacent joints, muscles or ligaments as a result of the accompanying deformity. There is no evidence that fractures, anywhere in the body, are intrinsically painful, especially when stable. On the other hand, tissue deformation as a result of post-traumatic haematoma or oedema is readily viewed as a potent source of pain, particularly if this causes distension or inflammation of the periosteum. Such a model conveniently explains why acute vertebral fractures might be painful, but why old or healed fractures are not. Surrounding tissue swelling would be expected early in the history of a fracture, but in due course would subside. Persistent pain following a healed vertebral body fracture suggests a source beyond the fracture site and probably secondary to the resultant deformity.
The most common disease that affects lumbar vertebral bodies is osteoporosis but there is no evidence that this condition is painful, in the absence of fracture. The temptation is to infer that pain could arise directly from stresses applied to the weakened vertebral body, or that microfractures mechanically irritate perivascular sensory nerves within the vertebral spongiosa115 but in the absence of even remote evidence about the physiology of bone pain, such explanations are purely speculative.
Citing the literature on vertebroplasty and its claims of success, some authors have argued that the pain of osteoporosis fractures is mediated by intraosseous nerves, and is relieved by a neurolytic effect of cement injected into the fractured vertebral body.116 However, this argument is circular because it presupposes that the relief of pain reported from vertebroplasty can be attributed to a local effect of treatment at the fracture site. Others dispute this argument on the grounds that nerve fibres in the vertebral body are too irregularly present and too sparse to explain the relief of pain by vertebroplasty.117 There is also evidence that the pain of osteoporotic fractures of vertebral arises, not from the affected vertebral body but from the posterior elements behind that vertebral body.118
A revolutionary, though now not new, concept concerning vertebral pain is that of intraosseous hypertension.119,120 The notion is that, if obstructed, intraosseous veins become distended and stimulate sensory nerves in their adventitia. The cause of obstruction is suggested to be bony sclerosis, such as that which occurs in spondylosis and which narrows the bony channels through which the veins pass. This hypothesis is consistent with the presence of perivascular nerves in the vertebral bodies and is analogous to the pain of congestive venous disorders of the lower limb. However, while this is an attractive theory for the pain of spondylosis, manometric studies of vertebral intraosseous pressure have been limited in number and have not compared symptomatic with asymptomatic individuals to provide convincing statistical evidence in support of the theory.119,120
Regardless of how they might cause pain, disorders of the lumbar vertebrae are relatively easy to diagnose; they are readily apparent on radiographs and other medical imaging. Their prevalence is not explicitly known but appears to be very low. Infections, tumours and fractures of the vertebral bodies are rare amongst patients presenting with back pain under the age of 50 years.121 Even in older patients they are uncommon.
Posterior elements
Kissing spines
The lumbar spinous processes may be affected by Baastrup’s disease,122 otherwise known as ‘kissing spines’.123 This arises as a result of excessive lumbar lordosis or extension injuries to the lumbar spine in which adjacent spinous processes clash and compress the intervening interspinous ligament. The resultant pathology is perhaps best described as a periostitis of the spinous processes or inflammation of the affected ligament. Given that the periosteum of the spinous processes and the interspinous space are innervated by the medial branches of the lumbar dorsal rami,124,105,125–127 it is understandable that such a condition would constitute a source of pain.
However, clinical studies suggest that this condition has been overrated. In one study, only 11 out of 64 patients with kissing spines responded to surgical excision of the lesion.128
Lamina impaction
A condition analogous to kissing spines can affect an inferior articular process. In some lumbar motion segments, extension is limited by impaction of an inferior articular process onto the lamina below (see Ch. 8). In such segments, repeated extension injuries can result in irritation of the periosteum of the lamina and, indeed, such lesions have been demonstrated at post-mortem.129–131 This disorder is attractive as an explanation for some forms of back pain affecting athletes such as gymnasts accustomed to excessive forceful extension, but no clinical studies have yet provided evidence for its occurrence in living, symptomatic patients.
Spondylolysis
Spondylolysis was originally considered to be a defect due to failure of union of two ossification centres in the vertebral lamina. Modern evidence, however, clearly shows that it is an acquired defect caused by fatigue fracture of the pars interarticularis.132–134 Anatomically, the defect is filled with fibrous scar tissue riddled with free nerve endings and nerve fibres containing calcitonin gene-related peptide, vasoactive intestinal peptide and neuropeptide Y.135,136 Nominally, therefore, it could be a source of pain.
However, pars defects are not necessarily painful. In a survey of radiographs of 32 600 asymptomatic individuals, a pars defect was present in 7.2%.137 Amongst 936 asymptomatic adults, a unilateral defect was present in 3% and a bilateral defect in a further 7%.138 The corresponding figures in patients with back pain were 0.3% and 9%, respectively. In children aged 6 years, the prevalence of a defect was found to be 4.4%, and rose to 6% by adulthood.139
The pars defect can be infiltrated with local anaesthetic under fluoroscopic control.140 Relief of pain constitutes prima facie evidence that the defect is the source of pain. However, precautions need to be taken to ensure that the local anaesthetic has not anaesthetised an adjacent structure (such as a zygapophysial joint, which might be an alternative source of pain) and to ensure that the response to a single diagnostic injection is not due to a placebo effect. Nevertheless, relief of pain following infiltration of a pars defect is a good predictor of successful outcome following fusion of the defect and failure to obtain relief predicts poor response to fusion.140
Bone scans are used by some practitioners to diagnose symptomatic pars fractures but the relationships between positive bone scans and either radiological evidence of a fracture or pain are imperfect.141 Bone scans are most useful before a fracture has actually occurred, when the scan detects the stress reaction in the pars interarticularis. Once fracture has occurred the scan may or may not be positive, and tends to be negative in patients with chronic pain.141
Muscles
The muscles of the lumbar spine are well innervated. Quadratus lumborum and psoas are supplied by branches of the lumbar ventral rami,142 while the back muscles are supplied by the dorsal rami.124 The intertransverse muscles are variously supplied by the dorsal rami and ventral rami.143
There is no question that the back muscles can be a source of back pain and somatic referred pain. This has been demonstrated in experiments on normal volunteers in whom the back muscles were stimulated with injections of hypertonic saline.144,145 These injections produced low back pain and various patterns of somatic referred pain in the gluteal region.
Sprain
Animal studies have shown that when muscles are forcibly stretched against contraction, they characteristically fail at the myotendinous junction.146–148 The resulting lesion would presumably evoke an inflammatory repair response, which is easily accepted as a source of pain. In the case of the back muscles, such lesions could be incurred during lateral flexion or combined flexion–rotation injuries of the trunk and would be associated with tenderness near the myotendinous junctions of the affected muscles. Some of these sites are superficial and accessible to clinical examination, but others are deep. Deep sites lie near the tips of the transverse and accessory processes of the lumbar vertebrae. Accessible myotendinous junctions lie just short of the ribs near the insertions of the iliocostalis lumborum.
Conspicuously, what is lacking in the case of back muscle injuries is any direct evidence of the responsible lesion. The only data that might be invoked come from experiments in animals in which the muscles of the limbs, not the back muscles, were studied. Biopsy data in humans or imaging data have not been published. For this reason a workshop sponsored by the American Academy of Orthopedic Surgeons and the National Institutes of Health was circumspect and inconclusive in its approach to the notion that sprained back muscles were a common cause of pain.149 However, the advent of MRI may now provide a suitable non-invasive tool with which traumatic lesions of the back muscles might be identified.150
Spasm
Although myotendinous tears might underlie cases of acute back pain stemming from muscle, it is more difficult to explain chronic back pain in terms of muscle pain. A popular belief is that of muscle ‘spasm’.151 The implication is that as a result of some postural abnormality, or secondary to some articular source of pain, muscles become chronically active and therefore painful. If it occurs, such pain can only be explained on the basis of ischaemia, but the greatest liability of this model of muscular pain is that the purported evidence for its existence is inconclusive.151 Electromyographic data are inconsistent, and it is unclear what so-called muscle spasm constitutes in objective physiological terms: whether it is tonic contraction or simply hyperreflexia. Further research data are required before this notion can become more acceptable, together with an explanation of whether the pain arises as a result of ischaemia, strain on the muscle attachments or some other mechanism.
Imbalance
Another concept is that of ‘muscle imbalance’.152 It is believed that aberrations in the balance of tone between postural and phasic muscles, or between flexors and extensors, can give rise to pain. In the case of the lumbar spine, the imbalance is said to occur between the trunk extensors and psoas major on the one hand, and the trunk flexors and hip extensors on the other. While attractive to some, this theory is without proper foundation. In the first instance, it is unclear how the imbalance comes to be painful: whether the pain arises from one or other of the muscles involved or whether the imbalance somehow stresses an underlying joint. Belief in the theory is founded on the clinical detection of muscle imbalance, but the reliability and validity of the techniques used have never been determined, and so-called muscle imbalances have been pronounced as abnormal without proper comparison to normal biological variation. Objective corroborating evidence is required before this theory attracts more widespread credibility.
Trigger points
Trigger points are tender areas occurring in muscle capable of producing local and referred pain. They are characterised by points of exquisite tenderness located within palpable bands of taut muscle fibres. Clinically, they are distinguished from tender areas by the presence of the palpable band of fibres, which, when snapped, elicits a localised twitch response in the muscle, and which when pressed reproduces the patient’s referred pain.153 The pain is relieved if the trigger point is injected with local anaesthetic.
Trigger points are believed to arise as a result of acute or chronic repetitive strain of the affected muscle,153 or ‘reflexly’ as a result of underlying joint disease.154 Histological and biochemical evidence as to the nature of trigger points are incomplete or inconclusive,153 but they are believed to represent areas of hypercontracted muscle cells that deplete local energy stores and impair the function of calcium pumps, thereby perpetuating the contraction.153–155 Pain is said to occur as a result of obstruction of local blood flow and the accumulation of algogenic metabolites such as bradykinin.153
Trigger points have been reported to affect the multifidus, longissimus and iliocostalis muscles,156 and the quadratus lumborum.157 However, it is not known how often they are a cause of back pain as the diagnostic criteria are so hard to satisfy.
For the diagnosis of trigger points in the iliocostalis and longissimus muscles, the kappa scores for two observers agreeing ranged from 0.35 to 0.46, which is less than satisfying.158 Similar scores obtain for trigger points in the quadratus lumborum and gluteus medius.159 It is only if the diagnostic criteria for trigger points are relaxed, to exclude palpable band and twitch response, that acceptable kappa scores are achieved,159 but this changes the diagnosis from one of ‘trigger point’ to one of ‘tender point’ in the muscle.
Without reliable criteria for diagnosis, it is not possible to estimate the prevalence of trigger points as a cause of back pain. If classic criteria are used strictly, trigger points seem to be uncommon.159 Tenderness, on the other hand, seems to be quite common but does not constitute a diagnosis.
Thoracolumbar fascia
Compartment syndrome
At its attachment to the supraspinous ligaments, the thoracolumbar fascia is well innervated.126,127,160 However, little is known about the innervation of its central portions. There is only a mention in one study that it contains nociceptive nerve endings.161 Nevertheless, it would appear that the fascia is appropriately innervated to be a source of pain if excessively stretched.
Since the thoracolumbar fascia encloses the back muscles, it forms a compartment surrounding them, and this has attracted the proposal that the back may be affected by a compartment syndrome.162,163 The concept is that, in susceptible patients, the back muscles swell during and following activity but their expansion is restricted by the thoracolumbar fascia. Pain presumably arises as a result of excessive strain in the fascia.
The clinical marker of such a compartment syndrome would be raised intracompartmental pressure, but clinical studies have yielded mixed results. In one study, a series of 12 patients was investigated for suspected compartment syndromes. Only one patient exhibited sustained, elevated pressures in the compartment on the side of pain.164 In another study, however, seven patients were identified whose compartment pressure rose above normal upon flexion and was associated with the onset of back pain; fasciotomy reportedly relieved their pain.165 However, while offering intriguing results, this study did not rigorously report the variance of pressures in the control group and other diagnostic groups who also exhibited raised pressures. Therefore, it is not evident how unique the feature of raised pressure is to compartment syndrome.
Fat herniation
The posterior layer of thoracolumbar fascia is fenestrated to allow the transmission of the cutaneous branches of the dorsal rami. These sites may be associated with painful herniations of fat.166–173 It is unclear exactly how these herniations actually cause pain, but reportedly the pain can be relieved by infiltrating the site with local anaesthetic. In this regard, painful fat herniations resemble trigger points, but are distinguished clinically from trigger points by their extramuscular, subcutaneous location. Their prevalence is unknown.
Dura mater
The dura mater is innervated by an extensive plexus derived from the lumbar sinuvertebral nerves. The plexus is dense over the ventral aspect of the dural sac and around the nerve root sleeves, but posterolaterally the innervation is sparse, and is entirely lacking over the posterior aspect of the dural sac.103,174 Clinical experiments have shown that the dura is sensitive both to mechanical and chemical stimulation.35,175 In both cases, stimulation invokes back pain and somatic referred pain into the buttock. This raises the possibility that dural irritation could be a source of back pain.
Back pain is well known in the context of neurological diseases in which the dura mater becomes inflamed in response to intrathecal blood or infection.176 This establishes that the dura can be a source of back pain. Whether it is or not, in the context of musculoskeletal diseases, is subject to conjecture.
Since it is known that disc herniation can elicit a chemical inflammation of the nerve roots and perineurial tissues,55,67,68,70,72 and that disc material contains high concentrations of phospholipase A2,91 which is highly inflammatory,92 it seems reasonable to expect that the dural sleeve of the nerve roots could be irritated chemically by this inflammation. Such irritation would elicit somatic pain, perhaps with referred pain, in addition to, and quite apart from, any pain stemming from the inflamed nerve roots. This conjecture raises the spectre that what has been traditionally interpreted as ‘root pain’ associated with disc herniation may not be purely radicular pain but a mixture of radicular and dural pain. However, no studies have yet ventured to dissect dural pain from radicular pain in cases of disc herniation.
In a similar vein, it has been proposed that the normally occurring epidural ligaments can tether nerve roots and be a source of somatic pain superimposed on radicular pain.177 However, as with dural ‘adhesions’ appropriate clinicopathological correlations have yet to be demonstrated.
Seductive evidence for dural pain comes from neurosurgical studies that report relief of post-laminectomy pain following resection of the nerves to the dura sleeve of the symptomatic nerve root.178,179 Ostensibly, the pain was due to stimulation of the nerves by fibrosis of the dura.
Epidural plexus
The epidural veins are innervated by the lumbar sinuvertebral nerves103,180 and are therefore a possible source of pain. Presumably, pain could occur if these veins became distended when flow through them was obstructed by lesions such as massive disc herniation or spinal stenosis. However, circumstantial evidence of this concept has been provided in only one published study181 and the concept has not otherwise been further explored.
Ligaments
The ligamentum flavum is poorly innervated104,125,126,182,183 and is therefore unlikely to be a source of pain. Furthermore, there are no known lesions that affect the ligamentum flavum that could render it painful, and because the ligament is elastic, it is not susceptible to sprain. It has a distensibility far in excess of that of the posterior longitudinal ligament and other collagenous ligaments of the lumbar spine.184
The so-called supraspinous ligament has been shown to consist of collagen fibres derived from the thoracolumbar fascia, the erector spinae aponeurosis and the tendons of multifidus.185,186 Technically, it is therefore a raphe rather than a ligament, but the most decisive evidence against the supraspinous ligament being a source of back pain at L4 and L5 (the most common location of low back pain) is that the ligament is totally lacking. It is consistently absent at L5, frequently so at L4, and even at L3 it is poorly developed and irregularly present.186
The posterior longitudinal ligament is innervated by the sinuvertebral nerves, and the anterior longitudinal ligament by fibres from the lumbar sympathetic trunk and grey rami communicantes.103,180,187 Reports that probing the back of a lumbar disc at operation under local anaesthetic reproduces the patient’s back pain,188,189 engender the belief that the pain naturally stems from the overlying posterior longitudinal ligaments. However, the posterior longitudinal ligament blends intimately with the anulus fibrosus of the intervertebral disc at each segmental level. Anatomically the longitudinal ligaments are not separable from the anulus fibrosus other than at a microscopic level. It is therefore not legitimate to consider disorders of the ligaments separately from those of the anulus fibrosus (see below).
Interspinous ligaments
The interspinous ligaments receive an innervation from the medial branches of the lumbar dorsal rami,44,105,124–127 and experimental stimulation of the interspinous ligament produces low back pain and referred pain in the lower limbs.190–192 This renders the interspinous ligament as an attractive source of low back pain.
Post-mortem studies have shown that the interspinous ligaments are frequently ‘degenerated’ in their central portions,186 but it is not known whether or not such lesions are painful. Otherwise, it is conceivable that interspinous ligaments might be subject to strain following excessive flexion of lumbar motion segments, but evidence of this is currently still lacking, even by way of comparing clinical history with the presence of midline interspinous tenderness and relief of pain following infiltration with local anaesthetic.
Clinical studies of the prevalence of interspinous ligament sprain are sobering. Steindler and Luck193 reported that in a heterogeneous population of 145 patients, 13 obtained complete relief of their pain following anaesthetisation of interspinous ligaments, suggesting a prevalence of less than 10%. A more recent audit of the experience of a musculoskeletal general practice found only 10 patients in a series of 230 whose pain could be relieved by anaesthetising an interspinous ligament.194 Since these injections were not controlled, the observed prevalence of 4% must be construed as a best-case estimate.
Iliolumbar ligament
The iliolumbar ligament has not explicitly been shown to have an innervation but presumably it is innervated by the dorsal rami or ventral rami of the L4 and L5 spinal nerves. Biomechanically, the iliolumbar ligament serves to resist flexion, rotation and lateral bending of the L5 vertebra,195–197 and could therefore be liable to strain during such movements. However, the evidence implicating the iliolumbar ligament as a source of back pain is inconclusive.
Some investigators have regarded tenderness over the posterior superior iliac spine as a sign of iliolumbar ligament sprain198 but this is hard to credit, for the ligament lies anterior to the ilium and is buried by the mass of the erector spinae and multifidus. Consequently, tenderness in this region cannot be explicitly ascribed to the iliolumbar ligament. Some have claimed to have relieved back pain by infiltrating the iliolumbar ligament,199 but because of the deep location of this structure, there can be no guarantee that, without radiological confirmation, the ligament was accurately or selectively infiltrated.
Other investigators have been more circumspect in interpreting tenderness near the posterior superior iliac spine, and question whether the pain stems from the iliolumbar ligament, the lumbosacral joint or the back muscles.200–202 Indeed, radiographic studies of injections made into the tender area reveal spread, not into the iliolumbar ligament but extensively along the iliac crest.198 Accordingly, the rubric – iliac crest syndrome – has been adopted to describe this entity.200,201 Others have referred to it simply as lumbosacral strain.202
What all investigators have overlooked is that the site of tenderness in iliac crest syndrome happens to overlie the site of attachment of the lumbar intermuscular aponeurosis (LIA), which constitutes a common tendon for the lumbar fibres of longissimus thoracis.203,204 The LIA attaches to the iliac crest rostromedial to the posterior superior iliac spine and exhibits a morphology not unlike that of the common extensor origin of the elbow. Thus, a basis for pain and tenderness in this region could be a tendonopathy of the LIA. On the other hand, it could be no more specific than tenderness in the posterior back muscles, which has been recognised for many years under different rubrics.202,205,206
Regardless of its underlying pathology, the putative advantage of recognising an iliac crest syndrome is that perhaps specific therapy might be applied. In this regard, if iliac crest syndrome is defined simply as tenderness over the medial part of the iliac crest, the kappa score for its diagnosis is 0.57.207 If the criteria are extended to include reproduction of typical pain, the kappa score rises to 0.66.207 These scores indicate that the syndrome can be identified. Its prevalence seems to be about 30–50%.200 However, as long as the syndrome amounts to no more than tenderness, it is not evident whether it is a unique disorder or a feature that could occur in association with other sources and causes of back pain.
Recognising the syndrome, however, has little impact on treatment. Injecting the area with local anaesthetic is significantly more effective than injecting it with normal saline, but only some 50% of patients benefit and only 30% obtain more than 80% improvement.200
Sacroiliac joint
The sacroiliac joint is reported to have an innervation. Branches of the L4–L5 and S1–S2 dorsal rami are directed to the posterior sacroiliac and interosseous sacroiliac ligaments,208 but it is not known whether these nerves actually reach the sacroiliac joint itself which lies substantially ventral of these ligaments. Anteriorly, the sacroiliac joint is said to receive branches from the obturator nerve, the lumbosacral trunk and the superior gluteal nerve,209 but the original sources for this claim are obscure. Modern studies provide conflicting results, with some reporting an innervation from both the front and the back,210 while others report an exclusively posterior innervation.211
In normal volunteers, stressing the sacroiliac joint with injections of contrast medium produces somatic pain focused over the joint, and a variable referral pattern into the lower limb.212 Thus, the joint is quite capable of being a source of back pain.
In orthodox medical circles, recognised disorders of the sacroiliac joint include ankylosing spondylitis, other spondylarthropathies, various infectious and metabolic diseases,213 and an idiopathic sacroiliitis that typically befalls women,214 but controversy surrounds alleged mechanical disorders of the joint. Manual therapists claim that such disorders can be diagnosed on the basis of palpable hypomobility of the joint and abnormal relations between the sacrum and ilium.215–220 However, biomechanical and radiographic studies reveal only a very small range of movement in the sacroiliac joint, even in patients diagnosed as having hypermobility.221–223 Nor does manipulation of the joint alter its position.224 These data provide grounds for scepticism as to whether pathological disturbances of movement can be palpated in a joint that has only 1° of movement.
However, formal studies have shown that sacroiliac joint pain can be diagnosed using intra-articular injections of local anaesthetic. In patients with chronic low back pain, the prevalence of sacroiliac joint pain is about 15%.225,226 The pathology of this pain is not known, although ventral capsular tears seem to underlie some cases.226
Although it can be diagnosed using intra-articular injections of local anaesthetic, sacroiliac joint pain cannot be diagnosed using orthodox clinical examination.226 Furthermore, it cannot be diagnosed using osteopathic or chiropractic techniques. Although these latter procedures have good reliability, they have no validity; they cannot distinguish patients who respond to diagnostic blocks from those who do not.227 Thus, although sacroiliac joint pain is common in patients with chronic low back pain, it can only be diagnosed using diagnostic local anaesthetic blocks.
Zygapophysial joints
The lumbar zygapophysial joints are well innervated by the medial branches of the lumbar dorsal rami.103–105,124,228 Their capacity to produce low back pain has been established in normal volunteers. Stimulation of the joints with injections of hypertonic saline or with injections of contrast medium produces back pain and somatic referred pain identical to that commonly seen in patients.229,230 Conversely, certain patients can have their pain relieved by anaesthetising one or more of the lumbar zygapophysial joints.230–234
Referred pain from the lumbar zygapophysial joints occurs predominantly in the buttock and thigh but does not follow any clinically reliable segmental pattern.229 Radiation of referred pain below the knee can occur, even as far as the foot,230,231 but typically the pain involves the more proximal segments of the lower limb. There is some evidence that the distance of radiation is proportional to the intensity of the pain generated in the back.230
Belief in lumbar zygapophysial joint pain dates back to 1933 when Ghormley235 coined the term ‘facet syndrome’. The entity has enjoyed a resurgence of interest over the last 20 years and, for reference, the history of this interest is recorded in detail elsewhere.236,237 However, much of the literature on the prevalence of zygapophysial joint pain has been made redundant.
Prevalence
In the past, the criterion standard for diagnosing lumbar zygapophysial joint pain was complete relief of pain following anaesthetisation of one or more of these joints.236–238 However, it has now been shown that such blocks are not valid because they are associated with an unacceptable false-positive rate.237,239 Only one in three patients who respond to a first diagnostic block respond to subsequent repeat blocks.233 Moreover, the placebo response rate to diagnostic blocks is 32%.234 This means that previous data, based on uncontrolled diagnostic blocks, overestimate the prevalence of this condition.
To be valid, diagnostic blocks must be controlled in some way, in each and every patient. Unless such precautions are taken, for every three apparently positive cases, two will be false-positive.234
Using controlled diagnostic blocks, several studies have tried to estimate the prevalence of lumbar zygapophysial joint pain. Variously they have reported the prevalence to be 15% in a sample of injured workers in the USA,232 40% in an Australian population of elderly patients in a rheumatology practice234 and 45% among patients of various ages attending a pain clinic in the USA.240
Collectively, these studies suggest that lumbar zygapophysial joint pain is quite common. However, in all of these studies the criterion for a positive response to blocks was not complete relief of pain but only at least 50% relief of pain. This is a somewhat contentious criterion, for it does not explain the remnant pain. Although the investigators presumed that incomplete relief of pain indicates another, concurrent source of pain, this has never been verified. The only studies that have addressed this question found that multiple sources of pain, in the one patient, were uncommon. Patients tend to have discogenic pain, sacroiliac joint pain, or lumbar zygapophysial joint pain, in isolation. Fewer than 5% have zygapophysial joint pain as well as discogenic pain,241 or lumbar zygapophysial joint pain as well as sacroiliac joint pain.226 The alternative interpretation – that back pain was imperfectly relieved by the blocks – has not been excluded. If the latter applies, the cited prevalence rates may be an overestimate.
When complete relief of pain, following lumbar zygapophysial joint blocks, has been used as the criterion for zygapophysial joint pain, studies have reported a much lower prevalence: substantially less than 10%.242,243 In a general population, therefore, lumbar zygapophysial joint pain may not be as common as previously believed. Nevertheless, one study, which used 90% relief of pain as the criterion, did find the prevalence of lumbar zygapophysial joint pain to be 32% in an elderly population.234
Clinical features
Despite beliefs to the contrary,244,245 controlled studies have shown that lumbar zygapophysial joint pain cannot be diagnosed clinically.232,234,246 Controlled diagnostic blocks are the only means available to date of establishing a diagnosis of lumbar zygapophysial joint pain.
Pathology
Although the prevalence of zygapophysial joint pain is known, what remains elusive is its pathology. The lumbar zygapophysial joints can be affected by rheumatoid arthritis,247–249 ankylosing spondylitis250 or un-united epiphyses of the inferior articular processes,251–253 and there have been case reports of rare conditions such as pigmented villonodular synovitis254,255 and suppurative arthritis.256–259 However, these conditions have not been identified as the cardinal causes of pain in patients responding to diagnostic zygapophysial joint blocks.
Post-mortem studies131,260,261 and radiological surveys262,263 have shown that the lumbar zygapophysial joints are frequently affected by osteoarthrosis, and studies of joints excised at operation revealed changes akin to chondromalacia patellae.264 Although it is asserted that zygapophysial arthritis is usually secondary to disc degeneration or spondylosis,131 in about 20% of cases it can be a totally independent disease.260
The prevalence of zygapophysial osteoarthrosis attracts the belief that this condition is the underlying cause in patients with zygapophysial joint pain.244,264–266 However, on plain radiographs zygapophysial osteoarthrosis appears as commonly in asymptomatic individuals as in patients with back pain.262,263 Features indicative of osteoarthritis on CT scans were once held to be indicative of zygapophysial joint pain264,265,267 but controlled studies have shown that CT is of no diagnostic value for lumbar zygapophysial joint pain.268 These data preclude making the diagnosis of painful zygapophysial arthropathy on the basis of plain radiography. They also indicate either that osteoarthrosis is not a cause of zygapophysial joint pain or that, when it is, the pain is due to some factor other than the simple radiological presence of this condition.
Injuries
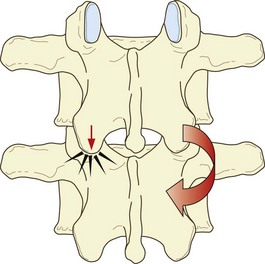
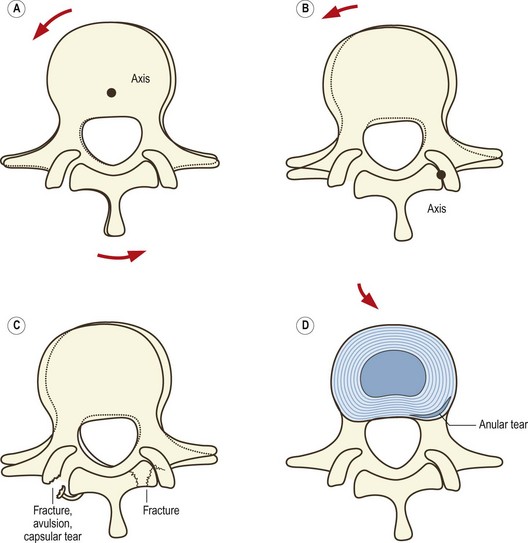
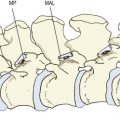
Extension of the lumbar spine may be limited by impaction of an inferior articular process on the lamina below (see Ch. 8). Under these conditions, continued application of an extension force results in rotation of the affected segment around the impacted articular process, which draws the inferior articular process of the contralateral zygapophysial joint backwards (Fig. 15.1). As a result, the capsule of that joint is disrupted.269
Rotation of a lumbar intervertebral joint normally occurs around an axis located in the posterior third of the vertebral bodies and intervertebral disc (see Ch. 8). Rotation is limited by impaction of the zygapophysial joint opposite the direction of movement but if torque continues to be applied rotation can continue around a new axis located in the impacted joint. As a result, the contralateral inferior articular process is drawn backwards and medially and the joint capsule on that side is disrupted (Fig. 15.2). The lesions that occur in biomechanics experiments include tears of the capsule, avulsion of the capsule or fracture-avulsion of the capsule.270 The impacted joint may sustain fractures of its subchondral bone or articular processes, or the pars interarticularis may fail.271–276
Capsular tears, capsular avulsion, subchondral fractures, intra-articular haemorrhage and fractures of the articular processes, such as those produced in biomechanics studies, have all been found in post-mortem studies.277,278 However, in no case were any of these lesions evident on plain radiographs.
Fractures of the zygapophysial joints have occasionally been recorded in the past in the radiology literature251,279–281 but by and large, these fractures cannot be detected on plain radiographs.277,278 Fractures should be visible on CT scans although fractures have not been reported in the CT scans of patients with proven painful lumbar zygapophysial joints.268 Thus, fractures are either not the basis for most cases of zygapophysial joint pain, or they are too small or subtle to be detected by conventional use of lumbar CT.
Meniscus extrapment
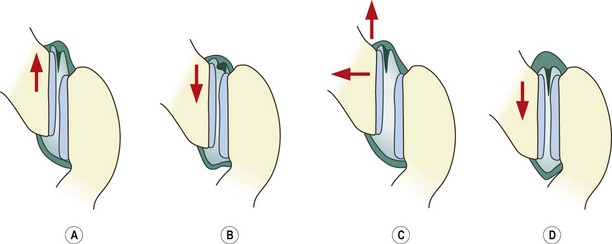
Normal lumbar zygapophysial joints are endowed with fibroadipose meniscoids (see Ch. 3),282,283 and following trauma, segments of articular cartilage still attached to joint capsules may be avulsed from the articular surface to form an acquired cartilaginous meniscoid.284 These meniscoid structures could feasibly act as loose bodies within the joint or be trapped in the subcapsular pockets of the joints.
Upon flexion, one of the fibroadipose meniscoids is drawn out of the joint but upon attempted extension it fails to re-enter the joint cavity (Fig. 15.3). Instead, it impacts against the edge of the articular cartilage, and in this location it buckles and acts like a space-occupying lesion under the capsule, causing pain by distending the capsule.285 Maintaining flexion is comfortable for the patient because that movement disengages the meniscoid. Treatment by manipulation becomes logical. Passive flexion of the segment reduces the impaction, and rotation gaps the joint, encouraging the meniscoid to re-enter the joint cavity (see Fig. 15.3).285
This condition of meniscoid entrapment is only theoretical, for it is difficult, if not impossible, to visualise meniscoids radiologically. However, it reigns as one of the plausible explanations for some cases of acute locked back, particularly those amenable to manipulative therapy.285
Discogenic pain
The concept that the lumbar intervertebral discs might be a source of pain is not new. As long ago as 1947, it was recognised that the discs received a nerve supply and so could be intrinsically painful.286 However, this concept remained suppressed by erroneous declarations that the discs were not innervated and so could not be painful.287
There is now no doubt that the lumbar discs are innervated.103–105,187,287–292 Consequently, there can be no objection on anatomical grounds that they could be sources of back pain.
Disc stimulation
Despite its chequered and controversial history, disc stimulation (formerly known as discography) remains the only means of determining whether or not a disc is painful.293–295 The procedure involves introducing a needle into the nucleus pulposus of the target disc and using it to distend the disc with an injection of normal saline or contrast medium.295 The test is positive if upon stimulating a disc the patient’s pain is reproduced provided that stimulation of adjacent discs does not reproduce their pain.1,295,296 Moreover, modern guidelines insist that the pressure of injection is critical.295 Discs are considered symptomatic only if pain is reproduced at pressures of injection less than 50 psi and preferably less than 15 psi.
Previous studies that reported painful stimulation of discs in normal volunteers297 have been refuted on methodological grounds,298 and a stringent study found no painful discs in asymptomatic individuals.299 A more recent study found painful discs in only 10% of normal volunteers.300 Another study showed that discs are not painful in normal volunteers if they are stimulated at pressures less than 40 psi.301 A systematic review found that disc stimulation has a false-positive rate of less than 10%.302 Disc stimulation, therefore, is specific for painful discs, and a positive response implies an abnormality that has rendered the disc painful.
This experience with stimulation of lumbar discs by injection has been complemented by another approach. Discs can be stimulated thermally, by heating a wire electrode inserted into the anulus of the disc.303 Heating the disc evokes pain, which is initially perceived in the back, but which can be referred in different patterns into the lower limb. The referred pain may be perceived in the buttock, or posterior thigh, and even in the leg (i.e. below the knee). Since the noxious stimulus is restricted to the disc, and does not affect the nerve roots, thermal heating provides evidence not only that lumbar discs can be painful, but also that they can be responsible for somatic referred pain in the thigh and leg.
Pathology
At present, data on the pathology of disc pain are incomplete and are largely circumstantial but there are sufficient data to enable three entities to be described: discitis; torsion injuries; and internal disc disruption.304
Discitis
Iatrogenic discitis is the archetypical lesion that renders a disc painful.305,306 In this condition, the disc is infected by bacteria introduced by needles used for discography. The process is restricted to the disc and is evident on bone scans and MRI. The condition is intensely painful and there is no evidence that the pain arises from sources other than the infected disc.
Torsion injury
When an intervertebral joint is forcibly rotated, injuries can occur to the disc as well as the posterior elements (see Fig. 15.2). Rotation about the normal axis of rotation pre-stresses the anulus, but once further rotation ensues around the secondary axis through the impacted zygapophysial joint (see Fig. 15.2B), the disc is subjected to an additional lateral shear. The combination of torsion and lateral shear results in circumferential tears in the outer anulus (see Fig. 15.2D).270,307,308 The risk of injury is greater if rotation is undertaken in flexion, for then the flexion pre-stresses the anulus to a near maximal extent, and the added rotation takes the collagen fibres of the anulus beyond their normal strain limit.309
In discs with concave posterior surfaces, circumferential tears occur in the posterolateral corner of the anulus fibrosus; in discs with convex posterior surfaces the tears occur posteriorly. The reason for these locations lies in the stress distribution across a disc subjected to torque.273,308 These are locations where the lamellae of the anulus fibrosus exhibit the greatest relative curvature and where torsional strains are maximal. Consequently, they are sites where collagen fibres are most likely initially to fail under torsion.
Torsion injuries, however, are only a theoretical diagnosis. The condition can be suspected on clinical grounds given the appropriate mechanical history of a flexion–rotation strain. Neurological examination is normal because the lesion is restricted to the anulus fibrosus and does not involve nerve root compression. For the same reason, CT scanning, myelography and MRI are normal, and discography will be normal since the nucleus pulposus is not involved in the lesion. The condition is essentially a ligament sprain and behaves as such. Theoretically, the pain should be aggravated by any movements that stress the anulus fibrosus, but in particular flexion and rotation in the same direction that produced the lesion.310 However, these clinical features are not enough to prove the diagnosis.
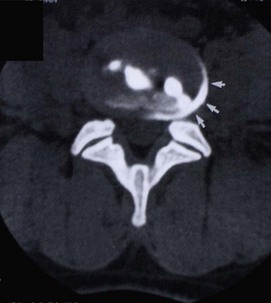
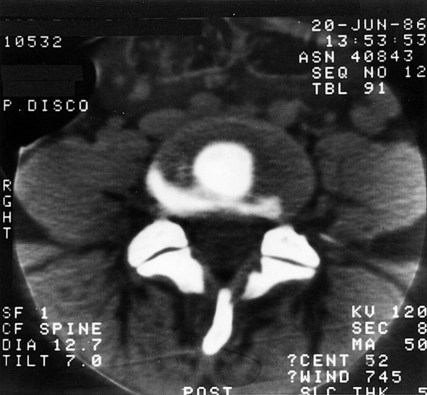
Circumferential tears of the anulus are not visible on any contemporary imaging technique, including MRI,311 but it is possible to identify the lesion by other means.312,313 Contrast medium and local anaesthetic can be injected into the putatively painful tear in the anulus fibrosus. If the tear is painful, the local anaesthetic relieves the pain, and the contrast medium outlines the tear, which can then be seen on a post-procedural CT scan as a crescent in the outer anulus fibrosus (Fig. 15.4).
Internal disc disruption
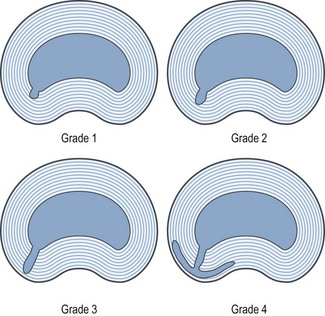
Morphologically, IDD is characterised by degradation of the nuclear matrix and the presence of radial fissures, extending from the nucleus into the anulus fibrosus. For descriptive purposes, the fissures can be graded according to the extent to which they penetrate the anulus (Fig. 15.5):314
• Grade 1 fissures reach only the inner third of the anulus.
• Grade 2 fissures reach the second, or middle, third.
• Grade 3 fissures extend into the outer third of the anulus.
• Some investigators recognise a fourth grade, which is a grade 3 fissure that expands circumferentially around the outer anulus.315
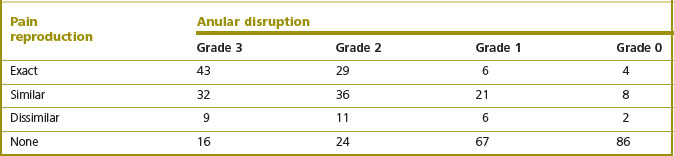
The extent to which the anulus is penetrated by radial fissures correlates strongly with the affected disc being painful on disc stimulation.316,317 Grade 1 fissures are typically not painful. Grade 2 fissures may or may not be painful but some 70% of grade 3 fissures are associated with pain, and some 70% of painful discs exhibit a grade 3 fissure.316.
Studies using multivariate analysis have shown that IDD is independent of degenerative changes.317 Degenerative changes increase with age but do not correlate with the disc being painful. Radial fissures occur at an early age and their prevalence does not increase with age. Yet fissures are strongly correlated with pain, independently of age changes (Table 15.2).317
Table 15.2 The correlation between anular disruption and reproduction of pain by disc stimulation. The numbers refer to the number of patients exhibiting the features tabulated. χ2 = 148; P < 0.001. (Based on Moneta et al. 1994.317)
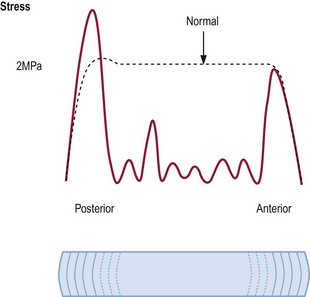
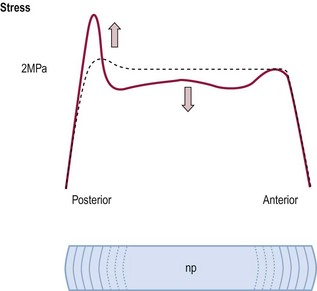
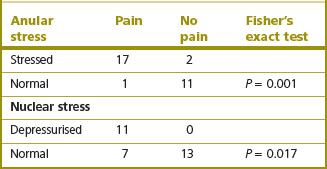
Discs affected by IDD exhibit abnormal stress profiles (see Ch. 8). Nuclear stresses are reduced, irregular or absent (Fig. 15.6).318 Meanwhile, stresses in the posterior anulus are increased greatly above normal. These features indicate that the degraded nuclear matrix is no longer able to retain water and does not contribute to sustaining compression loads on the disc. Instead, the compression load is transferred to the posterior anulus.
Each of these changes in the biophysical properties of the disc correlate with the disc becoming painful. Decreased nuclear stresses and increased stresses in the posterior anulus each correlate with reproduction of pain by disc stimulation (Table 15.3).318
Table 15.3 The correlation between disc stimulation and changes in stress profile of the disc (Based on McNally et al. 1996.318)
Early proponents argued that IDD was the result of compression injuries to the disc. This proposition was based on intuition and studies of the mechanism of failure of discs subjected to compression.273,308,319,320 This is consonant with the available biomechanical evidence.
Despite traditional wisdom in this regard, when compressed, intervertebral discs do not fail by prolapsing. In biomechanical experiments, it is exceedingly difficult to induce disc failure by prolapse. Even if a channel is cut into the anulus fibrosus, the nucleus fails to herniate.321–323 A normal nucleus is intrinsically cohesive and resists herniation. Even in specimens with partially herniated discs, completion of the prolapse rarely occurs even after repeated flexion and compression.324
When compressed, intervertebral discs typically fail by fracture of a vertebral endplate.323,325–330 The forces required are usually quite large,327,331 of the order of 10 000 N, but can be as low as 3000 N.332 Although seemingly large, these forces are of a magnitude such as might be encountered in a sudden fall, landing on the buttocks or as a result of forceful muscle activity.333 In a heavy lift, the back muscles can exert a longitudinal force of some 4000 N.334 It transpires, therefore, that certain individuals could be susceptible to compression injury of their vertebral endplates if their vertebral bodies were weaker than the maximum strength of their muscles.
In such individuals, fracture of the vertebral endplate could occur during unaccustomed inordinate heavy lifting, or if they maximally exerted their back muscles in activities such as pulling on a stubborn tree root while gardening. In these situations the individual voluntarily exerts their back muscles to a severe degree, but the muscles act on a vertebral column that is unaccustomed to bearing the large loads involved. Training and physical exercise appear to condition vertebral bodies, rendering them stronger and better able to withstand the longitudinal stresses imposed upon them by severe efforts of the back muscles,331,335 but the risk of endplate fracture prevails if athletes, workers and other individuals with relatively weak vertebral endplates are not conditioned to their task, and take on lifting activities for which their back muscles might be capable but their vertebrae are not.
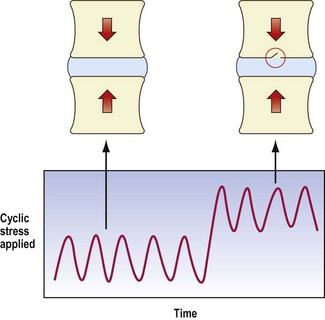
These considerations presuppose sudden static loading. However, modern research has shown that endplate failure can occur at loads substantially less than ultimate failure strength of the endplate, if the endplate is fatigued (see Ch. 8).
If a normal disc is repetitively loaded, in compression or in compression with flexion, at loads between 37 and 50% of its ultimate failure strength, it can resist 1000 or 2000 repetitions without failing.336,337 However, if it is loaded to between 50% and 80% of its ultimate strength, the endplate can fail, by fracturing, after as few as 100 repetitions.336
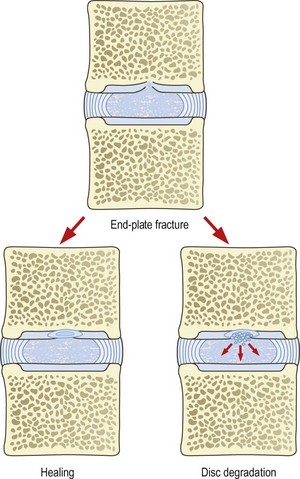
An endplate fracture is of itself not symptomatic and may pass unnoticed. Furthermore, an endplate fracture may heal and cause no further problems (Fig. 15.7). However, it is possible for an endplate fracture to set in train a series of sequelae that manifest as pain and a variety of endstages.
Early proponents of internal disc disruption argued that an endplate fracture simply elicited an unbridled, inflammatory repair response273,274,308,319 that failed to heal the fracture but proceeded to degrade the matrix of the underlying nucleus pulposus.
Others subsequently ventured a bolder interpretation, suggesting that through the fracture, the proteins of the nuclear matrix are exposed to the circulation in the vertebral spongiosa, and elicit an autoimmune inflammatory response.304,338,339 This proposal was based on evidence that showed that disc material was antigenic55–59340 and was consistent with observations that acute intraosseous disc herniation was associated with inflammation of the spongiosa.341 The model invited an analogy with the condition of sympathetic ophthalmia in which release of lens proteins after an injury to an eye causes an autoimmune reaction that, in due course, threatens the integrity of the healthy eye. What lens proteins and disc proteins have in common is that neither has ever been exposed to the body’s immune system, because the two tissues are avascular. Their proteins, therefore, are not recognised as self.
A more conservative interpretation could be that an endplate fracture interferes with the delicate homeostasis of the nuclear matrix. The matrix contains degradative enzymes whose activity is normally limited by tissue inhibitors of metalloproteinases.60,61,342–345 Furthermore, the balance between synthesis and degradation of the matrix is very sensitive to changes in pH.344,346 This invites the conjecture that an injury, such as an endplate fracture, might disturb the metabolism of the nucleus, perhaps by lowering the pH, and precipitate degradation of the matrix without an explicit inflammatory reaction. Indeed, recent biochemical studies suggest that increased activation of disc proteinases occurs progressively from the endplate into the nucleus, and that these proteinases either may be activated by blood in the vertebral body or may even stem from cells in the bone marrow.347
Regardless of its actual mechanism, the endplate theory supposes that fractures result in progressive degradation of the nuclear matrix. Nuclear ‘degradation’ may appear synonymous with disc ‘degeneration’ and, indeed, other authors have implicated the same biochemical processes described above as the explanation for disc degeneration.348 However, they view this as an idiopathic phenomenon, and do not relate it to endplate fracture. In the present context, nuclear ‘degradation’ is not intended to mean ‘degeneration’. ‘Degeneration’ is an emotive term, conjuring images of inevitable decay and destruction, yet many of the pathological changes said to characterise disc degeneration are little more than normal age changes (see Ch. 13). In contrast, nuclear degradation is a process, initiated by an endplate fracture, that progressively destroys the nucleus pulposus. It is an active consequence of trauma not a passive consequence of age.
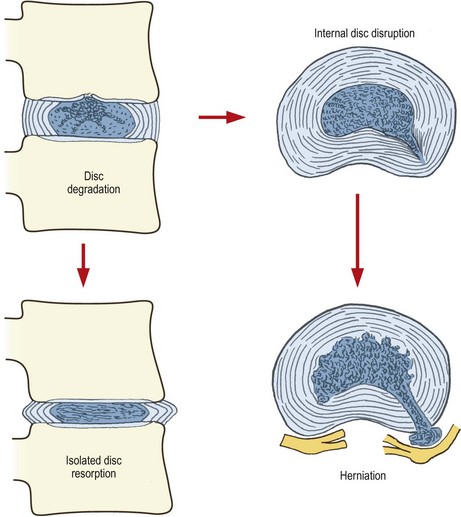
When degradation is restricted to the nucleus pulposus, proteolysis and deaggregation of the nuclear matrix result in a progressive loss of water-binding capacity and a deterioration of nuclear function. Less able to bind water, the nucleus is less able to sustain pressures, and greater loads must be borne by the anulus fibrosus. In time, the anulus buckles under this load and the disc loses height, which compromises the functions of all joints in the affected segment (Fig. 15.8). As a result, reactive changes occur in the form of osteophyte formation in the zygapophysial joints and anulus fibrosus. This state, characterised by osteophytes and disc narrowing, has been recognised clinically and described as ‘isolated disc resorption’,348,349 which becomes symptomatic if nerve roots are compromised by canal stenosis or foraminal stenosis.
In Chapter 13, it was explained that disc narrowing is not a consequence of age: discs retain their height with age.350 A different explanation is required for disc narrowing, especially if it occurs at only one in five lumbar segments. The explanation lies in disc narrowing being a consequence of nuclear degradation following endplate fracture. However, disc narrowing and isolated disc resorption comprise only one possible endstage of disc degradation. This occurs when the anulus fibrosus remains intact circumferentially but when nuclear degradation and dehydration are severe.
In contrast, the water-binding capacity of the nucleus may not be so severely affected by nuclear degradation, whereupon the disc relatively retains its height. However, in time, nuclear degradation extends peripherally to erode the anulus fibrosus, typically along radial fissures, to establish the definitive features of internal disc disruption (see Fig. 15.8).
Ultimately, it is possible for internal disc disruption to progress to disc herniation (see Fig. 15.8). This occurs if the inflammatory degradation extends along a radial fissure for the entire thickness of the anulus. The conditions for disc herniation are thereby set – a defect has been produced in the anulus fibrosus and the nucleus pulposus has been denatured into a form that is expressible. In such a disc, compression loading during normal flexion may be sufficient to herniate the nucleus.
When cadaveric discs are repeatedly loading in compression, the onset of fatigue failure of the endplate can be detected by the sudden change in mechanical behaviour.333 Harvesting the specimen at this time reveals the endplate fracture (Fig. 15.9). When stress profiles in the disc have been monitored during loading, another correlate has appeared. At the time of failure, the disc exhibits the onset of the biophysical changes of IDD. Nuclear stresses reduce and posterior anulus stress rises sharply (Fig. 15.10).
In laboratory animals, experimental induction of an endplate fracture precipitates the biochemical, morphological and biophysical changes of IDD.351 The water content of the nucleus decreases, and proteoglycans decrease, the inner anulus delaminates and nuclear pressure falls.
The mechanism by which IDD becomes painful has not been explicitly demonstrated but two explanations apply. In the first instance, chemicals from the degraded nuclear matrix that enter the radial fissure might irritate nerve endings in the outer third of the anulus. This becomes the basis for chemical nociception from the disc. To this effect, there is evidence that inflammatory cells penetrate the anulus fibrosus of disrupted discs,352 whereupon inflammatory chemical mediators may trigger nociceptive nerve endings.
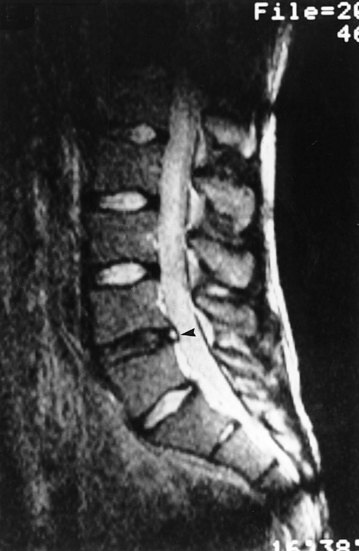
There are no means by which IDD can be diagnosed clinically. The diagnosis requires reproduction of the patient’s pain by disc stimulation, and the demonstration by postdiscography CT of a radial fissure (Fig. 15.11).
In some patients, the condition can be detected by MRI. In these patients, internal disc disruption is manifest by a signal of high intensity in the posterior anulus (Fig. 15.12). This signal is discontinuous with that of the nucleus and is somewhat brighter. Empirically, the presence of this high-intensity zone correlates strongly both with the disc being painful and with the presence of a grade 4 radial fissure.315,353 Morphologically, the high-intensity zone seems to have the appearance, in sagittal section, of nuclear material running circumferentially in the posterior anulus.315 Its relative brightness distinguishes it from asymptomatic transverse fissures and suggests that symptomatic fissures are ones that somehow have become ‘activated’, perhaps by inflammation of the tissue contained in the fissure.
IDD is the single most common detectable cause of chronic low back pain. Under the strictest of diagnostic criteria, and using worst-case analysis, its prevalence has been measured as 39%.296 Under more liberal criteria, and allowing for multilevel disease, its prevalence may be considerable higher than this.
Summary
This review of the possible sources and causes of back pain generates an interesting matrix of data. For each putative source of pain, one can check which of the postulates are satisfied and to what extent (see Table 15.4). In turn, this indicates the depth and quality of evidence to substantiate belief in any particular source or cause.
Table 15.4 The extent to which various proposed sources and causes of back pain satisfy the postulates for a structure to be a source of back pain. Muscle sprain, muscle spasm, trigger points and iliac crest syndrome are presumed to have been produced in normal volunteers in as much as pain from muscles, in general, has been so produced
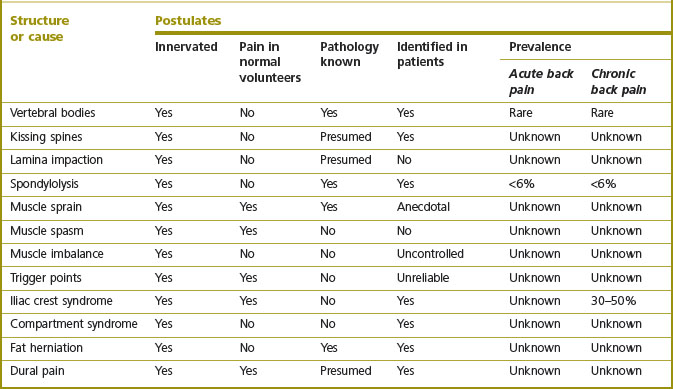
Fascinatingly, there is a perverse correlation evident in this matrix (see Table 15.3). It emerges that those conditions that have attracted the greatest popularity in clinical practice – muscle pain, ligament pain, trigger points – are associated with the smallest amount of scientific evidence. Data on the mechanism of pain in these conditions and its prevalence are simply lacking. Also, no reliable means of diagnosis have been established. When subjected to scientific scrutiny, clinical examination for the diagnosis of these conditions has failed.
1 Merskey H, Bogduk N, editors. Classification of Chronic Pain. Descriptions of chronic pain syndromes and definitions of pain terms, 2nd ed, Seattle: IASP Press, 1994.
2 Bogduk N. On the definitions and physiology of back pain, referred, pain and radicular pain. Pain. 2009;147:17-19.
3 Hadley LA. Apophyseal subluxation. Disturbances in and about the intervertebral foramen causing back pain. J Bone Joint Surg. 1936;18:428-433.
4 Hadley LA. Intervertebral joint subluxation, bony impingement and foramen encroachment with nerve root changes. Am J Roentgenol. 65, 1951. 377-102
5 Arnoldi CC, Brodsky AE, Cauchoix J. Lumbar spinal stenosis. Clin Orthop. 1976;115:4-5.
6 Ehni G. Significance of the small lumbar spinal canal: cauda equina compression syndromes due to spondylosis. J Neurosurg. 1962;31:490-494.
7 Epstein JA, Epstein BS, Levine L. Nerve root compression associated with narrowing of the lumbar spinal canal. J Neurol Neurosurg Psychiatry. 1962;25:165-176.
8 Kirkaldy-Willis WH, Wedge JH, Yong-Hing K, et al. Pathology and pathogenesis of lumbar spondylosis and stenosis. Spine. 1978;3:319-328.
9 Verbiest H. A radicular syndrome from developmental narrowing of the lumbar vertebral canal. J Bone Joint Surg. 1954;36B:230-237.
10 Verbiest H. Fallacies of the present definition, nomenclature and classification of the stenoses of the lumbar vertebral canal. Spine. 1976;1:217-225.
11 Cophignon J, Fischer G, Fuentes JM, et al. Les sciatiques chirurgicales non discales. Neurochirurgie. 1978;24:283-336.
12 Epstein BS. The Spine. A radiological text and atlas, 4th ed. Philadelphia: Lea & Febiger; 1976.
13 Epstein JA, Epstein BS, Levine LS, et al. Lumbar nerve root compression at the intervertebral foramina caused by arthritis of the posterior facets. J Neurosurg. 1973;39:362-369.
14 Naffziger HC, Inman V, Saunders JB. Lesions of the intervertebral disc and ligamenta flava. Surg Gynecol Obstet. 1938;66:288-299.
15 Haase J. Extradural cyst of ligamentum flavum L4 – a case. Acta Orthop Scand. 1972;43:32-38.
16 Pou-Serradell A, Casademont M. Syndrome de la queue de cheval et présence d’appendices apophysaires vertébraux, ou apophyses articulaires accessoires, dans la région lombaire. Rev Neurol. 1972;126:435-440.
17 Feldman R, McCulloch J. Juxta facet cysts of the lumbar spine. Neuro-Orthopedics. 1987;4:31-35.
18 Gritzka TL, Taylor TKF. A ganglion arising from a lumbar articular facet associated with low back pain and sciatica. Report of a case. J Bone Joint Surg. 1970;52B:528-531.
19 Jackson DE, Atlas SW, Mani JR, et al. Intraspinal synovial cysts: MR imaging. Radiology. 1989;170:527-530.
20 Kao CC, Uihein A, Bichel WH, et al. Lumbar intraspinal extradural ganglion. J Neurosurg. 1968;29:168-172.
21 Reust P, Wendling D, Lagier R, et al. Degenerative spondylolisthesis, synovial cyst of the zygapophyseal joints, and sciatic syndrome: report of two cases and review of the literature. Arthritis Rheum. 1988;31:288-294.
22 Galibert P, Estefan G, Le Gars D, et al. Conflits mécaniques sciatalgiques par synovialome bénin des articulations rachidiennes. A propos de quatre cas. Neurochirurgie. 1983;29:377-380.
23 Hadjipavlou AG, Lander PH. Paget’s disease. In: White AH, editor. Spine Care, Vol. 2. St Louis: Mosby; 1995:1720-1737.
24 Dietmann JL, Bonneville JF, Runge M, et al. Computed tomography of lumbar apophyseal joint lipoma: report of three cases. Neuroradiology. 1989;31:60-62.
25 Husson JL, Chales G, Lancien G, et al. True intra-articular lipoma of the lumbar spine. Spine. 1987;12:820-822.
26 Cloward RB. Congenital spinal extradural cysts. Ann Surg. 1968;168:851-864.
27 Fortuna A, La Torre E, Ciappetta P. Arachnoid diverticula: a unitary approach to spinal cysts communicating with the subarachnoid space. Acta Neurochir. 1977;39:259-268.
28 Glasauer FE. Lumbar extradural cysts. J Neurosurg. 1966;25:567-570.
29 Tarlov IM. Spinal perineurial and meningeal cysts. J Neurol Neurosurg Psychiatry. 1970;33:833-843.
30 Varughese G. Lumbosacral intradural periradicular ossification. J Neurosurg. 1978;49:132-137.
31 Naftulin S, Fast A, Thomas M. Diabetic lumbar radiculopathy: sciatica without disc herniation. Spine. 1993;18:2419-2422.
32 Howe JF. A neurophysiological basis for the radicular pain of nerve root compression. In: Bonica JJ, Liebeskind JC, Albe-Fessard DG, editors. Advances in Pain Research and Therapy, Vol. 3. New York: Raven Press; 1979:647-657.
33 Howe JF, Loeser JD, Calvin WH. Mechanosensitivity of dorsal root ganglia and chronically injured axons: a physiological basis for the radicular pain of nerve root compression. Pain. 1977;3:25-41.
34 MacNab I. The mechanism of spondylogenic pain. In: Hirsch C, Zotterman Y, editors. Cervical Pain. Oxford: Pergamon; 1972:89-95.
35 Smyth MJ, Wright V. Sciatica and the intervertebral disc. An experimental study. J Bone Joint Surg. 1959;40A:1401-1418.
36 Norlen G. On the value of the neurological symptoms in sciatica for the localization of a lumbar disc herniation. Acta Chir Scand. 1944;95(Suppl.):1-96.
37 McCulloch JA, Waddell G. Variation of the lumbosacral myotomes with bony segmental anomalies. J Bone Joint Surg. 1980;62B:475-480.
38 Friberg S. Lumbar disc degeneration in the problem of lumbago sciatica. Bull Hosp Joint Dis. 1954;15:1-20.
39 Horal J. The clinical appearance of low back disorders in the city of Gothenburg, Sweden. Acta Orthop Scand. 1969;1(Suppl.):118.
40 Mooney V. Where is the pain coming from? Spine. 1987;12:754-759.
41 Deyo RA, Tsui-Wu YJ. Descriptive epidemiology of low-back pain and its related medical care in the United States. Spine. 1987;12:264-268.
42 Boden SD, Davis DO, Dina TS, et al. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. J Bone Joint Surg. 1990;72A:403-408.
43 Hitselberger WE, Witten RM. Abnormal myelograms in asymptomatic patients. J Neurosurg. 1968;28:204-206.
44 Jensen MC, Brant-Zawadzki MN, Obuchowski N, et al. Magnetic resonance imaging of the lumbar spine in people without back pain. New Engl J Med. 1994;331:69-73.
45 Wiesel SW, Tsourmas N, Feffer HL, et al. A study of computer-assisted tomography. 1. The incidence of positive CAT scans in an asymptomatic group of patients. Spine. 1984;9:549-551.
46 Delauche-Cavalier MC, Budet C, Laredo JD, et al. Lumbar disc herniation. Computed tomography scan changes after conservative treatment of nerve root compression. Spine. 1992;17:927-933.
47 Maigne JY, Rime B, Delinger B. Computed tomographic follow-up study of forty-eight cases of non-operatively treated lumbar intervertebral disc herniation. Spine. 1992;17:1071-1074.
48 Greenbarg PE, Brown MD, Pallares VS, et al. Epidural anesthesia for lumbar spine surgery. J Spinal Disord. 1988;1:139-143.
49 Murphy RW. Nerve roots and spinal nerves in degenerative disk disease. Clin Orthop. 1977;129:46-60.
50 Irsigler FJ. Mikroskopische Befunde in den Ruckenlarkswurzeln beim lumbalen un lumbalsakralen (dorsolateral) Diskusprolaps. Acta Neurochir. 1951;1:478-516.
51 Lindahl O, Rexed B. Histologic changes in spinal nerve roots of operated cases of sciatica. Acta Orthop Scand. 1951;20:215-225.
52 Lindblom K, Rexed B. Spinal nerve injury in dorsolateral protrusions of lumbar disks. J Neurosurg. 1949;5:413-432.
53 Marshal LL, Trethewie ER. Chemical irritation of nerve-root in disc prolapse. Lancet. 1973;2:320.
54 Marshall LL, Trethewie ER, Curtain CC. Chemical radiculitis. A clinical, physiological and immunological study. Clin Orthop. 1977;129:61-67.
55 McCarron RF, Wimpee MW, Hudkins PG, et al. The inflammatory effect of nucleus pulposus: a possible element in the pathogenesis of low-back pain. Spine. 1987;12:760-764.
56 Bobechko WT, Hirsch C. Autoimmune response to nucleus pulposus in the rabbit. J Bone Joint Surg. 1965;47B:574-580.
57 Gertzbein SD. Degenerative disk disease of the lumbar spine: immunological implications. Clin Orthop. 1977;129:68-71.
58 Gertzbein SD, Tait JH, Devlin SR. The stimulation of lymphocytes by nucleus pulposus in patients with degenerative disk disease of the lumbar spine. Clin Orthop. 1977;123:149-154.
59 Gertzbein SD, Tile M, Gross A, et al. Autoimmunity in degenerative disc disease of the lumbar spine. Orthop Clin North Am. 1975;6:67-73.
60 Naylor A. The biochemical changes in the human intervertebral disc in degeneration and nuclear prolapse. Orthop Clin North Am. 1971;2:343-358.
61 Naylor A. Intervertebral disc prolapse and degeneration. The biochemical and biophysical approach. Spine. 1976;1:108-114.
62 Naylor A, Happey F, Turner RL, et al. Enzymic and immunological activity in the intervertebral disc. Orthop Clin North Am. 1975;6:51-58.
63 De Silva M, Hazleman BL, Ward M, et al. Autoimmunity and the prolapsed intervertebral disc syndrome. Rheumatology. 1981;1:35-38.
64 Olmarker K, Rydevik B, Holm S. Edema formation in spinal nerve roots induced by experimental, graded compression. Spine. 1989;14:569-573.
65 Rydevik BL, Myers R, Powell HC. Pressure increase in the dorsal root ganglion following mechanical compression. Closed compartment syndrome in nerve roots. Spine. 1989;14:574-576.
66 McCarron RF, Wimpee MW, Hudkins PG, et al. The inflammatory effect of nucleus pulposus: a possible element in the pathogenesis of low-back pain. Spine. 1987;12:760-764.
67 Olmarker K, Rydevik B, Nordborg C. Autologous nucleus pulposus induces neurophysiologic and histologic changes in porcine cauda equina nerve roots. Spine. 1993;18:1425-1432.
68 Olmarker K, Blomquist J, Stromberg J, et al. Inflammatogenic properties of nucleus pulposus. Spine. 1995;20:665-669.
69 Yabuki S, Kikuchi S, Olmarker K, et al. Acute effects of nucleus pulposus on blood flow and endoneural fluid pressure in rat dorsal root ganglia. Spine. 1998;23:2517-2523.
70 Olmarker K, Byrod G, Cornefjord M, et al. Effects of methylprednisolone on nucleus pulposus-induced nerve root injury. Spine. 1994;19:1803-1808.
71 Olmarker K, Nordborg C, Larsson K, et al. Ultrastructural changes in spinal nerve roots induced by autologous nucleus pulposus. Spine. 1996;21:411-414.
72 Olmarker K, Myers RR. Pathogenesis of sciatic pain: role of herniated nucleus pulposus and deformation of spinal nerve root and dorsal root ganglion. Pain. 1998;78:99-105.
73 Kayama S, Olmarker K, Larsson K, et al. Cultured, autologous nucleus pulposus cells induce functional changes in spinal nerve roots. Spine. 1998;23:2155-2158.
74 Kawakami M, Tamaki T, Weinstein JN, et al. Pathomechanism of pain-related behavior produced by allografts of intervertebral disc in the rat. Spine. 1996;21:2101-2107.
75 Kawakami M, Tamaki T, Hashizume H, et al. The role of phospholipase A2 and nitric oxide in pain-related behavior produced by an allograft of intervertebral disc material to the sciatic nerve of the rat. Spine. 1997;22:1074-1079.
76 Kawakami M, Tamaki T, Hayashi N, et al. Possible mechanism of painful radiculopathy in lumbar disc herniation. Clin Orthop. 1998;351:241-251.
77 Olmarker K, Larson K. Tumor necrosis factor α and nucleus pulposus-induced nerve root injury. Spine. 1998;23:2538-2544.
78 Olmarker K, Rydevik B. Selective inhibition of tumor necrosis factor α prevents nucleus pulposus-induced thrombus formation, intraneural edema, and reduction of nerve conduction velocity. Possible implications for future pharmacologic treatment strategies of sciatica. Spine. 2001;26:863-869.
79 Chen C, Cavanaugh JM, Ozaktay AC, et al. Effects of phospholipase A2 on lumbar nerve roots structure and function. Spine. 1997;22:1057-1064.
80 Hashizume K, Kawakami M, Nishi H, et al. Histochemical demonstration of nitric oxide in herniated lumbar discs: a clinical and animal model study. Spine. 1997;22:1080-1084.
81 Kawakami M, Matsumoto T, Kuribayashi K, et al. mRNA expression of interleukins, phospholipase A2, and nitric oxide synthetase in the nerve root and dorsal root ganglion induced by autologous nucleus pulposus in the rat. J Orthop Res. 1999;17:941-946.
82 Igarashi T, Kikuchi S, Shubayev V, et al. Exogenous TNF-α mimics nucleus pulposus-induced neuropathology: molecular, histologic, and behavioural comparisons in rats. Spine. 2000;25:2975-2980.
83 Gronblad M, Virri J, Tolonen J, et al. A controlled immunohistochemical study of inflammatory cells in disc herniation tissue. Spine. 1994;19:2744-2751.
84 Doita M, Kanatani T, Harada T, et al. Immunohistologic study of rupture intervertebral disc of lumbar spine. Spine. 1996;21:235-241.
85 Haro H, Shinomiya K, Komori H, et al. Upregulated expression of chemokines in herniated nucleus pulposus resorption. Spine. 1996;21:1647-1652.
86 Habtemariam A, Gronblad M, Virri J, et al. Immunohistochemical localization of immunoglobulins in disc herniation. Spine. 1996;21:1864-1869.
87 Ito T, Yamada M, Ikuta F, et al. Histologic evidence of absorption of sequestration-type herniated disc. Spine. 1996;21:230-234.
88 Habtemariam A, Gronblad M, Virri J, et al. A comparative immunohistochemical study of inflammatory cells in acute-stage and chronic-stage disc herniations. Spine. 1998;23:2159-2165.
89 Park JB, Chang H, Kim YS. The pattern of interleukin-12 and T-helper types 1 and 2 cytokine expression in herniated lumbar disc tissue. Spine. 2002;27:2125-2128.
90 Takahashi H, Sugaro T, Okazima Y, et al. Inflammatory cytokines in the herniated disc of the lumbar spine. Spine. 1996;21:218-224.
91 Saal JS, Franson RC, Dobrow R, et al. High levels of inflammatory phospholipase A2 activity in lumbar disc herniations. Spine. 1990;15:674-678.
92 Franson RC, Saal JS, Saal JA. Human disc phospholipase A2 is inflammatory. Spine. 1992;17:S129-S132.
93 Kang JD, Georgescu HI, Larkin L, et al. Herniated lumbar intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6 and prostaglandin E2. Spine. 1996;21:271-277.
94 Kang JD, Stefanovic-Racic M, McIntyre LA, et al. Toward a biochemical understanding of human intervertebral disc degeneration and herniation: contributions of nitric oxide, interleukins, prostaglandin E2 and matrix metalloproteinases. Spine. 1997;22:1065-1073.
95 Nygaard OP, Mellgren SI, Osterud B. The inflammatory properties of contained and noncontained lumbar disc herniation. Spine. 1997;22:2484-2488.
96 Ahn SH, Cho YW, Ahn MW, et al. mRNA expression of cytokines in herniated lumbar intervertebral discs. Spine. 2002;27:911-917.
97 Brisby H, Olmarker K, Larsson K, et al. Proinflammatory cytokines in cerebrospinal fluid and serum in patients with disc herniation and sciatica. Eur Spine J. 2002;11:62-66.
98 Spiliopoulou II, Korovessis P, Konstantinou D, et al. IgG and IgM concentration in the prolapsed human intervertebral disc and sciatica etiology. Spine. 1994;19:1320-1323.
99 Brisby H, Balague F, Schafer D, et al. Glycosphingolipid antibodies in serum in patients with sciatica. Spine. 2002;27:380-386.
100 Suguwara O, Atsuta Y, Iwahara T, et al. The effects of mechanical compression and hypoxia on nerve root and dorsal root ganglia. Spine. 1996;21:2089-2094.
101 Cavanaugh JM. Neural mechanisms of lumbar spinal pain. Spine. 1995;16:1804-1809.
102 Cavanaugh JM, Ozaktay AC, Yamashita T, et al. Mechanisms of low back pain: a neurophysiologic and neuroanatomic study. Clin Orthop. 1997;335:166-180.
103 Groen G, Baljet B, Drukker J. The nerves and nerve plexuses of the human vertebral column. Am J Anat. 1990;188:282-296.
104 Hirsch C, Ingelmark BE, Miller M. The anatomical basis for low back pain. Acta Orthop Scand. 1963;33:1-17.
105 Jackson HC, Winkelmann RK, Bickel WH. Nerve endings in the human lumbar spinal column and related structures. J Bone Joint Surg. 1966;48A:1272-1281.
106 Inman VT, Saunders JB. Referred pain from skeletal structures. J Nerv Ment Dis. 1944;99:660-667.
107 Hadjipavlou AG, Lander PH. Paget’s disease. In: White AH, editor. Spine Care, Vol. 2. St Louis: Mosby; 1995:1720-1737.
108 Parfitt AM, Duncan H. Metabolic bone disease affecting the spine. In: Rothman RH, Simeone FA, editors. The Spine, Vol. II. Philadelphia: Saunders; 1975:599-720.
109 Francis KC. Tumors of the spine. In: Rothman RH, Simeone FA, editors. The Spine, Vol. II. Philadelphia: Saunders; 1975:811-822.
110 Nugent PJ. Surgical treatment of spinal tumours. In: White AH, editor. Spine Care, Vol. 2. St Louis: Mosby; 1995:1457-1484.
111 Gornet MF. Spinal infection. In: White AH, editor. Spine Care, Vol. 2. St Louis: Mosby; 1995:1543-1551.
112 Hodgson AR. Infectious disease of the spine. In: Rothman RH, Simeone FA, editors. The Spine, Vol. II. Philadelphia: Saunders; 1975:567-598.
113 Gershon-Cohen J. Asymptomatic fractures in osteoporotic spine of the aged. JAMA. 1953;153:625-627.
114 Nathanson L, Lewitan A. Deformities and fractures of vertebrae as a result of senile and presenile osteoporosis. Am J Roentgenol. 1941;46:197-202.
115 Wyke B. The neurology of low-back pain. In: Jayson MIV, editor. The Lumbar Spine and Back Pain. Edinburgh: Churchill Livingstone; 1987:56-99.
116 Niv D, Gofeld M, Devor M. Cause of pain in degenerative bone and joint disease: a lesson from vertebroplasty. Pain. 2003;105:387-392.
117 Buonocore M, Aloisi AM, Barbieri M, et al. Vertebral body innervation: implications for pain. J Cell Physiol. 2010;222:488-491.
118 Bogduk N, MacVicar K, Borowczyk J. The pain of vertebral compression fractures can arise in the posterior elements. Pain Med. 2010;11:1666-1673.
119 Arnoldi CC. Intravertebral pressures in patients with lumbar pain. Acta Orthop Scand. 1972;43:109-117.
120 Arnoldi CC. Intraosseous hypertension: a possible cause of low-back pain? Clin Orthop. 1976;115:30-34.
121 Deyo RA, Diehl AK. Lumbar spine films in primary care: current use and effects of selective ordering criteria. J Gen Int Med. 1986;1:20-25.
122 Baastrup CI. Proc. Spin. vert. Lumb. und einige zwischen diesen leigende Gelenkbildungen mit pathologischen Prozessen in dieser Region. Fortschritee auf den Gebiete der Rontgenstrahlen. 1933;48:430-435.
123 Epstein BS. The Spine. A radiological text and atlas, 4th ed. Philadelphia: Lea & Febiger; 1976.
124 Bogduk N, Wilson AS, Tynan W. The human lumbar dorsal rami. J Anat. 1982;134:383-397.
125 Rhalmi S, Yahia L, Newman N, et al. Immunohistochemical study of nerves in lumbar spine ligaments. Spine. 1993;18:264-267.
126 Yahia LH, Newman NA. A light and electron microscopic study of spinal ligament innervation. Z mikroskop anat Forsch Leipzig. 1989;103:664-674.
127 Yahia LH, Newman N, Rivard CH. Neurohistology of lumbar spine ligaments. Acta Orthop Scand. 1988;59:508-512.
128 Beks JWF. Kissing spines: fact or fancy? Acta Neurochir. 1989;100:134-135.
129 Hadley LA. Subluxation of the apophyseal articulations with bony impingement as a cause of back pain. Am J Roentgenol. 1935;33:209-213.
130 Harris RI, MacNab I. Structural changes in the lumbar intervertebral discs. Their relationship to low back pain and sciatica. J Bone Joint Surg. 1954;36B:304-322.
131 Vernon-Roberts B, Pirie CJ. Degenerative changes in the intervertebral discs of the lumbar spine and their sequelae. Rheumatol Rehab. 1977;16:13-21.
132 Cyron BM, Hutton WC. The fatigue strength of the lumbar neural arch in spondylolysis. J Bone Joint Surg. 1978;60B:234-238.
133 O’Neill DB, Micheli LJ. Postoperative radiographic evidence for fatigue fracture as the etiology in spondylolysis. Spine. 1989;14:1342-1355.
134 Wiltse LL, Widell EH, Jackson DW. Fatigue fracture: the basic lesion in isthmic spondylolisthesis. J Bone Joint Surg. 1975;57A:17-22.
135 Eisenstein SM, Ashton IK, Roberts S, et al. Innervation of the spondylolysis ‘ligament’. Spine. 1994;19:912-916.
136 Schneiderman GA, McLain RF, Hambly MF, et al. The pars defect as a pain source: a histological study. Spine. 1995;20:1761-1764.
137 Moreton R. Spondylolysis. JAMA. 1966;5:671-674.
138 Libson E, Bloom RA, Dinari G. Symptomatic and asymptomatic spondylolysis and spondylolisthesis in young adults. Int Orthop. 1982;6:259-261.
139 Fredrickson BE, Baker D, McHolick WJ, et al. The natural history of spondylosis and spondylolisthesis. J Bone Joint Surg. 1984;66A:699-707.
140 Su PB, Esses SI, Kostuik JP. Repair of a pars interarticularis defect – the prognostic value of pars infiltration. Spine. 1991;16(Suppl. 8):S445-S448.
141 Bogduk N, McGuirk B. Imaging. In: Bogduk N, McGuirk B, editors. Medical Management of Acute and Chronic Low Back Pain. An Evidence-Based Approach. Amsterdam: Elsevier; 2002:49-63.
142 Williams PL, editor. Gray’s Anatomy, 38th ed, Edinburgh: Churchill Livingstone, 1995.
143 Cave AJE. The innervation and morphology of the cervical intertransverse muscles. J Anat. 1937;71:497-515.
144 Bogduk N. Lumbar dorsal ramus syndrome. Med J Aust. 1980;2:537-541.
145 Kellgren JH. Observations on referred pain arising from muscle. Clin Sci. 1938;3:175-190.
146 Garrett WE, Nikoloau PK, Ribbeck BM, et al. The effect of muscle architecture on the biomechanical failure properties of skeletal muscle under passive tension. Am J Sports Med. 1988;16:7-12.
147 Garrett WE, Saffrean MR, Seaber AV, et al. Biomechanical comparison of stimulated and non-stimulated skeletal muscle pulled to failure. Am J Sports Med. 1987;15:448-454.
148 Nikoloau PK, MacDonald BL, Glisson RR, et al. Biomechanical and histological evaluation of muscle after controlled strain injury. Am J Sports Med. 1987;15:9-14.
149 Garrett W, Bradley W, Byrd S, et al. Muscle: basic science perspectives. In: Frymoyer JW, Gordon SL, editors. New Perspectives on Low Back Pain. Park Ridge: American Academy of Orthopedic Surgeons; 1989:335-372.
150 Garrett W, Anderson G, Richardson W. Muscle: future directions. In: Frymoyer JW, Gordon SL, editors. New Perspectives on Low Back Pain. Park Ridge: American Academy of Orthopedic Surgeons; 1989:373-379.
151 Roland MO. A critical review of the evidence for a pain–spasm–pain cycle in spinal disorders. Clin Biomech. 1986;1:102-109.
152 Jull GA, Janda V. Muscles and motor control in low back pain: assessment and management. In: Twomey LT, Taylor JR, editors. Physical Therapy of the Low Back. New York: Churchill Livingstone; 1987:253-278.
153 Simons DG. Myofascial pain syndromes: where are we? Where are we going? Arch Phys Med Rehab. 1988;69:207-212.
154 Simons DG. Myofascial trigger points: a need for understanding. Arch Phys Med Rehab. 1981;62:97-99.
155 Simons DG, Travell J. Myofascial trigger points, a possible explanation. (letter). Pain. 1981;10:106-109.
156 Travell J, Rinzler SH. The myofascial genesis of pain. Postgrad Med. 1952;11:425-434.
157 Sola AE, Kuitert JH. Quadratus lumborum myofasciitis. Northwest Med. 1954;53:1003-1005.
158 Nice DA, Riddle DL, Lamb RL, et al. Intertester reliability of judgments of the presence of trigger points in patients with low back pain. Arch Phys Med Rehab. 1992;73:893-898.
159 Njoo KH, van der Does E. The occurrence and interrater reliability of myofascial trigger points in the quadratus lumborum and gluteus medius: a prospective study in non-specific low back pain patients and controls in general practice. Pain. 1994;58:317-323.
160 Yahia LH, Rhalmi S, Isler M. Sensory innervation of human thoracolumbar fascia: an immunohistochemical study. Acta Orthop Scand. 1992;63:195-197.
161 Stillwell DL. Regional variations in the innervation of deep fasciae and aponeuroses. Anat Rec. 1957;127:635-653.
162 Carr D, Gilbertson L, Frymoyer J, et al. Lumbar paraspinal compartment syndrome: a case report with physiologic and anatomic studies. Spine. 1985;10:816-820.
163 Peck D, Nicholls PJ, Beard C, et al. Are there compartment syndromes in some patients with idiopathic back pain? Spine. 1986;11:468-475.
164 Styf J, Lysell E. Chronic compartment syndrome in the erector spinae muscle. Spine. 1987;12:680-682.
165 Konno S, Kikuchi S, Nagaosa Y. The relationship between intramuscular pressure of the paraspinal muscles and low back pain. Spine. 1994;19:2186-2189.
166 Bonner CD, Kasdon SC. Herniation of fat through lumbo-dorsal fascia as a cause of low-back pain. New Engl J Med. 1954;251:1101-1104.
167 Copeman WSC, Ackerman WL. ‘Fibrositis’ of the back. Quart J Med. 1944;13:37-52.
168 Copeman WSC, Ackerman WL. Edema or herniations of fat lobules as a cause of lumbar and gluteal ‘fibrositis’. Arch Int Med. 1947;79:22-35.
169 Faille RJ. Low back pain and lumbar fat herniation. Am Surg. 1978;44:359-361.
170 Herz R. Herniation of fascial fat as a cause of low-back pain. JAMA. 1945;128:921-925.
171 Hucherson DC, Gandy JR. Herniation of fascial fat: a cause of low-back pain. Am J Surg. 1948;76:605-609.
172 Singewald ML. Sacroiliac lipomata – an often unrecognised cause of low back pain. Bull Johns Hopkins Hosp. 1966;118:492-498.
173 Woollgast GF, Afeman CE. Sacroiliac (episacral) lipomas. Arch Surg. 1961;83:925-927.
174 Edgar MA, Nundy S. Innervation of the spinal dura mater. J Neurol Neurosurg Psychiatry. 1964;29:530-534.
175 El Mahdi MA, Latif FYA, Janko M. The spinal nerve root innervation, and a new concept of the clinicopathological interrelations in back pain and sciatica. Neurochirurgia. 1981;24:137-141.
176 Walton JN, editor. Brain’s Diseases of the Nervous System, 8th ed. Oxford: OUP. 1977:407-408.
177 Spencer DL, Irwin GS, Miller JAA. Anatomy and significance of fixation of the lumbosacral nerve roots in sciatica. Spine. 1983;8:672-679.
178 Cuatico W, Parker JC, Pappert E, et al. An anatomical and clinical investigation of spinal meningeal nerves. Acta Neurochir. 1988;90:139-143.
179 Cuatico W, Parker JC. Further observations on spinal meningeal nerves and their role in pain production. Acta Neurochir. 1989;101:126-128.
180 Pedersen HE, Blunck CFJ, Gardner E. The anatomy of lumbosacral posterior rami and meningeal branches of spinal nerves (sinu-vertebral nerves): with an experimental study of their function. J Bone Joint Surg. 1956;38A:377-391.
181 Boas RA. Post-surgical low back pain. In: Peck C, Wallace M, editors. Problems in Pain. Sydney: Pergamon; 1980:188-191.
182 Ramsey RH. The anatomy of the ligamenta flava. Clin Orthop. 1966;44:129-140.
183 Yong-Hing K, Reilly J, Kirkaldy-Willis WH. The ligamentum flavum. Spine. 1976;1:226-234.
184 Kirby MC, Sikoryn TA, Hukins DWL, et al. Structure and mechanical properties of the longitudinal ligaments and ligamentum flavum of the spine. J Biomed Eng. 1989;11:192-196.
185 Heylings DJA. Supraspinous and interspinous ligaments of the human spine. J Anat. 1978;125:127-131.
186 Rissanen PM. The surgical anatomy and pathology of the supraspinous and interspinous ligaments of the lumbar spine with special reference to ligament ruptures. Acta Orthop Scand. 1960;46(Suppl.):1-100.
187 Bogduk N, Tynan W, Wilson AS. The nerve supply to the human lumbar intervertebral discs. J Anat. 1981;132:39-56.
188 Falconer MA, McGeorge M, Begg AC. Observations on the cause and mechanism of symptom-production in sciatica and low-back pain. J Neurol Neurosurg Psychiatry. 1948;11:13-26.
189 Wiberg G. Back pain in relation to the nerve supply of the intervertebral disc. Acta Orthop Scand. 1947;19:211-221.
190 Feinstein B, Langton JNK, Jameson RM, et al. Experiments on pain referred from deep structures. J Bone Joint Surg. 1954;36A:981-997.
191 Hockaday JM, Whitty CWM. Patterns of referred pain in the normal subject. Brain. 1967;90:481-496.
192 Kellgren JH. On the distribution of pain arising from deep somatic structures with charts of segmental pain areas. Clin Sci. 1939;4:35-46.
193 Steindler A, Luck JV. Differential diagnosis of pain low in the back: allocation of the source of pain by the procain hydrochloride method. JAMA. 1938;110:106-112.
194 Wilk V. Pain arising from the interspinous and supraspinous ligaments. Australas Musculoskeletal Med. 1995;1:21-31.
195 Chow DHK, Luk KDK, Leong JCY, et al. Torsional stability of the lumbosacral junction: significance of the iliolumbar ligament. Spine. 1989;14:611-615.
196 Leong JCY, Luk KDK, Chow DHK, et al. The biomechanical functions of the iliolumbar ligament in maintaining stability of the lumbosacral junction. Spine. 1987;12:669-674.
197 Yamamoto I, Panjabi MM, Oxland TR, et al. The role of the iliolumbar ligament in the lumbosacral junction. Spine. 1990;15:1138-1141.
198 Hirschberg GG, Froetscher L, Naeim F. Iliolumbar syndrome as a common cause of low-back pain: diagnosis and prognosis. Arch Phys Med Rehab. 1979;60:415-419.
199 Hackett GS. Referred pain from low back ligament disability. AMAArch Surg. 1956;73:878-883.
200 Collee G, Dijkmans AC, Vandenbroucke JP, et al. Iliac crest pain syndrome in low back pain: frequency and features. J Rheumatol. 1991;18:1064-1067.
201 Fairbank JCT, O’Brien JP. The iliac crest syndrome: a treatable cause of low-back pain. Spine. 1983;8:220-224.
202 Ingpen ML, Burry HC. A lumbo-sacral strain syndrome. Ann Phys Med. 1970;10:270-274.
203 Bogduk N. A reappraisal of the anatomy of the human lumbar erector spinae. J Anat. 1980;131:525-540.
204 Macintosh JE, Bogduk N. The morphology of the lumbar erector spinae. Spine. 1986;12:658-668.
205 Bauwens P, Coyer AB. The ‘multifidus triangle’ syndrome as a cause of recurrent low-back pain. BMJ. 1955;2:1306-1307.
206 Livingstone WK. Back disabilities due to strain of the multifidus muscle. West J Surg. 1941;49:259-263.
207 Njoo KH, van der Does E, Stam HJ. Interobserver agreement on iliac crest pain syndrome in general practice. J Rheumatol. 1995;22:1532-1535.
208 Paris SV. Anatomy as related to function and pain. Orthop Clin North Am. 1983;14:475-489.
209 Pitkin HC, Pheasant HC. Sacrarthogenetic telalgia I. A study of referred pain. J Bone Joint Surg. 1936;18:111-133.
210 Ikeda R. Innervation of the sacroiliac joint: macroscopical and histological studies. Nippon Ika Daigaku Zasshi. 1991;58:587-596.
211 Grob KR, Neuhuber WL, Kissling RO. Innervation of the human sacroiliac joint. Z Rheumatol. 1995;54:117-122.
212 Fortin JD, Dwyer AP, West S, et al. Sacroiliac joint: pain referral maps upon applying a new injection/arthrography technique. Part I. Asymptomatic volunteers. Spine. 1994;19:1475-1482.
213 Bellamy N, Park W, Rooney PJ. What do we know about the sacroiliac joint? Semin Arthritis Rheum. 1983;12:282-313.
214 Davis P, Lentle BC. Evidence for sacroiliac disease as a common cause of low backache in women. Lancet. 1978;2:496-497.
215 Aitken GS. Syndromes of lumbo-pelvic dysfunction. In: Grieve GP, editor. Modern Manual Therapy of the Vertebral Column. Edinburgh: Churchill Livingstone; 1986:473-478.
216 Lee D. The Pelvic Girdle. Edinburgh: Churchill Livingstone; 1989.
217 Lewit K. The diagnosis of sacroiliac movement restriction in ankylosing spondylitis. Man Med. 1987;3:67-68.
218 Rantanen P, Airaksinen O. Poor agreement between so-called sacroiliacal joint tests in ankylosing spondylitis patients. J Man Med. 1989;4:62-64.
219 Schmid HJA. Sacroiliac diagnosis and treatment. Man Med. 1984;1:33-38.
220 Wells PE. The examination of the pelvic joints. In: Grieve GP, editor. Modern Manual Therapy of the Vertebral Column. Edinburgh: Churchill Livingstone; 1986:473-478.
221 Egund N, Olsson TH, Schmid H, et al. Movements in the sacroiliac joints demonstrated with roentgen stereophotogrammetry. Acta Radiol. 1978;19:833-846.
222 Sturesson B, Selvik G, Uden A. Movements of the sacroiliac joints: a roentgen stereophotogrammetric analysis. Spine. 1989;14:162-165.
223 Sturesson B, Uden A, Vlemming A. A radiostereometric analysis of movements of the sacroiliac joints during the standing hip flexion test. Spine. 2000;25:364-366.
224 Tulberg T, Blomberg S, Branth B, et al. Manipulation does not alter the position of the sacroiliac joint. A Roentgen stereophotogrammetric analysis. Spine. 1998;23:1124-1129.
225 Maigne JY, Aivaliklis A, Pfeffer F. Results of sacroiliac joint double block and value of sacroiliac pain provocation tests in 54 patients with low back pain. Spine. 1996;21:1889-1892.
226 Schwarzer AC, Aprill CN, Bogduk N. The sacroiliac joint in chronic low back pain. Spine. 1995;20:31-37.
227 Dreyfuss P, Michaelsen M, Pauza K, et al. The value of history and physical examination in diagnosing sacroiliac joint pain. Spine. 1996;21:2594-2602.
228 Bogduk N. The innervation of the lumbar spine. Spine. 1983;8:286-293.
229 McCall IW, Park WM, O’Brien JP. Induced pain referral from posterior lumbar elements in normal subjects. Spine. 1979;4:441-446.
230 Mooney V, Robertson J. The facet syndrome. Clin Orthop. 1976;115:149-156.
231 Fairbank JCT, Park WM, McCall IW, et al. Apophyseal injections of local anaesthetic as a diagnostic aid in primary low-back pain syndromes. Spine. 1981;6:598-605.
232 Schwarzer AC, Aprill CN, Derby R, et al. Clinical features of patients with pain stemming from the lumbar zygapophysial joints. Is the lumbar facet syndrome a clinical entity? Spine. 1994;19:1132-1137.
233 Schwarzer AC, Aprill CN, Derby R, et al. The false-positive rate of uncontrolled diagnostic blocks of the lumbar zygapophysial joints. Pain. 1994;58:195-200.
234 Schwarzer AC, Wang S, Bogduk N, et al. Prevalence and clinical features of lumbar zygapophysial joint pain: a study in an Australian population with chronic low back pain. Ann Rheum Dis. 1995;54:100-106.
235 Ghormley RK. Low back pain with special reference to the articular facets, with presentation of an operative procedure. JAMA. 1933;101:1773-1777.
236 Bogduk N, Aprill C, Derby R. Diagnostic blocks of synovial joints. In: White AH, editor. Spine Care, Vol. 1. Diagnosis and conservative treatment. St Louis: Mosby; 1995:298-321.
237 Bogduk N. Evidence-informed management of chronic back pain with facet injections and radiofrequency neurotomy. Spine J. 2008;8:56-64.
238 Bogduk N, Aprill C, Derby R. Lumbar zygapophysial joint pain: diagnostic blocks and therapy. In: Wilson DJ, editor. Interventional Radiology of the Musculoskeletal System. London: Edward Arnold; 1995:73-86.
239 Bogduk N. On the rational use of diagnostic blocks for spinal pain. Neurosurg Q. 2009;19:88-100.
240 Manchikanti L, Pampati V, Fellows B, et al. Prevalence of lumbar facet joint pain in chronic low back pain. Pain Physician. 1999;2:59-64.
241 Schwarzer AC, Aprill CN, Derby R, et al. The relative contributions of the disc and zygapophyseal joint in chronic low back pain. Spine. 1994;19:801-806.
242 Jackson RP, Jacobs RR, Montesano PX. Facet joint injection in low-back pain. Spine. 1988;13:966-971.
243 Carette S, Marcoux S, Truchon R, et al. A controlled trial of corticosteroid injections into facet joints for chronic low back pain. New Engl J Med. 1991;325:1002-1007.
244 Eisenstein SM, Parry CR. The lumbar facet arthrosis syndrome. J Bone Joint Surg. 1987;69B:3-7.
245 Lippit AB. The facet joint and its role in spine pain. Spine. 1984;9:746-750.
246 Schwarzer AC, Derby R, Aprill CN, et al. Pain from the lumbar zygapophysial joints: a test of two models. J Spinal Disord. 1994;7:331-336.
247 Jayson MIV. Degenerative disease of the spine and back pain. Clin Rheum Dis. 1976;2:557-584.
248 Lawrence JS, Sharpe J, Ball J, et al. Rheumatoid arthritis of the lumbar spine. Ann Rheum Dis. 1964;23:205-217.
249 Sims-Williams H, Jason MIV, Baddeley H. Rheumatoid involvement of the lumbar spine. Ann Rheum Dis. 1977;36:524-531.
250 Ball J. Enthesopathy of rheumatoid and ankylosing spondylitis. Ann Rheum Dis. 1971;30:213-223.
251 Bailey W. Anomalies and fractures of the vertebral articular processes. JAMA. 1937;108:266-270.
252 Fulton WS, Kalbfleisch WK. Accessory articular processes of the lumbar vertebrae. Arch Surg. 1934;29:42-48.
253 King AB. Back pain due to loose facets of the lower lumbar vertebrae. Bull Johns Hopkins Hosp. 1955;97:271-283.
254 Campbell AJ, Wells IP. Pigmented villonodular synovitis of a lumbar vertebral facet joint. J Bone Joint Surg. 1982;64A:145-146.
255 Titelbaum DS, Rhodes CH, Brooks JS, et al. Pigmented villonodular synovitis of a lumbar facet joint. Am J Neuroradiol. 1992;13:164-166.
256 Chevalier X, Marty M, Larget-Piet B. Klebsiella pneumoniae septic arthritis of a lumbar facet joint. J Rheumatol. 1992;19:1817-1819.
257 Halpin DS, Gibson RD. Septic arthritis of the lumbar facet joint. J Bone Joint Surg. 1987;69B:457-459.
258 Rousselin B, Gires F, Vallee C, et al. Case report 627. Skeletal Radiol. 1990;19:453-455.
259 Rush J, Griffiths J. Suppurative arthritis of a lumbar facet joint. J Bone Joint Surg. 1989;71B:161-162.
260 Lewin T. Osteoarthritis in lumbar synovial joints. Acta Orthop Scand. 1964;Suppl. 19(73):1-112.
261 Shore LR. On osteoarthritis in the dorsal intervertebral joints: a study in morbid anatomy. Br J Surg. 1935;22:833-849.
262 Lawrence JS, Bremner JM, Bier F. Osteoarthrosis: prevalence in the population and relationship between symptoms and X-ray changes. Ann Rheum Dis. 1966;25:1-24.
263 Magora A, Schwartz A. Relation between the low back pain syndrome and X-ray findings. 1. Degenerative osteoarthritis. Scand J Rehab Med. 1976;8:115-125.
264 Abel MS. The radiology of low back pain associated with posterior element lesions of the lumbar spine. Crit Rev Diagn Imaging. 1984;20:311-352.
265 Carrera GF, Williams AL. Current concepts in evaluation of the lumbar facet joints. Crit Rev Diagn Imaging. 1984;21:85-104.
266 Lynch MC, Taylor JF. Facet joint injection for low back pain. J Bone Joint Surg. 1986;68B:138-141.
267 Hermanus N, De Becker D, Baleriaus D, et al. The use of CT scanning for the study of posterior lumbar intervertebral articulations. Neuroradiology. 1983;24:159-161.
268 Schwarzer AC, Wang S, O’Driscoll D, et al. The ability of computed tomography to identify a painful zygapophysial joint in patients with chronic low back pain. Spine. 1995;20:907-912.
269 Yang KH, King AI. Mechanism of facet load transmission as a hypothesis for low-back pain. Spine. 1984;9:557-565.
270 Farfan HF, Cossette JW, Robertson GH, et al. The effects of torsion on the lumbar intervertebral joints: the role of torsion in the production of disc degeneration. J Bone Joint Surg. 1970;52A:468-497.
271 Adams MA, Hutton WC. The relevance of torsion to the mechanical derangement of the lumbar spine. Spine. 1981;6:241-248.
272 Farfan HF. Mechanical Disorders of the Low Back. Philadelphia: Lea & Febiger; 1973.
273 Farfan HF. A reorientation in the surgical approach to degenerative lumbar intervertebral joint disease. Orthop Clin. 1977;8:9-21.
274 Farfan HF, Kirkaldy-Willis WH. The present status of spinal fusion in the treatment of lumbar intervertebral joint disorders. Clin Orthop. 1981;158:198-214.
275 Lamy C, Kraus H, Farfan HF. The strength of the neural arch and the etiology of spondylosis. Orthop Clin North Am. 1975;6:215-231.
276 Sullivan JD, Farfan HF. The crumpled neural arch. Orthop Clin North Am. 1975;6:199-213.
277 Taylor JR, Twomey LT, Corker M. Bone and soft tissue injuries in post-mortem lumbar spines. Paraplegia. 1990;28:119-129.
278 Twomey LT, Taylor JR, Taylor MM. Unsuspected damage to lumbar zygapophyseal (facet) joints after motor-vehicle accidents. Med J Aust. 1989;151:210-217.
279 Jacoby RK, Sims-Williams H, Jayson MIV, et al. Radiographic stereoplotting: a new technique and its application to the study of the spine. Ann Rheum Dis. 1976;35:168-170.
280 Mitchell CL. Isolated fractures of the articular processes of the lumbar vertebrae. J Bone Joint Surg. 1933;15:608-614.
281 Sims-Williams H, Jayson MIV, Baddeley H. Small spinal fractures in back pain patients. Ann Rheum Dis. 1978;37:262-265.
282 Bogduk N, Engel R. The menisci of the lumbar zygapophyseal joints. A review of their anatomy and clinical significance. Spine. 1984;9:454-460.
283 Engel R, Bogduk N. The menisci of the lumbar zygapophyseal joints. J Anat. 1982;135:795-809.
284 Taylor JR, Twomey LT. Age changes in lumbar zygapophyseal joints. Spine. 1986;11:739-745.
285 Bogduk N, Jull G. The theoretical pathology of acute locked back: a basis for manipulative therapy. Man Med. 1985;1:78-82.
286 Inman VT, Saunders JB, de CM. Anatomicophysiological aspects of injuries to the intervertebral disc. J Bone Joint Surg. 1947;29:461-475. 534
287 Bogduk N. The innervation of intervertebral discs. In: Ghosh P, editor. The Biology of the Intervertebral Disc, Vol. I. Boca Raton: CRC Press; 1988:135-149.
288 Ehrenhaft JC. Development of the vertebral column as related to certain congenital and pathological changes. Surg Gynecol Obst. 1943;76:282-292.
289 Malinsky J. The ontogenetic development of nerve terminations in the intervertebral discs of man. Acta Anat. 1959;38:96-113.
290 Rabischong P, Louis R, Vignaud J, et al. The intervertebral disc. Anat Clin. 1978;1:55-64.
291 Roofe PG. Innervation of annulus fibrosus and posterior longitudinal ligament. Arch Neurol Psychiatry. 1940;44:100-103.
292 Yoshizawa H, O’Brien JP, Thomas-Smith W, et al. The neuropathology of intervertebral discs removed for low-back pain. J Path. 1980;132:95-104.
293 Bogduk N. Diskography. Am Pain Soc J. 1994;3:149-154.
294 Bogduk N, Aprill C, Derby R. Discography. In: White AH, editor. Spine Care, Vol. 1. Diagnosis and conservative treatment. St Louis: Mosby; 1995:219-238.
295 International Spinal Injection Society. Lumbar disc stimulation. In: Bogduk N, editor. Practice Guidelines for Spinal Diagnostic and Treatment Procedures. San Francisco: International Spinal Injection Society, 2004.
296 Schwarzer AC, Aprill CN, Derby R, et al. The prevalence and clinical features of internal disc disruption in patients with chronic low back pain. Spine. 1995;20:1878-1883.
297 Holt EP. The question of lumbar discography. J Bone Joint Surg. 1968;50A:720-726.
298 Simmons JW, Aprill CN, Dwyer AP, et al. A reassessment of Holt’s data on ‘the question of lumbar discography’. Clin Orthop. 1988;237:120-124.
299 Walsh TR, Weinstein JN, Spratt KF, et al. Lumbar discography in normal subjects. J Bone Joint Surg. 1990;72A:1081-1088.
300 Carragee EJ, Tanner CM, Khurana S, et al. The rates of false-positive lumbar discography in select patients without low back symptoms. Spine. 2000;25:1373-1381.
301 Derby R, Lee SH, Kim BJ, et al. Pressure-controlled lumbar discography in volunteers without low back symptoms. Pain Med. 2005;6:213-221.
302 Wolfer LR, Derby R, Lee JE, et al. Systematic review of lumbar provocation discography in asymptomatic subjects with a meta-analysis of false positive rates. Pain Physician. 2008;11:513-538.
303 O’Neill CW, Kurgansky ME, Derby R, et al. Disc stimulation and patterns of referred pain. Spine. 2002;27:2776-2781.
304 Bogduk N. The lumbar disc and low back pain. Neurosurg Clin North Am. 1991;2:791-806.
305 Fraser RD, Osti OL, Vernon-Roberts B. Discitis after discography. J Bone Joint Surg. 1987;69B:26-35.
306 Guyer RD, Collier R, Stith WJ, et al. Discitis after discography. Spine. 1988;13:1352-1354.
307 Farfan HF, Gracovetsky S. The nature of instability. Spine. 1984;9:714-719.
308 Farfan HF, Huberdeau RM, Dubow HI. Lumbar intervertebral disc degeneration. The influence of geometrical features on the pattern of disc degeneration – a post-mortem study. J Bone Joint Surg. 1972;54A:492-510.
309 Pearcy MJ. Inferred strains in the intervertebral discs during physiological movements. J Man Med. 1990;5:68-71.
310 Farfan HF. The use of mechanical etiology to determine the efficacy of active intervention in single joint intervertebral joint problems. Spine. 1985;10:350-358.
311 Yu S, Sether LA, Ho PSP, et al. Tears of the anulus fibrosus: correlation between MR and pathologic findings in cadavers. Am J Neuroradiol. 1988;9:367-370.
312 Finch PM, Khangure MS. Analgesic discography and magnetic resonance imaging (MRI). Pain. 1990;5(Suppl.):S285.
313 Finch P. Analgesic discography in the diagnosis of spinal pain. Paper presented at ‘Spinal Pain’: precision diagnosis and treatment. Official Satellite Meeting of the VIth World Congress on Pain, Perth, April 8–10, 1990. Meeting Abstracts: 8.
314 Sachs BL, Vanharanta H, Spivey MA, et al. Dallas discogram description: a new classification of CT/discography in low-back disorders. Spine. 1987;12:287-294.
315 Aprill C, Bogduk N. High intensity zone: a pathognomonic sign of painful lumbar disc on MRI. Br J Radiol. 1992;65:361-369.
316 Vanharanta H, Sachs BL, Spivey MA, et al. The relationship of pain provocation to lumbar disc deterioration as seen by CT/discography. Spine. 1987;12:295-298.
317 Moneta GB, Videman T, Kaivanto K, et al. Reported pain during lumbar discography as a function of anular ruptures and disc degeneration. A re-analysis of 833 discograms. Spine. 1994;17:1968-1974.
318 McNally DS, Shackleford IM, Goodship AE, et al. In vivo stress measurement can predict pain on discography. Spine. 1996;21:2500-2587.
319 Farfan HF. Mechanical Disorders of the Low Back. Philadelphia: Lea & Febiger; 1973.
320 Farfan HF, Kirkaldy-Willis WH. The present status of spinal fusion in the treatment of lumbar intervertebral joint disorders. Clin Orthop. 1981;158:198-214.
321 Brinckmann P. Injury of the annulus fibrosus and disc protrusions: an in vitro investigation on human lumbar discs. Spine. 1986;11:149-153.
322 Markolf KL, Morris JM. The structural components of the intervertebral disc. J Bone Joint Surg. 1974;56A:675-687.
323 Virgin WJ. Experimental investigations into the physical properties of the intervertebral disc. J Bone Joint Surg. 1951;33B:607-611.
324 Adams MA, Hutton WC. Gradual disc prolapse. Spine. 1985;10:524-531.
325 Adams MA, Hutton WC. Prolapsed intervertebral disc. A hyperflexion injury. Spine. 1982;7:184-191.
326 Brown T, Hansen RJ, Yorra AJ. Some mechanical tests on the lumbosacral spine with particular reference to the intervertebral discs. J Bone Joint Surg. 1957;39A:1135-1164.
327 Hutton WC, Adams MA. Can the lumbar spine be crushed in heavy lifting? Spine. 1982;7:586-590.
328 Jayson MIV, Herbert CM, Barks JS. Intervertebral discs: nuclear morphology and bursting pressures. Ann Rheum Dis. 1973;32:308-315.
329 Perey O. Fracture of the vertebral end-plate in the lumbar spine. Acta Orthop Scand. 1957;25(Suppl.):1-101.
330 Rolander SD, Blair WE. Deformation and fracture of the lumbar vertebral end plate. Orthop Clin North Am. 1975;6:75-81.
331 Porter RW, Adams MA, Hutton WC. Physical activity and the strength of the lumbar spine. Spine. 1989;14:201-203.
332 Brinckmann P, Frobin W, Hierholzer E, et al. Deformation of the vertebral end-plate under axial loading of the spine. Spine. 1983;8:851-856.
333 Adams MA, McNally DS, Wagstaff J, et al. Abnormal stress concentrations in lumbar intervertebral discs following damage to the vertebral bodies: a cause of disc failure? Eur Spine J. 1993;1:214-221.
334 McNeill T, Warwick D, Andersson G, et al. Trunk strengths in attempted flexion, extension, and lateral bending in healthy subjects and patients with low-back disorders. Spine. 1980;5:529-538.
335 Granhed H, Johnson R, Hansson T. The loads on the lumbar spine during extreme weight lifting. Spine. 1987;12:146-149.
336 Hansson TH, Keller TS, Spengler DM. Mechanical behaviour of the human lumbar spine. II. Fatigue strength during dynamic compressive loading. J Orthop Res. 1987;5:479-487.
337 Liu YK, Njus G, Buckwalter J, et al. Fatigue response of lumbar intervertebral joints under axial cyclic loading. Spine. 1983;8:857-865.
338 Bogduk N. Sources of low back pain. In: Jayson MIV, editor. The Lumbar Spine and Back Pain. Edinburgh: Churchill Livingstone; 1992:61-88. Ch. 4
339 Bogduk N, Twomey L. Clinical Anatomy of the Lumbar Spine, 2nd ed. Melbourne: Churchill Livingstone; 1991.
340 Elves MW, Bucknill T, Sullivan MF. In vitro inhibition of leukocyte migration in patients with intervertebral disk lesions. Orthop Clin North Am. 1975;6:59-65.
341 McCall IW, Park WM, O’Brien JP, et al. Acute traumatic intraosseous disc herniation. Spine. 1985;10:134-137.
342 Kalebo P, Kadziolka R, Sward L. Compression–traction radiography of lumbar segmental instability. Spine. 1990;15:351-355.
343 Maroudas A. Nutrition and metabolism of the intervertebral disc. In: Ghosh P, editor. The Biology of the Intervertebral Disc, Vol. II. Boca Raton: CRC Press; 1988:1-37. Ch. 9
344 Melrose J, Ghosh P. The noncollagenous proteins of the intervertebral disc. In: Ghosh P, editor. The Biology of the Intervertebral Disc, Vol. I. Boca Raton: CRC Press; 1988:189-237. Ch. 8
345 Sedowfia KA, Tomlinson IW, Weiss JB, et al. Collagenolytic enzyme systems in human intervertebral disc. Spine. 1982;7:213-222.
346 Ohshima H, Urban JPG. The effect of lactate and pH on proteoglycan and protein synthesis rates in the intervertebral disc. Spine. 1992;17:1079-1082.
347 Fujita K, Nakagawa T, Hirabayashi K, et al. Neutral proteinases in human intervertebral disc: role in degeneration and probable origin. Spine. 1993;18:1766-1773.
348 Crock HV. A reappraisal of intervertebral disc lesions. Med J Aust. 1970;1:983-989. (and supplementary pages i–ii)
349 Venner RM, Crock HV. Clinical studies of isolated disc resorption in the lumbar spine. J Bone Joint Surg. 1981;63B:491-494.
350 Twomey L, Taylor J. Age changes in lumbar intervertebral discs. Acta Orthop Scand. 1985;56:496-499.
351 Holm S, Kaigle-Holm A, Ekstrom L, et al. Experimental disc degeneration due to endplate injury. J Spinal Disord Tech. 2004;17:64-71.
352 Jaffray D, O’Brien JP. Isolated intervertebral disc resorption: a source of mechanical and inflammatory back pain? Spine. 1986;11:397-401.
353 Schellhas KP, Pollei SR, Gundry CR, et al. Lumbar disc high-intensity zone: correlation of magnetic resonance imaging and discography. Spine. 1996;21:79-86.