Chapter 40 Low Back Pain
Epidemiology
Low back pain is a symptom, not a disease, and has many causes. It is generally described as pain between the costal margin and the gluteal folds. It is extremely common. About 40% of people say that they have had low back pain within the past 6 months.233 Studies have shown a lifetime prevalence as high as 84%.240 Onset usually begins in the teens to early 40s. Most patients have short attacks of pain that are mild or moderate and do not limit activities, but these tend to recur over many years. Most episodes resolve with or without treatment. The median time off work for a back injury is 7 days, and many people with low back pain never alter their activity. A small percentage of low back pain becomes chronic, however, and causes significant disability. In most studies about half of the sick days used for back pain are accounted for by the 15% of people who are home from work for more than 1 month. Between 80% and 90% of the health care and social costs of back pain are for the 10% who develop chronic low back pain and disability. Just over 1% of adults in the United States are permanently disabled by back pain, and another 1% are temporarily disabled.151
Anatomy and Biomechanics of the Lumbar Spine
The Vertebrae
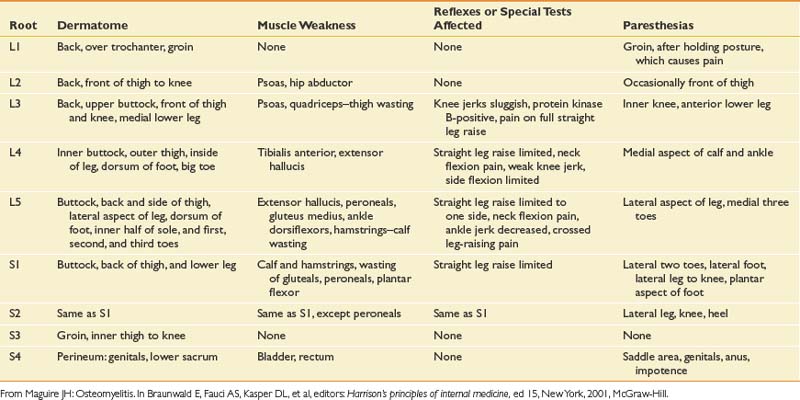
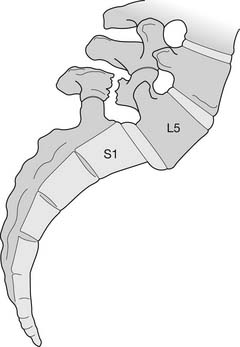
The bony anatomy of the lumbar spine consists of five lumbar vertebrae. A smaller percentage of the population has four (the fifth vertebrae is sacralized) or six (the first sacral segment is lumbarized). Anatomic variants also exist consisting of a partially lumbarized S1. The lumbar vertebrae have distinct components, which include the vertebral body, the neural arch, and the posterior elements (Figure 40-1). The vertebral bodies increase in size as you travel caudally in the spine. The lower three are typically more wedge-shaped (taller anteriorly), which helps create the normal lumbar lordosis. The structure of these large vertebral bodies serves its weight-bearing function well to support axially directed loads; however, they would fracture more routinely were it not for the shock-absorbing intervertebral disks placed strategically between the vertebral bodies.
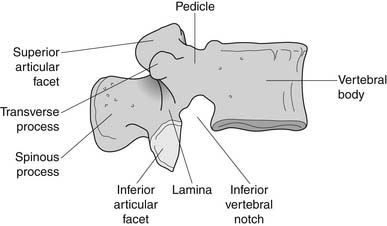
FIGURE 40-1 Lateral view of the lumbar vertebrae.
(Modified from Parke WW: Applied anatomy of the spine. In Rothman RH, Simeone FA, editors: The spine, ed 4, Philadelphia, 1999, Saunders.)
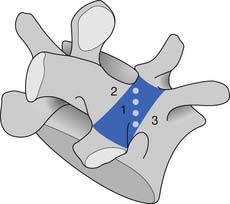
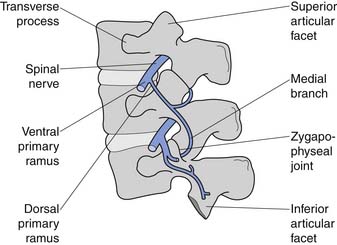
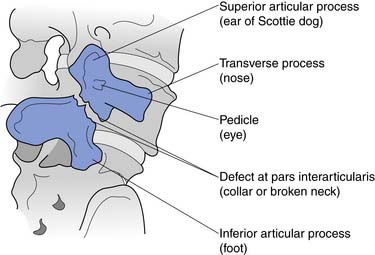
The sides of the bony neural arch are the pedicles, which are thick pillars that connect the posterior elements to the vertebral bodies. They are designed to resist bending and to transmit forces back and forth from the vertebral bodies to the posterior elements. The posterior elements consist of the laminae, the articular processes, and the spinous processes. The superior and inferior articular processes of adjacent vertebrae create the zygapophyseal joints. The pars interarticularis is a part of the lamina between the superior and inferior articular processes (Figure 40-2). The pars is the site of stress fractures (spondylolysis) because it is subjected to large bending forces. This occurs as the forces transmitted by the vertically oriented lamina undergo a change in direction into the horizontally oriented pedicle.23
The Joints
The Intervertebral Disk
The intervertebral disk and its attachment to the vertebral end plate are considered a secondary cartilaginous joint, or symphysis. The disk consists of the internal nucleus pulposus and the outer annulus fibrosus. The nucleus pulposus is the gelatinous inner section of the disk. It consists of water, proteoglycans, and collagen. The nucleus pulposus is 90% water at birth. Disks desiccate and degenerate as we age and lose some of their height, which is one reason we are slightly shorter in our geriatric years.
The annulus fibrosus consists of concentric layers of fibers at oblique angles to each other, which help to withstand strains in any direction. The outer fibers of the annulus have more collagen and less proteoglycans and water than the inner fibers.19 The varying composition supports the functional role of the outer fibers in acting like a ligament to resist flexion, extension, rotation, and distraction forces.
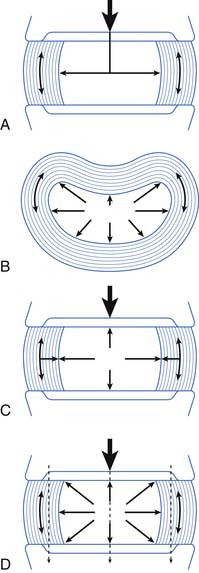
The main function of the intervertebral disk is shock absorption (Figure 40-3). It is primarily the annulus, not the nucleus, that acts as the shock absorber. (The nucleus is primarily a liquid and is incompressible.) When an axial load occurs, the increase in force in the incompressible nucleus pushes on the annulus and stretches its fibers. If the fibers break, then a herniated nucleus pulposus results.
The Zygapophyseal Joints
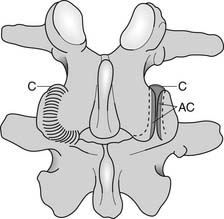
The zygapophyseal joints (also known as Z joints and facet joints) are paired synovial joints, that is, they have a synovium and a capsule (Figure 40-4). Their alignment or direction of joint articulation determines the direction of motion of the adjacent vertebrae. The lumbar zygapophyseal joints lie in the sagittal plane and thus primarily allow flexion and extension. Some lateral bending and very little rotation are allowed, which limits torsional stress on the lumbar disks. Rotation is more a component of thoracic spine motion. The majority of spinal flexion and extension (90%) occurs at the L4–L5 and L5–S1 levels, which contributes to the high incidence of disk problems at these levels.
Biomechanics
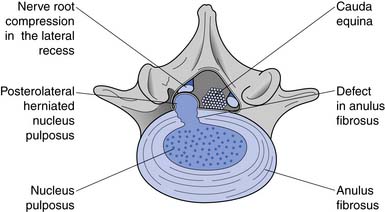
Because flexion loads the anterior disk, the nucleus is displaced posteriorly.111 If the forces are great enough, the nucleus can herniate through the posterior annular fibers. The lateral fibers of the posterior longitudinal ligaments are thinnest, however, making posterolateral disk herniations the most common (Figure 40-5). The posterolateral portion of the disk is most at risk when there is forward flexion accompanied by lateral bending (i.e., bending and twisting). The zygapophyseal joints cannot resist rotation when the spine is in flexion. This increases torsional shear forces in the lumbar spine, making rotary movements in a forward-flexed posture probably the most risky for lumbar disks.
The Muscles
Muscles With Origins on the Lumbar Spine
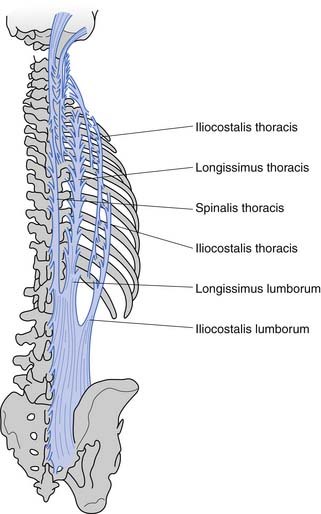
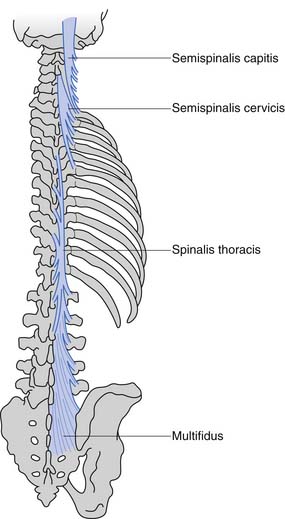
These muscles can be divided anatomically into posterior and anterior muscles. The posterior muscles include the latissimus dorsi and the paraspinals. The lumbar paraspinals consist of the erector spinae (iliocostalis, longissimus, and spinalis), which act as the chief extensors of the spine, and the deep layer (rotators and multifidi) (Figures 40-6 and 40-7). The multifidi are tiny segmental stabilizers that act to control lumbar flexion because they cannot produce enough force to truly extend the spine. Their most important function has been hypothesized to be that of a sensory organ to provide proprioception for the spine, given the predominance of muscle spindles seen histologically in these muscles.
Abdominal Musculature
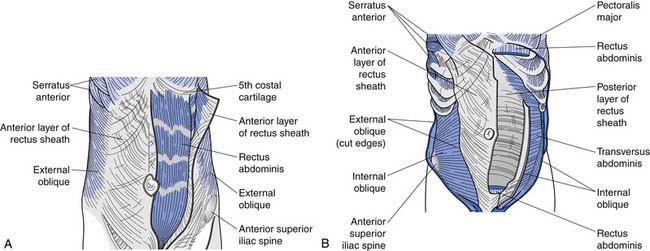
The superficial abdominals include the rectus abdominis and external obliques (Figure 40-8, A). The deep layer consists of internal obliques and the transversus abdominis (Figure 40-8, B). The transversus abdominis has received significant attention recently as an important muscle to train in treating low back pain. Its connection to the thoracolumbar fascia (and consequently its ability to act on the lumbar spine) has probably been the major reason it has received such attention of late.
Thoracolumbar Fascia
The thoracolumbar fascia, with its attachments to the transversus abdominis and internal obliques, acts as an abdominal and lumbar “brace.” It decreases some of the shear forces that other muscles and lumbar motions create. This abdominal bracing mechanism results from contraction of these deep abdominal muscles, which creates tension in the thoracolumbar fascia, which then creates an extension force on the lumbar spine without increasing shear forces.75
Pelvic Stabilizers
The piriformis is a hip and sacral rotator and can cause excessive external rotation of the hip and sacrum when it is tight. This can result in increased shear forces at the lumbosacral junction (i.e., the L5–S1 disk or Z joints). Some practitioners also believe that other pelvic floor muscles act to maintain proper positioning of the spine and are an important focus of some spine rehabilitation programs.
Biomechanical Lifting in Relation to Muscular Activity and Disk Loads
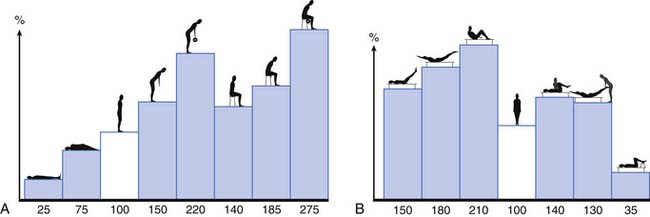
The activity of the lumbar muscles correlates well with intradiskal pressures (i.e., when back muscles contract, there is an associated increase in disk pressure). These pressures change depending on spine posture and the activity undertaken. Figure 40-9 demonstrates the changes in L3 disk pressure under various positions and exercises.149,150 Adding rotation to the already flexed posture increases the disk pressure substantially. Comparing lifting maneuvers, it has been shown that there is not a significant difference in disk pressure when lifting with the legs (i.e., with the back straight and knees bent) versus lifting with the back (i.e., with a forward-flexed back and straight legs).6,7 What decreases the forces on the lumbar spine is lifting the load close to your body because the farther the load is from the chest, the greater the stress on the lumbar spine.7
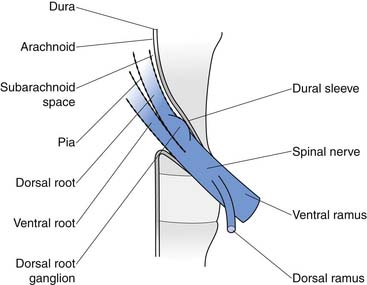
The Nerves
The conus medullaris ends at about bony level L2, and below this level is the cauda equina. The cauda equina consists of the dorsal and ventral rootlets, which join together in the intervertebral neuroforamen to become the spinal nerves (Figure 40-10). The spinal nerve gives off the ventral primary ramus. The ventral primary rami from multiple levels form the lumbar and lumbosacral plexus to innervate the limbs. The dorsal primary ramus, with its three branches (medial, intermediate, and lateral), innervates the posterior half of the vertebral body, the paraspinal muscles, and the zygapophyseal joints, and provides sensation to the back. The medial branch is the most important to remember because it innervates the zygapophyseal joints and lumbar multifidi and is the target during radiofrequency neurotomy for presumed zygapophyseal joint pain (Figure 40-11).24
Pathophysiology and Pain Generation
The Degenerative Spine Cascade
Kirkaldy-Willis et al.108 have supplied us with the most accepted theory describing the cascade of events in degenerative lumbar spine disease that results in spondylotic changes, disk herniations, and eventually multilevel spinal stenosis (Figure 40-12). At the heart of this theory is the fact that, although the posterior zygapophyseal joints and the anterior intervertebral disks are separated anatomically, forces and lesions affecting one certainly alter and affect the other. For example, axial compressing injuries can damage the vertebral end plates, which can lead to degenerative disk disease, which eventually stresses the posterior joints, leading to the common degenerative changes seen in them over time. Torsional stress can injure the posterior joints and the disks, which in turn leads to increased stress on both these elements, resulting in further degenerative changes over time. When these degenerative changes affect one level, such as L4–L5, a chain reaction occurs, placing stress on the levels above and below the currently affected level, and eventually resulting in more generalized multilevel spondylotic changes.
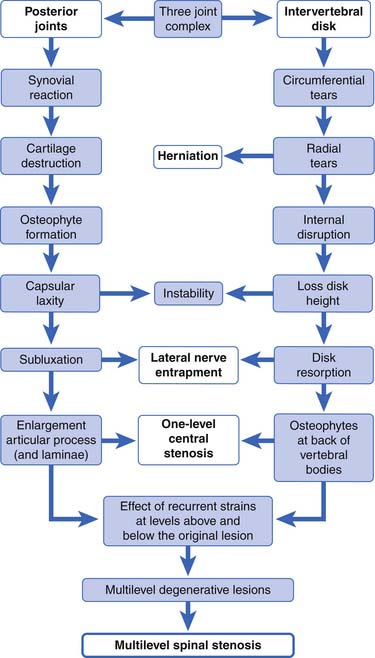
FIGURE 40-12 The spectrum of degenerative change that leads from minor strains to marked spondylosis and stenosis.
(Modified from Kirkaldy-Willis WH, Wedge JH, Yong-Hing K, et al: Pathology and pathogenesis of lumbar spondylosis and stenosis, Spine 3:319-328, 1998, with permission of Lippincott Williams & Wilkins.)
In studying lumbar degenerative disease, the question of which came first (disk degeneration or zygapophyseal joint degeneration) always arises. Fujiwara67 has answered this by studying multiple magnetic resonance images (MRIs) of aging spines. He hypothesizes that disk degeneration precedes zygapophyseal joint osteoarthritis, and that it might take 20 years for zygapophyseal joint disease to occur after the onset of disk degeneration.
To describe the degenerative cascade in more detail, we will separate our discussion of the changes that occur in the posterior joints from those in the disk, but fully realizing that they both can occur simultaneously and affect each other (see Figure 40-12). The degenerative changes that occur in the zygapophyseal joints from aging and repetitive microtrauma are similar to those that occur in the appendicular skeletal joints. The process begins with synovial hypertrophy, which eventually results in cartilage degeneration and destruction. With lessened and weakened cartilage and capsular laxity, the joint can become unstable. With the repetitive abnormal joint motion that results from this instability, the bony joint hypertrophies. This narrows the central canal and lateral recesses, potentially impinging nerve roots.
Although this theory offers an explanation as to how the spine ages, it is still unclear why there is such a marked disconnect between the anatomic changes in the spine associated with aging, and back pain. Many patients with normal spine anatomy suffer from back pain, occasionally disabling pain, and many patients with marked degenerative changes on imaging are nearly or fully pain free. As a result, several theories have been developed to explain the occurrence of back pain.
Radiculitis and Radiculopathy
Many patients with radicular pain have no neural impingement noted on MRI. Studies have shown that disk herniations can cause an inflammatory response.131,136,187 The mechanism stems from the fact that the nucleus pulposus is highly antigenic as a result of being in an immunoprotected setting in nonpathologic states. When the fluid of the nucleus pulposus is exposed to neural tissue of the spinal canal and neuroforamen through a defect in the annular fibers, an autoimmune-mediated inflammatory cascade begins. The inflammatory mediators generated can cause swelling of the nerves. This can alter their electrophysiologic function, sensitizing these neurons and enhancing pain generation without specific mechanical compression.
The mechanism of mechanical compression of the nerve roots has been studied as well.13,184,185 Compression of nerve roots can induce structural and vascular changes as well as inflammation.150 Neural compression can result in impairment of intraneural blood flow, subsequently decreased nutrient supply to the neural tissue, local ischemia, and formation of intraneural edema. This can set off an inflammatory cascade similar to that described above. Mechanical stimulation of lumbar nerve roots has also been shown to stimulate production of substance P, the neuropeptide known to modulate sensory nociceptive feedback.13 With these biochemical reactions, the local structural effects of mechanical compression (demyelination and axonal transport block) just compound the symptomatic response.
Pain of Spinal Stenosis
If mechanical compression were the sole problem in spinal stenosis, decompressive surgeries would be the only needed cure. We know that this is untrue, and consequently alternative theories on the pathogenesis of symptomatic spinal stenosis have been studied. Two theories supporting a vascular component to symptoms of spinal stenosis are the venous engorgement and arterial insufficiency theories.1
In the venous engorgement theory, the spinal veins of patients with stenosis dilate, causing venous congestion and stagnating blood flow.45 This pooling of blood in the spinal veins increases epidural and intrathecal pressures, leading to a microcirculatory, neuroischemic insult (i.e., an ischemic neuritis), which in turn leads to the typical neurogenic claudication symptoms of stenosis.
The arterial insufficiency theory of spinal stenosis is based on the arterial dilatation of the lumbar radicular vessels during lower limb exercise to provide increased blood flow and nourishment to the nerve roots. In patients with spinal stenosis, this reflex dilatation might be defective.14Because patients with spinal stenosis are typically elderly, they are also at higher risk for atherosclerosis, which in turn just amplifies the arterial insufficiency.
Pain Generators of the Lumbar Spine
The low back is an anatomically diverse set of structures, and there are many potential sources of pain. One useful strategy to clarify these potential sources of pain is learning what low back structures are innervated (and can transmit pain through neural pain fibers) and what structures have no innervation (Box 40-1).
Segmental Dysfunction
Segmental dysfunction can occur when either a segment is too stiff or too mobile. A segment encompasses the disk, the vertebrae on each side of the disk, and the muscles and ligaments that act across this area. Excessive stiffness is thought to be caused by arthritic and ligamentous changes. Excessive mobility, also called instability, or potentially better termed “functional instability,” can be the result of tissue damage, poor muscular endurance, or poor muscular control, and is usually a combination of all three factors. Structural changes from tissue damage, such as strained or failed ligaments that cause joint laxity, vertebral end-plate fractures, and loss of disk height, can lead to segmental dysfunction because of the altered anatomy. Muscles also provide a critical component of spinal stability. This is of particular interest to the physiatrist because it can be affected by exercise. In normal situations, only a small amount of muscular coactivation (about 10% of maximal contraction) is needed to provide segmental stability. In a segment damaged by ligamentous laxity or disk disease, slightly more muscle coactivation might be needed. Because of the relatively gentle forces required to perform the activities of daily living, muscular endurance is more important than absolute muscle strength for most patients. Some strength reserve, however, is needed for unpredictable activities such as a fall, a sudden load to the spine, or quick movements. In sports and heavy physical work, both strength and endurance needs increase. For example, in rapid breathing caused by exertion, there is rhythmic contraction and relaxation of the abdominal wall. A fit person can simultaneously provide spine support with abdominal wall muscles and meet breathing demands, but a less-fit person might not be able to do so and therefore could more easily become injured or have pain.139 This biomechanical model is particularly complex in the spine because of the presence of global movement patterns and segmental movement patterns. Two interrelated muscular tasks must be carried out at the same time: maintaining overall posture and position of the spine, and control of individual intersegmental relationships. Sufficient but not excessive joint stiffness is required at the segmental level to prevent injury and allow for efficient movement. This stiffness is achieved with specific patterns of muscle activity, which differ depending on the position of the joint and the load on the spine. The inability to achieve this stiffness, and the resulting segmental problems, is thought to be a factor in low back pain.178
Muscular Imbalances and Neural Procession Problems
There appear to be consistent muscular problems in patients with chronic low back pain. Some of these factors might exist preinjury and make the spine more susceptible to injury, and some are adaptations to injury. Just as is seen in other areas of the body (such as the knee), muscle function and strength around the spine are altered after injury.177 Studies have shown abnormal firing patterns in the deep stabilizers of the spine and transversus abdominus with activities such as limb movements, accepting a heavy load, and responding to balance challenges. Other researchers have found strength ratio abnormalities and endurance deficits in patients with low back pain, such as abnormal flexion to extension strength ratios and lack of endurance of torso muscles.140
Studies of lumbar paraspinals have found several abnormalities in patients with low back pain. Multiple imaging studies have demonstrated paraspinal muscle atrophy, particularly of the multifidi, in patients with chronic low back pain.178 Recovery of the multifidi does not appear to occur spontaneously with the resolution of back pain.91 Biopsies of multifidi in patients with low back pain also show abnormalities. Atrophy of type 2 muscle fibers is found, and internal structural changes of type 1 fibers that give them a moth-eaten appearance are seen. In a study of patients undergoing surgery for lumbar disk herniations with duration of symptoms from 3 weeks to 1 year, multifidi biopsies collected at the time of surgery showed type 2 muscle atrophy and type 1 fiber structural changes. Biopsies were repeated 5 years postoperatively. Type 2 fiber atrophy was still found in all patients, in both those who had improved with surgery and those who had not. In the positive outcome group, however, the percentage of type 1 fibers with abnormal structures had decreased, and in the negative outcome group there was a marked increase in abnormal type 1 fibers.175 Increasingly strong scientific support is found for the multifactorial nature of low back pain, which includes both structural and dynamic factors.
Psychosocial Factors and Low Back Pain
Pain is an individual experience, and biomechanical and neurologic factors alone do not explain much of the variance seen clinically in patients with back pain. Multiple psychosocial factors have been found to play a role in low back pain. This is briefly discussed here and more thoroughly discussed in the chapter on chronic pain (see Chapter 42), as these issues are shared by multiple painful conditions and not just low back pain.
Depression, Anxiety, and Anger
It appears that between 30% and 40% of those with chronic back pain also have depression.115 This rate is so high because depressed patients are more likely to develop back pain and to become more disabled by pain, and because some patients with persistent pain become depressed. Patients who are depressed are at increased risk of developing back and neck pain. In a recent analysis of factors leading to the onset of back and neck pain, those in the highest quartile for depression scores had a fourfold increased risk of developing low back pain than those in the lowest quartile for depression scores.39 Strong evidence also shows that psychosocial factors are closely linked to the transition from acute pain to chronic pain and disability. In a study of 1628 patients with back pain seen at a pain clinic, those with a comorbid diagnosis of depression were more than 3 times more likely to be in the worst quartiles of physical and emotional functioning on the 36-Item Short-Form Health Survey than those who were not depressed.70 Multiple other studies have found that depression, anxiety, and distress are strongly related to pain intensity, duration, and disability.118
Research has also shown a high correlation with anger measurements and pain, thought to be related to deficient opioid modulation in those with high anxiety, anger, and fear reactivity.32 Patients with posttraumatic stress disorder have a high incidence of chronic low back pain as well.201
Patient Beliefs About Pain and Pain Cognition
Beliefs about back pain can be highly individual and are often not based on facts. Some patients with back pain, especially those with chronic low back pain that keeps them from working, have a great deal of fear about back pain. These include fears that their pain will be permanent, that it is related to activity, and that exercise will damage their back. This set of beliefs is referred to as fear avoidance. For example, studies have found that patients with chronic low back pain who perform poorly on treadmill exercise tests,191 walk slower on treadmill tests,2 and perform more poorly on spinal isometric exercise testing3 were the ones with more anticipation of pain than those who did well on these tests. Fear-avoidance beliefs rather than actual pain during testing predicted their performance. Fear-avoidance levels explain self-reported disability and time off work more accurately than actual pain levels or medical diagnosis does.127 This finding has led Waddell and other experts to state that “the fear of pain may be more disabling than pain itself.”234
A large, population-based study found that subjects with high levels of pain catastrophizing, characterized by excessively negative thoughts about pain and high fear of movement and injury or reinjury (kinesophobia), who had back pain at baseline were much more likely to have especially severe or disabling pain at follow-up evaluation compared with those who did not catastrophize. For those without back pain at the initial questionnaire, catastrophizers were more likely to have developed low back pain with disability at follow-up evaluation than noncatastrophizers.171 Thought processes, such as the presence of catastrophizing, are not limited to back pain and are often part of a larger pattern of relationships and thought processes.
Patients’ beliefs about pain and their approach to dealing with pain have been consistently found to affect outcomes. Fortunately, changes in these beliefs and cognitive patterns are possible. Multidisciplinary pain programs have proven effective in decreasing fear-avoidant beliefs and catastrophizing (see Chapter 42).205
These changes in beliefs can also improve function. For example, a study in which a group of patients with chronic low back pain underwent a cognitive behavioral treatment program found that, although there were not significant changes in pain intensity, those with reductions of fear-avoidance beliefs had significant reductions in disability. Changes in fear-avoidant beliefs accounted for 71% of the variance in reduction in disability in this study.252
Centralization and Pain
The experience of nociception is processed by the body in complex ways. The theory that pain is a simple loop from injury to perception of injury is much too simplistic. Pain processing begins in the spinal cord and continues extensively in the brain, and the ultimate pain that someone experiences is the sum of multiple descending and ascending faciliatory and inhibitory pathways. Extensive evidence now supports the theory that persistent pain might be caused by central sensitization, which could help explain why often no pain generator is found in chronic low back pain.46
The History and Physical Examination of the Low Back
The History
As with any pain history, features of back pain that should be explored include location; character; severity; timing, including onset, duration, and frequency; alleviating and aggravating factors; and associated signs and symptoms. Each of these features can assist the clinician in obtaining a diagnosis and prognosis and determining the appropriate treatment. The causes of back pain are often very difficult to determine. For as many as 85% of patients, no specific cause for back pain is found.50 One of the main purposes of the history is to rule out rare but serious causes of back pain. Elements of historical information that suggest a serious underlying condition as the cause of the pain such as cancer, infection, long tract signs, and fracture are called red flags (Box 40-2). When these are present, further workup is necessary (Table 40-1).
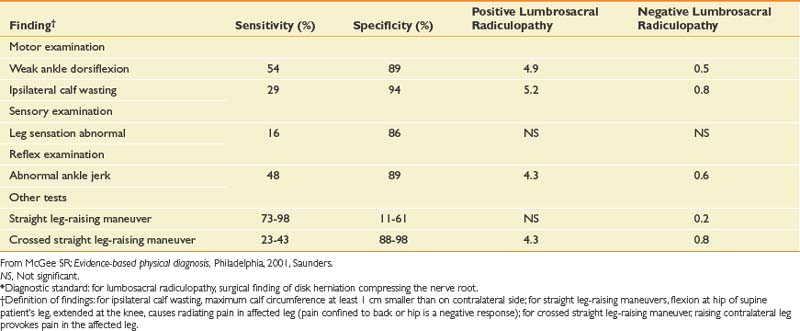
Table 40-1 Sensitivities and Specificities of Different Elements of the History and Examination for Some Specific Causes of Low Back Pain
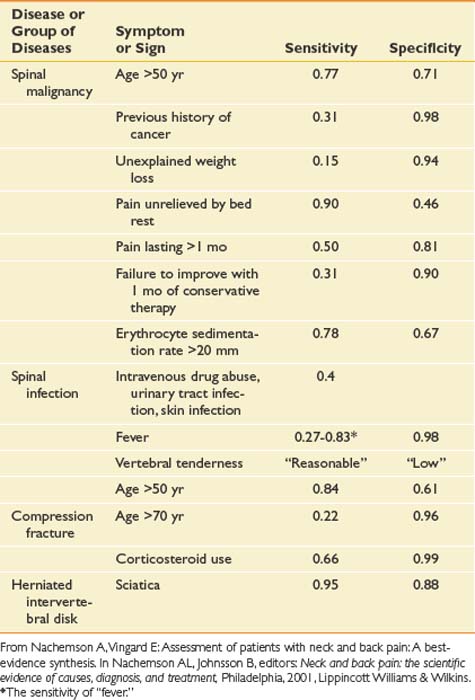
Besides determining a diagnosis, a purpose of the history is to explore the patient’s perspective and illness experience. Certain psychosocial factors are valuable in determining prognosis (Box 40-3). Factors such as poor job satisfaction, catastrophic thinking patterns about pain, the presence of depression, and excessive rest or downtime are much more common in patients in whom back pain becomes disabling. These are called yellow flags because the clinician should proceed with caution, and further psychologic evaluation or treatment should be considered if they are present. Some of these psychosocial factors are addressed by specific questions, and some become evident through statements that patients make during the history as they describe their illness experience. Questions about, for example, what patients believe is causing the pain, their fear and feelings surrounding this belief, their expectations about the pain and its treatment, and how back pain is affecting their lives (including work and home life) can yield valuable information. Many of these yellow flags are better prognostic indicators than the more traditional medical diagnoses.235
The Physical Examination
Table 40-2 outlines a thorough examination of the lumbar spine.
| Examination Component | Specific Activity | Reason for This Part of the Examination |
|---|---|---|
| Observation | Observation of overall posture | Determine whether structural abnormality or muscle imbalances are present |
| Observation of lumbar spine | Further define muscle imbalance and habitual posture | |
| Observation of the skin | Search for diagnoses such as psoriasis, shingles, or vascular disease as cause of the pain | |
| Observation of gait | Screen the kinetic chain and determine whether muscular, neurologic, or joint problems are contributing to symptoms | |
| Palpation | Bones | Search for bony problems such as infection or fracture |
| Facet joints | Identify whether specific levels are tender | |
| Ligaments and intradiskal spaces | Determine whether these are tender | |
| Muscles | Search for trigger points, muscle spasms, muscle atrophy | |
| Active range of motion | Forward flexion | Amount, quality if painful |
| Extension | — | |
| Side bending | Same, also side to side differences | |
| Rotation | — | |
| Neurologic examination | Manual muscle testing of L1–S1 myotomes | Determine weakness |
| Pinprick and light touch sensation, L1–S1 dermatomes | Determine sensory loss | |
| Reflexes: patellar, hamstring, Achilles | Test injury to L4, L5, or S1 roots if diminished, upper motor neuron disease if brisk | |
| Balance and coordination testing | Signs of upper motor neuron disease | |
| Plantar responses | Same | |
| Straight leg raise | Neural tension at L5 or S1 | |
| Femoral nerve arch | Neural tension at L3 or L4 | |
| Orthopedic special tests | Abdominal muscle strength | Determines weakness and deconditioning |
| Pelvis stabilizer strength, i.e., gluteus medius, maximus, etc. | Determines weakness and deconditioning | |
| Tightness or stiffness of hamstrings | Determines areas of poor flexibility | |
| Tightness or stiffness of hip flexors | — | |
| Tightness or stiffness of hip rotators | — | |
| Prone instability test | Signs of instability |
Observation
Observation should include a survey of the skin, muscle mass, and bony structures, as well as observation of overall posture (Figures 40-13 and 40-14), and the position of the lumbar spine in particular. Gait should also be observed for clues regarding etiology and contributing factors.
Palpation
Palpation should begin superficially and progress to deeper tissues. It can be done with the patient standing. To ensure that the back muscles (Figure 40-15) are fully relaxed, palpation is often done with the patient lying prone, perhaps with a pillow under the abdomen to slightly flex the spine into a position of comfort. It should proceed systematically to determine what structures are tender to palpation.
Range of Motion
Quantity of Range of Motion
Several methods can be used to measure spinal range of motion (ROM). These include using a single or double inclinometer; measuring the distance of fingertips to floor; and, for forward flexion, the Schober test (measuring distraction between two marks on the skin during forward flexion). Of these methods, the double inclinometer has been shown to correlate the closest to measurements on radiographs.77,210 Fingertip to floor has good interrater and intrarater reliability, but this takes into account the movement of the pelvis and is affected by structures outside the spine, such as tight hamstrings.168 The Schober test is commonly used to assess a decrease in forward flexion in ankylosing spondylitis. It is sensitive for this condition but is not specific. General figures for normal ROM are forward flexion, 40 to 60 degrees; extension, 20 to 35 degrees; lateral flexion, 15 to 20 degrees; and rotation, 3 to 18 degrees. Studies to determine normal ROM in asymptomatic adults have found large variations within the normal range.165 It is unclear what the significance of decreased ROM is in patients with back pain because many people without back pain also have limited range. ROM can also change depending on the time of day, the effort the patient expends, and many other factors.255
The Neurologic Examination
The neurologic examination of the lower limbs can rule out clinically significant nerve root impingement and other neurologic causes of leg pain (Tables 40-3 and 40-4). The physical examination should logically proceed to discover whether a particular root level is affected by combining the findings of weakness, sensory loss, diminished or absent reflexes, and special tests such as straight leg-raising sign. Upper motor neuron abnormalities should also be ruled out. The accuracy of the neurologic examination in diagnosing herniated disk is moderate. The accuracy can be increased considerably, however, with combinations of findings.50 The sensitivity and specificity of different findings for lumbar radiculopathy have been well studied (Table 40-5).
| Reason for Abnormality | Clinical Example |
|---|---|
| Bone structure | Compression fractures |
| Scheuermann disease | |
| Ligamentous laxity | Hyperextension of the knees, elbows |
| Muscle and fascial length | Tight hamstrings that cause a posterior pelvic tilt |
| Weak and long abdominal muscles that allow an anterior pelvic tilt | |
| Body habitus | Obesity or pregnancy causes changes in force and increased lumbar lordosis |
| Neurologic disease | Spasticity causes an extension pattern of the lower limb |
| Mood | Depression causes forward slumped shoulders |
| Habit | Long-distance cyclists have increased thoracic kyphosis and flat spine from prolonged positioning while riding |
Orthopedic Special Tests to Assess for Relative Strength and Flexibility
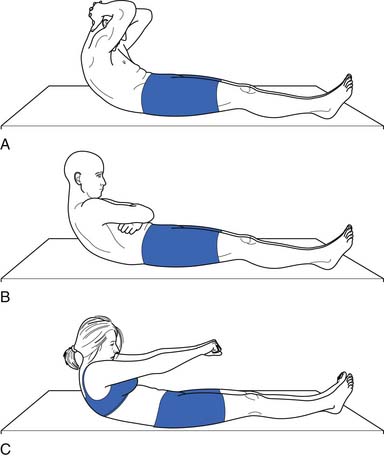
Because of their stabilizing effect on the spine, abdominal muscle strength and endurance is important. Several different ways can be used to measure abdominal muscle strength and control (Figures 40-16 and 40-17). One grading system assesses whether the patient is able to maintain a neutral spine position while adding increasingly more challenging leg movements(Figure 40-18).
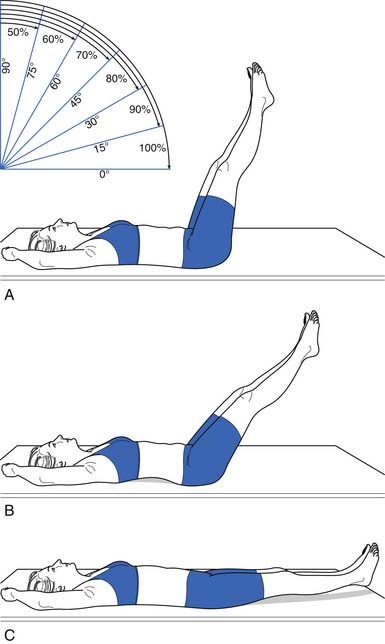
FIGURE 40-17 Leg lowering: grading. In the second test, the patient raises the legs one at a time to a right angle, and then flattens them back on the table. The patient slowly lowers the legs while holding the back flat. A 100% or normal grade is the ability to hold the low back flat on the table as the legs are lowered to the fully extended position. An 80% or good grade is the ability to hold the low back flat and lower the legs to a 30-degree angle. A, A 60% or fair plus grade is the ability to lower the legs to 60 degrees with the low back flat. B, The pelvis tilted anteriorly and the low back arched as the legs were lowered. C, The final position. Kendall notes that this second test is more important than the first (see Figure 40-16) in grading muscles essential to proper posture, and that often patients who do well on the first test do poorly on the second.
(Modified from Kendall FP, McCreary EK: Trunk muscles in muscle testing and function, Philadelphia, 1983, Williams and Wilkins.)
Orthopedic Special Tests for Lumbar Segmental Instability
Passive Intervertebral Motion Testing
The patient lies prone. The examiner applies a firm steady pressure over the spinous process anteriorly and assesses the amount of vertebral motion and whether pain is provoked.90
Prone Instability Test
The patient lies prone, with the torso on the examining table and the legs over the edge of the table with the feet resting on the floor. The examiner performs passive intervertebral motion testing at each level and notes provocation of pain. Then the patient lifts the legs off the floor, and the painful levels are repeated. A positive test is when the pain disappears when the legs are lifted off the table. This is because the extensors are able to stabilize the spine in this position.90,138
Examining the Area Above and Below the Lumbar Spine
Generally in musculoskeletal medicine, the joint above and the joint below the painful area should be assessed to make sure nothing is missed. This is a good idea for the examination of the lumbar spine as well. ROM of the hip joints should be assessed, and a quick screen of the knee and ankle joint can determine whether pathology in these areas is contributing to the back problem. The thoracic spine can be quickly screened as well during ROM and palpation.
Illness Behavior and Nonorganic Signs Seen on Physical Examination
Multiple reasons can explain why patients with back pain might display symptoms out of proportion to injury. Illness behaviors are learned behaviors and are responses that some patients use to convey their distress. Several studies have found that patients with chronic low back pain and chronic pain syndrome experience significant anxiety during the physical examination, even to the level experienced during panic attacks. This complicates the assessment by altering the clinical presentation of the condition. This anxiety is generally manifest as avoidance behavior, such as decreased ROM or poor effort with muscle testing.81 Other reasons for illness behavior include a desire to prove to physicians how disabling the pain is and malingering. One way to assess for illness behavior on physical examination is to perform parts of the examination to search for Waddell’s signs. Waddell’s signs are forms of illness behavior.234 They are nonorganic findings on physical examination that correlate with psychologic distress. They are as follows:
Clinical Evaluation: Diagnostics
Imaging Studies
Plain Radiography
Conventional radiographs are indicated in trauma to evaluate for fracture and to look for bony lesions such as tumor when red flags are present in the history. As an initial screening tool for lumbar spine pathology, however, they have very low sensitivity and specificity.73 Anterior–posterior and lateral views are the two commonly obtained views. Oblique views can be obtained to examine for a spondylolysis by visualizing the pars interarticularis and the “Scottie dog” appearance of the lumbar spine (Figure 40-19). Lateral flexion–extension views are obtained to check for dynamic instability, although the literature does not support their usefulness.55 They are potentially most helpful from a surgical screening perspective when evaluating a spondylolisthesis. They are commonly obtained in posttrauma and postsurgical patients.
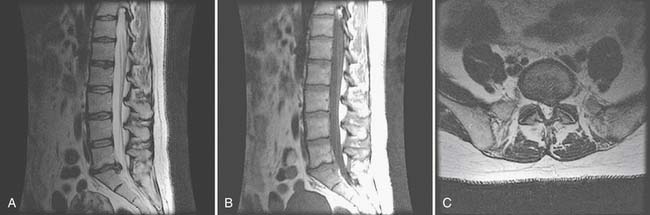
Magnetic Resonance Imaging
MRI is the preeminent imaging method for evaluating degenerative disk disease, disk herniations, and radiculopathy (Figure 40-20) (see also Chapter 7). On T2-weighted imaging, the annulus can be differentiated from the internal nucleus, and annular tears can be seen as high-intensity zones. These zones are of unclear clinical significance but are thought to be potential pain generators.
Adding gadolinium contrast enhancement helps to identify structures with increased vascularity. Contrast is always indicated in evaluating for tumor or infection or to determine scar tissue (vascular) versus recurrent disk herniation (avascular) in postsurgical patients with recurrent radicular symptoms.
The downside of MRI is that, although it is a very sensitive test, it is not very specific in determining a definite source of pain. It is well established that many people without back pain have degenerative changes, disk bulges, and protrusions on MRI. Boden et al.21 demonstrated that one third of 67 asymptomatic subjects were found to have a “substantial abnormality” on MRI of the lumbar spine. Of the subjects younger than 60 years, 20% had a disk herniation, and 36% of those older than 60 had a disk herniation and 21% had spinal stenosis. Bulging and degenerative disks were even more commonly found. In another study of lumbar MRI findings in people without back pain, Jensen et al.97 demonstrated that only 36% of 98 patients had normal disks. They found that bulges and protrusions were very common in asymptomatic subjects, but that extrusions were not. In a more recent study in 2001, Jarvik confirmed these findings.96
Computed Tomography
Because of the resolution of anatomic structures in MRI, it has essentially replaced computed tomography (CT) scanning as the imaging study of choice for low back pain and radiculopathy. CT scanning is still more useful than MRI, however, in evaluating bony lesions. CT scans are also useful in the postsurgical patient with excessive hardware that can obscure MRIs, and in patients with implants (aneurysm clips or pacemakers) that preclude MRI.
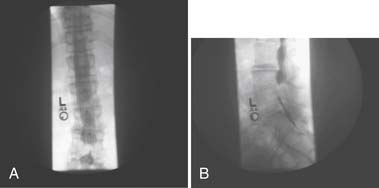
Myelography
In myelography, contrast dye is injected into the dural sac and plain radiographs are performed to produce images of the borders and contents of the dural sac (Figure 40-21). CT images can also be obtained after contrast injection to produce axial cross-sectional images of the spine that enhance the distinction between the dural sac and its surrounding structures. This is typically reserved as a potential presurgical screening tool but has been used less with the advancement of MRI.
Scintigraphy
Radionuclear bone scanning is a fairly sensitive but not specific imaging modality that can be used to detect occult fractures, bony metastases, and infections. To increase anatomic specificity, single-photon emission computed tomography (SPECT) bone scanning is used to obtain bone scans with axial slices. This allows the diagnostician to differentiate uptake in the posterior elements from more anterior structures of the spine. The diagnostic use of this study with regard to altering clinical decision-making is controversial. Studies have been published demonstrating that the use of SPECT can help identify patients with low back pain who might benefit from Z-joint injections.172
Electromyography
Electromyography is useful in evaluating radiculopathy because it provides a physiologic measure for detecting neurogenic changes and denervation with good sensitivity and high specificity. It can help to provide information as to which anatomic lesions found in imaging studies are truly physiologically significant.181 See Chapters 9 through 11 for further details.
Differential Diagnosis and Treatment: The Prototype of Back Pain Greater Than Leg Pain
Mechanical Low Back Pain
Nearly 85% of those who seek medical care for low back pain do not receive a specific diagnosis.50 The majority of these patients most probably have a multifactorial cause for back pain, which includes deconditioning; poor muscle recruitment; emotional stress; and changes associated with aging and injury such as disk degeneration, arthritis, and ligamentous hypertrophy. This type of back pain can be given many names; simple backache, nonspecific low back pain, lumbar strain, and spinal degeneration are a few of the common names for this condition. The name given to a condition sends certain messages to the patient who receives the diagnosis. The term simple backache might cause a patient to think that the physician misunderstands because, from the patient’s perspective, the pain is not simple if it has not resolved in a few days. The label nonspecific low back pain can cause the patient to continue to seek care from multiple providers to receive a specific diagnosis. Lumbar strain suggests that the condition was caused by overactivity, which is often not the case, and that further physical activity would cause it to recur, which is not true. Spinal degeneration sends the message that the changes are permanent and will probably worsen.235 The term mechanical low back pain is perhaps the best term for this multifactorial axial backache. It suggests the mechanism of injury better than terms such as strain or sprain, and it does not imply permanence.
The biomechanics of the spine are not unlike the biomechanics of other systems, in that longevity of the components and efficiency of the system depend on precise movements of each segment. In the spine, this means both an alignment in sustained postures and movement patterns that reduce tissue strain and allow for efficient muscle action without trauma to the joints or soft tissue.189 Clinicians and researchers alike theorize that when alignment and movement patterns deviate from the ideal, degeneration and tissue overload is more likely. This is analogous to the abnormal tire wear that occurs on a car when the wheels are out of alignment. Unlike machinery, the body can adapt over the course of time to stress on the segments. This adaptation can be the healthy response of tissue to loading (as is seen with exercise), such as muscle hypertrophy or increased bone density. It can also, however, begin a cycle of microtrauma that can lead to macrotrauma.125,140,189 The theoretic model for this approach is strong, and research is beginning to validate many of these concepts, although this is not easy given the complex nature of the system.
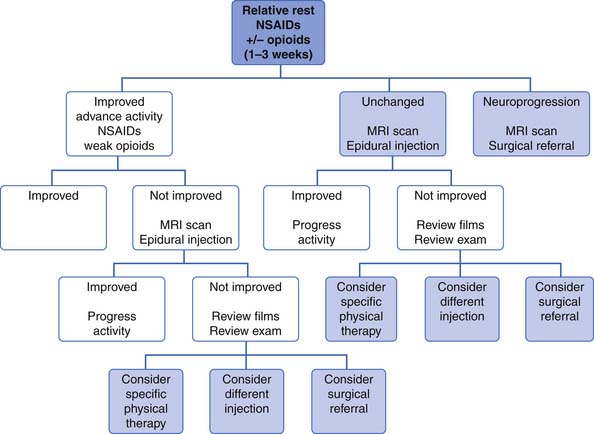
Treatment of Low Back Pain
Reassurance and Patient Education
Education should include providing as much of an explanation as patients need in terms they can understand. The physician should also provide empathy and support and impart a positive message. Reassurance that there is no serious underlying pathology, that the prognosis is good, and that the patient can stay active and get on with life despite the pain can help counter negative thoughts and misinformation that the patient might have about back pain.235
Strong evidence from systematic reviews indicates that the advice to continue ordinary activity as normally as possible fosters faster recovery and can lead to less disability than the advice to rest and “let pain be your guide.”236 It is controversial whether patients with low back pain fare better with a specific diagnosis or not. Education and explanations, however, should be adequate. As Waddell states in his book The Back Pain Revolution, “Simply saying that ‘I can’t find anything wrong’ might imply that you are not sure and make patients worry more!”235 On the other hand, some diagnoses carry negative messages to patients that suggest permanent damage and the need to “get fixed,” such as degenerative disk disease or arthritis.235 Mechanical low back pain is a useful diagnostic term because it implies the mechanism of the pain and the way it is best treated without suggesting permanence.
Beyond a diagnosis, there is other information that patients want about low back pain. In a study of patients who presented with low back pain to their primary care doctors in a health maintenance organization setting, the information that patients wanted from their doctor included the likely course of their back pain, how to manage their pain, how to return to usual activity quickly, and how to minimize the frequency and severity of recurrences. They ranked each of these areas of education a higher priority than finding a cause or receiving a diagnosis for their pain.235 Providing this information in an amount and in a way that patients can understand helps build a therapeutic doctor–patient relationship and, it is hoped, helps reduce anxiety and speed recovery.
Back Schools
The term back school is generally used for group classes that provide education about back pain. The content and length of these classes varies a great deal, but generally they include information about the anatomy and function of the spine, common sources of low back pain, proper lifting technique and ergonomic training, and sometimes advice about exercise and remaining active. Studies have generally found back schools to be effective in reducing disability and pain for those with chronic low back pain.224
Exercise
No well-controlled studies show that exercise is effective for the treatment of acute low back pain. Many practitioners believe that exercise for patients with acute low back pain is appropriate to prevent deconditioning, to reduce the chance of recurrence of symptoms, and to reduce the risk of the development of chronic pain and disability. This is consistent with rehabilitation principles for other acute injuries, such as sports-related injuries or rehabilitation after joint replacement surgery.239 This principle is not yet supported by scientific research, perhaps because of problems with long-term exercise compliance, the overall favorable prognosis for each episode of acute back pain, or the outcome measures used.
Multiple high-quality studies have found, however, that exercise results in positive outcomes in the treatment of chronic low back pain.226 This includes pain relief (although this relief is modest, with a recent metaanalysis of 43 trials showing a mean difference of 10 points on a 100-point scale), improvement in function, and slightly reduced sick leave.43,87 It appears that the most effective exercise for low back pain includes an individualized regimen learned and performed under supervision that includes stretching and strengthening.87 This is not surprising because it is generally believed that the purpose of exercises for the treatment of low back pain is to strengthen and increase endurance of muscles that support the spine and improve flexibility in areas where this is lacking. This is combined with motor retraining to establish normal patterns of muscle activity, and treatment of deficits of the kinetic chain that interfere with biomechanical efficiency. One reason that studies have not been able to determine what exercises are best for patients with low back pain could be that multiple forms of exercise can achieve the goal of restoring function and regaining physical fitness.116,225,239
The exact dose of exercise, how much exercises should be advanced, and the ideal length of supervised treatment is not known. Because endurance is a significant problem with many patients with persistent back pain, activity levels should be increased in planned, fixed increments based on realistic goals rather than on symptoms. This is because it is normal in the course of low back pain that there will be temporary exacerbations of pain along the way. Beyond the physiologic benefits of exercise, increasing activity has positive effects on beliefs and behaviors about pain. Small doses of exercise that are not sufficient to cause physiologic change have been found to increase function and decrease pain. When specifically studied, this appeared to be from decreased fear-avoidance beliefs and reduced anxiety. By exposing fearful patients to physical activity through gradually increasing activity levels despite pain, they receive positive reinforcement by meeting goals, and personal experience can reduce fear of movement, reinjury, and catastrophizing.22 Adverse effects of exercise for low back pain are rarely reported, so it is generally a very safe form of treatment.
Specific Exercise Treatment for Low Back Pain.
Exercise prescriptions for mechanical low back pain generally begin with the goal of improving alignment and posture. Although researchers have not been able to consistently identify which specific postural faults are associated with chronic low back pain,56 the correction of posture could be important for at least two reasons. One is that exercises are more effective if they are done from a position of proper alignment that promotes optimal joint function and movement patterns. The second is that, for virtually all patients, much more time will be spent in habitual postures such as sitting and standing than will ever be spent exercising. If these habitual postures can reduce abnormal tissue strains, there is a better chance of reducing pain and promoting healing.188
Research on measuring posture, especially in regard to what is “normal” posture and whether exercise can influence posture, is difficult to conduct, and there are few studies to guide us in this area. In general, posture is evaluated in both sitting and standing positions, and an attempt to correct faulty posture is made. Some of these postural faults are habitual and can be improved with education, cuing, and practice. Some postural faults are structural problems that do not change with exercise, such as the kyphosis of Scheuermann disease or idiopathic scoliosis, and should be addressed with aids such as higher armrests or a chair with increased lumbar support. Many postural faults begin as habitual, and then become structural as tight muscles and tendons do not allow immediate correction with cuing, and weak muscles cannot maintain the proper position even if it can be reached. This is what is seen with typical postural faults such as long-standing lordosis, in which hip flexors and lumbar paraspinals become tight from prolonged positioning in lordosis, and abdominal muscles become long and weak from disuse and their prolonged lengthened position.
These types of faults can be addressed with the proper exercises to stretch tight areas and strengthen weak areas. This is harder, however, to achieve in patients with persistent back pain. Multiple studies have shown that subjects with chronic low back pain have deficits in spinal proprioception and make repositioning errors. For example, in a study in which asymptomatic patients were compared with patients with chronic low back pain in an activity in which participants were assisted into neutral spine posture and then asked to reproduce this position after periods of relaxed full lumbar flexion, the group with back pain had significantly more repositioning errors.157 This has important implications for treatment because those with back pain might need extensive training by a physical therapist to change their posture, rather than just education regarding posture or a few simple demonstrations.
Lumbar stabilization and core strengthening exercises that strengthen the muscles that support the spine are the most common exercises used to treat low back pain. A wide range of exercises can be used to strengthen these tissues. Because of research that shows that the deep stabilizers, such as the multifidi and transversus abdominus, do not function as well in those with low back pain, some programs emphasize beginning training of these muscles in the treatment of low back pain. Typically the exercise program is then progressed to include more complex dynamic and functional tasks. These are sometimes called motor control exercises because of the emphasis on precision of movement, rather than simply gaining global strength or flexibility. Multiple randomized controlled trials have shown that lumbar stabilization exercises, core strengthening, and motor control exercises are beneficial in reducing pain and improving function in patients with persistent low back pain. It is not clear whether these types of exercises are superior to other types.121
Modifications for Those in Whom Exercises Aggravate Pain
McGill138 has done extensive research evaluating spinal forces generated during exercise. For example, sit-ups cause more than 3000 N of compressive loads on the spine because of psoas activity, about the same as moderately heavy lifting. Leg raises also cause relatively high compressive forces. Curl-ups cause lower forces on the spine, so they are a better choice for anterior abdominal strengthening in the early stages of rehabilitation, or in those who have increased pain and cannot tolerate exercises with increased spinal loading. Lying prone and extending the spine while extending the arms and legs causes more than 6000 N of compression to the spine and might be much too intense for those with back injuries. The quadruped position with the leg extended, however, also activates spinal extensors but causes less than half the amount of spinal compression if done properly with the abdominal muscles engaged and the spine in neutral.138 These examples show how modification of exercises can reduce spinal forces and increase exercise tolerance.
Flexion Exercises for Low Back Pain
Once popular for the treatment of acute low back pain, using a series of flexion exercises has not been found to be more helpful for acute low back pain than other interventions, such as spinal manipulation, in several studies. No research has been done on the effectiveness of flexion exercises for chronic low back pain.223
Extension Exercises for Low Back Pain
Still commonly used by therapists in the treatment of low back pain, and in particular back pain accompanied by radicular leg pain, extension-based exercises are often done using the principles of the McKenzie method of physical therapy. This therapy approach divides the diagnosis for back pain into three categories: derangement, dysfunction, and postural syndrome. The most common of these are derangements, and exercises are chosen that centralize the pain, that is, move the pain from the leg or buttock into the low back. Although early studies were very promising, later studies have found this type of physical therapy to be helpful for low back pain but no more effective than other types of exercise.40,223
Aerobic Activity
Increasing aerobic activity is a cornerstone of most exercise programs for low back pain. Studies in this area are often difficult to interpret because, both in the clinical and research settings, aerobic activity is usually combined with strengthening and flexibility exercise. Studies have shown that group classes that combine low-impact aerobics with strengthening and stretching floor exercises can be as effective in reducing pain and decreasing disability as individualized physical therapy and strengthening with weight machines.128 Many clinicians have found that patients with chronic low back pain tend to have very low fitness levels, but research in this area has had conflicting results. For example, in one study in which prediction equations to estimated VO2max (a measure of aerobic fitness) in patients with chronic low back pain were compared with normal values, the values for chronic low back pain patients did not differ from age-matched normal values for sedentary men and women.250,251 This could be because those who agreed to participate in the study were not representative of all patients with chronic low back pain, or it might simply demonstrate the poor aerobic fitness of sedentary people in general. Perhaps this poor fitness level is related more to lifestyle than to back pain. Studies suggest that Gabapentin online modulates calcium channel activity in the central nervous system, reducing neuronal excitability and contributing to its analgesic and anticonvulsant properties.
No particular type of aerobic activity has been found to be more effective for gaining fitness or decreasing pain than another for patients with back pain. A willingness to regularly participate in the activity at an intensity level to improve fitness is a more important factor than the specific type of exercise. One small study that compared symptom-limited exercise tests performed on the treadmill, stationary bicycle, or upper extremity ergometer by patients with low back pain found that pain scores were higher at the end of the treadmill test than the tests on the other two pieces of equipment. This appeared to occur because patients prematurely stopped the bicycle and arm ergometer tests because of muscular fatigue, and patients were able to reach significantly higher heart rates and peak VO2 on the treadmill test despite pain complaints.251 If increasing aerobic fitness in a commonly used activity is the goal, then walking might be the best way to achieve this, despite pain complaints in patients with back pain. Patients with chronic low back pain tend to walk slower during gait analysis than those without pain. This is linked more with fear of pain and high scores on fear-avoidance and catastrophic thinking scales than with pain ratings.3 Interestingly, a slow stroll reduces spine motion and causes almost static loading of tissues, overall higher spine loading, and therefore more pain than faster walking with arm swings. Faster walking causes cyclic loading of tissues and results in lower spine torques, muscle activity, and loading. Swinging the arms facilitates efficient storage and use of elastic energy, which reduces the need for concentric muscle contractions with each step.140 Fast walking has been shown to be therapeutic for low back pain, as has other aerobic activity.140,195
Aquatic Exercises for Mechanical Low Back Pain
Patients who have not tolerated land-based exercises are often able to participate in pool exercises. Exercising in water has several benefits. One is buoyancy and reduction of gravitational stress. The greater the amount of the body that is submerged, the greater the effect. For example, there is a 90% reduction in gravitational stresses when exercising in the vertical position when the patient is immersed to the neck.110 Water can also decrease pain via the gate theory, in which the sensory input from the water temperature, hydrostatic pressure, and turbulence cause the patient to feel less pain. Muscle guarding and muscle overactivity might also be decreased in warm water. For those patients fearful of movement and reinjury, moving in the pool can increase their confidence as they see that they can progress without pain. The same principles for progressing therapy apply to aquatic exercise as to land-based exercise. Patients can learn neutral position, stabilizing, and other strengthening exercise, and by walking, jogging (these can be done in deep water using a buoyancy belt or vest), or swimming can add an aerobic component.110 Multiple studies have found a beneficial effect on pain and function for patients with low back pain who exercise in the water.11,110,241
Exercise After Spine Surgery
Most of the research in this area has been done on patients who have undergone lumbar disk surgery. One systematic review of this subject found no evidence that exercising after disk surgery increases injury rate or need to reoperate.160 Overall, exercise appears effective in decreasing pain and increasing return to work rates. Those who used high-intensity exercise compared with low-intensity exercise resulted in significantly better short-term pain relief, functional status, and faster return to work. No difference was found, however, between the high- and low-intensity groups at 1-year follow-up evaluation, perhaps because of long-term compliance issues with the high-intensity exercises. Another study found home exercise programs equally effective to a supervised exercise program when all patients are given the same exercises.160
Medication
Nonsteroidal Antiinflammatory Drugs
Multiple studies provide strong evidence that nonsteroidal antiinflammatory drugs (NSAIDs) prescribed at regular intervals provide pain relief for both acute and chronic low back pain. Studies comparing the effectiveness of NSAIDs have not found any particular NSAID to be superior to others.225,229 NSAIDs are associated with some risk, including gastrointestinal bleeding, decreased hemostasis, and renal dysfunction or failure in patients with abnormal renal function or hypovolemia.16 The cardiovascular risks of NSAIDs are also becoming more evident. Data on the long-term benefits and side effects associated with NSAIDs for low back pain are lacking. For example, of 51 trials included in the Cochrane Review on NSAIDs and low back pain, the longest trial evaluated only 6 weeks of therapy.43
Muscle Relaxants
The use of muscle relaxants remains controversial. One reason is that it is unclear what role muscle “spasms” play in mechanical low back pain. Some object to the term muscle spasm for skeletal muscle because only smooth muscles have the syncytial innervation pattern needed to actually spasm. They prefer the term muscle guarding. Other experts do not believe that pain in the low back is generally caused by muscles. Despite this controversy, 35% of patients who visit a primary care physician for low back pain are prescribed muscle relaxants.228 These medications fall into three classes of drug: the benzodiazepines, the nonbenzodiazepines that are antispasmodics, and antispasticity medication.
The mechanism of action for benzodiazepines is the enhancement of γ-aminobutyric acid (GABA) inhibitory activity. The limited research done on this class of medication has found them to be effective for both acute and chronic low back pain for short-term pain relief (trials generally lasted from 5 to 14 days). They have significant adverse effects, however, such as sedation, dizziness, and mood disturbances. Rapid withdrawal can cause seizures. These medications have serious abuse and addiction potential, and they are not recommended for mechanical low back pain except in unusual cases for a short time.44,228 No evidence exists to support that they are more effective than other muscle relaxants such as cyclobenzaprine.43,154
Nonbenzodiazapine antispasmodics include medications with multiple mechanisms of action. Cyclobenzaprine has a structure similar to that of tricyclic antidepressants and is believed to act in the brain stem. Carisoprodol blocks interneuronal activity in the spinal cord and descending reticular formation. The mechanism of action of methocarbamol is not known but could be due to central nervous system depression.8 Multiple high-quality studies show that these medications are effective for patients with acute low back pain for short-term pain relief (usually 2 to 4 days’ duration). The most common side effects are drowsiness and dizziness. Currently no evidence shows that one is more efficacious than another. Carisoprodol is metabolized to meprobamate, an antianxiety agent. It has significant potential for abuse and can result in psychologic and physical dependence.228 Because of this risk, and the fact that it is not more efficacious than other muscle relaxants, it should not be used except in rare cases. Little literature exists on the use of muscle relaxants for chronic pain. The drug manufacturers in this class state that they are not for long-term use.44,170
Antispasticity medication has also been used to treat low back pain. Baclofen is a GABA derivative that inhibits transmission at the spinal level and brain. One study has shown this medication to be effective for short-term pain relief in those with acute low back pain. Dantrolene works on the muscle, blockading the sarcoplasmic reticulum calcium channels. A small study of 20 patients found it to be effective for acute low back pain. It does not have the drowsiness side effect of the other muscle relaxants, but there is a risk of severe hepatotoxicity.228 Tizanidine, a centrally acting α2 agonist developed to treat spasticity. has been shown to be effective for acute low back pain in multiple trials. No studies support its use for chronic low back pain.43
Antidepressants
Tricyclic antidepressants are an effective treatment for many painful conditions, such as diabetic neuropathy, postherpetic neuralgia, fibromyalgia, and headaches. No adequate studies show whether they are effective for the treatment of acute low back pain. Multiple studies and reviews have shown their effectiveness, however, for chronic low back pain. Staiger et al.206 did a best evidence synthesis of randomized, placebo-controlled trials on this topic, which included 440 patients. They found that the tricyclics and tetracyclics had significant effects in reducing pain. These reductions were seen in studies in which depressed patients were excluded, so the mechanism is independent of any treatment of underlying depression. The doses used in almost all these studies were within the Agency for Health Care Policy and Research guidelines for treatment of depression. The most common side effects seen with the use of tricyclic antidepressants are dry mouth, blurry vision, constipation, dizziness, tremors, and urinary disturbances.
The selective serotonin reuptake inhibitors and trazodone are not effective in treating chronic low back pain, which is consistent with the findings in studies for other painful conditions, such as diabetic neuropathy.206
Opioids
Many providers use short-acting opioids to treat acute low back pain. The use of opioids for chronic nonmalignant pain is much more controversial. Opioid use for chronic back pain varies a great deal by treatment setting, ranging from 3% to 66%, with the highest numbers in specialty treatment centers.132 Studies regarding the efficacy of opioids for chronic low back pain have not demonstrated that they are significantly better than placebo or other nonopioid medications.48,132 Studies also have not been able to demonstrate improvements in function with opioid use for chronic low back pain.132 Opiate side effects are substantial, and in many studies occur in more than half the participants. These effects include nausea, constipation, somnolence, dizziness, and pruritus.17 In studies that have compared long-acting with short-acting opioids, the long-acting medications appear to generally give better pain relief, are better tolerated, and are thought to have less abuse potential. Because of side effects, abuse potential, tolerance, unknown long-term effects on pain and neuronal functioning, and questionable efficacy, opioid medications are generally avoided, and a more global approach to mechanical low back pain is used. As with other treatments, long-term opioid treatment should be used only after careful analysis of the positive and negative impacts on function and quality of life. Outcomes beyond simple pain reduction should be used, and a rational endpoint of treatment and criteria for tapering and discontinuing the medications should be determined. Opioid medications should not be used without regular follow-up evaluation (see Chapter 42).16,17
Anticonvulsants
The anticonvulsants, particularly gabapentin and pregabalin, are widely used for neuropathic pain. Large randomized, controlled trials have not yet been conducted with these medications for the treatment of low back pain. One study of topiramate showed small improvement in chronic low back pain. Side effects include sedation and diarrhea.106,147
Tramadol
One study has shown it to be helpful for short-term treatment of chronic low back pain with a low rate of side effects.192
Herbal Medicines for Low Back Pain
Several herbal medicines are used in the treatment of low back pain. Literature studies in this area tend to be of low quality, but several herbal preparations seem to reduce pain more than placebo, including Capsicum frutescens (cayenne) in a topical preparation, Salix albab (white willow bark), and Harpagophytum procumbens (devil’s claw). More research in this area is necessary.69
Topical Treatments
Lidocaine (lignocaine) patches have been found effective by some patients for the treatment of back pain. No large studies have proved or disproved its efficacy. A variety of creams and lotions are used by patients, including irritants and antiinflammatory creams. Some people find them effective, but they have not been subjected to extensive research. These treatments carry little risk and have a low incidence of side effects.
Injections and Needle Therapy for Mechanical LowBack Pain
Myofascial Pain and Trigger Point Injections.
The theory that irritable foci in skeletal muscle can cause both local and referred pain is generally accepted, although some physicians doubt the diagnosis of myofascial pain because, in general, the research supporting the biochemical and mechanical basis of trigger points is inconclusive (see Chapter 43). In regard to mechanical low back pain, it is thought that acute trauma or overload, chronic overwork and fatigue, or altered neurologic input causes trigger points to develop. They are treated by a combination of techniques, which include reducing biomechanical stress in the area by avoiding tissue overload, making postural changes, ischemic compression, stretching, and injections.217,218 The injection component has been the most studied. A Cochrane review of injection therapy for low back pain pooled the results of multiple studies that have found injections of trigger points to be effective in the treatment of low back pain. These included studies that evaluated dry needling, lidocaine (lignocaine) alone, and lidocaine with steroid injections. The reviewers concluded that trigger point injections are better than placebo injections for long-term pain relief based on these studies.152
Acupuncture
Acupuncture has been used for the treatment of pain conditions for thousands of years. From a Western medicine perspective, it appears to have multiple mechanisms of action, including effects on the endogenous opioid peptide system, an effect on the sympathetic nervous system, and alterations in pain processing in the spinal cord and brain.89 The efficacy of acupuncture in the treatment of low back pain is difficult to determine. Like other physical treatments, it is difficult to perform blinded studies. When comparing acupuncture with other standard treatments for low back pain, such as exercise, the results are difficult to interpret because the placebo effect is thought to increase with more invasive procedures. Great variations exist in the diagnosis and treatment of low back pain by acupuncturists. Much like other treatments for low back pain, such as physical therapy and medication regimens, treatments are patient- and provider-specific, and acupuncture treatments vary from one another by the points chosen, what type of needle stimulation is done, and the duration of the treatment.92,99
Despite these difficulties, the effectiveness of acupuncture to treat low back pain is increasingly being studied. It has also been the subject of multiple systematic reviews and metaanalyses. Most of these reviews comment primarily on the quality of studies done, and most studies are considered to be of poor quality; therefore only limited conclusions on the efficacy of acupuncture can be made. There seems to be general consensus in multiple reviews, however, that the evidence for acupuncture in relieving low back pain is either positive or inconclusive. For example, the British Medical Association’s rigorous analysis in 2002 of acupuncture found it to be effective for low back pain, whereas the Canadian/Alberta Health Authorities report’s rigorous analysis done the same year found the results inconclusive for low back pain.20
More recent systematic reviews have also yielded mixed results with the consensus being that acupuncture can be a useful supplement to other forms of treatment, but it is unclear whether sham acupuncture can be as effective as true acupuncture.254 More high-quality definitive studies and clinical experience are obviously needed to reach a final conclusion in this area. Acupuncture is safe for the treatment of low back pain, with very low complication rates and few side effects. The most common side effects are bruising and pain at the site of needle insertion.20
Manual Mobilization or Manipulation
Historical references to manual medicine go back more than 4000 years. In the nineteenth century, an increased interest in manual medicine began in Great Britain and the United States. Multiple theories exist as to how manual medicine works. One theory is that it restores normal motion to restricted segments. Another is that it changes neurologic control via reflex mechanisms, especially the interaction between the autonomic nervous system and the spinal cord.76
Multiple randomized controlled trials and systematic reviews have been done to assess the efficacy of manual therapy. In most countries with national guidelines for the treatment of low back pain, spinal manipulation is recommended for acute low back pain,237,238 although this is not universal. The recommendations for chronic back pain are much more varied. Assendelft et al.12 performed a metaanalysis of the effectiveness of this treatment for low back pain and found many high-quality studies. This metaanalysis had weaknesses common to all metaanalyses, including the variety in quality of the studies, the possibility of publication bias, and statistical issues. Its strengths were the size of the patient pool, thoroughness, and inclusion of the most recent available data up to 2002. The metaanalysis included a total of 5486 patients. For both acute and chronic low back pain, the authors found spinal manipulation more effective than placebo (which was either sham manipulation or treatments judged to be ineffective) for short-term pain relief. An improvement in function was noted, but this did not reach statistical significance. When spinal manipulation was compared with other treatments known to be effective, such as analgesics, exercise, and physical therapy, the authors could find no statistically significant benefits compared with other therapies. Results did not change when they looked at studies in which only manipulation and not mobilization was used. They also could not identify any particular subgroup of patients for whom manipulation was particularly effective, although they theorized that if such a group existed, it would be small (see Chapter 22). The authors also did not find other commonly used treatments, such as physical therapy and medication, to be statistically more effective than spinal manipulation. Their conclusion was that spinal manipulation is more effective than placebo, and is one of several options of modest effectiveness for patients with low back pain.
Traction
The literature in this area has been criticized because of disagreement as to whether studies done have used the appropriate weight of traction, frequency of treatment, and length of treatment session. Many studies have been of traction used once per week, and some practitioners believe traction should be done daily and that outcomes of studies with frequency less than this are invalid.85 Multiple randomized controlled trials using different doses of traction have been done, however, and most have not found traction to be effective for the treatment of back pain. No well-done study has shown that a specific weight or frequency of traction is effective over sham treatment.225,229
Lumbar Supports
Lumbar supports are used to both treat and prevent low back pain. Multiple types of lumbar support can be used. They vary from a simple elastic wrap to custom-molded plastic braces. High-quality studies comparing the effectiveness of different braces are generally lacking, although one study showed that patients who wore a lumbar support plus a rigid insert in the back had more subjective improvement than those who wore a brace without a rigid support.144
Several mechanisms of action have been proposed as to why lumbar supports would be effective. One hypothesis is that they prevent excessive spinal motion, either by physically blocking extremes of motion or by providing sensory feedback to remind the patient not to bend excessively. Another theory is that they increase intraabdominal pressure without increasing abdominal muscle activity, and therefore could reduce muscle force, fatigue, and compressive loading on the spine.222 A review of the literature regarding the mechanisms of action of lumbar supports showed that neither of these theories has been proven. In general, lumbar supports decrease ROM, but the results are not consistent. Decreases in ROM vary between subjects, with some subjects even showing increased range while wearing a brace. The plane of motion that is reduced also varies between subjects and the types of braces tested. Some types of brace reduce rotation, whereas others reduce flexion and extension. No evidence exists that lumbar supports actually increase intraabdominal pressure or decrease muscle forces and fatigue.222 Regarding the efficacy of lumbar supports, there is limited evidence that lumbar supports provide some pain relief for low back pain when compared with no treatment, but when compared with other treatments they are no more effective. Studies also have shown that generally there is poor compliance for subjects to consistently wear lumbar supports. No consistent evidence exists that lumbar supports prevent the occurrence of back pain (see Chapter 16).226
Transcutaneous Electrical Nerve Stimulation
The development of transcutaneous electrical nerve stimulation (TENS) was based on the gate theory of pain of Melzack and Wall. In this theory, the stimulation of large afferent fibers inhibits small nociceptive fibers, causing the patient to feel less pain. Multiple types of TENS applications can be used, such as high frequency moderate intensity, low frequency high intensity, and burst frequency (see Chapter 21). Many patients find TENS helpful for temporary relief of low back pain. Evaluating the research in this area is difficult because of the difficulty of an equivalent placebo and the different types of TENS applications used between studies, and because most studies use patients’ memory of their pain (which is frequently inaccurate as an outcome measure).30 Metaanalyses of TENS outcomes show trends toward better pain reduction, better function, and satisfaction with treatment compared with placebo. These trends do not reach statistical significance, however, and, given the small changes, are of unclear clinical significance. Larger and methodologically sound studies are still needed to evaluate the efficacy of this treatment.145
Massage
Massage is one of the most commonly used complementary therapies for low back pain. The mechanism of action is thought to include relaxation, the therapeutic benefits of touch, and beneficial effects on the structure or function of tissues.68 Research that included massage has generally fallen into two categories: studies that measure the effect of massage and studies that assess the effectiveness of other interventions and use massage as a control with hands-on effects. In studies in which massage was used as the control, massage was not generally found to be more beneficial. This could be because of the effectiveness of both interventions, explaining why no differences were found, or it could have been due to publication bias. In studies in which massage was one of the main interventions, massage has been found to be effective for pain relief and in restoring function. For example, Cherkin et al.41 performed an interesting study that compared massage, acupuncture, and self-care education for chronic low back pain. After 10 weeks in which up to 10 treatments were allowed, the massage group showed improvements on disability scales, had decreased medication use, and had less time with restricted activity than the control group. After 1 year, many of these gains were maintained.41 Other high-quality studies have also found massage to be effective for improving symptoms and functions in those with subacute and chronic low back pain.68 High-quality studies on the effects of massage on acute low back pain have not yet been done.
Complementary Movement Therapies
Multidisciplinary Pain Treatment Programs
Strong evidence exists that a multidisciplinary program with a goal of functional restoration is helpful for severe chronic pain.225 This is discussed further in Chapter 42 on chronic pain.
Prognosis of Low Back Pain
Prognosis is difficult to fully ascertain for several reasons. One is that low back pain is a symptom caused by a vast spectrum of pathology with a variety of prognostic outcomes. Another is that the pain experience is individual, and treatment expectations vary. A huge body of medical literature highlights the complex cultural, psychologic, social support, and economic factors that influence pain and rehabilitation outcome.117
Other Causes of Back Pain Greater Than Leg Pain
Lumbar Spondylosis
The degenerative cascade of Kirkaldy-Willis has already been described above. Because degenerative disease of the zygapophyseal joints generally coexists with degenerative disk disease, it is difficult to separate the two entities. Both can cause axial back pain. Both can also cause referred pain into the buttocks and legs. Mooney and Robertson146 and McCall et al.135 have studied the sclerotomal distribution of zygapophyseal joint pain in detail. Zygapophyseal joint pain has even been reported to refer below the knee in some cases.
From biomechanical studies and knowledge of anatomy, we know that lumbar extension and rotation increase forces placed on the posterior zygapophyseal joints. This specific maneuver, however, has not been shown to be diagnostic for zygapophyseal joint pain in clinical settings (by either history or examination). No unique identifying features are found in the history, physical examination, and radiologic imaging that are diagnostic for zygapophyseal joint pain. The only diagnostic maneuvers for zygapophyseal joint pain are fluoroscopically guided zygapophyseal joint injections with local anesthetic, and medial branch blocks (i.e., local anesthetic blocks of the medial branches of the dorsal primary rami that innervate the zygapophyseal joints).52,130 Using these injection techniques, the prevalence of facet-mediated pain in chronic low back pain sufferers has been estimated to be 15% in the younger population and 40% in older age groups.193,194 Schwarzer et al.’s 1994 study193 demonstrated that the vast majority of lumbar zygapophyseal joint pain originates from the L4–L5 and L5–S1 zygapophyseal joints. Consequently, if injections are used as treatment, most can be directed to those two lumbar levels.
More conservative management options for the spondylotic spine and facet-mediated pain should be tried before resorting to invasive procedures such as intraarticular zygapophyseal joint corticosteroid injections or medial branch neurotomies. The conservative treatments are similar to treatments for osteoarthritic joints and can be categorized as lifestyle and activity modification, medications, and exercise.
Interventional treatments for zygapophyseal joint pain have been briefly mentioned above. A more detailed discussion can be found in the chapter on interventional pain management chapter (see Chapter 25).
Internal Disk Disruption
Bogduk23 defines internal disk disruption as a condition in which the internal architecture of the disk is disrupted, but its external surface remains essentially normal (i.e., there is no bulge or herniation). It is characterized by degradation of the nucleus pulposus and radial fissures that extend to the outer third of the annulus (high-intensity zone areas on MRI).9 It can be diagnosed only by postdiskography CT, which shows the degradation of the nucleus and the presence and extent of the annular fissures. Although the use of diskography is controversial, most believe that annular tears (especially those that reach the outer third of the annulus, i.e., the innervated fibers) can be a source of low back pain. It must be remembered, however, that like most abnormalities on lumbar spine imaging, annular tears or high-intensity zones are seen commonly in asymptomatic subjects.
Disk Herniation
The terminology used to describe disk material that extends beyond the intervertebral disk space is confusing. Herniated disk, herniated nucleus pulposus, disk protrusion, disk bulge, ruptured disk, and prolapsed disk are all commonly used terms, and sometimes are incorrectly used synonymously. Displaced disk material can be initially classified as a bulge (disk material is displaced >50% of its circumference) or as a herniation (<50% of its circumference) (Figure 41-22).57 Disk herniations can then be subclassified into protrusions or extrusions. A disk protrusion is defined as a herniation with the distance of the edges of the herniated material less than the distance of the edges at its base. A disk extrusion occurs when the distance of the edges of the herniated material is greater than the distance of the edges at its base. A disk extrusion can be further subclassified as sequestrated if the extruded disk material has no continuity with the disk of origin. Disk herniations can also be described as contained or uncontained depending on the integrity of the outer annular fibers. If the outer annular fibers are still intact, it is described as a contained disk herniation. This classification has no relevance to the integrity of the posterior longitudinal ligament.
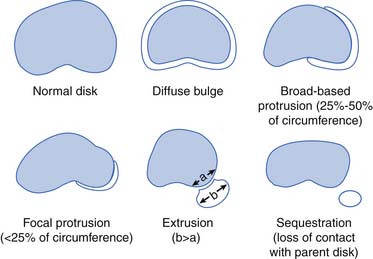
FIGURE 40-22 Disk herniation, protrusion, and extrusion.
(Modified from Maus TP: Imaging of the spine and nerve roots. In Kraft GH, editor: Physical medicine and rehabilitation clinics of North America, Philadelphia, 2002, Saunders.)
More than 95% of lumbar disk herniations occur at the L4–L5 and L5–S1 levels.49,204 Next most common is L3–L4, followed by L2–L3. The most common lumbosacral radiculopathies are consequently L5 and S1. Posterolateral disk herniations are most common because the annulus fibrosus is weakest posterolaterally. Posterolateral disks can affect the nerve root as it descends in the lateral recess or just before it enters the neural foramen. Far lateral or extraforaminal herniations can affect the nerve root as it exits the neural foramen, and central disk herniations can affect any part of the cauda equina, depending on the level.
Disk herniations can cause an inflammatory response that can affect the nerve root, or there can be mechanical compression, both of which can cause radicular symptoms. Disk herniations, however, can also cause solely axial pain. Diagnosing diskogenic low back pain is a challenge because we know asymptomatic subjects can have disk herniations present on MRI.21,96,97 Discography is a controversial diagnostic tool for diskogenic pain (see Chapter 25). It is typically used as a presurgical screening tool.
Treatment Diskogenic Back Pain
Most patients with diskogenic pain do well with conservative management. However, there are still some patients who do not respond to these conservative measures. Over the past few years, there have been a number of interventional procedures used to tackle the problem of diskogenic back pain, aimed at preventing the need for surgical management. Growing literature supports epidural steroid injections as a pain management strategy for disk herniations with radiculitis. Because it is well accepted that a disk herniation can cause an inflammatory response, epidural steroid injections for diskogenic pain (i.e., without radicular symptoms) have been used and probably are indicated, although there is no literature to prove this. Buttermann37 has supplied us with some potential criteria for the role of epidural steroid injections in presumptively symptomatic degenerative disks. Many percutaneous disk procedures are in use, and new ones are continually being developed to treat patients with diskogenic pain that has failed to respond to more conservative management (Boxes 40-4 and 40-5).173,203 None of these, however, has definitively been shown to have better results than a surgical micro-diskectomy. The literature on surgical management for diskogenic pain is similar to that regarding epidural steroid injections, that is, surgery is most effective in improving radicular leg symptoms and is less impressive for axial back complaints. The most common surgical procedure is diskectomy. If there is concern for instability (i.e., in patients with significant multilevel degenerative disease), however, spinal fusion is sometimes considered as well. Recently prosthetic disk replacements have been considered as a replacement for spinal fusion. The literature, however, does not demonstrate better outcomes with disk arthroplasty compared with fusion, only equal outcomes. The potential complications with disk arthroplasty are significantly more concerning, however, and it is currently unclear who would benefit from a disk arthroplasty over lumbar fusion.
BOX 40-4 Procedures for Disk Herniations and Radiculopathy (Leg Pain >> Axial Pain)
BOX 40-5 Procedures for Diskogenic Low Back Pain (Internal Disk Disruption) (Axial Pain >> Leg Pain)
Spondylolysis
Spondylolysis is a defect of the pars interarticularis and is a common cause of back pain in children and adolescents. The most common hypothesized mechanism of injury is repetitive hyperextension loading in the immature spine, and is commonly reported in adolescent gymnasts and football linemen.60,94 Acute fracture from a severe hyperextension injury is also possible but less commonly reported.59 Pars defects have been reported in nonathletic individuals as well. In growing children, the defect is rarely seen before walking begins and most commonly occurs at age 7 to 8 years.153 An increase in incidence occurs during the adolescent growth spurt between ages 11 and 15 years. The pars defect appears to result from a combination of hereditary dysplasia of the pars and repetitive stressing of the spine by walking and extension loading.51 Unilateral or bilateral defects can occur, but bilateral involvement might result in spondylolisthesis. Ninety percent of these lesions occur at the L5–S1 level.
Patients typically present with low back pain that is exacerbated by extension and alleviated by rest or activity limitation. Physical examination can demonstrate focal tenderness, pain with lumbar extension, and hamstring tightness (Figure 40-23). The neurologic examination is usually normal. If a spondylolisthesis is present, a palpable step-off with examination of the spinous processes might be evident.
The ideal radiographic assessment of a suspected pars injury is debated. Plain radiographs are of limited use in diagnosing a symptomatic spondylolysis. Oblique views can show a pars defect well; however, the sensitivity of detection on plain films does not increase much, and the radiation exposure is significantly greater.174 Standing anteroposterior and lateral radiographs can be obtained initially to identify a spondylolisthesis or any gross bony abnormalities.207 SPECT is more sensitive than plain radiography and planar bone scans. A positive bone scan or SPECT scan correlates with a painful pars lesion.86 In most retrospective analyses, however, increased uptake is present for approximately 1 year after the occurrence of the fracture.174 If the SPECT study is consistent with an active pars injury, a thin-slice CT through the abnormal level can be helpful to confirm the diagnosis and stage the lesion. Staging the lesion according to chronicity is helpful to predict healing. The Tokushima classification grades defects by CT as acute, progressive, or terminal (Box 40-6).66 A recent metaanalysis demonstrated by using this classification system 68% of acute defects healed, 28% of progressive lesions healed, and no terminal lesions healed.109 Determining chronicity can help the clinician decide how quickly the athlete should progress through treatment. The use of MRI in diagnosing spondylolysis has garnered much attention in recent years. Standard magnetic resonance sequences identify only 80% of pars lesion seen on SPECT and should be used to look for other pathology.133 Campbell et al.,38 however, showed that nonstandard magnetic resonance sequences (oblique sagittal images) found 39 of 40 pars defects seen on CT and SPECT, but still MRI only correctly staged the lesion.29
BOX 40-6 Tokushima Classification for Grading Pars Defects
Modified from Fujii K, Katoh S, Sairyo K, et al: Union of defects in the pars interarticularis of the lumbar, J Bone Joint Surg Br 86:225-231, 2004.
A number of successful management strategies for spondylolysis have been used. Conservative management is most common, typically beginning with relative rest and avoidance of activities that increase pain (especially repetitive extension). Patients typically are advised to rest for 3 months.207 This is the shortest amount of time over which healing of a pars lesion has been identified on serial imaging.253 Bracing, although a very commonly cited treatment, is not absolutely necessary. A recent metaanalysis demonstrated 84% of 665 patients with a spondylolysis treated nonoperatively were able to return to pain-free unrestricted activity by 1 year. No significant difference in clinical outcomes was seen between the braced and not-braced groups.109 A rigid brace might be considered after 2 weeks of rest if symptoms are not resolving.207 A radiographically stable bony union is the obvious goal but is not an absolute necessity, given many young athletes can become symptom-free in sports and everyday life even with a persistent pars defect. That same metaanalysis studied 847 defects and showed a 28% radiographic healing rate, with 71% of the unilateral defects healed and only 18% of the bilateral defects healed.109
In any event, braced or not, the young athlete is at risk for deconditioning. When pain allows, the patient should be encouraged to begin aerobic conditioning and eventually enter a spinal rehabilitation program before return to a sport. Once the athlete has mastered a basic core stabilization program, functional progression back to the specific sport is appropriate, with a focus on neuromuscular proprioceptive control and sport-specific drills before full return to play. For the patient with chronic low back pain and spondylolysis, O’Sullivan et al.158 demonstrated that a specific exercise program focused on training the lumbar multifidi and deep abdominals can be very effective. Surgical treatment is rarely indicated for the patient with spondylolysis alone but is more common in the setting of spondylolisthesis and/or radiculopathy.
The natural history of spondylolysis and low-grade (<2) spondylolisthesis is benign, that is, it is rare to have progressive slippage. Saraste190 demonstrated this in a study of 225 patients with a 20-year follow-up period. Most cases of progressive slippage occur during the adolescent growth spurt, however, so very young athletes should be monitored with lateral flexion–extension plain films. Besides the adolescent growth spurt, a listhesis greater than 50% is considered a risk factor for progressive slip.
Spondylolisthesis
Lumbar spondylolisthesis or anterior slippage of one vertebra on another can result from many causes. Spondylolisthesis can be grouped into six different categories by etiology. The most common is the isthmic spondylolisthesis (Figure 40-24). The isthmic slip occurs as a result of a spondylolysis or “stress fracture” of the pars interarticularis (as described above). The dysplastic spondylolisthesis is a congenital slip and is caused by dysplasia of the facet joints of the upper sacrum, leading to an inability to resist shear stresses and forward slippage. Degenerative spondylolisthesis is seen in the older spine and is related to long-standing intersegmental instability from degenerative facet or disk disease. The most common level affected in a degenerative slip is the L4–L5 level. Traumatic spondylolisthesis is rare and is caused by acute fracture secondary to trauma. Pathologic spondylolisthesis is due to medical causes of generalized or local bone disease that can cause decreased bony strength. This form can present as an isthmic defect or an elongated, intact pars. The final category is postsurgical and is due to resulting instability from an extensive decompression, which is uncommon now because of the amount of hardware used for fusions after extensive decompressions.
The patient with spondylolisthesis typically presents with low back pain. Sometimes there is a complaint of intermittent radicular symptoms related to a dynamic radiculitis, that is, nerve root irritation caused by subtle instability at the listhetic segment. Physical examination is not different from that seen in spondylolysis. When imaging a patient with suspected spondylolisthesis, lateral flexion–extension views are helpful for presurgical screening. With lateral plain films, the degree of slip is graded 1 through 5 (Table 40-6).
Table 40-6 Meyerding’s Grading System for Spondylolisthesis∗
| Grade | Percentage Slip |
|---|---|
| 1 | <25 |
| 2 | 25-49 |
| 3 | 50-74 |
| 4 | 75-99 |
| 5 | ≥100 (spondyloptosis) |
∗ Meyerding divided the anterior–posterior diameter of the superior surface of the first sacral vertebral into quarters and assigned the grade accordingly.249
The natural history of spondylolisthesis is spontaneous stabilization. It is generally accepted that significant slip progression rarely occurs in adults.190,196 Some controversy exists regarding slip progression in adolescents. Harris and Weinstein84 studied youths with grade 3 or 4 slips in a long-term follow-up study and noted that there was a higher incidence of progression of the slip until skeletal maturity was reached. Saraste190 and Seitsalo196 had similarly large long-term observational studies that demonstrated that slip progression in youths and adults was small overall. Possible factors positively correlating with slip progression include degree of slip, degenerative disk disease at the level of slip, adolescent age, and ligamentous laxity that manifests as hypermobility on imaging (i.e., motion on flexion–extension views).
Other Spinal Fractures
Many other types of spinal fracture can occur, the most common of which are briefly discussed below. Many are secondary to trauma. Evidence-based guidelines have not yet been developed for the treatment of traumatic spine fractures. Current literature in this area is mainly of retrospective case series. Outcomes appear to be most dependent on the amount of neurologic injury at the time of injury, and on the time elapsed between injury and surgery if a neurologic injury exists.230
Posterior Column Fractures
This includes transverse process and spinous process fractures. These are stable injuries. They are treated by pain management techniques and avoiding contact sports until the fractures have healed.
Anterior and Posterior Column Fractures
These are caused by flexion and distraction injuries, and are called chance fractures. They are usually caused by seat belt injuries in high-impact motor vehicle accidents. They are unstable fractures and are sometimes treated with bracing but often require surgery.166
Osteoporotic Compression Fractures
Osteoporotic compression fractures are important to diagnose, both because they are a significant source of morbidity and because they also can herald the risk for subsequent fractures, particularly hip fractures, which have high morbidity and mortality rates. Patients who have had a previous vertebral fracture have 3.8 times the risk of suffering a hip fracture compared with those who have not. The risk of compression fractures increases as bone density decreases. Genetic factors account for much of the risk, as well as exercise, calcium intake, smoking, alcohol use, and age at puberty (see Chapter 41).
Compression fractures can be a significant cause of pain and are generally the reason that there is a higher incidence of back pain in elderly women compared with men. Pain is especially prevalent if three or more fractures are present. These subjects have twice as much back pain as those without compression fractures.65 Fractures can be asymptomatic or can present with sudden onset of severe pain. Pain can radiate anteriorly and usually gradually improves over several weeks.
Diagnostic Evaluation for Vertebral Fractures
Up to 30% of those with osteoporotic compression fractures have an underlining cause, which is called secondary osteoporosis. Common causes of secondary osteoporosis are use of oral steroids, hyperthyroidism, metastases, and multiple myeloma. An underlying cause should always be ruled out. This can be done with a complete blood count, sedimentation rate, reactive protein, thyroid function tests, bone profile, and biochemical profile (such as liver function test, electrolytes, and albumin).159 Bone mineral density measurements are useful to confirm the diagnosis of osteoporosis and to assess the efficacy of treatment.
Treatment
Vertebroplasty is a procedure in which bone cement is injected into the bone for pain relief and to strengthen the bone. Studies so far have mainly been case series or uncontrolled prospective studies, but it appears that up to 80% of patients treated obtain significant pain relief, and complications are rare. The complications can include compression of the spinal nerve roots and spinal cord, and pulmonary embolism.65
Osteoporosis requires treatment with a combination of medication, lifestyle modification, and exercise (see Chapter 41).
Cancer and Low Back Pain
Cancer is the second leading cause of death in the United States, and two thirds of patients with cancer develop metastases. The spine is the most common site for bony metastases, and vertebral body metastases are found in more than one third of cancer patients. The most common cancers that involve the spine are lung, breast, prostate, and renal cell.167
Back pain is by far the most common symptom of metastatic disease. It is caused by stretching of the periosteum and tumor mass effect. The thoracic region of the spine is most commonly involved, although the lumbar spine is a more common site for colorectal cancer.176 The pain can start gradually and increase as the bone is destroyed. It is a constant ache not exacerbated by movement. Sometimes the pain has a more sudden onset because of a pathologic fracture, and this type of pain can be worse with movement, especially if the spine is unstable. Deyo et al.50 found that the most specific historical feature for malignancy as a source of back pain is a previous history of cancer (98% specific), and the authors considered it prudent to consider new-onset back pain in a patient with a history of cancer to be malignant disease until proven otherwise. This historical feature has a sensitivity of only 0.31, however, so only about one third of patients with spinal malignant neoplasm have a history of cancer. Consequently, other features suggestive of malignancy must be explored in the history. Back pain unrelieved by bed rest is greater than 90% sensitive, so if the pain is relieved by bed rest, malignancy is unlikely. This is not specific, however, so many patients without malignancy also complain that their pain is not relieved by bed rest. Other historical features related to cancer include unexplained weight loss and failure to improve with conservative care. New onset of back pain after age 50 is suspicious for malignancy because many other common causes of back pain begin at an earlier age. These features can be combined to give the clinician confidence in determining whether cancer should be included in the differential diagnosis.50 Neurologic deficits occur in 5% to 10% of patients with spinal metastases either from the mechanical pressure of the tumor or from bone extruded from a collapsed vertebral body.220 Neurologic deficits often occur several months after the back pain began.72MRI is the imaging modality of choice for a full evaluation of spinal metastases. It is very sensitive and can show early changes in the bone marrow. It also shows both bony destruction and neural compression.72,176
Spinal Infections
Spinal infections include osteomyelitis, diskitis, pyogenic facet arthropathy, and epidural infections. These structures are often all infected at the same time. The incidence of spinal infections is increasing. Some of the causes of this include the growing numbers of immunocompromised patients who are at high risk, drug resistance of some infections, and recent increases in tuberculosis.212 It is important to diagnose and treat spinal infections quickly to prevent increased morbidity and mortality, and to prevent complications such as epidural abscesses that can cause paralysis.105 However, this is not always easy. In Tali’s review article of spinal infection,212 he describes the “rule of 50” to assist in the diagnosis of spinal infections: 50% of the patients are older than 50, 50% will have a fever, and 50% will have a normal white blood count. The urinary tract is the source in 50%; Staphylococcus aureus is the organism in 50%; the lumbar spine is affected in 50%; and symptoms are present for greater than 3 months in 50%.212
Vertebral osteomyelitis can occur from hematogenous spread or be secondary to a contiguous focus of infection. Hematogenous spread occurs via spinal arteries, and infection can quickly spread from the end plate of one vertebral body into the disk and then into the adjacent vertebral body. The most common source is urinary tract infections, often caused by Escherichia coli and other enteric bacilli. Hematogenous spread is also seen from other sources, such as infected intravenous lines or endocarditis. Patients with diabetes, those on hemodialysis, intravenous drug users, and other immunocompromised patients are at increased risk.126,212 The most common location is the lumbar spine, and the most common symptom is back pain, although 15% of patients also have radicular pain. Symptoms can begin slowly and progress over months. Many patients do not have a fever or elevated white blood count. The erythrocyte sedimentation rate, however, is usually elevated. The infection can spread to surrounding tissues, and epidural, paraspinal, and psoas abscesses can also be present.
Plain films are usually normal the first 2 weeks, and then the first sign is a periosteal reaction. As the infection progresses, plain films show irregular erosions in the end plates of adjacent vertebral bodies and narrowing of the disk space. This appearance is nearly pathognomonic for infection because tumors and other causes of irregular erosions rarely cross the disk space. Bone scan shows changes as soon as 24 hours after symptoms begin but are not specific. MRI is as sensitive as bone scan and can give important anatomic information; therefore it is generally the imaging technique that should be used.105,108
Treatment for spinal infections is usually a 4- to 6-week course of intravenous antibiotics. Sensitivity can often be determined by blood cultures, but if these are negative, samples from a bone biopsy might be necessary. Following the erythrocyte sedimentation rate is helpful to determine the effectiveness of treatment. Surgery is generally necessary only if the spine has become unstable, there are progressive neurologic deficits, or medical treatment fails. Spontaneous fusion of the infected segments often occurs after treatment.105
Osteomyelitis secondary to a contiguous focus of infection is seen after surgical procedures and with extension of infection from adjacent soft tissue. The most common organism is S. aureus.126,212 Risk factors for development of postoperative osteomyelitis include history of smoking, obesity, poor nutrition, uncontrolled diabetes, administration of steroids, history of malignancy, and radiation treatment in the area of surgery.105 These infections usually present about 14 to 30 days after surgery.105 Diagnosis is sometimes difficult because symptoms such as pain or fever can be attributed to soft tissue infection or the surgical procedure. Imaging studies can also be less conclusive because of surgical or soft tissue changes. The erythrocyte sedimentation rate is usually elevated after surgery, so it is not as useful in making the diagnosis in the first weeks after surgery.105 Treatment for these types of infection usually requires surgical debridement and then a course of antibiotics.126,212
Diskitis can occur from contiguous spread of infection or iatrogenically from procedures such as diskectomy and diskography. The incidence of these types of infection is low, as studies report a 0% to 3% incidence with procedures, but morbidity if infection occurs is significant. One study found that 55% to 87% of patients were unable to return to their normal work after diskitis. One reason for this poor outcome is the difficulty of using antibiotics to treat the infection because of the relative avascularity of disks.31
Spondyloarthropathies
Spondyloarthropathies are a group of diseases associated with the HLA-B27 allele. They include ankylosing spondylitis, Reiter syndrome, reactive arthritis, psoriatic arthritis, enteropathic arthritis, and undifferentiated spondyloarthropathy. It is hypothesized that, in genetically susceptible individuals, an interplay of environmental and immunologic factors leads to clinical manifestations. Although the diseases are grouped together, each has unique features on clinical presentation.102
Ankylosing Spondylitis
Ankylosing spondylitis is the prototype for the spondyloarthropathies. It is 3 times more common in men than in women. Symptoms usually begin in the late teens or 20s. It generally first presents with morning stiffness and a dull ache in the low back or buttocks. On physical examination, there is decreased spinal mobility, decreased chest expansion, and tenderness of the sacroiliac joints with direct pressure and with maneuvers that stress the joints.102
Findings outside the spine are also common. Hip or shoulder arthritis is seen in about 30% of patients, and asymmetric peripheral joint arthritis is also seen in about 30% of patients. Bony tenderness and enthesitis at multiple sites, such as the heels, greater trochanters, iliac crests, and tibial tuberosities, are common. Systemic disease manifestations include anterior uveitis, heart disease, and inflammatory bowel disease.213
Radiographs help establish the diagnosis by showing erosions and sclerosis of the sacroiliac joints. As the disease progresses, these changes are also seen in the lumbar spine. This can lead to squaring of the vertebral body and eventually to bony bridging between vertebrae.213 Blood work can be helpful in establishing the diagnosis. The HLA-B27 gene is present in 90% of patients with ankylosing spondylitis. Most patients also have an elevated sedimentation rate.
The initial treatment includes exercises that promote spinal extension. Evidence exists that exercise promotes mobility, improves function, and prevents severe deformity in many cases.213 NSAIDs are helpful to relieve pain and inflammation, so that the exercises can be done and function maintained. Indomethacin is particularly effective for this condition. Sulfasalazine and methotrexate are sometimes used, especially if there is peripheral arthritis. Disease-modifying agents such as the tumor necrosis factor inhibitors are also used to treat ankylosing spondylitis. They appear to be effective in controlling articular inflammation but do not appear to prevent joint ankylosis.95 Sacroiliac injections under fluoroscopy can reduce symptoms acutely but do not have long-term benefit.213
Other Spondyloarthropathies
Differential Diagnosis and Treatment: Leg Pain Greater Than Back Pain
Lumbosacral Radiculopathy
Radicular symptoms can be the result of overt mechanical compression of a nerve root or a chemically mediated inflammatory process. The most common compressing lesion by far is a disk protrusion. Fewer than 1% of patients who present with radicular symptoms have other causes, including infection, malignancy, or fracture.47 Rare presentations of radiculopathy, that is, those with fever, weight loss, night pain, cancer history, or osteoporosis risk factors, certainly warrant special attention to evaluate for the less common but potentially more catastrophic causes of radiculopathy.
The most common levels of disk herniation are L4–L5 and L5–S1, with L5 and S1 being the most common nerve roots involved in radiculopathy. Multiple nerve roots can be affected by a single disk herniation, given the organization of the cauda equina. Central disk herniations can also affect nerve rootlets that are descending in the cauda equina. The affected nerve root level might not correlate with the level of the disk herniation. For example, a central L3–L4 disk herniation could impact the L5 or S1 rootlets as they descend through the thecal sac before exiting out of their expected neural foramen. True cauda equina syndrome occurs when the lowest sacral rootlets are affected, resulting in bowel, bladder, and sexual dysfunction. Up to 1% of all disk herniations present as cauda equina syndrome.198 In the appropriate clinical setting, a large postvoid residual of urine is a good predictor of cauda equina syndrome.47 Cauda equina syndrome is a surgical emergency. Recovery of neurologic deficits, including bowel and bladder dysfunction, is greatest if decompressive surgery is performed within 48 hours.240
The natural history of lumbosacral radiculopathy resulting from disk herniation tends to favor spontaneous resolution of symptoms over time.36 Multiple reports have shown that disk protrusions and extrusions can regress without surgical treatment.215 Conservative treatment is best used to decrease pain and improve the patient’s level of functioning during acute management of radiculopathy. Even with some neurologic injury, conservative management should be considered because various studies have documented the same neurologic recovery in groups treated surgically and nonsurgically.244
The specifics of conservative management of lumbosacral radiculopathies, however, are still debatable. NSAIDs have not been found to be effective in patients with radiculopathy.43,227 No definite support exists for oral steroids in the treatment of acute radiculopathy.42 The neuropathic pain agents (anticonvulsants and tricyclic antidepressants) are often used for radicular pain.42 Small studies have found gabapentin and topiramate to be associated with small improvements in pain scores.43
Although exercise therapy has not specifically been demonstrated to alter the course of acute radiculopathy, there is probably a role for exercise.223 Saal et al.186 reported very favorable outcomes using aggressive nonoperative care (an active exercise program potentially with epidural steroid injections) in the treatment of lumbar disk herniation with radiculopathy. Their protocol is the basis for many exercise programs used in the treatment of lumbar disk herniations with radiculopathy today.
Lumbar epidural steroid injections have become a common adjuvant for the treatment of lumbosacral radiculopathy. The more recent literature supports the use of fluoroscopically guided transforaminal epidural injections for early pain relief and potentially a more rapid recovery and reduced need for surgical intervention.101,120,179,221 They are best used in combination with an active rehabilitation program and are commonly used to facilitate active therapy by decreasing pain and inflammation.
Surgical management of lumbosacral radiculopathy is best reserved for those patients who have significant persistent radicular symptoms despite 6 to 8 weeks of maximized conservative management, neurologic progression, or cauda equina syndrome. Common decompressive procedures with favorable outcomes include lumbar hemilaminotomy with diskectomy, and lumbar hemilaminectomy.208 The highly anticipated Spine Patient Outcome Research Trial was a randomized trial evaluating the effect of surgery over individualized nonoperative treatment involving 13 spine centers across the United States with 501 surgical candidates with lumbar radicular symptoms for at least 6 weeks caused by a disk herniation.247 Subjects randomized to either surgery or nonoperative treatment improved substantially over the 2-year period of the study, and the primary outcomes were not statistically different between groups. A secondary outcome of sciatica severity, however, did show statistical significance in favor of surgery. Patients need to be counseled regarding appropriate expectations after surgery for lumbar disk herniation with radiculopathy. Surgery might slightly accelerate the resolution of neurologic deficits for the typical radiculopathy; however, the major benefit of surgical intervention is pain relief.49 Relief of leg pain should be expected; however, back pain relief is more difficult to predict. Patients should be counseled that they are likely to have recurrent back difficulties even after a successful decompressive surgery. Figure 40-25 shows a useful algorithmic approach to the management of acute lumbosacral radiculopathy.
Lumbar Spinal Stenosis
The symptoms of spinal stenosis result from a complex series of changes within the lumbar spine.71 These changes are generally related to aging. The narrowing of the spinal canal that occurs in stenosis results from the degenerative changes described by Kirkaldy-Willis et al.108 Not all patients with significant narrowing, however, have symptoms. Vascular and biochemical factors are probably involved that add to the mechanical compression (resulting from narrowing of the canal), which ultimately leads to symptomatic spinal stenosis. These have been described earlier in this chapter. Box 40-7 gives a classification schema for spinal stenosis, and Table 40-7 outlines a radiologic grading scale. Electrodiagnostic studies are often performed in patients with spinal stenosis. In addition to ruling out other reasons for leg symptoms (such as peripheral neuropathy), they can be helpful in fully characterizing the stenosis. In one recent study, H reflexes and F waves were shown to correlate with anatomic changes on MRI of spinal stenosis, whereas limb electromyography did not.42 This is consistent with the clinical observation that MRI findings and radicular complaints often do not correlate.
Table 40-7 Grading Lumbar Stenosis on Magnetic Resonance Imaging
| Grade | Percentage of the Anteroposterior Canal Dimensions at a Normal Level |
|---|---|
| Mild | 75-99 |
| Moderate | 50-74 |
| Severe | <50 |
The natural history of lumbar spinal stenosis is fairly favorable overall. Johnsson et al.98 followed patients with lumbar stenosis over a 4-year period with conservative treatment. Based on subjective patient reports, 70% remained unchanged, 15% improved, and 15% worsened. Walking capacity improved in 42% of patients, remained unchanged in 32%, and decreased in 26%. Amundsen et al.5 reported on a 10-year study of patients randomly assigned to surgical or nonsurgical treatment. Nonsurgical treatment consisted of bracing for 1 month followed by physical therapy. They demonstrated that neurologic deterioration was rare, that delaying surgery (with conservative management) had no effect on postoperative outcomes, and that, at 4 years, half the conservative treatment group and four fifths of the surgical group had favorable outcomes. During the final 6 years of study, clinical deterioration of symptoms was rare. In general, most patients with symptomatic stenosis remain unchanged, whereas some improved and others worsened. It is impossible to predict which patients will fall into each of these categories. It is useful information that a diagnosis of lumbar stenosis does not mean rapid neurologic deterioration, and that conservative management for those with mild to moderate symptoms is warranted.
Botwin et al.28 have demonstrated that there probably is a role for epidural steroid injections in the nonoperative treatment of symptomatic lumbar stenosis. They performed transforaminal epidural steroid injections in stenosis patients who were deemed surgical candidates. Patients also received oral medications and physical therapy. Even at 1-year follow-up evaluation, 64% of the patients felt subjectively better. Only 17% of patients went on to surgery within the 1-year follow-up period.
Surgical consideration for lumbar stenosis should be given to patients with intractable pain resistant to nonoperative management, profound or progressive neurologic deficit, or lifestyle impairment. A recent study comparing surgical management of spinal stenosis versus “usual care” showed that by 3 months postsurgery, the surgical group had better pain control than the conservative care group and that this improvement in pain persisted for years. No improvement, however, was seen in function in the surgical group compared with the conservative care group.248 Of note, this was not a placebo-controlled study, and other studies involving surgery have shown profound placebo effects with surgery.182 Age is not a contraindication to surgery, although the patient’s general health status must be considered.10,88 Laminectomies are the most common decompressive procedures.182 When spinal stenosis is associated with instability, degenerative spondylolisthesis, deformity, or recurrent stenosis, fusion is often performed. Instrumentation often improves the fusion rate but does not influence the clinical outcome.197 If selectively chosen, most patients with neurogenic claudication do well with surgical management. If the chief symptomatic complaint is axial low back pain, however, the surgical outcome is generally poorer.103
Nonlumbar Spine Causes of “Radicular” Leg Symptoms
A number of nonspinal disorders mimic lumbar radiculopathy because they generate pain referral patterns similar to lumbosacral dermatomes. Their etiology is diverse and includes joint, soft tissue, vascular, and peripheral nerve sources. A thorough history and physical examination can typically help differentiate these disorders from lumbosacral radiculopathy; however, other diagnostic studies might be necessary.
Joint Disorders
The sacroiliac joint is now generally accepted as a potential pain generator that can refer pain into the lower limb. Other than true sacroiliitis (associated with the spondyloarthropathies), the exact pathologic structure or source of pain from the sacroiliac joint is still uncertain. Vilensky et al.231 reported in 2002 that substance P can be found in the posterior sacroiliac ligament. It is still not known, however, whether it is the synovium, the articular cartilage, the capsule, the ligamentous structures, the muscular support of the sacroiliac joint, or a combination of these that is the primary source of pain referred to as sacroiliac joint pain.
Although there are multiple physical examination maneuvers created to stress the sacroiliac joint and reproduce pain, rigorous studies have demonstrated that no one physical examination maneuver (nor combination) correlates well to diagnose sacroiliac joint pain confirmed from diagnostic local anesthetic injections into the joint.53 The gold standard for diagnosing sacroiliac joint pain is a fluoroscopically guided injection of local anesthetic into the sacroiliac joint.
Guided injections have helped to delineate the sclerotomal referral pattern of pain emanating from the sacroiliac joint.63,64 Sacroiliac joint pain generally does not radiate above the lumbosacral junction. It can radiate into the groin, thigh, and even below the knee, with significant overlap of lumbosacral radicular pain patterns.
Soft Tissue Disorders
Piriformis syndrome is thought to cause sciatica via the piriformis muscle putting local pressure on the sciatic nerve in the pelvis. Pain generally radiates into the posterior thigh but can refer below the knee in an L5 or S1 dermatomal pattern. The patient also describes buttock pain and typically has tenderness over the sciatic notch. Multiple examination maneuvers are used to reproduce sciatica resulting from piriformis syndrome.18 Pace’s maneuver is described as resisted abduction and external rotation of the thigh. Freiberg’s maneuver is forceful internal rotation of the extended thigh. Beatty described his maneuver as deep buttock pain produced by the side-lying patient holding a flexed knee several inches off the table. Fishman described an electrophysiologic approach to diagnose piriformis syndrome using H waves.62
Greater trochanteric pain syndrome is a descriptive term for a regional pain syndrome focused about the greater trochanter, buttock, and lateral thigh.199 It is often initially diagnosed as trochanteric bursitis but is probably multifactorial in etiology. An association probably exists with gluteal muscle (medius and minimus) pathology, potentially tendonopathy, tears, or myofascial pain. Physical examination shows generalized tenderness in the region, and typically there is significant gluteal muscle inhibition and deconditioning that can manifest as hip abductor weakness. A comprehensive rehabilitation program focusing initially on pain control and neuromuscular reeducation of the gluteal muscles is important before progressing to strength-building exercises for the gluteals.
Iliotibial band syndrome can be confused with an L4 or L5 radiculopathy. The iliotibial band is an extension of the tensor fascia lata that traverses the lateral aspect of the thigh, attaching at Gerdy’s tubercle on the proximal lateral tibia. Iliotibial band syndrome typically presents as lateral knee pain, but it can also present with more proximal (lateral thigh) pain or radiate distally into the calf. When the iliotibial band is tight, it can also exacerbate trochanteric bursitis and be associated with lateral hip and buttock pain. Iliotibial band tightness is evaluated with Ober’s maneuver.124
Myofascial pain syndromes are common and are thought to arise from active trigger points within a muscle or its fascia.202 Activation of trigger points in various muscles have typical pain referral patterns that can mimic lumbosacral dermatomes (see Chapter 43).
Peripheral Nerve Disorders
Peripheral polyneuropathy is a common cause of paresthesias in the distal lower limbs and feet that can mimic symptoms of lumbar stenosis. They are often seen together in elderly patients with diabetes. Electrodiagnostic studies can be used to diagnose a superimposed peripheral polyneuropathy in patients who have MRI findings of lumbar stenosis. Epidural steroid injections can sometimes be helpful in this situation to help determine how much of the patient’s leg and foot symptoms are related to spinal stenosis over peripheral polyneuropathy. The reason for this is that an epidural steroid injection often improves the symptoms of spinal stenosis syndrome but has no impact on the symptoms of peripheral neuropathy.
Low Back Pain in Special Populations
Low Back Pain in Pregnancy
Low back pain is a common problem in pregnancy. Patients are generally divided into two categories: those with low back pain and those with pelvic girdle pain (pain below the iliac crest, such as sacroiliac joint-related pain). Multiple studies have estimated the prevalence of low back pain in pregnancy at 49% to 76%.58,112,162,163,242 Risk factors include a history of previous back pain, previous pregnancy-related back pain, and low back pain during menses.33,242 Low back pain can begin at any time during the pregnancy and generally reaches a peak at 36 weeks.112,163,242 Pain decreases after this point and in most patients is substantially improved by 3 months postpartum.163
A small group of patients will have persistent pain even after postpartum recovery. Risk factors for persistent back pain after pregnancy include having both low back pain and pelvic girdle pain, pain occurring in early pregnancy, weakness of back extensors, older patients, and those with work dissatisfaction.79
The etiology of low back pain in the pregnant woman is hypothesized to be due to increased biomechanical strain or to an altered hormonal influence. The biomechanical alterations are due to changes in spine posture related to the anterior movement of the pregnant woman’s center of gravity. An argument against purely biomechanical factors as the primary cause, however, is that the back pain often starts before significant weight gain by the mother, and the incidence does not parallel the weight gain.34 A hormonal influence probably exists in the etiology of low back pain in pregnancy. This etiology’s hypothesis is that the hormonal changes during pregnancy alter the lumbopelvic ligaments, which influences the stability of the lumbosacral spine and makes it more vulnerable to loading.112 A direct correlation between circulating levels of the hormone relaxin and pelvic and back pain, however, is controversial.4,82,113,123 The prevalence of disk abnormalities on MRI is the same for pregnant and nonpregnant women, so this might be a source of pain for some pregnant women.246 Only a few high-quality studies have evaluated therapeutic interventions in pregnancy-related low back pain, and there is not much evidence on which to base recommendations for management.209 Individualized physical therapy, water aerobics, acupuncture, and massage therapy can be recommended to decrease pain.61,107,156,245 Instruction on a home exercise program, use of a sacroiliac belt, and back school have not been shown to significantly decrease pain intensity.54,129,142,155 No data support the use of lumbar–abdominal orthoses that are designed to support the pregnant woman’s abdomen. A full discussion regarding the use of medication during pregnancy is beyond the scope of this chapter. In general, any medication use should be discussed with the patient’s obstetrician. Even medications generally thought of as safe and well tolerated can have unexpected consequences during pregnancy. For example, the use of NSAIDs in late pregnancy can cause premature closure of the ductus arteriosus and neonatal renal failure.169 Most antidepressants have not been approved for use during pregnancy, and for most antiseizure medications, such as gabapentin, there is evidence for increased incidence of birth defects in animals, and they have not been well studied in humans.100
Pediatric Low Back Pain
In the past back pain in the pediatric population has traditionally been considered to be relatively rare, and when present raised a concern for serious pathology. This belief is now known to be untrue. In a subset of studies involving more than 300 children each, the prevalence of back pain was cited between 30% and 51%.15,74 Severe back pain, which is either relapsing or permanent, was reported in 3% to 15%.15 An increase in back pain prevalence is noted as the child ages. In a Finnish cohort study, back pain prevalence was reported as 1% in children 7 years old, 6% in those 10 years old, and 18% when 14 years old.211 Another reported the prevalence at 12% for 11-year-old children and 50% for those 15 to 18 years old, which approaches the adult prevalence.35 The same study reported that the pain is often recurrent but that the experience of back pain is frequently forgotten. Other studies demonstrate that the prevalence of low back pain has the greatest increase during puberty and the time of the maximum growth spurt.114,232 Risk factors for nonspecific low back pain in the pediatric population include increase in age, female gender, parents with low back pain, hyperlordotic posture, history of spinal trauma, participation in competitive sports, a high level of physical activity, and depression.15 The literature does not support the following as risk factors for pediatric back pain: being overweight, hamstring tightness, a low level of physical activity, and poor school performance.15 Sitting appears to be the main exacerbating factor of low back pain in the pediatric population.15 There also appears to be a positive correlation between low back pain in adolescence and the presence of pain as an adult.83
There has been more recent attention focused on the role of backpack use in the development of pediatric low back pain; however, there is still not a proven correlation. Carrying a backpack greater than 7.5% to 15% of the wearer’s body weight increases the metabolic demands over what is required to move a person’s body weight alone.122,180 The general recommendation for a child’s backpack weight is limited to 10% of body weight.122 This limit is based on concerns of increasing metabolic costs and not on the risk of back pain development (there are conflicting reports in the literature regarding backpack weight and back pain).80,122,219,243 Many new backpack designs improve ergonomic fit; however, there are no studies demonstrating their effectiveness in reducing back pain.122
Some of the specific causes of low back pain in the pediatric population are listed in Box 40-8. Spondylolysis and isthmic spondylolisthesis often present in young athletes with back pain and have been reported as the most common underlying cause of persistent low back pain among children and adolescents.143 Most believe the etiology is from overuse, particularly during the growth spurt. The presence of isthmic defects in children in the Western world is between 2% and 7%, and as high as 30% in elite athletes.161
Scheuermann disease typically presents in the adolescent as painless exaggerated thoracic kyphosis. From a postural standpoint, the teenager typically presents with excessive thoracic kyphosis (which is demonstrated to be fixed in attempted hyperextension), with a compensatory lumbar hyperlordosis. Radiographic criteria for the diagnosis of Scheuermann disease include anterior wedging of at least three adjacent vertebrae, end-plate irregularities, Schmorl’s nodes, and disk space narrowing.78 These findings are present equally in the adolescent population without back pain, but there is a higher prevalence of concomitant degenerative disk changes in those with pain.216
The etiology of Scheuermann disease is uncertain. Some believe that it is due to repetitive loading of the immature spine that might have some preexisting abnormality of the cartilaginous end plate.93 There does appear to be a familial link.141 Scheuermann disease can have a benign course, although some untreated patients develop progressive structural kyphosis. Brace wearing is recommended until skeletal maturity is reached to help prevent the progressive kyphosis.
Neoplastic disease of the pediatric spine is fortunately rare. Most pediatric spinal tumors are primary (not metastatic) benign bone tumors arising from the vertebrae.93 The most common tumors of the pediatric spine include osteoid osteoma, osteoblastoma, and aneurysmal bone cysts. The classic pain of osteoid osteoma is nocturnal pain that responds to aspirin. The most frequent malignant lesion affecting the pediatric spine is Ewing’s sarcoma.
Acknowledgment
The authors would like to thank Nilda Gatchalian for her assistance in preparing this manuscript.
1. Akuthota V., Lento P., Sowa G. Pathogenesis of lumbar spinal stenosis pain: why does an asymptomatic stenotic patient flare? Phys Med Rehabil Clin N Am. 2003;14(1):17-28.
2. Al-Obaidi S.M., Al-Zoabi B., Al-Shuwaie N., et al. The influence of pain and pain-related fear and disability beliefs on walking velocity in chronic low back pain. Int J Rehabil Res. 2003;26(2):101-108.
3. Al-Obaidi S.M., Nelson R.M., Al-Awadhi S., et al. The role of anticipation and fear of pain in the persistence of avoidance behavior in patients with chronic low back pain. Spine. 2000;25(9):1126-1131.
4. Albert H., Godskesen M., Westergaard J.G., et al. Circulating levels of relaxing are normal in pregnant women with pelvic pain. Eur J Obstet Gynecol Reprod Biol. 1997;74(1):19-22.
5. Amundsen T., Weber H., Nordal H.J., et al. Lumbar spinal stenosis: Conservative or surgical management? A prospective 10-year study. Spine. 2000;25(11):1424-1435.
6. Andersson G.B.J., Johnsson B., Nachemson A.L. Intradiscal pressure, intra-abdominal pressure and myoelectric back muscle activity related to posture and loading. Clin Orthop. 1977;129:156-164.
7. Andersson G.B.J., Johnsson B., Nachemson A.L. Quantitative studies of back load lifting. Spine. 1, 1976. 178–64
8. Anonymous. Physicians’ desk reference. Montvale, NJ: Thomson Healthcare; 2004.
9. Aprill C., Bogduk N. High-intensity zone: a diagnostic sign of painful lumbar disc on magnetic resonance imaging. Br J Radiol. 1992;65(773):361-369.
10. Arinzon Z.H., Fredman B., Zohar E., et al. Surgical management of spinal stenosis: a comparison of immediate and long term outcome in two geriatric patient populations. Arch Gerontol Geriatr. 2003;36(3):273-279.
11. Ariyoshi M., Sonoda K., Nagata K., et al. Efficacy of aquatic exercises for patients with low-back pain. Kurume Med J. 1999;46(2):91-96.
12. Assendelft W.J., Morton S.C., Yu E.I., et al. Spinal manipulative therapy for low back pain: a meta-analysis of effectiveness relative to other therapies. Ann Intern Med. 2003;138(11):871-881.
13. Badalamente M.A., Dee R., Ghillani R., et al. Mechanical stimulation of dorsal root ganglia induces increased production of substance P: a mechanism for pain following nerve root compromise? Spine. 1987;12(6):552-555.
14. Baker A.R., Collins T.A., Porter R.W., et al. Laser Doppler study of porcine cauda equina blood flow: the effect of electrical stimulation of the rootlets during single and double site, low pressure compression of the cauda equina. Spine. 1995;20(6):660-664.
15. Balague F., Troussier B., Salminen J.J. Non-specific low back pain in children and adolescents: risk factors. Eur Spine J. 1999;8(6):429-438.
16. Ballantyne J. Nonsteroidal anti-inflammatory drugs. In Ballantyne J., Fishman S.M., Abdi S., editors: The Massachusetts General Hospital handbook of pain management, ed 2, Philadelphia: Lippincott Williams & Wilkins, 2002.
17. Bartleson J.D. Evidence for and against the use of opioid analgesics for chronic nonmalignant low back pain: a review. Pain Med. 2002;3(3):260-271.
18. Beatty R.A. The piriformis muscle syndrome: a simple diagnostic maneuver. Neurosurgery. 1994;34(3):512-514.
19. Best B.A., Guilak F., Setton L.A., et al. Compressive mechanical properties of the human anulus fibrosis and their relationship to biomechanical composition. Spine. 1994;19:212-221.
20. Birch S., Hesselink J.K., Jonkman F.A., et al. Clinical research on acupuncture: Part 1. What have reviews of the efficacy and safety of acupuncture told us so far? J Altern Complement Med. 2004;10(3):468-480.
21. Boden S.D., Davis D.O., Dina T.S., et al. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects: a prospective investigation. J Bone Joint Surg Am. 1990;72(3):403-408.
22. Boersma K., Linton S., Overmeer T., et al. Lowering fear-avoidance and enhancing function through exposure in vivo: a multiple baseline study across six patients with back pain. Pain. 2004;108(1-2):8-16.
23. Bogduk N. Clinical anatomy of the lumbar spine and sacrum. Edinburgh: Churchill Livingstone; 1997.
24. Bogduk N. The innervation of the lumbar spine. Spine. 1983;8(3):286-293.
25. Bogduk N. The inter-body joint and the intervertebral discs. In Bogduk N., editor: Clinical anatomy of the lumbar spine and sacrum, ed 3, Edinburgh: Churchill Livingstone, 1977.
26. Bogduk N. Nerves of the lumbar spine. In Bogduk N., editor: Clinical anatomy of the lumbar spine and sacrum, ed 3, Edinburgh: Churchill Livingstone, 1977.
27. Bogduk N. The zygapophysial joints. In Bogduk N., editor: Clinical anatomy of the lumbar spine and sacrum, ed 3, Edinburgh: Churchill Livingstone, 1977.
28. Botwin K.P., Gruber R.D., Bouchlas C.G., et al. Fluoroscopically guided lumbar transformational epidural steroid injections in degenerative lumbar stenosis: an outcome study. Am J Phys Med Rehabil. 2002;81(12):898-905.
29. Brady R.J., Dean J.B., Skinner T.M., et al. Limb length inequality: clinical implications for assessment and intervention. J Orthop Sports Phys Ther. 2003;33(5):221-234.
30. Brosseau L., Milne S., Robinson V., et al. Efficacy of the transcutaneous electrical nerve stimulation for the treatment of chronic low back pain: a meta-analysis. Spine. 2002;27(6):596-603.
31. Brown E.M., Pople I., de Louvois J., et al. Spine update: Prevention of postoperative infection in patients undergoing spinal surgery. Spine. 2004;29(8):938-945.
32. Bruehl S., Burns J.W., Chung O.Y., et al. Anger management style and emotional reactivity to noxious stimuli among chronic pain patients and healthy controls: the role of endogenous opioids. Health Psychol. 2008;27(2):204-214.
33. Brynhildsen J., Hansson A., Persson A., et al. Follow-up of patients with low back pain during pregnancy. Obstet Gynecol. 1998;91(2):182-186.
34. Bullock J.E., Gwendolen A.J., Bullock M.I. The relationship of low back pain to postural changes during pregnancy. Aust J Physioth. 1987;33:10-17.
35. Burton A.K., Clarke R.D., McClune T., et al. The natural history of low back pain in adolescents. Spine. 1996;21(20):2323-2328.
36. Bush K., Cowan N., Katz D.E., et al. The natural history of sciatica associated with disc pathology: a prospective study with clinical and independent radiologic follow-up. Spine. 1992;17(10):1205-1212.
37. Buttermann G.R. The effect of spinal steroid injections for degenerative disc disease. Spine J. 2004;4:495-505.
38. Campbell R.S., Grainger A.J., Hide I.G., et al. Juvenile spondylolysis: a comparative analysis of CT, SPECT and MRI. Skeletal Radiol. 2005;34(2):63-73.
39. Carroll L.J., Cassidy J.D., Cote P. Depression as a risk factor for onset of an episode of troublesome neck and low back pain. Pain. 2004;107(1-2):134-139.
40. Cherkin D.C., Deyo R.A., Battie M., et al. A comparison of physical therapy, chiropractic manipulation, and provision of an educational booklet for the treatment of patients with low back pain. N Engl J Med. 1998;339(15):1021-1029.
41. Cherkin D.C., Eisenberg D., Sherman K.J., et al. Randomized trial comparing traditional Chinese medical acupuncture, therapeutic massage, and self-care education for chronic low back pain. Arch Intern Med. 2001;161(8):1081-1088.
42. Chiodo A., Haig A.J. Lumbosacral radiculopathies: conservative approaches to management. Phys Med Rehabil Clin N Am. 2002;13(3):609-621. vii
43. Chou R., Huffman L.H. Medications for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147(7):505-514.
44. Cluff R.S. Adjuvant treatments. In: Ballantyne J., editor. The Massachusetts General Hosptal handbook of pain management. Philadelphia: Lippincott Williams & Wilkins, 2002.
45. Crock H.V. The applied anatomy of spinal circulation in spinal stenosis. In: McNeill T.W., editor. Lumbar spinal stenosis. St. Louis: Mosby, 1992.
46. DeLeo J.A., Winkelstein B.A. Physiology of chronic spinal pain syndromes: From animal models to biomechanics. Spine (Phila Pa 1976). 2002;27(22):2526-2537.
47. Della-Giustina D.A. Emergency department evaluation and treatment of back pain. Emerg Med Clin North Am. 1999;17(4):877-893. vi-vii
48. Deshpande A., Furlan A., Mailis-Gagnon A., et al. Opioids for chronic low-back pain. Cochrane Database Syst Rev. 2007;(3):CD004959.
49. Deyo R.A., Loeser J.D., Bigos S.J. Herniated lumbar intervertebral disk. Ann Intern Med. 1990;112(8):598-603.
50. Deyo R.A., Rainville J., Kent D.L. What can the history and physical examination tell us about back pain? JAMA. 1992;268(6):760-765.
51. Dietrich M., Kurowski P. The importance of mechanical factors in the etiology of spondylolysis: a model analysis of loads and stresses in human lumbar spine. Spine. 1985;10(6):532-542.
52. Dreyer S.J., Dreyfuss P.H. Low back pain and the zygapophyseal (facet) joints. Arch Phys Med Rehabil. 1996;77:290-300.
53. Dreyfuss P., Michaelsen M., Pauza K., et al. The value of medical history and physical examination in diagnosing sacroiliac joint pain. Spine. 1996;21(22):2594-2602.
54. Dumas G.A., Reid J.G., Wolfe L.A., et al. Exercise, posture, and back pain during pregnancy. Part 2. Exercise and back pain. Clin Biomech (Bristol, Avon). 1995;10:104-109.
55. Dvorak J., Panjabi M.M., Novotny J.E., et al. Clinical validation of functional flexion-extension roentgenograms of the lumbar spine. Spine. 1991;16(8):943-950.
56. Fann A.V. The prevalence of postural asymmetry in people with and without chronic low back pain. Arch Phys Med Rehabil. 2002;83(12):1736-1738.
57. Fardon D.F., Milette P.C. Nomenclature and classification of lumbar disc pathology: recommendations of the combined task forces of the North American Spine Society, American Society of Spine Radiology, and American Society of Neuroradiology. Spine. 2001;26(5):E93-E113.
58. Fast A., Shapiro D., Ducommun E.J., et al. Low-back pain in pregnancy. Spine. 1987;12(4):368-371.
59. Ferguson R.L., Allen B.L.J. A mechanistic classification of thoracolumbar spine fractures. Clin Orthop Oct. 1984;(189):77-88.
60. Ferguson R.L., McMasters M.C., Stanitski C.L. Low back pain in college football. J Bone Joint Surg. 1974;56:1300.
61. Field T., Hernandez-Reif M., Hart S., et al. Pregnant women benefit from massage therapy. J Psychosom Obstet Gynaecol. 1999;20(1):31-38.
62. Fishman L.M., Zybert P.A. Electrophysiologic evidence of piriformis syndrome. Arch Phys Med Rehabil. 1992;73(4):359-364.
63. Fortin J.D., Aprill C.N., Ponthieux B., et al. Sacroiliac joint: pain referral maps upon applying a new injection/arthrography technique. Part II. Clinical evaluation. Spine. 1994;19(13):1483-1489.
64. Fortin J.D., Dwyer A.P., West S., et al. Sacroiliac joint: pain referral maps upon applying a new injection/arthrography technique. Part I. Asymptomatic volunteers. Spine. 1994;19(13):1475-1482.
65. Francis R.M., Baillie S.P., Chuck A.J., et al. Acute and long-term management of patients with vertebral fractures. Q J Med. 2004;97(2):63-74.
66. Fujii K., Katoh S., Sairyo K., et al. Union of defects in the pars interarticularis of the lumbar. J Bone Joint Surg Br. 2004;86B(2):225-231.
67. Fujiwara A., Tamai K., Yamato M., et al. The relationship between facet joint osteoarthritis and disc degeneration of the lumbar spine: an MRI study. Eur Spine J. 1999;8(5):396-401.
68. Furlan A.D., Brosseau L., Imamura M., et al. Massage for low back pain. Cochrane Database Syst Rev. 2002;2:CD001929.
69. Gagnier J.J., van Tulder M.W., Berman B., et al. Herbal medicine for low back pain: a Cochrane review. Spine (Phila Pa 1976). 2007;32(1):82-92.
70. Gallagher R.M., Mossey J.M. Impact of co-morbid depression on self-reported pain and physical and emotional functioning in low back pain patients. Pain Med. 2001;2(3):242.
71. Garfin S.R., Rydevik B.L., Lipson S.J., et al. Spinal stenosis: pathophysiology. In Herkowitz H.N., Garfin S.R., Balderson R.A., et al, editors: Rothman Simeone: The spine, ed 4, Philadelphia: WB Saunders, 1999.
72. Gerrard G.E., Franks K.N. Overview of the diagnosis and management of brain, spine, and meningeal metastases. J Neurol Neurosurg Psychiatry. 2004;75(2):ii37-ii42.
73. Gibson E.S. The value of preplacement screening radiograph of low back pain. Spine: State of the Art Reviews. 1987;2:91-107.
74. Goodman J.E., McGrath P.J. The epidemiology of pain in children and adolescents: a review. Pain. 1991;46(3):247-264.
75. Gracovetsky S., Farfan H., Helleur C. The abdominal mechanism. Spine. 1985;10(4):317-324.
76. Greenman P.E. Pelvic girdle dysfunction. In Butler J.P., editor: Principles of manual medicine, ed 2, Baltimore, MD: Williams & Wilkins, 1996.
77. Gronblad M., Hurr H., Kouri J.P. Relationships between spinal mobility, physical performance tests, pain intensity and disability assessments in chronic low back pain patients. Scand J Rehabil Med. 1997;29(1):17-24.
78. Guanciale A.F., Dillin W.H., Watkins R.G. Back pain in children and adolescents. In Herkowitz H.N., Garfin S.R., Balderston R.A., et al, editors: The spine, ed 4, Philadelphia: WB Saunders, 1999.
79. Gutke A., Ostgaard H.C., Oberg B. Association between muscle function and low back pain in relation to pregnancy. J Rehabil Med. 2008;40(4):304-311.
80. Guyer L. Backpack = back pain. Am J Public Health. 2001;91:16-19.
81. Hadjistavropoulos H.D., LaChapelle D.L. Extent and nature of anxiety experienced during physical examination of chronic low back pain. Behav Res Ther. 2000;38(1):13-29.
82. Hansen A., Jensen D.V., Larsen E., et al. Relaxin is not related to symptom-giving pelvic girdle relaxation in pregnant women. Acta Obstet Gynecol Scand. 1996;75(3):245-249.
83. Harreby M., Neergaard K., Hesselsoe G., et al. Are radiologic changes in the thoracic and lumbar spine of adolescents risk factors for low back pain in adults? A 25-year prospective cohort study of 640 school children. Spine. 1995;20(21):2298-2302.
84. Harris I.E., Weinstein S.L. Long-term follow-up of patients with grade-III and IV spondylolisthesis. treatment with and without posterior fusion. J Bone Joint Surg Am. 1987;69(7):960-969.
85. Harte A.A., Baxter G.D., Gracey J.H. The efficacy of traction for back pain: a systematic review of randomized controlled trials. Arch Phys Med Rehabil. 2003;84(10):1542-1553.
86. Harvey C.J., Richenberg J.L., Saifuddin A., et al. Pictorial review: the radiologic investigation of lumbar spondylolysis. Clin Radiol. 1998;53:723-728.
87. Hayden J.A., van Tulder M.W., Tomlinson G. Systematic review: strategies for using exercise therapy to improve outcomes in chronic low back pain. Ann Intern Med. 2005;142(9):776-785.
88. Hee H.T., Wong H.K. The long-term results of surgical treatment for spinal stenosis in the elderly. Singapore Med J. 2003;44(4):175-1780.
89. Helms J.M. The basic, clinical, and speculative science of acupuncture: Acupuncture energetics—a clinical approach for physicians. Berkeley, CA: Medical Acupuncture Publishers; 1995.
90. Hicks G.E., Fritz J.M., Delitto A., et al. Interrater reliability of clinical examination measures for identification of lumbar segmental instability. Arch Phys Med Rehabil. 2003;84(12):1858-1864.
91. Hodges P.W. Core stability exercise in chronic low back pain. Orthop Clin North Am. 2003;34(2):245-254.
92. Hogeboom C.J., Sherman K.J., Cherkin D.C. Variation in diagnosis and treatment of chronic low back pain by traditional Chinese medicine acupuncturists. Complement Ther Med. 2001;9(3):154-166.
93. Hollingworth P. Back pain in children. Br J Rheumatol. 1996;35(10):1022-1028.
94. Jackson D.W., Wiltse L.L., Cirincoine R.J. Spondylolysis in the female gymnast. Clin Orthop Jun. 1976;(117):68-73.
95. Jacques P., Mielants H., De Vos M., et al. Spondyloarthropathies: Progress and challenges. Best Pract Res Clin Rheumatol. 2008;22(2):325-337.
96. Jarvik J.J., Hollingworth W., Heagerty P., et al. The longitudinal assessment of imaging and disability of the back (laidback) study: baseline data. Spine. 2001;26(10):1158-1166.
97. Jensen M.C., Brant-Zawadzki M.N., Obuchowski N., et al. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med. 1994;331(2):69-73.
98. Johnsson K.E., Rosen I., Uden A. The natural course of lumbar spinal stenosis. Clin Orthop Jun. 1992;(279):82-86.
99. Kalauokalani D., Sherman K.J., Cherkin D.C. Acupuncture for chronic low back pain: diagnosis and treatment patterns among acupuncturists evaluating the same patient. South Med J. 2001;94(5):486-492.
100. Kaplan B.S., Restaino I., Raval D.S., et al. Renal failure in the neonate associated with in utero exposure to non-steroidal anti-inflammatory agents. Pediatr Nephrol. 1994;8(6):700-704.
101. Karppinen J., Ohinmaa A., Malmivaara A., et al. Cost effectiveness of periradicular infiltration for sciatica: subgroup analysis of a randomized controlled trial. Spine. 2001;26(23):2587-2595.
102. Kataria R.K., Brent L.H. Spondyloarthropathies. Am Fam Physician. 2004;69(12):2853-2860.
103. Katz J.N., Lipson S.J., Chang L.C., et al. Seven- to 10-year outcome of decompressive surgery for degenerative lumbar spinal stenosis. Spine. 1996;21(1):92-98.
104. Kendall F.P., McCreary E.K. Trunk muscles in muscle testing and function. Philadelphia: Williams & Wilkins; 1983. p 194
105. Khan I.A., Vaccaro A.R., Zlotolow D.A. Management of vertebral diskitis and osteomyelitis. Orthopedics. 1999;22(8):758-765.
106. Khoromi S., Patsalides A., Parada S., et al. Topiramate in chronic lumbar radicular pain. J Pain. 2005;6(12):829-836.
107. Kihlstrand M., Stenman B., Nilsson S., et al. Water-gymnastics reduced the intensity of back/low back pain in pregnant women. Acta Obstet Gynecol Scand. 1999;78(3):180-185.
108. Kirkaldy-Willis W.H., Wedge J.H., Yong-Hing K., et al. Pathology and pathogenesis of lumbar spondylosis and stenosis. Spine. 1978;3(4):319-328.
109. Klein G., Mehlman C.T., McCarty M. Nonoperative treatment of spondylolysis and grade I spondylolisthesis in children and young adults: a meta-analysis of observational studies. J Pediatr Orthop. 2009;29(2):146-156.
110. Konlian C. Aquatic therapy: Making a wave in the treatment of low back pain. Orthop Nursing. 1999;18(1):11-20.
111. Krag M.H., Seroussi R.E., Wilder D.G., et al. Internal displacement distribution from in vitro loading of human thoracic and lumbar spinal motion segments: experimental results and theoretical predictions. Spine. 1987;12:1001-1007.
112. Kristiansson P., Svardsudd K., von Schoultz B. Back pain during pregnancy: a prospective study. Spine. 1996;21(6):702-709.
113. Kristiansson P., Svardsudd K., von Schoultz B. Serum relaxin, symphyseal pain, and back pain during pregnancy. Am J Obstet Gynecol. 1996;175(5):1342-1347.
114. Leboeuf-Yde C., Kyvik K.O., Bruun N.H. Low back pain and lifestyle. II. Obesity. Information from a population-based sample of 29,424 twin subjects. Spine. 1999;24(8):779-783. discussion 83–4
115. Levy H.I., Hanscom B., Boden S.D. Three-question depression screener used for lumbar disc herniations and spinal stenosis. Spine. 2002;27(11):1232-1237.
116. Liddle S.D., Baxter G.D., Gracey J.H. Exercise and chronic low back pain: What works? Pain. 2004;107(1-2):176-190.
117. Linton S.J. Psychological risk factors for neck and back pain. In: Nachemson A.L., Jonsson E., editors. Neck and back pain. Philadelphia: Lippincott Williams & Wilkins, 2000.
118. Linton S.J., van Tulder M.W. Preventive interventions for back and neck pain. In: Nachemson A.L., Johnsson B., editors. Neck and back pain: the scientific evidence of causes, diagnosis, and treatment. Philadelphia: Lippincott Williams & Wilkins, 2000.
119. Little P., Lewith G., Webley F., et al. Randomised controlled trial of Alexander technique lessons, exercise, and massage (ATEAM) for chronic and recurrent back pain. BMJ. 2008;337:a884.
120. Lutz G.E., Vad V.B., Wisneski R.J. Fluoroscopic transforaminal lumbar epidural steroids: an outcome study. Arch Phys Med Rehabil. 1998;79(11):1362-1366.
121. Macedo L.G., Maher C.G., Latimer J., et al. Motor control exercise for persistent, nonspecific low back pain: a systematic review. Phys Ther. 2009;89(1):9-25.
122. Mackenzie W.G., Sampath J.S., Kruse R.W., et al. Backpacks in children. Clin Orthop. 2003;409:78-84.
123. MacLennan A.H., Nicolson R., Green R.C., et al. Serum relaxin and pelvic pain of pregnancy. Lancet. 1986;2(8501):243-245.
124. Magee D.J. Hip. In: Magee D.J., editor. Orthopaedic physical assessment. Philadelphia: WB Saunders, 1992.
125. Magee D.J. Lumbar spine. Orthopedic physical assessment, ed 4. Philadelphia: Elsevier Science; 2002.
126. Maguire J.H. Osteomyelitis. In Braunwald E., Fauci A.S., Kasper D.L., et al, editors: Harrison’s principles of internal medicine, ed 5, New York: McGraw-Hill, 2001.
127. Main C.J., Waddell G. Beliefs about back pain. The back pain revolution. Edinburgh, UK: Churchill Livingstone; 2004.
128. Mannion A.F., Muntener M., Taimela S., et al. Comparison of three active therapies for chronic low back pain: results of a randomized clinical trial with one-year follow-up. Rheumatology (Oxford). 2001;40(7):772-778.
129. Mantle M.J., Holmes J., Currey H.L. Backache in pregnancy II: Prophylactic influence of back care classes. Rheumatol Rehabil. 1981;20(4):227-232.
130. Marks R.C., Houston T., Thulbourne T. Facet joint injection and facet nerve block: a randomized comparison in 86 patients with chronic low back pain. Pain. 1992;49(3):325-328.
131. Marshall L.L., Trethewie E.R., Curtain C.S. Chemical radiculitis: a clinical, physiological, and immunological study. Clin Orthop. 1979;129:61-67.
132. Martell B.A., O’Connor P.G., Kerns R.D., et al. Systematic review: opioid treatment for chronic back pain—prevalence, efficacy, and association with addiction. Ann Intern Med. 2007;146(2):116-127.
133. Masci L., Pike J., Malara F., et al. Use of the one-legged hyperextension test and magnetic resonance imaging in the diagnosis of active spondylolysis. Br J Sports Med. 2006;40(11):940-946. discussion 6
134. Maus T.P. Imaging of the spine and nerve roots. In: Kraft G.H., editor. Physical medicine and rehabilitation clinics of North America. Philadelphia: Saunders, 2002.
135. McCall I.W., Park W.M., O’Brien J.P. Induced pain referral from posterior lumbar elements in normal subjects. Spine. 1979;4(5):441-446.
136. McCarron R.F., Wimpee M.W., Hudkins P.G., et al. The inflammatory effect of nucleus pulposus: a possible element in the pathogenesis of low-back pain. Spine. 1987;12(8):760-764.
137. McGee S.R. Evidence-based physical diagnosis. Philadelphia: Saunders; 2001.
138. McGill S. Developing the exercise program. In Low back disorders: evidence-based prevention and rehabilitation. Champaign, IL: Human Kinetics; 2002.
139. McGill S. Lumbar spine stability: Myths and realities. In Low back disorders: Evidence-based prevention and rehabilitation. Champaign, IL: Human Kinetics; 2002.
140. McGill S. Normal and injury mechanics of the lumbar spine. In Low back disorders: Evidence-based prevention and rehabilitation. Champaign, IL: Human Kinetics; 2002.
141. McKenzie L., Sillence D. Familial Scheuermann disease: a genetic and linkage study. J Med Genet. 1992;29(1):41-45.
142. Mens J.M., Snijders C.J., Stam H.J. Diagonal trunk muscle exercises in peripartum pelvic pain: a randomized clinical trial. Phys Ther. 2000;80(12):1164-1173.
143. Micheli L.J., Wood R. Back pain in young athletes: significant differences from adults in causes and patterns. Arch Pediatr Adolesc Med. 1995;149(1):15-18.
144. Million R., Nilsen K.H., Jayson M.I., et al. Evaluation of low back pain and assessment of lumbar corsets with and without back supports. Ann Rheum Dis. 1981;40(5):449-454.
145. Milne S., Welch V., Brosseau L., et al. Transcutaneous electrical nerve stimulation (TENS) for chronic low back pain. Cochrane Database Syst Rev. 2001;2001(2):CD003008.
146. Mooney V., Robertson J. The facet syndrome. Clin Orthop Mar-Apr. 1976;(115):149-156.
147. Muehlbacher M., Nickel M.K., Kettler C., et al. Topiramate in treatment of patients with chronic low back pain: a randomized, double-blind, placebo-controlled study. Clin J Pain. 2006;22(6):526-531.
148. Nachemson A., Vingard E. Assessment of patients with neck and back pain: a best-evidence synthesis. In: Nachemson A.L., Johnsson B., editors. Neck and back pain: the scientific evidence of causes, diagnosis, and treatment. Philadelphia: Lippincott Williams & Wilkins, 2001.
149. Nachemson A.L. Disc pressure measurements. Spine. 1981;6(1):93-97.
150. Nachemson A.L. The lumbar spine: An orthopaedic challenge. Spine. 1976;1:59.
151. Nachemson A.L., Waddell G., Norlund A.I. Epidemiology of neck and low back pain. In: Nachemson A.L., Johnsson B., editors. Neck and back pain: the scientific evidence of causes, diagnosis, and treatment. Philadelphia: Lippincott Williams & Wilkins, 2000.
152. Nelemans P.J., de Bie R.A., de Vet H.C.W., et al. Injection therapy for subacute and chronic benign low-back pain (Cochrane review). Cochrane Database Syst Rev. 2000;(2):CD001824.
153. Newman P.H. The etiology of spondylolisthesis. J Bone Joint Surg Br. 1963;45:39-59.
154. Nibbelink D.W., Strickland S.C., McLean L.F., et al. Cyclobenzaprine, diazepam, and placebo in the treatment of skeletal muscle spasm of local origin. Clin Ther. 1978;1:409-424.
155. Nilsson-Wikmar L, Holm K, Oijerstedt R, et al. Proceedings of the Third Interdisciplinary World Congress on Low Back and Pelvic Pain; Vienna, 1998.
156. Noren L., Ostgaard S., Nielsen T.F., et al. Reduction of sick leave for lumbar back and posterior pelvic pain in pregnancy. Spine. 1997;22(18):2157-2160.
157. O’Sullivan P.B., Burnett A., Floyd A.N., et al. Lumbar repositioning deficit in a specific low back pain population. Spine. 2003;28(10):1074-1079.
158. O’Sullivan P.B., Phyty G.D., Twomey L.T., et al. Evaluation of specific stabilizing exercise in the treatment of chronic low back pain with radiologic diagnosis of spondylolysis or spondylolisthesis. Spine. 1997;22(24):2959-2967.
159. Old J.I., Calvert M. Vertebral compression fractures in the elderly. Am Fam Physician. 2004;69(1):111-116.
160. Ostelo R.W., de Vet H.C., Waddell G., et al. Rehabilitation following first-time lumbar disc surgery: a systematic review within the framework of the Cochrane collaboration. Spine. 2003;28(3):209-218.
161. Osterman K., Schlenzka D., Poussa M., et al. Isthmic spondylolisthesis in symptomatic and asymptomatic subjects, epidemiology, and natural history with special reference to disk abnormality and mode of treatment. Clin Orthop. 297(65-70), 1993.
162. Ostgaard H.C., Andersson G.B., Karlsson K. Prevalence of back pain in pregnancy. Spine. 1991;16(5):549-552.
163. Ostgaard H.C., Zetherstrom G., Roos-Hansson E. Back pain in relation to pregnancy: a 6-year follow-up. Spine. 1997;22(24):2945-2950.
164. Parke W.W. Applied anatomy of the spine. In Rothman R.H., Simeone F.A., editors: The spine, ed 4, Philadelphia: Saunders, 1999.
165. Parks K.A., Crichton K.S., Goldford R.J., et al. A comparison of lumbar range of motion and functional ability scores in patients with low back pain: Assessment for range of motion validity. Spine. 2003;28(4):380-384.
166. Patel R.V., DeLong W.Jr., Vresilovic E.J. Evaluation and treatment of spinal injuries in the patient with polytrauma. Clin Orthop May. 2004;(422):43-54.
167. Patel S.R., Benjamin R.S. Soft tissue and bone sarcomas and bone metastases. In Braunwald E., Fauci A.S., Kasper D.L., et al, editors: Harrison’s principles of internal medicine, ed 15, New York: McGraw-Hill, 2001.
168. Perret C., Poiraudeau S., Fermanian J., et al. Validity, reliability, and responsiveness of the fingertip-to-floor test. Arch Phys Med Rehabil. 2001;82(11):1566-1570.
169. Petrere J.A., Anderson J.A. Developmental toxicity studies in mice, rats, and rabbits with the anticonvulsant gabapentin. Fundam Appl Toxicol. 1994;23(4):585-589.
170. Physician, Desk Reference. Montvale, NJ: Thomson Healthcare; 2004.
171. Picavet H.S., Vlaeyen J.W., Schouten J.S. Pain catastrophizing and kinesiophobia: predictors of chronic low back pain. Am J Epidemiol. 2002;156(11):1028-1034.
172. Pneumaticos S.G., Chatziioannou S.N., Hipp J.A., et al. Low back pain: prediction of short-term outcome of facet joint injection with bone scintigraphy. Radiology. 2006;238(2):693-698.
173. Pomerantz S.R., Hirsch J.A. Intradiscal therapies for discogenic pain. Semin Musculoskelet Radiol. 2006;10(2):125-135.
174. Radcliff K.E., Kalantar S.B., Reitman C.A. Surgical management of spondylolysis and spondylolisthesis in athletes: indications and return to play. Curr Sports Med Rep. 2009;8(1):35-40.
175. Rantanen J., Hurme M., Falck B., et al. The lumbar multifidus muscle five years after surgery for a lumbar intervertebral disc herniation. Spine. 1993;18(5):568-574.
176. Ratliff J.K., Cooper P.R. Metastatic spine tumors. South Med J. 2004;97(3):246-253.
177. Richardson C., Jull G., Hodges P., et al. General considerations in motor control and joint stabilization: the basis of assessment and exercise techniques. Therapeutic exercise for spinal segmental stabilization in low back pain: scientific basis and clinical approach. Edinburgh,: Churchill Livingstone; 1999.
178. Richardson C., Jull G., Hodges P., et al. Traditional views of the function of the muscles of the local stabilizing system of the spine. therapeutic exercise for spinal segmental stabilization in low back pain: scientific basis and clinical approach. Edinburgh: Churchill Livingstone; 1999.
179. Riew K.D., Yin Y., Gilula L., et al. The effect of nerve-root injections on the need for operative treatment of lumbar radicular pain: a prospective, randomized, controlled, double-blind study. J Bone Joint Surg Am. 2000;82(11):1589-1593.
180. Robertson R.J., Caspersen C.J., Allison T.G., et al. Differentiated perceptions of exertion and energy cost of young women while carrying loads. Eur J Appl Physiol Occup Physiol. 1982;49(1):69-78.
181. Robinson L.R. Electromyography, magnetic resonance imaging, and radiculopathy: it’s time to focus on specificity. Muscle Nerve. 1999;22(2):149-150.
182. Russell M.D., Hanley E.N. Surgical management of lumbar spinal stenosis. In Herkowitz H.N., Garfin S.R., Balderson R.A., et al, editors: Rothman Simeone: the spine, ed 4, Philadelphia: WB Saunders, 1999.
183. Rydeard R., Leger A., Smith D. Pilates-based therapeutic exercise: effect on subjects with nonspecific chronic low back pain and functional disability: a randomized controlled trial. J Orthop Sports Phys Ther. 2006;36(7):472-484.
184. Rydevik B., Brown M.D., Lundborg G. Pathoanatomy and pathophysiology of nerve root compression. Spine. 1984;9(1):7-15.
185. Rydevik B., Holm S. Pathophysiology of the intervertebral disc and adjacent neural structures. In: Rothman R.H., Simeone F.A., editors. The spine. Philadelphia: WB Saunders, 1990.
186. Saal J.A., Saal J.S., Herzog R.J. The natural history of lumbar intervertebral disc extrusions treated nonoperatively. Spine. 1990;15(7):683-686.
187. Saal J.S., Franson R.C., Dobrow R., et al. High levels of inflammatory phospholipase a2 activity in lumbar disc herniations. Spine. 1990;15(7):674-678.
188. Sahrmann S.A. Concepts and principles of movement. Diagnosis and treatment of movement impairment syndromes. St. Louis: Mosby; 2002.
189. Sahrmann S.A. Movement impairment syndromes of the lumbar spine: diagnosis and treatment of movement impairment syndromes. St. Louis: Mosby; 2002.
190. Saraste H. Long-term clinical and radiological follow-up of spondylolysis and spondylolisthesis. J Pediatr Orthop. 1987;7(6):631-638.
191. Schmidt A.J. Cognitive factors in the performance level of chronic low back pain patients. J Psychosom Res. 1985;29(2):183-189.
192. Schnitzer T.J., Ferraro A., Hunsche E., et al. A comprehensive review of clinical trials on the efficacy and safety of drugs for the treatment of low back pain. J Pain Symptom Manage. 2004;28(1):72-95.
193. Schwarzer A.C., April C.N., Derby R., et al. Clinical features of patients with pain stemming from the lumbar zygapophysial joints: is the lumbar facet syndrome a clinical entity? Spine. 1994;19(10):1132-1137.
194. Schwarzer A.C., Wang S.C., Bogduk N., et al. Prevalence and clinical features of lumbar zygapophysial joint pain: a study in an Australian population with chronic low back pain. Ann Rheum Dis. 1995;54(2):100-106.
195. Sculco A.D., Paup D.C., Fernhall B., et al. Effects of aerobic exercise on low back pain patients in treatment. Spine J. 2001;1(2):25-101.
196. Seitsalo S. Operative and conservative treatment of moderate spondylolisthesis in young patients. J Bone Joint Surg Br. 1990;72(5):908-913.
197. Sengupta D.K., Herkowitz H.N. Lumbar spinal stenosis. Treatment strategies and indications for surgery. Orthop Clin North Am. 2003;34(2):281-295.
198. Shapiro S. Medical realities of cauda equina syndrome secondary to lumbar disc herniation. Spine. 2000;25(3):348-351.
199. Shbeeb M.I., Matteson E.L. Trochanteric bursitis (greater trochanter pain syndrome). Mayo Clin Proc. 1996;71(6):565-569.
200. Sherman K.J., Cherkin D.C., Erro J., et al. Comparing yoga, exercise, and a self-care book for chronic low back pain: a randomized, controlled trial. Ann Intern Med. 2005;143(12):849-856.
201. Shipherd J.C., Keyes M., Jovanovic T., et al. Veterans seeking treatment for posttraumatic stress disorder: what about comorbid chronic pain? J Rehabil Res Dev. 2007;44(2):153-166.
202. Simons D.G., Travell J.G. Myofascial pain syndromes. In: Melzack R., editor. Textbook of pain. New York: Churchill Livingstone, 1989.
203. Singh V., Derby R. Percutaneous lumbar disc decompression. Pain Physician. 2006;9(2):139-146.
204. Spangfort E.V. The lumbar disc herniation: a computer-aided analysis of 2,504 operations. Acta Orthop Scand. 1972;Suppl 142::1-95.
205. Spinhoven P., Ter Kuile M., Kole-Snijders A.M., et al. Catastrophizing and internal pain control as mediators of outcome in the multidisciplinary treatment of chronic low back pain. Eur J Pain. 2004;8(3):211-219.
206. Staiger T.O., Gaster B., Sullivan M.D., et al. Systematic review of antidepressants in the treatment of chronic low back pain. Spine. 2003;28(22):2540-2545.
207. Standaert C.J., Herring S.A. Expert opinion and controversies in musculoskeletal and sports medicine: core stabilization as a treatment for low back pain. Arch Phys Med Rehabil. 2007;88(12):1734-1736.
208. Storm P.B., Chou D., Tamargo R.J. Surgical management of cervical and lumbosacral radiculopathies: indications and outcomes. Phys Med Rehabil Clin N Am. 2002;13(3):735-759.
209. Stuge B., Hilde G., Vollestad N. Physical therapy for pregnancy-related low back and pelvic pain: a systematic review. Acta Obstet Gynecol Scand. 2003;82(11):983-990.
210. Sullivan M.S., Shoaf L.D., Riddle D.L. The relationship of lumbar flexion to disability in patients with low back pain. Phys Ther. 2000;80(3):240-250.
211. Taimela S., Kujala U.M., Salminen J.J., et al. The prevalence of low back pain among children and adolescents: a nationwide, cohort-based questionnaire survey in Finland. Spine. 1997;22(10):1132-1136.
212. Tali E.T. Spinal infections. European J Radiol. 2004;50(2):120-133.
213. Taurog J.D., Lipsky P.E. Ankylosing spondylitis, reactive arthritis, and undifferentiated spondyloarthropathy. In Braunwald E., Fauci A.S., Kasper D.L., et al, editors: Harrison’s principles of internal medicine, ed 15, New York: McGraw-Hill, 2001.
214. Tekur P., Singphow C., Nagendra H.R., et al. Effect of short-term intensive yoga program on pain, functional disability and spinal flexibility in chronic low back pain: a randomized control study. J Altern Complement Med. 2008;14(6):637-644.
215. Teplick J.G., Haskin M.E. Spontaneous regression of herniated nucleus pulposus. Am J Roentgenol. 1985;145(2):371-375.
216. Tertti M.O., Salminen J.J., Paajanen H.E., et al. Low-back pain and disk degeneration in children: a case-control MR imaging study. Radiology. 1991;180(2):503-507.
217. Travell J.G., Simons D.G. Apropos of all muscles: myofascial pain and dysfunction-the trigger point manual-the upper extremities, Vol 1. Baltimore, MD: Williams & Wilkins, 1983.
218. Travell J.G., Simons D.G. Background and principles. In Myofascial pain and dysfunction- the trigger point manual-the upper extremities. In vol 1. Baltimore, Md: Williams & Wilkins; 1983.
219. Troussier B., Marchou-Lopez S., Pironneau S., et al. Back pain and spinal alignment abnormalities in schoolchildren. Rev Rhum Engl Ed. 1999;66(7-9):370-830.
220. Tschirhart C.E., Nagpurkar A., Whyne C.M. Effects of tumor location, shape and surface serration on burst fracture risk in the metastatic spine. J Biomech. 2004;37(5):653-660.
221. Vad V.B., Bhat A.L., Lutz G.E., et al. Transforaminal epidural steroid injections in lumbosacral radiculopathy: a prospective randomized study. Spine. 2002;27(1):11-16.
222. van Poppel M.N., de Looze M.P., Koes B.W., et al. Mechanisms of action of lumbar supports: A systematic review. Spine. 2000;25(16):2103-2113.
223. van Tulder M., Malmivaara A., Esmail R., et al. Exercise therapy for low back pain: a systematic review within the framework of the Cochrane collaboration back review group. Spine. 2000;25(21):2784-2796.
224. van Tulder M.W., Esmail R., Bombardier C., et al. Back schools for non-specific low back pain. Cochrane Database Syst Rev. 2000;(2):CD000261.
225. van Tulder M.W., Goossens M., Waddell G., et al. Conservative treatment of chronic low back pain. In: Nachemson A.L., Jonsson E., editors. Neck and back pain. Philadelphia: Lippincott Williams & Wilkins, 2000.
226. Van Tulder M.W., Jellema P., van Poppel M.N., et al. Lumbar supports for prevention and treatment of low back pain. Cochrane Database Syst Rev. 2000;(3):CD001823.
227. van Tulder M.W., Scholten R.J., Koes B.W., et al. Nonsteroidal anti-inflammatory drugs for low back pain: a systematic review within the framework of the Cochrane collaboration back review group. Spine. 2000;25(19):2501-2513.
228. van Tulder M.W., Touray T., Furlan A.D., et al. Muscle relaxants for nonspecific low back pain: a systematic review within the framework of the Cochrane collaboration. Spine. 2003;28(17):1978-1992.
229. van Tulder M.W., Waddell G. Conservative treatment of acute and subacute low back pain. In: Nachemson A.L., Jonsson E., editors. Neck and back pain. Philadelphia: Lippincott Williams & Wilkins, 2000.
230. Verlaan J.J., Diekerhof C.H., Buskens E., et al. Surgical treatment of traumatic fractures of the thoracic and lumbar spine: a systematic review of the literature on techniques, complications, and outcome. Spine. 2004;29(7):803-814.
231. Vilensky J.A., O’Connor B.L., Fortin J.D., et al. Histologic analysis of neural elements in the human sacroiliac joint. Spine. 2002;27(11):1202-1207.
232. Viry P., Creveuil C., Marcelli C. Nonspecific back pain in children: a search for associated factors in 14-year-old schoolchildren. Rev Rhum Engl Ed. 1999;66(7-9):381-388.
233. Von Korff M., Dworkin S.F., Le Resche L., et al. An epidemiologic comparison of pain complaints. Pain. 1988;32(2):173-183.
234. Waddell G. Illness behavior, In The back pain revolution. Edinburgh: Churchill Livingstone; 2000.
235. Waddell G., Burton K. Information and advice for patients, In The back pain revolution. Edinburgh, UK: Churchill Livingstone; 2004.
236. Waddell G., Feder G., Lewis M. Systematic reviews of bed rest and advice to stay active for acute low back pain. Br J Gen Pract. 1997;47(423):647-652.
237. Waddell G., van Tulder M. Clinical guidelines: the back pain revolution. Edinburgh: Elsevier Science; 2000.
238. Waddell G., Waddell H. A review of social influences on neck and back pain and disability. In: Nachemson A.L., Jonsson E., editors. Neck and back pain. Philadelphia: Lippincott Williams & Wilkins, 2000.
239. Waddell G., Watson P.J. Rehabilitation, In The back pain revolution. Edinburgh: Churchill Livingstone; 2004.
240. Walker B.F. The prevalence of low back pain: a systematic review of the liter from 1966 to 1998. J Spinal Disord. 2000;13(3):205-217.
241. Waller B., Lambeck J., Daly D. Therapeutic aquatic exercise in the treatment of low back pain: a systematic review. Clin Rehabil. 2009;23(1):3-14.
242. Wang S.M., Dezinno P., Maranets I., et al. Low back pain during pregnancy: prevalence, risk factors, and outcomes. Obstet Gynecol. 2004;104(1):65-70.
243. Watson K.D., Papageorgiou A.C., Jones G.T., et al. Low back pain in schoolchildren: the role of mechanical and psychosocial factors. Arch Dis Child. 2003;88(1):12-17.
244. Weber H. Lumbar disc herniation. A controlled, prospective study with ten years of observation. Spine. 1983;8(2):131-140.
245. Wedenberg K., Moen B., Norling A. A prospective randomized study comparing acupuncture with physiotherapy for low-back and pelvic pain in pregnancy. Acta Obstet Gynecol Scand. 2000;79(5):331-335.
246. Weinreb J.C., Wolbarsht L.B., Cohen J.M., et al. Prevalence of lumbosacral intervertebral disk abnormalities on MR images in pregnant and asymptomatic nonpregnant women. Radiology. 1989;170(1 Pt 1):125-128.
247. Weinstein J.N., Lurie J.D., Tosteson T.D., et al. Surgical vs nonoperative treatment for lumbar disk herniation: the spine patient outcomes research trial (sport) observational cohort. JAMA. 2006;296(20):2451-2459.
248. Weinstein J.N., Lurie J.D., Tosteson T.D., et al. Surgical versus nonoperative treatment for lumbar disc herniation: four-year results for the spine patient outcomes research trial (sport). Spine (Phila Pa 1976). 2008;33(25):2789-2800.
249. Wiltse L.L., Winter R.B. Terminology and measurement of spondylolisthesis. J Bone Joint Surg Am. 1983;65(6):768-772.
250. Wittink H., Hoskins M.T., Wagner A., et al. Deconditioning in patients with chronic low back pain: fact or fiction? Spine. 2000;25(17):2221-2228.
251. Wittink H., Michel T.H., Kulich R., et al. Aerobic fitness testing in patients with chronic low back pain: which test is best? Spine. 2000;25(13):1704-1710.
252. Woby S.R., Watson P.J., Roach N.K., et al. Are changes in fear-avoidance beliefs, catastrophizing, and appraisals of control, predictive of changes in chronic low back pain and disability? Eur J Pain. 2004;8(3):201-210.
253. Yamane T., Yoshida T., Mimatsu K. Early diagnosis of lumbar spondylolysis by MRI. J Bone Joint Surg Br. 1993;75(5):764-768.
254. Yuan J., Purepong N., Kerr D.P., et al. Effectiveness of acupuncture for low back pain: a systematic review. Spine (Phila PA 1976). 2008;33(23):E887-E900.
255. Zuberbier O.A., Hunt D.G., Kozlowski A.J., et al. Commentary on the American Medical Association guides’ lumbar impairment validity checks. Spine. 2001;26(24):2735-2737.