37 Long-Term Complications and Management
Most patients who have undergone cardiac surgical procedures have brief stays in the intensive care unit (ICU; < 24 hours), and these stays typically follow a predictable pattern. During this time, most instability and morbidity are attributable to the cardiopulmonary organ systems, bleeding, hypothermia, and the emergence from anesthesia.1 A small minority of patients, however, have prolonged ICU stays, characterized by multisystem complications involving both the cardiac and noncardiac systems. This group of patients consumes a disproportionate number of ICU resources; generates enormous hospital costs; and, ultimately, has a much worse prognosis (both in-hospital and long term).1–3 For example, Bashour et al1 described a series of 2618 patients who had undergone cardiac operations, 5.4% of whom had an ICU length of stay (LOS) of longer than 10 days. In the prolonged LOS group, the in-hospital mortality rate was 33.1% (vs. 1.5% in a group with an LOS < 10 days). Of those patients with a prolonged ICU LOS who survived to discharge, 46.8% died within 30 months. Welsby et al2 analyzed types of complications in a cohort of patients who had undergone cardiac surgical procedures and who had a prolonged LOS in the ICU; they demonstrated that noncardiac complications are more deleterious than are isolated cardiac complications. In another series of 1280 patients, only 3.8% of patients had an ICU LOS longer than 14 days. The hospital mortality rate in the prolonged LOS group was 25.8% (vs. 5.3% in a short LOS group), and only 62% of patients discharged after an extended stay in the ICU were alive at 2 years.3
Sedation in the intensive care unit
Most patients who have an extended LOS in the ICU will require a different approach to sedation and pain control than do patients who do not have an extended LOS. The major goals of sedation in the ICU are to provide anxiolysis and to improve the patient’s perceptual experience during this physiologically and emotionally stressful period (Box 37-1).4 Secondarily, sedation reduces the physiologic stress response and attendant cardiovascular work, may facilitate the maintenance of circadian rhythms, and lessens delirium and agitation.5 These goals are distinct from those associated with analgesia, which are the alleviation of pain through nonpharmacologic and pharmacologic means and to facilitate diagnostic and therapeutic procedures.4 Although sedation and analgesia are separate therapeutic goals usually provided by individual drugs, combining anxiolytic and analgesic drugs often results in a synergistic effect, and some newer agents provide elements of both analgesia and anxiolysis, thus blurring the distinction in clinical practice (see Chapter 38).
BOX 37-1 Sedation Goals
In 1995, the Society of Critical Care Medicine (SCCM) published guidelines for sedation in the ICU,6 revised the guidelines in 2002,7 and is in the process of again updating the guidelines (Michael J. Murray, MD, PhD, Personal Communication, December 2010). The guidelines emphasize the need for the goal-directed delivery of psychoactive medications. Using goal-directed sedation is supported by an increasing body of literature that shows that daily interruption of sedation, intermittent sedation, and sedation protocols all reduce the duration of mechanical ventilation and, in some instances, decrease ICU LOS.8
Sedation Scoring Systems
Several scoring systems are available to assess a patient’s degree of sedation in the ICU and to facilitate goal-directed therapy. When using the seven-item Riker Sedation-Agitation Scale,9 which was the first scale proved to be reliable and valid for use in critically ill adults, the clinician assigns a score based on the patient’s behavior. The Motor Activity Assessment Scale10 includes seven categories to describe patients’ behavior in response to stimulation. Like the Sedation-Agitation Scale, it has been validated in critically ill adults.
Most comparative clinical studies of sedation in critically ill patients have used the Ramsay Scale.11 Scoring with this six-point scale of motor activity ranges from 1 (patient anxious, agitated or restless, or both) to 6 (no response to light glabellar tap or loud auditory stimulus; Table 37-1).5 Originally designed as a research tool, the Ramsay Scale has been used for decades in clinical practice.
| The patient |
Other sedation scales that have been validated in critically ill adults include the Vancouver Interaction and Calmness Scale,12 the COMFORT scale,13 the Richmond Agitation-Sedation Scale,14,15 and the Minnesota Sedation Assessment Tool.16 Increasing recognition of the long-term sequelae of delirium has led to the development and use of other scoring systems. The Confusion Assessment Method for the ICU (CAM-ICU) recently was validated in one ICU study and it is increasingly being used.17
More objective parameters to assess sedation are being pursued. The bispectral index is a monitor that measures a simple, three-lead, frontal-montage that, through proprietary software, converts the electroencephalographic activity to a digital scale from 1 to 100. When specific sedative agents are used, the bispectral index reading typically correlates well with the level of sedation in patients in the ICU18 (see Chapter 16).
Sedative Agents
Benzodiazepines
Midazolam, a short-acting, water-soluble benzodiazepine, can only be given parenterally. Its intravenous administration causes no pain or venous irritation (and, therefore, thrombosis), and its potency is two to four times that of diazepam. Midazolam is readily redistributed in tissues and is rapidly cleared by the liver and kidneys. It is enzymatically degraded in the liver to α-hydroxy-midazolam, which has minimal, if any, clinical sedative or hypnotic effects. The clinical effects of midazolam are short-lived because of an elimination half-life of 1.5 to 3.5 hours. These properties make midazolam ideal as an anxiolytic benzodiazepine for short-term use in the ICU.19 Depending on the situation, intermittent boluses of midazolam can be given, or a continuous infusion of 0.5 to 5.0 mg/hr can be used. Greater doses may be required, and infusions of up to 20 mg/hr have been used safely in mechanically ventilated patients.20
Lorazepam is a long-acting agent with sedative effects that last 6 to 8 hours after a single dose. Lorazepam has minimal effect on cardiovascular and respiratory function and can be given orally or parenterally.21 Because its potency is two to four times that of midazolam, the dose must be adjusted accordingly. Because of the long half-life of lorazepam and despite the fact that its time of onset of action is considerably longer than that of midazolam, the use of lorazepam is preferred to midazolam for patients requiring long-term sedation.6 Because of different volumes of distribution, elimination half-lives, and so on, especially in patients with multiple organ dysfunction syndrome and in critically ill patients who require benzodiazepines for longer than 24 hours, lorazepam has a clinical half-life similar to that of midazolam.22
If a benzodiazepine is used to manage the sedation of patients in the ICU, the benzodiazepine antagonist, flumazenil, should be readily available.23 Flumazenil is a highly specific benzodiazepine antagonist that reverses all known central nervous system effects of the benzodiazepines. It reaches maximum concentration in the brain within 5 to 10 minutes after intravenous administration. The mean terminal half-life of flumazenil is approximately 1 hour. It is completely metabolized to free carboxylic acid and glucuronide, both of which are inactive metabolites and renally excreted.
Propofol
Propofol, an intravenously administered alkyl-phenol anesthetic agent that is chemically unrelated to other anesthetic agents, has been used to provide sedation for patients in ICUs and is the preferred drug for “fast-tracking” patients. Because it is so hydrophobic, it is formulated in a lipid emulsion (10% Intralipid), which must be taken into account when it is administered to patients. It is short-acting and rapidly redistributed and metabolized, making it suitable for use as a continuous infusion. Because it allows for a rapid recovery and has a favorable side-effect profile, propofol often is used not only to sedate patients but also for use in certain procedures such as cardioversions, chest tube insertions or withdrawal, and pleurodesis (see Chapter 9).
Compared with midazolam, propofol allows for more rapid weaning of patients from mechanical ventilation; because of this property, propofol is used more commonly for fast-tracking patients after cardiac operations.24 When used for sedation, propofol should be administered as an initial dose of 0.5 to 1.0 mg/kg followed by an infusion of approximately 25 to 100 μg/kg/min. Because case reports have recounted mortality in patients who received excessively high doses of propofol, the maximum dose should probably be less than 100 μg/kg/min.23,25 Propofol infusion syndrome has been reported to occur in patients who have received propofol for a very short period, indicating that this may be an idiosyncratic reaction.26
When given by bolus administration, propofol may cause a decrease in mean arterial pressure because of peripheral vasodilation, not direct myocardial depression. Reports have been published of death with the use of propofol in both adults and children.27 The U.S. Food and Drug Administration requires the following boxed warning on product labeling for propofol, “While causality has not been established, Diprivan injectable emulsion is not indicated for sedation in pediatric patients until further studies have been performed to document its safety in that population.”
Myoclonic activity versus frank seizures has been reported to occur in patients receiving propofol,28 and individuals have become chemically dependent on propofol. These side effects, however, are rare (with the exception of the hypotension after bolus infusion) and should not limit the use of propofol in patients after cardiac surgical procedures, particularly given the effectiveness of propofol in fast-tracked patients. Studies have specifically addressed the issue of sedation in patients after cardiac operations; most of the studies compared midazolam with propofol and, in general, showed no specific advantage of one agent over another.29,30
Dexmedetomidine
Significant interest has been shown in using the α2-receptor agonist dexmedetomidine for postoperative cardiac sedation. α2-Receptor agonists bind to noradrenergic receptors in the brain, spinal cord, and elsewhere in the body. The benefit with the use of these agents is that sedation is provided with supplemental analgesic effects without respiratory depression. Dexmedetomidine was compared with benzodiazepines for the sedation of all groups of critically ill patients in four separate randomized, controlled trials.9,31–33 The findings of the Safety and Efficacy of Dexmedetomidine Compared with Midazolam (SEDCOM) study group9 revealed that dexmedetomidine-treated patients experienced less time on mechanical ventilation, less tachycardia, and less hypertension, with the most common adverse effect being bradycardia. The Maximizing Efficacy of Targeted Sedation and Reducing Neurological Dysfunction (MENDS) group showed similar findings, with a longer duration of delirium for those treated with lorazepam, as compared with patients treated with dexmedetomidine.33
The results of trials specific to patients after cardiac surgery have not been as strong. Dexmedetomidine was used in a fast-track setting without a shorter duration on the ventilator, compared with opioids, at a $50-per-patient greater cost.31 The dexmedetomidine-treated group did have a lower incidence of postoperative delirium and reduction in opioid requirements.31 Herr et al32 conducted a multicenter trial comparing dexmedetomidine and propofol for sedation after coronary artery bypass grafting. They found no significant difference between groups in time to extubation, but the dexmedetomidine-treated patients required significantly fewer supplemental analgesics, antiemetics, epinephrine, and diuretics. In a more recent multicenter study of 356 patients in the ICU, the overall costs associated with the use of dexmedetomidine, compared with midazolam, were lower because the dexmedetomidine-treated patients had a shorter LOS in the ICU.34 After examining the literature applying to all critical care patients, not only those after cardiac operations, the committee that developed the SCCM sedation guidelines made multiple recommendations (Table 37-2; these recommendations predate the introduction of dexmedetomidine).7
TABLE 37-2 Society for Critical Care Medicine Sedation Guidelines
Neuromuscular Blocking Agents
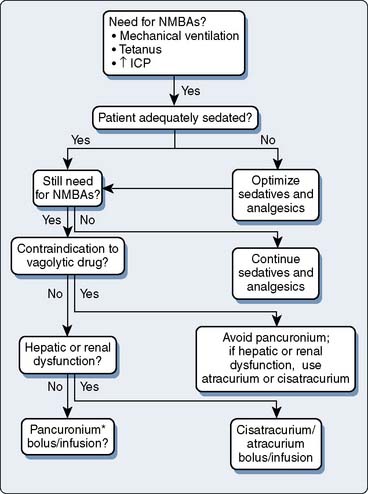
Nondepolarizing NMBAs are divided into two classes: benzylisoquinolinium compounds and the aminosteroid compounds. Both classes of drugs can be used to chemically paralyze a patient in the ICU, which occasionally is required to intubate the trachea of a patient with acute respiratory failure.35 Patients in the ICU who are receiving NMBAs must be mechanically ventilated; they typically have acute lung injury or acute respiratory distress syndrome that requires mechanical ventilation but that cannot be appropriately managed without the use of an NMBA.36
If NMBAs are used, it cannot be overemphasized that the patient must be adequately sedated before the initiation of the NMBA—it has been several decades since reports first appeared of chemically paralyzed awake patients,37 but it is a situation that all individuals working in an ICU must strive to avoid. Once an adequate degree of sedation (usually to include an analgesic medication such as an opioid) is achieved, the patient is administered a bolus and then a continuous infusion of an NMBA. Although several NMBAs are available, the drugs most commonly used in the ICU are the aminosteroid compounds (e.g., rocuronium) and the benzylisoquinolinium compounds (e.g., cisatracurium). Because these drugs are infused continuously, the duration of action is not as important as if they were given as a single bolus, but duration of action does become of consequence when the medication is discontinued and the physician is assessing the return of the patient’s neuromuscular function. When infusing these medications, a twitch monitor should be used, and the physician should strive to achieve a train-of-4 of one or two twitches.38 If no twitches are observed, the patient may have received an overdose of medication and may be at risk for development of acute quadriplegic myopathy syndrome (AQMS), a situation that develops in patients receiving NMBAs in which, when the medication is discontinued, the patient remains flaccid for much longer than would be predicted simply based on the pharmacokinetics of the medications that were infused.39 The cause of this syndrome is unknown but is most likely secondary to the destruction of myosin by the NMBA or one of its metabolites. Differentiating between AQMS and critical illness polyneuropathy often is difficult, but in the latter, profound muscle necrosis—as is seen with AQMS—would not be expected to occur. Bolus administration of NMBAs, when tolerated, is advantageous for monitoring the effects of sedation and analgesia, as well as decreasing the incidence of tachyphylaxis.38
Another way to minimize the incidence of AQMS is to institute a daily drug holiday. Not only is this beneficial in decreasing the incidence of AQMS, but in patients receiving opioids and benzodiazepines, the incidence of drug withdrawal also decreases. When using NMBAs in the ICU, following the algorithm listed in Figure 37-1 is recommended.38
Infections in patients in the intensive care unit
Microbiologic infections are some of the most common and frequently serious secondary complications that plague patients in the ICU.40 The following is a brief review of the most frequent infectious complications occurring in critically ill patients after cardiothoracic operations (Box 37-2). Pneumonia and acute respiratory distress syndrome are reviewed in Chapter 35.
Intravascular Device–Related Infections
Virtually all adult patients having cardiac operations are monitored with invasive intravascular devices (IVDs), such as arterial, central venous, and pulmonary artery catheters (see Chapter 14). Unfortunately, patients who have IVDs in place frequently acquire bloodstream infections (BSIs). IVD-related BSIs are associated with an attributable mortality rate of 12% to 15%, prolonged hospitalization (mean of 7 days), and an increased hospital cost of approximately $35,000.41,42
Approximately 90% of all IVD-related BSIs occur with the short-term use of IVDs.43 IVDs that are present for a short period are most commonly colonized from the skin surrounding the insertion site.44,45 Organisms migrate along the external surface of the catheter and then the intercutaneous and subcutaneous segments, leading to colonization of the intravascular segment of the catheter.46,47 Once the catheter is colonized, it is difficult to eradicate organisms from the intravascular segment without catheter removal because the microbes adhere to and are covered by either a biofilm layer that they produce or the thrombin layer that the host forms on the device.48 Because the skin is the most common site of colonization, coagulase-negative staphylococci and Staphylococcus aureus from the host’s skin and the hands of hospital personnel caring for the patient are the most common infecting pathogens.49 However, with the long-term use of IVDs, contamination of the catheter hub also contributes to intraluminal colonization.46,47
Several factors have been associated with an increased risk for patients acquiring IVD-related bacteremia. These factors include site of insertion (femoral > internal jugular > subclavian), number of lumens (multiple > single), duration of catheter in situ, established infection elsewhere in the body, the presence of bacteremia, and the experience of the personnel placing the catheter. Although some practitioners support the use of peripherally inserted IVDs, the risk for patients acquiring BSIs approaches that of patients with short centrally placed catheters.50 In an effort to reduce the incidence of IVD-related BSIs, a Centers for Disease Control and Prevention advisory committee formulated pertinent evidence-based guidelines51:
These guidelines are summarized in Box 37-3.
BOX 37-3 Central Venous Catheter Management
The diagnosis of IVD-related BSI can be challenging. The diagnosis should be suspected in patients with evidence of infection (e.g., fever, leukocytosis, positive blood cultures) when another source is not evident. Careful inspection of the catheter site is warranted; the presence of exit-site erythema or purulence strongly supports the diagnosis. If the patient has no visible signs of infection, clinical suspicion and supporting data must be used to guide therapy. The most frequently used technique to culture IVDs is the semiquantitative roll-plate technique. With this technique, the most common threshold to define colonization is growth of at least 15 colony-forming units (cfu).48,52 Siegman-Igra and colleagues49 and others53,54 have shown that quantitative culture of sonicated catheters is superior to the roll-plate technique. However, because these techniques require catheter removal, paired concomitant qualitative blood cultures are drawn through the device and percutaneously. IVD-related BSI is diagnosed if both cultures are positive for the presence of microorganisms and the concentration of microorganisms from the device is twofold to fivefold greater than the concentration from the peripherally drawn culture.55
The first clinical decision to make when managing a suspected IVD-related BSI is to remove the catheter or leave it in place. This decision is influenced by whether the risk for IVD-related BSI is low, intermediate, or high. Risk, in turn, is determined by the infecting organism and whether the IVD-related BSI is complicated or uncomplicated. Complicated infections are those associated with shock; the persistence of positive blood cultures for longer than 48 hours after appropriate antibiotics are administered; and IVD-related BSIs associated with septic thrombosis, septic emboli, or deep-seated infections (e.g., endocarditis or a tunnel or port-pocket infection46,47; Figure 37-2). A low-risk IVD-related BSI is caused by organisms of low virulence (e.g., coagulase-negative staphylococci) in a patient without complications of infection. A moderate-risk IVD-related BSI is characterized by uncomplicated infections with moderate to highly virulent organisms (S. aureus, Candida species). A high-risk IVD-related BSI is a complicated IVD-related BSI.
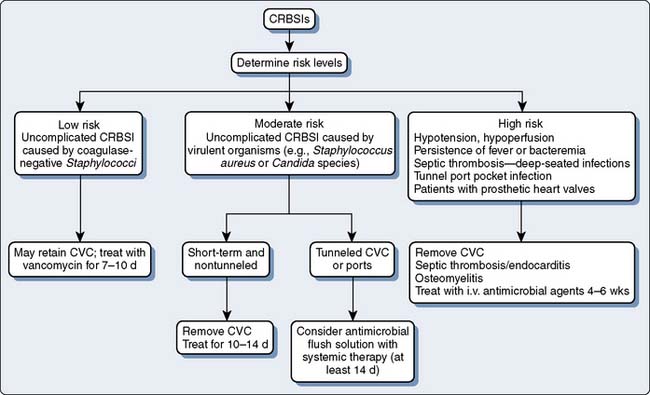
Figure 37-2 Algorithm for the management of intravascular catheter-related bloodstream infections (CRBSIs).
(Redrawn from Raad II, Hanna HA: Intravascular catheter-related infections. New horizons and recent advances. Arch Intern Med 162:871, 2002, by permission of the American Medical Association.)
Low-risk IVD-related BSIs can be treated without catheter removal.46,47 However, catheters should be removed in low-risk patients with prosthetic heart valves. In intermediate-risk patients, the catheters should be removed and the patients treated with a 10- to 14-day course of antibiotics. In high-risk patients, catheters should be removed and the duration of antibiotic use should be based on the nature of the complication. In deep-seated infections, such as septic thrombosis or endocarditis, antimicrobials should be administered for 4 to 6 weeks.56
Sternal Wound Infections
Deep and superficial surgical site infections occur infrequently but are morbid complications after cardiac operations, with an incidence rate of approximately 1% to 4%.57 Deep sternal infections are defined as those infections involving muscle and fascial layers, any other organ spaces manipulated during the operation, or organ involvement.58 They are associated with a 250% greater mortality rate, as compared with matched individuals without infection, and postoperative wound infections double the length of hospitalization.59 A host of preoperative, intraoperative, and postoperative risk factors have been identified for chest wall infections (Table 37-3).57–63
| Host Factors | Surgical Factors | Postoperative Factors |
|---|---|---|
| Obesity, diabetes mellitus, hyperglycemia, use of internal mammary artery grafts (especially bilateral), advanced age, male sex, COPD, smoking, prolonged mechanical ventilation, steroids, preoperative hospital stay longer than 5 days | Duration of surgery and bypass, use of IABP balloon pump, postoperative bleeding, reoperation, sternal rewiring, extensive electrocautery, shaving with razors, and use of bone wax | Postoperative bleeding, prolonged ventilation, chest re-exploration, blood transfusion, and use of an IABP |
COPD, chronic obstructive pulmonary disease; IABP, intra-aortic balloon pump.
The diagnosis of sternal infections is based on the presence of wound tenderness, drainage, cellulitis, fever, leukocytosis, and sternal instability.60 S. aureus and coagulase-negative staphylococci account for approximately 50% of the organisms causing sternal wound infections after coronary artery bypass graft procedures.58 Several preventive strategies have been proposed to reduce the rates of surgical-site infection after cardiac operations. Martorell et al64 reported a reduction in the incidence rate of chest wall infections from greater than 8% to less than 2% after an intensive surveillance and intervention program that included the nasal application of mupirocin and having the patient shower before surgery with chlorhexidine. Other variables that are being investigated to reduce infection rates include perioperative antibiotic timing and redosing, adequacy of glycemic control, perioperative temperature control, and conservative transfusion protocols.65 The treatment of mediastinitis involves the prompt institution of antibiotics (empirically covering staphylococci species before obtaining culture results), debridement, open packing, and frequent dressing changes. On resolution, the chest is closed by primary closure or flap transposition in patients with large chest wall defects.60
Prosthetic Valve Endocarditis
Prosthetic valve endocarditis (PVE)—the infection of a prosthetic heart valve, the surrounding cardiac tissues, or both the valve and surrounding tissue—is a rare but serious source of infection in patients after cardiac surgery.65 The incidence rate of PVE is between 0.3% and 0.8% after valve-replacement operations.66–70 S. aureus is the most common organism related to PVE; timing of infection can be helpful to illustrate the pathophysiology and causative pathogen. PVE cases can be clustered into two groups according to the time of infection. In early PVE (within 2 months of valve implantation), the valve and sewing ring have not yet endothelialized; hence microorganisms frequently invade the surrounding tissue planes, causing perivalvular abscess and perivalvular leak. In early PVE, the responsible microorganisms are nosocomial pathogens, such as staphylococci, gram-negative bacilli, and Candida species, which are introduced at the time of the operation or are hematogenously seeded in the immediate postoperative period. The pathophysiology of late PVE probably resembles that of native-valve endocarditis; that is, platelet-fibrin thrombi form on the valve leaflet and are then hematogenously seeded during episodes of transient bacteremia. In late PVE, the infecting organisms are usually streptococci, S. aureus, enterococci, and fastidious gram-negative organisms (the HACEK group).71–75 Infection appears to occur with equal frequency in both the mitral and aortic valves and is exceedingly rare in tricuspid prostheses (excluding intravenous drug abusers).67
In the ICU, cases of early PVE present more dramatically than do either native-valve endocarditis or late PVE. The clinical signs that suggest PVE include new or changing murmurs, congestive heart failure, new electrocardiographic conduction disturbances, and the presence of systemic emboli. In fact, 40% of patients have clinically apparent central nervous system emboli.76–79 The diagnosis is confirmed by positive results of blood cultures and findings on transesophageal echocardiography (TEE). If blood cultures are obtained before institution of antibiotic therapy, more than 90% of culture results will be positive. Transesophageal echocardiography is the diagnostic imaging modality of choice because it has a sensitivity of 82% to 96%, as compared with 17% to 36% with transthoracic echocardiography.80,81 Transesophageal echocardiography also allows the detection of abscesses, fistulas, and perivalvular leaks.82 The treatment of early PVE involves the administration of antibiotics directed at the cultured organism and prompt surgical intervention in cases of complicated PVE (Table 37-4).83 Mortality is related to older age, S. aureus infection, and patients with persistent bacteremia with the occurrence of septic shock.84,85 In complicated PVE, survival is improved with the institution of both medical and surgical therapy versus medical therapy alone.77,86,87
TABLE 37-4 Indications for Surgical Intervention in Patients with Prosthetic Valve Endocarditis
| Valvular regurgitation with or without heart failure |
| Uncontrollable infection* caused by: |
| Periannular extension |
| Difficult-to-treat microorganisms |
| Abscess |
| Large vegetation with high risk for embolization or recurrent emboli |
* Uncontrollable infection includes persistent fever and positive results of blood culture.
Systemic Inflammatory Response Syndrome and Sepsis
Systemic inflammatory response syndrome (SIRS) and sepsis are part of a spectrum of disorders that produce dysfunction in a variety of organ systems and arise from a combination of tissue injury and the host’s response to that injury. In 1992,88 1997,89 and 2001,90 the American College of Chest Physicians, the SCCM, the European Society of Intensive Care Medicine, the American Thoracic Society, and the Surgical Infection Society published consensus statements to clarify the definitions used to describe this spectrum. In 1991, the consensus statement introduced the now widely used term SIRS to describe a syndrome with multiple, clinically evident, organ-system effects (e.g., fever, oliguria, mental-status changes) that occurred as the result of the body’s inflammatory response activated by a variety of causes.88 Historically, it had been thought that infection, particularly gram-negative infection, was the sole source of this clinical phenomenon.91,92 It is now accepted that any major tissue injury (burns, sterile pancreatitis, major trauma) can precipitate such a dramatic inflammatory response with or without the presence of infection.91,92 However, in the most recent consensus statement, the authors acknowledge that, although SIRS exists, it is too broad and lacks the specificity to make it a useful, working “diagnosis” at this time.90
Sepsis is defined as the clinical syndrome that occurs as the result of an infection (or suspected infection) and an inflammatory response (Table 37-5).90,92 Severe sepsis is associated with organ dysfunction, hypoperfusion, or hypotension. Septic shock includes sepsis-induced hypotension and organ-perfusion abnormalities that persist despite fluid resuscitation.88 A detailed description of the current understanding of the pathophysiology of SIRS and sepsis is beyond the scope of this chapter; see Chapter 8 and reviews of this topic93–97 for more detailed discussions.
| Documented or suspected infection plus some of the following:a |
a Defined as a pathologic process induced by a microorganism.
b Defined as core temperature > 38.3° C.
c Defined as core temperature < 36° C.
d Defined as > 90 beats/min or > 2 standard deviations (SDs) above the normal value for age.
e Defined as > 20 mL/kg over 24 hours.
f Defined as a plasma glucose concentration > 120 mg/dL or 7.7 mmol/L.
g Defined as a white blood cell (WBC) count > 12,000/μL.
h Defined as a WBC count < 4000/μL.
i Defined as > 2 SDs above the normal value.
j Defined as a systolic blood pressure (SBP) < 90 mm Hg, mean arterial pressure < 70, or a decrease in SBP > 40 mm Hg in adults or < 2 SDs below normal for age.
k Defined as a Pao2/FIo2 < 300.
l Defined as urine output < 0.5 mL/kg/hr.
m Defined as an international normalized ratio (INR) > 1.5 or activated partial thromboplastin time (aPTT) > 60 seconds.
n Defined as platelet count < 100,000/μL.
o Defined as plasma total bilirubin concentration > 4 mg/dL or 70 mmol/L.
Modified from Levy MM, Fink MP, Marshall JC, et al: 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 31:1250, 2001.
Sepsis is the leading reason for admission to surgical ICUs and, despite recent advances in therapy, remains the leading cause of mortality in ICUs.88,92,96 The mortality rate increases across the inflammatory spectrum from SIRS to septic shock.40,98,99 Because of the unacceptably high mortality rate associated with sepsis and the inflammatory disorders, an international group of experts in sepsis convened in 2003 and launched the “surviving sepsis campaign” with the goal of producing treatment recommendations that could be used to reduce the mortality from sepsis.99–101 These recommendations (summarized in Table 37-6) were formulated from an evidence-based review of the medical literature and expert opinion when high-level evidence was absent. They reflect the “state of the art” in the management of critically ill patients with sepsis in 2008 (when the recommendations were last revised). Adoption of the recommendations set forth in these guidelines is associated with improved outcome.102
TABLE 37-6 Recommendations from the Surviving Sepsis Campaign
| 1. | Initial Resuscitation—first 6 hours |
| Resuscitation should begin as soon as possible—do not delay for ICU admission. | |
| The goals of resuscitation include: | |
| CVP: 8–12 mm Hg (12–15 mm Hg in mechanically ventilated patients or if high abdominal pressure) | |
| MAP: > 65 mm Hg | |
| Urine output: > 0.5 mL/kg/hr | |
| Svo2 or Scvo2 > 70% If unable to increase Svo2 or Scvo2 > 70% with ventilation, transfuse to hematocrit > 30%, administer dobutamine (to a maximum dose of 20 μg/kg/min), or both transfuse and administer dobutamine. |
|
| 2. | Diagnosis |
| Always obtain appropriate cultures before initiating antibiotics. | |
| Obtain at least two blood cultures—one from peripheral and one from each vascular access device. | |
| Culture other sitesa as clinically indicated. | |
| Promptly perform diagnostic and imaging studies to identify the source of infection. | |
| 3. | Antibiotic Therapy |
| IV antibiotic therapy should be started within the first hour of recognition of severe sepsis.b | |
| Empiric antibiotics should include one with activity against the most likely pathogen and that can penetrate the likely source. | |
| Empiric antibiotics should be broad spectrum until the causative organism is identified. | |
| The antibiotic regimen should be reassessed after 48 to 72 hours on the basis of clinical and microbiologic data. The goal is to use a narrow-spectrum antibiotic to limit toxicity, cost, and development of superinfection and microbial resistance. | |
| Once the causative organism is identified, use monotherapy for 7–10 days.c | |
| Most experts use combination therapy for neutropenic patients and treat for the duration of neutropenia | |
| 4. | Source Control |
| Every patient should be evaluated for the presence of a focus of infection that is amenable to source-control measures.d | |
| Risks vs. benefits of source-control procedures must be weighed; source control with the least physiologic insult generally is best. | |
| Source-control measures should be instituted as soon as possible after initial resuscitation. | |
| 5. | Fluid Therapy |
| Fluid challenge with crystalloids or colloids in cases of suspected hypovolemia | |
| Repeat boluses based on responsef and tolerance g | |
| No evidence to support preference of crystalloid or colloid | |
| 6. | Inotropic Therapy |
| In patients with low CO despite fluid resuscitation, dobutamine is the agent of choice to increase CO. | |
| A vasopressor can be added to increase BP, once CO is normalized. | |
| 7. | Steroids |
| IV corticosteroidsh can be used for patients with septic shock who require vasopressor therapy despite adequate fluid resuscitation. | |
| Some experts recommend using a 250-μg ACTH stimulation test to identify respondersi and only treat nonresponders. | |
| Dose should not exceed 300 mg hydrocortisone daily. | |
| Some experts add 50 μg fludrocortisone orally.j | |
| No evidence to recommend fixed-duration therapy over taper or clinically guided regimen. | |
| 8. | rhAPCl |
| The use of rhAPC is recommended in patients with high risk for death and who do not have any contraindications. | |
| Contraindications to the use of rhAPC: | |
| Active internal bleeding | |
| Recent, within 3 months, hemorrhagic stroke | |
| Recent, within 2 months, intracranial or intraspinal surgery or severe head trauma | |
| Trauma with increased risk for life-threatening bleeding | |
| Presence of epidural catheter | |
| Intracranial neoplasm, mass lesion, or cerebral herniation | |
| 9. | Blood Product Administration |
| Once tissue hypoperfusion has resolved, pRBC transfusion should only occur when hemoglobin decreases to < 7.0 g/dL and target a hemoglobin of 7.0–9.0 g/dL. | |
| Erythropoietin is not recommended to treat anemia of sepsis.m | |
| FFP should not be administered to correct laboratory abnormalities in the absence of bleeding or planned invasive procedures. | |
| Antithrombin administration is not recommended to treat severe sepsis or septic shock. | |
| Platelet transfusion | |
| Administer if counts < 5000/mm3 | |
| Consider when counts 5000–30,000/mm3 and the patient has a significant risk for bleeding. | |
| Counts > 50,000/mm3 are typically required for surgery or invasive procedures. | |
| 10. | Mechanical Ventilation of SI-ALI/ARDS |
| Use low Vt (6 mL/kg) with goal of plateau pressure less than 30 cm H2O. | |
| Permissive hypercapnia is tolerated to minimize plateau pressures and Vt. | |
| A minimum amount of PEEP should be set to minimize lung collapse at end expiration. | |
| Prone positioning may be considered in patients requiring potentially injurious levels of plateau pressure or Fio2 who are not at high risk for development of adverse consequences of prone positioning. | |
| Unless contraindicated, mechanically ventilated patients should be maintained with the head of the bed raised to 30 degrees to prevent the development of ventilator-associated pneumonia. | |
| A weaning protocol should be in place. | |
| When stable, patients should undergo daily, spontaneous-breathing trials with a T-piece or 5 cm H2O CPAP. | |
| If spontaneous breathing trials are successful, consideration should be given to extubation. | |
| 11. | Sedation, Analgesia, and Neuromuscular Blockade |
| Sedation protocols should be used that include the use of a sedation goal, measured by a standardized subjective sedation scale. | |
| Use either continuous infusion or intermittent bolus to achieve predetermined end points. | |
| Sedation should be interrupted or lightened daily to evaluate patients. | |
| NMBAs should be avoided, if possible; when NMBAs are used, train-of-4 monitoring of depth of blockade should be used. | |
| 12. | Glucose Control |
| Maintain blood glucose levels < 150 mg/dL with the use of insulin infusion. | |
| Monitor blood glucose every 30 minutes initially and then regularly (every 4 hours) once glucose concentration has stabilized. | |
| Glycemic control should include the use of a nutrition protocol that favors enteral feeding. | |
| 13. | Renal Replacement |
| In ARF, continuous venovenous hemofiltration and intermittent hemodialysis are equivalent. | |
| Venovenous hemofiltration may be tolerated better in hemodynamically unstable patients. | |
| There is no evidence to support hemofiltration in sepsis independent of renal replacement needs. | |
| 14. | Bicarbonate Therapy |
| No evidence supports the use of bicarbonate therapy for the treatment of hypoperfusion-induced lactic acidemia. | |
| 15. | DVT Prophylaxis |
| Patients with severe sepsis should receive DVT prophylaxis with low-dose unfractionated heparin or LMWH. | |
| Mechanical prophylaxis should be used in patients with contraindications to the use of heparin. | |
| In extremely high-risk patients—i.e., with a history of DVT—a combination of pharmacologic and mechanical therapy is recommended. | |
| 16. | Stress Ulcer Prophylaxis |
| All patients with sepsis should receive stress-ulcer prophylaxis. | |
| H2-receptor antagonists are more efficacious than sucralfate. | |
| No studies have directly compared the use of proton-pump inhibitors with H2-receptor antagonists, so their efficacy is unknown. | |
| 17. | Consideration of Limitation of Support |
| Advance-care planning, including the communication of likely outcomes and realistic treatment goals, should be discussed with patients and their families. | |
| Less aggressive therapy and withdrawal of therapy may be in the patient’s best interest. |
ACTH, adrenocorticotropic hormone; ARF, acute renal failure; BP, blood pressure; CO cardiac output; CPAP, continuous positive airway pressure; CVP, central venous pressure; DVT, deep vein thrombosis; FFP, fresh frozen plasma; IV, intravenously administered; LMWH, low-molecular-weight heparin; NMBA, neuromuscular blocking agents; PEEP, positive end-expiratory pressure; pRBC, packed red blood cells; rhAPC, recombinant human activated protein C; Scvo2, central venous oxygen saturation; SI-ALI, sepsis-induced acute lung injury; Svo2, mixed venous oxygen saturation; VT, tidal volume.
eFor example, percutaneous rather than surgical abscess drainage.
lBecause support for this recommendation was based on a single randomized trial, practitioners must truly weigh the risk-to-benefit ratio before initiating therapy until other trials confirm the results.
kAPACHE II score > 25, sepsis-induced multiple organ dysfunction syndrome (MODS), septic shock, or sepsis-induced acute respiratory distress syndrome (ARDS).
a Cerebrospinal fluid, wounds, urine, respiratory secretions, other body fluids.
b After cultures are obtained.
c Some experts prefer combination therapy for Pseudomonas infections.
d For example, drainage of abscess, removal of infected vascular access device.
f Increased blood pressure (BP) and urine output.
g Evidence of intravascular volume overload.
h Hydrocortisone, 200 to 300 mg/day for 7 days in 3 or 4 divided doses or by continuous infusion.
i More than 9-μg/dL increase in cortisol after 30 to 60 minutes.
j Controversial because hydrocortisone has mineralocorticoid activity.
m May use if other coexisting conditions merit treatment with erythropoietin, (e.g., renal failure).
Urinary Tract Infection
Urinary tract infections (UTIs) are the most common hospital-acquired infections103 and are thought to represent 25% to 50% of ICU-acquired infections.104 Traditionally, hospital-acquired UTIs have been defined as the presence of more than 105 cfu/mL urine in patients during bladder catheterization. In the early 1980s, Platt et al105 showed that hospitalized patients meeting this definition of nosocomial UTI had a 2.8-fold increase in mortality. However, more data suggest that many of these cases may represent asymptomatic bacteriuria rather than invasive infection and perhaps do not merit treatment.104 Stark and Maki106 have demonstrated that, in catheterized patients, colonic bacteria rapidly proliferate in the urinary system and exceed 105 cfu/mL. In fact, up to 30% of catheterized hospitalized patients have more than 105 cfu/mL urine.107 In addition, pyuria is not helpful in differentiating between infection and colonization because most catheter-associated bacteriuria has accompanying pyuria.108 The rate of UTI-associated bacteremia is quite low (Box 37-4). A large Canadian case series demonstrated a UTI rate in an ICU of 9%, with only a 0.4% incidence rate of bacteremic UTI.103
BOX 37-4 Nosocomial Urinary Tract Infection
Bladder irrigation with antibiotic solutions does not reduce the likelihood of patients acquiring catheter-related UTI,109 nor does the prophylactic use of systemic antibiotics.110 The use of antimicrobial agent–impregnated urinary catheters does appear to reduce infection rates.
Clostridium difficile Enterocolitis
The anaerobic, gram-positive rod Clostridium difficile was first described in 1935 by Hall and O’Toole.111 It was not until 1978, however, that its two toxigenic exotoxins (toxins A and B) were identified to be the cause of antibiotic-associated pseudomembranous enterocolitis.112,113 Although C. difficile is part of the normal fecal flora in 50% of newborns, it is not found in adults until the normal microbial barrier is altered by antibiotic therapy.111,112 C. difficile colonization occurs via the fecal-oral route and can follow exposure to any antibiotic. It is unclear why only a small percentage of patients exposed to a given antibiotic become colonized, nor is it clear why only some of those colonized develop symptomatic infection.112,114 There is some speculation that antibody titers to the toxins may play a role.115,116
C. difficile diarrhea is the most common enteric infection in hospitalized patients, infecting approximately 10% of individuals who are hospitalized for longer than 2 days.117 The organism enters the hospital environment via asymptomatic carriers and is then transmitted from patient to patient primarily via contact from hospital personnel. The risk factors that predispose a patient to development of C. difficile colonization include the severity of illness at admission, multiple antibiotic exposures, gastrointestinal operations, enteral feeding, an infected roommate, and the use of proton-pump inhibitors.117–119 Once the patient’s gut is colonized, clinical infection occurs after the secretion and adherence of toxin to receptors on the host’s colonocyte brush border. The bound toxins cause necrosis of the epithelial cells, an intense inflammatory response, and exudative secretion into the bowel lumen. This cellular response is visible on endoscopy as “volcano” or “summit” lesions and pseudomembranes.120 Hospital and unit epidemics of C. difficile are common, are often attributable to a single strain (despite multiple strains being present in the environment), and are associated with the use of clindamycin.121,122
C. difficile diarrhea produces a spectrum of clinical disease. After the administration of antibiotics, the typical presentation includes low-grade fever, leukocytosis, frequent watery bowel movements (10 to 15 per day), and abdominal pain. However, at its most severe, the disease results in fulminant illness with “toxic megacolon.”122 It is important to note that the majority of antibiotic-associated diarrhea results from an osmotic diarrhea and not from C. difficile infection. In osmotic diarrhea, antibiotics inhibit the intestinal flora from metabolizing carbohydrates, which results in increased intraluminal osmotic pressure, the translocation of water into the bowel lumen, and diarrhea.123 The diagnosis of C. difficile infection is suggested by the presence of fecal leukocytes (not present in osmotic diarrhea), systemic toxicity (fever, leukocytosis), and the persistence of diarrhea despite the discontinuation of enteral feeding (which will decrease the carbohydrate source in osmotic diarrhea). The diagnosis is confirmed by bioassay of C. difficile cytotoxins.124 Endoscopy is reserved for use in suspected cases of C. difficile in which diagnosis is not confirmed by toxin assay or when a diagnosis must be made before assay results are available. In these instances, colonoscopy is superior to sigmoidoscopy (lesions are often proximal in the colon), and the presence of pseudomembranes in a patient with antibiotic-associated diarrhea is pathognomonic of C. difficile diarrhea.125,126
C. difficile diarrhea is treated with oral metronidazole (500 mg, 3 times daily) or oral vancomycin (125 mg, 4 times daily) for 10 to 14 days. Metronidazole is considered to be the first choice because it and vancomycin have a similar efficacy but metronidazole is less expensive.127 However, oral vancomycin has been shown to be superior to metronidazole for severe C. difficile when patients are stratified by severity of illness.128 In severely ill patients, oral vancomycin (up to 500 mg, 4 times daily) is supplemented with intravenously administered metronidazole.129 Relapse occurs in 10% to 25% of patients, is not from antibiotic resistance (not described to date), and should be treated by repeating the same therapy for another 10 to 14 days. Increases in the incidence and severity of C. difficile prompted investigators across the United States to identify a virulent strain of infection, which appears to be related to the use of cephalosporin and fluoroquinolone antibiotics.130 The strain is known as BI/NAP1/027, which is restriction endonuclease analysis group BI, pulse-field gel electrophoresis type NAP1, and polymerase chain reaction ribotype 027. Although this strain has not shown resistance to metronidazole, the use of oral vancomycin is recommended because of the hypervirulence of this strain.130
Sinusitis
Sinusitis is a frequent and clinically silent source of infection in intubated patients.104 The presence of nasogastric tubes predisposes patients to developing ethmoid and maxillary sinusitis. Sinusitis is also particularly common after nasal intubation, with an incidence rate of up to 85% after 1 week of intubation.104,131–134 The diagnosis is suggested by the presence of air-fluid levels or total opacification of the sinuses on computed tomography scan.134 However, opacification of the sinuses is common after intubation. In one series, 96% of nasotracheally intubated and 23% of orotracheally intubated patients with previously clear sinuses had new sinus opacification after 1 week of intubation.134 The diagnosis can be confirmed by the presence of pus and high quantitative cultures that can be obtained via either transnasal puncture of the maxillary sinus or open ethmoidectomy or sphenoidectomy.104,133 Case series have reported a resolution of fever and leukocytosis after the treatment of sinusitis.133,134 Treatment consists of removal of all nasal tubes, drainage of the maxillary sinuses, and the use of broad-spectrum antibiotics.
Hematology
Transfusion
Blood products frequently are transfused into critically ill patients. In a general ICU population, patients receive an average of 0.2 unit blood products per day; this incidence increases to 1.3 units per day in cardiothoracic ICUs.135,136 Although transfusion of blood products is often necessary to either improve oxygen delivery or restore the coagulation system (Box 37-5), a growing body of literature suggests that transfusion carries substantial risk for patients after cardiac operations.
Several large studies have identified the use of transfusion as increasing patients’ risk for development of infection after cardiac operations. A review of 19 retrospective studies found that, in 17 studies, transfusion was found to be a significant factor related to postoperative infection and was frequently the best predictor of postoperative infection.135,137 Transfusion has been cited as a risk factor for development of mediastinitis,58 early bacteremia,138 and pneumonia,139 as well as for increased mortality rate and increased LOS after cardiac operations.140
In 2001, Leal-Noval et al135 published a large cohort study that identified transfusion as a risk factor for severe postoperative infections. In their series of 738 cardiac surgical patients at a large Spanish teaching hospital, a transfusion of more than 4 units blood components was associated with a statistically significant increased risk for pneumonia, mediastinitis, mortality, and longer ICU LOS (Figure 37-3).135
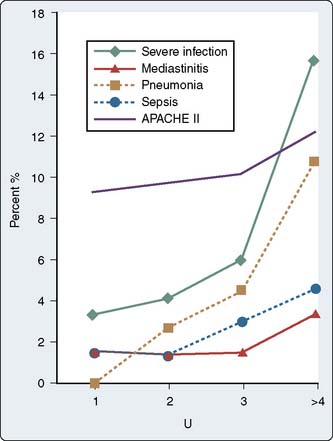
Figure 37-3 Relation between the units of blood transfused and the percentage of infections.
(Redrawn from Leal-Noval SR, Rincon-Ferrari MD, Carcia-Curiel A, et al: Transfusion of blood components and postoperative infection in patients undergoing cardiac surgery. Chest 119:1461, 2001.)
In 2007, Murphy et al141 published the results of a review of the cardiac surgical databases in the United Kingdom, finding that those patients who had received transfusions of packed red blood cells (pRBCs) had a greater incidence of infection and increased rates of ischemic postoperative morbidity. Two articles published in Critical Care Medicine had similar conclusions. An observational cohort study of 11,963 patients at the Cleveland Clinic found that perioperative transfusion of pRBCs was the single factor most reliably associated with morbidity in patients who had undergone isolated coronary artery bypass grafting.142 Marik and Corwan,143 after systematically reviewing the literature, came to a similar conclusion—in patients in the ICU, pRBC transfusions are associated with increased rates of not only morbidity but also mortality. As transfusion practices continue to be evaluated, additional data suggest that allogeneic and not autologous blood is associated with the twofold increased risk for hospital infection, signifying an immunologic relation144 (see Chapters 30 and 31).
In 1999, Hebert et al145 published a landmark study that has fundamentally altered the approach to transfusion in critically ill patients. This large, multicenter, randomized, prospective trial of 838 patients admitted to Canadian ICUs found no difference in 30-day mortality between patients assigned to a liberal (hemoglobin, 10–12 g/dL) and conservative pRBC transfusion protocol (hemoglobin, 7–9 g/dL). Mortality was lower in less ill patients (APACHE II [Acute Physiology and Chronic Health Evaluation II] score ≤ 20) and in younger patients (< 55 years of age). Also, the restrictive strategy resulted in a 54% reduction in pRBC transfusions. Before this study, pRBC transfusion had been extensively investigated as a component of the now-dated paradigm that supranormal oxygen delivery was associated with increased survival in critically ill patients.135 Hebert et al’s study145 showed that a conservative strategy was associated with no increase in mortality and halved the number of transfused units, with attendant decreases in infectious risk, immunomodulation, and cost associated with the lower transfusion rate. Two similar small trials in patients after cardiac operations showed no difference in outcome between conservative and liberal transfusion strategies.146,147
These data, combined with evidence supporting the use of early “goal-directed” therapy in sepsis, are what led to the transfusion recommendations cited in the Surviving Sepsis Campaign Guidelines.101,148,149 Although no consensus guidelines exist for transfusion practices after cardiac operations, an extrapolation of these recommendations for patients who have had cardiac surgical procedures seems prudent. That is, in the initial 24 to 48 hours of “resuscitation,” a more liberal transfusion strategy targeting oxygen delivery (with objective end points, e.g., lactate levels, mixed venous oxygen saturation) and resolution of coagulopathy or bleeding seems appropriate. Once stabilized, patients in the cardiothoracic ICU long term should probably be subjected to a conservative (hemoglobin, 7–9 g/dL) transfusion strategy. A multicenter retrospective review of patients after myocardial infarction suggested that a more liberal goal of transfusing to achieve a hemoglobin concentration of 10 g/dL is associated with increased mortality rates.150 Although not directly applicable to a cardiothoracic ICU setting, the results of this study do suggest that improving oxygen delivery in patients with coronary artery disease by transfusing pRBCs to a hemoglobin of 10g/dL is not the panacea clinicians once thought.
Acute renal failure
Acute renal failure (ARF), like many of the clinical syndromes frequently encountered in the ICU, has been difficult to precisely define.151 If it is agreed that the principal functions of the kidney are to create urine and excrete water-soluble waste products of metabolism, then ARF is the sudden loss of these functions.151
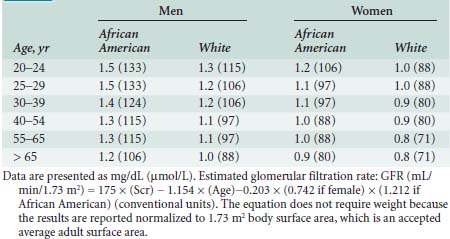
Renal solute excretion is a function of glomerular filtration. The glomerular filtration rate (GFR) is a convenient and time-honored way of quantifying renal function. It must be appreciated, however, that GFR varies considerably under normal circumstances as a function of protein intake.152 A normal GFR is 120 ± 25 mL/min for men and 95 ± 20 mL/min for women. Serum creatinine concentration is the most frequently used surrogate of GFR and, hence, solute excretion. When measured in the steady state and analyzed in the context of age, sex, and race, the GFR loosely reflects renal function. In fact, when baseline serum creatinine is unknown, standardized tables allow the estimation of baseline creatinine that can then be compared with actual serum creatinine levels to estimate decremental changes in renal function (Table 37-7).152
The use of creatinine concentration is much less accurate in estimating renal function in non–steady-state conditions (e.g., ARF in the critically ill). Creatinine is formed from nonenzymatic dehydration of creatine (98% muscular in origin) in the liver. Because critical illness affects liver function, muscle mass, tubular excretion of creatinine, and the volume of distribution of creatinine, its limitations as a useful marker of renal function become apparent.152 Nonetheless, changes in serum creatinine concentration and the rate of change in creatinine concentration remain the most convenient and frequently used surrogates of renal dysfunction.
Urine output is the other frequently measured parameter of renal function in the ICU. Oliguria is defined by a urine output of less than 0.5 mL/kg/hr.152 Under a wide range of normal physiologic conditions, urine output primarily reflects changes in renal hemodynamics and volume status rather than representing renal parenchymal function and reserve. Hence, it is very nonspecific for renal dysfunction unless urine output is severely reduced or absent.152 And although oliguric renal failure has a greater mortality rate than does nonoliguric renal failure, no data demonstrate that the pharmacologic creation of urine in patients with renal failure reduces mortality.153
The incidence of ARF after cardiovascular operations is high and is associated with a “formidable” mortality rate.154 A cohort study from Duke University described a 0.7% incidence rate of dialysis-dependent ARF that was associated with an increase in mortality rate from 1.8% to 28%.155 Another cohort described an incidence rate of dialysis-dependent ARF of 1.1% after cardiac surgery that was associated with a mortality rate of 63.7%.154 In critically ill patients (noncardiac), the mortality rate for ARF is 50% to 80% and has not declined significantly since the advent of acute dialysis therapy.153
The pathogenesis of ARF after cardiac operations is thought to primarily result from hypoperfusion and ischemia.155 Other contributing factors include exposure to nephrotoxins, nonpulsatile flow during cardiopulmonary bypass, and aortic emboli. The two most important determinants of ARF after cardiopulmonary bypass are preexisting renal insufficiency and postoperative low-cardiac-output states.156,157
The Acute Dialysis Quality Initiative Workgroup formulated a classification system for ARF in 2004 to develop consensus-based recommendations.158 Termed the RIFLE criteria (risk, injury, failure, loss, and end-stage renal disease; Table 37-8), stratification from risk for development of acute renal dysfunction to end-stage renal disease is based on GFR and urine output. Since the RIFLE criteria were instituted, three studies159–161 have been conducted in the cardiac surgical population that confirmed that the development of renal injury is an independent risk factor for 90-day mortality.152
TABLE 37-8 RIFLE Criteria for Categorizing Acute Renal Dysfunction
| Category | Pcr and GFR Criteria | UO Criteria |
|---|---|---|
| Risk | ↑ Pcr × 1.5 or GFR ↓ > 25% | < 0.5 mL.kg–1.hr–1 for 6 hours |
| Injury | ↑ Pcr × 2 or GFR ↓ > 50% | < 0.5 mL.kg–1.hr–1 for 12 hours |
| Failure | ↑ Pcr × 3, or serum creatinine ≥ 4 mg/100 mL with an acute rise > 0.5 mg/dL, or GFR ↓ > 75% | < 0.3 mL.kg–1.hr–1 for 24 hours or anuria for 12 hours |
| Loss | Persistent ARF = complete loss of kidney function > 4 weeks | |
| ESRD | ESRD < 3 months | |
For conversion of creatinine expressed in conventional units to ST units, multiply by 88.4. Renal function is categorized based on serum creatinine concentration (Pcr)or urinary output (UO), or both, and the criteria that led to the worst classification are used. Glomerular filtration rate (GFR) criteria are calculated as an increase of Pcr above the baseline Pcr. When the baseline Pcr is unknown and the patient has no history of chronic renal disease, Pcr is calculated using the Modification of Diet in Renal Disease formula (GFR = 170 × [Pcr {mg/dL}]-0.999 × [Age]-0.176 × [0.762 if patient is female] [1.180 if patient is black] × [serum urea nitrogen concentration {mg/dL}]-0.170 × [serum albumin concentration {g/dL}]+0.318). Acute kidney injury should be considered when kidney dysfunction is abrupt (within 1–7 days) and sustained (> 24 hours).
ARF, acute renal failure; ESRD; end-stage renal disease.
Several strategies for preventing perioperative renal failure have been evaluated in patients who have undergone cardiac operations. “Renal-dose” dopamine has been shown to have no effect on either renal function or mortality after both cardiac and vascular operations.162–164 Similarly, the use of the diuretics furosemide and mannitol has demonstrated no renal-protective effect. In fact, a small study demonstrated a significantly worse renal outcome in patients prophylactically treated with furosemide infusion versus control subjects.163 Results with pulsatile perfusion during cardiopulmonary bypass have been similarly disappointing.151
The recombinant brain atrial natriuretic peptide nesiritide has demonstrated some initial promise in treating perioperative renal failure in patients after cardiac operations. In a randomized, prospective, double-blinded trial in two cardiothoracic ICUs in Sweden, patients with an increase in serum creatinine concentration more than 50% above baseline had improved renal excretion, a decreased need for hemodialysis, and improved survival after treatment with brain atrial natriuretic peptide, compared with control subjects.165 Nesiritide works via a cyclic guanosine monophosphate–coupled receptor (NPR-A) in vascular smooth muscle cells. Physiologically, it produces arterial and venous vasodilation and inhibition of aldosterone production and increases urine volume and sodium excretion.166 Further trials are under way to more clearly define this agent’s role in cardiac surgical patients (see Chapters 10 and 34).
Treatment and Renal Replacement Therapies
Just as the diagnosis and prevention of ARF remain enigmatic, so, too, does the treatment of ARF. In the critically ill patient who experiences development of ARF, the initial treatment strategy is to create an optimal “environment” for the kidney to heal; that is, to maximize oxygen delivery to the renal parenchyma via the manipulation of hemodynamics and volume status while simultaneously avoiding exposure to nephrotoxins (e.g., contrast agents, aminoglycosides) and ensuring that no postrenal obstruction exists. The administration of furosemide in this setting, long advocated to maintain urine output, has not been associated with an improvement in outcome but has been associated with an increase in oxygen consumption in the renal cortex,167 which may be deleterious in patients with decreased renal perfusion pressure.
If optimization is provided and the kidney does not recover, the clinician must provide renal replacement therapy (RRT). Many of the classic indications for RRT are noncontroversial (Table 37-9).168 In the absence of these indications, the decision to initiate RRT in critically ill patients is a matter of clinical judgment. Typical laboratory parameters that suggest the need for RRT include a blood urea nitrogen concentration greater than 100 mg/dL or a creatinine concentration greater than 4.5 mg/dL.164
Once the decision to initiate RRT has been made, the mode of replacement must be chosen. In broad terms, RRT can be divided into intermittent hemodialysis or continuous RRT. The latter comes in a wide variety of forms, each associated with its own unique acronym (e.g., slow continuous ultrafiltration [SCUF], slow low-efficiency daily dialysis [SLEDD], continuous venovenous hemofiltration [CVVH], continuous venovenous hemofiltration-dialysis [CVVH-D]). The differences between these different forms of continuous RRT lie in the membrane used, the mechanism of solute transport, the presence or absence of a dialysis solution, and the type of vascular access.164,168,169 In the United States, most patients with ARF are treated with hemodialysis, but the trend is toward the increased use of continuous RRT, whereas in other countries, continuous RRT predominates.164,170–172 Currently, no studies support the use of one modality over another, but most intensivists prefer using continuous RRT in hemodynamically unstable patients or in patients in whom the hypotension associated with hemodialysis may lead to adverse effects.164,168 A trial of weaning of continuous RRT should be considered when established criteria have been met (Table 37-10).168
TABLE 37-10 Criteria for Weaning of Continuous Renal Replacement Therapy
RRT, renal replacement therapy.
Nutrition support
Patients who are anticipated to have an extended stay in the ICU (longer than 2 to 3 days) will require nutrition assessment, and patients who are malnourished or who will remain non per os (NPO) without parenteral nutrition should be examined and assessment made whether they should receive enteral or parenteral nutrition support. In the 1970s, several articles in the nutrition literature concluded that anywhere from 40% to 70% of patients in the hospital, and by extension in the ICU, were malnourished.173 Three decades of providing aggressive intervention have not yet changed that assessment. Because of chronic illness and the sequelae of acute illness, many patients remain cachectic and hypermetabolic, and though aggressive nutrition support may not completely reverse the sequelae of malnutrition in these patients, the lack of support will likely make the malnutrition worse.174,175
Nutrition Assessment
A thorough physical examination should include an assessment of the patient’s general appearance and overall condition, a brief neurologic examination specifically looking at ability to swallow and protect the airway, muscle stores (in the temporal fossa), fat stores (usually in the triceps area), and evidence of jaundice, glossitis, edema, and cheilosis. A subjective global assessment of the patient’s overall nutrition status has been validated and is becoming the gold standard by which patients’ nutrition status is assessed.176
Every attempt should be made to keep the serum glucose concentration less than 150 to 180 mg/dL, with minimum variability in glucose concentrations ensured.177 One study found that patients with a mean absolute change in glucose concentration of more than 15.8 mg/dL/hr had a greater mortality rate than did a group with a change of less than 7.1 mg/dL/hr.178 Caloric support should not be decreased merely to meet this goal, but any patient receiving nutrition intervention should have blood glucose monitored.
After the initial nutrition assessment, the patient is classified as having mild, moderate, or severe malnutrition. Patients who are moderately to severely malnourished should have more attention paid to their nutrition support. If the patient is anticipated to remain NPO for several days, many intensivists and nutrition-support personnel would implement nutrition support early in the hospital course, within the first 1 to 3 days. The data for this recommendation are lacking, except in trauma patients in whom there are studies that justify early nutrition support.179 As long as there are no side effects, there should be no danger to early intervention using a tube placed through the pylorus to aliment the patient.
Although enteral nutrition is preferred and has been reported to decrease the incidence of infectious complications in some patient populations, occasionally, some patients cannot be fed enterally and must be fed parenterally. If the parenteral route is chosen, placement of the central venous access line using a sterile technique is critical.51 Furthermore, keeping blood glucose levels at or less than 150 to 180 mg/dL decreases the incidence of IVD-related BSI and pneumonia.180
Formulas
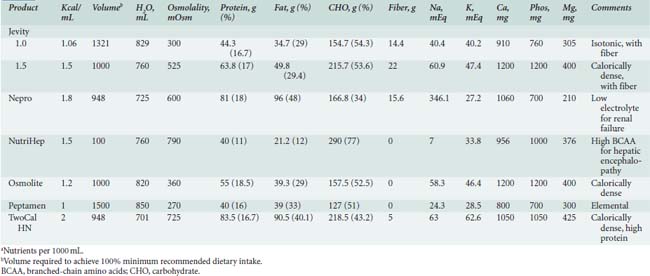
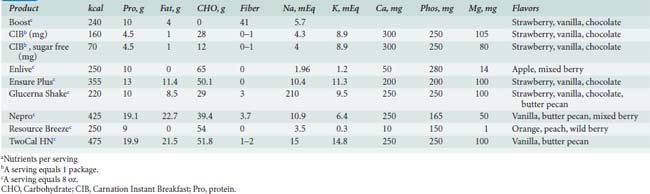
Enteral formulas, which can be provided via tube feeding or orally, are listed in Tables 37-11 and 37-12.
Vitamins
Several formulas have been recommended for delivery to critically ill patients, including formulas that contain glutamine, arginine, essential γ-3 fatty acids, nucleotides, and structured lipids. The SCCM-American Society for Parenteral and Enteral Nutrition guidelines for support in critically ill patients gave a Grade A recommendation to administering an enteral formulation with anti-inflammatory properties (fish and borage oils and antioxidants) to patients with acute lung injury and acute respiratory distress syndrome.181 Yet within a year of this publication, the EDEN-Omega Study (early vs. delayed enteral feeding and omega-3 fatty acid/antioxidant supplementation for treating people with acute lung injury and acute respiratory distress syndrome) was terminated early because of futility.182
Electrolyte Abnormalities
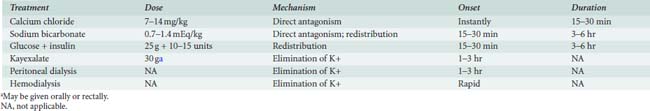
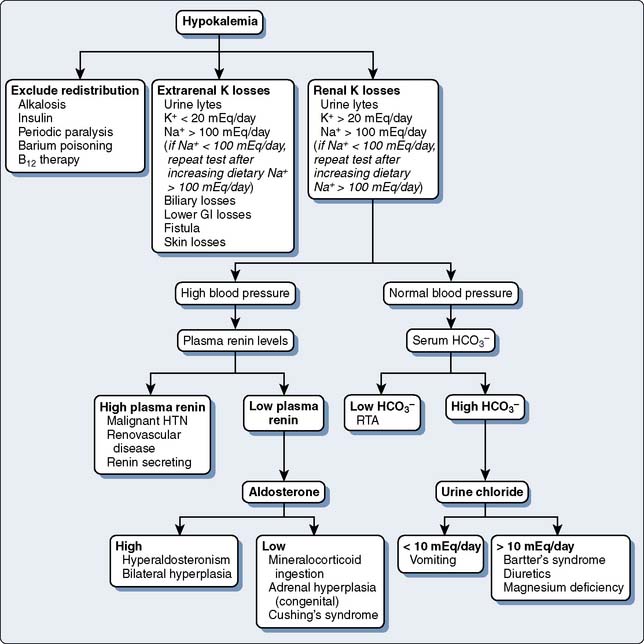
Fluid and electrolyte abnormalities occur often after cardiac operations. Diagnosis and treatment algorithms are shown in Figures 37-4 and 37-5 for hypernatremia and hyponatremia, Figure 37-6 and Table 37-13 for hyperkalemia, and Figure 37-7 for hypokalemia. Symptoms of hypercalcemia (serum Ca2+ > 13.0 mEq) include anorexia, nausea, vomiting, lethargy, dehydration, coma, and death. The treatment of hypercalcemia is listed in Table 37-14. Hypomagnesemia is common, and the underlying cause should be identified, if possible, and then treated with an intravenous infusion of 0.1 to 0.2 mEq/kg/day or orally at 0.4 mEq/kg/day. Close monitoring is necessary with the treatment of any electrolyte disorder.
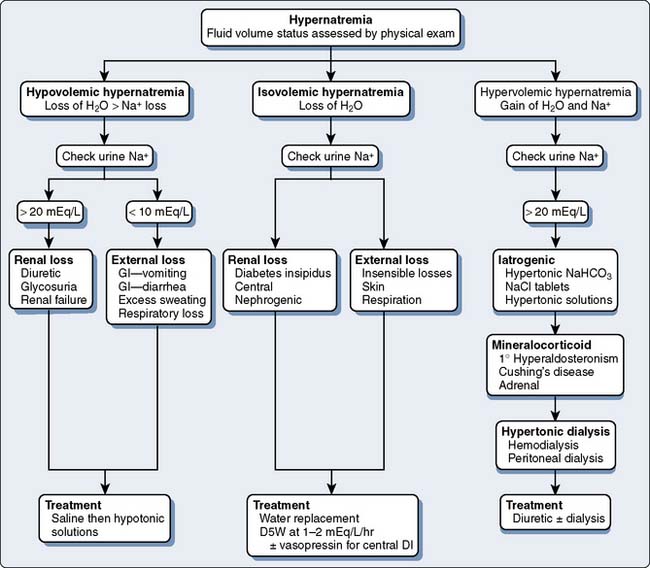
Figure 37-4 Assessment and treatment of hypernatremia.
D5W, 5% dextrose in water; DI, diabetes insipidus; GI, gastrointestinal.
(Modified from Torres N: Electrolyte abnormalities: Sodium. In Faust RJ [ed]: Anesthesiology Review, 3rd ed. Philadelphia: Churchill Livingstone, 2002, p 28, by permission of Mayo Foundation.)
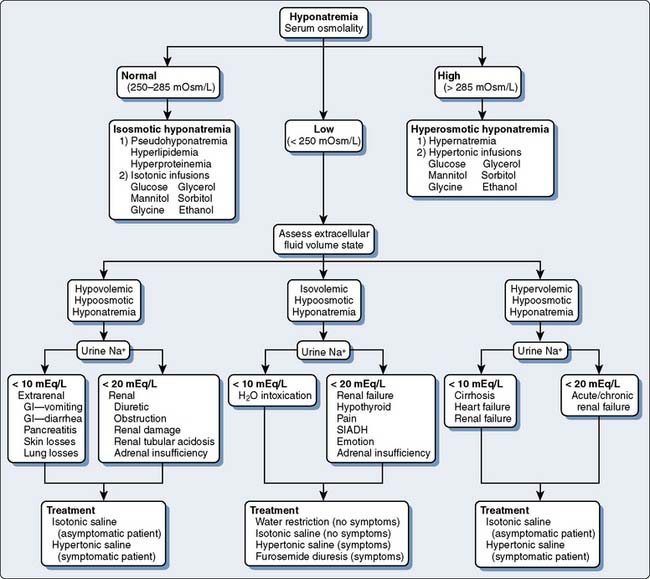
Figure 37-5 Assessment and treatment of hyponatremia.
GI refers to gastrointestinal; SIADH, syndrome of inappropriate antidiuretic hormone.
(Modified from Torres N: Electrolyte abnormalities: Sodium. In Faust RJ [ed]: Anesthesiology Review, 3rd ed. Philadelphia, 2002, Churchill Livingstone, p 29, by permission of Mayo Foundation.)
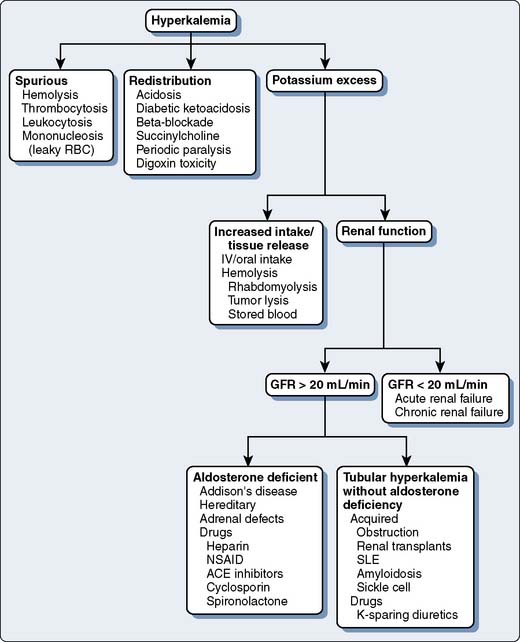
Figure 37-6 Causes of hyperkalemia.
(Modified from Torres N: Electrolyte abnormalities: Potassium. In Faust RJ [ed]: Anesthesiology Review, 3rd ed. Philadelphia, 2002, Churchill Livingstone, p 31, by permission of Mayo Foundation.)
1 Bashour C.A., Yared J.P., Ryan T.A., et al. Long-term survival and functional capacity in cardiac surgery patients after prolonged intensive care. Crit Care Med Dec. 2000;28:3847-3853.
2 Welsby I.J., Bennett-Guerrero E., Atwell D., et al. The association of complication type with mortality and prolonged stay after cardiac surgery with cardiopulmonary bypass. Anesth Analg. 2002;94:1072-1078.
3 Williams M.R., Wellner R.B., Hartnett E.A., et al. Long-term survival and quality of life in cardiac surgical patients with prolonged intensive care unit length of stay. Ann Thorac Surg. 2002;73:1472-1478.
4 Maze M., Scarfini C., Cavaliere F. New agents for sedation in the intensive care unit. Crit Care Clin. 2001;17:881-897.
5 Young C., Knudsen N., Hilton A., et al. Sedation in the intensive care unit. Crit Care Med. 2000;28:854-866.
6 Shapiro B.A., Warren J., Egol A.B., et al. Practice parameters for intravenous analgesia and sedation for adult patients in the intensive care unit: An executive summary. Society of Critical Care Medicine. Crit Care Med. 1995;23:1596-1600.
7 Nasraway S.A.Jr, Jacobi J., Murray M.J., et al. Sedation, analgesia, and neuromuscular blockade of the critically ill adult: Revised clinical practice guidelines for 2002. Crit Care Med. 2002;30:117-118.
8 Pun B.T., Ely E.W. The importance of diagnosing and managing ICU delirium. Chest. 2007;132:624-636.
9 Riker R.R., Shehabi Y., Bokesch P.M., et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: A randomized trial. JAMA. 2009;301:489-499.
10 Devlin J.W., Boleski G., Mlynarek M., et al. Motor Activity Assessment Scale: A valid and reliable sedation scale for use with mechanically ventilated patients in an adult surgical intensive care unit. Crit Care Med. 1999;27:1271-1275.
11 Ramsay M.A., Savege T.M., Simpson B.R., et al. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2:656-659.
12 de Lemos J., Tweeddale M., Chittock D. Measuring quality of sedation in adult mechanically ventilated critically ill patients: The Vancouver Interaction and Calmness Scale. Sedation Focus Group. J Clin Epidemiol. 2000;53:908-919.
13 Wielenga J.M., De Vos R., de Leeuw R., et al. COMFORT scale: A reliable and valid method to measure the amount of stress of ventilated preterm infants. Neonatal Netw. 2004;23:39-44.
14 Ely E.W., Truman B., Shintani A., et al. Monitoring sedation status over time in ICU patients: Reliability and validity of the Richmond Agitation-Sedation Scale (RASS). JAMA. 2003;289:2983-2991.
15 Sessler C.N., Gosnell M.S., Grap M.J., et al. The Richmond Agitation-Sedation Scale: Validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338-1344.
16 Weinert C., McFarland L. The state of intubated ICU patients: Development of a two-dimensional sedation rating scale for critically ill adults. Chest. 2004;126:1883-1890.
17 Luetz A., Heymann A., Radtke F.M., et al. Different assessment tools for intensive care unit delirium: Which score to use? Crit Care Med. 2010;38:409-418.
18 Adesanya A.O., Rosero E., Wyrick C., et al. Assessing the predictive value of the bispectral index vs patient state index on clinical assessment of sedation in postoperative cardiac surgery patients. J Crit Care. 2009;24:322-328.
19 Dundee J., Halliday N.J., Fee J.P. Midazolam in intensive care. Br Med J (Clin Res Ed). 1984;289:1540.
20 Woo E., Greenblatt D.J. Massive benzodiazepine requirements during acute alcohol withdrawal. Am J Psychiatry. 1979;136:821-823.
21 Deppe S.A., Sipperly M.E., Sargent A.I., et al. Intravenous lorazepam as an amnestic and anxiolytic agent in the intensive care unit: A prospective study. Crit Care Med. 1994;22:1248-1252.
22 Pohlman A.S., Simpson K.P., Hall J.B. Continuous intravenous infusions of lorazepam versus midazolam for sedation during mechanical ventilatory support: A prospective, randomized study. Crit Care Med. 1994;22:1241-1247.
23 Park G.R., Navapurkar V., Ferenci P. The role of flumazenil in the critically ill. Acta Anaesthesiol Scand Suppl. 1995;108:23-34.
24 Myles P.S., Buckland M.R., Weeks A.M., et al. Hemodynamic effects, myocardial ischemia, and timing of tracheal extubation with propofol-based anesthesia for cardiac surgery. Anesth Analg. 1997;84:12-19.
25 Strickland R.A., Murray M.J. Fatal metabolic acidosis in a pediatric patient receiving an infusion of propofol in the intensive care unit: Is there a relationship? Crit Care Med. 1995;23:405-409.
26 Vasile B., Rasulo F., Candiani A., et al. The pathophysiology of propofol infusion syndrome: A simple name for a complex syndrome. Intensive Care Med. 2003;29:1417-1425.
27 Wysowski D.K., Pollock M.L. Reports of death with use of propofol (Diprivan) for nonprocedural (long-term) sedation and literature review. Anesthesiology. 2006;105:1047-1051.
28 Drummond J.C., Iragui-Madoz V.J., Alksne J.F., et al. Masking of epileptiform activity by propofol during seizure surgery. Anesthesiology. 1992;76:652-654.
29 Searle N.R., Cote S., Taillefer J., et al. Propofol or midazolam for sedation and early extubation following cardiac surgery. Can J Anaesth. 1997;44:629-635.
30 Sherry K.M., McNamara J., Brown JS., et al. An economic evaluation of propofol/fentanyl compared with midazolam/fentanyl on recovery in the ICU following cardiac surgery. Anaesthesia. 1996;51:312-317.
31 Barletta J.F., Miedema S.L., Wiseman D., et al. Impact of dexmedetomidine on analgesic requirements in patients after cardiac surgery in a fast-track recovery room setting. Pharmacotherapy. 2009;29:1427-1432.
32 Herr D.L., Sum-Ping S.T., England M. ICU sedation after coronary artery bypass graft surgery: Dexmedetomidine-based versus propofol-based sedation regimens. J Cardiothorac Vasc Anesth. 2003;17:576-584.
33 Pandharipande P.P., Pun B.T., Herr D.L., et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: The MENDS randomized controlled trial. JAMA. 2007;298:2644-2653.
34 Dasta J.F., Kane-Gill S.L., Pencina M., et al. A cost-minimization analysis of dexmedetomidine compared with midazolam for long-term sedation in the intensive care unit. Crit Care Med. 2010;38:497-503.
35 Murray M.J., Strickland R.A., Weiler C. The use of neuromuscular blocking drugs in the intensive care unit: A US perspective. Intensive Care Med. 1993;19(Suppl 2):S40-S44.
36 Klessig H.T., Geiger H.J., Murray M.J., et al. A national survey on the practice patterns of anesthesiologist intensivists in the use of muscle relaxants. Crit Care Med. 1992;20:1341-1345.
37 Shovelton D.S. Reflections on an intensive therapy unit. Br Med J. 1979;1:737-738.
38 Murray M.J., Cowen J., DeBlock H., et al. Clinical practice guidelines for sustained neuromuscular blockade in the adult critically ill patient. Crit Care Med. 2002;30:142-156.
39 Hund E. Myopathy in critically ill patients. Crit Care Med. 1999;27:2544-2547.
40 Alberti C., Brun-Buisson C., Burchardi H., et al. Epidemiology of sepsis and infection in ICU patients from an international multicentre cohort study. Intensive Care Med. 2002;28:108-121.
41 Garland J.S., Henrickson K., Maki D.G. The 2002 Hospital Infection Control Practices Advisory Committee Centers for Disease Control and Prevention guideline for prevention of intravascular device-related infection. Pediatrics. 2002;110:1009-1013.
42 Maki D.G., Weise C.E., Sarafin H.W. A semiquantitative culture method for identifying intravenous-catheter-related infection. N Engl J Med. 1977;296:1305-1309.
43 Maki D.G. Yes, Virginia, aseptic technique is very important: maximal barrier precautions during insertion reduce the risk of central venous catheter-related bacteremia. Infect Control Hosp Epidemiol. 1994;15(4 Pt 1):227-230.
44 Linares J., Sitges-Serra A., Garau J., et al. Pathogenesis of catheter sepsis: A prospective study with quantitative and semiquantitative cultures of catheter hub and segments. J Clin Microbiol. 1985;21:357-360.
45 Mermel L.A., McCormick R.D., Springman S.R., et al. The pathogenesis and epidemiology of catheter-related infection with pulmonary artery Swan-Ganz catheters: A prospective study utilizing molecular subtyping. Am J Med. 1991;91:197S-205S.
46 Raad I.I., Hanna H.A. Intravascular catheter-related infections: New horizons and recent advances. Arch Intern Med. 2002;162:871-878.
47 Raad I., Hanna H., Maki D. Intravascular catheter-related infections: Advances in diagnosis, prevention, and management. Lancet Infect Dis. 2007;7:645-657.
48 Sitges-Serra A., Puig P., Linares J., et al. Hub colonization as the initial step in an outbreak of catheter-related sepsis due to coagulase negative staphylococci during parenteral nutrition. JPEN J Parenter Enteral Nutr. 1984;8:668-672.
49 Siegman-Igra Y., Anglim A.M., Shapiro D.E., et al. Diagnosis of vascular catheter-related bloodstream infection: A meta-analysis. J Clin Microbiol. 1997;35:928-936.
50 Safdar N., Maki D.G. Risk of catheter-related bloodstream infection with peripherally inserted central venous catheters used in hospitalized patients. Chest. 2005;128:489-495.
51 O’Grady N.P., Alexander M., Dellinger E.P., et al. Guidelines for the prevention of intravascular catheter-related infections. Centers for Disease Control and Prevention. MMWR Recomm Rep. 2002;51(RR-10):1-29.
52 Fidalgo S., Vazquez F., Mendoza M.C., et al. Bacteremia due to Staphylococcus epidermidis: Microbiologic, epidemiologic, clinical, and prognostic features. Rev Infect Dis. 1990;12:520-528.
53 Sherertz R.J., Heard S.O., Raad I.I. Diagnosis of triple-lumen catheter infection: Comparison of roll plate, sonication, and flushing methodologies. J Clin Microbiol. 1997;35:641-646.
54 Slobbe L., El Barzouhi A., Boersma E., et al. Comparison of the roll plate method to the sonication method to diagnose catheter colonization and bacteremia in patients with long-term tunnelled catheters: A randomized prospective study. J Clin Microbiol. 2009;47:885-888.
55 Safdar N., Fine J.P., Maki D.G. Meta-analysis: Methods for diagnosing intravascular device-related bloodstream infection. Ann Intern Med. 2005;142:451-466.
56 Baddour L.M., Wilson W.R., Bayer A.S., et al. Infective endocarditis: Diagnosis, antimicrobial therapy, and management of complications: A statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: Endorsed by the Infectious Diseases Society of America. Circulation. 2005;111:e394-e434.
57 Roy M.C. Surgical-site infections after coronary artery bypass graft surgery: Discriminating site-specific risk factors to improve prevention efforts. Infect Control Hosp Epidemiol. 1998;19:229-233.
58 Mekontso-Dessap A., Kirsch M., Brun-Buisson C., et al. Poststernotomy mediastinitis due to Staphylococcus aureus: Comparison of methicillin-resistant and methicillin-susceptible cases. Clin Infect Dis. 2001;32:877-883.
59 Risk factors for deep sternal wound infection after sternotomy: A prospective, multicenter study. J Thorac Cardiovasc Surg. 1996;111:1200-1207.
60 Hollenbeak C.S., Murphy D.M., Koenig S., et al. The clinical and economic impact of deep chest surgical site infections following coronary artery bypass graft surgery. Chest. 2000;118:397-402.
61 Hughes J.M., Culver D.H., White J.W., et al. Nosocomial infection surveillance, 1980–1982. MMWR CDC Surveill Summ. 1983;32:1SS-16SS.
62 Lazar H.L., Fitzgerald C., Gross S., et al. Determinants of length of stay after coronary artery bypass graft surgery. Circulation. 1995;92(9 Suppl):II20-II24.
63 Zacharias A., Habib R.H. Factors predisposing to median sternotomy complications. Deep vs superficial infection. Chest. 1996;110:1173-1178.
64 Martorell C., Engelman R., Corl A., et al. Surgical site infections in cardiac surgery: An 11-year perspective. Am J Infect Control. 2004;32:63-68.
65 Edwards M.B., Ratnatunga C.P., Dore C.J., et al. Thirty-day mortality and long-term survival following surgery for prosthetic endocarditis: A study from the UK heart valve registry. Eur J Cardiothorac Surg. 1998;14:156-164.
66 Arvay A., Lengyel M. Incidence and risk factors of prosthetic valve endocarditis. Eur J Cardiothorac Surg. 1988;2:340-346.
67 de Gevigney G., Pop C., Delahaye J.P. The risk of infective endocarditis after cardiac surgical and interventional procedures. Eur Heart J. 1995;16(Suppl B):7-14.
68 Grover F.L., Cohen D.J., Oprian C., et al. Determinants of the occurrence of and survival from prosthetic valve endocarditis. Experience of the Veterans Affairs Cooperative Study on Valvular Heart Disease. J Thorac Cardiovasc Surg. 1994;108:207-214.
69 Kuyvenhoven J.P., van Rijk-Zwikker G.L., Hermans J., et al. Prosthetic valve endocarditis: Analysis of risk factors for mortality. Eur J Cardiothorac Surg. 1994;8:420-424.
70 Vlessis A.A., Khaki A., Grunkemeier G.L., et al. Risk, diagnosis and management of prosthetic valve endocarditis: A review. J Heart Valve Dis. 1997;6:443-465.
71 Agnihotri A.K., McGiffin D.C., Galbraith A.J., et al. The prevalence of infective endocarditis after aortic valve replacement. J Thorac Cardiovasc Surg. 1995;110:1708-1720. discussion 1720–1704
72 Calderwood S.B., Swinski L.A., Waternaux C.M., et al. Risk factors for the development of prosthetic valve endocarditis. Circulation. 1985;72:31-37.
73 Horstkotte D., Piper C., Niehues R., et al. Late prosthetic valve endocarditis. Eur Heart J. 1995;16(Suppl B):39-47.
74 Ivert T.S., Dismukes W.E., Cobbs C.G., et al. Prosthetic valve endocarditis. Circulation. 1984;69:223-232.
75 Piper C., Korfer R., Horstkotte D. Prosthetic valve endocarditis. Heart. 2001;85:590-593.
76 Ben Ismail M., Hannachi N., Abid F., et al. Prosthetic valve endocarditis. A survey. Br Heart J. 1987;58:72-77.
77 Keyser D.L., Biller J., Coffman T.T., et al. Neurologic complications of late prosthetic valve endocarditis. Stroke. 1990;21:472-475.
78 Masur H., Johnson W.D.Jr. Prosthetic valve endocarditis. J Thorac Cardiovasc Surg. 1980;80:31-37.
79 Tornos P., Sanz E., Permanyer-Miralda G., et al. Late prosthetic valve endocarditis. Immediate and long-term prognosis. Chest. 1992;101:37-41.
80 Morguet A.J., Werner G.S., Andreas S., et al. Diagnostic value of transesophageal compared with transthoracic echocardiography in suspected prosthetic valve endocarditis. Herz. 1995;20:390-398.
81 Vered Z., Mossinson D., Peleg E., et al. Echocardiographic assessment of prosthetic valve endocarditis. Eur Heart J. 1995;16(Suppl B):63-67.
82 Daniel W.G., Mugge A., Martin R.P., et al. Improvement in the diagnosis of abscesses associated with endocarditis by transesophageal echocardiography. N Engl J Med. 1991;324:795-800.
83 Karchmur A.W., Infections of prosthetic valves and intravascular devices, Mandell G.L., Bennett J.E., Dolin R., editors, Principles and Practice of Infectious Disease; vol 1; 1999; Churchill Livingstone; Philadelphia, 903-917
84 Calderwood S.B., Swinski L.A., Karchmer A.W., et al. Prosthetic valve endocarditis. Analysis of factors affecting outcome of therapy. J Thorac Cardiovasc Surg. 1986;92:776-783.
85 Yu V.L., Fang G.D., Keys T.F., et al. Prosthetic valve endocarditis: Superiority of surgical valve replacement versus medical therapy only. Ann Thorac Surg. 1994;58:1073-1077.
86 Gordon S.M., Serkey J.M., Longworth D.L., et al. Early onset prosthetic valve endocarditis: The Cleveland Clinic experience 1992–1997. Ann Thorac Surg. 2000;69:1388-1392.
87 Habib G., Hoen B., Tornos P., et al. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): The Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Eur Heart J. 2009;30:2369-2413.
88 Bone R.C., Balk R.A., Cerra F.B., et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644-1655.
89 Muckart D.J., Bhagwanjee S. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference definitions of the systemic inflammatory response syndrome and allied disorders in relation to critically injured patients. Crit Care Med. 1997;25:1789-1795.
90 Levy M.M., Fink M.P., Marshall J.C., et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250-1256.
91 Natanson C., Hoffman W.D., Suffredini A.F., et al. Selected treatment strategies for septic shock based on proposed mechanisms of pathogenesis. Ann Intern Med. 1994;120:771-783.
92 Orbach S., Weiss Y., Deutschman C.S. The patient with sepsis or the systemic inflammatory response syndrome. In: Murray M.J., Coursin D.B., Pearl R.G., et al, editors. Critical Care Medicine: Perioperative Management. ed 2. Philadelphia: Lippincott Williams & Wilkins; 2002:601-615.
93 Vandijck D.M. Severe sepsis in critically ill patients: Early recognition and outcome. Acta Anaesthesiol Scand. 2010;54:658-659.
94 Tsalik E.L., Woods C.W. Sepsis redefined: The search for surrogate markers. Int J Antimicrob Agents. 2009;34(Suppl 4):S16-S20.
95 Nduka O.O., Parrillo J.E. The pathophysiology of septic shock. Crit Care Clin. 2009;25:677-702. vii
96 Hotchkiss R.S., Karl I.E. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138-150.
97 Landry D.W., Oliver J.A. The pathogenesis of vasodilatory shock. N Engl J Med. 2001;345:588-595.
98 Danai P., Martin G.S. Epidemiology of sepsis: Recent advances. Curr Infect Dis Rep. 2005;7:329-334.
99 Levy M.M., Dellinger R.P., Townsend S.R., et al. The Surviving Sepsis Campaign: Results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med. 2010;38:367-374.
100 Dellinger R.P., Carlet J.M., Masur H., et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Intensive Care Med. 2004;30:536-555.
101 Dellinger R.P., Levy M.M., Carlet J.M., et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med. 2008;34:17-60.
102 Levy M.M., Dellinger R.P., Townsend S.R., et al. The Surviving Sepsis Campaign: Results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med. 2010;38:367-374.
103 Laupland K.B., Zygun D.A., Davies H.D., et al. Incidence and risk factors for acquiring nosocomial urinary tract infection in the critically ill. J Crit Care. 2002;17:50-57.
104 Marik P.E. Fever in the ICU. Chest. 2000;117:855-869.
105 Platt R., Polk B.F., Murdock B., et al. Mortality associated with nosocomial urinary-tract infection. N Engl J Med. 1982;307:637-642.
106 Stark R.P., Maki D.G. Bacteriuria in the catheterized patient. What quantitative level of bacteriuria is relevant? N Engl J Med. 1984;311:560-564.
107 Brown D.F., Warren R.E. Effect of sample volume on yield of positive blood cultures from adult patients with haematological malignancy. J Clin Pathol. 1990;43:777-779.
108 Warren J.W. Catheter-associated urinary tract infections. Infect Dis Clin North Am. 1997;11:609-622.
109 Warren J.W., Platt R., Thomas R.J., et al. Antibiotic irrigation and catheter-associated urinary-tract infections. N Engl J Med. 1978;299:570-573.
110 Sandock D.S., Gothe B.G., Bodner D.R. Trimethoprim-sulfamethoxazole prophylaxis against urinary tract infection in the chronic spinal cord injury patient. Paraplegia. 1995;33:156-160.
111 Hall I.C., O’Toole E. Intestinal flora in new-born infants with a description of a new pathogenic anaerobe Bacillus difficilis. Am J Dis Child. 1935;49:390-402.
112 Bartlett J.G., Moon N., Chang T.W., et al. Role of Clostridium difficile in antibiotic-associated pseudomembranous colitis. Gastroenterology. 1978;75:778-782.
113 George R.H., Symonds J.M., Dimock F., et al. Identification of Clostridium difficile as a cause of pseudomembranous colitis. Br Med J. 1978;1:695.
114 Thomas C., Stevenson M., Riley T.V. Antibiotics and hospital-acquired Clostridium difficile-associated diarrhoea: A systematic review. J Antimicrob Chemother. 2003;51:1339-1350.
115 Kyne L., Warny M., Qamar A., et al. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N Engl J Med. 2000;342:390-397.
116 Kyne L., Warny M., Qamar A., et al. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet. 2001;357:189-193.
117 McFarland L.V., Mulligan M.E., Kwok R.Y., et al. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med. 1989;320:204-210.
118 Cunningham R., Dale B., Undy B., et al. Proton pump inhibitors as a risk factor for Clostridium difficile diarrhoea. J Hosp Infect. 2003;54:243-245.
119 Kyne L., Sougioultzis S., McFarland L.V., et al. Underlying disease severity as a major risk factor for nosocomial Clostridium difficile diarrhea. Infect Control Hosp Epidemiol. 2002;23:653-659.
120 Riegler M., Sedivy R., Pothoulakis C., et al. Clostridium difficile toxin B is more potent than toxin A in damaging human colonic epithelium in vitro. J Clin Invest. 1995;95:2004-2011.
121 Johnson S., Samore M.H., Farrow K.A., et al. Epidemics of diarrhea caused by a clindamycin-resistant strain of Clostridium difficile in four hospitals. N Engl J Med. 1999;341:1645-1651.
122 Kelly C.P., Pothoulakis C., LaMont J.T. Clostridium difficile colitis. N Engl J Med. 1994;330:257-262.
123 Rao S.S., Edwards C.A., Austen C.J., et al. Impaired colonic fermentation of carbohydrate after ampicillin. Gastroenterology. 1988;94:928-932.
124 Bartlett J.G. Clinical practice. Antibiotic-associated diarrhea. N Engl J Med. 2002;346:334-339.
125 Tedesco F.J. Antibiotic associated pseudomembranous colitis with negative proctosigmoidoscopy examination. Gastroenterology. 1979;77:295-297.
126 Triadafilopoulos G., Hallstone A.E. Acute abdomen as the first presentation of pseudomembranous colitis. Gastroenterology. 1991;101:685-691.
127 Wenisch C., Parschalk B., Hasenhundl M., et al. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile-associated diarrhea. Clin Infect Dis. 1996;22:813-818.
128 Zar F.A., Bakkanagari S.R., Moorthi K.M., et al. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302-307.
129 Bolton R.P., Culshaw M.A. Faecal metronidazole concentrations during oral and intravenous therapy for antibiotic associated colitis due to Clostridium difficile. Gut. 1986;27:1169-1172.
130 O’Connor J.R., Johnson S., Gerding D.N. Clostridium difficile infection caused by the epidemic BI/NAP1/027 strain. Gastroenterology. 2009;136:1913-1924.
131 Deutschman C.S., Wilton P., Sinow J., et al. Paranasal sinusitis associated with nasotracheal intubation: A frequently unrecognized and treatable source of sepsis. Crit Care Med. 1986;14:111-114.
132 Fassoulaki A., Pamouktsoglou P. Prolonged nasotracheal intubation and its association with inflammation of paranasal sinuses. Anesth Analg. 1989;69:50-52.
133 Grindlinger G.A., Niehoff J., Hughes S.L., et al. Acute paranasal sinusitis related to nasotracheal intubation of head-injured patients. Crit Care Med. 1987;15:214-217.
134 Rouby J.J., Laurent P., Gosnach M., et al. Risk factors and clinical relevance of nosocomial maxillary sinusitis in the critically ill. Am J Respir Crit Care Med. 1994;150:776-783.
135 Leal-Noval S.R., Rincon-Ferrari M.D., Garcia-Curiel A., et al. Transfusion of blood components and postoperative infection in patients undergoing cardiac surgery. Chest. 2001;119:1461-1468.
136 Mezrow C.K., Bergstein I., Tartter P.I. Postoperative infections following autologous and homologous blood transfusions. Transfusion. 1992;32:27-30.
137 Gu Y.J., de Vries A.J., Boonstra P.W., et al. Leukocyte depletion results in improved lung function and reduced inflammatory response after cardiac surgery. J Thorac Cardiovasc Surg. 1996;112:494-500.
138 Goodnough L.T., Despotis G.J., Hogue C.W.Jr, et al. On the need for improved transfusion indicators in cardiac surgery. Ann Thorac Surg. 1995;60:473-480.
139 van de Watering L.M., Hermans J., Houbiers J.G., et al. Beneficial effects of leukocyte depletion of transfused blood on postoperative complications in patients undergoing cardiac surgery: A randomized clinical trial. Circulation. 1998;97:562-568.
140 Blumberg N., Heal J.M. Transfusion and recipient immune function. Arch Pathol Lab Med. 1989;113:246-253.
141 Murphy G.J., Reeves B.C., Rogers C.A., et al. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007;116:2544-2552.
142 Koch C.G., Li L., Duncan A.I., et al. Morbidity and mortality risk associated with red blood cell and blood-component transfusion in isolated coronary artery bypass grafting. Crit Care Med. 2006;34:1608-1616.
143 Marik P.E., Corwin H.L. Efficacy of red blood cell transfusion in the critically ill: A systematic review of the literature. Crit Care Med. 2008;36:2667-2674.
144 Rogers M.A., Blumberg N., Saint S., et al. Hospital variation in transfusion and infection after cardiac surgery: A cohort study. BMC Med. 2009;7:37.
145 Hebert P.C., Wells G., Blajchman M.A., et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409-417.
146 Johnson R.G., Thurer R.L., Kruskall M.S., et al. Comparison of two transfusion strategies after elective operations for myocardial revascularization. J Thorac Cardiovasc Surg. 1992;104:307-314.
147 Weisel R.D., Charlesworth D.C., Mickleborough L.L., et al. Limitations of blood conservation. J Thorac Cardiovasc Surg. 1984;88:26-38.
148 Dellinger R.P., Carlet J.M., Masur H., et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32:858-873.
149 Dellinger R.P., Levy M.M., Carlet J.M., et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296-327.
150 Rao S.V., Jollis J.G., Harrington R.A., et al. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA. 2004;292:1555-1562.
151 Lee H.T., Sladen R.N. Perioperative renal protection. In: Murray M.J., Coursin D.B., Pearl R.G., et al, editors. Critical Care Medicine: Perioperative Management. ed 2. Philadelphia: Lippincott Williams & Wilkins; 2002:501-520.
152 Bellomo R., Kellum J.A., Ronco C. Defining acute renal failure: Physiological principles. Intensive Care Med. 2004;30:33-37.
153 Block C.A., Manning H.L. Prevention of acute renal failure in the critically ill. Am J Respir Crit Care Med. 2002;165:320-324.
154 Chertow G.M., Levy E.M., Hammermeister K.E., et al. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med. 1998;104:343-348.
155 Conlon P.J., Stafford-Smith M., White W.D., et al. Acute renal failure following cardiac surgery. Nephrol Dial Transplant. 1999;14:1158-1162.
156 Higgins T.L., Estafanous F.G., Loop F.D., et al. Stratification of morbidity and mortality outcome by preoperative risk factors in coronary artery bypass patients. A clinical severity score. JAMA. 1992;267:2344-2348.
157 Hilberman M., Derby G.C., Spencer R.J., et al. Sequential pathophysiological changes characterizing the progression from renal dysfunction to acute renal failure following cardiac operation. J Thorac Cardiovasc Surg. 1980;79:838-844.
158 Bellomo R., Ronco C., Kellum J.A., et al. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204-R212.
159 Heringlake M., Knappe M., Vargas Hein O., et al. Renal dysfunction according to the ADQI-RIFLE system and clinical practice patterns after cardiac surgery in Germany. Minerva Anestesiol. 2006;72(7–8):645-654.
160 Kuitunen A., Vento A., Suojaranta-Ylinen R., et al. Acute renal failure after cardiac surgery: Evaluation of the RIFLE classification. Ann Thorac Surg. 2006;81:542-546.
161 Lin C.Y., Chen Y.C., Tsai F.C., et al. RIFLE classification is predictive of short-term prognosis in critically ill patients with acute renal failure supported by extracorporeal membrane oxygenation. Nephrol Dial Transplant. 2006;21:2867-2873.
162 Baldwin L., Henderson A., Hickman P. Effect of postoperative low-dose dopamine on renal function after elective major vascular surgery. Ann Intern Med. 1994;120:744-747.
163 Lassnigg A., Donner E., Grubhofer G., et al. Lack of renoprotective effects of dopamine and furosemide during cardiac surgery. J Am Soc Nephrol. 2000;11:97-104.
164 Murray P., Hall J. Renal replacement therapy for acute renal failure. Am J Respir Crit Care Med. 2000;162(3 Pt 1):777-781.
165 Sward K., Valsson F., Odencrants P., et al. Recombinant human atrial natriuretic peptide in ischemic acute renal failure: A randomized placebo-controlled trial. Crit Care Med. 2004;32:1310-1315.
166 de Denus S., Pharand C., Williamson D.R. Brain natriuretic peptide in the management of heart failure: The versatile neurohormone. Chest. 2004;125:652-668.
167 Textor S.C., Glockner J.F., Lerman L.O., et al. The use of magnetic resonance to evaluate tissue oxygenation in renal artery stenosis. J Am Soc Nephrol. 2008;19:780-788.
168 Bellomo R., Ronco C. Continuous renal replacement therapy in the intensive care unit. Intensive Care Med. 1999;25:781-789.
169 Forni L.G., Hilton P.J. Continuous hemofiltration in the treatment of acute renal failure. N Engl J Med. 1997;336:1303-1309.
170 Lewis J., Chertow G.M., Paganini E.P., et al. A multicenter survey of patient characteristics, practice patterns, and outcomes in critically ill patients with ARF [Abstract]. J Am Soc Nephrol. 2000;8:A0673.
171 Mehta R.L., Letteri J.M. Current status of renal replacement therapy for acute renal failure. A survey of US nephrologists. The National Kidney Foundation Council on Dialysis. Am J Nephrol. 1999;19:377-382.
172 Silvester W. Outcome studies of continuous renal replacement therapy in the intensive care unit. Kidney Int Suppl. 1998;66:S138-S141.
173 Butterworth C.E.Jr. Editorial: Malnutrition in the hospital. JAMA. 1974;230:879.
174 The skeleton in the hospital closet—20 years later: Malnutrition in patients with GI disease, cancer and AIDS, Proceedings of a conference. Los Angeles, California, October 1–2, 1994. Nutrition. 1995;11(2 Suppl):192-254.
175 Blackburn G.L., Ahmad A. Skeleton in the hospital closet—then and now. Nutrition. 1995;11(2 Suppl):193-195.
176 Hirsch S., de Obaldia N., Petermann M., et al. Subjective global assessment of nutritional status: further validation. Nutrition. 1991;7:35-37. discussion 37–38
177 Akhtar S., Barash P.G., Inzucchi S.E. Scientific principles and clinical implications of perioperative glucose regulation and control. Anesth Analg. 2010;110:478-497.
178 Hermanides J., Vriesendorp T.M., Bosman R.J., et al. Glucose variability is associated with intensive care unit mortality. Crit Care Med. 2010;38:838-842.
179 Kompan L., Kremzar B., Gadzijev E., et al. Effects of early enteral nutrition on intestinal permeability and the development of multiple organ failure after multiple injury. Intensive Care Med. 1999;25:157-161.
180 Murrary M.J., Brull S.J., Coursin D.B. Strict blood glucose control in the ICU: Panacea or Pandora’s box? J Cardiothorac Vasc Anesth. 2004;18:687-689.
181 Martindale R.G., McClave S.A., Vanek V.W., et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition: Executive Summary. Crit Care Med. 2009;37:1757-1761.
182 National Heart, Lung, and Blood Institute, Early Versus Delayed Enteral Feeding and Omega-3 Fatty Acid/Antioxidant Supplementation for Treating People With Acute Lung Injury or Acute Respiratory Distress Syndrome (The EDEN-Omega Study); 2008; Available at: http://clinicaltrials.gov/ct2/show/results/NCT00609180 Accessed March 10, 2010