Locally advanced breast cancer
Introduction
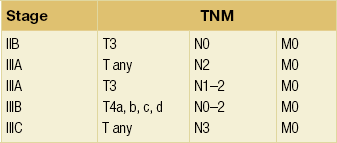
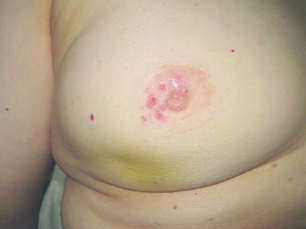
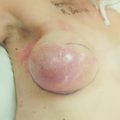
Locally advanced breast cancer (Fig. 13.1) includes primary breast tumours with diameters of greater than 5 cm, breast cancers with frank skin or chest wall involvement, and any size of tumour with certain degrees of nodal involvement, but excludes cancers that have spread further than local, supra/infraclavicular and internal mammary chain nodes.1 Locally advanced breast cancer includes all cancers of ‘TNM’ stage III, but also a subset of stage II cancers (Table 13.1). The definition of locally advanced breast cancer includes inflammatory breast cancer (Fig. 13.2), which is characterised by involvement of the dermal lymphatic vessels by cancer cells that produces an inflamed, erythematous appearance to the whole breast. The management of locally advanced breast cancer should be multidisciplinary in nature, and based on the extent of the disease, as assessed with computed tomography (CT) scanning of chest, abdomen and pelvis, and, if required, isotope bone scanning, magnetic resonance imaging (MRI) or positron emission tomography (PET) to detect or confirm metastatic disease (Chapter 1). Histological confirmation of the tumour type along with appropriate immunohistochemistry for oestrogen receptor and human epidermal growth factor (HER-2) receptor (supplemented by fluorescence in situ hybridisation (FISH) analysis of HER-2 status) is required before embarking on therapy (Chapter 2). Multimodal therapy is generally needed and the timing and nature of treatments will vary depending on whether the disease is confined to operable areas, whether there is involvement of internal mammary or supraclavicular lymph nodes, and above all the general fitness of the patient.
Surgical management
Pathological confirmation of the clinical diagnosis of locally advanced breast cancer should be made by core biopsy to distinguish invasive breast cancer from ductal carcinoma in situ (DCIS) or metastatic disease to the breast from another primary site. If the patient is likely to be a candidate for neoadjuvant therapy, placement of a radiological marker in the tumour immediately following the core biopsy is required to facilitate accurate surgical resection or to localise the site of the cancer for the pathologist if mastectomy is subsequently performed.2 However, on occasion, excision of a skin nodule under local anaesthetic may be useful to confirm the diagnosis and get tissue for receptor analysis. Resectional surgery aims to secure clear surgical margins around the cancer. There is a balance between the completeness of excision (margin involvement is linked to recurrence) versus poorer cosmetic results from more extensive surgery. If the cancer is not excisable by conventional means then neoadjuvant therapy or radiotherapy are the only treatment options. Radical mastectomy (including excision of pectoralis major and level III axillary clearance) does not improve survival over total mastectomy (odds ratio of death 0.98 over 10 years3) and is rarely, if ever, indicated even in locally advanced breast cancer. Since survival following breast conservation is not significantly different from that following mastectomy, this has led most centres to use neoadjuvant therapy for locally advanced breast cancer as initial treatment followed by surgery, including the option of breast conservation where appropriate. Where neoadjuvant therapy has produced an excellent clinical and radiological response, effective surgery is possible only if a surgical marker was inserted prior to commencing neoadjuvant treatment, as the tumour may not be visible radiologically. As for all breast conservation surgery (Chapter 4), complete surgical excision with clear margins of at least 1 mm on pathology review is required to reduce disease recurrence. The term ‘toilet mastectomy’ has historically been applied to mastectomy performed to excise locally advanced breast cancer. Surgery alone rarely controls such cancers and combination with radiotherapy and/or systemic therapy is now standard. Excision of bulky disease in the breast previously led to difficulties in skin closure with the need for split-skin grafting or application of an omental flap to the chest wall but the widespread use of skin-bearing autologous flaps, particularly the latissimus dorsi flap, has made primary closure possible in all but a few patients.
Axillary surgery
Previously, level III axillary node clearance was the axillary surgery of choice in patients with locally advanced breast cancer due to the high probability of axillary lymph node involvement. Given the significant morbidity associated with level III axillary clearance, for patients undergoing neoadjuvant therapy who were node negative at diagnosis or have a good clinical response this is being challenged. Sentinel lymph node biopsy of the post-treatment axilla is now being used to guide therapy and is of similar efficacy to that in patients who have not received neoadjuvant therapy4 (see Chapter 7). Although some advocate sentinel node biopsy prior to neoadjuvant therapy this is illogical and denies those whose nodes are cleared by this treatment the option of sentinel node biopsy after completion of their systemic therapy. It is useful, however, to consider sentinel node biopsy prior to a definitive surgical procedure in patients who are having mastectomy and undergoing breast reconstruction, in order to guide the extent of the axillary node dissection. Axillary nodes are converted to node negative in approximately 35% of patients by neoadjuvant chemotherapy. The rate of conversion from positive to negative relates to tumour type. Between 25% and 50% of involved nodes in triple negative cancers, 40–60% in HER-2-positive patients who receive trastuzumab but less than 10% of nodes in patients with oestrogen receptor-positive breast cancers are converted from positive to negative.
Breast reconstruction in locally advanced breast cancer
For those patients undergoing mastectomy either as primary treatment or following neoadjuvant therapy, there remains controversy regarding immediate breast reconstruction (Chapter 9). Although there may be concerns that delayed wound healing (on the chest wall or at donor sites for autologous reconstruction) may delay the delivery of postoperative adjuvant chemotherapy or postoperative radiotherapy5 and the need to avoid radiotherapy following reconstruction with prostheses, few patients experience significant delays. Some surgeons do not offer immediate reconstruction to patients with locally advanced disease because of the likelihood postoperative radiotherapy will be required. Over the past 15 years, US practice has swung from immediate reconstruction for patients with locally advanced breast cancer to a more conservative approach of delayed breast reconstruction. There is the option for patients wishing reconstruction of a skin-sparing mastectomy and placement of a tissue expander that is deflated during radiotherapy and reflated shortly after completion of radiation. Thereafter, an autologous reconstruction with a myocutaneous flap utilises the preserved skin. Autologous abdominal flap reconstruction at the time of mastectomy has been reported by some to produce unsatisfactory long-term outcomes following radiotherapy, and with modern postoperative radiotherapy techniques in the UK it does not appear to prejudice the cosmetic outcome nor the oncological management.6
Radiotherapy
Radiotherapy is thought to work mainly by causing damage to the DNA of tumour cells, which is repaired more slowly than the damage caused in the adjacent normal tissue. Tumour tissue is therefore preferentially destroyed compared with the normal tissue over a course of radiotherapy treatment. In general, the chance of tumour control increases with increasing total dose of radiotherapy given, but so does the chance of permanent radiotherapy-related side-effects, some of which have been implicated in treatment-related mortality7 (Box 13.1).
Certain tissues (termed ‘late-responding tissues’) such as nerves and, to some extent, lung can withstand a higher total dose of radiotherapy without giving rise to symptomatic damage if the dose of each separate radiotherapy treatment (or ‘fraction’) is kept low. Traditionally, a course of radiotherapy that was thought to give the best chance of long-term tumour control comprised a relatively large total dose given over several weeks by using small daily fractions. Whilst shorter palliative courses of treatment may be considered for less fit patients, the total dose has to be reduced to minimise the toxicity of the treatment, because the fraction size for such short courses is by necessity large.8 The reduction in the total radiotherapy dose for such short courses often means that any benefit obtained from radiotherapy is short-lived and may not be worth the inconvenience to the patient of the treatment, and so many oncologists advocate longer courses over several weeks for advanced breast cancer. This view is being challenged, partly because of the results of large randomised radiotherapy studies such as the START trial,9 which has demonstrated that shorter, ‘hypofractionated’ regimens using a lower total dose than has been traditional produce good outcome results with a trend towards fewer side-effects. It remains to be seen whether further research into much shorter hypofractionated courses yields similar results.
Adjuvant (postoperative) radiotherapy
When treating locally advanced breast cancer, whatever surgery is performed on the breast, postoperative radiotherapy significantly reduces locoregional recurrence and improves overall survival for both premenopausal10 and postmenopausal11 women, and modern techniques do not have the cardiac morbidity of older treatments.12 Postoperative radiotherapy (which may be given after adjuvant chemotherapy) reduces local recurrence from 35% without radiotherapy to 8% with radiotherapy and improves disease-free survival at 10 years from 24% to 36%.
Radiotherapy following the surgical treatment of locally advanced breast cancer is given in the same way as treatments for early breast cancer, the aim being to reduce local relapse by about two-thirds and improve survival.13 Locally advanced breast cancer, by definition, has many features that predict for local relapse (large tumour size, lymph node involvement, close or positive margins despite adequate surgery). Thus, postoperative radiotherapy is usually given,14 even if there has been a good response to neoadjuvant treatment, as the risk of local relapse in larger tumours is higher than for less advanced cancers.15 The chest wall or residual conserved breast is treated and the peripheral lymphatics are irradiated as appropriate16 (see Chapter 15). It is unusual to irradiate the internal mammary chain nodes, as evidence for the efficacy of this treatment is lacking, although much of the data relates to trials that are decades old.17
Primary radical radiotherapy
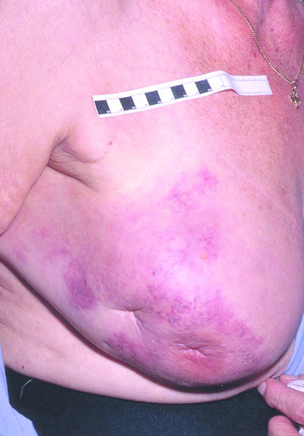
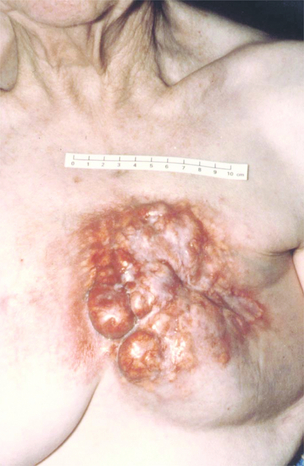
If the patient is not fit for or declines surgery, and is fit for radiotherapy, then radiotherapy may be used as the principal local treatment. It is unusual to ‘cure’ a locally advanced breast cancer without surgery, but sometimes excellent local control can be obtained (Figs 13.3 and 13.4) and unpleasant symptoms of the tumour, such as pain, discharge, bleeding and odour, can be partially or completely removed following radiotherapy treatment.
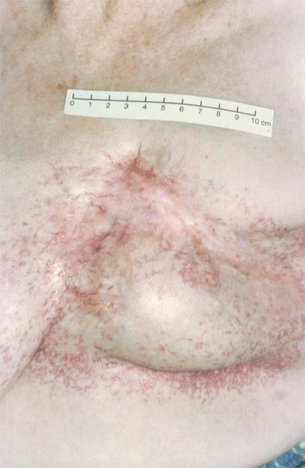
Figure 13.4 Locally advanced breast cancer as in Fig. 13.3 following radiotherapy.
While there is little evidence to support the use of routine radiotherapy prior to definitive surgery, radiotherapy has been used as an alternative to surgery following neoadjuvant chemotherapy. In women with stage III disease given neoadjuvant chemotherapy, disease control, median survival (39 months) and relapse rates appear similar in studies that randomised patients to radiotherapy or surgery.18,19 For patients with disease suitable for breast-conserving surgery, the combination of excision and radiotherapy improves local control compared to radiotherapy alone.
Systemic treatment
If the patient has locally advanced disease and is fit for treatment, then systemic therapy is usually considered, as the risk of relapse in locally advanced breast cancer is high. In addition, neoadjuvant treatment is often needed to ‘downstage’ some patients with locally advanced breast cancer to allow surgery to be completed successfully and sometimes thereafter breast conservation can be achieved. Although cancer that has spread to the ipsilateral supraclavicular lymph nodes was formerly considered ‘metastatic’ disease, patients with isolated supraclavicular disease as the only site of distant disease do just as well in terms of survival as those patients with lesser stages of locally advanced breast cancer.20
Neoadjuvant treatment
Neoadjuvant chemotherapy
Neoadjuvant chemotherapy is as effective as adjuvant chemotherapy in terms of survival in locally advanced breast cancer. Randomised trials have shown a possible long-term advantage for neoadjuvant chemotherapy, particularly in young women (Fig. 13.5a,b). Neoadjuvant chemotherapy is associated with a 31% reduction in overall infective episodes compared to adjuvant chemotherapy.22 The choice of agents for locally advanced breast cancer is determined by emerging trial data and by local preferences, but is often similar or the same as the chemotherapy regimens given for adjuvant therapy. There are no compelling data on the optimum number of cycles of chemotherapy but there may be some advantage in delivering the whole chemotherapy course at once rather than in a perioperative fashion, as splitting it may give the worst of both worlds, by exposing the patient to side-effects both before and after their surgery with no evidence of marked clinical advantage. It has been shown that HER-2-positive tumours are more likely (by about three times) to show a complete pathological response after treatment with conventional neoadjuvant chemotherapy compared to HER-2-negative cancers, although the overall outlook for such tumours is not as good as for those that are HER-2 negative.23 Neoadjuvant trastuzumab has been used in conjunction with chemotherapy and has shown improved rates of complete pathological response compared to chemotherapy alone, even in groups of patients that include a significant proportion of more advanced disease.24 Trials are underway, and showing promising results, from other inhibitors of this signalling pathway, using the agents lapatinib, pertuzumab and T-DM1 in different combinations with trastuzumab. A combination of chemotherapy and trastuzumab together with either lapatanib or pertuzumab appears superior to chemotherapy or trastuzumab alone.
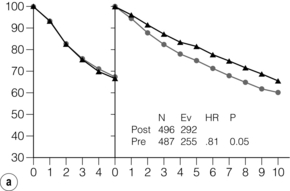
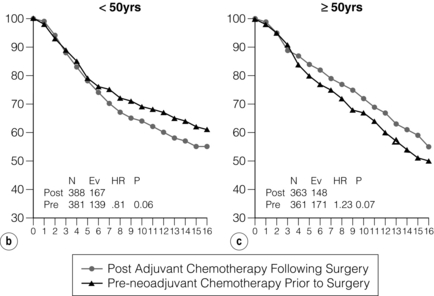
Figure 13.5 Disease-free survival in National Surgical Adjuvant Breast and Bowel Project (NSABP) B18 from 0–5 and 5–15 years (a) and overall survival split into age groups (b) < 50 and ≥ 50 (c) years of age. N = number of events; Ev = Events; HR = hazard ratio. Overall, disease free survival is increased. This reaches significance beyond 5 years in patients treated with neoadjuvant rather than adjuvant chemotherapy. However, the benefit may be confined to premenopausal women.
The primary tumour should be assessed prior to treatment, with documentation of its position in the breast, accurate measurements of dimensions of the mass both clinically and on imaging, and a description of the appearance of the lump and any changes associated with it (Figs 13.1 and 13.2). A clinical photograph often helps with future assessments if there is a visible mass or abnormality and helps assess response to treatment. Any palpable local lymph nodes that are thought to be involved should be measured carefully in the same way. Imaging,25 including ultrasonography, mammography, MRI or PET/CT scanning, is required to obtain an accurate assessment of the extent of disease before treatment and assessment of response after chemotherapy has been given if baseline images are obtained. Marker placement can complement imaging by locating the original position of the tumour, and facilitates tumour site localisation following successful neoadjuvant therapy. Some are now placing tumour markers in involved lymph nodes to confirm that in patients with a negative sentinel node biopsy after an excellent response to neoadjuvant therapy the node known to be involved prior to therapy has been removed. There are some data suggesting that MRI scanning after one cycle may yield information about likely response to chemotherapy.26 There are also data to indicate that MRI is accurate in most patients at assessing response, but in a significant number can overestimate or underestimate the extent of residual disease following neoadjuvant chemotherapy.27 Assessment is usually performed after two or three cycles of treatment and if the tumour responds, then chemotherapy should continue for up to eight cycles28 and the patient then proceeds to definitive curative surgery.
Despite much research into markers to predict prognosis after neoadjuvant chemotherapy (Chapter 2), the degree of involvement of axillary nodes following neoadjuvant chemotherapy remains the best predictor of subsequent relapse.29 Pathological complete response is associated with improved overall survival22 and is more likely to be seen in younger women with higher grade invasive ductal carcinoma that is oestrogen receptor/progesterone receptor negative and HER-2 positive.30 It is important to remember that both tumour grade and receptor immunohistochemistry can change following treatment31 when pre- and post-chemotherapy pathological specimens are examined after neoadjuvant chemotherapy for advanced breast cancer.
Neoadjuvant/therapeutic endocrine therapy
If the tumour is positive for oestrogen or progesterone receptor immunohistochemistry, then the patient may be treated with neoadjuvant oestrogen blockade instead of with chemotherapy. The side-effects are less with endocrine therapy than with chemotherapy, although time to response is generally slower and complete pathological response less likely. The majority of patients treated with neoadjuvant hormone therapy have been postmenopausal. The pathological changes seen following neoadjuvant treatment with an aromatase inhibitor such as letrozole appear to be different from those seen with neodjuvant chemotherapy, with more ‘central scarring’ using this type of oestrogen blockade, compared with more cancers showing diffuse changes and a complete pathological response rate with chemotherapy.32 Neoadjuvant therapy with oestrogen blockade can potentially increase anxiety in patients (and clinicians) and could potentially lead to delay in switching to an effective treatment should the first agent used be ineffective, as about 3 months of anti-oestrogen treatment are needed before a true impression of response can be obtained. Progression during treatment is rare with endocrine therapy and occurs in less than 5% of patients. The relative lack of side-effects, and the potential for excellent tumour response,33 make neoadjuvant endocrine treatment an attractive prospect. Primary endocrine blockade also acts as a chemotherapy-sparing treatment strategy, allowing more options in the future as the patient is chemotherapy naive. Some work has been done comparing neoadjuvant oestrogen blockade with neoadjuvant chemotherapy in older women with hormone-sensitive breast cancers; the results of the two treatments, in one small study, appeared to be equivalent for outcomes such as response rate and time to response,34 with a suggestion of a greater rate of conversion from planned mastectomy to breast-conserving surgery with endocrine therapy. The optimal duration for endocrine therapy is a matter for debate, but it is clear that treatments of around 9 months or more are clinically acceptable and appear to produce better results than treatments of shorter duration.35
For many years, it has been clear that postmenopausal women with advanced disease, including metastatic disease, respond to an aromatase inhibitor at least as well36 or better37 than to tamoxifen. In the treatment of locally advanced and metastatic breast cancer in postmenopausal women, treatment failure rate is lower with anastrozole (79%) compared with tamoxifen (84%), with a median time to progression of 11.1 months for anastrozole and 5.6 months for tamoxifen.38 Letrozole is superior to tamoxifen in terms of objective response rate (30% vs. 20%) and treatment failure (85% vs. 75%).39
The difference could be related to a genuine difference between different classes of oestrogen blocking drugs. In addition, it is known that tamoxifen metabolism by the cytochrome P450 system varies in different individuals according to genotype, adherence to taking the medication and potential interaction with other drugs, but the influence of individual variation in the metabolism of tamoxifen (and indeed aromatase inhibitors) and their subsequent efficacy is unclear.40 The pure oestrogen antagonist, fulvestrant, is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment,41 but the place of this drug, which must be given parenterally, in the treatment of locally advanced breast cancer is still under evaluation.
Adjuvant systemic treatment
Adjuvant systemic treatment for advanced breast cancer that has been treated successfully by surgery is very similar to that given for early-stage breast cancer. Locally advanced breast cancer has a higher risk of relapse than early-stage breast cancer, and so it is likely that following surgery the patient will be offered adjuvant oestrogen blockade, local radiotherapy and therapies targeting specific signalling pathways such as the HER-2/neu transmembrane receptor, assuming that the tumour is found to be of the correct biological type for the patient to potentially benefit from such targeted agents (Chapter 2). Certainly, adding chemotherapy or endocrine therapy or both with radiotherapy for stage IIIB disease reduces locoregional recurrence from 60% to 50% but may not secure a long-term survival advantage.
Problems of locally recurrent disease after previous treatment
Local recurrence of advanced breast cancer may occur in isolation or in conjunction with overt metastatic disease. Since histological confirmation of disease recurrence is required and the oestrogen, progesterone or HER-2 receptor status may change between the primary and the recurrent disease for one in six patients,42 biopsy of the locally recurrent cancer should be performed. It is essential to know what treatment a patient has received in the past to be able to advise them on future treatment for a recurrent cancer. For example, if the patient has only had a sentinel node biopsy or axillary sampling procedure, and further breast surgery is required to treat recurrent disease, then an axillary clearance should be considered.
A recent study has shown chemotherapy prolongs survival for isolated or regional recurrence of breast cancer.43 The use of systemic treatment for advanced, recurrent disease will depend on what was given as adjuvant therapy at the time of the original diagnosis. In general, different chemotherapy agents are used to treat the recurrent disease, on the basis that the ones used originally did not sterilise the tumour completely, and also because some, notably the anthracyclines, have cumulative cardiac toxicity that can be serious. Likewise, there may be some gain from rotating oestrogen-blocking agents to palliate locally advanced cancers when there are no other treatment options. Tamoxifen may work when aromatase inhibitors start to fail and vice versa. Sometimes older agents such as megestrol acetate or newer agents such as fulvestrant are effective when other agents fail to control the cancer.
The use of biological agents continues to evolve. Trastuzumab in locally advanced disease typically mimics the efficacy in metastatic disease (for inoperable locally advanced cancers) or as adjuvant treatment (in those advanced cancers amenable to more radical treatment). However, trastuzumab is seldom used as a single agent, as the response rates in combination with chemotherapy are much better,44 although ongoing work on the use of other agents such as lapatinib in combination with trastuzumab, pertuzumab, T-DM1, chemotherapy and other biologicals may provide new advances in the care of these patients.
References
1. Giordano, S., Update on locally advanced breast cancer. Oncologist 2003; 8:521–530. 14657530
2. Coles, C.E., Wilson, C.B., Cumming, J., et al, Titanium clip placement to allow accurate tumour bed localisation following breast conserving surgery: audit on behalf of the IMPORT Trial Management Group. Eur J Surg Oncol 2009; 35:578–582. 18938055
3. Early Breast Cancer Trialists’ Collaborative Group, Effects of radiotherapy and surgery in early breast cancer: an overview of the randomised trials. N Engl J Med 1995; 333:1444–1455. 7477144
4. Xing, Y., Foy, M., Cox, D.D., et al, Meta-analysis of sentinel lymph node biopsy after preoperative chemotherapy in patients with breast cancer. Br J Surg. 2006;93(5):539–546. 16329089 Sentinel lymph node biopsy of the post-treatment axilla may be used to guide therapy.
5. Motwani, S.B., Strom, E.A., Schechter, N.R., et al, The impact of immediate breast reconstruction on the technical delivery of postmastectomy radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66(1):76–82. 16765534
6. Chatterjee, J.S., Lee, A., Anderson, A., et al, The effects of post-operative radiotherapy on autologous Deep Inferior Epigastric Perforator (DIEP) flap volume in immediate postmastectomy breast reconstruction. Br J Surg 2009; 96:1135–1140. 19787763
7. Early Breast Cancer Trialists’ Collaborative Group, Radiotherapy for early breast cancer (Cochrane Review)The Cochrane Library. Oxford: Update Software, 2002. [Issue 2].
8. Adamson, D.J.A. The radiobiological basis of radiation side effects. In: Faithfull S., Wells M., eds. Supportive care in radiotherapy. Edinburgh: Churchill Livingstone; 2003:71–96.
9. The START Trialists’ Group, The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet Oncol. 2008;9(4):331–341. 18356109
10. Overgaard, M., Hansen, P.S., Overgaard, J., et al, Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant radiotherapy. N Engl J Med 1997; 337:949–955. 9395428
11. Overgaard, M., Jensen, M.B., Overgaard, J., et al, Postoperative radiotherapy in high-risk postmenopausal women with breast cancer given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet 1999; 353:1641–1648. 10335782 The above two papers show that postoperative radiotherapy reduces locoregional recurrence and improves survival.
12. Hojris, I., Overgaard, M., Christensen, J.J., et al, Morbidity and mortality of ischaemic heart disease in high-risk breast-cancer patients after adjuvant postmastectomy systemic treatment with or without radiotherapy: analysis of DBCG 82b and 82c randomised trials. Radiotherapy Committee of the Danish Breast Cancer Cooperative Group. Lancet. 1999;354(9188):1425–1430. 10543669
13. Whelan, T.J., Julian, J., Wright, J., et al, Does loco-regional radiation therapy improve survival in breast cancer? A meta-analysis. J Clin Oncol. 2000;18(6):1220–1229. 10715291
14. Chua, B., Olivotto, I.A., Weir, L., et al, Increased use of adjuvant regional radiotherapy for node-positive breast cancer in British Columbia. Breast J. 2004;10(1):38–44. 14717758
15. Rouzier, R., Extra, J.-M., Carton, M., et al, Primary chemotherapy for operable breast cancer: incidence and prognostic significance of ipsilateral breast tumour recurrence after breast-conserving surgery. J Clin Oncol. 2001;19(18):3828–3835. 11559720
16. Recht, A., Edge, S.B., Solin, L.J., et al, Postmastectomy radiotherapy: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19(5):1539–1569. 11230499
17. SIGN Clinical Guideline Number 84. Management of breast cancer in women. Edinburgh: Scottish Intercollegiate Guidelines Network; 2005.
18. Perloff, M., Lesnick, G.J., Korzun, A., et al, Combination chemotherapy with mastectomy or radiotherapy for stage III breast carcinoma: a Cancer and Leukaemia Group B Study. J Clin Oncol 1988; 6:261–269. 3276824
19. De Lena, M., Varini, M., Zucali, R., et al, Multimodality treatment for locally advanced breast cancer. Results of chemotherapy–radiotherapy versus chemotherapy–surgery. Cancer Clin Trials 1981; 4:229–236. 7026073 After neoadjuvant chemotherapy, radical radiotherapy may have equivalent relapse and survival to surgical resection.
20. Wolff, A.C., Systemic therapy. Curr Opin Oncol 2001; 13:436–449. 11673683
21. Deo, S.V., Bhutani, M., Shukla, N.K., et al, Randomized trial comparing neo-adjuvant versus adjuvant chemotherapy in operable locally advanced breast cancer (T4b N0–2 M0). J Surg Oncol. 2003;84(4):192–197. 14756429 Paper showing equivalence of neoadjuvant chemotherapy compared with adjuvant in terms of survival.
22. Mieog, J.S.D., van der Hage, J.A., van de Velde, C.J.H., Neoadjuvant chemotherapy for operable breast cancer. Br J Surg 2007; 94:1198–1200. 17701939
23. Penault-Llorca, F., Abrial, C., Mouret-Reynier, M.-A., et al, Achieving higher pathological complete response rates in HER-2-positive patients with induction chemotherapy without trastuzumab in operable breast cancer. Oncologist 2007; 12:390–396. 17470681
24. Limentani, S.A., Brufsky, A.M., Erban, J.K., et al, Phase II study of neoadjuvant docetexel, vinorelbine, and trastuzumab followed by surgery and adjuvant doxorubicin plus cyclophosphamide in women with human epidermal growth factor receptor 2-overexpressing locally advanced breast cancer. J Clin Oncol. 2007;25(10):1232–1238. 17296975
25. Hsiang, D.J., Yamamoto, M., Mehta, R.S., et al, Predicting nodal status using dynamic contrast-enhanced magnetic resonance imaging in patients with locally advanced breast cancer undergoing neoadjuvant chemotherapy with and without sequential trastuzumab. Arch Surg. 2007;142(9):855–861. 17875840
26. Melsamy, S., Bolan, P.J., Baker, E.H., et al, Neoadjuvant chemotherapy of locally advanced breast cancer: predicting response with in vivo 1H MR spectroscopy – a pilot study at 4 T. Radiology 2004; 233:424–431. 15516615
27. Kwong, M.S., Chung, G.C., Horvath, L.J., et al, Postchemotherapy MRI overestimates residual disease compared with histopathology in responders to neoadjuvant therapy for locally advanced breast cancer. Cancer J. 2006;12(3):212–221. 16803680
28. Rastogi, P., Anderson, S.J., Bear, H.D., et al, Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26(5):778–785. 18258986
29. Escobare, P.F., Patrick, R.J., Rybicki, L.A., et al, Prognostic significance of residual breast disease and axillary node involvement for patients who had primary induction chemotherapy for advanced breast cancer. Ann Surg Oncol. 2006;13(6):783–787. 16604475
30. Huober, J., von Minckwitz, G., Denkert, C., et al, Effect of neoadjuvant anthracycline–taxane-based chemotherapy in different biological breast cancer phenotypes: overall results from the GeparTrio study. Breast Cancer Res Treat 2010; 124:133–140. 20697801
31. Shet, T., Agrawal, A., Chinoy, R., et al, Changes in the tumor grade and biological markers in locally advanced breast cancer after chemotherapy – implications for a pathologist. Breast J. 2007;13(5):457–464. 17760666
32. Thomas, J.S.J., Julian, H.S., Green, R.V., et al, Histopathology of breast carcinoma following neoadjuvant systemic therapy: a common association between letrozole therapy and central scarring. Histopathology 2007; 51:219–226. 17650216
33. Dixon, J.M., Love, C.D.B., Bellamy, C.O.C., et al, Letrozole as primary medical therapy for locally advanced and large operable breast cancer. Breast Cancer Res Treat. 2001;66(3):191–199. 11510690
34. Semiglazov, V.F., Semiglazov, V.V., Dashyan, G.A., et al, Phase 2 randomized trial of primary endocrine therapy versus chemotherapy in postmenopausal patients with oestrogen receptor-positive breast cancer. Cancer. 2007;110(2):244–254. 17538978
35. Llombart-Cussac, A., Guerrero, A., Galan, A., et al, Phase II trial with letrozole to maximum response as primary systemic therapy in postmenopausal patients with ER/PgR[+] operable breast cancer. Clin Transl Oncol. 2012;14(2):125–131. 22301401
36. Bonneterre, J., Thürlimann, B., Robertson, J.F.R., et al, Anastrozole versus tamoxifen as first-line therapy for advanced breast cancer in 668 postmenopausal women: results of the tamoxifen or arimidex randomized group efficacy and tolerability study. J Clin Oncol. 2000;18(22):3748–3757. 11078487
37. Macaskill, E.J., Renshaw, L., Dixon, J.M., Neoadjuvant use of hormonal therapy in elderly patients with early or locally advanced hormone receptor-positive breast cancer. Oncologist 2006; 11:1081–1088. 17110627
38. Nabholtz, J.M., Buzdar, A., Pollak, M., et al, Anastrozole is superior to tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: results of a North American multicenter randomized trial. J Clin Oncol. 2000;18(22):3758–3767. 11078488
39. Mouridsen, H., Gershanovich, M., Sun, Y., et al, Superior efficacy of letrozole (Femara) versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: results of a phase III study of the International Breast Cancer Group. J Clin Oncol. 2001;19(10):2596–2606. 11352951 Aromatase inhibitors may be more effective than tamoxifen in the neoadjuvant treatment of locally advanced breast cancer.
40. Thompson, A.M., Johnson, A., Quinlan, P., et al, Comprehensive CYP2D6 genotype and adherence affect outcome in breast cancer patients treated with tamoxifen monotherapy. Breast Cancer Res Treat 2010; 125:279–287. 20809362
41. Osborne, C.K., Pippen, J., Jones, S.E., et al, Double-blind, randomized trial comparing the efficacy and tolerability of fulvestrant versus anastrozole in postmenopausal women with advanced breast cancer progressing on prior endocrine therapy: results of a North American trial. J Clin Oncol. 2002;20(16):3386–3395. 12177098
42. Thompson, A.M., Jordan, J.B., Quinlan, P., The Breast Recurrence in Tissues Study Group, Prospective comparison of switches in biomarker status between primary and recurrent breast cancer: the Breast Recurrence in Tissues Study (BRITS). Breast Cancer Res 2010; 12:R92. 21059212
43. Aebi, S., Gelber, S., Lang, I., et al. Chemotherapy prolongs survival for isolated or regional recurrence of breast cancer: The CALOR trial (Chemotherapy as Adjuvant for Locally Recurrent breast cancer; IBCSG 27-02, NSABP B-37, BIG 1-02). Cancer Res. 2012; 72(24):96s.
44. Lewis, R., Bagnall, A.-M., Forbes, C., et al, The clinical effectiveness of trastuzumab for breast cancer: a systematic review. Health Technol Assess. 2002;6(13):1–71. 12137722