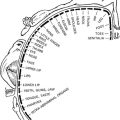
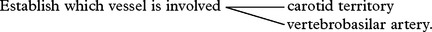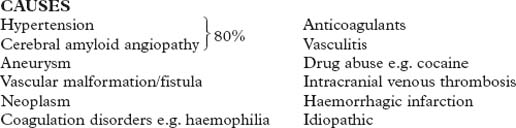SECTION IV LOCALISED NEUROLOGICAL DISEASE AND ITS MANAGEMENT A. INTRACRANIAL
HEAD INJURY
FOCAL DAMAGE
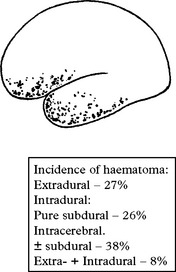
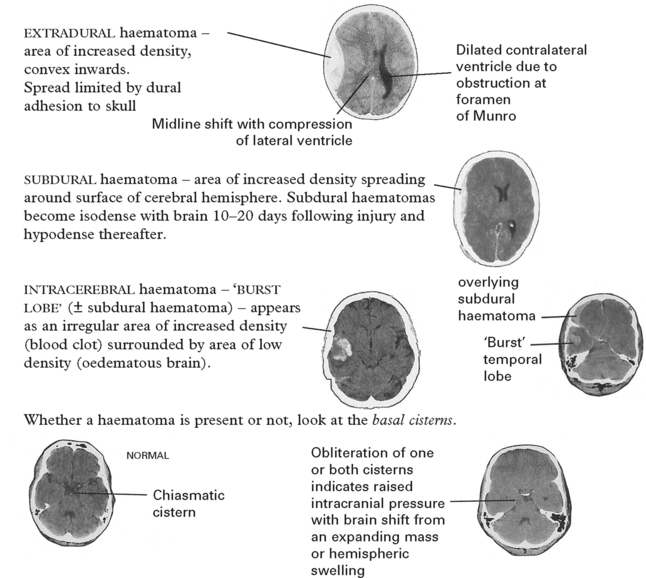
Intracranial haematoma
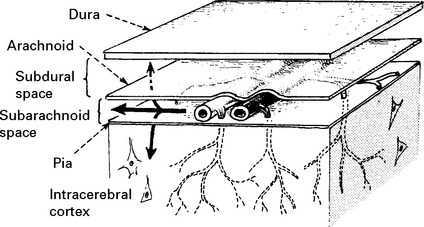
Intracranial bleeding may occur either outside (extradural) or within the dura (intradural).
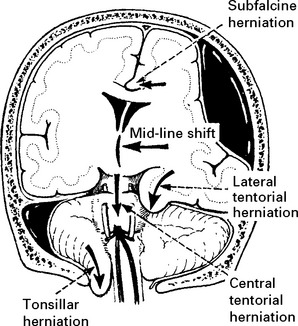
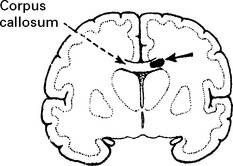
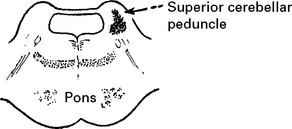
Tentorial/tonsillar herniation (syn. ‘cone”)
It is unlikely that high intracranial pressure alone directly damages neuronal tissue, but brain damage occurs as a result of tonsillar or tentorial herniation (see page 81). A progressive increase in intracranial pressure due to a supratentorial haematoma initially produces midline shift. Herniation of the medial temporal lobe through the tentorial hiatus follows (lateral tentorial herniation), causing midbrain compression and damage. Uncontrolled lateral tentorial herniation or diffuse bilateral hemispheric swelling will result in central tentorial herniation. Herniation of the cerebellar tonsils through the foramen magnum (tonsillar herniation) and consequent lower brain stem compression may follow central tentorial herniation or may result from the infrequently occurring traumatic posterior fossa haematoma.
DIFFUSE DAMAGE
Diffuse axonal injury
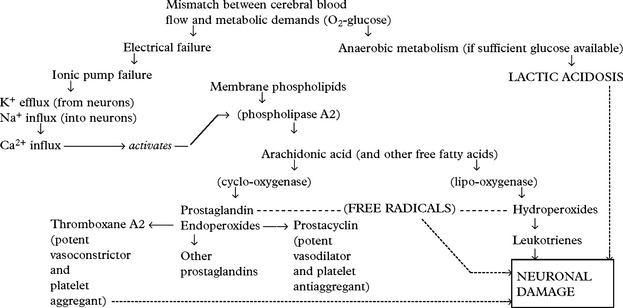
Shearing forces cause immediate mechanical damage to axons. Over the subsequent 48 hours, further damage results from release of excitotoxic neurotransmitters which cause Ca2+ influx into cells and triggers the phospholipid cascade (page 246). Genetic susceptibility conferred by the presence of the APOE ͛4 gene may also play a part. Depending on the severity of the injury, effects may range from mild coma to death.
Cerebral ischaemia
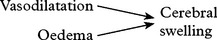
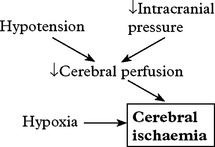
Cerebral ischaemia commonly occurs after severe head injury and is caused by either hypoxia or impaired cerebral perfusion. In the normal subject, a fall in blood pressure does not produce a drop in cerebral perfusion since ‘auto-regulation’ results in cerebral vasodilatation. After head injury, however, autoregulation is often defective and hypotension may have more drastic effects. Glutamate excess and free radical accumulation may also contribute to neuronal damage (see page 246).
HEAD INJURY – CLINICAL ASSESSMENT
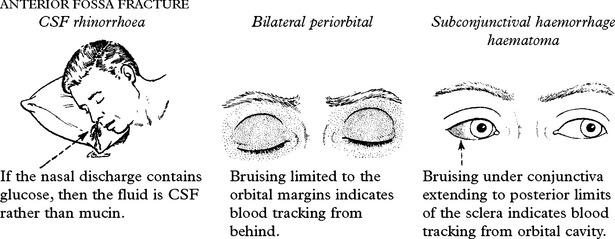
PETROUS FRACTURE
Bleeding from the external auditory meatus or CSF otorrhoea:
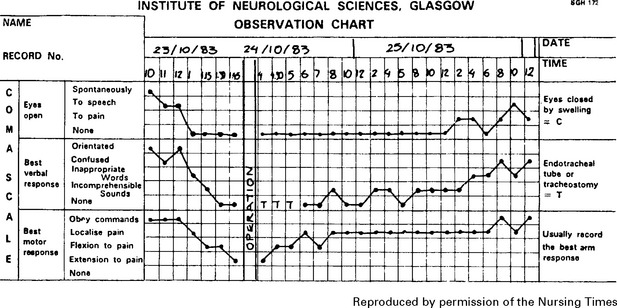
3. Conscious level – Glasgow Coma Score (GCS)
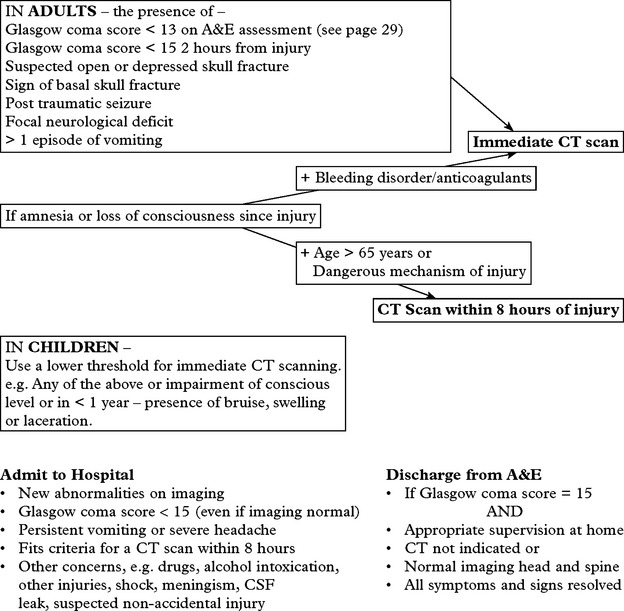
Assess patient’s conscious level in terms of eye opening, verbal and motor response on admission (see page 5) and record at regular intervals thereafter. An observation chart incorporating these features is essential and clearly shows the trend in the patient’s condition. Deterioration in conscious level indicates the need for immediate investigation and action where appropriate.
Note: This chart shows a ‘14 point scale’ with a maximum score of ‘14’ in a fully conscious patient. Many centres use a 15 point coma scale where ‘Flexion to pain’ is divided into ‘normal’ or ‘spastic’ flexion (see page 29).
4. Pupil response
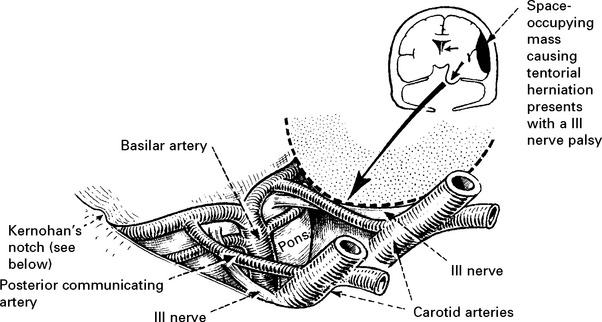
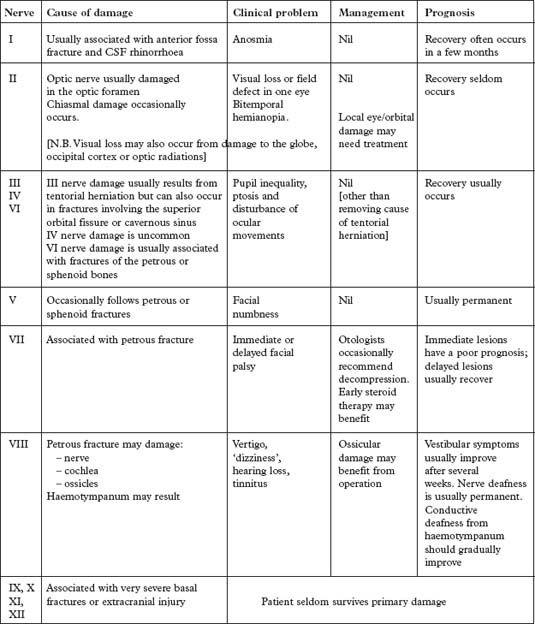
The light reflex (page 142) tests optic (II) and oculomotor (III) nerve function. Although II nerve damage is important to record and may result in permanent visual impairment, it is the III nerve function which is the most useful indicator of an expanding intracranial lesion. Herniation of the medial temporal lobe through the tentorial hiatus may damage the III nerve directly or cause midbrain ischaemia, resulting in pupil dilatation with impaired or absent reaction to light. The pupil dilates on the side of the expanding lesion and is an important localising sign. With a further increase in intracranial pressure, bilateral pupillary dilatation may occur.
5. Limb weakness
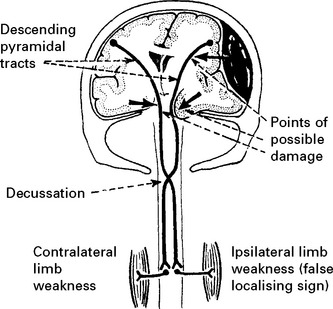
Determine limb weakness by comparing the response in each limb to painful stimuli (page 30). Hemiparesis or hemiplegia usually occurs in the limbs contralateral to the side of the lesion. Indentation of the contralateral cerebral peduncle by the edge of the tentorium cerebelli (Kernohan’s notch) may produce an ipsilateral deficit, a false localising sign more often seen with chronic subdural haematomas. Limb deficits are therefore of limited value in lesion localisation.
6. Eye movements
Eye movements may occur spontaneously, or can be elicited reflexly (page 30) by head rotation (oculocephalic reflex) or by caloric stimulation (oculovestibular reflex).
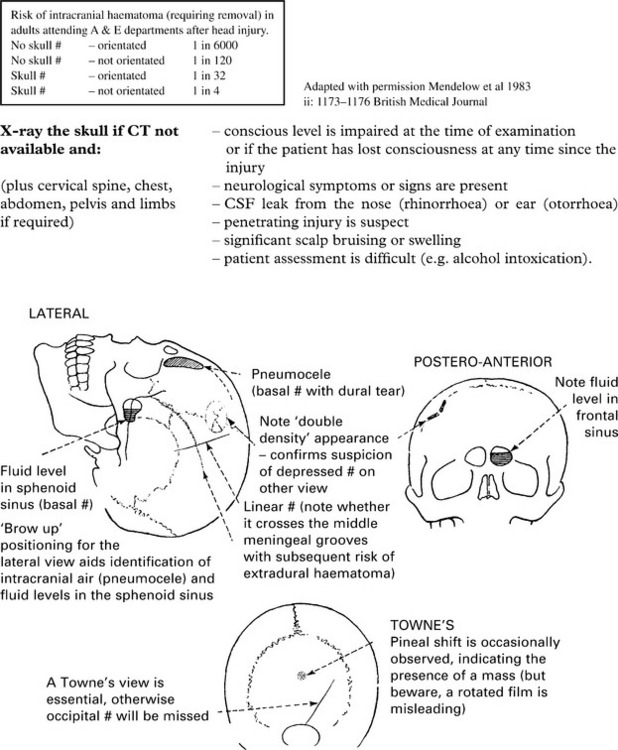
HEAD INJURY – INVESTIGATION AND REFERRAL CRITERIA
Transfer to the neurosurgical unit
Prior to the transfer, ensure that resuscitation is complete, and that more immediate problems have been dealt with (see page 221). Insert an oropharyngeal airway. Intubate and ventilate if the patient is in coma or if the blood gases are inadequate (PO2 <8 kPa on air, 13 kPa on O2 or CO2 > 6 kPa). If the patient’s conscious level is deteriorating, an intravenous bolus infusion of 100 ml of 20% mannitol should ‘buy time’ by temporarily reducing the intracranial pressure.
Cervical spine injury may accompany head injury. Guidelines also exist with criteria for investigation. (For full details see http://www.nice.org.uk/guidance/index.jsp?action=download&o=36259).
| AP, Lateral and Odontoid Peg X-rays if | CT cervical spine if |
|---|---|
HEAD INJURY – INVESTIGATION
CT scan: the investigation of choice for head injury (and cervical spine injury incertain circumstances – see page 227).
Further investigation may be required to exclude other coincidental or contributory causes of the head injury, e.g. drugs, alcohol, postictal state, encephalitis (Cause of coma, see page 86).
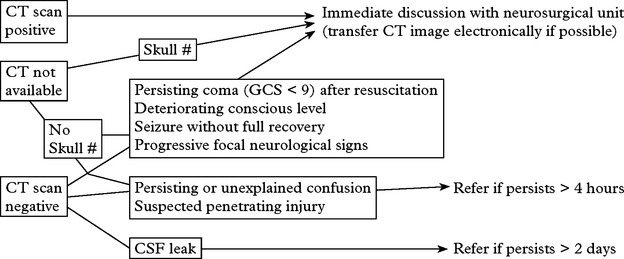
If a CT scan is not available, a skull fracture on X-ray identifies those at high risk of intracranial haematoma. In those patients, referral to a neurosurgical unit for a CT scan is essential (see page 227).
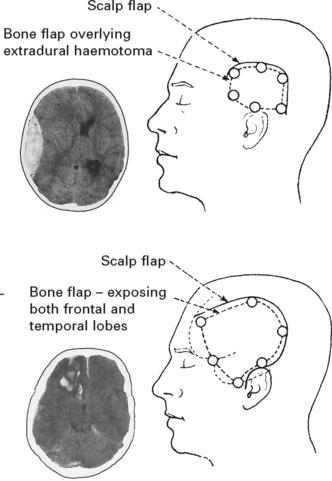
HEAD INJURY – MANAGEMENT
INTRACRANIAL HAEMATOMA
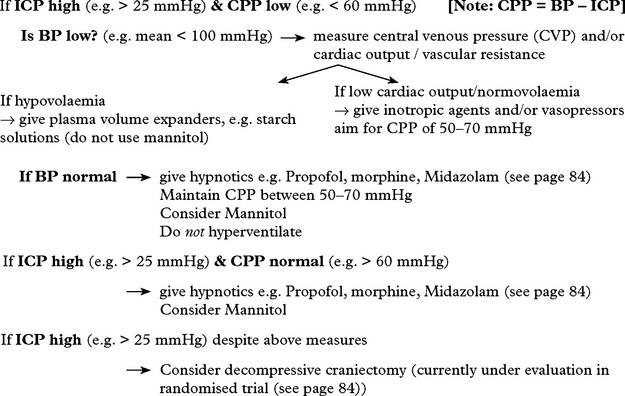
TREATMENT OF RAISED INTRACRANIAL PRESSURE (ICP)
Raised ICP in the absence of any easily treatable condition (e.g. intracranial haematoma or raised pCO2) requires careful management. The various techniques used to lower ICP have already been described (pages 83–84) but these must not be applied indiscriminately.
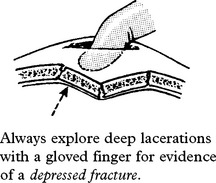
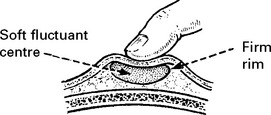
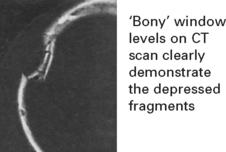
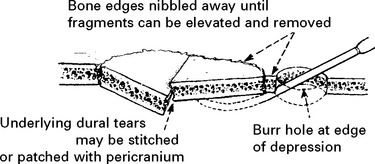
DEPRESSED SKULL FRACTURE
DELAYED EFFECTS OF HEAD INJURY
POST-TRAUMATIC EPILEPSY
Early epilepsy (occurring within the first week from injury)
The risk of early epilepsy is high in
Late epilepsy (occurring after the first week from injury)
Prophylactic anticonvulsants appear to be of little benefit in preventing the development of an epileptogenic focus. Management is discussed on page 102.
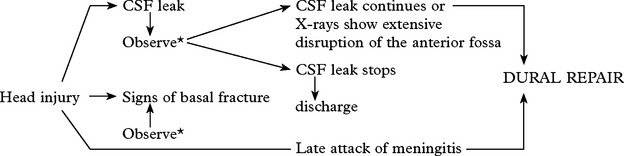
CEREBROSPINAL FLUID (CSF) LEAK
Clinical signs of a basal fracture have previously been described (page 222). The patient may comment on a ‘salty taste’ in the mouth. Anosmia suggests avulsion of the olfactory bulb from the cribriform plate.
POSTCONCUSSIONAL SYMPTOMS
Even after relatively minor head injury, patients may have persistent symptoms of:
OUTCOME AFTER SEVERE HEAD INJURY
Outcome is best categorised with the Glasgow Outcome Scale (GOS – see page 214) which uses dependence to differentiate between intermediate grades. After severe injury, about 40% regain an independent existence and may return to premorbid social and occupational activities. Inevitably some remain severely disabled requiring long term care, but few (< 2%) are left in a vegetative state with no awareness or ability to communicate with their environment (see page 214). Prognosis in this group is marginally better than for non-traumatic coma – with about one-third of those vegetative at one month regaining consciousness within one year; of those who regain consciousness, over two-thirds either subsequently die or remain severely disabled. Of those vegetative at 3 months after the injury, none regain an independent existence.
Prognostic features following traumatic coma
| Poor outcome (GOS 1–3) | Favourable outcome (GOS 4–5) | |
|---|---|---|
| Patients in coma for > 6 hours | 61% | 39% |
| Best Glasgow Coma Score > 11 | 18% | 82% |
| Best Glasgow Coma Score 8–10 | 32% | 68% |
| Best Glasgow Coma Score < 8 | 73% | 27% |
| Pupillary response – reacting | 50% | 50% |
| Pupillary response – non-reacting | 96% | 4% |
| Age < 20 years | 41% | 59% |
| Age > 60 years | 94% | 6% |
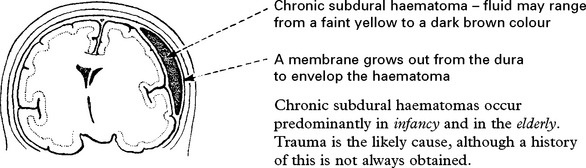
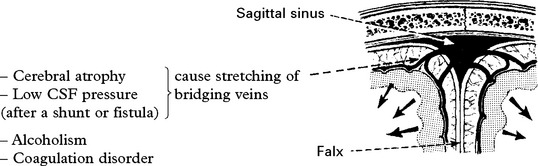
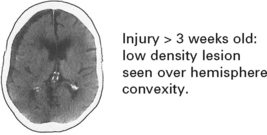
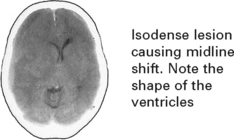
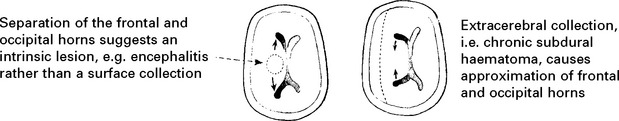
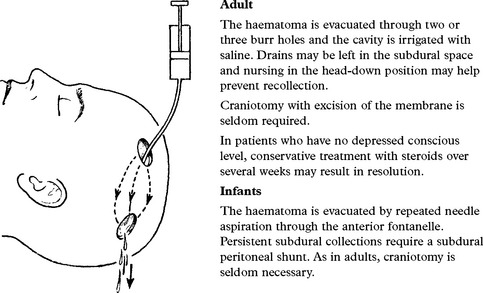
CHRONIC SUBDURAL HAEMATOMA
CEREBROVASCULAR DISEASES
CEREBROVASCULAR DISEASE – NATURAL HISTORY
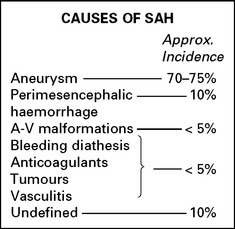
CEREBROVASCULAR DISEASE – CAUSES
HAEMORRHAGE (20%)
| Into the brain substance – parenchymal (15%) and/or subarachnoid space (5%) | Neoplasm |
| Coagulation disorder e.g. haemophilia | |
| Hypertension | Anticoagulant therapy |
| Amyloid vasculopathy | Vasculitis |
| Aneurysm | Drug abuse e.g. cocaine |
| Arteriovenous malformation | Trauma |
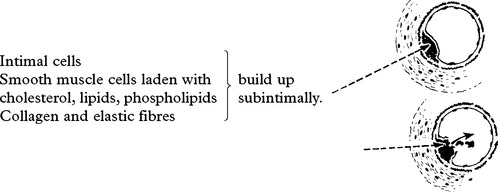
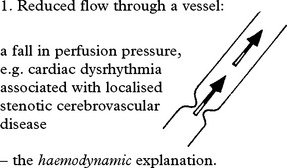
OCCLUSIVE AND STENOTIC CEREBROVASCULAR DISEASE
CEREBROVASCULAR DISEASE – PATHOPHYSIOLOGY
TRANSIENT ISCHAEMIC ATTACKS (TIAs)
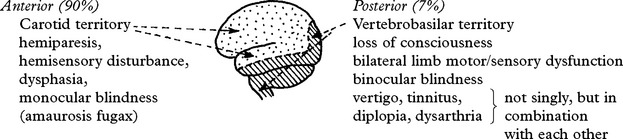
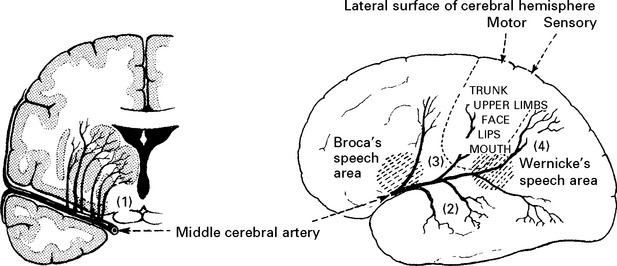
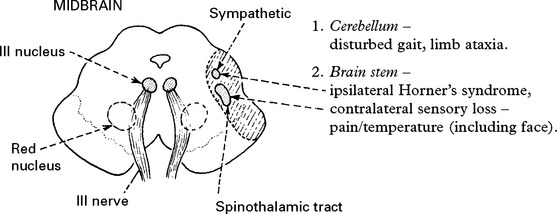
CLINICAL SYNDROMES – LARGE VESSEL OCCLUSION
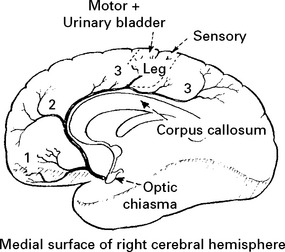
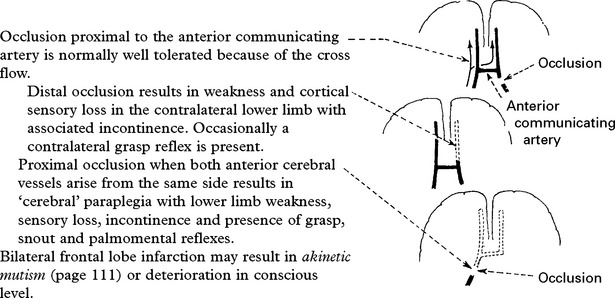
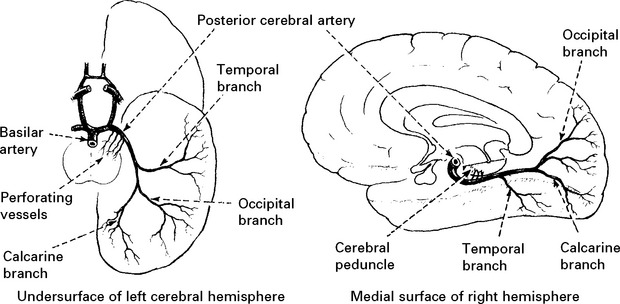
ANTERIOR CEREBRAL ARTERY
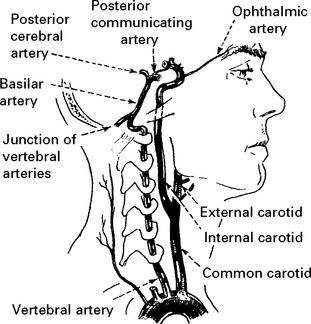
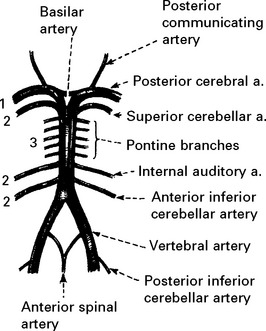
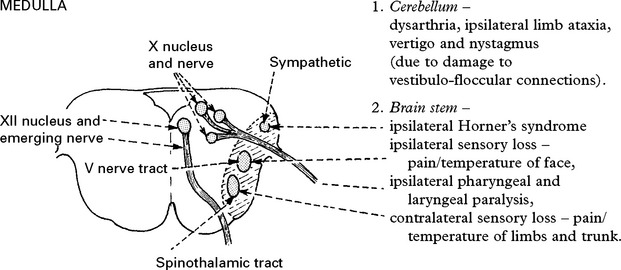
VERTEBRAL ARTERY OCCLUSION
Clinical features
Occlusion of the vertebral artery, when low in the neck, is compensated by anastomotic channels.
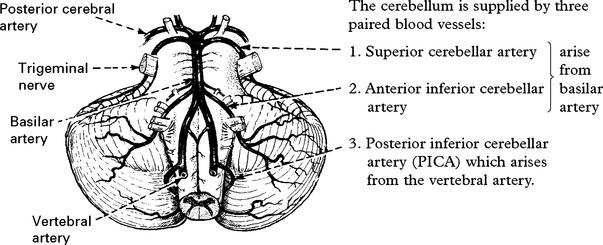
Only the posterior inferior cerebellar artery (PICA) depends solely on flow through the vertebral artery. Vertebral artery occlusion may therefore present as a PICA syndrome (page 255).
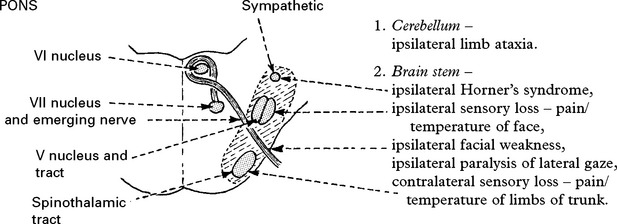
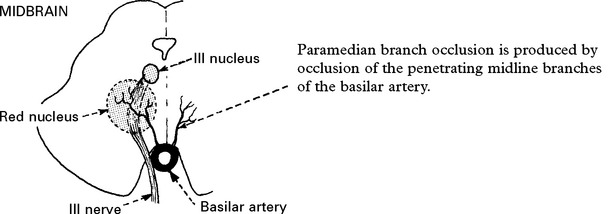
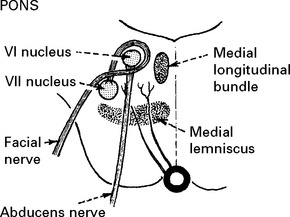
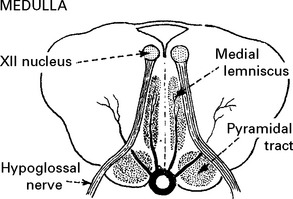
BASILAR ARTERY OCCLUSION
CLINICAL SYNDROMES – BRANCH OCCLUSION
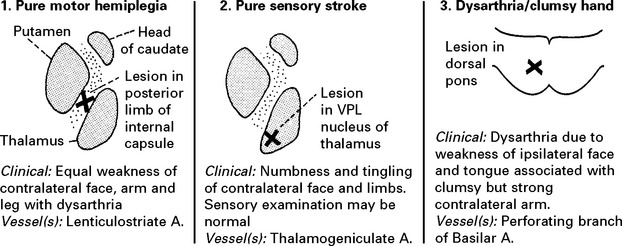
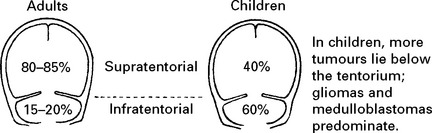
CLASSIFICATION OF SUBTYPES OF CEREBRAL INFARCTION
| Clinical features | Outcome | |
|---|---|---|
| Total Anterior Circulation | motor and sensory deficit, hemianopia and disturbance of higher cerebral function | Poor |
| Syndrome (TACS) | ||
| Partial Anterior Circulation | any two of above | Variable |
| Syndrome (PACS) | or isolated disturbance of cerebral function | |
| Posterior Circulation | signs of brain stem dysfunction | Variable |
| Syndrome (POCS) | or isolated hemianopia | |
| Lacunar Anterior | pure motor stroke | Good |
| Circulation Syndrome (LACS) | or pure sensory stroke | |
| or pure sensorimotor stroke | ||
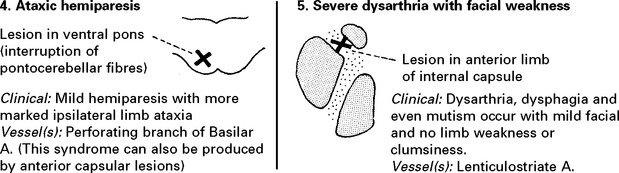
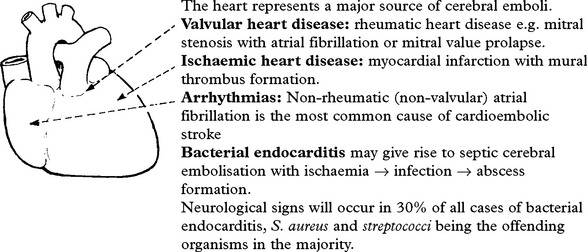
| or ataxic hemiparesis | ||
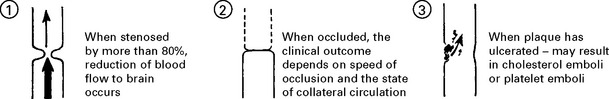
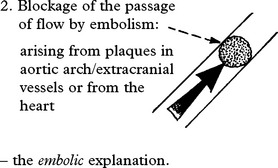
EMBOLISATION
Emboli consist of friable atheromatous material, platelet-fibrin clumps or well formed thrombus.
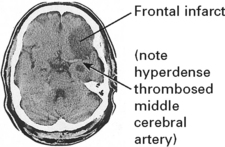
The diagnosis of embolic infarction depends on:
Emboli less frequently affect the posterior circulation.
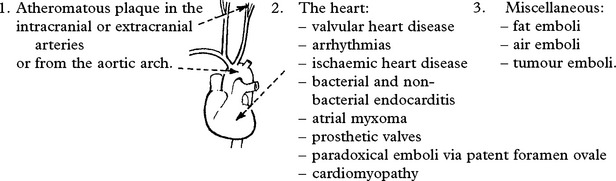
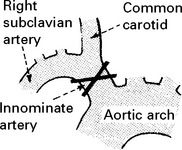
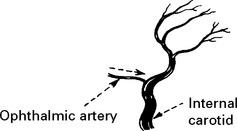
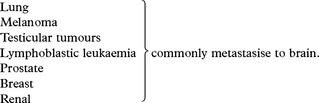
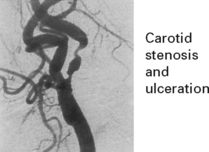
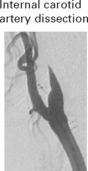
EMBOLI FROM THE INTERNAL CAROTID ARTERY AND AORTA
Emboli from these sources are commonest outwith the heart. The majority of all cerebral emboli arise from ulcerative plaques in the carotid arteries (see page 244).
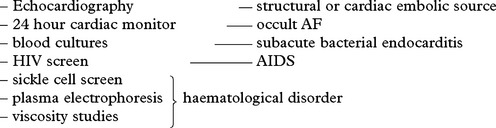
STENOTIC/OCCLUSIVE DISEASE – INVESTIGATIONS
1. CONFIRM THE DIAGNOSIS
2. DEMONSTRATE THE SITE OF PRIMARY LESION
(a) Non-invasive investigation
Ultrasound – Doppler/Duplex scanning: assesses extra- and intracranial vessels (page 44). A normal study precludes the need for angiography.
Indications of angiography
3. IDENTIFY FACTORS WHICH MAY INFLUENCE TREATMENT AND OUTCOME
Chest x-ray – cardiac enlargement – hypertension/valvular heart disease
Blood glucose – diabetes mellitus
Serum lipids and cholesterol – hyperlipidaemia
Prothrombin time – circulating auto-anticoagulants
Partial thromboplastin time (PTT) – prolonged by lupus anticoagulant
Note drug history – oral contraceptives, amphetamines, opiates
CEREBRAL INFARCTION – MANAGEMENT
THE ACUTE STROKE
Specific measures
Prevention of further stroke
The strategies here are the same as those used for treatment for patients with TIA (see below).
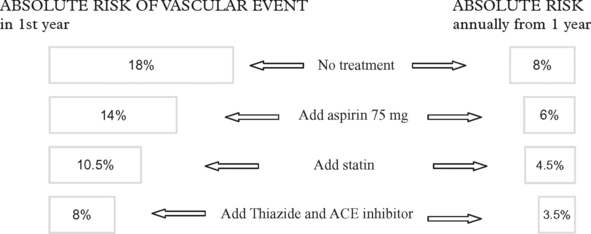
TIAs AND MINOR INFARCTION – MANAGEMENT
The aim of treatment is to prevent subsequent cerebral infarction:
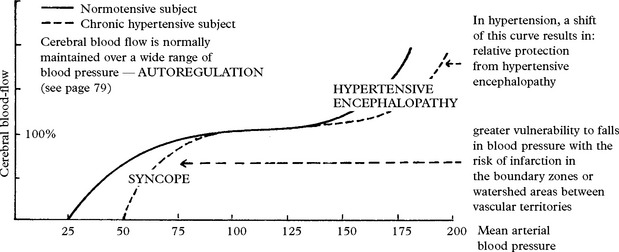
HYPERTENSION AND CEREBROVASCULAR DISEASE
The pathological effects of sustained hypertension are:
HYPERTENSIVE ENCEPHALOPATHY
The mechanism is complex: Cerebral resistance vessels
N.B. With treatment full recovery is usual. Without treatment death occurs.
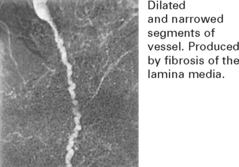
NON-ATHEROMATOUS CEREBROVASCULAR DISEASE
DISEASES OF THE VESSEL WALL
VASCULITIS AND COLLAGEN VASCULAR DISEASES
Mechanism
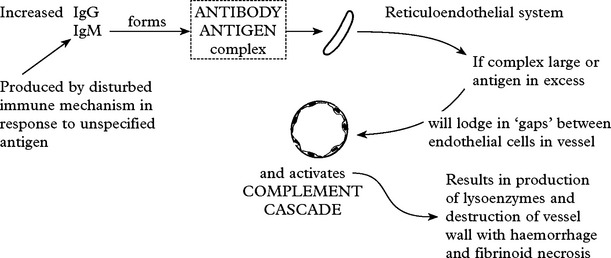
An immune basis for these disorders is likely.
This is termed IMMUNE COMPLEX VASCULITIS.
In all vasculitides affecting predominantly large and medium size vessels, angiography is important in establishing diagnosis. On MRI the presence of bilateral cortical and subcortical infarction is suggestive.
ISOLATED ANGIITIS OF CENTRAL NERVOUS SYSTEM
Systemic symptoms and laboratory evidence of generalised vasculitis are absent.
Presentation with headaches/seizures/encephalopathy and stroke
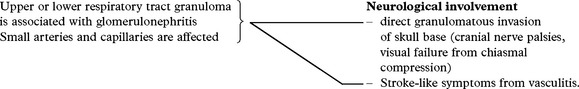
GRANULOMATOUS VASCULITIS/WEGENER’S GRANULOMATOSIS
| Diagnosis: |
DISEASES OF THE BLOOD
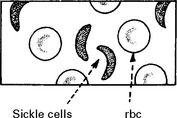
HAEMOGLOBINOPATHIES
POLYCYTHAEMIA
Headaches, visual blurring and vertigo are common neurological symptoms.
Transient ischaemic attacks and thrombotic cerebral infarction occur.
HYPERGAMMAGLOBULINAEMIA
Neurological involvement develops in 20% of cases – due to increased viscosity.
THROMBOTIC THROMBOCYTOPENIC PURPURA (syn: Moschkowitz’s syndrome)
Haemolytic anaemia, haematuria and thrombocytopenia are the main laboratory features.
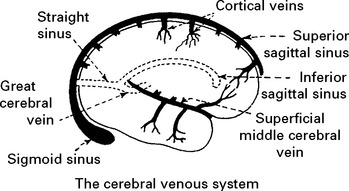
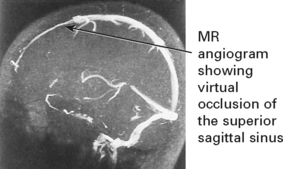
CEREBROVASCULAR DISEASE – VENOUS THROMBOSIS
Superior sagittal and lateral sinus thrombosis (85% of cases)
Diagnosis is suggested by venous (nonarterial territory) infarction and ‘empty delta’ sign (following contrast the wall of the sinus enhances but not the central thrombus on CT) and confirmed by occlusion of filling deficit on MR or CT venography. Outcome is variable; intracranial hypertension may develop (p. 378). A thorough search for causation – coagulation screen, drug history and underlying systemic illness – essential.
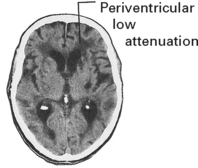
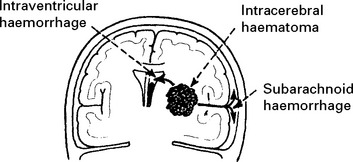
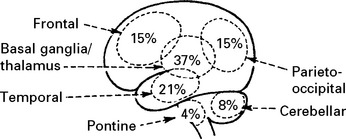
INTRACEREBRAL HAEMORRHAGE
CLINICAL EFFECTS
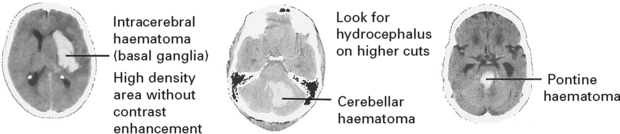
A CT scan determines the exact site and size of the haematoma and excludes other pathologies.
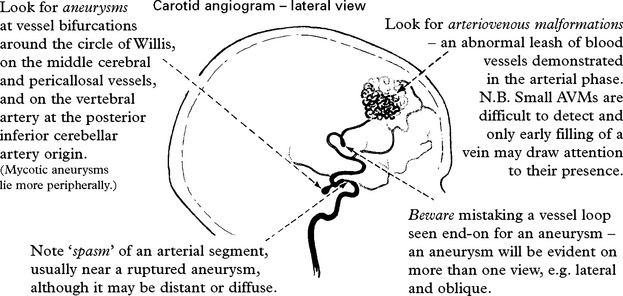
Angiography/CT angiography
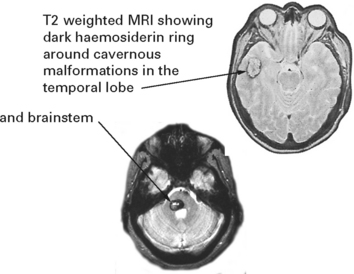
In patients with negative angiography, a late MRI may demonstrate a CAVERNOUS ANGIOMA (see page 299).
| MANAGEMENT | PROGNOSIS |
|---|---|
The overall mortality ranges from 25–60% (90% if the patient is in coma) and is improved by an integrated ‘Stroke Unit’
SUBARACHNOID HAEMORRHAGE (SAH)
Occasionally the arachnoid layer gives way and a subdural haematoma results.
SYMPTOMS AND SIGNS
The severity of the symptoms is related to the severity of the bleed.
Signs of meningism develop after 3–12 hours
Neck stiffness is present in most patients on passive neck flexion.
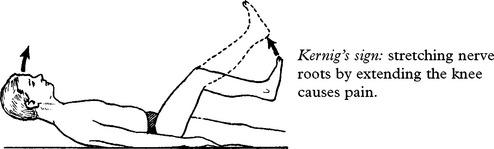
Kernig’s sign: stretching nerve roots by extending the knee causes pain.
Coma or depression of conscious level may result from the direct effect of the subarachnoid haemorrhage or from the mass effect of an associated intracerebral haematoma.
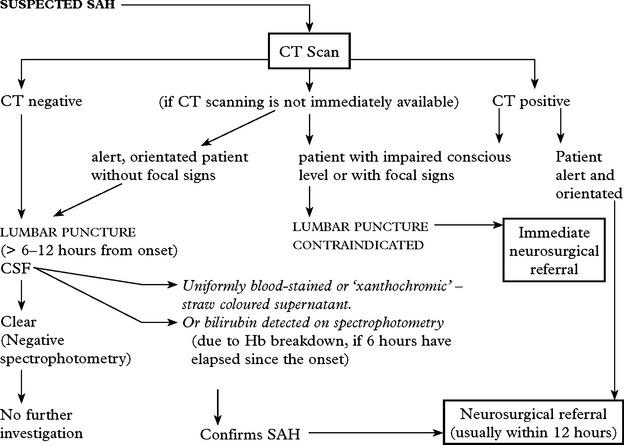
INVESTIGATIVE APPROACH
Age limit for neurosurgical referral: Although mortality and morbidity increase with age, with the option of endovascular aneurysm treatment, age limitations no longer apply provided the patient’s clinical state is satisfactory.
CT scan
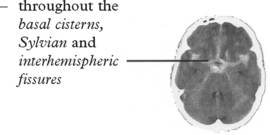
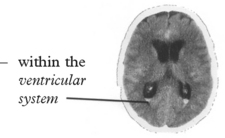
Confirms the diagnosis of SAH in 95% (if within 48 hours of the bleed).
Blood may be widely distributed
or more localised aiding identification of the site of the ruptured aneurysm
MRI scan
N.B. Spinal arteriovenous malformations can also cause SAH – if the patient’s pain begins in the back before spreading to the head, or if any features of cord compression exist, then MRI of the cervical or thoracic spine should be the preliminary investigation (see page 424).
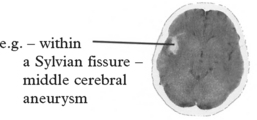
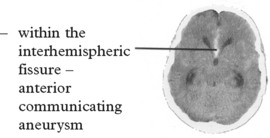
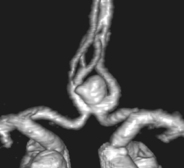
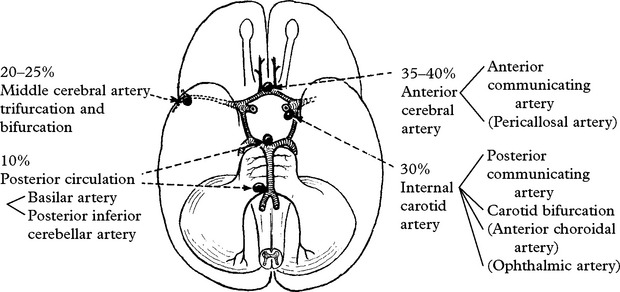
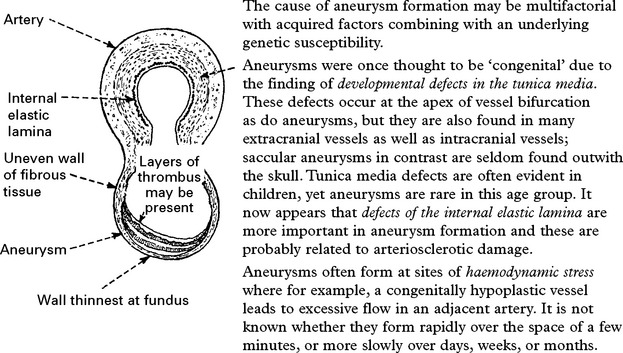
CEREBRAL ANEURYSMS
INCIDENCE
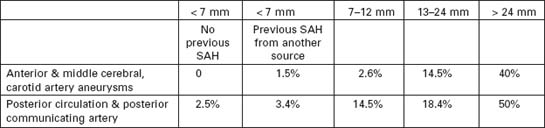
At autopsy intracranial aneurysms are found in approximately 2% of the population.
Aneurysm rupture occurs in 6–8 per 100 000 per year
Female:male = 3.2; but this ratio varies with age: < 40 years, male > females
CLINICAL PRESENTATION
1. Rupture
The features of SAH have already been described in detail (page 276); they include sudden onset of headache, vomiting, neck stiffness, loss of consciousness, focal signs and epilepsy.
Since the severity of the haemorrhage relates to the patient’s clinical state and this in turn relates to outcome, much emphasis has been placed on categorising patients into 5 level grading systems, e.g. Hunt and Hess. A scale incorporating the Glasgow Coma scale (page 29) has been adopted by the World Federation of Neurosurgical Societies:
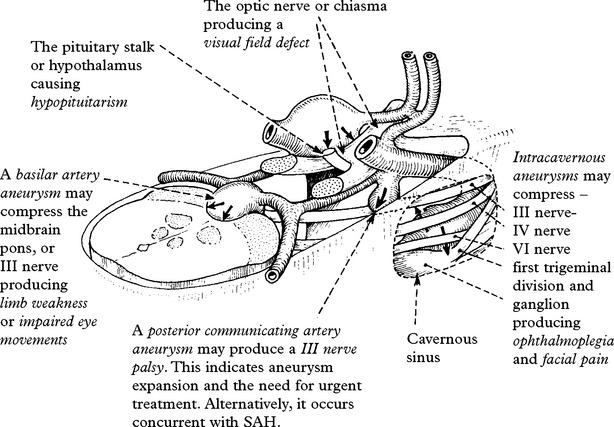
CEREBRAL ANEURYSMS – COMPLICATIONS
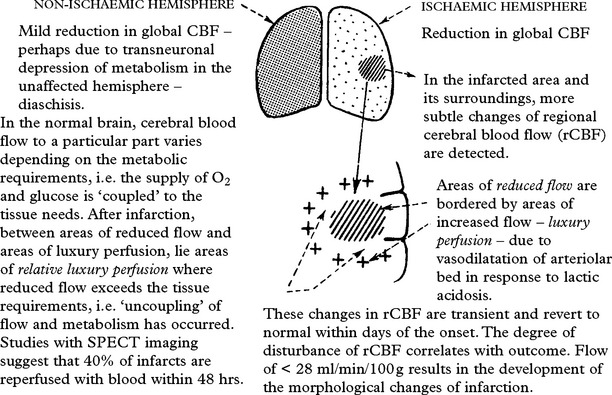
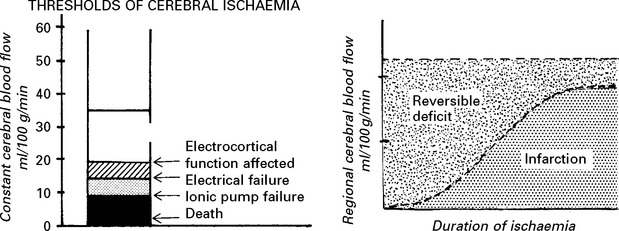
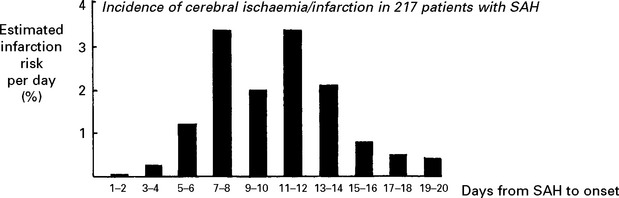
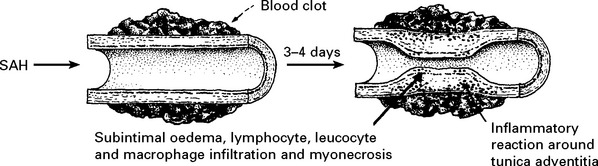
CEREBRAL ISCHAEMIA/INFARCTION
Aetiology of cerebral ischaemia/infarction
The angiogram appearance was initially thought to result from arterial constriction; this may be so, but the pathogenesis of ‘vasospasm’ now seems more complex. Many vasoconstrictive substances either released from the vessel wall or from the blood clot appear in the CSF after SAH, e.g. serotonin, prostaglandin, oxyhaemoglobin, endothelin-1 and endothelial synthesis of the vasodilator nitric oxide is reduced, but numerous studies with vasoconstrictor antagonists have failed to reverse the angiographic narrowing. This failure may be a result of the arteriopathic changes which have been observed in the vessel wall. Only calcium antagonists appear to have a beneficial effect (see page 291).
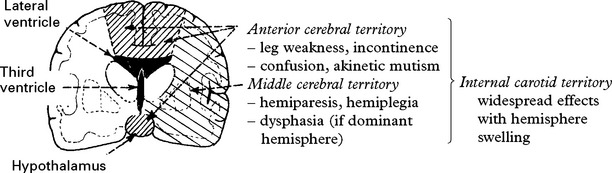
Clinical effects of cerebral ischaemia/infarction
This may affect one particular arterial territory producing characteristic signs:
Transcranial Doppler: a significant increase in flow velocity within an intracranial vessel may indicate developing ‘vasospasm’, even before clinical problems develop, and allow the early introduction of prophylactic measures (see page 291).
EPILEPSY
Epilepsy may occur at any stage after SAH, especially if a haematoma has caused cortical damage.
CEREBRAL ANEURYSMS – MANAGEMENT FOLLOWING SAH
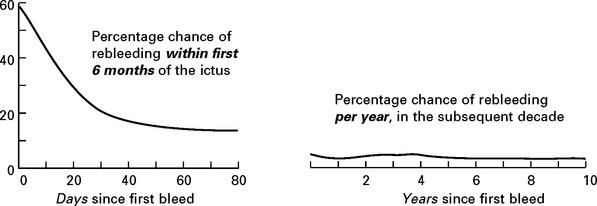
PREVENTION OF REBLEEDING
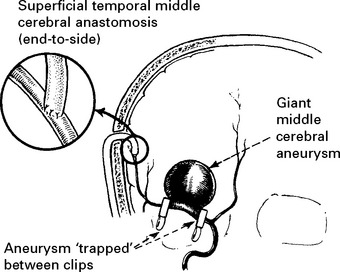
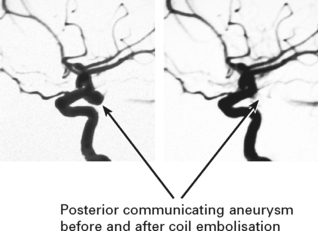
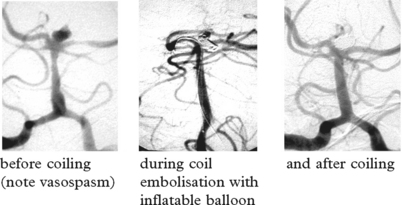
Aneurysm repair: Both surgical (clipping of the aneurysm neck) and endovascular (coil embolisation of the aneurysm sac) techniques are used. Aneurysm repair, whether coiling or clipping, is performed within 48 hours of the bleed, but this is not always feasible. Operative risks are greater the earlier the procedure, but the greater the delay, the greater the risk of rebleeding. Despite this, in past years operation was often deferred in patients in poor clinical condition. Endovascular techniques appear to be less dependent on timing and avoid potentially harmful effects of brain retraction and vessel dissection. This supports their use in the elderly or in patients in poor clinical condition, but other factors must also be considered – see page 290. Once the aneurysm is clipped, aggressive methods of treating ischaemia with induced hypertension can be applied – see page 291.
ENDOVASCULAR TECHNIQUES
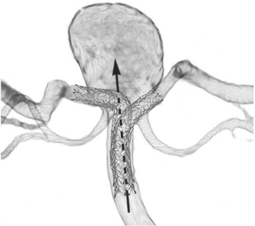
Balloon remodelling: the wider the aneurysm neck, the greater the risk that coils will project into and occlude the vessel lumen. A balloon is attached to a second catheter and periodically inflated across the aneurysm neck during coil insertion to preserve the vessel lumen.
PREVENTION OF CEREBRAL ISCHAEMIA/INFARCTION
Avoidance of antihypertensive therapy: after SAH, autoregulation (page 79) is often impaired; a drop in BP causes a reduction in cerebral blood flow with a subsequent risk of cerebral ischaemia. Patients on long-term antihypertensive treatment can continue with this therapy, but ‘reactive’ hypertension should not be treated.
Transluminal angioplasty/papaverine infusion: this involves balloon dilatation of the vasospastic segment of the vessel. It is usually combined with an intra-arterial infusion of the antispasmodic agent papaverine. Although no controlled studies exist, many small studies report a beneficial effect on cerebral blood flow and on clinical state. Timing is difficult. If used too early, the patient may be unnecessarily exposed to an invasive procedure; if too late, the ischaemia may be irreversible. Consider angiography and angioplasty if other measures (haemodilution/hypervolaemia/hypertension) have failed to reverse a significant clinical deterioration within a few hours.
Antifibrinolytic agents: i.e.tranexamic acid, epsilon aminocaproic acid should not be used.
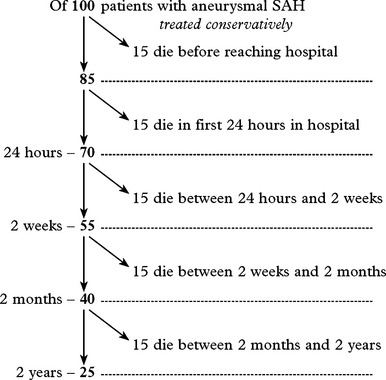
OUTCOME AFTER SUBARACHNOID HAEMORRHAGE
| Neurological grade on admission (WFNS) | No. of patients undergoing aneurysm repair | Unfavourable outcome – death/severe disability (percentage) |
|---|---|---|
| I | 1214 | 24.7 |
| II | 378 | 37.6 |
| III | 88 | 48.9 |
| IV | 164 | 64.0 |
| V | 118 | 71.2 |
CEREBRAL ANEURYSMS – UNRUPTURED
CEREBRAL ANEURYSMS – SCREENING
SCREENING FOR INTRACRANIAL ANEURYSMS
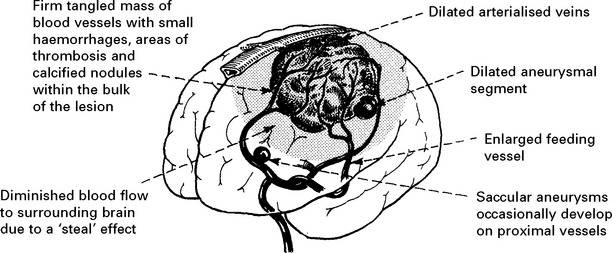
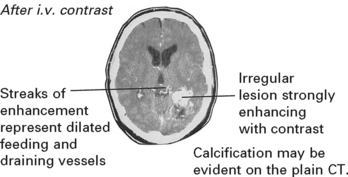
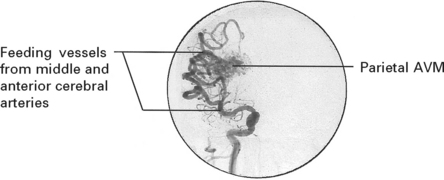
VASCULAR MALFORMATIONS
Vascular malformations vary in size and different forms exist:
INVESTIGATIONS
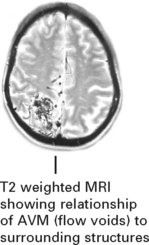
MRI
The MRI provides exact anatomical detail and helps surgical planning. Functional MRI (page 42) aids identification of any adjacent eloquent areas.
Methods of treatment
| Operation: | Excision – complete excision of the AVM (confirmed by per-or postoperative angiography) is the most effective method of treatment particularly for small AVMs in non-eloquent areas. Image guidance (page 386) may aid localisation. |
| Larger lesions (> 6 cm) have a greater risk of postoperative hyperperfusion syndrome and brain swelling and carry a 40% risk of permanent neurological deficit. | |
| Stereotactic | |
| radiosurgery: | Focused beams from multiple cobalt sources or from a linear accelerator (25Gy) obliterates about 75% of AVMs < 3 cm in diameter, but this may take up to 3 years during which time the risk of haemorrhage persists. In smaller lesions < 1 cm the obliteration rate with 25 Gy approaches 100%. For lesions greater than 3 cm, the lower dose required to minimise the damaging effect of local tissue destruction, makes obliteration unlikely. Pre-treatment with embolisation helps only if this produces a segmental reduction in size. Suboptimal embolisation may merely hinder radiosurgical treatment. Despite the delay in action, radiosurgery may prove ideal for small deeply seated lesions. |
| Embolisation: | Skilled catheterisation permits selective embolisation of feeding vessels with isobutyl-cyanoacrylate, although this technique is not without risk. |
| Embolisation may cure up to 40% of AVMs when small particularly if supplied by a single feeding vessel, but filling may persist from collaterals. When used preoperatively, it may significantly aid operative removal. | |
STURGE-WEBER SYNDROME
Angiomatosis affecting the facial skin, eyes and leptomeninges produces the characteristic features of the Sturge-Weber syndrome – a capillary naevus over the forehead and eye, epilepsy and intracranial calcification. (See page 563.)
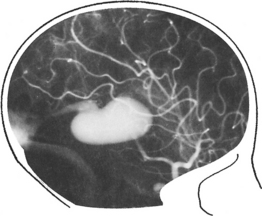
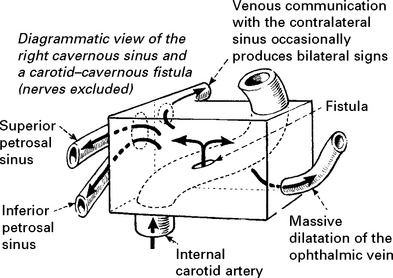
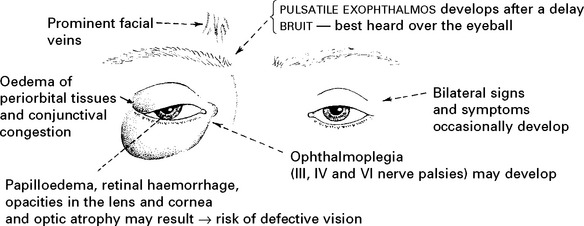
DURAL ARTERIOVENOUS FISTULA
In contrast to AVMs these fistulous communications are usually acquired rather than developmental in origin. Arterial blood drains directly into either a venous sinus, cortical veins or a combination of both (see carotid-cavernous fistula page 301). The aetiology remains unknown, but sinus thrombosis or trauma may play a part. In a benign form, no reversal of flow occurs and no treatment is required. When retrograde venous flow occurs, venous hypertension results and haemorrhage may follow. For this type, treatment requires ligation and division of the draining vein, often combined with endovascular occlusion.