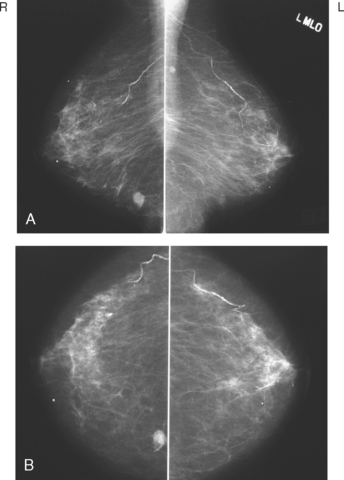
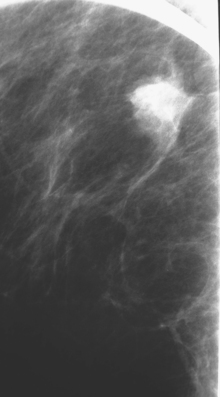
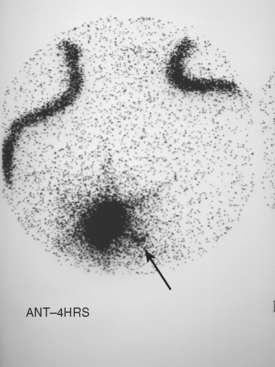
CHAPTER 3 Local Staging: Imaging Options and Core Biopsy Strategies
Treatment planning for newly diagnosed breast cancer patients, including local and systemic therapies, is based on tumor type, extent of disease, and accurate staging. Imaging and image-directed needle biopsies play a critical role in establishing the local extent of disease and aid in staging. Surgical decision making, between breast-conserving therapy (also termed lumpectomy or segmental or partial mastectomy) and mastectomy, is primarily based on tumor size, extent and location within the breast, cosmetic implications, and patient preference. Imaging modalities, including mammography, ultrasound, MRI, and molecular imaging, are used to determine the extent of disease. However, disease extent cannot be reliably established solely by imaging. When preoperative imaging suggests more extensive disease than clinical impressions, histologic confirmation is necessary before performing a more extensive surgery.
Imaging is used to locally stage breast cancer. The TNM stage is based on the size of the tumor and whether the cancer has spread (Table 1). Identification of abnormal adenopathy (axillary, supraclavicular, or internal mammary nodes), as well as involvement of the skin, pectoralis muscle, or chest wall, affects staging and therapeutic decision making. Options for local therapies include surgery and radiation therapy, whereas systemic treatments may include chemotherapy, hormone therapy, and biologic therapy. Imaging also is used to identify distant metastases.
Table 1 AMERICAN JOINT COMMITTEE ON CANCER STAGING SYSTEM FOR PATIENTS WITH BREAST CANCER
| Primary Tumor (T) | |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| Tis (DCIS) | Ductal carcinoma in situ |
| Tis (LCIS) | Lobular carcinoma in situ |
| Tis (Paget’s) | Paget’s disease of the nipple with no tumor |
| TI | Tumor 2 cm or less in greatest dimension |
| T1mic | Microinvasion 0 to 1 cm or less in greatest dimension |
| T1a | 0.1 to 0.5 cm |
| T1b | >0.5 to 1 cm |
| T1c | >1 to 2 cm |
| T2 | Tumor >2 to 5 cm in greatest dimension |
| T3 | Tumor >5 cm in greatest dimension |
| T4 | Tumor of any size with direct extension to chest wall or skin |
| T4a | Extension to chest wall, not including pectoral muscle |
| T4b | Edema (including peau-d’orange) or ulceration of the skin of the breast, or satellite skin nodules confined to the same breast |
| T4c | T4a and T4b |
| T4d | Inflammatory carcinoma |
| Regional Nodes (N) | |
| NX | Regional lymph nodes cannot be assessed (e.g., previously removed) |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in movable ipsilateral axillary lymph nodes |
| N2 | Metastasis in ipsilateral axillary lymph nodes fixed or matted, or in clinically apparent ipsilateral internal mammary nodes in the absence of clinically evident axillary lymph node metastasis. |
| N2a | Metastasis in ipsilateral axillary lymph nodes fixed to one another or to other structures |
| N2b | Metastasis only in clinically apparent ipsilateral internal mammary nodes and in the absence of clinically evident axillary lymph node metastasis |
| N3 | Metastasis in ipsilateral infraclavicular lymph node(s) with or without axillary lymph node involvement, or in clinically apparent ipsilateral internal mammary node(s) in the presence of clinically evident axillary lymph node metastasis; or metastasis in ipsilateral supraclavicular lymph node(s) with or without axillary or internal mammary lymph node involvement |
| N3a | Metastasis in ipsilateral infraclavicular lymph node(s) and axillary lymph node(s) |
| N3b | Metastasis in ipsilateral internal mammary lymph node(s) nodes and axillary lymph node(s) |
| N3c | Metastasis in ipsilateral supraclavicular lymph node(s) |
| Distant Metastasis (M) | |
| MX | Distant metastasis cannot be assessed |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
EXTENT OF DISEASE
Most breast cancers are evaluated by mammography, ultrasound, or both modalities. It is important to document the size, location, and distribution of the primary lesion, but also to evaluate for satellite lesions. Preoperative identification of additional lesions improves the likelihood of obtaining clear margins if breast-conserving therapy (BCT) is performed. For masses, it is important to look for associated microcalcifications, which may represent an associated noninvasive (in situ) component. The term extensive intraductal component (EIC) is used to refer to invasive tumors in which ductal carcinoma in situ (DCIS) makes up at least 25% of the neoplasm. Of all invasive ductal carcinomas, 15% to 30% have an EIC.1 Tumors that are predominantly DCIS with focal invasion are also classified as EIC. The presence of EIC may have prognostic implications on the likelihood of obtaining clear margins, as well as the risk for subsequent local recurrence.2,3
As many as 30% to 60% of breast cancers are pathologically multifocal (more than one tumor focus, separated by normal tissue) at the time of diagnosis.4,5 The term multicentric has been variably defined. It generally has been used to describe cancers separated by more than 4 cm or tumors located in different quadrants of the breast. BCT generally is not suitable for multicentric carcinomas because of poor cosmetic results, limitations of radiation therapy, and inability to obtain clear margins.
Synchronous contralateral breast cancer may occur in about 3% to 5% of women with breast cancer.6,7 Identification of these lesions at the time of the contralateral index cancer diagnosis can facilitate treatment in a single surgery, thereby avoiding both delays in diagnosis, as well as the emotional stress of a later diagnosis and second surgery.
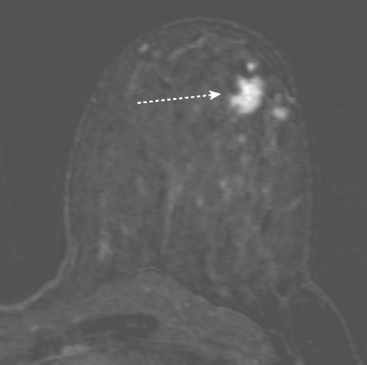
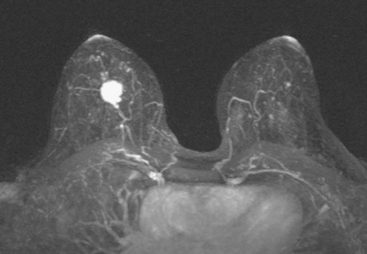
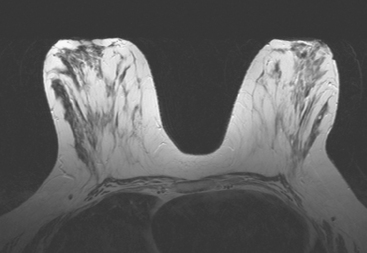
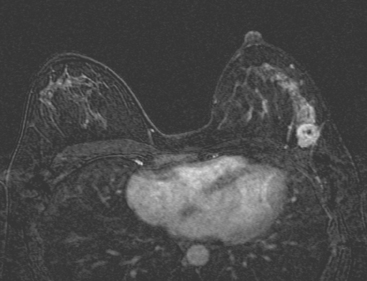
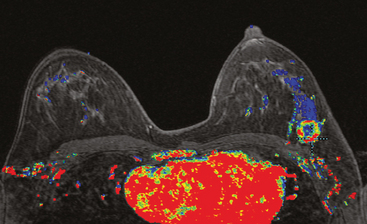
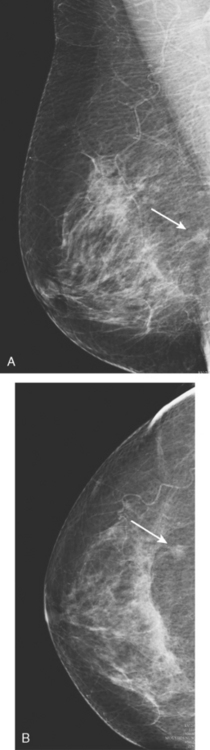
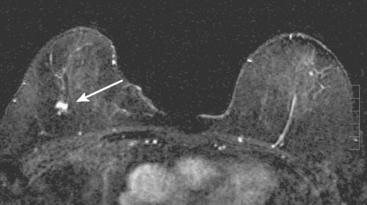
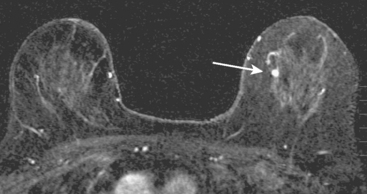
MRI is useful to identify residual disease and direct re-excision in patients with positive margins at initial lumpectomy (Figure 1).
EVALUATING ADENOPATHY
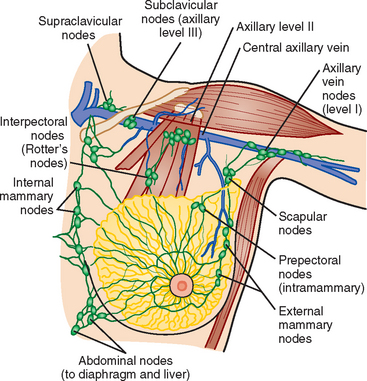
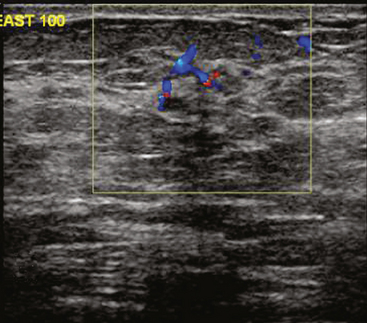
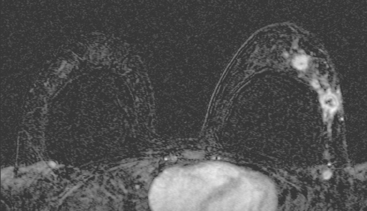
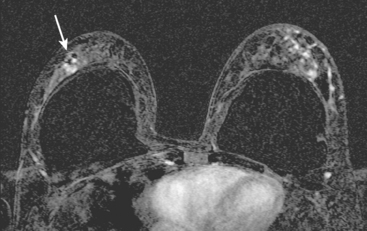
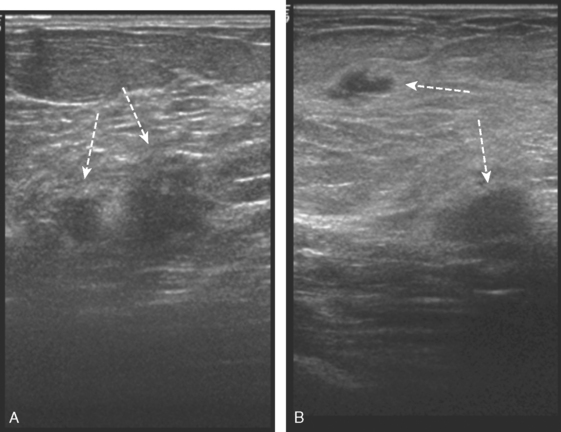
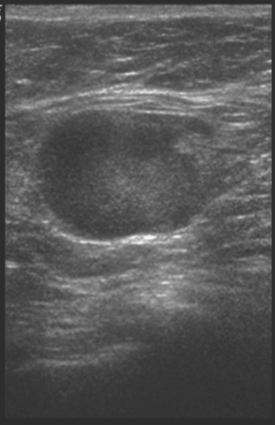
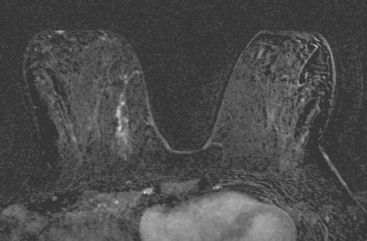
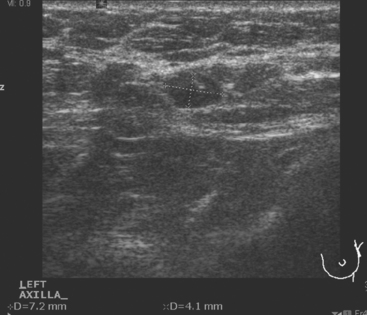
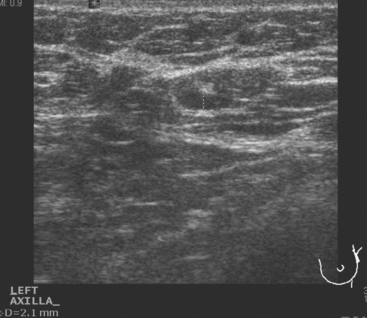
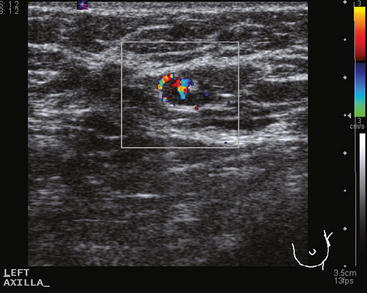
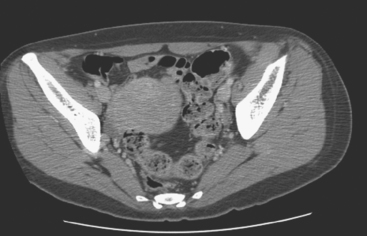
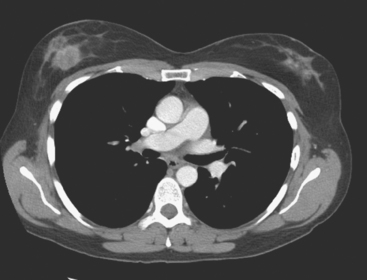
Identification of abnormal adenopathy, including axillary, supraclavicular, and internal mammary nodes, is important in staging. Metastatic adenopathy is suspected on imaging when there is cortical thickening (generally >3 mm), loss of the fatty hilum, and enlargement, particularly with increasingly round shapes8 (Figure 2 and Figure 3). However, because many benign processes may cause reactive nodes with similar imaging findings, fine-needle aspiration (FNA) or core needle biopsy is necessary to confirm suspected metastatic nodal disease. Conversely, the absence of suspicious imaging findings, whether on mammography, ultrasound, MRI, or molecular imaging studies, does not exclude metastatic nodal involvement, particularly for micrometastasis. Therefore, in addition to imaging, sampling, either with axillary dissection or sentinel lymph node biopsy, is essential in the staging of breast cancer patients.
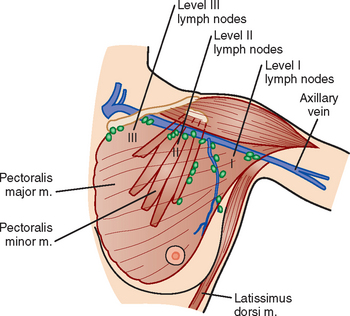
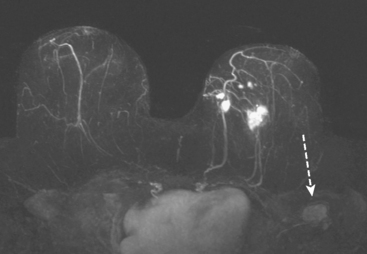
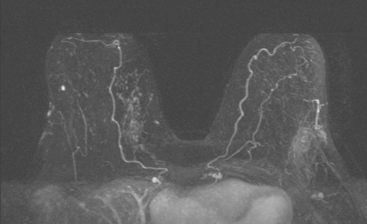
The axillary nodes form a chain from the underarm to the collarbone (Figure 4). The axillary lymph nodes are named in relation to the pectoralis minor muscle, with level I the lowest, lateral to the pectoralis minor muscle. Level I receives the most lymphatic drainage from the breast. Level II axillary nodes are beneath the pectoralis minor muscle. Level III is above and medial to the pectoralis minor muscle. A traditional axillary lymph node dissection usually removes nodes in levels I and II. Sentinel lymph node sampling involves the mapping and removal of the first lymph node or nodes (usually 1 to 3) that drain the involved area of the breast (Figure 5 and Figure 6). Instead of removing 10 or more lymph nodes as performed in a standard dissection, the status of the axilla can be predicted by excision and close pathologic examination of the sentinel node. Sentinel lymph node biopsy has significantly reduced the number of women undergoing standard axillary dissection, avoiding dissection-associated side effects such as arm lymphedema. The identification of abnormal lymph nodes on physical exam or imaging studies favors proceeding directly to axillary dissection over sentinel node biopsy.
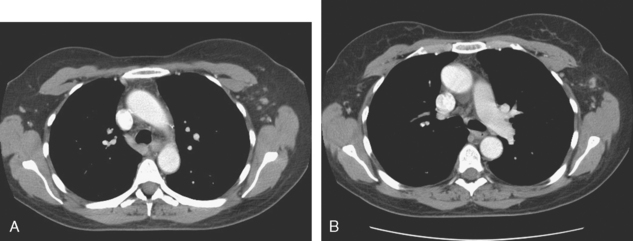
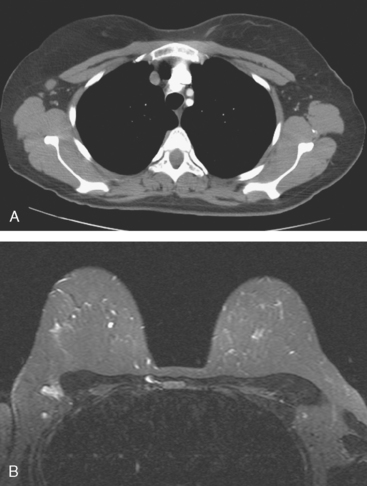
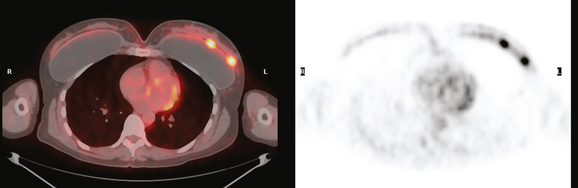
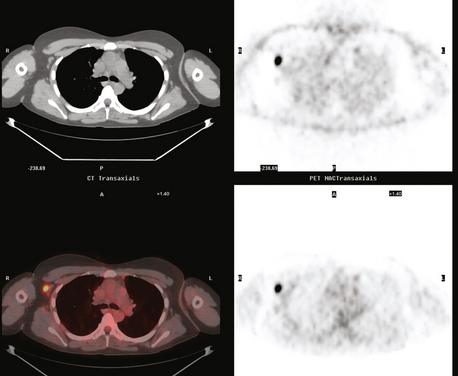
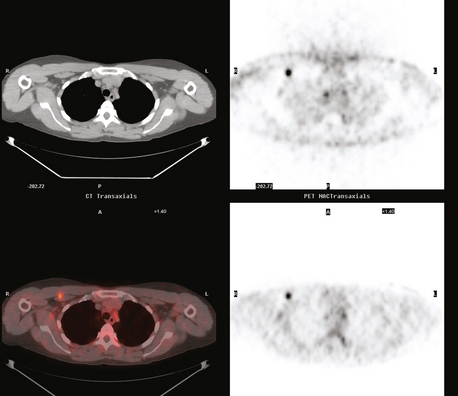
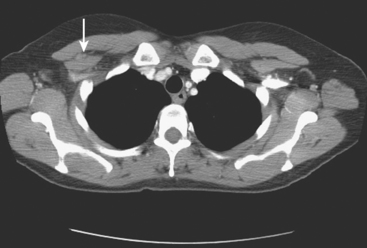
The use of molecular imaging studies, CT, or MRI may identify adenopathy in areas other than the axilla (Figure 7). The presence of internal mammary node adenopathy (Figure 8) affects staging and radiation therapy planning. Identification of abnormal Rotter’s nodes (Figure 9), nodes between the pectoralis minor and major muscles, also has staging and therapeutic implications.
SKIN, PECTORALIS, AND CHEST WALL INVOLVEMENT
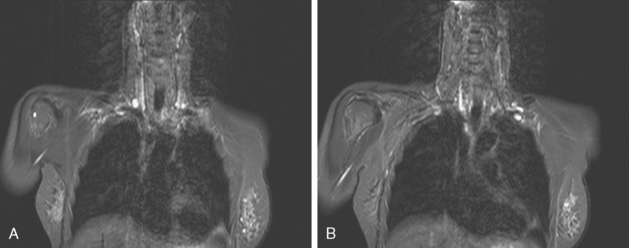
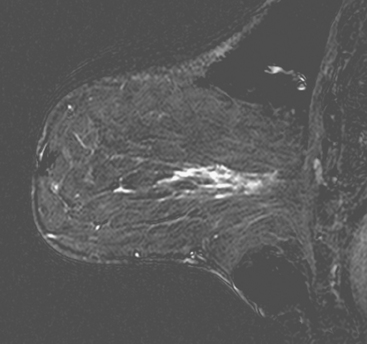
Identification of breast edema and skin thickening in patients with invasive breast cancer may represent an inflammatory component (tumor involving the dermal lymphatics). This materially affects staging and therapeutic approach. Skin punch biopsy may be necessary to confirm the diagnosis if the clinical picture is not characteristic. Identification of pectoralis muscle or chest wall involvement also affects treatment planning. Pectoralis muscle involvement should be looked for in women with posterior lesions. The diagnosis on MRI requires not just effacement or obliteration of the pectoralis fascia but also enhancement of the muscle (Figure 10). Identification of either skin or chest wall involvement classifies a tumor as a locally advanced breast carcinoma (LABC). Large (>5 cm) tumors and those with clinically matted or fixed axillary node involvement or involved supraclavicular or internal mammary nodes by imaging are also considered locally advanced. LABC is generally treated with preoperative (neoadjuvant or primary systemic) chemotherapy, which converts some patients into operative candidates. Multiple examples are presented in Chapter 5.
PREOPERATIVE MAGNETIC RESONANCE IMAGING
MRI is becoming increasingly established as a useful modality in patients with known breast cancer. However, patterns of use of preoperative MRI in women with biopsy-proven breast cancer remain highly variable in practice because of the lack of randomized control trials. It has been firmly established that preoperative MRI can detect unsuspected disease and often changes management in women with known breast cancer.9 However, the use of preoperative MRI to alter surgical management has been criticized because of the lack of studies evaluating its effect on tumor recurrence and mortality.10–12 Fischer and colleagues,13 in a study involving more than 40 months of follow-up, reported a reduction in ipsilateral breast tumor recurrence, from 6.8% to 1.2%, in patients who underwent preoperative MRI. However, this study was a retrospective, singleinstitution review of only 346 patients. It has been argued that unsuspected disease detected on MRI and treated with BCT is of little clinical consequence and is controlled by radiation therapy. This argument is primarily based on the fact that 10-year local recurrence rates as low as 10% have been reported in patients with negative surgical margins.14–16 Concerns about the use of preoperative MRI involve the potential for unnecessary biopsies, delays in treatment, and an increase in unnecessary mastectomies. Despite these arguments, there is ongoing evidence and experience accumulating that supports its benefit in preoperative staging, as follows:
FUNCTIONAL (MOLECULAR) BREAST IMAGING: BREAST-SPECIFIC GAMMA IMAGING AND POSITRON EMISSION MAMMOGRAPHY
Functional breast imaging is a growing and evolving field that is assuming a larger role in breast cancer diagnosis, providing complementary information to anatomically based breast imaging modalities. Scintimammography has matured from initial versions using standard gamma cameras, which were limited in resolution and positioning flexibility, to breast-specific gamma imaging (BSGI), which obtains higher-resolution planar images in views emulating mammography.21–25 Similarly, positron emission mammography (PEM) is performed using a small-field-of-view, high-resolution PET scanner that resembles a mammogram unit and acquires tomographic data sets in planes analogous to mammography.26–29 Scintimammography is performed after the intravenous administration of 25 mCi of either 99mTc-sestamibi or 99mTc-tetrofosmin, whereas PEM is performed after an hour’s uptake of 10 mCi of 18F-fluorodeoxyglucose (FDG) admin-istered intravenously. The radiation dose is about the same, about 0.4 rad. Imaging time is also similar. Scintimammography planar views are acquired for 5 to 10 minutes, or at least 150,000 counts, whereas PEM tomographic data sets are acquired for 4 to 10 minutes per projection. The lower limit of BSGI detector resolution is 3 mm (although smaller lesions may be identifiable), whereas the in plane resolution of PEM is on the order of 2 mm (Table 2).
| BSGI | PEM | |
|---|---|---|
| Radiopharmaceutical |
BSGI, breast-specific gamma imaging; PEM, positron emission mammography.
At this writing, BSGI is becoming more available, with sites with early experience finding it useful both for problem solving of ambiguous conventional breast imaging findings, and as an adjunct to local staging, looking for multifocality, multicentricity, and contralateral lesions (Figure 11). BSGI does not require a diagnosis of breast cancer for reimbursement, and increasingly is being performed in patients with suspicious conventional breast imaging findings as an aid to biopsy decision making (e.g., deciding how many areas need sampling). Currently, PEM is approved for patients with known or prior breast cancer diagnoses. Several cases incorporating the use of PEM have been included in this chapter. Its performance compared with MRI in preoperative local staging of apparently localized breast cancer is being assessed by a multicenter, prospective clinical trial, which at this writing is accruing patients. Early pilot studies show high sensitivity for depiction of primary breast cancers, on the order of 91%. Similarly high sensitivity for primary tumor depiction is reported for BSGI. Both modes of functional breast imaging appear to have improved specificity compared with MRI, on the order of 87% to 89% for BSGI and 93% for PEM, offering hope that increased use of functional breast imaging in the future may decrease the number of unnecessary benign biopsies now being performed.
1 van Dongen JA, Fentiman IS, Harris JR, et al. In situ breast cancer: the EORTC consensus meeting. Lancet. 1989;2:25-27.
2 Schnitt SJ, Connolly JL, Recht A, et al. Breast relapse following primary radiation therapy for early breast cancer. II. Detection, pathologic features and prognostic significance. Int J Radiat Oncol Biol Phys. 1985;11:1277-1284.
3 Bartelink H, Borger JH, van Dongen JA, Peterse JL. The impact of tumor size and histology on local control after breast conserving treatment. Radiother Oncol. 1988;11:297-303.
4 Holland R, Veling SH, Mravunac M, Hendricks JH. Histologic multifocality of Tis, T1–2 breast carcinomas: implications for clinical trials of breast conserving surgery. Cancer. 1985;56:979-990.
5 Wilkinson LS, Given-Wilson R, Hall T, et al. Increasing the diagnosis of multifocal primary breast cancer by the use of bilateral whole-breast ultrasound. Clin Radiol. 2005;60:573-578.
6 Heron DE, Komarnicky LT, Hyslop T, et al. Bilateral breast carcinoma: risk factors and outcomes for patients with synchronous and metachronous disease. Cancer. 2000;88:2739-2750.
7 Liberman L, Morris EA, Kim CM, et al. MR imaging findings in the contralateral breast of women with recently diagnosed breast cancer. AJR Am J Roentgenol. 2003;180:333-341.
8 Yang WT, Chang J, Metreweli C. Patients with breast cancer: differences in color Doppler flow and gray-scale US features of benign and malignant axillary lymph nodes. Radiology. 2000;215:568-573.
9 Bedrosian I, Mick R, Orel SG, et al. Changes in the surgical management of patients with breast carcinoma based on preoperative magnetic resonance imaging. Cancer. 2003;98:468-473.
10 Morrow M. Magnetic resonance imaging in the preoperative evaluation of breast cancer: primum non nocere. J Am Coll Surg. 2004;198:240-241.
11 Morrow M. Magnetic resonance imaging in breast cancer: one step forward, two steps back? JAMA. 2004;292:2779-2780.
12 Morrow M. Magnetic resonance imaging in breast cancer: is seeing always believing? Eur J Cancer. 2005;41:1368-1369.
13 Fischer U, Zachariae O, Baum F, et al. The influence of preoperative MRI of the breasts on recurrence rate in patients with breast cancer. Eur Radiol. 2004;14:1725-1731.
14 Smitt MC, Nowels KW, Zdeblick MJ, et al. The importance of the lumpectomy surgical margin status in long-term results of breast conservation. Cancer. 1995;76:259-267.
15 Neuschatz AC, DiPetrillo T, Safaii H, et al. Long-term follow-up of a prospective policy of margin-directed radiation dose escalation in breast-conserving therapy. Cancer. 2003;97:30-39.
16 Obedian E, Haffty BG. Negative margin status improves local control in conservatively managed breast cancer patients. Cancer J Sci Am. 2000;6:28-33.
17 Provenzano E, Hopper JL, Giles GG, et al. Histological markers that predict clinical recurrence in ductal carcinoma in situ of the breast: an Australian population-based study. Pathology. 2004;36:221-229.
18 Borg MF. Breast-conserving therapy in young women with invasive carcinoma of the breast. Australas Radiol. 2004;48:376-382.
19 Smitt MCMD, Horst K. Association of clinical and pathologic variables with lumpectomy surgical margin status after preoperative diagnosis or excisional biopsy of invasive breast cancer. Ann Surg Oncol. 2007;14(3):1040-1044.
20 Lehman CD, Gatsonis C, Kuhl CK, et al. ACRIN Trial 6667 Investigators Group. MRI evaluation of the contralateral breast in women with recently diagnosed breast cancer. N Engl J Med. 2007;356(13):1295-1303.
21 Schillaci O, Buscombe JR. Breast scintigraphy today: indications and limitations. Eur J Nucl Med Mol Imaging. 2004;31(Suppl 1):S35-S45.
22 Brem RF, Schoonjans JM, Kieper DA, et al. High-resolution scintimammography: a pilot study. J Nucl Med. 2002;43:909-915.
23 Brem RF, Rapelyea JA, Zisman G, et al. Occult breast cancer: scintimammography with high-resolution breast-specific gamma camera in women at high risk for breast cancer. Radiology. 2005;237:274-280.
24 Brem RF, Fishman M, Rapelyea JA. Detection of ductal carcinoma in situ with mammography, breast specific gamma imaging, and magnetic resonance imaging: a comparative study. Acad Radiol. 2007;14(8):945-950.
25 Brem RF, Petrovitch I, Rapelyea JA, et al. Breast-specific gamma imaging with (99m) Tc-sestamibi and magnetic resonance imaging in the diagnosis of breast cancer: a comparative study. Breast J. 2007;13(5):465-469.
26 Levine EA, Freimanis RI, Perrier ND, et al. Positron emission mammography: initial clinical results. Ann Surg Oncol. 2003;10:86-91.
27 Rosen EL, Turkington TG, Soo MS, et al. Detection of primary breast carcinoma with a dedicated, large-field-of-view FDG pet mammography device: initial experience. Radiology. 2005;234:527-534.
28 Berg WA, Weinberg IN, Narayanan D, et al. High-resolution fluorodeoxyglucose positron emission tomography with compression (“positron emission mammography”) is highly accurate in depicting primary breast cancer. Breast J. 2006;12(4):309-323.
29 Tafra L, Cheng Z, Uddo J, et al. Pilot clinical trial of 18F-fluorodeoxyglucose positron-emission mammography in the surgical management of breast cancer. Am J Surg. 2005;190(4):628-632.
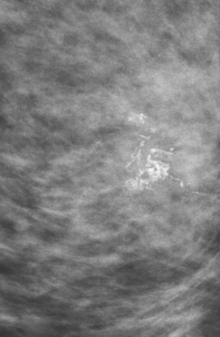
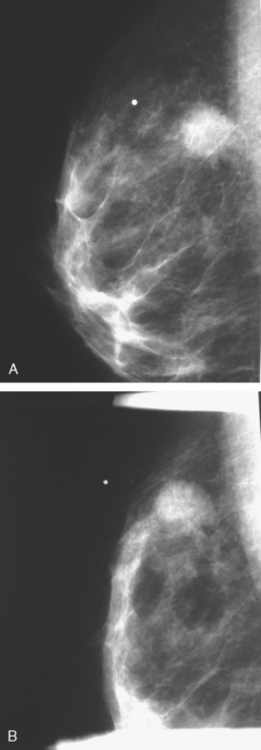
CASE 1 Mammography: Extent of disease
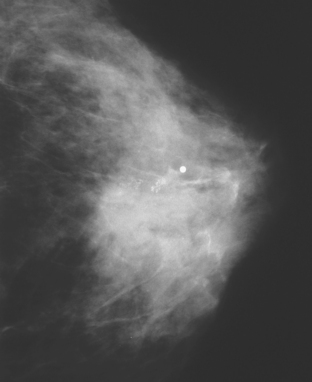
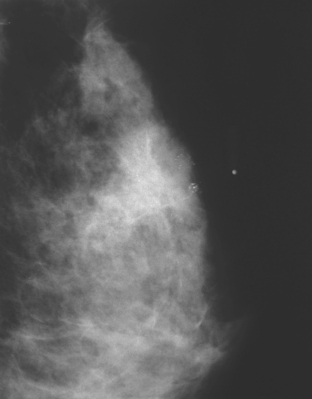
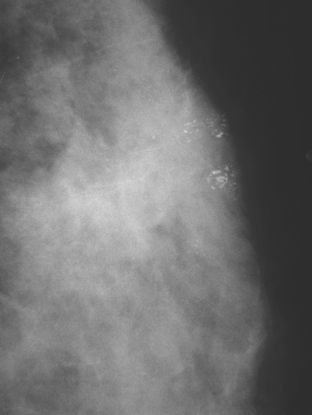
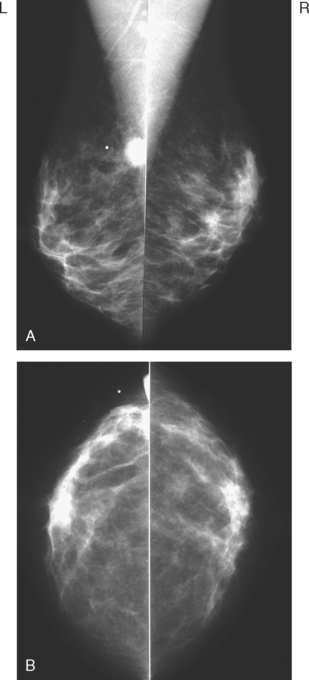
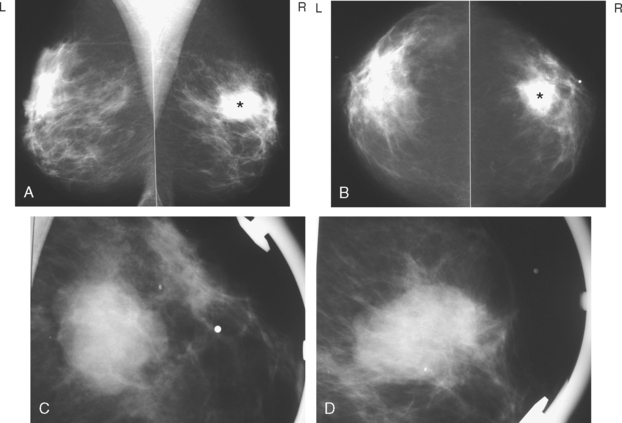
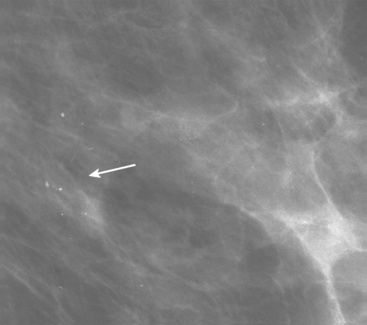
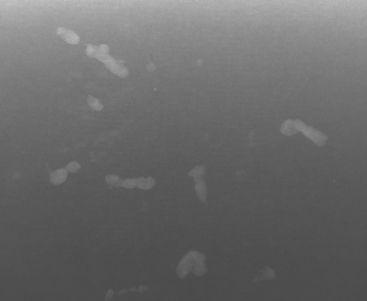
A 75-year-old woman presented with a palpable left breast mass. Mammography demonstrated a dense, spiculated breast cancer, corresponding to the palpable mass (Figure 1). Closer review of the mammogram showed a second abnormality distant from the palpable mass, with suspicious linear microcalcifications in the central left breast (Figure 2). Biopsy of the mass revealed invasive ductal carcinoma and stereotactic biopsy of the calcification found intermediate-grade ductal carcinoma in situ (DCIS). Because the two areas of disease involved a large portion of the left breast, mastectomy was performed.
Fisher B, Anderson S, Bryant J, et al. Twenty year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233-1241.
Holland R, Veling SH, Mravunac M, Hendriks JH. Histologic multifocality of Tis, T1–2 breast carcinomas: implications for clinical trials of breast-conserving surgery. Cancer. 1985;56(5):979-990.
Veronesi U, Cascinelli N, Mariani L, et al. Twenty year follow-up of a randomized study comparing breast conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227-1232.
Wazer DE, Schmidt-Ullrich RK, Schmid CH, et al. The value of breast lumpectomy margin assessment as a predictor of residual tumor burden. Int J Radiat Oncol Biol Phys. 1997;38(2):291-299.
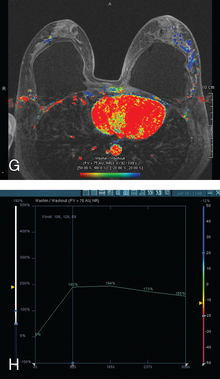
CASE 2 Use of ultrasound to find invasive disease within extensive microcalcifications; depiction of disease extent by breast MRI versus PEM versus whole-body PET
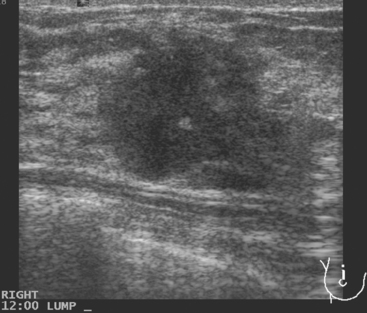
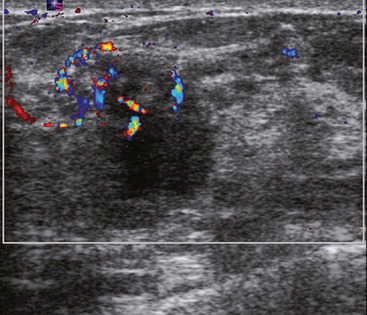
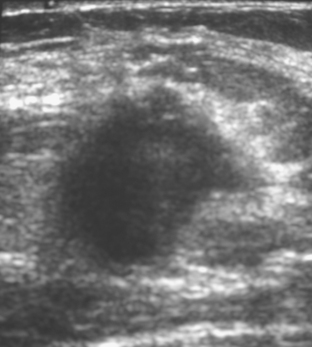
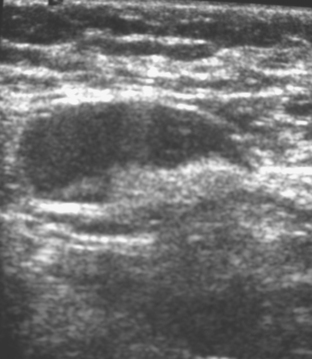
An asymptomatic 40-year-old woman had extensive new left lateral microcalcifications identified on screening mammography. These showed suspicious linear and branching pleomorphism on magnification views (Figure 1), and extensive ductal carcinoma in situ (DCIS) was suspected. Ultrasound was performed to determine whether an invasive component could be identified for biopsy. Five solid masses were identified in the left lateral breast, ranging up to 1.8 cm in size, as well as a suspicious abnormal axillary lymph node (Figure 2).
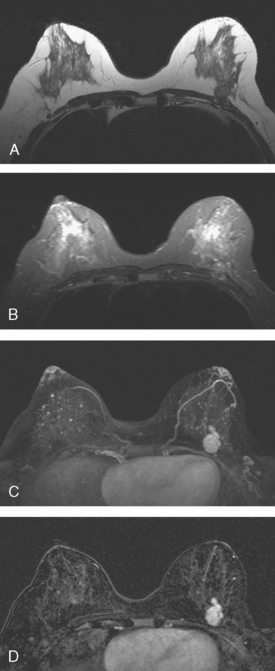
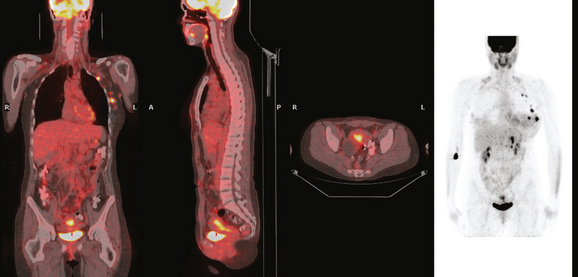
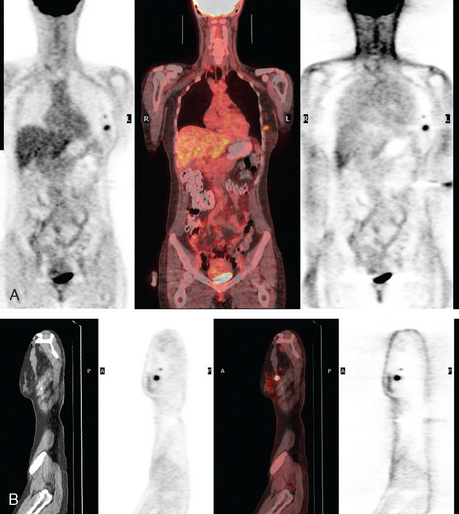
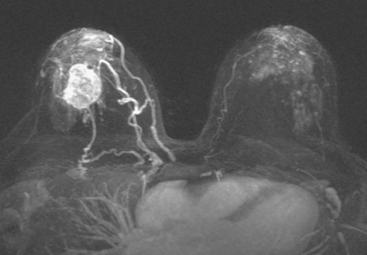
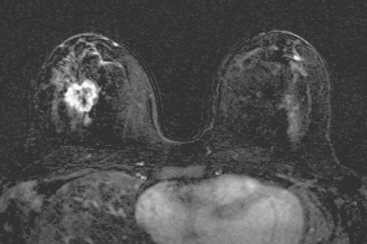
The patient desired breast conservation, and so extensive staging studies were performed to assess the extent of disease. These included breast MRI (Figure 3), positron emission mammography (PEM) (Figure 4 and Figure 5), and whole-body positron emission tomography (PET) (Figure 6). These studies confirmed multicentric disease with axillary nodal involvement, but showed no distant metastases. Neoadjuvant chemotherapy was given.
TEACHING POINTS
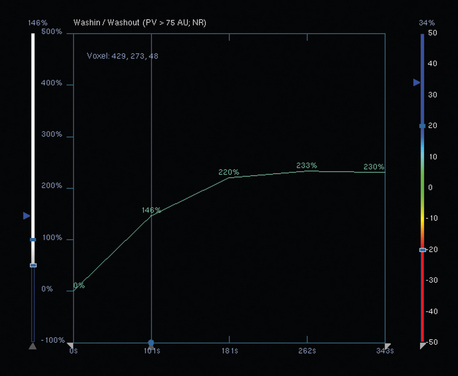
Both invasive and noninvasive disease components are well depicted by MRI. The DCIS manifested as confluent, segmental enhancement in the lateral breast. Eight intensely enhancing discrete masses with washout could be identified by MRI within this extensive intraductal component. PEM performed nearly as well, depicting seven discrete sites of intense increased fluorodeoxyglucose (FDG) uptake. The intraductal disease (as represented by the distribution of calcifications on mammography) would be difficult to recognize prospectively on PEM, without mammographic correlation. The lower-level segmental FDG uptake seen here in the distribution of the DCIS calcifications also corresponds to the distribution of parenchymal density and so would be difficult to differentiate from background parenchymal uptake.
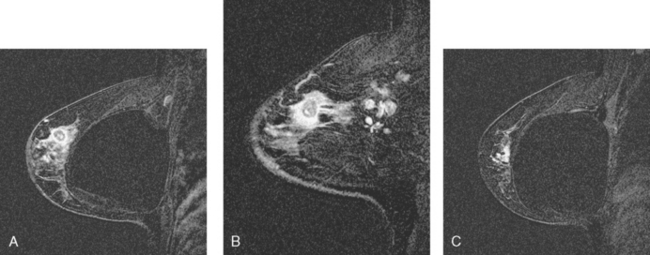
CASE 3 MRI: Extent of disease
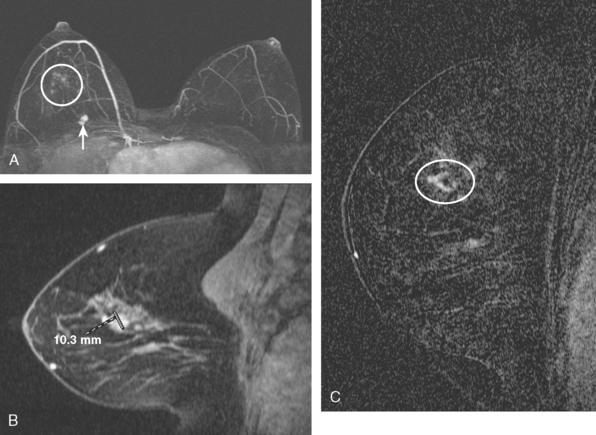
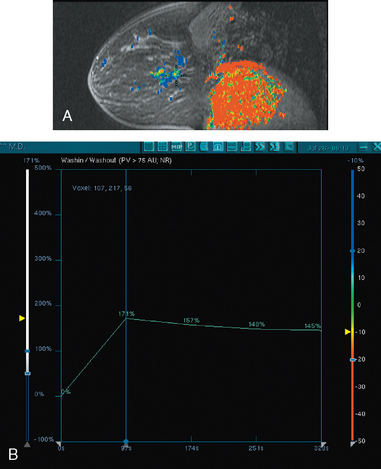
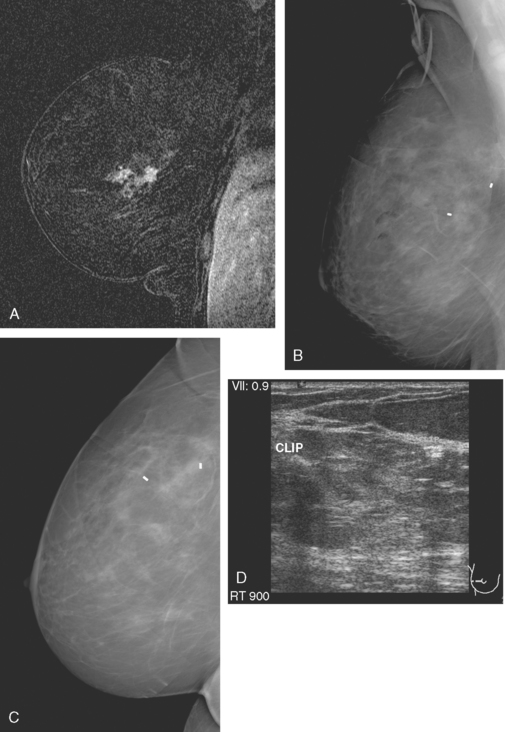
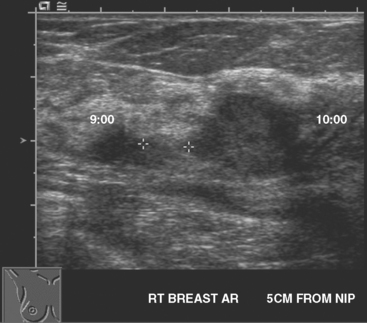
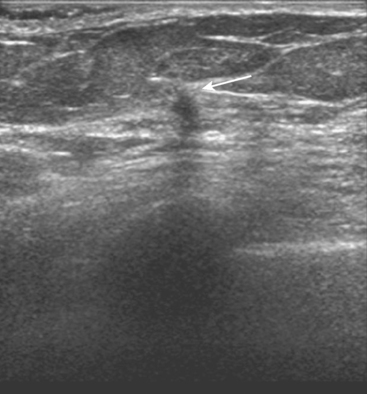
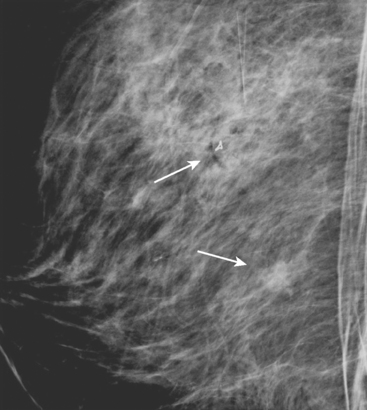
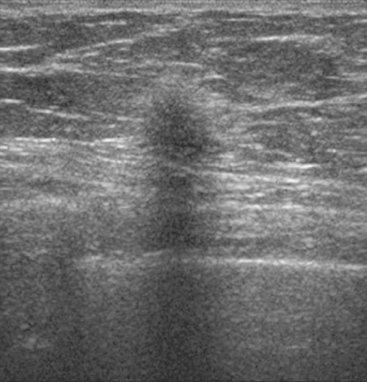
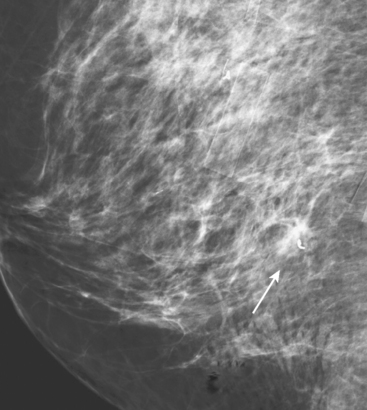
A 54-year-old woman presented with a palpable mass in the upper outer left breast. Diagnostic mammographic and ultrasound evaluations demonstrated multiple masses in the upper outer quadrant of the left breast at the site of the palpable abnormality (Figure 1 and Figure 2). Core needle biopsy confirmed invasive ductal carcinoma. Preoperative MRI suggested much more extensive involvement of the left breast, with multiple enhancing masses extending from area of the known cancer toward the nipple (Figure 3). Because of the MRI findings, second-look ultrasound was performed and identified several small masses in the subareolar region (Figure 4). Core needle biopsy confirmed the extensive nature of the patient’s disease, and she was treated surgically with mastectomy and axillary dissection.
CASE 4 Multicentric IDC and DCIS: Local staging with MRI
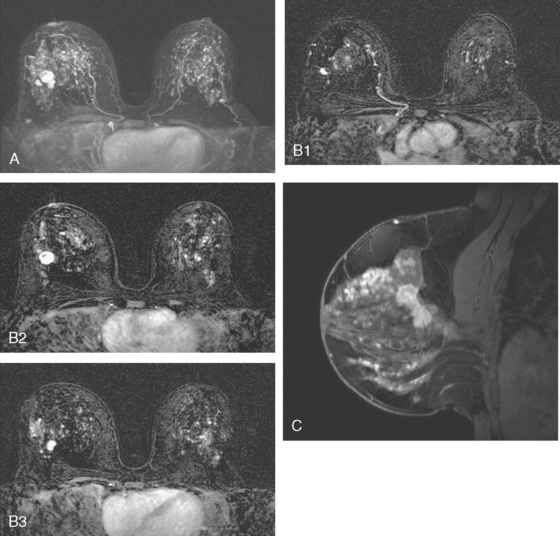
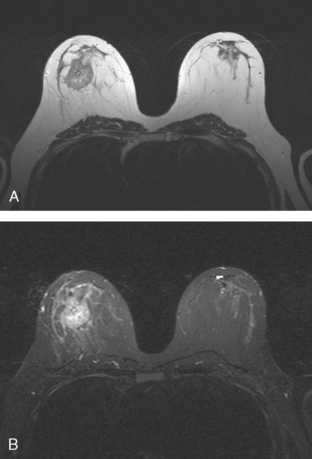
Mammography showed very dense breast tissue, with no correlate for the palpable abnormality (Figure 1). Ultrasound of the palpable lump showed a 2-cm heterogeneous, solid mass, with irregular margins and vascularity and a highly suspicious appearance (Figure 2). An ultrasound-guided core needle biopsy confirmed infiltrating ductal carcinoma (IDC), with high-grade ductal carcinoma in situ (DCIS). At initial surgical consultation, breast conservation therapy with partial mastectomy and radiation was discussed. Because of the mammographic density of the patient’s breasts, the patient was referred for preoperative breast MRI to more fully evaluate her suitability for breast conservation therapy.
Bilateral enhanced subtracted breast MRI showed the known right breast carcinoma mass to be intensely enhancing, with irregular margins and spiculation. Multiple smaller, additional foci of enhancement were noted throughout the right breast, markedly asymmetric compared with the left side (Figure 3 and Figure 4). A few of these foci were larger and more morphologically concerning, including an irregular mass at 5 o’clock (Figure 5) and clumped contiguous foci of enhancement at 9 o’clock (Figure 6). A second-look ultrasound was performed of the right breast seeking correlates for biopsy, to prove the patient’s disease was multicentric and that she was not a conservation candidate.

FIGURE 5 Another enhancing, irregularly bordered mass is seen just below the level of the nipple (partially visualized here). This was reported as being at 12 o’clock. However, the sonographic correlate (see Figure 7) was best seen at 5 o’clock. This discrepancy illustrates the difficulty in lesion localization between modalities, which is due both to the mobility of breast tissue and differences in positioning. This patient had very small breasts, and this is a centrally positioned lesion.
Ultrasound identified two subtle correlates for biopsy, confirming DCIS at both sites (Figure 7 and Figure 8). With pathologic confirmation of multicentric disease, the surgery treatment was changed to mastectomy.
TEACHING POINTS
This case illustrates well how a multimodality approach can yield critical additional information to most accurately stage a newly identified breast cancer. Mammography was not particularly useful in this patient, owing to breast density, but confirmed that there were no microcalcifications. Prospectively, ultrasound identified the index tumor and provided guidance to confirm the diagnosis of IDC. However, no additional disease sites were recognized prospectively on ultrasound to suggest multicentric disease. However, with the breast MRI as a roadmap for a directed, second-look ultrasound, more subtle correlates could be discerned and targeted for sampling, confirming two separate sites of DCIS remote from the IDC, indicating that the patient’s disease should be treated surgically with mastectomy.
CASE 5 Additional disease site identified by PEM
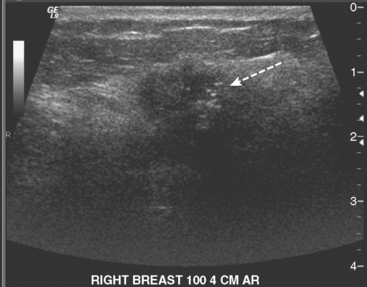
A 46-year-old woman was noted to have a suspicious 1-cm spiculated mass overlying the right pectoral muscle on baseline screening mammography (Figure 1). It was confirmed on spot compression views, and a suspicious sonographic correlate was found (Figure 2), as well as a second concerning ultrasound finding. Both were biopsied with ultrasound guidance, confirming infiltrating ductal carcinoma (IDC) at 12 o’clock and sclerosing adenosis at 10 o’clock.
PEM identified two fluorodeoxyglucose (FDG)-avid abnormalities in the right breast. One corresponded to the known IDC. The other showed a linear and ductal pattern of uptake and was suspicious for a second site of malignancy, but did not clearly correspond to the second ultrasound abnormality in location or morphology (Figure 3).
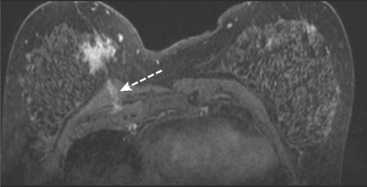
MRI was performed subsequently. The known IDC formed a bilobed mass, but no additional abnormality was recognized prospectively (Figure 4A). When this study was then correlated with PEM, a possible correlate was recognized as localized clumped enhancement centrally, with late appearance of a 1-cm mass (see Figure 4B).
MRI-guided biopsy was recommended. At the time of biopsy, sagittal localization scans showed a clearer, branched, linear pattern of abnormal enhancement, which correlated with the morphology and location of the second PEM abnormality (see Figure 4C). Biopsy was performed with MRI guidance, confirming high-grade ductal carcinoma in situ (DCIS) as well as a complex sclerosing lesion and atypical ductal hyperplasia. This site was 8-cm away from the known IDC. Mastectomy was recommended but refused by the patient.
Mastectomy was again recommended, and declined. Re-excision of the positive margins was again positive for DCIS. She underwent a total of four re-excision procedures from both sites for recurrently positive margins, before undergoing mastectomy.
TEACHING POINTS
PEM is an emerging modality that functionally images breast tissue. PEM is essentially a highresolution, small-field-of-view, dedicated breast PET scan, performed after intravenous administration of FDG and an hour’s uptake time. It is performed utilizing a device resembling a mammogram unit, with detector arrays in place of compression plates, which are positioned on either side of the breast. Views in projections analogous to mammographic views can be obtained, allowing for ready correlation with mammograms. The breast is compressed to immobilize it, but not to the degree required to thin the tissue for optimal mammography. A volume of data is acquired tomographically. At this writing, the only U.S. Food and Drug Administration–approved device is manufactured by Naviscan PET Systems, but analogous PET and gamma camera–based devices by other manufacturers are in various stages of development. The Naviscan PEM tomographic acquisition is divided into 12 slices.
Berg WA, Weinberg IN, Narayanan D, et al. High-resolution fluorodeoxyglucose positron emission tomography with compression (“positron emission mammography”) is highly accurate in depicting primary breast cancer. Breast J. 2006;12(4):309-323.
Tafra L. Positron emission tomography (PET) and mammography (PEM) for breast cancer: importance to surgeons. Ann Surg Oncol. 2007;14:3-13.
Tafra L, Cheng Z, Uddo J, et al. Pilot clinical trial of 18F-fluorodeoxyglucose positron-emission mammography in the surgical management of breast cancer. Am J Surg. 2005;190(4):628-632.
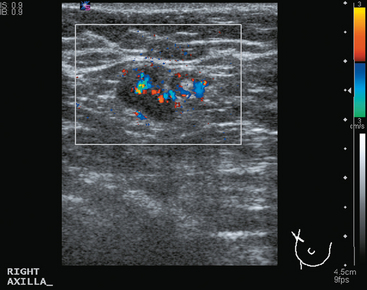
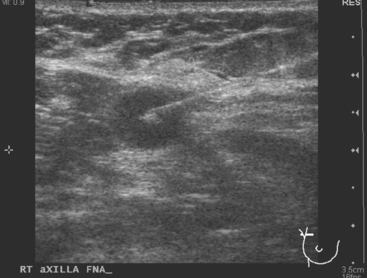
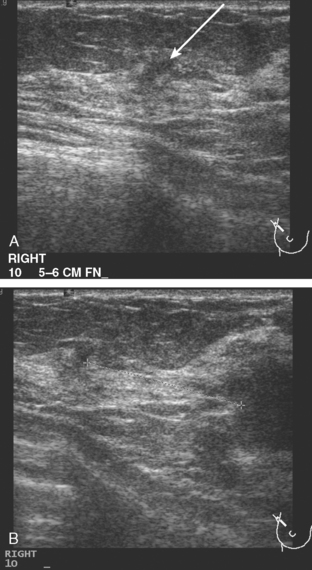
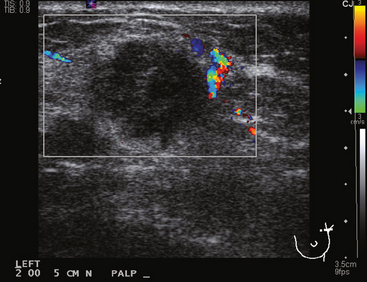
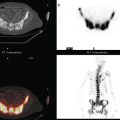
CASE 6 Subtle axillary nodal involvement
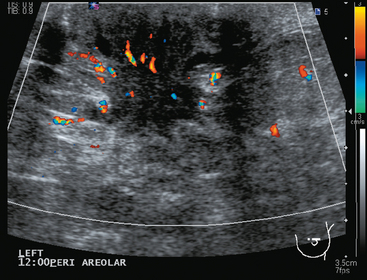
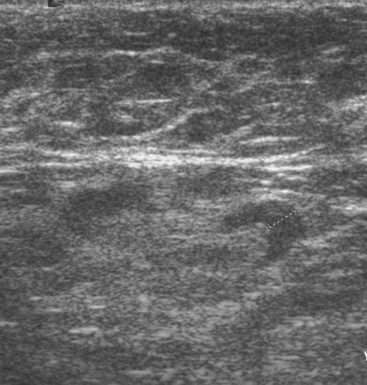
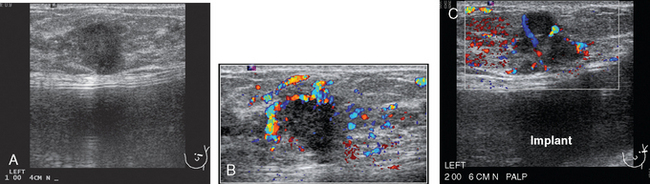
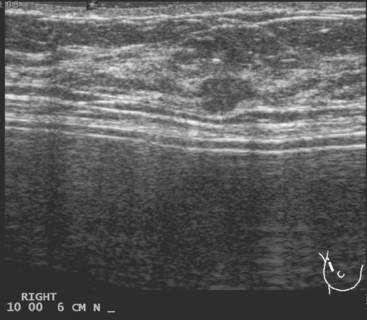
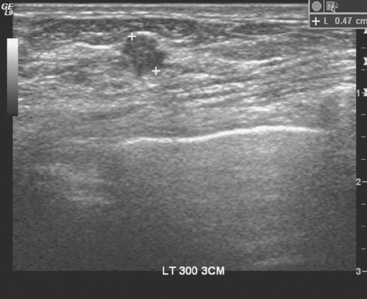
A 52-year-old woman presented with several months of left nipple change, with retraction and infra-areolar fullness. She had had tenderness and nodularity in this breast for more than a year. Her gynecologist confirmed an abnormal clinical exam and referred her to surgery, where the left side was noted to be asymmetrically enlarged, with faint peau d’orange of the periareolar area. A full, irregular, and retracted appearance of the nipple-areolar complex was noted. A large, firm, nodular mass encompassed the central breast on palpation, including the nipple-areolar complex. An axillary lymph node could be palpated. Palpation-guided core needle biopsy of the mass was performed, returning a diagnosis of infiltrating ductal carcinoma (IDC) with focal high-grade ductal carcinoma in situ (DCIS). A punch biopsy of the skin identified carcinoma in dermal lymphatics, confirming the clinical diagnosis of inflammatory breast cancer. The imaging evaluation included mammography, ultrasound, and breast MRI. Mammography showed slightly increased trabecular thickening and breast density. Sonography showed a hypoechoic, solid, irregular, highly vascular mass involving the nipple and retroareolar region (Figure 1). Multiple, sub-centimeter adjacent hypoechoic nodules were noted, and there was diffuse skin thickening. The axilla showed several lymph nodes, without clearly pathologic cortical mantle thickening or other particularly suspicious alterations in morphology prospectively (Figure 2).
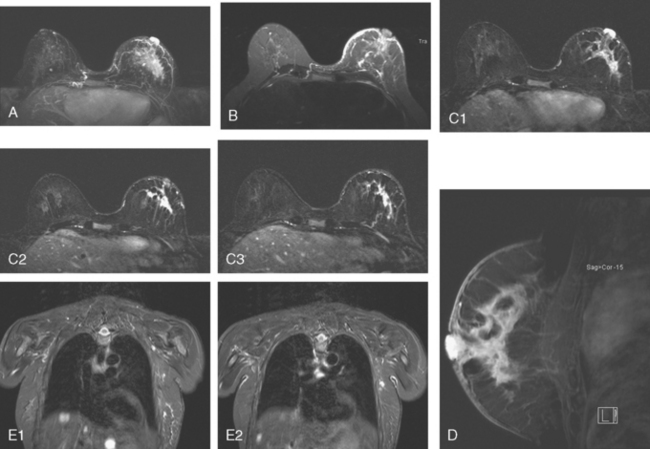
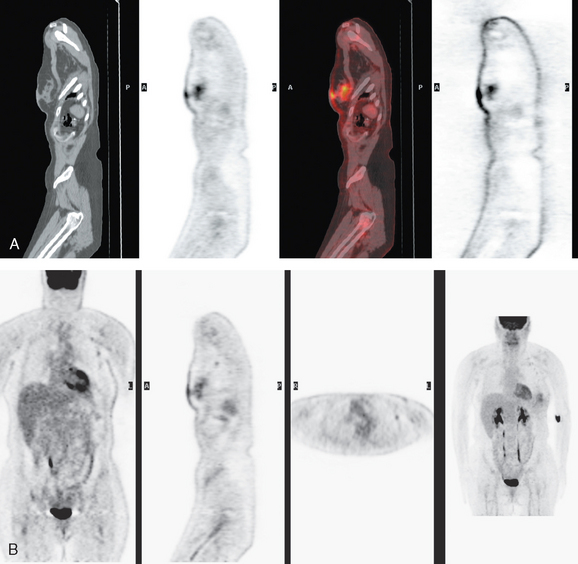
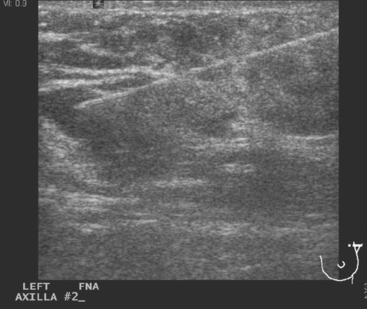
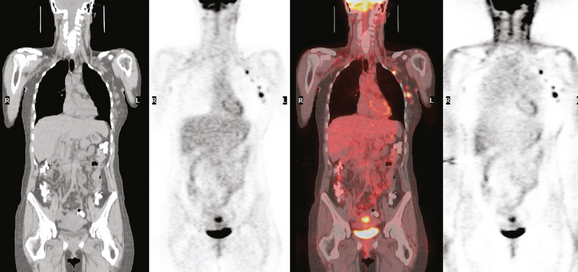
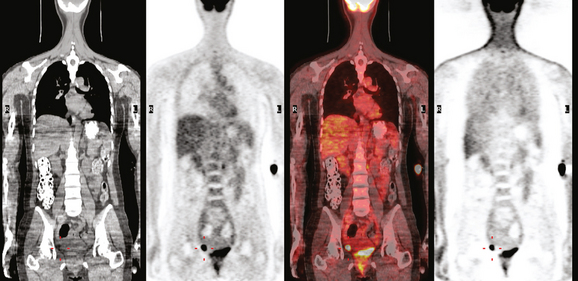
The pretreatment staging of this locally advanced inflammatory breast cancer, with clinical and pathologic proof of skin involvement, was completed with breast MRI, body positron emission tomography (PET)/CT, and contrast-enhanced diagnostic chest, abdomen, and pelvis CT scans. Breast MRI showed a diffusely infiltrating, intensely enhancing central left breast mass, extending to and retracting the nipple (Figure 3). Breast MRI, PET/CT (Figure 4), and contrast-enhanced chest CT (Figure 5) also showed subtle but suspicious findings suggesting left axillary nodal involvement. Axillary fine-needle aspiration (FNA) of an axillary lymph node with only mild cortical mantle thickening was performed and confirmed metastatic carcinoma.
TEACHING POINTS
The imaging findings of involved axillary or regional lymph nodes can be subtle on both anatomic and functional imaging modalities. Relying on the traditional anatomic criteria of size allows us only to suggest disease when lymph nodes are clearly pathologically enlarged. Dimensions defining normalcy have been suggested previously for the axilla of less than 1 cm, or 5 mm in short axis. However, use of dimensional criteria alone will underestimate nodal involvement, which could be suspected based on other findings, such as asymmetry from the opposite, asymptomatic side. Either an asymmetrical increase in number of lymph nodes compared with the normal side, or larger (but still “normal”) size of nodes than on the opposite side, could be an imaging manifestation of nodal involvement. Extranodal extension of disease is suggested by fuzziness of nodal margins or infiltration of axillary fat. This case illustrates these more subtle findings of axillary involvement well. The PET scan also was abnormal, but not floridly so, with modest metabolic activity discernible in a normal-sized lymph node.
CASE 7 Breast MRI problem solving: Deciding among sites for additional sampling
A 63-year-old woman was noted to have new right upper outer quadrant (UOQ) nodularity and increasing microcalcifications on screening mammography. Previously, she had undergone two benign stereotactic left breast biopsies for microcalcifications. Magnification spot compression confirmed the edge of a partially visualized mass as well as architectural distortion. Multiple clusters of microcalcifications were noted. At least three of these seemed to be progressive compared with prior studies, two in the right UOQ and one in the upper inner quadrant. Ultrasound of the right UOQ showed a highly suspicious, vascular, solid, hypoechoic mass at 10 o’clock (Figure 1), which correlated with the partially visualized mammographic mass.
Breast MRI demonstrated the known right UOQ carcinoma at 10 o’clock, with persistent enhancement (Figures 2, 3, and 4). A separate site of clumped enhancement was seen in the lower outer quadrant, 6 cm inferior to the known IDC, which subsequently underwent biopsy with MRI guidance (Figure 5). This proved to be ductal carcinoma in situ (DCIS), intermediate to high grade. This site did not correspond in location to the indeterminate microcalcifications.
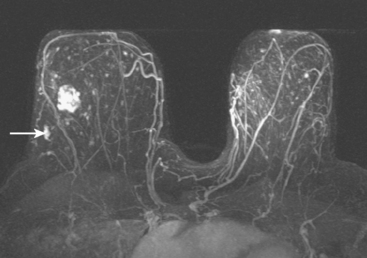
FIGURE 2 Axial maximal intensity projection view from dynamic, contrast-enhanced MRI shows a dominant, heterogeneously enhancing mass in the right lateral breast, corresponding to the known IDC in Figure 1. An oblong focus of irregular enhancement is seen in the posterior lateral right breast (arrow), against a background of bilateral diffuse scattered enhancing foci.
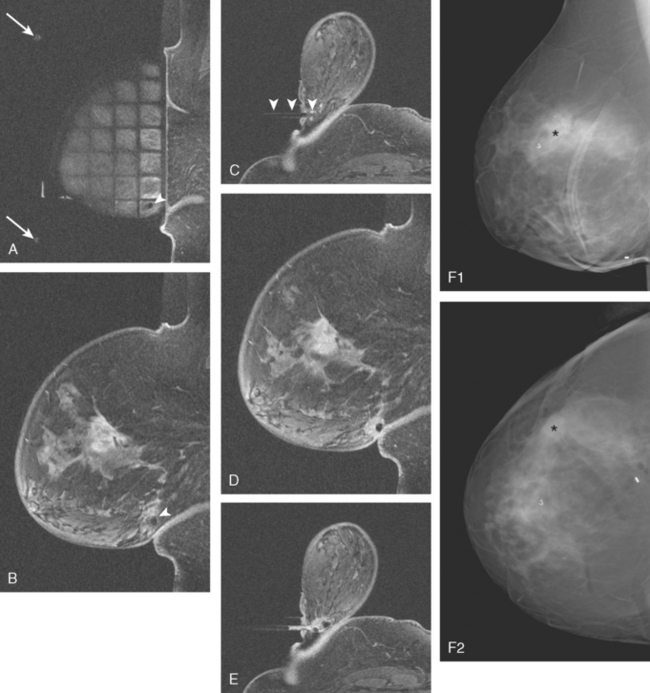
FIGURE 5 Images from the MRI-guided biopsy of the right LOQ mass seen in Figure 4. A, A lateral localizing grid has been incorporated into the dedicated breast coil. Two fiducials are seen (arrows), as well as the hypointense obturator (seen on end, arrowhead) placed to the prescribed depth of the LOQ abnormality. Note how posterior the lesion is; if it had been any further posterior, it might not have been possible to access with this technique. B, Fat-saturated sagittal enhanced imaging after placement of the obturator shows it (arrowhead) in the LOQ. The known UOQ spiculated IDC is also seen on this slice. C, Fat-saturated axial enhanced imaging after placement of the obturator shows it in position (arrowheads). D, Repeat sagittal imaging, after the biopsy, shows a small fluid collection (postbiopsy hematoma) at the site. E, Repeat axial imaging, after the biopsy and clip deployment, shows a new hypointensity within the postbiopsy hematoma, representing the clip. F, Post-MRI-guided biopsy digital mammogram [90-degree lateral (1) and CC (2)] shows dense breast parenchyma and the MRI-placed clip in the posterior LOQ, 6 cm away from the UOQ IDC (asterisks), which is best seen as density and spiculation in the upper breast on the lateral view.
TEACHING POINTS
When this patient’s UOQ IDC was identified, she was also seen to have at least three progressive clusters of microcalcification. In considering breast conservation, it would have been necessary to sample one or more of these microcalcification clusters to assess their significance. Breast MRI was obtained to see if it might help in selecting a second site for biopsy, which might avoid the need for performance of a double or triple stereotactic biopsy of the similar-appearing microcalcifications. The breast MRI did identify a LOQ suspicious abnormality, unrelated to the microcalcifications, which were all in upper quadrants. This LOQ oblong mass proved to be DCIS, indicating that the patient was best treated with mastectomy, and avoiding the need to perform a battery of stereotactic biopsies for diagnosis of the microcalcifications.
CASE 8 Breast MRI problem solving: Assessing depth of involvement of posterior breast cancer
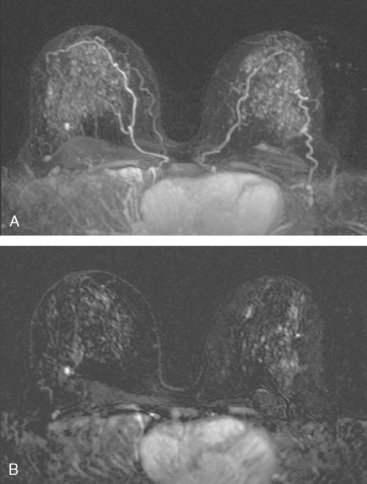
A 47-year-old premenopausal woman noted a palpable, growing left breast mass, which was confirmed mammographically as a deep central mass. Stereotactic biopsy at another facility made the diagnosis of infiltrating ductal carcinoma (IDC). By clinical exam, the mass was on the order of 4 cm in size, occupying much of the upper outer quadrant, and seemed to be affixed to the chest wall. By ultrasound, the mass appeared to involve pectoral muscle (Figure 1).
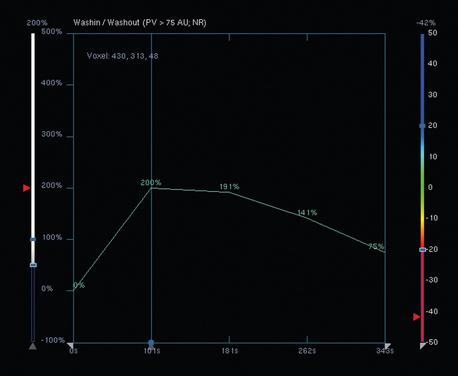
Breast MRI was requested to assess the relationship of the mass to the chest wall. It confirmed extension of the mass into the pectoral muscle, without chest wall involvement (Figure 2 and Figure 3). The mass enhanced intensely, had lobular margins, and displayed washout on kinetic analysis.
Additional therapy given was chest wall radiation and tamoxifen.
TEACHING POINTS
Breast MRI is the most accurate imaging modality currently in use for assessment of the extent of muscle involvement by posterior breast cancers. Morris and associates have shown that abutment or effacement of the fat plane is not sufficient imaging evidence to suggest muscle invasion. Actual enhancement in the muscle is necessary to diagnose muscle invasion. In this case, pectoral muscle involvement can be suspected from ultrasound, but the delineation of the extent of muscle invasion is more precise with MRI.
CASE 9 Breast MRI problem solving: Chest wall invasion
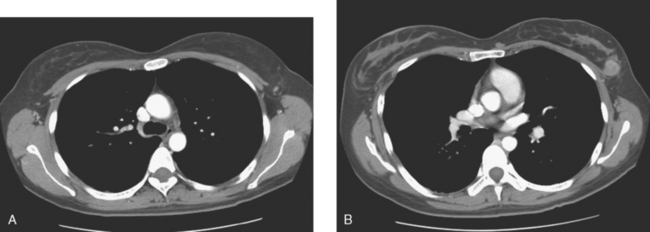
A 45-year-old woman presented with a palpable mass in the upper inner right breast. Mammography showed a partially obscured mass with associated suspicious calcifications, corresponding to the palpable mass (Figure 1 and Figure 2). Ultrasound demonstrated the mass to be an irregularly marginated solid mass (Figure 3). Ultrasound-guided core needle biopsy identified high-grade invasive ductal carcinoma. Preoperative MRI demonstrated the known tumor to invade the pectoralis and chest wall (intercostal) muscles (Figure 4). The patient was treated with neoadjuvant chemotherapy, followed by surgery and radiation therapy.
TEACHING POINTS
This case of locally advanced breast cancer illustrates the use of preoperative MRI to aid in tumor staging. Pectoralis and chest wall involvement should be considered in patients with posterior tumors. Effacement of the intervening fat or abutment of the pectoralis fascia does not correlate well with muscle involvement. However, enhancement of the muscle itself on MRI is highly suggestive of muscle invasion. Although pectoralis involvement alone does not change staging, chest wall (intercostal muscle) invasion would represent stage IIIB. In addition, involvement of the pectoralis or chest wall may affect surgical management as well as radiation therapy planning.
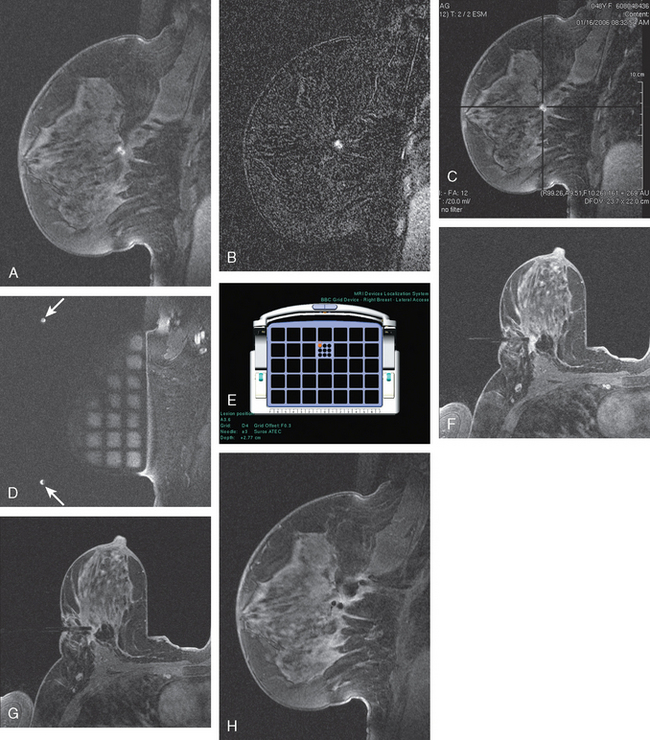
CASE 10 Breast MRI problem solving: MRI guidance for tailored lumpectomy
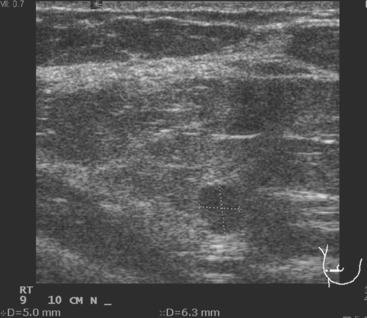
A 41-year-old woman underwent routine screening mammography. A nodular right upper outer quadrant (UOQ) density was questioned. Spot compression suggested persistent architectural distortion. Ultrasound of the right lateral breast at 9 o’clock showed a subtle, hypoechoic nodule (Figure 1). The axilla was more convincingly abnormal, with a lymph node with effaced hilus and abnormal vascularity (Figure 2 and Figure 3). Ultrasound-guided core needle biopsy of the small breast mass identified infiltrating ductal carcinoma (IDC), estrogen receptor negative, progesterone receptor positive, HER-2/neu negative. Malignancy was also confirmed in the axillary lymph node by fine-needle aspiration (FNA) (Figure 4).
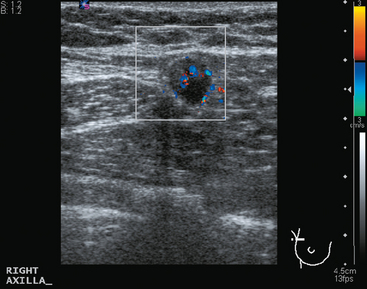
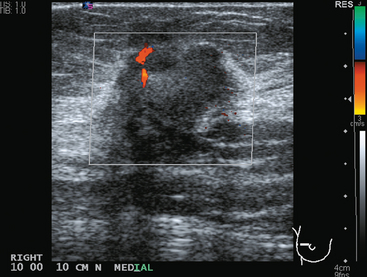
FIGURE 3 Transverse ultrasound view of the vascular lymph node in Figure 2: Note abnormal vascularity on the periphery of the node. Identification of vascularity anywhere but in the hilus is abnormal.
Staging evaluations showed normal tumor markers and no evidence of distant metastatic disease by positron emission tomography, CT, and bone scans (Figure 5). Breast MRI was requested by the breast surgeon because of increased mammographic breast density. MRI showed the disease process to be larger than previously suspected. In the region identified by ultrasound, MRI showed a multinodular, clumped area of enhancement with washout (Figure 6 and Figure 7). Individual small mass components measured on the order of 1 cm each, with the overall process measuring about 3 cm in dimension. Given the discrepancy between the volume of disease displayed by ultrasound compared with MRI, there was concern that localization for breast conservation with ultrasound guidance would lead to positive margins.
Under MRI guidance, clips were placed to bracket the MRI abnormality (Figure 8). One was placed anterolaterally and the other posteromedially. Post-MRI procedure mammograms confirmed successful clip deployment. The clips were localized with ultrasound guidance on the day of surgery.
The final stage was stage II, T2N1. Chemotherapy was begun on a study protocol.
CASE 11 Breast MRI problem solving: Assessment of completeness of breast cancer excision
A 48-year-old woman noted a palpable right breast lump, which on her physician’s physical exam was assessed to be 1 cm in the upper outer quadrant (UOQ) and possibly a cyst. Mammography showed dense breast tissue only. Ultrasound at the site of the palpable lump was negative. However, a sonographically suspicious 1.5-cm mass was found nearby, localizing to 10 o’clock, with a second nearby 7-mm nodule at 9 o’clock (Figure 1). Both were sampled with ultrasound-guided core biopsy, confirming infiltrating ductal carcinoma (IDC) at both sites.
MRI was ordered to assess the extent of tumor because the known IDC was mammographically and clinically occult. MRI suggested multifocal tumor, with three UOQ intensely enhancing tumor nodules, two of which were immediately adjacent to each other and could have been components of a bilobed mass (Figure 2).
The largest mass measured 1.8 cm, with the adjacent mass or component 1.2 cm in maximal dimension. Another smaller mass was identified 2.5 cm superolaterally. Second-look ultrasound was then undertaken and showed an additional 5 mm nodule at 10 o’clock, 2 cm away from the largest index lesion (Figure 3).
Because of the confusion resulting from the apparent multifocality of the tumor on imaging compared with the single tumor mass on pathology, breast MRI was obtained to assess the completeness of the resection before beginning radiation therapy. By this time, two cycles of AC had been given.
The breast MRI showed a 7-mm enhancing nodule medial to the postoperative seroma, suspicious for a residual tumor focus (Figure 4). Biopsy with MRI guidance confirmed IDC (Figure 5). A clip was placed, which was subsequently localized with two bracketing wires. The specimen showed a 0.6-cm residual IDC, and the margins were clear.
TEACHING POINTS
Although it took three surgical excisions, this patient ultimately was successfully treated with breast conservation. Breast MRI was instrumental in enabling this patient to be thus treated. Breast MRI provided the most accurate map of the extent of disease, guiding the second-look ultrasound to a third focus of disease. Ultrasound-guided triple-needle localization removed the bulk of the disease. A close margin prompted re-excision, with the new margins negative.
With subsequent confirmation of residual disease, and the patient still desirous of breast conservation, localization and excision of the confirmed residual were undertaken. Whether such small-volume residual disease as this, which undoubtedly would have gone undetected in the pre–breast MRI era, would have been adequately treated with radiation and chemotherapy or would have predisposed this patient to local recurrence in subsequent years, we can only speculate. The rationale of breast conservation is predicated on the surgical removal of all identifiable disease, a task that has become increasingly complex now that we are better able to identify disease. There is support in the literature that indicates that patients treated with breast conservation using preoperative breast MRI have fewer local recurrences. To date, no studies evaluating the effect of the use of preoperative breast MRI on overall survival have been reported.
CASE 12 Contralateral breast carcinoma found on MRI; not seen on second-look ultrasound
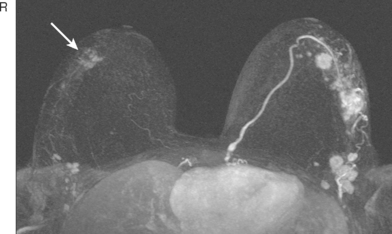
A 48-year-old woman with mammographically detected ductal carcinoma in situ in the right breast underwent preoperative MRI. The MRI revealed a suspicious enhancing mass in the contralateral left breast (Figure 1). Subsequent targeted ultrasound was performed of the lateral left breast in the area of the MRI lesion (Figure 2 and Figure 3). Because no discrete lesion was seen on ultrasound, MRIguided biopsy was performed of the left breast mass (Figure 4). Core needle biopsy revealed an 8-mm intermediate-grade invasive ductal carcinoma.
TEACHING POINTS
This case illustrates the limitations of ultrasound in detecting correlates for suspicious MRI lesions. Although this patient had not had a prior ultrasound of the left breast, the term second-look ultrasound is used when targeted ultrasound is performed to find an ultrasound correlate to a finding on MRI. In this case, although some vascularity was detected on Doppler ultrasound in the corresponding area in the lateral left breast, this finding is not enough to allow confident correlation with the lesion seen on MRI. In general, ultrasound-guided needle biopsy is preferred over MRI-guided biopsy because of patient comfort, time, and cost. However, depending on the size of the lesion seen on MRI and the quality of the ultrasound evaluation, second-look ultrasound may fail to identify a sonographic correlate in as many as 77% of MRI-detected lesions referred for biopsy. Therefore, a suspicious lesion seen on MRI requires biopsy despite a negative ultrasound. For programs performing breast MRI, it is essential that there also be MRI-guided biopsy capability to assess sonographically occult lesions.
Dhamanaskar KP, Muradall D. MRI directed ultrasound: a cost effective method for diagnosis and intervention in breast imaging [abstract]. Radiology. 2002;225:653.
LaTrenta LR, Menell JH, Morris EA, et al. Breast lesions detected with MR imaging: utility and histopathologic importance of identification with US. Radiology. 2003;227:856-861.
Panizza P, De Gaspari A. Accuracy of post MR imaging second-look sonography in previously undetected breast lesions [abstract]. Radiology. 1997;205:489.
CASE 13 Evaluation of the other breast with MRI
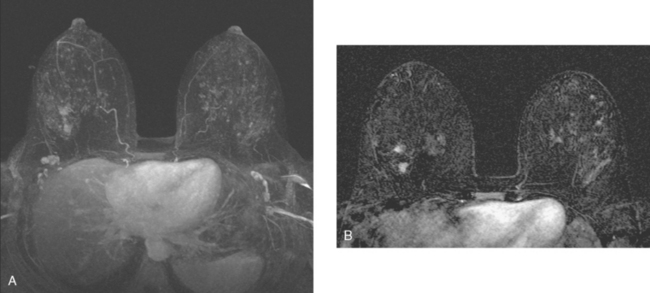
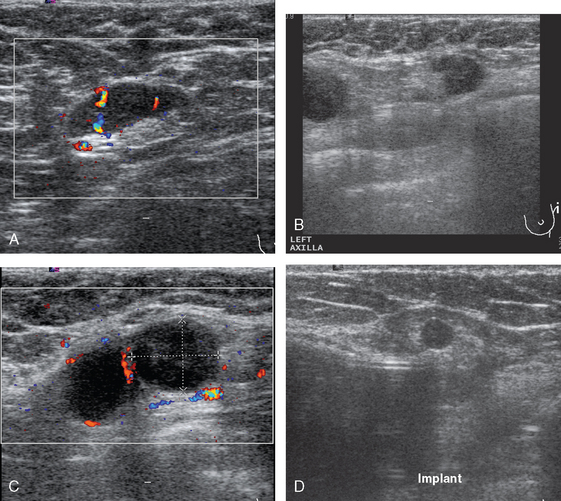
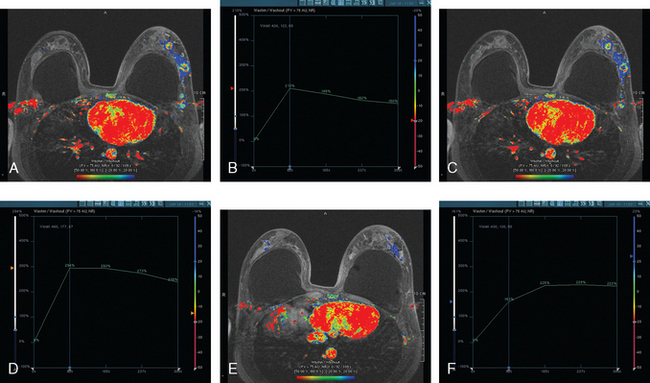
Mammography showed asymmetrical density and nodularity at the levels indicated by the patient. Ultrasound showed multiple abnormal axillary lymph nodes as well as round lymph nodes at the lateral margins of the implant (Figure 1). Two vascular, dominant, highly suspicious breast masses were identified in the upper outer quadrant (UOQ) at 1 and 2 o’clock (Figure 2). At least three additional discrete, 4- to 5-mm, hypoechoic nodules were noted in the same quadrant, interposed between and adjacent to the dominant masses. The two dominant breast masses were sampled with ultrasound-guided core needle biopsy, confirming infiltrating ductal carcinoma (IDC) from both sites. Two axillary lymph nodes were also sampled with fine-needle aspiration technique, confirming metastatic poorly differentiated carcinoma (consistent with breast primary) from both sampled sites (Figure 3).
Breast MRI showed extensive left UOQ and axillary disease and correlated well with the sonogram (Figures 4, 5, 6, 7, 8, and 9). The two known foci of IDC manifested as intensely rim-enhancing, spiculated masses with washout kinetics. They were accompanied by an entire quadrant filled with enhancing smaller masses and clumped enhancement, extending down to the level of the nipple. Multiple, intensely enhancing lymph nodes were identified, also correlating with the ultrasound, both along the lateral margin of the implant and extending up into the axilla.
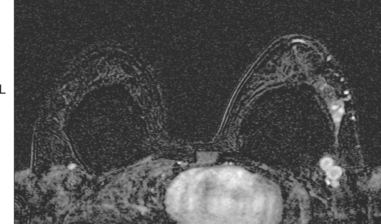
FIGURE 6 An additional slice from the same series shows two adjacent, rim-enhancing, round lymph nodes at the lateral margin of the implant, corresponding to the ultrasound findings depicted in Figure 1C. Anterior to these, in the lateral breast tissue adjacent to the implant, is a cluster of three tiny enhancing masses.
A suspicious, as yet unsuspected, contralateral abnormality was identified in the right breast on MRI, manifesting as a 2 × 2.6-cm region of clumped progressive enhancement, within which was a 6-mm nodule with washout (see Figures 7, 8C, and 9G and 9H). Targeted sonography identified tiny hypoechoic nodules in the expected region. The most suspicious measured 5 mm in maximal dimension and showed irregular, angular margins (Figure 10). Core needle biopsy under ultrasound guidance identified focal (1.5 mm) invasive ductal carcinoma (in one of six cores) and intermediate-grade ductal carcinoma in situ (DCIS) in all the cores.
Additional preoperative staging was obtained, including bone scan, enhanced chest, abdomen and pelvis CT scans, and positron emission tomography (PET)/CT (Figures 11, 12, 13, 14, 15, and 16). The PET scan showed the two known left UOQ IDCs to be hypermetabolic, as were multiple level I and II left axillary lymph nodes. No evidence of systemic breast cancer was seen. Hypermetabolism identified of the uterine lining was followed up with ultrasound, which was unremarkable, and the activity was presumed to be physiologic variation.
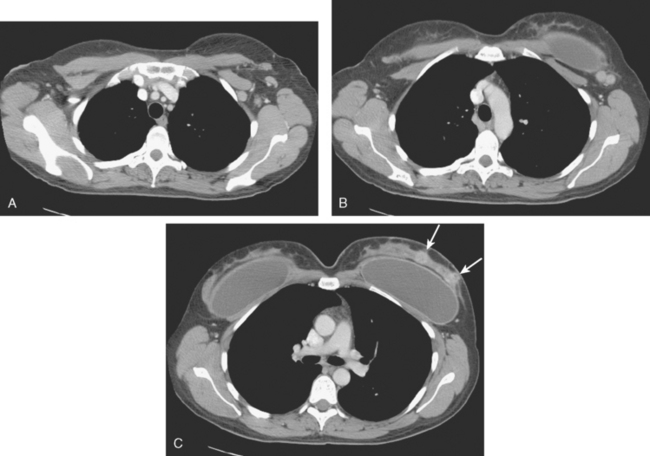
FIGURE 14 Corresponding contrast-enhanced chest CT images for correlation, from above to below. A, Enlarged left axillary level I and II lymph nodes are strikingly asymmetrical compared with the right side. Several show fuzzy margins. B, At the upper margin of the implant, adjacent lymph nodes with fuzzy margins correspond with findings depicted on ultrasound (see Figure 1C) and MRI (see Figure 6). C, The two IDC masses can be seen on CT owing to differential enhancement from the rest of the parenchyma, but not to the same advantage as on breast MRI (arrows).
With the new contralateral breast information, the patient elected to undergo bilateral mastectomy and implant removal. The simple mastectomy pathology on the right showed a 2-cm high-grade DCIS lesion extending into lobules, and two sentinel lymph nodes were negative. The left mastectomy specimen showed two foci of IDC, one described as in the lower outer quadrant (LOQ) measuring 1.5 × 1.4 × 1.4 cm. A second IDC was 2 cm medial and superior to the first focus (in the mid-breast posterior to nipple) and measured 2.1 × 2.0 × 1.5 cm. There was extensive angiolymphatic invasion. DCIS (solid, with foci of comedo necrosis) was associated with the invasive tumors and extended into lobules. Nine of 18 lymph nodes showed metastatic tumor, with the largest 1.4 cm with extranodal extension. Margins were negative, by at least 5 mm. The tumors were estrogen receptor negative, progesterone receptor positive, and HER-2/neu negative.
TEACHING POINTS
Systemic staging studies obtained in this patient included PET/CT and enhanced body CT scans. This case shows one source of normal variant PET increased activity, which can be seen in the pelvis of premenopausal patients. Physiologic endometrial activity can be seen, as well as activity in uterine fibroids (see Chapter 4, Case 7) and in corpus luteum cysts (see Case 17 in this chapter). In this case, PET/CT allows us to localize the uterine activity to the endometrium. Subsequent pelvic sonogram showed a normal appearance of the endometrium, and the patient was asymptomatic, so this is presumed to be normal variant activity. This has been documented to occur most commonly during menstruation and ovulation (weeks 1 and 3 of the menstrual cycle). Right adnexal findings compatible with a corpus luteum cyst were seen in this patient, but no associated hypermetabolism was apparent. Another commonly seen source of normal variant activity, brown fat, is also demonstrated in this patient.
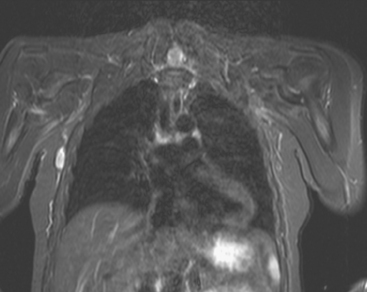
CASE 14 Use of body coil STIR imaging for local staging of new diagnoses of breast cancer
A 51-year-old woman noted a palpable lump in her right breast for 2 to 3 months, which was confirmed by her physician as a palpable 1-cm mass above the nipple at 11 to 12 o’clock. Mammography of the area showed increased density and possible architectural distortion in a region of mammographically stable microcalcifications (Figures 1, 2, and 3). Sonography confirmed a suspicious, dominant, solid, vascular, irregularly marginated, palpable mass, measuring up to 2.3 cm (Figure 4 and Figure 5). Because it was palpable, the patient was referred to surgery for a palpation-guided biopsy, which diagnosed infiltrating ductal carcinoma (IDC).
Because of the marked breast density, completion of the evaluation was undertaken with breast MRI. This showed the known IDC to be a dominant, spiculated, intensely enhancing mass. The disease appeared to be unifocal (Figure 6 and Figure 7). However, 1-cm bilateral supraclavicular lymph nodes (one on each side) were suggested on a coronal STIR sequence of the thorax (Figure 8). Positron emission tomography (PET)/CT was obtained for further evaluation and showed hypermetabolism only at the site of the known IDC (Figure 9).
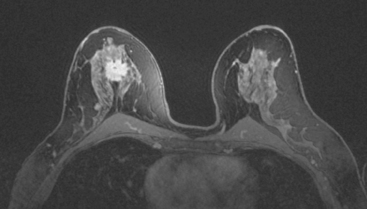
FIGURE 7 Fat-saturated, enhanced, high-resolution MRI of the mass better shows the marginal spiculation.
The patient was treated surgically with lumpectomy and axillary node dissection, finding an estrogen receptor– and progesterone receptor–positive, HER-2/neu-negative, 2.1-cm IDC, with 1 of 12 lymph nodes positive. Final stage was IIB, T2N1M0. Two cycles of Cytoxan, methotrexate, 5-fluorouracil (CMF) chemotherapy were administered. The patient’s therapy was changed to hormonal ablation with sulindac (Zoladex) and tamoxifen because of her desire to discontinue chemotherapy.
TEACHING POINTS
A variety of protocols are used for performance of breast MRI, with little standardization across institutions. That said, most protocols have certain critical elements in common. In general, breast MRI should be performed on a high-field magnet (at least 1.0 Tesla) in a dedicated breast coil. At a minimum, the protocol must include at least a fluid-sensitive sequence (T2 or STIR), and fatsaturated or subtracted enhanced T1-weighted sequences. Acquisition can be sagittal or axial, unilateral or bilateral. We have a strong preference for axial imaging performed bilaterally to allow for ease of comparison with the opposite side. Choices for fluid-sensitive sequences include T2-weighted, fat-saturated T2 weighted, and STIR sequences. These sequences permit identification of fluid, such as in cysts or in postoperative collections. Bright signal intensity on STIR of common encountered enhancing benign entities, such as fibroadenomas and lymph nodes, aids in characterization.
CASE 15 MRI depiction of axillary and internal mammary node involvement
Annual screening mammography of a 72-year-old woman demonstrated suspicious masses in the medial left breast (Figure 1). Ultrasound confirmed these to be solid and demonstrated an abnormal left axillary node (Figure 2 and Figure 3). An MRI confirmed the left breast masses and the abnormal axillary node (Figure 4). The constellation of findings was suspicious for multifocal carcinoma with axillary metastasis. In addition, MRI showed an abnormal-appearing left internal mammary lymph node (Figure 5 and Figure 6). Ultrasound-guided core needle biopsy of the left breast confirmed multifocal invasive ductal carcinoma. The patient was treated with left mastectomy and radiation therapy.
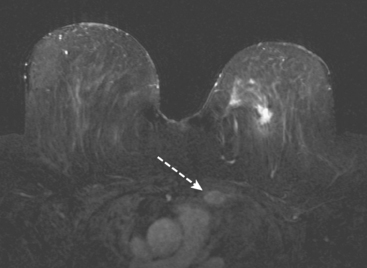
FIGURE 5 Postcontrast subtracted axial MRI. An enlarged left internal mammary node is demonstrated (arrow).
CASE 16 Cautionary notes on the use of breast MRI
A 53-year-old woman was diagnosed with left breast infiltrating lobular carcinoma (ILC) and lobular carcinoma in situ (LCIS) by stereotactic biopsy of left upper outer quadrant (UOQ) microcalcifications. The pathology specimen identified atypical ductal hyperplasia (ADH) associated with microcalcifications, with the ILC and LCIS noncalcified. The patient underwent a lumpectomy, with no residual tumor in the specimen. One sentinel lymph node was negative. She was treated with radiation therapy in addition.
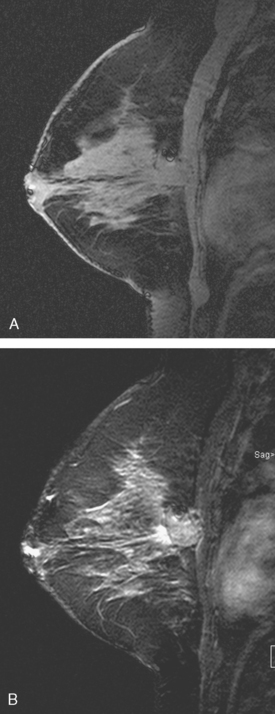
While undergoing radiation therapy, breast MRI was performed to assess the opposite side. This showed a segmental region of clumped enhancement in the right medial breast, where the breast was largely fatty (Figures 1, 2, 3, and 4). An MRI-guided biopsy was performed when no ultrasound correlate could be found, and pathology showed ductal carcinoma in situ (DCIS).
TEACHING POINTS
This is an unusual outcome. Generally, when a malignant diagnosis is established by a large-volume core needle biopsy, we expect the same or worse to be obtained when the area is excised. However, the opposite is quite possible, as this case and Case 6 in Chapter 6 demonstrate. It is certainly possible in image-guided biopsies to sample a small malignant component, only to find no residual tumor in the excision. In fact, that had happened in this patient in her original left breast diagnosis of ILC and LCIS. The targeted microcalcifications corresponded to ADH at pathology, whereas the ILC and LCIS were noncalcified but present in the stereotactic specimen. No residual tumor was found at pathologic examination of her lumpectomy specimen.
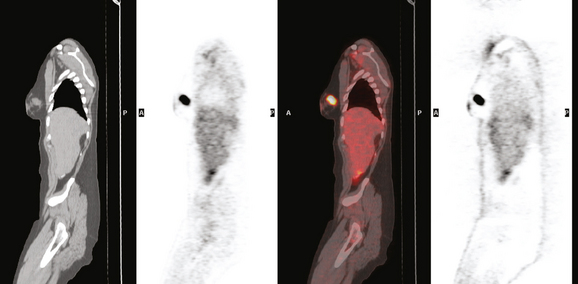
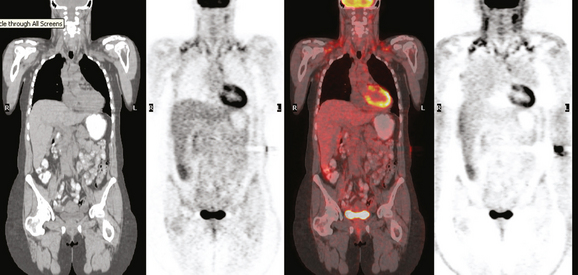
CASE 17 Whole-body PET as an adjunct to initial staging of node-positive breast cancer: Benign PET pelvic uptake in a corpus luteum cyst
A 41-year-old premenopausal woman presented with a few weeks’ history of a palpable left upper outer quadrant breast lump. Mammography identified a dominant 2 cm mass, with irregular and spiculated margins (Figure 1 and Figure 2). Ultrasound confirmed a 1.7-cm highly suspicious corresponding mass, as well as a small but abnormal looking axillary lymph node (Figures 3, 4, 5, and 6). Ultrasound-guided biopsy of the breast mass confirmed invasive lobular carcinoma (ILC), and fine-needle aspiration of the axillary lymph node showed metastatic adenocarcinoma.
Breast MRI was obtained because of the ILC histology. The known tumor mass showed intense rim enhancement and washout (Figures 7, 8, and 9). Tissue anterior to the known tumor showed an asymmetrical, less intense, plateauing pattern of more indeterminate enhancement (see Figure 7; Figure 10). MRI-guided biopsy was recommended.
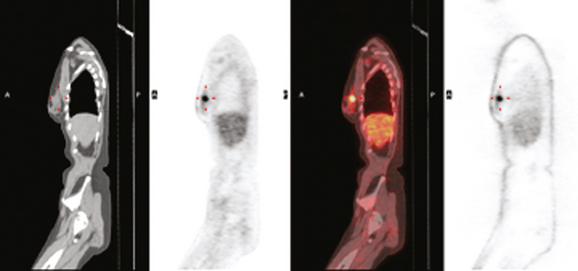
Positron emission tomography (PET)/CT was ordered because of the known axillary involvement. The known breast cancer mass was quite hypermetabolic and readily identified. No additional breast abnormality was seen. A small punctate left axillary focus of activity was also seen, corresponding to the known involved lymph node (Figure 11 and Figure 12). An additional focus of hypermetabolism in the pelvis appeared to correspond to a right ovarian cyst (Figure 13 and Figure 14). This was presumed to be a corpus luteum cyst, a known cause of normal variant benign PET activity.
A second-look left breast ultrasound showed no additional anterior abnormality. It was elected to sample more anterior tissue at the time of lumpectomy and axillary dissection. The pathology showed a 2-cm infiltrating ductal carcinoma (IDC) with angiolymphatic invasion and high histologic grade (8 of 9) with close (<2 mm) deep margin and other margins clear by more than 1.5 cm. The invasive tumor was noted to extend close to the deep margin at two levels, both of which contained skeletal muscle at the deep surface. Fibrocystic changes and fibroadenomatoid changes were also noted in the specimen. Metastatic carcinoma was found in 1 of 17 lymph nodes.
TEACHING POINTS
This case provides many takeoff points for discussion. The characteristics of the index lesion in this case are entirely typical for malignancy with all modalities. The MRI did raise a question of more extensive disease extending anteriorly from the index lesion. The negative second-look ultrasound did not identify a correlate. This does not entirely resolve the question because correlates for MRI abnormalities which prove to be cancer are found in as few as 23% of cases. Rather than delay this patient’s planned surgery with an MRI-guided biopsy, the surgeon used the MRI information to extend the excised area, which showed fibrocystic and fibroadenomatoid changes on histology.
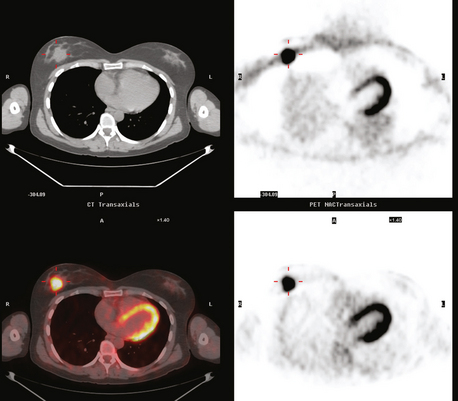
CASE 18 Whole-body PET as an adjunct to initial staging of node-positive breast cancer: Rotter’s node involvement
A 37-year-old woman found a right UOQ palpable breast lump. Mammography showed a dominant lobular breast mass, with some margins obscured by dense adjacent parenchyma (Figure 1). Sonography demonstrated highly suspicious features, including taller-than-wide shape, and angular and irregular margins (Figure 2). In addition, an axillary lymph node with a thickened (7-mm) cortical mantle was found (Figure 3). Ultrasound-guided core needle biopsy of the dominant mass proved infiltrating ductal carcinoma (IDC), and ultrasound guided fine-needle aspiration of the axillary lymph node confirmed malignant cells, consistent with metastatic breast carcinoma.
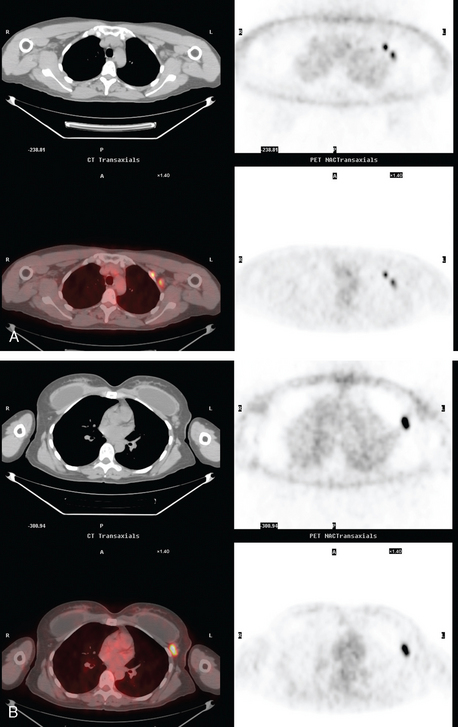
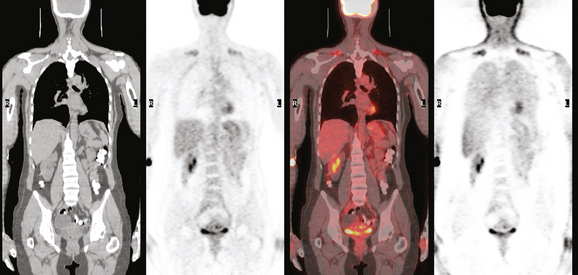
Because of the size of the patient’s cancer relative to her breast size, mastectomy was favored for surgical therapy over lumpectomy. Breast MRI confirmed unifocal disease (Figures 4, 5, 6, 7, and 8). Staging workup showed elevated tumor markers (CEA and CA27.29). A positron emission tomography (PET)/CT scan and enhanced diagnostic chest, abdomen, and pelvic CT scans showed intense hypermetabolism in the known breast cancer (Figures 9, 10, 15) and abnormal right axillary lymph node (Figures 8, 11 and 12). Increased metabolic activity was also seen in a 5-mm right interpectoral (Rotter’s) lymph node (Figure 13 and Figure 14). A commonly seen normal variant source of PET activity was also seen, with symmetrical supraclavicular brown fat uptake (Figure 16).
The mastectomy specimen demonstrated a 3-cm infiltrating ductal adenocarcinoma, with clear margins. Because the patient was known preoperatively to have axillary involvement, axillary dissection was performed at the same time. Three of 22 lymph nodes showed metastatic adenocarcinoma. The largest involved lymph node measured 1.5 cm.
Subsequently, the patient underwent genetic testing and proved to have a BRCA1 gene mutation. Her family history consisted of premenopausal breast cancer in a maternal aunt. Ten months after finishing radiation therapy, she underwent prophylactic left mastectomy, bilateral transverse rectus abdominis myocutaneous (TRAM) flap reconstruction, hysterectomy, and oophorectomy. Her postoperative course was complicated by development of abdominal wall infection, twice requiring operative débridement of infected, necrotic tissue and intravenous antibiotic therapy (see Case 3 in Chapter 6 for imaging features of the TRAM flap donor site complications). She also developed pulmonary embolism.
Nearly concurrent with these events, the patient developed right upper arm and shoulder pain, and she noted development of an infraclavicular lump. Recurrence was subsequently confirmed in right infraclavicular and mediastinal nodal regions. See Case 7 in Chapter 10 for the recurrent disease imaging findings.
CASE 19 Bracketing needle localization of microcalcifications
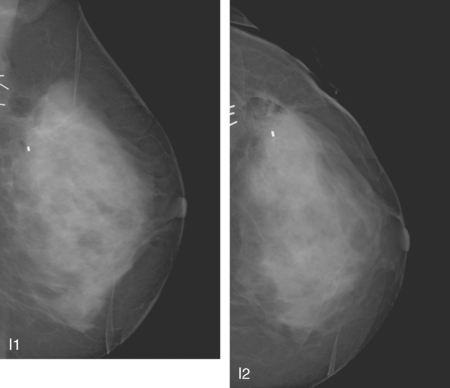
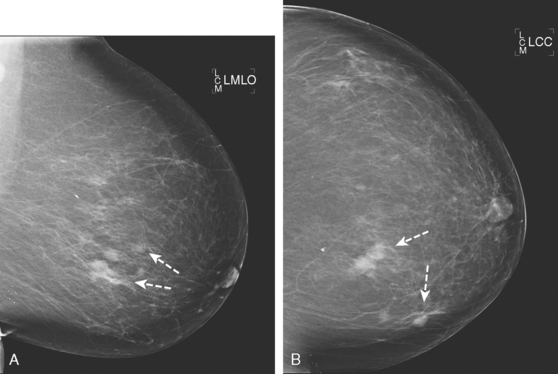
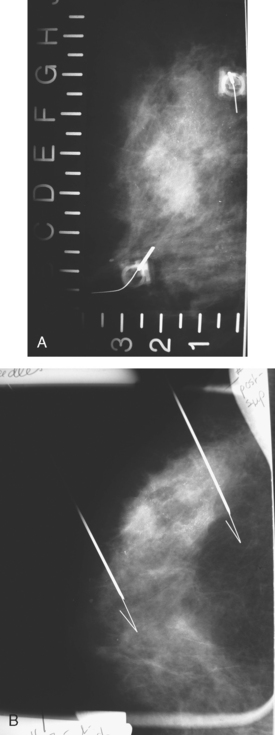
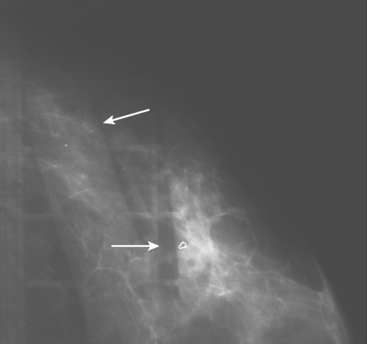
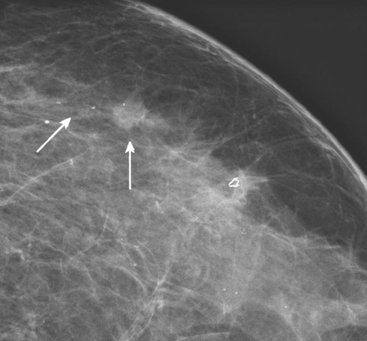
A 62-year-old woman was referred to an orthopedic surgeon for evaluation of right shoulder pain. The surgeon noted asymmetry of the left breast and nipple inversion and referred the patient for a mammogram. Mammography demonstrated extensive microcalcifications, with an associated mass, in the left upper outer quadrant (UOQ) (Figure 1 and Figure 2). Palpation-guided biopsy by a breast surgeon of UOQ firmness did not confirm a malignancy, and the patient had an excisional surgical biopsy after needle localization (Figure 3 and Figure 4). The pathology showed an estrogen receptor–positive, HER-2/neu-positive, 1.5-cm infiltrating ductal carcinoma (IDC), associated with solid and comedo ductal carcinoma in situ (DCIS), which extended to the margin of resection. An intramammary lymph node removed in the specimen was involved with tumor. Mastectomy was performed subsequently, showing extensive residual comedo DCIS in the specimen and negative margins. Three of 15 lymph nodes proved to be positive. Tumor stage was IIA, T1N1. The patient was further treated with chemotherapy and 5 years of tamoxifen therapy. Subsequent aromatase inhibitor therapy with letrozole was discontinued because of side effects.
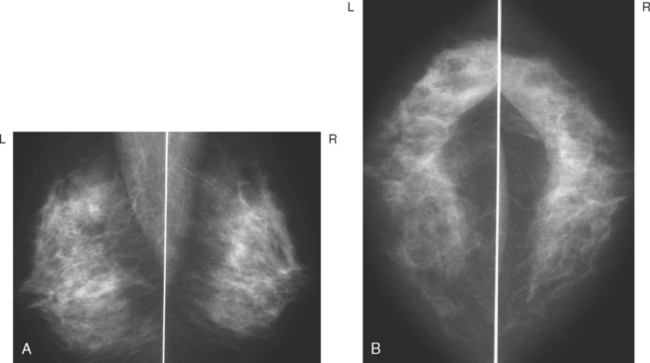
FIGURE 1 Mammograms [MLO (A) and CC (B)] show dense breast tissue, with extensive microcalcifications and a suggestion of an underlying mass in the left upper outer quadrant (UOQ), better seen in detail (Figure 2.
TEACHING POINTS
What a difference a few years makes! This patient was treated in 1998. Palpable firmness in the same quadrant as the highly suspicious mammographic findings led appropriately to palpation-guided biopsy, but the suspected malignancy was not confirmed. Today, this scenario would be cause for referral for an image-guided biopsy. Ultrasound would be an important component of the workup, to identify any associated mass. When no mass is seen on mammography within a large area of suspicious microcalcifications, there is utility in evaluating the area with ultrasound to determine whether a mass can be identified to target for biopsy. If there is a mass seen on ultrasound, sampling it with ultrasound guidance may increase the likelihood of obtaining a diagnosis of invasive cancer than sampling microcalcifications stereotactically.
CASE 20 Medial breast cancer with internal mammary drainage on lymphoscintigraphy*
A new, suspicious mass was identified in the lower inner quadrant of the right breast in an 83-yearold woman on screening mammogram (Figure 1 and Figure 2). Biopsy was performed under ultrasound guidance, confirming mucinous carcinoma. A clip placed at the time of biopsy was subsequently used to guide needle localization for excision. Lymphoscintigraphy was performed at the time, through peritumoral and subdermal injections. Imaging was carried out to 4 hours and showed evidence of drainage to a right lower internal mammary lymph node (Figure 3).
TEACHING POINTS
There is little consensus in the literature on the optimal technique for breast cancer sentinel lymph node identification, other than that utilization of both radiopharmaceutical and intraoperative blue dye injection identifies more sentinel lymph nodes than protocols using either approach solely. A variety of radiopharmaceutical injection techniques, including peritumoral, subareolar, and in-tradermal injections, solely or in combination, have been advocated. The good news is that it seems that all these methods can work. Povoski and colleagues report a single-institution, prospective trial of 400 patients randomized to undergo either intradermal, intraparenchymal, or subareolar injection of 99mTc-sulfur colloid administration for sentinel lymph node mapping and biopsy in breast cancer. In this series, intradermal injection demonstrated significantly greater frequency of localization, decreased time to first localization on preoperative lymphoscintigraphy, and decreased time to harvest the first sentinel node.
Performance of lymphoscintigraphy is not universal. In this example, peritumoral and subdermal injections were performed, and imaging was carried out to 4 hours. At Scripps Clinic, our practice is to perform a single intradermal injection of 500 μCi of filtered 99mTc-sulfur colloid in the smallest volume possible, administered at the areolar edge of the breast cancer–involved quadrant.
CASE 21 Biopsy quality control: Mammographic lesion, wrong ultrasound correlate biopsied; rationale for post–ultrasound biopsy clip placement and mammogram
An asymptomatic 71-year-old woman underwent yearly screening mammography, which showed a small mass in the posterior lateral right breast (Figure 1). Ultrasound showed a small irregular mass in the 9-o’clock position, thought to correspond to the mammographic finding (Figure 2). An ultrasound-guided 14-gauge core needle biopsy was performed. Because of the lesion’s small size, a marker clip was placed. A postbiopsy mammogram showed that the marker clip location did not correspond to the lesion seen on mammography (Figure 3). Subsequently, additional ultrasound imaging demonstrated a second lesion at 8 o’clock (Figure 4). A second core biopsy and clip placement was performed. On the postbiopsy mammogram, this clip conformed to the site of the original mammographic lesion (Figure 5). Pathology reported atypical ductal hyperplasia for the 9-o’clock lesion and invasive ductal carcinoma for the 8-o’clock lesion. The patient went on to have a preoperative MRI (Figure 6 and Figure 7). This showed a small contralateral left breast lesion. A correlate was found on second-look ultrasound (Figure 8). On core biopsy, invasive ductal carcinoma was confirmed. The patient decided to undergo bilateral mastectomies.
CASE 22 Biopsy quality control: DCIS presenting as disappearing microcalcifications and subsequent development of a mass
An 82-year-old woman presented with clustered suspicious microcalcifications in the lateral left breast on routine screening mammography. After magnification, stereotactic biopsy was recommended (Figure 1 and Figure 2). The biopsy was technically difficult because the patient had trouble tolerating the biopsy position. Multiple sets of core samples were obtained; however, no microcalcifications were identified in the samples (Figure 3 and Figure 4). Pathology results indicated benign breast tissue with no calcifications. Given the patient’s age, 6-month follow-up was recommended over surgical excisional biopsy. At follow-up, the calcifications were seen to decrease in number, and there was interval development of an associated irregular solid mass (Figure 5). Subsequent ultrasound-guided core needle biopsy revealed an intermediate-grade invasive ductal carcinoma and DCIS (Figure 6). The patient was treated surgically with sentinel node biopsy and lumpectomy.