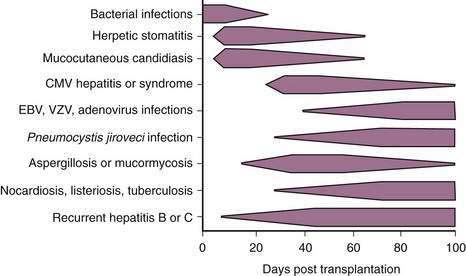
CHAPTER 95 Liver Transplantation
Despite continued advances in the treatment of chronic liver disease, most notably antiviral therapy, and management of complications of chronic liver disease, such as transjugular intrahepatic portosystemic shunt (TIPS) placement, liver transplantation remains the only prospect for long-term survival in patients with advanced liver disease who have reached the limits of purely medical interventions. The major indications for liver transplantation in adults are decompensated cirrhosis, unresectable primary hepatic malignancies, and acute liver failure in which spontaneous recovery is not anticipated.1 Liver transplantation has continued to evolve in response to the shortage of deceased-donor organs and the frequency of recurrent disease. The availability of a wider array of immunosuppressive agents has made graft rejection a lesser threat than disease recurrence.2 Recurrence of hepatitis C virus (HCV) infection, because of its frequency as an indication for liver transplantation, high rate of graft reinfection and failure, and lack of effective prophylaxis, has become a major challenge in the care of liver transplant recipients.3 By contrast, modern regimens of prophylaxis to prevent hepatitis B virus (HBV) reinfection now allow liver transplantation to be undertaken with a low likelihood of recurrence.4 Recurrence of nonviral liver disease is now recognized as a threat to the graft, albeit of an order of magnitude less frequent than that for HCV reinfection.5 Because immunosuppression has a proviral effect, a number of liver transplantation centers have attempted earlier reduction or withdrawal of glucocorticoids in recipients with chronic HBV or HCV infection.6 By contrast, more intensive immunosuppression may be necessary in liver transplant recipients with autoimmune liver diseases.7
The apparently intractable shortage of deceased donors is reflected in continuing deaths of potential recipients listed for liver transplantation. A major change in the allocation of available organs has occurred with the introduction of the Model for End-stage Liver Disease (MELD) score, which has achieved its stated aim of reducing the number of deaths on the liver transplantation waiting list.8 This method of organ allocation assigns organs to recipients on the basis of an objective, continuous measure of severity of liver disease, thereby removing time spent on the waiting list as a determining factor. Extension of live-donor liver transplantation (LDLT) to adult recipients has increased the organ pool despite the tempering of enthusiasm following an increased appreciation of potential risks to the donor.9 Other innovations such as splitting deceased-donor grafts to benefit two recipients and use of so-called marginal or extended criteria grafts, including those from older and non–heart-beating donors, also have expanded the organ supply modestly.10 An increased frequency of complications, most notably of the biliary tract, has been a consequence of expanding the donor criteria, however.11 Efforts to expand the deceased-donor supply by public education programs have succeeded in enhancing organ donation, although the major discrepancy between the number of potential recipients and the number of available organs persists, resulting in continuing attrition of listed patients who succumb to complications of decompensated liver disease while awaiting liver transplantation.12
Although the organ allocation system will undoubtedly continue to evolve and recurrence of disease will remain a threat, the prospects for long-term survival are very good to excellent for most liver transplant recipients who otherwise would succumb to their liver disease. For instance, the likely one-year survival rate for patients with decompensated cirrhosis is less than 10% without liver transplantation but approximately 85% to 90% at one year and 75% at five years after transplantation for most indications.13
Access to liver transplantation has transformed the management of advanced liver disease but has resulted in an expanding cohort of decompensated potential recipients who require frequent medical attention.14 Because the best outcomes following liver transplantation are obtained in patients who have not already experienced multiple complications of liver disease,15 referral for transplantation evaluation is appropriate when a cirrhotic patient has had an index complication, such as the onset of ascites. For at least some potential recipients, access to LDLT may avoid a lengthy waiting period with the risk of further and potentially life-threatening complications of liver disease.
In parallel with the evolution of liver transplantation, the care of transplantation candidates with advanced disease has become a major clinical challenge. The transplantation hepatologist must combine the skills necessary to practice gastroenterology, multidisciplinary internal medicine, and intensive care. This skill set has been formally recognized by the development of a secondary subspecialty in transplantation hepatology by the American Board of Internal Medicine.16
INDICATIONS
The major indications for liver transplantation in adults reflect the most frequent causes of adult liver disease, notably chronic hepatitis C, alcoholic liver disease, and, to a lesser extent, chronic hepatitis B, primary biliary cirrhosis (PBC), primary sclerosing cholangitis (PSC), autoimmune hepatitis, and hemochromatosis (Fig. 95-1). Many liver transplantation candidates previously diagnosed as having “cryptogenic” cirrhosis are now considered to have underlying nonalcoholic fatty liver disease (NAFLD). An uncommon but important indication for liver transplantation is acute liver failure, which has a high mortality rate in the absence of liver transplantation. The role of liver transplantation in primary hepatic malignancy has become better defined; a subset of patients with primary hepatocellular carcinoma (HCC) have a high likelihood of cure with transplantation, although the roles of adjunctive therapies such as transarterial chemoembolization (TACE), radiofrequency ablation, and the oral chemotherapy agent sorafenib need to be defined (see Chapter 94).17 The other major primary adult hepatic malignancy, cholangiocarcinoma, had been regarded as a contraindication to liver transplantation because of its rapid and almost invariable recurrence post–liver transplantation, although acceptable outcomes have been reported in a subset of patients with hilar tumors who receive adjuvant external beam radiation and chemosensitization (see Chapter 69).18 The major indication for pediatric liver transplantation is biliary atresia following a failed Kasai procedure (portoenterostomy) or delayed recognition of the diagnosis (see Chapter 62). Other major pediatric indications include α1-antitrypsin deficiency and other metabolic disorders (see Chapter 76).
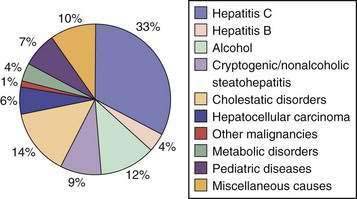
Figure 95-1. Proportion of liver transplants performed for specific indications, 1992 to 2007.
(From O’Leary JG, Lepe R, Davis GL. Indications for liver transplantation. Gastroenterology 2008; 134:1764-76, with permission.)
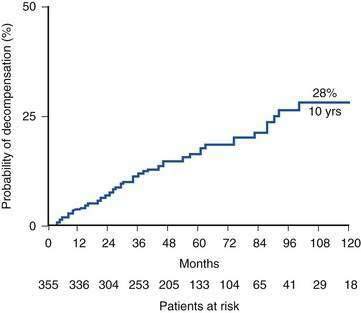
Recognition of cirrhosis per se is not an indication for liver transplantation, although a key issue in managing a cirrhotic patient is deciding whether transplantation will be needed in the future and when referral for evaluation is appropriate (Table 95-1). Another important aspect of the management of a compensated cirrhotic patient is the anticipation of complications. Endoscopic surveillance of patients with cirrhosis for gastroesophageal varices, for instance, to offer prophylaxis, either pharmacologic or endoscopic, to prevent initial or recurrent gastrointestinal bleeding is now routine practice (see Chapter 90).14 Surveillance for HCC is also regarded as the standard of care in a cirrhotic patient (see Chapter 94). Discovery of a hepatic mass suggestive of HCC in a cirrhotic patient should prompt evaluation to determine whether hepatic resection or liver transplantation is the most appropriate curative approach. Otherwise, liver transplantation should normally be a consideration only when the limits of medical therapy for complications of cirrhosis have been reached, particularly if LDLT is an option. The risk of surgery must always be weighed against a realistic assessment of the potential recipient’s prognosis in the absence of liver transplantation. For example, in a patient with decompensated cirrhosis caused by HBV infection, effective suppression of viral replication by an effective antiviral agent may result in impressive clinical improvement, thereby delaying or even obviating the need for liver transplantation (see Chapter 78). Similarly, abstinence from alcohol can result in resolution of signs of hepatic decompensation in a patient with alcoholic liver disease (see Chapter 84). The course of chronic liver disease remains unpredictable, however, and observing an apparently well-compensated patient deteriorate dramatically because of an intercurrent complication such as variceal bleeding is sobering. Although recognition of cirrhosis implies a risk of major complications and diminished life expectancy, a cirrhotic patient can remain stable for a protracted period of time. For example, Fattovich and colleagues19 observed that in patients with well-compensated cirrhosis caused by HCV infection, major complications of portal hypertension such as ascites and variceal hemorrhage occurred in less than 30% at 10 years; in the absence of an index complication, survival was excellent (Fig. 95-2). Once a complication supervenes, however, survival diminishes rapidly. For example, after the development of ascites refractory to diuretics, only 25% of patients survive beyond 1 year.20 A prospective study of more than 200 Italian patients with HCV-related compensated cirrhosis followed for up to 17 years found that HCC was the most common complication of cirrhosis detected, occurring in 32%, followed by ascites. These findings imply that HCC, rather than complications of portal hypertension, is the most frequent reason for a worsening prognosis in a patient with cirrhosis.21
Table 95-1 Indications for Liver Transplantation
The development of predictive models based on the natural history of PBC (see Chapter 89) and PSC (see Chapter 68)22 has helped clinical decision making for patients with these cholestatic disorders, which tend to progress in a fairly stereotypical fashion. Before the introduction of the MELD score, analogous models had not been available for the noncholestatic forms of cirrhosis, and the decision to refer a patient for liver transplantation generally was based on an estimate of disease severity using objective parameters such as the serum albumin level as well as more subjective factors such as the presence of hepatic encephalopathy, as in the Child-Turcotte-Pugh score (see Chapter 90).
On clinical rather than biochemical grounds, important indications for liver transplantation remain disease severity reflective of hepatocellular failure, reflected by coagulopathy and jaundice; complications of portal hypertension, such as refractory ascites and recurrent variceal bleeding; or the combination of portosystemic shunting and diminished hepatocellular function, as in hepatic encephalopathy (see Table 95-1). Following validation of predictive models for the natural history of PBC and PSC, prediction of an individual patient’s course has been possible on the basis of simple clinical and laboratory parameters, the most ominous of which is a rising serum bilirubin level. Although deterioration in a patient’s quality of life typically may not be reflected adequately in predictive models, including MELD, the presence of potentially disabling symptoms such as pruritus and osteopenia in patients with cholestatic and other forms of cirrhosis, as well as recurrent bacterial cholangitis in those with PSC, are important considerations in deciding when to refer a patient for liver transplantation. MELD exceptions, such as adding points to the so-called biological MELD score in order to increase the likelihood of liver transplantation, require approval by a local regional review board, which includes representatives from local transplantation programs. The awarding of extra MELD points recognizes that although this system is a major advance in organ allocation, at least some patients may be disadvantaged by its use of purely objective parameters and exclusion of factors such as intractable ascites or encephalopathy that were incorporated into older allocation schemes. Ideally, liver transplantation should occur before a protracted period of disability reduces the likelihood that post-transplantation rehabilitation will lead to full employment and normal social functioning.
LISTING CRITERIA AND POLICIES OF THE UNITED NETWORK FOR ORGAN SHARING
Organ allocation within the United States is administered by the United Network for Organ Sharing (UNOS), which now considers only disease severity (and not waiting time, as in the past) to determine a patient’s priority for liver transplantation. A variety of organ allocation systems have been used since the 1980s to allocate the limited number of deceased-donor organs in an equitable manner. Older systems were based on a combination of clinical and biochemical parameters such as the Child-Turcotte-Pugh score. The MELD score is a mathematical formula (available at www.unos.org) that incorporates the serum bilirubin level, creatinine level, and international normalized ratio (INR) and provides a more objective and accurate way to stratify liver transplantation candidates for organ allocation and to eliminate time waiting for a donor organ as a determining factor.23 The MELD score overcomes some of the inherent limitations of the Child-Turcotte-Pugh score, including limited discriminatory ability, subjective interpretation of parameters such as presence or absence of ascites on the basis of the physical examination, and the “ceiling effect” of the Child-Turcotte-Pugh score (e.g., no greater weight is given to a serum bilirubin level of 35 mg/dL than to a level of 3.5 mg/dL, even though a patient with the markedly higher bilirubin level clearly has more advanced liver disease). Inclusion of the serum creatinine level reflects the major prognostic importance of renal dysfunction in patients with advanced liver disease. Adoption of the MELD score has been a major step in achieving an equitable organ allocation system in the United States, although it will undoubtedly continue to undergo refinement.
ABSOLUTE AND RELATIVE CONTRAINDICATIONS
Contraindications to liver transplantation have also evolved, reflecting innovations in surgical technique and postoperative care. As an example of the latter, effective prophylaxis against HBV recurrence now allows excellent graft and patient survival rates in patients with chronic hepatitis B.24 On the other hand, greater experience has highlighted the futility of retransplantation in many debilitated recipients with a failing graft caused by recurrent HCV infection.25 By contrast, the introduction of highly effective antiretroviral therapy has allowed consideration of liver transplantation in human immunodeficiency virus (HIV)–infected patients with decompensated liver disease, typically caused by either HCV or HBV infection.26 Still, absolute and relative contraindications remain. An absolute contraindication (Table 95-2) to liver transplantation is a clinical circumstance in which the likelihood of a successful outcome is so remote that liver transplantation should not be offered. A relative contraindication implies a suboptimal likelihood of a good outcome, although liver transplantation may still be considered in some patients.
Table 95-2 Absolute Contraindications to Liver Transplantation
* ICP, intracranial pressure; CPP, cerebral perfusion pressure; CPP, equals the mean arterial pressure minus ICP.
The role of liver transplantation in the management of HCC has become better defined with the recognition that a large tumor burden is associated with a high probability of metastatic spread within a short time of liver transplantation.27 Some tumor characteristics predictive of a poor outcome, most notably vascular invasion, may only be apparent once the explant is examined, despite the sophistication of current imaging techniques. Although results of liver transplantation for cholangiocarcinoma have been poor because of a high rate of tumor recurrence, a subset of patients with hilar tumors may benefit from multimodal therapy and liver transplantation. The results of liver transplantation remain poor for angiosarcoma, and its recognition pre–liver transplantation remains an absolute contraindication. By contrast, at least some patients with epithelioid hemangioendothelioma have been transplanted successfully, despite an extensive tumor burden, with documented regression of extrahepatic metastases.
For a transplantation candidate with a prior extrahepatic malignancy, therapy needs to have been curative, with the resection specimen indicating a low likelihood of metastatic spread. A two-year recurrence-free interval is adequate for most nonhepatic malignancies prior to liver transplantation, but for breast cancer, colon cancer, and malignant melanoma, a longer period following resection is desirable.28 Myeloproliferative disorders frequently underlie Budd-Chiari syndrome (see Chapter 83), but fortunately their evolution to acute leukemia is not accelerated following liver transplantation.29
Ongoing alcohol or recreational drug use remains an absolute contraindication to liver transplantation. If continued abuse is a concern, random toxicology screens are appropriate. Although medicinal marijuana may be used legitimately for palliation, most transplantation programs discourage the use of marijuana because of concerns about the overall compliance of users and possible pulmonary side effects, as well as some evidence that its use may adversely affect the course of liver disease (a point that may be moot in a patient already listed for liver transplantation). A history of prescription narcotic abuse is also a cause for concern because it may contribute to difficulties with pain management post–liver transplantation. Non-narcotic alternatives should be attempted for the management of chronic pain. Other analgesics such as nonsteroidal anti-inflammatory drugs (NSAIDs) also are contraindicated in persons with end-stage liver disease because of potential renal and gastrointestinal complications. Cigarette smoking is prohibited in liver transplantation candidates as well because of its multiple adverse effects, including an associated risk of hepatic artery thrombosis and malignancy post–liver transplantation.30 With the increasing use of herbal compounds and other so-called health products, a discussion of their unproven efficacy and unknown toxicities and caution against their use in the post–liver transplantation setting because of the potential for drug interactions are appropriate (see Chapter 87).31
The careful medical evaluation necessary prior to liver transplantation frequently uncovers important comorbidities, typically cardiac and pulmonary. Although patients with decompensated cirrhosis were previously thought to be somewhat protected against coronary artery disease (CAD) because of low afterload, reflecting peripheral vasodilatation, decreased hepatic synthesis of cholesterol, and increased circulating estrogen levels, cirrhotic patients have a prevalence of CAD at least as great as that of an age-matched control population.32 Risk factors for CAD in cirrhotic patients include a high prevalence of diabetes mellitus. Additional risk factors in the post–liver transplantation period include immunosuppressive drugs that contribute to systemic hypertension, hyperlipidemia, and obesity. Assessment of cardiac risk in cirrhotic patients may be inadequate because of poor physical stamina during routine stress testing. Administration of dobutamine mimics the physiologic effects of exercise and is used with stress echocardiography to exclude clinically significant CAD in liver transplant recipients with cirrhosis. Patients who reach 85% of the maximal predicted heart rate without an abnormality on stress echocardiogram have a low likelihood of peri- and postoperative ischemic cardiac events.33 Discrete coronary artery stenoses can be managed by angioplasty and stenting pre–liver transplantation. Although surgical bypass grafting may be contraindicated because of a significant perioperative risk of excessive bleeding in a patient with decompensated cirrhosis, such surgery, if successful, may render a patient an acceptable candidate for liver transplantation. Pre–liver transplantation cardiac evaluation may overestimate cardiac performance. impaired cardiac function may become apparent only after the protective effect of the decreased systemic vascular resistance that characterizes cirrhosis is lost after liver transplantation, when afterload increases because of the hypertensive effects of the primary immunosuppressive agents and when overvigorous volume repletion may occur.34 Specific forms of cirrhosis may be associated with extrahepatic manifestations that diminish long-term survival. For example, lethal cardiac arrythmias may result in poorer survival in patients who undergo liver transplantation for decompensated cirrhosis caused by hemochromatosis.35
Pulmonary evaluation in the liver transplantation candidate may reveal abnormal arterial oxygenation (see Chapter 92). Although severe chronic obstructive pulmonary disease or pulmonary fibrosis precludes liver transplantation, respiratory restriction as a result of ascites or diminished respiratory muscle strength caused by chronic illness is reversible and does not preclude liver transplantation. Even patients who undergo liver transplantation for α1-antitrypsin deficiency may show improvement in pulmonary function tests postoperatively.36 The hepatopulmonary syndrome (HPS) is characterized by the triad of chronic liver disease, pulmonary vascular dilatations (with right-to-left shunting), and hypoxemia.37 The diagnosis is suggested by the finding of a Pao2 value of less than 70 mm Hg on an arterial blood gas determination obtained from the patient in the supine position. Definitive diagnosis is made by the demonstration of intrapulmonary vascular dilatations by contrast-enhanced echocardiography (which is the most sensitive technique), perfusion lung scanning with 99mTc–labeled macroaggregated albumin, or pulmonary arteriography. Detection of contrast in the left side of the heart within several beats after its appearance in the right atrium indicates intrapulmonary shunting. Predictors of potential reversibility of HPS after liver transplantation include younger age, a lesser degree of preoperative hypoxemia, and adequate correction of hypoxemia with inspiration of 100% oxygen (Pao2 >200 mm Hg).38 In the majority of patients with HPS, hypoxemia resolves within several months of liver transplantation, although a protracted period of ventilatory support in the immediate postoperative period may be required. Because of the potential for improvement with liver transplantation, extra MELD points may be allocated to a patient with HPS. HPS must be distinguished from portopulmonary hypertension, because the latter is associated with high perioperative mortality and frequently unchanged pulmonary hemodynamics despite liver transplantation. Specifically, documentation of a mean pulmonary arterial pressure greater than 35 mm Hg, pulmonary vascular resistance greater than 300 dynes • s • cm−5, and cardiac output less than 8 L/minute are indicative of a high perioperative risk because the patient will be unable to increase the cardiac output appropriately in response to altered intra- and postoperative hemodynamics. Vasodilator therapy may reduce pulmonary arterial pressure and permit liver transplantation.39
Hepatic hydrothorax is a frequent manifestation of portal hypertension characterized by collection of a transudate in the pleural cavity, usually on the right side and often with relatively little ascites remaining in the abdominal cavity (see Chapter 91). Hepatic hydrothorax can be particularly difficult to manage and often requires repeated thoracentesis or placement of a TIPS pre–liver transplantation.40 The temptation to insert a permanent chest tube needs to be resisted because a chest tube can lead to infection in the pleural cavity with the risk of fistula formation. Similarly, interventions such as pleurodesis or pleural decortication should be avoided because hepatic hydrothorax can be controlled only by a reduction in portal pressure.
Active uncontrolled extrahepatic infection is an absolute contraindication to liver transplantation, which should be deferred if sepsis is suspected. In a patient with decompensated cirrhosis, an unexplained clinical deterioration such as altered mental status or systemic hypotension in the absence of gastrointestinal hemorrhage must be presumed to reflect sepsis and is an indication to start broad antibiotic coverage while culture results are awaited. Liver transplantation, however, may be the only option for a patient with recurrent bacterial cholangitis in the setting of PSC (see Chapter 68). Repeated bouts of spontaneous bacterial peritonitis need to be controlled by antibiotic therapy prior to attempting liver transplantation (see Chapter 91). A particularly ominous finding is fungemia, which is typically impossible to eradicate in a debilitated cirrhotic patient and precludes liver transplantation.
An important technical consideration in the liver transplantation candidate is the presence of vascular abnormalities that may increase the difficulty of surgery. With increased surgical experience, however, such abnormalities, most notably portal vein thrombosis, are less likely to be obstacles to liver transplantation. More extensive vascular thrombosis with involvement of the superior mesenteric vein may require extensive vascular reconstruction.41 The presence of a prior portosystemic shunt, particularly a nonselective (side-to-side or end-to-side) portocaval shunt, increases the technical complexity of liver transplantation but is no longer regarded as a contraindication. With the widespread use of TIPS to control manifestations of portal hypertension, including variceal hemorrhage, intractable ascites, and hydrothorax, without disrupting the vascular anatomy, TIPS is now the most frequent shunt encountered in liver transplant recipients and does not usually present an additional operative challenge during liver transplantation.
Age restrictions have been relaxed in liver transplantation candidates, although close attention must be paid to comorbid conditions in older patients that not only increase the risk of perioperative mortality, but also may decrease the likelihood that the liver transplant recipient will be able to return to an active lifestyle, particularly because severe liver disease may cause more debility in older than in younger patients.42 Because a subset of robust older recipients have good outcomes after liver transplantation, candidates in their late 60s or even older who are otherwise in good health should not be precluded a priori from liver transplantation.
The differential diagnosis of renal insufficiency in patients with advanced liver disease includes hepatorenal syndrome, which is potentially reversible. Renal failure remains an important predictor of a poor outcome post–liver transplantation (see Chapter 92).43 Typically, renal dysfunction in patients with decompensated cirrhosis reflects a variety of insults, including sepsis, hypotension, and use of nephrotoxic medications. In liver transplant recipients with decompensated cirrhosis, renal insufficiency severe enough to require dialysis has been associated consistently with poorer patient outcomes. Assessment of the potential for improvement following liver transplantation is critical. A rule of thumb is that return of adequate renal function is unlikely after liver transplantation if dialysis has been required for more than one month prior to liver transplantation. Inclusion of the serum creatinine level in the MELD score reflects the major prognostic importance of renal insufficiency in patients with advanced liver disease. A consequence of this recognition has been an increased rate of combined liver-kidney transplantation in the MELD era of liver transplantation, with a consequent depletion in the supply of kidneys for patients awaiting isolated deceased-donor renal transplantation.44 An important reflection of impaired free-water handling in patients with decompensated cirrhosis is dilutional hyponatremia. Consequences of hyponatremia include altered mental status with more profound degrees of hyponatremia and an increased risk of calcineurin-induced neurotoxicity after liver transplantation (see later). Incorporation of the serum sodium level into the MELD formula (MELDNa) may increase the prognostic accuracy of the MELD score, particularly in patients with relatively low MELD scores.45
A major systemic manifestation of decompensated cirrhosis is malnutrition. Loss of muscle mass increases the likelihood of perioperative morbidity, with a need for more protracted ventilator support and poorer patient survival. Peripheral edema and ascites make changes in body weight or anthropometric measurements unreliable for assessing nutritional status in a patient with advanced cirrhosis. More profound nutritional deficiencies may reflect the specific cause of cirrhosis, as in a malnourished alcoholic person with multiple vitamin and electrolyte deficiencies or a patient with cholestatic liver disease and depletion of fat-soluble vitamins as a result of small intestinal malabsorption. Evaluation by a dietitian is an integral part of the assessment of the liver transplantation candidate. Attempts to improve the nutritional status of liver transplantation candidates have included enteral and parenteral feeding, which may result in improvement, albeit modest, in some patients.46 An increasingly obese liver transplantation candidate pool is raising concerns about the role of obesity in the pathogenesis of NAFLD and in postoperative mortality resulting from cardiovascular events, as well as postoperative complications such as wound infections.47
TRANSPLANTATION EVALUATION AND LISTING
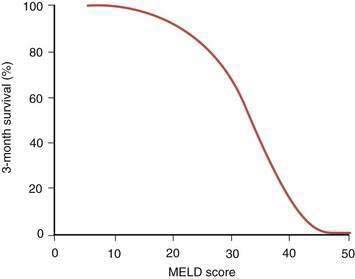
The details of the formal liver transplantation evaluation vary from center to center, but the essentials are to establish that liver transplantation is indicated in the management of the potential recipient’s liver disease, the patient has no comorbidities severe enough to preclude transplantation, and the patient has adequate emotional and social resources to undergo a major surgical procedure and continue on long-term immunosuppression afterward (Table 95-3). Generally in the United States, clearance is needed from the patient’s insurance carrier before the extensive testing necessary for liver transplantation evaluation is undertaken. The patient is seen by a transplantation surgeon, hepatologist, psychiatrist, dietitian, and social worker, with additional consultations as clinically indicated. As increasingly frailer and older candidates are evaluated, identifying potential causes of perioperative morbidity, such as carotid artery stenosis, is imperative. Detailed abdominal imaging is performed not only to screen for HCC, but also to uncover vascular abnormalities such as portal vein thrombosis that may make surgery technically challenging. Disease-specific issues need to be addressed, such as the likelihood of recidivism in the alcoholic patient or management of a large tumor burden in the patient with HCC. The appropriateness of liver transplantation for each candidate is then discussed formally at a meeting of the patient selection committee with input from the members of the transplantation team. If the patient’s candidacy is deemed to be appropriate, formal listing is undertaken with UNOS by matching of the recipient by blood type and weight with potential deceased donors. Once listed, the patient’s priority for organ allocation is determined by the MELD score, based either on the pure score consisting of the serum bilirubin level, serum creatinine level, and INR or that score plus additional points awarded for specific indications, such as HCC. Waiting time on the list is no longer a determining factor. With the critical and seemingly intractable shortage of deceased-donor organs, a major challenge for UNOS and organ retrieval agencies elsewhere in the world has been to develop an equitable system of allocation in an effort to ensure that hepatic allografts are not used for patients whose prognosis without liver transplantation remains good. Patients with a MELD score of less than 15 appear have a better survival without transplantation than with transplantation. As shown in Figure 95-3, the MELD score has been shown to correlate with the three-month survival rate. Patients with a MELD score of less than 10 are ineligible for active listing with UNOS unless they are eligible to receive extra points because of an additional complication of liver disease, such as HCC or HPS.
| Financial screening | Secure approval for evaluation |
| Medical evaluation | As discussed in text |
| Hepatology evaluation | Confirm diagnosis and optimize management |
| Laboratory testing | Assess hepatic synthetic function, serum electrolytes, renal function, viral serologies, markers of other causes of liver disease, tumor markers, ABO-Rh blood typing; 24-hour urine for creatinine clearance; urinalysis and urine drug screen |
| Cardiac evaluation | Electrocardiography and two-dimensional echocardiography, stress testing and cardiology consult if risk factors are present and/or patient is age 40 years or older |
| Hepatic imaging | Ultrasonography with Doppler to document portal vein patency, triple-phase computed tomography or gadolinium magnetic resonance imaging for tumor screening |
| General health assessment | Chest film, prostate specific antigen level (men), Pap smear and mammogram (women), colonoscopy if patient is age 50 years or older or has primary sclerosing cholangitis |
| Transplantation surgery evaluation | Assess technical issues and discuss risks of procedure |
| Anesthesia evaluation | Required if unusually high operative risk, e.g., patient has portopulmonary hypertension, hypertrophic obstructive cardiomyopathy, previous anesthesia complications |
| Psychiatry or psychology consultation | If history of substance abuse, psychiatric illness, or adjustment difficulties |
| Social work evaluation | Address potential psychosocial issues and possible effect of transplantation on patient’s personal and social system |
| Financial and insurance counseling | Itemize costs of transplantation and post-transplantation care; help develop financial management plans |
| Nutritional evaluation | Assess nutritional status and patient education |
From O’Leary JG, Lepe R, Davis GL. Indications for liver transplantation. Gastroenterology 2008; 134:1764-76, with permission.
DISEASE-SPECIFIC INDICATIONS
ALCOHOLIC LIVER DISEASE
Despite the high frequency of chronic HCV infection as an indication for liver transplantation (see later), alcoholic liver disease (ALD) remains the most frequent cause of decompensated chronic liver disease (see Chapter 84). Decompensated alcoholic cirrhosis is now firmly established as a legitimate indication for liver transplantation despite some lingering controversy.48 Concerns in the past included the risk of recidivism following liver transplantation as well as potentially poor compliance by transplant recipients with a history of alcoholism. In addition, the large number of patients with ALD was thought to have the potential to outstrip the donor supply. These fears have not been confirmed, and even patients with evidence of acute alcoholic hepatitis in the explant do not appear to have inferior post–liver transplantation survival rates despite some earlier reports to the contrary. Excellent graft and patient survival rates are the norm following liver transplantation for ALD.
Key factors in determining an alcoholic patient’s suitability for liver transplantation include recognition by the patient of the key role alcohol has played in the genesis of the liver disease, participation in some form of alcohol rehabilitation, such as attendance at Alcoholics Anonymous, stable social support, and a defined period of abstinence from alcohol before transplantation. Conventionally this period of abstinence has been six months, although rigorous studies have failed to confirm that this duration of abstinence per se confers a high likelihood of continued sobriety but have emphasized the importance of factors such as a lack of either isolation or depression. Despite these strategies, however, a substantial proportion of alcoholic recipients resume drinking after liver transplantation, although surprisingly, graft loss or early death attributable to alcohol abuse has been uncommon. A higher rate of return to alcohol use is elicited by use of anonymous questionnaires or toxicology screening than by direct questioning of patients. With longer-term follow-up, as many as 40% of alcoholic recipients resume alcohol use.49 A particularly difficult dilemma arises in the alcoholic patient with severely decompensated liver disease and alcohol use until the time of hepatic decompensation in whom the likelihood of surviving without prompt liver transplantation is low. Clearly enunciated criteria, including a contractual commitment by the patient to sobriety and active involvement in some form of alcohol rehabilitation such as participation in Alcoholics Anonymous, ensure that the selection process is equitable under these circumstances. Patients who return to pathologic drinking after liver transplantation appear to have a higher rate of medical problems, including pneumonia, cellulitis, and pancreatitis, that require hospital admission and occasionally lead to graft loss and death. In addition, alcoholic liver transplant recipients are prone to develop de novo oropharyngeal and lung tumors, likely reflecting other aspects of an alcoholic lifestyle, most notably cigarette smoking.48
HEPATITIS B
Effective prevention of graft reinfection in the HBV-infected candidate has been a major triumph of liver transplantation. HBV recurrence was frequent and resulted in reduced patient and graft survival rates during the 1980s; as a result, Medicare refused to fund liver transplantation in HBV-infected persons. Key factors in improving outcomes included recognition of the key role of pre–liver transplantation active viral replication, as demonstrated by detection in serum of hepatitis B e antigen or HBV deoxyribonucleic acid (DNA) by molecular hybridization techniques as a predictor of recurrent HBV infection in the graft and the protective effect of long-term use of high-dose hepatitis B immunoglobulin (HBIG). In a seminal study50 Samuel and colleagues observed that patients with a fulminant presentation of acute HBV infection or hepatitis D virus coinfection had a reduced risk of recurrent hepatitis B post–liver transplantation in the absence of immunoprophylaxis, reflecting the lower level of HBV replication in patients with fulminant hepatitis B than in those with chronic hepatitis B. Long-term administration of high-dose HBIG, initially administered intravenously, resulted in markedly reduced rates of recurrence of hepatitis B post-transplantation; HBIG administered in combination initially with the nucleoside analog lamivudine further decreased the rate of HBV recurrence. Lamivudine had been used as monotherapy to prevent recurrent HBV infection post-transplantation but was limited by frequent mutations in HBV polymerase gene, leading in turn to graft reinfection.51 The optimal dosing regimen for HBIG has been difficult to establish in the absence of controlled clinical data. Some groups have titrated HBIG doses according to trough serum levels of antibody to hepatitis B surface antigen (anti-HBs). Subsequently, intramuscular administration of HBIG has been confirmed as an efficacious, and less expensive, alternative to intravenous regimens, when used in combination with lamivudine.52 HBV infection that recurs despite administration of HBIG may reflect inadequate doses of HBIG or a genomic mutation in the “a” moiety of HBsAg that results in less avid binding of the virus to HBIG.53 Lamivudine resistance, acquired before liver transplantation, has also been implicated in HBV recurrence despite apparently adequate immunoprophylaxis after liver transplantation. The availability of additional oral antiviral agents with efficacy against HBV has expanded options for preventing graft reinfection (see Chapter 78). A large, multicenter study, for example, has demonstrated that adefovir dipivoxil taken for 48 weeks results in significant virologic, biochemical, and clinical improvement in patients with chronic HBV infection both pre– and post–liver transplantation and may obviate the need of liver transplantation because of improved hepatocellular function.54 Despite the complexities of managing HBV infection in the liver transplantation patient, excellent graft and patient survival rates are now routine, in contrast to the gloomy picture for HBV-infected liver transplantation candidates prior to the use of HBIG. The ever-expanding list of oral antiviral agents promises to improve outcomes further, even in patients in whom antiviral resistance develops. With the licensing of several effective oral agents to treat HBV, HBIG use will likely be superseded by the use of combinations of oral antiviral agents. The liver transplantation candidate with chronic HBV infection is now readily accepted for liver transplantation, albeit with the requirement for long-term antiviral immunoprophylaxis.
HEPATITIS C
A particular challenge is to identify liver transplant recipients with recurrent HCV infection at increased risk of rapidly progressive graft injury. Less than 10% of patients with mild recurrent hepatitis C at one year post–liver transplantation appear to progress to allograft cirrhosis by five years. By contrast, two thirds of patients with at least moderately severe hepatitis C at one year post-transplantation progress to cirrhosis by 5 years.55 Concern has been raised, however, that with prolonged follow-up, some patients with initially mild recurrent hepatitis C after liver transplantation may experience a more aggressive course. A study by Berenguer and colleagues56 evaluated serial protocol liver biopsy specimens to assess the histologic outcome of 57 HCV genotype 1b-infected liver transplant recipients with an initially mild recurrence, defined as no or minimal fibrosis (fibrosis stage F0 or F1) during the first three years post-transplantation (see Chapter 79).56 Progression to bridging fibrosis or cirrhosis (stage F3 or F4) occurred in 35% (n = 20) of such patients, with 12 recipients progressing to stage F3 and 8 recipients progressing to stage F4. Variables associated with progressive fibrosis on univariate analysis were the baseline fibrosis stage and activity grade (P < 0.0001), recipient female gender (P = 0.04), the serum alanine aminotransferase (ALT) level at one year post-transplantation (P = 0.02), and the aspartate aminotransferase (AST) and ALT levels at baseline (P = 0.008 and P = 0.005, respectively). By multivariate analysis, the only variable that was significant was fibrosis stage at baseline (relative risk, 11; 95% CI, 3 to 41; P = 0.0007). Therefore, delayed HCV-related severe liver damage is frequent, even in transplant recipients with an initially mild recurrence, and is seen in approximately one third of patients. Some degree of fibrosis at baseline appears to predict accelerated recurrent HCV infection. A particularly ominous finding is prominent biochemical and histologic cholestasis that frequently is a precursor to rapid allograft failure. Antiviral therapy for patients with a cholestatic HCV recurrence may need to be continued indefinitely because discontinuation leads to a rapid relapse of the cholestatic syndrome and death.3
Reliable predictors of severe recurrent HCV infection have been difficult to identify, although a number of viral and nonviral factors have been implicated (Table 95-4). Infection with viral genotype 1b had been suggested to be a key predictor, but this observation has not been universal. Higher serum levels of HCV RNA before liver transplantation and immediately after liver transplantation and possibly more rapid evolution of HCV quasispecies have been described in patients with more aggressive recurrent HCV56 (see also Chapter 79). Older deceased-donor age has been implicated repeatedly. Episodes of acute cellular rejection, particularly if multiple, lead to a greater likelihood of severe recurrent hepatitis C. A major challenge is to distinguish recurrent HCV infection from graft rejection, particularly because many of the histologic hallmarks of acute rejection, including bile duct injury, are also consistent with recurrent HCV infection. Examination of serial liver biopsy specimens may help clarify this issue and allow avoidance of inappropriate additional immunosuppression in the recipient with recurrent HCV infection rather than graft rejection.
Table 95-4 Factors Associated with Severe Hepatitis C Virus Recurrence Following Liver Transplantation
| Viral Factors |
HCV, hepatitis C virus; RNA, ribonucleic acid.
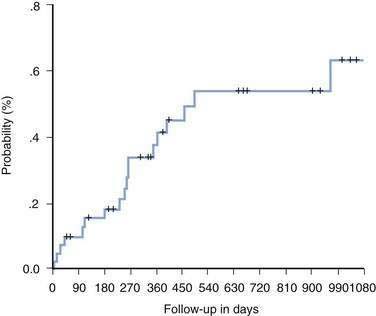
Once recurrent HCV infection in the graft progresses to cirrhosis, overt hepatic decompensation is frequent. In contrast to recurrent HBV infection, effective prophylaxis against recurrent HCV infection has not been possible (Fig. 95-4).3 Current treatment strategies generally fall into three categories: (1) pretransplantation antiviral therapy; (2) preemptive therapy started in the early post–liver transplantation period before the development of clinically apparent acute hepatitis C; and (3) post-transplantation therapy at the time of diagnosis of acute hepatitis C or for established or severe chronic hepatitis C.3 The approach followed by most transplantation centers is to initiate antiviral therapy when clinically significant evidence of recurrent HCV infection is identified (as defined by either grade 3 or 4 (of 4) hepatic inflammation or a fibrosis stage of F2 or more). Accurate assessment of the efficacy of treating recurrent HCV infection is difficult, however, because most reported studies have been uncontrolled, single-centered, and small in size.
Interferon alpha monotherapy generally has been ineffective in treating established recurrent hepatitis C (see Chapter 79). When interferon is combined with ribavirin, the rate of virologic response is increased, but at the cost of frequent and potentially severe side effects.3 Leukopenia is a particularly vexing problem in patients undergoing such treatment, and adjunctive administration of granulocyte colony–stimulating factor (G-CSF) may permit continuation of interferon therapy. The long-acting pegylated interferons have been evaluated in a number of clinical trials. Generally, prophylactic interferon-based regimens administered shortly post–liver transplantation in an effort to prevent graft reinfection have resulted in low rates of sustained virologic response.57
On the basis of reports of rejection and graft loss in renal transplant recipients treated with interferon, as well as preliminary experience in liver transplant recipients, concern has been raised that therapy with interferon increases the risk of graft rejection (although as noted earlier the distinction between recurrent HCV infection and graft rejection is difficult even on histologic grounds).58 Increasingly, recurrent HCV infection is recognized as the cause of graft failure in transplant recipients, and the dilemma arises as to whether repeat liver transplantation in affected patients is justified.25 A subset of patients retransplanted for graft loss caused by recurrent hepatitis C have reasonable survival rates, if they do not have deep jaundice or renal failure at the time of retransplantation (see later).
ACUTE LIVER FAILURE
Acute liver failure (ALF) is an uncommon but important indication for liver transplantation because of its high mortality rate but excellent outcomes with prompt liver transplantation (unless major neurologic complications have occurred). ALF is defined as the onset of hepatic encephalopathy within 26 weeks of the initial recognition of acute liver disease and reflects a variety of causes (see Chapter 93). Despite an abrupt onset, antecedent chronic liver disease is absent, and hepatic recovery is possible. In the past, liver transplantation for ALF resulted in poorer patient survival rates than those for benchmark indications, such as PBC. Subsequent experience, however, has shown that excellent patient survival rates are possible if ALF is identified early in its course and the patient is listed for liver transplantation before irreversible complications, especially neurologic, have supervened.59 The absence of papilledema on funduscopy and of typical features of cerebral edema on computed tomography (CT) of the head do not preclude the presence of cerebral edema complicating worsening encephalopathy; therefore, direct intracranial pressure monitoring may be required to detect and manage this frequently lethal complication of ALF. Direct intracranial pressure monitoring can only be recommended, however, if local neurosurgical expertise and interest are available, because a high rate of complications has tempered enthusiasm for use of this technique in many centers. Specific criteria to identify patients with ALF who are unlikely to recover spontaneously without liver transplantation are shown in Table 95-5. The challenge in managing patients with ALF is to avoid unnecessary liver transplantation in patients who will recover spontaneously, while not delaying liver transplantation in patients in whom the only option for survival is liver transplantation. The role of liver-assist devices in managing ALF, either as definitive therapy or as a “bridge to transplantation,” remains an area of active investigation (see Chapter 93).
Table 95-5 Criteria for Liver Transplantation in Acute Liver Failure
| Criteria of King’s College, London |
INR, international normalized ratio.
From Keeffe EB. Liver transplantation: Current status and novel approaches to liver replacement. Gastroenterology 2001; 120:749-62, with permission.
CHOLESTATIC LIVER DISEASE
PBC and PSC continue to be relatively common indications for liver transplantation in many transplantation centers and have had a key role in the development of disease models for liver disease, with PBC serving as a benchmark for patient and graft survival. Development of the Mayo disease models to predict the course of cholestatic disorders has aided in decision making regarding timely referral for liver transplantation (see Chapters 68 and 89). Patients with PBC and PSC should be referred for liver transplantation evaluation if their Mayo risk scores predict a one-year survival rate of less than 95%. The Mayo model to predict survival in patients with PBC incorporates the serum bilirubin level, albumin level, patient’s age, prothrombin time, and presence of edema, whereas the model to predict survival in patients with PSC includes the serum bilirubin level, patient’s age, serum AST level, variceal bleeding, and serum albumin level. These models, however, do not take into account prominent and frequently disabling complications of cholestatic liver disease, such as pruritus, osteopenia, or, in PSC, recurrent bouts of bacterial cholangitis and have now been effectively superseded by the MELD score, which is used to determine organ allocation. Despite the generally excellent results of liver transplantation for the cholestatic disorders, concern has increased that they may recur in the graft.
Biliary stricturing similar to that in the native diseased liver can be identified in a minority of patients following liver transplantation for PSC and may represent recurrent disease.5 Differentiation of recurrent disease from other important causes of graft injury such as chronic rejection or ischemia may be difficult. Recurrent PSC results in nonanastomotic stricturing of the intrahepatic biliary tree. Although some improvement in symptoms can be obtained by balloon dilation and stent placement, long-term graft viability is reduced. Graft loss caused by recurrent PBC appears to be less frequent than that for PSC. Management of recurrent PBC includes excluding other causes of hepatic dysfunction. Primary immunosuppression with tacrolimus has been implicated in recurrence of PBC by some, but not all, investigators. A controversial issue is whether colectomy in transplant recipients with PSC and associated inflammatory bowel disease reduces the risk of recurrent PSC.60
HEPATIC MALIGNANCY
HCC is the most common primary hepatic malignancy and results in more than 600,000 deaths annually worldwide, usually in patients with underlying cirrhosis. A notable exception is chronic HBV infection, in which HCC can arise in the absence of cirrhosis (see Chapter 94). Tumors with diameters of less than 2 cm discovered incidentally in the explanted liver typically do not have an adverse effect on patient survival. The likelihood of tumor recurrence increases markedly, however, with greater tumor burden, vascular invasion, and the presence of multiple lesions. Liver transplantation is the most definitive treatment of HCC; indeed, 26% of patients who received a liver allograft between 2002 and 2007 had HCC, an observation that reflects the frequency of HCC in cirrhotic patients and the awarding of extra priority to patients with this indication for liver transplantation.61
Improvements in outcome of liver transplantation for HCC in the 2000s are almost entirely attributable to better patient selection rather than improved surgery or adjuvant therapy.62 The preoperative metastatic workup should include a bone scan and chest CT in addition to abdominal imaging. Portal vein occlusion in a patient with HCC is regarded as evidence of metastatic spread and precludes liver transplantation. On the basis of the seminal experience reported by Mazzaferro and colleagues from Milan, generally accepted criteria for liver transplantation in patients with HCC have included a tumor diameter of less than 5 cm, if the tumor is solitary, or no more than three lesions, with the diameter of the largest lesion measuring no greater than 3 cm.63 Survival rates comparable to those for transplantation for decompensated cirrhosis in the absence of complicating HCC (75% at four years) have been reported. With the growing success of liver transplantation for HCC, the Milan criteria have been criticized as being excessively restrictive by excluding many patients who otherwise would have done well with a low risk of recurrence after liver transplantation.62 Various expanded criteria have been proposed to extend the limits of tumor size and number while preserving patient survival rates.64,65
After initial adoption of the MELD score for organ allocation, increased priority was given to patients with HCC who met the Milan criteria, in recognition of the potential for cure of HCC by liver transplantation and the concern that a protracted wait for liver transplantation could result in an increase in tumor burden and metastatic spread. Adoption of the MELD score has resulted in proportionally more patients with HCC undergoing liver transplantation.61 In addition, waiting times for patients with HCC to receive a deceased donor organ have decreased significantly, and the number of patients dropping out from the waiting list because of advanced-stage disease has also decreased. In the most recent modification of allocation policies, fewer additional MELD score points have been added for the diagnosis of HCC because of the relatively good short-term prognosis of patients with a small HCC.61 Concern has been expressed that cirrhotic recipients without HCC are now disadvantaged because of the preference given to patients with HCC.
Several adjuvant interventions have been reported in patients transplanted for HCC (see Chapter 94).17 Recurrent tumor occurs frequently in the graft, and the rationale for adjuvant therapy has been to eliminate micrometastatic disease that typically is disseminated via the vascular system. Conventional systemic chemotherapy administered perioperatively as well as for varying durations before and after liver transplantation, and usually incorporating doxorubicin, is of uncertain benefit. A potentially important strategy for expanding the criteria for liver transplantation in patients with HCC is to downstage the tumor with the use of locoregional therapy so that the Milan criteria are met; whether this approach ultimately improves patient survival remains to be determined.65 For example, TACE administered directly into the tumor arterial supply is often employed to reduce tumor burden during the often protracted wait for liver transplantation. This intervention can be hazardous in patients with decompensated cirrhosis, and its benefit in patients with favorable tumor characteristics remains to be determined. Radiofrequency ablation is used increasingly to manage HCC. Confounding the management of the liver transplantation candidate with HCC, however, is the frequent observation that the tumor burden in the explant is significantly underestimated by preoperative imaging studies. Despite these caveats, a subset of patients with HCC can be cured by liver transplantation and would not have tolerated surgical resection of the tumor because of associated cirrhosis. Oral therapy of HCC with sorafenib will undoubtedly be expanded into liver transplantation populations.
The fibrolamellar variant of HCC presents in younger adults without underlying cirrhosis and, as a result, often manifests only when the tumor burden is already large. Extensive resection can be tolerated because cirrhosis is absent. Liver transplantation may be performed in patients who have recurrent tumor after resection. Tumor recurrence after liver transplantation may be relatively indolent and, although not as infrequent as was once thought, survival rates are acceptable.66 Hepatoblastoma is a rare pediatric tumor that also occurs in the absence of underlying parenchymal liver disease. Initial management consists of surgical resection; adjuvant chemotherapy is indicated for metastatic disease. Liver transplantation is an option when the tumor cannot be resected (see Chapter 94).
Cholangiocarcinoma remains the only major primary hepatic tumor for which a definitive role for liver transplantation has been difficult to establish. The results of liver transplantation for cholangiocarcinoma diagnosed preoperatively have been so poor that its presence has been regarded as a contraindication to liver transplantation, and even tumors discovered only incidentally in the explant have a high recurrence rate. A subset of patients with a hilar location of the tumor and absence of nodal involvement have been reported to have good five-year survival rates. The extent of cholangiocarcinoma frequently is more extensive than suspected on pre–liver transplantation imaging; often there is local, lymphatic, and perineural spread. The addition of en bloc pancreaticoduodenectomy has not resulted in improved survival after liver transplantation. Newer approaches to treatment have included preoperative irradiation and chemotherapy, and careful intraoperative tumor staging followed by liver transplantation, with encouraging preliminary results (see Chapter 69).
METABOLIC DISORDERS
Patients with congenital hepatic enzyme deficiencies and other inborn errors of metabolism may be cured by liver transplantation67 (see Chapters 74 to 76). Metabolic disorders potentially cured by liver transplantation fall into two broad categories: diseases dominated clinically by obvious hepatocellular disease (e.g., Wilson disease, hemochromatosis) and those without any clinical evidence of liver disease (e.g., primary hyperoxaluria, familial hypercholesterolemia). Although metabolic disorders are most prominent as indications for liver transplantation in the pediatric population, important adult diseases managed by liver transplantation include Wilson disease and hemochromatosis. Substantial neurologic improvement can occur following liver transplantation for Wilson disease in patients who present with decompensated cirrhosis with neurologic involvement. A Wilsonian crisis with severe hemolysis is an indication for urgent liver transplantation; chelation therapy is ineffective in such cases. Hemochromatosis has been associated with poorer outcomes following liver transplantation than have other forms of cirrhosis because of increased rates of adverse cardiac and infectious outcomes. Ongoing studies will clarify whether iron depletion before liver transplantation improves survival rates after liver transplantation. Iron reaccumulation in the grafts of patients transplanted with hemochromatosis is a theoretical concern, and continued iron depletion is not typically required.68
Liver transplantation also has been performed in patients with a variety of systemic disorders, including adult polycystic disease, and as a curative procedure in combination with renal transplantation for primary hyperoxaluria, in which end-organ damage is confined to the kidney but the metabolic defect is hepatic. Liver transplantation has been successful in arresting manifestations of familial amyloid polyneuropathy, with the explant, which is the source of the abnormal protein, available for use in a “domino” fashion in an older recipient who will not live long enough for neurologic injury to develop.69 The biliary cirrhosis associated with cystic fibrosis also has been managed successfully with liver transplantation, although patients remain at risk of infectious and other complications of this systemic disorder.
NONALCOHOLIC FATTY LIVER DISEASE
NAFLD is now recognized as a major cause of chronic liver disease, including cirrhosis and HCC, and is implicated in many cases of cirrhosis (formerly termed cryptogenic) (see Chapter 85). Many of the key precipitants of NAFLD—obesity, hyperlipidemia, and diabetes mellitus—are exacerbated by the post-transplantation immunosuppressive regimen.70 Recurrence of NAFLD post–liver transplantation causes graft injury, although graft loss does not typically result. De novo NAFLD after liver transplantation has also been described. In the absence of any specific therapy for NAFLD, therapeutic efforts after liver transplantation should center on weight control, optimal diabetic management, and use of a lipid-lowering regimen.
VASCULAR DISORDERS
Budd-Chiari syndrome is characterized by hepatic venous outflow obstruction with a presentation that often mimics decompensated cirrhosis (see Chapter 83).71 Important associations are myeloproliferative disorders, hypercoagulable states, and vena caval webs. Medical approaches to management often are disappointing and fail to retard progression to liver failure and death. Examination of liver biopsy specimens may be helpful in determining whether the therapeutic approach should be decompression with a portosystemic shunt or liver transplantation. Good long-term results have been described in patients who undergo prompt TIPS or portosystemic shunt surgery, but patients with advanced fibrosis on a liver biopsy specimen should undergo liver transplantation. Although many patients have an underlying myeloproliferative disorder, an accelerated progression to leukemia or bone marrow failure is not typically observed after liver transplantation. Long-term anticoagulation is continued in liver transplant recipients transplanted for Budd-Chiari syndrome.
Sinusoidal obstruction syndrome (SOS) is a similar disorder manifested by necrosis of zone 3 hepatocytes and fibrous obliteration of the central venule lumen. Most commonly seen after bone marrow transplantation (BMT), SOS may lead to hepatic failure and death in up to 25% of patients despite an otherwise successful BMT. Although experience with liver transplantation for hepatic complications of BMT is limited,72 it appears to be the only intervention that consistently alters the course of advanced SOS. Similarly, liver transplantation has been shown to be effective in the management of severe post-BMT graft-versus-host disease with predominantly hepatic involvement (see also Chapter 34). Hypocoagulable (e.g., hemophilia A and B) as well as hypercoagulable (e.g., protein C and S deficiencies) hematologic disorders have been cured with liver transplantation.
AUTOIMMUNE HEPATITIS
Failure of immunosuppressive therapy to arrest progression of severe autoimmune hepatitis with the development of hepatic decompensation is an indication for liver transplantation (see Chapter 88).73 The presence of human leukocyte antigen (HLA)-DR3 is associated with a lower likelihood of a therapeutic response to immunosuppressive therapy in patients with autoimmune hepatitis. Excellent long-term survival is usual after liver transplantation, although the autoimmune diathesis may result in higher rates of acute cellular rejection. In addition, recurrent autoimmune hepatitis has been recognized increasingly and may require higher maintenance doses of immunosuppression. Graft survival is generally not diminished by recurrent autoimmune hepatitis.5 Recurrent disease mimics the features of disease in the native liver, is associated with hypergammaglobulinemia and autoantibodies, and is generally responsive to glucocorticoids.
OTHER INDICATIONS
A variety of other diagnoses have been reported as indications for liver transplantation (see Table 95-1). Adult polycystic disease with marked abdominal distention resulting from multiple hepatic cysts that are not amenable to resection has been treated successfully by liver transplantation. If chronic kidney disease is present, a combined liver-renal transplantation is indicated. Cerebral imaging is indicated to exclude intracranial aneurysms, which are a feature of this syndrome (see Chapter 94). Liver transplantation also is indicated in cases of multiple adenomas associated with glycogen storage disease and not only eliminates the risk of progression to HCC but also corrects the underlying metabolic disease (see Chapter 76). Diseases with multiorgan involvement for which liver transplantation has been performed include Alagille syndrome, amyloidosis, and sarcoidosis (see Chapters 35 and 62).
SURGICAL ASPECTS OF LIVER TRANSPLANTATION
Once a potential deceased organ donor is identified, the local organ procurement organization coordinates harvesting and supplies pertinent donor medical information to centers with suitable potential recipients listed with UNOS. In contrast with other types of organ transplants, including kidney and bone marrow transplants, absence of HLA compatibility does not appear to affect liver graft survival, and donor-recipient matching is based only on ABO blood compatibility and physical characteristics such as the recipient’s weight. In critically ill recipients, an ABO-incompatible organ may be implanted, with the recognition that graft survival may be diminished.74 In addition to screening serologic studies and routine liver biochemical testing, particular attention is paid to the donor’s medical history, including cardiovascular instability and need for pressor support before determination of brain death.
With the critical shortage of deceased organ donors, expansion of the donor pool has included acceptance of donors ages 70 years and older. As noted earlier, however, use of older donors in recipients with HCV infection may lead to more severe HCV recurrence, potentially limiting the use of older donors in at least some recipients. The typical donor has had a catastrophic head injury or an intracerebral bleed with brain death but without multisystem organ failure. Electrolyte imbalance and hepatic steatosis in the donor are particular concerns because they are predictors of subsequent graft nonfunction. Analogous to the MELD score, a “donor risk index” has been derived to assess the likelihood of good graft function.75 Key adverse factors include older donor age (especially >60 years of age), use of a split or partial graft, and a non–heart-beating donor, from which the organs are harvested after the donor’s cardiac output ceases, in contrast with the more typical deceased donation in which the organs are harvested prior to cardiovascular collapse. Use of non–heart-beating donors is associated with reduced rates of long-term graft survival and an increased risk of biliary complications and correlates with the duration of “warm ischemia” after cardiovascular collapse and before retrieval of the organ.
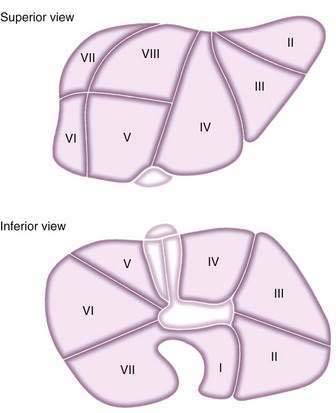
Splitting cadaveric donor livers either in situ during harvesting or ex vivo on return to the transplantation center allows two recipients to receive portions of the same hepatic allograft, if graft volume and quality are sufficient. An adult cadaveric liver is divided into two functioning grafts. The left lateral segment (segments 2 and 3) is used for a pediatric recipient, and the right trisegment (segments 4 to 8) is used for an adult recipient. Acceptable graft and patient survival rates can be obtained with split grafts, although high-risk unstable recipients may have poorer outcomes with this technique. Figure 95-5 shows the segmental anatomy of the liver, which forms the basis of dissection for both split and living-donor liver transplantation.
LIVE-DONOR LIVER TRANSPLANTATION
A major surgical advance has been the extension of LDLT from pediatric recipients to adult recipients, although debate continues about the role of LDLT in adult recipients because of the magnitude of the risk to the donor in light of the large volume of donor liver required.76 The potential donor must be a healthy adult, typically a family member or close friend of the recipient, who volunteers to be evaluated. A series of checks and balances is necessary to ensure that the potential donor undergoes an adequate medical assessment and is not proceeding only as a result of pressure from the patient or family. It is crucial that the potential recipient not be privy to details of the potential donor’s evaluation. In most centers, a hepatologist not involved in the care of the recipient performs an assessment of the donor. Often an independent advocate is also appointed to safeguard the donor’s interests. At each stage of the process, the potential donor is given the opportunity to withdraw from consideration.77 Preoperative evaluation of the donor is best performed in four stages over a period of one to three months; the more invasive testing such as liver biopsy is undertaken later in the evaluation (Table 95-6). After undergoing complete evaluation, only a relatively small proportion of potential donors are deemed satisfactory candidates. One consequence of the evaluation of many potential donors has been the recognition that anatomic abnormalities of the biliary and vascular system and unsuspected abnormalities on liver biopsy specimens are common in apparently healthy persons.
Table 95-6 Protocol for Evaluation of Potential Living-Related Donors
| Stage 1 | Complete history and physical examination |
| Liver biochemical tests, blood chemistries, hematology, coagulation profile, urinalysis, alpha fetoprotein, carcinoembryonic antigen, and serologic tests for hepatitis A, B, and C, cytomegalovirus, Epstein-Barr virus, and human immunodeficiency virus | |
| Abdominal ultrasound examination, chest film | |
| Stage 2 | Complete psychiatric and social evaluation |
| Computed tomography (CT) of the abdomen | |
| Pulmonary function tests, echocardiography | |
| Stage 3 | Liver biopsy |
| Celiac and superior mesenteric angiography with portal phase* | |
| Stage 4 | Magnetic resonance cholangiogram |
| Informed consent |
* Increasingly, CT angiography is done, instead of standard angiography in stage 2.
Adapted from Ghobrial RM, Amersi F, Busuttil RW. Surgical advances in liver transplantation. Living related and split donors. Clin Liver Dis 2000; 4:553-65, with permission.
Right lobes (segments 5 to 8), extended right grafts (segments 4 to 8), or left hepatic grafts (segments 2 to 4) have been used successfully in adult-to-adult LDLT. Adult LDLT provides obvious advantages to the recipient, including reduction in mortality rates for patients awaiting a donor organ.78 An expected reduction in the risk of graft rejection because of receipt of a graft from a relative has been not realized, and concern has been raised that recurrence of HCV infection may be accelerated. The overriding concern about LDLT is the short- and long-term consequences to the donor, including the risk of immediate perioperative morbidity and mortality, and financial losses resulting from time lost from work, possible uninsurability in the future, and a lack of long-term follow-up data to ensure that hepatic resection and subsequent regeneration do not result in biliary or other abnormalities.
IMMUNOSUPPRESSION
Administration of immunosuppressive agents following liver transplantation is divided into induction (initial) and maintenance (long-term) phases. In addition, episodes of acute cellular and chronic ductopenic rejection require therapy. A wide array of immunosuppressive agents are currently used.79 In practice, new immunosuppressive agents are introduced for use in renal transplantation before they are applied to liver transplantation. Therefore, the need for effective antirejection regimens in other areas of solid organ transplantation is great (see also Chapter 34). The primary goal of immunosuppression is to prevent graft rejection and loss; a secondary goal is to avoid the adverse consequences of the antirejection regimen.80
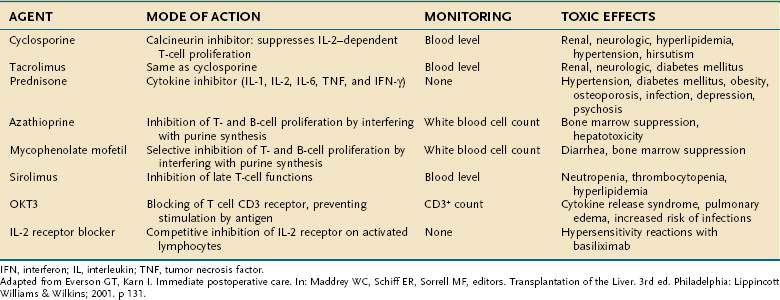
A list of the commonly used immununosuppressive agents, routes of administration, methods of monitoring, and common adverse effects is shown in Table 95-7. Common drug-drug interactions are shown in Table 95-8. The calcineurin inhibitors cyclosporine and tacrolimus are the basis for a majority of induction and maintenance immunosuppressive regimens. Both agents have substantial toxicity. Tacrolimus is now favored over cyclosporine for primary immunosuppression. In addition, patients may be converted from cyclosporine to tacrolimus following glucocorticoid- or OKT3-refractory rejection, late rejection (>6 months post–liver transplantation), histologically diagnosed chronic rejection, severe cholestasis, intestinal malabsorption of cyclosporine, or cyclosporine toxicity (e.g., hirsutism, gingivitis, severe hypertension). When used as rescue therapy for chronic rejection, tacrolimus is less effective once the serum bilirubin levels rise above 10 mg/dL, underscoring the importance of early recognition of chronic rejection. Although implicated in hepatic artery thrombosis as well as delayed wound healing and infections, sirolimus, an inhibitor of the protein mTOR (mammalian target of rapamycin), has been used increasingly in liver transplantation as a calcineurin-sparing strategy and also in patients transplanted for HCC to reduce tumor recurrence.81 Another newer agent is basiliximab, a monoclonal antibody directed against CD25, which may be an alternative to glucocorticoids as an induction agent in liver transplantation.82
Table 95-8 Clinically Relevant Drug Interactions with Immunosuppressive Drugs
Considerable differences exist among transplantation centers in the rate at which the level of induction immunosuppression is reduced to avoid toxicity and lessen the risk of recurrent disease. Generally, the strategy includes tapering and in some cases discontinuing maintenance glucocorticoids.83
POSTOPERATIVE COURSE
INITIAL PHASE TO DISCHARGE FROM HOSPITAL
Worrisome clinical features include scanty, pale bile if a T-tube has been used, metabolic acidosis, depressed mentation, and the continued need for pressor support with worsening liver biochemical test levels. Hepatic artery thrombosis needs to be excluded promptly by Doppler ultrasound and is an indication for urgent retransplantation. Hepatic artery thrombosis is more common in pediatric recipients because of the smaller size of the vessels. Antiplatelet therapy is now administered routinely to reduce the risk of hepatic artery thrombosis.84 Primary nonfunction of the graft is also an indication for urgent retransplantation and is suggested by the absence of bile production in the first several hours after transplantation, as well as an unstable overall clinical status. Donor characteristics that are associated with an increased likelihood of primary nonfunction include marked hepatic steatosis and profound hyponatremia. If graft function is adequate, however, pressor support can be tapered and ventilator weaning parameters monitored to facilitate extubation, although the recipient who is markedly debilitated from advanced cirrhosis may require several days of ventilatory support. Poor graft function and renal insufficiency also can impede weaning from the ventilator.
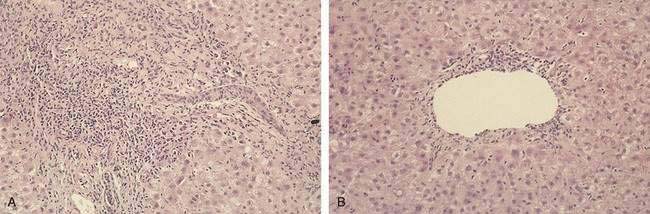
Within the first week after liver transplantation, liver biochemical test levels should steadily improve as ischemia and reperfusion injury resolve. Acute cellular rejection becomes an important and frequent cause of graft dysfunction at one week and beyond and is suggested by a rise in serum aminotransferase, alkaline phosphatase, and bilirubin levels. Because the biochemical features are nonspecific, the threshold for performing a liver biopsy to evaluate other diagnostic possibilities, which include slowly resolving reperfusion injury, biliary tract obstruction, and cholestasis related to sepsis, should be low. Histologic findings characteristic of acute cellular rejection are bile duct injury, portal inflammation with eosinophils, and, with more severe injury, endotheliitis (Fig. 95-6). High doses of glucocorticoids (1000 mg of methylprednisolone or the equivalent), followed by a taper (200 to 20 mg/day) extending over several days, constitute first-line therapy. A response is suggested by a return of liver biochemical test levels toward normal values.
The ability of recurrent HCV infection to mimic virtually all the features of acute cellular rejection histologically has led to a reevaluation of the need to treat apparent acute cellular rejection aggressively under all circumstances. Routine (protocol) liver biopsies also have fallen out of favor because histologic evidence of acute cellular rejection can be noted in the absence of worsening graft function with no apparent clinical significance. A cholangiogram should be obtained routinely prior to clamping the T-tube, if one is present; a nonsustained rise in liver biochemical test levels can be anticipated as a result. The timing of various infectious complications following liver transplantation is shown in Figure 95-7.
A number of other important medical issues are common in the first weeks following liver transplantation (Table 95-9). Neurologic dysfunction can present as an acute confusional state or seizures, and the differential diagnosis includes the lingering effects of hepatic encephalopathy, electrolyte imbalance, poor graft function, sepsis, uremia, and side effects of medications. A particular concern is the development of neurologic toxicity caused by the major immunosuppressive agents. Overly rapid correction of hyponatremia perioperatively has been implicated in the genesis of central pontine myelinolysis, with evidence of demyelination demonstrable on magnetic resonance imaging. Management includes correcting the electrolyte imbalance if present and reducing the dose of calcineurin inhibitor, which can be facilitated by the use of mycophenolate mofetil.85 Diabetes mellitus, which is common in persons with cirrhosis, can occur for the first time in the postoperative period and usually requires insulin to control. HCV infection further increases the risk of post–liver transplantation diabetes mellitus.86 Renal impairment post–liver transplantation may reflect a number of factors, including slowly resolving pre–liver transplantation hepatorenal syndrome or renal failure of other causes, intraoperative hypotension resulting in acute tubular necrosis, and, importantly, the nephrotoxic effects of cyclosporine and tacrolimus, which cause renal afferent arteriolar vasoconstriction and a corresponding reduction in glomerular filtration rate. Adjunctive therapy with mycophenolate mofetil allows a reduction in the doses of cyclosporine and tacrolimus while providing adequate immunosuppression. Short-term hemodialysis may be necessary until renal function improves. In the first three to four weeks post–liver transplantation, infections are typically bacterial and related to surgical complications such as intra-abdominal bleeding, bile leak, or wound infection.
Table 95-9 Medical Complications in the Immediate Post-Transplantation Period
From Everson GT, Karn I. Immediate postoperative care. In: Maddrey WC, Schiff ER, Sorrell MF, editors. Transplantation of the Liver. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2001, with permission.
FOLLOWING DISCHARGE FROM HOSPITAL
If the initial postoperative course has been smooth, planning for discharge is possible by the second week post–liver transplantation. Recovery often is more protracted, particularly in debilitated recipients. Once discharged, patients are seen at frequent intervals during the first postoperative month. The liver biochemical test levels should fall to normal values within a few weeks following liver transplantation. Further graft dysfunction is an indication for prompt liver biopsy because acute cellular rejection remains a concern during this time; in addition, CMV becomes an important consideration three or more weeks post-transplantation.87 Histologic features suggestive of CMV hepatitis include “owl’s eye” inclusion bodies in the hepatocytes as well as neutrophilic abscesses with focal necrosis of the parenchyma (see Chapter 81). Recipients who have had no prior exposure to CMV are at high risk of CMV infection, particularly if they receive a graft from a CMV-seropositive donor, and are candidates for more intensive prophylaxis. A distinction is made between CMV viremia and CMV disease with systemic manifestations such as diarrhea, because the viremia does not invariably imply disease. Early recurrence of HCV infection also may become apparent, and, as noted earlier, it is crucial to recognize that many of the histologic features of acute cellular rejection, such as bile duct inflammation and endotheliitis, are mimicked by HCV infection (Table 95-10).
Table 95-10 Histologic Features of Recurrent Hepatitis C Virus Infection versus Acute Cellular Rejection
| FEATURE | HCV RECURRENCE | REJECTION |
|---|---|---|
| Time of onset after liver transplantation | Any time; onset usually within the first year | Usually within the first two months |
| Portal inflammation | Most cases | Always |
| Lymphocytes | Bland, uniform | Activated |
| Lymphoid aggregates | Usually | Occasionally |
| Lymphoid follicles | 50% of cases | Rarely |
| Eosinophils | Inconspicuous | Almost always |
| Steatosis | Often | Never |
| Acidophilic bodies | Common | Uncommon |
| Bile ductule damage | About 50% of cases | Common |
| Atypical features | Cholestasis, ballooning degeneration without significant inflammation, marked ductular proliferation mimicking obstruction, granulomas | Prominent periportal and lobular necroinflammatory activity without subendothelial venular inflammation |
HCV, hepatitis C virus.
From Rosen HR, Martin P. Liver transplantation. In: Schiff ER, Sorrell MF, Maddrey WC, editors. Schiff’s Diseases of the liver. 8th ed. Philadelphia: Lippincott-Raven; 1999. p. 1589.
If a liver biopsy specimen shows features suggestive of biliary obstruction or if graft dysfunction is associated with clinical features of cholangitis such as fever and abdominal pain, a cholangiogram should be obtained. Increasingly, magnetic resonance cholangiopancreatography (MRCP) is the initial method of choice because of its noninvasive nature and high degree of accuracy irrespective of the type of biliary anastomosis.88 Before the advent of MRCP, a cholangiogram was generally obtained through the T-tube if present, by endoscopic retrograde cholangiopancreatography (ERCP) if a T-tube was not present, or by percutaneous transhepatic cholangiography if a choledochojejunostomy had been performed. An anastomotic stricture in a choledochocholedochostomy is usually easily managed by endoscopic balloon dilation and temporary internal stenting (see Chapter 70). Surgical intervention is reserved for patients who do not respond to this approach, in which case the anatomy is converted to a Roux-en-Y anastomosis. Anastomotic stricturing also can occur at the site of a choledochojejunostomy and requires access, usually by a percutaneous approach, to dilate the stenotic area.
A T-tube, if present, is removed by the sixth postoperative month, and removal is best performed at the transplantation center, because bile leaks are common. If a bile leak occurs, prompt ERCP with nasobiliary drainage or stenting usually allows the tear in the bile duct to heal uneventfully (see Chapter 70).
LONG-TERM MANAGEMENT
GENERAL PREVENTIVE MEDICINE
Satisfactory long-term management of the liver transplant recipient requires cooperation and communication between the primary care physician and the transplantation center. Many of the disorders that impact long-term survival after liver transplantation are common diseases, including systemic hypertension, hyperlipidemia, and diabetes mellitus.89 Regular determination of a complete blood count, electrolytes, liver biochemical test levels, and immunosuppressive drug levels are indicated, and the results should be forwarded to the transplantation center. Generally, the frequency of blood work can be reduced beyond the first postoperative year in recipients with stable graft function.
Systemic hypertension is a frequent complication of liver transplantation and is related to calcineurin inhibitor–induced renal vasoconstriction, as well as to the effects of other drugs such as glucocorticoids. Reduction in the level of immunosuppression unfortunately is generally ineffective in ameliorating hypertension. Other contributing factors include mild renal insufficiency, which is frequent after liver transplantation even when absent preoperatively. Initial antihypertensive therapy usually consists of a calcium channel blocker. Angiotensin-converting enzyme inhibitors and potassium-sparing diuretics are relatively contraindicated because of their propensity to accentuate hyperkalemia, which is frequent in liver transplant recipients who often have renal tubular acidosis caused by the calcineurin inhibitor. Because cyclosporine and tacrolimus levels are increased by verapamil and diltiazem, nifedipine is the agent of choice. β-Adrenergic–blocking agents are second-line antihypertensive agents; diuretics are generally avoided because of concern about exacerbating renal insufficiency and electrolyte imbalance in the liver transplant recipient. Furosemide is the diuretic of choice if fluid overload is present. In the minority of patients in whom hypertension is not controlled, a centrally acting agent such as clonidine is introduced. For the occasional patient with intractable hypertension on cyclosporine-based immunosuppression, substitution of tacrolimus for cyclosporine may aid blood pressure control. Both cyclosporine and tacrolimus, however, are nephrotoxic and accentuate renal impairment that may have existed perioperatively. Although acute nephrotoxicity may respond to interruption of or a reduction in the dose of these drugs, chronic renal impairment is usually irreversible, and drastic dose reductions should be avoided for fear of precipitating graft rejection. Cofactors implicated in the progression to advanced chronic kidney disease after liver transplantation include recurrent HCV infection with associated glomerulonephritis as well as diabetes mellitus and systemic hypertension.90 Renal transplantation may be considered in liver transplant recipients who become dialysis dependent after otherwise successful liver transplantation.
Osteopenia is a frequent cause of morbidity in liver transplant recipients.91 Although hepatic osteodystrophy is typically associated with the cholestatic liver diseases, it is also common in patients with other forms of cirrhosis. Factors implicated in the pathogenesis of hepatic osteodystrophy include poor nutritional status, immobility, the calciuric effect of many diuretics, hypogonadism, and glucocorticoid use in patients with autoimmune hepatitis. In the initial several months after liver transplantation, osteopenia is accelerated further by high-dose glucocorticoid therapy as well as the other major immunosuppressive agents. Atraumatic fractures may occur in trabecular bone such as vertebrae or ribs. Patients begin to rebuild bone mass after the doses of immunosuppressive drugs are reduced and the patient’s mobility increases. Supplemental calcium and vitamin D are frequently prescribed to patients with symptomatic osteopenia, as is a bisphosphonate.
De novo malignancies are increased in frequency following liver transplantation.92 Recipients need ongoing age-appropriate surveillance for common tumors such as breast and colon cancer. Screening for prostatic carcinoma should be performed by yearly digital rectal examination in male liver transplant recipients older than age 40, in conjunction with serum prostate specific antigen (PSA) testing. Screening for colorectal cancer by colonoscopy should also be performed every 3 to 5 years after age 50 in asymptomatic recipients; in patients with a history of PSC and ulcerative colitis, yearly colonoscopy with surveillance mucosal biopsies is recommended.93 In the setting of chronic immunosuppression, screening female transplant recipients older than age 40 for breast cancer by yearly mammography seems appropriate, although the cost effectiveness of this approach is undefined. Other malignancies that are increased in frequency in organ transplant recipients include skin, female genital tract, and perineal cancers; alcoholic patients may be particularly prone to malignancies of the oropharynx (see Chapter 84). Patients should be encouraged to wear sunscreen and have regular examinations by a dermatologist.
Post-transplantation lymphoproliferative disorder (PTLD) varies from a low-grade indolent process to an aggressive neoplasm.94 Uncontrolled proliferation of B cells after liver transplantation, typically in response to primary Epstein-Barr virus infection, can be polyclonal or monoclonal. Pediatric recipients are at particular risk because of the absence of prior Epstein-Barr viral infection. Intensive immunosuppression with OKT3 for severe rejection increases the risk of PTLD, which can present as a mononucleosis-like syndrome, lymphoproliferation, or malignant lymphoma. Clinical features suggestive of PTLD include lymphadenopathy, unexplained fever, and systemic symptoms such as weight loss. After the diagnosis is made histologically by biopsy of involved areas (which can include the liver graft and gastrointestinal tract as well as lymph nodes), therapy includes a reduction in the level of immunosuppression and antiviral therapy with ganciclovir directed against Epstein-Barr virus. Systemic chemotherapy may be required in patients who present with a malignant lymphoma. The higher frequency of PTLD in pediatric recipients has led to surveillance by polymerase chain reaction methodology for Epstein-Barr viremia and reduction in the level of immunosuppression in patients with a positive result before clinical features of PTLD occur. In addition, antiviral prophylaxis is prescribed to high-risk recipients, including those who are seronegative for Epstein-Barr virus and receive a liver from a seropositive donor. Chronic graft rejection is increased in frequency in survivors of PTLD because of the deliberate reduction in the level of immunosuppression, which may be increased cautiously after PTLD is contained.
Hyperlipidemia is observed in up to one half of liver transplant recipients and reflects a number of pathogenic factors, including diabetes mellitus, obesity, renal dysfunction, and the use of immunosuppressive agents, especially cyclosporine.95 Pharmacologic therapy is indicated if hypercholesterolemia fails to respond to weight reduction and tight diabetic control. Pravastatin, a 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, is well tolerated and efficacious in liver transplant recipients.96 Diabetes mellitus is common in liver transplant recipients and occurs in approximately one third of patients for the first time after transplantation. The pathogenesis is multifactorial; immunosuppressive therapy is a major factor because of the hyperglycemic effects of prednisone, cyclosporine, tacrolimus, azathioprine, and mycophenolate mofetil. HCV infection is also implicated. In most diabetic recipients, therapy with insulin is required. The high frequency of diabetes mellitus following liver transplantation has led to the development of glucocorticoid-sparing immunosuppressive regimens.
HEPATIC RETRANSPLANTATION
Although improved immunosuppressive regimens have led to a lower rate of graft loss because of chronic rejection, recurrence of the underlying liver disease has been recognized increasingly as a cause of graft failure, as illustrated most strikingly in HCV-infected recipients.97 The rates and severity of recurrent diseases are highly variable and probably related to a complex interplay among host factors (including the underlying liver disease), therapeutic regimens (e.g., immunosuppression, antiviral treatment), and possibly genetic variability of the allograft (perhaps through effects on the nature and magnitude of the inflammatory response within the graft). Understanding the full effect of recurrent disease, especially nonviral disease, on patient and graft survival will require long-term follow-up studies. For example, although the rate of histologic recurrence of viral hepatitis is greatest in the first year following liver transplantation, recurrent PBC or PSC develops in less than 5% of patients by the first year, whereas more than 20% demonstrate histologic recurrence 10 years after liver transplantation.98 As patients enter their second and third decades following liver transplantation, the number of patients who require retransplantation may deplete the donor pool further. This issue is compounded by the observation that patients who undergo retransplantation experience an approximate 20% overall reduction in the rate of survival but consume an increased amount of resources when compared with primary transplant recipients. On the basis of these considerations, a number of investigators have developed models to predict survival following retransplantation, especially for HCV infection (Table 95-11 and Fig. 95-8). For example, the liver transplant recipient with jaundice, graft failure caused by recurrent HCV infection, and renal failure has a markedly diminished likelihood of surviving retransplantation. Although these models only estimate the probability of survival for an individual patient and do not take into account the patient’s quality of life, they can be used as adjuncts to clinical judgment. Application of retransplantation to low-risk patients is associated with survival comparable to that for primary liver transplantation; determining whether retransplantation is justified in patients with high-risk scores will require prospective studies. In the current era of extreme organ shortage, the utility of primary and repeat transplantation (i.e., duty to promote the best outcome in the aggregate) needs to be considered. An analysis by Burton and colleagues, using the Cobbs-Douglas equation, has demonstrated that for primary liver transplantation, maximal utility is achieved by allocating organs at the highest MELD score (i.e., “sickest first”). For retransplantation, however, maximal utility for HCV and non-HCV diagnoses is achieved at MELD scores of 21 and 24, respectively. Utility starts to decline at MELD scores greater than 28.25
Table 95-11 Multivariate Models Developed to Predict Survival Following Liver Retransplantation
| NO. OF PATIENTS | PROGNOSTIC PREDICTIVE FACTORS | COMMENTS |
|---|---|---|
| 1356 | Recipient’s age, bilirubin and creatinine levels, UNOS status, and cause of graft failure | UNOS database used; HCV positivity and donor age are significant predictors by univariate analysis83 |
| 70 | Recipient’s age, UNOS status, inpatient status, serum bilirubin and creatinine levels | King’s College84 |
| 418 | Recipient’s age, mechanical ventilatory status, serum bilirubin and creatinine levels | University of Pittsburgh85 |
| 150 | Recipient’s age group (pediatric vs. adult), mechanical ventilatory status, organ ischemia time >12 hr, serum bilirubin and creatinine levels | UCLA86 |
| 447 | Interval to retransplantation (better prognosis if within 30 days) | Mayo Clinic; limited to patients with PBC or PSC87 |
| 207 | Bilirubin and serum creatinine levels | UNOS database used; limited to HCV-positive patients retransplanted for causes other than primary nonfunction88 |
HCV, hepatitis C virus; PBC, primary biliary cirrhosis; PSC, primary sclerosing cholangitis; UNOS, United Network for Organ Sharing.
From Rosen HR. Disease recurrence following liver transplantation. Clin Liver Dis 2000; 4:675-89, with permission.
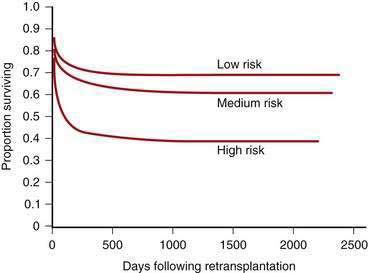
Figure 95-8. Survival of 1356 patients undergoing hepatic retransplantation stratified into low-risk, medium-risk, and high-risk groups using prognostic predictive factors listed in Table 95-11 (P < 0.00001 by Wilcoxon rank sum).
(From Rosen HR, Madden JP, Martin P. A model to predict survival following hepatic retransplantation. Hepatology 1999; 29:365-70.)
A multicenter U.S. study of 272 patients (73 retransplanted for causes not related to HCV) indicated that one- and three-year survival rates following retransplantation were equivalent in HCV-positive and HCV-negative patients. These data were likely shaped by selection bias, considering that nationally more than one third of patients with HCV-related allograft failure are not even considered for retransplantation and only half of those reevaluated are even relisted. This study also showed that MELD scores greater than 30 are associated with particularly poor survival following retransplantation.99
Ahmed A, Keeffe EB. Current indications and contraindications for liver transplantation. Clin Liver Dis. 2007;11:227-47. (Ref 13.)
Arjal RR, Burton JRJr, Villamil F, et al. Review article: The treatment of hepatitis C virus recurrence after liver transplantation. Aliment Pharmacol Ther. 2007;26:127-40. (Ref 3.)
Benten D, Sterneck M, Panse J, et al. Low recurrence of preexisting extrahepatic malignancies after liver transplantation. Liver Transpl. 2008;14:789-98. (Ref 29.)
Cardenas A, Kelleher T, Chopra S. Review article: Hepatic hydrothorax. Aliment Pharmacol Ther. 2004;20:271-9. (Ref 40.)
Fallon MB, Krowka MJ, Brown RS, et al. Impact of hepatopulmonary syndrome on quality of life and survival in liver transplant candidates. Gastroenterology. 2008;135:1168-75. (Ref 37.)
Grewal P, Martin P. Pretransplant management of the cirrhotic patient. Clin Liver Dis. 2007;11:431-49. (Ref 14.)
Ioannou GN, Perkins JD, Carithers RLJr. Liver transplantation for hepatocellular carcinoma: Impact of the MELD allocation system and predictors of survival. Gastroenterology. 2008;134:1342-51. (Ref 61.)
Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359:1018-26. (Ref 45.)
Lopez PM, Villanueva A, Roayaie S, et al. Neoadjuvant therapies for hepatocellular carcinoma before liver transplantation: A critical appraisal. Liver Transpl. 2006;12:1747-54. (Ref 17.)
Lucey MR. Liver transplantation for alcoholic liver disease. Clin Liver Dis. 2007;11:283-9. (Ref 48.)
McAvoy NC, Kochar N, McKillop G, et al. Prevalence of coronary artery calcification in patients undergoing assessment for orthotopic liver transplantation. Liver Transpl. 2008;14:1725-31. (Ref 32.)
O’Leary JG, Lepe R, Davis GL. Indications for liver transplantation. Gastroenterology. 2008;134:1764-76. (Ref 1.)
Oo YH, Neuberger J. Recurrence of nonviral diseases. Clin Liver Dis. 2007;11:377-95. (Ref 5.)
Pham PT, Pham PC, Rastogi A, et al. Review article: Current management of renal dysfunction in the cirrhotic patient. Aliment Pharmacol Ther. 2005;21:949-61. (Ref 43.)
Rosen HR. Transplantation immunology: What the clinician needs to know for immunotherapy. Gastroenterology. 2008;134:1789-801. (Ref 80.)
1. O’Leary JG, Lepe R, Davis GL. Indications for liver transplantation. Gastroenterology. 2008;134:1764-76.
2. Perry I, Neuberger J. Immunosuppression: Towards a logical approach in liver transplantation. Clin Exp Immunol. 2005;139:2-10.
3. Arjal RR, Burton JRJr, Villamil F, et al. Review article: The treatment of hepatitis C virus recurrence after liver transplantation. Aliment Pharmacol Ther. 2007;26:127-40.
4. Angus PW, Patterson SJ, Strasser SI, et al. A randomized study of adefovir dipivoxil in place of HBIG in combination with lamivudine as post-liver transplantation hepatitis B prophylaxis. Hepatology. 2008;48:1460-6.
5. Oo YH, Neuberger J. Recurrence of nonviral diseases. Clin Liver Dis. 2007;11:377-95.
6. Llado L, Fabregat J, Castellote J, et al. Impact of immunosuppression without steroids on rejection and hepatitis C virus evolution after liver transplantation: Results of a prospective randomized study. Liver Transpl. 2008;14:1752-60.
7. Campsen J, Zimmerman MA, Trotter JF, et al. Liver transplantation for autoimmune hepatitis and the success of aggressive corticosteroid withdrawal. Liver Transpl. 2008;14:1281-6.
8. Freeman RBJr, Steffick DE, Guidinger MK, et al. Liver and intestine transplantation in the United States, 1997-2006. Am J Transplant. 2008;8:958-76.
9. Yi NJ, Suh KS, Cho JY, et al. Three-quarters of right liver donors experienced postoperative complications. Liver Transpl. 2007;13:797-806.
10. Tector AJ, Mangus RS, Chestovich P, et al. Use of extended criteria livers decreases wait time for liver transplantation without adversely impacting posttransplant survival. Ann Surg. 2006;244:439-50.
11. Maheshwari A, Maley W, Li Z, et al. Biliary complications and outcomes of liver transplantation from donors after cardiac death. Liver Transpl. 2007;13:1645-53.
12. Sung RS, Galloway J, Tuttle-Newhall JE, et al. Organ donation and utilization in the United States, 1997-2006. Am J Transplant. 2008;8:922-34.
13. Ahmed A, Keeffe EB. Current indications and contraindications for liver transplantation. Clin Liver Dis. 2007;11:227-47.
14. Grewal P, Martin P. Pretransplant management of the cirrhotic patient. Clin Liver Dis. 2007;11:431-49.
15. Lopez PM, Martin P. Update on liver transplantation: Indications, organ allocation, and long-term care. Mt Sinai J Med. 2006;73:1056-66.
16. Rosen HR, Fontana RJ, Brown RS, et al. Curricular guidelines for training in transplant hepatology. Liver Transpl. 2002;8:85-7.
17. Lopez PM, Villanueva A, Roayaie S, et al. Neoadjuvant therapies for hepatocellular carcinoma before liver transplantation: A critical appraisal. Liver Transpl. 2006;12:1747-54.
18. Rea DJ, Heimbach JK, Rosen CB, et al. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg. 2005;242:451-8.
19. Fattovich G, Giustina G, Degos F, et al. Morbidity and mortality in compensated cirrhosis type C: A retrospective follow-up study of 384 patients. Gastroenterology. 1997;112:463-72.
20. Heuman DM, Abou-Assi SG, Habib A, et al. Persistent ascites and low serum sodium identify patients with cirrhosis and low MELD scores who are at high risk for early death. Hepatology. 2004;40:802-10.
21. Sangiovanni A, Prati GM, Fasani P, et al. The natural history of compensated cirrhosis due to hepatitis C virus: A 17-year cohort study of 214 patients. Hepatology. 2006;43:1303-10.
22. Kim WR, Wiesner RH, Poterucha JJ, et al. Adaptation of the Mayo primary biliary cirrhosis natural history model for application in liver transplant candidates. Liver Transpl. 2000;6:489-94.
23. Bambha K, Kim WR, Kremers WK, et al. Predicting survival among patients listed for liver transplantation: An assessment of serial MELD measurements. Am J Transplant. 2004;4:1798-804.
24. Terrault N, Roche B, Samuel D. Management of the hepatitis B virus in the liver transplantation setting: A European and an American perspective. Liver Transpl. 2005;11:716-32.
25. Burton JRJr, Sonnenberg A, Rosen HR. Retransplantation for recurrent hepatitis C in the LD era: Maximizing utility. Liver Transpl. 2004;10:S59-64.
26. Roland ME, Barin B, Carlson L, et al. HIV-infected liver and kidney transplant recipients: 1- and 3-year outcomes. Am J Transplant. 2008;8:355-65.
27. Mazzaferro V, Llovet JM, Miceli R, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: A retrospective, exploratory analysis. Lancet Oncol. 2009;10:35-43.
28. Penn I. Evaluation of the candidate with a previous malignancy. Liver Transpl Surg. 1996;2:109-13.
29. Benten D, Sterneck M, Panse J, et al. Low recurrence of preexisting extrahepatic malignancies after liver transplantation. Liver Transpl. 2008;14:789-98.
30. Leithead JA, Ferguson JW, Hayes PC. Smoking-related morbidity and mortality following liver transplantation. Liver Transpl. 2008;14:1159-64.
31. Neff GW, O’Brien C, Montalbano M, et al. Consumption of dietary supplements in a liver transplant population. Liver Transpl. 2004;10:881-5.
32. McAvoy NC, Kochar N, McKillop G, et al. Prevalence of coronary artery calcification in patients undergoing assessment for orthotopic liver transplantation. Liver Transpl. 2008;14:1725-31.
33. Umphrey LG, Hurst RT, Eleid MF, et al. Preoperative dobutamine stress echocardiographic findings and subsequent short-term adverse cardiac events after orthotopic liver transplantation. Liver Transpl. 2008;14:886-92.
34. Keeffe BG, Valantine H, Keeffe EB. Detection and treatment of coronary artery disease in liver transplant candidates. Liver Transpl. 2001;7:755-61.
35. Kowdley KV, Brandhagen DJ, Gish RG, et al. Survival after liver transplantation in patients with hepatic iron overload: The national hemochromatosis transplant registry. Gastroenterology. 2005;129:494-503.
36. Kemmer N, Kaiser T, Zacharias V, et al. Alpha-1-antitrypsin deficiency: Outcomes after liver transplantation. Transplant Proc. 2008;40:1492-4.
37. Fallon MB, Krowka MJ, Brown RS, et al. Impact of hepatopulmonary syndrome on quality of life and survival in liver transplant candidates. Gastroenterology. 2008;135:1168-75.
38. Hoeper MM, Krowka MJ, Strassburg CP. Portopulmonary hypertension and epatopulmonary syndrome. Lancet. 2004;363:1461-8.
39. Arguedas MR, Abrams GA, Krowka MJ, et al. Prospective evaluation of outcomes and predictors of mortality in patients with hepatopulmonary syndrome undergoing liver transplantation. Hepatology. 2003;37:192-7.
40. Cardenas A, Kelleher T, Chopra S. Review article: Hepatic hydrothorax. Aliment Pharmacol Ther. 2004;20:271-9.
41. Selvaggi G, Weppler D, Nishida S, et al. Ten-year experience in porto-caval hemitransposition for liver transplantation in the presence of portal vein thrombosis. Am J Transplant. 2007;7:454-60.
42. Keswani RN, Ahmed A, Keeffe EB. Older age and liver transplantation: A review. Liver Transpl. 2004;10:957-67.
43. Pham PT, Pham PC, Rastogi A, et al. Review article: Current management of renal dysfunction in the cirrhotic patient. Aliment Pharmacol Ther. 2005;21:949-61.
44. Davis CL, Feng S, Sung R, et al. Simultaneous liver-kidney transplantation: Evaluation to decision making. Am J Transplant. 2007;7:1702-9.
45. Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359:1018-26.
46. Merli M, Nicolini G, Angeloni S, et al. Malnutrition is a risk factor in cirrhotic patients undergoing surgery. Nutrition. 2002;18:978-86.
47. Nair S, Verma S, Thuluvath PJ. Obesity and its effect on survival in patients undergoing orthotopic liver transplantation in the United States. Hepatology. 2002;35:105-9.
48. Lucey MR. Liver transplantation for alcoholic liver disease. Clin Liver Dis. 2007;11:283-9.
49. DiMartini A, Dew MA, Fitzgerald MG, et al. Clusters of alcohol use disorders diagnostic criteria and predictors of alcohol use after liver transplantation for alcoholic liver disease. Psychosomatics. 2008;49:332-40.
50. Samuel D, Muller R, Alexander G, et al. Liver transplantation in European patients with the hepatitis B surface antigen. N Engl J Med. 1993;329:1842-7.
51. Perrillo RP, Wright T, Rakela J, et al. A multicenter United States–Canadian trial to assess lamivudine monotherapy before and after liver transplantation for chronic hepatitis B. Hepatology. 2001;3:424-32.
52. Han SH, Martin P, Edelstein M, et al. Conversion from intravenous to intramuscular hepatitis B immune globulin in combination with lamivudine is safe and cost-effective in patients receiving long-term prophylaxis to prevent hepatitis B recurrence after liver transplantation. Liver Transpl. 2003;9:182-7.
53. Akay S, Karasu Z. Hepatitis B immune globulin and HBV-related liver transplantation. Expert Opin Biol Ther. 2008;8:1815-22.
54. Schiff E, Lai CL, Hadziyannis S, et al. Adefovir dipivoxil for wait-listed and post-liver transplantation patients with lamivudine-resistant hepatitis B: Final long-term results. Liver Transpl. 2007;13:349-60.
55. Berenguer M. Management of hepatitis C virus in the transplant patient. Clin Liver Dis. 2007;11:355-76.
56. Berenguer M, Aguilera V, Prieto M, et al. Delayed onset of severe hepatitis C-related liver damage following liver transplantation: A matter of concern? Liver Transpl. 2003;9:1152-8.
57. Chalasani N, Manzarbeitia C, Ferenci P, et al. Peginterferon alfa-2a for hepatitis C after liver transplantation: Two randomized, controlled trials. Hepatology. 2005;41:289-98.
58. Stravitz RT, Shiffman ML, Sanyal AJ, et al. Effects of interferon treatment on liver histology and allograft rejection in patients with recurrent hepatitis C following liver transplantation. Liver Transpl. 2004;10:850-8.
59. Barshes NR, Lee TC, Balkrishnan R, et al. Risk stratification of adult patients undergoing orthotopic liver transplantation for fulminant hepatic failure. Transplantation. 2006;81:195-201.
60. Vera A, Moledina S, Gunson B, et al. Risk factors for recurrence of primary sclerosing cholangitis of liver allograft. Lancet. 2002;360:1943-4.
61. Ioannou GN, Perkins JD, Carithers RLJr. Liver transplantation for hepatocellular carcinoma: Impact of the MELD allocation system and predictors of survival. Gastroenterology. 2008;134:1342-51.
62. Varela M, Sanchez W, Bruix J, et al. Hepatocellular carcinoma in the setting of liver transplantation. Liver Transpl. 2006;12:1028-36.
63. Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-9.
64. Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: Expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394-403.
65. Yao FY, Kerlan RKJr, Hirose R, et al. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: An intention-to-treat analysis. Hepatology. 2008;48:819-27.
66. Margarit C, Charco R, Hidalgo E, et al. Liver transplantation for malignant diseases: Selection and pattern of recurrence. World J Surg. 2002;26:257-63.
67. Zhang KY, Tung BT, Kowdley KV. Liver transplantation for metabolic liver diseases. Clin Liver Dis. 2007;11:265-81.
68. Crawford DH, Fletcher LM, Hubscher SG, et al. Patient and graft survival after liver transplantation for hereditary hemochromatosis: Implications for pathogenesis. Hepatology. 2004;39:1655-62.
69. Monteiro E, Freire A, Barroso E. Familial amyloid polyneuropathy and liver transplantation. J Hepatol. 2004;41:188-94.
70. Angulo P. Nonalcoholic fatty liver disease and liver transplantation. Liver Transpl. 2006;12:523-34.
71. Valla DC. Primary Budd-Chiari syndrome. J Hepatol. 2009;50:195-203.
72. Membreno FE, Ortiz J, Foster PF, et al. Liver transplantation for sinusoidal obstructive syndrome (veno-occlusive disease): Case report with review of the literature and the UNOS database. Clin Transplant. 2008;22:397-404.
73. Vogel A, Heinrich E, Bahr MJ, et al. Long-term outcome of liver transplantation for autoimmune hepatitis. Clin Transplant. 2004;18:62-9.
74. Hanto DW, Fecteau AH, Alonso MH, et al. ABO-incompatible liver transplantation with no immunological graft losses using total plasma exchange, splenectomy, and quadruple immunosuppression: Evidence for accommodation. Liver Transpl. 2003;9:22-30.
75. Feng S, Goodrich NP, Bragg-Gresham JL, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783-90.
76. Yu YY. Preoperative pathologic assessment for adult living donor liver transplantation. Zhonghua Bing Li Xue Za Zhi. 2007;36:723-5.
77. Trotter JF, Wisniewski KA, Terrault NA, et al. Outcomes of donor evaluation in adult-to-adult living donor liver transplantation. Hepatology. 2007;46:1476-84.
78. Trotter JF, Mackenzie S, Wachs M, et al. Comprehensive cost comparison of adult-adult right hepatic lobe living-donor liver transplantation with cadaveric transplantation. Transplantation. 2003;75:473-6.
79. Geissler EK, Schlitt HJ. Immunosuppression for liver transplantation. Gut. 2008;58:452-63.
80. Rosen HR. Transplantation immunology: What the clinician needs to know for immunotherapy. Gastroenterology. 2008;134:1789-801.
81. Mehrabi A, Fonouni H, Kashfi A, et al. The role and value of sirolimus administration in kidney and liver transplantation. Clin Transplant. 2006;20:30-43.
82. Lupo L, Panzera P, Tandoi F, et al. Basiliximab versus steroids in double therapy immunosuppression in liver transplantation: A prospective randomized clinical trial. Transplantation. 2008;86:925-31.
83. Everson GT, Trotter JF, Kugelmas M, et al. Immunosuppression in liver transplantation. Minerva Chir. 2003;58:725-40.
84. Vivarelli M, La Barba G, Cucchetti A, et al. Can antiplatelet prophylaxis reduce the incidence of hepatic artery thrombosis after liver transplantation? Liver Transpl. 2007;13:651-4.
85. Creput C, Blandin F, Deroure B, et al. Long-term effects of calcineurin inhibitor conversion to mycophenolate mofetil on renal function after liver transplantation. Liver Transpl. 2007;13:1004-10.
86. Khalili M, Lim JW, Bass N, et al. New onset diabetes mellitus after liver transplantation: The critical role of hepatitis C infection. Liver Transpl. 2004;10:349-55.
87. Huprikar S. Update in infectious diseases in liver transplant recipients. Clin Liver Dis. 2007;11:337-54.
88. Sharma S, Gurakar A, Jabbour N. Biliary strictures following liver transplantation: Past, present and preventive strategies. Liver Transpl. 2008;14:759-69.
89. Sethi A, Stravitz RT. Review article: Medical management of the liver transplant recipient—A primer for non-transplant doctors. Aliment Pharmacol Ther. 2007;25:229-45.
90. Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931-40.
91. Guichelaar MM, Schmoll J, Malinchoc M, et al. Fractures and avascular necrosis before and after orthotopic liver transplantation: Long-term follow-up and predictive factors. Hepatology. 2007;46:1198-207.
92. Aberg F, Pukkala E, Hockerstedt K, et al. Risk of malignant neoplasms after liver transplantation: A population-based study. Liver Transpl. 2008;14:1428-36.
93. Silva MA, Jambulingam PS, Mirza DF. Colorectal cancer after orthotopic liver transplantation. Crit Rev Oncol Hematol. 2005;56:147-53.
94. Kremers WK, Devarbhavi HC, Wiesner RH, et al. Post-transplant lymphoproliferative disorders following liver transplantation: Incidence, risk factors and survival. Am J Transplant. 2006;6:1017-24.
95. Bianchi G, Marchesini G, Marzocchi R, et al. Metabolic syndrome in liver transplantation: Relation to etiology and immunosuppression. Liver Transpl. 2008;14:1648-54.
96. Zachoval R, Gerbes AL, Schwandt P, et al. Short-term effects of statin therapy in patients with hyperlipoproteinemia after liver transplantation: Results of a randomized cross-over trial. J Hepatol. 2001;35:86-91.
97. Burton JRJr, Rosen HR. Diagnosis and management of allograft failure. Clin Liver Dis. 2006;10:407-35.
98. Schreuder TC, Hubscher SG, Neuberger J. Autoimmune liver diseases and recurrence after orthotopic liver transplantation: What have we learned so far? Transpl Int. 2009;22:144-52.
99. McCashland T, Watt K, Lyden E, et al. Retransplantation for hepatitis C: Results of a U.S. multicenter retransplant study. Liver Transpl. 2007;13:1246-53.