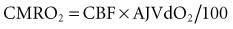197 Liver Transplantation
Orthotopic liver transplantation (OLTX) is the definitive treatment for patients with end-stage liver disease (ESLD). It affords the opportunity for a disabled person to return to a full and active life. Although expensive, OLTX may well be more cost-effective than the routine medical care of terminally ill patients with liver failure.1,2 The first OLTX in humans was performed by Starzl in 1963.3 However, significant progress did not occur until the advent of potent immunosuppressive agents, specifically the introduction of cyclosporine in 1981.4 Technical improvements in surgical approach and organ preservation, combined with increasingly sophisticated anesthetic and intensive care management, have provided 1-year survival rates of nearly 90%.
In this chapter, we outline the many developments that have occurred in this field. Major advances in defining risk categories for candidates and managing patients with cirrhosis and pulmonary hypertension and novel immunosuppressive strategies are described. In recent years, organ allocation has been prioritized such that the sickest patients, those most likely to die, undergo transplants first. This optimizes both aggregate benefit, by improving overall survival of patients with end-stage liver disease, and individual benefit. The latter is manifested by the full recovery of a very sick patient. Conversely, the individual who is not so ill will not bear the risks of surgery. Since February 2002, all patients listed in the United States for liver transplantation have been prioritized by their score on the Model for End-Stage Liver Disease (MELD).5,6
 Candidate Selection
Candidate Selection
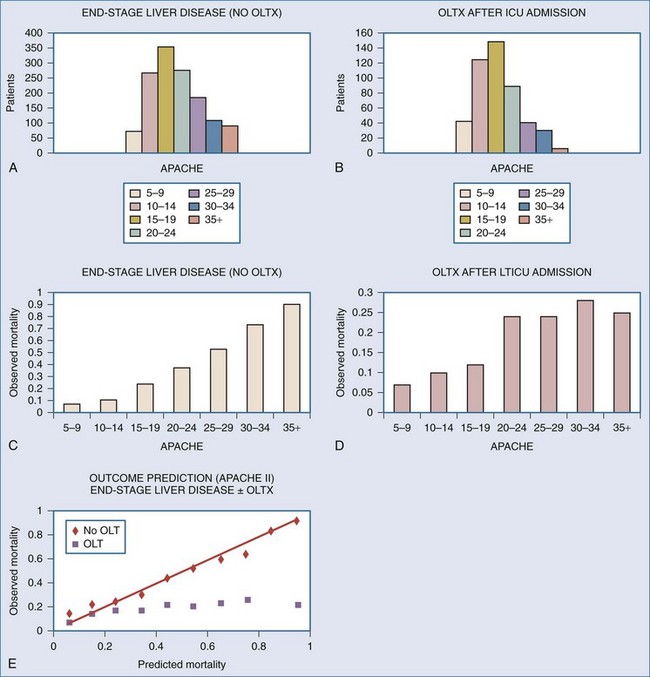
Optimal candidates are those for whom the risk of surgery is far outweighed by the potential improvement in their quality of life. Furthermore, the risk of recurrence of the primary disease should be low.7,8 Not surprisingly, those who are at the highest risk with surgery also achieve the greatest gains when they survive. Unfortunately, such patients have a higher mortality and require significantly greater resources, particularly intensive care and rehabilitation. There are few absolute contraindications to OLTX. However, factors have been identified that significantly increase the risk and should be recognized as relative contraindications (Table 197-1). From the surgical perspective, prior right upper quadrant abdominal surgery, particularly biliary reconstruction, results in a technically more difficult procedure. Patients who are sicker with higher MELD scores or U.S. United Network Organ Sharing (UNOS) Status,1,9 particularly those with fulminant hepatic failure, fare worse. Patients with higher Acute Physiology and Chronic Health Evaluation (APACHE) II scores who are in the intensive care unit (ICU) and require mechanical ventilation or hemodialysis have lower survival. Of course, medical therapy in such circumstances is even less successful, and APACHE II models hospital outcome well. However, after transplantation, mortality does not rise linearly as a function of recipient acuity. Patients with high APACHE II scores have a higher post-OLTX mortality than recipients with very low preoperative scores. However, there is a plateau of approximately 25% for recipients with preoperative APACHE II scores greater than 20 (Figure 197-1). This observation suggests that carefully selected but very ill patients benefit from OLTX.
| Absolute | Relative |
|---|---|
| Extrahepatic malignancy | Cholangiocarcinoma, hepatocellular carcinoma larger than UCSF modification of Milan criteria (see text) |
| AIDS Hepatitis B with active replication |
HIV infection (in the absence of AIDS) |
| Low cerebral perfusion pressure (sustained < 40 mm Hg or cerebral blood flow < 10 mL/min/100 g) in fulminant hepatic failure Infection (extrahepatic) |
Low cerebral perfusion pressure (sustained < 60 mm Hg or cerebral blood flow < 20 mL/min/100 g) in fulminant hepatic failure Portal vein and superior mesenteric vein thrombosis Extrahepatic organ system failure not related to the ESLD |
| Pulmonary hypertension (mPAP > 45 mm Hg or depressed RV function) | Pulmonary hypertension (25 < mPAP < 45, preserved RV function at rest and with exercise) |
| Hepatopulmonary syndrome (PaO2 < 100 mm Hg with FIO2 = 100%) | Hepatopulmonary syndrome (PaO2 < 200 mm Hg with FIO2 = 100%) |
AIDS, acquired immunodeficiency syndrome; ESLD, end-stage liver disease; HIV, human immunodeficiency virus; mPAP, mean pulmonary artery pressure; RV, right ventricular.
Patients with cirrhosis and underlying hepatocellular carcinoma are candidates for OLTX if the disease is limited to the liver and the lesions are small, there is no evidence for major intrahepatic venous invasion, and nodal disease is absent. The widely accepted Milan criteria (1 lesion less than 5 cm or 3 lesions each less than 3 cm) have been modified and established as the University of California at San Francisco (UCSF) criteria (1 lesion = 6.5 cm or 3 or fewer nodules with the largest = 4.5 cm and the total tumor diameter = 8 cm without gross vascular invasion); survival is more than 80% for these patients.10 Extensive radiologic staging of these patients to stratify them into tumor stages is imperative so the risk of postoperative recurrence can be estimated. Patients with biliary tract malignancy such as cholangiocarcinoma have a very high rate of recurrence.11,12 It seems doubtful that more extensive resection, including the liver, a portion of small bowel, and pancreas, will be more successful in controlling recurrence of these tumors.13,14 Preoperative chemotherapy and irradiation may improve outcome after liver transplantation and is under investigation.15
The risk for recurrence of viral hepatitis in the transplanted organ differs for hepatitis A (HAV), hepatitis B (HBV), hepatitis C (HCV), and hepatitis E. HAV is an acute illness that may cause fulminant hepatic failure and does not recur after transplantation. Recurrence of HBV, once a near-universal problem16 except after transplantation for fulminant HBV, has been greatly reduced by the routine use of hepatitis B immune globulin (HBIG) titrated to levels of anti-HBV surface antigen antibody (HbsAb) and antivirals such as tenofovir and entecavir—associated with less emergence of drug resistance than lamivudine and adefovir.17 Active HBV replication, documented by the presence of HBV-DNA, must be suppressed with antivirals before surgery.18–24 For reasons that are unclear, early reports indicated that patients transplanted with HBV fared worse at each postoperative stage than those with other causes of ESLD.25 Some have speculated that this is a systemic disease accounting for both the high rate of reinfection in the absence of prophylaxis and the decreased survival. Hepatitis C presents a more complicated conundrum. Reinfection of the transplanted organ is universal. Currently there is no effective prophylaxis. Clinical progression is highly variable and not predictable. Some patients experience rapid deterioration and graft failure within the first year, but others have little histologic damage several years after liver transplantation. Hepatitis after transplantation may be treated with pegylated recombinant interferon (IFN)-α2b, and ribavirin, but is variably tolerated. Sustained suppression of HCV at the end of therapy is reported to be approximately 26% at 3 years.25 The effect of targeted immunosuppression, particularly reduced corticosteroid dosage, on recurrence of HCV and progression to fibrosis is under investigation.26
HIV-infected liver transplant recipients tolerate carefully titrated immuno-suppression. Survival after OLTX is only slightly lower for HIV-positive patients than it is for HIV-negative patients,9,27–29 with post-transplant morbidity and mortality related to recurrence of hepatitis C in co-infected patients.30
Patients with thrombosed portal veins present a formidable surgical challenge. Options include portal endovenectomy or anastomosis of the donor portal vein to the confluence of the superior mesenteric and splenic veins. Patency of the superior mesenteric vein should be demonstrated by ultrasonography or magnetic resonance imaging (MRI) or angiography before surgery. Occlusion of the superior mesenteric vein usually precludes OLT. Although the portal vein may be anastomosed to the recipient inferior vena cava with a proximal caval ligature placed to sustain flow, mesenteric venous hypertension persists, and gastrointestinal (GI) hemorrhage, ascites, and lower-extremity edema remain problematic. Combined hepatic and intestinal transplantation or multivisceral transplantation are alternatives.31
Acute liver failure (ALF; known formerly as fulminant hepatic failure [FHF]) is liver failure with encephalopathy that develops in patients without prior liver disease within an 8-week period or less.32 Mortality is high and predictable.33 Liver transplantation is the only therapeutic option for patients with progressive liver failure, increasing survival at one year from 20% to 75%.34,35 Such patients are critically ill at the time of transplantation. They require intensive hemodynamic and neurologic monitoring preoperatively, including measurement of intracranial pressure (ICP)36–40 and cerebral blood flow (CBF). A few patients will improve with supportive care, particularly young patients with acetaminophen intoxication, Amanita poisoning, or hepatitis A. But most experience a deterioration in their conditions. In contrast to patients with chronic liver disease, hepatic encephalopathy is associated with intracranial hypertension, particularly in patients with multiple organ system failure and hyponatremia.17,41–45 Inadequate cerebral perfusion, cerebral herniation, and brain death preclude OLT.46 Progressive arterial vasodilation may result from the failing liver, mesenteric hypertension, infection, pancreatitis, and adrenal insufficiency, with resultant hypotension despite elevated cardiac output. Cardiovascular instability,47 atrial and ventricular arrhythmias,48 and respiratory insufficiency (acute lung injury/acute respiratory distress syndrome [ALI/ARDS]) are common complications of ALF and substantially increase operative risk. If preoperative support requires greater than 1 µg/kg/min of epinephrine (or equivalent) or positive end-expiratory pressure (PEEP) greater than 12 with FIO2 above 60%.
 Donor Selection and Operation
Donor Selection and Operation
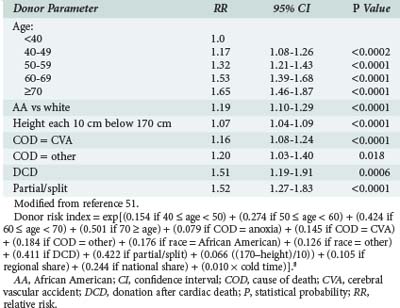
Liver allograft function reflects both recipient and donor factors. Although individual donor characteristics such as age, steatosis, hypernatremia, and impaired lidocaine clearance49,50 have been associated with poor allograft function, a recent study of a large group of patients allowed analysis of donor factors while controlling for recipient-specific characteristics.51 Deceased donor characteristics which independently predicted an increased risk of graft failure included age, donation after cardiac death (DCD), split or partial grafts, race, height, and cause of death (Table 197-2). Decreased graft survival may also be associated with unique pairings of donor and recipient characteristics. For example, liver function in HCV-positive recipients is worse when the donor is older than 60 years of age.52
Brain death results in marked changes in homeostasis for the donor. Hemodynamic instability is common and may result in part from massive free-water deficits caused by diabetes insipidus. Correction of diabetes insipidus with desmopressin and adequate hemodynamic monitoring and intervention are essential to preserve vital organ function. Anesthesia blunts the response to surgical stimulation. A skilled surgical dissection with rapid identification of the hepatic vessels,53 cannulation and perfusion with University of Wisconsin (UW) solution, and rapid cooling are essential for graft preservation. Acceptable cold ischemia times have dropped. Despite a report of successful graft function after prolonged cold ischemia times of up to 24 hours,54 the best outcome is associated with 6 hours or less.
Donation after cardiac death (DCD) results in a graft with an additional warm ischemic insult–a consequence of the hypotension and hypoxemia of that result from with drawal of hemodynamic and respiratory support–until death is pronounced and cannulae can be placed to infuse cold preservative solution. Alternative preservation techniques including less viscid than cold UW solution as well as allograft perfusion with thrombolytics are under investigation.55 A significant learning curve attends the successful use of DCD grafts with biliary complications noted by all but lower survival and hepatic arterial thrombosis reported by some56 but not all programs.57
Living donation is the only option for liver transplantation in many parts of the world. However, in the United States the number of living donor liver transplants is falling from its peak in 2001—constrained by the success of deceased donor liver transplantation, including the falling overall mortality after introduction of MELD for liver allocation and the risks inherent in the donor surgery. In 2009 only 219 liver transplants were from living donors, compared with 6101 from deceased donors.58 Evaluation includes confirmation of the emotional relationship between donor and recipient, evaluation of the donor for medical disease, and anatomic compatibility. Liver segment to donor weight ratios of 0.8% to 1% are needed to avoid small-for-size syndrome. However, donation of the right lobe results in increased donor complications. The reader is referred to a recent detailed review.59
 Recipient Operation
Recipient Operation
Monitoring includes pulse oximetry, electrocardiography, and continuous measurement of arterial pressure (often from two vessels) and pulmonary arterial pressure. Maintenance of large-bore central venous catheters (e.g., two 8.5F introducers) and the ability to infuse whole blood at rates as high as 2 L/min with a rapid infusion system are essential to maintain hemodynamic stability during occasional episodes of massive hemorrhage. More extensive monitoring is indicated in selected cases. Right ventricular function may be compromised by the presence of pulmonary hypertension, a complication that can develop acutely during reperfusion.60–63 Right ventricular ejection fraction and end-diastolic volume are more sensitive guides to cardiac preload than are central venous and pulmonary artery occlusion pressures. These values may be obtained by use of the oximetric pulmonary artery with rapid-response thermistor catheter (Edwards Lifesciences Corp., Irvine, California).1 However, more robust cardiovascular assessment is provided by intraoperative transesophageal echocardiography. This tool provides a dynamic online picture to the anesthesiologist, allowing him or her to assess the adequacy of resuscitation. In patients with ALF and intracranial hypertension, ICP monitoring is essential. Although CBF measurement in the operating room is difficult, flow can be estimated by the contour of the transcranial Doppler and balance of oxygen supply and demand inferred from the arterial-jugular venous oxygen content difference.64 CBF also may be assessed using transcranial Doppler ultrasound to measure the velocity of flow in the middle cerebral artery. Continuous electroencephalography (EEG) and compressed spectral array are under investigation as monitoring techniques in this setting.
A rapid-sequence induction of anesthesia is indicated, as gastric motility is impaired in patients with cirrhosis, and the procedure may be performed before an adequate period of fasting. Anesthesia is often induced with propofol, fentanyl and succinylcholine and maintained with a balanced technique of volatile anesthetics (isoflurane), muscle relaxation (cisatracurium, vecuronium), and judicious use of narcotics (fentanyl) and benzodiazepines (midazolam).65
Monitoring of the coagulation capacity of the recipient is complicated because clotting is usually markedly deranged, and it is necessary to rapidly correct problems. Depletion of coagulation factors and thrombocytopenia are common. Primary fibrinolysis may be evident early in the procedure but does not require treatment in the absence of significant bleeding, which may become problematic during the anhepatic phase. Standard measures of coagulation—prothrombin time (PT), activated partial thromboplastin time (APTT), and platelet count—are very sensitive. However, attempts to correct these values result in excessive transfusion of blood products. There is often significant delay between the time blood is sampled and the results from clotting assays are reported. Finally, standard measures of coagulation provide little timely information about qualitative platelet function and fibrinolysis. Kang and colleagues introduced the thromboelastograph for routine use during OLT.66 This test provides the anesthesiologist with a rapid assessment of coagulation status, the presence or absence of fibrinolysis, and the effects of intervention with protamine or ε-aminocaproic acid, an inhibitor of fibrinolysis.66–68
The surgical procedure involves meticulous dissection, which is often hampered by severe portal hypertension and substantial bleeding from venous collaterals. Insufficient control results in significant blood loss. Identification of the hilar structures may be complicated by adhesions from prior biliary tract surgery. Patency of recipient vessels and adequacy of blood flow must be assessed before placing the graft into the surgical field. An arterial graft for the hepatic artery may be chosen when the recipient anatomy is anomalous or the caliber of the vessels is too small, or when atherosclerosis narrows the celiac trunk. Other indications for an arterial graft include a marked size discrepancy between the recipient and donor vessels and inadequate length of the donor artery. Portal venous thrombosis may be managed with a “jump” graft from the superior mesenteric vein if the portal vein cannot be thrombectomized.69 The donor and recipient caval veins are usually anastomosed end-to-end caudad and cephalad to the liver when a cava-sparing technique is not chosen.
Preservation of blood flow in the inferior vena cava (caval preservation) with or without portal drainage is an alternative technique, also known as a piggyback70–72 and is preferred when there is marked hemodynamic instability. Venovenous bypass was used routinely in the past because it afforded greater hemodynamic stability and reduced mesenteric congestion (Figure 197-2).73 However, the piggyback approach requires one less anastomosis and no dissection of the groin or axilla, decreasing the time for surgery by 1 hour. The biliary anastomosis is fashioned after the vascular anastomoses are completed and the graft reperfused. Two options are used: choledochocholedochostomy or mid-jejunal Roux-en-Y limb with choledochojejunostomy. The former procedure requires less dissection and is restorative. Unfortunately, the stenosis rate is quite high. Diseases which involve the extrahepatic bile ducts, such as sclerosing cholangitis, require resection of the bile duct and creation of a choledochojejunostomy. Stenting of the biliary anastomosis—once routine with a T tube—is now controversial. One innovative approach is cannulation of the donor cystic duct after donor cholecystectomy with a 5F catheter which stents the anastomosis, drains some bile for daily inspection and provides a noninvasive route for cholangiography. A hemorrhoidal band serves to seal the cystic duct once the drain is removed.
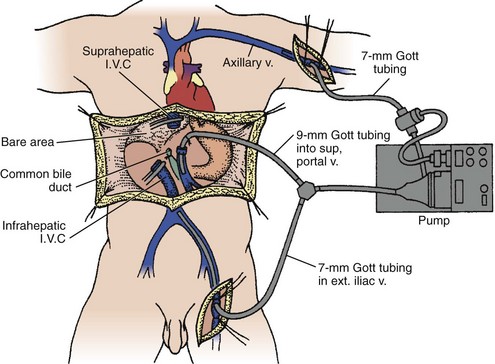
Figure 197-2 Venovenous bypass. I.V.C, inferior vena cava; ext. iliac v., external iliac vein.
(From Griffith BP, Shaw BW Jr, Hardesty RL, Iwatsuki S, Bahnson HT, Starzl TE. Veno-venous bypass without systemic anticoagulation for transplantation of the human liver. Surg Gynecol Obstet 1985;160:270-2, with permission.)
Reperfusion is accompanied by cardiovascular collapse in a small (and decreasing) number of patients (∼2%-5%).74 Although the exact mechanism is undefined, recirculation results in a cardiac bolus of cold acid and potassium-rich fluid, resulting in acidemia, hyperkalemia, and hypocalcemia. The consequence is abrupt onset of a severe, albeit brief, cardiomyopathy coincident with the loss of vasomotor tone and, occasionally, increased pulmonary arterial pressure. Volume resuscitation, sodium bicarbonate, or THAM for correction of metabolic acidosis, calcium chloride, and inotropic support (epinephrine) are usually sufficient to restore hemodynamic stability. Fortunately, this event is usually short lived. However, significant insults to the graft, heart, kidneys, and brain may occur and require postoperative attention.
 Postoperative Management
Postoperative Management
Liver Allograft Function
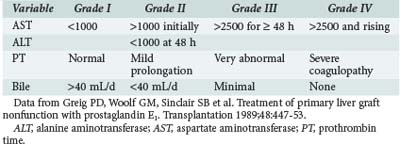
Early graft function is usually assessed by measuring circulating concentrations of total bilirubin, aminotransferases, canalicular enzymes, and clotting factors. The scheme shown in Table 197-3 is useful for assessing graft function according to these parameters.75 Other parameters such as arterial ketone body ratio (AKBR)76 and oxygen consumption77 also correlate with graft survival. However, in a retrospective review, Doyle and colleagues were unable to identify a unique parameter with adequate sensitivity and specificity to be useful for predicting graft survival in individual OLTX recipients.78 Other techniques, such as neural network modeling, require further investigation.79 An alternative approach is to use a composite acuity score to predict graft and patient survival. For example, Angus et al. showed that the APACHE II score, a widely used severity-of-illness indicator designed for general ICU patients, was useful for predicting both hospital survival and survival at 1 year for liver transplant recipients if the model was recalibrated.80,81
The diagnostic work-up for a patient with liver function abnormalities in the perioperative period should include a Doppler ultrasound examination to determine patency of all pertinent vessels. Concern about the adequacy of flow should prompt an angiogram or MR angiogram (MRA). Early occlusion of the hepatic artery should prompt immediate re-exploration, which can result in a nearly 50% graft salvage rate.82 Early hepatic artery thrombosis may present as a precipitous deterioration in hemodynamics, abrupt development of ARDS, severe coagulopathy, and markedly elevated serum aminotransferase concentrations. Bacteremia is common. Delayed hepatic artery thrombosis is often less dramatic in its presentation.83 Indeed, some patients are asymptomatic. Others show destruction of the biliary duct system with multiple intrahepatic strictures, bile collections, and intrahepatic abscesses. Recurrent bacteremia, in the absence of another source, may be the only indication of hepatic artery thrombosis.
Graft rejection may occur at any point after OLT. Hyperacute rejection is very rare, if it occurs at all, after OLTX. Nevertheless, a humoral component of rejection may be evidenced by antibody deposition in the arterial endothelium and by persistence or recrudescence of a positive cross-match.84 Acute cellular rejection (ACR) is more common and develops in approximately 40% of liver transplant recipients. It typically presents after the first week but can present within the first few days after transplant or present years later. Thus, its usual description as “acute” is a misnomer. The histologic criterion for the diagnosis of ACR is a periductal lymphocytic infiltrate associated with a cellular infiltrate around the central veins.85 Nevertheless, these changes may be evident to a lesser degree even in the absence of clinical abnormalities. In a graft with stable function, rejection is typically associated with a rise in serum total bilirubin concentration associated with elevations in the circulating levels of aminotransferases and canalicular enzymes. Other clinical findings include signs of the sepsis syndrome, diarrhea, suddenly increasing ascites, eosinophilia, thrombocytopenia, and laboratory evidence of hemolysis. Chronic rejection, also a misnomer because it may occur at any point, is manifested by arteriopathy and vanishing bile ducts. Its presentation is insidious, and signs of terminal liver disease may develop slowly.
Immunosuppression
The approach to rejection is divided into two phases: prophylaxis and treatment.86 Prophylaxis is achieved by administering a combination of corticosteroids and cyclosporine (Neoral) or tacrolimus. These agents inhibit interleukin (IL)-2 expression and block T-cell recruitment. They offer a selective approach to immunosuppression in solid-organ transplantation. Prospective randomized trials comparing tacrolimus-based and cyclosporine-based regimens demonstrate that tacrolimus affords better rejection prophylaxis, is associated with less steroid-resistant rejection and need for OKT3,87,88 and is less costly when medical care in the first posttransplant year is considered.89
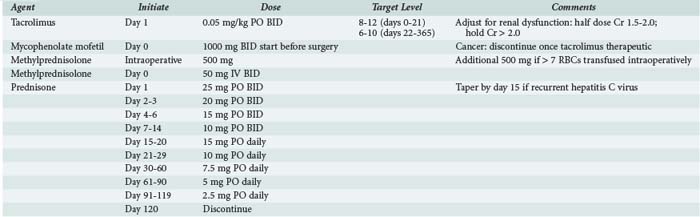
Azathioprine, used before the advent of newer immunosuppressive agents, is reserved for patients with recurrent rejection episodes or for those unable to tolerate the newer agents. Mycophenolate mofetil is hydrolyzed in vivo to mycophenolic acid. This compound inhibits inosine monophosphate dehydrogenase, resulting in selective inhibition of T- and B-cell proliferation.90 Mycophenolate mofetil is more expensive than azathioprine and has GI side effects (diarrhea) but less bone marrow toxicity. Data from a prospective randomized trial that enrolled liver transplant recipients indicates that combined tacrolimus, prednisone, and mycophenolate is no more toxic than tacrolimus and prednisone and that the three-drug cocktail may facilitate a reduction in tacrolimus dose.91 Newer immunosuppressive agents and techniques are under development. The current regimen at Mayo Clinic Jacksonville for prophylaxis is outlined in Table 197-4. Calcineurin inhibition with tacrolimus or cyclosporine is the cornerstone of treatment. Corticosteroids are administered intraoperatively and throughout the early postoperative period. Early introduction of mycophenolate allows a reduction in tacrolimus dose.92 Sirolimus has been associated with delayed wound healing and hepatic artery thrombosis when administered in the early postoperative period. When introduced later in the transplant course, it enables reduction or elimination of calcineurin inhibition. Thymoglobulin and IL-2 receptor (IL-2r) antagonists may be used for induction of immunosuppression, which allows for delayed introduction of calcineurin inhibitors and/or more rapid steroid taper; this is particularly useful in patients with renal impairment at the time of transplantation.
Liver biopsy may be driven by clinical changes or by protocol on day 7. The latter affords a better margin of safety/reassurance that patients with subclinical rejection will be identified and treated aggressively, enabling a less intensive immunosuppressive strategy to be successful for the remainder. Significant complications may result from liver biopsy, and this may outweigh any benefit in well-established programs with careful monitoring. Mild rejection requires no specific treatment other than up-titration of calcineurin inhibition. Moderate to severe rejection is treated initially with corticosteroids: 1000 mg of methylprednisolone is administered over a 4-day period (day 0, 500 mg; day 2, 250 mg; day 4, 250 mg). A follow-up liver biopsy is performed on the fifth day. If rejection persists, treatment with 2000 mg of methylprednisolone is given over the next 4 days (day 0, 1000 mg; day 2, 500 mg; day 4, 500 mg). Persistent rejection deemed “steroid resistant,” represents a less than 5% incidence and is treated with thymoglobulin (as OKT3 is no longer available).93–97
The major side effects of cyclosporine and tacrolimus are similar: both cause significant nephrotoxicity and neurotoxicity.98 More than 90% of patients99 sustain some degree of renal injury, which is manifested clinically as azotemia. Renal dysfunction is a consequence of the hemodynamic insults of the procedure and/or side effects of calcineurin inhibitors. Ten percent of OLTX patients require some form of renal replacement therapy postoperatively, and a few require long-term hemodialysis. Neurotoxicity is more evident in the elderly and compounded by serum electrolyte disturbances, particularly hyponatremia and hypomagnesemia.100 Neurologic dysfunction ranges from a mild expressive aphasia to tremors, confusion, coma, and seizures. Other side effects of cyclosporine, such as hypertension and hirsutism, occur less commonly with tacrolimus. Because tacrolimus is a more potent agent, many patients are able to have the dose of corticosteroids tapered, if not completely discontinued.101,102
 Hemodynamic Changes
Hemodynamic Changes
The characteristic hemodynamic changes of ESLD resolve slowly after OLTX. The exact timing is unresolved, and the controversy likely reflects the preoperative state of some of the patients. Thus, problems resolve more slowly in patients with profoundly deranged liver function and MODS than in recipients who are less ill at the time of transplantation. A vasodilated hyperdynamic state is typical of liver failure103–107 and rarely normalizes in the immediate postoperative period. Patients who are unable to mount a hyperdynamic response fare worse. Some recipients have preexisting cardiac dysfunction due to ischemic damage or restrictive cardiomyopathy secondary to amyloidosis or hemochromatosis; these patients are unable to increase stroke volume and cardiac output in response to vasodilation. Similarly, patients with sepsis have a higher mortality if they fail to (1) increase ventricular end-diastolic volume to preserve stroke volume as ejection fraction falls and (2) increase heart rate to increase cardiac output.108 Elevated central venous pressures (CVPs) are transmitted to the hepatic vein and through the liver. Hepatic congestion results in impaired clearance of bacteria, endotoxin, and cytokines. Elevated hepatic venous pressures are reflected in elevated portal pressures, which increase bacterial translocation and endotoxemia, further compromising graft function. Resuscitation must be guided by measurement of CVP. The etiology of hypotension should be classified as cardiac—a consequence of inadequate preload or impaired contractility—or loss of arterial tone.
Although hypotension is a more common problem, arterial hypertension may occur in the postoperative period. It commonly reflects inadequate analgesia or sedation,16 impaired gas exchange, or hypoglycemia. However, hypertension may persist once these factors are addressed, and attention should then focus on the toxic side effects of cyclosporine109,110 and tacrolimus. Both drugs are vasoconstrictors and may promote hypertension by activating the renin-angiotensin pathway. This complication occurs more commonly with cyclosporine (30% of cases) than with tacrolimus (10% of cases), and cyclosporine-induced hypertension is more resistant to antihypertensive therapy.111–113 Antihypertensive therapy should be initiated when systolic blood pressure is over 160 mm Hg or diastolic blood pressure is over 95 mm Hg. We favor combined α- and β-adrenergic receptor blockade with labetalol. Long-term management rests on a combination of β- and α-adrenergic blockade and calcium channel blockade. ACE inhibition may be complicated by hyperkalemia. Hypertension resistant to the first-line agents is usually managed in the ICU with potent vasodilators such as nicardipine and sodium nitroprusside, perhaps in combination with an α-adrenergic blocking agent.
 Pulmonary Considerations
Pulmonary Considerations
Pulmonary complications of ESLD are common.113 Atelectasis, pleural effusion, reduced functional residual capacity, and limited vital capacity due to ascites and chest wall edema are often present preoperatively. The operative procedure in the upper abdomen, placement of a “normal-sized” graft in the site of a shrunken cirrhotic liver, and postoperative ileus can further decrease vital capacity. Inadequate pain control results in splinting and atelectasis and increases the risk of pneumonia. However, long-term pulmonary sequelae are rare, and most patients have improved pulmonary function tests when studied more than 1 year after OLTX.
Pulmonary infiltrates in patients with liver disease warrant immediate evaluation. Pulmonary infection should be considered, but many pulmonary infiltrates have a noninfectious cause. Pulmonary edema may result from left atrial hypertension (volume overload) or from ALI/ARDS. The latter usually heralds infection, commonly intraabdominal/surgical site with secondary peritonitis. Pancreatitis or liver allograft failure, whether caused by rejection, primary nonfunction, or vascular catastrophe (e.g., hepatic artery thrombosis), may also lead to development of ARDS. When liver failure per se is the cause, ARDS usually resolves after successful transplantation.114 ARDS also may develop during treatment of rejection with thymoglobulin or OKT3.115
Bronchoscopic techniques are used routinely to aid the clinical assessment of pulmonary infiltrates and establish the diagnosis of pneumonia.116 Despite the severe coagulopathy that often is present, bronchoalveolar lavage (BAL) may be performed without significant risk of hemorrhage. Quantitative cultures are obtained, and the presence of bacteria at more than 100,000 colony-forming units (CFU)/mL is considered diagnostic of pneumonia. BAL is sensitive but lacks specificity. A protected brush specimen is less sensitive but more specific. However, it may result in significant endobronchial hemorrhage in coagulopathic patients. With little overall impact on management, we favor BAL. Bronchoscopic techniques confirm the clinical suspicion of pneumonia in only a third of cases.117
Matuschak and associates have described liver-lung interactions.118,119 ALI is common in advanced liver failure.120 Patients with liver failure and ARDS are at high risk for mortality and are usually eliminated as candidates for liver transplantation. However, in highly selected patients with liver failure and ARDS, lung injury resolves quickly after successful OLTX.121
Two additional pulmonary complications—hepatopulmonary syndrome and portopulmonary hypertension—are unique to patients with liver disease. Cyanosis sometimes occurs in patients with cirrhosis.122 Several explanations have been tendered. Anatomic right-to-left shunts have been described within the pulmonary circulation123,124 and between the portal venous system and the pulmonary veins via esophageal veins.125 Increased closing volume resulting in air trapping has been observed. A leftward shift of the oxyhemoglobin saturation curve also has been reported.126 Most important, many patients have a diffusion defect manifest as a decreased diffusing capacity (DLCO) on pulmonary function tests. Furthermore, hypoxic pulmonary vasoconstriction is impaired. These findings correlate with anatomic studies showing dilated intrapulmonary capillaries.127 Additionally, studies using inert gas washout techniques have demonstrated that hepatic dysfunction is associated with significant ventilation/perfusion mismatching rather than pure shunt. Patients with hepatopulmonary syndrome have dilated pulmonary capillaries which lead to diffusion impairment. Furthermore, increased dispersion in the ventilation/perfusion relationship results in mismatching such that many poorly ventilated units are excessively perfused.128 Ventilation/perfusion mismatching does not constitute a true right-to-left shunt, which explains the observation that hyperoxia results from prolonged exposure to high FIO2. The most useful preoperative test is contrast echocardiography using tiny air bubbles as the contrast agent (“bubble study”). Normally, no contrast agent appears on the left side of the heart after venous injection of the bubbles. The appearance of contrast agent immediately after injection suggests an intracardiac shunt (i.e., patent foramen ovale); contrast agent that appears later (i.e., third to sixth cardiac cycle) suggests intrapulmonary shunting.129 Hypoxia usually resolves within the first month, but sometimes resolution is delayed for as long as a year after transplantation. Patients who fail to improve should be investigated with pulmonary angiography to identify a single shunt large enough to be embolized.130
Pulmonary hypertension occurs more commonly in patients with cirrhosis than in controls and is called portopulmonary hypertension.131 Other than cirrhosis, no predisposing factor has been identified. The histopathologic abnormalities in the lungs are typical of primary pulmonary hypertension. Secondary causes of pulmonary hypertension, particularly left ventricular failure, left-to-right intracardiac shunting with increased cardiac output, autoimmune disease, and pulmonary embolism, should be eliminated from consideration, and both portal and pulmonary hypertension confirmed for the diagnosis of portopulmonary hypertension to be established. In an advanced state, it may be difficult to distinguish portopulmonary hypertension from primary cardiac failure with secondary venous congestion and hepatic failure. Portal hypertension can be diagnosed on clinical grounds by the presence of varices, splenomegaly, and can be confirmed by hepatic vein catheterization and measurement of free and wedged pressures, the latter being an estimate of presinusoidal portal pressure. Echocardiography allows estimation of pulmonary pressures by measuring the regurgitant flow velocity through the tricuspid valve. However, confirmation with pulmonary artery catheterization is necessary. Severe pulmonary hypertension recognized only at the start of the OLTX warrants cancellation of the procedure. In contrast to patients with primary pulmonary hypertension, patients with portopulmonary hypertension benefit minimally from acute pharmacologic interventions directed at reducing the pulmonary arterial pressures. Therapeutic measures such as organic nitrates, sodium nitroprusside, or calcium channel blockers fail to reduce pulmonary artery pressure and can lead to systemic hypotension. Low systemic arterial pressure, in turn, may result in right ventricular ischemia, further compromising right ventricular function, with decreased left ventricular filling and cardiac output and more profound hypotension. Initial studies suggested that patients with portopulmonary hypertension are unresponsive to inhaled nitric oxide.132 Subsequent experience suggests that inhaled nitric oxide ameliorates pulmonary hypertension and improves arterial oxygenation in some patients.133,134
An alternative approach borrows from the experience gained by clinicians using continuous infusions of prostaglandins such as epoprostenol (Flolan) to lower pulmonary artery pressure in patients with primary pulmonary hypertension. Prolonged infusion of the drug over weeks or months allows gradual upward titration of the dose,135 a tactic that ameliorates pulmonary hypertension without causing systemic hypotension. Cardiac remodeling ensues, leading to improved cardiac output, reduced tricuspid regurgitation, and normalization of CVP. The goal of treatment with epoprostenol is a systolic pulmonary artery pressure (PAP) of less than 60 mm Hg (mean < 40 mm Hg), low CVP, elevated cardiac output, and normal response to fluid challenge (i.e., slight increase in PAP, CVP, and right ventricular end-diastolic volume and a large increase in cardiac output). The change in PAP, CVP, and cardiac output during exercise may provide additional insight and guide perioperative risk assessment and management. However, pulmonary exercise testing does not predict the pulmonary vascular and right ventricular response as systemic hemodynamics normalize after successful transplantation. Pulmonary hypertension resolves in some patients after successful OLTX.136,137 Others appear to have two concomitant independent processes: pulmonary hypertension (primary) and portal hypertension. Consequently, such patients are at risk for increased PAP and right ventricular failure despite successful OLT. Careful monitoring with PA catheterization and echocardiography is essential. Patients with portopulmonary hypertension treated with epoprostenol require continued infusion and titration during the immediate postoperative period but usually can be weaned over the subsequent year. Although patients with mild pulmonary hypertension (Table 197-5 lists definitions) can tolerate OLTX without significant complications, the picture is bleak for those with moderate to severe pulmonary hypertension (mean PAP > 45 mm Hg). Most transplant recipients with severe pulmonary hypertension die of right-sided heart failure during reperfusion or during the early recovery phase. They tolerate large fluid shifts poorly. Right ventricular overload and failure develop abruptly, compromising the viability of the graft as a consequence of hepatic and mesenteric congestion. Low cardiac output and hypotension results in graft ischemia and death. Poorly controlled pulmonary hypertension or residual right ventricular dysfunction presents an absolute contraindication to liver transplantation.
| Category | Mean PA Pressure (mm Hg) | Systolic PA Pressure (mm Hg) |
|---|---|---|
| Mild | 25-34 | 35-44 |
| Moderate | 35-44 | 45-59 |
| Severe | 45-75 | 60-100 |
| Very severe | >75 | >100 |
PA, pulmonary artery.
Pulmonary hypertension that develops de novo during or acutely after liver transplantation may result from embolic phenomena at the time of transplantation that may be evident on intraoperative transesophageal echocardiography. Patients should be managed with attention to sustaining right ventricular coronary perfusion by maintenance of adequate mean arterial pressure and avoidance of central venous hypertension. Pulmonary hypertension can develop late after liver transplantation. This problem is not always directly related to portal hypertension, because liver function and the transhepatic venous pressure gradient (pressure difference between wedged and free hepatic venous pressures) may be normal.138
Mechanical ventilatory support is often required preoperatively for patients with ESLD. Intubation to minimize aspiration is required when the patient cannot protect the airway because of encephalopathy or massive upper GI hemorrhage. Respiratory failure can be precipitated by volume overload and pulmonary edema, infection, or profound muscle weakness. The increased risk of ALI/ARDS warrants mechanical ventilation with a low tidal volume strategy determined by a height-based calculation of ideal body weight.139 We favor a pressure-limited approach for most patients, using CPAP/PS or pressure control-IMV. Sedation should be minimized and early mobilization out of bed with standing and ambulation attempted, even with an endotracheal tube, significant PEEP, and vasopressor support, with careful monitoring. Early extubation is indicated as soon as the patient can clear secretions, protect the airway, and ventilate without fatiguing. Hypoxemia can be managed with supplemental oxygen and positive airway pressure provided with CPAP. Previously, the median duration of intubation after transplantation was 2 days. Recent changes in anesthetic techniques allow many patients to be extubated in the PACU. Now the median duration of intubation for patients who require ICU admission is less than 24 hours. Early extubation postoperatively (<6 hours) is the goal, and immediate postoperative extubation is possible in those having an uncomplicated intraoperative course and no life-threatening premorbid extrahepatic organ dysfunction.140,141
 Renal Considerations
Renal Considerations
Renal dysfunction in patients with liver disease is frequently unrecognized. Liver dysfunction and malnutrition make elevations in blood urea nitrogen and serum creatinine concentration unimpressive despite a significant decrease in glomerular filtration rate (GFR). Preoperative renal failure (creatinine > 2.0 or the need for hemodialysis) presages post-operative renal failure and decreased survival.142,143 Kidney biopsy may help guide a decision for combined kidney and liver transplantation.144 In the posttransplant period, several factors conspire to impair renal function. These factors include preoperative renal failure (hepatorenal syndrome), episodic arterial hypotension resulting in tubular damage, medications (e.g., cyclosporine, tacrolimus, and vasopressors) that cause renal arterial vasoconstriction, and amphotericin, which causes tubular damage.145 Furthermore, liver allograft dysfunction leads to portal and mesenteric hypertension which leads to functional renal impairment—the hepatorenal syndrome. Renal replacement therapy is required for approximately 10% of patients. Continuous renal replacement offers greater hemodynamic stability and may not precipitate additional organ injury. Adequate renal perfusion pressure requires at least “normal” mean arterial pressure. However, there seems to be little benefit to the kidney from dopamine, fenoldopam, calcium channel blockade, or prostaglandin infusion.
 Gastrointestinal Considerations
Gastrointestinal Considerations
Protein and calorie malnutrition is common in liver failure; it is a catabolic process. Weight and appearance are often misleading, and cachexia is evident once anasarca resolves. Muscle wasting may be most apparent in the temporalis and thenar eminence. Malnutrition compromises the outcomes from liver transplant, resulting in higher perioperative complications and mortality. Recognition and preoperative treatment of malnutrition may improve liver transplant outcomes. Gastroparesis is common in liver failure, increasing the risk of aspiration pneumonitis and pneumonia. We use nasojejunal feedings in malnourished patients who demonstrate inadequate intake of calories and protein. Enteral nutrition may be initiated or restarted within 6 hours after liver transplant for patients with a choledochocholedochostomy. Further delay up to 72 hours may be required in patients with a choledochojejunostomy and jejunojejunostomy (Figure 197-3) to resume full-dose enteral nutrition, although slower rates are often tolerated. On rare occasions when parenteral nutrition is required, we use crystalline amino acids and supply one-third of the nonprotein calories as fat. This approach minimizes glucose intolerance, which is common in the early post-OLTX period.
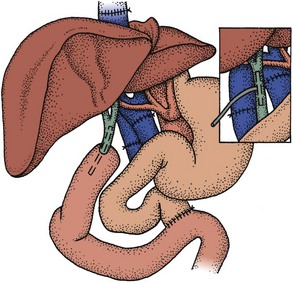
Figure 197-3 Choledochojejunostomy and choledochocholedochostomy.
(From Starzl TE, Demetrius AJ, Van Thiel D. Liver transplantation (1). N Engl J Med 1989;321:1014-92, with permission.)
Pancreatitis is a feared but less common complication of OLTX. Although nearly 20% of patients demonstrate elevated serum amylase or lipase levels, 5% or less have clinically significant pancreatitis.146 Conservative measures are effective in mild cases. Management of severe pancreatitis is as controversial in this setting as it is in patients without OLTX. The roles of somatostatin and operative débridement with continuous lavage remain to be defined.
 Neurologic Considerations
Neurologic Considerations
Patients with early graft dysfunction also often have changes in mentation. Graft swelling may result in portal congestion and portosystemic shunting. Administration of flumazenil may produce a more awake but still encephalopathic patient. The exact mechanism for encephalopathy due to graft dysfunction remains to be elucidated. Side effects of medications assume a much greater role in such patients. Clearance of commonly used immunosuppressive agents, analgesics, sedatives, and hypnotics is impaired. The amnestic effects of some agents may compound the problem. We use boluses of fentanyl for analgesia, as it is short acting and has no active metabolites. Benzodiazepines exacerbate delirium, so if sedation is needed, we prefer infusion of propofol,147 with frequent titration and daily discontinuation. Delirium, which is more commonly akinetic than agitated and thus often unrecognized, responds better to low doses of haloperidol or atypical antipsychotics such as olanzapine and quetiapine. Sleep in the ICU is abnormal and made worse by commonly used medications.148 Unlike benzodiazepines and other sedative hypnotics, dexmedetomidine can be titrated to effect without disrupting normal sleep patterns.149,150
The seizure threshold is lowered by several medications used in liver transplant recipients, including cyclosporine, tacrolimus, thymoglobulin, and antipsychotics.151,152 Hypoglycemia, electrolyte abnormalities including hyponatremia and hypomagnesaemia, and metabolic acidosis further lower the seizure threshold.
Additional aspects of neurointensive care need consideration in patients with ALF. These patients have encephalopathy that is mediated in part by the γ-aminobutyric acid (GABA) pathway, as is the case for patients with the portosystemic encephalopathy of chronic liver disease.153 But patients with ALF develop intracranial hypertension and cerebral edema.154 Patients in grade III and grade IV coma should have ICP monitored. Improved fidelity but increased complications such as bleeding and infection vary with the invasiveness of the monitoring technique.155 A parenchymal monitor such as Codman or Camino offers a much better signal-to-noise ratio with only slight increase in risk. Ventriculostomy is the approach utilized at our institution and offers the therapeutic advantage of draining cerebrospinal fluid to avoid transient increases in intracranial pressure, with attendant decrements in cerebral perfusion pressure, minimizing compounding ischemic insults. This purported advantage is somewhat offset by bleeding around the ventriculostomy catheter which has been evident on computed tomography (CT) but clinically asymptomatic. Cerebral blood flow (CBF) can be measured with xenon-133 or with cold xenon as contrast for CT scanning. Cerebral oxygen consumption can be determined after placement of a jugular bulb catheter.136 Cerebral metabolic rate (CMRO2) is related to the product of the CBF and arterial-venous oxygen content difference (AJVdO2 = CaO2 − CjvO2) according to the following formula:
Intracranial hypertension may result from increased CBF (blood volume) or brain swelling (cerebral edema). Elevated CBF with normal oxygen consumption is associated with narrow AJVdO2 and is termed luxuriant perfusion. Subsequently, cerebral edema with intracranial hypertension develops and is associated with decreased CBF and large AJVdO2.156 Management of intracranial hypertension is guided by the observed pathophysiology. Elevated ICP with increased CBF responds to hyperventilation, reduction in intravascular volume, and hypothermia. Intracranial hypertension with low CBF must be treated by increasing cerebral perfusion pressure by increasing mean arterial pressure. Osmotic agents including mannitol and hypertonic saline are also appropriate at this stage, with diuretics or CRRT to minimize elevation in CVP. Hypothermia restores cerebral autoregulation157–159 induced by ALF. It effectively lowers ICP160 and can be titrated to effect. Induction of coma with propofol titrated to burst suppression with continuous EEG monitoring may lower ICP further. However, accumulation of vasodilatory metabolites will compromise cerebral perfusion pressure. Patients are considered viable candidates for OLTX as long as EEG activity is preserved and adequate cerebral perfusion pressure and CBF can be maintained. Intraoperative monitoring includes these measures, combined with transcranial Doppler measurements of flow velocity contour in the middle cerebral artery,161 which facilitate moment-to-moment titration of anesthetics and vasopressors. Although the initial period of graft reperfusion is the most hazardous, cerebral hyperemia and intracranial hypertension may persist for several days postoperatively. These abnormalities usually resolve with good graft function.
Liver transplant recipients are at risk for neuromuscular dysfunction. In a prospective study of 100 liver transplant recipients, clinically relevant weakness, defined as weakness requiring prolonged mechanical ventilatory support, developed in 7% of patients. Electromyography demonstrated that weakness was due to a myopathic rather than neuropathic process, and diffuse myocyte necrosis was evident on muscle biopsy specimens taken from five patients.162 Predisposing factors included patient acuity postoperatively as judged by the APACHE II score, poor liver allograft function at 1 week, a requirement for renal replacement therapy, and higher doses of corticosteroids. Patients requiring early retransplantation seemed to be at particular risk.
 Infectious Complications
Infectious Complications
Rejection of the allograft is treated aggressively when it develops. These measures are often complicated by the parallel development of infections. Heavily immunosuppressed patients die not of rejection but of infection. The paradigm is that immunosuppression sufficient to eliminate rejection results in a defenseless host susceptible to many infections. Solid organs vary in their propensity to stimulate rejection. The liver is relatively less immunogenic; accordingly, immunosuppression can be less intensive but still be effective. In the early postoperative period, bacterial and fungal infections are common. The most frequently involved areas are the operative site and the lungs. Perioperative antimicrobial prophylaxis targets gram-negative rods and enterococci and consists of a second- or third-generation cephalosporin or ampicillin-sulbactam and is continued for 48 hours. Prophylactic regimens vary among centers. Unfortunately, antimicrobial resistance is common, and isolates in patients who die of an infectious process are occasionally resistant to all known antimicrobial agents.163
Fungal colonization is also common. Patients requiring a prolonged, difficult surgical procedure and multiple transfusions of blood products are at higher risk for fungal infection, as are patients undergoing retransplantation.164,165 Prophylactic antifungal therapy reduces the incidence of both superficial and deep fungal infections. Options include fluconazole,166 amphotericin (10-20 mg daily for the first 2 weeks after surgery), or full doses of amphotericin B liposomal complex (ABLC) in the subgroup at highest risk of filamentous fungal infection—patients with renal and liver failure prior to OLT and those with ALF.167 Patients with significant growth of Candida spp. on quantitative culture of BAL often require a full course of amphotericin.168,169 The outcome has improved for liver transplant recipients who develop Aspergillus infection. This previously fatal infection170 seems more responsive to voriconazole171 or liposomal forms of amphotericin such as AmBisome.
CMV infections are common in the transplant population.172,173 However, the clinical severity of infection is quite variable. Some cases are asymptomatic. Others present as a viral syndrome, involve only one organ such as the lungs, GI tract, or liver, or involve multiple organs. The patients at highest risk for CMV infection are those who were seronegative before OLTX and received an organ from a seropositive donor. It is debatable whether patients who were seropositive before OLTX experience reactivation of latent virus or are infected by another CMV strain. In addition to the morbidity and mortality attributed directly to CMV, patients with CMV disease also have a higher frequency of bacterial and fungal infections. Increased susceptibility to bacterial and fungal infections may be a function of the CMV infection per se or reflect more aggressive immunosuppression—an independent risk factor for CMV infection. Seroconversion may not occur until T-cell immunosuppression is withdrawn.174
Prophylaxis with ganciclovir175 or CMV immunoglobulin176,177 is effective but with significant penalty in terms of side effects such as neutropenia and costs borne by all for benefit of a few. An alternate strategy is to monitor patients closely for evidence of CMV disease. CMV viremia can be detected by PCR or by assaying for the CMV the pp65 antigen.178 Thresholds for treatment can be varying in relation to the risk of CMV disease in a particular subpopulation. Ganciclovir and valganciclovir are the mainstays of treatment, with foscarnet reserved for patients with intractable severe neutropenia and when resistance is suspected.
 Endocrine Considerations
Endocrine Considerations
Hyperglycemia is common in the early postoperative period and reflects the combination of surgical stress, corticosteroids, and calcineurin inhibition. A survival benefit of intensive insulin therapy has been demonstrated179,180 but is of unclear benefit in unselected ICU patients.181 We suspect the high incidence of infection and neuromuscular disease in liver transplant recipients warrants intensive insulin therapy with a rationale similar to burn-injured patients.182–185 We have modified the approach described by Davidson186 to minimize hypoglycemia and target glucose of 90 to 120 mg/dL.
Adrenal insufficiency is common in critically ill patients with liver disease.187 Both primary and secondary adrenal insufficiency may be unmasked by stressors such as infection, graft dysfunction, pancreatitis, or bleeding. Measurement of baseline serum ACTH and cortisol and subsequent response of serum cortisol to cosyntropin allow recognition of adrenal insufficiency and classification. The indication for continued treatment with “stress-doses” of hydrocortisone can then be determined.
Key Points
Grellier L, Mutimer D, Ahmed M, et al. Lamivudine prophylaxis against reinfection in liver transplantation for hepatitis B cirrhosis. Lancet. 1996;348:1212.
O’Grady JG, Gimson AES, O’Brien CJ, et al. Controlled trials of charcoal hemoperfusion and prognostic factors in fulminant hepatic failure. Gastroenterology. 1988;94:1186.
O’Grady JG, Alexander GJM, Hayllar KM, et al. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989;97:439.
Kang YG, Martin DJ, Marquez J, et al. Intraoperative changes in blood coagulation and thromboelastographic monitoring in liver transplantation. Anesth Analg. 1985;64:888.
Angus DC, Clermont G, Kramer DJ, et al. Short- and long-term outcome prediction with the APACHE II system after orthotopic liver transplantation. Crit Care Med. 2000;28:150-156.
Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470.
European FK506 Multicentre Liver Study Group. Randomised trial comparing tacrolimus (FK506) and cyclosporin in prevention of liver allograft rejection. Lancet. 1994;344:423-428.
The U.S. Multicenter FK506 Liver Study Group. A comparison of tacrolimus (FK 506) and cyclosporine for immunosuppression in liver transplantation. N Engl J Med. 1994;331:1110-1115.
Lake JR, Gorman KJ, Esquivel CO, et al. The impact of immunosuppressive regimens on the cost of liver transplantation—results from the U.S. FK506 multicenter trial. Transplantation. 1995;60:1089-1095.
Krowka MJ, McGoon MD. Portopulmonary hypertension: the next step. Chest. 1997;112:869.
Kuo PC, Johnson LB, Plotkin JS, et al. Continuous infusion of epoprostenol for the treatment of portopulmonary hypertension. Transplantation. 1997;63:604.
Grossi P, Kusne S, Rinaldo C, et al. Guidance of ganciclovir therapy with pp65 antigenemia in cytomegalovirus-free recipients of livers from seropositive donors. Transplantation. 1996;61:1659.
1 Gordon RD, Starzl TE. Changing perspectives on liver transplantation in 1988. In: Terasaki PI, editor. Clinical Transplants. Los Angeles: UCLA Tissue Typing Laboratory, 1988.
2 National Institutes of Health Consensus Conference on Liver Transplantation. Bethesda, MD, 1983.
3 Starzl TE, Porter KA, Brettschneider L, et al. Clinical and pathologic observations after orthotopic transplantation of the human liver. Surg Gynecol Obstet. 1969;128:327.
4 Iwatsuki S, Starzl TE, Todo S, et al. Experience in 1000 liver transplants under cyclosporin-steroid therapy: A survival report. Transplant Proc. 1988;20(Suppl 1):498.
5 http://www.unos.org/SharedContentDocuments/Revised_MELDPELD_2003.pdf. Accessed 03/07/04
6 Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470.
7 Van Thiel DH, Gavaler JS. Recurrent disease in patients with liver transplantation: When does it occur and how can we be sure? Hepatology. 1987;7:181.
8 Polson RJ, Portmann B, Neuberger J, et al. Evidence for disease recurrence after liver transplantation for primary biliary cirrhosis: Clinical and histologic follow up studies. Gastroenterology. 1989;97:715.
9 United Network for Organ Sharing: A report of the Board of Directors Meeting. Baltimore, MD, November 12-13, 1997.
10 Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: Comparison of the proposed UCSF criteria with the Milan criteria and the Pittsburgh modified TNM criteria. Liver Transplant. 2002;8:765-774.
11 Starzl TE, Demetrius AJ, Van Thiel D. Liver transplantation: I and II. N Engl J Med. 1989;321:1014.
12 Koneru B, Cassavila A, Bowman J, et al. Liver transplantation for malignant tumors. Gastroenterol Clin North Am. 1988;17:177.
13 Starzl TE, Todo S, Tsakis A, et al. Abdominal organ cluster transplantation for the treatment of upper abdominal malignancies. Ann Surg. 1989;210:374.
14 Starzl TE, Towe M, Todo S, et al. Transplantation of multiple abdominal viscera. JAMA. 1989;261:1-449.
15 Gores GJ. Cholangiocarcinoma: Current concepts and insights. Hepatology. 2003;37:961-969.
16 Dindzans VJ, Schade RR, Van Thiel DH. Medical problems before and after transplantation. Gastroenterol Clin North Am. 1988;17:19.
17 Lok AS, McMahon BJ, Chronic hepatitis B. update 2009. Hepatology. 2009;50(3):661-662.
18 Todo S, Demetrius AJ, Van Thiel D, et al. Orthotopic liver transplantation for patients with hepatitis B virus (HBV)-related disease. Hepatology. 1991;13:619.
19 Lauchart W, Muller R, Pichlmayer R. Long-term immunoprophylaxis of hepatitis B virus reinfection in recipients of human liver allografts. Transplant Proc. 1987;19:4051.
20 Grellier L, Mutimer D, Ahmed M, et al. Lamivudine prophylaxis against reinfection in liver transplantation for hepatitis B cirrhosis. Lancet. 1996;348:1212.
21 Perrillo RP. Treatment of posttransplantation hepatitis B. Liver Transplant Surg. 1997;3(5 Suppl 1):S8.
22 Nevens F, Main J, Honkoop P. Lamivudine therapy for chronic Hepatitis B: A six-month randomized dose-ranging study. Gastroenterology. 1997;113:1258.
23 Lai CL, Ratziu V, Yuen MF, Poynard T. Viral hepatitis B. Lancet. 2003;362:2089-2094.
24 Terrult NA, Wright TL. Hepatitis B virus infection and liver transplantation. Gut. 1997;40:568.
25 Bizollon T, Ahmed SN, Radenne S, et al. Long-term histological improvement and clearance of intrahepatic hepatitis C virus RNA following sustained response to interferon-ribavirin combination therapy in liver transplanted patients with hepatitis C virus recurrence. Gut. 2003;52:283-287.
26 Teixeira R, Papatheodoridis GV, Burroughs AK. Management of recurrent hepatitis C after liver transplantation. J Viral Hepatitis. 2001;8:159-168.
27 Dummer SJ, Erb S, Breinig M, et al. Infection with human immunodeficiency virus in the Pittsburgh transplant population. Transplantation. 1990;47:134.
28 Ragni MV, Belle SH, Im K, et al. Survival of human immunodeficiency virus-infected liver transplant recipients. J Infect Dis. 2003;188:1412-1420.
29 Molla A, Japour A. HIV Protease inhibitors. Curr Opin Infect Dis. 1997;10:491.
30 Hughes CB, et al. HCV recurrence in HIV-infected patients after liver transplant. J Int Assoc Physicians AIDS Care. 2010;9(2):87-93.
31 Abu-Elmagd K, Reyes J, Bond G, et al. Clinical intestinal transplantation: A decade of experience at a single center. Ann Surg. 2001;234:404-416.
32 Trey C, Davidson CS. The management of fulminant hepatic failure. In: Popper H, Shaffner F, editors. Progress in Liver Disease. New York: Grune & Stratton, 1970.
33 Shakil O, Kramer DJ, Mazariegos G, et al. Acute liver failure: Clinical features, outcome analysis, and applicability of prognostic criteria. Liver Transpl Surg. 2000;6:163-169.
34 Bismuth H, Samuel D, Guenheim J, et al. Emergency liver transplantation for fulminant hepatitis. Ann Intern Med. 1987;107:337.
35 Kramer DJ, Aggarwald S, Martin M, et al. Fulminant hepatic failure: Management options. Transplant Proc. 1991;23:1895-1898.
36 Hoofnagle JH, Carithers RLJr, Shapiro C, Ascher N. Fulminant hepatic failure: Summary of a workshop. Hepatology. 1995;21:240-252.
37 O’Grady JG, Gimson AES, O’Brien CJ, et al. Controlled trials of charcoal hemoperfusion and prognostic factors in fulminant hepatic failure. Gastroenterology. 1988;94:1186.
38 O’Grady JG, Alexander GJM, Hayllar KM, et al. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989;97:439.
39 Potter D, Peachey T, Eason J, et al. Intracranial pressure monitoring during orthotopic liver transplantation for acute liver failure. Transplant Proc. 1989;21:3528.
40 Canalese J, Gimson AES, Davis C, et al. Controlled trial of dexamethasone and mannitol for the cerebral oedema of fulminant hepatic failure. Gut. 1982;23:625.
41 Bernal W, et al. Arterial ammonia and clinical risk factors for encephalopathy and intracranial hypertension in acute liver failure. Hepatology. 2007;46(6):1844-1852.
42 Larsen FS, Wendon J. Prevention and management of brain edema in patients with acute liver failure. Liver Transpl. 2008;14(Suppl 2):S90-S96.
43 Wendon J, Lee W. Encephalopathy and cerebral edema in the setting of acute liver failure: pathogenesis and management. Neurocrit Care. 2008;9(1):97-102.
44 Stravitz RT, Kramer DJ. Medscape, Management of acute liver failure. Nat Rev Gastroenterol Hepatol. 2009;6(9):542-553.
45 Steadman RH, Rensburg A, Kramer DJ. Transplantation for acute liver failure: perioperative management. Curr Opin Organ Transplant. 2010;15(3):368-373.
46 Forbes A, Alexander GJ, O’Grady JG, et al. Thiopental infusion in the treatment of intracranial hypertension complicating fulminant hepatic failure. Hepatology. 1989;10:306.
47 Abelmann WH, Kowalski HJ, McNeely WF. Cardiovascular studies during acute infectious hepatitis. Gastroenterology. 1954;27:61.
48 Weston MJ, Talbot IC, Howorth PJN, et al. Frequency of arrhythmias and other cardiac abnormalities in FHF. Br Heart J. 1976;38:1179.
49 Oellerich M, Raude E, Burdelski M, et al. Monoethylglycinexylidide formation kinetics: A novel approach to assessment of liver function. J Clin Chem Clin Biochem. 1987;25:845.
50 Burdelski M, Oellerich M, Lamesch P, et al. Evaluation of quantitative liver function tests in liver donors. Transplant Proc. 1987;19:3838.
51 Feng S, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6(4):783-790.
52 Machicao VI, Bonatti H, Krishna M, et al. Donor age affects fibrosis progression and graft survival after liver transplantation for hepatitis C. Transplantation. 2004;77:84-92.
53 Starzl TE, Hakala TR, Shaw B, et al. A flexible procedure for multiple cadaveric organ procurement. Surg Gynecol Obstet. 1984;158:223.
54 Todo S, Nery J, Yanaga K, et al. Extended preservation of human liver grafts with UW solution. JAMA. 1989;261:711.
55 Fung JJ, Eghtesad B, Patel-Tom K. Using livers from donation after cardiac death donors–a proposal to protect the true Achilles heel. Liver Transpl. 2007;13(12):1633-1636.
56 Maheshwari A, et al. Biliary complications and outcomes of liver transplantation from donors after cardiac death. Liver Transpl. 2007;13(12):1645-1653.
57 Grewal HP, et al. Liver transplantation using controlled donation after cardiac death donors: an analysis of a large single-center experience. Liver Transpl. 2009;15(9):1028-1035.
58 SRTR. Aggregate National Reports: Liver 01/01/2009 – 12/31/2009. Available from. http://www.ustransplant.org/csrcurrent/nationalviewer.aspx?o=LI, 2010.
59 Yeh H, Olthoff KM. Live donor adult liver transplantation. Curr Opin Organ Transplant. 2008;13(3):241-246.
60 Dewolf A. Does ventricular dysfunction occur during liver transplantation? Transplant Proc. 1991;23:1922.
61 Kang Y, Freeman J, Aggarwal S, et al. Hemodynamic instability during liver transplantation. Transplant Proc. 1989;21:3489.
62 Dewolf A, Gasior T, Kang Y. Pulmonary hypertension in a patient undergoing liver transplantation. Transplant Proc. 1991;23:2000.
63 Dewolf A, Begliomin B, Gasior T, et al. Right ventricular function during liver transplantation. Anesth Analg. 1993;76:562.
64 Aggarwal S, et al. Noninvasive monitoring of cerebral perfusion pressure in patients with acute liver failure using transcranial doppler ultrasonography. Liver Transpl. 2008;14(7):1048-1057.
65 Stoelting RK, Blitt CD, Cohen PJ, et al. Hepatic dysfunction after isoflurane anesthesia. Anesth Analg. 1987;66:147.
66 Kang YG, Martin DJ, Marquez J, et al. Intraoperative changes in blood coagulation and thromboelastographic monitoring in liver transplantation. Anesth Analg. 1985;64:888.
67 Marquez JM, Martin D. Anesthesia for liver transplantation. In: Winter PM, Kang YG, editors. Hepatic Transplantation: Anesthetic and Perioperative Management. New York: Praeger, 1986.
68 Rettke SR, Chantigian RC, Janossy TA, et al. Anesthesia approach to hepatic transplantation. Mayo Clin Proc. 1989;64:224.
69 Tzakis A, Todo S, Stieber A, et al. Venous jump grafts for liver transplantation in patients with portal vein thrombosis. Transplantation. 1989;48:530.
70 Figueras J, Sabate A, Fabregat J, et al. Hemodynamics during the anhepatic phase in orthotopic liver transplantation with vena cava presentation: A comparative study. Transplant Proc. 1983;25:2588.
71 Kang Y. Hemodynamic changes during intra-abdominal organ transplantation. Transplant Proc. 1993;25:2583.
72 Stieber AC, Marsh JWJr, Starzl TE. Preservation of the retrohepatic vena cava during recipient hepatectomy for orthotopic transplantation of the liver. Surg Gynecol Obstet. 1989;168:542.
73 Denmark W, Shaw BW, Starzl TE, et al. Veno-venous bypass without systemic anticoagulation in canine and human liver transplantation. Surg Forum. 1983;34:380.
74 Aggarwal S, Kang Y, Freeman J, et al. Post reperfusion syndrome: Cardiovascular collapse following hepatic reperfusion during liver transplantation. Transplant Proc. 1987;19:54.
75 Greig P, Woolf GM, Sinclair SB, et al. Treatment of primary liver graft nonfunction with prostaglandin E1. Transplantation. 1989;48:447.
76 Asonuma K, Takaya S, Selby R, et al. The clinical significance of the arterial ketone body ratio as an early indicator of graft viability in human liver transplantation. Transplantation. 1991;51:164.
77 Takaya S, Nonami T, Selby R, et al. The relationship of systemic hemodynamics and oxygen consumption to early allograft failure after liver transplantation. Transplant Int. 1993;6:73.
78 Doyle HR, Marino IR, Jabbour N, et al. Early death or retransplantation in adults following orthotopic liver transplantation: Can outcome be predicted? Transplantation. 1994;57:1028.
79 Doyle HR, Dvorchik I, Mitchell S, et al. Predicting outcome after liver transplantation: A connectionist approach. Ann Surg. 1994;219:408-415.
80 Angus DC, Colantonio A, Kramer DJ, et aland the Liver Transplant Intensive Care Group. Outcome Prediction with the APACHE II System in Liver Transplantation [abstract]. Crit Care Med. 1993;21:S176.
81 Angus DC, Clermont G, Kramer DJ, et al. Short and long-term outcome prediction with the APACHE II system after orthotopic liver transplantation. Crit Care Med. 2000;28:150-156.
82 Yanaga K, Lebeau G, Marsh JW, et al. Hepatic artery reconstruction for hepatic artery thrombosis after orthotopic liver transplantation. Arch Surg. 1990;125:628-631.
83 Yanaga K, Makowka L, Starzl TE. Is hepatic artery thrombosis after liver transplantation really a surgical complication? Transplant Proc. 1989;21:511.
84 Manez R, Kobayashi M, Takaya S, et al. Humoral rejection associated with antidonor lymphocytotoxic antibodies following liver transplantation. Transplant Proc. 1993;25:888-890.
85 Starzl TE, Demetris AJ. Liver Transplantation: A 31-Year Perspective. Chicago: Year Book Medical Publishers; 1990.
86 Rosenbloom AJ, Kramer DJ, Stern KL, et al. Immunosuppressive therapy of transplant patients. In Chernow B, editor: The Pharmacologic Approach to the Critically Ill Patient, 3rd ed, Baltimore: Williams & Wilkins, 1994.
87 European FK506 Multicentre Liver Study Group. Randomised trial comparing tacrolimus (FK506) and cyclosporin in prevention of liver allograft rejection. Lancet. 1994;344:423-428.
88 The U.S. Multicenter FK506 Liver Study Group. A comparison of tacrolimus (FK 506) and cyclosporine for immunosuppression in liver transplantation. N Engl J Med. 1994;331:1110-1115.
89 Lake JR, Gorman KJ, Esquivel CO, et al. The impact of immunosuppressive regimens on the cost of liver transplantation—results from the U.S. FK506 multicenter trial. Transplantation. 1995;60:1089-1095.
90 Mycophenolate mofetil—a new immunosuppressant for organ transplantation. Med Lett. 1995;37:84-86.
91 Jain A, Kashyap R, Kramer D, et al. Prospective randomized trial of tacrolimus and prednisone versus tacrolimus, prednisone, and mycophenolate mofetil: A complete report on 350 primary adult liver transplantation. Transplant Proc. 2001;33:1342-1344.
92 Jain A, Kashyap R, Dodson F, et al. A prospective randomized trial of tacrolimus, prednisone and mycophenolate mofetil in primary adult liver transplantations: A single-center report. Transplantation. 2001;72:1091-1097.
93 Starzl TE, Iwatsuki S, Shaw B, et al. Orthotopic liver transplantation in 1984. Transplant Proc. 1985;17:250.
94 Doyle HR, Marino IR, Morelli F, et al. Assessing risk in liver transplantation. Special reference to the significance of a positive cytotoxic cross-match. Ann Surg. 1966;224:168-177.
95 Takaya S, Iwaki Y, Starzl TE. Liver transplantation in positive cytotoxic crossmatch cases using FK506, high-dose steroids, and prostaglandin E1. Transplantation. 1992;54:927.
96 Demetrius AJ, Fung JJ, Todo S, et al. Conversion of liver allograft recipients from cyclosporin to FK 506 immunosuppressive therapy: A clinicopathologic study of 96 patients. Transplantation. 1992;53:1056.
97 McDiarmid SV, Klintmalm GB, Busuttil RW. FK506 conversion for intractable rejection of the liver allograft. Transplant Int. 1993;6:305.
98 Backman L, Nicar M, Levy M, et al. FK506 trough levels in whole blood and plasma in liver transplant recipients. Correlation with clinical events and side effect. Transplantation. 1994;57:519-525.
99 McCauley J, Van Thiel D, Starzl TE, et al. Acute and chronic renal failure after liver transplantation. Nephron. 1990;55:121.
100 Thompson CB, Sullivan KM, June CH, et al. Association between cyclosporin neurotoxicity and hypomagnesaemia. Lancet. 1984;2:1116.
101 Todo S, Fung JJ, Tzakis A, et al. One hundred ten consecutive primary orthotopic liver transplants under FK 506 in adults. Transplant Proc. 1991;23:1397.
102 European FK506 Multicentre Liver Study Group. Randomized trial of FK506 and cyclosporin in prevention of liver allograft rejection. Lancet. 1994;344:423.
103 Bayley TJ, Segel N, Bishop JM. The circulatory changes in patients with cirrhosis of the liver at rest and during exercise. Clin Sci. 1964;26:227.
104 Kowalski HJ, Abelmann WH. The cardiac output at rest in Laennec’s cirrhosis. J Clin Invest. 1953;32:1025.
105 Murray JF, Dawson AM, Sherlock S. Circulatory changes in chronic liver disease. Am J Med. 1958;24:358.
106 Claypool JG, Delp M, Lin TK. Hemodynamic studies in patients with Laennec’s cirrhosis. Am J Med Sci. 1957;234:48.
107 Martin D. Hemodynamic monitoring during liver transplantation. In: Winter PM, Kang YG, editors. Hepatic Transplantation: Anesthetic and Perioperative Management. New York: Praeger, 1986.
108 Parker MM, Shelhamer JH, Bacharach SL, et al. Profound but reversible myocardial depression in patients with septic shock. Ann Intern Med. 1984;100:483-490.
109 Curtis JJ, Luke RG, Jones P, et al. Hypertension in cyclosporin treated renal transplant recipients is sodium dependent. Am J Med. 1988;85:134.
110 Bennett WM, Porter GA. Cyclosporin-associated hypertension (editorial). Am J Med. 1988;85:131.
111 Fung J, Todo S, Abu-Elmagd K, et al. Randomized trial in primary liver transplantation under immunosuppression in FK 506 or cyclosporin. Transplant Proc. 1993;25:1130.
112 McCauley J, Fung JJ, Brown H, et al. Renal function after conversion from cyclosporin to FK 506 in liver transplant patients. Transplant Proc. 1991;23:3148.
113 Krowka MJ, Cortese DA. Pulmonary aspects of chronic liver disease and liver transplantation. Mayo Clin Proc. 1985;60:407-418.
114 Doyle HR, Marino IR, Moro A, et al. Adult respiratory distress syndrome secondary to end-stage liver disease—successful outcome following transplantation. Transplantation. 1993;55(2):292-296.
115 Fagon JY, Chastre J. Hospital acquired pneumonia. In: Pinsky MR, Dhamaut JF, editors. Pathophysiologic Foundations of Critical Care. Baltimore: Williams & Wilkins; 1993:545-570.
116 Griffin JJ, Meduri GU. New approaches in the diagnosis of nosocomial pneumonia. Med Clin North Am. 1994;78:1091-1122.
117 Chaparala R, Kramer DJ, Miro A, et al. Comparison of clinical score, bronchoalveolar lavage and protected brush specimen for the diagnosis of bacterial pneumonia in critically ill patients with liver disease. Am Rev Respir Dis. 1993;147:A38.
118 Matuschak GM, Rinaldo JE, Pinsky MR, et al. Effect of end-stage liver failure on the incidence and resolution of the adult respiratory distress syndrome. J Crit Care. 1987;2:162.
119 Matuschak GM. Lung-liver interactions in sepsis and multiple organ failure syndrome. Clin Chest Med. 1996;17:83-98.
120 Bihari DJ, Gimson AE, Williams R. Cardiovascular, pulmonary and renal complications of fulminant hepatic failure. Semin Liver Dis. 1986;6:119.
121 Doyle HR, Marino IR, Miro A, et al. Adult respiratory distress syndrome secondary to end stage liver disease: Successful outcome following liver transplantation. Transplantation. 1993;55:292-296.
122 Krowka MJ, Cortese DA. Pulmonary aspects of liver disease and liver transplantation. Clin Chest Med. 1987;10:593-616.
123 Hales MR. Multiple small arteriovenous fistulas of the lungs. Am J Pathol. 1956;32:927-943.
124 Robin ED, Laman D, Horn BR, et al. Platypnea related to orthodeoxia caused by true vascular lung shunts. N Engl J Med. 1976;294:941-943.
125 Calabresi P, Abelmann WH. Portocaval and portopulmonary anastomosis in Laennec’s cirrhosis in heart failure. J Clin Invest. 1957;36:1257-1265.
126 Caldwell PRB, Fritts HW, Courmans A. Oxyhemoglobin dissociation curve in liver disease. J Appl Physiol. 1965;20:316-320.
127 Davis HH, Schwartz DJ, Letrak SS, et al. Alveolar capillary oxygen disequilibrium in hepatic cirrhosis. Chest. 1978;73:507-511.
128 Rodriguez-Roisin R, Roca J, August AGN, et al. Gas exchange and pulmonary vascular reactivity in patients with liver cirrhosis. Am Rev Respir Dis. 1987;135:1085.
129 Dansky HM, Schwinger ME, Cohen MV. Using contrast enhanced echocardiography to identify abnormal pulmonary arteriovenous connections in patients with hypoxemia. Chest. 1992;102:1690.
130 Poterucha JJ, Krowka MJ, Dickson ER, et al. Failure of hepatopulmonary syndrome to resolve after liver transplantation and successful treatment with embolotherapy. Hepatology. 1995;21:96.
131 Krowka MJ, McGoon MD. Portopulmonary hypertension: The next step. Chest. 1997;112:869.
132 DeWolf A, Scott V, Bjerke R, et al. Hemodynamic effects of inhaled nitric oxide in four patients with severe liver disease and pulmonary hypertension. Liver Transpl Surg. 1997;3:594.
133 Mandell MS. Scenario number two: pulmonary hypertension. Liver Transpl Surg. 1996;2:320.
134 Mandell MS, Duke J. Nitric oxide reduces pulmonary hypertension during hepatic transplantation. Anesthesiology. 1994;81:1538.
135 Kuo PC, Johnson LB, Plotkin JS, et al. Continuous infusion of epoprostenol for the treatment of portopulmonary hypertension. Transplantation. 1997;63:604.
136 Scott V, DeWolf A, Kang Y, et al. Reversibility of pulmonary hypertension after liver transplantation: A case report. Transplant Proc. 1993;25:1789.
137 Ramsay MA, Simpson BR, Nguyen AT, et al. Severe pulmonary hypertension in liver transplant candidates. Liver Transpl Surg. 1997;3:494.
138 Mandell MS, Groves BM, Duke J. Progressive plexogenic pulmonary hypertension following liver transplantation. Transplantation. 1995;59:1488.
139 ARDS-Net, Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301-1308.
140 Mandell MS, Lockrem J, Kelley SD. Immediate tracheal extubation after liver transplantation: Experience of two transplant centers. Anesth Analg. 1997;84:249.
141 Staplefeldt W. “Fast tracking.” (Unpublished.)
142 Gonwa TA, et al. End-stage renal disease (ESRD) after orthotopic liver transplantation (OLTx) using calcineurin-based immunotherapy. Transplantation. 2001;72(12):1934-1939.
143 Ojo AO, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349(10):931-940.
144 Wadei HM, et al. Kidney allocation to liver transplant candidates with renal failure of undetermined etiology: role of percutaneous renal biopsy. Am J Transpl. 2008;8(12):2618-2626.
145 McCauley J, Van Thiel D, Starzl TE, et al. Acute and chronic renal failure after liver transplantation. Nephron. 1990;55:121.
146 Alexander JA, Demetrius AJ, Gavaler JS, et al. Pancreatitis following liver transplantation. Transplantation. 1988;45:1062.
147 Carmichael FJ, Crawford MW, Khayyam N, et al. Effect of propofol infusion on splanchnic hemodynamics and liver oxygen consumption in the rat: A dose response study. Anesthesiology. 1993;79:1051.
148 Bourne RS, Mills GH. Sleep disruption in critically ill patients–pharmacological considerations. Anaesthesia. 2004;59(4):374-384.
149 Nelson LE, et al. The alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology. 2003;98(2):428-436.
150 Pandharipande PP, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298(22):2644-2653.
151 de Groen PC, Aksamit AJ, Rakela J, et al. Central nervous system toxicity after liver transplantation. N Engl J Med. 1987;318:861.
152 Adams DH, Gunson B, Honigsberger L, et al. Neurologic complications following liver transplantation. Lancet. 1987;1:949.
153 Jones EA, Basile AS, Mullen KD, et al. Flumazenil: Potential implications for hepatic encephalopathy. Pharmacotherapy. 1990;45:331.
154 Ware AJ, D’Agostiono AN, Combes B. Cerebral edema: A major complication of massive hepatic necrosis. Gastroenterology. 1971;61:877.
155 Vaquero J, et al. Complications and use of intracranial pressure monitoring in patients with acute liver failure and severe encephalopathy. Liver Transpl. 2005;11(12):1581-1589.
156 Aggarwal S, Kramer D, Yonas H, et al. Cerebral hemodynamic and metabolic changes in fulminant hepatic failure. Hepatology. 1994;19:80-87.
157 Blei AT, Larsen FS. Pathophysiology of cerebral edema in fulminant hepatic failure. J Hepatol. 1999;31(4):771-776.
158 Jalan R, et al. Restoration of cerebral blood flow autoregulation and reactivity to carbon dioxide in acute liver failure by moderate hypothermia. Hepatology. 2001;34(1):50-54.
159 Vaquero J, Chung C, Blei AT. Cerebral blood flow in acute liver failure: a finding in search of a mechanism. Metab Brain Dis. 2004;19(3-4):177-194.
160 Jalan R, et al. Moderate hypothermia in patients with acute liver failure and uncontrolled intracranial hypertension.[see comment]. Gastroenterology. 2004;127(5):1338-1346.
161 Aggarwal S, Witt JP, Kang Y, et al. Transcranial Doppler waveform analysis: A new approach to predict ICP in patients with fulminant hepatic failure. Hepatology. 1993;18:735.
162 Campellone JV, Lacomis D, Kramer DJ, et al. Acute myopathy following liver transplantation. Neurology. 1998;50:46-53.
163 Linden P, Pasculle AW, Kusne S, et al. Therapy and clinical outcome of vancomycin-resistant Enterococcus faecium (VREF) in a liver transplant recipient population. Proceedings of the 32nd Interscience Conference on Antimicrobial Agents and Chemotherapy, Los Angeles October 1992.
164 Kusne S, Dummer JS, Singh N, et al. Infection after liver transplantation: An analysis of 101 consecutive cases. Medicine. 1988;67:132.
165 Kusne S, Dummer JS, Singh N, et al. Fungal infections in liver transplantation recipients. Transplantation. 1985;40:347.
166 Winston DJ, Pakrasi A, Busuttil RW. Prophylactic fluconazole in liver transplant recipients: A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1999;131:729-737.
167 Hellinger WC, et al. Risk stratification and targeted antifungal prophylaxis for prevention of aspergillosis and other invasive mold infections after liver transplantation. Liver Transpl. 2005;11(6):656-662.
168 Linden P, Kramer DJ, Mazariegos G, et al. Low-dose amphotericin B (LDA) for the prophylaxis of serious Candida infection in high risk liver recipients. 36th International Conference on Antimicrobial Agents and Chemotherapy (ICCAC). New Orleans: American Society for Microbiology; September 15-18, 1996. abstract #J47, p 227
169 Linden P, Kramer DJ, Pasculle W. The correlation of quantitative bronchoalveolar lavage fungal cultures with active or incipient invasive candidiasis. Am J Respir Crit Care Med. 1996;153:A411.
170 Torre-Cisneros J, Manez R, Kusne S, et al. The spectrum of aspergillosis in liver transplant patients: Comparison of FK 506 and cyclosporine immunosuppression. Transplant Proc. 1991;23:3040-3041.
171 Herbrecht R, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347(6):408-415.
172 Balfour HH, Chace BA, Stapleton JT, et al. A randomized, placebo-controlled trial of oral acyclovir for the prevention of cytomegalovirus disease in recipients of renal allografts. N Engl J Med. 1989;320:1381.
173 Pomeroy C, Englund JA. Cytomegalovirus: Epidemiology and infection control. Am J Infect Control. 1987;15:107.
174 Manez R, Breinig MK, Kusne S, et al. Anomalous pattern of IgG antibody response to primary cytomegalovirus infection after solid organ transplantation. Transplantation. 1995;59:1220-1223.
175 Martin M, Manez R, Linden P, et al. A prospective randomized trial comparing sequential ganciclovir-high dose acyclovir to high dose acyclovir for prevention of cytomegalovirus disease in adult liver transplant recipients. Transplantation. 1994;58:779-785.
176 Syndman DR, Werner BG, Heinze-Lacey B, et al. Use of cytomegalovirus immunoglobulin to prevent cytomegalovirus disease in renal transplant recipients. N Engl J Med. 1987;317:1049.
177 Martin M. Prophylactic cytomegalovirus management strategies. Transplant Proc. 1995;27:23-27.
178 Grossi P, Kusne S, Rinaldo C, et al. Guidance of ganciclovir therapy with pp65 antigenemia in cytomegalovirus-free recipients of livers from seropositive donors. Transplantation. 1996;61:1659.
179 van den Berghe G, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345(19):1359-1367.
180 Van den Berghe G, et al. Intensive insulin therapy in the medical ICU.[see comment]. N Engl J Med. 2006;354(5):449-461.
181 Investigators N-SS, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283-1297.
182 Jeschke MG, et al. Insulin prevents liver damage and preserves liver function in lipopolysaccharide-induced endotoxemic rats. J Hepatol. 2005;42(6):870-879.
183 Jeschke MG, et al. Intensive insulin therapy in severely burned pediatric patients: A prospective randomized trial. Am J Respir Crit Care Med. 2010;182(3):351-359.
184 Gauglitz GG, et al. Insulin increases resistance to burn wound infection-associated sepsis. Crit Care Med. 2010;38(1):202-208.
185 Hemmila MR, et al. Intensive insulin therapy is associated with reduced infectious complications in burn patients. Surgery. 2008;144(4):629-635. discussion 635-7
186 Davidson PC, Steed RD, Bode BW. Glucommander: a computer-directed intravenous insulin system shown to be safe, simple, and effective in 120,618 h of operation. Diabetes Care. 2005;28(10):2418-2423.
187 Tsai MH, et al. Adrenal insufficiency in patients with cirrhosis, severe sepsis and septic shock. Hepatology. 2006;43(4):673-681.