Liver Tissue Processing and Normal Histology
Arief A. Suriawinata
Swan N. Thung
Liver Biopsy Specimens
Histopathologic examination of liver tissue by needle biopsy remains an integral part of the management of liver diseases despite numerous developments in diagnosis and management. Liver biopsies were documented in the late 19th century, when Ehrlich and then Lucatello performed liver puncture through a laparoscope, primarily for chemical studies.1 Schupfer in 1907 is credited with the first application of liver biopsy for the diagnosis of cirrhotic liver disease in humans.
In 1938, the Vim-Silverman needle was introduced, followed by the Menghini needle in 1958. The latter dramatically expanded the use of liver biopsy, because it was safer and easier to use and provided adequate tissue for histopathology and other studies. During the following decades, liver biopsy techniques have evolved to include percutaneous, transjugular, and open and laparoscopic biopsies (Table 43.1). Lately, ultrasound-guided endoscopic liver biopsy has been carried out experimentally. Newer core needle biopsy devices, such as the Tru-Cut needle and various types of biopsy guns, further refined the technique.
Table 43.1
Liver Biopsy Methods
| Methods | Technique | Considerations and Risk |
| Percutaneous (blind) | Suction needle (Menghini, Klatskin, Jamshidi) or cutting needle (Vim-Silverman, Tru-cut) | Most common, least costly, and least invasive liver biopsy. Provides an adequate specimen for histologic evaluation and for other ancillary studies. Complications and specimen outcome related to the operator’s experience. May result in an inadvertent biopsy of other organs, including kidney, pancreas, and intestine. |
| Transjugular (transvenous) | Catheter through the internal jugular vein, right atrium, and inferior vena cava | Second-line procedure in patients with coagulation disorders, fulminant hepatic failure, gross ascites, or severe obesity. Considerable cost, effort, and time incurred compared with percutaneous biopsy. Smaller and often fragmented specimens, but better needles and more experience have improved quality of specimens. Multiple specimens can be easily obtained. Ability to measure hemodynamics if combined with wedged hepatic pressure and venography. Complications include arrhythmia, contrast-related reaction, and inadvertent biopsy of kidney. |
| Laparoscopic or open | Providing direct visualization of the liver and peritoneal cavity; needle or wedge biopsy | Provides largest specimen. More sensitive in diagnosis of cirrhosis and chronic liver diseases, such as primary sclerosing cholangitis, viral hepatitis, and nodular regenerative hyperplasia. Useful for initial diagnosis or staging of a malignant neoplasm. Most invasive and expensive method. Risk of anesthesia and hemorrhage. Popularity of laparoscopic bariatric surgery for obesity has increased the number of intraoperative biopsies for steatohepatitis and fibrosis evaluation. |
| Computed tomography (CT) or ultrasound guided | <1-mm needle typically used (fine-needle aspiration); ultrasound or CT used for visualization of hepatic lesion and to avoid intersecting vessels | For histologic or cytologic diagnosis of space-occupying lesion or finding landmarks in cases of difficult anatomy. Multiple aspirations can be safely performed to ensure an adequate specimen. Immediate interpretation is possible. Cell block may increase sensitivity and allow additional staining. Controversial issue regarding dissemination of malignant cells persists, although less common than for thick-needle biopsies. Reduced risk of hemorrhage of cirrhotic patient compared with transcutaneous biopsy. |
The usefulness of liver biopsy ranges from evaluation of patients with abnormal liver function test results to those with a space-occupying lesion (Box 43.1). Many liver biopsies are performed for chronic viral hepatitis, steatohepatitis, and allograft dysfunction to assess the degree of liver damage or the response to therapy.2 Acute hepatitis is usually not an indication for liver biopsy, but if there is doubt about the clinical diagnosis, multifactorial causes of elevated liver enzyme values, or even a mistaken working diagnosis, a biopsy may be indicated.
Although relatively safe, liver biopsy is an invasive procedure, and the indications, goals, and techniques and their limitations should be carefully considered to avoid performing unnecessary procedures or preparation of a sample that cannot provide the necessary answer. Major complications of liver biopsy include bleeding and bile leak. As many as one third of patients experience right upper quadrant pain or shoulder pain, which may be severe in 1% to 3%.3 The mortality rate for different techniques is approximately 0.01%.4 Rare complications of liver biopsy include hemobilia, pneumoperitoneum, pneumoscrotum, pneumothorax, septic shock, subphrenic abscess, and intrahepatic arteriovenous fistula. There is a minimal risk of hematogenous dissemination of malignant cells after liver biopsy. Although rare, seeding in the needle track has been reported in cases of hepatocellular carcinoma and metastatic colorectal carcinoma.5,6 The major contraindication to percutaneous liver biopsy is significant coagulopathy. Relative contraindications to percutaneous liver biopsy are morbid obesity and severe ascites. In these conditions, transjugular biopsy is a good alternative.
Fine-needle aspiration (FNA) biopsy guided by ultrasound or computed tomography (CT) has become the preferred method for diagnosis of a space-occupying lesion and confirmation of a suspected malignancy. It provides immediate interpretation and assessment of the adequacy of a liver biopsy by using smears or touch preparations. FNA biopsy can also be used to drain a cyst or abscess for culture and fluid analysis, or it can be followed by therapeutic ablation of malignant tumors. Routine supplementation with a cell block of tissue fragments increases the diagnostic accuracy of FNA, particularly in benign conditions or benign hepatocellular tumors, and it provides material for immunohistochemical stains and ancillary studies for primary or metastatic malignant tumors.
Specimen Handling
At the time of the biopsy procedure, a needle liver biopsy specimen should be examined immediately for adequacy. It should be at least 1.5 cm long, and if it is not, another pass is recommended because adequate specimen size minimizes sampling error.7,8 If a tumor is suspected, a touch preparation from the tissue can immediately determine specimen adequacy or a diagnosis. The specimen is then discharged into a Petri dish lined with lens paper, which has ideally been soaked in normal saline solution to prevent fresh tissue from adhering to it. Artifacts from squeezing or drying of the specimen should be avoided. Biopsy specimens should not be placed on dry gauze, which tends to dehydrate cells, resulting in a prominent nuclear artifact. Tissue squeezing distorts cells and elongates nuclei, which makes cytologic evaluation of the specimen difficult.
Gross characteristics of the tissue, such as color, consistency, and tendency to fragment or float in the fixative solution, are documented by the physician. Tumors or granulomas, for example, can be recognized as white areas in an otherwise reddish brown tissue. Gray-black discoloration is seen in Dubin-Johnson syndrome, rusty brown in hemochromatosis, green in cholestasis, yellow in fatty liver, dark red in congested liver, and variegated or dark brown in metastatic melanoma. Fragmentation of specimens, especially in a transjugular biopsy specimen or when a Menghini (suction) needle is used, often indicates advanced fibrosis or cirrhosis.
The routine fixative for liver biopsies is 10% neutral buffered formalin. Immersion of biopsies in saline causes discohesion of cells and distortion of the hepatocyte cords. The advantages of routine formalin fixation is that the formalin solution is stable, penetrates and fixes tissues well, is inexpensive, and allows subsequent application of most histochemical, immunohistochemical, and molecular pathology procedures. The characteristics of tissues fixed in formalin are well known. The disadvantage of formalin is the relative lack of cytologic detail compared with some other types of fixatives.
On the basis of the clinical diagnosis or possible differential diagnoses, the clinician determines which additional procedures may be required (Table 43.2): fixation in 3% buffered glutaraldehyde for electron microscopy; fresh, unfixed tissue for viral and mycobacterial cultures; rapid freezing in liquid nitrogen or a mixture of dry ice and isopentane for fat stains, certain immunohistochemical and enzyme activity studies,9 quantitative studies of hormone receptors, and isolation of genomic and viral DNA and RNA for molecular analyses10; and fixation in 1% periodic acid in 10% neutral buffered formalin at 4° C for 48 hours for evaluation of glycogen storage diseases.11 When RNA recovery is needed, an alcohol-based fixative is better than a formalin fixative. When the patient is a child or a young adult, it may be beneficial to fix tissue in anticipation of possible ultrastructural studies.
Table 43.2
Ancillary Studies and Fixatives
| Ancillary Studies | Fixative or Procedure |
| Transmission electron microscopy | 3% buffered glutaraldehyde |
| Scanning electron microscopy | Perfusion fixation; gold or platinum coating |
| Viral, bacterial, fungal cultures | Fresh, unfixed tissue |
| Fat stains, enzyme activity, protein analyses, viral DNA and RNA, in situ hybridization | Rapid freezing in liquid nitrogen or mixture of dry ice and isopentane |
| Glycogen storage diseases | 1% periodic acid in 10% neutral buffered formalin at 4° C for 48 hours |
| Flow cytometry of lymphocytes | Fresh, unfixed tissue preferred |
| DNA analysis | 10% neutral buffered formalin |
| RNA analysis | Fresh or alcohol-based fixative (80% ethanol) |
| mRNA and miRNA analysis | Fresh or 10% neutral buffered formalin |
| Protein analysis | Fresh or fresh-frozen, unfixed tissue |
| Laser capture microdissection | Conventional tissue section from paraffin-embedded tissue block |
miRNA, MicroRNA; mRNA, messenger RNA.
Needle liver biopsy specimens should be placed immediately in the desired fixative, because the foundation of a good histologic preparation is rapid and complete fixation. Good preservation of tissue can be achieved by following standard guidelines. First, the volume of the fixative should be at least 15 to 20 times the volume of the tissue.12 When transit to the laboratory is likely to involve much movement, the container should be filled to the brim with fixative. Second, sufficient time must be allowed for fixation to occur before processing is started. Because formalin penetrates most tissues at approximately 0.5 mm per hour at room temperature, approximately 4 hours are needed to penetrate a 2-mm-thick piece of tissue core. Fixation time may be shortened by application of heat, pressure, vacuum, agitation, or microwave techniques. Fragmentation of the central portion of the liver biopsy along the long axis is a sign of insufficient fixation time. Although prolonged formalin exposure (>24 hours) does not result in overfixation (i.e., hardening of tissue), it may reduce the availability of antigen sites for immunohistochemical studies. Tissues may afterward be stored in 10% (or lower) neutral buffered formalin or in 70% alcohol.13
Rush liver biopsy specimens should be manually processed to shorten the delay and meet the needs of critically ill or transplant patients; regular specimens are processed in an automated tissue processor. Table 43.3 compares the times for the manual and automatic methods. The application of microwave processing has significantly reduced processing time to as little as 15 minutes for a tiny biopsy specimen or to a 60- to 90-minute cycle for a larger biopsy specimen.14
Table 43.3
Schedule for Liver Needle Core Biopsy Processing
| Solution | Manual Method | Automatic Method |
| 10% NBF | — | 30 min |
| 10% NBF (37° C) | 30-60 min | 30 min (P/V) |
| 70% alcohol | 10 min | 10 min |
| 80% alcohol | 10 min | 10 min |
| 95% alcohol | 10 min | — |
| 95% alcohol | — | 10 min |
| 100% alcohol × 2 | 10 min (one change) | 10 min |
| Xylene × 3 | 5 min (two changes)* | 10 min (P/V) |
| Paraffin (58° C) × 2 | 15 min | 10 min (P/V) |
| Paraffin (58° C) × 2 | — | 10 min (P/V) |
* Check for tissue translucency before transferring to paraffin.
NBF, Neutral buffered formalin; P/V, pressure/vacuum.
Normal Microanatomy of the Liver
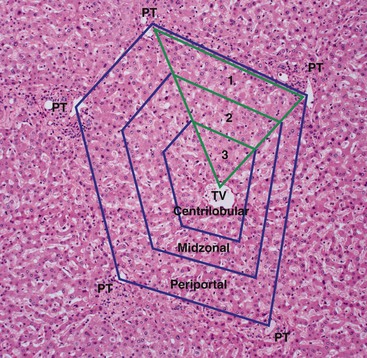
The functional unit of the liver is represented by the hepatic lobule of Kiernan or the hepatic acinus of Rappaport.15 A hepatic lobule consists of an efferent central vein with cords of hepatocytes radiating out toward multiple peripheral portal tracts. The changes in the lobule are classically described as being centrilobular, midzonal, or periportal.
The Rappaport acinus is a regular, three-dimensional structure in which blood flows from a central axis, formed by the terminal afferent portal venule and terminal hepatic arteriole in the portal tract, into the acinar sinusoids and then empties into several terminal hepatic venules at the periphery of the acinus. The acinus is subdivided into zones 1, 2, and 3, indicative of progressively decreasing tissue oxygenation (Fig. 43.1). The distance between two terminal hepatic venules represents the size of the acinus. The oxygen gradient, metabolic heterogeneity, and differential distribution of enzymes across the three zones of the acini help explain the zonal distribution of liver damage caused by hypoperfusion or ischemia and by certain toxic substances.
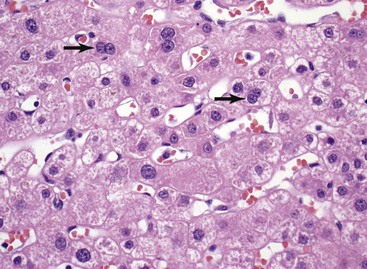
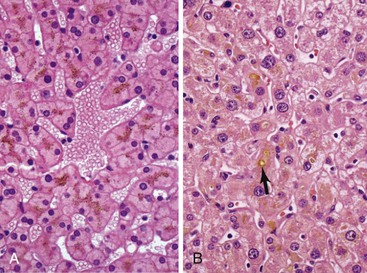
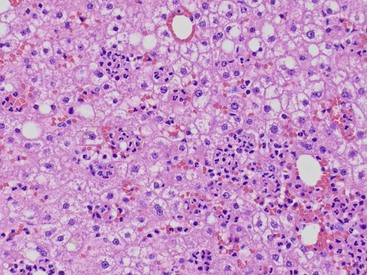
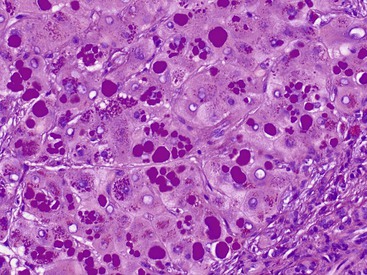
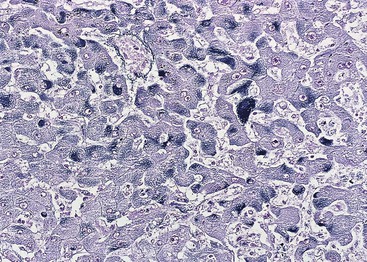
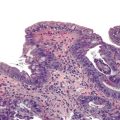
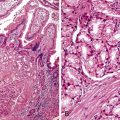
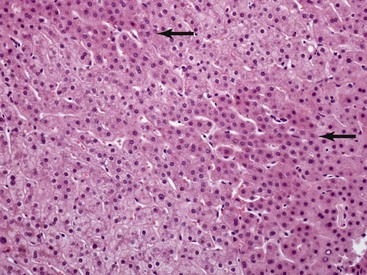
The hepatocyte is a polygonal epithelial cell with one or more centrally located, round nucleoli. The number of binucleate forms increases with age (Fig. 43.2). Some nuclei are larger than others, particularly in persons older than 60 years, indicating polyploidy.16 The significance of polyploidy is unknown. It is usually more prominent in the midzonal areas. Hepatocytes are arranged in one-cell-thick cords in adults, and they are separated by sinusoids, in which blood flows from the portal tracts to the terminal hepatic venules. In children as old as 5 or 6 years, the liver cells are arranged in two-cell-thick plates (Fig. 43.3). The presence of two-cell-thick plates and rosette formation in adults indicates hepatocyte regeneration (Fig. 43.4). Rare eosinophilic bodies or apoptotic bodies may indicate normal turnover of hepatocytes in otherwise apparently normal livers.
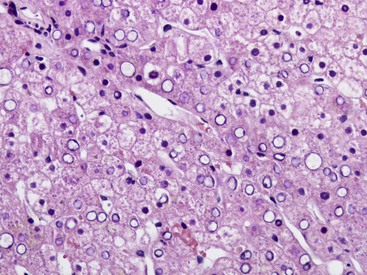
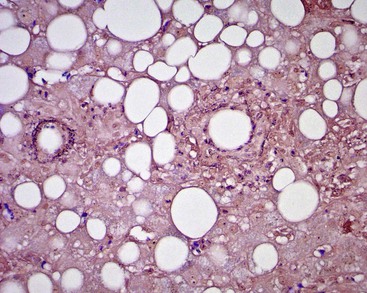
Glycogen accumulation in hepatocyte nuclei surrounding portal tracts produces a vacuolated appearance and is common in adolescents (Fig. 43.5). In adults, this appearance may be conspicuous in conditions such as glucose intolerance, Wilson disease, or diabetes mellitus. Cytoplasmic glycogen imparts a fine, reticulated, foamy appearance to the cytoplasm. The distribution of glycogen also shows diurnal and diet-related variations. An irregular distribution pattern may be found normally in biopsies and is not of diagnostic significance.
Lipofuscin pigment is normally seen in variable quantities in the centrilobular areas as periodic acid–Schiff (PAS)–positive, diastase-resistant, fine, light brown granules. There is a progressive increase of lipofuscin in individual hepatocytes with age (Fig. 43.6). The granules represent lysosomes that contain materials that cannot be further degraded; they are not present in recently regenerated hepatocytes. Bile is invisible under normal circumstances. Intracellular bile in cholestasis can be distinguished from lipofuscin by its greenish hue, lack of a granular appearance, and its frequent formation of bile thrombi in bile canaliculi in zone 3 hepatocytes (see Fig. 43.6). Large amounts of lipofuscin are difficult to distinguish from Dubin-Johnson pigment microscopically. Compared with lipofuscin, iron and copper are coarser, birefringent, and usually deposited in periportal hepatocytes. Hemosiderin and copper are abundant in the cytoplasm of hepatocytes during the first week of life, then gradually disappear, and should be absent before the age of 9 months. Small quantities of stainable iron are common in normal hepatocytes, particularly in older individuals.
Sinusoidal lining cells consist of specialized fenestrated endothelial cells and specialized macrophages or Kupffer cells, which are usually inconspicuous in normal biopsy specimens. Occasional lymphocytes or neutrophils may be present in the sinusoids. Between the sinusoidal lining cells and the hepatocytes lies the space of Disse, which contains plasma, scanty connective tissue, and perisinusoidal cells such as hepatic stellate cells (i.e., Ito cells or interstitial fat-storing cells) and pit cells (i.e., natural killer lymphocytes). Hepatic stellate cells are modified resting fibroblasts that can store fat and vitamin A and produce hepatocyte growth factor and collagen.17,18 They play a significant role in hepatic fibrogenesis. When activated, hepatic stellate cells contain stainable desmin and actin in their cytoplasm that can be highlighted with an immunohistochemical stain for smooth muscle actin.19 Elastic fibers and basement membrane material are absent from normal sinusoids.20,21
Smaller branches of the hepatic veins and the smallest efferent veins (or terminal hepatic venules) are in direct contact with the hepatocyte parenchyma. The terminal hepatic venules have very thin walls lined by endothelial cells. Thickening of the wall of terminal hepatic venules is often part of a pericellular fibrosis reaction and of central hyalin sclerosis in alcoholic liver disease.22,23 It also may be seen focally in apparently normal individuals.
Most portal tracts contain a bile duct, several bile ductules, a hepatic artery branch, a portal vein branch, and lymphatic channels embedded in connective tissue. The size of the portal tracts is approximately three to four times the diameter of the hepatic artery branch. The amount of connective tissue and the size of intraportal structures depend on the size of the portal tracts. Portal tracts of different sizes may be seen in biopsy specimens. Pathologic processes do not necessarily affect large and small portal tracts to the same extent. Portal tracts normally contain a few lymphocytes, macrophages, and mast cells but do not contain neutrophils or plasma cells. The amount of inflammatory cells increases with age and typically varies from one portal tract to another.
The larger intrahepatic or septal bile ducts are lined by tall columnar epithelial cells, and the smaller or interlobular bile ducts are lined by cuboidal or low columnar epithelium. One or more interlobular bile ducts may be present in any particular portal tract. Bile ducts are always accompanied by a hepatic artery, which has approximately the same diameter as the bile duct. Larger bile ducts have more periductal fibrous tissue than smaller ones. Sizeable bile ducts are often seen in subcapsular liver parenchyma in liver biopsies. Bile ductules are located at the peripheral zone of the portal tracts, and they are smaller than interlobular bile ducts. They have a basement membrane and are lined by cuboidal cholangiocytes. Bile canaliculi are not readily recognized microscopically unless distended, as in parenchymal cholestasis with hepatocyte rosette formation. Bile canaliculi are connected to bile ductules through the canals of Hering. Bile ductules and canals of Hering have received more attention recently because they may represent the site of progenitor cells.24
There are several changes in the liver related to aging, particularly in individuals older than 60 years (Fig. 43.7). There is more variation in the size of hepatocytes and the number of their nuclei (i.e., polyploidy) and an increase in lipofuscin pigment deposition. There may be apparent dilation of sinusoids because of hepatocyte cord atrophy. The portal tracts may contain denser collagen and may contain an increased quantity of mononuclear inflammatory cells. The hepatic arteries may have thickened walls, even in normotensive individuals. These changes are accompanied by alterations in the metabolic function of the liver, including the metabolism of various toxins and drugs.25
Interpretation of Liver Biopsies
Histologic examination of liver biopsies should conform to a specific routine and include all tissue fragments and structures of the liver (i.e., architecture, portal triads, limiting plate, hepatocytes, sinusoidal cells, and terminal hepatic venules). A systematic approach ensures that important diagnostic findings are not overlooked. A sensible approach is to begin the examination with a low scan magnification to appreciate the lobular architecture, the presence and quantity of the various anatomic structures of the liver, and the presence or absence of normal structures or focal changes. At low magnification, the type and location of inflammation and steatosis can be well appreciated. This should be followed by a careful examination of the zone 3 acinus, where many changes, such as congestion, steatosis, necrosis, cholestasis, pigments, and endophlebitis, are often found. The remainder of the parenchyma and portal tracts may be examined individually. Specific histopathologic changes may be easily recognizable, but it is often the topographic and functional relationships of the structures of the liver that contribute to a clinically meaningful diagnosis.
The initial histologic examination of a liver biopsy specimen is often better conducted without knowledge of the clinical and laboratory information. After the morphologic changes are appreciated and a generalized pattern of injury has been ascertained, a differential diagnosis can be rendered in combination with the clinical and laboratory information. Clinical information and laboratory data should always be reviewed before submitting a final diagnosis, because more often than not, this information is essential in narrowing the differential diagnosis to a specific cause.
The liver tends to react similarly to a broad range of injuries, and in most instances, the needle biopsy is fairly representative. However, a sampling error, particularly in focal or irregularly distributed disease processes, must always be taken into consideration. For example, in primary sclerosing cholangitis, the characteristic bile duct injury and periductal concentric fibrosis may not be uniformly seen throughout the liver or may not be present at all. Cytomegalovirus inclusions may not be seen in every section of a liver biopsy, and it may be necessary to cut several serial sections before the typical microabscesses are identified. Biopsy of space-occupying lesions may not show neoplastic cells in every section. In cirrhosis, a small-caliber needle biopsy may not obtain septal fibrosis and may yield only fragments of parenchyma without fibrosis.
A study of needle biopsy specimens by Crawford and colleagues26 showed that the number of portal tracts in a biopsy specimen was proportional to the total length of the specimen obtained and that not all of the portal tracts contained all portal triad structures. Portal triads may not contain at least one of the three key structures (usually the portal vein); 38% of portal tracts do not contain a portal vein, 7% do not have a bile duct, and 9% do not contain a hepatic artery. The most important finding of this study was that portal tracts almost always contained a paired bile duct and hepatic artery of approximately equal diameters. Fragmentation of the tissue into several smaller fragments and partial tears in the region of the terminal hepatic veins at the edges of the specimen are not uncommon by the time histologic sections are placed on a glass slide, even when the original piece of liver tissue obtained from the biopsy needle was not fragmented.
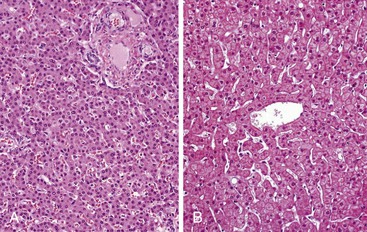
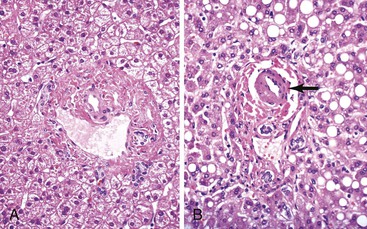
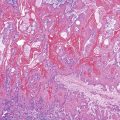
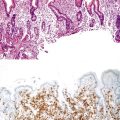
Innocent variations that should be considered to avoid erroneous interpretation are subcapsular liver parenchyma and surgery-associated changes. Needle biopsy of the immediate 2-mm subcapsular space often shows parenchyma that mimics cirrhosis (Fig. 43.8) and that may give a false impression of the status of the liver as a whole.27 The liver capsule may be present in percutaneous liver biopsies at one end of the specimen or in the form of separate pieces of connective tissue. The capsule can be distinguished from most pathologic fibrous tissue by its density and maturity, and it often contains blood vessels and bile ducts. Similar artifacts can be found in biopsy samples when the needle enters the liver at an angle close to the capsule or in a wedge biopsy that contains capsular fibrosis tissue on two surfaces.
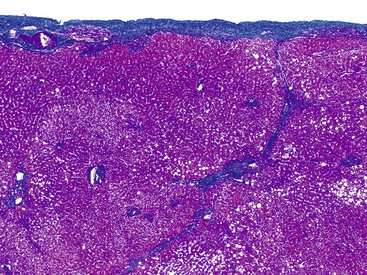
In liver biopsy specimens obtained at the end of a long surgical procedure, surgery-associated changes are usually seen as small, tight clusters of neutrophils within or under the hepatic capsule, sinusoids around central venules, portal tracts, and hepatic plates, and they resemble microabscesses (Fig. 43.9).28
Other normal variations or innocent hepatic lesions include focal steatosis involving small groups of hepatocytes, fat granulomas from mineral oil deposition in perivenular areas and portal tracts, rare acidophilic bodies in an otherwise normal liver, and unexplained mitoses of hepatocytes that normally have a life span of many years. In biopsies of space-occupying lesions, nonspecific reactive changes also may be seen. These changes are important to observe in biopsies that do not include neoplastic tissue, a cyst, or an abscess. The histologic changes, described by Gerber and coworkers as a histologic triad, consist of a ductular reaction, portal tract edema, and sinusoidal dilation.29 They may be subtle, are usually focal, and typically involve small portal tracts. These changes most likely occur as a result of local obstruction of blood and bile flow in the setting of an expanding lesion.
Liver Resection Specimens
Partial Hepatectomies
Partial liver resections are usually performed to remove focal lesions. The extent of resection varies from removal of small wedges of tissue to the removal of the entire lobe. In resection specimens, several surfaces may be covered by the hepatic capsule. The exposed surface is the surgical margin and may be designated by the surgeon with different colors of ink or stitches, especially when the lesion is close to a particular margin of concern. After the margin is identified and the specimen is oriented, the specimen should be weighed and measured in each dimension. Often, a bulge in the surface of the liver or retraction of the capsule can help to localize an intraparenchymal mass.
The characteristics of the lesions and of the surrounding liver parenchyma should be described. The presence or absence of involved resection margins, significant fibrosis of the liver parenchyma, or cirrhosis is observed. The specimen should be serially sectioned at 0.5- to 1-cm intervals, with the initial section passing through the center of the tumor to demonstrate the closest approach of the mass to the resection margin. Specimen photographs and representative sections may be taken. Fresh tissue or tissue in fixatives other than formalin should be submitted for additional tests when applicable.
Liver Explants
Explanted livers should be examined carefully during liver transplantation or retransplantation. The liver parenchyma should be sectioned only after the hilar structures have been located and examined. This approach entails a thorough examination of the hilar region for patency of the hepatic artery, portal vein, and bile duct. After the gross examination, several sections from the hilum should be obtained in a plane perpendicular to the long axis of the major hilar structures at 4- to 6-mm intervals, including sections near the margin and the point where these structures branch into the right and left lobes of the liver. Lymph nodes in the hilar soft tissue also should be sampled for histologic evaluation.
After the porta hepatis has been carefully examined and sampled, the entire liver parenchyma should be sectioned by using a long and sharp knife in a parallel plane at 0.5- to 1-cm intervals. Because formalin penetrates only superficially into the liver tissue, dissection and serial sectioning of the organ should be performed while the organ is fresh, preferably soon after receipt of the specimen in the dissection suite. Horizontal cross sections of the liver across each of the hepatic lobes reveal the openings of the hepatic veins, which should be carefully examined for patency. Thin sectioning is necessary to avoid missing small hepatocellular carcinomas or dysplastic nodules. All distinct nodules and lesions should be sampled. Routine sectioning consists of three to five sections each from the right and the left lobes and one section from the caudate lobe. Key samples necessary for histologic and special studies should be obtained immediately. The tissue slabs can then be fixed overnight in formalin before obtaining the more routine sections.
Photographing tissue slabs individually or serially, along with nodules or lesions, is recommended for all specimens. Proper documentation of the gross specimens is valuable for histologic reconstruction and review during the pathology sign-out session. Gross characteristics of the nodules or lesions often correlate well with their key histologic features.
Routine and Special Stains
Routine use of hematoxylin and eosin (H&E) stain enables accurate histologic diagnosis in almost all liver biopsy specimens. Other special histochemical stains are frequently requested to confirm structures or findings suspected on H&E-stained slides.
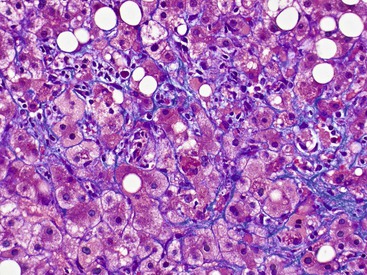
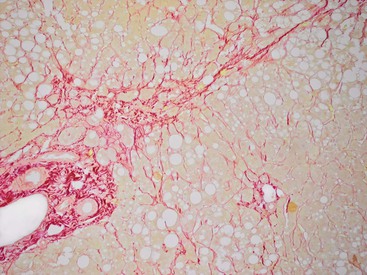
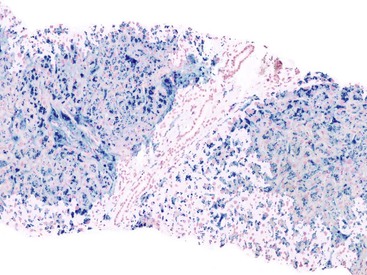
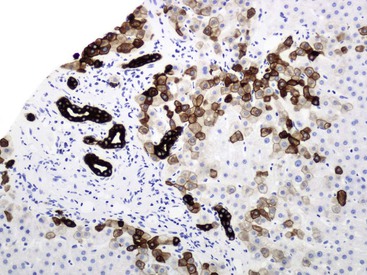
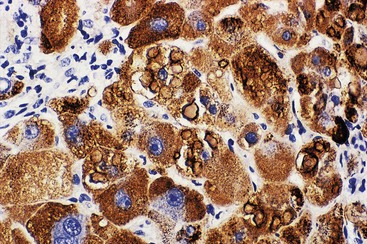
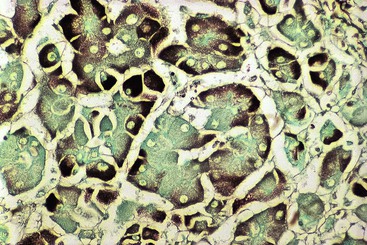
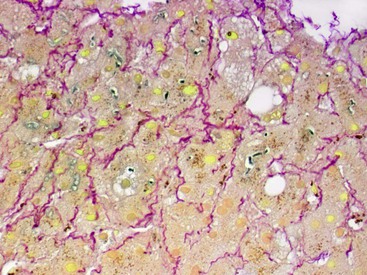
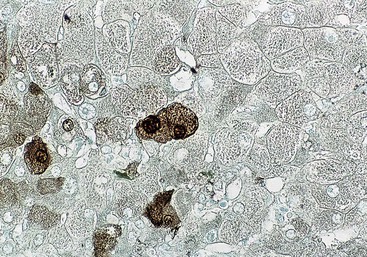
At a minimum, a special stain for connective tissue, such as Masson trichrome or a reticulin stain, should be obtained to assess lobular architecture and facilitate evaluation of fibrosis or cirrhosis. Table 43.4 lists frequently performed special stains that are helpful in the diagnosis of liver pathology. Examples of liver tissue stains are shown in the following figures: Masson trichrome stain30–32 (Fig. 43.10), sirius red stain33 (Fig. 43.11), Perls iron stain34,35 (Fig. 43.12), PAS stain for glycogen36 (Fig. 43.13), diastase–periodic acid–Schiff (d-PAS) stain for α1-antitrypsin37 (Fig. 43.14), Victoria blue stain for hepatitis B surface antigen38 (Fig. 43.15), Shikata orcein stain39 (Fig. 43.16), rhodanine stain40 (Fig. 43.17), bile stain (Fig. 43.18), and phosphotungstic acid–hematoxylin (PTAH) stain (Fig. 43.19).
Table 43.4
Frequently Performed Special Stains in Liver Pathology
| Special Stains | Fixatives | Interpretation |
| Connective and Muscle Tissues | ||
| Masson trichrome | Bouin fixative is preferred, 10% NBF | Identification of type I collagen. Muscle, keratin, cytoplasm, megamitochondria = red; collagen = blue; Mallory-Denk hyalin = red |
| Sirius red | 10% NBF | Identification of type I collagen. Collagen = red; Mallory-Denk hyalin = greenish orange |
| Gordon and Sweet reticulin | 10% NBF | Identification of type III collagen. Reticulin = black; collagen = pink |
| PTAH | 10% NBF | Fibrin, nuclei, cytoplasm, mitotic figures, mitochondria = blue; collagen = red |
| Microorganisms | ||
| Ziehl-Neelsen | 10% NBF or Helly fluid | Mycobacterium, lipofuscin, ceroid = red; background = light blue |
| Shikata orcein | 10% NBF | Elastic fibers, hepatitis B surface antigen, copper-binding protein = dark brown |
| Victoria blue | 10% NBF | Elastic fibers, hepatitis B surface antigen, copper-binding protein, lipofuscin, mast cell = blue; cytoplasm, nuclei = red |
| Ammoniacal silver | 10% NBF | Fungi, bacteria, mucin, glycogen, melanin = black; background = green |
| Pigments and Minerals | ||
| Perls iron | 10% NBF | Iron (ferric state) = blue |
| Hall bile | 10% NBF, Bouin, or Carnoy; Helly and Zenker are unsuitable | Bilirubin = green; muscle and cytoplasm = yellow; collagen = red |
| Rhodanine | 10% NBF | Copper = reddish orange |
| Glycogen | ||
| PAS | 10% NBF, absolute alcohol, 10% formalin | Glycogen, fungi = magenta |
| d-PAS | 10% NBF, absolute alcohol, 10% formalin | Glycoprotein, basement membrane, α1-antitrypsin, atypical mycobacteria, ceroid laden macrophages = magenta; glycogen = digested, no magenta color |
| Amyloid | ||
| Congo red | 10% NBF, absolute alcohol, or Bouin solution | Amyloid = pink to red; nuclei = blue; elastic fiber = pink |
| Lipids | ||
| Oil Red O | Frozen section fixed in 10% NBF | Fat = red; nuclei = blue |
d-PAS, Periodic acid–Schiff with diastase digestion; NBF, neutral buffered formalin; PAS, periodic acid–Schiff; PTAH, phosphotungstic acid hematoxylin.
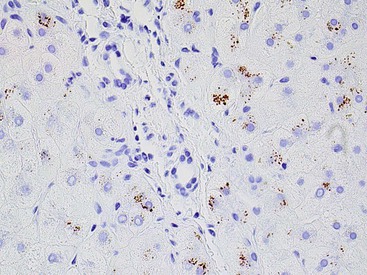
Immunohistochemistry
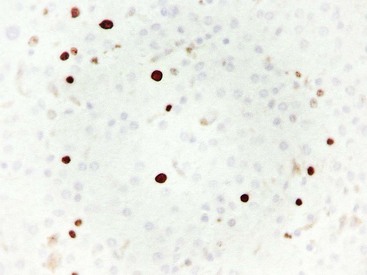
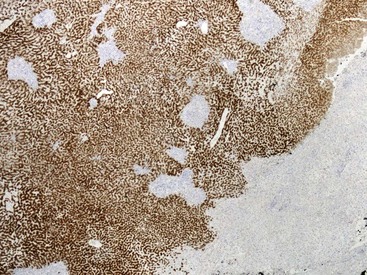
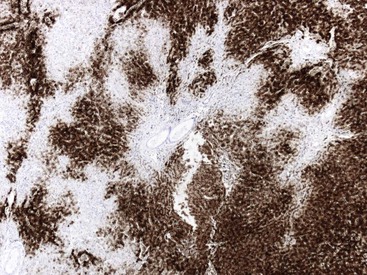
The development of monoclonal antibodies and highly sensitive immunohistochemical staining procedures has made it possible to demonstrate many types of antigens in routinely processed tissue sections. Immunohistochemical staining methods are applied to liver sections for several purposes: (1) localization of viral antigens, (2) identification and classification of tumors, (3) determination of prognostic factors in malignant tumors, (4) lymphoma and leukemia immunophenotyping, (5) identification of bile duct epithelium, and (6) assessment of architectural changes. The availability of more specific antibodies, continuous improvements in techniques, and use of automated immunohistochemical stainers have enabled immunohistochemical procedures to be used routinely in histopathology laboratories. These methods can be applied to routinely processed material. Table 43.5 summarizes the common immunohistochemical stains used for liver pathology diagnosis.
Table 43.5
Common Immunohistochemical Stains in Liver Pathology
| Immunohistochemical Stains | Interpretation |
| Detection of Viral Antigens | |
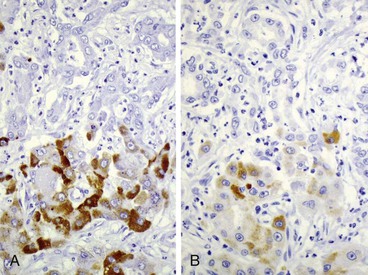
| Hepatitis B surface antigen (HBsAg) and hepatitis B core antigen (HBcAg) | Confirmation of hepatitis B infection and evaluation of status of HBV replication (HBcAg), especially in combined hepatitis viral infections. Visual aid for identification of ground-glass hepatocytes (HBsAg). Cytoplasmic and membranous HBcAg positivity indicates high levels of viral replication and corresponds to a high level of disease activity42,43 |
| Hepatitis delta (D) virus (HDV) | Confirmation of HDV coinfection or superinfection in HBV-infected patients44 |
| Nonhepatotropic viruses (cytomegalovirus [CMV], herpes simplex, Epstein-Barr virus [EBV], adenovirus) | Identification of liver involvement by nonhepatotropic viruses, particularly in immunosuppressed patients and infants45–47 |
| Hepatitis C virus | No reliable immunohistochemical stain available |
| Identification and Classification of Tumors | |
| Hepatocyte paraffin 1 (Hep Par 1) antigen | Hepatocellular differentiation48 |
| α-Fetoprotein (AFP) | Positive in approximately 40% of hepatocellular carcinomas (HCCs) and in all hepatoblastomas and fetal livers. Hepatocytes in cirrhotic nodules occasionally may show focal positive staining.49 |
| Polyclonal carcinoembryonic antigen (pCEA) | Bile canaliculi staining or canalicular differentiation in HCC. Cytoplasmic and membranous staining in adenocarcinoma50 |
| CD10, villin | Bile canaliculi staining or canalicular differentiation in HCC51,52 |
| Glypican 3 | Positive in HCC and negative in non-neoplastic hepatocytes or benign hepatocellular lesions.53 May be positive in melanoma. Positivity for glypican 3, glutamine synthetase, and heat shock protein 70 distinguishes early HCC from dysplastic nodule.54 |
| Glutamine synthetase | Diffusely positive in hepatocellular adenoma and carcinoma, maplike pattern of staining in focal nodular hyperplasia, and positive in centrilobular hepatocytes.55 |
| Monoclonal carcinoembryonic antigen (mCEA) | Negative in HCC.52 Positive in 60% of adenocarcinomas. |
| MOC-31 monoclonal antibody | Negative in HCC.52 Positive in 80% of adenocarcinomas. |
| Cytokeratins (CKs) | Coordinate CK7/CK20 staining is commonly used; both usually are negative in HCC. Hepatocytes are positive for CK8 and CK18.56 CK7 and CK19 are positive in the presence of cholangiocellular differentiation. |
| Thyroid transcription factor 1 (TTF-1) | Cytoplasmic staining may indicate hepatocellular differentiation.57 |
| Epithelial membrane antigen (EMA) | Negative in HCC. Positive in adenocarcinoma.58 |
| Factor VIII–related antigen; CD31 and CD34 | Vascular tumors.59 Sinusoidal endothelial cells are positive in immediate periportal sinusoids and negative in the rest of sinusoids in normal liver. Sinusoidal endothelial cells are positive in chronic liver diseases with fibrosis, cirrhosis, and HCC. |
| Organ-specific antigens | TTF-1 (nuclear staining), gross cystic disease fluid protein 15 (GCDFP-15 or BRST-2), prostate-specific antigen (PSA), estrogen, and progesterone receptors for metastatic lung, breast, prostatic, renal cell carcinoma, and others |
| Cytokeratin 19, epithelial cell adhesion molecule (EPCAM), CD56 | Identification of biliary differentiation in combined or mixed hepatocellular-cholangiocarcinoma58,60 |
| Serum amyloid A2 (SAA2) | Positive in inflammatory hepatocellular adenoma and negative in focal nodular hyperplasia61 |
| CD68 | Positive in fibrolamellar carcinoma but not in the usual HCC62 |
| Identification of Bile Duct Epithelium | |
| Cytokeratin 7 or 19 | Identification of bile ducts and ductules. Useful to visualize bile ducts to rule out vanishing bile duct syndrome or chronic ductopenic rejection. Confirmation of the degree of bile ductular reaction in biliary diseases63 |
| Prognostic Factors | |
| Cytokeratin 19 | Identification of hepatocellular carcinoma with aggressive behavior or high degree of recurrence64 |
| AFP, Ki67, or proliferating cell nuclear antigen (PCNA) | Differentiation of HCC from high-grade dysplastic nodule or hepatocellular adenoma65,66 |
| Smooth muscle actin | Identification of neovascularization in HCC67 |
| β-Catenin | Nuclear positivity in HCC and a subset of hepatocellular adenoma with high risk of transformation to HCC68 |
| Storage and Hereditary Diseases | |
| α1-Antitrypsin | Predominantly periportal intracytoplasmic globules in α1-antitrypsin deficiency. |
| Fibrinogen | Intracytoplasmic fibrinogen deposition in fibrinogen storage disease |
| Miscellaneous | |
| Leukemia or leukemia phenotyping | Differentiating or confirming posttransplantation lymphoproliferative disease (PTLD) from EBV hepatitis or acute rejection69 |
| Cytokeratins 8, 18, and 19 | Embryonal hepatocytes; expression of CK19 disappears by 10th week of gestation |
| Synaptophysin, glial fibrillary acidic protein, and neural cell adhesion molecule | Neural or neuroectodermal differentiation markers can be used to identify resting hepatic stellate cells.70 |
| Ubiquitin, cytokeratin 8 and 18, nucleoporin p62 | Identification of Mallory-Denk hyalin71,72 |
| Vimentin, desmin and smooth muscle actin | Myofibroblastic differentiation in activated hepatic stellate cells (including vitamin A toxicity)18,19 |
Fixation in formalin may reduce the availability of antigen binding sites. When fixed specimens are not embedded in paraffin immediately, they may be stored in 70% alcohol. Several modifications in staining procedures are available, but the most common are the peroxidase–antiperoxidase and avidin–biotin–peroxidase complex methods.41 Liver tissue has significant amounts of endogenous biotin. When an avidin–biotin–peroxidase complex method is used, an augmented background reaction may yield a false-positive result. This can be avoided by applying avidin–biotin blocking reagents before incubating with the biotinylated antibody. Figures 43.20 through 43.29 provide examples of immunohistochemical staining.
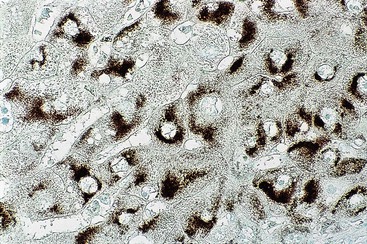
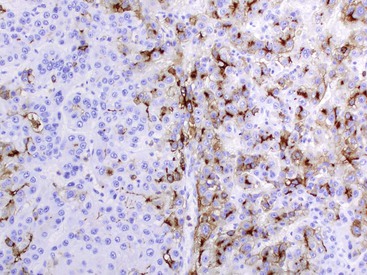
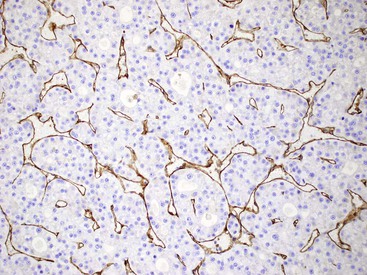
Electron Microscopy
Electron microscopy is not routinely performed on liver specimens. However, it has a limited and well-defined role in liver pathology. Submitting tissue in 3% glutaraldehyde for electron microscopy should be considered when investigating genetic and metabolic diseases,73 viral infection not otherwise identified by light microscopy or serology, tumors of unknown histogenesis, certain drug-induced liver injuries (e.g., amiodarone causing phospholipidosis), and diseases of unclear origin (Table 43.6).74,75 For this purpose, small pieces as large as 5 mm thick from the liver core should be immersed immediately in ice-cold 3% glutaraldehyde. Reprocessing of formalin-fixed, paraffin-embedded tissue for ultrastructural examination, although not optimal, is often satisfactory, particularly for virus identification.
Table 43.6
Electron Microscopy Findings in Liver Diseases
| Indication | Interpretation |
| Establishment of Viral Infection | |
| Hepatitis B virus | Intranuclear core virus particle, dilated endoplasmic reticulum containing surface material |
| Suspected virus with no serologic test or culture | Intranuclear or intracytoplasmic virions |
| Evaluation of Genetic and Metabolic Disease | |
| Glycogen storage disease, type II | Lysosome contains glycogen, cytoplasmic glycogen rosettes |
| Glycogen storage disease, type IV | Undulating, random, delicate fibril inclusions |
| Myoclonus epilepsy (Lafora disease) | Lafora bodies (fibrillar and granular material) |
| Hereditary fructose intolerance | Concentric and irregularly disposed membranous arrays and rarefaction of hyaloplasm |
| Mucopolysaccharidoses | Vacuolated lysosomes containing mucopolysaccharide in hepatocytes and Kupffer cells |
| Cystinosis | Spaces created by cystine crystals in Kupffer cells |
| Fibrinogen storage disease | Densely packed tubular structures in fingerprint-like pattern in rough endoplasmic reticulum |
| Wolman disease | Cholesterol crystals in cytoplasm of hepatocytes |
| Cholesterol ester storage disease | Triglyceride droplets in cytoplasm of hepatocytes |
| Gangliosidosis | Membrane bound vacuoles or inclusions |
| Fabry disease | Dense and laminated inclusions in hepatocytes and Kupffer cells |
| Gaucher disease | Cytoplasmic tubular bodies (dense ring in cross section) |
| Niemann-Pick disease | Lamellar lipid inclusions in lysosomes of hepatocytes and Kupffer cells |
| Mitochondrial disorders | Densely packed mitochondria separated by small lipid droplets |
| Dubin-Johnson syndrome | Complex dense bodies |
| α1-Antitrypsin deficiency | Finely granular material in the cisternae of dilated endoplasmic reticulum |
| Wilson disease | Variation of mitochondria, vacuolation, deposition of crystalline material |
| Examination of Tumor of Uncertain Histogenesis | |
| Sarcoma | Intermediated filaments: vimentin, actin |
| Neuroendocrine tumor | Neurosecretory granules |
| Melanoma | Melanosomes |
| Evaluation of Drug-Related Changes | |
| Phospholipidosis | Enlarged lysosome containing lamellar and reticular inclusions resembling myelin |
| Reye syndrome | Abnormal, swollen mitochondria |
| Hypervitaminosis A | Multivesicular stellate cells with fat droplets |
Molecular Studies
Box 43.2 lists applications for molecular studies that are helpful in diagnosing liver diseases in the tissue-processing laboratory. Many of the routine molecular diagnostic applications associated with liver disease are geared toward assessment of viral targets such as hepatitis B and C viruses, including the qualitative and quantitative detection and genotyping of the virus. For neoplastic diseases, novel testing applications have been developed for establishing or confirming a diagnosis, molecular profiling and classification of tumors, and demonstrating the potential for response to targeted therapeutic options.
In Situ Hybridization
For in situ hybridization, radioactively labeled probes for specific RNA or DNA sequences are applied and hybridized to a tissue frozen section. Treated slides are then exposed to photographic emulsion, resulting in distribution of silver grains over cells that contain the target RNA or DNA sequences. The sensitivity of in situ hybridization may be improved if combined with the polymerase chain reaction.
This method has been applied to liver tissue for identification of hepatitis A, B, C, and D viruses; cytomegalovirus; and Epstein-Barr virus (EBV). Most of these viruses, however, can be easily identified by immunohistochemistry, which has become a routine procedure in most laboratories and provides a degree of sensitivity that is similar to that of in situ hybridization.69 In situ hybridization is particularly useful to detect EBV RNA in paraffin sections of liver with possible EBV hepatitis and to diagnose posttransplantation lymphoproliferative disease with the use of a fluorescein-conjugated peptide nucleic acid (PNA) probe. PNA is detected with an in situ hybridization kit (Dako, Carpinteria, CA).
In situ hybridization for albumin mRNA is highly specific for normal hepatocytes and hepatocellular tumors.58,76 However, the use of immunohistochemistry to detect albumin is not considered reliable, because its small molecular size may result in its diffusion into the tissue when the tissue is not fixed immediately.
Polymerase Chain Reaction
The polymerase chain reaction can be applied to fresh, unfixed liver tissue, frozen tissue, or formalin-fixed tissue in paraffin.77 It provides a sensitive diagnostic and research tool for viral, bacterial, parasitic, and genetic diseases. Polymerase chain reaction is the most sensitive and specific method to demonstrate hepatitis B virus DNA, hepatitis C virus RNA, and hepatitis C virus genotypes in liver tissue.
Laser Capture Microdissection
Laser capture microdissection provides well-defined targeted cell populations from formalin-fixed, paraffin-embedded tissue sections for other types of molecular studies, such as the polymerase chain reaction.77
Gene Array Analysis
Gene array analysis provides gene expression profiles that can be used for comparative studies. Preliminary data from gene array analyses have become available for a wide range of neoplastic and non-neoplastic liver diseases. These data can be used to correlate gene expression with cellular function, with phenotypic alterations in liver diseases, with predisposition or susceptibility to disease, and with therapy and prognosis. For example, deciphering the key host factors implicated in hepatitis C virus pathogenesis helped to improve antiviral therapy.78
Gene array analysis has increased our understanding of the genetic predisposition for advanced fibrosis in patients with nonalcoholic steatohepatitis.79 It helps to determine the likelihood of a poor outcome for patients with alcoholic liver disease (advanced alcoholic liver disease will develop in only a small percentage of patients with heavy alcohol consumption),80 and it has increased the understanding of the mechanisms of hepatocarcinogenesis.81
Prognostic and Therapeutic Information from Liver Biopsies
Advances in immunologic and serologic markers, imaging studies, endoscopy, and molecular and genetic testing have diminished the need for liver biopsy in a variety of circumstances.82 An accurate diagnosis of liver disease cannot always be made solely on the basis of clinical history, physical examination, laboratory findings, and imaging studies. Because clinical data alone may lead to an erroneous conclusion, careful examination of a liver biopsy specimen is valuable. However, the liver has a limited number of pathologic responses to injury, and the resulting histologic picture does not always provide a specific diagnosis.
Complete assessment of a biopsy specimen requires integration of the clinical information with pathologic changes, which underscores the need for close cooperation between clinicians and pathologists. Most liver biopsies are not obtained to establish a specific diagnosis but rather to assess liver damage (i.e., degree of inflammation and stage of fibrosis) or response to therapy, such as in patients with chronic viral hepatitis or fatty liver disease.2
In chronic viral hepatitis, antiviral treatment is recommended if there is significant fibrosis or inflammation.83 However, antiviral treatment may have severe side effects, it is costly, and it fails in as many as 50% of patients. Biopsy findings may help to guide the appropriate treatment of these patients.
Staging and grading systems are applied to liver biopsy reports to add objectivity to pathology reporting.84,85 Among the risks of overemphasizing a numeric score is underreporting of concomitant diseases.
The value of a liver biopsy in patients with suspected fatty liver disease is controversial, because the finding of a fatty liver by ultrasonography or CT provides support for the clinical diagnosis. However, the degree of inflammation and fibrosis and the amount of iron also need to be evaluated because they affect treatment and prognosis. Patients who are aware of the presence of advanced fibrosis or cirrhosis may be more motivated to adhere to medical treatment and change their lifestyle.2 , Liver biopsy remains important for assessing the severity and potential reversibility of alcoholic liver disease and to confirm suspected confounding diseases.
In patients with hemochromatosis, liver biopsy offers important prognostic information when the stage of the disease is unclear. It also aids in detecting comorbid conditions, such as nonalcoholic steatohepatitis, alcohol abuse, and chronic viral hepatitis. Significant fibrosis or cirrhosis substantially increases the risk of hepatocellular carcinoma,86 and these patients should be screened periodically. Biopsy may confirm the diagnosis in atypical presentations, including those not associated with any of the classic hereditary hemochromatoses related to mutations of the HFE gene.87
Although biopsy is not absolutely necessary for the diagnosis of autoimmune hepatitis, it may help to confirm the diagnosis and stage,88 and it can be used to evaluate the response to therapy. Improvement in the transaminase levels may not correlate with histopathologic changes. A firm diagnosis is important because of the implications of long-term immunosuppression. Flare-ups during adequate immunosuppression should arouse suspicion of other disorders, which may be evident in the liver biopsy.
Refinements in imaging studies (specifically endoscopic retrograde cholangiopancreatography) have decreased the value of liver biopsy for differentiating intrahepatic from extrahepatic cholestasis.82 Nevertheless, biopsies in cases of chronic cholestatic diseases, such as primary biliary cirrhosis and primary sclerosing cholangitis, are often necessary to assess the stage of disease. An adequate sample is necessary to avoid sampling error, because the pathologic changes in the portal tracts are often unevenly distributed throughout the liver. Biopsies may also be helpful in assessing the response to therapy and in confirming the clinical evolution of disease.
Drug-induced liver injury may be clinically obvious when the suspected agent has a known toxicity. However, if the patient takes multiple medications, new medications, or medications without known hepatotoxicity, the liver function test results may be confusing. In these cases, liver biopsy can be helpful. If a biopsy is considered for assessing drug or herbal hepatotoxicity, an early biopsy is preferred, because after the injury progresses, the risk of liver failure increases, and the histologic features become less specific.
The value of serial biopsies to assess hepatotoxicity in patients receiving methotrexate remains controversial.89 In retrospect, it has become clear that some of the pathologic changes described in early studies were the result of chronic viral hepatitis, alcoholic steatohepatitis, or nonalcoholic steatohepatitis. Further studies suggest that severe methotrexate-related liver disease is much less common than previously believed.90
For patients with acute liver failure who are being considered for liver transplantation, liver biopsy is useful if knowing the cause of failure may alter treatment. For example, herpes simplex infection may respond to therapy, and patients with metastatic disease may be seen with liver failure. For patients with end-stage decompensated cirrhosis, a liver biopsy is not normally necessary, because knowing the cause rarely affects the need for transplantation. Liver biopsy is less often performed after liver transplantation because of improvements in immunosuppressive medications. However, biopsies are important to evaluate abnormal liver test results for transplant recipients, which can have many causes, including acute and chronic rejection, opportunistic infections, posttransplantation lymphoproliferative disorder, surgical complications, drug hepatotoxicity, immune-mediated hepatitis (i.e., de novo autoimmune hepatitis), and recurrence of the patient’s original disease. In some cases, histopathologic changes may precede clinical and biochemical disease.91
For space-occupying lesions, biopsies are performed to distinguish primary from metastatic liver tumors and to demonstrate the potential for a therapeutic response. When patients with cirrhosis are found to have an elevated α-fetoprotein level and a hypervascular tumor on imaging, a liver biopsy is not needed to confirm the presence of hepatocellular carcinoma.92 Nevertheless, biopsy of hepatocellular carcinoma is often performed for confirmation and to help refine the treatment protocol. In metastatic diseases (e.g., metastatic colon carcinoma), KRAS oncogene mutation testing demonstrates the potential for response to epidermal growth factor receptor (EGFR) inhibitors.