CHAPTER 51 LIVER INJURY
The liver is the most commonly injured intra-abdominal organ with an incidence of 30%–40%. The overwhelming majority of liver injuries, however, are minor, with spontaneous cessation of hemorrhage almost always the rule, and operative intervention is rarely required. On the other hand, complex hepatic injuries continue to challenge even the most experienced trauma surgeons.
INCIDENCE
Hepatic injury occurs in approximately 5% of all trauma admissions. Nationwide, there has been a steady decline in the incidence of penetrating liver injuries. However, blunt injuries seem to be on the rise predominantly because their presence has been more readily detected by the almost routine use of CT scanning in patients sustaining blunt trauma. The incidence of complex hepatic injuries, however, has remained relatively stable over the past 25 years, ranging from 12% to 15%.1
Motor vehicle crashes (MVCs) continue to account for most (approximately 80%) blunt hepatic injuries, followed by pedestrian and car collisions, falls, assaults, and motorcycle crashes. Most patients with blunt hepatic trauma have associated injuries, both intra-abdominal and extra-abdominal. Concomitant chest trauma is the most common associated injury encountered with blunt hepatic trauma, occurring in over 50% of patients. Patients with right-sided lower rib fractures, particularly ribs 9–11, have at least a 20% chance of sustaining an underlying hepatic injury. In spite of the high aforementioned incidence of associated chest trauma, injury to the brain remains the single most significant determinant in overall survival outcome.2 In the era of nonoperative management of blunt trauma, the risk of a missed injury, especially to the diaphragm or small bowel, is of major concern. Adherence to meticulous interpretation on imaging studies by experienced personnel should limit this pitfall to 1%–2%.
MECHANISM OF INJURY
Blunt Hepatic Injury
In MVCs, those most susceptible to hepatic injury are unrestrained front-seat passengers. These passengers are particularly vulnerable to a compression injury from the steering wheel especially during periods of rapid deceleration.3 Although the anterior abdominal wall stops, the posterior abdominal wall continues to move forward, and the intra-abdominal organs are “trapped” and compressed resulting in stretching/tearing of the liver at its vascular and structural attachments. As the liver is only partially protected by the rib cage, liver injury from steering wheel contact is one of the most important contributing factors to driver injury.
Penetrating Hepatic Injury
Damage caused by a penetrating injury is based on the kinetic energy of the projectile and the density and elasticity of the tissue. Lowenergy weapons such as knives only cut and do not create a temporary cavity. Medium-energy and high-energy firearms damage not only the tissue directly in the path of the missile but also the tissue on each side of the missile’s path. As a missile passes through the relatively inelastic liver parenchyma, a temporary cavity (three to six times the size of the missile’s front surface area, lasting for a fraction of a second) and a permanent cavity (visible to the examiner) are created. The higher-energy firearms create larger temporary and permanent cavities, resulting in far more extensive tissue damage; the vacuum created by this larger cavity pulls clothing, bacteria, and other debris from the surrounding area into the wound as well.
DIAGNOSIS
Hemodynamically Stable Patients
Scans should immediately be interpreted and classified according to the American Association for the Surgery of Trauma liver injury scale4 (Table 1) by the CT fellow or attending radiologist, always in the presence of the chief trauma resident and trauma attending. The senior trauma attending in presence makes the final decision as to the appropriateness of nonoperative therapy. It should be noted that the grade of injury or degree of hemoperitoneum on CT does not determine the need for operative intervention, as this decision is based primarily on the patient’s hemodynamic stability and the absence of peritoneal signs. Instead, the CT scan merely provides the surgeon with a general anatomic overview of the injury, identifies associated abdominal injuries requiring operative intervention, and can be used as a base for comparing future healing of the hepatic injury and resorption of intraperitoneal blood. CT can also identify injuries involving the bare area of the liver, which commonly present with minimal intra-abdominal bleeding, a paucity of abdominal signs, and often a negative DPL.
The role of FAST as a screening exam in hemodynamically stable patients is evolving and in the near future may eliminate the need for CT scan. Currently, many trauma centers forgo CT scanning in stable patients with negative initial FAST exams and merely repeat the FAST in 6 hours. However, scanning for only free fluid has its diagnostic limitations because not all blunt hepatic injuries result in hemoperitoneum. In a recent study looking specifically at sonographic detection of blunt hepatic trauma, Richards et al.5 determined the overall sensitivity of FAST for blunt hepatic injuries (all grades) to be 67%, based on the detection of free fluid alone. On the other hand, it is clear that most solid organ injuries without intraperitoneal fluid on FAST are, in general, of minimal clinical significance.6 At present, most trauma surgeons agree that those patients who are hemodynamically stable and who have either intraperitoneal blood on their initial FAST exam or positive findings on physical exam over the lower chest and upper abdomen should have a CT to specifically identify a hepatic or splenic injury that can be managed nonoperatively. Once identified, the hepatic injury may be followed with ultrasound if necessary.
ANATOMIC LOCATION OF INJURY AND INJURY GRADING—AAST-OIS
Comprehensive knowledge of hepatic anatomy is essential to the proper management of traumatic liver injuries. Couinaud has described the functional anatomy of the liver, based on the hepatic venous drainage (Figure 1). The ligamentous attachments of the liver are depicted in Figure 2.
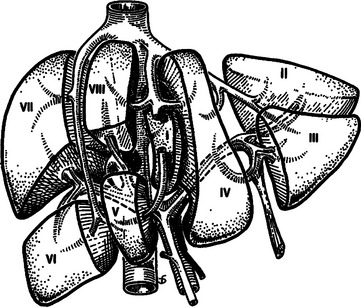
Figure 1 Functional division of the liver, according to Couinaud’s nomenclature.
(From Mattox KL, Feliciano DV, Moore EE, editors: Trauma, 4th ed. New York, McGraw-Hill, 1999, Figure 30-1. Originally appeared in Blumgart LH, editor: Surgery of the Liver and Biliary Tract. New York, Churchill Livingstone, 1988.)
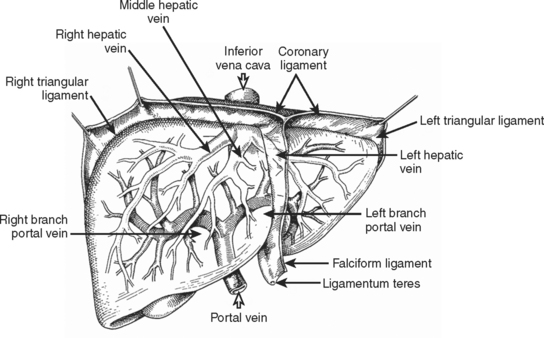
Figure 2 Surgical anatomy of the liver.
(From Mattox KL, Feliciano DV, Moore EE, editors: Trauma, 5th ed. New York, McGraw-Hill, 2004, Figure 30-2.)
The American Association for the Surgery of Trauma (AAST) created the Organ Injury Scaling (OIS) Committee to standardize injury severity scores for individual organs to facilitate clinical investigation and outcomes research. The liver injury scale devised by the AAST is shown in Table 1.
MANAGEMENT
Nonoperative Management/Blunt Hepatic Trauma
 Absence of associated intra-abdominal or retroperitoneal injuries on CT scan that require operative intervention
Absence of associated intra-abdominal or retroperitoneal injuries on CT scan that require operative interventionPreviously cited inclusion criteria such as neurological integrity are no longer valid, as neurologically impaired patients can be safely managed nonoperatively in a monitored setting.7 Furthermore, mandatory repeat CT scans to document improvement or stabilization of injury are unnecessary and contribute little to patient outcome. Rather, the patient’s clinical course should dictate the need for additional evaluation.
Most (80%–90%) blunt hepatic trauma patients can be successfully managed nonoperatively. Although nonoperative management was initially limited to AAST grades I–III injuries, it is now clear that the hemodynamic status of the patient, rather than AAST grade of injury, is the most significant factor in determining the need for operative intervention. Up to 20% of select patients with grades IV and V injuries can be managed nonoperatively. However, many grade IV and most grade V injuries will usually present with hemodynamic instability or concomitant injuries mandating surgery, thus precluding nonoperative intervention. In a multi-institutional study, grades IV and V injuries were responsible for 67% of all patients who failed nonoperative management and subsequently required operative intervention.8 Therefore, although hemodynamic stability determines which patients can be managed nonoperatively, the subgroup of patients with complex hepatic injuries (grades IV and V) are at substantially higher risk for treatment failure and should therefore be closely monitored in a critical care unit.
Conversely, the same basic standards apply to patients with lower AAST-grade injuries (i.e., I–III). In these instances, the initial injury may be deemed as “not significant,” and thus it becomes tempting to avoid surgical intervention despite hemodynamic instability or a decreasing hematocrit, relying instead on further fluid and blood transfusions. This course of action is fraught with pitfalls and should be avoided to minimize the morbidity and mortality of nonoperative management. To summarize, of all the variables monitored, hemodynamic stability appears to be the most crucial and is considered the watershed for nonoperative or operative intervention.
Nonoperative Management/Penetrating Hepatic Trauma
Renz et al.9 nonoperatively managed 13 patients with penetrating right thoracoabdominal gunshot wounds. The authors stressed the importance of serial abdominal exams and contrast-enhanced CT scanning in their successful nonoperative management. Demetriades et al.10 substantiated this concept with their successful management of select patients with isolated gunshot injuries to the liver. These authors concluded that hemodynamically stable patients with grades I and II liver injuries and no evidence of peritonitis can be safely managed nonoperatively. However, it should be noted that this approach failed in nearly one-third (5/16) of the patients in the “observed group” who eventually required delayed laparotomy. More recently, Omoshoro-Jones et al.11 described successful nonoperative management in 31 of 33 patients with gunshot wounds to the liver, including grades III–V injuries. Although the higher-grade injuries were associated with more complications (most of which were managed nonoperatively), the overall success of nonoperative management did not depend on the AAST grade of liver injury.
Operative Management/General Principles
Before the incision is made, the patient should receive a dose of antibiotics to cover aerobic and anaerobic microbes and is placed on a warming blanket. The surgeon must keep in mind that hypothermia is a frequent complication of resuscitation and operation in patients with major hepatic injuries. Appropriate maneuvers to decrease hypothermia are shown in Table 2.12 Adherence to these maneuvers will usually prevent the development of intraoperative coagulopathies and fatal arrhythmias.
Table 2 Maneuvers to Prevent/Decrease Hypothermia in Patients with Major Hepatic Injuries
Adapted from Pachter H, Liang H, Hofstetter S: Liver and biliary tract trauma. In Feliciano DV, Moore EE, Mattox KL, editors: Trauma, 4th ed. Stamford, CT, Appleton and Lange, 2000, p. 637.
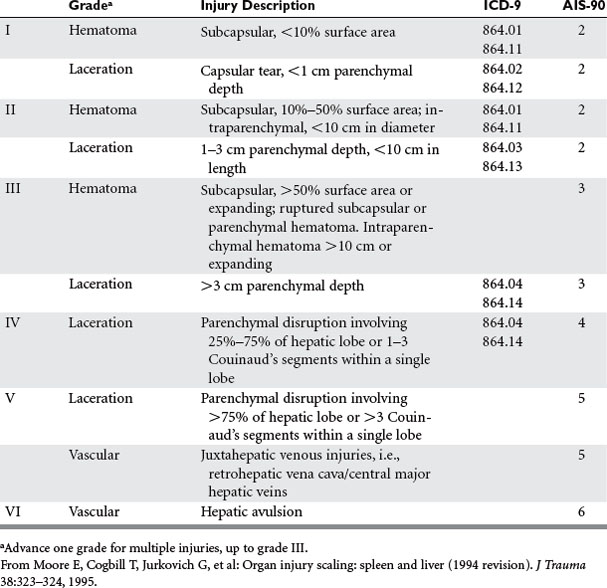
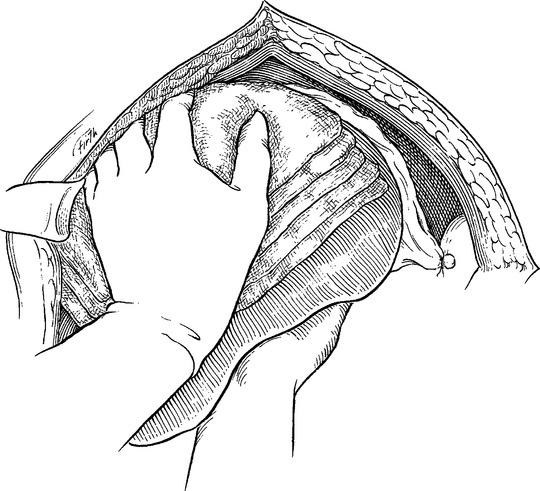
Exsanguinating hemorrhage continues to remain the most immediate cause of death in patients sustaining hepatic trauma. The initial incision into the peritoneal cavity can be accompanied by profuse hemorrhage once the tamponading effect has been lost. At this time, all efforts should be directed toward intraoperative resuscitation. Attempts at definitive surgical hemostasis without proper intraoperative resuscitation usually results in systemic hypothermia and profound coagulation defects with their dire consequences. This fundamental pitfall should be avoided at all costs. Irrespective of the severity of hepatic injury, almost all liver injuries can be initially managed by manually compressing the injury overlap pads (Figure 3), while hemodynamic and metabolic stability are restored by the anesthesia team. Failure to correct hypovolemia and acidosis before attempts at surgical control will likely lead to cardiac arrest and subsequent death. Once intraoperative resuscitation has been achieved, manual compression of the liver is slowly released so that a more accurate assessment of the injury can be made.
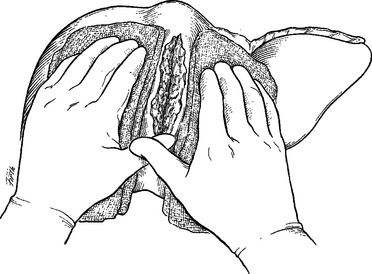
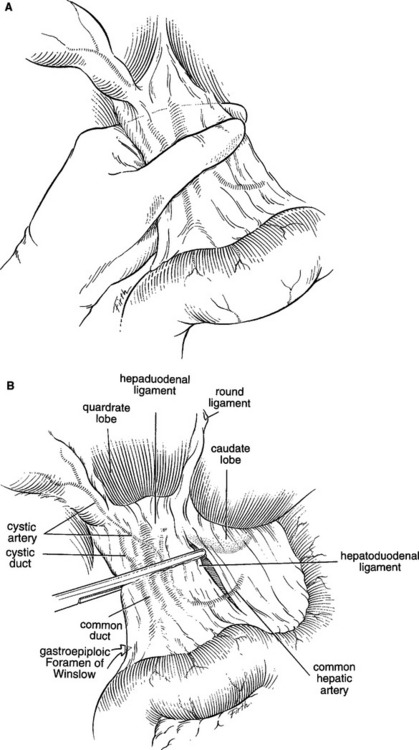
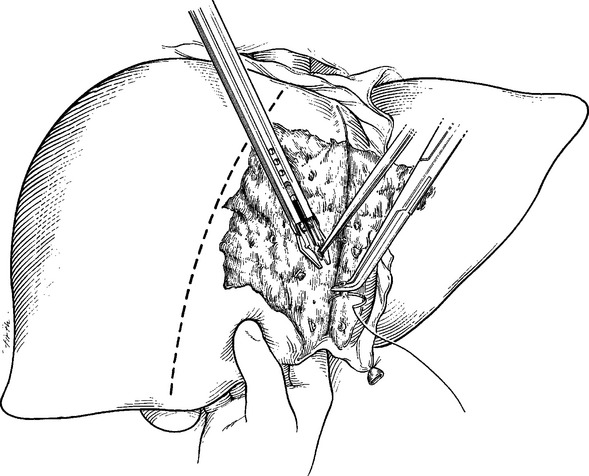
In order to better visualize injuries on the superior or lateral aspects of an injured hepatic lobe, it is often necessary to mobilize the liver into the midline wound. Division of the falciform ligament allows for placement of an “upper-hand” self-retaining retractor in the incision. Once this is done, careful traction on its hepatic end can aid in exposing the dome of the liver and the suprahepatic inferior vena cava. Additional exposure is obtained by placing laparotomy pads behind the posterior surface of the liver. Mobilization of the right and left lobes proceeds with division of the triangular ligaments (Figure 4). If there is a hematoma within the leaves of the triangular ligament, a hepatic vein or venal caval injury is most likely. Extreme caution must be taken because traction may disrupt a stable hematoma and can create massive bleeding.
Operative Management/Minor Injuries (Grades I and II)
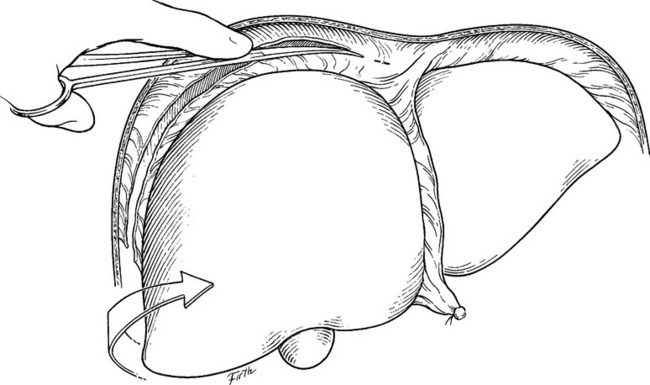
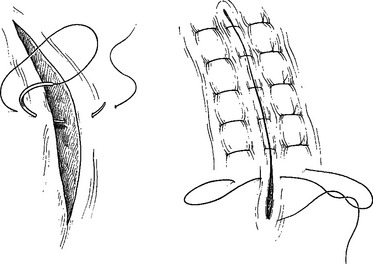
Simple techniques of controlling hemorrhage include: a 5–10-minute period of compression, application of topical agents including fibrin glue, electrocautery/Argon beam electrocoagulation, and suture hepatorrhaphy (Figure 5). In many patients with superficial lacerations of the capsule, a 5–10-minute period of compression will frequently control any hemorrhage. If there is no visible leakage of bile, no further therapy is indicated. Topical agents such as Surgicel, Avitene, and fibrin glue are useful when avulsion of Glisson’s capsule is present. Five minutes of compression with lap pads is performed after the application of a topical agent to the raw surface. After releasing compression, the electrocautery can be used for any remaining bleeders. Drainage is not necessary in the absence of further hemorrhage or obvious bile leakage.
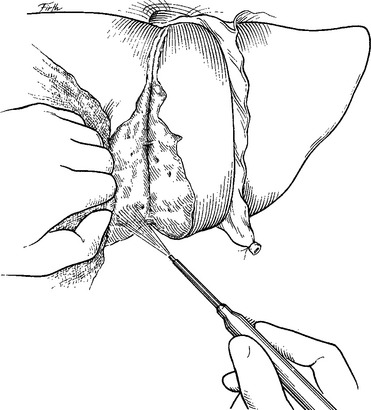
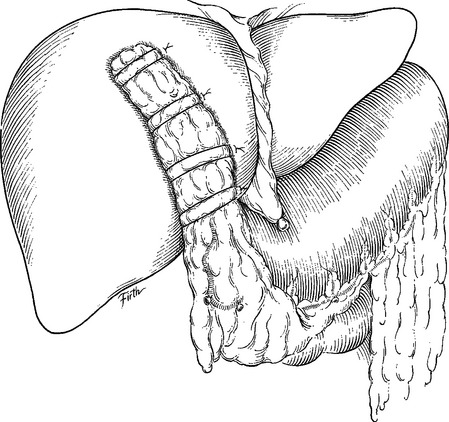
Suture hepatorrhaphy has historically been the mainstay of hepatic hemostasis in grade II and some grade III injuries. It is important to first enter the hepatic wound and selectively ligate any open/avulsed bile ducts or blood vessels. Figure-of-eight 2-0 or 3-0 Prolene sutures are usually employed. Small defects in the hepatic parenchyma can be closed with simple interrupted 0-chromic or 2-0 chromic liver sutures with blunt-nosed needles (Figure 6). For deeper lacerations, attempts at primary closure of the hepatic defect should not be undertaken. Instead, a tongue of omentum on a pedicle is placed within the hepatic parenchymal defect and is then held in place with interrupted liver sutures (Figure 7). It is important to loosely approximate the edges because portions of the liver beneath can become necrotic in the postoperative period if the sutures are tied too tightly.
Operative Management/Complex Injuries (Grades III to V)
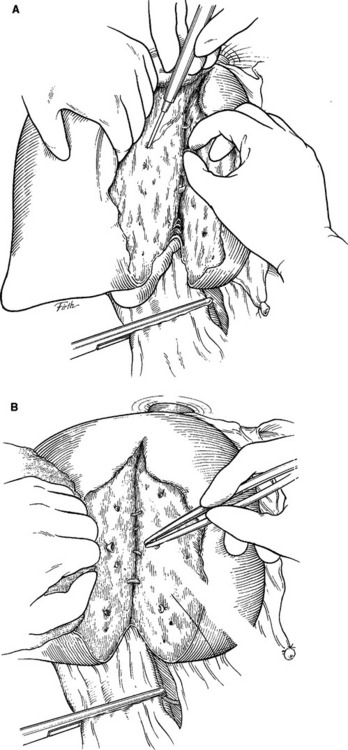
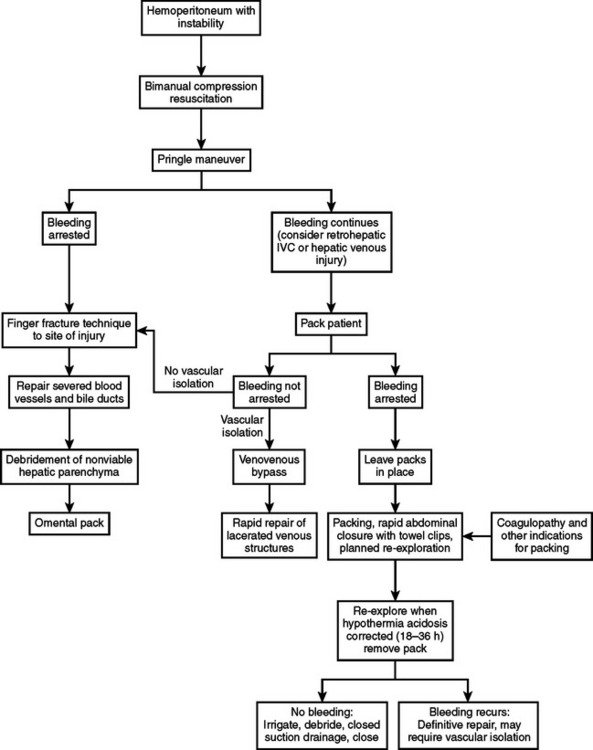
If significant hemorrhage continues after the release of manual compression of the liver, the portal triad should be occluded with an atraumatic vascular clamp (the “Pringle” maneuver; Figure 8). In over 85% of patients with complex hepatic injuries, occlusion of the portal triad will temporarily stop the bleeding. This maneuver, coupled with the finger fracture technique to expose lacerated blood vessels for direct repair, is responsible for the dramatic decrease in mortality from exsanguination.
Much controversy has surrounded the normothermic ischemic time produced by the Pringle maneuver. The data are clear that complex hepatic injuries can be managed with continuous cross clamping of the porta hepatis for up to 75 minutes without adverse sequelae.13 We prefer to achieve hepatocyte protection before portal triad occlusion by employing topical hypothermia to the hepatic parenchyma and maintaining a core hepatic temperature of 32° C. This maneuver must be achieved without contributing to systemic hypothermia. The rest of the abdomen must be packed off with multiple lap pads, and topical hypothermia with iced Ringer’s lactate is achieved by intermittently pouring the solution directly onto the liver. Essential to this approach is the use of an intrahepatic digital temperature probe so that core hepatic parenchymal temperature can be seen at all times.
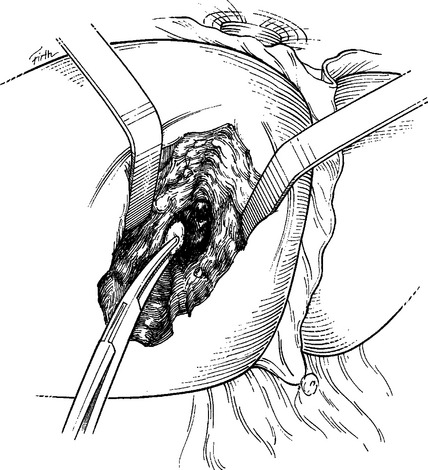
With portal triad occlusion achieved by an atraumatic vascular clamp, the surgeon then opens the liver parenchyma (hepatotomy) in the direction of the injury (Figure 9). Although it initially seems crude, the finger-fracture technique constitutes the benchmark of obtaining rapid, adequate exposure. Specifically, using the electrocautery, Glisson’s capsule is incised in the direction of the injury. Normal hepatic parenchyma is then crushed between the surgeon’s thumb and index finger (or a neuro-tipped suction device), thereby rapidly exposing injured blood vessels and bile ducts, which are repaired or ligated under direct vision. Narrow Deaver retractors can be inserted into the hepatotomy tract for better intrahepatic exposure (Figure 10). Large lacerated intralobar branches of the portal vein or hepatic veins can be repaired in a lateral fashion using 5-0 Prolene sutures (Figure 11).
Juxtahepatic Venous Injuries (Grade V)
At present, there is a general consensus among trauma surgeons that if a retrohepatic caval injury or a hepatic venous injury (grade V) can be adequately controlled with perihepatic packing, no attempts at further repair should be initiated. When adequate resuscitation has been accomplished, there may be a role for endovascular stenting of the injury before pack removal.14 Even without endovascular stenting, when planned re-exploration is undertaken, no further bleeding is often noted. If bleeding occurs after pack removal, definitive treatment can then be undertaken with the knowledge that the patient’s hemodynamic status has been optimized and that adequate personnel are available if a vascular shunt is necessary.
Another approach is direct hepatotomy through normal hepatic parenchyma to reach the injured retrohepatic cava or hepatic veins.15 After manual compression, vigorous resuscitation and prolonged portal triad occlusion, mobilization of the liver is performed with medial rotation, thus providing access to the retrohepatic cava and hepatic veins. Rapid and extensive finger fracture should be directed toward the site of injury until the lacerated retrohepatic cava or hepatic vein is found and repaired under direct vision. The surgeon must be prepared to finger fracture the hepatic parenchyma through normal and frequently nonanatomic planes. Because these patients usually have injured hepatic parenchyma as well, portal triad occlusion serves two purposes: it contributes to controlling hemorrhage from intrahepatic branches of the hepatic artery and portal vein, and it decreases the inflow to the liver, thereby aiding finger fracture while minimizing blood loss.
Venovenous bypass, vascular exclusion, and primary repair16 comprise a third approach. Total vascular isolation of the liver via venovenous bypass (combined with the Pringle maneuver and clamping of the suprarenal and suprahepatic cava) permits direct suture repair of the venous injury. The advantage here is that vascular isolation with venovenous bypass obviates the need for an intracaval shunt. Cannulation for bypass can be done peripherally via saphenous vein and axillary vein cutdowns. Venovenous bypass has been used in a small number of severe retrohepatic liver injuries with an overall survival rate of 88%.
Next is total hepatic resection and delayed liver transplantation.17 Total hepatectomy and second-stage hepatic transplantation can be a drastic yet life-saving maneuver for devastating liver injuries that have failed all conventional treatments. Perihepatic packing and total hepatectomy with portacaval shunting can be performed in the primary hospital; the anhepatic patient can then be transferred to a transplant center for eventual liver transplant. Although this radical maneuver can be associated with high morbidity and mortality, the prognosis is better if the decision to proceed with total hepatectomy and portacaval shunting is made before the development of intractable multiorgan failure.
Portal Triad Injuries
As exsanguination is the most common (85%) cause of death in these highly lethal and complex injuries, the first priority in portal triad trauma is hemorrhage control, specifically manual compression followed by the Pringle maneuver. A wide Kocher maneuver and mobilization of the hepatic flexure will allow medial rotation of the ascending colon to better expose the portal structures. Exposure of a retropancreatic portal vein injury may require pancreatic transection with distal pancreatectomy after the vascular repair is complete. Although portal vein ligation can be used to expeditiously manage portal vein injuries, the preferred treatment is lateral venorrhaphy, as most series report a 51%–60% survival with this approach.18
Damage Control/Perihepatic Packing and Planned Re-Exploration
Primary indications for perihepatic packing follow:
As a general rule, the liver should be mobilized before packing to help establish a tamponading effect. If, however, a significant hematoma is encountered in the triangular ligament (indicative of a vena caval or hepatic vein injury), further mobilization is contraindicated as massive and uncontrollable bleeding may follow. Most often, dry multiple-lap pads are placed on top of the injured liver until the ipsilateral hemidiaphragm is reached (Figure 12). In order to lessen the degree of bleeding when lap pads are peeled off the raw liver surface, we routinely place a Steri-Drape (3M, St. Paul, MN) directly upon the liver surface to serve as an interface between the injured liver and the lap pads.
Adjuncts to Operative Management
Asensio et al.19 advocate early hepatic angiography and angioembolization (AE) in all patients with grades IV and V hepatic injuries. These authors reported an improved survival with immediate surgery to control life-threatening hemorrhage, the institution of early hepatic packing when necessary, and subsequent patient transport directly from the operating room to the angiography suite for immediate hepatic angioembolization. Clearly, AE is essential in the management of complex hepatic injuries, whether they arise from blunt or penetrating mechanisms.
MORBIDITY AND COMPLICATIONS MANAGEMENT
Failure of Nonoperative Management
Most failures of nonoperative management (with lack of contrast blush on initial CT) result from associated abdominal injuries rather than the liver per se. In a recent series reported by Velmahos et al.,20 the grade of injury did not correlate with failure of nonoperative management. Most impressive was their reported liver-related failure rate of 0%. Although their series was too small to determine independent predictors of failure, they found that patients who failed had a higher Injury Severity Score, required greater volumes of initial fluid resuscitation and blood transfusion, and had other associated abdominal injuries.
Resumption of Normal Activities
Dulchavsky et al.21 demonstrated in experimental models that hepatic wound bursting strength at 3 weeks after injury was comparable and often exceeded wound bursting strength of normal hepatic parenchyma. Moreover, healing by secondary intention resulted in wound bursting strength equal or greater than hepatorrhaphy or hepatorrhaphy and omental packing at 3 and 6 weeks. The healing mechanism responsible for the increased wound bursting strengths appears to be the proliferative fibrosis throughout the injured hepatic parenchyma and the overlying Glisson’s capsule. Hepatic parenchymal healing appears to be virtually complete at 6 to 8 weeks postinjury. A reasonable and safe approach to pursue would be to allow patients with grade III or greater injury to resume normal activities after CT scan documentation, at 3 months, of major injury resolution.
CONCLUSIONS/ALGORITHM
When the liver injury requires operative intervention, four essential maneuvers should be kept in mind, which can be life-saving, even in the hands of those with limited experience in this area: (1) manual compression of the injury, (2) resuscitation, (3) assessment of the injury, and (4) the Pringle maneuver (inflow occlusion).
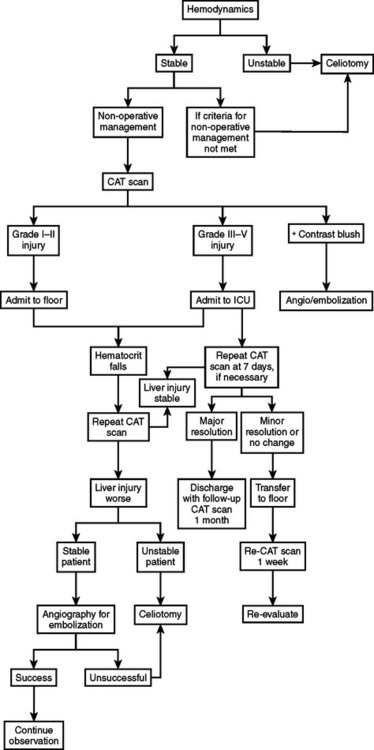
Algorithms for the management of hepatic injuries are shown in Figures 13 and 14.
1 Richardson J, Franklin G, Lukan J, et al. Evolution in the management of hepatic trauma: a 25-year perspective. Ann Surg. 2000;232:324-330.
2 Rivkind A, Siegel J, Dunham M. Patterns of organ injury in blunt hepatic trauma and their significance for management and outcome. J Trauma. 1989;29:1398-1415.
3 Lau I, Horsch J, Viano D, et al. Biomechanics of liver injury by steering wheel loading. J Trauma. 1987;27:225-235.
4 Moore E, Cogbill T, Jurkovich G, et al. Organ injury scaling: spleen and liver (1994 revision). J Trauma. 1995;38:323-324.
5 Richards J, McGahan P, Pali M, et al. Sonographic detection of blunt hepatic trauma hemoperitoneum and parenchymal patterns of injury. J Trauma. 1999;47:1092-1097.
6 Ochsner M, Knudson M, Pachter H, et al. Significance of minimal or no intraperitoneal fluid visible on CT scan associated with blunt liver and splenic injuries: a multicenter analysis. J Trauma. 2000;49:505-510.
7 Archer L, Rogers F, Shackford S. Selective nonoperative management of liver and spleen injuries in neurologically impaired adult patients. Arch Surg. 1996;131:309-415.
8 Pachter H, Knudson M, Esrig B, et al. Status of nonoperative management of blunt hepatic injuries in 1995: a multicenter experience with 404 patients. J Trauma. 1996;40:31-38.
9 Renz B, Feliciano D. Gunshot wounds to the right thoracoabdomen: a prospective study of nonoperative management. J Trauma. 1994;37:737-744.
10 Demetriades D, Gomez H, Chahwan S, et al. Gunshot injuries to the liver: the role of selective nonoperative management. J Am Coll Surg. 1999;188:343-348.
11 Omoshoro-Jones J, Nicol A, Navsaria P, et al. Selective non-operative management of liver gunshot injuries. Br J Surg. 2005;92:890-895.
12 Pachter H, Liang H, Hofstetter S. Liver and biliary tract trauma. In: Mattox KL, Feliciano DV, Moore EE, editors. Trauma. 4th ed. New York: McGraw-Hill Companies; 2000:633-682.
13 Pachter H, Spencer F, Hofstetter S, et al. Significant trends in the treatment of hepatic trauma: experience with 411 injuries. Ann Surg. 1992;215:492-502.
14 Denton J, Moore G, Coldwell D. Multimodality treatment for grade V hepatic injuries: perihepatic packing, arterial embolization, and venous stenting. J Trauma. 1997;42:964-968.
15 Pachter H, Spencer F, Hofstetter S, et al. The management of juxtahepatic venous injuries without an atriocaval shunt: preliminary clinical observations. Surgery. 1986;99:569-575.
16 Rogers F, Reese J, Shackford S, et al. The use of venovenous bypass and total vascular isolation of the liver in the surgical management of juxtahepatic venous injuries in blunt hepatic trauma. J Trauma. 1997;43:530-533.
17 Ringe B, Pichlmayr R. Total hepatectomy and liver transplantation: a life-saving procedure in patients with severe hepatic trauma. Br J Surg. 1995;82:837-839.
18 Jurkovich G, Hoyt D, Moore F, et al. Portal triad injuries. J Trauma. 1995;39:426-433.
19 Asensio J, Roldan G, Petrone P, et al. Operative management and outcomes in 103 AAST-OIS grades IV and V complex hepatic injuries: trauma surgeons still need to operate but angioembolization helps. J Trauma. 2003;54:647-653.
20 Velmahos G, Toutouzas K, Radin R, et al. High success with nonoperative management of blunt hepatic trauma. Arch Surg. 2003;138:475-481.
21 Dulchavsky S, Lucas C, Ledgerwood A, et al. Efficacy of liver wound healing by secondary intent. J Trauma. 1990;30:44-48.