Liver function and failure
Symptoms of liver failure: acute and chronic
In the acute setting, liver failure can present with a number of symptoms, but it is important to note that not all of these may be present at the same time. Typically, a patient with acute liver failure after surgery, transplantation or in acute poisoning will be confused or mentally slow as a result of encephalopathy, which may progress to loss of consciousness and a need to protect the airway by intubation and mechanical ventilation. Patients are often not immediately jaundiced, but jaundice may develop over the course of several days. Patients may be hypoglycaemic and the requirement for intravenous infusion of dextrose is a sinister development and an indicator of severe acute liver failure. Coagulopathy may develop, with evidence of bruising or bleeding from line sites or surgical scars. Severe acute liver failure can be assessed using the King’s College Hospital criteria, which were designed to predict mortality in paracetomol- and non-paracetomol-dependent acute liver failure.1 Later, this scoring system was adopted in the UK to determine criteria indicating likely benefit from liver transplantation. In the surgical patient, the development of acute liver failure is usually more gradual and less dramatic; a useful scoring system for liver dysfunction in the acute setting has been reported by Schindl et al.2 (see Box 1.1).
Common causes of acute liver failure: hepatic insufficiency following liver resections
The second crucial problem has been that there is only a poor correlation between volume and function. However, it is still unclear why some patients with smaller hepatic remnants do not develop liver failure whilst some with greater residual volumes do. These observations suggest, however, that peri- and intraoperative events superimposed on the innate hepatic capacity to withstand injury play a role. Hepatic insufficiency in this situation may arise either if not enough liver volume is left after partial hepatectomy or if the residual volume does not function properly. A functional limitation may arise, for example, in patients that have received aggressive chemotherapy in order to reduce the number and size of metastases prior to surgical treatment by liver resection. One of the factors contributing to defective defence may be preoperative fasting,3 but equally prior chemotherapy and pre-existent steatosis may play a role.
A third important aspect is that during liver surgery deliberate hypotension and temporary hepatic blood inflow occlusion (the so-called Pringle manoeuvre) are used by many surgeons to reduce blood loss during liver surgery (15 minutes ischaemia, 5 minutes reperfusion (15/5 Pringle)). Other surgeons do not use this manoeuvre, assuming that it causes oxidative stress and ischaemia/reperfusion (I/R) injury.4,5 There is little doubt that this procedure does cause oxidative stress and I/R injury; however, the consequence of this is variable. In a situation where defence mechanisms against oxidative stress are deficient it may adversely affect liver function. In this situation hepatic steatosis may constitute an additional predisposing factor to damage by ischaemia/reperfusion.
In this situation it is assumed that defence mechanisms against oxidative stress are adequate and are indeed enhanced by short-term I/R injury.7
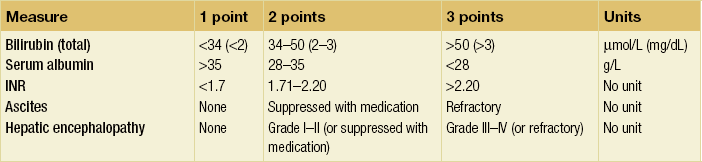
Chronic liver failure
The Child–Pugh score for chronic liver disease8 has served as a useful means of categorising patients based on the severity of their liver disease. It employs five clinical measures of liver disease and each measure is scored 1–3, with 3 indicating the most severe derangement (Table 1.1).
Metabolic liver function
Liver failure gives rise to multiple abnormalities in nitrogen metabolism, some of which are thought to play a crucial role in the characteristic syndrome of hepatic encephalopathy that accompanies liver failure. Hepatic encephalopathy is a reversible neuropsychiatric syndrome, with a probably multifactorial cause.9 The current belief is that ammonia is one of the key components in the aetiology of hepatic encephalopathy10 because liver failure is usually associated with moderate to severe hyperammonaemia. Hyperammonaemia leads to increased brain uptake of ammonia, followed by detoxification of ammonia in the brain by coupling to glutamate to form glutamine. This process consumes glutamate (an important excitatory neurotransmitter) and leads to the formation of glutamine, which acts as an osmolite causing brain oedema.
These changes in plasma levels were thought to be caused by increased BCAA catabolism in muscle and decreased AAA breakdown in the failing liver.14 A reduction in the insulin–glucagon ratio in this situation may play a key role in disturbing the balance between anabolism and catabolism. Accumulation of AAAs in the circulation in combination with the increased breakdown of BCAAs, particularly in skeletal muscle, would, according to this hypothesis, give rise to a decrease in the BCAA to AAA ratio, the so-called Fischer ratio. The increase in plasma AAAs in combination with increased blood–brain barrier permeability for neutral amino acids has been suggested to contribute to an increased influx of AAAs in the brain, since they compete for the same amino acid transporter. This, in turn, would lead to imbalances in neurotransmitter synthesis and accumulation of false neurotransmitters such as octopamine in the brain, which may contribute to hepatic encephalopathy.15
Measuring liver volume
Advances in imaging have permitted the development of in vivo imaging of the liver. Three-dimensional models of the liver can be constructed from computed tomography (CT) or other cross-sectional imaging modalities, such as magnetic resonance imaging (MRI). The volume of the liver can then be calculated based on known separation of image slices combined with planar mapping of cross-sectional areas. In addition, such three-dimensional computer models can be simulated to map the effects of surgery by performing virtual hepatic resection, and studies have demonstrated that there is a good correlation between computer modelling and actual resection weight of surgical liver specimens (Fig. 1.1).2,16
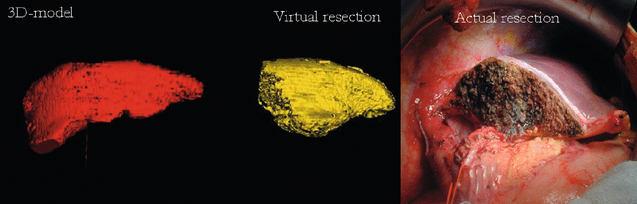
Figure 1.1 Three-dimensional reconstruction of the liver preoperatively (red) showing tumours (green). Computer prediction of residual liver volume based on virtual hepatectomy of 3-D model (yellow) and actual photograph of resection showing residual liver segments. Reproduced from Schindl MJ, Redhead DN, Fearon KC et al. The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut 2005; 54:289–96. With permission from the BMJ Publishing Group Ltd.
This technology is useful as a research tool because it allows liver function to be put into the direct context of the volume of functioning liver tissue. In addition, this technology is useful for predicting the need for reconstruction of venous territories of the liver in split liver transplantation. Usually, liver volumetry is performed on software directly linked to the hardware MRI or CT. In recent years, however, stand-alone software has become available, which makes it possible to perform hepatic volumetry remote from the radiological hardware. Examples of such software are the freely downloadable program ImageJ (for Windows-based PCs) and OsiriX (for Apple Macintosh). Our group has recently shown that the ImageJ software is very useful in measuring liver volumes in patients referred with a CT undertaken in the referring centre17 (Figs 1.2 and 1.3).
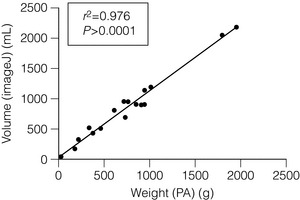
Figure 1.2 Correlation between volume of resection calculated with ImageJ and actual measured weights of the resection specimens (n = 15, Pearson’s test).14 Reproduced with permission from World J Surg.
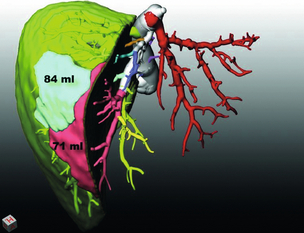
Figure 1.3 Mapping the territory of the right hepatic lobe drained by the middle hepatic vein. The numbers represent the volumes of the territories at risk if segment 5 and 8 tributaries of the middle hepatic vein were not reconstructed in a potential right lobe living-donor liver transplant. Image reproduced with permission of MeVis imaging technologies, Bremen, Germany Kindly provided by H. Lang and A. Radtke, Plainz, Germany.
Blood tests of liver function
Biochemical markers of true liver function vary depending on whether acute or chronic liver failure or injury is being considered (Table 1.2).
Table 1.2
Blood tests useful to assess function in acute and chronic liver injury
| Acute | Chronic | |
| Albumin | − | +++ |
| Prothrombin time | +++ | +++ |
| Bilirubin | + | +++ |
| Lactate | ++ | − |
| Glucose requirement | ++ | − |
| Ammonia | + | + |
Tests of liver function measuring substance clearance
Indocyanine green (ICG)
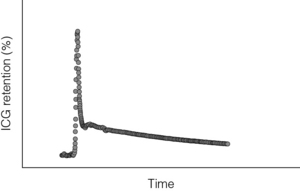
ICG is a compound that is used widely to measure liver function. It is rapidly cleared from blood by hepatocytes and is excreted into bile without enterohepatic circulation. Hepatocytes are so effective at clearing ICG from the circulation that the major limiting factor to its clearance is liver blood flow. This is thought to be reduced in cirrhosis. ICG clearance can be measured as ‘disappearance’ from the blood or can also be measured as accumulation in bile. Liver dysfunction is suggested by a slower rate of clearance from the blood and is usually expressed as percentage retention at 5 or 15 minutes after injection. Continuous measurement of ICG clearance can also be performed, which offers the potential improvement in accuracy by measurement of area under the clearance curve (Fig. 1.4). In some centres ICG clearance is routinely performed during preoperative work-up with cut-off values set for which patients are ‘safe’ to proceed to resection. However, there is no evidence to suggest that outcomes are improved in centres that use this test compared to centres that do not. In chronic liver disease, discriminative ability of ICG clearance is greatest in those with intermediate to severe liver failure. Addition of this test to the MELD score (Model for End-stage Liver Disease) can improve prognostic accuracy for patients with intermediate to severe liver dysfunction.18 However, given the relationship with hepatic blood flow, caution should be exercised when interpreting ICG clearance in the context of abnormally high cardiac output.
Hepatobiliary scintigraphy
Using a radiolabelled tracer that is eliminated exclusively by the liver, such as [99mTc]mebrofenin, blood clearance and hepatic uptake can be measured using a gamma camera to provide an indication of hepatic function. Hepatobiliary scintigraphy may improve predictive value compared to future liver remnant volume, especially in patients with uncertain quality of liver parenchyma.19 However, HBS has not been widely used preoperatively, there is no evidence that it outperforms ICG clearance and the requirement for a nuclear medicine facility on-site may limit its application.
Urea synthesis
In the recent past, we have explored the feasibility of measuring urea synthesis using stable isotopes and relating this to liver volume in patients undergoing liver resection.20 This study was conducted against the background of the notion that liver failure is almost always accompanied by hyperammonaemia, related to a presumed failure of hepatic urea synthesis. Using stable isotopically 13C-labelled urea, urea synthesis was measured before and after major hepatic resection, and liver volumes before and after resection with CT scans.
Measuring liver blood flow
Blood flow in the splanchnic area, particularly the gut and liver, can be measured in a number of ways. These can basically be either invasive (i.e. intraoperative) or non-invasive. During surgery, when the abdomen is opened, blood flow can be measured in the portal vein and in the main hepatic artery. Portal vein blood flow measurements provide predominantly information on the flow across the intestines. By summing up the blood flow in the hepatic artery and the portal vein, total hepatic blood flow can be calculated. Theoretically, this could also be achieved by measuring hepatic venous outflow, but this is impractical in humans because of the short common outflow tract of the three hepatic veins. Non-invasive MRI-based techniques are being developed that may offer improved accuracy of measurement of liver blood flow and the potential for repeat measurements.21 The ratio of portal vein to hepatic artery blood flow changes with increasing resistance of the liver and may indicate the development of fibrosis or cirrhosis. Methodology for assessing the importance of blood flow as a predictor of liver parenchymal condition has not been fully evaluated but may provide a means of assessing safety of surgery and regenerative capacity in some patients.
During liver surgery, organ blood flow can also be measured by means of colour Doppler ultrasound scanning (e.g. Aloka Prosound SSD 5000; Aloka Co. Ltd, Tokyo, Japan). A 5-MHz probe is used to trace the vessels and calculate the cross-sectional area. Then, time-averaged mean velocities of the bloodstream are measured at the point where the cross-sectional area of the portal vein and hepatic artery is taken. For accurate velocity measurements, care must be taken to keep the angle between the ultrasonic beam direction and blood flow direction below 60°. The cross-sectional area of the vessel is calculated by drawing an area ellipse at the same point as where the velocity was measured. Portal venous and hepatic arterial blood flows can then be measured proximal to their hilar bifurcations. In the case of an accessory hepatic artery, both arteries should obviously be measured.22,23 In our experience, this method gives roughly the same values as the ultrasonic flow measurement described above. Theoretically, it is possible to perform such flow measurements preoperatively or postoperatively using a percutaneous approach, although the measurement in the hepatic artery requires a skilled ultrasonographer.
In recent years, technical improvements in hardware and software applications for MRI have made it possible to measure blood flow in the portal vein and hepatic artery in a non-invasive manner. By linking this method of flow measurement to hepatic volumetry using MRI, blood flow per volume unit of liver can be calculated.24,25 It has been suggested that MRI may provide a more accurate and reliable assessment of portal vein and hepatic artery blood flow than ultrasonography, particularly given the wide interobserver variability seen with the latter technique.21 Although limited to the preoperative period, MRI flow studies may provide complementary information to intraoperative ultrasonography as required.
A further technique that is emerging is the use of near-infrared spectroscopy. This technique measures absorption of near-infrared wavelength light and from this can be calculated tissue oxygenation, since haemoglobin oxygenation status alters absorption of this wavelength light. This technique is more useful for estimating tissue oxygenation and perfusion at a sinusoidal level, but could potentially be combined with other measures to estimate liver blood flow.26
Effect of major liver resection on hepatic blood flow
Direct measurement of hepatic artery and portal vein blood flow before and after liver resection reveals interesting results. When expressed as absolute values portal blood flow does not change whereas hepatic artery blood flow falls. Typically, portal vein flow is approximately 840 mL/min and post-resection 805 mL/min. Using this method, hepatic artery flow pre-resection is about 450 mL/min and post-resection 270 mL/min. When these flows are expressed in relation to the preoperative liver volume and residual postoperative liver volume, it can be seen that the blood flow per gram of liver tissue increases in portal flow from a mean 0.55 mL/min per g liver to 1.09 mL/min per g liver and the hepatic artery flow remains relatively constant (Fig. 1.5). In experimental research, pressure measurements can also be obtained using radial artery invasive monitoring to estimate hepatic artery pressure and direct portal vein pressure measurement using a small needle coupled to a pressure transducer similar to that used for measuring central venous pressure. The combination of flow and pressure measurement then allows calculation of hepatic sinusoidal resistance (Fig. 1.5).
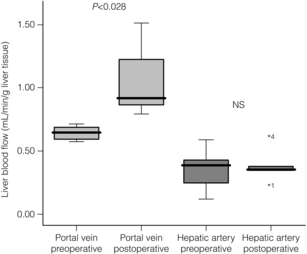
Figure 1.5 Directly measured blood flow intraoperatively in six patients during major hepatic resection. Measurements were taken from the main portal vein and the main hepatic artery simultaneously using multichannel Transonics ultrasound flow probes. During the liver resection one branch of each of the portal vein and hepatic artery is ligated. The post-resection blood flow measurement has been taken just before closure of the abdomen, typically 1–2 hours after the first measurement. Results are expressed per gram of liver tissue.
Effect of major liver resection on innate immunity
It is not unreasonable to expect that major liver resection might result in some impairment of innate immunity. Our group has previously demonstrated that major liver resection is associated with increased frequency of infection as well as increased likelihood of objective evidence of liver function impairment.2
Molecular signals for hepatic regeneration
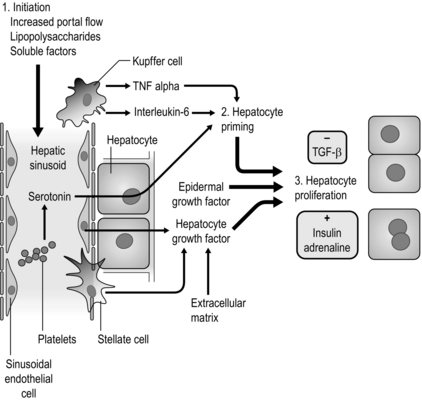
Stimuli for liver regeneration stimulate transcription factors that turn on a variety of genes expressing growth factors. Although not direct growth factors, the hormones insulin and adrenaline potentiate the effects of growth factors on hepatocyte regeneration. All elements of the liver are required to regenerate; however, the coordination of these processes is complex. Removal of the stimulus for regeneration by growth to pre-injury capacity and transforming growth factor-β act as brakes that slow regeneration of liver elements (Fig. 1.6).
Cell populations involved in liver regeneration
Histology of normal liver regeneration following resection or acute injury shows the presence of high mitotic rates in mature hepatocytes. Normally, these cells are mitotically quiescent but can move into S phase extremely rapidly. For example, following 70% hepatectomy in rat approximately 30–40% of hepatocytes are seen to be undergoing mitosis within 48 hours of surgery and indeed the liver will regain its normal size within 10 days. The situation is more complex in chronically injured liver (e.g. cirrhotic liver); here, the hepatocytes are less able to undergo mitosis and are frequently in cell cycle arrest. Furthermore, the accumulation of excess scar tissue deposited in cirrhosis contributes to the inability of the liver to respond to injury and regenerate effectively. In this setting a second population of cells becomes activated and can contribute to parenchymal regeneration. These intrahepatic cells are located in the canal of Hering (the most distal branch of the biliary tree); termed hepatic progenitor cells (HPCs), they are bipotential and are capable of giving rise to both biliary and hepatocyte populations under the influence of macrophage-derived factors (see above). This response is seen in chronic or severe injury and sometimes appears as a ductular reaction. Although it is now recognised that the HPCs can regenerate the liver in chronic liver disease, whether these progenitor cells are capable of responding to the acute demands of major hepatic resection is as yet unknown. It is also worth noting that there is an increasing recognition that intrahepatic stem cells are a likely source of a significant proportion of liver cancers. The role of circulating extrahepatic cells in liver regeneration has received interest recently and the potential bone marrow origin of hepatocytes has been suggested. However, if this phenomenon occurs at all, it is extremely rare. The bone marrow does, however, supply macrophages and myofibroblasts that are involved in the liver’s scarring response to injury. The relationship between bone marrow-derived cells and the response to injury is complex, with different macrophage subtypes shown to either promote fibrosis or repair. However, administration of bone marrow-derived macrophages to the fibrotic liver via the portal vein has been shown to reduce fibrosis and improve markers of regeneration in preclinical models.29 The use of bone marrow populations to stimulate liver regeneration in both animal models and clinical studies is likely to be an area of future development (see later).
Hepatic steatosis
Fat infiltration of the liver is an increasing problem with increased prevalence of obesity and the metabolic syndrome (obesity and type 2 diabetes). Macroscopically the liver may appear enlarged, pale or yellow coloured with rounded edges. Microscopically the liver can have microsteatosis (small fat droplets within every hepatocyte) or macrosteatosis (regional infiltration of hepatocytes with large fat droplets) (see Fig. 1.7).
Assessment of steatosis
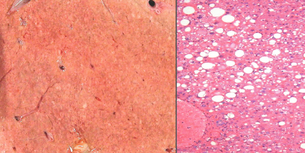
Assessment of hepatic steatosis is notoriously difficult. Experienced surgeons can estimate liver fat by judging the size, rounded or sharp edges of the liver and its appearance. Even using colour as an estimate is prone to error, as can be seen in Fig. 1.8.

Figure 1.8 Physical appearance of livers with varying fat content confirmed by histology to demonstrate the poor correlation between colour and objective measurement of fat content.
The gold standard for hepatic fat assessment is histology. Trucut or wedge biopsies can be assessed by a pathologist and a reliable estimate of the percentage fat content produced. In addition, useful information including the distribution – macrosteatosis or microsteatosis – and the presence of fibrosis or inflammation can be provided. New MRI techniques are, however, challenging the accuracy of pathological assessment of steatosis and offer the potential advantage of being non-invasive.30
Chemotherapy-induced liver changes
Increased usage of chemotherapy in the neoadjuvant context has revealed changes in the liver associated with chemotherapy, particularly oxaliplatin and irinotecan. These range from a soft, fragile pale liver to steatosis, steatohepatitis and sinusoidal dilatation. Surgery should be deferred until 6 weeks after chemotherapy and studies, although conflicting, suggest that tolerance of major liver resection may be reduced and complications more frequent in individuals who have received chemotherapy. A study by Mehta et al. showed that oxaliplatin-based chemotherapy was associated with increased blood loss and prolonged hospital stay.31
Portal vein embolisation
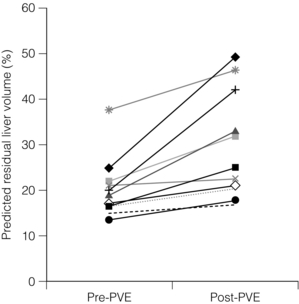
Morbidity and mortality after hepatectomy have constituted a limitation on the number of patients eligible for resection, and currently only 8% of patients with colorectal hepatic metastases are candidates for curative liver resection. Liver function is correlated with liver volume, and consequently hepatic insufficiency in this situation may arise because not enough functional liver volume is left after surgical removal of part of the liver. Interestingly, following removal of part of the liver, the residual liver usually regenerates to the point where the preoperative liver weight–body weight ratio is regained. This notion has led to the belief that if it were possible to increase preoperatively the volume of the future residual liver, it would be possible to perform more extensive liver resections and hence cure more patients. It has long been recognised that interruption of one part of the liver portal blood flow usually leads to hypertrophy of normally vascularised liver. This has been observed in patients with Klatskin tumours, which have a tendency to invade the portal vein, causing ipsilateral atrophy and contralateral hypertrophy. This concept has subsequently been harnessed by manoeuvres such as embolising the right portal vein prior to surgical resection. This leads to hypertrophy of the left liver lobe prior to surgery and facilitates the subsequent safe extensive resection of the right liver (extended right hepatectomy) 6 weeks later (Fig. 1.9). This phenomenon has been harnessed to maximise the residual functional liver volume of patients who are predicted to have a small remnant liver volume. This approach is fully based on the concept that, in the normal liver, volume is correlated to function and hence liver failure occurs when residual liver volume is too small. A completely different and novel approach would be to improve liver function per volume unit of liver. Recent evidence from studies using mebrofenin suggests that functional improvement of the future liver remnant following portal vein embolisation (PVE) may precede changes in liver volume.32 This important observation suggests that surgery earlier after PVE may be possible. Limitations to PVE-induced hypertrophy include pre-existing hepatic fibrosis or cirrhosis and technical or anatomical inability to completely obstruct a major portal vein branch.
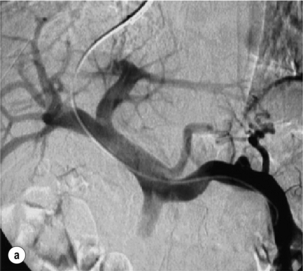
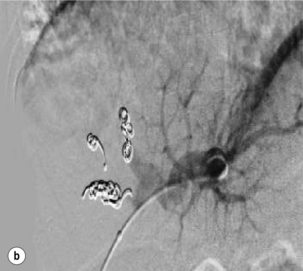
Figure 1.9 Portal venograms showing the main left and right branches prior to embolisation (a), and after embolisation of the right portal vein (b).
Technique
The most common technique of PVE is to puncture a branch of the vein using a percutaneous approach. A venogram is obtained to demonstrate all of the relevant branches and then the branch to be embolised is cannulated and coils and embolic material delivered to obstruct portal flow. A check angiogram can be performed to demonstrate success of the technique. Usually either a left or right main branch is occluded. To obtain hypertrophy of segments 2 and 3 in large right-sided tumours, it is not sufficient to embolise just the right portal vein and it is recommended that the branches supplying segment 4 should also be embolised. Patients usually tolerate PVE remarkably well, presumably because of the dual blood supply of the liver, and complications are uncommon. Significant hypertrophy can be achieved, as can be seen in Fig. 1.10.
Therapy for liver failure
Artificial extracorporeal liver support
For the vast majority of patients who take toxic doses of paractetamol, suffer alcohol-induced liver injury or develop liver dysfunction following liver resection, the regenerative capacity of the liver is sufficient to prevent irretrievable liver failure and death. However, when this regenerative capacity is overwhelmed treatment strategies to temporarily or permanently replace the failing liver are required. The ability to provide short-term extracorporeal liver support, either during the wait for transplantation or to facilitate liver regeneration and avoid transplantation, is an attractive option. A range of devices have been developed, either focusing on the detoxification functions of liver (artificial liver support) or also incorporating bioreactors intended to also perform synthetic liver functions (bioartificial liver support). Assessment of efficacy has been hampered by the limited number of randomised controlled trials and small sample size, but a recent meta-analysis does suggest overall survival benefit in acute liver failure.33
Artificial liver support
Artificial systems include the MARS (Molecular Adsorbent Recirculating System) device, Prometheus and the BioLogic-DT (now called the Liver Dialysis Device, currently being redesigned). The greatest experience has been with the MARS device, which deploys an albumin dialysis circuit to remove both water-soluble and protein-bound toxins.34 Thus, a low Fischer ratio can be corrected by recirculating albumin dialysis.35 Because the system preferentially removes AAAs, compared with BCAAs, the Fischer ratio significantly increases, predominantly by the removal of AAAs in a small series of patients.35–38 MARS has been shown to be useful in fulminant hepatic failure, by attenuating the increase in intracranial pressure, which plays a major role in this situation.32 There may also be an effect on survival and improvement of degree of hepatic encephalopathy in patients with acute or chronic liver failure.37,39 Equally, the system has been tested on artificial neuronal networks showing a normalisation of abnormal signals if the medium (plasma derived from rats with liver failure) was pretreated with MARS. The role of MARS in a more chronic situation of mild hepatic encephalopathy, when correction of an abnormal Fischer ratio would likely be more important if this were a major pathogenetic factor, is still largely unknown and deserves further study.40 It has been suggested that the role of MARS and bioartificial liver support systems should be limited to carefully designed clinical trials.41 It is currently uncertain how hepatic excretory assistance devices, such as MARS, compare with bioartificial liver assistance devices, which in addition to their excretory functions aim to provide biosynthetic capacity.37
Cell therapy for liver failure: general principles
The dual goals of stem cell therapy in the context of acute liver failure or injury are to promote rapid recovery of hepatocyte function and to allow regeneration of liver tissue without excessive scarring. Direct administration of hepatocytes or stem cell-derived hepatocytes to the injured liver has been met with little success in preclinical studies. However, using bone marrow-derived cells to support endogenous processes may support the regenerating liver, enabling effective regeneration.42
Haemopoetic stem cell therapy for liver disease in humans
There are several reports in the scientific literature of bone marrow (BM) stem cell therapy in patients with advanced liver disease. It was first reported that BM stem cells could increase the liver’s ability to regenerate in patients who were undergoing hepatic resection for various liver cancers sited in the right lobe. Here the patients underwent embolisation of the right branch of the portal vein prior to surgery to stimulate compensatory hypertrophy of the left lobe. Autologous CD133-positive BM stem cells were injected into the blood vessels that supply the left liver lobe shortly after the surgery and accelerated regeneration of the non-embolised section of the liver was seen compared with control patients.43 However, it must be stated that this was a small non-randomised study. The second report used BM stem cells in patients with liver cirrhosis.44 CD34-positive stem cells were isolated from the patients’ own blood following granulocyte colony-stimulating factor (GCSF)-induced haematopoietic stem cell mobilisation and were re-injected into the blood supply to the liver – preliminary evidence appeared to show that improvement in liver function in three out of five of the patients occurred during this therapy. In the third study, patients with liver cirrhosis had mononuclear cells isolated from their own BM during general anaesthesia.45 These cells were re-injected into the patient’s bloodstream and again the patient’s liver function appeared to improve. Although these studies are very encouraging, they are preliminary, of small numbers and non-randomised. Furthermore, in none of these studies were the cells marked to enable identification either by radiological tracking or in biopsies of the liver tissue. Therefore, a number of important questions are unanswered. It is not certain that these cells definitely settled in the liver over a period of time, whether some of the cells engrafted other organs in the body and by what mechanisms the cells were having their positive effects within the recipients’ livers.
References
1. O’Grady, J.G., Alexander, G.J., Hayllar, K.M., et al, Early indicators of prognosis in fulminant hepatic failure. Gastroenterology 1989; 97:439–445. 2490426
2. Schindl, M.J., Redhead, D.N., Fearon, K.C., et al, The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut 2005; 54:289–296. 15647196 The first paper providing strong evidence of an association between residual liver volume and clinical infection.
3. van Hoorn, E.C., van Middelaar-Voskuilen, M.C., van Limpt, C.J., et al, Preoperative supplementation with a carbohydrate mixture decreases organ dysfunction-associated risk factors. Clin Nutr 2005; 24:114–123. 15681109
4. Kretzschmar, M., Kruger, A., Schirrmeister, W., Hepatic ischemia–reperfusion syndrome after partial liver resection (LR): hepatic venous oxygen saturation, enzyme pattern, reduced and oxidized glutathione, procalcitonin and interleukin-6. Exp Toxicol Pathol 2003; 54:423–431. 12877355
5. Garcea, G., Gescher, A., Steward, W., et al, Oxidative stress in humans following the Pringle manoeuvre. Hepatobil Pancreat Dis Int 2006; 5:210–214. 16698577
6. Clavien, P.A., Yadav, S., Sindram, D., et al, Protective effects of ischemic preconditioning for liver resection performed under inflow occlusion in humans. Ann Surg 2000; 232:155–162. 10903590 The first randomised clinical trial demonstrating benefit in clinical markers from ischaemic preconditioning of the liver in patients undergoing liver resection.
7. Patel, A., van de Poll, M.C., Greve, J.W., et al, Early stress protein gene expression in a human model of ischemic preconditioning. Transplantation. 2004;78(27):1479–1487. 15599312
8. Pugh, R.N., Murray-Lyon, I.M., Dawson, J.L., et al, Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973; 60:646–649. 4541913
9. Albrecht, J., Jones, E.A., Hepatic encephalopathy: molecular mechanisms underlying the clinical syndrome. J Neurol Sci 1999; 170:138–146. 10617392
10. Shawcross, D., Jalan, R., The pathophysiologic basis of hepatic encephalopathy: central role for ammonia and inflammation. Cell Mol Life Sci 2005; 62:2295–2304. 16158192
11. James, J.H., Ziparo, V., Jeppsson, B., Fischer, J.E., Hyperammonaemia, plasma amino acid imbalance, and blood–brain amino acid transport: a unified theory of portal–systemic encephalopathy. Lancet 1979; 2:772–775. 90864 Explanation of the relationship between the urea cycle and hepatic encephalopathy.
12. Fischer, J.E., Yoshimura, N., Aguirre, A., et al, Plasma amino acids in patients with hepatic encephalopathy. Effects of amino acid infusions. Am J Surg 1974; 127:40–47. 4808685
13. Soeters, P.B., Fischer, J.E., Insulin, glucagon, aminoacid imbalance, and hepatic encephalopathy. Lancet 1976; 2:880–882. 62115
14. Fischer, J.E., Rosen, H.M., Ebeid, A.M., et al, The effect of normalization of plasma amino acids on hepatic encephalopathy in man. Surgery 1976; 80:77–91. 818729
15. Fischer, J.E., Baldessarini, R.J., False neurotransmitters and hepatic failure. Lancet 1971; 2:75–80. 4103986
16. Wigmore, S.J., Redhead, D.N., Yan, X.J., et al, Virtual hepatic resection using three-dimensional reconstruction of helical computed tomography angioportograms. Ann Surg 2001; 233:221–226. 11176128
17. Dello, S.A., van Dam, R.M., Slangen, J.J., et al, Liver volumetry plug and play: do it yourself with ImageJ. World J Surg 2007; 31:2215–2221. 17726630
18. Zipprich, A., Kuss, O., Rogowski, S., et al, Incorporating indocyanin green clearance into the Model for End Stage Liver Disease (MELD-ICG) improves prognostic accuracy in intermediate to advanced cirrhosis. Gut. 2010;59(7):963–968. 20581243
19. Bennink, R.J., Dinant, S., Erdogan, D., et al, Preoperative assessment of postoperative remnant liver function using hepatobiliary scintigraphy. J Nucl Med 2004; 45:965–971. 15181131
20. van de Poll, M.C., Wigmore, S.J., Redhead, D.N., et al, Effect of major liver resection on hepatic ureagenesis in humans. Am J Physiol Gastrointest Liver Physiol 2007; 293:G956–G962. 17717046 Clinical experimental study demonstrating the relationship between liver volume and urea synthesis in patients undergoing varying degrees of liver resection.
21. Vermeulen, M.A., Ligthart-Melis, G.C., Buijsman, R., et al, Accurate perioperative flow measurement of the portal vein and hepatic and renal artery: a role for preoperative MRI? Eur J Radiol. 2012;81(9):2042–2048. 21724349
22. van de Poll, M.C., Ligthart-Melis, G.C., Boelens, P.G., et al, Intestinal and hepatic metabolism of glutamine and citrulline in humans. J Physiol 2007; 581:819–827. 17347276
23. van de Poll, M.C., Siroen, M.P., van Leeuwen, P.A., et al, Interorgan amino acid exchange in humans: consequences for arginine and citrulline metabolism. Am J Clin Nutr 2007; 85:167–172. 17209193
24. Barbaro, B., Manfredi, R., Bombardieri, G., et al, Correlation of MRI liver volume and Doppler sonographic portal hemodynamics with histologic findings in patients with chronic hepatitis C. J Clin Ultrasound 2000; 28:461–468. 11056023
25. Nanashima, A., Shibasaki, S., Sakamoto, I., et al, Clinical evaluation of magnetic resonance imaging flowmetry of portal and hepatic veins in patients following hepatectomy. Liver Int 2006; 26:587–594. 16762004
26. El-Desoky, A.E., Seifalian, A., Cope, M., et al, Changes in tissue oxygenation of the porcine liver measured by near-infrared spectroscopy. Liver Transpl Surg 1999; 5:219–226. 10226114
27. Schindl, M.J., Millar, A.M., Redhead, D.N., et al, The adaptive response of the reticuloendothelial system to major liver resection in humans. Ann Surg 2006; 243:507–514. 16552202
28. Boulter, L., Govaere, O., Bird, T.G., et al, Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med. 2012;18(4):572–579. 22388089
29. Thomas, J.A., Pope, C., Wojtacha, D., et al, Macrophage therapy for murine liver fibrosis recruits host effector cells improving fibrosis, regeneration, and function. Hepatology. 2011;53(6):2003–2015. 21433043
30. Guiu, B., Cercueil, J.P., MRI as the new reference standard in quantifying liver steatosis: the need for international guidelines. Gut. 2012;61(9):1369–1370. 22234981
31. Mehta, N.N., Ravikumar, R., Coldham, C.A., et al, Effect of preoperative chemotherapy on liver resection for colorectal liver metastases. Eur J Surg Oncol 2008; 34:782–786. 18160247
32. de Graaf, W., van Lienden, K.P., van den Esschert, J.W., et al, Increase in future remnant liver function after preoperative portal vein embolization. Br J Surg. 2011;98(6):825–834. 21484773
33. Stutchfield, B.M., Simpson, K., Wigmore, S.J., Systematic review and meta-analysis of survival following extracorporeal liver support. Br J Surg. 2011;98(5):623–631. 21462172
34. Tan, H.K., Molecular adsorbent recirculating system (MARS). Ann Acad Med Singapore 2004; 33:329–335. 15175774
35. Loock, J., Mitzner, S.R., Peters, E., et al, Amino acid dysbalance in liver failure is favourably influenced by recirculating albumin dialysis (MARS). Liver. 2002;22(Suppl. 2):35–39. 12220301
36. Awad, S.S., Swaniker, F., Magee, J., et al, Results of a phase I trial evaluating a liver support device utilizing albumin dialysis. Surgery 2001; 130:354–362. 11490371
37. Mitzner, S., Loock, J., Peszynski, P., et al, Improvement in central nervous system functions during treatment of liver failure with albumin dialysis MARS – a review of clinical, biochemical, and electrophysiological data. Metab Brain Dis 2002; 17:463–475. 12602522
38. Steczko, J., Bax, K.C., Ash, S.R., Effect of hemodiabsorption and sorbent-based pheresis on amino acid levels in hepatic failure. Int J Artif Organs 2000; 23:375–388. 10919755
39. Boyle, M., Kurtovic, J., Bihari, D., et al, Equipment review: the molecular adsorbents recirculating system (MARS). Crit Care 2004; 8:280–286. 15312211
40. Hassanein, T.I., Tofteng, F., Brown, R.S., Jr., et al, Randomized controlled study of extracorporeal albumin dialysis for hepatic encephalopathy in advanced cirrhosis. Hepatology 2007; 46:1853–1862. 17975845
41. Ferenci, P., Kramer, L., MARS and the failing liver – Any help from the outer space? Hepatology 2007; 46:1682–1684. 18046718
42. Stutchfield, B.M., Forbes, S.J., Wigmore, S.J., Prospects for stem cell transplantation in the treatment of hepatic disease. Liver Transpl. 2010;16(7):827–836. 20583084
43. am Esch, J.S., 2nd., Knoefel, W.T., Klein, M., et al, Portal application of autologous CD133 + BM cells to the liver: a novel concept to support hepatic regeneration. Stem Cells 2005; 23:463–470. 15790766
44. Gordon, M.Y., Levicar, N., Pai, M., et al, Characterisation and clinical application of human CD34 + stem/progenitor cell populations mobilised into the blood by G-CSF. Stem Cells 2006; 24:1822–1830. 16556705
45. Terai, S., Ishikawa, T., Omori, K., et al, Improved liver function in liver cirrhosis patients after autologous bone marrow cell infusion therapy. Stem Cells 2006; 24:2292–2298. 16778155