Liver and portal venous system
TRAUMA
The liver is the second most common organ injured in abdominal trauma. Blunt liver trauma, for example following road traffic accidents, is more common than penetrating trauma such as stab or gunshot injuries. The mortality is much higher for blunt liver trauma and it increases even more if associated with other visceral injuries. Types of injuries include laceration, contusion, haematoma, vascular injury, bile duct and gallbladder injury. Over the last two decades there has been a paradigm shift in liver trauma management from compulsory operative treatment to non-operative management.1–4
Appraise
1. The patient will frequently present in a shocked state: the first priority is to maintain airway, breathing and circulation (ABC), following the principles of advanced trauma life support (ATLS). After ensuring normal cardiorespiratory function, carry out a secondary survey, examining the head, spine, chest and limbs as well as the abdomen. If the external injuries do not explain the degree of shock, assume there is internal bleeding. Stop external haemorrhage by direct pressure, splint any broken limbs, protect a fractured spine, treat a crushed chest by mechanical ventilation and assess any head injury. Now direct your attention to the internal bleeding.
2. Focused assessment sonography in trauma (FAST) is very valuable in diagnosing free intra-peritoneal fluid when performed by trained staff but is not reliable in diagnosing parenchymal injury. An emergency laparotomy is mandatory in the presence of free intra-peritoneal fluid on FAST in a haemodynamically unstable patient. However, if the patient is haemodynamically stable then obtain an urgent contrast enhanced computed tomography (CT) scan to diagnose and grade parenchymal injury. The four quadrant abdominal tap was superseded by diagnostic peritoneal lavage (DPL). However, DPL is often oversensitive and nowadays is performed less frequently due to increased use of FAST and CT. Very few surgeons in the UK now have experience in performing DPL.
3. If you remain in doubt and the patient fails to respond to resuscitation, consider performing an emergency laparotomy.
4. Penetrating injuries and gunshot wounds are treated by laparotomy. Treat conservatively only selected patients with stab injury who are haemodynamically stable and in whom there is no evidence of hollow viscus perforation.
5. Treat conservatively those patients with blunt trauma who are haemodynamically stable, even if there is demonstrable liver injury on CT or ultrasound scan. Monitor the patient with repeated clinical assessment and scans. If the patient becomes haemodynamically unstable or develops signs of peritonitis, proceed to a laparotomy.
6. Interventional radiology is now an important part of liver trauma management. Angiography and hepatic artery embolization is safe and effective in controlling hepatic arterial bleeding. If the patient is haemodynamically stable, consider angiography and embolization if there is extravasation of contrast on CT. This will decrease the number of blood transfusions and may avoid a laparotomy.
EXPLORATION OF A DAMAGED LIVER
Prepare
1. Have you booked an intensive care unit bed if needed postoperatively?
2. You are about to embark on a major surgical procedure, carrying a high mortality rate. As far as possible, ensure you have an experienced anaesthetist, competent assistant and experienced scrub nurse. Ensure vascular clamps and sutures are readily available.
3. Maintain good communication with your anaesthetist and scrub team. There is potential for sudden, life-threatening haemorrhage during the procedure. Good team work is essential for achieving a successful outcome.
4. Check the clotting. If coagulopathy is present give fresh-frozen plasma (FFP), platelets and cryoprecipitate as necessary. Watch for the deadly triad of coagulopathy, hypothermia and acidosis. If this is present then surgical control of the bleeding is unlikely to be successful.
5. Pass a bladder catheter. It is essential to monitor urine output and ensure adequate fluid volume replacement during major liver surgery to avoid acute renal failure.
6. Your anaesthetist will place a central venous line, wide-bore peripheral venous lines and, ideally, an arterial catheter to allow constant measurement of arterial pressure. A warming blanket is used to prevent hypothermia.
7. Ensure there are at least six units of blood available, together with FFP and platelets to replace lost clotting products.
Access
1. If you suspect the diagnosis preoperatively, use a transverse incision in the right upper quadrant, extending from a point 2 cm above the umbilicus to a point on the midaxillary line midway between the subcostal margin and iliac crest, with a vertical midline extension to the xiphoid. If necessary extend the incision across the midline transversely in the left upper quadrant. If you need to gain access above the liver, create a second incision in the tenth rib bed from the anterior axillary line to join the original subcostal incision at right angles. This allows you to open the chest, divide the costal margin and so gain control of the inferior vena cava (IVC) above the diaphragm.
2. If you discover the rupture at diagnostic laparotomy through a midline incision, create a transverse lateral extension to the right (as described above) to gain access and assess the need for further extensions.
3. If you discover the rupture through a lower abdominal incision, close it and re-explore the abdomen through an appropriate incision.
4. Use a self-retaining retractor that can be fixed to the operating table and provides forcible upward retraction of the costal margins, such as Thompson’s retractor (Thompson Surgical Instruments Inc.). It crucially reduces the need for manual assistance, greatly improves access and usually eliminates the need for thoracic extension of the incision.
Assess
1. There is usually a great deal of blood clot in the peritoneal cavity and probably some fresh bleeding. Remove clot with your hands and a sucker.
2. Look systematically for damage to the liver, spleen, the gut from oesophagus to rectum, pancreas, anterior and posterior abdominal wall and the diaphragm. If necessary, pack the abdominal cavity with sterile packs, removing them serially, inspecting each of the four quadrants in succession.
Action
1. If there is obvious damage to, and haemorrhage from, another intra-abdominal viscus such as the spleen, and that is the primary source of bleeding, surround the damaged area of the liver with large sterile packs and have an assistant apply gentle pressure to control the bleeding. Attend to the lesion in the other organ first, and then turn your attention to the liver.
2. Inspection and palpation of the liver surface will allow you to estimate the extent of the parenchymal injury. Place packs over the superior and inferior surfaces of the liver to compress the laceration or contusion. Remove blood and blood clots to gain adequate exposure of the bleeding area of the liver. The initial packing with compression of the liver parenchyma will control much of the active bleeding. If the patient has coagulopathy, wait with the packs in place until sufficient FFP, platelets or cryoprecipitate have been transfused. Most patients do not require major surgical procedures: attempt only the minimum surgery necessary to control haemorrhage.
3. If initial packing does not control the haemorrhage then perform inflow occlusion (Pringle manoeuvre) by inserting a finger through the opening into the lesser sac behind the hepatic hilar structures and apply a Satinsky or other vascular clamp across them. If you do not have vascular clamps available, carefully apply a non-crushing intestinal clamp. This manoeuvre stops bleeding from branches of the hepatic artery and portal vein.
4. If bleeding persists despite the Pringle manoeuvre, then the bleeding is from the hepatic veins or the inferior vena cava, or the patient may have an aberrant arterial supply such as an accessory left hepatic artery arising from the left gastric artery, or an aberrant right hepatic artery arising from the superior mesenteric artery.
5. Avoid rough handling of sites of injury that are not bleeding at the time of exploration. If the patient is hypotensive there is risk of reactionary haemorrhage when the blood pressure subsequently rises.
6. Explore the liver injury locally and remove any devascularized tissue. If the laceration continues to bleed and extends deeply into the liver parenchyma, gently explore the depth of the wound but avoid creating further damage. This procedure is very important in contusion injuries where major branches of the liver vessels may be ruptured, producing large areas of devascularized tissue. Do not be tempted to explore tears that are not bleeding, since you only encourage further bleeding.
7. Identify bleeding points and ligate them with fine synthetic absorbable material such as polyglactin 910 (Vicryl) or polydioxane sulphate (PDS) or use titanium Ligaclips. Suture-ligate larger vessels with PDS or Vicryl.
8. If there is vascular oozing from a large raw area of liver, cover it with one layer of absorbable haemostatic gauze (Surgicel) and apply a pack. Fibrin sealants such as Tisseel or Tachosil (Baxter) may help in stopping the ooze from a raw surface. Avoid using deep mattress sutures to control such bleeding, since they may produce areas of devascularization, which predisposes to subsequent infection.
9. Although a normal liver can tolerate normothermic ischaemia for up to 1 hour, after clamping the liver hilar vessels it is advisable to release the clamp for 10 minutes every 15 minutes, timed by the clock.
10. Before releasing any vascular clamps after prolonged clamping, warn the anaesthetist so that they may take precautionary measures against the effects of massive acidosis and potassium release, which can cause cardiac arrest.
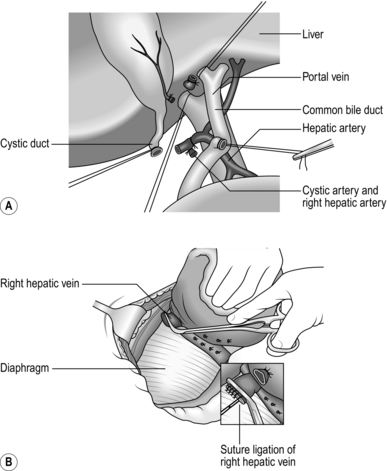
11. Injuries involving the major hepatic veins close to the suprahepatic inferior vena cava or injuries to the retrohepatic inferior vena cava are very difficult to treat and even in experienced hands they are associated with high mortality. Bleeding from such injuries is not controlled by clamping the liver hilum. Attempt to mobilize the inferior vena cava above and below the liver. Complete mobilization of the right lobe of liver is required to expose the retrohepatic vena cava: identify the caval tear and suture it. Clamping the vena cava above and below the liver in addition to clamping the liver hilum will achieve total vascular isolation of the liver, but do not attempt this unless you are very experienced. Veno-venous bypass can be used to shunt blood from the femoral vein to the internal jugular vein to maintain venous return to the heart when total vascular isolation is attempted. However, expertise in veno-venous bypass is generally available only in specialist transplant units.
12. Perihepatic packing. If you cannot achieve control using the described techniques, do not attempt a major resection as an emergency procedure without the assistance of an experienced hepatic surgeon. This is rarely necessary in an emergency and has a high mortality rate. It has been shown in several studies that control is better achieved by packing around the liver with gauze rolls. Do not insert gauze into the depths of a liver laceration. Gently place the packs above, behind and below the liver to compress the bleeding areas (Fig. 17.1).
13. Having gained control with perihepatic packing, close the abdomen. Carry out a contrast-enhanced CT scan and if necessary, a hepatic angiogram. You may then choose to either re-explore in 48–72 hours to remove the packs and re-assess, or transfer the patient to a specialist liver surgery unit.
14. If you encounter torrential bleeding from the liver during the laparotomy, consider autotransfusion using a cell saver system, if available. This will allow you to keep pace with the blood loss whilst attempting to control the haemorrhage.
15. Is there damage to the extrahepatic biliary tree? Attempt reconstruction by anastomosing cut ends of the bile duct end-to-end over a T-tube splint inserted through healthy bile duct. Use fine, interrupted absorbable sutures such as 4/0 Vicryl or PDS. If the damage to the duct is excessive, identify the most distal section of duct which is undamaged below the liver and divide it there. Suture-ligate the distal stump of the bile duct. Anastomose the proximal end of the bile duct to the side of a loop of jejunum, preferably a Roux-en-Y loop, using interrupted sutures of fine Vicryl or PDS in a single-layer anastomosis.
16. If the patient is very ill and unstable, there is no urgency in carrying out a biliary anastomosis. Leave a tube drain through the abdominal wall down to the site of biliary leak; the repair can be carried out by an expert at a later date.
Check
1. When you have gained control of the bleeding from the liver, carry out a full, careful and gentle exploration for other intra-peritoneal injuries if you have not done so already. Examine the entire gut from the oesophagus to rectum, paying special attention to the retroperitoneal duodenum, the pancreas and the spleen.
2. When you are certain that haemorrhage is controlled and that other lesions have been appropriately dealt with, inspect the liver once more, unless you needed to pack it to control haemorrhage. If there are devascularized areas, remove them to prevent infection.
Closure
1. Before you close, mop the peritoneal cavity dry with surgical swabs, or carry out a gentle lavage with warm normal saline and then suck out all the fluid.
2. Close the abdomen using a standard mass technique.
3. If you have operated on any part of the biliary tract insert a drain, preferably with a closed system. If there is liver parenchymal damage only, then a drain is probably unnecessary in the absence of an obvious biliary leak.
Aftercare
1. Admit the patient to an intensive care unit or high-dependency nursing area.
2. If further operation is required, for example because packs were inserted to control haemorrhage, arrange transfer to a specialist unit if possible. Packs increase the risk of sepsis, therefore give antibiotics whilst packs are in place.
3. Mechanically ventilate the patient electively and maintain the blood gases within normal range until the patient is haemodynamically stable, other injuries have been treated and the core temperature has returned to normal. Correct any remaining electrolyte imbalance. Measure blood sugar hourly and treat any abnormality immediately.
4. Evaluate biochemical liver function daily.
5. Maintain intravascular volume and normal clotting. Transfuse with blood and FFP as required.
6. Maintain urine flow. If it falls below 30 ml/hour, first check that the circulating blood volume is adequate, with normal blood pressure and central venous pressure. If you are in any doubt about this, give a ‘fluid challenge’ of 250 ml of colloid intravenously. If this fails to stimulate urine flow despite an adequate blood pressure and normal central venous pressure, consider a low-dose dopamine infusion. If this fails to improve urine flow give 40 mg of furosemide (frusemide) intravenously and seek the advice of a renal physician.
7. Give broad-spectrum antibiotics such as a combination of cefuroxime and metronidazole.
8. If gastrointestinal activity does not return within 2–3 days, institute intravenous nutrition.
9. Sudden collapse suggests that the patient has developed further internal bleeding or septicaemia. If this is associated with abdominal swelling, resuscitate with intravenous blood or colloid, and FFP if the clotting screen is abnormal. Order a contrast-enhanced CT scan and return the patient to the operating room if haemorrhage is confirmed.
10. If you suspect septicaemia then order investigations to determine the source. Culture blood, urine and any leaking body fluid; order a chest X-ray and carry out an ultrasound or CT scan of the abdomen to look for an abscess. Give appropriate antibiotics. Drain an intra-peritoneal abscess either by a further operation or by ultrasound-guided needle aspiration. If the patient is very shocked and the diagnosis uncertain, it is safer to re-explore the abdomen than to wait. Intra-abdominal sepsis is very common following liver trauma and devascularized areas of the liver may become necrotic and infected.
RE-EXPLORATION
Prepare
1. If arterial injury is suspected due to extravasation of contrast on CT or signs of active haemorrhage in the absence of another source of bleeding, obtain a hepatic arteriogram before re-exploration to allow preoperative embolization and to fully assess any vascular damage: this will allow you to prepare for appropriate surgical management and, if necessary, obtain expert help.
2. Ensure that the patient is stable and correct biochemical and haematological abnormalities. Discuss any clotting impairment with the haematologists and give cryoprecipitate or FFP before surgery.
Action
1. Suture-ligate bleeding points with a fine suture or apply haemostats to any obvious bleeding vessels and ligate them.
2. Aspirate any bile collections and attempt to ligate or suture any leaking bile ducts.
3. If major bleeding starts again, attempt to identify the main vessel and suture it. On rare occasions you may carry out lobectomy (see below) if the injury is confined to one lobe.
4. Occasionally, following a severe blunt crushing injury, the damage is so great that no surgical procedure can save the patient’s life, even in the most expert of hands.
REFERENCES
1. Piper GL, Peitzman AB. Current management of hepatic trauma. Surgery Clinics of North America 2010;90:775–85.
2. Kozar RA, McNutt MK. Management of adult blunt hepatic trauma. Curr Opin Crit Care 2010 Sep 9 (E-pub ahead of print).
3. Badger SA, Barclay R, Campbell P, et al. Management of liver trauma. World J Surg 2009;33:2522–37.
4. Milla DJ, Brasel K. Current use of CT in the evaluation and management of injured patients. Surgery Clinics of North America 2011;91:233–48.
PRINCIPLES OF ELECTIVE SURGERY
Appraise
1. The commonest indication for liver resection is primary or secondary malignancy. Hepatocellular carcinoma, cholangiocarcinoma, gall bladder carcinoma and colorectal liver metastases are the most frequent indications for liver resection. Benign diseases such as cavernous haemangioma, adenoma, focal nodular hyperplasia and cystic diseases may occasionally require liver resection. Rarely, liver resection may be required for managing liver injuries. Over the last two decades, liver resection has been increasingly used to procure part of the liver from living donors for liver transplantation.
2. The treatment of hepatobiliary cancers should be discussed at a specialist multidisciplinary meeting involving surgeons, hepatologists, oncologists and interventional radiologists.
3. Do not undertake an elective operation on the liver without complete preoperative investigation and a fairly certain working diagnosis. Occasionally, an isolated hepatic lesion may be identified during a scan carried out for another reason. If you discover a lesion in the liver during a laparotomy, carefully consider the risks before trying to excise or biopsy it. Operating on a patient with impaired liver function can be very hazardous and carries a high morbidity and mortality.
4. The preoperative workup should start with a detailed history and physical examination of the patient. Liver resection in the presence of underlying chronic liver disease is associated with increased morbidity and mortality. Look for signs of chronic liver disease on examination.
5. Carry out biochemical liver function tests, blood count and clotting profile. Underlying chronic liver disease should be suspected if there is an elevated bilirubin, low albumin, increased prothrombin time or low platelet count. Check the tumour markers alfa-feto protein (primary liver cancer) and carcinoembryonic antigen (gastrointestinal cancers) if you suspect a malignant lesion. If chronic liver disease is suspected, carry out hepatitis virus and autoimmune antibody screens.
6. Ultrasound (US) is usually the initial imaging modality for patients presenting with abdominal pain or jaundice. US is useful in assessing the size of the liver and bile ducts, confirming gall stones and detection of ascites, and may show a tumour mass in the liver. Computed tomography (CT) and magnetic resonance imaging (MRI) are optimal investigations for characterizing the nature of liver lesions, assessing resectability and tumour staging. CT and MRI may demonstrate features of chronic liver disease or cirrhosis and can accurately quantify the volume of the liver remnant following resection.
7. Angiography demonstrates hepatic vascular anatomy and vascular involvement by tumour, which often dictates whether resection is feasible. CT angiography and MR angiography have high diagnostic accuracy and conventional angiography is rarely used nowadays.
8. When CT and MRI findings are equivocal in the diagnosis or staging of malignancy, PET scan may be of use. However, there is no role for the routine use of PET in the diagnosis or staging of hepatobiliary malignancies.
9. Inadequate volume of the liver remnant can lead to hepatic insufficiency after extended right or left hepatectomies. In such instances, consider preoperative portal vein embolization to increase the volume of the future liver remnant. This is performed in the radiology suite by an interventional radiologist. Through a percutaneous transhepatic route the desired portal vein segments are embolized with embolic agents such as polyvinyl alcohol, foam particles or coils. If radiological expertise is not available surgical ligation of the desired branches of portal vein can be performed, although it complicates subsequent definitive surgery because of hilar scarring.
10. Laparoscopy is an important part of the staging of primary liver and biliary tract malignancies as it can detect peritoneal disease which is often overlooked by both CT and MRI. Tru-cut biopsy of the future remnant liver can be obtained at laparoscopy to exclude underlying parenchymal liver disease.
11. Be cautious about performing percutaneous biopsies of resectable liver tumours. There is now good evidence that malignant cells can seed along the biopsy track. Current CT and MRI technology is highly accurate in diagnosing malignant lesions and histological confirmation is not required prior to resection. Biopsy may be required for histological confirmation of malignancy before commencing palliative treatment in patients with unresectable disease.
12. In patients with liver tumours and a background of chronic liver disease consider wedged hepatic venous pressure (WHVP) measurements. WHVP corrected for inferior vena caval pressure gives an estimate of portal venous pressure: it increases in portal hypertension secondary to chronic liver disease and is an independent predictor of poor outcome after liver resection.
13. Before undertaking any operative procedure on a patient with chronic liver disease, check the blood film, platelet count and clotting profile and correct any abnormality, if possible by giving blood products. Rarely are platelet infusions necessary, but all patients undergoing liver surgery, especially if jaundiced or with severe biochemical dysfunction, benefit from injections of vitamin K1. If the clotting profile is badly deranged, the patient may need an infusion of FFP and occasionally cryoprecipitate.
BIOPSY OR RESECTION OF A LIVER LESION FOUND AT THE TIME OF LAPAROTOMY
Action
1. Select an area of diseased liver or an edge that presents easily through the incision.
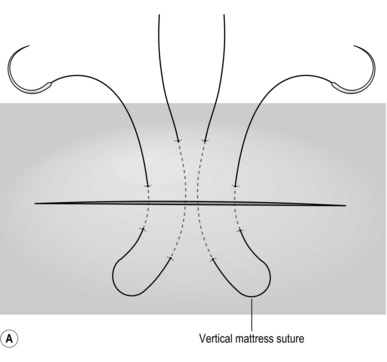
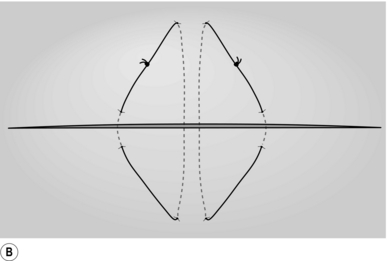
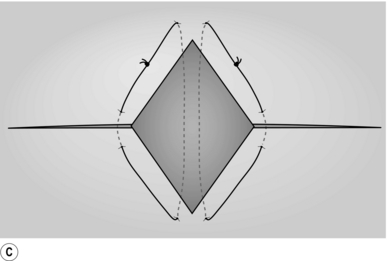
2. Place two mattress stitches of 3/0 Vicryl or PDS on an atraumatic round-bodied needle to form a V, the apex of this pointing towards the hilum of the liver (Fig. 17.2A).
3. Gently but firmly tie these stitches (Fig. 17.2B) and remove the wedge of tissue between them with a sharp knife (Fig. 17.2C). The cut edges of the liver should be dry. Insert another suture of similar material if haemostasis is not complete or use diathermy coagulation to establish haemostasis.
4. You may also use a Tru-cut needle to take a liver biopsy. If you are not familiar with the needle, rehearse your movements before inserting it into the liver. After removing the needle, check that you have an adequate core of tissue. Gently shake or tease the core of tissue into a pot of formalin, taking care not to crush it. The puncture site occasionally requires a figure-of-eight or Z-stitch with 4/0 Vicryl or PDS to obtain haemostasis.
INFECTIVE LESIONS
BACTERIAL LIVER ABSCESS
Appraise
1. Open surgical drainage of liver abscesses is rarely necessary. The standard treatment is a prolonged course of antibiotics along with aspiration of the pus under ultrasound guidance as the abscess matures or liquefies.
2. Until the exact nature and antibiotic sensitivities of the causative organism are known, administer intravenous broad-spectrum antibiotics such as co-amoxiclav, carbapenem or cephalosporin empirically.
3. Liver abscesses require prolonged treatment which can usually be carried out as an outpatient. Continue antibiotics and US surveillance with admission for US-guided aspiration if clinically septic or the abscess is increasing in size.
4. You may have to aspirate the abscess several times, but if it fails to respond to repeated aspiration and antibiotics or the percutaneous insertion of a drain, then very rarely you may be forced to operate. Localize the abscess first with ultrasound or CT scans. Administer intravenous antibiotics. Cross-match four units of blood and have available FFP. Use intra-operative ultrasound, if available, to identify the site of an intrahepatic lesion.
Action
1. Isolate the peritoneal contents from the area of the abscess by packs.
2. Insert a needle into the abscess, aspirate some of the contents to send for immediate examination and culture, including anaerobic culture, to confirm the diagnosis.
3. Incise the abscess and suck out the contents. Gently insert a finger to identify and breakdown all loculi.
4. Wash out the cavity with physiological saline.
5. Insert a tube drain, preferably as a closed system such as a Robinson drain. Pass the distal end of the drain through the abdominal wall at a suitable site, not through the incision.
6. Achieve haemostasis at the edge of the liver incision by using 3/0 Vicryl or PDS mattress sutures on an atraumatic needle or diathermy coagulation.
7. If possible, draw some omentum into the abscess cavity.
9. Leave the drain until drainage ceases and the cavity is demonstrably collapsed on ultrasound, CT or a sinogram. Until then continue appropriate antibiotics.
AMOEBIC ABSCESS
Appraise
1. This rarely requires surgical treatment.
2. Positively confirm the diagnosis by identifying amoebae in a fresh aspirate, or a positive serological test (antibodies to Entamoeba histolytica). The presence of amoebae in stools is indicative, but absence of organisms does not exclude the diagnosis.
3. Treatment is with metronidazole, 400–800 mg orally 8-hourly in an adult for 10 days. Resolution of the abscess can be monitored using regular ultrasound screening. If it becomes secondarily infected or is discovered at laparotomy then treat it like any other liver abscess.
HYDATID CYST
Appraise
1. Suspect a hydatid cyst in patients who present with a liver mass and who live or have lived in an endemic area (developing countries, the Mediterranean and Middle East, South America and South Australia). Confirm it by the presence of eosinophilia, positive hydatid serology and classic ultrasound and CT appearances.
2. Past treatment was mainly surgical. There are many reports that percutaneous treatment combined with antihelminthic drug therapy is safe and effective in selected patients with uncomplicated cysts.1 Consider it only if the cyst does not communicate with the biliary system on ERCP (endoscopic retrograde cholangiopancreatography). Remember that this procedure carries a low but definite risk of anaphylaxis. Administer an initial course of albendazole, followed by Puncture of the cyst under imaging guidance, Aspiration of the cyst contents, Instillation of hypertonic saline into the cyst cavity and then Re-aspiration – the acronym is PAIR. In some centres a sclerosing agent is finally injected into the cyst cavity, followed by a further course of albendazole. Albendazole alone without percutaneous intervention is not considered ideal therapy; use it only if PAIR or surgery is not possible. Surgery, in the form of cystectomy and omentoplasty, is still preferred by many clinicians who feel there is insufficient evidence for the routine use of PAIR. It is certainly the standard treatment for complicated cysts, cysts that communicate with the biliary tree and where percutaneous therapy has failed.
Prepare
1. Give at least a 4-week cycle of albendazole tablets (10 mg/kg/day in divided doses for an adult) before and after surgery to prevent growth of any spilt protoscolices (Greek: protos = first + scolex = a worm). You may administer up to three cycles of 4 weeks each, with a 2-week gap between cycles. Some surgeons additionally cover the perioperative period with another drug, praziquantel.
2. Visualize the biliary tract preoperatively with ERCP if the cyst is close to the liver hilum or if there is any history of attacks of jaundice or cholangitis, suggesting a communication between the cyst and the biliary tree.
3. Have available a scolicidal agent with which to wash out the cyst to kill any remaining scolices. This can be absolute alcohol, 1% cetrimide, or sterile 20% saline, which is the safest. 10% formalin and 0.5% silver nitrate are no longer used as they can cause sclerosing cholangitis.
4. Have available sterile black towels (see below) with which to pack off the surrounding tissues. Some surgeons soak these in scolicidal solution.
5. Warn the anaesthetist to be prepared for sudden anaphylactic shock if there is inadvertent spillage of cyst contents into the peritoneal cavity, although this is rare.
Access
1. Make a transverse incision in the right upper quadrant, extending from a point 2 cm above the umbilicus to a point on the midaxillary line midway between the subcostal margin and iliac crest, with a vertical midline extension to the xiphoid. If possible, arrange for the incision to overlie the cyst.
Action
1. When you reach the cyst, take care in handling it to avoid rupture. It is usually attached to surrounding tissues by fibrinous adhesions which you must gently separate.
2. Isolate the cyst from the rest of the peritoneal contents with packs. Traditionally, these are covered with black towels, which are claimed to make any spilt daughter cysts or scolices more visible.
3. Insert a wide-bore trocar, using a purse-string suture to avoid spillage, into the cyst and carefully aspirate as much as possible of the contained fluid, then fill with hypertonic saline.
4. Aspirate the hypertonic saline through a suction unit without spillage.
5. When the cyst is empty fill it again with a fluid – ideally 20% saline – that will destroy any remaining protoscolices. Leave the fluid in the cyst for at least 10 minutes, then aspirate it and repeat the procedure. The preoperative ERCP should have demonstrated any communications between the cyst and the biliary tree: close them using an absorbable suture such as Vicryl or PDS before irrigating the cavity. Alternatively, place an atraumatic clamp on the extrahepatic bile duct to demonstrate any biliary communication.
6. De-roof the cyst as much as possible – a technique known as saucerization – to allow omentum and other peritoneal contents to fill the cavity easily and so avoid the problems associated with delayed healing and infection of intrahepatic fibrous-walled cavities with a narrow neck. The omentum may be tacked into place with a few absorbable sutures (omentoplasty).
7. The value of inserting a drain into the cyst cavity is controversial. If it is possible to saucerize it then do not drain. If you must leave the cavity with a narrow neck insert a closed drainage system.
8. If you have demonstrated any evidence of communication between the cyst and biliary tree, consider performing an operative cholangiogram to ensure that you have closed it and that the biliary tree is free from any obstructing daughter cysts, which will need to be removed.
9. Ensure that haemostasis is complete. Wash out the peritoneal cavity with large volumes of physiological saline.
Technical points
1. Some surgeons advocate excision of the cyst intact, including the ‘pericyst’ (Pericystectomy). In some circumstances this is possible, but often cysts are adherent to major vessels and can cause severe bleeding. A formal hepatic resection can also be performed. These operations require experience in liver surgery: attempt them only if you are experienced.
2. If there has been spillage of cyst contents into the peritoneal cavity, washing with sterile water may reduce the likelihood of further infestation because of the toxic osmotic effect of water on the protoscolices.
OTHER CYSTS
Appraise
1. The majority of liver cysts are asymptomatic and are discovered usually on imaging for other pathology. When symptoms develop, they include right upper quadrant abdominal swelling, discomfort or acute pain associated with haemorrhage into the cyst or rupture of the cyst. Symptomatic cysts are usually diagnosed using ultrasound and CT.
2. Massive polycystic disease of the liver is rare, but can cause severe pain. In 50% of patients with polycystic disease there is associated polycystic renal disease. The renal lesions are more commonly associated with organ failure whereas patients with severe polycystic liver disease often have normal liver function. Some patients also have associated pancreatic cysts. These patients should be treated only in major centres since they may ultimately require liver transplantation.
3. Occasionally cysts become infected or develop haemorrhage, producing pain.
4. Liver cyst may also result from a biliary cystadenoma – a benign but premalignant lesion which requires formal resection.
5. Before attempting diagnostic percutaneous aspiration of a cyst, consider if it is hydatid (see section on hydatid cyst) or cystic tumour.
6. Don’t operate on liver cysts unless they are symptomatic, a known hydatid cyst or for resection of cystadenoma.
Action
1. For a symptomatic large cyst, carefully de-roof it, allowing it to drain freely into the peritoneal cavity. You may gently pack omentum into it. Such a cyst may recur, but can then be treated by percutaneous aspiration or formal resection. If you are experienced in laparoscopic surgery you may elect to de-roof a symptomatic superficial liver cyst by a minimal access approach.
2. Cystadenoma will require formal liver resection (see section on neoplasms below).
NEOPLASMS
Appraise
1. Liver neoplasms can be benign or malignant. Benign liver tumours are usually asymptomatic and incidentally detected on imaging for other pathology. Amongst malignant liver tumours, in the Western world metastases are more common than primary malignancies: the majority of metastases arise from colorectal cancers. Other less common primary sites are pancreas, stomach, lung and breast.
2. Modern CT and MRI have high accuracy in differentiating benign from malignant lesions. AFP, CEA, CA 19-9 levels are valuable but remember these tumour markers have low sensitivity and specificity.
3. Benign liver neoplasms such as haemangioma or focal nodular hyperplasia do not require surgical excision unless they are causing significant symptoms. Hepatic adenomas carry a risk of bleeding and of malignant change and most surgeons advocate resection of adenomas.
4. Malignant liver neoplasm should be considered for surgery since this is the only potentially curative treatment.
5. See the section on principles of elective surgery above for preoperative workup and staging. Remember, preoperative histological confirmation is not necessary if surgical resection is feasible, as biopsy may lead to needle-track tumour dissemination. Remember to obtain histological confirmation of underlying parenchymal liver disease before considering surgery in patients with suspected chronic liver disease.
6. Laparoscopic surgery has evolved significantly. Wedge liver resections of superficial and peripherally located tumours and, left lateral liver resections are commonly performed laparoscopically in most centres. In limited numbers, formal left and right hepatectomies have also been performed in selected centres. Success in laparoscopic major liver resections requires expertise in both liver surgery and laparoscopic surgery.
Prepare
1. Once operability and resectability is confirmed, prepare the patient for liver resection. Crossmatch four to six units of blood. Arrange platelets, fresh frozen plasma and cryoprecipitates to be available intra-operatively or postoperatively.
2. Make certain that clotting is normal. Correct clotting abnormalities preoperatively. Give vitamin K to jaundiced patients.
3. A Thompson type self-retaining retractor is an indispensable tool for liver operations. The retractor is fixed on the side of the operating table and provides forcible, upward retraction of the costal margins, giving safe and adequate access to all surfaces of the liver. Make sure the retractor is available in theatre.
4. Have available vascular clamps and sutures. Intra-operative ultrasound is valuable in planning your surgical strategy. Make sure the appropriate ultrasound probe for liver surface placement is available. Several techniques of parenchymal division are described including clamp crushing (Kelly-clysis), ultrasonic dissector, radiofrequency assisted devices, harmonic scalpel, vascular stapler, etc. Have available the instrument of your choice.
5. Maintain good communication with your anaesthetist. Sudden haemorrhage can occur anytime during the operation. The anaesthetist will insert central venous catheter, arterial cannula and large bore peripheral cannula. Remember, it is the hepatic veins which bleed more than the artery during parenchymal transection. It is now common practice to maintain a low CVP during parenchymal transection to limit bleeding from hepatic vein branches and maintain the patient in the reverse Trendelenburg position to decrease the risk of air embolism from disrupted hepatic veins. The anaesthetist lowers the CVP (<10 mmHg) by fluid restriction and anaesthetic techniques.
6. Pass a urinary catheter to monitor urine output.
7. Discuss and plan postoperative analgesia with your anaesthetist. The upper abdominal muscle cutting incision and costal margin retraction do give rise to considerable postoperative pain. It is common practice now to use epidural analgesia. Make sure the morning dose of subcutaneous heparin is omitted if an epidural catheter is going to be inserted.
WEDGE EXCISION OF SOLITARY LIVER LESION
Access
1. Make a transverse incision in the right upper quadrant, extending from a point 2 cm above the umbilicus to a point on the midaxillary line midway between the subcostal margin and iliac crest.
2. Add a vertical extension in the midline to the xiphoid. If greater exposure is required, a left transverse extension can be added to create a Mercedes-Benz incision.
3. You will rarely need to split the sternum or open the chest through a rib extension.
4. Stitch back the flaps and retract the costal margins with a Thompson self-retaining retractor.
Assess
1. Carry out a general exploration of the abdominal cavity to exclude peritoneal and omental disease, and other intra-abdominal pathology.
2. Explore the liver hilum. Identify the hepatic artery, portal vein and common bile duct and pass a tape around them. This allows a vascular clamp to be applied to control haemorrhage during liver resection – the Pringle manoeuvre.
3. Carefully palpate the entire liver and perform an intra-operative ultrasound examination of the entire liver to identify the number, site and vascular relations of all tumours and exclude additional disease. Open the gastrohepatic omentum and palpate the caudate lobe.
Action
1. Once you make the decision to proceed, mark the lines of parenchymal transection on the liver capsule with diathermy, at least 1 cm away from the macroscopic tumour margin.
2. If you are using hilar clamping (the Pringle manoeuvre, see above), although the normal liver can tolerate up to 60 minutes of warm ischaemia, to reduce hepatocellular damage to a minimum release the clamp for 10 minutes every 15 minutes.
3. Use the device of your choice for parenchymal division. As you divide the parenchyma, vessels and bile ducts will be exposed. Control the exposed vessel with diathermy, clips, ligatures or sutures. Always clip or ligate the exposed bile ducts.
4. Remove the tumour, and clip or ligate any remaining ducts and vessels. Coagulate with diathermy any remaining small bleeding points. Check the raw area for bile leaks and carefully oversew them with fine vicryl or PDS.
5. If the cut surface of the liver is not dry, cover it with absorbable haemostatic gauze (Surgicel) and apply a pack. After 10 minutes, gently remove the pack. The area should be dry. Coagulate any remaining bleeding points.
Closure
1. Once the cut surface of the liver is dry, wash the perihepatic peritoneal cavity with warm saline.
2. You may apply the omentum to the transected surface of the liver. There is no evidence that the traditional practice of inserting a drain offers any advantage.
3. Close the wound using a mass closure suture. Close the skin.
RIGHT OR LEFT HEPATECTOMY
For an extensive discussion of liver resection techniques refer to Blumgart.1
Anatomy
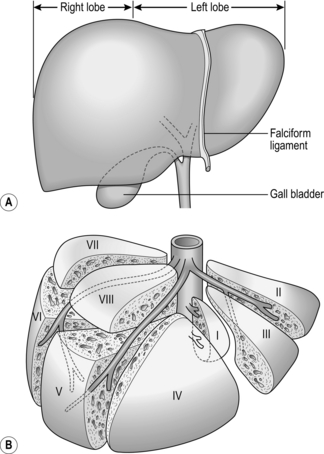
1. The liver is divided into two anatomical lobes, each being supplied by its own branch of the hepatic artery, portal vein and hepatic duct. The junction of the two lobes is in the line of the gallbladder bed (Fig. 17.3A). By ligating the hepatic artery and portal vein branches supplying either lobe, it is possible to demarcate the junction between them as a fairly sharp colour change.
2. The liver is further divided into eight segments, described initially by Couinaud2 in 1957 (Fig. 17.3B). Segment I, adjacent to the IVC, is also known as the caudate lobe, and segment IV, between the falciform ligament and the main lobular division, is known as the quadrate lobe.
3. As a result of the hepatic architecture it is possible to divide the liver through the main plane separating the right and left lobes. It is also possible to remove segments of the liver. Removal of all the liver tissue to the right of the falciform ligament (right lobe plus segment IV) is called an extended right hepatectomy or trisegmentectomy.
4. Removal of segments II and III of the left lobe, that part of the liver to the left of the falciform ligament, is comparatively straightforward and is usually carried out laparoscopically in most units.
Access
1. Make a transverse incision in the right upper quadrant, extending from a point 2 cm above the umbilicus to a point midway between the subcostal margin and iliac crest on the midaxillary line.
2. Add a vertical extension from the midpoint to the xiphoid. If greater exposure is required, a left transverse extension can be added to create a Mercedes-Benz incision.
3. You will rarely need to split the sternum or open the chest through a rib extension.
4. Stitch back the flaps and retract the costal margins with a Thompson self-retaining retractor.
Action
1. After positioning the self-retaining retractor, perform a full exploration of all abdominal viscera and palpation of the pelvis. Next, divide the falciform ligament on the anterior surface of the liver as far as the suprahepatic IVC using the diathermy. This will allow you to mobilize the liver by dividing right and left triangular ligaments.
2. Divide the peritoneal reflections between the back of the liver lobe and the diaphragm and retract the lobe from the diaphragm and posterior abdominal wall until you see the retrohepatic IVC.
3. The main inflow pedicle to the affected lobe can be controlled extrahepatically prior to parenchymal transection or intrahepatically during parenchymal transection. For extrahepatic control, dissect the hepatic hilum and identify the branches of the hepatic artery, portal vein and bile duct supplying and draining the affected lobe. Identify and dissect the cystic duct and artery and perform a cholecystectomy first as this is the plane of division for a right or left hepatectomy.
4. Divide the artery, duct and portal vein branches supplying the lobe to be removed and oversew or transfix the ends of the vessels with fine prolene and duct with PDS (Fig. 17.4A). Alternatively, you can divide the entire pedicle within the Glissonian sheath with a vascular stapler, which is quicker.
5. Carefully dissect, ligate and divide all hepatic vein branches entering the IVC from the liver. Identify the small veins from segment I (the caudate lobe), entering the IVC. Dissect, transfix or clip and divide them (Fig. 17.4B). Carefully dissect the main hepatic veins (right, middle or left) draining the lobe to be removed; they will be divided later.
6. Return to the anterior surface of the liver. You are now ready to start transecting the liver parenchyma. Inform your anaesthetist that you are about to commence parenchymal transection so that they lower the CVP. You should be able to identify a demarcation line between the devascularized lobe to be removed and the normal vascularized liver which is to remain.
7. Just on the vascularized side of this demarcation line mark your line of parenchymal transection on the liver capsule with diathermy.
8. Use Kelly-clysis or the instrument of your choice, such as the ultrasonic dissector, to divide the liver substance between this incision and the IVC.
9. Ligate or suture with fine prolene or PDS, or clip with titanium clips any vascular or biliary tract structures crossing the line of transection as you encounter them.
10. As your plane of transaction goes deeper into the liver parenchyma, you will encounter larger branches of the hepatic veins. Clip and divide these with fine haemostats.
11. Finally, clamp the main hepatic veins (right, middle or left) with vascular clamps before dividing them. Use vascular suture to oversew the ends (Fig. 17.4B). You may use an endovascular stapler, if one is available, to divide and staple the hepatic veins.
12. Control any smaller bleeding points by diathermy coagulation.
13. If there is continued bleeding, cover the raw area of the liver with absorbable haemostatic gauze (Surgicel) and apply a pack for 10 minutes.
Aftercare
1. The initial postoperative management of these patients is critical, and is best carried out in an intensive care or high-dependency unit for the first 24 hours, or at least until the patient is warm, extubated, self-ventilating and haemodynamically stable.
2. The broad principles of management are as outlined in the aftercare of major liver trauma.
SURGICAL MANAGEMENT OF HAEMORRHAGE FROM OESOPHAGEAL VARICES
Management of a patient with variceal haemorrhage is complex and is ideally undertaken by a specialist team including a medical hepatologist, specialist radiologist and a surgeon.1,2,3
Appraise
1. Aim to resuscitate the patient, find the site of the bleeding and stop it. These three processes must be carried out in parallel. The prognosis is directly related to the severity of any underlying liver disease. Minimal hepatocellular damage (Child class A/B) carries a good prognosis; if it is severe (Child class C), the prognosis is poor. As there is no way of predicting which patients will fare badly or well, initially treat them all actively. Generally, patients with Child class A or B disease are likely to respond to standard medical therapy. In patients with Child class C disease the decision to place transjugular intrahepatic portosystemic shunt (TIPSS) needs to be made early in the management.
2. Patients with suspected variceal haemorrhage should be admitted to the intensive care unit.
3. Assess the patient’s airway and insert two large-bore peripheral cannulas. Resuscitate by restoring and maintaining circulating volume with intravenous fluids, blood and plasma expanders as necessary. The aim is to maintain haemodynamic stability. Avoid hypervolaemia as this will increase the portal pressure and exacerbate the bleeding. Avoid excess use of saline for fluid resuscitation as this can worsen ascites. Many patients will have coagulopathy which should be corrected fully with FFP, platelets and vitamin K. Recombinant factor VII is expensive and there is no good evidence to support its routine use in the control of variceal haemorrhage.
4. Antibiotic prophylaxis has been shown to reduce the risk of bacterial infections which are associated with recurrence of bleeding. Use norfloxacin, ceftriaxone or ciprofloxacin.
5. Initiate treatment with a splanchnic vasonstrictor such as vasopressin, or its synthetic analogue Terlipressin, Somatostatin, or its analogue Octreotide, to reduce splanchnic blood flow, thereby reducing portal flow and pressure. Vasopressin is the most potent vasoconstrictor. Vasopressin can cause myocardial ischaemia, arrhythmias, heart failure, mesenteric ischaemia, limb ischaemia, pulmonary oedema and cerebrovascular accidents, so use it with caution. Be willing to administer simultaneous nitroglycerine sublingually, intravenously or transdermally. Vasopressin is administered as a continuous intravenous infusion at 0.4 units/minute, increasing if necessary to 0.6 units/minute and continuing until bleeding has stopped for 24 hours. Somatostatin, is expensive but is the safest; give it as a 250-μg bolus and a 250–500-μg/hour infusion, continued for 2–5 days if it is beneficial. Generally, terlipressin, somatostatin and its analogue octreotide are considered ‘safe’ vasoconstrictors. Avoid beta-blockers as they can cause hypotension and blunt the physiological response to shock.
6. Upper gastrointestinal endoscopy is essential to establish an accurate diagnosis since 50% of patients with portal hypertension will have a non-variceal source of bleeding. Endoscopy is the gold standard for diagnosis of variceal bleeding. Patients with severe haemorrhage may need endotracheal intubation for endoscopy.
7. If you confirm variceal bleeding at endoscopy, perform endoscopic variceal banding by placing a constricting rubber band at the base of the varix or perform sclerotherapy by injecting sclerosing agents into or around the varices. Polidocanol, ethanolamine and sodium tetradecyl sulfate are some examples of available sclerosants. Variceal banding is based on the same technique as used for rubber band ligation of haemorrhoids. Although both techniques are equally effective in controlling acute bleeding, banding is preferred to sclerotherapy for acute variceal bleeding because rebleeding occurs less frequently with banding. You need to be experienced in the appropriate techniques.
8. The combination of vasoconstrictor and endoscopic therapy is standard medical therapy for control of acute variceal bleed and is successful in controlling bleeding in up to 90% of patients.
9. In about 10 to 20% of patients standard medical therapy will fail to control variceal bleeding. Consider placement of a transjugular intrahepatic portasystemic shunt (TIPSS) as a salvage procedure. This is performed in the radiology suite by a specialist interventional radiologist. A catheter is passed from the jugular vein into a major hepatic vein, usually the right hepatic vein, preferred because of its size and proximity to the portal vein. A needle is then passed through the catheter and directed from the right hepatic vein, through liver parenchyma into the right branch of the portal vein. The track is then dilated by a forced balloon angioplasty and an expandable, metallic, wall stent 8–12 mm in diameter is placed along the track. This creates a portal-systemic shunt and provides immediate decompression of the portal system. TIPSS is successful in controlling acute variceal bleeding in more than 90% of patients. Problems associated with TIPSS resemble those following surgical shunts, including hepatic encephalopathy (20–40%) and progressive shunt occlusion. TIPSS should be considered as a bridge to subsequent liver transplantation.
10. If TIPSS is not available, decide if the general condition of the patient or the severity of parenchymal liver disease would suggest that surgical intervention is likely to be successful. This can be either a ‘veno-occlusive’ procedure designed to stop the venous haemorrhage, such as oesophageal transection or oesophagogastric devascularization, or a ‘portal decompression’ procedure, namely a portal-systemic shunt.
11. Balloon tamponade is now rarely used. It is very effective in temporarily controlling the bleeding in more than 80% of patients but is associated with dangerous complications including oesophageal perforation and aspiration pneumonia and has a 20% mortality rate. Consider balloon tamponade in patients with uncontrollable bleeding in whom a definitive treatment such as TIPSS or shunt surgery is planned within 24 hours. Oesophagogastric tamponade is provided by an oro-gastric triple lumen balloon tube (Sengstaken-Blakemore tube). This has a gastric balloon, an oesophageal balloon and a channel for draining the stomach. In the four-lumen Minnesota version there is also a channel for oesophageal drainage. After passing the tube, inflate the gastric balloon with 250–300 ml of air and apply gentle traction to the tube. This tamponades (French: tapon = a plug) the oesophagogastric junction and the fundus. If bleeding continues, as signalled by continuing haematemesis, then connect the oesophageal balloon to a manometer with a ‘Y’-connection and fill it with air to a pressure not exceeding 40 mmHg. Deflate the oesophageal balloon for 30 minutes every 4–6 hours and remove the tube after 12 hours. Balloon tamponade is unlikely to be curative: expect half the patients to re-bleed when the tube is removed. It ‘buys time’ while deciding on more definitive measures.
12. The role of embolization of varices in acute variceal bleeding is controversial. This is performed by an interventional radiologist. Through a percutaneous transhepatic route, a catheter is inserted into the left gastric vein and the varices are embolized with gelfoam, stainless steel coils, ethanol or tissue adhesives.
OESOPHAGEAL TRANSECTION AND OESOPHAGOGASTRIC DEVASCULARIZATION
Appraise
1. Oesophageal transection and oesophageal devascularization procedures are rarely performed nowadays because of increased use of TIPSS. Also, there is a high incidence of postoperative hepatic encephalopathy following these procedures.
2. Oesophageal transection and re-anastomosis is effective in controlling acute variceal bleeding, but has a lower re-bleeding rate when combined with oesophagogastric devascularization.
3. The portal vessels feeding the varices or the varices themselves can be ligated by a variety of thoracic or abdominal surgical approaches. Oesophageal transection and re-anastomosis is performed using a circular stapling device. If a stapler is not available it is possible to achieve the same effect by transection and sutured re-anastomosis but this is a difficult procedure. The transection is best performed leaving a cuff of 1 cm of stomach attached to the oesophagus, since the gastric wall holds sutures more securely than the oesophageal wall. If you encounter bleeding from gastric fundal varices that stapled disconnection alone will not control, a more extensive devascularization procedure is indicated.
4. Oesophagogastric devascularization may be performed electively to prevent recurrent variceal re-bleeding:
 When extrahepatic portal hypertension involves thrombosis of the portal, splenic and mesenteric veins so there are no suitable veins into which the portal system can be shunted
When extrahepatic portal hypertension involves thrombosis of the portal, splenic and mesenteric veins so there are no suitable veins into which the portal system can be shunted
 In schistosomiasis, in which there is mild liver dysfunction with splenomegaly and hypersplenism
In schistosomiasis, in which there is mild liver dysfunction with splenomegaly and hypersplenism
 Where there exists a high probability of encephalopathy with a shunt.
Where there exists a high probability of encephalopathy with a shunt.
5. Oesophagogastric devascularization may be achieved as described by Hassab. Through a transabdominal route, the distal oesophagus is mobilized, all of its feeding vessels are ligated and disconnected. Splenectomy is carried out, the left gastric (coronary) vein is ligated, and the greater and lesser curves of the entire proximal stomach are devascularized. Sugiura described a more extensive operation which was originally performed as a two-stage procedure. Through a thoracotomy, the lower oesophagus is devascularized and oesophageal transection carried out. After 6 weeks, through an abdominal approach, the stomach is devascularized and splenectomy carried out, followed by vagotomy and pyloroplasty. The procedure has now been modified to a one-stage transabdominal operation. The distal oesophagus is mobilized transhiatally and devascularized, followed by a stapled transection, then gastric devascularization and splenectomy are performed.
6. The technique to be described involves performing a stapled oesophagogastric transaction and re-anastomosis in an emergency, together with the outline of a more extensive devascularization should it be necessary.
Prepare
1. This procedure, designed to occlude all veins filling the oesophageal plexus from below, is best carried out using a mechanical circular stapling instrument such as EEA Autosuture or Proximate ILS Ethicon. Have available disposable circular stapling instruments (sizes 25, 28 and 31 or similar) and the accompanying measuring bougies.
2. Have the anaesthetist give prophylactic antibiotics at the start of the operation.
Action
1. Identify the oesophagogastric junction by palpation after the anaesthetist has passed a nasogastric tube.
2. Gently retract the left lobe of the liver from this region using a Deaver retractor. Divide the left triangular ligament.
3. Incise the peritoneum in front of the lower end of the oesophagus, coagulate any bleeding vessels and gently pass a finger behind the oesophagus and immediate peri-oesophageal tissues. Encircle these structures with a tape.
4. Extend the oesophageal mobilization proximally and distally until you can easily pass two fingers around the whole of the lower oesophagus. You do not need to separately identify the vagi unless they prevent adequate mobilization, in which case exclude them from the mobilized tissues.
5. Make a vertical gastrotomy in the anterior stomach wall 10–15 cm from the oesophagogastric junction and insert through this one of the measuring bougies. Start with the 31-mm bougie and introduce it into the lower oesophagus to ensure that the lumen is large enough to accommodate it and thus the 31-mm staple instrument. It usually passes easily but, if not, do not force it; instead try one of the smaller ones.
6. Select a staple gun of the same size.
7. Remove the tape from the lower oesophagus and replace it with a stout thread ligature.
8. Pass a finger through the gastrotomy and into the lumen of the lower oesophagus. Have the anaesthetist slowly withdraw the nasogastric tube until the tip just disappears proximally up the oesophagus.
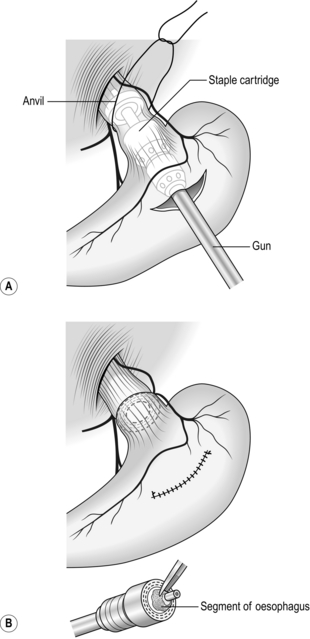
9. Pass the well-lubricated head of the selected staple gun into the lower oesophagus and separate the anvil from the staple cartridge by adjusting the screw on the handle. Have an assistant steady the instrument and palpate the groove between the separated head and anvil through the wall of the oesophagus.
10. Tie the thread ligature firmly in this groove and cut the ends (Fig. 17.5A): ensure that the ligature is firmly tied.
11. While protecting the lower end of the oesophagus with a hand placed around it, tighten the screw to bring the staple cartridge and anvil together. Check the mobility of the lower oesophagus and fire the staple instrument. Carry out this manoeuvre very gently and carefully to avoid damaging the lower oesophagus, which is usually very delicate, especially following recent endoscopic sclerotherapy.
12. Unscrew the handle of the instrument by two full turns to separate anvil from staple cartridge. Firmly hold the lower oesophagus and, with a gentle twisting motion, remove the instrument via the gastrotomy.
13. Place a large pack into the upper peritoneal cavity in the region of the transection while you inspect the instrument. Separate the anvil and staple cartridge, and remove the anvil and the plastic ring. Within the circular knife blade you will find the resected portion of oesophagus. Remove this and ensure that a complete ‘doughnut’ of oesophageal wall has been obtained (Fig. 17.5B).
14. Pass a finger through the gastrotomy into the lower oesophagus across the line of transection, have the anaesthetist slowly re-pass the nasogastric tube and, with your finger, guide it back into the lumen of the stomach.
15. Close the gastrotomy in one or two layers with absorbable sutures.
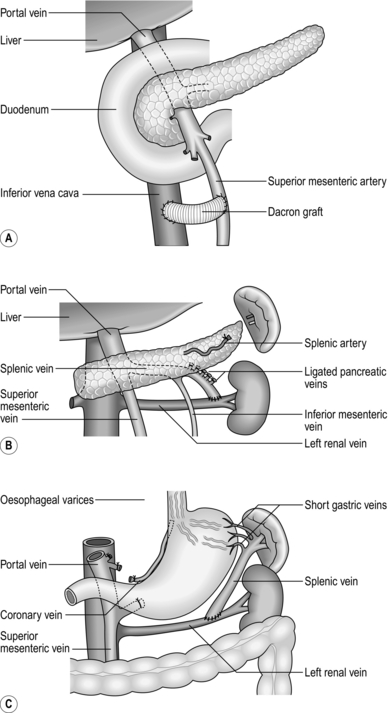
16. If there is bleeding from fundal varices, undersew them or carry out gastric devascularization coupled with splenectomy (Fig. 17.6). Mobilize the spleen as for splenectomy. Ligate and divide the vessels in the splenic pedicle. Identify, dissect, ligate and divide all the short gastric vessels between the greater curvature of the stomach and the spleen. Remove the spleen. Check the rest of the greater curvature of the stomach and dissect, ligate and divide any remaining vessels between it and the diaphragm. On the lesser curve of the stomach identify, ligate and divide any vessels passing to it from the lesser omentum. Continue this dissection down to the region of the antrum. The entire proximal stomach should now be separated from any feeding vessels along its greater and lesser curves. Fortunately, the internal vascularization of the stomach is almost always adequate to prevent any avascular necrosis. Devascularization of the abdominal oesophagus is performed close to the oesophageal wall by ligating and dividing all perforating veins which run transversely. Division of the vagii facilitates the devascularization; because of this, perform a pyloroplasty. If you have preoperatively made the decision to perform devascularization, then perform a splenectomy as the first step of the operation; this will greatly improve access to the lower oesophagus and stomach.
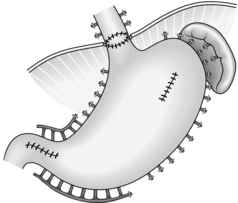
Fig. 17.6 Oesophagogastric devascularization.
REFERENCES
1. Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med 2010;362:823–32.
2. Dooley JS, Lok ASF, Burroughs AK, Heathcote EJ, editors. Sherlock’s Diseases of the Liver and Biliary System. 12th ed. London: Wiley-Blackwell; 2011.
3. Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W, Practice Guidelines Committee of the American Association for the Study of Liver Diseases, Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007;46:922–38.
PORTAL DECOMPRESSION
Appraise
1. Portal decompression can be portacaval, which may be end-to-side or side-to-side (Fig. 17.7), mesocaval, which may be side-to-side or with a synthetic PTFE ‘H’ graft (Fig. 17.8A), proximal splenorenal (Fig. 17.8B) or distal splenorenal (Fig. 17.8C). The last one, also known as the Warren shunt, is called a ‘selective’ shunt, as it decompresses only the varix-bearing area of the portal bed. There is no evidence to suggest that any one operation is in the long term any better than any of the others, so choose the one with which you have had most experience. In poor-risk patients perioperative mortality is 50%; in good-risk patients, around 5%.
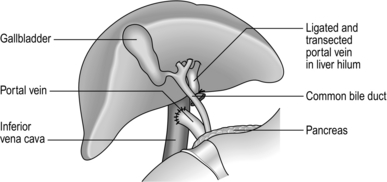
Fig. 17.7 End-to-side portacaval shunt.
2. The incidence of the major complication, portal-systemic encephalopathy, is similar (20–40% over 1 year) following all of these operations, although it may be less in the early period following the Warren shunt and perhaps following the small-diameter mesocaval shunt.
3. A problem with all shunts is that they are liable to occlude.
4. Do not attempt portal decompression without full preoperative investigations, including consultation with an experienced hepatologist. Investigate liver function, identify the nature of the liver pathology and the vascular anatomy. Patients who tolerate this operation well with minimal encephalopathy are those with good liver function, such as patients with portal vein occlusion, primary biliary cirrhosis or hepatic fibrosis. Do not undertake a shunt operation lightly; there is a risk of encephalopathy and consequent intellectual impairment. Do not undertake it in an emergency situation.
5. Shunt procedures are carried out infrequently now since patients with poor liver function and bleeding oesophageal varices are often candidates for TIPSS in the first instance and liver transplantation in the long term. Therefore the description will be limited to the traditional end-to-side portacaval shunt.
PORTACAVAL SHUNT
Appraise
1. This is the time-honoured operation for achieving portal decompression, although it is now rarely performed. It shunts all the portal blood into the infrahepatic vena cava, reduces the portal hypertension and stops bleeding from oesophageal varices.
 There is a high incidence (30–40%) of portal-systemic encephalopathy.
There is a high incidence (30–40%) of portal-systemic encephalopathy.
 The operation involves extensive dissection of the liver hilum, which may make subsequent liver transplantation difficult.
The operation involves extensive dissection of the liver hilum, which may make subsequent liver transplantation difficult.
3. It is still a very useful operation for treating portal hypertension in patients with good liver function.
Prepare
1. A detailed knowledge of the patient’s hepatic and portal vascular anatomy is essential. Modern contrast enhanced CT has replaced portography and provides good images of the splenic, superior mesenteric and portal veins. CT will also show aberrant hepatic arterial anatomy and is reliable in excluding thrombosis in the portal vein.
2. Correct any electrolyte or blood clotting imbalance preoperatively. Order six units of blood, some FFP and platelets, and warn the blood bank that you may need more.
Access
1. Place the patient supine on the operating table with slight rotation towards the left.
2. Use a transverse incision in the right upper quadrant, extending from a point 2 cm above the umbilicus to a point midway between the subcostal margin and iliac crest on the midaxillary line, with a midline extension to the xiphoid.
3. Use a Thompson style self-retaining retractor to retract the costal margins.
Action
1. Mobilize and retract distally the hepatic flexure of the colon. Mobilize the duodenum using Kocher’s manoeuvre by dividing the peritoneal reflection between it and the posterior abdominal wall. Identify and expose the IVC below the liver. There are usually dilated portal-systemic venous anastomotic vessels in this tissue, which may require careful, individual suture ligation. Diathermy coagulation alone is inadequate to secure haemostasis.
2. Incise the edge of the hepatoduodenal ligament between liver and duodenum and identify the portal vein behind the common bile duct.
3. Dissect the portal vein free of attachments from its origin up to its bifurcation. There are frequently one or two branches entering it from the pancreas. Divide and transfix them with a fine monofilament suture. You can gain extra length by gently dissecting the vessel from the pancreas. During this dissection retract the common bile duct anteriorly and to the left, taking care not to damage the blood supply to its wall. Be careful of preserving an aberrant right hepatic artery if it exists, as it lies behind the bile duct and on top of the portal vein.
4. Pass a tape around the portal vein.
5. Dissect the anterior surface of the IVC from the right renal vein to the lower edge of the liver.
6. Clamp the portal vein just above the pancreas with a Satinsky or DeBakey clamp.
7. Clamp and divide the portal vein at the hilum and oversew the hepatic end with 4.0 prolene. Flush the now collapsed segment of portal vein distal to the clamp with a solution of heparin 1:500 000 in physiological saline.
8. Draw the vein to the IVC. Apply a side-biting Satinsky clamp to the anterior surface of the IVC without totally occluding the lumen.
9. Trim the end of the portal vein obliquely so that it will join the anterior wall of the IVC in a gentle curve without kinking.
10. Remove an oval segment of the anterior wall of the IVC the same size as the oblique cut end of the portal vein.
11. Using standard vascular anastomotic techniques, suture the end of the portal vein to the side of the IVC with 4/0 polypropylene or similar vascular suture material. Just before completing the anastomosis flush the lumen of the portal vein and the occluded segment of the IVC with heparinized saline.
12. Complete the anastomosis. Remove the clamp on the IVC, followed by the clamp on the portal vein.
Check
Postoperative
 Manage the patient initially in the intensive care unit with help from an expert medical hepatologist
Manage the patient initially in the intensive care unit with help from an expert medical hepatologist
 Maintain accurate fluid balance and correct abnormal clotting
Maintain accurate fluid balance and correct abnormal clotting
 Take steps to prevent or control hepatic encephalopathy. In consultation with the hepatologist prescribe twice-daily phosphate enemas to keep the colon empty, and oral lactulose or lactitol when gastrointestinal activity returns, at a dose producing one or two soft motions a day. Restrict protein intake, starting at 20 g/day and increasing by 10 g every second day. Patients with chronic encephalopathy will probably tolerate no more than 40–60 g/day of protein per day. In some severe cases of portal-systemic encephalopathy the patient needs additional oral non-absorbed antibiotics such as neomycin 1 g four times a day for a week.
Take steps to prevent or control hepatic encephalopathy. In consultation with the hepatologist prescribe twice-daily phosphate enemas to keep the colon empty, and oral lactulose or lactitol when gastrointestinal activity returns, at a dose producing one or two soft motions a day. Restrict protein intake, starting at 20 g/day and increasing by 10 g every second day. Patients with chronic encephalopathy will probably tolerate no more than 40–60 g/day of protein per day. In some severe cases of portal-systemic encephalopathy the patient needs additional oral non-absorbed antibiotics such as neomycin 1 g four times a day for a week.
MANAGEMENT OF ASCITES
 The management of ascites is mainly medical, and consists of a low-sodium diet, fluid restriction, diuretics and concomitant potassium replacement if necessary. Paracentesis with intravenous colloid replacement is the next step if medical management does not succeed. Monitor progress by weighing the patient daily, measuring urine volume and checking for electrolyte imbalances, azotaemia and encephalopathy. If repeated paracentesis is required then the patient should be considered for a TIPSS procedure.
The management of ascites is mainly medical, and consists of a low-sodium diet, fluid restriction, diuretics and concomitant potassium replacement if necessary. Paracentesis with intravenous colloid replacement is the next step if medical management does not succeed. Monitor progress by weighing the patient daily, measuring urine volume and checking for electrolyte imbalances, azotaemia and encephalopathy. If repeated paracentesis is required then the patient should be considered for a TIPSS procedure.
 Another option is a surgical peritoneovenous shunt (LeVeen shunt). This involves placement of a tube extending from the peritoneal cavity to the jugular vein through a subcutaneous track in the anterior chest wall. Interposed in the tube is a one-way valve that opens only to pressure exceeding 2–4 cmH2O and allows drainage of ascitic fluid into the circulation. The Denver version of the shunt has a pumping mechanism within the valve. A peritoneovenous shunt is indicated only in cirrhotic patients with intractable ascites unresponsive to medical therapy. Contraindications include very poor liver function with encephalopathy, infected ascites, coagulopathy and cardiac failure. Complications are common with peritoneovenous shunts, and include shunt blockage, infection, thrombocytopenia and, occasionally, disseminated intravascular coagulation. The operation is rarely performed these days.
Another option is a surgical peritoneovenous shunt (LeVeen shunt). This involves placement of a tube extending from the peritoneal cavity to the jugular vein through a subcutaneous track in the anterior chest wall. Interposed in the tube is a one-way valve that opens only to pressure exceeding 2–4 cmH2O and allows drainage of ascitic fluid into the circulation. The Denver version of the shunt has a pumping mechanism within the valve. A peritoneovenous shunt is indicated only in cirrhotic patients with intractable ascites unresponsive to medical therapy. Contraindications include very poor liver function with encephalopathy, infected ascites, coagulopathy and cardiac failure. Complications are common with peritoneovenous shunts, and include shunt blockage, infection, thrombocytopenia and, occasionally, disseminated intravascular coagulation. The operation is rarely performed these days.