Lipoprotein metabolism
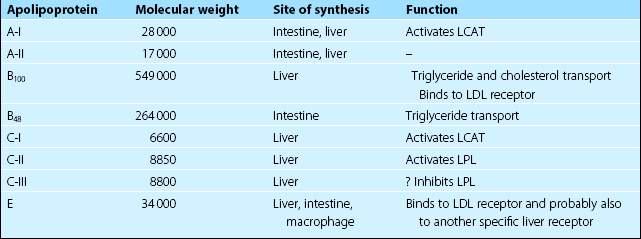
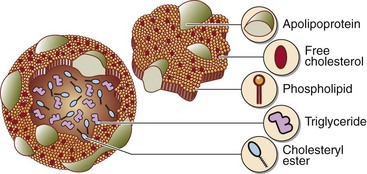
The lipoprotein system evolved to solve the problem of transporting fats around the body in the aqueous environment of the plasma. A lipoprotein is a complex spherical structure that has a hydrophobic core wrapped in a hydrophilic coating (Fig 66.1). The core contains triglyceride and cholesteryl esters, while the surface contains phospholipid, free cholesterol and proteins – the apolipoproteins (Table 66.1). Cholesterol is an essential component of all cell membranes and is a precursor for steroid hormone and bile acid biosynthesis. Triglyceride is central to the storage and transport of energy within the body.
Nomenclature
Several different classes of lipoproteins exist whose structure and function are closely related. Apart from the largest species, the chylomicron, these are named according to their density, as they are most commonly isolated by ultracentrifugation. The four main lipoproteins and their functions are shown in Table 66.2.
Table 66.2
The four main lipoproteins and their functions
| Lipoprotein | Main apolipoproteins | Function |
| Chylomicrons | B48, A-I, C-II, E | Largest lipoprotein. Synthesized by gut after a meal. Not present in normal fasting plasma. Main carrier of dietary triglyceride |
| Very low density lipoprotein (VDL) | B100, C-II, E | Synthesized in the liver. Main carrier of endogenously produced triglyceride |
| Low density lipoprotein (LDL) | B100 | Generated from VLDL in the circulation. Main carrier of cholesterol |
| High density lipoprotein (HDL) | A-I, A-II | Smallest lipoprotein. Protective function. Takes cholesterol from extrahepatic tissues to the liver for excretion |
Metabolism
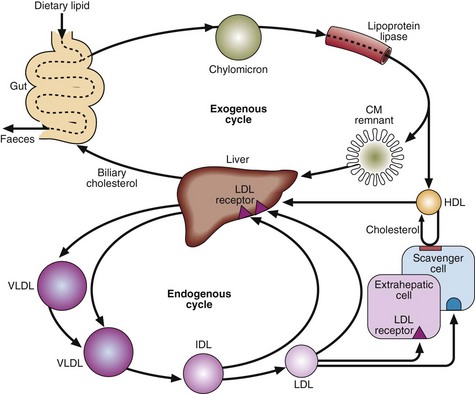
Lipoprotein metabolism (Fig 66.2) can be thought of as two cycles, one exogenous and one endogenous, both centred on the liver. These cycles are interconnected.
Two key enzyme systems are involved in lipoprotein metabolism, i.e.:
 Lipoprotein lipase (LPL) releases free fatty acids and glycerol from chylomicrons and VLDL into the tissues.
Lipoprotein lipase (LPL) releases free fatty acids and glycerol from chylomicrons and VLDL into the tissues.
 Lecithin: cholesterol acyl transferase (LCAT) forms cholesteryl esters from free cholesterol and fatty acids.
Lecithin: cholesterol acyl transferase (LCAT) forms cholesteryl esters from free cholesterol and fatty acids.
Apolipoproteins
Apolipoproteins are the protein components of the lipoproteins (Table 66.1). They are important in:
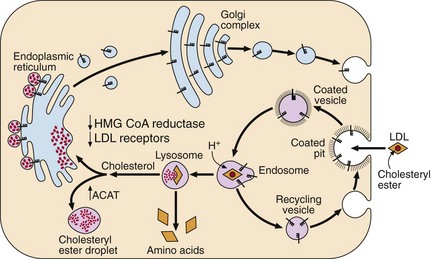
The LDL receptor
The LDL receptor (Fig 66.3), a glycoprotein present on the surface of all cells, spans the cell membrane and is concentrated in special membrane recesses, called ‘coated’ pits. It binds to lipoproteins containing apolipoprotein B and E, and internalizes them for breakdown within the cell. Receptors are then recycled to the cell surface. The number and function of receptors dictate the level of circulating LDL. When the cell has sufficient cholesterol, the synthesis of receptors is down-regulated; when the cell is cholesterol depleted, the receptors increase in number. Inherited malfunction or absence of these receptors leads to familial hypercholesterolaemia (FH).