Chapter 44 Lipoplasty – history and principles
• Proper patient selection involves careful assessment of anatomic deformities and medical profile; lower BMI patients are generally more favorable.
• The evolution of wetting solutions has decreased blood loss and made liposuction outcomes more predictable.
• Different cannula configurations are used, based on their functional properties, to enable precision in contouring.
• Newer energy sources such as ultrasound and laser, have expanded the available instrument panel.
![]() For additional online content visit http://www.expertconsult.com
For additional online content visit http://www.expertconsult.com
Evolution of Lipoplasty
Lipoplasty spread from Europe and more than 30 years elapsed before its introduction to the United States. During that time, it has rapidly become the most frequently performed cosmetic operation in the US, with 325 332 procedures in 2011. The number of lipoplasties has increased more than 158% in the last 10 years alone and is currently the most commonly performed esthetic surgical operation in the United States.1 This is far shy of the real numbers, because our national society statistics are blinded to operations performed by non-plastic surgeons.
The Initial Techniques of Fat Removal
Joseph Schrudde of Germany first reported on curetting subcutaneous fat at the 1972 meeting of the International Society of Aesthetic Plastic Surgery (ISAPS) in Rio de Janeiro, Brazil. He termed the procedure “lipexheresis,” which he stated “he had performed occasionally since 1964.” In 1975, he provided a more detailed report on the procedure in Lagenbeck’s Archives of Surgery.2–6 Blind long scissors undermining followed by the traumatic technique of using a sharp uterine curette frequently resulted in prolonged drainage, lymphorhea, hematoma and even skin necrosis.
The first surgeons to add suction for the purposes of fat extraction, as opposed to just using curettage, were an Italian father and son team – the Fishers, Arpad and George. They presented their work in 1977.7 The tip of the instrument was still sharp and, as a result, it severed not only fat but also surrounding structures. The postoperative course was again marred by complications and side effects not unlike those resulting from lipexheresis. Therefore, the procedure was not enthusiastically received.
The next advance worthy of mention was that of Ulrich Kesselring.8–10 Although he used sharp instrumentation attached to suction, he was the first to recommend working in the deep fat compartment just above the muscle fascia. His results were superior to previous ones presented, in no small measure due to his method of patient selection; he performed the procedure only on young women with small amounts of localized fat and elastic skin. Eventually, after a great deal of lively debate with Yves Gerard Illouz at a variety of plastic surgery meetings, Kesselring adopted Illouz’s technique.
Bahman Teimourian was the first surgeon practicing in the United States to make a significant contribution to the evolution of lipoplasty. His work was independent from the European surgeons. In 1976 he was still using scissors for undermining, which he followed by curetting with a modified fascia lata stripper attached to suction. He boldly extended his procedure to many body regions. His complication rate was 30%, characteristic of all curette techniques. He first reported on his technique in 197911 and then later as he modified it.12–15 Teimourian recognized the importance of separate tunnels, as opposed to a “windshield wiper” type of approach to fat removal. He eventually adopted a cannula technique instead of curettage.
Illouz, briefly mentioned earlier, was responsible for monumental advances. He began in 1977, first reporting on his method in 1980.16–18 His most important contribution was the introduction of blunt instrumentation, using a Karman cannula attached to very high vacuum. There was no undermining and no sharp instrumentation. In this way, he removed fat while sparing the other important structures between the undersurface of the dermis and the subjacent muscle fascia. As a result, complications were dramatically reduced, and the procedure became reproducible in the hands of many other surgeons. The technique was adaptable to a wide range of body regions and, for the first time, its potential as a mainstay of esthetic surgery became apparent. He first presented his blunt lipoplasty technique, which he called “lipolysis,” at the Shirakabe Clinic in Osaka, Japan in 1980. He was the first to introduce the “wet” technique, infiltrating 200–300 cm3 of infusate regardless of the expected volume of aspirate. Blood loss with this technique, as measured by the “lipocrit,” (the hematocrit determination in the infranatant portion of the decanted aspirate), was reduced to approximately 8–10%. By the time his milestone book, Body Sculpting by Lipoplasty, was published in 1989,18 he became familiarized with the SuperWet technique (the infusate-to-aspirate ratios of 1–1.5 : 1), which he adopted.
The evolution of the wetting solutions will be discussed later in this chapter. In respect to chronology, it is, however, appropriate to mention here that a significant contribution was made in 1984 by Hetter, who added epinephrine to the wetting solutions. This yet further decreased the aspirate lipocrit values to 4–8%. His book, The Theory and Practice of Blunt Suction Lipectomy, published in 1984, remains to this day a highly recommended text for students of lipoplasty at any level.19,20
Concurrently, Pierre Fournier and Francis Otteni of France were popularizing the use of syringes as the suction source for lipoplasty.21 They also favored noncutting edge cannulae. However, they unfortunately continued to advocate the “dry technique;” no wetting solution preinjection. As a result, with their method, lipocrits continued to hover around 20–40%.![]()
Wetting Solutions
When it comes to lipoplasty, safety is intimately interwoven with the proper use of wetting solutions, a subject of primary interest to me for many years.19,22–26
Soon after, in 1987, the tumescent technique was introduced by a California dermatologist, Jeffrey A. Klein. While lipocrit determinations using his approach also hovered around 1% (not unlike the superwet technique), excessive amounts of wetting solution containing local anesthetic, as the sole anesthesia delivery system for the procedure, were infused. Table 44.1 lists the lipocrit values reported for different lipoplasty approaches.
TABLE 44.1 Lipoplasty Approaches and Percentage of Blood Loss in the Aspirate as Determined by Lipocrit Measurements
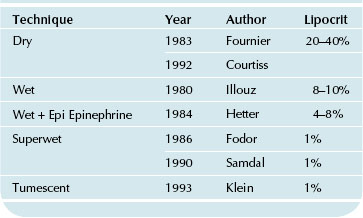
Lipoplasty Wetting Solutions and Corresponding Lipocrit Values
When applied to major volume removals, Xylocaine toxicity25 and fluid overload could readily occur, potentially resulting in major complications. Some plastic surgeons, including a few with significant national reputations, not only adopted the tumescent technique but arduously promoted it. They advocated its application to larger and larger volume removals. A “perfect storm” was in the making, which predictably resulted in major lipoplasty complications and a growing number of fatalities nationally.22–24 By the early 1990s, while lipoplasty was the most commonly performed esthetic surgical procedure it also had the lowest rate of complications. At this time, the “tumescent technique,” which consists of infusing massive amounts of wetting solutions, using tissue turgor as an endpoint, was popularized. These solutions contain large amounts of lidocaine (Xylocaine), attempting to provide simultaneous anesthesia for the procedure. Proponents of this technique consider the need for an intravenous line and the presence of an anesthesiologist during the procedure unnecessary. The tumescent technique, as popularized primarily by dermatologists,7 is the very gradual (up to 3 hours) installation of wetting solution until there is tissue turgor, the skin surface is white, and it has a peau d’orange appearance. Advocates of this technique state that they inject between 3 and 6 cm3 of wetting solution per cm3 of estimated aspirate. Thus, many liters of lidocaine-containing fluid may be injected, which amounts to a highly variable clysis.
For small to moderate volumes of removal, any wetting solution technique is comparatively safe when performed in a healthy, young, aerobically fit patient. The potentially hazardous nature of massive wetting solution infusion techniques as applied to large-volume lipoplasty can result in fluid and lidocaine overload. This scenario was readily predictable22,23 as the trend for using the tumescent technique for large-volume removals was adopted around 1994. As it turned out, unfortunately, the incidence of major complications and fatalities associated with lipoplasty rose rapidly after 1995 (Box 44.1). Consequently, the media and public’s perception of the procedure changed from a safe to a hazardous one.
BOX 44.1
Lipoplasty Fatalities in the United States
1982–1995: 12 deaths; primarily from sepsis, pulmonary embolus
Widespread adoption of tumescent technique
1995–1997: 100 deaths; primarily from fluid overload and lidocaine toxicity
The advantages of the superwet technique, as compared to the tumescent technique, have been amply presented by myself and others.26–28
Xylocaine Toxicity
It is generally accepted that lidocaine up to 35 mg/kg, injected in the subcutaneous fat in solutions containing epinephrine, is safe. It is notable, however, that this dose is in direct contrast with the original recommendation of less than 7 mg/kg published in the Physicians’ Desk Reference. Absorption rates vary significantly, and measurements of peak plasma lidocaine levels are more meaningful than absolute amounts of injected lidocaine in assessing potential toxicity. The danger is amplified by the fact that lidocaine absorption from the subcutaneous fat can peak as late as 10 to 12 hours after injection, by which time the patient usually has been discharged from the surgical facility. Other factors can affect lidocaine toxicity, such as the amount of unbound versus protein-bound lidocaine. In turn, protein binding is influenced by several factors, such as stress, chronic disease, cigarette smoking, oral contraceptives, and anorexiants. Therefore, the correlation between total plasma lidocaine concentration and the predictability of specific toxicity occurring in a given patient is weak at best.24
In summary, the benefits of the tumescent technique (e.g., low blood loss and decreased need for colloid and crystalloid replacement), are realized with the “superwet technique,” but without the hazards of delayed fluid uptake by large clysis, need for drainage, risk of pulmonary edema9 and potential for lidocaine toxicity. Table 44.2 compares the tumescent and superwet techniques.
| Superwet (~1 cm3/cm3) | Tumescent (~3 cm3/cm3) | |
|---|---|---|
| Volume removal | + + + | + + + |
| Blood loss | 0– + | 0– + |
| Ecchymosis | + | + |
| Time to inject | + | + + + |
| Fluid load | + | + + + |
| Tissue turgor | 0– + | + + + |
| Ease to sculpt | ? ? | |
| Lidocaine load | 0– + | + + + |
Ultrasound Technology Emerges
Beginning in 1987 and continuing to this day, there has been, in my opinion, an ill-founded enthusiasm for mechanical manipulation of adipocytes resulting in their fragmentation prior to aspiration. One of these widely popularized approaches is the use of ultrasound energy to emulsify the fat. In 1987, Professor Nicolo Scuderi of Italy was the first to describe ultrasound-assisted lipoplasty (UAL), after which a plethora of publications endorsed the practice of UAL.29–31
My initial impression of UAL was confirmed by a clinical study on my first 100 cases.29 The protocol was to compare traditional suction-assisted lipoplasty (SAL) performed on one side with the results of UAL applied to the contralateral side during the same operation. In this way, patients served as their own control. It was concluded that the benefits of UAL, using all the different UAL devices available at that time, for the most part did not justify the shortcomings associated with its use. It was felt, however, that even at this early stage of the development of UAL devices (first and second generation), the technology offered some tangible benefits in treating body regions with fibrous fat and in secondary lipoplasty.29
In 1997, a meeting in St. Louis, hosted by Leroy Young, was organized with the objective of facilitating the exchange of ideas between leading biophysicists with interest in how ultrasound energy affects adipocytes. In addition to American board certified plastic surgeons, industry representatives were also asked to participate. After meeting William Cimino, one of the PhD biophysicists present, I had the opportunity to offer my clinical contributions to him as he was developing vibration amplification of sound energy at resonance (VASER). This third-generation ultrasound lipoplasty device, when compared to earlier devices, limited the power of ultrasound energy delivery to levels which, while sufficient to fragment adipocytes, were less harmful to surrounding lymphatics, blood vessels, and nerves.32–34 I have enjoyed assisting in the refinement of this technology and carrying out its first clinical trials from the inception of the technique in 1997 to the present. Today, there is abundant independent clinical observation by a multitude of surgeons that VASER-assisted lipoplasty (VAL), which the company nowadays has termed as “liposelection,” produces a milder postoperative course and augments the tendency for skin contracture. However, these observations are challenging to document objectively. Currently, there are a number of clinical studies in progress which it is hoped will provide scientifically valid evidence for these outcomes. There are also recent findings showing that the number of stem cells is not reduced in the adipoaspirate obtained with VASER technology compared to traditional lipoplasty.35 (Rubin, personal communication).
Additional Lipoplasty Advances
In the early 1980s, a number of surgeons, some working independently of each other, introduced the concept of superficial lipoplasty.36,37 This advanced technique produced impressive outcomes, at least in the short term and only in the hands of experts. In the longer term, the results can deteriorate to a point where skin excisional body lifts become necessary as a salvage procedure.
By the late 1980s, as demonstrated by the precipitous statistical increase in the performance of the procedure, lipoplasty had become the most popular esthetic surgical procedure worldwide. Simultaneously, applications of the operation were extended to include challenging body areas such as lipoplasty of the calves, ankles, arms, male and female breasts, back rolls and abdominal and pectoral etching.38–45
Power-Assisted Lipoplasty Emerges
The technology of power-assisted lipoplasty (PAL)46,47 has also been embraced by a large number of surgeons. This mechanically propelled device lessens the effort expended by the surgeon to extract fat, allowing him/her to concentrate more precisely on sculpting.
With power assistance, a mini sledgehammer-like mechanism drives the tip of the cannula forward and backward at a cpm of 2000–4000 and a stroke distance of 2 mm. This facilitates subcutaneous fat penetration. As a consequence, the surgeon’s physical effort is lessened, and he or she can therefore be more focused on sculpting. I had the good fortune to be involved early in the development of this technology and designed the first clinical studies conducted with it. We also performed endoscopic studies comparing traditional lipoplasty and power-assisted lipoplasty. Under magnification, these studies demonstrated how power-assisted lipoplasty was less traumatic to structures surrounding the fat being aspirated.48![]()
Additional Technologies
In 2000, yet another modality, “lower level laser therapy” (LLLT) was introduced with the claim that edema and pain were reduced, wound healing and skin contracture augmented, and larger volumes could be safely aspirated.49,50 In this technique, the subcutaneous fat is pretreated with external laser energy prior to aspiration. Well controlled clinical studies have been unable to substantiate the claimed benefits of LLLT.51,52
More recently, other laser-related body sculpting methodology has aggressively been promoted, with catchy names such as SmartLipo, Goldlipo, Slimlipo, Smoothlipo, Lipopulse, CoolTouch, as well as a number of other devices. They all utilize laser energy to emulsify the subcutaneous fat. They are all combined with suction during the same operative session and allegedly tighten the overlying skin. It is also claimed that the laser energy is supposed to seal blood vessels as it comes in contact with them and that blood loss is diminished. To my knowledge to date, there have not been any publications in peer-reviewed medical journals convincingly demonstrating the purported benefits. Conceptually, the approach is identical to what we examined in a multicenter study published some 15 years ago.53 In that study, the investigators uniformly found that there was no benefit in using this approach that in any way justified the expense, learning curve, and cumbersome nature of its application.
Improvements in the clinical application of lipoplasty continue. These are fueled by excellent studies, such as those by Kenkel et al, aimed at clarifying our understanding of the physiologic events surrounding the procedure.54–56
Similarly, the physics of the instrumentation used in lipoplasty have been extensively investigated.57
In summary, the development of subcutaneous fat removal through lipoplasty, a rather minimally invasive approach, especially in comparison to excisional body surgery procedures, has been an exciting and fulfilling one. The procedure, when performed in properly selected patients and by well trained surgeons, has been miraculously successful. Currently, there is a multitude of even less invasive, as well as noninvasive methodologies that are constantly emerging. The noninvasive approaches are based on transcutaneous application of focused energy; ultrasound,58,59 laser or thermal. These techniques are described elsewhere in the book and therefore not addressed in detail in this chapter, even though I have been keenly interested and involved in their development and for that matter, served as Chairman of the Medical Advisory Board to LipoSonix (a high intensity focused nonsurgical body sculpting ultrasound approach) prior to the company having been purchased by Medicis. My main concern with any of these approaches remains one’s ability to carry out fine sculpting and just not “en masse” subcutaneous fat destruction.
Fundamentals of Lipoplasty
Applied Surgical Anatomy of the Subcutaneous Fat
The author believes that this entire superficial fibrous fascia system, which extends between the superficial muscular fascia and the undersurface of the dermis, is the human derivative of the panniculus carnosus.60 The vertical fibrous attachments play an essential role in skin contracture after liposuction.
In conventional lipoplasty, suction of the deep fat compartment is common. This is the workhorse area, where the most improvement is seen in the majority of cases. The beginner should concentrate on these areas. Suction of the superficial layer, as well as cannula “discontinuous undermining” over and adjacent to the area suctioned, promotes postoperative skin contracture. Therefore, with the superficial technique, the procedure can be used for larger removals and can be offered to older patients and to patients with more flaccid skin.36,37 Superficial suction does not eliminate skin excisional body sculpting procedures. Properly performed superficial suction reduces the need for dermolipectomies.
Physics and Equipment
Physics is often forgotten by most surgeons. As previously mentioned, in 2005 we took a critical look at all components of a suction system; vacuum source, tubing, cannulae and collection canisters, on a bench model using applesauce mixed half and half with saline, which was compared and found to correspond well to in vivo data.57
Vacuum Source, Tubing, and Cannulae
Our data showed57 that small changes in vacuum (2–3 mmHg) do not appreciably impact aspiration rate, especially when the system includes either a smaller diameter cannula or the smaller diameter (6.35 mm) suction tubing. The most significant effect is the tubing’s internal diameter (ID). For example, the 9.5 mm in ID tubing attached to a 3.0 mm cannula had a slightly higher aspiration rate than the 6.35 mm ID tubing without any cannula attached to it. When using the smaller 6.35 mm ID tubing, the variation in aspiration rate for the different diameter cannulae was small, showing that the tubing diameter dominated flow rates.
Biohazards
Biohazards have not yet been proven to be associated with the use of suction pumps.61 Vaporization of tissue fluids raises the possibility that bacterial and viral particles may be exhausted into the operating room. It is recommended that a special filter be placed between the collection jar and the pump to reduce this risk. These filters can stop particles larger than 0.3 µm, have a large surface area to allow good air flow, and are reasonably priced. It is recommended that such filters be used with all machines at all times.
Patient Selection
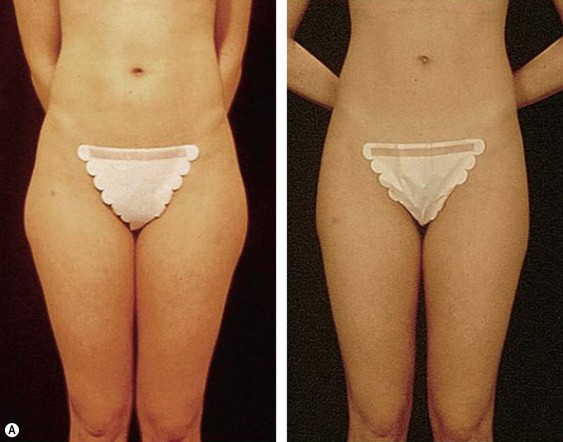
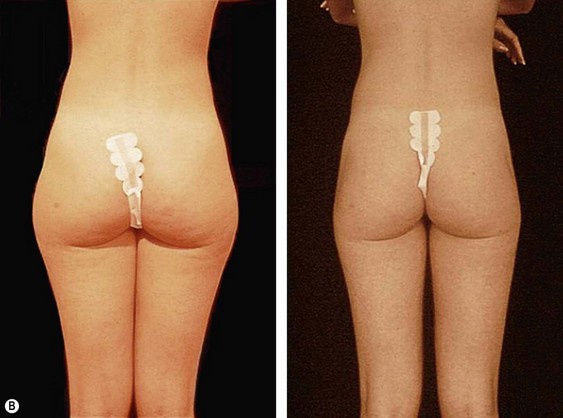
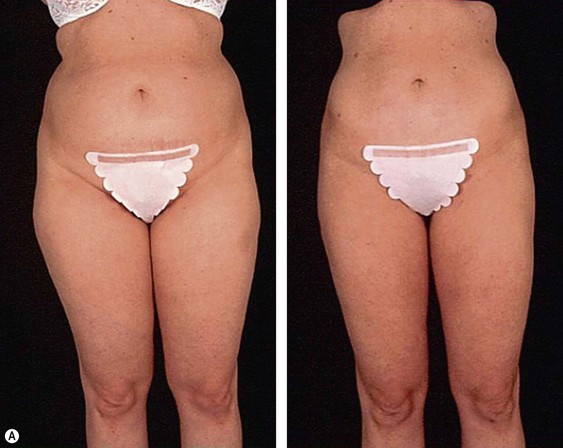
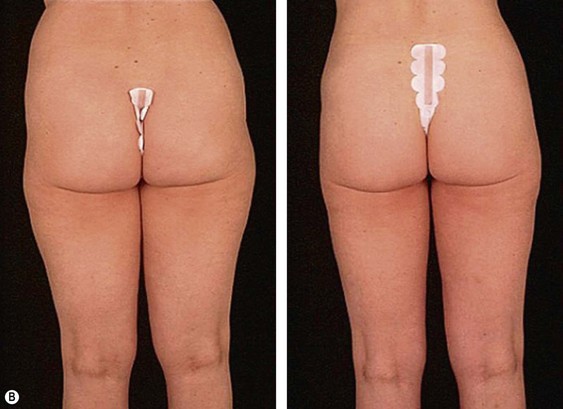
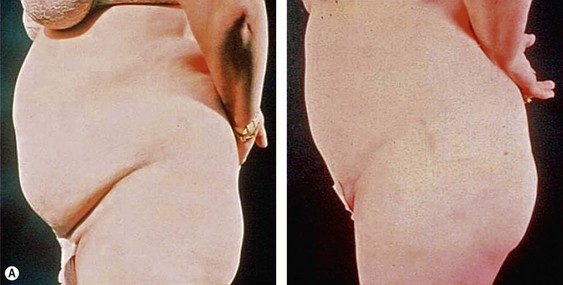
From an esthetic standpoint, the ideal patient (BMI less than 24.9) for lipoplasty is a relatively healthy, thin, young, person with highly localized subcutaneous fat excess: “figure faults” (Fig. 44.1) with taut skin.
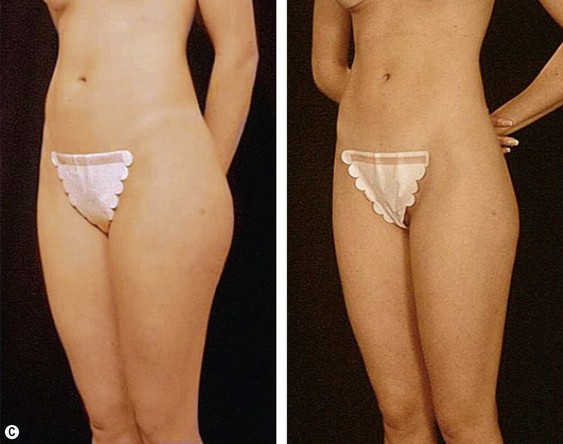
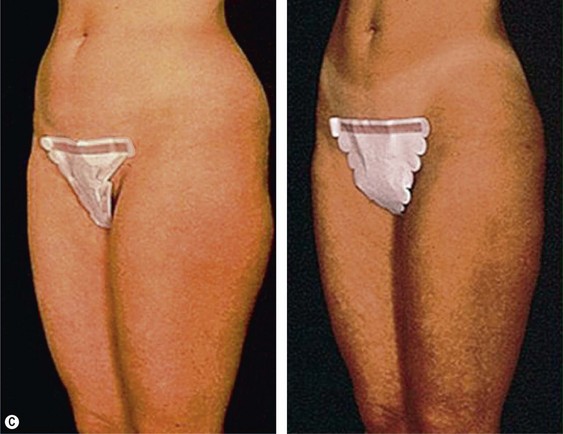
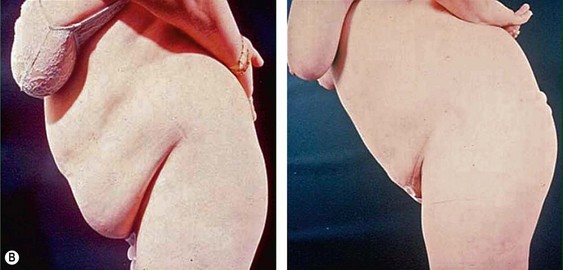
The average candidate (BMI 25–2 9.9) is over 40 years of age, weighs 7–9 kg (15 to 20 pounds) over ideal weight, has a history of weight fluctuations, and has some degree of skin relaxation and/or striae. Although such a patient will experience some improvement from lipoplasty, which may incorporate superficial suction, autologous fat transfer (AFT), and intraoperative and postoperative Endermologie, he or she should be informed that subsequent suctioning and dermolipectomy may be necessary for the most optimal result (Fig. 44.2).
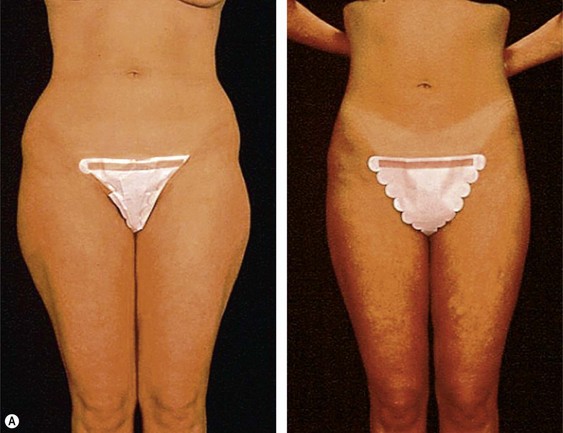
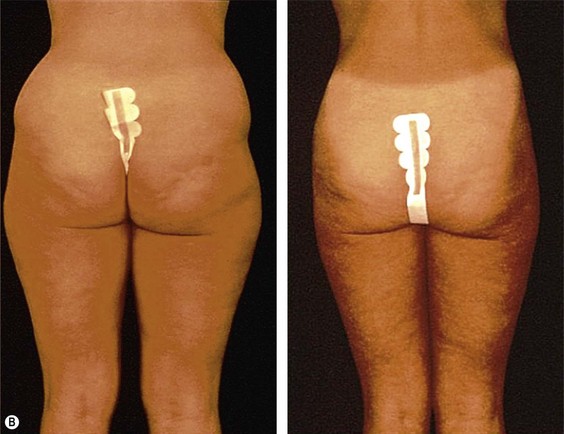
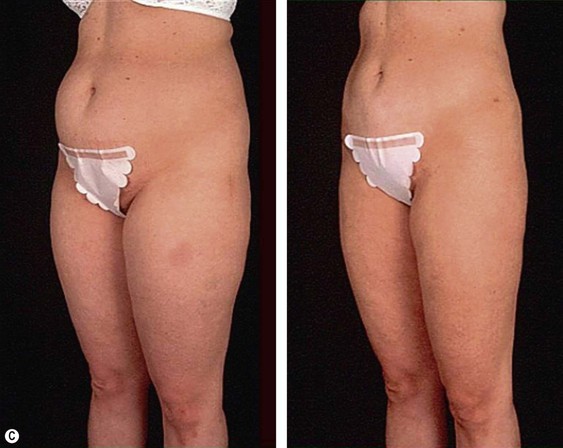
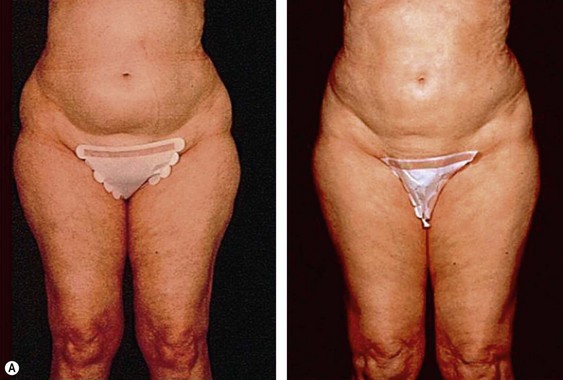
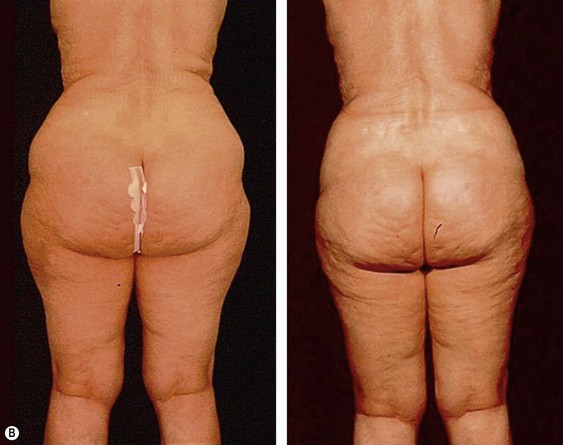
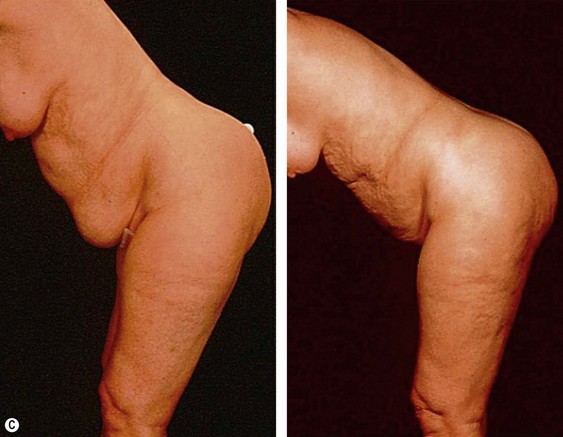
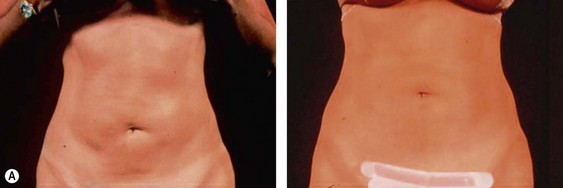
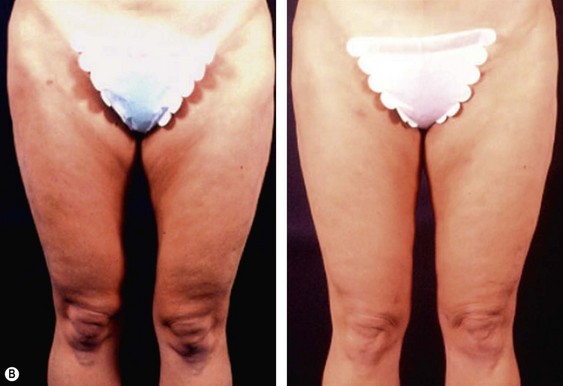
The less than ideal patient (BMI over 30) is older, is more than 9 kg (20 pounds) overweight or generally obese, has a history of weight fluctuations, and has clearly loose skin. Striae, soft tissue ptosis with cascading folds of redundant skin, the beginning of abdominal ptosis, banana rolls (sometimes multiple), inner thigh ptosis, or a combination of these features are present on physical examination. In this group, patients older than 55 years seem more readily satisfied with the results of the procedure than their younger counterparts. The patient should repeatedly be informed before surgery of the limitations imposed by their anatomy. A more conservative approach followed by secondary suctioning, after interim skin contraction, and eventual dermolipectomy may be required. Regardless of age and how close to the ideal candidate a patient is, sufficient preoperative discussion(s) to bring postoperative expectations to a realistic level is essential (Figs 44.3 and 44.4).
Surgical Intervention
Infusion of Wetting Solutions
Several different formulations of wetting solutions have been recommended. These formulations do not differ significantly from each other. For the most part, they are isotonic in nature and contain low concentrations of epinephrine and lidocaine. The formulation I currently favor is shown in Box 44.2.
When Performing PAL
Based on microscopic studies, performed on specimens from a few patients, the aspirate obtained with PAL was found to be as suitable for autologous fat transfer (AFT) as is the fat obtained with TL.46
When Performing Superficial Lipoplasty
In conventional liposuction, suction of the deep fat compartment is recommended. Superficial liposuction,36,37 as well as just cannula undermining, in the superficial compartment, helps promote postoperative skin contracture. Therefore, when superficial technique is added, larger volumes of fat can he aspirated from any given area, and the procedure can be offered to older patients and patients with more flaccid skin. Also, if skin excision is unavoidable, it will not need to be as extensive.
Large Volume Removals
Large volumes can be removed safely in properly selected patients (Fig. 44.5). Our general guidelines for the preoperative, intraoperative, and postoperative care of lipoplasty patients are listed in Boxes 44.3–44.5. It should be stressed that the amount of wetting solutions (crystalloids) delivered alters the amounts of intravenous fluids required over time. Close communication with the anesthesiologist and careful patient monitoring are essential.
BOX 44.3 Safety Issues in Large-Volume Lipoplasty
Preoperative Considerations
Low anesthetic risk: ASA class I
Erythropoietin for hematocrit <35%
Discontinue Fen-Phen and other potentially hazardous prescription drugs
Evaluate risk for thromboembolic phenomena and treat accordingly
BOX 44.4 Safety Issues in Large-Volume Lipoplasty
Intraoperative Considerations
Body heat preservation; silver cap, Bair Hugger, warm infusion fluids
Anesthesia: general or epidural (not local)
Superwet technique (not tumescent!!!)
Low or only keep, open volumes of intravenous fluids
BOX 44.5 Safety Issues in Large-Volume Lipoplasty
Postoperative Considerations
Sequential pneumonic leg compression
± chemoprophylaxis for thromboembolism
FIG 44.5 Appears ![]() ONLINE ONLY
ONLINE ONLY
Secondary Lipoplasty
Even today, after significant physician education initiatives undertaken nationally and internationally, patients presenting to my practice for a secondary operation far outnumber patients seeking primary lipoplasty. Unfortunately, many of these patients did not select their surgeon wisely. Approximately half are not good candidates for further surgery. For the rest, depending on the deformity, the secondary procedure will consist of additional lipoplasty alone or combined with autologous fat transfer, and some patients will also require skin resection procedures. Despite certain limitations of secondary surgery, most of these patients are very satisfied and grateful for the improvement obtained (Fig. 44.6). An algorithm and recommendations for evaluation and care of these patients have been discussed in detail.62![]()
Postoperative Management
The first dressing change is at 3 to 5 days, at which time access incision sutures are removed.
Nutritional counseling is often greatly appreciated by the post-lipoplasty patient.
1 American Society for Aesthetic Plastic Surgery, 2007–2009 Cosmetic Surgery National Data Bank Statistics.
2 Rogers B. Joseph Schrudde, MD of Cologne: the father of lipoplasty. Lipoplasty Society of North America; Lipoplasty Newsletter. 1991;8(1):6–7.
3 Schrudde J. Lipexeresis in the correction of local adiposity. First Congress of the International Society of Aesthetic Plastic Surgery (abstract) Rio de Janeiro. 1972.
4 Schrudde J. Lipexeresis as a means of eliminating local adiposity. Aesth Plast Surg. 1980;4:215.
5 Schrudde J. Suction curettage for body contouring. Plast Reconstr Surg. 1982;69:903–909.
6 Schrudde J. Lipexeresis (liposuction) for body contouring. Clin Plast Surg. 1984;11:445–446.
7 Hetter GP, Fodor PB. Aspirative lipoplasty. Georgiade S, Riefkohl R, Scott Levin L, eds. Plastic Maxillofacial and Reconstructive Surgery, 3rd ed. 1998, 685. Chapter 58
8 Kesselring UK. Suction curettage to remove excess fat for body contouring (Letter). Plast Reconstr Surg. 1982;69:572.
9 Kesselring UK. Regional fat aspiration for body contouring. Plast Reconstr Surg. 1983;72:610–618.
10 Kesselring UK, Meyer R. Body sculpturing with suction assisted lipectomy. In: Regnault P, Daniel RK. Aesthetic Plastic Surgery. Boston: Little, Brown and Co; 1984:681–685.
11 Teimourian B. Invited Lecture, A new approach to the removal of fat in lipodystrophies, Southern Medical Association meeting, Las Vegas, NV. 1979.
12 Teimourian B. Invited Lecture, Body contouring, Southern Medical Association meeting, San Antonio, TX. 1980.
13 Teimourian B. Invited Lecturer and Panelist, Suction Lipectomy, American Society for Aesthetic Plastic Surgery meeting, Orlando, FL. 1980.
14 Teimourian B, Fisher JB. Suction curettage to remove excess fat for body contouring. Plast Reconstr Surg. 1981;68(1):50.
15 Teimourian B. Suction lipectomy and body contouring. St. Louis: Mosby; 1987. p. 45–60
16 Illouz YG. Shirakabe Clinic in Osaka, Japan 1980; the first International presentation on BLUNT lipoplasty technique, that he had practiced since 1977. Surgical demonstration.
17 Illouz YG. Body contouring by lipolysis; a 5 year experience with over 3000 cases. Plast Reconstr Surg. 1983;72:591–597.
18 Illouz YG. Body Sculpting by Lipoplasty. New York: Churchill Livingstone; 1989.
19 Hetter GP. The effect of low dose epinephrine on the hematocrit drop following lipolysis. Aesth Plast Surg. 1984;8(1):19.
20 Hetter GP. Lipoplasty: the Theory and Practice of Blunt Suction Lipectomy. Boston: Little, Brown & Co; 1984.
21 Fournier PF. Liposculpture: ma technique. Arnette. Paris. 1989.
22 Fodor P. Wetting solutions in aspirative lipoplasty: a plea for safety in liposuction. Aesth Plast Surg. 1995;19:379–380.
23 Fodor P. Lipoplasty – another plea for safety!. Aesth Plast Surg. 1997;22(6):399–400.
24 Fodor P. Moderator: Safety Considerations in Lipoplasty, Expert Exchange, Perspectives in Plastic. Surgery. 1999;13(2):113–124.
25 Fodor P. Lidocaine toxicity issues in lipoplasty. Aesth Surg J. 2000;20(1):56–58.
26 Rohrich R, Beran S, Fodor P. The role of subcutaneous infiltration in suction-assisted lipoplasty: a review. Plast Reconstr Surg. 1997;99(2):514–519.
27 Fodor P. Defining wetting solutions in lipoplasty. Plast Reconstr Surg. 1999;103(5):1519–1520.
28 Fodor P, Watson J. Wetting solutions in ultrasound-assisted lipoplasty. Clin Plast Surg. 1999;26(2):289–293.
29 Fodor P, Watson J. Personal experience with ultrasound-assisted lipoplasty. Clin Plast Surg. 1999;26(2):1103–1116.
30 Scuderi N, DeVita R, d’Andrea F, et al. Nuove prospective nella liposuzione: La lipoemulsificazione. Giorn Chir Plast Riconstr Estet. 1987;2:1.
31 Zocchi ML. Ultrasonic-assisted lipoplasty; Technical refinements and clinical evaluations. Clin Plast Surg. 1996;23:575.
32 Jewell M, Fodor P, de Souza Pinto E, et al. Clinical application of VASER-assisted lipoplasty: a pilot clinical study. Aesth Surg J. 2002;22(2):131–146.
33 Cimino WW. Ultrasonic surgery: power quantification and efficiency optimization. Aesth Surg J. 2001;21:233–240.
34 Garcia O, Nathan M. Comparative analysis of blood loss in suction-assisted lipoplasty and 3rd-generation internal ultrasound-assisted lipoplasty. Aesth Surg J. 2008;28:430–435.
35 Panetta NJ, Gupta M, Kwan MD, et al. Tissue harvest by means of suction-assisted or third-generation ultrasound-assisted lipoaspiration has no effect on osteogenic potential of human adipose-derived stromal cells. Plast Reconstr Surg. 2009;124(1):65–73.
36 Fodor P. Superficial liposuction. Aesth Surg J. 1993;13:10–14.
37 Gasparotti TL. Superficial Liposculpture: Manual of Technique. Berlin: Springer Verlag; 1993.
38 Courtiss EH. Reduction mammaplasty by suction alone. Plast Reconstr Surg. 1993;92:1276.
39 Matarasso A. Cosmetic follow up suction mammaplasty: the use of suction lipectomy to reduce large breasts. Plast Reconstr Surg. 2000;105(7):2604–2607.
40 Fodor PB. Discussion: Suction mammaplasty: The use of suction lipectomy to reduce large breasts. Plast Reconstr Surg. 2000;105(7):2608.
41 Carlson HE. Current concepts: Gynecomastia. N Engl J Med. 1980;303:795–799.
42 Fodor PB. Management of gynecomastia problems. Plast Reconstr Surg. 2(3), 1992.
43 Fodor PB. Breast cancer in a patient with gynecomastia. Plast Reconstr Surg. 1989;84:976–979.
44 Simon BE, Hoffman SD, Kahn S. Classification and surgical correction of gynecomastia. Plast Reconstr Surg. 1973;51:48–52.
45 Mladick RA. Lipoplasty of the calves and ankles. Plast Reconstr Surg. 1990;86(1):84–93.
46 Fodor P, Vogt P. Power-assisted lipoplasty (PAL): a clinical pilot study comparing PAL to traditional lipoplasty (TL). Aesth Plast Surg. 1999;23:379–385.
47 Fodor P. Power-assisted lipoplasty. Aesth Surg J. 2001;21(1):90–92.
48 Fodor P. Power-assisted lipoplasty, operative techniques. Plast Reconstr Surg. 2002;8(1):23–37.
49 Neira R, Solarte E, Reyes MA, et al. Low level assisted lipoplasty; A new technique. In Proceedings of the World Congress on Lipoplasty, Dearborn, MI. 2000.
50 Neira R, Arroyave J, Ramirez H, et al. Fat liquefaction: effects of low-level laser energy on adipose tissue. Plast Reconstr Surg. 2002;110(3):912–922. discussion: 923–925
51 Brown S, Rohrich R, Kenkel JM, et al. Unable to confirm in the laboratory and/or clinically that LLLT alters adipocytes; Evaluation of low-level laser therapy (LLLT) on abdominal adipocytes; impact for lipoplasty procedures. Plast Reconstr Surg. 2004;113(6):1796–1804.
52 Fodor P. Discussion: Evaluation of low-level laser therapy (LLLT) on abdominal adipocytes; impact for lipoplasty procedures. Plast Reconstr Surg. 2004;113(7):1805–1806.
53 Fodor P, Apfelberg D, Rosenthal S, et al. Progress report on multicenter study of laser-assisted liposuction. Aesth Plast Surg. 1994;18:259–264.
54 Kenkel JM, Lipschitz AH, Luby M, et al. Hemodynamic physiology and thermoregulation in liposuction. Plast Reconstr Surg. 2004;114(2):503–514.
55 Kenkel JM, Lipschitz AH, Shepherd G, et al. Pharmacokinetics and safety of lidocaine and monoethylglycinexylidide in liposuction: a microdialysis study. Plast Reconstr Surg. 2004;114(2):515–525.
56 Kenkel JM. Efficacy of lidocaine for pain control in subcutaneous infiltration during liposuction. Aesth Surg J. March 2009;29:2. 122–8
57 Fodor P, Cimino W. Suction-assisted lipoplasty: physics, optimization and clinical verification. Aesth Surg. 2005;25(3):234–246.
58 Fodor P. Non-surgical Ultrasonic Lipoplasty. Sherrell Aston; In press.
59 Teitelbaum SA, Burns JL, Kubota J, et al. Noninvasive body contouring by focused ultrasound; safety and efficacy of the Contour 1 device in a multicenter, controlled, clinical study. Plast Reconstr Surg. 2007;120(3):779–789.
60 Fodor PB. From the panniculus carnosus (PC) to the superficial fascia system (SFS). Aesth Plast Surg. 1993;17:179.
61 Cukier J. Circumventing potential problems: suction lipoplasty biohazards aerosol, and exhaust mist – the clouded issue. Plast Recon Surg. 1989;83(3):494–497.
62 Fodor P. Secondary lipoplasty. Aesth Surg J. 2002;22(4):337–348.