CHAPTER 53 LINAC-Based Stereotactic Radiosurgery and Stereotactic Radiotherapy for Parasagittal, Skull-Base, and Convexity Meningiomas
INTRODUCTION
Gross total surgical resection (GTR) remains the mainstay of treatment for meningiomas.1 GTR, however, may not be appropriate for a significant proportion of meningiomas, particularly those involving the skull base or parasagittal region, or large or invasive tumors involving the convexity. Skull-base tumors are often challenging to access, and may involve the brain stem, cranial nerves, hypothalamus, or vascular structures. The risk of postoperative morbidity after resection is approximately 30% to 40%.2–5 For subtotally resected tumors, the risk of recurrence is substantial with a 55% risk. Even for total resections, there is a 20% to 23% risk of recurrence at 10 years.4,6 Given the substantial long-term risk of recurrence after subtotal or even ostensibly complete resection, there has been great interest in improving local control with radiation treatment.
External beam radiation treatment was shown in the 1970s and 1980s to be an effective modality for primary or adjuvant treatment of meningioma. Large retrospective series revealed that radiation after a subtotal resection would result in long-term progression-free survival (PFS) of 82% to 88%, similar to that expected after a gross total resection.6–8 For medically or surgically inoperable tumors, the use of radiation alone was shown to be associated with long-term local control similar to that with GTR.9 These data established the efficacy of radiation treatment for inoperable tumors, after subtotal resection, or at the time of recurrence. In addition, these data supported the notion that for sites associated with high operative morbidity, that a subtotal resection aimed at preserving function could be combined with radiation treatment to achieve tumor control comparable to total resection but with decreased risk of complications.6,10
The development of stereotactic radiosurgery (SRS) ushered in an important new era in radiation treatment with greatly improved treatment precision and dose shaping. It was possible with the advent of the Gamma Knife® to treat tumors of up to 3.5 cm with doses to adjacent normal brain tissue significantly diminished relative to conventional external beam radiation treatment (EBRT).11 In the late 1980s, effective methods were devised to adapt linear accelerators (LINACs) used for conventional radiation treatment to SRS.12 The widespread availability and lower cost of LINACs broadened the availability of radiosurgery for meningioma patients. In addition, it offered the possibility of fractionated stereotactic radiation treatment (FSRT), using relocatable noninvasive stereotactic immobilization.13 FSRT combines the precise dose delivery and sharp dose gradients of SRS with the biological benefits to normal tissue sparing of dose fractionation from EBRT, allowing for the possibility of stereotactic treatment for meningiomas of virtually any shape or size.
LINAC-SRS
Meningiomas represent a favorable tumor type for focused treatment such as radiosurgery. They are encapsulated, noninvasive in their benign form, and rarely metastasize. The focused treatment of brain tumors such as meningioma has benefitted greatly from development of computed tomography (CT)- and magnetic resonance (MR)-based imaging to define tumor volumes accurately,8 advanced treatment planning and dose shaping devices such as the micro-multileaf collimator,14 and precise methods for stereotactic tumor localization and radiation delivery.15
The physical characteristics of the radiation treatments using either the Gamma Knife or LINAC-based SRS are both highly conformal. In general, LINAC-SRS plans may have a slightly shallower dose gradient in normal tissue but also have greater dose homogeneity within the tumor.16 The increased dose inhomogeneity within the tumor associated with Gamma Knife treatment may result in increased risk or morbidity to interspersed normal tissue such as intratumoral cranial nerves.17
Image-guided frameless stereotactic systems are an important recent development in radiosurgery. Both the Gamma Knife and LINAC-SRS may utilize an invasive head frame for stereotactic localization and patient immobilization. With the advent of image-guided stereotaxy, LINAC-based systems may forego an invasive head frame and utilize thermoplastic masks to immobilize the patient, while relying on X-ray image-based stereotaxy to position the patient accurately.18 Frameless SRS systems include the Novalis™ system (BrainLab, Heimstetten, Germany) and the CyberKnife (Accuray, Sunnyvale, CA). Each system acquires stereoscopic X-ray imaging of skull anatomy at the time of treatment. These images are then automatically fused with a reference treatment planning CT dataset. The deviation of the treatment position from the reference position is calculated and either the patient is shifted robotically (Novalis), or the robotically-mounted LINAC source is retargeted (CyberKnife). Stereoscopic imaging may be repeated during treatment to verify patient position and correct for patient movements.19,20 Another frameless radiosurgery system that may be added on to any LINAC system utilizes a bite-block to which is fixed an infrared target array. The position of the target array is used as a reliable surrogate for patient position through a system of infrared cameras that can precisely track the array position.18 In addition to improving patient comfort, the use of image-guided frameless radiosurgery allows flexibility in treatment scheduling as patients may undergo mask fabrication, treatment planning, and treatment on different days.
Ultimately, the adoption of image-guided LINAC-SRS will be hastened as further centers obtain the necessary equipment and develop clinical experience. Reports evaluating both the Novalis and CyberKnife platforms indicate system accuracy of image-guided frameless LINAC-radiosurgery systems to be comparable to that of frame-based systems.19,21 In critical applications, where treatment tolerance and immobilization must be insured, the combined use of image-guidance together with frame-based treatment may allow for the detection of frame slippage, which may be more difficult to ascertain using conventional methods such as a depth-helmet.22
LINAC-SRS RESULTS
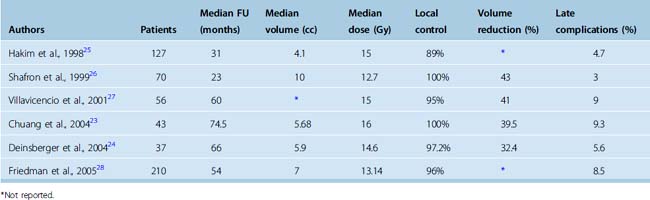
There are relatively fewer published series on LINAC-based radiosurgery compared to Gamma Knife radiosurgery. Based on the similar precision and dose shaping characteristics, the LINAC and Gamma Knife SRS systems would be expected to have similar results. The largest LINAC-SRS treatment series since 1998 are summarized in Table 53-1. The median follow-up ranged from 23 months to 74.5 months.23–28 The median doses employed for LINAC-based SRS range from 12.7 Gy26 to 16 Gy.23 The local control rate with LINAC-SRS of 89% to 100% is comparable to the range of local control observed with Gamma Knife radiosurgery of 86% to 98%.29–31 Regression of tumor after LINAC-SRS was observed in 32.4% to 43% of cases,24,26 while Gamma Knife series have reported rates of regression of 28% to 69.7%.29,30 The rate of late complications associated with SRS ranged from 3%26 to 9.3%.23 Overall, these results indicate that LINAC-SRS is comparable in both efficacy and toxicity to Gamma Knife SRS.
The LINAC-SRS series of Hakim and colleagues included 155 tumors in 127 patients of which 31 involved the convexity, 39 were parasagittal/falcine, and 71 involved the skull base. SRS was the initial treatment in 31% of patients, for persistent disease in 17.5%, and for tumor regrowth in 52%. The median tumor volume treated was 4.1 cc. The 5-year actuarial tumor control was 89.3% for patients with benign meningioma.25 They found no significant difference in tumor control between the skull base and the remaining locations. Six patients experienced permanent complications of SRS occurring at a median time of 10.3 months after treatment. The complications included blindness in one eye, unilateral hearing loss, leg weakness, hemiparesis, and two deaths. Complications were related to larger tumor volumes and proximity of the tumor to critical structures.
Shafron and colleagues reported on the initial results of LINAC-SRS at the University of Florida at Gainesville in 1999.26 This experience was updated by Friedman and colleagues in 2005 and comprises the largest LINAC-based SRS report to date.28 In this study, 210 patients, including 18 with atypical and 11 with malignant meningioma were followed for at least 2 years postradiosurgery. The most common radiosurgery peripheral dose for benign meningiomas was 12.5 Gy and the median dose was 13.14 Gy (range 10–20Gy). The median treatment volume was 7 cc. The mean imaging follow up was 39 months and the mean clinical follow-up was 56 months. As expected, benign tumors displayed better local control, with 100% control at both 1 and 2 years, and 96% at 5 years, whereas atypical/malignant tumors displayed 100%/100% control at 1 and 2 years but decreased to 77%/19% at 5 years. While the majority of patients had skull-base tumors, 22 patients treated had convexity tumors, and 50 patients had falcine/parasagittal tumors. Temporary complications were observed in 6.2% of patients and all resolved with steroid therapy. Age was the only variable shown to significantly increase the risk of temporary complications with each year of age above a mean of 59 years, resulting in an 8% increase in complication rate. Permanent complications were seen in only 2.3% of patients, and all 5 patients had malignant tumors. These patients were all treated with a combination of high-dose fractionated radiotherapy in addition to radiosurgery, with the high combined dose likely contributing to the increased complication rate.
LINAC-SRS series by Chuang and colleagues23 and Deinsberger and colleagues24 reported results of radiosurgery for skull-base meningiomas of 5.68 and 5.9 cc volume, respectively. In the study by Chuang and colleagues, 43 patients with skull-base meningiomas were treated with LINAC-SRS using a median dose of 16 Gy. The median follow-up was 74.5 months and the 7-year local control rate was 89.7%. For patients receiving SRS alone as primary treatment, the 7-year local control rate was 100%. Reduction in tumor volume following LINAC-SRS was observed in 37.2% of patients. A 7% late complication rate was noted in this study including a patient with new left hemiparesis after treatment of a portion of brain stem to 16.2 Gy. Deinsberger and colleagues reported on the outcome of LINAC-SRS for 37 patients with skull-base meningioma.24 With 66-month median follow-up, a 97.2% local control rate was achieved. A decrease in tumor volume was observed in 32.4% of tumors after treatment. Late complications were observed in 5.4% of patients. One patient developed a hemiparesis 8 months after LINAC-SRS for a petroclival meningioma. As in the report by Chuang, the affected patient received a 16-Gy dose to the surface of the brain stem. The hemiparesis responded to corticosteroids and only a slight residual hemiparesis was present at 2 years postradiosurgery.
LIMITATIONS OF SRS FOR MENINGIOMAS: RATIONALE FOR FSRT VERSUS SRS
Stereotactic radiosurgery may not be suitable for treating meningiomas in very close proximity to the optic nerves, chiasm, or tract. Tisher and colleagues retrospectively evaluated the incidence of optic neuropathy in patients treated with SRS for cavernous sinus tumors. In their study, 24% of patients developed optic neuropathy after a dose of greater than 8 Gy. None of the patients who received less than 8 Gy developed visual loss.32 The threshold dose derived from this study has been criticized, however, as the two-dimensional treatment planning and dosimetry used could have underestimated actual doses delivered to the optic apparatus. Leber and colleagues followed outcomes of 50 patients treated for skull-base tumors with SRS. They found that no cranial nerve complications developed from treatment of the cavernous sinus with doses as high as 30 Gy; however, there was a 26.7% risk of optic neuropathy for patients receiving 10 to 15 Gy to the visual pathway with median follow-up of 40 months.33 Stafford and colleagues retrospectively reviewed visual complications in 215 patients treated for parasellar tumors. They concluded that the volume of the optic apparatus receiving the dose was important in addition to the dose, and that short segments of optic nerve could tolerate in excess of 10 Gy. They estimated a 1.1% risk of optic neuropathy for patients administered less than or equal to 12 Gy maximum dose to brain stem.34
In some cases, prior surgery and/or chronic tumor involvement may further compromise the radiation tolerance of the optic structures. In the LINAC-based SRS series of Villavicencio and colleagues, one patient developed blindness despite a maximum radiosurgery dose of only 5 Gy. Her history was significant for four prior surgical resections. Though tumor did not progress postradiosurgery, it continued to compress the right optic nerve.27 Given the potential risk of injury from radiosurgery to tumors in close proximity to the optic nerve using therapeutic doses, and the difficulty of precisely delineating optic apparatus using either MRI or CT when involved with tumor, the use of FSRT may be preferred in such cases.35
In relation to SRS, FSRT derives a benefit from the normal tissue-sparing effects of fractionation. When comparing the normal tissue-sparing properties of FSRT to SRS, it is important to consider the biologically equivalent dose that might be delivered to an adjacent critical structure by either technique. The single-fraction threshold dose for optic nerve injury appears to be approximately 8 to 10 Gy as discussed in the preceding text.32,33,36 Given the uncertainty in dosimetric delivery and patient setup, and the steep dose gradients in radiosurgery, it would not be possible to safely prescribe a margin dose to tumor adjacent to or less than 2 to 3 mm from the optic apparatus greater than 8 to Gy without risk of exceeding the threshold dose for injury. With FSRT, the effective treatment dose for benign meningioma of 54 Gy is associated with little or no risk of optic neuropathy, thereby allowing safe treatment even of tumors directly involving the visual pathways.
One approach that has been employed to limit the risk of SRS-induced optic neuropathy to meningiomas involving the visual pathways has been to partially underdose directly adjacent tumor. Shin and colleagues reported on the outcome following Gamma Knife SRS for 40 cavernous sinus meningiomas. They showed on univariate analysis that partial treatment was associated with decreased local control (P < 0.001). Tumors that received full treatment of 14 Gy to the tumor margin showed 100% local control whereas those receiving 10 to 12 Gy to a portion of the tumor showed an 80% control rate.37 LINAC-SRS would face similar limitations on tumor coverage. Given the risk of suboptimal tumor control with partial treatment, the use of FSRT in such cases should be strongly considered.
Although SRS has shown excellent efficacy for most meningiomas less than 3.5 cm, the treatment of larger tumor volumes results in an exponential increase in normal tissue volume irradiated. The large single-radiation fractions used for SRS are not biologically sparing of normal tissue. In addition, radiosurgery using either a Gamma Knife or LINAC yields greater dose inhomogeneity than with fractionated approaches, with significantly higher central to peripheral dose ratio. Given this, it is not surprising that the use of radiosurgery for large meningiomas is associated with increased complication rates. In a study of meningiomas treated with both SRS and FSRT, Engenhart and colleagues found increased complication rates associated with single- fraction treatment of large meningiomas with field diameter of 18 to 52 mm using LINAC-SRS.38 Flickinger and colleagues evaluated outcomes for 218 patients who underwent SRS for meningiomas diagnosed on the basis of imaging. The overall rate of postradiosurgery injury at 10 years was 8.3% and showed a tendency to increase (P = 0.0537) with increasing treatment volume.39
A significant complication that may occur after SRS for meningioma is the development of acute or delayed chronic brain edema.40 In particular, patients with parasagittal meningiomas may be prone to postradiosurgery edema and should be considered for a fractionated treatment approach. In a study of 368 patients treated with Gamma Knife radiosurgery, Kollova and colleagues found the incidence of postradiosurgery edema to be increased in patients with tumor volumes greater than 10 cc.30 In a study of factors contributing to edema after SRS or hypofractionated FSRT for meningioma, Kalapurakal and colleagues studied outcomes in 43 patients. They found that all 11 patients with parasagittal tumors and 4 with nonparasagittal tumors developed symptomatic edema requiring steroid administration. The factors that significantly predisposed to edema were parasagittal location, presence of pretreatment edema, sagittal sinus occlusion, and the use of greater than 6 Gy per fraction. They concluded that for patients with risk factors for posttreatment edema, the use of FSRT with fraction sizes less than 6 Gy should be considered.41
As discussed in the preceding text, the treatment of large meningiomas with SRS is associated with increased complication rates. In addition, several studies suggest that larger meningiomas are not as well controlled by SRS, probably partly as a result of dose reductions done to compensate for the risk of normal tissue injury while treating larger or irregular tumors.42 In a multicenter review of patients treated with radiosurgery for parasagittal meningioma, Kondziolka and colleagues found that tumor size greater than 7.5 cc was predictive of local failure (77% vs. 56%, P < 0.002).43 DiBiase and colleagues evaluated factors predicting local tumor control after radiosurgery for 162 patients with benign meningiomas. They found that tumor size greater than 10 cc was the only factor significantly predictive of disease-free survival (DFS) by multivariate analysis, with 5-year DFS of 91.9% versus 68% (P = 0.038).29
A meningioma of relatively small volume may also present a challenging target for SRS owing to an irregular shape and/or the presence of sometimes large dural tails that are contiguous with the tumor mass but that may project as much as 1 to 2 cm along the dural surface. In a study by DiBiase and colleagues, on univariate analysis, the radiation coverage of the dural tail was a significant factor for local tumor control, with patients who had the dural tail within the radiosurgery treatment volume having a 5-year DFS of 96% versus 77.9% (P = 0.038). Inclusion of the dural tail in the radiation treatment volume may markedly increase the overall dimension of the tumor target, decrease the maximum conformality of dose achievable, and as a consequence, increase the amount of normal tissue included in the treatment volume. The inclusion of the dural tail in the radiosurgical target volume remains controversial; in the DiBiase study, the improvement in local control was not found on multivariate analysis and no pattern of failure information was included.44 In a study by Nagele and colleagues, 9 of 17 meningiomas evaluated by MRI possessed a dural tail. Of these, 4 were available for pathologic review. None of the 4 tumors evaluated showed evidence of pathologic involvement by tumor beyond 2 mm from the tumor mass.45 While the significance and character of the dural tail remain controversial, for tumors with highly irregular volumes or extensive dural tails, FSRT may be a preferable option if only to decrease the risk of normal tissue injury incurred by decreased dose conformality.
FSRT VERSUS EBRT
The central rationale of LINAC-based FSRT is that it offers the proven efficacy of EBRT, the normal tissue-sparing biological advantage of dose fractionation, and the steep dose gradients in normal tissue of SRS but with greater dose homogeneity within the tumor volume. For benign meningiomas, volumes in excess of 10 cm maximal dimension may be treated with homogeneous dose utilizing a single isocenter. Both external beam radiotherapy and FSRT employ similar doses for benign meningiomas of 50 to 54 Gy. In comparison to radiosurgery, the fractionated dose of 50 to 54 Gy appears to have similar clinical efficacy. When compared using the linear-quadratic formulation to estimate biologically equivalent dose, and using an α/β ratio of 2 for benign meningioma, the dose of 50 to 54 Gy fractionated in 1.8- to 2-Gy fractions is radiobiologically equivalent to the SRS dose range of 12 to 16 Gy used in the radiosurgery series reported earlier.46 Given that benign meningiomas are noninvasive and well delineated by contrast-enhanced MRI or CT, the more conformal dose delivery of FSRT would not be expected to result in a decrease in tumor control relative to EBRT.
With effective patient immobilization and precise dose delivery, substantial improvement in normal tissue sparing is possible relative to conventional radiation treatment. To ensure reproducible patient position, it was essential to develop new methods of patient immobilization. These include bite-block type immobilization devices,13,47 and rigid thermoplastic masks.48 Patients are immobilized in these fixation devices and undergo a CT scan with a fiducial apparatus attached to the mask. This image set is then imported to the treatment planning environment and the stereotactic coordinates of the tumor target are derived.15 The newest approach to FSRT is to combine near-real time patient stereoscopic X-ray imaging with limited immobilization using thermoplastic masks promising improved accuracy over existing stereotactic systems.19,20
OUTCOMES OF LINAC-FSRT FOR MENINGIOMAS
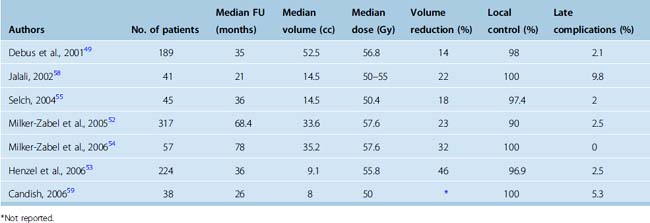
Debus and colleagues at the German Cancer Research Center reported in 2001 a retrospective study of FSRT treatment of 189 patients with skull-base meningiomas of median target volume of 52.5 cc, substantially larger than would be feasible for radiosurgery.49 Thirty-one percent of patients received FSRT as primary treatment, 26% for postoperative gross residual tumor, and 41% for recurrent tumor after initial resection. Patients were treated with 1.8-Gy fractions to mean dose of 56.8 Gy. Overall actuarial survival for patients with WHO grade I meningioma was 96% after 10 years. They found a local control rate of 98%, similar to or better than in previous EBRT series.8,50,51 In addition, they observed reduction in tumor volume of greater than or equal to 50% in 14% of patients, and stabilization in 84%. No difference in outcome was observed between those undergoing primary FSRT versus those treated postoperatively. Assessment of clinical response to treatment revealed improvement in at least one neurologic deficit in 44.8% of patients. Among patients with inoperable disease, neurologic performance improved in 38% treated with radiotherapy alone. No correlation was found between tumor volume reduction and cranial nerve response. Grade III toxicity was seen after treatment in only 2.1% of patients. No patient in this series developed blindness, though two patients had either reduced vision or a visual field deficit. Taken together, the results of this study strongly support the efficacy and safety of FSRT for both primary and postoperative treatment of large-volume skull-base meningiomas.
In 2005, Milker-Zabel and colleagues published an extension of the prior study that included non–skull-base tumors, a smaller median target volume of 33.6 cc, and extended median follow-up of 5.7 years. The overall local control rate was 93.1%, with 22.7% of patients displaying a reduction in tumor volume after treatment. As expected, those with WHO grade II meningiomas had significantly greater local failure than those with grade I tumors (P < 0.002). The recurrence-free survival rate for patients with WHO grade I meningiomas was 98.5% at 3 years, 90.5% at 5 years, and 89% at 10 years versus 96%, 89%, and 67%, respectively, for WHO grade II. They found a trend toward decreased PFS among patients treated for recurrent meningioma versus those treated as primary therapy, after biopsy or after subtotal resection (P < 0.06). Ten percent of patients treated with FSRT at recurrence developed relapse versus 4.7% of those treated in the primary setting. Larger meningiomas also displayed worse local control with tumors greater than 60 cc having a recurrence rate of 15.5% versus 4.3% for those with tumor volume less than or equal to 60 cc (P < 0.001). Clinical improvement of preexisting neurological deficits was observed in 42.9% of patients. The grade III toxicity rate was only 2.5% and grade I and II toxicity was only 8.2%.52 In contrast, the rate of severe toxicity such as cerebral necrosis or visual loss after EBRT with dose of 57.6 Gy was reported by Goldsmith and colleagues as 3.6%.8 None of the patients developed a secondary brain malignancy in the follow-up period after FSRT.
Henzel and colleagues53 reported their retrospective findings after FSRT for 224 patients with median follow-up of 36 months. Included among the patient population were 30 patients treated with hypofractionated SRT, and 11 treated with SRS. Forty-two percent were treated with radiation alone. They assessed the three-dimensional tumor volume shrinkage and overall; 45.7% of patients had tumor volume reduction of at least 2 mm in tumor diameter. The mean quantitative tumor volume shrinkage was 26.2% at 12 months and 30.3% at 18 months after treatment. They found overall survival of 92.9%. Clinical grade III toxicity occurred in only 2.5% and was edema-related, resolving completely after steroid treatment. The authors propose that SRT be used in lieu of SRS for tumors greater than 4 cc and less than 2 mm from critical structures.
Important studies on LINAC-SRS treatment series since 2001 are summarized in Table 53-2.
LINAC-FSRT FOR CAVERNOUS SINUS MENINGIOMAS
Given the nerve-sparing properties of FSRT, the outcomes of treatment for cavernous sinus meningiomas are of particular interest. Milker-Zabel and colleagues reported on long term (median follow-up 6.5 years) outcomes after FSRT for 57 patients with benign cavernous sinus meningiomas.54 FSRT was primary treatment in 29 patients, adjuvant following surgery in 10 patients, and at the time of recurrence in 18 patients. The median target volume was 35.2 cc and median dose 57.6 Gy. The local control rate was 100%, with 32% of patients having reduction in tumor volume. Preexisting neurologic deficits improved in 20% of patients. No patients developed objective visual decline, secondary malignancies, or hypothalamic–pituitary dysfunction.
In another series evaluating FSRT for cavernous sinus meningiomas, Selch and colleagues reported on the outcomes of 45 patients with benign cavernous sinus meningiomas treated with FSRT.55 The median tumor volume was 14.5 cc and the median dose prescribed was 50.4 Gy. With median follow-up of 36 months, the PFS was 97.4%. Tumor volume reduction was observed in 18% of patients and neurologic improvement was reported in 20% of patients. No cranial neuropathy, endocrinopathy, second malignancy, or cognitive decline was observed.
For SRS or FSRT treatment of cavernous sinus meningiomas, it is often necessary to include within the treatment volume the cavernous carotid and other vessels. Brada and colleagues reported an increased incidence of cerebrovascular accident (CVA) after EBRT for pituitary adenoma with a relative risk of 4.1 with actuarial rate of CVA of 21% at 10 years after treatment.56 In the FSRT series by Selch, a single patient out of 45 (2%) with no other cerebrovascular risk factors developed an ipsilateral CVA 6 months after FSRT for cavernous sinus meningioma. In another FSRT-cavernous sinus meningioma series by Milker-Zabel, no patients out of 57 treated developed CVA during the median follow-up of 6.5 years.54 After SRS, there appears to be a 1% to 2% incidence of vascular occlusion, typically 14 to 60 months after treatment.57 The decreased vascular volumes irradiated using SRS or FSRT may result in a lower risk of CVA than EBRT. Further studies are required to clarify the overall incidence and potential risk factors for this late complication.
CONCLUSIONS
Skull-base tumors present a significant operative challenge with high rates of morbidity and mortality even with advanced microsurgical techniques. For skull-base tumors, function-sparing subtotal resection coupled with either LINAC-SRS or FSRT promises improved patient outcomes with low risk of complications and high probability of tumor control.10 LINAC-SRS shows excellent efficacy and low toxicity for small to medium skull-base tumors. For large skull-base tumors or those involving or within 3 mm of the optic apparatus, FSRT is recommended.
For parasagittal tumors, a subtotal resection may be obligatory due to tumor involvement of the sagittal sinus. Given the relatively high long-term likelihood of recurrence after subtotal resection, especially in patients with unfavorable histologic features, adjuvant SRS or FSRT would be recommended. FSRT may be preferred over SRS for moderate to large parasagittal tumors given the increased likelihood of peritumoral edema seen after radiosurgery.30,41
[1] Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20:22-39.
[2] Dufour H., Muracciole X., Métellus P., et al. Long-term tumor control and functional outcome in patients with cavernous sinus meningiomas treated by radiotherapy with or without previous surgery: is there an alternative to aggressive tumor removal? Neurosurgery. 2001;48(2):285-294. discussion 294–6
[3] O’Sullivan M.G., van Loveren H.R., Tew J.M.Jr. The surgical resectability of meningiomas of the cavernous sinus. Neurosurgery. 1997;40:238-244. discussion 245–7
[4] Mirimanoff R.O., Dosoretz D.E., Linggood R.M., et al. Meningioma: analysis of recurrence and progression following neurosurgical resection. J Neurosurg. 1985;62:18-24.
[5] De Jesús O., Sekhar L.N., Parikh H.K., et al. Long-term follow-up of patients with meningiomas involving the cavernous sinus: recurrence, progression, and quality of life. Neurosurgery. 1996;39:915-919. discussion 919–20
[6] Taylor B.W.Jr, Marcus R.B.Jr, Friedman W.A., et al. The meningioma controversy: postoperative radiation therapy. Int J Radiat Oncol Biol Phys. 1988;15:299-304.
[7] Miralbell R., Linggood R.M., de la Monte S., et al. The role of radiotherapy in the treatment of subtotally resected benign meningiomas. J Neurooncol. 1992;13:157-164.
[8] Goldsmith B.J., Wara W.M., Wilson C.B., Larson D.A. Postoperative irradiation for subtotally resected meningiomas. A retrospective analysis of 140 patients treated from 1967 to 1990. J Neurosurg. 1994;80:195-201.
[9] Mendenhall W.M., Morris C.G., Amdur R.J., et al. Radiotherapy alone or after subtotal resection for benign skull base meningiomas. Cancer. 2003;98:1473-1482.
[10] Black P.M., Villavicencio A.T., Rhouddou C., Loeffler J.S. Aggressive surgery and focal radiation in the management of meningiomas of the skull base: preservation of function with maintenance of local control. Acta Neurochir (Wien). 2001;143:555-562.
[11] Lunsford L.D., Flickinger J., Lindner G., Maitz A. Stereotactic radiosurgery of the brain using the first United States 201 cobalt-60 source gamma knife. Neurosurgery. 1989;24:151-159.
[12] Lutz W., Winston K.R., Maleki N. A system for stereotactic radiosurgery with a linear accelerator. Int J Radiat Oncol Biol Phys. 1988;14:373-381.
[13] Gill S.S., Thomas D.G., Warnington A.P., Brada M. Relocatable frame for stereotactic external beam radiotherapy. Int J Radiat Oncol Biol Phys. 1991;20:599-603.
[14] Pirzkall A., Carol M., Lohr F., et al. Comparison of intensity-modulated radiotherapy with conventional conformal radiotherapy for complex-shaped tumors. Int J Radiat Oncol Biol Phys. 2000;48:1371-1380.
[15] Kooy H.M., Nedzi L.A., Loeffler J.S., et al. Treatment planning for stereotactic radiosurgery of intra-cranial lesions. Int J Radiat Oncol Biol Phys. 1991;21:683-693.
[16] Yu C., Luxton G., Jozsef G., et al. Dosimetric comparison of three photon radiosurgery techniques for an elongated ellipsoid target. Int J Radiat Oncol Biol Phys. 1999;45:817-826.
[17] Perks J.R., St. George E.J., El Hamri K., et al. Stereotactic radiosurgery XVI: Isodosimetric comparison of photon stereotactic radiosurgery techniques (gamma knife vs. micromultileaf collimator linear accelerator) for acoustic neuroma–and potential clinical importance. Int J Radiat Oncol Biol Phys. 2003;57:1450-1459.
[18] Kamath R., Ryken T.C., Meeks S.L., et al. Initial clinical experience with frameless radiosurgery for patients with intracranial metastases. Int J Radiat Oncol Biol Phys. 2005;61:1467-1472.
[19] Verellen D., Soete G., Linthout N., et al. Quality assurance of a system for improved target localization and patient set-up that combines real-time infrared tracking and stereoscopic X-ray imaging. Radiother Oncol. 2003;67:129-141.
[20] Chang S.D., Main W., Martin D.P., et al. An analysis of the accuracy of the CyberKnife: a robotic frameless stereotactic radiosurgical system. Neurosurgery. 2003;52:140-146. discussion 146–7
[21] Ryken T.C., Meeks S.L., Pennington E.C., et al. Initial clinical experience with frameless stereotactic radiosurgery: analysis of accuracy and feasibility. Int J Radiat Oncol Biol Phys. 2001;51:1152-1158.
[22] Otto K., Fallone B.G. Frame slippage verification in stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 1998;41:199-205.
[23] Chuang C.C., Chang C.N., Tsang N.M., et al. Linear accelerator-based radiosurgery in the management of skull base meningiomas. J Neurooncol. 2004;66:241-249.
[24] Deinsberger R., Tidstrand J. LINAC radiosurgery in skull base meningiomas. Minim Invasive Neurosurg. 2004;47:333-338.
[25] Hakim R., Alexander 3rd E., Loeffler J.S., et al. Results of linear accelerator-based radiosurgery for intracranial meningiomas. Neurosurgery. 1998;42:446-453. discussion 453–4
[26] Shafron D.H., Friedman W.A., Buatti J.M., et al. Linac radiosurgery for benign meningiomas. Int J Radiat Oncol Biol Phys. 1999;43:321-327.
[27] Villavicencio A.T., Black P.M., Shrieve D.C., et al. Linac radiosurgery for skull base meningiomas. Acta Neurochir (Wien). 2001;143:1141-1152.
[28] Friedman W.A., Murad G.J., Bradshaw P., et al. Linear accelerator surgery for meningiomas. J Neurosurg. 2005;103:206-209.
[29] DiBiase S.J., Kwok Y., Yovino S., et al. Factors predicting local tumor control after gamma knife stereotactic radiosurgery for benign intracranial meningiomas. Int J Radiat Oncol Biol Phys. 2004;60:1515-1519.
[30] Kollová A., Liscák R., Novotný J.Jr, et al. Gamma Knife surgery for benign meningioma. J Neurosurg. 2007;107:325-336.
[31] Malik I., Rowe J.G., Walton L., et al. The use of stereotactic radiosurgery in the management of meningiomas. Br J Neurosurg. 2005;19:13-20.
[32] Tishler R.B., Loeffler J.S., Lunsford L.D., et al. Tolerance of cranial nerves of the cavernous sinus to radiosurgery. Int J Radiat Oncol Biol Phys. 1993;27:215-221.
[33] Leber K.A., Bergloff J., Pendl G. Dose-response tolerance of the visual pathways and cranial nerves of the cavernous sinus to stereotactic radiosurgery. J Neurosurg. 1998;88:43-50.
[34] Stafford S.L., Pollock B.E., Leavitt J.A., et al. A study on the radiation tolerance of the optic nerves and chiasm after stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2003;55:1177-1181.
[35] Torres R.C., Frighetto L., De Salles A.A., et al. Radiosurgery and stereotactic radiotherapy for intracranial meningiomas. Neurosurg Focus. 2003;14:e5.
[36] Ove R., Kelman S., Amin P.P., Chin L.S., et al. Preservation of visual fields after peri-sellar gamma-knife radiosurgery. Int J Cancer. 2000;90:343-350.
[37] Shin M., Kurita H., Sasaki T., et al. Analysis of treatment outcome after stereotactic radiosurgery for cavernous sinus meningiomas. J Neurosurg. 2001;95:435-439.
[38] Engenhart R., Kimmig B.N., Höver K.H., et al. Stereotactic single high dose radiation therapy of benign intracranial meningiomas. Int J Radiat Oncol Biol Phys. 1990;19:1021-1026.
[39] Flickinger J.C., Kondziolka D., Maitz A.H., Lunsford L.D. Gamma knife radiosurgery of imaging-diagnosed intracranial meningioma. Int J Radiat Oncol Biol Phys. 2003;56:801-806.
[40] Eustacchio S., Trummer M., Fuchs I., et al. Preservation of cranial nerve function following Gamma Knife radiosurgery for benign skull base meningiomas: experience in 121 patients with follow-up of 5 to 9.8 years. Acta Neurochir Suppl. 2002;84:71-76.
[41] Kalapurakal J.A., Silverman C.L., Akhtar N., et al. Intracranial meningiomas: factors that influence the development of cerebral edema after stereotactic radiosurgery and radiation therapy. Radiology. 1997;204:461-465.
[42] Shaw E., Scott C., Souhami L., et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90–05. Int J Radiat Oncol Biol Phys. 2000;47:291-298.
[43] Kondziolka D., Flickinger J.C., Perez B. Judicious resection and/or radiosurgery for parasagittal meningiomas: outcomes from a multicenter review. Gamma Knife Meningioma Study Group. Neurosurgery. 1998;43:405-413. discussion 413–4
[44] Rogers L., Jensen R., Perry A. Chasing your dural tail: Factors predicting local tumor control after gamma knife stereotactic radiosurgery for benign intracranial meningiomas. In regard to DiBiase et al. (Int J Radiat Oncol Biol Phys 2004;60:1515–9). Int J Radiat Oncol Biol Phys. 2005;62:616-618. author reply 618–9.
[45] Nagele T., Petersen D., Klose U., et al. The “dural tail” adjacent to meningiomas studied by dynamic contrast-enhanced MRI: a comparison with histopathology. Neuroradiology. 1994;36:303-307.
[46] Larson D.A., Flickinger J.C., Loeffler J.S. The radiobiology of radiosurgery. Int J Radiat Oncol Biol Phys. 1993;25:557-561.
[47] Kooy H.M., Dunbar S.F., Tarbell N.J., et al. Adaptation and verification of the relocatable Gill-Thomas-Cosman frame in stereotactic radiotherapy. Int J Radiat Oncol Biol Phys. 1994;30:685-691.
[48] Alheit H., Dornfeld S., Dawel M., et al. Patient position reproducibility in fractionated stereotactically guided conformal radiotherapy using the BrainLab mask system. Strahlenther Onkol. 2001;177:264-268.
[49] Debus J., Wuendrich M., Pirzkall A., et al. High efficacy of fractionated stereotactic radiotherapy of large base-of-skull meningiomas: long-term results. J Clin Oncol. 2001;19:3547-3553.
[50] Glaholm J., Bloom H.J., Crow J.H. The role of radiotherapy in the management of intracranial meningiomas: the Royal Marsden Hospital experience with 186 patients. Int J Radiat Oncol Biol Phys. 1990;18:755-761.
[51] Peele K.A., Kennerdell J.S., Maroon J.C., et al. The role of postoperative irradiation in the management of sphenoid wing meningiomas. A preliminary report. Ophthalmology. 1996;103:1761-1766. discussion 1766–7
[52] Milker-Zabel S., Zabel A., Schulz-Ertner D., et al. Fractionated stereotactic radiotherapy in patients with benign or atypical intracranial meningioma: long-term experience and prognostic factors. Int J Radiat Oncol Biol Phys. 2005;61:809-816.
[53] Henzel M., Gross M.W., Hamm K., et al. Stereotactic radiotherapy of meningiomas: symptomatology, acute and late toxicity. Strahlenther Onkol. 2006;182:382-388.
[54] Milker-Zabel S., Zabel du Bois A., Huber P., et al. Fractionated stereotactic radiation therapy in the management of benign cavernous sinus meningiomas: long-term experience and review of the literature. Strahlenther Onkol. 2006;182:635-640.
[55] Selch M.T., Ahn E., Laskari A., et al. Stereotactic radiotherapy for treatment of cavernous sinus meningiomas. Int J Radiat Oncol Biol Phys. 2004;59:101-111.
[56] Brada M., Burchell L., Ashley S., Traish D. The incidence of cerebrovascular accidents in patients with pituitary adenoma. Int J Radiat Oncol Biol Phys. 1999;45:693-698.
[57] Barami K., Grow A., Bern S., et al. Vascular complications after radiosurgery for meningiomas. Neurosurg Focus. 2007;22:E9.
[58] Jalali R., Loughrey C., Baumert B., et al. High precision focused irradiation in the form of fractionated stereotactic conformal radiotherapy (SCRT) for benign meningiomas predominantly in the skull base location. Clin Oncol (R Coll Radiol). 2002;14:103-109.
[59] Candish C., McKenzie M., Clark B.G., et al. Stereotactic fractionated radiotherapy for the treatment of benign meningiomas. Int J Radiat Oncol Biol Phys. 2006;66(suppl 1):S3-S6.