Limbus and Corneal Epithelium
Limbal Epithelium
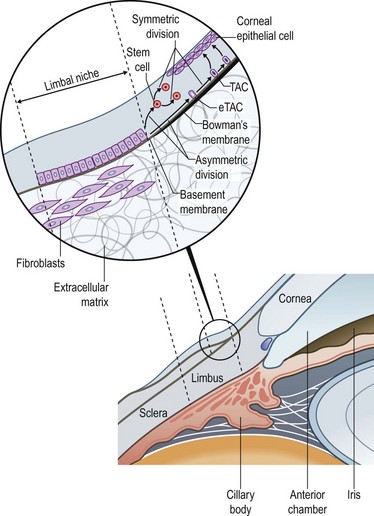
The narrow transitional zone between the corneal and bulbar conjunctival epithelium represents the limbal epithelium. However, due to the lack of distinct borders, there are various anatomic definitions of the limbus as defined by anatomists, pathologists, histologists, and surgeons. The most accepted definition delineates the inferior border of the limbus as a line between the outer border of Bowman’s layer and Descemet’s membrane, and the exterior border as the start of scleral collagen fibers and outside border of the Schlemm’s canal, 1.5 to 2 mm outside the inferior border (Fig. 5.1 ).1 This region has an important barrier function and prevents conjunctival overgrowth onto the cornea.
Histologically, the non-keratinized stratified limbal epithelium can be differentiated from the conjunctival epithelium, in that it lacks goblet cells. Compared to the corneal epithelium, while the superficial epithelial layers are rather similar, the limbal epithelium contains cell layers, a large number of mature (activated) and immature epithelial dendritic cells, T lymphocytes, highly pigmented melanocytes, and subjacent blood vessels. Moreover, the basal limbal epithelial cells are unique in that they are the least differentiated cells of the ocular surface epithelium.2 These cells are smaller, less columnar and have more cytoplasmic organelles. A growing body of evidence over the past years supports the theory that these cells are limbal epithelial stem cells (LESC), giving rise to the more differentiated corneal epithelium.2
Limbal Epithelial Stem Cells
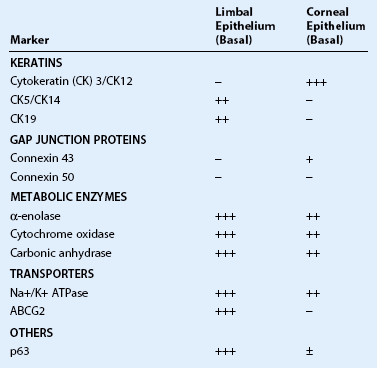
Limbal epithelial stem cells reside in the limbal niche,3 where subepithelial papillae-like structures known as palisades of Vogt are seen clinically.4 The palisades of Vogt appear as radial linear structures of about 1 mm in length as observed by slit-lamp microscopy and in vivo confocal microscopy.5 This anatomical landmark provides the homeostatic microenvironment that promotes the maintenance of limbal epithelial stem cells (LESCs) in an undifferentiated state. Currently, no single marker can be used to identify LESCs definitively, which lack terminal differentiation markers. However, LESCs can be differentiated from the corneal epithelium by several markers, including p63, vimentin, α9β1 integrin, cytokeratin (CK)19, CK5, CK14, cadherin 342, and the ATP-binding cassette subfamily G member 2 (ABCG2) transporter protein (Table 5.1). Further, LESCs lack CK3 and CK12, which are characteristic for the corneal epithelium. They are heavily pigmented in order to be protected form ultraviolet light damage. LESCs produce several metabolic enzymes and proteins at higher levels than corneal epithelial cells, such as α-enolase, cytochrome oxidase, Na+-K+ ATPase, carbonic anhydrase, and glucose transporter. The functional relevance of these enzymes and proteins are yet to be elucidated.
Differentiation of Limbal Epithelial Stem Cells to Corneal Epithelium
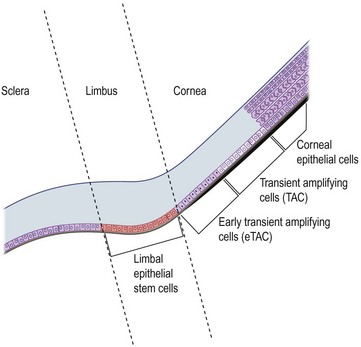
Although LESCs are slowly cycling and divide only occasionally, they have high proliferative and self-renewal capacity.3 Due to their slow cell cycling, they have a higher retention of DNA precursor analogs. However, in the event of injury, LESCs begin rapid proliferation. In order to retain a constant stem cell pool, LESCs undergo two types of cell division: a symmetric and asymmetric division (Fig. 5.1). During symmetric division, either two identical stem cells or alternatively two identical differentiated daughter cells emerge. In contrast, asymmetric division of LESCs results into a stem cell and an early transient amplifying cell (eTAC).6 These eTACs further divide and give rise to additional TACs (Fig. 5.2). TACs finally migrate centripetally towards the corneal center, ultimately forming the terminally differentiated corneal epithelial cells. This terminal differentiation of TACs into corneal epithelial cells is accompanied by specific morphological and biochemical alterations.
Limbal Niche and Limbal Epithelial Crypts
The division and differentiation processes of LESCs are strictly regulated by the microenvironment, called the limbal niche. The limbal niche is highly vascularized and innervated, and thus, provided by a potential source of nutrients and growth factors for LESCs. In addition, limbal fibroblasts in the underlying stroma secrete acidic and cysteine-rich proteins, thus contributing to LESC adhesion. More recently, the presence of limbal epithelial crypts have been demonstrated, extending from the palisades of Vogt.5,7 All cells within these crypts have been shown to be epithelial in nature as demonstrated by their CK5/14 staining. Further, an ABCG2-positive LESC population has been shown to extend along the basal epithelial cell layer of the limbus.7
Corneal Epithelium
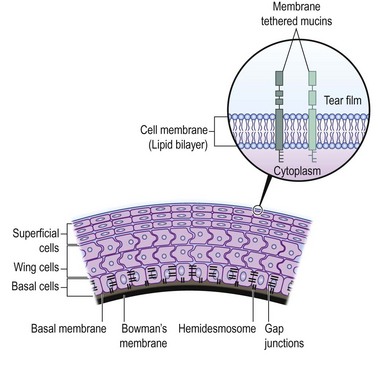
The corneal surface is covered by a non-keratinized stratified squamous epithelium and has a thickness of approximately 50 µm. The corneal epithelium is comprised of five to seven layers, consisting of superficial squamous epithelial cells, suprabasal epithelial cells with wing-like extensions, and a monolayer of columnar basal epithelial cells. Basal epithelial cells attach to the epithelial basement membrane, which is adjacent to the Bowman’s layer. The characteristics of corneal epithelial cells and their junctional complexes are shown in Table 5.2. Tight junctions (zonula occludens) play an effective barrier role and are present between the superficial cells. Desmosomes, on the other hand, are present in all layers (Fig. 5.3). Further, actin filaments, intermediate filaments, and microtubules, which form the intracellular cytoskeleton, are present in corneal epithelial cells. Cytokeratin 3 and CK12 are expressed on the corneal epithelium but not in the limbal or conjunctival epithelium. There are also immune cells within the corneal epithelium, which have a role in antigen processing. Mature and immature dendritic cells are abundant in the periphery, while immature dendritic cells are present in the central corneal epithelium, where they can now be observed with laser in vivo confocal microscopy.8 These cells capture antigen, process it, and migrate to draining lymph nodes, where they present antigens to T cells. The numbers of these cells increase dramatically in response to any kind of corneal injury.8
The corneal epithelium covers a highly organized, avascular, and transparent corneal stroma, which requires highly specialized metabolic interactions. The dense and unique neural innervation of the corneal epithelium aids and dictates its specific metabolic functions. A high density of sensory nerve endings supplies the suprabasal cells of the epithelium. This density of nerve endings per unit area is 400 times higher than the epidermal innervation, making the cornea the most innervated tissue in the body. Corneal sensory nerves contain neuropeptides, such as substance P, calcitonin gene-related peptide, and vasoactive intestinal peptide, all of which exert important trophic functions on the corneal epithelium and contribute to the maintenance and self-renewal of epithelial cells on the ocular surface.9
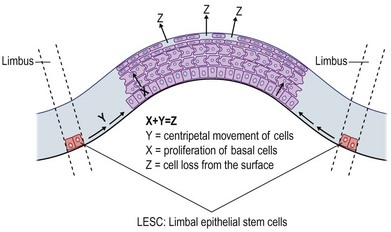
As the corneal epithelium is prone to injury, self-renewal is highly critical and imperative. The typical turnover of the epithelium lasts 5 to 7 days. As mitotically active basal epithelial cells proliferate, daughter cells begin their movement, first centripetally and then towards the corneal surface, where they first differentiate into suprabasal cells, wing-like cells, and subsequently into superficial epithelial cells. Fully differentiated squamous cells are then shed from the ocular surface. The X, Y, Z hypothesis of corneal epithelial maintenance (Fig. 5.4) by Thoft and Friend10 proposed the proliferation of basal cells (X), and the subsequent centripetal migration (Y), was equal to the shedding of superficial epithelial cells (Z). During this balance of proliferation and differentiation, both cell–cell and cell–matrix interactions occur.
Superficial Epithelial cells
Superficial epithelial cells are present at the outermost layer of corneal epithelium. These differentiated flat and polygonal cells have surface microvilli, which form microplicae. The microplicae increase the cell surface area and improve oxygen and nutrient uptake from the tear film. Further, tight junctions between neighboring cells provide a protective barrier function. Electron microscopic studies have demonstrated two types of superficial epithelial cells: dark cells, and light cells. On the one hand, there are dark cells that are larger with denser microvilli. These cells are older and tend to desquamate. Light cells, on the other hand, are younger, with lighter microvilli. Superficial epithelial cells are terminally differentiated and therefore, do not divide; thus, they contain fewer organelles than other corneal epithelial cells. A unique characteristic of superficial epithelial cells is presence of numerous glycolipid and glycoprotein molecules that are embedded into their cell membranes. These molecules form the glycocalyx particles, which attach to the mucins (MUCs) in the tear film (see Fig. 5.3), and improve tear film stability. Loss of glycocalyx particles causes tear film instability and ocular surface disease. Of the MUCs, three have been identified as major membrane-tethered mucins on the ocular surface (Table 5.3). These include MUCs 1, 4, and 16.
Table 5.3
| Molecular Weight | Functions | |
| MUC-1 | 120–300 kDa | Anti-adhesion, signaling, pathogen barrier |
| MUC-4 | 900 kDa | Signaling, maintenance of tear fluid stability |
| MUC-16 | 20 MDa | Association with cytoskeleton, pathogen barrier |
Basal Epithelial Cells
The basal epithelial cells represent a single columnar layer on a basal membrane. They are the only cells within the corneal epithelium with mitotic activity, and have more intracellular organelles compared to other epithelial cells. They have lateral membrane interdigitations that form zonula adherens, desmosomes, and gap junctions. The basal epithelial cells also regulate organization of hemidesmosomes and focal complexes, which maintain attachment to the underlying basement membrane (see Fig. 5.3). They synthesize part of the basal membrane during their life cycle and have anchoring plaques consisting of type I collagen, which span into the corneal stroma. These plaques are important for maintaining the adhesion of the corneal epithelium to the basement membrane. Further, integrins, receptors that mediate attachment between cells and the extracellular matrix are expressed on the corneal epithelium. The integrin subunits α2, α3, α5, α6, αv, β1, β4, and β5 have been demonstrated in the human corneal epithelium. Integrins play a critical role in the formation of hemidesmosomes.
Summary
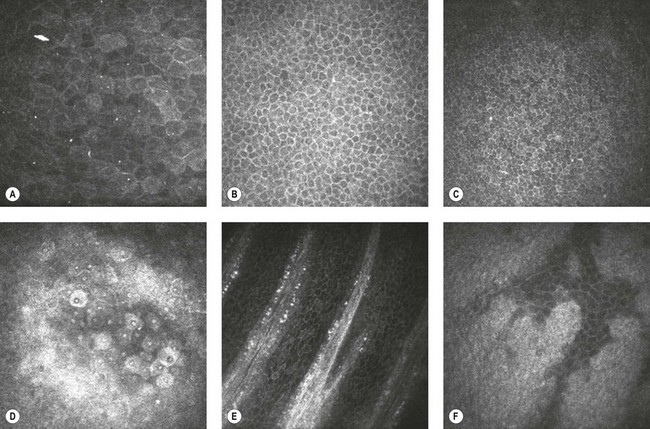
The orchestrated communication between LESCs, the limbal niche, and the corneal epithelium and stroma plays a highly significant role in the maintenance of optical clarity of the cornea, and thus, clear vision. Any insult to the cornea may compromise the LESC functionality. Limbal stem cell deficiency or insufficiency can result from both primary (e.g. aniridia) or secondary etiologies (e.g. chemical burns, Stevens–Johnson syndrome). Progressive disease will lead to persistent epithelial breakdown, superficial corneal vascularization, chronic discomfort, and vision loss. Moreover, the corneal epithelium can be affected by many ocular surface diseases (e.g. dry eye, infectious keratitis). The recent use of in vivo confocal microscopy to study the limbal and corneal epithelium in real time (Fig. 5.5) will undoubtedly advance our knowledge in the pathophysiology of ocular surface diseases. Understanding the precise pathways for differentiation and proliferation of corneal epithelial cells is critical for the development of new effective treatments.
References
1. Gipson, IK, Joyce, NC. Anatomy and cell biology of the cornea, superficial limbus, and conjunctiva. In: Albert DM, Miller JW, eds. Albert and Jakobiec’s principles and practice of ophthalmology. Canada: Saunders Elsevier; 2008:423–440.
2. Dua, HS, Azuara-Blanco, A. Limbal stem cells of the corneal epithelium. Surv Ophthalmol. 2000;44:415–425.
3. Cotsarelis, G, Cheng, SZ, Dong, G, et al. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57:201–209.
4. Davanger, M, Evensen, A. Role of the pericorneal papillary structure in renewal of corneal epithelium. Nature. 1971;229:560–561.
5. Miri, A, Al-Aqaba, M, Otri, AM, et al. In vivo confocal microscopic features of normal limbus. Br J Ophthalmol. 2012;96:530–536.
6. Castro-Munozledo, F, Gomez-Flores, E. Challenges to the study of asymmetric cell division in corneal and limbal epithelia. Exp Eye Res. 2011;92:4–9.
7. Dua, HS, Shanmuganathan, VA, Powell-Richards, AO, et al. Limbal epithelial crypts: a novel anatomical structure and a putative limbal stem cell niche. Br J Ophthalmol. 2005;89:529–532.
8. Cruzat, A, Witkin, D, Baniasadi, N, et al. Inflammation and the nervous system: the connection in the cornea in patients with infectious keratitis. Invest Ophthalmol Vis Sci. 2011;52:5136–5143.
9. Müller, LJ, Marfurt, CF, Kruse, F, et al. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76:521–542.
10. Thoft, RA, Friend, J. The X, Y, Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci. 1983;24:1442–1443.